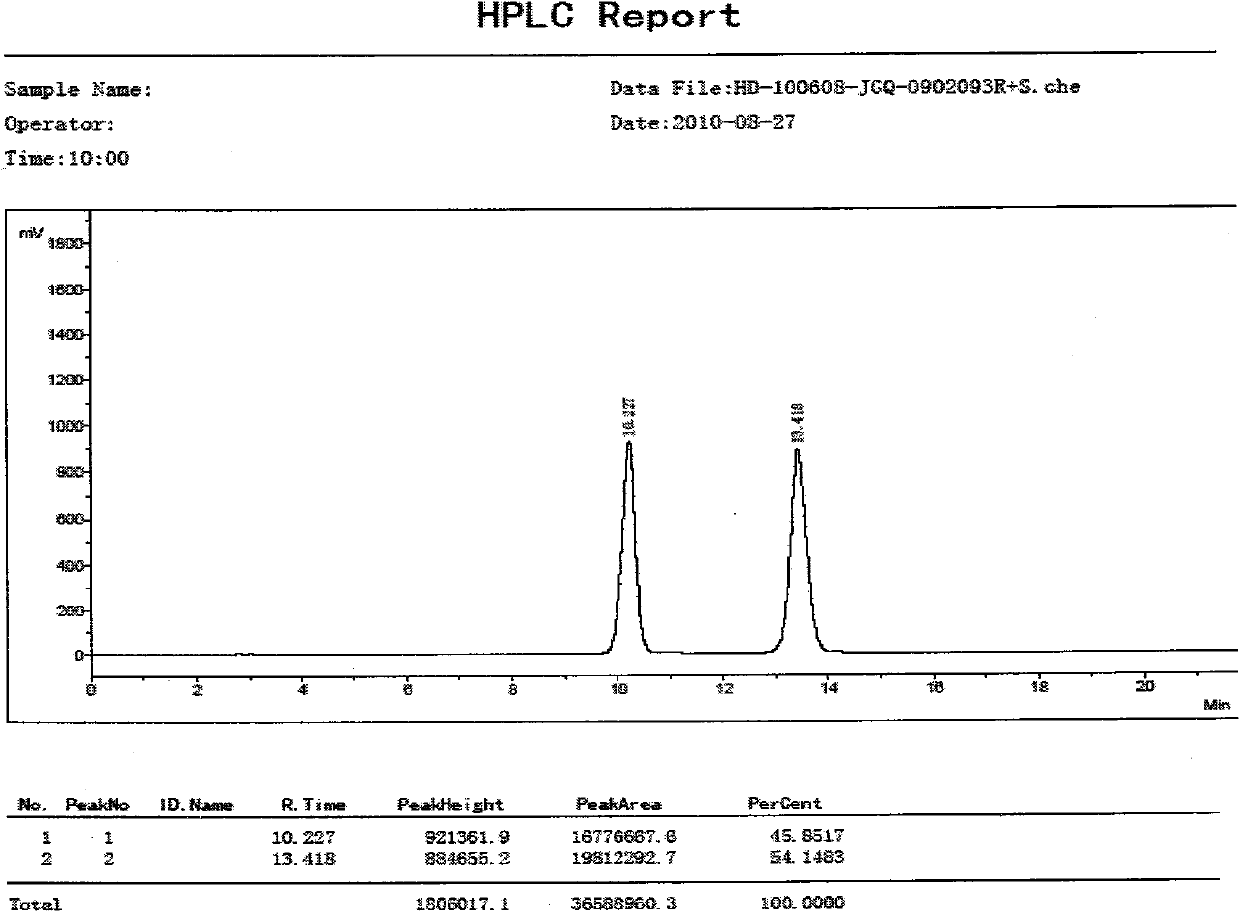
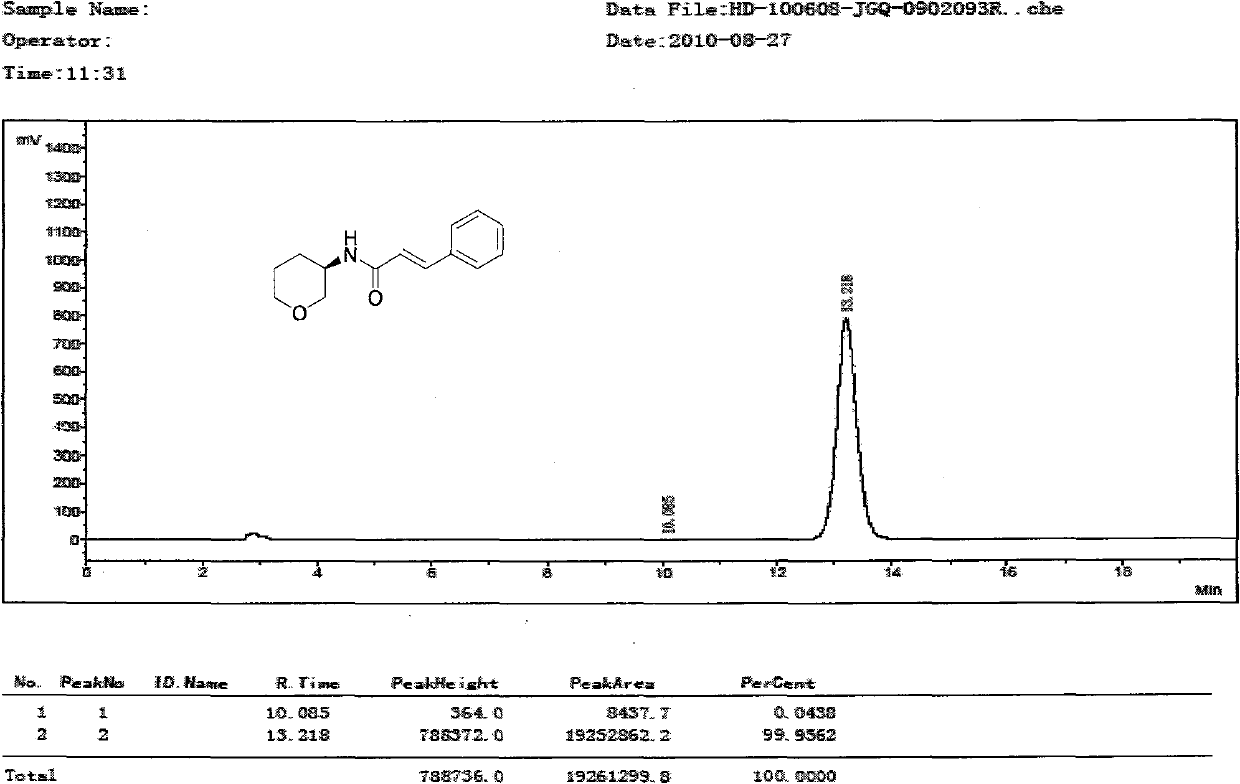
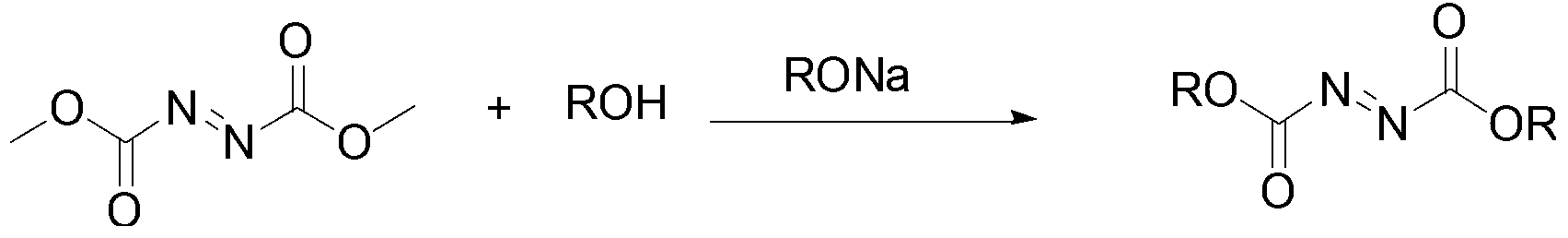
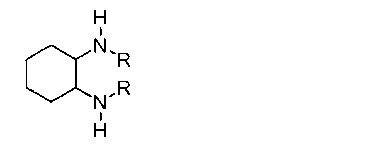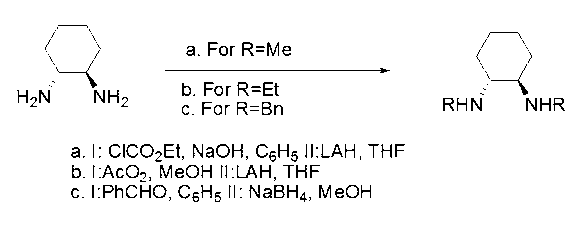Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
55 results about "Diethyl azodicarboxylate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
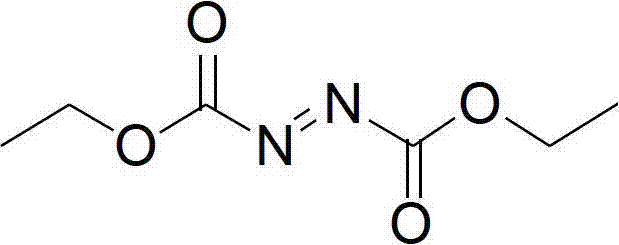
Diethyl azodicarboxylate, conventionally abbreviated as DEAD and sometimes as DEADCAT, is an organic compound with the structural formula CH₃CH₂O₂CN=NCO₂CH₂CH₃. Its molecular structure consists of a central azo functional group, RN=NR, flanked by two ethyl ester groups. This orange-red liquid is a valuable reagent but also quite dangerous and explodes upon heating. Therefore, commercial shipment of pure diethyl azodicarboxylate is prohibited in the United States and is carried out either in solution or on polystyrene particles.
