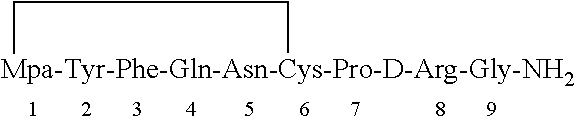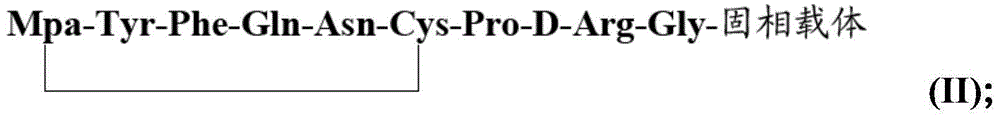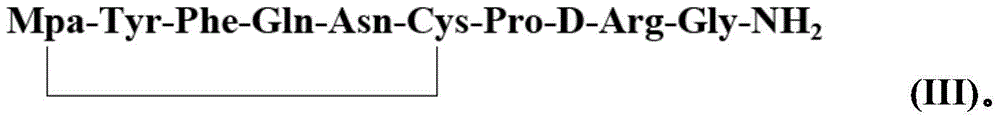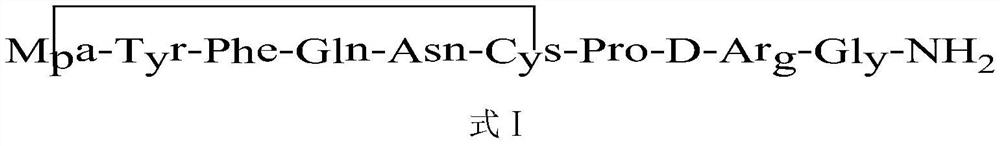Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
45 results about "Desmopressin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Desmopressin is used to control the amount of urine your kidneys make.
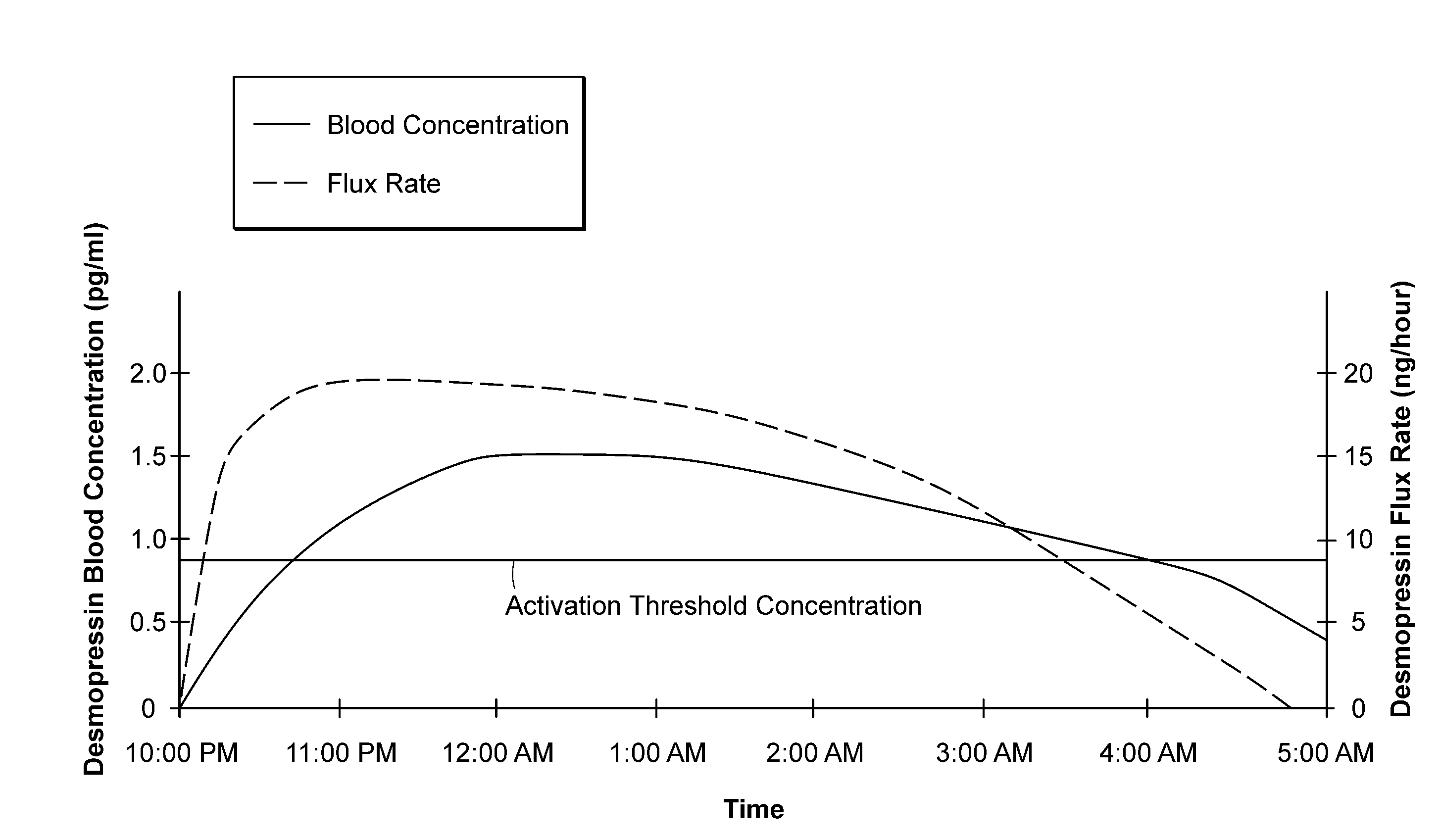
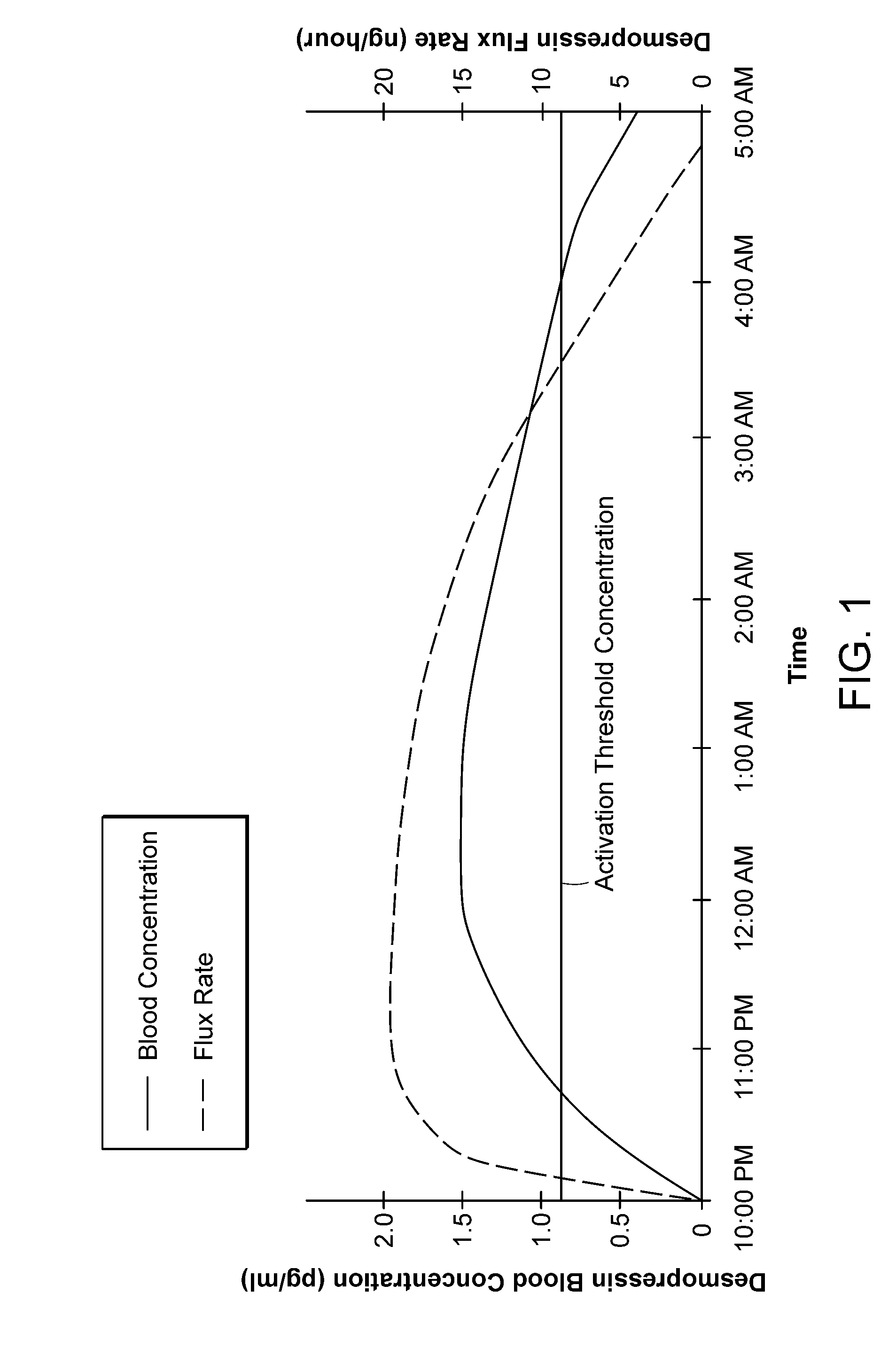
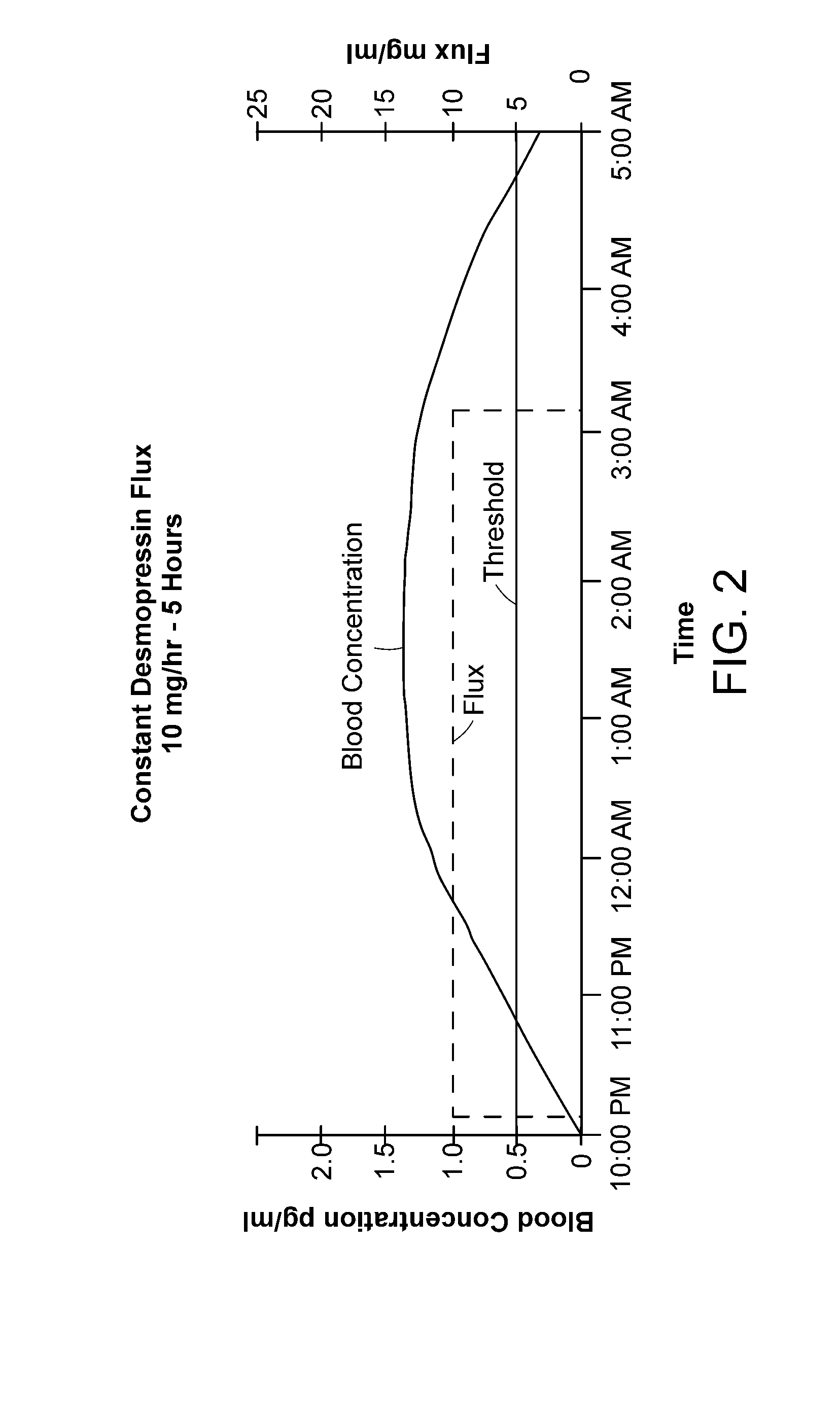
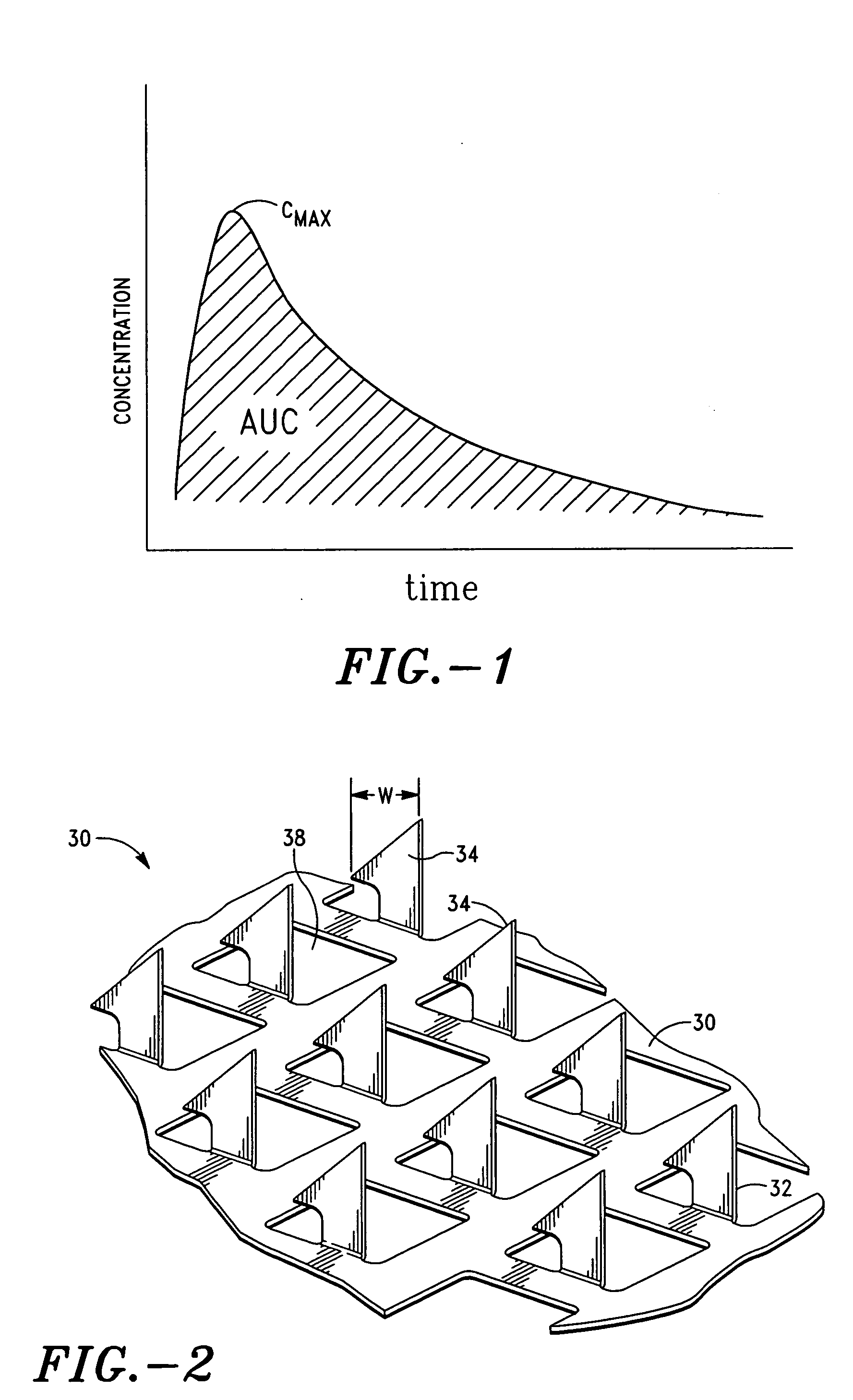
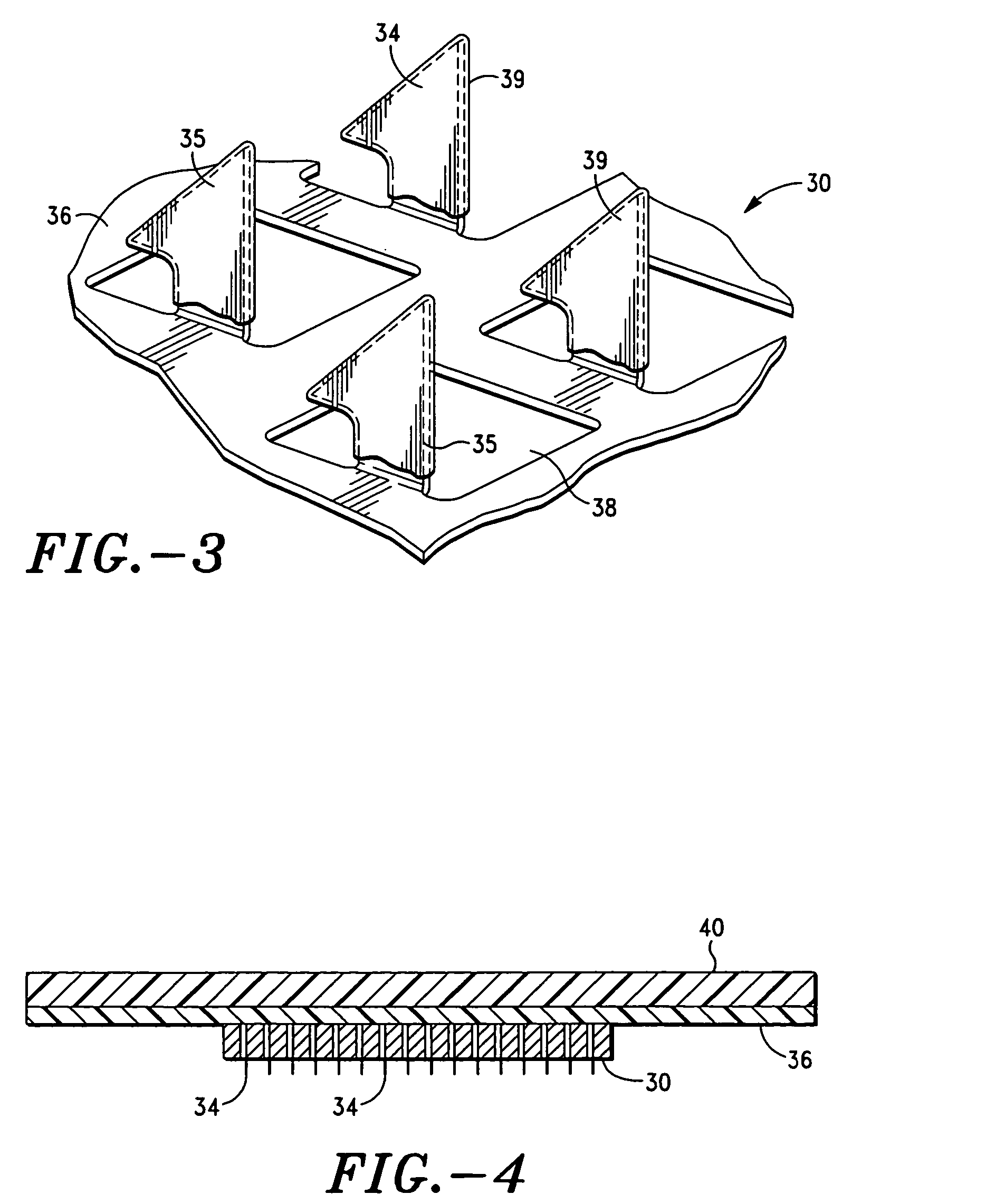
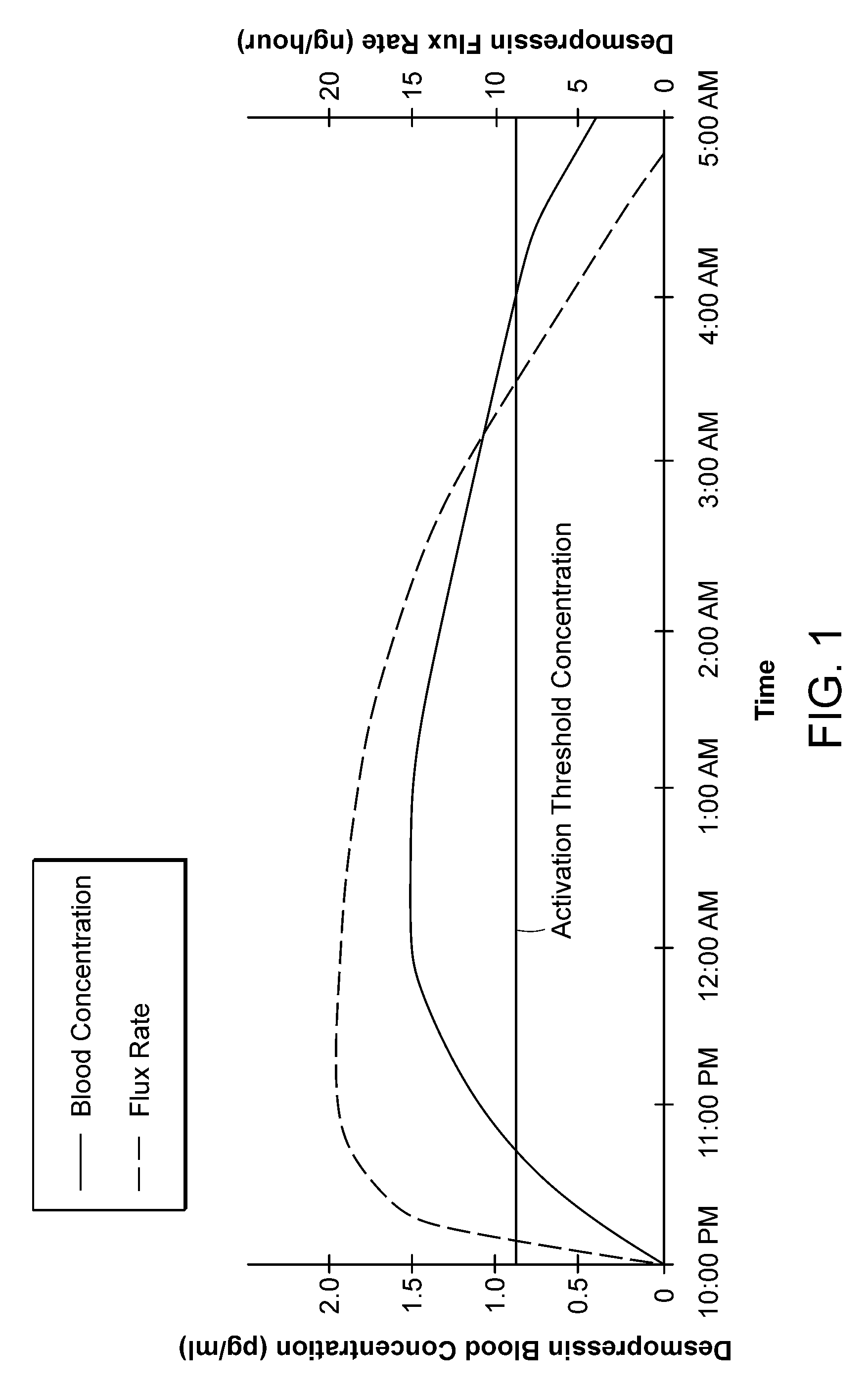
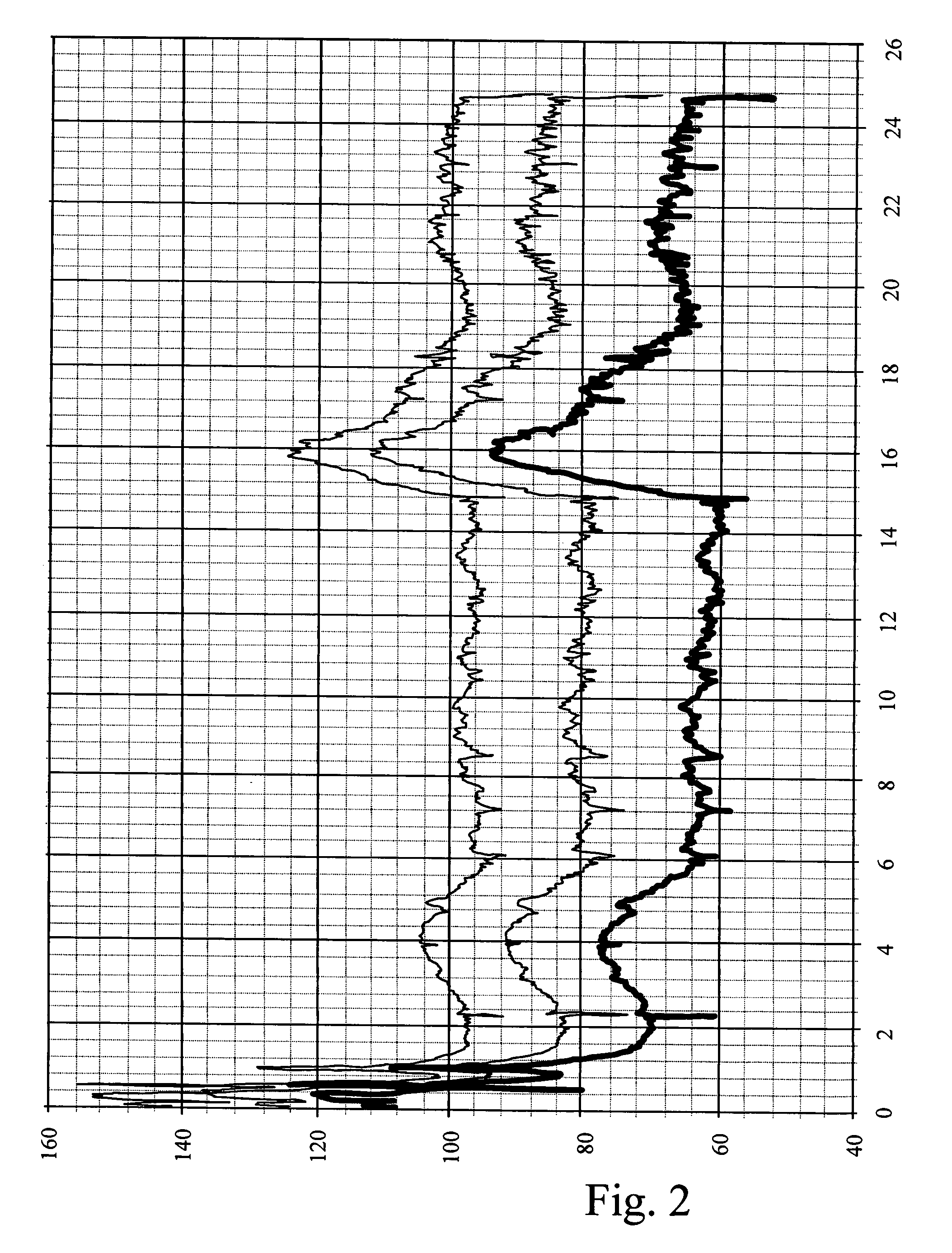
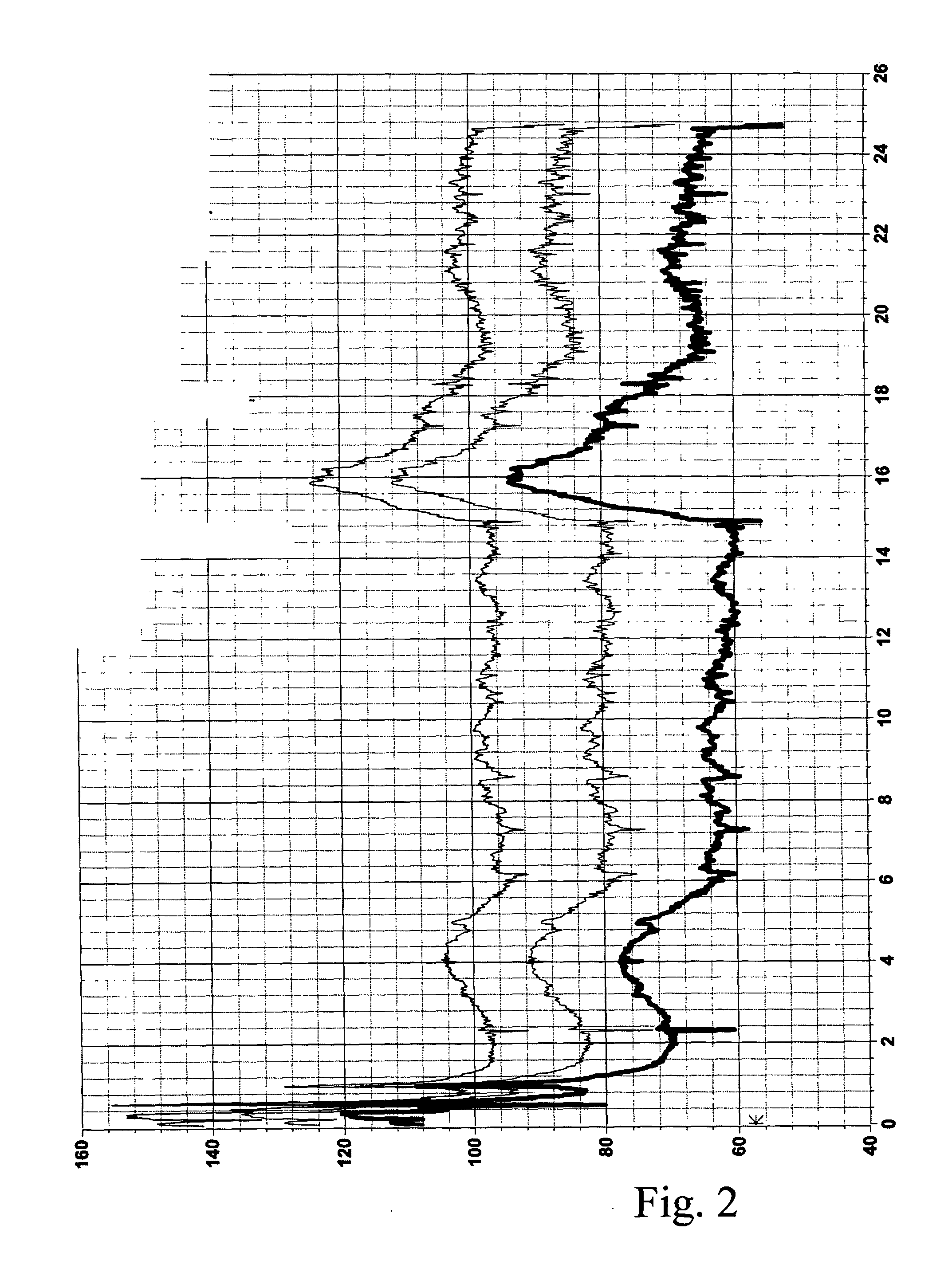
Methods and devices for desmopressin drug delivery
InactiveUS20090042970A1Reduce urine productionRestore normal urine productionBiocidePowder deliveryDecreased sodiumSide effect
Disclosed are devices for urine voiding postponement, and methods for treating conditions such as central diabetes insipidus, enuresis, nocturia, urinary frequency or incontinence. The devices deliver a desmopressin flux through the skin of a patient in a low dose amount just necessary to achieve a desired anti-diuretic effect without undesirable side effects such as hyponatremia. The devices are designed to permit a state of normal urinary production to return quickly after the desmopressin flux is terminated.
Owner:SERENITY PHARMA CORP
Apparatus and method for transdermal delivery of desmopressin
InactiveUS20060093658A1Minimize and eliminate bleedingMinimize and eliminate and irritationMicroneedlesMedical devicesDermisDesmopressin
An apparatus and method for transdermally delivering desmopressin comprising a delivery system having a microprojection member (or system) that includes a plurality of microprojections (or array thereof) that are adapted to pierce through the stratum corneum into the underlying epidermis layer, or epidermis and dermis layers. In one embodiment, the desmopressin is contained in a biocompatible coating that is applied to the microprojection member.
Owner:ALZA CORP
Stable aqueous composition of a peptide
InactiveUS20030216302A1Improve anti-corrosion performanceGood curative effectBiocideInorganic non-active ingredientsPreservativeBuffering agent
The present invention relates to a stable aqueous composition comprising desmopressin or its other pharmaceutically acceptable salts in a pharmaceutically acceptable carrier, wherein the carrier comprises a buffering agent, a parahydroxybenzoate preservative, and a cosolvent.
