Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
77 results about "Bicalutamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Bicalutamide is used with another drug (LHRH-type such as leuprolide, goserelin) to treat prostate cancer.
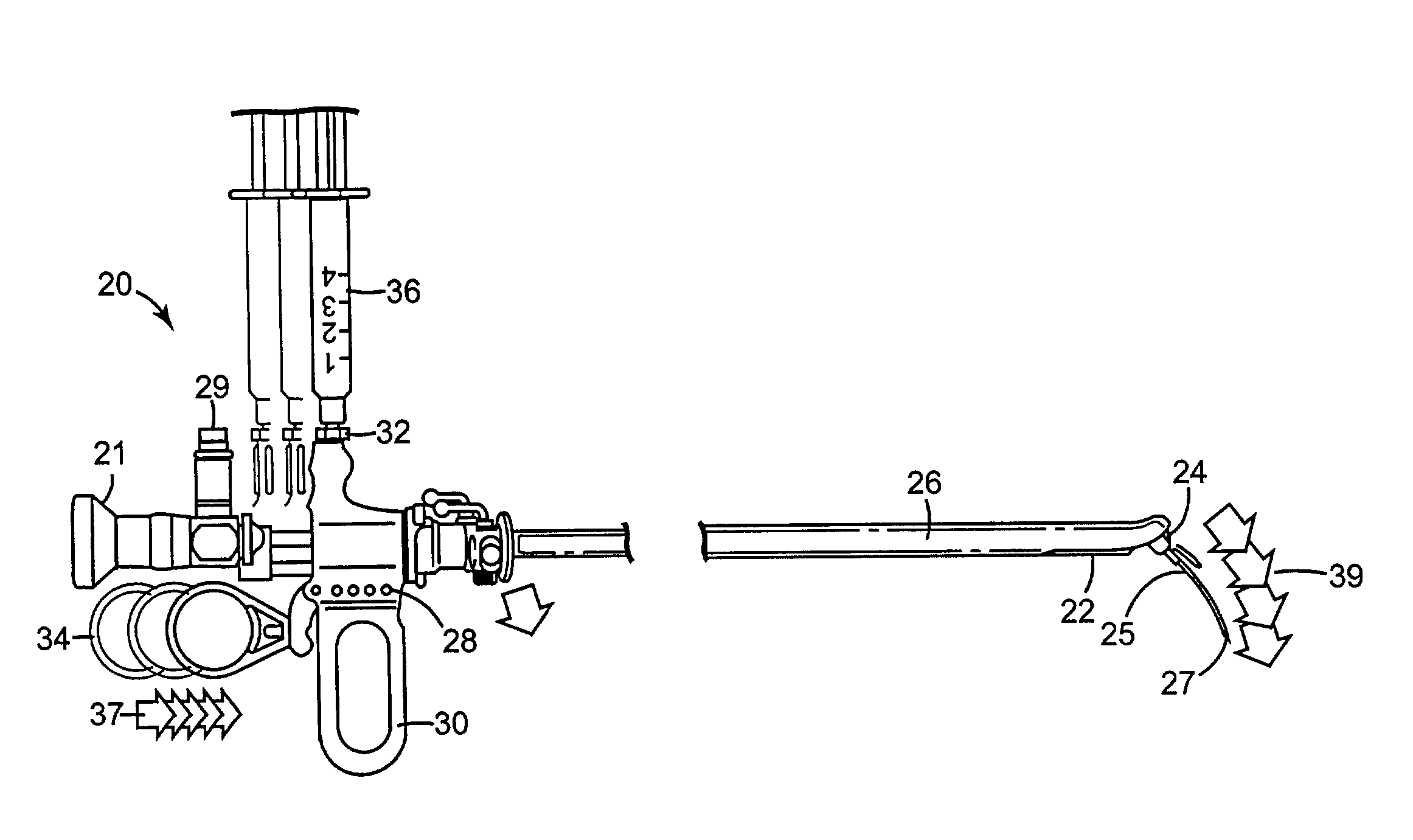
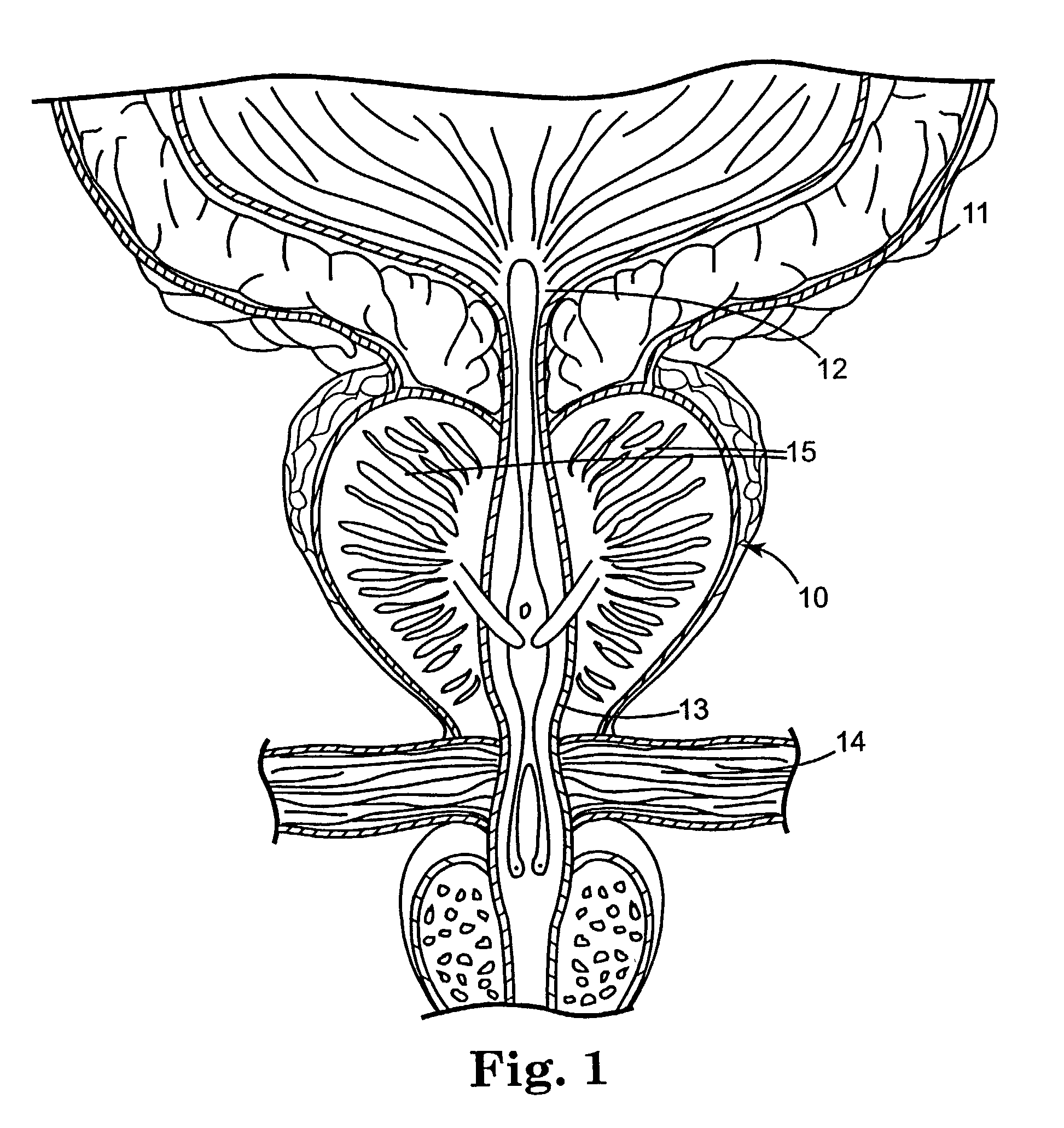
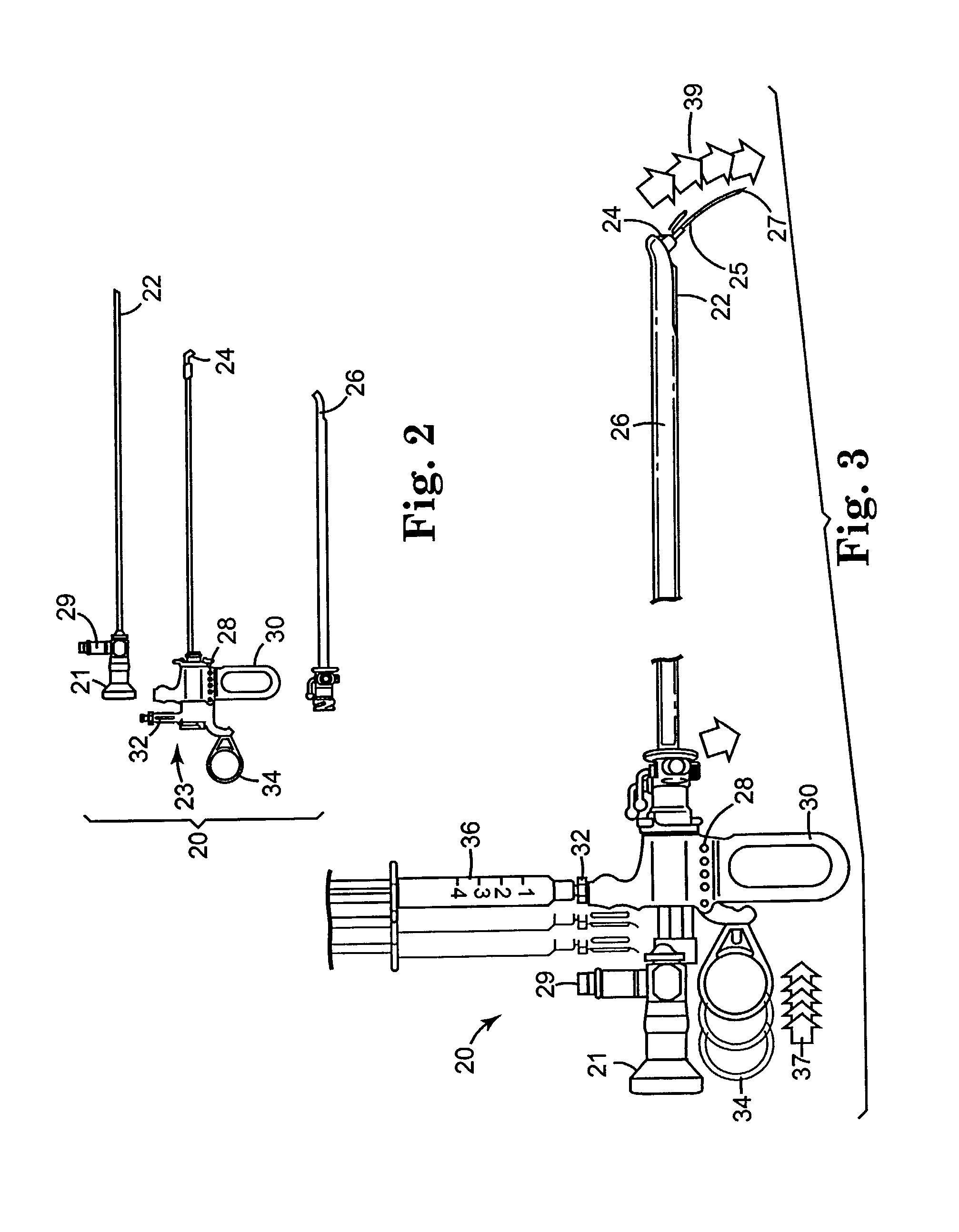
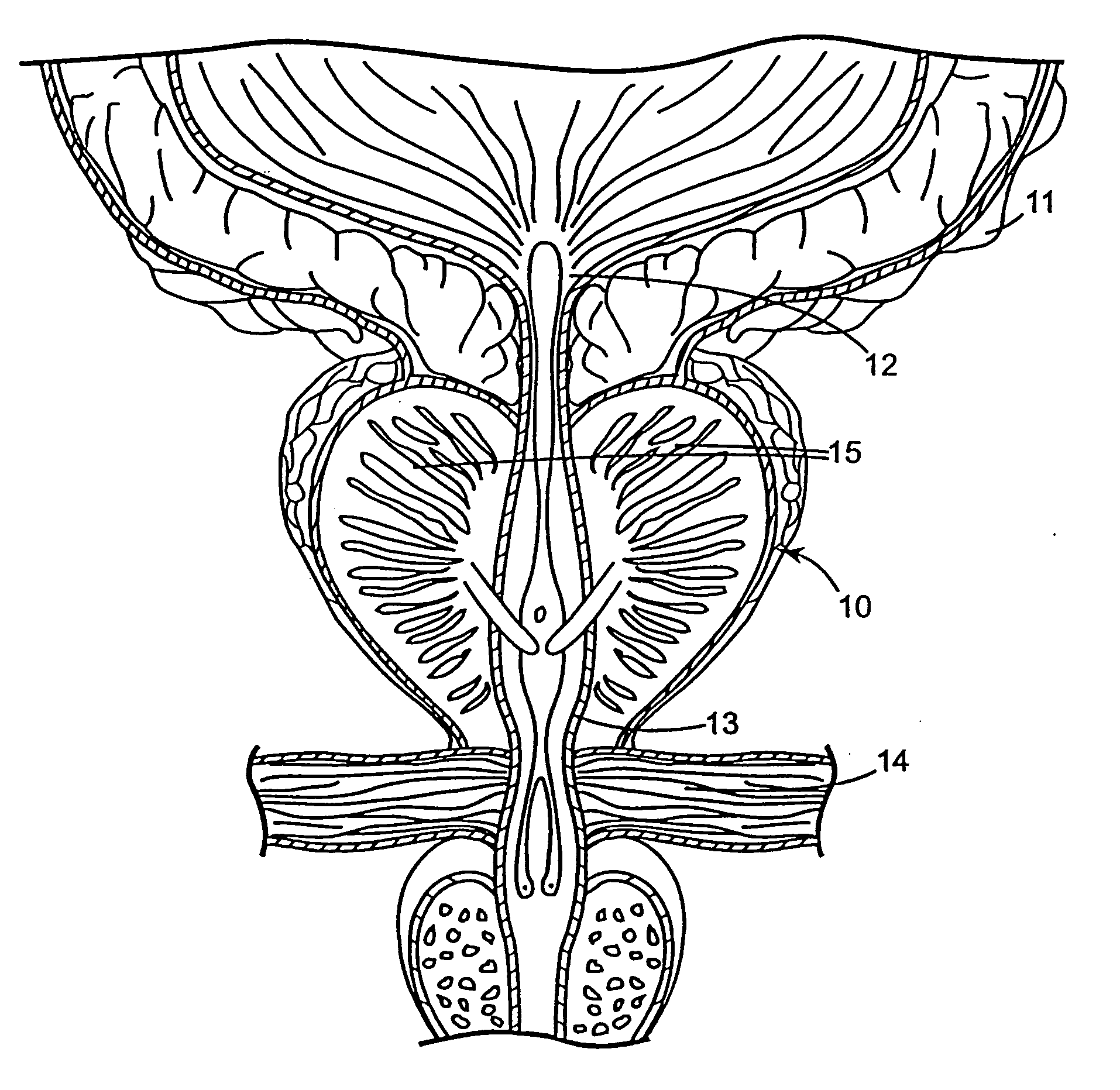
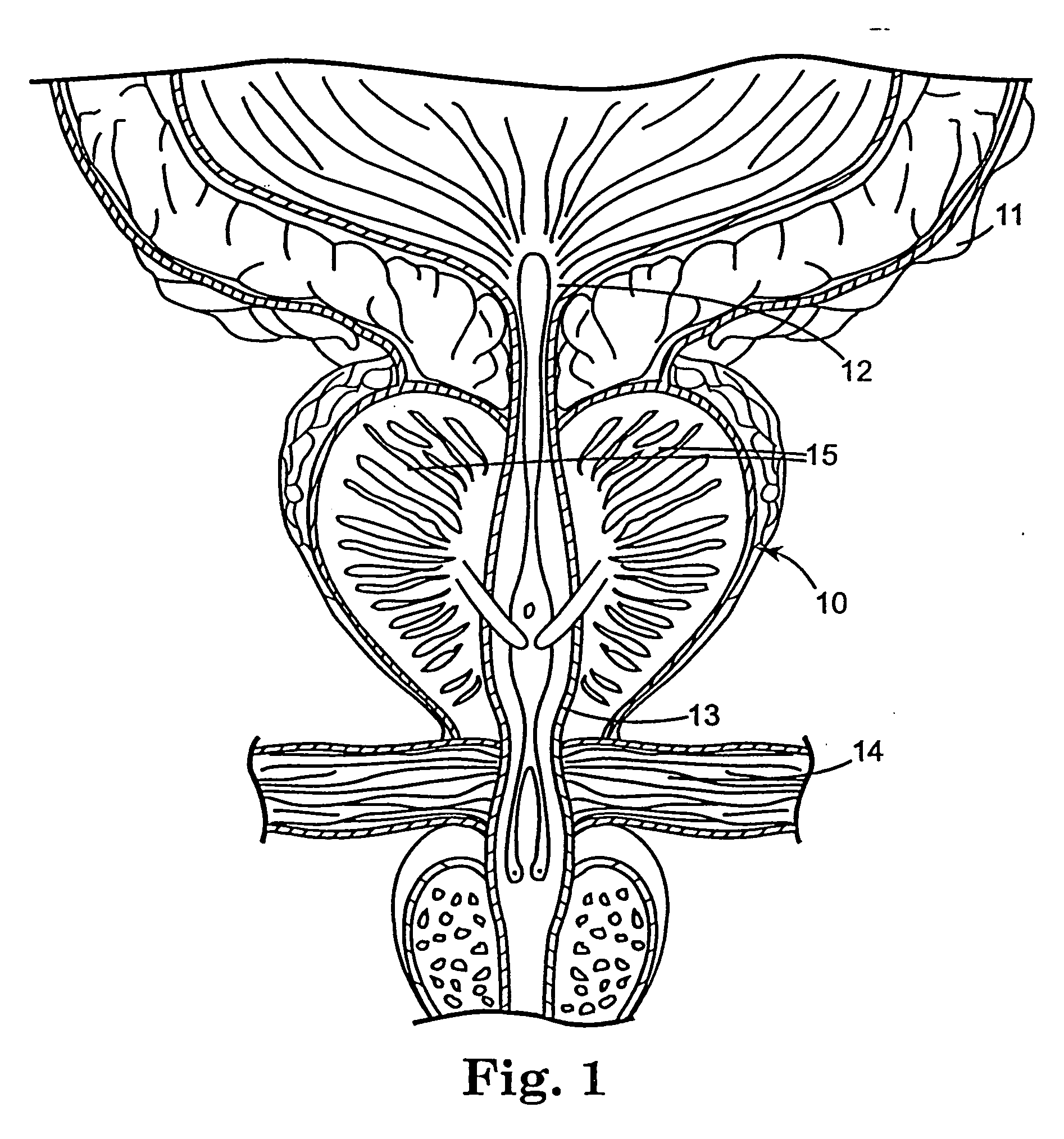
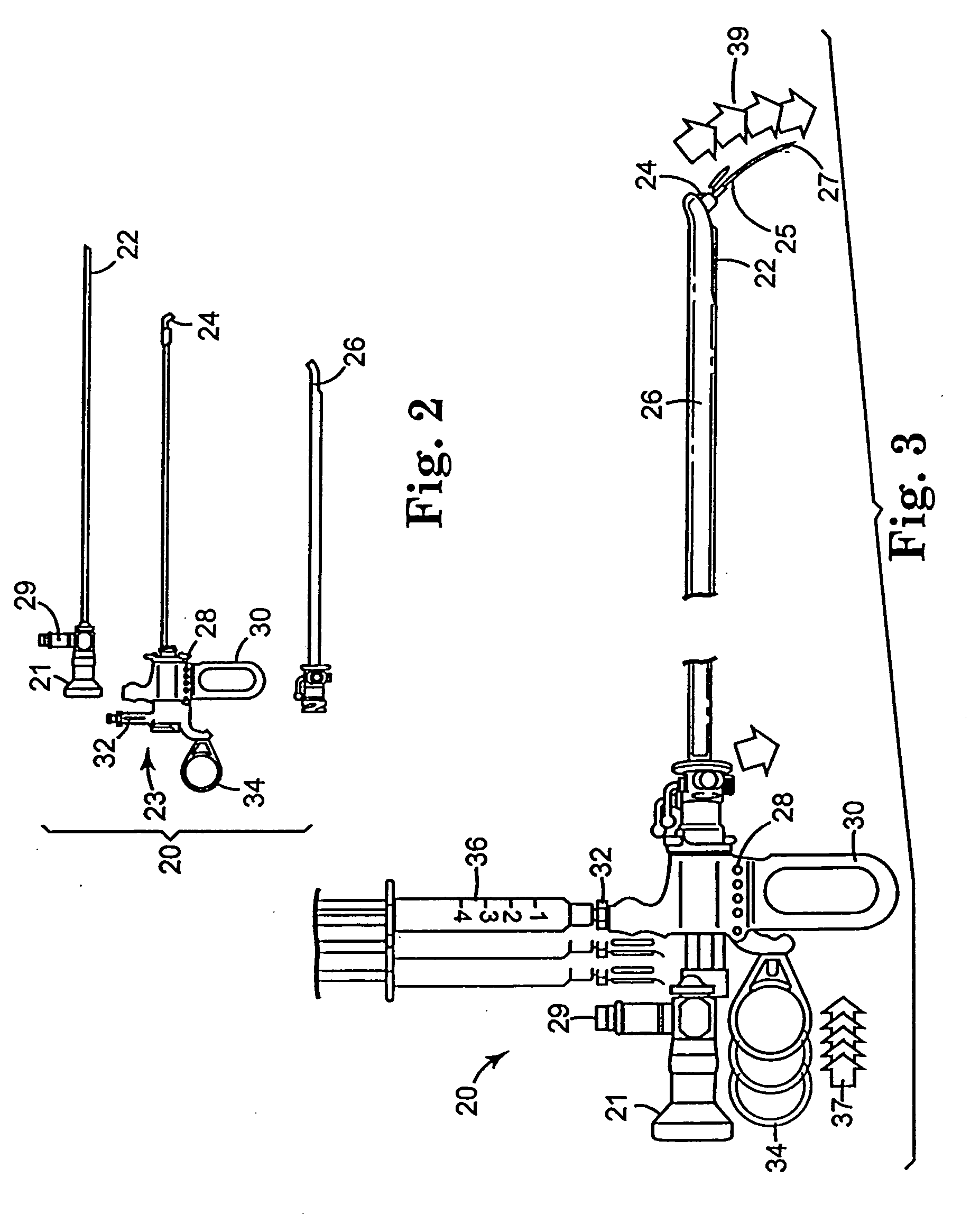
Regimen for treating prostate tissue and surgical kit for use in the regimen
InactiveUS7015253B2Decreasing prostate sizeSmall sizeBiocideHydroxy compound active ingredientsSteroidal antiandrogenRegimen
