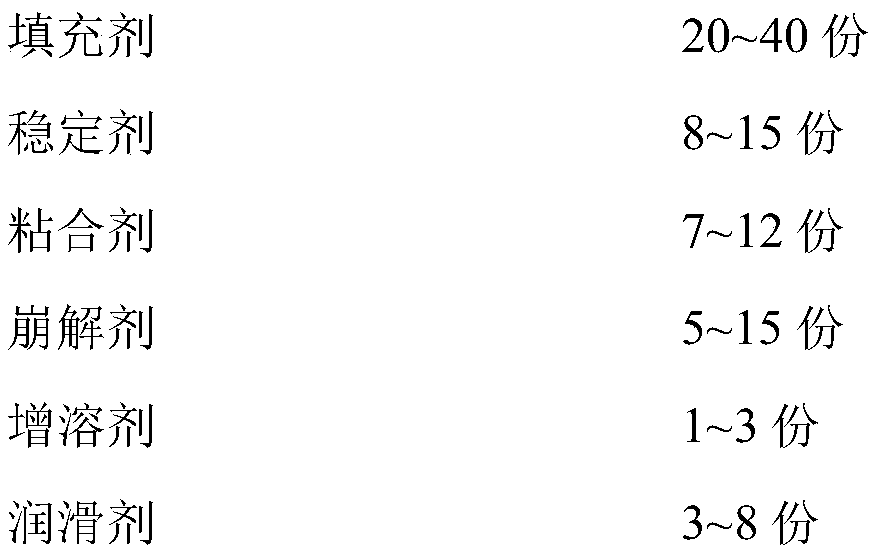Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
41 results about "Benserazide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Benserazide (also called Serazide or Ro 4-4602) is a peripherally acting aromatic L-amino acid decarboxylase or DOPA decarboxylase inhibitor, which is unable to cross the blood–brain barrier.
Modulaton of cellular DNA repair activity to intercept malignancy
InactiveUS20160038444A1Preventing malignancyBiocideMicrobiological testing/measurementBenserazideMalignancy
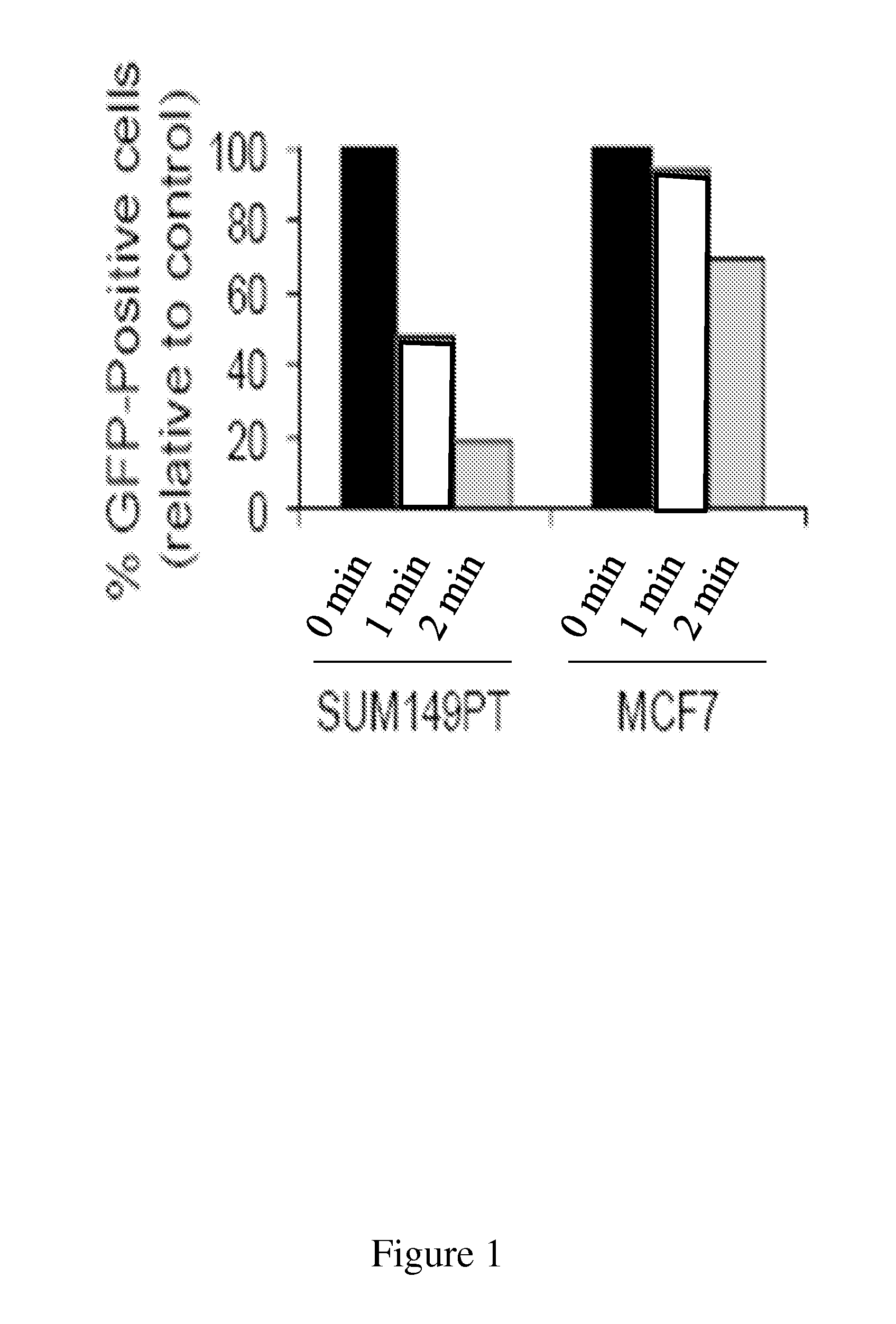
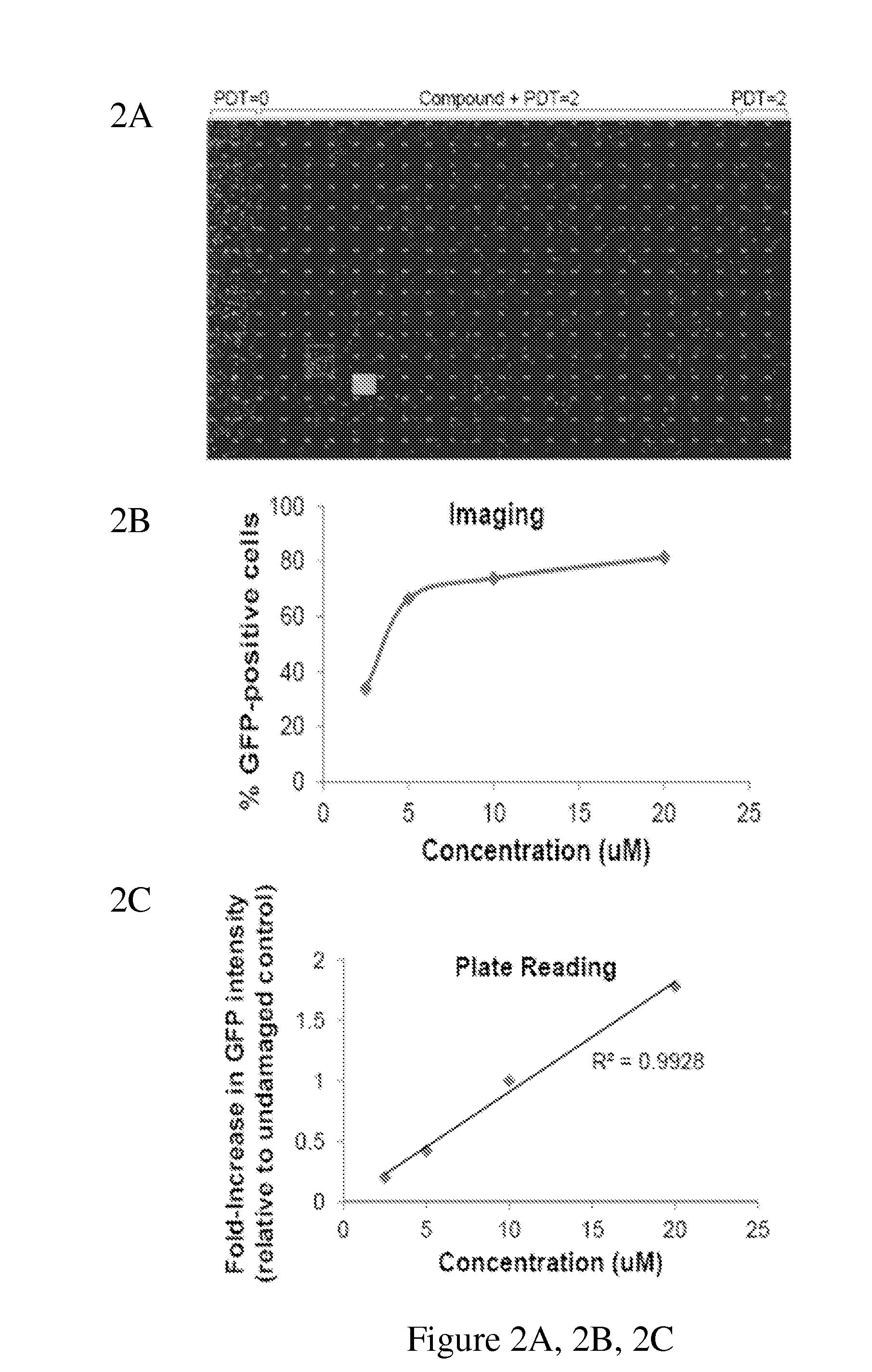
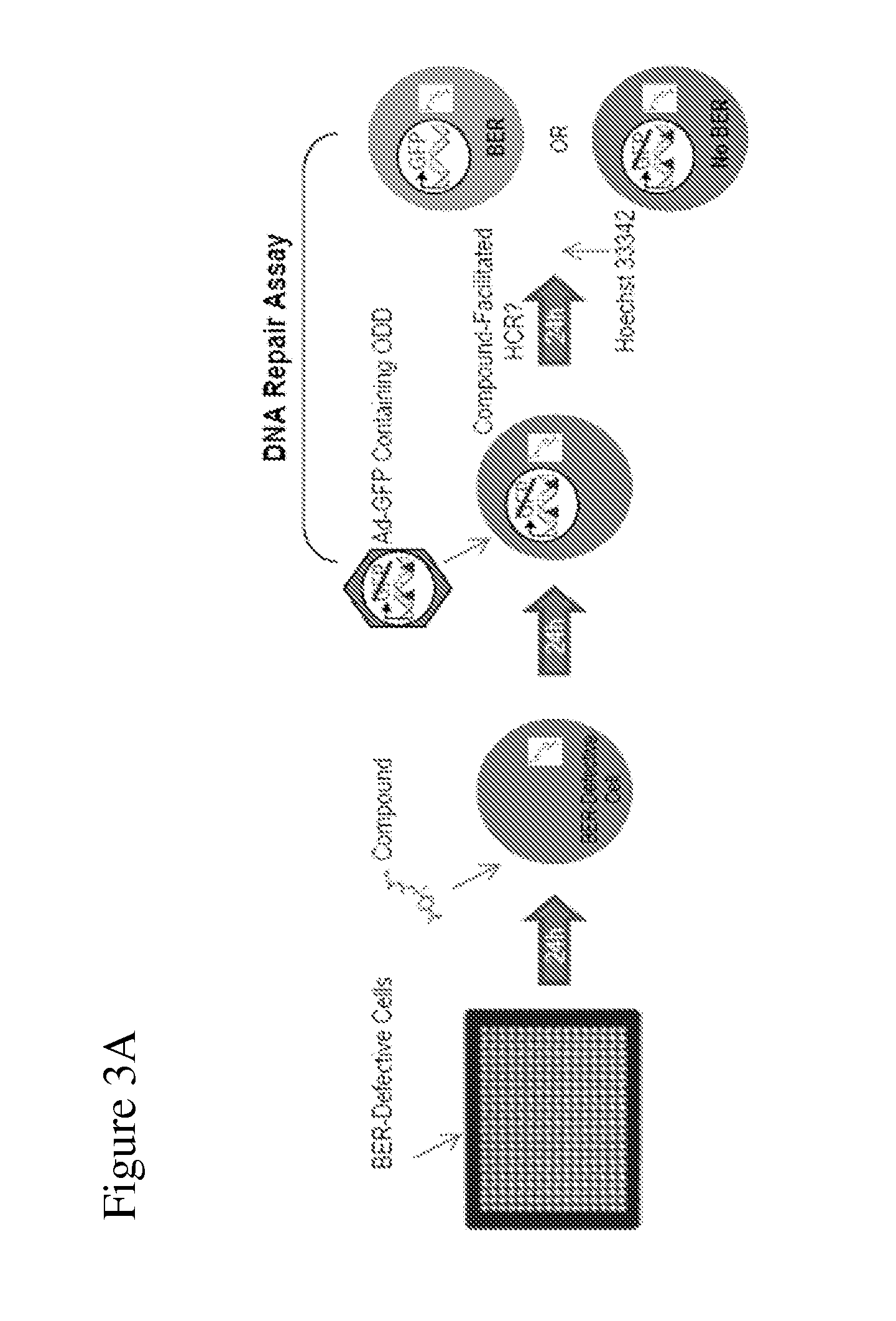
Disclosed herein are methods for identifying compounds that enhance base excision repair, as well as compounds identified thereby and methods of using such compounds in the interception of malignancy, i.e. the prevention of progression of a disease from a state of susceptibility to active disease. Exemplified compounds are acetohexamide and related compounds, as well as benserazide and analogs thereof. Exemplified malignancies are those of human breast cells carrying mutations, in particular, SUM149 cells and HCC1937 cells, which cells carry BRCA1 mutations.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
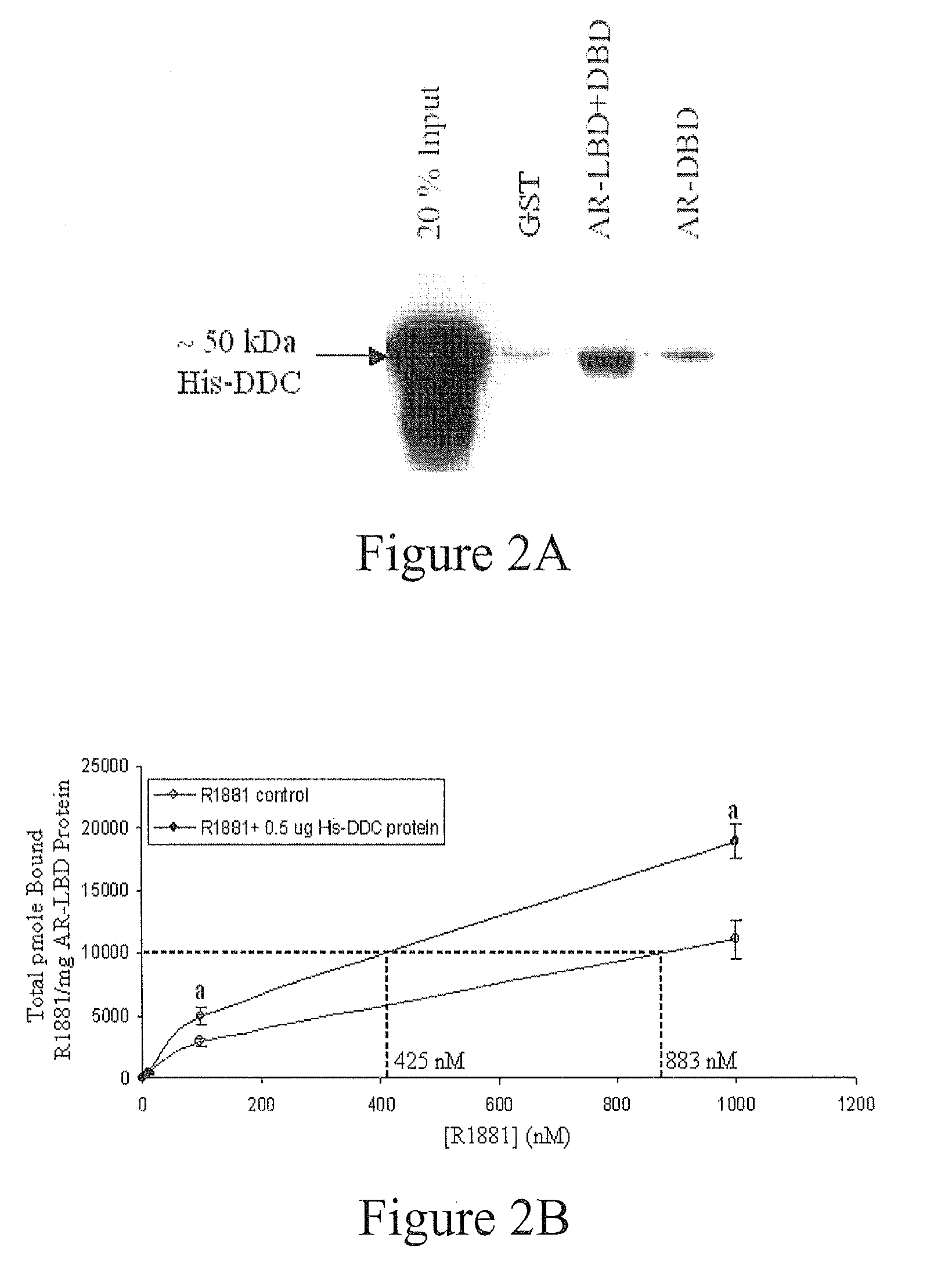
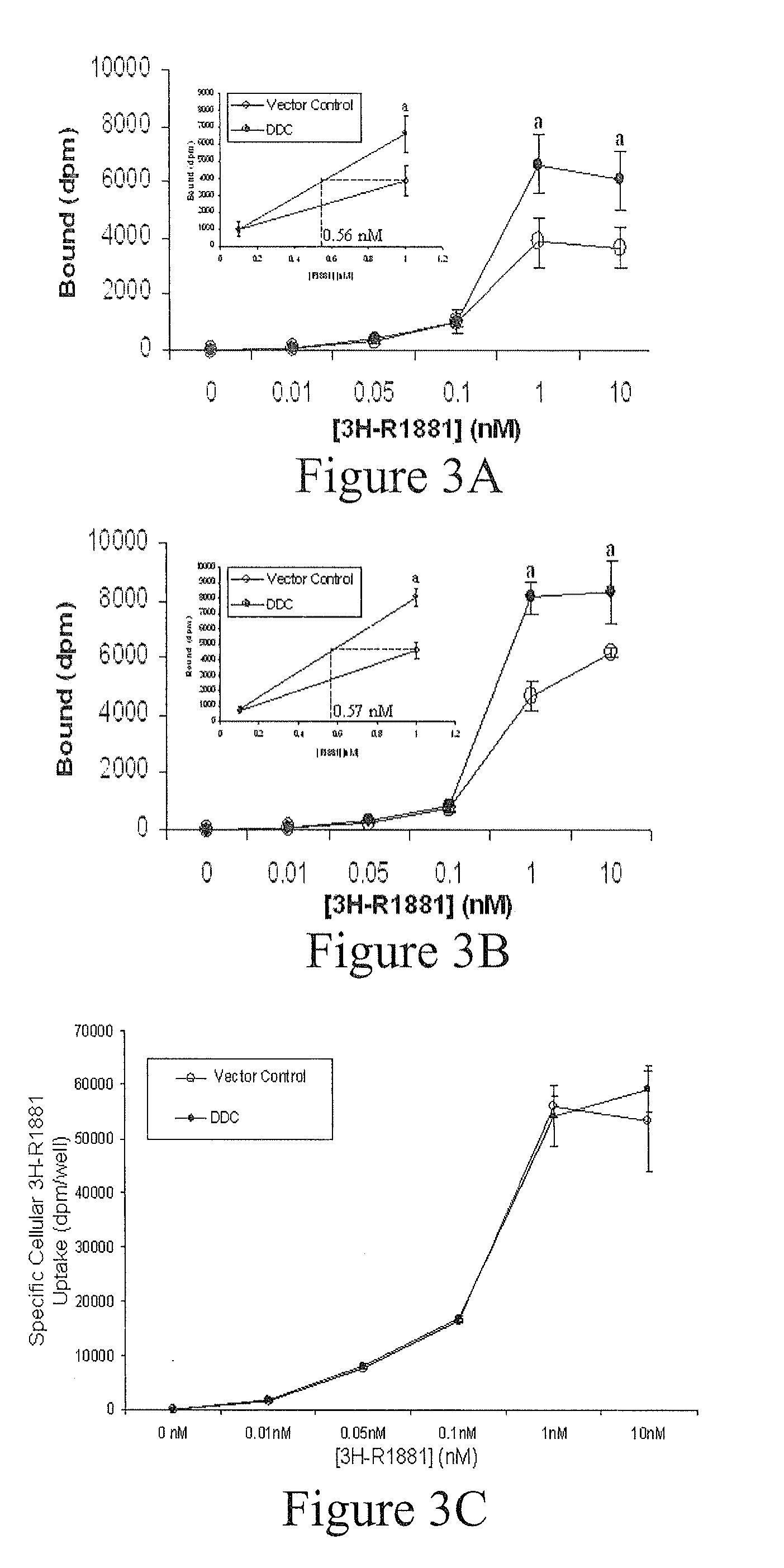
Treatment of Prostate Cancer with DDC Inhibitor
ActiveUS20100048709A1Low affinityBiocidePeptide/protein ingredientsMultiple formsDihydroxyphenylalanine
Owner:THE UNIV OF BRITISH COLUMBIA
Levodopa/benserazide orally disintegrating tablet
InactiveCN101797244BReasonable formulaGood compatibilityNervous disorderPharmaceutical non-active ingredientsBenserazideOrally disintegrating tablet
Owner:QINGDAO UNIV OF SCI & TECH
Method for improving stability of benserazide hydrochloride oral administration solid composition
PendingCN112741808AImprove liquidityReduce collisionOrganic active ingredientsNervous disorderBenserazideBENSERAZIDE HYDROCHLORIDE
The invention belongs to the field of drug preparations, and particularly relates to a method for improving the stability of an oral administration solid composition containing benserazide hydrochloride. The benserazide hydrochloride is a peripheral decarboxylase inhibitor, and the benserazide hydrochloride and levodopa are always prepared into a compound preparation and are combined to be mainly used for treating the Parkinson' s disease (essential paralysis agitans), and postencephalitic or the symptomatic Parkinson' s syndrome (non-drug-induced paralysis agitans syndrome) combined with cerebral arteriosclerosis. The property of the benserazide hydrochloride is extremely unstable, and the benserazide hydrochloride is extremely sensitive to change of PH, ray, temperature and humidity. Since the ways of wet granulation and vacuum drying are adopted, the stability of the benserazide hydrochloride in an oral administration solid preparation containing the benserazide hydrochloride can be improved.
