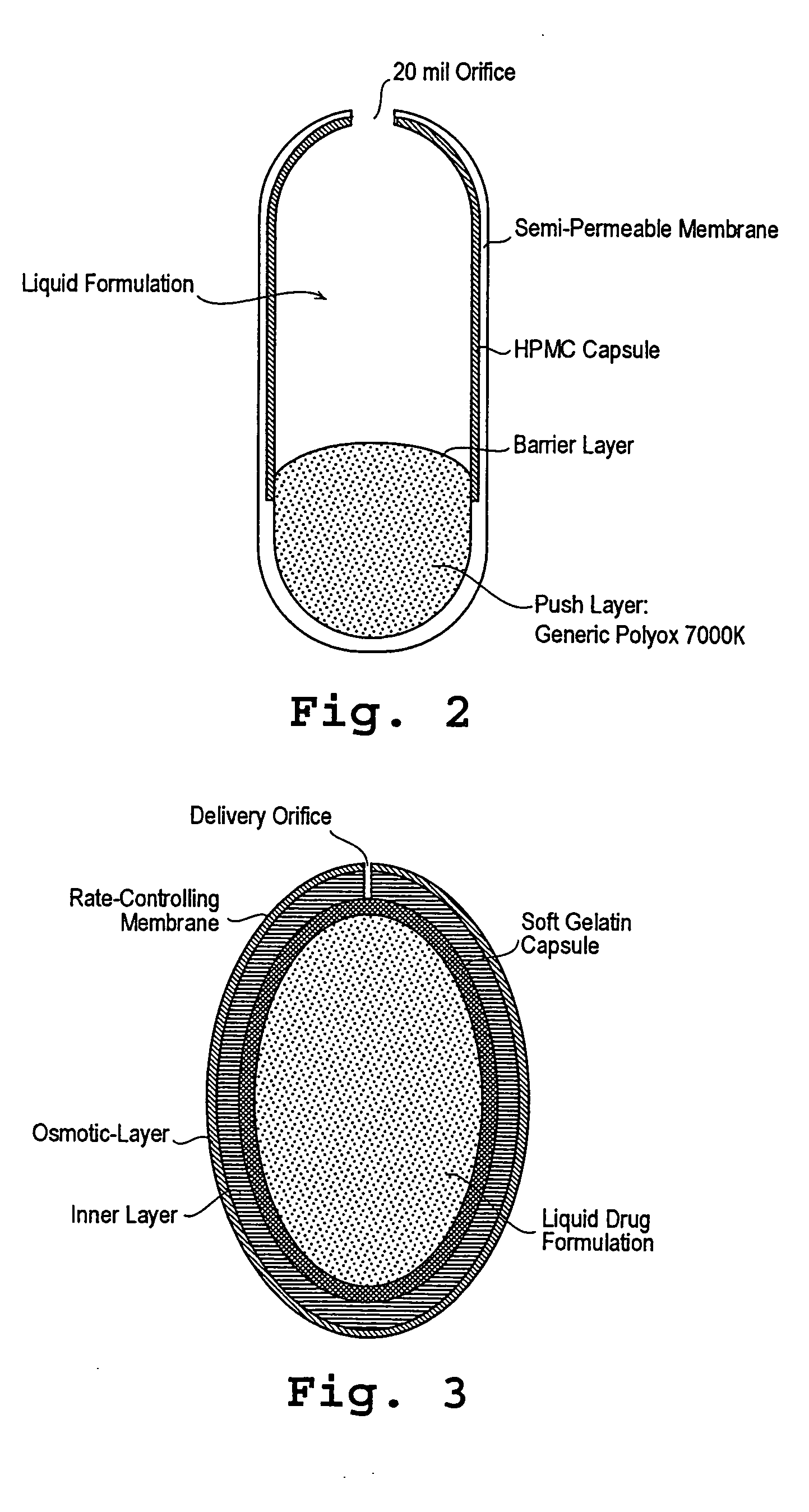
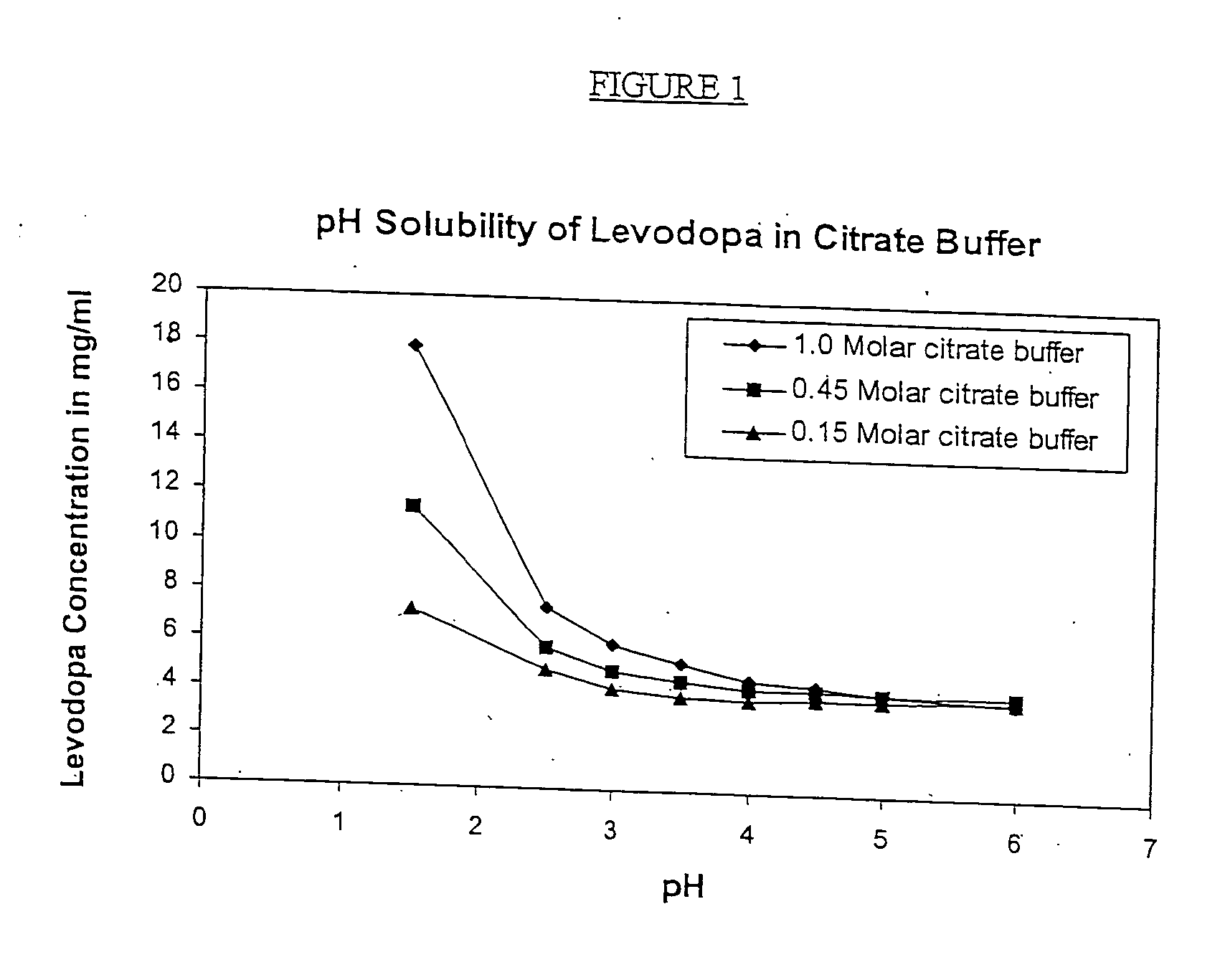
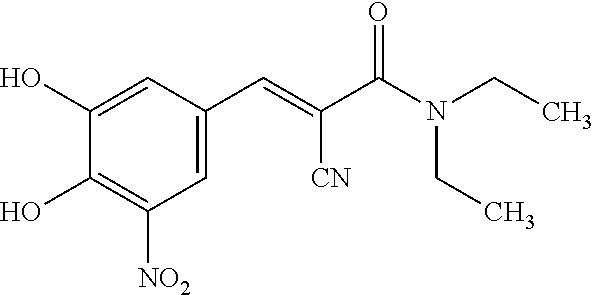
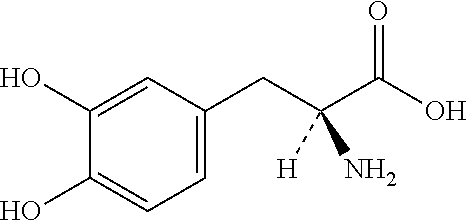
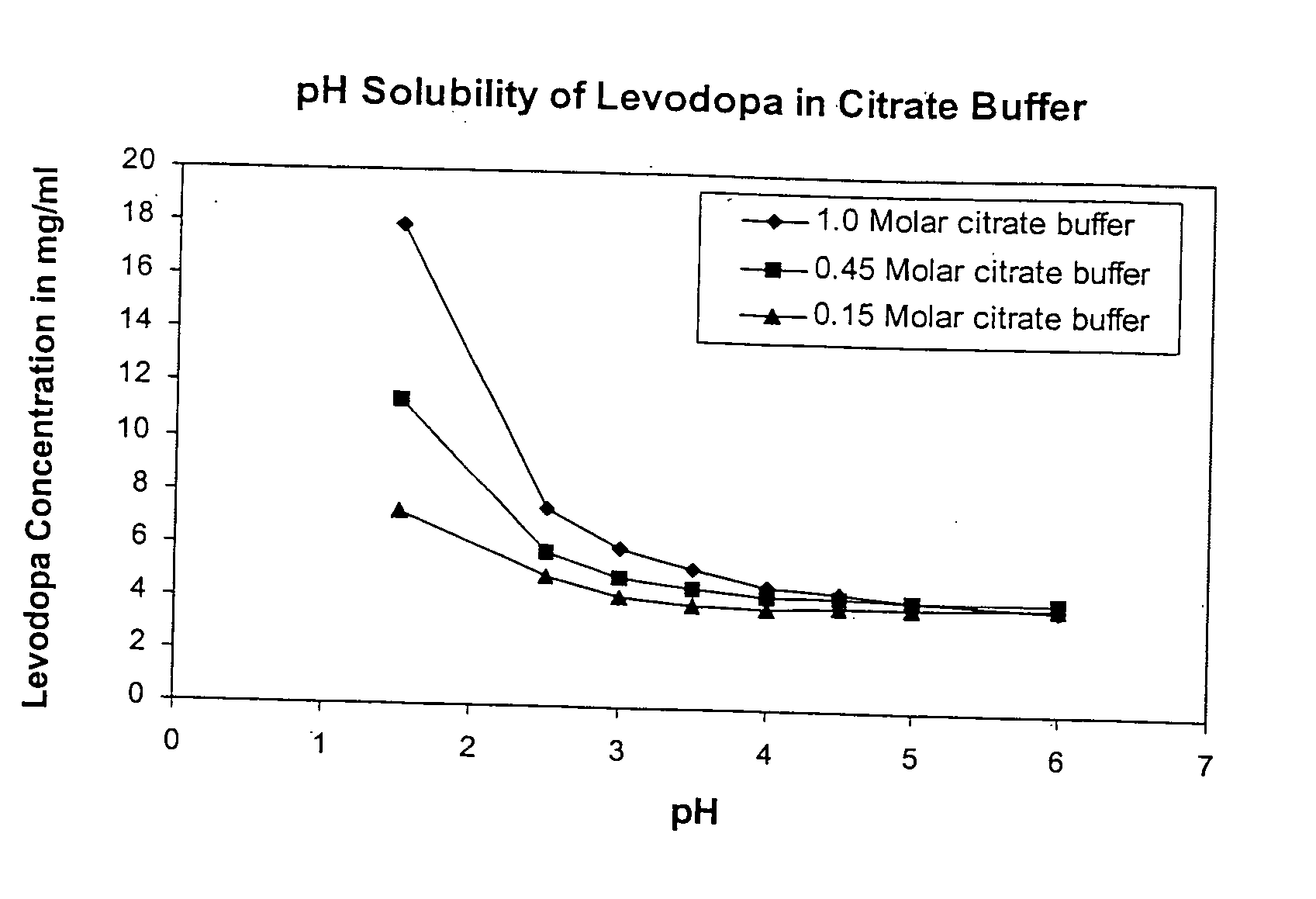
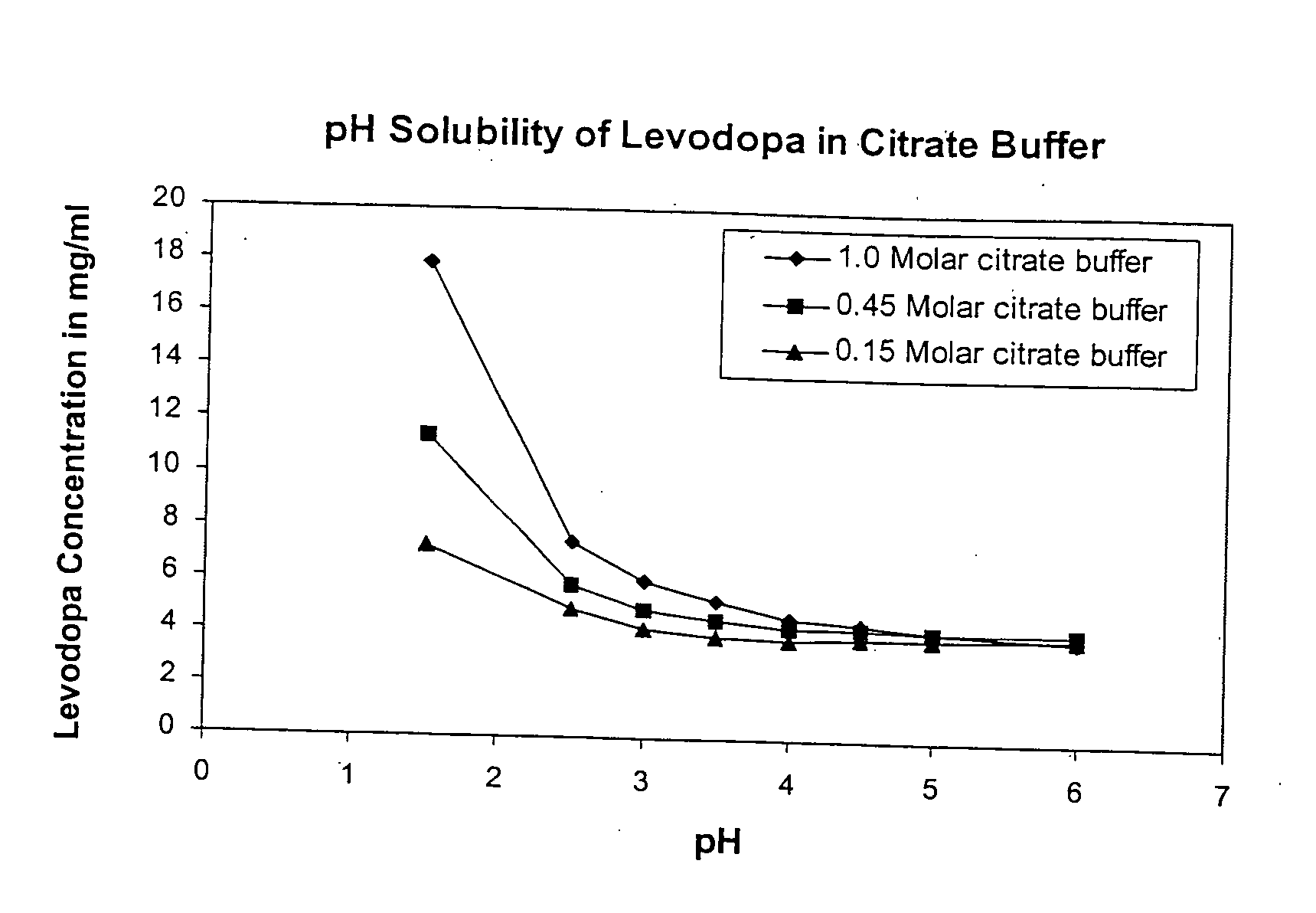
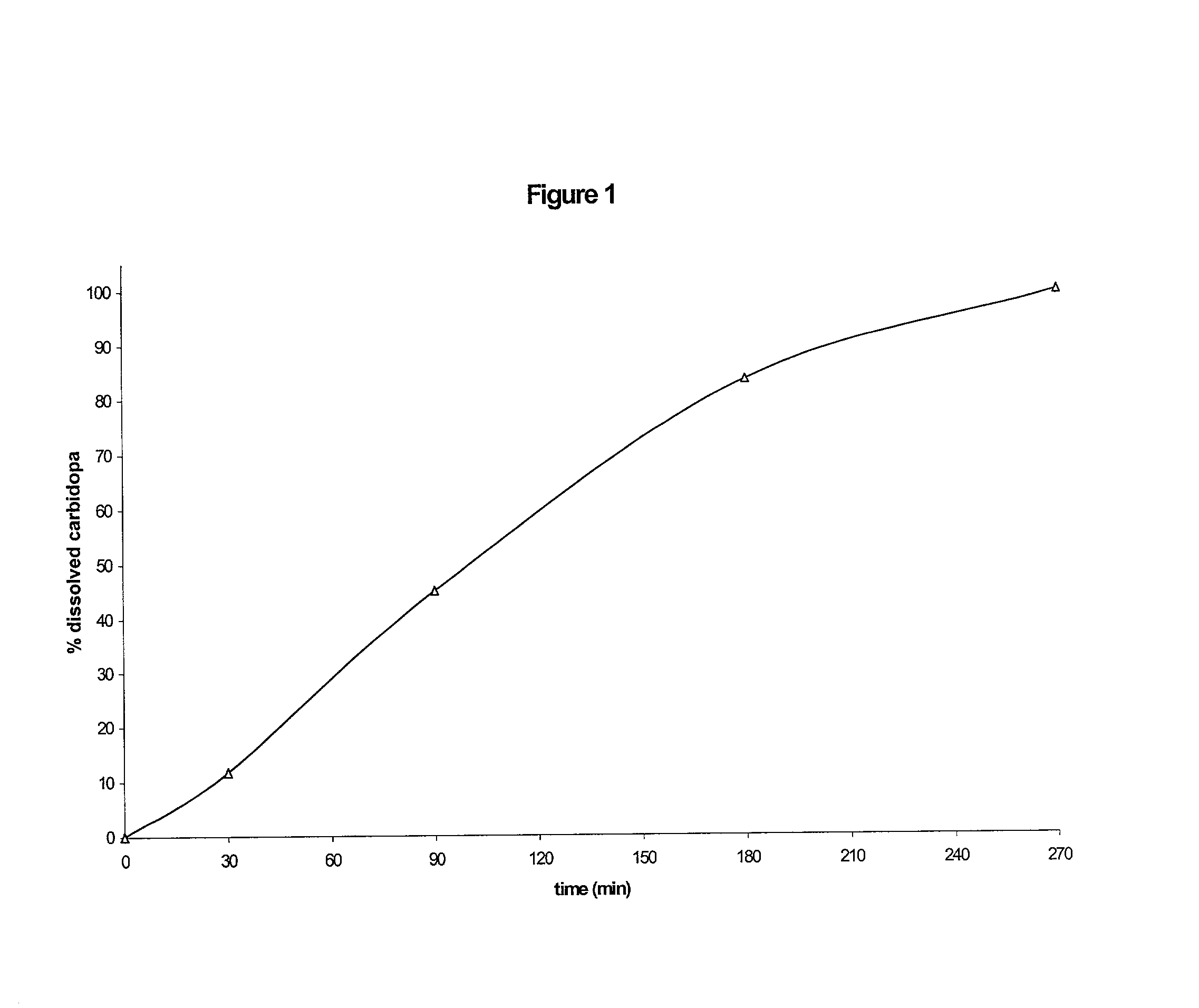
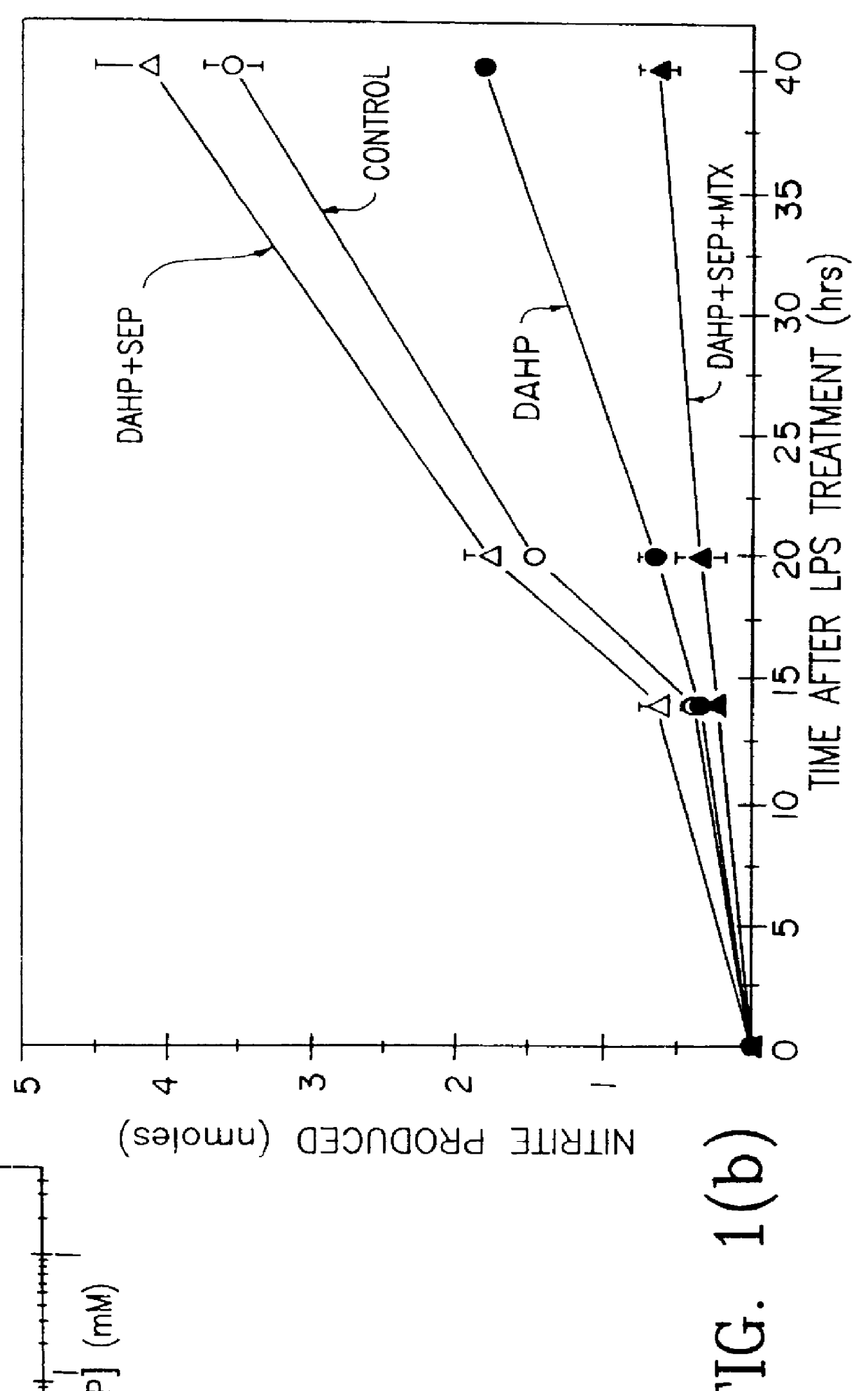
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
104 results about "Carbidopa" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used with a combination levodopa/carbidopa product to treat symptoms of Parkinson's disease or Parkinson-like symptoms (such as shakiness, stiffness, difficulty moving).
Pharmaceutical compositions for treatment of parkinson's disease and related disorders
The invention relates to the improvement in the treatment of certain neural disorders / diseases, such as Parkinson's disease and other motor disorders. The invention relates to drug compositions and dosage forms comprising said drug composition; methods