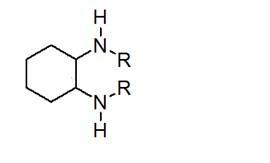
Method for synthesizing enantiomorphous pure symmetric trans-dialkyl cyclohexylamine
InactiveCN102531918ASimple and fast operationMild reaction conditionsOrganic compound preparationAmino compound preparationDiisopropyl azodicarboxylateHexamethylenediamine
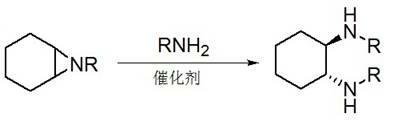
The invention discloses a method for synthesizing enantiomorphous pure symmetric trans-dialkyl cyclohexylamine, belonging to the field of chemistry. The method comprises the following steps of: carrying out an airtight or reflux reaction on cyclohexene oxide and an aqueous solution of alkylamine for reacting at 80-120 DEG C for 1.5-5h to obtain 2-alkyl-amino cyclohexanol, dropwise adding DEAD (Diethyl Azodicarboxylate) or DIAD (Diisopropyl Azodicarboxylate) to triphenylphosphine, 2-alkyl-amino cyclohexanol and a solvent under an ice bath for reacting for 5-20h at room temperature to obtain N-alkyl-7-azabicyclo[4, 1, 0]heptane, adding a catalyst to the N-alkyl-7-azabicyclo[4, 1, 0]heptane and the aqueous solution of alkylamine at 100-120 DEG C to carry out the airtight or reflux reaction to obtain trans-N,N'-dialkyl-1,2 cyclohexanamine, dissolving the trans-N,N'-dialkyl-1,2 cyclohexanamine in an alcoholic solvent, and adding a tartaric acid type resolving agent with the equivalent weight of 0.5 to the alcoholic solvent to resolve so as to obtain the enantiomorphous pure symmetric trans-dialkyl cyclohexylamine.
Owner:ANYANG INST OF TECH
Method for synthesizing enantiomorphous pure symmetric trans-dialkyl cyclohexylamine
InactiveCN102531918BSimple and fast operationMild reaction conditionsOrganic compound preparationAmino compound preparationDiisopropyl azodicarboxylateHexamethylenediamine
The invention discloses a method for synthesizing enantiomorphous pure symmetric trans-dialkyl cyclohexylamine, belonging to the field of chemistry. The method comprises the following steps of: carrying out an airtight or reflux reaction on cyclohexene oxide and an aqueous solution of alkylamine for reacting at 80-120 DEG C for 1.5-5h to obtain 2-alkyl-amino cyclohexanol, dropwise adding DEAD (Diethyl Azodicarboxylate) or DIAD (Diisopropyl Azodicarboxylate) to triphenylphosphine, 2-alkyl-amino cyclohexanol and a solvent under an ice bath for reacting for 5-20h at room temperature to obtain N-alkyl-7-azabicyclo[4, 1, 0]heptane, adding a catalyst to the N-alkyl-7-azabicyclo[4, 1, 0]heptane and the aqueous solution of alkylamine at 100-120 DEG C to carry out the airtight or reflux reaction to obtain trans-N,N'-dialkyl-1,2 cyclohexanamine, dissolving the trans-N,N'-dialkyl-1,2 cyclohexanamine in an alcoholic solvent, and adding a tartaric acid type resolving agent with the equivalent weight of 0.5 to the alcoholic solvent to resolve so as to obtain the enantiomorphous pure symmetric trans-dialkyl cyclohexylamine.
Owner:ANYANG INST OF TECH
Synthesis method of diethyl azodicarboxylate and intermediate of diethyl azodicarboxylate
ActiveCN102898328ANo pollution in the processIn line with the concept of modern green chemistryHydrazide preparationDiisopropyl azodicarboxylateDistillation
The invention discloses a synthesis method of diethyl azodicarboxylate. The synthesis method comprises the following steps that (1) under the effect of sodium ethoxide, diethyl carbonate and ethyl carbazate are heated for reaction for 1 to 6 hours, the pH of solution is regulated to 3 to 8, white crystals are separated out, the recrystallization is carried out, and hydrogenated diethyl azodicarboxylate is obtained; and (2) the hydrogenated diethyl azodicarboxylate is added at minus 15 DEG C to 45 DEG C, in the acid solution, bromine or hydrobromic acid or sodium bromide and potassium bromide are used as catalysts, excessive hydrogen peroxide is dripped, the reaction is carried out for 1 to 10 hours, the extraction is carried out, organic solvents are removed through distillation, and saffron diethyl azodicarboxylate is obtained. The invention also provides a hydrogenated diethyl azodicarboxylate intermediate and a synthesis method of the hydrogenated diethyl azodicarboxylate intermediate. The synthesis method has the advantages that diethyl carbonate is used as raw materials, cleanness and environment protection are realized, no pollution exists, raw materials can be cyclically utilized, the economy is better, the operation is simple, the reaction temperature range is wide, the reaction is stable, the energy consumption is low, the yield is high, and the industrial production is favorably realized.
Owner:SHANDONG NORMAL UNIV
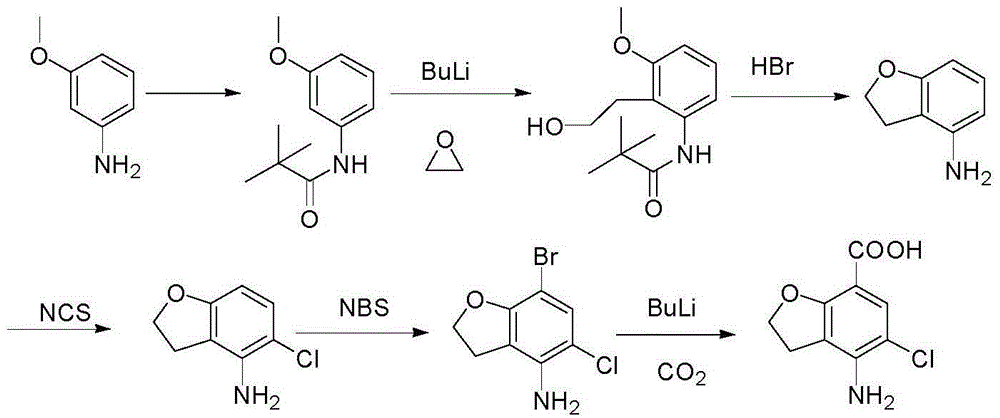
Method for synthesizing 4-amino-5-chloro-2,3-dihydro benzofuran-7-carboxylic acid
InactiveCN104016949AEasy to operate in industrialized productionHigh yieldOrganic chemistryCarboxylic acidSolvent
The invention discloses a method for synthesizing a prucalopride midbody 4-amino-5-chloro-2,3-dihydro benzofuran-7-carboxylic acid. The method comprises the following steps: firstly, adding methyl 4-(acetyl amino)-2-hydroxy-3-(2-hydroxy ethyl) benzoate into an organic solvent, adding triphenylphosphine and azo dioctyl phthalate diethyl ester, performing cyclization to obtain a methyl 4-acetamido-2,3-dihydro benzofuran-7-formate rough product, directly chloridizing the rough product by using N-chloro succinimide to obtain a rough product methyl 4-acetamide amino-5-chloro-7-benzofuran formate, and performing hydrolysis and purification to obtain a 4-amino-5-chloro-2,3-dihydro benzofuran-7-carboxylic acid pure product. Compared with a conventional method, the method simplifies the industrial production difficulty and remarkably improves the yield, so that the method disclosed by the invention is easy in industrial production operation, nearly no organic and inorganic waste solvents are used, the total yield is relatively high, and the industrial on-scale production is facilitated.
