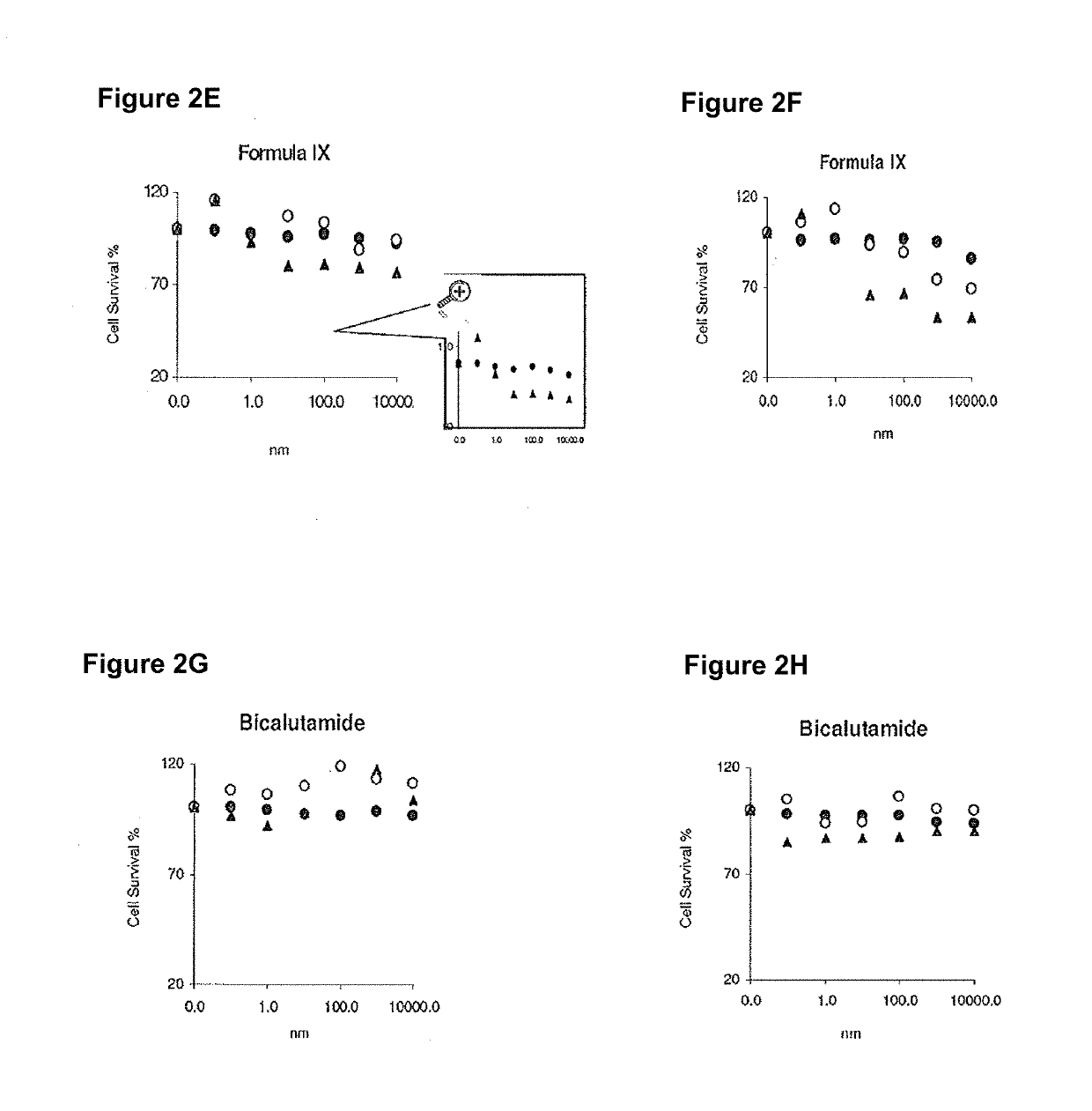
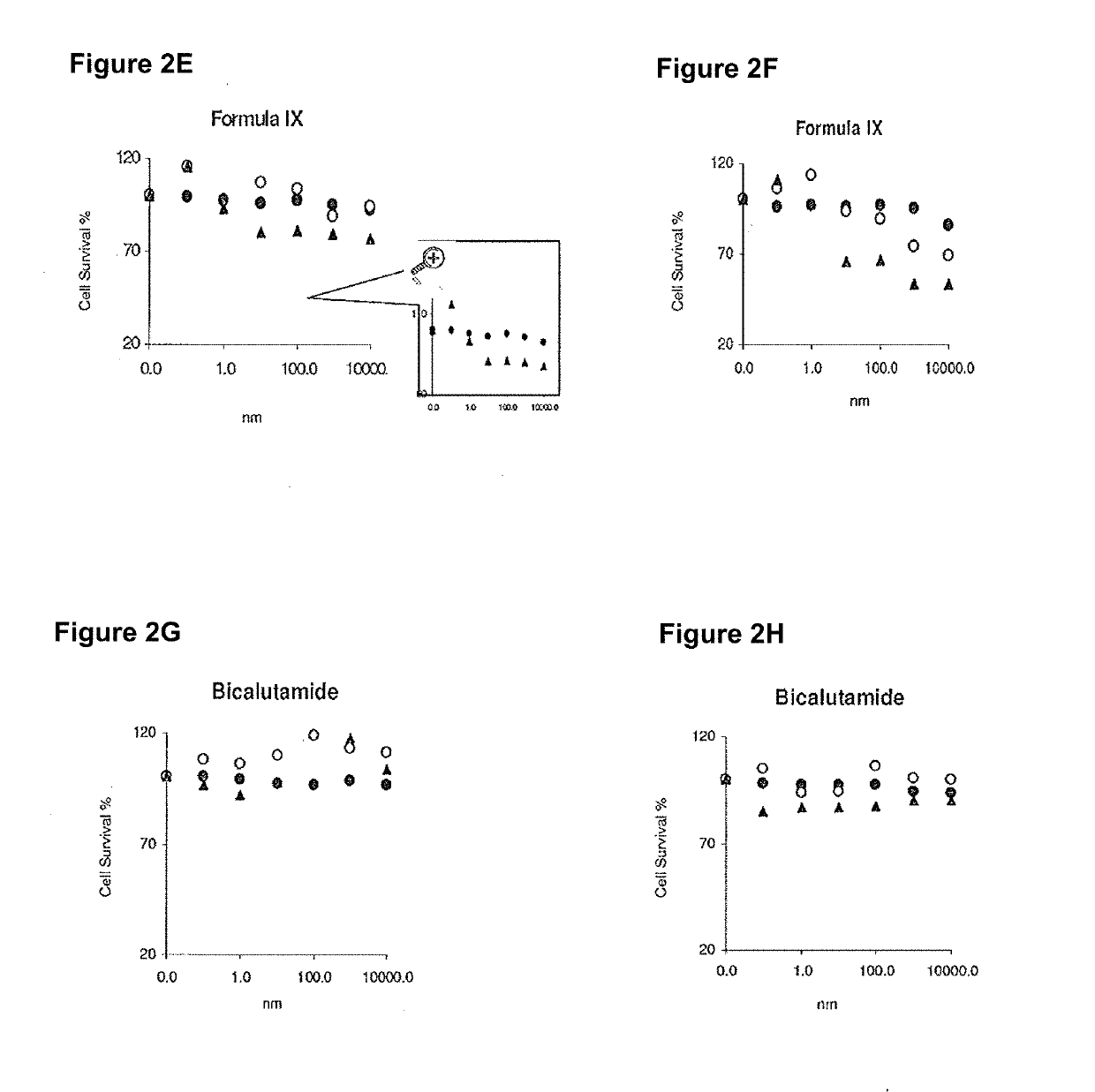
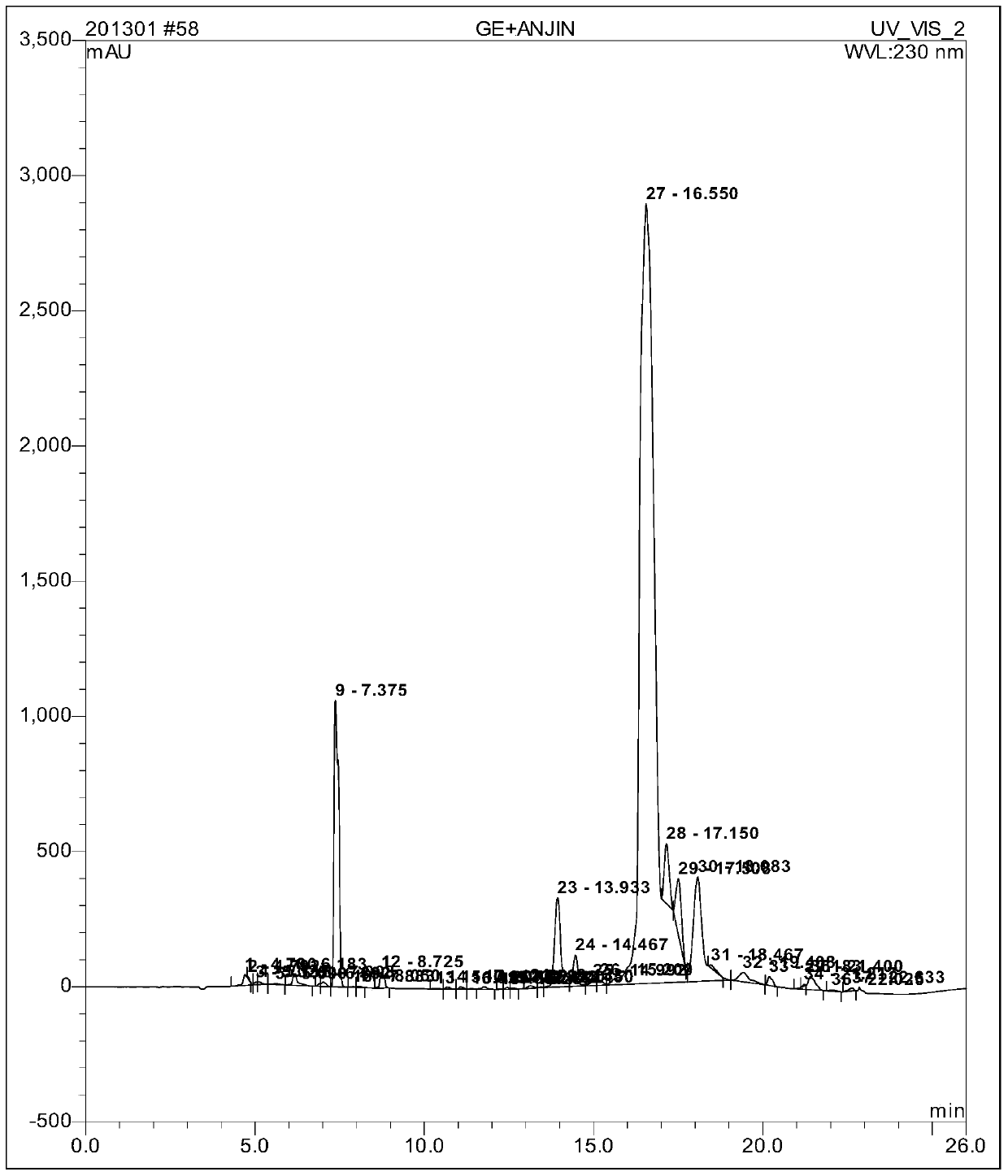
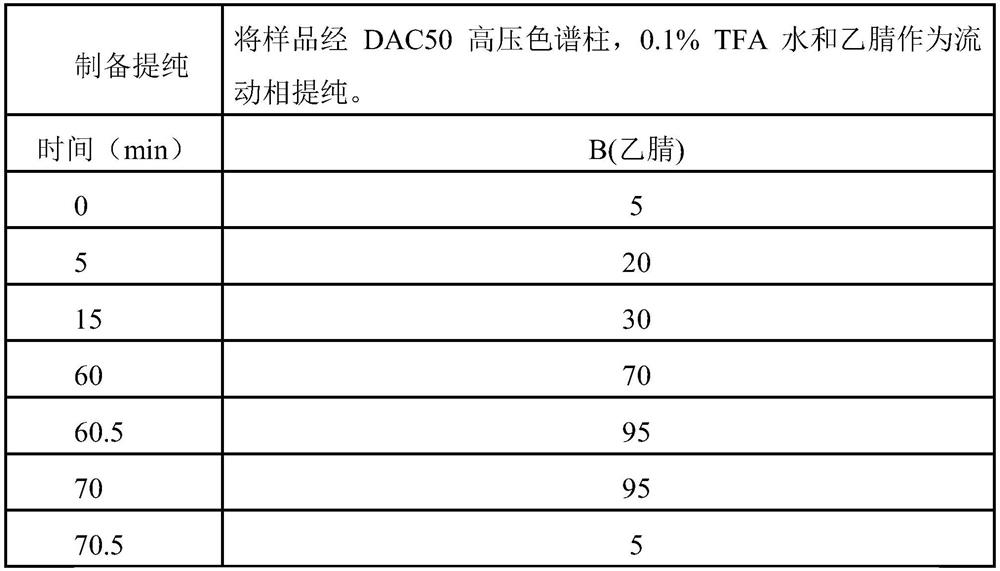
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
48 results about "Goserelin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Goserelin is used in men to treat prostate cancer. It is used in women to treat certain breast cancers or a certain uterus disorder (endometriosis). It is also used in women to thin the lining of the uterus (endometrium) in preparation for a procedure to treat abnormal uterine bleeding.
