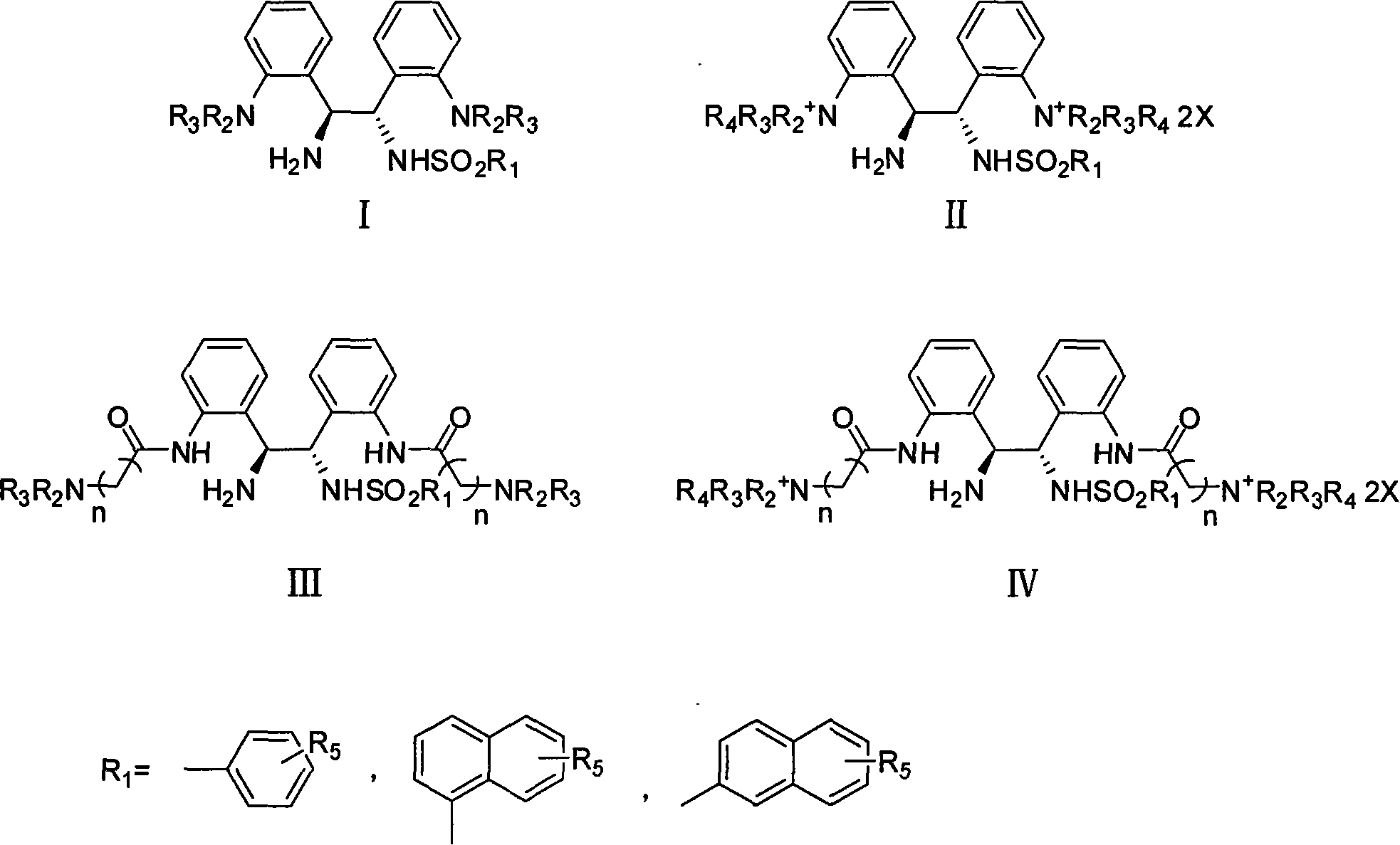
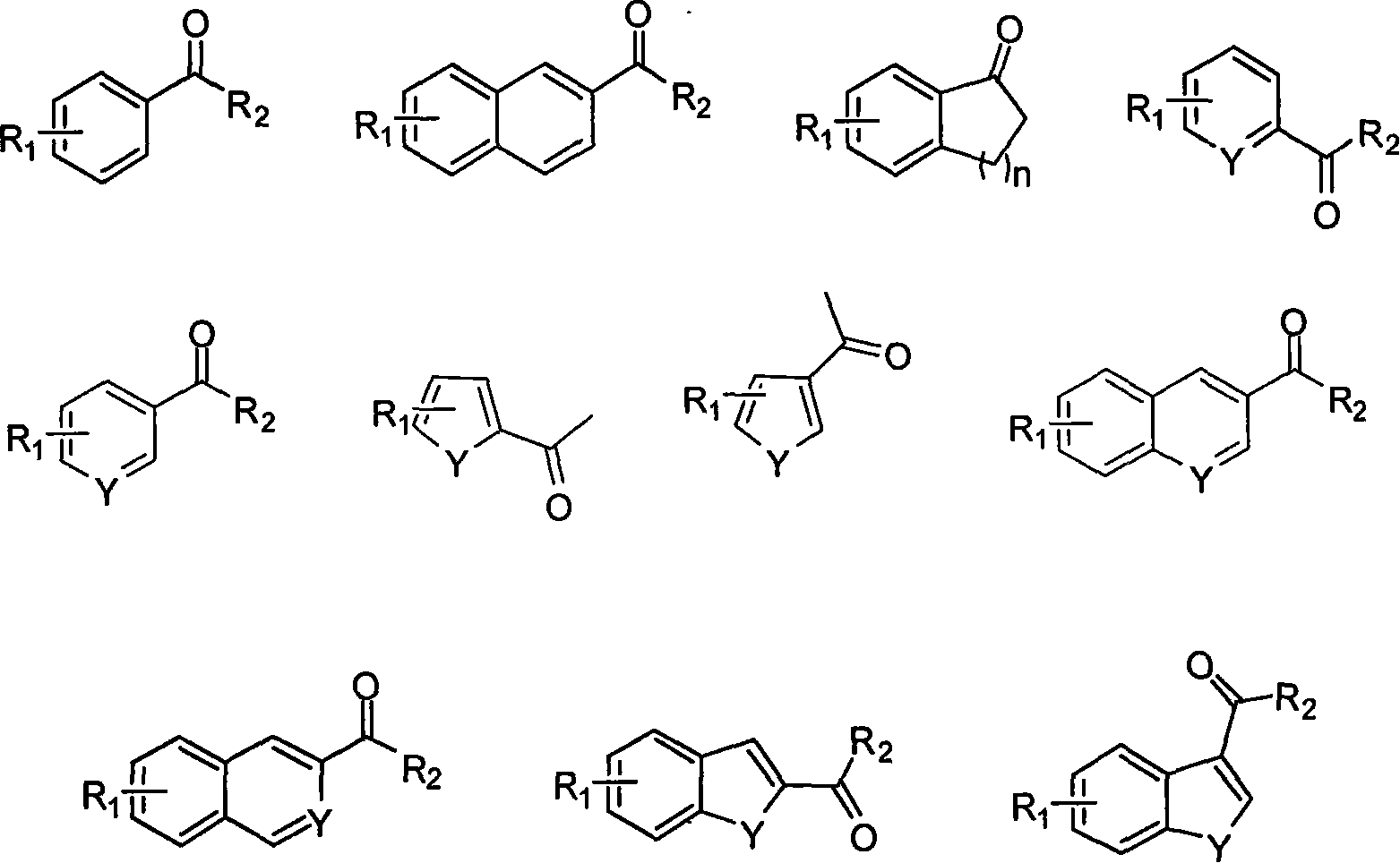
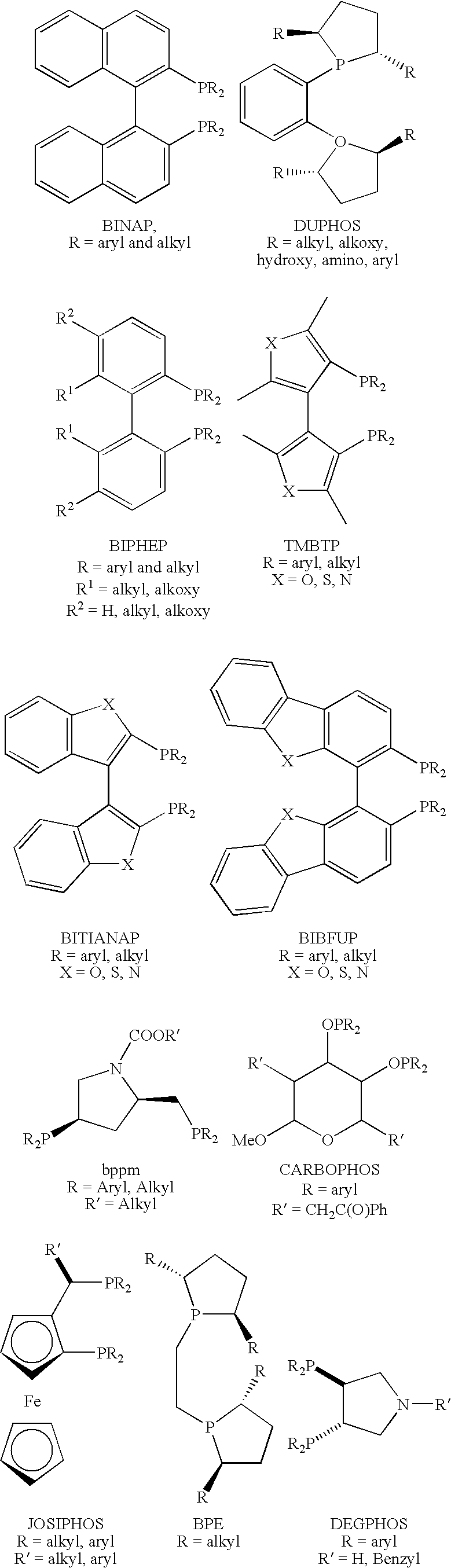
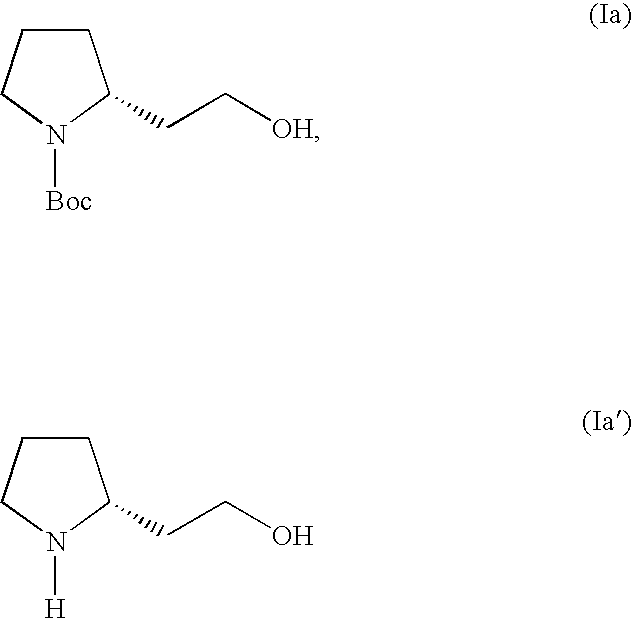
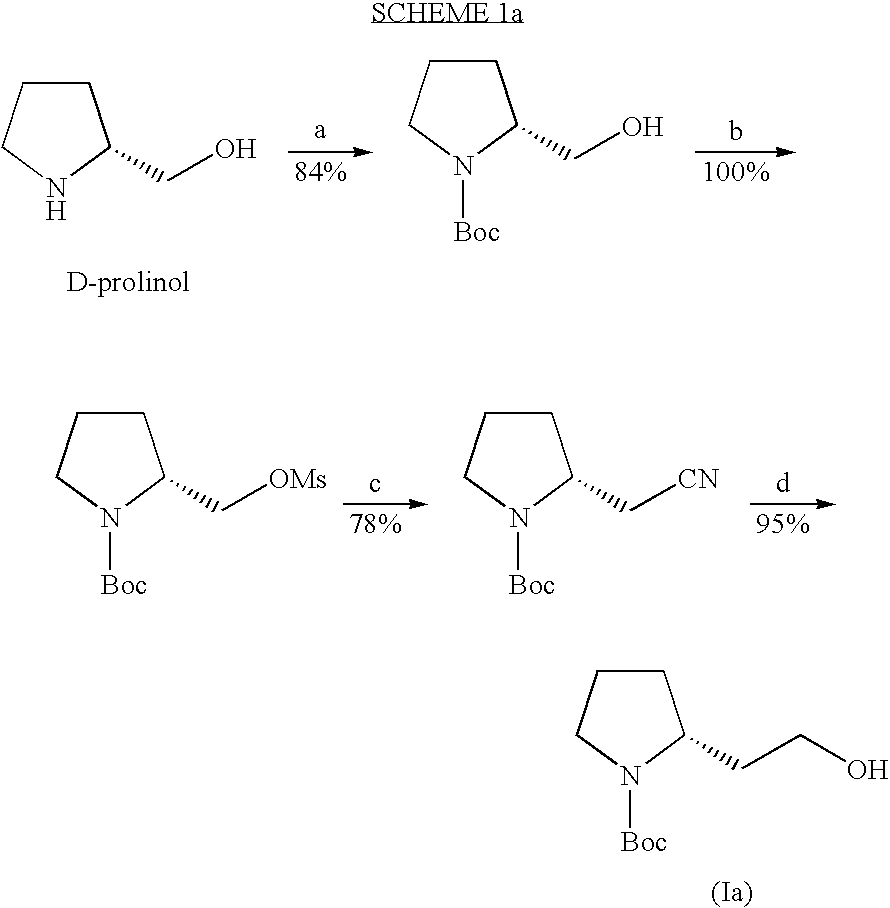
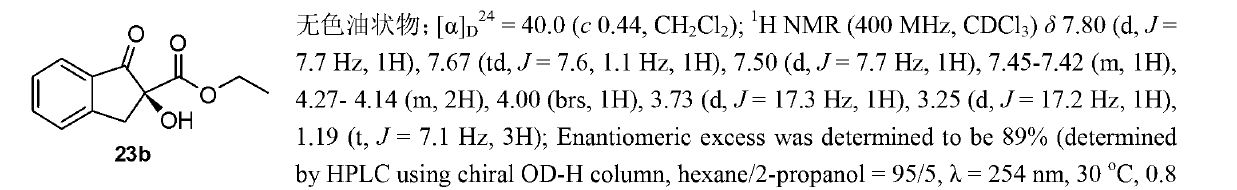
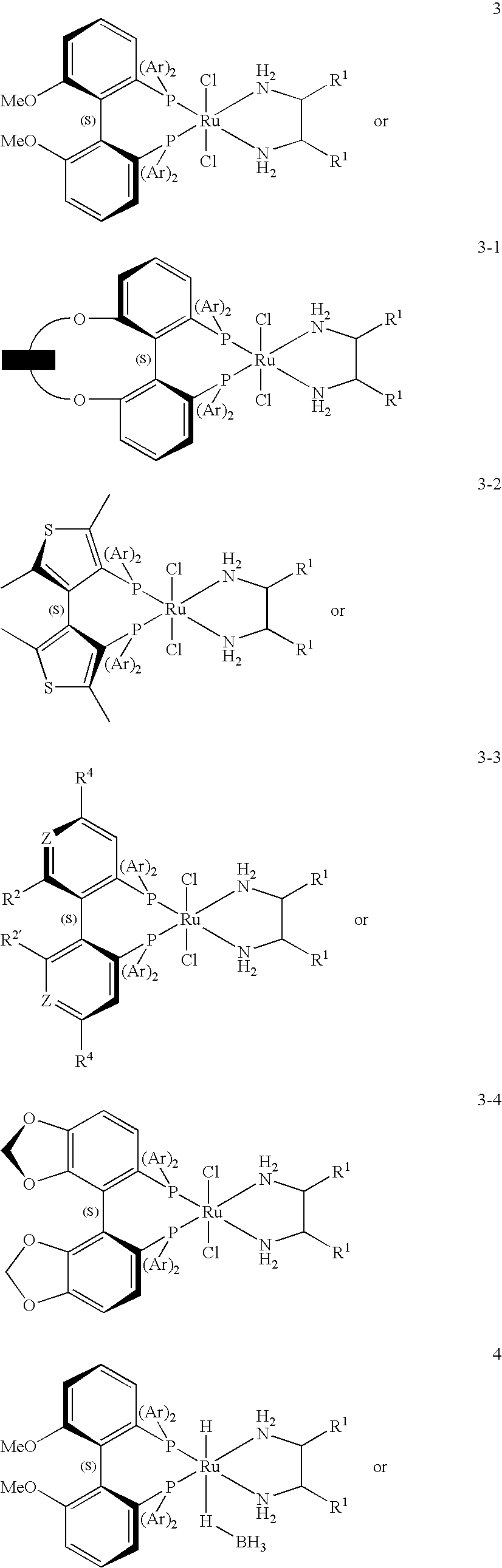
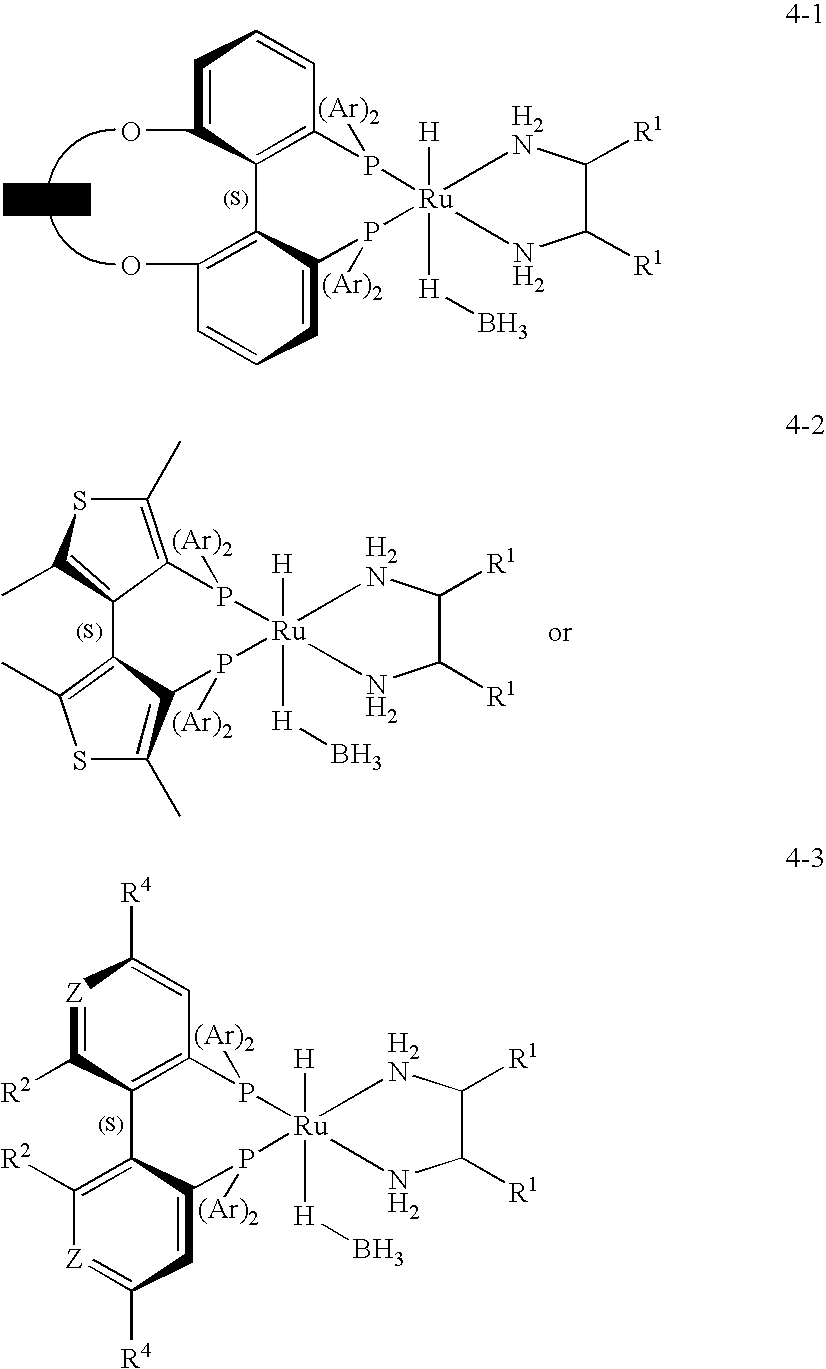
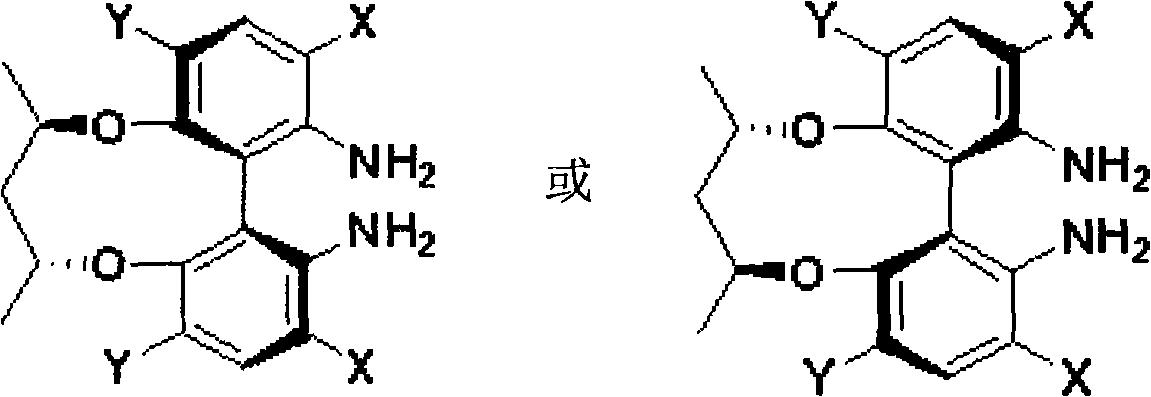
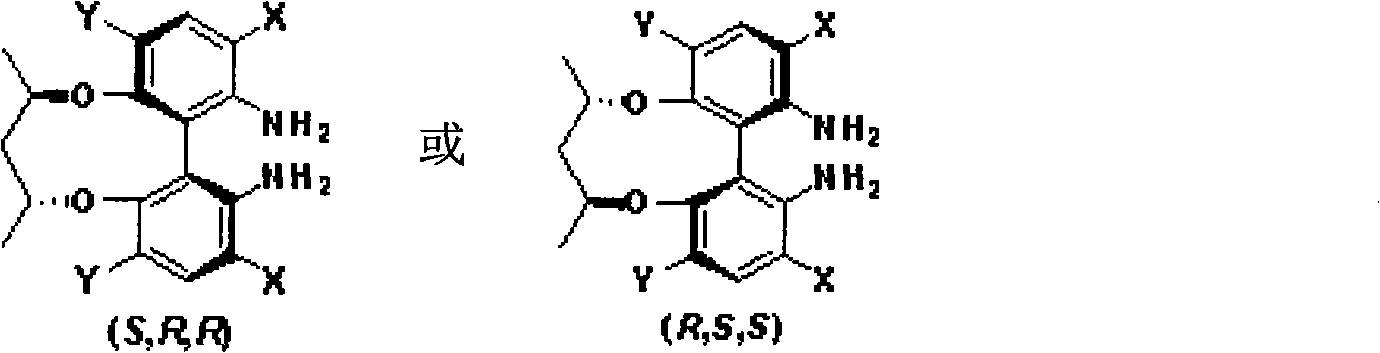
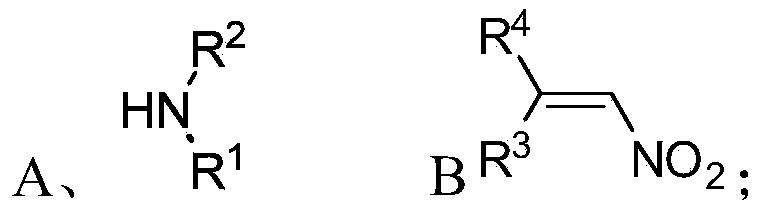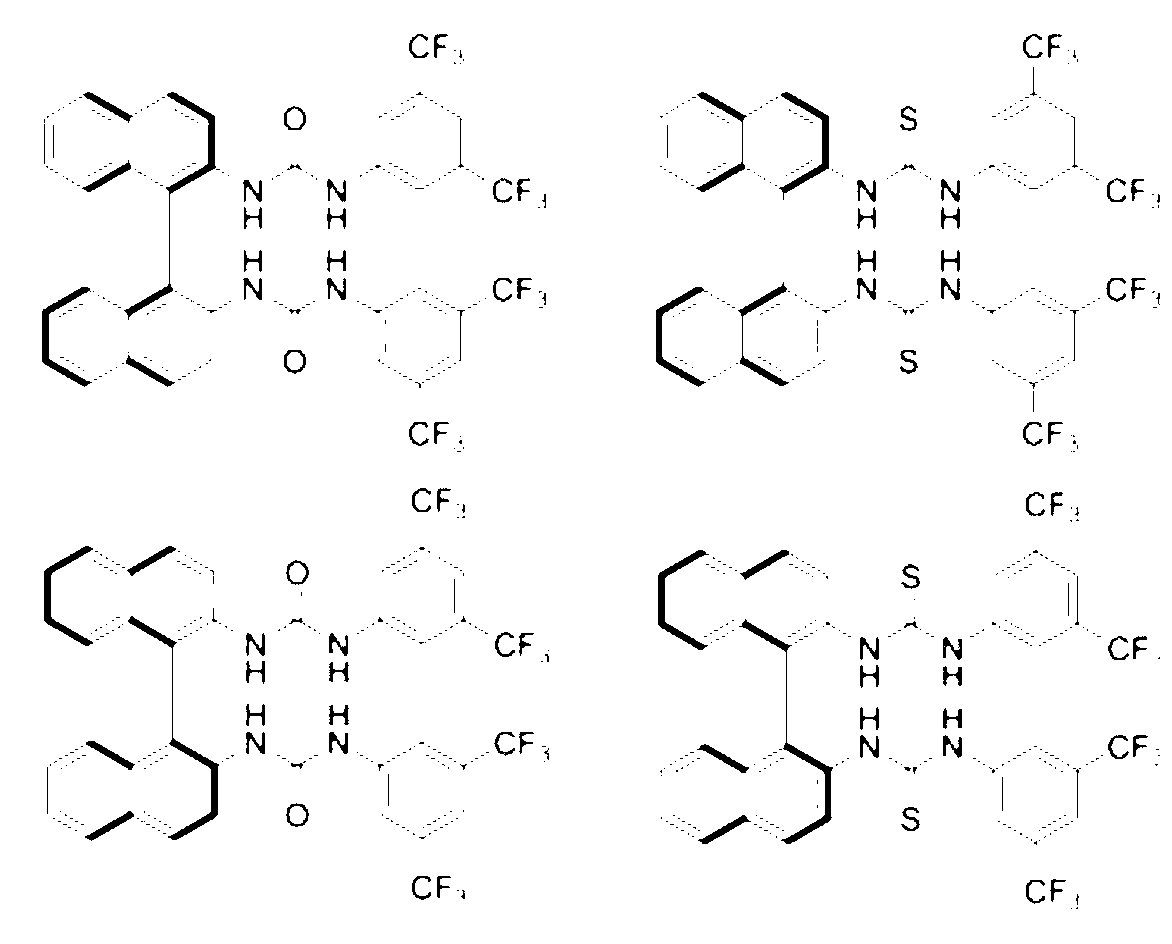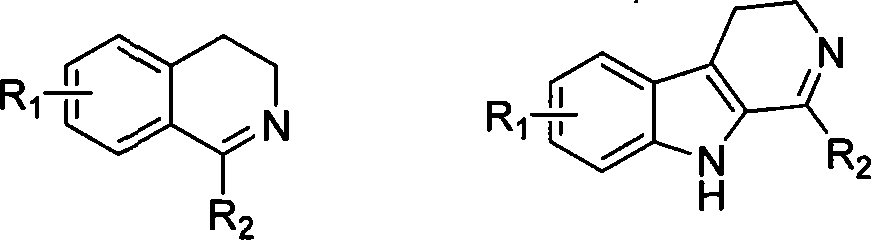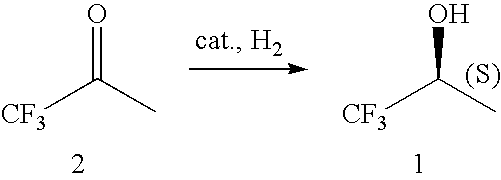Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
79 results about "Chiral diamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
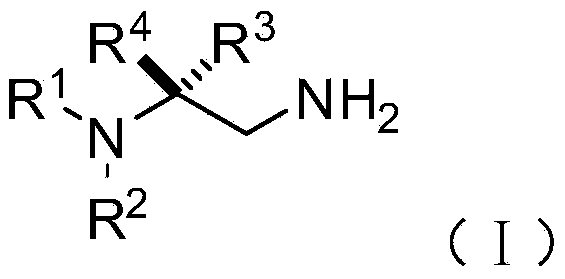
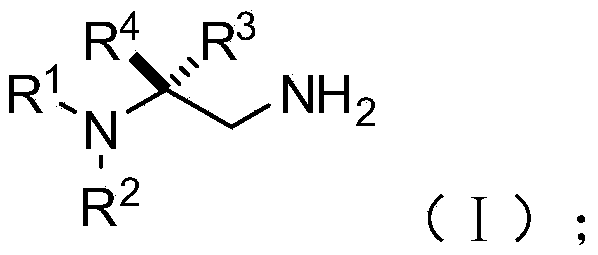
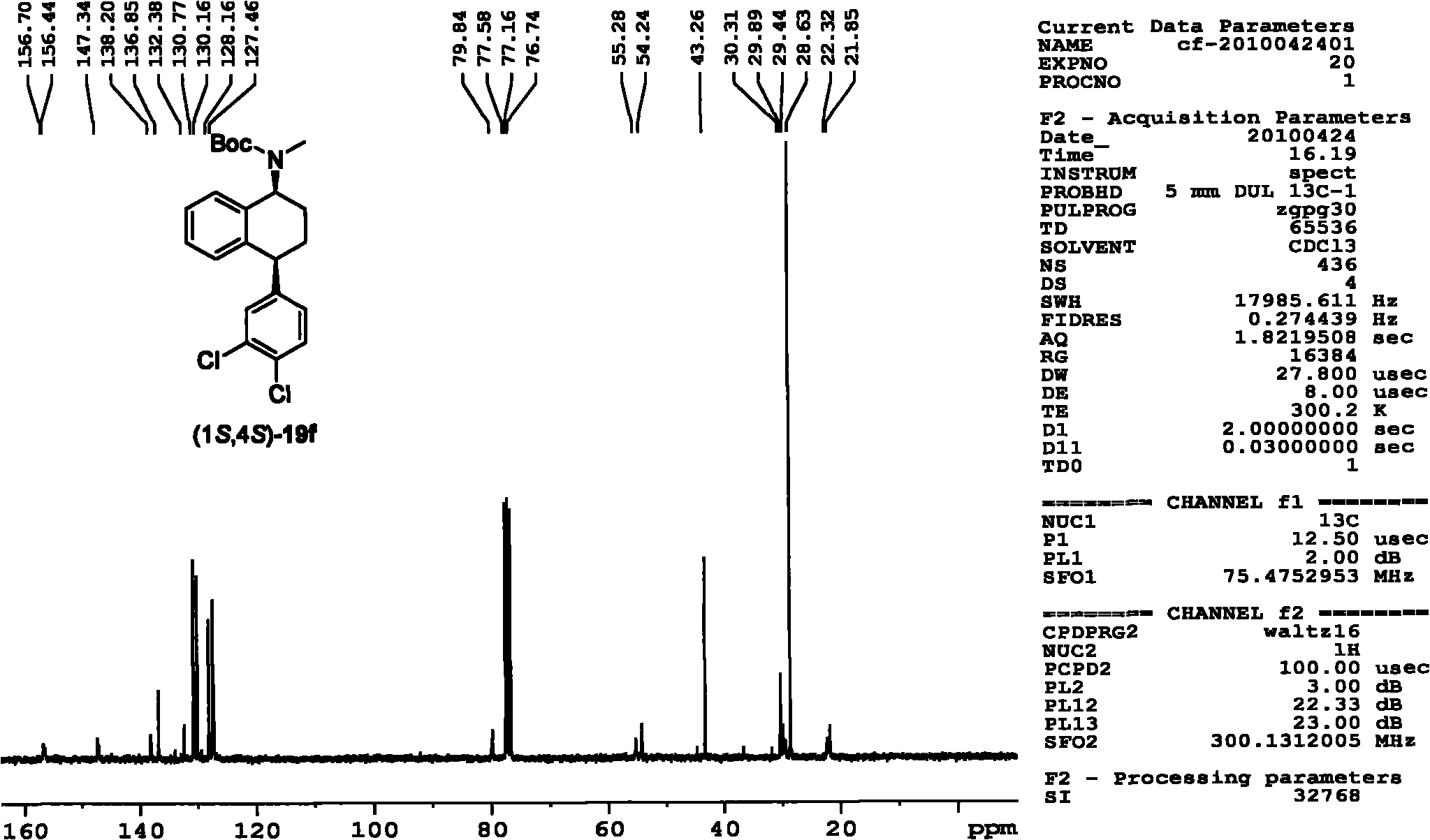
Chiral 1,2-diamine compound and preparation method and application thereof
ActiveCN105367427AAchieve strict metal-freeThe reaction process is safe and controllableOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCompound aNitroalkene
The invention discloses a chiral 1,2-diamine compound and a preparation method and application thereof. The molecular structure general formula of the chiral 1,2-diamine compound is shown as the general formula (I) in the specification. The preparation method of the chiral 1,2-diamine compound comprises the steps that an amine compound A and a nitroolefin compound B are added to a reaction system containing an n-heterocyclic carbine catalyst, an alkali reagent, a proton additive and a dewatering reagent for reacting and other steps. The chiral 1,2-diamine compound comprises chiral quaternary carbon atoms containing electrondrawing groups and amino groups, and can be widely used for synthesizing drug intermediates, especially heterocyclic ring structure compounds, and preparing functional materials. The preparation method is simple in process and low in requirement for reaction conditions, the reaction process is safe and controllable, the atom utilization rate and production efficiency are high, meanwhile, the enantioselectivity of products is efficiently guaranteed, and the environment pollution pressure of the methodology is low through the introduction of the small organic molecule asymmetric catalysis concept.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
