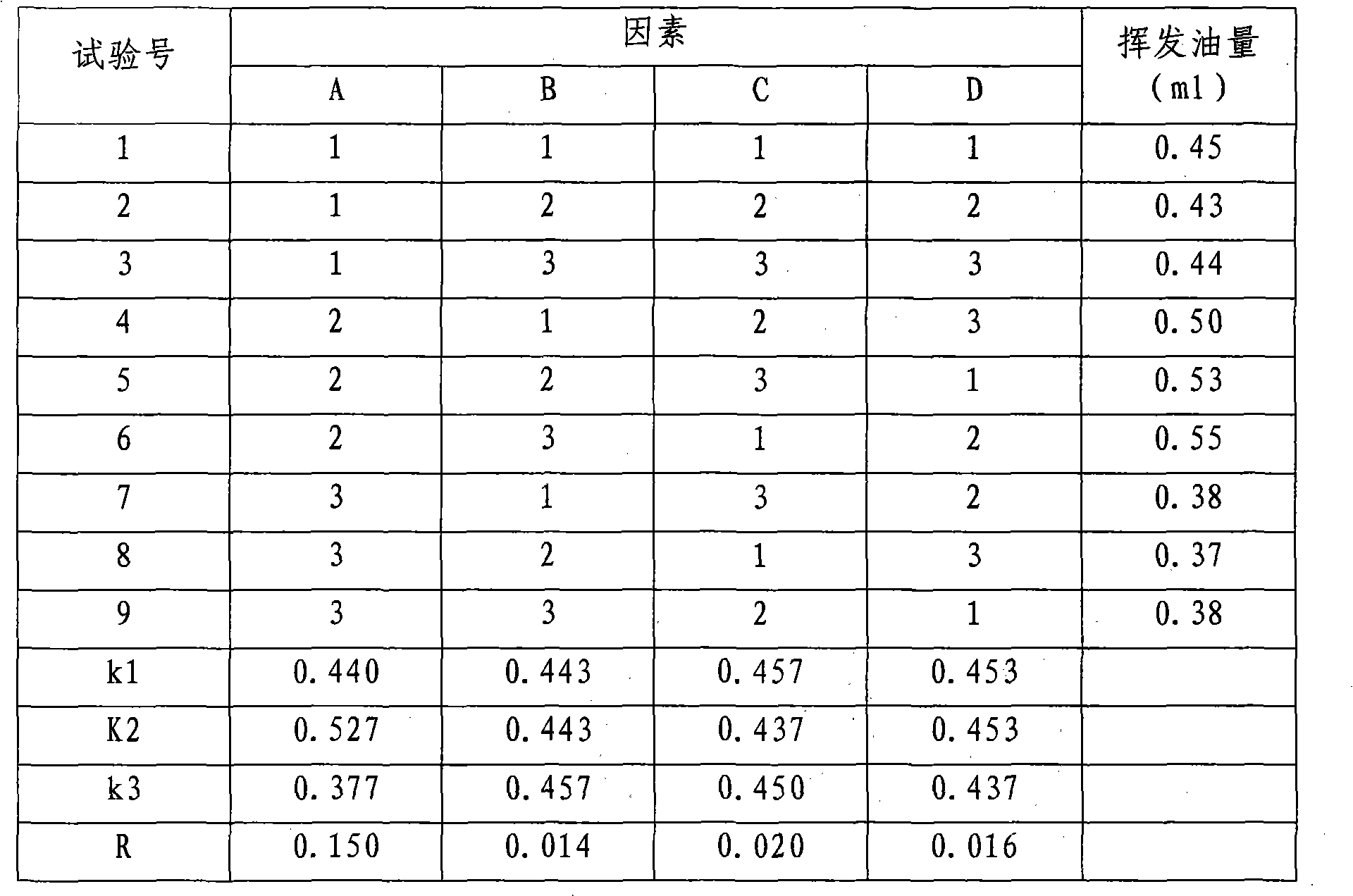
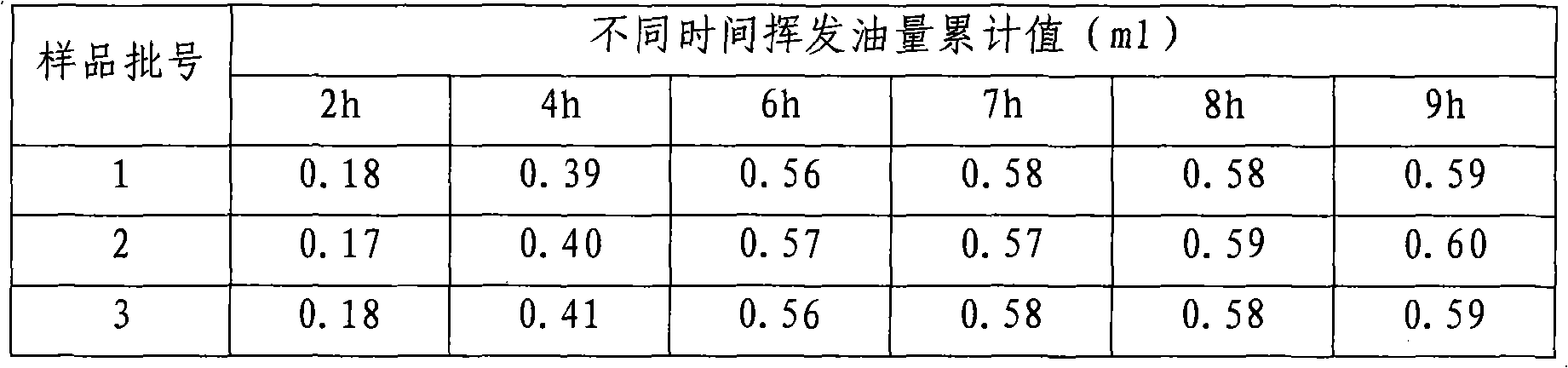
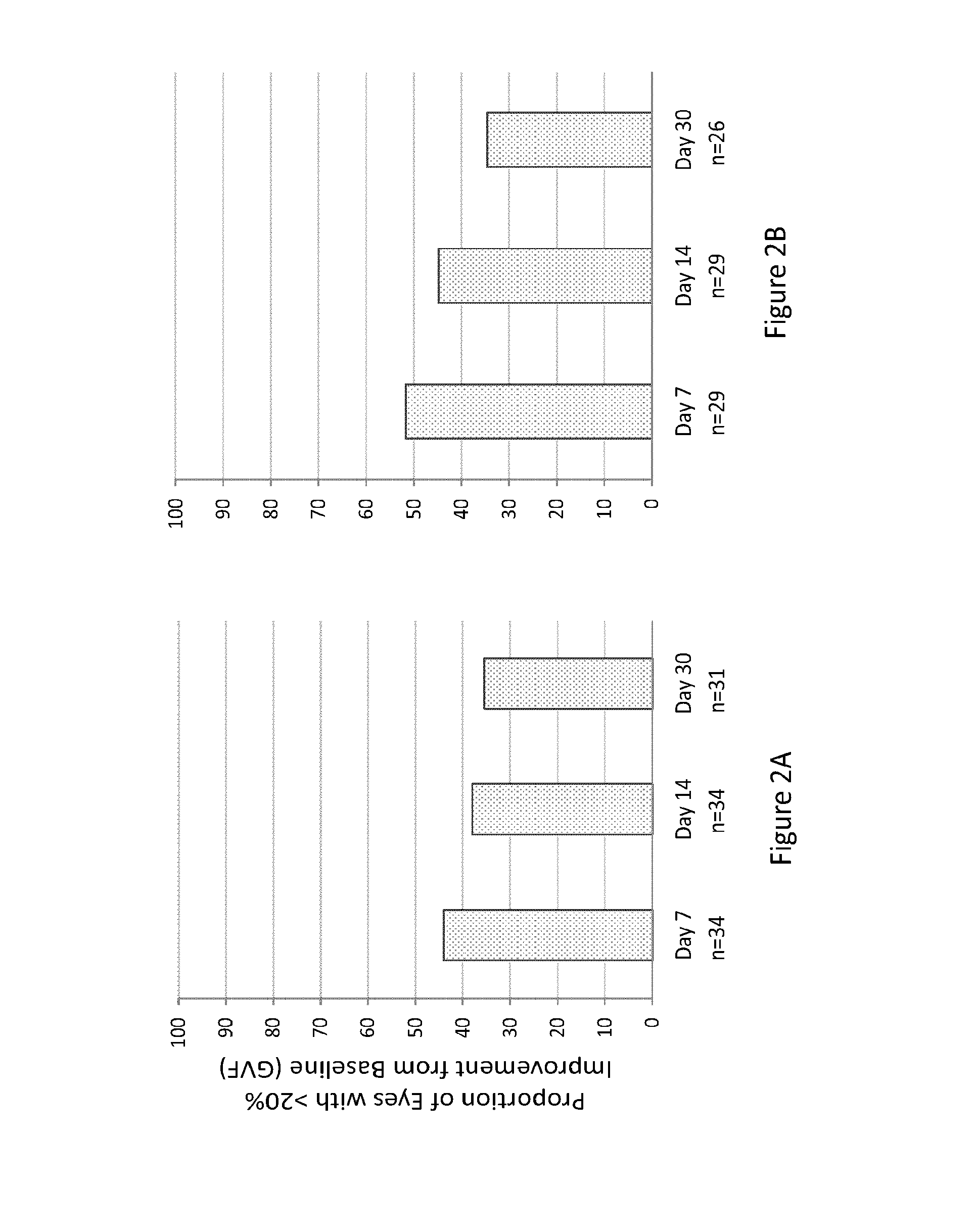
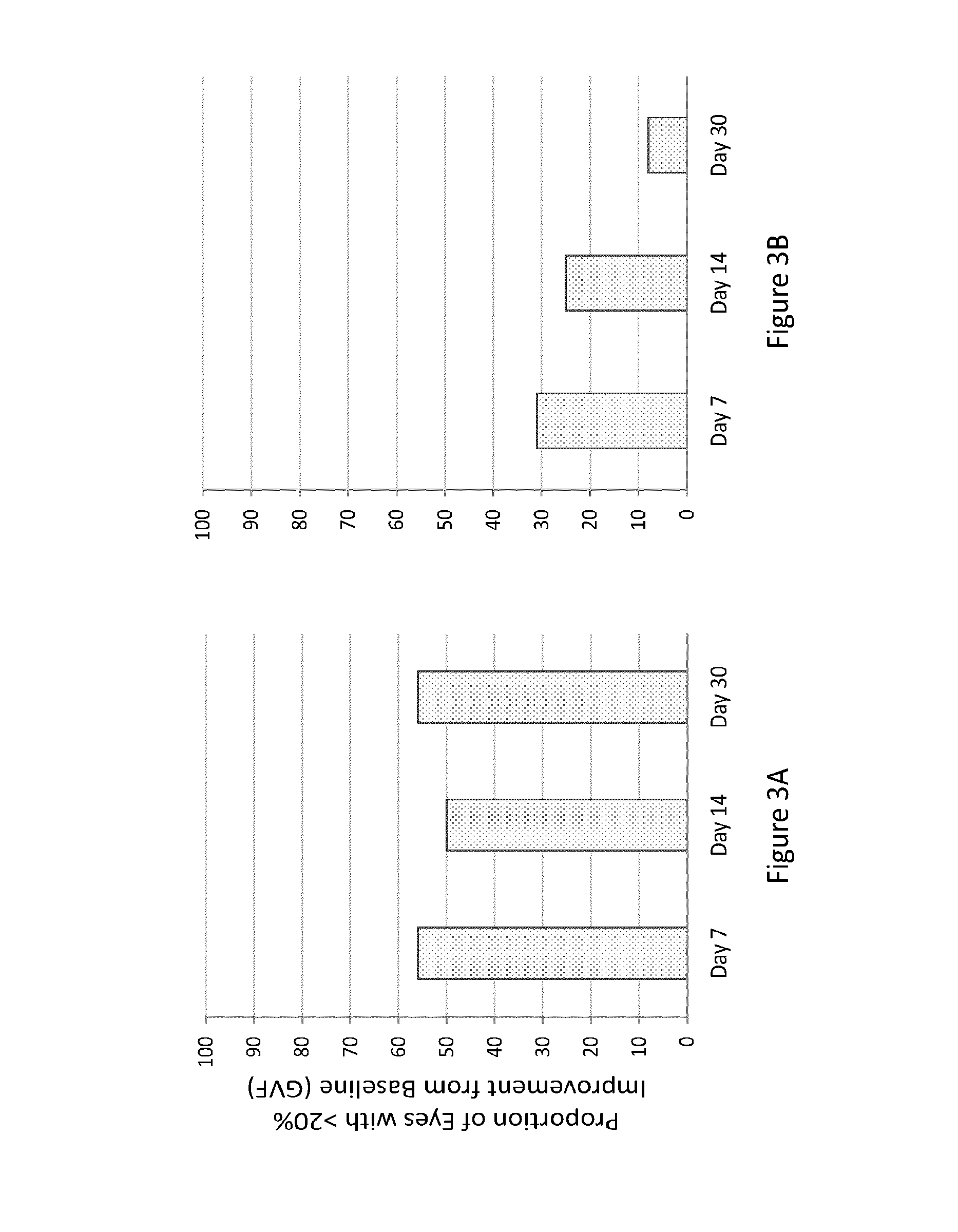
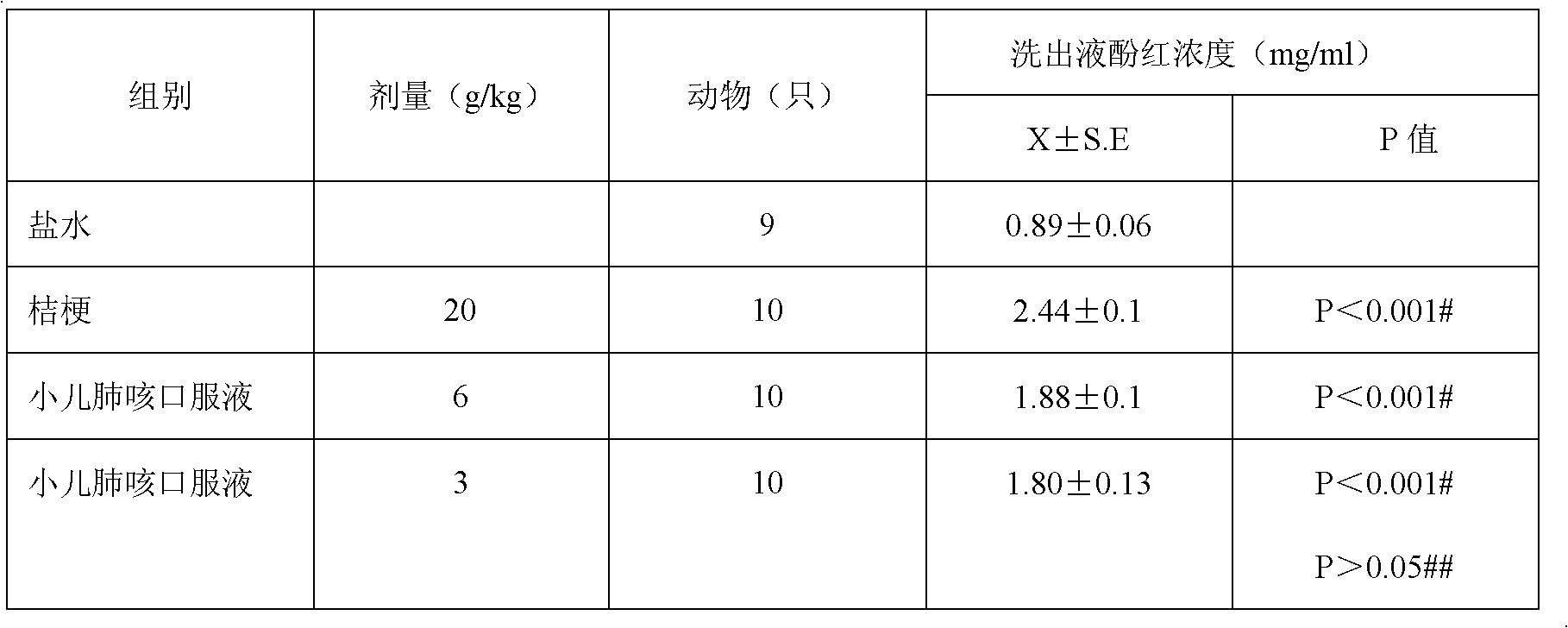
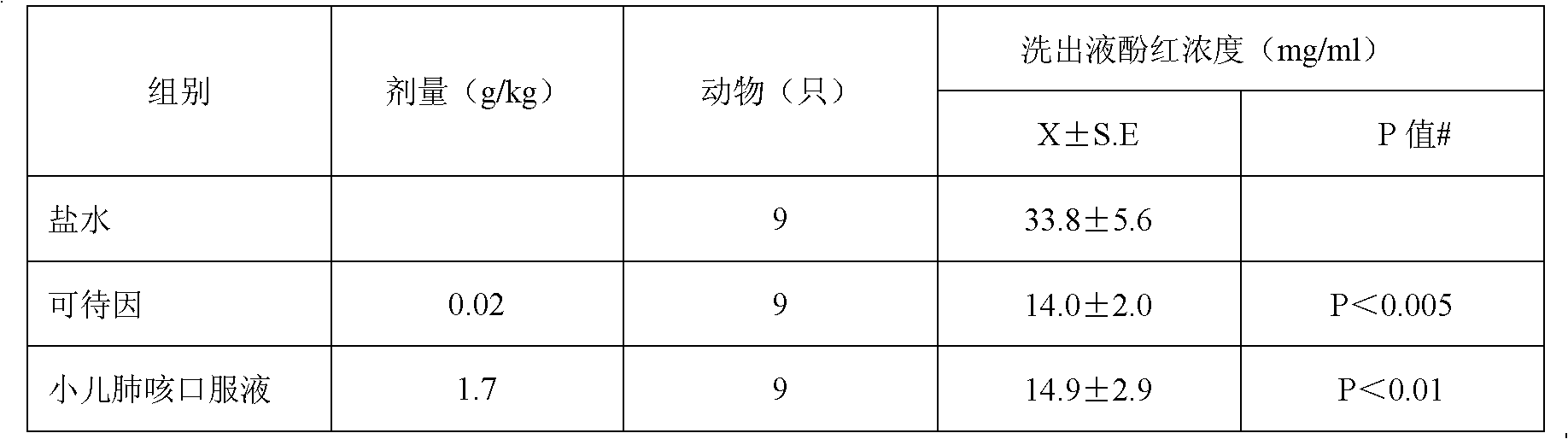
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Divided dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
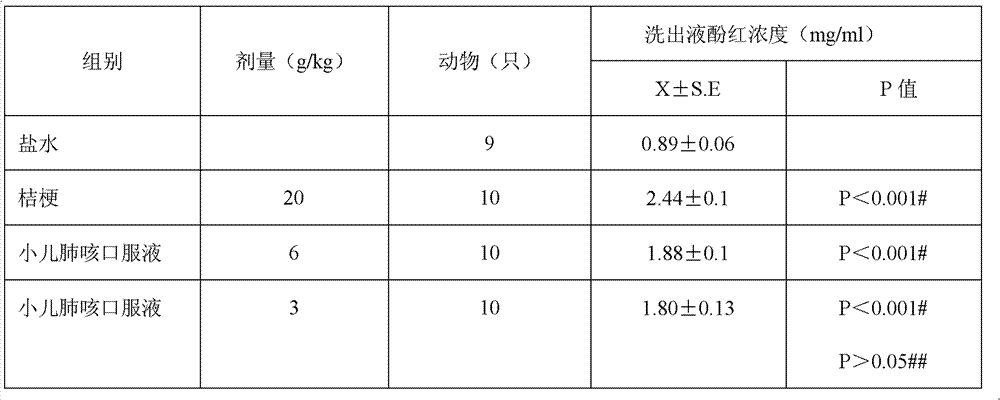
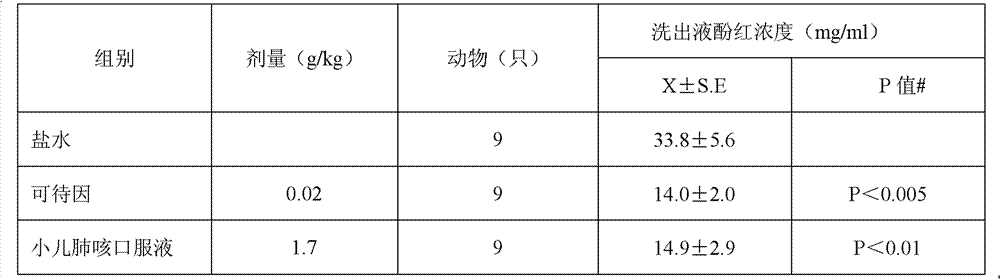
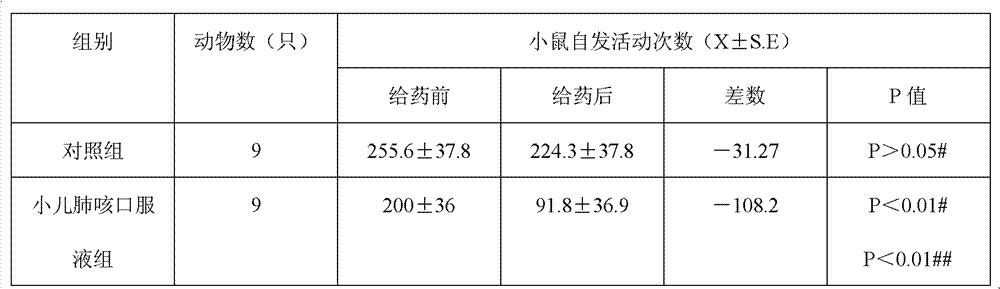
Divided doses is the term used when they want to specify a max dose per 24 hours, as maybe when a total dose given all at once might not be safe (toxicity, perhaps) or because its half-life is so short that there won't be an effective blood level over the course of the day and it has to stay on board all day and all night.
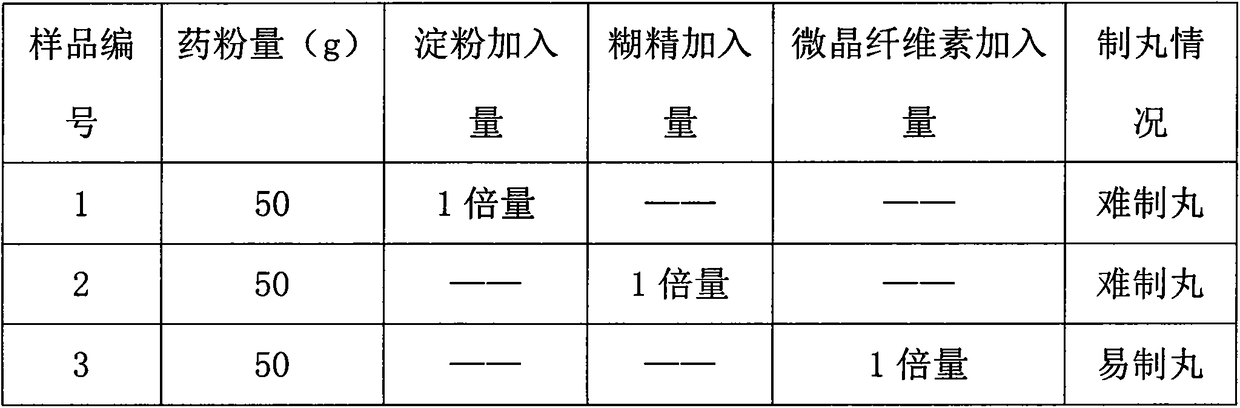
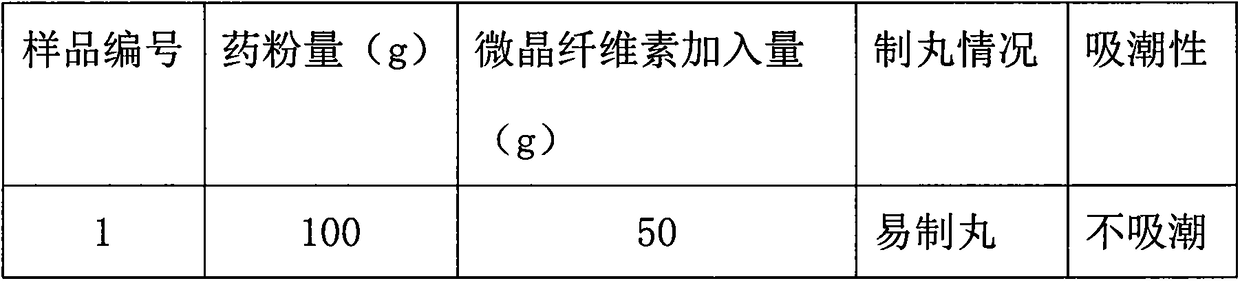
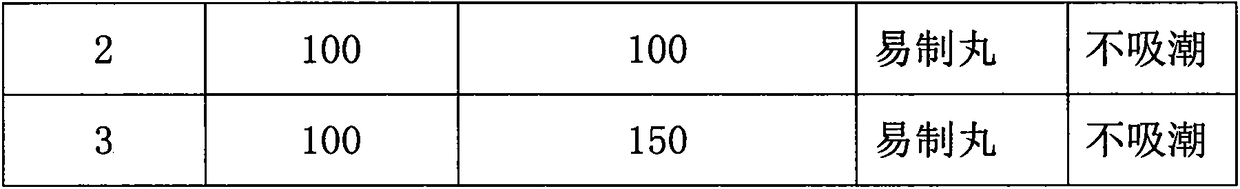
Preparation method of pellet-type formula granules
InactiveCN107744510ASimple preparation processImprove compliancePharmaceutical product form changeCoatingsMedicineMoisture absorption
The invention discloses a preparation method of pellet-type formula granules. The method comprises the following steps: (1) selecting at least one of the traditional Chinese medicinal materials; (2) respectively extracting the medicinal materials selected in step (1) for obtaining extracts, or respectively grinding the medicinal materials, or respectively carrying out partial extraction and partial grinding, thus obtaining pretreated materials; and (3) preparing the pretreated materials obtained in step (2) into pellets, thus obtaining the pellet-type formula granules. According to the preparation method provided by the invention, on the basis of the prior art, the preparation process of the formula granules is innovated, the formula granules are innovated into pellets, the fluidity and moisture absorption resistance are good, the friability is small, the shape is round and uniform as well as regular, the content uniformity is small, and the divided dose is accurate.