Owner:TIANJIN WEIJIE TECH
LDPE (Low-density polyethylene)/EVA (ethylene-vinyl acetate copolymer)/CPE (chlorinated polyethylene) composite foam material and preparation method thereof
ActiveCN103102567AImprove interfacial bond strengthHigh tensile strengthLow-density polyethylenePhosphoric Acid Esters
The invention discloses an LDPE (low-density polyethylene) / EVA (ethylene-vinyl acetate copolymer) / CPE (chlorinated polyethylene) composite foam material. The foam material is prepared from the following raw materials in parts by weight: 80-100 parts of LDPE, 30-40 parts of EVA, 20-30 parts of CPE, 10-20 parts of talcum powder, 10-15 parts of nanometer potassium feldspar powder, 2-3 parts of bis(dioctyl pyrophosphate) ethylene titanate, 1-2 parts of vinyl tri(beta-methoxyethoxy) silane, 5-10 parts of zinc borate, 5-8 parts of diethyl azodicarboxylate, 5-8 parts of tributyl citrate, 1-1.5 parts of di-tert-butyl peroxide, 2-3 parts of zinc oxide, 1-2 parts of stearic acid, 1-2 parts of tribasic lead sulfate, 2-3 parts of polyethylene wax, 3-5 parts of modified wood ash, 1-2 parts of ultraviolet light absorber UV-531 and 2-3 parts of antioxidant 1035. The produced LDPE foam material is light and soft, has high strength, good resilience, an ideal foaming effect, uniform, fine and dense pores and good flame retardance, is resistant to chemical corrosion and aging and is durable in use.
Owner:HEFEI LANGSHENG NOVEL MATERIAL CO LTD
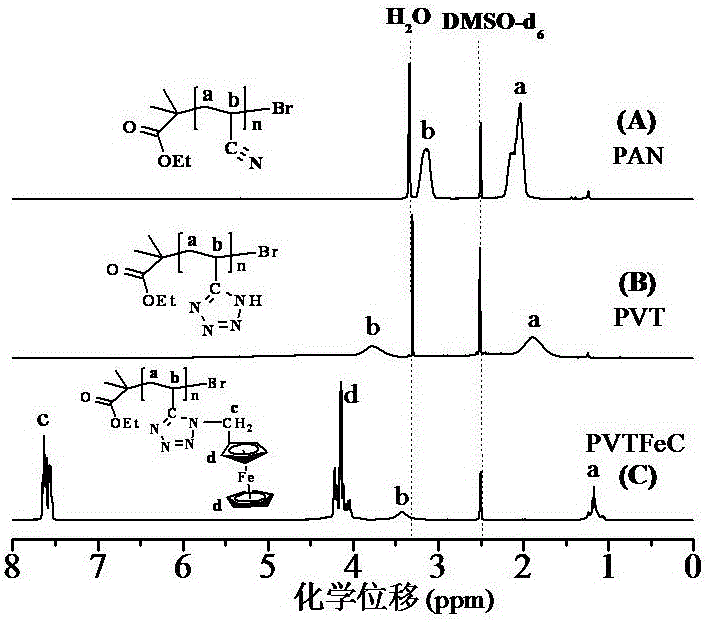
Novel method for preparing ferrocenyl polymer from controllable type polyacrylonitrile resin
The invention discloses a novel method for preparing a ferrocenyl polymer from controllable type polyacrylonitrile resin. The reaction of the novel method comprises the following steps of (1) preparing the polyacrylonitrile resin into a reaction system of polyvinyl tetrazolium (PVT) through a nitrile based click chemistry reaction, and enabling polyacrylonitrile, sodium azide, a catalyst and a solvent to be subjected to a reaction at certain temperature for a certain time so as to obtain an intermediate product PVT; and (2) preparing a reaction system of the ferrocenyl polymer (PVT-FeC) through a Mitsunobu reaction, and enabling the intermediate product PVT prepared in the step (1), ferrocenemethanol, triphenylphosphonium and diethyl azodicarboxylate to be subjected to a reaction at appropriate temperature for a certain time so as to obtain the ferrocenyl polymer. The molecular weight of the obtained polymer can be conveniently designed, besides, the end group of the polymer can still have activity, and the polymer can be used for synthesizing some other functional copolymers with a topological structure.
Owner:LUDONG UNIVERSITY
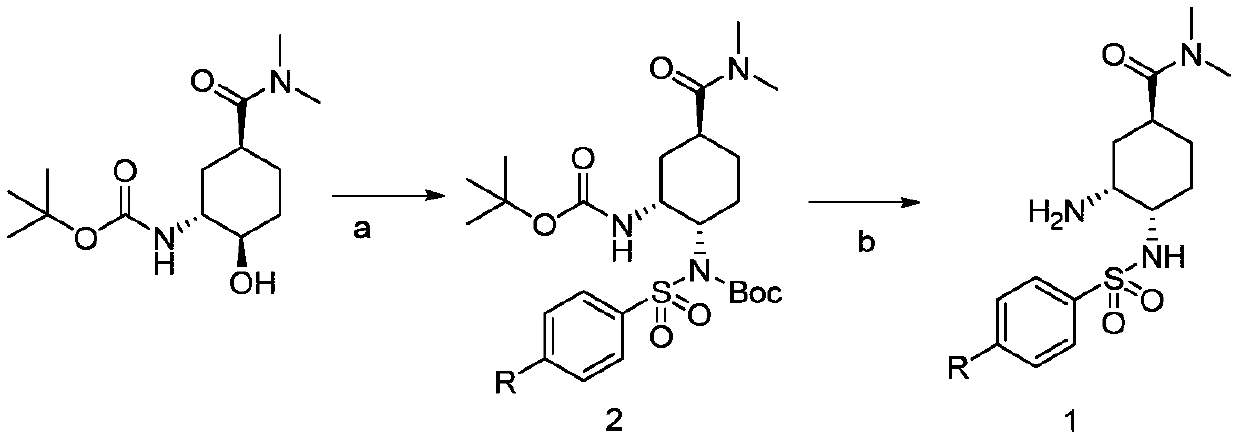
Preparation method of Edoxaban tosylate intermediate and intermediate compound
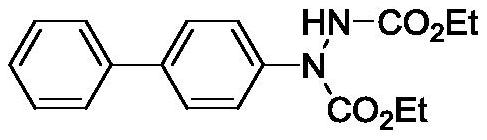
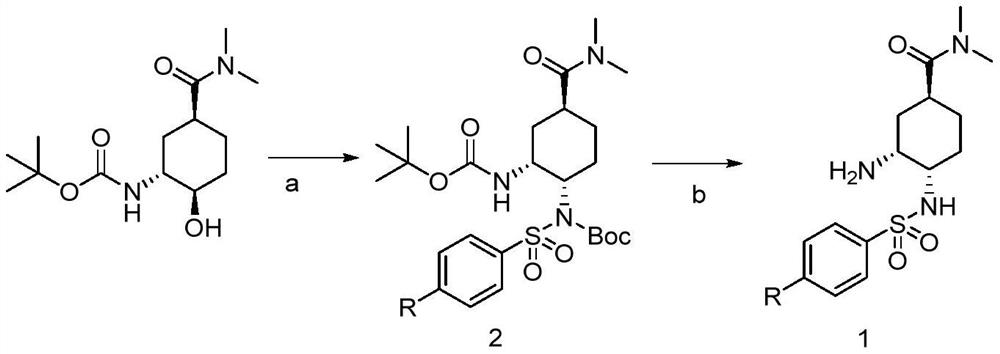
ActiveCN109836360AEasy to introduceHigh reaction conversion rateSulfonic acid amide preparationBulk chemical productionTert-Butyloxycarbonyl protecting groupSodium azide
The invention provides a preparation method of an Edoxaban tosylate intermediate, the method comprises the following reaction steps: step a, under a certain temperature condition, adding dimethylformamide substituted cyclohexane, triphenylphosphine (PPh3)) and diethyl azodicarboxylate (DEAD) in a solvent, and then adding N-(t-butyloxycarboryl) p-nitrobenzenesulfonamide or N-(t-butyloxycarboryl) p-toluene sulfonamide for reacting to obtain a chirally-turned compound 2; and step b, removing Boc under a strong acid condition so as to obtain a compound 1; the invention further provides intermediate compounds of the compound 1 and the compound 2 in the preparation of the Edoxaban tosylate intermediate; the amino introduction process in the preparation method is simple, the reaction conversion rate is relatively high, and industrialization is easy to realize, so that the use of hazardous chemical sodium azide is avoided, the process safety is improved, and the production yield is improved.
