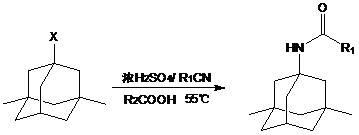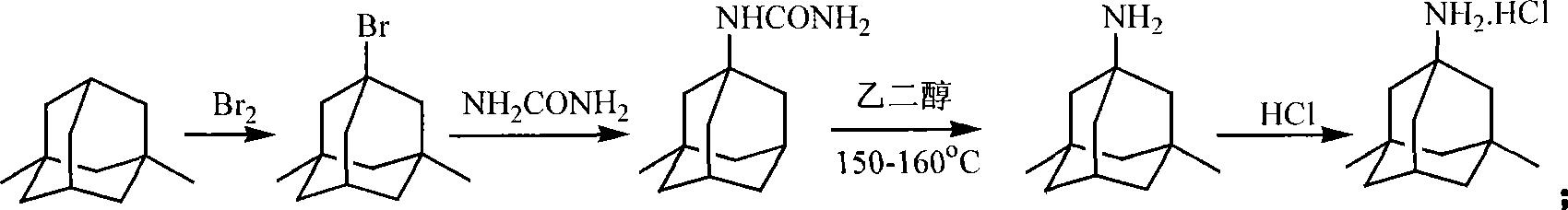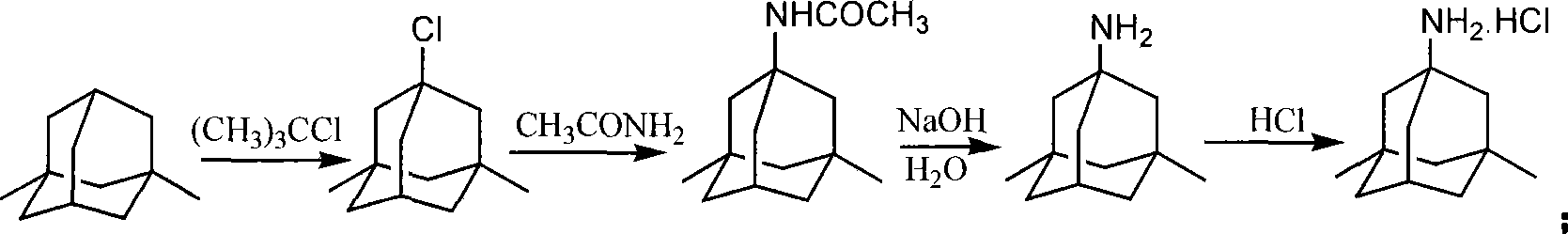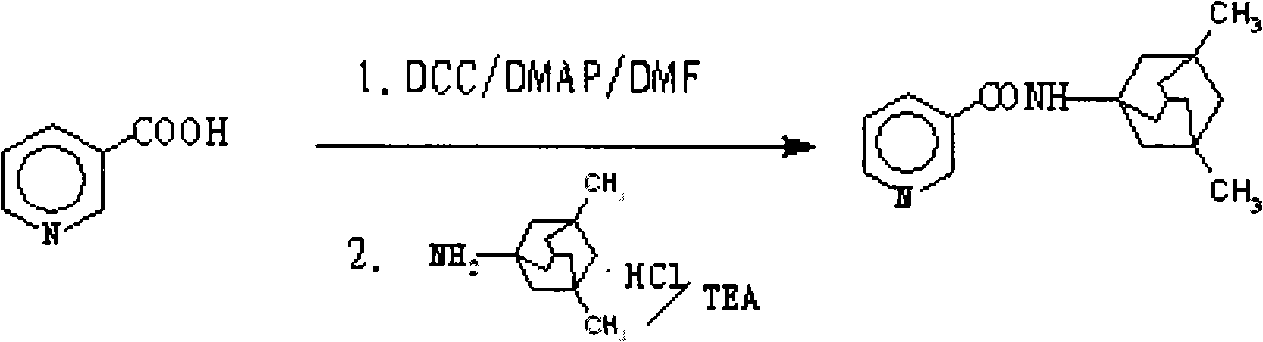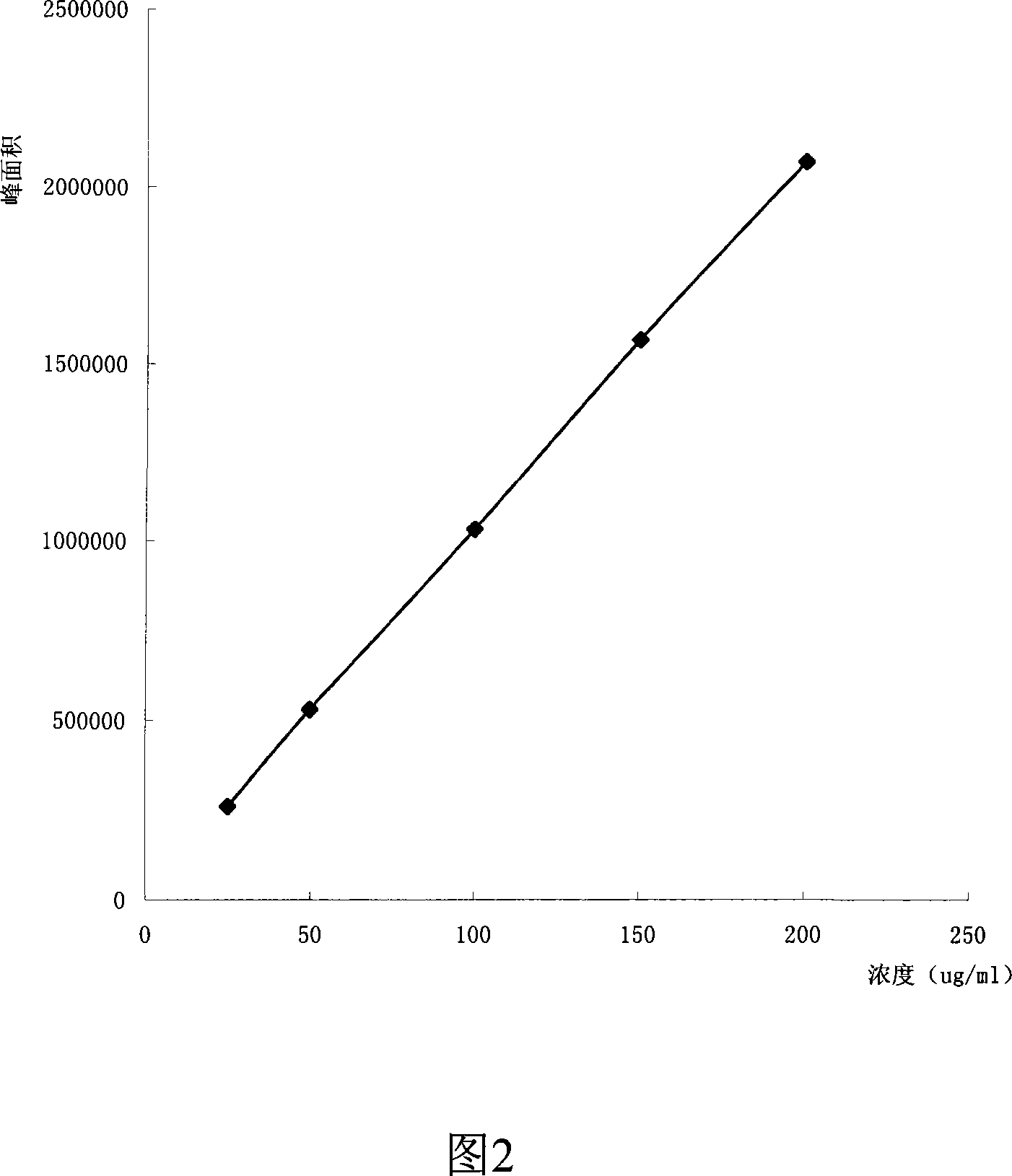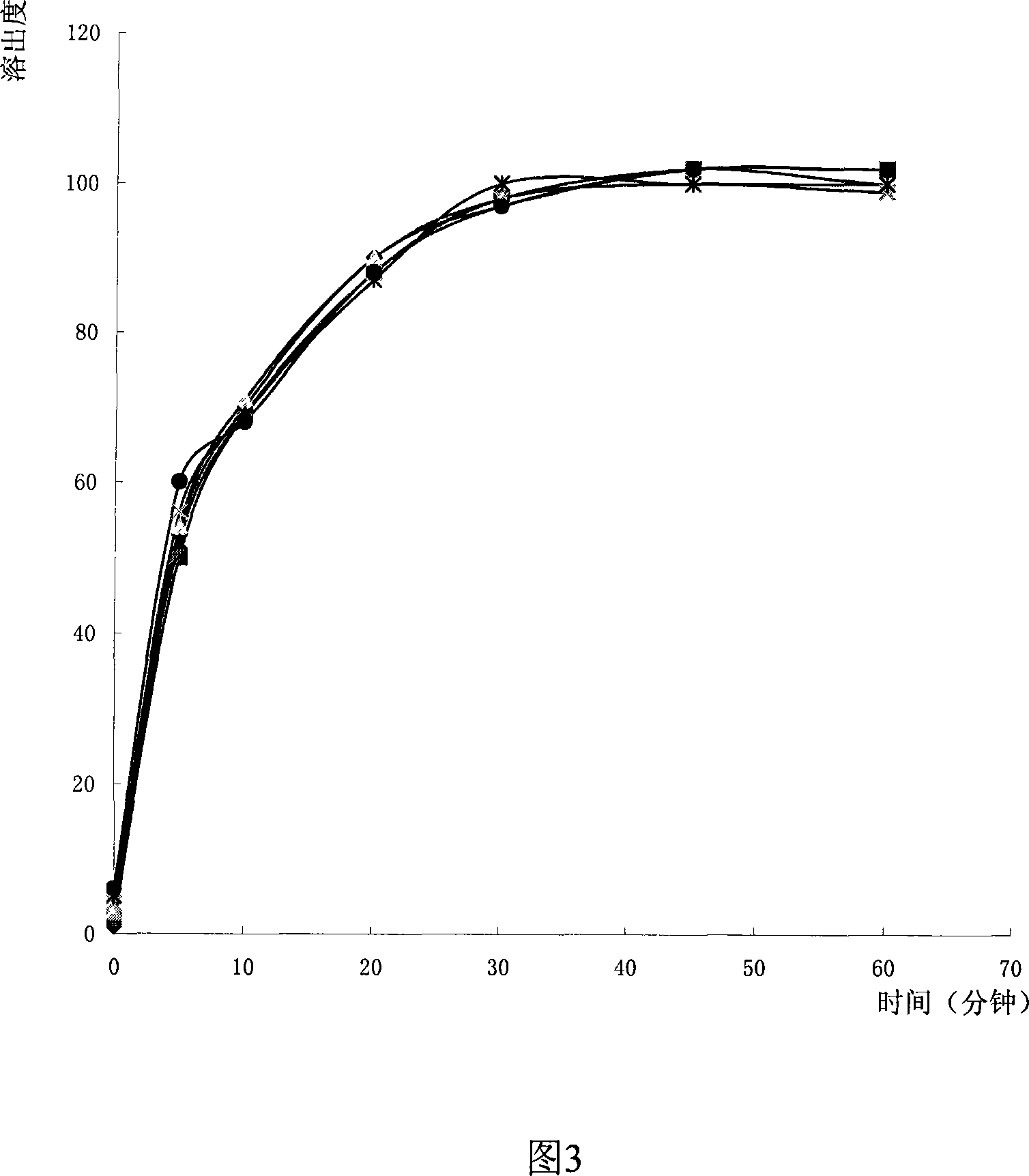Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
121 results about "Memantine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Namenda (memantine hydrochloride) is an orally active NMDA receptor antagonist used to treat moderate to severe Alzheimer's type dementia.
Memantine hydrochloride orally disintegrating tablet and its preparing method
InactiveCN1709229AExpand the application range of dosage formsImprove bioavailabilityNervous disorderPill deliveryMemantine HydrochlorideOrally disintegrating tablet
The present invention relates to a memantine hydrochloride oral disintegrant tablet and its preparation method. Its composition includes (by wt%) 2-20% of memantine hydrochloride as active component, 20-92% of filling agent, 5-30% of disintegrant agent and 1-30% of corrective agent.
Owner:FUKANGREN BIO PHARMA
Preparation method of memantine hydrochloride
InactiveCN1400205AGet stableOperational securityNervous disorderOrganic compound preparationMemantine HydrochlorideSolvent
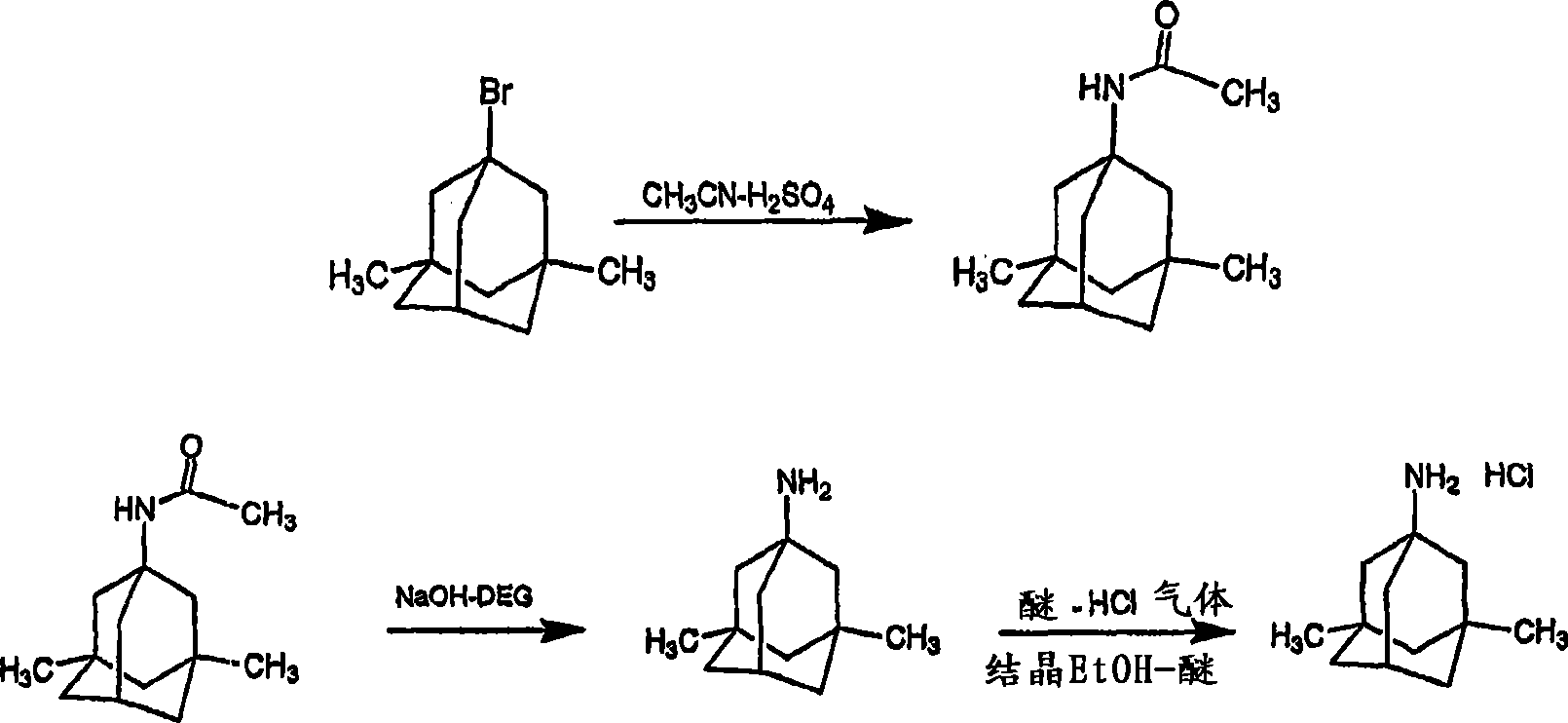
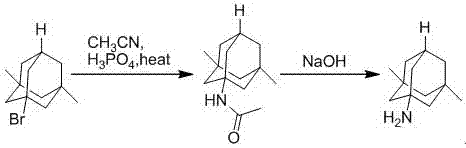
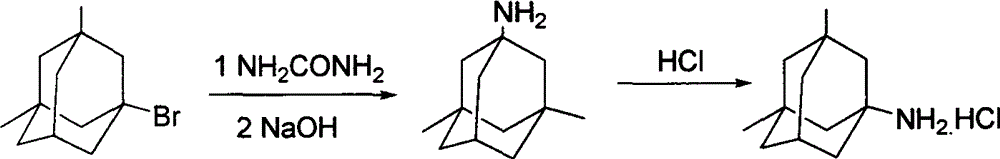
The preparation method of medicine for curing dementia, N-methyl-D-aspartic acid (NMDA) receptor antagonist memantine hydrochloride includes the following steps: using polybasic alcohol as solvent; making 1-bromo-3,5-dimethyl adamantane and urea according to the mole ratio of 1:0.25-10 react for 0.5-48 hr. at 20-200 deg.C; after the reaction is completed, adding sodium hydroxide into the reactionsolution according to that the mole ratio of 1-bromo-3,5-dimethyl adamantane and sodium hydroxide is 1:0.1-10, making alcoholysis at 50-200 deg.C, chloroform extraction, concentration, acidification with hydrochloric acid and salt-forming so as to obtain the invented menantine hydrochloride. It features safe and simple operation and low cost, etc.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
Taste-masked orally disintegrating tablets of memantine hydrochloride
The present invention relates to a solid pharmaceutical composition comprising memantine, which dissolves or disintegrates in the oral cavity preferably within about 60 seconds. The present invention further discloses orally disintegrating tablets of taste-masked memantine of optimal mechanical strength comprising memantine along with a taste-masking agent and pharmaceutically acceptable excipients.