Goserelin release microsphere preparation, preparation method and detecting method thereof
InactiveCN101721370AImprove complianceMedication conveniencePowder deliveryPeptide/protein ingredientsMicrosphereMolecular materials
The invention discloses a Goserelin release microsphere preparation, wherein the Goserelin microsphere contains Goserelin or salt thereof which is 0.1% to 40% of the weight of the microsphere, high molecular material which is biodegradable and biocompatible, has the molecular weight ranged from 5000 to 100,000 daltons and is 60% to 99.9% of the weight of the microsphere, and other medical acceptable auxiliary materials which are 0% to 15% of the weight of the microsphere. The Goserelin or salt thereof is packed or embedded in the high molecular material. The size of the microsphere is 1 to 10 microns and the average size is 5 to 50 microns. The invention further discloses a preparation method and a detecting method for the Goserelin release microsphere preparation. The Goserelin release microsphere preparation of the invention can work for days, weeks, even months by just injecting for one time so the injection times is reduced, the compliance of patient is promoted, and the clinical use is convenient. Furthermore, the patient is convenient to take medicine.
Owner:RESEARCH INSTITUTE OF TSINGHUA UNIVERSITY IN SHENZHEN
Therapy of Prostate Cancer With Ctla-4 Antibodies and Hormonal Therapy
InactiveUS20080279865A1Peptide/protein ingredientsAntibody ingredientsHistrelinAntiendomysial antibodies
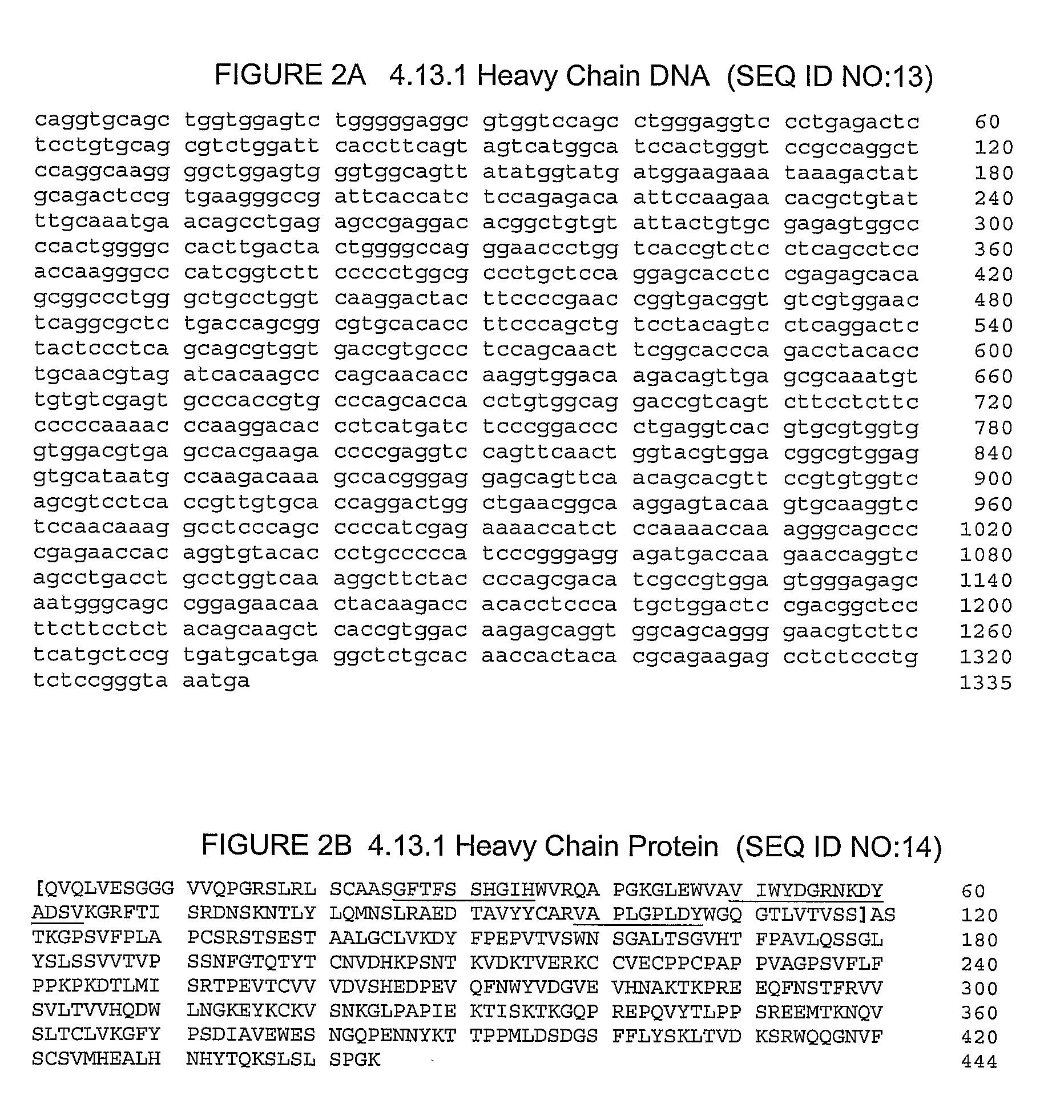
The invention relates to methods for treating prostate cancer comprising administration of an anti-CTLA4 antibody, or an antigen-binding portion thereof, particularly a human antibody to human CTLA4, e.g., antibody 3.1.1, 4.1.1, 4.8.1, 4.10.2, 4.13.1, 4.14.3, 6.1.1, ticilimumab (also known as 11.2.1), 11.6.1, 11.7.1, 12.3.1.1, 12.9.1.1, and ipilimumab (also known as MDX-010 and 10D1), in combination with hormonal therapy. Hormonal therapy agents include, inter alia, an anti-androgen (e.g., megestrol, cyproterone, flutamide, nilutamide, and bicalutamide), a GnRH antagonist (e.g., abarelix and histrelin), and a LH-RH agonist (e.g., leuprolide, goserelin, and buserelin). The invention relates to neoadjuvant therapy, adjuvant therapy, therapy for rising PSA, first-line therapy, second-line therapy, and third-line therapy of prostate cancer, whether localized or metastasized.
