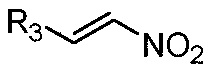
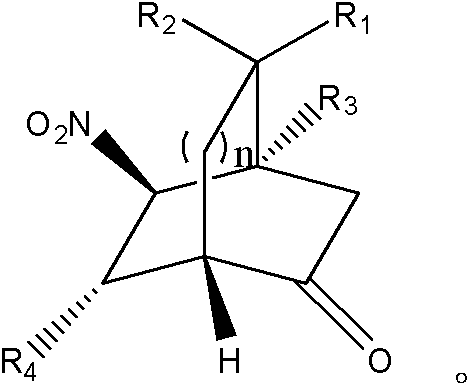
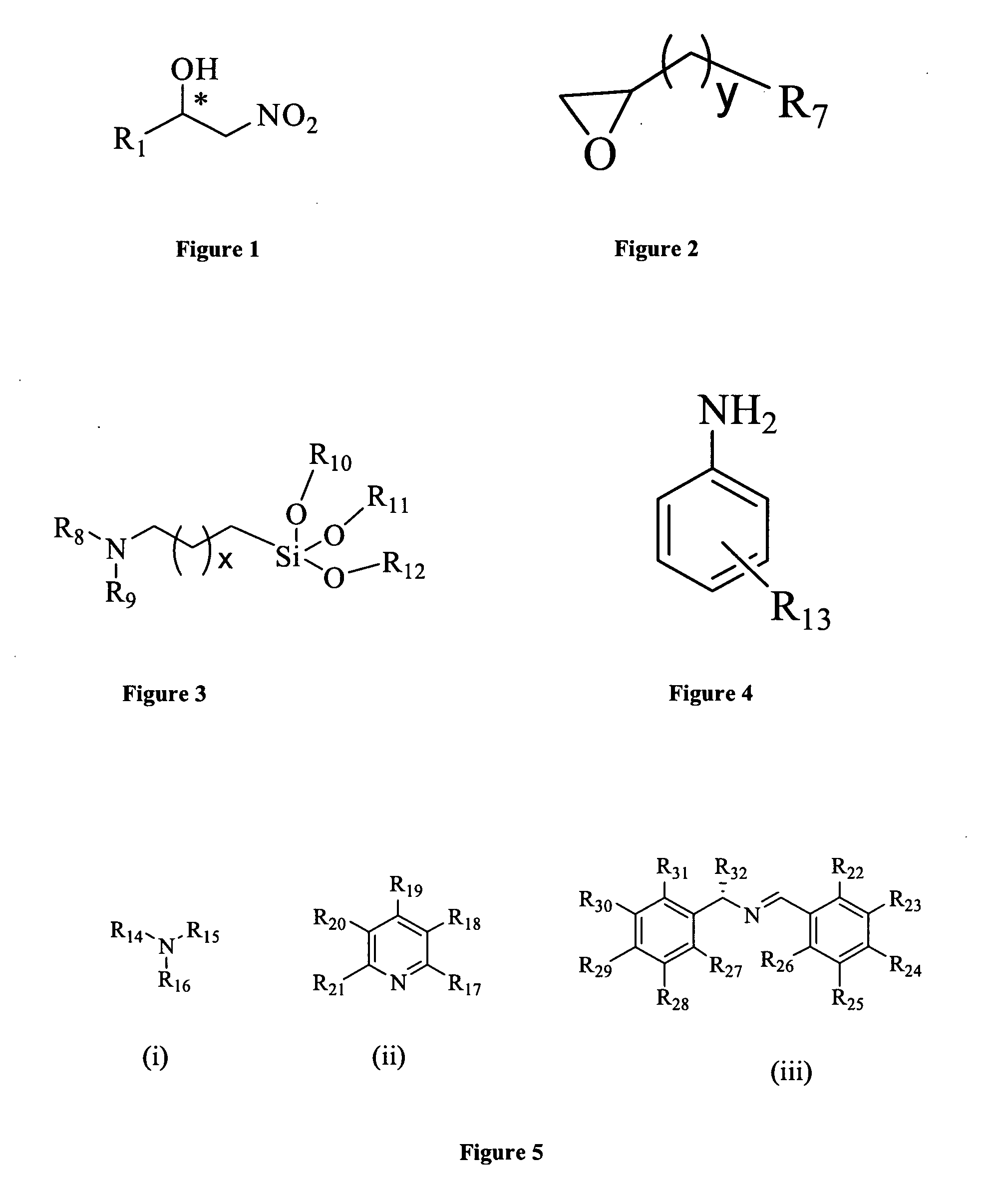
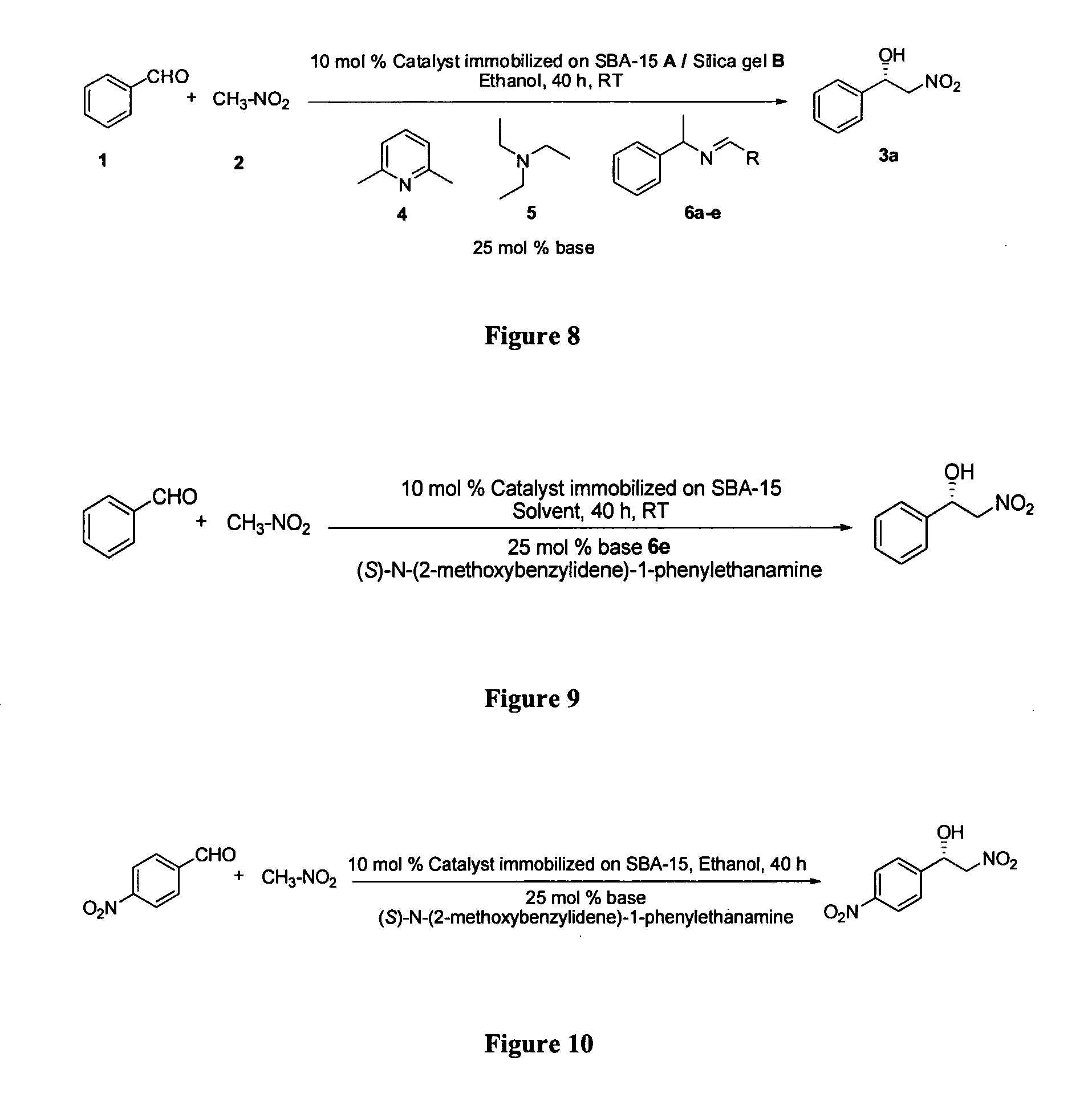
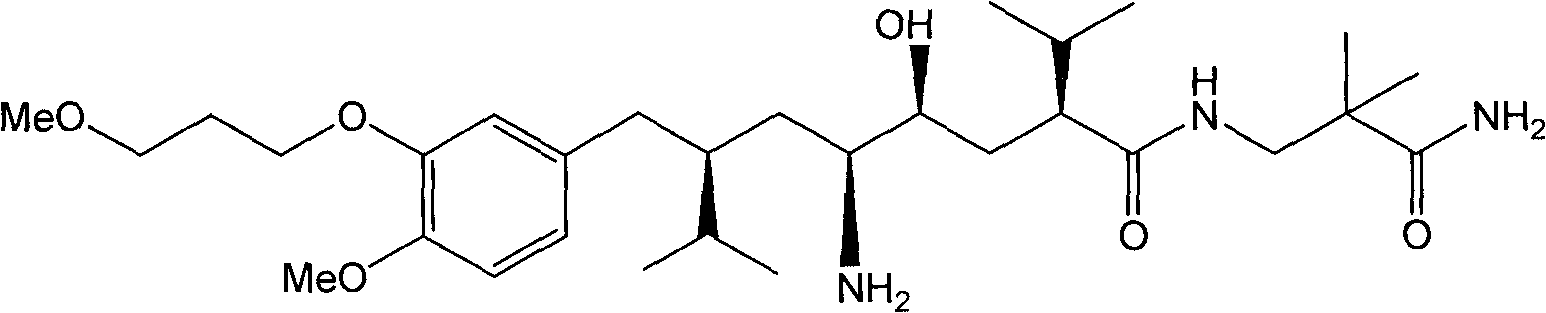
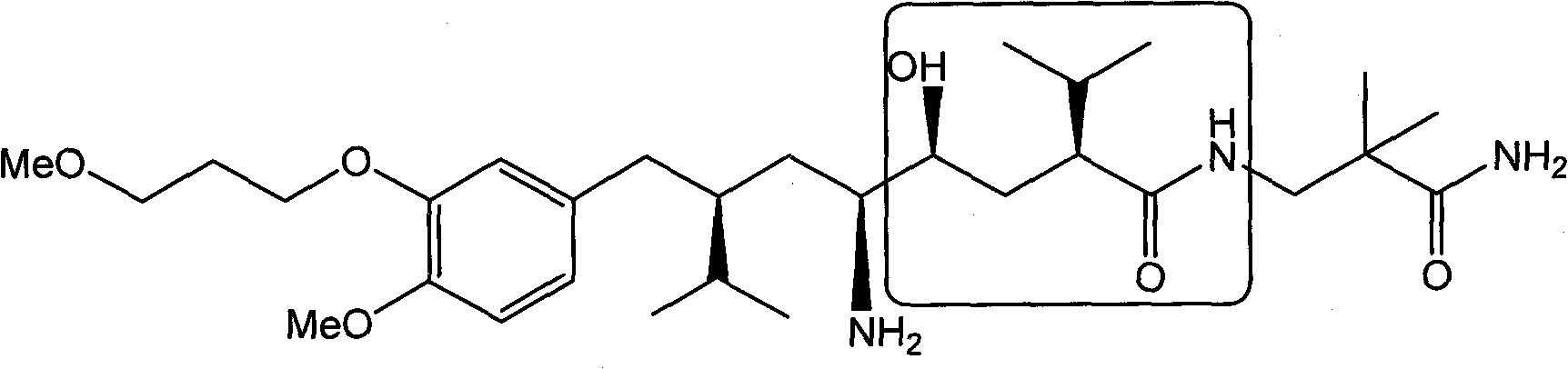
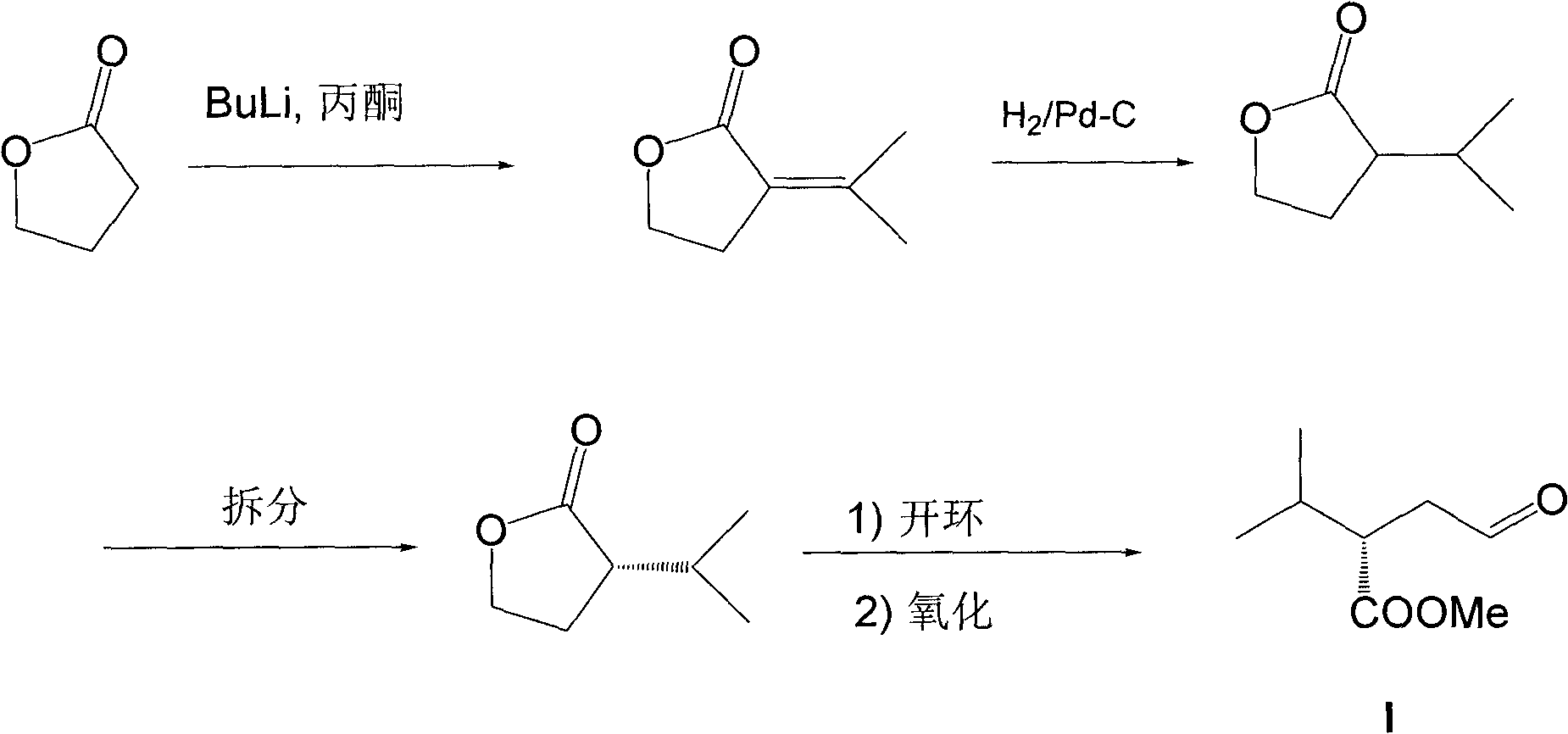
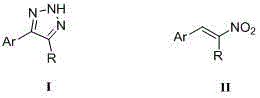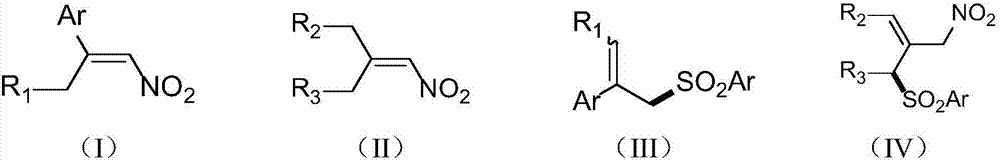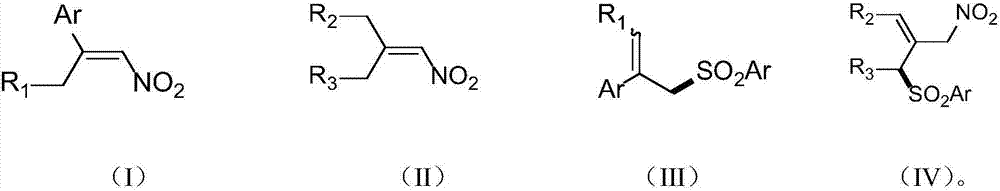Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
78 results about "Nitroalkene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
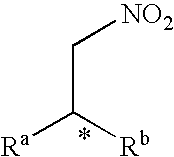
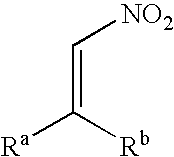
A nitroalkene, or nitro olefin, is a functional group combining the functionality of its constituent parts, an alkene and nitro group, while displaying its own chemical properties through alkene activation, making the functional group useful in specialty reactions such as the Michael reaction or Diels-Alder additions.