Owner:RUBICON RES PTY LTD
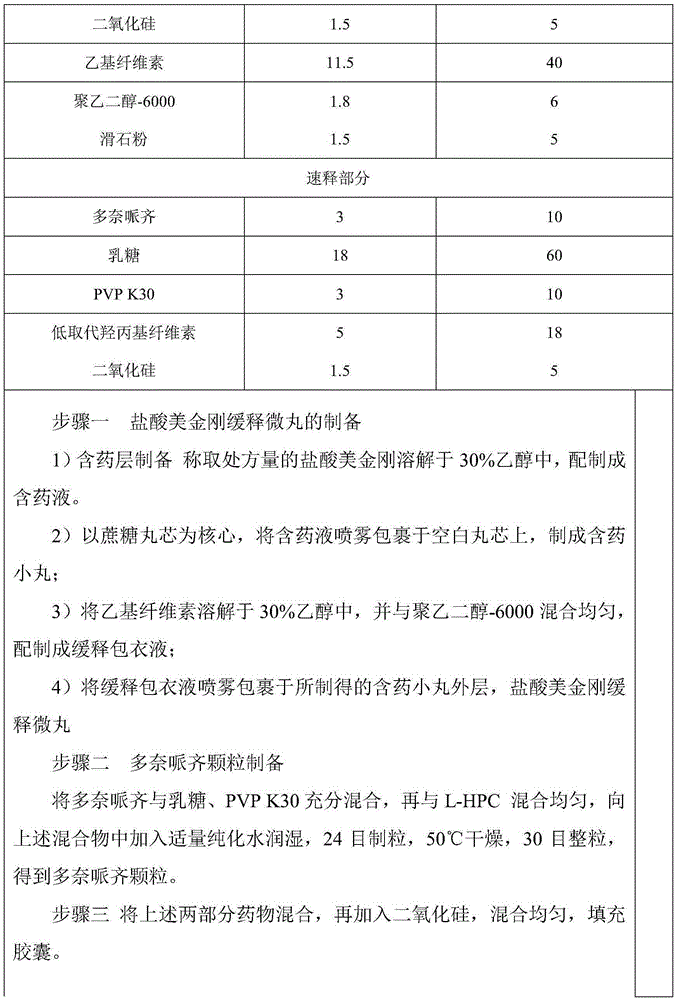
Memantine hydrochloride slow-release pill and preparation method thereof
ActiveCN104013592ASolve the problem of easy releaseReduce the risk of sudden releaseNervous disorderPharmaceutical product form changeMemantine HydrochlorideCurative effect
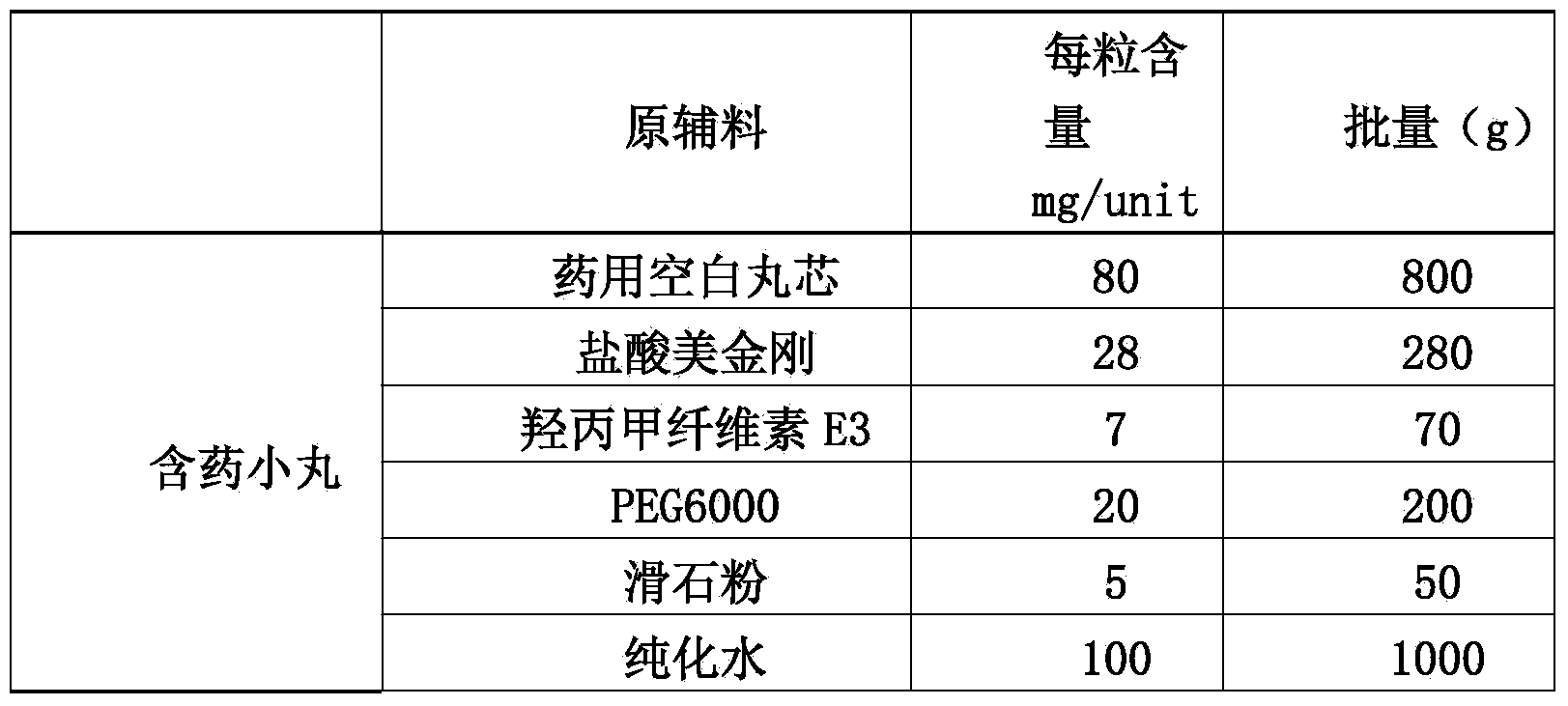
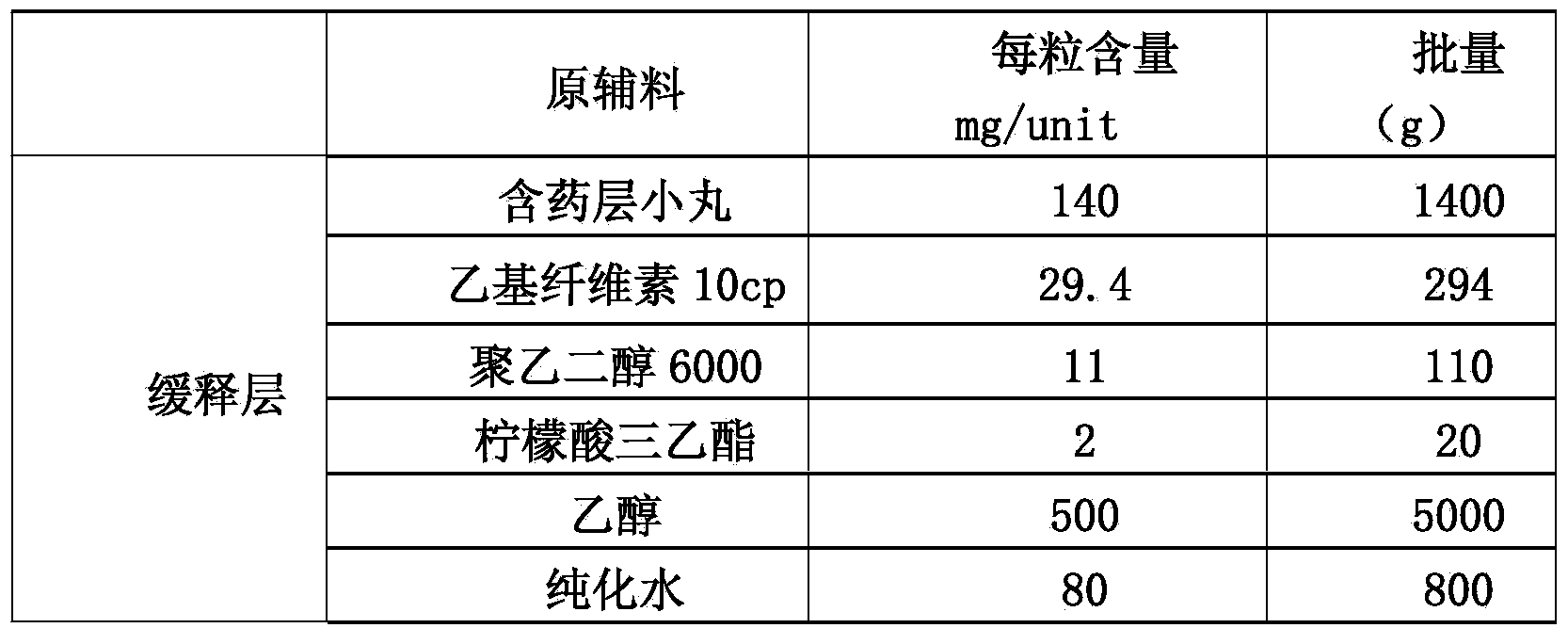
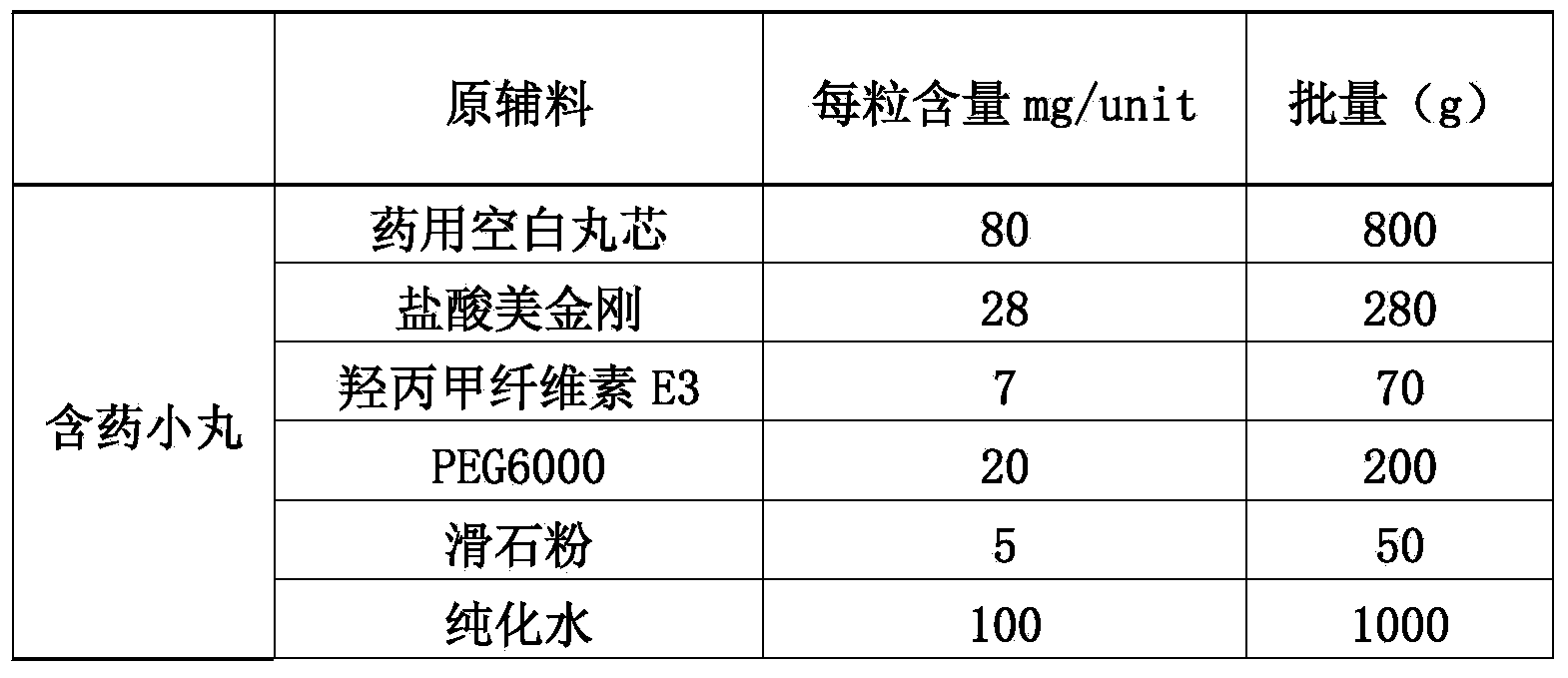
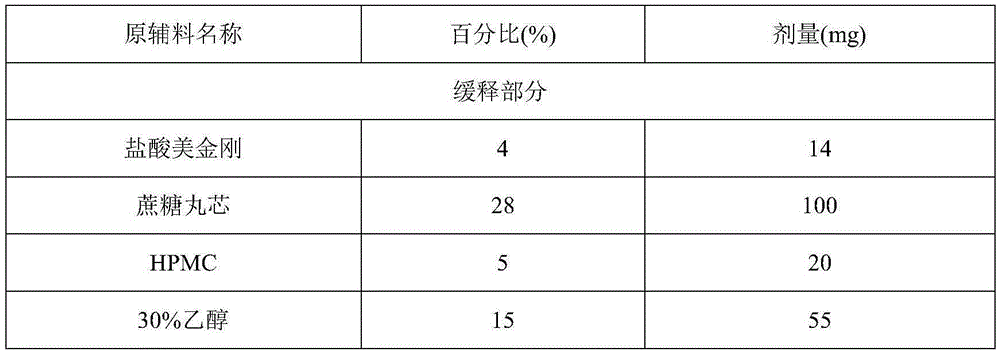
The invention discloses a memantine hydrochloride slow-release pill and a preparation method thereof. Packing inside a capsule is a memantine hydrochloride slow-release pill; the memantine hydrochloride slow-release pill sequentially comprises a hollow pill core, a main medicine layer containing memantine hydrochloride, an isolated coating layer and a slow-release coating layer from inside to outside. The memantine hydrochloride slow-release pill is good in stability and free of an initial burst release phenomenon, the condition that the medicine is lastingly, evenly and slowly released is ensured, and the curative effect of the product is improved.
