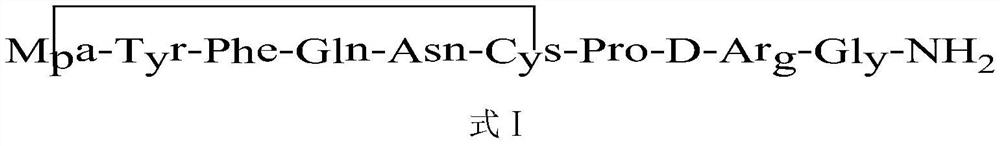Synthetic method of desmopressin
A technology for desmopressin and a synthesis method, which is applied in the field of desmopressin synthesis, can solve the problems of low atom utilization rate, waste of amino acids, low yield of desmopressin, etc., and achieves improved atom utilization rate. , the effect of reducing production costs and improving utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: peptide resin synthesis
[0031] Weigh Rink Amide resin (52g, substitution degree is 0.48mmol / g) and add it to the solid-phase reaction column, wash it twice with DMF, then swell the resin with DMF for 30 minutes, add DBLK for deprotection (5min+7min), and use the resin DMF washed 6 times.
[0032] Weigh Fmoc-Gly-OH (22.3g, 75mmol) and HOBT (12.159g, 90mmol) into DMF (250mL) to dissolve, cool in an ice-water bath to 0-5°C, add DIPCDI (12.75mL, 75mmol) to activate for 5min, Add this activation solution into the reaction column, blow nitrogen at room temperature for 2 hours, and detect the end of the reaction with ninhydrin (if the resin is colorless and transparent, stop the reaction; if the resin develops color, prolong the reaction for 1 hour). After the reaction is over, wash the resin with DMF for 3 times, add DBLK for deprotection for 5min + 7min, wash the resin with DMF for 6 times, and detect the color of the resin with ninhydrin.
[0033]Weigh Fmo...
Embodiment 2
[0041] Example 2: Cleavage of Peptide Resin
[0042] Add 57.65 g of the peptide resin obtained in Example 1 into a 2L single-necked bottle, and add the lysate (TFA:H 2 O: PhOMe: thioanisole=90:5:4:1 (V:V), 600ml), stirred at room temperature for 4 hours, filtered the resin, and collected the filtrate. The resin was washed with a small amount of TFA, and the filtrates were combined. The filtrate was slowly added to 6L of glacial ether for precipitation. Centrifuge, wash with glacial ether for 5 times, and blow dry with nitrogen to obtain 27.80 g of crude peptide with a purity of 88.9% and a yield of 99.9%.
Embodiment 3
[0043] Example 3: Liquid Oxidative Coupling of Disulfide Bonds
[0044] Add 27.80 grams of the crude peptide obtained in Example 2 into a 1000ml three-necked flask, add 500ml of acetonitrile and 8.2g (25mmol) of tetrabutylammonium tetrafluoroboride in sequence, and then insert a platinum electrode (anode: 15mm×15mm×0.3mm , cathode: 15mm×15mm×0.3mm)), controlled current 12mA, stirred and reacted at 35°C for 48 hours. After the reaction is over, stop the power supply.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com