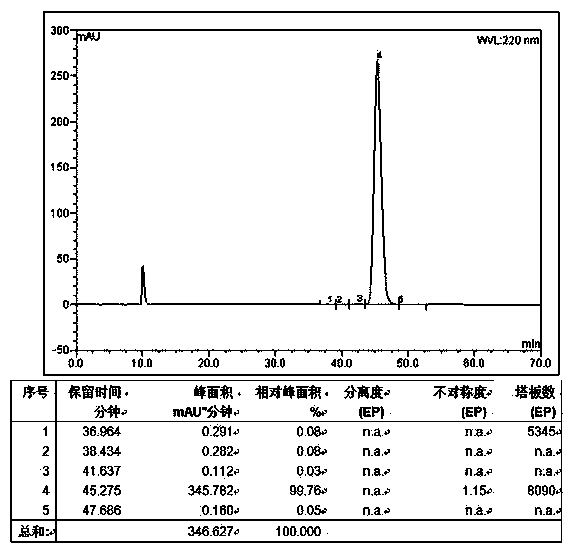
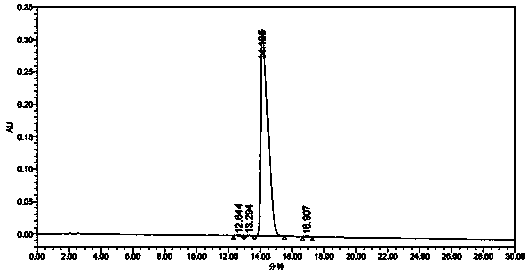
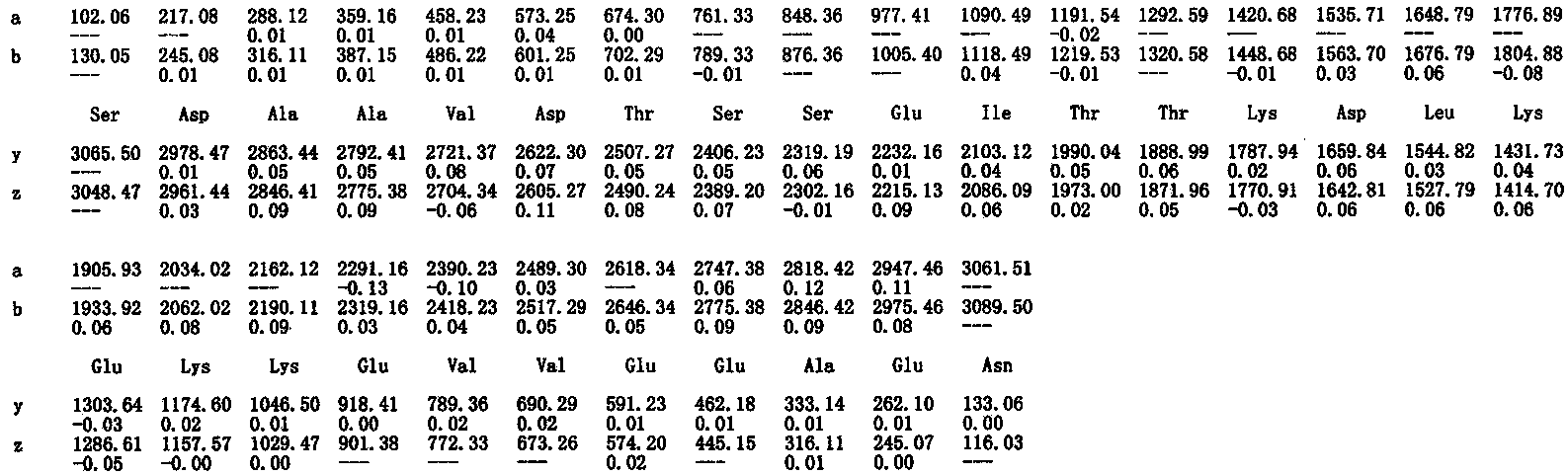
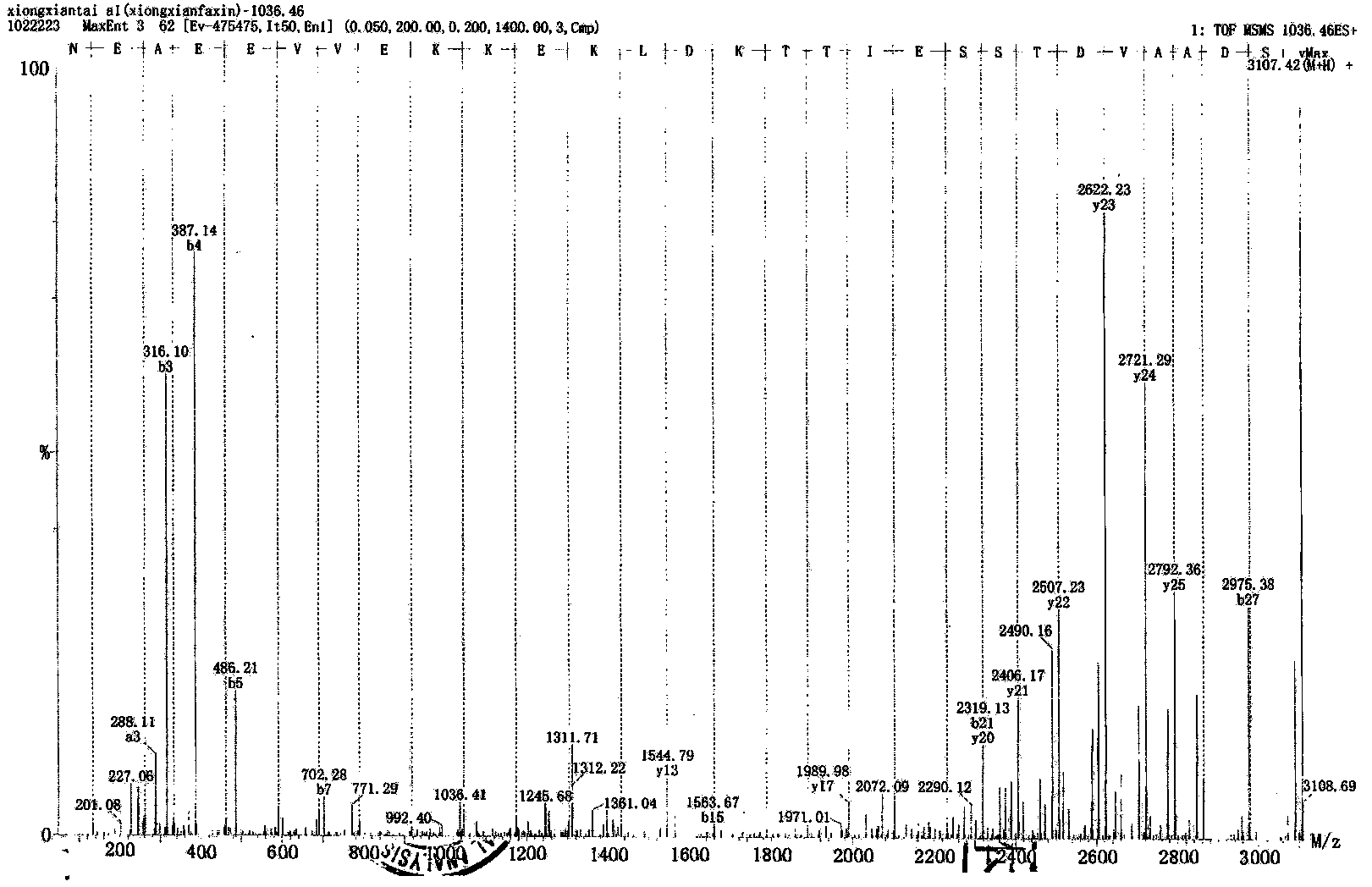
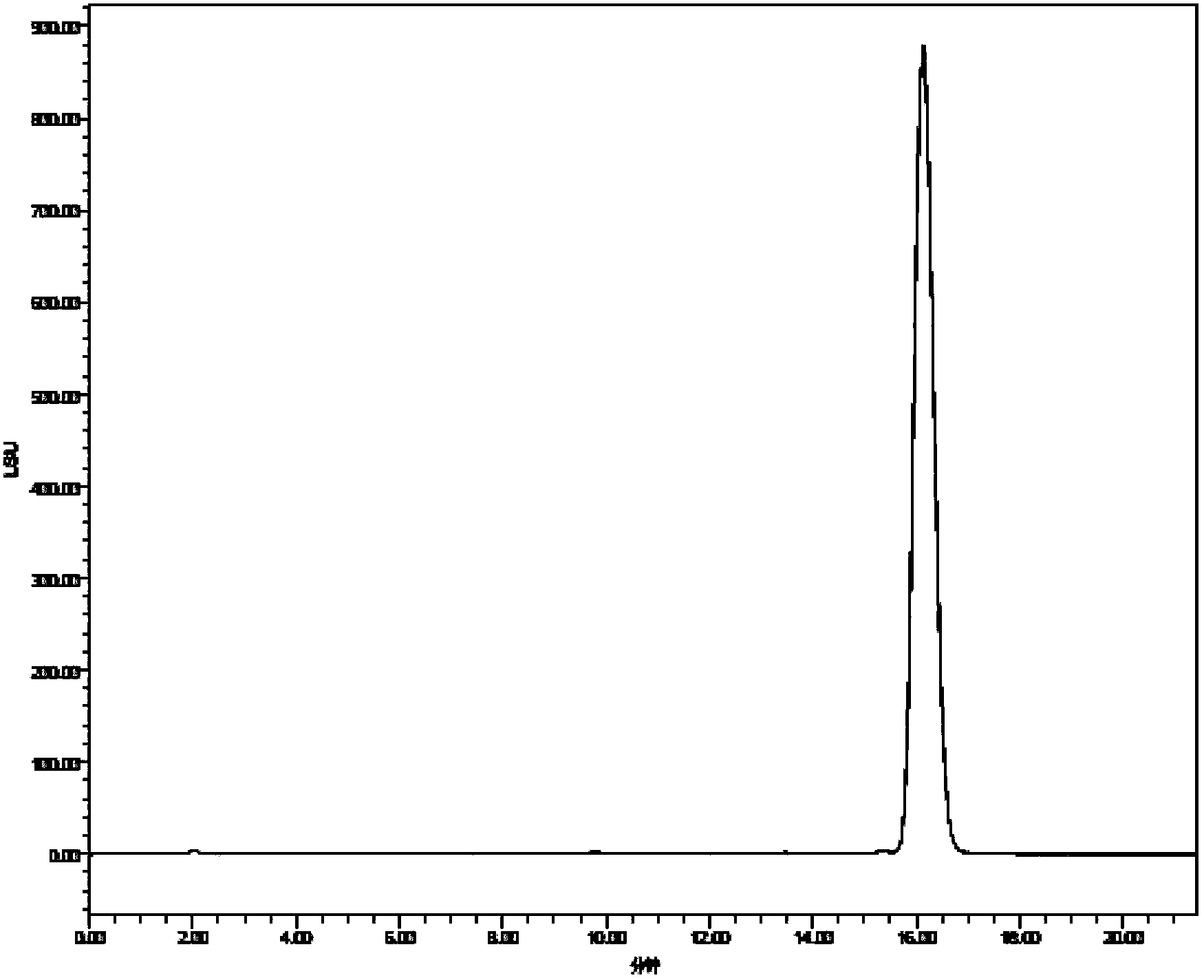
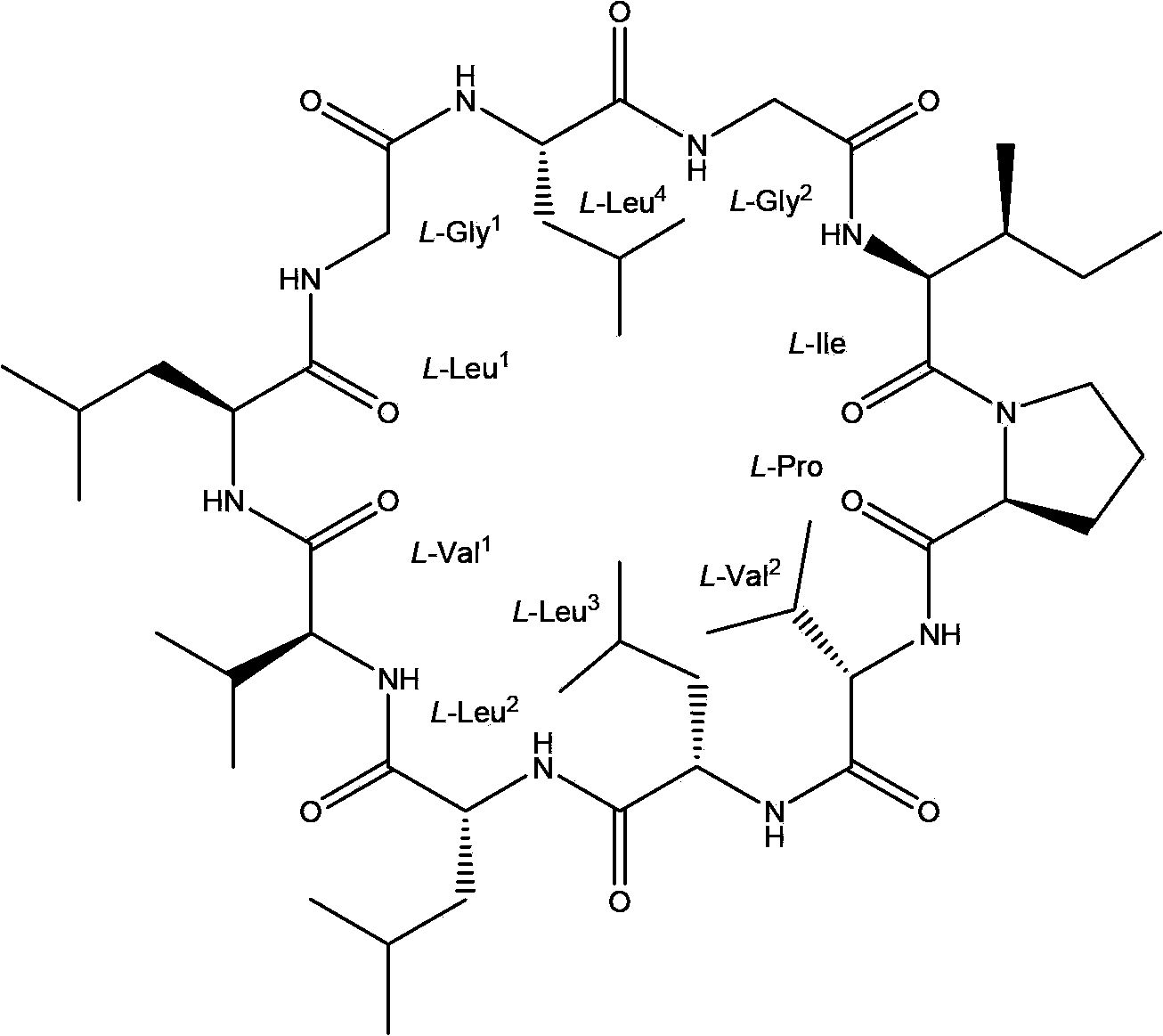
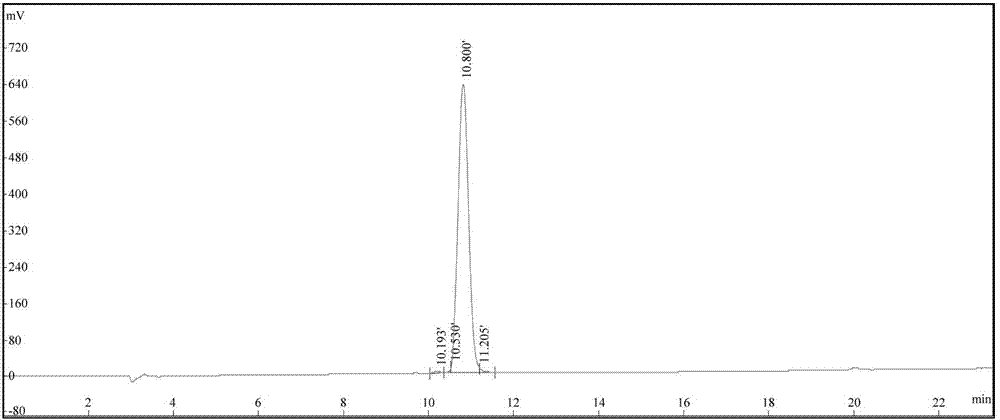
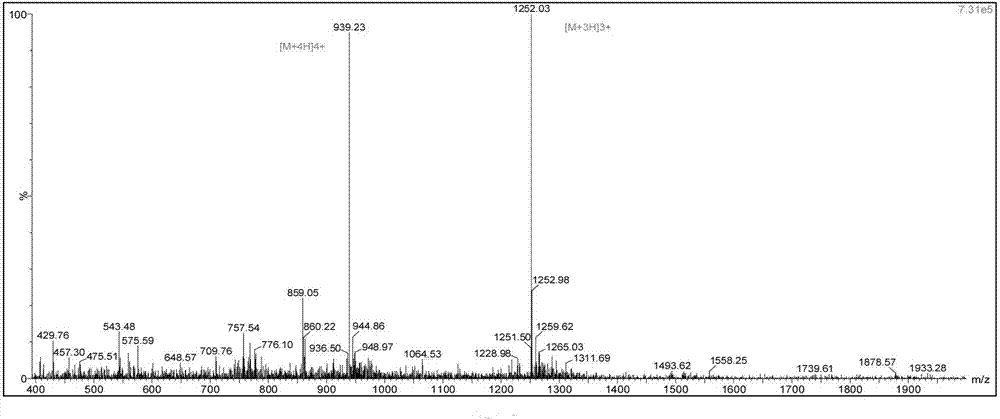
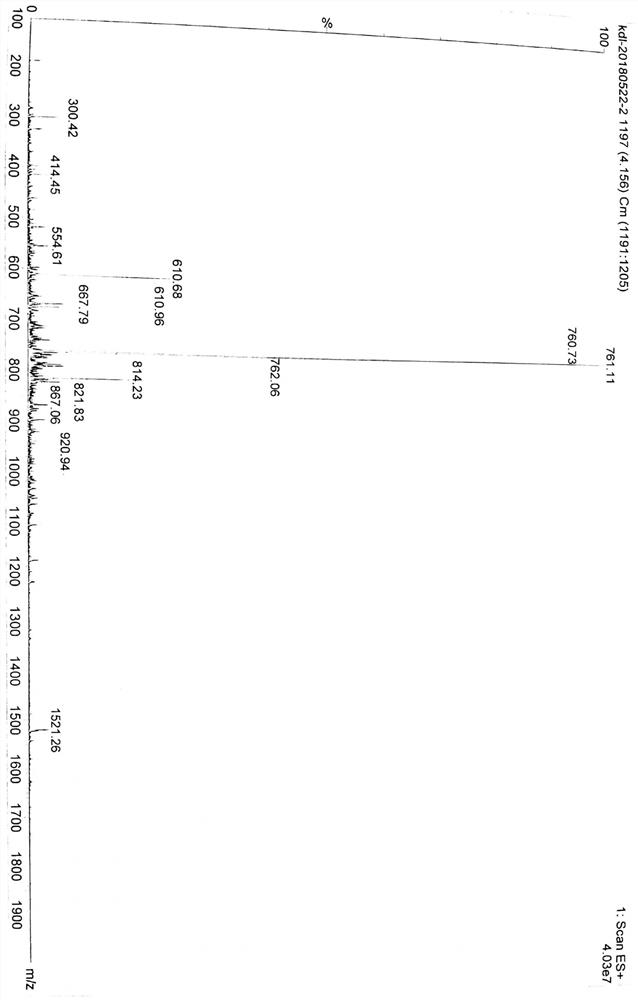
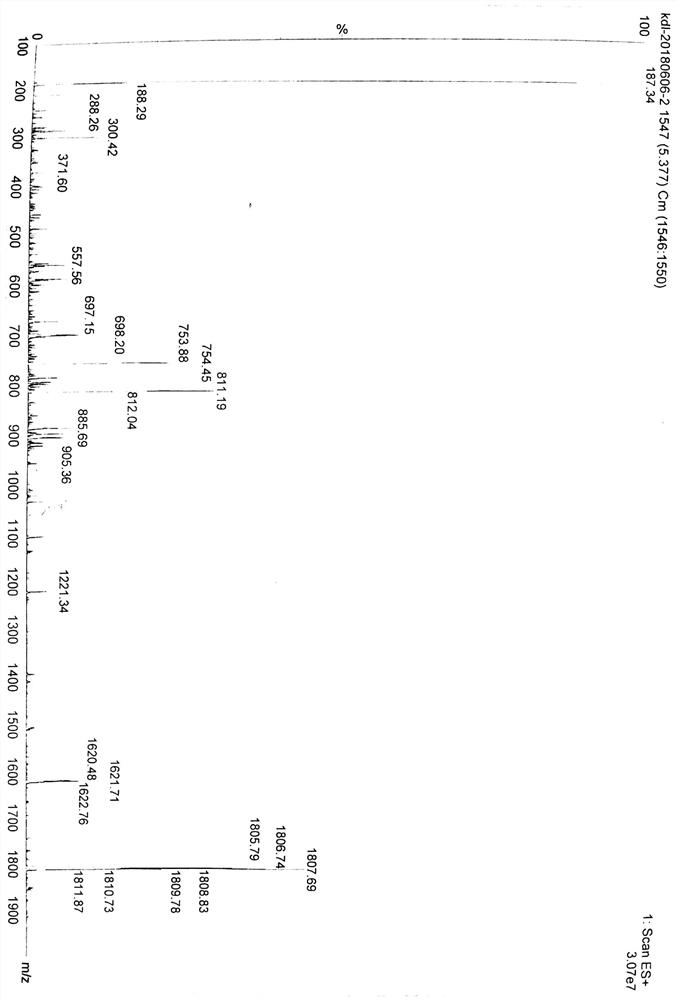
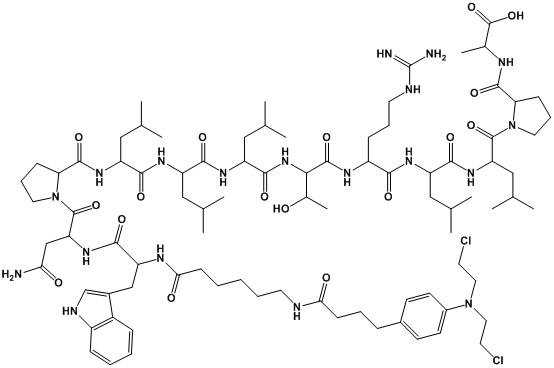
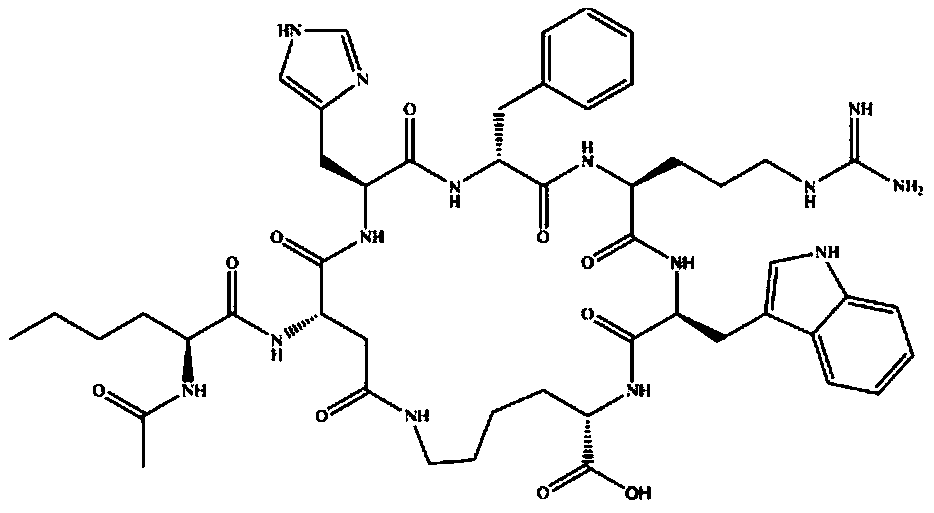
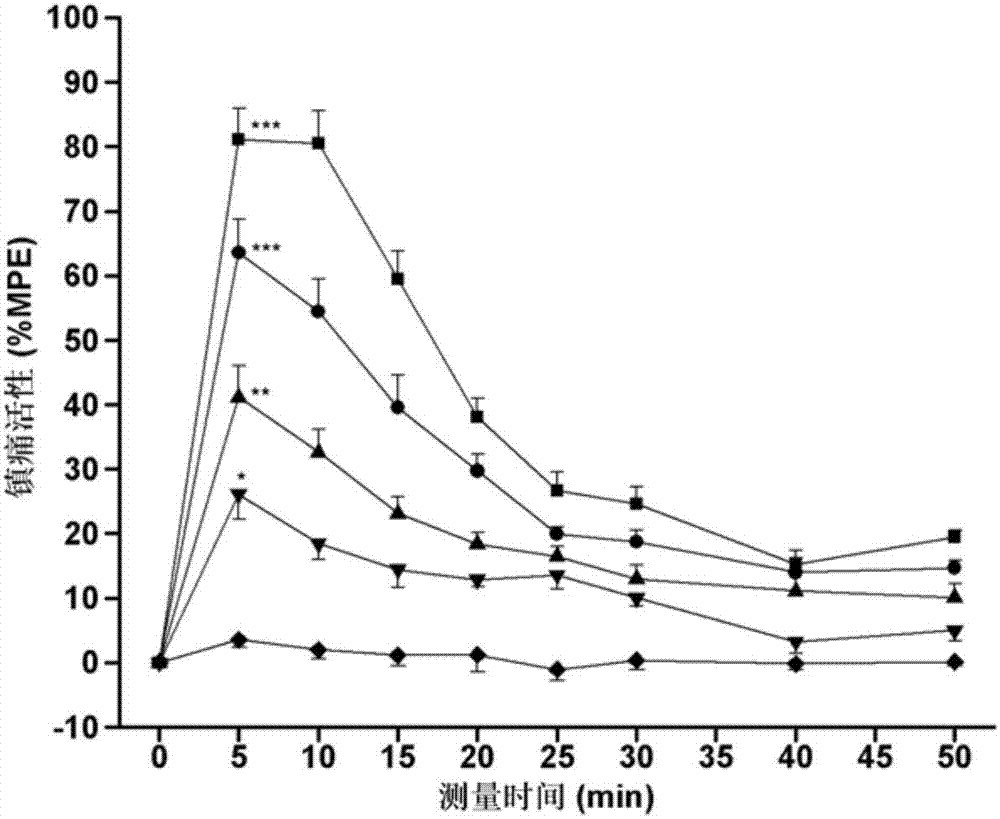
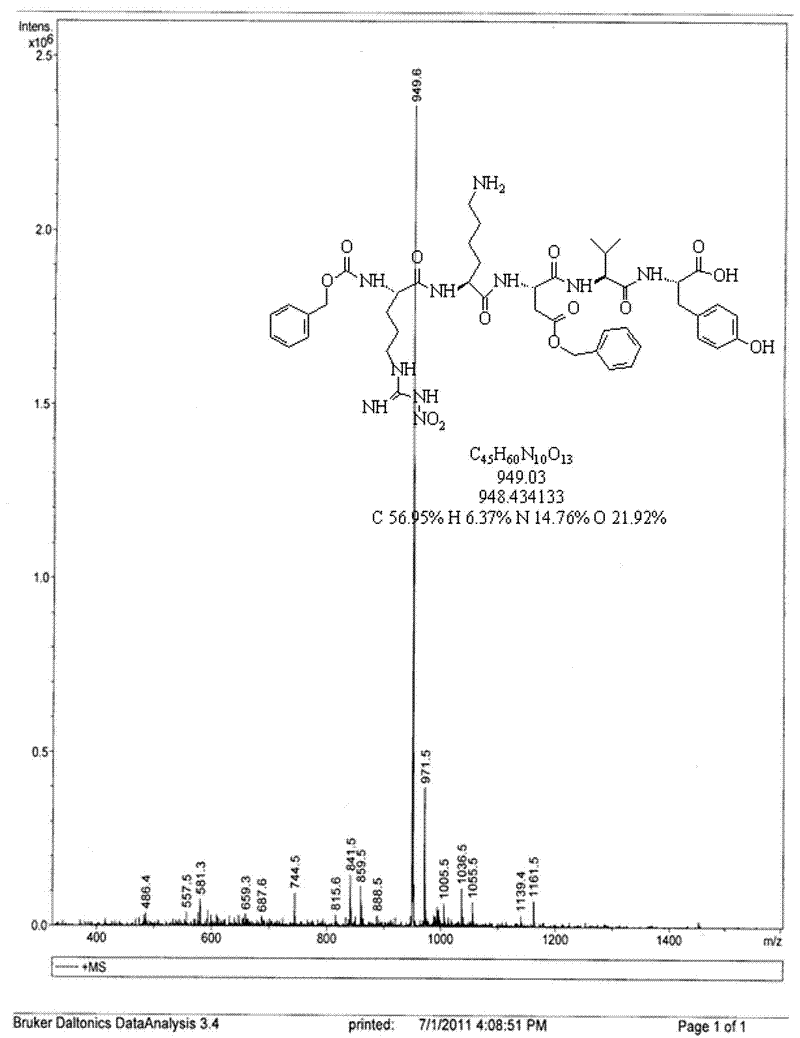
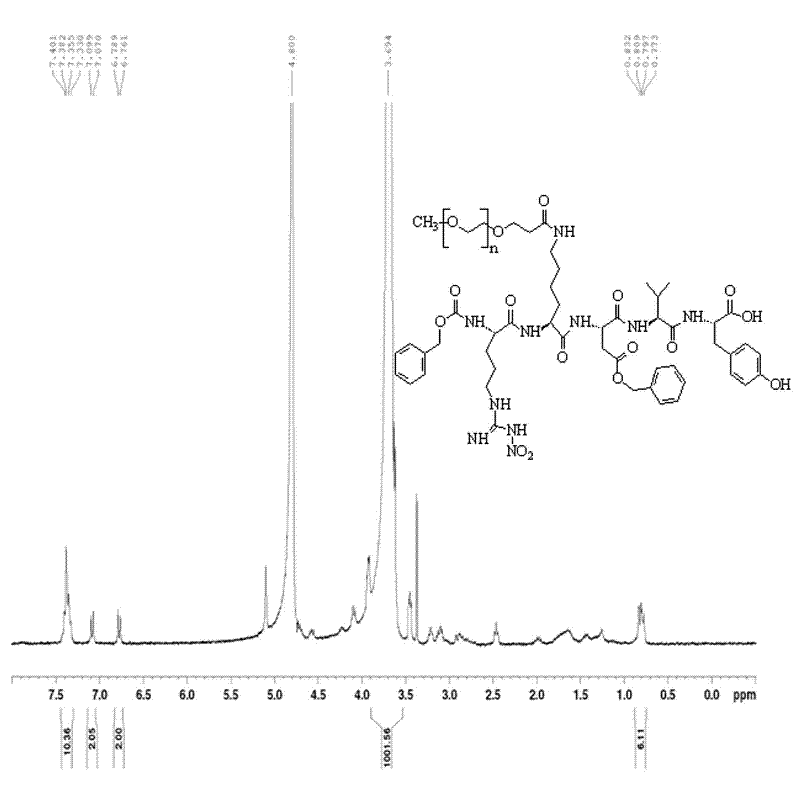
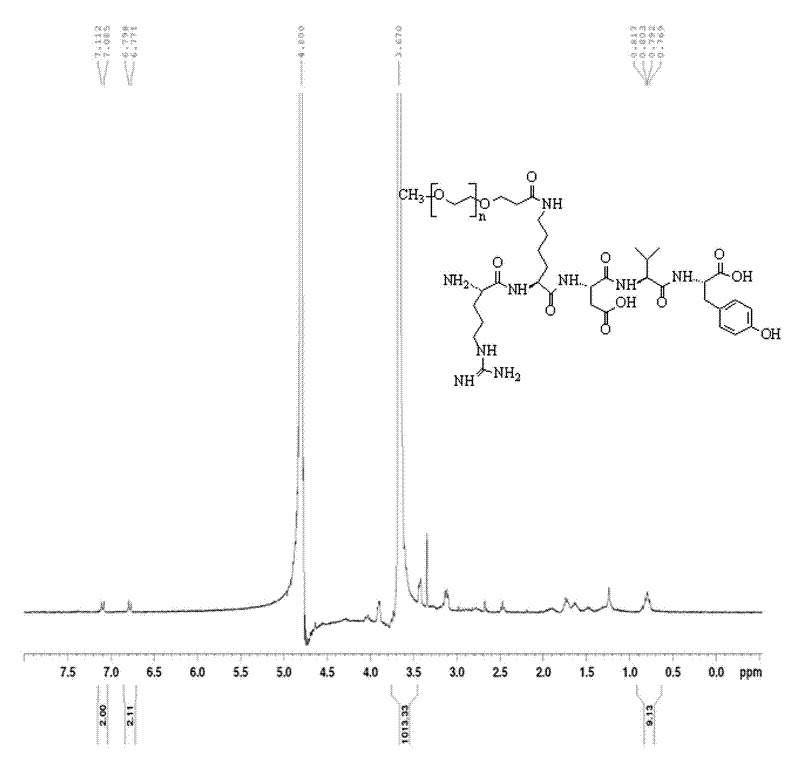
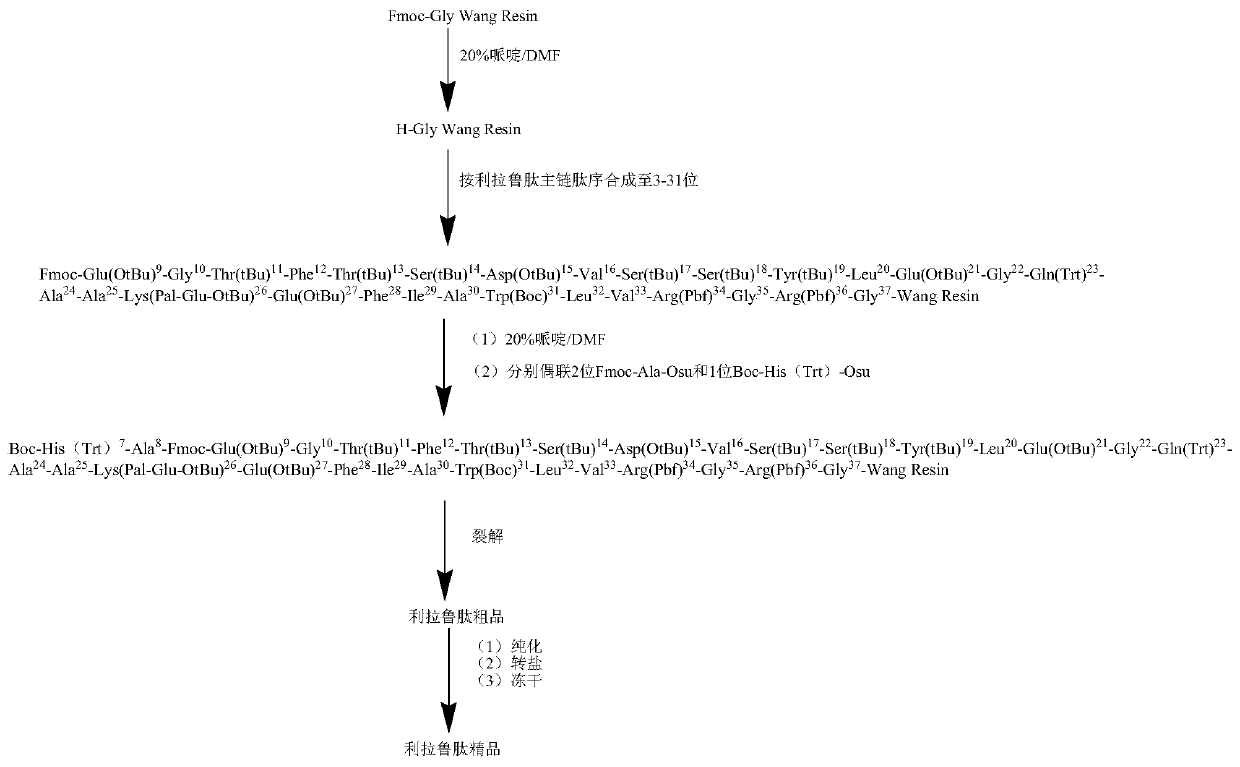
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
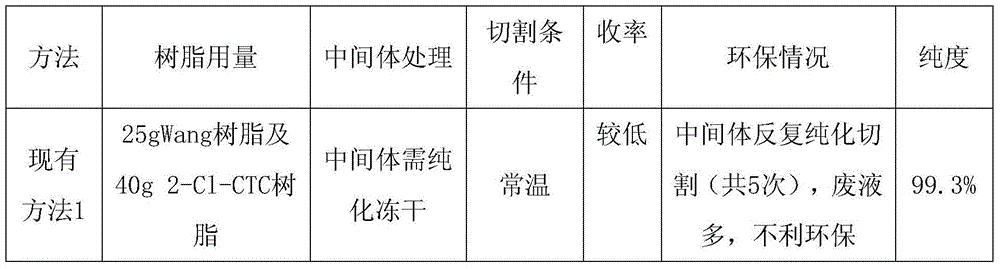
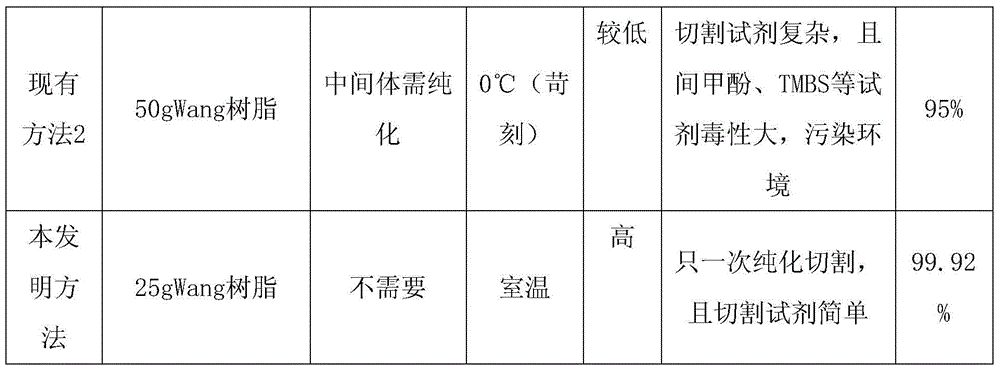
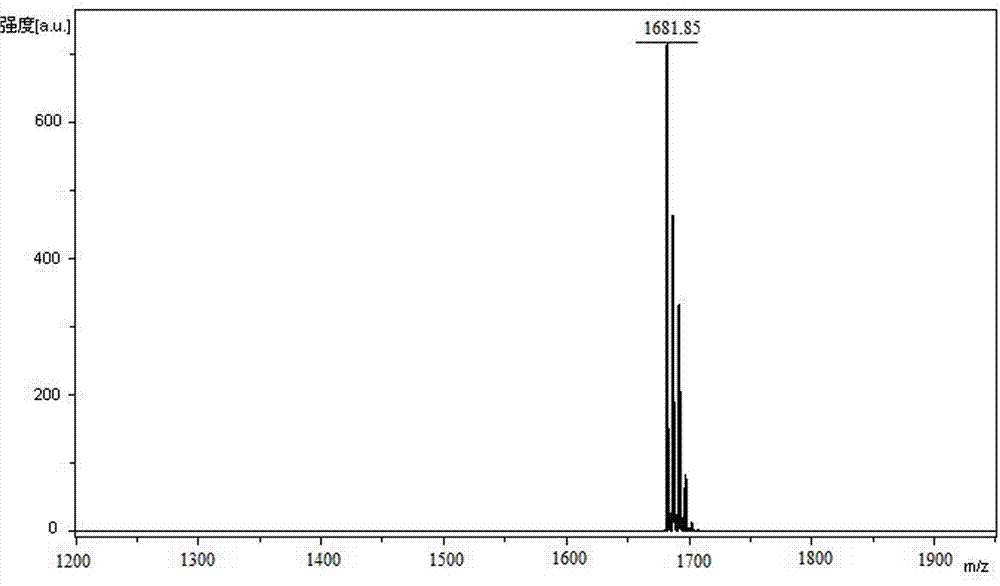