Owner:ZHEJIANG JINGXIN PHARMA
Method for preparing memantine hydrochloride
InactiveCN1488622AGood crystal formHigh purityNervous disorderPreparation by rearrangement reactionsMemantine HydrochlorideTert-Butyl chloride
The invention is a manufacturing method of diamante amine hydrochlorate. The invention adopts 1, 3-dimethyl adamantine to reacts with tert-butylchlorine and gets 1-chlorine-3, 5-dimethyl adamantine; then reacts with acetamide directly, the educt from water reacts with sodium hydroxide in ethanediol or glycerine solvent, extracts by acetic ester, condenses, and blow in dry hydrochloride gas and gets the coarse product. There gets the pure product through alcohol-acetic ester recrystallization.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Memantine hydrochloride capsule sustained-release preparation and preparation method for same
InactiveCN102552218ALarge distribution areaImprove bioavailabilityNervous disorderPharmaceutical delivery mechanismControl layerSide effect
The invention discloses a memantine hydrochloride capsule sustained-release preparation, wherein the preparation is composed of two parts, namely, immediate-release grains and sustained-release grains; the sustained-release part is composed of a blank pellet core, a medicine layer and a release control layer; and the immediate-release part is composed of a blank pellet core and a medicine layer. The capsule is uniform in content, good in release effect, stable in blood concentration, good for reducing the toxic and side effects of medicines, and is capable of being used for treating moderate-to-severe Alzheimer-type dementia, and the medicine-taking times of the patient are reduced,.
Owner:无锡万全医药技术有限公司
Memantine hydrochloride sustained-release capsule and preparation method thereof
ActiveCN103181914ASustained releaseSmooth releaseNervous disorderPharmaceutical non-active ingredientsSustained release pelletsMemantine Hydrochloride
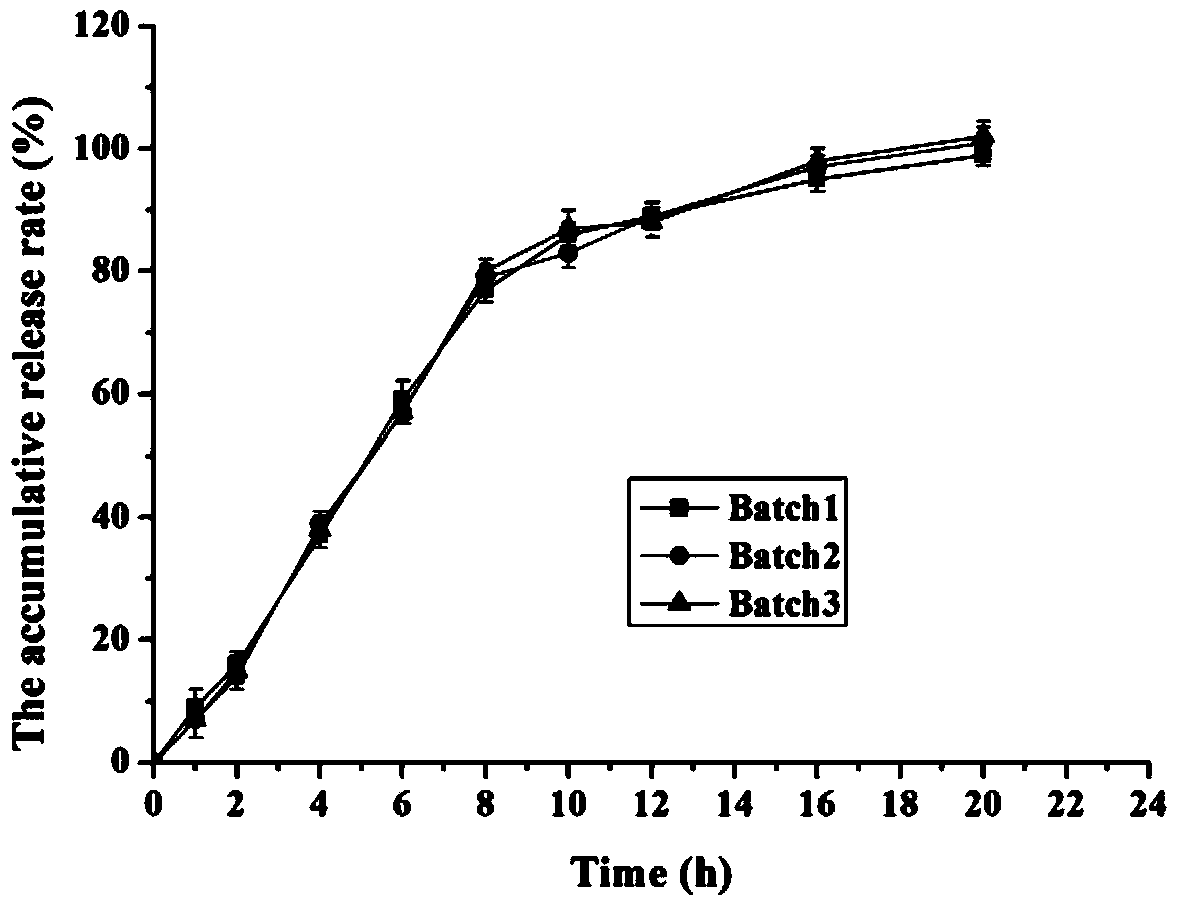
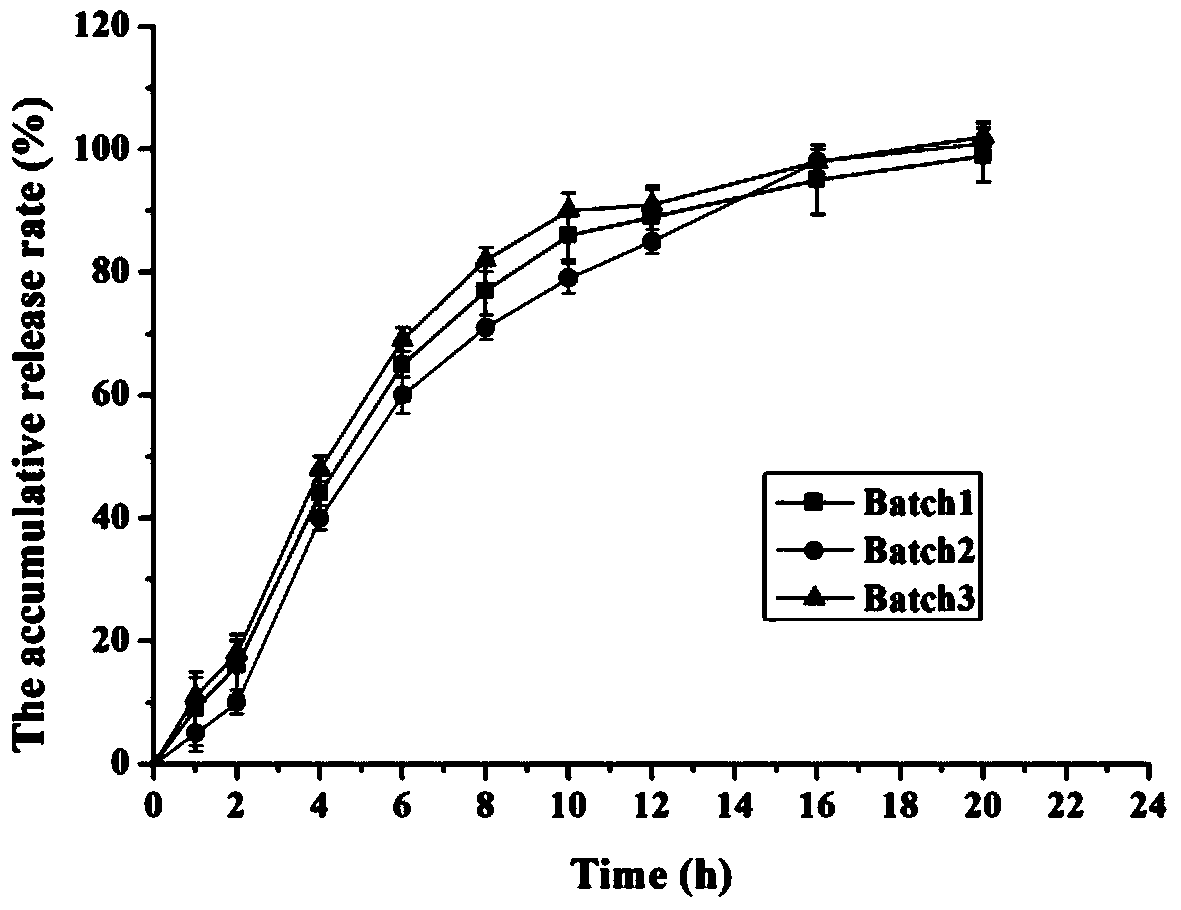
The invention provides a preparation method of a memantine hydrochloride sustained-release preparation. According to the present invention, sustained-release pellets are obtained by sustained-release coating of drug-containing pellets containing memantine hydrochloride. The drug-containing pellets are obtained by extrusion spheronization or solution medicine-feeding or suspension medicine-feeding, and are nearly circular in shape. The shape and granularity-controllable drug-containing pellets are subjected to sustained-release coating and the thickness of the film formed by coating can also be controlled. The invention realizes the controllability of the coating film and the spherical pellets, and reproducibility of stability of releasing the memantine hydrochloride is controlled under the circumstance of guaranteeing nonoccurrence of crystal form of memantine hydrochloride. The preparation of the invention can provide sustained release in the form of a single dose within 24 hours. The drug penetrates and diffuses to the release medium through the film pores. Because the size is small, the medicine taking is less susceptible to foods and the efficacy is improved. The production method of the present invention is simple, is suitable for industrial production, and has a great application value.
Owner:SHANGHAI FOSUN PHARMA DEV CO LTD
Memantine hydrochloride sustained release-donepezil quick release compound capsule
InactiveCN105326837AQuality is easy to controlHigh yieldNervous disorderPharmaceutical delivery mechanismSustained release pelletsMemantine Hydrochloride
Owner:BEIJING VENTUREPHARM BIOTECH
Synthetic method of memantine hydrochloride
InactiveCN102432473AShort timeSolve the difficulty of stirringOrganic compound preparationAmino compound preparationMemantine HydrochlorideMethylene Dichloride
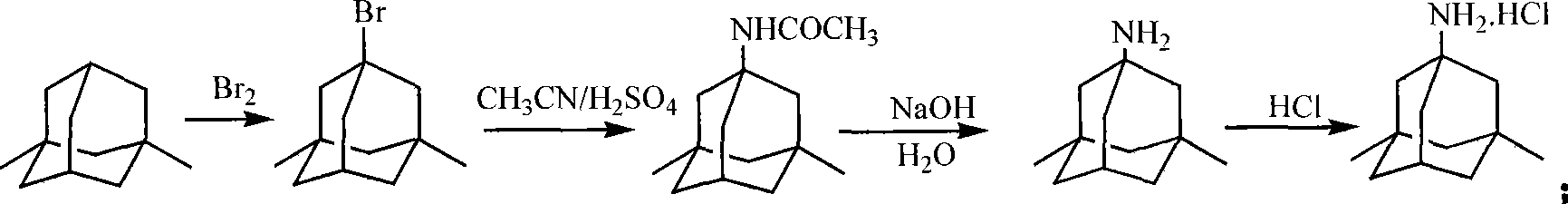
The invention discloses a synthetic method of memantine hydrochloride serving as a medicament for treating dementia. The method comprises the following steps of: reacting halogenated dimethyladamantane with nitrile, concentrated sulfuric acid and organic acid to obtain 1-acetamido-3,5-dimethyladamantane crystal serving as an intermediate for synthesizing memantine hydrochloride; hydrolyzing 1-acetamido-3,5-dimethyladamantane with alcohol and alkali to generate raw memantine; and extracting and salting to obtain memantine hydrochloride. In the method, the organic acid is taken as a solvent, so that the problems of low yield, difficulty in stirring and a series of problems caused by difficulty in stirring are solved; compared with an original process, the method has the advantages that: five steps for distilling the solvent under reduced pressure, adding water, extracting methylene dichloride, recovering the solvent under reduced pressure and recrystallizing are reduced, the reaction post-treatment operation is simplified greatly, and reaction post-treatment is easy and convenient; operation is easier and more convenient, so that the time consumption of a memantine hydrochloride synthesizing process is lowered, and the efficiency is increased; compared with an original process, the method has the advantages that: the step for extracting methylene dichloride serving as an organic solvent is eliminated, so that higher economic efficiency and environmental friendliness are achieved; and more importantly, the acetamide reaction yield is increased from lower than 70 percent to over 90 percent.
