Dihydroarteannuin-memantine diad compounds, and synthesis method and application thereof
A technology of dihydroartemisinin and bromoalkyldihydroartemisinin, which is applied in the field of medicinal chemistry, can solve the problems of lack of therapeutic drugs, etc., and achieve the effect of simple preparation process and excellent curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
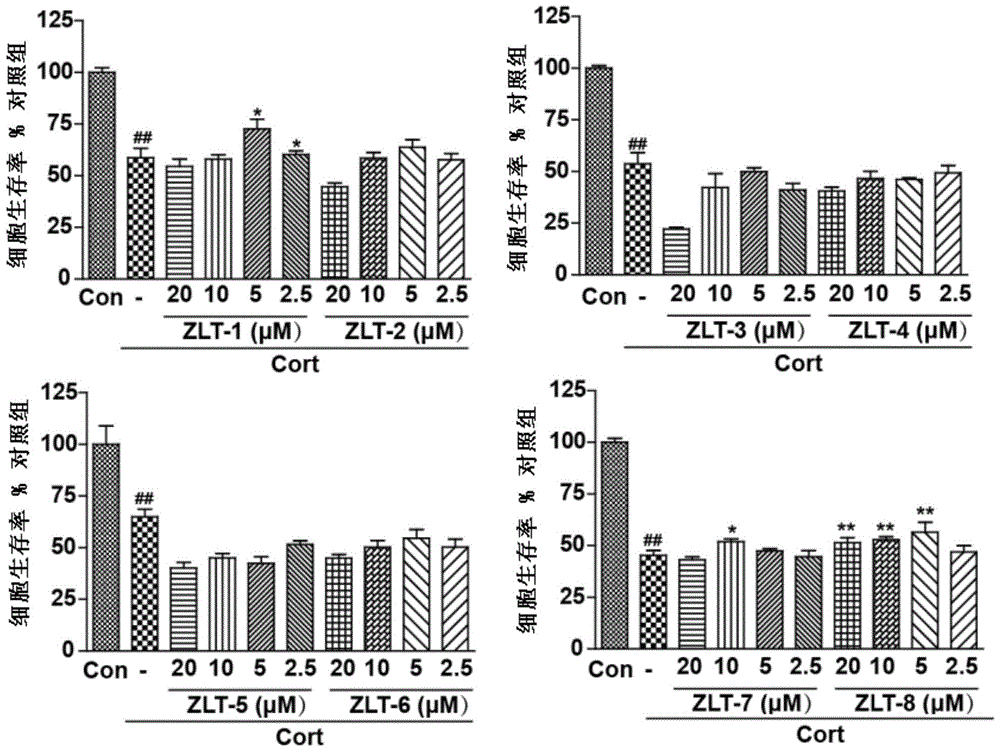
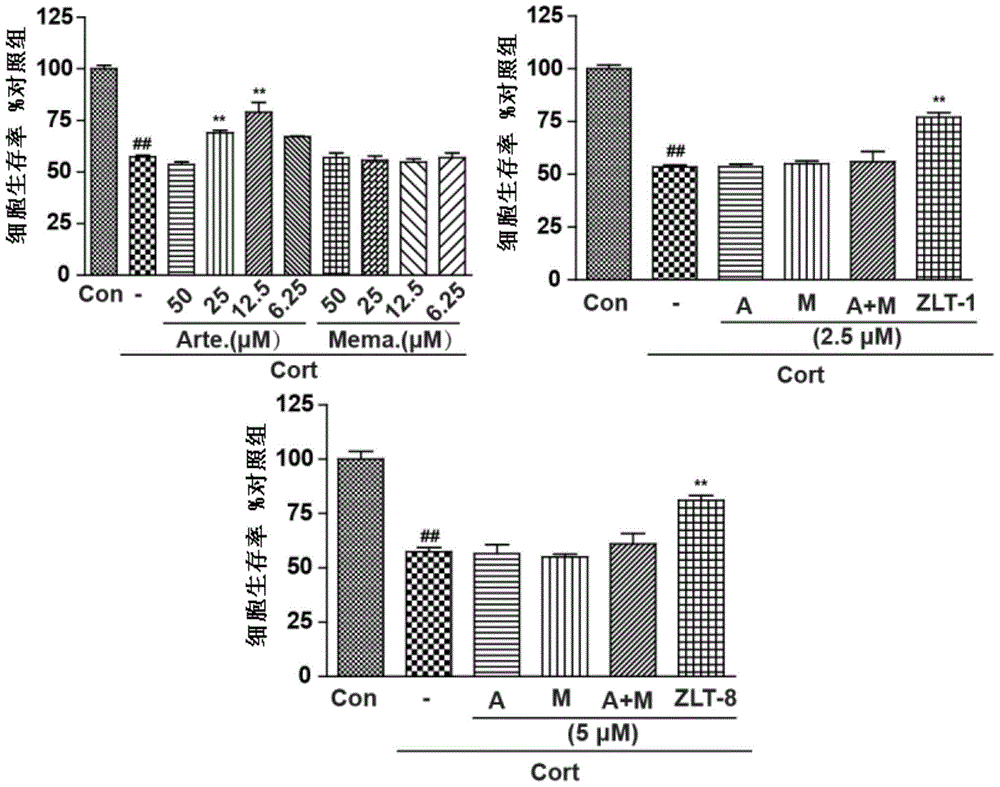
[0046] The synthesis of 12-beta-12-O-(2-memantine amino) ethyl dihydroartemisinin (ZLT-1) comprises the following three steps:
[0047] (1) Preparation of dihydroartemisinin: Dissolve artemisinin (565mg, 2.0mmol) in 50mL methanol, pre-cool in ice bath for 5min, then add NaBH in batches 4 (151mg, 4.0mmol), the reaction was continued for 3h under ice-bath conditions. After the reaction, neutralize excess NaBH with 50% AcOH / MeOH under ice-bath condition 4 , adjust the pH value to 6-7 until no more bubbles are produced. Remove methanol, add cold water, stir for 15 minutes, filter with suction, and wash the filter cake with water. The product was dissolved in DCM and dried over anhydrous sodium sulfate to obtain 482 mg of flocculent white solid with a yield of 85%.
