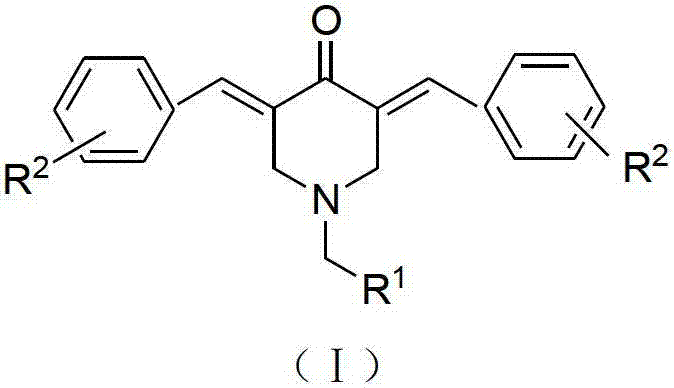
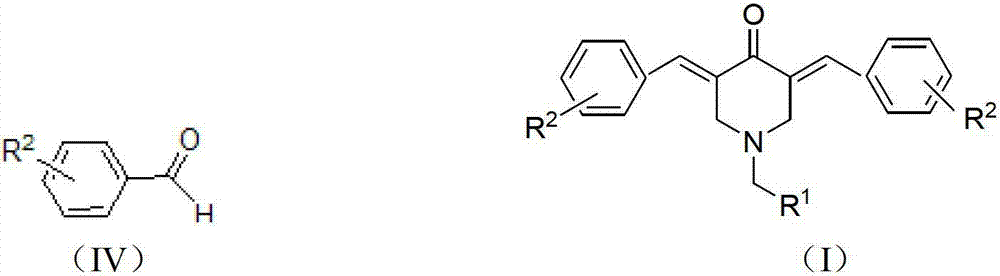
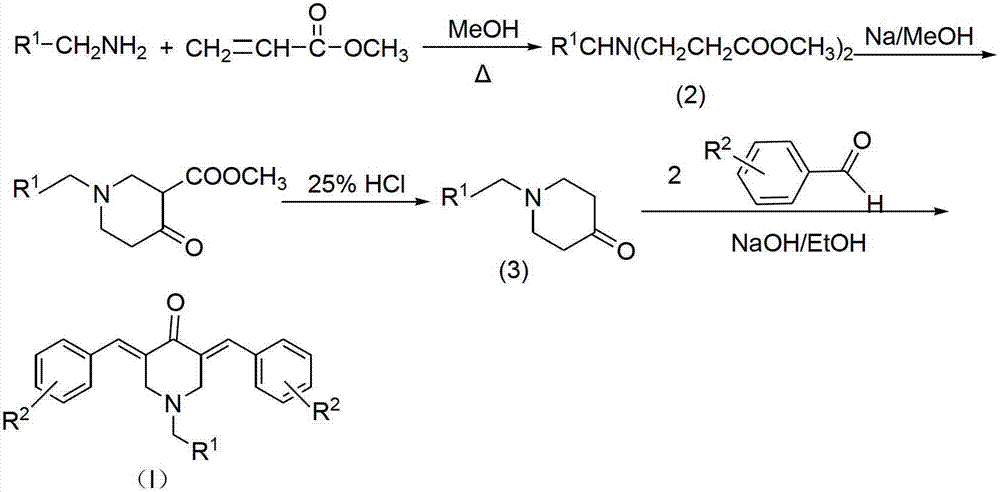
N-substituted methyl-3,5-disubstituted benzylidene base-4-piperidone and preparation method and application thereof
A technology of piperidone and double substitution, applied in the field of chemical drugs, can solve the problems of high toxicity and low selectivity, and achieve the effect of effectively inhibiting proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 N-(4-Methoxybenzyl)-3,5-bis(4-cyanobenzylidene)-4-piperidone (Ia)
[0044] In an ice-water bath at 0°C, add 0.4 mol of methyl acrylate and 7 mL of methanol into a 100 mL three-necked flask. After stirring for 30 minutes, slowly add 0.1 mol of 4-methoxybenzylamine and 10 mL of methanol to the mixed solution to control the drop. Acceleration so that the system temperature does not exceed 50°C (the dropping rate is about 1 drop / sec). After the addition is complete, stir at room temperature for 30 minutes, continue to raise the temperature to 65°C, reflux, and thin layer chromatography (TLC) to track the reaction progress. After reacting for about 8 hours, the reaction was stopped, and methanol and unreacted methyl acrylate were recovered by distillation under reduced pressure.
[0045] A pale yellow oily liquid intermediate (2a) was obtained.
[0046] At room temperature, add 15 mL of anhydrous toluene and 0.1 mol of metallic sodium (cut into blocks) into a 250 mL dry...
Embodiment 2
[0050] Example 2 N-(4-Methoxybenzyl)-3,5-bis(3,4-dichlorobenzylidene)-4-piperidone (Ib)
[0051] (2b) and (3b) are prepared by the same method as (2a) and (3a).
[0052] Add intermediate N-4-methoxybenzyl-4-piperidone (3b) (0.005mol) and 3,4-dichlorobenzaldehyde (0.01mmol) in a 50mL round bottom flask, 15mL of absolute ethanol and 10% (mass fraction) sodium hydroxide 1mL. Stir at room temperature (15~25℃) for 1h, and solids will precipitate out. Filter by suction, then wash the product with absolute ethanol, or recrystallize with ethyl acetate and petroleum ether to obtain the target compound (Ib).
[0053] Yield: 80.2%; yellow solid, mp 131-133°C; 1 H NMR(400MHz, CDCl 3 )δ3.65(s,2H), 3.71(s,3H), 3.85(s,4H), 7.02(d,J=8.7Hz,2H), 7.13(d,J=8.7Hz,2H), 7.24( d,J=8.0Hz,4H),7.36(d,J=8.0Hz,2H),7.71(s,2H); IR(KBr): 2805,2745,1667,1615,1575,1480,1260,1185, 1085,821cm -1 ;Anal.calcd.for C 27 H 21 Cl 4 NO 2 C%60.81,H%3.97,N%2.63;Found:C%60.72H%3.95,N%2.68
Embodiment 3
[0054] Example 3 N-(4-Methoxybenzyl)-3,5-bis(2,4-dichlorobenzylidene)-4-piperidone (Ic)
[0055] Prepare (2c) and (3c) in the same way as (2a) and (3a).
[0056] Add intermediate N-4-methoxybenzyl-4-piperidone (3c) (0.005mol) and 3,4-dichlorobenzaldehyde (0.01mmol) in a 50mL round bottom flask, 15mL of absolute ethanol and 10% (mass fraction) sodium hydroxide 1mL. Stir at room temperature (15~25℃) for 1h, solids will precipitate out, filter with suction, then wash the product with absolute ethanol, or recrystallize with ethyl acetate and petroleum ether to obtain the target compound (Ic).
[0057] Yield: 73.7%; yellow solid, mp 183-185°C; 1 H NMR(400MHz, CDCl 3 )δ3.62(s, 2H), 3.75(s, 3H), 3.82(s, 4H), 7.03(d, J=8.7Hz, 2H), 7.12(d, J=8.7Hz, 2H), 7.22( d,J=8.0Hz,2H),7.28(d,J=8.0Hz,4H),7.73(s,2H);;IR(KBr):2805,2743,1664,1603,1574,1487,1262,1185 ,1094,821cm -1 ;Anal.calcd.for C 27 H 21 Cl 4 NO 2 C%60.81,H%3.97,N%2.63;Found:C%60.72H%3.95,N%2.68
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com