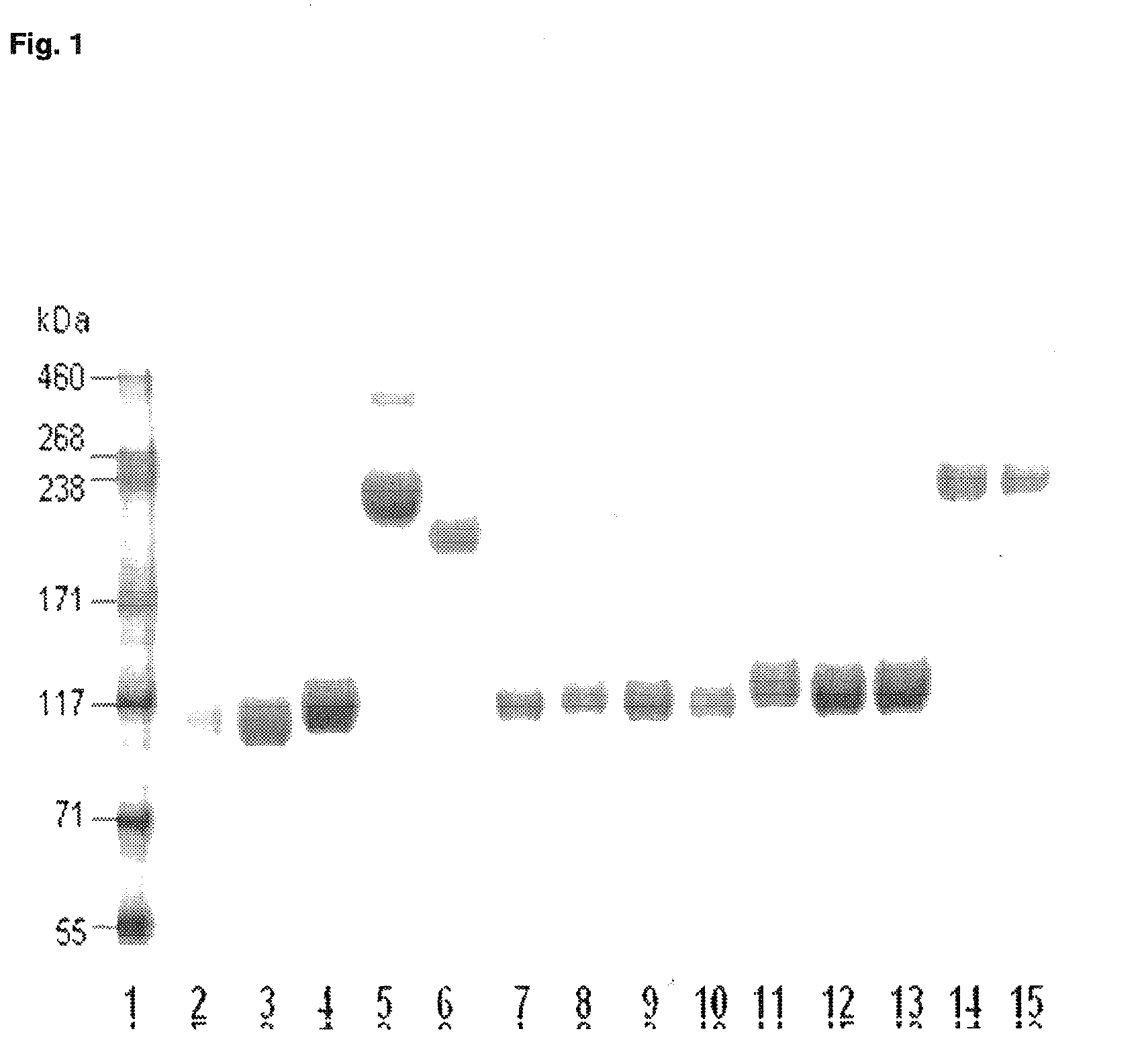
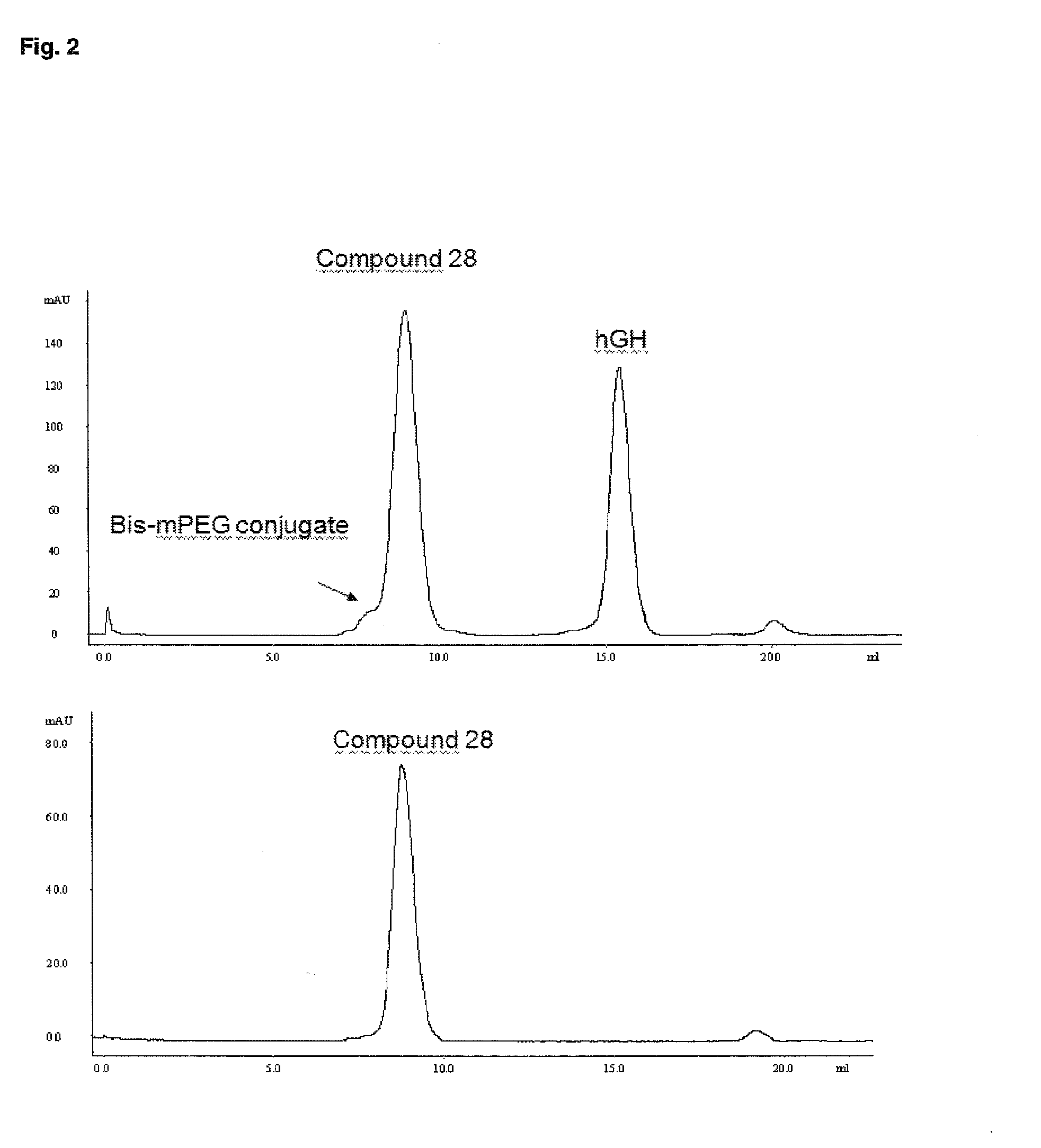
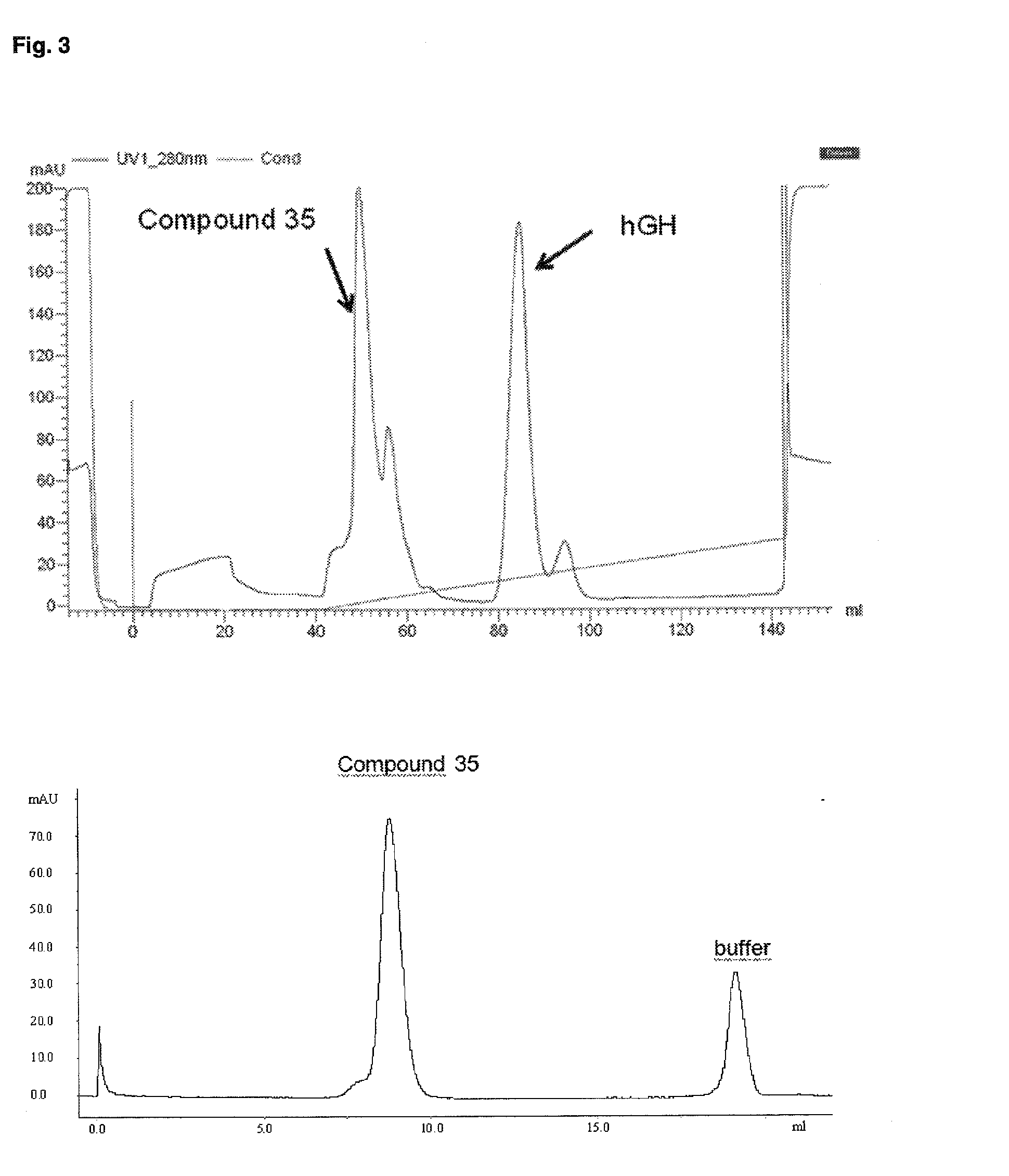
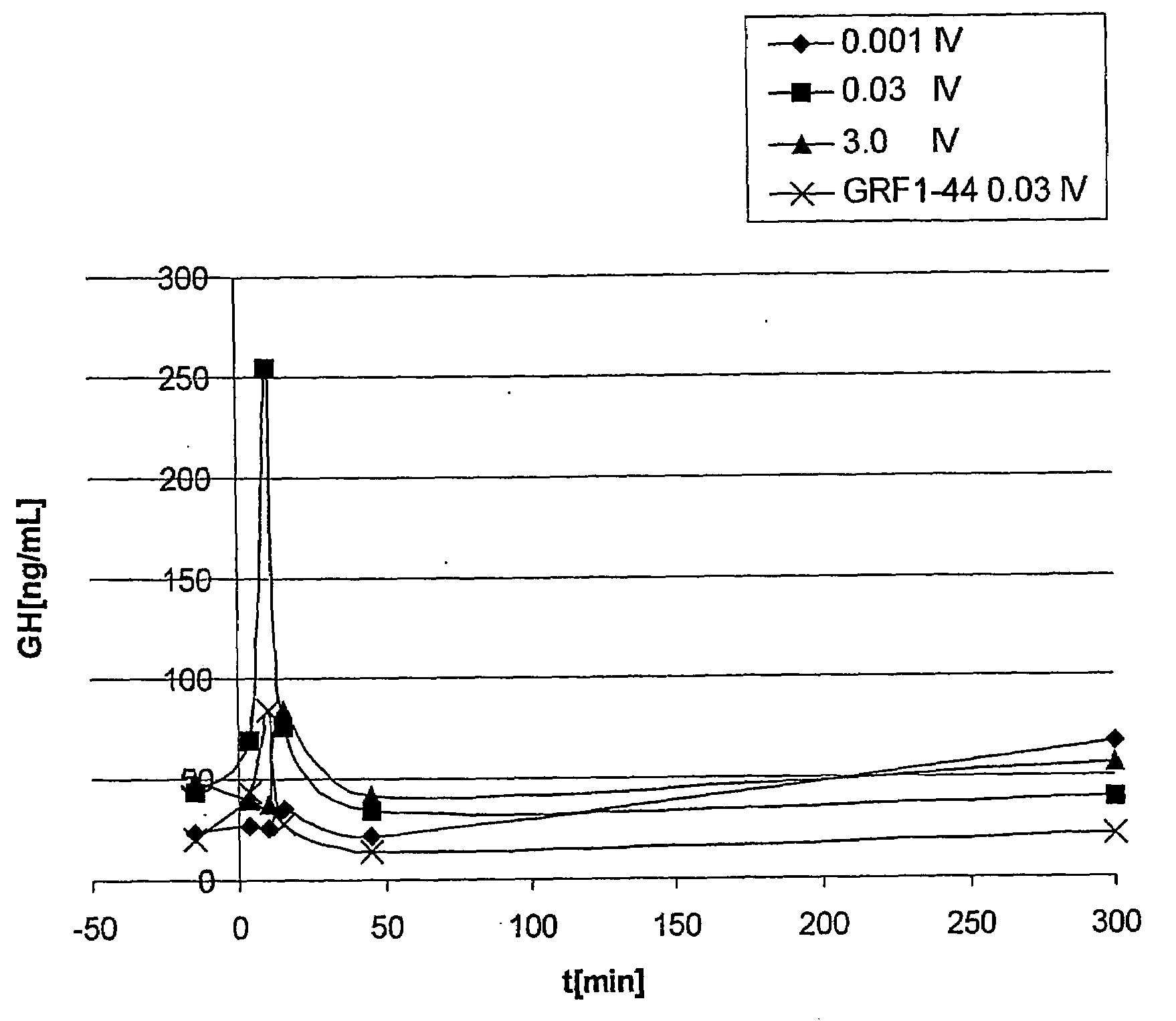
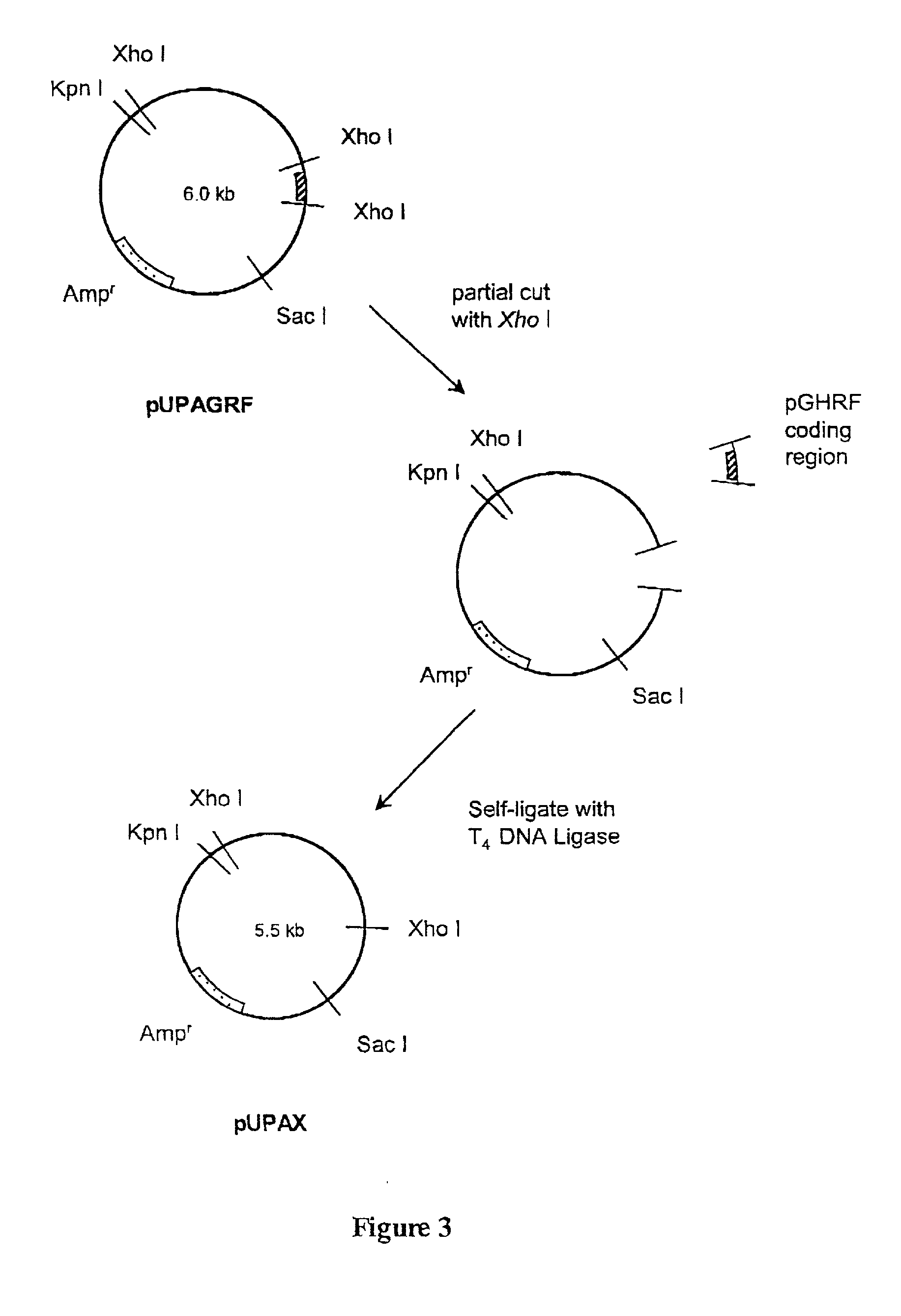
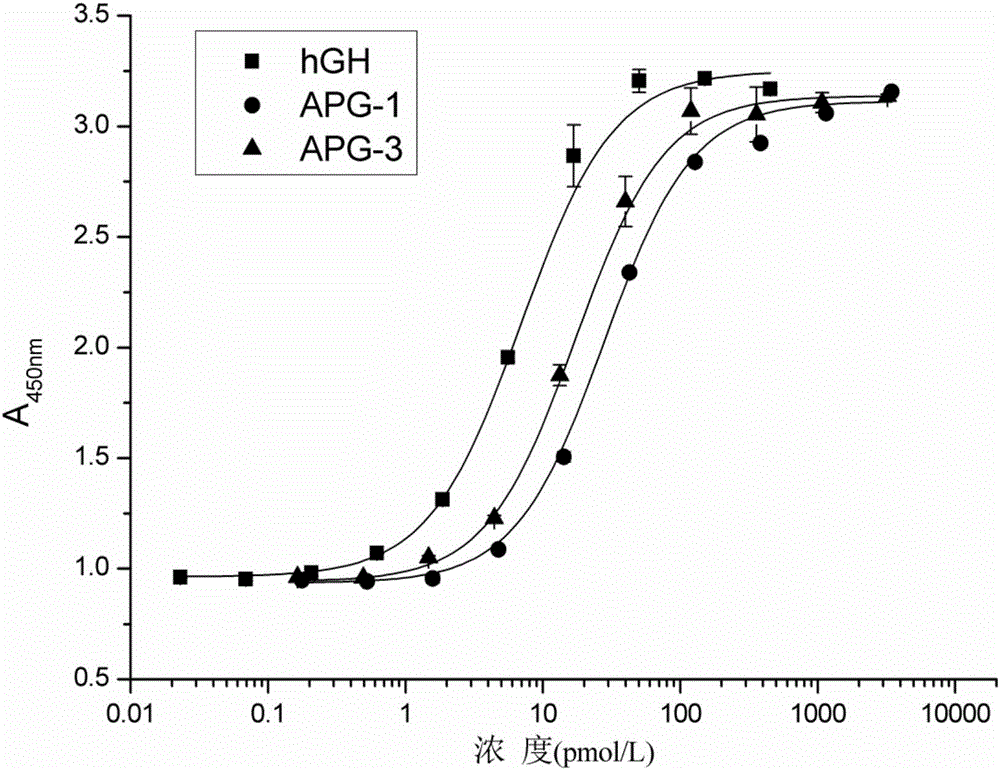
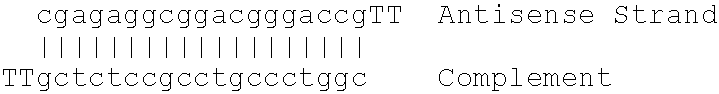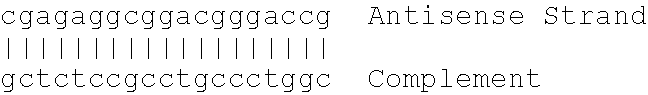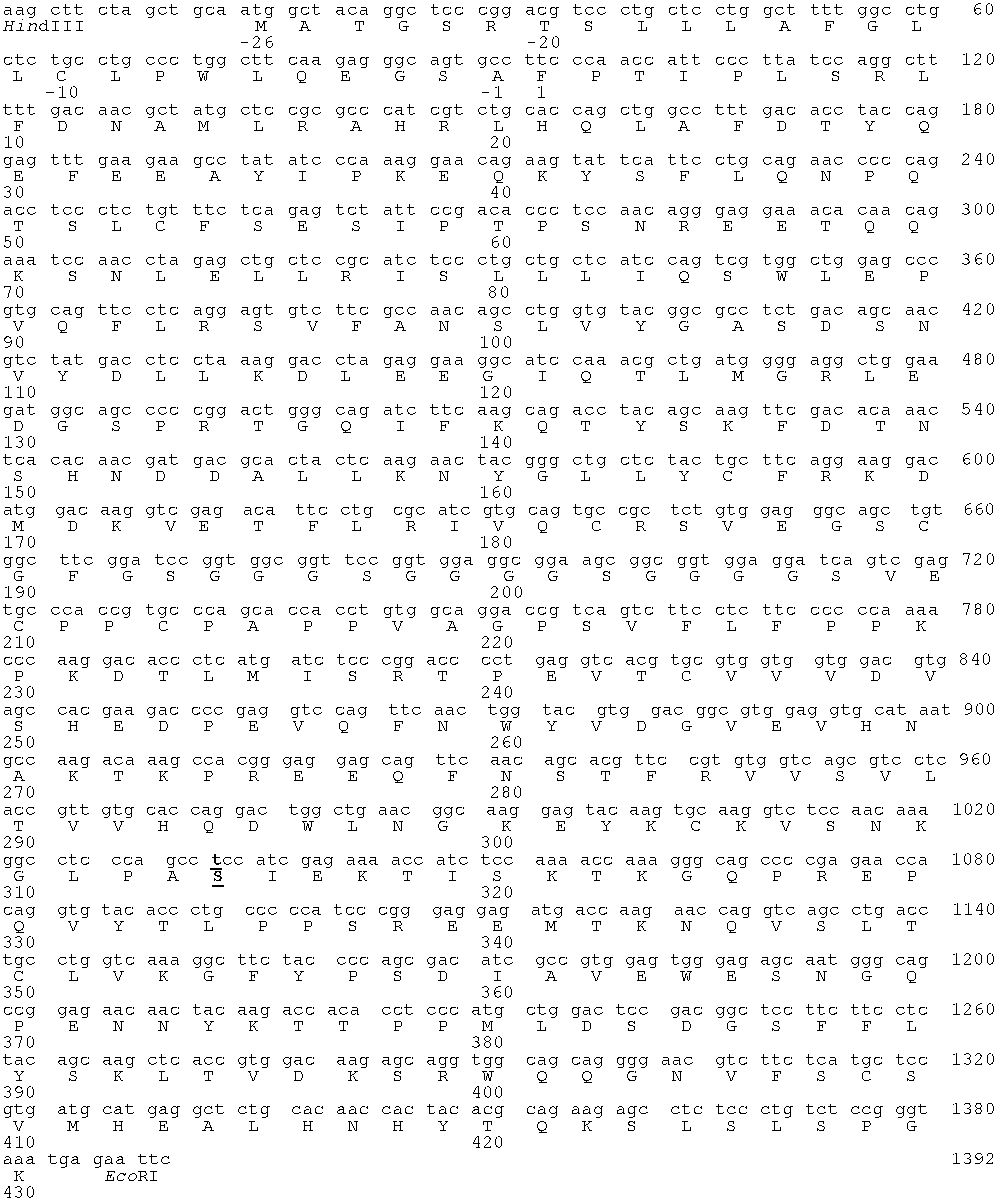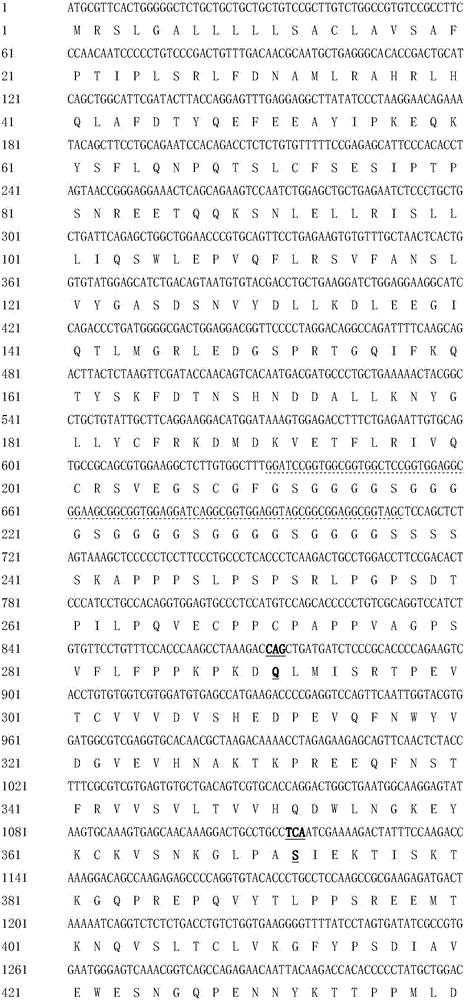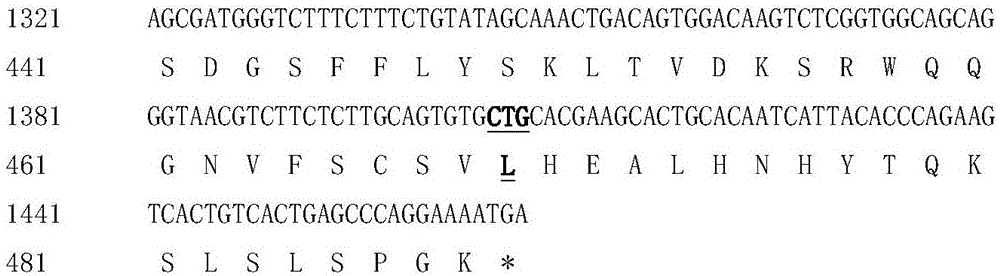Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
128 results about "Somatropin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A recombinant form of endogenous human growth hormone (GH), a polypeptide produced by the anterior lobe of the human pituitary gland. GH exhibits growth-promoting effects and metabolic effects on carbohydrate, fat, protein and bone metabolism. GH stimulates protein synthesis and the uptake of amino acids into cells, and induces lipolysis in adipose tissues. The secretion of GH increases with sexual maturation and then declines steadily.