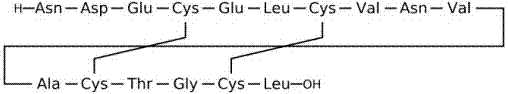
75 results about "Wang resin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sigma-Aldrich Online Catalog Product List: Wang Resins
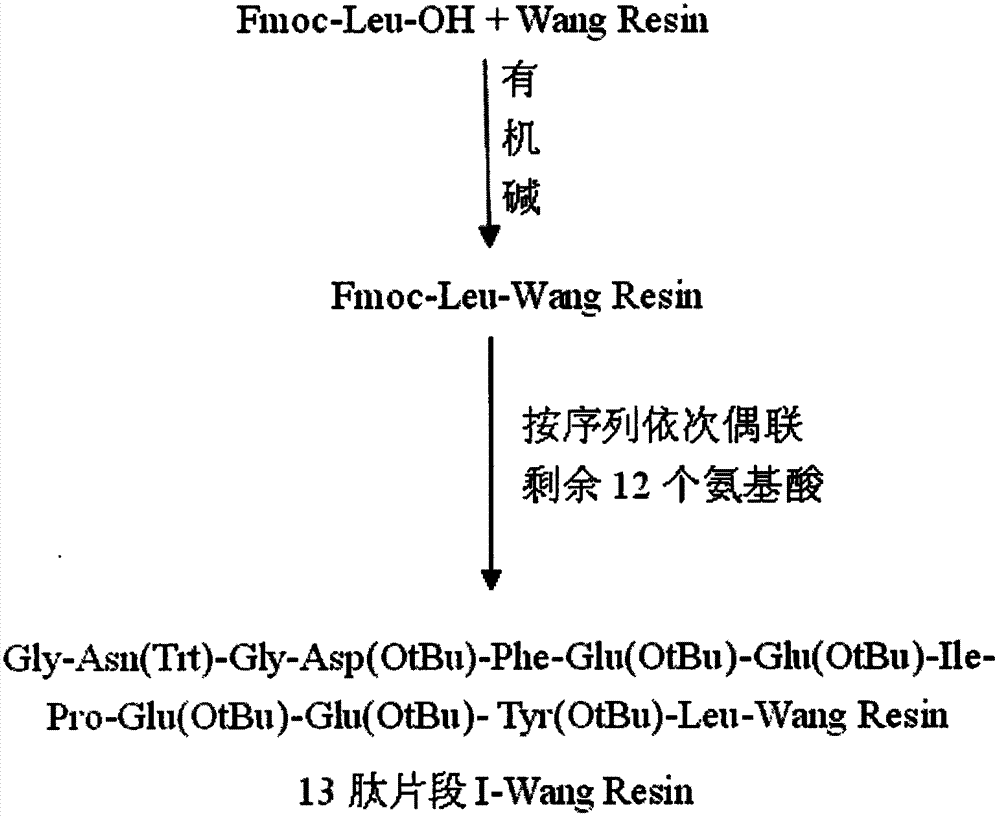
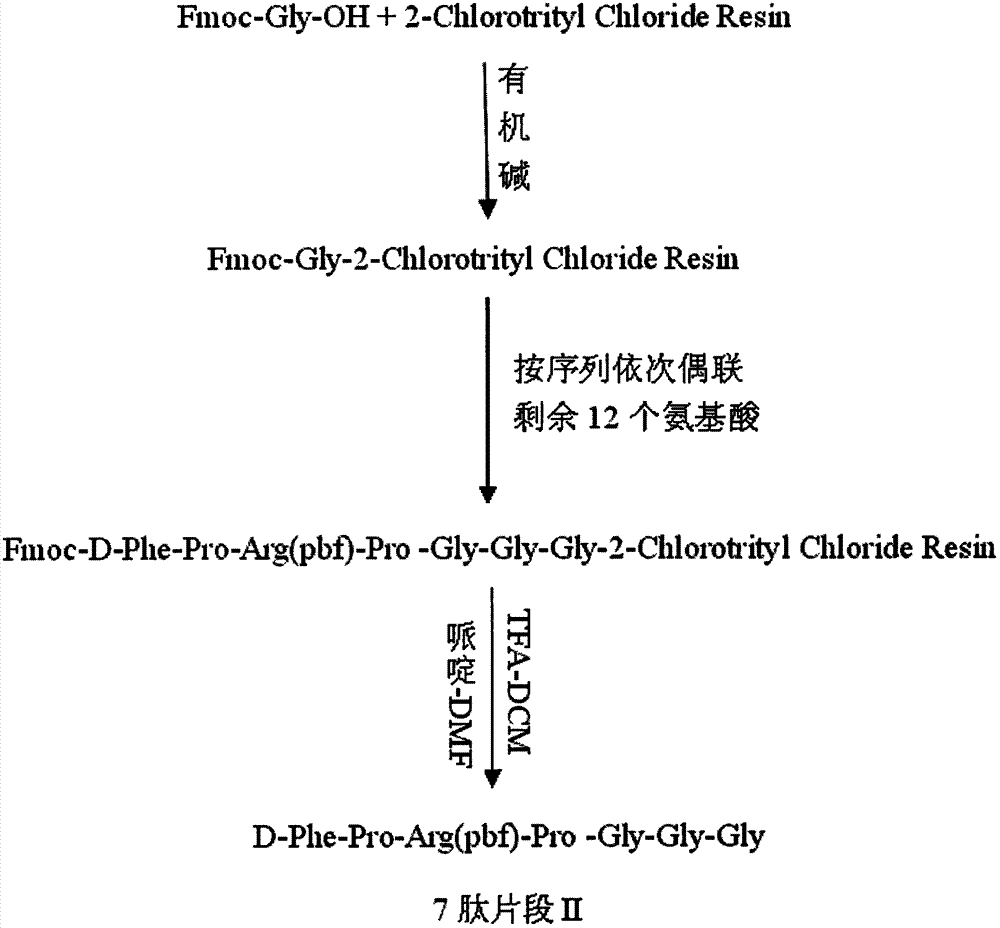
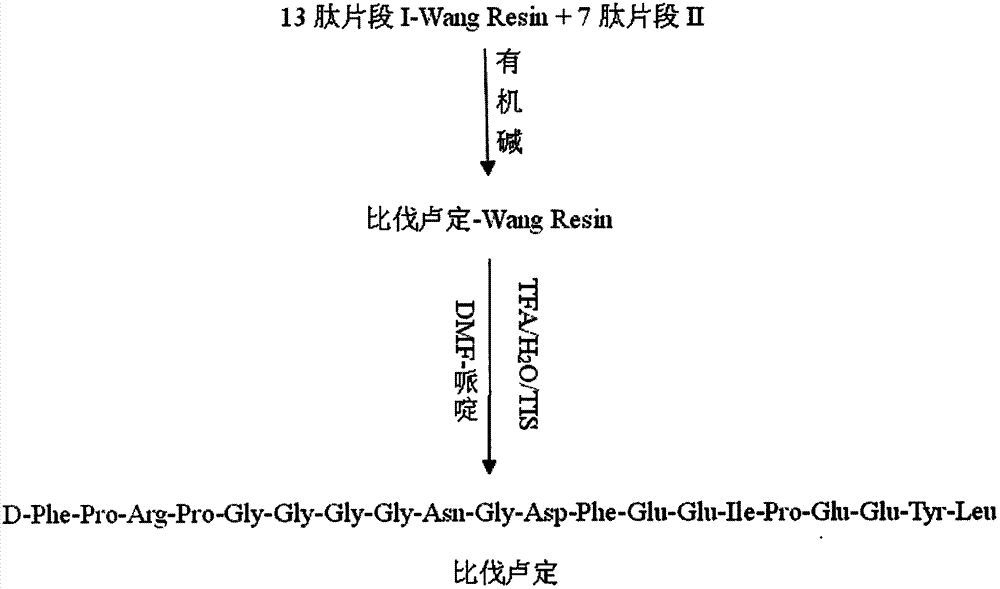
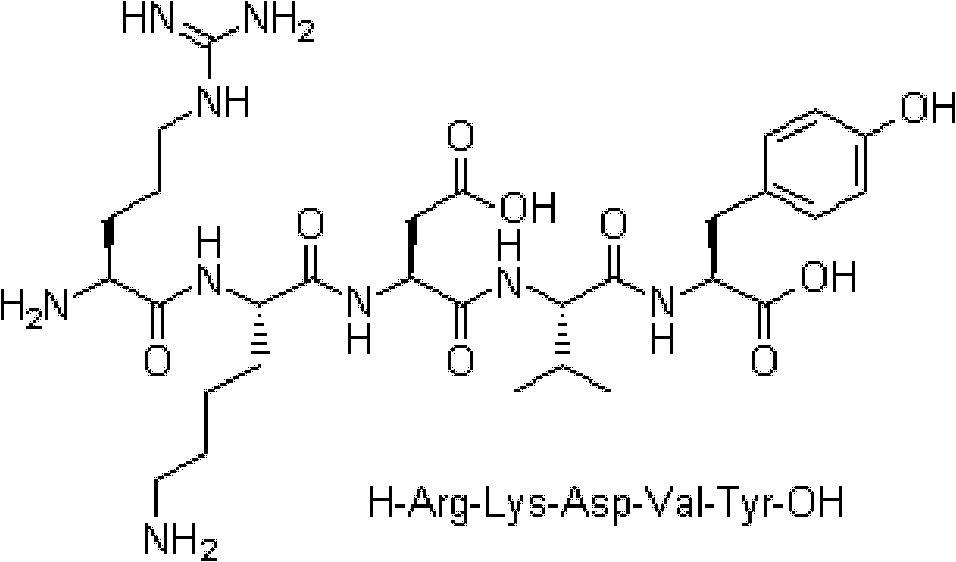
Preparation method of synthesizing bivalirudin from solid phase polypeptide
ActiveCN101033249AConvenient sourceReduce usagePeptide-nucleic acidsPeptide preparation methodsSide chainWang resin
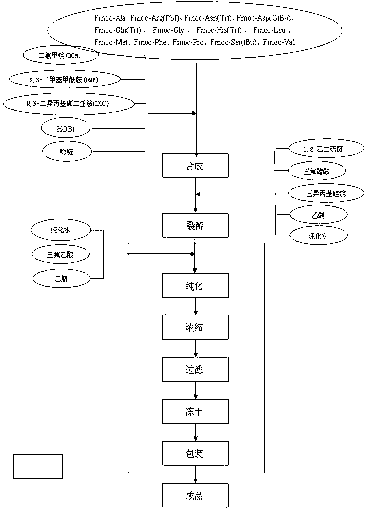
This invention discloses a preparation method of solid-phase peptide synthesizing bivalirudin. It includes the following steps: taking any one of triphenyl methyl chloride resin, 4-methyl-triphenyl methyl chloride resin, 4-methoxy-triphenyl methyl chloride resin, 2-chlorine-triphenyl methyl chloride resin, or Wang