Owner:GUANGDONG LUOFUSHAN SINOPHARM
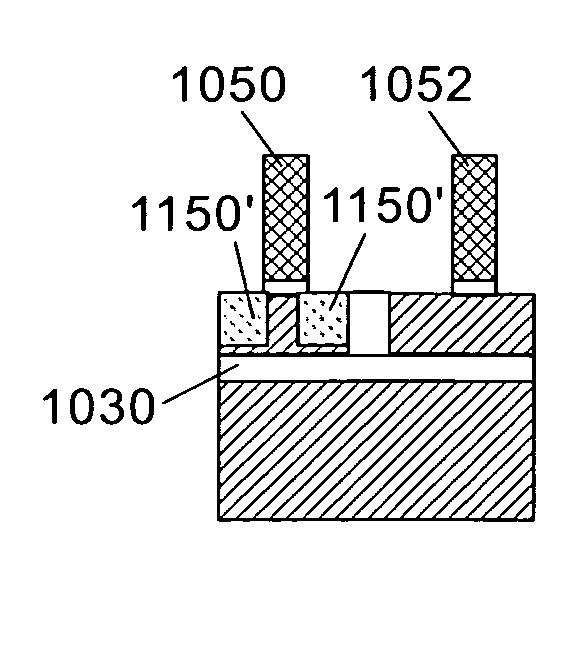
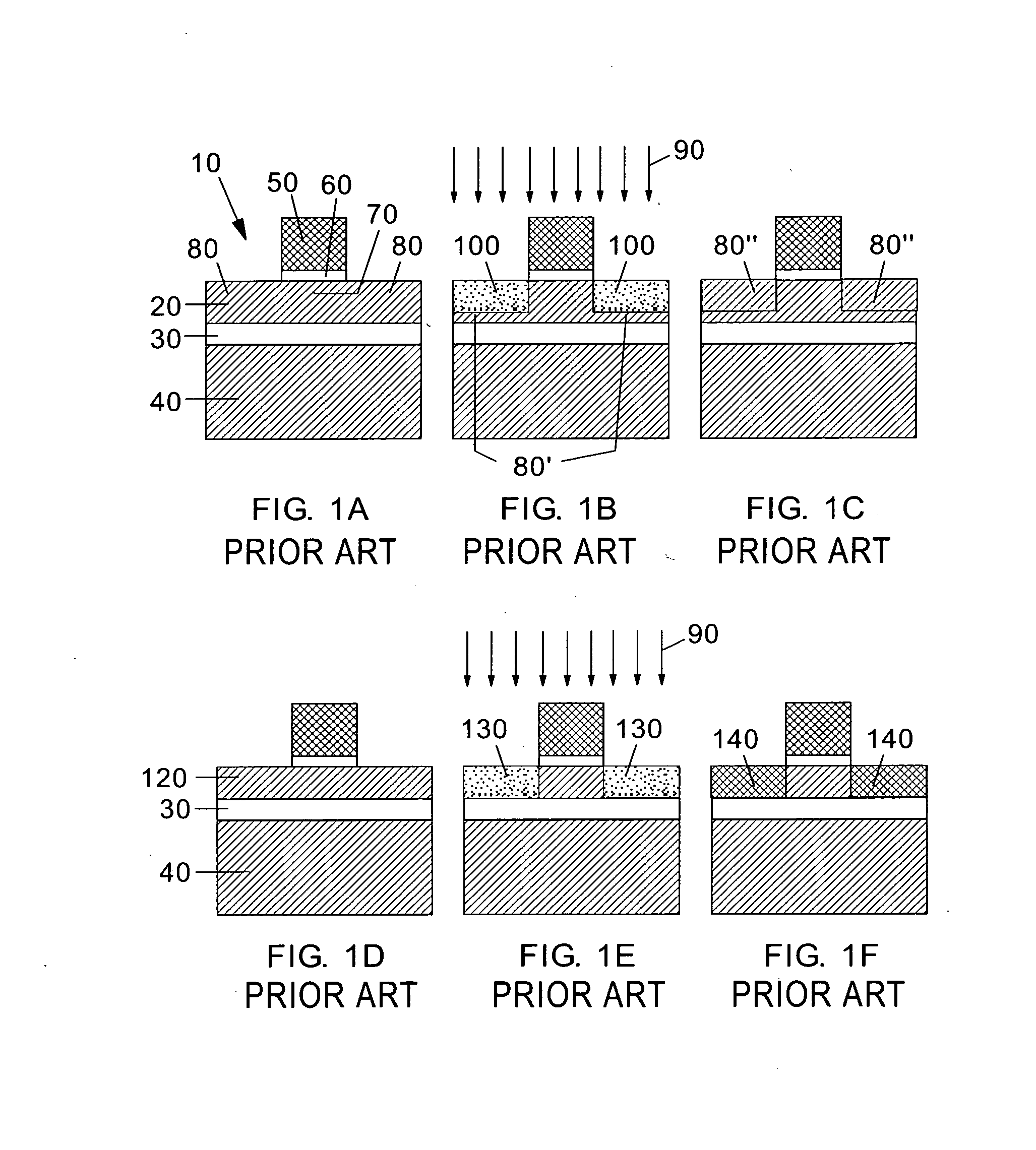
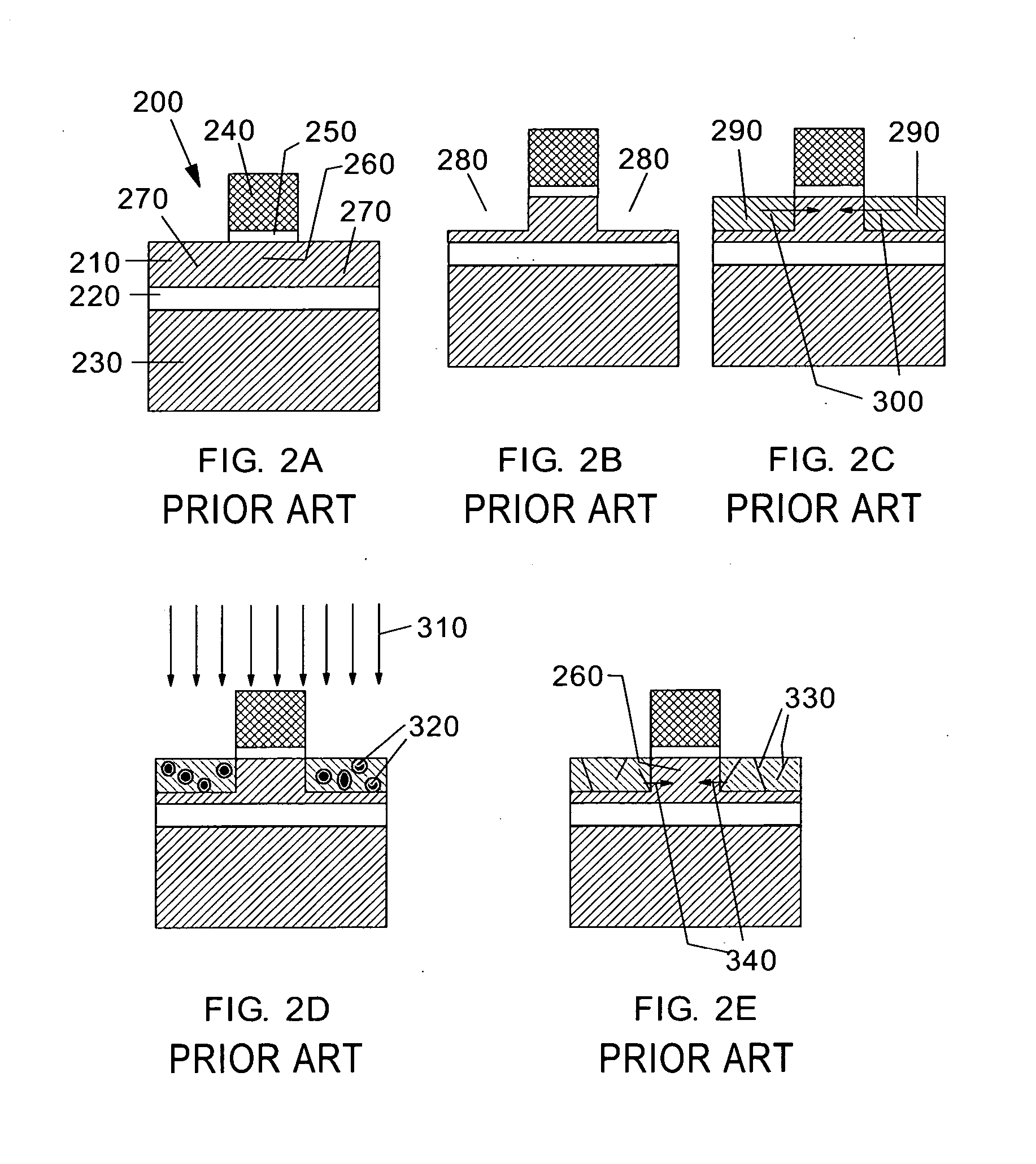
Ion implantation combined with in situ or ex situ heat treatment for improved field effect transistors
InactiveUS20070257315A1Reduce and avoidReduce harmSolid-state devicesSemiconductor/solid-state device manufacturingLattice mismatchField-effect transistor
This invention teaches methods of combining ion implantation steps with in situ or ex situ heat treatments to avoid and / or minimize implant-induced amorphization (a potential problem for source / drain (S / D) regions in FETs in ultrathin silicon on insulator layers) and implant-induced plastic relaxation of strained S / D regions (a potential problem for strained channel FETs in which the channel strain is provided by embedded S / D regions lattice mismatched with an underlying substrate layer). In a first embodiment, ion implantation is combined with in situ heat treatment by performing the ion implantation at elevated temperature. In a second embodiment, ion implantation is combined with ex situ heat treatments in a “divided-dose-anneal-in-between” (DDAB) scheme that avoids the need for tooling capable of performing hot implants.
Owner:GLOBALFOUNDRIES INC
Levodopa/carbidopa compound sustained-release suspension and preparation method thereof
InactiveCN103622942AImprove stabilitySolving Difficult to Swallow ProblemsOrganic active ingredientsNervous disorderMulti unitLarge dose
The invention discloses a levodopa / carbidopa compound sustained-release suspension and a preparation method thereof. The preparation comprises the following components in percentage by weight: 10-50% of levodopa, 10-50% of carbidopa and 10-50% of accessory realizing a sustained-release effect. Compared with a solid preparation, the suspension disclosed by the invention has the advantages that the drug is coated in resin so that the drug stability is improved; the suspension can be taken in divided dose, so that the problem that the oral solid sustained-release preparation prepared from large-dose drugs is hard to swallow is solved; in a multi-unit drug release system, the drug release action is the total of the drug release actions of particles, and the reproducibility and consistency are better; the phenomenon of excessively high local concentration often caused after the oral solid sustained-release preparation is ground can be avoided; the bad smell of the drug can be covered, and the palatability of the preparation is improved. The sustained-release preparation disclosed by the invention is to be clinically used as an anti-paralysis agitans drug.
Owner:JIANGSU UNIV
Ion implantation combined with in situ or ex situ heat treatment for improved field effect transistors
InactiveUS20080258220A1Reduce and avoidReduce harmSolid-state devicesSemiconductor/solid-state device manufacturingLattice mismatchField-effect transistor
This invention teaches methods of combining ion implantation steps with in situ or ex situ heat treatments to avoid and / or minimize implant-induced amorphization (a potential problem for source / drain (S / D) regions in FETs in ultrathin silicon on insulator layers) and implant-induced plastic relaxation of strained S / D regions (a potential problem for strained channel FETs in which the channel strain is provided by embedded S / D regions lattice mismatched with an underlying substrate layer). In a first embodiment, ion implantation is combined with in situ heat treatment by performing the ion implantation at elevated temperature. In a second embodiment, ion implantation is combined with ex situ heat treatments in a “divided-dose-anneal-in-between” (DDAB) scheme that avoids the need for tooling capable of performing hot implants.
Owner:GLOBALFOUNDRIES INC
Fractional dose production method for children drugs, production device and products
The invention discloses a fractional dose production method for children drugs, a production device and products. The production device is formed by an arrangement type quantitative system, a forming thermal sealing system and a cutting system; the arrangement type quantitative system is provided with corresponding feeding column types according to different fractional dose requirements; the problems that the thermal sealing quality of the drugs is poor due to dust pollution and gases inside bags cannot be escaped due to reduction of bag openings prevents materials entering into the bags are solved. The production device has the advantages of improving accuracy and flexibility of the fractional dose of the children drugs, being simple in production, easy to process and multifunctional, confirming to requirements of specific children drugs and the like.