Owner:GUANGZHOU BOJI MEDICINE SERVICES
Method for synthesizing memantine hydrochloride
InactiveCN101412678AAvoid pollutionAvoid it happening againNervous disorderAmino preparation by functional substitutionAcetic acidMemantine Hydrochloride
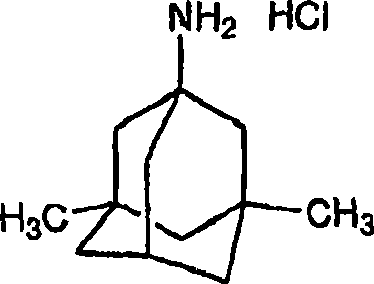
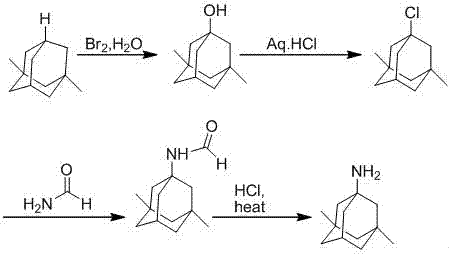
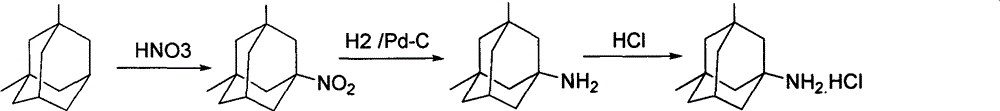
The invention discloses a method for preparing memantine hydrochloride, which comprises the following steps: using 1, 3-dimethyl adamantane as a raw material; nitrifying and then reducing the 1, 3-dimethyl adamantane to synthesize an intermediate 1-amino-3, 5-dimethyl adamantane; and acidifying the intermediate by hydrochloric acid and recrystallizing the intermediate by ethanol-ethyl acetate to obtain the memantine hydrochloride through purification,. The method has the advantages of moderate reaction condition, simple post treatment, good safety, low cost and little environment pollution, and suitability for industrialized production.
Owner:SUZHOU MACWELL BIOLOGICAL MEDICINE SCI & TECH
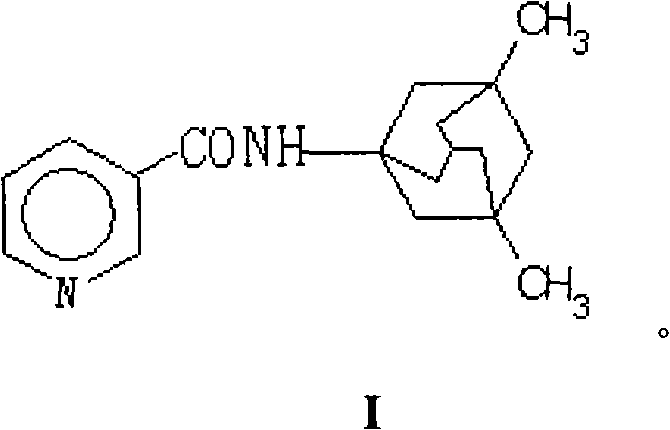
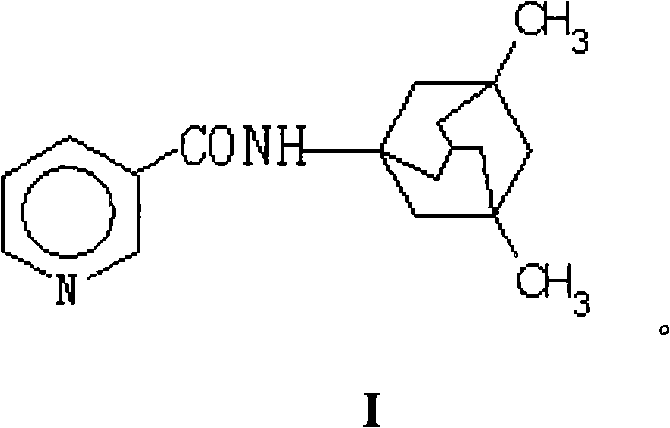
N-(3-pyridine formyloxy)-3,5-dimethyl-1-amantadine for curing senile dementia or pharmaceutical salt thereof
The invention relates to a N-(3-pyridine methoxy)-3,5-dimethyl-1-amine diadamantane for treatment of Alzheimer's Disease or a salt thereof for medicinal purpose, and also relates to the method for synthesizing the compound or the salt thereof for medicinal purpose, as well as the application in the preparation of drugs for treatment of Alzheimer's Disease, particularly, the compound is a synergetic prodrug produced through chemical coupling of amido link between nicotinic acid and amine diadamantane hydrochloric, on one hand, the prodrug not only avoids the side effects caused by nicotinic acid which is orally taken, but also implements cooperative dual effects on prevention and treatment of Alzheimer's disease by nicotinic acid and amine diadamantane, thereby improving the treatment effect of amine diamantine hydrochloric on Alzheimer's Disease; on the other hand, the enhanced molecular solubility of amine diadamantane in lipid facilitates the passage of the prodrug through a blood-brain barrier, increases the distribution concentration of the prodrug in the cerebrospinal fluid, thereby accomplishing the aim of reducing the peripheral side effects of the drug.
Owner:XIAN LIJUN PHARMA CO LTD
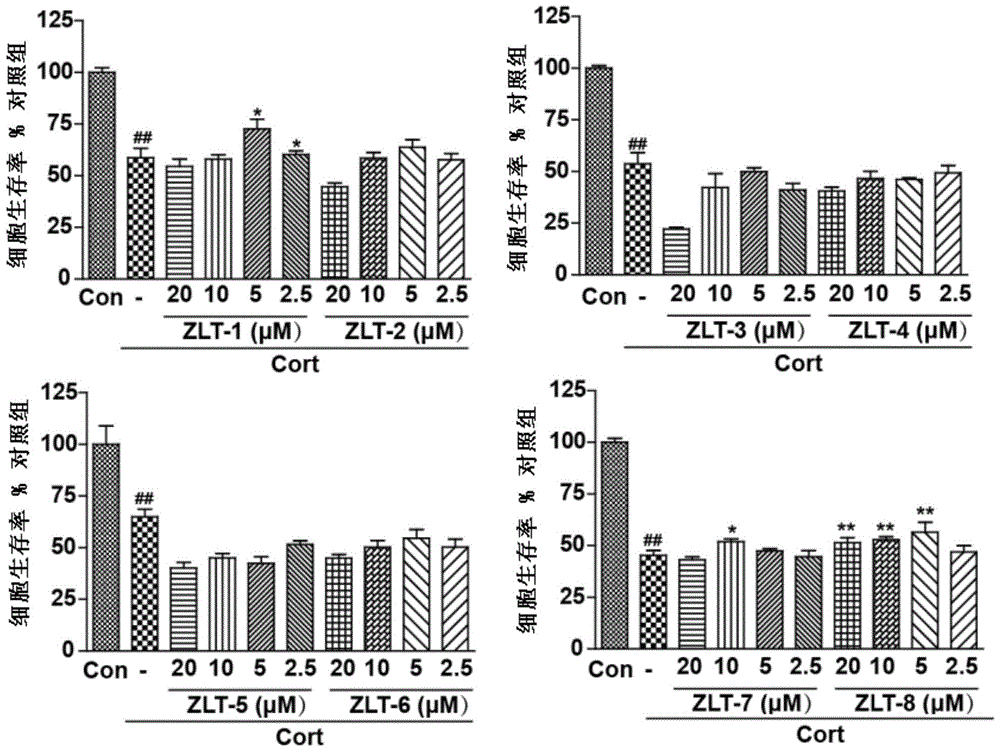
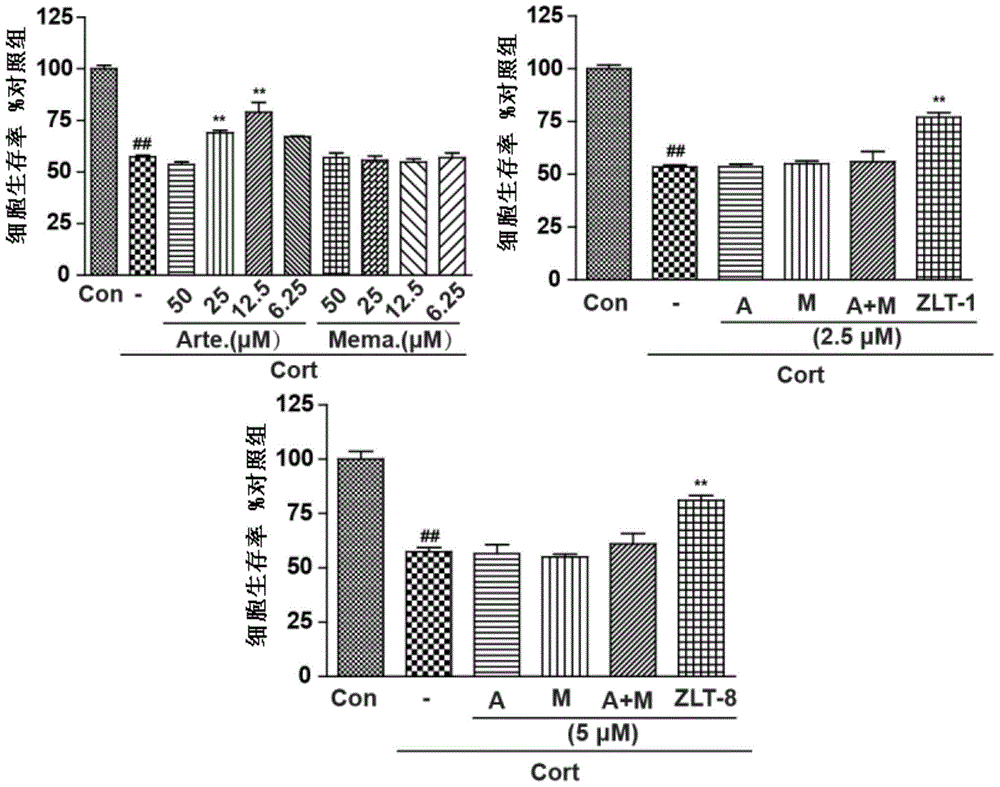
Dihydroarteannuin-memantine diad compounds, and synthesis method and application thereof
ActiveCN105732654ASimple preparation processSignificant effectOrganic active ingredientsNervous disorderMemantine HydrochlorideTreatment effect
The invention discloses dihydroarteannuin-memantine diad compounds, and a synthesis method and application thereof. The structure of the compounds is disclosed as Formula I. The synthesis method comprises the following steps: reducing arteannuin to obtain dihydroarteannuin, carrying out acetalation reaction on the dihydroarteannuin and 2-bromoethanol under the catalytic action of Lewis acid, and carrying out reaction on the acetalation reaction product and memantine hydrochloride to obtain the dihydroarteannuin-memantine diad compounds. The compounds are reported for the first time, have an therapeutic effect on neurodegenerative diseases, and can be used for preparing drugs for treating neurodegenerative diseases. Compared with other prior arts, the compounds disclosed by the invention have the advantages of simple preparation technique and better curative effect than memantine.
Owner:JINAN UNIVERSITY
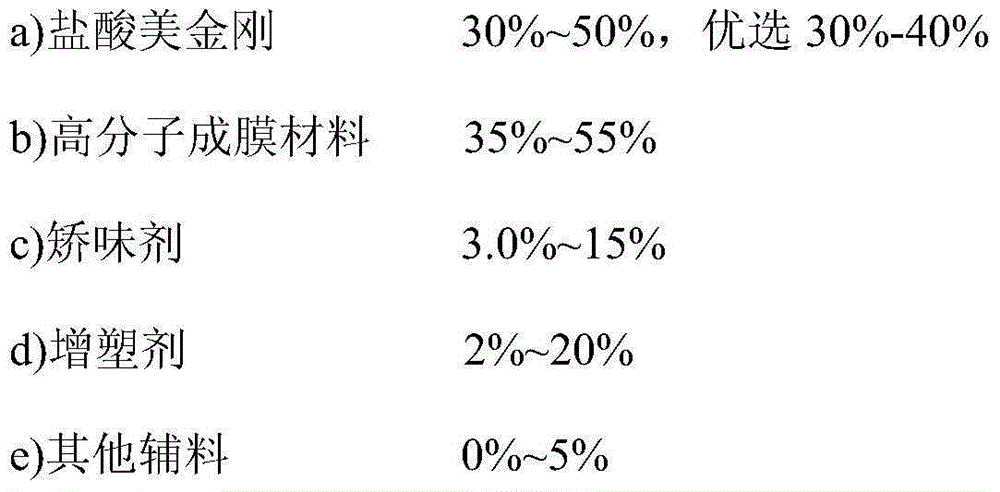
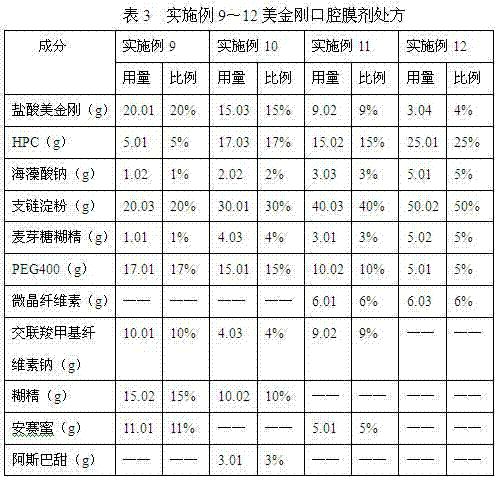
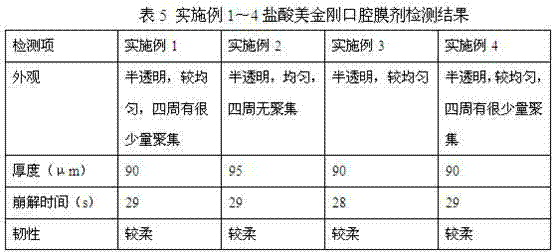
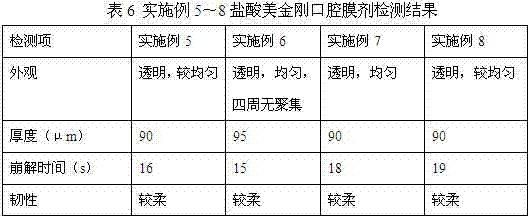
Memantine hydrochloride oral-dissolving film preparation and preparation method and application of preparation
The invention discloses a memantine hydrochloride oral-dissolving film preparation. The preparation shields a stimulated flavor and is good in appearance. The memantine hydrochloride oral-dissolving film preparation comprises, by weight, 30-50% of memantine hydrochloride, preferentially, 30-40% of memantine hydrochloride, 35-55% of macromolecule film-forming materials, 3.0-15% of flavor corrective, 2-20% of plasticizers and 0-5% of other auxiliary materials. The other auxiliary materials comprise one or two of pigment and flavoring agents. Through increasing the content of the raw material chemicals, selecting the appropriate flavor corrective and the film-forming materials and controlling the grain size of the raw material chemicals, the film preparation free of the stimulated flavor, smooth in surface, even in content of chemicals, high in disintegration speed and rapid in absorption is obtained; the film preparation is convenient to take and improves the compliance of a patient. The invention further provides a method of preparing the film preparation and an application of the film preparation in treating the Alzheimer disease.