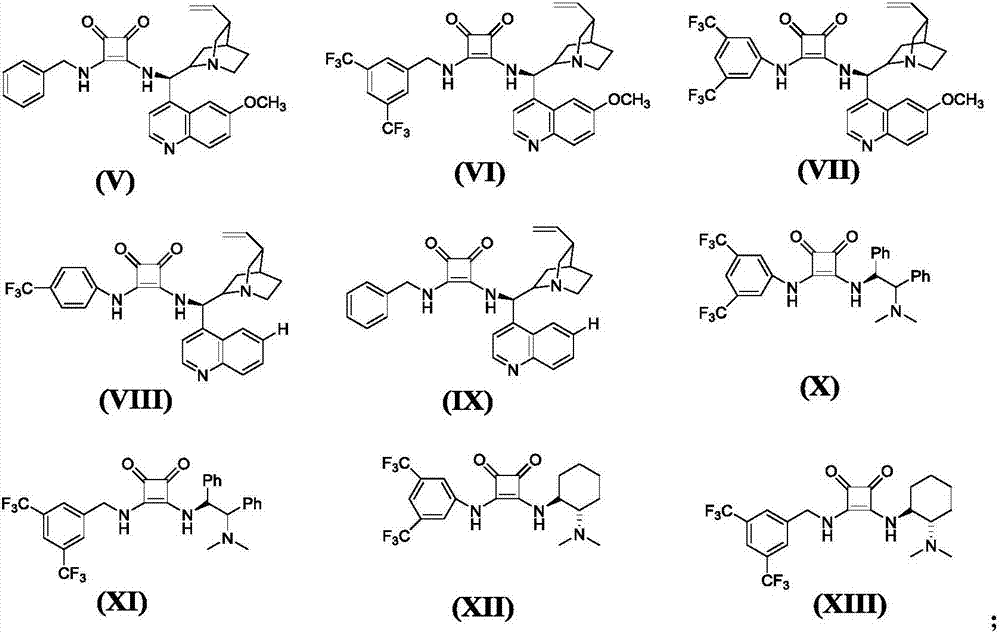
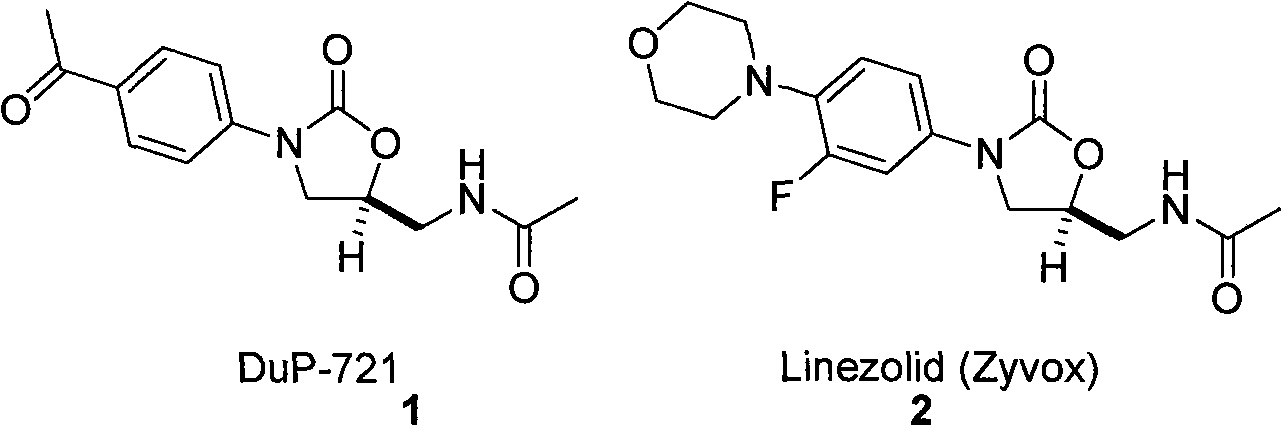
Methods Of Use Of Nitroalkene Compositions In Dermatologic Applications
Owner:PERRICONE NICHOLAS V
Methods Of Use Of Nitroalkane Compositions In Dermatologic Applications To Prevent or Treat Skin Aging
Topical compositions comprising an effective amount of a nitroalkene and a carrier are used to prevent or treat skin aging.
Owner:N V PERRICONE
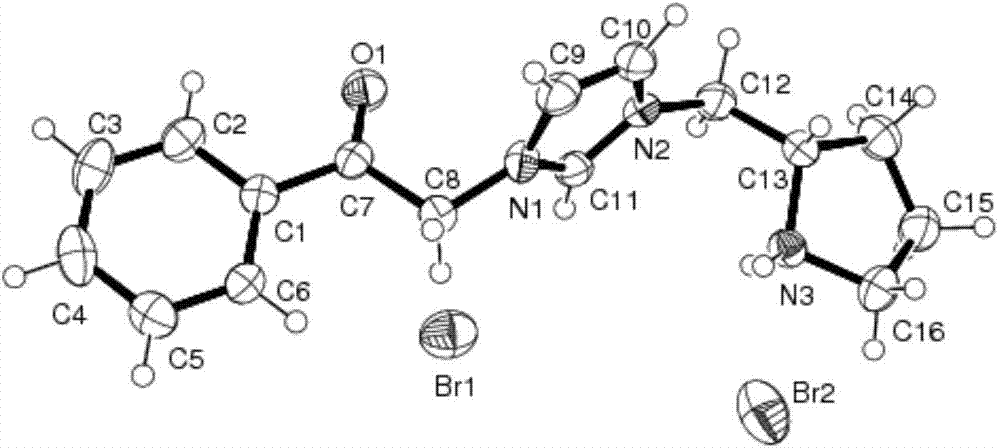
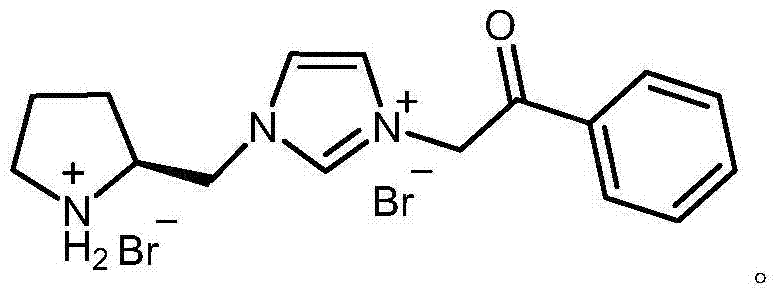
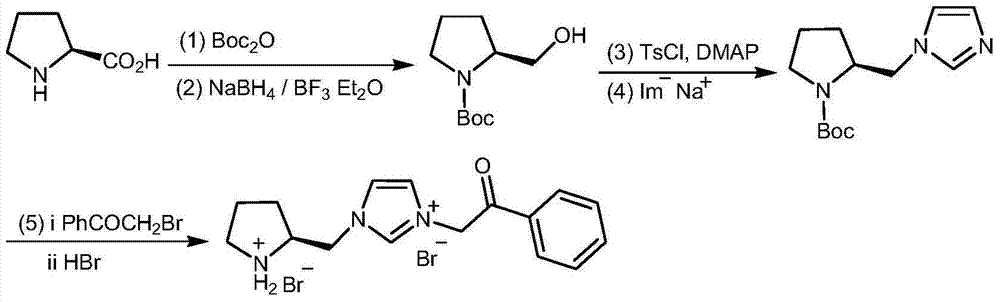
Chiral pyrrolidine functionalized imidazolium salt, and preparation method and application thereof
InactiveCN103570691ARaw materials are easy to getEasy to manufactureOrganic chemistryOrganic compound preparationCyclohexanoneHydrobromide
The invention provides a chiral pyrrolidine functionalized imidazolium salt, and a preparation method and an application thereof. The chiral pyrrolidine functionalized imidazolium salt is bromized 1-[2-(S)-(pyrrolidyl)methyl]-3-benzoyl methyl imidazole hydrobromide. The preparation method of the chiral pyrrolidine functionalized imidazolium salt comprises the following steps of: by taking natural amino acid L-proline as the starting raw material, obtaining the chiral pyrrolidine functionalized imidazolium salt through a plurality of conventional organic synthetic reactions such as Boc acylation, carboxylic acid reduction, hydroxyl sulfonylation, nucleophilic substitution of imidazole anions, quaternization of halohydrocarbon and de-Boc protection. The chiral pyrrolidine functionalized imidazolium salt provided by the invention is capable of catalyzing asymmetric Michael addition reaction of cyclohexanone and nitroolefin, and has extremely high diastereoselectivity and enantioselectivity.