resin as the starting raw materials, connecting amino acids in turn according to the method of solid-phase synthesis, to get a protective 28-peptide resin, removing Fmoc-protective group in turn, side-chain protecting group and cutting the peptide to get a crude, then purifying the crude through C18 (or C8) high-pressure column to get bivalirudin exquisite article. In this invention, the peptide yield of every step is more than 99%, and the total yield is 14%.
Owner:SHANGHAI SOHO YIMING PHARMA
Solid-phase synthesis method of liraglutide
InactiveCN103087181ALess usableSmall quantityPeptide preparation methodsBulk chemical productionSide chainWang resin
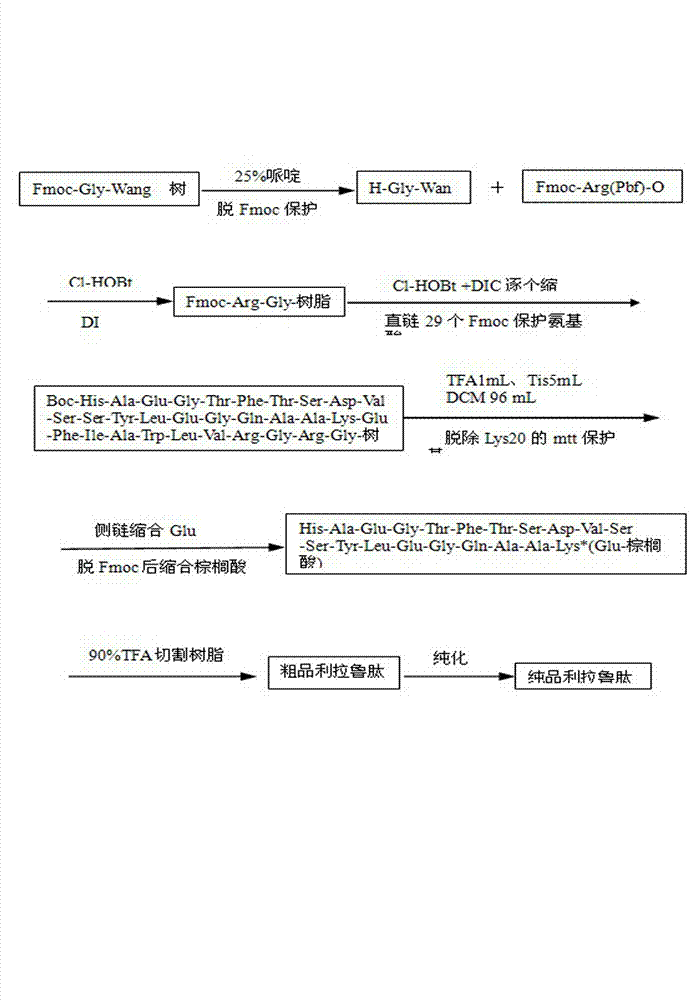
The invention discloses a method for synthesizing liraglutide. The method comprises the following steps of: 1, selecting Fmoc-Gly-Wang resin and N terminal Fmoc protected and side chain protected amino acid as raw materials, wherein lysine on a 26th site adopts Fmoc-Lys(Mtt)-OH or Fmoc-Lys(Mmt)-OH, histidine on an N terminal adopts Boc-His(Trt)-OH or Boc-His(Boc)-OH; 2, selectively removing a Mtt or Mmt protecting group on the lysine by adopting 1 percent TFA (Trifluoroacetic Acid); 3, sequentially coupling one g-glutamic acid and palmitic acid on a side chain; and 4, cutting resin by the TFA to obtain a crude product of the liraglutide, and carrying out preparative liquid chromatography purification and lyophilization to obtain a pure product of the liraglutide. The method for synthesizing the liraglutide is simple in steps, saves time and labor, is less in difficult sequences in a coupling process, simple and easy to operate during side chain de-protection, less in byproducts and high in yield, and is suitable for industrialized production.