The present invention provides treatment regimens for treating diseased prostate tissue, including the steps of chemically ablating prostate tissue and coadministering an antiandrogen. In some embodiments, prostate tissue is chemically ablated by injection of ethanol, or an injectable gel comprising ethanol, into prostate tissue. Steroidal and non-steroidal antiandrogens are suitable antiandrogens. One suitable non-steroidal antiandrogen is bicalutamide. The treatment regimen is suitable for treatment of prostate tissue diseases including benign prostatic hyperplasia and prostatic carcinoma. The invention further provides a treatment regimen for treating benign prostatic hyperplasia, including the steps of damaging prostate tissue and coadministering an antiandrogen. Also provided by the present invention is a kit for treating a human male, including a means for necrosing prostate tissue, an antiandrogen drug, and a means for administering the antiandrogen drug. A kit including a first surgical device for delivering a chemoablation fluid to prostate tissue transurethrally, an antiandrogen drug such as bicalutamide, and a second surgical device for administering the antiandrogen drug, is further provided.
Owner:BOSTON SCI SCIMED INC
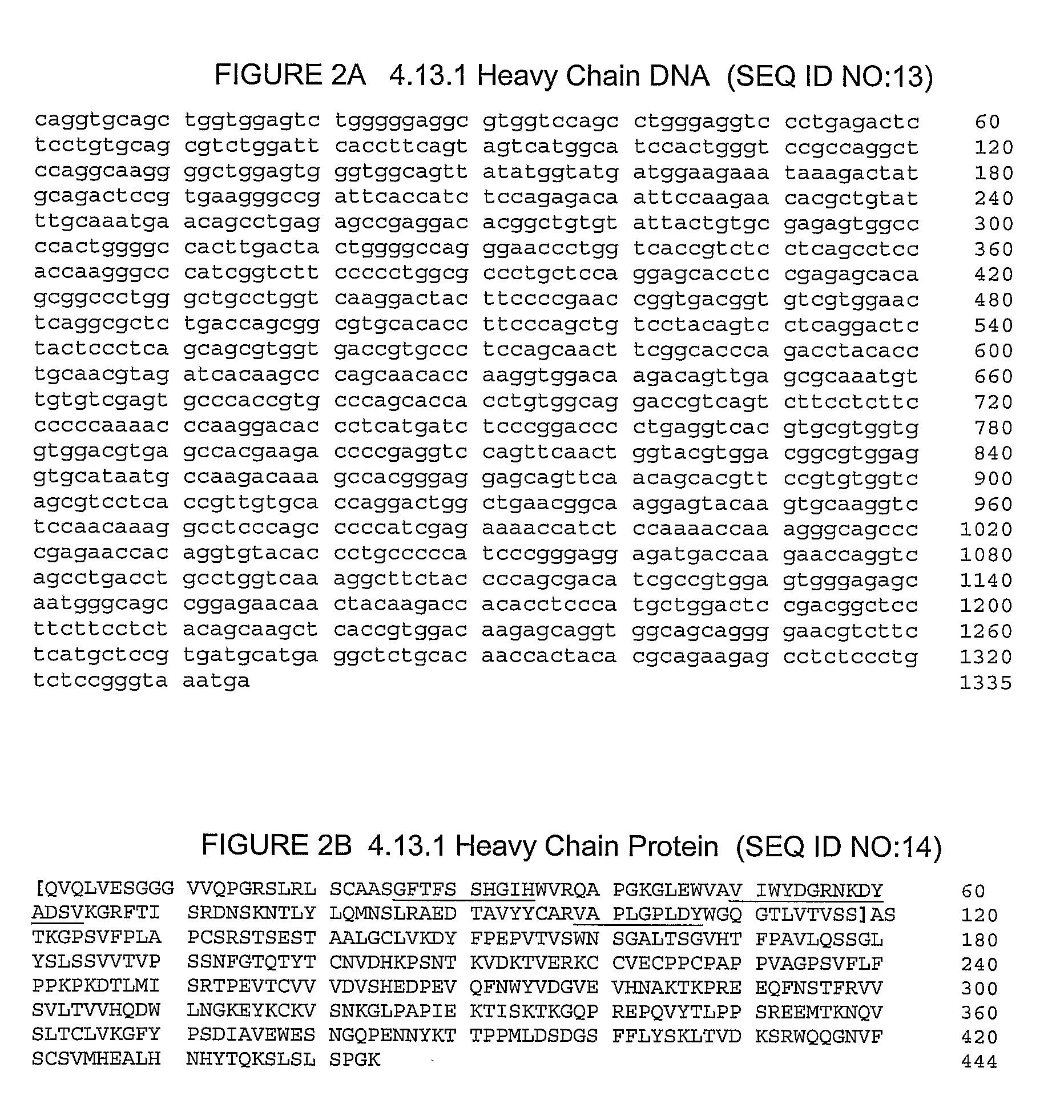
Therapy of Prostate Cancer With Ctla-4 Antibodies and Hormonal Therapy
InactiveUS20080279865A1Peptide/protein ingredientsAntibody ingredientsHistrelinAntiendomysial antibodies
The invention relates to methods for treating prostate cancer comprising administration of an anti-CTLA4 antibody, or an antigen-binding portion thereof, particularly a human antibody to human CTLA4, e.g., antibody 3.1.1, 4.1.1, 4.8.1, 4.10.2, 4.13.1, 4.14.3, 6.1.1, ticilimumab (also known as 11.2.1), 11.6.1, 11.7.1, 12.3.1.1, 12.9.1.1, and ipilimumab (also known as MDX-010 and 10D1), in combination with hormonal therapy. Hormonal therapy agents include, inter alia, an anti-androgen (e.g., megestrol, cyproterone, flutamide, nilutamide, and bicalutamide), a GnRH antagonist (e.g., abarelix and histrelin), and a LH-RH agonist (e.g., leuprolide, goserelin, and buserelin). The invention relates to neoadjuvant therapy, adjuvant therapy, therapy for rising PSA, first-line therapy, second-line therapy, and third-line therapy of prostate cancer, whether localized or metastasized.
