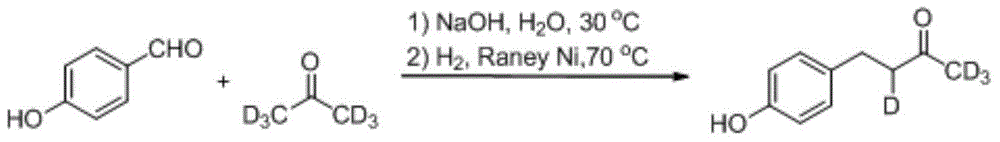Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
215 results about "Aldol reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The aldol reaction is a means of forming carbon–carbon bonds in organic chemistry. Discovered independently by the Russian chemist Alexander Borodin in 1869 and by the French chemist Charles-Adolphe Wurtz in 1872, the reaction combines two carbonyl compounds (the original experiments used aldehydes) to form a new β-hydroxy carbonyl compound. These products are known as aldols, from the aldehyde + alcohol, a structural motif seen in many of the products. Aldol structural units are found in many important molecules, whether naturally occurring or synthetic. For example, the aldol reaction has been used in the large-scale production of the commodity chemical pentaerythritol and the synthesis of the heart disease drug Lipitor (atorvastatin, calcium salt).
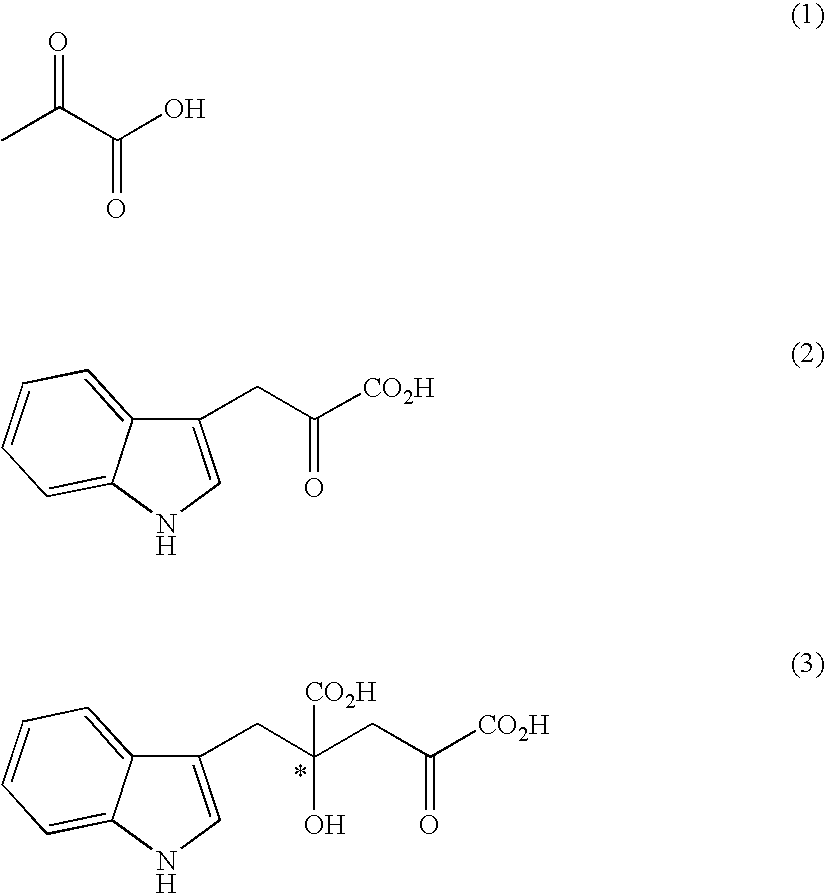
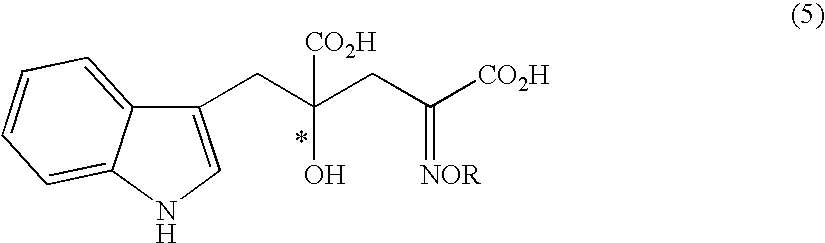
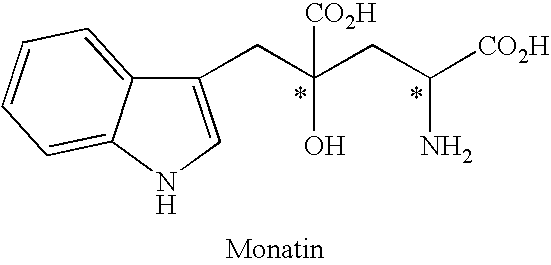
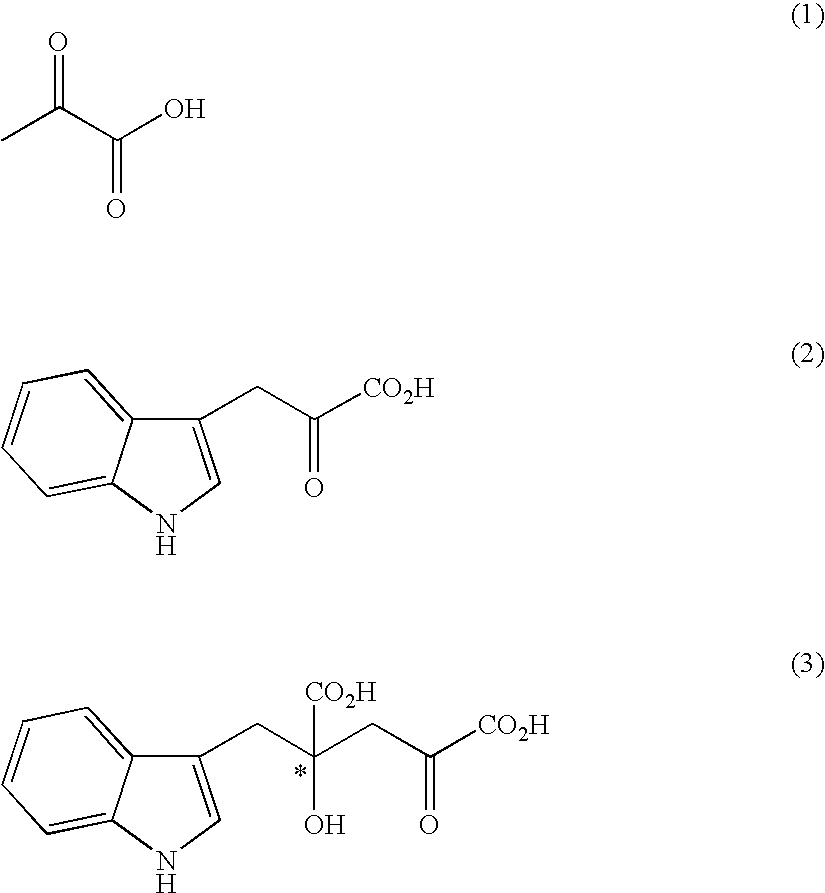
Process for producing an optically active compound
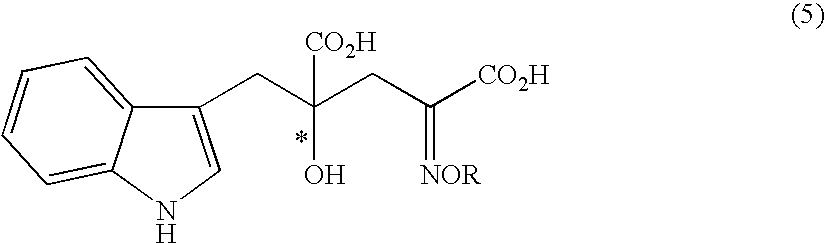
The present invention provides a process for selectively producing an enantiomer at position 4 of an optically active compound in the cross aldol reaction of pyruvic acid and indole-3-pyruvic acid. The process comprises the step of reacting pyruvic acid with indole-3-pyruvic acid in the presence of an optically active α-amino acid containing a secondary amine and a metal ion.
Owner:AJINOMOTO CO INC
Process for producing an optically active compound
Owner:AJINOMOTO CO INC
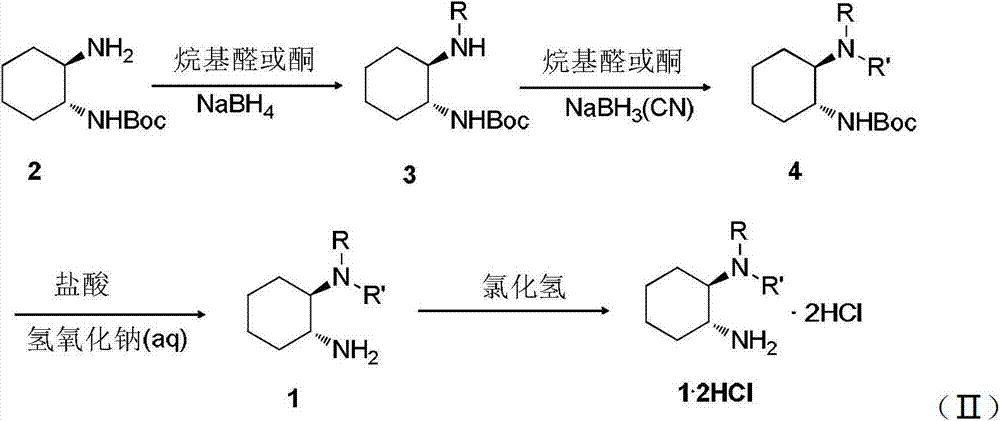
Chirality covalent organic framework and synthesis method and application thereof
ActiveCN105622579ALarge specific surface areaRegular pore structureOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsTert-Butyloxycarbonyl protecting groupSynthesis methods
The invention discloses a synthesis method of a chirality covalent organic framework. The method includes the following steps that firstly, a chirality precursor and benzenetricarboxaldehyde are catalyzed by acetic acid under the inert atmosphere to react and generate a solid product, wherein the structural formula of the chirality precursor can be seen in the description, and Boc is t-butyloxycarboryl; secondly, t-butyloxycarboryl is removed from the solid product obtained in the first step to obtain the chirality covalent organic framework material. The chirality covalent organic framework is successfully synthesized, has a large specific area and a regular pore structure, has good catalytic activity and circulation for the asymmetric Aldol reaction of ketone and aldehyde, and is a good multiphase chirality catalyst, and the application range of the molecular sieve material in the field of chirality catalysis is greatly expanded.
Owner:LANZHOU UNIVERSITY
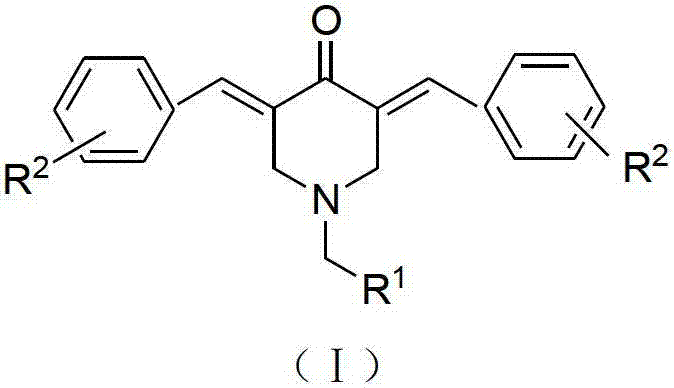
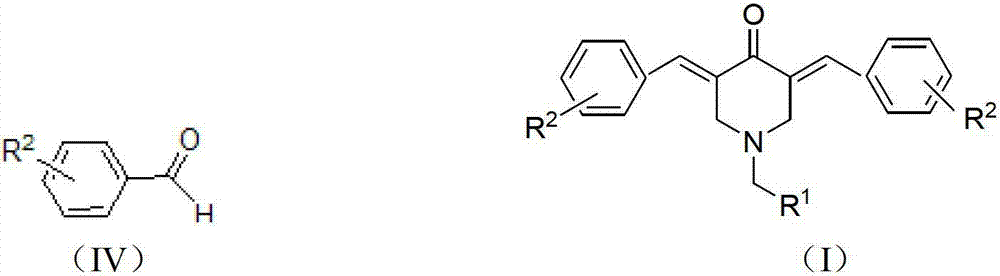
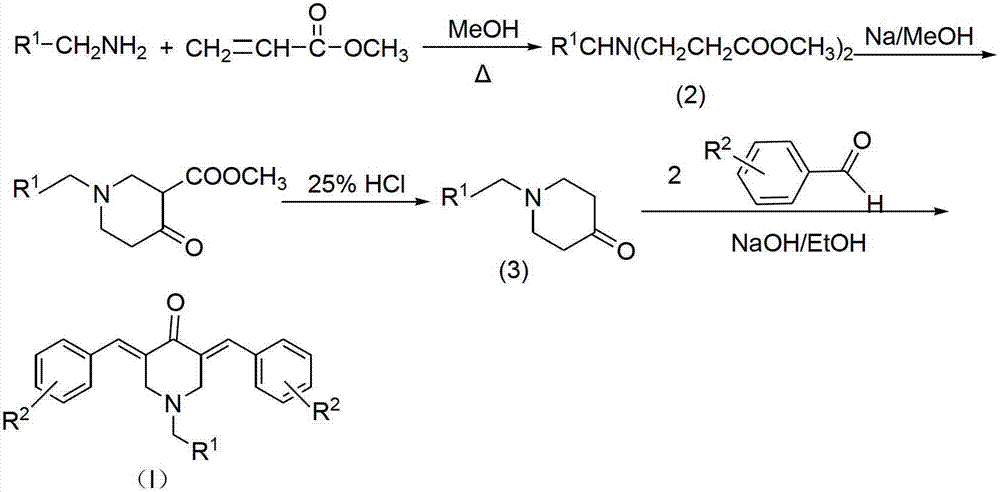
N-substituted methyl-3,5-disubstituted benzylidene base-4-piperidone and preparation method and application thereof
InactiveCN102863376AInhibit biological activitySmall side effectsOrganic chemistryAntineoplastic agentsCarcinoma cell lineCancer cell
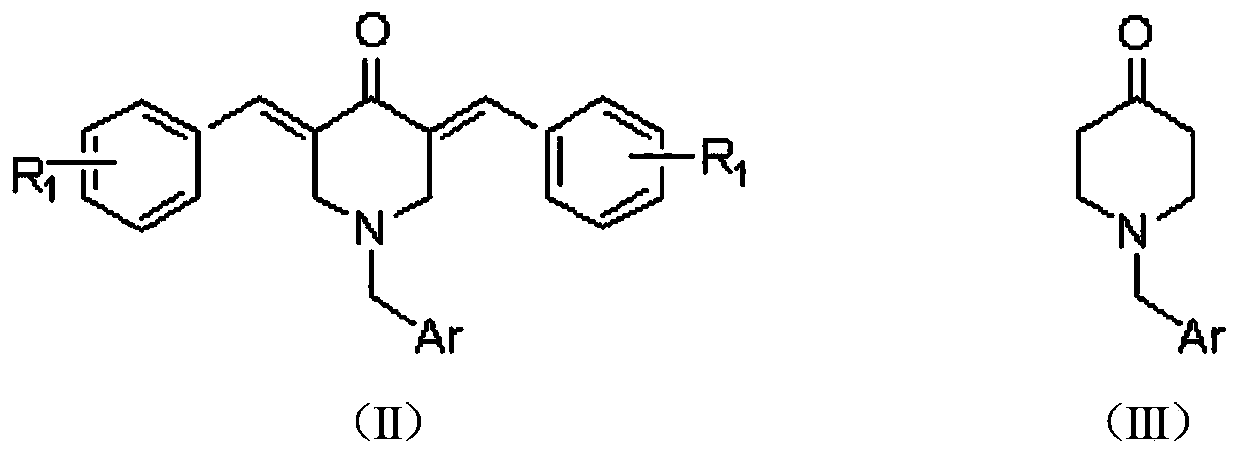
The invention relates to the field of organic synthesis and medicine, and discloses a preparation method for N-substituted methyl-3,5-disubstituted benzylidene base-4-piperidone and biological activity for efficiently inhibiting cell line proliferation such as leukemia, ovarian cancer, breast cancer, liver cancer and esophagus cancer. The method includes: starting from various substituted methylamine and methyl acrylate, sequentially going through Michael addition, Dieckmann condensation, acidolysis and decarboxylation to obtain N-substituted methyl-4-piperidone, and subjecting the N-substituted methyl-4-piperidone to aldol reaction with substituted benzaldehyde to obtain a target compound N-substituted methyl-3,5-disubstituted benzylidene base-4-piperidone. The target compound can selectively and efficiently inhibit cell line proliferation such as leukemia, ovarian cancer, breast cancer, liver cancer and esophagus cancer, and activity of inhibiting carcinoma cell line proliferation is obviously higher than conventional chemotherapeutic 5-fluorouracil.