Owner:QILU PHARMA
Process for the preparation of 1-amino-3,5-dimethyladamantane hydrochloride
InactiveCN101102996AOrganic compound preparationCarboxylic acid amides preparationMemantine HydrochlorideMedicinal chemistry
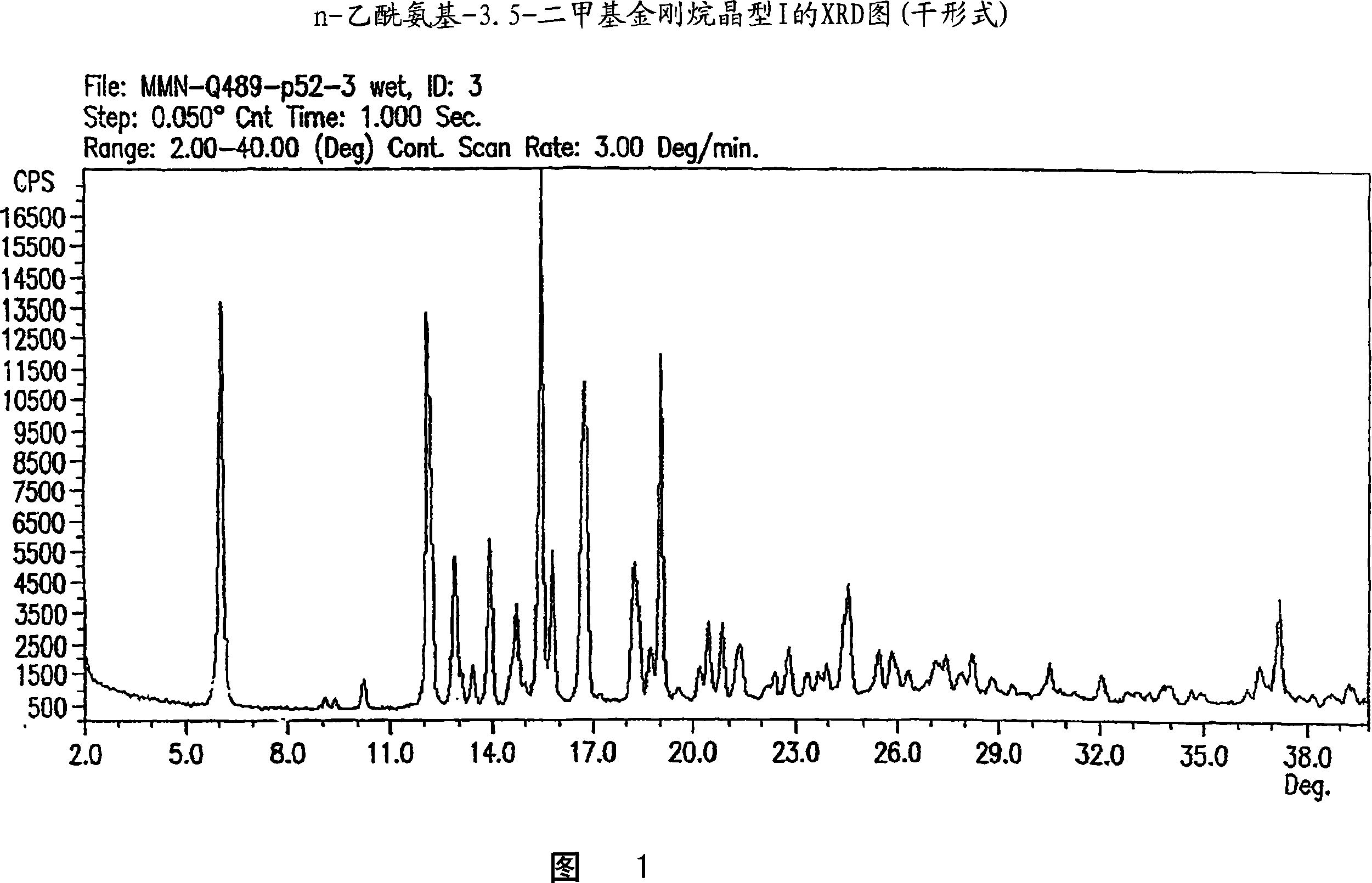
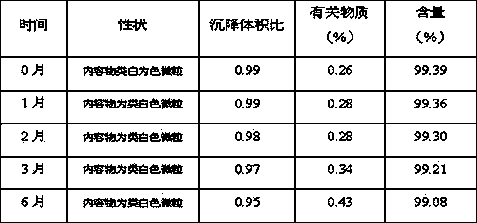
A crystalline Form II of memantine hydrochloride, pharmaceutical compositions containing crystalline Form II, and methods of preparing crystalline Forms I and II of memantine hydrochloride are provided.
Owner:TEVA PHARMA FINE CHEMI
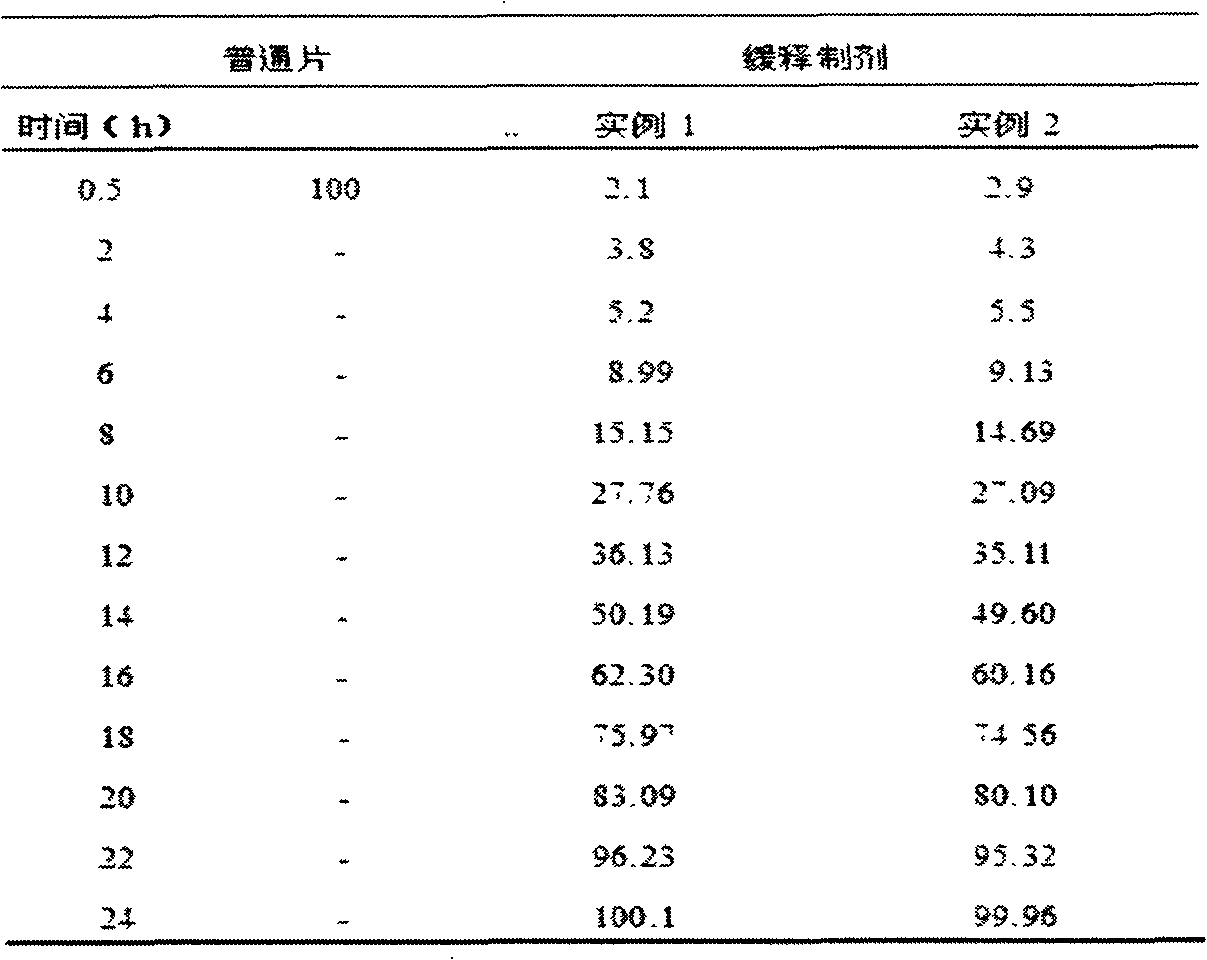
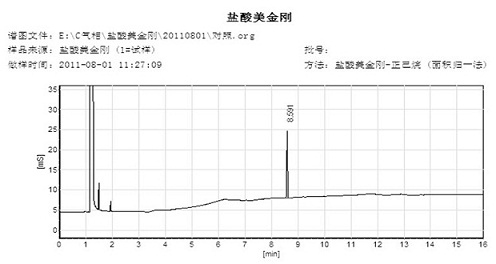
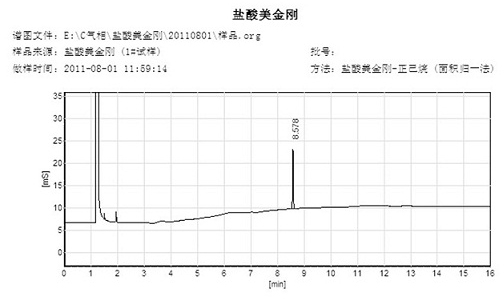
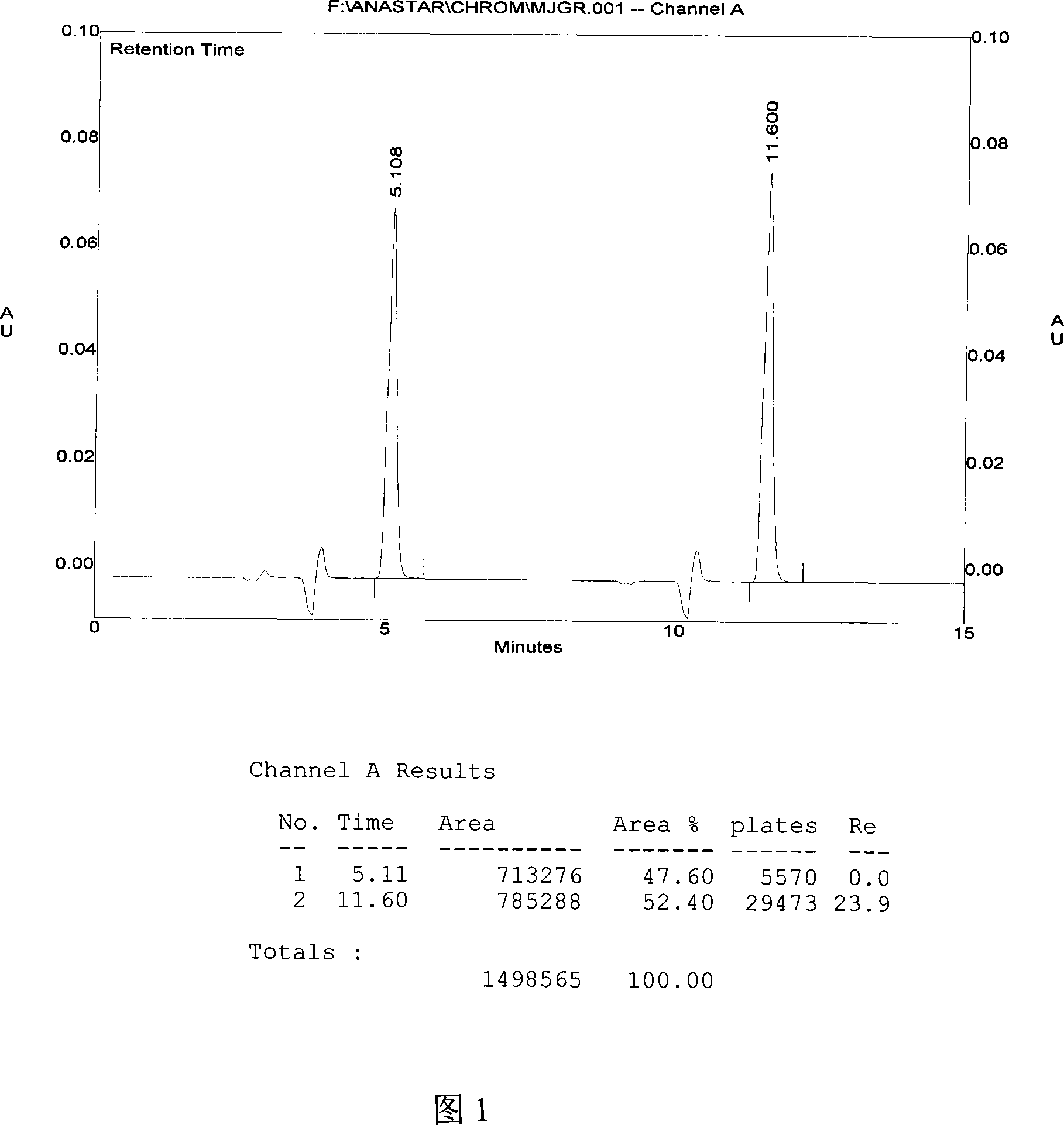
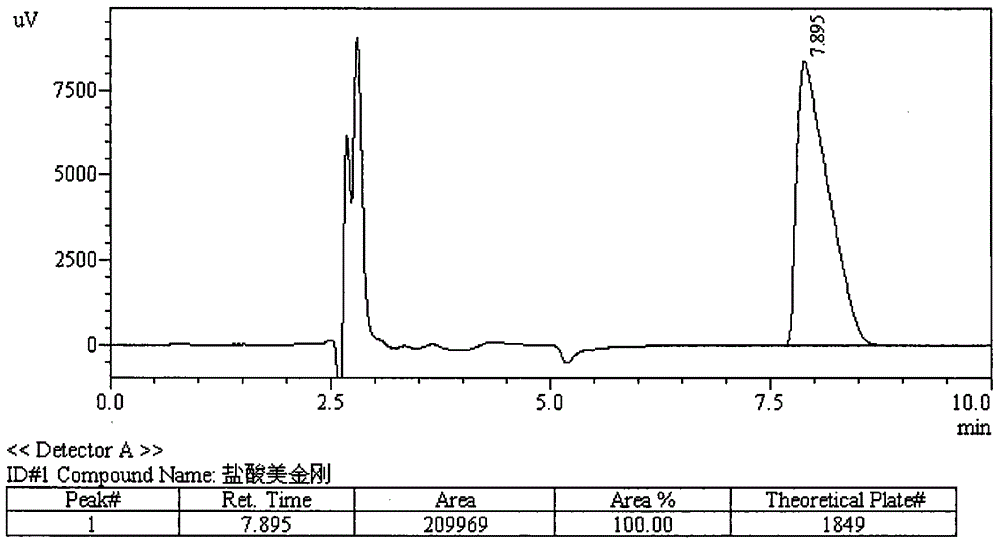
Method for detecting dissolution of memantine hydrochloride related preparations
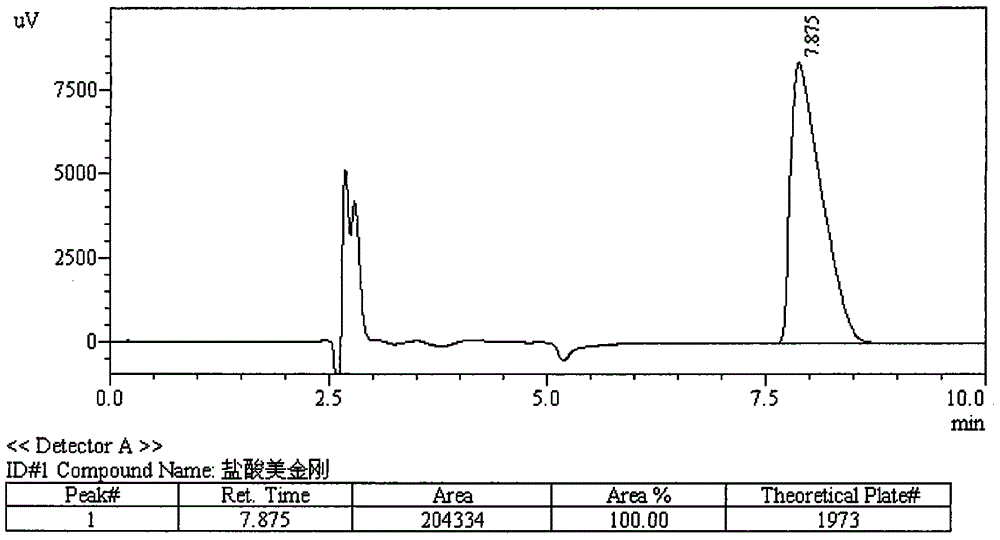
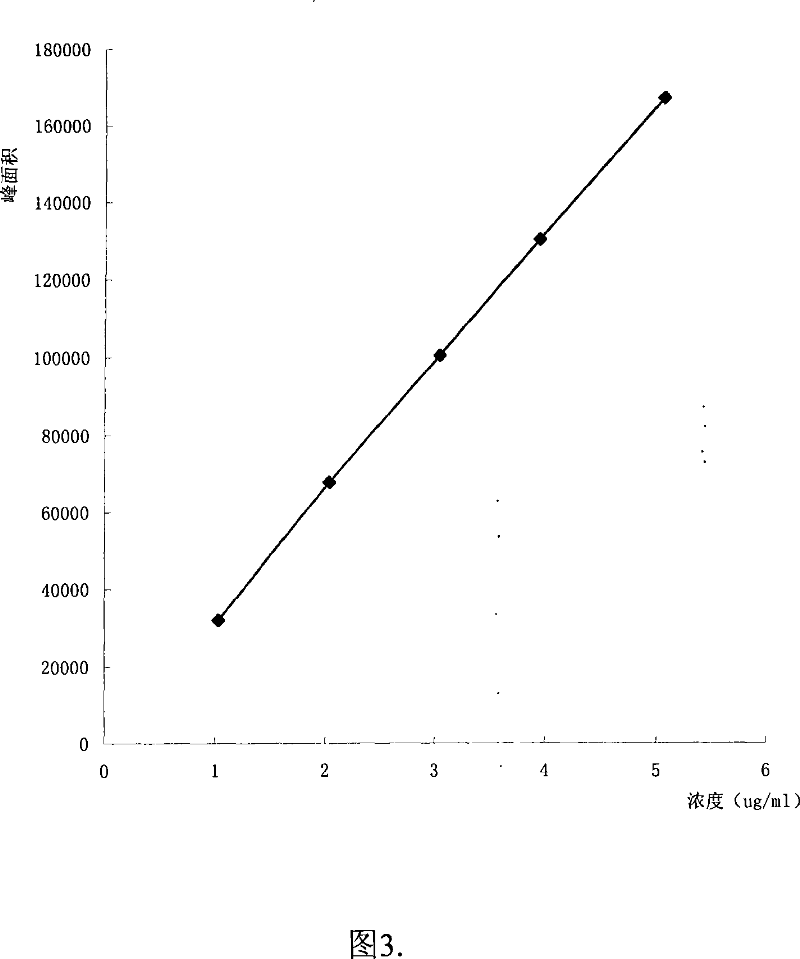
ActiveCN101034084AHigh detection sensitivityThe detection method is accurateComponent separationTesting medicinal preparationsMemantine HydrochloridePropanoic acid
This invention relates to a dissolution test method of hydrochloric acid memantine correlation preparation. The invention adopts high performance liquid chromatograph - evaporation light scattering detector, takes methanol - 0.05 percent penta- fluorine propanoic acid solution as mobile phase. The invention gets memantine correlation preparation six unit, and take 0.1 mol / L hydrochloric acid solution 100 ml as solvent, by 30 minutes, then take right amount solution to filter out as examining solution. Add water into controlling article to prepare 0.1 mg / ml solution for controlling article solution. Taking each kind solution 20 mu L to inject to liquid phase chromatograph, recording chromatogram. By external standard method and peak area to calculate dissolution degree of hydrochloric acid memantine. This invention overcome the shortcoming of that hydrochloric acid memantine structure is more stable and can not carry out effective detection without absorbing at ultraviolet region. The invention can just use simple mobile phase condition to accuracy detect hydrochloric acid memantine dissolution of correlation preparation, possess quickly and precise merit.