Method for secretory production of human growth hormone
InactiveUS6436674B1Increase productionDecreased tendency for lysisBacteriaHydrolasesHuman growth hormoneA-DNA
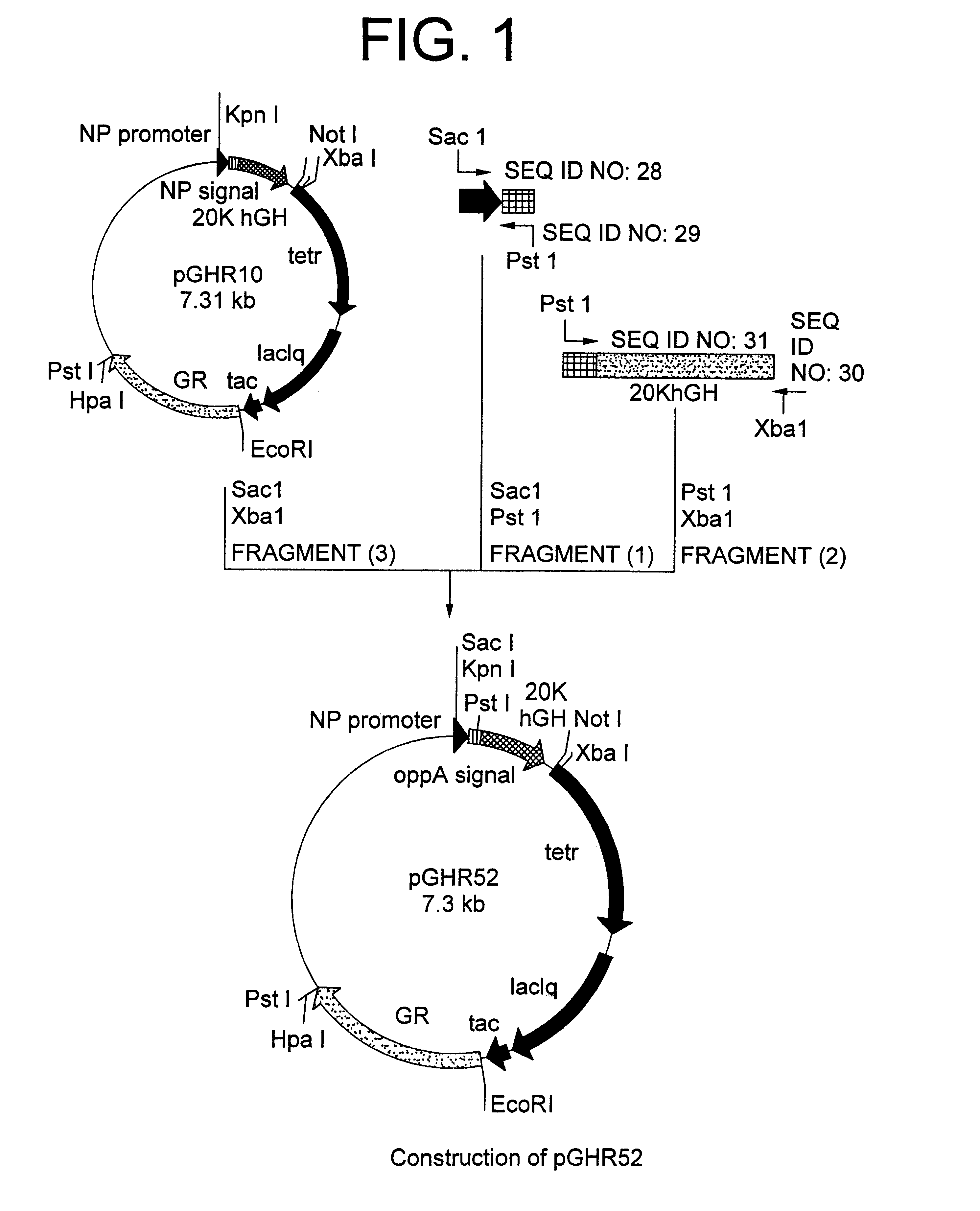
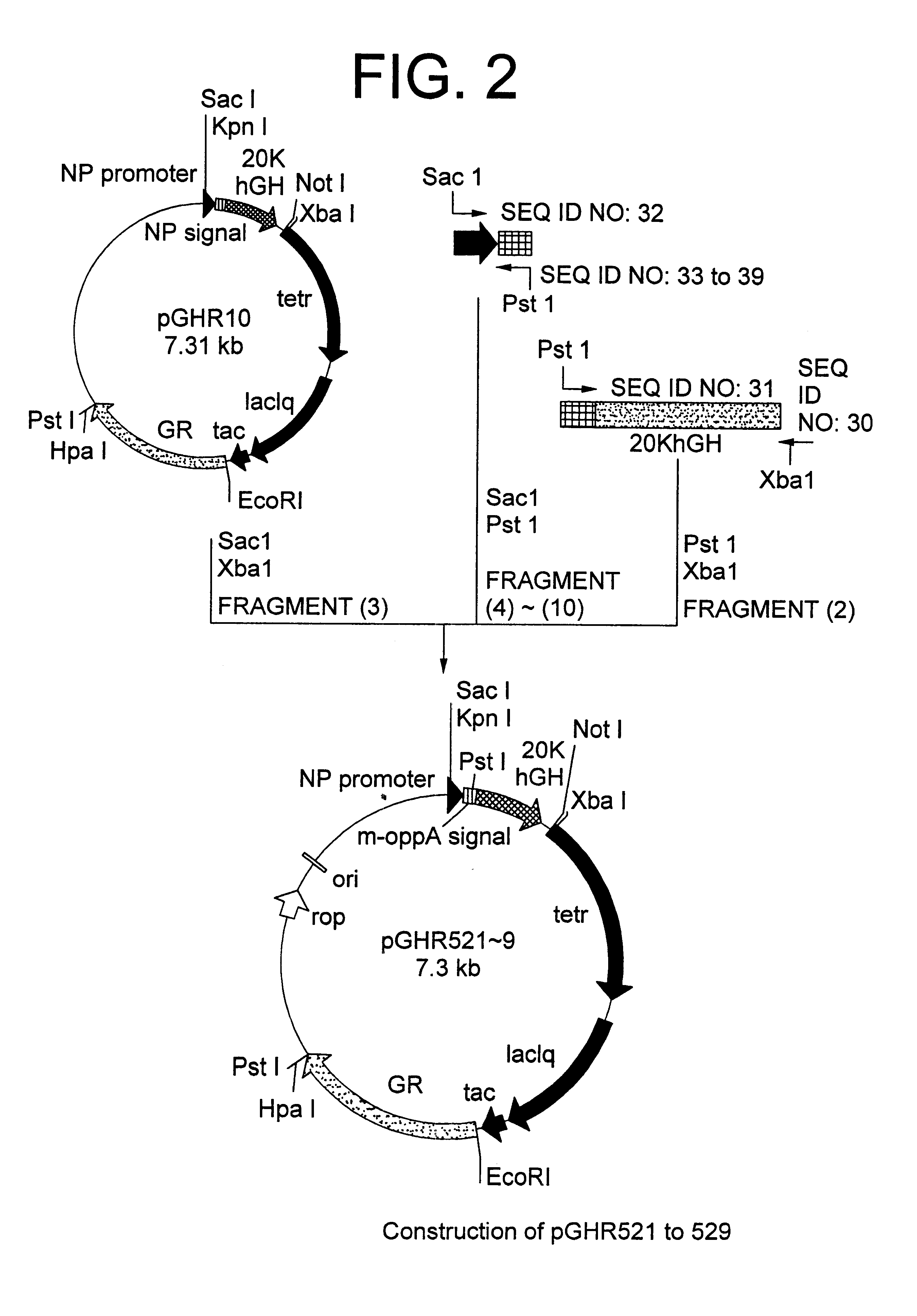
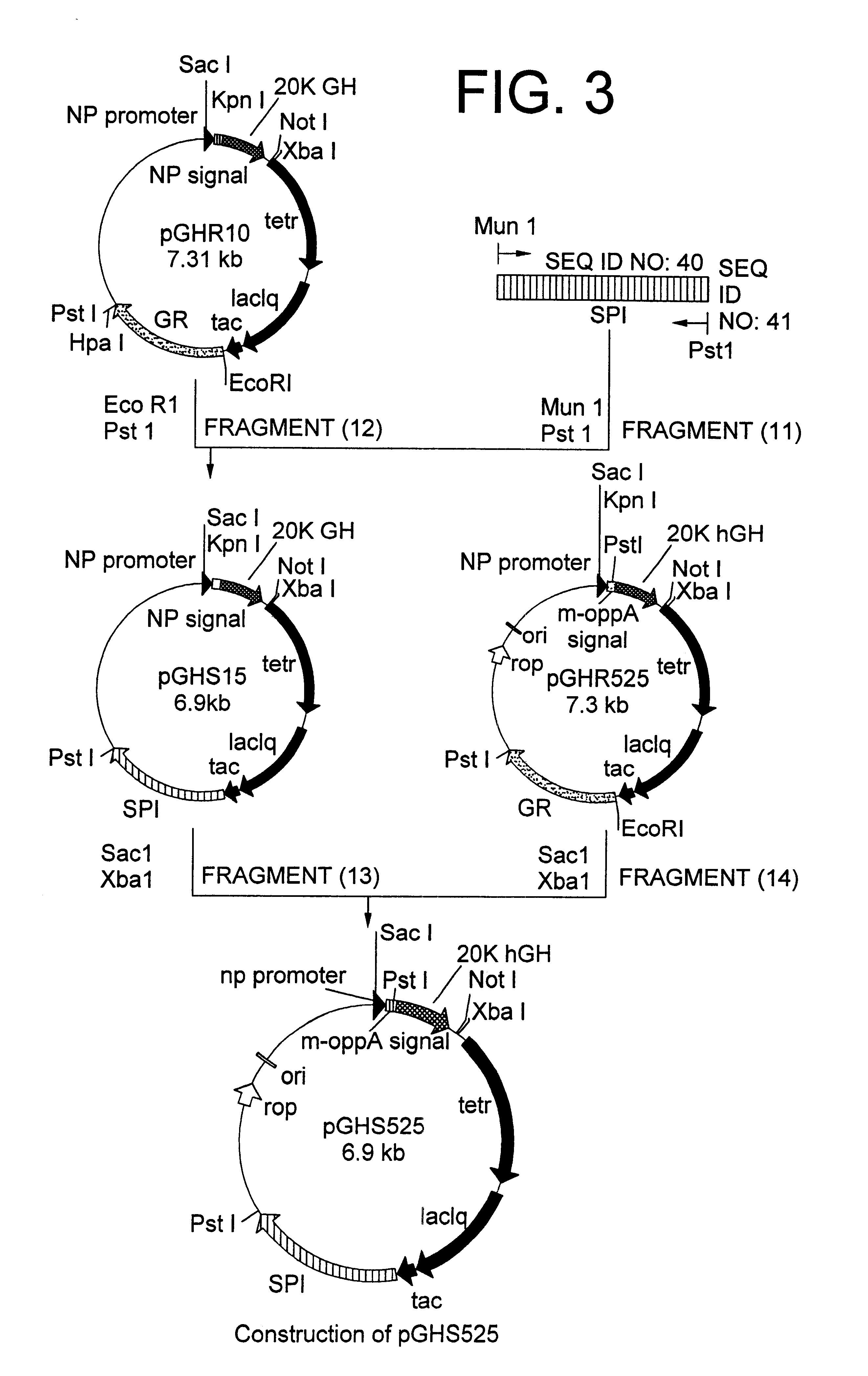
A DNA encoding 20K hGH is connected directly to a gene encoding Escherichia coli OppA protein secretion signal, or a modified form thereof, and a DNA encoding signal peptidase 1 to construct a recombinant plasmid, E. coli is transformed by the plasmid and cells of the resulting E. coli transformant strain are cultured for secretory production of the 20K hGH in the E. coli periplasm. This method enables efficient secretory production of 20K hGH and easy isolation and purification of 20K hGH from the periplasm fraction because the level of impure proteins in the E. coli periplasm is low.