Owner:刘卫 +1
Preparation method for linaclotide
InactiveCN104628826AGuaranteed stabilitySuitable for oxidation capacityPeptide preparation methodsBulk chemical productionSide chainWang resin
The invention provides a preparation method for linaclotide. The method includes: utilizing a standard Fmoc technology to connect a side chain protected amino acid with Wang resin, adding a condensing agent HBTU and alkali DIPEA, carrying out condensation reaction in a DMF solvent, employing a DMF solution containing 20% hexahydropiperidine to perform de-Fmoc protection, then conducting cutting from the solid phase resin, adding elemental iodine into a sodium phosphate buffer solution to carry out Cysteine oxidation, thus obtaining linaclotide. The preparation method for linaclotide provided by the invention is the technology for highyield synthesis of linaclotide, simplifies the cyclization process and enhances the cyclization yield, and the final product yield reaches 30%-60%. The preparation method has the advantages of simplicity, mild reaction conditions, high yield, and high product purity, is a feasible preparation method for industrialization of linaclotide, and provides good prospects for industrial production.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for preparing linaclotide
InactiveCN104163853AHigh purityHigh yieldPeptide preparation methodsBulk chemical productionCrystallographyWang resin
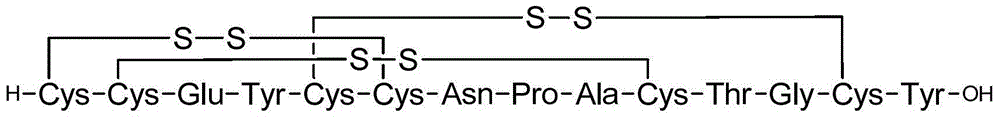
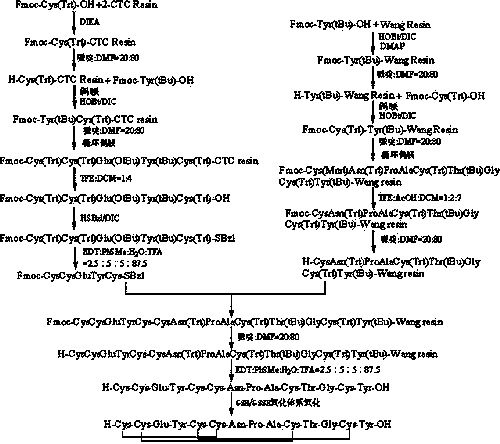
The invention relates to a method for preparing linaclotide. The method comprises the following steps of A, synthesizing a pentapeptide fragment I of Fmoc-CysCysGluTyrCys-SBzl by a solid-liquid phase method, B, a nonapeptide fragment resin II of H-CysAsn(Trt)ProAlaCys(Trt)Thr(tBu)GlyCys(Trt)Tyr(tBu)-Wang resin by the solid-liquid phase method, C, adding the pentapeptide fragment I into the nonapeptide fragment resin II, carrying out thioester exchange and S-to-N acyl transfer to obtain a novel tetrakaideca-peptide fragment resin, removing a Fmoc protective group, and carrying out pyrolysis to obtain high-purity linaclotide linear peptide, and D, carrying out oxidation by a GSH / GSSH oxidation system to obtain linaclotide. Through thioester exchange and S-to-N acyl transfer, the method for preparing linaclotide is realized and has the advantages of mild synthesis conditions, high product purity, high yield and large-scale production feasibility.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Solid-phase synthesis method of teriparatide
InactiveCN104530218AEase of mass productionReduce generationPeptide preparation methodsParathyroid hormonesSide chainEther
The invention provides a solid-phase synthesis method of teriparatide. The method comprises the following steps that Wang resin is used as a resin solid-phase carrier and is coupled with phenylalanine (Fmoc-Phe-OH) protected by N-end Fmoc to obtain Fmoc-Phe-resin; HOBT / DIC or HBTU / DIEA or TBTU / DIEA serves as a condensating agent, and through the solid-phase synthesis method, amino acid which has the N-end Fmoc protection and side chain protection is sequentially coupled according to a main chain peptide sequence of the teriparatide to obtain teriparatide resin; the teriparatide resin is split, a protecting group and the resin are removed, absolute ether is added into resin for precipitation, and crude teriparatide is obtained; c cartridges are adopted for separation and purification, lyophilization is carried out, and the teriparatide is obtained.
Owner:哈尔滨吉象隆生物技术有限公司
Solid phase polypeptide synthesis preparation method for leuprorelin
ActiveCN1865280AConvenient sourceReduce usagePeptide preparation methodsBulk chemical productionHydrogen fluorideLeuprorelin
The invention discloses a bright-ala-ruilin preparing method of solid-phase polypeptide, which comprises the following steps: adopting Wang resin or CTC resin as original material to connect amino with protective group to produce protective nonapeptide resin; removing Fmoc-protective group sequently; proceeding side-chain protective group synchronizingly and cutting peptide; connecting ethylamine through ethylamine-to-HOBT to produce crude product; proceeding separation and purifying through C18 (or C8) pillar to produce fine bright-ala-ruilin. The invention avoids the utility of poisonous agent, which improves the purifying, peptide connecting and obtaining rate.
Owner:SHANGHAI SOHO YIMING PHARMA
Method for solid phase synthesis of plecanatide by means of secondary cyclization
InactiveCN107383171AImprove orientation efficiencyHigh yieldPeptide preparation methodsBulk chemical productionPlecanatideSide chain
The invention provides a synthetic method of plecanatide. The method comprises the following sequential steps of: performing a programmed reaction on Wang resin as a solid phase carrier, performing condensation reaction successively to connect 16 protective amino acids to obtain linear peptide fully protected resin with 16 amino acids, wherein in two groups of Cys forming a disulfide bond, Cys in the same group are connected to Mmt or Acm at the same time, and the protecting groups connected by Cys in different groups are different; and successively removing the protecting groups of the two groups of Cys, successively performing directional cyclization reactions to form a dithio cyclic bond, and removing the protecting groups on the side chain and cutting resin to obtain plecanatide containing two dithiorings. The method provided by the invention has the beneficial effects that the method can generate few byproducts, and is high in directional efficiency and simple; the product is favorably purified, and the product yield is high.
Owner:SUZHOU UNIV OF SCI & TECH
Method for synthesis of bivalirudin in solid-phase fragment approach
ActiveCN102731624AHigh yieldIncrease costPeptide preparation methodsBulk chemical productionFreeze-dryingWang resin
The invention provides a method for synthesis of bivalirudin in a solid-phase fragment approach. The method comprises: first employing a solid phase stepwise method to synthesize a 13-peptide fragment I-Wang Resin, and a 7-peptide fragment II with the terminal N protected by Fmoc, coupling the two fragments in the solid-phase fragment approach to synthesize bivalirudin -Wang Resin; and finally conducting deprotection, cracking, purification, and freezing drying so as to obtain pure bivalirudin. Compared with existing solid phase stepwise method or liquid phase fragment method, the method of the invention has the advantages of simple technological operation, high yield and purity, small environmental pollution, and easy large-scale production, etc.
Owner:YANCHENG KAILI PHARMA
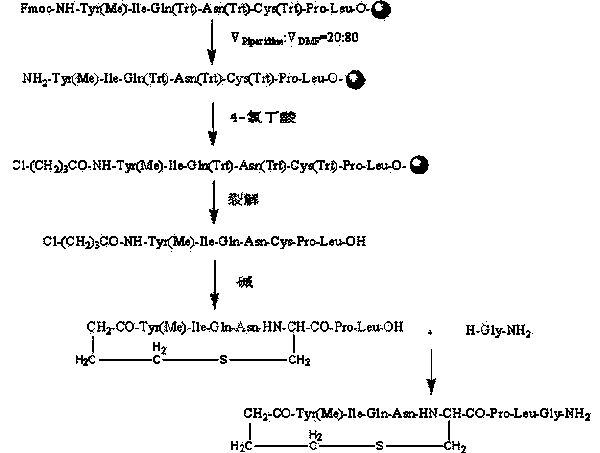
Carbetocin synthesis method
InactiveCN103992390AHigh purityHigh yieldOxytocins/vasopressinsPeptide preparation methodsSynthesis methodsFreeze-drying
The invention relates to a carbetocin synthesis method. The carbetocin synthesis method solves the problem that the prior art has a high cost, low purity and more impurities. The carbetocin synthesis method comprises the following steps of orderly coupling seven amino acids to wang resin, wherein the coupled amino acids orderly comprise Fmoc-Leu-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Ile-OH and Fmoc-Tyr(Me)-OH, removing a protective group Fmoc, connecting 4-chlorobutyric acid, carrying out cracking to obtain an acylated carbetocin seven-peptide linear peptide, carrying out liquid cyclizing under alkaline conditions to obtain a carbetocin seven-peptide crude cyclopeptide, carrying out purification and freeze-drying on the crude cyclopeptide to obtain a carbetocin seven-peptide fine cyclopeptide, and coupling the fragment of the carbetocin seven-peptide fine cyclopeptide subjected to purification and freeze-drying and H-Gly-NH2.HCl to obtain carbetocin. The invention provides the carbetocin synthesis method having a low cost and a high yield and suitable for large-scale production.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Method for synthesizing icatibant
InactiveCN104072585AHigh yieldThe synthesis process is simplePeptide preparation methodsBulk chemical productionSide chainArginine
The invention relates to the field of medicine synthesis and discloses a method for synthesizing icatibant. The method comprises the following steps: protection arginine of a protecting group is coupled on the N end and the side chain of arginine; the protection arginine and a resin carrier are subjected to esterification reaction under the action of a coupling reagent and a reactivate reagent to obtain peptide resin 1; the resin carrier is Wang resin or HMP; according to amino acid sequence from C end to N end of icatibant and starting from the peptide resin 1, at presence of a condensation reagent and the reactivate reagent, the protection amino-acid are coupled in sequence in an extending manner to obtain icatibant resin eventually; then the icatibant resin is subjected to acid decomposition to obtain the crude icatibant; the crude icatibant is purified to obtain the pure icatibant. According to the invention, as proper Wang resin or HMP is selected, the whole synthesis process is optimized and the total yield of the icatibant is improved significantly; compared with the prior art, the total yield is increased over 20 %.
