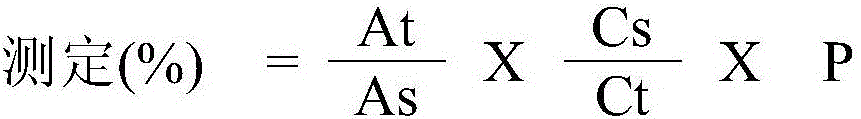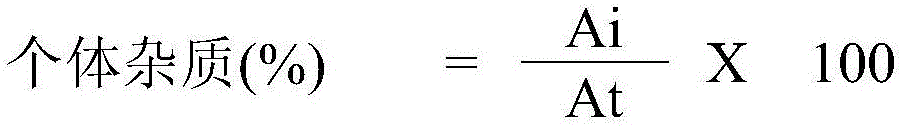Stabilized desmopressin
A desmopressin and stabilizer technology, which is applied in the field of stabilized desmopressin, and can solve problems such as instability of desmopressin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、2
[0051] Total impurities (embodiment 1,2,3) and determination (embodiment 1)
[0052] test solution – Examples 1, 2, 3
[0053] Put 1 mg desmopressin acetate equivalent of the membrane prepared according to the preparation example into a 10 ml volumetric flask. This was mixed with the mobile phase as listed below for the HPLC conditions until the solution reached the 10 ml mark. Put the solution into a centrifuge tube and centrifuge for 20 minutes. The solution was filtered with a 0.2 μm filter (hydrophilic polytetrafluoroethylene (PTFE)). Completion of these steps yielded test solutions (0.1 mg / ml).
[0054] Excipient solution – Example 2, 3
[0055] Each excipient (HPC, TiO 2 , gum) into a 10ml volumetric flask. The mobile phase as listed below for the HPLC conditions was poured into the bottle until the solution reached the 10 ml mark. Place the solution in a centrifuge tube and centrifuge for 20 minutes. The solution was filtered using a 0.2 μm filter (hydroph...
Embodiment 1
[0085] LOD (Example 1, 2)
[0086] 0.5 g of the film prepared as described in the preparation above was tested as follows:
[0087] - Dry the glass bottle in a 105° C. chamber for 1 chamber, then cool in a desiccator (room temperature) for 30 minutes.
[0088] - Weigh the cooled glass bottle from the desiccator. The film samples were then rolled or folded and, without undue delay, placed into standing glass jars. The glass bottle with the sample is accurately weighed.
[0089] - Incubate the glass vials with the membrane samples in a chamber at 105°C for 4 hours.
[0090] - After 4 hours, the glass bottle was cooled in a desiccator (room temperature) for 30 minutes.
[0091] - The cooled glass jars are then weighed without undue delay.
[0092] - To calculate the LOD value, divide the reduced weight of the film sample by the weight of the first film sample.
[0093] Example 1
[0094] Determining Conditions for Membrane Drying
[0095] Membrane preparation soluti...
Embodiment 2
[0109] The stabilizing effect of gum
[0110] Membranes were prepared according to the method as described in the preparation, wherein the components and amounts are given in Table 4. The membrane was dried at 80°C for 30 minutes.
[0111] Table 4
[0112]
[0113] The viscosity of the solution was measured using a Brookfield viscometer. The results of the stability measurements are shown as total impurities (%). Total Impurities (%) Determine the total amount of impurities of desmopressin measured after 2-4 weeks under accelerated conditions (40±2° C., relative humidity 75±5%).
[0114] The standard deviation of the LOD values was ±0.5% and there were no significant differences between the test groups.
[0115] As a result, as can be seen in Table 4, over the entire weight ratio range of desmopressin acetate to gum from 10:1 to 1:50, compared to the same composition without any gum (control), A gum (in this example xanthan gum) as a stabilizer resulted in a sign...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com