Asymmetric catalytic hydrogenation method for ketone-derived N-alkylimine
InactiveCN102050688AHigh enantioselectivityCarbamic acid derivatives preparationOrganic compound preparationAsymmetric hydrogenationKetone
The invention discloses a method for performing the asymmetric catalytic hydrogenation of ketone-derived N-alkylimine. In the method disclosed by the invention, the catalytic hydrogenation of the ketone-derived N-alkylimine is performed in the presence of a chiral catalyst formed by a chiral diamine ligand and a transitional metal to obtain a chiral amine product with high yield and high enantioselectivity. The enantiomeric excess of a chiral amine product formed by the hydrogenation of an acyclic N-alkylimine substrate may reach 98 percent ee; and the enantiomeric excess of a chiral amine product formed by the hydrogenation of an exocyclic N-alkylimine substrate may reach over 99 percent ee. The method disclosed by the invention realizes the asymmetric catalytic hydrogenation of ketone-derived N-alkylimine with high enantioselectivity, and the obtained chiral amine product is an important medicinal and material intermediate and has a bright actual application prospect.
Owner:INST OF CHEM CHINESE ACAD OF SCI
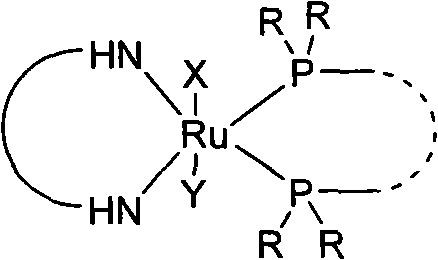
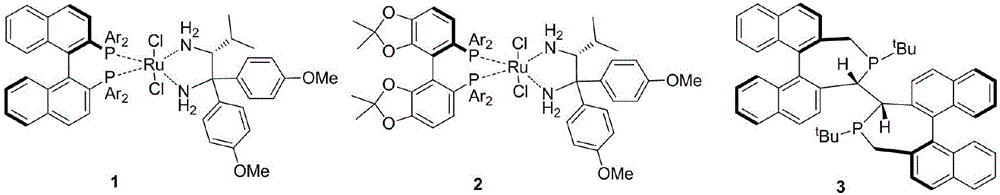
Chiral ruthenium catalyst and use thereof in asymmetric hydrogenation of ketone
InactiveCN102000606AControl chiral environmentLow costOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric hydrogenationKetone
