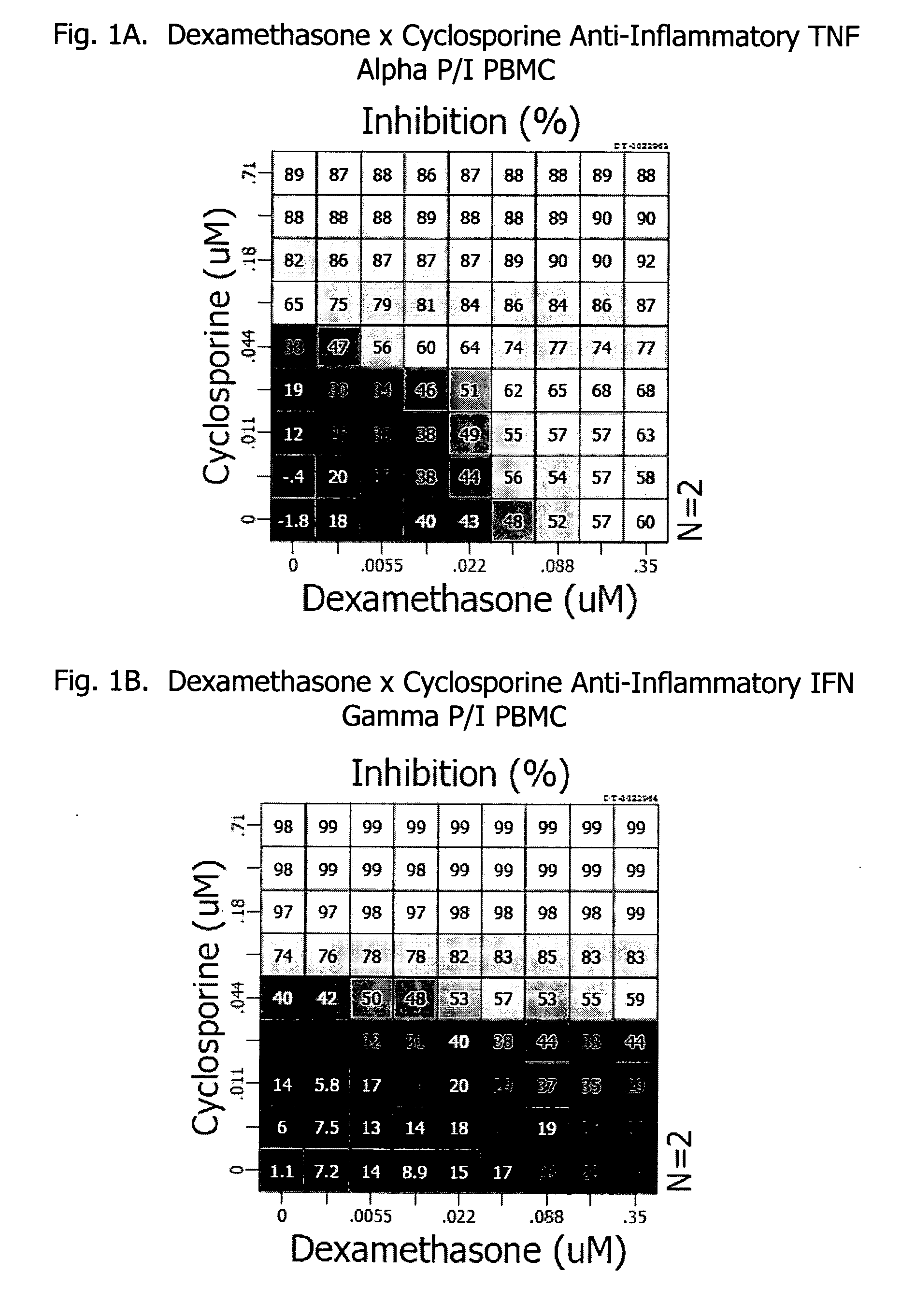
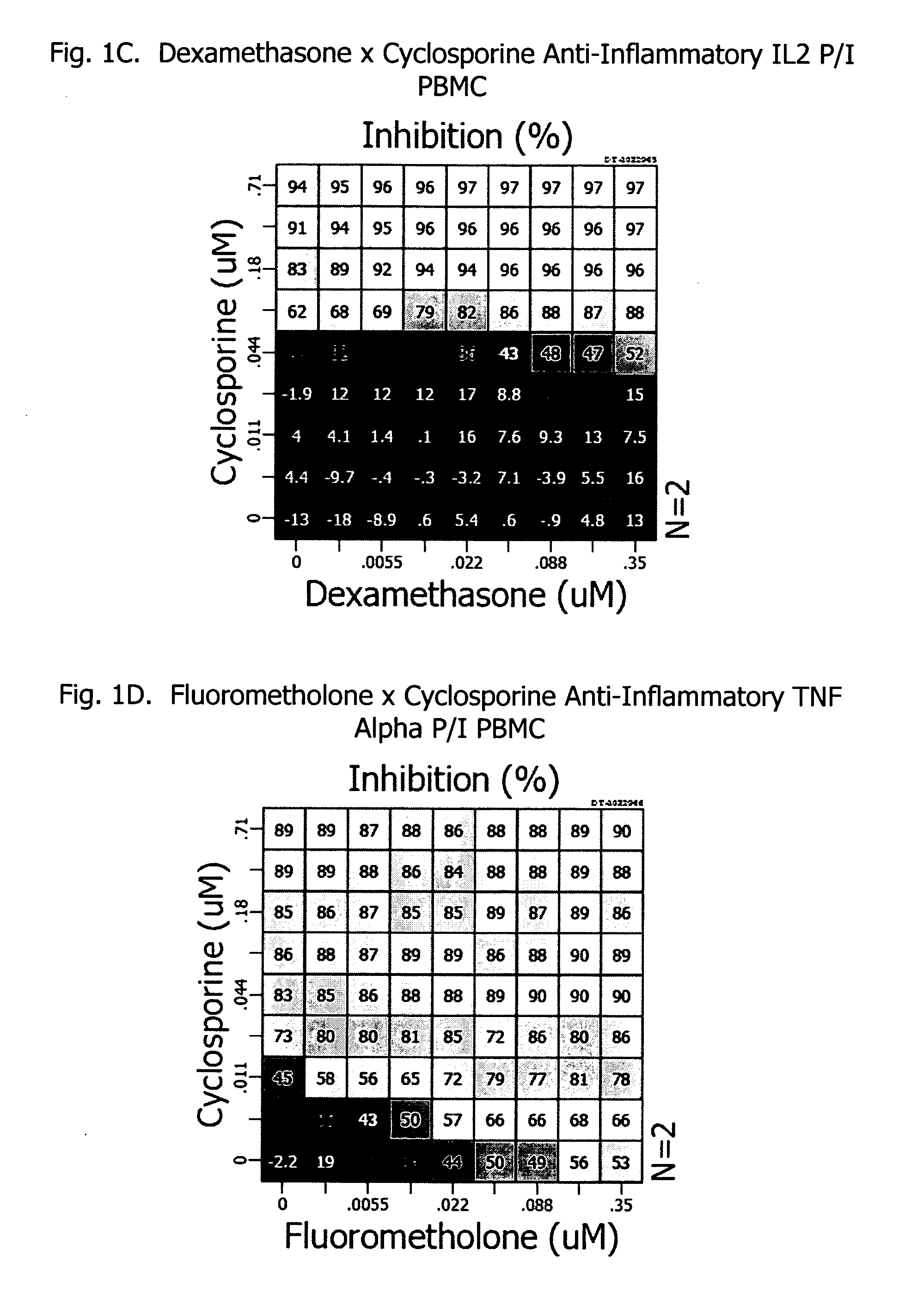
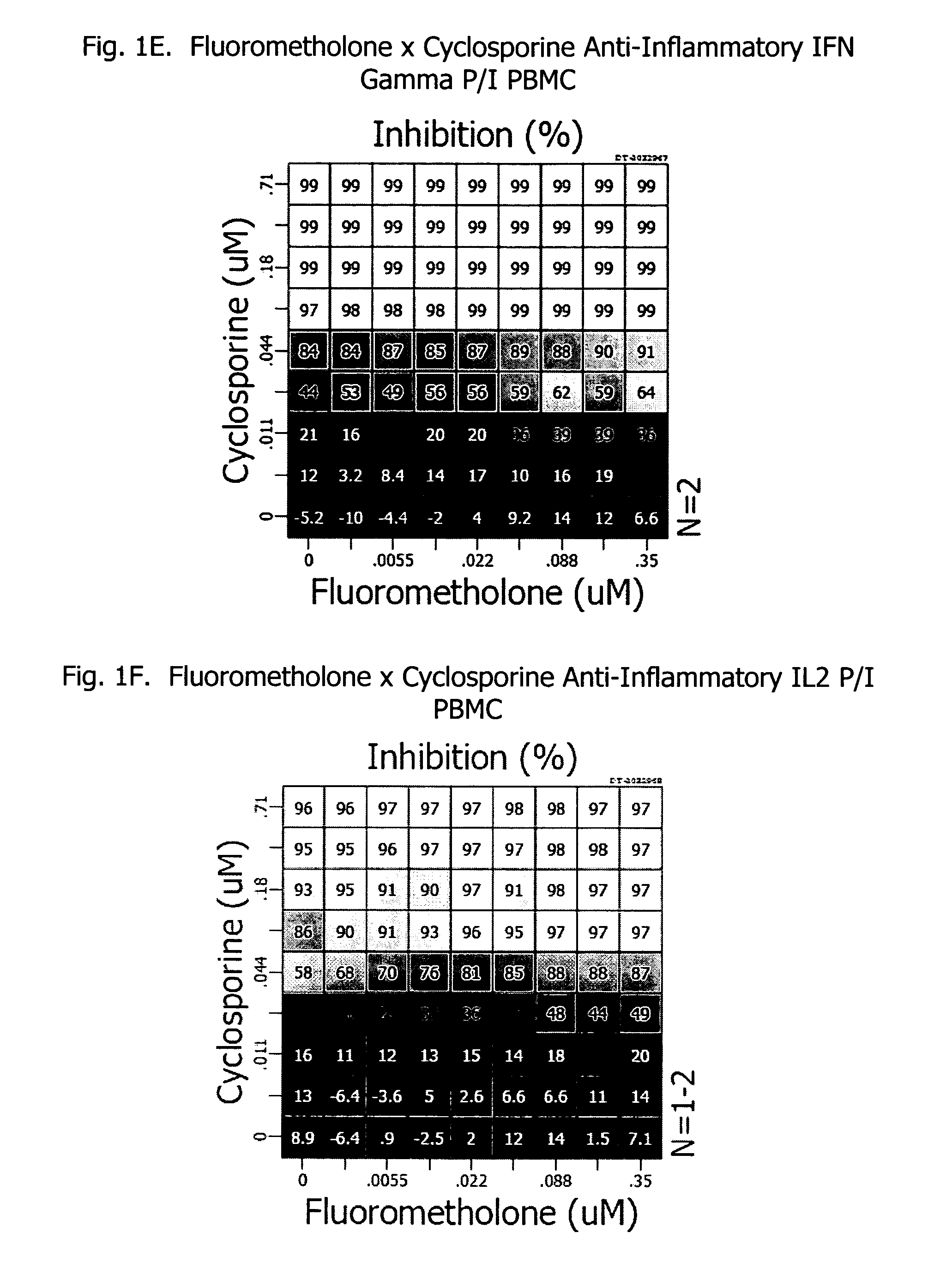
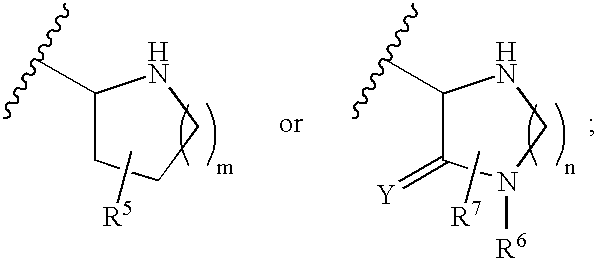Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
597 results about "Non steroidal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A nonsteroidal compound is a drug that is not a steroid nor a steroid derivative.