Owner:NANJING GRITPHARMA CO LTD
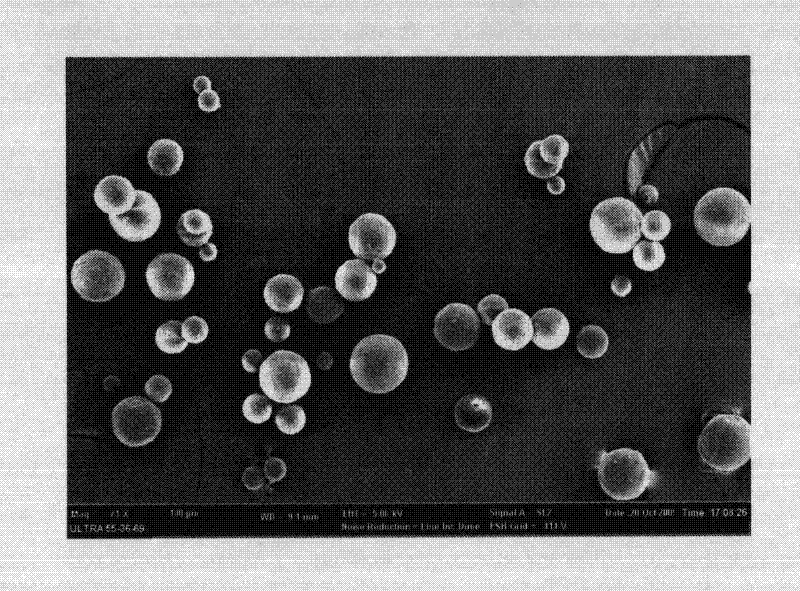
Microsphere combination medicament containing antiparkinsonism drug and application thereof
InactiveCN101879143AGood synergyLower AIM scoreOrganic active ingredientsNervous disorderDiseaseMicrosphere
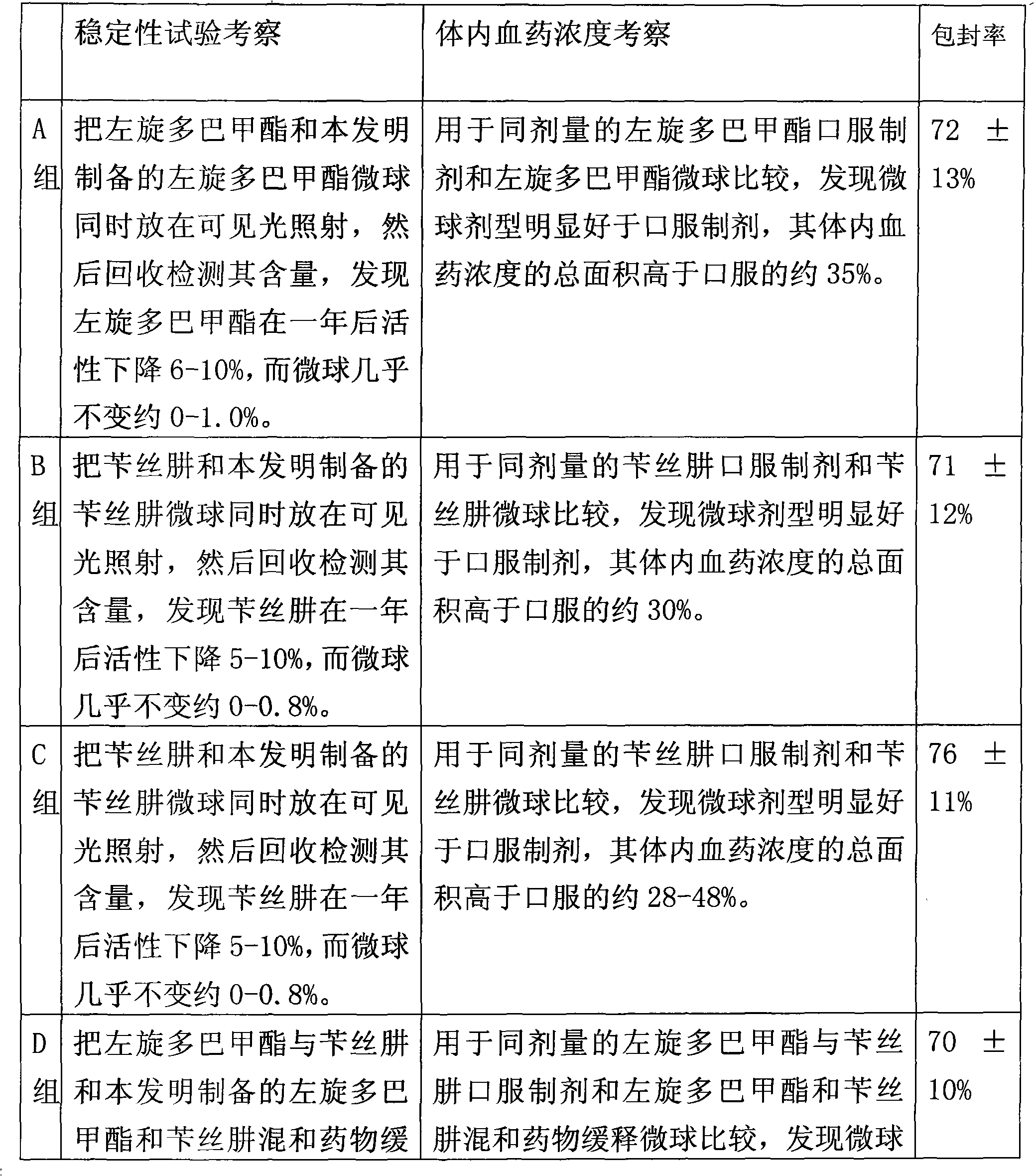
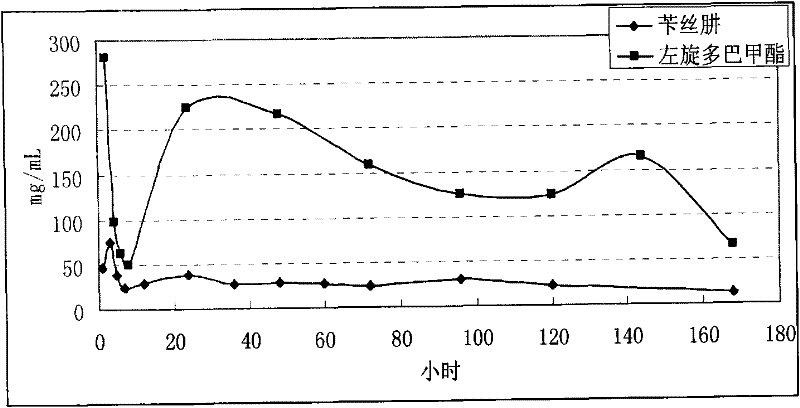
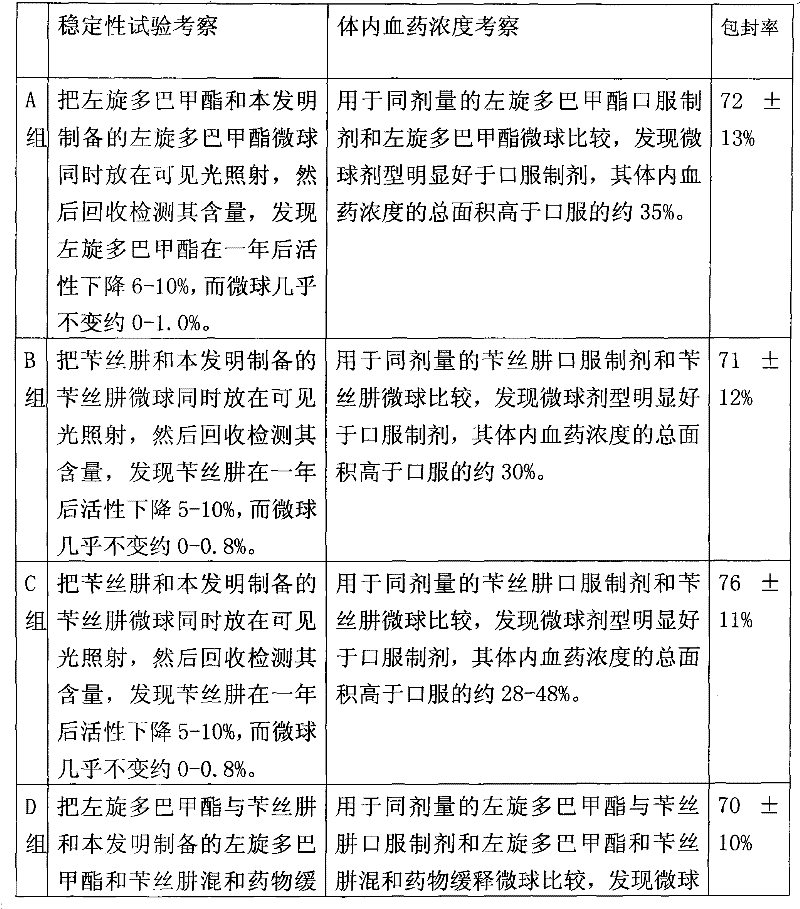
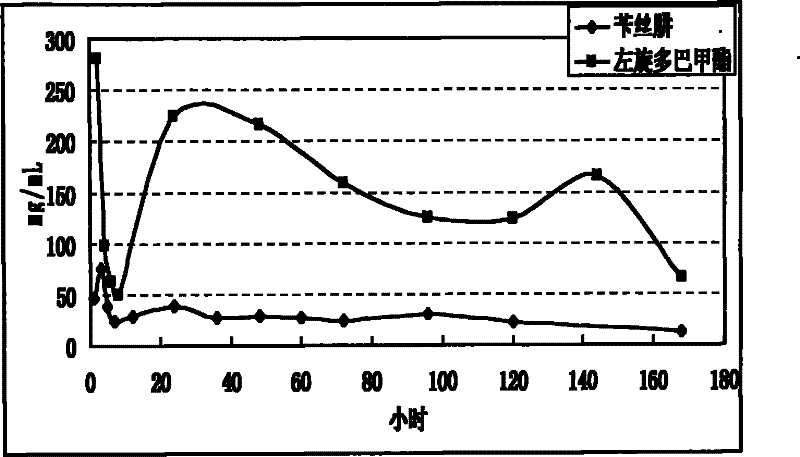
The invention relates to an application of a microsphere composition containing an antiparkinsonism drug to preparation of a medicine for preventing and treating parkinson diseases and complicating diseases of parkinson diseases. The microsphere composition containing the antiparkinsonism drug is selected from benserazide microspheres, L-dopa methyl ester microspheres, the L-dopa methyl ester microspheres and the benserazide microspheres, or L-dopa methyl ester and benserazide mixing drug microspheres. The invention also provides a microsphere combination medicament containing the antiparkinsonism drug. The invention explores novel medical application for the microsphere combination medicament containing the antiparkinsonism drug and develops a novel application field. The microsphere combination medicament containing the antiparkinsonism drug of the invention is safe and nontoxic, has strong pharmacological action and indicates good medicinal prospect.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Compositions comprising blockers of L-DOPA renal cell transfer for treatment of Parkinson's disease
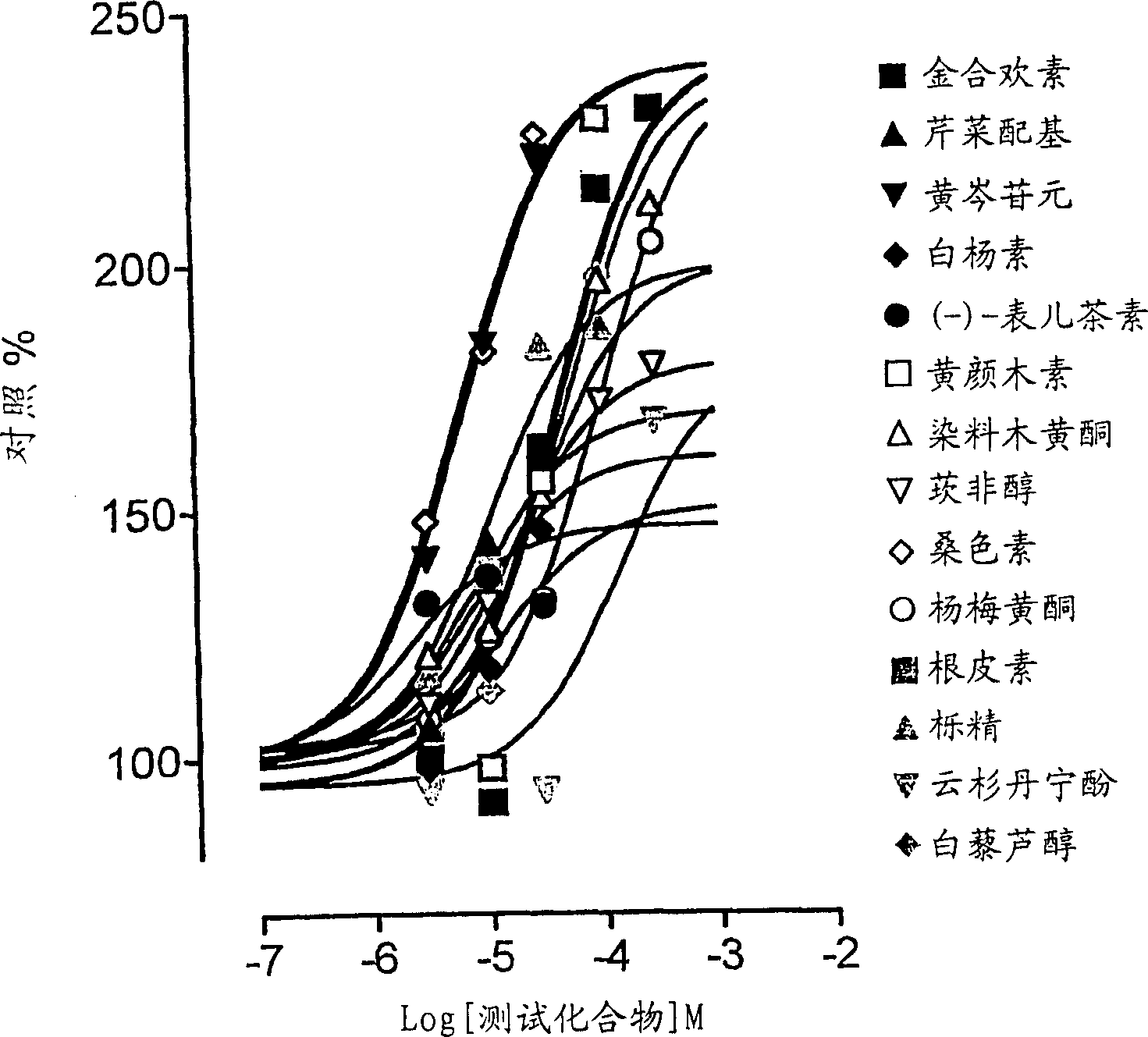
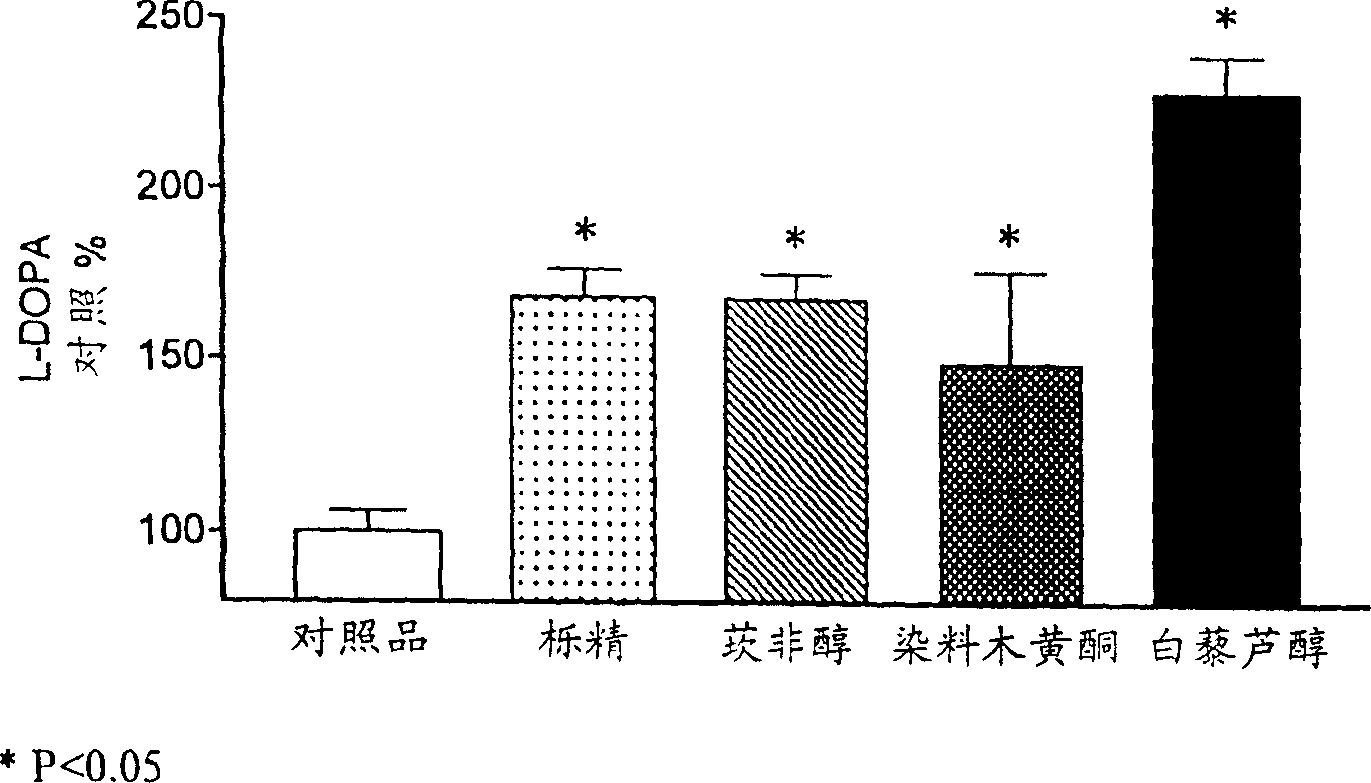
A pharmaceutical composition for the treatment of Parkinson's disease comprises L-DOPA and at least one compound capable of blocking the L-DOPA renal cell outward transfer pathway; said blocking compound being chose from (a) a flavonoid phenyl benzopyran derivative; (b) a trans-stilbene derivative; or (c) phloretin [3-(4-hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)-1-propanone]. The composition may also comprise an inhibitor of the enzyme amino acid decarboxylase (AADC), such a carbidopa or benserazide, and / or an inhibitor of the enzyme catechol-O-methyl transferase (COMT), such as entacapone or tolcapone. The composition is preferably administered in soli form and the L-DOPA may be administered simultaneously or sequentially with the L-DOPA renal cell outward transfer blocking compound.
