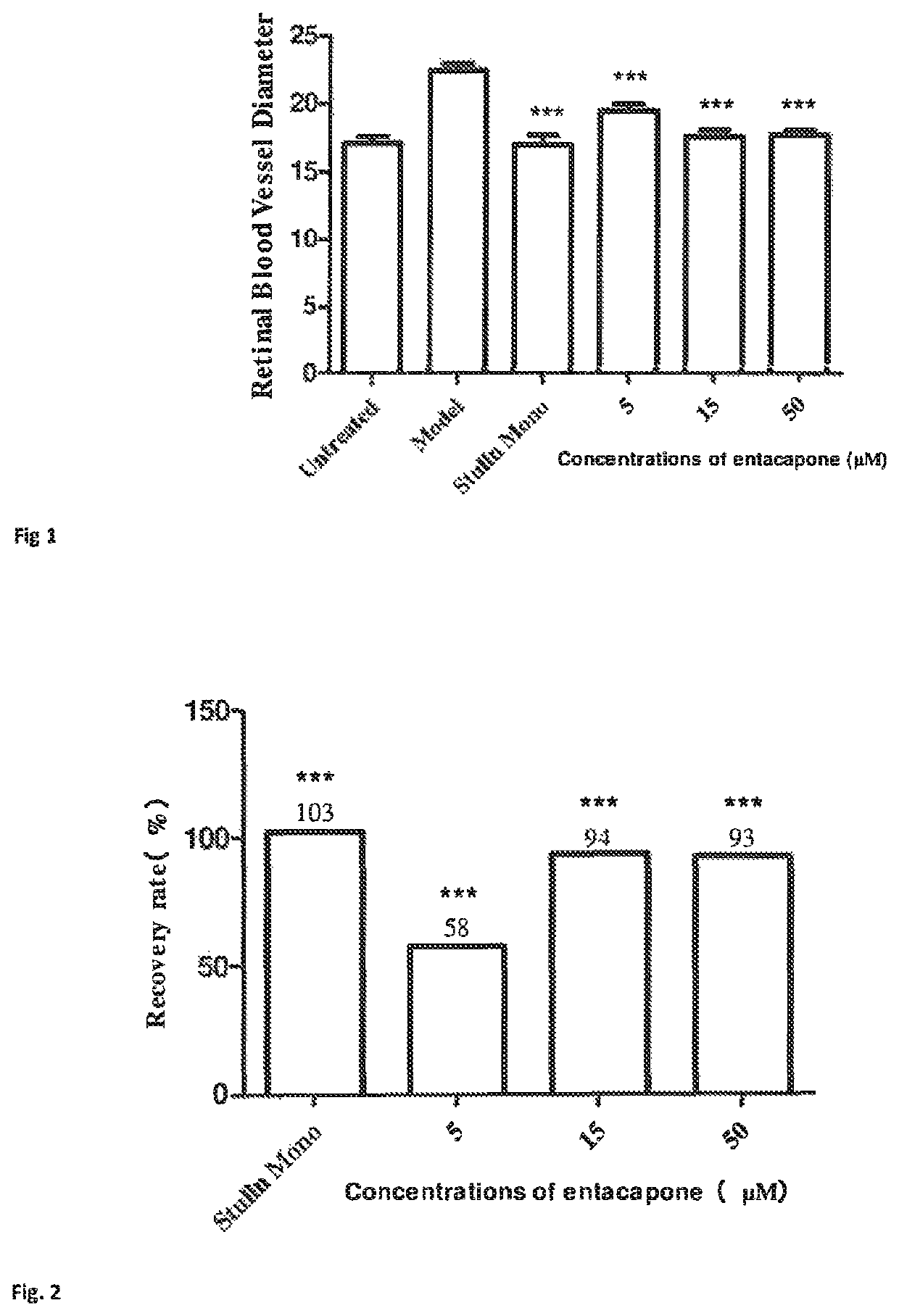
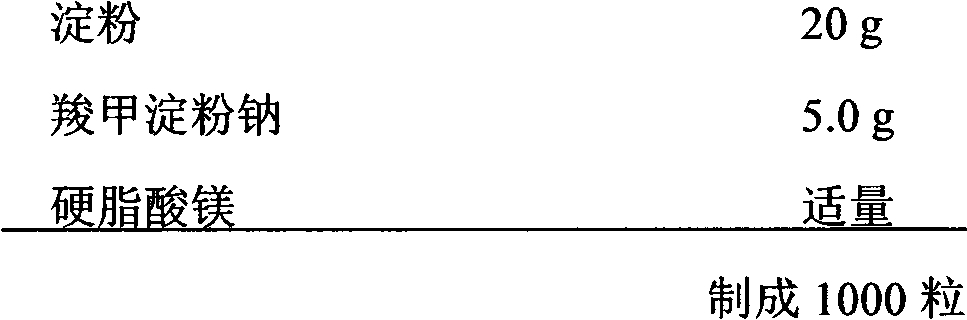Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
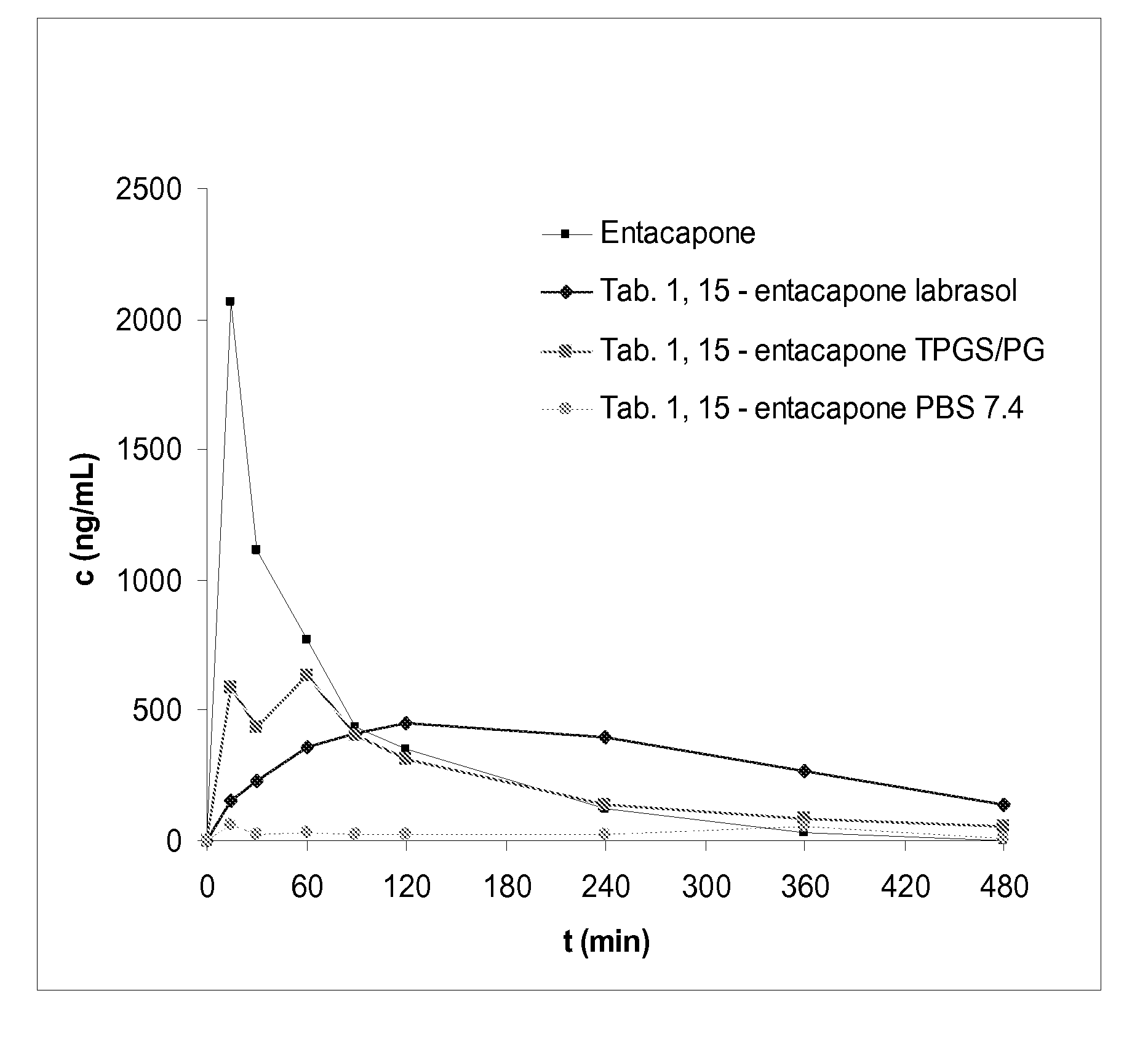
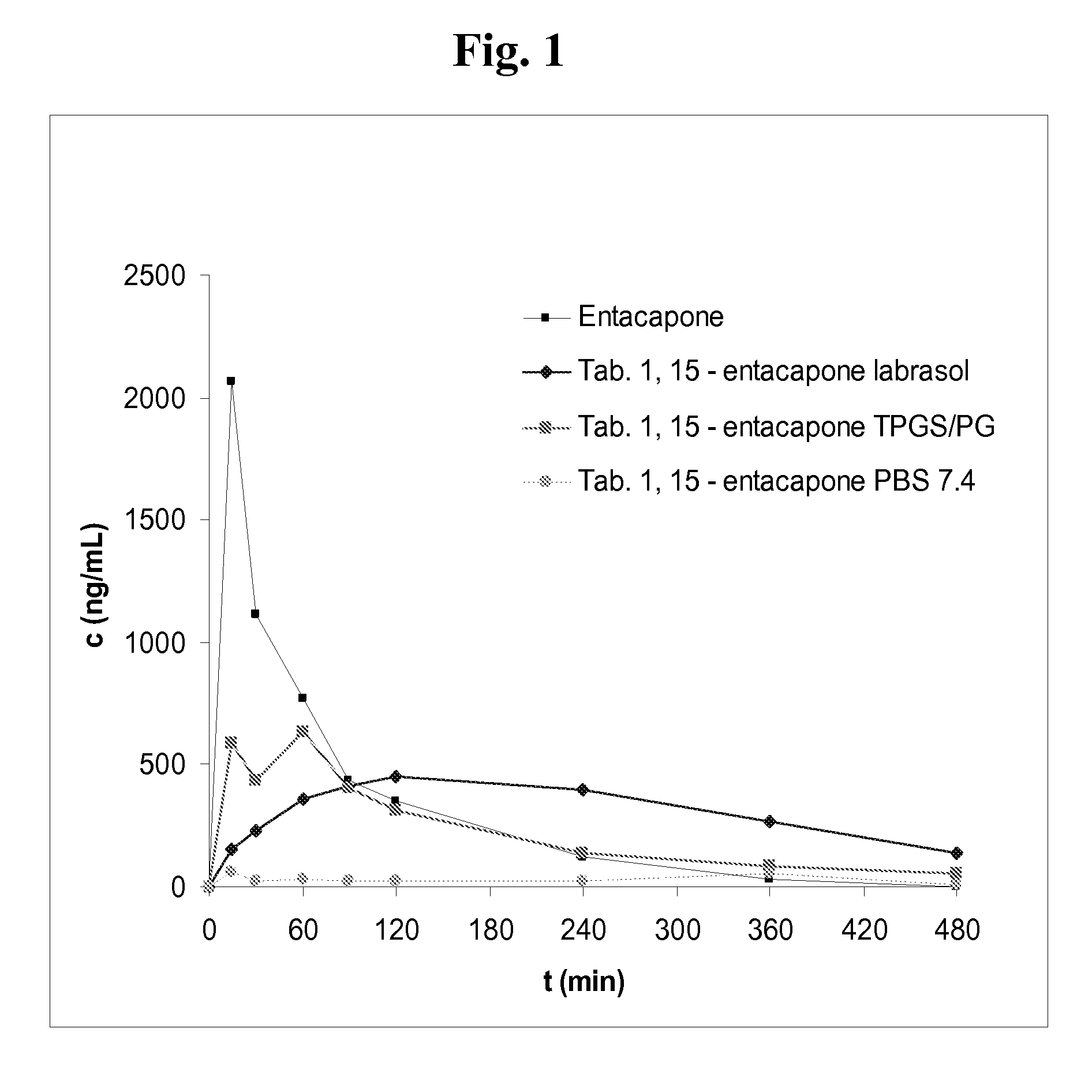
57 results about "Entacapone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
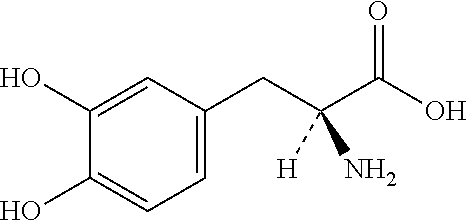
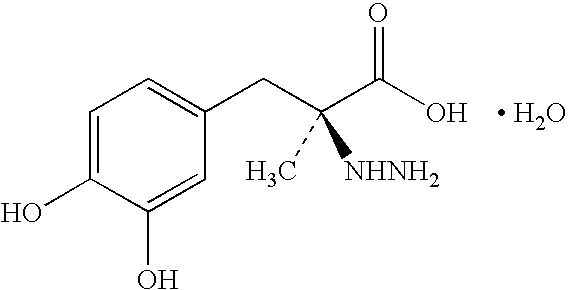
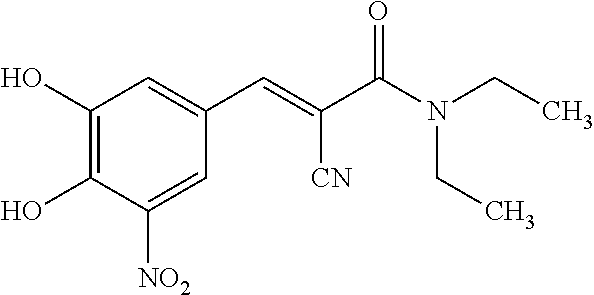
This medication is used with other medications (levodopa/carbidopa) to treat Parkinson's disease.
