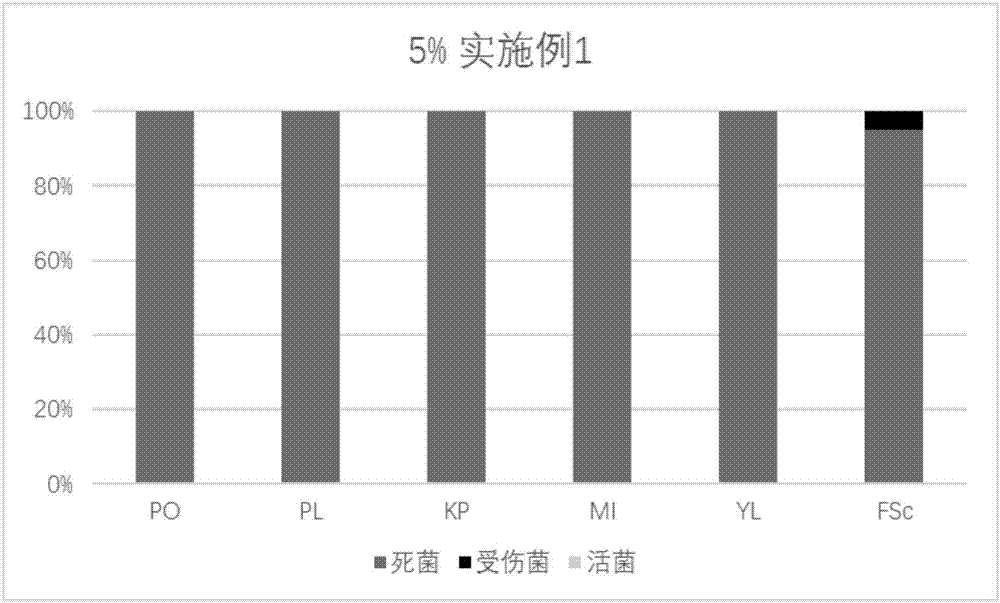
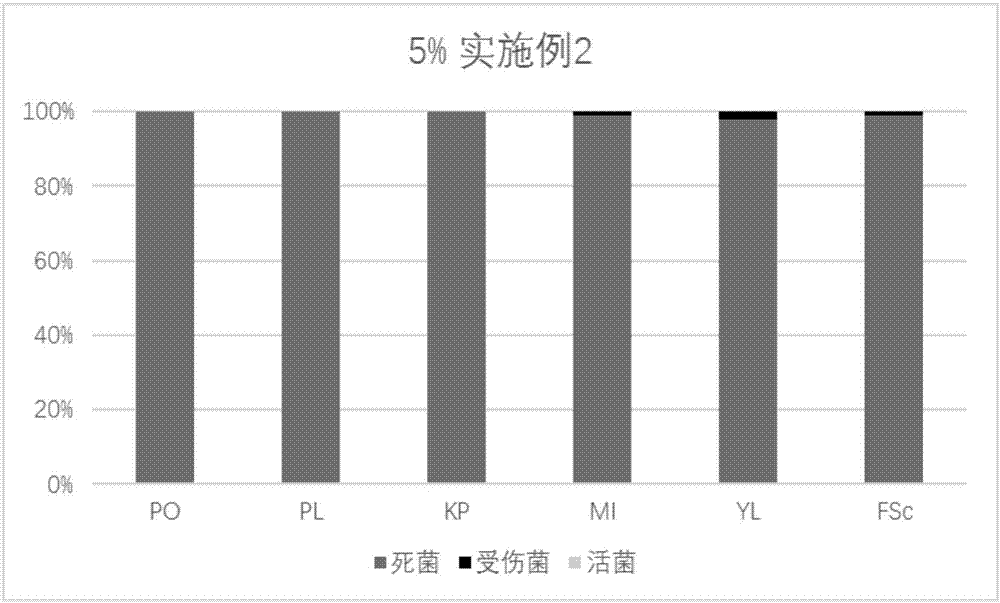
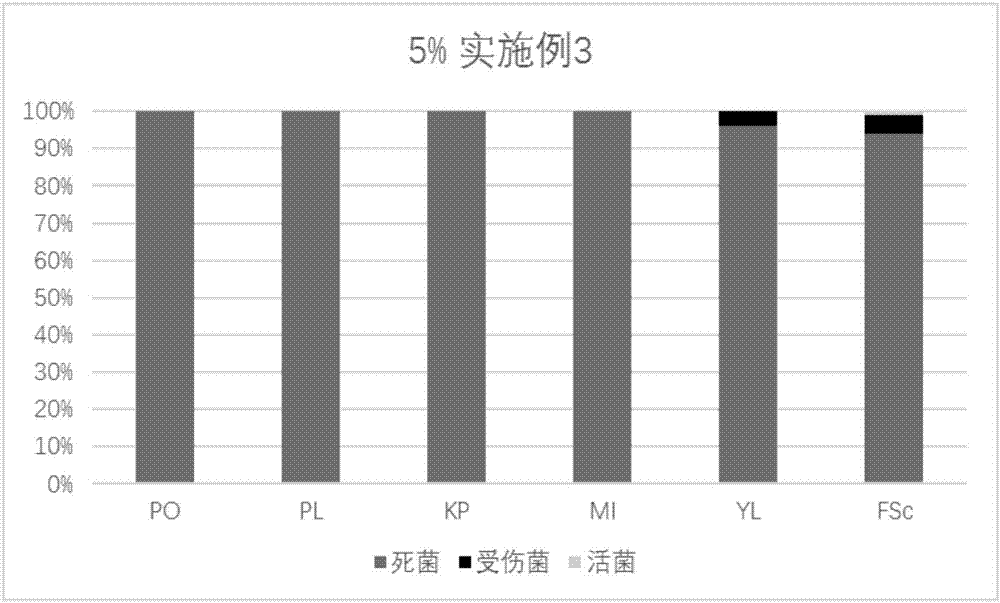
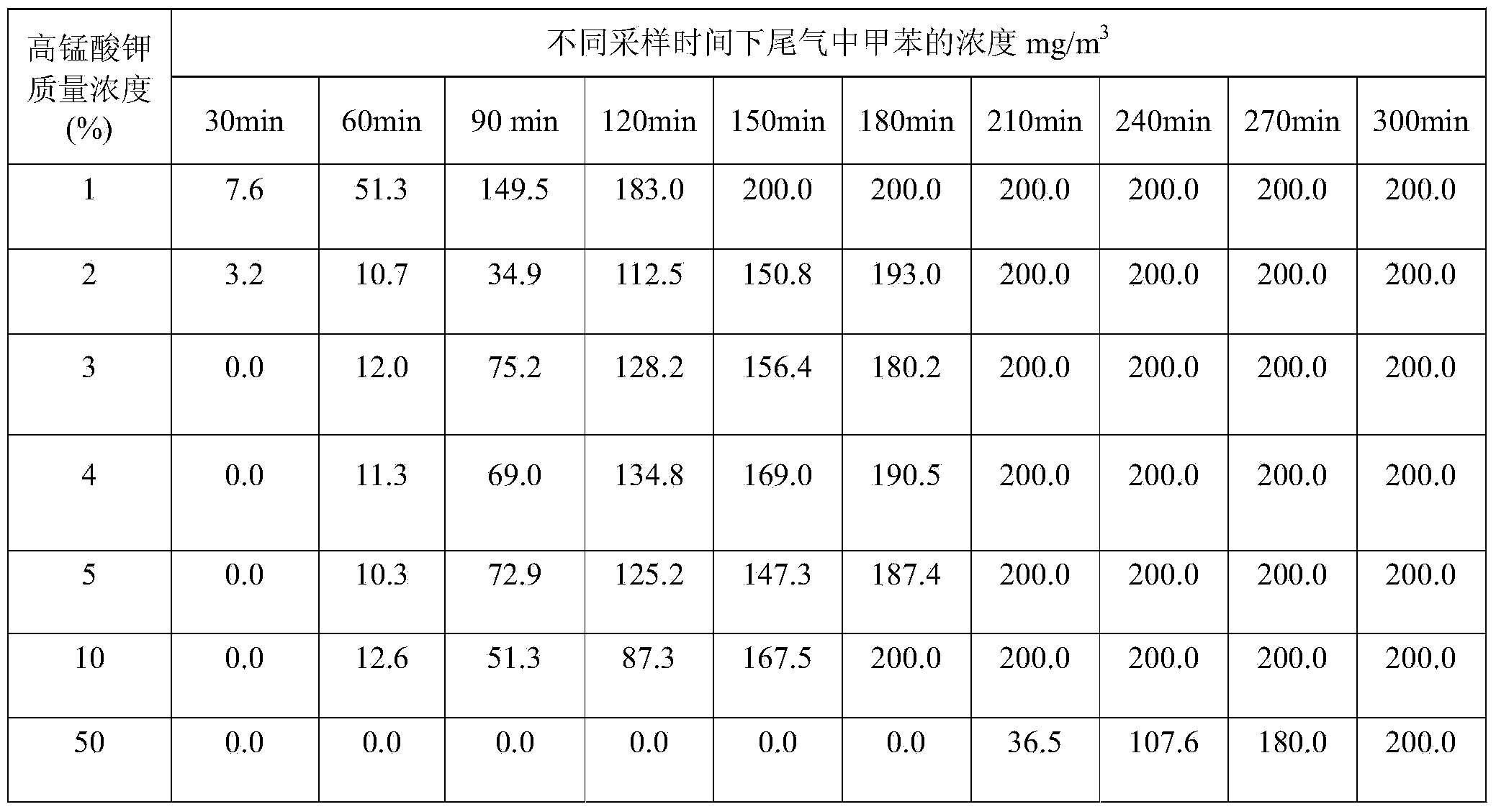
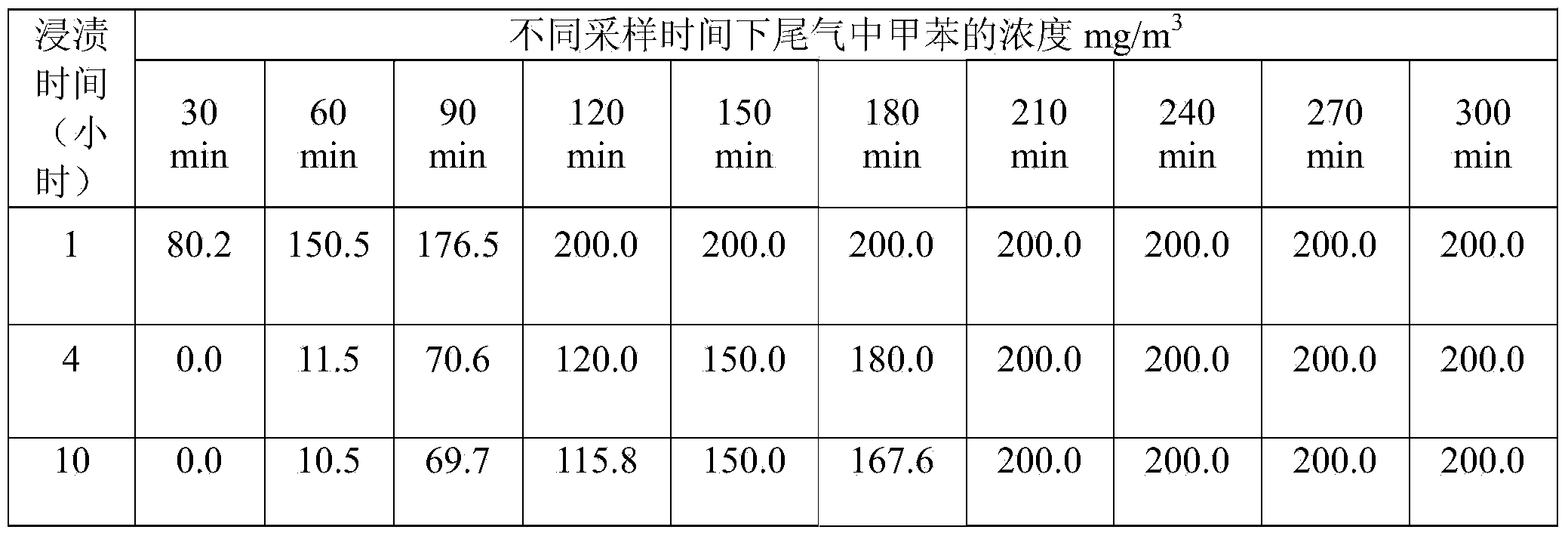
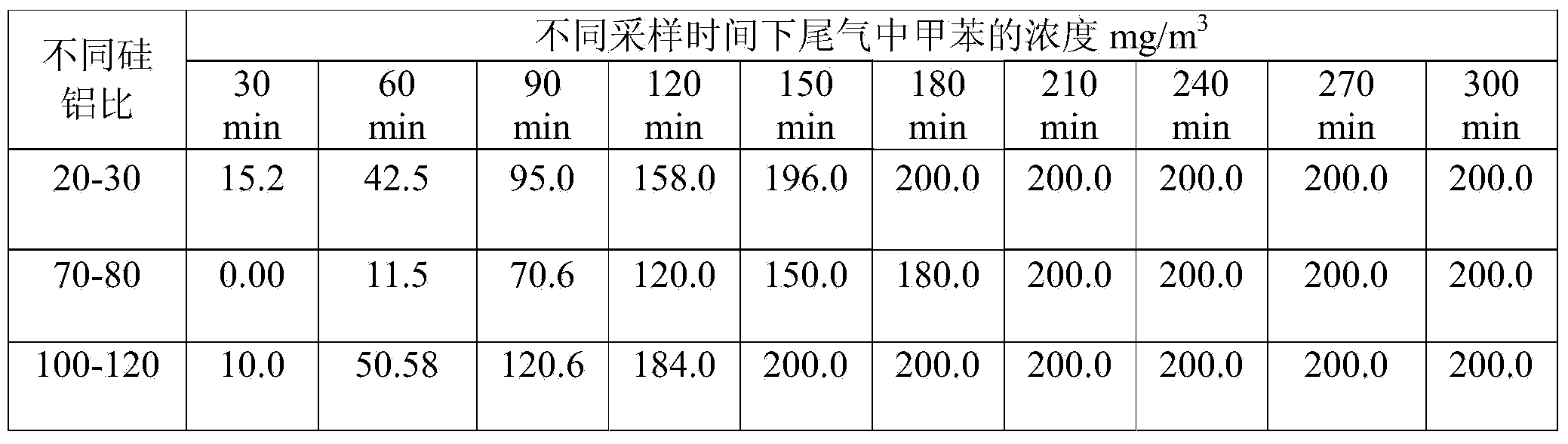
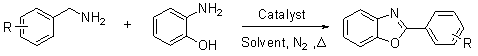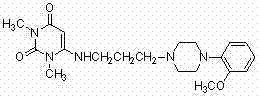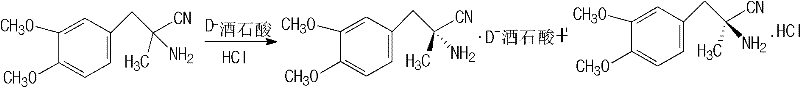Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
473results about How to "In line with the concept of green chemistry" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of starch and fatty acid compound
The invention belongs to the technical field of natural polymer modification and discloses a preparation method of a starch and fatty acid compound. The method comprises the following steps: adding de-ionized water into starch and balancing moisture in a closed container; carrying out heat treatment to obtain the starch subjected to wet and hot treatment; then adding a buffering solution to prepare a starch solution; after pre-heating, adding 2 to 5u / g pullulanase liquid to react for 4 to 5 hours; after carrying out enzyme deactivation, centrifuging and separating to obtain enzyme treatment modified starch; then stirring and gelatinizing the starch in a boiled water bath for 25 to 45 minutes and cooling to 60 to 90 DEG C to obtain gelatinized starch; then transferring the gelatinized starch into a homogenizing machine and adding fatty acid to carry out homogenization and mixing; and keeping the heat of the water bath to synthesize for 30 to 40 minutes to obtain the starch and fatty acid compound. According to the preparation method, a greener and more environment-friendly new way is provided for synthesizing the starch and fatty acid compound through wet and hot treatment and controlled enzymolysis treatment by adopting a gelatinizing method.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method and application of carbon-based metal organic frame (MOF) compound derivative material
The invention discloses preparation method and application of a carbon-based metal organic frame (MOF) compound derivative material, and belongs to the technical field of preparation of a functional nanometer material. The preparation method comprises the steps of placing a carbon fiber / polyacrylonitrile (PAN) thin film in an MOF precursor solution, achieving self-assembly of different morphologies of an MOF on different substrates at a room temperature, mixing the obtained product and an appropriate amount of melamine, and then performing thermal reduction on in-situ catalytic growth carbon nanotube (CNT) in an inert atmosphere to obtain the carbon-based MOF derivative material. The function nanometer material prepared by the method has the physical