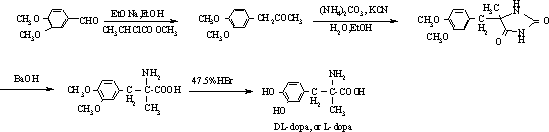
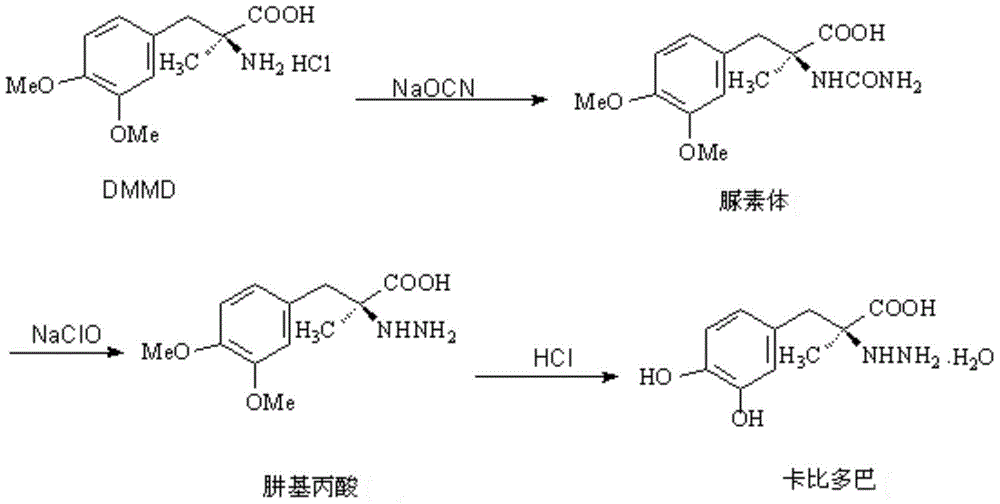
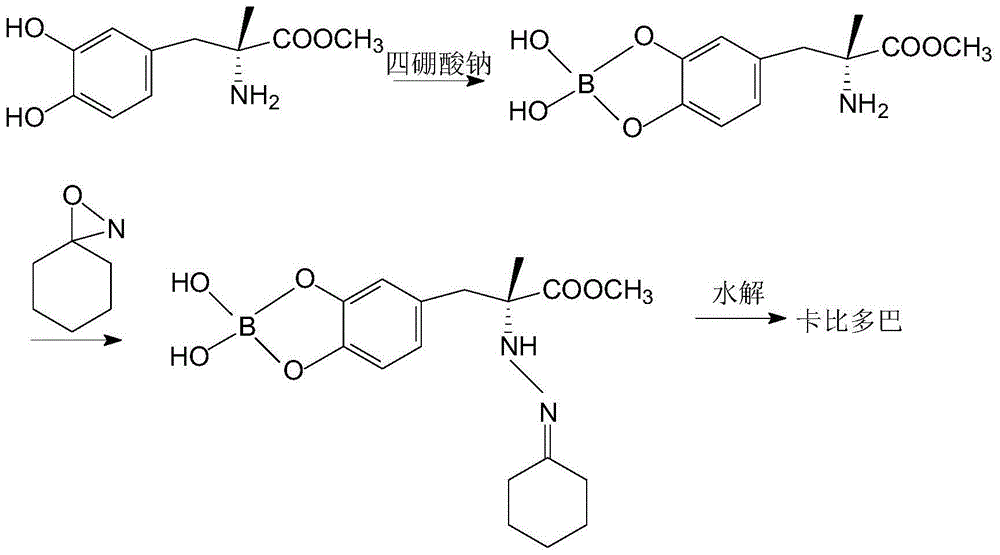
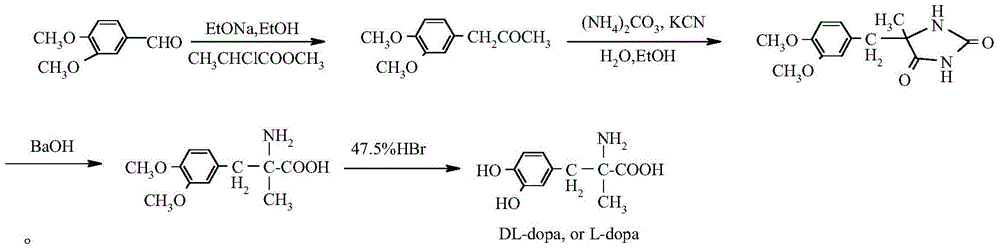
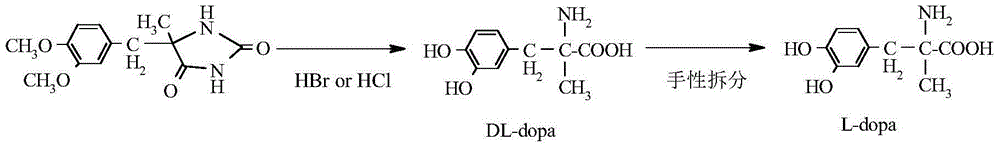
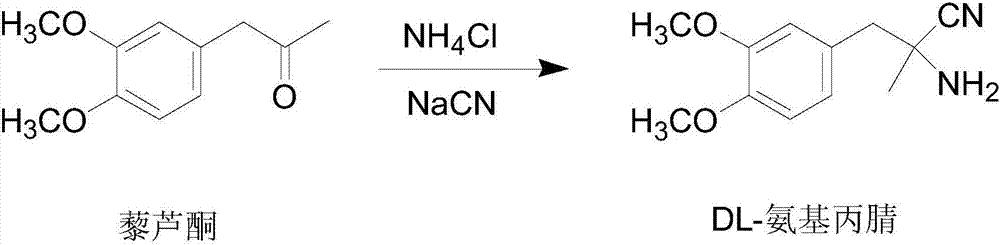
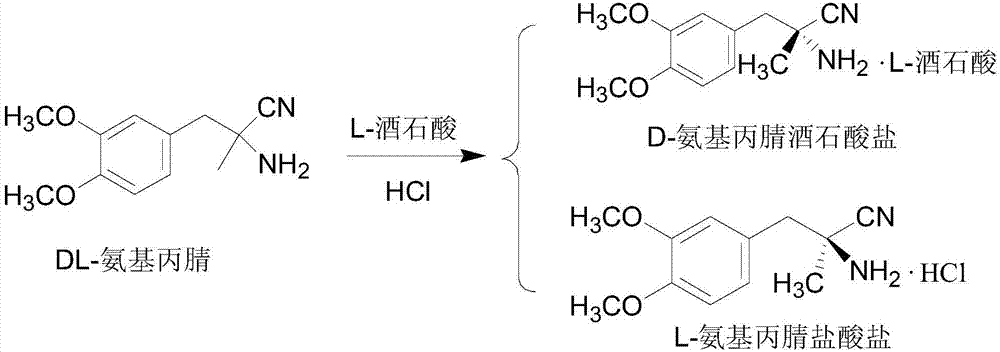
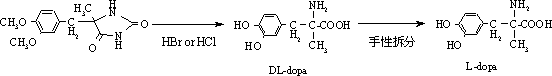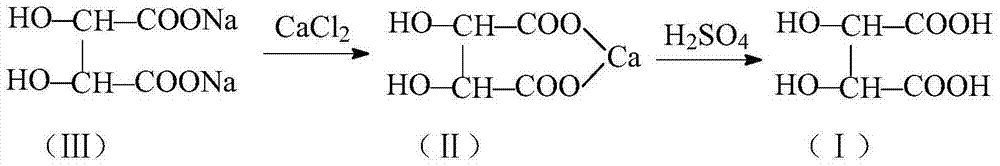Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
36 results about "Methyldopa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used alone or with other medications to treat high blood pressure (hypertension).
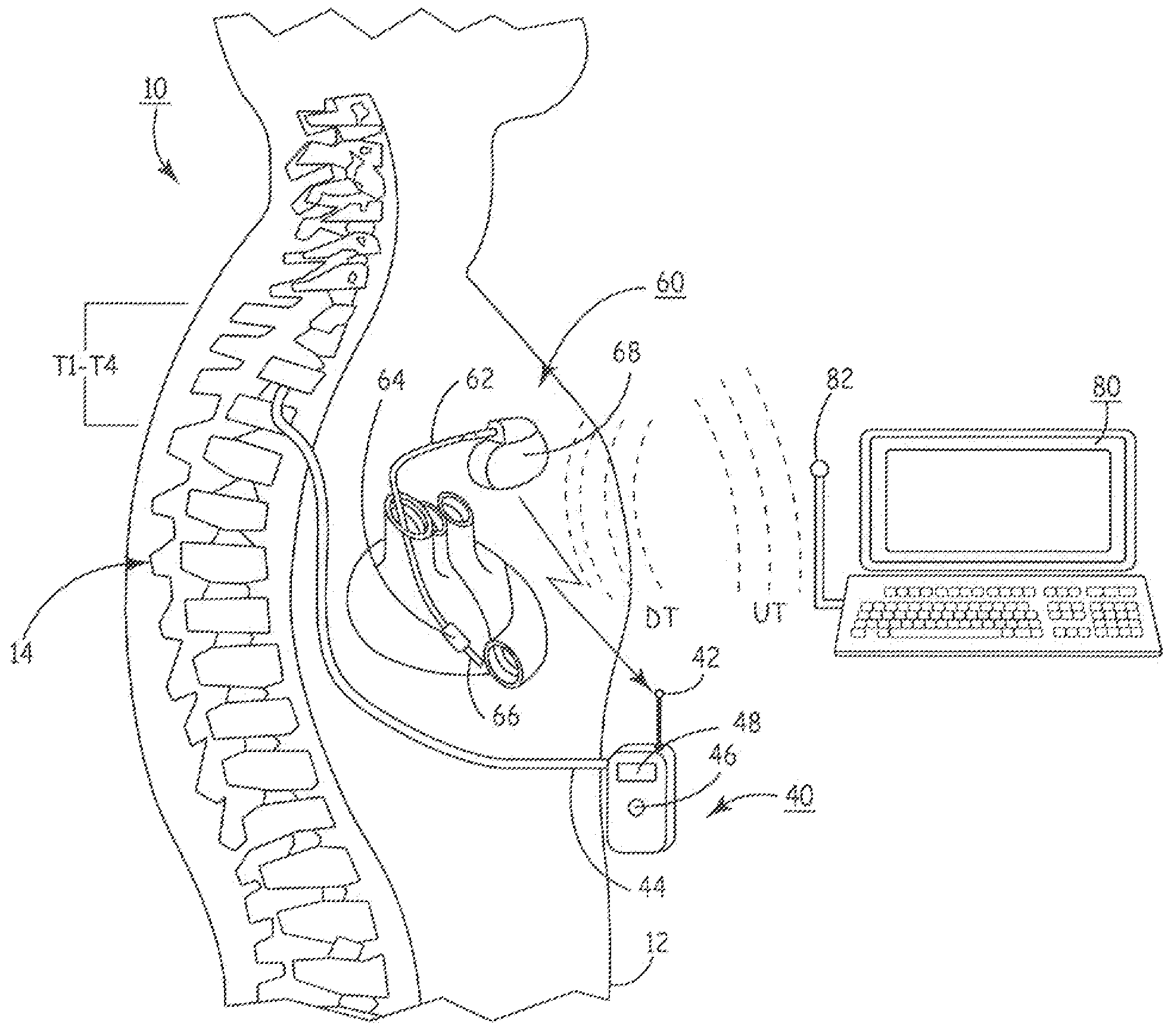
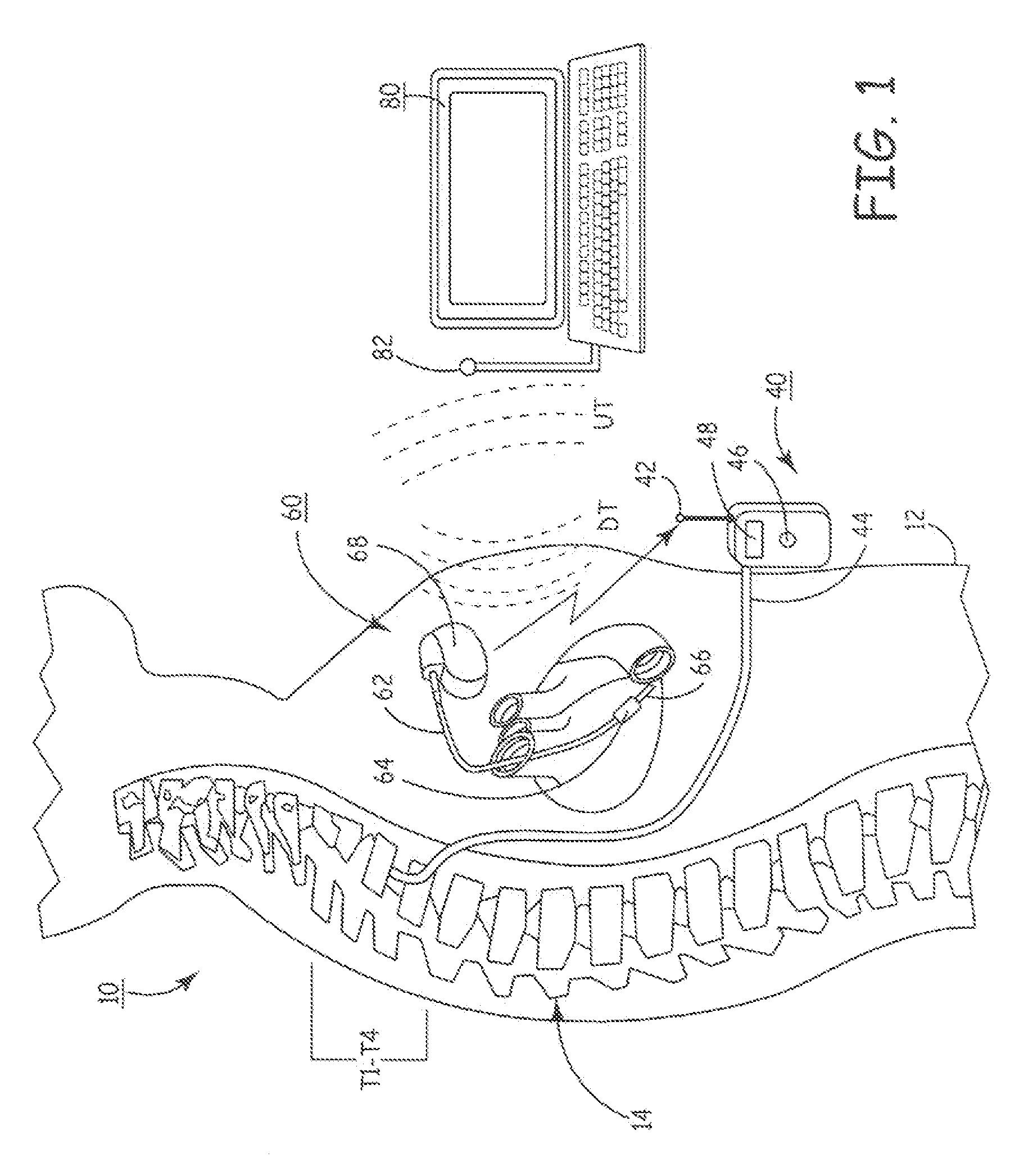
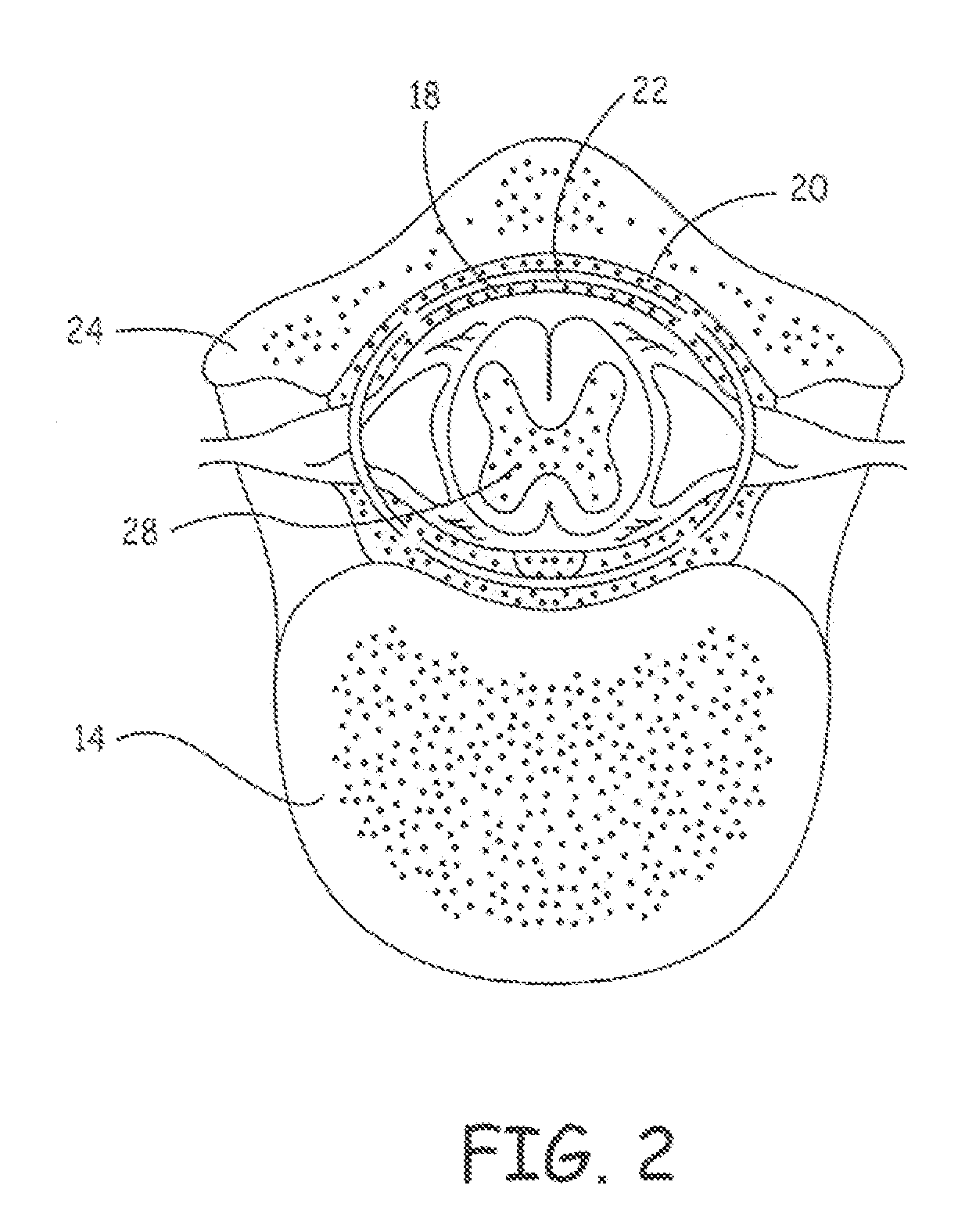
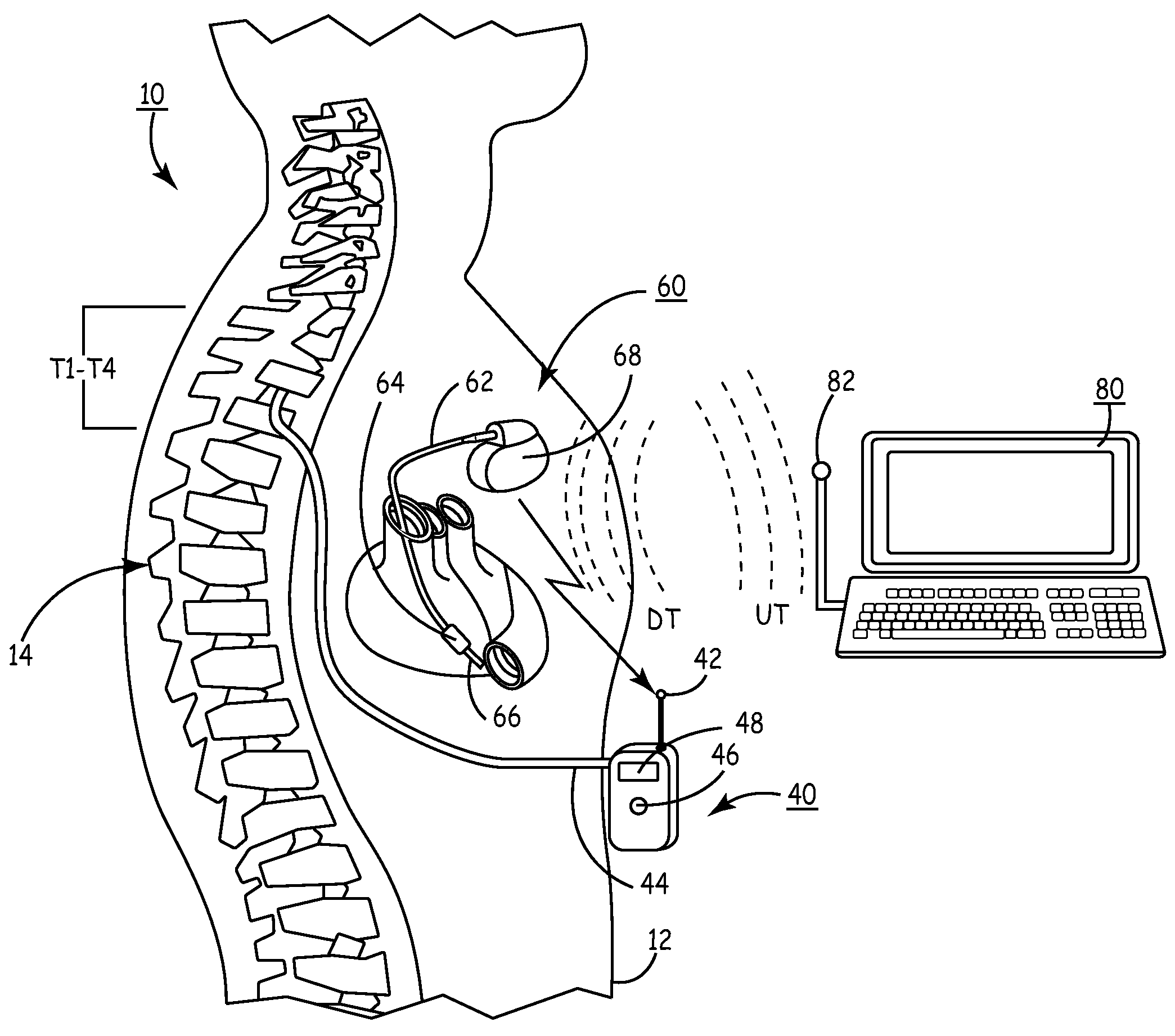
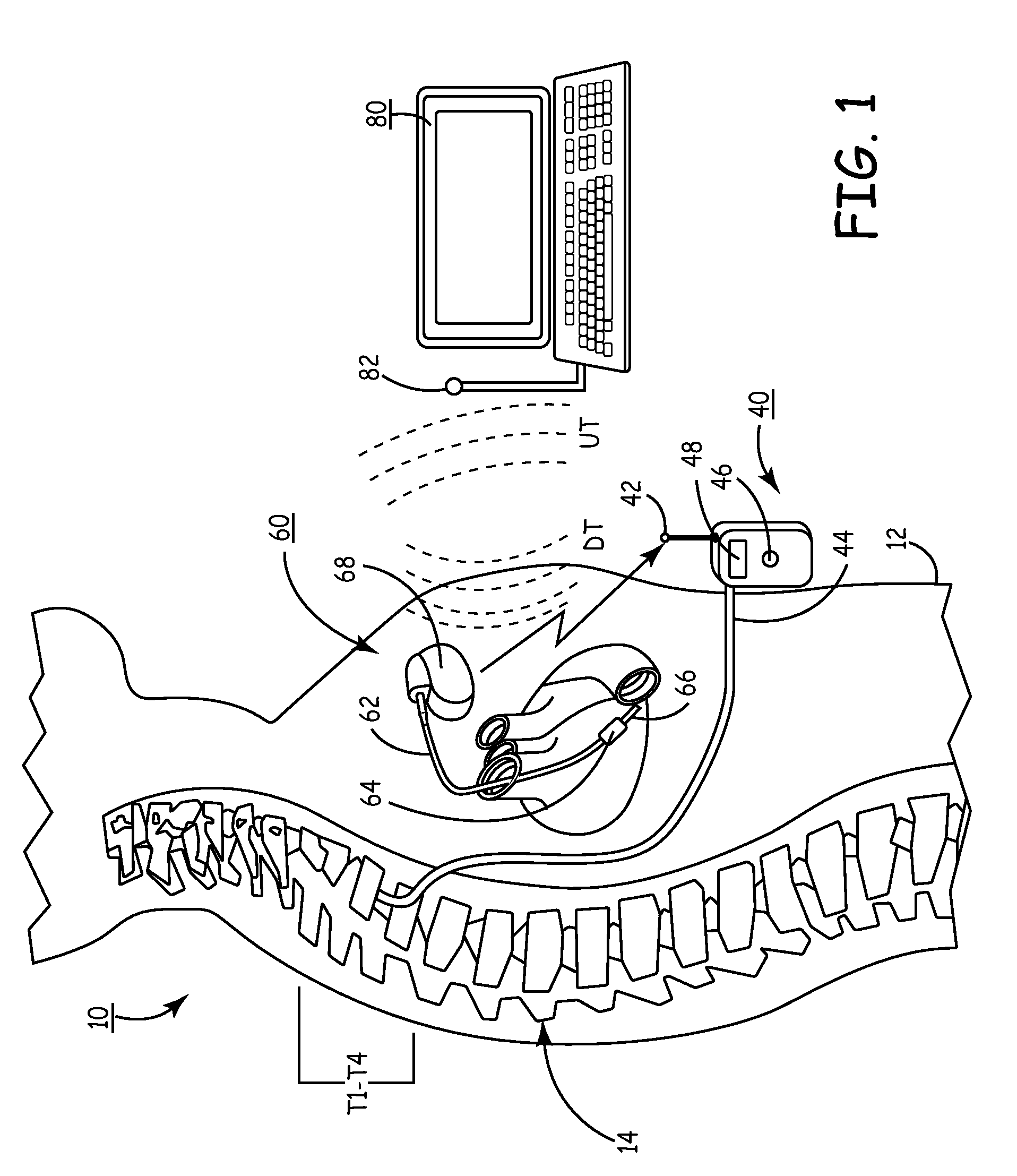
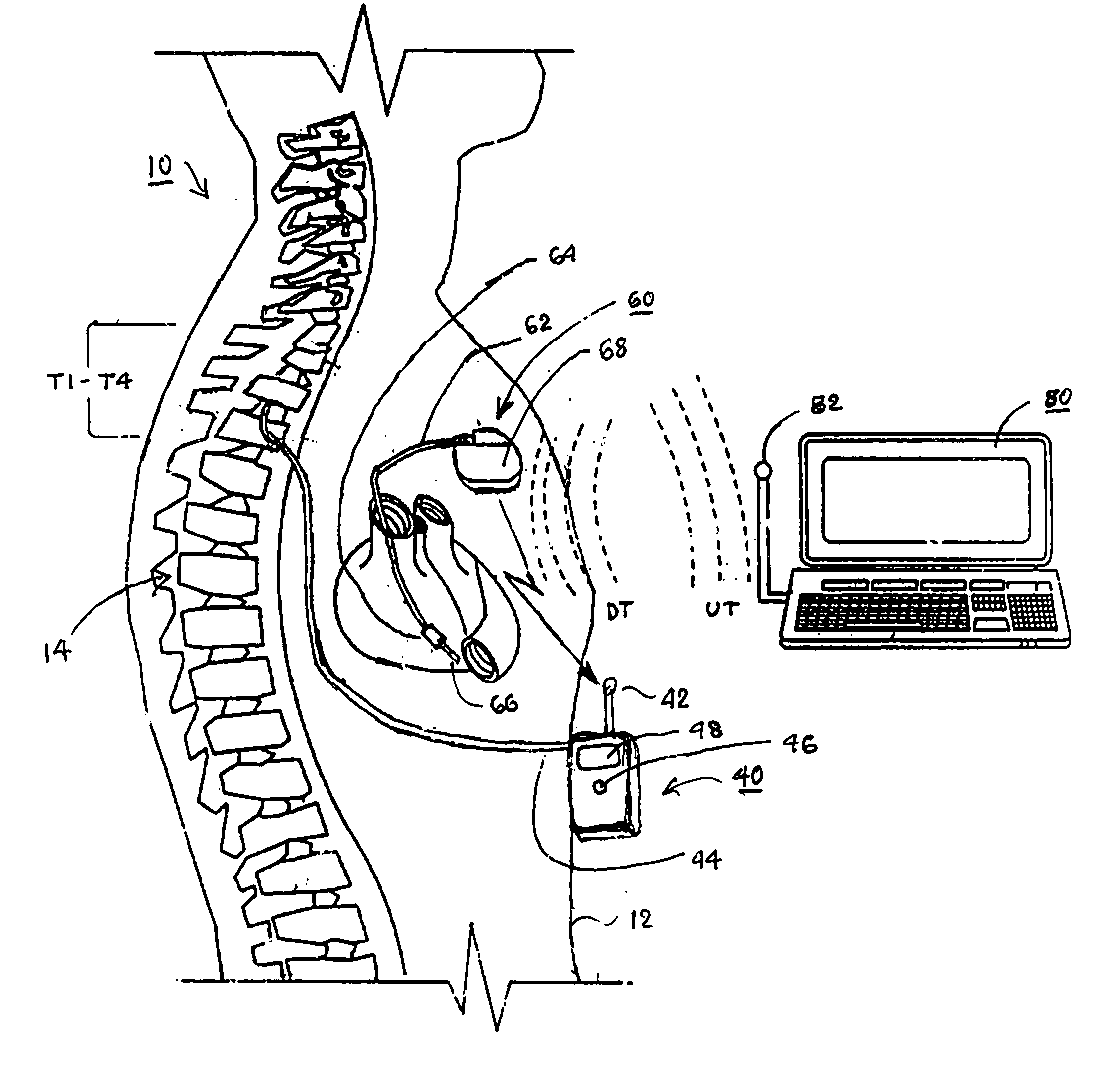
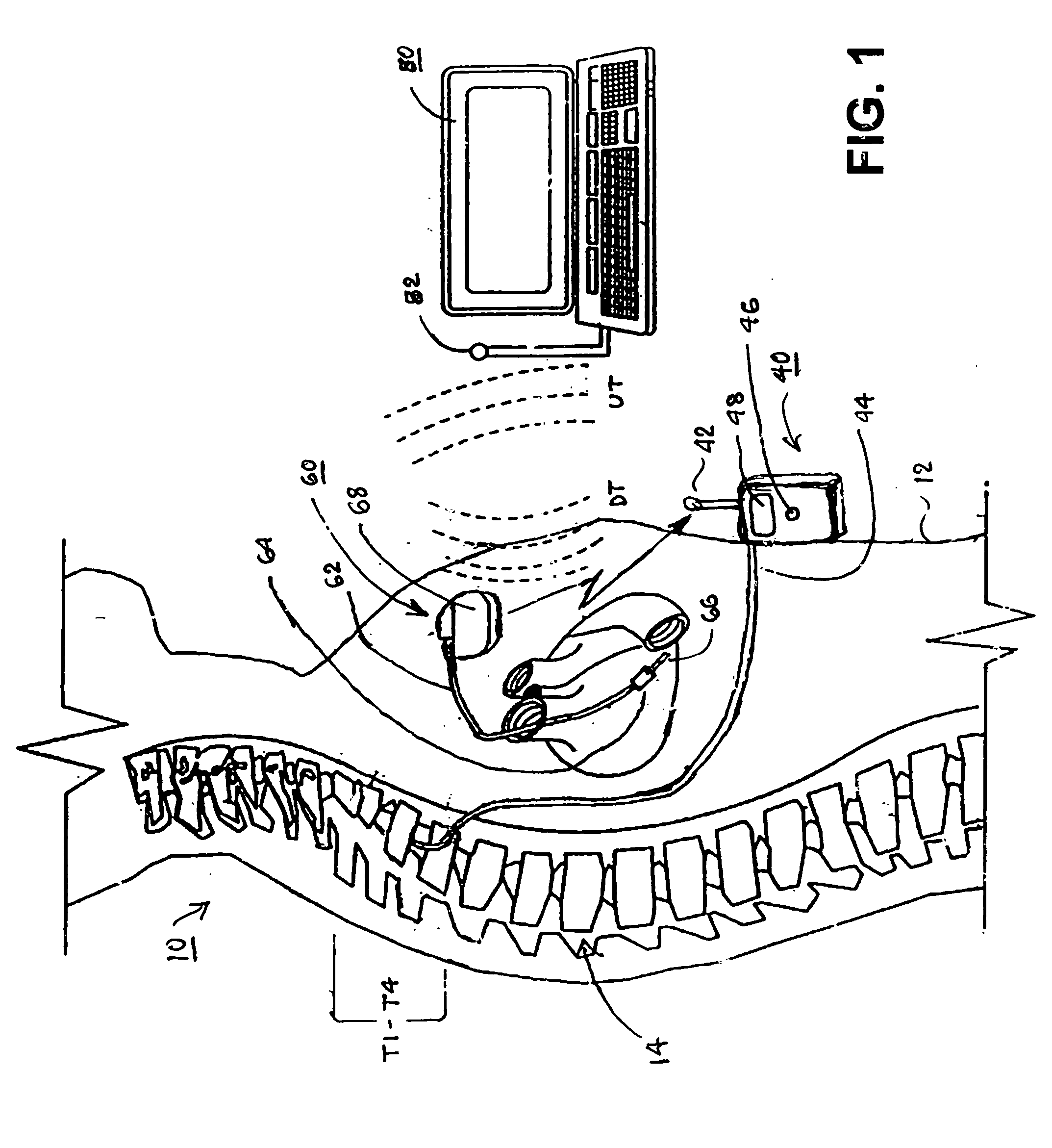
Delivery of a sympatholytic cardiovascular agent to the central nervous system
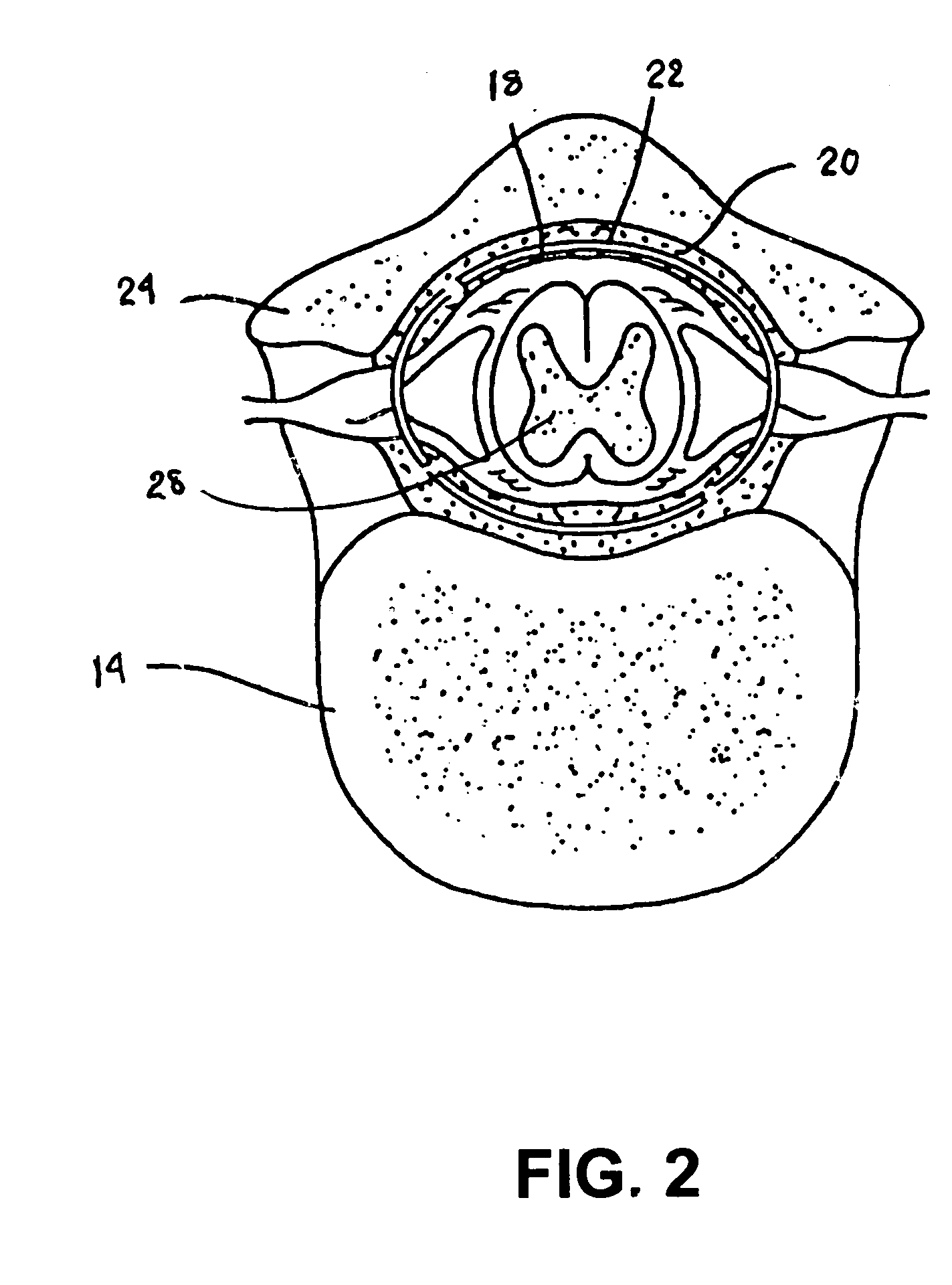
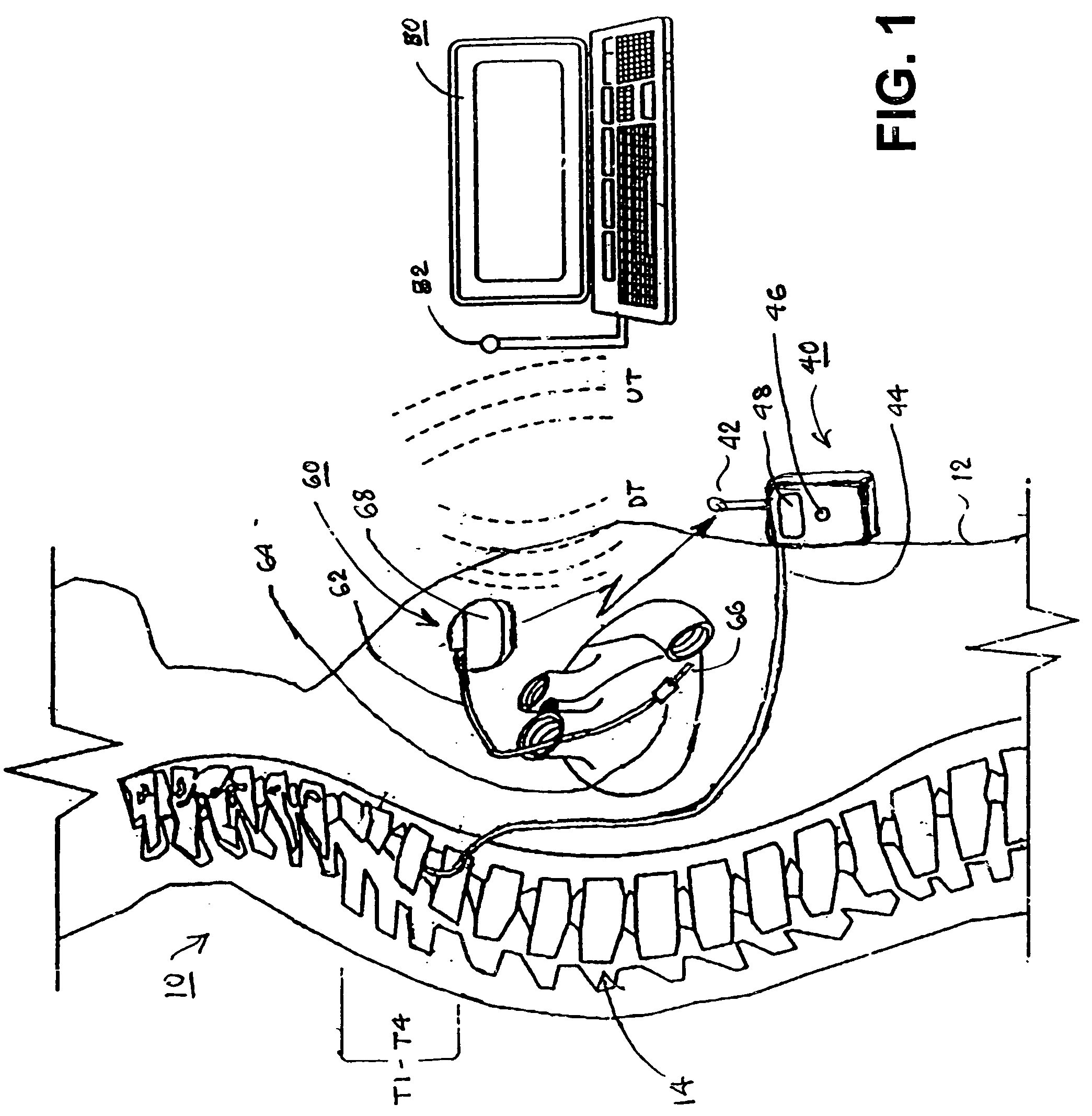
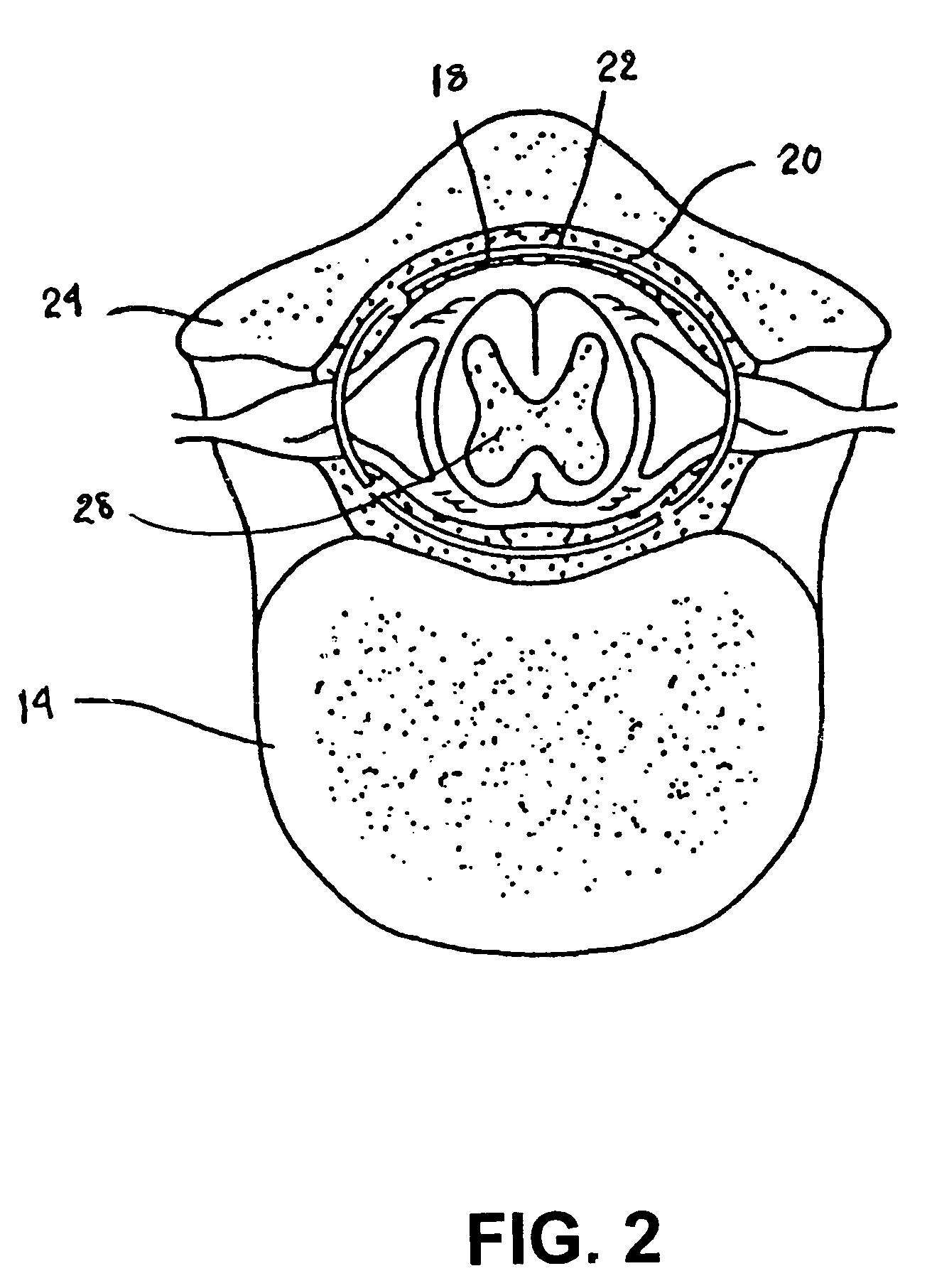
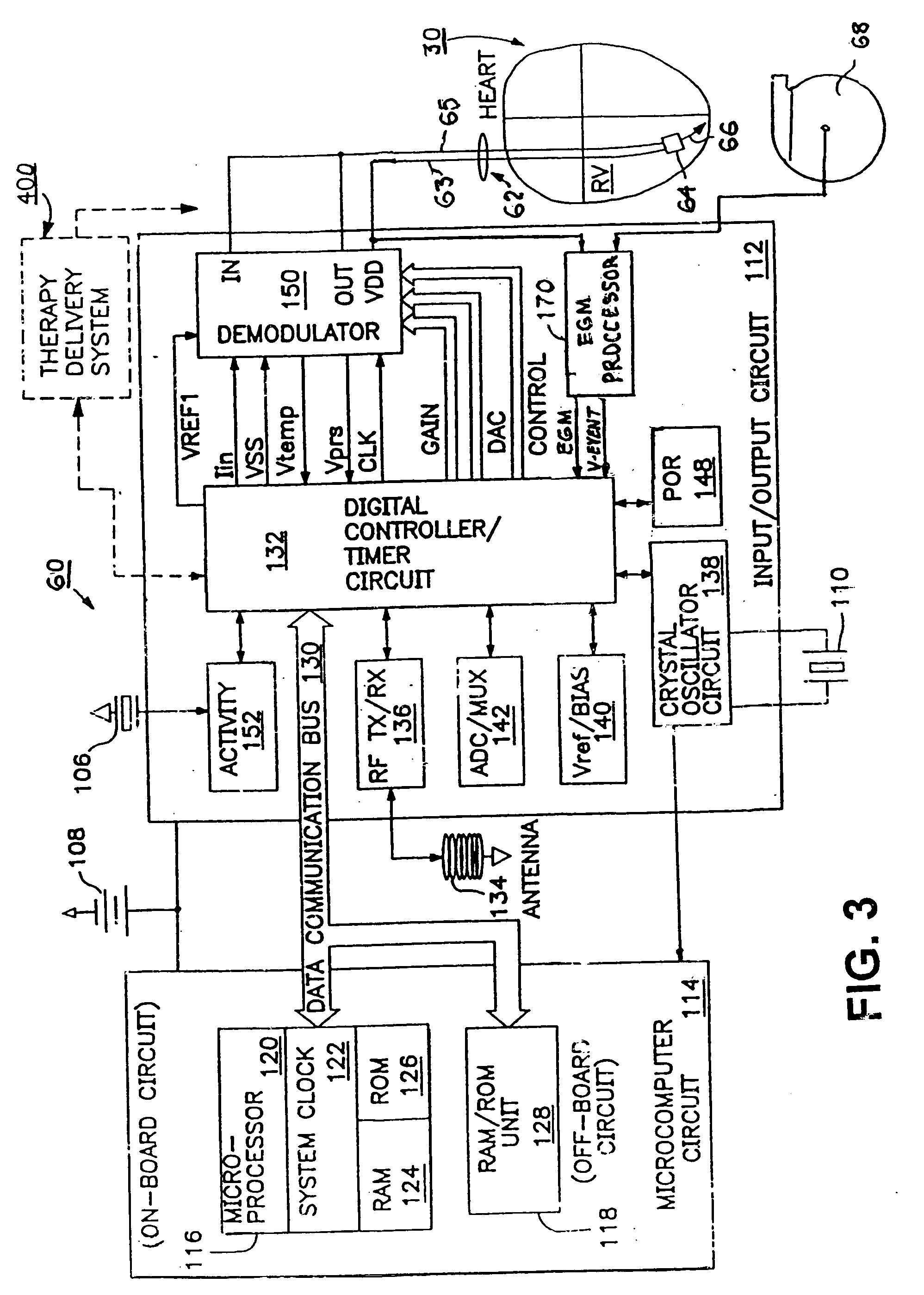