Extended Release Pharmaceutical Composition Of Entacapone Or Salts Thereof
InactiveUS20110229561A1Reducing “ wearing off ” phenomenonReduce wearBiocideNervous disorderTriple combinationCarbidopa
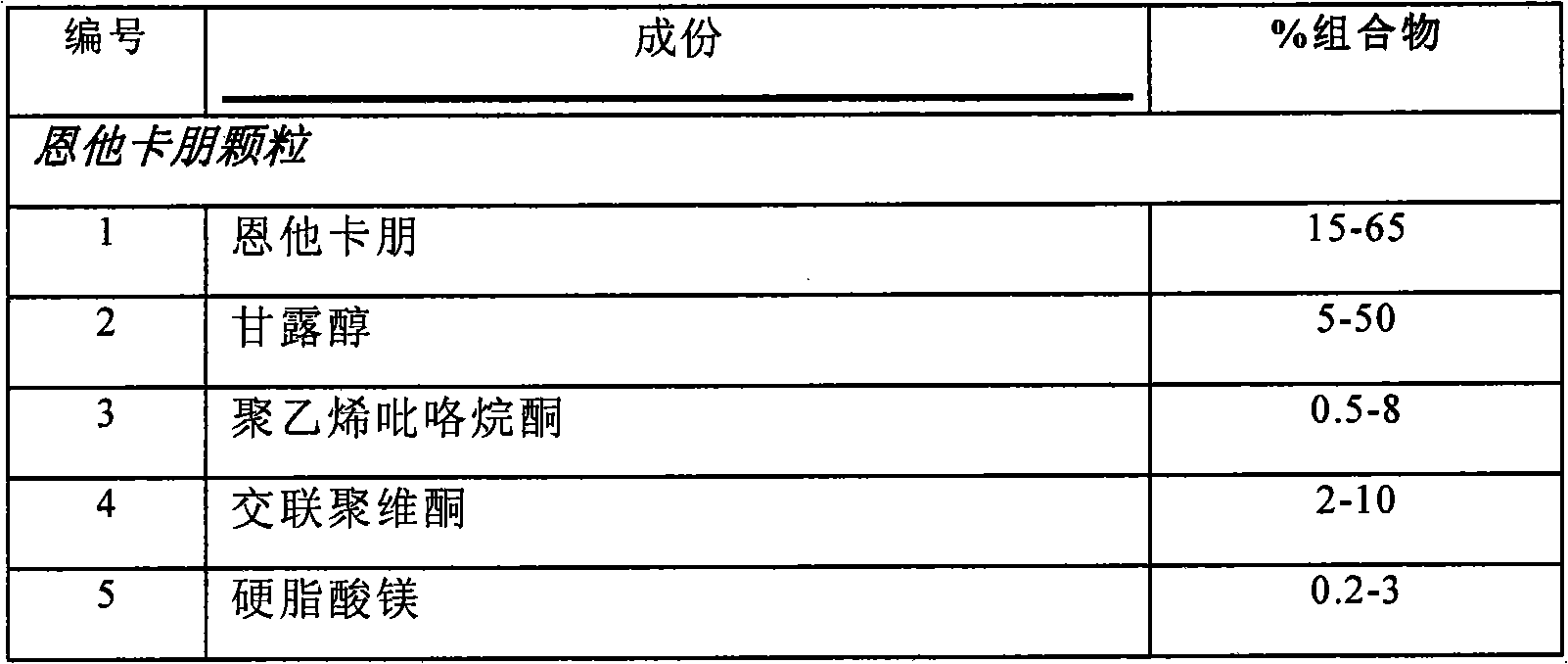
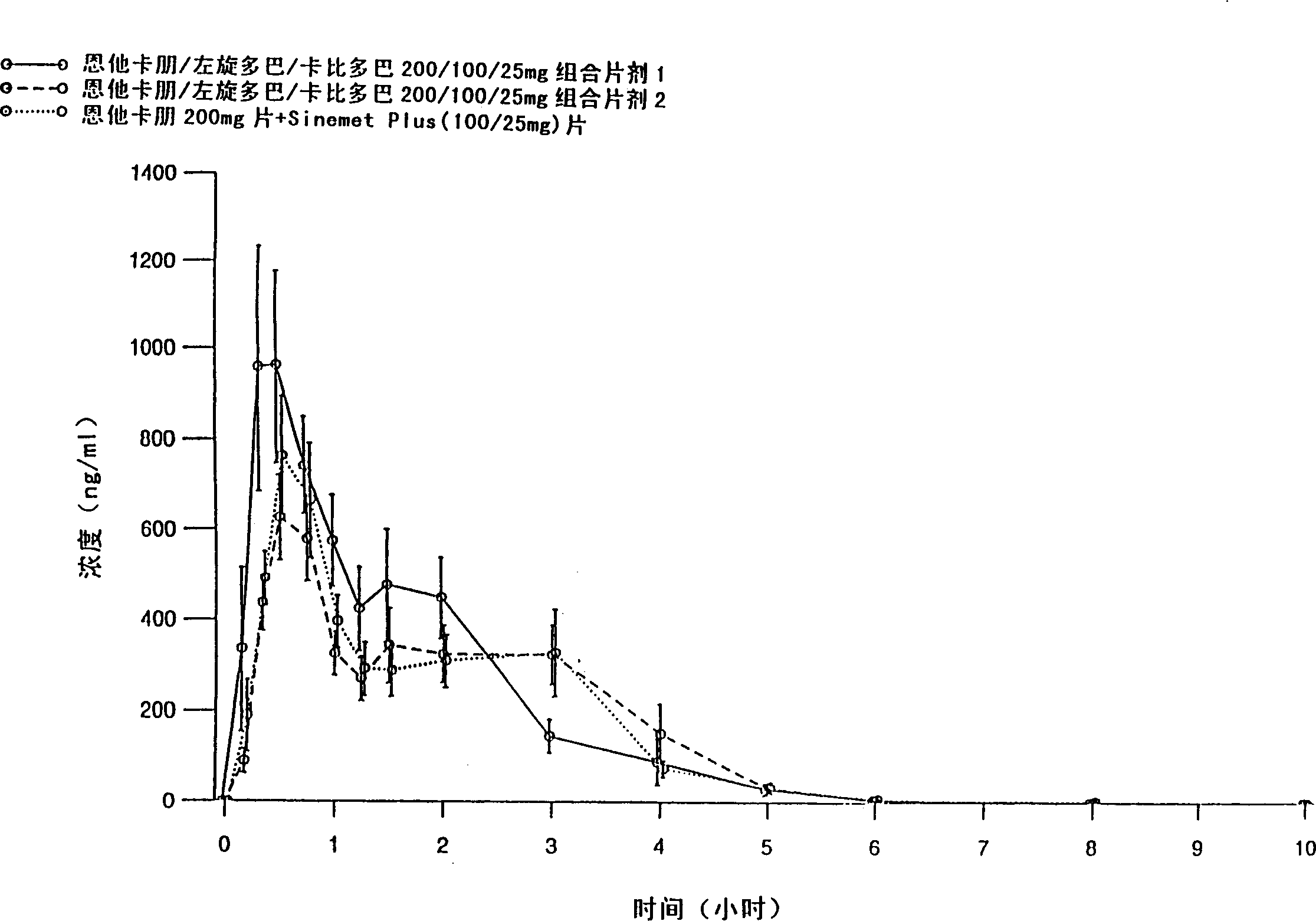
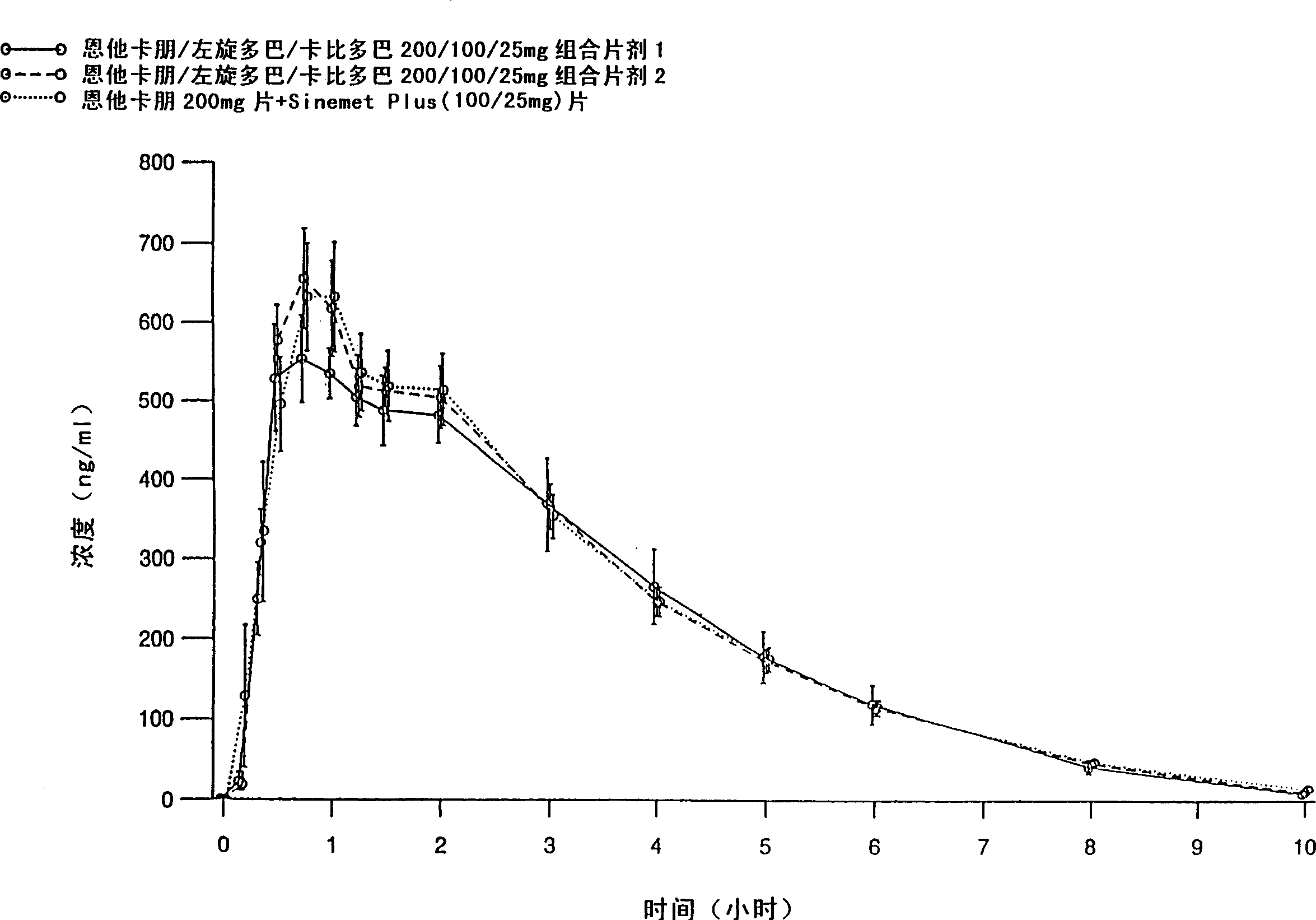
There is provided an extended release pharmaceutical composition comprising from about 200 mg to about 1000 mg of entacapone or salts thereof, optionally with other pharmaceutically acceptable excipients. The invention also provides an extended release pharmaceutical composition comprising triple combination of from about 30 mg to about 300 mg of levodopa, 10 mg to about 100 mg of carbidopa and 200 mg to about 1000 mg of entacapone or salts thereof, optionally with other pharmaceutically acceptable excipients. The invention also relates to process of preparation of such compositions.
Owner:WOCKHARDT LTD
Continuous Administration of L-Dopa, Dopa Decarboxylase Inhibitors, Catechol-O-Methyl Transferase Inhibitors and Compositions for Same
ActiveUS20140051755A1Effectively treating movementImprove efficiencyBiocideNervous disorderTolcaponeCarbidopa
Provided herein, in part, is a method of treating a neurological or movement disorder in a patient in need thereof, comprising subcutaneously administering to said patient a pharmaceutically acceptable composition comprising levodopa and optionally carbidopa and optionally entacapone or tolcapone, or pharmaceutically acceptable salts thereof, wherein said composition is administered substantially continuously, and compositions that can be used in the disclosed methods.
Owner:NEURODERM
Pharmaceutical composition and preparation method thereof
InactiveUS20060222703A1Small sizeDisintegrates quicklyBiocideOrganic active ingredientsEquilibrium moisture contentExcipient
The invention relates to an oral solid pharmaceutical composition comprising pharmacologically effective amounts of entacapone, levodopa and carbidopa, or a pharmaceutically acceptable salt or hydrate thereof, and one or more pharmaceutically acceptable excipients, wherein the excipients are long-chain polymers having an equilibrium moisture content of at least 2%, and to a preparation method thereof. The compositions can be used for the treatment of Parkinson's disease.
Owner:IPRBOX
Pharmaceutical compositions of entacapone
InactiveUS20100104634A1Small particle sizeSmall sizeBiocideOrganic active ingredientsPharmacologyEntacapone
The invention relates to pharmaceutical compositions comprising entacapone or pharmaceutically acceptable salts thereof. The invention also relates to processes for the preparation of such compositions.
Owner:KALANTRI MAHESH RAMESHWAR
Method for treatment of parkinson's disease
The present invention provides a method for treatment of a neurological or movement disorder, e.g., Parkinson's disease, in an individual in need thereof, by parenteral administration of a composition comprising carbidopa and levopoda, or pharmaceutically acceptable salts thereof, and concomitant oral administration of a catechol-O-methyl transferase (COMT) inhibitor, e.g., entacapone or tolcapone.
Owner:NEURODERM
Pharmaceutical composition containing levodopa, entacapone and carbidopa
The present invention refers to a solid pharmaceutical composition of entacapone, levodopa and carbidopa or pharmaceutically acceptable salts thereof characterized in that entacapone is in the form of granules and it is added separately to levodopa and carbidopa. In addition, this invention provides the process for its preparation.
Owner:LAB LESVI SL
Entacapone-derivatives
Pharmaceutical composition comprising one or more entacapone derivatives and one or more pharmaceutically acceptable carriers, a process for producing the pharmaceutical composition, specific entacapone derivatives, a process for the preparation of entacapone derivatives, and the use of the entacapone derivatives for the preparation of a medicament.
Owner:SCHWARZ PHARM AG
Continuous administration of l-dopa, dopa decarboxylase inhibitors, catechol-o-methyl transferase inhibitors and compositions for same
ActiveUS20140249228A1Improve efficiencyReduce the daily dosage of levodopaBiocideNervous disorderTolcaponeCarbidopa
Provided herein, in part, is a method of treating a neurological or movement disorder in a patient in need thereof, comprising subcutaneously administering to said patient a pharmaceutically acceptable composition comprising levodopa and optionally carbidopa and optionally entacapone or tolcapone, or pharmaceutically acceptable salts thereof, wherein said composition is administered substantially continuously, and compositions that can be used in the disclosed methods.
Owner:NEURODERM
Continuous administration of l-dopa, dopa decarboxylase inhibitors, catechol-o-methyl transferase inhibitors and compositions for same
ActiveUS20140249229A1Improve efficiencyReduce the daily dosage of levodopaBiocideNervous disorderTolcaponeCarbidopa
Provided herein, in part, is a method of treating a neurological or movement disorder in a patient in need thereof, comprising subcutaneously administering to said patient a pharmaceutically acceptable composition comprising levodopa and optionally carbidopa and optionally entacapone or tolcapone, or pharmaceutically acceptable salts thereof, wherein said composition is administered substantially continuously, and compositions that can be used in the disclosed methods.
Owner:NEURODERM
Levodopa/carbidopa/entacapone pharmaceutical preparation
The invention relates to an oral solid fixed dose composition comprising pharmacologically effective amounts of entacapone, levodopa, and carbidopa, or a pharmaceutically acceptable salt or hydrate thereof, and comprising at least one pharmaceutically acceptable excipient. The composition of the invention can be used e.g. for the treatment of Parkinson's disease.