[0048]The analytical data of the product are as follows: 1 HNMR (300MHz, CDCl 3 )δ:0.91-0.95(m,12H),1.22-1.25(m,1H),1.25-1.29(m,2H),1.29-1.32(m,1H),1.41(d,J=3.0Hz,6H) ,1.45-1.52(m,3H),1.63-1.68(m,2H),1.68-1.91(m,...
Embodiment 2
[0055] The synthesis of 12-β-12-O-(4-memantine amino) n-butyldihydroartemisinin (ZLT-2) comprises the following four steps:
[0056] (1) Preparation of dihydroartemisinin: steps are the same as step (1) in Example 1;
[0057] (2) Preparation of 4-bromobutanol: Dissolve 1,4-butanediol (1.8g, 20mmol) in 60mL of benzene, add HBr (2.4mL, 20mmol), and reflux for 2h. After the reaction, the benzene in the benzene layer was removed by rotary evaporation. The resulting substance was subjected to column chromatography [V (petroleum ether): V (ethyl acetate) = 5:1] to obtain 0.3 g of a colorless oil. Yield 10%.
[0058] The analytical data of the product are as follows: 1 HNMR (CDCl 3 ,300MHz)δ:1.56-1.68(m,2H),1.80-1.92(m,2H),3.37(t,J=6.9Hz,2H),3.57(t,J=6.6Hz,2H),3.98(s ,1H). The above data prove that this compound is 4-bromobutanol;
[0059] (3) Preparation of 12-β-O-(4-bromo-n-butyl)dihydroartemisinin: the steps are the same as step (2) in Example 1 to obtain a colorless oily l...