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms of acute or chronic cardiac insult or impaired cardiac performance. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with cardiac insult or impaired cardiac performance and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist (e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine).
Owner:MEDTRONIC INC
Delivery of a sympatholytic cardiovascular agent to the central nervous system to counter heart failure and pathologies associated with heart failure
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms and otherwise treat heart failure (HF) and pathologies associated with HF. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with HF (or pathologies associated with HF) and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist, e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine.
Owner:MEDTRONIC INC
Delivery of a sympatholytic cardiovascular agent to the central nervous system to counter heart failure and pathologies associated with heart failure
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms and otherwise treat heart failure (HF) and pathologies associated with HF. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with HF (or pathologies associated with HF) and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist, e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine.
Owner:MEDTRONIC INC
Delivery of a sympatholytic cardiovascular agent to the central nervous system
A sympatholytic cardiovascular agent delivered by a drug delivery pump to a central nervous system site to alleviate symptoms of acute or chronic cardiac insult or impaired cardiac performance. The drug delivery pump can be external or implantable infusion pump (IIP) coupled with a drug infusion catheter extending to the site. A patient activator can command delivery of a dosage and / or an implantable heart monitor (IHM) coupled with a sensor can detect physiologic parameters associated with cardiac insult or impaired cardiac performance and trigger dosage delivery. The IIP and IHM can be combined into a single implantable medical device (IMD) or can constitute separate IMDs that communicate by any of known communication mechanisms. The sympatholytic cardiovascular agent is one of the group consisting of an alpha-adrenergic agonist and an alpha2-adrenergic agonist (e.g., clonidine, p-aminoclonidine, guanabenz, lidamidine, tizanidine, moxonidine, methyldopa, xylazine, guanfacine, detomidine, medetomidine, and dexmedetomidine).
