Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
155 results about "Spinal muscular atrophy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A genetic neuromuscular disorder that affects motor neurons in the spinal cord causing progressive muscle degeneration and weakness.
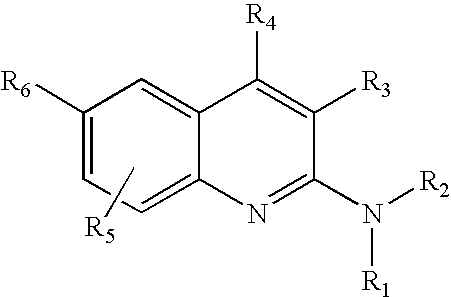
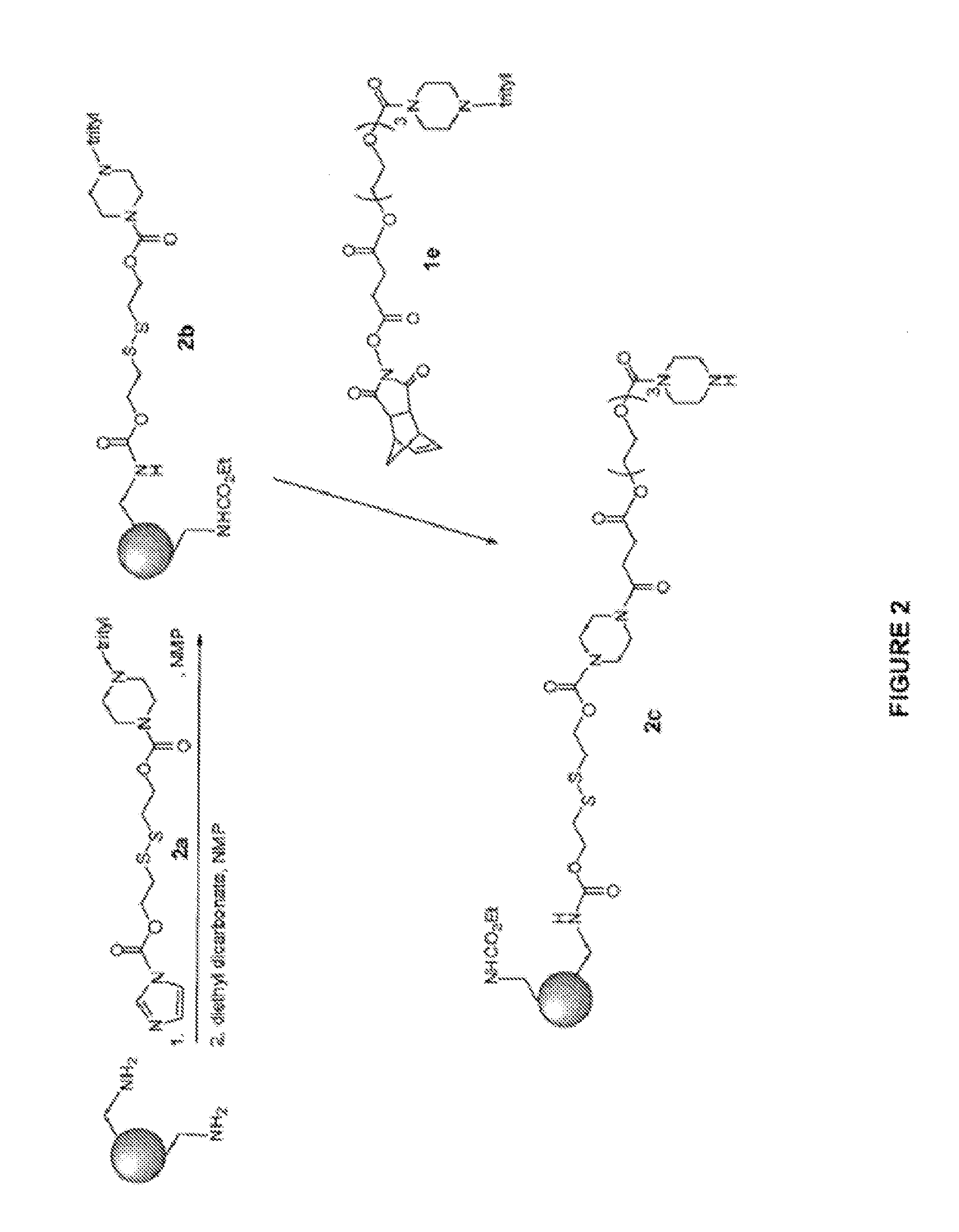
4-Aminoquinoline compounds
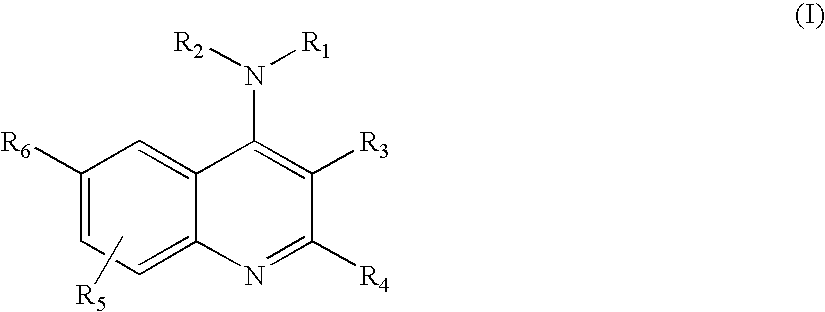
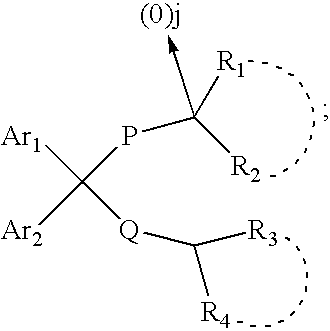
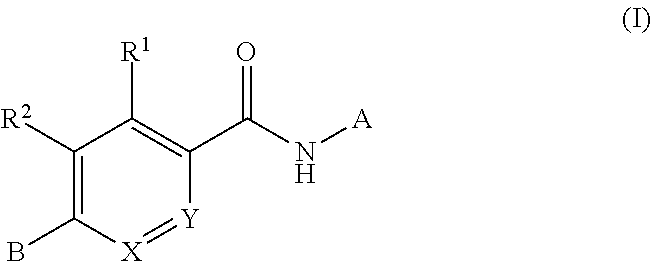
The present invention is concerned with compounds of the general Formula I: and pharmaceutically acceptable salts thereof, which are useful as melanin concentrating hormone receptor antagonists, particularly MCH-1R antagonists. As such, compounds of the present invention are useful for the treatment or prevention of obesity or eating disorders associated with excessive food intake and complications thereof, osteoarthritis, certain cancers, AIDS wasting, cachexia, frailty (particularly in elderly), mental disorders stress, cognitive disorders, sexual function, reproductive function, kidney function, locomotor disorders, attention deficit disorder (ADD), substance abuse disorders and dyskinesias, Huntington's disease, epilepsy, memory function, and spinal muscular atrophy. Compounds of formula I may therefore be used in the treatment of these conditions, and in the manufacture of a medicament useful in treating these conditions. Pharmaceutical formulations comprising one of the compounds of formula (I) as an active ingredient are disclosed, as are processes for preparing these compounds.
Owner:DEVITA ROBERT J +5
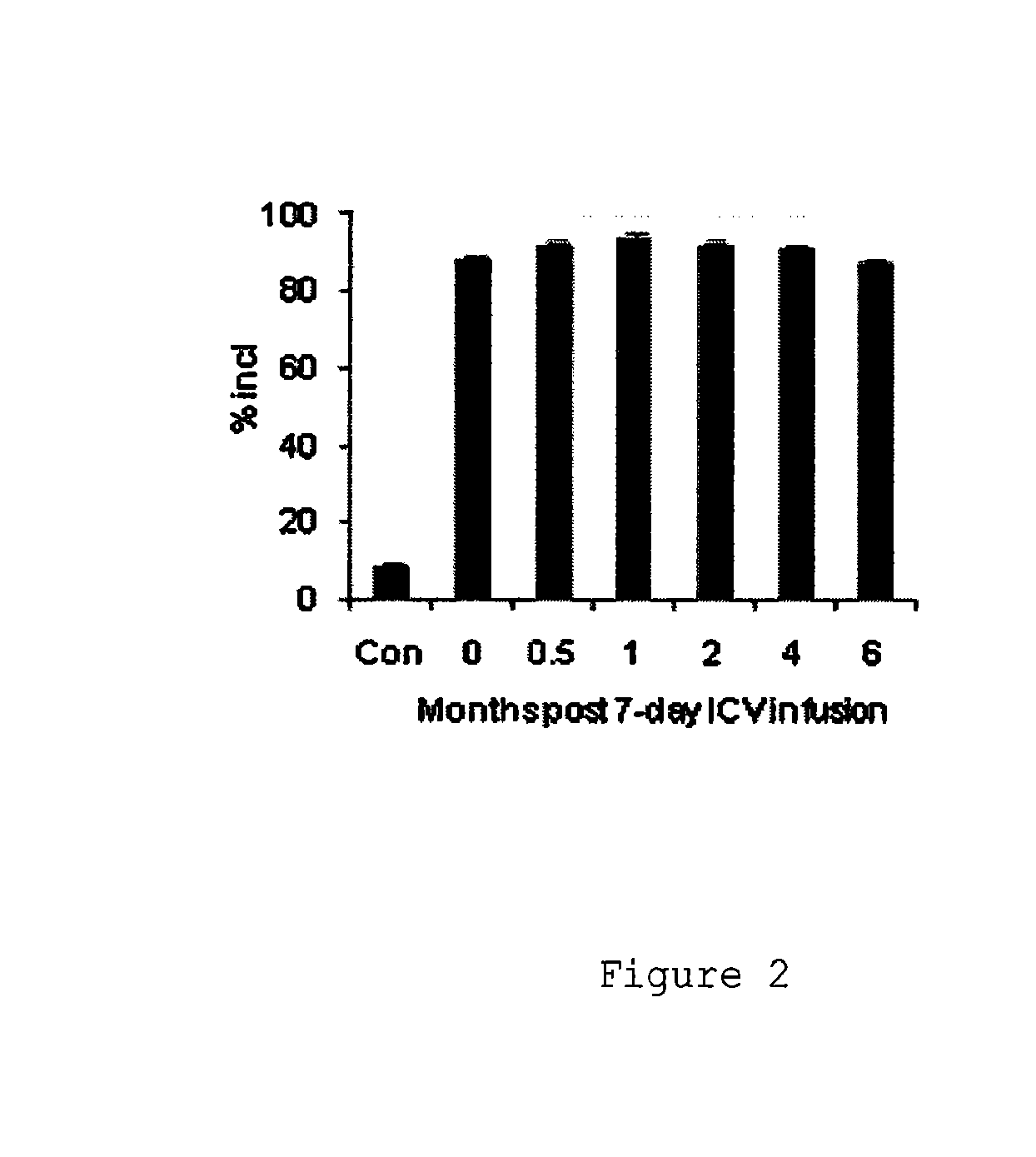
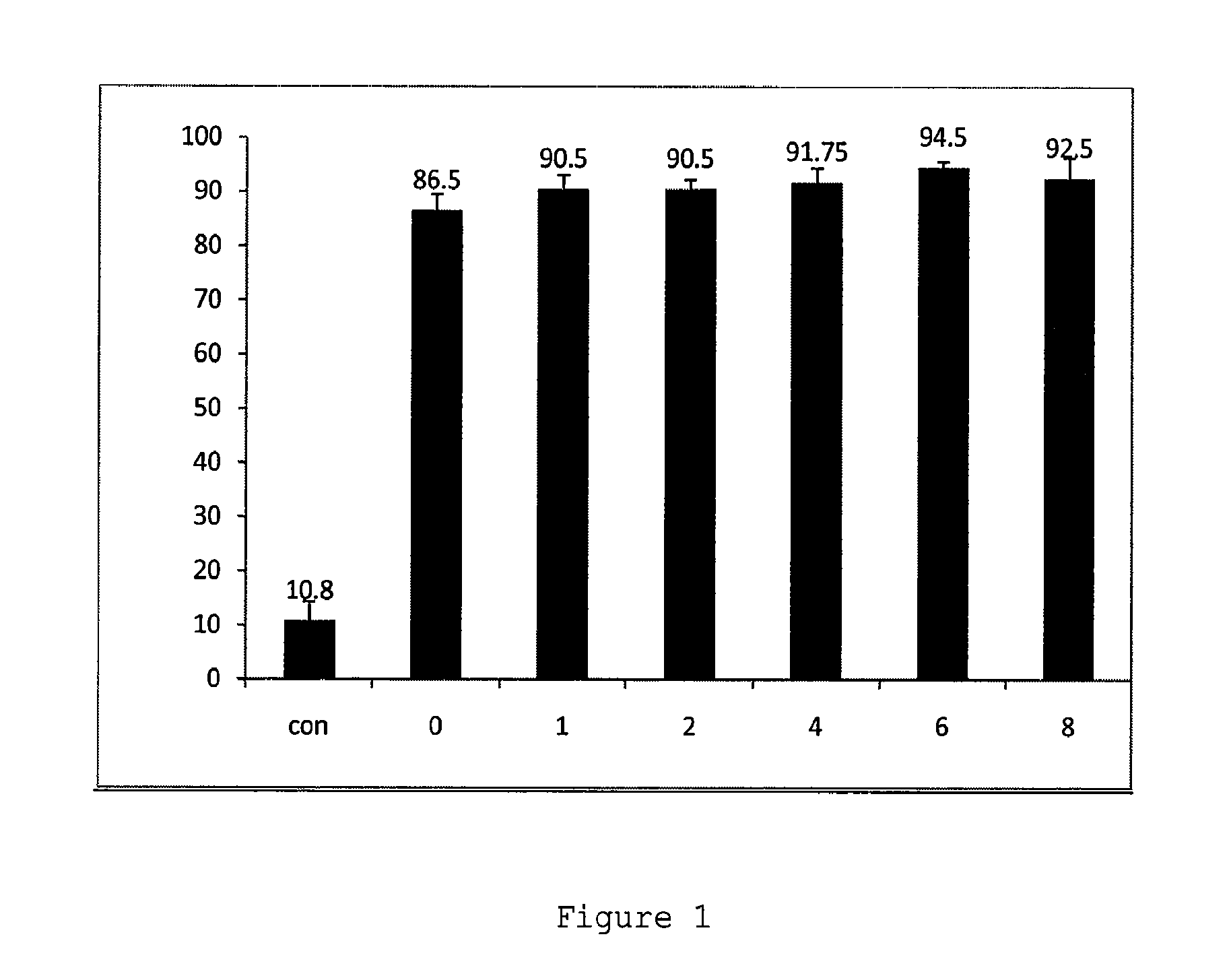
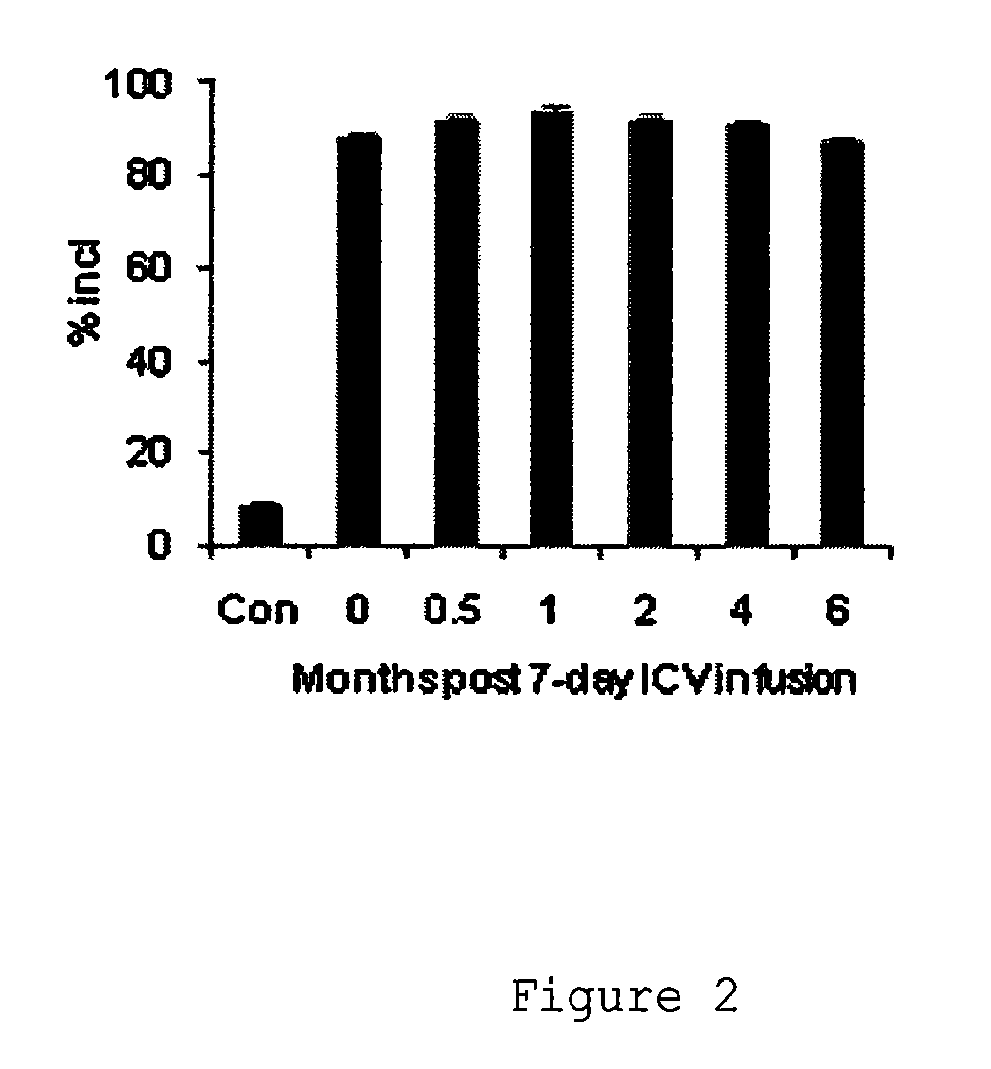
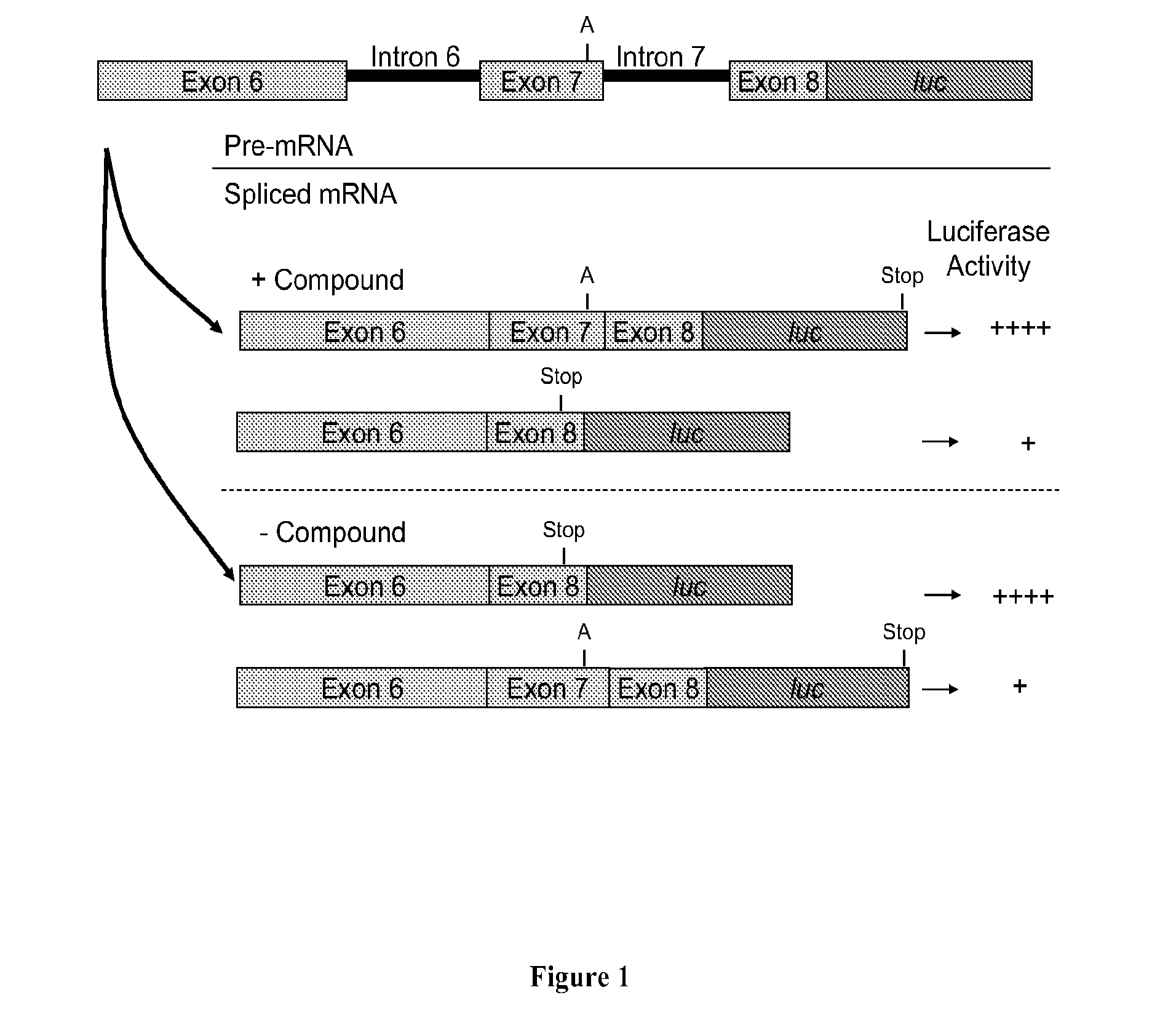
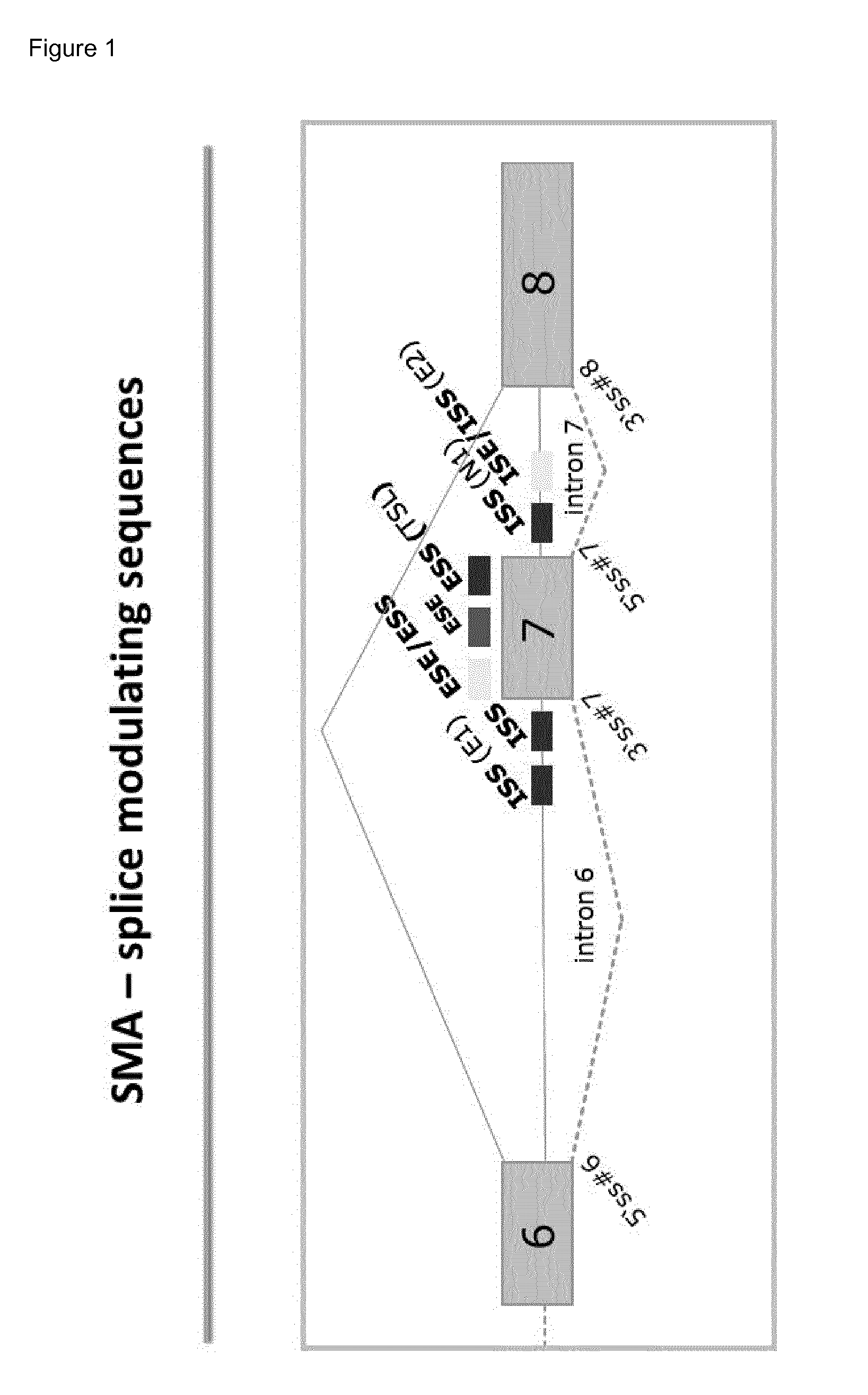
Compositions and methods for modulation of smn2 splicing in a subject
ActiveUS20120190728A1Increase inclusivenessOrganic active ingredientsSplicing alterationDiseasePharmaceutical drug
Disclosed herein are compounds, compositions and methods for modulating splicing of SMN2 mRNA in a subject. Also provided are uses of disclosed compounds and compositions in the manufacture of a medicament for treatment of diseases and disorders, including spinal muscular atrophy.
Owner:BIOGEN MA INC +1
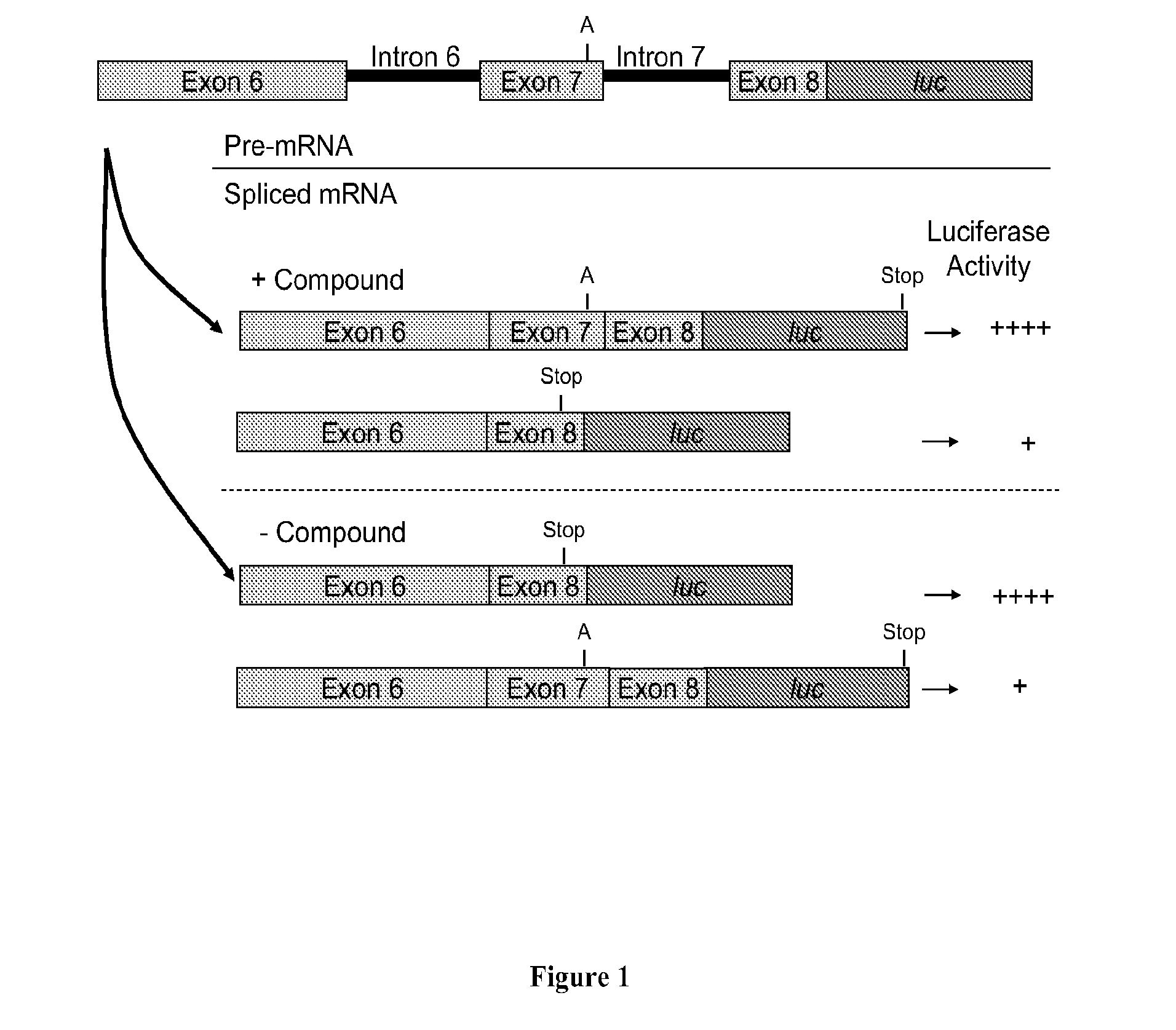
Methods for treating spinal muscular atrophy
InactiveUS20110086833A1Increase inclusivenessImprove the level ofBiocideNervous disorderExonNucleic acid
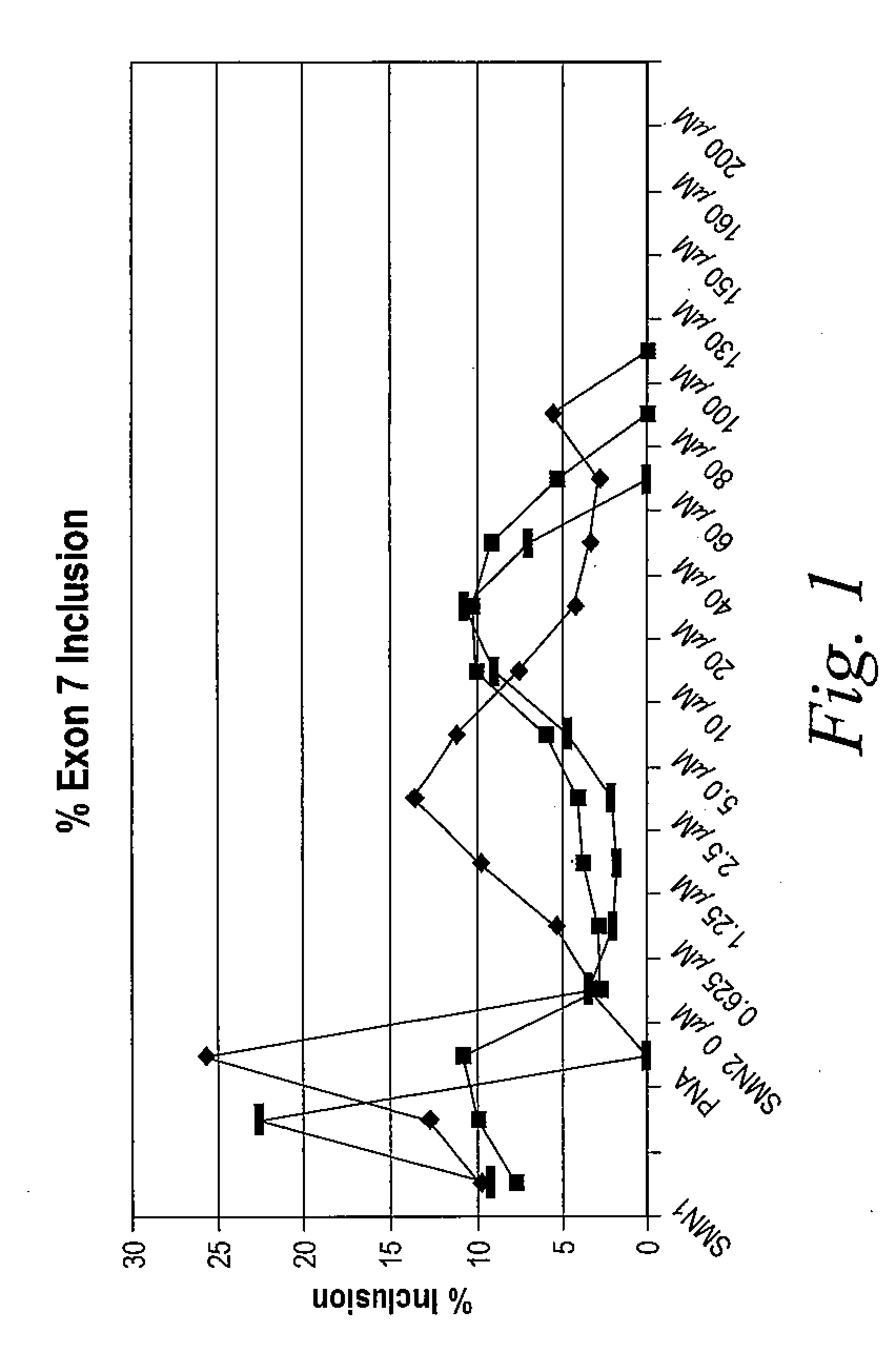
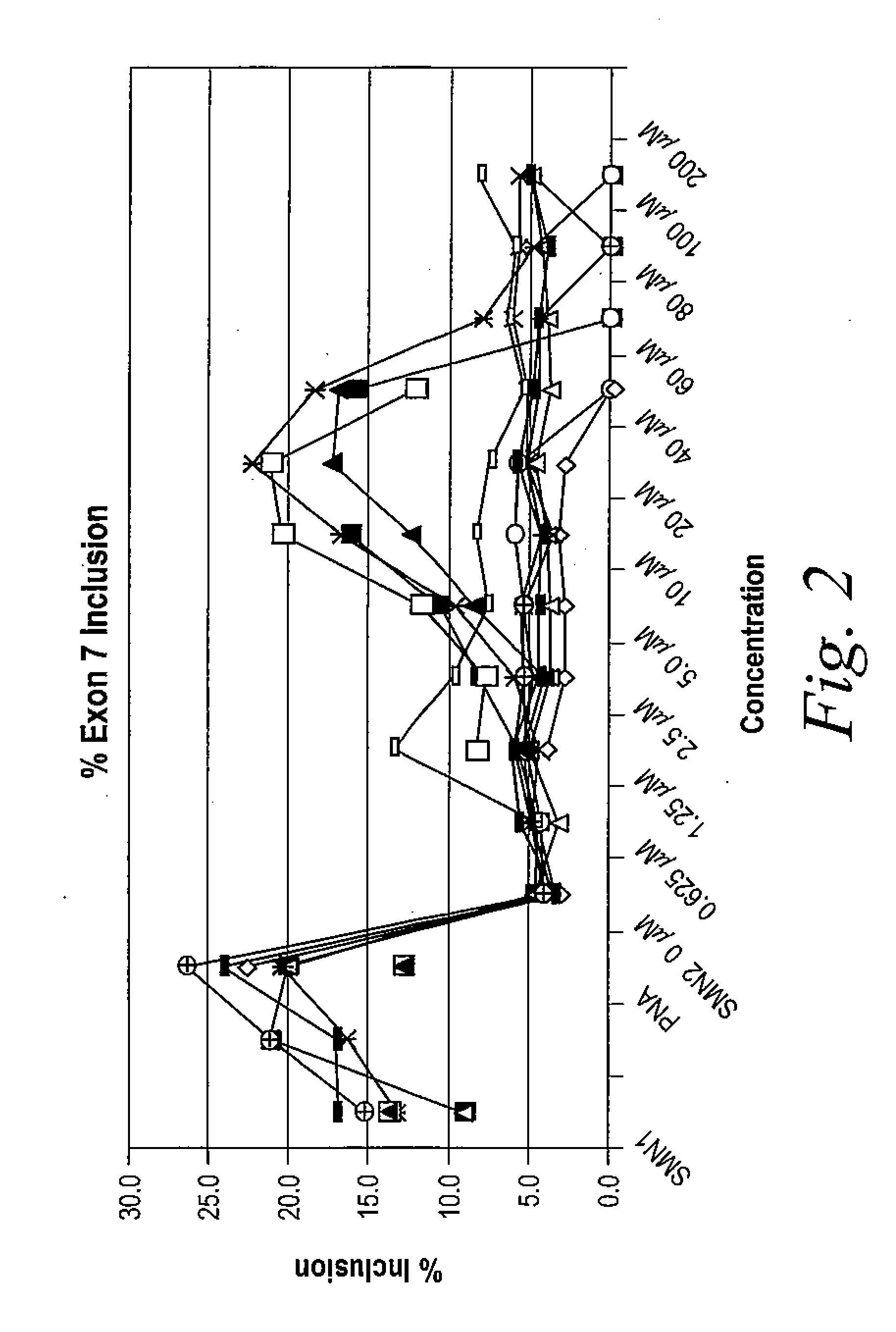