Owner:SHANGHAI NORMAL UNIVERSITY
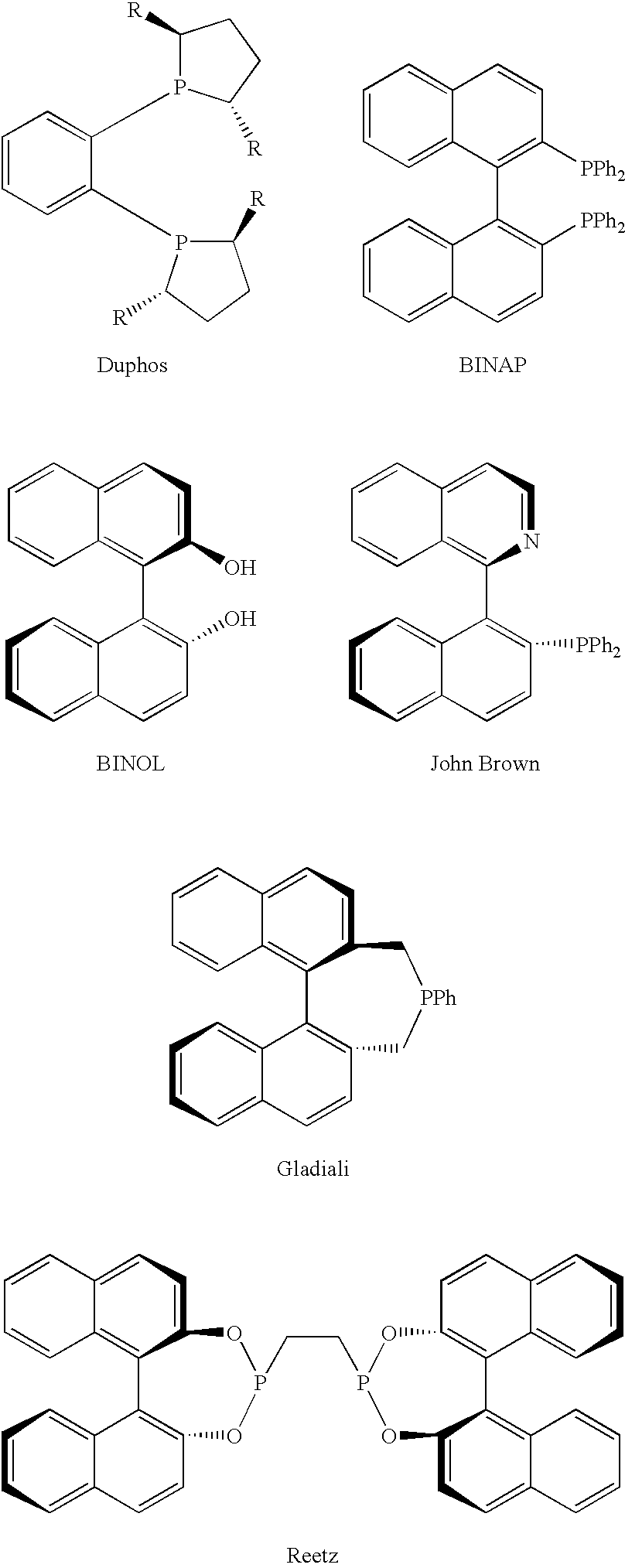
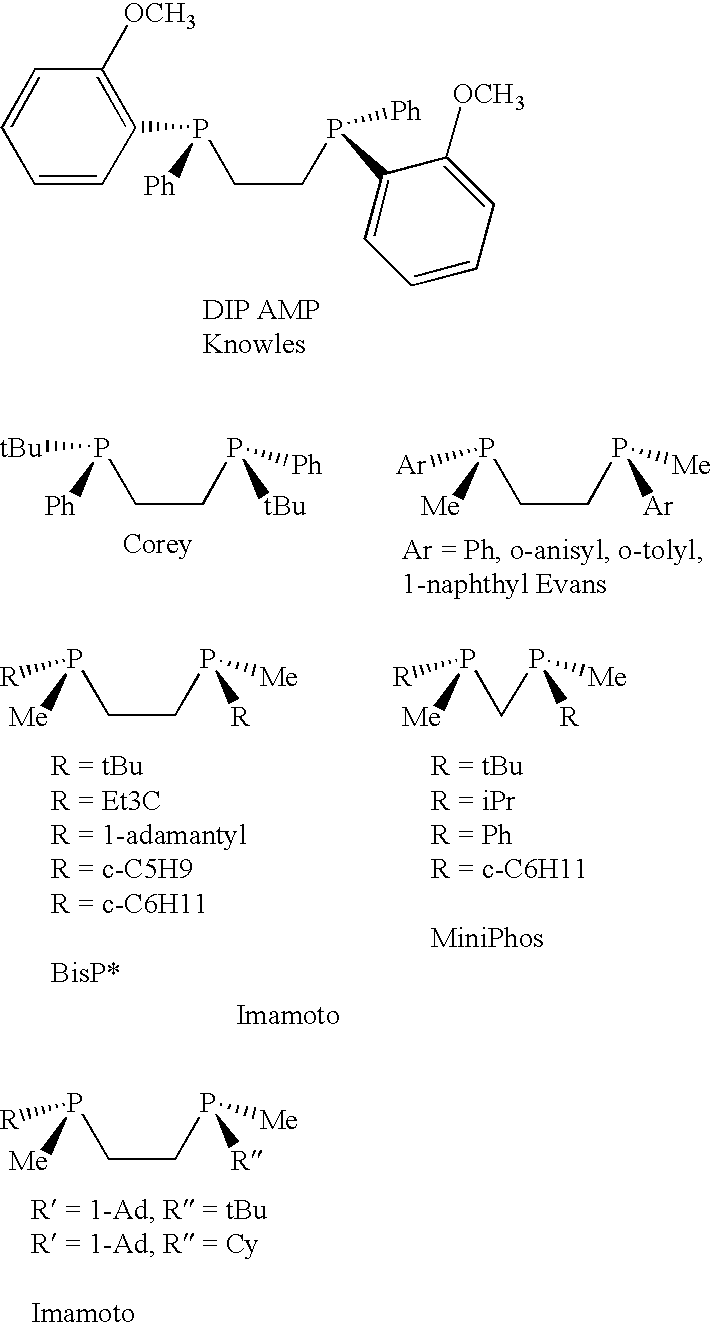
Chiral ligands, transition-metal complexes thereof and uses thereof in asymmetric reactions
InactiveUS6525210B1Carboxylic acid amides optical isomer preparationPreparation by carbon monoxide reactionIsomerizationHydrosilylation
Chiral ligands and transition metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The chiral ligands include phospholanes, P,N ligands, N,N ligands, biphenols, and chelating phosphines. The ferrocene-based irridium (R,R)-f-binaphane complex reduces imines to the corresponding amines with 95-99.6% enantioselectivity and reduces beta-substituted-alpha-arylenamides with 95% enantioselectivity. The transition metal complexes of the chiral ligands are useful in asymmetric reactions such as asymmetric hydrogenation of imines, asymmetric hydride transfer reactions, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, allylic alkylation, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition and epoxidation reactions.
Owner:PENN STATE RES FOUND
L-prolinamide derivative, preparation method and application of same
InactiveCN101891668AHigh selectivityEasy to operateCarboxylic acid nitrile preparationOrganic compound preparationHydrazine compoundTrifluoroacetic acid
The invention relates to L-prolinamide derivative of the general formula I, a preparation method and application of the same, belonging to the chemical field. The preparation method comprises following steps of: coupling N-Boc-L-Pro to mono terminal protecting (R,R)-1,2-cyclohexanediamine of the general formula II to obtain intermediate of the general formula III, processing the obtained intermediate by using trifluoroacetic acid to remove a Boc protecting group or by using hydrazine hydrate and ethanol to remove a mono terminal protecting group to obtain the L-prolinamide derivative of the general formula I. The L-prolinamide derivative can be used as the catalyst of the intermolecular asymmetric direct Aldol reaction, has the advantages of high efficiency, high selectivity, easy operation, recycling and the like and is suitable for large-scale industrial application. The invention further discloses a method for catalyzing the intermolecular asymmetric direct Aldol reaction by using the L-prolinamide derivative.
Owner:SOUTHWEST UNIVERSITY
Preparation method of 1, 3-butylene glycol
InactiveCN109422635AImprove economyTemperature controlOrganic compound preparationChemical/physical/physico-chemical microreactorsHydrogenation reactionFixed bed
The invention provides a preparation method of 1, 3-butylene glycol. The preparation method comprises following steps: A, acetaldehyde is introduced into a microchannel reactor, under the effect of abasic ionic liquid catalyst, aldol reaction is carried out so as to obtain 3-hydroxybutyraldehyde; and B, 3-hydroxybutyraldehyde is subjected to continuous hydrogenation reaction in a fixed bed reactor so as to obtain 1, 3-butylene glycol. According to the preparation method, the traditional kettle type reaction technology is improved; the microchannel reactor is adopted, at the same time, the basic ionic liquid catalyst is adopted to replace conventional liquid alkali (such as sodium hydroxide) catalysts, and in the step of hydrogenation reduction, a supported nickel hydrogenation catalyst isadopted, and hydrogenation reduction is carried out in the fixed bed reactor. The preparation method is capable of solving problems in the prior art product quality is poor, product yield is low, andtechnology process is complex; relatively high reaction conversion rate and yield are achieved; no neutralizing or desalting process is needed in reaction process; and great improvement of traditional 1, 3-butylene glycol preparation technology is realized. The prepared 1, 3-butylene glycol is colorless and smelless, is high in purity, and is capable of satisfying application requirements of highgrade industries such as cosmetic.
Owner:DONGYING HI TECH SPRING CHEM IND
Heterogeneous catalyst for catalyzing asymmetric Aldol reaction and preparation method thereof
InactiveCN105498839AOptimal Control StructureMild reaction conditionsOrganic compound preparationOrganic chemistry methodsSilicon oxidePyridine
The invention discloses a heterogeneous catalyst for catalyzing an asymmetric Aldol reaction, and comprises immobilization of a dipyridine proline derivative onto a metal modified bimodal mesoporous silicon oxide nano-material carrier. The invention discloses a preparation method of the heterogeneous catalyst. The preparation method comprises the following steps: preparing a bimodal mesoporous silicon oxide nano-material, preparing the metal modified bimodal mesoporous silicon oxide nano-material carrier, and immobilizing the dipyridine proline derivative. The immobilization of the dipyridine proline derivative and metal of the metal modified bimodal mesoporous silicon oxide nano-material carrier is carried out by coordination, so that the preparation technology is simple, cost is low, the structure of the catalyst is controllable, and the reaction condition is mild; the catalyst can be recycled and recovered, and is easy to separate.
Owner:BEIJING UNIV OF TECH
Nanogel modified by ionic liquid and loaded with chiral catalyst and preparing method and application thereof
InactiveCN105131170AHigh selectivityGood dispersionOrganic chemistryOrganic compound preparationFunctional monomerSolvent
The invention discloses nanogel modified by ionic liquid and loaded with a chiral catalyst and a preparing method and application thereof. The nanogel is characterized in that polymerization of functional monomers, a crosslinking agent, sodium dodecyl sulfate, chiral monomers and the imidazolium ionic liquid is initiated by initiator ammonium persulfate in solvent water to form a crosslinking copolymer, and the nanogel modified by the ionic liquid and loaded with the chiral catalyst is obtained through dialysis. The invention further discloses the preparing method of the nanogel modified by the ionic liquid and loaded with the chiral catalyst and application of the nanogel to an asymmetric Aldol reaction. The ionic liquid is introduced into preparation of chiral nanometer hydrogel, and a simple and effective path is provided for preparing a novel chiral catalyst which is high in catalytic activity and selectiveness, easy to separate, capable of being used repeatedly in an aqueous solution.