of manufacturing the drug compositions and dosage forms; and methods of treatment, comprising administering the drug composition and dosage form to an individual. In certain embodiments, the drug compositions and dosage forms comprise carbidopa and levodopa in a formulation suitable for once-daily administration.
Owner:COMBINATORX
Liposomal formulation for oral administration of glutathione (reduced)
ActiveUS20060099244A1Promote absorptionPrevents and slows degradationBiocideOrganic active ingredientsOral medicationCompound (substance)
The invention is a composition administrable orally to provide systemic glutathione (reduced) and a method for providing systemic glutathione by oral administration of glutathione (reduced) in a liposome encapsulation. The administration of a therapeutically effective amount of oral liposomal glutathione (reduced) results in improvement of symptoms in disease states related to glutathione deficiency such as Parkinson's disease and cystic fibrosis. Compounds enhancing the effect of the liposomal glutathione are contemplated such as Selenium, EDTA, carbidopa, and levodopa.
Owner:YOUR ENERGY SYST
Extended Release Pharmaceutical Composition Of Entacapone Or Salts Thereof
InactiveUS20110229561A1Reducing “ wearing off ” phenomenonReduce wearBiocideNervous disorderTriple combinationCarbidopa
There is provided an extended release pharmaceutical composition comprising from about 200 mg to about 1000 mg of entacapone or salts thereof, optionally with other pharmaceutically acceptable excipients. The invention also provides an extended release pharmaceutical composition comprising triple combination of from about 30 mg to about 300 mg of levodopa, 10 mg to about 100 mg of carbidopa and 200 mg to about 1000 mg of entacapone or salts thereof, optionally with other pharmaceutically acceptable excipients. The invention also relates to process of preparation of such compositions.