Transdermal drug delivery compositions and topical compositions for application on the skin
InactiveUS20090053290A1Improve biological activityEliminate side effectsPowder deliveryCosmetic preparationsRadio frequencyOtic Agents
Transdermal delivery compositions and topical compositions for application to the skin are provided. The transdermal delivery composition includes at least two penetrants working synergistically but by disparate biochemical pathways. In one embodiment, the transdermal delivery system includes benzyl alcohol and lecithin organogel. The transdermal delivery compositions are used in a variety of topical compositions as a means of transdermally delivering and topically administering different drugs and agents, including compositions promoting collagen biosynthesis, retinoids and skin lighteners, chemical denervation agents such as BOTOX®, anti-fungal agents, anesthetics and non-steroidal anti-inflammatory drugs (NSAIDs). In addition, these topical compositions may be used in combination with non-ablative treatment modalities, such as microdermabrasion, laser-based skin remodeling and radio-frequency-based skin remodeling.
Owner:NUVIANCE
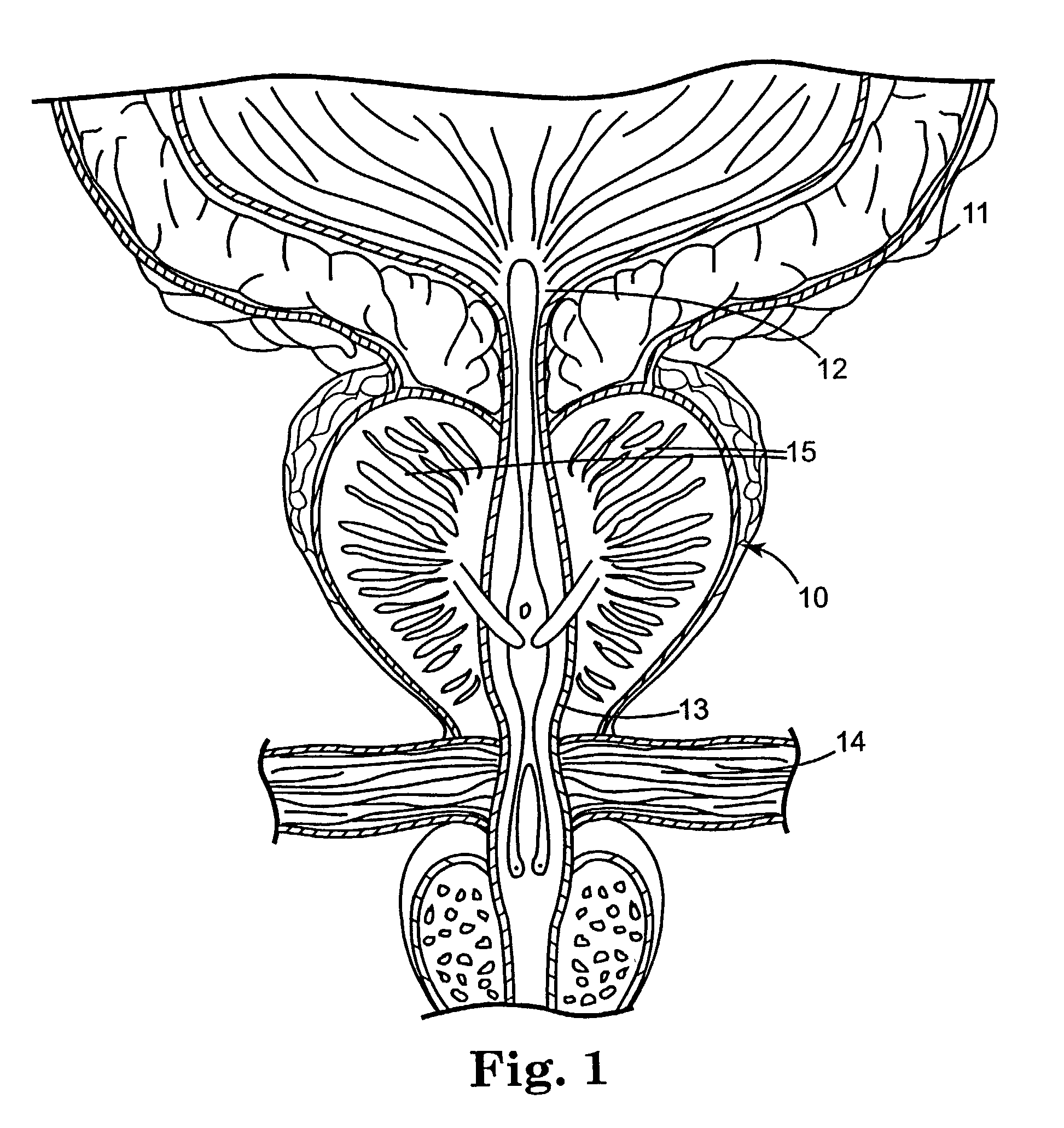
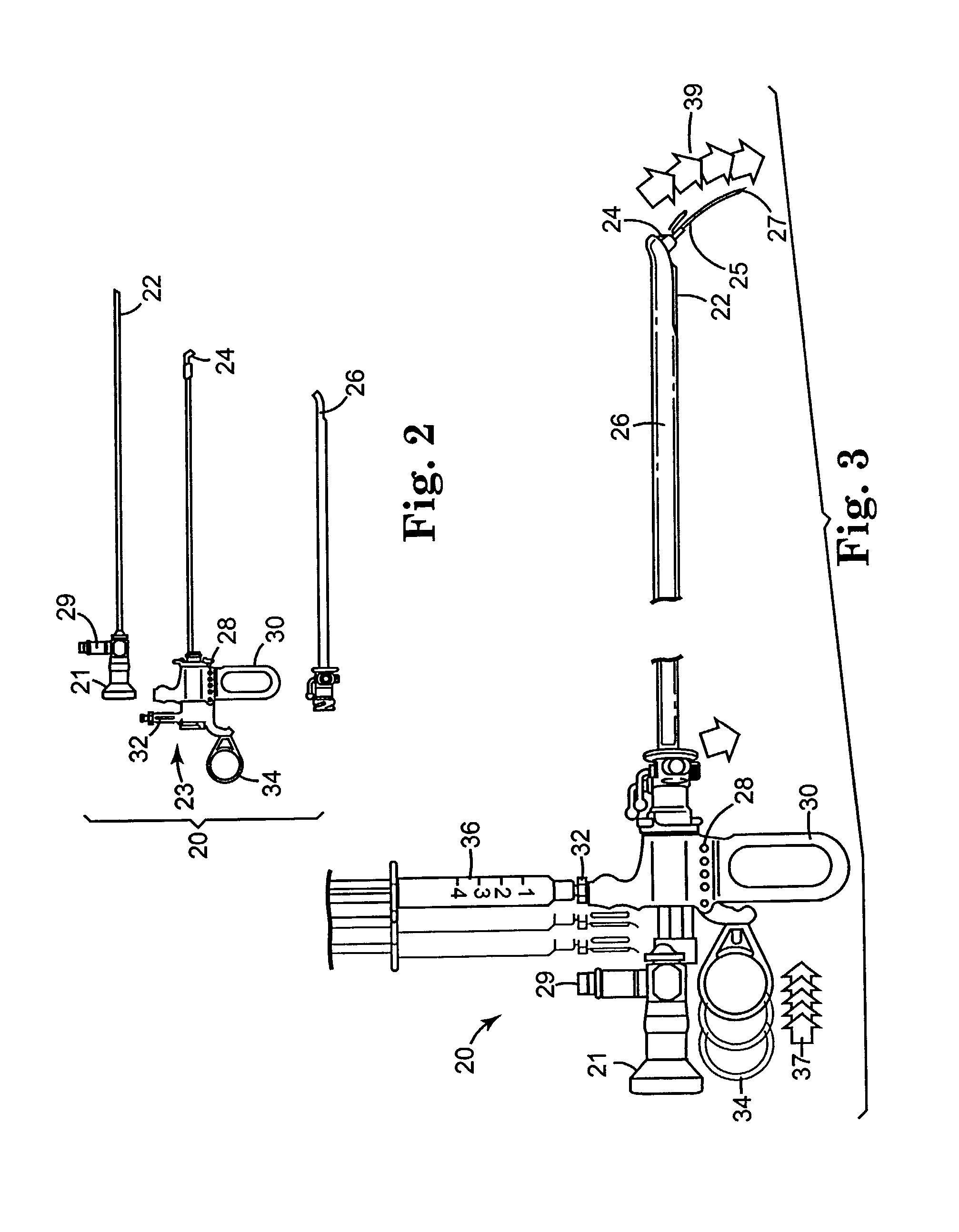
Regimen for treating prostate tissue and surgical kit for use in the regimen
InactiveUS7015253B2Decreasing prostate sizeSmall sizeBiocideHydroxy compound active ingredientsSteroidal antiandrogenRegimen
The present invention provides treatment regimens for treating diseased prostate tissue, including the steps of chemically ablating prostate tissue and coadministering an antiandrogen. In some embodiments, prostate tissue is chemically ablated by injection of ethanol, or an injectable gel comprising ethanol, into prostate tissue. Steroidal and non-steroidal antiandrogens are suitable antiandrogens. One suitable non-steroidal antiandrogen is bicalutamide. The treatment regimen is suitable for treatment of prostate tissue diseases including benign prostatic hyperplasia and prostatic carcinoma. The invention further provides a treatment regimen for treating benign prostatic hyperplasia, including the steps of damaging prostate tissue and coadministering an antiandrogen. Also provided by the present invention is a kit for treating a human male, including a means for necrosing prostate tissue, an antiandrogen drug, and a means for administering the antiandrogen drug. A kit including a first surgical device for delivering a chemoablation fluid to prostate tissue transurethrally, an antiandrogen drug such as bicalutamide, and a second surgical device for administering the antiandrogen drug, is further provided.
Owner:BOSTON SCI SCIMED INC
Synthesis of selective androgen receptor modulators
InactiveUS6995284B2Decreased libidoAlteration in mood and cognitionOrganic active ingredientsCarbamic acid derivatives preparationAging maleProstate cancer incidence
The present invention relates to a synthetic process for the preparation of a novel class of androgen receptor targeting agents (ARTA) which demonstrate androgenic and anabolic activity of a nonsteroidal ligand for the androgen receptor. The agents define a new subclass of compounds which are selective androgen receptor modulators (SARM) which are useful for a) male contraception; b) treatment of a variety of hormone-related conditions, for example conditions associated with Androgen Decline in Aging Male (ADAM), such as fatigue, depression, decreased libido, sexual dysfunction, erectile dysfunction, hypogonadism, osteoporosis, hair loss, anemia, obesity, sarcopenia, osteopenia, osteoporosis, benign prostate hyperplasia, alterations in mood and cognition and prostate cancer; c) treatment of conditions associated with Androgen Decline in Female (ADIF), such as sexual dysfunction, decreased sexual libido, hypogonadism, sarcopenia, osteopenia, osteoporosis, alterations in cognition and mood, depression, anemia, hair loss, obesity, endometriosis, breast cancer, uterine cancer and ovarian cancer; d) treatment and / or prevention of chronic muscular wasting; e) decreasing the incidence of, halting or causing a regression of prostate cancer; f) oral androgen relacement and / or other clinical therpauetic and / or diagnostic areas. The process of the present invention is suitable for large-scale preparation, since all of the steps give rise to highly pure compounds, thus avoiding complicated purification procedures which ultimately lower the yield. Thus the present invention provides methods for the synthesis of non-steroidal agonist compounds, that can be used for industrial large-scale synthesis, and that provide highly pure products in high yield.