Owner:SHANXI UNIV
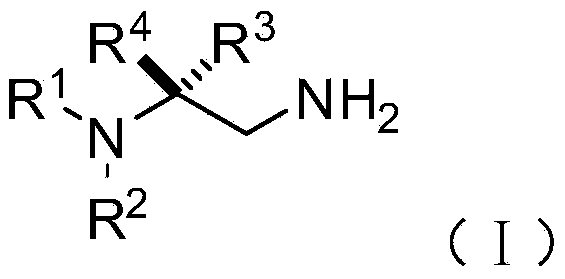
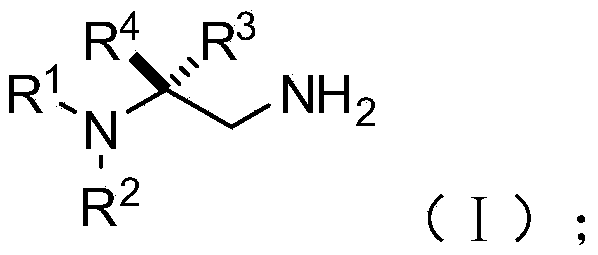
Chiral 1,2-diamine compound and preparation method and application thereof
ActiveCN105367427AAchieve strict metal-freeThe reaction process is safe and controllableOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCompound aNitroalkene
The invention discloses a chiral 1,2-diamine compound and a preparation method and application thereof. The molecular structure general formula of the chiral 1,2-diamine compound is shown as the general formula (I) in the specification. The preparation method of the chiral 1,2-diamine compound comprises the steps that an amine compound A and a nitroolefin compound B are added to a reaction system containing an n-heterocyclic carbine catalyst, an alkali reagent, a proton additive and a dewatering reagent for reacting and other steps. The chiral 1,2-diamine compound comprises chiral quaternary carbon atoms containing electrondrawing groups and amino groups, and can be widely used for synthesizing drug intermediates, especially heterocyclic ring structure compounds, and preparing functional materials. The preparation method is simple in process and low in requirement for reaction conditions, the reaction process is safe and controllable, the atom utilization rate and production efficiency are high, meanwhile, the enantioselectivity of products is efficiently guaranteed, and the environment pollution pressure of the methodology is low through the introduction of the small organic molecule asymmetric catalysis concept.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
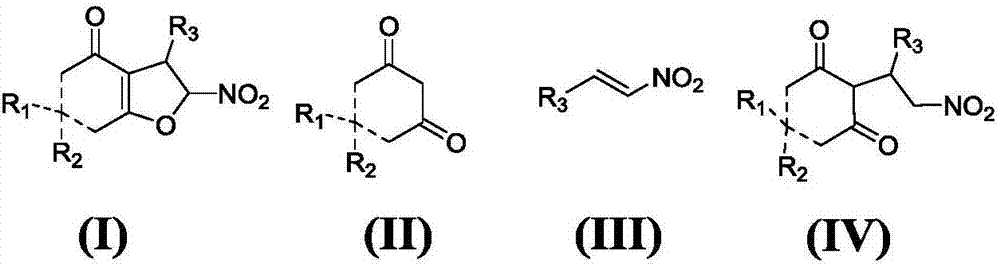
Asymmetric synthesis method for chiral dihydrofuran compound
The invention provides an asymmetric synthesis method for a chiral dihydrofuran compound shown as formula (I). The synthesis method is carried out according to the following steps: 1,3-cyclohexanedione compound shown as formula (II), nitroolefin compound shown as formula (III), chiral squaric acid catalyst and organic solvent A are mixed and thoroughly react under negative 20 DEG C to negative 60 DEG C, so that a compound shown as formula (IV) is obtained; iodine source additive, an alkaline substance and organic solvent B are added into the compound shown as formula (IV) and thoroughly react under negative 20 DEG C to negative 60 DEG C, and after reaction solution is post-treated, the chiral dihydrofuran compound shown as formula (I) is obtained. the asymmetric synthesis method has the advantages of mild reaction conditions, high product yield and excellent selectivity; the prepared chiral dihydrofuran compound is chiral, and the core framework structure is novel. (img file='DDA00012852165300000II.TIF' wi='1549' he='406' / ).
Owner:ZHEJIANG UNIV OF TECH
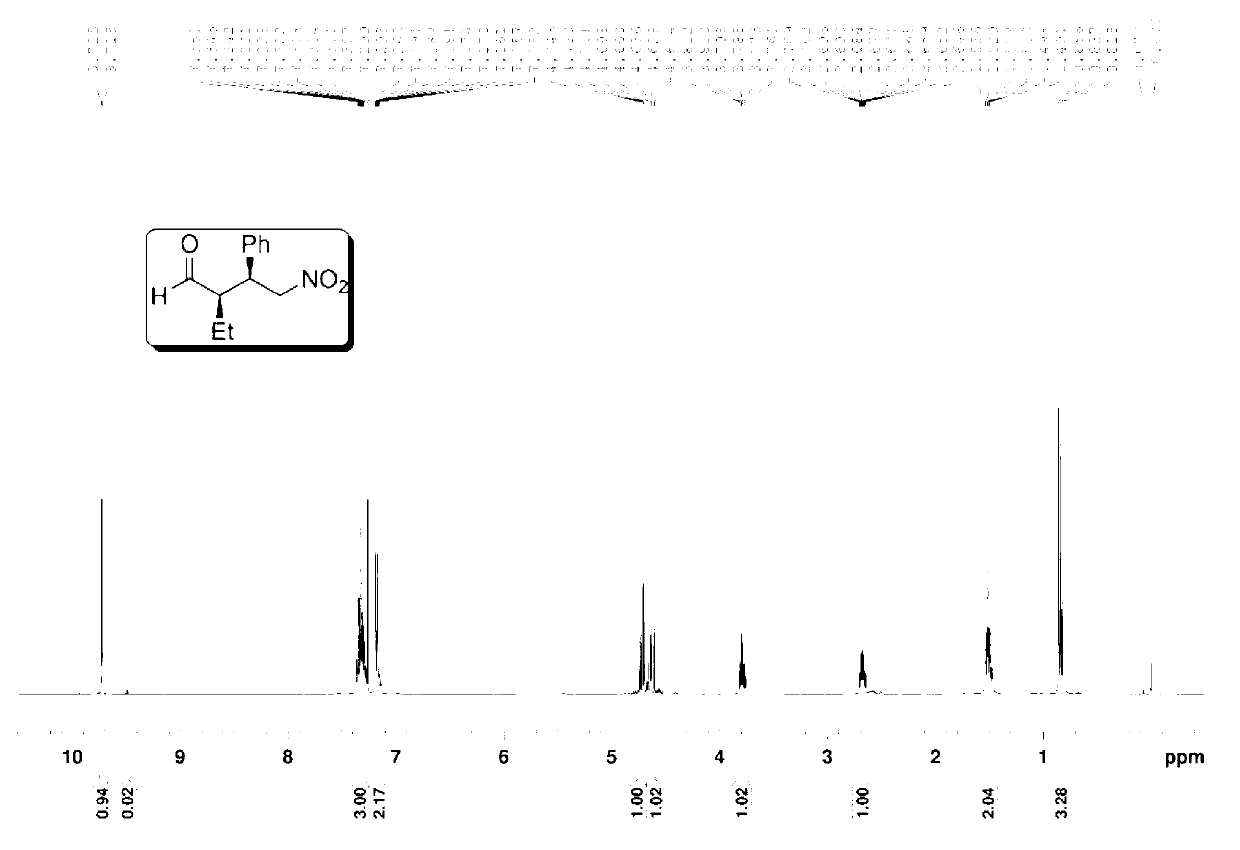
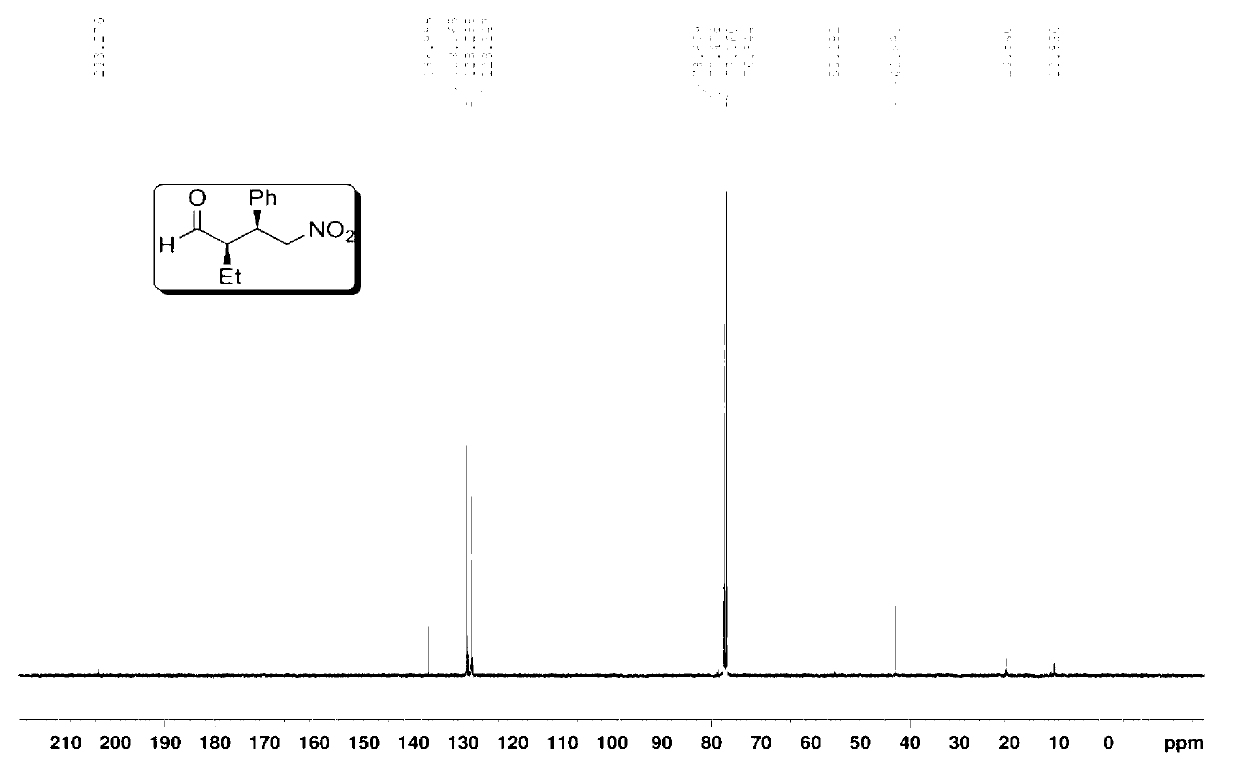
Preparation method of addition product of aldehyde and nitroolefin
ActiveCN102942430AHigh stereoselectivitySimple structureOrganic compound preparationAsymmetric synthesesLithiumNitroalkene
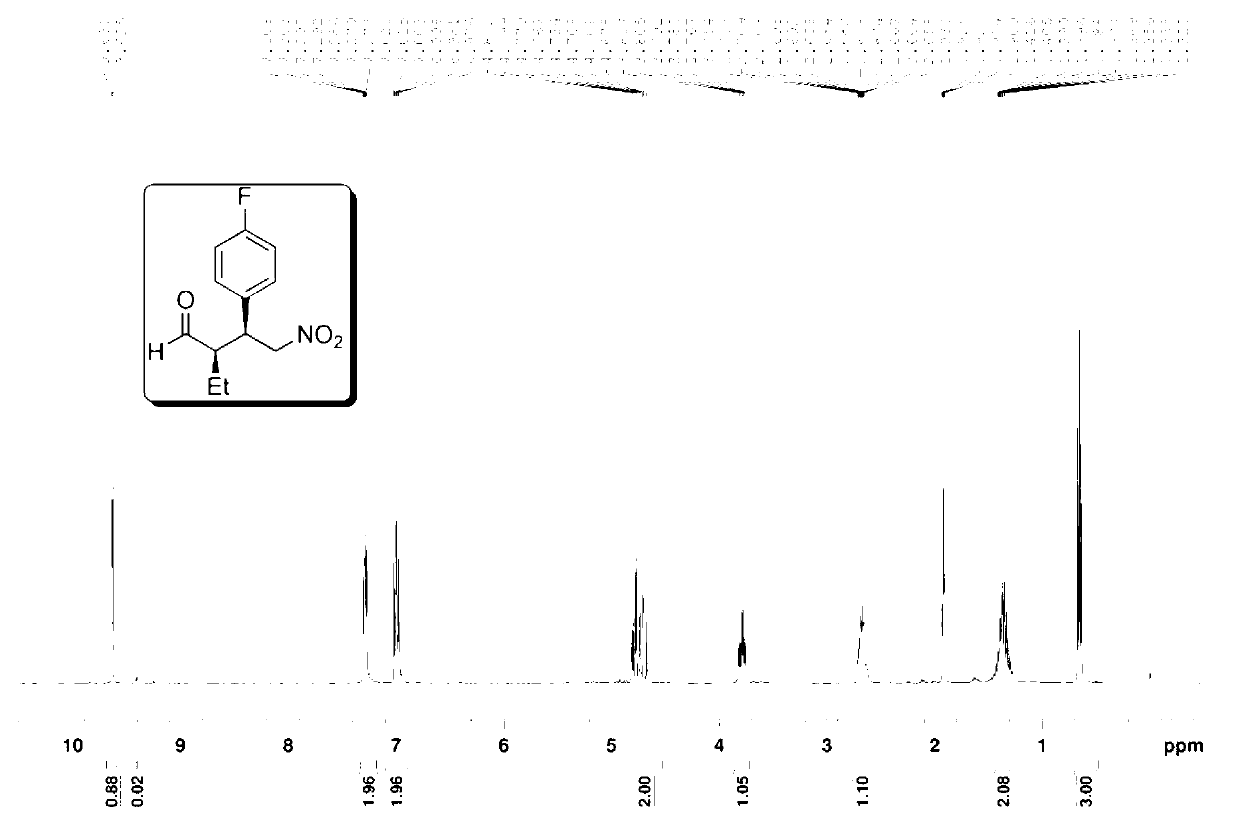
The invention provides a preparation method of addition product of aldehyde and nitroolefin. (S)-proline lithium or (R)-proline lithium is used as a chiral catalyst to katalyze the asymmetric Michael addition reaction between aldehyde and nitroolefin. The (S)-proline lithium or (R)-proline lithium has high catalytic efficiency; and the obtained addition product of aldehyde and nitroolefin has the advantages of high stereoselectivity and simple structure, is cheap and accessible, and can easily implement large-scale production.