Owner:WOCKHARDT LTD
Extended release solid pharmaceutical composition containing carbidopa and levodopa
InactiveUS20070275060A1Improve aestheticsImprove bioavailabilityOrganic active ingredientsBiocideExtended release tabletsImmediate release
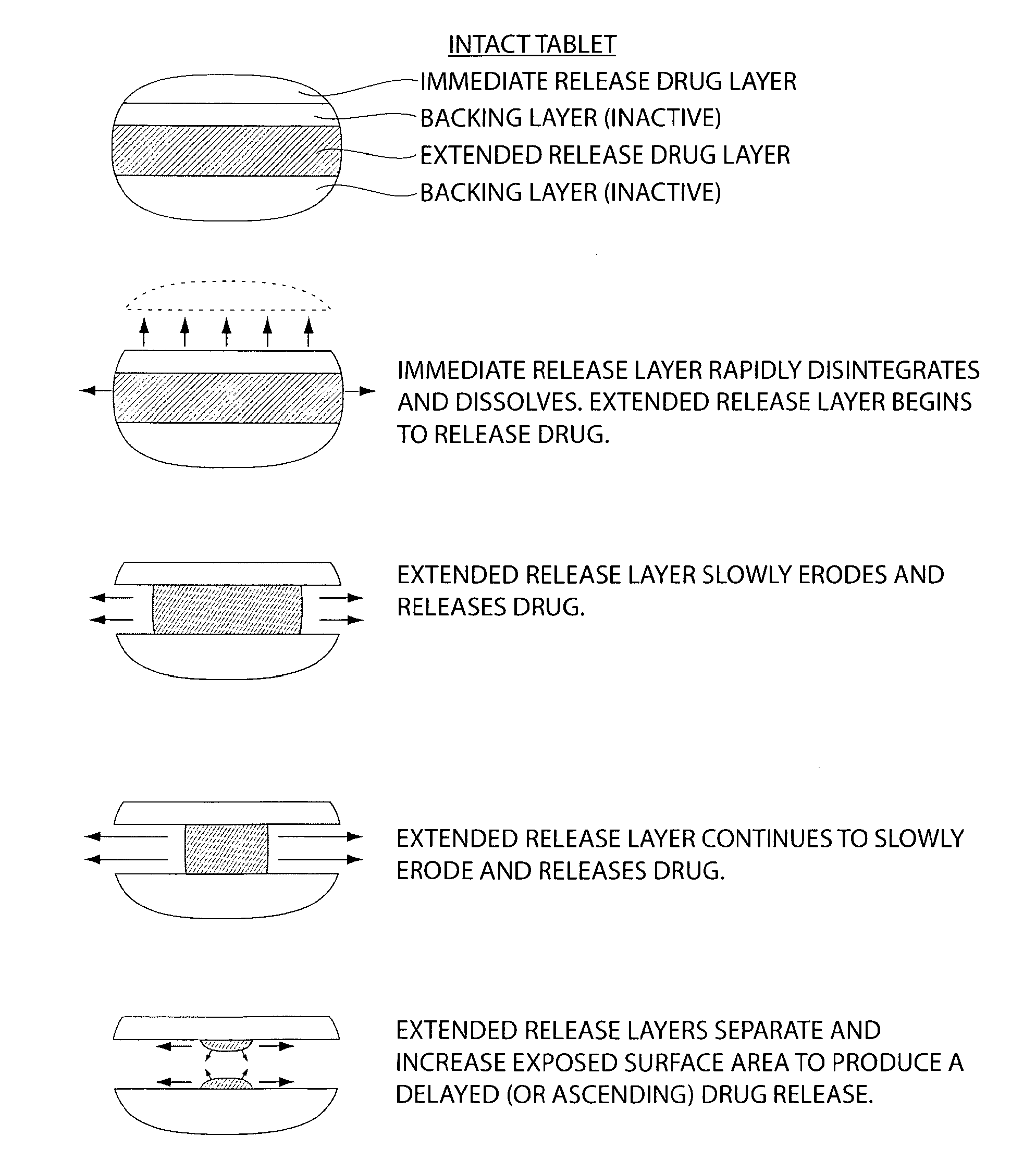
The invention provides a compressed tablet that provides a extended release tablet containing a extended release form of carbidopa and a extended release form of levodopa. The tablet optionally further comprises an immediate or rapid release composition of carbidopa and / or levodopa. The extended release composition in the tablet excludes a release rate-controlling polymer, and a release rate-controlling coating; however, the release of the carbidopa and / or levodopa is independently optionally delayed for a lag time. The invention also provides a tablet having a extended release form of levodopa and a rapid or immediate release form of carbidopa. A tablet can contain levodopa present in extended release form and rapid or immediate release form, and carbidopa present in extended release form and rapid or immediate release form. The tablet is used to treat Parkinson's disease and other movement related disorders, diseases or syndromes.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
Oral disintegrating dosage forms
InactiveUS20050147670A1Good compressibilityImprove rapid dispersion characteristicOrganic active ingredientsBiocideImmediate releaseOrally disintegrating tablet
The invention is directed to pharmaceutical dosage forms having immediate release via rapid oral disintegration, specifically, orally disintegrating tablets containing levodopa and carbidopa. The invention further provides formulations containing relatively increased amounts of carbidopa than previously available, including, for example, formulations containing carbidopa-levodopa ratios of about 1:1 to about 1:3.
Owner:IMPAX LAB INC
Composition with sustained release of levodopa and carbidopa
Owner:COVIS PHARM GMBH
Combination immediate release controlled release levodopa/carbidopa dosage forms
The present invention relates to dosage forms of a combination of carbidopa and levodopa comprising both immediate release and controlled release components for the treatment of ailments associated with depleted amounts of dopamine in a patient's brain tissue.
Owner:IMPAX LAB INC
Continuous Administration of L-Dopa, Dopa Decarboxylase Inhibitors, Catechol-O-Methyl Transferase Inhibitors and Compositions for Same
ActiveUS20140051755A1Effectively treating movementImprove efficiencyBiocideNervous disorderTolcaponeCarbidopa
Provided herein, in part, is a method of treating a neurological or movement disorder in a patient in need thereof, comprising subcutaneously administering to said patient a pharmaceutically acceptable composition comprising levodopa and optionally carbidopa and optionally entacapone or tolcapone, or pharmaceutically acceptable salts thereof, wherein said composition is administered substantially continuously, and compositions that can be used in the disclosed methods.
Owner:NEURODERM
Composition with sustained release of levodopa and carbidopa
The invention relates to a pharmaceutical composition comprising a therapeutically effective amount of levodopa and of carbidopa, dispersed in a hydrophilic matrix, said composition further comprising an organic acid. A subject of the invention is also a process for preparing the composition, comprising granulation, in particular in a fluidized bed, of the various components and compression of the granules obtained.
Owner:COVIS PHARM GMBH
Pharmaceutical composition and preparation method thereof
InactiveUS20060222703A1Small sizeDisintegrates quicklyBiocideOrganic active ingredientsEquilibrium moisture contentExcipient
The invention relates to an oral solid pharmaceutical composition comprising pharmacologically effective amounts of entacapone, levodopa and carbidopa, or a pharmaceutically acceptable salt or hydrate thereof, and one or more pharmaceutically acceptable excipients, wherein the excipients are long-chain polymers having an equilibrium moisture content of at least 2%, and to a preparation method thereof. The compositions can be used for the treatment of Parkinson's disease.
