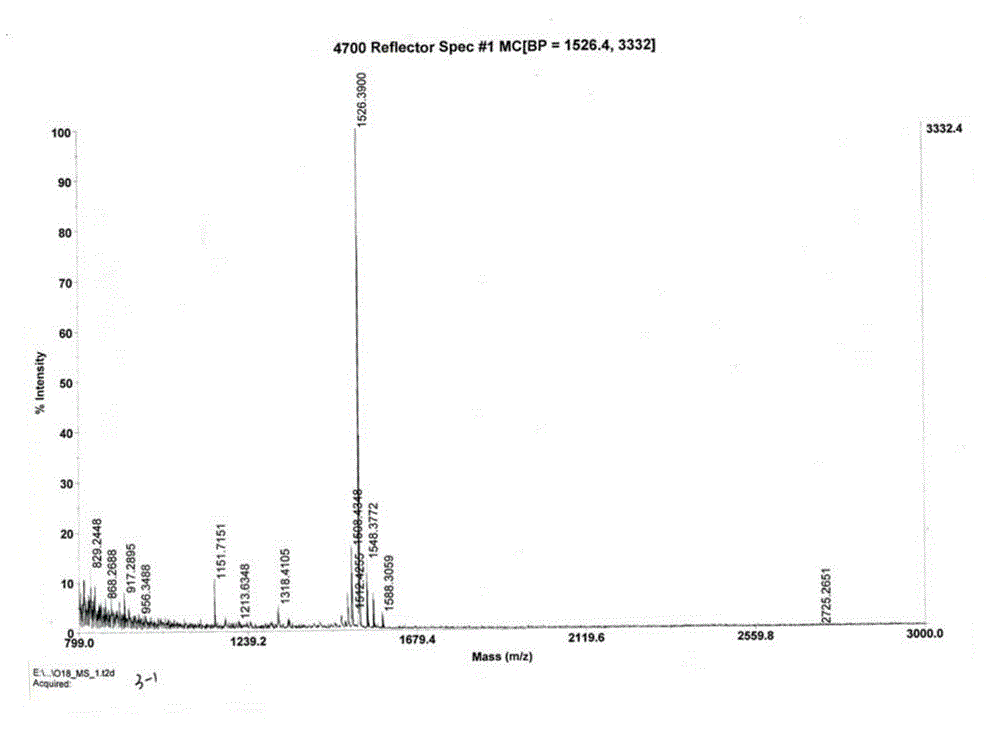
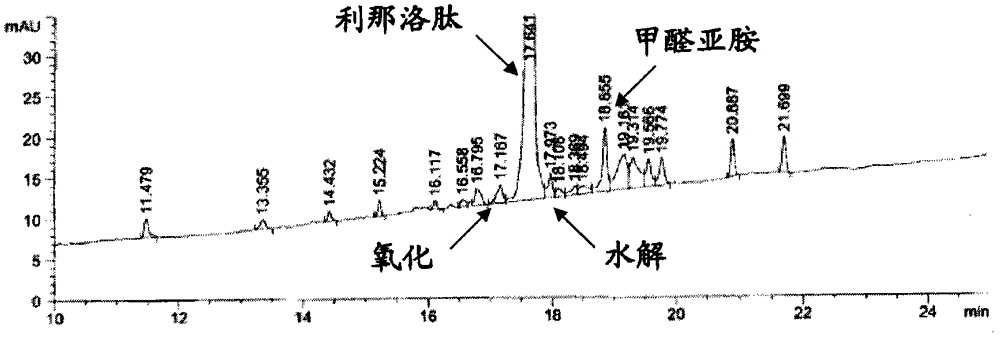
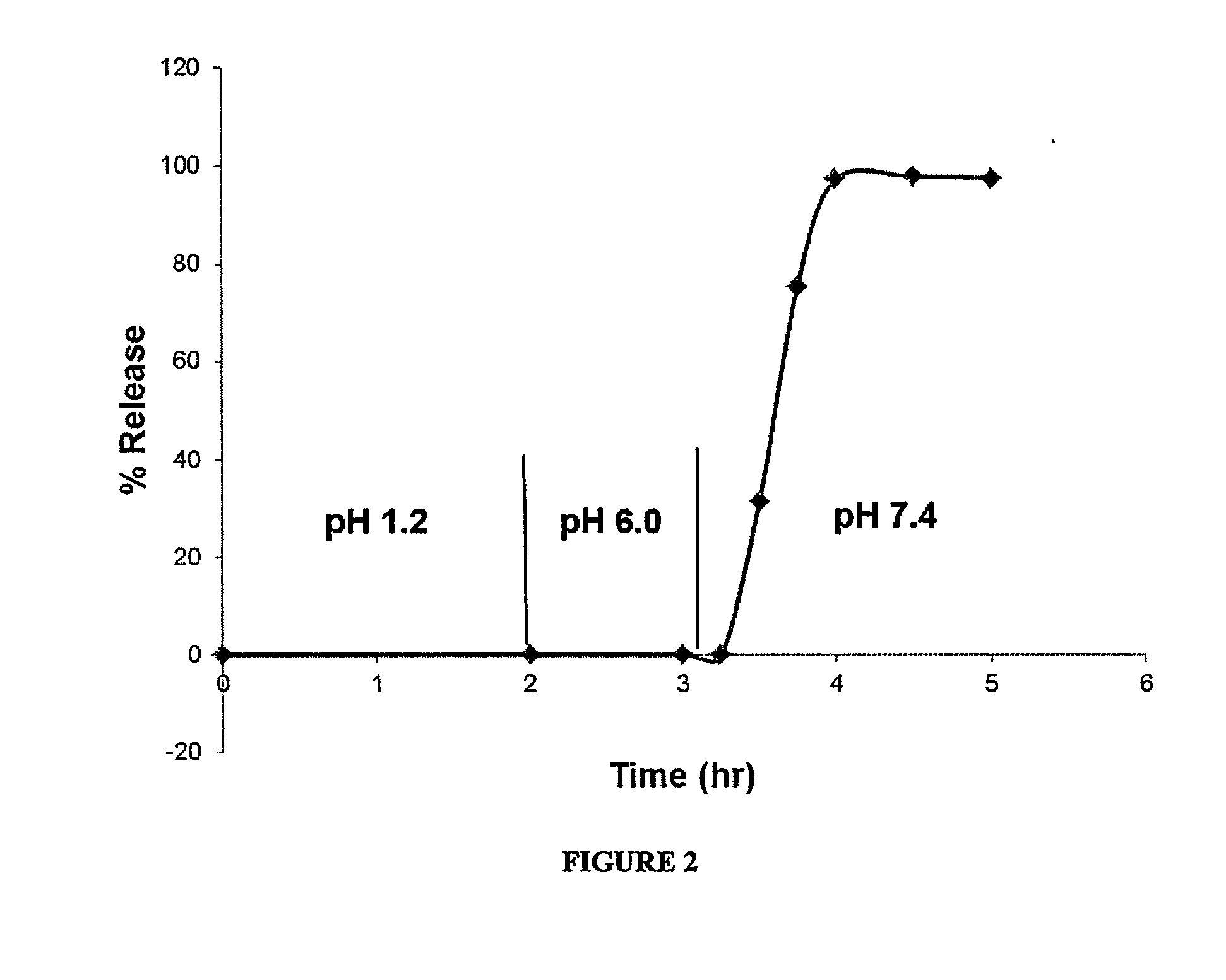
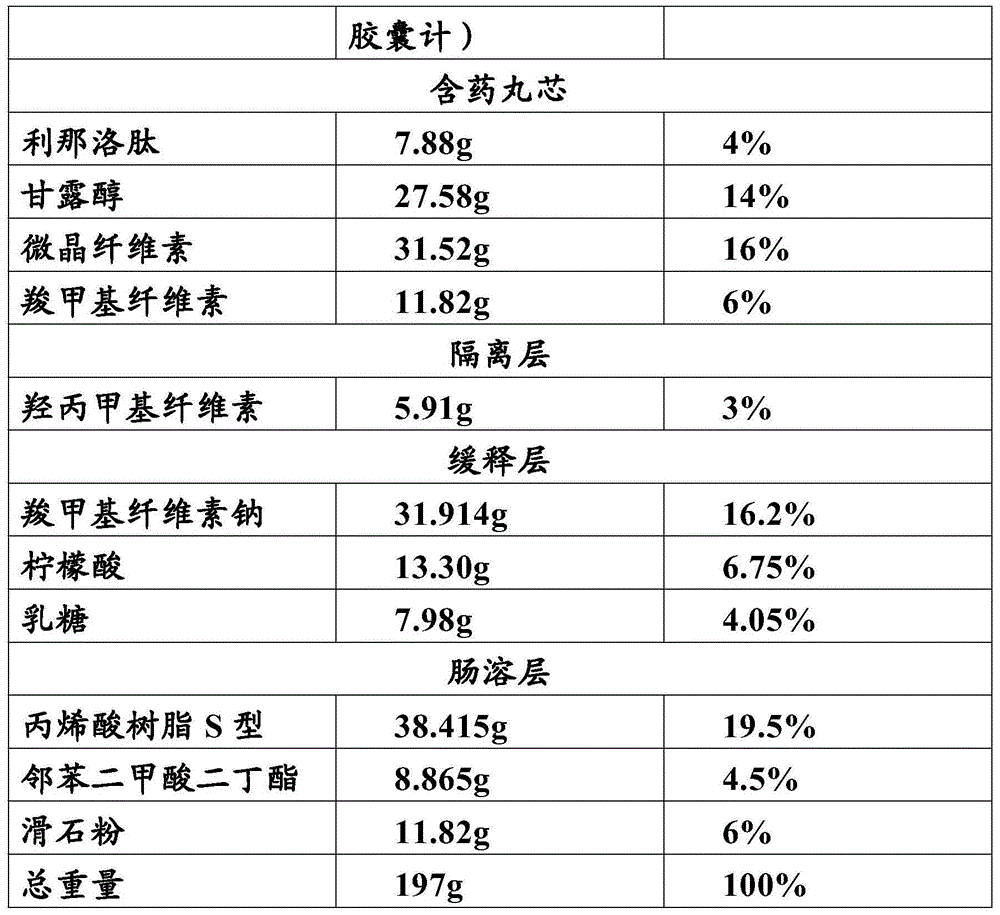
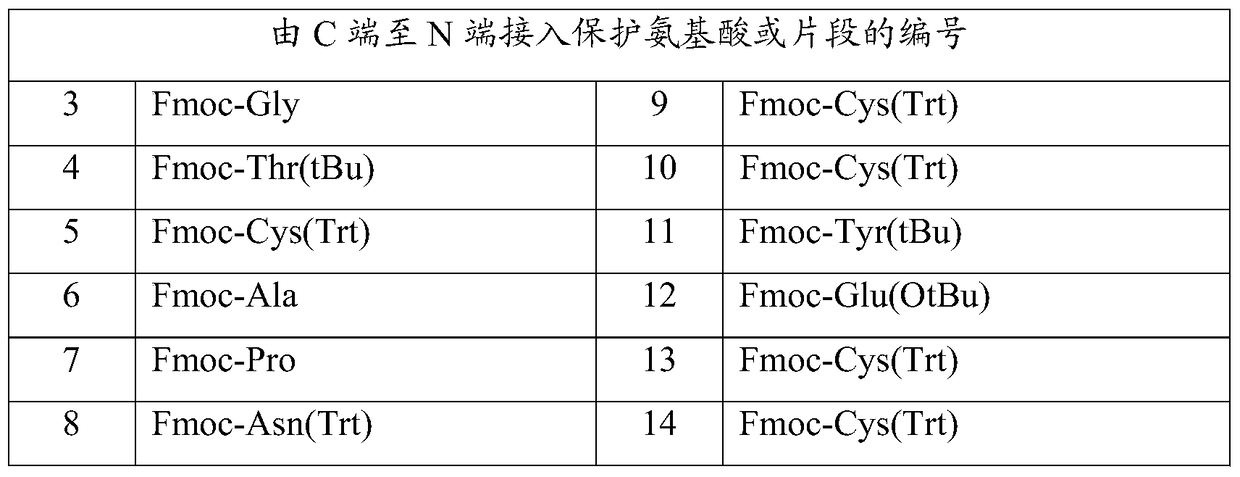
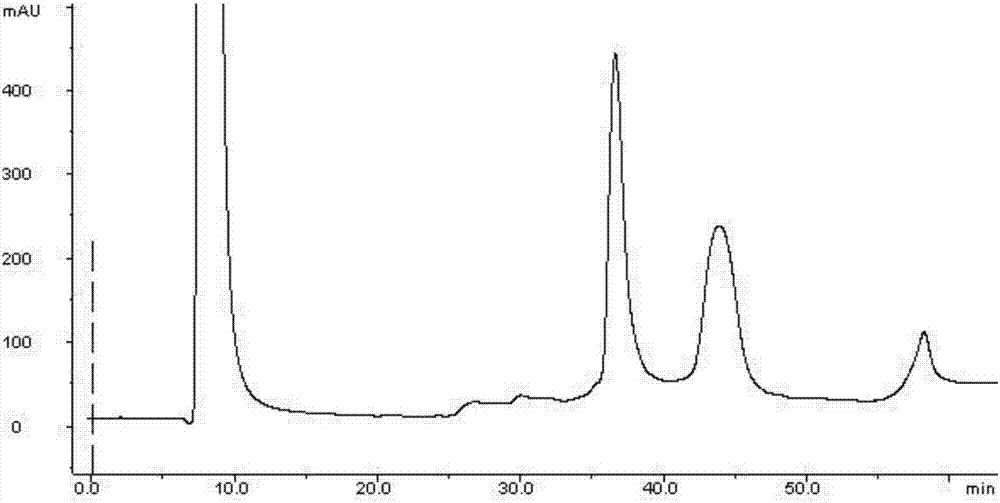
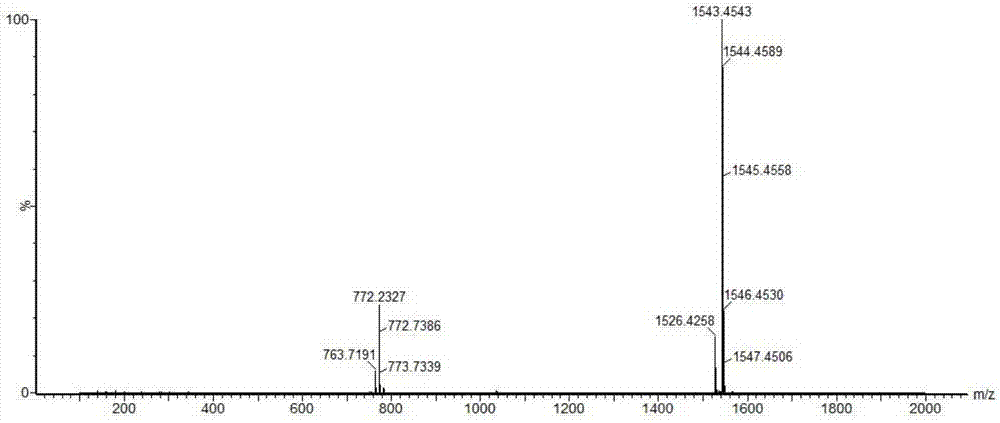
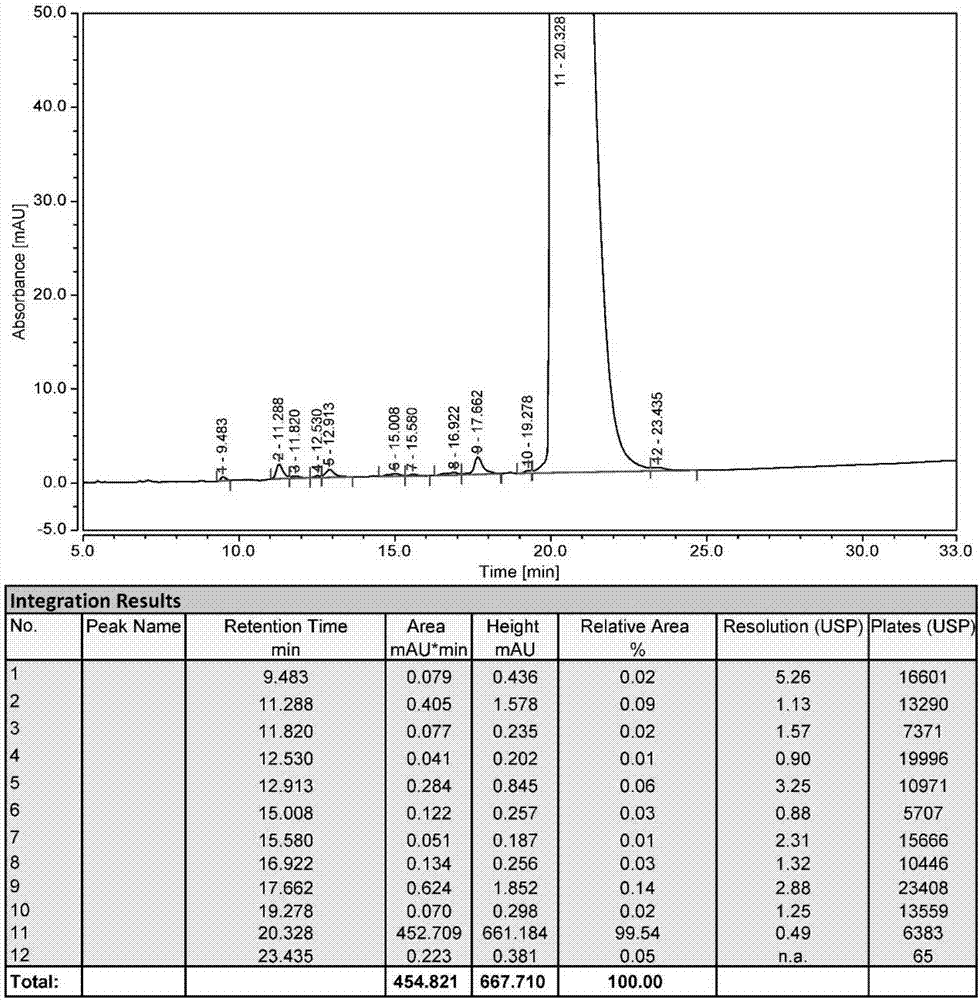
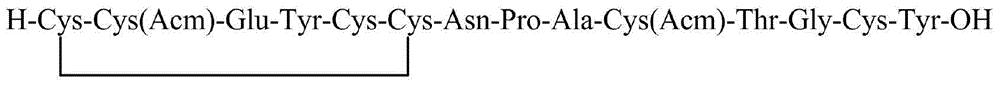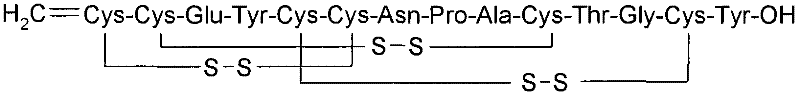Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
62 results about "Linaclotide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
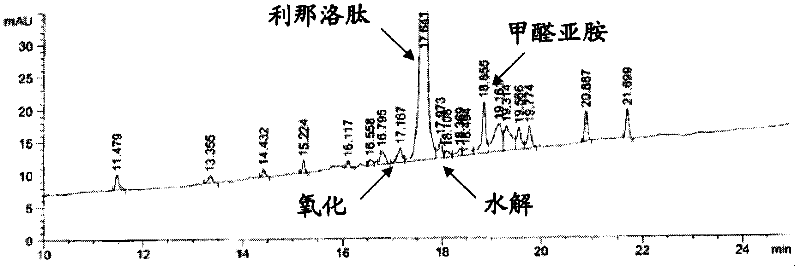
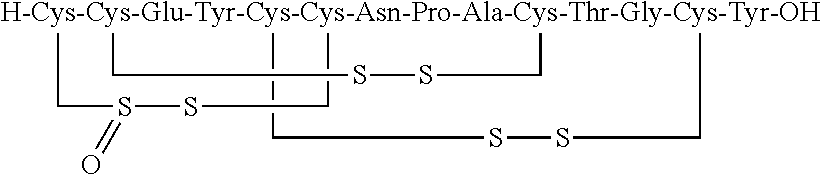
Linaclotide (marketed under the trade name Linzess in the US and Mexico, and as Constella elsewhere) is a drug used to treat irritable bowel syndrome with constipation and chronic constipation with no known cause. It has a black box warning about the risk of serious dehydration in children in the US; the most common adverse effects in others are gastrointestinal.
Linaclotide synthesis method