Owner:UNIV OF TENNESSEE RES FOUND
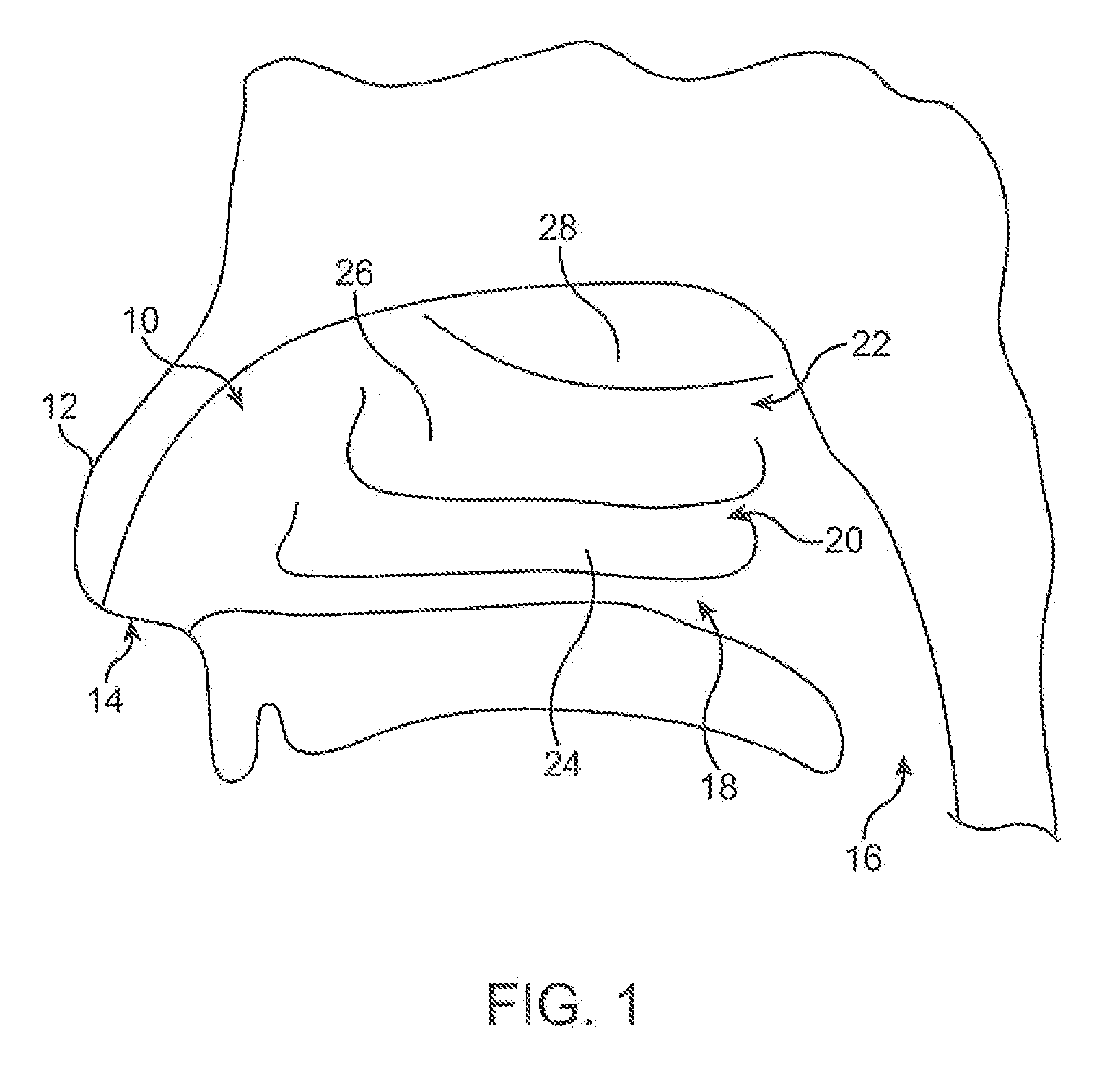
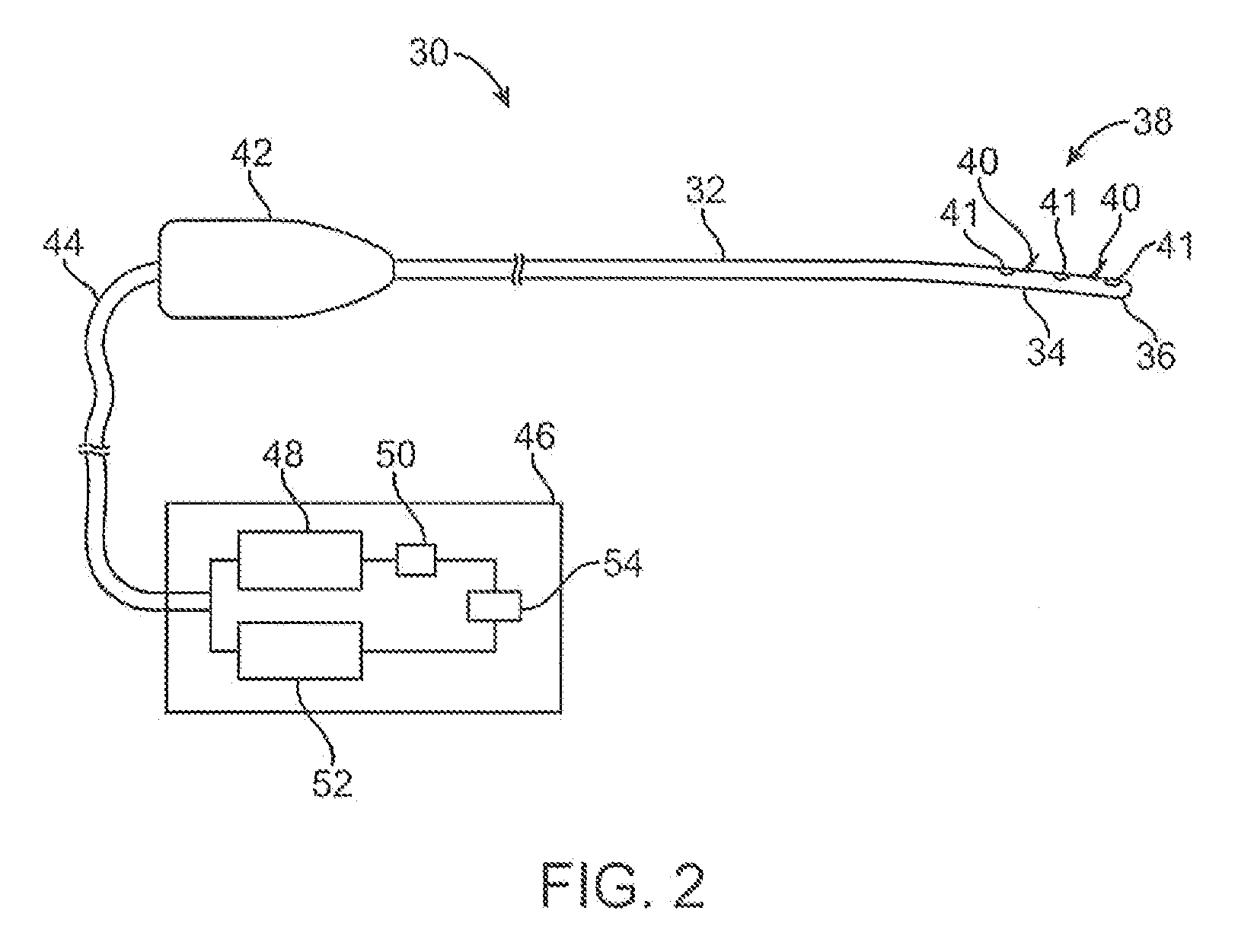
Systems for treatment of nasal tissue
InactiveUS20080027423A1Small sizeIncrease airflowUltrasound therapySurgical instrument detailsPain managementAnalgesic agents
Systems for the treatment of nasal tissue, particularly the nasal turbinates, are described. One method for reducing the size of the inferior nasal turbinate is to apply ultrasound energy to the tissue regions beneath the surface of the turbinate tissue. One instrument may be used to deliver ultrasound energy and provide an infusion or injection of a fluid directly into the turbinate being treated, e.g., to bulk up the size of the turbinate to ensure that the ultrasound energy is properly delivered directly into the intended turbinate tissue. Fluids containing anesthetics, fluids infused with analgesics, etc. may be used for pain management while other medications, such as non-steroidal drugs, steroidal drugs, anti-inflammatory drugs, anti-histamines, anti-bacterial drugs, etc., can also be used. Such assemblies can also be utilized with other instruments as a system. For example, such a probe can be used with nasal speculums or imaging instrument in treating tissue.
