Memantine insoluble salt sustained-release injection and preparation method thereof
A technology of sustained-release injections and memantine, applied in the field of medicine, to achieve excellent sustained-release effects, reduce social burdens, and facilitate industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
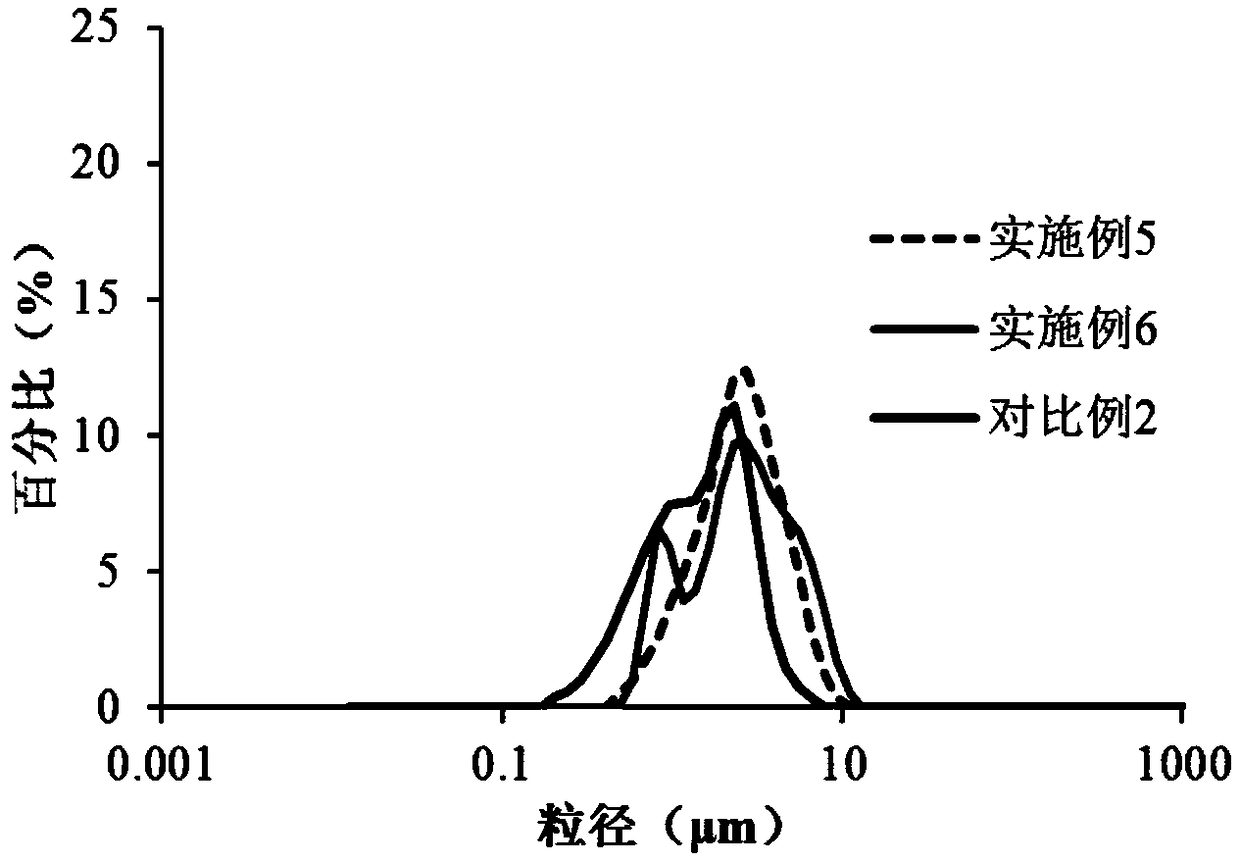
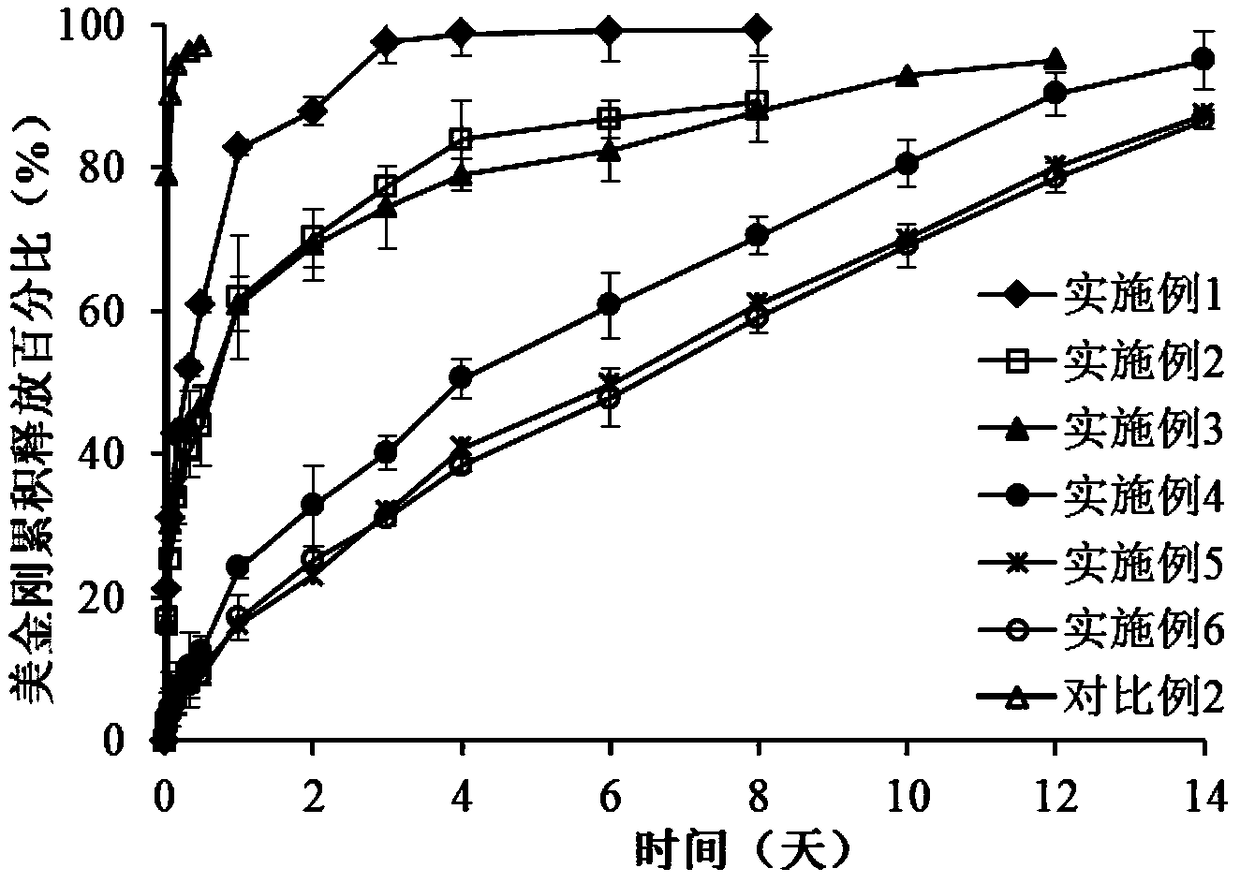
Image
Examples
Embodiment 1
[0034] Preparation of memantine-stearate sustained-release injection:
[0035] A. Synthesis of memantine-stearate
[0036] Weigh an appropriate amount of sodium stearate, disperse it into 100mL of 75°C distilled water, stir to dissolve, and prepare a sodium stearate aqueous solution with a concentration of 0.1mol / L; weigh an appropriate amount of memantine hydrochloride, dissolve it in 100mL of 75°C distilled water, A memantine hydrochloride aqueous solution with a concentration of 0.1 mol / L was obtained. At 75°C, under stirring, add the above memantine hydrochloride aqueous solution into the sodium stearate aqueous solution dropwise, centrifuge at 10,000r / min for 10min, filter with suction, collect the precipitate, wash with hot water three times, and vacuum-dry the obtained filter cake at 40°C for 24h , that is, memantine-stearate.
[0037] B. Preparation of Memantine-Stearate Sustained Release Injection
[0038] 25g of memantine-stearate (calculated as memantine) is disp...
Embodiment 2
[0040] Preparation of memantine-palmitate extended-release injection:
[0041] A. Synthesis of memantine-palmitate
