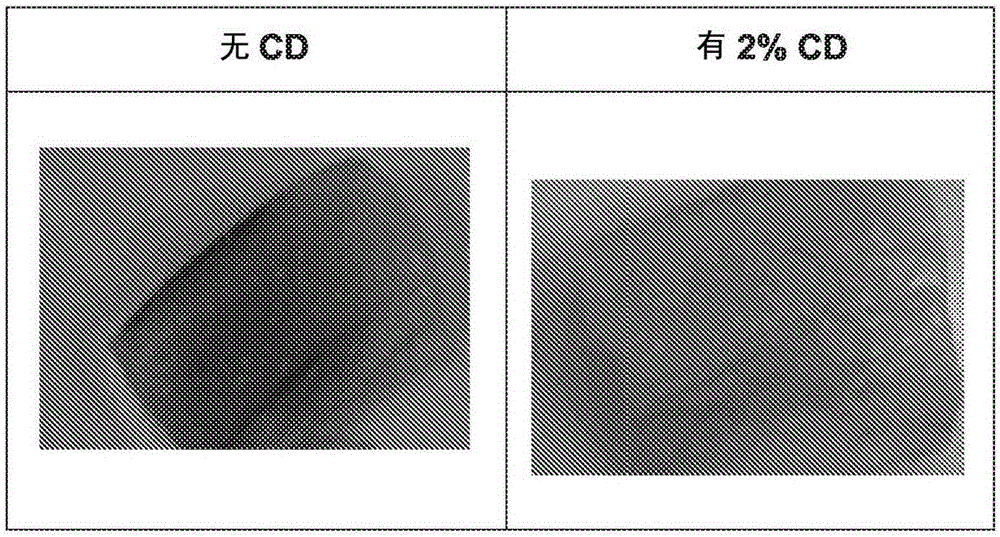
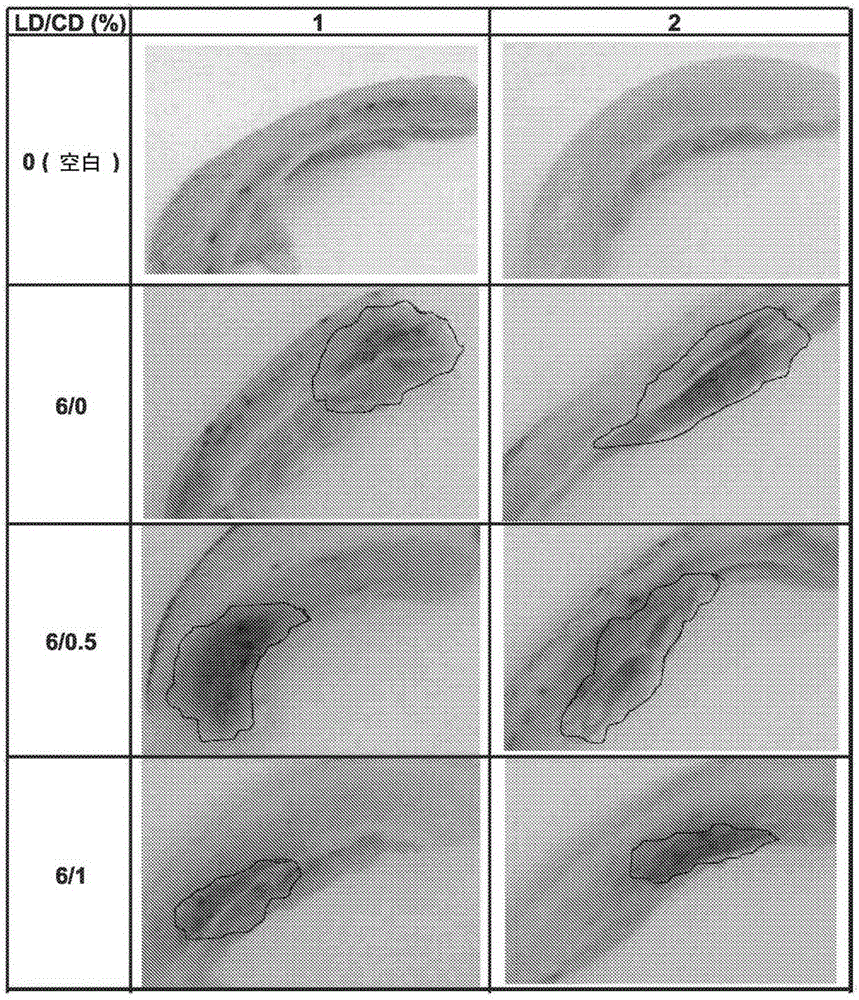
Method for treatment of Parkinson's disease
一种帕金森病、组合物的技术,应用在帕金森病的治疗领域,能够解决没有提供帕金森病最佳治疗等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1. Preparation of solutions / formulations for subcutaneous administration
[0063] A. A 2% carbidopa solution / formulation was prepared by adding a pre-warmed 0.1% sodium bisulfite solution to carbidopa [ASSIAL Ltd.]. Arginine (Merck) was added to obtain a final molar ratio of CD(carbidopa):Arg(arginine) of 1:1.2. The mixture was stirred at 60 °C until complete dissolution was obtained. Heating was stopped and the preparation was allowed to cool to RT; pH of 8.5. The solution was filtered through a sterile 0.22 μM PVDF membrane.
[0064] B. Prepare a 10% tolcapone solution / formulation as follows: by adding the corresponding amount of H 2 O was added to tolcapone (Synfine Research), a solution containing 10% tolcapone was prepared and arginine was added slowly with stirring to obtain a final molar ratio of 1:1. Stir the mixture until complete dissolution is obtained. After cooling, the pH of the solution was 7.8.
[0065] C. A solution containing 10% entacapo...
Embodiment 2
[0070] Embodiment 2. preparation preparation process
[0071] Levodopa (LD) and carbidopa (CD) formulations can be prepared as follows. However, as shown in Table A, the method of preparation has a significant impact on the final physical and chemical stability of the composition.