Owner:南京恩泰医药科技有限公司
Composite foaming agent for rigid PVC foam material
The invention discloses a composite foaming agent for a rigid PVC foam material. The foaming agent comprises the following raw materials, by weight: 5-10 parts of azodicarbonamide, 2-4 parts of diethyl azodicarboxylate, 2-3 parts of OBSH, 0.5-1.5 parts of lauryl sodium sulfate, 1-2 parts of sodium bicarbonate, 1-2 parts of zinc carbonate, 1-3 parts of urea, 0.5-0.8 part of lead stearate, 2-4 parts of citric acid, and 15-20 parts of glycol propyl ether. According to the invention, raw materials including urea, zinc carbonate, sodium bicarbonate, sodium dodecyl sulfate are adopted for modification of azodicarbonamide to prepare the composite foaming agent for rigid PVC foam material; and the composite foaming agent has advantages of decomposition temperature within the range of molding processing of rigid PVC foam material, basically balanced heat absorption-release, smooth decomposition rate, large deflating amount and no abruptness. The rigid PVC material prepared from the composite foaming agent has good mechanical properties, uniform and fine pores, thin pore wall, easily controlled processing conditions, and no emergence of burning.
Owner:安徽艾柯泡塑股份有限公司
Organic silicon pressure sensitive adhesive
The invention discloses an organic silicon pressure sensitive adhesive which is prepared by reacting the following substances in percentage by mass: 45-50% of organic silicon adhesive, 2-4% of hydroxyl polysiloxane, 2-5% of stannous octoate, 2-3% of isopropyl tri(dodecylbenzene phenylsulfonyl) titanate, 0.5-5% of alkynol, 15-30% of tackifying resin and 15-25% of azo-compound foaming agent, wherein the azo-compound foaming agent is any one of azodicarbonamide, azodiisobutyronitrile, azo-dioctyl phthalate diisopropyl ester, azo-dioctyl phthalate diethyl ester, diazo-aminobenzene or azo-dibarium formate; the hydroxyl polysiloxane is alpha,omega-dyhydroxy polysiloxane; the tackifying resin is a MQ resin. The structural size of the micro pore structure of the organic silicon adhesive can be adjusted, the micro pores can be arranged densely or sparsely, and the problem that the conventional organic silicon adhesive is not smooth in gas discharge is solved.
Owner:SUZHOU SIDIKE NEW MATERIALS SCI & TECH
Toughened reinforced environment-friendly flame-retardant PBT/PET (polybutylene terephthalate/polyethylene terephthalate) alloy
InactiveCN104672809AImprove notched impact strengthIncrease stiffnessElastomerPolyethylene terephthalate glycol
The invention discloses a toughened reinforced environment-friendly flame-retardant PBT / PET (polybutylene terephthalate / polyethylene terephthalate) alloy which is composed of the following components in percentage by mass: 20-60% of PBT, 10-40% of PET, 10-25% of glass fiber, 5-15% of halogen-free flame retardant, 5-15% of nano calcium carbonate, 5-10% of elastomer, 0.5-2% of maleic anhydride, 0.1-1% of diethyl azodiformate, 1-5% of plasticizer, 0.1-1% of composite nucleating agent, 0.1-1% of composite antioxidant and 0.1-2% of lubricant. Compared with the prior art, the nano calcium carbonate subjected to surface modification pretreatment and the elastomer interact and generate grafting reaction to form the core-shell particles using the nano calcium carbonate as the core and the elastomer as the shell, and the core-shell particles are used for performing toughening modification on the PBT / PET alloy, thereby greatly enhancing the notch impact strength, rigidity, heat resistance and corrosion resistance of the alloy system and keeping excellent mechanical, chemical and electric properties; the toughened reinforced environment-friendly flame-retardant PBT / PET alloy has the advantages of favorable flowability, high gloss, no halogen, flame retardancy, high safety, low smoke, no toxicity, high efficiency and low cost, achieves the flame retardancy grade UL94V-0, and can be widely used in the fields of home appliances, electronic and electric appliances, automobile industry, mechanical equipment, textiles and the like.
Owner:QINGDAO JIAYIYANG IND & TRADE
Core-shell toughened and anti-fogging preservation master batch for PET film
The invention discloses a core-shell toughened and anti-fogging preservation master batch for a PET film. The master batch comprises the following components in percentage by mass: 10-30 percent of a PET resin, 20-40 percent of nano calcium carbonate, 20-40 percent of an elastomer, 2-6 percent of methylacrylic acid, 1-3 percent of diethyl azodicarboxylate, 3-10 percent of a dripping agent, 3-10 percent of an antifogging agent, 1-3 percent of a stabilizer, 0.5-2 percent of a nucleating agent, 0.5-3 percent of a coupling agent and 0.1-2 percent of an antioxidant. The nano calcium carbonate is treated and interacts with the elastomer so as to carry out a grafting reaction, a core-shell structure in which the elastomer is chemically coated with nano calcium carbonate is formed, and the synergistic toughening effect is enhanced. Meanwhile, the prepared master batch has the characteristics of anti-fogging performance, aging resistance, heat resistance, chemical resistance and the like and can be directly added and used in production of PET films, the process is simple, the operation is convenient, the product quality can be improved, and the master batch has high practical values and application prospects.
Owner:QINGDAO JIAYIYANG IND & TRADE
Method for synthesizing spermidine hydrochloride
ActiveCN111302953AGet efficientlySimple process conditionsAmino compound purification/separationCarbamic acid derivatives preparationMeth-Diisopropyl azodicarboxylate
The invention relates to a method for synthesizing spermidine hydrochloride. The reaction formula is shown as the following formula (I) which is described in the specification. In the formula (I), theprotective group R1 of amino in 4-amino-1-butanol of a compound 2 is one of tert-butyloxycarboryl Boc-, triphenylmethyl Trt- and p-methoxytriphenylmethyl Mmt-, and a protective group R2 of propane diamine of the compound is one of tert-butyloxycarbonyl Boc-, triphenylmethyl Trt- and p-methoxytriphenylmethyl (Mmt-). A reagent used in the Mitsunobo reaction condition is one of triphenylphosphine, di-tert-butyl azodicarboxylate, diethyl azodicarboxylate and diisopropyl azodicarboxylate. The preparation method disclosed by the invention is simple in required process condition, mild in reaction condition, capable of effectively obtaining spermidine hydrochloride, relatively short in synthesis step and relatively high in synthesis yield.