Owner:HENAN NORMAL UNIV
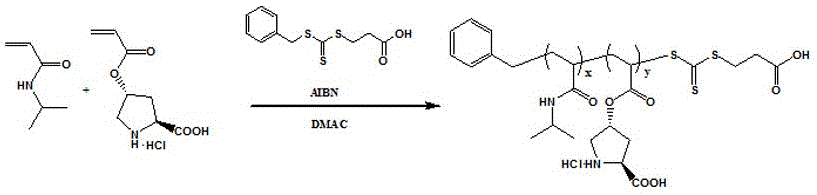
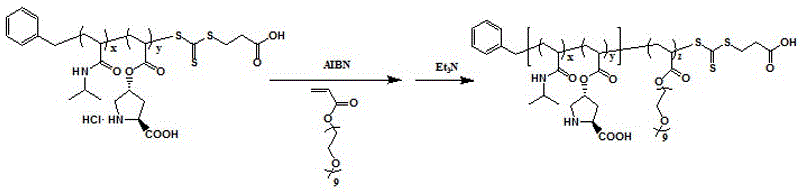
Preparation method of L-proline-loaded temperature/magnetism double responsive functional hybrid microspheres
ActiveCN106423292AIncrease local concentrationIncrease the rate of catalytic reactionsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydrophilic monomerMicrosphere
The invention discloses a preparation method of L-proline-loaded temperature / magnetism double responsive functional hybrid microspheres. The technical point is as follows: a temperature-sensitive amphipathic block polymer is obtained by polymerizing functional L-proline ProlA, a temperature-sensitive monomer N-isopropylacrylamide NIPAM and a hydrophilic monomer oligomeric ethylene glycol acrylate OEGA by virtue of a reversible addition fragmentation chain transfer (RAFT) polymerization method and is loaded to magnetic Fe3O4 nanoparticles by virtue of a ''click chemistry'' method so as to obtain the temperature / magnetism double responsive hybrid microspheres. The hybrid microspheres can be used for catalyzing direct asymmetric Aldol reaction in a water phase, and the temperature of a reaction system is higher than a lower critical solution temperature (LCST) of the block polymer, so that the improvement of the catalytic efficiency of the Aldol reaction and the release of organic products are benefited; and by virtue of the magnetism, the hybrid microspheres can be efficiently separated and recycled and repeatedly used.
Owner:TAIYUAN UNIV OF TECH
Preparation method of ordered mesoporous catalyst for synthesizing acrylic acid by acetic acid and methanol
ActiveCN105772057ARaw materials are easy to getReduce riskMolecular sieve catalystsOrganic compound preparationWater bathsActive matter
The invention discloses a preparation method of an ordered mesoporous catalyst for synthesizing acrylic acid by acetic acid and methanol. The preparation method includes synthesizing ordered mesoporous molecular sieves SBA-15 according to a hydrothermal synthesis method; dissolving sodium salt, boric acid and assistant in water and mixing evenly to obtain precursor steeping liquor; adding the carrier SBA-15 into the precursor steeping liquor for steeping, evaporating the mixed liquor in water bath to dryness, and putting the mixed liquor in a drying oven; baking the mixed liquor in a muffle furnace at the temperature of 400-600 DEG C for 2-8 hours to obtain the ordered mesoporous catalyst. The preparation method has the advantages that toxic hazard reagents or organic solvents are not used during catalyst preparation, and conditions are easy to control; raw materials are easy to get, production cost is low, and the preparation method is suitable for industrial mass production; due to well-developed ordered mesoporous passages of the prepared catalyst, the catalyst activity of acid-base thermometal active matter to aldol reaction is high, and selectivity and catalyst stability are also high.
Owner:JIANGSU SOPO CHEM +1
Chiral phosphines, transition metal complexes thereof and uses thereof in asymmetric reactions
InactiveUS6576772B1High enantioselectivityEnantioselectivity decreaseOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsIsomerizationHydrosilylation
Chiral ligands and transition metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The chiral ligands include (R,S,S,R)-DIOP*. The ruthenium complex reduces enamide to the corresponding amine with up to 99% enantioselectivity. The transition metal complexes of the chiral ligands are useful in asymmetric reactions such as asymmetric hydrogenation, hydride transfer, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, hydrocarboxylation, isomerization, allylic alkylation, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition and epoxidation reactions.
Owner:PENN STATE RES FOUND
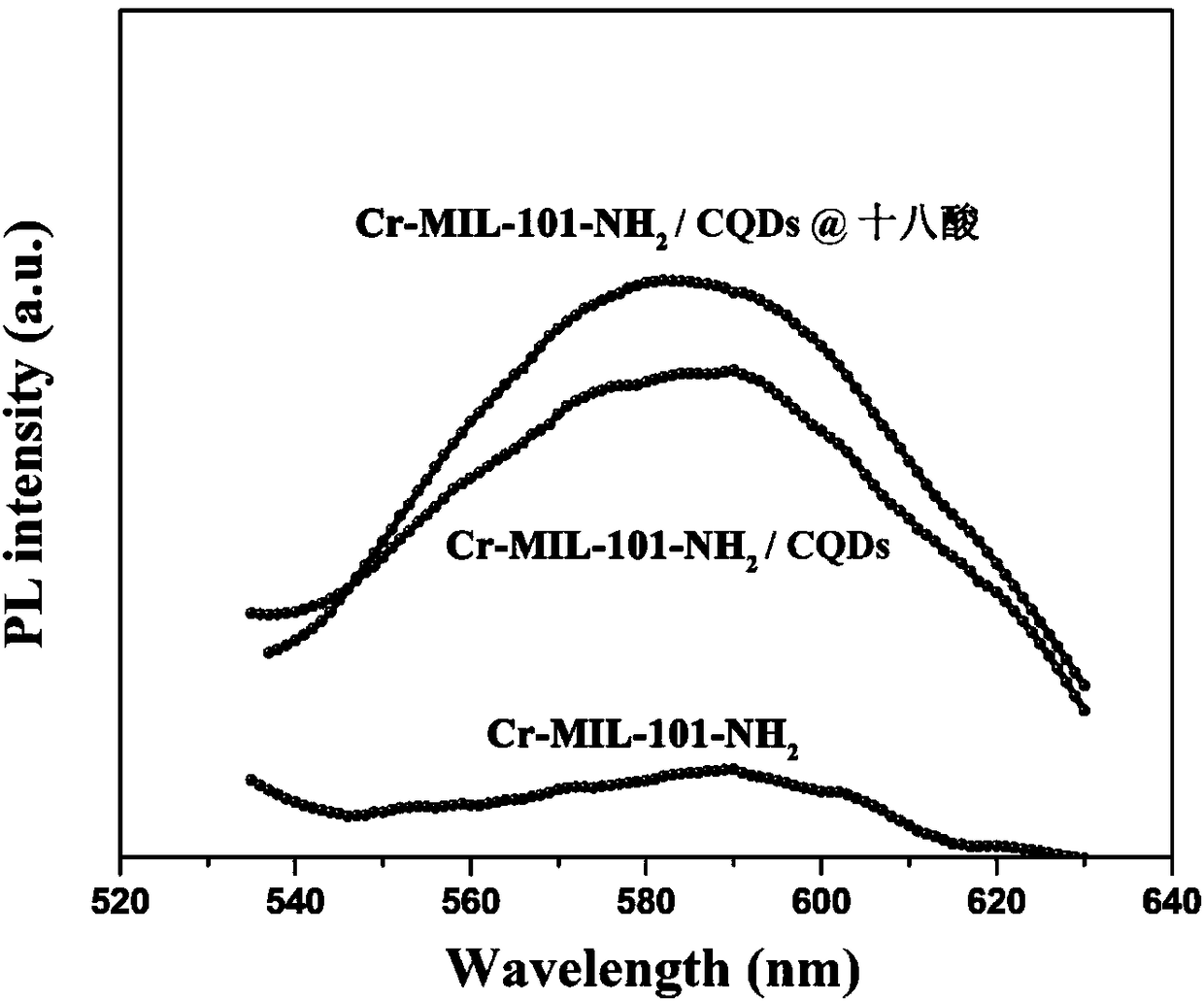
Preparation method of fluorescence phase-change material
The invention relates to a preparation method of a fluorescence phase-change material, and belongs to the field of composite phase-change materials. The method comprises the steps of firstly, at roomtemperature, utilizing soluble alkali and organic ketone or aldehyde for carrying out Aldol reaction, and preparing CQDs powder through adjusting types and contents of cross-linking agents; secondly,dispersing a certain mass of the CQDs powder into a solvent containing soluble metal salt and an organic carboxylic acid ligand, stirring at room temperature, reacting at 25 to 250 DEG C for 2 to 36h,filtering and washing for multiple times, and drying in a drying oven to obtain an MOFs / CQDs porous carrier material; finally, adopting a solution impregnation method for preparing a soluble phase-change core material into a solution, dispersing the prepared MOFs / CQDs carrier material into the prepared phase-change material solution, utilizing a pore-passage structure of MOFs / CQDs for adsorbing and limiting the phase-change core material in a pore passage, and drying at the temperature higher than the phase-change temperature to obtain a MOFs / CQDs-based fluorescence phase-change material. According to the material provided by the invention, the selection of core materials is diverse, leakage and corrosion can be effectively prevented, the pore-passage structure is adjustable, and the fluorescence phase-change material is enable to have a favorable heat performance of the phase-change material, and also has a multicolor fluorescence function.
Owner:UNIV OF SCI & TECH BEIJING
Preparation method of fatty group water reducing agent with high water-reducing rate
The invention discloses a preparation method of a fatty group water reducing agent with high water-reducing rate. The method comprises the following steps: adopting a low sulfonation degree formaldehyde-acetone prepolymerized sulfonating compound prepared at low temperature; adopting an acetone-cyclohexanone presulfonates compound sulfonated at medium temperature; automatically increasing the reaction temperature through the control of material dropwise adding sequence and speed, and carrying out aldol reaction at the high temperature at the last stage of the reaction; initiating free radical polymerization through adding slight persulfate salt, and improving the molecular weight of the product. Through the adoption of the implementation manner, the fatty group water reducing agent with high water-reducing rate can be obtained.
Owner:科之杰新材料集团河南有限公司
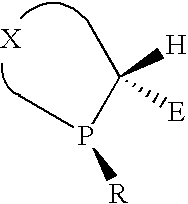
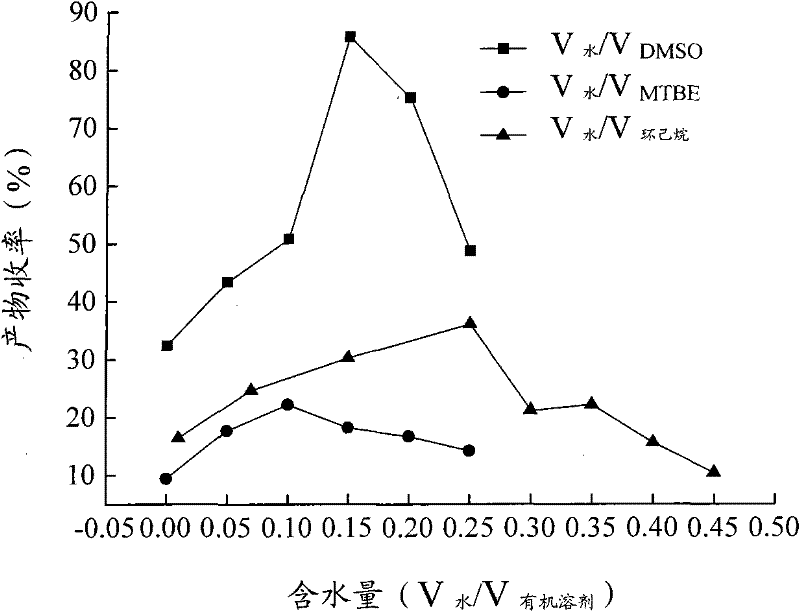
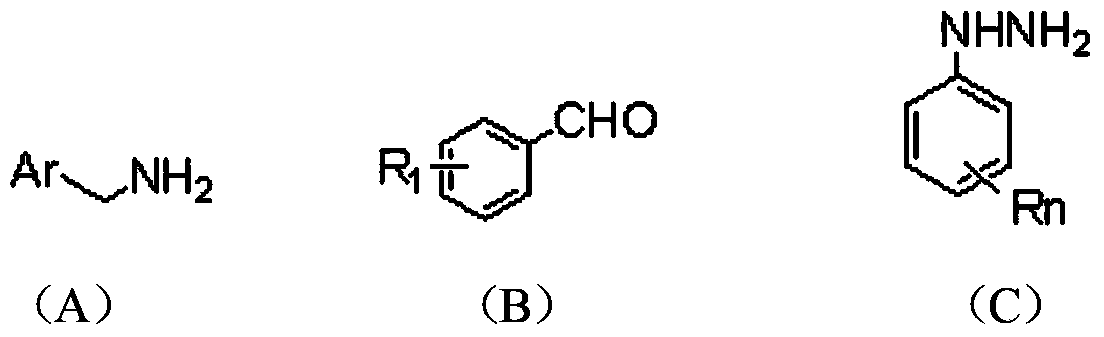
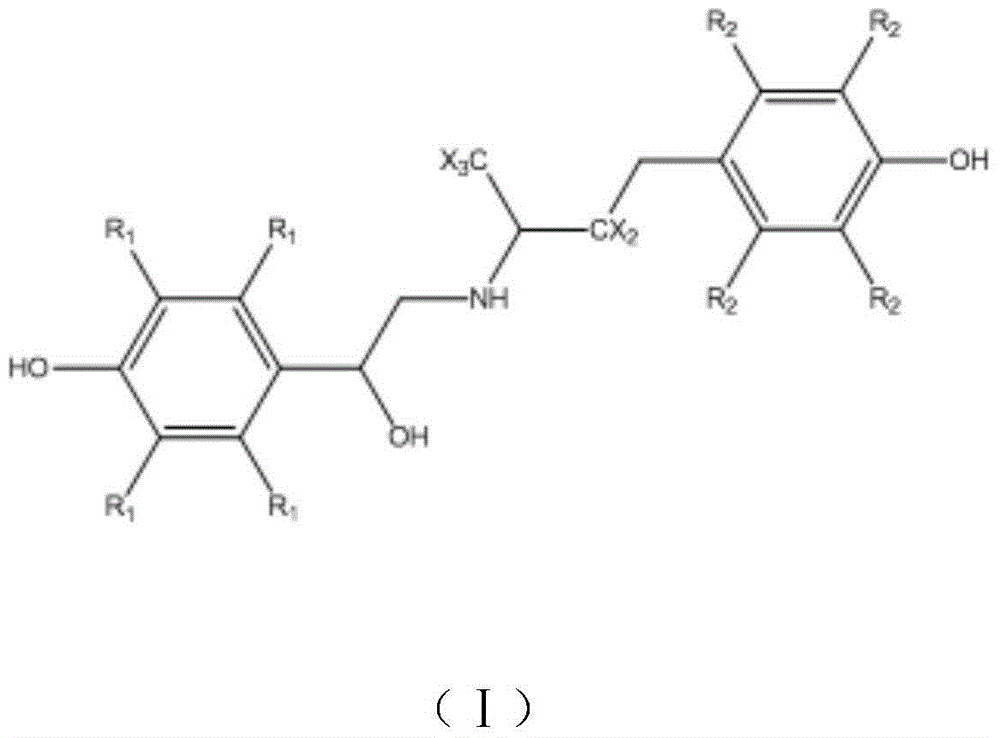
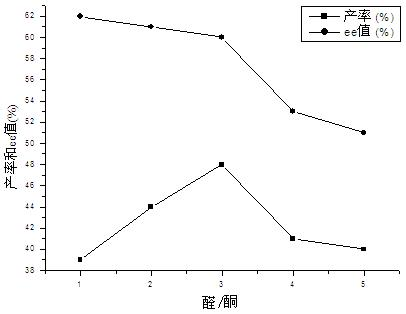
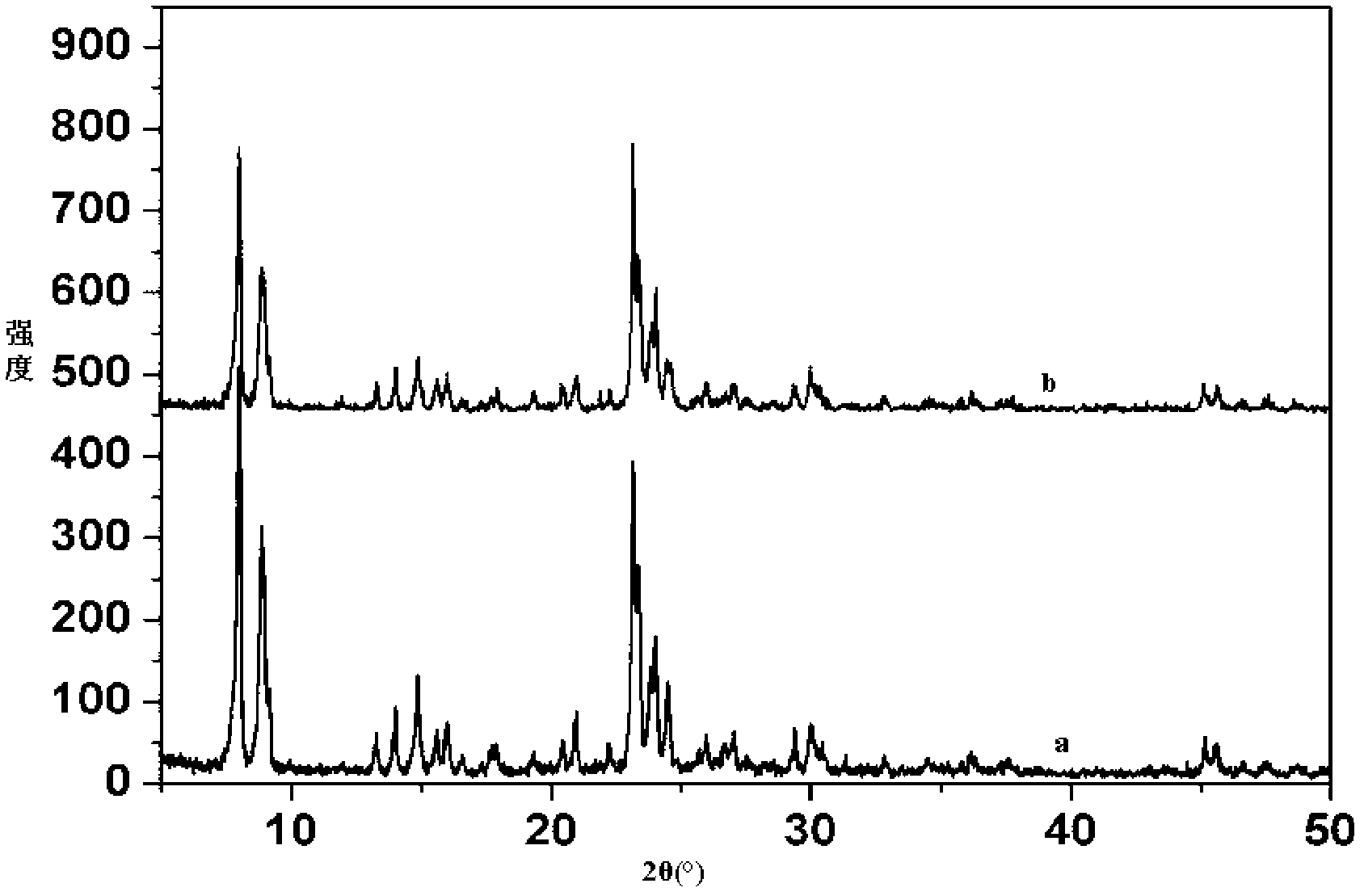
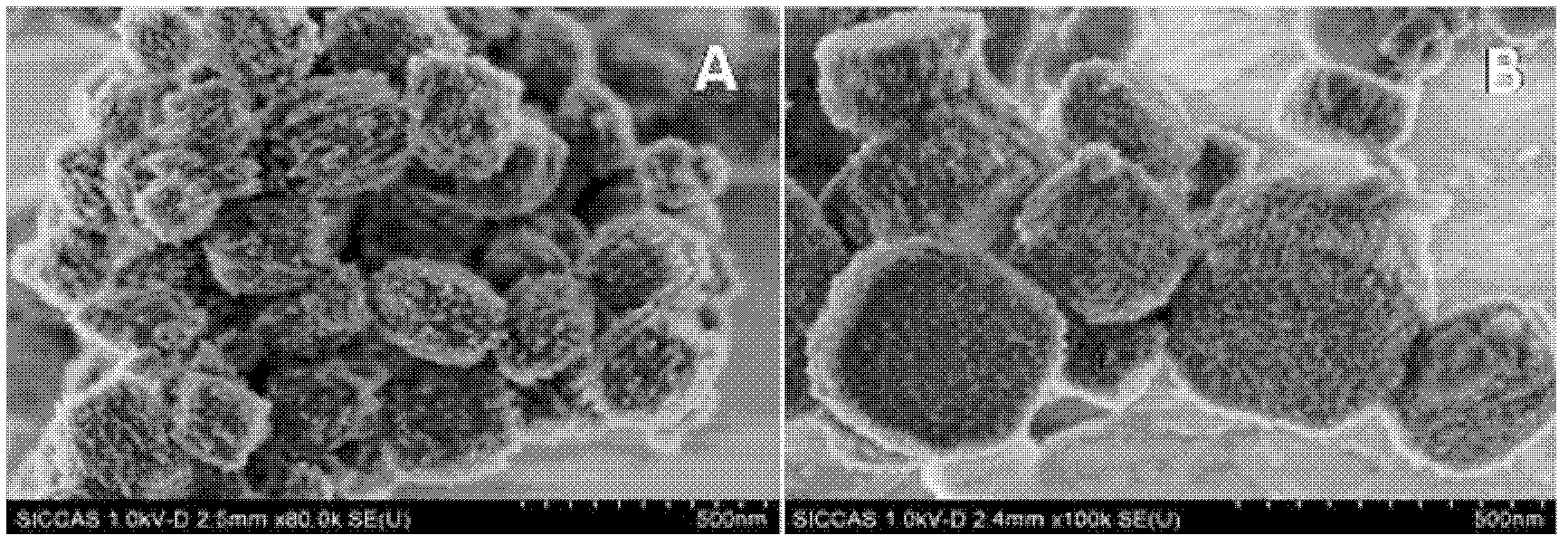