The invention provides a chiral ruthenium catalyst, which has a relatively low cost but the same high efficiency. The catalyst is characterized in that a chiral diamine and an achiral phosphorus are used as ligands, and has a structural formula below. In the formula, NH=NH represents the chiral diamine, X and Y are negative ions, Ru is a metal ruthenium element and PR2=PR2 represents the achiral phosphorus ligand. The chiral ruthenium catalyst is used in the asymmetric hydrogenation of ketone and has very high activity and enantioselectivity.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for measuring optical purity of chiral carboxylic acid
InactiveCN103293176AEasy to synthesizeEasy to operateAnalysis using nuclear magnetic resonanceCarboxylic acidReagent
The invention provides a method for measuring the optical purity of chiral carboxylic acid by use of a chiral chemical shift reagent. A series of chiral diamines such as 1,2-diphenylethylenediamine, cyclohexyldiamine and dithio (diurea) derivatives of binaphthyldiamine are used as chiral shift reagents, and the optical purity of the chiral carboxylic acid is quickly detected by a nuclear magnetic resonance spectrometer. The method provided by the invention has the advantages of easy synthesis of shift reagents and simplicity in operation, and is a quick, efficient, convenient and practical detection means.
Owner:FUJIAN INST OF RES ON THE STRUCTURE OF MATTER CHINESE ACAD OF SCI
Optical activity di(heteto)aryl methanol and asymmetric synthesis method thereof
InactiveCN106831550AEasy to synthesizeLow priceOrganic compound preparationHydroxy compound preparationIridiumSynthesis methods
The invention relates to optical activity di(heteto)aryl methanol and an asymmetric synthesis method thereof. The method comprises the following steps: taking mono-sulfonyl chiral diamine and a complex of metals ruthenium, rhodium and iridium as catalysts, taking sodium formate or formic acid / triethylamine or isopropanol as a hydrogen source, carrying out an asymmetric transfer hydrogenation reaction of di(heteto)aryl ketone first, thereby obtaining the optical activity di(heteto)aryl methanol. The method is mild in reaction conditions, easy and convenient to operate, readily available in raw materials, wide in substrate application range and high in enantioselectivity and has important application prospects in the aspects of synthesis of antihistamine chiral drugs such as diphenhydramine, methyldiphenhydramine, carbinoxamine and bepotastine.