Owner:CHENGDU SHENGNUO BIOPHARM
Coupling oligoarginine and 2 site substituted endomorphin-1 analogue as well as synthetic method and application thereof
InactiveCN104650182AEnhanced enzymatic stabilityImprove permeabilityPeptide/protein ingredientsAntipyreticSide effectCoupling
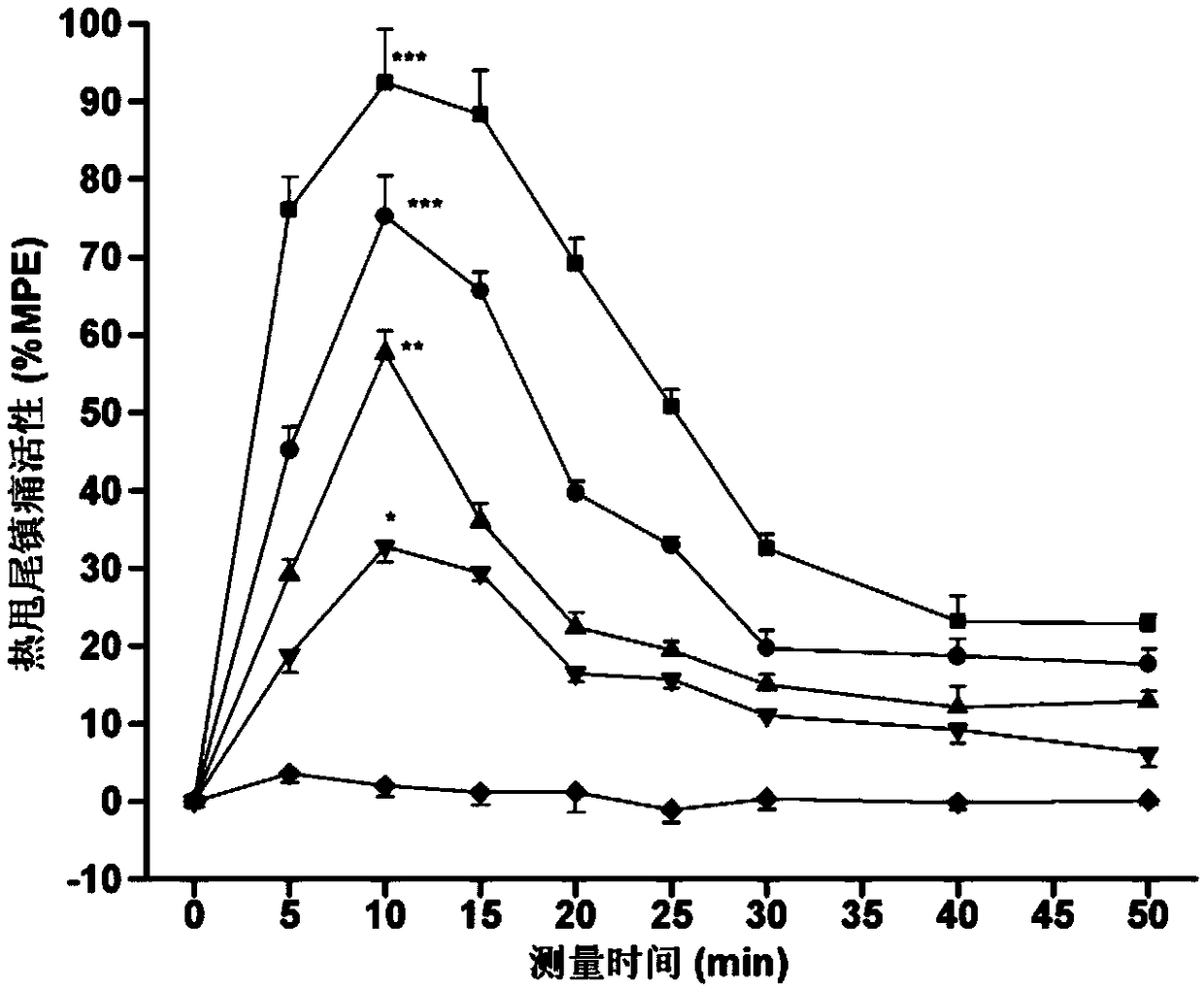
The invention discloses a coupling oligoarginine and 2 site substituted endomorphin-1 analogue as well as a synthetic method and application thereof, and relates to an endomorphin-1 analogue as well as a synthetic method and application thereof. The coupling oligoarginine and 2 site substituted endomorphin-1 analogue aims at solving the problems that endomorphin-1 is relatively low in biological stability and peripheral administration analgesic activity and has relatively large side effects on the gastrointestinal tract. The synthetic method comprises the following steps: I, pre-treating Wang resin which is protected by 'Fmoc'; II, removing 'Fmoc' protection groups; III, performing amino acid condensation reaction; IV, extending a peptide chain; V, cutting the peptide chain from the resin; and VI, desalting and purifying crude peptides. According to the endomorphin-1 analogue disclosed by the invention, the opioid affinity and agonist activity can be maintained; and meanwhile, the endomorphin-1 analogue has higher biological stability and peripheral administration analgesic activity than that of maternal endomorphin-1, also has the advantages of reducing the side effects of the gastrointestinal tract, and can be used for preparing polypeptide analgesic medicines.
Owner:HARBIN INST OF TECH
Preparation method of thymopentapeptide
InactiveCN1534042AGet twice the result with half the effortThe method of protection is simple and easyThymopoietinsTrifluoroacetic acidSide chain
A process for preparing thymopentapeptide (TP-5) includes the reaction between Wang resin and fluorenylmethyloxycarbonyl (FMOC) protected tyrosine to obtain tyrosine resin, condensation reaction on FMOC protected amino acid to obtain protected TP-5 resin, and cracking by trifluoroacetic acid to obtain coarse TP-5 product. Its advantages are high output rate and easy purification.
Owner:上海丽珠制药有限公司
Method for preparing icatibant
ActiveCN103992383AResolving incomplete couplingLow synthetic yieldPeptide preparation methodsBulk chemical productionSide chainCoupling
The invention relates to a method for preparing icatibant. The method specifically comprises the following steps: A) synthesizing fragment Boc-D-Arg-Arg-OH.2HCl by a liquid phase; B) sequentially coupling amino acids having an N-terminal Fmoc protecting group and a side chain protecting group in accordance with the main chain peptide sequence of icatibant by solid-phase synthesis method, wherein coupling of the last two amino acids is performed by the fragment Boc-D-Arg-Arg-OH.2HCl, and the Wang resin is taken as a starting resin; C) cleaving the peptide from the resin, purifying, desalinating and lyophilizing to obtain icatibant, wherein the content of both of deletion peptide impurities des-D-Arg1-icatibant and des-Arg2-icatibant is less than 0.1%. The invention provides a method for preparing icatibant with the advantages of high purity, low cost and capability of large-scale production; the content of impurities, namely, des-D-Arg1-icatibant and des-Arg2-icatibant can be effectively controlled under the premise of the yield of icatibant is not affected by virtue of the method.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Method for preparing high-purity thymalfasin
ActiveCN103880945AExtended reaction timeShort reaction timeThymosin peptidesPeptide preparation methodsWang resinThymalfasin
The invention discloses a method for preparing high-purity thymalfasin. The preparation method comprises: firstly preparing a first resin polypeptide fragment, a second resin polypeptide fragment, a third resin polypeptide fragment, a fourth resin polypeptide fragment and a fifth resin polypeptide fragment respectively; cleaving the second resin polypeptide fragment, the third resin polypeptide fragment, the fourth resin polypeptide fragment and the fifth resin polypeptide fragment to obtain crude peptide fragments; purifying the obtained second, third, fourth and fifth crude polypeptide fragments respectively; linking each purified polypeptide fragment to the first resin polypeptide fragment; carrying out an acetylation reaction on resin polypeptide fragments to obtain thymalfasin wang resin; cleaving to obtain the thymalfasin crude product; then purifying the obtained thymalfasin crude product twice; and collecting the mobile phase containing thymalfasin in a collection and purification process, evaporating to dryness under reduced pressure and centrifuging to obtain thymalfasin, and vacuum drying to obtain thymalfasin products. The thymalfasin is prepared by the method disclosed by the invention, the reaction time is effectively shortened, the reaction yield and the quality of the final product are improved; and the purity of thymalfasin prepared is more than 99%.
Owner:郑州大明药物科技有限公司
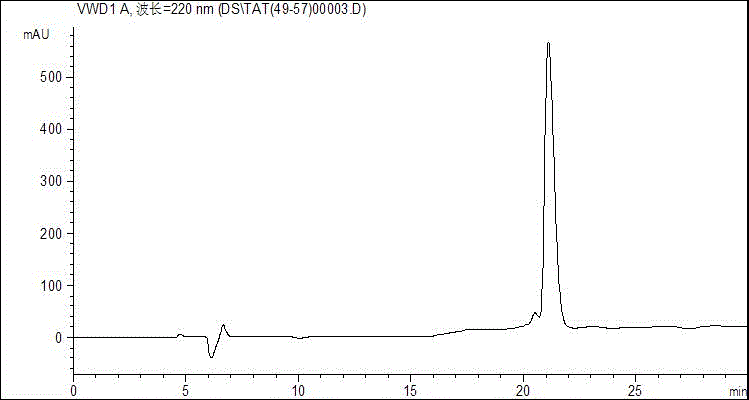
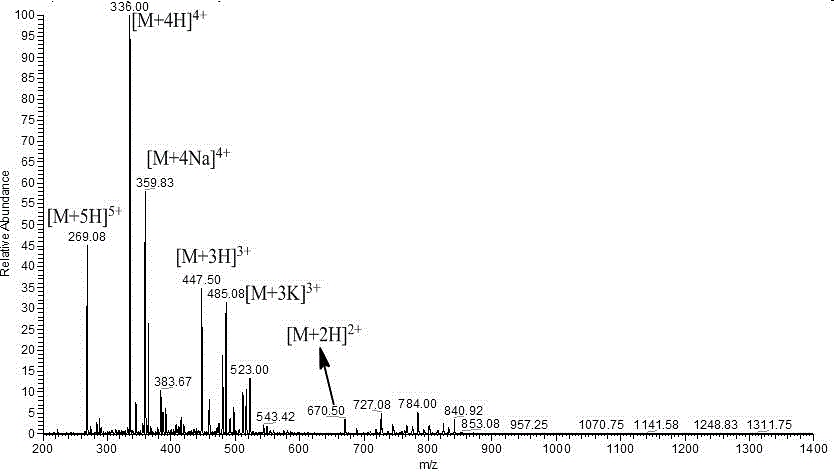
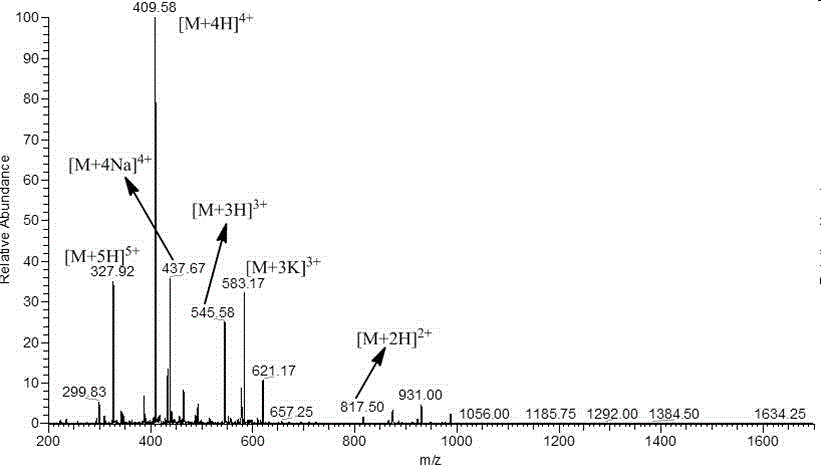
Antimicrobial peptide based on cell penetrating peptide Tat (49-57) and synthetic method of antimicrobial peptide
InactiveCN106749559AGood inhibition efficiencyHigh suppression efficiencyAntibacterial agentsVirus peptidesEscherichia coliAmino acid side chain
The invention belongs to the field of antimicrobial peptides, and discloses an antimicrobial peptide based on a cell penetrating peptide Tat (49-57) and a synthetic method of the antimicrobial peptide. The antimicrobial peptide is Tat (YG), Tat (YY), Tat (FG) or Tat (FF), and a sequence of a peptide chain is successively as shown by SEQ ID No. 2 to No. 5. The antimicrobial peptide is synthesized by adopting Wang resin as a carrier, adopting Fmoc as an amino acid side chain protection base, adopting a DMF solution of piperidine as a deprotection reagent and adopting HBTU, HOBT and DIEA as amino acid condensing agents in a solid-phase synthetic method. The four derivative antimicrobial peptides have good inhibition efficiency for escherichia coli, salmonella typhimurium, Bacillus subtilis and staphylococcus aureus, and the inhibition rate for some bacteria can be higher than that of the cell penetrating peptide Tat (49-57); and moreover, the hemolytic activity of the four derivative microbial peptides is small, and the concentration when the bacteriostatic activity is played cannot reach the lowest hemolytic concentration.
Owner:ZHENGZHOU UNIV +1
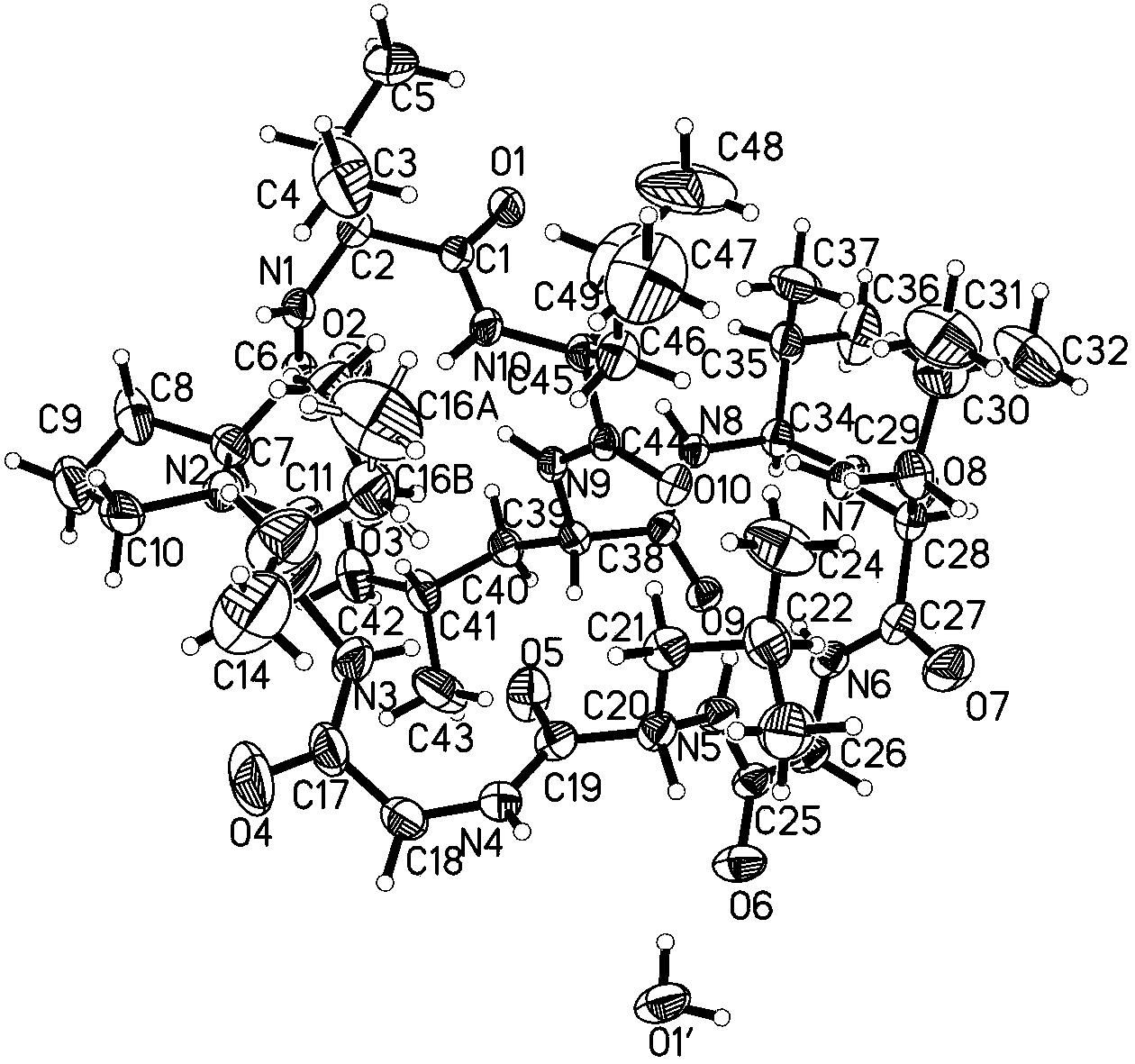
Chemical preparation method of cyclic decapeptide compound GG-110824
InactiveCN103665115AThree-dimensional structure guaranteeSimple preparation stepsPeptide preparation methodsChromatographic separationX-ray
The invention belongs to the field of pharmacy, and relates to a chemical preparation method of a cyclic decapeptide compound GG-110824. The cyclic decapeptide compound GG-110824 is formed by connecting ten alpha-L-amino acids end to end in sequence, has a structure shown by a formula (I) and is expressed as cyclo(Gly-Leu-Val-Leu-Leu-Val-Pro-Ile-Gly-Leu). The preparation method of the GG-110824 is a chemical preparation method of a solid phase synthesis straight chain peptide precursor and a liquid phase closed ring by taking Wang resin as a solid carrier, HATU / HOAt (2-(7-Aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate / 1-Hydroxy-7-azabenzotriazole) as a condensating agent and N,N-dimethylfomamide as a solvent; the GG-110824 is obtained through chromatographic separation of reaction products, and the structure of a prepared product is conformed through monocrystal X-ray diffraction. The preparation method disclosed by the invention is simple in step and high in yield and has a very high practical value.