ActiveCN102875655AAvoid impuritiesHigh purityPeptide preparation methodsBulk chemical productionOxidized GlutathioneSide chain
The invention relates to the field of pharmaceutical synthesis and discloses a linaclotide synthesis method. The linaclotide synthesis method includes: performing solid-phase synthesis to obtain linaclotide resin with an N terminal, a Thr side chain, a Cys side chain, an Asn side chain, a Tyr side chain and a Glu side chain of an amino acid sequence shown in SEQ ID NO:1 coupled with protecting groups and with a C terminal coupled with a resin solid-phase carrier, cracking to remove the protecting groups and the resin solid-phase carrier prior to carrying out oxidizing reaction by the aid of a GSH (glutathione) / GSSH (oxidized glutathione) oxidization system to obtain a crude linaclotide product, and purifying the crude linaclotide product so that linaclotide is obtained. By the method, an Mmt protecting group is used for protecting a cysteine side chain, crude linear linaclotide peptide is synthesized by a one-by-one coupling mode, and the linaclotide is obtained by oxidization by the aid of the GSH / GSSH oxidization system. Compared with existing methods, the linaclotide synthesis method has the advantages that purity of the crude linear peptide is improved, the oxidization step can be performed without purification, and purity and yield of the crude linaclotide product are remarkably improved.
Owner:HYBIO PHARMA
Method for preparing linaclotide
InactiveCN103626849AEasy to operatePost-processing is simplePeptide preparation methodsBulk chemical productionDisulfide bondPeptide
The invention belongs to the technical field of medicine synthesis, and discloses a method for synthesizing linaclotide by three pairs of total selectively formed disulfide bonds. By using the process, formation of three pairs of disulfide bonds in linaclotide can be sequentially completed in the same solution system, so that the operation method of the process is simple, the yield of final fine peptide of linaclotide can be greatly increased, and large-scale production can be realized.