Owner:CHINA THREE GORGES UNIV
Soluble chiral diamino derivative, its production and use
InactiveCN101074207AGood water solubilityQuick responseOrganic-compounds/hydrides/coordination-complexes catalystsSulfonic acid amide preparationEthylenediamineOrganic solvent
The invention relates to a soluble chiral diamino derivative, use and a preparation thereof, belongs to the organic chemistry field. A water-soluble chiral diamine-pure optical N-monosulfonated acylation-1,2-2-(2'-amino-phenyl)-1,2-ethylenediamine and its derivative or its salt, their uses and productions are disclosed. It's fast, simple, no need for other organic solvents or additives and has better effect than organic phase. Complex formed of the soluble chiral diamine and metal can be used for ketone and imine asymmetric catalytic hydrogenating reduction in aqueous phase.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
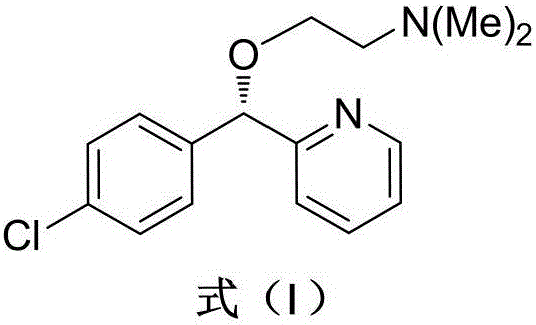
Asymmetric syntheses method of ophthalmologic drug bepotastine besilate
The invention relates to an asymmetric synthesis method of an ophthalmologic drug pylistramine besylate. The method concretely comprises the following steps: oxidizing (4-chlorophenyl)(2-pyridyl)methanone to obtain (4-chlorophenyl)(2-pyridyl)ketone-N-oxide; carrying out asymmetric transfer hydrogenation reduction on the (4-chlorophenyl)(2-pyridyl)ketone-N-oxide through using a complex of monosulfonyl chiral diamine and metallic ruthenium, rhodium and iridium as a catalyst and a sodium formate or formic acid and triethylamine mixture or isopropanol as a hydrogen source in order to prepare (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide; reducing the (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide to prepare (S)-(4-chlorophenyl)(2-pyridyl)methanol; and condensing the (S)-(4-chlorophenyl)(2-pyridyl)methanol and ethyl 4-(4-bromopiperidine-1-yl)butyrate to obtain the ophthalmologic drug pylistramine besylate. The total yield is 61.6%.
