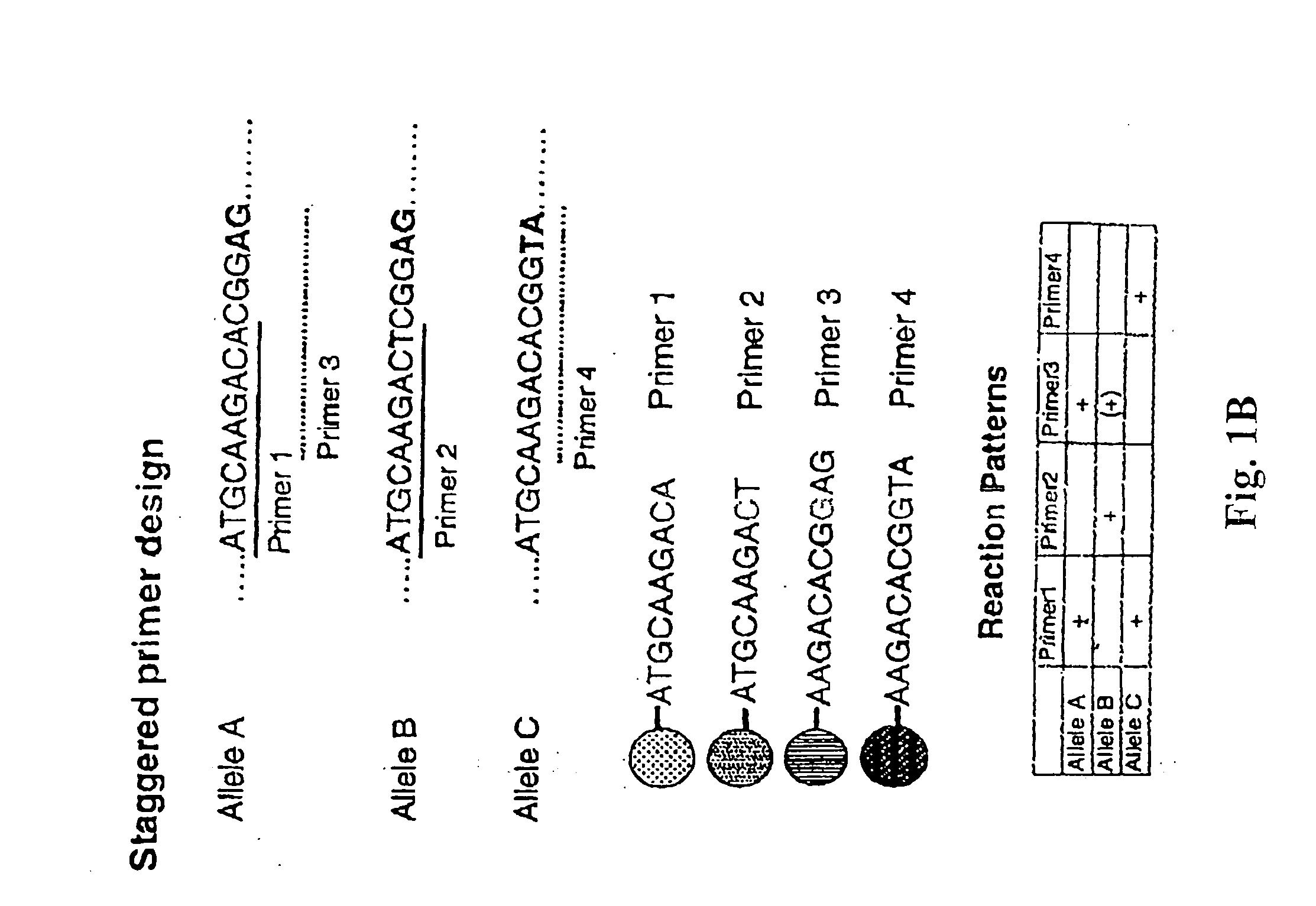
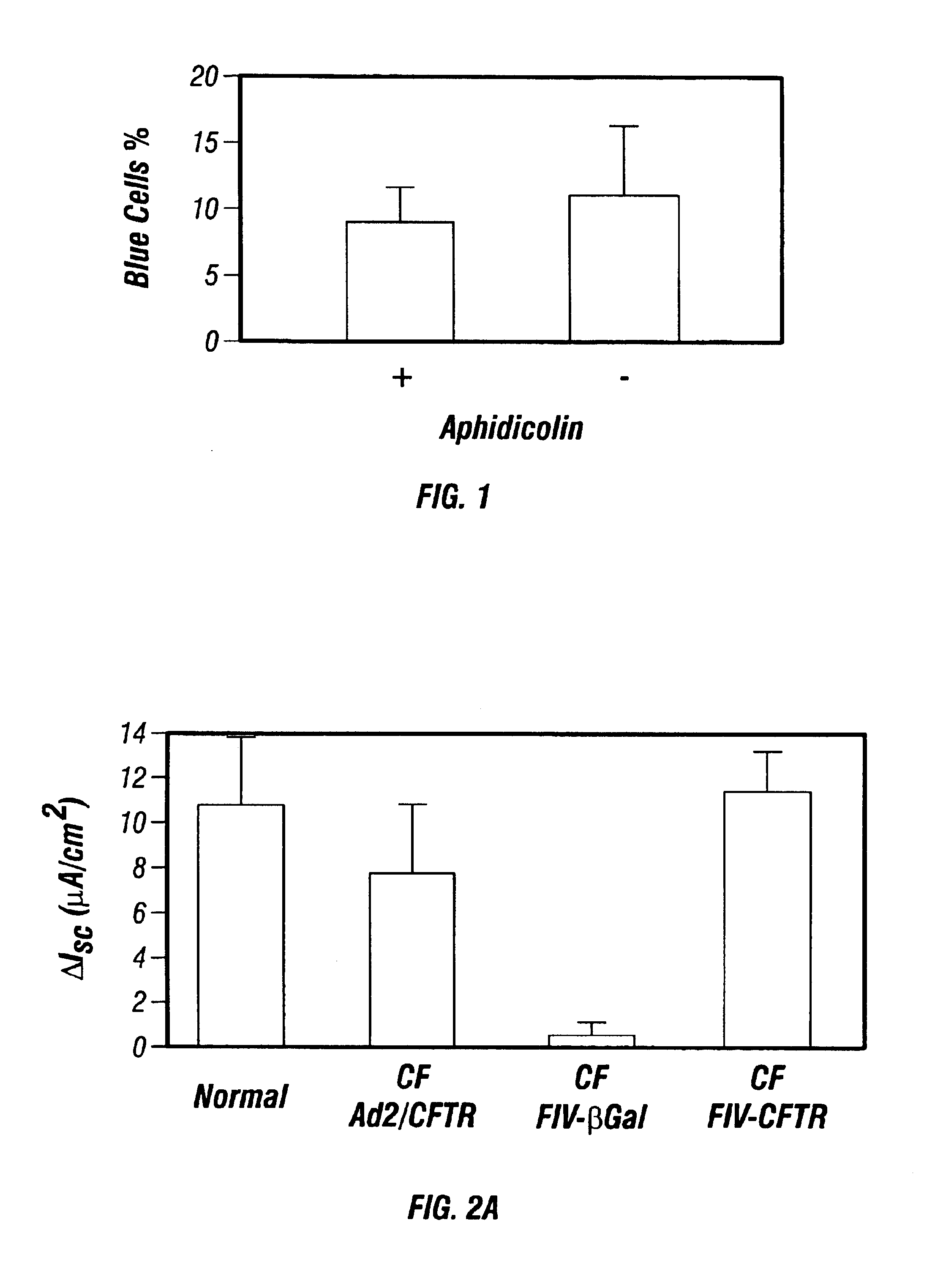
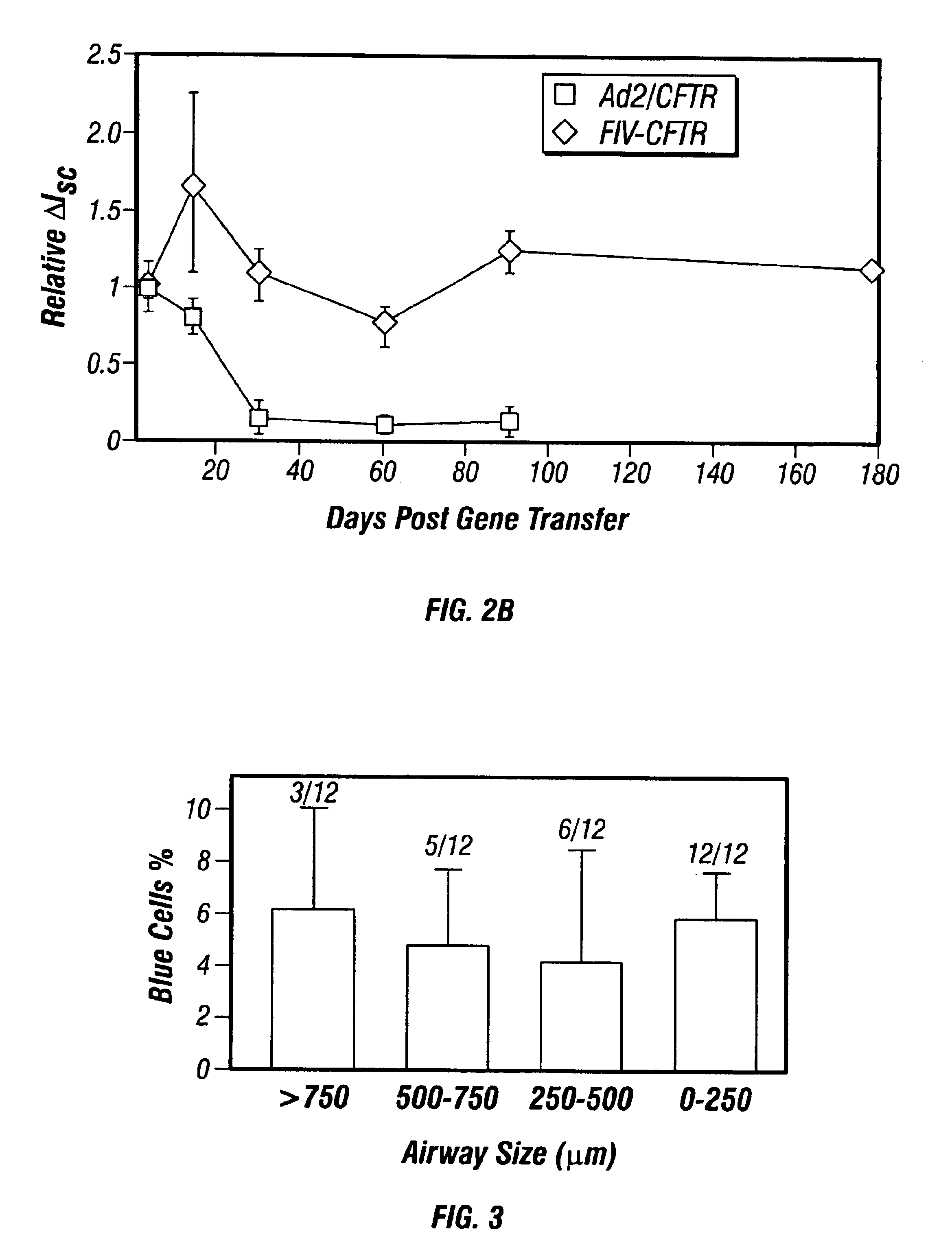
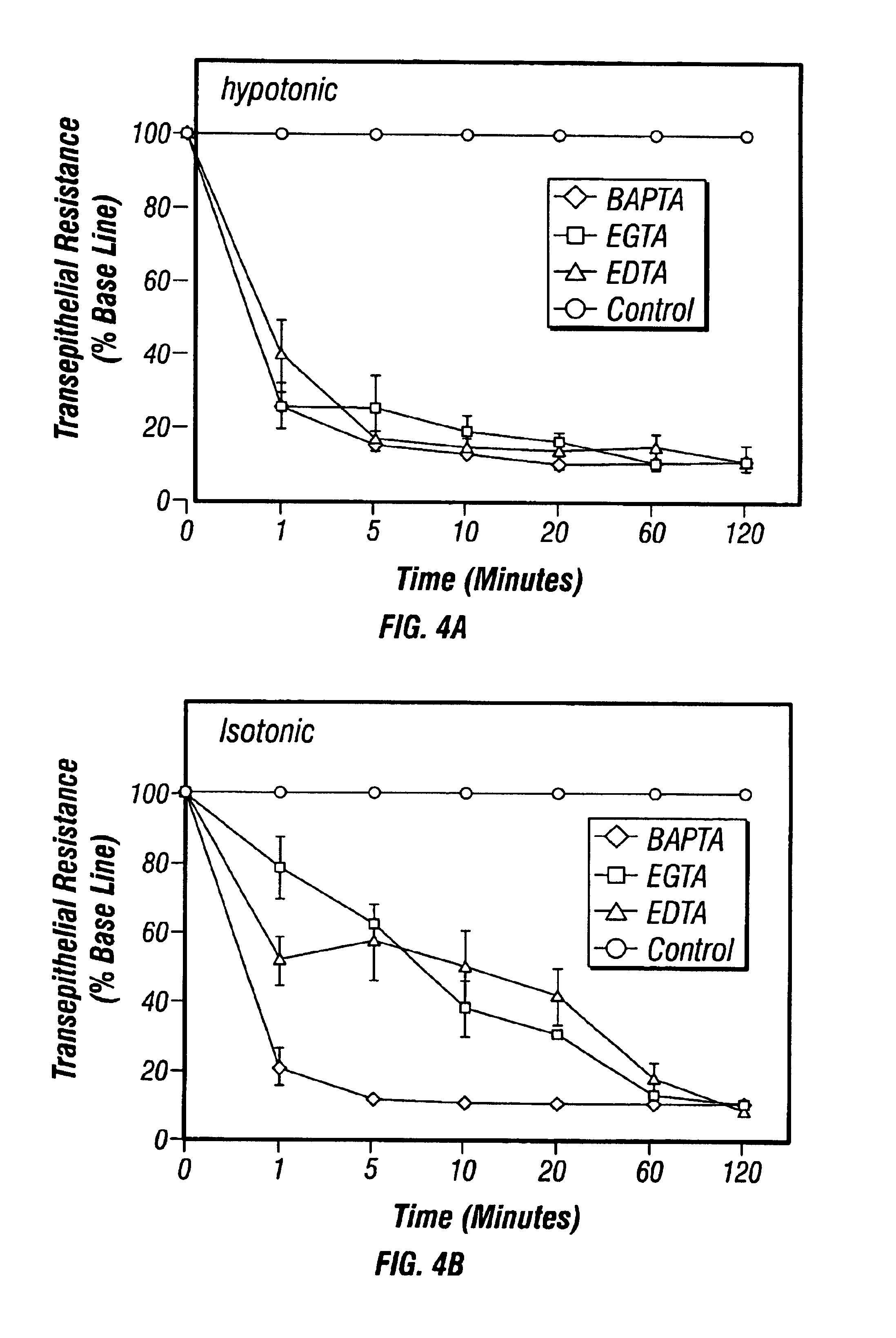
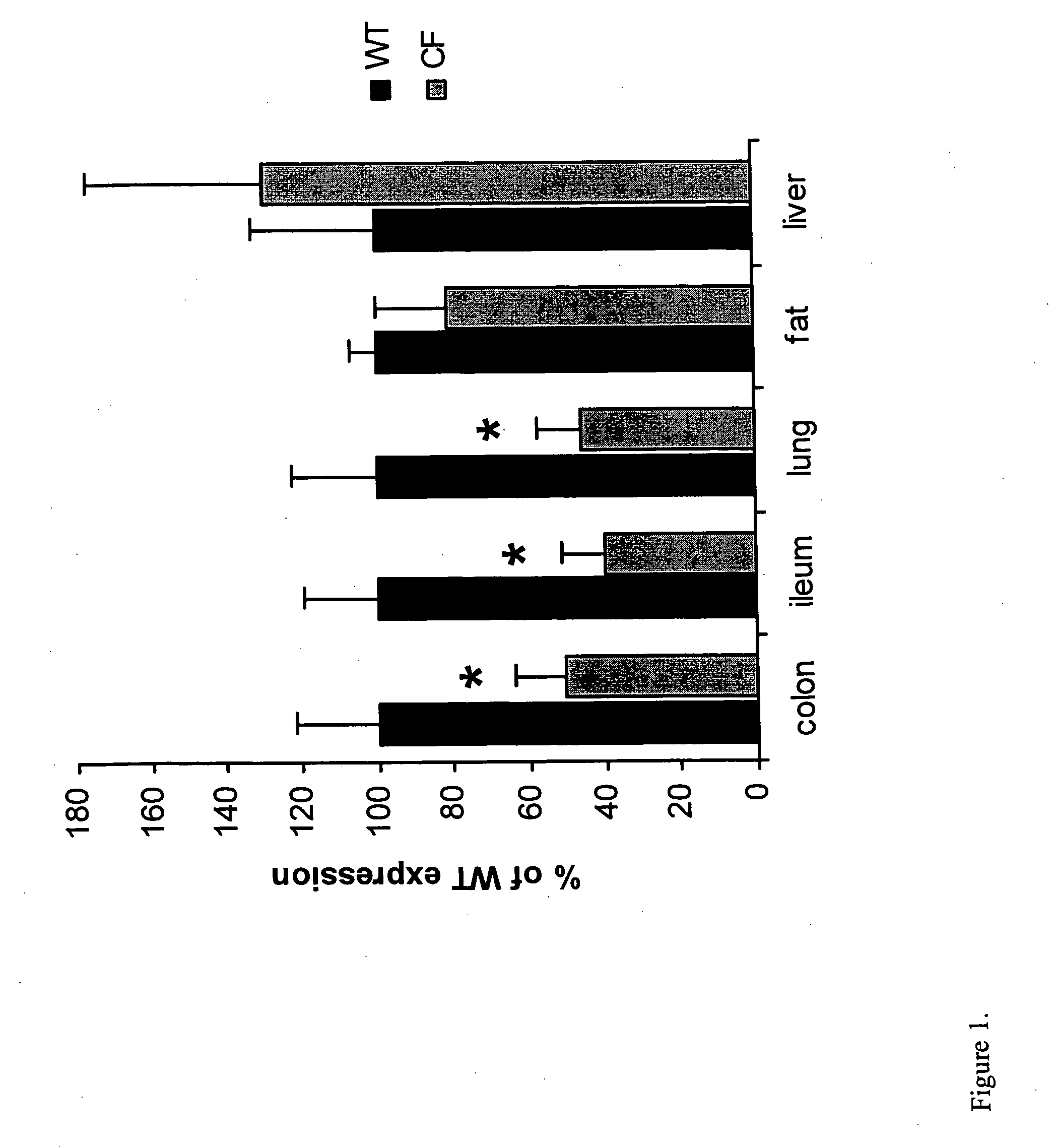
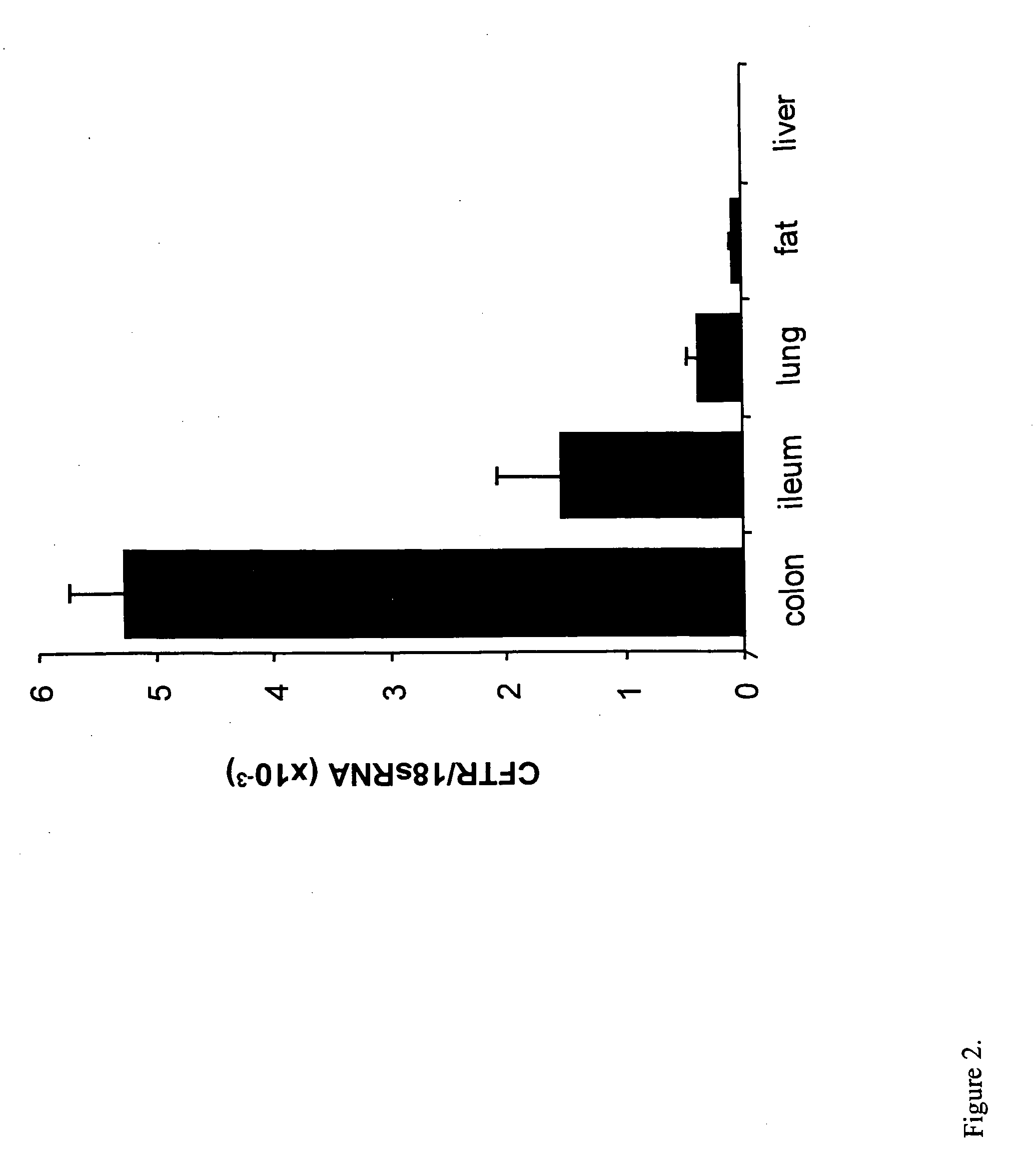
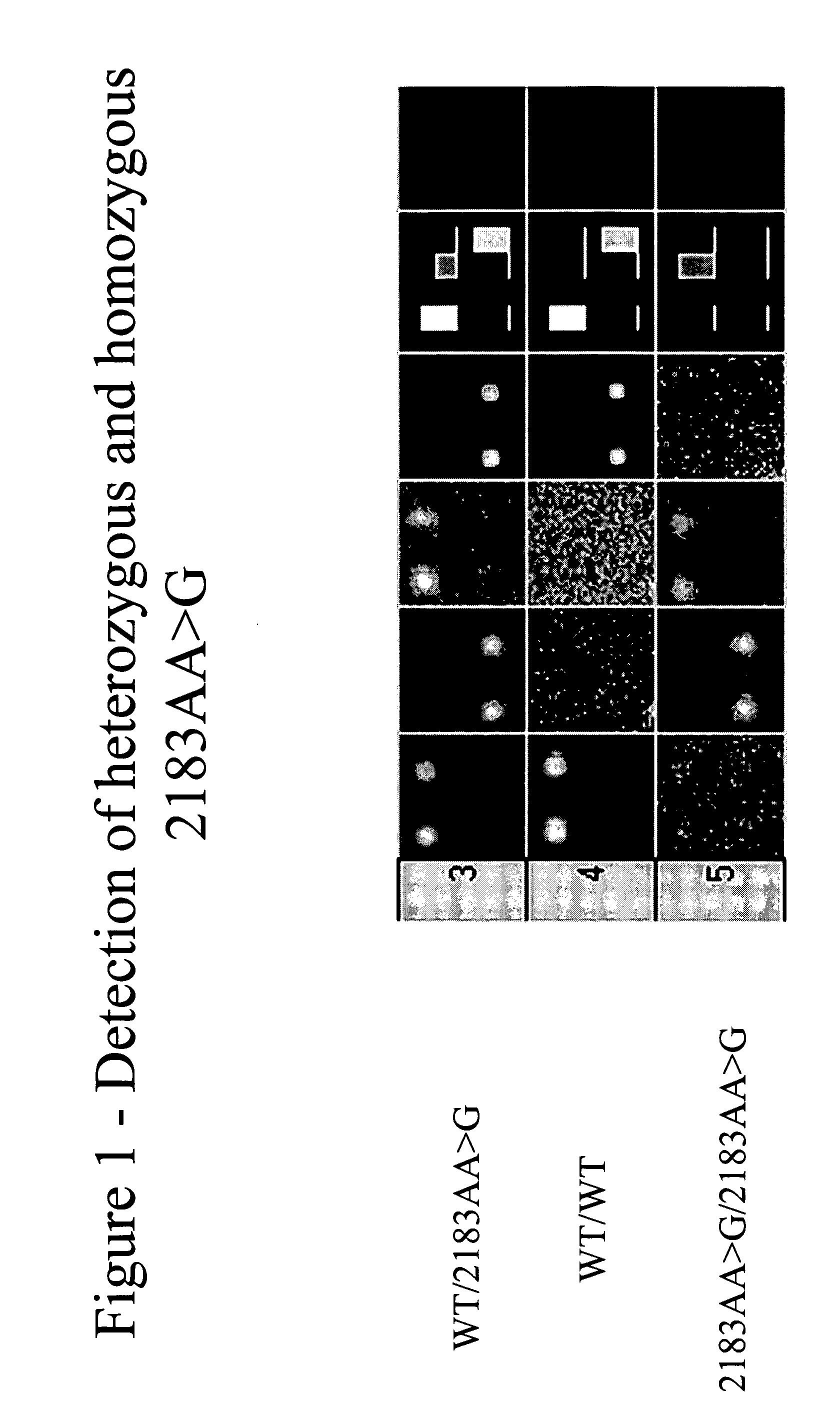
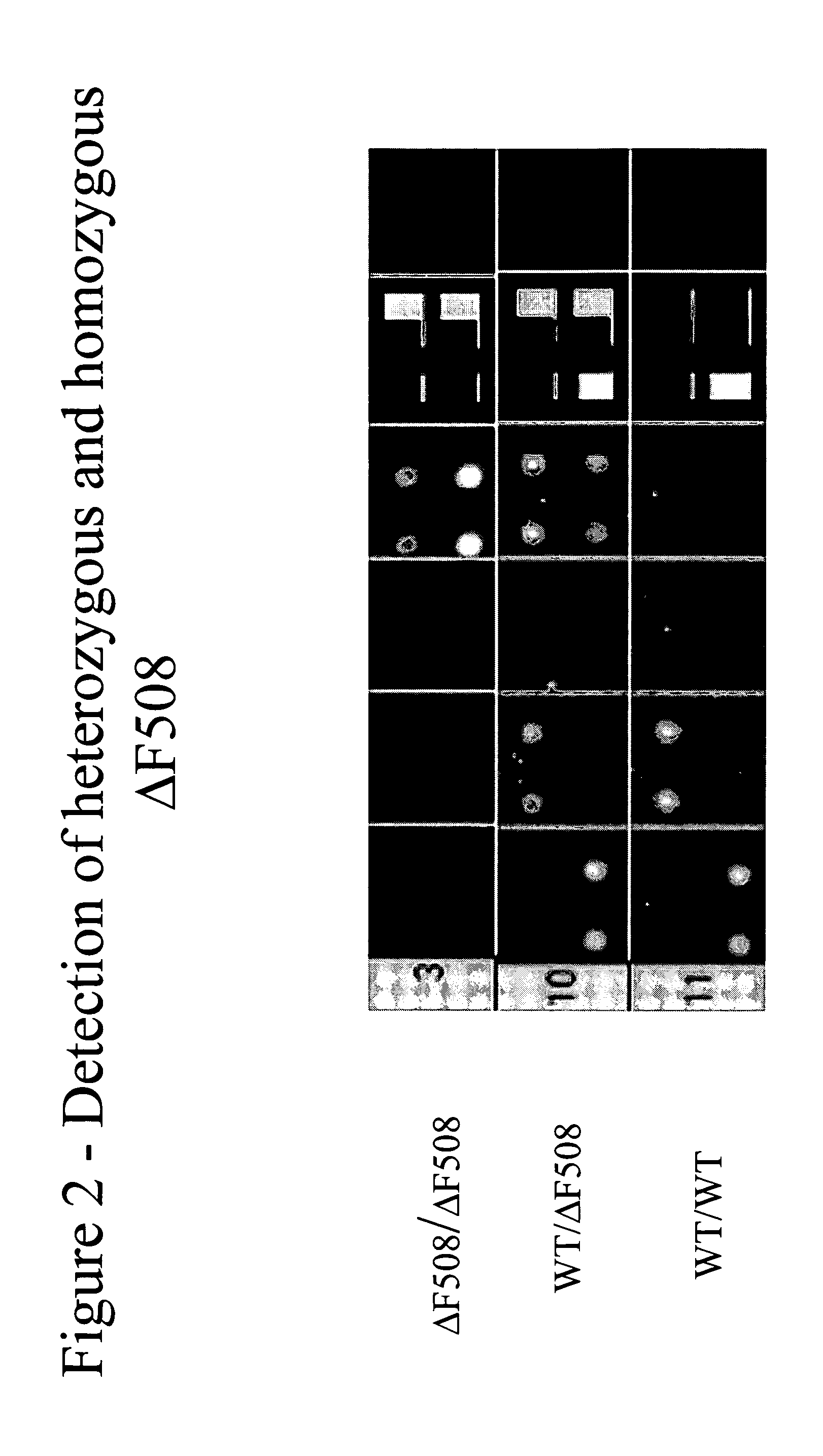
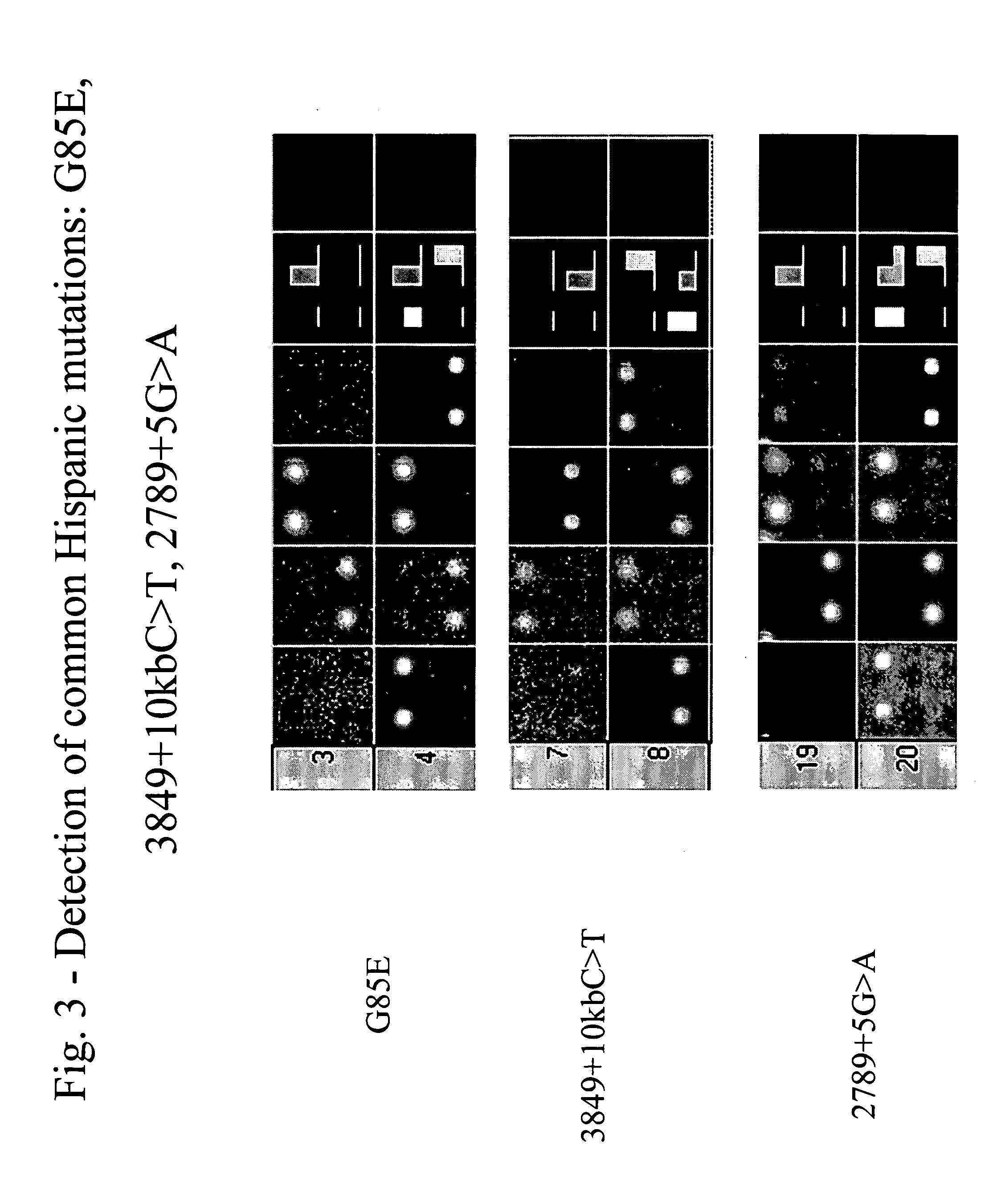
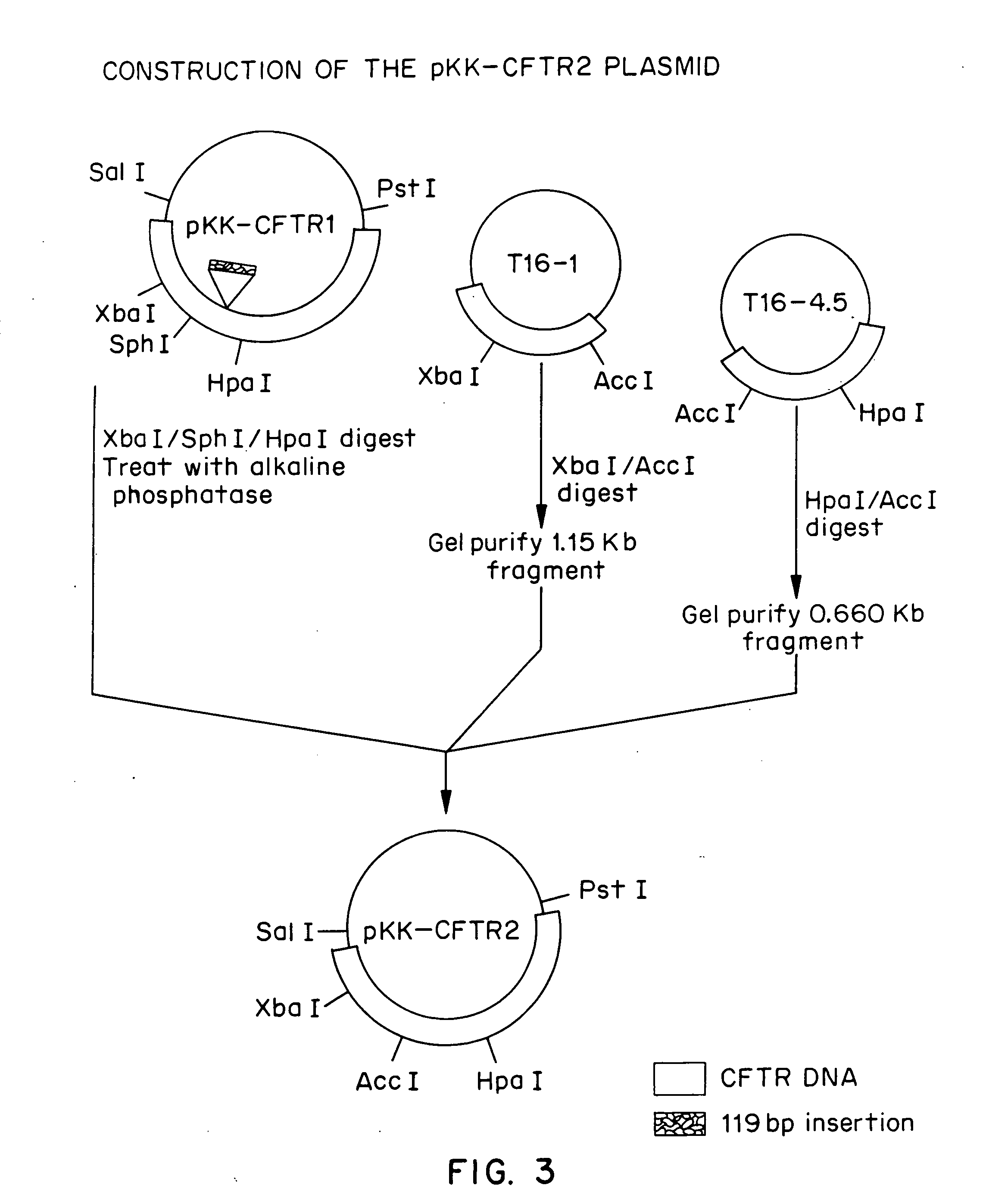
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Cftr gene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