Owner:南京康立瑞生物科技有限公司
Antibacterial and mildew-proof PP foam board and preparation method thereof
The invention discloses an antibacterial and mildew-proof PP foam board and a preparation method thereof. The antibacterial and mildew-proof PP foam board is prepared by, by weight, 72-94 parts of polypropylene, 41-63 parts of chlorothalonil, 24-46 parts of cyanoethyl cellulose, 3-6 parts of dichlorophenyl dimethyl urea, 13-19 parts of tricresyl phosphate, 17-23 parts of magnetic wave stone, 10-15 parts of glass fibers, 4-8 parts of oxidized polyethlene wax, 2-3 parts of zinc isooctanoate, 15-20 parts of opal shale, 34-48 parts of waste bauxite cement, 1-2 parts of magnesium isooctanoate, 1.5-2.5 parts of diethyl azodicarboxylate, 26-39 parts of alum dregs, 2-4 parts of oxine-copper, 12-18 parts of tributyl citrate and 4-7 parts of ammonium carbonate. The antibacterial and mildew-proof PP foam board has the advantages that the chlorothalonil and the cyanoethyl cellulose are used to modify the polypropylene, the dichlorophenyl dimethyl urea, the tricresyl phosphate, the oxine-copper, the magnetic wave stone, the opal shale and the glass fibers are added for compositing, the antibacterial and mildew-proof performance of the foam board can be increased, and the water resistance and weather resistance of the foam board can also be increased.
Owner:郭云鹏
Redox induced pH-responsive type methacrylate fluorine-containing monomer as well as synthetic method and application thereof
ActiveCN107721888AThe synthesis method is simpleQuality improvementRadioactive preparation carriersSulfonic acid amide preparationProtonationSulfonyl chloride
The invention relates to a synthetic method and application of a redox induced pH-responsive type methacrylate fluorine-containing monomer. The general formula is shown in the specification, wherein a, b, c and d are respectively independent integers. The synthetic method comprises the following steps: with organic alkali as an acid-binding agent, enabling a methacrylate monomer containing a primary amine end group to react with 2,4-dinitrobenzene sulfonyl chloride in anhydrous tetrahydrofuran so as to obtain a sulfamide monomer; and with diethyl azodicarboxylate and triphenylphosphine as catalysts, enabling the sulfamide monomer to have a Mitsunobu reaction with a fluorine alcohol monomer, separating and purifying, and assembling in an aqueous solution. A strong electron withdrawing group2,4-dinitro-benzsulfamide exists in the structure disclosed by the invention and can rapidly have a nucleophilic substitution reaction with mercapto group so as to produce a secondary amine group, and the secondary amine group is protonized under corresponding pKa, so that redox induced pH response is realized. Development of fluorine-containing small molecules is facilitated, and the developmentneed of a 19FMRI probe is met. The synthetic method disclosed by the invention is simple, and the monomer is stable in quality and high in yield. The structural formula is as shown in the specification.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Synthetic method of betamethasone or prednisolone intermediate
The invention discloses a synthetic method of 11 beta,17 alpha-dihydroxy-1,4-diene pregne-3,20-diketone-21-acetate. The invention employs 11 alpha-hydroxy-1,4-diene-16,17-epoxy progesterone (ii) as a starting material, which together with methyl p-toluenesulfonate is subjected to a Mitunobu reaction for inversion under the catalysis of diethyl azodiformate and triphenyl phosphine to obtain 11 beta-p-toluenesulfonyl-16,17 epoxy-1,4-diene progesterone (iii), namely a 11 beta-hydroxy protector; the (iii) is subjected to protective group removal in the presence of HBr, and 16,17-epoxy addition to obtain 11 beta, 17 alpha-dihydroxy-16 beta-bromo-1,4-allyl progesterone (iv); the (iv) is subjected to 3 steps to synthesize 11 beta,17 alpha-dihydroxy-1,4-diene pregne-3,20-diketone (v), which is added with iodine to obtain 11 beta,17 alpha-dihydroxy-1,4-diene pregne-3,20- diketone-21-iodine (vi); and the (vi) is subjected to replacement with potassium acetate to obtain the 11 beta,17 alpha-dihydroxy-1,4-diene pregne-3,20-diketone-21-acetate (i). The method provided by the invention can avoid complex strain breeding, has the advantages of simple operation, high yield, little environmental pollution, and has good industrial application prospect.
Owner:SHANGHAI NEW HUALIAN PHARMA
Insulating packaging material for fresh food and preparation method thereof
InactiveCN110358183ABoth antibacterial and sterilizingBoth cushioning and shock absorptionFlexible coversWrappersFiberChlorogenic acid
The invention discloses an insulating packaging material for fresh food and a preparation method thereof. The packaging material comprises a four-layered structure which successively comprises an antibacterial layer, a shock absorbing layer, an insulating layer and a protecting layer, wherein the antibacterial layer is prepared from 1-5 parts of lysozyme, 1-3 parts of chlorogenic acid, 3-5 parts of tea polyphenol, 10-15 parts of a tourmaline powder, 3-7 parts of microcrystalline graphene and 30-50 parts of chitosan. The shock absorbing layer is prepared from 20-25 parts of a modified wheat straw powder, 20-30 parts of waste paper pulp, 10-15 parts of linen fibers, 5-10 arts of aluminum borate crystal whiskers, 4-6 parts of diethyl azobarboxylate, 30-40 parts of a vinyl-vinyl acetate copolymer and 1-2 parts of a silane coupling agent. The insulating layer is prepared from 15-25 parts of diatom ooze, 5-15 parts of sepiolite amianthine, 15-20 parts of a silicon dioxide aerogel and 30-40 parts of a silica sol. The packaging material plays roles of being antibacterial and sterilizing, buffering and absorbing shock, insulating and isolating heat, and being waterproof and dampproof simultaneously, so that the preservation time and the goods shelf supply period of fresh food are prolonged greatly.
Owner:淮北市硕华机械设备有限公司
Synthesis method of Lupinus luteus wighteone
InactiveCN103936706ALow costEasy post-processingOrganic chemistryDimethylaniline N-oxideSynthesis methods
The invention discloses a synthesis method of Lupinus luteus wighteone. The method provided by the invention toakes genistein as a raw material to undergo four-step reaction so as to obtain Lupinus luteus wighteone. In the second-step reaction of the method, isoamylene bromide, potassium carbonate, and a DMF system are employed to replace isopentenyl alcohol, diethyl azodicarboxylate, triphenylphosphine and a THF system, the cost is greatly reduced, the after-treatment is much more convenient, and the yield is enhanced from 83% to 92%. In the third-step reaction, diethyl aniline, dimethylaniline, decahydronaphthalene and other high-boiling point solvents are adopted as the solvents, and under heating reflux conditions, para Claisen rearrangement of isopentenyl is completed to replace Eu(fod)3 catalyzed rearrangement adopted by original methods, thus saving the precious metal catalyst, greatly reducing the cost, and increasing the yield from original 68% to 87%.
Owner:CHANGZHOU UNIV
High-tensile strength cable protective casing and production method
InactiveCN105968688AHas excellent high tensile strengthExcellent high tensile strengthInsulated cablesInsulated conductorsPolyvinyl chlorideNano al2o3
The invention discloses a high tensile strength cable sheath tube, which is composed of raw materials: acrylonitrile-butadiene-styrene copolymer, polyvinyl chloride, chicken feather hydrolyzed powder, sodium pentanesulfonate, stearic acid decanoate Tetraalkyl ester, γ‑aminoethylaminopropyltrimethoxysilane, diethyl azodicarboxylate, polypropylene glycol dioxirane methyl ether, nano silicon carbide, nano magnesium nitride, nano aluminum oxide , Gum Arabic, Silica. The invention also discloses the production method of the above-mentioned sheath pipe, and the produced sheath pipe has the advantage of excellent high tensile strength, and at the same time, has good flame-retardant performance.