Owner:YICHANG HUMANWELL PHARMA +1
Catalysts
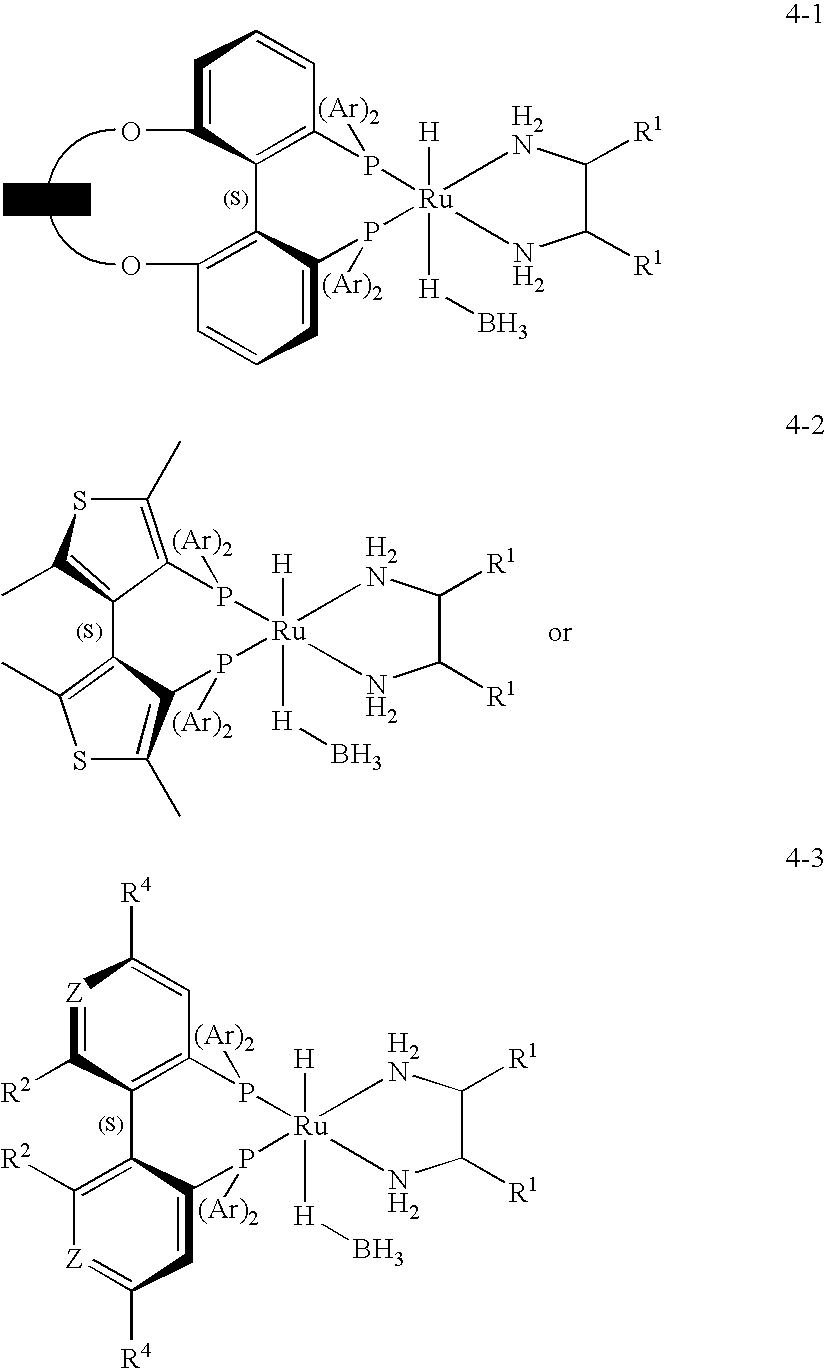
InactiveUS20100197976A1High enantiomeric excessLarge structureRuthenium organic compoundsOrganic compound preparationArylCompound a
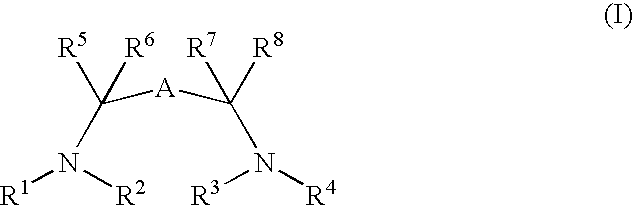
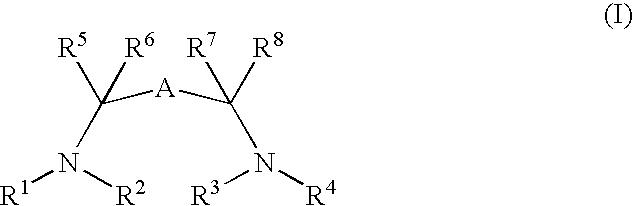
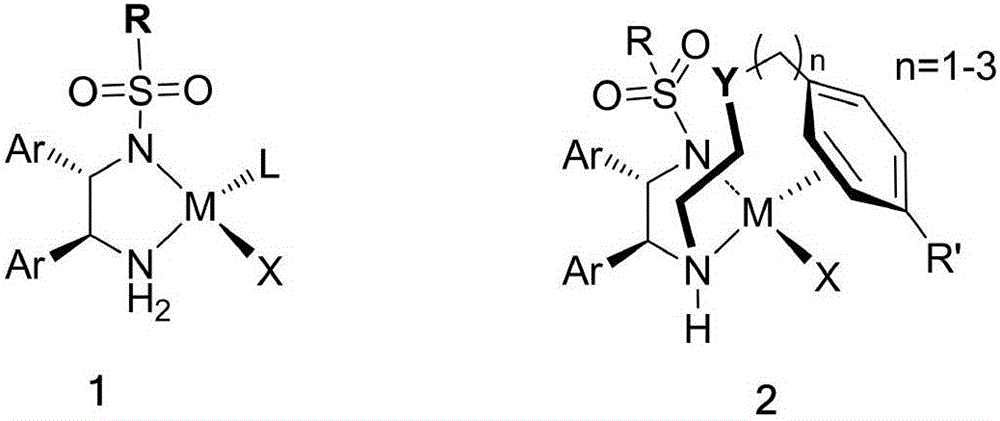
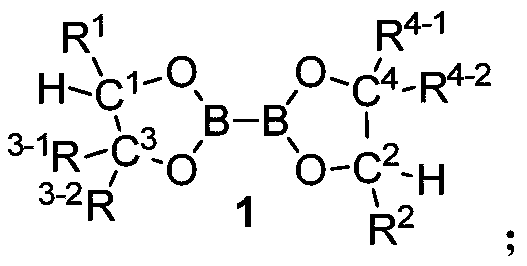
Catalysts suitable for asymmetric hydrogenation reactions is described comprising the reaction product of a group 8 transition metal compound a chiral phosphine and a chiral diamine of formula (I)in which R1, R2, R3 and R4 are independently hydrogen, a saturated or unsaturated alkyl or cycloalkyl group, an aryl group or a urethane or sulphonyl group and R5, R6, R7 and R8 are independently hydrogen, a saturated or unsaturated alkyl or cycloalkyl group, or an aryl group, at least one of R1, R2, R3 and R4 is hydrogen and A is a linking group comprising one or two substituted or unsubstituted carbon atoms.
Owner:JOHNSON MATTHEY PLC
Synthesis by chiral diamine-mediated asymmetric alkylation
ActiveUS20060069250A1Improve efficiencyHigh enantioselectrivityOrganic chemistrySparteineAlkyl transfer
Chiral synthesis from an achiral starting material by chiral diamine-mediated, such as sparteine-mediated, intermolecular asymmetric alkylation with a strained cyclic ether in the presence of a Lewis acid.
Owner:JANSSEN PHARMA NV
Chiral guanidine catalysts based on tartaric acid skeleton, preparation method and application thereof
InactiveCN103272638AHigh enantioselectivityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsArylThiourea
The invention relates to a series of chiral guanidine catalysts based on a tartaric acid skeleton and a preparation method thereof, and asymmetric catalytic application thereof, and belongs to the field of a catalyst technology and preparation methods of catalysts. The chiral guanidine catalysts have a structure shown in a general formula I or II; the basic skeletons heptatomic ring and five-membered ring respectively contain guanidyl and ketal structure units; and alpha, alpha'- of guanidyl on the heptatomic ring are respectively connected with two aryl groups. The invention provides a method for preparing chiral guanidine of the tartaric acid skeleton from chiral thiourea based on the tartaric acid skeleton and amine under promotion of cuprous chloride. The reaction temperature is 40 DEG C; the reaction time is 4-72 hours; and the yield is 50-99%. The chiral thiourea is prepared from tartrate by the steps of condensation, grignard reaction, chloro, azidation and reduction into chiral diamine and reaction with carbon disulfide.
Owner:DALIAN UNIV OF TECH
Chiral diamine diphosphine metal compound catalysts as well as preparation method and application thereof
InactiveCN102553646AOrganic compound preparationGroup 5/15 element organic compoundsDiphosphinesPhosphine
The invention discloses chiral diamine diphosphine metal compound catalysts as well as a preparation method and an application thereof and relates to heteroatomic ring phosphine group-containing chiral diamine diphosphine late transition metal compound catalysts and a preparation method thereof. PCl2(NEt2) is selectively synthesized from low-price PCl3 beginner by reaction with NEt2H, then PR2Cl containing two heteroatomic ring groups (R represents different heteroatomic ring groups shown in the patent) is synthesized by twice group exchange reactions, and finally a series of diamine diphosphine chiral ligands containing different heteroatom-substituted cyclic aryl phosphine groups and late transition metal compound catalysts thereof can be synthesized from PR2Cl as precursor. The catalysts can be used for asymmetric transfer hydrogenation of aromatic ketone. The transformation rate and the enantioselectivity of the catalysts in catalytic transformation of chiral aromatic alcohols from aromatic ketone are up to 97.1% and 99.5% respectively.
Owner:XIAMEN UNIV
Dicyclopentadienyl iron pocket ligand possessing central chirality and planar chirality, synthesis method and uses
InactiveCN1733782AHigh yieldHigh enantioselectivityGroup 5/15 element organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsPlanar chirality
The invention relates to a ferrocene ligand having central chirality and planar chirality, method for synthesizing and use thereof. The ligand has the central chirality of diamines, and is obtained through condensation of ferrocene phosphine aldehyde and chiral diamine, and further reduction, the ligand can be purified through recrystallization and column chromatography. The ligand can be used as catalyst in asymmetric alkylation reaction.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Fluorinated quinolylamide foldamer, preparation method, chirality recognition method and application
ActiveCN109705036AOrganic chemistryAnalysis by subjecting material to chemical reactionAbsolute configurationAlcohol
The invention discloses a fluorinated quinolylamide foldamer, a preparation method, a chirality recognition method of the fluorinated quinolylamide foldamer and an application of the fluorinated quinolylamide foldamer. The fluorinated quinolylamide foldamer has a structural formula represented by a formula (1) shown in the description, wherein R1 and R4 represent alkoxy of any length and may be the same or different, X is an O or N atom, R2 is an electron withdrawing group, for example nitro or cyano, R3 is alkyl of any length, and n is a random positive integer. The fluorinated quinolylamidefoldamer disclosed by the invention can be applied to the recognition and ee value determination of absolute configurations such as chiral amines, chiral diamines, chiral amine alcohols and chiral amino-acid esters. According to the fluorinated quinolylamide foldamer, the preparation method, the chirality recognition method and the application, a chiral group is linked to the quinolylamide foldamer in a covalent bond mode in a manner that amino attacks the fluorinated quinolylamide foldamer under alkaline conditions, so that chirality is transferred to the quinolylamide foldamer, and absoluteconfigurations and ee values of chiral guest molecules are detected according to CD signals of corresponding quinolylamide foldamers.
