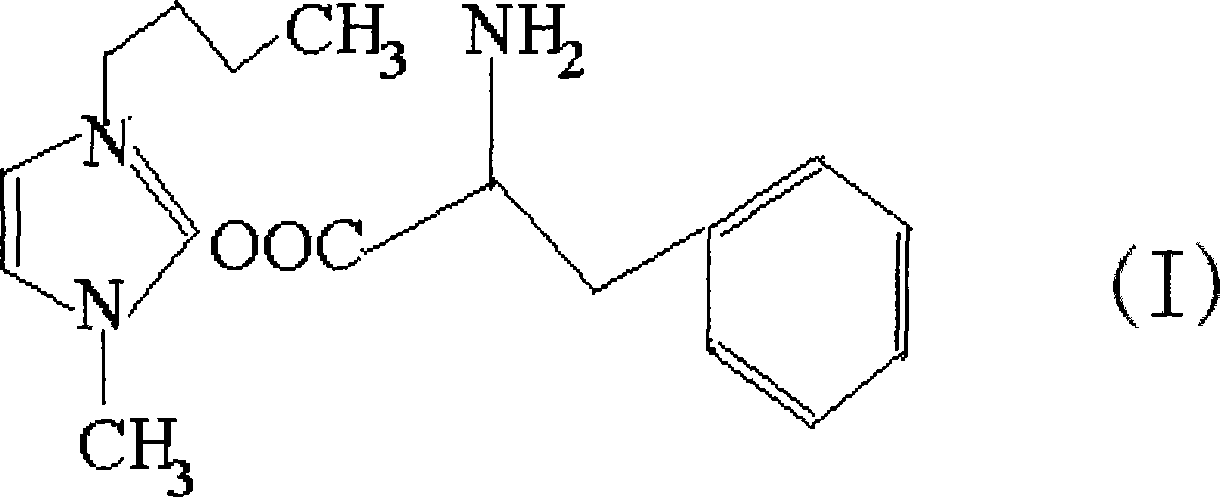
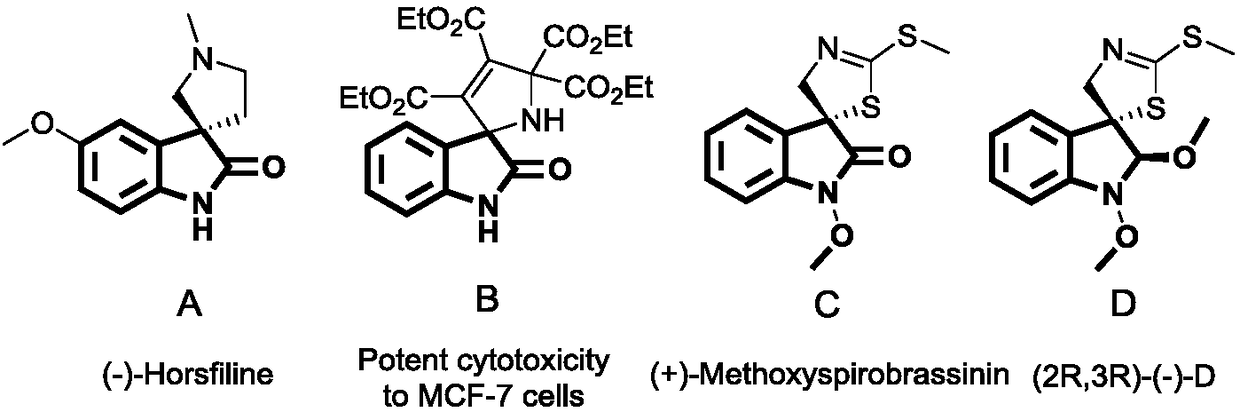
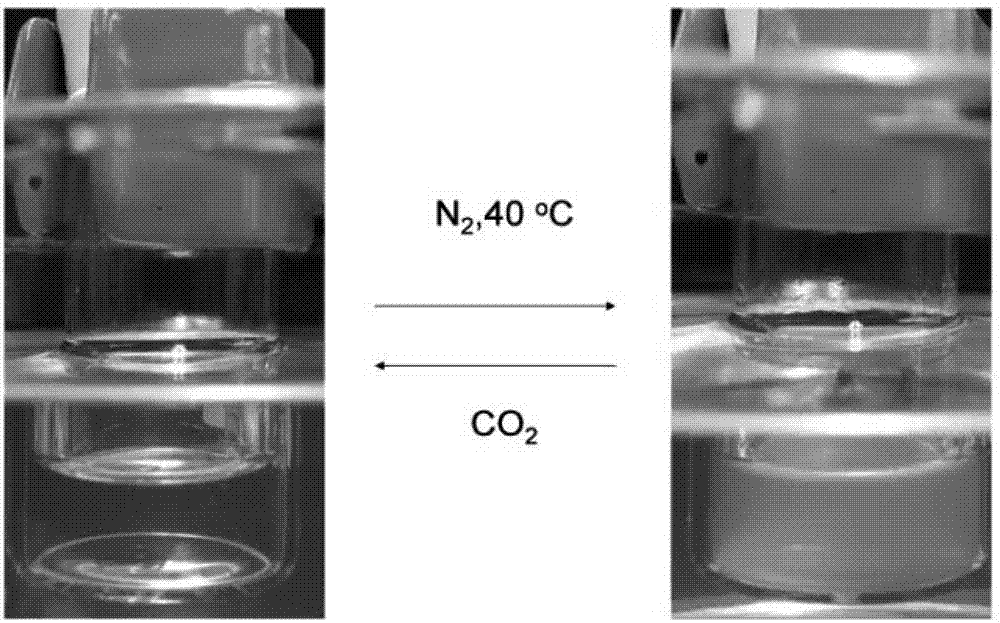
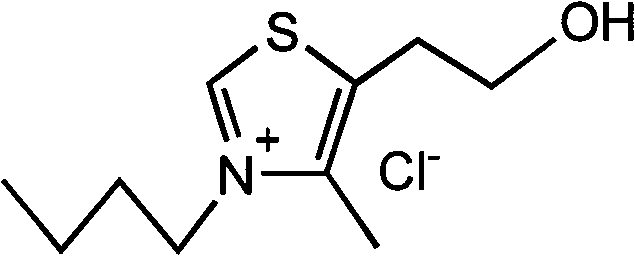
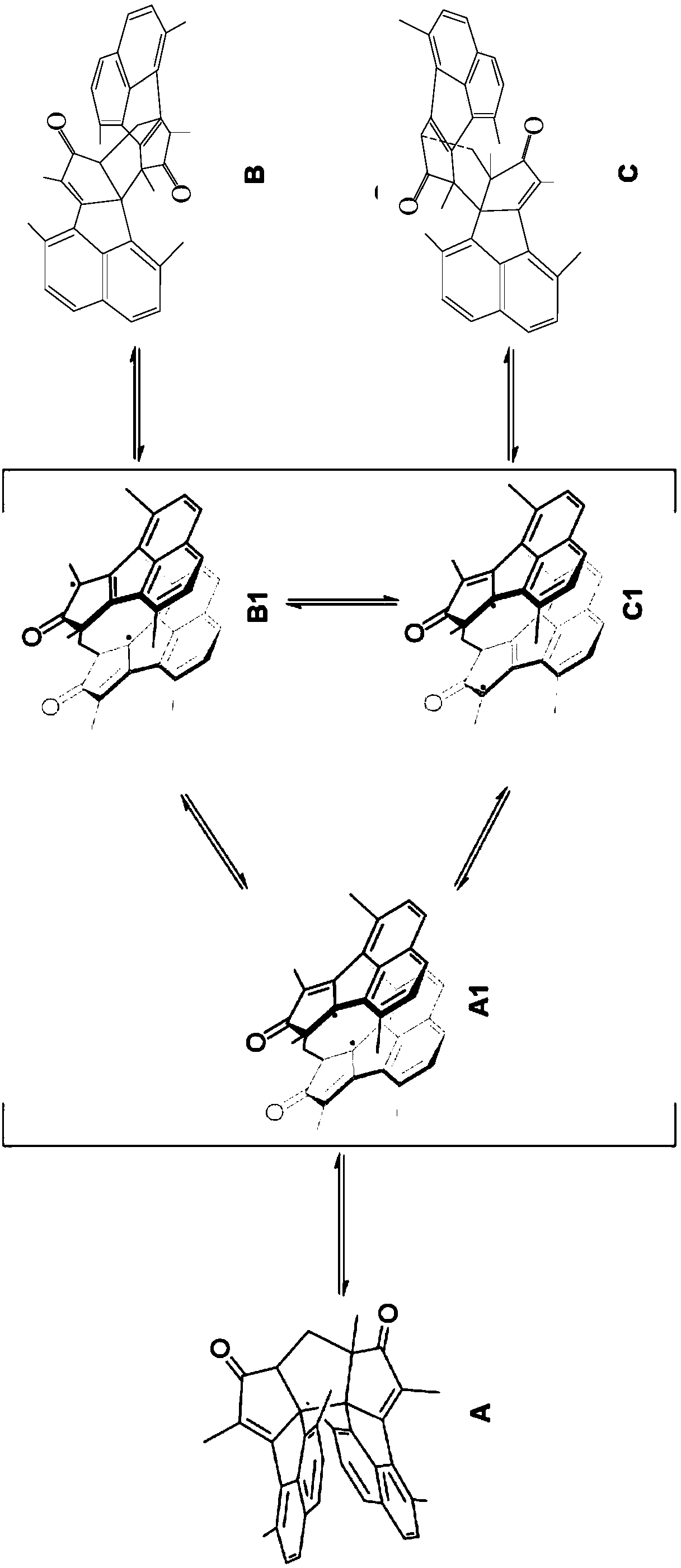
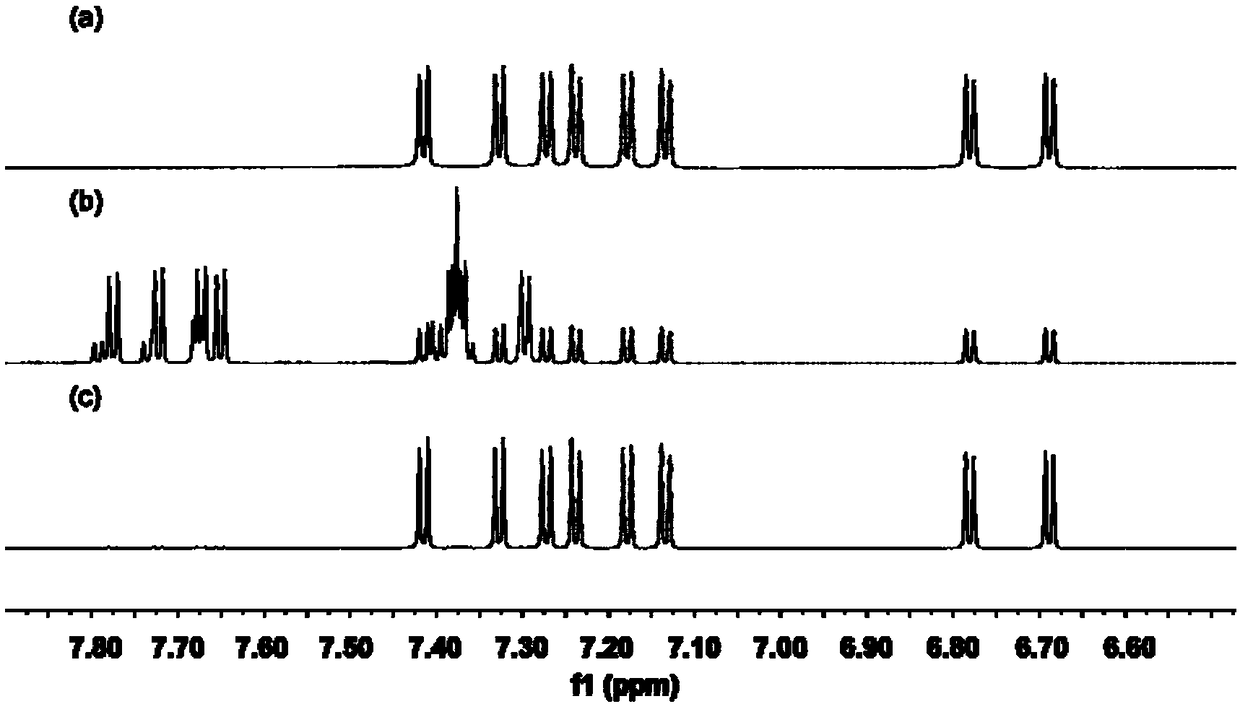
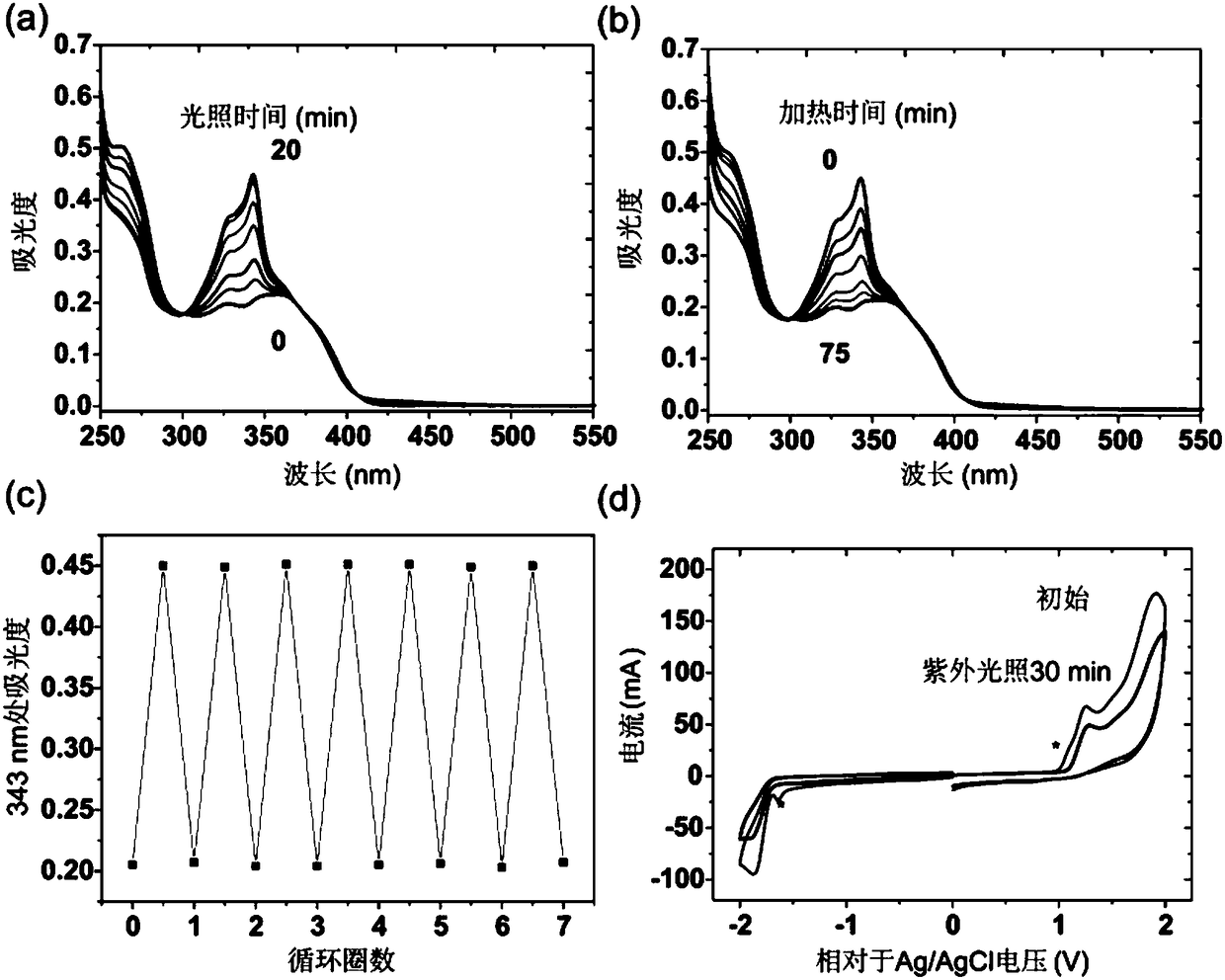
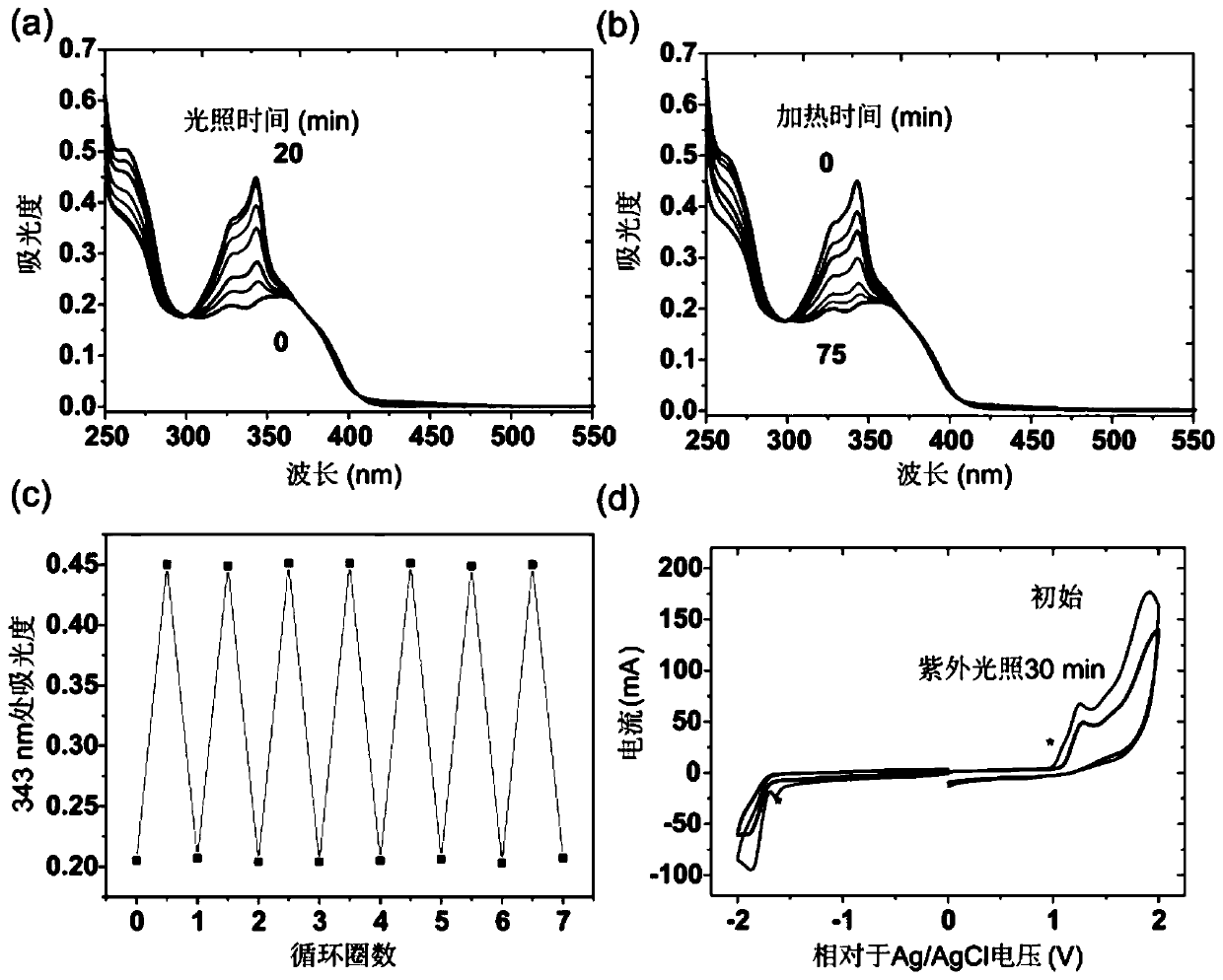
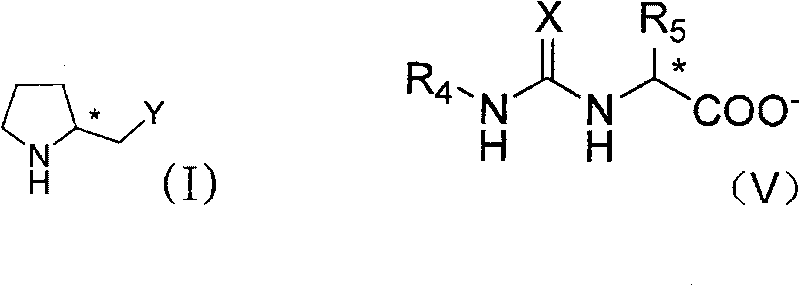
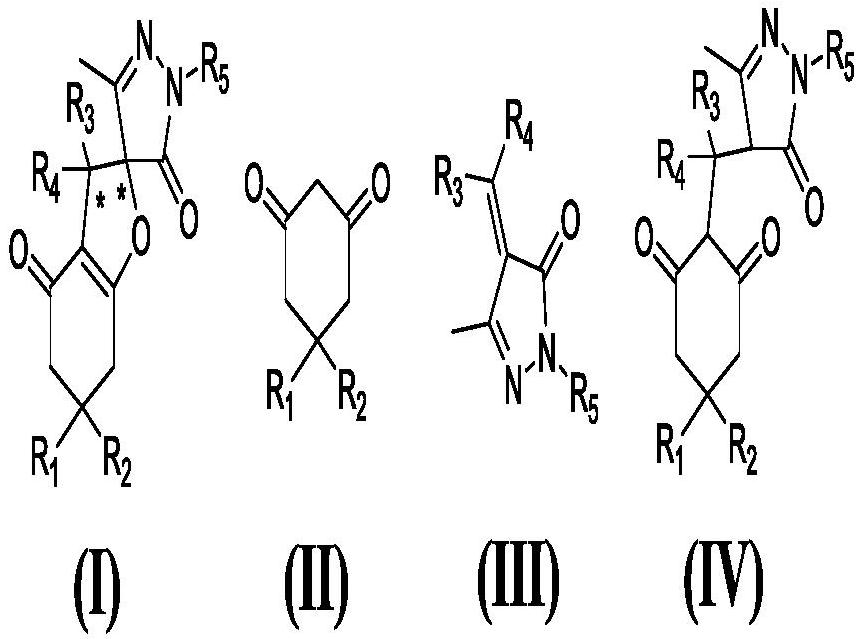
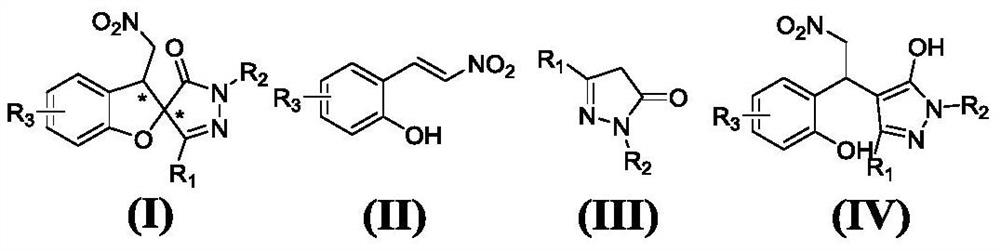
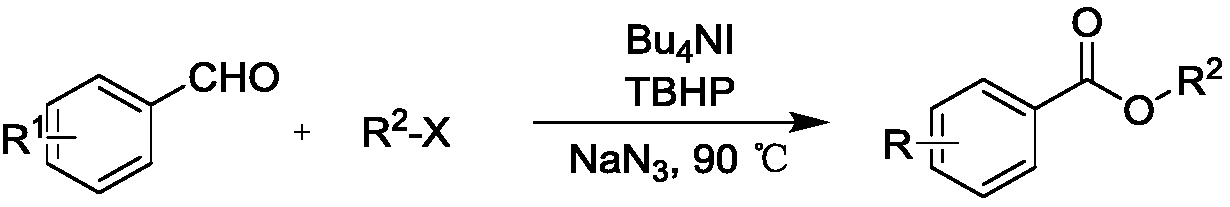Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32results about How to "Chiral" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phenylalanine-1-methyl-3-butyl-imidazole amino acid ionic liquid and preparing method thereof
This invention relates to a Phe-1-methyl-3-butyl imidazole amino acid ionic liquid and its preparation method including: chloro normal butane and N-methyl imidazole are heated and flown back to be synthesized to BMIC, acetic acid, ethyl and acetonit rile solution are heated and flown back to filter the BMIC, ions in anion exchange resin are exchanged to synthesize BMIOH, titration is done to BMIOH with HCl to take the BMIOH solution and add it into D-Phe solution to be mixed for 24h in icy water to eliminate the water and dry it for 48h under 80deg.C to be cooled and added with absolute methyl alcohol and acetonit rile solution to be sealed and beated up for 12h acutely, then placed at 0-10deg.C to pick up the un-reacted D-Phe and depress and filter them, rotate and evaporate to eliminate methyl alcohol and acetonit rile and get the product, which is dried under 80deg.C.