Owner:HYBIO PHARMA
Preparation method for linaclotide
ActiveCN104231051AAvoid toxicityEasy to purifyPeptide preparation methodsBulk chemical productionPeptide sequenceDisulfide bond
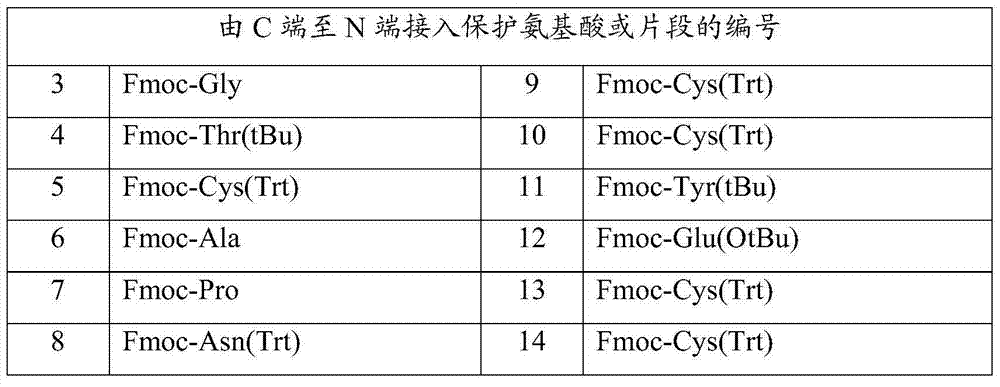
Belonging to the technical field of pharmaceutical synthesis, the invention discloses a preparation method for linaclotide. During synthesis of linear peptides, the carrier resin adopts Wang Resin, which has stable nature relative to chlorine resin and makes peptide difficult to fall off. Meanwhile, during synthesis of linear peptides, five of six cysteines in a peptide sequence adopt relatively cheap Fmoc-Cys(Trt)-OH as the reaction raw material, thus greatly reducing the synthesis cost and being beneficial to industrial production. In addition, during formation of three disulfide bonds, the one-step oxidation method is employed to oxidize crude peptide directly so as to form correctly paired three disulfide bonds. The method has the advantages of simple and practical operation, high yield and low cost, and is in favor of industrial production. The linaclotide prepared by the method provided by the invention can have a linear crude peptide weight yield up to 103.9%, purity up to 82.9%, refined peptide purity stabilized at 99.12-99.30%, and a total yield up to 32.15%.
Owner:HYBIO PHARMA
Linaclotide solid-phase synthesis method
InactiveCN104974229AGood effectPrecise positioningPeptide preparation methodsBulk chemical productionFreeze-dryingSolid-phase synthesis
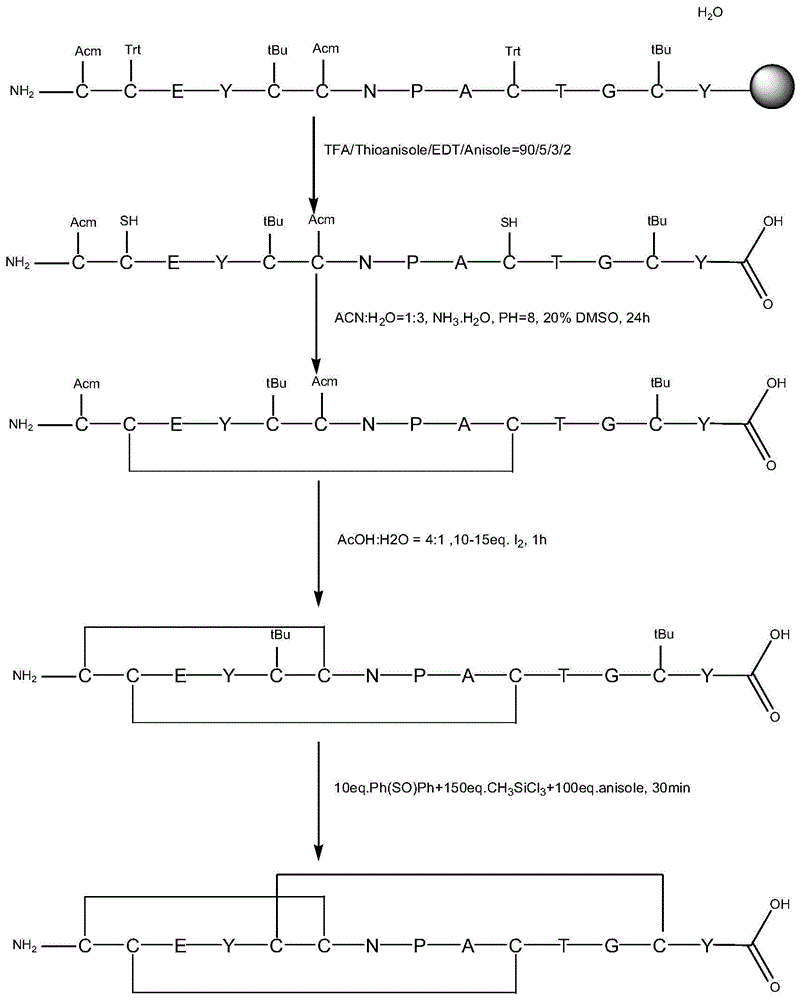
The invention discloses a linaclotide solid-phase synthesis method, and belongs to the biochemical technical field. The method includes the following steps: (1) preparation of linaclotide resin; (2) cutting the linaclotide linear peptide resin obtained in the step (1), to obtain a protection group linear peptide containing Cys(Acm) and Cys(tBu); (3) oxidizing to form a first disulfide bond, to obtain a monodisulfide cyclopeptide; (4) removing an Acm protection group in the monodisulfide cyclopeptide, to obtain a dual disulfide cyclopeptide; (5) removing a tBu protection group of the dual disulfide cyclopeptide, to obtain a trisdisulfide cyclopeptide; and (6) purifying the trisdisulfide cyclopeptide by HPLC, and freeze-drying to obtain linaclotide. The process has the characteristics of simple reaction operation, easy post-processing, low cost, high yield, and considerable economic and practical values, and besides, has wide application prospect in the polypeptide drug design and synthesis field.
Owner:NANTONG SHIMEIKANG PHARMA CHEM
Method for improving stability of polypeptide active pharmaceutical ingredients
ActiveCN106188218AChange particle shapeChange moistureOxytocins/vasopressinsThymosin peptidesOxytocinBivalirudin
The invention discloses a method for improving the stability of polypeptide active pharmaceutical ingredients. The polypeptide active pharmaceutical ingredients comprise but are not limited to bivalirudin, octreotide acetate, lanreotide acetate, eptifibatide or cetrorelix acetate, ganirelix acetate, degarelix, liraglutide, oxytocin, thymosin alpha1, leuprolide acetate, goserelin acetate, terlipressin or linaclotide. The method comprises the steps that after polypeptide drugs are salified, a polypeptide solution containing compensation ions is obtained, and a target polypeptide product is prepared through an ultralow temperature vacuum freeze-drying method. According to the method for improving the stability of the polypeptide active pharmaceutical ingredients, the problem that the polypeptide active pharmaceutical ingredients are prone to degradation after being placed for a long time is solved, the uniformity of the product is improved, and the drug risk is reduced.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Preparation method for linaclotide
InactiveCN104628826AGuaranteed stabilitySuitable for oxidation capacityPeptide preparation methodsBulk chemical productionSide chainWang resin
The invention provides a preparation method for linaclotide. The method includes: utilizing a standard Fmoc technology to connect a side chain protected amino acid with Wang resin, adding a condensing agent HBTU and alkali DIPEA, carrying out condensation reaction in a DMF solvent, employing a DMF solution containing 20% hexahydropiperidine to perform de-Fmoc protection, then conducting cutting from the solid phase resin, adding elemental iodine into a sodium phosphate buffer solution to carry out Cysteine oxidation, thus obtaining linaclotide. The preparation method for linaclotide provided by the invention is the technology for highyield synthesis of linaclotide, simplifies the cyclization process and enhances the cyclization yield, and the final product yield reaches 30%-60%. The preparation method has the advantages of simplicity, mild reaction conditions, high yield, and high product purity, is a feasible preparation method for industrialization of linaclotide, and provides good prospects for industrial production.