Owner:WUYI UNIV +1
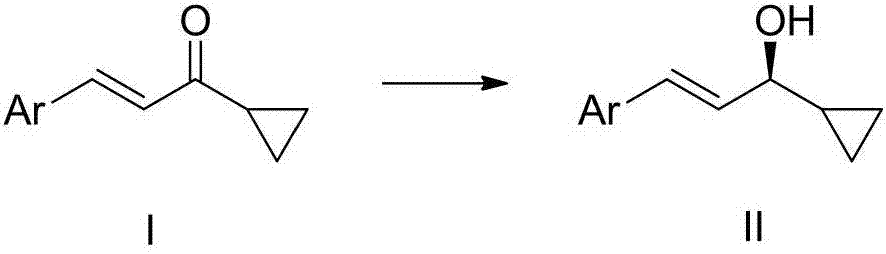
Cyclopropyl substituted allyl alcohol and asymmetric synthesis method thereof
ActiveCN107473941AEasy to synthesizeLow priceEther separation/purificationOrganic compound preparationIridiumSynthesis methods
The invention relates to cyclopropyl substituted allyl alcohol and an asymmetric synthesis method thereof. The method comprises the following steps: by taking monosulfonyl chiral diamine and a complex of metals ruthenium, rhodium and iridium as a catalyst, carrying out an asymmetric transfer hydrogenation reaction, thereby obtaining the cyclopropyl substituted allyl alcohol. The method is mild in reaction conditions, simple and convenient to operate, readily available in raw materials, wide in substrate application range and high in enantioselectivity and has important application prospects in the aspect of synthesizing a psoriasis treatment drug calcipotriol.
Owner:CHINA THREE GORGES UNIV
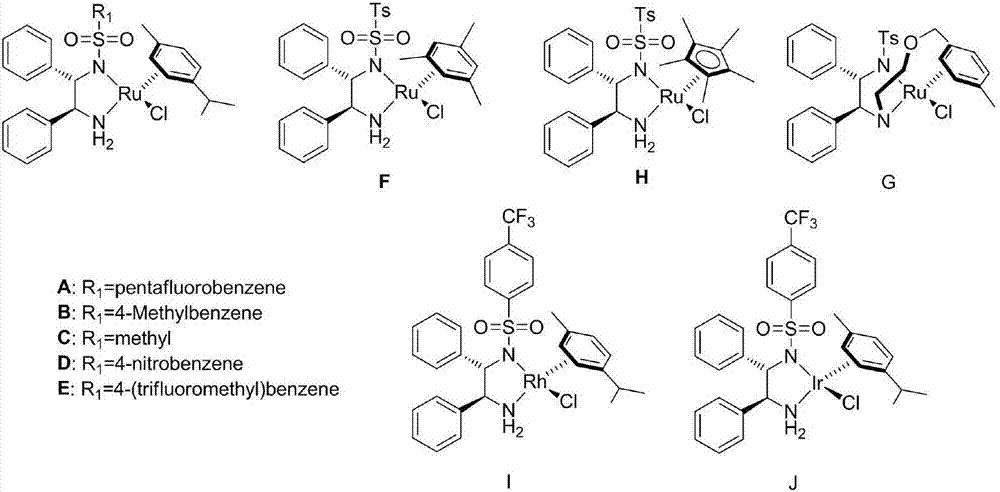
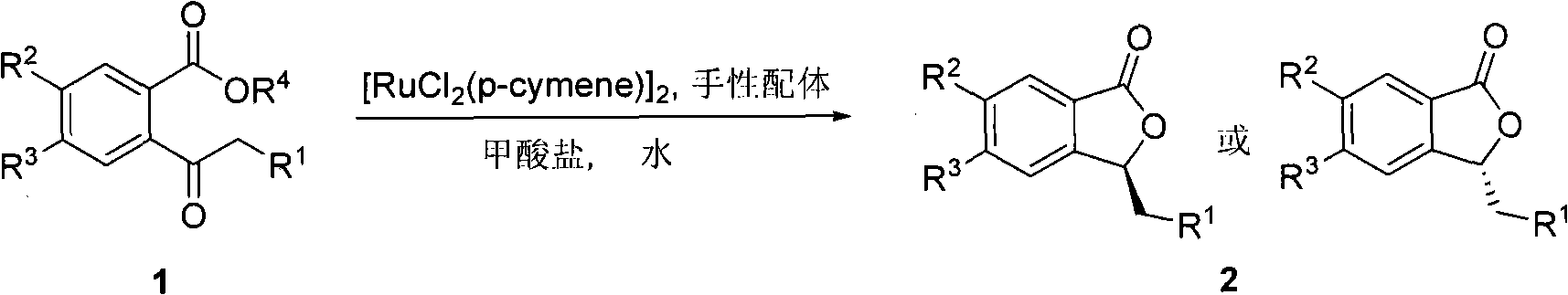
Method for preparing high optical purity 3-substituted chiral phthalide compounds
ActiveCN101337955AOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesChloridePhthalide
The invention relates to a method for preparing 3-substitution chirality phthalide compound with high optical purity, which is realized by utilizing asymmetric hydrogen transfer reaction which is performed between chiral diamine ligand and (p-methyl-isopropyl) phenyl ruthenous chloride dimer, and is catalyzed by complexes in aqueous phase. The reaction condition is mild, the operation is simple and convenient, the applicability of zymolyte is good, no secondary reaction occurs nearly, the 3-substitution chirality phthalide compound with high optical purity can be prepared in high yield in a highly stereoselective manner, and a certain industrial application prospect is possessed.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD +1
Asymmetric synthesis method for anti-allergy drug carbinoxamine
The invention relates to an asymmetric synthesis method for an anti-allergy drug carbinoxamine. The method specifically comprises the following steps: oxidizing (4-chlorophenyl)(2-pyridyl)ketone to obtain (4-chlorophenyl)(2-pyridyl)ketone-N-oxide; by taking monosulfonylated chiral diamine and metal ruthenium / rhodium / iridium complex as catalysts and sodium formate or a formic acid and triethylamine mixture or isopropanol as a hydrogen source, reducing the (4-chlorophenyl)(2-pyridyl)ketone-N-oxide through asymmetry transfer hydrogenation to prepare (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide; reducing the (S)-(4-chlorophenyl)(2-pyridyl)methanol-N-oxide to obtain (S)-(4-chlorophenyl)(2-pyridyl)methanol; and performing etherification reaction on the (S)-(4-chlorophenyl)(2-pyridyl)methanol and 2-chloro-N,N-dimethylethylamine to obtain the product, wherein the overall yield is 74.6%.
Owner:CHINA THREE GORGES UNIV
Novel method for preparing chiral dicarbonyl derivative by catalysis
InactiveCN101880233AEasy to makeCheap manufacturingOrganic chemistryOrganic compound preparationRoom temperatureToluene
The invention relates to a novel method for preparing a chiral dicarbonyl derivative by catalysis, needs to overcome various defects in the conventional method for preparing the chiral dicarbonyl derivative and provides an environment-friendly, economic, efficient and nontoxic method for preparing the chiral dicarbonyl derivative. The method of the invention is characterized in that a chiral diamine derivative is used as a catalyst, the molar ratio of the catalyst to raw materials is 1:10-20 and the target product, namely the chiral dicarbonyl derivative is obtained by performing reaction at the normal pressure and the room temperature in a non-polar solvent; the raw materials are an achiral dicarbonyl derivative and a nitroolefin derivative and the molar ratio of the using amount of the achiral dicarbonyl derivative to the using amount of the nitroolefin derivative is 1:1; the non-polar solvent is toluene; and the catalyst is one of the following catalysts from a to i.
Owner:HANGZHOU NORMAL UNIVERSITY
Asymmetric hydrogenation of 1,1,1- trifluoroacetone
ActiveUS20080027249A1High chemical purityHigh isomerical purityOrganic compound preparationOrganic chemistry methodsPropanolHydrogen
The invention relates to the preparation of enantiomerically pure (S)-1,1,1-trifluoro-2-propanol by asymmetric hydrogenation of 1,1,1-trifluoroacetone which process comprises hydrogenating 1,1,1-trifluoroacetone in the presence of a ruthenium phosphine complex catalyst represented by formulaRu(E)(E)(L)(A)wherein E, E′ are both chloro or E is hydrogen and E′ is BH4;L is a chiral diphosphine ligand; andA is an optionally chiral diaminewherein hydrogenation occurs in the presence of a weak base, with or without an additive, when E and E′ are both chloro orb) in the absence of a base and an additive when E and E′ are hydrogen and BH4.