Owner:ORION CORPORATION
Method for treatment of Parkinson's disease
Owner:NEURODERM
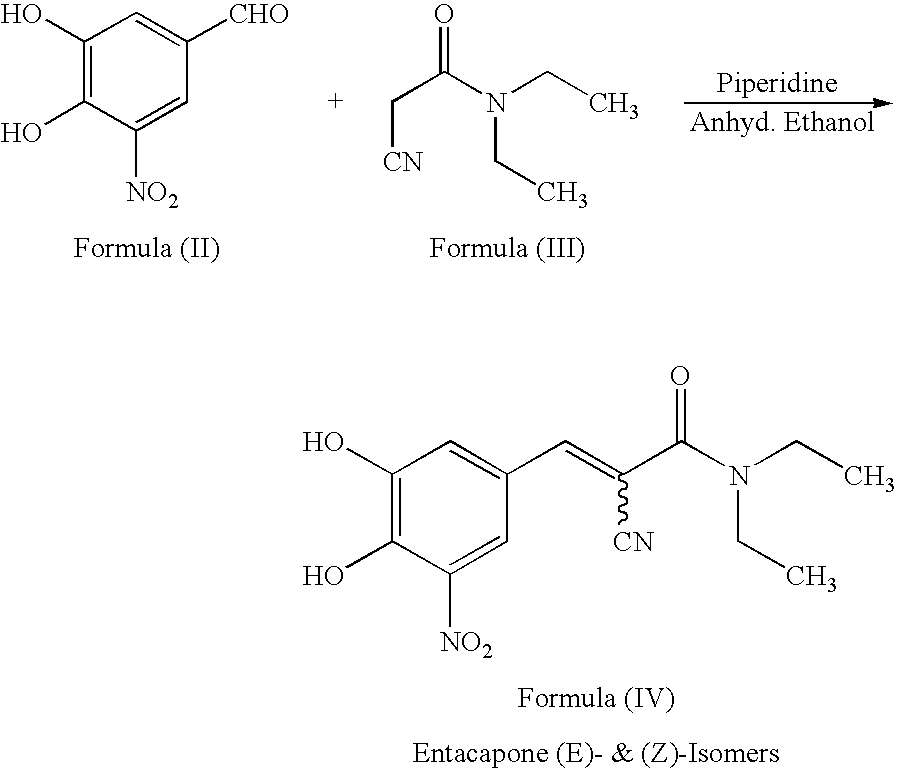
A process for the preparation of entacapone form-A
InactiveCN101460451ACarboxylic acid nitrile preparationOrganic compound preparationCorrosive acidSolvent
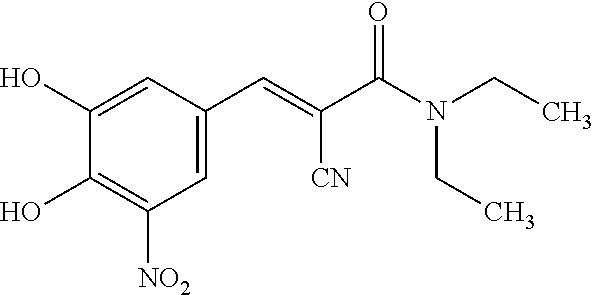
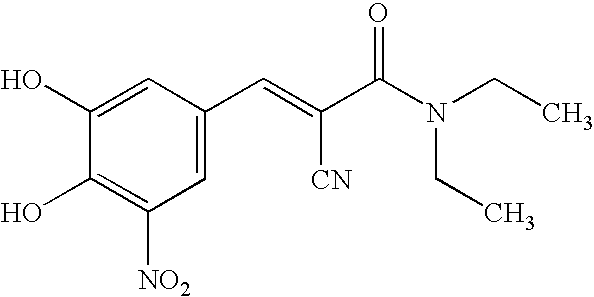
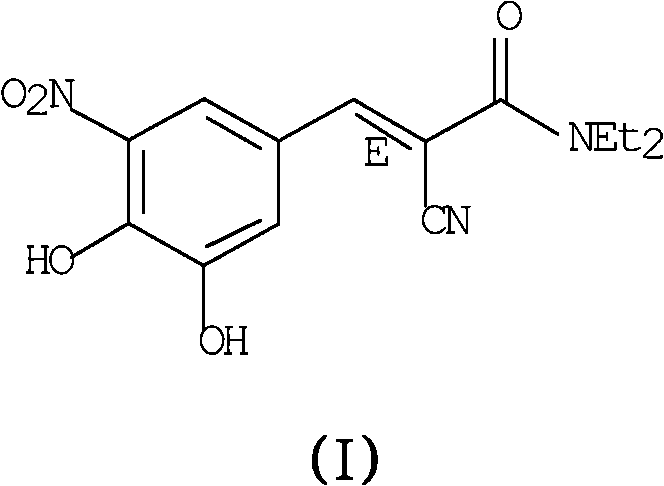
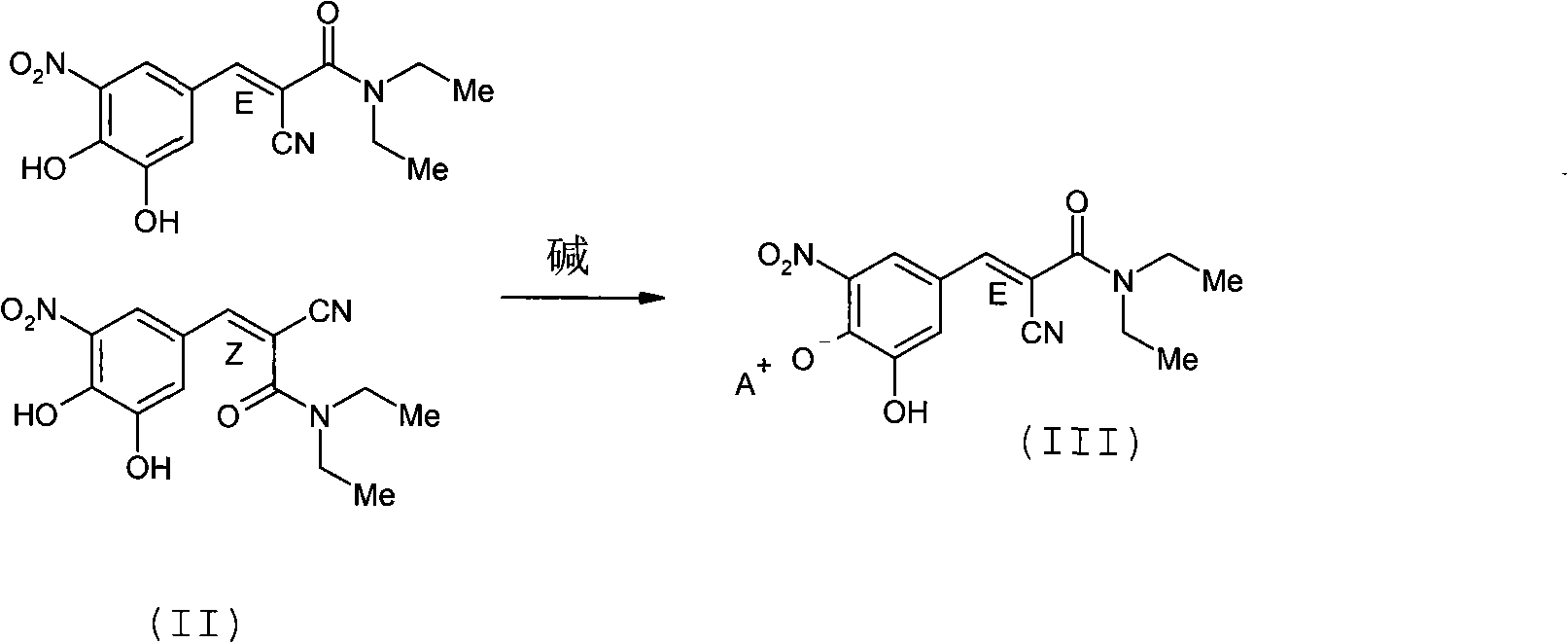
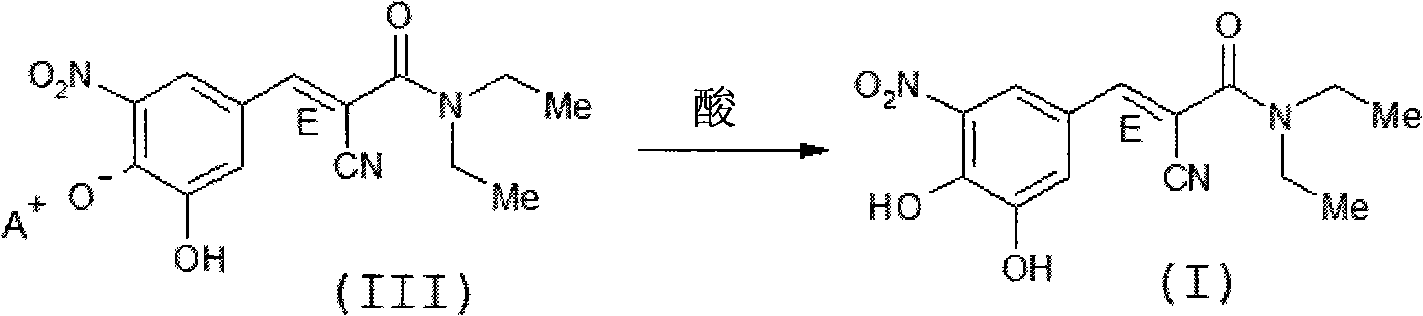
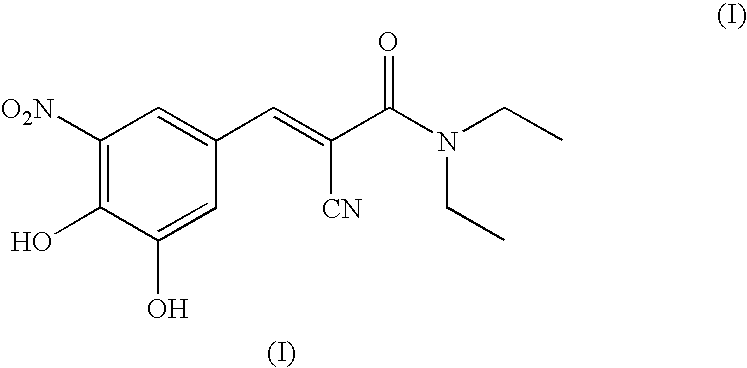
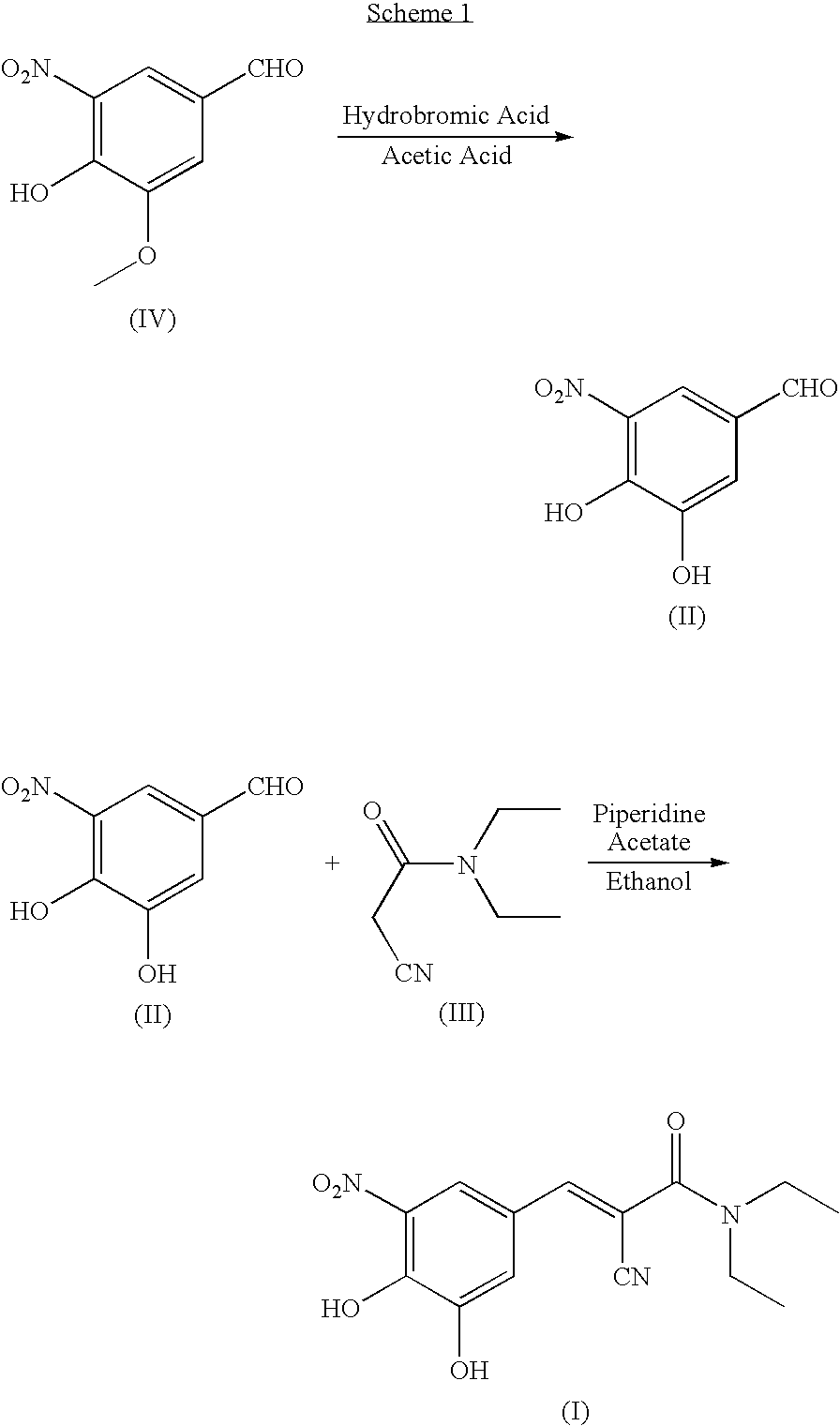
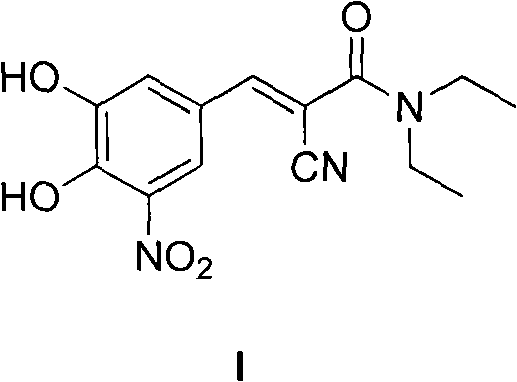
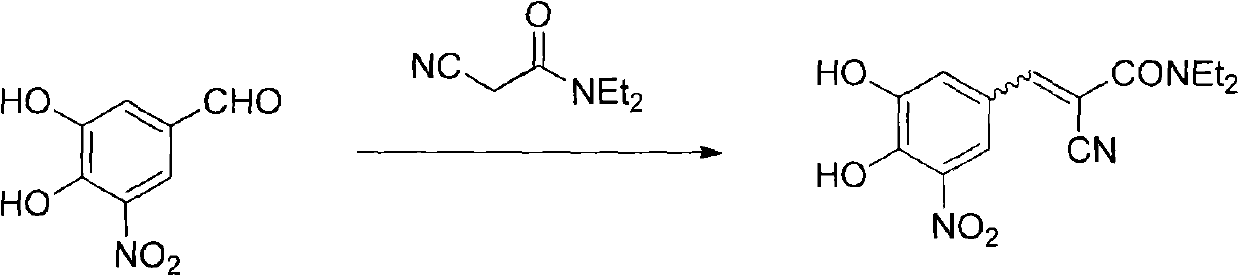
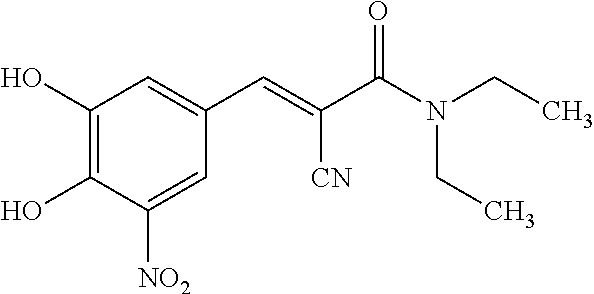
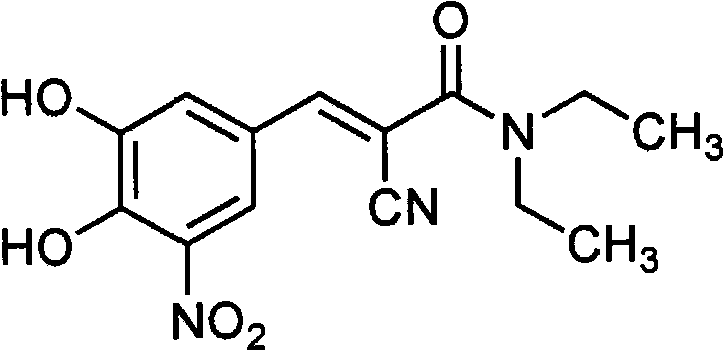
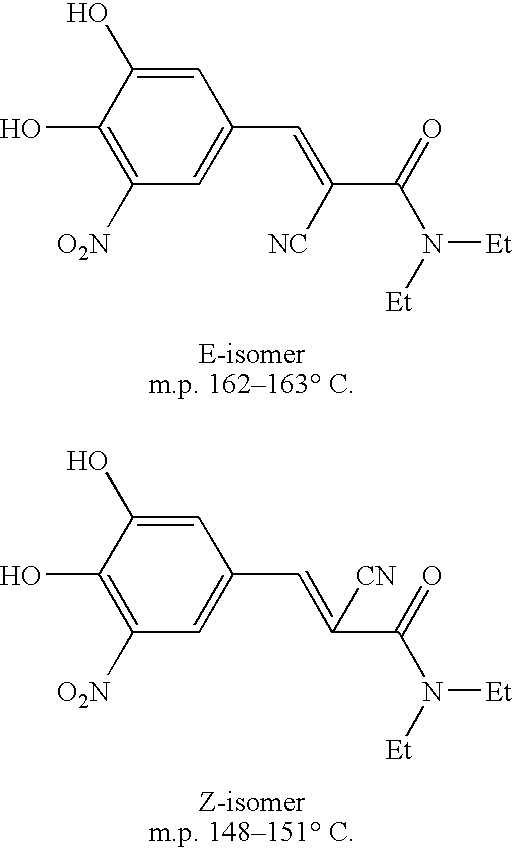
A process to prepare (2E) -2-cyano-3- (3 , 4-dihydroxy-5-nitrophenyl) -N, N-diethyl-2- propenamide (Entacapone) eliminating corrosive acids in the purification, with more than 99.5 % purity with a Z-isomer content of less than 0.1% comprising condensation of 3, 4-Dihydroxy{5-nitrobenzaldehyde with N,N-diethyl cyanoacetamide in the presence of a base selected from cyclic and acyclic secondary amines and a mixture of solvents, to obtain a crude product, stirring the crude product in a halogenated solvent, filtering and finally crystallization of polymorph A of Entacapone in a solvent.