Owner:ANHUI HUAXING CABLE GROUP
Preparation method of high-optical-purity pitavastatin calcium key intermediate
ActiveCN102174039BHigh optical purityHigh E-stereoselectivityOrganic chemistryButyl acetateDiisopropyl azodicarboxylate
The invention belongs to the technical field of blood fat reducing medicaments and relates to a preparation method of a high-optical-purity pitavastatin calcium key intermediate. The preparation method comprises the following steps of: performing a Mitsunobu reaction on (4R,6S)-6-hydroxymethyl-2,2-dimethyl-1,3-dioxane-4-butyl acetate II, a compound III, triphenylphosphine and diisopropyl azodicarboxylate (DIAD) or diethyl azodicarboxylate (DEAD) in a solvent to obtain a compound IV; performing an oxidation reaction to obtain a sulfone compound V; reacting the sulfone compound V with a compound VI under an alkaline environment to obtain the high-optical-purity pitavastatin calcium key intermediate. By the preparation method, the technical problems of low optical purity, low E type stereoselectivity, high separation and purification difficulty and low yield in the conventional pitavastatin calcium intermediate are solved.
Owner:JIANGSU WANBANG BIOPHARMLS +1
Method for synthesizing chiral pharmaceutical intermediate 3-amino tetrahydropyrane and salt thereof
InactiveCN102030734AEasy to manufactureHigh optical activityOrganic chemistryBulk chemical productionDiisopropyl azodicarboxylateReaction intermediate
The invention relates to a new method for synthesizing chiral pharmaceutical intermediate 3-amino tetrahydropyrane and salt thereof, in particular to a method for synthesizing racemic pharmaceutical intermediate 3-amino tetrahydropyrane and salt thereof. The method is characterized by comprising a step C of: cooling glycol 3 and triphenyl phosphorus to the temperature of below 0 DEG C in an ice bath under the protection of nitrogen; slowly dropwise adding diisopropyl azodicarboxylate or diethyl azodicarboxylate into a mixture of the glycol 3 and the triphenyl phosphorus; reacting at the temperature of between 10 and 30 DEG C after dripping until the reaction is performed completely; and concentrating and drying to obtain a white solid 4 rough product, wherein the molar ratio of the glycol to the triphenyl phosphorus to the diisopropyl azodicarboxylate or the diethyl azodicarboxylate is 1:(1-3):(1-3); the used raw materials are cheap and readily available; a synthesis route is short; all reaction intermediates and a final product are not required to be subjected to column chromatographic purification; and a large amount of R or S-type 3-amino tetrahydropyrane with high optical activity and racemate thereof can be conveniently prepared from natural L or D-type glutamic acid or racemate thereof serving as a raw material. The method has the advantages of low cost and higher efficiency; and an obtained product has high chemical purity and optical purity.
Owner:苏州汉德创宏生化科技有限公司
Esterification method for preparing azodicarbonic acid
ActiveCN103193686ANo pollution in the processRaw materials are easy to getOrganic chemistryAlcoholDistillation
The invention discloses a method for preparing dialkyl azodicarbonic acid. The method comprises the steps of: mixing dimethyl azodicarbonic acid or diethyl azodicarbonic acid with sodium alkoxide solution, heating and reacting for 1-24h, and carrying out transesterification reaction between the dimethyl azodicarbonic acid or diethyl azodicarbonic acid and alcohol under the catalytic action of the sodium alkoxide; after the reaction is completed, regulating the pH value of the solution to 6-8, extracting an organic phase for 2-3 times with dichloromethane, combining the organic phases, drying for 8-12h with anhydrous sodium sulfate, and then performing reduced pressure distillation or recrystallization to obtain dialkyl azodicarbonic acid ROOCN=NCOOR, wherein R is alkyl containing 2-16 carbon atoms. According to the method, the dimethyl azodicarbonic acid or diethyl azodicarbonic acid is selected as raw materials, and is easily available; and the production cost is low, the economy is good, and pollution to the environment does not exist; the method for preparing the dialkyl azodicarbonic acid through esterification is simple to operate, short in process route, mild in reaction conditions, and high in yield, as well as suitable for industrialized development.
Owner:SHANDONG NORMAL UNIV
Preparation method of ketorolac tromethamine