Owner:MEDTRONIC INC
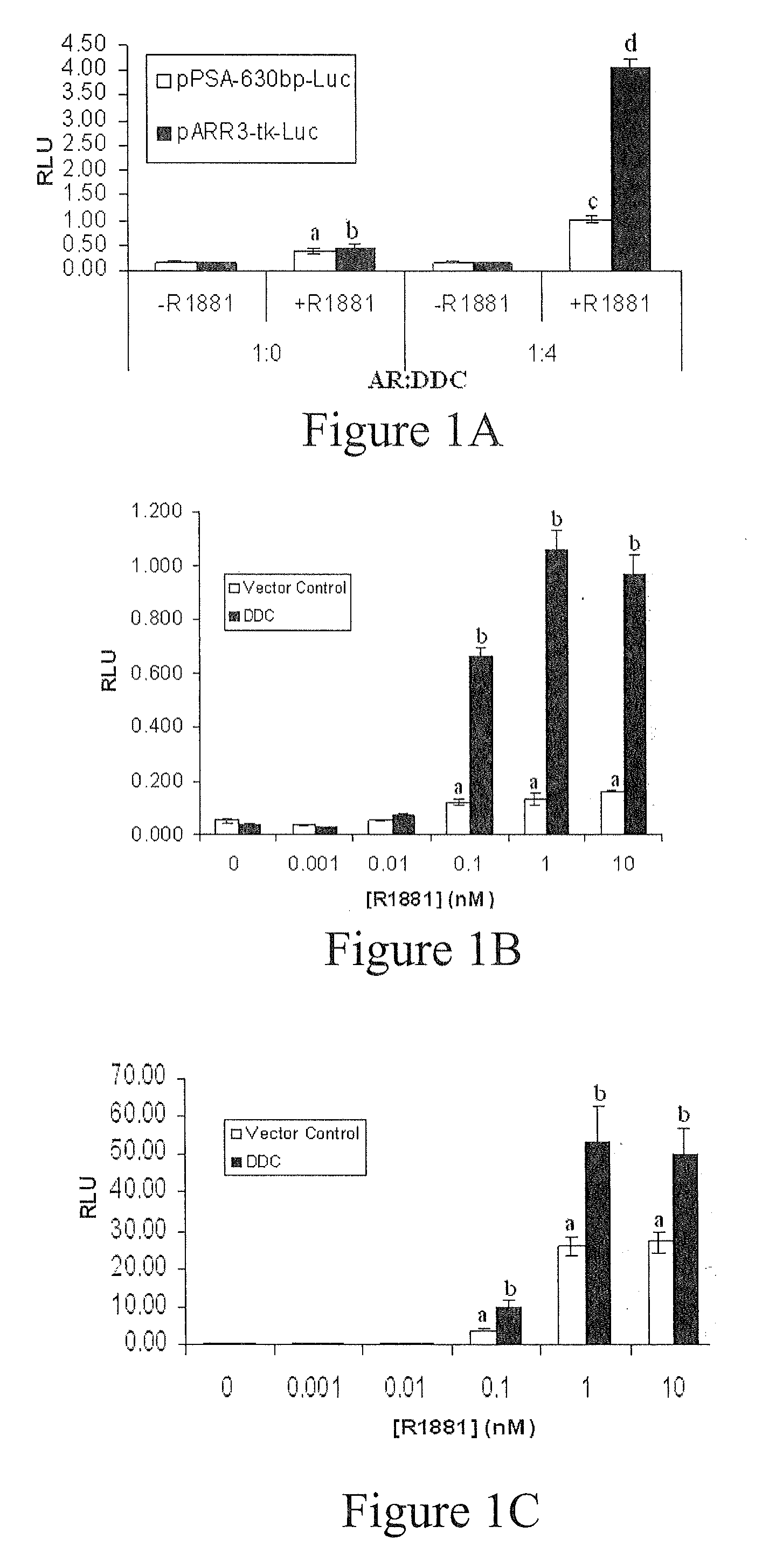
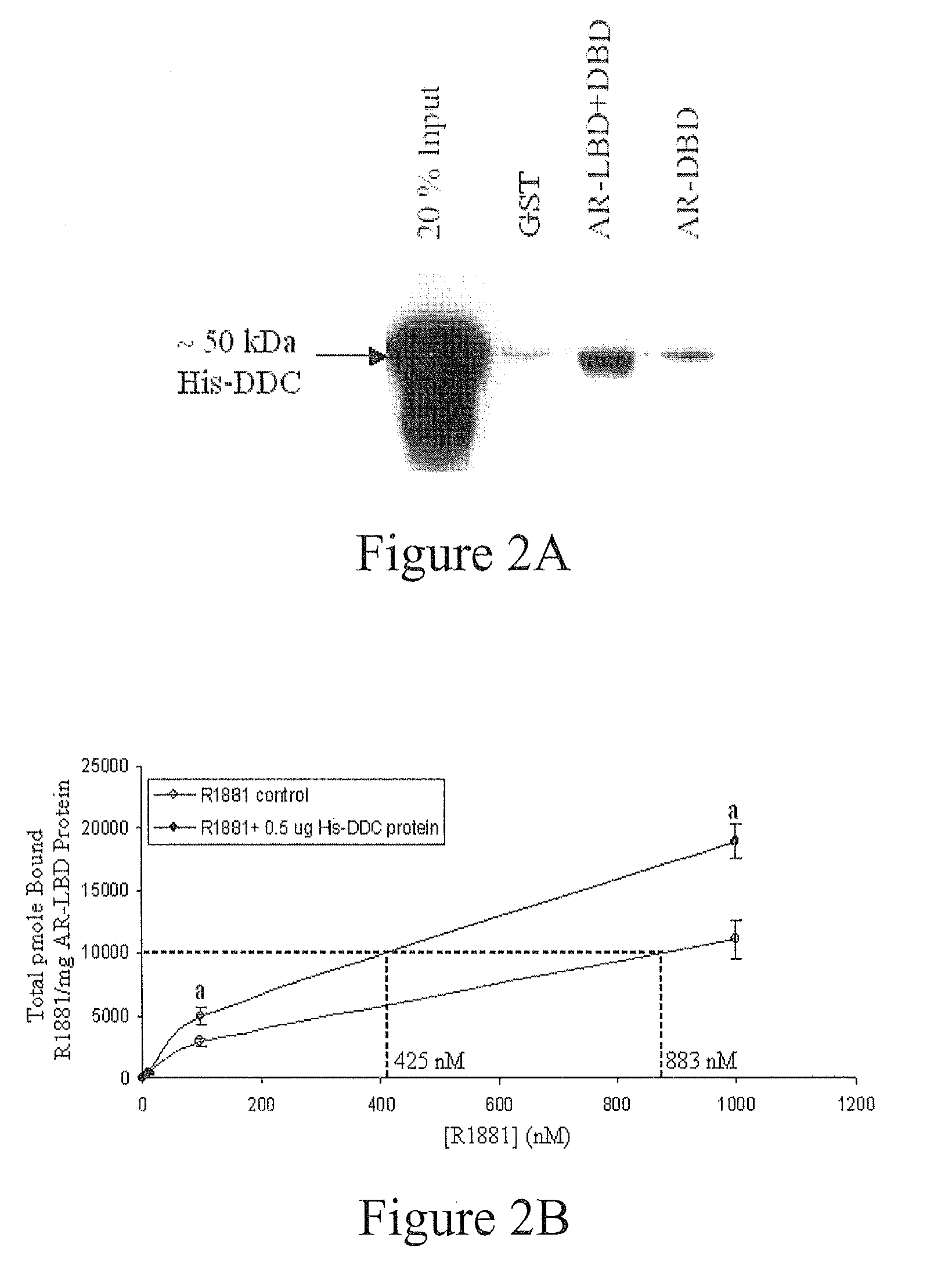
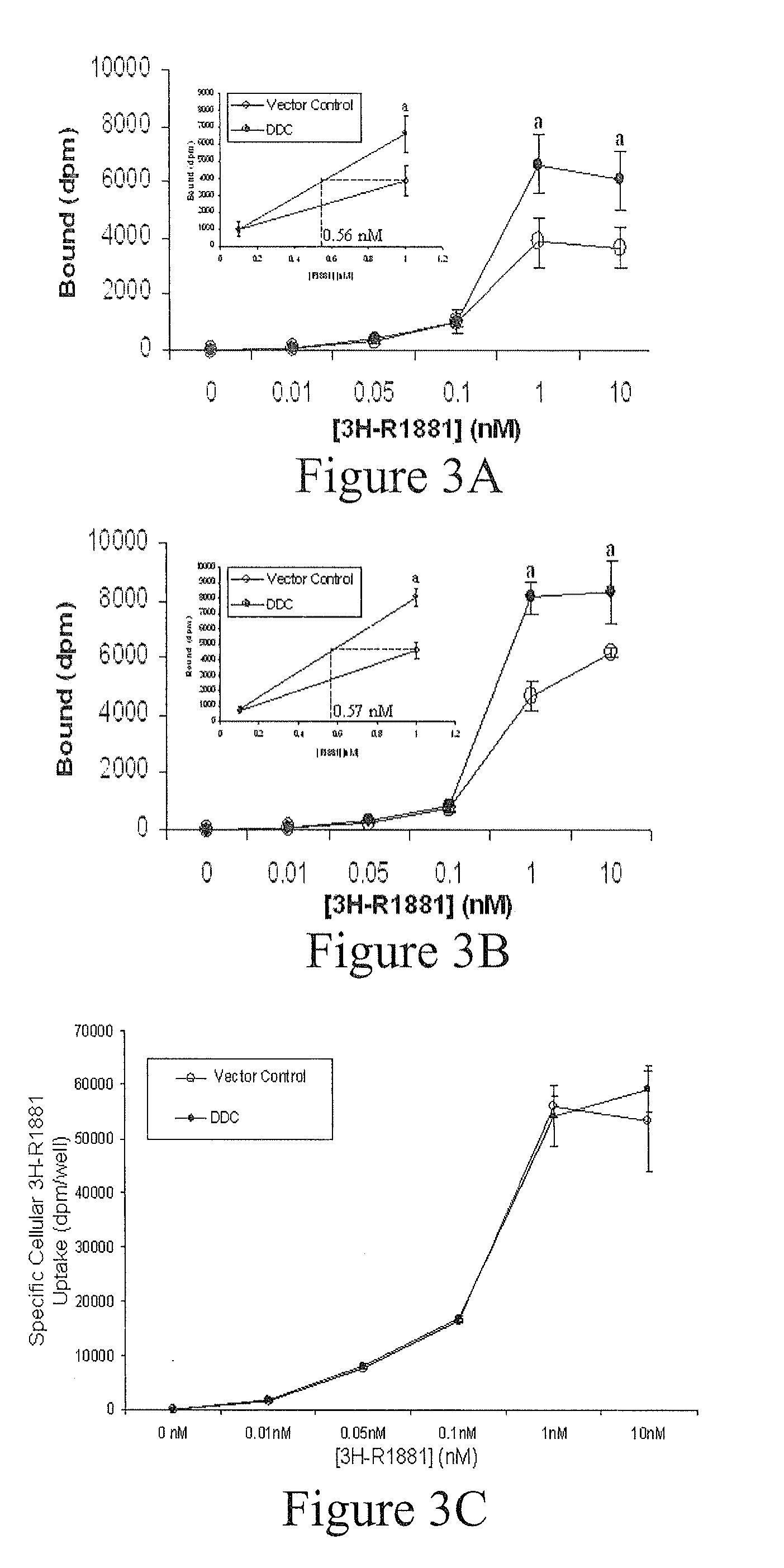
Treatment of Prostate Cancer with DDC Inhibitor
ActiveUS20100048709A1Low affinityBiocidePeptide/protein ingredientsMultiple formsDihydroxyphenylalanine
Owner:THE UNIV OF BRITISH COLUMBIA
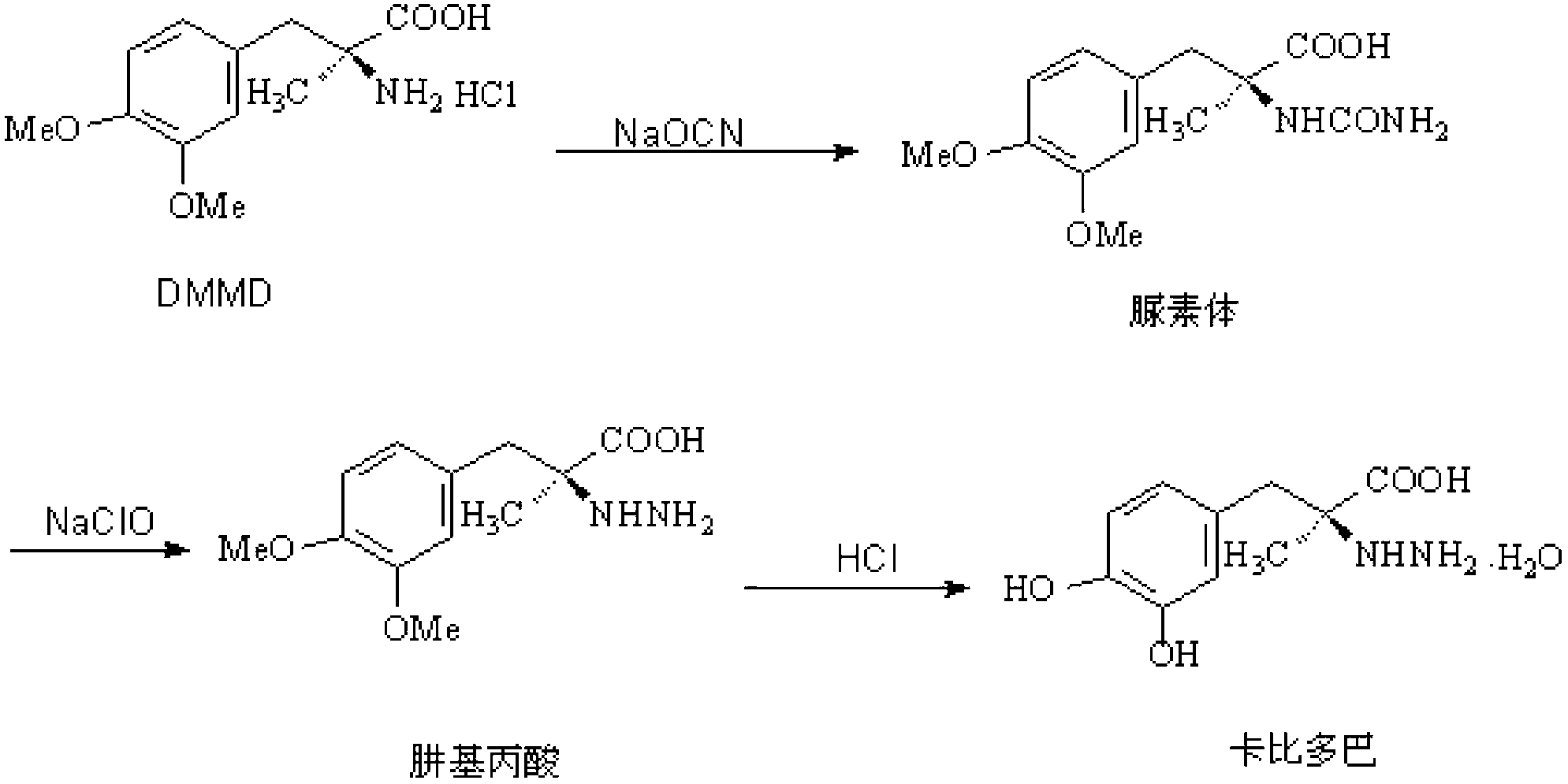
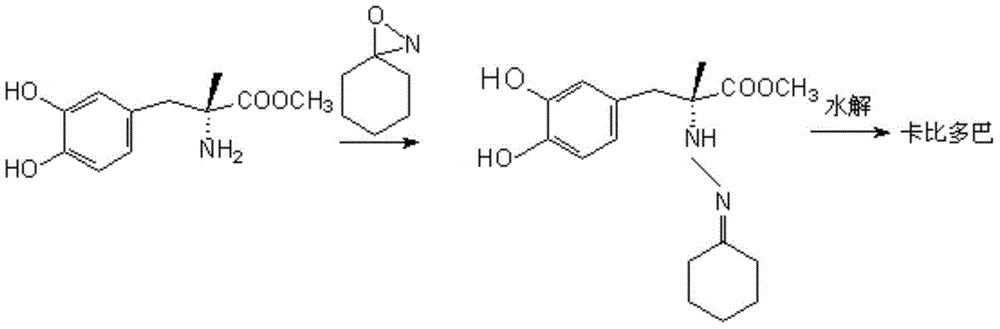
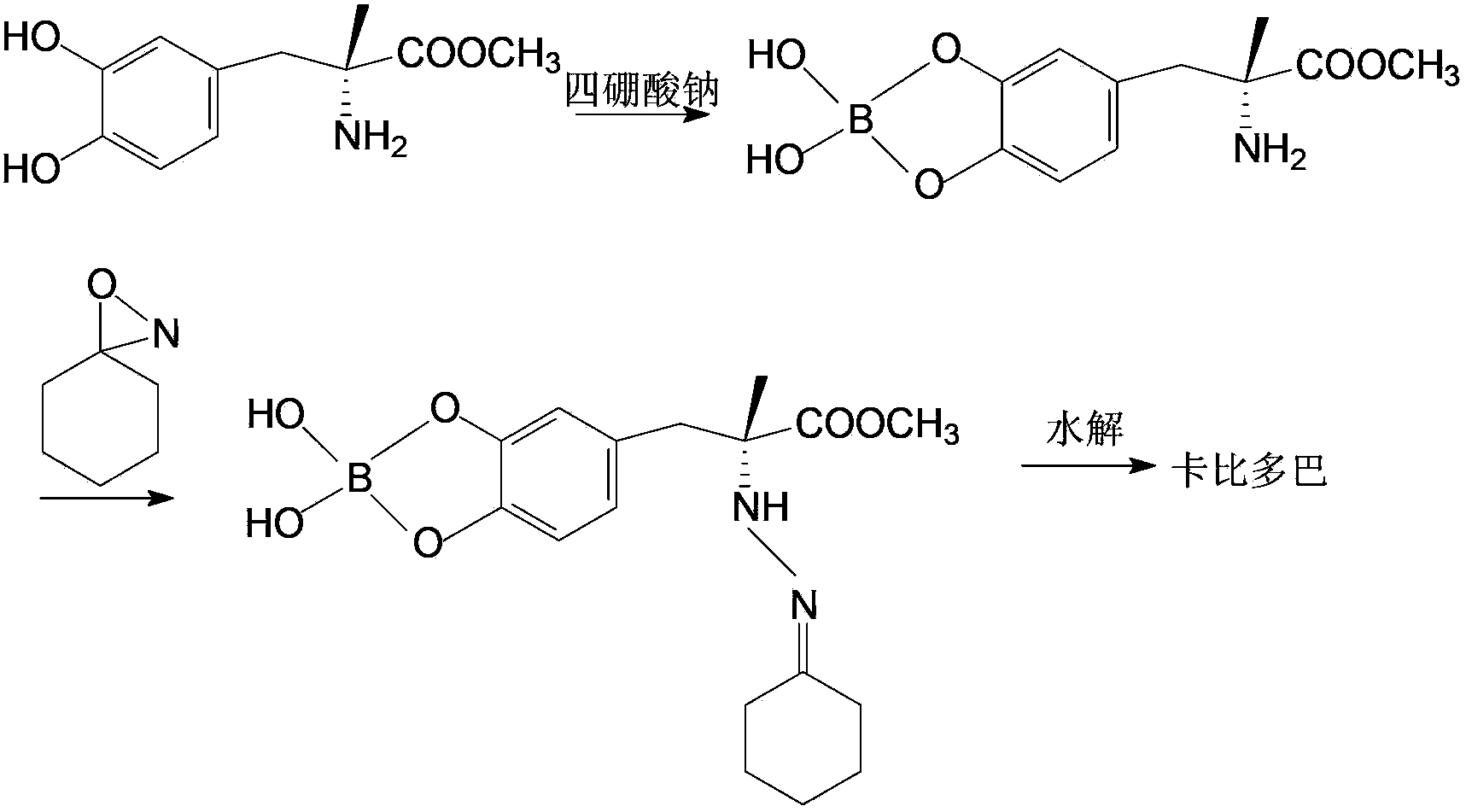
Method for synthesizing carbidopa
ActiveCN102702019AHigh yieldImprove product qualityHydrazine preparationChemical synthesisMethyldopate
The invention belongs to the field of chemical synthesis, and in particular relates to a method for synthesizing carbidopa. The method is characterized by comprising the following steps of: reacting oxaziridine with methyldopa ester to obtain methyldopa imido ester, and hydrolyzing to obtain the carbidopa. The method has the advantages that the carbidopa is prepared by a brand-new process, the used raw materials are favorable for synthesizing a medicine, and the prepared carbidopa is high in yield and quality.