The present invention provides nucleic acid constructs, methods for identifying and validating compounds that increase the inclusion of exon 7 of SMN2 into mRNA transcribed from the SMN2 gene, compounds and pharmaceutical compositions that increase levels of SMN protein produced from the SMN2 gene, and methods for use thereof in treating of SMA.
Owner:PTC THERAPEUTICS INC
2-Aminoquinoline compounds
The present invention is concerned with compounds of the general Formula I: and pharmaceutically acceptable salts thereof, which are useful as melanin concentrating hormone receptor antagonists, particularly MCH-1R antagonists. As such, compounds of the present invention are useful for the treatment or prevention of obesity or eating disorders associated with excessive food intake and complications thereof, osteoarthritis, certain cancers, AIDS wasting, cachexia, frailty (particularly in elderly), mental disorders stress, cognitive disorders, sexual function, reproductive function, kidney function, locomotor disorders, attention deficit disorder (ADD), substance abuse disorders and dyskinesias, Huntington's disease, epilepsy, memory function, and spinal muscular atrophy. Compounds of formula I may therefore be used in the treatment of these conditions, and in the manufacture of a medicament useful in treating these conditions. Pharmaceutical formulations comprising one of the compounds of formula (I) as an active ingredient are disclosed, as are processes for preparing these compounds.
Owner:MERCK SHARP & DOHME CORP
Methods for Treating Spinal Muscular Atrophy Using Tetracycline Compounds
Methods for using tetracycline compounds for the treatment of spinal muscular atrophy are described.
Owner:MINTZ LEVIN COHN FERRIS GLOVSKY & POPEO PC
Compositions and methods for modulation of SMN2 splicing
Disclosed herein are compounds, compositions and methods for modulating splicing of SMN2 mRNA in a cell, tissue or animal. Also provided are uses of disclosed compounds and compositions in the manufacture of a medicament for treatment of diseases and disorders, including spinal muscular atrophy.
Owner:BIOGEN MA INC +1
2-Aminoquinoline compounds
The present invention is concerned with compounds of the general Formula I:and pharmaceutically acceptable salts thereof, which are useful as melanin concentrating hormone receptor antagonists, particularly MCH-1R antagonists. As such, compounds of the present invention are useful for the treatment or prevention of obesity or eating disorders associated with excessive food intake and complications thereof, osteoarthritis, certain cancers, AIDS