Owner:CHOI GEORGE Y
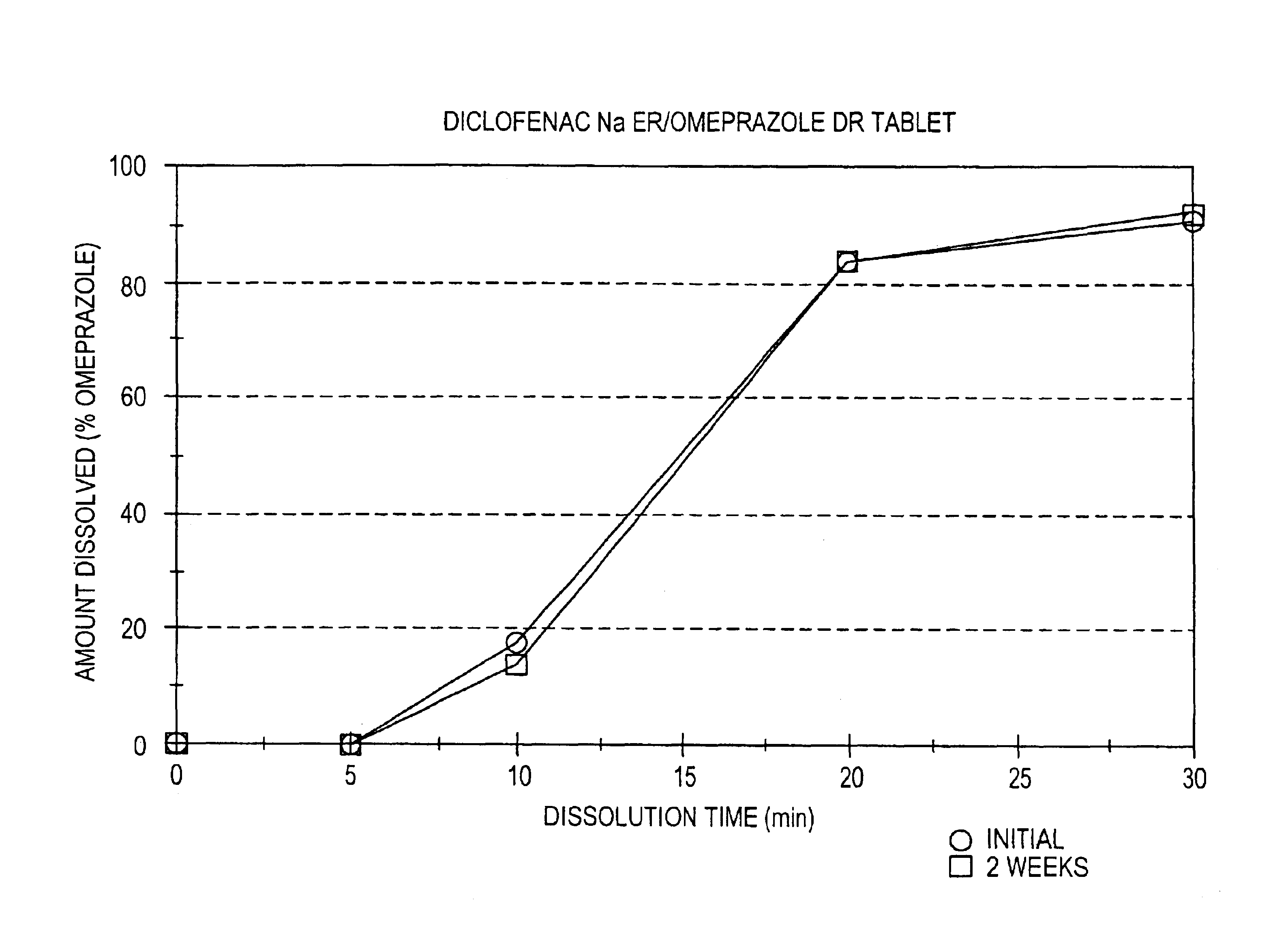
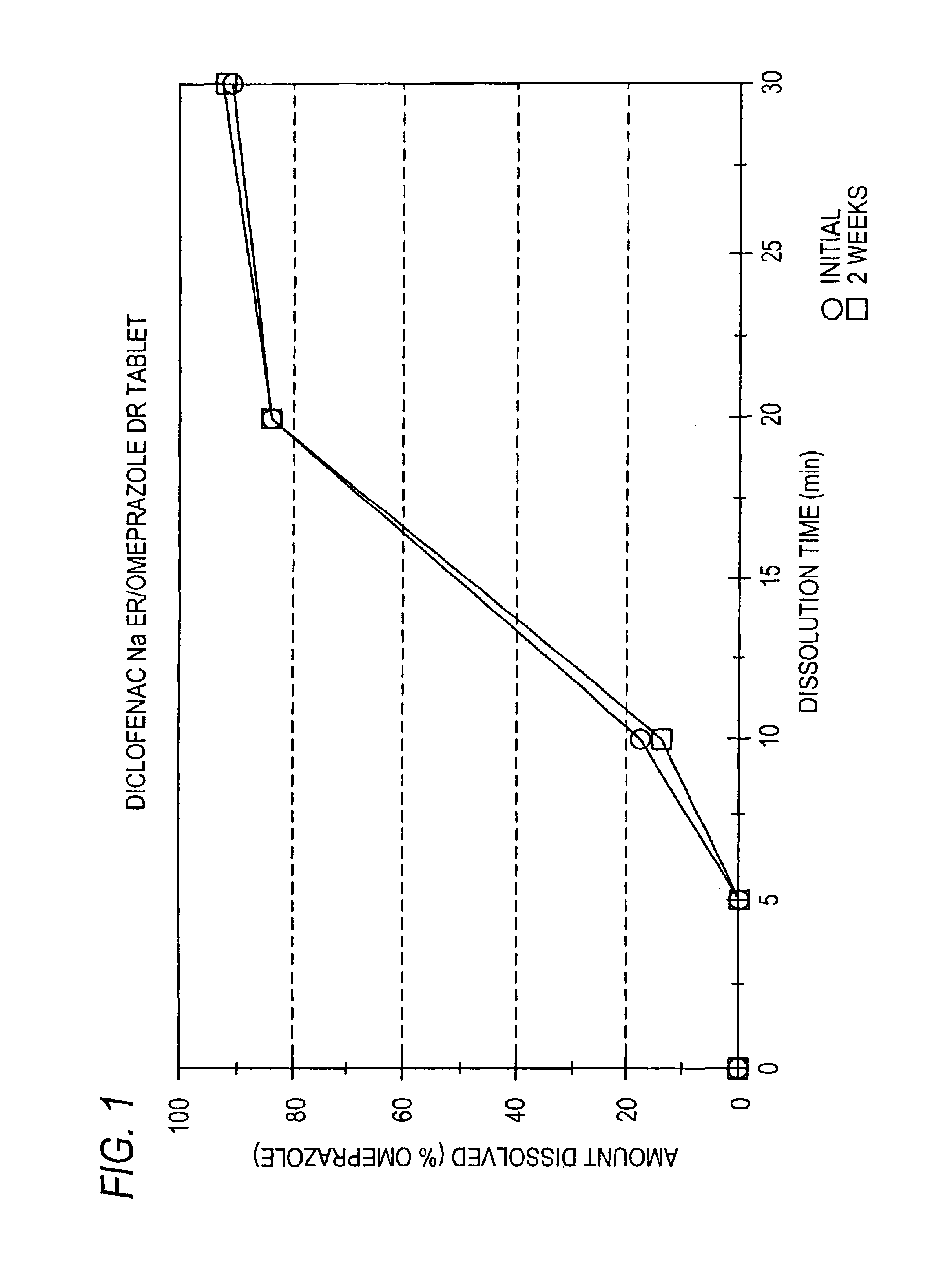
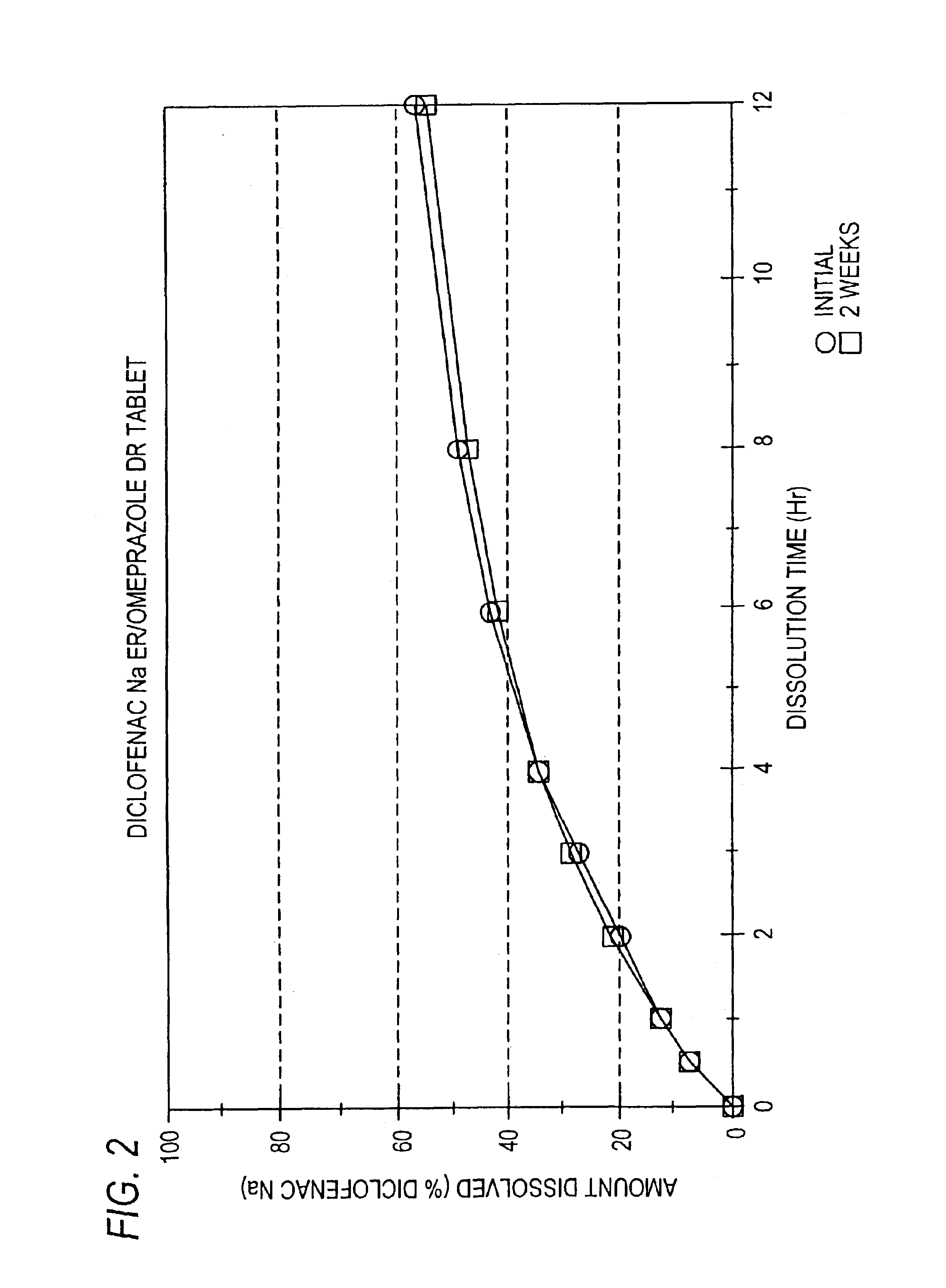
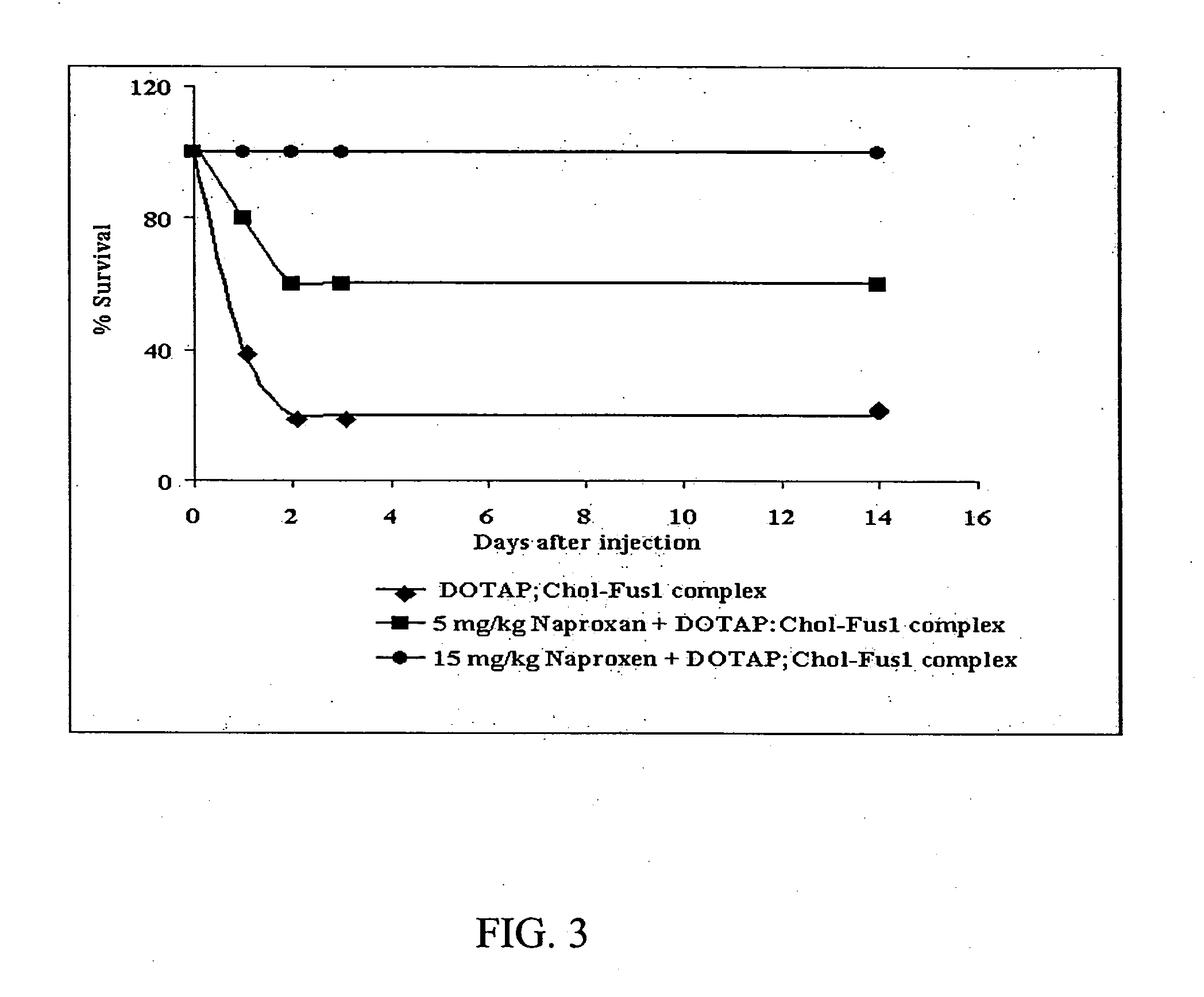
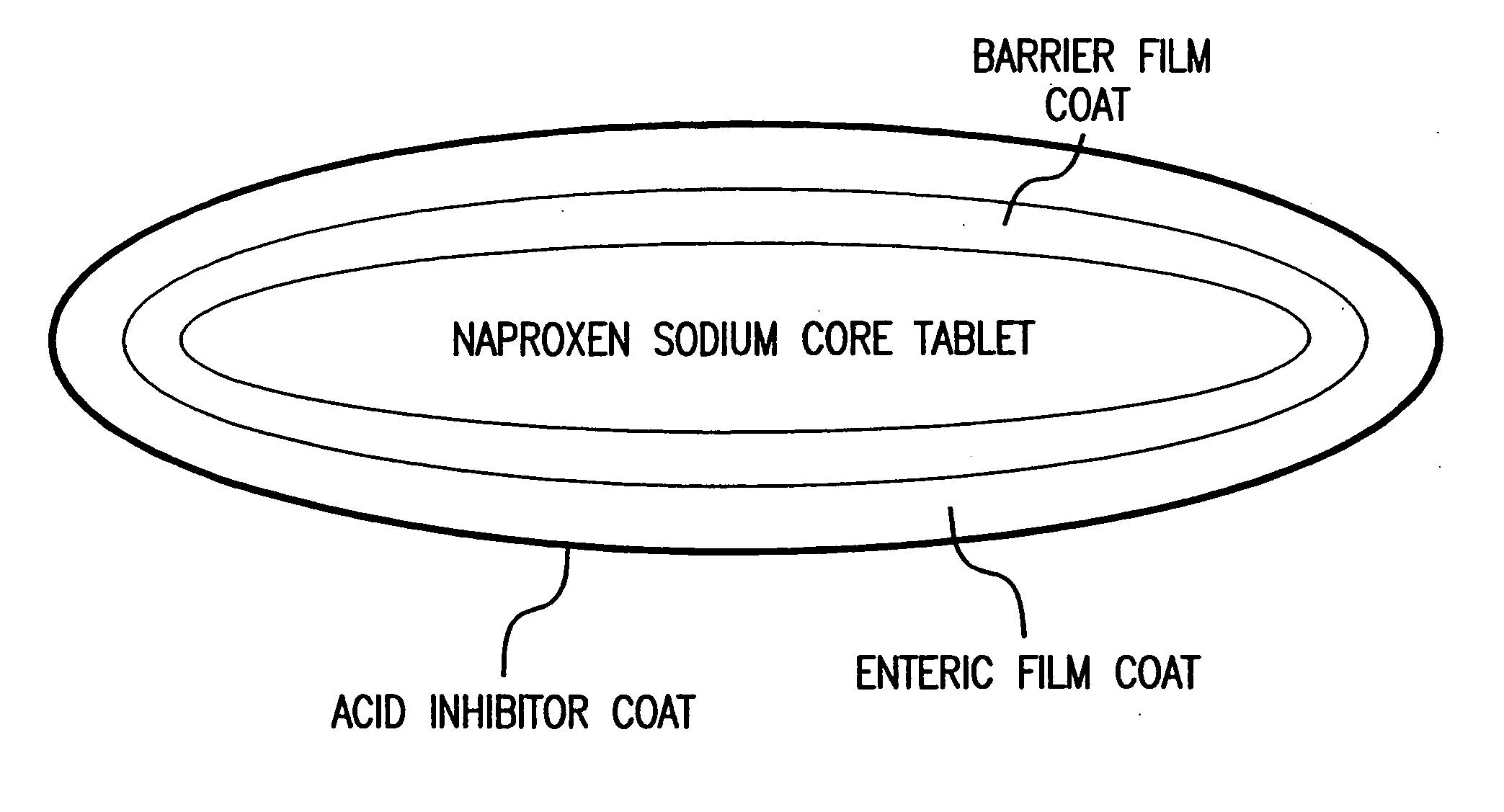
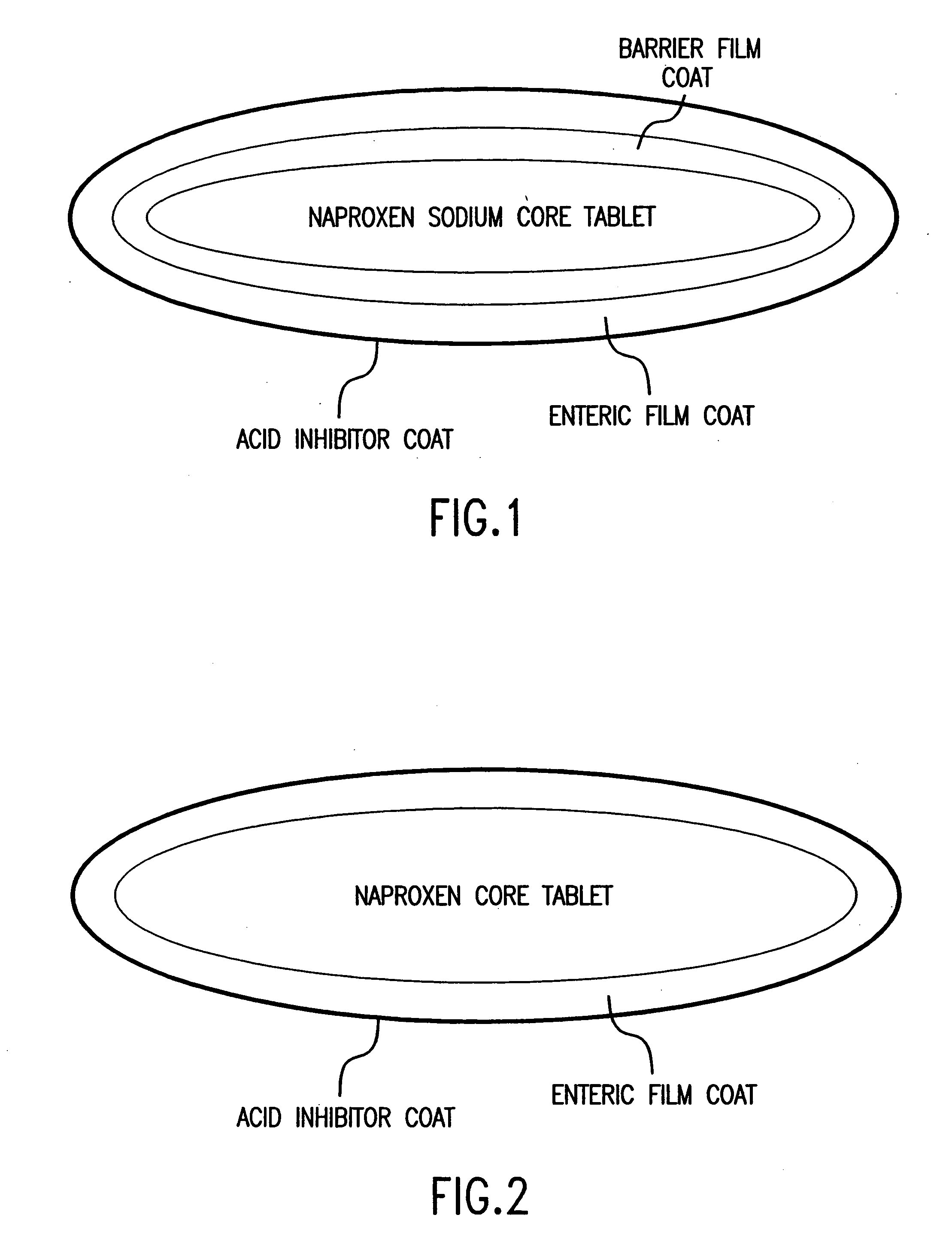
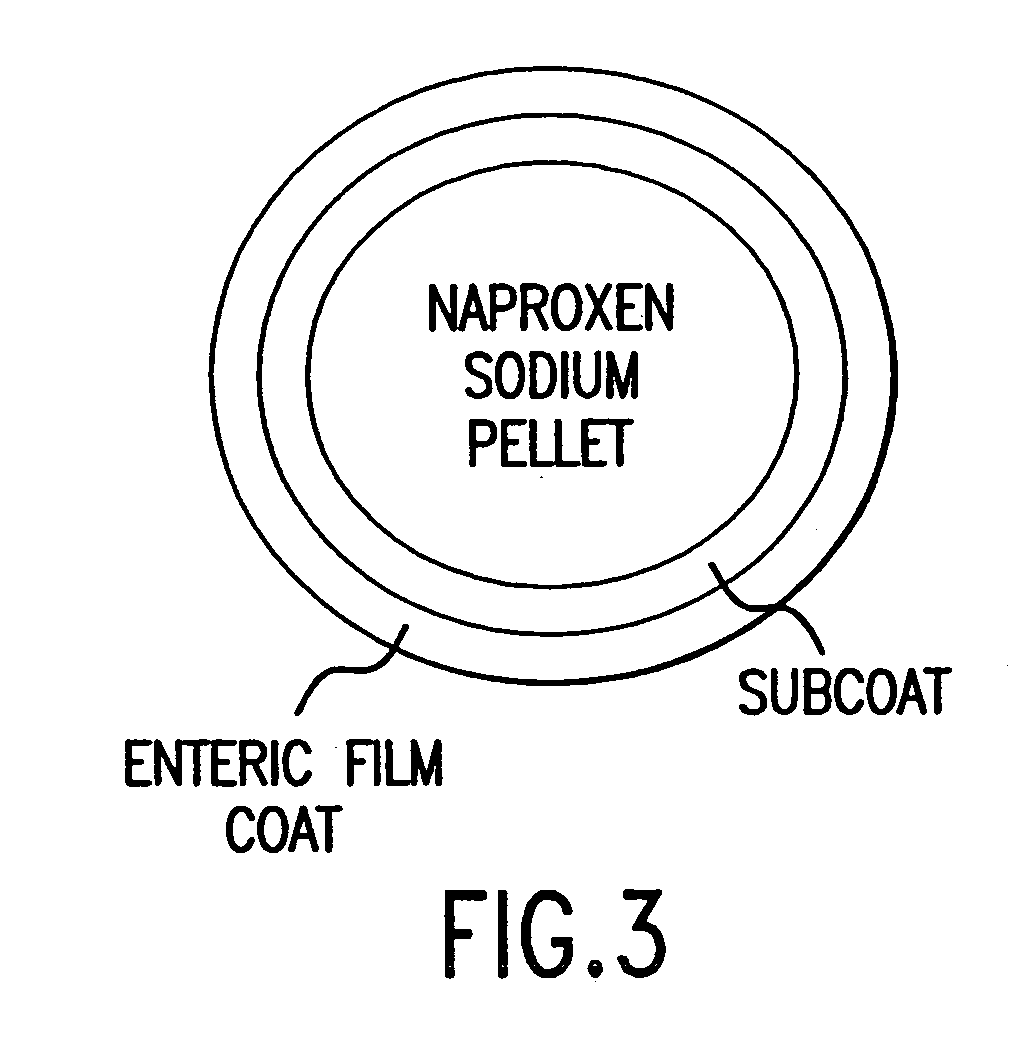
Pharmaceutical formulations containing a non-steroidal antiinflammatory drug and a proton pump inhibitor
InactiveUS6869615B2Decrease risk of development and exacerbationGood curative effectPowder deliveryAntipyreticSide effectDepressant
An oral solid dosage form includes a therapeutically effective amount of an NSAID and a proton pump inhibitor in an amount effective to inhibit or prevent gastrointestinal side effects normally associated with the NSAID. Also disclosed is a method of treating a human patient in need of antiinflammatory, analgesic and / or antipyretic therapy, comprising orally administering to the patient an oral pharmaceutical dosage form which includes a therapeutically effective amount of an NSAID and an amount of a proton pump inhibitor effective to substantially inhibit gastrointestinal side effects of the NSAID. The invention is further related to a method of prophylactically treating a human patient who is on a therapy known to have significant gastrointestinal side effects or is about to begin such a therapy, via concurrent administration of an NSAID and a proton pump inhibitor in a combination (single) oral dosage form.
Owner:ANDRX LABS
Diacylhydrazine ligands for modulating the expression of exogenous genes in mammalian systems via an ecdysone receptor complex
The present invention relates to non-steroidal ligands for use in nuclear receptor-based inducible gene expression system, and a method to modulate exogenous gene expression in which an ecdysone receptor complex comprising: a DNA binding domain; a ligand binding domain; a transactivation domain; and a ligand is contacted with a DNA construct comprising: the exogenous gene and a response element; wherein the exogenous gene is under the control of the response element and binding of the DNA binding domain to the response element in the presence of the ligand results in activation or suppression of the gene.
