Process for the preparation of 1-amino-3,5-dimethyladamantane hydrochloride
A technology of dimethyladamantane and dimethyladamantine, applied in the field of preparation of 1-amino-3,5-dimethyladamantane hydrochloride, which can solve problems such as danger
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
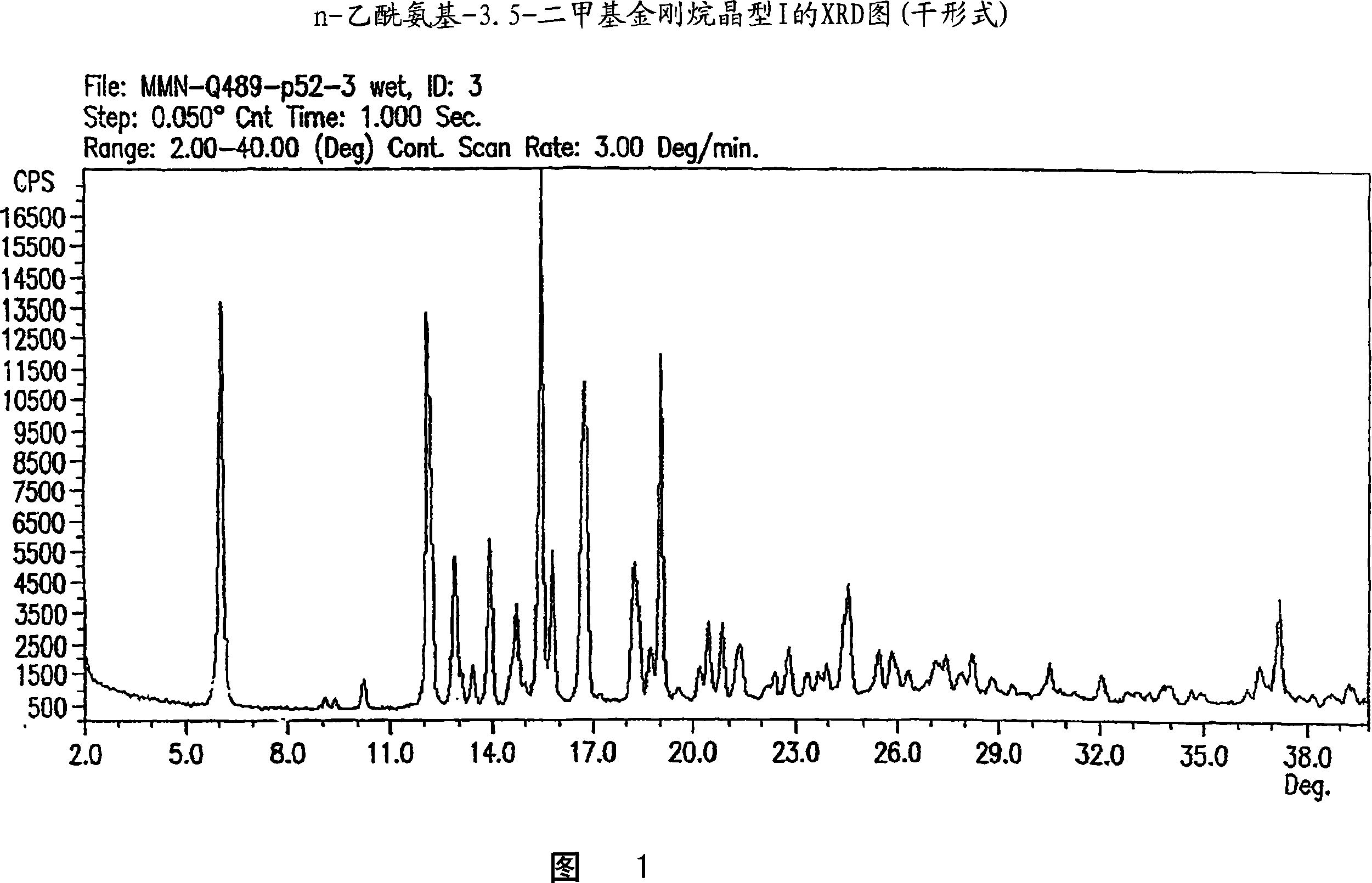
Image
Examples
Embodiment 1-1
[0068] Example 1-Synthesis of 1-Acetylamino-3,5-Dimethyladamantane
[0069] The following examples are used to illustrate the embodiments of the present invention.