Owner:PFIZER PFIZER PRODS
Pharmaceutical formulation
The present invention relates to a pharmaceutical formulation comprising bicalutamide and an enteric polymer having a pKa from 3 to 6. The invention also relates to a daily pharmaceutical dose of bicalutamide provided by such a formulation. In addition, the invention relates to the use of such an enteric polymer in solid dispersion with bicalutamide for increasing the bioavailability of the bicalutamide; for reducing inter-patient variability in plasma concentrations of bicalutamide; or for treating and / or reducing the risk of prostate cancer in a patient.
Owner:ASTRAZENECA AB
Bicalutamide compositions
InactiveUS20050008691A1Good drug release properties/profilesSimple structurePowder deliveryBiocideMedicineDissolution
A bicalutamide pharmaceutical composition having a high content of bicalutamide is provided. The composition can be made from micronized bicalutamide in order to enhance the speed of dissolution and is preferably made from a granulate of bicalutamide that contains at least 50 (w / w)% of bicalutamide
Owner:SYNTHON IP
Novel process for preparing and isolating rac-bicalutamide and its intermediates
InactiveUS20040044249A1Increase ratingsGood reproducibilityLithium organic compoundsOrganic compound preparationPropionatePropanoic acid
The present invention relates to a new process for the synthesis of racemic and optically active bicalutamide starting from ethyl pyruvate and methyl methacrylate. The present invention discloses processes of preparing bicalutamide intermediates including ethyl-[2-{4-fluorophenyl sulfone}]-2-hydroxy propionate, 1,2-epoxy-2-methyl propionate and 2-hydrox-2-methyl-3-(4-fluorophenylthio) propionic acid. The present invention further discloses micronized rac-bicalutamide and the preparation thereof. The present invention further discloses a new process for the isolation and purification of racemic and optically active bicalutamide.
Owner:TEVA PHARM USA INC +1
Crystals of bicalutamide and process for their production
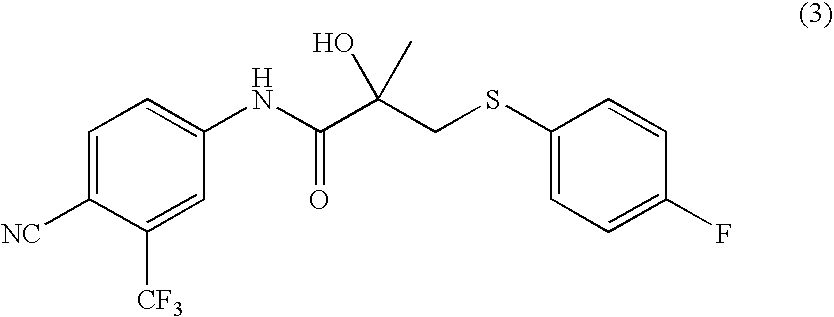
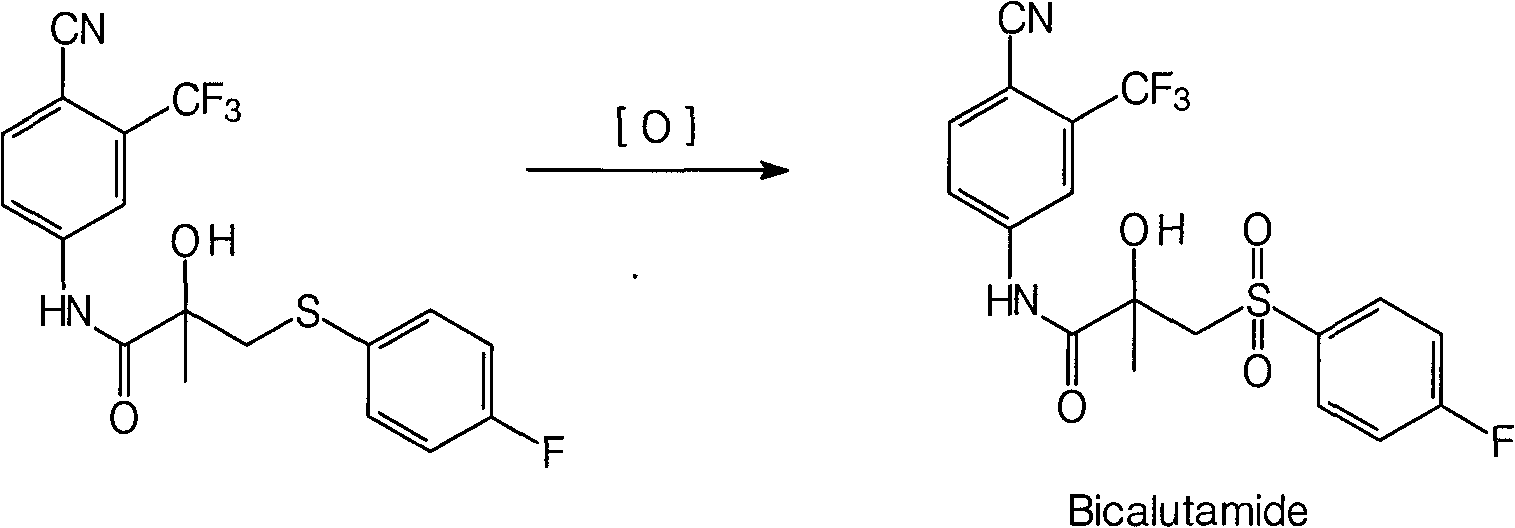
The present invention relates to a crystal of bicalutamide having a defined form, as well as economical and industrially practical production methods of bicalutamide and a crystal thereof, which are superior in environmental benignity and safety. Accordingly, the present invention provides a production method of bicalutamide represented by the formula (I): which includes at least a step of reacting a compound represented by the formula (3): with an oxidizing agent, a production method of a crystal of bicalutamide, as well as a crystal form of bicalutamide as defined by X-ray diffraction (XRD) or solid <13>C NMR measurement.
Owner:SUMITOMO CHEM CO LTD
Process for producing bicalutamide and method of purifying intermediate thereof
InactiveUS20060183934A1Organic chemistryOrganic compound preparationPurification methodsCarboxylic acid
The present invention provides a process for producing bicartamide of the formula (4); which comprises Step A comprising reacting Compound (1) of the formula (1); with peroxycarboxylic acid to obtain Compound (2) of the formula (2); Step B comprising reacting said Compound (2) with 4-fluorothiophenol to obtain crude crystals of Compound (3) of the formula (3); dissolving the crude crystals in a solvent and crystallizing to obtain purified crystals of Compound (3), and Step C comprising reacting Compound (3) and percarboxylic acid to obtain bicalutamide, and also provides a method for purifying crystals of Compound (3) which comprises dissolving crude crystals of Compound (3) in a solvent and crystallizing.
Owner:SUMITOMO CHEM CO LTD
Process for preparation of bicalutamide
A process for preparation of Bicalutamide of formula (I),comprising oxidation of compound of formula (II),with potassium permanganate in presence of water or a mixture of water and water miscible solvent and isolating Bicalutamide of formula (I) thereof.
