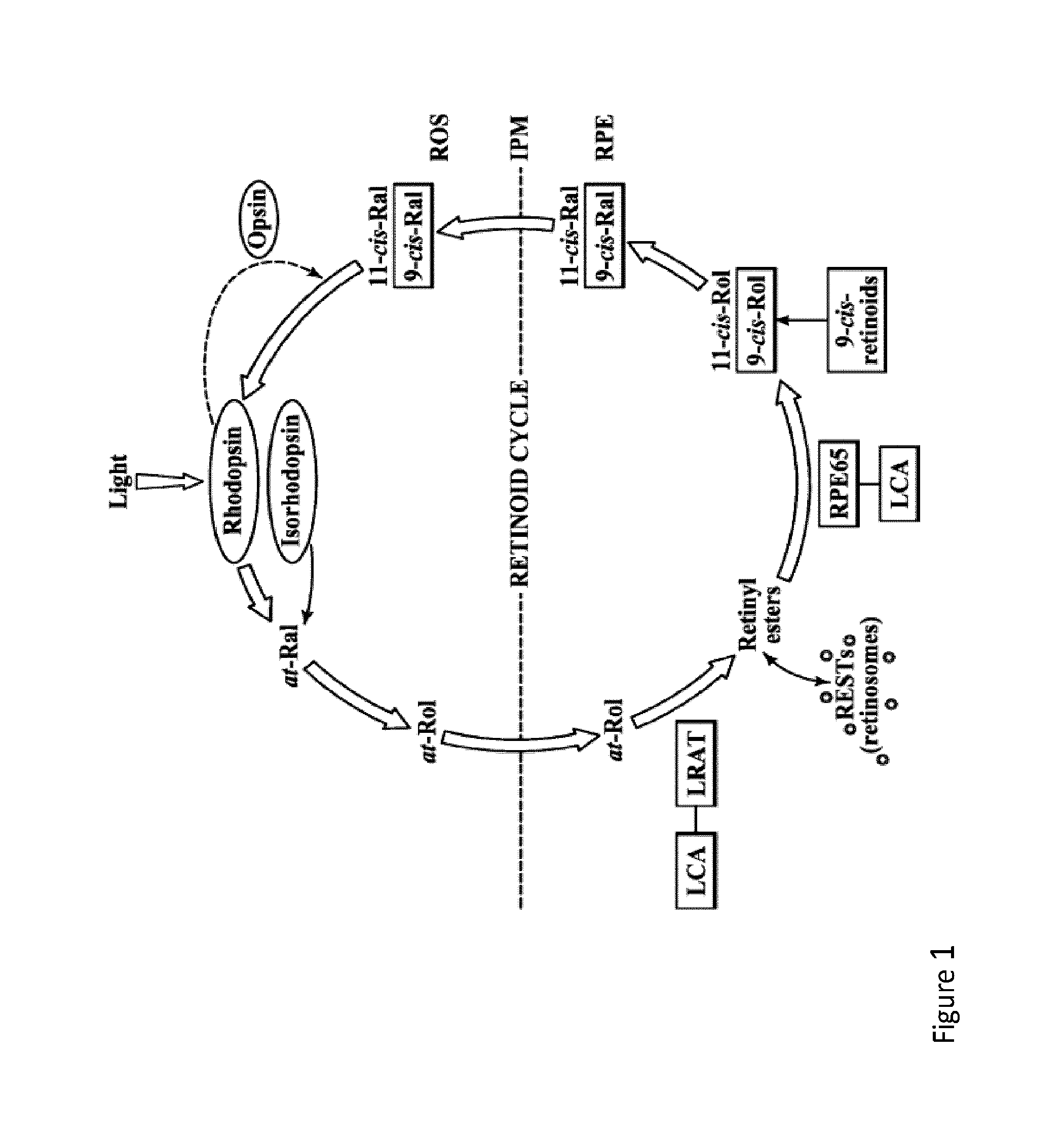
Therapeutic regimens and methods for improving visual function in visual disorders associated with an endogenous retinoid deficiency
a technology of endogenous retinoid and therapeutic regimens, applied in the field of therapeutic regimens and methods for improving visual function, can solve the problems of severe visual impairment and childhood blindness, affect the synthesis of 11-cis-retinal, so as to reduce the severity of toxic side effects, improve the effect of vision, and improve the effect of visual function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Safety Study
[0319]An open-label, repeat dose escalation study of an orally-delivered pharmaceutically acceptable composition of the disclosure was conducted in twenty (20) healthy human volunteers to determine the safety and tolerability of repeat daily oral doses of a composition comprising 9-cis-retinyl acetate ((2E, 4E, 6Z, 8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl) nona-2,4,6,8-tetraen-1-yl acetate) and butylated hydroxyanisole (BHA) dissolved in soybean oil (USP). The concentration of 9-cis-retinyl acetate in the composition was adjusted such that the volume to be administered was convenient. For the dosing range of the study, compositions of 1.25 mg / mL, 5.0 mg / mL and 20 mg / mL 9-cis-retinyl acetate were prepared, containing 0.10% w / w BHA in Soybean oil (USP). Six dose cohorts of healthy subjects received escalating daily doses of the composition orally from 1.25 mg / m2 up to 40 mg / m2, i.e., 1.25, 2.5, 5, 10, 20 and 40 mg / m2.