Owner:HAINAN HONZ PHARMA
Oral ulcer mucoadhesive film and preparation method thereof
InactiveCN106551979AGood flexibilityHigh tensile strengthHydroxy compound active ingredientsTetracycline active ingredientsSide effectOral ulcers
The invention provides an oral ulcer mucoadhesive film and a preparation method thereof. The oral ulcer mucoadhesive film is prepared by honeysuckle flower, menthol, vitamin B [2], borneol, tetracaine hydrochloride, sorbic acid, prednisone acetate, chlortetracycline hydrochloride, glycerin, stevioside, ethanol PVA 17-88 and purified water. Compared with the prior art, the film has good flexibility and tensile strength, can rapidly being expended and dissolved when meets water, a high-density protection drug film layer is formed on the oral cavity mucous membrane, and the effect for rapidly relieving swelling and pain with long term can be reached; oral cavity mucous membrane is employed for direction drug administration, the drug can be directly effected on the disease, simultaneous treatment of principal and subordinate symptoms can be reached, curative effect is fully performed; a lattice impression technology is employed on the technology, administration with divided dose is accurate, curative effect is increased, quality is stable, the oral ulcer mucoadhesive film has no toxic and side effect for oral cavity, and is safe and effective; and has the advantages of small volume, light weight, and convenient carrying and usage.
Owner:刘从荡
Butylphthalide fat emulsion injection and preparation process thereof
InactiveCN105796486AGood solubilization effectAccurate doseOrganic active ingredientsPharmaceutical non-active ingredientsSide effectMicrometer
The invention discloses a butylphthalide fat emulsion injection and a preparation process thereof.The butylphthalide fat emulsion injection is prepared from 0.1-1.0% of refined butylphthalide, 10.0-20.0% of oil for injection, 1.0-5.0% of a compound emulsifier, 0.5-2.5% of an isotonic regulator and the balance water for injection, wherein the grain size of an obtained milk white emulsion solution ranges from 0.1 micrometer to 1.0 micrometer.Butylphthalide is dissolved in an oil phase, a better solubilization effect can be achieved after the oil phase and a water phase are fully emulsified under the action of the compound emulsifier at a specific proportion, the divided dose is accurate, and no solubilizer or cosolvent needs to be added; meanwhile, stability is improved, and the toxic and side effects of the butylphthalide fat emulsion injection are reduced.
Owner:NANJING TIANXIANG PHARMA TECH
Chinese medicine preparation for treating stomach-ache and its preparation process
The invention relates to a Chinese materia medica preparation for treating gastralgia and the preparation method thereof, which is composed of acceptable carriers on medical components and / or pharmacy, wherein the said medical components comprises bupleurum, corydalis tuber, bitter orange, cyperirhizome, white peony root, glycyrrhiza, with effects of dispersing the liver depression and gastric analgesia. The preparation mainly refers to pellet, concentrated pill, and sustained-release preparation. The preparation has advantages of large gastrointestinal distribution area, high bioavailability, small excitability, good fluidity, even size, easy to coat and divide dose comparing with the other oral dosages.
Owner:BEIJING YINKERUISI BIOLOGICAL PODUCTS RES INST
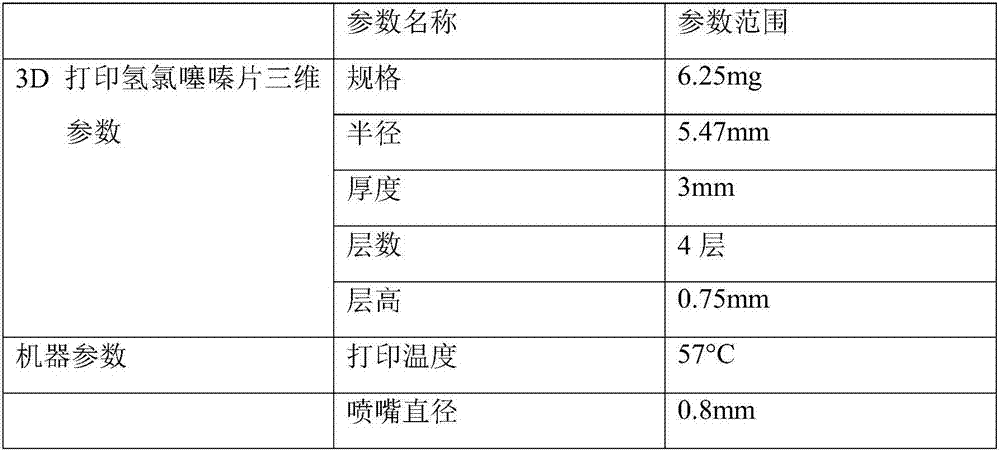
3D (Three dimensional) printed hydrochlorothiazide tablets and preparation method thereof
ActiveCN107412177AAccurate volumeBeautiful appearanceOrganic active ingredientsAdditive manufacturing apparatusHydrochlorothiazidePatient compliance
The invention provides 3D (three dimensional) printed hydrochlorothiazide tablets and a preparation method thereof. The preparation method comprises the following steps: performing accurate divided dose on commercially available hydrochlorothiazide tablets by using a 3D hot melt extrusion technology, firstly, preparing the commercially available hydrochlorothiazide tablets and auxiliary materials into 3D printed hydrochlorothiazide tablet powder, then generating the tablet models with different sizes through the modeling software to determine the dose contained in the tablets, and finally setting the corresponding parameters in a 3D printer to print and prepare the 3D printed hydrochlorothiazide tablets. The 3D printed hydrochlorothiazide tablets prepared by the invention can precisely perform divided dose on the commercially available hydrochlorothiazide tablets to enable the hydrochlorothiazide tablets to be safer in combination with drugs for the treatment of hypertension; aesthetic appearance and integrity are realized, and patient compliance and easier preservation and transportation are improved.
Owner:GUANGDONG PHARMA UNIV
Esomeprazole magnesium suspension tablet and preparation method thereof
InactiveCN104027320APrecise split doseSuitable for childrenOrganic active ingredientsDigestive systemEsomeprazole PillSuspending Agents
The invention provides an esomeprazole magnesium suspension tablet which is prepared by mixing and tabletting an esomeprazole pill, a filler, corrigent, a disintegrating agent, a suspending agent, a lubricant and the like, wherein the esomeprazole pill is prepared by carrying the medicine in a pill core, wrapping an isolating layer and wrapping an enteric coating. Because of nicking, the esomeprazole magnesium suspension tablet can be torn apart for taking; a small pill tabletting process is adopted, the divided dose is accurate, the enteric effect of the pill is not affected after the tablet is torn apart, the tablet can be reasonably taken according to the age and the weight of children, and the clinical compliance is improved. The esomeprazole magnesium suspension tablet provided by the invention can be rapidly dispersed into suspension, is beneficial for distribution of the medicine in gastrointestinal tracts, can improve the bioavailability of the medicine, and is also applicable to patients with dysphagia.
Owner:杭州新诺华医药有限公司
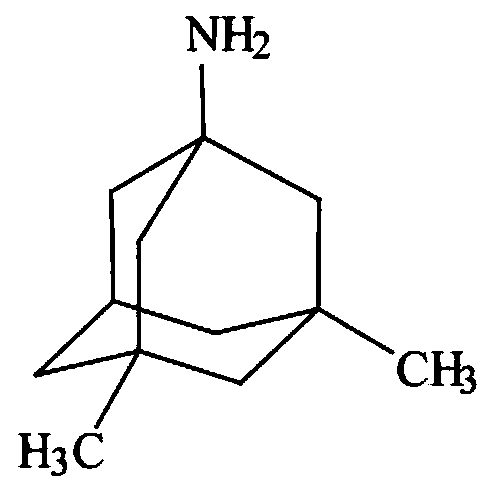
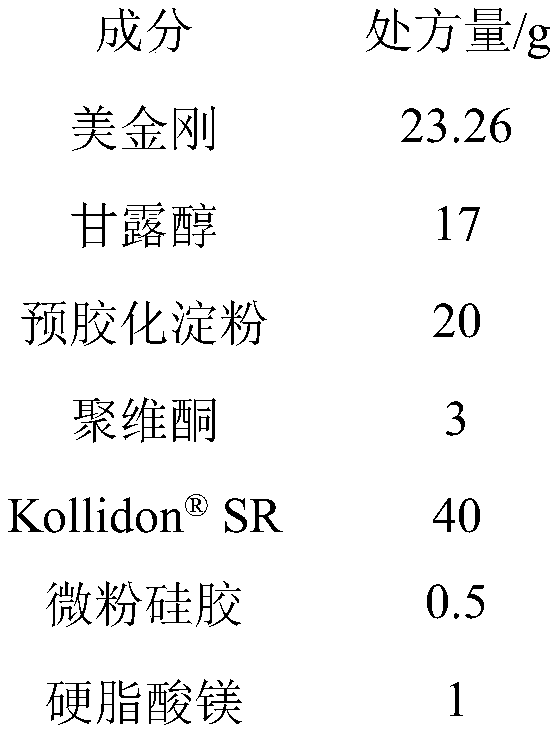
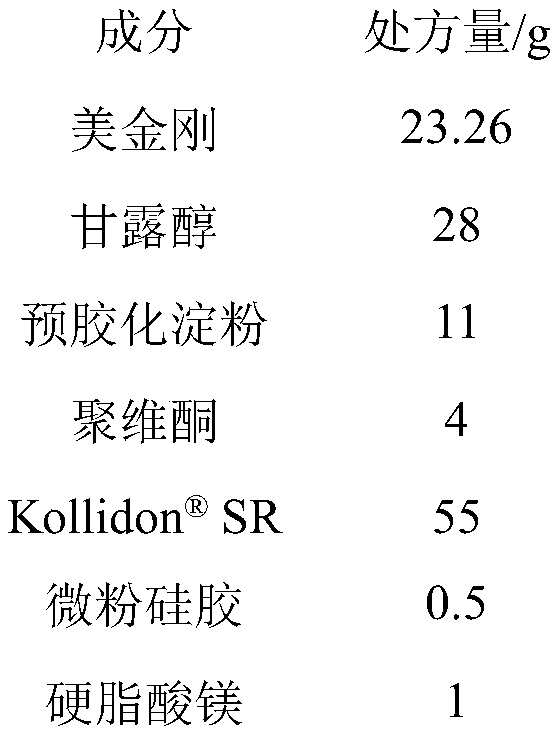
Memantine slow-release micro-tablet capsules and preparation method thereof
InactiveCN109833309ASimple production processGood reproducibility of releaseNervous disorderPharmaceutical delivery mechanismDiseaseSolubility
The invention belongs to the technical field of a medicine slow-release preparation, and particularly relates to slow-release micro-matrix-tablet capsules containing active components namely memantineand a preparation method of the slow-release micro-matrix-tablet capsules. When hydrochloric acid memantine slow-release capsules are taken, the dosage needs to be increased progressively, high dosage requirements are needed, the slow-release micro-matrix-tablet capsules are small in size, each of the slow-release micro-matrix-tablet capsules is about 20-30mg, 4 micro tablets are contained, 4 divided dose units are contained, and the 4 micro tablets can be loaded into small No.4 capsules, so that the slow-release micro-matrix-tablet capsules have great compliance to old people suffering fromAlzheimer's Disease( Alzheimer's Disease, AD). Therefore, if the hydrochloric acid memantine slow-release capsules can be successfully developed, the hydrochloric acid memantine slow-release capsulescan obtain good clinical advantages. Based on the reasons, the recipe of the hydrochloric acid memantine slow-release capsules is firstly researched, but the raw material of hydrochloric acid memantine is good in water solubility, development of matrix slow-release micro tablets is difficult, and the hydrochloric acid memantine is considered to be reduced to memantine (base), and then is preparedinto the memantine slow-release micro-tablet capsules, so that the difficulty of the recipe and technological design can be basically solved, and the memantine slow-release micro-tablet capsules can be successfully developed.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Medicament for treating innominate pyogenic infections
InactiveCN101890105AGood effectAntinoxious agentsDermatological disorderCurative effectHeating furnace
The invention discloses a medicament for treating innominate pyogenic infections. The medicament is implemented by the following technical schemes of: placing 30g of gypsum into a heating furnace for heating for 10 to 15min at the temperature of 600 to 800 DEG C; then immediately placing the heated gypsum into water for burst cooling to obtain fine powder; removing the water to remain the fine powder and drying; heating the fine powder in a drying box at the temperature of 100 to 120 DEG C; preserving the heat for 5 to 10min; taking the fine powder out and thoroughly cooling the fine powder; mixing the fine powder, 30g of lonicera flower, 15g of fresh rehmannia root, 9g of achyranthes root and 5g of coptsir root, and decocting the mixture with water for 15min; filtering liquor out; decocting the mixture with water again for 20min; removing dregs; uniformly mixing the liquor obtained by the two steps; and taking the liquor in divided doses. By the method, the medicament can obviously improve the curative effect.
