Analgesics for nasal administration
analgesics and compositions, applied in the field of analgesic compositions, can solve the problems of prolonged analgesia, and increase the residence time of analgesic in the nasal cavity, and achieve the effects of rapid uptake, increased residence time of analgesic in the nasal cavity, and fast onset of analgesia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
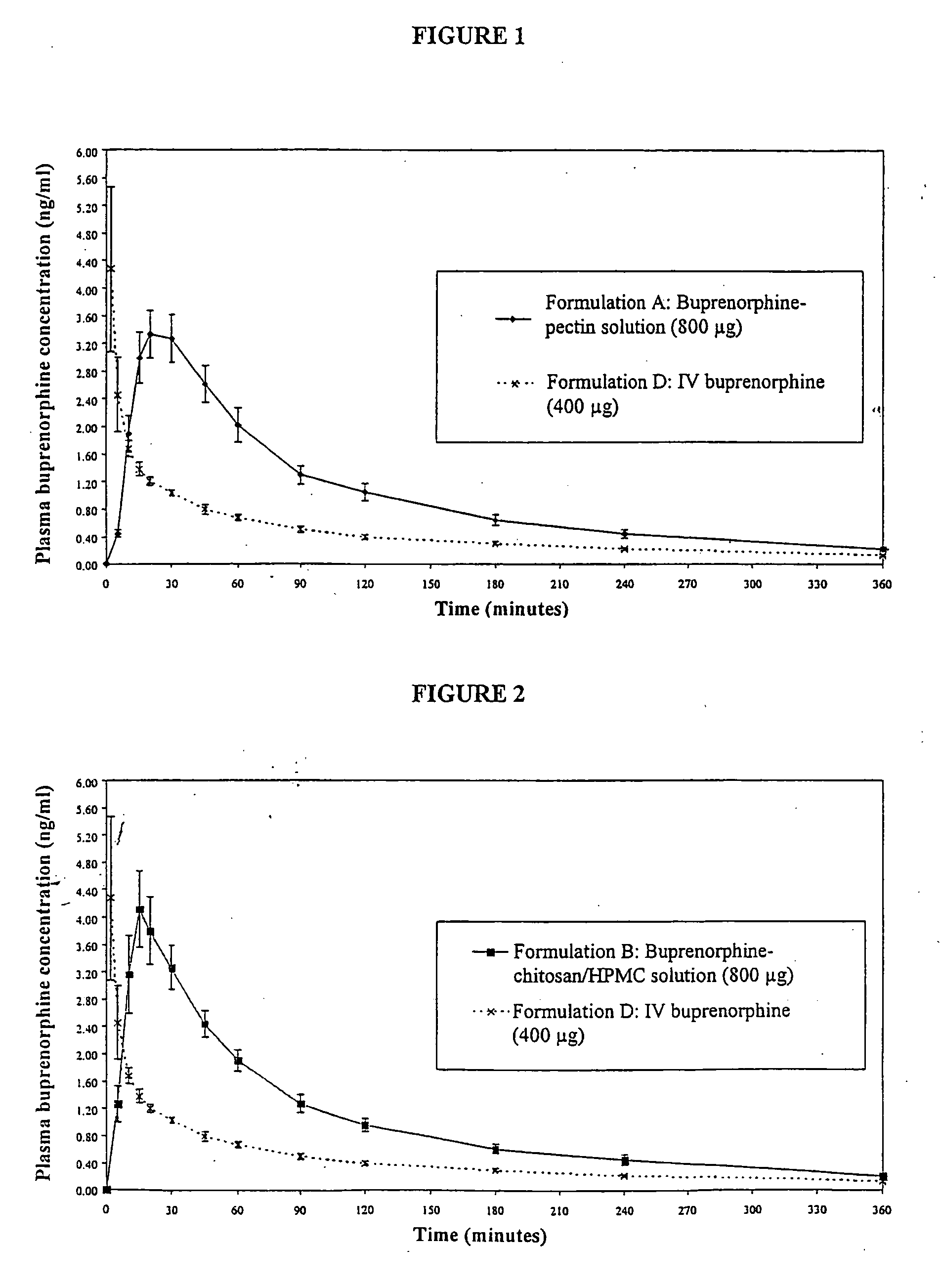
Nasal Solution Containing Buprenorphine (4 mg / ml) and Pectin
[0168] 5 g of pectin (SLENDID (trade mark) 100, CP Kelco, Denmark) was dissolved by stirring into approximately 180 ml of water for injection (WFI) (Baxter, UK). 1075 mg of buprenorphine hydrochloride (MacFarlan Smith, UK) and 12.5 g of dextrose (Roquette) were dissolved into the pectin solution. 1.25 ml of phenylethyl alcohol (R. C. Treat, UK) and 50 mg of propyl hydroxybenzoate (Nipa, UK) were dissolved into the pectin / buprenorphine solution. The solution was adjusted to 250 ml using WFI. 1M hydrochloric acid (BDH, UK) was added to adjust the pH to 3.6.