Owner:UNIV OF SCI & TECH OF CHINA
Method for catalytically synthesizing chiral curcumin analogs
ActiveCN102115446ANovel structureUnique structureOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystThiourea
The invention relates to an asymmetric chemical reaction process of catalytic conjugate addition, in particular to a method for catalytically synthesizing chiral curcumin analogs. The method comprises the steps of: taking nitroolefin and curcumin analogs as raw materials; taking tertiary amine-thiourea organic catalyst as a catalyst system; reacting in dissolvent, wherein the reaction time is 0.5-15 days, and the reaction temperature is -40-40 DEG C; and generating a conjugate addition product. The reaction general formula is shown in the description: in the formula, R1 and R2 are aliphatic series group and aromatic series group. The structural formula of the tertiary amine-thiourea organic catalyst organic catalyst is shown in the description: in the formula, R1 is tertiary amine-containing quindenary derivative, R2 and R3 are different or same aromatic series substituent groups respectively, and R4 is sulfonyl substituent group. The tertiary amine-thiourea organic catalyst organic catalyst is high in catalytic activity and stereoselectivity in the Michael addition reaction between the nitroolefin and the curcumin analogs, wherein the enantioselectivity is highest to 97%, the yield is highest to 96%, and the reaction substrate is wide in range.
Owner:EAST CHINA UNIV OF SCI & TECH
Recyclable chiral catalyst for asymmetric nitroaldol reaction and process for the preparation thereof
InactiveUS20150368181A1High yieldWithout loss yieldOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystNitroalkene
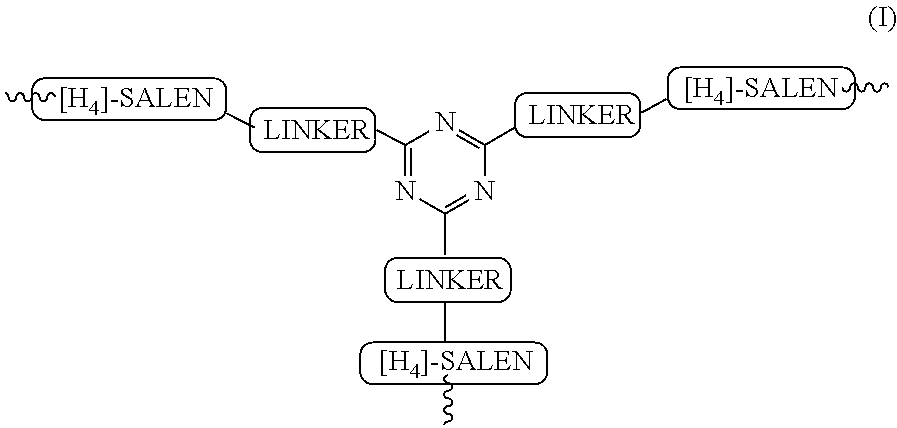
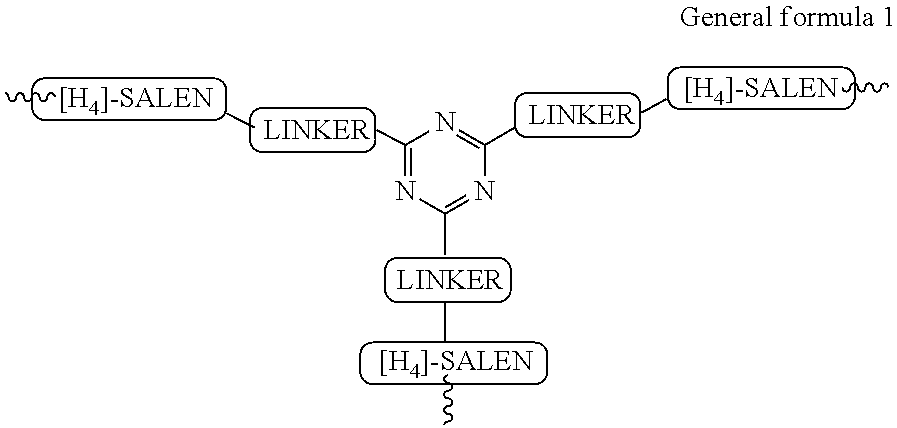
The present invention relates to preparation of highly efficient chiral recyclable homogeneous catalysts generated in situ by the reaction of chiral oligomeric [H4] ligands and a metal salt taken in 1:1 molar ratio for asymmetric nitroaldol reaction, wherein nitroaldol reactions of various aldehydes such as aromatic, aliphatic α,β-unsaturated aldehydes, alicyclic aldehydes and nitroalkenes were carried out to produce optically active β-nitroalcohols in high yield and with moderate to excellent enantioselectivity (ee up to >95%) in presence of a base and an optically active chiral recyclable homogeneous catalyst represented by the following formula (I).
Owner:COUNCIL OF SCI & IND RES
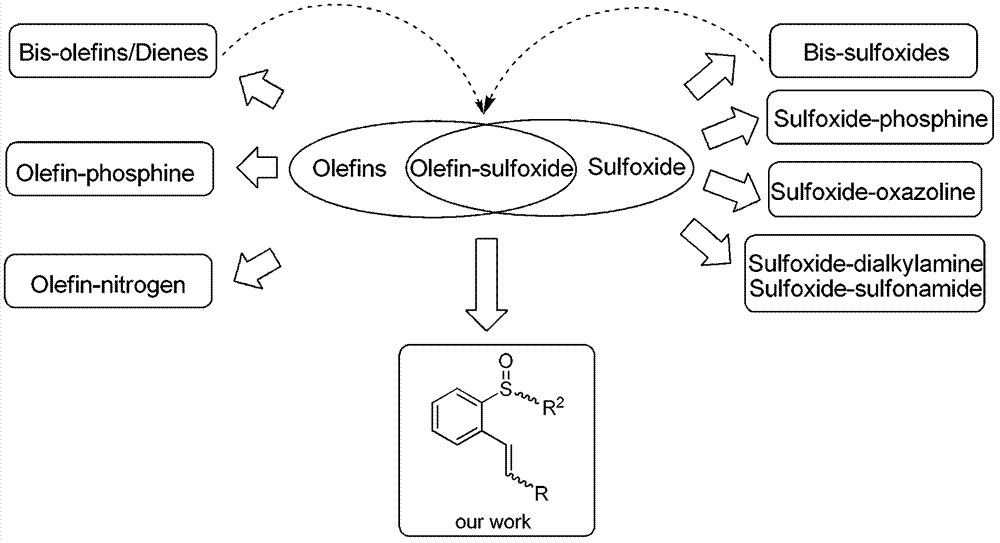
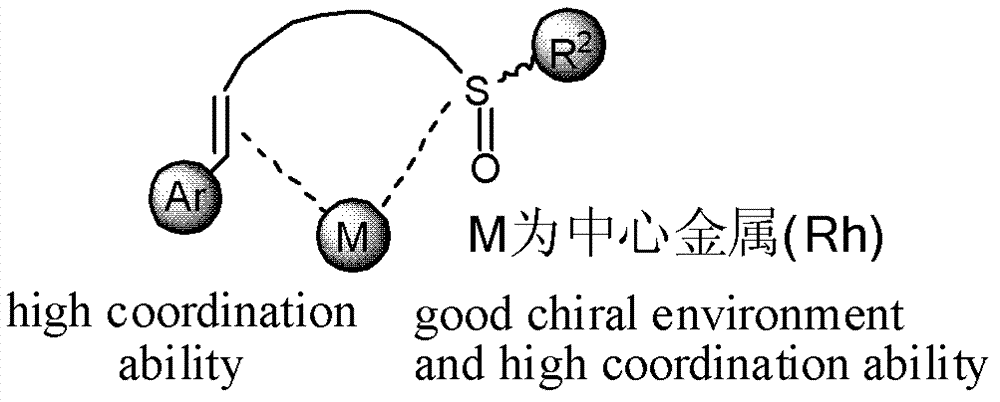
Chiral sulfoxide alkene ligand, preparation method and application thereof
InactiveCN102786453AImprove catalytic performanceStable in natureCarboxylic acid nitrile preparationOrganic compound preparationDiethyl phosphatePhosphorous acid
The invention relates to a chiral sulfoxide alkene ligand, a preparation method and an application thereof. According to the present invention, cheap and available 2-bromobenzyl bromide is adopted as a start material to obtain 2-bromobenzyl diethyl phosphate; then a Horner-Emmons reaction is adopted to introduce an alkene group; and finally lithiation modification is performed on bromine, and a corresponding chiral sulfoxide group is introduced. With the present invention, the target product is conveniently and rapidly synthesized through three steps; coordinate of the novel chiral sulfoxide alkene ligand and rhodium can be well used for catalysis of conjugate addition reactions on cyclic ketene, nitroalkene, and cyano alkene by aryl boronic acid, especially for an addition reaction on cyclic ketene by aryl boronic acid, wherein a good catalysis effect of the addition reaction on the cyclic ketene by the aryl boronic acid is provided.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for the effective synthesis of optically active oxazoline-2-ketone derivative
The invention discloses a method for the effective synthesis of optically active oxazoline-2-ketone derivative. The optically active oxazoline-2-ketone derivative has a structure represented by the formula (I), wherein, R is phenyl, para-methoxyphenyl, para-chlorophenyl, para-fluorophenyl, ortho-chlorophenyl, ortho-methoxyphenyl, meta-bromophenyl, 1-naphthyl, para-bromophenyl, para-nitrophenyl, para-methylphenyl or 3,4-difluorophenyl; R is phenyl, para-methylphenyl, para-methoxyphenyl, para-fluorophenyl, para-chlorophenyl, para-bromophenyl or 2-furyl. Such compounds can be obtained by means of high diastereoselectivity and high enantioselectivity of tandem reaction of organically catalytic C2 symmetrical chiral sulfur ylide and nitroolefin.
Owner:HUAZHONG NORMAL UNIV
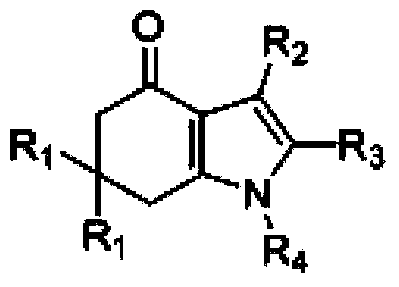
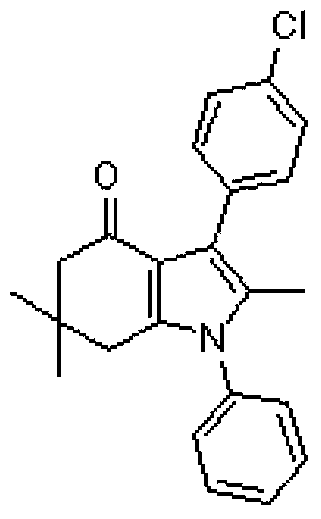
Tetrahydroindole compound, and preparation method and application thereof
InactiveCN103467356AHigh yieldHigh purityOrganic chemistryAntineoplastic agentsChemical synthesisSolvent
The invention discloses a tetrahydroindole compound, and a preparation method and an application thereof, and belongs to the technical field of the chemical synthesis. Raw materials comprising a 1,3-cyclohexanedione compound, nitroalkene and amine undergo a one-pot process under microwave radiation in water as a solvent under the action of L-proline as a catalyst to prepare the tetrahydroindole compound in high yield. The catalyst used in the invention is a non-transition metal catalyst having a low price, so the synthesis cost is substantially reduced; the reaction condition in a catalysis system is mild and can be easily controlled, and the product can be obtained through a domino cyclized multi-component one-step reaction; and the method has the advantages of green and pollution-free experiment program, simple and effective experiment operation, and diversified product structure. The method which uses water as a solvent has the characteristics of simple post-treatment, small pollution to the environment, no damages to the health of the body of an operation worker, and easy realization of the industrialized production.
Owner:SHAOXING UNIVERSITY
Method for synthesizing beta-iodo-nitroolefin compound
InactiveCN106083505AHigh yieldMild reaction conditionsNitro/nitroso group formation/introductionNitro compound preparationNitroalkeneNitrite
The invention provides a method for synthesizing a beta-iodo-nitroolefin compound as shown in a formula (III). The method comprises: mixing substituted alkyne shown in a formula (I), an iodine source and tert-butyl nitrite shown in a formula (II) in a solvent for completely reacting to obtain reaction liquid, and after reacting for 4 to 6h at a temperature of 25 to 80 DEG C, processing the reaction liquid to obtain the beta-iodo-nitroolefin compound, wherein the solvent is tetrahydrofuran, acetonitrile, acetone or 1,2-dichloroethane, and a ratio of quantities of fed substances, i.e., the substituted alkyne shown in the formula (I), the iodine source and the tert-butyl nitrite shown in the formula (II), is 1 to (0.5 to 1) to (2 to 5). The method provided by the invention is high in substrate adaptability and mild in reaction condition, does not relate to use of an oxidizing agent and a metal catalyst, is safe and environmental-friendly, is simple in reaction operation, and is more beneficial to application in medicine synthesis. The formulas are shown in the description.