Owner:孙一凡
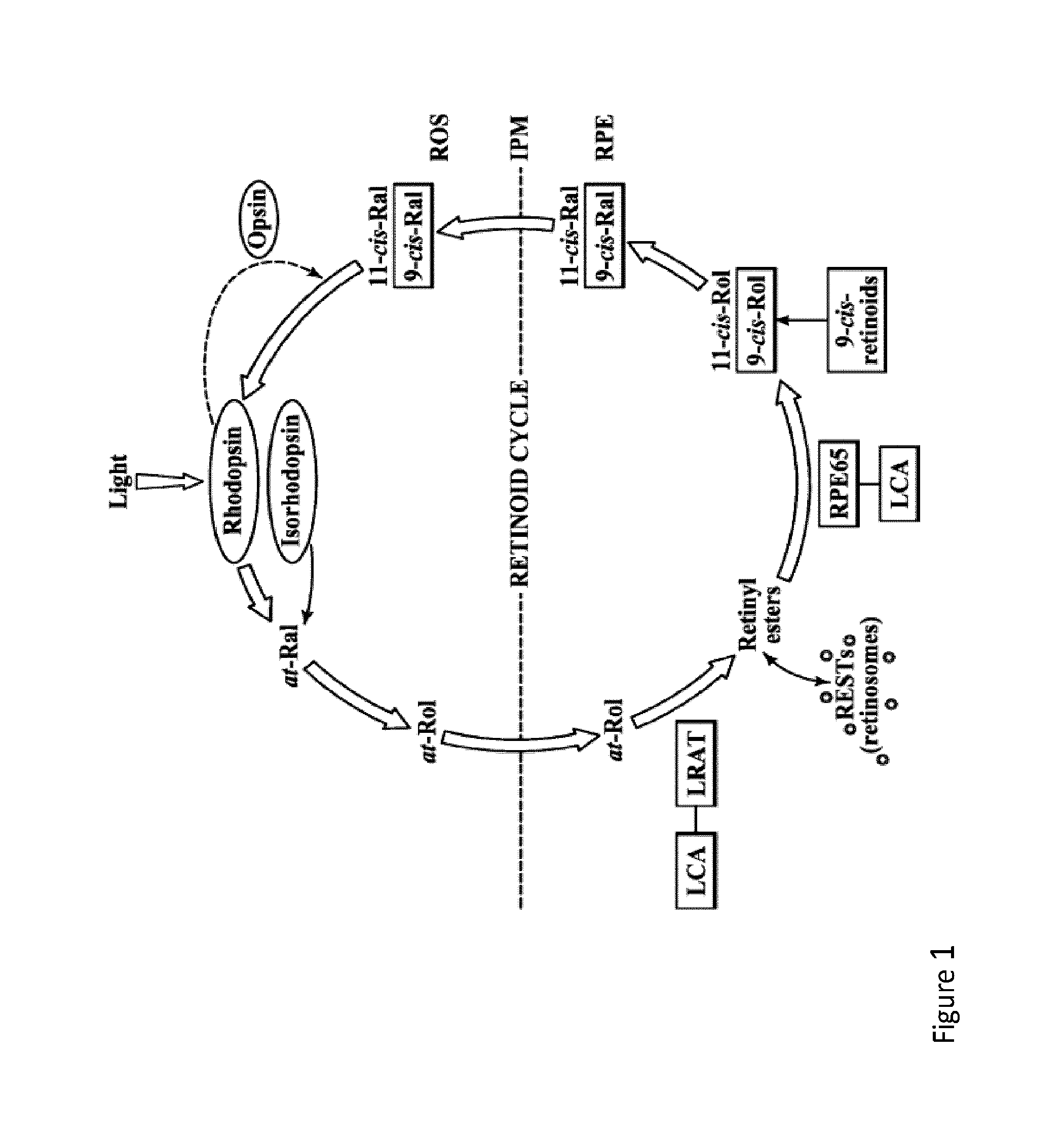
Use of Opioid Antagonists for the Preparation of a Medicament in the Treatment of Retinal Degenerative Diseases
Retinal degenerative diseases affect the delicate layer of retina tissue that lines the inside back of the eye leading to gradual vision loss. The use of opioid anatgonists for the preparation of a medicament for the selective blocking of the body's opioid receptors sites is a method of treating this human suffering by daily administration to the patient of from about 0.5 to about 10 mg of drugs such as naltrexone, naloxone or nalmefene. They act primarily by normalizing derangements in the human complement system that may occur in the disease etiology. Therapeutical approach for macular degeneration and retinitis pigmentosa is primarily intended. Oral, parenteral uses as well as topical applications may all be considered. They may be administered in one or divided doses daily for the best receptor blocking activity, preferably in the evening hours. Low Dose Naltrexone suitable for oral administration is most preferred regimen at about 4.5 mg / day.
Owner:IMUNEKS FARMA ILAC SANAYI VE TICARET
Biochemical oral liquid and preparation method thereof
InactiveCN102114236AOvercome the disadvantage of oral inconvenientEasy to acceptAntipyreticAnalgesicsIrritationAdditive ingredient
The invention discloses biochemical oral liquid, which takes Chinese angelica, Szechuan lovage rhizome, peach seed, dried ginger and liquoric root as active pharmaceutical ingredients, the active pharmaceutical ingredients are prepared into liquid extract by adopting the modern process, and a flavoring agent, a surfactant and a preservative are further added for preparing the oral liquid. A modern pharmaceutical technology is applied for changing biochemical pills to the oral liquid formulation which is easy to accept by women, thereby overcoming the shortcoming of being inconvenient to take orally of the original formulation; and the biochemical oral liquid is fast in medicament release speed, high in bioavailability, small in irritation, accurate in divided dose, small in volume and convenient to take and carry. The quality standard of the biochemical oral liquid is comprehensively improved on the original basis, a scientific qualitative and quantitative control method is provided, and the product quality is ensured.
Owner:SHANXI ZHENDONG PHARMA
Therapeutic regimens and methods for improving visual function in visual disorders associated with an endogenous retinoid deficiency
InactiveUS20160296478A1Meaningful improvement of visionAcceptable safety profileSenses disorderPharmaceutical delivery mechanismDiseaseRetinoid
Therapeutic regimes for improving visual function in a subject having a deficiency in endogenously produced 11-cis retinal comprising administering the synthetic retinal derivative as a divided dose over 2-7 days then providing a resting period of 7-28 days after which the second dose of the synthetic retinal derivative is administered. Preferred synthetic retinal derivatives are 9-or 11-cis-retinyl esters. Disorders associated with deficiency in endogenously produced 11-cis retinal include retinitis pigmentosa and Leber congenital amaurosis
Owner:NOVELION THERAPEUTICS INC
Medicament for treating cyclomastopathy
The invention discloses a method for preparing a medicament for treating cyclomastopathy. The technical scheme comprises the following steps of: soaking pinellia tuber with ginger juice for 1 hour and mixing at the same time; baking the mixture over a tile; soaking common bletilla pseudobulb with artemisia argyi-vinegar soup for 1 hour, and airing; adding water into common bletilla pseudobulb, dandelion, rhizoma anemarrhenae, Chinese honeylocust spine and frankincense, decocting for 15 minutes and filtering to obtain decoction; adding water, decocting for 20 minutes and removing the residues; and uniformly mixing the decoction obtained twice and taking in divided doses. The method can obviously improve the curative effect.
Owner:苗丽华
Compound oral solution for treating infantile cough and preparation method thereof
ActiveCN102205104AGreat tasteStable traitsPharmaceutical delivery mechanismMammal material medical ingredientsDiseaseMonkshoods
The invention discloses a compound oral solution for treating infantile cough, which is prepared from the following raw materials in parts by weight through active ingredient extraction, purification and formation: 21 parts of ginseng, 19.5 parts of tuckahoe, 9 parts of bighead atractylodes rhizome, 18.5 parts of dried orange peel, 19 parts of gizzard pepsin, 11.5 parts of rhubarb roasted with wine, 18.5 parts of turtle shell, 22 parts of wolfberry bark, 38 parts of radix glehniae, 11.5 parts of roasted licorice, 28 parts of sweet wormwood, 38 parts of ophiopogon root, 9 parts of cassia twig,9 parts of dried ginger, 7 parts of prepared monkshood, 28 parts of snakegourd fruit, 19 parts of coltsfoot flower, 21 parts of aster, 24 parts of mulberry bark, 7 parts of arisaema cum bile, 21 parts of milk vetch and 19 parts of medlar. The active ingredients in the compound oral solution disclosed by the invention disperse in the medium in a molecular or granular state, thus the compound oral solution has the advantages of large dispersion degree and high absorption rate and quickly exerts the treatment effect; and simultaneously, the compound oral solution is easy to realize the divided dose, is convenient to take, reduces the irritation of medicines, has better taste and is especially suitable for old people and children. The compound oral solution disclosed by the invention has the advantages of stable property, high clarity, long shelf life (2 years) and high bioavailability, and has good treatment effect and no toxic side effect when used for treating infantile cough.
Owner:天圣制药集团重庆药物研究院有限公司
Powder for external application for defervescence
InactiveCN100515442CPromote absorptionImprove permeabilityPowder deliveryAntipyreticExternal applicationGardenia
The invention discloses an antipyretic powder for external application, which is characterized in that it is prepared by crushing, grinding, sieving, mixing uniformly, and packaging in divided doses of the following traditional Chinese medicines in parts by weight: 1.5-2.5 parts of gypsum, 1.5-2.5 parts of alum, 1.5-2.5 parts, Artemisia annua 0.5-1.5 parts, Gardenia 1.5-2.8 parts, Rhubarb 1.5-2.8 parts, Xuan Ming powder 0.5-1.5 parts. In the present invention, the above-mentioned various traditional Chinese medicines are compatible, which can effectively eliminate fever caused by exogenous pathogenic factors, lung heat, stomach fire, etc., and can be reconciled with egg white, which can strengthen local skin absorption, is simple and easy to implement, has a large effect, reliable curative effect, and is easy to accept by the crowd .