Owner:IPRBOX
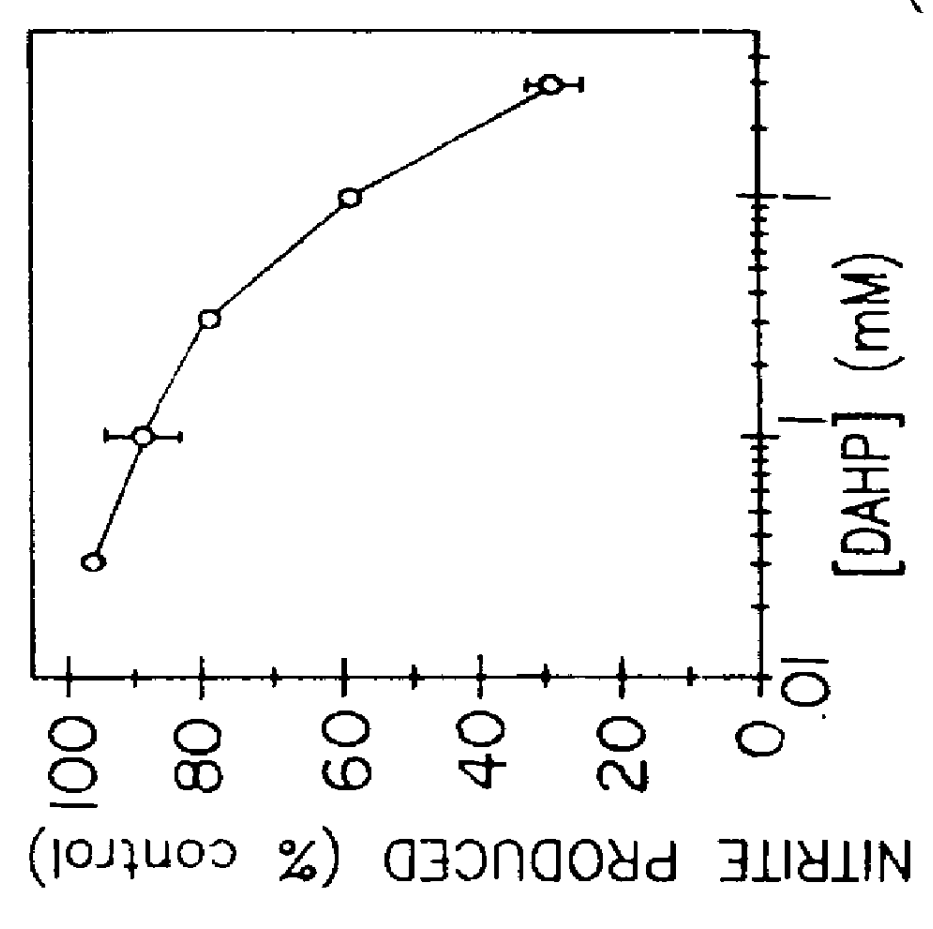
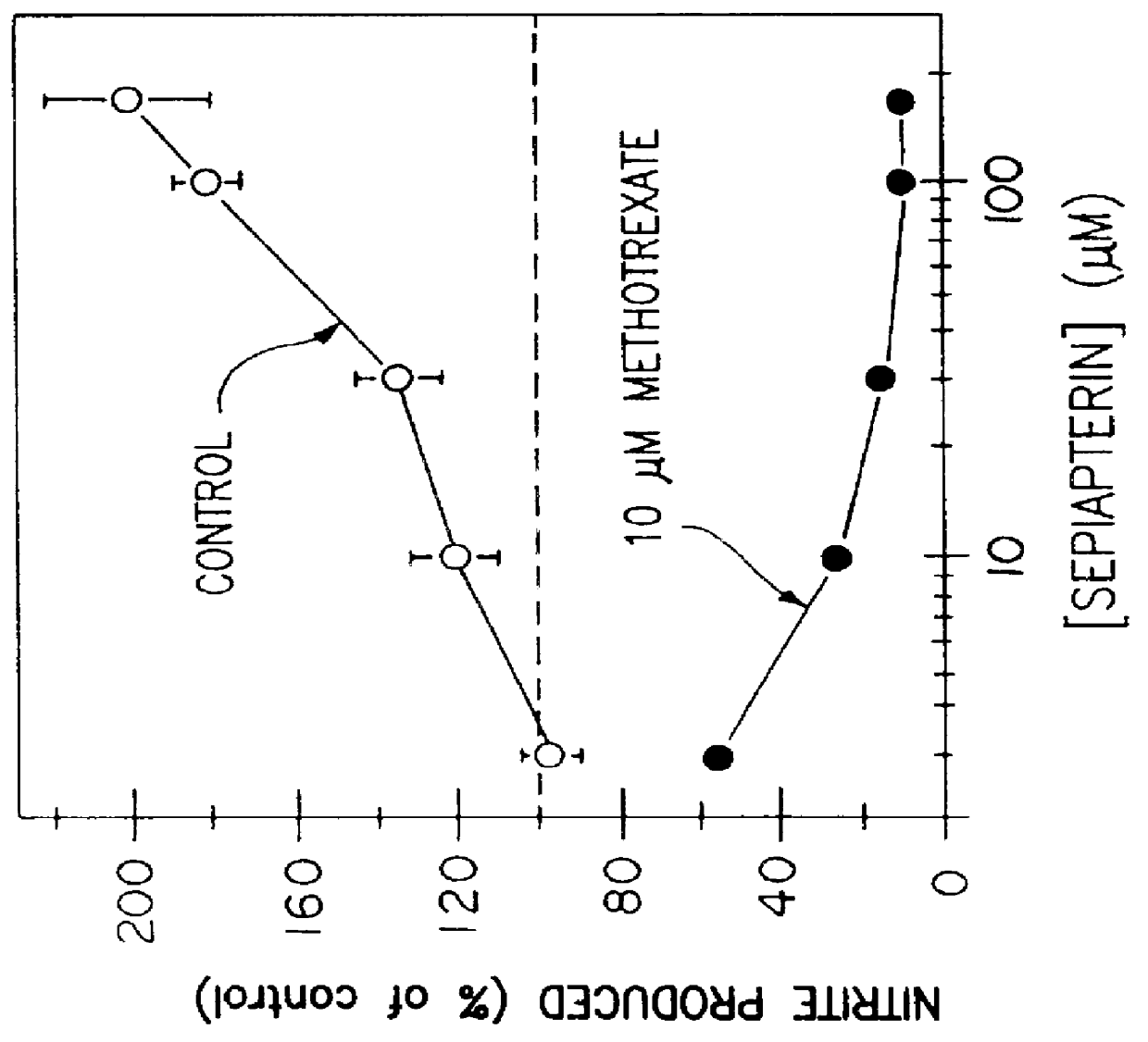
Blocking induction of tetrahydrobioterin to block induction of nitric oxide synthesis
InactiveUS6153615AReduce inhibitionRestore sensitivityBiocidePeptide/protein ingredientsSide effectTreatment effect
Guanosine triphosphate pathway tetrahydrobiopterin synthesis antagonist and / or pterin salvage pathway tetrahydrobiopterin synthesis antagonists are administered to inhibit nitric oxide synthesis from arginine in vascular cells in a subject in need of such inhibition (e.g., for prophylactic or curative effect for endotoxin- or cytokine-induced hypotension or for restoration of vascular contractile sensitivity to pressor agents in the treatment of such hypotension). The tetrahydrobiopterin synthesis antagonist may be administered with alpha 1-adrenergic agonist or with nitric oxide synthase inhibitor. The tetrahydrobiopterin synthesis antagonists are also administered to attenuate inflammation caused by induced nitric oxide production in immune cells. Unwanted counterproductive or side effects can be eliminated or ameliorated by administration additionally of levodopa with or without carbidopa and L-5-hydroxytryptophane.
Owner:CORNELL RES FOUNDATION INC
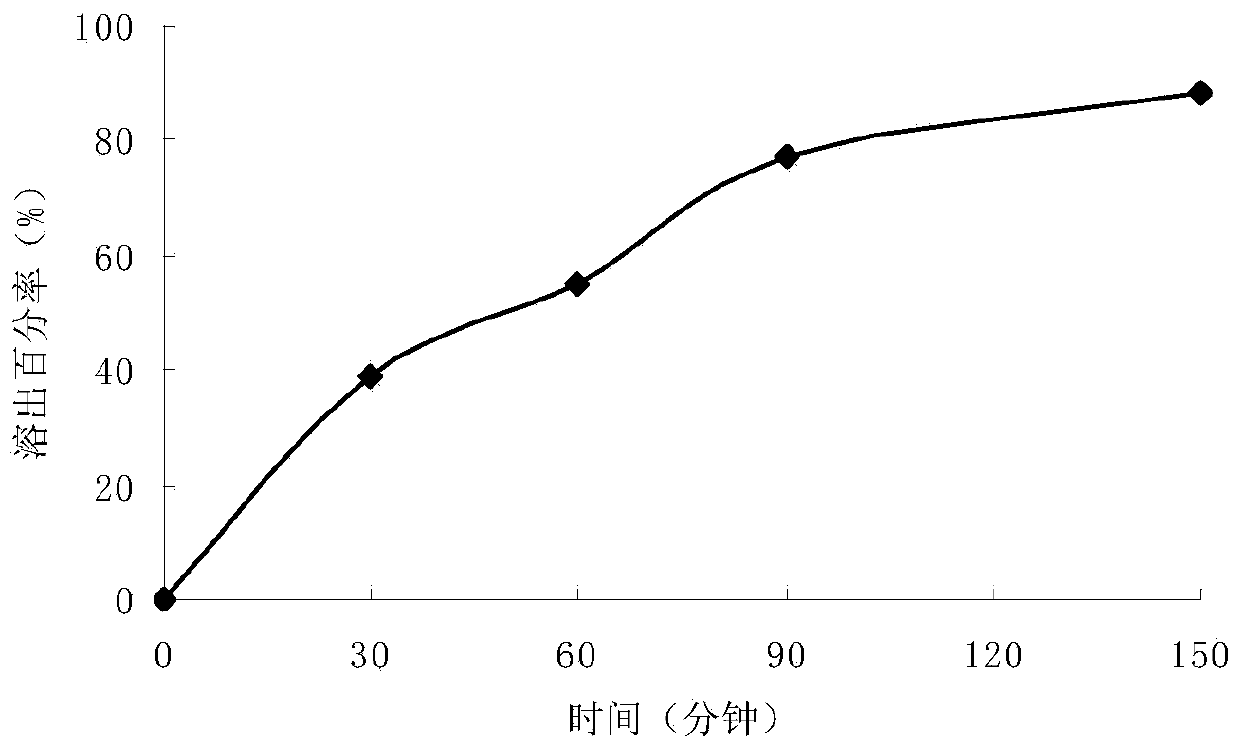
Carbidopa/lipodopa gastroretentive drug delivery
A gastroretentive drug formulation for the sustained release of an active agent in the gastrointestinal tract comprises an internal layer or compartment comprising an active agent and one or more pharmaceutical excipients, of which at least one is a polymer and two membranes forming together an envelope around the inner membrane, each membrane comprising at least one polymeric combination of an enteric polymer which is not soluble in gastric juice, and an hydrophilic swelling polymer, and at least one plasticizer.
Owner:INTEC PHARMA
Controlled release pharmaceutical compositions of carbidopa and levodopa
The present invention relates to controlled release pharmaceutical compositions of carbidopa and levodopa that include a combination of different molecular weight cellulose ethers and in particular, hydroxypropyl cellulose ether.
Owner:RANBAXY LAB LTD
Extended release pharmaceutical dosage forms of carbidopa and levodopa and process of preparation thereof
The present invention relates to an extended-release pharmaceutical dosage form of carbidopa and levodopa comprising (i) an immediate-release unit of carbidopa and levodopa; (ii) an extended-release unit of carbidopa and levodopa; and (iii) an immediate or extended-release unit of a carboxylate salt. The present invention further provides a process of preparation thereof.
Owner:RANBAXY LAB LTD
Method for treatment of parkinson's disease
The present invention provides a method for treatment of a neurological or movement disorder, e.g., Parkinson's disease, in an individual in need thereof, by parenteral administration of a composition comprising carbidopa and levopoda, or pharmaceutically acceptable salts thereof, and concomitant oral administration of a catechol-O-methyl transferase (COMT) inhibitor, e.g., entacapone or tolcapone.
Owner:NEURODERM
Pharmaceutical composition containing levodopa, entacapone and carbidopa
The present invention refers to a solid pharmaceutical composition of entacapone, levodopa and carbidopa or pharmaceutically acceptable salts thereof characterized in that entacapone is in the form of granules and it is added separately to levodopa and carbidopa. In addition, this invention provides the process for its preparation.
Owner:LAB LESVI SL
Carbidopa and L-Dopa Prodrugs and Methods of Use
The present disclosure relates to (a) carbidopa prodrugs, (b) pharmaceutical combinations and compositions comprising a carbidopa prodrug and / or an L-dopa prodrug, and (c) methods of treating Parkinson's disease and associated conditions comprising administering a carbidopa prodrug and an L-dopa prodrug to a subject with Parkinson's disease.