Owner:ACTAVIS GRP PTC EHF
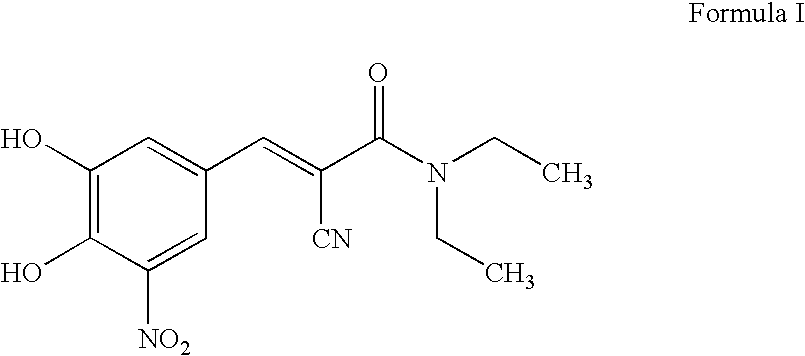
Application of entacapone to prevention or treatment of obesity and other metabolic syndrome
ActiveCN103845317AMetabolism disorderMicrobiological testing/measurementDiseaseLow density lipoprotein cholesterol
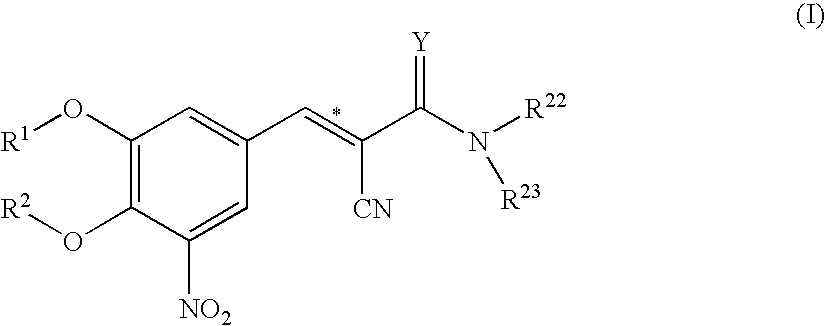
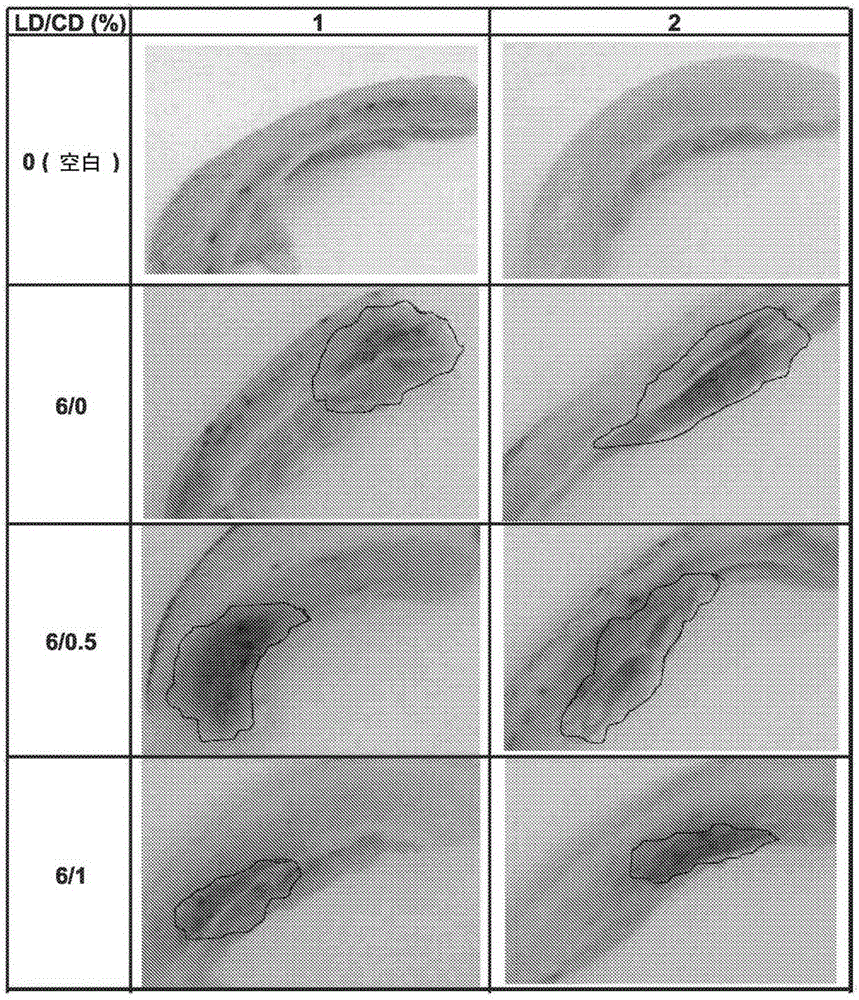
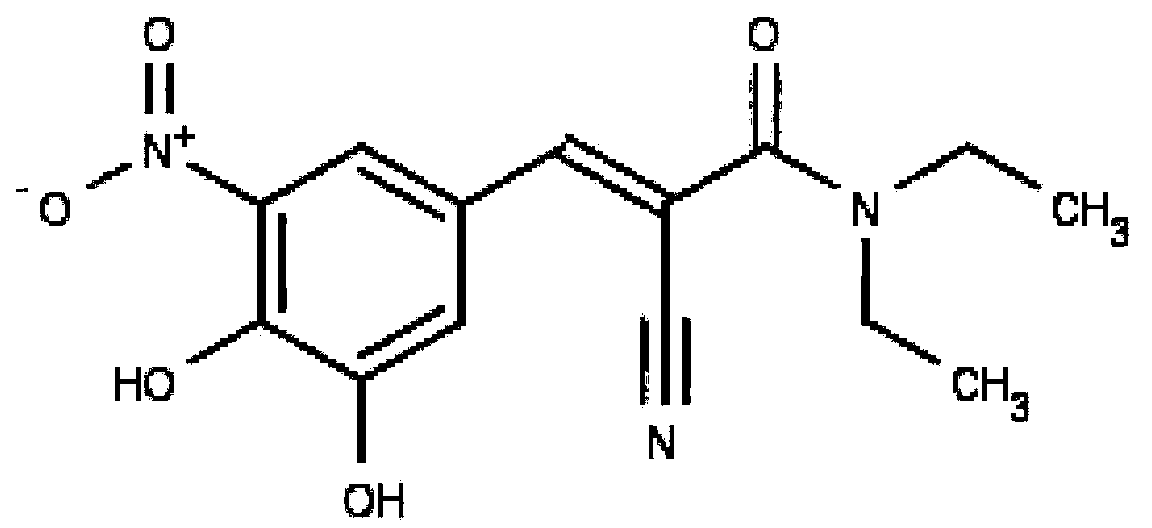
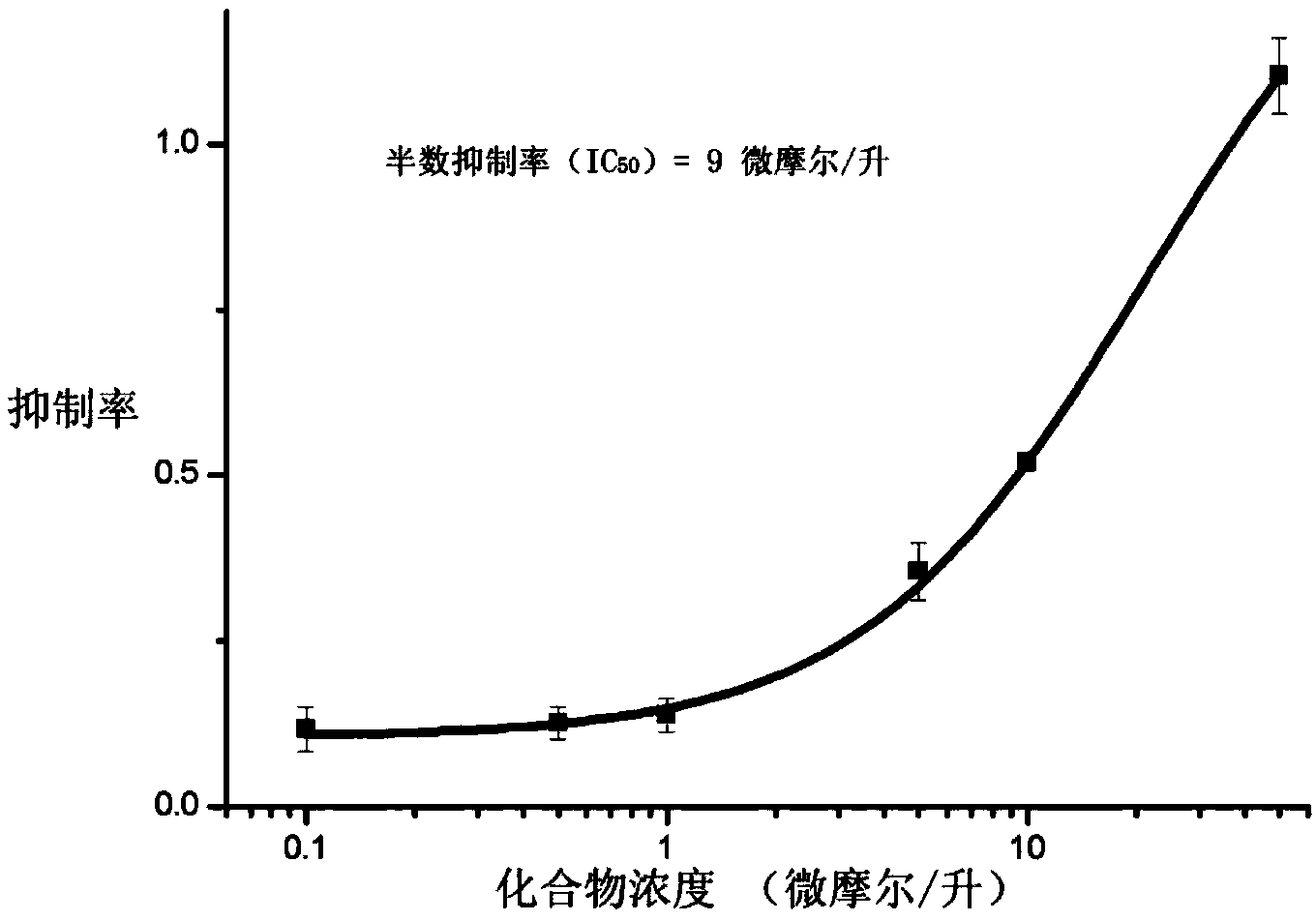
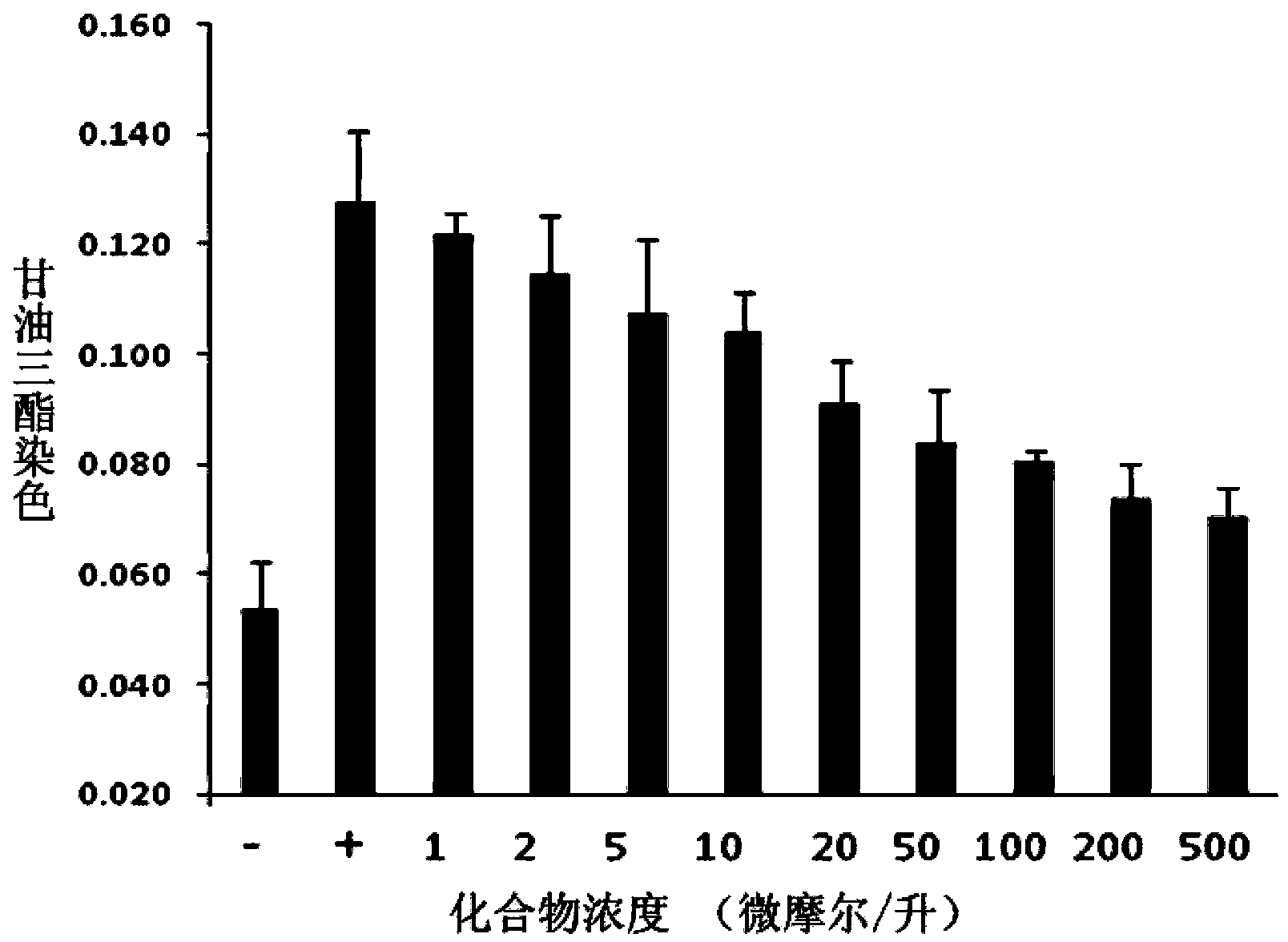
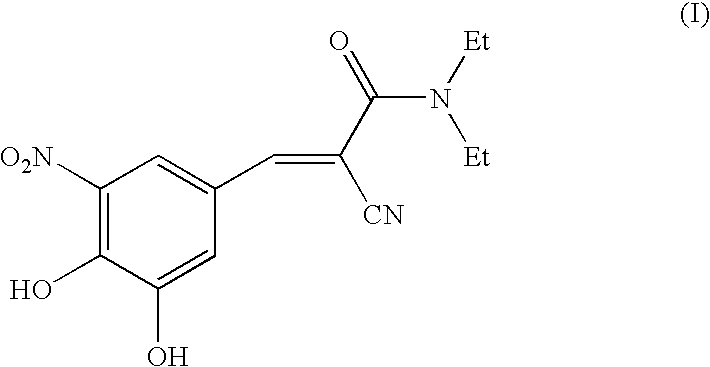
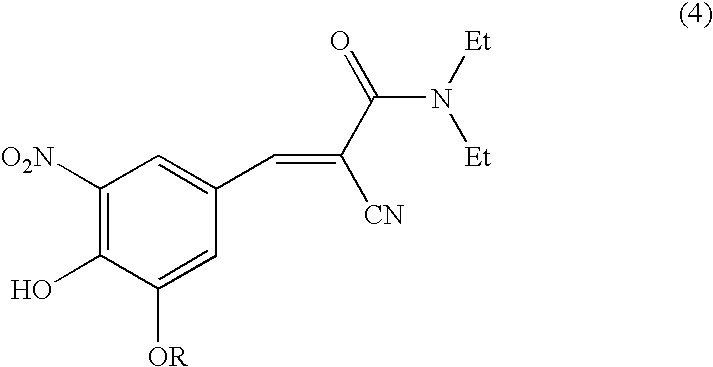
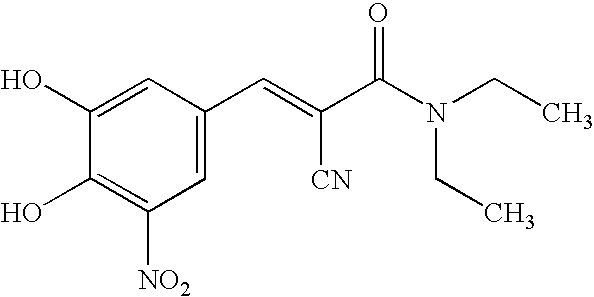
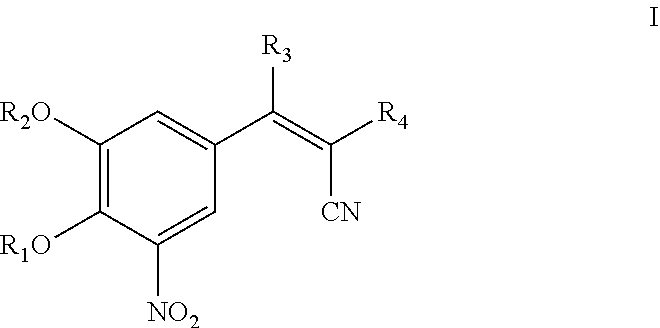
The invention discloses application of entacapone to prevention or treatment of obesity and other metabolic syndromes. Specifically, the invention discloses application of entacapone as shown in the formula I or a pharmaceutically acceptable salt thereof to preparation of medicaments for the prevention and / or treatment of metabolic diseases, cardiovascular and cerebrovascular diseases, as well as liver, kidney and thyroid diseases induced therefrom. The metabolic disease is selected from obesity, diabetes and hyperlipidemia; the cardiovascular and cerebrovascular disease is selected from hypertension, coronary heart disease, and atherosclerosis. The prevention and / or treatment of obesity refers to inhibition of body weight increase or promotion of weight loss. The blood lipid is selected from the group consisting of low density lipoprotein, low density lipoprotein-cholesterol, cholesterol and triglyceride. The renal disease is selected from diabetic nephropathy. (img file = 'DDA00002485677700011.TIF' wi = '1113' he = '654' / ).