Owner:SUN PHARMA INDS
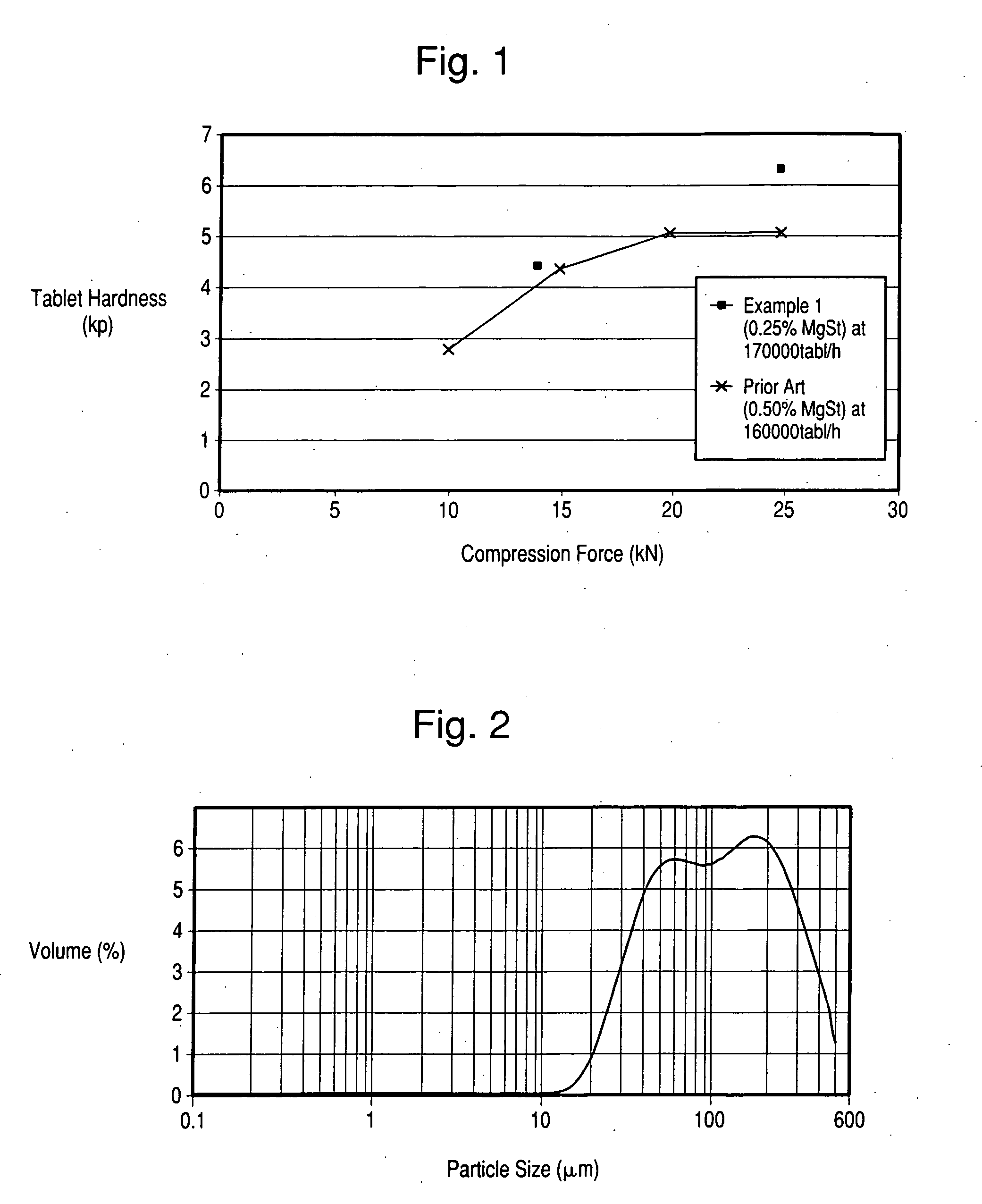
Pharmaceutical composition as solid dosage form and method for manufacturing thereof
Owner:FERRING B B
Pharmaceutical composition as solid dosage form and method for manufacturing thereof
ActiveUS7094545B2Improve manufacturing speedIncrease speed and capacityOrganic active ingredientsPeptide/protein ingredientsSolid Dose FormBULK ACTIVE INGREDIENT
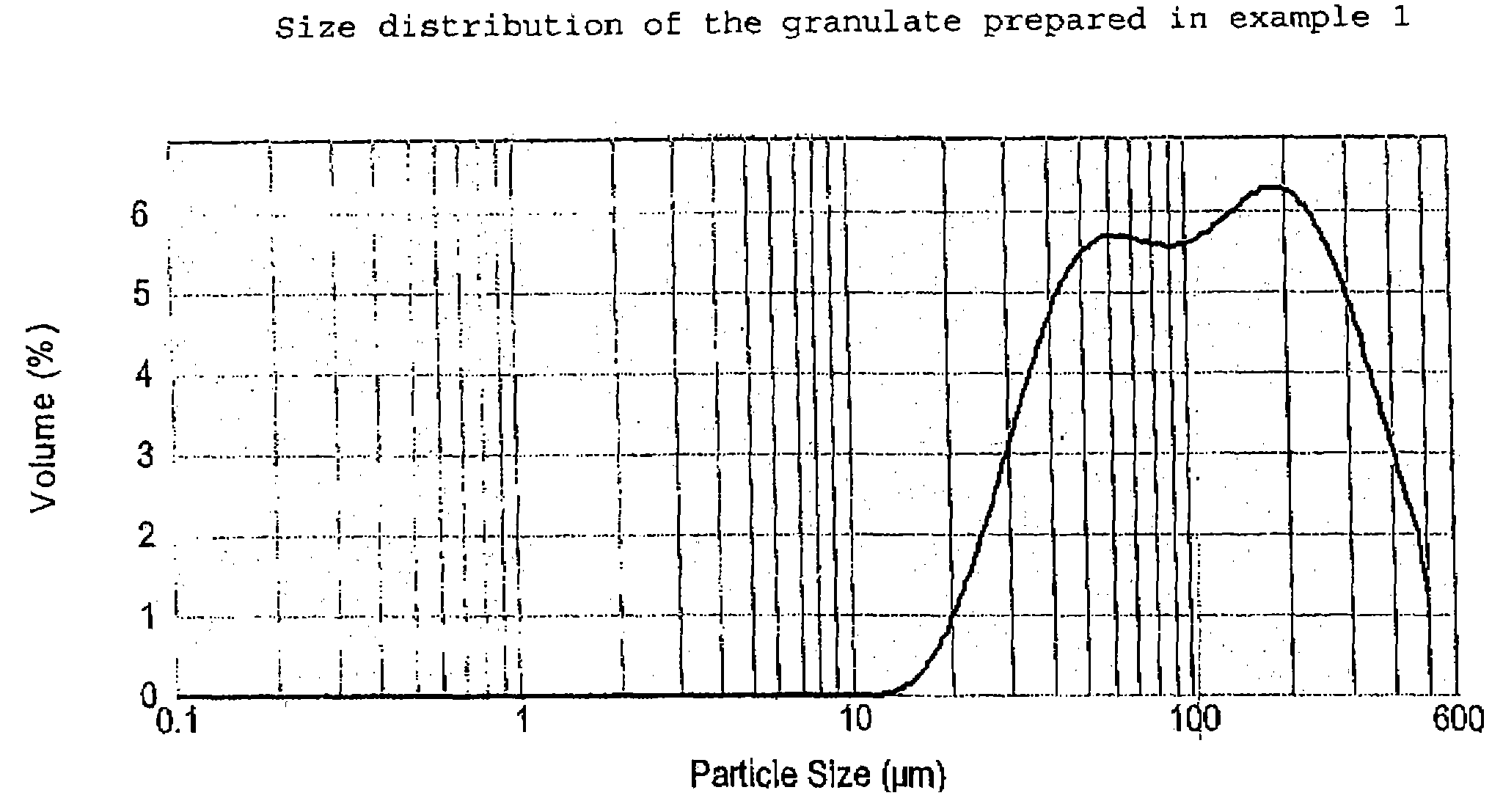
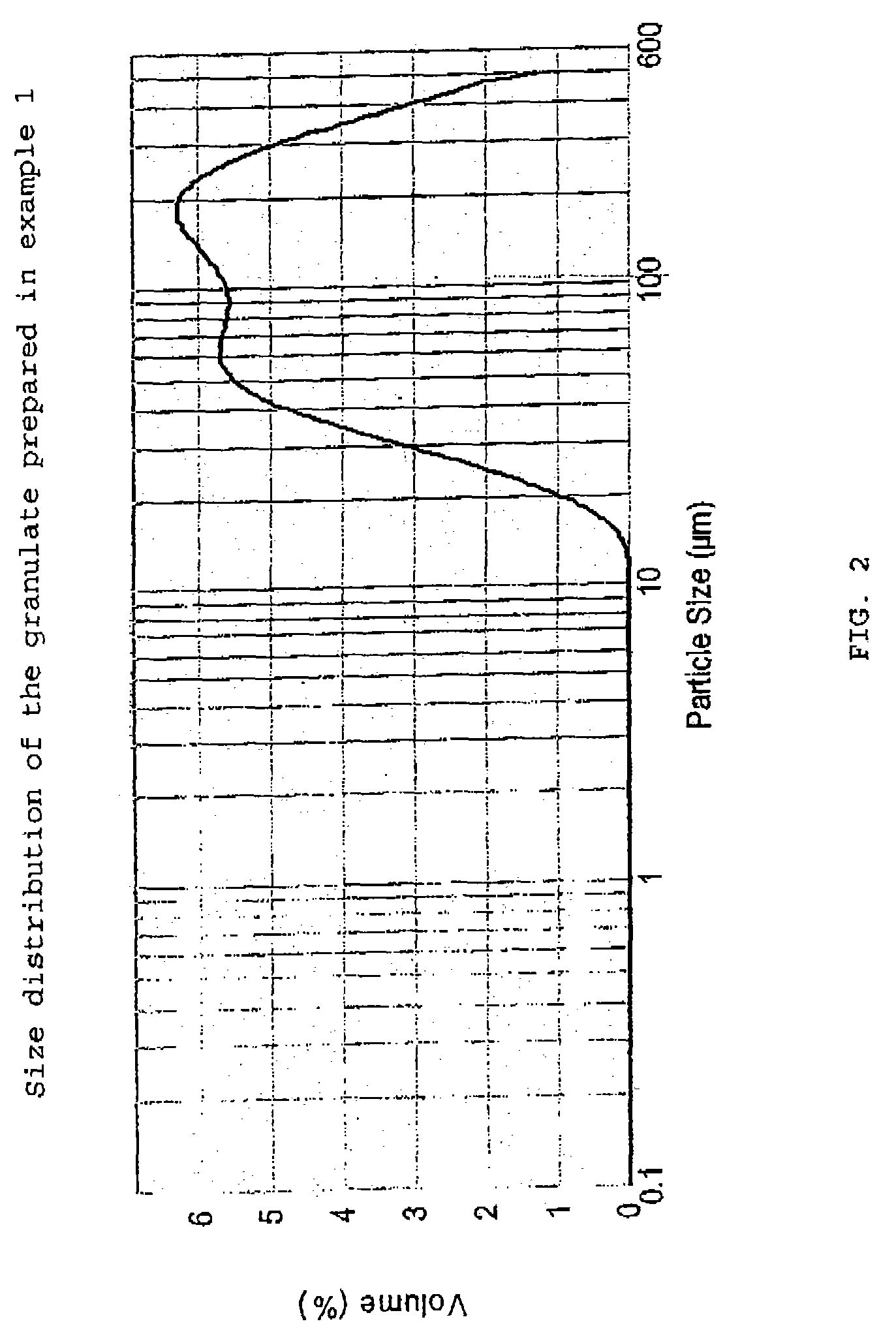
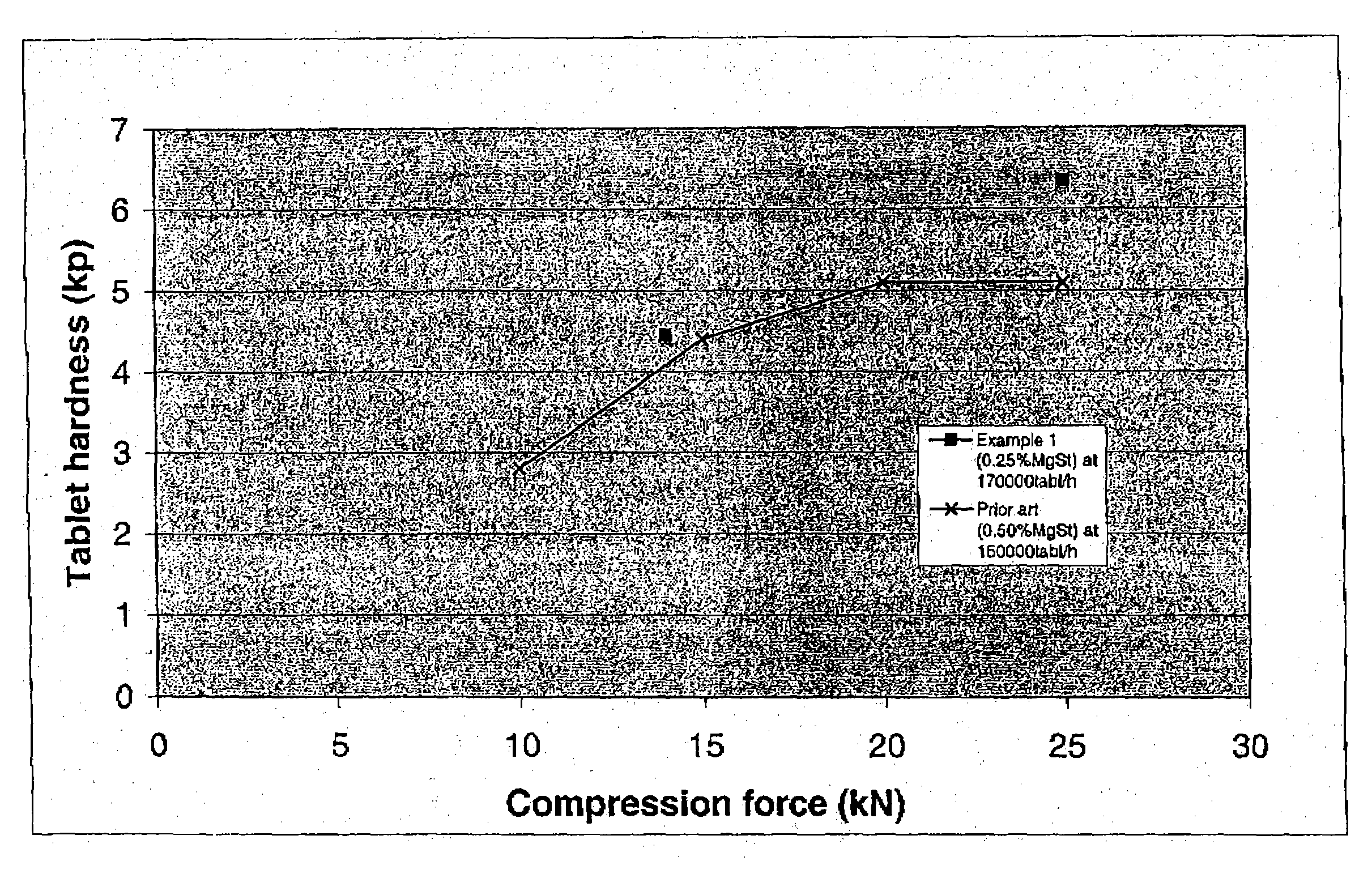
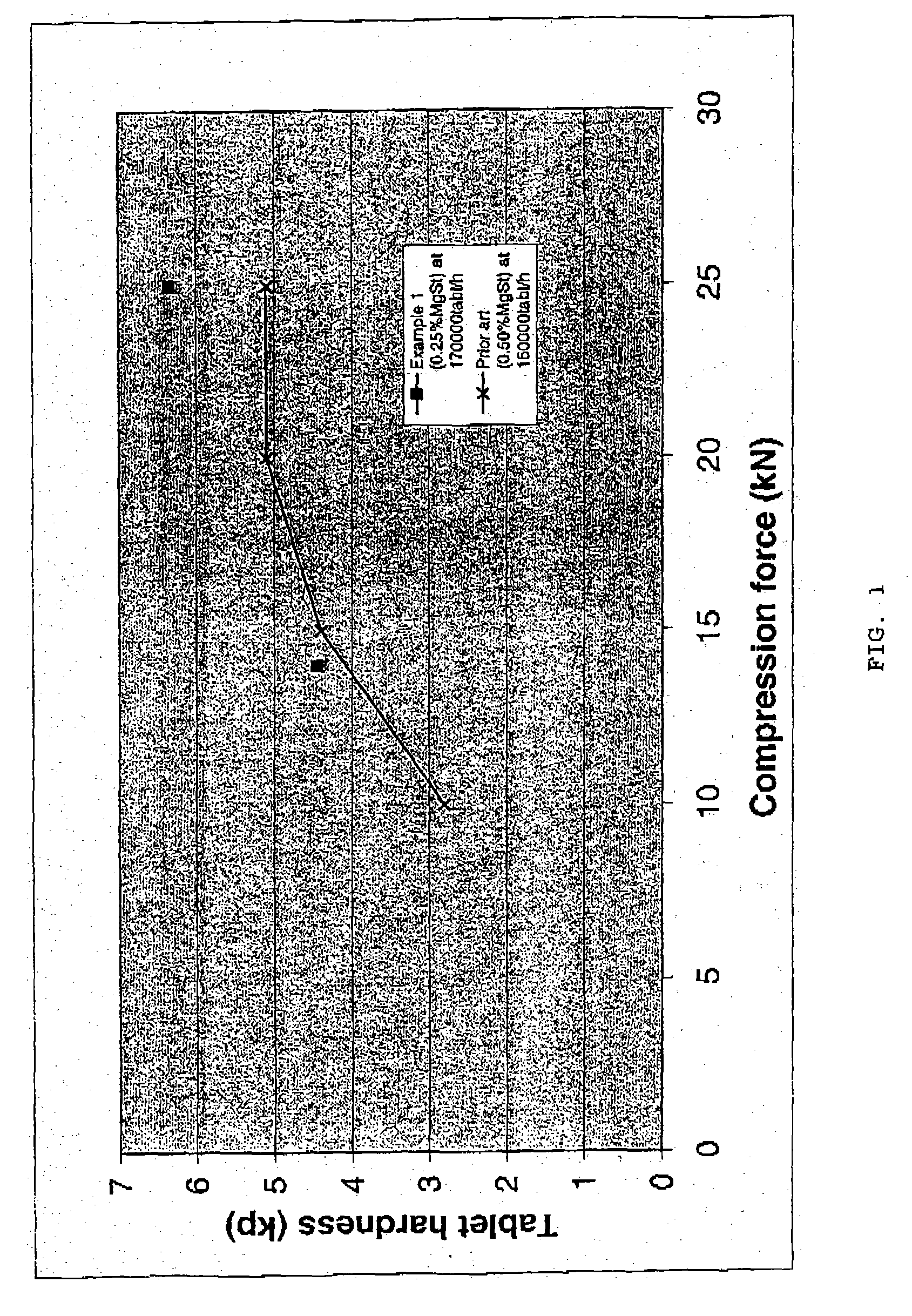
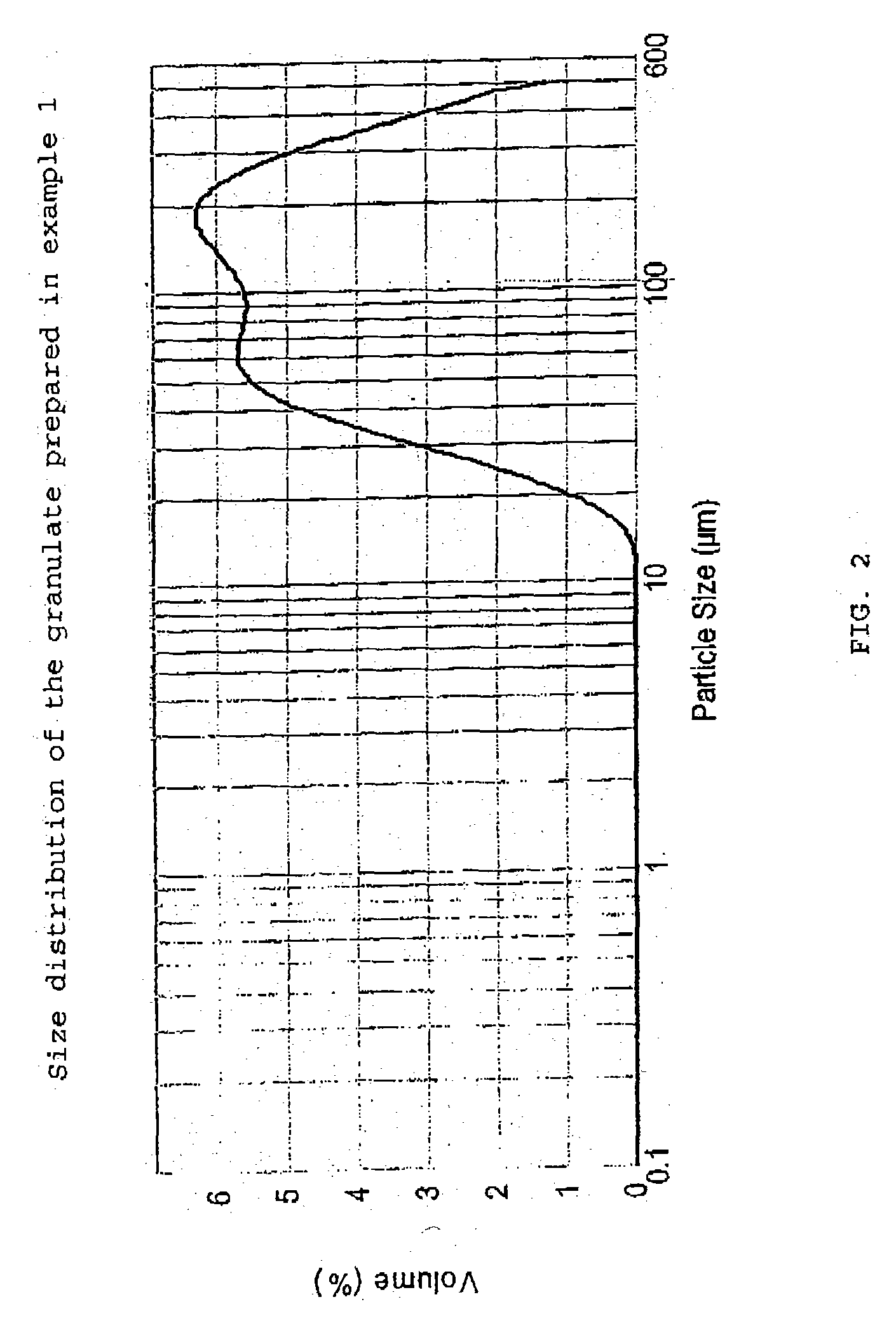
The present invention relates to a novel pharmaceutical composition as a solid dosage form comprising desmopressin as a therapeutically active ingredient, and to a method for manufacturing thereof. The invention relates to a pharmaceutical composition as a solid dosage form comprising desmopressin, or a pharmaceutically acceptable salt thereof, as a therapeutically active ingredient together with a pharmaceutically acceptable excipient, diluent or carrier, or mixture thereof, wherein at least one of said excipient, diluent and carrier is a substance selected from a monosaccharide, disaccharide, oligosaccharide and a polysaccharide, wherein the said substance has an average particle size in the range of from 60 to 1,000 μm. A method according to the present invention provides an improved production of solid dosage forms of desmopressin.
Owner:FERRING BV
Pharmaceutical composition as solid dosage form and method for manufacturing thereof
InactiveUS20050019392A1Reduce wearPeptide/protein ingredientsPharmaceutical non-active ingredientsDiluentBULK ACTIVE INGREDIENT
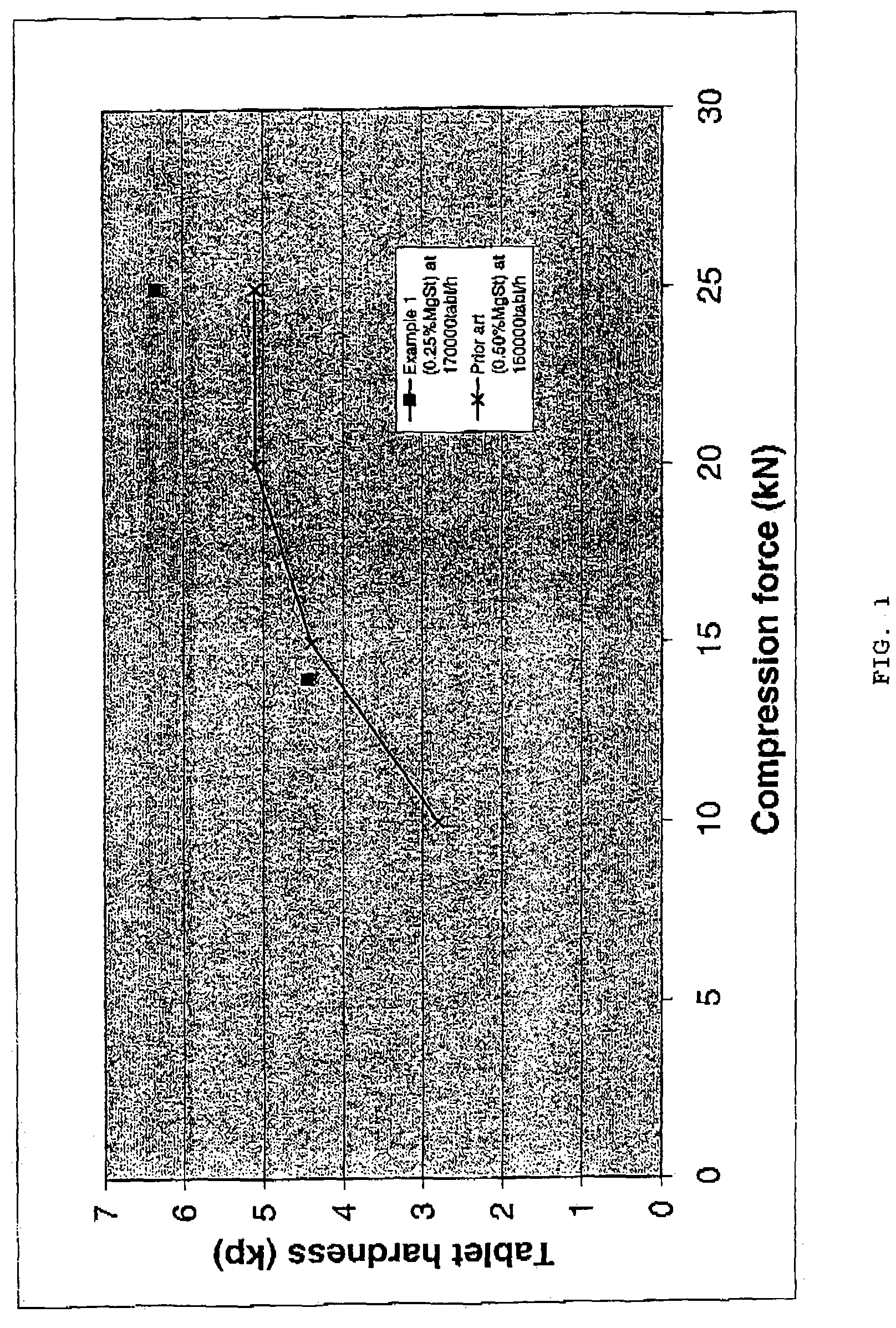
The present invention relates to a novel pharmaceutical composition as a solid dosage form comprising desmopressin as a therapeutically active ingredient, and to a method for manufacturing thereof. The invention relates to a pharmaceutical composition as a solid dosage form comprising desmopressin, or a pharmaceutically acceptable salt thereof, as a therapeutically active ingredient together with a pharmaceutically acceptable excipient, diluent or carrier, or mixture thereof; wherein the pharmaceutical composition is composed of a compressed granulate and contains lubricant in an amount of from 0.05 to less than 0.50 percent by weight of said pharmaceutical composition.
Owner:FERRING B B
Method for preparing solid dosage form of desmopressin
InactiveUS20050158378A1Simple preparation processShort processing timePeptide/protein ingredientsWood working apparatusSolid Dose FormUrology
The present invention relates to a novel method for the preparation of a solid dosage form of desmopressin, or a pharmaceutically acceptable salt thereof, comprising providing a desmopressin containing granulate suitable for compression to a pharmaceutically acceptable tablet, as well as to solid dosage forms, preferably tablets, obtainable by said method.
Owner:FERRING BV
Pharmaceutical composition as solid dosage form and method for manufacturing thereof
ActiveUS20040220080A1Organic active ingredientsPeptide/protein ingredientsBULK ACTIVE INGREDIENTSolid Dose Form