Owner:FRESENIUS KABI ONCOLOGY LTD
Therapy of prostate cancer with CTLA4 antibodies and hormonal therapy
InactiveCN101146552APeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsHistrelinFlutamide
The present invention relates to a method for treating prostate cancer, which comprises administering an anti-CTLA4 antibody, or an antigen-binding portion thereof, especially a human antibody against human CTLA4, such as antibodies 3.1.1, 4.1.1, 4.8.1, 4.10.2 , 4.13.1, 4.14.3, 6.1.1, ticilimumab (also known as 11.2.1), 11.6.1, 11.7.1, 12.3.1.1, 12.9.1.1, and ipilimumab (also known as MDX-101 and 10D1) , in combination with hormone therapy. Hormone therapy agents include, inter alia, antiandrogens (e.g., megestrol, cyproterone, flutamide, nilutamide, and bicalutamide), GnRH antagonists (e.g., ababa Rick and histrelin), and LH-RH agonists (eg, leuprolide, goserelin, and buserelin). The present invention relates to neoadjuvant therapy, adjuvant therapy, treatment of elevated PSA, first-line treatment, second-line treatment and third-line treatment of localized or metastatic prostate cancer.
Owner:PFIZER PROD INC
Preparation method of medicine (R)-Bicalutamide for resisting prostatic cancer
ActiveCN101863806AHigh yieldReduce pollutionOrganic chemistryOrganic compound preparationAnilineMethyl group
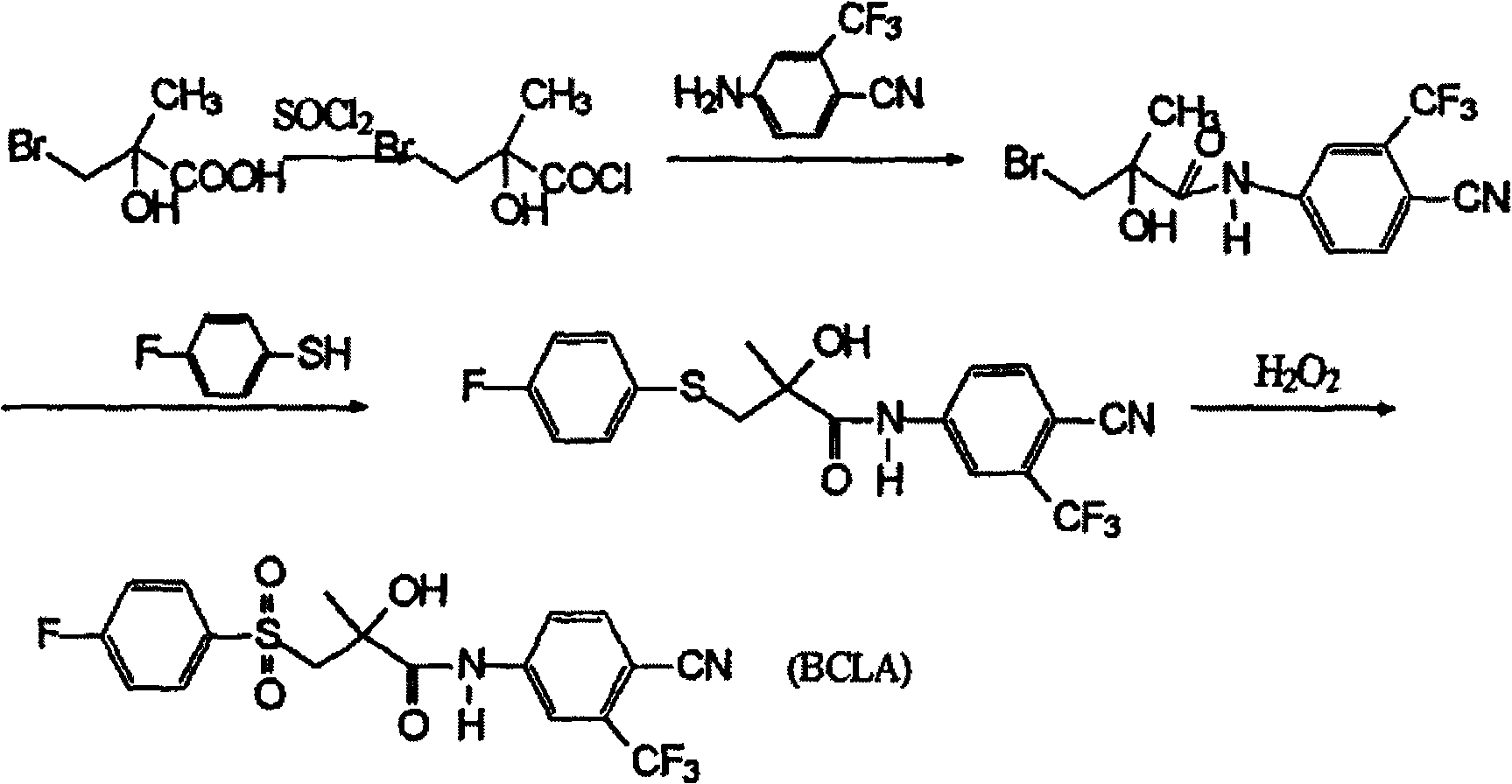
The invention relates to a preparation method of a medicine (R)-Bicalutamide for resisting prostatic cancer, which comprises the following steps: taking (R)-3-bromine-2-hydroxy-2-methylpropionate as a raw material; carrying out acylation reaction, condensation reaction and oxidizing reaction to obtain the (R)-Bicalutamide, wherein the acylation reaction is carried out in the following steps: dissolving the (R)-3-bromine-2-hydroxy-2-methylpropionate, 4-cyan-3-(trifluoro-methyl)aniline and 4-dimethylamino pyridine into N, N- dimethyl acetamide, dropwise adding thionyl chloride at temperature between minus 10 and minus15 DEG C, then preserving the temperature and reacting for 1-5h after finishing adding the thionyl chloride, heating to room temperature for reaction for 8-12h, and purifying to obtain the (R)-3-bromine-2-hydroxy-2-methyl-N-[4-cyan-3-(trifluoro-methyl phenyl)] propionamide. The preparation method is simple in operation, has short reaction time and high yield, and is suitable for industry production.
Owner:湖北省医药工业研究院有限公司
New process for preparing epoxide intermediate of Bicalutamide by utilizing air oxidation
The invention discloses a new process for preparing an epoxide intermediate of Bicalutamide by utilizing air oxidation. The process comprises the following steps: using N-(4-cyan-3-trifluoromethylphenyl)-2-methacrylamide as the compound raw material, dissolving the N-(4-cyan-3-trifluoromethylphenyl)-2-methacrylamide in a reaction solvent, adding aldehyde, a free-radical initiator and a catalyst, and carrying out air oxidation reaction at negative 20-70 DEG C for 5-45 hours, thereby obtaining the epoxide intermediate of the Bicalutamide. The mass amount of the reaction solvent is 3-15 times of that of the compound raw material; the mol ratio of the aldehyde to the compound raw material is 0.1-4.0; the mol ratio of the free-radical initiator to the compound raw material is 0.05-5%; and the mol ratio of the catalyst to the compound raw material is 0.01-2%. The process only consumes oxygen in the air is consumed, the economical efficiency of atoms is greatly enhanced, and the cost is greatly lowered; the equipment and instruments required by the reaction are simple and easy to operate, and are convenient for after treatment; the product yield is 80-95%, the purity reaches up to 99%, and the product has good color; the process is environment-friendly, since acid generated in the reaction is recyclable, no environmental pollution is caused; and the process has the advantages of no explosion hazard and high safety, and is suitable for industrial production.
Owner:宁波人健化学制药有限公司
Process for making bicalutamide and intermediates thereof
Bicalutamide and / or its intermediates are made by the use of p-fluorobenzenesulfinic acid salt as a reagent.