wasting, cachexia, frailty (particularly in elderly), mental disorders stress, cognitive disorders, sexual function, reproductive function, kidney function, locomotor disorders, attention deficit disorder (ADD), substance abuse disorders and dyskinesias, Huntington's disease, epilepsy, memory function, and spinal muscular atrophy. Compounds of formula I may therefore be used in the treatment of these conditions, and in the manufacture of a medicament useful in treating these conditions. Pharmaceutical formulations comprising one of the compounds of formula (I) as an active ingredient are disclosed, as are processes for preparing these compounds.
Owner:MERCK SHARP & DOHME CORP
Compositions and methods for modulation of SMN2 splicing in a subject
Owner:BIOGEN MA INC +1
Compositions and methods for modulation of smn2 splicing in a subject
Disclosed herein are compounds, compositions and methods for modulating splicing of SMN2 mRNA in a subject. Also provided are uses of disclosed compounds and compositions in the manufacture of a medicament for treatment of diseases and disorders, including spinal muscular atrophy.
Owner:BIOGEN MA INC
Compositions and methods for modulation of smn2 splicing
ActiveUS20100216238A1Increase in exon inclusionSplicing alterationSugar derivativesMedicineDisease cause
Disclosed herein are compounds, compositions and methods for modulating splicing of SMN2 mRNA in a cell, tissue or animal. Also provided are uses of disclosed compounds and compositions in the manufacture of a medicament for treatment of diseases and disorders, including spinal muscular atrophy.
Owner:BIOGEN MA INC +1
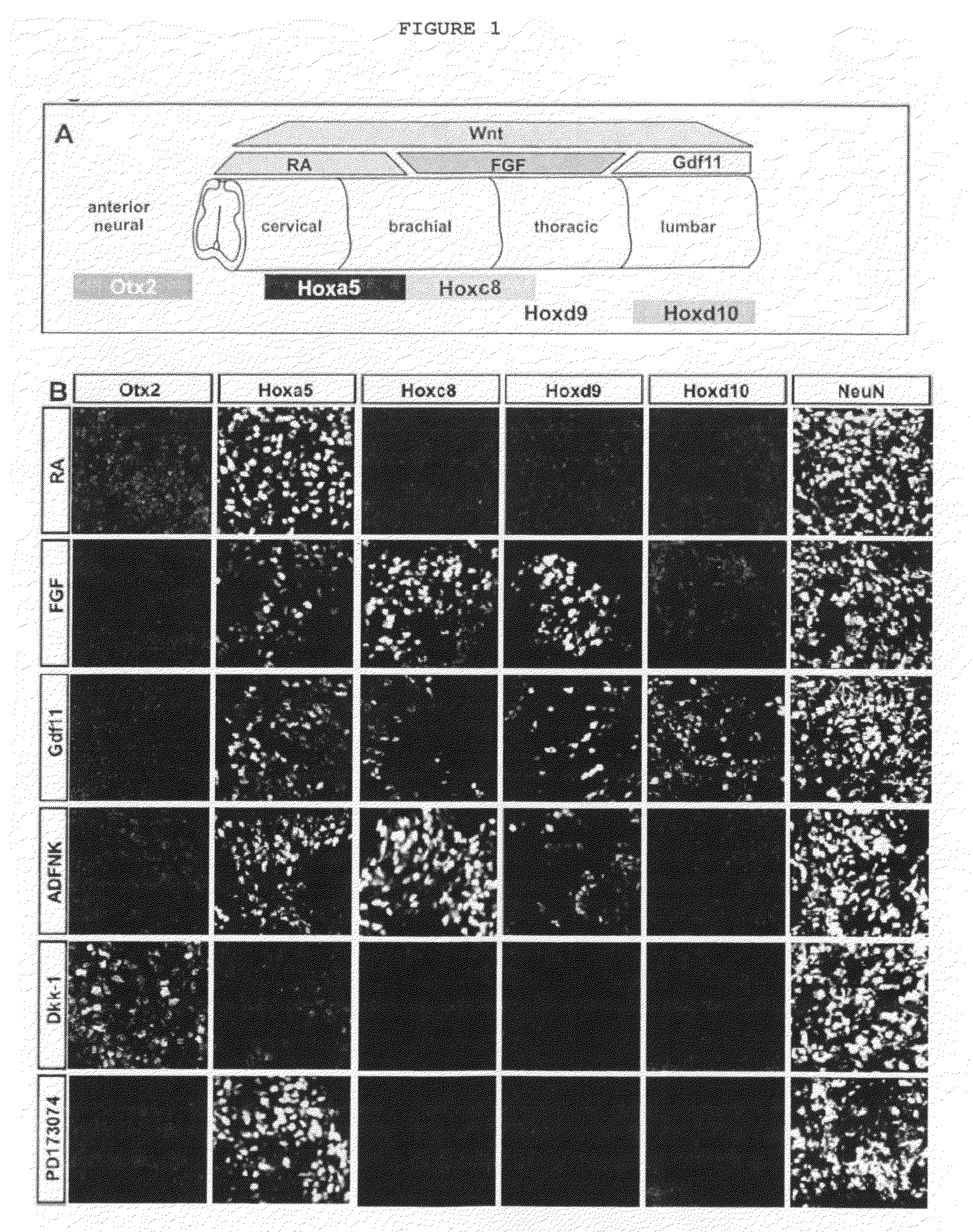
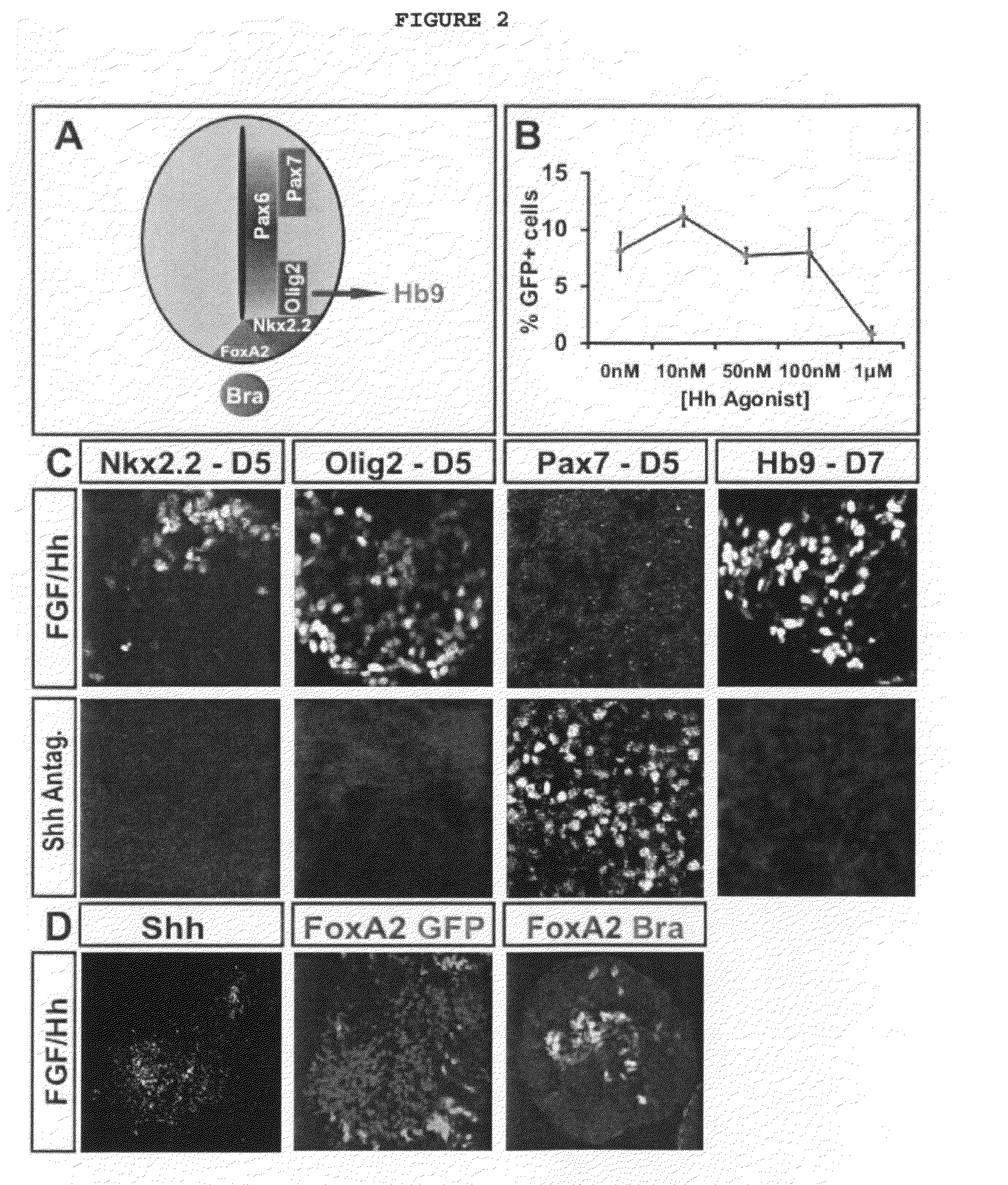
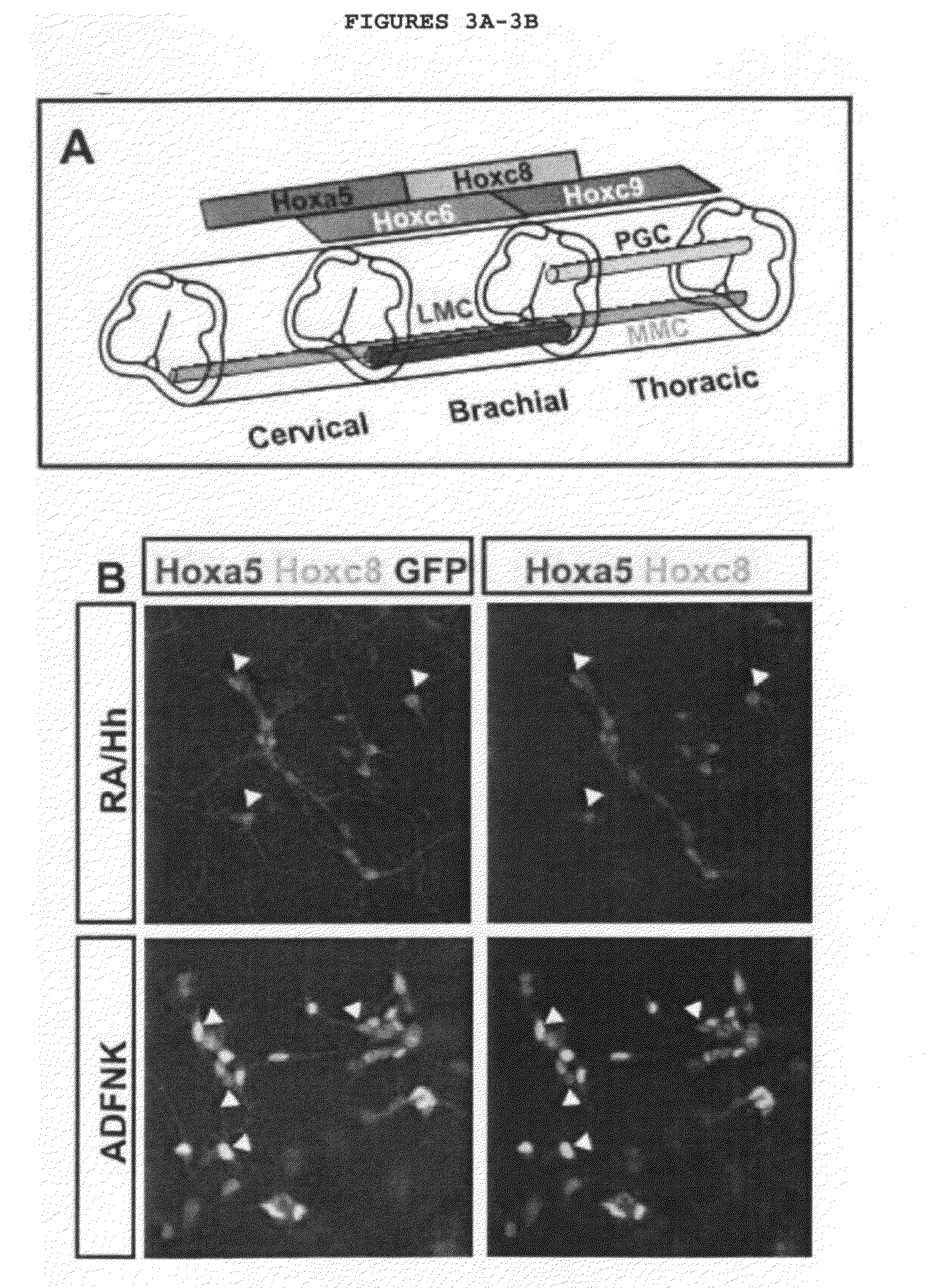
Generation of brachial, thoracic and lumbar spinal motor neurons from embryonic stem cells in the absence of all-trans retinoic acid supplement
InactiveUS20100196332A1Increase the number ofAvoid signalingBiocidePeptide/protein ingredientsFOXP1Retinoid
Disclosed are methods for generating a neuron expressing Hoxc8 transcription factor or a caudal motor neuron comprising culturing an embryonic stem cell in a composition which is essentially free of retinoids and comprises an isotonic salt solution, so as to generate the neuron which expresses Hoxc8 transcription factor or the caudal motor neuron. Disclosed are also methods for generating a caudal brachial motor neuron, a thoracic motor neuron, or a lumbar motor neuron from an embryonic stem cell in a composition essentially free of retinoids and comprising ADFNK medium, an amount of FGF-2, or