The present invention relates to a novel pharmaceutical composition as a solid dosage form comprising desmopressin as a therapeutically active ingredient, and to a method for manufacturing thereof. The invention relates to a pharmaceutical composition as a solid dosage form comprising desmopressin, or a pharmaceutically acceptable salt thereof, as a therapeutically active ingredient together with a pharmaceutically acceptable excipient, diluent or carrier, or mixture thereof, wherein at least one of said excipient, diluent and carrier is a substance selected from a monosaccharide, disaccharide, oligosaccharide and a polysaccharide, wherein the said substance has an average particle size in the range of from 60 to 1,000 mum. A method according to the present invention provides an improved production of solid dosage forms of desmopressin.
Owner:FERRING BV
Pharmaceutical composition as solid dosage form and method for manufacturing thereof
The present invention relates to a novel pharmaceutical composition as a solid dosage form comprising desmopressin as a therapeutically active ingredient, and to a method for manufacturing thereof. The invention relates to a pharmaceutical composition as a solid dosage form comprising desmopressin, or a pharmaceutically acceptable salt thereof, as a therapeutically active ingredient together with a pharmaceutically acceptable excipient, diluent or carrier, or mixture thereof, wherein the pharmaceutical composition is composed of a compressed granulate and contains lubricant in an amount of from 0.05 to less than 0.50 percent by weight of said pharmaceutical composition.
Owner:FERRING BV
Composition Comprising Desmopressin
InactiveUS20100273709A1Extended shelf lifeSuppression of the level of residual oxidising agent(Peptide/protein ingredientsInorganic active ingredientsDesmopressinSolid Dose Form
The present invention relates to a novel pharmaceutical composition as a solid dosage form comprising desmopressin as a therapeutically active ingredient. It addresses means for providing increased shelf-life for said active ingredient in said dosage form.
Owner:FERRING BV
Pharmaceutical composition as solid dosage form and method for manufacturing thereof
InactiveUS20060252696A1Improve manufacturing speedIncrease speed and capacityBiocideOrganic active ingredientsSolid Dose FormBULK ACTIVE INGREDIENT
The present invention relates to a novel pharmaceutical composition as a solid dosage form comprising desmopressin as a therapeutically active ingredient, and to a method for manufacturing thereof. The invention relates to a pharmaceutical composition as a solid dosage form comprising desmopressin, or a pharmaceutically acceptable salt thereof, as a therapeutically active ingredient together with a pharmaceutically acceptable excipient, diluent or carrier, or mixture thereof, wherein at least one of said excipient, diluent and carrier is a substance selected from a monosaccharide, disaccharide, oligosaccharide and a polysaccharide, wherein the said substance has an average particle size in the range of from 60 to 1,000 μm. A method according to the present invention provides an improved production of solid dosage forms of desmopressin.