Owner:MITSUI CHEM INC
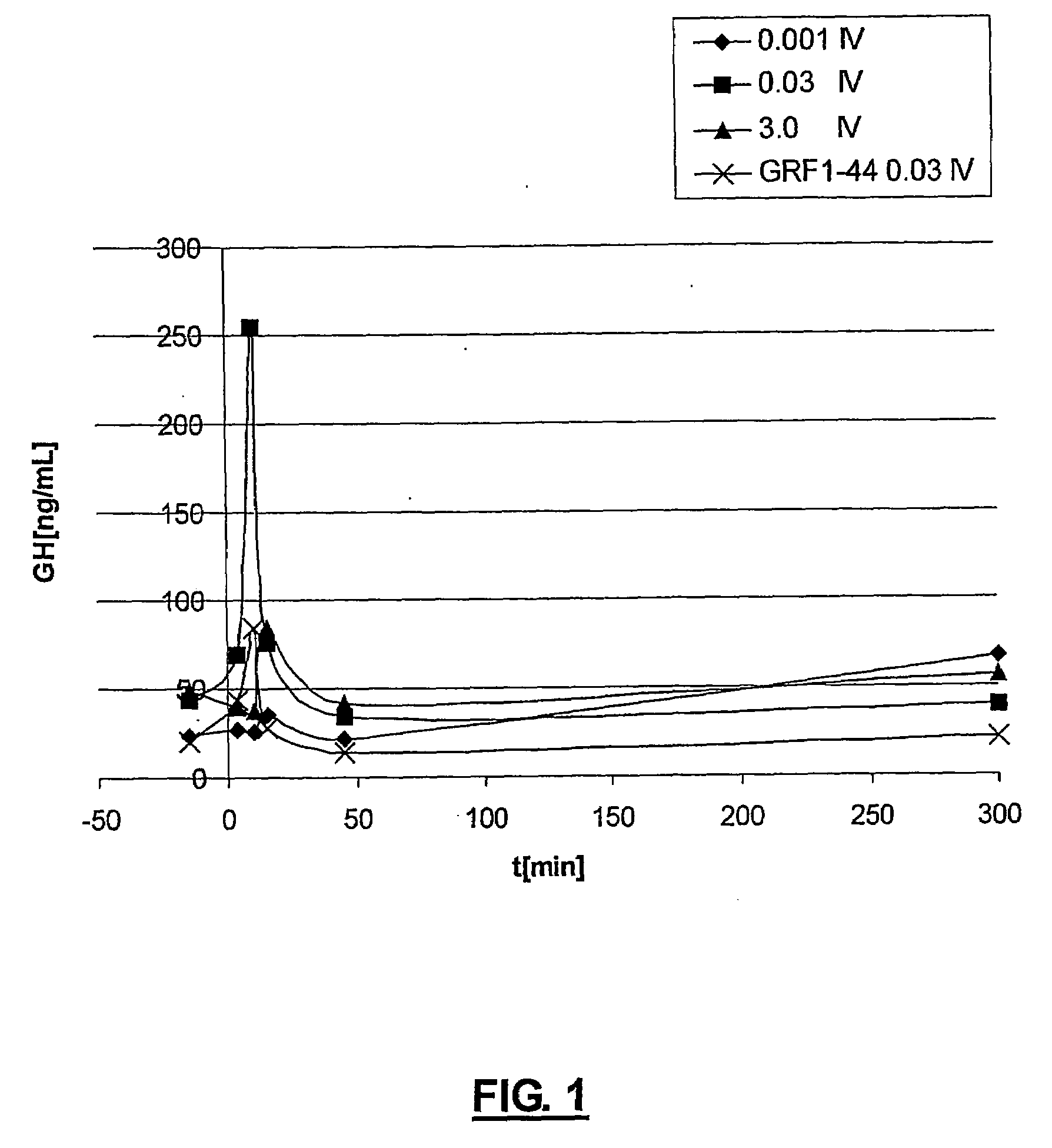
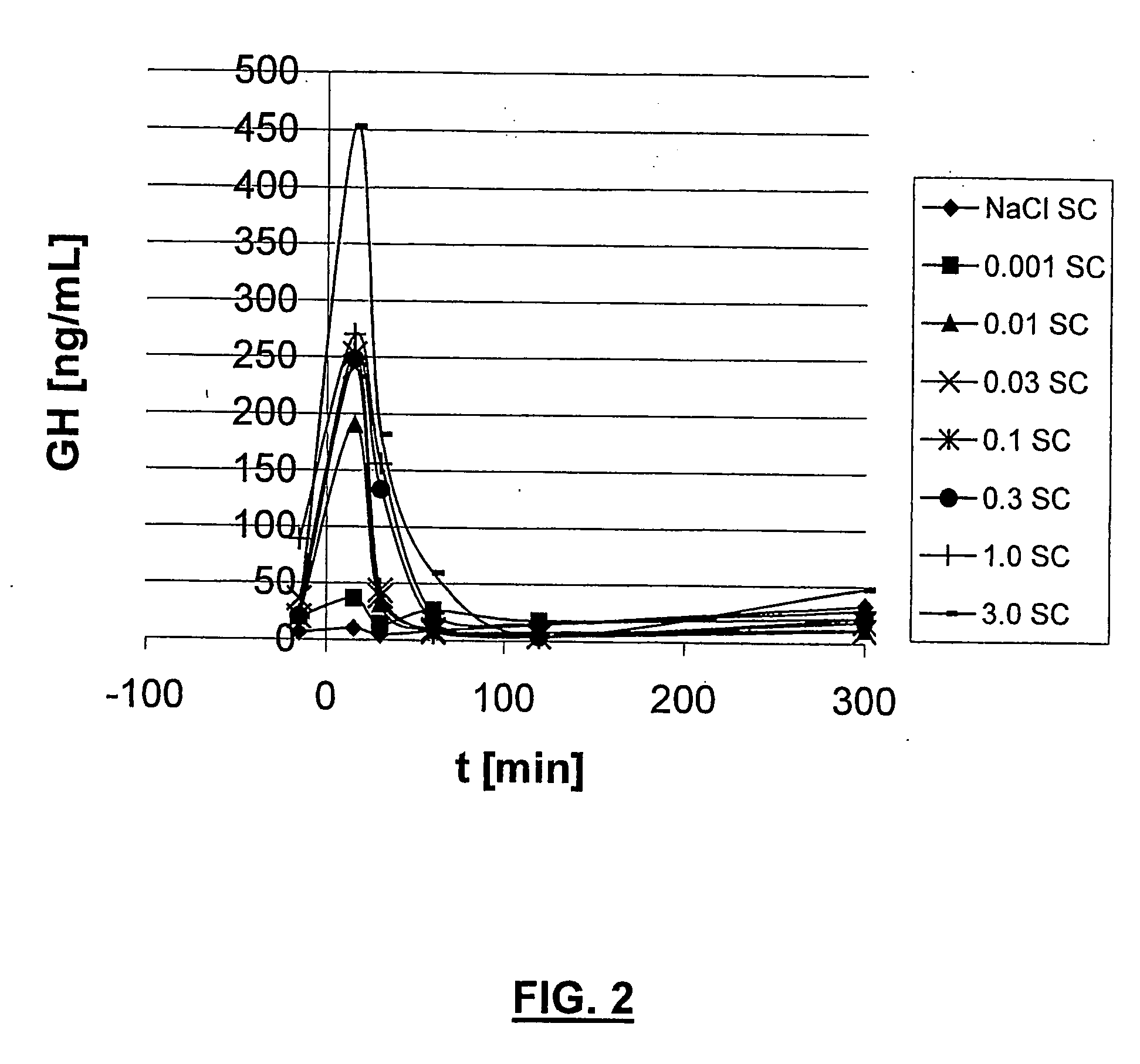
Long lasting growth hormone releasing factor derivatives
InactiveUS7268113B2Prolong half-life in vivoPeptide/protein ingredientsMuscular disorderHalf-lifeIn vivo
This invention relates to growth hormone releasing factor (GRF) derivatives. In particular, this invention relates to GRF peptide derivatives having an extended in vivo half-life, for promoting the endogenous production or release of growth hormone in humans and animals.
Owner:CONJUCHEM
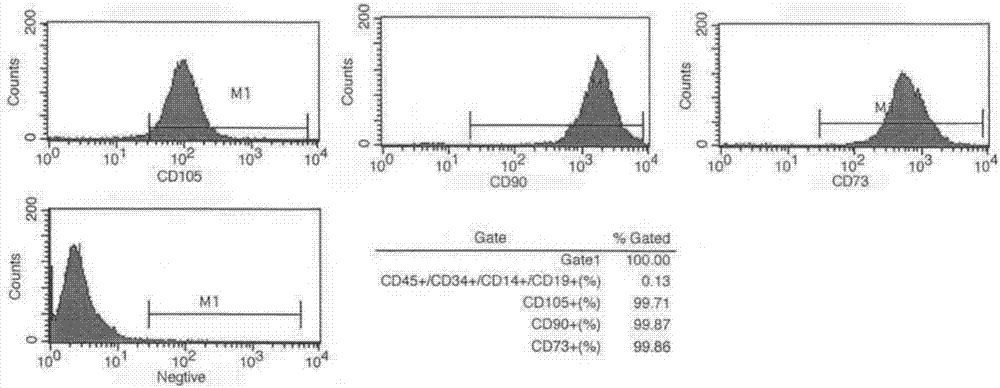
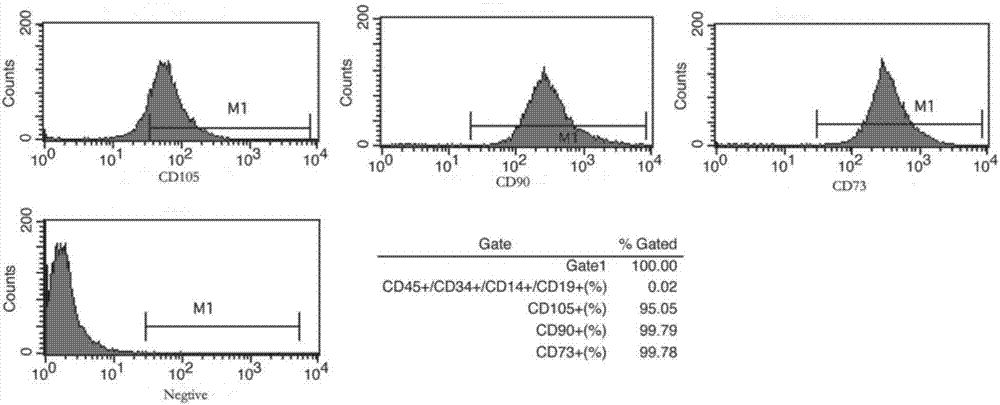
Serum-free medium for culturing placenta mesenchymal stem cells
ActiveCN103805562AIncrease growth rateMaintain stem cell propertiesSkeletal/connective tissue cellsFibroblast growth factor receptor 2Cell culture media
The invention discloses a serum-free medium for culturing placenta mesenchymal stem cells. The serum-free medium takes a DMEM (Dulbecco Modified Eagle Medium) culture solution as a basis and also contains a fibroblast growth factor receptor 2, growth hormone, insulin, transferrin, glutathione, BMP-4, L-glutamine, sodium pyruvate, non-essential amino acids and beta-mercaptoethanol. According to various serum-free media provided by the invention, growth and proliferation of the placenta mesenchymal stem cells in a serum-free medium system can be effectively promoted, the placenta mesenchymal stem cells have higher growth and proliferation rate in the serum-free medium system compared with a serum cell culture medium, the characteristics of the stem cells are preserved, the serum-free medium has multiple differential potentials, and the stem cells can be directionally induced into fat cells and osteoblasts.
