Therapy of prostate cancer with CTLA4 antibodies and hormonal therapy
A technology of prostate cancer and hormone therapeutic agent, applied in the field of treating prostate cancer with CTLA4 antibody and hormone therapy, can solve problems such as damage to physiological functions, destruction of normal cells, and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
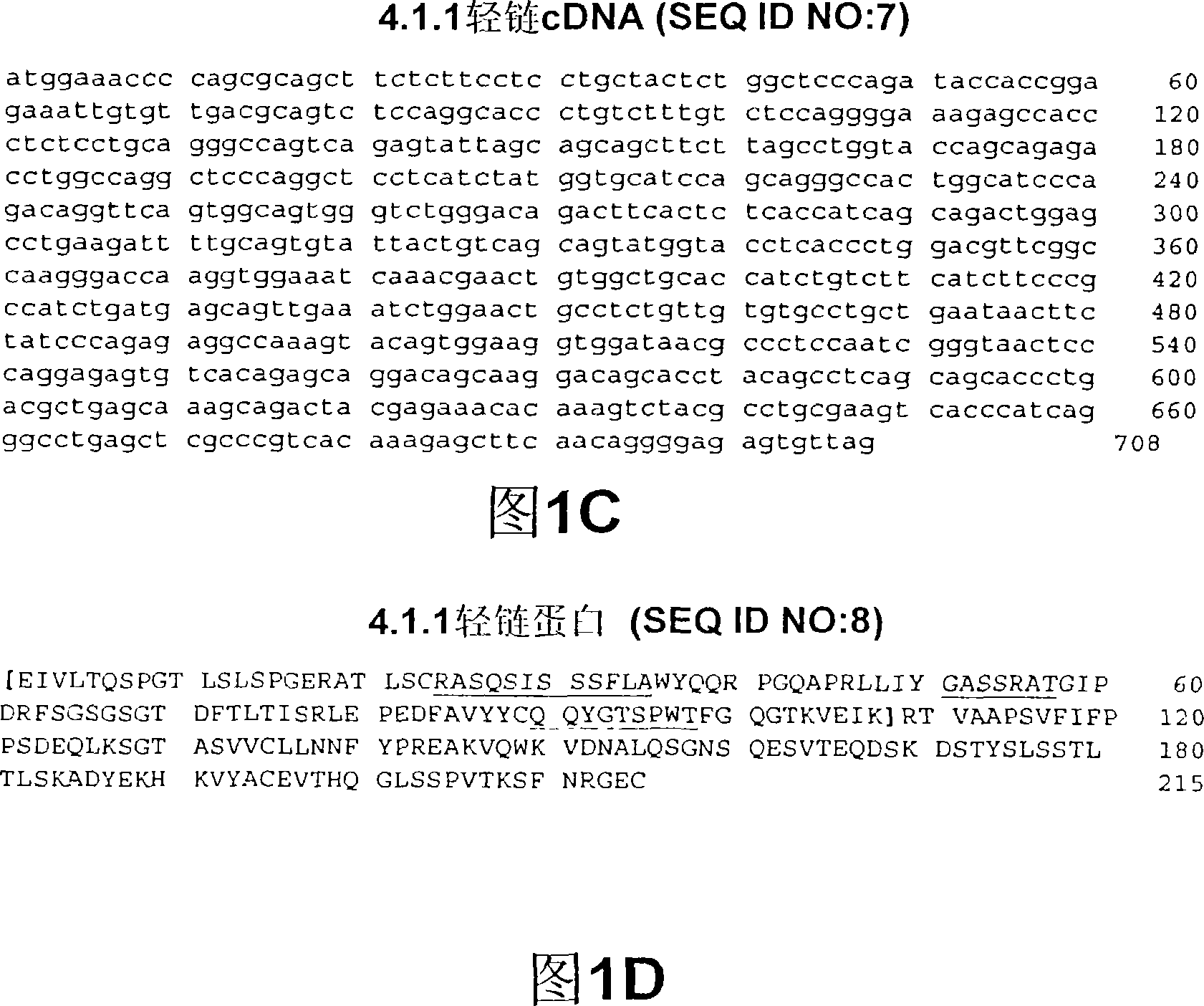
Image
Examples
preparation example Construction
[0176] The present invention includes the preparation and use of a pharmaceutical composition comprising an anti-CTLA4 antibody or antigen-binding portion thereof, and at least two hormones independently selected from, for example, anti-androgens, GnRH antagonists, and LH-RH agonists therapeutic agent. Such a pharmaceutical composition may consist of either only the antibody or only the hormone therapeutic agent (e.g., an effective dose of anti-CTLA4, an effective dose of at least one hormone therapeutic agent) each in a form suitable for administration to a subject, or the pharmaceutical combination The composition may comprise an antibody or hormone therapeutic agent and one or more pharmaceutically acceptable carriers, one or more other (active and / or inactive) ingredients, or other combinations of these ingredients.
[0177] In one embodiment, the antibody is administered parenterally (e.g., intravenously) as an aqueous solution, while the hormonal therapy (e.g., antiandro...
Embodiment 1
[0204] Combination of anti-CTLA4 and at least two hormones in neoadjuvant therapy for high-risk prostate cancer hormone therapy
[0205] Patients with prostate cancer who are candidates for radical prostatectomy and who are at intermediate or high risk of recurrence according to current medical criteria are candidates for combined neoadjuvant antibody-hormone therapy. The target group for this study was defined using the risk of biochemical recurrence rather than the likelihood of pathologically localized cancer. This approach is supported by the observation that patients with disease confined to the prostate can have biochemical relapses despite 'definitive' local therapy. Therefore, this patient should be defined more broadly. Considering recent data showing that time to PSA relapse of less than 2 years is predictive of later relapse, patients at high risk for early PSA relapse are candidates for neoadjuvant (and adjuvant) antibody-hormone combination therapy as disclos...
Embodiment 2
[0227] Combined use of anti-CTLA4 in the adjuvant treatment of clinically localized prostate cancer Two Hormone Therapies
[0228] Patients with clinically localized prostate cancer are given (before and during radiation therapy) goserelin (ZOLADEX) and flutamide (EULEXIN) on each hormone therapy regimen, e.g. EULEXIN 250 mg 3 times daily (tid ), and 3.6 mg ZOLADEX was subcutaneously administered monthly for a total of 4 months (2 months before radiotherapy and 2 months during radiotherapy). Optionally, the patient received ZOLADEX for an additional 24 months. Such a dosing regimen for adjuvant therapy, typically with radiotherapy, but not with or after surgery, is described, for example, in Hanks et al., J Chin Oncology 21:3972-3978 (2003).
[0229] The patient is further treated with a single IV infusion of ticilimumab (100 mL / hr) as described herein at a dose of approximately 3 mg / kg or 6 mg / kg or 10 mg / kg or 15 mg / kg. Prophylactic antidiarrheal drugs are given as nee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com