Owner:NAT INST OF BIOLOGICAL SCI BEIJING
Process for preparing entacapone substantially free of z-isomer, synthesis intermediates thereof and a new crystalline form
The present invention relates to a new process for preparing Entacapone substantially free of Z-isomer from 3, 4-dihydroxy-5-Nitrobenzaldehyde and N, N-Dimethylcyano acetamide, or directly from a mixture of (E) - and (Z) - isomers of Entacapone, by formation of organic or inorganic salts, specially piperidine and sodium ones. A new crystalline form G of Entacapone can be obtained from this method in a fast, efficient, and simple way and substantially free of Z-isomer. Another object of the invention is a pharmaceutical composition comprising it.
Owner:凯默伊比利亚公司
Methods for the preparation of Entacapone
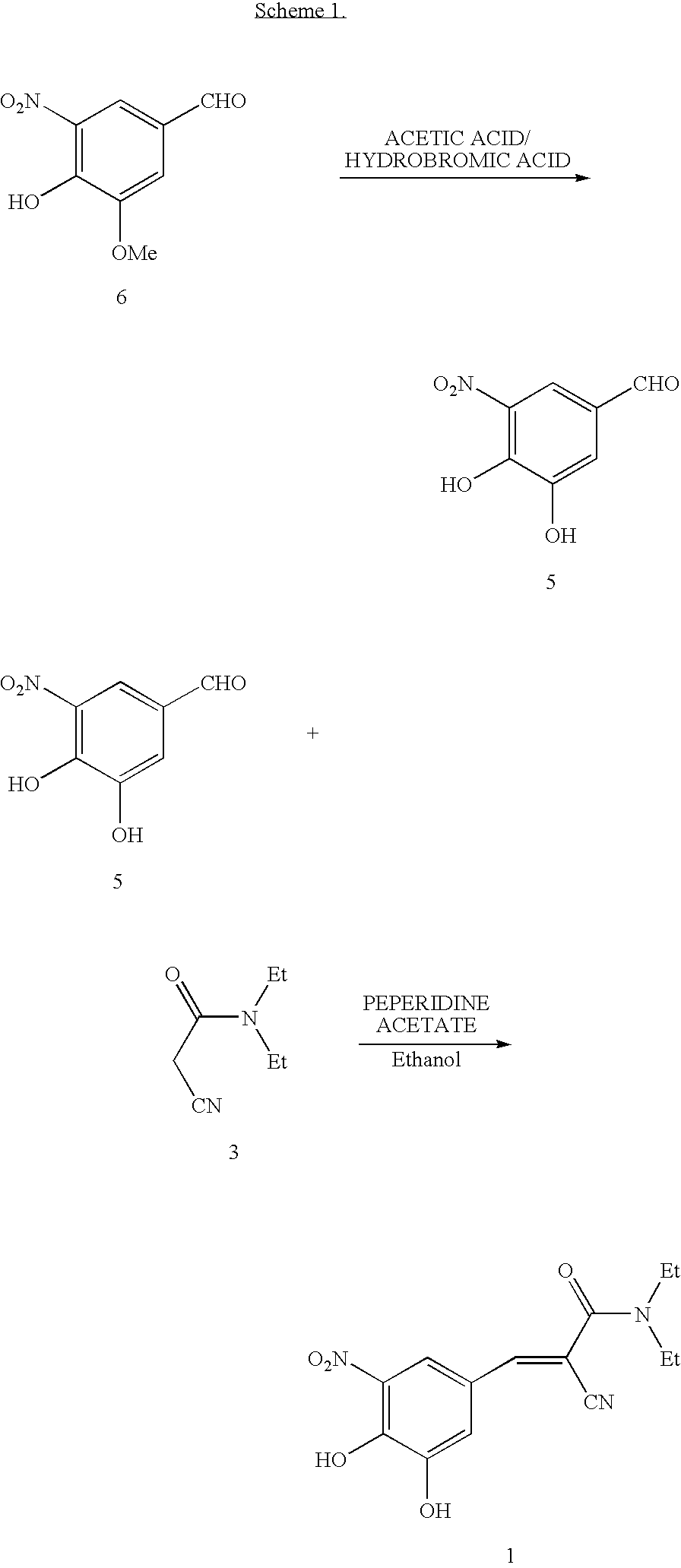
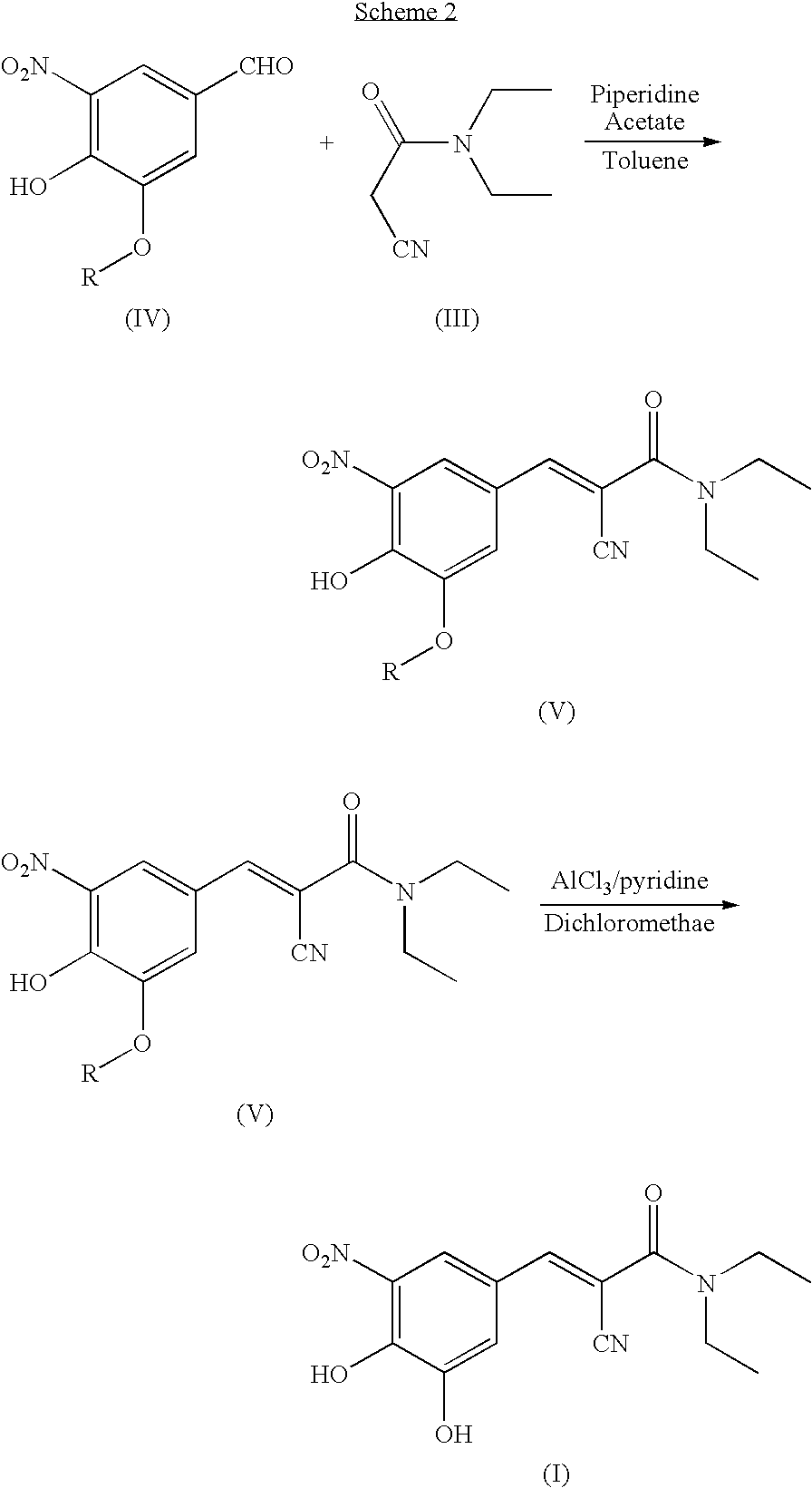
ActiveUS7385072B2Good effectEasy to controlCarboxylic acid nitrile preparationOrganic compound preparationAlkoxy groupOrganic base
The invention disclosed in this application relates to an improved process for the preparation of Entacapone. In one embodiment the process involves: (i) reacting a 3-alkoxy-4-hydroxy-5-nitrobenzadehyde with N,N-diethylaminocyanoacetamide in the presence of a mild acid catalyst and a solvent, to provide a 3-O-alkylated Entacapone, and treating the 3-O-alkylated Entacapone with an acid catalyst in the presence of an organic base to provide Entacapone.