Owner:梁进
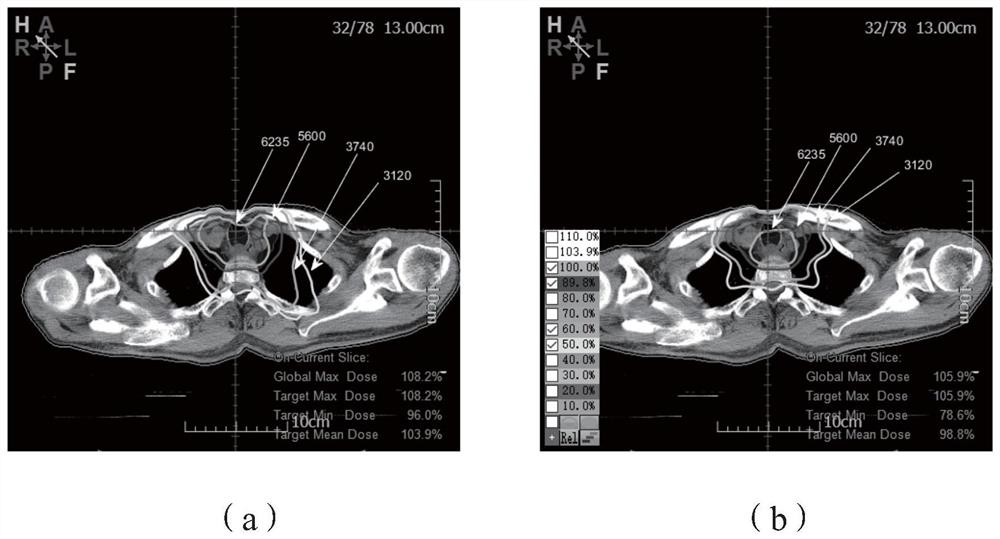
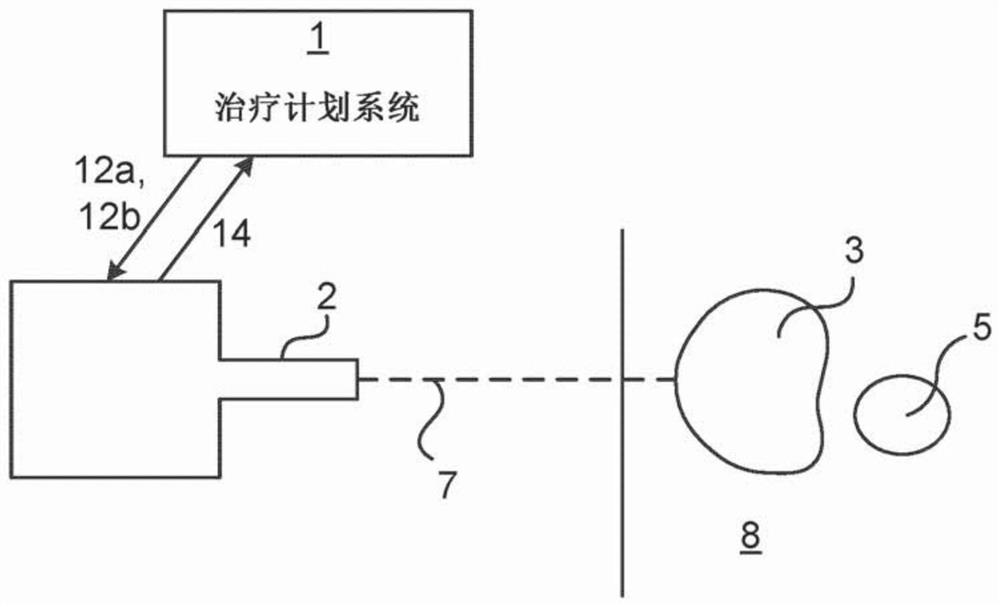
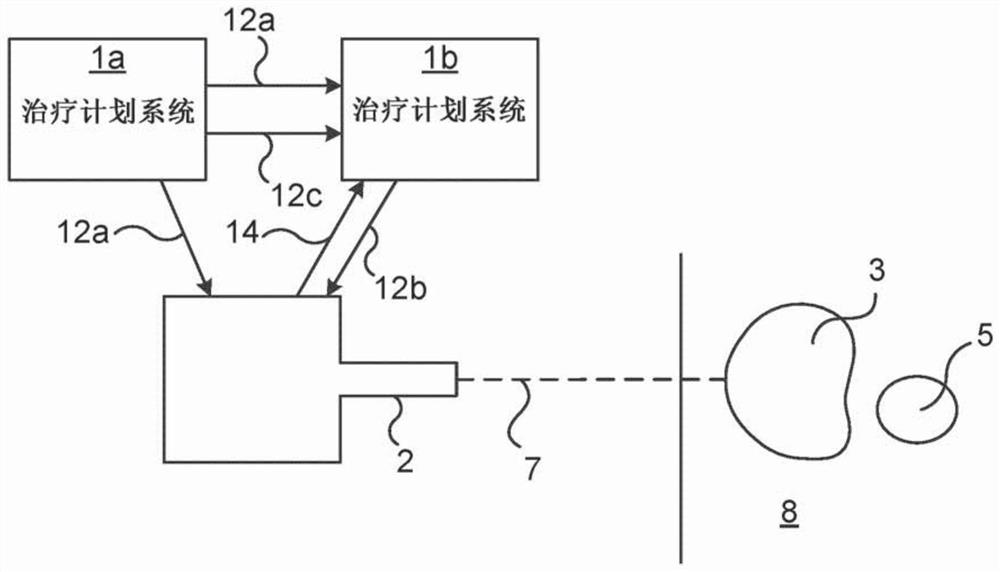
Knowledge based treatment planning corrected for biological effects
Solutions are provided herein that specifically accounts for biological effects of tissue during radiation planning (such as treatment planning). In one or more embodiments, the biological effects may be calculated by accessing a knowledge base to determine reference data comprising at least one biological characteristic corresponding to the at least one organ, predicting a biological effect for the plurality of identified structures based on the biological characteristic corresponding to the at least one organ, and generating or modifying a radiation plan based on the biological effect. By incorporating biological data and fraction dose information, dose-estimation models can be created and trained to more accurately estimate dose absorption and effectiveness. Moreover, existing estimation models may be adapted to create dose estimations that account for the biological efficiency of target structures.
Owner:VARIAN MEDICAL SYST INT AG
Powder medicine for treating throat diseases
The invention discloses a throat powder, which is characterized in that it is prepared from the following traditional Chinese medicines in parts by weight: 6 parts of Prunella vulgaris, 2 parts of Bellflower, 2 parts of Ophiopogon japonicus and licorice 1 serving. One pair of throat powder of the present invention is 10-15g, and different amounts are taken depending on the patient's condition, and the patient can directly soak and drink with boiled water repeatedly, one pair a day, generally 20-30 pairs can fully recover. The invention combines the above-mentioned various traditional Chinese medicines to have the effects of detoxifying and purging fire, moistening the lungs and resolving phlegm, relieving throat and relieving cough, relieving stagnation and resolving stagnation, and can be drunk as tea.
Owner:梁进
A traditional Chinese medicine pellet preparation for regulating menstruation and removing freckles and its preparation method
ActiveCN105288173BEnsure beneficial effectsReasonable workmanshipUnknown materialsGranular deliveryCvd riskSolvent
The invention discloses a traditional Chinese medicine pellet preparation for regulating menstruation and removing freckles and a preparation method thereof and belongs to preparation technology of Chinese patent medicine. The traditional Chinese medicine pellet preparation is characterized in that the pellet preparation is a pellet composed of dry extract powder of active ingredient water extract, crude drug powder and microcrystalline cellulose, grain size of the pellet is smaller than 2.5mm, and a weight ratio of the dry extract powder and the crude drug powder to microcrystalline cellulose is 1 to (0.5-1.0). The inventor performs studying on production process, screening of excipient type and solvent amount proportion experimenting, so that the traditional Chinese medicine pellet preparation has the advantages of high medicine releasing speed, high bioavailability, little irritancy, accurate divided dose and convenience for administration and carrying. Through dosage form updating, high cost and safety risk of existing production processes are improved, requirements of enterprises on convenience and safety of production process are met, and a novel medicine preparation which is more convenient to take and cheaper in price is provided for patients.
Owner:北京斯利安药业有限公司
A kind of preparation method of stem and leaf deep processing product of Moringa oleifera
ActiveCN105211871BStay nourishedKeep the tasteClimate change adaptationFood preservationMoringaDivided dose
Owner:张亚飞
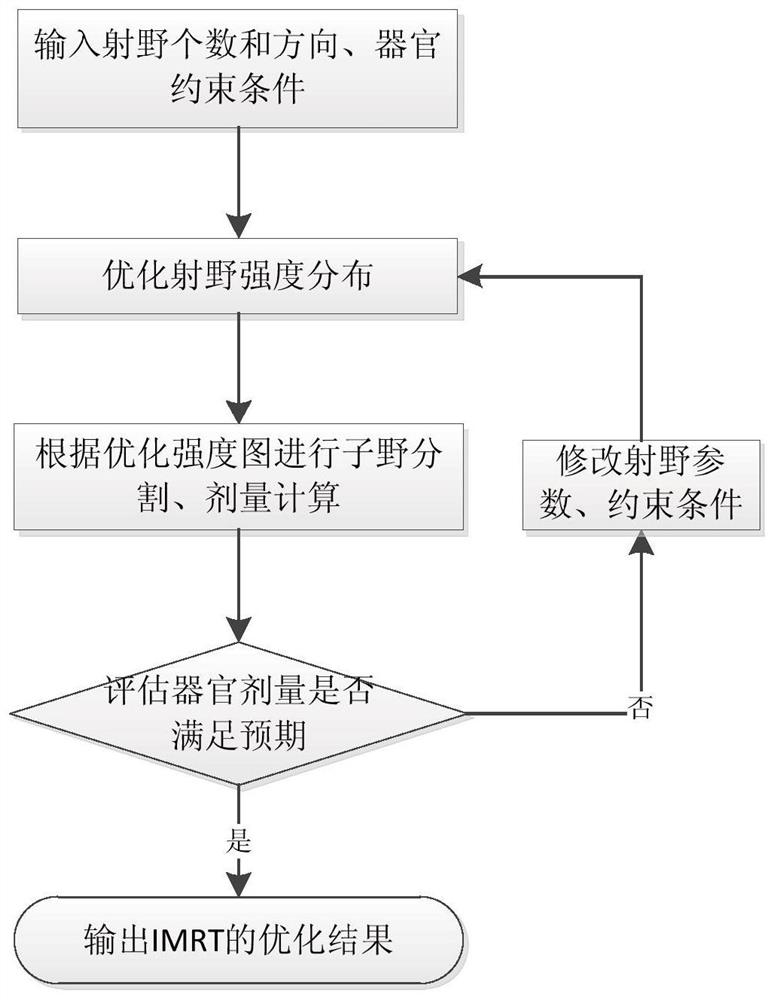
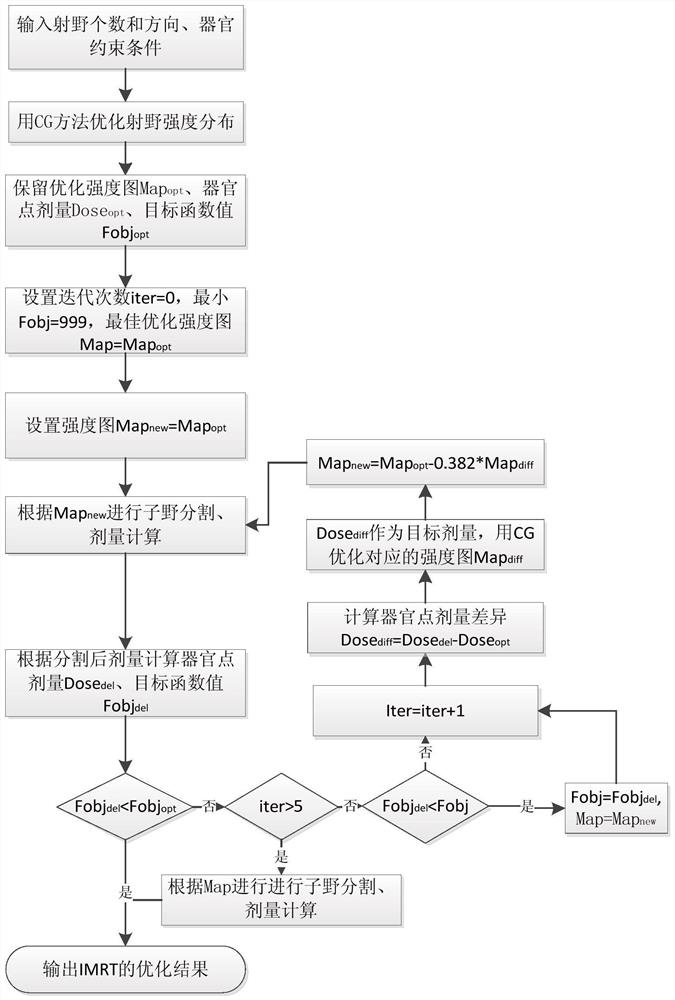
An optimal dose-guided TPS automatic iterative optimization method
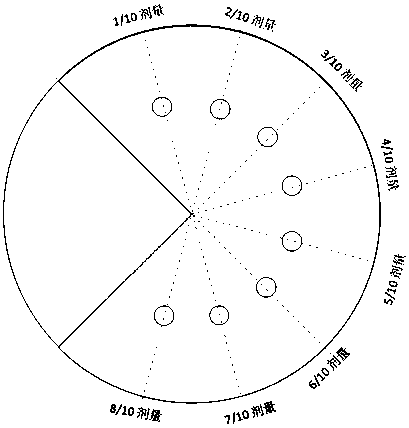
The invention discloses a TPS automatic iterative optimization method guided by optimized dose, which includes the following content: first, calculate the difference between the dose of each point in each organ and the optimization result according to the divided dose, and use it as the input of the optimization engine to obtain the difference part The contribution of the intensity map; then, subtract the intensity map of the difference part from the original optimized intensity map, and use it as the input of the intensity map of the sub-field segmentation to obtain the iterated sub-field and calculate the dose; finally, repeat the whole process to make the final The dose distribution is close to the optimized dose, and the expected dose distribution is obtained. By applying the present invention, the subfield segmentation step is considered in the whole optimization process, and the dose deviation generated by subfield segmentation is continuously corrected, which can significantly improve the planning quality and speed up the planning production efficiency.