Owner:LIAONING UNIVERSITY
Asymmetric synthesis method of chiral benzofuran spirooxindole compound
The invention provides an asymmetric synthesis method of a chiral benzofuran spirooxindole compound as shown in a formula (I). The synthesis method is performed through the following steps of mixing ao-hydroxy nitroolefin compound as shown in the formula (II), with an oxoindole compound as shown in a formula (III), a chiral hydrogen key catalyst and an organic solvent, and performing a reaction at minus 40-60 DEG C for 1-240h so as to obtain a compound as shown in the formula (IV); and adding an iodine source additive and an oxidizer to the compound as shown in the formula (IV), performing areaction at minus 40-60 DEG C for 1-48h, and performing after-treatment on reaction liquid so as to obtain the chiral benzofuran spirooxindole compound as shown in the formula (I). According to the asymmetric synthesis method disclosed by the invention, the reaction condition is mild, the yield of products is high, and the selectivity is excellent. (As shown in the description).
Owner:ZHEJIANG UNIV OF TECH
Inorganic semiconductor nanometer material and preparation method thereof
The invention provides an inorganic semiconductor nanometer material and a preparation method thereof. The method comprises the following steps of: firstly, dissolving a cation coordination agent and a chiral stabilizer into the same solvent and adjusting the pH value to be 9-12 to enable the mixture to generate a chiral metal complex; and secondly, adding an anion coordination agent and heating at a temperature of 60-120DEG C to generate a kernel. In the invention, the chiral stabilizer is added into the raw materials, so that the obtained inorganic semiconductor nanometer material has a chiral characteristic; the adopted method is simple; and the prepared chiral nanometer material has diversified components and properties and wide application range.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Chiral CO2 responsive vinyl amino acid polymer and preparation method thereof
ActiveCN107501451AHighlight substantiveGood biocompatibilityPharmaceutical non-active ingredientsSodium azideOmega
The invention belongs to the field of intelligent polymer materials and relates to a chiral CO2 responsive vinyl amino acid polymer and a preparation method thereof. The polymer e is obtained by reacting a compound a with a compound b under the action of a catalyst to obtain a chiral monomer c, and polymerizing the monomer c in a solvent III, wherein the compound a is vinyl amino acid benzyl ester, and the compound b is an alpha-primary amine-omega-tertiary amine small molecule. The preparation method provided by the invention does not need a sodium azide reaction and a click reaction and is mild in reaction conditions; the polymer obtained by the preparation method disclosed by the invention has chirality; in addition, along with alternative introduction of CO2 and N2, the polymer has hydrophilic and hydrophobic transformation, which shows that the polymer has obvious CO2 response performance; besides, amino contained in the terminal of the polymer has remarkable advantages in DNA (Deoxyribonucleic Acid) compression and gene transfer; the chiral CO2 responsive vinyl amino acid polymer has a wider application prospect in the fields of drug controlled release, genetic engineering, chiral separation and catalysis.
Owner:BEIFANG UNIV OF NATITIES
Thiazole amino acid salt type ionic liquid and preparation method thereof
InactiveCN102153523AImprove conductivityLow melting pointOrganic compound preparationAmino-carboxyl compound preparationSolubilityThiazole
The invention relates to a thiazole amino acid salt type ionic liquid and a preparation method thereof. The ionic liquid has the structural general formula of A<+>B<->, wherein A<+> has a structural general formula containing R1, R2 and R3, and R1, R2 and R3 in the general formula comply to the following rules: (a) at least containing one carbon atom; and (b) at most containing 20 carbon atoms; and B<-> is one of amino acid anions. The preparation method comprises the following steps of: preparing a thiazole non-amino-acid-salt-type ionic liquid; converting the thiazole non-amino-acid-salt-type ionic liquid into a thiazole hydroxide type ionic liquid through anion exchange reaction; and performing neutralization reaction between amino acid and the thiazole hydroxide type ionic liquid to prepare the thiazole amino acid salt type ionic liquid. The thiazole amino acid salt type ionic liquid which has the advantages of environmental friendliness, diversity, strong dissolvability, biocompatibility and the like is a new generation of ionic liquid which is more environmentally friendly and greener and has wide application prospects in pharmaceutical chemistry, organic chemistry and industrial chemistry.
Owner:TIANJIN POLYTECHNIC UNIV
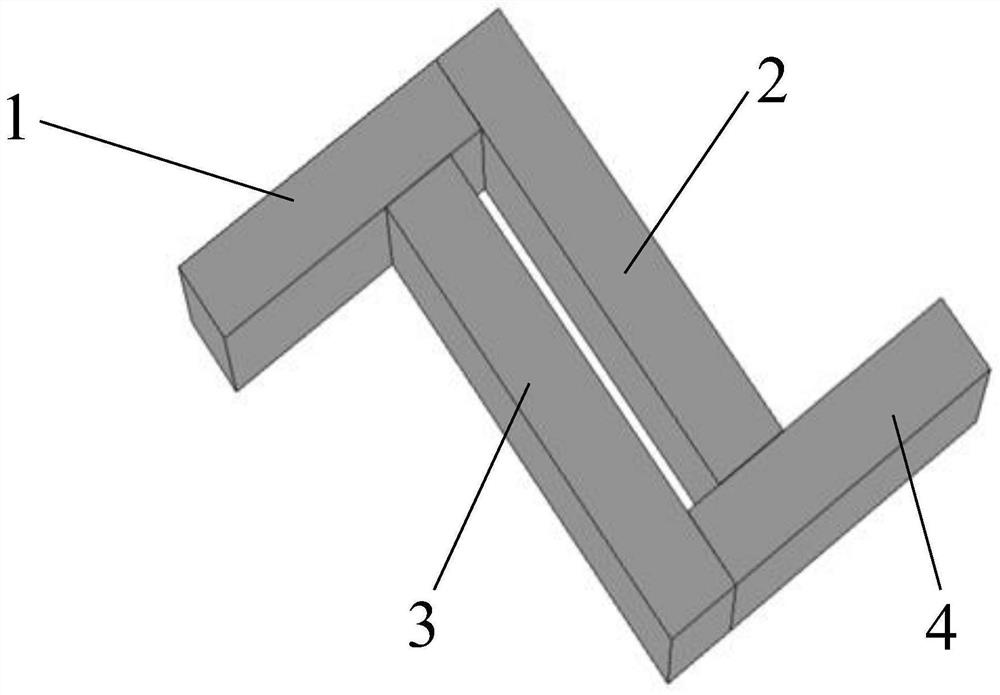
Micro-nano optical structure
InactiveCN110208186AChiralEnhanced circular dichroismTelevision system detailsImpedence networksMicro nanoCircular dichroism
The invention relates to the technical field of micro-nano optics, in particular to a micro-nano optical structure. The structure comprises a first metal strip, a second metal strip, a third metal strip and a fourth metal strip; one end of the second metal strip is vertically connected with one end of the first metal strip; the third metal strip is vertically connected with a non-endpoint positionat the other end of the first metal strip; the second metal strip and the third metal strip are located on a same side of the first metal strip; one end of the fourth metal strip is vertically connected with the other end of the third metal strip; and the other end of the second metal strip is vertically connected with a non-endpoint position at the other end of the fourth metal strip. The micro-nano optical structure has chirality, is capable of generating relatively large circular dichroism, generating an excited electric field on the surface under the excitation of incident light, generating different absorptions for left polarized light and right polarized light, forming magnetic dipoles and electric dipoles and generating different phases so as to generate circular dichroism signals,and can be combined with natural molecules to enhance the chirality and facilitate the detection.