Owner:EAST CHINA UNIV OF SCI & TECH
Treatments of gastrointestinal disorders
InactiveUS20140005128A1Stable low-dose pharmaceutical compositionStable pediatric pharmaceutical compositionDigestive systemCyclic peptide ingredientsDiseaseMedicine
The present invention relates to stable pharmaceutical compositions comprising linaclotide or pharmaceutically acceptable salts thereof, as well as to various methods and processes for the preparation and use of the compositions.
Owner:IRONWOOD PHARMA
Method for preparing linaclotide
InactiveCN105017387AAvoid it happening againHigh purityPeptide preparation methodsBulk chemical productionDisulfide bondImpurity
The invention relates to the field of polypeptide synthesis, and in particular to a method for preparing linaclotide. The method adopts a fragment method to perform synthesis of peptide chains of linaclotide, can be used for forming three pairs of disulfide bonds by three steps in a completely selective manner, and specifically comprises the following steps: (a) synthesizing linear peptide of a segment I; (b) forming the first pair of disulfide bonds to obtain oxidized peptide of the segment I; (c) synthesizing peptide resin of a segment II; (d) synthesizing linaclotide crude peptide containing a pair of disulfide bonds; (e) synthesizing the second pair of the disulfide bonds; and (f) synthesizing the third pair of the disulfide bonds. The method adopts a process for forming the three pairs of the disulfide bonds by three steps in a completely selective manner to prepare linaclotide, isomer impurities with mismatched disulfide bonds can be avoided, the difficulty of a purification process is reduced, and meanwhile, the segment method reduces the difficulty for forming the first pair of the disulfide bonds, so that the purity and yield of the finally obtained crude peptide are relatively high, the operation process is simple and convenient, and the method is suitable for large-scale production.
Owner:JINAN KANGHE MEDICAL TECH
Method for synthesizing linaclotide
ActiveCN104844693AHigh purityIncrease productivityPeptide preparation methodsBulk chemical productionAcid hydrolysisSide chain
The invention relates to the field of medicinal synthesis and discloses a method for synthesizing linaclotide. The method comprises the following steps: synthesizing a linaclotide resin coupled with protecting groups on side chains Thr, Cys, Asn, Tyr and Glu of an amino acid sequence shown as SEQ ID NO:1 and coupled with a resin carrier at the terminal C; performing acid hydrolysis to remove the protecting groups and resin carrier, obtaining a linaclotide linear crude peptide, cyclizing the linear crude peptide by adopting a cysteine / DMSO buffer solution, obtaining a crude linaclotide product, purifying and converting the crude product into acetate, thereby obtaining the finished product. According to the method disclosed by the invention, starting from a cyclizing system, the linaclotide synthesis method is improved, the total yield of linaclotide is improved by a stage by virtue of simple and rapid process steps, and the purity can reach a high level. Meanwhile, the later-stage cyclizing time is greatly shortened, and compared with the prior art, the method has high practical value and application prospects.
Owner:CHENGDU SHENGNUO BIOTEC CO LTD
Method for synthesizing and purifying linaclotide
InactiveCN106167514AReduce volumeHigh purityPeptide preparation methodsBulk chemical productionSide chainCombinatorial chemistry
The invention relates to the field of medicine synthesis, in particular to a method for synthesizing linaclotide. An amino acid sequence as shown in SEQ ID NO:1 by solid-phase synthesis is adopted, protecting groups are coupled to side chains of Thr, Cys, Asn, Tyr and Glu, and linaclotide resin of a resin carrier is coupled to a C-terminal; linaclotide resin pyrolysis is performed to remove all the protecting groups and the resin carrier, uncyclized linaclotide linear crude peptides are obtained, a guanidine hydrochloride oxidation system of Cystine / Cysteine performs oxidation reaction on the linaclotide linear crude peptides, three disulfide bonds are formed from an N-terminal to the C-terminal, crude linaclotide is obtained, and linaclotide is obtained after purification. The method starts from the aspect of the oxidation system, the method for producing linaclotide is improved, the total yield of linaclotide is improved through simple, convenient and rapid process steps, the total yield is 38% or above, the product purity is stabilized to be 99% or above, and single purities are controlled below 0.1%.
Owner:ZHEJIANG PEPTITES BIOTECH CO LTD
Orally Disintegrating Compositions of Linaclotide
The present invention relates to orally disintegrating or dissolving pharmaceutical compositions comprising linaclotide or pharmaceutically acceptable salts thereof, as well to various methods and processes for the preparation and use of the compositions.
Owner:IRONWOOD PHARMA +1
Synthetic method of linaclotide
ActiveCN106892968ALow costEasy to operatePeptide preparation methodsOxidoreductasesCouplingDrugs synthesis
The invention belongs to the technical field of drug synthesis and discloses a method for synthesizing linaclotide from three pairs of completely selectively formed disulfide bonds. The method comprises the followings steps: (1) synthesizing linaclotide precursor resin in a solid phase manner; (2) carrying out solid-phase oxidation, so as to form a first pair of disulfide bonds; (3) carrying out liquid-phase oxidation, so as to form a second pair of disulfide bonds; and (4) removing methyl from methyl-protected cysteine, and simultaneously carrying out oxidative coupling on the third pair of disulfide bonds, so as to obtain linaclotide. The method has the beneficial effects that reaction conditions are mild, the cost is low, the yield is high, the product purity is high, and the process is simple, stable and suitable for large-scale production.