[0042] Weigh an appropriate amount of sodium palmitate, disperse it into 100mL of 75°C distilled water, stir to dissolve, and prepare a sodium palmitate aqueous solution with a concentration of 0.1mol / L; weigh an appropriate amount of memantine hydrochloride, dissolve it in 100mL of 75°C distilled water, and obtain the concentration 0.1mol / L memantine hydrochloride aqueous solution. At 75°C, under stirring, add the above-mentioned memantine hydrochloride aqueous solution into the sodium palmitate aqueous solution dropwise, centrifuge at 10,000r / min for 10min, filter with suction, collect the precipitate, wash with hot water three times, and vacuum-dry the obtained filter cake at 40°C for 24h. That is, memantine-palmitate.
[0043] B. Preparation of memantine-palmitate sustained-release injection
[0044] Disperse 25g of memantine-palmitate (calculated as mema...
Embodiment 3
[0046] Preparation of Memantine-Oleate Sustained Release Injection:
[0047] A. Synthesis of memantine-oleate
[0048] Weigh an appropriate amount of sodium oleate, disperse it into 100mL of 75°C distilled water, stir to dissolve, and prepare a sodium oleate aqueous solution with a concentration of 0.1mol / L; weigh an appropriate amount of memantine hydrochloride, dissolve it in 100mL of 75°C distilled water, and obtain the concentration 0.1mol / L memantine hydrochloride aqueous solution. At 75°C, under stirring, add the above-mentioned memantine hydrochloride aqueous solution into the sodium oleate aqueous solution dropwise, centrifuge at 10000r / min for 10min, collect the oily viscous liquid in the upper layer, wash it with hot water three times, and vacuum the obtained oily viscous liquid at 40°C Dry for 24 hours to obtain memantine-oleate.
[0049] B. Preparation of Memantine-Oleate Sustained Release Injection
[0050] Disperse 25 g of memantine-oleate (calculated as meman...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com