Owner:F HOFFMANN LA ROCHE INC
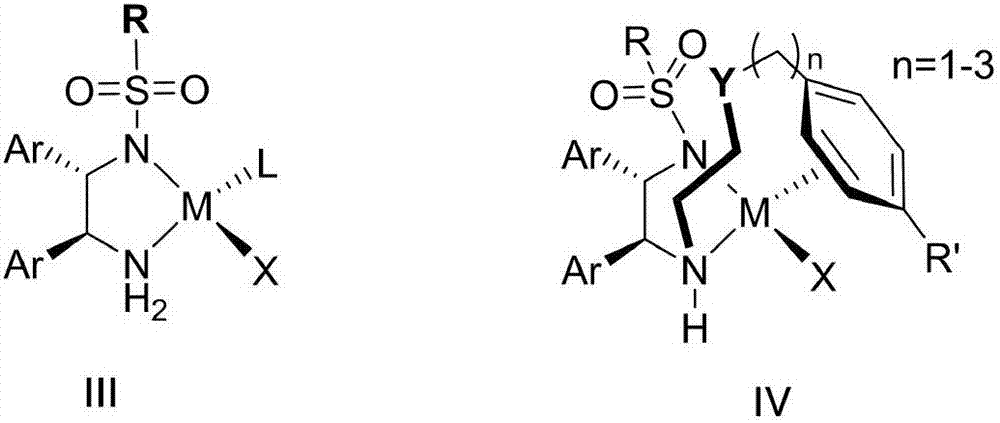
Preparation method and intermediate of chiral diamine compound
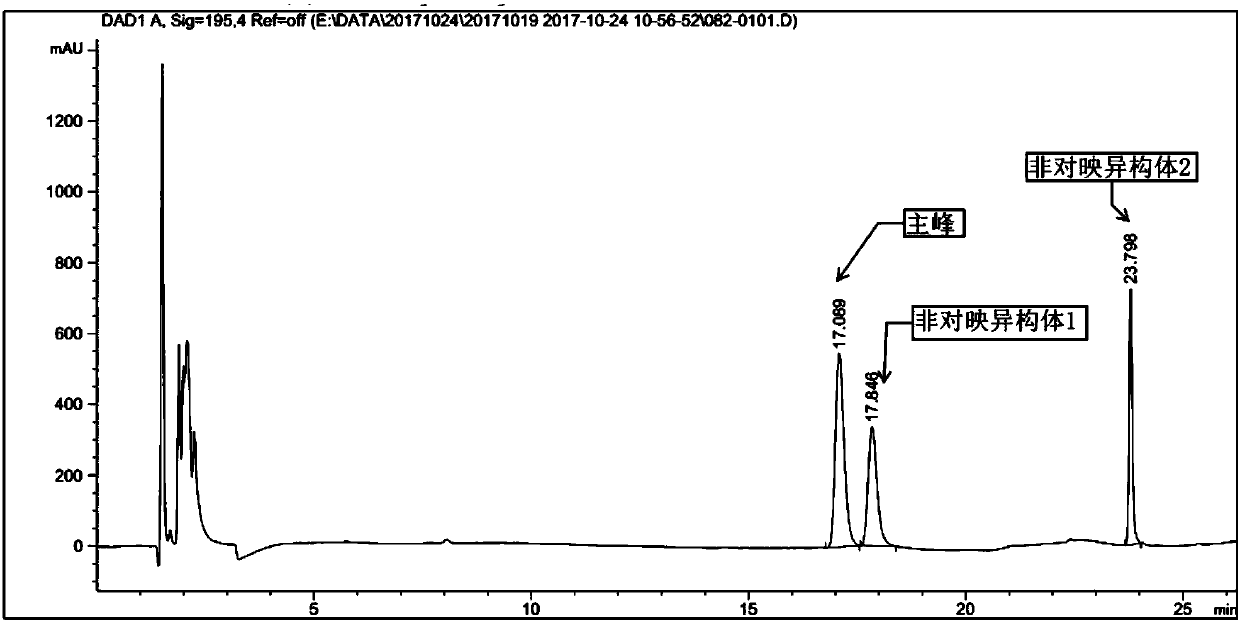
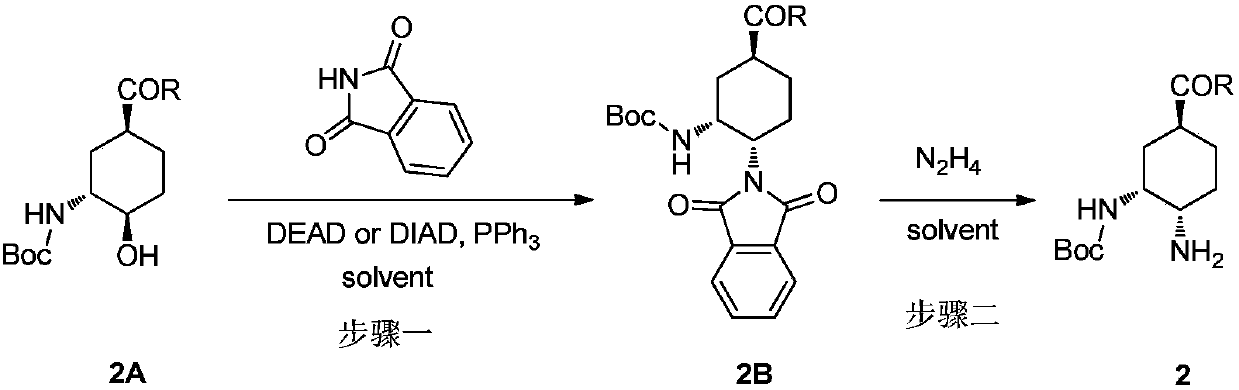
InactiveCN109912493AReduce security risksSuitable for industrial productionCarbamic acid derivatives preparationOrganic compound preparationDiamineOral anticoagulant
The present invention relates to a preparation method and an intermediate of a chiral diamine compound, wherein the chiral diamine compound is used for preparing the key intermediate of a small molecule oral anticoagulant edoxaban. According to the present invention, the steps of the preparation method of the chiral diamine compound 2 and the key intermediate 2B are defined in the specification, wherein the group R is preferably selected from methoxy, ethoxy and dimethylamino.
Owner:SHANGHAI SYNCORES TECH INC +1
Method for preparing optically active poly(alkyl-aryl) silane
The invention relates to an organic high molecular compound, in particular to a method for preparing optically active poly(alkyl-aryl) silane. As the traditional method for synthesizing optically active poly(alkyl-aryl) silane is extremely limited, the invention provides a novel method for preparing optically active poly(alkyl-aryl) silane. Polysilane which is not optically active is used as a raw material in an organic solvent A, alkyl lithium is a reactant, chiral diamine is used as a chiral inductor, and saturated ammonium chloride is used for quenching reaction to prepare the optically active poly(alkyl-aryl) silane. The preparing method has the advantages of convenience for operation and low cost.
Owner:HANGZHOU NORMAL UNIVERSITY
Asymmetric hydrogenation of 1,1,1-trifluoroacetone
The invention relates to the preparation of enantiomerically pure (S)-1,1,1-trifluoro-2-propanol by asymmetric hydrogenation of 1,1,1-trifluoroacetone which process comprises hydrogenating 1,1,1-trifluoroacetone in the presence of a ruthenium phosphine complex catalyst represented by formulaRu(E)(E′)(L)(A)wherein E, E′ are both chloro or E is hydrogen and E′ is BH4;L is a chiral diphosphine ligand; andA is an optionally chiral diaminewherein hydrogenation occurs in the presence of a weak base, with or without an additive, when E and E′ are both chloro orb) in the absence of a base and an additive when E and E′ are hydrogen and BH4.
Owner:F HOFFMANN LA ROCHE & CO AG
Axial chirality diamine compound induced by central chirality and synthetic method
Axial chirality diamine compound induced by central chirality, in formula, X and Y are hydrogen, bromine, chlorine, iodine, methyl or aryl. The invention comprises the following steps: reacting 2-iodine-3-nitrophenol with 2,4-pentanediol with central chirality or derivate to gain 2,4-bi[(2-iodine-3-nitryl)phenoxy]pentane; coupling and restoring to gain (S)-[6,6'-((R,R)-2,4-pentanediol oxygen)]-2,2'-biamino-1,1'-biphenyl or (R)-[6,6'-((S,S)-2,4-pentanediol oxygen)]-2,2'-biamino-1,1'-biphenyldiamine; reacting the axial chirality biphenyldiamine induced by central chirality with the halogenating reagent to gain enantiomer pure 3,3'-dihalo or 3,3', 5,5'-tetrahalo axial chirality biphenyldiamine; and then Suzuki coupling enantiomer pure 3,3'-dihalo or 3,3', 5,5'-tetrahalo axial chirality biphenyldiamine to produce enantiomer pure 3,3'-dimethyl or aryl or 3,3', 5,5'-tetramethyl or aryl axial chirality biphenyldiamine. The invention has advantages of easily-gained raw material and simple operation. A series of enantiomer pure diamine produced by the invention can be applied to a series of asymemetric reaction.
Owner:王春江
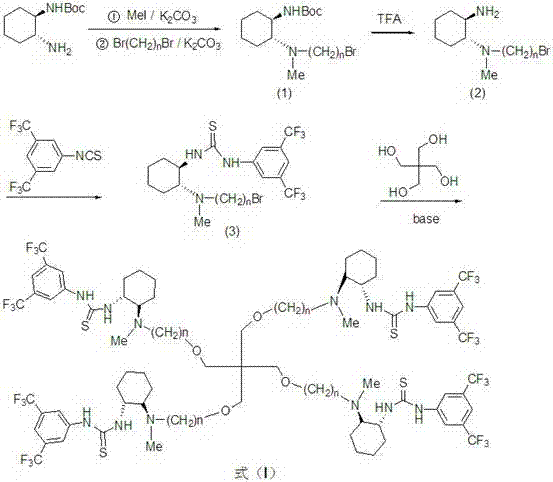
Pentaerythritol loaded chiral diamine derivative thiourea catalyst, preparation method and application thereof
ActiveCN107469858AThe synthetic route is simpleHigh yieldCarbamic acid derivatives preparationOrganic compound preparationPentaerythritolThiourea
The invention discloses a pentaerythritol loaded chiral diamine derivative thiourea catalyst, a preparation method and an application thereof. The structure thereof is as shown in a formula (I). The preparation method of the pentaerythritol loaded chiral diamine derivative thiourea catalyst comprises the following steps: a) reacting S,S-N-Boc-cyclohexanediamine with CH3I under room temperature in the presence of acetonitrile and K2CO3 and then reacting with Br(CH2)nBr, thereby acquiring an intermediate (1); b) reacting the intermediate (1) with F3CCO2H, thereby acquiring an intermediate (2); c) reacting the intermediate (2) with 3,5-bi(trifluoromethyl) isothiocyanate under room temperature, acquiring an intermediate (3); and d) reacting the intermediate (3) with pentaerythritol under the existence of alkali and phase transfer catalyst, thereby acquiring the pentaerythritol loaded chiral diamine derivative thiourea catalyst shown as formula (I). The pentaerythritol loaded chiral diamine derivative thiourea catalyst can be applied to the dissymmetric Michael addition reaction. The pentaerythritol loaded chiral diamine derivative thiourea catalyst can be simply separated and purified and can be reused.
Owner:东营睿港招商服务有限责任公司
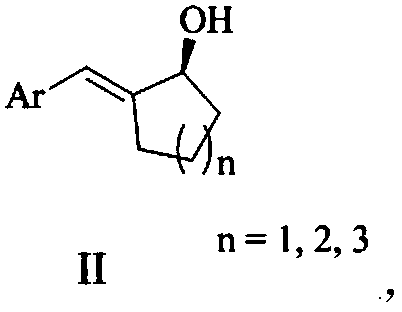
Chiral 2-aromatic methylene naphthenic alcohol and asymmetric synthesizing method thereof
ActiveCN109111334AEasy to synthesizeLow priceOrganic compound preparationHydroxy group formation/introductionIridiumTransfer hydrogenation
The invention relates to chiral 2-aromatic methylene naphthenic alcohol and an asymmetric synthesizing method thereof. A specific structure of the chiral 2-aromatic methylene naphthenic alcohol is shown as II. According to the method, mono-sulfonyl chiral diamine and metal ruthenium, rhodium and iridium coordination compound are utilized as catalysts, formic acid / triethylamine azeotrope is utilized as a hydrogen source, and chemical-selection asymmetrical transfer hydrogenation is performed on 2-aromatic methylene naphthenone (I) under the mild conditions to prepare the chiral 2-aromatic methylene naphthenic alcohol (II). The method has convenience in operation, raw materials are easy to obtain, and yield and enantioselectivity are both higher. (The formulas are shown in the description).