Owner:SUVEN LIFE SCI LTD
Compositions comprising blockers of L-DOPA renal cell transfer for treatment of Parkinson's disease
A pharmaceutical composition for the treatment of Parkinson's disease comprises L-DOPA and at least one compound capable of blocking the L-DOPA renal cell outward transfer pathway; said blocking compound being chose from (a) a flavonoid phenyl benzopyran derivative; (b) a trans-stilbene derivative; or (c) phloretin [3-(4-hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)-1-propanone]. The composition may also comprise an inhibitor of the enzyme amino acid decarboxylase (AADC), such a carbidopa or benserazide, and / or an inhibitor of the enzyme catechol-O-methyl transferase (COMT), such as entacapone or tolcapone. The composition is preferably administered in soli form and the L-DOPA may be administered simultaneously or sequentially with the L-DOPA renal cell outward transfer blocking compound.
Owner:波特拉和康潘希亚股份有限公司
Process for the preparation of entacapone
InactiveUS20080146829A1Avoid high temperature heatingCarboxylic acid nitrile preparationOrganic compound preparationEntacaponeCrystallization
A process for the preparation of entacapone, in particular as the polymorphic form A, comprising the preparation of a compound of formula (V), as herein defined, by condensation of N,N-diethyl-cyano-acetamide with a compound of formula (IV), as herein defined, in the presence of a strong basic agent; the dealkylation of said compound of formula (V) to obtain entacapone and the crystallization thereof to the polymorphic form A.
Owner:DIPHARMA FRANCIS
Pharmaceutical compositions comprising entacapone, levodopa, and carbidopa
The present invention relates to stable pharmaceutical compositions comprising entacapone, levodopa and carbidopa, or pharmaceutically acceptable salts or hydrates thereof. The invention also relates to processes for the preparation of such compositions.
Owner:WOCKHARDT RES CENT (IN)
New preparation method of (2E)-2-cyano-3-(3,4-dihydroxy-5-nitrobenzene)-N,N-diethyl-2-acrylamide
ActiveCN102120726AShort stepsShort reaction timeCarboxylic acid nitrile preparationOrganic compound preparationEnantiomerNitrobenzene
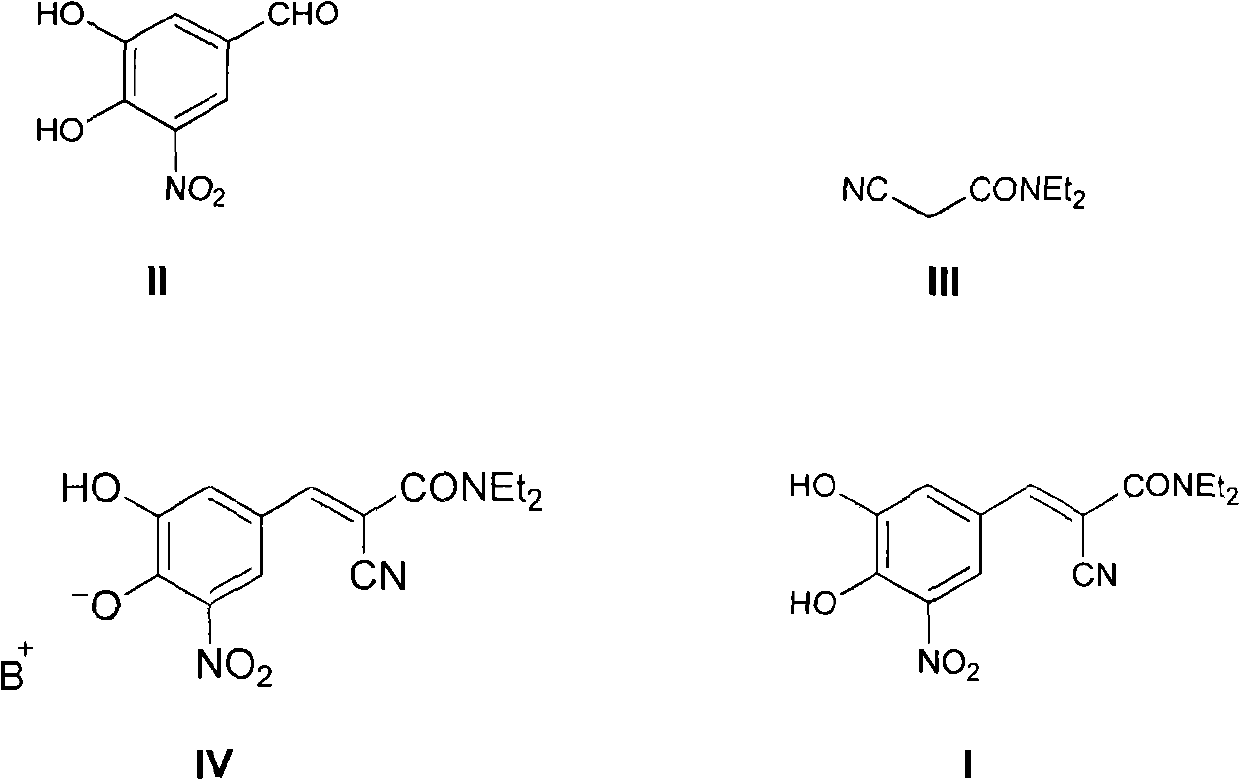
The invention discloses a new method for preparing (2E)-2-cyano-3-(3,4-dihydroxy-5-nitrobenzene)-N,N-diethyl-2-acrylamide (I) of which purity is more than 99.9% and Z-isomer content is 0.01%. The method is as follows: 3,4-dihydroxy-5-nitrobenzaldehyde (II) performs condensation with N,N-diethylcyanoacetamide (III) in the presence of mixed solvent and alkali (B) to obtain entacapone salt (IV), and the obtained compound (IV) performs acid dissociation in proper solvent to obtain entacapone (I) with pure enantiomers. The method disclosed by the invention has high yield, environmental friend and mild reaction conditions and is suitable for large-scale industrial production.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +1
Pharmaceutical Compositions of Entacapone, Levodopa and Carbidopa with Improved Bioavailability
The present invention relates to single oral dose pharmaceutical compositions comprising a combination of entacapon, levodopa and carbidopa, or salts thereof along with one or more sugar alcohols, wherein the entacapone is co-micronized with one or more sugar alcohols. The composition of the invention exhibits bioequivalence to commercially available entacapone, levodopa and carbidopa combination formulation marketed under the trade name Stalcvo200®. The invention also relates to processes for making such compositions.
Owner:WOCKHARDT LTD
Pharmaceutical compositions of entacapone co-micronized with sugar alcohols
The present invention relates to pharmaceutical compositions comprising entacapone or pharmaceutically acceptable salts thereof along with one or more sugar alcohols; wherein the entacapone is co-micronized with one or more sugar alcohols. The invention also relates to processes of making such compositions.
Owner:WOCKHARDT RES CENT (IN)
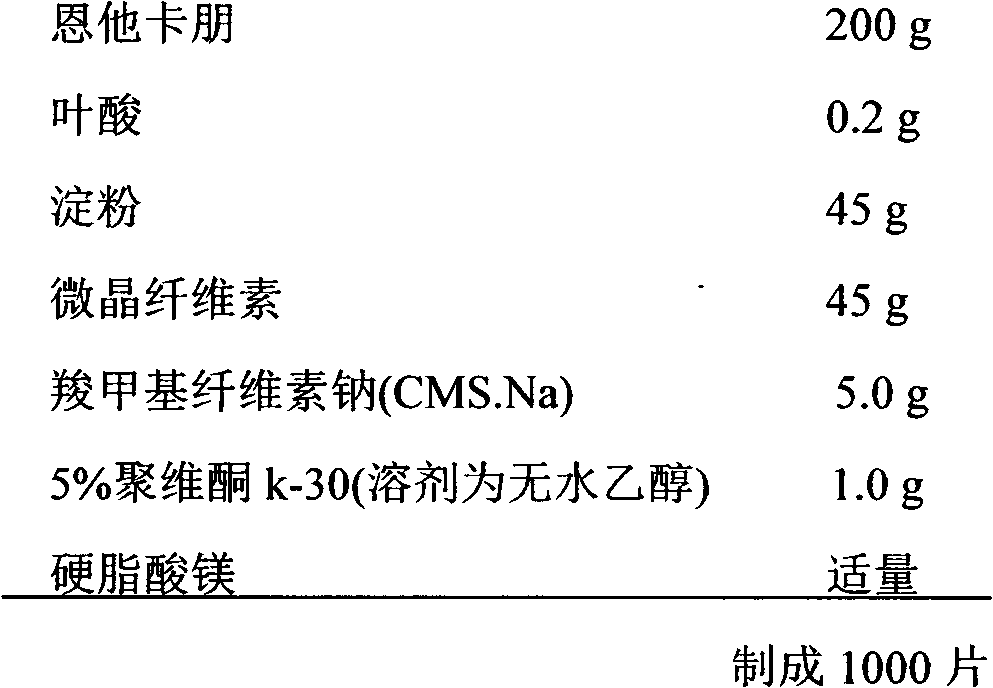
Entacapone/folic acid compound medicine composition and application thereof
InactiveCN103127123AIncrease the curative effect of the diseaseGood curative effectNervous disorderNitrile/isonitrile active ingredientsLife qualityRisk stroke
The invention relates to an entacapone / folic acid compound medicine composition and application thereof and belongs to the technical field of pharmacy. The medicine composition contains officinal dose of entacapone, officinal dose of folic acid compound and a carrier acceptable in the pharmacy. The dose of the entacapone is 100-300mg, and the dose of the folic acid compound is 0.2-1.6mg. The entacapone / folic acid compound medicine composition has the advantages of strengthening curative effect on treatment of Parkinson's disease through multiple-target-point synergistic effect and improving the life quality of patients. The medicine composition still can effectively prevent and reduce the risk that Parkinson's disease patients are subjected to brain stroke through high homocysteine (Hcy) target points. In addition, the entacapone / folic acid compound medicine composition can be conveniently taken by the patients.
Owner:SUZHOU FAMO BIOLOGICAL TECH
Entacapone-related compounds to treat macular degeneration
ActiveUS10980766B2Senses disorderPharmaceutical delivery mechanismPharmaceutical medicinePerylene derivatives
The invention provides use of entacapone, an entacapone derivative or a stereoisomer, hydride, or pharmaceutically-acceptable salt thereof, in a person in need thereof, to treat or inhibit macular degeneration or age-related macular degeneration, and related compostions.