characteristics of high conductivity, rapid ion transmission passage, good flexibility, favorable self-support structure and the like and shows long service lifetime, high-capacity electric storage performance and excellent electrochemicalstability during energy storage and conversion; and the preparation process of the whole material is simple, no toxic product during reaction is generated, and the material is green and environmental-friendly and is suitable for industrial production on a large scale.
Owner:NANJING UNIV OF TECH
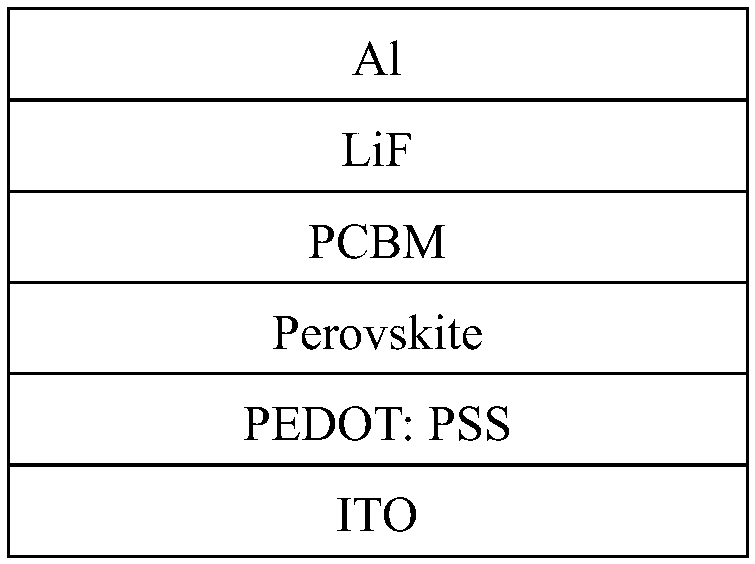
Stable and high-efficiency two-dimensional layered perovskite solar cell and preparation method therefor
ActiveCN108365102AIn line with the concept of green chemistryLarge grainSolid-state devicesSemiconductor/solid-state device manufacturingPerovskite solar cellSolvent
The invention discloses a method for preparing a stable and high-efficiency two-dimensional layered perovskite solar cell, and the method is characterized in that the method achieves the growth of micron-order perovskite crystalline grains in the air through the continuous heating in the whole spin coating process, and achieves the preparation of a flat high-quality perovskite film and a stable and high-efficiency perovskite photovoltaic device. A continuous heating method in the whole spin coating process, which is disclosed by the invention, enables a solvent to quickly volatilize to speed up the growth and conversion of the crystalline grains, also facilitates the growth of two-dimensional layered perovskite in a direction perpendicular to a substrate, and finally forms a flat and denseuniform perovskite film. The method disclosed by the invention does not need inert atmosphere for protection, facilitates the preparation of the large-area perovskite, and reduces the cost.
Owner:NANJING TECH UNIV
Carbon aerogel prepared from superfine nano aerogel obtained through TEMPO (2,2,6,6-tetramethylpiperidinooxy) oxidation and preparation method of carbon aerogel
The invention discloses carbon aerogel prepared from superfine nano aerogel obtained through TEMPO (2,2,6,6-tetramethylpiperidinooxy) oxidation. The superfine nano aerogel is prepared with a TEMPO oxidation method, then, preoxidation at the high temperature is performed, and the carbon aerogel is prepared with a carbonization method under the inert gas shielding condition. Used TEMPO is an environment-friendly solvent and has high safety, and therefore, the prepared carbon aerogel cannot have toxicity and cannot corrode instrument equipment or pollute the environment. TEMPO as an oxidizing agent can be recycled, and the condition of environment pollution caused by metal ions serving as a catalyst can be avoided.
