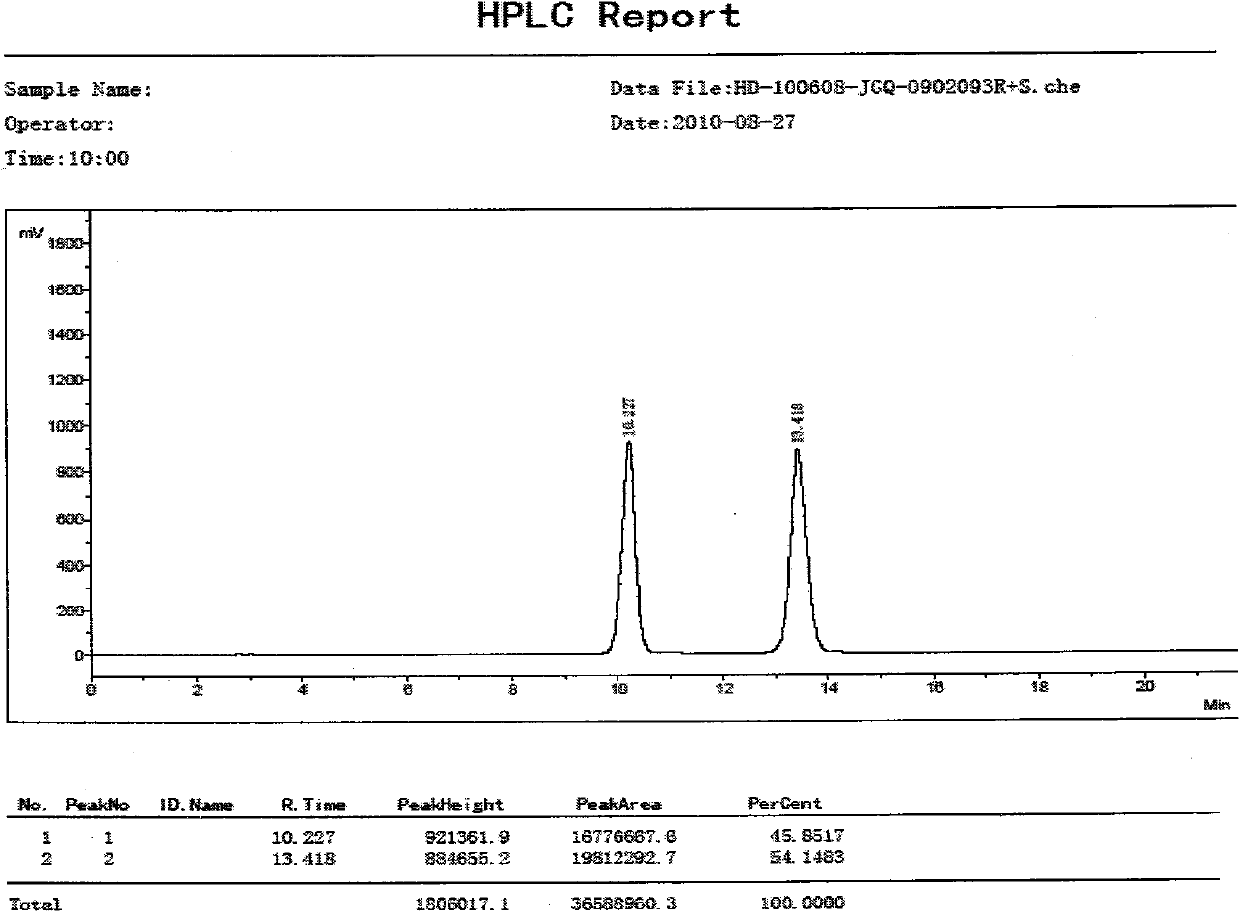
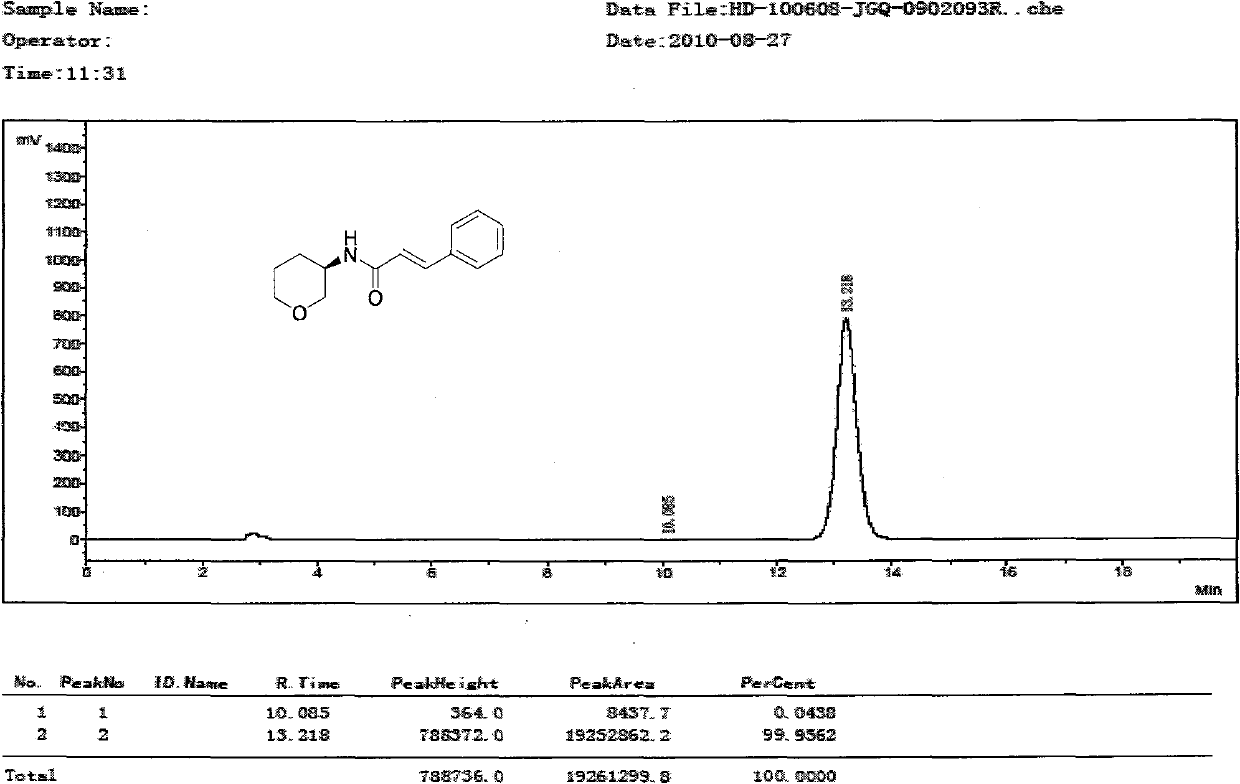
Method for synthesizing chiral pharmaceutical intermediate 3-amino tetrahydropyrane and salt thereof
A technology of aminotetrahydropyran and synthesis method, which is applied in the field of synthesis of chiral pharmaceutical intermediate 3-aminotetrahydropyran (and its salts), can solve the difficulty of splitting racemates, the high cost, and the development of , The production has no problems such as literature reports, and achieves the effects of low cost, short synthesis route and reduced production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of 1,5-(2-dibenzylamino)glutarate bisbenzyl ester (2a) (Method A)
[0043] Add 1200 milliliters of water, 1.6mol sodium hydroxide (65 grams), 2.2mol potassium carbonate (306 grams), 1.2mol glutamic acid (180 grams), 4.8mol benzyl bromide (2457 g), after dropping, reflux and stir for 30 minutes, cool to 10-30°C, add 1200 ml of ethyl acetate for extraction, wash the organic phase with 600 ml of brine, dry over anhydrous sodium sulfate, filter off the desiccant, and concentrate to obtain 300 1 g of crude product was directly used in the next reaction. 1 H NMR (400MHz, CDCl3) δ7.55-7.21 (m, 20H), 5.30 (d, J=12.2Hz, 1H), 5.25-5.13 (m, 1H), 5.07-4.96 (m, 2H), 3.92 ( d, J=13.7Hz, 2H), 3.54(d, J=13.7Hz, 2H), 3.44(q, J=7.3Hz, 1H), 2.54(dt, J=16.6, 7.1Hz, 1H), 2.44- 2.32(m, 1H), 2.18-2.05(m, 2H).MS(ESI)M / Z508[M+H] +
[0044] Synthesis of 1,5-(2-dibenzylamino)pentanediol (3)
[0045] Add 300 ml of tetrahydrofuran into a three-necked flask with a stirrer, and add 0.1...