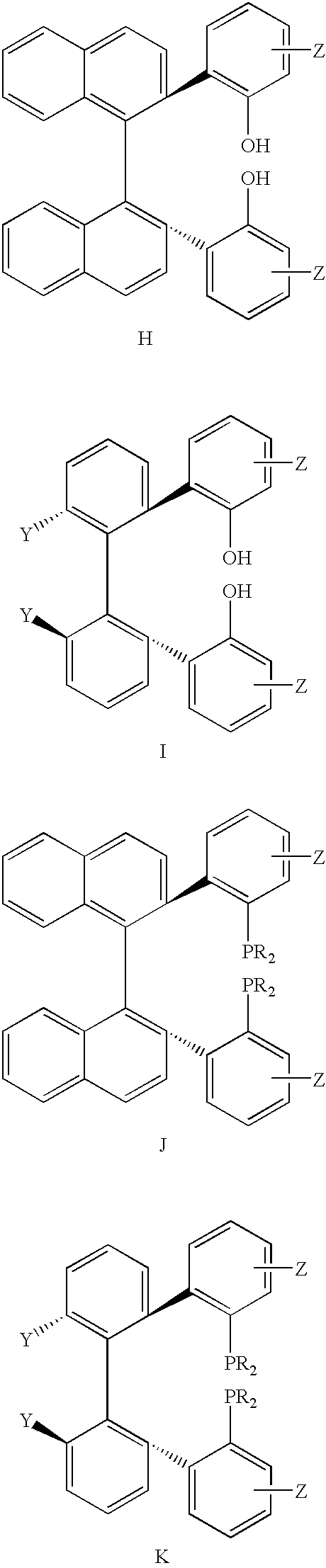
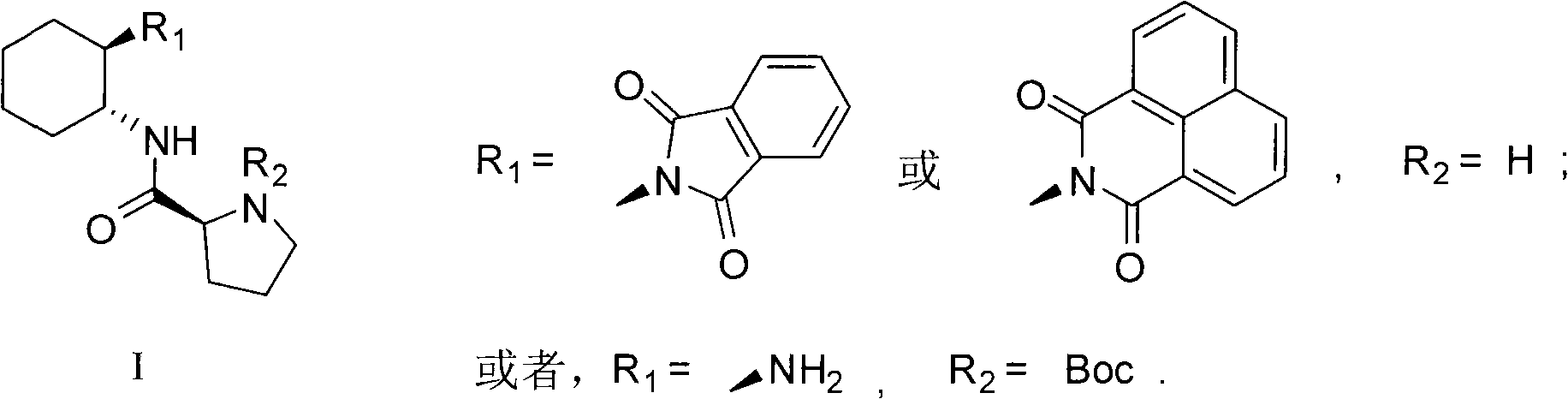
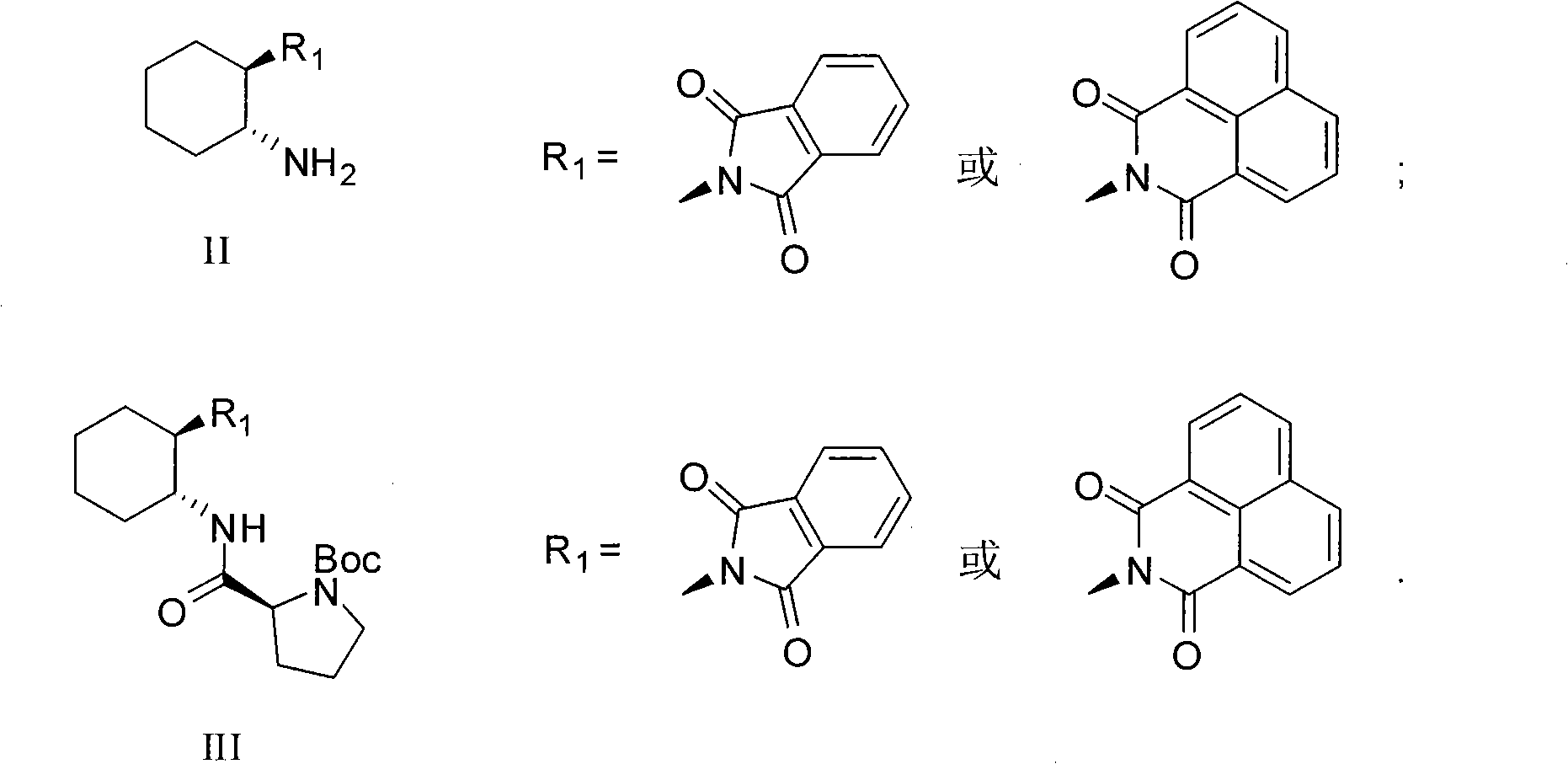
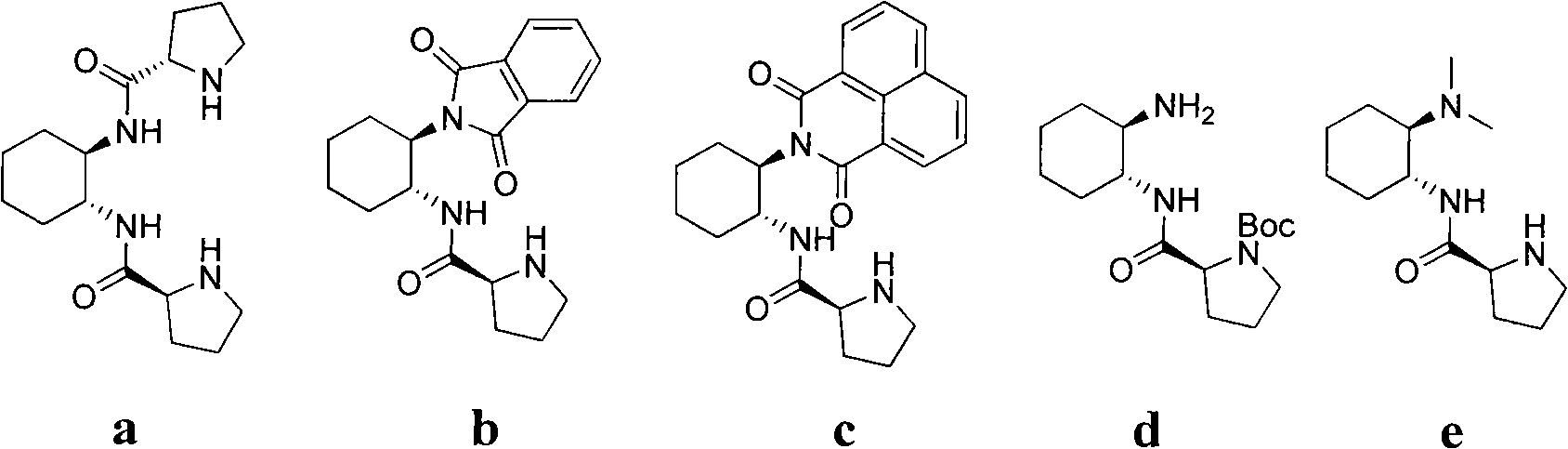
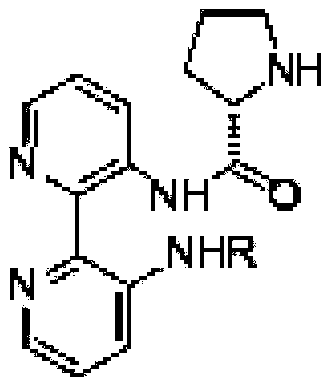
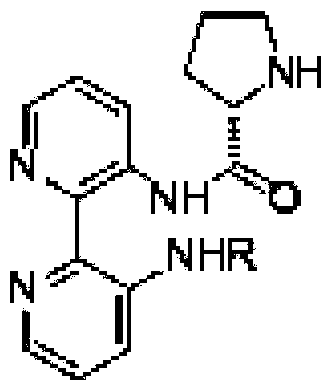
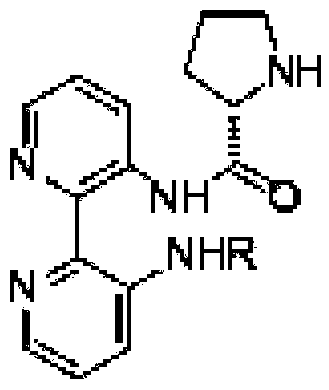
Calix [4] proline derivative and green catalytic asymmetric Aldol reaction method thereof
ActiveCN103467351AEffectively combine and exert supramolecular recognition functionBinding and exerting supramolecular recognition functionCarboxylic acid nitrile preparationOrganic compound preparationReaction temperatureSODIUM SULFATE ANHYDROUS
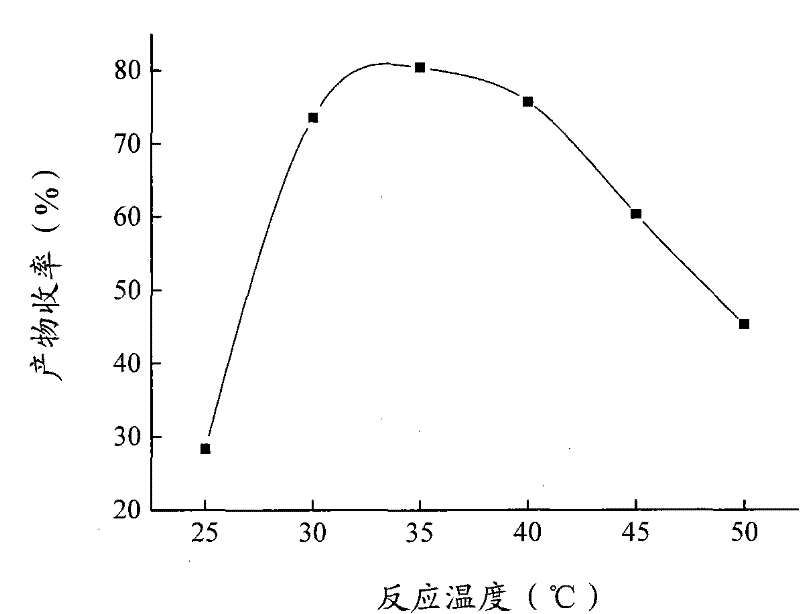
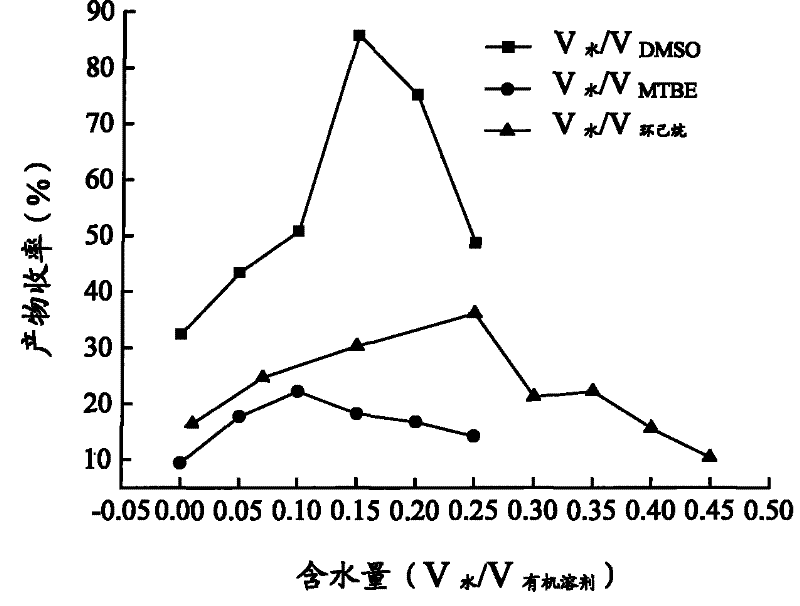
The invention relates to a calix [4] proline derivative and a green catalytic asymmetric Aldol reaction method thereof. The method comprises the steps of using aromatic aldehyde and naphthenone as raw materials, the calix [4] proline derivative as a phase transfer catalyst, and water as a solvent for Aldol catalytic reaction at 10-35 DEG C for 12-96h, adding dichloromethane after the reaction, performing extraction separation on an organic phase, washing with a saturated saline solution, drying with anhydrous sodium sulfate, and performing separation and purification through column chromatography, wherein a mole ratio of aromatic aldehyde to naphthenone is 1:(1-3); a mole ratio of aromatic aldehyde to the water is 1:(10-50); and the use level of catalyst is 1-5mol% of aromatic aldehyde. A synthesis technology of the calix [4] proline derivative is mild in condition and high in catalytic efficiency; the catalytic asymmetric Aldol reaction of the calix [4] proline derivative uses the water as the solvent and is green and environment-friendly; a good dr (anti / syn) value and a good ee (enantiomeric excess) value can be obtained by the catalytic reaction under a room temperature condition; and the calix [4] proline derivative has a wide application prospect.
Owner:枣庄市新星钢结构有限公司
P-chiral phospholanes and phosphocyclic compounds and their use in asymmetric catalytic reactions
InactiveUS7169953B2SelectiveGroup 1/11 organic compounds without C-metal linkagesAsymmetric synthesesIsomerizationCycloaddition
Owner:PENN STATE RES FOUND
Reaction device suitable for aldol reaction
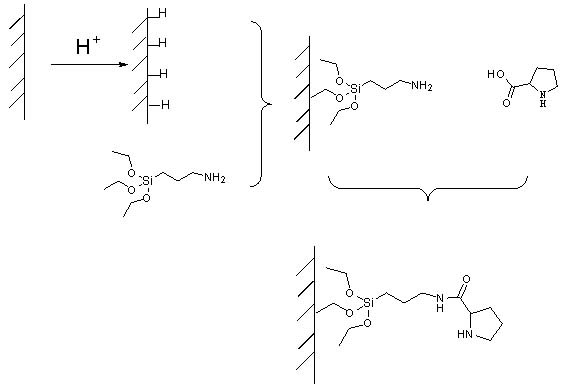
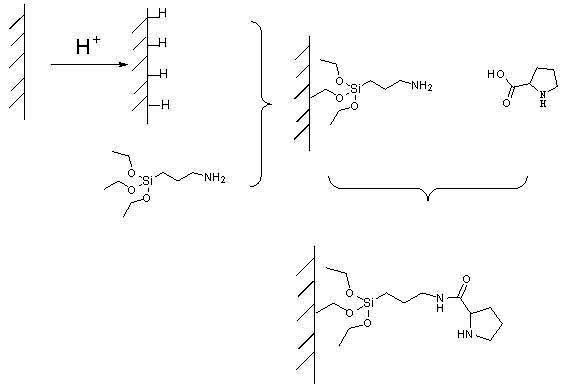
ActiveCN102101035AEasy to separateSimple methodCarboxylic acid nitrile preparationOther chemical processesStationary phaseTriethoxysilane
The invention belongs to the field of an aldol reaction device, and relates to a chromatographic column which can be used for fixing a catalytic agent on a stationary phase, wherein the chromatographic column is a reaction device suitable for aldol reaction. The reaction device is the chromatographic column, the stationary phase in the chromatographic column is silicon dioxide, the surface of thesilicon dioxide is connected with catalytic agent praline, and a coupling agent which connects the silicon dioxide and the catalytic agent praline is 3-azyl propyltriethoxysilane. The catalytic agentis fixed on the stationary phase, and mobile-phase reaction raw materials can repeatedly flow through the catalytic agent, so that the reaction can be completely performed; and the catalytic agent can not be retained in a reaction product, so that the reaction product can be conveniently separated.