Owner:INST OF WOOD INDUDTRY CHINESE ACAD OF FORESTRY
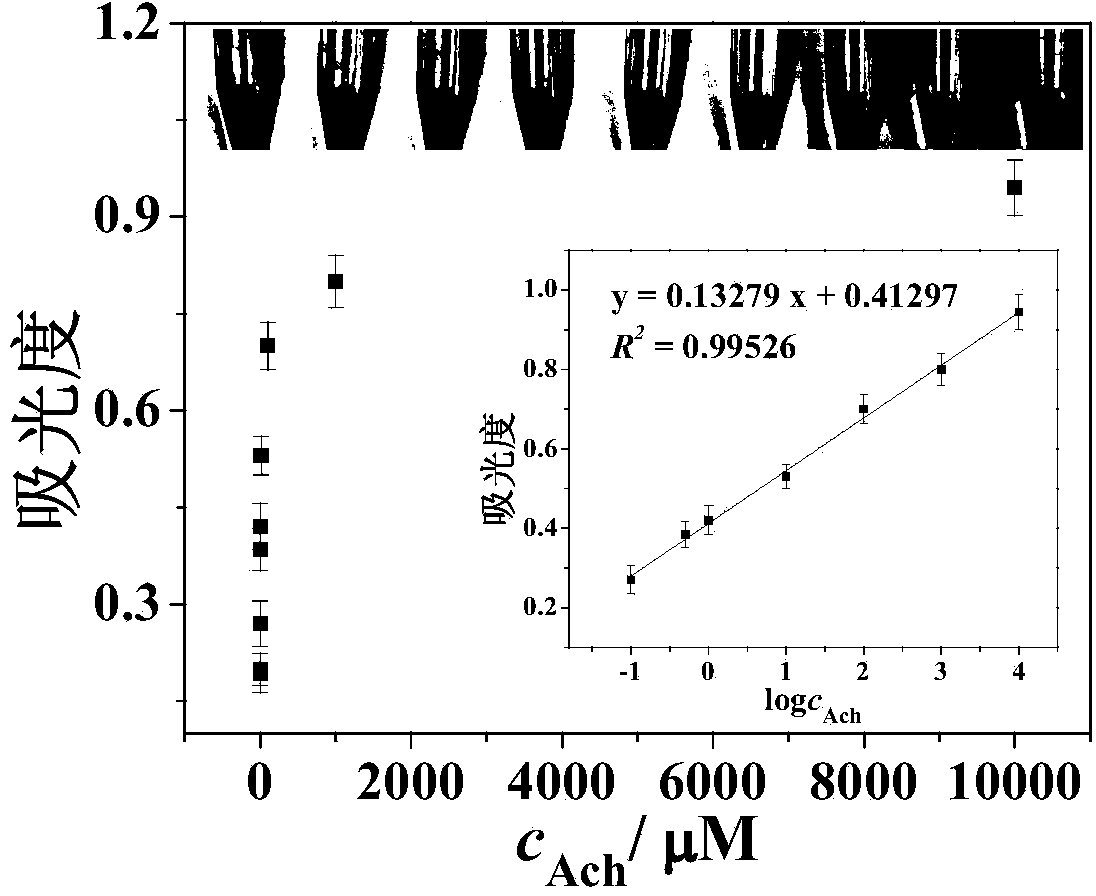
Magnetic graphene enzyme-mimicking property-based acetylcholine visual-detection method
InactiveCN103712983ASmall particle sizeEvenly distributedMaterial analysis by observing effect on chemical indicatorPeroxidaseAcetylhomocholine
The invention relates to a magnetic graphene enzyme-mimicking property-based acetylcholine visual-detection method, belonging to the technical field of biosensing. A hydrothermal method is used for synthesizing Fe3O4 / rGO by one step, H2O2 (hydrogen peroxide) is catalyzed to oxidize a substrate TMB (tetramethylabenzidine) to generate blue by utilizing the peroxidase-like property of the Fe3O4 / rGO, and the characteristic absorption intensity of an oxidation product of the TMB at the wavelength of 652nm is detected to realize the detection of hydrogen peroxide; furthermore, the H2O2 can be catalyzed to oxidize and convert the colorless TMB into a corresponding blue product based on the Fe3O4 / rGO, and the blue product is combined with acetyl choline to decompose H2O2 in the presence of both acetylcholinesterase and choline oxidase, and a novel acetylcholine visual-sensing method is established. The invention aims to provide an acetylcholine colorimetric detection method, and according to the method, the operation is convenient and flexible, the detection cost is low, the sensitivity is high, and instruments and equipment are simple.
Owner:JIANGSU UNIV
Nano silver-polyvinylidene fluoride composite separation membrane and preparation method thereof
InactiveCN102302903AIncrease water fluxHigh retention rateSemi-permeable membranesNanoparticlePolyvinylidene difluoride
The invention discloses a nano silver-polyvinylidene fluoride composite separation membrane and a preparation method thereof. The separation membrane is obtained by modifying a polyvinylidene fluoride membrane with nano silver. According to the invention, two dispersion methods namely a physical method and a chemical method are simultaneously adopted in the preparation process, so that nano particle reaches an ideal dispersion effect in the polyvinylidene fluoride separation membrane and a reliable guarantee is provided for the excellent property of the membrane. In the invention, a noble metal nano silver the price of which is relatively low is added, and the amount of nano silver is regulated, so that the mechanical property, antibiotic property and pollution resistance of the polyvinylidene fluoride separation membrane are greatly improved, the water flux and trapping rate of the polyvinylidene fluoride separation membrane are also improved, the service life of the membrane is prolonged, and the membrane has high performance-to-price ratio.
Owner:UNIV OF JINAN
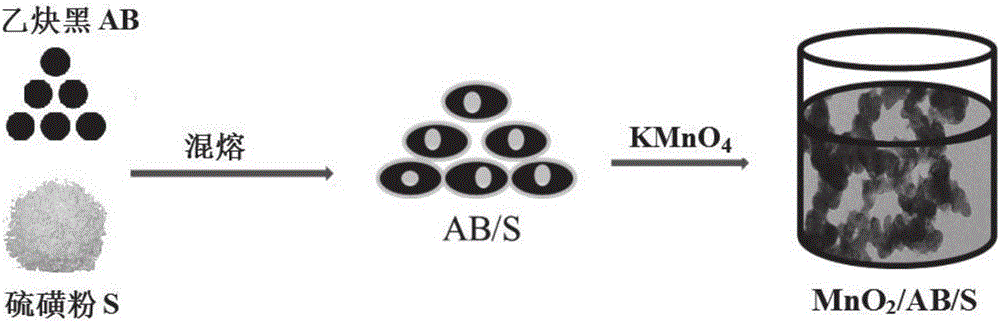
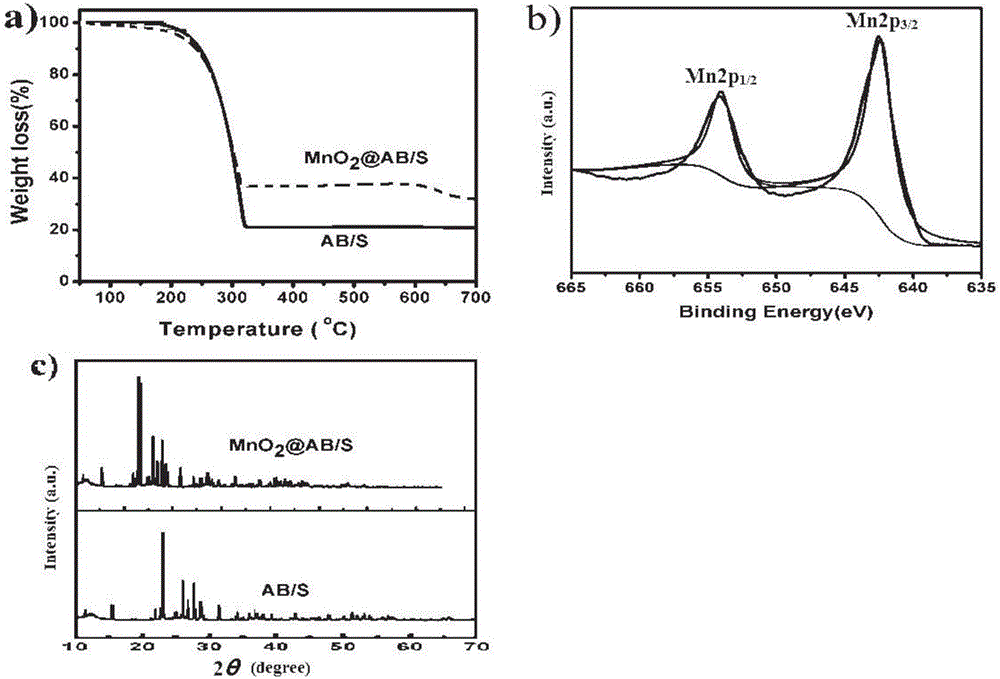
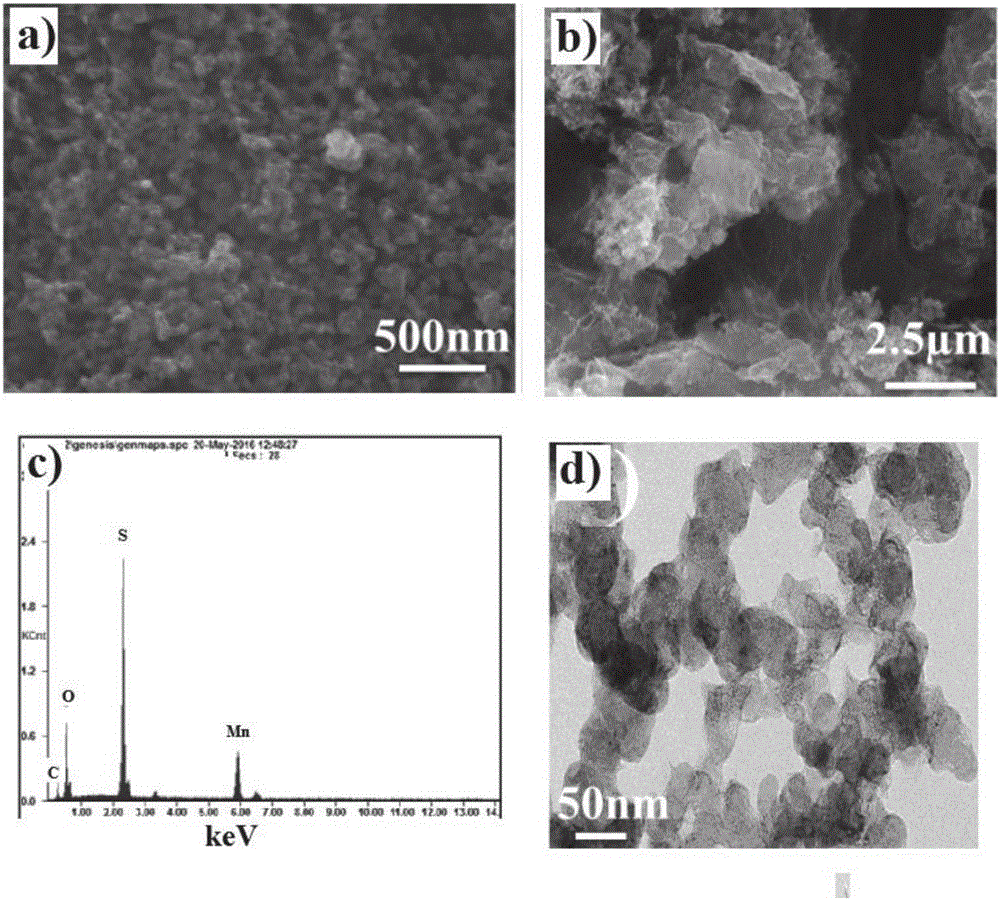
Manganese dioxide nanosheet coated carbon/sulphur compound, preparation method and application thereof
InactiveCN106129384AWrappedIncrease the speed of electron transferCell electrodesLi-accumulatorsSulphur compoundCathode material
The invention discloses a method for preparing a manganese dioxide nanosheet coated carbon / sulphur (MnO2@C / S) compound. The method comprises the following steps: firstly heating and melting a carbon material and sulphur simple substance to obtain a carbon / sulphur compound, then adding permanganate solution into aqueous phase dispersion liquid of the carbon / sulphur compound, heating, stirring, reacting, then carrying out solid-liquid separation, washing, and drying, so that the manganese dioxide nanosheet coated carbon / sulphur compound is obtained. Besides, the invention also discloses the manganese dioxide nanosheet coated carbon / sulphur compound obtained by adopting the method and application thereof. When the manganese dioxide nanosheet coated carbon / sulphur compound is applied to a lithium sulphur battery cathode material, and initial discharge capacity, cycle performance and rate capability of the obtained battery are respectively greatly improved.
