Preparation method and intermediate of chiral diamine compound
A technology of chiral diamines and compounds, applied in the field of preparation of chiral diamine compounds and their intermediates, can solve the problems of lower conversion efficiency, difficult separation of diastereoisomers, harsh reaction conditions, etc., and achieve high yield High, good chiral purity, low safety risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
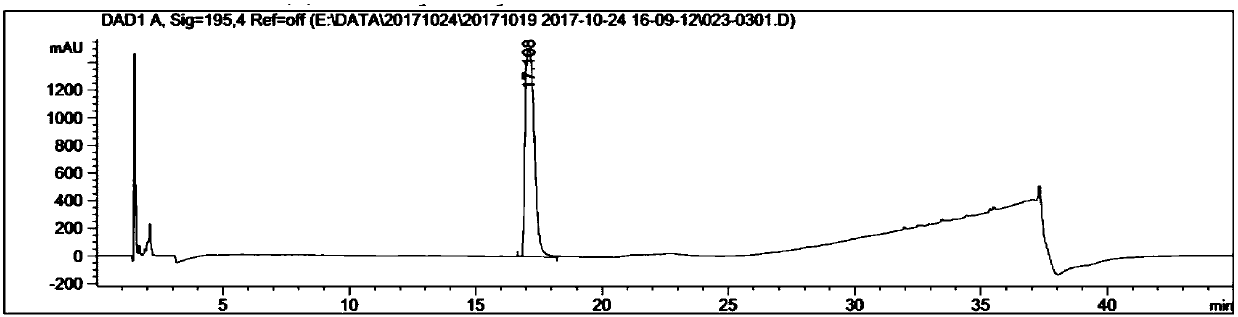
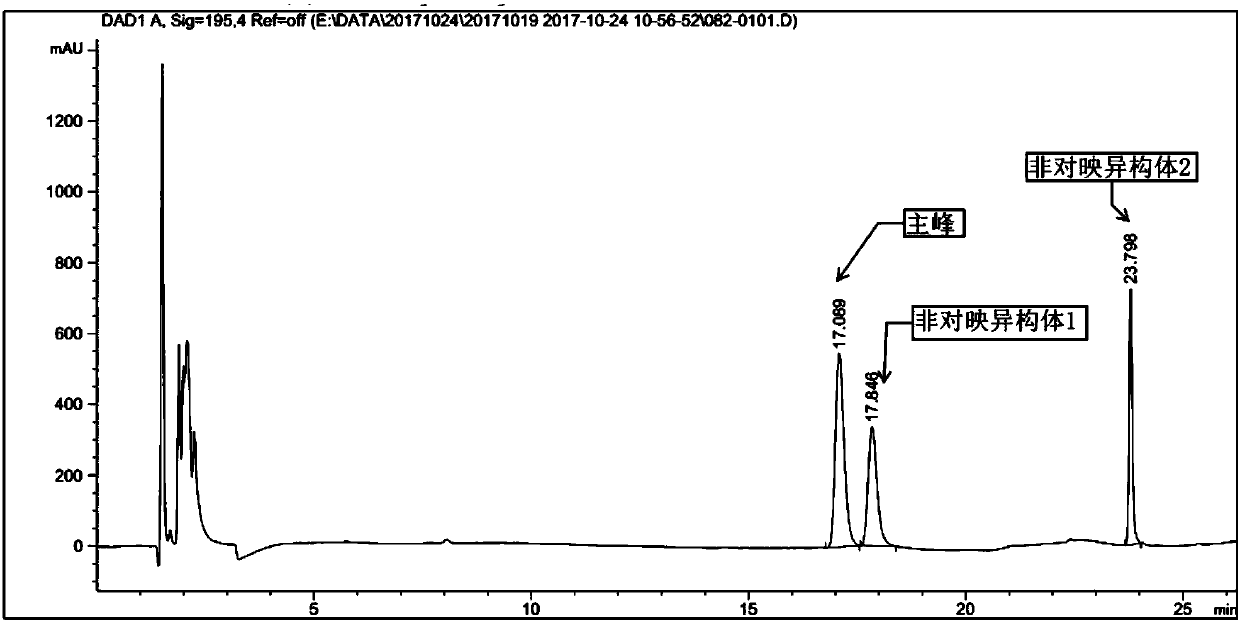
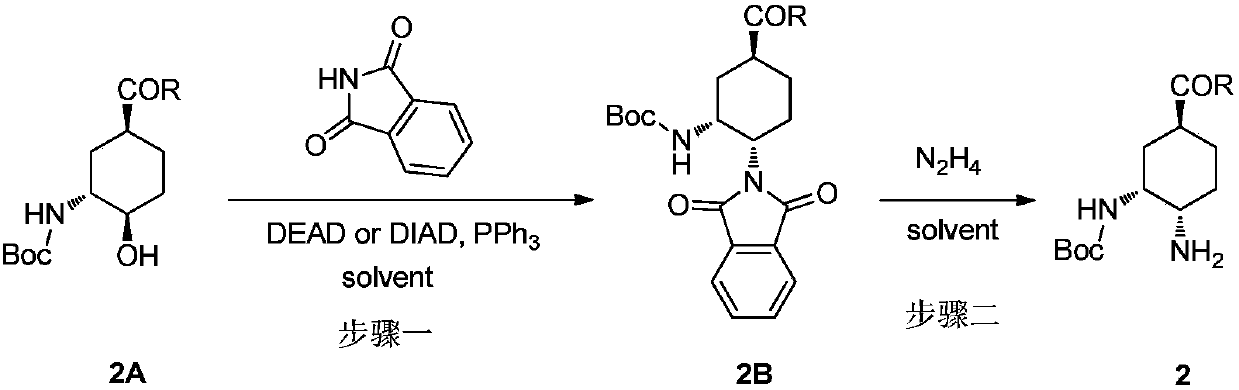
[0029] Example 1: Preparation of ethyl (1S,3R,4S)-3-(tert-butoxycarbonylamino)-4-(1,3-dioxoisoindoline-2-yl)cyclohexylcarboxylate
[0030]
[0031] In a three-necked flask equipped with mechanical stirring and a thermometer, add 930 mL of anhydrous tetrahydrofuran, ethyl (1S,3R,4R)-3-((tert-butoxycarbonylamino)-4-hydroxycyclohexanecarboxylate (46.5 g, 1.0 eq), phthalimide (28.5g, 1.2eq), triphenylphosphine (51.0g, 1.2eq), N 2 After the replacement, the temperature of the system was lowered to 0-10° C., and diisopropyl azodicarboxylate (42.3 g, 1.5 eq) was slowly added dropwise, and N 2 After replacement, it was raised to room temperature for 2 hours, and further raised to 40-50° C. for 12 hours. TLC monitoring showed that the reaction was complete. After cooling down to room temperature, the tetrahydrofuran was concentrated to dryness, and 280 mL of methyl tert-butyl ether was added for slurry at about 10°C for 2 hours, and an off-white solid was obtained by filtration. T...
Embodiment 2
[0032] Example 2: Preparation of ethyl (1S,3R,4S)-3-(tert-butoxycarbonylamino)-4-(1,3-dioxoisoindoline-2-yl)cyclohexylcarboxylate