Owner:SHANDONG XINHUA PHARMA CO LTD
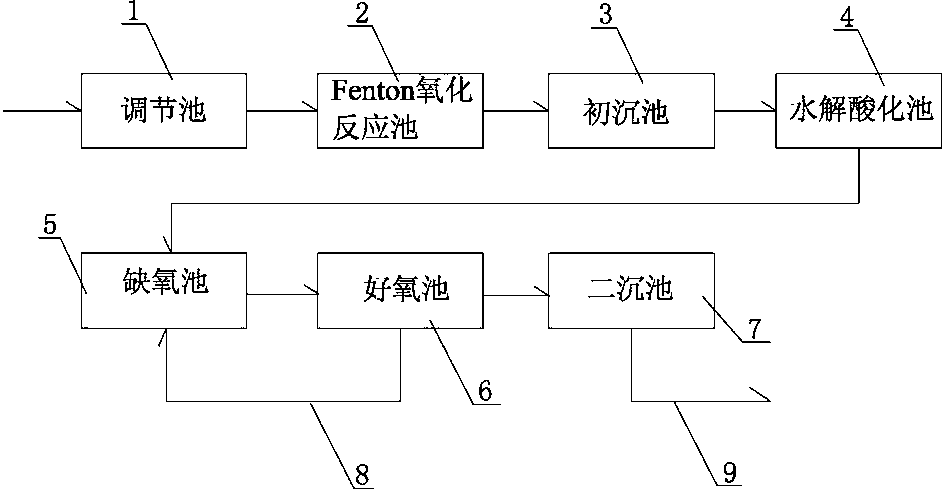
Processing equipment and processing method for methyldopa sewage
InactiveCN103319044AEfficient degradationReduce processing costsMultistage water/sewage treatmentWater/sewage treatment by oxidationProcess equipmentNitrogen
The invention relates to processing equipment and a processing method for methyldopa sewage. The equipment comprises a regulating tank, a Fenton oxidation reaction tank, a primary sedimentation tank, a hydrolysis acidification tank, an anoxia tank, an aerobic tank, and a secondary tank, wherein, the regulating tank, the Fenton oxidation reaction tank, the primary sedimentation tank, the hydrolysis acidification tank, the anoxia tank, the aerobic tank, and the secondary tank are connected in sequence and form integrated processing equipment. The processing equipment and the processing method for methyldopa sewage combine the advantages of advanced oxidation and biological processing together. Ammonia and nitrogen of methyldopa sewage processed by the processing equipment can be effectively degraded, and chroma and COD are decreased. Therefore the methyldopa sewage is processed to meet the demand of discharging into a pipe net. The processing equipment and the processing method for methyldopa sewage have the advantages of low processing cost, simple operation and easiness in control.
Owner:SHAOXING HEQIAO JIANGBIN WATER TREATMENT
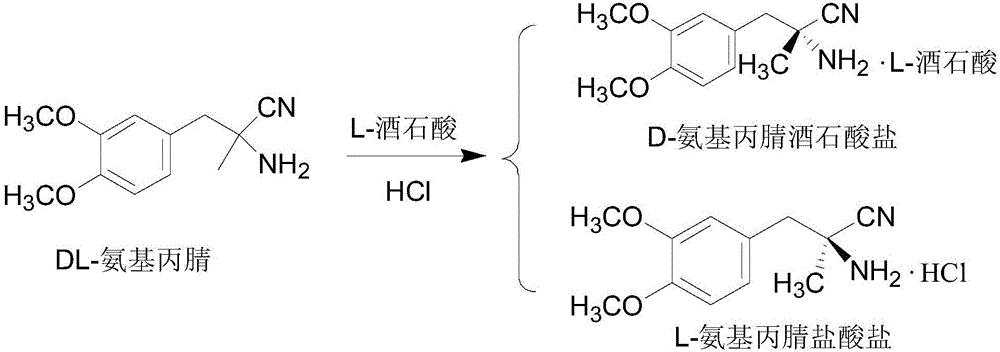
Recycling method and application of L-methyldopa intermediate
ActiveCN105906520AReduce ammonia nitrogenReduce CODOrganic compound preparationAmino-carboxyl compound preparationPropionitrileGreen chemistry
The invention discloses a recycling method and application of an L-methyldopa intermediate. The recycling method comprises the following steps: hydrolyzing L-3-(3,4-dimethoxy phenyl)-2-amino-2-methyl propionitrile hydrochloride by utilizing hydrochloric acid, and filtering to obtaining L-3-(3,4-dimethoxy phenyl)-2-methyl alanine hydrochloride and mother liquor, adding haloid salt or ammonium salt into the mother liquor, carrying out vacuum distillation, concentrating until volume is less than or equal to 1 / 3 of the original volume, and filtering, wherein a filter cake is namely the recycled 3-(3,4-dimethoxy phenyl)-2-methyl alanine hydrochloride solid. The recycling method disclosed by the invention has the advantages that L-methyldopa can be recycled from 3-(3,4-dimethoxy phenyl)-2-methyl alanine hydrochloride production waste liquor, and ammonia nitrogen and COD in wastewater can also be reduced, so that the recycling method accords with the green chemistry concept.
Owner:ZHEJIANG WILD WIND PHARMA
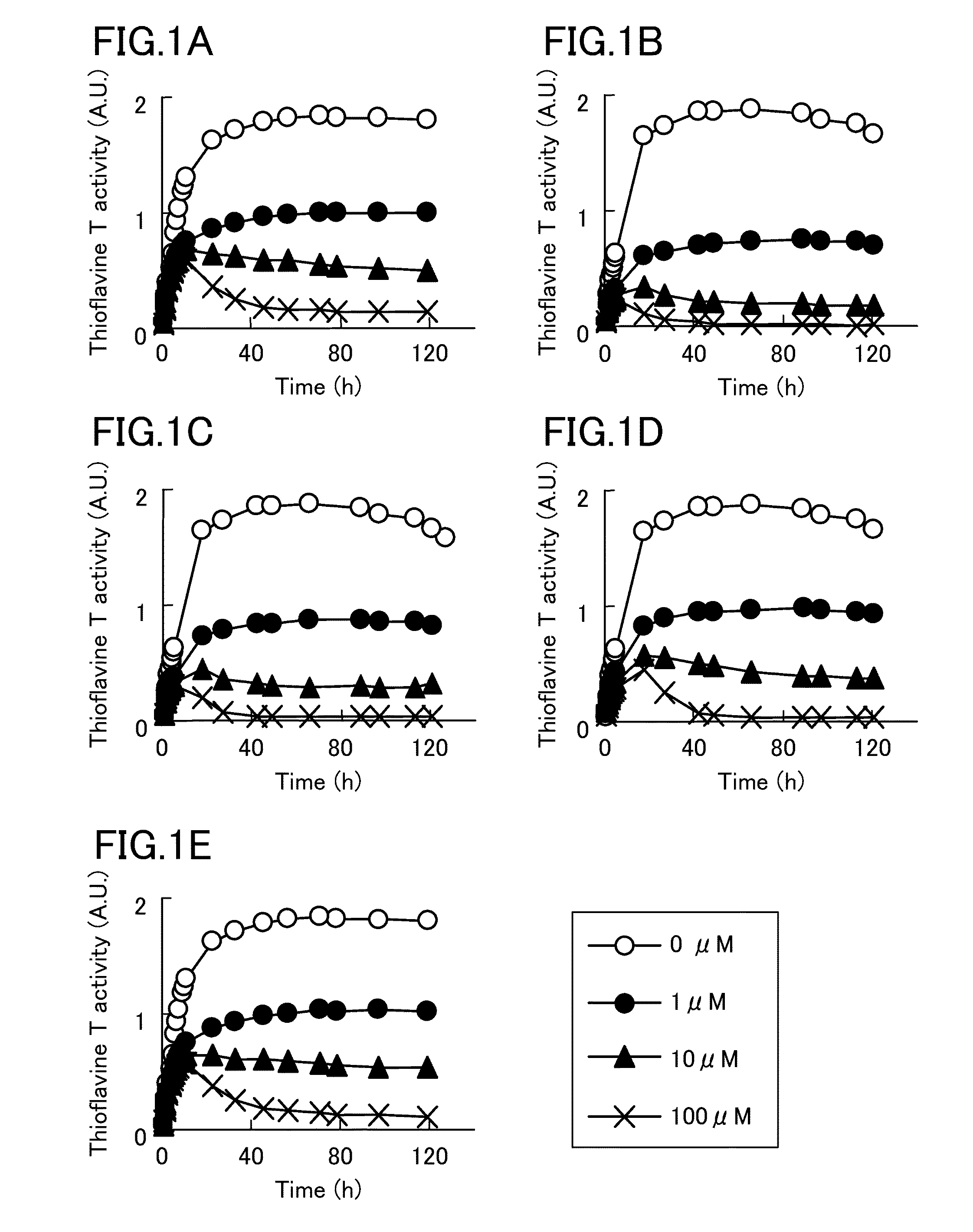
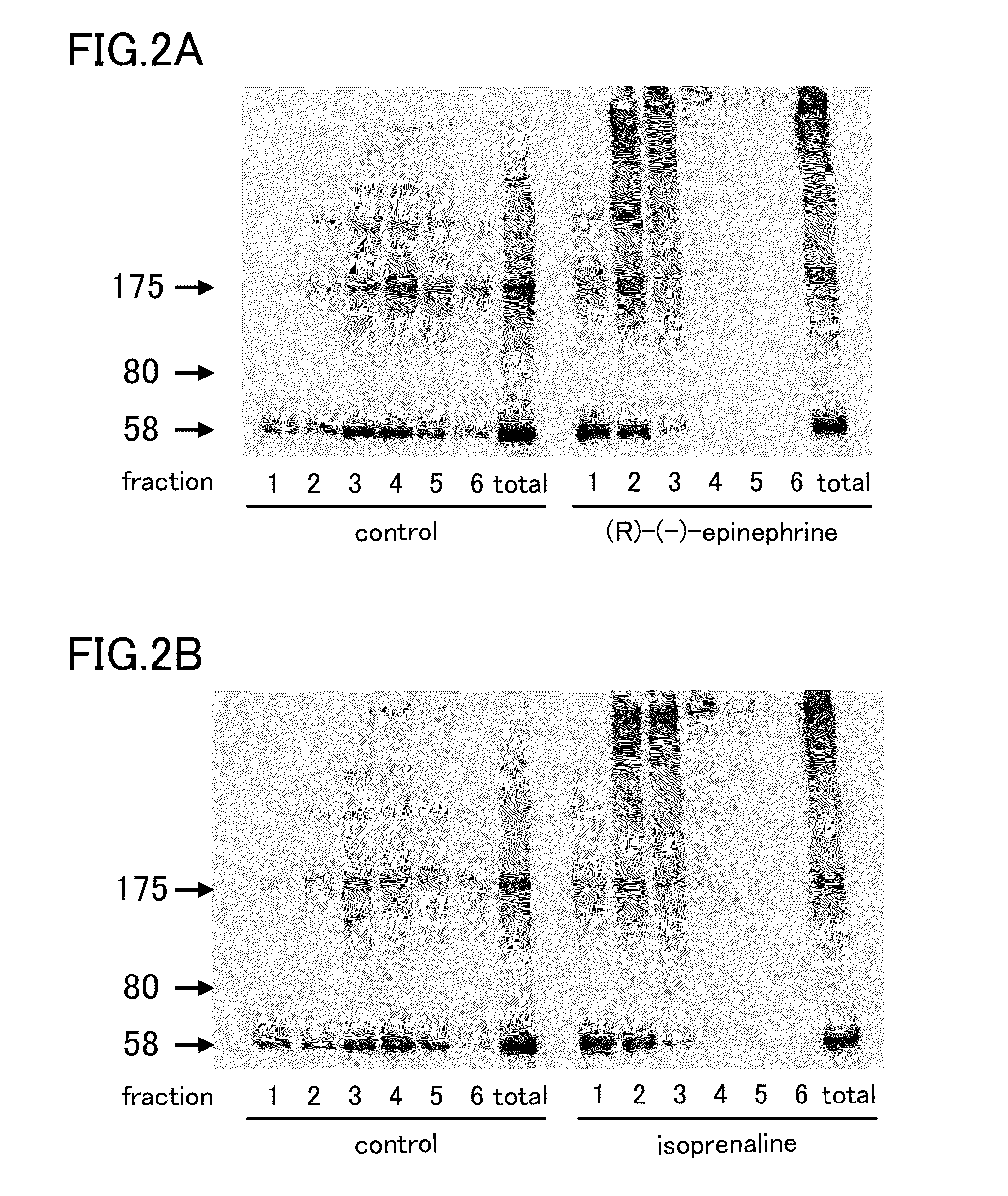
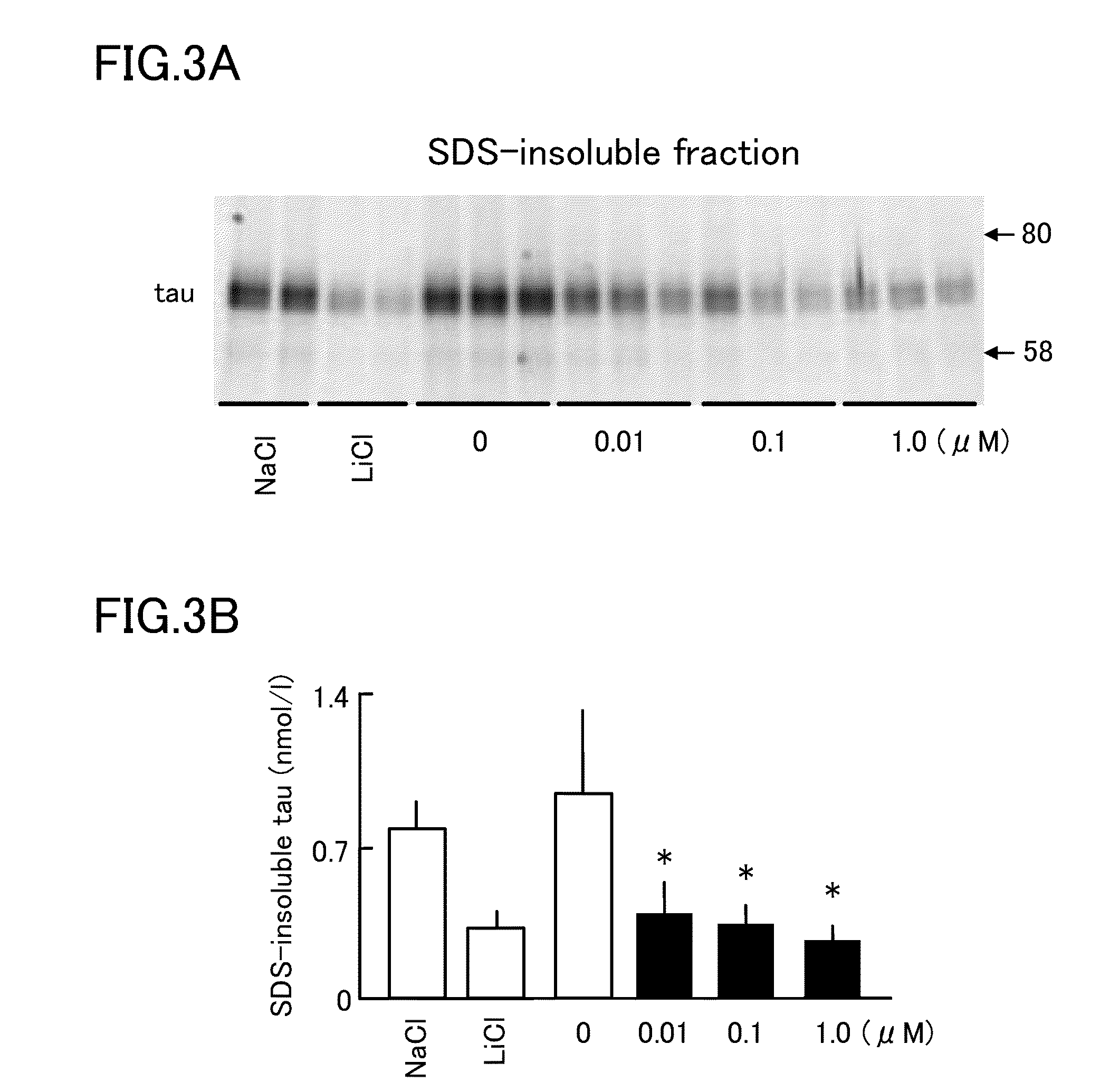
Tau aggregation inhibitor
InactiveUS20140249180A1Reducing aggregation of TauEffective contributionUrea derivatives preparationBiocideDobutamineDepressant
A tau aggregation inhibitor reduces tau aggregation in cells. The tau aggregation inhibitor can include a catechol structure-containing compound or a salt thereof, and the catechol structure-containing compound can be one of isoprenaline, dopamine, dobutamine, levodopa, levodopa / carbidopa, trimetoquinol, hexoprenaline, methyldopa, and droxidopa. One example of the catechol structure-containing compound is isoprenaline, which can be d-enantiomer of isoprenaline or d / l-racemic mixture of isoprenaline. Tauopathies to be prevented or treated by the inhibitor include AD, Down's syndrome, Pick's disease, corticobasal degeneration, and progressive supranuclear palsy.