Owner:波特拉和康潘希亚股份有限公司
Levodopa preparation and application thereof
InactiveCN105078952AImproving the disturbance of consciousnessOvercome the chillsOrganic active ingredientsNervous disorderReperfusion injuryCerebral resuscitation
The invention relates to levodopa preparation and application thereof. The levodopa preparation comprises, by weight percentage, 70-90% of levodopa and 10-30% of benserazide. The levodopa preparation has the advantages that the levodopa preparation contains the levodopa and the benserazide of fixed content, is applicable to rehabilitation treatment of cerebral dysfunction after cerebral resuscitation, and provides medicine for treating cerebral dysfunction caused by hypoxia-ischemia brain damage after cardio-pulmonary resuscitation; the preparation can be used to prepare medicine for repairing cerebral ischemia-reperfusion injury, medicine for the rehabilitation treatment of cerebral dysfunction after cerebral resuscitation, medicine for treating hypoxia-ischemia brain damage and medicine for replenishing body dopamine; meanwhile, a new purpose of levodopa is provided.
Owner:CHINA REHABILITATION RES CENT
Levodopa / benserazide hydrochloride compound sustained-release suspension and preparation method thereof
InactiveCN103948579ASolving Difficult to Swallow ProblemsEasy to takeOrganic active ingredientsNervous disorderBenserazideBENSERAZIDE HYDROCHLORIDE
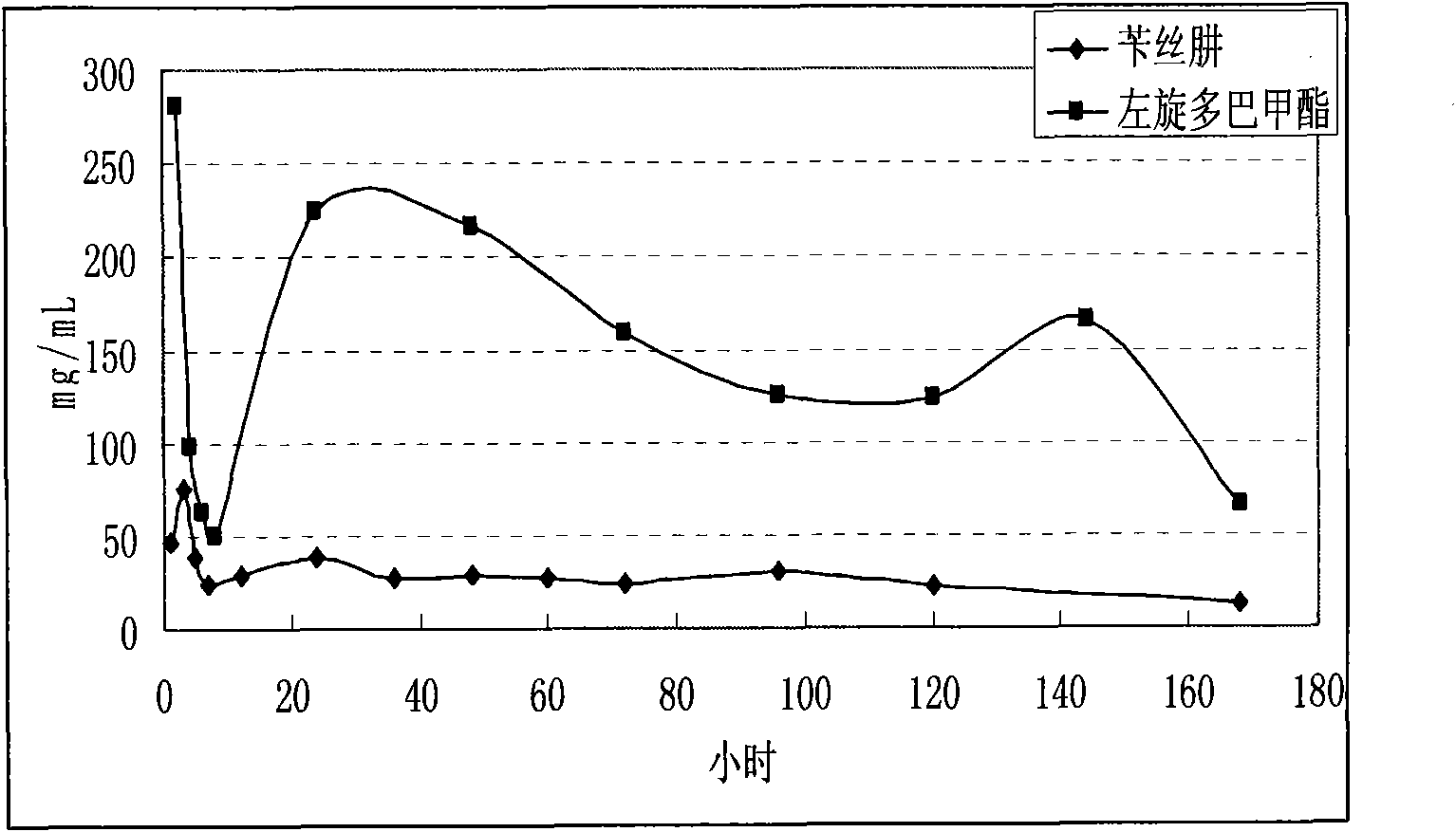
The invention discloses a levodopa / benserazide hydrochloride compound sustained-release suspension. The preparation contains by weight 10-50% of levodopa, 10-50% of benserazide hydrochloride and 10-50% of accessories. Compared with a solid preparation, the invention has the following advantages: the drug is coated in resin to improve drug stability; the preparation can be taken by doses to solve the problem that a large dose of drug prepared into an oral solid sustained release preparation is difficult to swallow; a multiple unit drug delivery system, in which drug release behavior is the sum of the drug release behavior of each particle, has better reproducibility and consistency; the preparation can avoid the situation of high local concentration caused by fragmentation of the oral solid sustained release preparation, and can cover the adverse taste of drugs and increase palatability. The sustained release preparation provided by invention can be used clinically as an anti-parkinsonian drug.