Owner:ZHEJIANG UNIV OF TECH
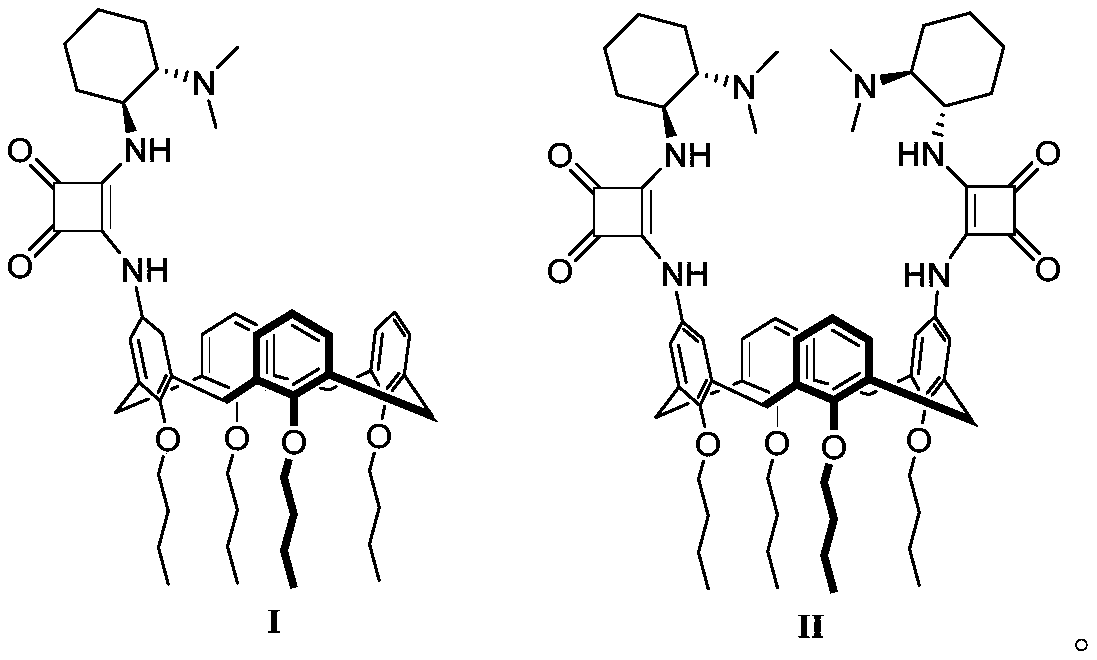
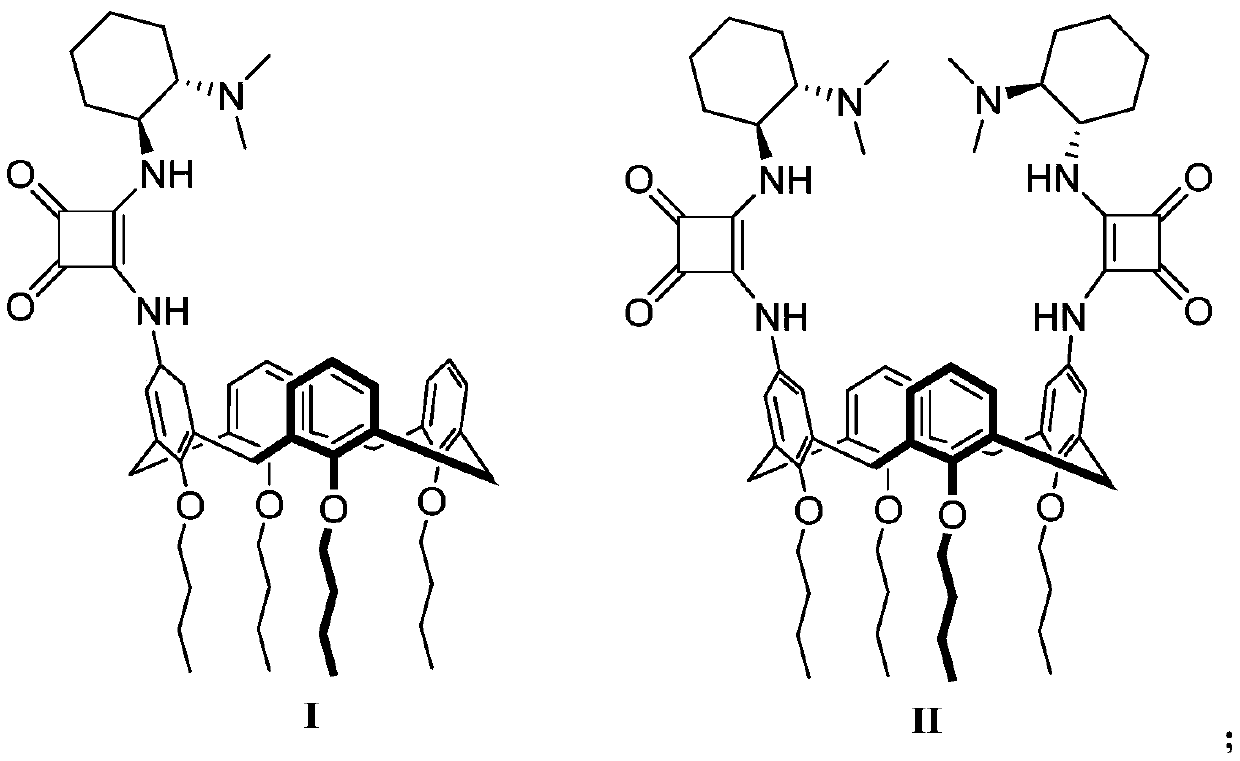
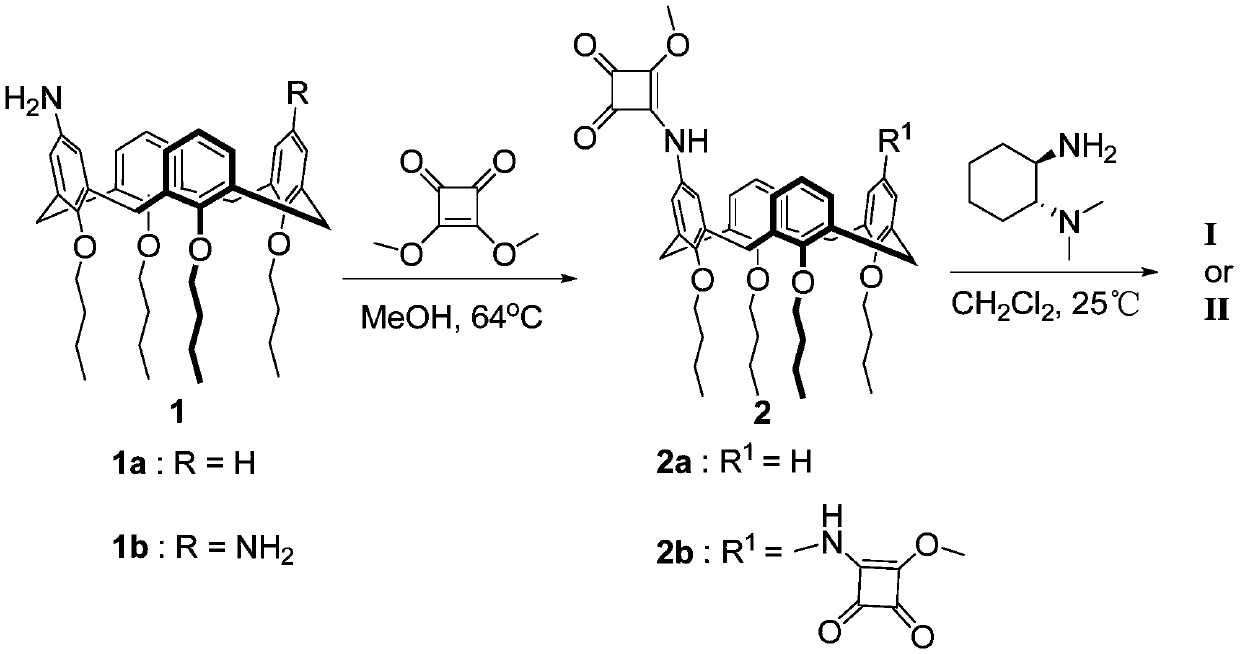
Calix[4]thiourea diaminocyclohexane derivatives and method thereof for catalyzing asymmetric Michael addition
InactiveCN108727241AEffective Phase Transfer CatalysisSynthetic process conditions are mildOrganic chemistryOrganic compound preparationChromatographic separationNitroalkene
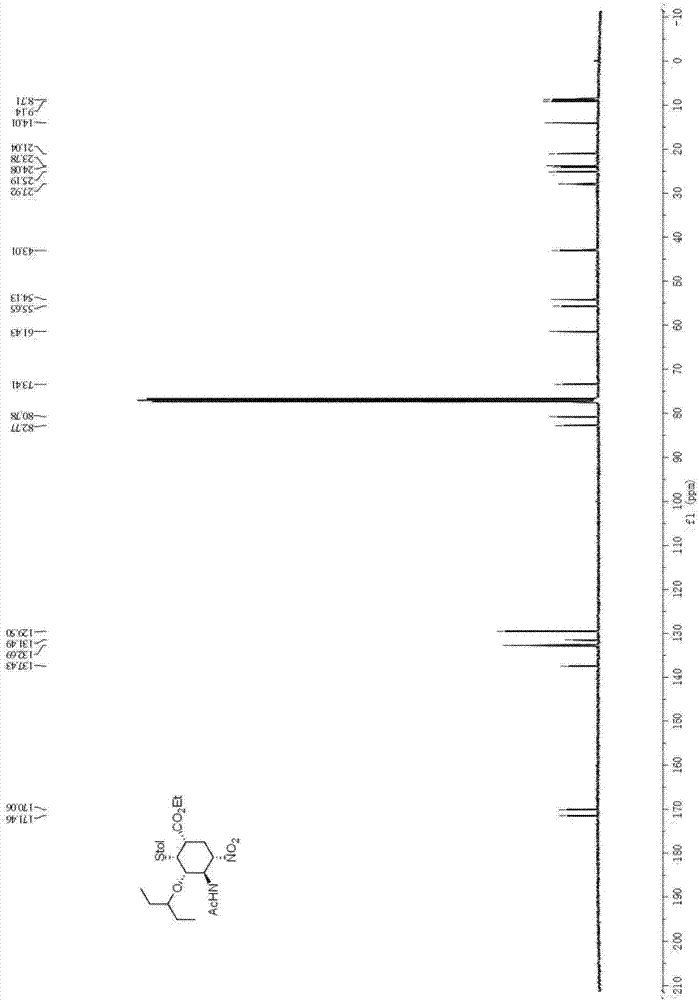
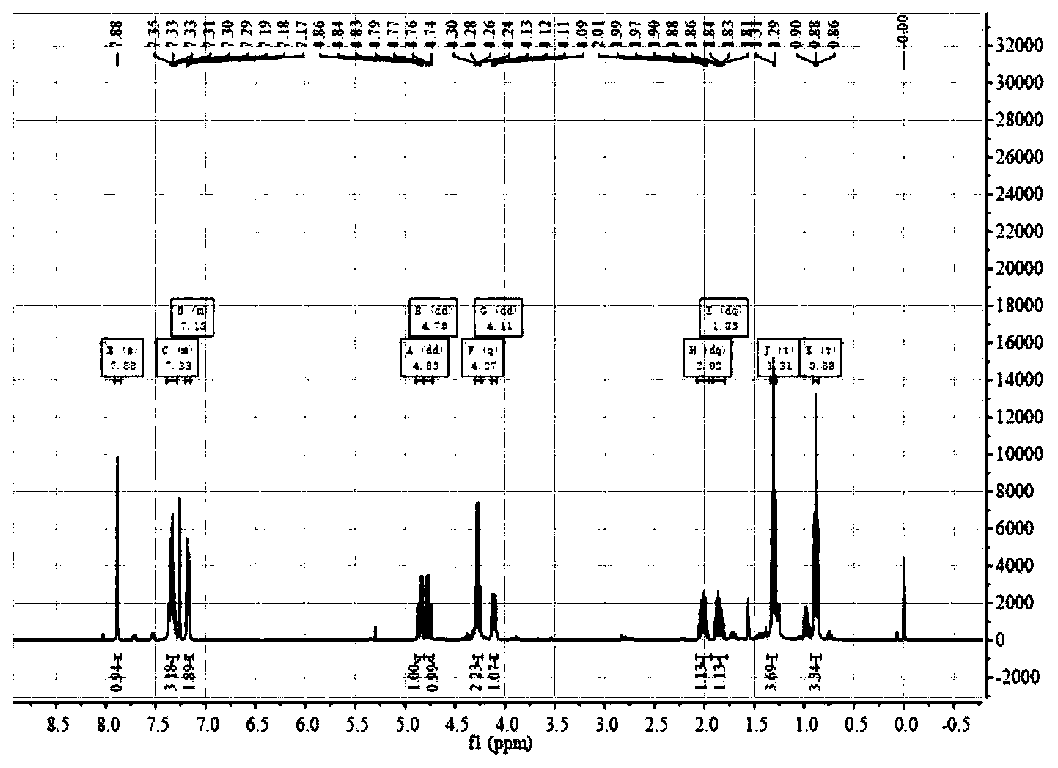
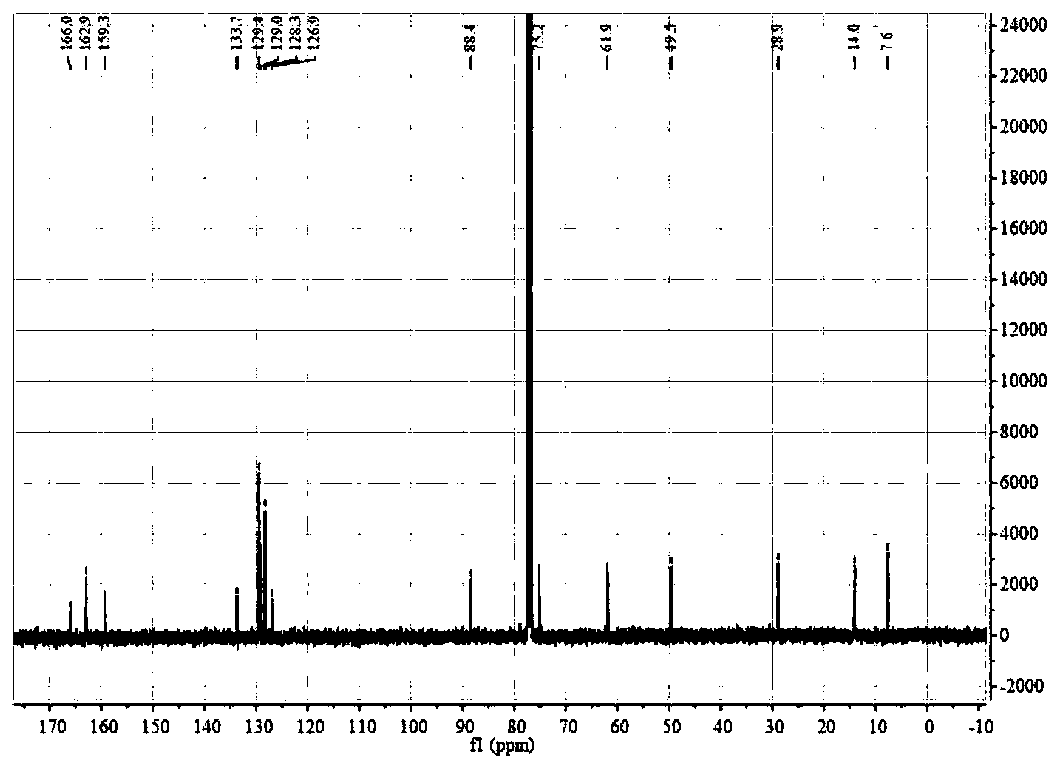
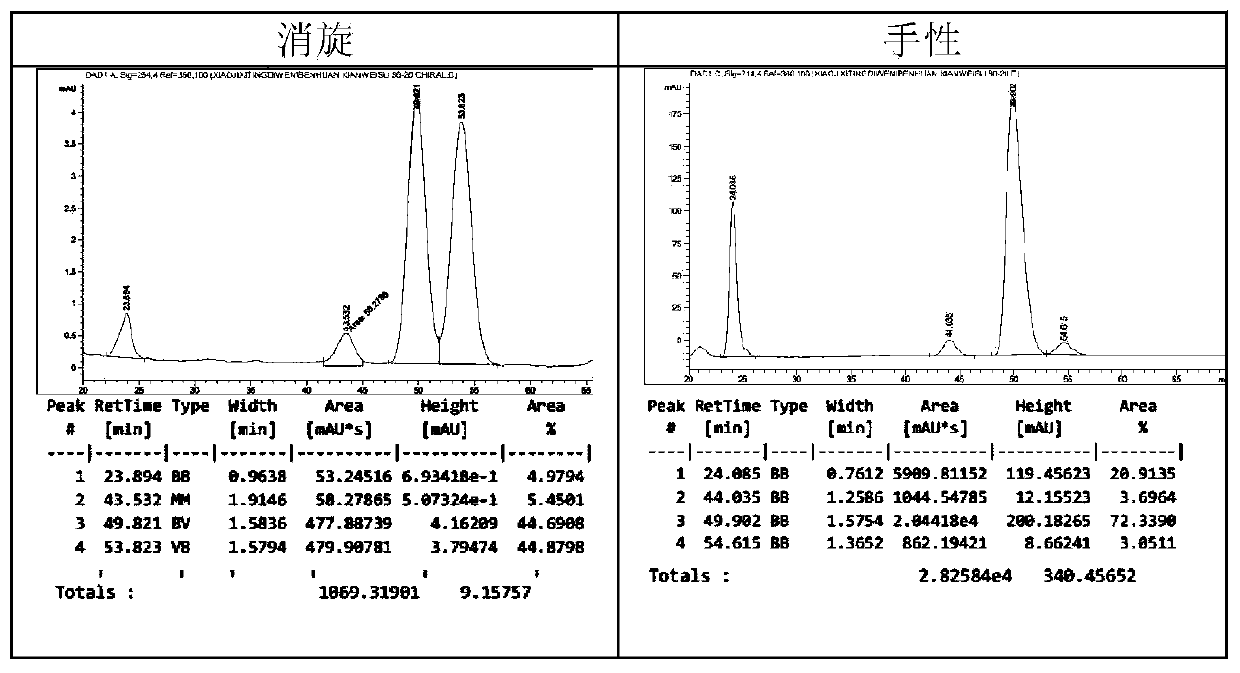
The invention relates to calix[4]thiourea diaminocyclohexane derivatives and a method thereof for catalyzing asymmetric Michael addition. An Michael addition catalytic reaction is performed on nitroolefin and 1,3-dicarbonyl ketone as raw materials, the calix[4]thiourea diaminocyclohexane derivatives as a phase transfer catalyst and water / toluene as a solvent, after the reaction, the solvent is concentrated, and a product is obtained by column chromatographic separation on silica gel. The calix[4]thiourea diaminocyclohexane derivatives adopt mild synthesis process conditions and are high in catalysis efficiency, water / toluene is taken as the solvent for asymmetric Michael addition catalyzed by the derivatives, reaction speed and yield can be increased greatly by water addition, and the catalyst has a phase transfer catalysis function. Better ee value can be obtained by the catalytic reaction at room temperature, and the derivatives have broad application prospect.
Owner:CHANGZHOU UNIV
Method for catalyzing asymmetric Michael addition reaction and catalyst
ActiveCN110372514ARich variety of chemical modificationSynthetic raw materials are readily availableOrganic compound preparationOrganic chemistry methodsChromatographic separationNitroalkene
The invention belongs to the technical field of catalytic organic synthesis and particularly relates to a method for catalyzing an asymmetric Michael addition reaction and a catalyst. Nitroolefin and1,3-dicarbonyl ketone are taken as raw materials, a calix[4]squaramide-diaminocyclohexane derivative is taken as a catalyst, dichloromethane is taken as a solvent, the Michael addition catalytic reaction is conducted, after the reaction is completed, the solvent is concentrated, and a product is obtained through silica gel column chromatographic separation. The calix[4]squaramide-diaminocyclohexane catalyst is mild in synthetic process condition and high in catalytic efficiency, the good ee value can be obtained through the catalytic reaction under the room temperature condition, and wide application prospects are realized.
Owner:CHANGZHOU UNIV
Preparation method of nitroolefin derivative with nitrate as nitro source
ActiveCN110003011ARaw materials are cheap and easy to getHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationTrimethylsilyl chlorideRotary evaporator
The invention relates to a nitroolefin derivative with nitrate as nitro source and a preparation method thereof. Under the atmosphere of nitrogen, an olefin compound, nitrate, trimethylchlorosilane (TMSC1) and copper salt are stirred in acetonitrile at 0-30 DEG C; in addition, the reaction degree is monitored by using a TLC point plate; after the olefin compound is completely consumed, alkali is added to an obtained mixture to be stirred for 20-30 min; then after a solvent is removed from an obtained mixture by using a rotary evaporator, the nitroolefin derivative can be obtained through silicagel column purification. Compared with the prior art, the nitroolefin derivative with the nitrate as the nitro source, provided by the invention, has the advantages of mild reaction condition, high yield, high E type selectivity and the like.
Owner:SHANGHAI NORMAL UNIVERSITY
Synthesis method of oseltamivir
InactiveCN107304171AShort routeOrganic compound preparationOrganic chemistry methodsSynthesis methodsOrganic synthesis