Owner:NAT CENT FOR GERIATRICS & GERONTOLOGY +1
Method for refining carbidopa
ActiveCN102432496AReduce manufacturing costHigh yieldHydrazine preparationProduct systemNitrogen gas
The invention discloses a method for refining carbidopa, which comprises the following steps: diluting a crude carbidopa product with water, regulating the pH value to below 6.5 with acid, and heating to 50-110 DEG C under the nitrogen protection or / and reducer addition condition, thereby obtaining a crude product system; adding a decolorant into the crude product system to decolorize at 50-110 DEG C for 5-60 minutes; after decolorizing, filtering while the crude product system is hot to obtain a filtrate, stirring and cooling the filtrate to 0-30 DEG C, and continuing stirring at 0-30 DEG C for 0.5-5 hours to obtain a refined system; and filtering the refined system, washing the filter cake with water, and carrying out vacuum drying to obtain the finished carbidopa product. The method disclosed by the invention has the advantages of simple preparation method, favorable controllability, strong operability, high methyldopa (impurity) removal rate, high yield and low production cost, can easily implement industrial production, has high economic benefit in the industrial production, can be easily popularized, and has wide application prospects.
Owner:ZHEJIANG CHIRAL MEDICINE CHEM
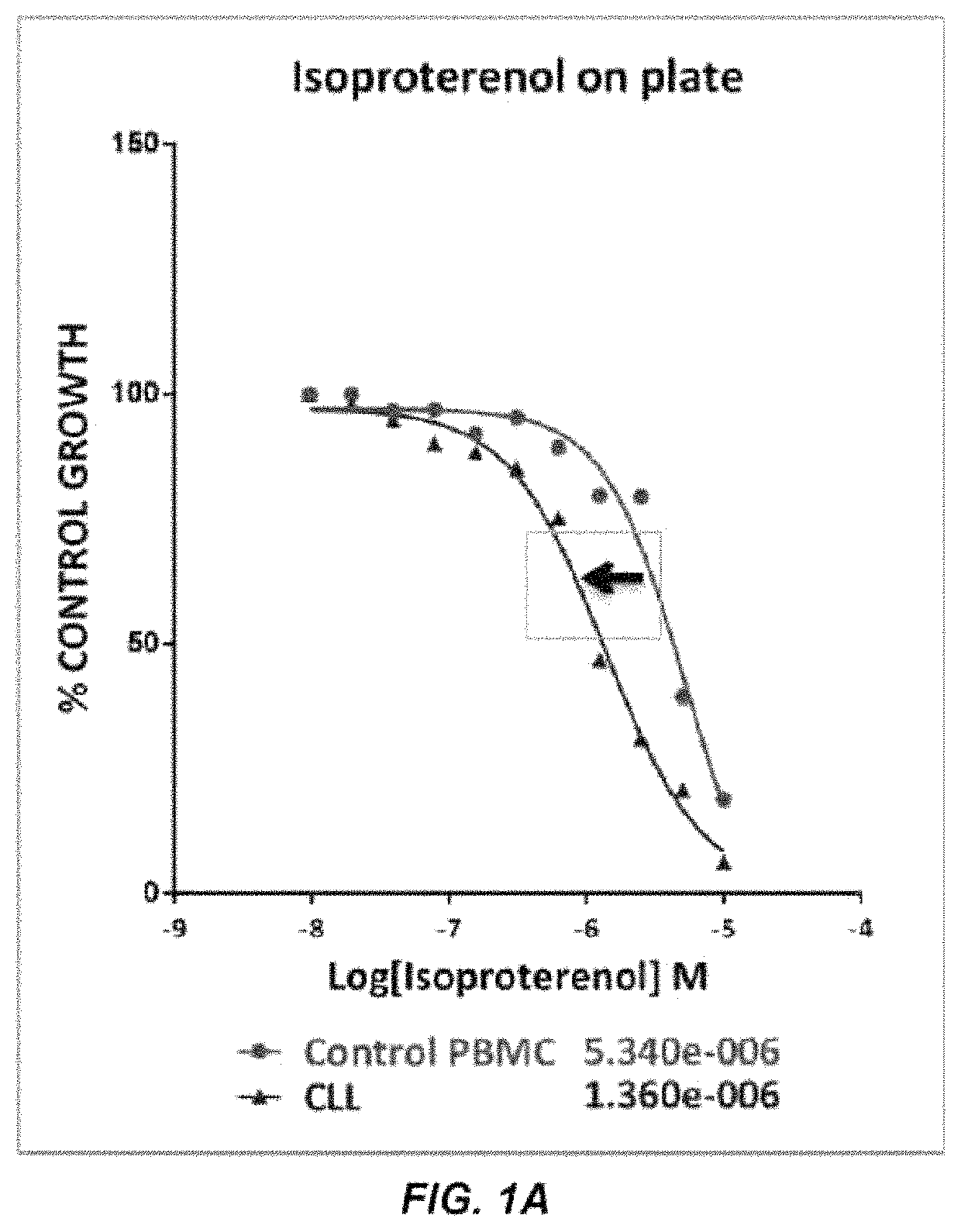
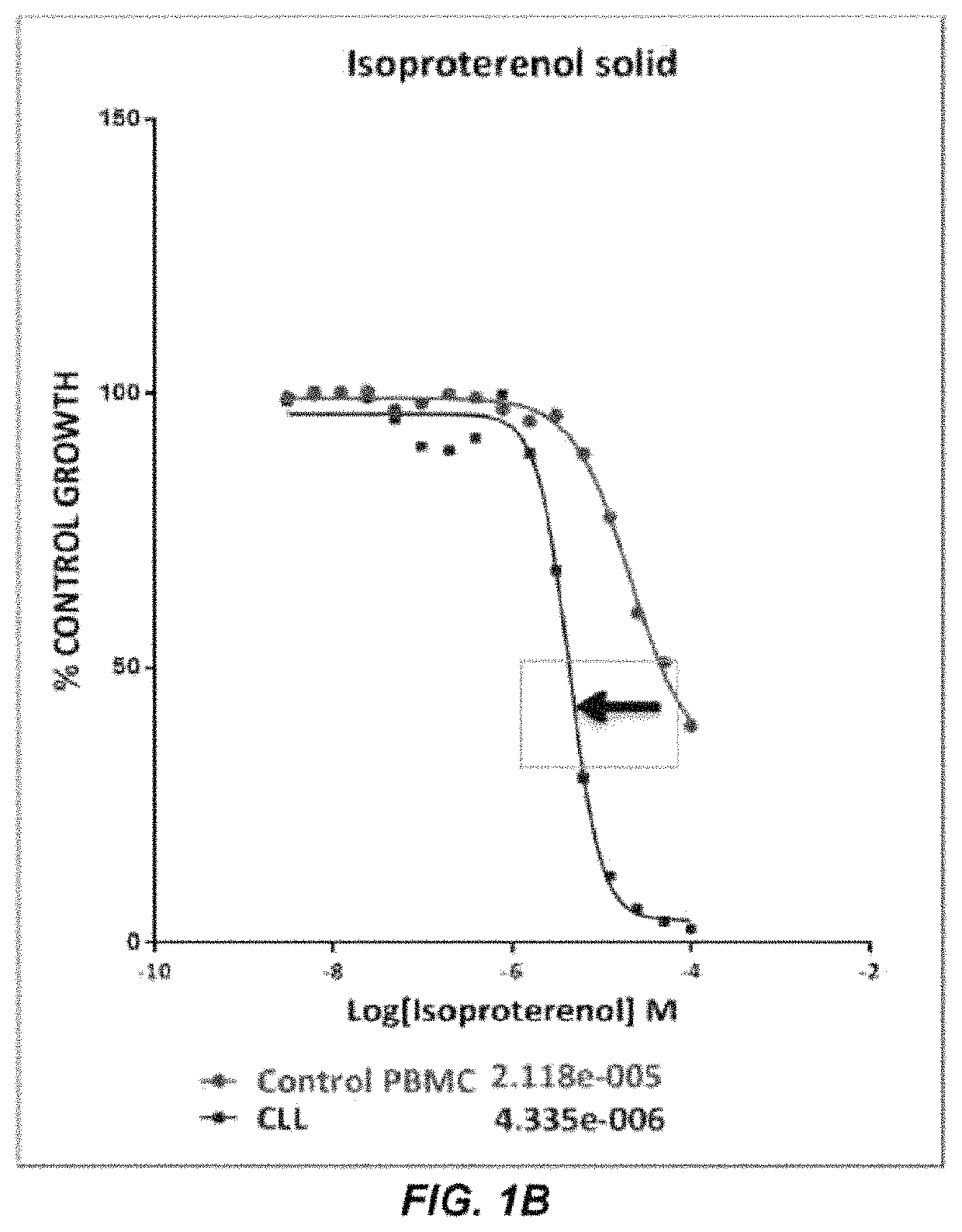
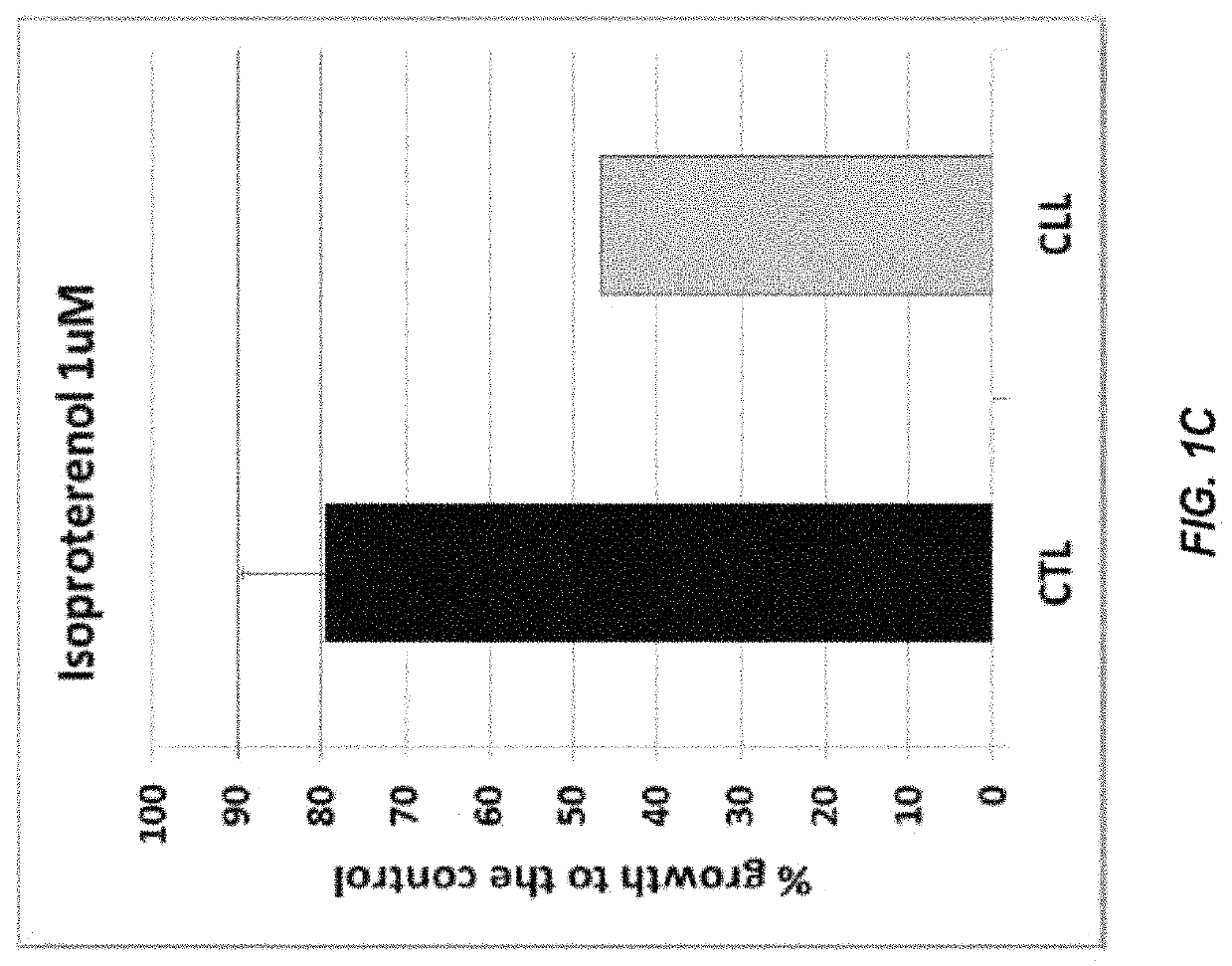
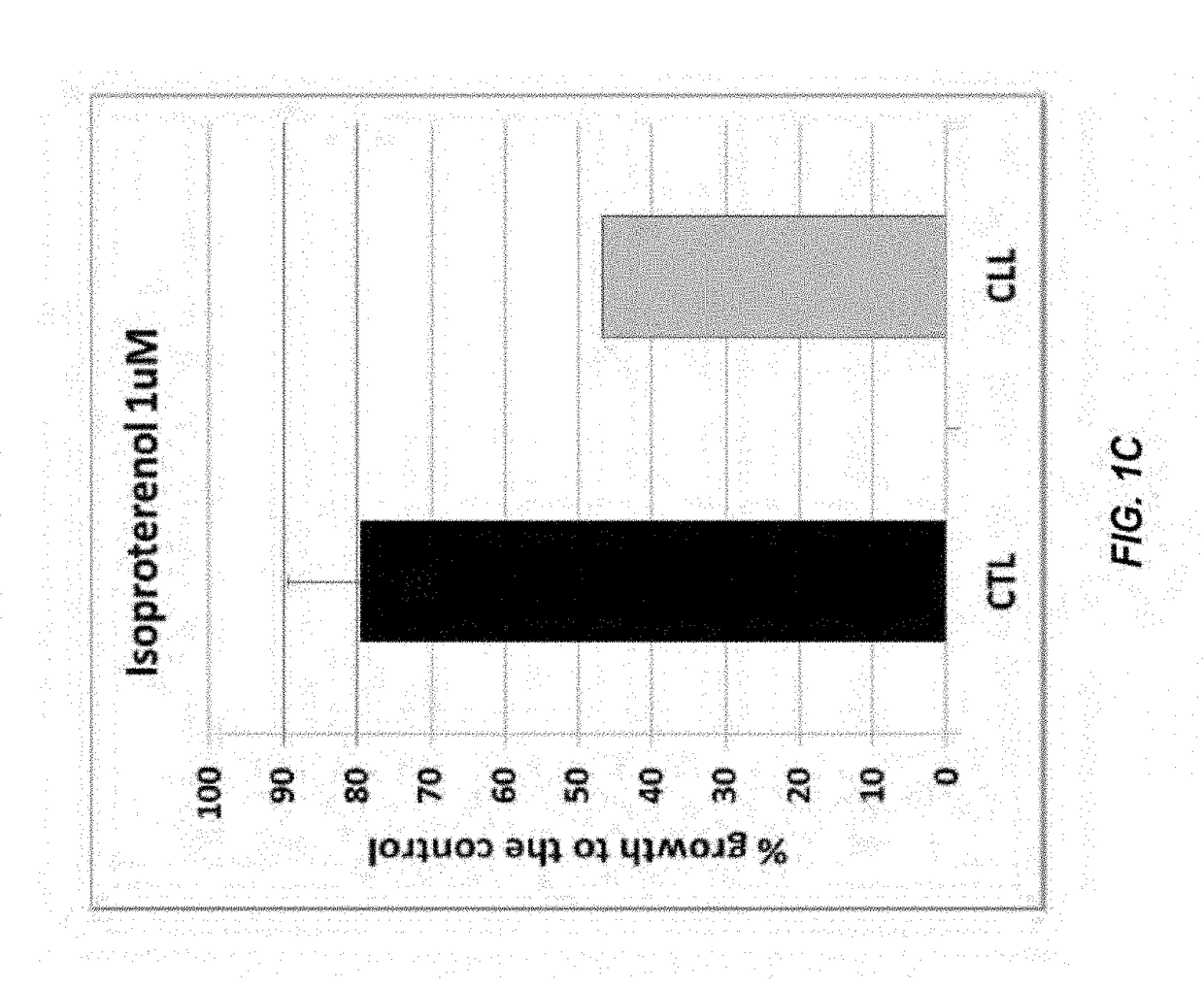
Method for treating leukemia
ActiveUS10639382B2Reduce in quantityOrganic active ingredientsPharmaceutical non-active ingredientsSerotoninAntiendomysial antibodies
The present disclosure relates to methods of reducing the number of abnormal PBMC cells in a leukemia patient. The methods may include administering an effective amount of a drug, which may not be indicated for leukemia, or an antibody-drug conjugate. The antibody-drug conjugate includes an antibody selected from the group consisting of an anti-α2-adrenoreceptor antibody, an anti-β adrenoceptor antibody, an anti-trace amine-associated receptor 1 antibody, an anti-dopamine receptor antibody, and an anti-serotonin receptor antibody; a drug selected from the group consisting of isoproterenol, methyldopa, olanzapine, and a derivative thereof; and a linker that conjugates the antibody and the drug.