Owner:章毅 +10
Modulation of growth hormone receptor expression and insulin-like growth factor expression
Compounds, compositions and methods are provided for modulating the expression of growth hormone receptor and / or insulin like growth factor-I (IGF-I). The compositions comprise oligonucleotides, targeted to nucleic acid encoding growth hormone receptor. Methods of using these compounds for modulation of growth hormone receptor expression and for diagnosis and treatment of disease associated with expression of growth hormone receptor and / or insulin-like growth factor-I are provided. Diagnostic methods and kits are also provided.
Owner:ANTISENSE THERAPEUTICS LTD +1
Modified human growth hormone
InactiveUS20060189529A1Good water solubilityImprove solubilityAntibacterial agentsSugar derivativesHuman growth hormoneSomatropin
Owner:AMBRX
Monopegylated growth hormone proteins
The present invention relates to novel methods of making soluble proteins having free cysteines in which a host cell is exposed to a cysteine blocking agent. The soluble proteins produced by the methods can then be modified to increase their effectiveness. Such modifications include attaching a PEG moiety to form pegylated proteins.
Owner:BOLDER BIOTECH
Modified Human Growth Hormone
InactiveUS20080102124A1Good water solubilityImprove solubilityBiocidePowder deliveryHuman growth hormoneSomatropin
Owner:AMBRX
Pegylated recombinant human growth hormone compounds
ActiveUS20110112021A1Reduce dosing frequencyReduced activityNervous disorderAntipyreticLipoatrophyDisease
A chemically modified human Growth Hormone (rhGH) prepared by attaching a transient linker which comprises a polyethylene glycol. The chemically modified protein may have a much longer lasting rhGH activity than that of the unmodified rhGH, enabling reduced dose and scheduling opportunities and the modified rhGH may not cause lipoatrophy. Also includes methods of use for the treatment and / or prevention of diseases or disorders in which use of growth hormone is beneficial.
Owner:ASCENDIS PHARM AS
Human growth hormone formulations
InactiveUS20080095837A1Organic active ingredientsPeptide/protein ingredientsDiseaseBuccal administration
The present invention relates to dosage forms of human growth hormone, the use of an absorption enhancer to allow absorption of human growth hormone into the systemic circulation in a biologically active form, in particular after oral administration, as well as the use of oral dosage forms comprising human growth hormone and an absorption enhancer for the treatment of human growth hormone deficiencies and disorders associated therewith.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
GHRH analogues
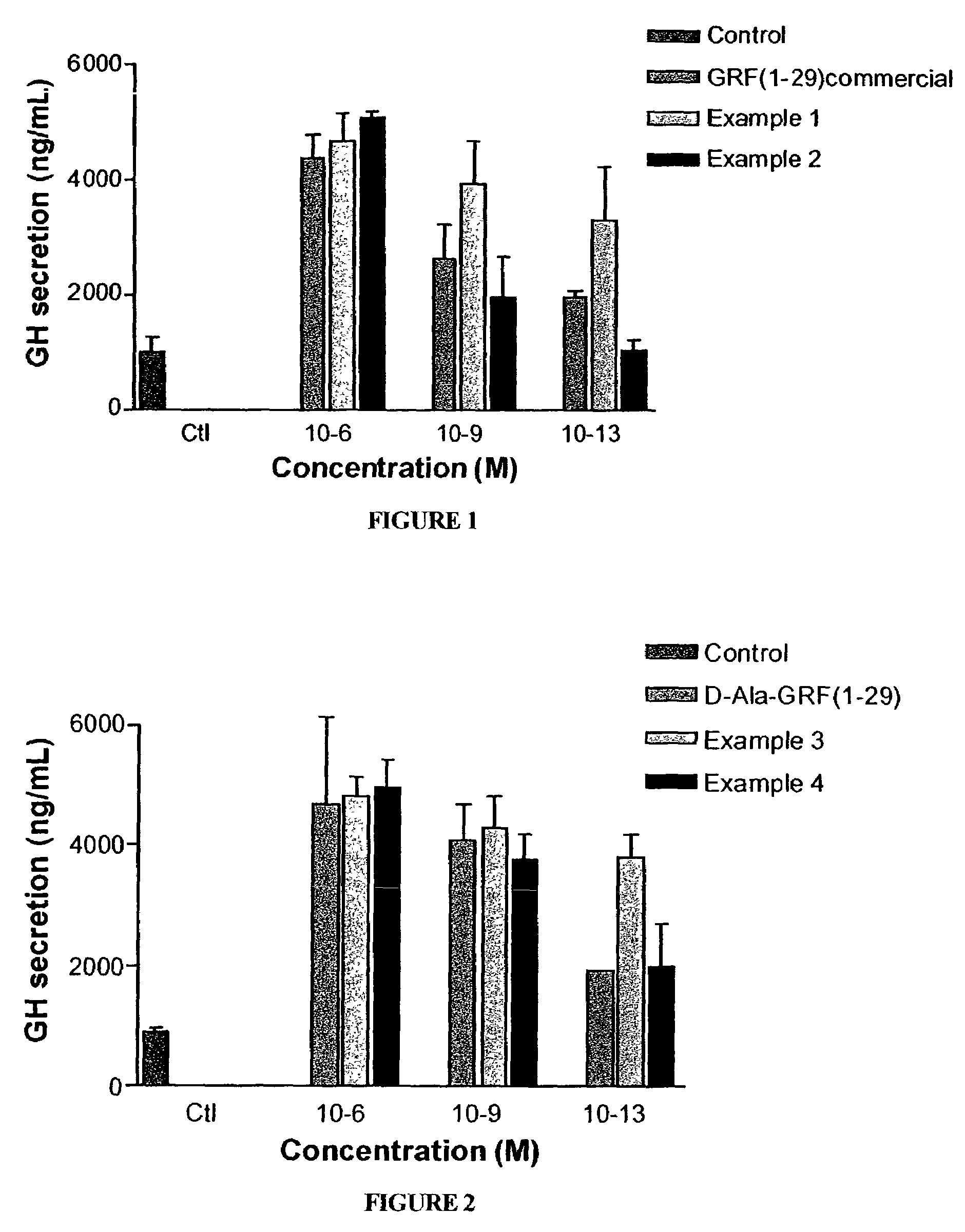
The present invention relates to growth hormone-releasing hormone (GHRH) analogues. More particularly, the invention relates to synthetic GHRH analogues of amino acids or more, exhibiting concomitantly an increased resistance to proteolysis and high binding affinity to human GHRH receptor in in vitro studies, in comparison with human native GHRH (1-29)NH2. The present invention also relates to a pharmaceutical composition comprising any one of said GHRH analogues and to the use of these analogues for specific stimulation of in vivo GH release as well as preparation of a drug in the treatment of GH deficiency-related conditions. The present invention also provides for a method for initiating GHRH-induced biological actions in a mammal.
Owner:CENT HOSPITALER DE LUNIV DE MONTREAL CHUM
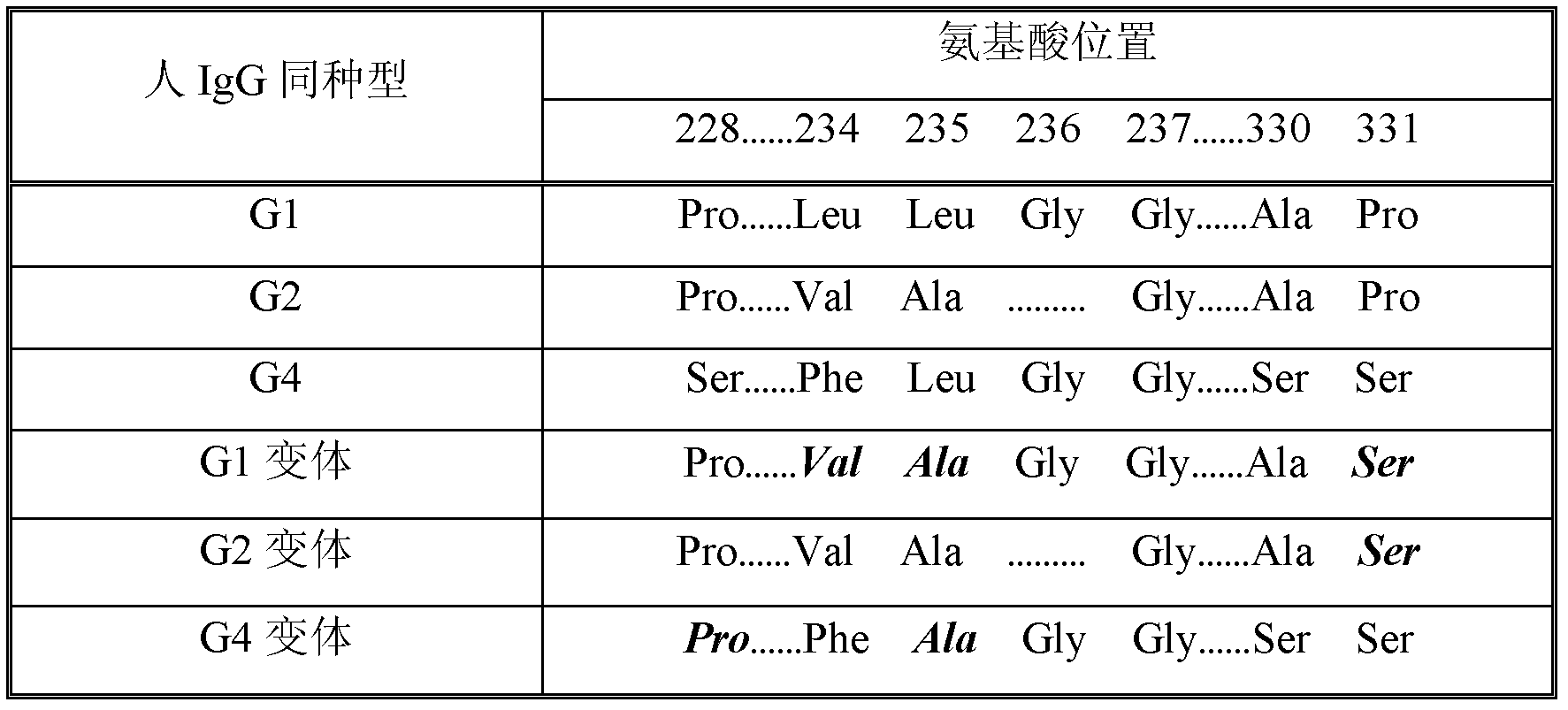
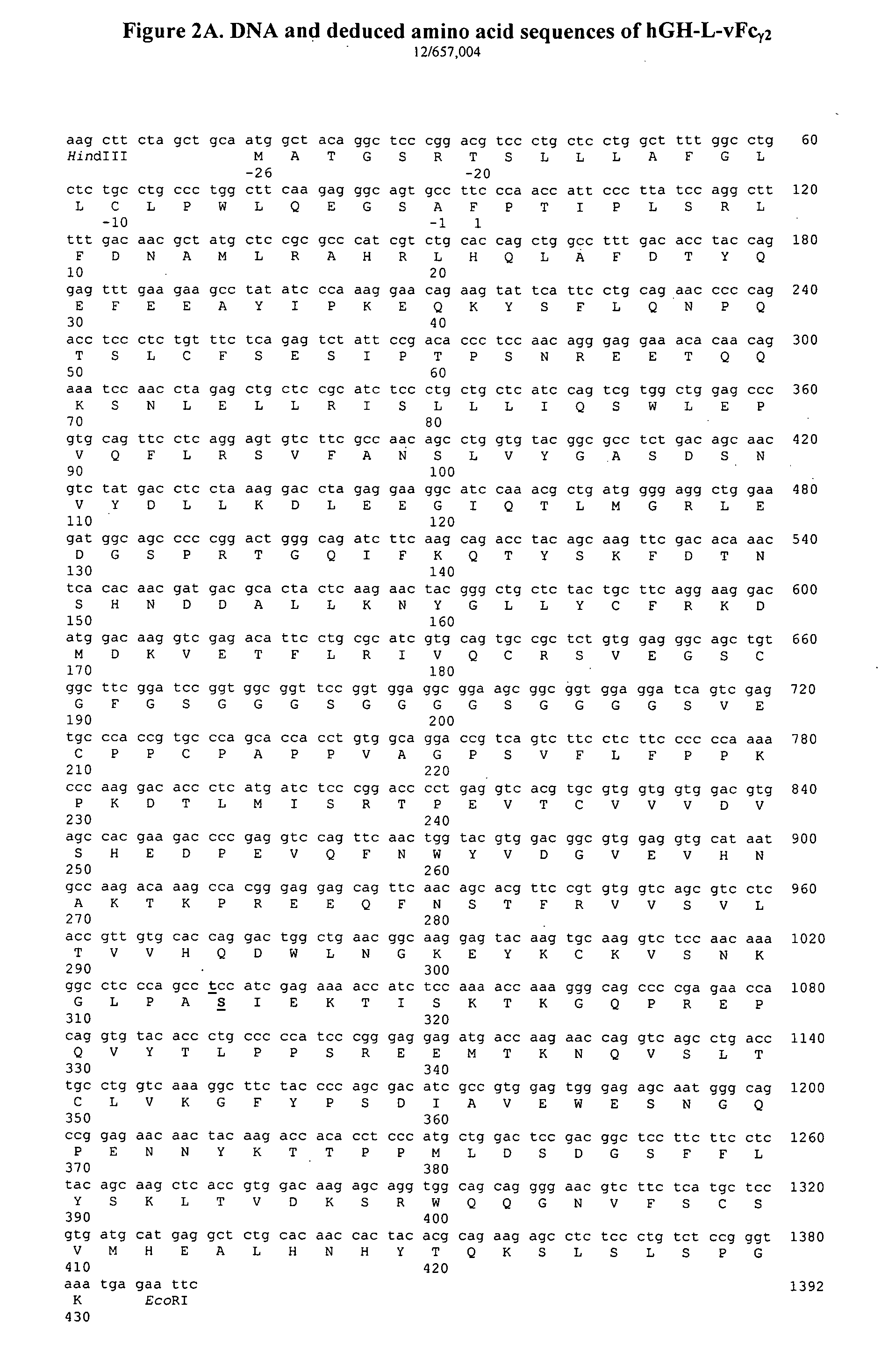
Fc fusion protein of long-acting recombinant human growth hormone
The invention discloses a Fc fusion protein of long-acting recombinant human growth hormone. The Fc fusion protein (hGH-L-vFc fusion protein) disclosed herein contains human growth hormone, flexible peptide linker of about 2-20 amino acids, and human IgG Fc mutant. The Fc mutant is not lytic and has tiny side effect of adverse Fc-mediator. The invention further discloses a method for preparing or generating the fusion protein with high expression level. The hGH-L-vFc fusion protein disclosed herein has prolonged serum half-life period and increased biological activity, so as to improve the pharmacokinetics and drug efficacy, and needs few times of injection required in treatment.