[0169] The final product was a slightly turbid solution 4.3 mg / ml buprenorphine hydrochloride (corresponding to 4 mg / ml buprenorphine), 20 mg / ml pectin, 50 mg / ml dextrose, 5 μl / ml phenylethyl alcohol and 0.2 mg / ml propyl hydroxybenzoate. The pH of the solution was 3.6, as mentioned above. The osmolality of the solution was 0.46 osmol / kg.
[0170] Single dose nasal spray dev...
example 2
Nasal Solution Containing, Buprenorphine (2 mg / ml) and Pectin
[0171] 5 g of pectin is dissolved by stirring into approximately 180 ml of WFI. 538 mg of buprenorphine hydrochloride and 12.5 g of dextrose are dissolved into the pectin solution. 1.25 ml of phenylethyl alcohol and 50 mg of propyl hydroxybenzoate are dissolved into the pectin / buprenorphine solution. The solution is adjusted to 250 ml using WFI.
[0172] The final product is a slightly turbid solution containing 2.16 mg / ml buprenorphine hydrochloride (corresonding to 2 mg / ml buprenorphine), 20 mg / ml pectin, 50 mg / ml dextrose, 5 μl / ml phenylethyl alcohol and 0.2 mg / mil propyl hydroxybenzoate.
[0173] 123 μl of the above solution is filled into a Valois Monospray single dose nasal spray device (Pfeiffer, Germany). Actuation of the device will deliver a dose of 100 μl of liquid containing 200 μg of buprenorphine and 2 mg of pectin.