Owner:SHAANXI NORMAL UNIV
Series catalytic preparation method of chiral pyrazospirofurin compound
ActiveCN108997365AHigh yieldMild reaction conditionsOrganic chemistry methodsOrganic solventSynthesis methods
The invention provides a series catalytic preparation method of a chiral pyrazospirofurin compound represented by the formula (I). The synthesis method is carried out according to the following steps:mixing an O-hydroxy nitroolefin compound represented by the formula (II) with a pyrazolone compound represented by the formula (III), a chiral squaric acid catalyst and an organic solvent, and carrying out a reaction for 1 to 48 h at the temperature of -20 to 60 DEG C, to obtain a compound represented by the formula (IV); and adding an iodine source additive and an oxidant to the compound represented by the formula (IV), carrying out a reaction for 1 to 48 h at the temperature of -20 to 60 DEG C, and post-treating the reaction liquid to obtain the chiral pyrazospirofurin compound representedby the formula (I). The reaction conditions are mild, the yield of the product is high and the selectivity is excellent. The prepared chiral pyrazospirofurin compound has chirality, and the skeleton structure has novelty.
Owner:ZHEJIANG UNIV OF TECH
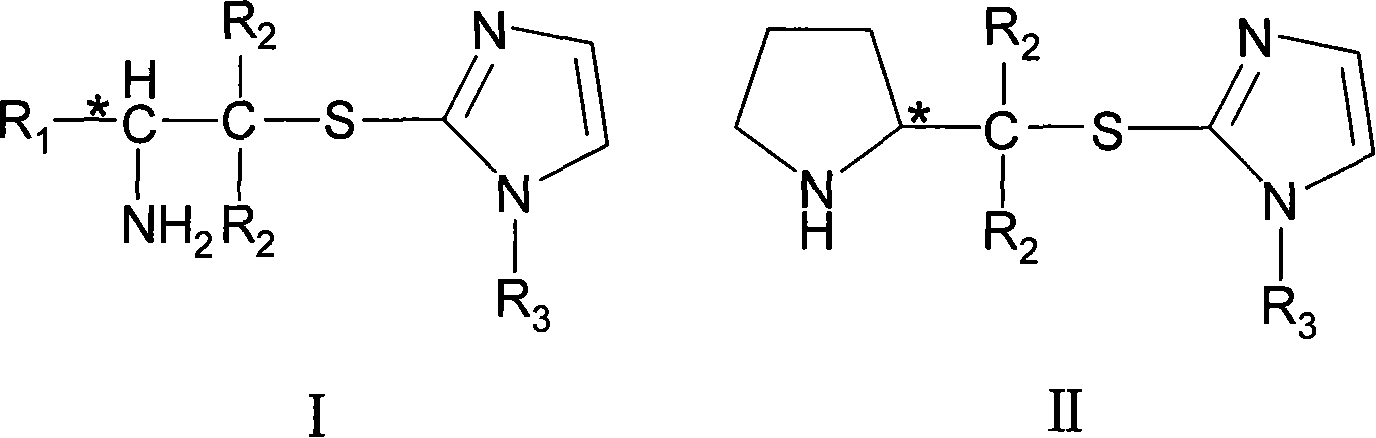
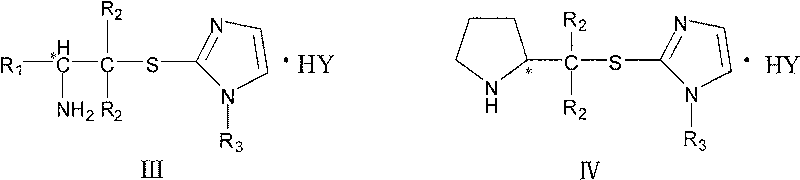
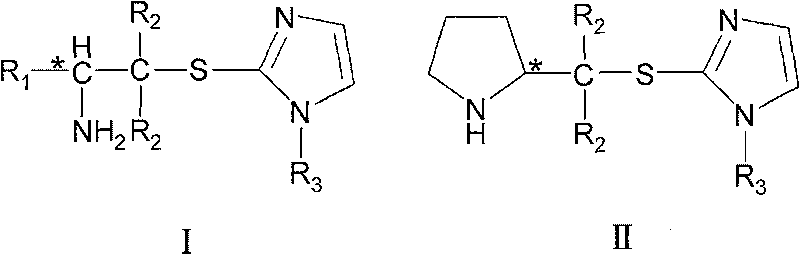
Chirality amine protonic acid salt containing imidazole sulfur ether structure and preparation method and usage thereof
ActiveCN101041637AChiralGood chiral induction propertiesOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesOrganic solventSulfur Ethers
The invention discloses a making method chiral amine protonic acid salt with general formula as III and IV, which comprises the following steps: dissolving chiral amine with imidazole sulfide structure as general formula I or II in the organic solvent; adding the solution of protonic acid HY into chiral amine solution; stirring to react completely; removing solvent; obtaining the product as chiral agent, catalyst and chiral material or ionic liquid.
Owner:ZHEJIANG UNIV OF TECH
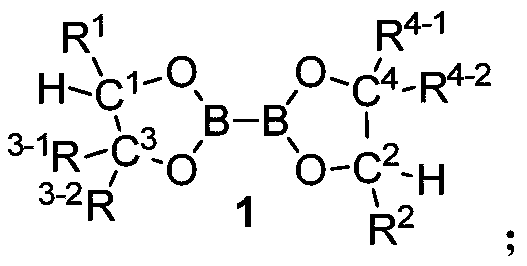
Diboron glycol ester as well as preparation method, intermediate and application thereof
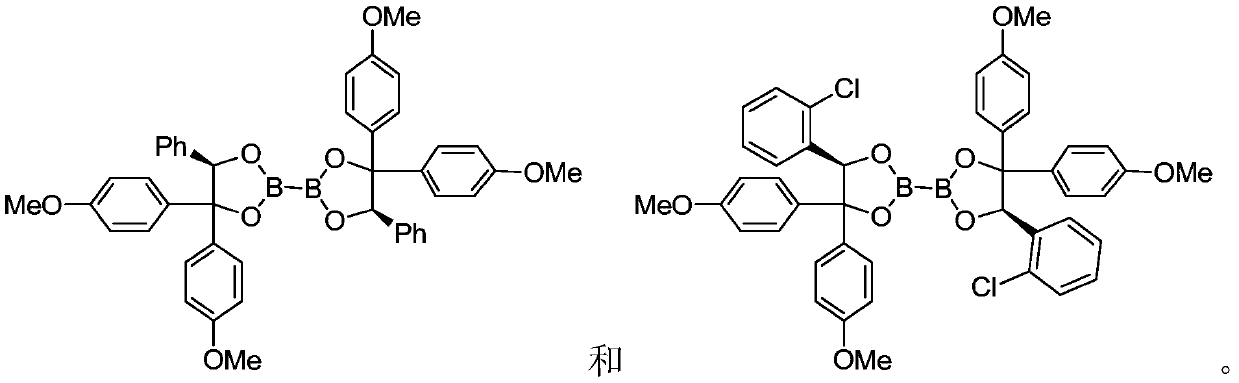
ActiveCN111440205AChiralEasy to manufactureOrganic compound preparationGroup 3/13 element organic compoundsCombinatorial chemistryDiol
The invention discloses diboron glycol ester as well as a preparation method, an intermediate and application thereof. The diboron glycol ester can be used for inducing reductive coupling reaction with imine as a substrate, and the substrate can be obtained by reaction of aldehyde and ammonia and is very easy to obtain and quite low in cost. The product can be separated from a reaction system onlyby acid-base operation without column chromatography purification, and the post-treatment mode is convenient and easy to operate. The yield of the obtained product is high, and protective group operation is not needed. The diboron glycol ester has chirality, the stereoselectivity of the reductive coupling reaction is generally excellent, and 99% ee chiral diamine can be obtained only through simple recrystallization. The diboron glycol ester can be obtained by reacting diol with diboron glycol ester, the diol is convenient to prepare and easy to amplify, the diol can be recycled from a reaction solution through simple acid-base operation, the recovery rate reaches 95%, and the preparation cost is further saved.