Owner:PFIZER PFIZER PRODS
Method for synthesizing goserelin
ActiveCN101759777AShort synthesis cycleHigh yieldPeptide preparation methodsSynthesis methodsGoserelin
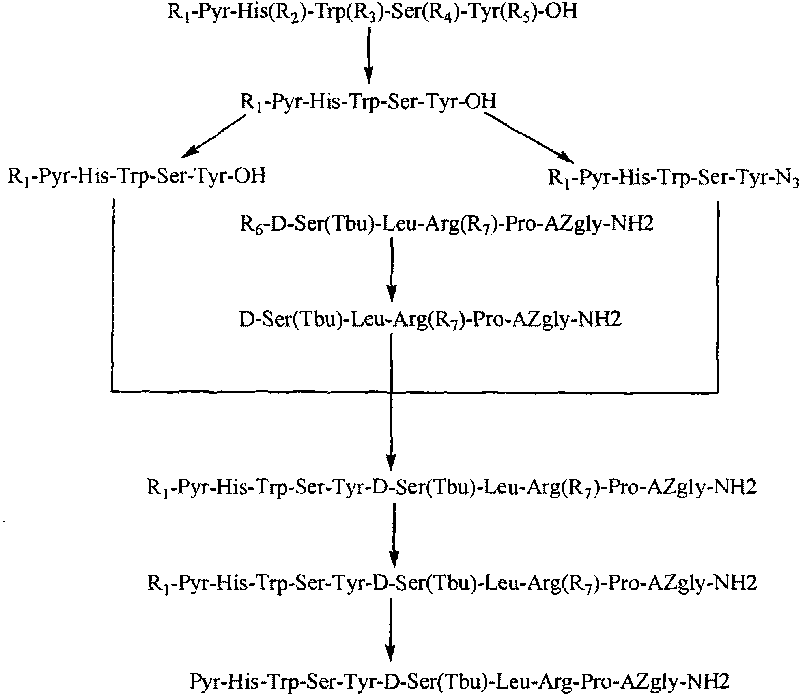
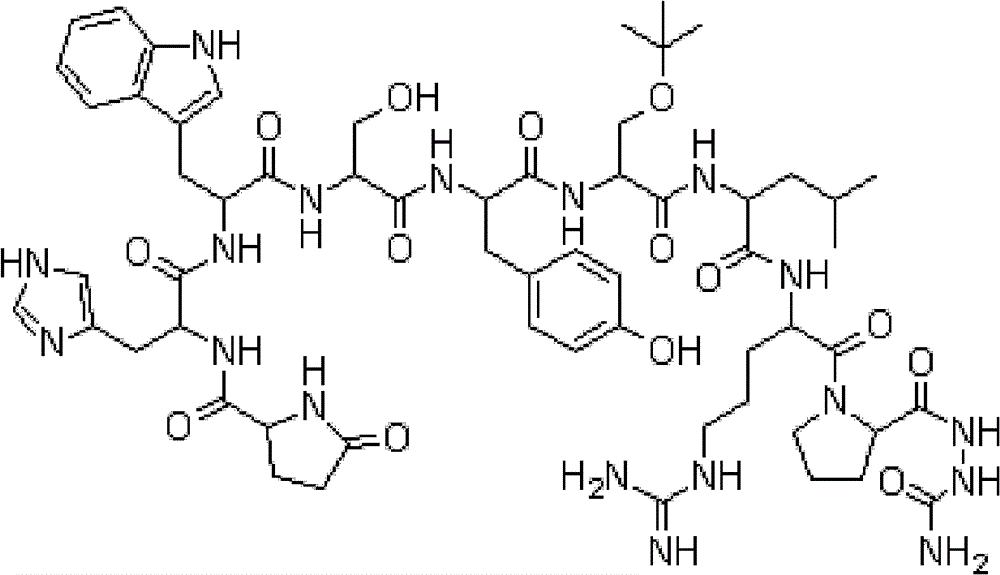
The invention belongs to the field of the synthesis of medicines, which particularly relates to a novel method for synthesizing goserelin by a pure liquid-phase fragment method. In the method for synthesizing the goserelin, the goserelin Pyr-His-Trp-Ser-Tyr-D-Ser(Tbu)-Leu-Arg-Pro-AZgly-NH2 is formed by condensing a pentapeptide fragment Pyr-His-Trp-Ser-Tyr-OH and a tetrapeptide fragment D-Ser(Tbu)-Leu-Arg-Pro-AZgly-NH2 under the existence of a condensing agent. The invention adopts a 5+4 fragment synthesis method and can directly condense the pentapeptide fragment Pyr-His-Trp-Ser-Tyr-OH and the tetrapeptide fragment D-Ser(Tbu)-Leu-Arg-Pro-AZgly-NH2 into the goserelin under the existence of the condensing agent. The method shortens the synthesis period, avoids rigorous reaction conditions in the traditional method, has high yield, good product purity, low cost and mild reaction condition and is suitable for industrialized production.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Enhanced nasal composition of active peptide
InactiveUS20090035260A1Prevent oxidationImprove stabilityPeptide/protein ingredientsMetabolism disorderNasal cavityGoserelin
A pharmaceutical composition has a therapeutically effective amount of at least one of: a pharmaceutically active nasal peptide, its pharmaceutically acceptable salt and its peptidic fragment. The composition also contains an absorbefacient effective amount of THAM in a pharmaceutically acceptable, aqueous liquid diluent or carrier. The composition is provided in a convenient form for nasal administration. In one embodiment, the peptidic fragment may be selected physiologically active lymphokines and monokines, peptidic enzymes, proteic vaccines, peptidic toxoids and personalized proteins derived from genoma. In another embodiment, the peptidic fragment may be selected from the peptide hormones and hormone antagonists buserelin, desmopressin, vasopressin, angiotensin, felypressin, octreotide, somatropin, thyrotropin (TSH), somatostatin, gosereline, thryptorelin and insulin selected from the group consisting of cow and pig, synthetic and recombinant.