Owner:ABBVIE INC
Continuous administration of l-dopa, dopa decarboxylase inhibitors, catechol-o-methyl transferase inhibitors and compositions for same
ActiveUS20140249228A1Improve efficiencyReduce the daily dosage of levodopaBiocideNervous disorderTolcaponeCarbidopa
Provided herein, in part, is a method of treating a neurological or movement disorder in a patient in need thereof, comprising subcutaneously administering to said patient a pharmaceutically acceptable composition comprising levodopa and optionally carbidopa and optionally entacapone or tolcapone, or pharmaceutically acceptable salts thereof, wherein said composition is administered substantially continuously, and compositions that can be used in the disclosed methods.
Owner:NEURODERM
Continuous administration of l-dopa, dopa decarboxylase inhibitors, catechol-o-methyl transferase inhibitors and compositions for same
ActiveUS20140249229A1Improve efficiencyReduce the daily dosage of levodopaBiocideNervous disorderTolcaponeCarbidopa
Provided herein, in part, is a method of treating a neurological or movement disorder in a patient in need thereof, comprising subcutaneously administering to said patient a pharmaceutically acceptable composition comprising levodopa and optionally carbidopa and optionally entacapone or tolcapone, or pharmaceutically acceptable salts thereof, wherein said composition is administered substantially continuously, and compositions that can be used in the disclosed methods.
Owner:NEURODERM
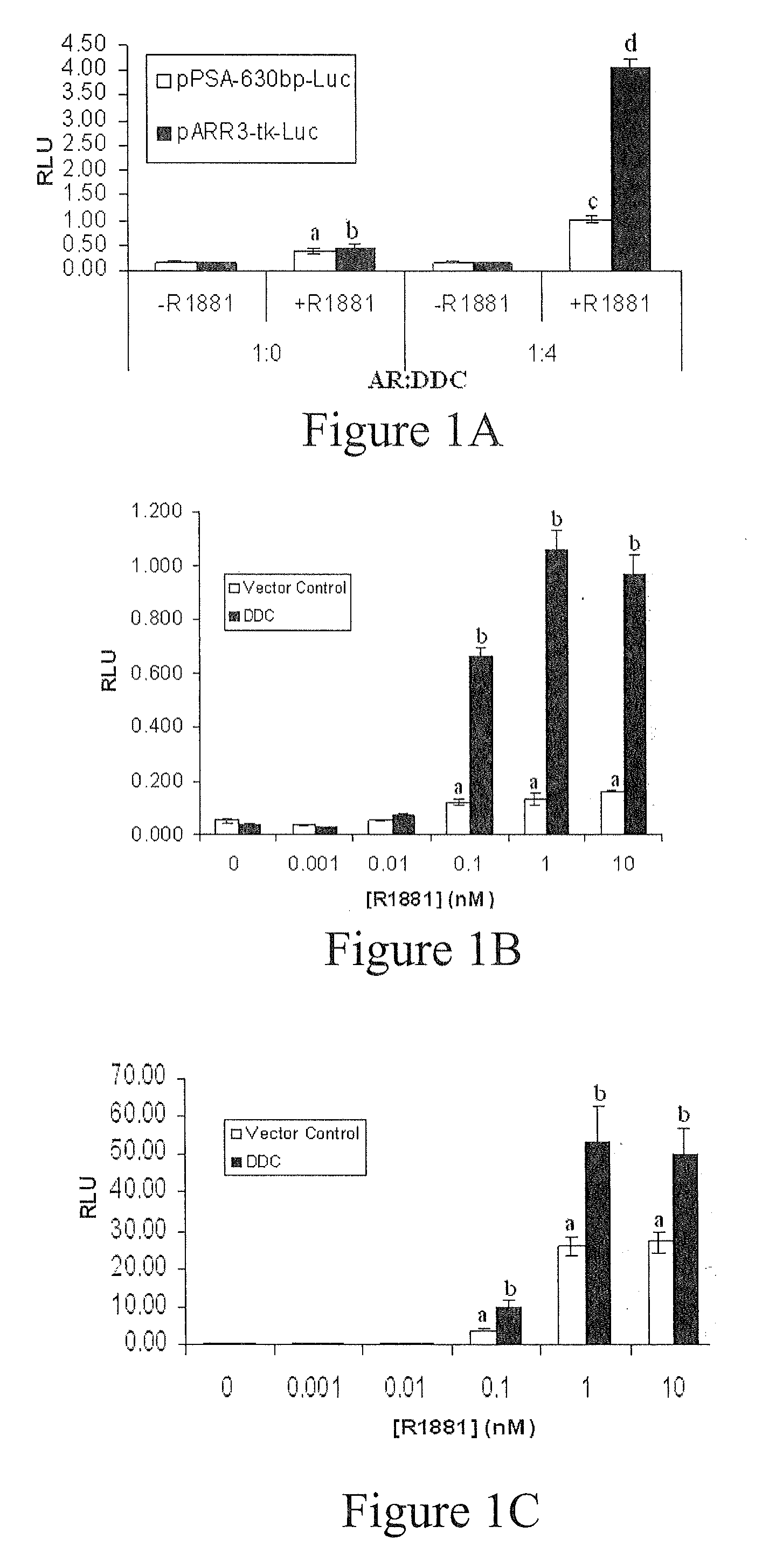
Treatment of Prostate Cancer with DDC Inhibitor
ActiveUS20100048709A1Low affinityBiocidePeptide/protein ingredientsMultiple formsDihydroxyphenylalanine
Owner:THE UNIV OF BRITISH COLUMBIA
Levodopa/carbidopa compound sustained-release suspension and preparation method thereof
InactiveCN103622942AImprove stabilitySolving Difficult to Swallow ProblemsOrganic active ingredientsNervous disorderMulti unitLarge dose
The invention discloses a levodopa / carbidopa compound sustained-release suspension and a preparation method thereof. The preparation comprises the following components in percentage by weight: 10-50% of levodopa, 10-50% of carbidopa and 10-50% of accessory realizing a sustained-release effect. Compared with a solid preparation, the suspension disclosed by the invention has the advantages that the drug is coated in resin so that the drug stability is improved; the suspension can be taken in divided dose, so that the problem that the oral solid sustained-release preparation prepared from large-dose drugs is hard to swallow is solved; in a multi-unit drug release system, the drug release action is the total of the drug release actions of particles, and the reproducibility and consistency are better; the phenomenon of excessively high local concentration often caused after the oral solid sustained-release preparation is ground can be avoided; the bad smell of the drug can be covered, and the palatability of the preparation is improved. The sustained-release preparation disclosed by the invention is to be clinically used as an anti-paralysis agitans drug.
Owner:JIANGSU UNIV
Levodopa/carbidopa/entacapone pharmaceutical preparation
The invention relates to an oral solid fixed dose composition comprising pharmacologically effective amounts of entacapone, levodopa, and carbidopa, or a pharmaceutically acceptable salt or hydrate thereof, and comprising at least one pharmaceutically acceptable excipient. The composition of the invention can be used e.g. for the treatment of Parkinson's disease.