example 3
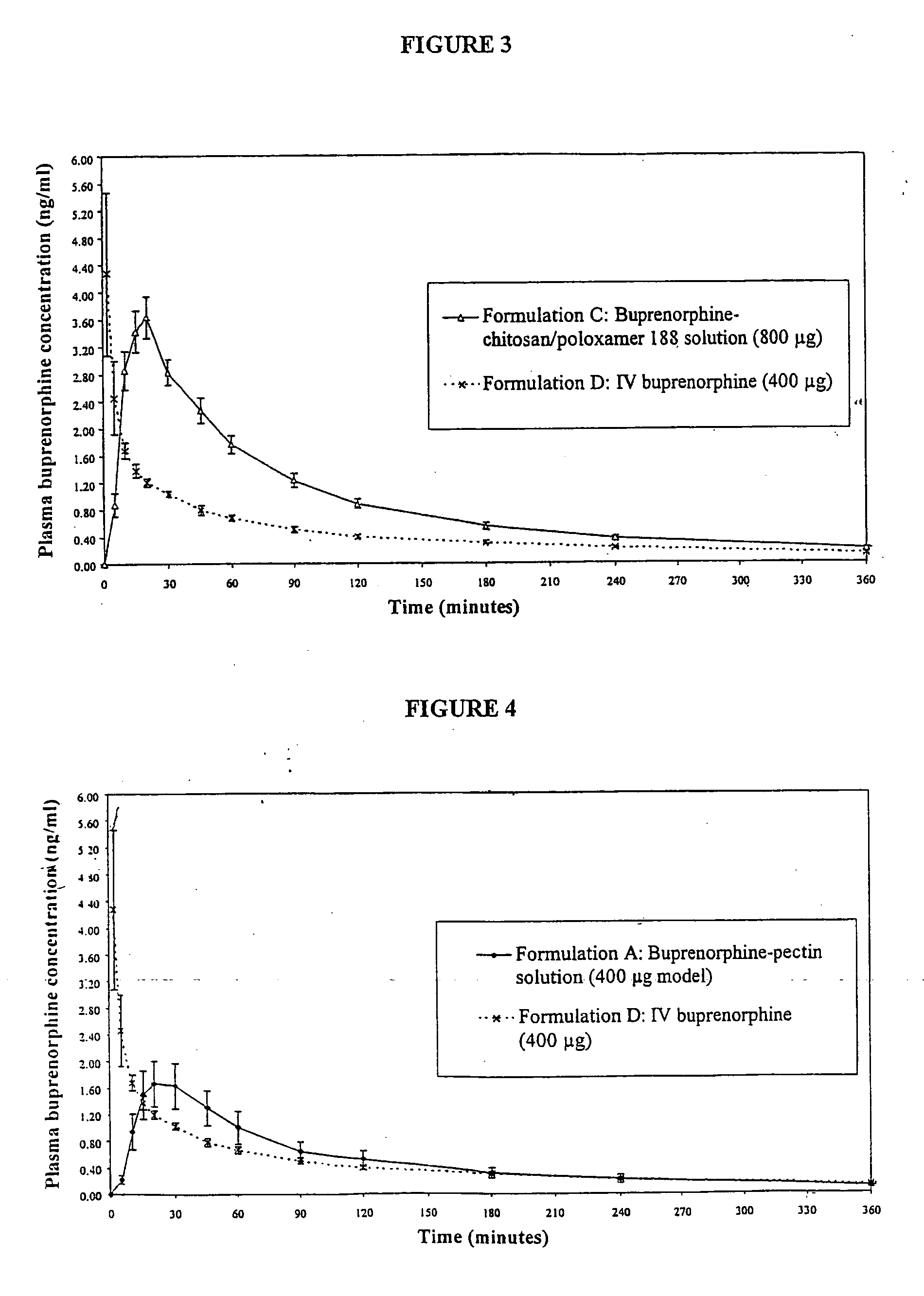
Nasal Solution Containing Buprenorphine (4 mg / ml), Chitosan and HPMC
[0174] 0.75 g of HPMC (Methocel (trade mark) E4M, Colorcon, UK) was dispersed into approximately 125 ml of pre-heated (70-80° C.) water for injection (WFI) (Baxter, UK). The HPMC dispersion was stirred in an ice bath until a clear solution had formed. 1.25 g of chitosan glutamate (Protosan (trade mark) UPG213, Pronova, Norway) was dissolved in the HPMC solution. 75 mg of 50% w / w benzalkonium chloride solution (Albright and Wilson, UK) was dispersed in. 10 ml of WFI and transferred with an additional 40 ml of WFI to a 250 ml volumetric flask. 1075 mg of buprenorphine hydrochloride (MacFarlan Smith, UK) and 12.5 g of dextrose (Roquette, UK) were transferred into the volumetric flask. The chitosan / HPMC solution and an additional 40 ml of WFI were added to the flask. The solution was adjusted to pH 3.4 using 1M hydrochloric acid solution (BDH, UK) and the flask contents adjusted to 250 ml using WFI.
[0175] The final pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com