Celecoxib preparation process
A celecoxib, reaction technology, applied in the field of medicine, can solve the problems of prolonging the reaction time, increasing the cost, increasing the operation process, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
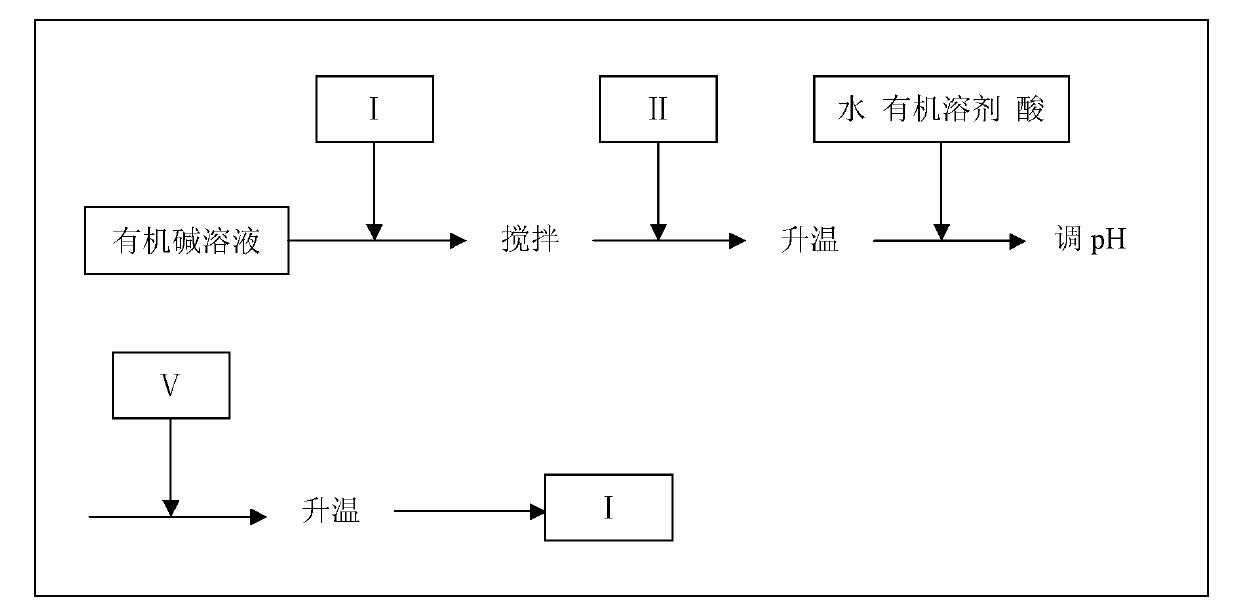
[0027] Under nitrogen protection, 80 mL of dry methanol and 1.5 g (65.2 mmol) of sodium metal were added to a four-neck flask equipped with a reflux condenser and a mechanical stirrer to prepare a sodium methoxide solution. At room temperature, 20 mL of methanol solution containing 13.9 mL (96.2 mmol) of ethyl trifluoroacetate was slowly added dropwise, after stirring, 15 mL of methanol solution containing 10.5 mL (77.0 mmol) of (III) was added, and the temperature was raised to 40°C. Slowly raise the temperature to 70°C, at this temperature, continue the reaction for 2h. Heating was stopped, and 30 mL of cold water, 10% dilute hydrochloric acid and ethyl acetate were added, and the pH=2 was measured at this time. 18.5 g (77.0 mmol) of p-aminosulfonylphenylhydrazine hydrochloride was added thereto, and the temperature was raised to reflux with stirring. After the reaction was completed, the temperature was lowered to room temperature, and an off-white solid was precipitated. ...
Embodiment 2
[0029] Under nitrogen protection, 160 mL of 20% (474.1 mmol) sodium ethoxide solution was added to a four-neck flask equipped with a reflux condenser and a mechanical stirrer. Under stirring at room temperature, 60 mL of ethanol solution containing 58.4 g (447.1 mmol) of methyl trifluoroacetate was slowly added dropwise, and after stirring for 0.5 h, 60 mL of ethanol solution containing 50.9 g (372.6 mmol) of (III) was added. Slowly raise the temperature to 90°C and react for 2h. The temperature was lowered to 30° C., and 10% dilute hydrochloric acid and ethyl acetate were added to the system, and the pH=1 was measured at this time. Add 80 g (357.0 mmol) of 4-sulfanitrophenylhydrazine hydrochloride to it, stir and heat up to reflux. After the reaction was completed, the temperature was lowered to room temperature, and an off-white solid was precipitated. The white celecoxib solid was obtained by suction filtration, dried in vacuo, and weighed 114.3 g (HPLC: I 99.05%, VI 0.95...
Embodiment 3
[0031] Under nitrogen protection, 46.8 g (323.0 mmol) of ethyl trifluoroacetate and 50 mL of tetrahydrofuran were added to a four-neck flask equipped with a reflux condenser and a mechanical stirrer. Stir and cool down to below 5°C, slowly add 130g of 60% (323.0mmol) sodium hydride, and after stirring for 0.5h, add 36.9g (270.0mmol) of (III). Stop refrigeration, rise to room temperature, and then slowly heat until the system is in a reflux state. At this temperature, the reaction was 2h. Add cold water and lower the temperature to below 30°C. 10% dilute hydrochloric acid and ethyl acetate were added to the system, and the pH=1 was measured at this time. 62.0 g (260.0 mmol) of 4-aminosulfonylnitrophenylhydrazine hydrochloride was added thereto, and the temperature was raised to reflux with stirring. After the reaction was completed, the temperature was lowered to room temperature, and an off-white solid was precipitated. The white celecoxib solid was obtained by suction fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com