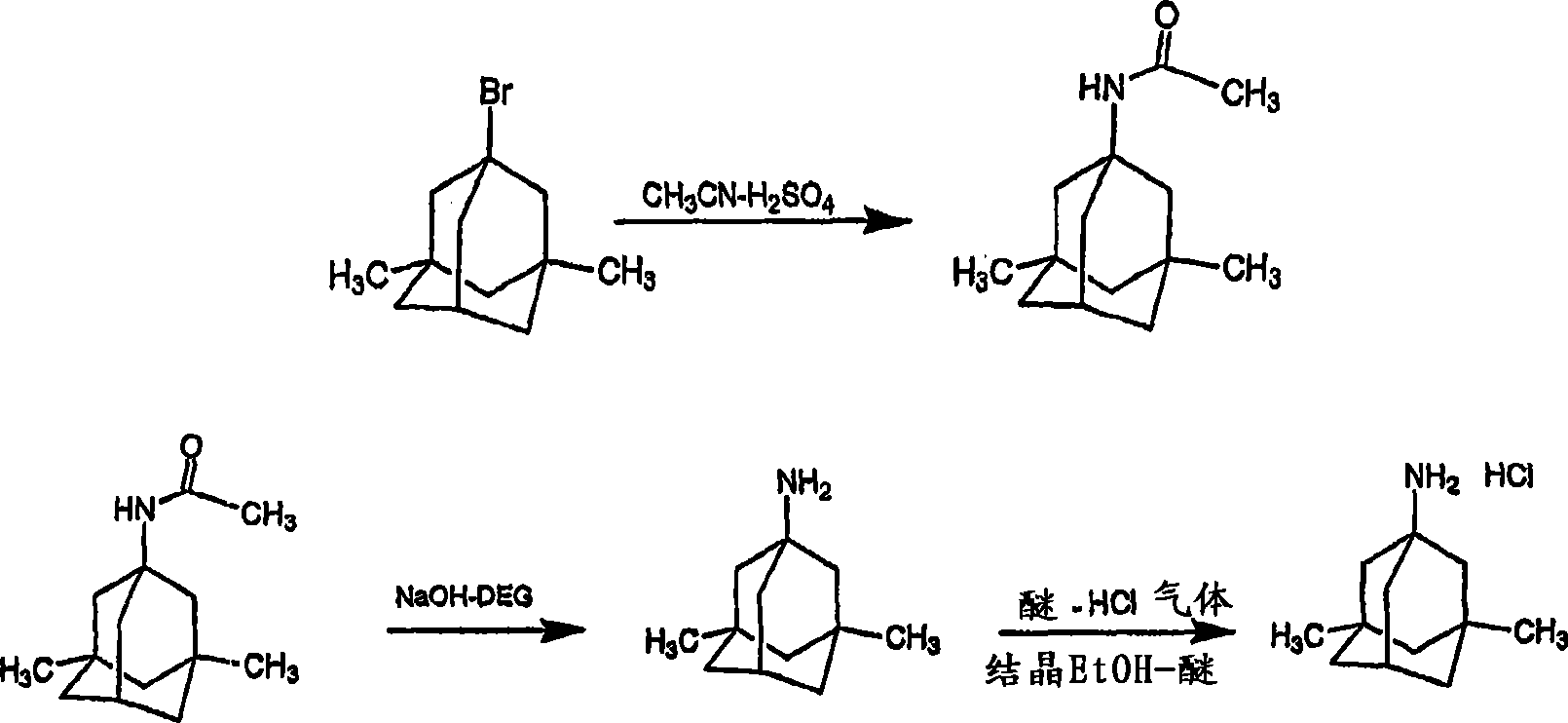
[0070] The first step in the synthesis of 1-acetylamino-3,5-dimethyladamantane follows the following synthesis scheme.
[0071]
[0072] At room temperature, 500 g (2.056 moles) of 1-bromo-3,5-dimethyladamantane and 500 ml of 393 g (9.6 moles) of acetonitrile are introduced into a three-necked round bottom equipped with a thermometer, a condenser and a mechanical stirrer In the flask. Then, 806 grams of 75% phosphoric acid (6.2 moles) was added dropwise in about 15 minutes, keeping the temperature below 40°C. The resulting two-phase mixture was heated to 87±2°C and kept at this temperature for 3 hours.
[0073] 1000 ml of n-butanol and 770 ml of water are added to produce a homogeneous solution. Sodium hydroxide 30% (337.5 grams) was added to create a two-phase system. The phases were separated, and 350 ml of wate...
Embodiment 3
[0075] Example 3-Synthesis of memantine free base from n-acetyl-memantine
[0076] At room temperature, 10 g (0.045 mol) of n-acetyl-memantine, 75 ml of n-butanol and 10.5 g of sodium hydroxide flakes (0.263 mol) were introduced into a three-necked round bottom flask equipped with a thermometer, condenser and mechanical stirrer in. The resulting suspension was heated to 120°C and kept at 120°C for 10 hours. The reaction mixture was cooled to 80°C, and 25 ml of water was added. Separate the phases, add 25 ml of water to the rich organic phase, and adjust the pH to 10.5-11 with 37% hydrochloric acid. After stirring, the phases are separated, and 25 ml of water is added to the rich organic phase. After stirring, the phases were separated again. The resulting organic phase of butanol was concentrated in vacuo at an internal temperature of 70°C until an oily residue (10.5 g) was obtained to obtain memantine free base.
[0077] (Optionally add 100 ml of ethyl acetate to the oily resid...
Embodiment 4
[0079] Example 4-Synthesis of Memantine Hydrochloride from n-Acetyl Memantine
[0080] According to the following synthesis scheme, steps 2 and 3 of synthesizing 1-amino-3,5-dimethyladamantane synthesize memantine hydrochloride.
[0081]
[0082] At room temperature, 50 g (0.2259 mol) of n-acetyl-memantine, 200 ml of n-butanol, and 80.29 g of 89.9% potassium hydroxide flakes (1.288 mol) were introduced into the three-neck equipped with thermometer, condenser and mechanical stirrer Round bottom flask. The resulting suspension was heated to 130±2°C and kept at 130±2°C for 10-11 hours. The reaction mixture was cooled to 50°C, and 150 ml of water was added. Separate the phases, add 75 ml of water to the rich organic phase, and adjust the pH to 10.5-11 with 37% hydrochloric acid. After stirring, separate the phases and add 75 ml of water to the organic rich phase. After stirring, the phases were separated again.
[0083] The resulting solution was filtered, and 23.1 g of 37% hydroc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com