Owner:JIANGSU UNIV
Levodopa/benserazide orally disintegrating tablet
InactiveCN101797244AReasonable formulaGood compatibilityNervous disorderPill deliveryBenserazideOrally disintegrating tablet
The invention discloses a levodopa / benserazide orally disintegrating tablet and a preparation method thereof. The tablet comprises the main drugs including levodopa and benserazide and the auxiliary materials including microcrystalline cellulose, mannitol, lactose, crospolyvinylpyrrolidone, low-substituted hydroxypropyl cellulose, sodium cyclamate, menthol, aerosol and magnesium stearate. The tablet of the invention can effectively treat the Parkinson disease or Parkinsonism syndrome, is convenient to take, good in mouthfeel, quick in disintegration, fast in absorption and high in bioavailability and provides convenience for the Parkinson disease or Parkinsonism syndrome patients who have difficulty in taking the whole tablet or feel inconvenient to take the tablet.
Owner:QINGDAO UNIV OF SCI & TECH
Levodopa methyl ester and benserazide mixed medicament slow-release microsphere composition and preparation method thereof
InactiveCN101879153ANo pollutionNon-adhesiveOrganic active ingredientsNervous disorderMicrosphereFreeze-drying
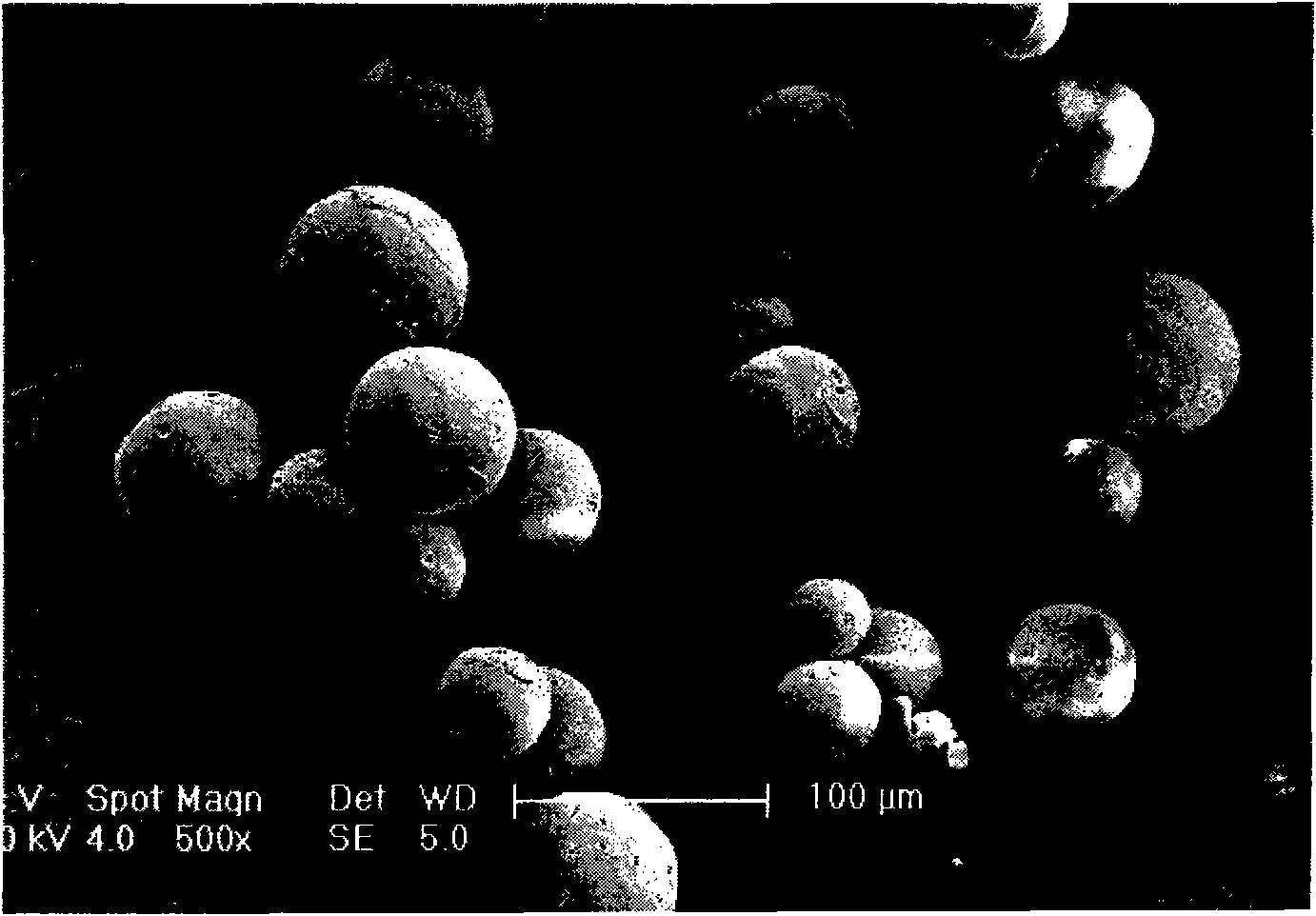
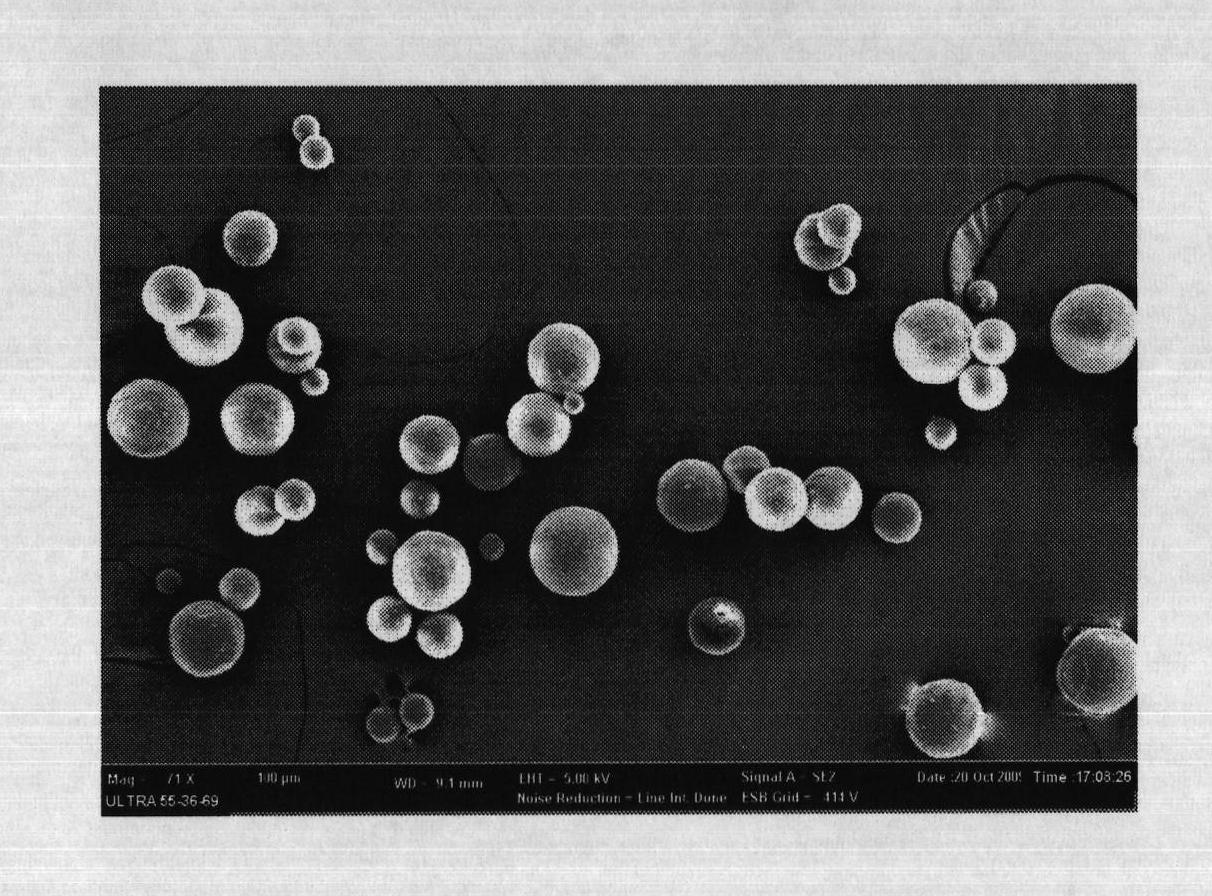
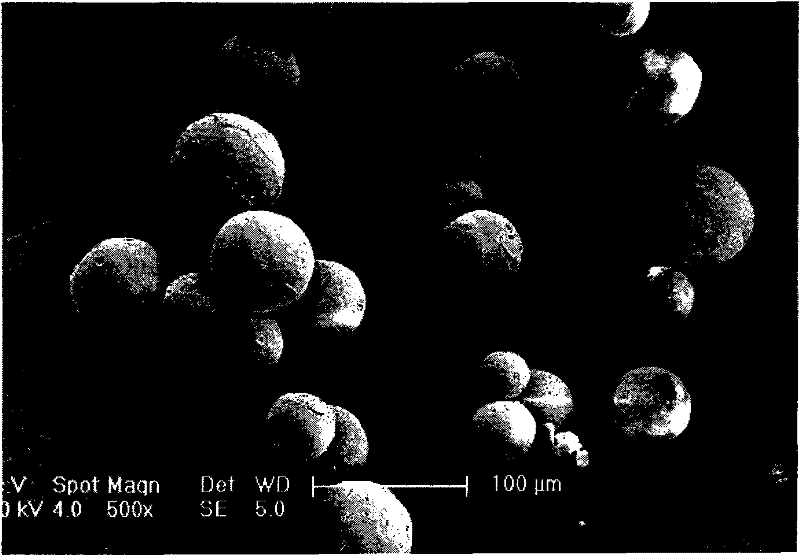
The invention relates to a levodopa methyl ester and benserazide mixed medicament slow-release microsphere composition. The composition comprises the following components in percentage by weight: 50 to 99 percent of degradable hydrophobic polymer and 1 to 50 percent of levodopa methyl ester and benserazide mixed medicament, wherein the weight ratio of the levodopa methyl ester to the benserazide is 1:1-4:1. The invention also provides a preparation method for the mixed medicament slow-release microsphere composition. The invention overcomes the disadvantage of unavailable effective treatment due to frequent missing of administration caused by the conventional single oral administration scheme and frequent oral administration requirement and provides a W / O / W preparation method. The particle diameter of the composition can be regulated and controlled from 1 to 500mu m according to different requirements without causing environmental pollution; the composition can prevent the influence on the treatment effect of the levodopa methyl ester and benserazide mixed medicament; the solution has the advantages of smooth and round surface, regularity and no adhesion; and the freeze-dried powder of the composition is white, fine, loose, non-collapse and non-adhesive and has high dispersibility.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
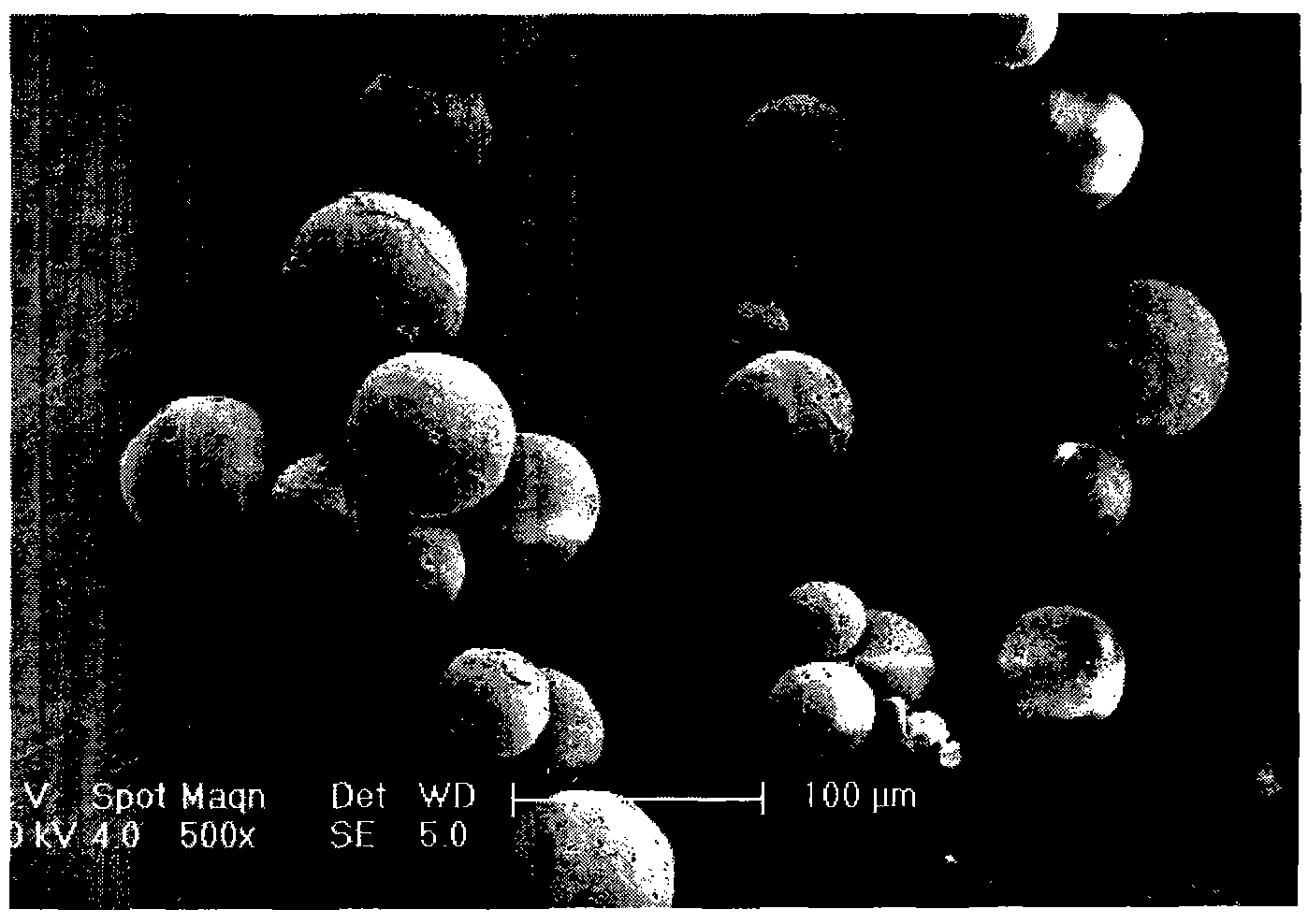
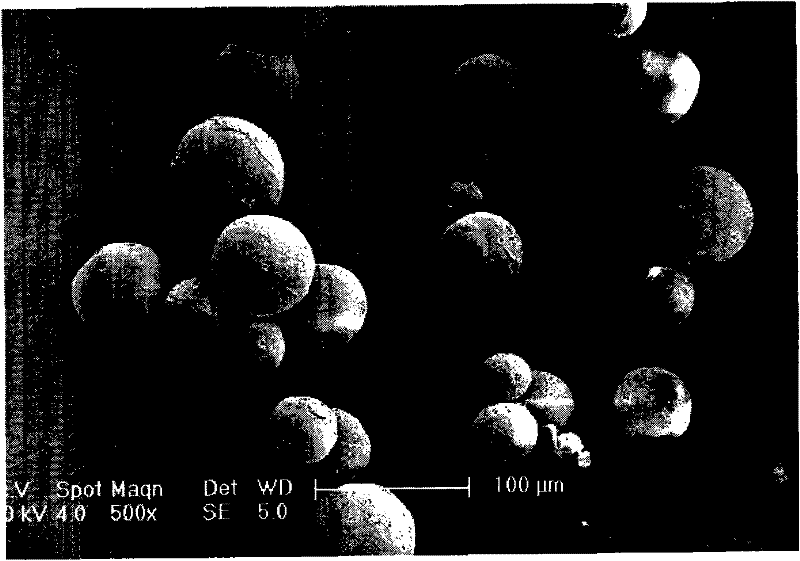
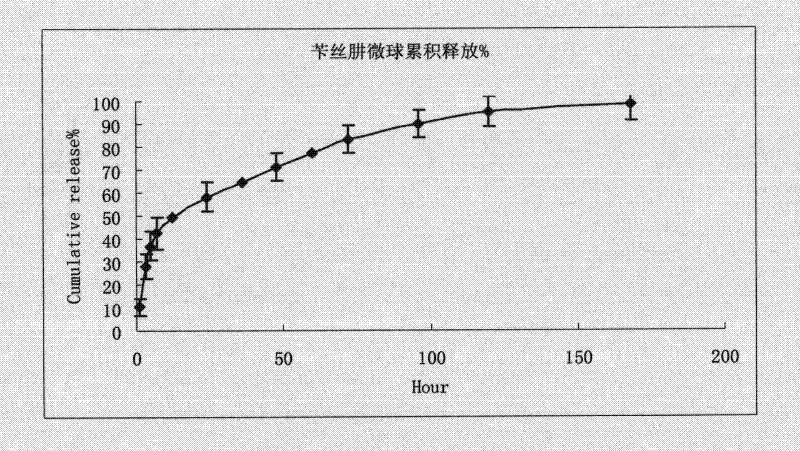