Owner:NAT GUARD HEALTH AFFAIRS +2
Method for manufacturing carbon nano tube-based electrochemical transducer for simultaneously detecting methyldopa and hydrodiuril
The invention relates to a manufacturing method for an electrochemical transducer, and particularly relates to the manufacturing method for a novel carbon nano tube-based electrode. Compared with non-modified electrodes, the modified electrode of the transducer prepared by the method has obviously enhanced volt-ampere response of MD (methyldopa) on the surface and is successfully used for simultaneously detecting the MD and HCT (hydrodiuril) for the first time, and the oxidation potential of the MD is below 220mV. The transducer has high stability, high sensitivity, long-term use stability, and remarkable volt-ampere repeatability. The detection result to practical samples shows that the prepared transducer has very good practicability.
Owner:WUXI BAILING SENSING TECH
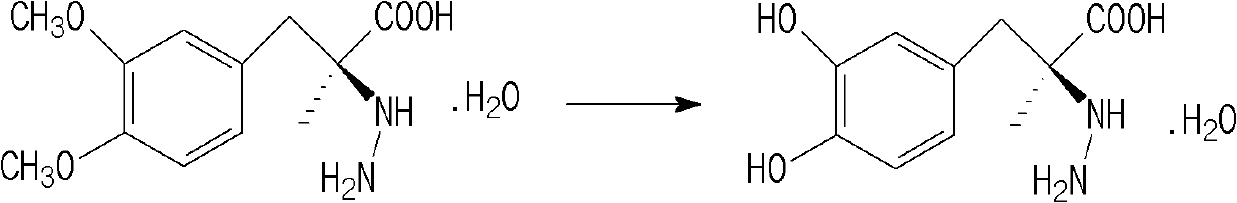
Method for preparing methyldopa by directly hydrolyzing 5-methyl-5-(3,4-dimethoxybenzyl)hydantoin with acid
ActiveCN102911072AOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisPharmaceutical drug
The invention belongs to the technical field of one of key steps of chemical synthesis of medicine methyldopa [alpha-methyl-(3,4-dihydroxyphenyl]-alpha-alanine] or synthesis of chiral medicine L-methyldopa [L-alpha-methyl-(3,4-dihydroxyphenyl]-alpha-alanine]: reaction for preparing methyldopa by hydrolyzing 5-methyl-5-(3,4-dimethoxybenzyl)hydantoin. The invention relates to a method for preparing methyldopa or L-methyldopa by directly hydrolyzing 5-methyl-5-(3,4-dimethoxybenzyl)hydantoin with acid at appropriate temperature. When disacidification is carried out under reduced pressure after the reaction finishes, N2 is introduced to protect the product from easy oxidative stain and accelerate the disacidification. In order to protect the product from deterioration, the vacuum drying temperature of the product is generally carried out at 50 DEG C for 5 hours.
Owner:ZHEJIANG CHIRAL MEDICINE CHEM
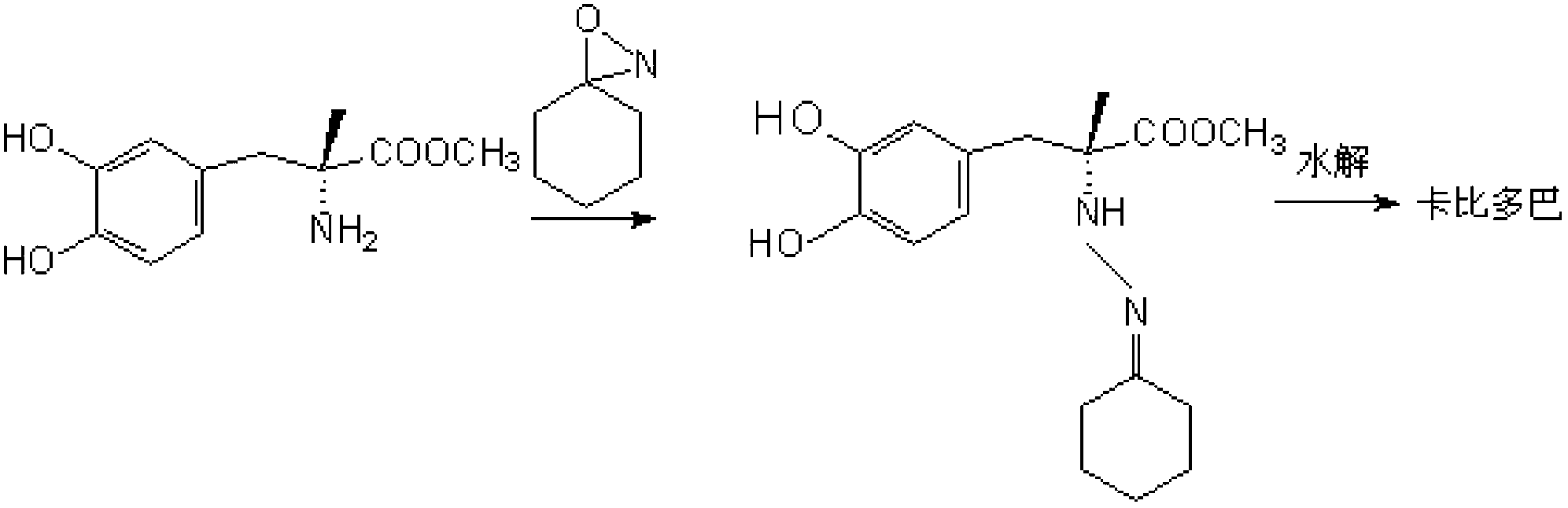
Method for synthesizing Carbidopa
The invention belongs to the field of chemical synthesis and particularly relates to a method for synthesizing Carbidopa. The method is characterized by subjecting Oxaziridine to a reaction with methyldopate so as to produce imide methyldopate, and then, carrying out hydrolysis, thereby producing the Carbidopa. The method has the advantages that the Carbidopa is prepared by adopting a bran-new process route, all the raw materials used are suitable for pharmaceutical synthesis, and the prepared Carbidopa is high in yield and good in product quality.
Owner:QINGDAO HUANGDAO HOSPITAL OF TRADITIONAL CHINESE MEDICINE
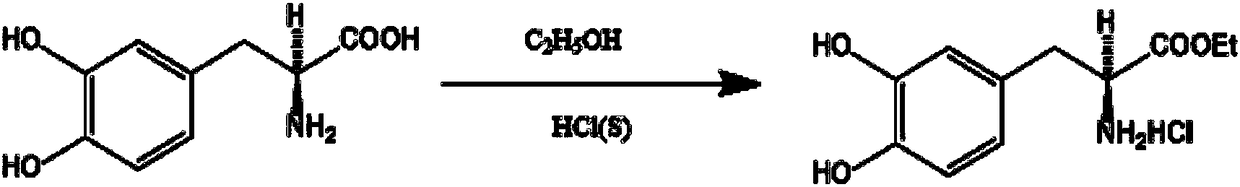
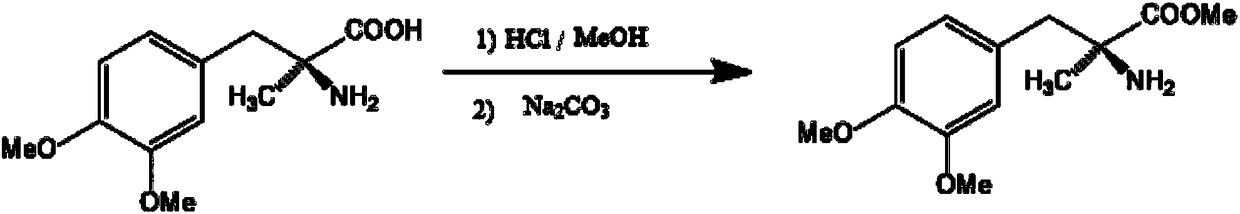
Preparation process of L-methyldopa methyl ester
InactiveCN108147974ALess side effectsLow costOrganic compound preparationOrganic chemistry methodsChemical synthesisCentrifugation
The invention relates to a preparation process of L-methyldopa methyl ester and belongs to the field of organic chemical synthesis. The preparation process of the L-methyldopa methyl ester comprises the following steps of 1, sequentially adding L-methyldopa and methanol and introducing hydrogen chloride; 2, at 0-45 DEG C, performing esterification reaction for 10-72 H; 3, after esterification reaction is completed, performing filtering, dissolution, neutralization, crystallization, centrifugation and drying to obtain a finished product. The preparation process of the L-methyldopa methyl estercan provide increased yield and reduced energy consumption, and meanwhile, through circulated application of a mother solution, the cost can be reduced and good environment friendliness can be provided.
Owner:SHANDONG XINHUA PHARMA CO LTD
Treatment of prostate cancer with DDC inhibitor
Prostate cancer comes in various forms and has proven difficult to treat. Provided herein are various methods and compositions for treating all forms of prostate cancers with dopa decarboxylase (DDC) inhibitors. These dopa decarboxylase inhibitors include carbidopa (α-Methyl-dopahydrazine), MFMD (α-monofluoromethyldopa), NSD-1015 (3-hydroxybenzylhydrazine), Methyldopa (L-α-Methyl-3,4-dihydroxyphenylalanine) or benserazide, and the inhibitors may be used in combination. DDC inhibitors may also be used to inhibit the progression of prostate cancer to androgen-independence and neuroendocrine prostate cancer.
Owner:THE UNIV OF BRITISH COLUMBIA
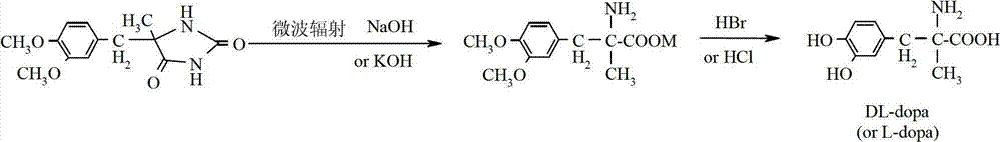
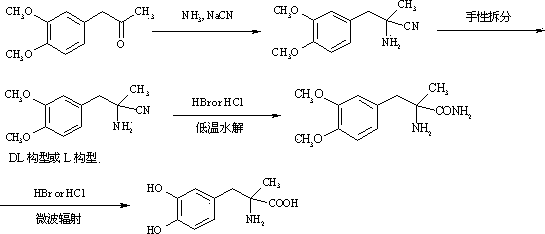
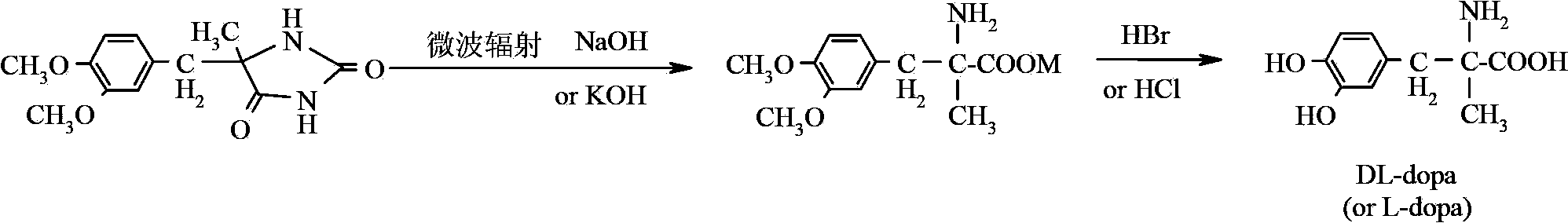
Method for preparing methyldopa through microwave basic hydrolysis 5-methyl-5-(3,4-dimethoxy benzyl) hydantoin
ActiveCN102775319AOrganic compound preparationAmino-carboxyl compound preparationMicrowave powerMethyl group
The invention relates to a method for preparing methyldopa or L-methyldopa through microwave basic hydrolysis 5-methyl-5-(3,4-dimethoxy benzyl) hydantoin. Various alkaline aqueous solutions with different concentrations are adopted to hydrolyze 5-methyl-5-(3,4-dimethoxy benzyl) hydantoin under different microwave power and radiation times to prepare DL-alpha-methyl-(3,4-dimethoxy benzyl)-alpha-aminopropionic acid salt which is hydrolyzed in an acid aqueous solution to prepare methyldopa[DL-alpha-methyl-(3,4-dimethoxy benzyl)-alpha-aminopropionic acid] or L-methyldopa[L-alpha-methyl-(3,4-dimethoxy benzyl)-alpha-aminopropionic acid]. When the method is used for hydrolyzing DL-alpha-methyl-(3,4-dimethoxy benzyl)-alpha-aminopropionic acid (or L-alpha-methyl-(3,4-dimethoxy benzyl)-alpha-aminopropionic acid), the molar ratio of a substrate and hydrogen bromide (Hbr) is 1:10-20, and the molar ratio of the substrate and chlorine hydride (HCl) is 1:10-30. The hydrolysis reaction is smooth when acid using amount is big, and disacidify pressure after the hydrolysis reaction is reduced when the acid using amount is small.
Owner:ZHEJIANG CHIRAL MEDICINE CHEM
Method of treating leukemia
ActiveUS20180296692A1Reduce in quantityOrganic active ingredientsImmunoglobulins against animals/humans5-HT receptorTrace amine associated receptor 1
The present disclosure relates to methods of reducing the number of abnormal PBMC cells in a leukemia patient. The methods may include administering an effective amount of a drug, which may not be indicated for leukemia, or an antibody-drug conjugate. The antibody-drug conjugate includes an antibody selected from the group consisting of an anti-α2-adrenoreceptor antibody, an anti-β adrenoceptor antibody, an anti-trace amine-associated receptor 1 antibody, an anti-dopamine receptor antibody, and an anti-serotonin receptor antibody; a drug selected from the group consisting of isoproterenol, methyldopa, olanzapine, and a derivative thereof; and a linker that conjugates the antibody and the drug.
Owner:NAT GUARD HEALTH AFFAIRS +2
Method for recovering and preparing L(+)-2, 3-dihydrobutanedioic acid from L-methyldopa production wastewater
InactiveCN103483183AAvoid pollutionEasy to operatePreparation from carboxylic acid saltsFiltrationNuclear chemistry
The invention belongs to the field of wastewater treatment and specifically relates to a method for recovering and preparing L(+)-2, 3-dihydrobutanedioic acid from L-methyldopa production wastewater. The method comprises the steps of adding an inorganic acid salt of calcium into the L-methyldopa production wastewater to obtain the precipitate of L(+)-2, 3-dihydrobutanedioic acid calcium (II), suspending the precipitate with water, further reacting with an inorganic acid to obtain a water solution of the L(+)-2, 3-dihydrobutanedioic acid (I), decoloring with activated carbon, and performing hot filtration, reduced-pressure concentration, crystallization and centrifugation to prepare a crude product of the L(+)-2, 3-dihydrobutanedioic acid (I), and refining to prepare a fine product of the L(+)-2, 3-dihydrobutanedioic acid (I). The method disclosed by the invention has the advantages of simplicity in operation, mild reaction conditions, easiness in control and high yield; the purity of the obtained product is high; the environmental pollution caused by L(+)-2, 3-dihydrobutanedioic acid sodium in the L-methyldopa production wastewater is thoroughly avoided, cyclic economy is realized, production cost is lowered, and the process is stable; therefore the method is suitable for industrial production.
Owner:SHANDONG XINHUA PHARMA CO LTD
Method for preparing methyldopa by directly hydrolyzing 5-methyl-5-(3,4-dimethoxybenzyl)hydantoin with acid
ActiveCN102911072BOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisMethyl group
The invention belongs to the technical field of one of key steps of chemical synthesis of medicine methyldopa [alpha-methyl-(3,4-dihydroxyphenyl]-alpha-alanine] or synthesis of chiral medicine L-methyldopa [L-alpha-methyl-(3,4-dihydroxyphenyl]-alpha-alanine]: reaction for preparing methyldopa by hydrolyzing 5-methyl-5-(3,4-dimethoxybenzyl)hydantoin. The invention relates to a method for preparing methyldopa or L-methyldopa by directly hydrolyzing 5-methyl-5-(3,4-dimethoxybenzyl)hydantoin with acid at appropriate temperature. When disacidification is carried out under reduced pressure after the reaction finishes, N2 is introduced to protect the product from easy oxidative stain and accelerate the disacidification. In order to protect the product from deterioration, the vacuum drying temperature of the product is generally carried out at 50 DEG C for 5 hours.
Owner:ZHEJIANG CHIRAL MEDICINE CHEM
Medicine for treating cardiovascular and cerebrovascular disease
Disclosed is a medicament for treating cardiovascular and cerebrovascular diseases which comprises the components of (by weight ratio) sodium ascorbate 2-3, chlorhydric pyridoxine 0.1-0.15, thiamine 0.1-0.15, methyldopa 0.05-0.1, Urapidil 0.05-0.1, puerarin 0.1-0.15, xinkeshu tablet 0.05-0.1, xuekang capsule 0.05-0.1, ginkgo leaf tablet 0.1-0.15, fresh vegetable and fruit maceration extract 20-40, and distilled water 77.3-56.
Owner:邱季端 +1
A kind of recovery method and application of L-methyldopa intermediate
ActiveCN105906520BReduce CODEasy to prepareOrganic compound preparationAmino-carboxyl compound preparationPropionitrileGreen chemistry
The invention discloses a recycling method and application of an L-methyldopa intermediate. The recycling method comprises the following steps: hydrolyzing L-3-(3,4-dimethoxy phenyl)-2-amino-2-methyl propionitrile hydrochloride by utilizing hydrochloric acid, and filtering to obtaining L-3-(3,4-dimethoxy phenyl)-2-methyl alanine hydrochloride and mother liquor, adding haloid salt or ammonium salt into the mother liquor, carrying out vacuum distillation, concentrating until volume is less than or equal to 1 / 3 of the original volume, and filtering, wherein a filter cake is namely the recycled 3-(3,4-dimethoxy phenyl)-2-methyl alanine hydrochloride solid. The recycling method disclosed by the invention has the advantages that L-methyldopa can be recycled from 3-(3,4-dimethoxy phenyl)-2-methyl alanine hydrochloride production waste liquor, and ammonia nitrogen and COD in wastewater can also be reduced, so that the recycling method accords with the green chemistry concept.