Owner:HYBIO PHARMA
Stable solid formulation of a GC-C receptor agonist polypeptide suitable for oral administration
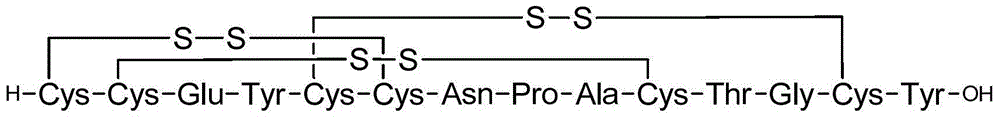
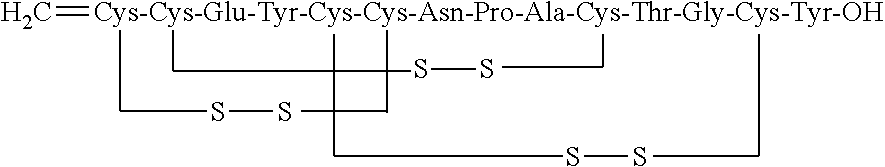
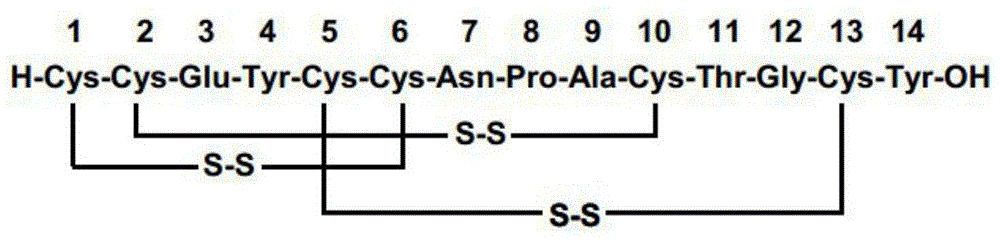
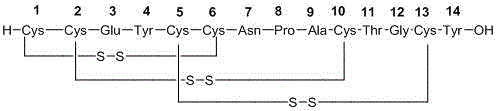
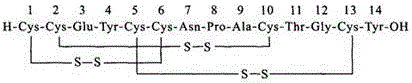
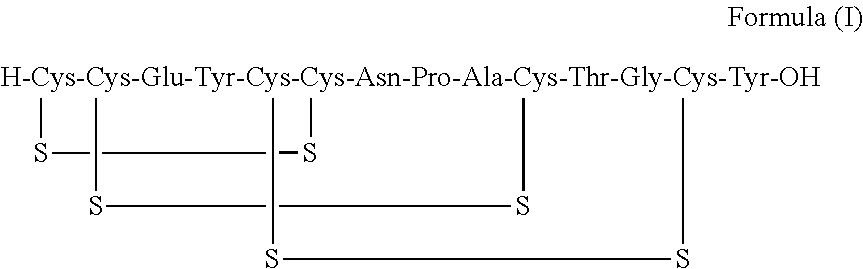
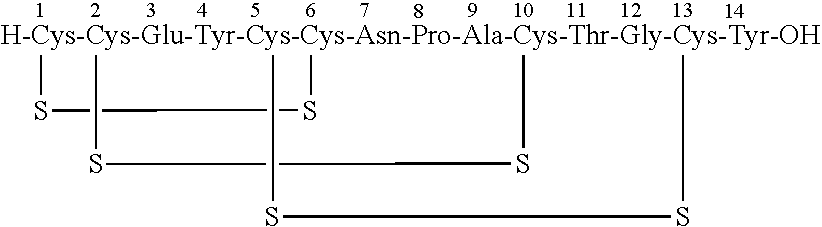
Solid, stable formulations of linaclotide suitable for oral administration are described herein as are methods for preparing such formulations. The formulations described herein contain a polypeptide consisting of the amino acid sequence Cys Cys GIu Tyr Cys Cys Asn Pro Ala Cys Thr GIy Cys Tyr ("linaclotide") or a pharmaceutically acceptable salt thereof. The linaclotide formulations described herein are stable and have a sufficient shelf life for manufacturing, storing and distributing the drug.
Owner:硬木药品公司
Method for preparing linaclotide
InactiveCN106008674AHigh purityAvoid mismatchPeptide preparation methodsBulk chemical productionSynthesis methodsFreeze-drying
The invention belongs to the technical field of biochemical polypeptide synthesis and relates to a method for preparing linaclotide. The method mainly solves the problem that the existing synthesis method has complicated steps of synthesis, high raw material cost, long synthesis period, low yield and more impurity and is unsuitable for industrial production. The method comprises that (1) Fmoc-Tyr(tBu)-OH and a carrier resin undergo a reaction to produce Fmoc-Tyr(tBu)-resin, (2) the Fmoc-Tyr(tBu)-resin and other amino acids with Fmoc protecting groups are coupled one by one through an activator so that linaclotide linear peptide resin is obtained, (3) the linaclotide resin is subjected to deprotection and cracking agent-based cracking to form linaclotide linear peptide, (4) three disulfide bonds of the linaclotide linear peptide is cyclized in an ammonium hydroxide / DMSO system to form linaclotide crude peptide, and (5) the linaclotide crude peptide is purified in an alkaline buffer solution and then is subjected to freeze-drying so that pure linaclotide is obtained.
Owner:CS BIO SHANGHAI
Linaclotide synthesis method
ActiveCN102875655BHigh purityHigh yieldPeptide preparation methodsBulk chemical productionOxidized GlutathioneSide chain
The invention relates to the field of pharmaceutical synthesis and discloses a linaclotide synthesis method. The linaclotide synthesis method includes: performing solid-phase synthesis to obtain linaclotide resin with an N terminal, a Thr side chain, a Cys side chain, an Asn side chain, a Tyr side chain and a Glu side chain of an amino acid sequence shown in SEQ ID NO:1 coupled with protecting groups and with a C terminal coupled with a resin solid-phase carrier, cracking to remove the protecting groups and the resin solid-phase carrier prior to carrying out oxidizing reaction by the aid of a GSH (glutathione) / GSSH (oxidized glutathione) oxidization system to obtain a crude linaclotide product, and purifying the crude linaclotide product so that linaclotide is obtained. By the method, an Mmt protecting group is used for protecting a cysteine side chain, crude linear linaclotide peptide is synthesized by a one-by-one coupling mode, and the linaclotide is obtained by oxidization by the aid of the GSH / GSSH oxidization system. Compared with existing methods, the linaclotide synthesis method has the advantages that purity of the crude linear peptide is improved, the oxidization step can be performed without purification, and purity and yield of the crude linaclotide product are remarkably improved.
Owner:HYBIO PHARMA
Method for synthesizing linaclotide
InactiveCN105884864APurity unchangedThe cyclization reaction is completePeptide preparation methodsHydroquinone CompoundAmmonium carbonate
The invention discloses a method for synthesizing linaclotide, and relates to the field of medicine synthesis. The method mainly comprises the following steps: improving a linear cyclizing system of linaclotide, cyclizing crude linear peptide by using a ammonium carbonate / DMSO / aqueous solution together with hydroquinone or TCEP so as to obtain a crude linaclotide product, and purifying the crude linaclotide product, thereby obtaining a finished product. By improving the cyclizing system, the yield of linaclotide is increased, the cyclizing time is shortened, the cost of raw materials is lowered, and the method is applicable to industrial production.
Owner:JIANGSU SKYRUN PHARMA CO LTD
Orally Disintegrating Compositions of Linaclotide
The present invention relates to orally disintegrating or dissolving pharmaceutical compositions comprising linaclotide or pharmaceutically acceptable salts thereof, as well as to various methods and processes for the preparation and use of the compositions.
Owner:FOREST LAB HLDG LTD +1
Low-cost method for preparing high-purity linaclotide
The invention discloses a low-cost method for preparing high-purity linaclotide. According to the technical scheme, the low-cost method comprises the following steps: 1) synthesizing linear linaclotide by utilizing resin carriers including CTC resin or Wang resin and the like through a Fmoc method or obtaining the linear linaclotide by utilizing a segment method or a liquid-phase synthesis method;2) obtaining a linaclotide crude product which is accurately matched by adopting a one-step oxidization method; 3) obtaining the high-purity linaclotide through purifying by adopting high performanceliquid chromatography. The method disclosed by the invention is started from the aspects including an oxidization system and the like, and a method for accurately matching a linaclotide disulfide bond is improved; the method is simple and stable in technology and low in cost, and the linaclotide crude product can be obtained through one-step cyclization; the purity of a purified product can be greater than 99 percent and the content of a single impurity can be controlled to be 0.1 percent or lower. Compared with the prior art, the method has better economic benefits and application prospect.