Gdf11 respectively. Disclosed are also methods of transplanting a motor neuron into a subject comprising generating the motor neuron and transplanting the motor neuron into the subject. Disclosed is also a population of motor neuron cells enriched for motor neuron cells expressing Foxp1 and expressing a gene associated with Spinal Muscular Atrophy (SMA) or Amyotrophic Lateral Sclerosis (ALS).
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Compositions and methods for modulation of smn2 splicing
InactiveUS20120149757A1Increase in exon inclusionOrganic active ingredientsNervous disorderDiseaseMedicine
Disclosed herein are compounds, compositions and methods for modulating splicing of SMN2 mRNA in a cell, tissue or animal. Also provided are uses of disclosed compounds and compositions in the manufacture of a medicament for treatment of diseases and disorders, including spinal muscular atrophy.
Owner:KRAINER ADRIAN R +4
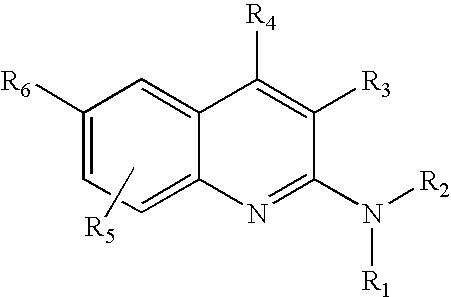
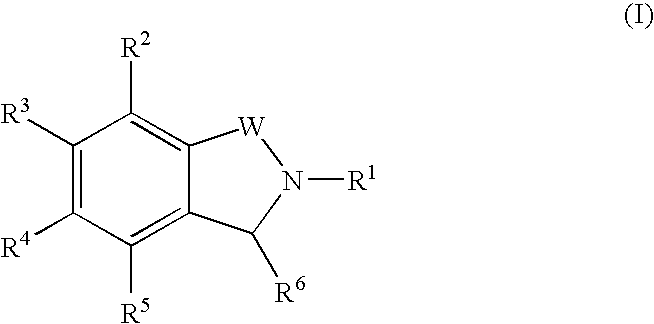
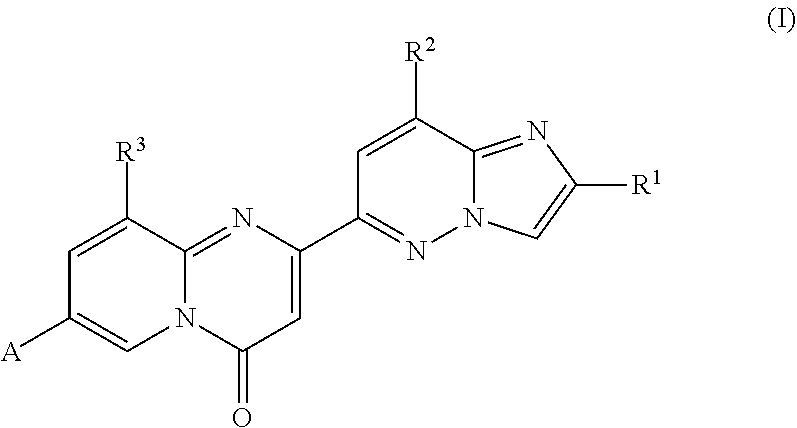
Isoindoline compounds for the treatment of spinal muscular atrophy and other uses
InactiveUS20100267712A1High expressionBiocideNervous disorderTranslational terminationSpinal muscular atrophy
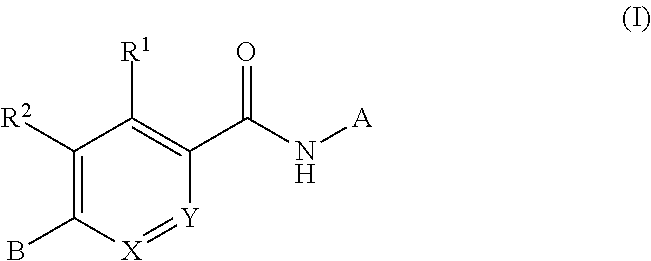
Disclosed is a compound of Formula (I) in which W and R1-R6 are defined herein. Also disclosed is a method of treating spinal muscular atrophy, as well as methods of using such compounds to increase SMN expression, increase EAAT2 expression, or increase the expression of a nucleic acid that encodes a translational stop codon introduced directly or indirectly by mutation or frameshift.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Methods for treating spinal muscular atrophy
InactiveUS8633019B2Increase inclusivenessImprove the level ofBiocideNervous disorderExonNucleic acid
The present invention provides nucleic acid constructs, methods for identifying and validating compounds that increase the inclusion of exon 7 of SMN2 into mRNA transcribed from the SMN2 gene, compounds and pharmaceutical compositions that increase levels of SMN protein produced from the SMN2 gene, and methods for use thereof in treating of SMA.
Owner:PTC THERAPEUTICS INC
Compositions and methods for modulation of smn2 splicing
Disclosed herein are compounds, compositions and methods for modulating splicing of SMN2 mRNA in a cell, tissue or animal. Also provided are uses of disclosed compounds and compositions in the manufacture of a medicament for treatment of diseases and disorders, including spinal muscular atrophy.