Owner:宁波赜军医药科技有限公司
Chiral dicyclic compound and asymmetric syntheses method thereof
ActiveCN102531911AChiralImprove response characteristicsOrganic chemistryOrganic compound preparationNitroalkeneOrganic solvent
The invention discloses a chiral dicyclic compound and an asymmetric syntheses method thereof, and the chiral dicyclic compound has a structure as shown in formula (I). According to the invention, a cyclohexenone derivative with a structure as shown in formula (II) and a nitroalkene derivative with a structure as shown in formula (III) are used as substrates, and a reaction is performed in an organic solvent with the catalysis of a chiral secondary amine catalyst, a polyglycol-series compound and an acid so as to obtain the chiral dicyclic compound with a structure as shown in formula (I). The chiral dicyclic compound synthesized in the invention has chirality, can be used as a synthetic intermediate of chiral compounds, and has wide application prospects.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing chiral poly-fluorene helical nano-fibers
ActiveCN104593896AChiralWith characteristicsMonocomponent synthetic polymer artificial filamentFiberPolyfluorene
The invention discloses a method for preparing chiral poly-fluorene helical nano-fibers. The method comprises the following steps: adding 9,9-dioctyl fluorene polymer into a (R)-(+)-limonene or (S)-(-)-limonene solution, and heating at the temperature of 70-90 DEG C to be dissolved so as to be prepared into a 0.1-0.4 mg / mL limonene solution; then cooling the limonene solution to room temperature; and finally, putting the limonene solution at the low temperature and self-assembling to obtain the chiral poly-fluorene helical nano-fibers. The influence of freezing time on polymer circular dichroism spectrum (CD), ultraviolet visible (UV-vis) spectrum and fluorescent (FL) spectrum is observed; after an assembly body is stabilized, the solution containing the chiral poly-fluorene helical nano-fibers is obtained. According to the method, a solvent chiral transfer technology is used for preparing chiral poly-fluorene helical nano-fibers by utilizing non-chiral polyfluorene at a first time, so that the problems of expensive cost and complicated synthetic steps of the preparation of spiral nano-fiber chiral reagents in a traditional synthetic method of the chiral polymer are solved.
Owner:苏州吉尼尔机械科技有限公司
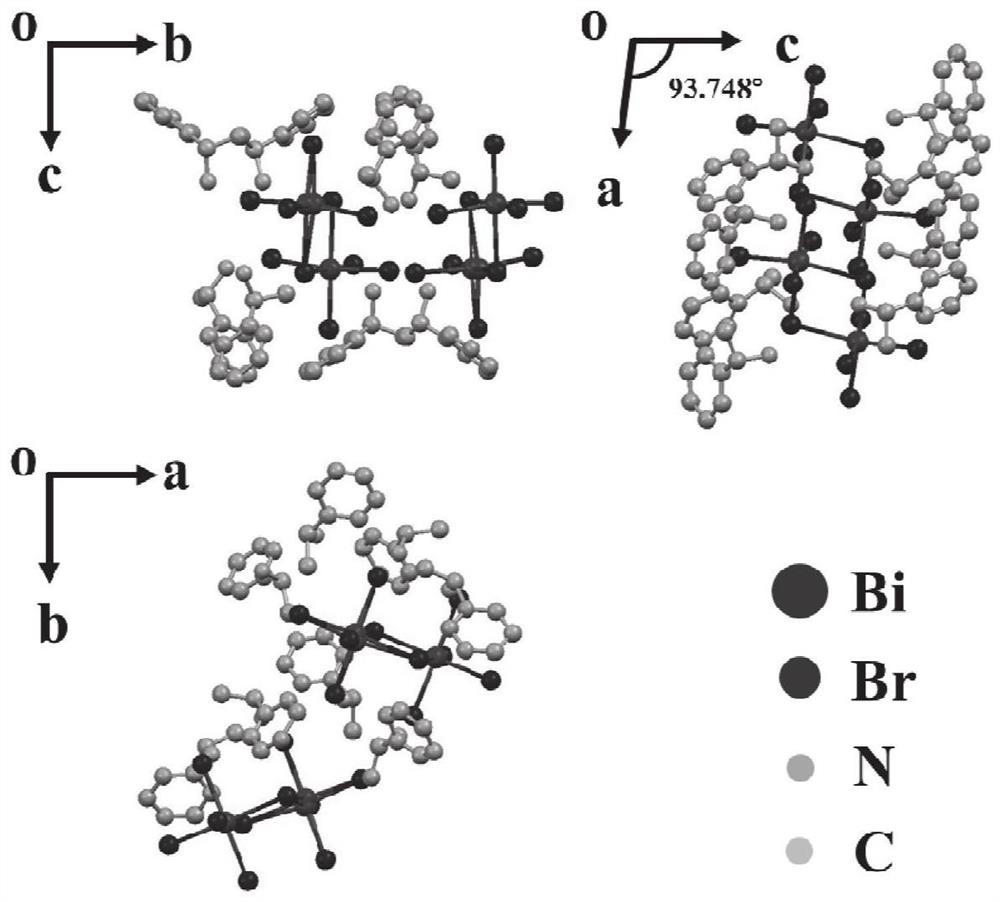
Preparation method and application of organic-inorganic hybrid lead-free piezoelectric crystal
ActiveCN114108069ALow costGood crystallization performancePolycrystalline material growthFrom normal temperature solutionsElectricityOxide
The invention relates to a preparation method of an organic-inorganic hybrid lead-free piezoelectric crystal, which comprises the following steps: uniformly mixing bismuth oxide, hydrobromic acid and R (+)-alpha-methylbenzylamine, heating in a drying oven, and cooling twice to obtain the organic-inorganic hybrid lead-free piezoelectric crystal. Compared with a pure organic piezoelectric material, the piezoelectric property is improved. Bismuth atoms are adopted to replace lead atoms to serve as central atoms, preparation of the lead-free material is achieved, and the method is more environmentally friendly.
Owner:SHANDONG UNIV
Chirality amine containing imidazole sulfur ether structure and preparation method and usage thereof
ActiveCN101041638AChiralGood chiral induction propertiesOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsOrganic solventSulfur Ethers
The invention discloses a making method chiral amine protonic acid salt with general formula as I and II, which comprises the following steps: dissolving halogenated aliphatic amine haloid acid salt of chiral amino acid derivant as formula III or IV and mercapto imidazole substituted by N-R3 as formula V into organic solvent to do substituted reaction; neutralizing the reacting liquid; obtaining the product as chiral agent, catalyst and chiral material.
Owner:ZHEJIANG UNIV OF TECH
Prepn of three-effect automobile tail gas purifying catalyst on foamed ceramic carrier
InactiveCN1120752CChiralEffective absorptionCatalyst carriersDispersed particle separationMicrowaveSilicate
The present invention provides one kind of tail gas purifying three-effect catalyst with foamed ceramic carrier. Microwave absorbing carrier and gamma-Al2O3 powder are adhered together by using ethyl silicate so as to load catalyst onto the microwave absorbing carrier. The catalyst has chiral features, can absorb microwave energy effectively for homogeneous heating, has active coating prepared through new technological process, and may be used to purify automobile tail gas effectively under the action of microwave with excellent cold start effect.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
Preparation method and application of glycosyl metal porous coordination polymer
InactiveCN109851803ASynthetic method greenThe synthesis method is simpleWater/sewage treatment by ion-exchangePorous carbonCarbonization
The invention discloses a preparation method and application of a glycosyl porous coordination coordination polymer. The preparation method comprises the following steps: performing ball milling on anorganic compound with chiral carbon atoms such as monosaccharide, disaccharide or polysaccharide having a skeleton containing or partially containing a hydroxyl group, an aldehyde group or a ketone group and salts containing various metal ions for a polymerization reaction to form the porous coordination polymer, performing heating to 400-1200 DEG C, and performing carbonization to obtain a porous carbon material based on the porous coordination polymer. The porous carbon material based on the chiral porous coordination polymer provided by the invention has the advantages of a multi-stage pore structure, an adjustable pore size, low costs, a simple preparation process, good stability and good safety, is easy to use, and can be recycled and be used in the fields of gas adsorption, chiral separation, catalysis and gas storage.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Photochromic compounds, preparation method thereof and photochromic products
ActiveCN108359439ALarge structural changesLarge dipole changeOrganic compound preparationCarbonyl compound preparationHalogenCarbon atom
The invention provides photochromic compounds. The structure of the photochromic compounds is shown as general formula I-I or general formula I-II in the description, wherein R1 is H or an alkyl groupwith 1-10 carbon atoms; R2 is an alkyl group with 1-10 carbon atoms, an alkoxy group with 1-10 carbon atoms, halogen or nitro group; R3, R4, R5 and R6 are independently selected from hydrogen, alkylgroups, alkoxy groups, cyano groups,-B(OH)2, groups shown in the description, halogen or other chromophores. The invention further provides a preparation method of the photochromic compounds and an application of the photochromic compounds to photochromic products.