Owner:FERRING BV
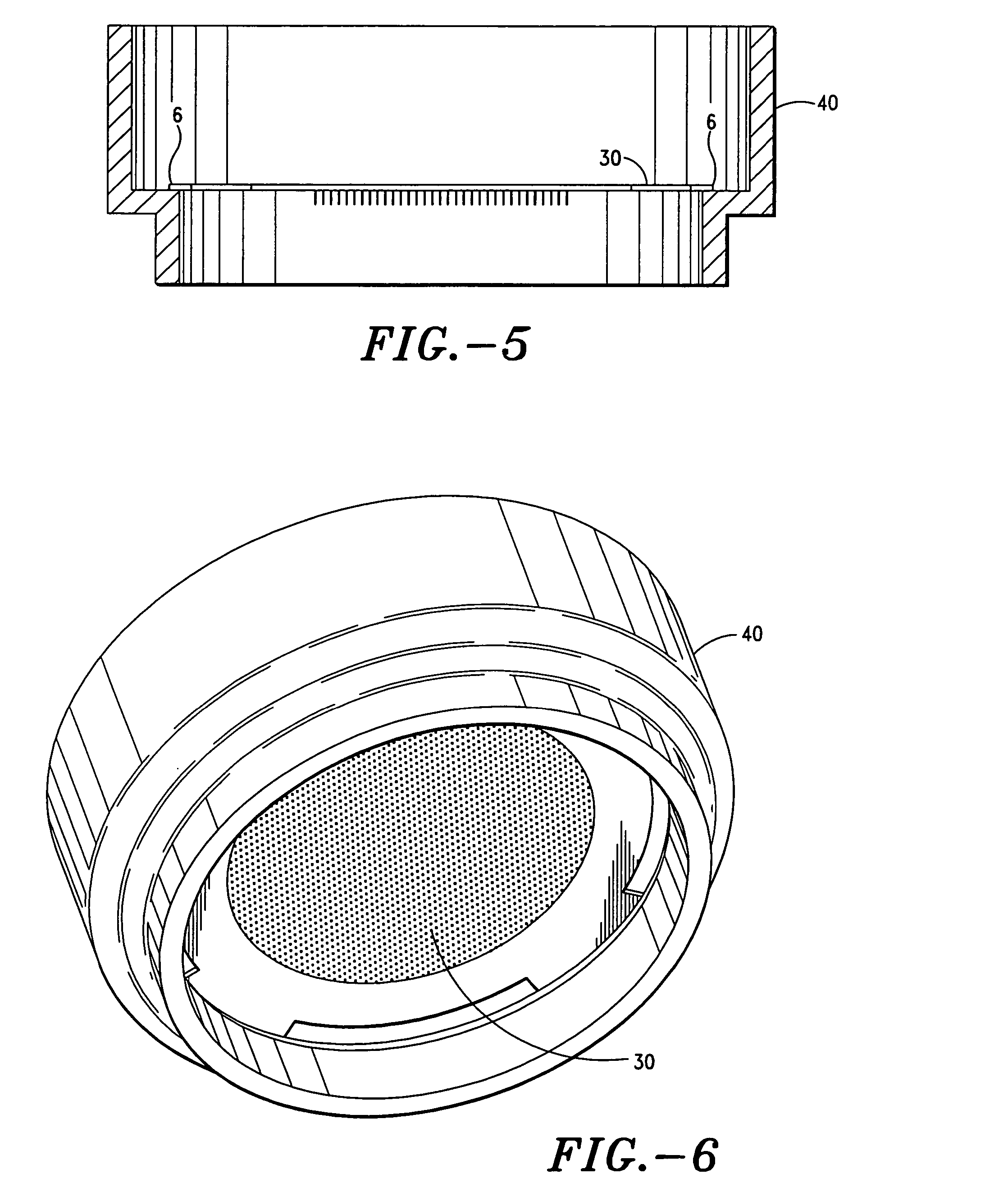
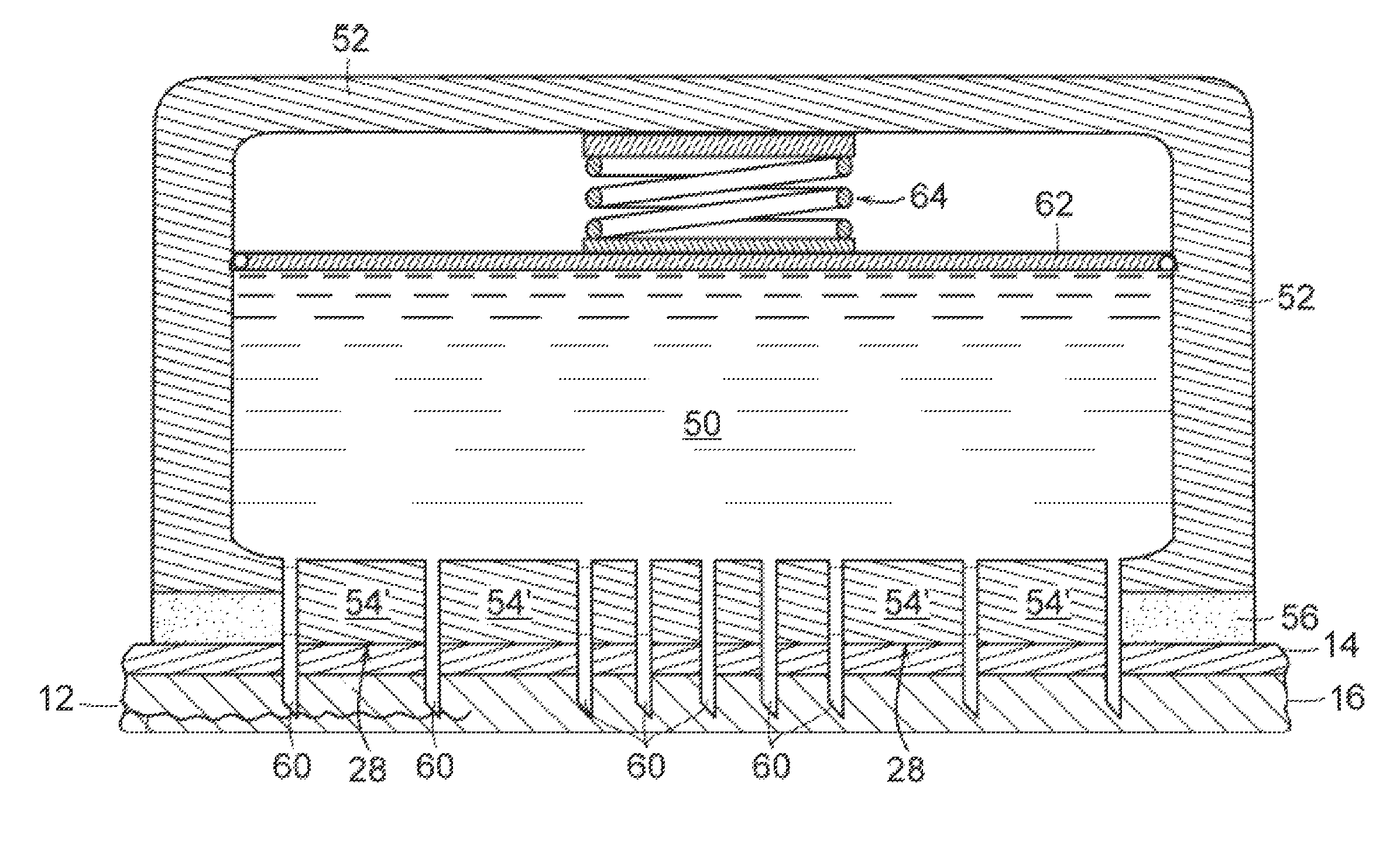
Blister pack and solid dosage form therefor
ActiveUS20070117759A1Prevent degradationSimple preparation processSmall article dispensingPowder deliveryAdjuvantBlister pack
The present invention relates to a blister pack for pharmaceutical use comprising blisters containing a solid dosage form of desmopressin, or a pharmaceutically acceptable salt thereof, and to say solid dosage form. In one embodiment it specifically relates to a blister pack for pharmaceutical use comprising blisters containing a solid dosage form of desmopressin, or a pharmaceutically acceptable salt thereof, in association with a pharmaceutically acceptable adjuvant, diluent and / or carrier, wherein said solid dosage form is adapted to prevent moisture related degradation of said desmopressin.
Owner:FERRING BV
Enhanced nasal composition of active peptide
InactiveUS20090035260A1Prevent oxidationImprove stabilityPeptide/protein ingredientsMetabolism disorderNasal cavityGoserelin
A pharmaceutical composition has a therapeutically effective amount of at least one of: a pharmaceutically active nasal peptide, its pharmaceutically acceptable salt and its peptidic fragment. The composition also contains an absorbefacient effective amount of THAM in a pharmaceutically acceptable, aqueous liquid diluent or carrier. The composition is provided in a convenient form for nasal administration. In one embodiment, the peptidic fragment may be selected physiologically active lymphokines and monokines, peptidic enzymes, proteic vaccines, peptidic toxoids and personalized proteins derived from genoma. In another embodiment, the peptidic fragment may be selected from the peptide hormones and hormone antagonists buserelin, desmopressin, vasopressin, angiotensin, felypressin, octreotide, somatropin, thyrotropin (TSH), somatostatin, gosereline, thryptorelin and insulin selected from the group consisting of cow and pig, synthetic and recombinant.
Owner:THERAPICON SRL
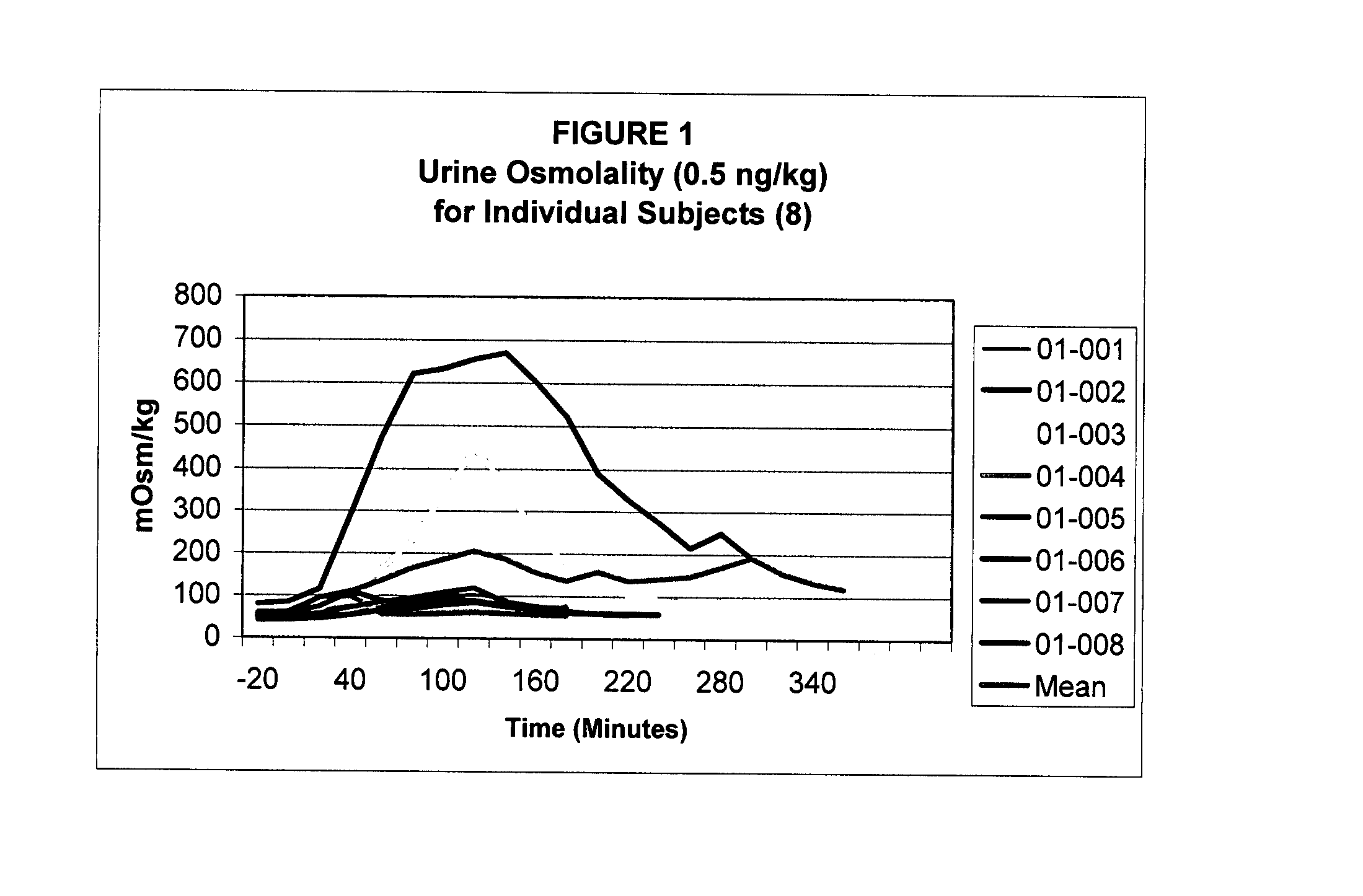