Owner:COLD SPRING HARBOR LAB INC +1
Spinal muscular atrophy diagnostic methods
The present invention relates to the discovery of the human survival motor-neuron gene or SMD gene, which is a chromosome 5-SMA (Spinal Muscular Atrophy) determining gene. The present invention further relates to the nucleotide sequence encoding the SMN gene and corresponding amino acid sequence, a vector containing the gene encoding the SMN protein or a DNA sequence corresponding to the gene and transformant strains containing the SMN gene or a DNA sequence corresponding to the gene.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
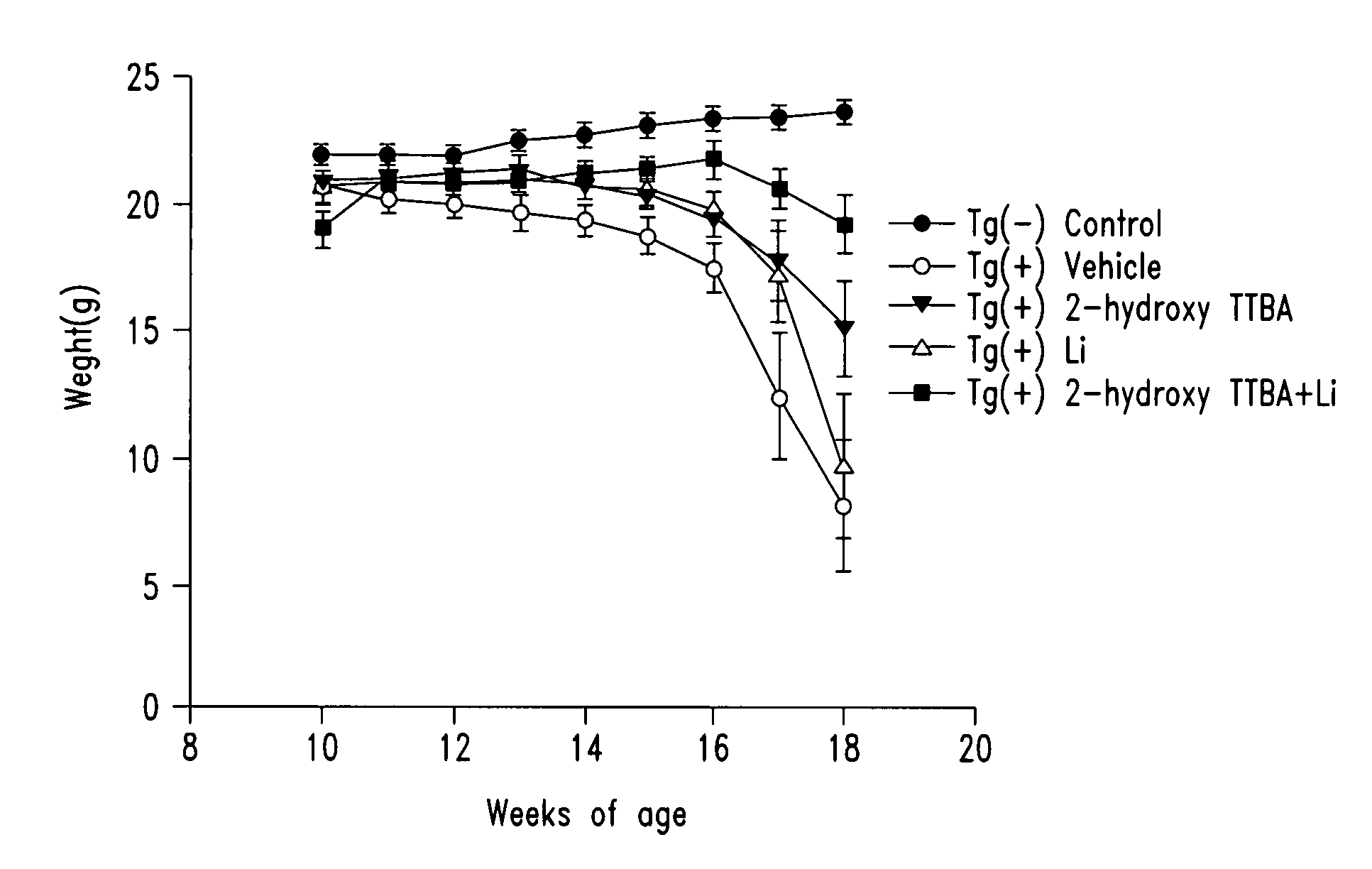
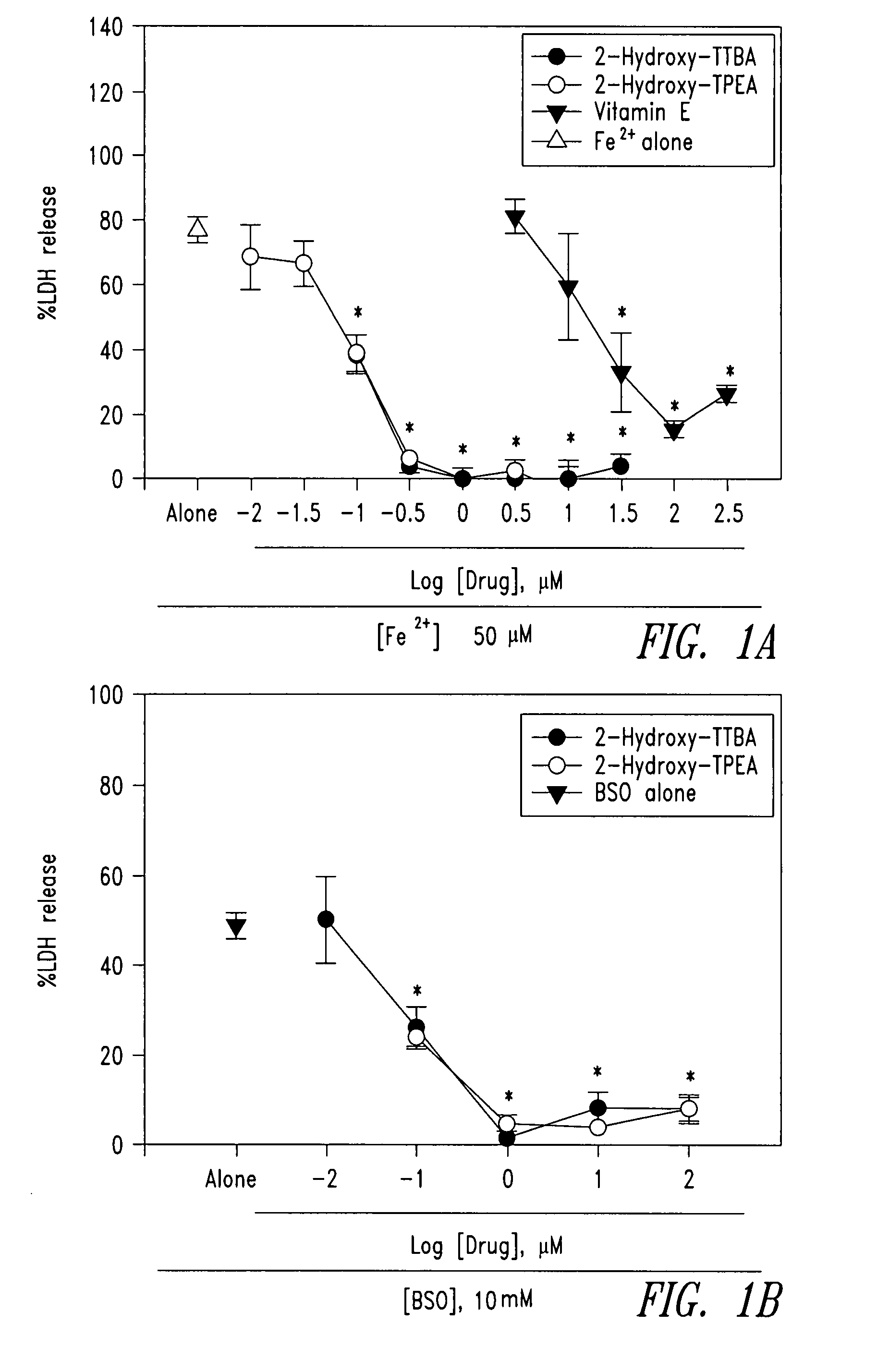
Compounds and compositions for treating neuronal death or neurological dysfunction
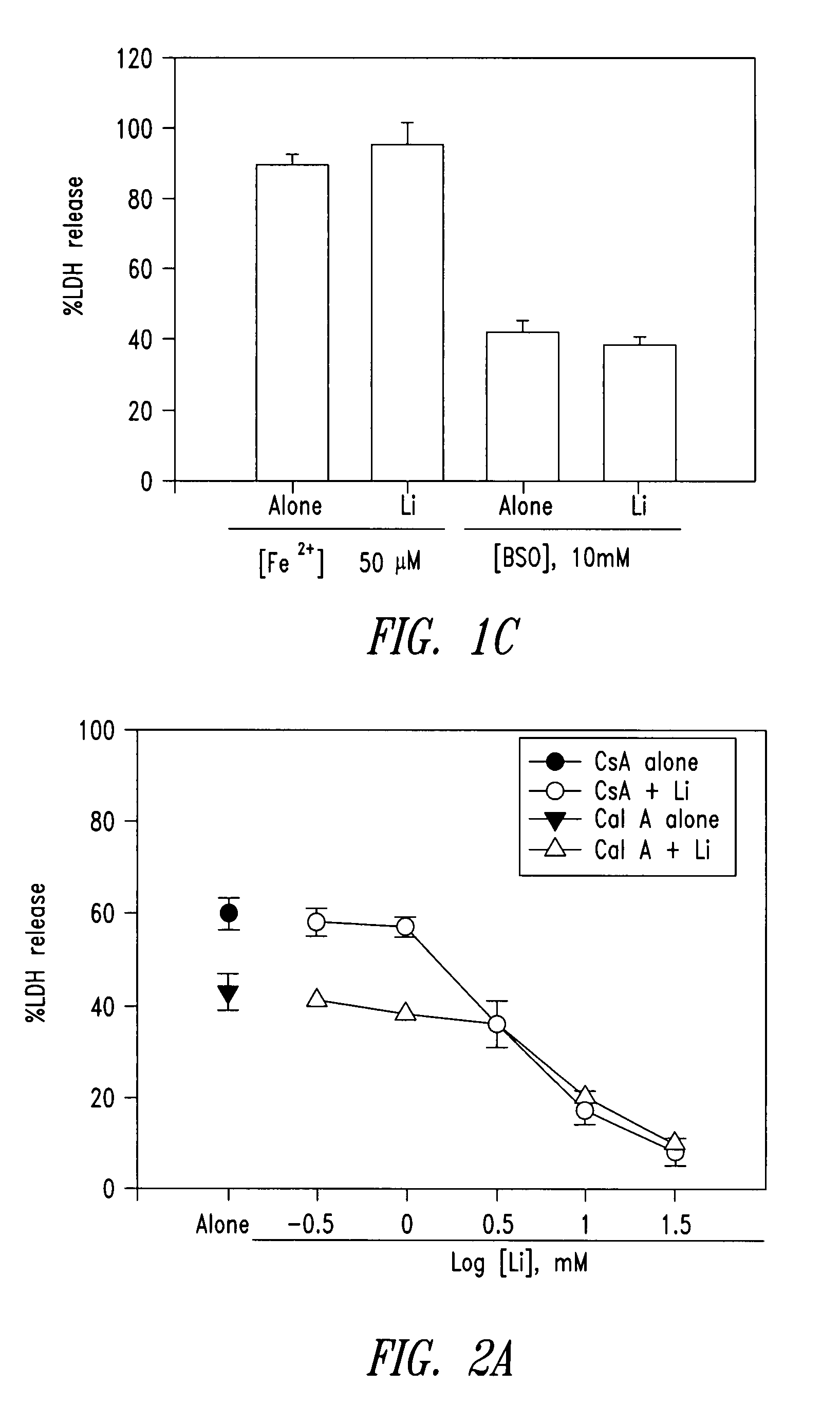
InactiveUS20070298129A1Avoid necrosisReduced infarct volumeBiocideSalicyclic acid active ingredientsDiabetic retinopathyBenzoic acid
The present invention relates to 2-hydroxy-alkylamino-benzoic acid derivatives and to a combination of cell necrosis inhibitor and lithium, process for the preparation of the derivatives or the combination, pharmaceutical formulation containing the derivatives or the combination, and use of the derivatives or the combination by either concomitant or sequential administration for improvement of treatment of neuronal death or neurological dysfunction. The derivatives and the combination of the present invention are useful for treating neurological diseases, such as amyotrophic lateral sclerosis (ALS, Lou Gehrig's disease), spinal muscular atrophy, Alzheimer's disease, Parkinson's disease, Huntington's disease, stroke, traumatic brain injury or spinal cord injury; and for treating ocular diseases such as glaucoma, diabetic retinopathy or macular degeneration.
Owner:NEUROTECH PHARMA
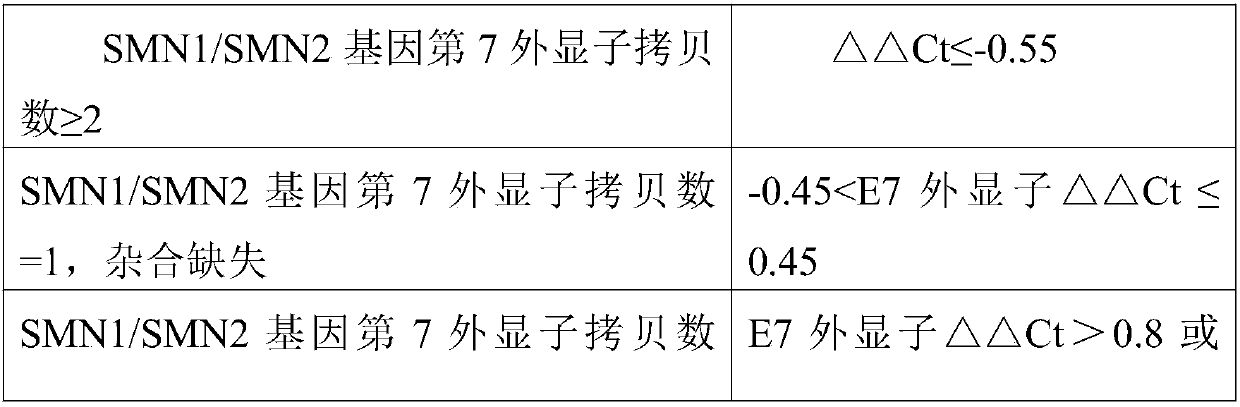
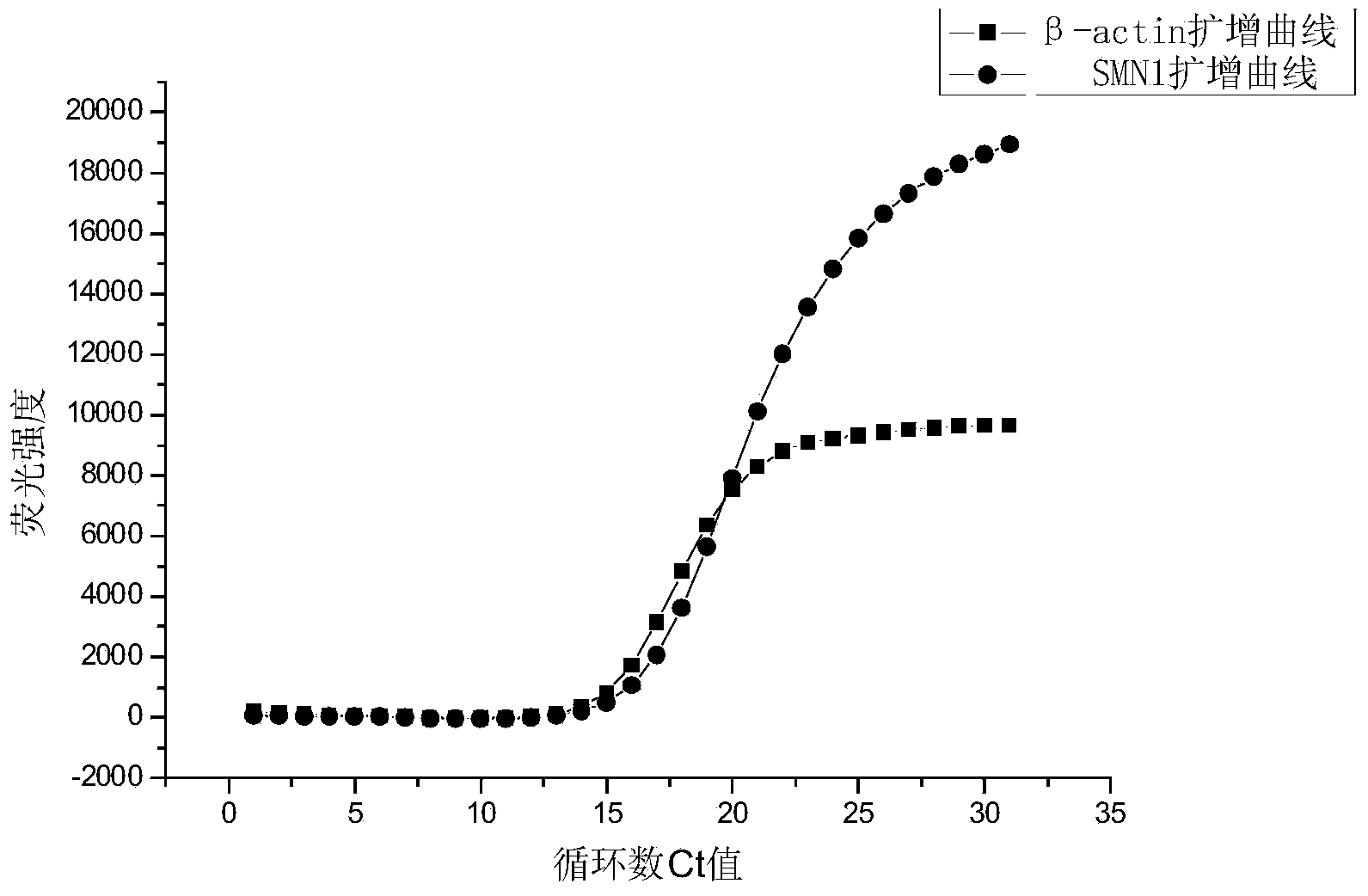
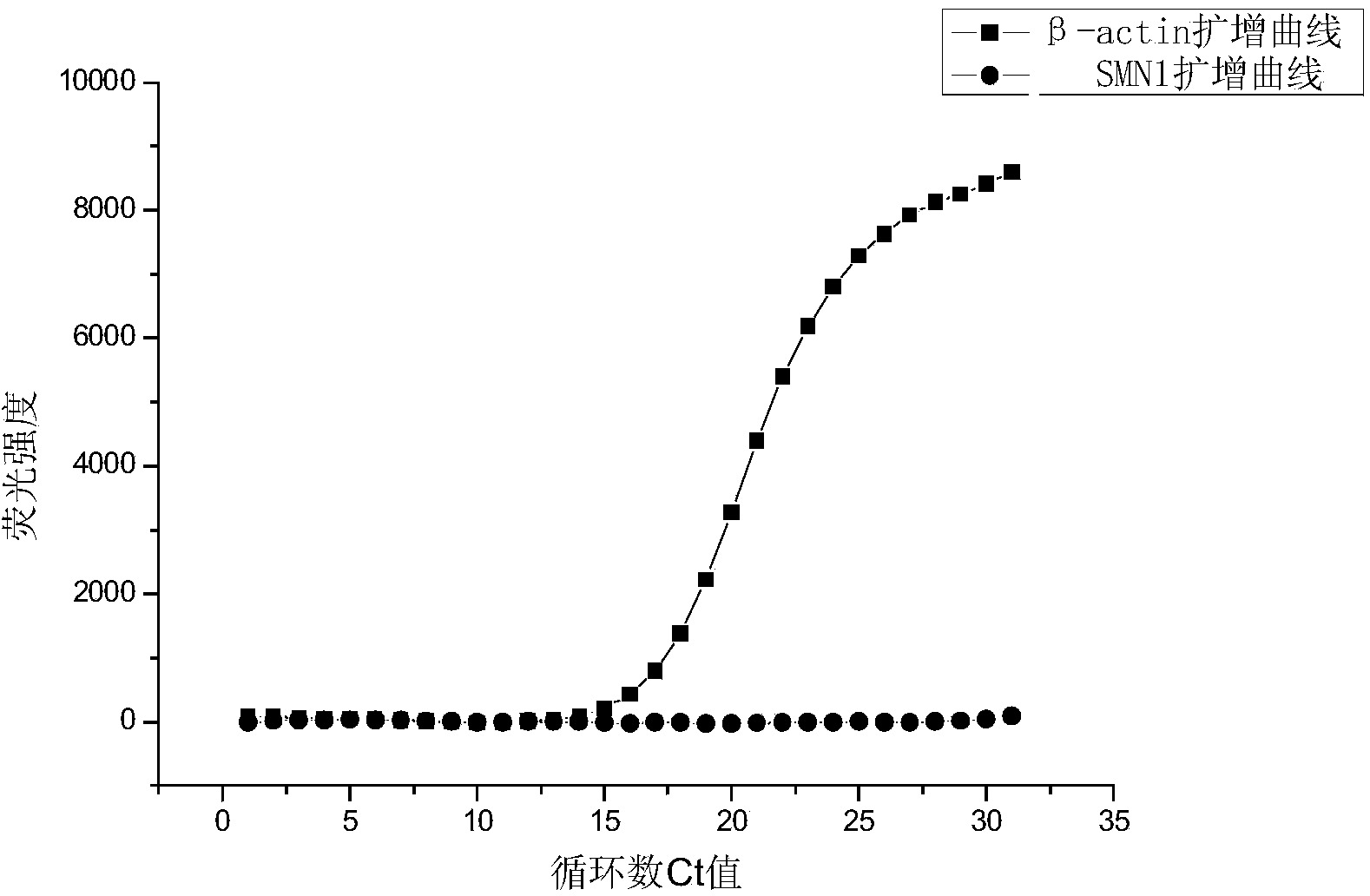
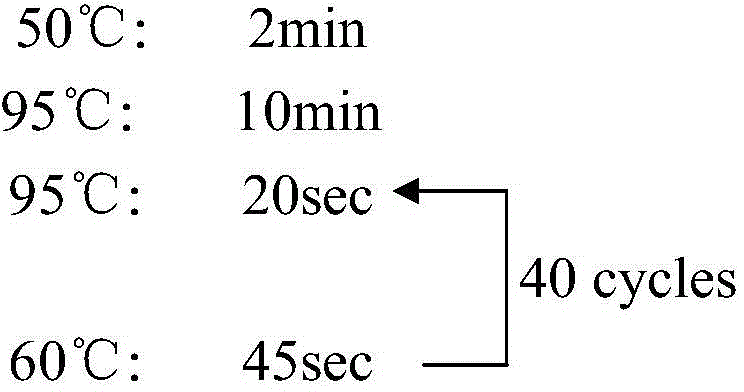
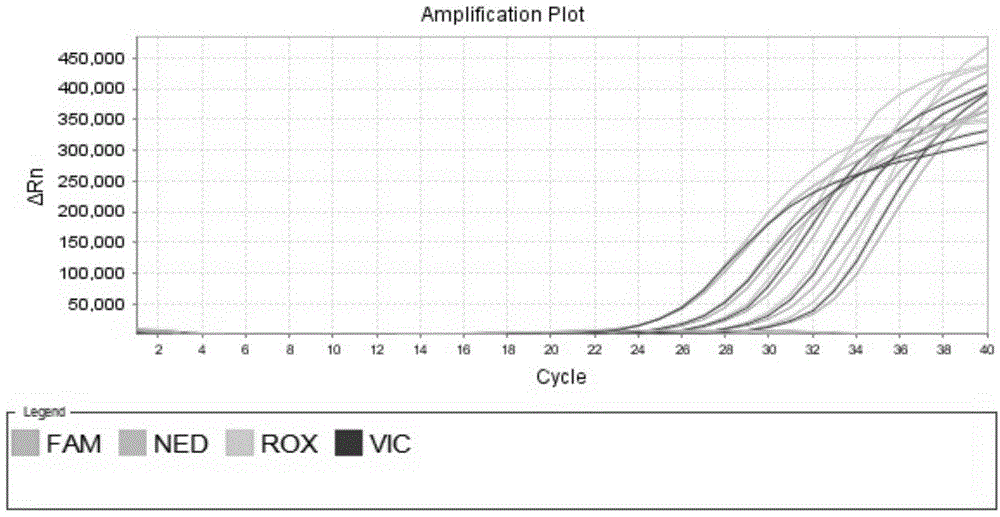
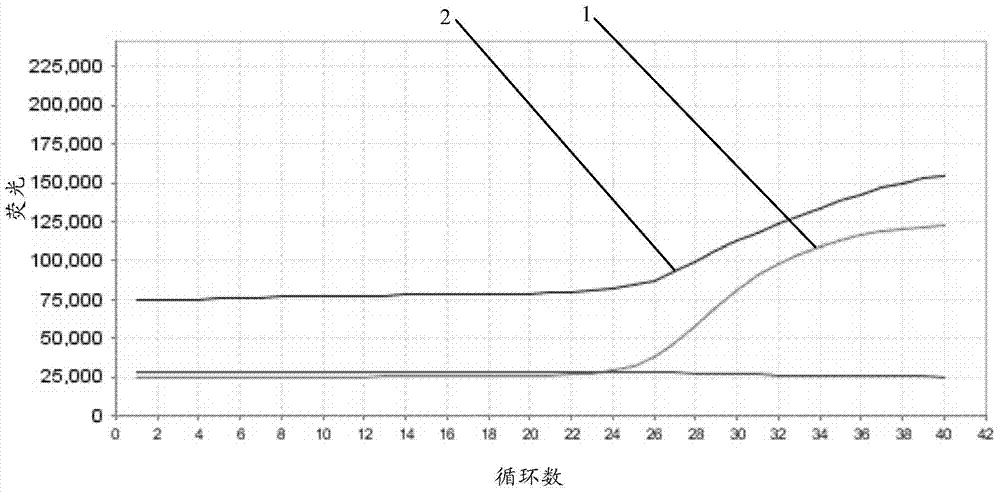
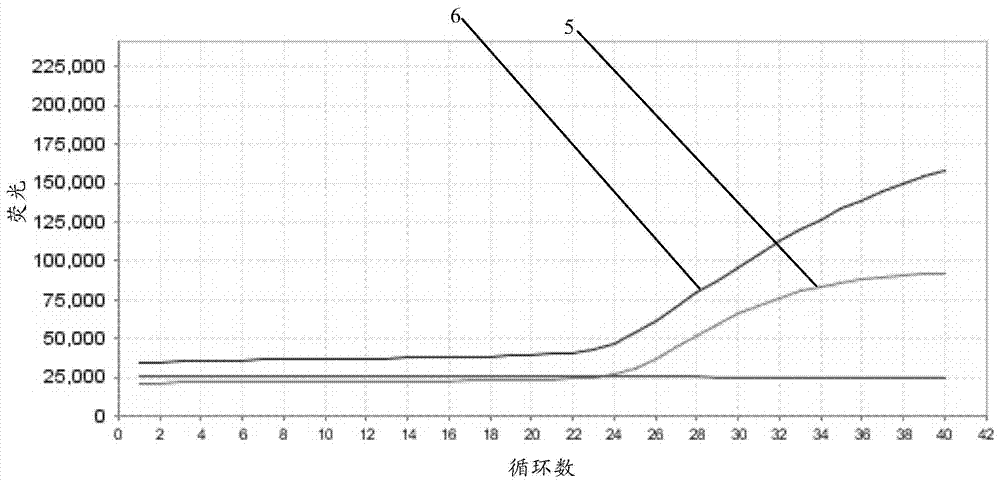
Fluorescent quantitative PCR kit for detecting copy number of virulence gene of human spinal muscular atrophy
InactiveCN108048548AAvoid interferenceEliminate interfering fluorescent signalsMicrobiological testing/measurementDNA/RNA fragmentationReference genesVirulent characteristics