Owner:杭州肽佳生物科技有限公司
A synthetic method for linaclotide
ActiveCN109311941AAvoid repeated foldingAvoid it happening againPeptide preparation methodsBulk chemical productionN-BromosuccinimideAmino acid side chain
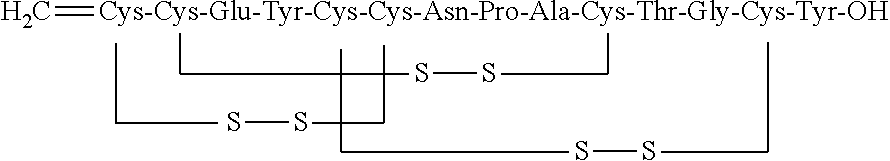
The invention relates to the field of pharmaceutical synthesis, and discloses a synthetic method for linaclotide. The method uses a solid phase one-step cyclization method to prepare linaclotide, andthe linaclotide linear peptide resin is directly cyclized by a N-X-substituted succinimide solution oxidation system without cleavage to obtain linaclotide resin, the resin is cleaved, purified and lyophilized to give linaclotide. The N-X-substitured succinimide is one of N-chlorosuccinimide, N-bromosuccinimide, N-iodosuccinimide, and N-hydroxy thiosuccinimide. The method has the following advantages that: 1) solid phase cyclization is adopted, firstly, the pseudo-dilution effect is achieved, repeated folding of the peptide chain is avoided, and the cyclization reaction can be carried out at ahigher concentration, which can greatly improve the production efficiency; secondly, the linear peptide resin is not cleaved before cyclization, avoiding the production of a large amount of impurities and improving the efficiency of linaclotide cyclization; 2) one-step cyclization using N-X-substituted succinimide can avoid multi-step purification of the intermediates, reduce the composition of the intermediate purification step, and improve the total yield of linaclotide; and 3) a specific amino acid side chain protecting group is adopted, thus positioning a pair of disulfide bonds in the cyclization process, reducing the formation of mismatch by-products, improving the purity of linaclotide, greatly improving production efficiency, and reducing the manufacturing cost.
Owner:SHENZHEN JYMED TECH
A process for the preparation of linaclotide
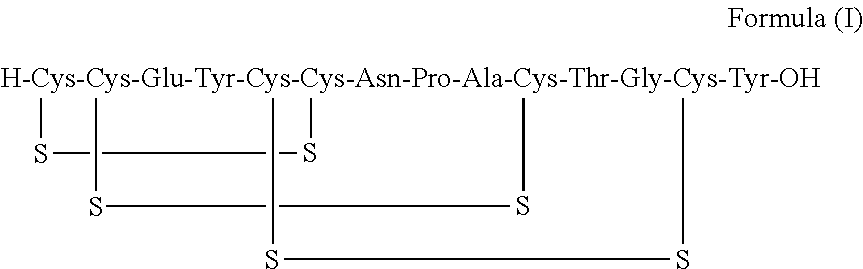
ActiveUS20170240599A1Simple, industrially applicable and robustHigh yieldDigestive systemSolid sorbent liquid separationOxidizing agentPhotochemistry
The present invention relates to a process for the preparation of Linaclotide by oxidizing linear Linaclotide of formula (II) using combination of air and oxidizing agent followed by purification using RP-HPLC.
Owner:AURO PEPTIDES
Method for preparing Linaclotide
PendingCN106831950AProcess reaction route is shortRaw materials are cheap and easy to getPeptidesBulk chemical productionEnergy consumptionDisulfide bond
The invention discloses a method for preparing Linaclotide. The method includes the step: performing oxidation reaction for Linaclotide intermediates containing two pairs of disulfide bonds to obtain the Linaclotide. The Linaclotide intermediates containing the two pairs of disulfide bonds are disulfide bonds with second Cys (cysteine) and tenth Cys and disulfide bonds with fifth Cys (cysteine) and thirteenth Cys. According to the method, a reaction route is short, raw materials are low in cost and easy to obtain, reaction conditions are mild, energy consumption is reduced, and production cost is reduced. Compared with a route published by a predecessor, the route is high in reaction selectivity and conversion rate, waste of the raw materials is decreased, and the route has high economy. According to the method, optimal reaction time is 30min, the purity of prepared products can reach 85%, and discharge of waste gas, waste water and solid waste is decreased, and the method is green and safe.
Owner:NANJING UNIV OF TECH
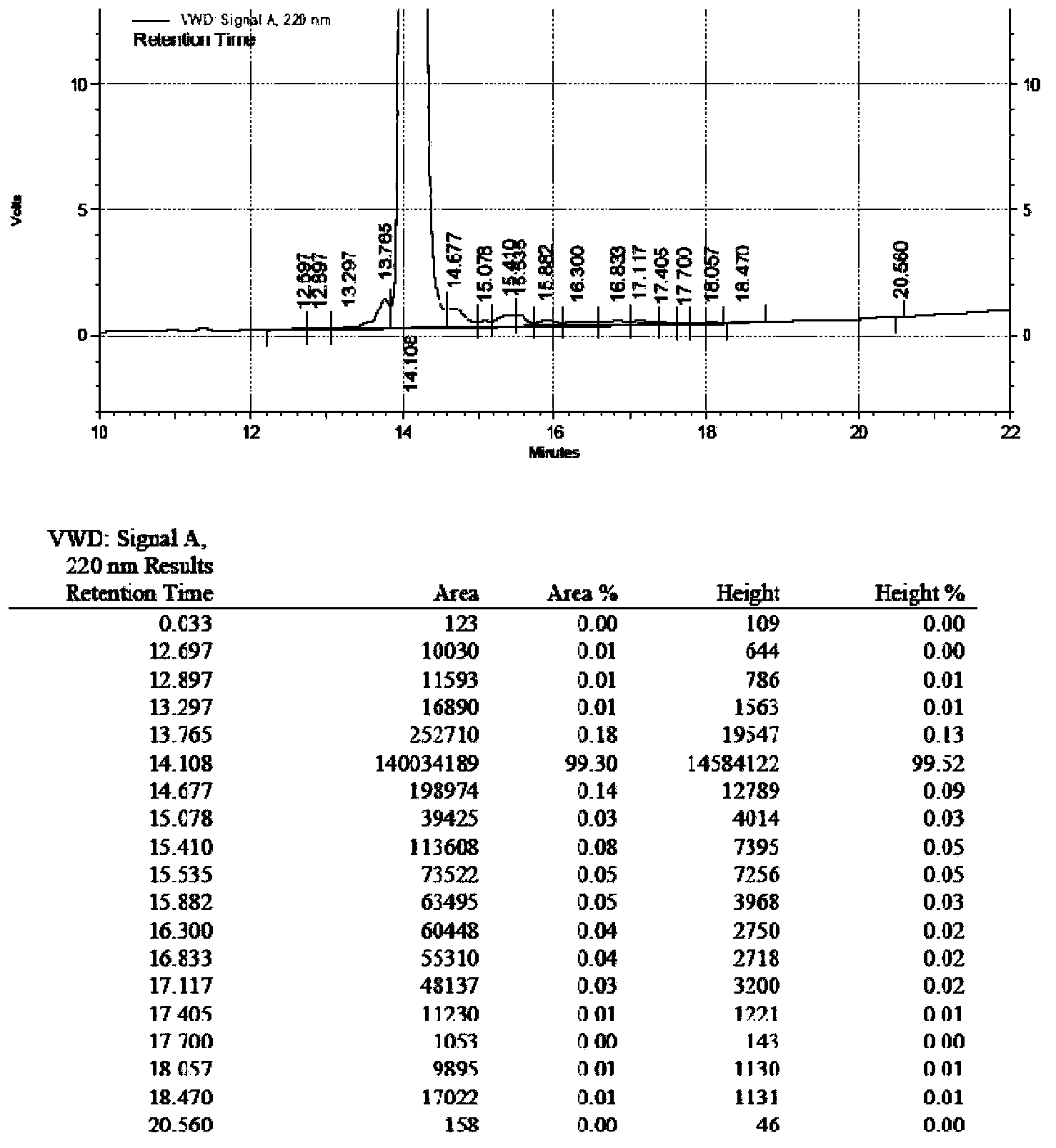
Purification method of linaclotide
ActiveCN105153284AHigh purityHigh yieldPeptide preparation methodsIsocratic elutionTrifluoroacetic acid
The invention relates to a purification method of linaclotide. The purification method is characterized by comprising following steps: (1), pH of a solution of a linaclotide crude product is regulated by trifluoroacetic acid to be 3.5 plus or minus 0.2; (2), gradients are set according to volume fraction, 50% of a flowing phase B is used for washing a reverse phase filler column for 10 min, and 5% of the flowing phase B is adopted to perform isocratic elution balancing for 10 min; (3), the solution in the step (1) is loaded to reverse phase filler; (4), gradients are set according to volume fraction, the initial-state flowing phase B of the elution gradients is 5% and kept for 5 min, the proportion of the flowing phase B is increased to 16% in one minute, the proportion of the flowing phase B is increased to 26% in 80 minutes, and elution fractions are collected; (5), the elution fractions are transformed into salt and subjected to concentration and freeze-drying, and fine linaclotide is obtained.