CFTR is an autosomal recessive gene. This means that you must inherit two defective genes, one from each parent, to have cystic fibrosis. A carrier of cystic fibrosis has one CFTR defective gene. A carrier of cystic fibrosis does not present with any symptoms of the condition, but he can pass on one defective gene to his offspring.
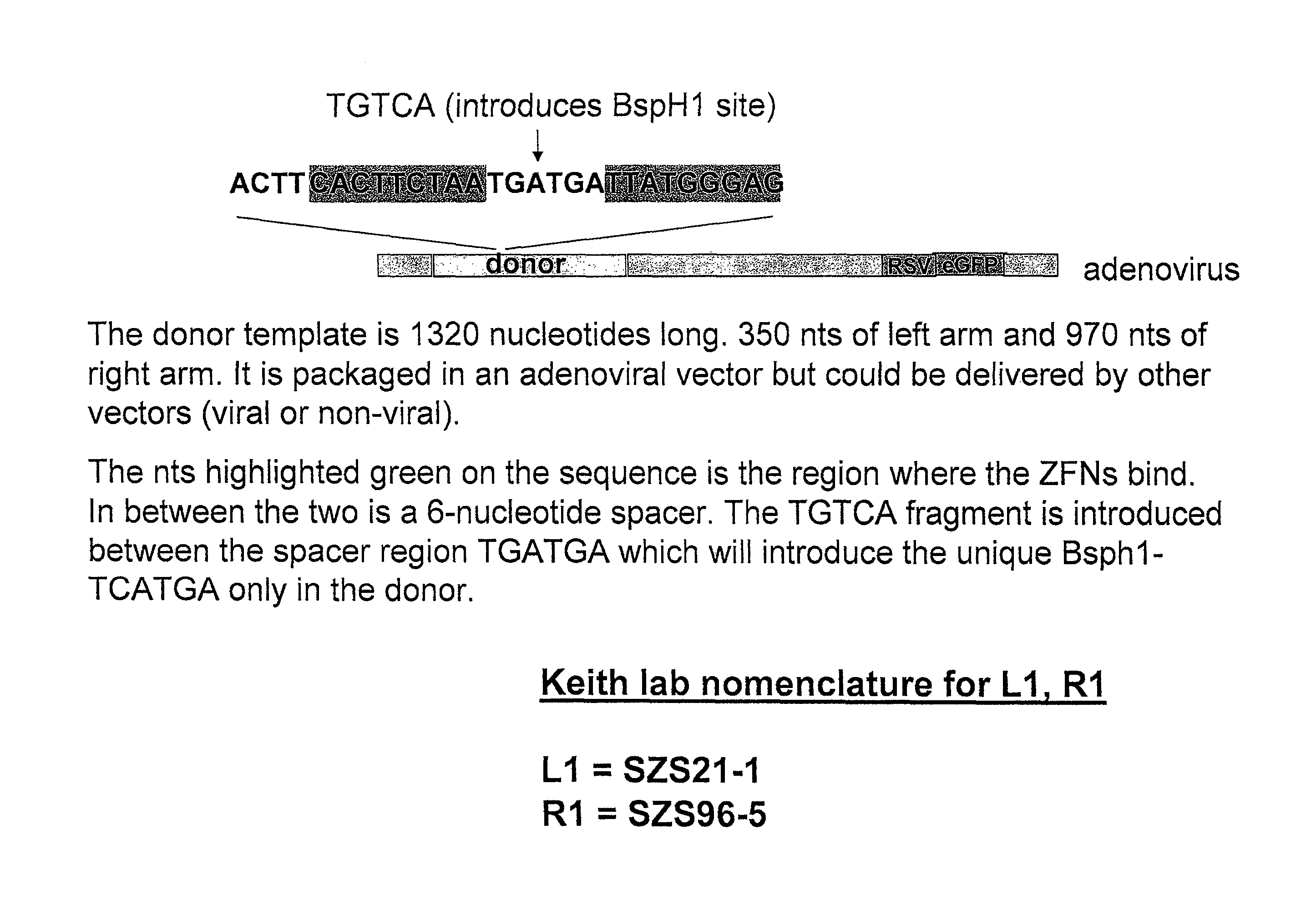
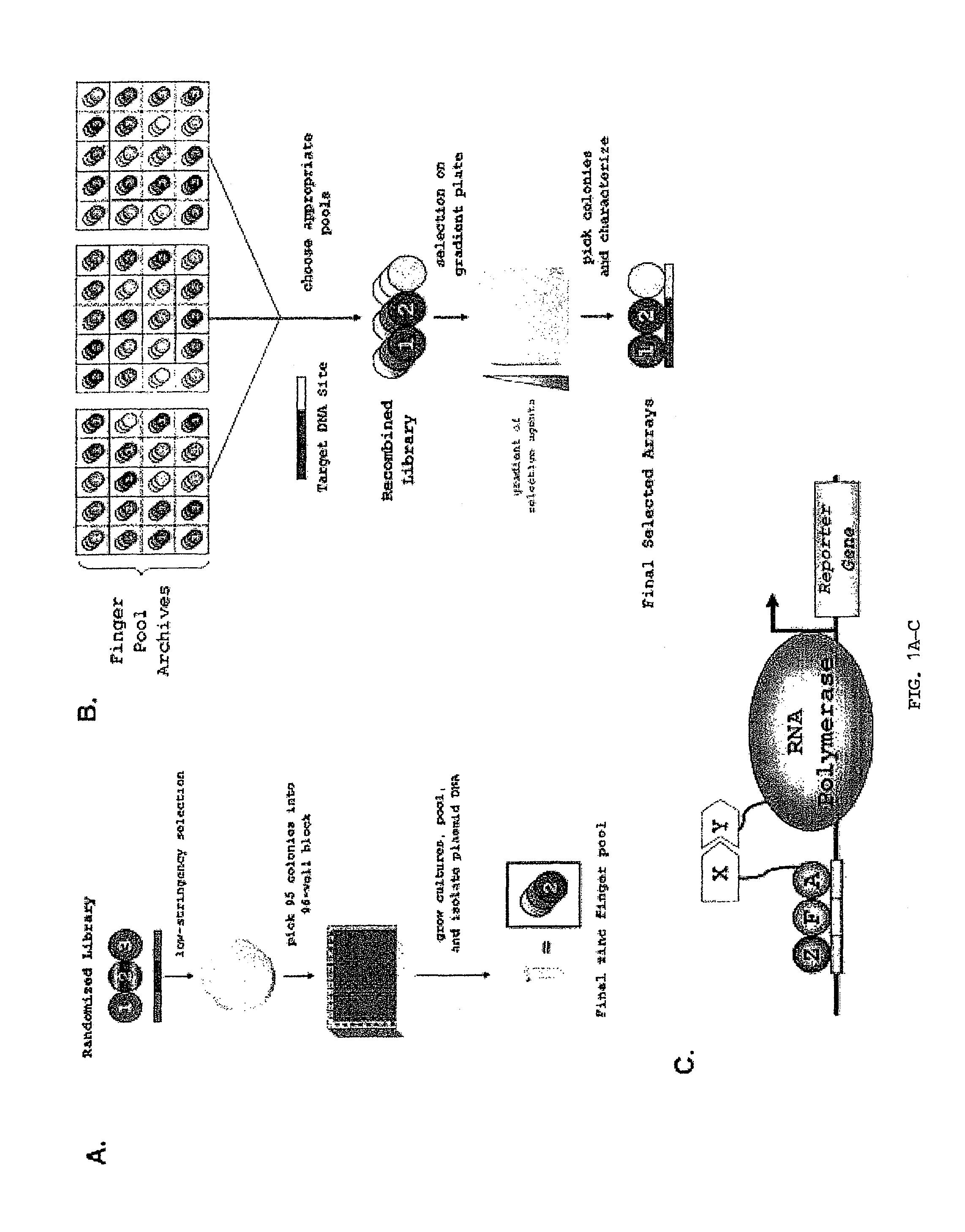
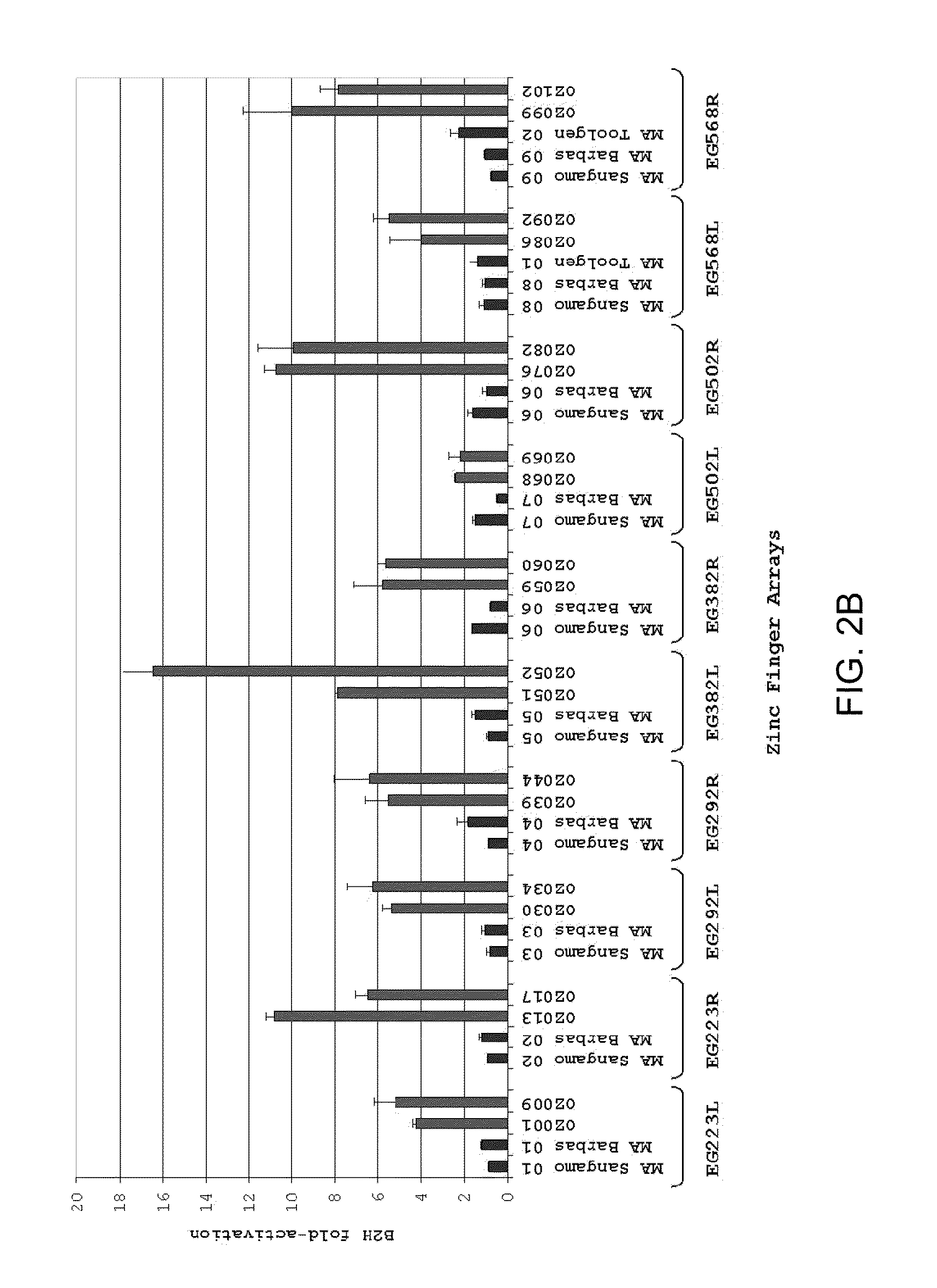
Zinc finger nuclease for the CFTR gene and methods of use thereof
Owner:UNIV OF IOWA RES FOUND +1
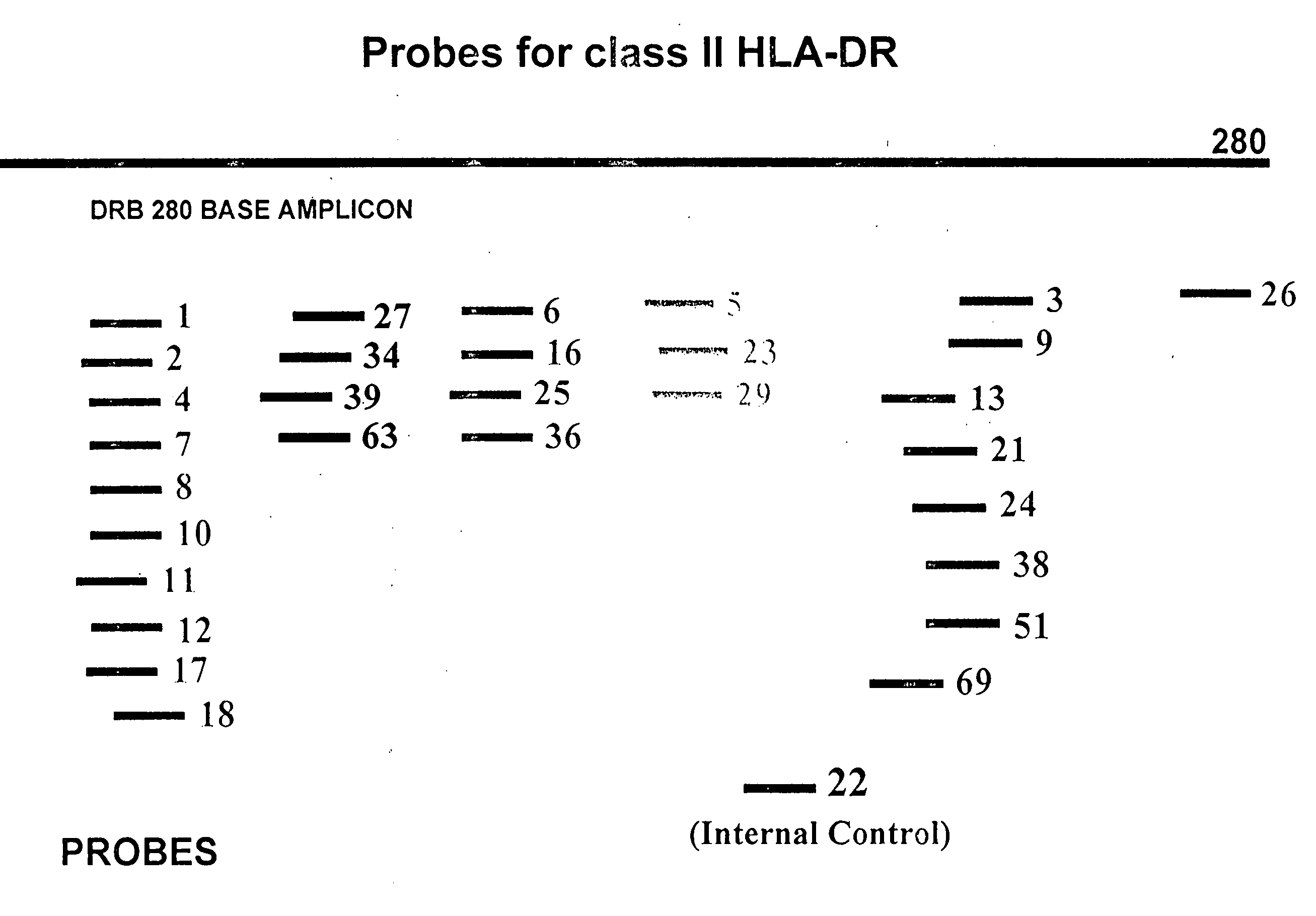
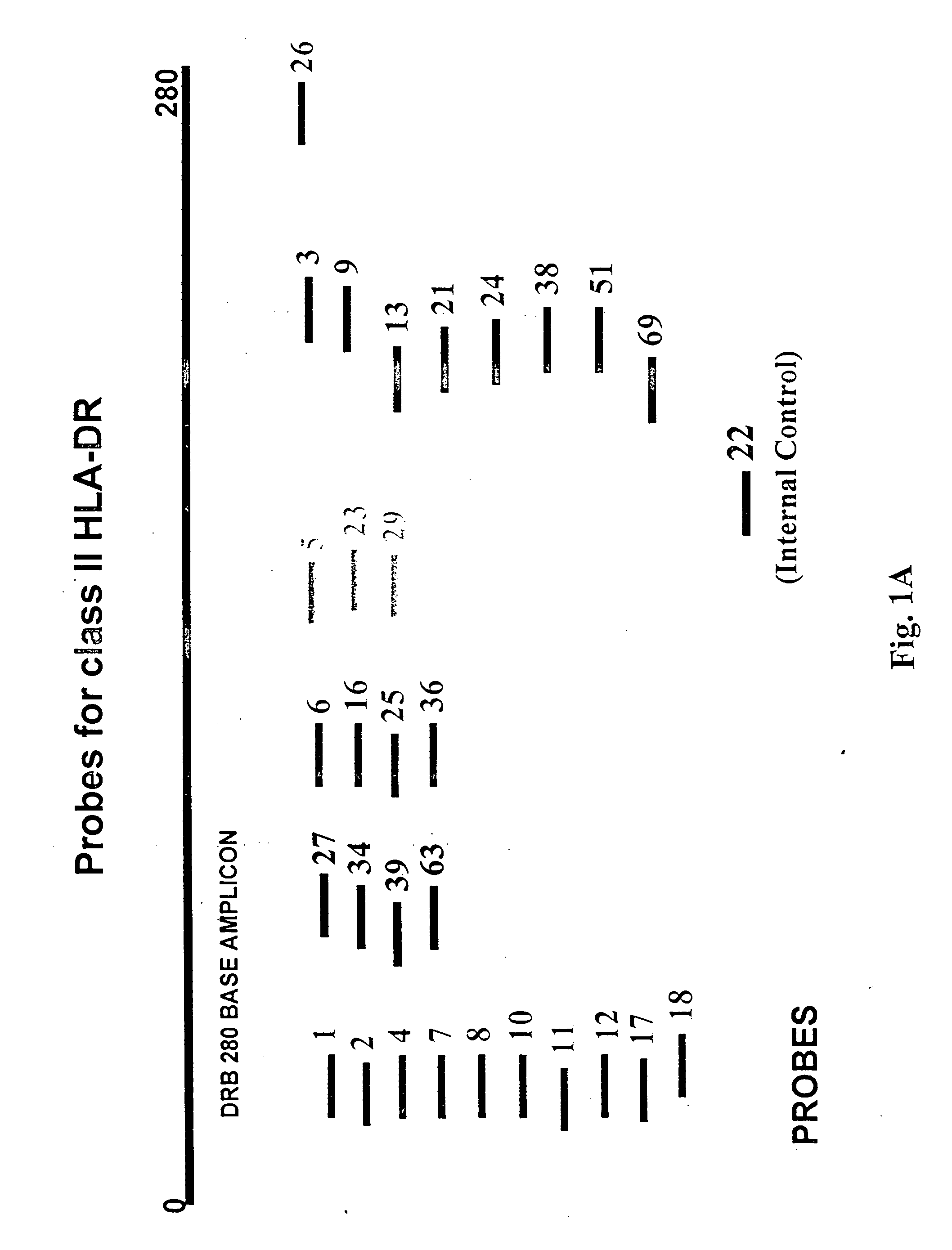