The invention discloses a fluorescent quantitative PCR kit for detecting the copy number of a virulence gene of human spinal muscular atrophy. The fluorescent quantitative PCR kit comprises amplimersand fluorescent probes, wherein the amplimers consist of a pair of shared primers for specific amplification of the seventh exon of SMN1 and SMN2 genes, a pair of shared primers for specific amplification of the eighth exon of the SMN1 and SMN2 genes, and a pair of specific primers for specific amplification of a reference gene, i.e., a CFTR gene; and the fluorescent probes consist of a fluorescent probe for specific detection of the seventh exon of the SMN1 gene, a fluorescent probe for specific detection of the eighth exon of the SMN1 gene, a fluorescent probe for specific detection of the seventh exon of the SMN2 gene, a fluorescent probe for specific detection of the eighth exon of the SMN2 gene and a fluorescent probe for specific detection of the reference gene CFTR. The fluorescentquantitative PCR kit for detecting the copy number of the virulence gene of human spinal muscular atrophy is directed at problems and insufficiencies in quantitative detection of the copy numbers of human motoneuron genes and is rapid and simple in detection and reliable in detection results.
Owner:北京华瑞康源生物科技发展有限公司
Compounds for treating spinal muscular atrophy
ActiveUS20150080383A1Increase inclusivenessImprove the level ofBiocideNervous disorderAnesthesiaSpinal muscular atrophy
Provided herein are compounds, compositions thereof and uses therewith for treating spinal muscular atrophy.
Owner:PTC THERAPEUTICS INC
Compounds for treating spinal muscular atrophy
Owner:F HOFFMANN LA ROCHE & CO AG +1
Compounds for the modulation of smn2 splicing
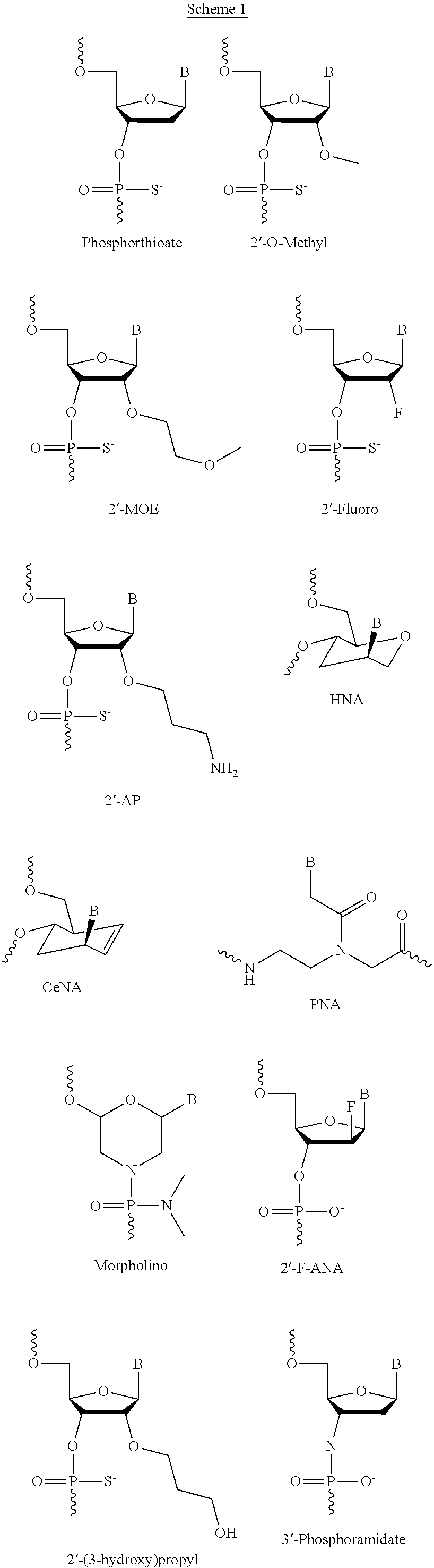
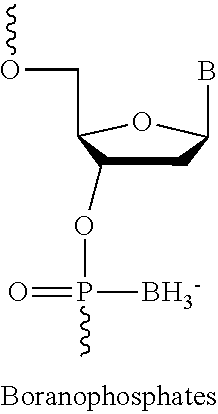
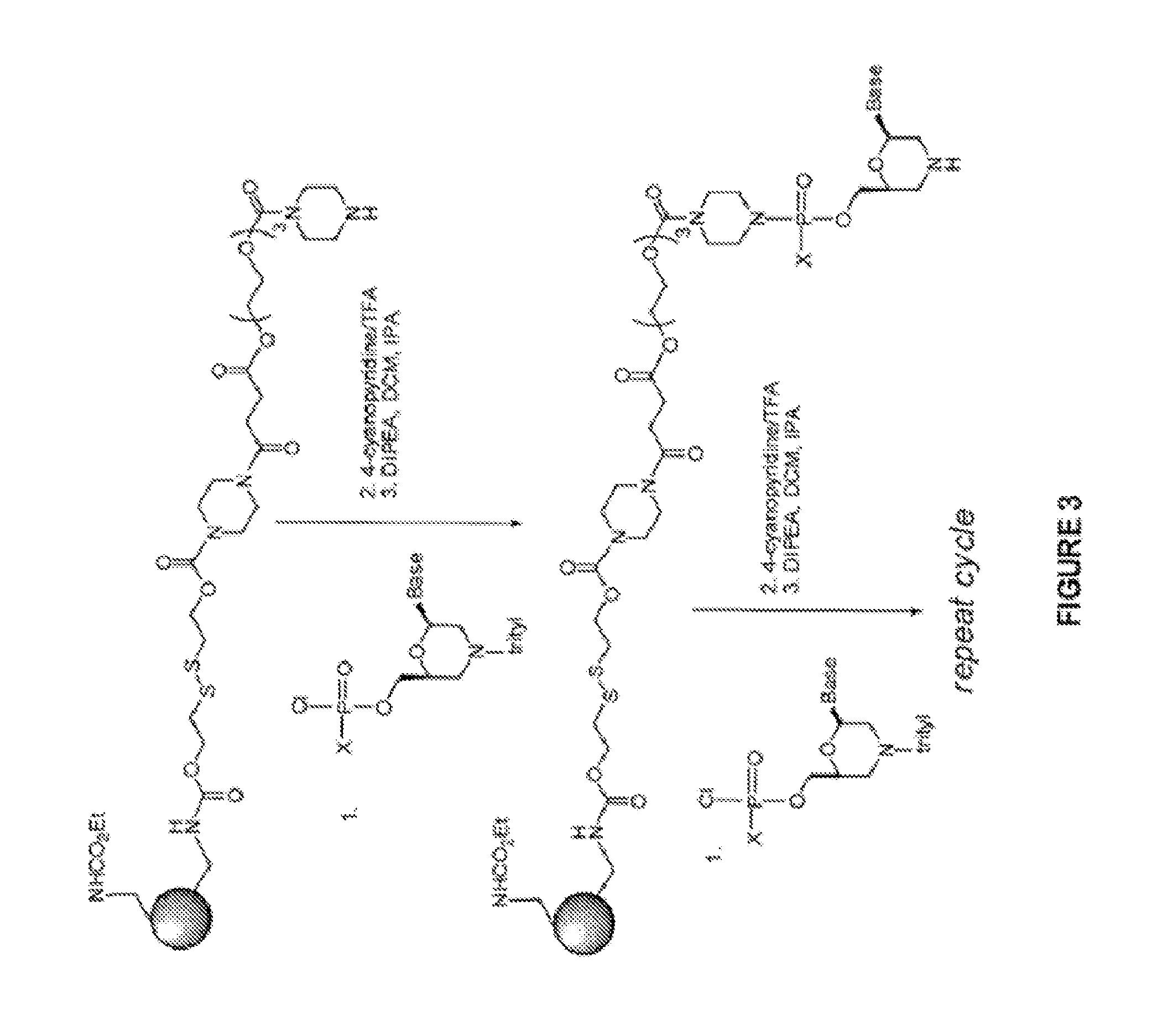
The present invention relates to oligomer compounds (oligomers) which target nucleic acids encoding human SMN2 in a cell, leading to modulation of SMN2 mRNA splicing which favors full length SMN2 mRNA rather than the poorly functional truncated transcript, SMN2 Δ7. Reduction of SMNA7 mRNA expression and / or increase in full length SMN2 mRNA expression are beneficial for the treatment of diseases or disorders associated with overexpression or undesirably high levels of aberrant forms of SMN2, particularly SMN2 Δ7, such as spinal muscular atrophy (SMA).