Owner:NAT INST OF BIOLOGICAL SCI BEIJING
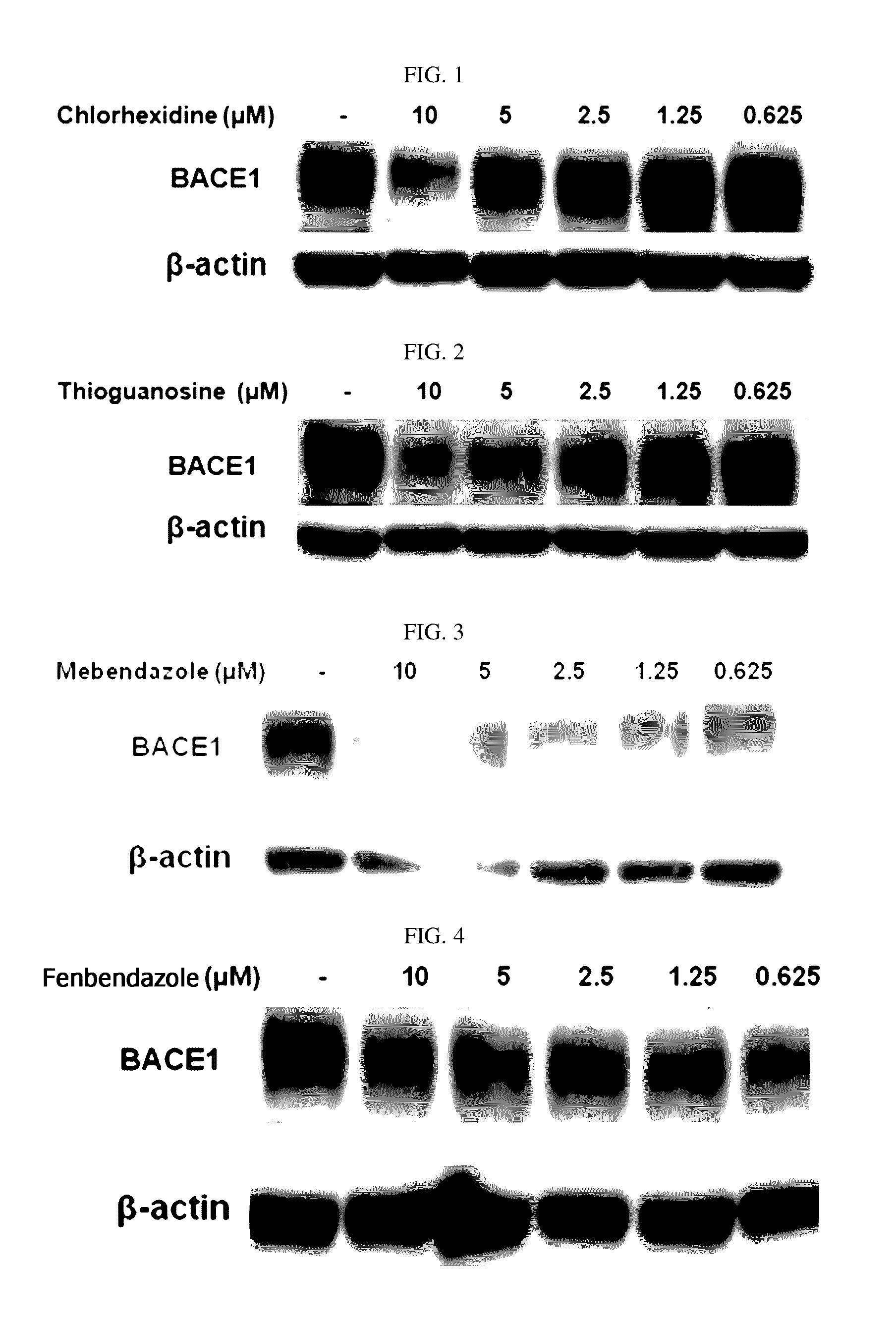
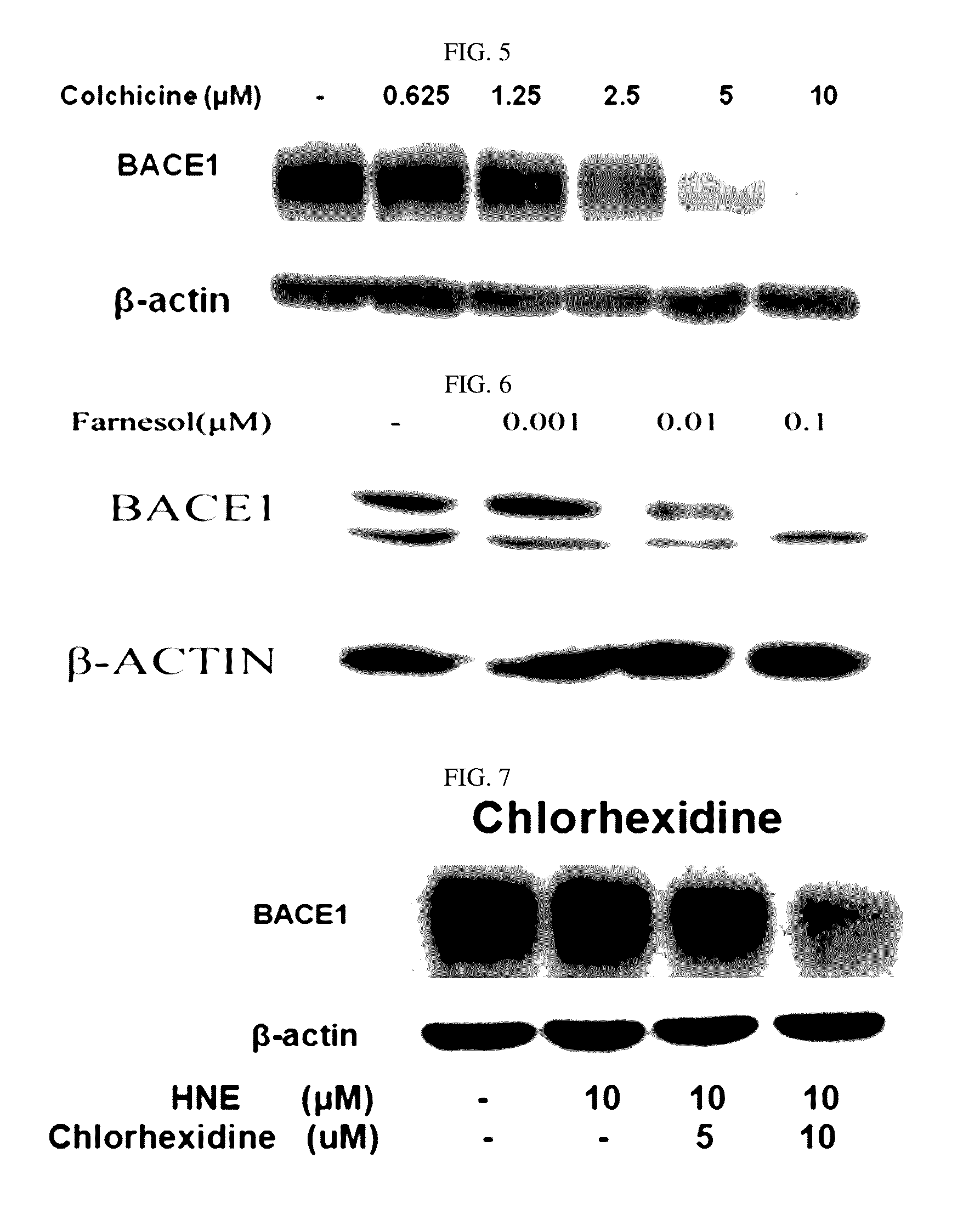
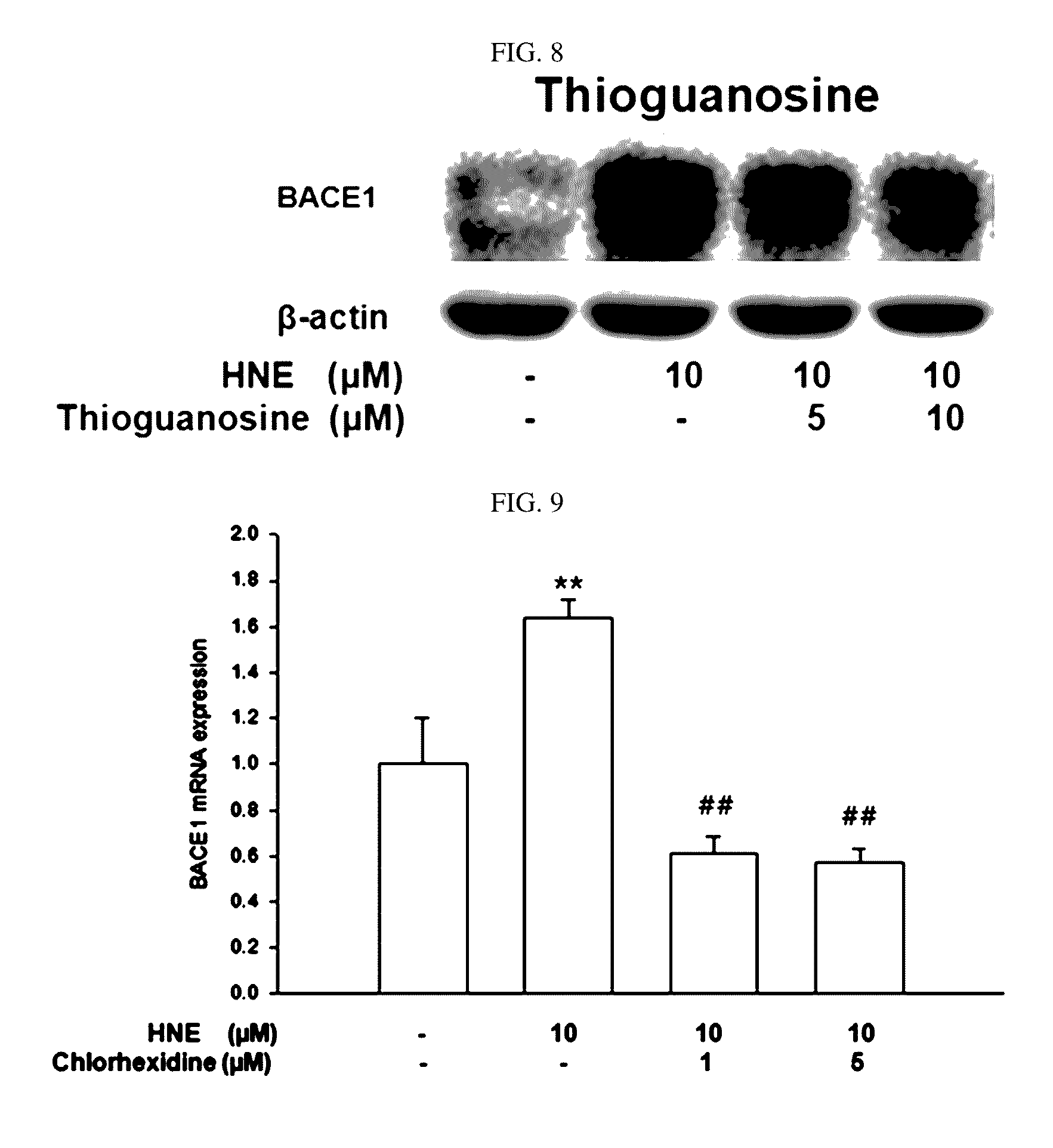
Composition for preventing or treating degenerative brain diseases including compound downregulating expression of BACE1 proteins
ActiveUS9549942B2Suppress generationBiocideHydroxy compound active ingredientsRaloxifene HydrochlorideDisease injury
Owner:RES & BUSINESS FOUND SUNGKYUNKWAN UNIV
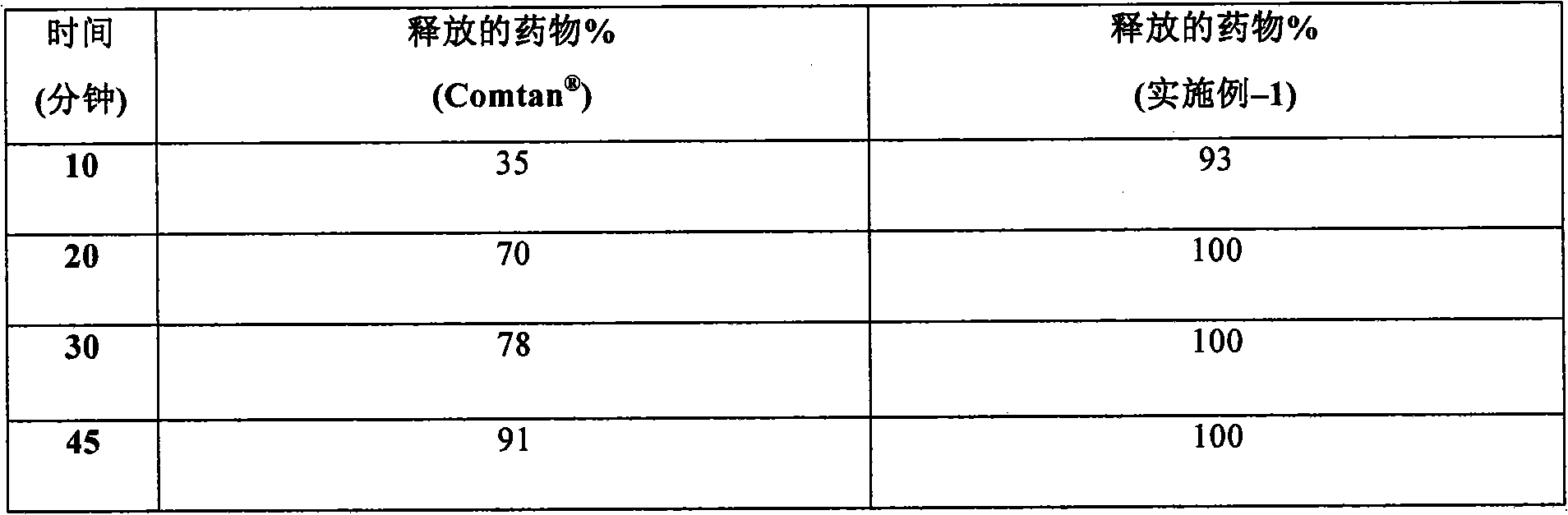
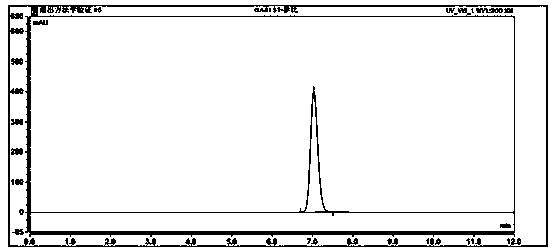
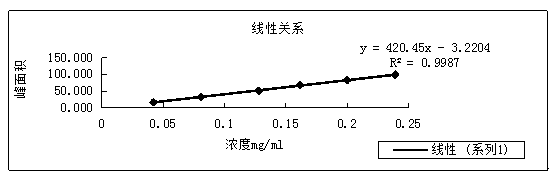
Method for measuring entacapone tablet dissolution rate through utilization of high performance liquid chromatography
ActiveCN110441421AEffective evaluationAccurate evaluationComponent separationUltraviolet detectorsTablet dissolution
The invention provides a method for measuring an entacapone tablet dissolution rate through utilization of high performance liquid chromatography. An ultraviolet detector is employed and elution is carried out through utilization of reversed-phase high performance liquid chromatography. A sample solution and a standard solution are prepared through utilization of a dissolution apparatus. The sample solution and the standard solution are injected into a chromatograph, and chromatograms are recorded. Computing is carried out based on a peak area according to an external standard method, and theentacapone tablet dissolution rate is measured. According to the method, the vacancy that no analysis method for measuring the entacapone tablet dissolution rate through utilization of the high performance liquid chromatography exists at present is filled up, research, development and production requirements can be satisfied, and relatively strict and effective control over the entacapone tablet dissolution rate can be carried out.
Owner:聊城高新生物技术有限公司 +1
Efficient method for the manufacture of (E) -Entacapone polymorphic Form A
The present invention describes an improved method for the preparation of Entacapone, (E)-N,N-diethyl-2-cyano-3-(3,4-dihydroxy-5-nitrophenyl)acrylamide, polymorphic Form A, which is an inhibitor of catechol-O-methyltransferase (COMT) enzyme. The method provides pure (E)-isomer of Entacapone Form A.
Owner:WOCKHARDT LTD
Extended Release Pharmaceutical Composition Of Entacapone Or Salts Thereof
InactiveUS20160015645A1Reducing ‘ wearing off ’ phenomenonAvoid large absorptionBiocideNervous disorderTriple combinationCarbidopa
There is provided an extended release pharmaceutical composition comprising from about 200 mg to about 1000 mg of entacapone or salts thereof, optionally with other pharmaceutically acceptable excipients. The invention also provides an extended release pharmaceutical composition comprising triple combination of from about 30 mg to about 300 mg of levodopa, 10 mg to about 100 mg of carbidopa and 200 mg to about 1000 mg of entacapone or salts thereof, optionally with other pharmaceutically acceptable excipients. The invention also relates to process of preparation of such compositions.
Owner:WOCKHARDT LTD
Levodopa/carbidopa/entacapone pharmaceutical preparation
The invention relates to an oral solid fixed dose composition comprising pharmacologically effective amounts of entacapone, levodopa, and carbidopa, or a pharmaceutically acceptable salt or hydrate thereof, and comprising at least one pharmaceutically acceptable excipient. The composition of the invention can be used e.g. for the treatment of Parkinson's disease.