Owner:SUZHOU HAOLI CULTURE MEDIA TECH CO LTD
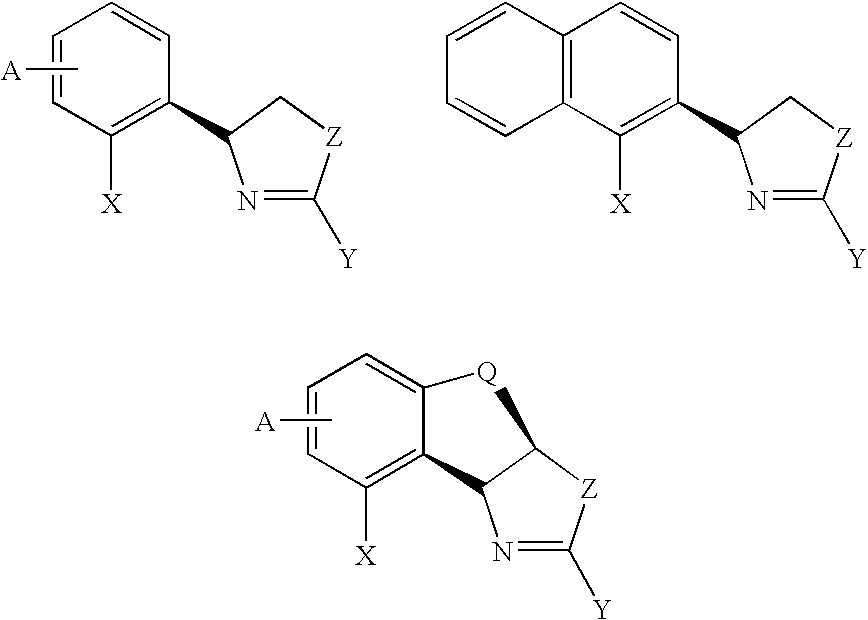
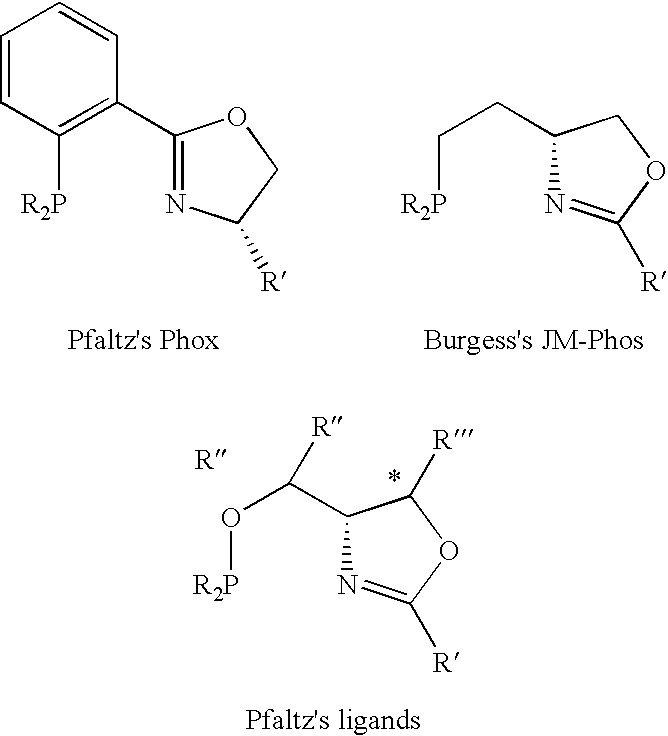
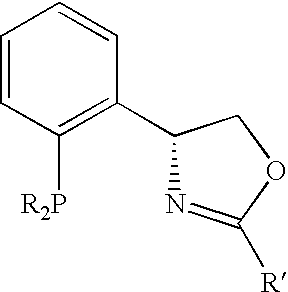
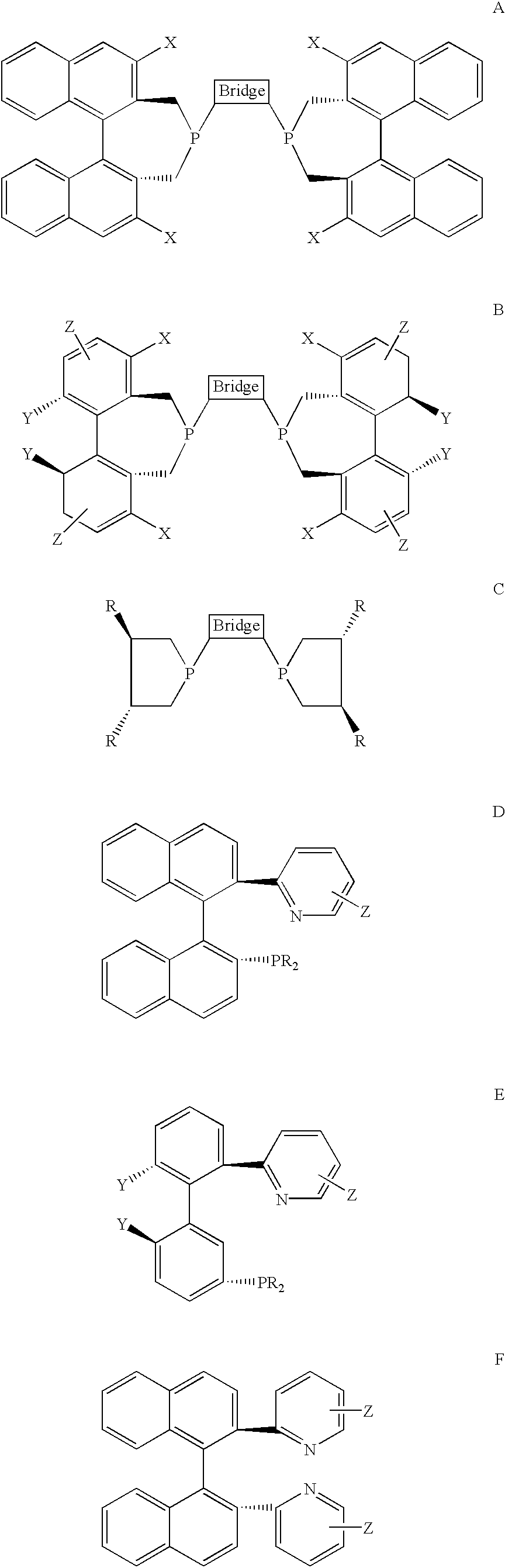
New oxazoline ligands for asymmetric catalysis
InactiveUS20050137403A1Organic compound preparationGroup 8/9/10/18 element organic compoundsEnantiomerHydrosilylation
A chiral ligand represented by the formula and its enantiomer: wherein A, X, Y and Z are as defined in the specification is provided. Also provided is a process of making the chiral ligands and catalysts prepared from these ligands and a transition metal, a salt thereof or a complex thereof. In addition, a method of preparing an asymmetric compound by a transition metal catalyzed asymmetric reaction, such as, hydrogenation, hydride transfer reaction, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, hydrocarboxylation, allylic alkylation, epoxidation, cyclopropanation, Diels-Alder reaction, Aldol reaction, ene reaction, Heck reaction and Michael addition is provided.
Owner:PENN STATE RES FOUND
Application of bacillus licheniformis alkali protease as asymmetrical direct Aldol reaction catalyst
The invention discloses application of bacillus licheniformis alkali protease as an asymmetrical direct Aldol reaction catalyst, which has the characteristics of high efficiency, high selectivity, recycle, economy, environment protection and the like and has good application prospects.
Owner:SOUTHWEST UNIVERSITY
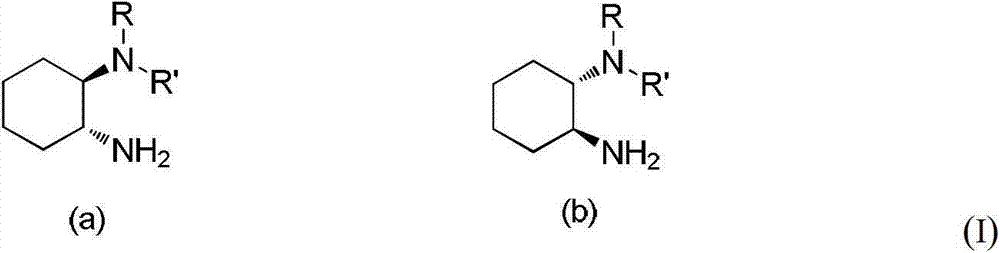
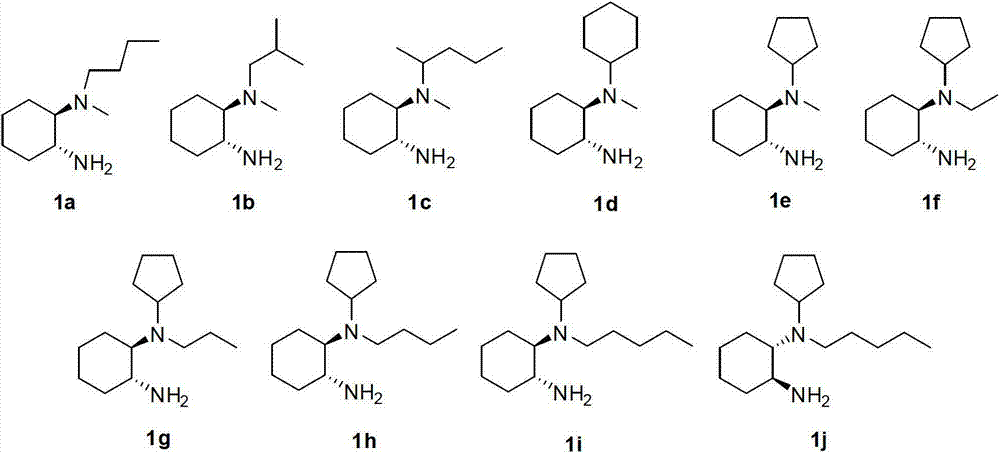
Chirality N, N-dialkyl-1, 2-diaminocyclohexane catalyst as well as preparation method and application thereof
ActiveCN103111323AHigh stereoselectivityCarboxylic acid nitrile preparationOrganic compound preparationOrganic chemistryAldehyde
The invention discloses a chirality N, N-dialkyl-1, 2-diaminocyclohexane catalyst (I), a preparation method of the catalyst and also an application of the catalyst in asymmetric aldol reaction, belonging to the technical field of catalysts.
Owner:SOUTHEAST UNIV
L-proline immobilized temperature-responsive core-shell microgel and preparation and application thereof
InactiveCN109225324AReduce agglomeration effectActivity controlOrganic chemistryOrganic compound preparationCross-link4-Hydroxy-L-proline
The invention discloses an L-proline immobilized temperature-responsive core-shell microgel. Methyl methacrylate is cross-linked with 4-hydroxy-L-proline to form a hydrophobic core microgel P (MMA-co-L-ProlA) core layer, and a temperature-responsive monomer, namely N-isopropylacrylamide, is cross-linked on the core layer to form a shell layer so as to obtain the core-shell microgel adopting a P (MMA-co-L-ProlA)@PNIPAM structure. The microgel has the L-proline immobilizing amount of 0.3-0.6mmol / g, has the number average particle size of 1.55-1.80 microns, not only can efficiently and highly selectively catalyze a direct asymmetric Aldol reaction in an aqueous phase environment, but also can be recycled through simple centrifugal separation.
Owner:TAIYUAN UNIV OF TECH
Chiral ligands, transition-metal complexes thereof and uses thereof in asymmetric reactions
InactiveUS20030163003A1Preparation by oxidation reactionsCarboxylic acid amides optical isomer preparationIsomerizationHydrosilylation
Chiral ligands and transition metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The chiral ligands include phospholanes, P,N ligands, N,N ligands, biphenols, and chelating phosphines. The ferrocene-based irridium (R,R)-f-binaphane complex reduces imines to the corresponding amines with 95-99.6% enantioselectivity and reduces beta-substituted-alpha-arylenamides with 95% enantioselectivity. The transition metal complexes of the chiral ligands are useful in asymmetric reactions such as asymmetric hydrogenation of imines, asymmetric hydride transfer reactions, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, allylic alkylation, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition and epoxidation reactions.
Owner:PENN STATE RES FOUND
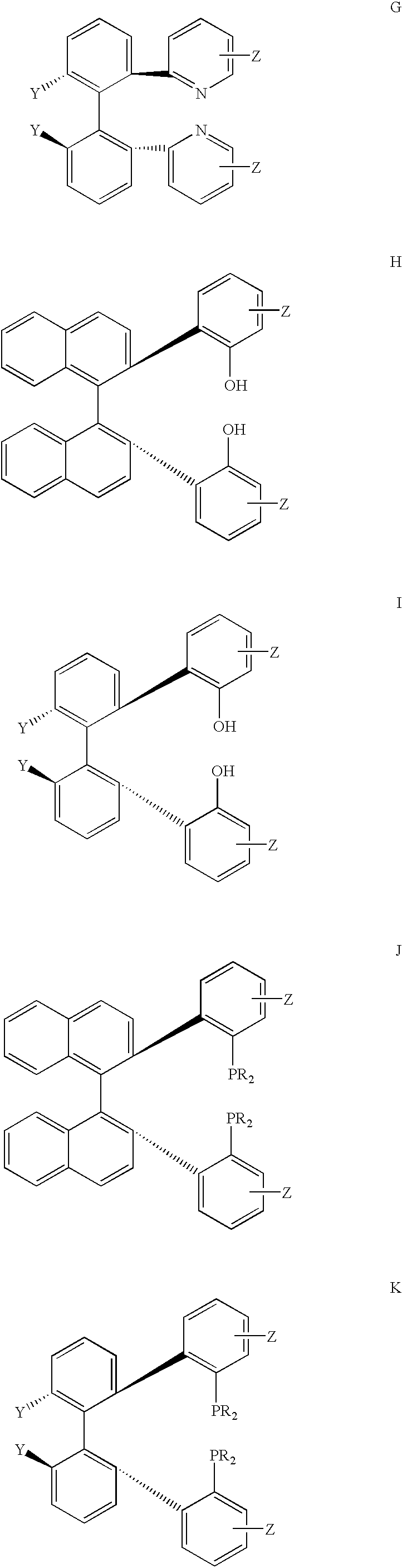
Recyclable chiral catalyst for asymmetric nitroaldol reaction and process for the preparation thereof
InactiveUS20150368181A1High yieldWithout loss yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystNitroalkene
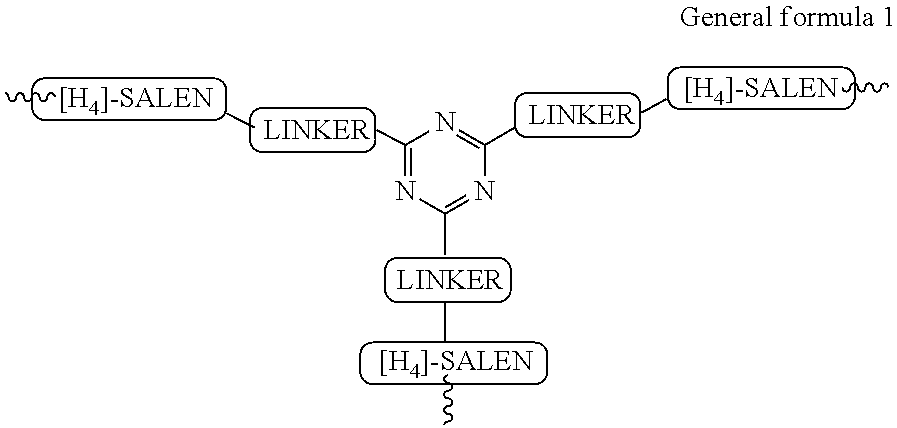
The present invention relates to preparation of highly efficient chiral recyclable homogeneous catalysts generated in situ by the reaction of chiral oligomeric [H4] ligands and a metal salt taken in 1:1 molar ratio for asymmetric nitroaldol reaction, wherein nitroaldol reactions of various aldehydes such as aromatic, aliphatic α,β-unsaturated aldehydes, alicyclic aldehydes and nitroalkenes were carried out to produce optically active β-nitroalcohols in high yield and with moderate to excellent enantioselectivity (ee up to >95%) in presence of a base and an optically active chiral recyclable homogeneous catalyst represented by the following formula (I).