[0320]Eighteen subjects received all 7 days...
example 2
Study in RP Patients
[0323]Study Protocol
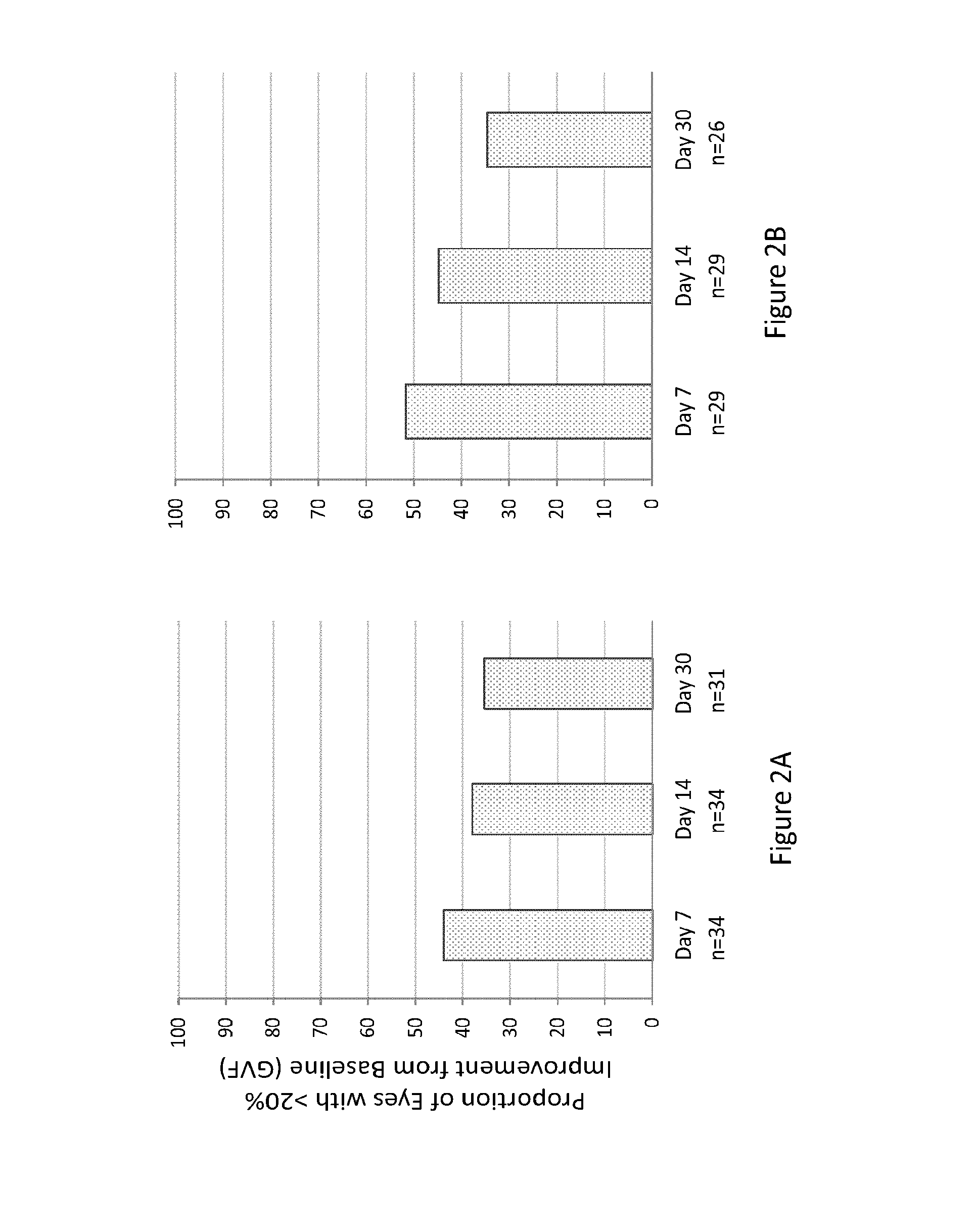
[0324]A study was designed to determine the efficacy of the composition of Example 1 orally administered to human RP subjects having RP caused by mutations in either LRAT or RPE65 (also known as early-onset RP). Seventeen RP subjects received a once-daily dose of the composition orally (40 mg / m2) for 7 days. Both eyes of each RP subject were evaluated separately. Protocol-defined assessments of visual function included: best-corrected visual acuity testing using Early Treatment Diabetic Retinopathy Study (ETDRS), visual field testing, full-field electroretinogram (ERG); retinal sensitivity (FST), dynamic pupillometry, nystagmus testing, OCT and FAF, and subject questionnaire.