The invention belongs to the technical field of organic synthesis, aims at solving the problems that an existing synthesis method of oseltamivir is multiple in reaction steps, low in overall yield and high in cost. The invention provides a synthesis method of oseltamivir. The method comprises the following steps of (1) adopting 3-pentyloxy acetaldehyde and nitroolefin as substrates and reacting under catalysis of a catalyst in the presence of lewis acid to obtain an aldehyde intermediate A; (2) reacting the obtained aldehyde intermediate A with 2-diethoxy oxygen-phosphorus acrylic acid ethyl ester under the action of an alkali catalyst to obtain a cyclohexenyl intermediate B; (3) reacting the cyclohexenyl intermediate B with thiocresol to obtain a cyclohexane intermediate C; (4) preparing a compound D from the cyclohexane intermediate C under the action of zinc powder and trimethylchlorosilane; and (5) obtaining a final product oseltamivir from the intermediate D obtained in the step (4) under the action of an ammonia gas and potassium carbonate. The method has the characteristics that the route is short and the catalyst is easy to recover.
Owner:HANGZHOU NORMAL UNIVERSITY
Preparation method of 1, 3-dimethyl-pentylaminehydrochloride
ActiveCN103467303AReduce manufacturing costEasy to operateOrganic compound preparationAmino compound preparationPotassium borohydridePtru catalyst
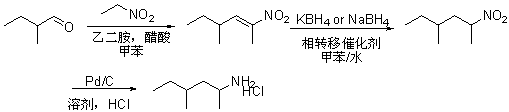
The invention discloses a preparation method of 1, 3-dimethyl-pentylaminehydrochloride. The preparation method comprises the following steps: performing condensation and dehydration on 2-methylbutyraldehyde used a starting material and nitroethane under the catalyzing of an acetic acid / ethylenediamine composite catalyst in a methylbenzene solvent to obtain a nitroolefin compound; reducing through potassium borohydride or sodium borohydride; then reducing through palladium carbon and acidizing through hydrochloric acid to obtain 1, 3-dimethyl-pentylaminehydrochloride. The preparation method of 1, 3-dimethyl-pentylaminehydrochloride has the advantages that the raw materials are simple and easy to obtain; the reaction condition is mild; the total yield is high; the industrial production can be performed well.
Owner:上海倍殊生物科技有限公司
Preparation method of beta-trans-nitroolefin
ActiveCN111217707AImprove toleranceHigh yieldEnergy efficient lightingNitro compound preparationNitroalkeneDrugs synthesis
The invention relates to a preparation method of beta-trans-nitroolefin. The method comprises: sequentially adding an olefin compound, a nitration reagent and a solvent into a reaction container, mixing the substances uniformly, and carrying out constant temperature reaction for 18h under an illumination condition to obtain a reaction solution; and sequentially carrying out drying, concentration and column chromatography treatment on the reaction solution to obtain the beta-trans-nitroolefin compound. The method is simple and practicable, low in cost and high in product yield, can realize large-scale production, and has good industrial application prospects in the aspects of functional organic material, bioactive compound and drug synthesis.
Owner:NORTHWEST NORMAL UNIVERSITY
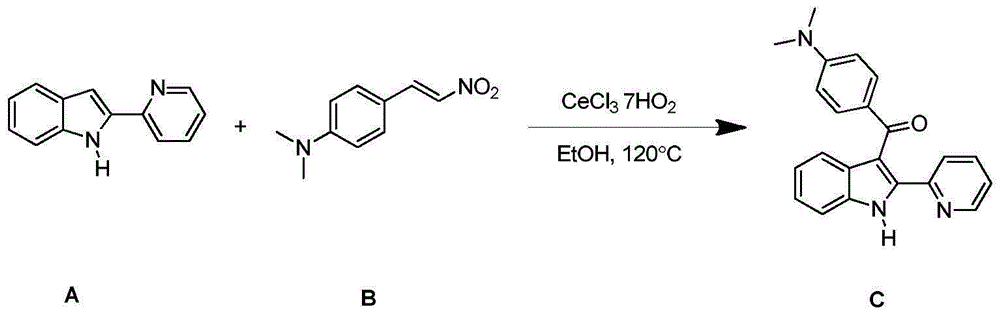
Synthesizing method for 3-aroyl indole compound
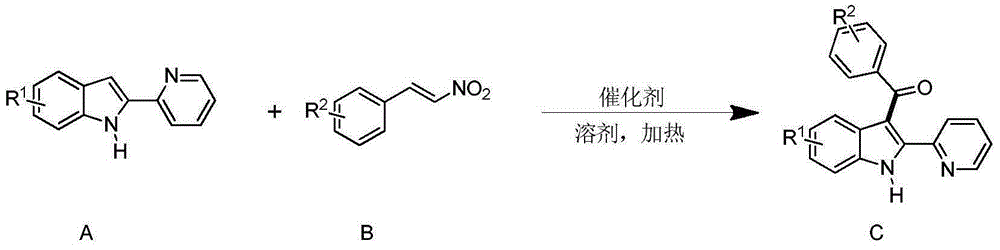
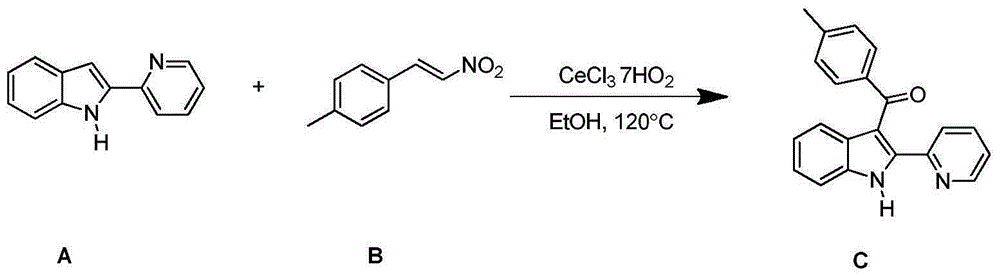
The invention discloses a synthesizing method for a 3-aroyl indole compound as shown in the following formula (C). The synthesizing method is characterized in that R1 replacing indole (A) and R2 replacing nitroalkene serve as raw materials, cerium salt serves as a catalyst, and in an air environment, acylation reaction is performed. According to the synthesizing method for the 3-aroyl indole compound, the more stable and cheaper cerium salt is adopted to serve as the catalyst, absolute non-water processing does not need to be performed in the reaction, inert gases are not needed for protection, that is, the acylation reaction can be directly performed, the reaction condition is milder, and the yield is high.