Owner:CHINA THREE GORGES UNIV
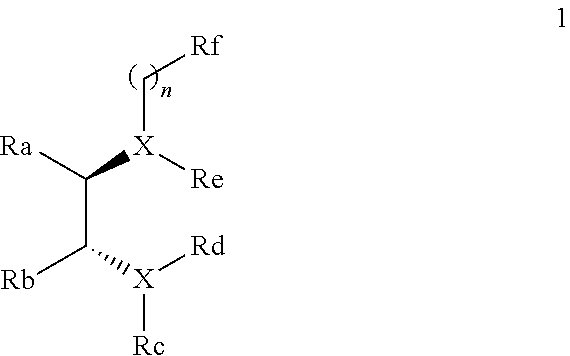
Chiral diamine compounds for the preparation of chiral alcohols and chiral amines
A process for the stereoselective preparation of a chiral alcohol or a chiral amine, the process comprising reacting a first prochiral reactant selected from the group consisting of a ketone, an aldehyde, and an imine, with a second reactant comprising a Grignard reagent, in the presence of a chiral trans-diamine of formula (1) as defined herein:Also provided is the use of the chiral trans-diamine of formula (1) in a Grignard reaction and the chiral trans-diamines per se.
Owner:UNIV COLLEGE DUBLIN NAT UNIV OF IRELAND DUBLIN
Preparation method of optically active N-benzyl phenylephrine
InactiveCN102381990AImprove conversion rateHigh yieldOrganic compound preparationAsymmetric synthesesHydrogenAsymmetric hydrogenation
The invention discloses a preparation method of optically active N-benzyl phenylephrine, which is shown as a formula 1. The preparation method includes steps that due to alkali assistant and catalysis of chiral diphosphine- chiral diamine transition metal asymmetric hydrogenation catalyst, compound II and hydrogen realize asymmetric hydrogenation in liquor to complete a preparation process, wherein, '*' stands for chiral carbon, namely R or S configuration. The preparation method is high in product yield, higher in both chemical purity and optical purity, low in cost and simple in aftertreatment.
Owner:CHIRAL QUEST (SUZHOU) CO LTD
Polysubstituted chiral (1-ethoxy)benzene and asymmetric synthesis method thereof
ActiveCN108046995ARaw materials are easy to getSimple and fast operationOrganic compound preparationOrganic chemistry methodsHydration reactionKetone
The invention relates to polysubstituted chiral (1-ethoxy)benzene. The specific structure of the polysubstituted chiral (1-ethoxy)benzene is as shown in a formula II. The invention also discloses a 'two-step one-pot' synthetic method of the polysubstituted chiral (1-ethoxy)benzene. The 'two-step one-pot' synthetic method is characterized by taking polyacetylenyl substituted benzene (I) as a raw material, and comprises the following steps: step (1) taking fluorine-containing alcohol and water as solvents, carrying out hydration reaction under the catalysis of trifluoromethanesulfonic acid, thusgenerating an intermediate-ketone; step (2) directly adding a complex of mono-sulfonyl chiral diamine and metal ruthenium, rhodium or iridium as a catalyst in a reaction system, adding alkali, supplementing hydrogen, and carrying out asymmetric hydrogenation reaction, thus obtaining a product II; or directly adding the complex of the mono-sulfonyl chiral diamine and the metal ruthenium, rhodium or iridium as the catalyst in the reaction system, using a mixture of sodium formate or formic acid and triethylamine as a hydrogen source, and carrying out the asymmetric transfer hydrogenation reaction, thus obtaining the product II. According to the 'two-step one-pot' synthetic method disclosed by the invention, the operation is simple, the raw material is easy to obtain, and enantioselectivityand diastereoselectivity are very high. (The formula II is shown in the description).
Owner:CHINA THREE GORGES UNIV
Method for direct conversion of aromatic alkyne into chiral alcohol through one-pot process
ActiveCN108101740ASimple and fast operationMild reaction conditionsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsHydration reactionIridium
The invention relates to a method for direct conversion of aromatic alkyne into chiral alcohol through a one-pot process. The method uses cheap and easily-available alkyne I as a raw material, adoptsa two-step one-pot strategy for direct synthesis of chiral alcohol II, and comprises the following concrete steps: step 1) with fluorine-containing alcohol and water as solvents, allowing the alkyne Ito generate a hydration reaction under the catalysis of trifluoromethanesulfonic acid so as to generate an intermediate namely ketone; and step 2) directly adding a complex of monossulfonyl chiral diamine and metal ruthenium or rhodium or iridium as a catalyst into a reaction system, and with a mixture of a sodium formate aqueous solution or formic acid-triethylamine as a hydrogen source, carrying out an asymmetric transfer hydrogenation reaction so as to obtain a product II. The method provided by the invention has the following advantages: operation is simple and convenient; reaction conditions are mild; and a substrate has wide application range and high enantioselectivity. Concretely, the method provided by the invention has a general reaction formula which is described in the specification.
Owner:CHINA THREE GORGES UNIV
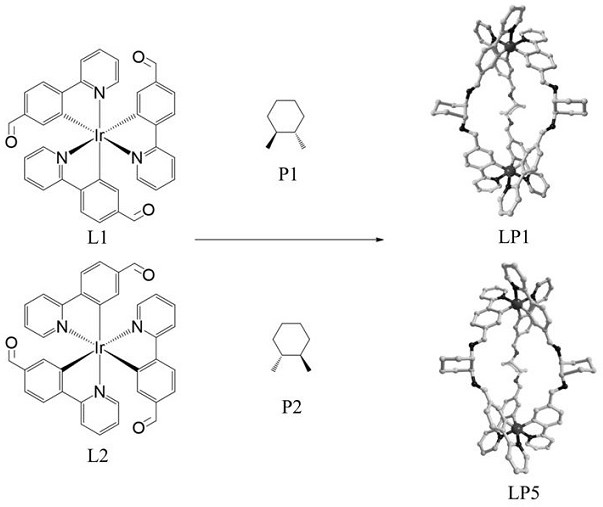
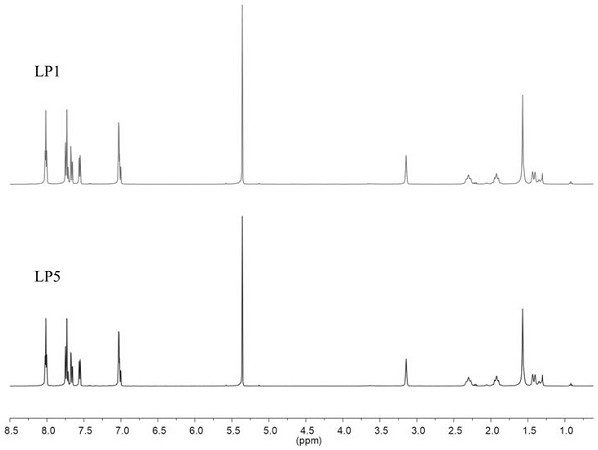
Ir (III)-based chiral metal-organic porous material with splitting function as well as preparation method and application of Ir (III)-based chiral metal-organic porous material
ActiveCN114213465AChirality is maintained for a long timeRich chiral informationIndium organic compoundsOrganic compound preparationCrystalline materialsAdsorption separation
The invention discloses an Ir (III)-based chiral metal-organic porous material with a resolution function as well as a preparation method and application thereof, and belongs to the technical field of chiral compound resolution. According to the material, chiral Ir (III)-based complexes L1 and L2 are used as construction modules and are respectively subjected to Schiff base reaction with chiral diamines P1 and P2 to generate a chiral metal-organic porous material; the method comprises the following steps: by taking 1, 1 '-co-2-naphthol as a to-be-resolved molecule, adding a solvent, taking the obtained multi-chiral helical compound as a solid resolving agent, and realizing adsorption separation of a corresponding single chiral molecule through solid-liquid stirring. The Ir (III) complex enables the chirality of the target material to be maintained for a long time; the introduction of chiral diamine enriches the chiral information of the pore channel of the target crystalline material, realizes the strict matching with chiral molecules, and realizes the efficient resolution of 1, 1 '-co-2-naphthol (ee is as high as gt, 99%).
Owner:DALIAN UNIV OF TECH
Diboron glycol ester as well as preparation method, intermediate and application thereof
ActiveCN111440205AChiralEasy to manufactureOrganic compound preparationGroup 3/13 element organic compoundsCombinatorial chemistryDiol
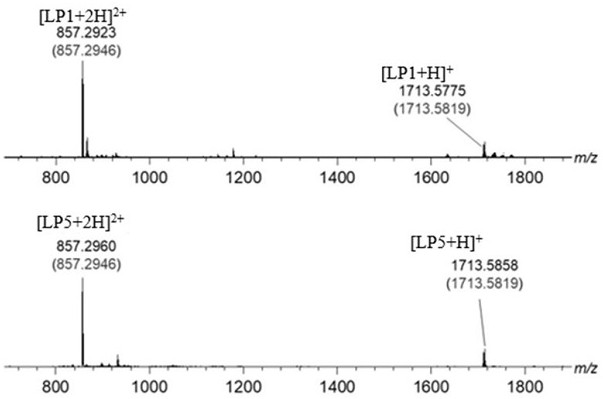
The invention discloses diboron glycol ester as well as a preparation method, an intermediate and application thereof. The diboron glycol ester can be used for inducing reductive coupling reaction with imine as a substrate, and the substrate can be obtained by reaction of aldehyde and ammonia and is very easy to obtain and quite low in cost. The product can be separated from a reaction system onlyby acid-base operation without column chromatography purification, and the post-treatment mode is convenient and easy to operate. The yield of the obtained product is high, and protective group operation is not needed. The diboron glycol ester has chirality, the stereoselectivity of the reductive coupling reaction is generally excellent, and 99% ee chiral diamine can be obtained only through simple recrystallization. The diboron glycol ester can be obtained by reacting diol with diboron glycol ester, the diol is convenient to prepare and easy to amplify, the diol can be recycled from a reaction solution through simple acid-base operation, the recovery rate reaches 95%, and the preparation cost is further saved.
Owner:宁波赜军医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com