[0033] Add 240 mL of anhydrous toluene, ethyl (1S,3R,4R)-3-((tert-butoxycarbonylamino)-4-hydroxycyclohexanecarboxylate (12.0 g, 1.0 eq), phthalimide (12.3g, 2.0eq), triphenylphosphine (21.9g, 2.0eq), N 2 After the replacement, the temperature of the system was lowered to 0-10° C., and diisopropyl azodicarboxylate (21.8 g, 3.0 eq) was slowly added dropwise, and N 2 After the replacement, rise to room temperature and react for 2 hours, and further rise to 70-80° C. for 6 hours. TLC monitoring shows that the reaction is complete. Reduce to about 10°C and beat for 2 hours, filter to obtain off-white solid. Dissolve the solid in 144 mL of dichloromethane, add 72 mL of water and wash once. Separate the organic phase, concentrate the dichloromethane to dryness, add 84mL of ethanol, heat up to 60°C to dissolve, then cool down to 0-10°C to crystallize t...
Embodiment 3
[0034]Example 3: Preparation of (1S,3R,4S)-3-(tert-butoxycarbonylamino)-4-(1,3-dioxoisoindoline-2-yl)cyclohexylcarboxylic acid methyl ester
[0035]
[0036] Add 240 mL of anhydrous methyl tert-butyl ether, (1S,3R,4R)-3-((tert-butoxycarbonylamino)-4-hydroxycyclohexanecarboxylic acid methyl ester into a three-necked flask equipped with mechanical stirring and a thermometer (12.0g, 1.0eq), phthalimide (12.9g, 2.0eq), triphenylphosphine (23.0g, 2.0eq), N 2 After the replacement, the temperature of the system was lowered to 0-10° C., and diethyl azodicarboxylate (22.9 g, 3.0 eq) was slowly added dropwise, and N 2 After replacement, it was raised to room temperature for 2 hours, and further raised to 40-50° C. for 12 hours. TLC monitoring showed that the reaction was complete. Reduce to about 10°C and beat for 2 hours, filter to obtain off-white solid. Dissolve the solid in 144 mL of dichloromethane, add 72 mL of water and wash once. Separate the organic phase, concentrate th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com