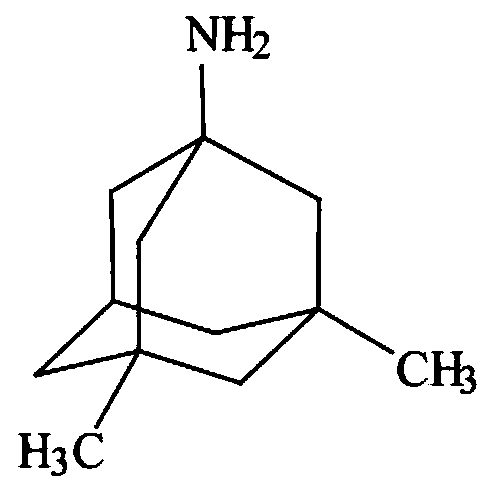
Memantine slow-release micro-tablet capsules and preparation method thereof
A technology of sustained-release microtablets and memantine, which is applied in pharmaceutical formulations, medical preparations with inactive ingredients, drug delivery, etc. and production difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
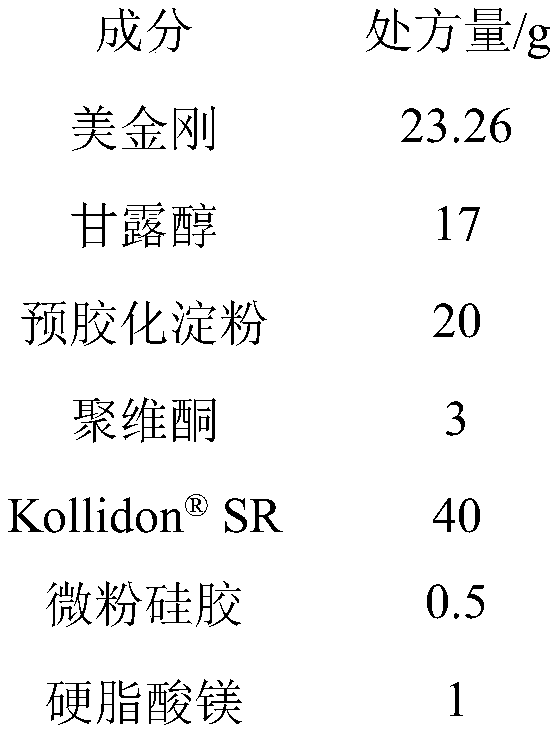
[0034]
[0035] Preparation method: (1) first carry out inspection, the raw material memantine should be able to pass through a 150-mesh sieve; (2) mix the raw material memantine, mannitol, and pregelatinized starch evenly, and granulate with povidone solution; (3) ) dry the wet granules in an oven at 50°C; (4) sieve the granules, and add the prescribed amount of Mix SR, micronized silica gel and magnesium stearate, and press into tablets with a micro-punch of 3-3.5mm; (5) Put the micro-tablets into No. 4 capsules, and each capsule contains 4 micro-tablets.
Embodiment 2
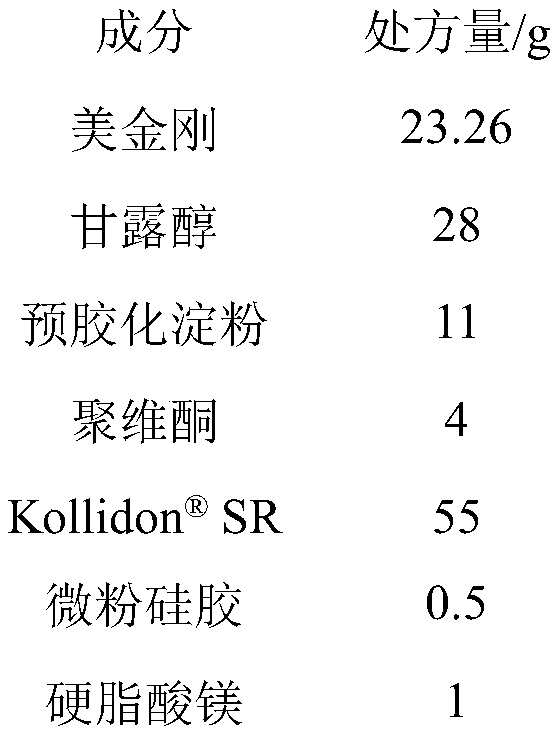
[0037]
[0038] Preparation method: with embodiment 1.
Embodiment 3
[0040]
[0041] Preparation method: with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com