Owner:SANTARIS PHARMA AS
Fluorescent quantitative PCR (Polymerase Chain Reaction) kit for diagnosing human spinal muscular atrophy
ActiveCN103614477AAvoid differencesMicrobiological testing/measurementNeuronal Apoptosis-Inhibitory ProteinFluorescence
The invention relates to a fluorescent quantitative PCR (Polymerase Chain Reaction) kit for diagnosing human spinal muscular atrophy. The fluorescent quantitative PCR kit comprises amplification primers and fluorescent probes. The fluorescent quantitative PCR kit is characterized in that the amplification primers include a pair of shared primers used for specifically amplifying SMN1 (Survival Motor Neuron 1) and SMN2 (Survival Motor Neuron 2) genes, a pair of primers used for specifically amplifying an NAIP (Neuronal Apoptosis Inhibitory Protein) gene and a pair of primers used for specifically amplifying a reference sequence; the fluorescent probes include a fluorescent probe used for specifically detecting the SMN1 gene, a fluorescent probe used for specifically detecting the SMN2 gene, a fluorescent probe used for specifically detecting the NAIP gene and a fluorescent probe used for specifically detecting the reference sequence; the reference sequence is as shown in SEQ NO.14,and is positioned in a GRCh37 / hg19chr5:71107506-71107618 interval positioned on the 78kb position of the telomere side of the NAIP gene. The fluorescent quantitative PCR kit disclosed by the invention remarkably enhances the accuracy and stability of diagnosis by taking the DNA (Desoxvribose Nucleic Acid) sequence positioned at the telomere side of the NAIP gene as the reference sequence.
Owner:SOUTHERN MEDICAL UNIVERSITY
Primer and probe for screening spinal muscular atrophy (SMA) genes and using method of primer and probe
InactiveCN103555835ADifferentiate severityMicrobiological testing/measurementDNA/RNA fragmentationGenotypePrimary motor neuron
The invention discloses a primer and a probe for screening spinal muscular atrophy (SMA) genes and a using method of the primer and probe, belonging to the technical field of biology. The invention discloses eight combinations of the primer and probe for screening survival motor neuron genes 1 (SMN1), and the primer and probe can be effectively applied to screening the SMN1. Meanwhile, the invention also discloses sixteen groups of combinations of the primer and probe for screening survival motor neuron genes 2 (SMN2), as well as application in fetal SMA gene screening by utilizing the primer and probe for screening the SMN1 and the primer and probe for screening the SMN2. According to the primer and probe provided by the invention, the SMA genotypes of adults and fetuses can be detected at high efficiency.
Owner:曾骥孟
Primer, probe and kit for fluorescent quantitative detection on genes of spinal muscular atrophy (SMA)
ActiveCN104480206AEasy to detectAvoid false negativesMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceSmn gene
The invention relates to the field of gene detection, and particularly relates to a primer, a probe and a kit for fluorescent quantitative detection on genes of spinal muscular atrophy (SMA). The kit comprises the primer and the probe for fluorescent quantitative detection on genes of SMA, wherein the primer includes 9 pairs of primers; the probe includes 15 probes. According to the primer, the probe and the kit for fluorescent quantitative detection on genes of SMA, a fluorescent quantitative PCR technology is utilized, common mutation site detection which is not found in existing patent technologies is added, so that, 16 types of SMA patients caused by SMN gene deletion, transformation and site mutation can be specifically detected in one time, and classification of SMA patients can be realized.
Owner:亚能生物技术(深圳)有限公司
Spinal muscular atrophy-related gene mutation detection method, related detection probe composition and detection kit as well as related application
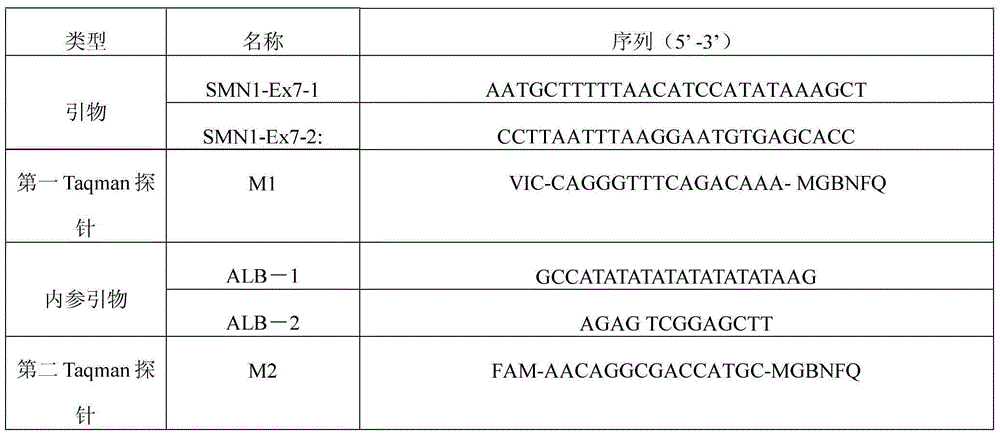
InactiveCN103789440AAvoid pollutionIngenious designMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceMutation detection
The invention provides a spinal muscular atrophy-related gene mutation detection method which comprises the following steps: extracting genome DNA (deoxyribonucleic acid) of a detection object; performing fluorescent quantitative PCR (polymerase chain reaction) on the genome DNA by adopting a combination of two PCR primer pairs and two Taqman MGB fluorescent probes respectively, wherein the PCR primer pairs are nucleotide sequences shown in SEQ ID NO:4 and SEQ ID NO:5 as well as SEQ ID NO:7 and SEQ ID NO:8 respectively, and nucleotide sequences of the Taqman MGB fluorescent probes are nucleotide sequences shown in SEQ ID NO:6 and SEQ ID NO:9 respectively; analyzing whether two loci of a spinal muscular atrophy-related gene in the genome DNA mutate according to a real-time amplification curve formed by fluorescent signals collected from the fluorescent quantitative PCR. The invention further relates to a related detection probe composition and a detection kit, as well as a related application. According to the method, the design is skilful, the closed pipe detection is adopted, the sample pollution can be effectively avoided, the detection is quick, accurate, easy and convenient, detection results can serve as references for diagnosis, treatment and drug use of doctors, and large-scale popularization and application are facilitated.