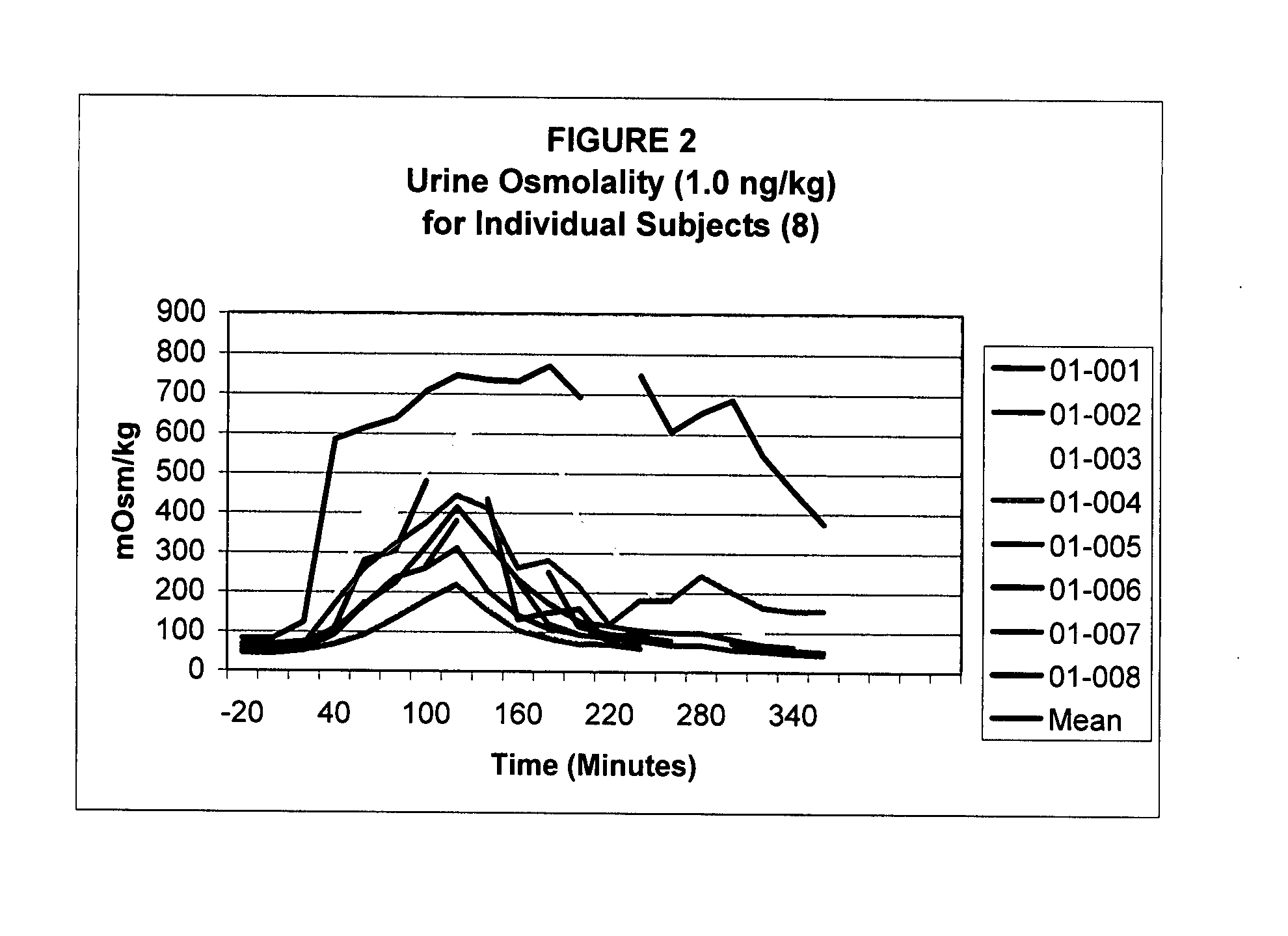
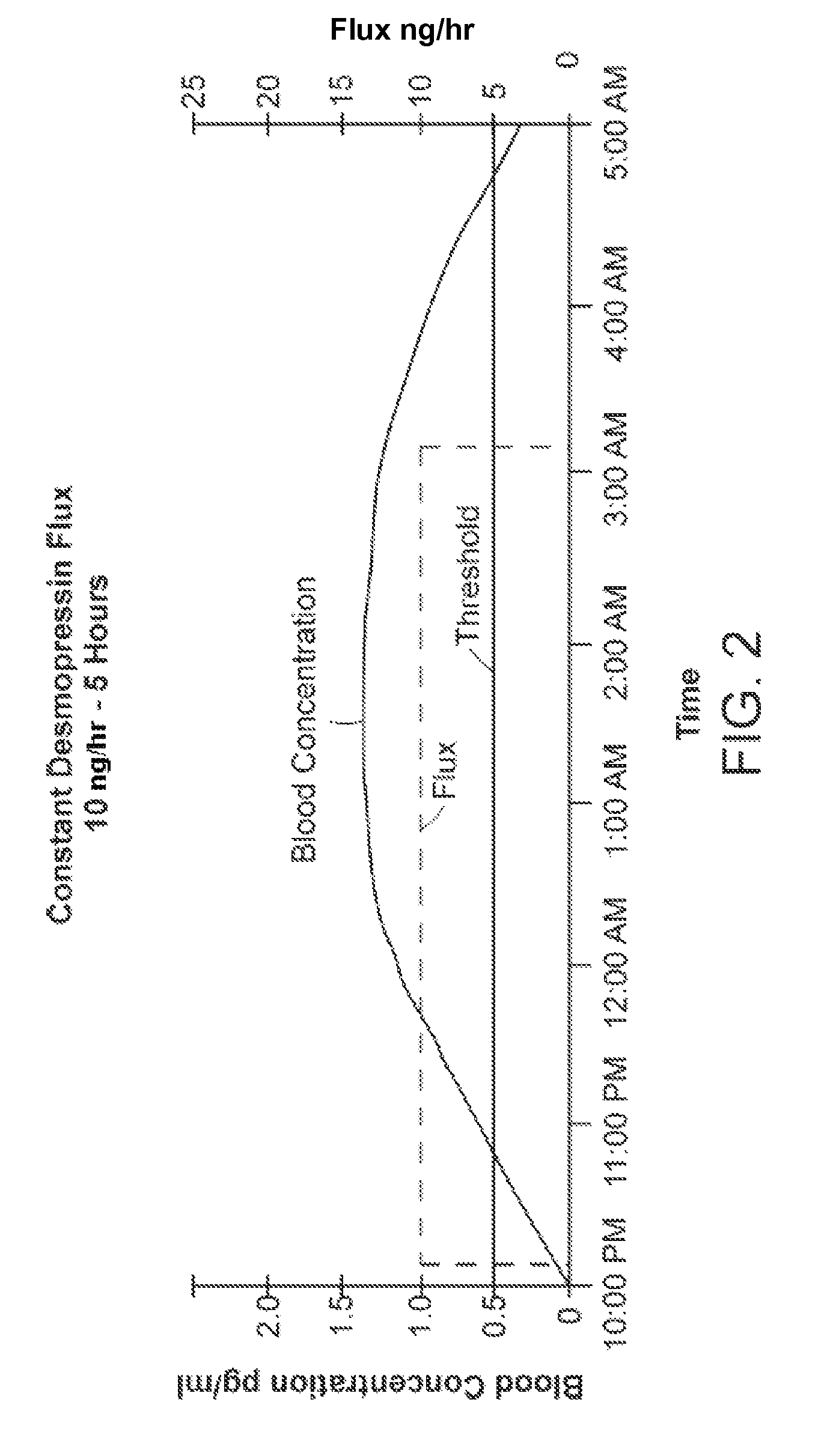
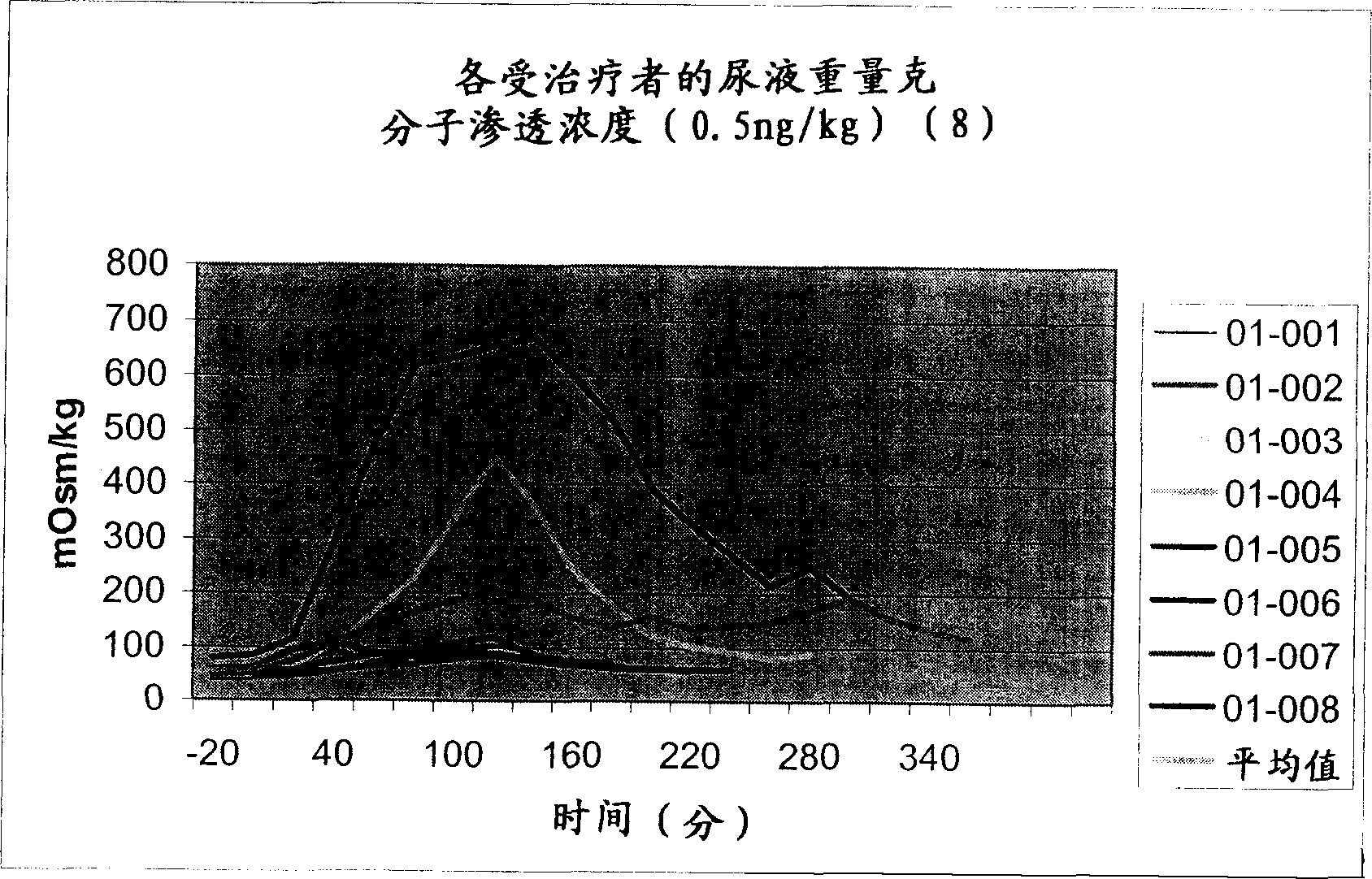
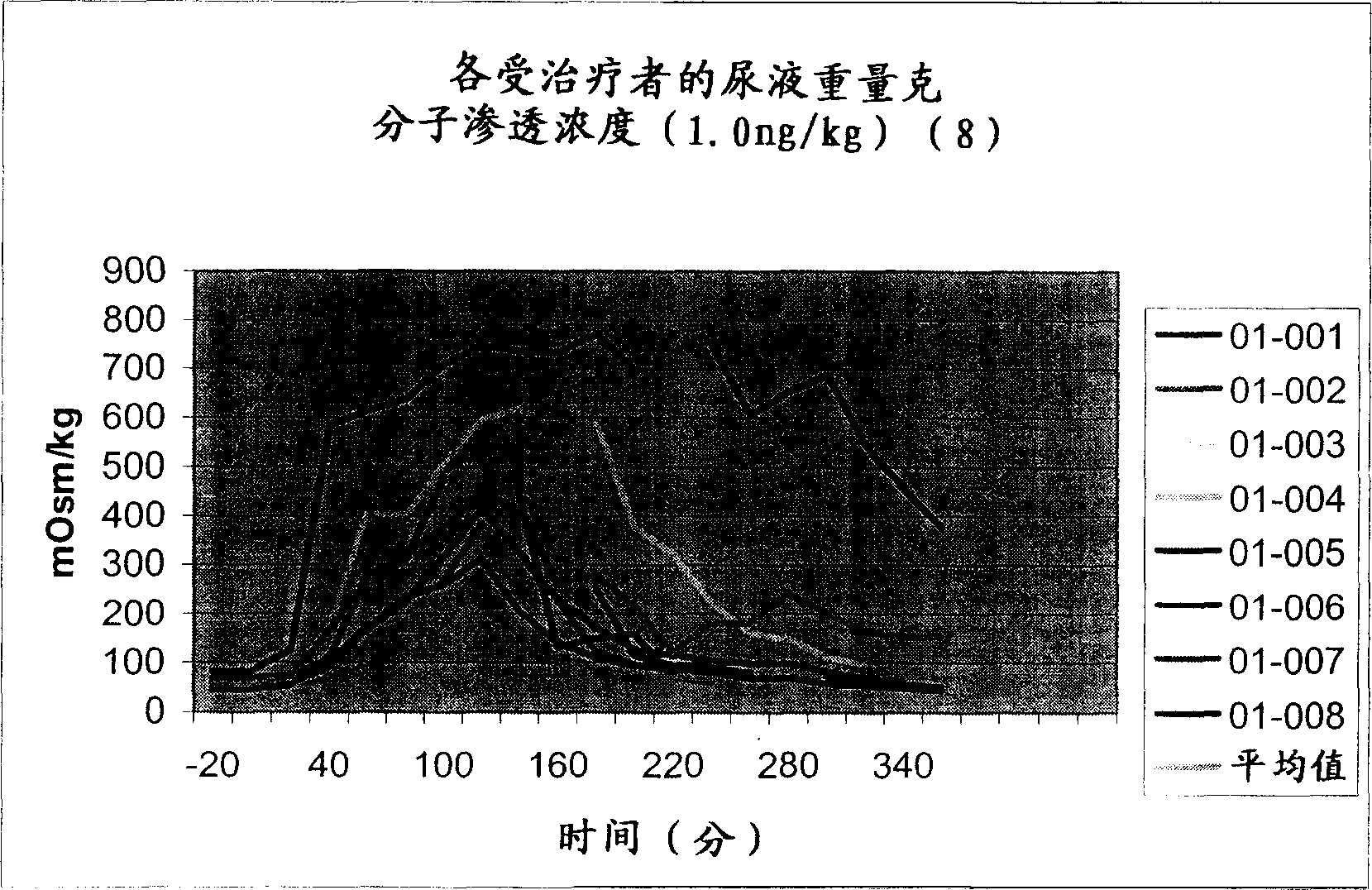
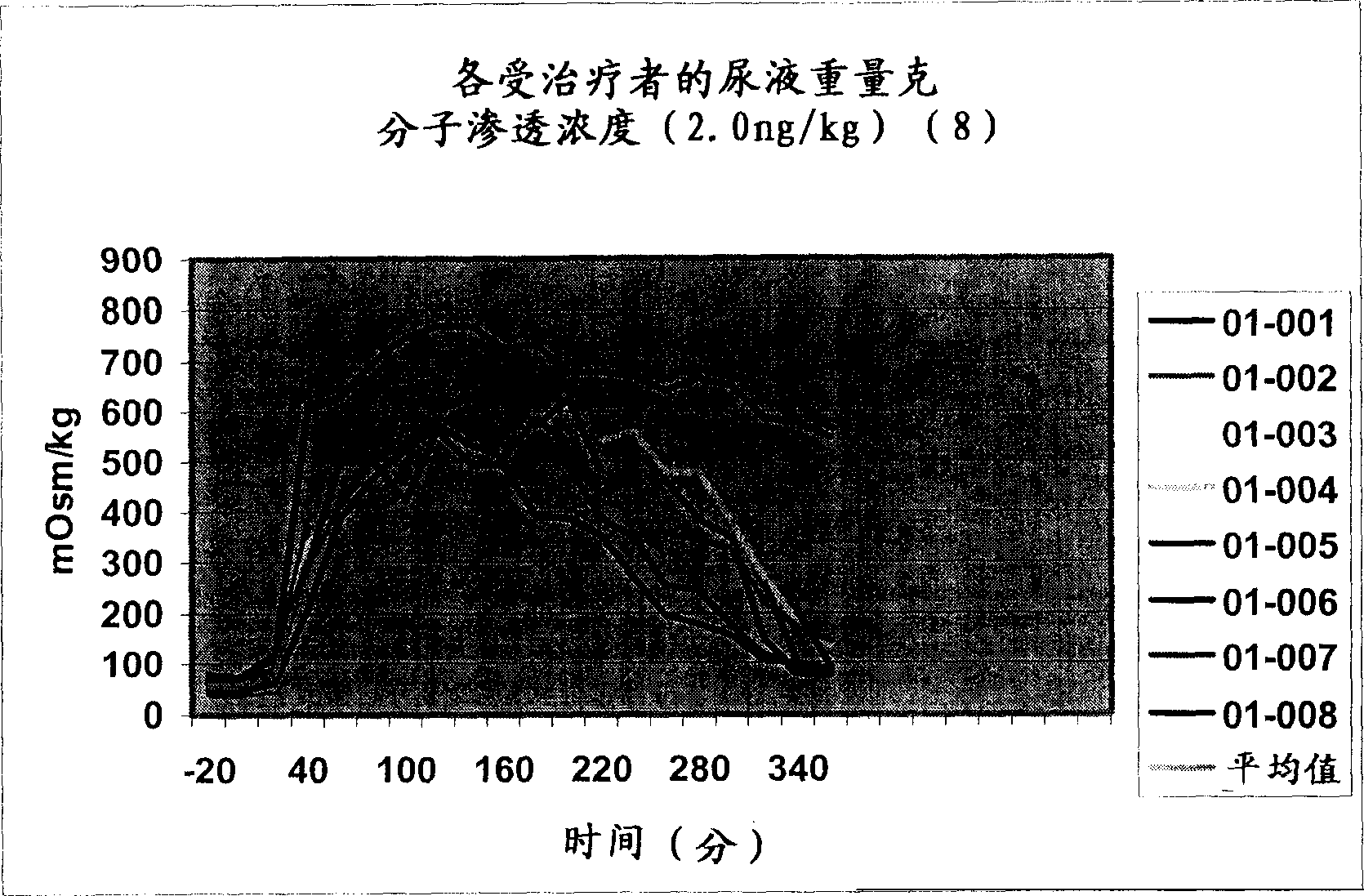
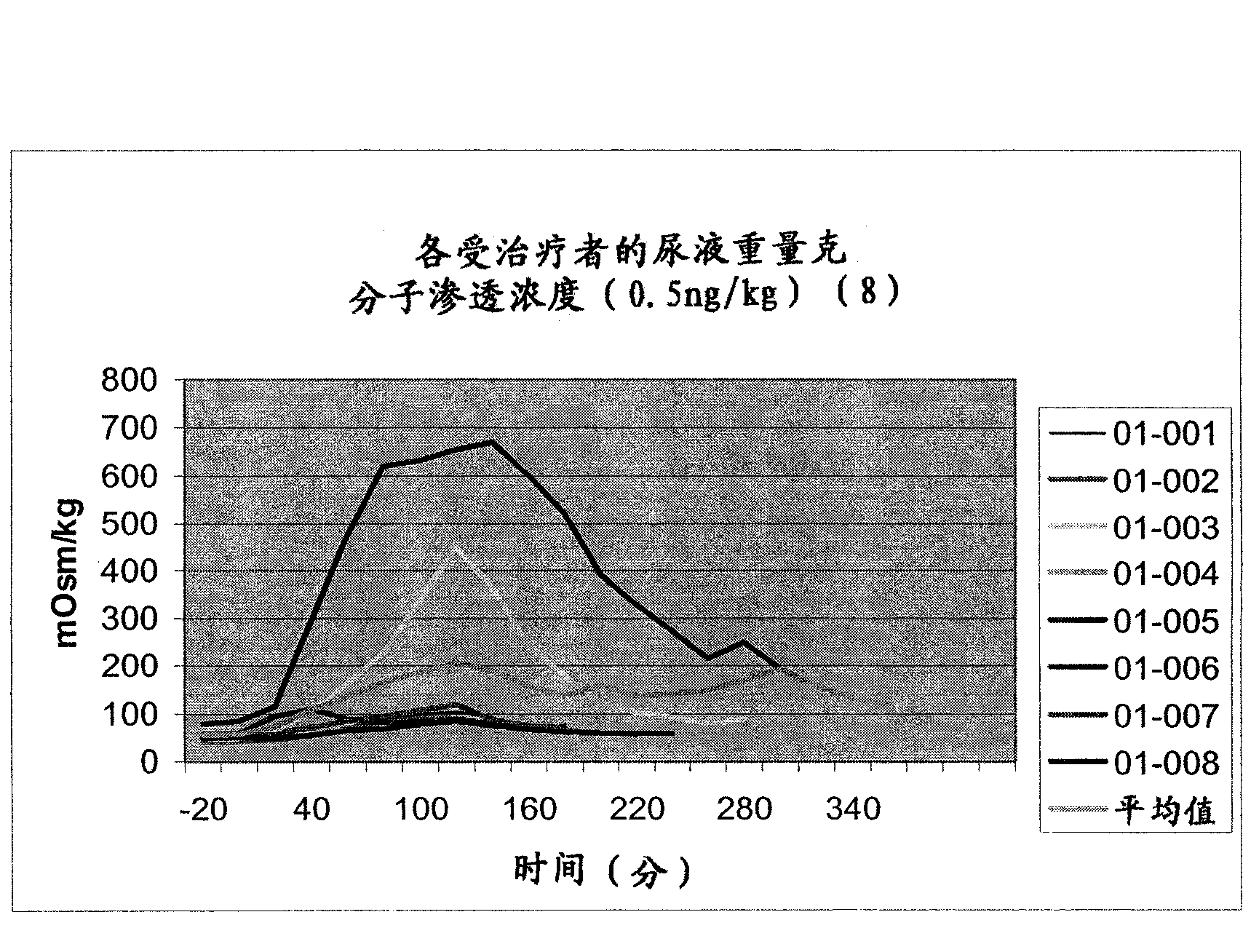
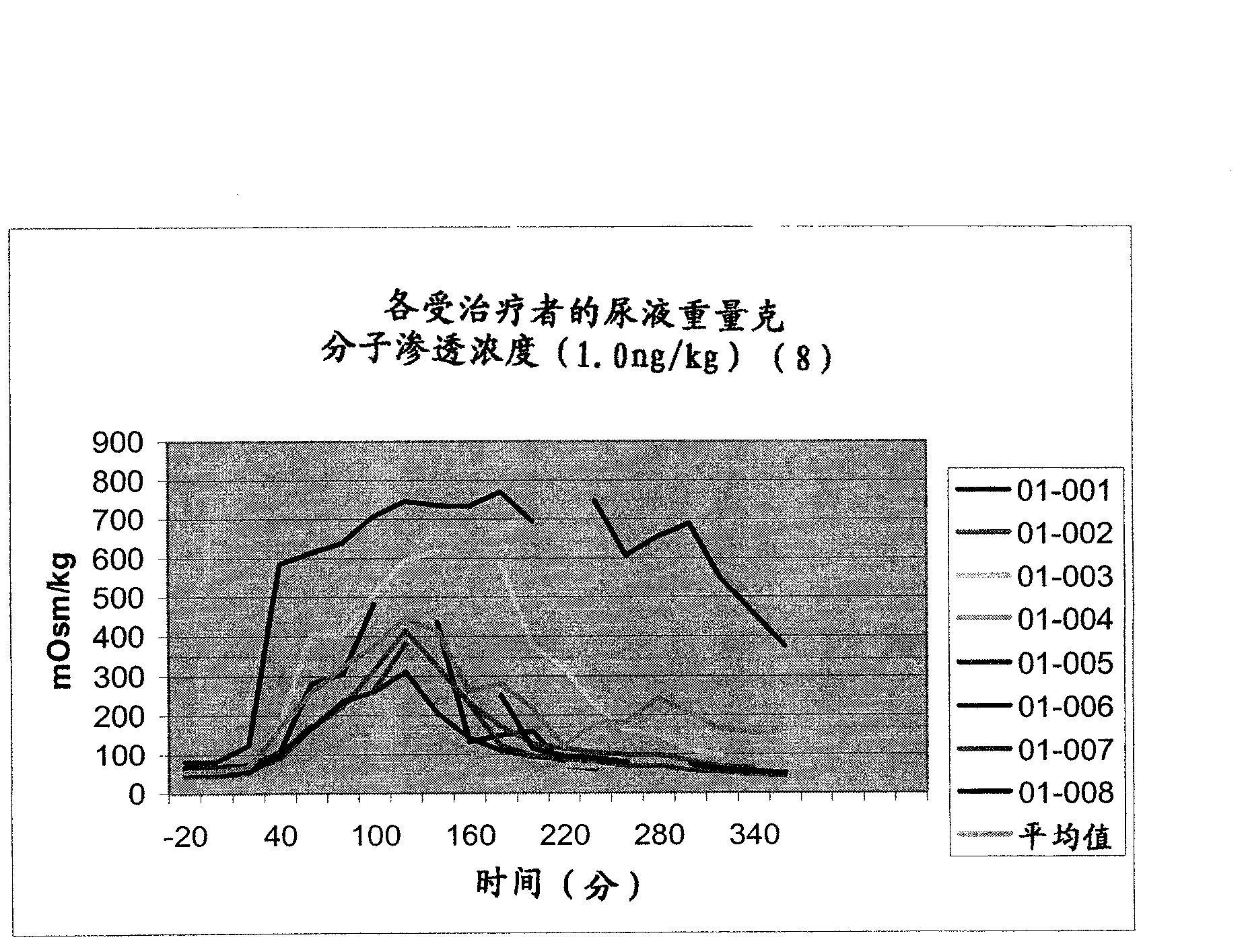
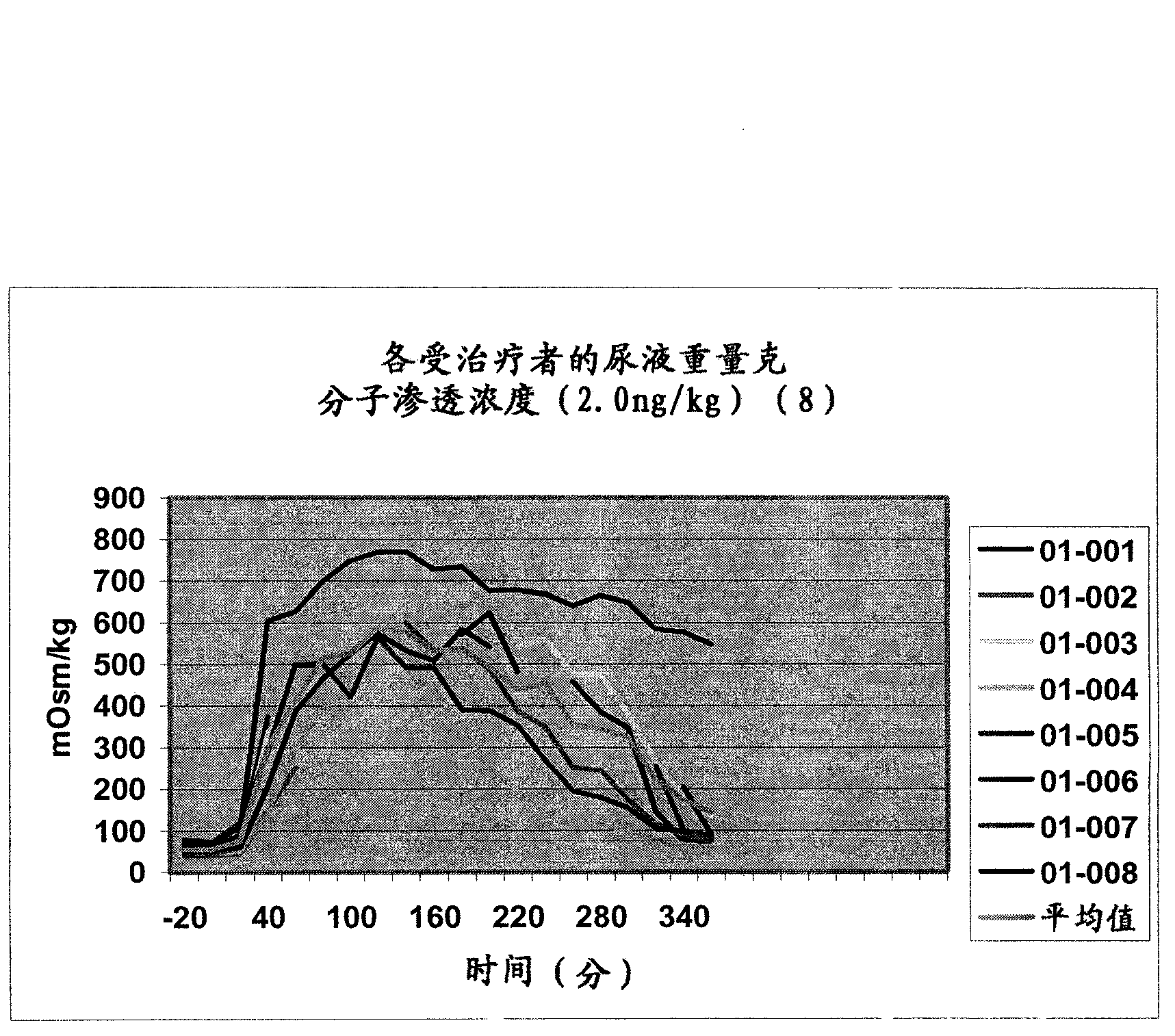
Pharmaceutical Compositions Including Low Dosages of Desmopressin
The present invention is directed to a pharmaceutical composition comprising 0.5 ng to 20 μg desmopressin and a pharmaceutically acceptable carrier. The present invention is also directed to a pharmaceutical composition comprising desmopressin and a pharmaceutically acceptable carrier, wherein the pharmaceutical composition is effective to establish a steady plasma / serum desmopressin concentration in the range of from about 0.1 picograms desmopressin per mL plasma / serum to about 10.0 picogram desmopressin per mL plasma / serum. Articles of manufacture and methods of using the above invention are also disclosed.
Owner:ACERUS PHARM USA LLC
Methods and devices for desmopressin drug delivery
InactiveUS8399410B2Prevent adverse side effectsReduce productionElectrotherapyMicroneedlesDecreased sodiumSide effect
Disclosed are devices for urine voiding postponement, and methods for treating conditions such as central diabetes insipidus, enuresis, nocturia, urinary frequency or incontinence. The devices deliver a desmopressin flux through the skin of a patient in a low dose amount just necessary to achieve a desired anti-diuretic effect without undesirable side effects such as hyponatremia. The devices are designed to permit a state of normal urinary production to return quickly after the desmopressin flux is terminated.
Owner:SERENITY PHARMA CORP
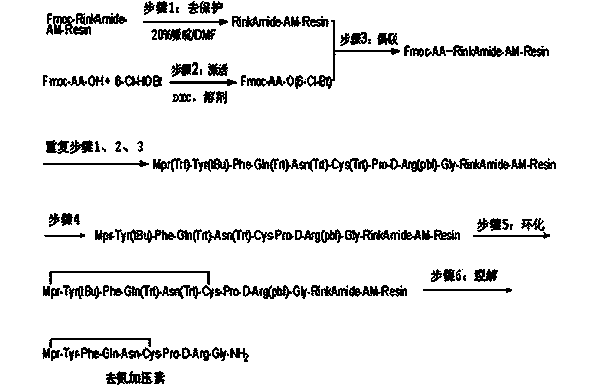
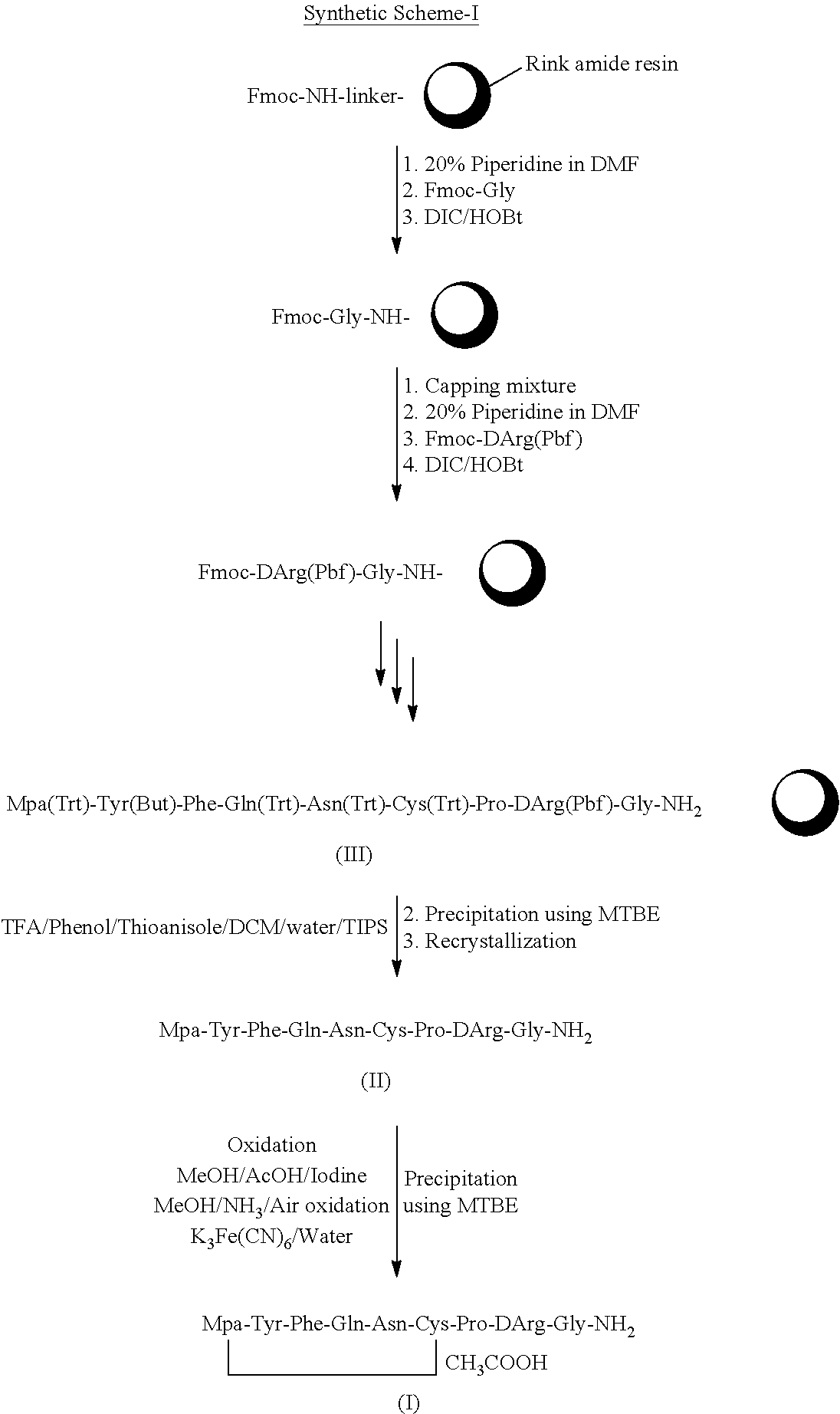
Method for solid cyclizing synthesis of desmopressin
ActiveCN103992389AEasy to operateLow costOxytocins/vasopressinsPeptide preparation methodsIodineOxidizing agent