Owner:XIAMEN UNIV
Chirality amine containing imidazole sulfur ether structure and preparation method and usage thereof
ActiveCN101041638BChiralGood chiral induction propertiesOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsOrganic solventSulfur Ethers
The invention discloses a making method chiral amine protonic acid salt with general formula as I and II, which comprises the following steps: dissolving halogenated aliphatic amine haloid acid saltof chiral amino acid derivant as formula III or IV and mercapto imidazole substituted by N-R3 as formula V into organic solvent to do substituted reaction; neutralizing the reacting liquid; obtainingthe product as chiral agent, catalyst and chiral material.
Owner:ZHEJIANG UNIV OF TECH
Chiral amine protonic acid salt containing imidazole sulfur ether structure and preparation method and usage thereof
ActiveCN101041637BChiralGood chiral induction propertiesOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesSulfur EthersOrganic solvent
The invention discloses a making method chiral amine protonic acid salt with general formula as III and IV, which comprises the following steps: dissolving chiral amine with imidazole sulfide structure as general formula I or II in the organic solvent; adding the solution of protonic acid HY into chiral amine solution; stirring to react completely; removing solvent; obtaining the product as chiral agent, catalyst and chiral material or ionic liquid.
Owner:ZHEJIANG UNIV OF TECH
A kind of preparation method of chiral metal micro-nano helical structure
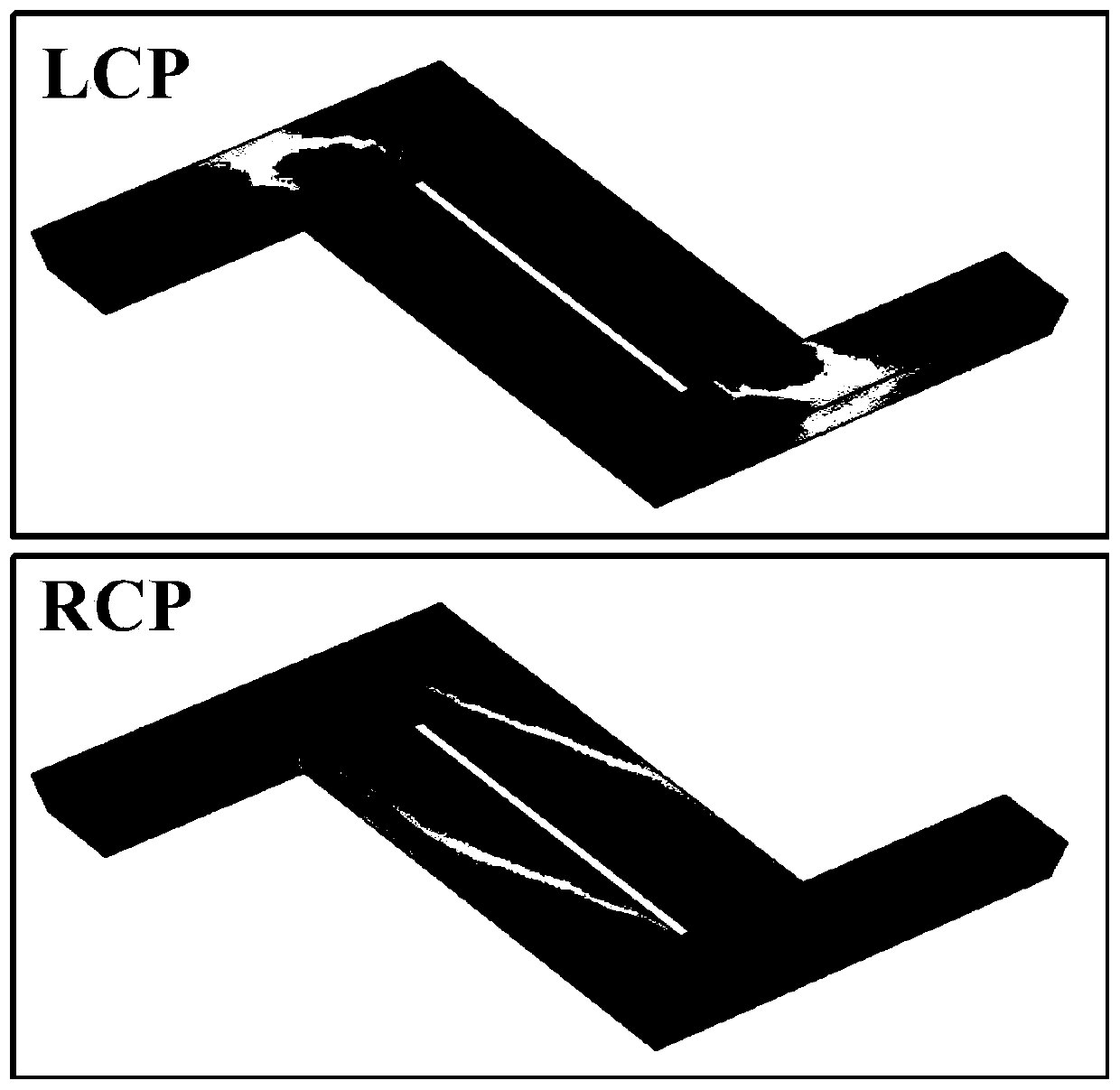
ActiveCN109594047BChiralSmall chiralityVacuum evaporation coatingSputtering coatingNano structuringMetallic materials
The invention relates to the field of preparation of a chiral metal micro-nano structure, and particularly relates to a preparation method of a chiral metal micro-nano-spiral structure, wherein the metal micro-nano-spiral structure takes polystyrene spheres as a substrate, an inclined evaporation insulating material is rotated clockwise or counterclockwise on the substrate to form a spiral structure, and then a metal material is perpendicularly evaporated on the spiral structure to finally form a metal spiral structure. The preparation method is simple and easy to operate, has low requirementsfor experimental equipment, and the prepared chiral metal micro-nano-spiral structure is strong in circular dichroism signal.
Owner:山东未来城建筑工程有限公司
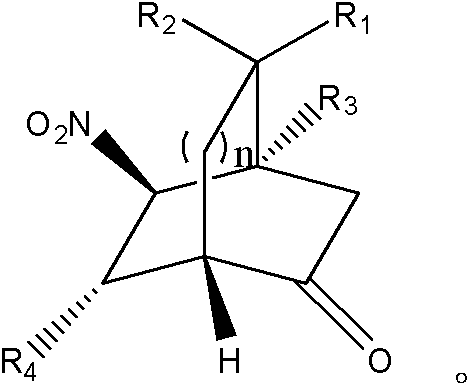
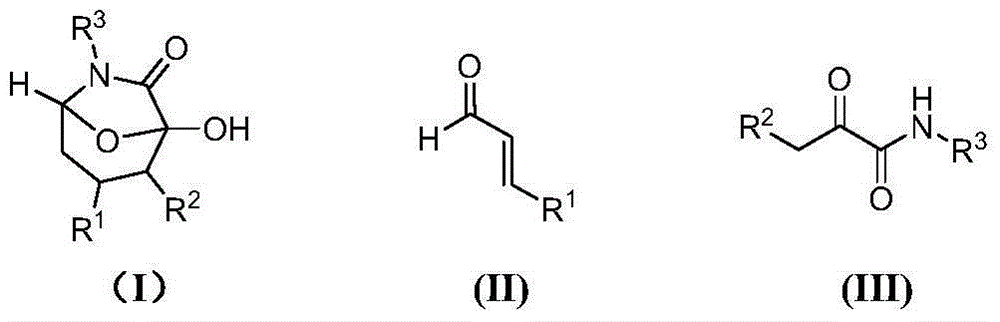
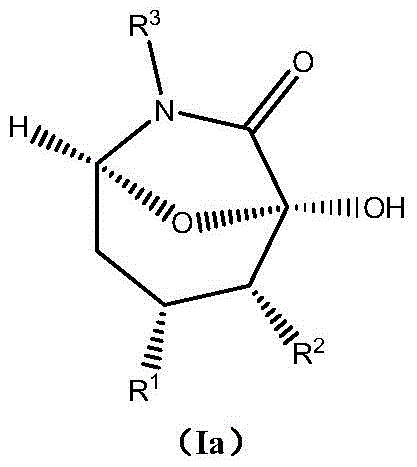
Asymmetric synthesis method of chiral bicyclo-caprolactam compound
The invention provides an asymmetric synthesis method of a chiral bicyclo-caprolactam compound as shown in a formula (I). The asymmetric synthesis method comprises the following steps of: mixing a chiral secondary amine catalyst, an alkaline substance and an organic solvent; adding alpha,beta-unsaturated carbonyl compound as shown in a formula (II) and an alpha-carbonyl amide ramification as shown in a formula (III) under the condition of stirring, and reacting at -20 DEG C -60 DEG C for 12-84 hours; and after the reaction is finished, carrying out post treatment on reaction liquid to obtain the chiral bicyclo-caprolactam compound as shown in a formula (I). The chiral bicyclo-caprolactam compound has chirality; the asymmetric synthesis method provided by the invention omits the purifying step through adoption of a one-pot method, enhances the product yield, is especially suitable for industrial production and can be applied to the fields of organic synthesis, materials and the like.