Owner:PRECIGEN INC
Methods and compositions for improved non-viral gene therapy
Methods to prevent or reduce inflammation secondary to administration of a lipid-nucleic acid complex in a subject, that include administering to the subject a non-steroidal anti-inflammatory agent, a salicylate, an anti-rheumatic agent, an antihistamine, or an immunsuppressive agent with the lipid-nucleic acid complex are disclosed. Also disclosed are methods of screening for inhibitors of the inflammatory response associated with administration of a lipid-nucleic acid complex to a subject, including providing a candidate substance suspected of preventing or inhibiting the inflammation associated with administration of a lipid-nucleic acid complex to the subject. Also disclosed are compositions that include a lipid, a nucleic acid, and a non-steroidal anti-inflammatory agent, a salicylate, an anti-rheumatic agent, an antihistamine, or an immunosuppressive agent.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Pharmaceutical compositions for the coordinated delivery of NSAIDs
InactiveUS20050249811A1Improve complianceReduce in quantityBiocideAntipyreticGastrointestinal InjuryArthritis
The present invention is directed to drug dosage forms that release an agent that raises the pH of a patient's gastrointestinal tract, followed by a non-steroidal anti-inflammatory drug. The dosage form is designed so that the NSAID is not released until the intragastric pH has been raised to a safe level. The invention also encompasses methods of treating patients by administering this coordinated release, gastroprotective, antiarthritic / analgesic combination unit dosage form to achieve pain and symptom relief with a reduced risk of developing gastrointestinal damage such as ulcers, erosions and hemorrhages.
Owner:NUVO PHARMA IRELAND DESIGNATED ACTIVITY CO +1
Endometriosis treatment protocol
The endometriosis treatment protocol provides for administering to a female patient in need of treatment for endometriosis a pharmaceutical composition in a form suitable for vaginal or rectal delivery having a pharmaceutically effective amount of an aromatase inhibitor, which may be either a steroid or non-steroidal. The pharmaceutical composition may be formed as a vaginal suppository, a rectal suppository, a vaginal gel, a rectal gel, a vaginal cream or a rectal cream. The pharmaceutical composition may optionally have pharmaceutically effective amounts of progesterone and calcitriol, and may be administered in combination with an oral COX-2 inhibitor. Alternatively, the pharmaceutical composition comprises an aromatase inhibitor administered vaginally or rectally and is administered in combination with oral calcitriol and the oral COX-2 inhibitor. The aromatase inhibitor is either steroidal or non-steroidal.
Owner:SHIPPEN EUGENE R
Pharmaceutical formulations containing a non-steroidal antiinflammatory drug and an antiulcerative drug
InactiveUS20050163847A1Reduce gastrointestinal side effectsEliminate side effectsBiocideSalicyclic acid active ingredientsNonsteroidal Antiinflammatory Drugs/NSAIDsAntiinflammatory drug
Disclosed is a pharmaceutical dosage form including a therapeutically effective amount of an NSAID and an antiulcerative agent.
Owner:ANDRX PHARMA INC
Tricyclic quinolinone and tricyclic quinoline androgen receptor modulator compounds and methods
Owner:LIGAND PHARMA INC
Synthesis of selective androgen receptor modulators
InactiveUS20060009529A1Decreased libidoAlteration in mood and cognitionBiocideOrganic active ingredientsDiseaseProstate cancer incidence
The present invention relates to a synthetic process for the preparation of a novel class of androgen receptor targeting agents (ARTA) which demonstrate androgenic and anabolic activity of a nonsteroidal ligand for the androgen receptor. The agents define a new subclass of compounds which are selective androgen receptor modulators (SARM) which are useful for a) male contraception; b) treatment of a variety of hormone-related conditions, for example conditions associated with Androgen Decline in Aging Male (ADAM), such as fatigue, depression, decreased libido, sexual dysfunction, erectile dysfunction, hypogonadism, osteoporosis, hair loss, anemia, obesity, sarcopenia, osteopenia,osteoporosis, benign prostate hyperplasia, alterations in mood and cognition and prostate cancer; c) treatment of conditions associated with Androgen Decline in Female (ADIF), such as sexual dysfunction, decreased sexual libido, hypogonadism, sarcopenia, osteopenia, osteoporosis, alterations in cognition and mood, depression, anemia, hair loss, obesity, endometriosis, breast cancer, uterine cancer and ovarian cancer; d) treatment and / or prevention of chronic muscular wasting; e) decreasing the incidence of, halting or causing a regression of prostate cancer; f) oral androgen relacement and / or other clinical therpauetic and / or diagnostic areas. The process of the present invention is suitable for large-scale preparation, since all of the steps give rise to highly pure compounds, thus avoiding complicated purification procedures which ultimately lower the yield. Thus the present invention provides methods for the synthesis of non-steroidal agonist compounds, that can be used for industrial large-scale synthesis, and that provide highly pure products in high yield.
Owner:UNIV OF TENNESSEE RES FOUND
Diaclhydrazine ligands for modulating the expression of exogenous genes in mammalian systems via an ecdysone receptor complex
The present invention relates to non-steroidal ligands for use in nuclear receptor-based inducible gene expression system, and a method to modulate exogenous gene expression in which an ecdysone receptor complex comprising: a DNA binding domain; a ligand binding domain; a transactivation domain; and a ligand is contacted with a DNA construct comprising: the exogenous gene and a response element; wherein the exogenous gene is under the control of the response element and binding of the DNA binding domain to the response element in the presence of the ligand results in activation or suppression of the gene.
Owner:PRECIGEN INC
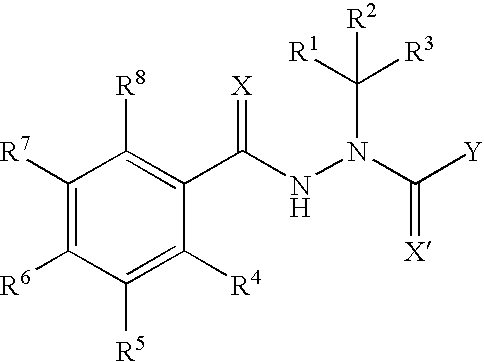
Phthalimide derivatives of non-steroidal Anti-inflammatory compounds and/or tnf-alpha modulators, method for producing same, pharmaceutical compositions containing same and uses thereof for the treatment of inflammatory diseases
InactiveUS20120115817A1Inhibit inflammationMinimizing major limitation and complicationBiocideMonoazo dyesNon steroidal anti inflammatoryRheumatoid arthritis
The present invention relates to phthalimide derivatives of non-steroidal and / or TNF-α modulating anti-inflammatory compounds as well as the process of obtaining the so-called derivatives, pharmaceutical compositions containing such derivatives and their uses, including use in the treatment of inflammatory diseases, especially those related to chronic inflammatory processes, such as rheumatoid arthritis and intestinal inflammatory diseases (for instance, Chron's disease) and the use of the referred to pharmaceutical compositions as antipyretic, analgesic and platelet antiaggregating medications.
Owner:EMS +1
Apparatus and methods for treatment of nasal tissue
InactiveUS20070244529A1Small sizeIncrease airflowUltrasound therapyElectrotherapyPain managementAnalgesic agents
Apparatus and methods for the treatment of nasal tissue, particularly the nasal turbinates, are described herein. One method for reducing the size of the inferior nasal turbinate is to apply ultrasound energy to the tissue regions beneath the surface of the turbinate tissue. One instrument may be used to deliver ultrasound energy and to provide an infusion or injection of a fluid directly into the turbinate being treated. The injected fluid can be used to bulk up the size of the turbinate to ensure that the ultrasound energy is properly delivered directly into the intended turbinate tissue. Accordingly, fluids containing anesthetics, fluids infused with analgesics, etc. may be used for pain management while other medications, such as non-steroidal drugs, steroidal drugs, anti-inflammatory drugs, anti-histamines, anti-bacterial drugs, etc., can also be used.
Owner:CHOI GEORGE Y
Dimethicone-containing sustained release injection formulation
InactiveUS20070053943A1Prevention and treatment of problemBiocidePharmaceutical delivery mechanismDrugAnalgesic agents
A sustained release formulation by using dimeticone as the dispersion medium, which includes active ingredient (e.g., drugs against parasites, insecticides, NSAIDs, antibiotics, sex hormone like agents or oily soluble vitamins) and dimeticone as the medium. Suitable stabilizer, antioxidant, local analgesics and material for sustained release may be added. The formulation is bio-compatible, stable and injectable.
Owner:WANG YUWAN +2
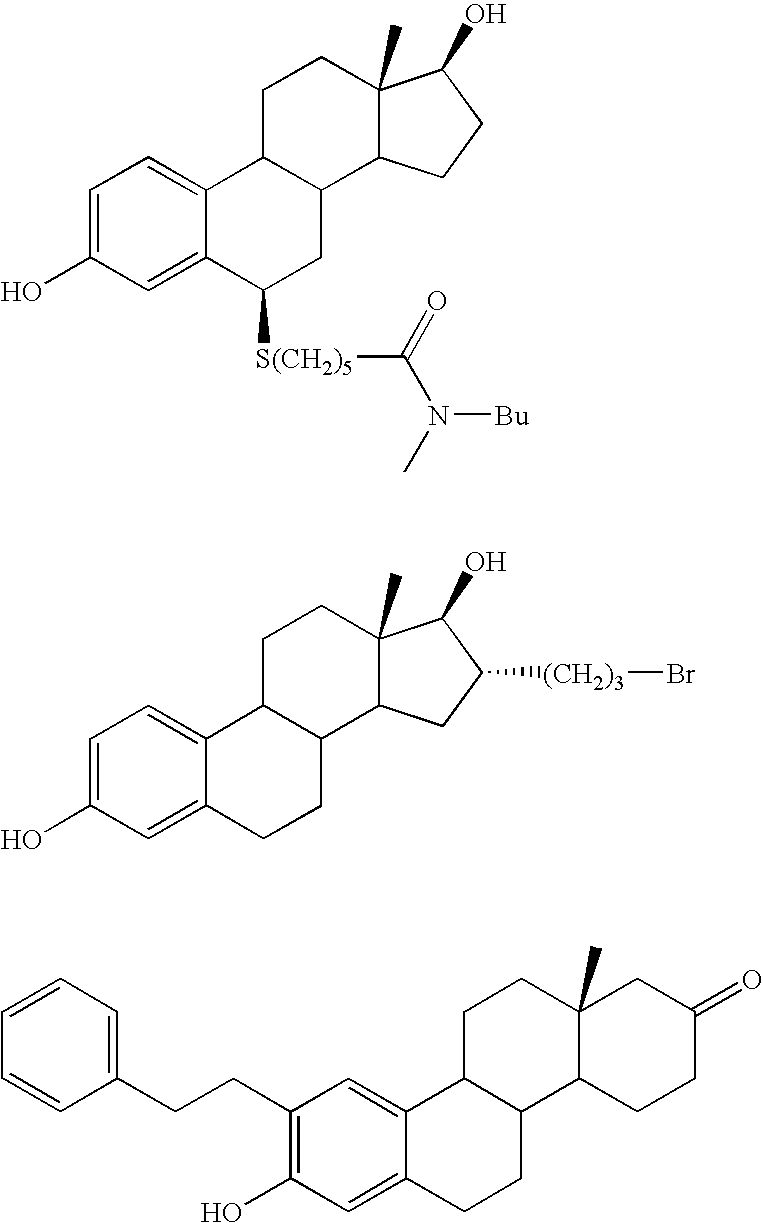
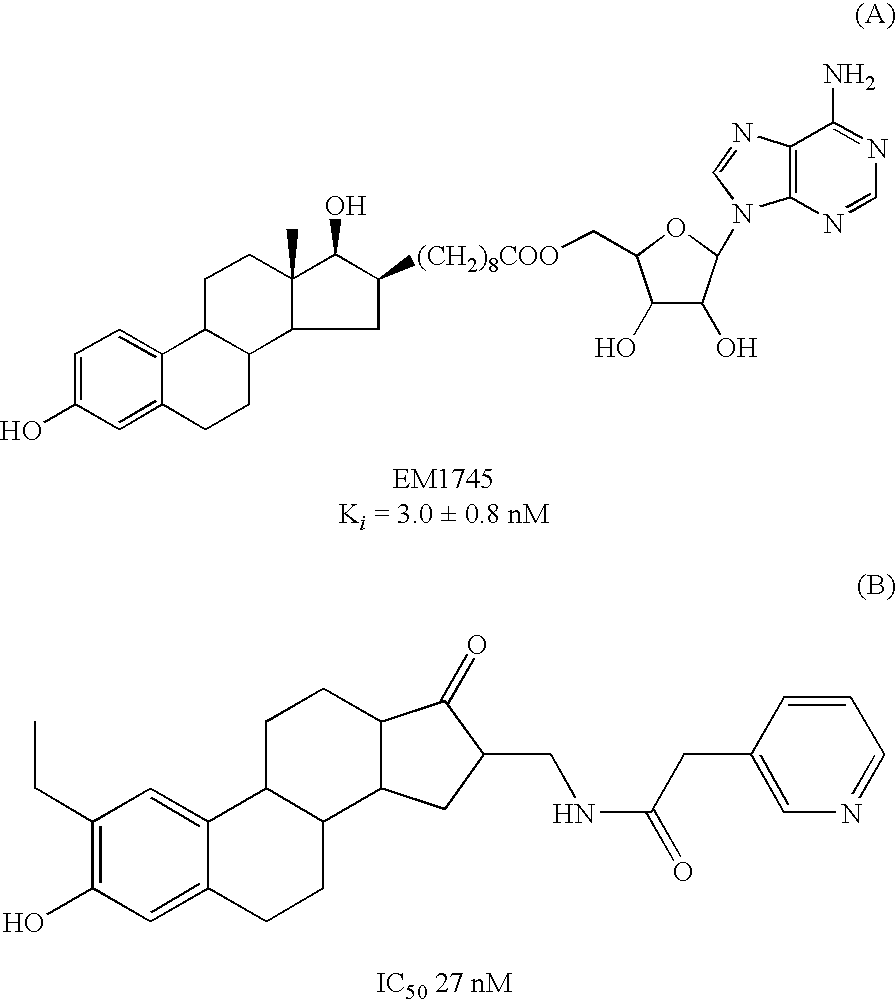
17Beta-Hydroxysteroid Dehydrogenase Type 1 Inhibitors for the Treatment of Hormone-Related Diseases
The invention relates to the use of non-steroidal 17beta-hydroxysteroid dehydrogenase type 1 inhibitors for the treatment and prophylaxis of hormone-dependent, particularly estrogen-dependent, diseases. The invention further relates to suitable inhibitors and to a method for the production thereof.
