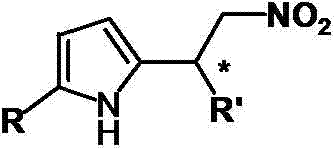
Catalytic synthesized beta-nitropyrrole derivative and synthetic method thereof
A technology of nitropyrrole and nitropyrrole is applied in the field of synthesis of optically pure β-nitropyrrole compounds, can solve the problems of less research on asymmetric alkylation of pyrrole ring and the like, achieves convenient preparation, low price, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Synthesis of 2-(2-nitro-1-phenylethyl)-1H-pyrrole
[0019] Preparation of a solution of chiral trimetallic Pd / Sm / Pd complex catalyst: To a stirred solution of ligand (4.15 mg, 0.01 mmol) in 0.4 ml THF, solid Pd(OAc) was added in portions at room temperature 2 (2.24 mg, 0.01 mmol), the mixture was stirred for 15min to obtain a yellow suspension, and Sm(OTf) was added in portions 3 (2.99 mg, 0.005 mmol), the yellow suspension immediately turned into a yellow transparent liquid, and the stirring was continued at room temperature for 15 min to obtain a solution of the chiral trimetallic Pd / Sm / Pd complex catalyst.
[0020] Add 1H-pyrrole (0.60 mmol) and 2-nitrostyrene (0.20 mmol) successively to the above catalyst solution, and stir at room temperature for 65 h. After the reaction, the resulting mixture is separated by column chromatography to obtain a pure product, a yellow solid, 1 H NMR (400 MHz, CDCl 3 ): δ 7.84 (bs, 1H), 7.38-7.29 (m, 3H), 7.25-7.23...
Embodiment 2
[0023] Example 2: Synthesis of 2-[1-(4-bromophenyl)-2-nitroethyl]-5-ethyl-1H-pyrrole
[0024] To a stirred solution of ligand (4.15 mg, 0.01 mmol) in 0.4 ml THF, solid Pd(OAc) was added in portions at room temperature 2 (2.24 mg, 0.01 mmol), the mixture was stirred for 15min to obtain a yellow suspension, and Sm(OTf) was added in portions 3 (2.99 mg, 0.005 mmol), the yellow suspension immediately turned into a yellow transparent liquid. Continue to stir at room temperature for 15 min to obtain a catalyst solution, add 2-ethyl-1H-pyrrole (0.60 mmol) and 4-bromo-2-nitrostyrene (0.20 mmol) successively to the solution, and stir at room temperature for 65 h , after the reaction, the resulting mixture was separated by column chromatography to obtain pure product, brown oil (58.0 mg, 90%). 1 H NMR (400 MHz, CDCl 3 ): δ 7.54 (bs, 1H), 7.50 (d, J = 8.0 Hz, 2H), 7.14 (d, J = 8.0 Hz, 2H), 5.96 (t, J = 4.0 Hz, 1H), 5.86 (t, J = 4.0 Hz, 1H), 4.97 (dd, J = 12.0, 4.0 Hz, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com