Benserazide sustained-release microspherical composition and preparation method thereof
InactiveCN101884622AAvoid instabilityGood treatment effectNervous disorderPharmaceutical non-active ingredientsChemical compositionMedicine
The invention relates to a benserazide sustained-release microspherical composition, which comprises the following components by weight percent: 40%-99% of a degradable hydrophobic polymer and 1%-60% of benserazide. The invention also provides a preparation method of the benserazide sustained-release microspherical composition. The invention can overcome the instability of benserazide during the storage process and improve the pharmaceutical therapeutic effect on patients. The method for preparing the microspherical composition can avoid the defects of low entrapment rate of the conventional W / O and W / O / W, and environmental pollution of S / O / O caused by serious burst release. In the invention, the grain diameter of the microspherical composition prepared by the method can be controlled according to different demands without environmental pollution, and the influence on the therapeutic effect of benserazide can be prevented. The surfaces of the obtained granules are smooth and rounded, the granules are regular without adhesion, the grain diameter can be regulated and controlled from 1mum to 5mum as required, and freeze-drying powder of the granules is white, exquisite and loose, is not collapsed and adhered, and has good redispersibility.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
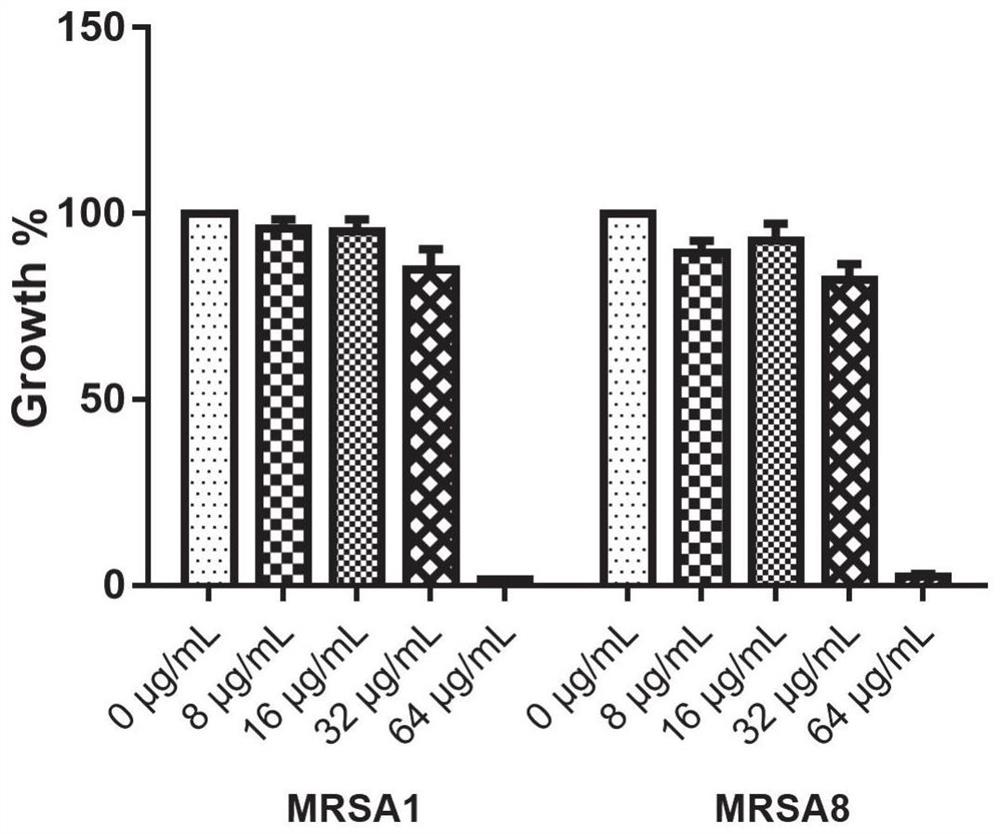
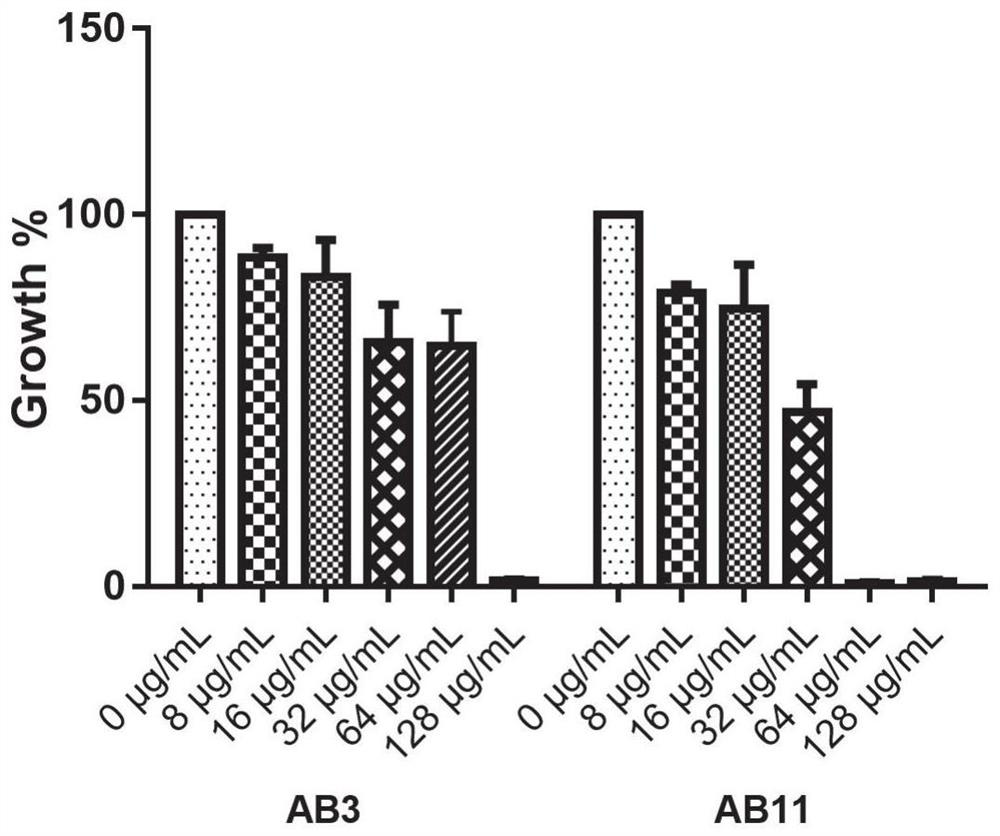
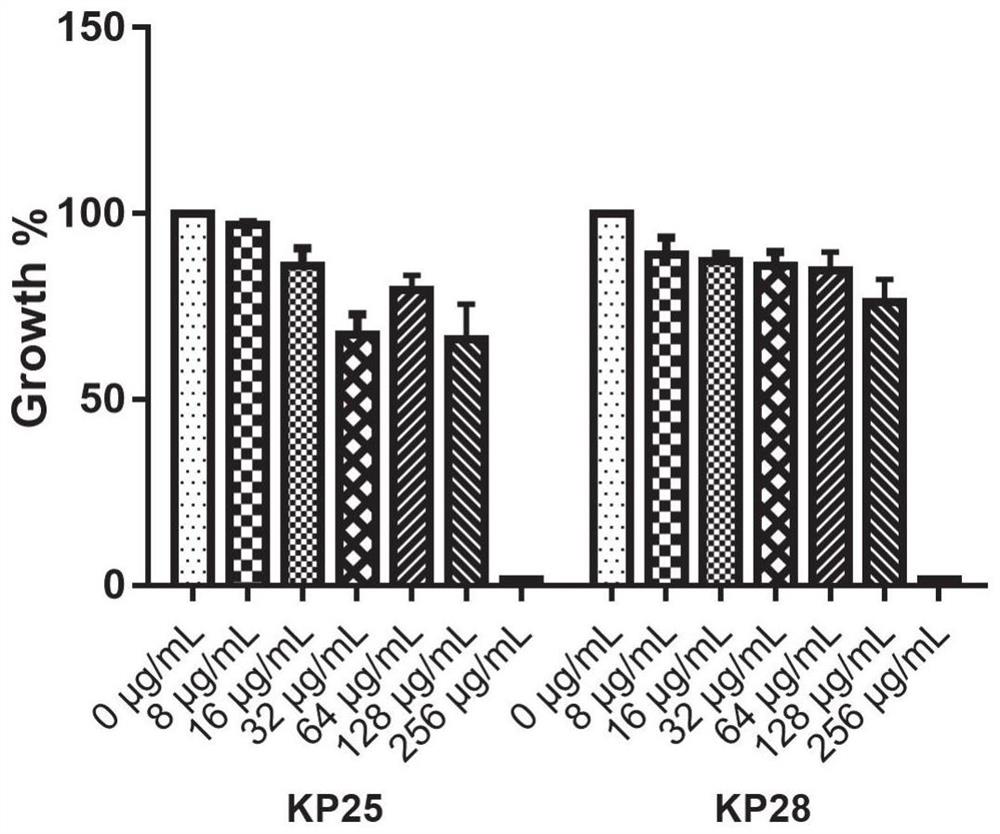
Application of benserazide as antibacterial agent
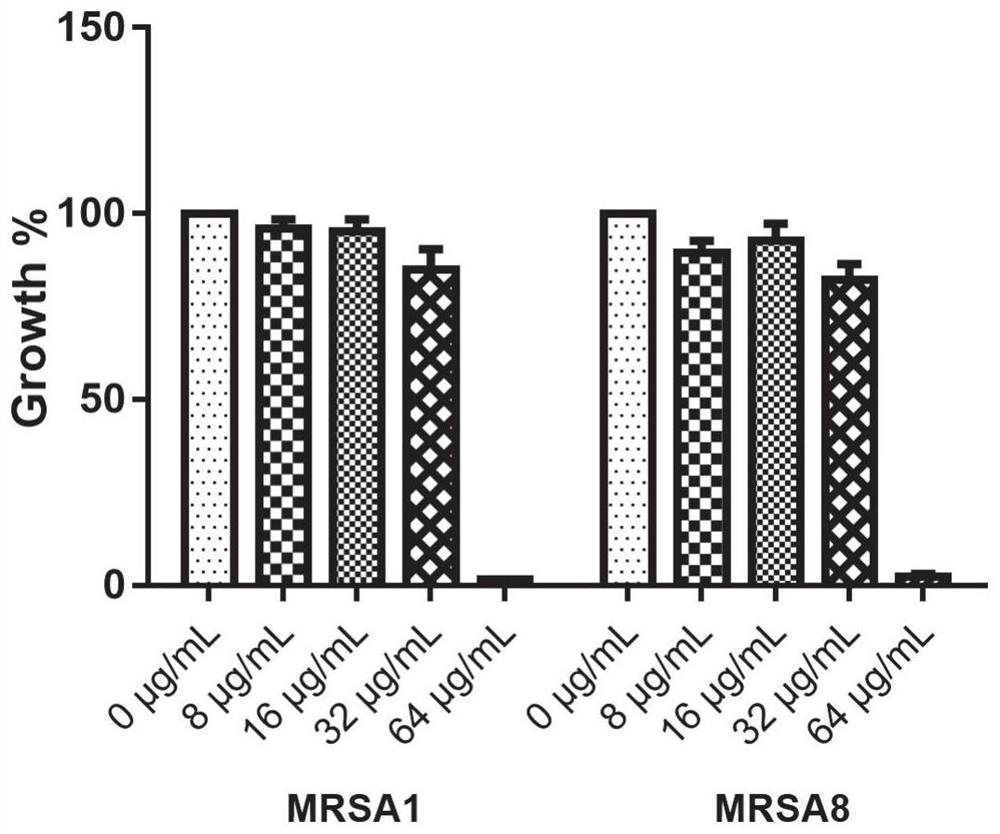
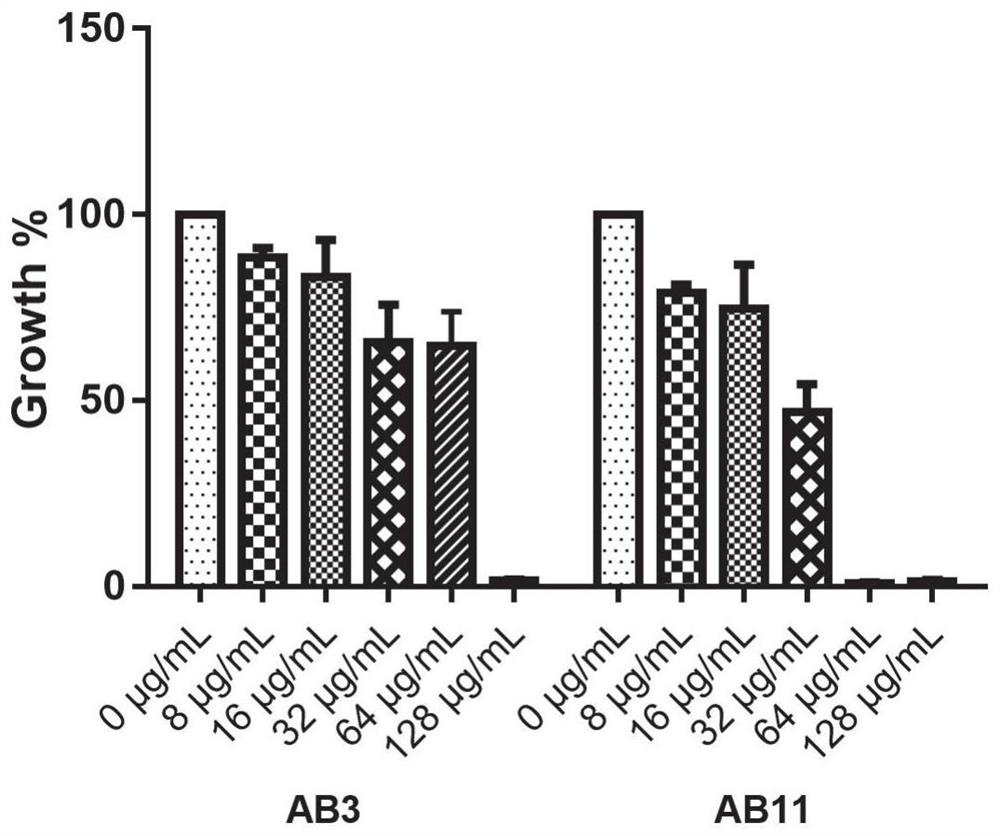
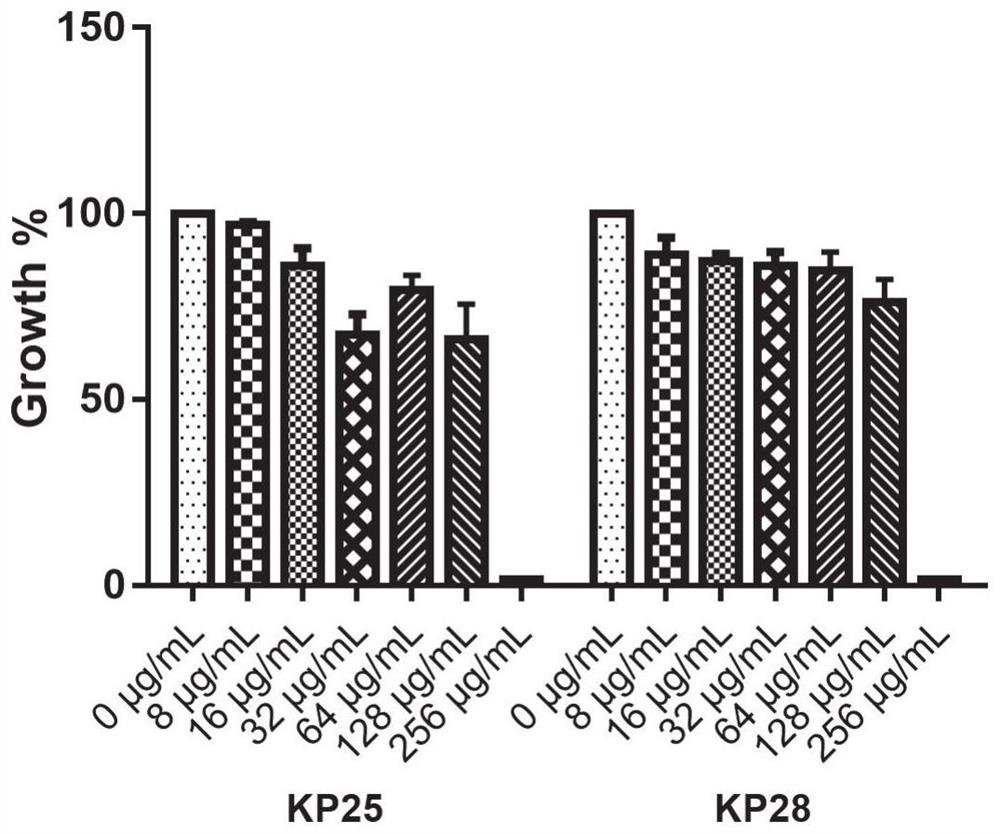
ActiveCN112076184AEnhanced inhibitory effectAvoid infectionAntibacterial agentsBiocideAntimicrobial drugBenserazide
The invention particularly relates to application of BZE as an antibacterial agent. Aiming at the current situations that drug-resistant bacteria are increased year by year, the research and development period of antibacterial drugs is long, and the marketing speed is low at present, non-antibiotic drugs with an antibacterial effect are developed in the field and used for coping with the troublesome current situation that the drug-resistant bacteria are difficult to treat. Based on the technical thought, BZE is screened and verified to have a good antibacterial effect, and has a good in-vitrocomplete inhibition effect on MRSA, MDRAB and CRKP with high drug resistance. Based on the research result, the BZE is expected to be developed as an effective component of a broad-spectrum antibacterial drug, and has good clinical treatment and economic significance.