[0072] Method 1 (L-Arg solution) . L-Arg and Na-Bis (sodium bisulfate) were dissolved in water. The solution was added to the LD and CD powders. The mixture was heated and stirred at 75 °C for 13 min until complete dissolution. The LD / CD solution was kept at RT for 10 min to cool down.
[0073] Method 2 (all powders together) . All powders (LD, CD and L-Arg) were weighed and water containing Na-Bis was added. The suspension was heated and stirred at 75 °C for 13 min until completely dissolved. The LD / CD solution was kept at RT for 10 min to cool down.
[0074] Method 3 (same as 2 but without Na-Bis preheat) . All powders (LD, CD and L-Arg) were weighed together and water was added. ...
Embodiment 3
[0087] Example 3. Effect of arginine on the long-term stability of levodopa and levodopa / carbidopa compositions
[0088] Prepare liquid formulations with levodopa, carbidopa and arginine using the procedure outlined in Example 2, and compare studies prepared with different concentrations of arginine, and / or amino sugars (e.g. meglumine) , and / or sugar (eg dextrose), and / or base (NaOH), or other basic amino acids (eg lysine, histidine) formulations. The results are shown in Table B.
[0089] Form B
[0090]
[0091] * Lys - lysine; His - histidine; Arg - arginine; Dex - dextrose; Meg - methylglumine; NA - not available.
[0092] Table B shows that arginine forms stable solutions with high concentrations of levodopa and carbidopa (>2.5%) at molar ratios <1:2.5, whereas with other basic amino acids, LD Not even soluble. At molar ratios of LD / CD to arginine of 1:<2, solutions have no long-term stability unless meglumine or another counterion is used, and meglumine can be u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com