Owner:ANHUI UNIV OF SCI & TECH
Method for synthesizing NH-1,2,3-triazole
InactiveCN104311495AHigh synthetic yieldMild reaction conditionsOrganic chemistryHydrogen atomNitroalkene
The invention discloses a method for synthesizing NH-1,2,3-triazole shown by a formula (I). Under the catalytic action of a Lewis acid catalyst, nitroalkene shown by a formula (II) and hydrazoic acid salt undergo 1,3-dipolar cycloaddition, wherein Ar is phenyl, substituted phenyl, naphthyl, furyl, thienyl, indolyl or styryl; and R is hydrogen atom, phenyl, substituted phenyl or C1-C6 alkyl. The method is mild in reaction condition, low in production cost, wide in substrate application range and high in yield, and provides a new path for synthesizing NH-1,2,3-triazole compounds with medicinal activity.
Owner:NORTHWEST UNIV
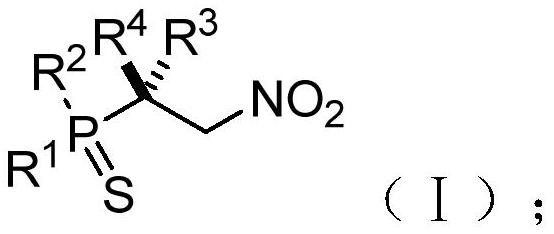
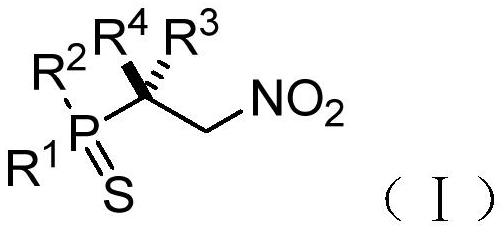
Chiral phosphorus-sulfur compound and Michael addition method thereof
PendingCN112409408AAchieve Michael bonusHigh enantioselectivityOrganic chemistry methodsGroup 5/15 element organic compoundsSimple Organic CompoundsNitroalkene
The invention relates to the technical field of organic compound synthesis, and provides a chiral phosphorus-sulfur compound and a Michael addition method thereof. The Michael addition method of the chiral phosphorus-sulfur compound comprises the following steps: respectively providing a phosphorus-sulfur compound A and a nitroolefin compound B with the following structures: A: B: adding the phosphorus-sulfur compound A and the nitroolefin compound B into a reaction system containing an N-heterocyclic carbene catalyst, a proton additive, an alkali reagent and a water absorption additive; performing a reaction under the condition that the temperature is -80 - 25 DEG C to obtain the chiral beta-nitro phosphorus-sulfur compound shown as the following structural general formula (I), thereby completing the Michael addition of the chiral phosphorus-sulfur compound.
Owner:SHENZHEN BAY LAB PINGSHAN TRANSLATIONAL MEDICINE CENT
Preparation method of indolocarbazole compound
InactiveCN110483524AMild reaction conditionsReaction conditions are readily availableOrganic chemistryNitroalkeneMetal catalyst
The invention provides a preparation method of an indolocarbazole compound, and the indolocarbazole compound is prepared through steps of: with substituted indole and nitroolefin as initial raw materials, performing a multi-component reaction under a room-temperature acidic condition. The preparation method provided by the invention has the advantages of mild reaction conditions, simple operation,no need of a metal catalyst, easily available reaction raw materials, simultaneous introduction of a plurality of substituents, easy separation of products and the like, and has a wide substrate application range. According to the method, various substrate structures can tolerate the reaction conditions, the application range is wide, and a simple and feasible method is provided for synthesizingthe indolocarbazole compound. The structural formula of the indolocarbazole compound is shown in the specification.
Owner:ZHEJIANG UNIV
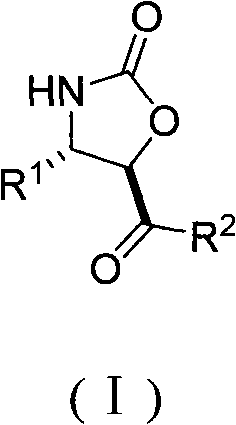
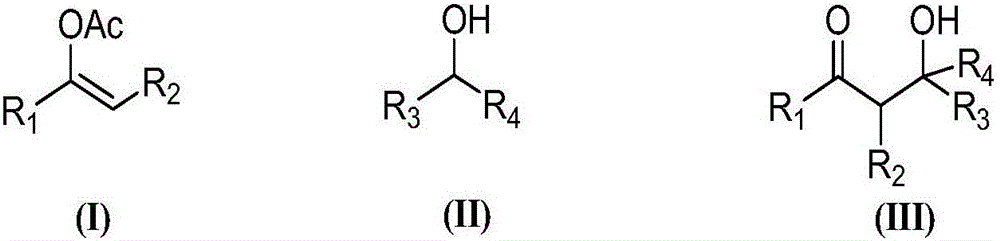
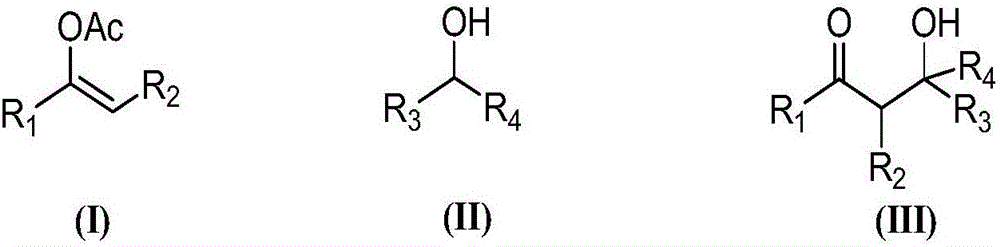
Chiral dicyclic compound and asymmetric syntheses method thereof
ActiveCN102531911AChiralImprove response characteristicsOrganic chemistryOrganic compound preparationNitroalkeneOrganic solvent
The invention discloses a chiral dicyclic compound and an asymmetric syntheses method thereof, and the chiral dicyclic compound has a structure as shown in formula (I). According to the invention, a cyclohexenone derivative with a structure as shown in formula (II) and a nitroalkene derivative with a structure as shown in formula (III) are used as substrates, and a reaction is performed in an organic solvent with the catalysis of a chiral secondary amine catalyst, a polyglycol-series compound and an acid so as to obtain the chiral dicyclic compound with a structure as shown in formula (I). The chiral dicyclic compound synthesized in the invention has chirality, can be used as a synthetic intermediate of chiral compounds, and has wide application prospects.
Owner:ZHEJIANG UNIV OF TECH
Chiral Heterogeneous Catalyst for Assymmetric Nitroaldol Reaction
InactiveUS20130096333A1Without loss yieldHigh enantioselectivityGroup 4/14 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsNitroalkeneHomogeneous catalysis
The present invention relates to the preparation of highly efficient chiral heterogeneous catalyst for asymmetric nitroaldol reaction, wherein Henry reactions of various aldehydes such as aromatic, aliphatic, α,β-unsaturated aldehydes, alicyclic aldehydes and nitroalkenes were carried out to produce optically active β-nitroalcohols in high yield, with moderate to excellent enantioselectivity (ee up to >99%) in presence of a base and an optically active chiral heterogeneous catalyst.
Owner:COUNCIL OF SCI & IND RES
Method for selectively synthesizing allylic sulfone compound by utilizing nonmetal catalyst
InactiveCN107417583AConvenient and effective synthesisIncrease reaction rateOrganic chemistryOrganic compound preparationNitro compoundReaction rate
The invention relates to a method for selectively synthesizing an allylic sulfone compound. The method comprises the following steps: under the conditions of taking nitroolefin and sodium sulfinate stable and convenient to operate as raw materials and having participation of a non-metal catalyst and an oxidant, adding a solvent for heating and stirring, and processing to obtain the allylic sulfone compound. The method has the beneficial effects that by adopting low-cost and easily available I2 or TBAI as a catalyst, nitroolefin is activated by iodine, and sulfonate can be used as a mild lewis base in reaction. The key step of the reaction is that lewis base can promote the balance of nitroolefin and ally-nitro compound, and through the step, stable nitroolefin is converted into ally-nitro compound, and due to the compounding of ally-nitro, free radical addition reaction can occur easily, so that the reaction is more easily conducted under mild conditions, the reaction rate is increased, the reaction time is shortened, and the compound of allylic sulfone compound structure can be more conveniently and effectively synthesized.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
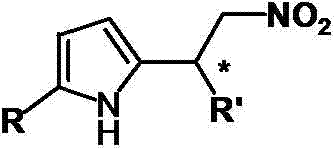
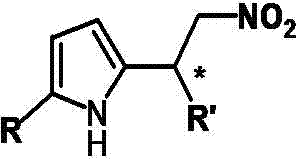
Catalytic synthesized beta-nitropyrrole derivative and synthetic method thereof
The invention relates to a catalytic synthesized beta-nitropyrrole derivative and a synthetic method thereof, a general formula of the catalytic synthesized beta-nitropyrrole derivative is shown as follows. The preparation method comprises the following steps: a solution of a chiral trimetal Pd / Sm / Pd complex catalyst is performed with in-situ preparation in a solvent, a pyrroles compound and a nitro olefines compound are added while stirring, and a mixture is separated by a column chromatography to obtain a pure product which is the beta-nitro pyrroles derivative. The method has the advantage that trimetal complex is taken as a catalyst for synthesizing the beta-nitro pyrroles derivative, and the method has the advantages of convenient preparation, low price, high yield and realization of large-batch synthesis.
Owner:JINGCHU UNIV OF TECH
Chiral gamma, gamma-disubstituted butenolide compound and preparation method thereof
The invention discloses a chiral gamma, gamma-disubstituted butenolide compound and a preparation method thereof. Chiral gamma, gamma-disubstituted butenolide compounds widely exist in various naturalproducts, have important biological activity, and are also important intermediates for constructing polyterpene compounds and medicines. According to the application, a series of chiral gamma, gamma-disubstituted butenolide compounds with the yield as high as 98% and the stereoselectivity greater than 20: 1dr and 99% ee are synthesized through a vinylogous Michael addition reaction of gamma-dimerfuranone catalyzed by a bifunctional thiourea catalyst and alpha, beta-unsaturated nitroolefin. According to the method, the functional gamma, gamma-disubstituted butenolide compound with multiple chiral centers can be conveniently, quickly and efficiently obtained.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
Method for producing an optically active nitro compound
InactiveUS20070037976A1Organic compound preparationCarboxylic acid amides optical isomer preparationNitro compoundHydrogen atom
An optically active nitro compound having two hydrogen atoms on its α-cabon atom and having β-asymmetric carbon atom can be produced by making α, β-unsaturated nitroolefin having a hydrogen atom on its α-cabon atom react with at least two organosilicon compounds having at lest one silicon-hydrogen bond in the molecule in the presence of an asymmetric copper complex, or react with an organosilicon compound having at least one silicon-hydrogen bond in the molecule in the presence of an asymmetric copper complex and water.