Owner:SHANDONG INST OF PHARMA IND
Memantine hydrochloride slow release-donepezil quick release compound capsule
InactiveCN106727439AReduce the peak and valley phenomenon of blood drug concentrationReduce the frequency of takingNervous disorderAmine active ingredientsMemantine HydrochlorideFluidized bed
The invention provides a memantine hydrochloride slow release-donepezil quick release compound capsule. The compound capsule is prepared by jointly filling a capsule with memantine hydrochloride slow release pellets and donepezil quick release granules; the memantine hydrochloride slow release pellets are prepared by using medicinal empty pellet cores as original cores, dissolving memantine hydrochloride serving as main drug and proper excipients to form coating liquid, spraying the coating liquid to the bottom of a fluidized bed to form a drug-containing layer and sequentially coating the fluidized bed with an isolating layer and a slow release layer from inside to outside; the donepezil quick release granules are formed by subjecting donepezil hydrochloride serving as main drug and proper excipients to wet-process granulation, and the slow release pellets and quick release granules are filled into one same capsule according to a certain proportion of the main drug.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Memantine oral film agent and preparation method thereof
ActiveCN103800306AUniform appearanceUniform and complete appearanceNervous disorderPharmaceutical non-active ingredientsCelluloseMemantine Hydrochloride
Owner:CHONGQING PHARMA RES INST
Memantine hydrochloride sustained release preparation and preparing method thereof
ActiveCN103816135ASimple production equipmentEase of industrial productionNervous disorderPharmaceutical delivery mechanismMemantine HydrochlorideMini tablets
The invention discloses a memantine hydrochloride sustained release preparation. The memantine hydrochloride sustained release preparation comprises a memantine hydrochloride sustained release mini-tablet formed by a tablet core and a coating film. The weight of the coating film is 0-30% of the weight of the tablet core. Equipment for producing the sustained release mini-tablet is simple and is similar to equipment for producing common tablets, besides changes of the diameter size of a punch, and therefore industrial production of the sustained release mini-tablet can be easily achieved. After process parameters of the mini-tablet are determined, the sizes of obtained tablets are largely the same, in-batch differences or differences among batches are almost nonexistent, and the quality is easy to control.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Method suitable for industrial production of memantine hydrochloride
InactiveCN107365255AMild responseHigh yieldAmino compound purification/separationOrganic compound preparationAluminium chlorideIce water
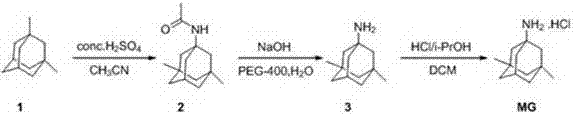
The invention relates to a method suitable for industrial production of memantine hydrochloride. 1,3-dimethyl adamantane is used as a starting material, and a Ritter reaction, hydrolysis, salt forming and refining are used for preparing; during the Ritter reaction, acetonitrile is directly used as an acetylation reagent, concentrated sulfuric acid is used as a catalyst and a solvent, and the reaction is carried out at 70-80 DEG C; after the reaction ends, water is directly added into ice water, dichloromethane is used for extraction and drying by distillation, n-hexane is used for recrystallization, and 1-acetamido-3, 5-dimethyladamantane is directly obtained by one step; 1-acetamido-3, 5-dimethyladamantane is hydrolyzed under an alkaline condition in order to obtain 1-amino-3, 5-dimethyladamantane, a memantine crude product is obtained by salt forming of 1-amino-3, 5-dimethyladamantane under the condition of dichloromethane solvent acidifying, water is used as a solvent in order to carry out recrystallization of the crude product, and a refined product is obtained. In the first acetyl amination process, concentrated sulfuric acid is used as a catalyst and a solvent, tedious industrial post-treatment due to usage of anhydrous aluminium chloride as a catalyst is avoided, an extractant employs dichloromethane, in order to avoid usage of chloroform of high toxicity, and recrystallization employs water as a solvent with environmental protection and cost saving.
Owner:CHANGZHOU PHARMA FACTORY
Menantine hydrochloride soft capsule and preparing method
InactiveCN1742712AMask bad smellDisintegrates quicklyNervous disorderCapsule deliveryDiseaseMemantine Hydrochloride
The present invention relates to a memantine hydrochloride soft capsule for curing senile dementia and its preparation method. It is made up by using memantine hydrochloride as raw material and adding a certain auxiliary material according to conventional process.
Owner:FUKANGREN BIO PHARMA
Method for detecting content, dissolution rate and releasing rate of memantine hydrochloride or analogues thereof in medicinal agent
InactiveCN104950047AEasy to handleSimple processComponent separationMemantine HydrochlorideFluid phase
The invention relates to the chemistry field and in particular relates to a method for detecting memantine hydrochloride or analogues thereof in a medicinal agent. A method for detecting the content, the dissolution rate and the releasing rate of memantine hydrochloride or analogues thereof in medicinal agent adopts the liquid chromatogram conditions as follows: a detector is a differential refraction detector, a chromatographic column takes octyl silane bonded silica gel as a filler, and the moving phase is methanol-0.05mol / L monopotassium phosphate solution. The method is rapid, convenient and accurate and has strong operational practicability.
Owner:ZHUHAI UNITED LAB
Memantine insoluble salt sustained-release injection and preparation method thereof
InactiveCN108969478AReduce solubilityExtended release timePowder deliveryNervous disorderSolubilityMemantine Hydrochloride
The invention discloses a memantine insoluble salt sustained-release injection and a preparation method thereof. The memantine insoluble salt sustained-release injection is characterized by being prepared from the following materials by mass percentage: 1% to 30% of memantine insoluble salt as an active ingredient, 0.1% to 10% of stabilizer , 0% to 1% of pH adjuster, 0.1% to 5% of isotonic regulator and 60% to 98% of injection water; the memantine insoluble salt includes one of memantine-oleate, memantine-linoleate, memantine-linolenate, memantine-arachidonate, memantine-stearate, memantine-laurate, memantine-tannate, memantine palmitate, memantine-pamoate, and memantine-furanate. An insoluble salt technology is adopted to prepare the memantine insoluble salt, and the drug release time isprolonged by reducing the solubility of the drug. Compared with memantine hydrochloride, the solubility of the memantine insoluble salt is reduced by 40 to 600 times, and the memantine insoluble saltcan be slowly released for several days to several weeks.
Owner:JIANGNAN UNIV
Synthesis of memantine hydrochloride
InactiveCN102942490AShort reaction timeLow reaction temperatureOrganic compound preparationCarboxylic acid amides preparationMemantine HydrochlorideSynthesis methods
The invention relates to a medicine-memantine hydrochloride synthesized from 1-chloro-3,5-dimethyladamantane as an initial raw material. At present, many synthesis methods for preparing memantine hydrochloride, such as urea method, digestive catalytic reduction method, acetamide method and acetonitrile method, have the problems of high bromide / urea reaction temperature, long time, great environmental pollution and the like to different degrees. The invention aims to develop a synthesis technique of the medicine memantine hydrochloride. The technical scheme is as follows: the technique comprises the following steps: by using 1-chloro-3,5-dimethyladamantane as the initial raw material and formamide as an aminating agent, carrying out amination reaction to generate a key intermediate 1-formamido-3,5-dimethyladamantane, hydrolyzing with concentrated hydrochloric acid, salifying, and carrying out vacuum drying to obtain the memantine hydrochloride. The reaction between the formamide and 1-chloro-3,5-dimethyladamantane shortens the reaction time, lowers the reaction temperature, and provides higher product yield and purity (the product yield is higher than 76%, and the purity is higher than 99%).
Owner:陕西方舟制药有限公司
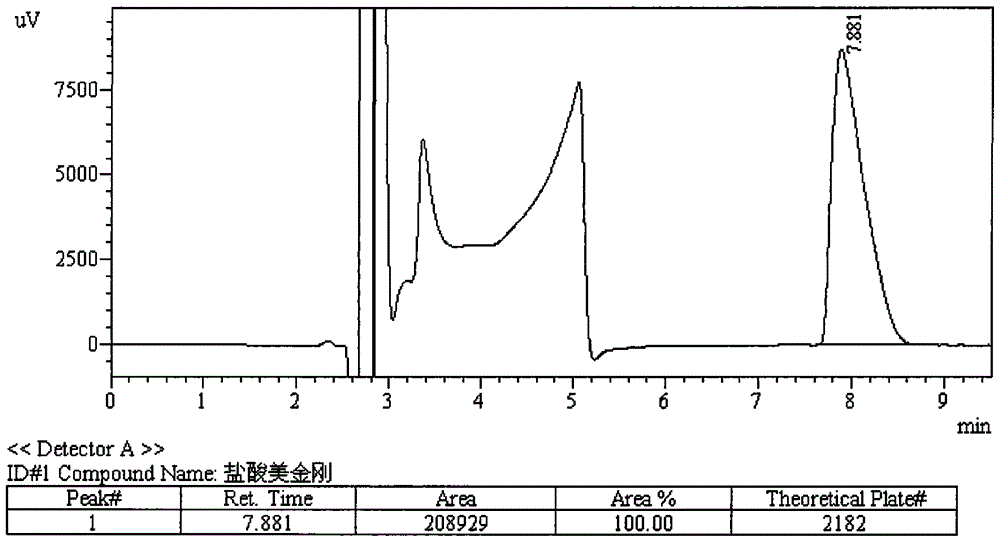
HPLC-ELSD measuring method for correlated matter in memantine hydrochloride
ActiveCN101038280AHigh detection sensitivityMeet checkComponent separationTesting medicinal preparationsMemantine HydrochlorideLocal structure
The present invention discloses a method used for detecting memantine hydrochloride as well as related matters thereof, specifically, a HPLC-ELSD detector is utilized and a methanol-acetonitrile-isopropanol-triethylamine solution is adopted as a mobile phase. The mobile phase is added to an appropriate amount of memantine hydrochloride as well as related preparations containing memantine hydrochloride to prepare a solution containing 0.3 mg memantine hydrochloride per 1 ml acting as a testing solution. The testing solution is then diluted to a concentration of 6 mu g memantine hydrochloride per 1 ml to act as a reference solution, using the mobile phase. The sample is introduced respectively to insure that the peak area sum of a variety of impurities in the testing solution is not more than the main peak area of the reference solution. the method used for detecting memantine hydrochloride as well as related preparations containing memantine hydrochloride in accordance with the present invention overcomes the shortcoming that it is difficult to perform an effective detection due to that the local structure is stable and there is no absorption in the ultraviolet region, and is capable of detecting the impurity in the emantine hydrochloride and conditions of degradation products, quickly and correctly. Said method is capable of better controlling product quality, with simple and convenient operations and high sensitivity.