Owner:THERAPICON SRL
Goserelin slow release microsphere preparation and preparation method thereof
The invention discloses a goserelin slow release microsphere preparation and a preparation method thereof. The slow release microsphere preparation comprises, based on the weight of microspheres, 0.5% to 30% (w / w) of goserelin or a salt thereof, 70% to 99.5% of a biodegradable and biocompatible high-molecular material with a molecular weight of 5, 000 to 300, 000 Dalton and 0.1% to 10% of other pharmaceutically acceptable accessories. The slow release microspheres in the invention have an average particle size of 5 to 40 mu m and an entrapment rate of greater than 80%. The slow release microspheres have a slow release period as long as a plurality of days or months, obviously reduce usage frequency of the preparation, improve bioavailability of goserelin, reduce toxic and side effects of the preparation and benefit clinical treatment.
Owner:SHENZHEN JYMED TECH
Therapy of prostate cancer with CTLA4 antibodies and hormonal therapy
InactiveCN101146552APeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHistrelinFlutamide
The present invention relates to a method for treating prostate cancer, which comprises administering an anti-CTLA4 antibody, or an antigen-binding portion thereof, especially a human antibody against human CTLA4, such as antibodies 3.1.1, 4.1.1, 4.8.1, 4.10.2 , 4.13.1, 4.14.3, 6.1.1, ticilimumab (also known as 11.2.1), 11.6.1, 11.7.1, 12.3.1.1, 12.9.1.1, and ipilimumab (also known as MDX-101 and 10D1) , in combination with hormone therapy. Hormone therapy agents include, inter alia, antiandrogens (e.g., megestrol, cyproterone, flutamide, nilutamide, and bicalutamide), GnRH antagonists (e.g., ababa Rick and histrelin), and LH-RH agonists (eg, leuprolide, goserelin, and buserelin). The present invention relates to neoadjuvant therapy, adjuvant therapy, treatment of elevated PSA, first-line treatment, second-line treatment and third-line treatment of localized or metastatic prostate cancer.
Owner:PFIZER PROD INC
Solid Phase Peptide for the Production of Goserelin
InactiveUS20100311946A1Peptide/protein ingredientsLuteinising hormone-releasing hormoneSide chainGoserelin
The present invention provides a process for the production of goserelin. In particular, the process of the invention allows the use of side chain protecting groups during synthesis of the peptide, and the addition of the azaglycine moiety of the peptide.
Owner:MALLINCKRODT INC
Synthesis method of goserelin
InactiveCN102746383AHigh reactivityEasy to synthesizeLuteinising hormone-releasing hormonePeptide preparation methodsSynthesis methodsTrifluoroacetic acid
The invention relates to a solid phase synthesis method of goserelin. The method consists of: first reacting RinkAmide MBHA Resin with carbonyldiimidazole (CDI) to obtain an intermediate product, which then reacts with Fmoc-NH-NH2 to obtain Fmoc-NH-NH-CO-NH-Resin, then conducting a programmed reaction, carrying out a condensation reaction in order to connect corresponding amino acids, thus obtaining goserelin resin; performing cutting with a low concentration trifluoroacetic acid solution so as to obtain a crude peptide solution, conducting purification to obtain the goserelin. According to the invention, by reacting a high-activity reactant carbonyldiimidazole with Fmoc-NH-NH2 and Rink Amide MBHA Resin to obtain Fmoc-NH-NH-CO-NH-Resin, the reaction efficiency is greatly improved, so that the reaction can be completed rapidly and efficiently, thus completely solving the problem of difficult synthesis of Fmoc-NH-NH-CO-NH-Resin, and improving the reaction efficiency and yield effectively.
Owner:HANGZHOU JIUYUAN GENE ENG
Sustained release pharmaceutical compositions
The present invention provides a sustained release microsphere composition comprising(i) microspheres comprising (A) a biodegradable polymer which is a homopolymer of lactic acid or a copolymer of lactic acid and glycolic acid having a monomer ratio in the range of about 1:1 to about 3:1, and (B) a therapeutically effective amount of goserelin or a pharmaceutically acceptable salt thereof, and(ii) pharmaceutically acceptable excipients,which when injected intramuscularly, delivers goserelin or a pharmaceutically acceptable salt thereof, for a period of at least one month.
Owner:SUN PHARMA INDS
Synthesis method of goserelin
ActiveCN106589072AHigh yieldHigh purityLuteinising hormone-releasing hormonePeptide preparation methodsSynthesis methodsGoserelin
The invention relates to a synthesis method of goserelin. The synthesis method comprises the following steps: respectively synthesizing 1st to 5th pentapeptide fragments by adopting solid phases, and then inducing D-Ser(tBu), thus forming 1st to 6th hexapeptide fragments; synthesizing 7th to 9th tripeptide fragments by using a solid phase or a liquid phase; inducing semicarbazide in the 7th to 9th tripeptide fragments in a liquid phase; coupling the 1st to 6th hexapeptide fragments and the 7th to 9th tripeptide fragments into the goserelin in the liquid phase, thus obtaining a goserelin crude product. According to the synthesis method disclosed by the invention, the yield and the purity of the goserelin are remarkably increased, catalytic reduction is not needed, the synthesis method just involves the technologies of condensation and deprotection of amino acid, the reaction is simple and controllable, and the synthesis method is suitable for industrial production.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Synthetic method for goserelin
InactiveCN104004054AAvoid Cutting UnsafelySimplify the tedious processPeptide preparation methodsPolymer resinGoserelin
The invention discloses a synthetic method for goserelin and belongs to the technical field of chemical pharmacy. The synthetic method comprises: taking 2-chlorotritylchloride polymer resin as a raw material, linking 2-chlorotritylchloride polymer resin with carboxyl of fluorenylmethyloxycarbonyl-protected proline under an alkaline condition, so as to obtain fluorenylmethyloxycarbonyl-protected proline-2-chlorotritylchloride polymer resin, then successively linking with residual amino acids of goserelin through peptide linking connections, using a full-protection cutting reagent for cracking and obtain a full-protection nine-peptide fragment, then performing ethylamination to obtain a full-protection goserelin fragment, and finally cracking the full-protection goserelin fragment to obtain a goserelin crude product. Goserelin is synthesized by employing a solid-liquid combination method and the yield reaches 84.1%. Also, the synthetic method helps to avoid the problems that a pure solid phase is not safe and convenient to cut from resin, the synthetic time is shortened, the tedious process for synthesizing a pure liquid phase is simplified, the production cost is substantially reduced, and the synthetic method is suitable for industrialized popularization.