Owner:ORION CORPORATION
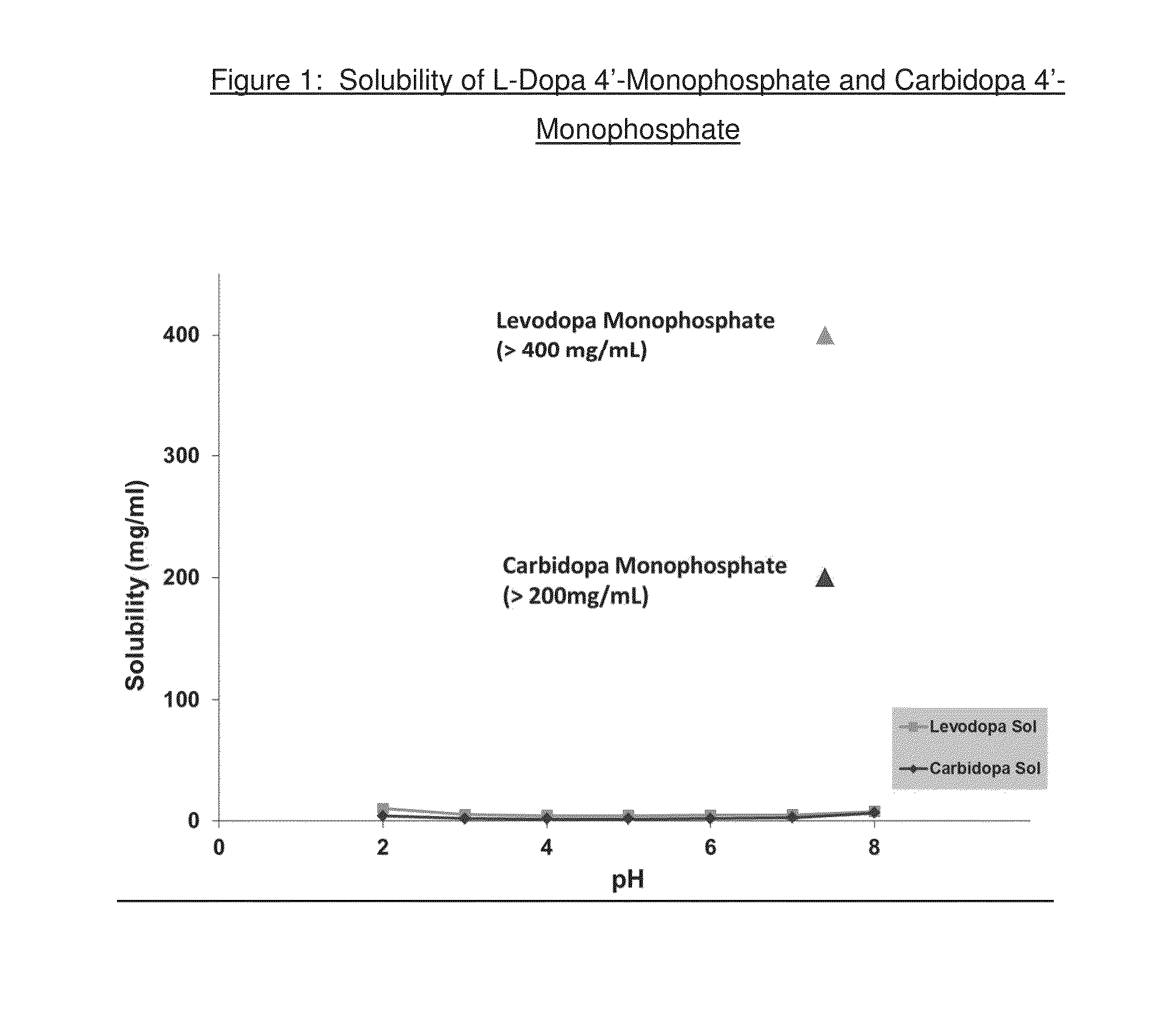
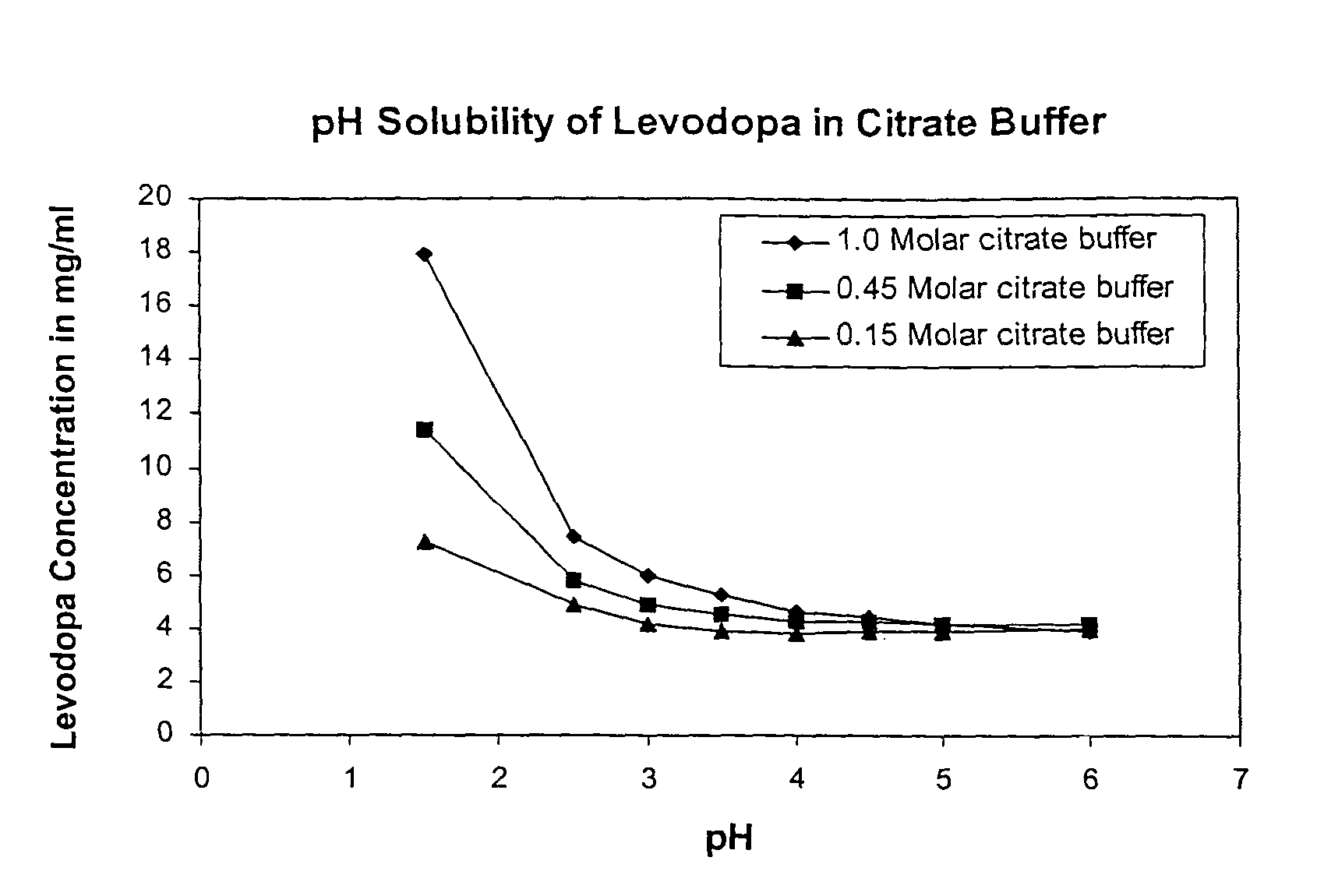
Pharmaceutical compositions and method of using levodopa and carbidopa
The present invention relates to stable dosage forms and compositions of levodopa and carbidopa for the treatment of patients suffering from Parkinson's disease. The dosage forms and compositions comprise both solid and liquid formulations and result in stable pharmaceutical products. Such dosage forms and compositions comprise a metal chelator and a levodopa concentration from about 1 mg / mL to about 30 mg / mL.
Owner:JANSSEN BIOTECH INC
Continuous administration of dopa decarboxylase inhibitors and compositions for same
ActiveUS9040589B2Improve efficiencyReduce daily dosageBiocideNervous disorderDiseaseHuntingtons chorea
Disclosed herein are compositions that include for example the arginine salt of carbidopa, and methods for treating neurological or movement diseases or disorders such as restless leg syndrome, Parkinson's disease, secondary parkinsonism, Huntington's disease, Parkinson's like syndrome, PSP, MSA, ALS, Shy-Drager syndrome and conditions resulting from brain injury including carbon monoxide or manganese intoxication, using substantially continuous administration of carbidopa or salt thereof together with administration of levodopa.
Owner:NEURODERM
