Memantine hydrochloride sustained-release capsule and preparation method thereof
A technology of memantine hydrochloride and sustained-release capsules, applied in microcapsules, capsule delivery, pharmaceutical formulations, etc., can solve problems such as adverse events, poor compressibility of lipid substances, and reduced patient compliance, so as to improve curative effect, uniform and slow The effect of simple and easy release and production method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] First crush memantine hydrochloride to particle size D with a jet mill 90 Less than 10 μm, micronized memantine hydrochloride was obtained.
[0046] (1) Dosing: Dissolve 50g of micronized memantine hydrochloride and 50g of ethyl cellulose in 400g of ethanol, and use Glatt's fluidized bed to spray the drug-containing solution to the On the core of 400g sucrose balls, obtain memantine hydrochloride drug-containing pellets.
[0047] (2) Coating: Evenly disperse 50g of talcum powder in 183g of water to form a uniform suspension of talc powder, and mix it with 167g of Eudragit NE30D (copolymer of ethyl acrylate and methyl methacrylate (2:1), Solid content is 30% water dispersion) water dispersion is stirred and mixed uniformly, gets 400g from the medicine-containing pellet that step (1) makes, uses the fluidized bed of Glatt to spray coating with this coating liquid at 25 ℃ of temperature to obtain coated sustained-release pellets.
[0048] (3) Aging: Mix the slow-release...
Embodiment 2
[0053] (1) drug application: 50g memantine hydrochloride and binder 75g ethyl cellulose are dissolved in 500g of ethanol, and the solvent spray is applied to the 375g sucrose ball core at a temperature of 28°C with the fluidized bed of Glatt, Dry at 28°C for 5-10 minutes to obtain the drug-coated pellets.
[0054] (2) Coating: Evenly disperse 36g of talc powder and 7.2g of triethyl citrate in 237g of water to form a uniform suspension of talc powder, with 108g of polyacrylic acid resin polymer - Eudragit RS 30D and 12g of Eudragit RL30D (both are water dispersions with a solid content of 30%) stirring and mixing evenly, spray the coating solution onto 420g drug-loaded pellets with the fluidized bed of Glatt at a temperature of 25°C-30°C, to obtain sustained-release pellets.
[0055] (3) Preparation of capsules: mix the slow-release pellets and talcum powder evenly in a weight ratio of 50:1, sieve, sieve off excess talcum powder, and pack about 167 mg of pellets into hard gelat...
Embodiment 3
[0058] First crush memantine hydrochloride to particle size D with a jet mill 90 Less than 10 μm, micronized memantine hydrochloride was obtained.
[0059] (1) Suspension dosing: 50g of micronized memantine hydrochloride and 100g of binder polyvinylpyrrolidone, 25g of anti-sticking agent talcum powder are evenly dispersed in 800g of water, and the fluidized bed of Glatt is used at a temperature of 40°C The solvent is sprayed onto the core of 325g sucrose pellets to obtain the pellets.
[0060] (2) Coating: the ethyl cellulose (10cps) of 60g and the hydroxypropyl methylcellulose of 15g are dissolved in ethanol, spray on the 425g medicine pellet with this coating liquid at the temperature of 28 ℃, obtain Slow-release pellets.
[0061] (3) Preparation of capsules: the sustained-release pellets were packed into hard gelatin capsules in a conventional manner.
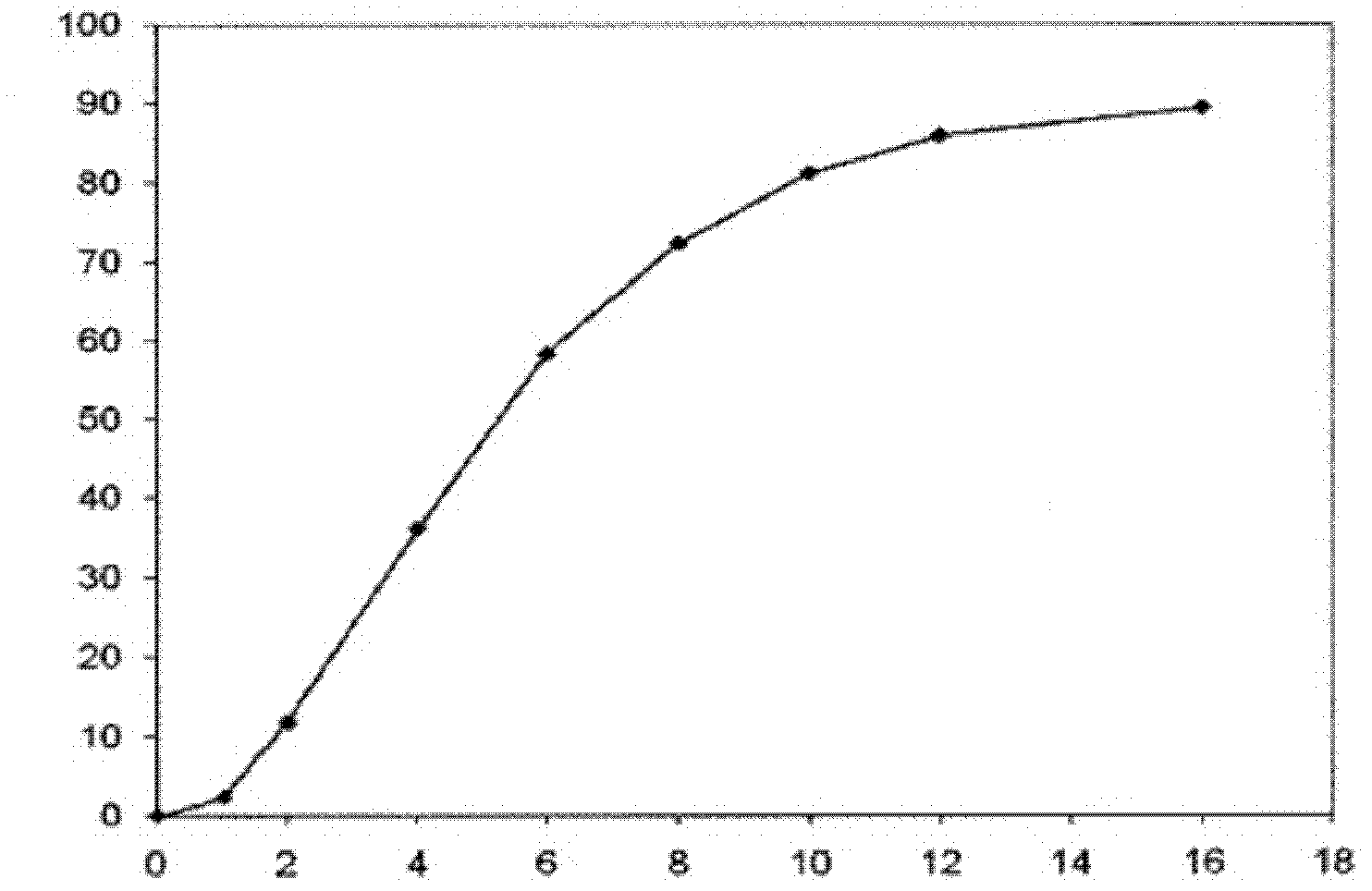
[0062] Content of Memantine Hydrochloride in Shangyao Pellets
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com