Owner:SUMITOMO CHEM COMPANY LIMITED PARTIAL INTEREST
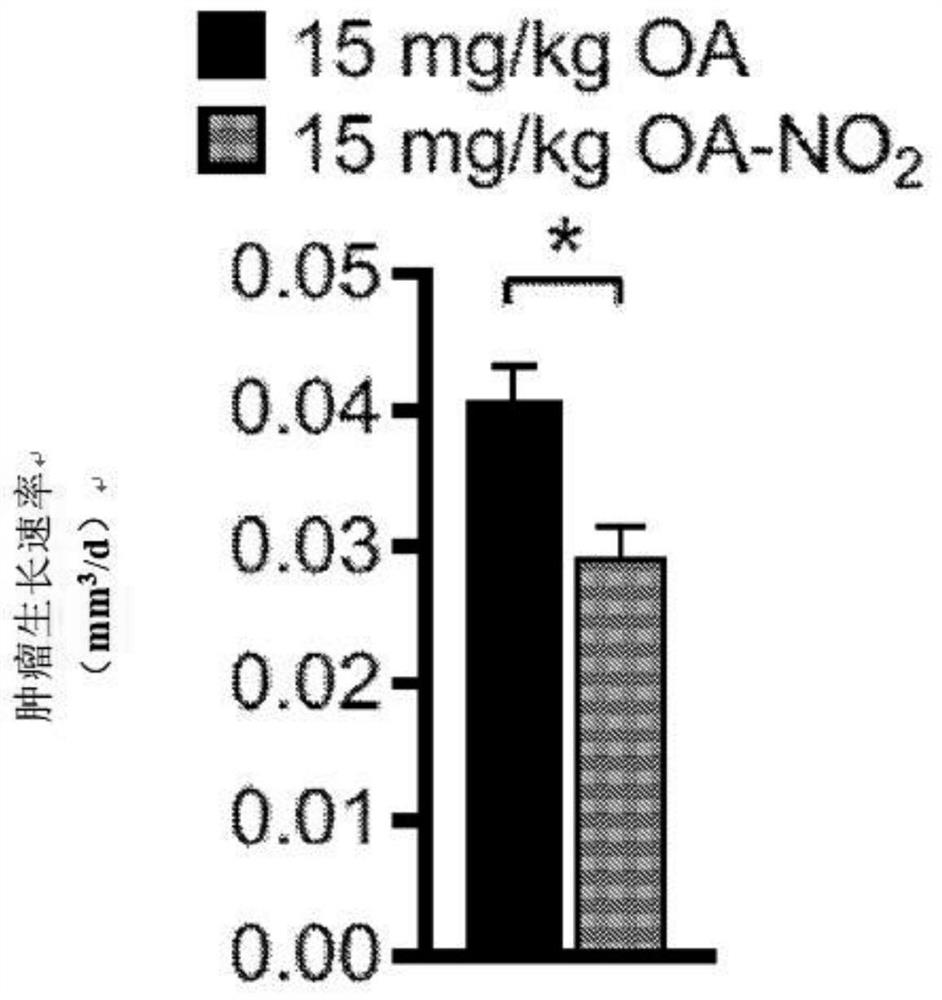
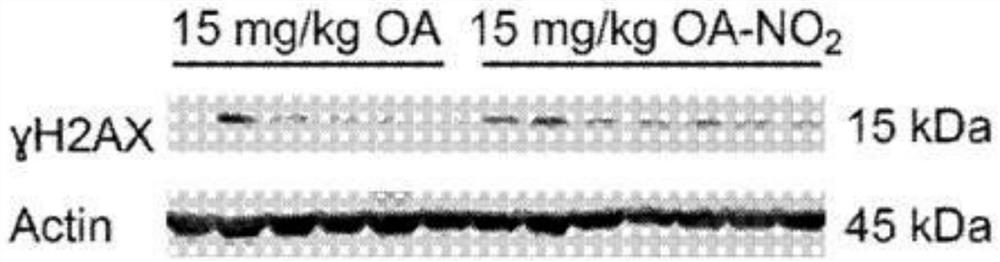
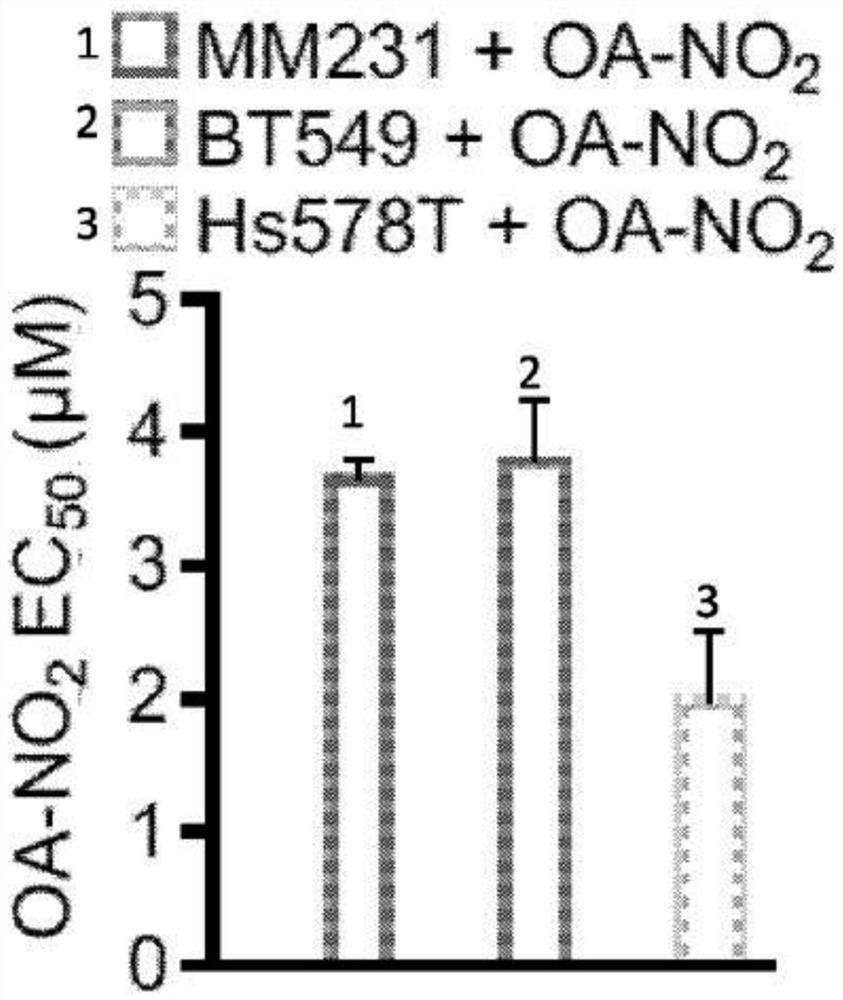
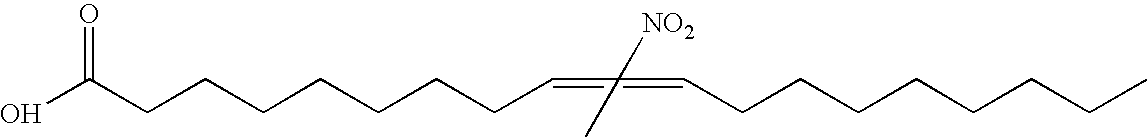
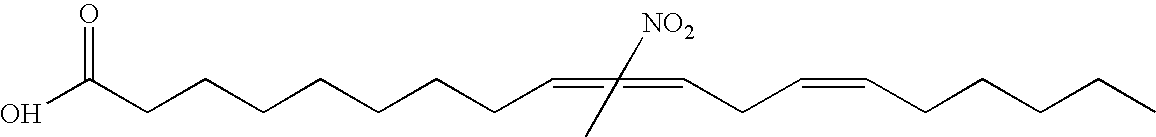
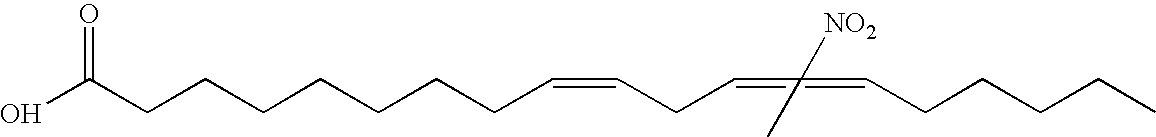
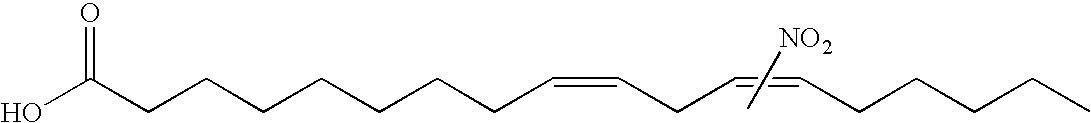
Electrophiles and electrophile pro-drugs as rad51 inhibitors
A method comprising co-administering to a subject having cancer, suspected of having cancer, or at risk of developing cancer: a therapeutically effective amount of at least one compound (a) selected from (a)(i) a nitroalkene fatty acid, (a)(ii) an unsaturated fatty acid having an electron withdrawing group, a leaving group, and a carbon-carbon double bond disposed between the electron withdrawing group and the leaving group, (a)(iii) a thiolated nitro fatty acid, or (a)(iv) a dicarboxylic acid compound containing an electron withdrawing group; and a therapeutically effective amount of at least one anti-neoplastic agent (b), wherein the cancer is a cancer with hereditary etiology of defects in DNA repair genes, a cancer with a high rate of spontaneous genomic instability, a cancer that responds well to DNA damaging agent(s), or a cancer that responds well to a combination of DNA damaging agent(s) with immunotherapy.
Owner:UNIVERSITY OF PITTSBURGH
Preparation method of aliskiren intermediate
ActiveCN102432466ARaw materials are easy to getAvoid splittingOrganic compound preparationCarboxylic acid esters preparationPentanalNitromethane
The invention relates to a preparation method of a compound, more specifically to a preparation method of an important intermediate (S)-2-isopropyl-4-oxobutyric acid methyl ester for synthesis of aliskiren. The preparation method provided by the invention comprises the following steps of: using isobutyraldehyde and nitromethane as raw materials, performing a Henry reaction and an elimination reaction to obtain corresponding conjugated nitroolefin, performing a Michael addition reaction between conjugated nitroolefin and acetaldehyde in the presence of a chiral catalyst to obtain (S)-4-methyl-3-(nitro methyl)pentanal, oxidizing the intermediate to obtain (S)-2-isopropyl-4-oxobutanoic acid, followed by methyl esterification to obtain (S)-2-isopropyl-4-oxobutyric acid methyl ester. The preparation method provided by the invention requires no low temperature, metal organic reagents or chiral separation, has advantages of easily obtained and low-priced raw materials and reagents, few synthesis steps, mild reaction condition and high yield, and is more suitable for industrial production.
Owner:WUHAN WUYAO PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
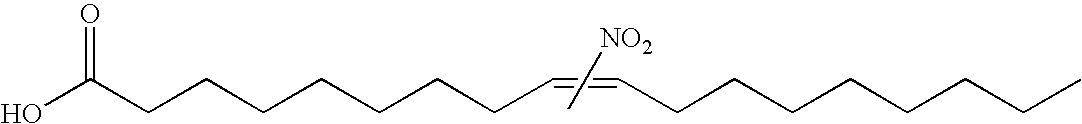
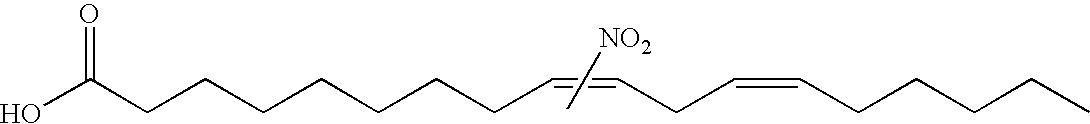
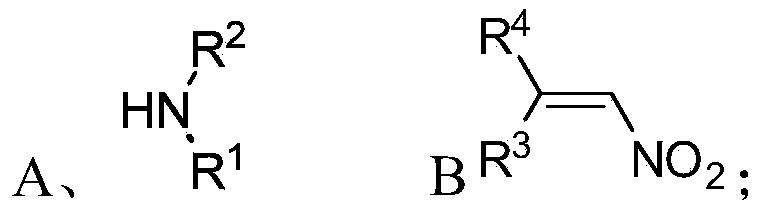
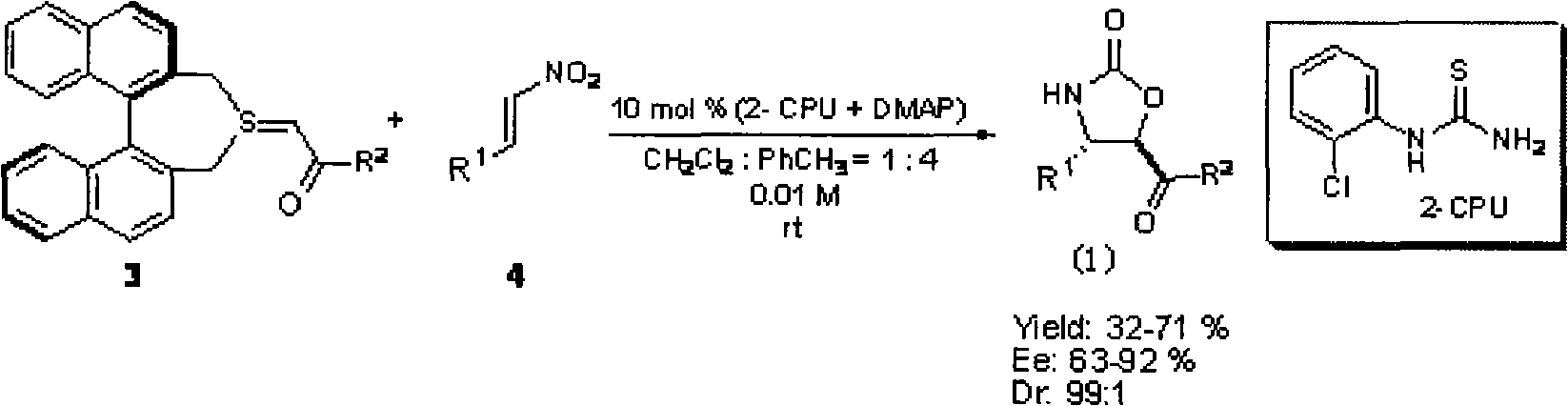
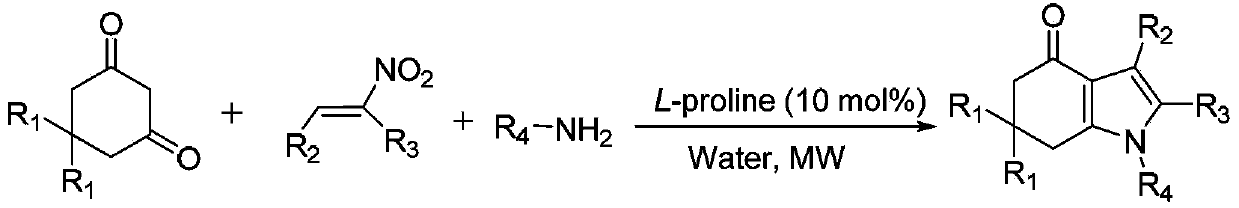
![Calix[4]thiourea diaminocyclohexane derivatives and method thereof for catalyzing asymmetric Michael addition Calix[4]thiourea diaminocyclohexane derivatives and method thereof for catalyzing asymmetric Michael addition](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7c46d7ac-c168-4c43-b645-f55ee7583e0b/BDA0001668876310000021.png)
![Calix[4]thiourea diaminocyclohexane derivatives and method thereof for catalyzing asymmetric Michael addition Calix[4]thiourea diaminocyclohexane derivatives and method thereof for catalyzing asymmetric Michael addition](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7c46d7ac-c168-4c43-b645-f55ee7583e0b/BDA0001668876310000041.png)
![Calix[4]thiourea diaminocyclohexane derivatives and method thereof for catalyzing asymmetric Michael addition Calix[4]thiourea diaminocyclohexane derivatives and method thereof for catalyzing asymmetric Michael addition](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/7c46d7ac-c168-4c43-b645-f55ee7583e0b/BDA0001668876310000071.png)