Owner:ORION CORPORATION
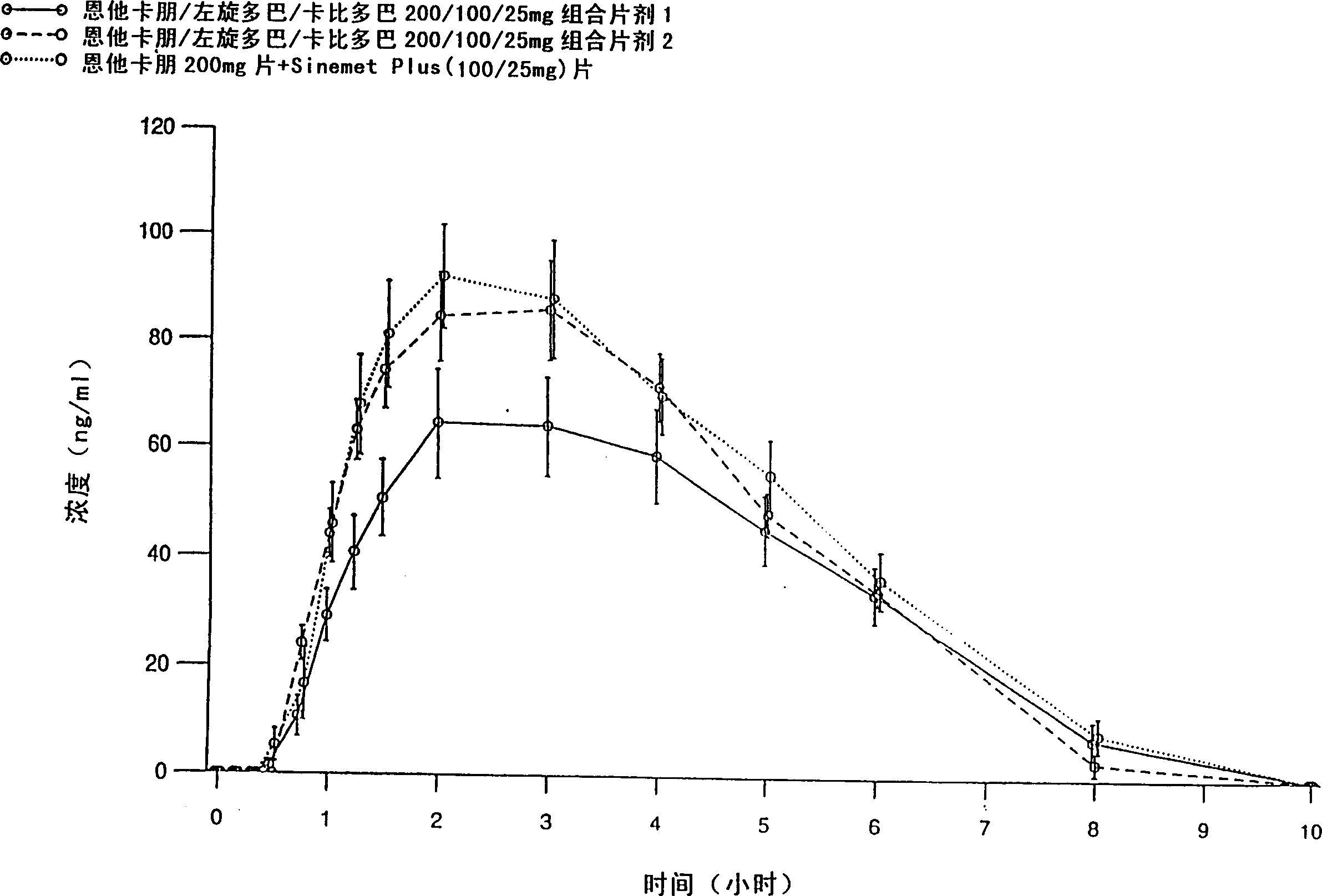
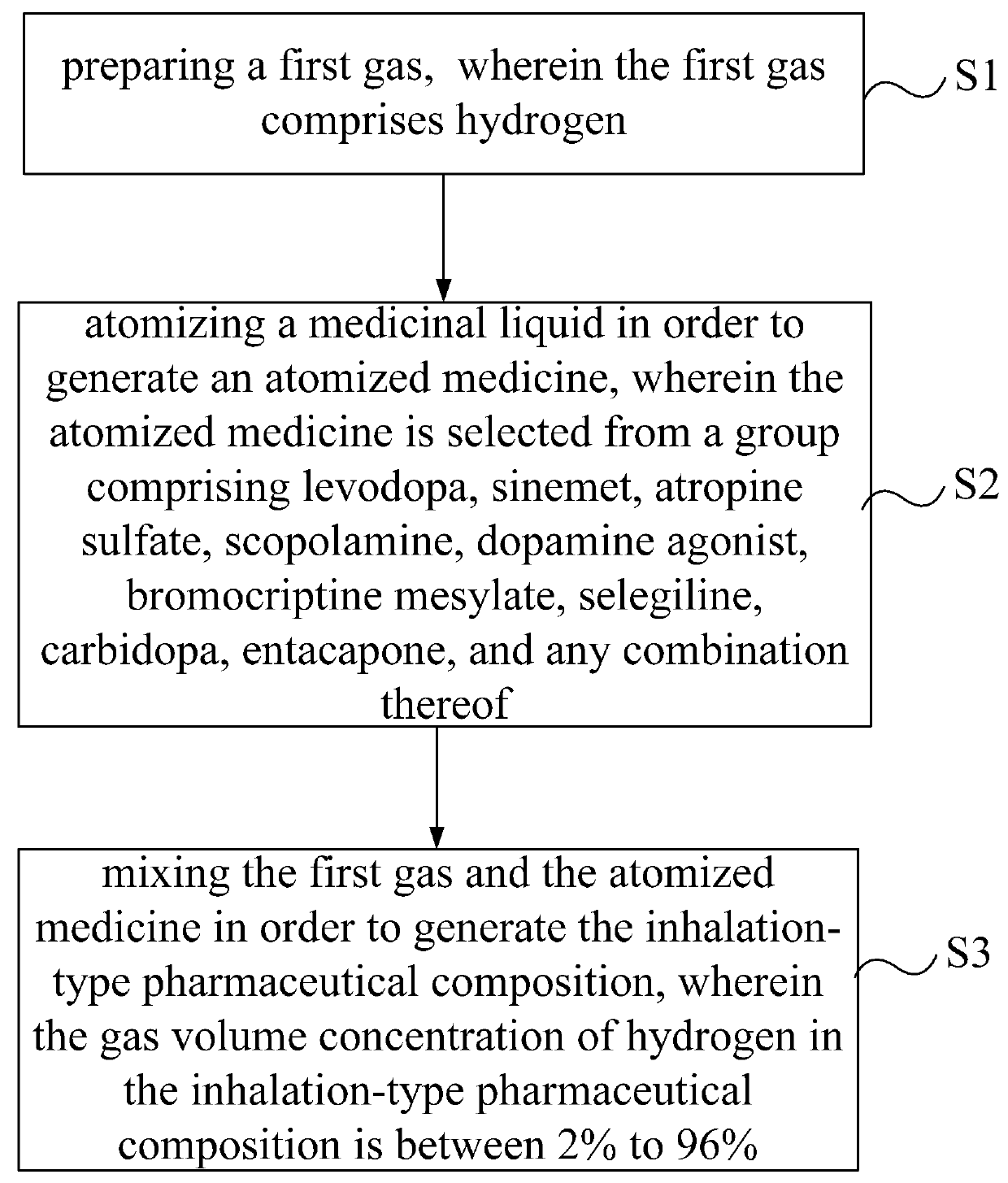
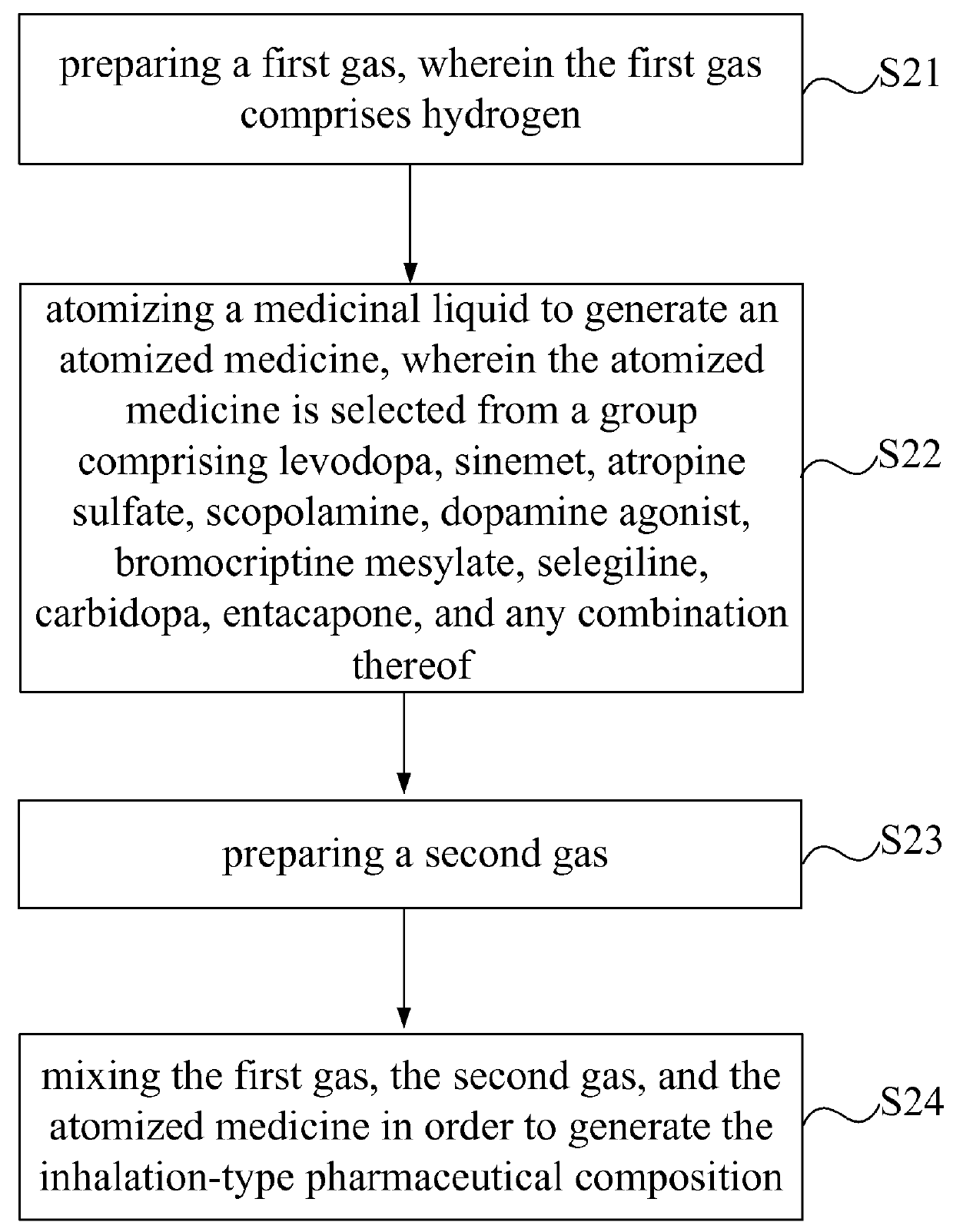
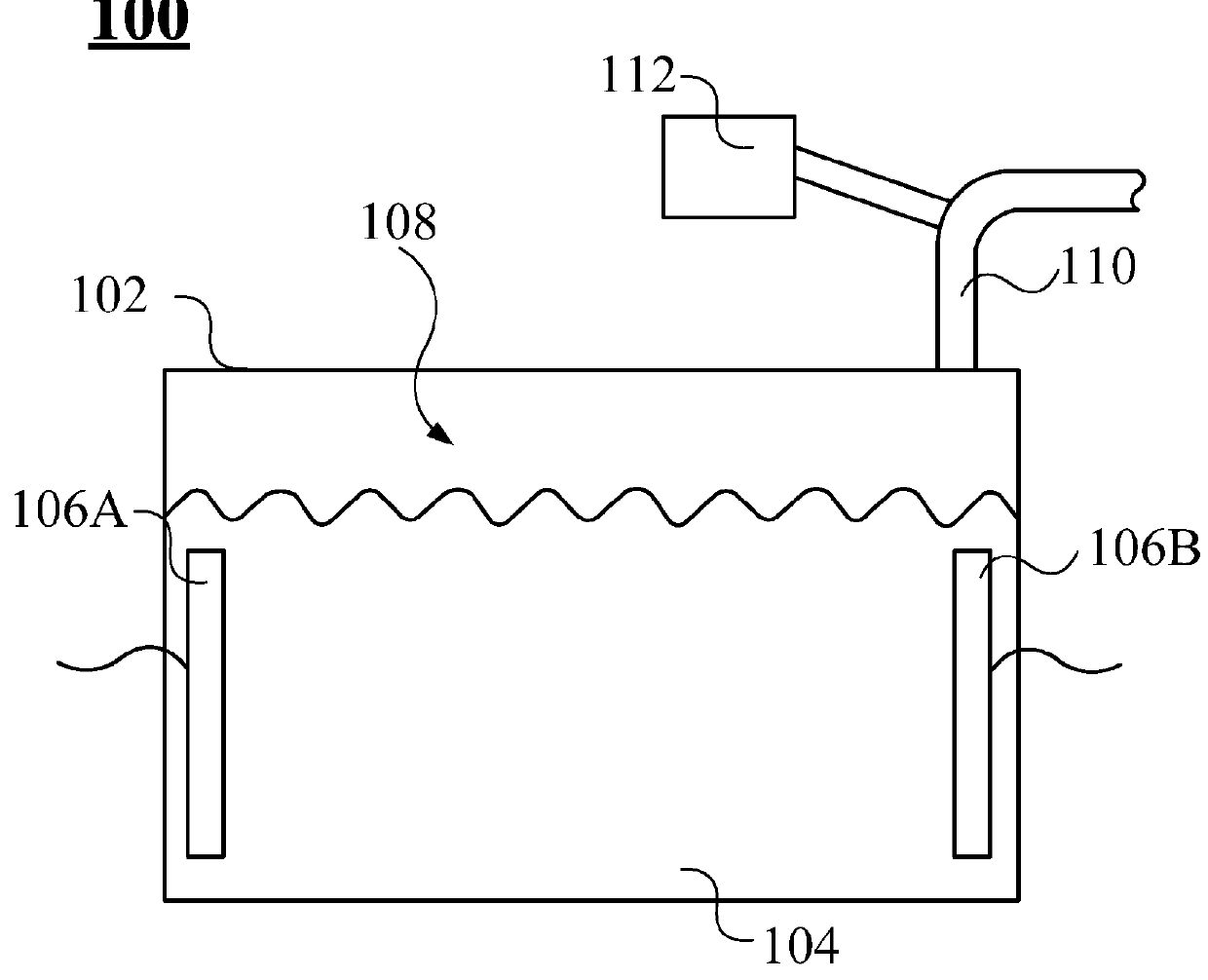
Inhalation-type pharmaceutical composition for the treatment of Parkinson's disease and preparation method thereof
ActiveUS9381152B2Promote absorptionGood curative effectPowder deliveryNervous disorderCarbidopaAbsorption effect
Owner:LIN HSIN YUNG
Pharmaceutical compositions of entacapone co-micronized with sugar alcohols
Owner:WOCKHARDT LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com