Owner:PHARMAB
Liquid formulation of long-acting human growth hormone conjugate
InactiveUS20130115231A1Good storage stabilityIncreased durabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsFactor iiAlcohol sugars
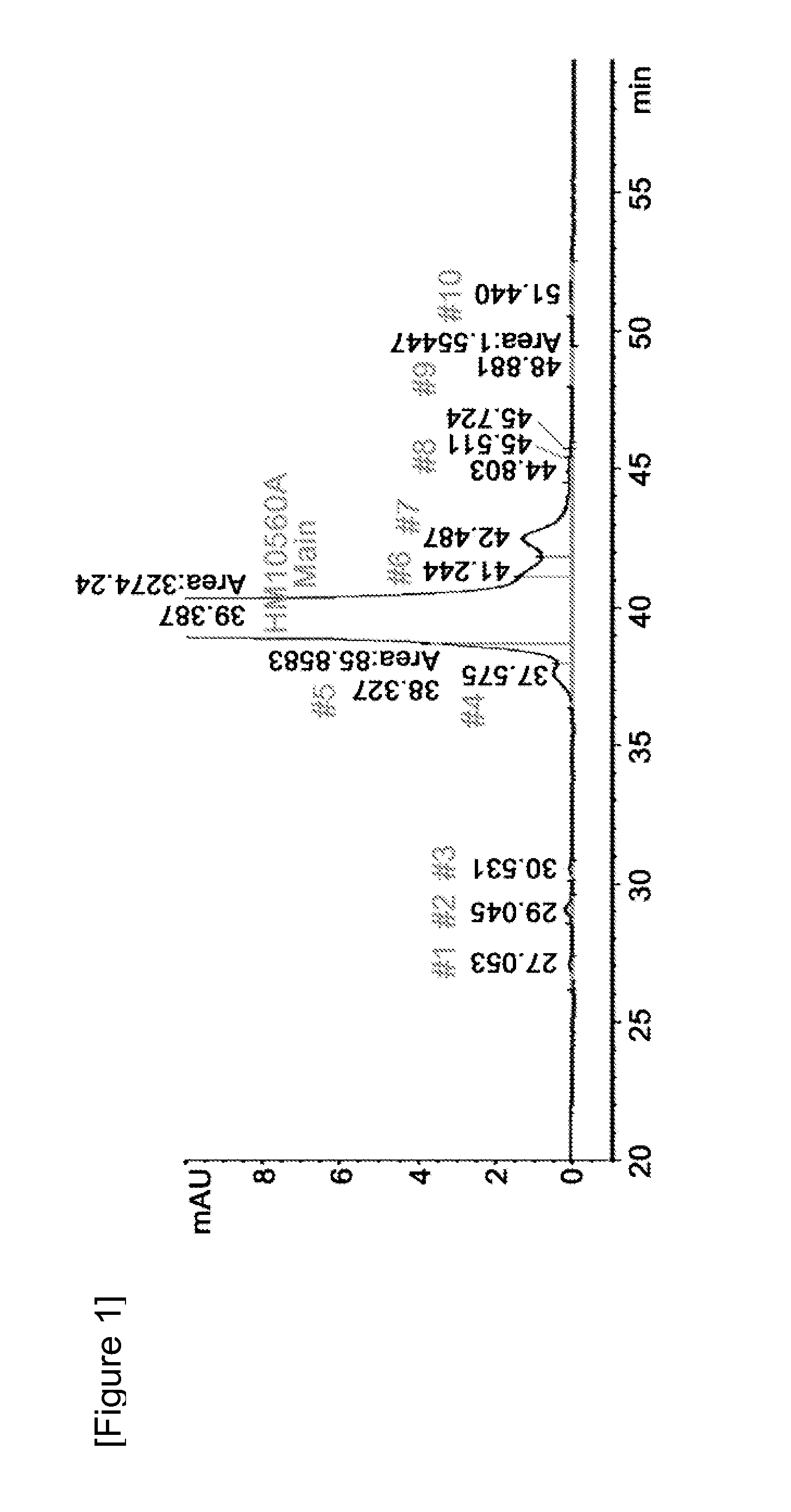
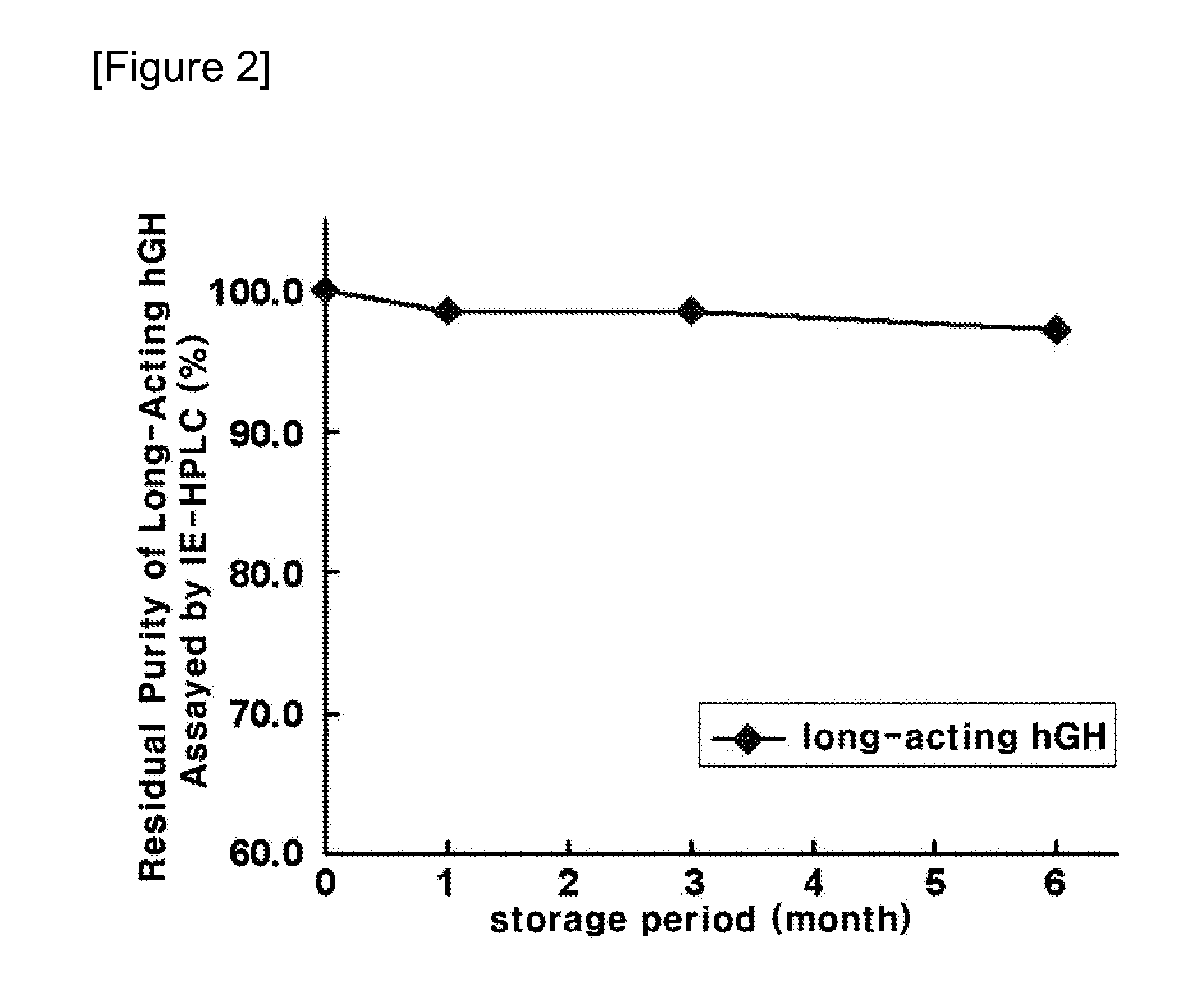
Disclosed is a liquid formulation of long-acting human growth hormone (hGH) conjugate, free of albumin, which can guarantee the stability of the long-acting hGH conjugate when stored over a long period of time, wherein the long-acting human growth hormone conjugate includes a human growth hormone linked to an immunoglobulin Fc region, and has a prolonged in vivo stability compared to the native form. The liquid formulation of hGH conjugate including a pH 5.0˜6.0 buffer, a sugar alcohol, a salt and a non-ionic surfactant is free of human serum albumin and other hazardous factors which are potentially contaminated with viruses, and can provide excellent storage stability customized for a long-acting hGH conjugate composed of an hGH polypeptide and an immunoglobulin Fc region which has higher molecular weight and in vivo durability, compared to the native.
Owner:HANMI SCI CO LTD
Enhanced nasal composition of active peptide
InactiveUS20090035260A1Prevent oxidationImprove stabilityPeptide/protein ingredientsMetabolism disorderNasal cavityGoserelin
A pharmaceutical composition has a therapeutically effective amount of at least one of: a pharmaceutically active nasal peptide, its pharmaceutically acceptable salt and its peptidic fragment. The composition also contains an absorbefacient effective amount of THAM in a pharmaceutically acceptable, aqueous liquid diluent or carrier. The composition is provided in a convenient form for nasal administration. In one embodiment, the peptidic fragment may be selected physiologically active lymphokines and monokines, peptidic enzymes, proteic vaccines, peptidic toxoids and personalized proteins derived from genoma. In another embodiment, the peptidic fragment may be selected from the peptide hormones and hormone antagonists buserelin, desmopressin, vasopressin, angiotensin, felypressin, octreotide, somatropin, thyrotropin (TSH), somatostatin, gosereline, thryptorelin and insulin selected from the group consisting of cow and pig, synthetic and recombinant.
Owner:THERAPICON SRL
Growth hormone variants
InactiveUS8637646B2Improve effectivenessBacteriaPeptide/protein ingredientsHuman growth hormoneAmino acid substitution
The invention provides variants of human growth hormone having an amino acid substitution at amino acid residue R77, numbered from the N-terminus of 191-amino add human growth hormone as well as nucleic acid molecules encoding these variants.