The invention relates to a method for solid cyclizing synthesis of desmopressin. The method solves the technical problem that the existing air liquid-phase oxidation method produces a large amount of waste liquid, utilizes behindhand processes, has low efficiency and produces more by-products because of oxidation adopting iodine as an oxidizing agent. The method comprises the following steps of orderly coupling nine amino acids to a RinkAmideAm resin, wherein the coupled amino acids orderly comprise Fmoc-Gly-OH, Fmoc-D-Arg(pbf)-OH, Fmoc-Pro-OH, Fmoc-Cys(Trt)-OH, Fmoc-Asn(Trt)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Phe-OH, Fmoc-Tyr(tBu)-OH and Mpa(Trt)-OH, removing protective groups Trt on Cys(Trt) and Mpa(Trt) by TFA / DCM having content of 1%, carrying out solid cyclizing synthesis by an oxidation reagent to obtain a cyclopeptide resin and carrying out cracking to obtain desmopressin. The invention provides the method for solid cyclizing synthesis of desmopressin and the method has simple processes, a low cost and a high yield and is suitable for large-scale production.
Owner:上海飞腾医药科技有限公司
System for manufacture of foam sheets rigidized with polymer infiltration
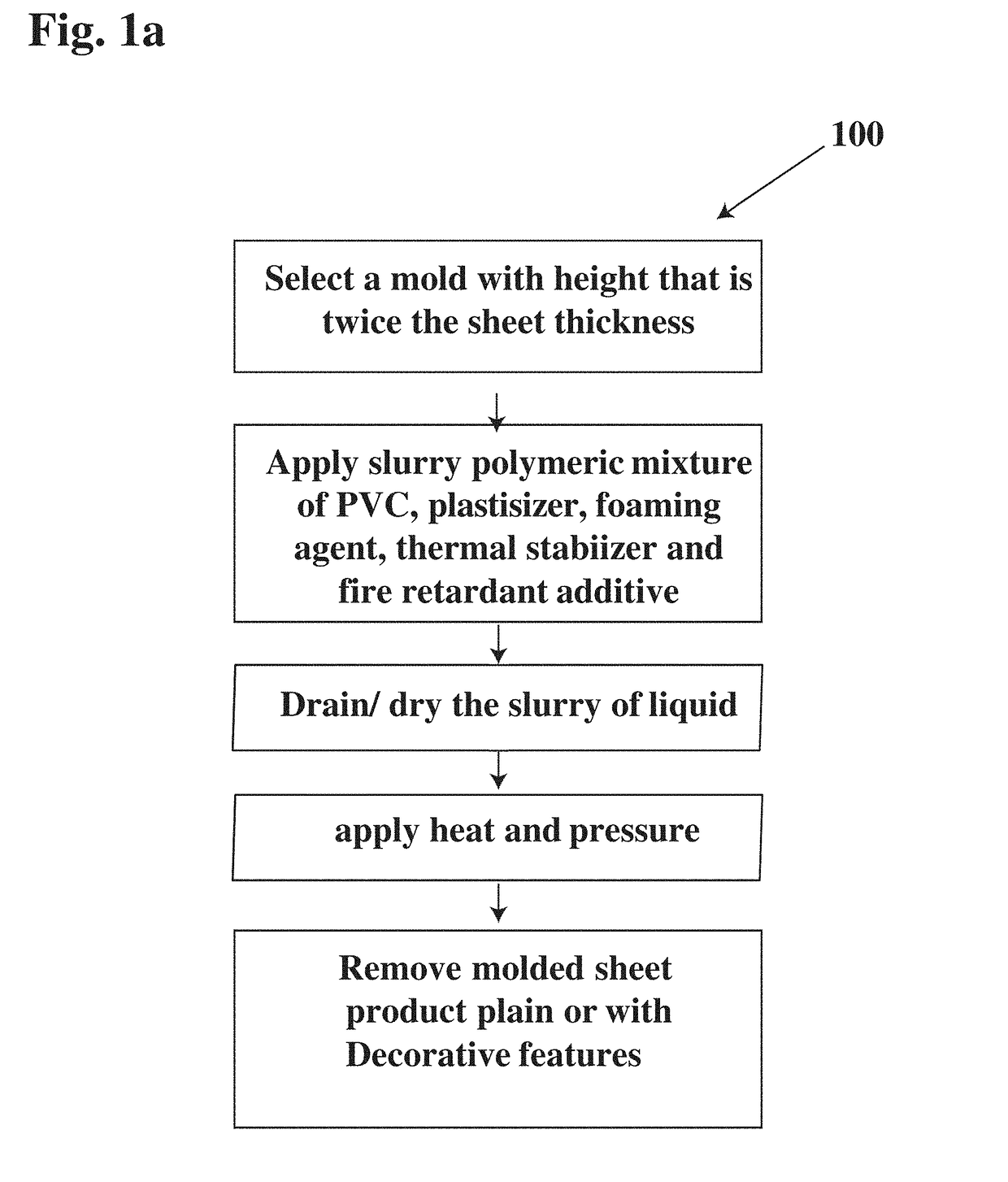
InactiveUS20180257757A1High strengthIncreased bend capabilityConstruction materialWeight reductionFiberPolyvinyl chloride
An eco-wood formulation finds new uses as construction materials. A rigid polymer material sheet for use in building construction comprises a polymer mixture of ultrafine particles of polyvinylchloride (PVC) impact modifier, plant fiber, coupling agent, smoke suppressant, activated clay, lubricant, an activator, environmentally friendly flame retardant, heat stabilizers, odorless crosslinking agent, foaming agent, desmopressin agent. Alternatively, the rigid polymer material sheet is composed of: a polymer mixture of PVC, plasticizer, nitrile rubber, PCC, stearate, zinc oxide, retardant heat, heat stabilizers, crosslinking agent, vesicant; whereby said rigid polymer material sheet provides enhanced thermal resistance and sound attenuation properties for use in building construction, aviation and marine industries.