Owner:COUNCIL OF SCI & IND RES
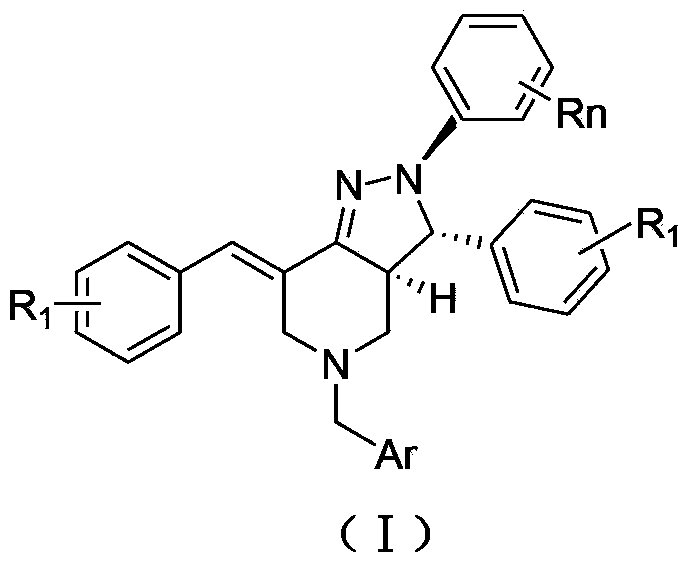
2, 3, 5, 7-tetrasubstituted dihydro-pyrazolo piperidine derivative and preparation method and application thereof
The invention provides 2, 3, 5, 7-tetrasubstituted dihydro-pyrazolo piperidine derivative and a preparation method and application thereof. The derivative is 2, 3-bis(substituted phenyl)-5-subsituted arylmethyl-7-substituted benzylidene dihydro-pyrazolo piperidine derivative, having the following formula (I). The preparation method includes using substituted arylmethyl amine and methyl acrylate as raw materials; subjecting the materials to Michael addition, Dieckmann condensation and hydrolysis-decarboxylation sequentially; allowing for Aldol reaction with substituted benzaldehyde to obtain intermediate N-substituted arylmethyl-3, 5-bis(substituted benzylidene)-4-piperidone; allowing for condensation with substituted phenylhydrazine to obtain a compound according to the formula (I). The derivative is efficient in inhibiting multiplication of various carcinoma cell lines such as leukemia, esophagus cancer, ovarian cancer and breast cancer in human, is well stably metabolic in liver microsomes of human and rat, is free of direct and competitive inhibition on five enzymes of liver microsomes, such as CYP3A4, CYP2D6, CYP2C9, CYP1A2 and CYP2C19, is highly bioavailable, is low in toxicity to normal cells, and is available for the preparation of drugs for the cancers.
Owner:SHANGHAI NORMAL UNIVERSITY
P-chiral phospholanes and phosphocyclic compounds and their use in asymmetric catalytic reactions
InactiveUS7105702B2SelectiveGroup 1/11 organic compounds without C-metal linkagesAsymmetric synthesesIsomerizationCycloaddition
Chiral ligands and metal complexes based on such chiral ligands useful in asymmetric catalysis are disclosed. The metal complexes according to the present invention are useful as catalysts in asymmetric reactions, such as, hydrogenation, hydride transfer, allylic alkylation, hydrosilylation, hydroboration, hydrovinylation, hydroformylation, olefin metathesis, hydrocarboxylation, isomerization, cyclopropanation, Diels-Alder reaction, Heck reaction, isomerization, Aldol reaction, Michael addition; epoxidation, kinetic resolution and [m+n] cycloaddition. Processes for the preparation of the ligands are also described.
Owner:PENN STATE RES FOUND
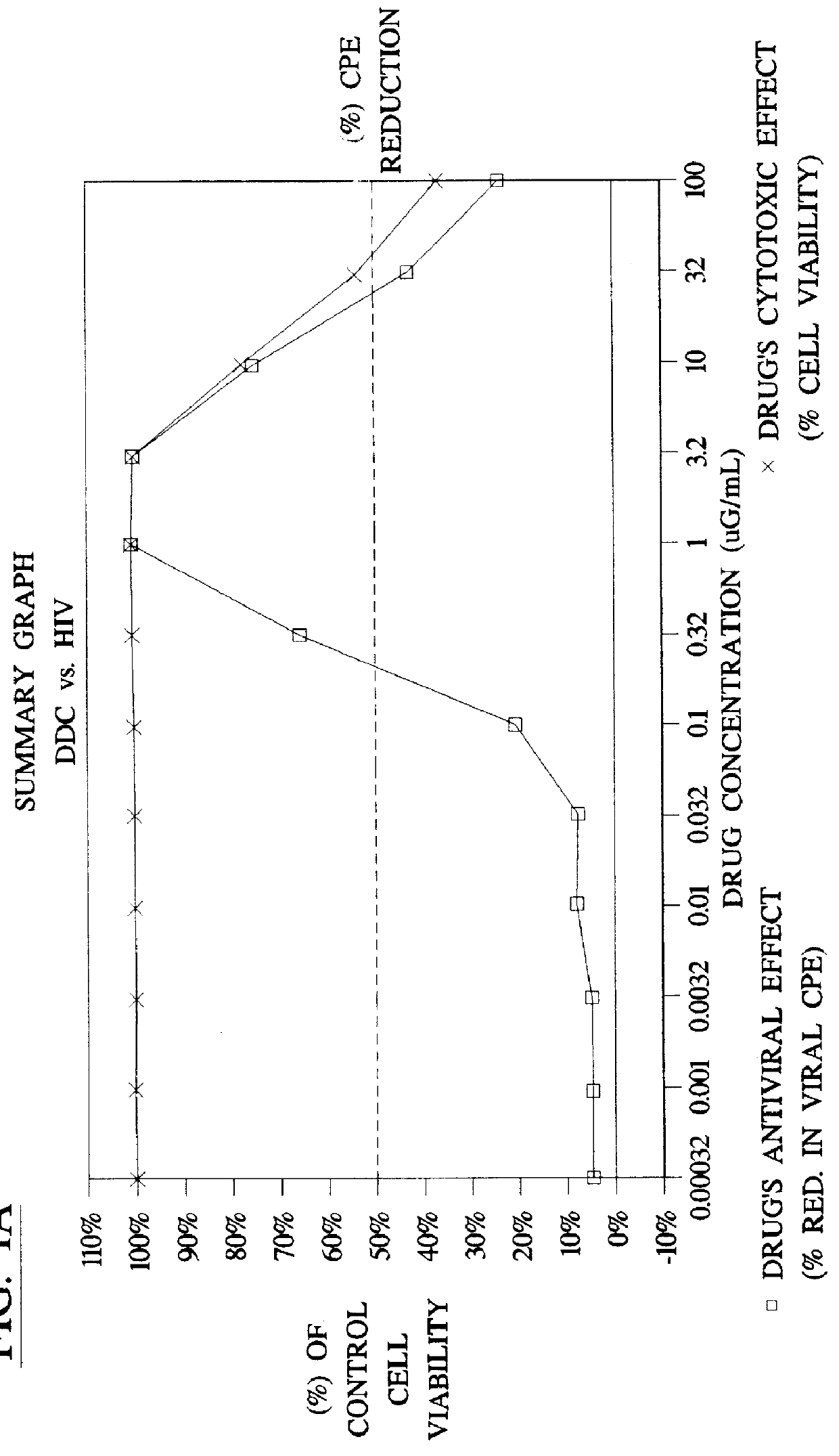
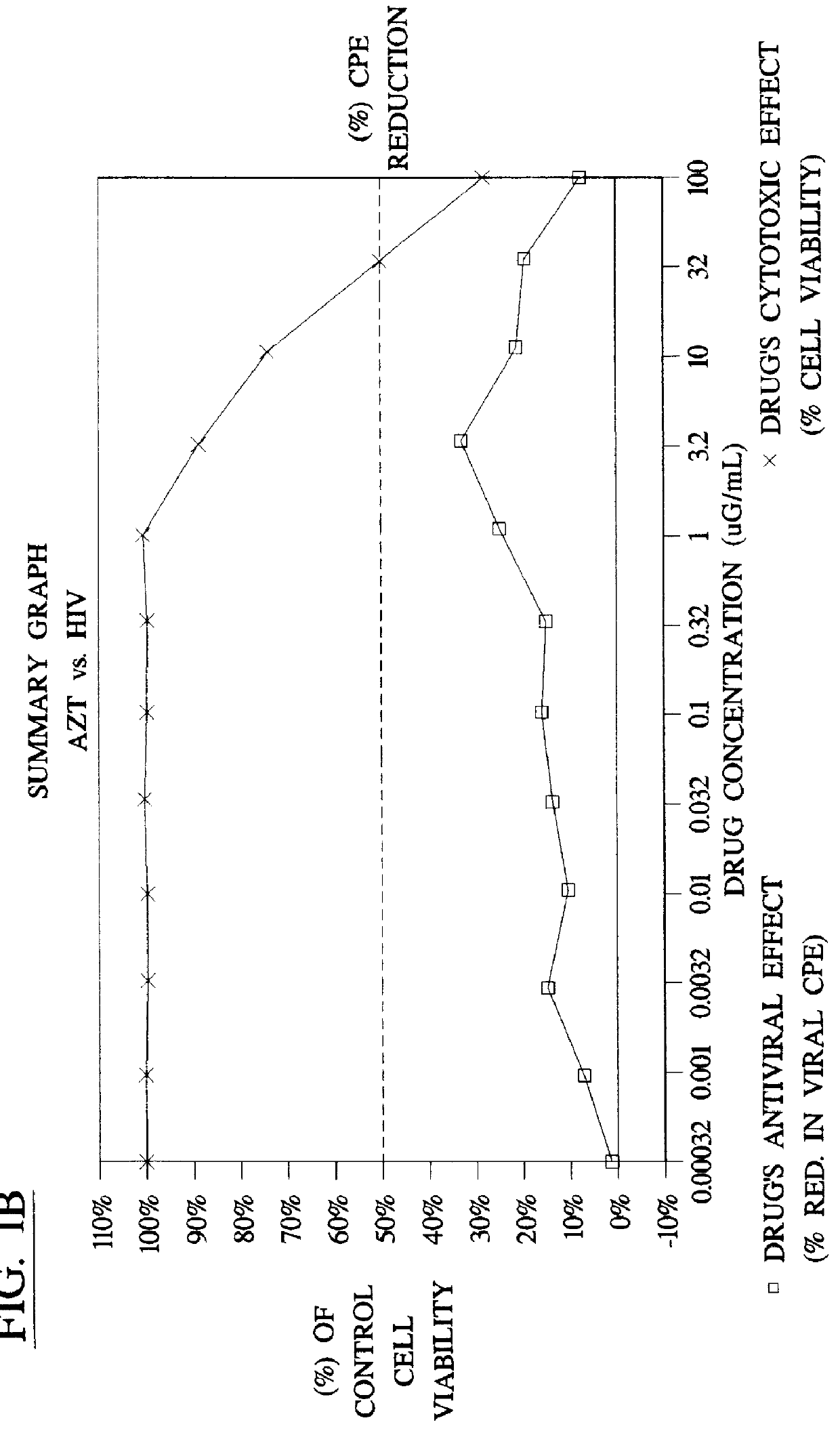
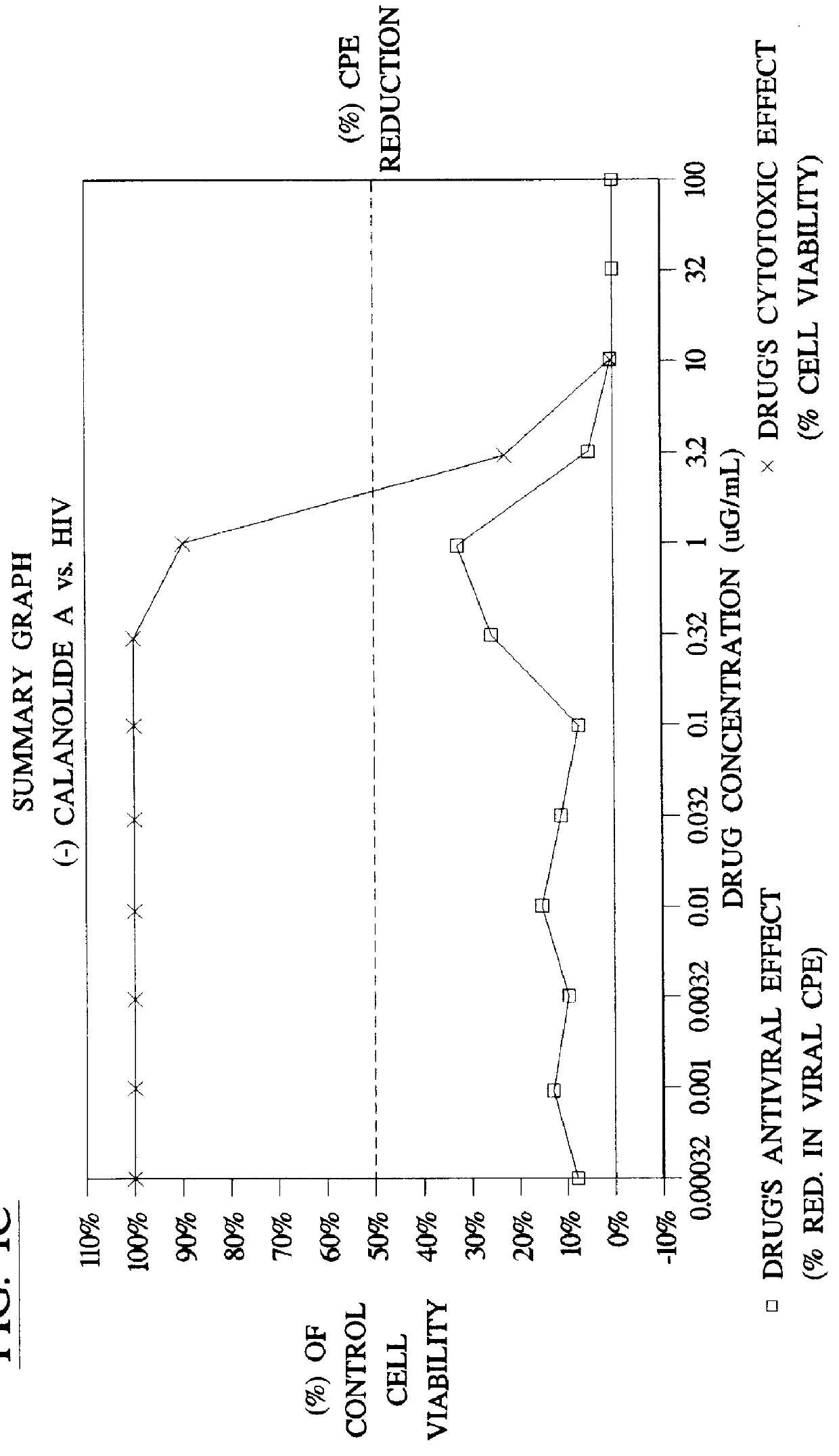
Method for the preparation of (+/-)-calanolide A and intermediates thereof
InactiveUS6043271APractical and convenientHigh yieldBiocideOrganic chemistryHydrolysisEnzyme inhibitor
A method of preparing (+ / -)-calanolide A, 1, a potent HIV reverse transcriptase inhibitor, from chromene 4 is provided. Useful intermediates for preparing (+ / -)-calanolide A and its derivatives are also provided. According to the disclosed method, chromene 4 intermediate was reacted with acetaldehyde diethyl acetal or paraldehyde in the presence of an acid catalyst with heating, or a two-step reaction including an aldol reaction with acetaldehyde and cyclization either under acidic conditions or neutral Mitsunobu conditions, to produce chromanone 7. Reduction of chromanone 7 with sodium borohydride, in the presence of cerium trichloride, produced (+ / -)-calanolide A. A method for resolving (+ / -)-calanolide A into its optically active forms by a chiral HPLC system or by enzymatic acylation and hydrolysis is also disclosed. Finally, a method for treating or preventing a viral infections using (+ / -)-calanolide or (-)-calanolide is provided.