Owner:GENENTECH INC
Mesenchymal stem cell serum-free culture medium
ActiveCN106190964ANormal growthQuality is easy to controlSkeletal/connective tissue cellsOsteoblastCulture mediums
The invention discloses a mesenchymal stem cell serum-free culture medium. The culture medium takes an L-DMEM, DMEM-F12 or alpha-MEM culture medium as a basic culture medium and also includes the following addition components: fibronectin, laminin, transferrin, trypsin, aprotinin, growth hormone, insulin, hydrocortisone, dexamethasone, bFGF, EGF, B27, IGF-I, IGF-II, choline, linoleic acid, sodium selenite, phosphorylethanolamine, glutathione, vitamin C, vitamin E, vitamin B12 and biotin. The culture medium has the advantages of defined composition and controllable quality, and mesenchymal stem cells cultured by the culture medium grow normal; and after multiple passages, the 'dryness' of the mesenchymal stem cells and the ability of the mesenchymal stem cells to be differentiated into osteoblasts, chondrocytes, adipocytes, neurons and other types of cells are still kept.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
Modified human growth hormone
InactiveUS20050020494A1Modify characteristicMagnitude is largePeptide/protein ingredientsImmunoglobulinsHuman growth hormoneIn vivo
The invention relates to the modification of human growth hormone (high) to result in human growth hormone proteins that are substantially non-immunogenic or less immunogenic than any non-modified counterpart when used in-vivo. The invention relates, furthermore, to T-cell epitome sequences deriving from high, which are immunogenic.
Owner:MERCK PATENT GMBH
Novel growth hormone releasing hormone analog peptides and application thereof in preparing medicines for treating infertility
The invention discloses novel growth hormone releasing hormone analog peptides and an application thereof in preparing medicines for treating infertility. Experiments discover that 2D, 2E or 2F peptide has obviously high hypophysis GH releasing activity and hypophysis hormone releasing specificity. Tested by the conjugation reaction of an in-vitro GHRH dimer peptide and a hypophysis GHRH receptor, 2D, 2E and 2F dimer peptides have extremely high hypophysis receptor binding activity, wherein the 2F dimer peptide has the maximum binding activity. With the 2F peptide as the representative, infertility model treatment finds out that, compared with a normal saline group and a pure cyclophosphamide control group, in the 2F dimer peptide group, the spermatocytes and the spermatogonia in the seminiferous tubules are obviously increased, the seminiferous tubule cells are arranged in order and large in volume, the cavities of the seminiferous tubules are reduced and even disappear, and dose dependency is shown. All the facts indicate that the GHRH peptides with the 2F peptide as the representative have an obvious effect of stimulating the proliferation and the maturation of spermatogonia / oogonia, thereby promoting reproduction; and as a result, the novel growth hormone releasing hormone analog peptides can be applied to medicines for treating infertility.
Owner:深圳纳福生物医药有限公司
Fc fusion proteins of human growth hormone
ActiveUS20120116056A1Improve biological activityImproved pharmacodynamicsAnimal cellsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human growth hormone with good biological activities relative to rhGH on a molar basis are disclosed. The hGH-L-vFc fusion protein comprises hGH, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such hGH-L-vFc fusion proteins exhibit extended or prolonged serum half-life and / or good biological activities relative to that of rhGH on a molar basis, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:LONGBIO PHARM (SUZHOU) CO LTD
Serum-free cryoprotectant, and application thereof in cryopreservation of mesenchymal stem cells
ActiveCN107494517AImprove survival rateIncreased clinical allergen riskDead animal preservationUmbilical cordResuscitation
A serum-free cryoprotectant includes: 8-15 v / v% of DMSO, 85-92 v / v% of a DMEM basic culture medium, and a nutritional additive. The nutritional additive includes: fibroblast growth factors, insulin, growth hormone, transferrin, bone morphogenetic protein 4, glutamine, sodium pyruvate, beta-mercaptoethanol, human epidermal growth factor, sodium selenite, and various amino acids and vitamins. The cryoprotectant is free of animal sourced serum and avoids pollution and risk of allergen, and has better clinical safety. The serum-free cryoprotectant is suitable for cryopreservation of human placenta sourced, umbilical cord sourced and cord blood sourced mesenchymal stem cells; compared with common serum cryoprotectants, perinatal mesenchymal stem cells preserved in the cryoprotectant have high cell survival rate after resuscitation, have excellent adherence growth status and maintain biological characters well.
Owner:章毅 +7
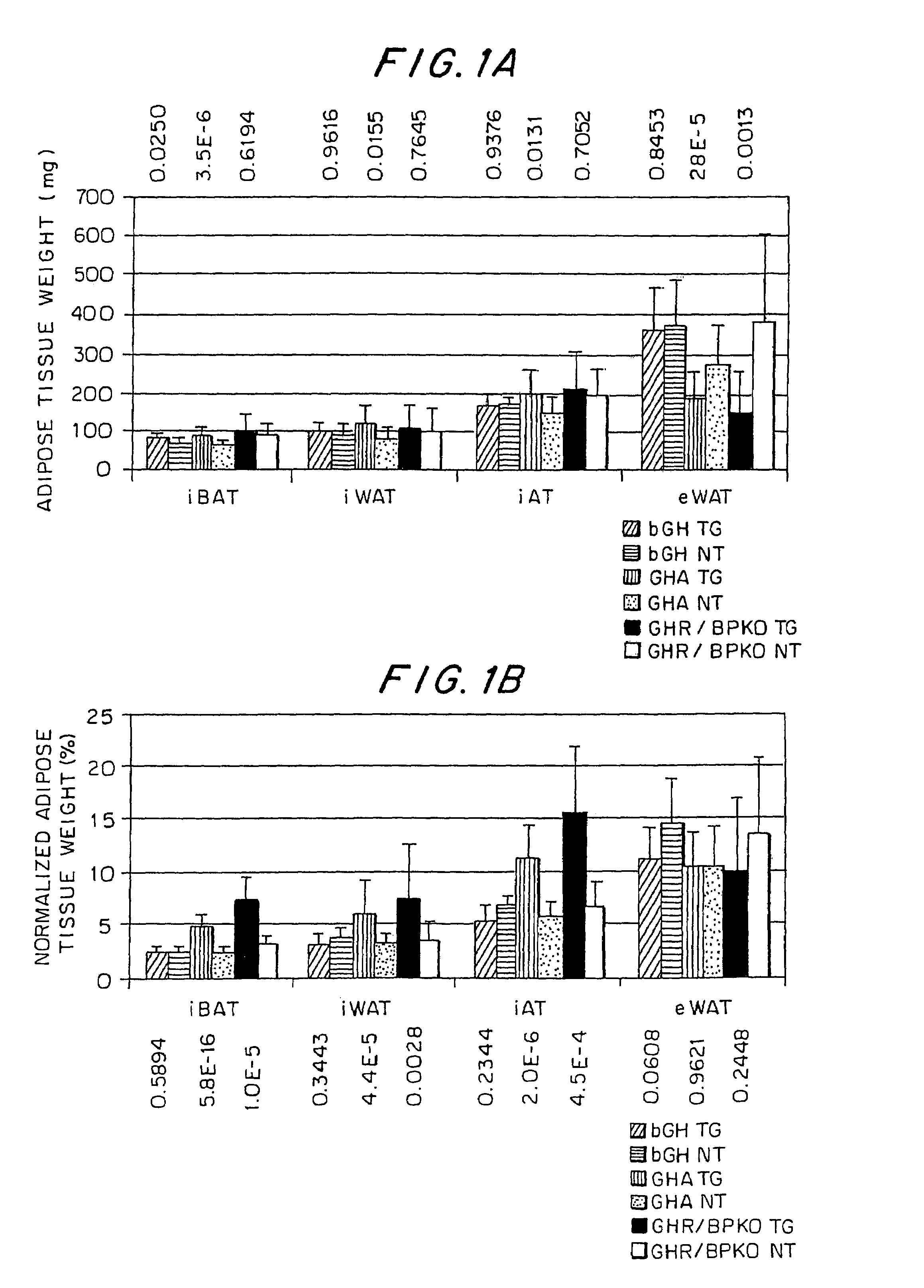
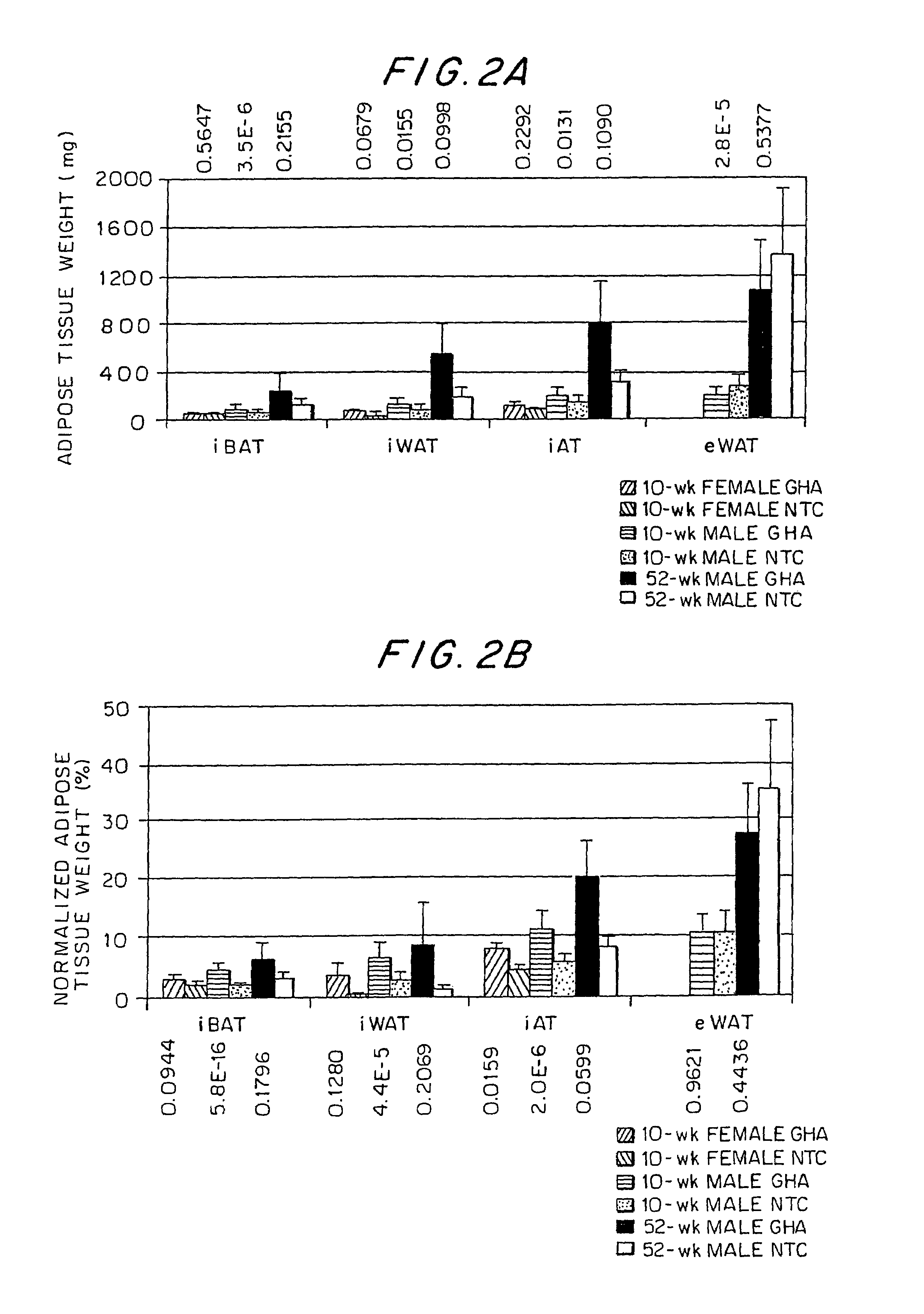
Growth hormone-regulatable brown adipose tissue genes and proteins and uses thereof
InactiveUS7060437B1High expressionSugar derivativesMicrobiological testing/measurementBrown adipose tissueFhit gene
Growth hormone-regulatable brown adipose tissue genes and proteins have been identified. They may be used as diagnostic markers of pathologies of adipose tissue.