Owner:UNIV DES SAARLANDES
Extended cycle multiphasic oral contraceptive method
ActiveUS8063030B2Reduce in quantityQuantity minimizationBiocideOrganic active ingredientsGynecologyObstetrics
A multiphasic method of contraception comprising the steps of sequentially administering to a female of child bearing age a Phase I composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg norethindrone acetate and an estrogen in an amount equivalent to about 5 to about 15 mcg of ethinyl estradiol for about 7 to about 14 days; a Phase II composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 10 to about 25 mcg of ethinyl estradiol for about 14 to about 22 days; a Phase III composition containing a progestogen in an amount equivalent to about 0.3 to about 1.5 mg of norethindrone acetate and an estrogen in an amount equivalent to about 15 to about 35 mcg of ethinyl estradiol for about 20 to about 31 days; and an optional Phase IV composition containing (i) an estrogen in an amount equivalent to about 5 to about 20 mcg of ethinyl estradiol, or (ii) a placebo or a non-steroidal component, or (iii) a combination of (i) and (ii), for about 2 to about 8 days. The ethinyl estradiol equivalent amount of estrogen in each of the successive Phases II and III is at least 5 mcg greater than the ethinyl estradiol equivalent amount of estrogen in the immediately-preceding phase.
Owner:APTALIS PHARMA
Analgesics for nasal administration
InactiveUS20050142072A1Rapid uptakeRapid onsetPowder deliveryBiocideNasal Cavity EpitheliumBlood plasma
An analgesic and a delivery agent are combined in a pharmaceutical composition such that, on introduction into the nasal cavity of a patient to be treated, the analgesic may be delivered to the bloodstream to produce within 30 minutes a therapeutic plasma concentration, Cther, of 0.2 ng / ml or greater which is maintained for a duration Tmaint of at least 2 hours. The analgesic may be an opioid analgesic or a non-steroidal anti-inflammatory drug.
Owner:IONIX PHARMA +1
Composition and method for treating the effects of diseases and maladies
InactiveUS6841544B2Prevent relapseGood effectBiocideInorganic active ingredientsDiseaseNon steroidal anti inflammatory
A medicinal composition for treating pain resulting from an inflammatory response comprises at least one pain relieving and anti-inflammatory pharmaceutical and at least one nutraceutical in a pharmaceutically acceptable base. The pharmaceutical is preferably acetaminophen or a non-steroidal anti-inflammatory drug (NSAID). The nutraceutical is preferably an immune booster, an anti-oxidant, a liver protectant, or a joint relief agent. Methods of using these compositions to treat pain caused by inflammation are also disclosed.
Owner:BIOSELECT INNOVATIONS
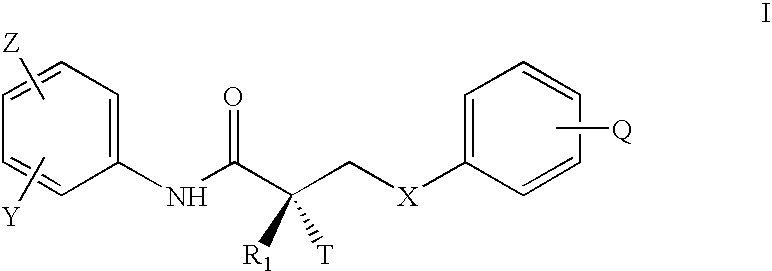
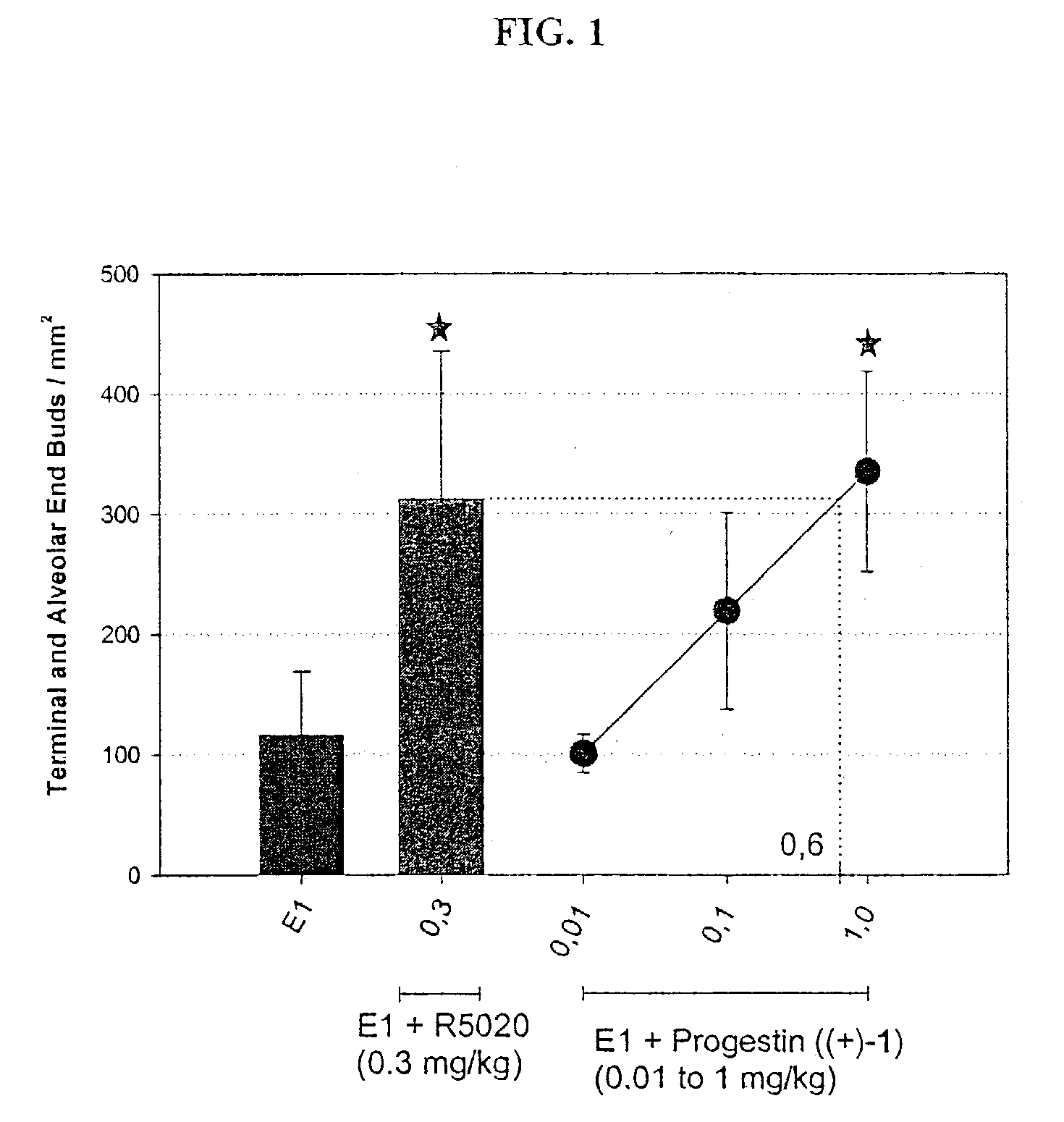
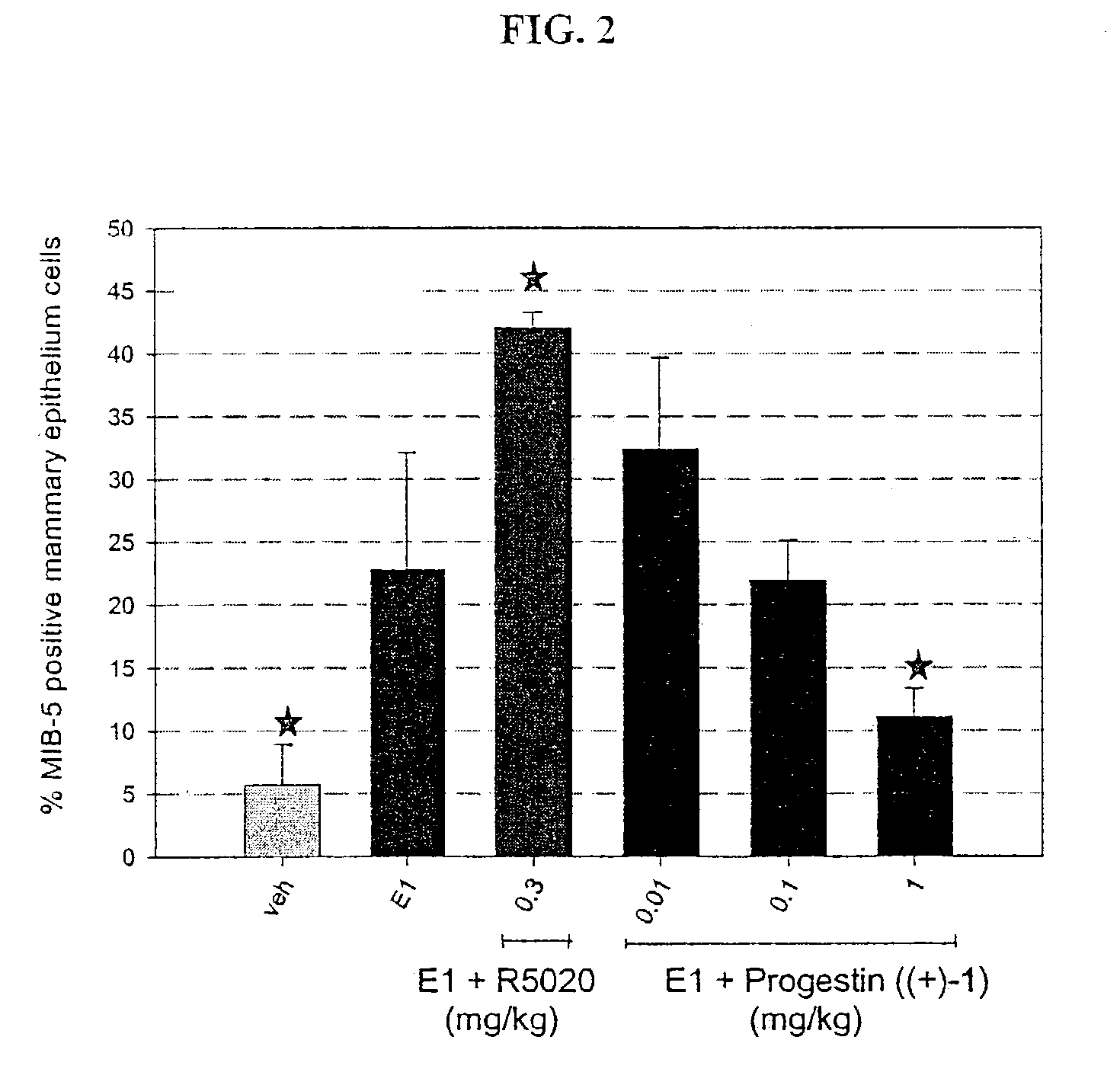
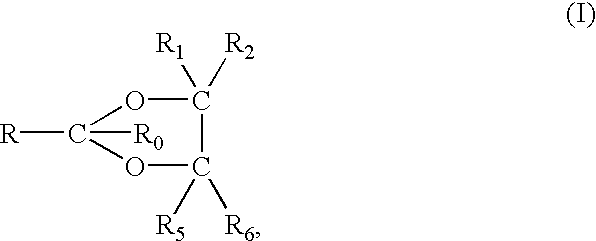
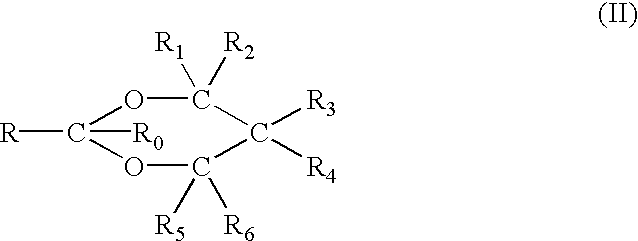
Non-steroidal progesting
InactiveUS7388006B2Suitable for useBiocideOrganic chemistryPR - Progesterone receptorOral contraceptive drug
The present invention relates to non-steroidal progestins of the general formula (I)whereinR1 and R2 are independently of each other —H or —F,R3 is —CH3 or —CF3, andAr isor a pharmaceutically acceptable derivative or analogue thereof. These progestins are suitable for selectively modulating progesterone receptor mediated effects in different target tissues, particularly in uterine tissue versus breast tissue. Therefore, the progestins of the present invention, optionally in combination with estrogens, may be used for contraception (in particular in estrogen-free oral contraceptives), hormone replacement therapy and the treatment of gynecological disorders. The present invention furthermore relates to methods for selectively modulating progesterone receptor mediated effects in different target tissues or organs.