Owner:SUZHOU LINATECH MEDICAL SCI & TECH CO LTD
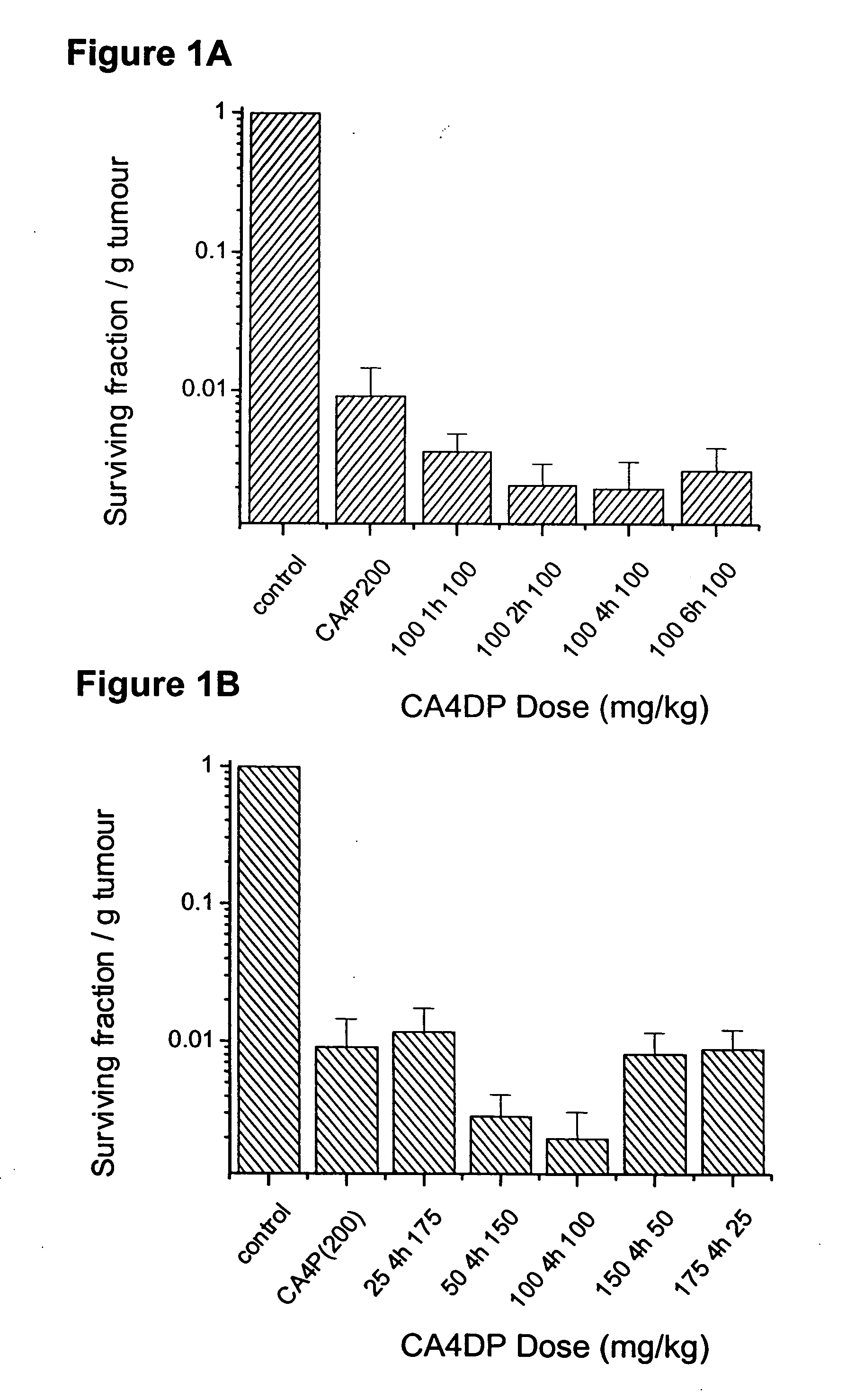
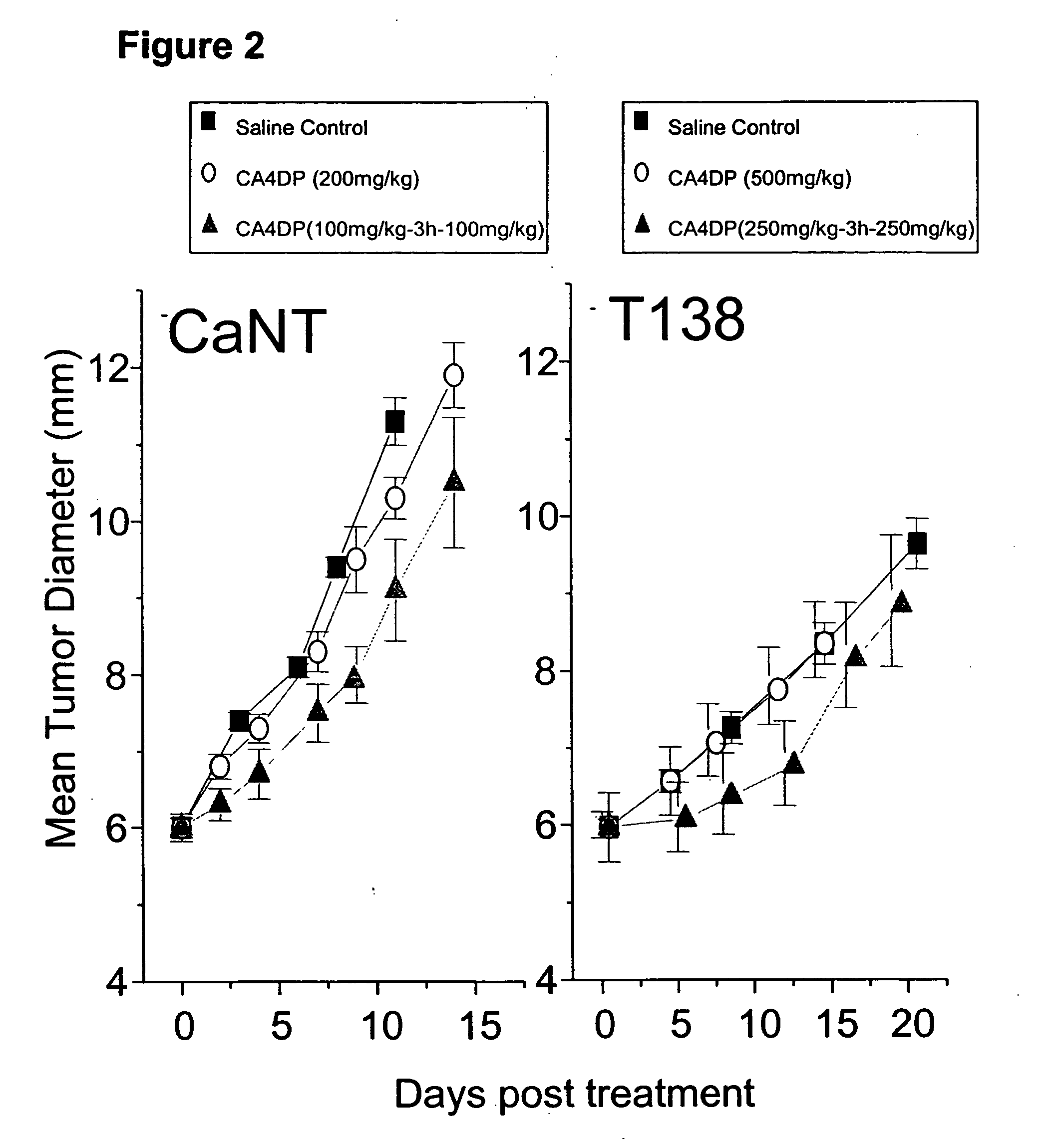
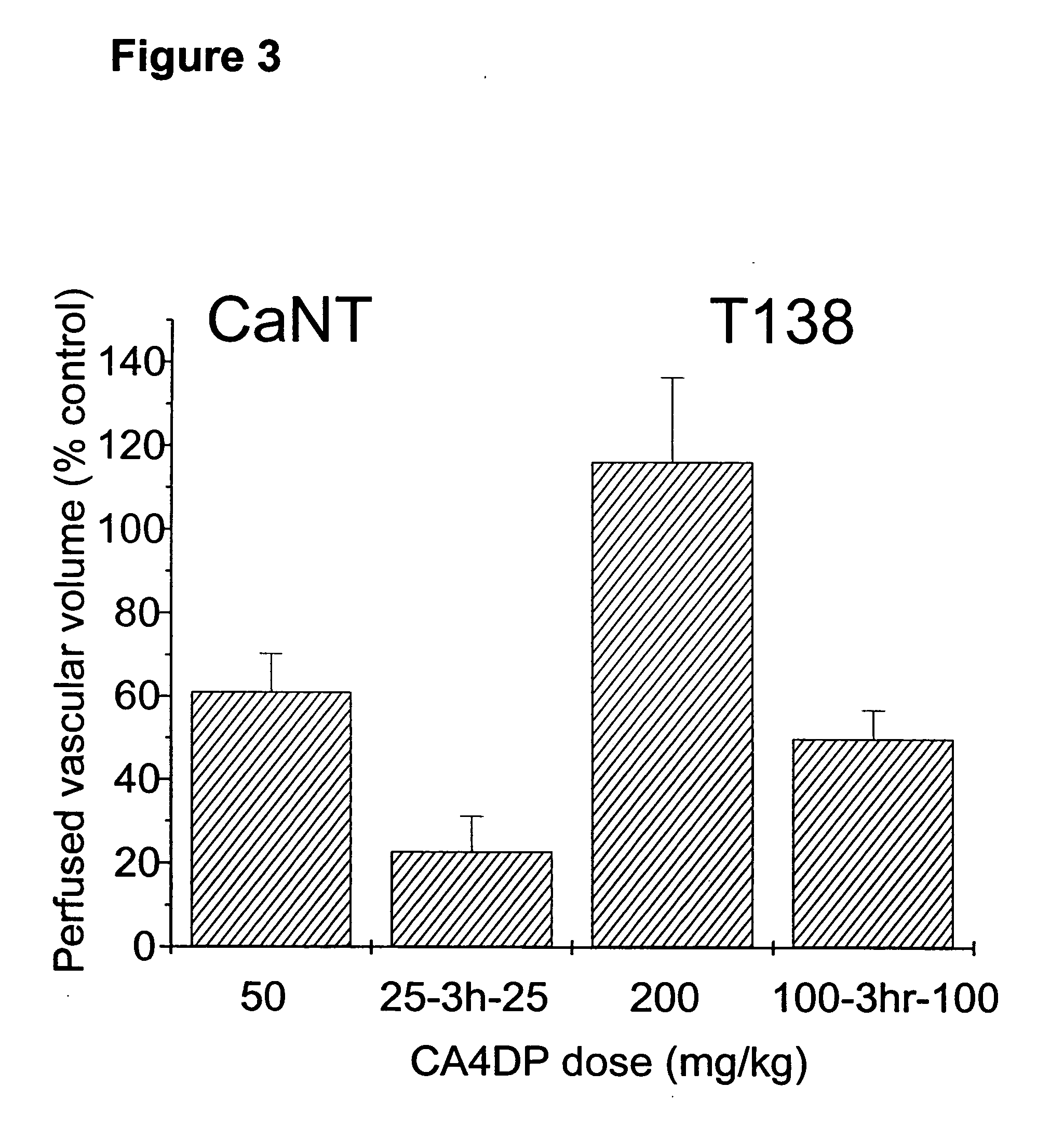
Method of administering split doses of a vascular targeting agent
The present invention is directed to the use of vascular targeting agents or pharmaceutically acceptable salts thereof for administration in divided doses to a warm-blooded animal, such as a human. Also disclosed is a medicament comprising two or more fraction of doses of a vascular targeting agent, or a pharmaceutically acceptable salt thereof, which together add up to a total daily dose, or administration in divided doses for use in a method of treating a human or warm-blooded animal. A kit comprising two or more fractions of doses of a vascular targeting agent or a pharmaceutically acceptable salt thereof, which together add up to a total daily dose, for administration in divided doses is also disclosed.
Owner:OXIGENE
Pen injector with drive member and reducer arm set
PendingUS20200353174A1Simple and easy and safeAccuracy to doseInfusion syringesMedical devicesPen InjectorPharmacy medicine
A portable self-administrable medication delivery device with “End of Life” feature includes a housing, a drive member within said housing, a locking element and a medicine filled container. The device further provides a “Partial Dose Prevention” feature to deliver an accurate amount of dose with precision. The device is capable of delivering multiple doses of a liquid medicament contained therein without the need of priming the injector prior to administration and allows for repeated administration of a dose of medicament in a simple, easy, safe and accurate manner.
Owner:AUROBINDO PHARMA LTD
Compound oral solution for treating infantile cough and preparation method thereof
ActiveCN102205104BGreat tasteStable traitsPharmaceutical delivery mechanismMammal material medical ingredientsDiseaseMonkshoods
The invention discloses a compound oral solution for treating infantile cough, which is prepared from the following raw materials in parts by weight through active ingredient extraction, purification and formation: 21 parts of ginseng, 19.5 parts of tuckahoe, 9 parts of bighead atractylodes rhizome, 18.5 parts of dried orange peel, 19 parts of gizzard pepsin, 11.5 parts of rhubarb roasted with wine, 18.5 parts of turtle shell, 22 parts of wolfberry bark, 38 parts of radix glehniae, 11.5 parts of roasted licorice, 28 parts of sweet wormwood, 38 parts of ophiopogon root, 9 parts of cassia twig, 9 parts of dried ginger, 7 parts of prepared monkshood, 28 parts of snakegourd fruit, 19 parts of coltsfoot flower, 21 parts of aster, 24 parts of mulberry bark, 7 parts of arisaema cum bile, 21 parts of milk vetch and 19 parts of medlar. The active ingredients in the compound oral solution disclosed by the invention disperse in the medium in a molecular or granular state, thus the compound oral solution has the advantages of large dispersion degree and high absorption rate and quickly exerts the treatment effect; and simultaneously, the compound oral solution is easy to realize the divided dose, is convenient to take, reduces the irritation of medicines, has better taste and is especially suitable for old people and children. The compound oral solution disclosed by the invention has the advantages of stable property, high clarity, long shelf life (2 years) and high bioavailability, and has good treatment effect and no toxic side effect when used for treating infantile cough.