Owner:SHANDONG INST OF PHARMA IND
Process for producing memantine hydrochloride
ActiveCN103965058AHigh yieldLess side effectsOrganic compound preparationAmino compound preparationMemantine HydrochlorideHydrolysis
The invention belongs to the technical field of medicine, and in particular relates to a process for producing memantine hydrochloride. The process for producing the memantine hydrochloride comprises the steps of performing acetyl amination reaction on 1-bromo-3,5-dimethyladamantane and acetonitrile at 5-10 DEG C under the action of concentrated sulfuric acid to obtain 1-acetamino-3,5-dimethyladamantane; performing hydrolysis reaction on the 1-acetamino-3,5-dimethyladamantane and polyol which does not contain an ether bond under an alkali condition to obtain 1-amino-3,5-dimethyladamantane; and acidifying the 1-amino-3,5-dimethyladamantane with hydrochloric acid, and performing re-crystallization to obtain the high-purity memantine hydrochloride. The process for producing the memantine hydrochloride, disclosed by the invention, has the advantage that the product yield of the memantine hydrochloride is increased by controlling process parameters and changing process conditions based on an existing synthetic process.
Owner:CHENGDU YILUKANG MEDICAL TECH & SERVICE
Method for detecting releasing rate of amantadine hydrochloride sustained release tablets
InactiveCN104237407AOvercoming Hard-to-Detect ShortcomingsHigh detection sensitivityComponent separationMemantine HydrochloridePhotochemistry
The invention discloses a method for detecting the releasing rate of amantadine hydrochloride sustained release tablets. The method comprises the steps of under chromatographic conditions that a high performance liquid chromatograph-evaporative light-scattering detector is adopted and methyl alcohol-acetonitrile-isopropanol-aqueous solution (in a ratio of 50:20:20:10) is taken as a flowing phase, processing six units of amantadine hydrochloride sustained release tablets with 100ml of 0.1mol / L hydrochloric acid solution as a releasing medium at a rotating speed of 75r / min according to a dissolution rate measurement method, taking 5ml of solution after one hour, two hours, four hours and eight hours respectively, filtering the extracted solution while supplementing the solution by 5ml in time, and measuring subsequent filtrate to obtain a test solution; adding water into a contrast to prepare a 0.1mg / ml solution serving as a contrast solution; and recording chromatogram maps of the two solutions, wherein the volume of each solution is 20 microliter. According to the releasing rate detection method, the defect that memantine hydrochloride with a relatively stable structure cannot be effectively detected as being free of absorption in an ultraviolet region is overcome; under a simple flowing phase condition, the dissolving rate of memantine hydrochloride can be accurately detected; the releasing rate detection method has the advantages of simplicity, quickness and accuracy.
Owner:SHANDONG INST OF PHARMA IND
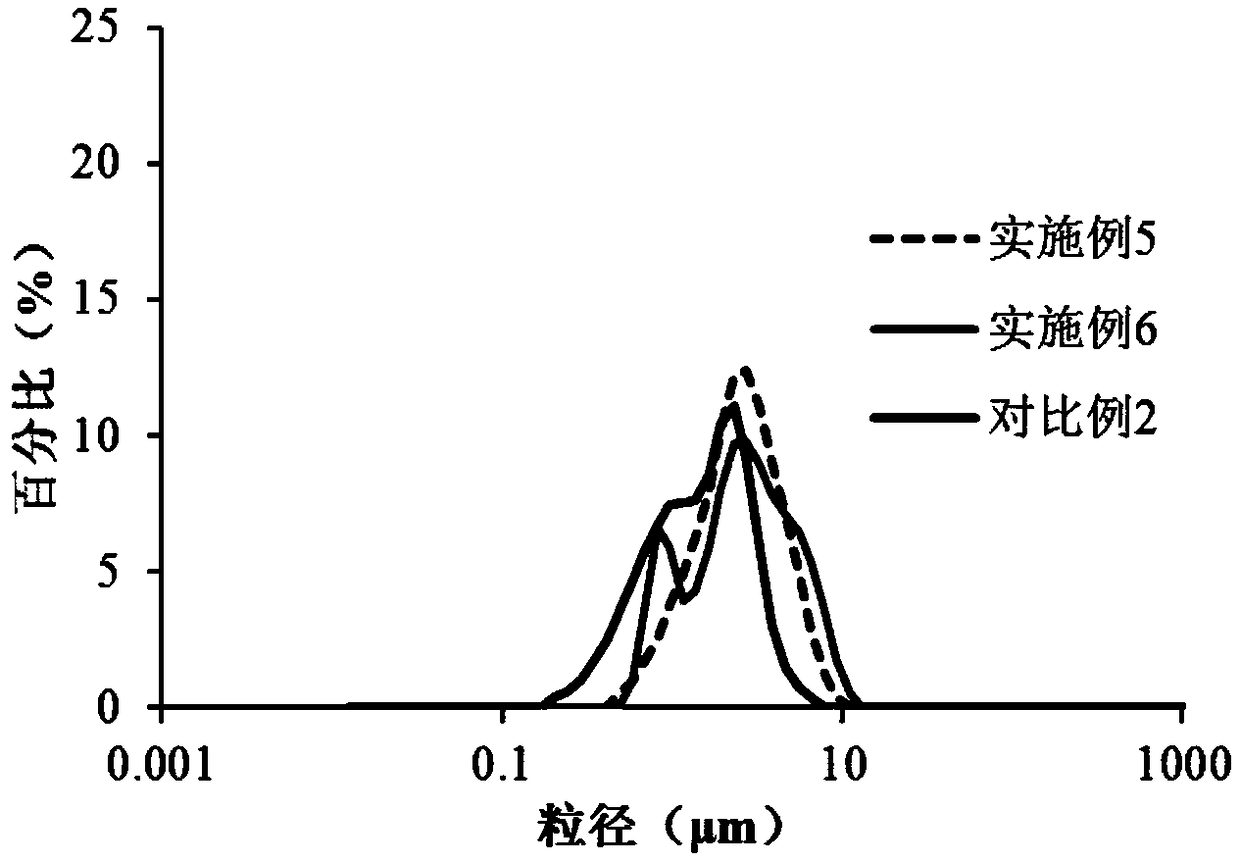
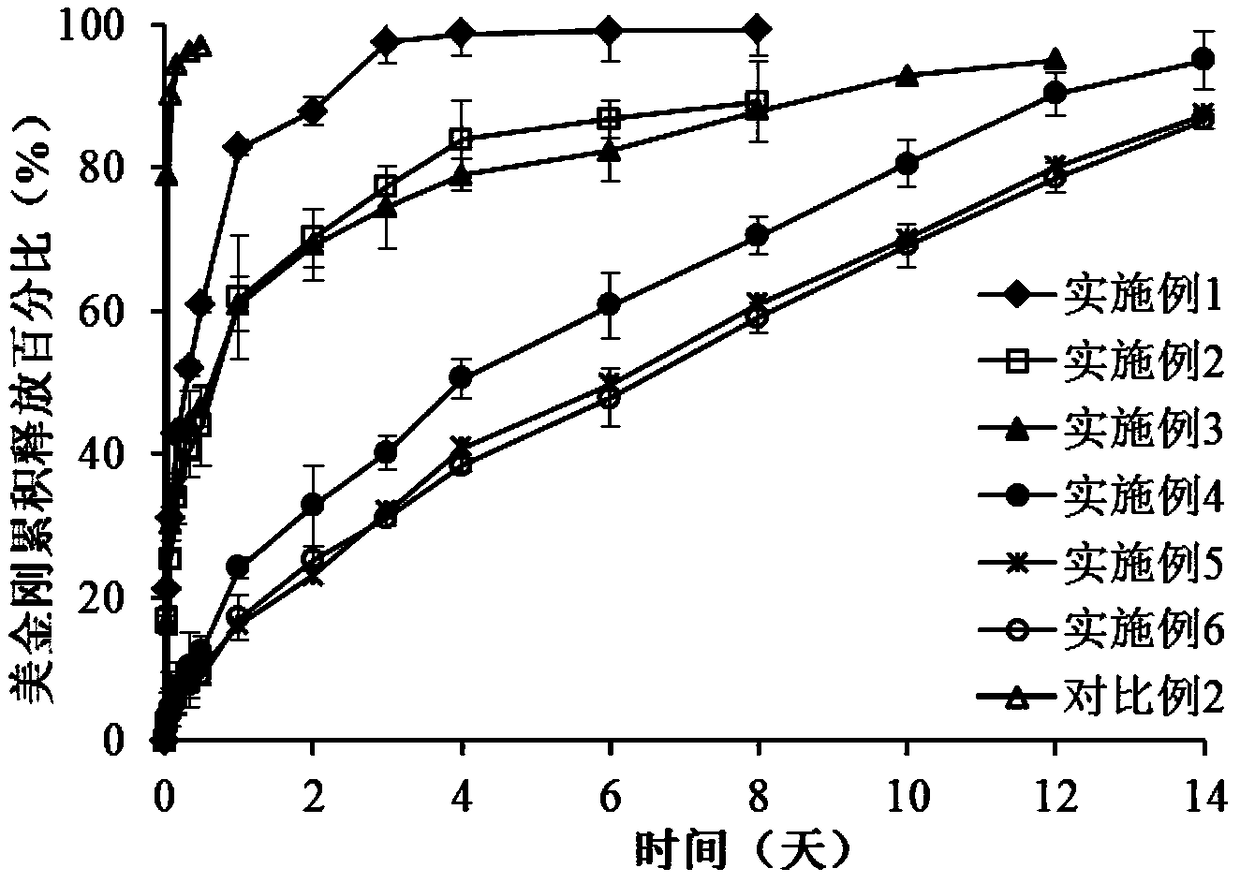
Memantine hydrochloride slow-release dry suspension and preparation method thereof
ActiveCN103417483ASimple preparation processEasy to operatePowder deliveryPharmaceutical product form changeDrug release rateMemantine Hydrochloride
The invention relates to a memantine hydrochloride slow-release dry suspension and a preparation method thereof. The memantine hydrochloride slow-release dry suspension comprises the following materials, by weight: 5-20% of memantine hydrochloride, 10-40% of ion exchange resin, 0.5-10% of a hydrophilic or water-soluble accessory, 5-20% of a dressing material and 10-50% of other accessories. The preparation technology of the memantine hydrochloride slow-release dry suspension is simple, easy for operation and control, and suitable for industrialized large production. The obtained memantine hydrochloride slow-release dry suspension has stable drug release rate and high accumulation drug release rate; after administration, patients can obtain stable plasma concentration level, so as to effectively reduce administration frequency and toxic and side effects; meanwhile, the memantine hydrochloride slow-release dry suspension solves the problems of medication validity, security and compliance for the elderly.
Owner:HAINAN PULIN PHARMA +1
Sustained release preparation containing memantine hydrochloride and huperzine A and preparation method thereof
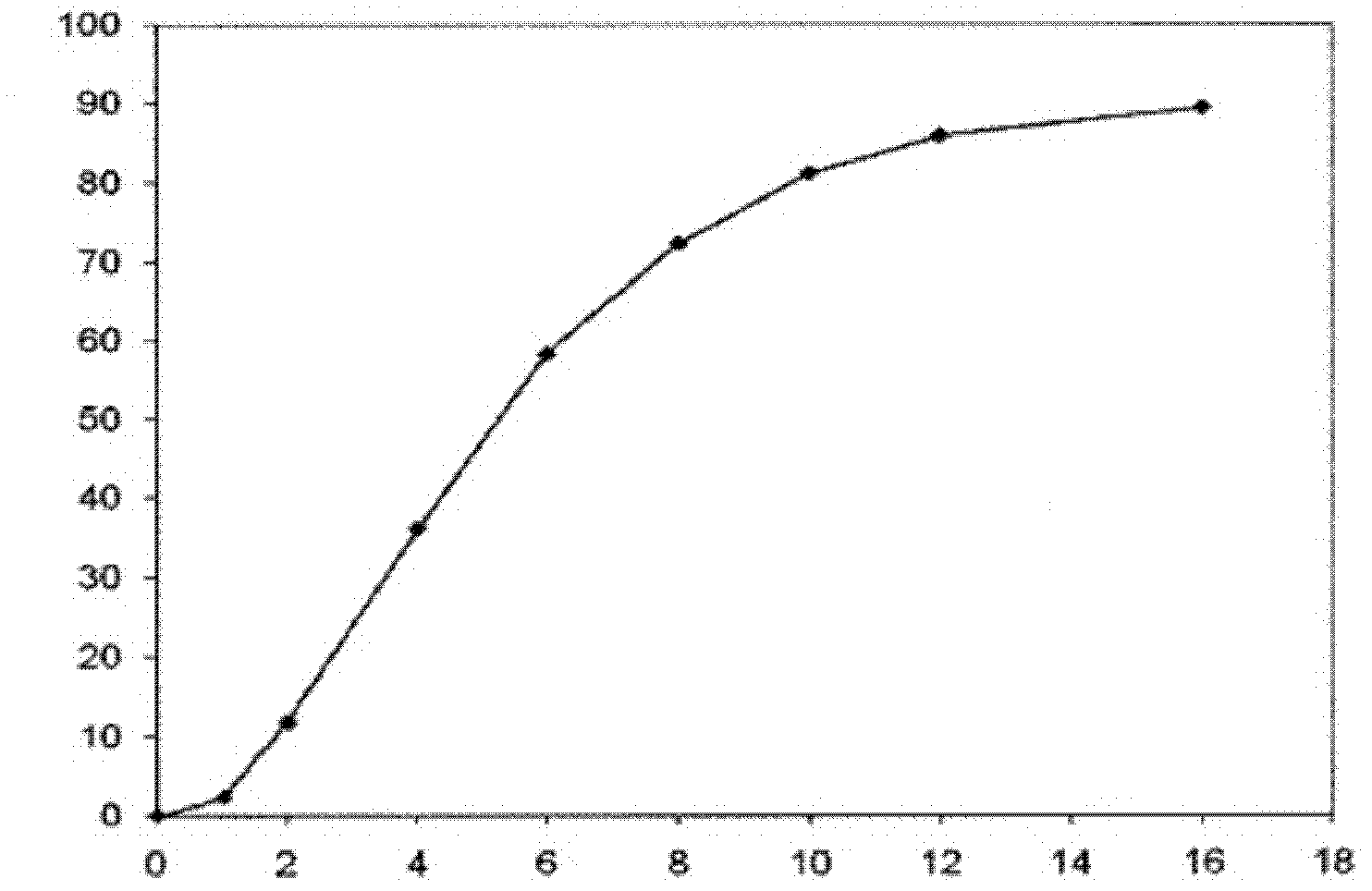
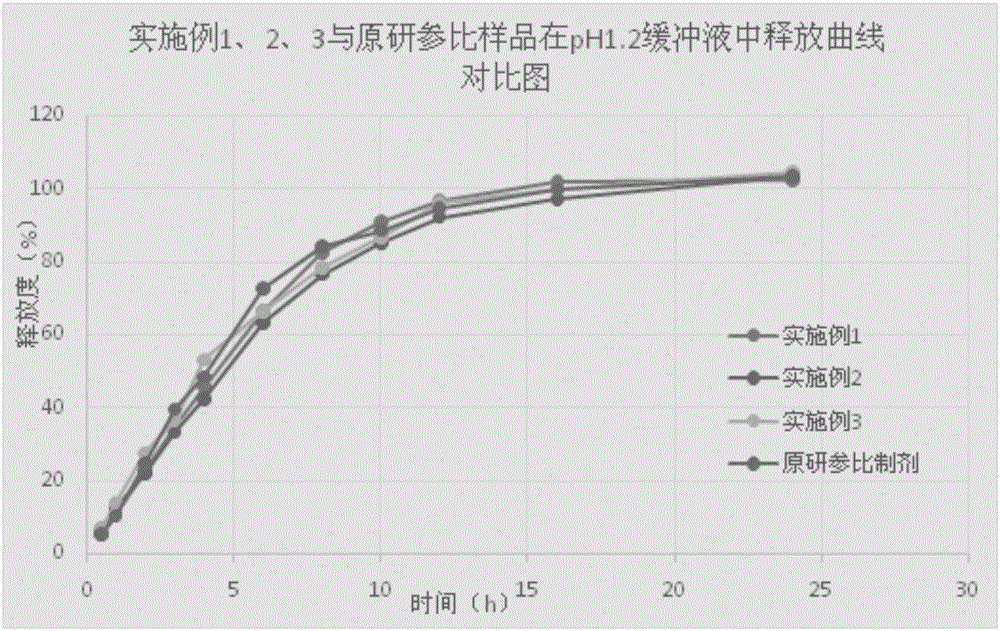
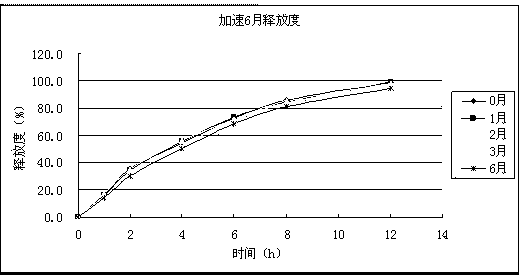
InactiveCN101756970ANervous disorderAmine active ingredientsMemantine HydrochlorideImmediate release
The invention relates to sustained release preparation containing memantine hydrochloride and huperzine A and preparation method thereof. The sustained release preparation includes quick release component and slow release component, wherein the memantine hydrochloride is the quick release component which represents the characteristic of quick release in vitro dissolution test and can be released by more 80% 30min later, while huperzine A represents the characteristic of slow release in vitro dissolution test and is released by 10-30% in the first hour, by 40-60% in 6 hours, by 60-80% in 12 hours and by more than 80% in 18 hours. The sustained release preparation is mild, has long-term effect and can reduce the application time (only once a day) and improve the compliance of the patient. The invention further discloses the vitro release characteristic and the preparation method of the sustained release preparation.