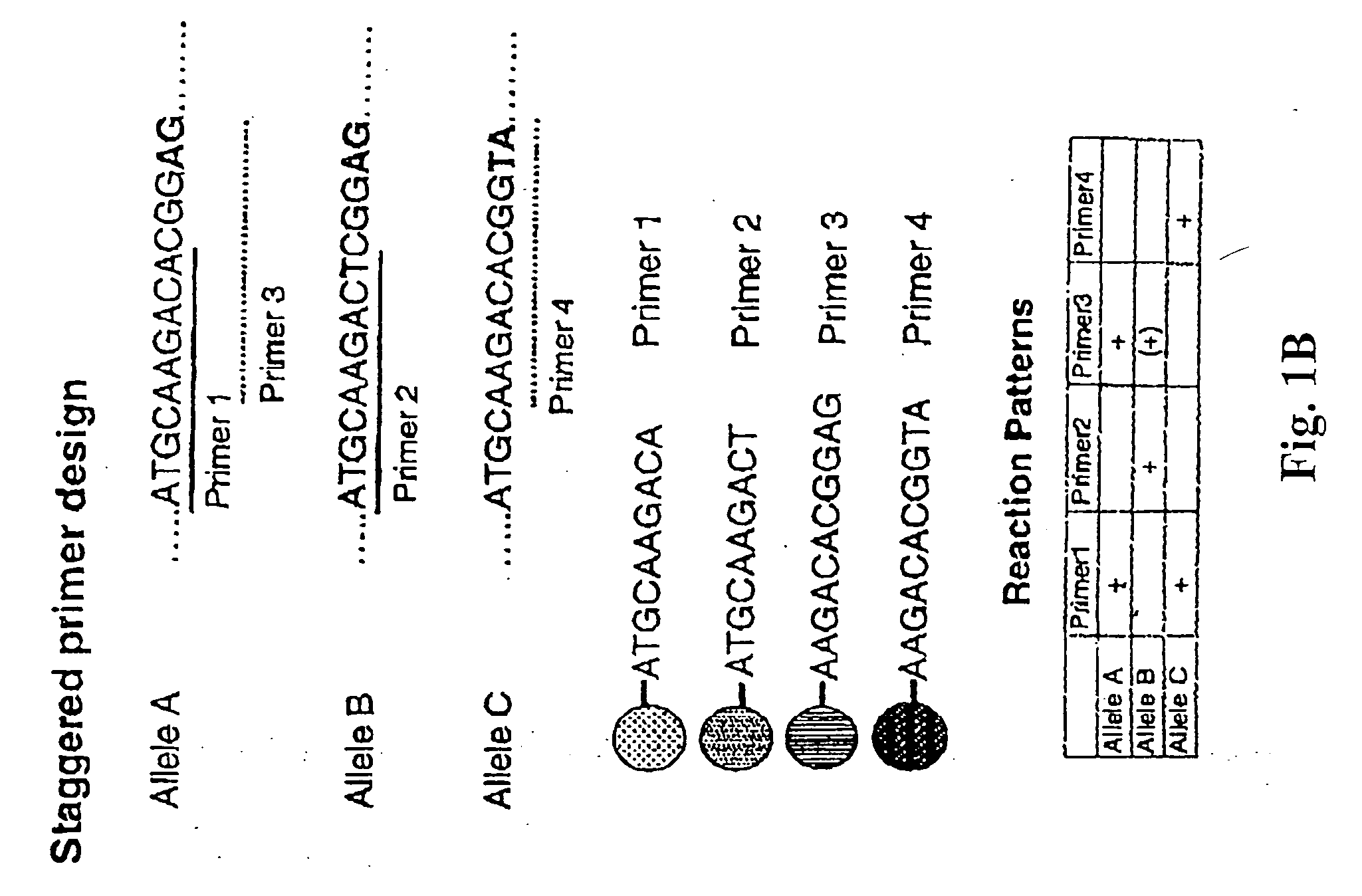
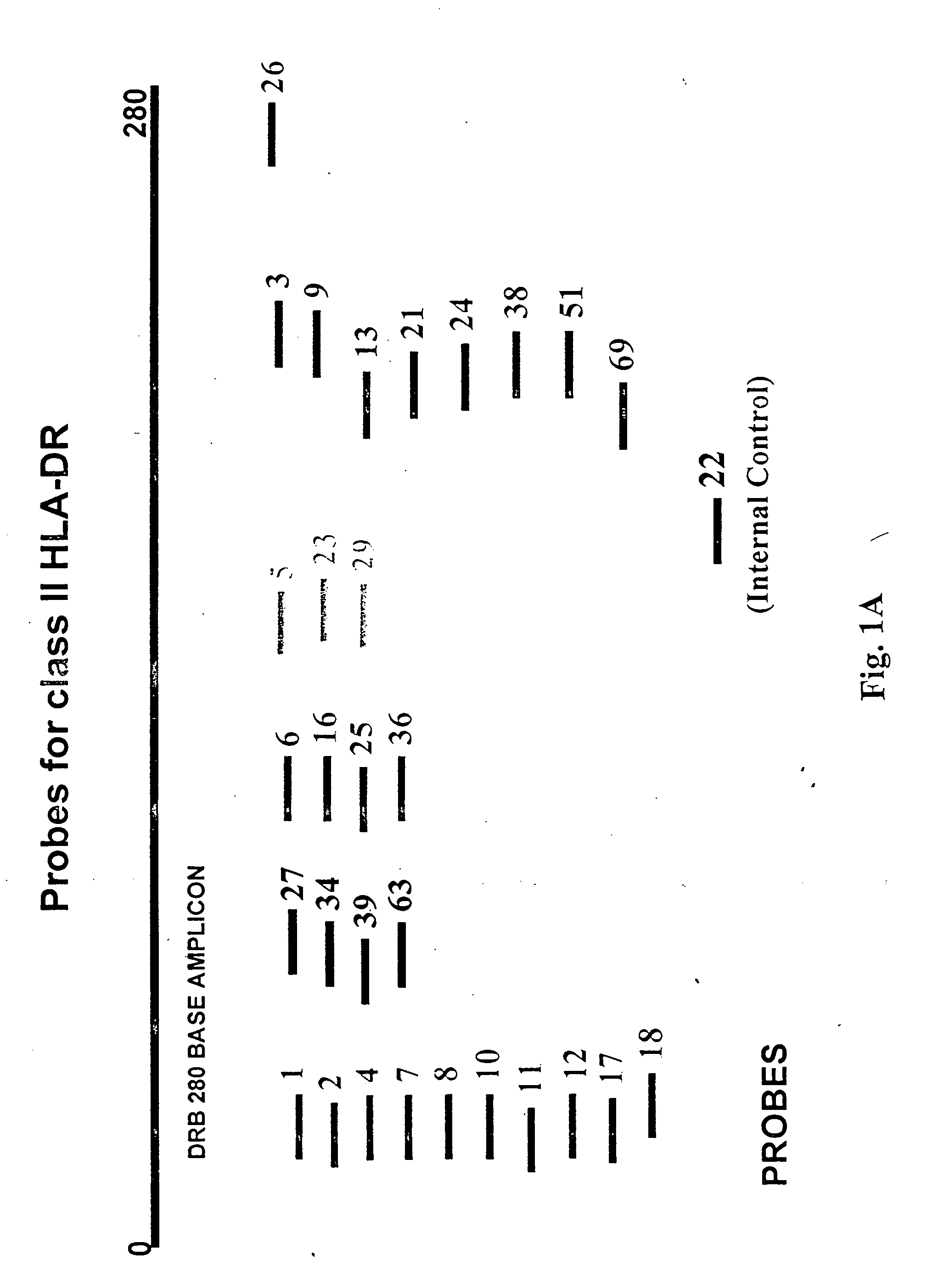
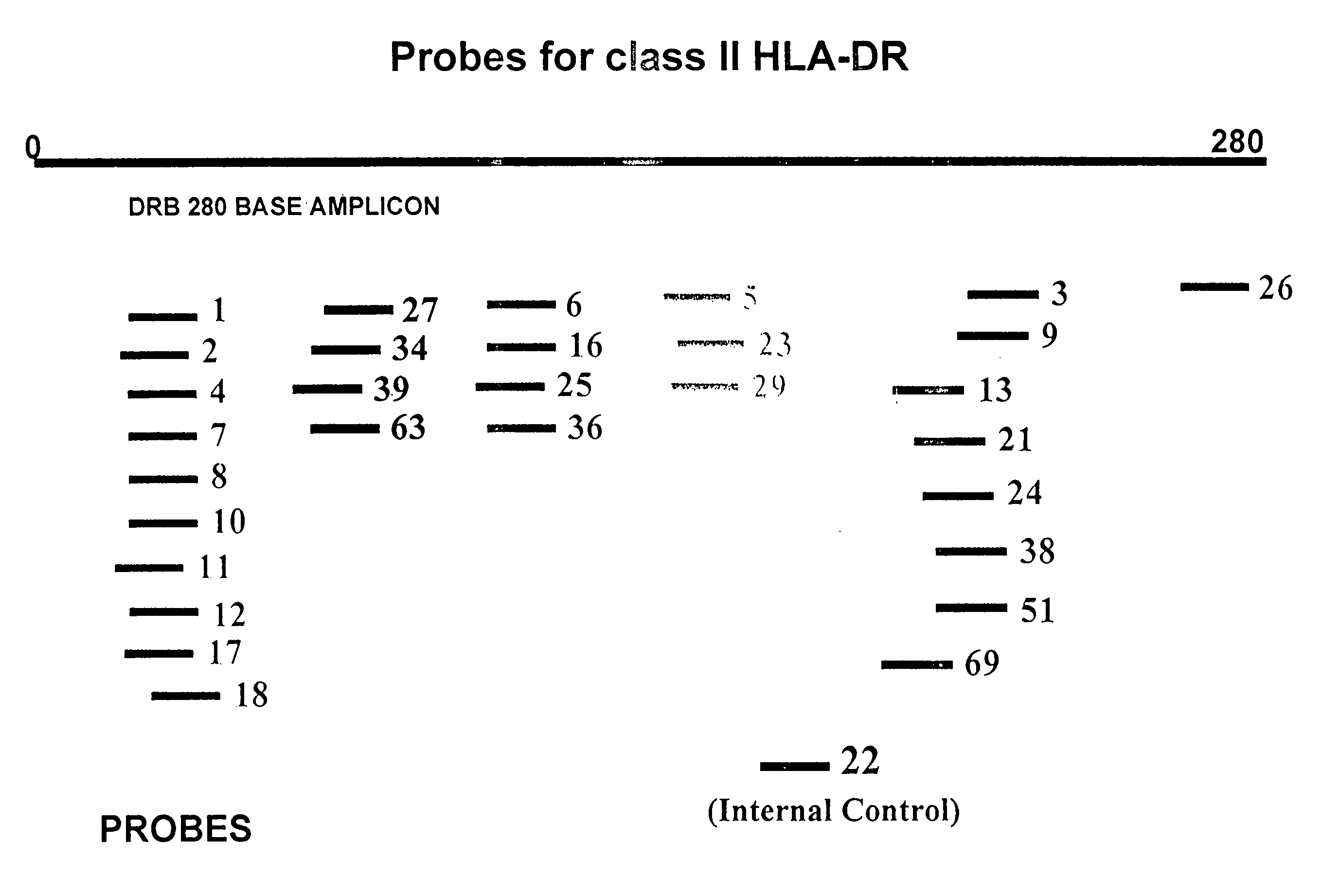
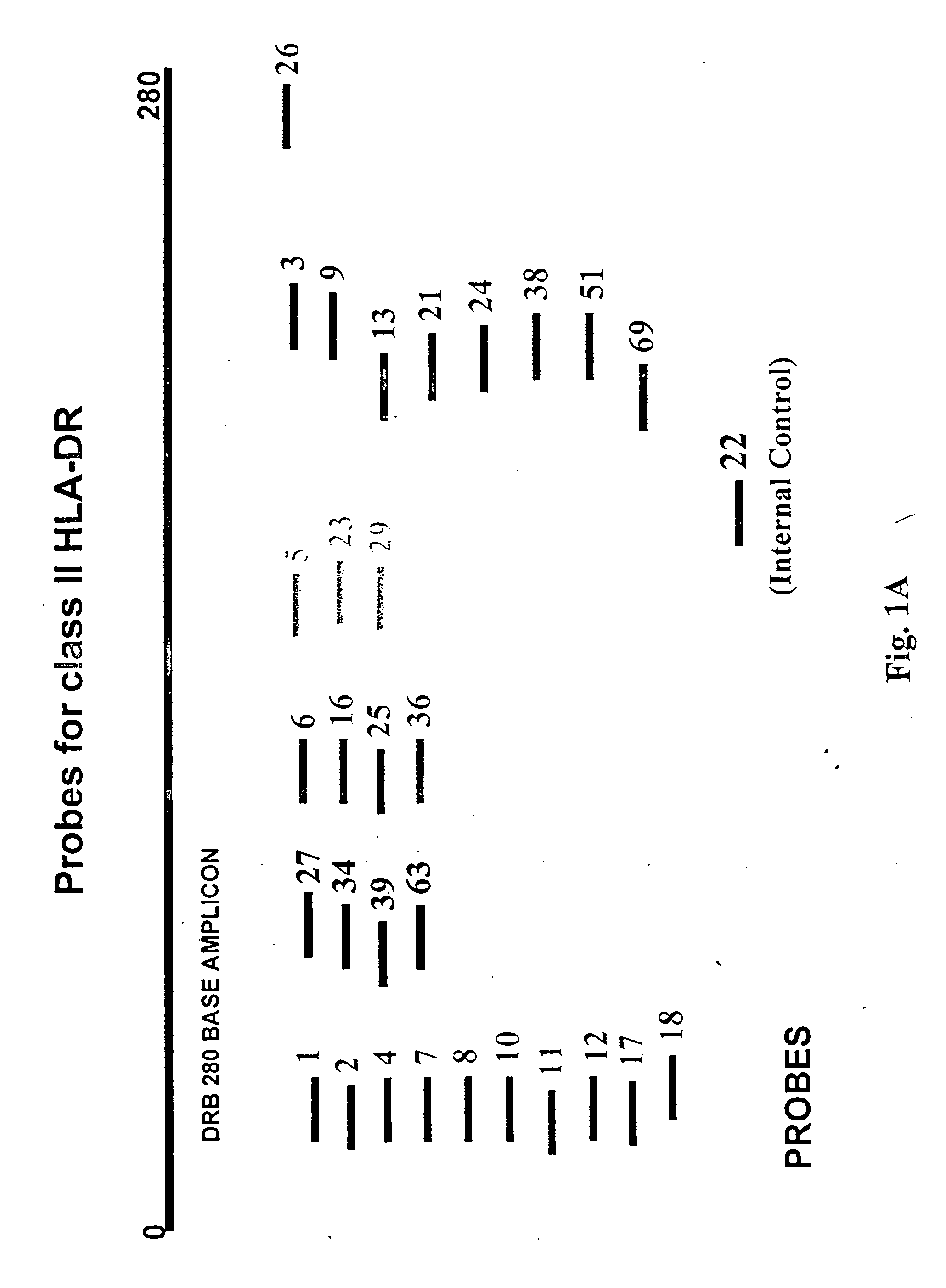
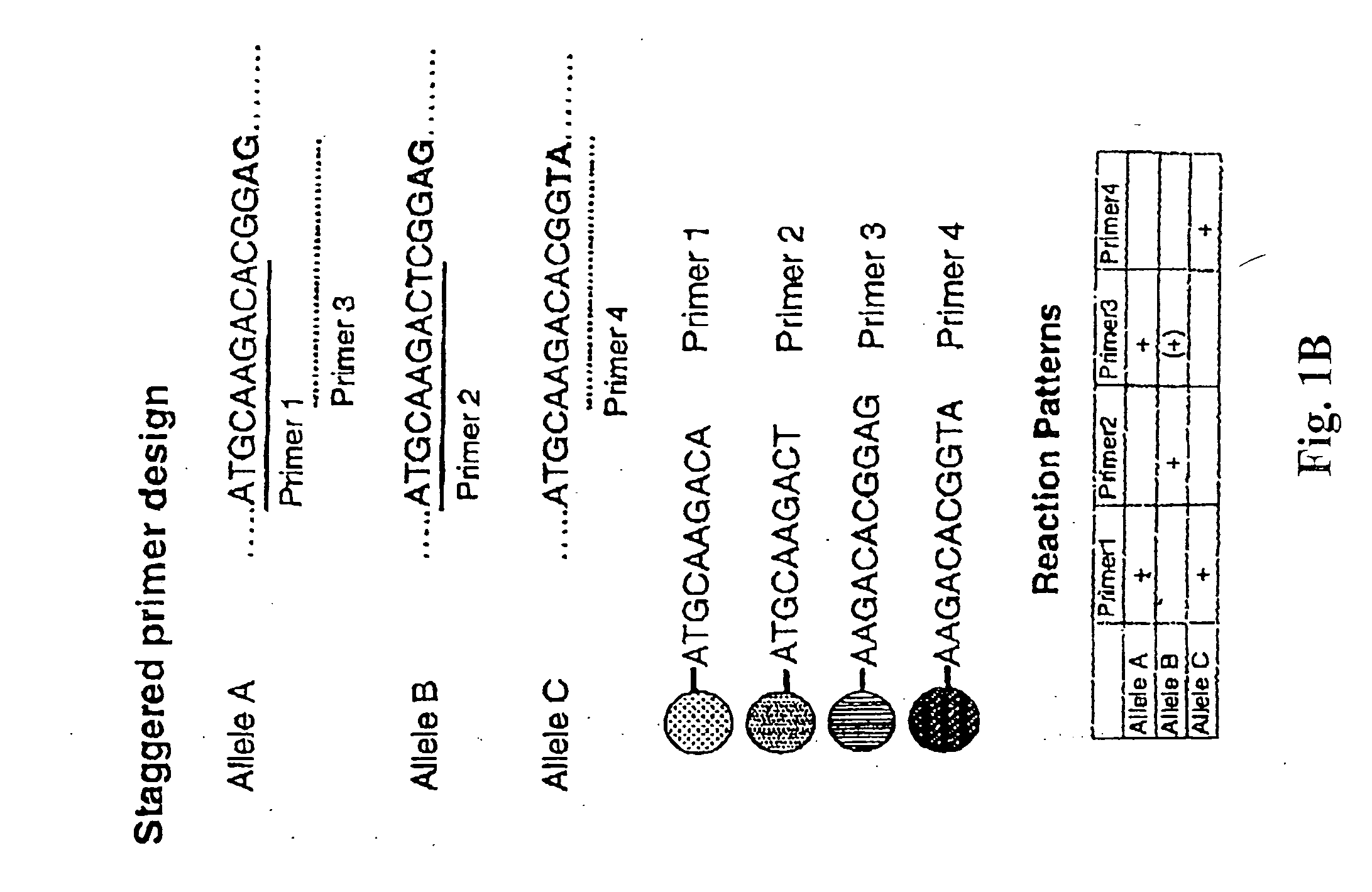
Multiplexed analysis of polymorphic loci by concurrent interrogation and enzyme-mediated detection
InactiveUS20080167195A1Strong specificityDegree of reductionMicrobiological testing/measurementLibrary member identificationHla genesEnzyme
The invention provides methods and processes for the identification of polymorphisms at one or more designated sites, without interference from non-designated sites located within proximity of such designated sites. Probes are provided capable of interrogation of such designated sites in order to determine the composition of each such designated site. By the methods of this invention, one or more mutations within the CFTR gene and the HLA gene complex can be can be identified.
Owner:BIOARRAY SOLUTIONS
Methods and compositions for increasing the infectivity of gene transfer vectors
InactiveUS6855549B1Increased susceptibilityImprove breathabilityPowder deliveryBiocideGene transferCystic fibrosis lungs
The present invention involves methods and compositions for increasing the susceptibility of target cells to transduction by gene transfer vectors. Specifically, it is proposed that increasing intracellular permeability in epithelial tissue increases the percentage of input vector that will transduce that target tissue. Specific examples show that receptors for retrovirus are preferentially accessible on the basolateral surface of airway epithelia, and permeabilizing such tissues results in greater infection with retrovirus. This has important implications in gene therapy, for example, to treat cystic fibrosis with the CFTR gene.