Owner:PERO III MICHAEL A
Use of blood coagulation factor XIII for treating hemophilia A
InactiveUS20070021340A1Peptide/protein ingredientsMicrobiological testing/measurementBlood coagulation factor XIIIHaemophilia
A patient having hemophilia A is treated by administering factor XIII generally in conjunction with factor VIII or desmopressin.
Owner:ZYMOGENETICS INC
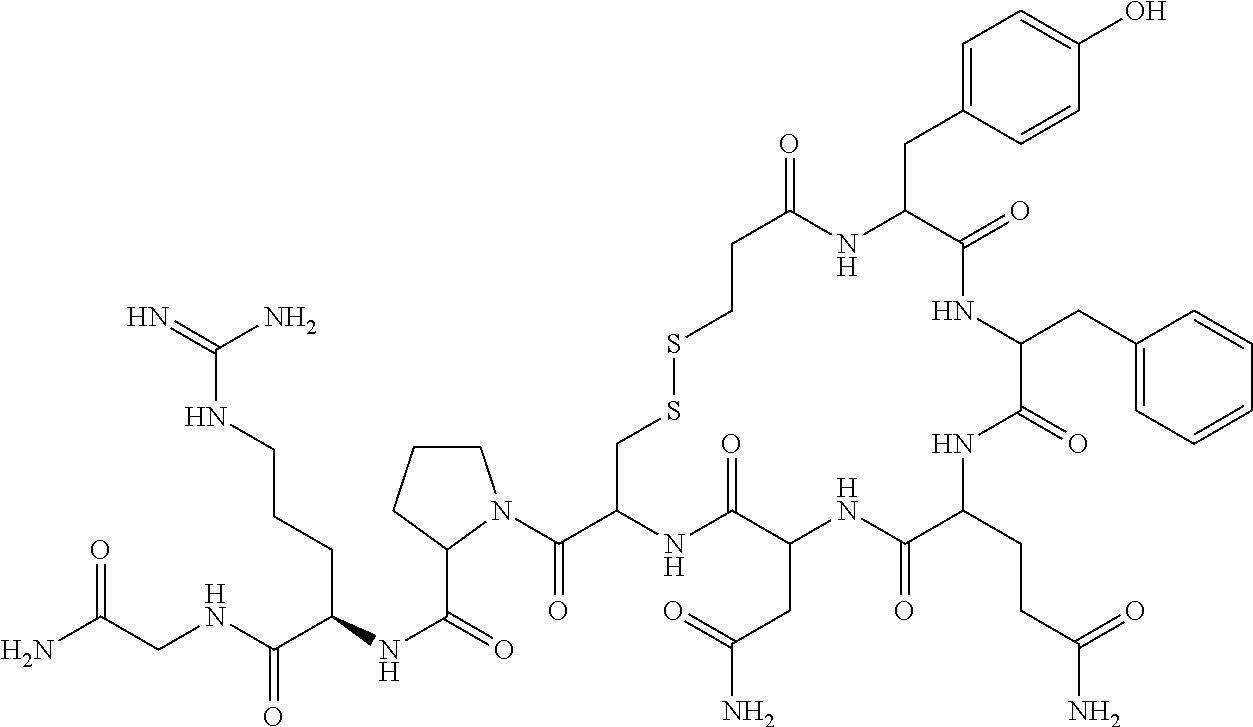
Improved process for the preparation of desmopressin or its pharmaceutically acceptable salts
InactiveUS20120094910A1Oxytocins/vasopressinsPeptide/protein ingredientsD-ArginineMedicinal chemistry
The present invention relates to a novel and improved process for the preparation of 1-deamino-8-D-arginine vasopressin (Desmopressin) or its pharmaceutically acceptable salts thereof and also relates to an improved process for the purification of Desmopressin or its pharmaceutically acceptable salts. Further, the present invention also relates to pharmaceutical composition of Desmopressin or its pharmaceutically acceptable salts thereof.
Owner:MYLAN LAB
Pharmaceutical compositions including low dosages of desmopressin
The present invention is directed to a pharmaceutical composition comprising 0.5 ng to 20 g desmopressin and a pharmaceutically acceptable carrier. The present invention is also directed to a pharmaceutical composition comprising desmopressin and a pharmaceutically acceptable carrier, wherein the pharmaceutical composition is effective to establish a steady plasma / serum desmopressin concentration in the range of from about 0.1 picograms desmopressin per mL plasma / serum to about 10.0 picogram desmopressin per mL plasma / serum. Articles of manufacture and methods of using the above invention are also disclosed.
Owner:ALLERGAN INC
Synthesis method of desmopressin
InactiveCN104926927ALow costEasy to operateOxytocins/vasopressinsPeptide preparation methodsOrganic solventSynthesis methods
The invention provides a synthesis method of desmopressin. The method comprises the following steps: coupling amino acid monomers and Mpa onto a solid carrier, from which the protective groups have been moved, in a water solution according to a sequence of PG-Gly-OH, PG-D-Arg-OH, PG-Pro-OH, PG-Cys(Trt)-OH, PG-Asn-OH, PG-Gln-OH, PG-Phe-OH, PG-Tyr-OH, and Mpa, wherein the PG represents a water-soluble amino protective group, oxidizing, cracking, and purifying. The provided synthesis method does not use any organic solvent, the cost is saved, the environment is protected, moreover, part of the side-chain functional groups in the peptide sequence does not need to be protected, the operation is simple, the cost is low, the yield is high, and thus the provided synthesis method can generate considerable economic benefits, is very practical, and has a wide application prospect.
Owner:HYBIO PHARMA
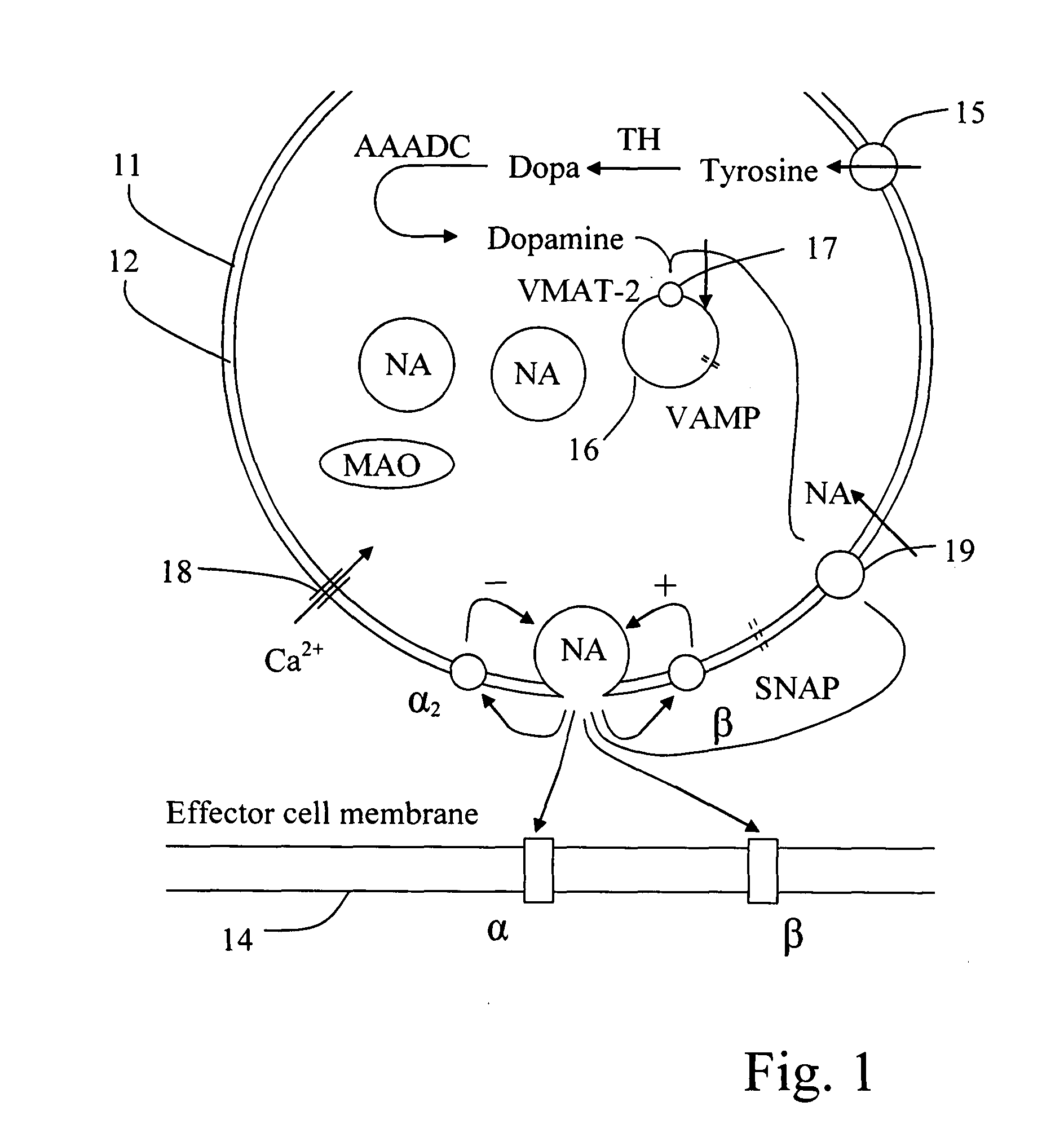
Composition comprising nor-adrenaline and a net inhibitor for administering to a brain-dead, heart-beating potential organ donor
InactiveUS20110270215A1Mitigate, alleviate or eliminate oneBiocideNervous disorderTricyclic antidepressantHydrocortisone
A composition, an infusion solution, a method for treatment, and a kit for intravascular administration for treatment of a brain-dead, heart-beating, respirated, potential organ donor. The composition has a non-adrenaline and NET inhibitor for noradrenaline. The NET inhibitor may be cocaine or an analogue thereof or a tricyclic antidepressant. The composition may in addition include adrenaline, hydrocortisone, thyroxin, insulin, triiodotyronine, dopamine, a vasopressor agent, such as desmopressin, and methylprednisolone. The ratio between the NET inhibitor and nor-adrenaline is about 1:1. The composition may be dissolved in pure water, Ringer's acetate solution or physiological sodium chloride solution. The composition is infused in such an amount as to maintain a mean arterial pressure of about 60 mmHg. The composition may be infused by a pump at a rate of about 1.7 ml / hour decreasing dose-dependent to about 0.4 ml / hour after 24 hours.
Owner:XVIVO PERFUSION
Medical fluid, a method of treatment and use of the fluid
InactiveUS20130065218A1Mitigate, alleviate or eliminate oneDead animal preservationRed blood cellMethylprednisolone
A medical fluid for a harvested organ, tissue or parts thereof, for evaluation and / or preservation. The fluid includes cocaine or a stimulating analogue thereof; nor-adrenalin; and / or adrenaline. In addition, the fluid includes an oncotic agent, such as dextran; hormones, such as thyroxin; triiodotyronine; cortisone, insulin; and electrolytes and optionally nutrients in substantially physiological concentrations in a physiologically acceptable medium. In addition, the medical fluid further includes albumin in a concentration not exceeding 5.0%, and an oxygen carrier, such as erythrocytes. Further components may be dopamine; hydrocortisone; methylprednisolone; and a vasopressor agent, such as desmopressin. The cocaine; adrenalin; and noradrenaline are present in concentrations of each about 0.010 μM to 0.100 μM, for example in a ratio of 1:1:1.
Owner:XVIVO PERFUSION
Composition comprising nor-adrenaline and amphetamine for administering to a brain-dead, heart-beating potential organ donor
InactiveUS20110281794A1Mitigate, alleviate or eliminate oneBiocideNervous disorderHydrocortisoneMethylprednisolone
A composition, an infusion solution, a method for treatment, and a kit for intravascular administration for treatment of a brain-dead, heart-beating, respirated, potential organ donor. The composition includes a non-adrenaline and amphetamine or an amphetamine-like substance. The composition may in addition include adrenaline, hydrocortisone, thyroxin, insulin, triiodotyronine, dopamine, a vasopressor agent, such as desmopressin, and methylprednisolone. The composition may be dissolved in pure water, Ringer's acetate solution or physiological sodium chloride solution. The composition is infused in such an amount as to maintain a mean arterial pressure of about 60 mmHg.
Owner:VIVOLINE MEDICAL
Method for treating von willebrand's disease
Use of factor XIII for treating von Willebrand's disease. A patient having von Willebrand's disease is treated by administering factor XIII generally in conjunction with factor VIII concentrate, 1-desamino-8-D-arginine vasopressin (DDAVP) or desmopressin.
Owner:ZYMOGENETICS INC
Stabilized desmopressin
InactiveUS20170128521A1Improve drug stabilityPeptide/protein ingredientsUrinary disorderBULK ACTIVE INGREDIENTActive ingredient
The present disclosure relates generally to pharmaceutical compositions comprising an active ingredient and a stabilizing agent, wherein the active ingredient is desmopressin or a pharmaceutically acceptable salt thereof, and wherein the stabilizing agent is one or more gums. The present disclosure further relates to methods of increasing the stability of a pharmaceutical composition comprising desmopressin or a pharmaceutically acceptable salt thereof as an active ingredient; methods for preparing orally disintegrating films comprising desmopressin or a pharmaceutically acceptable salt thereof and orally disintegrating films prepared thereby; and methods of treating or preventing nocturnal enuresis or nocturnal polyuria by administering stabilized pharmaceutical compositions comprising desmopressin or a pharmaceutically acceptable salt thereof as an active ingredient.
Owner:FERRING BV
Synthetic method of desmopressin
ActiveCN112062813AReduce usageIncrease profitOxytocins/vasopressinsPeptide preparation methodsDisulfide bondingFluid phase
The invention discloses a synthetic method of desmopressin. The synthetic method comprises the following steps: 1) selecting solid phase carrier resin; 2) synthesizing main chain peptide resin; 3) conducting cracking to remove resin and protective groups; and 4) conducting liquid phase electrochemical oxidation and coupling disulfide bonds to obtain the desmopressin. According to the method, the problem of specific selectivity of reaction can be avoided, the utilization rate of fragment peptide resin, the utilization rate of main chain fragments and the atom utilization rate can be increased,so that the production cost is reduced, the use of chemical oxidants iodine and hydrogen peroxide is avoided, and the method is more environment-friendly and more conforms to the concept of green chemistry.
Owner:HYBIO PHARMA WUHAN CO LTD +1
Use of Blood Coagulation Factor XIII for Treating Hemophilia A
InactiveUS20090036361A1Peptide/protein ingredientsBlood disorderFactor iiBlood coagulation factor XIII
Owner:ZYMOGENETICS INC
Stabilized desmopressin
ActiveCN106456706APeptide/protein ingredientsPharmaceutical non-active ingredientsBULK ACTIVE INGREDIENTActive ingredient
The invention is related to a pharmaceutical composition comprising an active ingredient and a stabilizing agent, wherein the active ingredient is desmopressin or a pharmaceutical acceptable salt thereof, and the stabilizing agent is at least one gum. The invention relates to uses of one or more gums to increase the stability of a pharmaceutical composition comprising desmopressin or a pharmaceutical acceptable salt thereof as an active ingredient against denaturation, a method for preparing an orally disintegrating film comprising desmopressin or a pharmaceutically acceptable salt thereof as well as the orally disintegrating film obtainable thereby.
Owner:FERRING BV
Medical composition containing low-dosage desmopressin
The invention relates to a medical composition containing 0.5-20 microgrammes of desmopressin and a pharmaceutically acceptable carrier. The invention further relates to a medical composition containing desmopressin the pharmaceutically acceptable carrier, wherein the medical composition can effectively establish stable blood plasma / blood serum desmopressin concentration from 0.1 micromicrograms of desmopressin / mL blood plasma / blood serum to 10.0 micromicrograms of desmopressin / mL blood plasma / blood serum. The invention further discloses a product made from the medical composition and the preparation method of the product.
Owner:ALLERGAN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com