[0325]Baseline visual function tests were performed, within 21 days of Day 0 of the study, including spectral-domain OCT in low light conditions to determine if there were viable photoreceptors in the retina. On Day 0 each RP subject received the first dose of the composit...
example 3
Safety and Efficacy Study for LCA Subjects
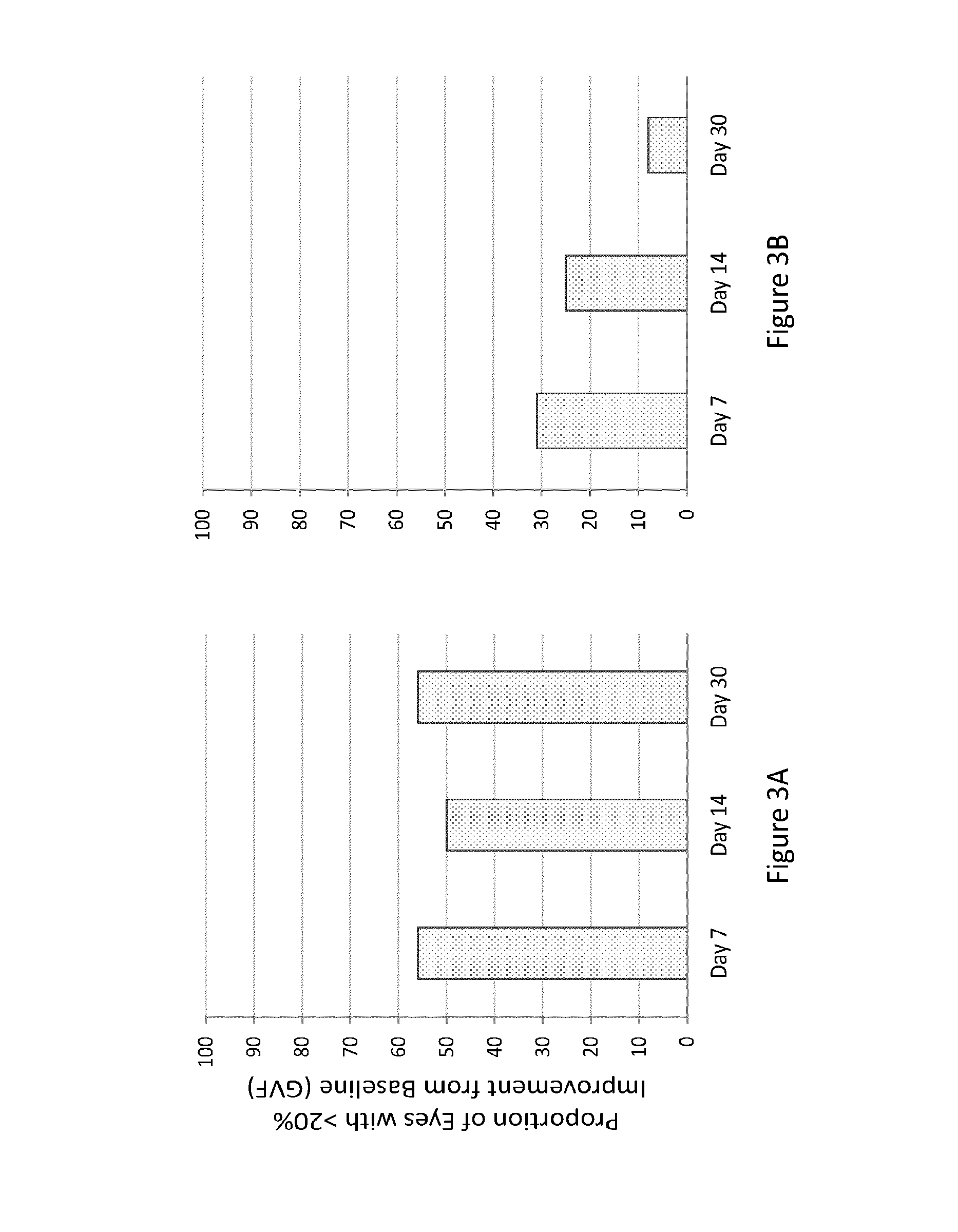
[0344]The study of Example 2 was also designed to determine the efficacy of the composition of Example 1 orally administered to human subjects having LCA (caused by mutations of either LRAT or RPE65). Subjects received a once-daily loading dose of the composition orally (40 mg / m2) for 7 days. Subjects were treated on an outpatient basis, but they received study treatment in the research clinic under medical supervision for each day of treatment. Both eyes of each subject were evaluated separately. Protocol-defined assessments of visual function included: best-corrected visual acuity testing using Early Treatment Diabetic Retinopathy Study (ETDRS) testing followed by low / high contrast Smith-Kettlewell Institute Low Luminance (SKILL) charts; visual field testing using Goldmann perimetry; full-field electroretinogram (ERG); and full-field stimulus threshold testing (FST). Baseline ERGs, ETDRS, and SKILL tests were repeated twice. During and aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com