ActiveCN101575340BQuality improvementShort reaction timeOrganic chemistryAntipyreticReducerEthyl ester
The invention provides a preparation method of ketorolac tromethamine. 5-benzoyl-2, 3-dihydro-1H-dilazine-1 and 1-diethyl azodicarboxylate are the starting materials and are prepared into salt by alkaline hydrolysis and acidification when reducers exist, and finally, the salt is prepared into ketorolac tromethamine. The method does not need complicated decarboxylation process, and effectively solves the problems of low purity, poor color and the like caused by the oxidizability of ketorolac. The invention has the advantages of simple operation, high yield and high purity (the total yield in two steps reaches 95% and the purity reaches more than 99.9%); thus, the preparation method is suitable for large-scale commercial process.
Owner:LUNAN PHARMA GROUP CORPORATION
Redox-induced pH-responsive methacrylate fluorine-containing monomer, synthesis method and application
ActiveCN107721888BThe synthesis method is simpleQuality improvementRadioactive preparation carriersSulfonic acid amide preparationMethacrylateSulfonyl chloride
The invention relates to a synthetic method and application of a redox induced pH-responsive type methacrylate fluorine-containing monomer. The general formula is shown in the specification, wherein a, b, c and d are respectively independent integers. The synthetic method comprises the following steps: with organic alkali as an acid-binding agent, enabling a methacrylate monomer containing a primary amine end group to react with 2,4-dinitrobenzene sulfonyl chloride in anhydrous tetrahydrofuran so as to obtain a sulfamide monomer; and with diethyl azodicarboxylate and triphenylphosphine as catalysts, enabling the sulfamide monomer to have a Mitsunobu reaction with a fluorine alcohol monomer, separating and purifying, and assembling in an aqueous solution. A strong electron withdrawing group2,4-dinitro-benzsulfamide exists in the structure disclosed by the invention and can rapidly have a nucleophilic substitution reaction with mercapto group so as to produce a secondary amine group, and the secondary amine group is protonized under corresponding pKa, so that redox induced pH response is realized. Development of fluorine-containing small molecules is facilitated, and the developmentneed of a 19FMRI probe is met. The synthetic method disclosed by the invention is simple, and the monomer is stable in quality and high in yield. The structural formula is as shown in the specification.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
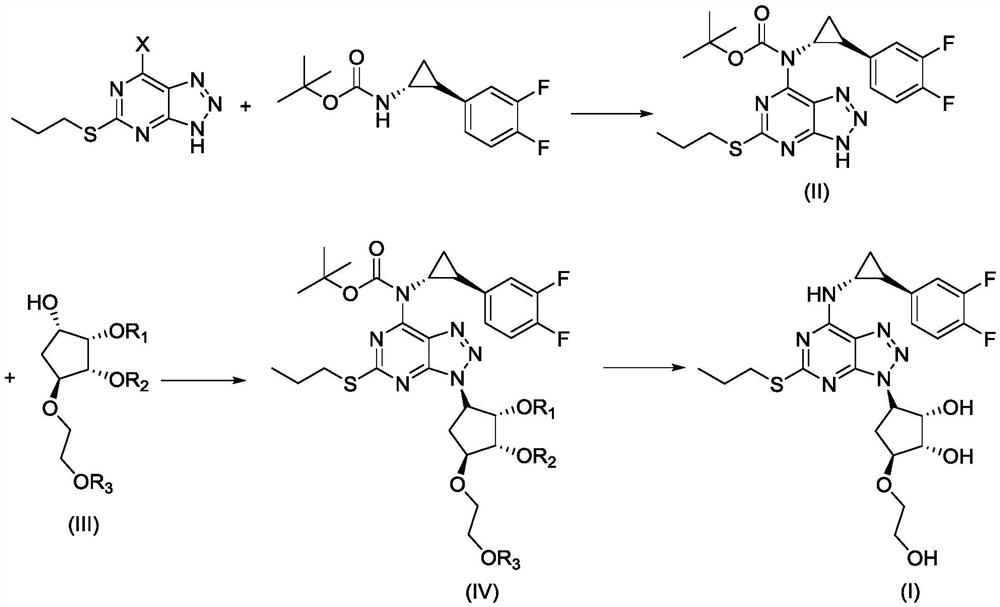
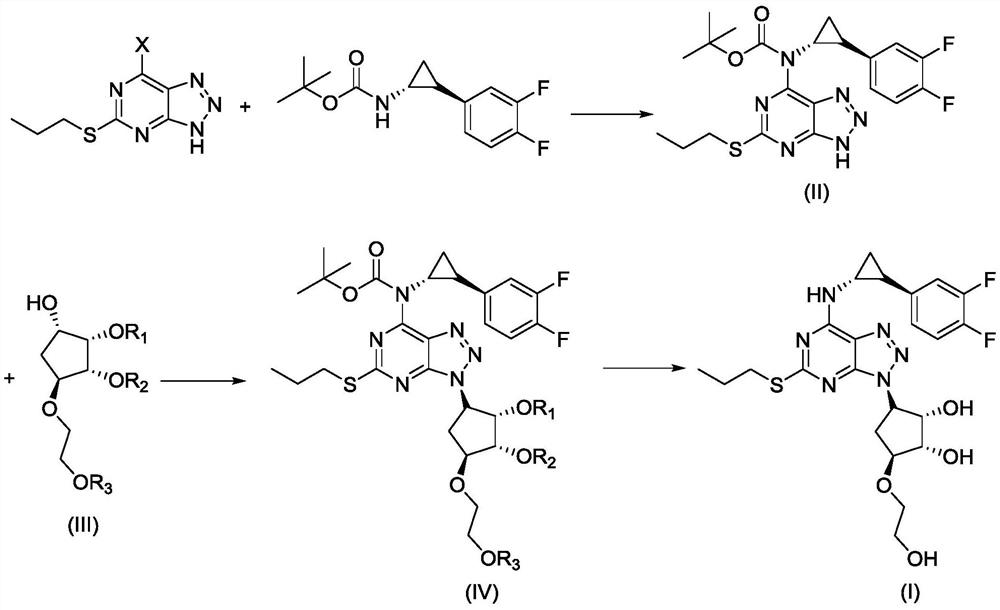
A kind of synthetic method of ticagrelor
ActiveCN111978328BReduce manufacturing costShort reaction stepsOrganic chemistry methodsCarbamateTicagrelor
The invention discloses a synthetic method of ticagrelor, which comprises the following steps: (a) using 6-halo-2-propylthio-8-azapurine as a raw material, in an alkali and an organic solvent, at room temperature , react with (1R,2S)-2-(3,4-difluorophenyl)cyclopropylcarbamate tert-butyl ester to obtain intermediate (II); (b) at room temperature, intermediate (II ) is dissolved in an organic solvent with compound (III), and reacts under the effect of triphenylphosphine and diethyl azodicarboxylate to obtain intermediate (IV); (c) intermediate (IV) in acid Under the action of deprotection, ticagrelor (I) was obtained. The method has the advantages of cheap and easy-to-obtain raw materials, low production cost, short reaction steps, mild reaction conditions, convenient post-treatment, high yield, and is more suitable for industrial production.
Owner:南京一心和医药科技有限公司 +1
Synthesis method based on amination reaction of aryl siloxane
ActiveCN113336677ABiologically activeMild reaction conditionsOrganic chemistryChlorobenzeneDiethyl azodicarboxylate
The invention relates to a synthesis method based on amination reaction of aryl siloxane. The method comprises the following steps: adding AgF, a reactant I, a reactant II, a butanone solution and a number 5 magneton into a reactor in sequence, connecting a condenser pipe, introducing condensate water from bottom to top, placing the reactor in an oil bath pan at 30-70 DEG C, heating, stirring and reacting for 10-12 hours, terminating the reaction, and purifying the product to obtain an aryl siloxane amination product, wherein the reactant I is optionally selected from phenyl triethoxy silane, p-tert-butyl phenyl triethoxy silane, p-methoxy phenyl triethoxy silane, p-methyl phenyl triethoxy silane, p-chlorphenyl triethoxy silane or 4-triethoxy silicon biphenyl; and the reactant II is diethyl azodicarboxylate. The method is mild in reaction condition, high in selectivity, relatively high in yield and environment-friendly; and the compound has certain biological activity and can be used in the field of synthesis of drugs, pesticides and paint dyes.