Owner:ZHEJIANG WILD WIND PHARMA
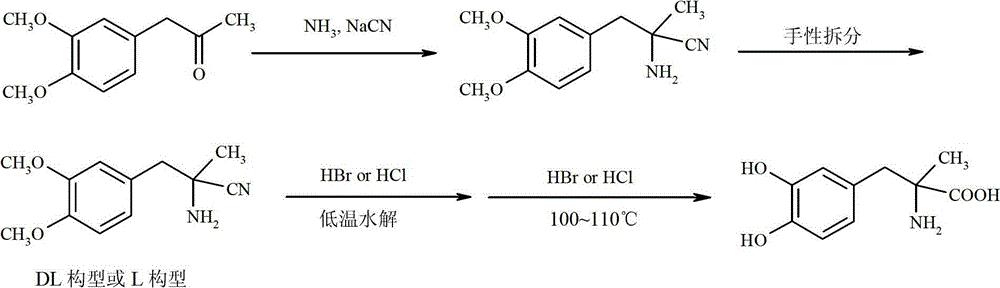
Method for preparing methyldopa from alpha-methyl-(3,4-dimethoxy phenyl)-alpha-aminopropionitrile by microwave hydrolysis method
ActiveCN102702004BEmission reductionHigh purityOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisChemical reaction
The invention belongs to the technical field of one critical step in microwave nitrile hydrolysis and demethylation hydrolysis of chemical synthesis medicine methyldopa [alpha-methyl-(3,4-dyhydroxy phenyl)-alpha-aminopropionic acid, similarly hereinafter] or synthetic chiral medicine L-methyldopa [L-alpha-methyl-(3,4-dyhydroxy phenyl)-alpha-aminopropionic acid, similarly hereinafter], i.e. the chemical reaction for preparing methyldopa or L-methyldopa from alpha-methyl-(3,4-dimethoxy phenyl)-alpha-aminopropionitrile or L-alpha-methyl-(3,4-dimethoxy phenyl)-alpha-aminopropionitrile through microwave nitrile hydrolysis and demethylation hydrolysis. The microwave hydrolysis uses low-concentration hydrochloric acid or hydrobromic acid, so the energy sources and the economic cost are reduced, the acid wastewater discharge is reduced, by-products are reduced, and the purity and the yield of products are improved.
Owner:ZHEJIANG CHIRAL MEDICINE CHEM
Method for preparing methyldopa through microwave basic hydrolysis 5-methyl-5-(3,4-dimethoxy benzyl) hydantoin
ActiveCN102775319BOrganic compound preparationAmino-carboxyl compound preparationMicrowave powerMethyl group
Owner:ZHEJIANG CHIRAL MEDICINE CHEM
Preparation of Methyldopa by Hydrolysis of α-Methyl-(3,4-Dimethoxyphenyl)-α-aminopropionitrile by Two-step Hydrolysis
InactiveCN102786428BEmission reductionReduce energy costsOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisHigh concentration
Owner:ZHEJIANG UNIV
Method for synthesizing carbidopa
ActiveCN102702019BHigh yieldImprove product qualityHydrazine preparationChemical synthesisMethyldopate
The invention belongs to the field of chemical synthesis, and in particular relates to a method for synthesizing carbidopa. The method is characterized by comprising the following steps of: reacting oxaziridine with methyldopa ester to obtain methyldopa imido ester, and hydrolyzing to obtain the carbidopa. The method has the advantages that the carbidopa is prepared by a brand-new process, the used raw materials are favorable for synthesizing a medicine, and the prepared carbidopa is high in yield and quality.
Owner:SHANDONG XINHUA PHARMA CO LTD
Pharmaceutical composition for intraocular or oral administration for treatment of retinal diseases
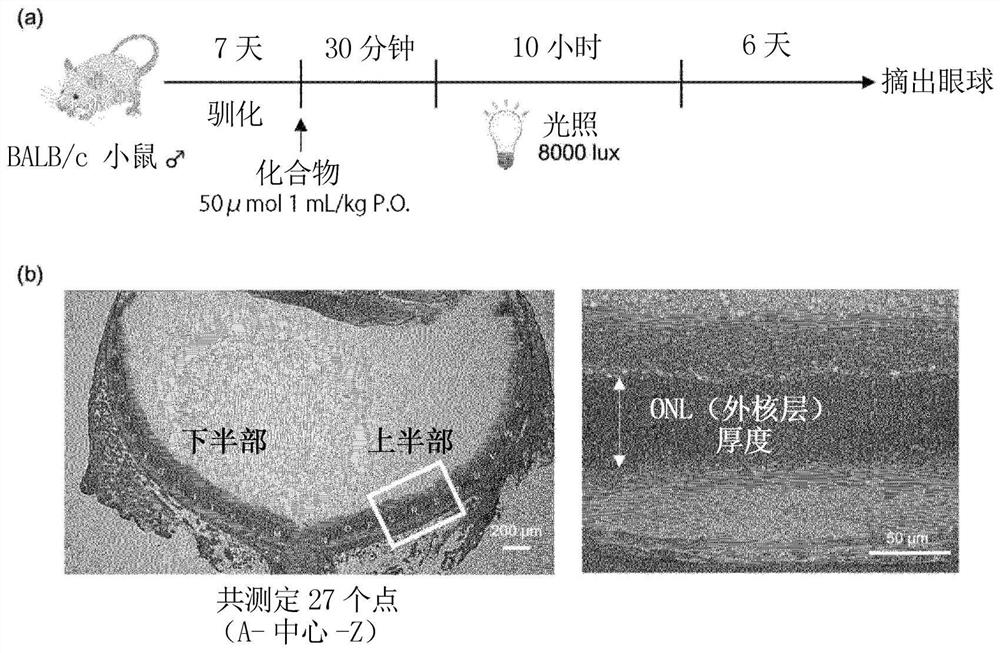
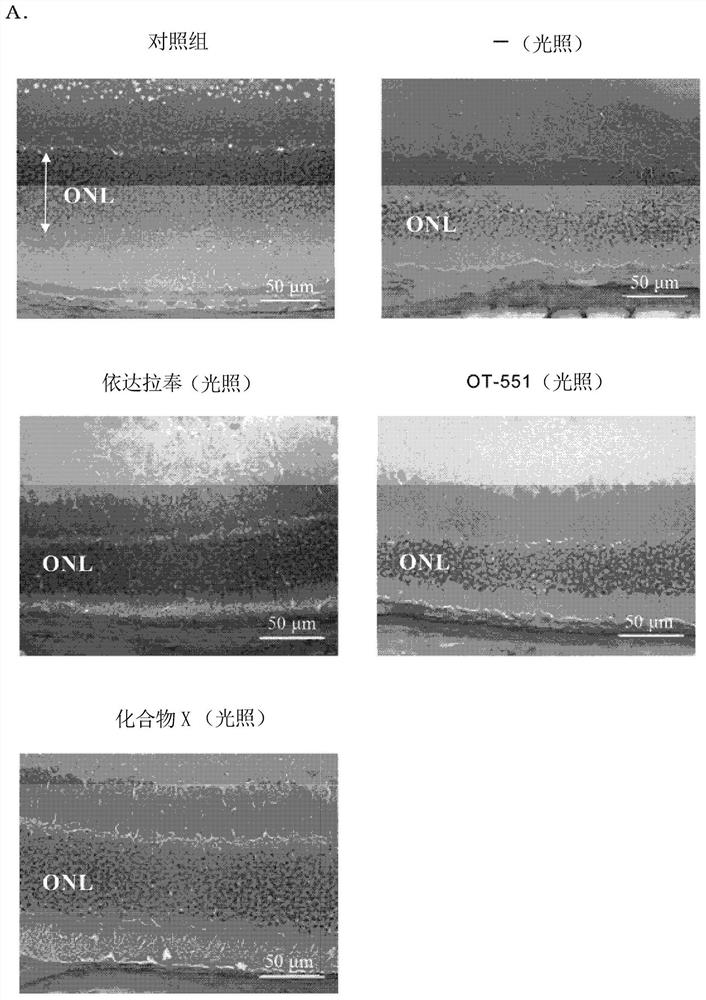
The present invention provides: a pharmaceutical composition that is to be intraocularly or orally administered for preventing or treating a retinal disease (e.g., age-related macular degeneration) of a patient who needs to be treated, or inhibiting the progression of the disease, and that contains a pharmaceutically-acceptable carrier and an effective amount of at least one compound selected from the group consisting of apomorphine, eseroline, ethoxyquin, methyldopa, olanzapine, indapamide, and the like; and a method.
Owner:FUSO PHARMA INDS +1
Method for refining carbidopa
ActiveCN102432496BReduce manufacturing costHigh yieldHydrazine preparationProduct systemEconomic benefits
The invention discloses a method for refining carbidopa, which comprises the following steps: diluting a crude carbidopa product with water, regulating the pH value to below 6.5 with acid, and heating to 50-110 DEG C under the nitrogen protection or / and reducer addition condition, thereby obtaining a crude product system; adding a decolorant into the crude product system to decolorize at 50-110 DEG C for 5-60 minutes; after decolorizing, filtering while the crude product system is hot to obtain a filtrate, stirring and cooling the filtrate to 0-30 DEG C, and continuing stirring at 0-30 DEG C for 0.5-5 hours to obtain a refined system; and filtering the refined system, washing the filter cake with water, and carrying out vacuum drying to obtain the finished carbidopa product. The method disclosed by the invention has the advantages of simple preparation method, favorable controllability, strong operability, high methyldopa (impurity) removal rate, high yield and low production cost, can easily implement industrial production, has high economic benefit in the industrial production, can be easily popularized, and has wide application prospects.
Owner:ZHEJIANG CHIRAL MEDICINE CHEM
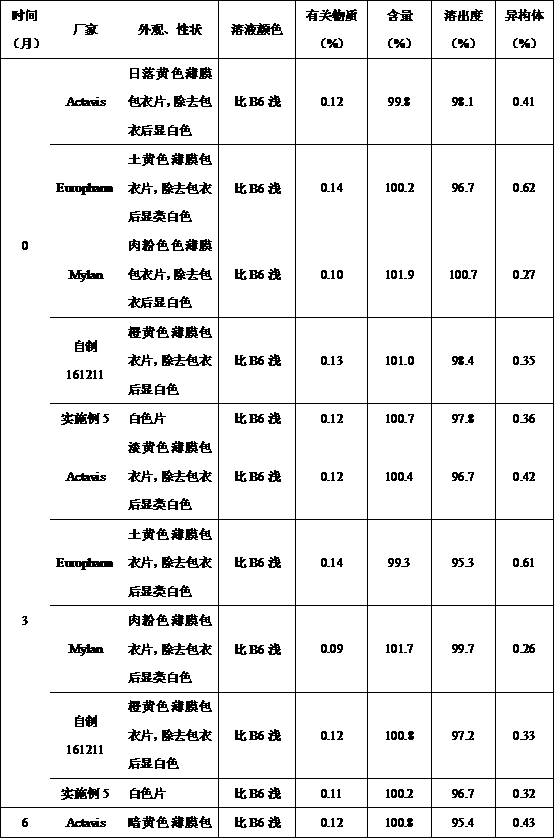
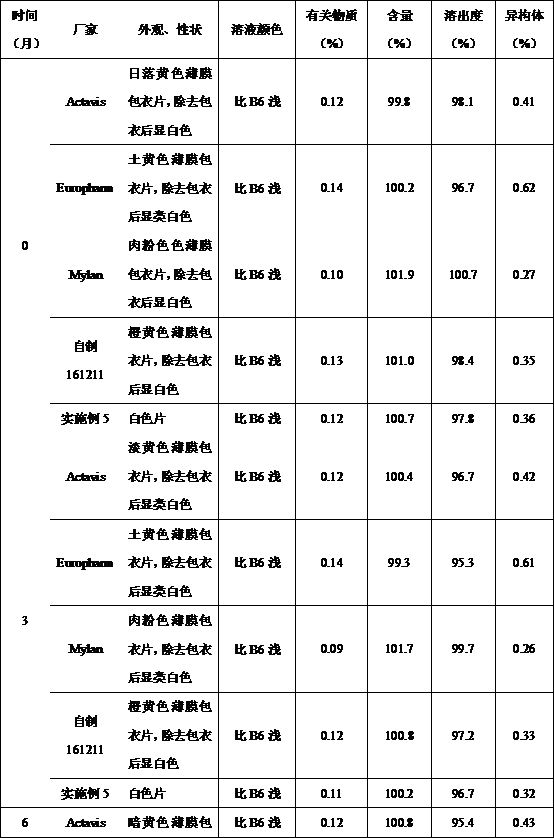
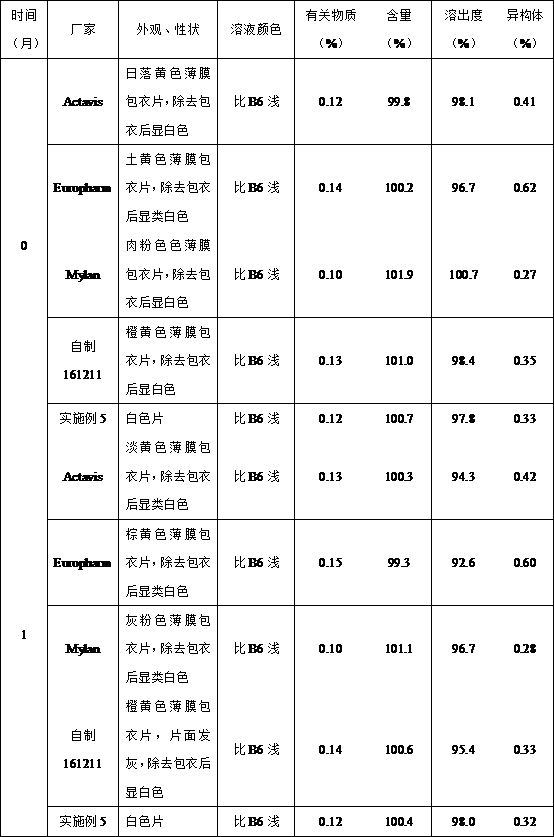
Methyldopa composition and methyldopa tablets as well as preparation methods thereof
ActiveCN108379233AGuaranteed stabilityNo significant changeOrganic active ingredientsPharmaceutical non-active ingredientsCompressibilitySilicon dioxide
The invention relates to a methyldopa-silicon dioxide-stearyl alcohol pharmaceutical composition and a preparation method thereof. The pharmaceutical composition is mainly applied to the preparation of methyldopa tablets. The pharmaceutical composition is prepared by carrying out polymerization on methyldopa, stearyl alcohol and colloidal silicon dioxide by using a spray drying technology; the Hausner ratio range of particles is 1.10-1.18, and the Carr index range of the particles is 10-15%; the pharmaceutical composition has good fluidity and compressibility, so that the compressibility and the tablet formation property are improved; furthermore, the medicine part is prevented from being oxidized and discoloring after being combined with water, so that the storage stability of the preparation is obviously improved.
Owner:南京泽恒医药技术开发有限公司
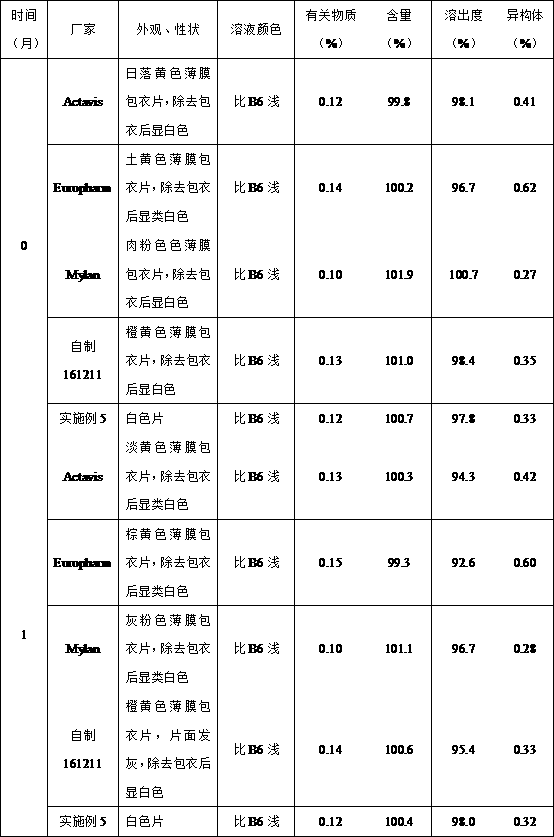
A kind of methyldopa composition, tablet and preparation method
ActiveCN108379233BGuaranteed stabilityNo significant changeOrganic active ingredientsPill deliveryPharmaceutical drugSpray dried
The invention relates to a methyldopa-silicon dioxide-stearyl alcohol pharmaceutical composition and a preparation method thereof. The pharmaceutical composition is mainly applied to the preparation of methyldopa tablets. The pharmaceutical composition is prepared by carrying out polymerization on methyldopa, stearyl alcohol and colloidal silicon dioxide by using a spray drying technology; the Hausner ratio range of particles is 1.10-1.18, and the Carr index range of the particles is 10-15%; the pharmaceutical composition has good fluidity and compressibility, so that the compressibility and the tablet formation property are improved; furthermore, the medicine part is prevented from being oxidized and discoloring after being combined with water, so that the storage stability of the preparation is obviously improved.
Owner:南京泽恒医药技术开发有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com