Medicine composition containing myricetrin or/and myricetin and application of medicine composition in preparation of medicine used for treating Parkinson
InactiveCN103211832AEnhance anti-inflammatoryImproves antioxidant activityOrganic active ingredientsNervous disorderLiver and kidneyAglycone
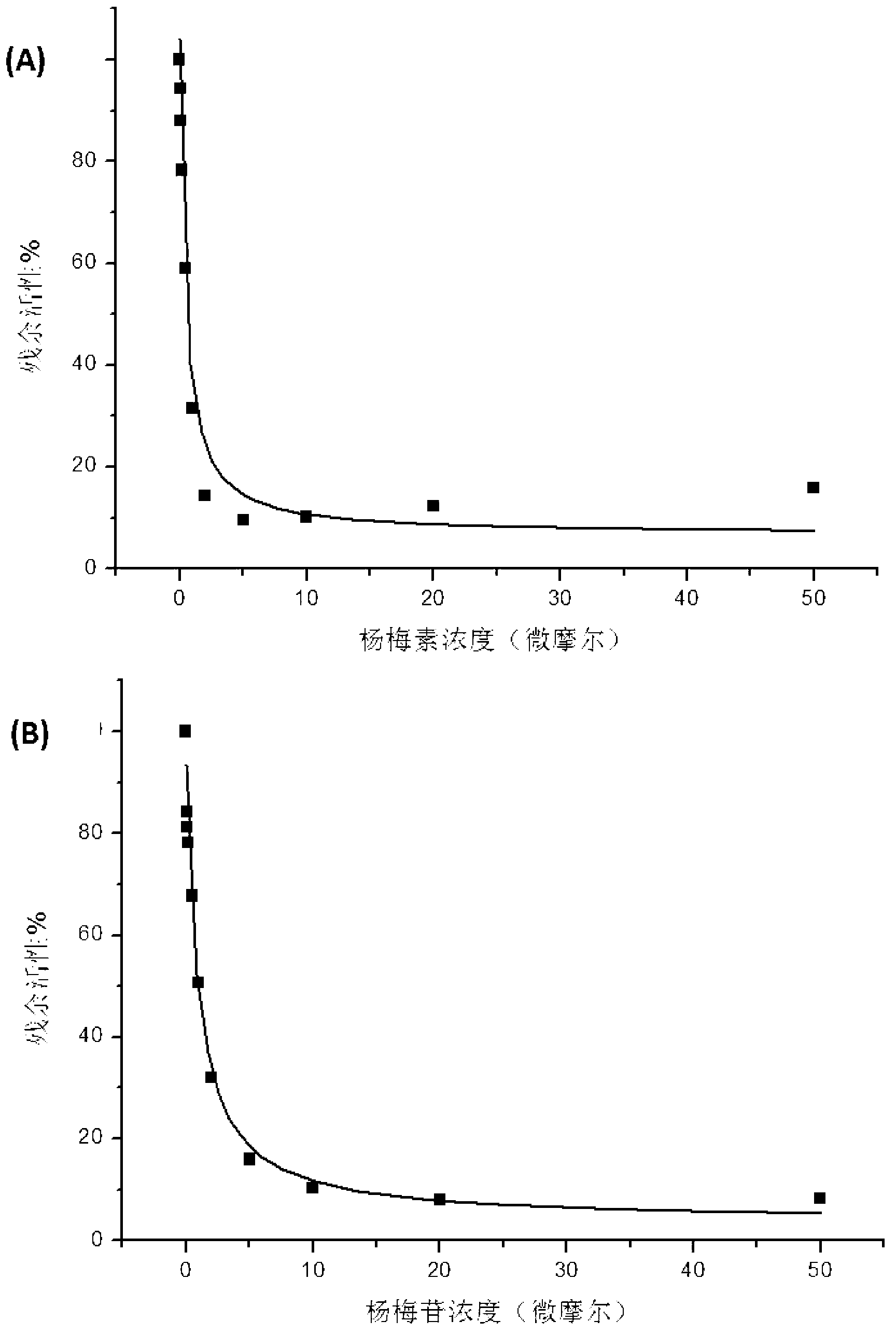
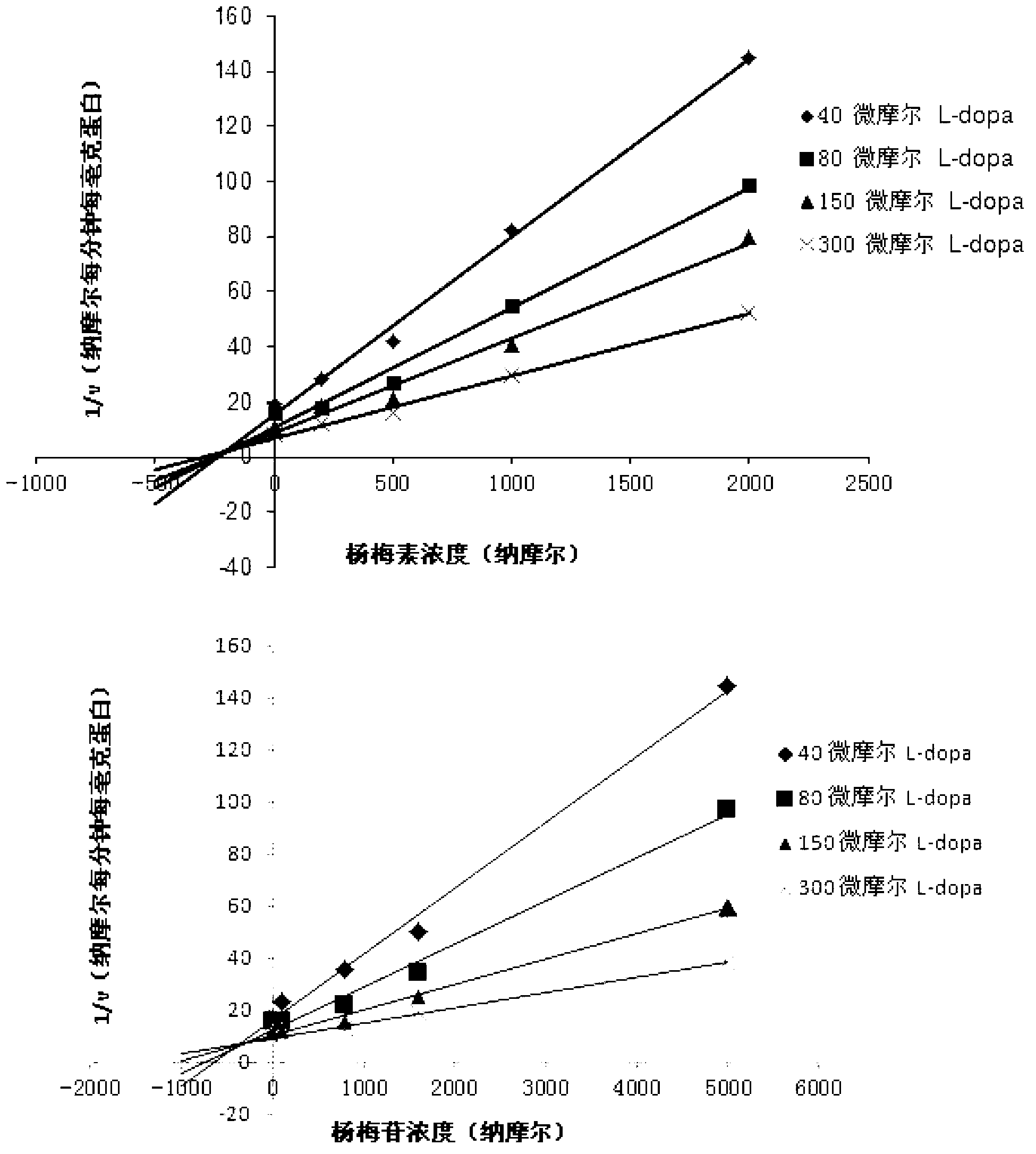
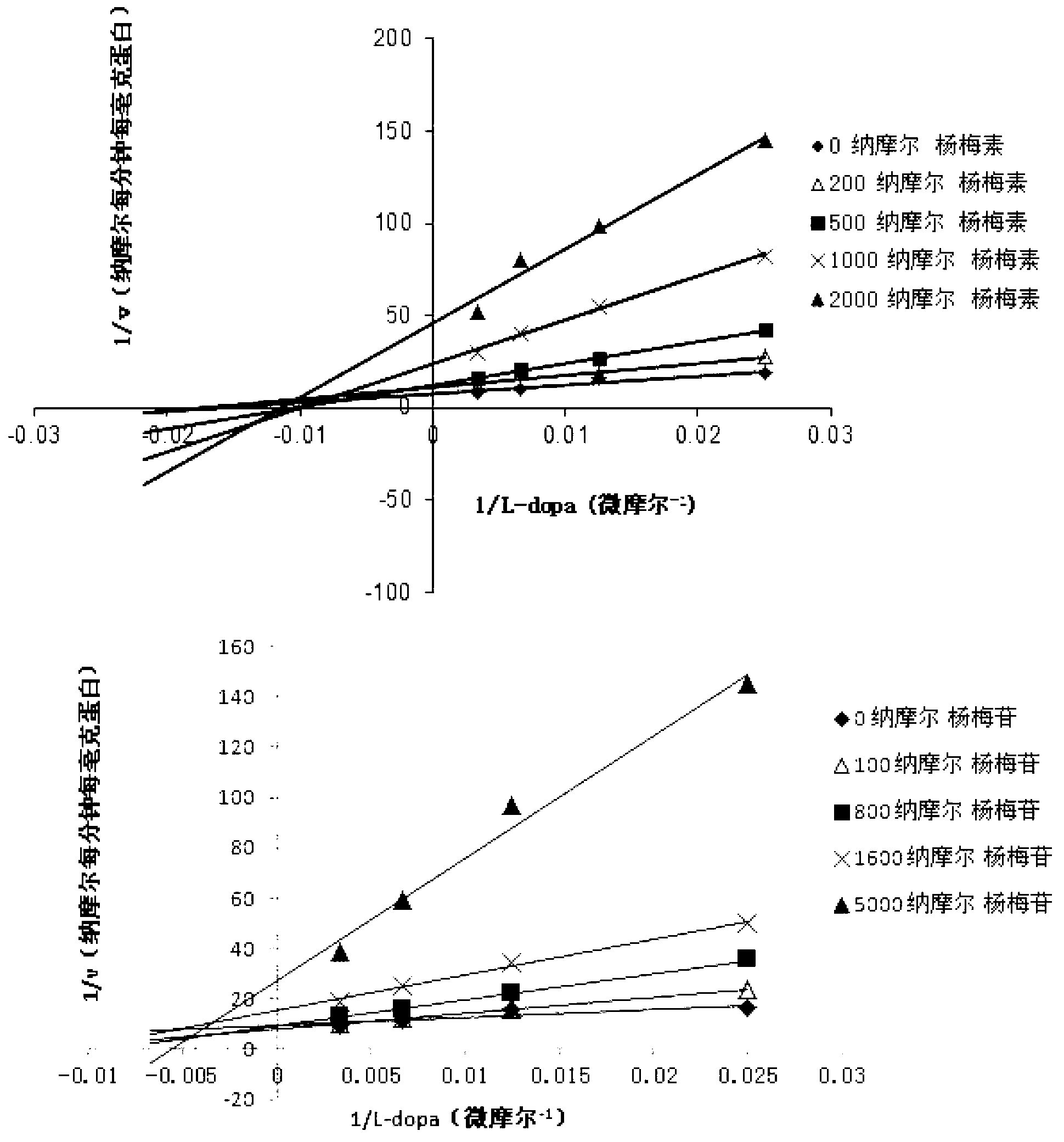
The invention relates to a medicine composition of myricetrin or / and myricetin aglycone thereof and levodopa or of levodopa and carbidopa and an application of myricetrin or / and myricetin aglycone thereof as a synergist in preparation of a medicine used for treating Parkinson. In vitro activity determination finds that IC50 of myricetrin and myricetin inhibiting catechol-O-methyltransferase (COMT) can be a micromole-nanomole scale. Rat overall pharmacokinetic study finds that blood concentration of levodopa can be increased after myricetrin and myricetin aglycone thereof and levodopa are used jointly; and myricetrin and myricetin aglycone thereof are high in safety, no obvious toxicity is produced to main visceral organ cells of a human body, and no active intermediate is produced by virtue of metabolism activation and further no damage done to organs such as liver and kidney is initiated, so that the myricetrin and myricetin aglycone thereof have a good application prospect in adjuvant therapy of Parkinson.
Owner:无锡艾德美特生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com