Owner:CENT SOUTH UNIV
Method for preparing 2-substituted benzoxazole compound
The invention discloses a method for preparing a 2-substituted benzoxazole compound. According to the method, a benzylamine compound or a benzaldehyde compound or a benzyl alcohol compound, and o-toluidine-N-methyl-o-phenylenediamine, ortho-aminophenol and o-aminobenzenethiol serve as raw materials, metals palladium, platinum or ruthenium serves as a catalyst, and N,N-dimethylformamide, N,N-dimethylacetamide or N-methylpyrrolidone serves as a solvent. The method comprises the following preparation steps: (1) mixing the raw materials; (2) reacting; (3) separating and extracting; and (4) drying and concentrating. An oxidant and a hydrogen acceptor are not required in the whole preparation process, and the used partial catalysts can be recycled. The method is high in atom economy, simple in aftertreatment and mild in reaction conditions and has a certain industrial application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH
Porous carbon material for alkali lignin-based supercapacitor as well as preparation method and application thereof
ActiveCN106744793AReduce equipment lossMaximize utilizationHybrid capacitor electrodesCarbon preparation/purificationChemistryTube furnace
The invention discloses a porous carbon material for an alkali lignin-based supercapacitor as well as a preparation method and application thereof. The preparation method comprises the following steps: mixing alkali lignin coarsely purified from papermaking black liquid with a three-block polymer Pluronic Fl 27 and Mg(CH3COO)2.4H2O, and adding formaldehyde and hydrochloric acid for full and uniform stirring to obtain a mixed solution; placing the mixed solution into a drying oven for drying, so as to obtain a black brown solid; then putting the black brown solid into a tubular furnace for carbonization reaction, continuously feeding nitrogen or inert gas at the end of the reaction, and cooling to a room temperature; finally carrying out acid pickling, washing and drying to obtain the black powdered porous carbon material. The obtained material can be applied to a double-electrode-layer supercapacitor; in the carbonization process, no strong-causticity chemical substances such as strong acid and strong alkali are used, so that the loss of equipment is reduced to the maximum extent; the used raw materials are by-products in the pulp and paper making industry, so that the maximum use of resources is realized; moreover, lignin is wide in source and low in cost, so that the production cost is reduced.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
Preparation method of modified nano calcium borate lubricant additive
InactiveCN103332701AHigh boron calcium ratioGood product purityMaterial nanotechnologyAdditivesChemistryCalcium borate
The invention discloses a preparation method of a modified nano calcium borate lubricant additive, which comprises the following steps of: a) weighing boric acid and calcium oxide, adding water respectively to prepare a boric acid aqueous solution and lime milk emulsion, b) stirring and mixing the boric acid aqueous solution and the lime milk emulsion, adding a surfactant, after heating for reaction, performing suction filtration, drying and grinding to obtain nano CaB6O10 powder, c) adding water to the nano CaB6O10 powder, then adding oleic acid, performing ultrasonic stirring, standing to separate an oil phase from a water phase, taking an upper oil phase, drying, and obtaining the oleic acid modified nano CaB6O10 lubricant additive. The additive prepared by the method is high in boron-calcium ratio, and small in particle size, and particle are distributed uniformly (10-15nm). According to the method, a washing technology is removed, and the whole production process from raw materials to the product is environment-friendly and non-pollution, and accords with a green chemical concept.
Owner:HEBEI UNIVERSITY
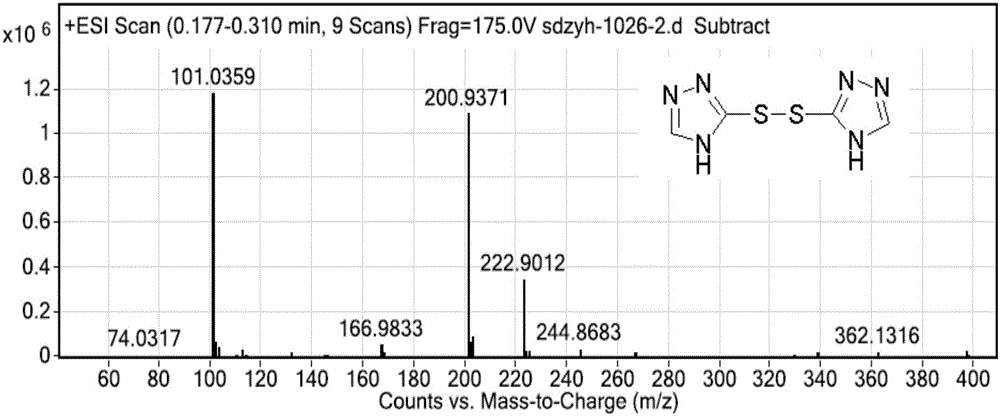
Intermediate compound, and synthetic method of prothioconazole
ActiveCN106749057ASynthetic raw materials are cheap and easy to obtainReduce manufacturing costOrganic chemistryPropanolGreen chemistry
The invention discloses an intermediate compound, and a synthetic method of prothioconazole. The method comprises the following steps: carrying out a substation reaction on 5,5'-dithio-bis(1,2,4-triazole) and 2-(1-chlorocyclopropyl)-3-chloro-1-(2-chlorophenyl)-2-propanol to obtain the key intermediate compound; and reducing the key intermediate compound to obtain the target product prothioconazole. The synthetic method has the advantages of high conversion rate, high selectivity, cheap and easily available synthesis raw materials, reduction of the production cost, mild and easily controlled technologic reaction conditions, simplicity in operation, easiness in product purification, obtaining of the product through direct re-crystallization, simple and accurate control method of intermediates in all steps, high product yield, good atom economy, avoiding of tedious post-treatment, large competition advantages and industrial production utilization values, avoiding of strong alkalis and other raw materials, extremely low three wastes, and according with the green chemistry idea.
Owner:NANJING TECH UNIV
Reinforcing method utilizing waterlogged wood relics of nanocellulose
ActiveCN106799781AHigh mechanical strengthEasy to cleanWood treatment detailsWood impregnation detailsDesalinationAcid corrosion
The invention discloses a reinforcing method utilizing waterlogged wood relics of nanocellulose. The reinforcing method comprises the following steps of: mixing nanocellulose with a filling agent to obtain a reinforcing agent; and performing dehydrating and desalination on the waterlogged wood relics, and adding the waterlogged wood relics into the reinforcing agent to soak, and performing drying for reinforcing. The waterlogged wood relics obtained by treatment of the reinforcing method are bright in color, are good in stability, do not easily separate out salts, and are especially free of secondary acid degradation. The reinforcing method disclosed by the invention can be used for effectively clearing and protecting the reinforcing method, the dried and reinforced wood is stabilized to a pH neutral state, acid corrosion and salt separation are effectively restrained, and mechanical strength of highly rotten wood is greatly reinforced.
Owner:INST OF WOOD INDUDTRY CHINESE ACAD OF FORESTRY
Construction method and application of surface protein-embossed self-energized biological fuel cell sensor
InactiveCN106525943ARealize specific identification detectionEasy to operateMaterial analysis by electric/magnetic meansExternal energyCarbon nanotube
The invention discloses a construction method and application of a surface protein-embossed self-energized biological fuel cell sensor. The method comprises the steps that the surface of a carbon electrode is coated with a molecularly-imprinted polymer, specific protein is labeled with a phenylboronic acid-bilirubin oxidase-carbon nanotube nanocomposite after being adsorbed to the surface of the molecularly-imprinted polymer, and then a surface protein-embossed biological cathode is obtained; the surface of a carbon electrode is modified with a thionine-graphene-glucose dehydrogenase compound, and then a biological anode is obtained; the surface protein-embossed biological cathode, the biological anode and parts including a PMDS electrolytic tank and external resistors are assembled, and then the biological fuel cell sensor is obtained. According to the sensor, the high selectivity and sensitivity to specific glycoprotein are achieved in a compound system where multiple proteins exist to generate interference, external energy supply is not needed during molecular recognition, and large-scale production and application requirements are met.