Owner:FUDAN UNIV
Low-cost method for preparing high-purity linaclotide
The invention discloses a low-cost method for preparing high-purity linaclotide. According to the technical scheme, the low-cost method comprises the following steps: 1) synthesizing linear linaclotide by utilizing resin carriers including CTC resin or Wang resin and the like through a Fmoc method or obtaining the linear linaclotide by utilizing a segment method or a liquid-phase synthesis method;2) obtaining a linaclotide crude product which is accurately matched by adopting a one-step oxidization method; 3) obtaining the high-purity linaclotide through purifying by adopting high performanceliquid chromatography. The method disclosed by the invention is started from the aspects including an oxidization system and the like, and a method for accurately matching a linaclotide disulfide bond is improved; the method is simple and stable in technology and low in cost, and the linaclotide crude product can be obtained through one-step cyclization; the purity of a purified product can be greater than 99 percent and the content of a single impurity can be controlled to be 0.1 percent or lower. Compared with the prior art, the method has better economic benefits and application prospect.
Owner:杭州肽佳生物科技有限公司
Solid phase synthetic method of liraglutide
InactiveCN107022021AAvoid it happening againReduce formationPeptide preparation methodsBulk chemical productionSide chainCombinatorial chemistry
The invention discloses a method for preparing liraglutide. The technical defects that heavy metal catalysts are used, synthetic steps are complicated, purification is difficult and the like in the prior art are overcome. The technical scheme of the invention comprises the following steps: 1) with Fmoc-Gly-Wang resin as a carrier, coupling 2nd to 12th amino acids one by one by adopting an Fmoc solid phase synthetic method to obtain polypeptide resin I; 2) removing an mmt protecting group of the 11th Lys side chain through acidolysis, modifying Pal-gamma-Glu on the resin, and removing a Dde protecting group through hydrazinolysis to obtain polypeptide resin II; 3) continuously coupling 13th to 31st amino acids to obtain polypeptide resin III; 4) cutting the polypeptide resin III, splitting polypeptide from the resin and simultaneously removing the protecting group of the side chain to obtain a liraglutide crude product; carrying out high-efficient liquid chromatography separation and purification on the liraglutide crude product, and then lyophilizing the liraglutide curde product to obtain liraglutide. The process method for preparing liraglutide adopted by the invention has the characteristics of simple operation, high purity of crude peptide and high comprehensive yield.
Owner:GL BIOCHEM SHANGHAI
Hexapeptide for inhabiting angiotensin transferase and preparation method thereof
InactiveCN102311484AAvoid side effectsMild preparation conditionsPeptide preparation methodsFermentationGraft reactionThreonine
Owner:SOUTH CHINA UNIV OF TECH
Polypeptide drug conjugate with tumor targeting, and preparation method of polypeptide drug conjugate
ActiveCN112386707ASolve the technical core problems of chemical synthesisFirmly connectedOrganic active ingredientsPharmaceutical non-active ingredientsTumor targetingCytotoxicity
The invention discloses a preparation method and application of a polypeptide drug conjugate with tumor targeting. The polypeptide drug conjugate with the tumor targeting structurally comprises tumortargeting peptide WA1, a non-splitting connector and a cytotoxicity anti-tumor drug. The preparation method of the polypeptide drug conjugate (PDC-WA1) with the tumor targeting adopts a Fmoc solid-phase synthesis method and comprises the following main synthesis steps of: taking Fmoc-Ala-Wang resin as a raw material, after the tumor targeting peptide is synthesized, connecting the synthesized tumor targeting peptide with Fmoc-Acp-OH and chlorambucil in sequence, and carrying out purification by HPLC to obtain the polypeptide drug conjugate with the tumor targeting and anti-tumor activity. Thepreparation method has the advantages of low production cost, low environment pollution, few reaction by-products and low purification difficulty and is simple in reaction operation.
Owner:辽宁医学诊疗科技研发中心有限公司
Solid-liquid phase synthesis method of Bremelanotide
ActiveCN110950933AHigh purityLow costPeptide preparation methodsBulk chemical productionDipeptideFluid phase
The invention discloses a solid-liquid phase synthesis method of Bremelanotide. The method comprises the following steps: synthesizing an AC-Nle-Asp-O-2-Phipr dipeptide fragment by virtue of a liquidphase method; coupling a main chain and a side chain of the Lys respectively to prolong a peptide sequence, obtaining Fmoc-His(Boc)-D-Phe-Arg(pbf)-Trp(Boc)-Lys(AC-Nle-Asp-O-2-Phipr)-Wang resin, and after the protecting group is removed, cyclizing the main chain and the side chain. The head and the tail are prevented from being folded into a ring, the problem of large steric hindrance of the foldedring is solved, and the influence of the formed peptide on the non-formed peptide is also reduced to the minimum due to the fact that the ring forming sites are closer. In addition, alkali is not adopted for hydrolysis, and corresponding impurities cannot be generated. The Bremelanotide synthesized by the synthesis method is high in purity, low in cost and suitable for large-scale production.
Owner:SUZHOU UNIV OF SCI & TECH
Chimeric peptide based on endomorphin-1 and neurotensin (8-13) and synthesis method and application thereof
ActiveCN107964047AHigh clinical application valueLittle side effectsNervous disorderPeptide/protein ingredientsSide effectSynthesis methods
The invention a chimeric peptide based on endomorphin-1 and neurotensin (8-13) and a synthesis method and application thereof. The chimeric peptide solves the problem that the existing endomorphin-1 has short lasting time of easing pain, low pain-easing activity based on peripheral administration and analgesic tolerance and gastrointestinal side effects. The chimeric peptide has an amino acid sequence of Tyr-Pro-Trp-Phe-Gly-Gly-Arg-Arg-Pro-Tyr-Ile-Leu. The synthesis method comprises 1, Fmoc-protective Wang resin pretreatment, 2, removal of a Fmoc-protective gene, 3, amino acid condensation reaction, 4, peptide chain extension, 5, cutting of the peptide chain from resin, and 6, crude peptide desalting and purification. The chimeric peptide has long duration of easing pain and has the advantages of no pain easing tolerance and low side effects on the gastrointestinal tract. The chimeric peptide is used for preparation of polypeptide pain easing drugs.
Owner:黑龙江省工研院资产经营管理有限公司
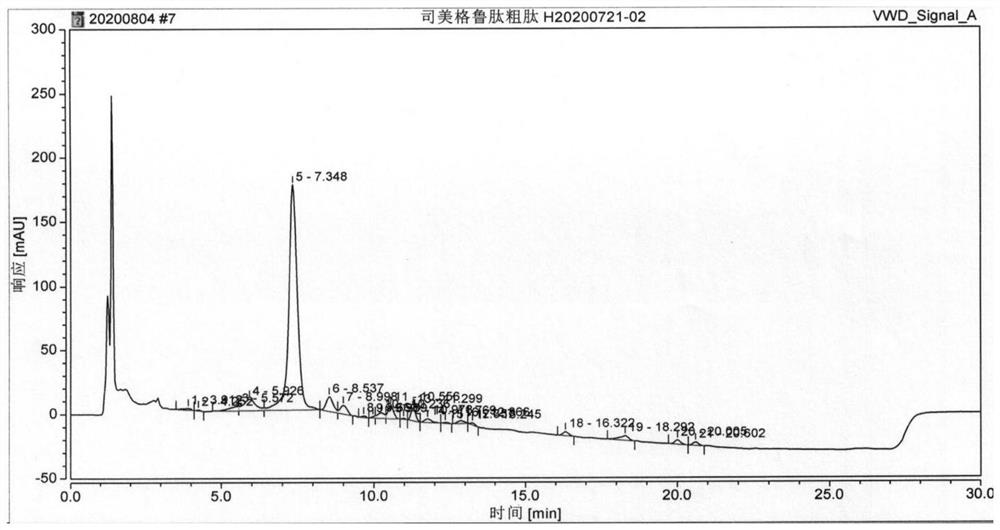
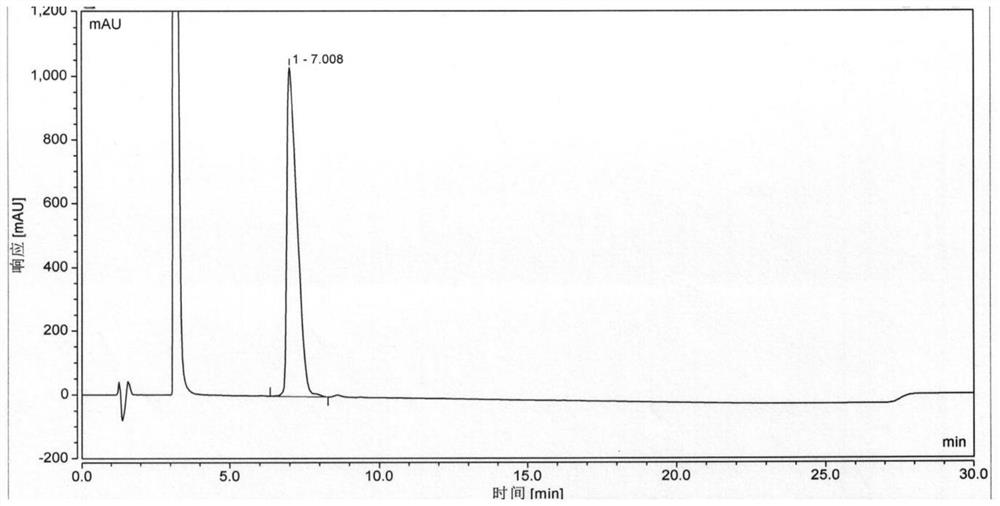
Synthesis method of semaglutide
InactiveCN112028986AHigh purityAvoid efficiencyPeptide preparation methodsBulk chemical productionAmino acid side chainDipeptide
The invention discloses a synthesis method of semaglutide. The synthesis method comprises the following steps: (1) taking Fmoc-Gly-Wang resin as a solid-phase carrier and removing an Fmoc protection group; gradually coupling amino acids from a C end to an N end in sequence; coupling to 20 amino acid R-Lys (Fmoc)-OH at the N end of a main chain; (2) removing a side chain Fmoc protection groupof the 20 amino acid at the N end of the main chain and coupling side chain amino acids in sequence; (3) removing a 20 amino acid R protection group at the N end of the main chain and coupling residual amino acids of the main chain in sequence according to a peptide sequence to obtain semaglutide full-protection peptide resin, wherein dipeptide fragments are selected as 18 -19 amino acids at the N end and 1 -2 <nd> amino acids at the N end; and (4) cracking and precipitating the semaglutide full-protection peptide resin through a cracking solution to obtain semaglutide crudepeptide. The method disclosed by the invention is simple and convenient; the prepared semaglutide has high purity and industrial production is facilitated.