Owner:HANGZHOU HEZE PHARMA TECH
Stable solid formulations of guanylate cyclase-c receptor agonist polypeptides suitable for oral administration
Solid, stable formulations of linaclotide suitable for oral administration are described herein as are methods for preparing such formulations. The formulations described herein contain a polypeptide consisting of the amino acid sequence Cys Cys Glu Tyr Cys Cys Asn Pro Ala Cys Thr Gly Cys Tyr (“linaclotide”; SEQ ID NO:1) or a pharmaceutically acceptable salt thereof. The linaclotide formulations described herein are stable and have a sufficient shelf life for manufacturing, storing and distributing the drug.
Owner:硬木药品公司
Delayed Release Compositions of Linaclotide
The present invention relates to delayed release pharmaceutical compositions comprising linaclotide or pharmaceutically acceptable salts thereof, as well as to various methods and processes for the preparation and use of the compositions.
Owner:FOREST LAB HLDG LTD +1
Process for the preparation of guanylate cyclase 2c agonist
InactiveUS20190055278A1Easy to handleEconomical and simplePeptide preparation methodsAgonistOrganic chemistry
The present invention relates to an improved process for the preparation of Linaclotide of Formula I. The process disclosed in the present invention is simple, economical and eco-friendly with reduced reaction times.
Owner:CIPLA LTD
Linaclotide enteric controlled-release pellet capsule preparation and preparing method and application thereof
InactiveCN105412904AReduce stimulationDoes not affect burstPeptide/protein ingredientsDigestive systemControl releaseIsolation layer
The invention relates to a linaclotide enteric controlled-release pellet capsule preparation. The linaclotide enteric controlled-release pellet capsule preparation is composed of linaclotide enteric controlled-release pellets and a hollow capsule, wherein the linaclotide enteric controlled-release pellets comprise pellet cores, isolation layers, controlled-release layers and enteric layers. The invention further relates to a method for preparing the linaclotide enteric controlled-release pellet capsule preparation and application of the linaclotide enteric controlled-release pellet capsule preparation in preparing medicine for treating a constipation type irritable bowel syndrome.
Owner:HYBIO PHARMA
Method for preparing linaclotide through solid-liquid combination
ActiveCN111499693AHigh purityHigh yieldPeptide preparation methodsBulk chemical productionDipeptideCombinatorial chemistry
The invention relates to the field of polypeptide synthesis, in particular to a method for preparing linaclotide through solid-liquid combination, thereby well avoiding the generation of impurity peptides, improving the purity of crude peptides and reducing the production cost. According to the preparation method disclosed by the invention, the linaclotide is synthesized by adopting dipeptide monomers Fmoc-Gly-Cys (tButhio)-OH and Fmoc-Pro-Ala-OH for the first time in a solid phase manner; meanwhile, a strategy of protecting cysteine sulfydryl by stable tButhio under a TFA cracking condition is introduced, so that the purity of the linear peptide can be effectively improved, the yield of a final product is improved, the amplification of a synthesis scale is facilitated, and the productioncost is reduced.
Owner:汉肽生物医药集团有限公司
Method for synthesizing linaclotide by solid-liquid phase combination
InactiveCN107936094AReduce generationHigh synthesis efficiencyPeptide preparation methodsBulk chemical productionSynthesis methodsPeptide fragment
The invention relates to a method for synthesizing linaclotide which is a product in the medicine field. The method adopts a solid-liquid phase combination process to synthesize the linaclotide product. A 6+8 synthesizing mode is adopted, firstly six peptide fragments are synthesized, then the fragments are coupled to solid-phase resin, selective synthesis of three dithio-rings is completed on thesolid-phase resin through protecting groups of Cys in different positions, and then the dithio-rings are cut from the resin and are directly purified, so that the synthesizing efficiency and the synthesizing yield are greatly improved, the production of the three wastes (waste water, waste solid and waste gas) is greatly reduced, and furthermore, the production cost is greatly lowered.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
A method for synthesizing linaclotide
ActiveCN104844693BHigh purityIncrease productivityPeptide preparation methodsBulk chemical productionSynthesis methodsSide chain
The invention relates to the field of medicinal synthesis and discloses a method for synthesizing linaclotide. The method comprises the following steps: synthesizing a linaclotide resin coupled with protecting groups on side chains Thr, Cys, Asn, Tyr and Glu of an amino acid sequence shown as SEQ ID NO:1 and coupled with a resin carrier at the terminal C; performing acid hydrolysis to remove the protecting groups and resin carrier, obtaining a linaclotide linear crude peptide, cyclizing the linear crude peptide by adopting a cysteine / DMSO buffer solution, obtaining a crude linaclotide product, purifying and converting the crude product into acetate, thereby obtaining the finished product. According to the method disclosed by the invention, starting from a cyclizing system, the linaclotide synthesis method is improved, the total yield of linaclotide is improved by a stage by virtue of simple and rapid process steps, and the purity can reach a high level. Meanwhile, the later-stage cyclizing time is greatly shortened, and compared with the prior art, the method has high practical value and application prospects.
Owner:CHENGDU SHENGNUO BIOTEC CO LTD
Linaclotide purifying method
InactiveCN107266535ALong-term purificationStable purificationPeptide preparation methodsFreeze-dryingGradient elution
The invention mainly relates to a linaclotide purifying method and belongs to the technical field of bioseparation. According to the linaclotide purifying method, an ion exchange chromatography method and a high-performance liquid chromatography method are combined, an anion exchange column is adopted as a stationary phase, a Tris HCl buffering solution is adopted as a mobile phase A, a NaCl-contained Tris-HCl buffering solution is adopted as a mobile phase B, a gradient eluting method is adopted to treat a crude linaclotide solution, eluate is subjected to desalination and acetate changing by means of the high-performance liquid chromatography method, a C18 column is adopted as a separating medium, after a sample is loaded, gradient elution is performed by adopting an aqueous acetic acid solution as the mobile phase A and acetonitrile as the mobile phase B, and eluate is subjected to freeze-drying to obtain pure linaclotide. The linaclotide purifying method is simple to operate, low in separation cost, high in yield and suitable for large-scale linaclotide production; purity of the obtained pure linaclotide can reach to above 99%, so that the obtained pure linaclotide is low in impurity content and has favorable economic and practical values and an extensive application prospect.
Owner:NANJING UNIV OF TECH +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com