Owner:BAYER SCHERING PHARMA AG
Non-steroidal antiinflammatory drug formulations for topical application to the skin
InactiveUS20030082226A1Improve performanceSignificant positive effectOrganic active ingredientsAntipyreticSkin penetrationAntiinflammatory drug
Topical alcoholic or aqueous alcoholic gels containing ibuprofen or other NSAIDs, such as, naproxen, in substantially neutral salt form, have enhanced penetration through skin and may provide rapid pain / inflammation relief by including in the formulation 2-n-nonyl-1,3-dioxolane or other hydrocarbyl derivative of 1,3-dioxolane-or 1,3-dioxane or acetal, as skin penetration enhancing compound. The amount of propylene glycol may be varied to adjust the initial flux of the NSAID through the skin, especially for ibuprofen, naproxen, and ketorolac.
Owner:SAMOUR CARLOS M +2
Method for synthesizing loxoprofen sodium
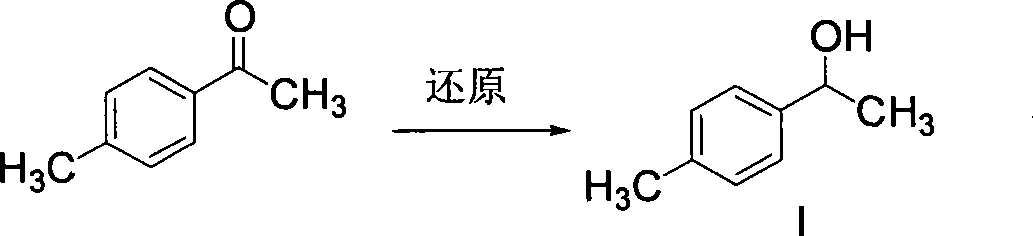
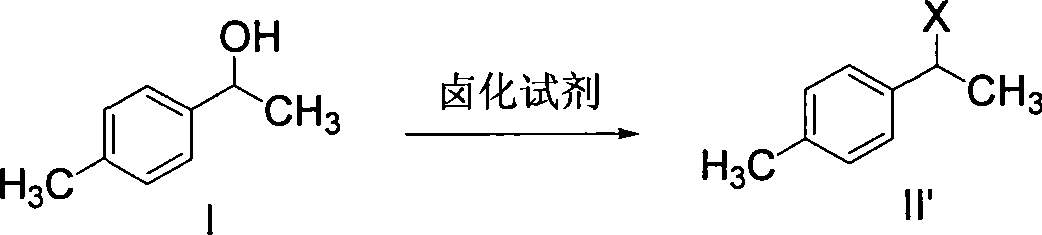
ActiveCN101412670ARaw materials are easy to getUnique craftOrganic compound preparationCarboxylic compound preparationSolventHydrolysis
The invention discloses a synthetic method for loxoprofen sodium, which is prepared by taking methyl acetophenone as an initial raw material through reduction, acylation or halogen substituent, cyanation, hydrolysis, bromination, condensation, decarboxylation and salifying. The method has the advantages of easily obtained raw material, unique technology, simple and stable operation, and high productive rate in each step of reaction; and all solvents used in the synthesis process can be recycled, so the production cost is reduced greatly. Tests show that the obtained product has reliable quality and stable performance, and can be further used for making preparation of non-steroidal anti-inflammatory drugs such as the loxoprofen sodium.
Owner:ZHEJIANG APELOA JIAYUAN PHARMA +1
Pharmaceutical preparations for external use containing non-steroidal anti-inflammatory and analgesic agents
InactiveUS6635674B1Excellent percutanous absorptionReduce effectBiocidePeptide/protein ingredientsAnti-inflammatorySolvent
The present invention relates to an anti-inflammatory and analgesic pharmaceutical preparation for external use having excellent percutaneous absorption and applicability. The pharmaceutical preparations for external use of this invention comprise NSAIDs and, as a percutaneous absorption promoting agent, oleic acid, oleyl alcohol or a mixture thereof, in a pharmaceutically acceptable aqueous alcoholic solvent comprised of a monohydric saturated aliphatic alcohol of 1-4 carbon atoms, a polyhydric alcohol selected from the group consisting of saturated aliphatic glycols of 2-4 carbon atoms and glycerol, and water.
Owner:NOVARTIS AG
COX-2-targeted imaging agents
InactiveUS20050002859A1X-ray constrast preparationsRadioactive preparation carriersSubject matterImaging agent
The presently disclosed subject matter provides a method for synthesizing a radiological imaging agent by reacting a COX-2-selective ligand with a compound comprising a detectable group, wherein the COX-2-selective ligand is a derivative of a non-steroidal anti-inflammatory drug (NSAID) comprising an ester moiety or a secondary amide moiety. Also provided are compositions that are synthesized using the method, as well as methods of using the compositions of the presently disclosed subject matter.
Owner:VANDERBILT UNIV
Zonisamide and nsaid nanoparticulate formulations
The present invention is directed to compositions comprising zonisamide, or a salt or derivative thereof, and at least one nanoparticulate NSAID. The zonisamide and NSAID combination preferably includes nanoparticulate NSAID particles of the composition with an effective average particle size of less than about 2000 nm. The zonisamide and NSAID combination is useful in the treatment of migraine and acute migraine pain and related conditions.
Owner:ELAN PHRMA INT LTD
Oxadiazoline ligands for modulating the expression of exogenous genes via an ecdysone receptor complex
The present invention relates to non-steroidal ligands for use in nuclear receptor-based inducible gene expression system, and a method to modulate exogenous gene expression in which an ecdysone receptor complex comprising: a DNA binding domain; a ligand binding domain; a transactivation domain; and a ligand is contacted with a DNA construct comprising: the exogenous gene and a response element; wherein the exogenous gene is under the control of the response element and binding of the DNA binding domain to the response element in the presence of the ligand results in activation or suppression of the gene.
Owner:PRECIGEN INC
Method and composition for skin inflammation and discoloration
InactiveUS20090306025A1BiocideSalicyclic acid active ingredientsNon steroidal anti inflammatoryInflammation
The invention provides a method and compound for treating darkness and / or swelling / inflammation of the skin of humans. An antihistamine compound and a non-steroidal anti-inflammatory drug (NSAID) compound in combination have been found to effectively treat under eye darkness, swelling and puffiness in particular, when applied topically to the affected skin.
Owner:FAIRFIELD CLINICAL TRIALS
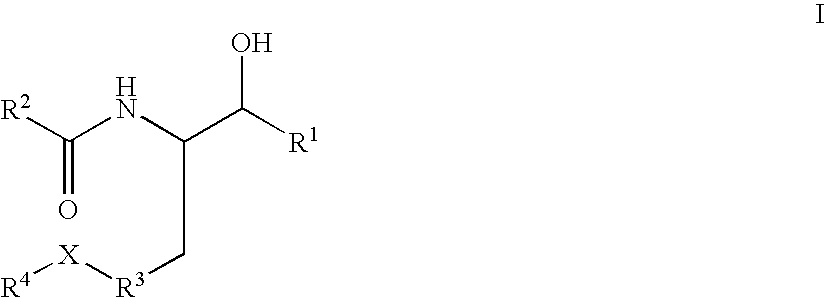
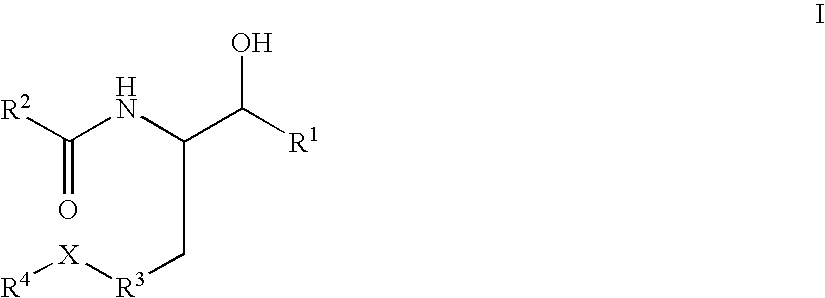
Substituted amide beta secretase inhibitors
Disclosed are novel compounds of the formula or a pharmaceutically acceptable salt or solvate thereof, wherein R1, R2, R3, R4 and X are as defined in the specification. Also disclosed are pharmaceutical compositions comprising the compounds of formula I. Also disclosed are methods of treating cognitive or neurodegenerative diseases such as Alzheimer's disease. Also disclosed are methods of treating a cognitive or neurodegenerative disease comprising administering to a patient I need of such treatment a combination of at least one compound of formula I and at least one compound selected from the group consisting of β-secretase inhibitors other than those of formula I, HMG-CoA reductase inhibitors, gamma-secretase inhibitors, non-steroidal anti-inflammatory agents, N-methyl-D-aspartate receptor antagonists, cholinesterase inhibitors and anti-amyloid antibodies.
Owner:MERCK SHARP & DOHME LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com