Owner:MATERNAL & CHILD HEALTH CARE HOSPITAL OF SHANDONG PROVINCE SHANDONG UNIV
Method for treatment of parkinson's disease
Disclosed is a method for the treatment of a neurological or movement disorder, e.g., Parkinson's disease, in an individual in need thereof, by parenteral administration of levodopa and a dopa decarboxylase inhibitor (DDCI), such as carbidopa, benserazide or any combination thereof, concomitantly with oral administration of levodopa, a DDCI, such as carbidopa, benserazide, or any combination thereof.
Owner:NEURODERM
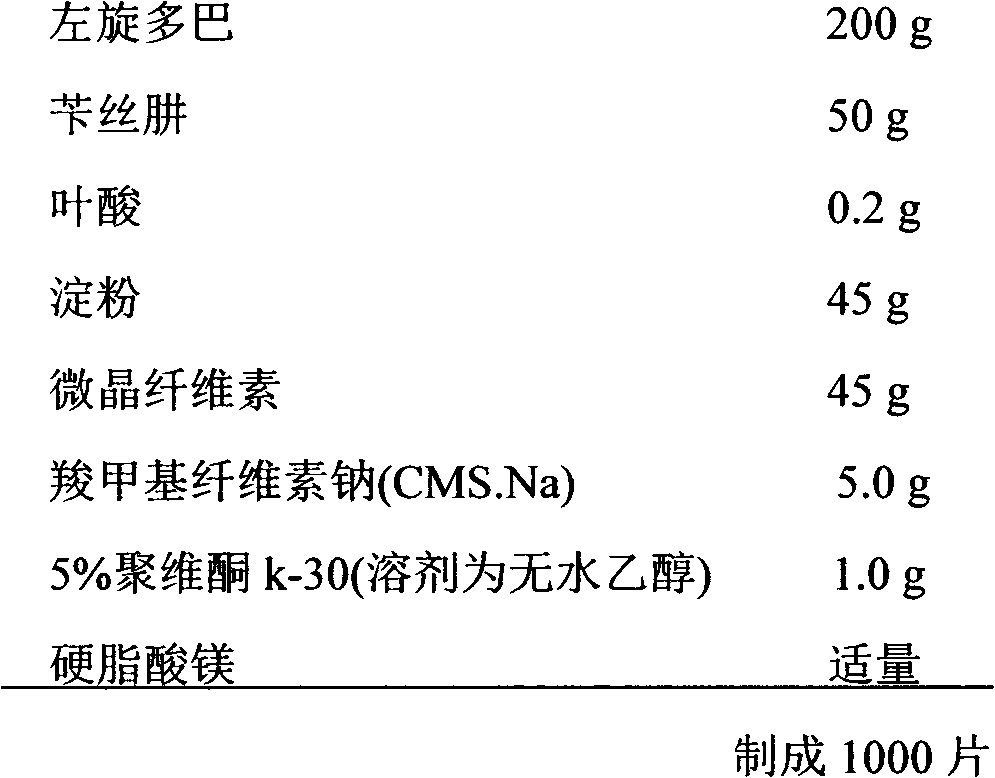
Pharmaceutical composition of levodopa/benserazide/folic acid compounds and purpose thereof
InactiveCN103127117AGood curative effectPrevent and reduce the risk of strokeOrganic active ingredientsNervous disorderPharmacyBenserazide
The invention relates to a pharmaceutical composition of levodopa / benserazide / folic acid compounds and a purpose thereof, and belongs to the technical field of pharmacy. The pharmaceutical composition comprises the levodopa with medical dose, the benserazide with the medical dose, the folic acid compounds with the medical dose and a carrier which can be accepted in the pharmacy. The dose of the levodopa is 100-300 mg, the dose of the benserazide is 30-70 mg, and the dose of the folic acid compounds is 0.2-1.6 mg. The pharmaceutical composition of the levodopa / the benserazide / the folic acid compounds and the purpose thereof have the advantages of enhancing the curative effect of Parkinson disease resistance and improving the quality of life of a patient through the synergistic effect of multiple target points; being capable of effectively preventing and lowering the occurring risk of cerebral apoplexy of a Parkinson disease patient through a homocysteine (Hcy) target point; and in addition, enabling the patient to take medicine conveniently.
Owner:SUZHOU FAMO BIOLOGICAL TECH
Levodopa methyl ester and benserazide mixed medicament slow-release microsphere composition and preparation method thereof
InactiveCN101879153BNo pollutionAvoid the effects of treatmentOrganic active ingredientsNervous disorderMicrosphereHydrophobic polymer
The invention relates to a levodopa methyl ester and benserazide mixed medicament slow-release microsphere composition. The composition comprises the following components in percentage by weight: 50 to 99 percent of degradable hydrophobic polymer and 1 to 50 percent of levodopa methyl ester and benserazide mixed medicament, wherein the weight ratio of the levodopa methyl ester to the benserazide is 1:1-4:1. The invention also provides a preparation method for the mixed medicament slow-release microsphere composition. The invention overcomes the disadvantage of unavailable effective treatment due to frequent missing of administration caused by the conventional single oral administration scheme and frequent oral administration requirement and provides a W / O / W preparation method. The particle diameter of the composition can be regulated and controlled from 1 to 500mu m according to different requirements without causing environmental pollution; the composition can prevent the influence on the treatment effect of the levodopa methyl ester and benserazide mixed medicament; the solution has the advantages of smooth and round surface, regularity and no adhesion; and the freeze-dried powder of the composition is white, fine, loose, non-collapse and non-adhesive and has high dispersibility.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
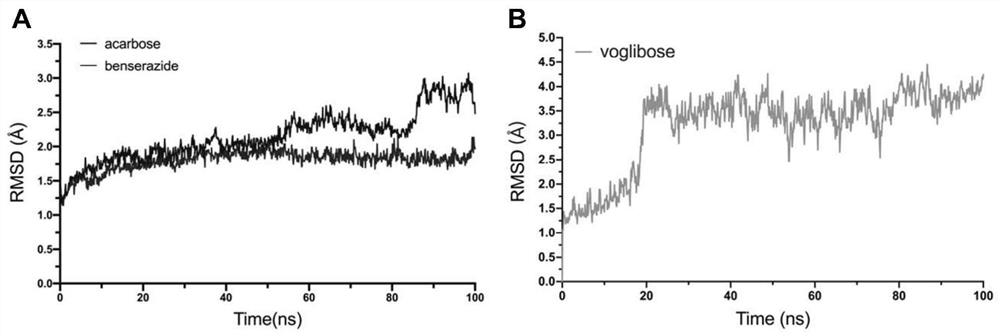
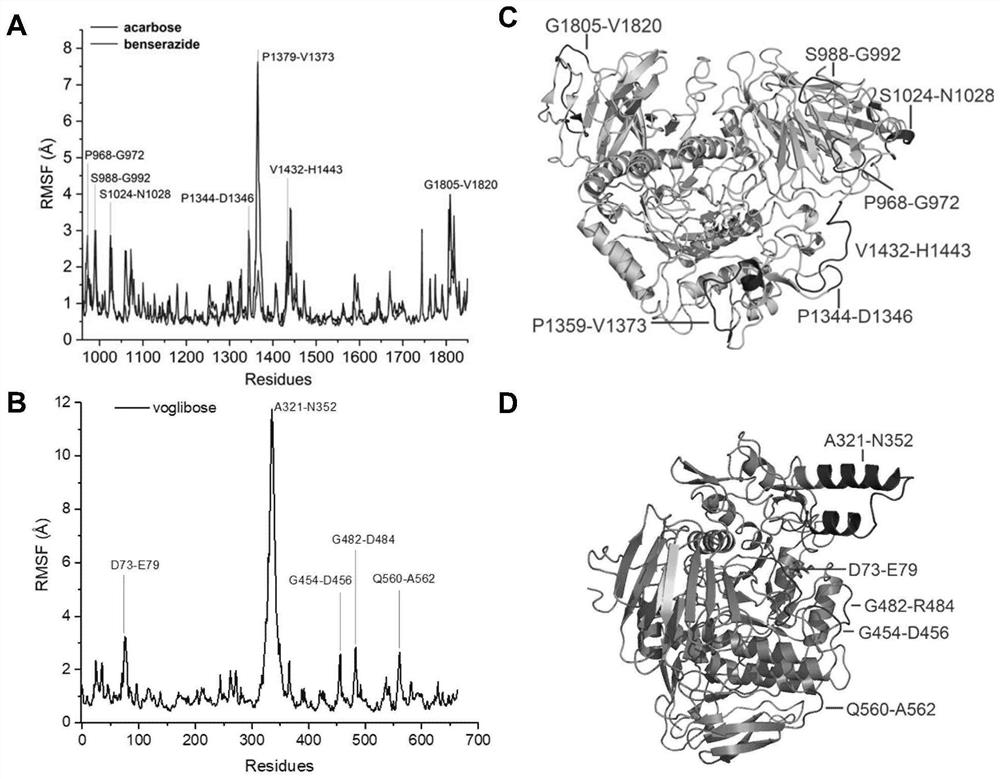
Application of benserazide as alpha-glucosidase inhibitor
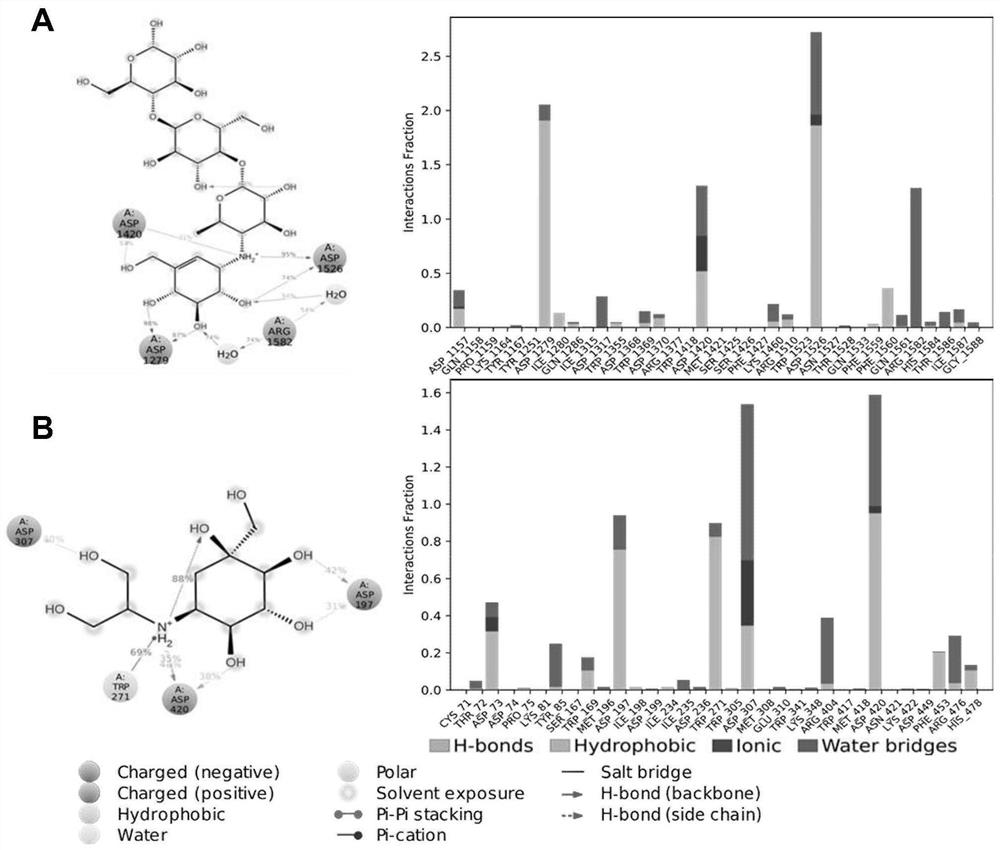
PendingCN111627504AImprove bindingPromote rapid developmentChemical property predictionComputational theoretical chemistryBenserazidePharmacophore
The invention belongs to the technical field of medicine. The invention in particular relates to the applicatoin of benserazide (The International Nonproprietary Names for Pharmaceutical Substances (INN) is Benserazide) as an alpha-glucosidase inhibitor, and a structure-based computational simulation screening method. Benserazide can be stably combined with an active site of alpha-glucosidase to inhibit the activity of alpha-glucosidase, so that the effect of reducing blood sugar is achieved. According to the invention, based on a thought of new use of old drugs, FDA-approved drugs are subjected to pharmacophore-based virtual screening, molecular docking and molecular dynamics simulation processes, and it is found that benserazide can be stably combined at an active site of alpha-glucosidase, and in-vitro experiments show that benserazide is an alpha-glucosidase inhibitor, so that the new application of the benserazide in reducing blood sugar is excavated. The invention not only provides a new angle for benserazide as a hypoglycemic drug, but also provides an example for rapid development of a novel alpha-glucosidase inhibitor.
Owner:SHENYANG PHARMA UNIVERSITY
Method for treatment of parkinson's disease
Disclosed is a method for the treatment of a neurological or movement disorder, e.g., Parkinson's disease, in an individual in need thereof, by parenteral administration of levodopa and a dopa decarboxylase inhibitor (DDCI), such as carbidopa, benserazide or any combination thereof, concomitantly with oral administration of levodopa, a DDCI, such as carbidopa, benserazide, or any combination thereof.