Owner:OHIO UNIV
Growth hormones with prolonged in-vivo efficacy
ActiveUS8779109B2Improve absorption rateWeak affinityPeptide/protein ingredientsComponent separationIn vivoAlbumin
Owner:NOVO NORDISK HEALTH CARE AG
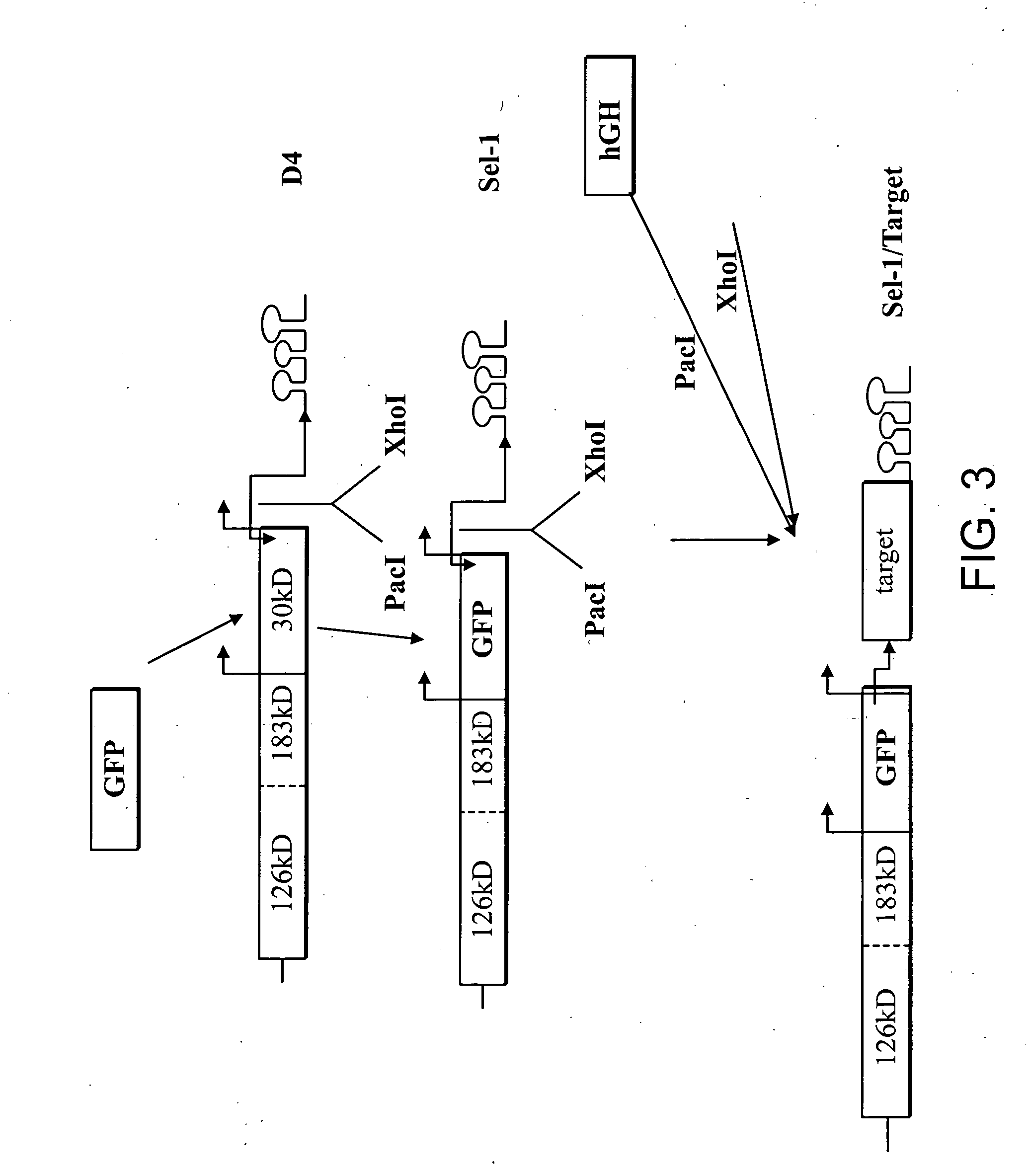
Preparations of growth hormone
The present invention relates to compositions and systems for expressing pharmaceutically active gene products in plants. In particular, the invention provides compositions, systems and methods for the production of human growth hormone in plants. Provided are nucleic acid and protein sequences, expression and vector constructs, host cells and plants capable of expressing human growth hormone, and compositions and kits comprising produced human growth hormone. Additionally provided are methods for production and use of the compositions of the invention. Therapeutic use of the produced human growth hormone is also provided
Owner:FRAUNHOFER USA +1
Modified methylotrophic Pichia pastoris yeast which secretes human growth hormone
In the present system an adequate expression system for the production and secretion of biologically active human growth hormone (HGH) in its natural form in which a methylotrophic yeast such as Pichia pastoris is used as host organism has been developed. This invention includes a methylotrophic yeast transformed with at least one copy of a functional cDNA sequence encoding HGH, which is functionally associated with a second DNA sequence encoding the S. cerevisae alpha factor pre-pro sequence (including the proteolytic processing site: lys-arg), and in which both DNA sequences are under the regulation of a methylotrophic yeast gene promoter which is inducible with methanol. Methylotrophic yeasts containing in their genome at least one copy of the DNA sequence efficiently produce and secrete mature, correctly processes and biologically active HGH, into the culture medium.
Owner:UNIV AUTONOMA DE NUEVO LEON
Recombinant oral protein TAT-GH of tilapia, preparation method for recombinant oral protein TAT-GH and application of recombinant oral protein TAT-GH
ActiveCN102703483AGuaranteed accuracyIntegrity guaranteedBacteriaAnimal feeding stuffBiotechnologyEscherichia coli
The invention discloses recombinant oral protein TAT-GH of tilapia, a preparation method for the recombinant oral protein TAT-GH and application of the recombinant oral protein TAT-GH. The method comprises the following steps of: extracting total messenger ribonucleic acid (mRNA) from a pituitary gland of male tilapia, performing reverse transcription-polymerase chain reaction (RT-PCR) to obtain complementary deoxyribonucleic acid (cDNA), performing PCR amplification to obtain a growth hormone (GH) gene of the tilapia, and amplifying a TAT-GH gene by a PCR overlap extension method; constructing a recombinant plasmid pET-32a(+)-TAT-GH; and transforming Escherichia coli BL21 (DE3) by using the recombinant plasmid pET-32a(+)-TAT-GH, efficiently expressing fusion protein TAT-GH in the form of an inclusion body under the induction of isopropyl thiogalactoside (IPTG), and purifying and renaturing to obtain active protein. The protein can effectively promote the growth and development of fries of the tilapia after being fed to the fries of the tilapia.
Owner:HUBEI TAIYANGHONG BIOLOGICAL TECH CO LTD
Leptin-resistance amerliorating agents
InactiveUS20030036512A1Improve securityReduce resistancePeptide/protein ingredientsMetabolism disorderNeurotrophic factorsLeptin resistance
Leptin resistance ameliorating agents comprising as the active ingredient a neurotrophic factor such as BDNF or a trk receptor ligand, namely, agents for ameliorating, treating or preventing hypertension, sterility, obesity, glucose metabolic disorders, ischemic diseases, growth hormone secretion insufficiency, growth hormone hyposecretion, immune function disorder, etc. caused by leptin resistance.
Owner:SUMITOMO DAINIPPON PHARMA CO LTD
Gene treatment to enhance feed efficiency and growth rate of livestock
InactiveUS20020137701A1Improve feed conversion efficiencyPromote growth rateBiocidePeptide/protein ingredientsBiotechnologyLivestock
This invention relates to the enhancement of feed efficiency and growth rate, and the reduction of fit accumulation to produce better quality meat by administration of an exogenous gene sequence comprising the complementary DNA sequence of growth hormone releasing factor to stimulate the production of the endogenous hormone peptide growth hormone. This invention further relates to methods for producing such gene sequence.
Owner:LEADERGENE
Hyperglycosylated human growth hormone fusion protein and preparation method and application thereof
InactiveCN106256835AImprove stabilityLow immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeImmunoglobulin Fc Fragments
The invention discloses hyperglycosylated human growth hormone fusion protein. The human growth hormone fusion protein sequentially contains a human growth hormone (hGH), a flexible peptide joint (L), human chorionic gonadotropin beta-carboxyl terminal rigid peptide (CTP) and a human immunoglobulin Fc fragment from the N terminal to the C terminal. The invention further discloses a method for efficiently preparing the fusion protein. Compared with a recombinant hGH, the built fusion protein has more excellent in-vivo drug efficacy, the in-vivo circulation half-life period is prolonged, the administration frequency is greatly decreased, and the bioavailability is improved; meanwhile, the production process is simpler and more efficient.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
Use of cytochrome p450-metabolized drugs and grf molecules in combination therapy
InactiveUS20100267636A1Affect their pharmacokineticsBiocidePeptide/protein ingredientsDrug interactionCytochrome P450
Combination therapies comprising a drug metabolized by cytochrome P450 and a growth hormone (GH)-inducing compound (such as a GRF molecule) are described, in which there are no or substantially no drug interactions.
Owner:THERATECHONOLGIES INC
Long-acting growth hormone and methods of producing same
InactiveCN104010650AIncrease weightPeptide/protein ingredientsMetabolism disorderNucleotidePolynucleotide
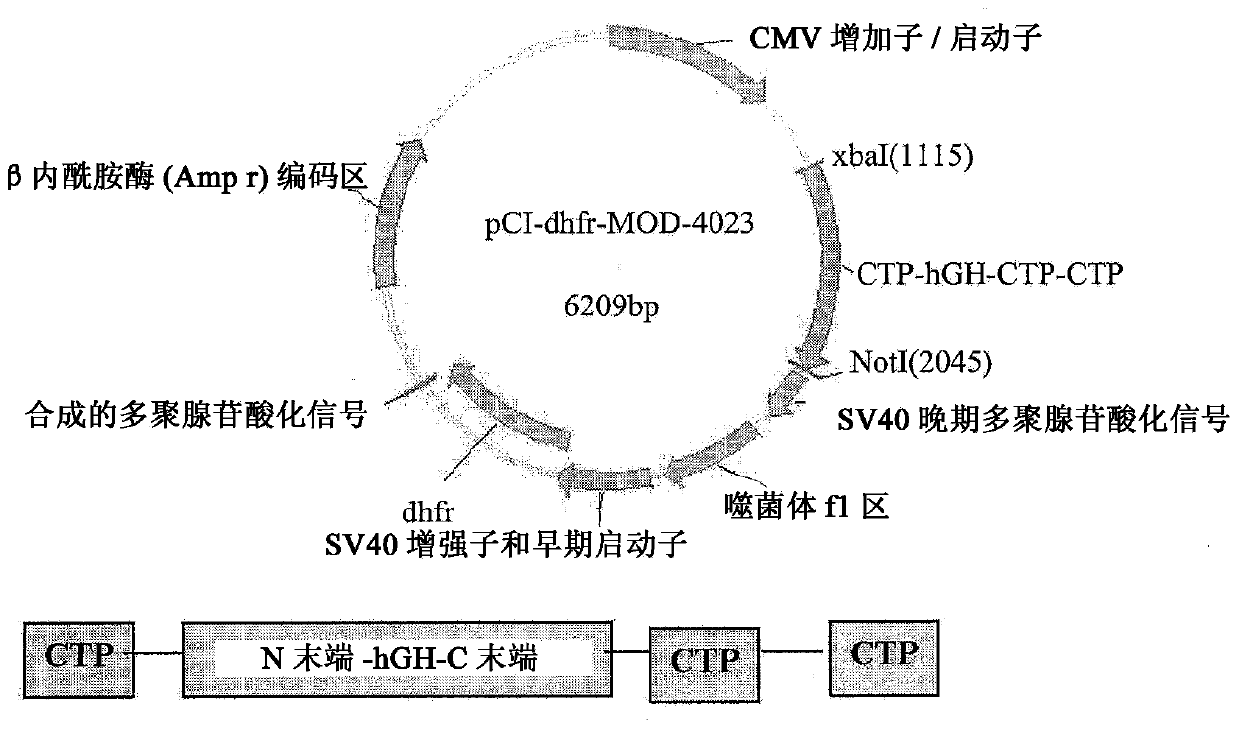
Use of a growth hormone protein and polynucleotides encoding the same comprising an amino-terminal carboxy-terminal peptide (CTP) of chorionic gonadotrophin and two carboxy-terminal chorionic gonadotrophin CTPs attached to the growth hormone in methods of inducing weight loss or body fat reduction, methods of increasing insulin-like growth factor (IGF-1) levels, and methods of reducing the dosing frequency of a growth hormone in a human subject are disclosed. Pharmaceutical compositions comprising the growth hormone and polynucleotides encoding the growth hormone of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Methods And Compositions For The Treatment Of Prolactin-Receptor Related Disorders
The present invention provides methods and compositions for the treatment, diagnosis, prevention, or amelioration of one or more symptoms of a prolactin receptor-related condition, including, for example, a cancer such as breast cancer or prostate cancer, by administering a growth hormone-based prolactin receptor antagonist and zinc, or administering a growth hormone-based prolactin receptor antagonist to a tissue with an effective local concentration of zinc. The invention further provides pharmaceutical compositions of growth hormone-based prolactin receptor antagonists and zinc useful in the methods of the invention.
Owner:TERCICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com