Methyldopa composition and methyldopa tablets as well as preparation methods thereof
A technology of methyldopa tablets and methyldopa, which is applied in the directions of drug combination, pill delivery, pharmaceutical formulation, etc., can solve the problem of harsh production conditions and can not improve the oxidative discoloration of methyldopa drugs and the graying of varieties. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
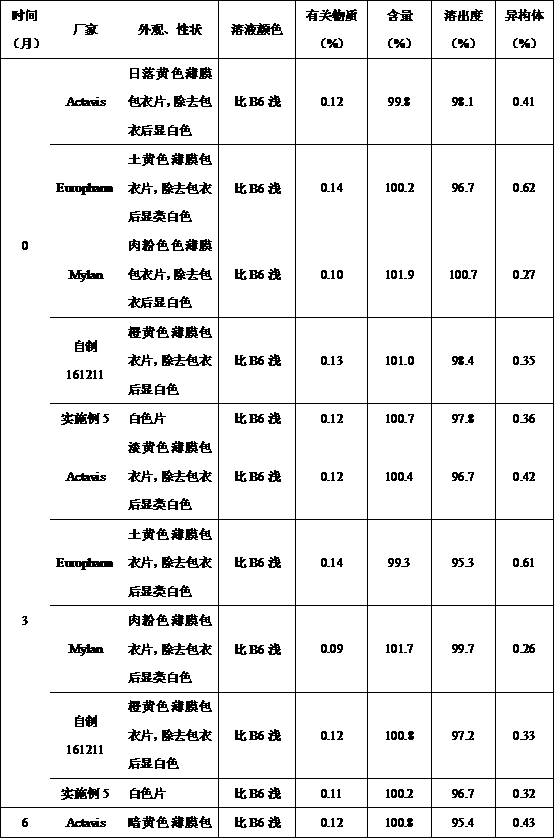
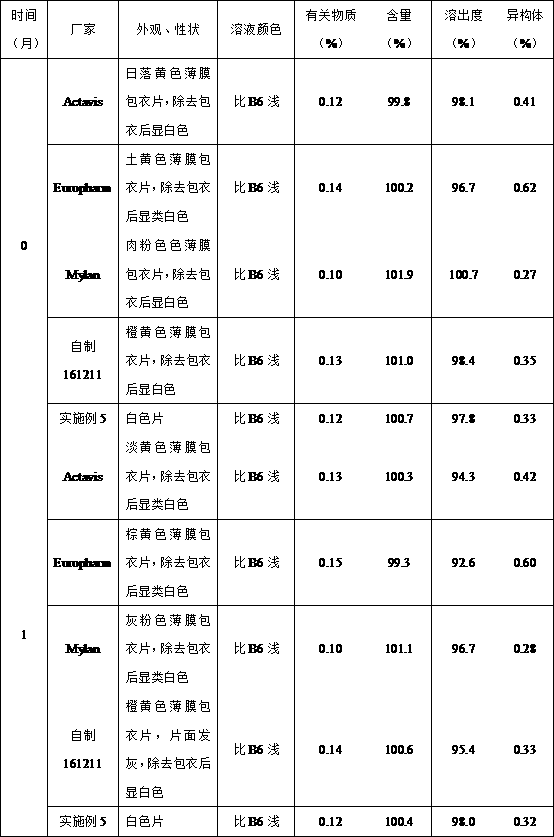
Image
Examples
Embodiment 1
[0042] Example 1 Methyldopa-Silicon Dioxide-Stearyl Alcohol Pharmaceutical Composition 1
[0043] Methyldopa (water)...960.0g
[0044] Colloidal silicon dioxide…………30.0g
[0045] Stearyl alcohol………………10.0g
[0046] 98% ethanol……………490g
[0047] ——A total of 1000g
[0048] Dissolve the prescribed amount of stearyl alcohol in 98% ethanol to prepare a stearyl alcohol solution for subsequent use; weigh the prescribed amount of methyldopa and colloidal silicon dioxide, place them in a fluidized bed, and mix them evenly. Spray stearyl alcohol solution, keep the temperature of the material at 25~35°C during the spraying period, and dry for 5~10min at the inlet air temperature of 30~35°C after spraying. After drying, pass the material through a 30-mesh sieve to obtain the methyldopa-silicon dioxide-stearyl alcohol pharmaceutical composition.
Embodiment 2
[0049] Example 2 Methyldopa-Silicon Dioxide-Stearyl Alcohol Pharmaceutical Composition 2
[0050] Methyldopa (water)...980.0g
[0051] Colloidal silicon dioxide…………14.0g
[0052] Stearyl alcohol………………6.0g
[0053] 95% ethanol………………300g
[0054] ——A total of 1000g
[0055] Preparation process: Dissolve the stearyl alcohol of the prescribed amount in 95% ethanol, prepare stearyl alcohol solution, and set aside; weigh the methyldopa and colloidal silicon dioxide of the prescribed amount, place them in a fluidized bed, mix uniform. Spray stearyl alcohol solution, keep the temperature of the material at 25~35°C during the spraying period, and dry for 5~10min at the inlet air temperature of 30~35°C after spraying. After drying, pass the material through a 24-mesh sieve to obtain the methyldopa-silicon dioxide-stearyl alcohol pharmaceutical composition.
Embodiment 3
[0056] Example 3 Methyldopa-Silicon Dioxide-Stearyl Alcohol Pharmaceutical Composition 3
[0057] Methyldopa (water)………970.0g
[0058] Colloidal silicon dioxide……………16.0g
[0059] Stearyl alcohol…………………… 14.0g
[0060] 95% ethanol………………450g
[0061] ——A total of 1000g
[0062] Preparation process: Dissolve the stearyl alcohol of the prescribed amount in 95% ethanol, prepare stearyl alcohol solution, and set aside; weigh the methyldopa and colloidal silicon dioxide of the prescribed amount, place them in a fluidized bed, mix uniform. Spray stearyl alcohol solution, keep the temperature of the material at 25~35°C during the spraying period, and dry for 5~10min at the inlet air temperature of 30~35°C after spraying. After drying, pass the material through a 24-mesh sieve to obtain the methyldopa-silicon dioxide-stearyl alcohol pharmaceutical composition.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com