Owner:ANHUI RUBIOX VISION BIOTECH
Anticancer sustained-release preparation loaded with anti-cancer medicine and synergist thereof
InactiveCN101380308AGood treatment effectLow toxicitySolution deliveryEmulsion deliveryGoserelinSuspending Agents
An anticarcinogenic slow release formulation carrying an anticancer drug and a synergist is a slow release injection or a slow release implant, and the slow release injection is made from slow release microspheres and a dissolvant. The slow release microspheres comprise anticancer active components and a slow adjuvant, and the dissolvant is a special dissolvant containing a suspending agent which is selected from sodium carboxymethyl cellulose and the like, and the viscosity of the suspending agent is 80cp-3,000cp (at room temperature). The anticancer active components are alkylating agents such as melphalan, ifosfamide and the like, purine analogues such as O6-BG and the like, and / or hormones anticancer drugs selected from triptorelin, goserelin, leuprorelin and a composition selected from epothilone (A-F) and derivatives thereof; the slow release adjuvant is chosen from one of or the copolymer or the mixture of polylactic acid and a copolymer thereof, polifeprosan and the copolymer or the mixture of polylactic acid and sebacic acid copolymer; the slow release implant and the slow release injection are injected or put in tumors or around the tumors, which is beneficial to diffusing the drug in the solid tumors, maintaining high concentration, reducing drug tolerance, being capable of mutual synergy and enhancing curative effects of chemotherapy and / or radiotherapy.
Owner:JINAN SHUAIHUA PHARMA TECH
Temperature controlled sustained-release injection containing steroids anti-cancer drugs
InactiveCN101273963APharmaceutical delivery mechanismPharmaceutical non-active ingredientsGoserelinTherapeutic effect
The invention relates to a temperature-controlled sustained-release injection containing a hormone anti-cancer drug, which comprises the anti-cancer drug, an amphiphilic block copolymer, a solvent and a certain amount of drug release regulator, wherein, the mixture of the amphiphilic block copolymer and a solvent without organic solvent has the temperature-sensitive gelatinization feature, which is flowable liquid in the environment that is lower than the body temperature and can be automatically converted to the water-insoluble gel that can not flow and be biodegradable for absorption in an endotherm, and the water-insoluble gel can allow the contained hormone anti-cancer drug to have the local sustained release in a tumor and maintain the effective drug concentration for a plurality of weeks to a plurality of months; the viscosity of the temperature-controlled sustained-release injection is 10cp to 3000cp ( at 5 DEG C to 30 DEG C ), and the gelatinization temperature is 35 DEG C to 37 DEG C. The sustained-release injection can be injected in the tumor or the tumor periphery or be arranged in the postoperative tumor cavity, thus significantly reducing the systemic reaction of the drug, selectively strengthening the treatment effects of chemotherapy, radiotherapy and other non-surgical therapies, and being used for the treatment of the tumors in different stages. The anti-cancer drug can be triptorelin, goserelin, leuprorelin, anastrozole, idoxifene, tamoxifen and other hormone anti-cancer drugs.
Owner:SHANDONG LANJIN PHARMA +1
Method for synthesizing goserelin impurities
InactiveCN110128505AInhibit side effectsHigh yieldPeptide preparation methodsBulk chemical productionTrifluoroacetic acidSide chain
The invention discloses a method for synthesizing goserelin impurities. According to the technical scheme, the method comprises the following steps: firstly synthesizing a first fragment: Pyr-His-Trp-Ser-Tyr-D-Ser (tBu)-Leu-Arg-Pro-OH, coupling the first fragment and Boc-NH-NH2 under a coupling agent to obtain a goserelin impurity crude product, removing side chain protection under the condition of trifluoroacetic acid, and purifying and freeze-drying to obtain the pure product of the goserelin impurities. The method has the advantages of simplicity in reaction operation, convenience in post-treatment and high yield, and has high reference value for the synthesis of goserelin. In addition, the scientific evaluation, pharmacological research, pharmacokinetics and the like of the goserelin are provided.
Owner:TLC NANJING PHARMA RANDD CO LTD
Preparation method of buserelin or goserelin
ActiveCN107540727AHigh purityHigh yieldLuteinising hormone-releasing hormonePeptide preparation methodsFreeze-dryingGoserelin
The invention relates to a preparation method of buserelin or goserelin. The method comprises the following steps: 1) preparing Fmoc-Pro-resin by using 2-CTC resin as a carrier; 2) preparing fully-protected 9 peptide resin by a solid-phase synthesis technology; 3) pyrolyzing the fully-protected 9 peptide resin to obtain a fully-protected 9 peptide; 4) reacting the fully-protected 9 peptide with ethylamine hydrochloride under the action of a coupling agent to obtain fully-protected buserelin, or reacting the fully-protected 9 peptide with semicarbazide hydrochloride under the action of the coupling agent to obtain fully-protected goserelin; 5) carrying out a palladium-carbon catalyzed hydrogenolysis reaction on the fully-protected buserelin or the fully-protected goserelin in a solvent Z, and filtering out the palladium-carbon after the reaction is finished in order to obtain a buserelin solution or a goserelin solution; and 6) purifying and freeze-drying the buserelin solution or the goserelin solution to obtain the buserelin or goserelin, wherein the solvent Z in step 5) is a methanol solution containing 5% of pyridine hydrochloride or an aqueous solution containing 85-95% of acetic acid.
Owner:HYBIO PHARMA
Anticancer medicament and synergist simultaneously carrying anticancer sustained release agent
InactiveCN1969821AGood treatment effectLow toxicityOrganic active ingredientsPharmaceutical delivery mechanismAdjuvantTreatment effect
Disclosed is an anti-cancer slow release agent in the form of slow release injection or slow release implantation agent carrying both anti-cancer drugs and synergistic agent, the slow release injection comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The suspending agent is selected from carboxymethylcellulose, the viscosity of the suspension adjuvant is 80-3000cp (at room temperature). The anticancer active constituent being the combination of alkylating agent such as Melphalan and isoendoxan, purine analogues such as 06-BG and / or hormone group anti-cancer drugs selected from Triptorelin, Goserelin Leuprorelin and Epothilone and its derivatives (Epothilone A-F), the slow release auxiliary materials are selected from polylactic acid and its copolymer, Polifeprosan, polylactic acid copolymer or mixture, sebacylic acid copolymer or mixture. The slow release injection and slow release implanting agent can be used independently for effectively suppressing tumor accretion, or used in combination with non-operative methods such as chemotherapy and / or radiotheraphy with the function of improving their treatment effects.