Owner:ZHEJIANG UNIV OF TECH
Asymmetric synthesis method of chiral bicyclocaprolactam compounds
ActiveCN104059083BChiralReduced purification stepsOrganic chemistryOrganic solventEnantioselective synthesis
The invention provides an asymmetric synthesis method of a chiral bicyclo-caprolactam compound as shown in a formula (I). The asymmetric synthesis method comprises the following steps of: mixing a chiral secondary amine catalyst, an alkaline substance and an organic solvent; adding alpha,beta-unsaturated carbonyl compound as shown in a formula (II) and an alpha-carbonyl amide ramification as shown in a formula (III) under the condition of stirring, and reacting at -20 DEG C -60 DEG C for 12-84 hours; and after the reaction is finished, carrying out post treatment on reaction liquid to obtain the chiral bicyclo-caprolactam compound as shown in a formula (I). The chiral bicyclo-caprolactam compound has chirality; the asymmetric synthesis method provided by the invention omits the purifying step through adoption of a one-pot method, enhances the product yield, is especially suitable for industrial production and can be applied to the fields of organic synthesis, materials and the like.
Owner:ZHEJIANG UNIV OF TECH
A micro-nano optical structure
InactiveCN110208186BChiralEnhanced circular dichroismTelevision system detailsImpedence networksMetal stripsNano structuring
The present invention relates to the field of micro-nano optics technology, in particular to an optical micro-nano structure, comprising a first metal strip, a second metal strip, a third metal strip and a fourth metal strip, one end of the second metal strip is vertically connected to the first metal strip One end of the strip, the third metal strip is vertically connected to the non-end position of the other end of the first metal strip, the second metal strip and the third metal strip are located on the same side direction of the first metal strip, and one end of the fourth metal strip is vertically connected to the first metal strip The other end of the three metal strips and the other end of the second metal strip are vertically connected to the non-end position of the other end of the fourth metal strip. The micro-nano optical structure of the present invention has chirality and can produce large circular dichroism. Under the excitation of incident light, the surface generates an exciting electric field, which produces different absorption for left-handed polarized light and right-handed polarized light, forming a magnetic dipole Muons and electric dipoles produce different phases, thereby generating circular dichroism signals, which can be combined with natural molecules to enhance their chirality and facilitate detection.
Owner:SHAANXI NORMAL UNIV
A kind of synthetic method of carboxylic acid ester compound
ActiveCN106588644BChiralBroad application spaceOrganic compound preparationCarboxylic acid esters preparationStrong acidsCombinatorial chemistry
Owner:HANGZHOU NORMAL UNIVERSITY
A kind of azo chiral metal complex, its synthesis method and application
InactiveCN104529817BSimple preparation conditionsEasy to operateTenebresent compositionsImino compound preparationSynthesis methodsFunction group
The present invention discloses azo chiral metal coordination compounds, a synthesis method and applications thereof, and relates to a variety of azobenzene group-containing chiral Schiff base metal coordination compounds substituted with different substituents. According to the present invention, the obtained metal coordination compounds have characteristics of reversible photochromism property of the azobenzene function group, light-emitting property of the Schiff base metal coordination compound, and the chiral characteristic of the molecule, and have good application potential in the photochromism field, and the preparation method has characteristics of simple preparation conditions, easy operation, mild reaction conditions and easily available raw materials, and is suitable for popularization and application.
Owner:XIAMEN UNIV
A kind of asymmetric synthesis method of chiral benzofuran spiro indole oxide compounds
The invention provides an asymmetric synthesis method of a chiral benzofuran spiro indole compound represented by formula (I), and the synthesis method is carried out according to the following steps: The nitroalkene compound is mixed with the oxidized indole compound represented by the formula (III), a chiral hydrogen bond catalyst, and an organic solvent, and the reaction is carried out at ‑40~60° C. for 1~240h to obtain the compound represented by the formula (IV), Add iodine source additive and oxidant to the compound represented by formula (IV), react at -40~60°C for 1~48h, the reaction solution is post-treated to obtain the chiral benzofuran spiro indole represented by formula (I) compound; the reaction conditions of the present invention are mild, the product yield is high, and the selectivity is excellent.
Owner:ZHEJIANG UNIV OF TECH
Photochromic compound and its preparation method and photochromic article
ActiveCN108359439BLarge structural changesLarge dipole changeOrganic compound preparationCarbonyl compound preparationHydrogenHalogen
The invention provides photochromic compounds. The structure of the photochromic compounds is shown as general formula I-I or general formula I-II in the description, wherein R1 is H or an alkyl groupwith 1-10 carbon atoms; R2 is an alkyl group with 1-10 carbon atoms, an alkoxy group with 1-10 carbon atoms, halogen or nitro group; R3, R4, R5 and R6 are independently selected from hydrogen, alkylgroups, alkoxy groups, cyano groups,-B(OH)2, groups shown in the description, halogen or other chromophores. The invention further provides a preparation method of the photochromic compounds and an application of the photochromic compounds to photochromic products.
Owner:XIAMEN UNIV
Ionic compound containing chiral amine-thiourea (urea) and its preparation method and application
ActiveCN101143862BChiralReduce dosageOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesThioureaOrganic synthesis
Owner:ZHEJIANG UNIV OF TECH
A kind of tandem catalytic preparation method of chiral pyrazole spirofuran compound
The invention provides a tandem catalytic preparation method of chiral pyrazole spirofuran compounds shown in formula (I), the synthesis method is carried out as follows: the o-hydroxyl nitroalkene shown in formula (II) Compounds and pyrazolone compounds shown in formula (III), chiral squaraine catalysts, and organic solvents are mixed, and reacted at -20 to 60°C for 1 to 48 hours to obtain compounds shown in formula (IV). Add an iodine source additive and an oxidizing agent to the compound shown in (IV), react at -20 to 60°C for 1 to 48 hours, and the reaction solution is post-treated to obtain the chiral pyrazole spirofuran compound shown in the formula (I); the present invention The reaction conditions are mild, the product yield is high, and the selectivity is excellent; the prepared chiral pyrazole spirofuran compound has chirality, and the core skeleton structure is novel.
Owner:ZHEJIANG UNIV OF TECH
A kind of iodine medium preparation method of chiral pyrazole spirofuran compound
ActiveCN109020987BMild reaction conditionsGood choiceOrganic chemistry methodsOrganic-compounds/hydrides/coordination-complexes catalystsFuranPtru catalyst
Owner:ZHEJIANG UNIV OF TECH
Chiral dark blue fluorescent material and preparation method thereof
ActiveCN111349013AChiralHigh fluorescence intensityOrganic compound preparationOrganic chemistry methodsBoronic acidULTRAMARINE BLUE
The invention discloses a class of chiral dark blue fluorescent materials and a preparation method thereof. In the structural general formula of the fluorescent material, R1 represents methyl methyl ether or C1-C3 alkyl, and R2 represents any one of H, phenyl, C1-C2 alkoxy substituted phenyl, C1-C3 alkyl substituted phenyl and triphenylamino. According to the preparation method, hydroxyl-protected3,3'-iodo-o-dinaphthol and triphenylamine-4-boric acid are utilized to synthesize an o-dinaphthol intermediate, and then a Suzuki coupling reaction is utilized to synthesize the final fluorescent material. The fluorescent material provided by the invention realizes dark blue fluorescence luminescence characteristics and chirality in one molecular skeleton at the same time, can be used for preparing organic light emitting devices and chiral light emitting devices, has strong fluorescence intensity in an organic solvent and water, has high solid-state luminous efficiency, and can realize imaging of cells, tissues and bacteria, glycosylation analysis of proteins, detection of ions and the like.
Owner:SHAANXI NORMAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com