Owner:SARAWAK MEDICHEM PHARMA
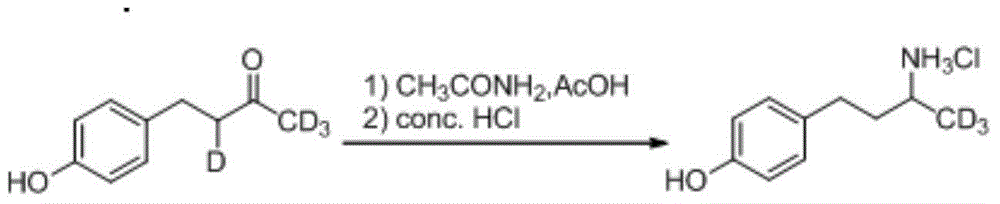
Synthesis method for deuterium marked ractopamine
InactiveCN104311436AThe synthesis steps are simpleEasy to control temperatureOrganic compound preparationAmino-hyroxy compound preparationChemical synthesisSynthesis methods
The invention belongs to the technical field of chemical synthesis, and particularly relates to a synthesis method for ractopamine marked by stable isotope deuterium. The method comprises the steps of firstly, carrying out aldol reaction on deuterium marked or non-marked p-hydroxy benzaldehyde and deuterium marked or non-marked acetone so as to generate deuterium marked raspberry ketone, then carrying out reductive amination, carrying out nucleophilic substitution reaction on deuterium marked or non-marked Omega-bromine-hydroxyacetophenone, reducing so as to prepare the deuterium marked ractopamine. The synthesis method has simple and efficient steps, the synthesized deuterium marked ractopamine has the purity of more than 99%, and the mark point isotope abundance is greater than 99%. Furthermore, the synthesis method has high finished product yield and high product yield, and effectively lowers the production cost. The deuterium marked ractopamine prepared by the synthesis method can be used for detection of residue of forbidden veterinary drug in food safety field and research of metabolic mechanism of ractopamine.
Owner:SHANGHAI INST OF MEASUREMENT & TESTING TECH
Application of porcine pancreatic lipase as catalyst of asymmetric aldol reaction of heterocyclic ketone and aromatic aldehyde
InactiveCN102517353AHigh catalytic activityHigh enantioselectivityFermentationPancrelipasePtru catalyst
The invention discloses an application of porcine pancreatic lipase as a catalyst of asymmetric aldol reaction of heterocyclic ketone and aromatic aldehyde. The aromatic nucleus of the aromatic aldehyde is connected with an electron withdrawing substituent, and the aromatic aldehyde has the advantages of good catalytic activity, high enantiomeric selectivity, environmental friendliness, good economical performance and the like. The invention also provides a method of using the porcine pancreatic lipase to catalyze the asymmetric aldol reaction of the heterocyclic ketone and the aromatic aldehyde, the yield is high, and the ee (enantiomeric excess) value is high. The scope of application of the porcine pancreatic lipase is broadened, a potentially excellent catalyst is provided for the asymmetric aldol reaction, and the porcine pancreatic lipase has broad application prospects in the field of asymmetric synthesis.
Owner:SOUTHWEST UNIVERSITY
Preparation method and application of Al2O3-modified SO4<2->/SnO2 solid acid catalyst
InactiveCN108722443AReduce pollutionExtension of timeOrganic chemistryOrganic compound preparationAir atmospherePhosphoric acid
The invention discloses a preparation method of an Al2O3-modified SO4<2-> / SnO2 solid acid catalyst and application thereof to an aldol reaction. At present, in the aldol reaction of isopentenyl aldehyde and isopentenyl alcohol, an acid-catalyzed reaction is performed by using liquid acid such as phosphoric acid, so that operating units are increased and more wastewater is produced. The preparationmethod of the solid acid catalyst comprises the following steps: a), dissolving soluble aluminum salt and soluble tin salt in water, and dispersing in an alcohol compound; b), dropwise adding an alkaline compound, leaving to stand for aging at room temperature, and filtering; c), immersing in sulfuric acid, and then filtering; d), calcinating in an air atmosphere. Through use of the solid acid catalyst, the time of the condensation reaction is significantly shortened and the conversion rate is significantly increased; in addition, the catalyst can be reused after being filtered, and the original activity thereof is still maintained without a deactivation phenomena, so that not only the production efficiency is greatly improved, but also the environmental pollution is reduced.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
Method for preparing hierarchical pore molecular sieve materials
InactiveCN103130239AImprove performanceHigh catalytic activityCrystalline aluminosilicate zeolitesMolecular sieveSurface-active agents
The invention discloses a method for preparing hierarchical pore molecular sieve materials. The method comprises the following steps: reaction premedicant liquid is prepared; high polymer subgrain is prepared in an aging mode; the hierarchical pore molecular sieve materials are prepared; and separation, washing, drying and calcination are conducted. A cationic surface active agent serves as a template agent, and the hierarchical pore molecular sieve materials with good performance are prepared through a single-step hydrothermal method. The problem in the prior art that split-phase exists when the cationic surface active agent serves as the template agent is solved effectively, and meanwhile the prepared materials have good catalytic activity in a macromolecule aldol reaction, hydrothermal stability is good, high catalytic activity is displayed when flavanone and chalcone drug intermediate are prepared, and the heterogeneous catalyst is beneficial for isolation and purification of products, and therefore the method for preparing the hierarchical pore molecular sieve materials has an industrial application value.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com



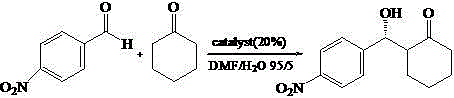
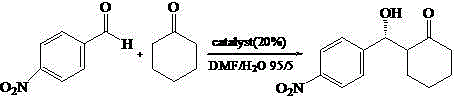
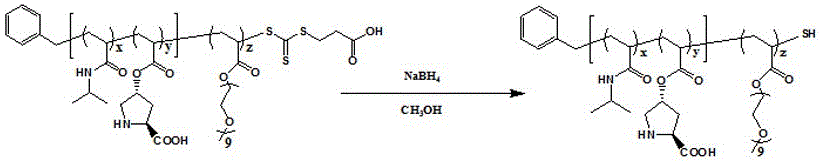
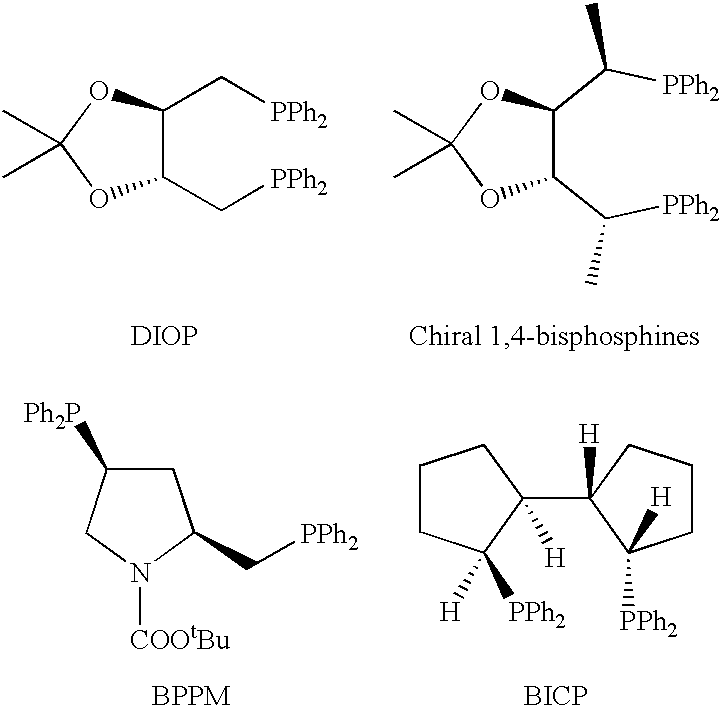

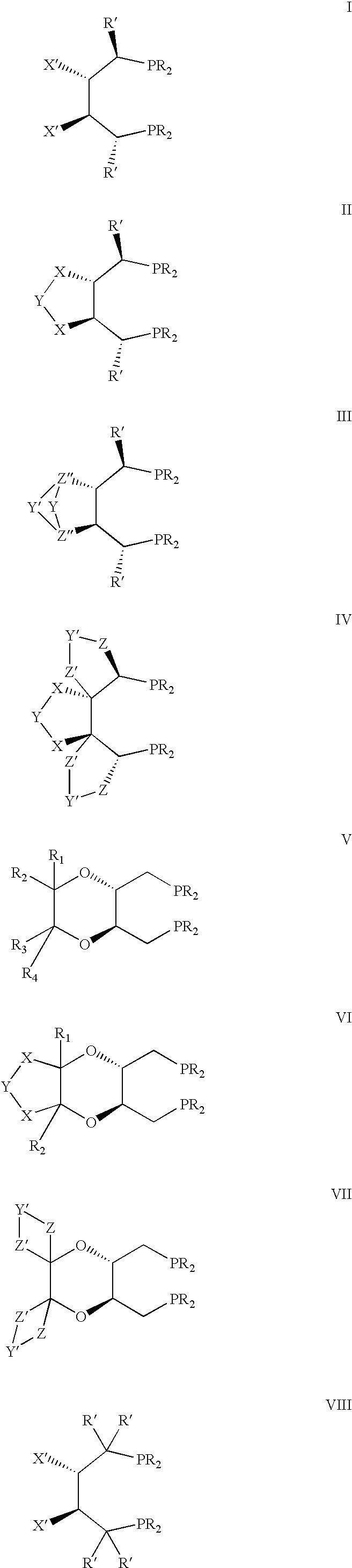

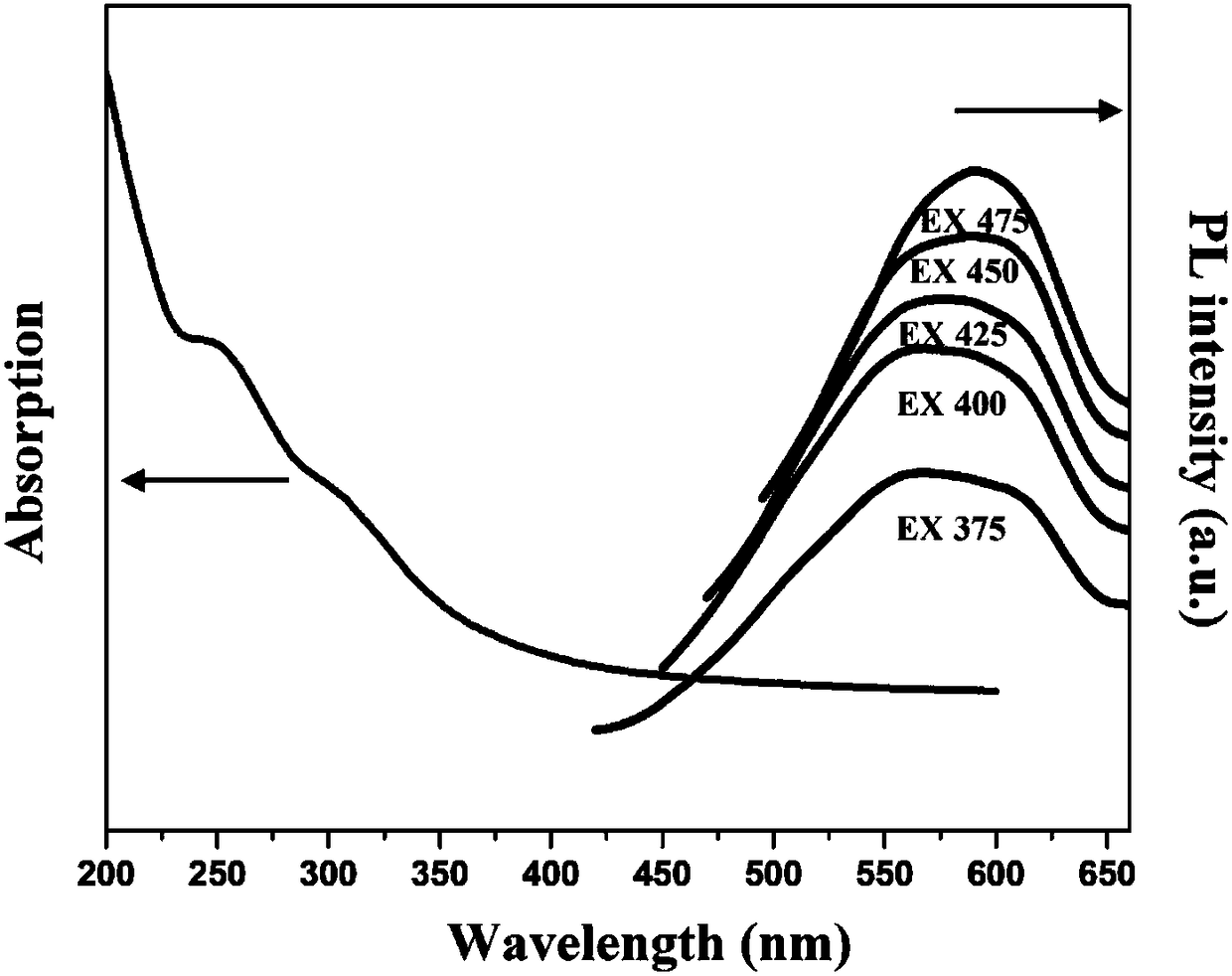
![Calix [4] proline derivative and green catalytic asymmetric Aldol reaction method thereof Calix [4] proline derivative and green catalytic asymmetric Aldol reaction method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5e1d06ec-f980-4701-839e-42573c84c511/BDA0000374506790000031.png)
![Calix [4] proline derivative and green catalytic asymmetric Aldol reaction method thereof Calix [4] proline derivative and green catalytic asymmetric Aldol reaction method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5e1d06ec-f980-4701-839e-42573c84c511/FDA0000374506780000011.png)
![Calix [4] proline derivative and green catalytic asymmetric Aldol reaction method thereof Calix [4] proline derivative and green catalytic asymmetric Aldol reaction method thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5e1d06ec-f980-4701-839e-42573c84c511/BDA0000374506790000011.png)