Owner:JINAN SHUAIHUA PHARMA TECH
Method for synthesizing goserelin with fragment method
ActiveCN108383896ALuteinising hormone-releasing hormonePeptide preparation methodsGoserelinPhotochemistry
The invention discloses a method for synthesizing goserelin with a fragment method. According to the technical scheme, the method includes the following steps that firstly, a first fragment H-Arg-Pro-Azagly-NH2 and a second fragment Pyr-His-Trp-Ser-Tyr-D-Ser(tBu)-Leu-OH are synthesized respectively, then, the first fragment and the second fragment are coupled under the coupling agent condition toobtain crude goserelin, and purification and lyophilization are carried out to obtain pure goserelin. The method has the advantages of simple reaction operation, convenient post-treatment, no catalytic reduction, high yield, low cost and the like, has considerable practical application prospects, and is applicable to industrial production.
Owner:CHINESE PEPTIDE CO
Method of treating er mutant expressing breast cancers with selective androgen receptor modulators (SARMS)
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; and / or treating a subject suffering from ER mutant expressing breast cancer, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Pharmaceutical compositions of goserelin sustained release microspheres
InactiveUS20160022584A1Narrow distributionUniform particle sizeHormone peptidesPowder deliveryMicrosphereLactide
A composition of goserelin sustained release microspheres is provided. The microspheres comprise goserelin, at least one poly(lactide-co-glycolide) and poloxamer or PEG. The sustained release microspheres have comparatively high bioavailability, which promotes the drug taking its full effect and have entrapment efficiency over 90%.
Owner:SHANDONG LUYE PHARMA CO LTD
Goserelin sustained release microsphere freeze-dried powder and preparation method thereof
ActiveCN109394705AReduce the number of dosesImprove compliancePowder deliveryPeptide/protein ingredientsMicrosphereFreeze-drying
The invention relates to freeze-dried powder, in particular to goserelin sustained release microsphere freeze-dried powder and a preparation method thereof in the technical field of water-soluble polypeptide drug sustained release long-acting injections. Each microsphere in the freeze-dried powder comprises a shell, each shell wraps a core, each core is temperature-sensitive gel, when the temperature of the temperature-sensitive gel is lower than the body temperature of human bodies, the mobility of the temperature-sensitive gel is good, after the temperature of the temperature-sensitive gel is increased to be equal to the body temperature of the human bodies, the mobility is reduced, then the temperature-sensitive gel is gelatinized to form a gel core, and each shell is a biodegradable material. The goserelin sustained release microsphere freeze-dried powder has the advantages that the encapsulation efficiency reaches 90% or above, drug release is jointly controlled by the internal temperature-sensitive gel cores and external biodegradable sustained release framework materials, and the goserelin sustained release microsphere freeze-dried powder is a double-layered drug release system.
Owner:SHENYANG PHARMA UNIVERSITY
Sustained-release microsphere preparation of goserelin composition
InactiveCN104436169AHigh acceptanceReduce dosing frequencyPeptide/protein ingredientsGranular deliverySide effectMedicine
The invention belongs to the field of a pharmaceutical preparation, and relates to a sustained-release microsphere preparation of a goserelin composition and a preparation method of the sustained-release microsphere preparation. Specifically, the goserelin composition includes goserelin and a polypeptide composition capable of enhancing immunity, wherein the polypeptide capable of enhancing immunity comprises thymalfasin, thymopentin and thymosin beta4. The sustained-release microsphere consists of 0.1-40% (w / w) of goserelin and polypeptide capable of enhancing immunity in terms of the total weight of the microsphere, 60-99.9% of a biodegradable and biocompatible high polymer material which is 5,000-200,000Dalton in molecular weight in terms of the weight of the microsphere, and 0-10% of other pharmaceutically acceptable accessories in terms of the weight of the microsphere. The sustained-release microsphere disclosed by the invention is 5-20microns in average grain size and encapsulation efficiency is more than 80%. The sustained-release duration of the sustained-release microsphere can last for several days or several months, so that administration frequency is obviously reduced, bioavailability is improved, the toxic and side effects of medicine are reduced, and the sustained-release microsphere is conducive to clinic treatment. The production process of the finished product is good in reproducibility and good in feasibility.
Owner:SHENZHEN JYMED TECH
Preparation method of goserelin
InactiveCN104744569AEasy to makeReduce manufacturing costLuteinising hormone-releasing hormoneGoserelinDimethylformamide
The invention relates to a preparation method of goserelin, wherein the production method comprises the following specific steps: suspending 1.0.2 mol of 5-oxoPro-His-NHNH2 in a mixed solvent of 0.9 mL of dimethylformamide and 0.7 mL of dimethylsulfoxide, at the temperature of 0 DEG C and under stirring, adding 0.8 mmol of dioxane solution with 5.7 mol / L hydrochloric acid, vigorously stirring for 5 min, and thus obtaining a clarified solution. The preparation method of goserelin is simple in preparation process and low in production cost.
Owner:李磊
Non-invasive method of evaluating breast cancers for selective androgen receptor modulator (SARM) therapy
This invention relates to the treatment of breast cancer in a subject, for example a female subject. Including methods of: treating metastatic breast cancer; refractory breast cancer; AR-positive breast cancer; AR-positive refractory breast cancer; AR-positive metastatic breast cancer; AR-positive and ER-positive breast cancer; triple negative breast cancer; advanced breast cancer; breast cancer that has failed selective estrogen receptor modulator (SERM) (tamoxifen, toremifene, raloxifene), gonadotropin-releasing hormone (GnRH) agonist (goserelin), aromatase inhibitor (AI) (letrozole, anastrozole, exemestane), cyclin-dependent kinase 4 / 6 (CDK 4 / 6) inhibitor (palbociclib (Ibrance), ribociclib (Kisqali), abemaciclib (Vorzenio)), mTOR inhibitor (everolimus), trastuzumab (Herceptin, ado-trastuzumab emtansine), pertuzumab (Perjeta), lapatinib, neratinib (Nerlynx), olaparib (Lynparza) (an inhibitor of the enzyme poly ADP ribose polymerase (PARP)), bevacizumab (Avastin), and / or fulvestrant treatments; metastasis in a subject suffering from breast cancer; HER2-positive; treating a subject suffering from ER mutant expressing breast cancer and / or treating breast cancer in a subject, by first determining the 18F-16β-fluoro-5α-dihydrotestosterone (18F-DHT) tumor uptake and identifying said subject as having AR-positive breast cancer based on 18F-DHT tumor uptake, comprising administering to the subject a therapeutically effective amount of a selective androgen receptor modulator (SARM) compound.