Owner:哈尔滨吉象隆生物技术有限公司
Method for synthetizing fullerene bis-addition polypeptide by combining liquid phase and solid phase
InactiveCN104478995AImprove solubilityImprove stabilityPeptide preparation methodsCarboxyl radicalArginine
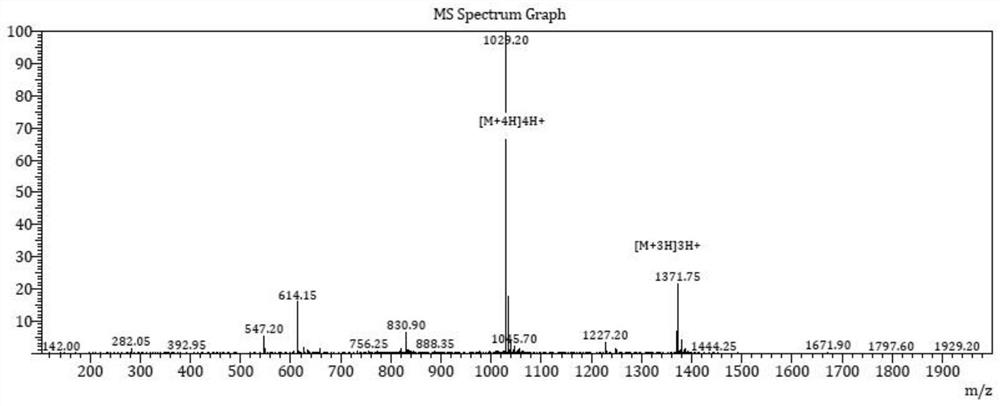
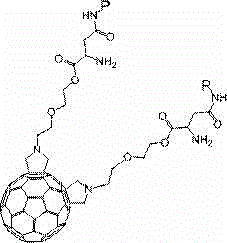
The invention discloses a method for synthetizing fullerene bis-addition polypeptide by combining a liquid phase and a solid phase. N-substitutive fullerene bis-addition pyrrolidine and Fmoc-Asp-OtBu with protected alfa-amino and beta-carboxyl have a condensation reaction with a liquid-phase synthesis method in the presence of DCC (dicyclohexylcarbodiimide), HOBt (hydroxybenzotriazole ) and DMAP (dimethylaminopyridine); after the reaction, separation and purification are conducted through silica-gel column chromatography, and fullerene bis-addition aspartic acid is obtained after deprotection of a product; an alpha-carboxyl of fullerene bis-addition aspartic acid and activated amino on arginine in Wang resin-polypeptide have a condensation reaction with a solid-phase synthesis method, and fullerene bis-addition polypeptide is obtained after deprotection and decomposition of a product. Fullerene bis-addition polypeptide has good anti-tutor activity and capacity for scavenging free radicals, improves the stability of peptide and water solubility of fullerene, can be used for connecting different polypeptide and obtains different biological activity with changing of functional peptide.
Owner:ZHENGZHOU UNIV
Method for synthesizing monopegylation fixed point modification thymopoietin
InactiveCN102443049AAvoid the disadvantage of difficult operationAvoid steric hindrancePeptide preparation methodsBulk chemical productionSide chainThymopentin
The invention provides a method for synthesizing monopegylation fixed piont modification thymopoietin, which belongs to the field of biomedicine. According to the method, firstly, a solid phase is adopted for synthesizing TP5 containing protecting groups, the defect that the fluorenylmethyl chloroformate (FMOC) unit removal operation of the method in the prior art is difficult to carry out is avoided, then, the modified polyethylene glycol is connected onto the lysine side chain amino at the fixed point in a liquid phase, the goal of fixed point modification is reached, simultaneously, the space steric hindrance of the solid phase wang resin is avoided through the liquid phase environment, finally, all protecting groups are removed in one step through palladium carbon catalytic hydrogenation to obtain the pegylation thymopoietin with the long-action effect, the synthesis process is simple and convenient, the cost is low, the yield is high (the total yield is higher than 20 percent), and the industrial production is convenient. The pegylation thymopoietin synthesized by the method can not be easily hydrolyzed by esoteric protease, the renal clearance can be obviously reduced because the molecular weight is heavy, and the internal circulation time is greatly prolonged, so the goals of obviously prolonging the half-life period and improving the bioavailability of organisms are reached.
Owner:倪京满 +1
Method for synthesizing liraglutide
InactiveCN110835369AReduce generationHigh purityPeptide preparation methodsBulk chemical productionWang resinOrganic chemistry
The invention discloses a method for synthesizing liraglutide and belongs to the technical field of polypeptide synthesis. The method of the invention comprises the steps: with Fmoc-Gly-Wang Resin serving as a solid-phase synthesis starting support, performing activation coupling on 29 amino acids from positions 37 to 9 (sequence C end to N end) of liraglutide by means of a novel condensing agentPyBrop (bromotrispyrrolidinophosphoniumhexafluophosphate), wherein Fmoc-Lys(Pal-Glu-OtBu)-OH is used as a raw material in the position 26; Boc-His(Trt)-Osu and Fmoc-Ala-Osu are used to perform condensing in the positions 7 and 8 to obtain a liraglutide all-protected peptide, and cracking precipitation is then performed to obtain liraglutide. The method has the advantages that amino acid coupling efficiency is improved, racemic peptides and deleted peptide impurities generated in the synthesis process of liraglutide are greatly reduced, racemic impurities (D-His7 and D-Ala8) which are very similar to the product in property are particularly effectively inhibited or reduced, the purity of the crude product is improved, the purification difficulty is reduced, and industrial scaled productionis facilitated.
Owner:苏州天马医药集团天吉生物制药有限公司
Multi-site modified enkephalin and neurotensin (8-13) coupled cycled hybrid peptide, compounding method and application thereof
ActiveCN109232748AImprove resistance to enzymatic hydrolysisImprove transportation capacityNervous disorderPeptide/protein ingredientsMulti siteWang resin
The invention provides a multi-site modified enkephalin and neurotensin (8-13) coupled cycled hybrid peptide, a compounding method and an application thereof and relates to a cycled hybrid peptide, the compounding method and the application thereof. The invention aims to solve the problems of inferior anti-enzymolysis capacity and non-ideal anti-neuropathic pain effect of present opioids. The hybrid peptide is a hybrid peptide 1, a hybrid peptide 2, a hybrid peptide 3 or a hybrid peptide 4. The preparation method comprises the following steps: 1) pre-treating 'Fmoc' protected Wang resin; 2) removing 'Fmoc' protecting groups; 3) triggering condensation reaction of amino acid; 4) prolonging peptide chain; 5) forming a disulfide bond; 6) cutting peptide chain from the resin; 7) desalting andpurifying crude peptide. According to the invention, the biological stability of hybrid peptide can be enhanced and an anti-neuropathic pain effect can be endowed, through multi-site unnatural amino acid substitution and cyclizing modification. The hybrid peptide provided by the invention can be used for preparing drugs for relieving neuropathic pain.
Owner:黑龙江省工研院资产经营管理有限公司
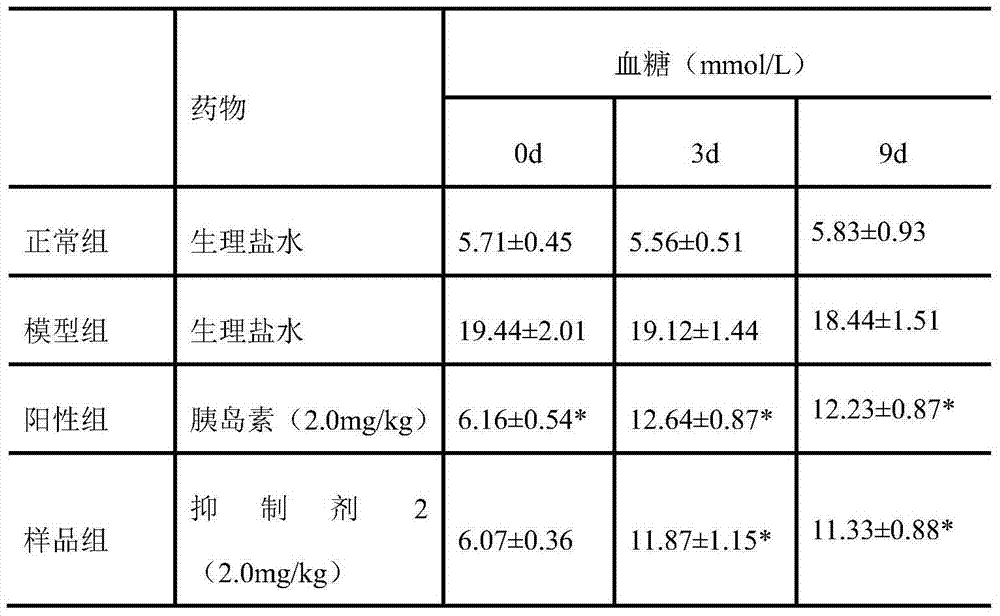
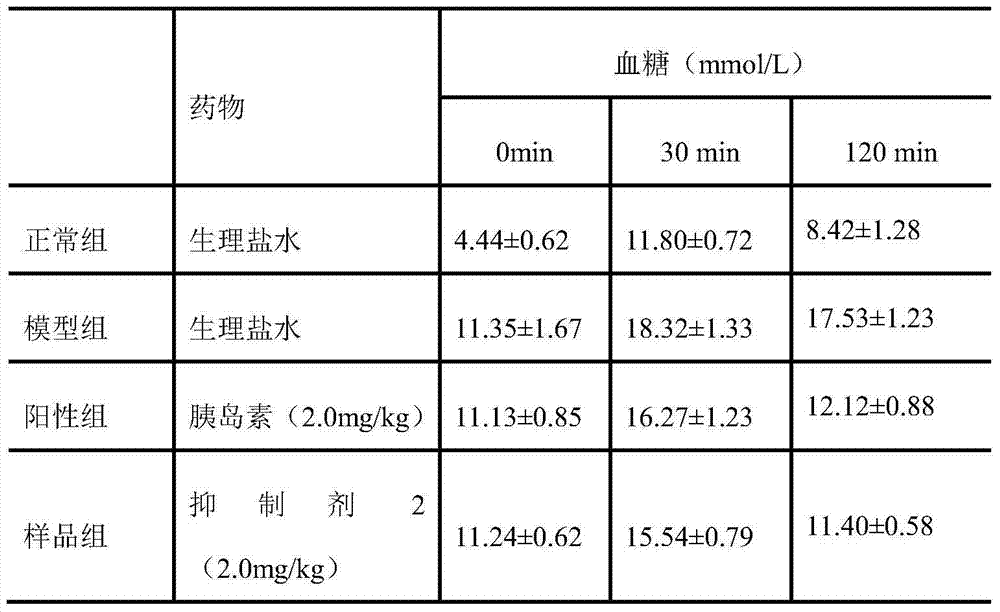
Insulin amyloid polypeptide inhibitor, preparation method and application thereof
Insulin amyloid polypeptide inhibitor, preparation method and application thereof. The invention relates to the field of drugs and particularly relates to an inhibitor 1 compound which can treat diabetes. A sequence of the inhibitor 2 is VLSVAALNHLDKATPIESH. A preparation method of the inhibitor 2 is carried out through Fmoc-protected solid-phase synthetic technology, with Rink amide MBHA resin and Fmoc-Gly-Wang resin as supporters, with 6-chloro-1-hydroxylbenzotriazole and N,N-diisopropyl carbodiimide as condensing agents, and with trifluoroacetic acid as a cutting reagent. The invention also provides an application of the inhibitor 2 for preventing or treating diabetes and complications thereof, wherein the complications comprise diabetic nephropathy, diabetic hypertension, diabetic eye diseases and diabetic neurogenic lesion. The inhibitor 2 can be employed for preventing or treating the diabetes and the complications thereof through various dosing methods, such as subcutaneous injection or intramuscular injection, intravenous injection or intravenous drip, and oral medication, such as pills, capsules, nasal spray agents and the like. The islet amyloid polypeptide inhibitor 2 can targetedly inhibit generation of islet amyloid polypeptide, thereby preventing or treating diabetes.
Owner:刘旭
Preparation method of human vascular endothelial inhibitory peptide
InactiveCN103183733AReduce racemizationImprove product qualityPeptide preparation methodsAnimals/human peptidesVascular endotheliumWang resin
The invention provides a solid-phase synthesis process of a human vascular endothelial inhibitory peptide: H-His-Ser-His-Arg-Asp-Phe-Gln-Pro-Val-Leu-His-Leu-Val-Ala-Leu-Asn-Ser-Pro-Leu-Ser-Gly-Gly-Met-Arg-Gly-Asp-Arg-Gly-Arg. The process includes: taking Fmoc-Asp(OtBu)-Wang resin as a raw material and putting it in a polypeptide synthesis reactor, using N, N-dimethylformamide (DMF) as a solvent, taking 20% piperidine (PIP) as an alpha-amino deprotection agent, adopting N, N-diisopropyl carbodiimide (DIC) as a condensation agent, and introducing protected amino acid (Fmoc-AA) in order to perform a programmed reaction; then carrying out cracking, precipitation by anhydrous ether, and oxidation to obtain a crude product; and subjecting the crude product to purification, desalination and freeze-drying so as to obtain a refined end product.
Owner:HARBIN PHARMA GROUP BIOLOGICAL ENG +1
Method for preparing thymopentin
The invention relates to the preparation of polypeptide medicines, in particular to a method for preparing an anti-tumor adjuvant medicine, namely thymopentin. The method for preparing the thymopentin comprises the following steps of: mixing an amino acid resin, namely polyethylene glycol (PEG)-wang resin-Tyr(tbu)-OH and a super absorbent resin; dissolving Fmoc-Val-OH into a dichloromethane (DCM) / dimethylformamide (DMF) solution, and adding the obtained solution into the mixed resin together with a 1-hydroxybenzotriazole (HOBt) / DMF / diisopropylcarbodiimide (DIC) mixed solution; sequentially washing by using DMF, ethanol and DCM to finish the connection of the Fmoc-Val-OH; and sequentially connecting Fmoc-Asp(OtBu)-OH, Fmoc-Lys(Boc)-OH and Fmoc-Arg-OH by the same method, performing pyrolysis by using a pyrolysis reagent in which the ratio of trifluoroacetic acid (TFA) to ethylenediamine tartrate (EDT) to water is 95:3:2 after connection, filtering the resin, adding a filtrate into absolute ether, sucking supernatant out after settlement, adding purified water of which the volume is the same as that of a precipitate into the precipitate, shaking, standing, demixing, extracting an aqueous layer by using the absolute ether, washing, concentrating, and freeze-drying a concentrated solution to obtain the thymopentin.
Owner:HAINAN ZHONGHE PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com