Owner:CHIRON CORP +1
Methods for modulating PPAR biological activity for the treatment of diseases caused by mutations in the CFTR gene
This invention features methods for treating diseases associated with mutations in the CFTR gene by administering PPAR agonists, specifically PPARγ, PPARα, and PPARδ agonists, PPAR inducers, and / or antioxidants. Also disclosed are screening methods for identifying therapeutically useful candidate compounds.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
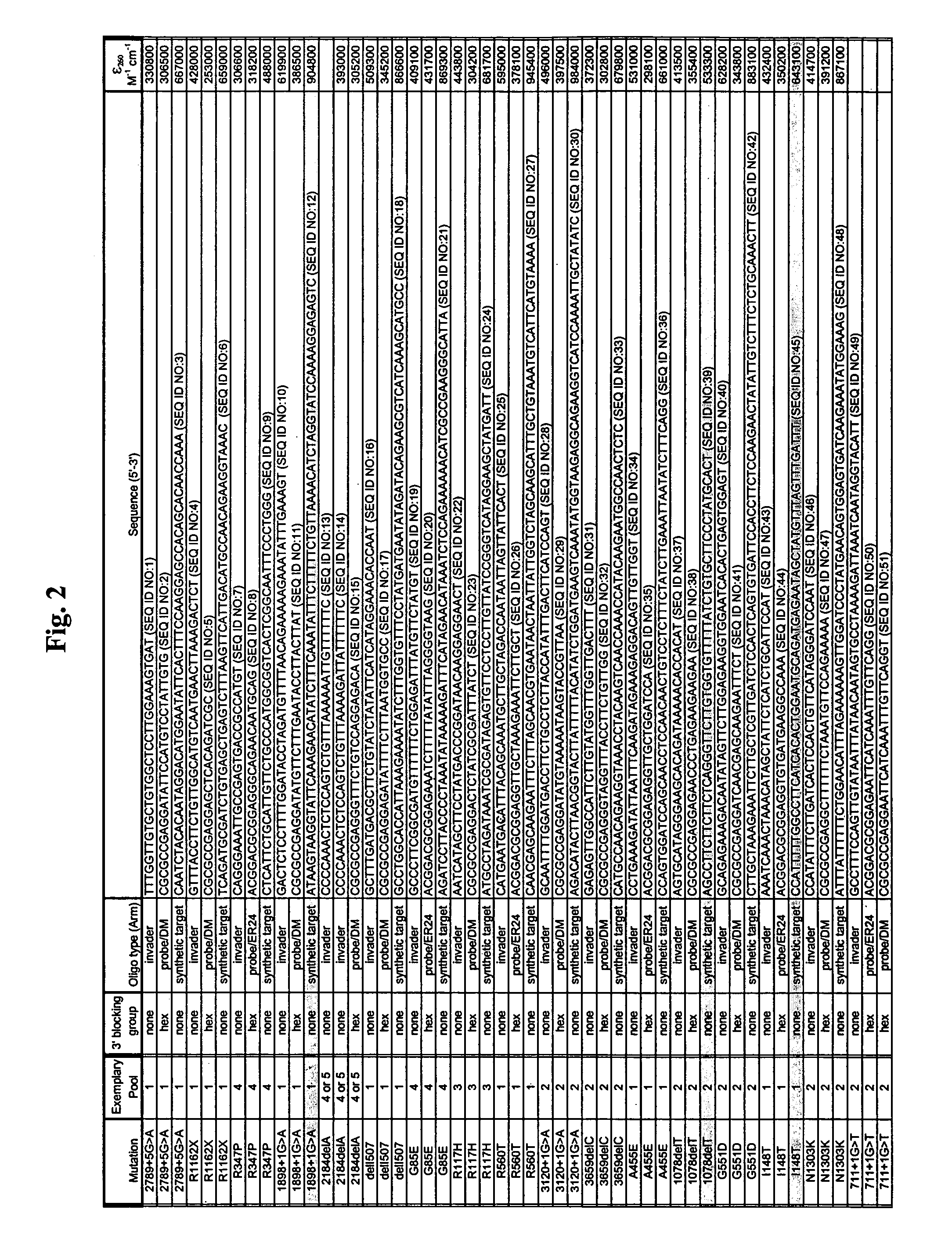
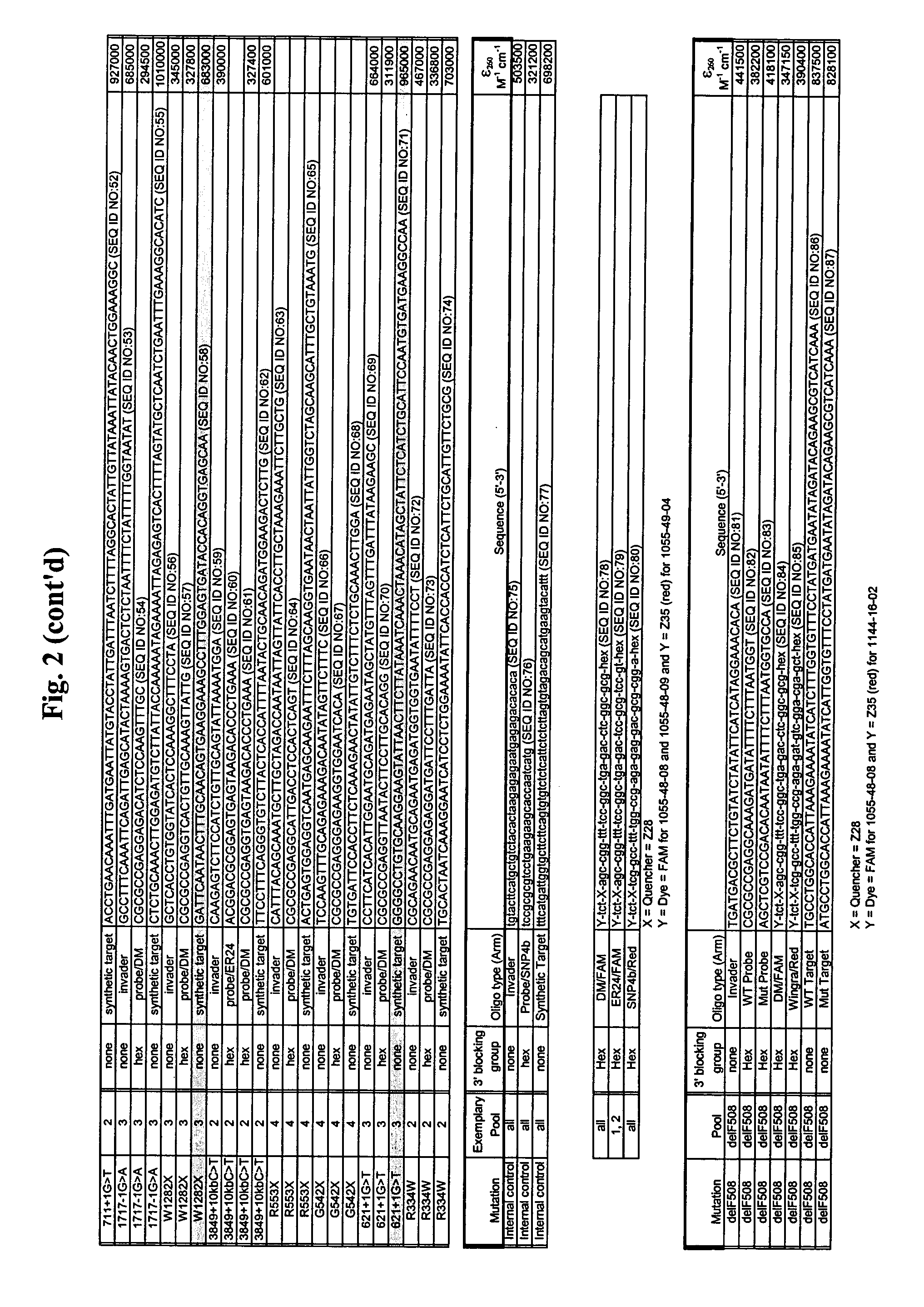
CFTR allele detection assays
InactiveUS7312033B2Reducing agentSugar derivativesMicrobiological testing/measurementAlleleCystic fibrosis lungs
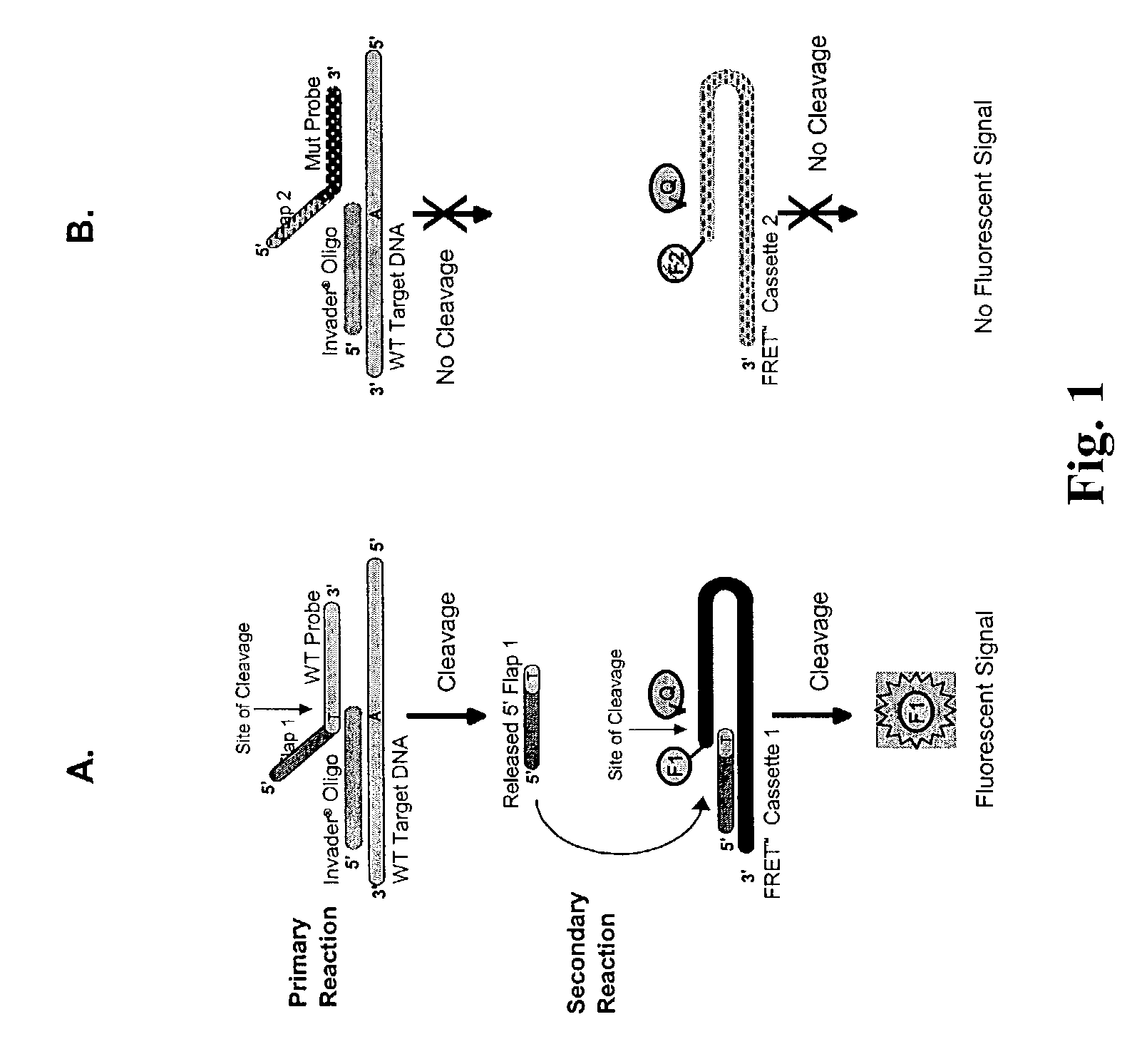
The present invention provides compositions and methods for the detection and characterization of mutations associated with cystic fibrosis. More particularly, the present invention provides compositions, methods and kits for using invasive cleavage structure assays (e.g. the INVADER assay) to screen nucleic acid samples, e.g., from patients, for the presence of any one of a collection of mutations in the CFTR gene associated with cystic fibrosis. The present invention also provides compositions, methods and kits for screening sets of CFTR alleles in a single reaction container.
Owner:GEN PROBE INC
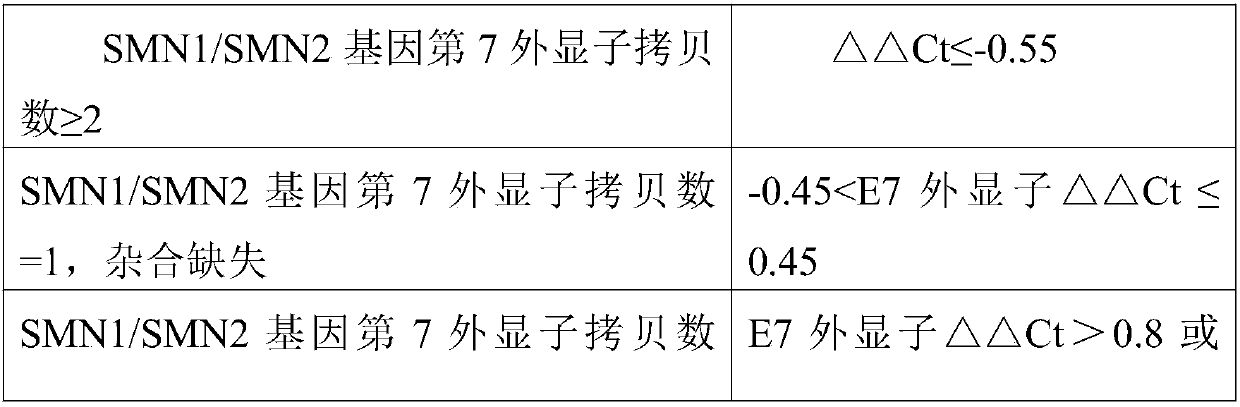
Fluorescent quantitative PCR kit for detecting copy number of virulence gene of human spinal muscular atrophy
InactiveCN108048548AAvoid interferenceEliminate interfering fluorescent signalsMicrobiological testing/measurementDNA/RNA fragmentationReference genesVirulent characteristics
The invention discloses a fluorescent quantitative PCR kit for detecting the copy number of a virulence gene of human spinal muscular atrophy. The fluorescent quantitative PCR kit comprises amplimersand fluorescent probes, wherein the amplimers consist of a pair of shared primers for specific amplification of the seventh exon of SMN1 and SMN2 genes, a pair of shared primers for specific amplification of the eighth exon of the SMN1 and SMN2 genes, and a pair of specific primers for specific amplification of a reference gene, i.e., a CFTR gene; and the fluorescent probes consist of a fluorescent probe for specific detection of the seventh exon of the SMN1 gene, a fluorescent probe for specific detection of the eighth exon of the SMN1 gene, a fluorescent probe for specific detection of the seventh exon of the SMN2 gene, a fluorescent probe for specific detection of the eighth exon of the SMN2 gene and a fluorescent probe for specific detection of the reference gene CFTR. The fluorescentquantitative PCR kit for detecting the copy number of the virulence gene of human spinal muscular atrophy is directed at problems and insufficiencies in quantitative detection of the copy numbers of human motoneuron genes and is rapid and simple in detection and reliable in detection results.
Owner:北京华瑞康源生物科技发展有限公司
Methods for modulating PPAR biological activity for the treatment of diseases caused by mutations in the CFTR gene
This invention features methods for treating diseases associated with mutations in the CFTR gene by administering PPAR agonists, specifically PPARγ, PPARα, and PPARδ agonists, PPAR inducers, and / or antioxidants. Also disclosed are screening methods for identifying therapeutically useful candidate compounds.