Owner:北京利乐生制药科技有限公司
Method for preparing adamantine disulfide tetroxide derivative
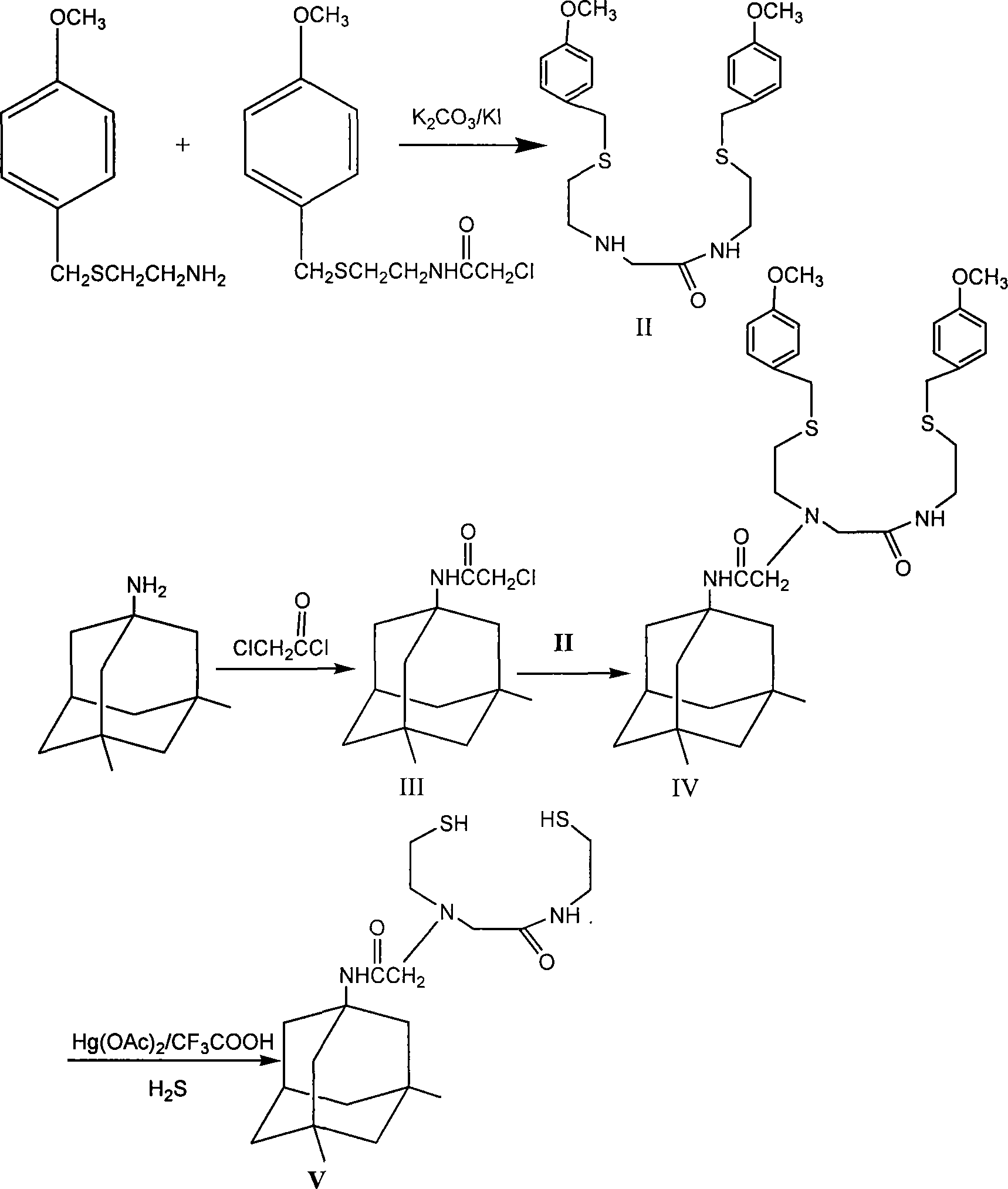
InactiveCN101157644AImprove permeabilityImprove stabilityThiol preparationRadioactive preparation carriersLipid formationSolubility
The invention relates to a preparative method of derivative of memantine hydrochloride alkyl 2-sulfer 2-nitrogen, and belongs to the technical field of synthesis of marking precursor compound for radiopharmaceutical. The invention provides a preparative method of N-[-(N-2-thiolethy)) amino acetyl]-(2- thiolethy)-3, 5-NCAM to prepare the radiopharmaceutical of 99m<Tc-NCAM marked by technetium which is used for image display of brain receptor. Being approved by experiment, the NCAM meets the requirements of permeability about blood brain barrier(BBB) in the aspects such as electroneutrality, molecule size and lipid solubility(LogP). After being mixed with other adjuvant drug and frozen, the NACM is easy to product a medicine box with good stability and a marking rate of above 95 percent. The prepared 99m<Tc-NCAM is tested by an animal distribution in mouse and radiation self-developing test, and the result is that the 99m<Tc-NCAM concentrates on the striaturn and hippocampus of the mouse. With high safety in animal test, the invention is a potential developer of the brain receptor.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Memantine hydrochloride sustained release micro-pill tablets and preparation method thereof
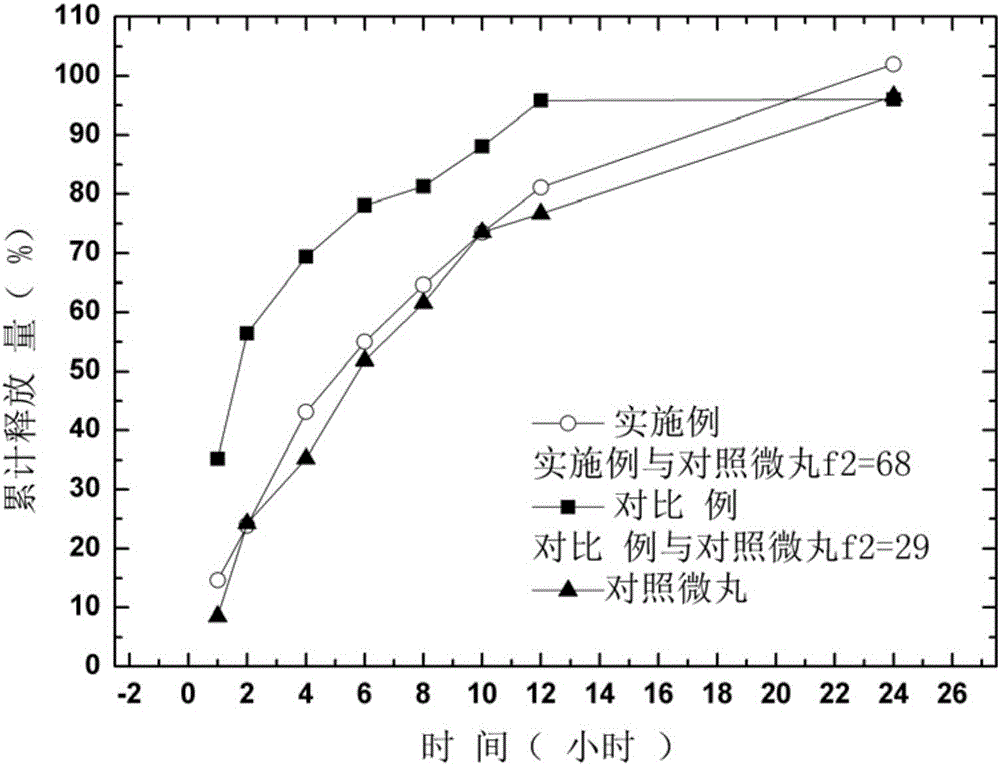
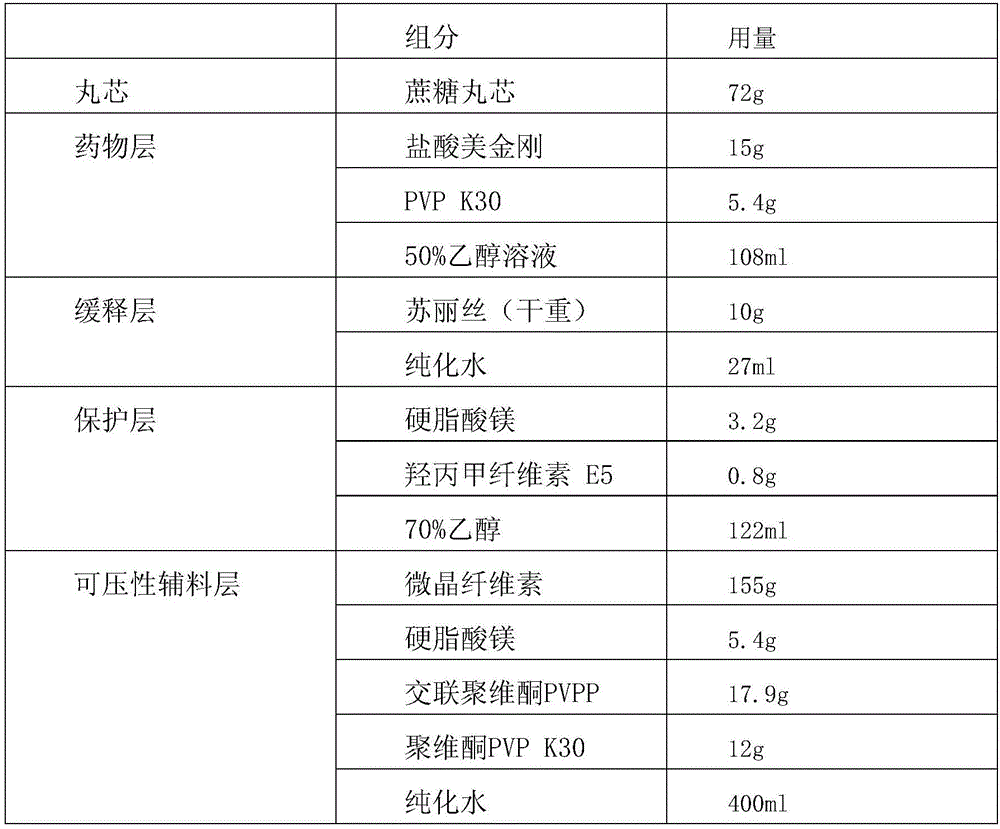
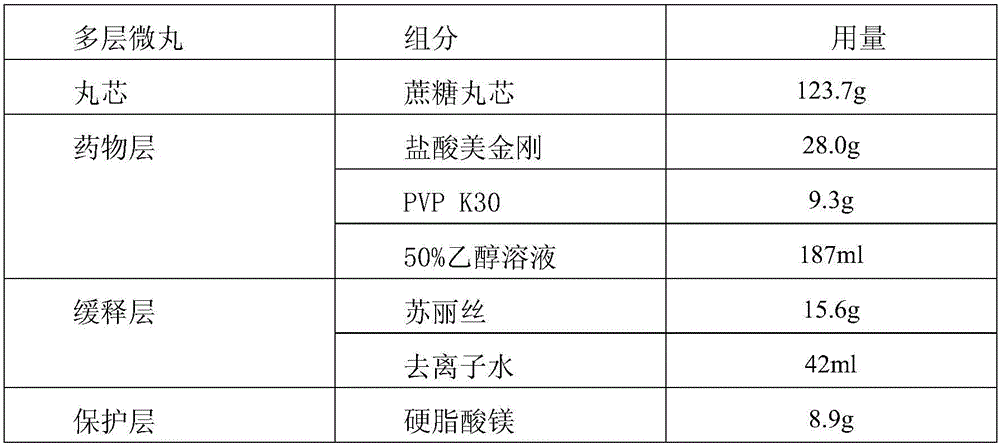
ActiveCN105769794AIntegrity guaranteedGood drug sustained release performanceNervous disorderPill deliveryMemantine HydrochlorideDrug content
The invention provides memantine hydrochloride sustained release micro-pill tablets and a preparation method thereof. The memantine hydrochloride sustained release micro-pill tablets are prepared by directly pressing self-protected multi-layer micro-pills, and a filling agent, a disintegrating agent and other auxiliary tablet materials do not need to be added. The self-protected multi-layer micro-pill is composed of a pill core, a medicine layer, a sustained release layer, a protective layer and a compressible composite auxiliary material layer, can tolerate extrusion of a certain tabletting pressure, and contributes to keeping the integrity of a micro-pill sustained release coating and good sustained release properties of the medicine. The self-protected multi-layer micro-pill also has high compressibility, disintegration and lubricating property and can be directly pressed into tablets, and the prepared memantine hydrochloride sustained release micro-pill tablets have good drug content uniformity.
Owner:BEIJING UNIV OF CHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com