Owner:CENT SOUTH UNIV
Preparation and application of graphene based metal compound nano array material
ActiveCN106914244ASimple methodEasy accessCell electrodesMetal/metal-oxides/metal-hydroxide catalystsElectrochemistryCalcination
The invention discloses a preparation and application of a graphene based metal compound nano array material, and belongs to the technical field of preparation of functional nano materials. A carbon precursor and a metal salt mixture are ground and then coat a foam nickel substrate, the coated foam nickel substrate is subjected to heat treatment in an inert atmosphere, and a graphene array material is obtained by washing. The graphene based metal compound nano array material is obtained from the graphene array material by solution growth or electrochemical deposition and calcination and other ways. The nano array material prepared by the method has high electric conductivity, fast ion transfer channels, high active sites and other physical properties, and shows electricity storage performance of long service life and high capacity and excellent electrochemical catalytic activity and stability in energy storage and conversion and electrochemical catalysis reactions. The whole material preparation process is simple, no toxic products are generated in the reaction, and the preparation method has the advantages of low energy consumption, and green and environmental protection, and is suitable for large-scale industrial production.
Owner:NANJING UNIV OF TECH
Large-scale preparation and lithium battery application of vanadium pentoxide and carbon nano composite thereof
InactiveCN106654186AWide variety of sourcesEasy accessFinal product manufactureCell electrodesFreeze-dryingCarbon nanotube
The invention discloses large-scale preparation and lithium battery application of vanadium pentoxide and carbon nano composite thereof, and belongs to the technical field of functional nano material preparation. The preparation method comprises the following steps: dissolving vanadium pentoxide solid powder in pure water, mixing with hydrogen peroxide, obtaining a scarlet solution, and preserving the temperature of the solution for a period of time to become a colloidal sol; carrying out freeze drying treatment on the colloidal sol to obtain a three-dimensional self-supporting solid; and taking partial solid to be calcinated under nitrogen atmosphere so as to obtain a vanadium pentoxide nano material. The corresponding solid is added into composite carbon nano tube and graphene as long as the scarlet solution is obtained, and the mixture is dispersed uniformly without changing remaining operation. The lithium battery positive electrode material prepared in the method is long in service life, high in capacity and stable in cycle performance. The whole technological process is simple, the price of raw materials is low, no toxic product is generated, the energy consumption is low, and the material is green and environmentally-friendly, so that the disadvantage that existing lithium battery materials are high in production cost, complex in process and great in byproduct toxicity is overcome, and the material is applicable to industrial large-scale production.
Owner:NANJING UNIV OF TECH
Inverse phase transfer catalysis preparation method for urapidil
InactiveCN102295607BShort reaction timeHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsOrganic synthesisUracil
The invention discloses a preparation method for urapidil, and relates to an inverse phase transfer catalysis method for preparing urapidil, and belongs to the technical field of organic synthesis. The method comprises the following steps that: water is adopted as a solvent; an inorganic alkaline substance is adopted as an acid binding agent; 6-(3-chloropropyl)-1,3-dimethyluracil (I) reacts with 1-(2-methoxyphenyl)piperazine hydrochloride (II) in the presence of an inverse phase transfer catalyst to generate 6-[[3-[4-(2-methoxyphenyl)-1-piperazinyl]-propyl]-amino]-1,3-dimethyl-uracil (III, urapidil). With the method, the high-selectivity and high-yield preparation of the urapidil can be realized; the prepared urapidil has high purity, wherein the purity of the urapidil is more than 99.5%;the conversion rate is high; the yield can reach 80%; the reaction conditions are mild; the selectivity is good; the post-treatment is simple; the method is environmental-friendly, and is suitable for the industrial production.
Owner:ZHENGZHOU UNIV +1
Method for preparing cyclic carbonate employing catalysis of carbon dioxide and epoxide with haloid
InactiveCN104725344AReduce manufacturing costLow initial reaction pressureOrganic chemistryPtru catalystOrganic synthesis
The invention discloses a method for preparing cyclic carbonate employing catalysis of carbon dioxide and epoxide with haloid, and belongs to the field of organic synthesis catalysis. According to the method, with haloid employing halogen serving as an anion as a catalyst, cycloaddition reaction employing carbon dioxide and epoxide as raw materials is catalyzed by adopting haloid which uses halogen serving as an anion as a catalyst. The catalyst is cheap and available; the production cost is reduced; the method is simple to operate in synthesis of the cyclic carbonate; the catalyst is cheap and available; the reaction condition is mild; the lowest initial reaction pressure can be as low as 0.25MPa; the catalyst is high in activity; the yield of ethylene carbonate is 99.5% under the optimal reaction condition; and with relatively environment-friendly alcohols as a solvent, the method conforms to the idea of green chemistry, and is an excellent system for preparing the cyclic carbonate.
Owner:DALIAN UNIV OF TECH
Unsymmetrical hydrogen migration synthesizing method for (R, R)-formoterol
InactiveCN101468954AIn line with the concept of green chemistryLow costOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsPhotochemistry
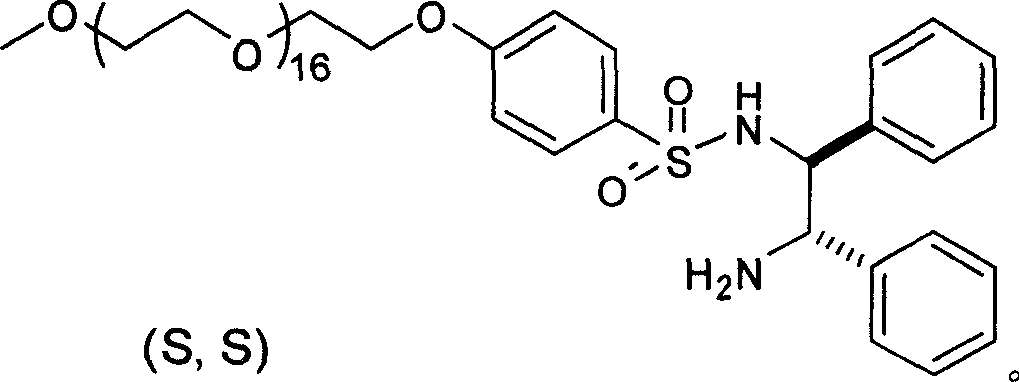
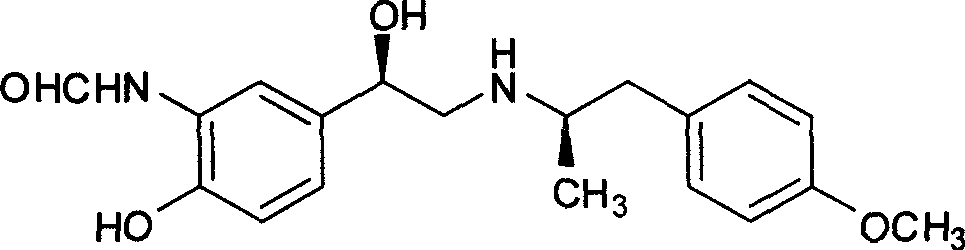
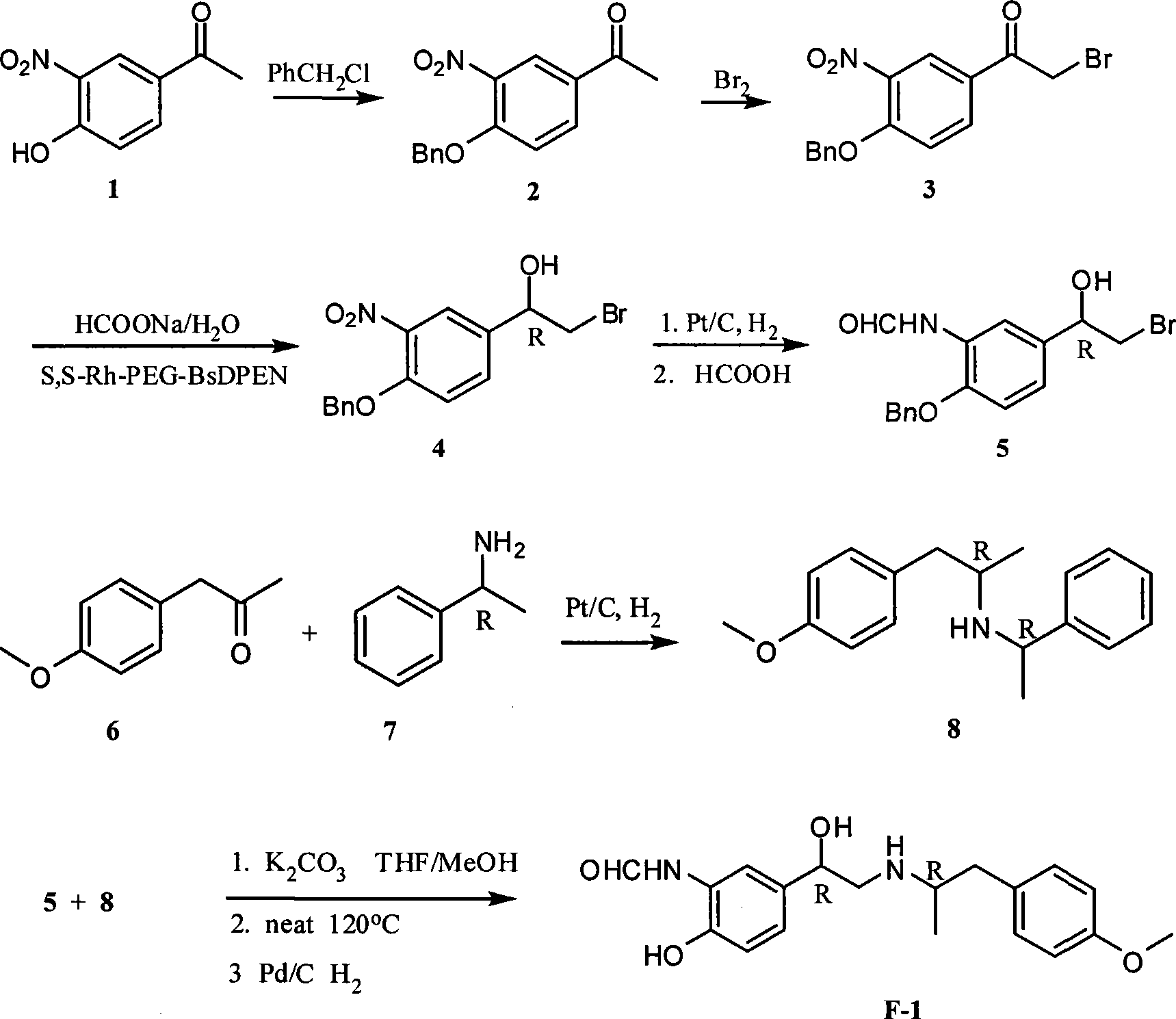
The invention relates to an asymmetric hydrogen transfer synthesis method for (R,R)-formoterol, and relates to a novel method for synthesizing an optical pure beta 2-adrenoreceptor excitant, namely formoterol. The method comprises: firstly, taking 4-hydroxyl-3 nitroacetophenone as a raw material, using benzyl groups to protect phenolic hydroxyl groups, and obtaining alpha-bromo keto after bromination; secondly, taking (S,S)-Rh-PEG-BsDPEN as a catalyst and formic acid and derivatives of the formic acid as hydrogen sources, and synthesizing chiral alcohol intermediate by an asymmetric hydrogen transfer method; thirdly, using (R)-alpha-methyl phenylethylamine and methoxyl phenylacetone to generate imine compounds, and obtaining chiral amine intermediate through hydrogenation reduction under the catalysis of Pt / C; and fourthly, reacting and coupling the chiral alcohol intermediate and the chiral amine intermediate, removing protective groups, and obtaining the (R,R)-formoterol. The invention uses the asymmetric hydrogen transfer method and a chiral auxiliary reagent to synthesize the (R,R)-formoterol, and has high yield and good ee value. Compared with a method for synthesizing chiral formoterol through chemical splitting, the method has the advantages of high total yield, mild reaction conditions, low cost and so on, and is favorable for industrial production.