Embodiment 2
[0053] Synthesis of dimethyl 1,5-(2-tert-butoxycarbonylamino)pentanoate (2b) (Method B)
[0054] Add 7 liters of methanol into a three-necked flask with a stirrer, cool to 0°C, slowly drop in 12.1 mol trimethylchlorosilane (1320 g), stir for 30 minutes, then add 4.8 mol glutamic acid (700 g ), react at room temperature until the reaction is complete. After cooling to 0°C, 25.6mol triethylamine (2600g) and 5.76mol di-tert-butyl dicarbonate (1257g) were slowly added dropwise in sequence, and stirred at 10-30°C until the reaction was complete after dropping. Concentrate, pour the residue into 5 liters of water, extract twice with 9 liters of ethyl acetate, combine the organic phases, wash with 4 liters of brine, dry over anhydrous sodium sulfate, filter off the desiccant, and concentrate the filtrate to obtain 1300 grams of light yellow oil is the product. 1 H NMR (400MHz, CDCl 3 )δ5.09(s, 1H), 4.33(s, 1H), 3.75(s, 3H), 3.68(s, 3H), 2.52-2.31(m, 2H), 2.19(td, J=13.5, 7.4Hz , ...
Embodiment 3
[0064] Synthesis of 3-Dibenzylaminotetrahydropyran (4)
[0065] N 2Under protection, add 2.38mol 1,5-(2-dibenzylamino)pentanediol (780 grams), 5 liters of dichloromethane and 9.99mol triphenylphosphine (873 gram), then add 9.99mol diisopropyl azodicarboxylate (DIAD, 673 grams) to the reaction mixture, and stir until the reaction is complete. Concentration gave 720 g of a white crude product, that is, 3-dibenzylaminotetrahydropyran. MS(ESI)M / Z 282[M+H] + .
[0066] All the other steps are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com