Owner:NEURODERM
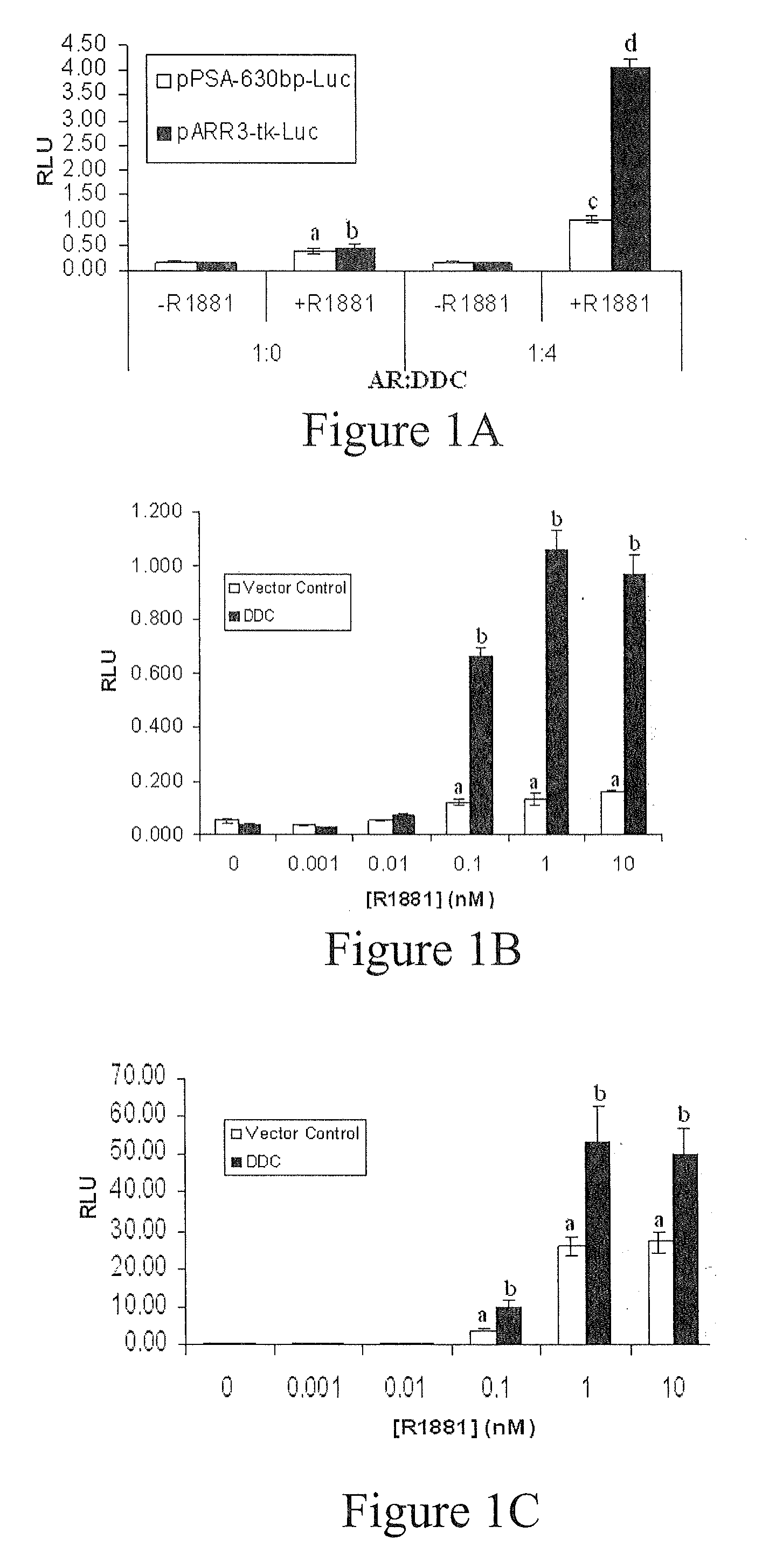
Treatment of prostate cancer with DDC inhibitor
Prostate cancer comes in various forms and has proven difficult to treat. Provided herein are various methods and compositions for treating all forms of prostate cancers with dopa decarboxylase (DDC) inhibitors. These dopa decarboxylase inhibitors include carbidopa (α-Methyl-dopahydrazine), MFMD (α-monofluoromethyldopa), NSD-1015 (3-hydroxybenzylhydrazine), Methyldopa (L-α-Methyl-3,4-dihydroxyphenylalanine) or benserazide, and the inhibitors may be used in combination. DDC inhibitors may also be used to inhibit the progression of prostate cancer to androgen-independence and neuroendocrine prostate cancer.
Owner:THE UNIV OF BRITISH COLUMBIA
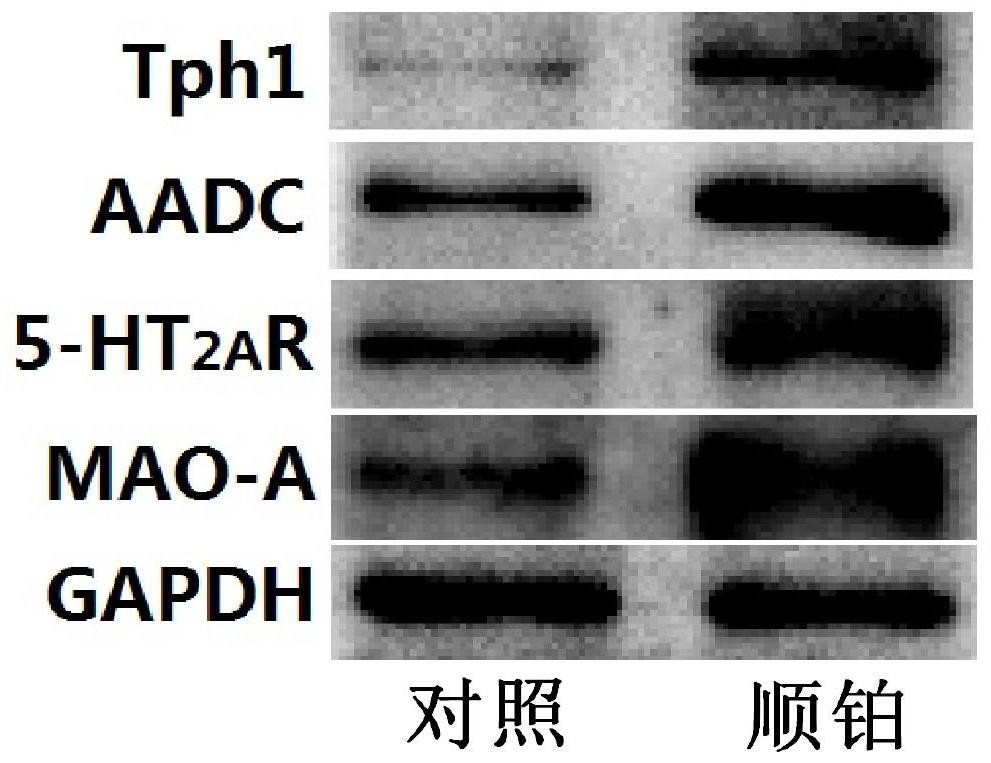
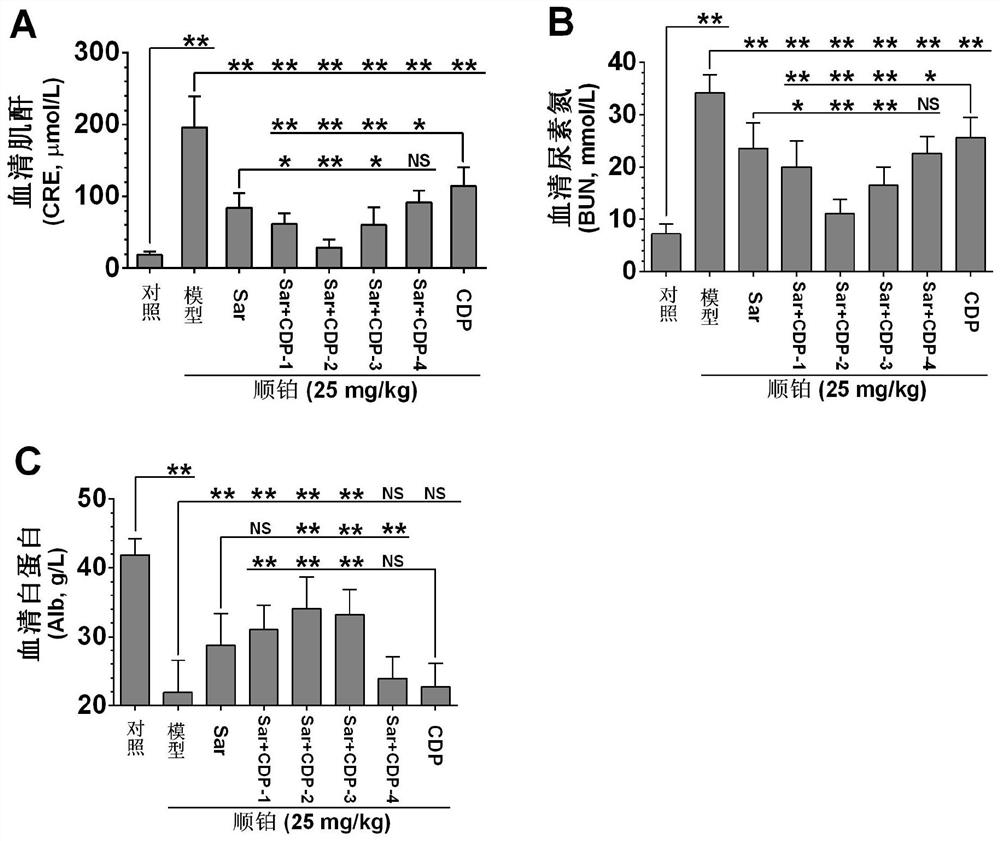
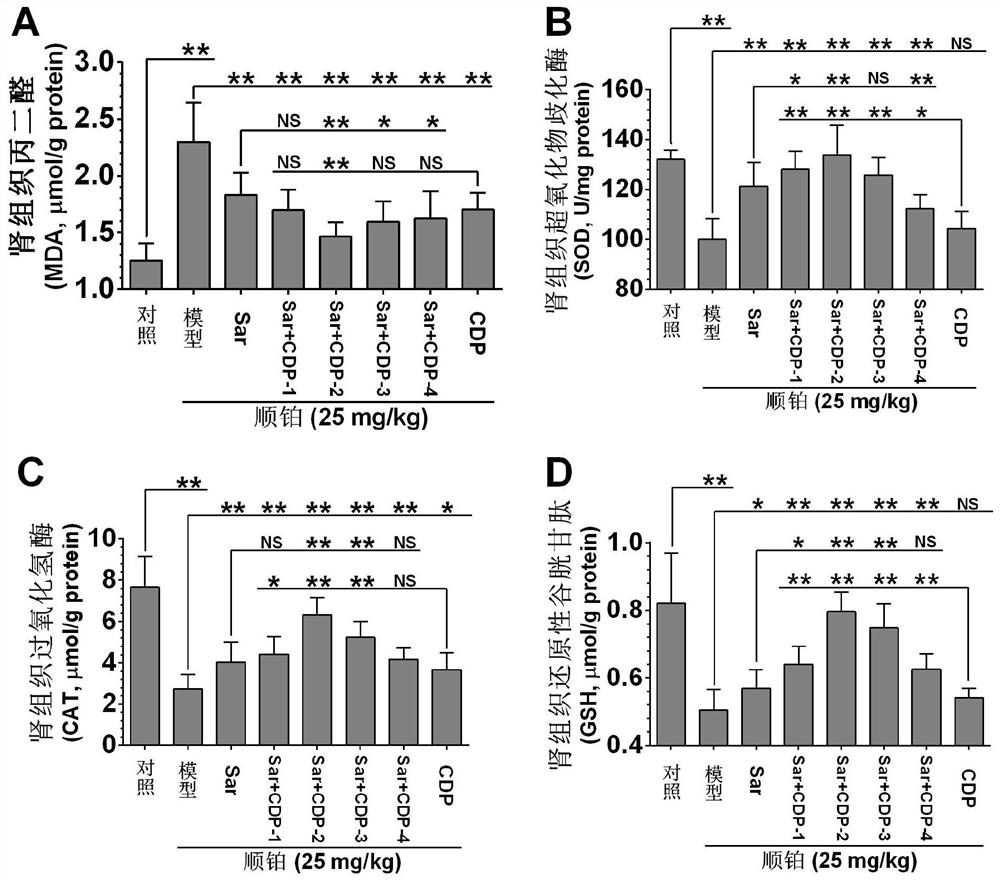
Application of a compound pharmaceutical composition in preparation of medicine for treating acute kidney injury
ActiveCN110548150BInhibit 5-HT synthesisInhibit synthesisOrganic active ingredientsUrinary disorderSarpogrelateBenserazide
The invention discloses the application of a compound pharmaceutical composition in the preparation of medicines for treating acute kidney injury. The compound pharmaceutical composition in the invention uses sarcogrelate and a 5-serotonin synthesis inhibitor as active ingredients, and the 5-serotonin synthesis inhibitor Carbidopa or benserazide. Sagrelate and carbidopa or benserazide can inhibit the synthesis of 5-HT 2 receptors and 5-HT in kidney cells, and have an obvious synergistic effect on the treatment of acute kidney injury, and have a significant effect on the treatment of acute kidney injury. Effect.
Owner:CHINA PHARM UNIV
Piribedil, levodopa and benserazide compound sustained-release three-layer tablet and preparation method thereof
PendingCN111084777AOvercome the lack of mutual influenceEasy to administerOrganic active ingredientsNervous disorderProlonged-release tabletBenserazide
The present invention discloses a piribedil, levodopa and benserazide compound sustained-release three-layer tablet and a preparation method thereof. The piribedil, levodopa and benserazide compound sustained-release three-layer tablet is characterized in that the tablet is a three-layered sustained-release tablet, an active ingredient of a first layer is piribedil, a second layer is an isolationlayer, and active ingredients of a third layer are levodopa and benserazide. The preparation process is as follows: wet granulation of the first layer, dry granulation of the second layer and the third layer, tableting by a three-layer tableting machine, sustained-release semi-permeable membrane covering, perforating, and then quick-release coating film wrapping. The piribedil, levodopa and benserazide compound sustained-release three-layer tablet can be prepared, feasibility of simultaneous administration is realized, stability of each active ingredient can be guaranteed and at the same timestable release of the effective ingredients is ensured.
Owner:山西卫生健康职业学院
Benserazide sustained-release microspherical composition and preparation method thereof
InactiveCN101884622BAvoid instabilityGood treatment effectNervous disorderPharmaceutical non-active ingredientsChemical compositionHydrophobic polymer
The invention relates to a benserazide sustained-release microspherical composition, which comprises the following components by weight percent: 40%-99% of a degradable hydrophobic polymer and 1%-60% of benserazide. The invention also provides a preparation method of the benserazide sustained-release microspherical composition. The invention can overcome the instability of benserazide during the storage process and improve the pharmaceutical therapeutic effect on patients. The method for preparing the microspherical composition can avoid the defects of low entrapment rate of the conventional W / O and W / O / W, and environmental pollution of S / O / O caused by serious burst release. In the invention, the grain diameter of the microspherical composition prepared by the method can be controlled according to different demands without environmental pollution, and the influence on the therapeutic effect of benserazide can be prevented. The surfaces of the obtained granules are smooth and rounded, the granules are regular without adhesion, the grain diameter can be regulated and controlled from 1mum to 5mum as required, and freeze-drying powder of the granules is white, exquisite and loose, is not collapsed and adhered, and has good redispersibility.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
Use of benserazide as an antibacterial agent
ActiveCN112076184BEnhanced inhibitory effectAvoid infectionAntibacterial agentsOrganic active ingredientsAntimicrobial drugBenserazide
The present invention specifically relates to the use of BZE as an antibacterial agent. In view of the current situation that drug-resistant bacteria are increasing year by year, and the research and development cycle of antibacterial drugs is long, and the speed of marketing is slow, this field develops non-antibiotic drugs with antibacterial effects to deal with the difficult situation that drug-resistant bacteria are difficult to treat. Based on this technical idea, the present invention screens and verifies that BZE has a good antibacterial effect, and has a good in vitro complete inhibitory effect on MRSA, MDRAB and CRKP with high drug resistance. Based on the results of this study, BZE is expected to be developed as an active ingredient of a broad-spectrum antibacterial drug, which has good clinical and economic significance.