Owner:HUBEI UNIV OF TECH
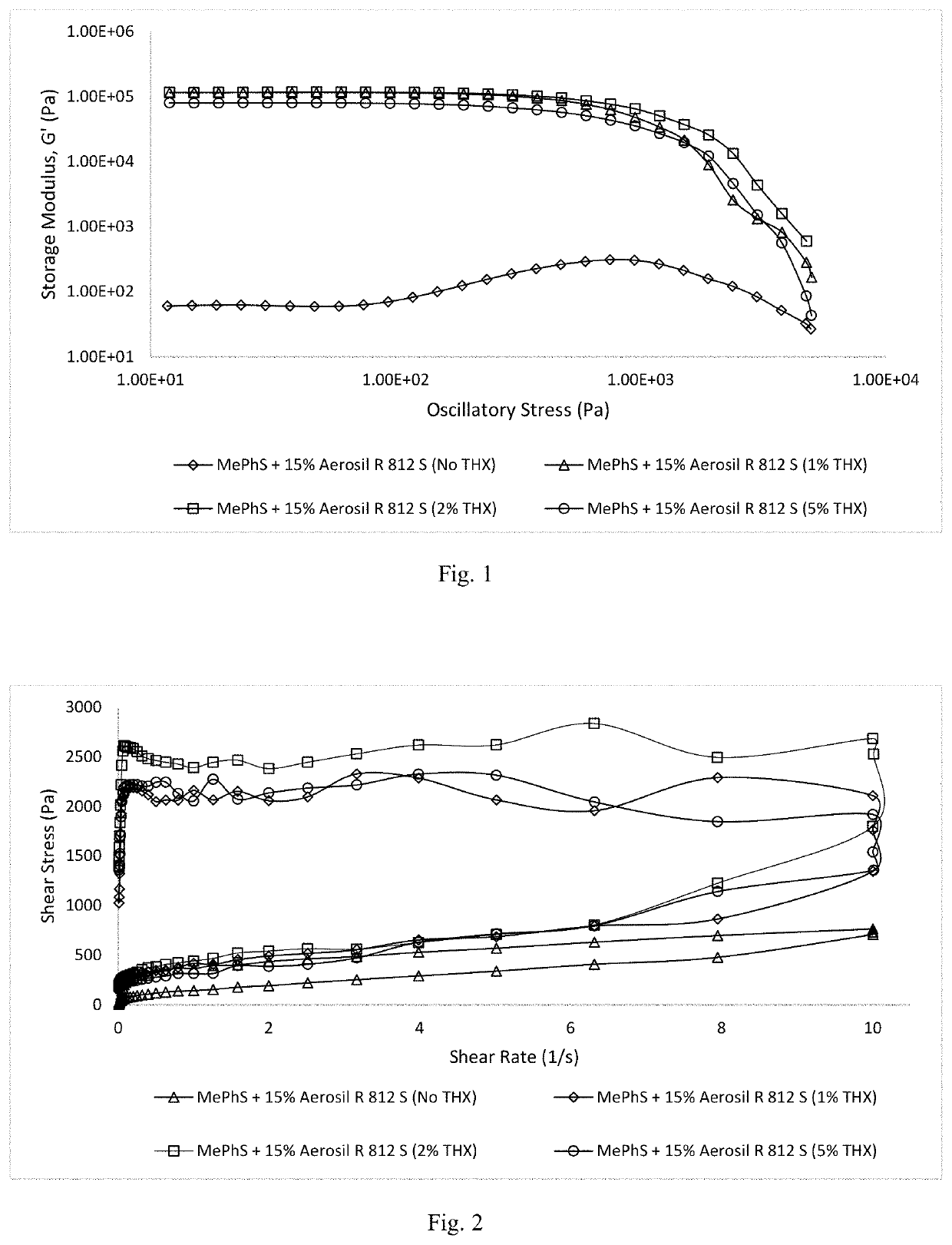
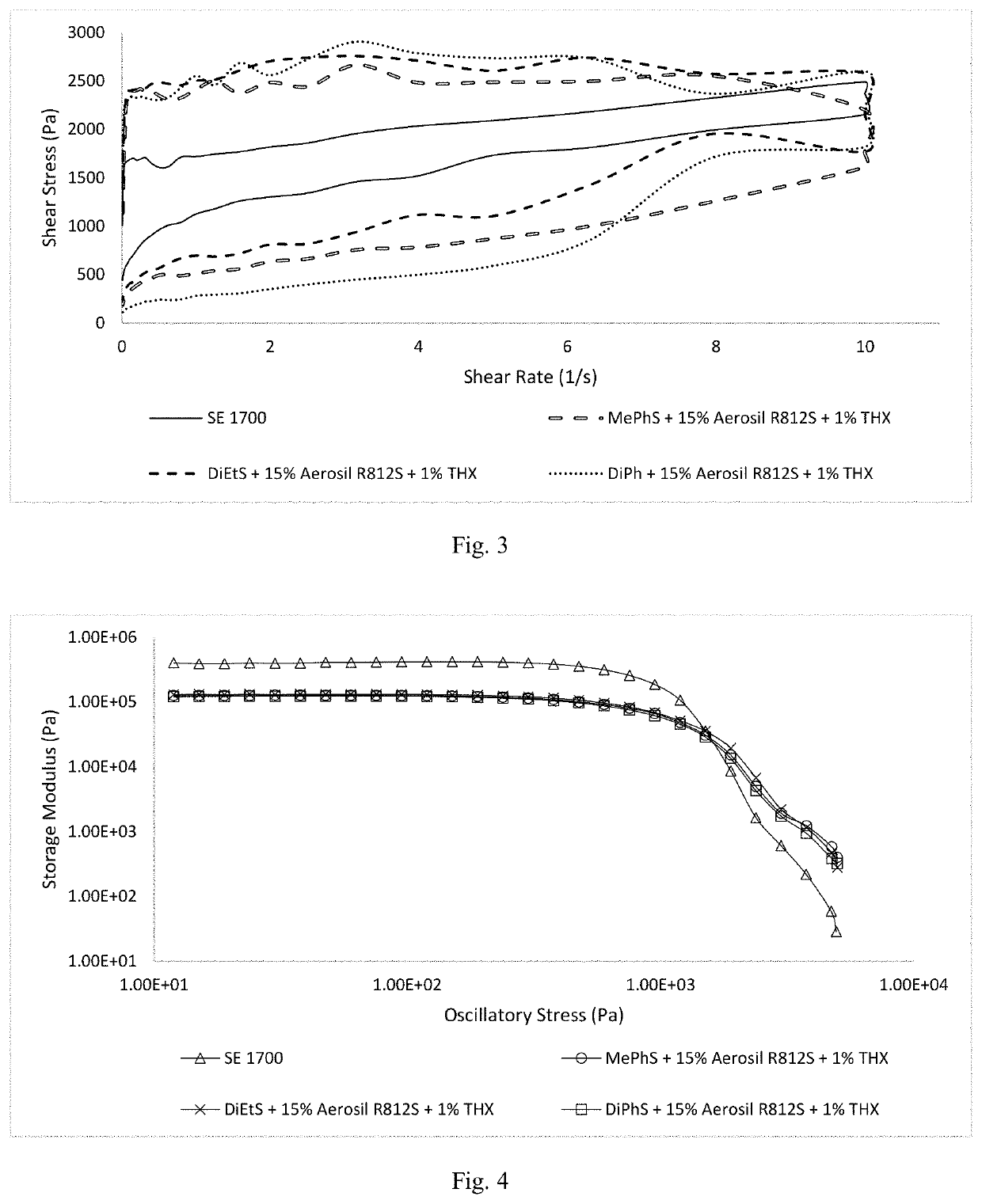
Thixotropic polysiloxane pastes for additive manufacturing
Shelf-stable, rapid crosslinking, “all-in-one” pastes useful as “inks” in additive manufacturing are provided. These pastes exhibit desirable rheological flow properties and crosslinking upon exposure to UV light. The pastes are based on vinylsilyl-functionalized, completely amorphous, linear terpolysiloxanes containing predominantly dimethylsiloxy- repeat units with small amounts of diphenylsiloxy-, methylphenylsiloxy-, diethylsiloxy-, and / or methyltrifluoroalkylsiloxy- crystallization disruptors. The base polymers are preferably compounded with a trimethylsilylated-hydrophobic silica filler, thixotropic flow agent, hydrosilyl-functionalized oligomeric crosslinker, and a catalytic system comprising platinum(II) acetylacetonate or trimethyl(methylcyclopentadienyl)-platinum(IV), and diethyl azodicarboxylate.
Owner:HONEYWELL FED MFG & TECHNOLOGI
Foaming agent for EPDM sponge rubber
A foaming agent for an EPDM sponge rubber is disclosed by the invention, and consists of the following raw materials in parts by weight: 5-10 parts of 4, 4'-oxybis(benzenesulfonyl hydrazide), 4-6 parts of N,N'-dinitroso pentamethylene tetramine, 2-5 parts of diethyl azodiformate, 1-3 parts of p-toluenesulfonyl semicarbazide, 4-6 parts of ammonium bicarbonate, 2-5 parts of coconut oil monoethanolamide, 8-12 parts of ethylenediamine, 20-30 parts of propylene glycol, 2-5 parts of benzoic acid, 1-2 parts of alum, and 0.5-1.5 parts of zinc oxide. The foaming agent of the invention is excellent in foaming power, high in foaming ratio, steady in process, low in cost and convenient to use, and the generated foams has good temperature adaptability and disperse uniformity. The EPDM sponge rubber material prepared by the foaming agent is good in mechanical properties, low in density, fine in micropore and uniform in dispersion.
Owner:中山市红喜橡塑五金制品有限公司
A kind of preparation method of edoxaban tosylate intermediate and intermediate compound
ActiveCN109836360BEasy to introduceHigh reaction conversion rateSulfonic acid amide preparationBulk chemical productionTert-Butyloxycarbonyl protecting groupNitrobenzene
Owner:南京恩泰医药科技有限公司
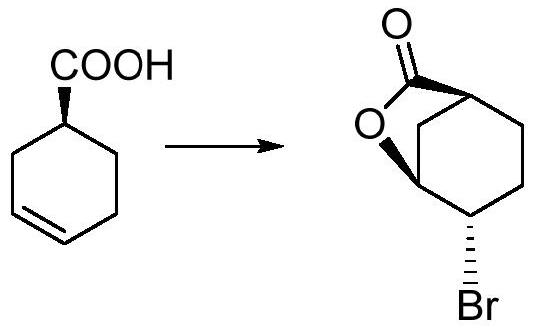
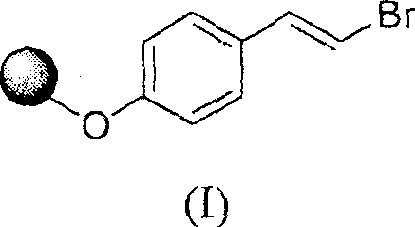
Method for preparing trans-4-(beta-bromoethyl) phenoxy-benzylic resin (I)
The invention belongs to the technical field of functional macromolecules, and in particular relates to a preparation method for synthesizing trans-4-(β-bromovinyl)phenoxy benzyl resin (I). The present invention adopts trans-4-(β- Bromovinyl) phenol and Wang resin were used as raw materials, and trans-4-(β-bromoethylene containing bromoethylene active groups was synthesized at room temperature in tetrahydrofuran, triphenylphosphine, and diethyl azodicarboxylate system. base) phenoxy benzyl resin. The trans-4-(beta-bromovinyl) phenoxy benzyl resin synthesized by this method takes vinyl bromide as a reactive group, and can be used as a starting material for the combinatorial chemical synthesis of many types of compounds for use in medicines, pesticides, Synthesis of physiologically active natural products, liquid crystals, functional materials, etc.
Owner:CHIZHOU DONGSHENG PHARMA
Orlistat preparation method
The present invention relates to an orlistat preparation method, which concretely comprises: dissolving (3S, 4S)-3-hexyl-4-[(R)-2-hydroxy tridecyl]-2-oxetanone, triphenyl phosphorous and N-formyl-L-leucine in tetrahydrofuran, adding diethyl azodicarboxylate in a dropwise manner under stirring, stirring overnight, taking out the organic phase, carrying out pressure reducing concentration, and carrying out silica gel column chromatography on the remaining material so as to obtain the product orlistat. The orlistat preparation method of the present invention has characteristics of simple preparation process and low production cost.
Owner:李磊
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com