Owner:SYNTHON IP
Ratio karoo amine slice and preparing technique thereof
InactiveCN101199486ASuitable for production processOrganic active ingredientsUrinary disorderDissolutionBicalutamide
The invention provides a bicalutamide tablet and the related preparation process. The invention adopts new tablet formula and new technique and overcomes the shortcomings in prior art. The technique of the invention is favorable for scale-type industrial production and the product is improved in quality and is good in dissolution.
Owner:SHANGHAI FOSUN PHARMA (GROUP) CO LTD +1
Crystal of bicalutamide and production method thereof
InactiveUS7132560B2Superior in environmental benignityImprove securityOrganic compound preparationOrganic chemistry methodsChemical compoundBicalutamide
The present invention relates to a crystal of bicalutamide having a defined form, as well as economical and industrially practical production methods of bicalutamide and a crystal thereof, which are superior in environmental benignity and safety.Accordingly, the present invention provides a production method of bicalutamide represented by the formula (I):which includes at least a step of reacting a compound represented by the formula (3):with an oxidizing agent, a production method of a crystal of bicalutamide, as well as a crystal form of bicalutamide as defined by X-ray diffraction (XRD) or solid 13C NMR measurement.
Owner:SUMITOMO CHEM CO LTD
Method for preparing bicalutamide by oxidization
InactiveCN102321000AReduce pollutionImprove pollutionOrganic chemistryOrganic compound preparationPotassiumSolvent
The invention relates to a method for preparing bicalutamide by oxidization of N-(4-cyan-3-trifluoromethylphenyl)-3-(4-fluorophenylthio)-2-methyl-2-hydroxylpropionamide. The method comprises the step of carrying out oxidization reaction on N-(4-cyan-3-trifluoromethylphenyl)-3-(4-fluorophenylthio)-2-methyl-2-hydroxylpropionamide which is used as a raw material in a solvent in the present of an oxidant so as to prepare the bicalutamide. The method is characterized in that the oxidant is potassium monopersulfate or a complex salt of potassium monopersulfate. Compared with the prior art, the method provided by the invention has the advantages that the bicalutamide is prepared by using potassium monopersulfate or the complex salt of potassium monopersulfate as the oxidant; the oxidant can be abundantly supplied on the market, is cheap in price and available, and cost is greatly reduced; and reaction conditions are mild, and no wastes which can pollute environment are generated.
Owner:宁波人健药业集团股份有限公司
Pharmaceutical formulation comprising bicalutamide
Owner:ASTRAZENCA UK LTD
Bicalutamide polymorphs
The invention provides crystalline form of bicalutamide and amorphous bicalutamide. The invention also provides methods for their preparation and pharmaceutical compositions containing the new forms of bicalutamide.
Owner:HETERO DRUGS LTD
Process for production of bicalutamide
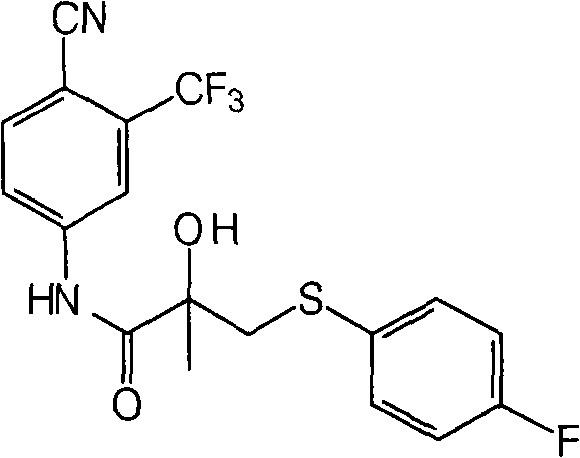
A process which includes the reacting of sodium perborate with N-[4-cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methylpropanamide to form bicalutamide. The process is efficient, inexpensive, environmentally friendly and produces bicalutamide in good yield.
Owner:APOTEX PHARMACHEN INC
Novel Process for Preparation of Bicalutamide
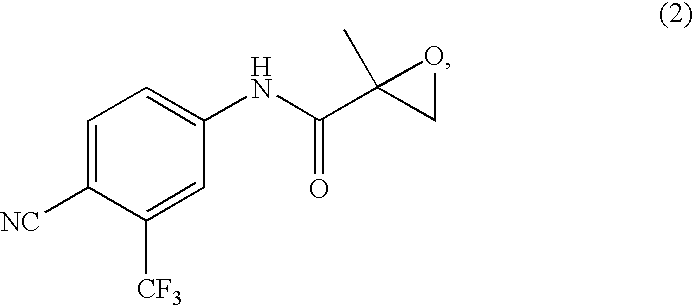
InactiveUS20080177109A1Improved and industrially viable and cost-effective processHigh yieldOrganic compound preparationOrganic chemistry methodsThio-Hexane
The present invention discloses a process for the synthesis of N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulphonyl]-2-hydroxy-2-methyl propanamide (Form I). The invention discloses a reagent for oxidation of N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)thio]-2-hydroxy-2-methyl propanamide to N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulphonyl]-2-hydroxy-2-methyl propanamide. More particularly, the invention discloses a method of purification of N-[4-Cyano-3-(trifluoromethyl)phenyl]-3-[(4-fluorophenyl)sulphonyl]-2-hydroxy-2-methyl propanamide in a mixture of methylethyl ketone and hexane giving form (I). This form (I) is useful as an active pharmaceutical and has antiandrogenic activity.
Owner:USV LTD
Process for preparing and isolating rac-bicalutamide and its intermediates
InactiveUS7102026B2High dissolution rateImproved and stable profileBiocideLithium organic compoundsPropionatePropanoic acid
The present invention relates to a new process for the synthesis of racemic and optically active bicalutamide starting from ethyl pyruvate and methyl methacrylate. The present invention discloses processes of preparing bicalutamide intermediates including ethyl-[2-{4-fluorophenyl sulfone}]-2-hydroxy propionate, 1,2-epoxy-2-methyl propionate and 2-hydrox-2-methyl-3-(4-fluorophenylthio) propionic acid. The present invention further discloses micronized rac-bicalutamide and the preparation thereof. The present invention further discloses a new process for the isolation and purification of racemic and optically active bicalutamide.
Owner:TEVA PHARM USA INC +1
New process of preparing bicalutamide by utilizing air for oxidizing bicalutamide thioether intermediate
ActiveCN101817768AHigh purityReduce pollutionOrganic chemistryOrganic compound preparationFiltrationReaction temperature
The invention discloses a new process of preparing bicalutamide by utilizing air for oxidizing a bicalutamide thioether intermediate, and the process comprises the following steps: taking N-(4-cyano-3-trifluoromethylphenyl)-3-(4-fluorophenylthio)-2-methyl-hydroxy-propionamide as a compound raw material, dissolving in a reaction solvent, further adding aldehyde, a free radical initiator and a catalyst, using the air for carrying out oxidation for 1-25h at the reaction temperature of -20-50 DEG C, and finally preparing the bicalutamide; the mass using quantity of the reaction solvent is 3-10 times of the mass of the compound raw material; the ratio of the molar using quantity of the aldehyde to the molar using quantity of the compound raw material is 0.1-5.0; the ratio of the molar using quantity of the free radical initiator to the molar using quantity of the compound raw material is 0.05-5%; and the ratio of the molar using quantity of the catalyst to the molar using quantity of the compound raw material is that: the metal catalyst is 0.01-1%; and the non-metal catalyst is 0.01-2%. The new process has the advantages of only consuming oxygen in the air, improving atom economy, reducing cost, not producing environmental pollution and achieving green environmental protection; the post-treatment is simple and easy to operate, and the produced acid can be recycled; a product can be obtained by direct filtration, the yield is near to 100%, the HPLC purity can be more than 99%, and the safety is high, thereby being suitable for industrial production.