Owner:SUN YAT SEN UNIV
Method for preparing psoralen and isopsoralen or extract containing psoralen and isopsoralen
The invention relates to a method for preparing psoralen and isopsoralen or an extract containing psoralen and isopsoralen. Particularly the method comprises the steps of (i) conducting backflow extraction on fructus psoraleae medicinal materials by using water, and concentrating a combined extracting solution to obtain a concentrated solution; (ii) adding acid in the obtained concentrated solution, heating and hydrolyzing; (iii) eluting hydrolysate on a macroporous adsorption resin by using water to remove impurities, eluting by using ethanol water solution with concentration larger than 50%, collecting an ethanol eluant, and concentrating or optionally drying further to obtain the extract containing psoralen and isopsoralen; and (iv) optionally subjecting the extract obtained in the step (iii) to a silicagel column, eluting by using an organic solvent, collecting psoralen fraction and isopsoralen fraction, and removing the solvent to respectively obtain psoralen and isopsoralen. The method is simple in operation, short in production period, high in yield, less in pollution and applicable to industrialization mass production.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA
Composite fungicide and metal cutting fluid with same and preparation method of composite fungicide
ActiveCN107125249AImprove the bactericidal effectOvercoming the problem of poor sterilization effectBiocideDead animal preservationKetoneLubrication
The invention provides a composite fungicide and metal cutting fluid with the same and a preparation method of the composite fungicide. The composite fungicide comprises 1,2-benzisothiazole-3-ketone, epsilon-polylysine and water-soluble organic amino. In the composite fungicide provided by the invention, the three components, namely the 1,2-benzisothiazole-3-ketone, the epsilon-polylysine and the water-soluble organic amino are synergistic, so that the sterilization effect of the composite fungicide is improved; and the metal cutting fluid with the composite fungicide is good in sterilization effect, remarkable in biological stability, high in security and wide in application prospect, meets on-site process requirements such as lubrication, cooling, emulsion stability and corrosion resistance, and meets ideas of green chemicals.
Owner:QUAKER CHEM CHINA
Adsorbent with adsorption and oxidation synergistic effects on indoor toluene and preparation method and application of adsorbent
InactiveCN104226241AEfficient removalPromote oxidationOther chemical processesAluminium silicatesBenzoic acidSorbent
The invention relates to the technical field of indoor air purification and discloses an adsorbent with adsorption and oxidation synergistic effects on indoor toluene, and the loading capacity of potassium permanganate on an H-ZSM-5 molecular sieve is 0.05 to 1.The adsorbent has good oxidation performance and excellent absorption effect, toluene can be directly oxidized into benzoic acid, and the technical problem of vacant adsorption of indoor low-concentration toluene is solved. The invention further discloses a preparation method of the adsorbent with the adsorption and oxidation synergistic effects on the indoor toluene, H-ZSM-5 is used as an adsorption carrier, potassium permanganate is loaded on the adsorption carrier by virtue of dipping, and then drying is performed by a nitrogen blowing instrument. The preparation method has the advantages of simplicity, short consumed time and low cost, conforms to the concept of green chemistry and has a wide application prospect. The invention further relates to application of the adsorbent to indoor toluene treatment. An effective method is provided for indoor pollution gas treatment, and an important application value is realized in the aspect of life safety.
Owner:杭州朱庇特环境科技有限公司
Porous polymer adsorption material containing triazine ring and azo bond functional groups, and porous polymer catalysis material, and preparation methods and applications thereof
The invention discloses a porous polymer adsorption material containing triazine ring and azo bond functional groups, and a porous polymer catalysis material, and preparation methods and applicationsthereof. 2,4,6-tris(4'-aminophenyl)-1,3,5-triazine and diphenol and / or trisphenol, which are used as raw materials, undergo diazo coupling polymerization cross-linking to obtain the porous polymer adsorption material. The porous polymer adsorption material has the charateristics of high specific surface area, high nitrogen element content, high micro-pore proportion, good affinity to carbon dioxide and good adsorption performance, and a metal ion supported porous polymer adsorption material obtained after loading metal ions on the porous polymer adsorption material has the advantages of mild reaction conditions, short reaction time, realization of the yield reaching 95% or above, and no obvious reduction of the yield using the recycled catalyst when used to catalyze a reaction of carbon dioxide and epoxypropane for generating propylene carbonate, and is expected to be used in actual industrial production.
Owner:CENT SOUTH UNIV
Preparation method of L-methyldopa
InactiveCN102531939AIncreased overall molar yieldReduce unit consumptionOrganic compound preparationAmino-carboxyl compound preparationMother liquorOrganic solvent
The invention discloses a preparation method of L-methyldopa. The key points of the method are as follows: veratone is recycled from the L-methyldopa production waste liquor, the mother liquor synthesized from DL-aminopropionitrile or the racemic mother liquor of D-aminopropionitrile is mixed with an organic solvent, the mixture is heated to 40-110 DEG C to react for 1-24 hours, and the obtained solution is stood and layered after the reaction to obtain a water layer and an organic layer; the organic solvent is a non-solvent of water and a good solvent of veratone; or alkali and water are added in the splitting waste liquor of DL-aminopropionitrile, the pH is adjusted to 7-14, the solution is heated to 40-110 DEG C to react for 1-24 hours, and the obtained solution is stood and layered after the reaction to obtain a water layer and an organic layer; and the solvent is recycled from the obtained organic layer, and high vacuum distillation is performed under the condition that the pressure is no more than 5kPa to obtain veratone for reuse. By adopting the preparation method of L-methyldopa, the main raw material veratone can be recycled from the L-methyldopa production waste liquor, the chemical oxygen demand (COD) of wastewater can be reduced and the concept of green chemistry can be realized.
Owner:ZHEJIANG CHIRAL MEDICINE CHEM
Preparation method and application of super-hydrophilic/underwater super-oleophobic polyvinylidene fluoride composite membrane
ActiveCN108771975ALow priceWide variety of sourcesMembranesSemi-permeable membranesIonPolyvinylidene fluoride
The invention provides a preparation method for a super-hydrophilic / underwater super-oleophobic polyvinylidene fluoride composite membrane, belonging to the field of preparation technologies of environmental functional materials. The preparation method comprises the following concrete steps: preparing a PVDF membrane and a PVDF@PDA membrane at first; dissolving nickel nitrate hexahydrate, cobalt nitrate hexahydrate and urea in deionized water to obtain a mixed solution, pouring the mixed solution into ethanol, adding the PVDF@PDA membrane and carrying out soaking; and then placing the PVDF@PDAmembrane in a reaction vessel for a reaction, cleaning the membrane by using deionized water and ethanol and then carrying out drying so as to prepare the PVDF@PDA@NiCo2(OH)6 composite membrane. According to the invention, membrane separation technology is adopted; process flow is short; operation is easy to control; resources are saved; no secondary pollution is produced; and the preparation comforms to the concept of green chemistry and is suitable for wide promotion and application.
Owner:JIANGSU UNIV
ZnIn2S4 nano-sheet coated beta-Bi2O3 core-shell heterogeneous compound photocatalyst, as well as preparation method and application thereof
ActiveCN109589989ARich sourcesGood chemical stabilityMaterial nanotechnologyPhysical/chemical process catalystsMethyl orangeElectron
The invention belongs to the field of environmental water pollution abatement, and discloses a ZnIn2S4 nanosheet coated beta-Bi2O3 core-shell heterogeneous compound photocatalyst, as well as a preparation method and application thereof. According to the method, the ZnIn2S4 nanosheet coated beta-Bi2O3 core-shell heterogeneous compound photocatalyst is prepared by using ZnIn2S4 and synthetic beta-Bi2O3 monomer as raw materials through a simple self-aggregation effect. The binary ZnIn2S4 / beta-Bi2O3 compound nano-photocatalyst can be applied to catalytic degradation of tetracycline hydrochloride and methyl orange under visible light, shows excellent photo-induced electron separation efficiency, and can be used for improving the visible light utilization and integral photocatalytic activity. The ZnIn2S4 nanosheet coated beta-Bi2O3 core-shell heterogeneous compound photocatalyst is prepared from nontoxic raw materials, has simple operation and mild reaction condition, accords with the concept of environment-friendly chemistry, and has a wide application prospect in the aspect of solving water environmental pollution.
