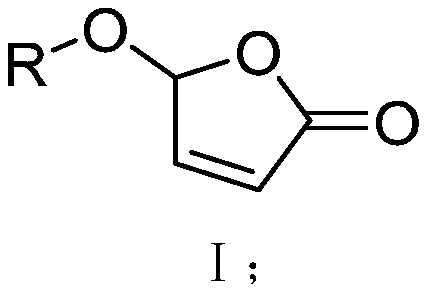
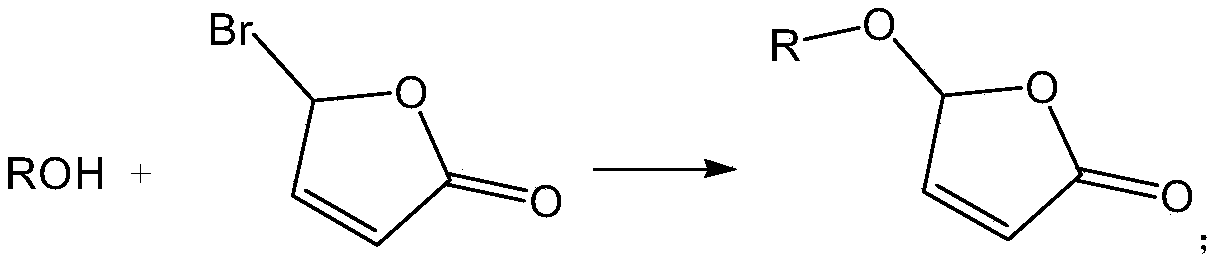
2(5H)-furan-2-one derivative, preparation method thereof and application of 2(5H)-furan-2-one derivative in inhibition of rice tillering
A technology of derivatives and furan, which is applied in the field of 2(5H)-furan-2-one derivatives and its preparation and in the application field of inhibiting rice tillering, and achieves the effect of low preparation price and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
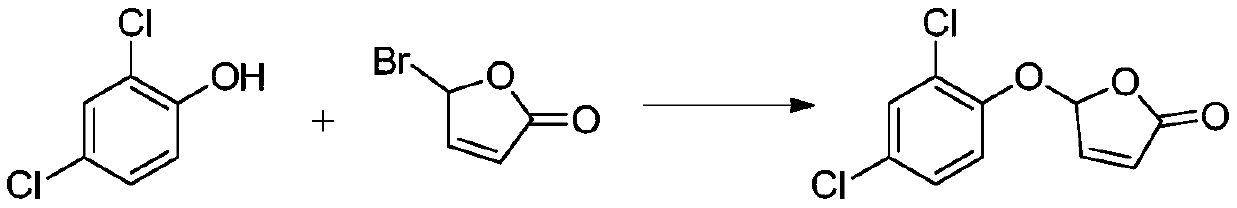
[0037] Weigh K 2 CO3 3 (2.5mmol, 1.25equiv) and tetra-n-butylammonium bromide (0.2mmol, 0.1equiv), add water (10ml), stir until completely dissolved, add and dissolve in CH 2 Cl 2 (5ml) in 2,4-dichlorophenol (2mmol, 1equiv), stirred at room temperature for 10min, dissolved in CH 2 Cl 2 5-Bromo-furan-2(5H)-one (2mmol, 1 equiv) in (5ml) was added dropwise to the above system, stirred at room temperature for 8h, and the reaction solution was washed with CH 2 Cl 2 (3×5ml) extraction, dried with anhydrous sodium sulfate, distilled off the solvent under reduced pressure, mixed with silica gel in a dry method, loaded the sample, and used (petroleum ether / ethyl acetate=6:1v / v) as the eluent to separate Compound 1 (white solid, yield 84%), identified as 5-(2,4-dichlorobenzene)furan-2(5H)-one.
[0038] 1 H NMR (400MHz, CDCl 3 )δ7.57(dd, J=5.6,1.2Hz,1H),7.37(d,J=8.1Hz,2H),7.13(t,J=8.1Hz,1H),6.45(s,1H),6.39( dd,J=5.6,1.0Hz,1H). 13 C NMR (101MHz, CDCl 3 )δ151.6, 149....
Embodiment 2
[0040]
[0041] With 2,6-dichlorophenol instead of 2,4-dichlorophenol in Example 1, others are the same as in Example 1 to obtain compound 2 (white solid, yield 81%), identified as 5-(2, 6-Dichlorophenoxy)furan-2(5H)-one.
[0042] 1 H NMR (400MHz, CDCl 3 )δ7.48(dd, J=5.7,1.2Hz,1H),7.43(d,J=2.3Hz,1H),7.28–7.24(m,2H),6.39(dd,J=5.7,1.1Hz,1H ),6.35(d,J=1.1Hz,1H). 13 C NMR (101MHz, CDCl 3 )δ169.9, 149.6, 148.2, 129.3, 126.8, 125.4, 103.0.
Embodiment 3
[0044]
[0045] Replace 2,4-dichlorophenol in Example 1 with 2-bromophenol, and others are the same as in Example 1 to obtain compound 3 (white solid, yield is 96%), identified as 5-(2-bromophenoxy base) furan-2(5H)-one.
[0046] 1 H NMR (400MHz, CDCl 3 )δ7.53(d, J=7.6Hz, 1H), 7.44(dd, J=5.7, 1.2Hz, 1H), 7.27–7.26(m, 2H), 7.00–6.96(m, 1H), 6.33(s ,1H),6.29(dd,J=5.7,1.1Hz,1H). 13 C NMR (101MHz, CDCl 3 )δ169.8, 152.9, 149.8, 133.6, 128.9, 125.4, 118.6, 113.5, 101.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com