Owner:SHANGHAI CHROMYSKY MEDICAL RES
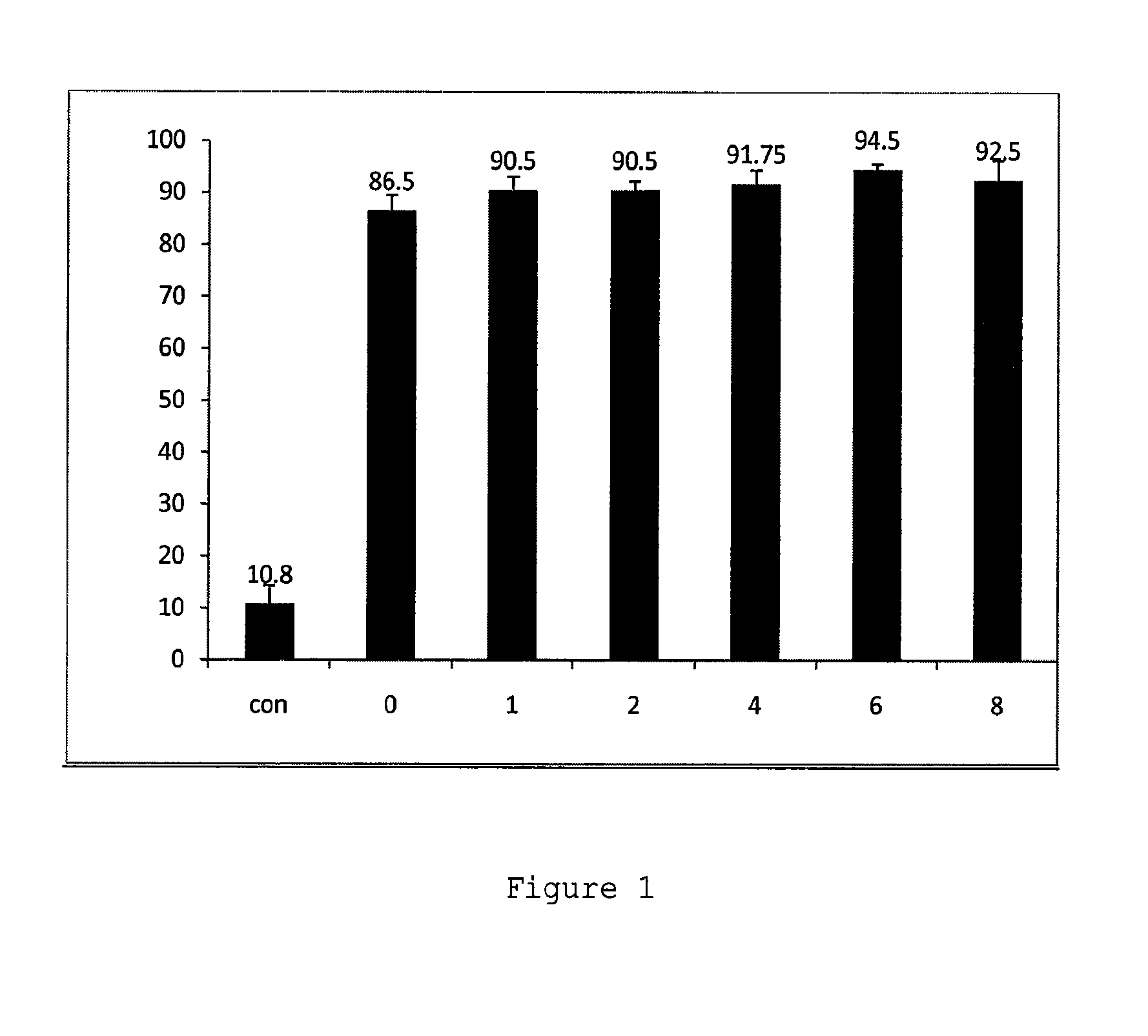
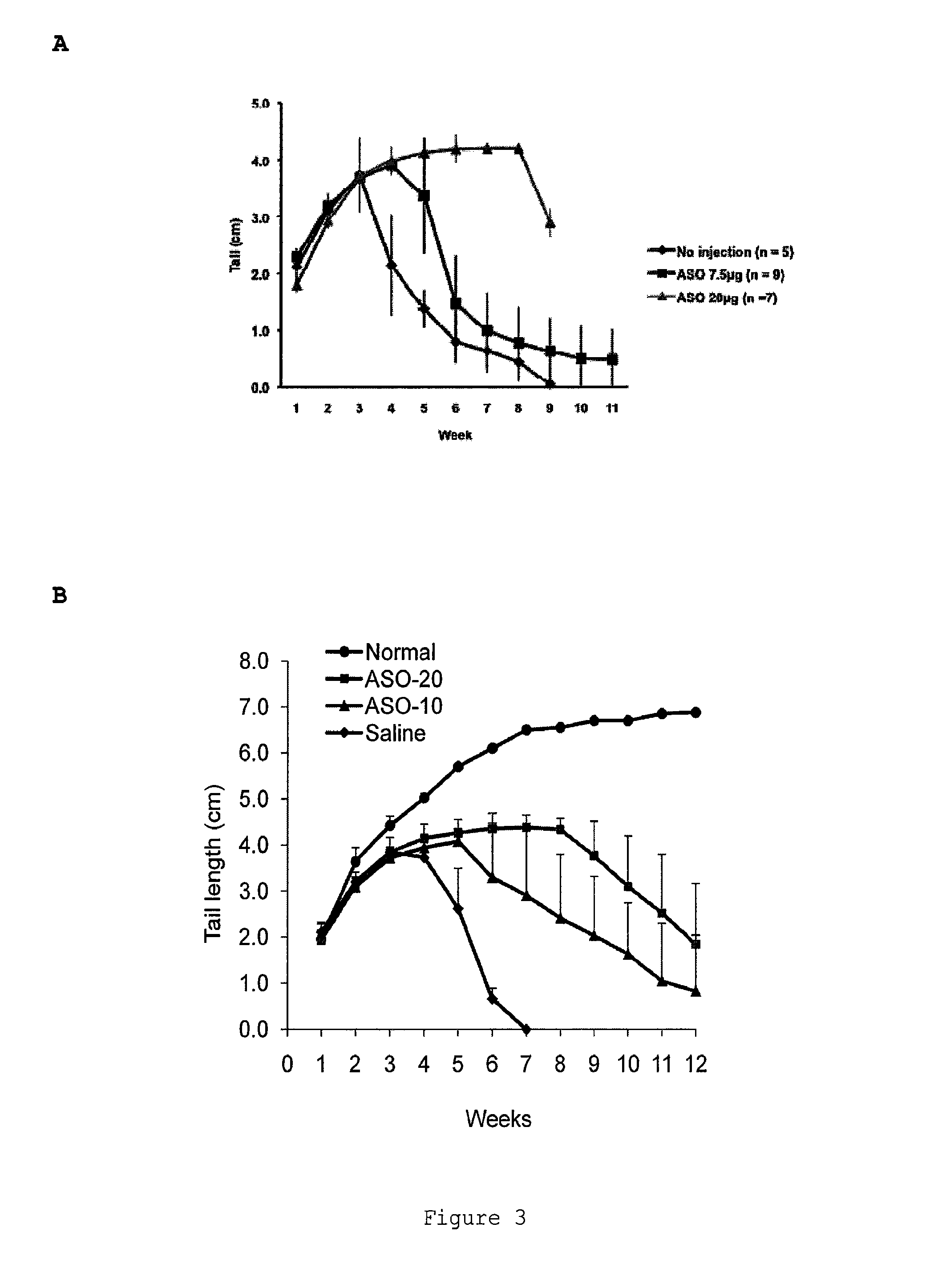
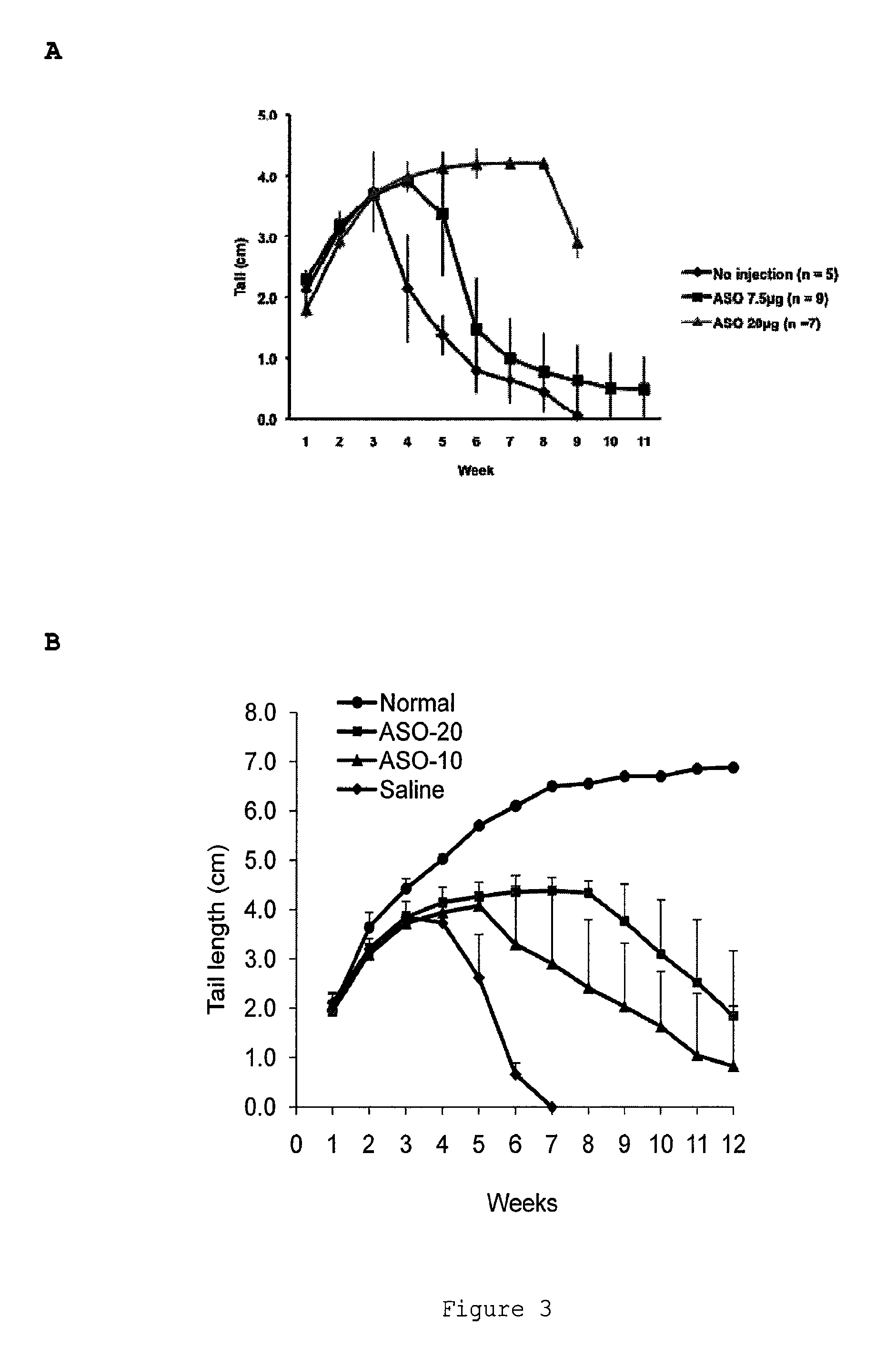
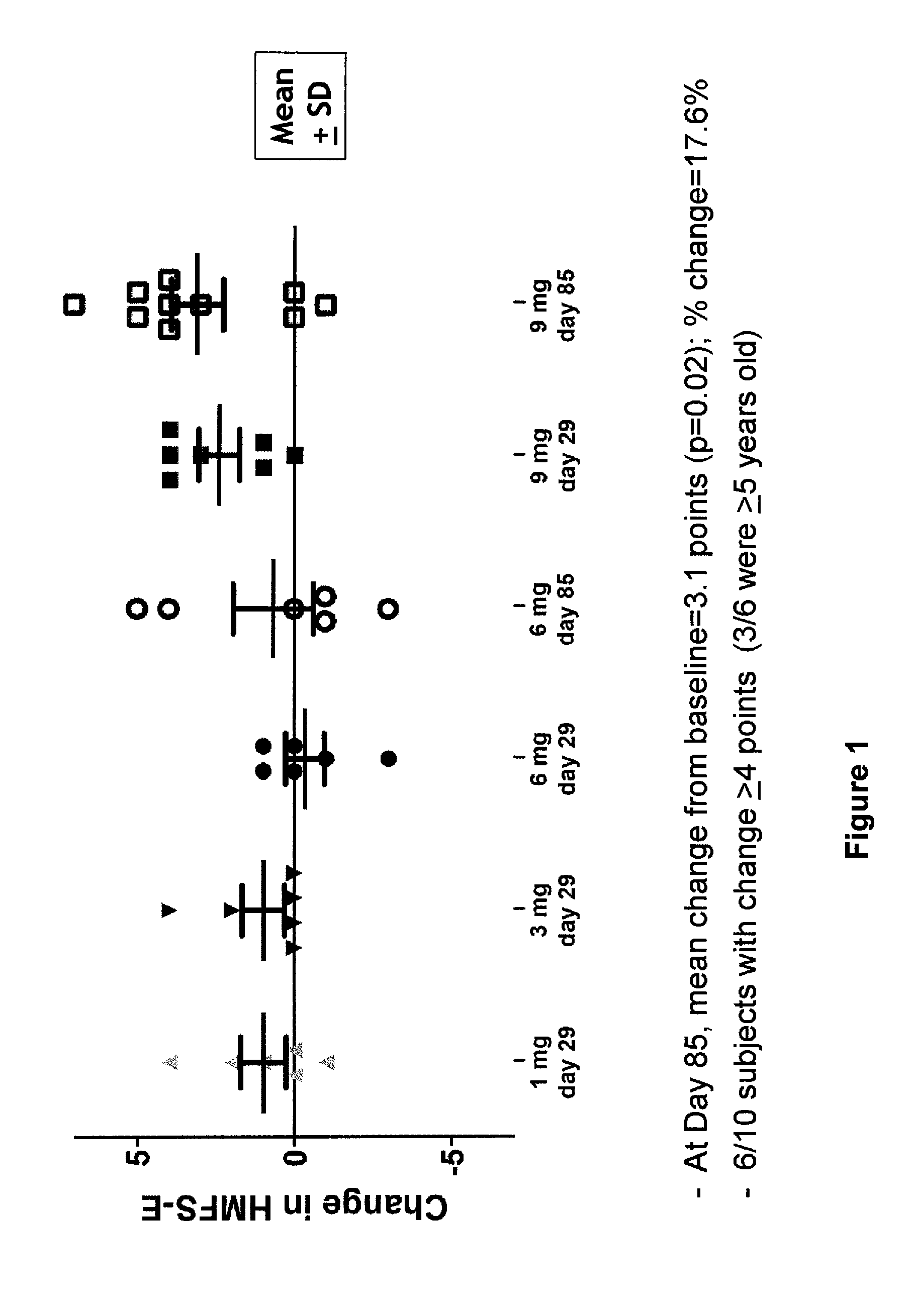
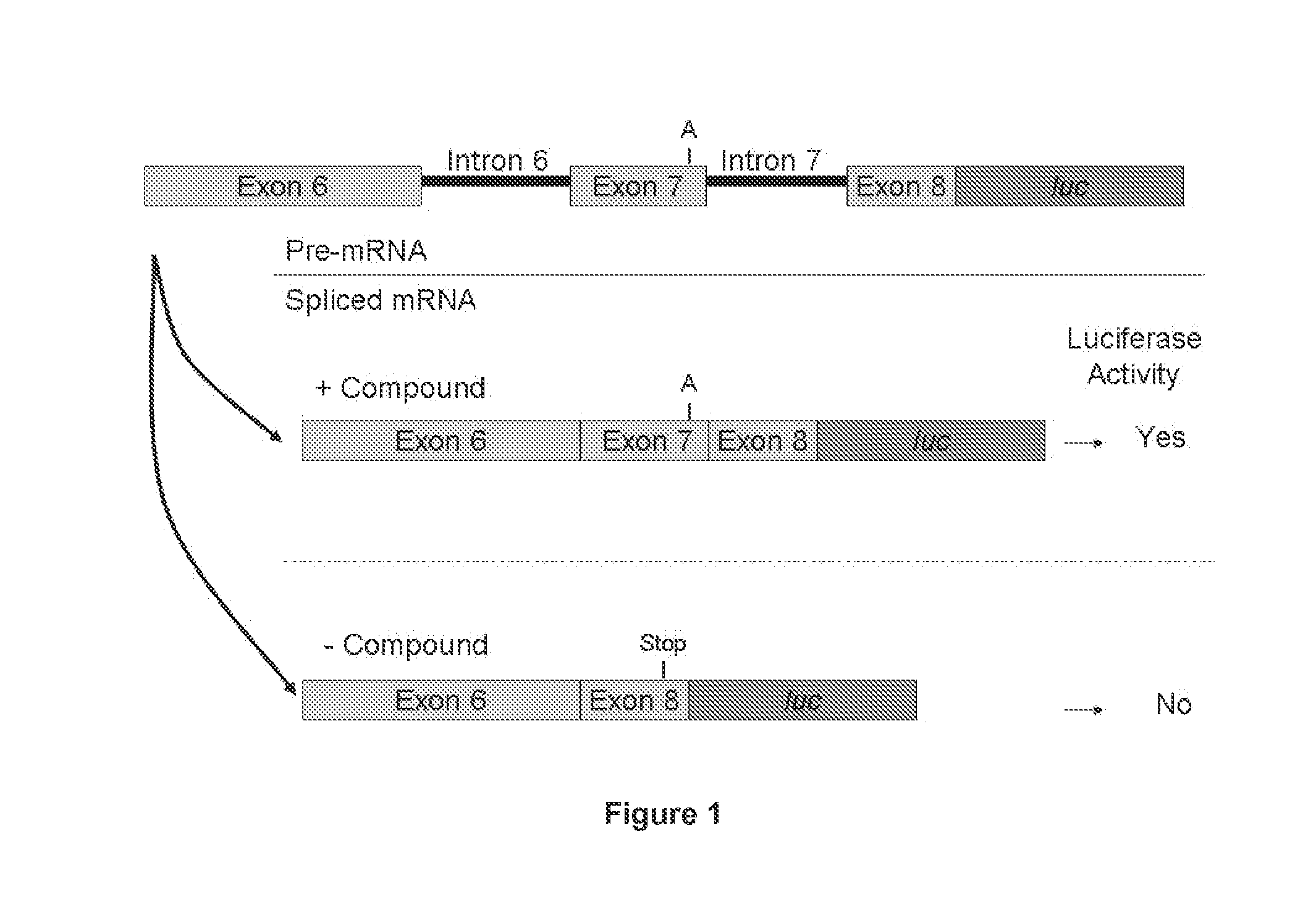
Induced exon inclusion in spinal muscle atrophy
ActiveUS20140329772A1Improve the level ofEnhances cellular uptakeOrganic active ingredientsBiocideMedicinePrimary motor neuron
The invention relates to the use of an antisense compound for inducing exon inclusion as a treatment for Spinal Muscle Atrophy (SMA). More particularly it relates to inducing inclusion of exon 7 to restore levels of Survival Motor Neuron (SMN) protein encoded by the Survival Motor Neuron (SMN) gene.
Owner:SAREPTA THERAPEUTICS INC
Methods and compositions for development of drug screening procedures and diagnostic tools
This invention defines novel research and clinical laboratory methodology and compositions related thereto appropriate for use in (a) determining the presence of a neurodegenerative disease selected from the group limited solely to Charcot-Marie-Tooth disease, familial Alzheimer's disease, familial Parkinson's disease, Huntington's disease, spinal muscular atrophy, Friedreich'a ataxia, giant axon neuropathy, juvenile ceroid-lipofuscinosis, familial motor neuron diseases, juvenile diabetic polyneuropathy and Down's syndrome, (b) monitoring the ongoing status of the physiological expression of said disease and (c) screening candidate therapeutic drug agents for possible effectiveness. The invention is based on the new and novel observation that the presence of a neurodegenerative disease can be characterized in part by the expression in cultured fibroblasts obtained from the patient of one or more proteins which are not the product of a defective disease-inducing gene, but which are stress proteins, one or more other proteins modified by conditions of oxidative stress or one or more other disease-related proteins. The invention depends on living cell material, namely fibroblasts, which are readily and, if necessary, repeatedly available from a patient. When adapted as a method and composition useful for the screening candidate therapeutic drug agents for possible effectiveness, this technology offers advantages in terms of (a) providing research opportunities which, in some cases, never existed before, (b) cost effectiveness when compared to alternative technologies, (c) ability to be used readily on a large scale, (d) ability to generate meaningful data in a comparatively short period of time, and (e) providing an early stage opportunity to obtain information based on direct interaction of a candidate drug and a living tissue disease model. Various aspects of diagnostic methods and compositions are also disclosed.
Owner:SHAPIRO HOWARD K
Treatment of ALS and variants thereof consisting of primary lateral sclerosis (PLS) or spinal muscular atrophy (SMA)
ActiveUS7659243B2Enhance cell viabilityNervous disorderPeptide/protein ingredientsDisease phenotypeNeuronal damage
The invention relates to the use of angiogenin, or a fragment or variant thereof, to treat diseases or conditions characterised by neuronal injury or death, or axonal degeneration, especially neurodegenerative diseases such as Amyotrophic Lateral Sclerosis (ALS). The invention also describes a plurality of mutations of the human angiogenin gene which are associated with a neurodegenerative disease phenotype, and particularly a ALS phenotype. Also described is a method of assessing whether an individual is afflicted with, or generically predisposed to develop, a disease or condition characterised by neuronal injury or death, or axonal degeneration.
Owner:ROYAL COLLEGE OF SURGEONS & IRELAND
Compounds for treating spinal muscular atrophy
Owner:F HOFFMANN LA ROCHE & CO AG +1
Kit for screening spinal muscular atrophy virulence gene carrier and application of kit
InactiveCN105039318AImprove throughputGood repeatabilityMicrobiological testing/measurementDNA preparationPcr methodGene carrier
The invention discloses a kit for screening a spinal muscular atrophy (SMA) virulence gene carrier and application of the kit, and belongs to the field of gene detection. The copy number of exon7 of a main virulence gene SMN1 of spinal muscular atrophy is quantitatively detected by a fluorescent quantitation PCR method based on a Taqman probe, so that distinguishing between the spinal muscular atrophy virulence gene carrier and a non carrier is realized. The kit can be quickly and conveniently applied to screening the spinal muscular atrophy virulence gene carriers from a large scale of people, is particularly suitable for screening on antenatal, premarital and pre-pregnant people and gene diagnosis on patients and is high in repetitiveness and accurate in result.
Owner:SUZHOU VOCATIONAL UNIV +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com