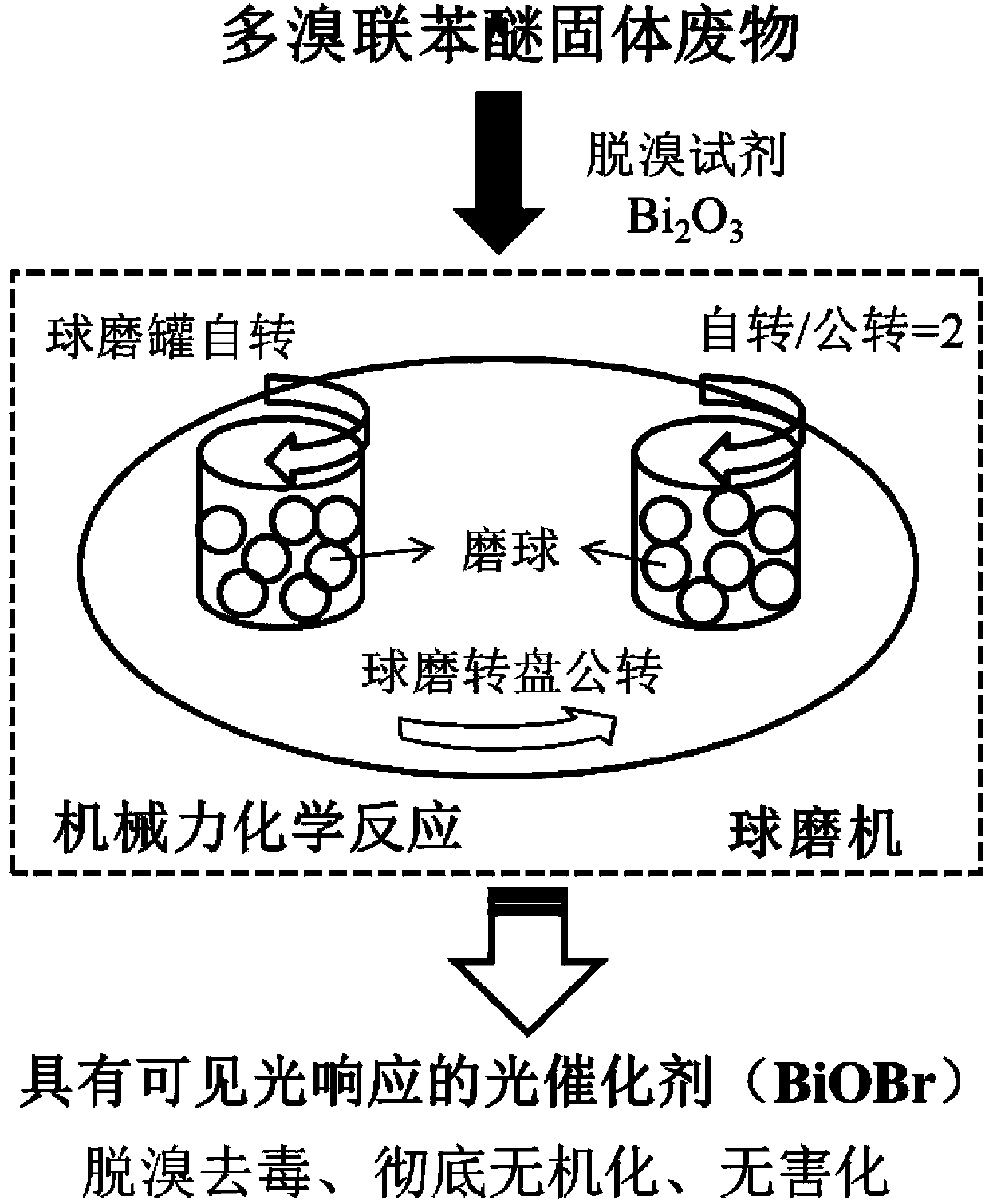
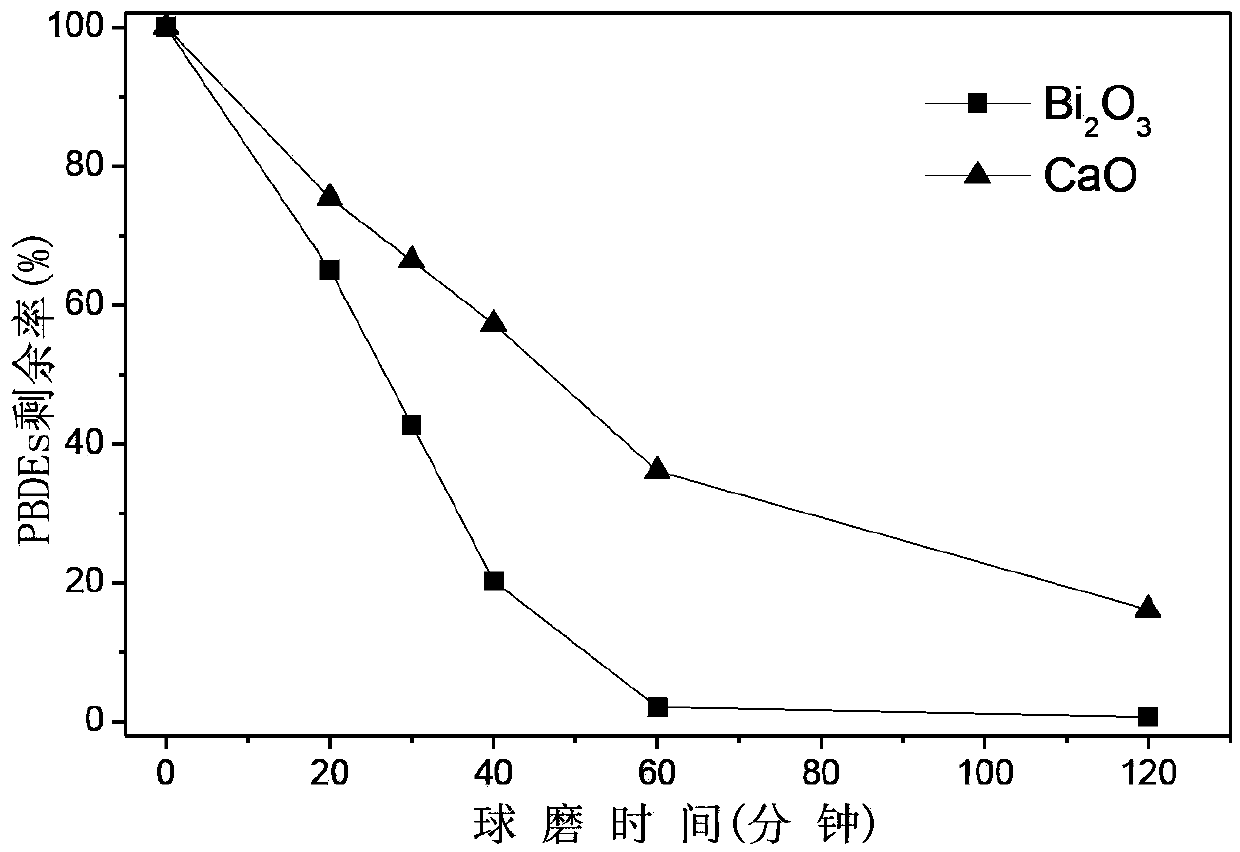
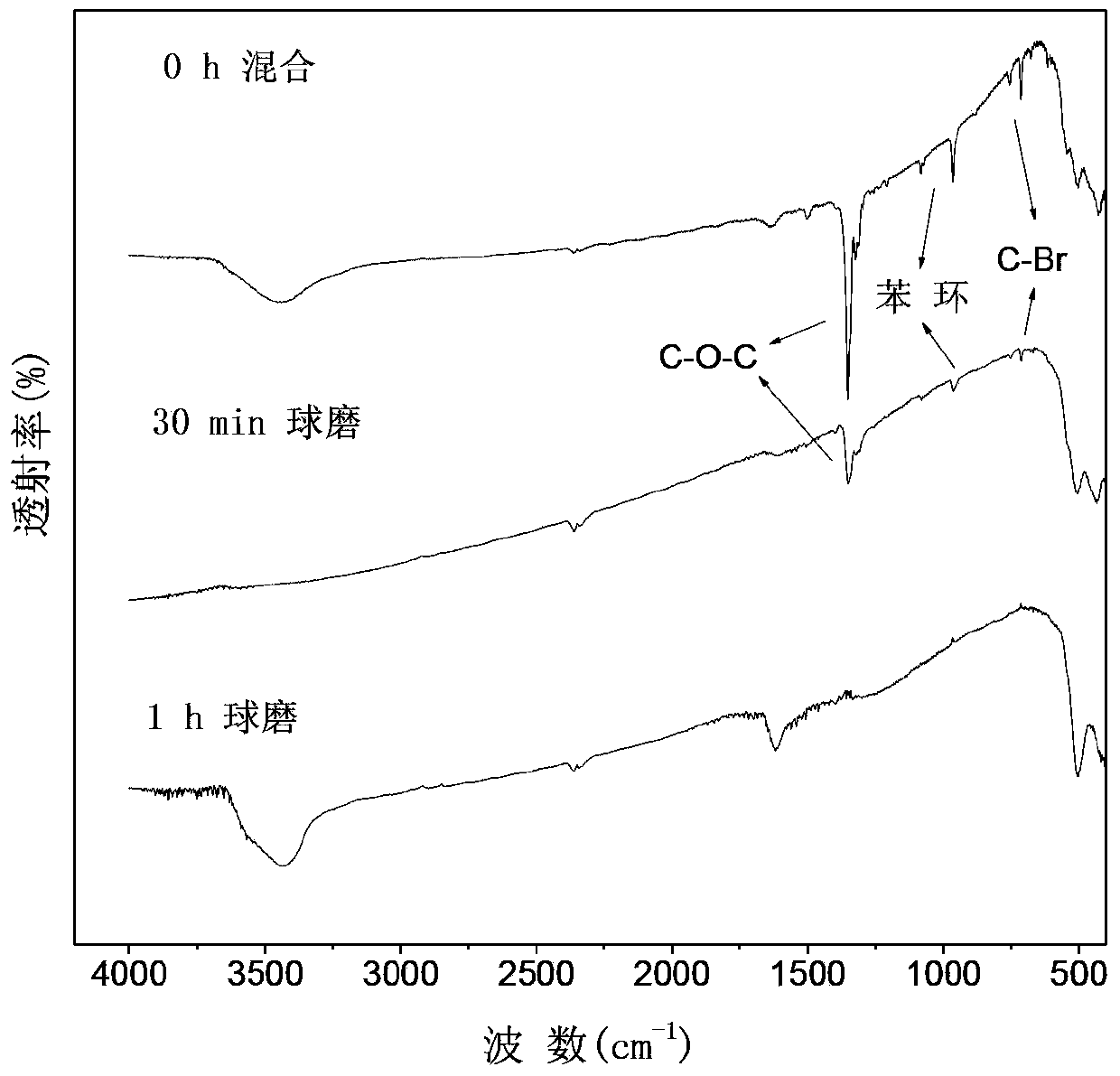
Method for preparing photocatalyst with visible light response by mechanochemical treatment on polybrominated diphenyl ether solid waste
ActiveCN103386314APromote decompositionAchieve reusePhysical/chemical process catalystsPtru catalystChemical reaction
The invention belongs to the technical field of environmental pollution waste treatment and novel material preparation and particularly relates to a method for preparing a photocatalyst with visible light response by mechanochemical treatment on polybrominated diphenyl ether solid waste. The method comprises the following steps: mixing polybrominated diphenyl ether solid waste with a debromination reagent under conditions of normal temperature and normal pressure, putting the mixture in a planetary high-energy ball mill reactor so that efficient degradation and debromination of polybrominated diphenyl ether can be realized through a mechanochemical reaction and the bromine element can be recycled at the same time, thus preparing the novel photocatalyst with visible light response. The method provided by the invention has the following advantages that the technological process is simple, the reaction conditions are mild (normal temperature and normal pressure), the target pollutants are completely decomposed, the finally generated product is a bromine-containing catalyst with visible light response; no excessive ball milling reagent is added and no secondary pollution is caused in the process, thereby meeting the conception of environmentally-friendly chemistry.