Owner:MATERNAL & CHILD HEALTH CARE HOSPITAL OF SHANDONG PROVINCE SHANDONG UNIV
Microsphere combination medicament containing antiparkinsonism drug and application thereof
InactiveCN101879143BGood synergyLower AIM scoreOrganic active ingredientsPharmacologic actionBenserazide
The invention relates to an application of a microsphere composition containing an antiparkinsonism drug to preparation of a medicine for preventing and treating parkinson diseases and complicating diseases of parkinson diseases. The microsphere composition containing the antiparkinsonism drug is selected from benserazide microspheres, L-dopa methyl ester microspheres, the L-dopa methyl ester microspheres and the benserazide microspheres, or L-dopa methyl ester and benserazide mixing drug microspheres. The invention also provides a microsphere combination medicament containing the antiparkinsonism drug. The invention explores novel medical application for the microsphere combination medicament containing the antiparkinsonism drug and develops a novel application field. The microsphere combination medicament containing the antiparkinsonism drug of the invention is safe and nontoxic, has strong pharmacological action and indicates good medicinal prospect.
Owner:XIN HUA HOSPITAL AFFILIATED TO SHANGHAI JIAO TONG UNIV SCHOOL OF MEDICINE
L-dopa and/or dopa decarboxylse inhibitors conjugated to sugar for the treatment of dopamine-responsive disorders
PendingUS20220016253A1Hydrazine preparationOrganic active ingredientsMedical disorderCarboxyl radical
The present invention provides conjugates comprising a sugar such as mannitol and one or more L-DOPA and / or DOPA decarboxylse inhibitors including, inter alia, L-DOPA, carbidopa, benserazide, or a combination thereof, wherein the sugar is conjugated to the carboxyl group of the L-DOPA and / or DOPA decarboxylse inhibitor / s) via a hydroxyl group of the sugar. The present invention further provides related pharmaceutical compositions and methods of producing the conjugates, as well as methods of use for treating medical disorders responsive to dopamininergic stimulation such as movement disorders including, inter alia, Parkinson's Disease.
Owner:B G NEGEV TECH & APPL LTD
Application of benserazide and its composition with fluconazole in the preparation of antifungal products
ActiveCN111700885BRich in clinical use purposesHigh economic valueOrganic active ingredientsAntimycoticsBenserazideFluconazole
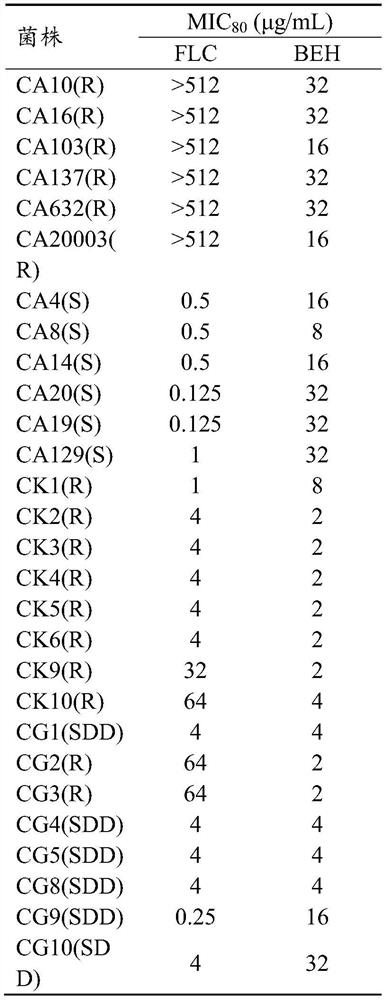
The invention specifically relates to the application of benserazide and its composition with fluconazole in the preparation of antifungal products. In recent years, the rate of fungal infection and the resulting mortality have shown an upward trend, among which Candida is the most serious threat to the body. At present, azole drugs are the first-line drugs for antifungal treatment, but the treatment effect is not satisfactory due to the continuous emergence of drug-resistant bacteria. Aiming at the current situation, the present invention provides the antifungal effect of benserazide, which enriches the compound entities with antifungal effect. Further, the research of the present invention also shows that benserazide can cooperate with fluconazole to treat Candida albicans, Gram The more harmful Candida such as Candida tenida and Candida glabrata have a good inhibitory effect, improve the drug sensitivity of drug-resistant strains, and reduce the dosage of drugs.
Owner:QIANFOSHAN HOSPITAL OF SHANDONG
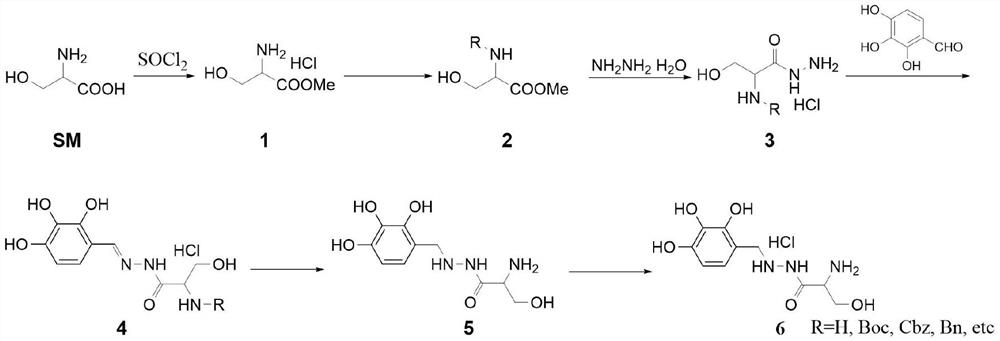
Method for synthesizing benserazide hydrochloride by utilizing fixed bed hydrogenation equipment
PendingCN112876379AReduce dosageEasy to purifyCarbamic acid derivatives preparationOrganic compound preparationPtru catalystBenserazide
The invention discloses a method for synthesizing benserazide hydrochloride by using fixed bed hydrogenation equipment, which is characterized in that a compound 1 reacts with an amino protective agent to carry out an amino protection reaction, so that the subsequent purification is easy, and the dosage of hydrazine hydrate in the synthesis method is small; according to the method, fixed bed hydrogenation equipment is used for synthesis, a fixed bed reactor is filled with a solid catalyst or a solid to realize a heterogeneous reaction process, the catalyst in the fixed bed reactor is relatively fixed, a reaction liquid flows through a bed layer, and the reaction liquid flows through the bed layer by adjusting the flow rate and the reaction pressure; qualified products can be obtained after reaction liquid flows out of the fixed bed, continuous production can be achieved, product deterioration caused by long-time contact of the products and the catalyst can be avoided, the same amount of products can be produced due to continuous production, the reactor size can be very small, the safety problem caused by high-pressure reaction is greatly reduced, the amount of the catalyst used in the reaction is less, and the production cost is reduced.
Owner:HEFEI LIFEON PHARMA
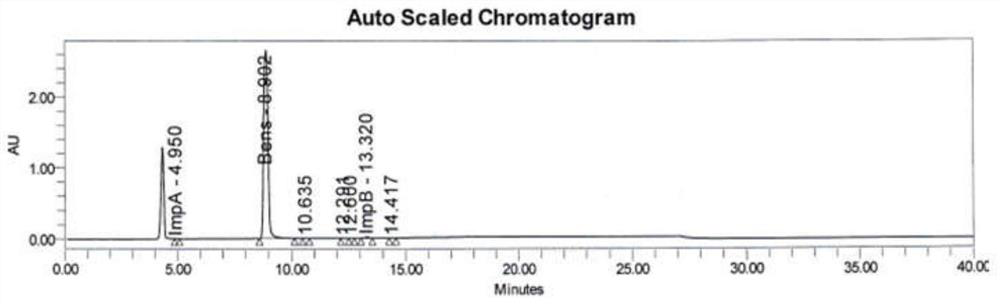
Method for analyzing benserazide impurity A in levodopa and benserazide hydrochloride compound preparation
PendingCN111812227AFast, effective and accurate detectionEasy to separateComponent separationBenserazideBENSERAZIDE HYDROCHLORIDE
The invention provides a method for detecting the content of a benserazide impurity A in a levodopa and benserazide hydrochloride compound preparation. According to the method, a hydrophilic chromatographic column is adopted, an aqueous solution containing sodium heptanesulfonate and monopotassium phosphate and having a pH value of 1.7-9.0 is adopted as a mobile phase A, methanol is adopted as a mobile phase B, and a staged gradient elution method is adopted to separate a benserazide impurity A and determine the content of the benserazide impurity A in a levodopa and benserazide hydrochloridecompound preparation. The method is good in separation effect, simple to operate, high in recovery rate, high in sensitivity and wide in linear range, and has important practical significance in the aspect of quality control in the preparation process of the preparation.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Blood brain barrier high-permeability hexokinase inhibitor as well as synthesis method and application thereof
PendingCN114539204AImprove permeabilityRegulated hexokinaseNervous disorderCarbamic acid derivatives preparationBenserazideDepressant
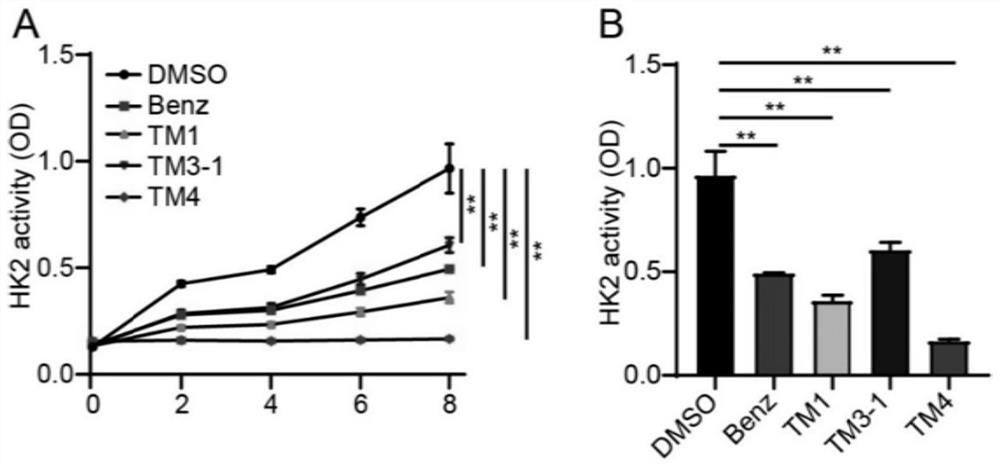
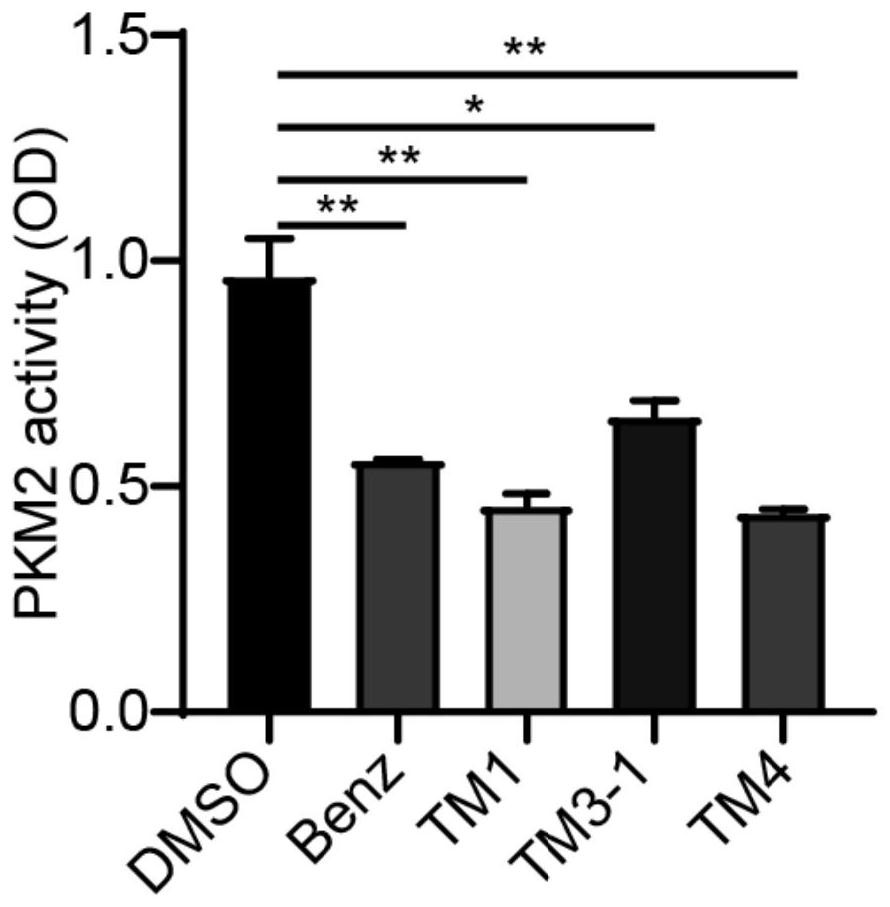
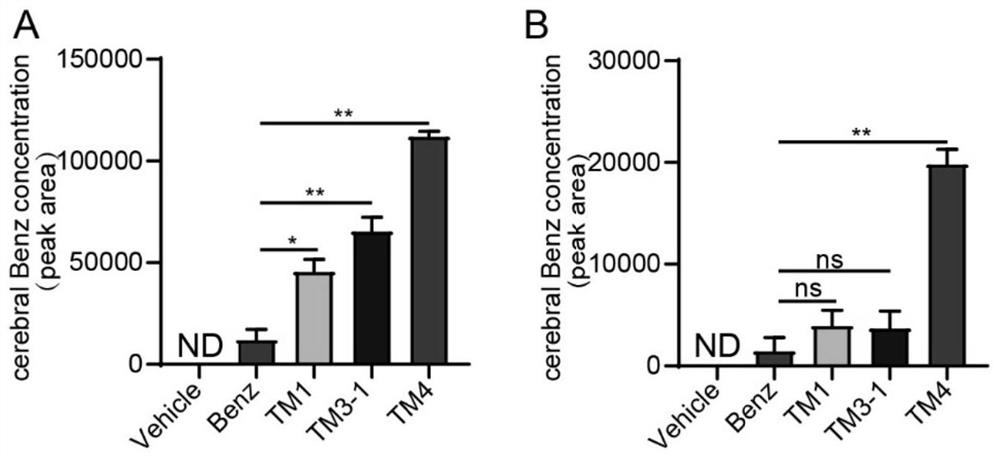
The invention discloses a blood-brain barrier high-permeability hexokinase inhibitor as well as a synthesis method and application thereof. Wherein the inhibitor has a structure as shown in a formula I. The compound provided by the invention can solve the problems that benserazide molecules have high polarity and have a plurality of phenolic hydroxyl groups, so that the bioavailability is low and the benserazide is difficult to penetrate through a blood-brain barrier. Therefore, the inhibitor disclosed by the invention has the advantages that the cell permeability and bioavailability are remarkably improved, so that the effect of treating the Alzheimer's disease, the Parkinson's disease and the multiple sclerosis is achieved by penetrating through the blood brain barrier, and the inhibitor has a wide application prospect in the field of central nervous system diseases.
Owner:福建金兰厚普生物科技有限公司
Method for treatment of parkinson's disease
Disclosed is a method for the treatment of a neurological or movement disorder, e.g., Parkinson's disease, in an individual in need thereof, by parenteral administration of levodopa and a dopa decarboxylase inhibitor (DDCI), such as carbidopa, benserazide or any combination thereof, concomitantly with oral administration of levodopa, a DDCI, such as carbidopa, benserazide, or any combination thereof.
Owner:NEURODERM
Method for treatment of Parkinson's disease
Disclosed is a method for the treatment of a neurological or movement disorder, e.g., Parkinson's disease, in an individual in need thereof, by parenteral administration of levodopa and a dopa decarboxylase inhibitor (DDCI), such as carbidopa, benserazide or any combination thereof, concomitantly with oral administration of levodopa, a DDCI, such as carbidopa, benserazide, or any combination thereof.
Owner:NEURODERM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com