Embodiment 3
[0065] The synthesis of 12-β-12-O-(5-memantine amino) n-pentyldihydroartemisinin (ZLT-3) comprises the following four steps:
[0066] (1) Preparation of dihydroartemisinin: the steps are the same as step (1) in Example 1.
[0067] (2) Preparation of 5-bromo-n-pentanol: Dissolve (300mg, 2mmol) 1,5-pentanediol in 10mL of toluene, add HBr (0.3mL, 2.4mmol), and reflux for 36h. Spin-dry the toluene layer to obtain Colorless oily liquid.
[0068] The analytical data of the product are as follows: 1 HNMR (CDCl 3 ,300MHz)δ:1.32-1.50(m,4H),1.69-1.81(m,2H),3.30(t,J=6.6Hz,2H),3.48(t,J=6.3Hz,2H),4.21(s ,1H). The above data prove that the compound is 5-bromo-n-pentanol.
[0069] (3) Preparation of 12-β-O-(5-bromo-n-pentyl)dihydroartemisinin: the steps are the same as step (2) in Example 1 to obtain a colorless oily liquid.
[0070] The analytical data of the product are as follows: 1 HNMR (CDCl 3 ,300MHz)δ:0.82(d,J=7.2Hz,3H),0.88(d,J=6.0Hz,3H),1.07-1.21(m,2H),1.22-1.29(m,1H),1.33-1.3...
PUM
| Property | Measurement | Unit |
|---|---|---|
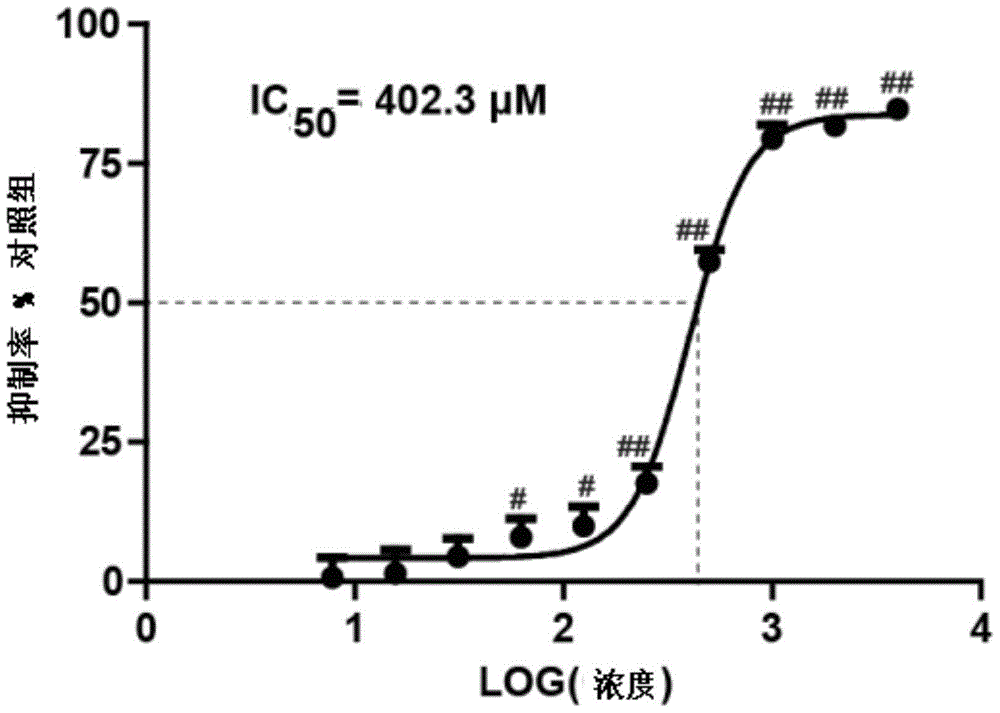
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com