Owner:天圣制药集团重庆药物研究院有限公司
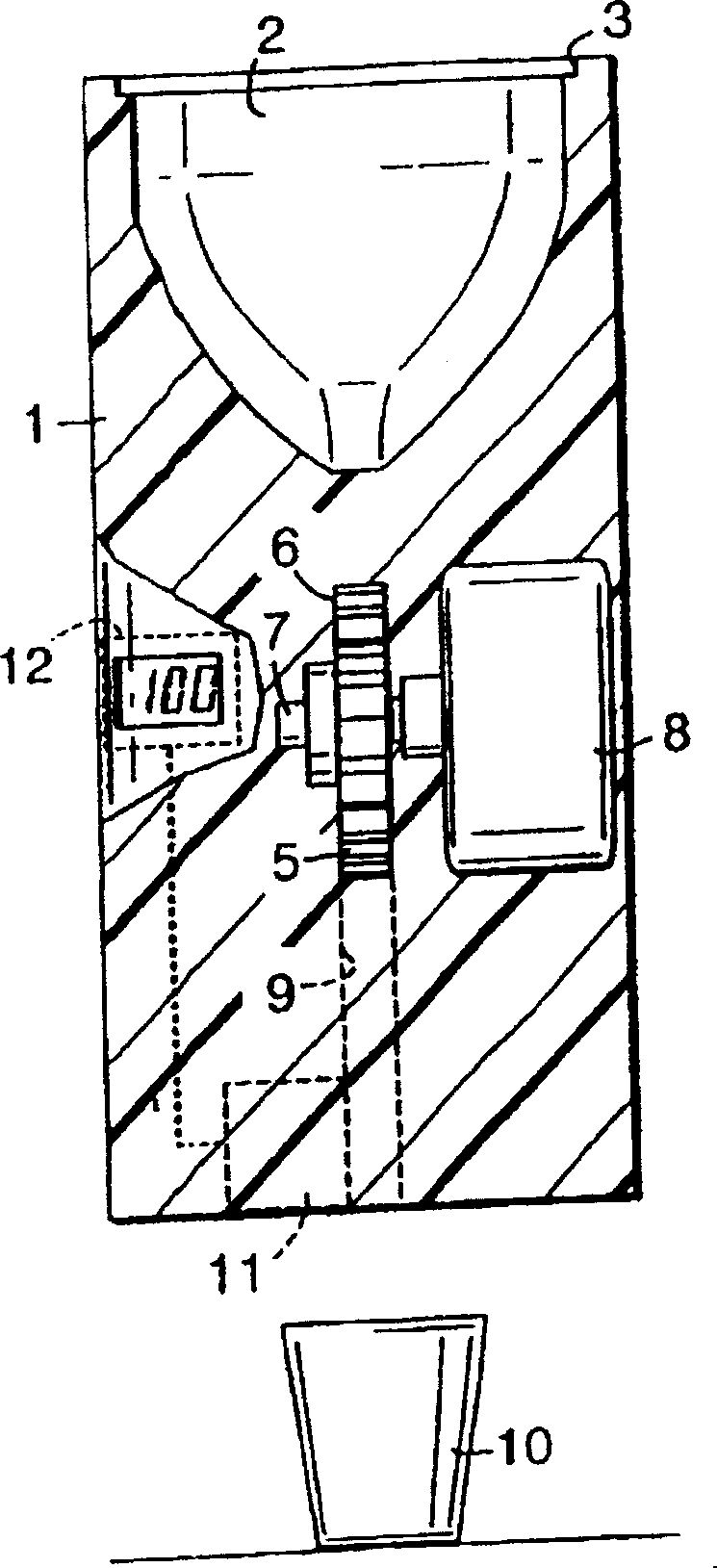
Method and apparatus for dosing medical preparation
The invention has reference to a procedure and a device for the dosing of a medicine to a consumer. In accordance with the invention, a medicine is supplied in the form of a number of small units, partial doses, containing a determined, equal quantity of the active medical substance, and a number of these units, which correspond to a predetermined dose quantity, total dose, of the active medical substance are taken out and dispensed to the consumer. A device for the carrying out of the procedure in accordance with the invention comprises devices (4, 11, 12) for counting and feeding-out of a number of units, partial doses of the medicine, which correspond to a predetermined dose quantity of the active medical substance and for transport (9) of these units to a dispensing device (9) for dispensing of the above-mentioned dose quantity, total dose, to a consumer.
Owner:SENSIDOSE
Diabetes-treating medicament Gliclazide tablet convenient for dividing dose and method for producing the same
InactiveCN101439023AAvoid wastingAvoid lostOrganic active ingredientsMetabolism disorderAdhesiveMedicine
The invention relates to a medicine treating diabetes-gliclazide tablet which is convenient for distributing dose and a production method. The gliclazide tablet comprises gliclazide, filler, disintegrant and lubricant. The invention adopts the production method that after the gliclazide, the filler and the disintegrant which are of 80 meshes or 100 meshes are mixed, soft materials and particles are made by adding adhesive, dryness and pelletization are carried out by adding the adhesive, uniform mixing is carried out after the lubricant is added, the tablet weight is ensured by testing content, a flat cross stamping tablet is adopted, and the gliclazide tablet can be obtained by packing. On the one hand, the invention solves the problems of accurate tablet and convenient dose distribution, and can conveniently divides the tablet into a one half tablet and a one fourths tablet; on the other hand, the invention solves the problems of the prior same tablet of complex process and high manufacture cost, saves the medicine cost and medicine resources, and finally lowers the therapy cost of a diabetic patient.
Owner:SICHUAN MEDCO PHARML
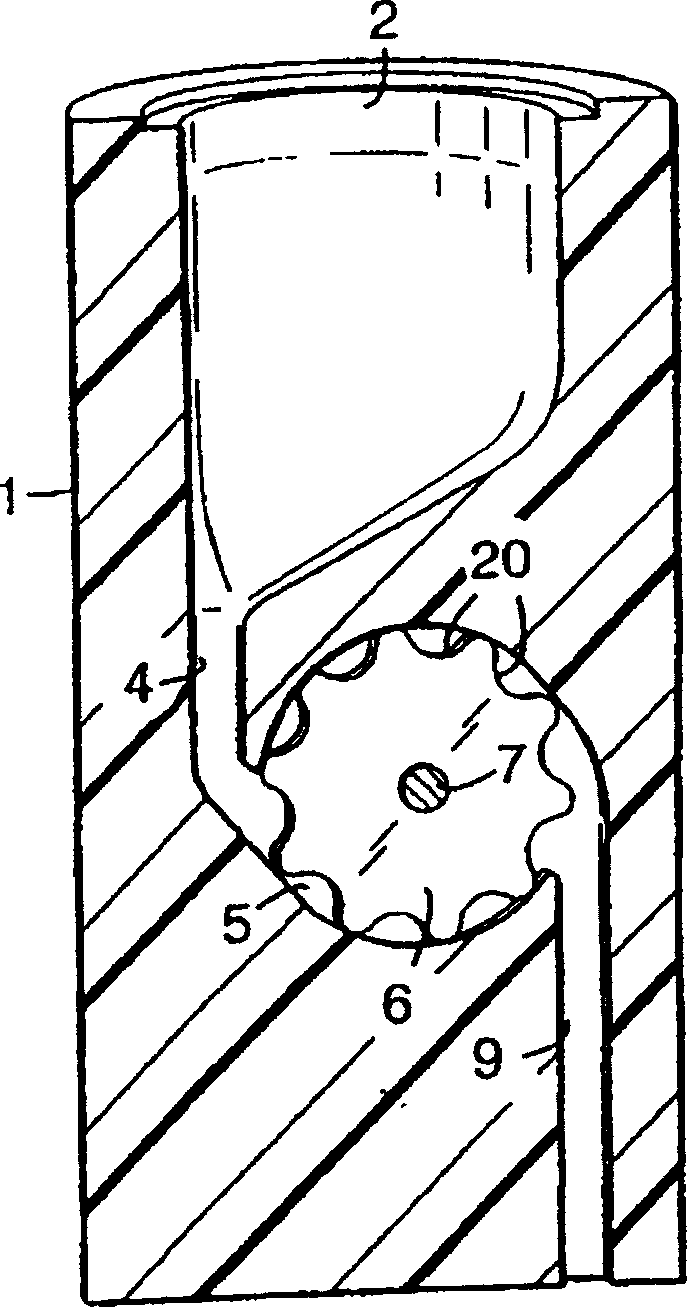
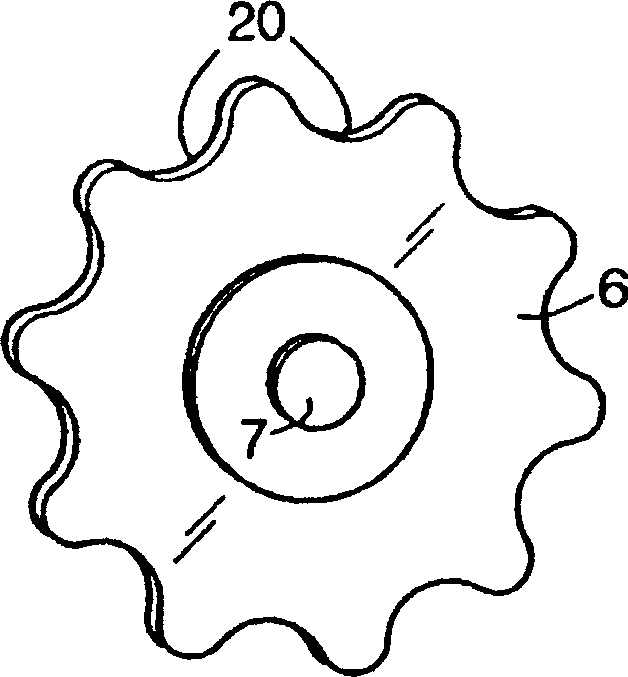
Packaging device for storing mini-pill medicine and use method of packaging device
PendingCN107814054AImprove complianceImprove securitySmall article dispensingInternal fittingsMedicinePatient compliance
The invention discloses a packaging device for storing mini-pill medicine and additionally further discloses a use method of the packaging device. The packaging device comprises a pill storage chamber, a primary dose dividing area, a secondary dose dividing area and a medicine administration area from top to bottom. The pill storage chamber and the primary dose dividing area are separated througha control plate, and the mini-pill medicine in the pill storage chamber is controlled to flow into the primary dose dividing area. The primary dose dividing area and the secondary dose dividing area are separated through a partition plate, and the secondary dose dividing area and the medicine administration area are separated through a partition plate. According to the packaging device and the usemethod, divided dose medicine taking can be conducted at any moment according to the clinical requirements, patient compliance can be improved, the dose dividing safety can be improved, and the dosedividing operative difficulty degree can be decreased; and the working procedure of divided dose packaging in the practical production process is omitted, and the problem that as for an existing mini-pill packaging system, dose dividing operation is tedious is solved, so that the medicine administration flexibility is improved.
Owner:上海合全医药有限公司
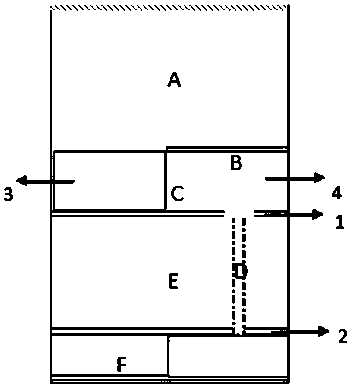
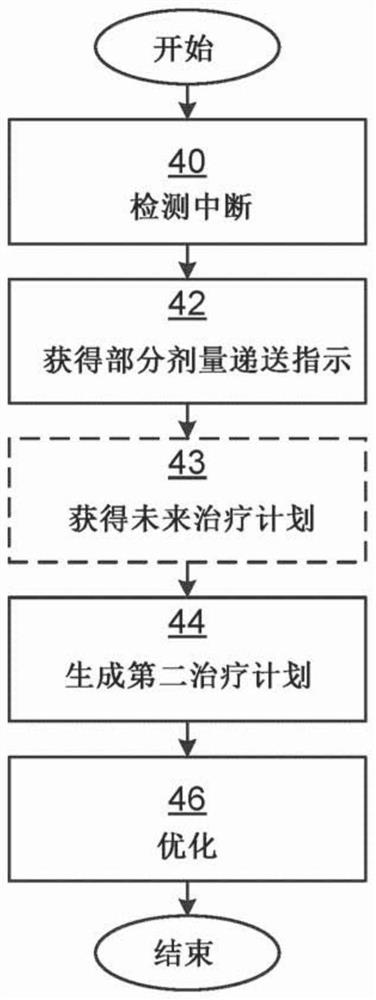
Providing treatment plans for radiotherapy when delivery is interrupted
A method is provided for providing a treatment plan for radiotherapy when delivery is interrupted, the method being performed by a treatment planning system. The method comprises the steps of: detecting that delivery of a first treatment plan has been interrupted; obtaining an indication of delivery of a partial dose representative of a portion of the first treatment plan delivered prior to the interruption; generating a second treatment plan, wherein the partial dose delivery is input to the second treatment plan generation as background dose formation; and optimizing the second treatment plan while taking into account the plurality of scenarios.
Owner:RAYSEARCH LAB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com