Owner:宁波人健化学制药有限公司
Compound drug capable of resisting prostatic cancer
ActiveCN110327465AGood effectEnhanced inhibitory effectAntineoplastic agentsNitrile/isonitrile active ingredientsCompounding drugsSide effect
The invention provides a compound drug capable of resisting the prostatic cancer. The compound drug consists of a polymethoxyflavone drug and an androgen receptor inhibitor drug, wherein the mass ratio of the polymethoxyflavone drug to the androgen receptor inhibitor drug is 1:1, the polymethoxyflavone drug is nobiletin, and the androgen receptor inhibitor drug is bicalutamide. The polymethoxyflavone drug and the androgen receptor inhibitor anti-cancer drug are jointly used, a drug function is obviously superior to single drug utilization, and therefore, the compound drug has a good inhibitioneffect on inhibiting the prostatic cancer and preventing the prostatic cancer to develop to malignance. The drug provided by the invention is low in a use dosage, and lowers the concentration of single drug utilization so as to reduce the toxic and side effects of the drug and reduce harm on the human body, the economic burden of a patient is further reduced through mixed utilization, and the compound drug performs a better effect than single utilization.
Owner:WUYI UNIV
UPLC (ultra-high performance liquid chromatography) method for simultaneously determining six related substances in bicalutamide
InactiveCN104897818AEfficient separationAccurate detectionComponent separationGradient elutionColumn temperature
The invention relates to a UPLC (ultra-high performance liquid chromatography) method for simultaneously determining six related substances in bicalutamide. The method includes the steps of firstly, preparing sample solution; secondly, preparing control solution; thirdly, performing UPLC determination, to be more specific, performing determination analysis on the control solution and the sample solution under the chromatographic conditions, wherein the chromatographic conditions include that a chromatographic column is ACQUITY UPLC BEH C18 (1.7 micrometers, 2.1mm*50mm), the flow phase A is 0.01% (v / v) trifluoroacetic acid aqueous solution, the flow phase B is 0.01% (v / v) trifluoroacetic acid acetonitrile solution, gradient elution is performed, flow speed is 0.5ml.min<-1>, column temperature is 25 DEG C, and detecting wavelength is 270 nanometers. The method has the advantages that under the conditions of the method, the bicalutamide and the six related substances are effectively separated, and methodology results conform to analyzing and determination requirements; the impurities and degradation products of the bicalutamide can be detected fast and accurately, and the method is simple to operate, good in reproducibility, high in flexibility and capable of well controlling bicalutamide product quality.
Owner:CHONGQING MEDICAL & PHARMA COLLEGE
Combination therapy for prostate cancer using botanical compositions and bicalutamide
InactiveCN103327994APeptide/protein ingredientsPill deliverySalvia miltiorrhizaCombined Modality Therapy
Botanical compositions comprising non-alcoholic organic extracts of Ganoderma lucidum, Salvia miltiorrhiza, and Scutellaria barbata for use in conjunction with bicalutamide therapy fpr cancer therapy, are provided. Methods for treatment or therapy of prostate cancer in a human is provided, the method comprising: administering an effective amount of a botanical composition that is effective for reducing androgen receptor protein expression; and administering concurrently an effective amount of a compound having anti-androgen activity, wherein the concurrent administration of the compound and the botanical composition achieves a therapeutic effect that is more effective than either agent alone.
Owner:GENYOUS BIOMED INT
(Heteroarylmethyl) Thiohydantoins as anticancer drugs
The invention refers to the use of androgen receptor antagonists for the treatment and / or prevention of fibroids, also known as uterine leiomyoma, leiomyomata. Particularly, the invention refers to the use of an androgen receptor antagonist being any one of the compounds according to the following list: cyproterone acetate, oxendolone, chlormadinone acetate, spironolactone, osaterone acetate, dienogest, flutamide, hydroxyflutamide, nilutamide, bicalutamide, RU 58841, LGD-2226, MDV3100, BMS-641988, BMS-779333, or 4-(3-{[6-(2-hydroxy-2-methylpropoxy)pyridin-3-yl]methyl}-4,4-dimethyl-5-oxo-2-thioxoimidazolidin-1-yl)-2-(trifluoromethyl)benzonitrile (thioxoimidazolidine derivative) for the treatment of fibroids.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
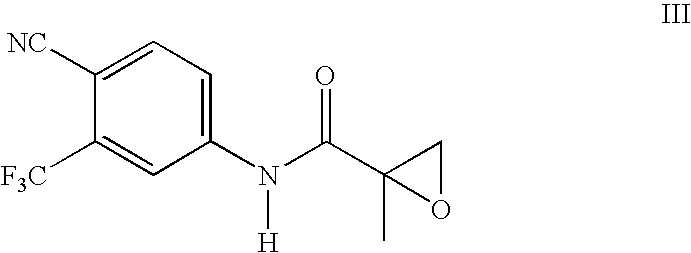
Preparation method of bicalutamide intermediate
The invention provides a preparation method of bicalutamide intermediate shown in the formula (II) and the method is more reasonable and more suitable for industrial production. The method comprises the following steps: adding compound (I), potassium carbonate and p-fluorophenol in reaction solvent, stirring while slowly heating the mixture to reaction temperature 30-80 DEG C, reacting for 12-18 hours, extracting, and purifying. The method of the invention is performed under mild conditions, the reagent potassium carbonate is accessible, the price is cheap and the method does not have burning and blast hazards, and the use security is high. Agents are added at room temperature without the protection of nitrogen, the reaction solvent does not need special treatment, the reaction yield is more than 75%, the raw material cost is greatly reduced and the method is more suitable for industrial production.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Pharmaceutical preparation
InactiveCN1503662APowder deliveryPharmaceutical non-active ingredientsProstate cancerBioavailability
The present invention relates to a pharmaceutical formulation comprising bicalutamide and an enteric polymer having a pKa from 3 to 6. The invention also relates to a daily pharmaceutical dose of bicalutamide provided by such a formulation. In addition, the invention relates to the use of such an enteric polymer in solid dispersion with bicalutamide for increasing the bioavailability of the bicalutamide; for reducing inter-patient variability in plasma concentrations of bicalutamide; or for treating and / or reducing the risk of prostate cancer in a patient.
Owner:ASTRAZENECA AB
Methods and compositions for producing anti-androgenic effects
The present invention relates to a preparation for oral use, which contains bicalutamide and has a modified release mode. The composition is suitable for adopting a dosage regimen which is three times a week, twice a week and once a week, and the composition is used for producing an anti-androgenic effect.
Owner:PANACEA BIOTEC
Nanoparticulate bicalutamide formulations
The present invention is directed to compositions comprising an acylanilide, such as bicalutamide, having improved solubility in water. The bicalutamide particles of the composition have an effective average particle size of less than about 2000 nm, and are useful in the treatment of prostate cancer.
Owner:ELAN PHRMA INT LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com