Owner:BETH ISRAEL DEACONESS MEDICAL CENT INC
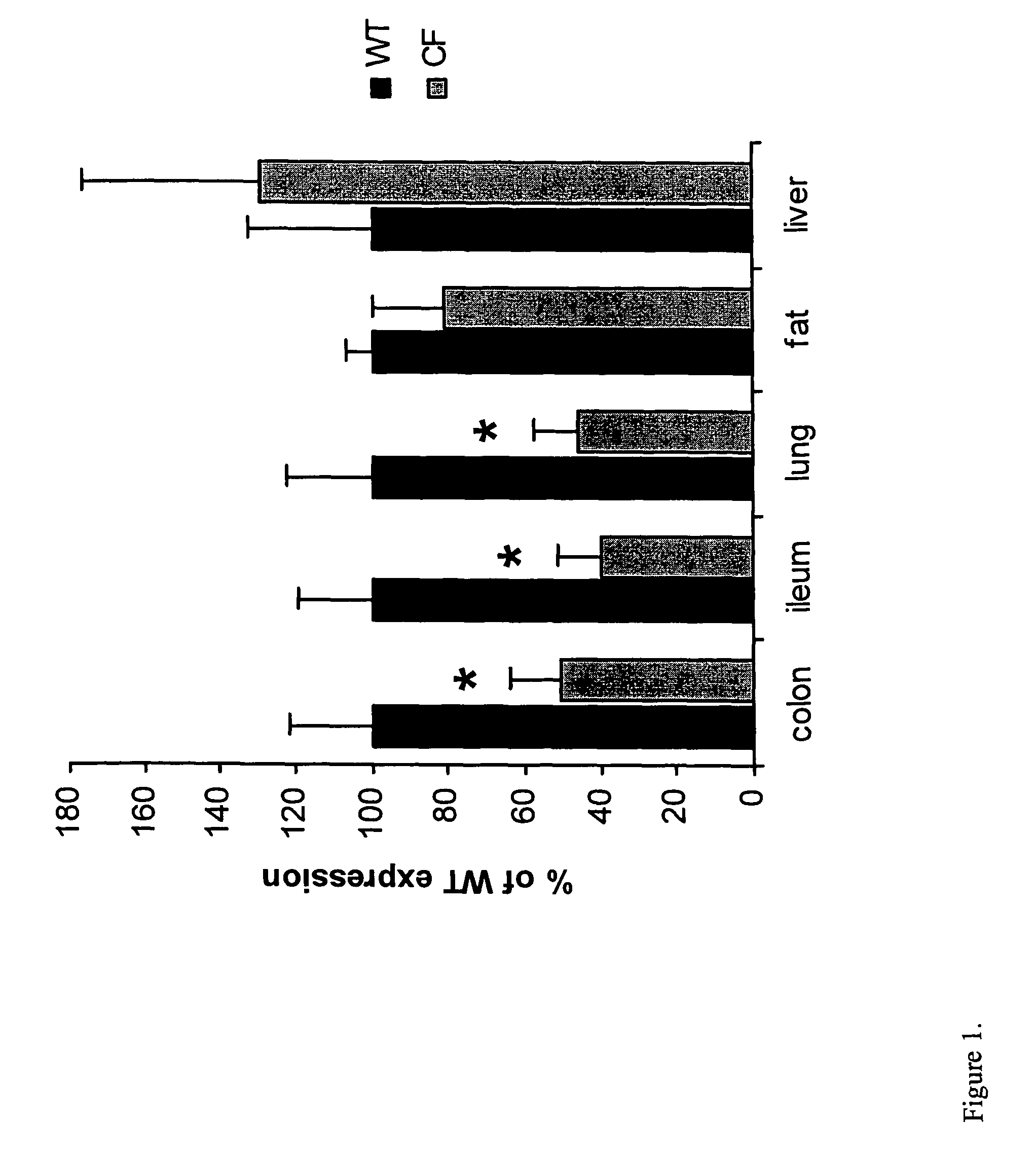
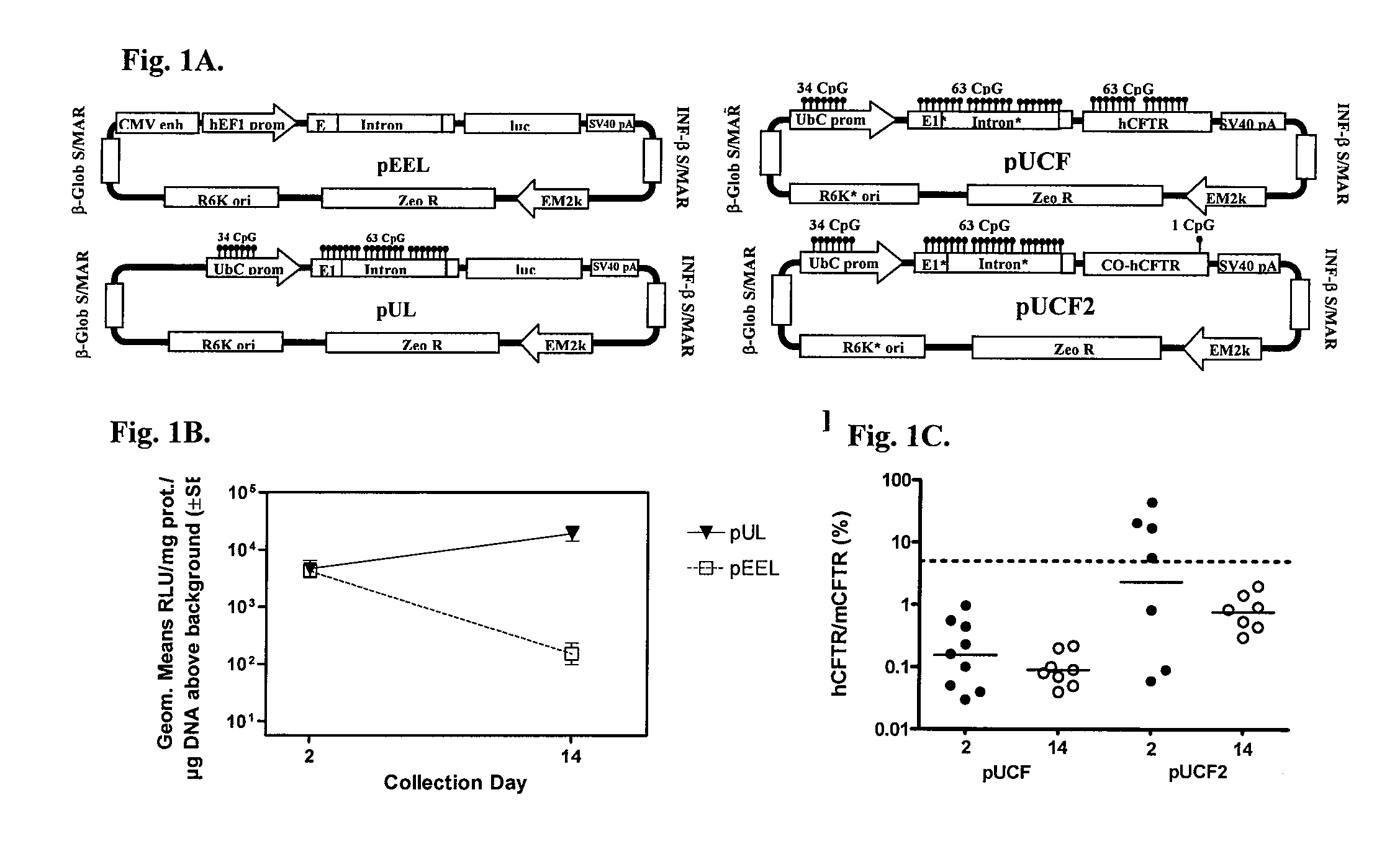
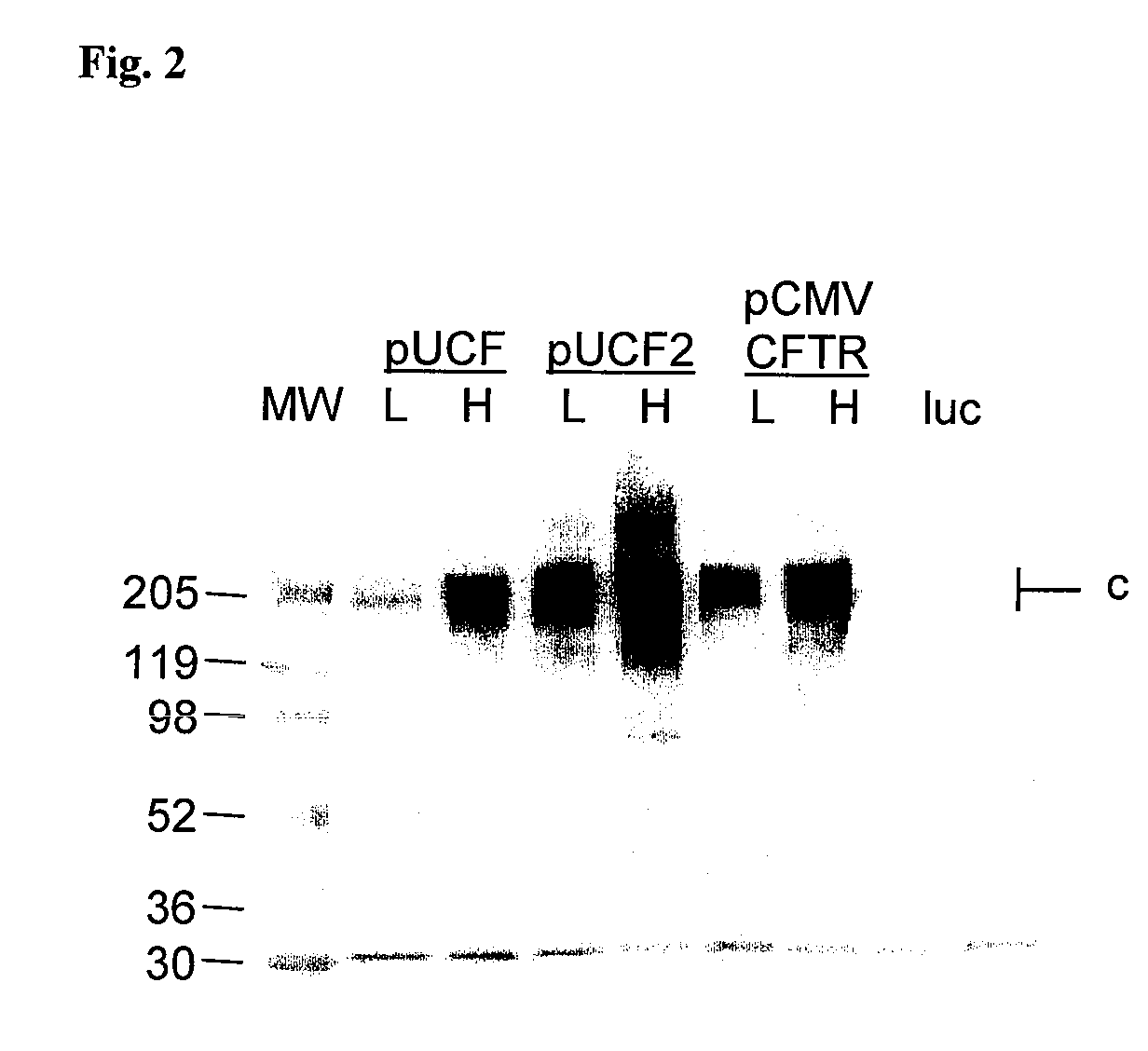
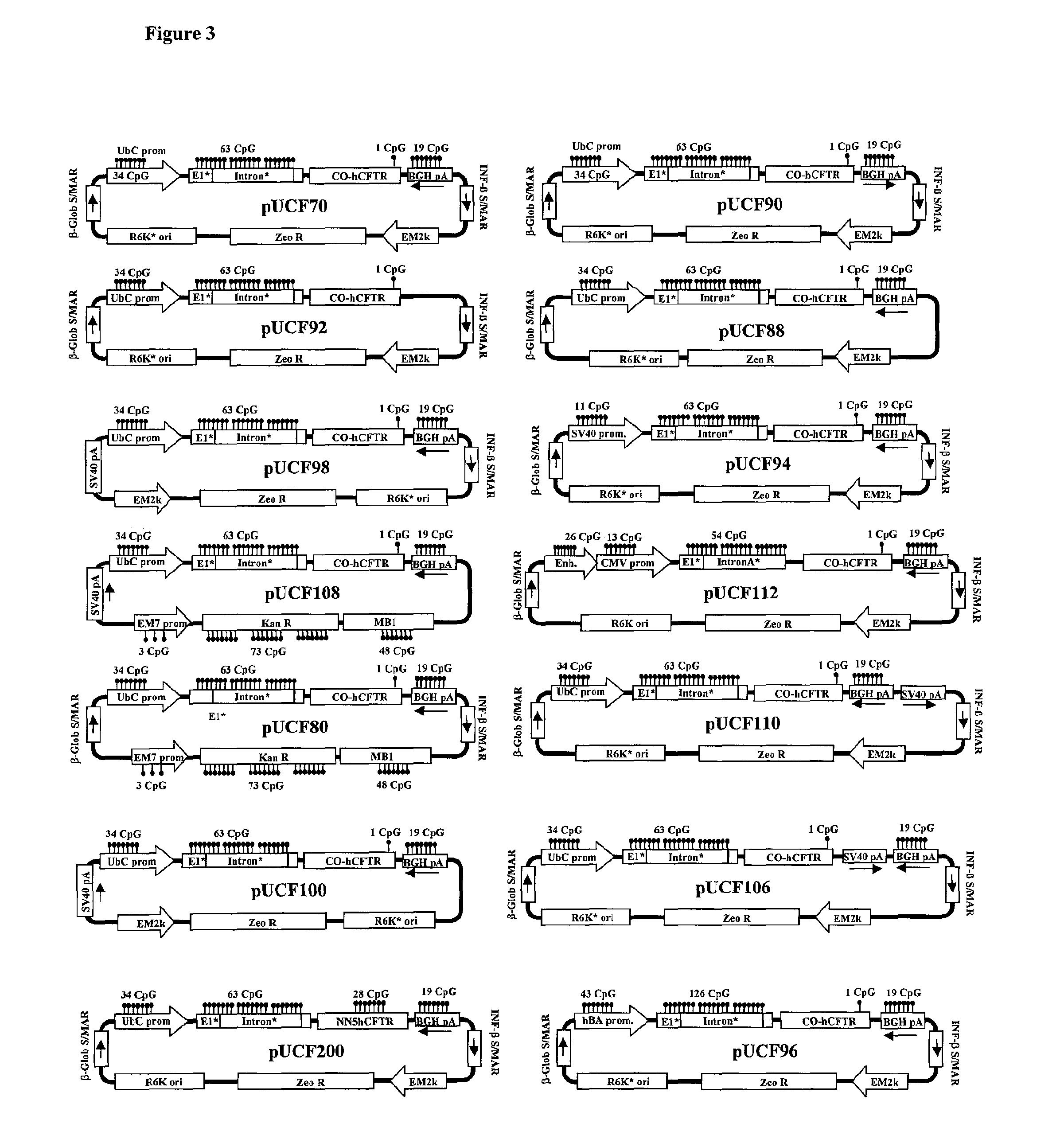
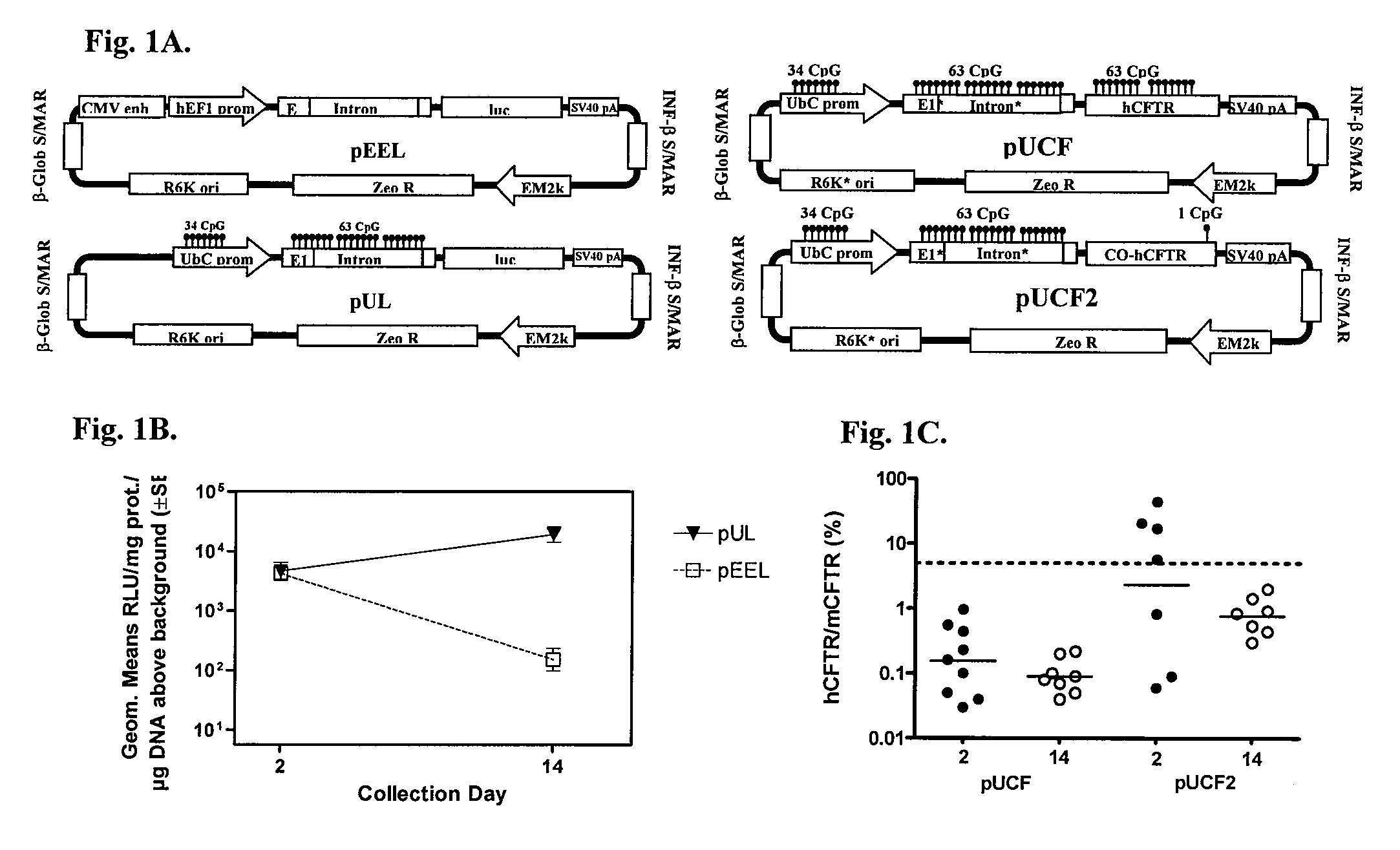
Long-term in vivo transgene expression
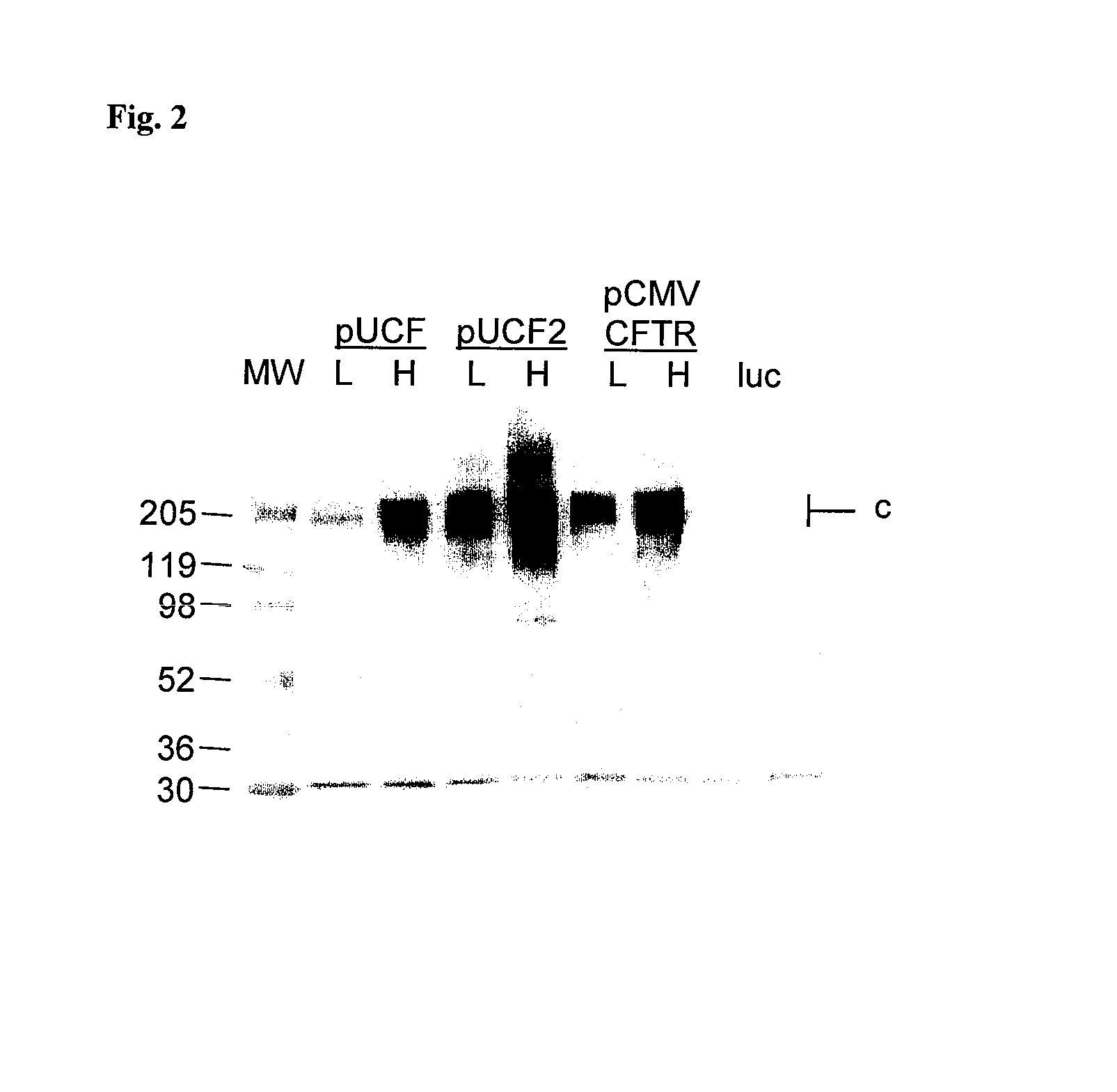
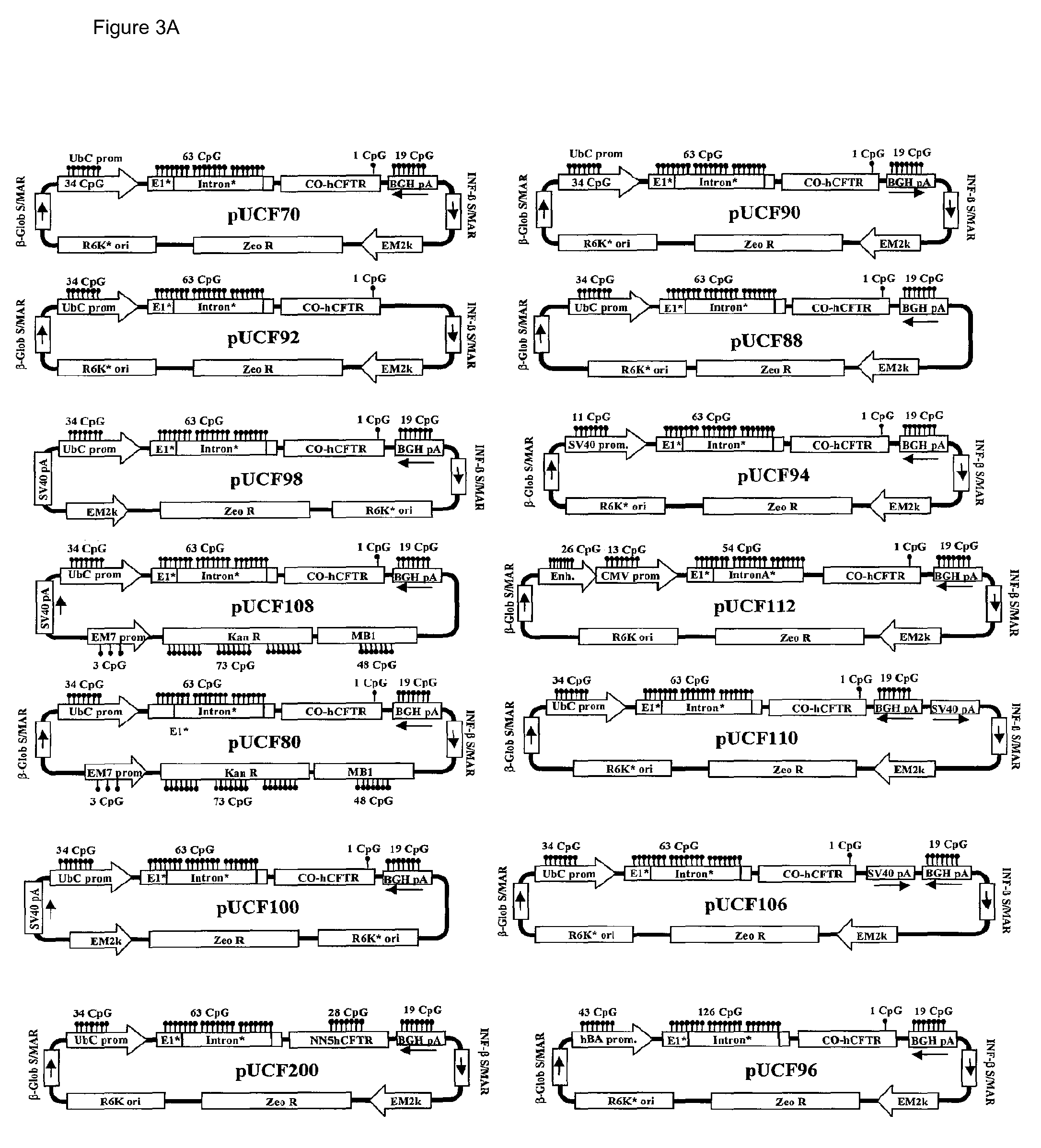
Efficient and prolonged hCFTR expression is one of the major obstacles for cystic fibrosis lung therapy. hCFTR mRNA expression levels depend on eukaryotic expression cassette components, prokaryotic backbone elements, and the gene transfer method may also influence transcriptional silencing mechanisms. A codon-optimized and CpG-reduced human CFTR gene (CO-CFTR) was made. Various vector modifications were tested to facilitate extended duration of CO-CFTR expression. Insertion of an extended 3′BGH transcribed sequence (712 bp) in an inverted orientation produced prolonged expression of CO-CFTR expression at biologically relevant levels. Further studies revealed that prolonged CO-CFTR expression is dependant on the orientation of the extended BGH 3′ BGH transcribed sequence and its transcription, is not specific to the UbC promoter, and is less dependent on other vector backbone elements.
Owner:COPERNICUS THERAPEUTICS
Methods and compositions for determining whether a subject carries a cystic fibrosis transmembrane conductance regulator (CFTR) gene mutation