Owner:苏州清初环境科技有限公司
Carbon dioxide and water processing methane catalyst and preparation method and application thereof
ActiveCN107890870AWide variety of sourcesLow priceHydrocarbon from carbon oxidesCatalystsWater processingMethane
The invention discloses a carbon dioxide and water processing methane catalyst and a preparation method and application thereof, and belongs to the technical field of chemical engineering. The catalyst is mainly prepared from metal simple substances and a supported nickel-based catalyst. The metal simple substances include Zn, Fe, Al, Mn, Ni, Co and Mg; the nickel-based catalyst is Ni / C, wherein Cis a carrier including Al2O3, SiO2, TiO2, ZrO2, CeO2 and La2O3. The total weight of the catalyst is adopted as the reference, the mass percent of the metal simple substances is 20-90%, and the mass percent of the nickel-based catalyst is 10-80%. According to the preparation process of the catalyst, dipping is adopted, sol-gel is adopted, a sediment or sedimentation method is adopted, and then drying, roasting and reducing are performed to obtain the catalyst. The reaction raw materials are carbon dioxide and water, sources are wide, the price is low, the catalyst preparation method is simple,easy to implement and low in cost, and the catalyst has the certain application prospect. The catalyst is mainly applied to thermo-catalysis instead of photo-catalysis or electro-catalysis.
Owner:TAIYUAN UNIV OF TECH
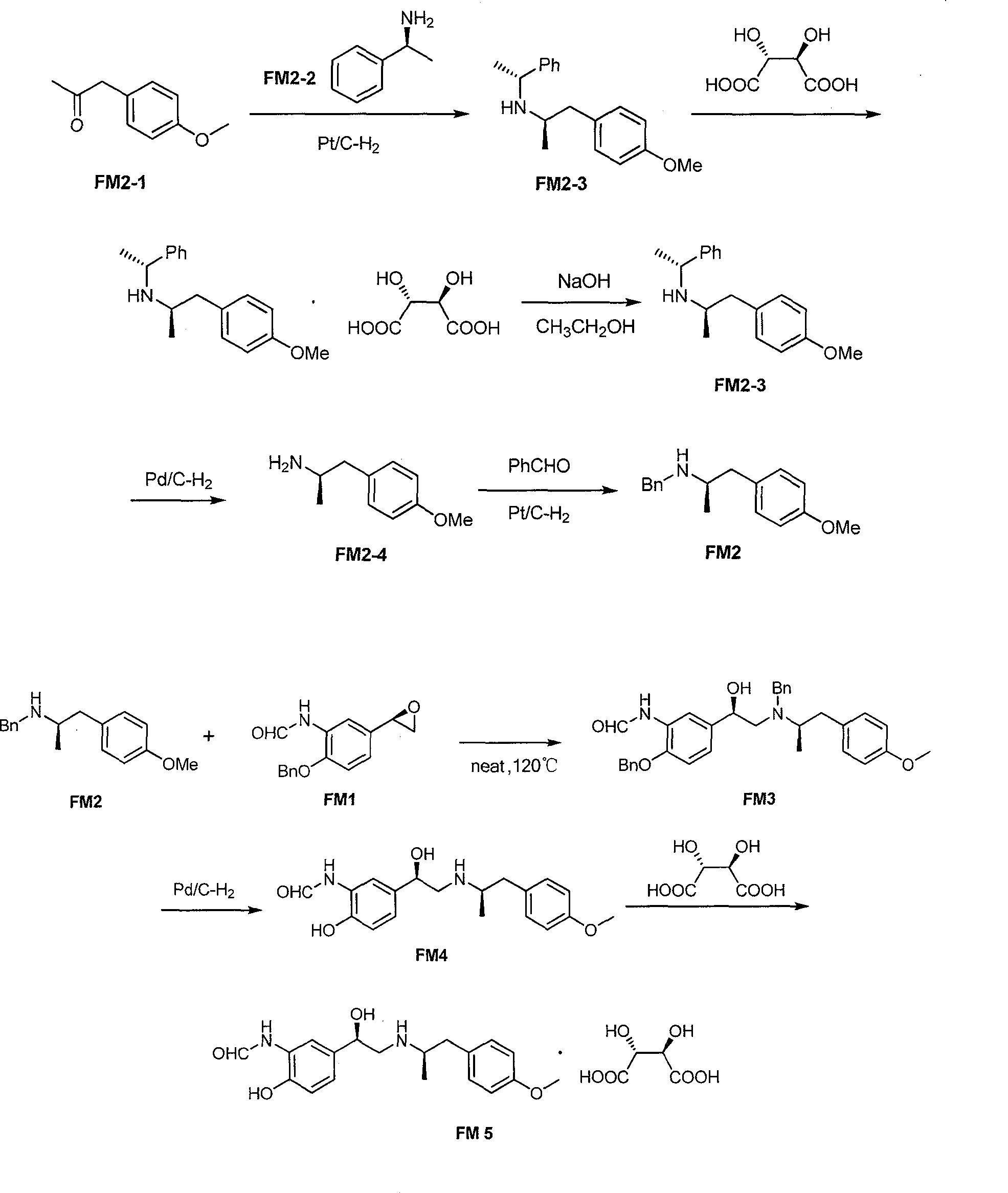
Asymmetric synthesis method of (R,R)-formoterol tartrate
InactiveCN103664677AHigh yieldHigh enantioselectivityCarboxylic acid amides optical isomer preparationCarboxylic acid salt preparationSynthesis methodsBenzaldehyde
The invention relates to an asymmetric synthesis method of (R,R)-formoterol tartrate, which comprises the following steps: by taking (S,S)-CsDPEN and transition metal complex as a catalyst, performing asymmetric hydrogen transfer reaction on alpha-bromoketone used as a raw material, thus obtaining a chiral alcohol intermediate compound; performing reaction steps of nitro-reduction, formylation, cyclization and the like, thus obtaining a key intermediate compound FM 1; by taking Pt / C as a catalyst and alpha-methylphenylethylamine as a chiral assistant, synthesizing an intermediate compound FM 2-3; performing tartaric acid salification, ionization and alpha-methylphenethyl removal, and reacting with benzaldehyde, thus preparing a chiral amine intermediate compound FM 2; reacting and coupling the two key intermediate compounds, and performing protective group removal to obtain (R,R)-formoterol FM 4; and performing tartaric acid salification on the FM 4, thus preparing the target product (R,R)-formoterol tartrate FM 5. According to the invention, the (R,R)-formoterol is synthesized through an asymmetric hydrogen transfer method by means of the chiral assistant, and high yield and favorable ee value are achieved. Compared with a chemical resolution method for synthesizing chiral formoterol, the method provided by the invention has the advantages of high overall yield, mild reaction conditions, low cost and the like, thereby being beneficial to industrial production.
Owner:SUN YAT SEN UNIV +1
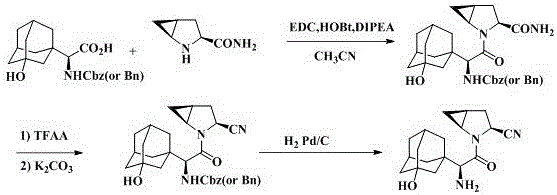
Amino-protected 3-hydroxy adamantane glycine benzothiazole-2-thiol active ester as well as preparation method and application thereof
ActiveCN106349185AIncrease structural diversityCheap and easy to getOrganic chemistryChemical recyclingSaxagliptinChemical structure
The invention relates to an amino-protected 3-hydroxy adamantane glycine benzothiazole-2-thiol active ester as well as a preparation method and application thereof. The thiol active ester is prepared by virtue of reaction between amino-protected 3-hydroxy adamantane glycine and dibenzothiazyl disulfide. The invention further discloses application of the compound in the preparation of a saxagliptin intermediate and saxagliptin. The invention provides a brand new chemical structure. The preparation method is simple and low in cost; the compound is applicable to the preparation of saxagliptin, so that the preparation process can be effectively simplified; and the reaction is mild, and the compound has a wide generalization prospect.
Owner:艾博仕医药科技石家庄有限公司
Novel packaging paper
InactiveCN105133432AWide range of raw materialsMild responseFlexible coversWrappersGlass fiberEngineering
The invention discloses novel packaging paper, which is prepared from the following components by weight: glycerin, corn starch, glass fibers, cunninghamia lanceolata powder and bentonite. According to the present invention, the novel packaging paper has advantages of wide raw materials, mild reaction, no toxicity, environmental protection and the like, and the green chemistry concept in the modern requirement is met; compared with the general packing paper, the produced packaging paper of the present invention has high tensile strength and high folding resistance, and is suitable for a variety of occasions; and the produced packaging paper has high printing adaptability, is suitable for a variety of surface printing, and further has characteristics of extremely high tensile resistance, complete degradation, and significant bacterial inhibition effect.
Owner:HEFEI LONGFA PACKING CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com