Owner:UNIV OF TENNESSEE RES FOUND
Method for preparing goserelin slow-release implant
ActiveCN102755627BLow boiling pointSpeed up evaporationPeptide/protein ingredientsAntipyreticAcetic acidOrganosolv
The invention provides a method for preparing a goserelin slow-release implant, which comprises the step of preparing acetic acid and an organic solvent of which the boiling point is lower than that of the acetic acid into a mixed solvent, thereby dissolving goserelin and a pharmaceutically-acceptable carrier. The goserelin slow-release implant prepared by the method has the advantage that medicaments are stable, are uniformly distributed and can be stably released for a long time; and moreover, the time consumed by the preparation method is short, so that the time cost is reduced. In addition, the invention also provides the medical implant prepared by the method.
Owner:SALUS PHARMA TECH SHANGHAI
Fragment-process synthesis method of goserelin
ActiveCN111233980AShort synthesis timeHigh purityLuteinising hormone-releasing hormonePeptide preparation methodsFluid phaseCombinatorial chemistry
The invention synthesizes goserelin by a synthesis method of combining a solid-phase method and a liquid-phase method. Fragments II and III are easy to synthesize and purify and high in purity; different fragments can be synthesized simultaneously, so that the synthesis time of the goserelin is effectively shortened, and the preparation efficiency is improved; and finally, the two fragments are butt-jointed by the liquid-phase method to obtain a goserelin precursor, low-cost coupling is realized, and industrial enlarged preparation is facilitated.
Owner:NANJING LEEWE BIOPHARMACEUTICAL CO LTD
Solid-phase synthesis of goserelin
ActiveCN104910257BTo solve such a drawback that cannot be monitoredLow costLuteinising hormone-releasing hormonePeptide preparation methodsPhosphoniumSide chain
Owner:苏州天马医药集团天吉生物制药有限公司
Controlled release agent of containing fluorouracil and synergist
InactiveCN1957919AEasy injectionIncrease drug concentrationOrganic active ingredientsPeptide/protein ingredientsWhole bodyMicrosphere
A slowly-release anticancer medicine in the form of injection or implant is disclosed. Said slowly-release injection is composed of a special solvent containing suspending aid and the slowly-release microballs consisting anticance medicine, iterstitial hydrolyte and slowly-releasing auxiliary. Said anticancer medicine is chosen from 5-FU, vincristine, etc. Said interstitial hydrolyte is chosen from collagenase, relaxin, etc. Said slowly-releasing auxiliary is chosen from polylactic acid, FAD, polyethanediol, etc.
Owner:SHANDONG LANJIN PHARMA +1
Synthesis method of goserelin
InactiveCN113461784AOvercome the problem of low synthesis yieldShorten the production cycleLuteinising hormone-releasing hormonePeptide preparation methodsFluid phaseBiochemical engineering
The invention provides a synthesis method of goserelin. The synthesis method comprises the following steps: generating pGlu-His (Trt)-Trp (Boc)-Ser (tBu)-Tyr (tBu)-OH and Fmoc-D-Ser (tBu)-Leu-Arg (NO2)-Pro-OH by virtue of a solid-phase synthesis method; carrying out Fmoc removal on the generated Fmoc-D-Ser (tBu)-Leu-Arg (NO2)-Pro-OH, so as to generate NH2-D-Ser (tBu)-Leu-Arg (NO2)-Pro-AzaGly-NH2; carrying out liquid phase coupling on two fragments, namely pGlu-His (Trt)-Trp (Boc)-Ser (tBu)-Tyr (tBu)-OH and NH2-D-Ser (tBu)-Leu-Arg (NO2)-Pro-AzaGly-NH2, and then removing an-NO2 protecting group, so as to obtain a goserelin crude product; and preparing a refined goserelin product through RP-HPLC (Reverse Phase-High Performance Liquid Chromatography) purification and freeze-drying. The method has the beneficial effects that the problem that the synthesis yield is low when a conventional Fmoc / tBu solid-phase reaction system is adopted is solved, the production period is shortened, the method is more suitable for large-scale production. Meanwhile, the production cost is also greatly reduced.
Owner:SUZHOU IBIO TECH CO LTD
4-(dihydroxyacetyl-L-serine)-goserelin as well as preparation method and application thereof
The invention provides a novel compound as well as a preparation method and application thereof. Specifically, the invention relates to a novel compound, namely 4-(dihydroxyacetyl-L-serine)-goserelin, and a goserelin long-acting preparation containing 4-(dihydroxyacetyl-L-serine)-goserelin with the weight percentage being less than 1%. The invention also discloses an application of the 4-(dihydroxyacetyl-L-serine)-goserelin as a reference substance in impurity detection of a goserelin long-acting preparation.
Owner:SHANDONG LUYE PHARMA CO LTD
Method for the treatment of prostate cancer
A method for the treatment of advanced prostate cancer comprises administering to a patient suffering from advanced prostate cancer an androgen suppressing amount of a luteinizing hormone releasing hormone agonist analog and an amount of calcitriol sufficient to enhance the effectiveness of the luteinizing hormone releasing hormone agonist analog against the cancer relative to treatment with the luteinizing hormone releasing hormone agonist analog alone. Preferably the calcitriol is in the form of a stabilized, injectable solution of calcitriol in isotonic saline containing about 1 to about 30 milligrams per milliliter of calcitriol and a sufficient quantity of nonionic surfactant to solubilize the calcitriol therein. Preferably the a luteinizing hormone releasing hormone agonist analog is a nonapeptide or decapeptide agonist, such as leuprolide, goserelin or salts thereof. The method of the present invention affords a surprisingly improved efficacy for treatment of advanced prostate cancer such as androgen-independent prostate cancer (AIPC) or hormone refractory prostate cancer (HRPC) in comparison to treatment with a luteinizing hormone releasing hormone agonist analog alone.
Owner:GENIX THERAPEUTICS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com