Methods are provided for determining whether a subject carries a CFTR gene mutation. In practicing the subject methods, an array comprising a plurality of CFTR gene mutation probes is contacted with a nucleic acid sample from the subject, and the presence of any resultant surface bound target nucleic acids is detected to determine whether the subject carries a CFTR gene mutation. In addition, reagents and kits thereof that find use in practicing the subject methods are provided.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Mutation detection in highly homologous genomic regions
InactiveUS20140302501A1High resolution thermal melting curveMinimize amplicon sizeBioreactor/fermenter combinationsBiological substance pretreatmentsMutation detectionExon
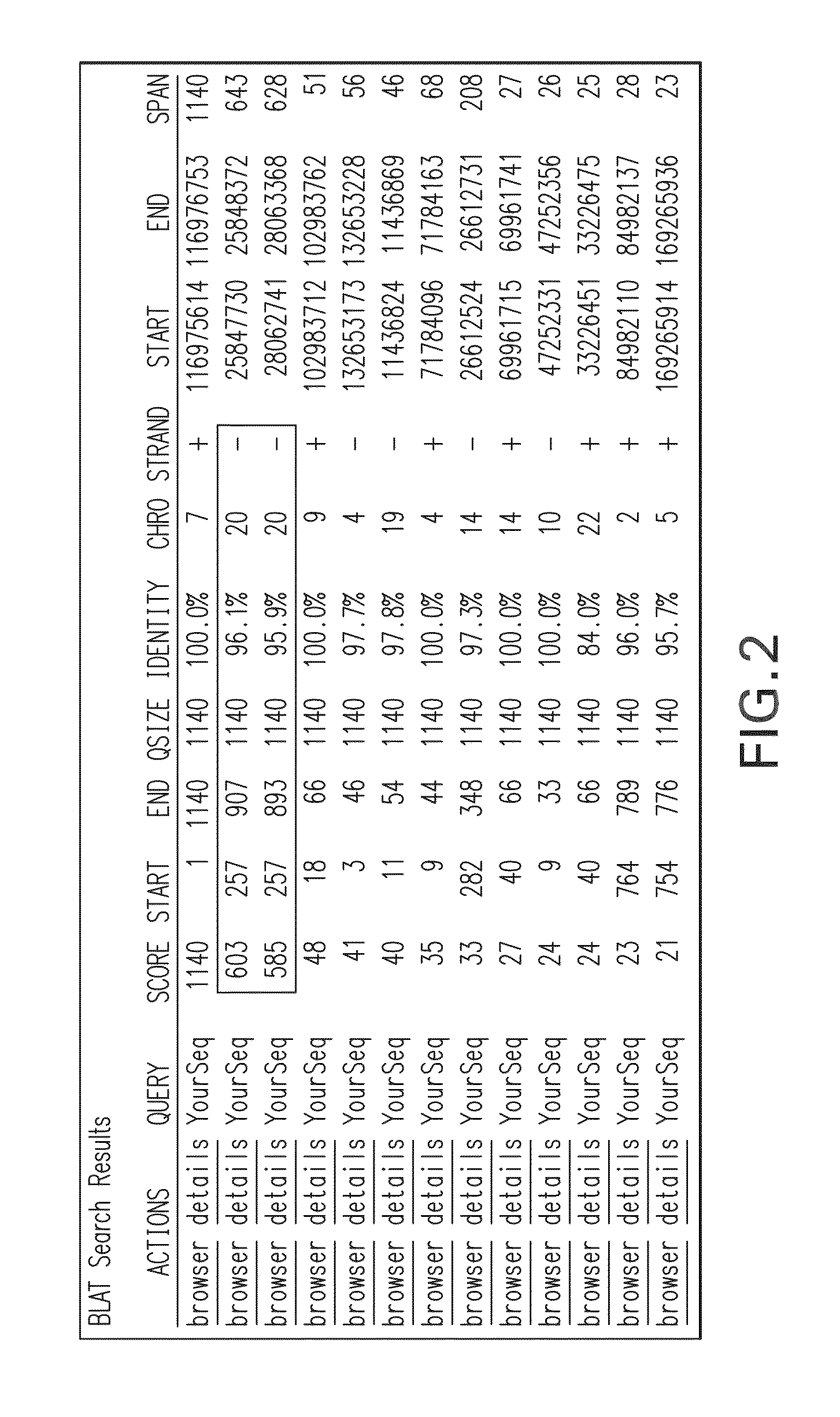
The present invention relates to methods, kits, primers and probes for use in distinguishing highly homologous genomic regions and detecting variants in a locus of interest. In one aspect, the present invention is useful for distinguishing Exon 9 of the CFTR gene from a large homologous region of chromosome 20 in order to determine the presence of the A455E variant.
Owner:CANON US LIFE SCIENCES INC
Restoration Of The CFTR Function By Splicing Modulation
ActiveUS20150211010A1Modulating splicingRestoring and enhancing functionOrganic active ingredientsSplicing alterationPrecursor mRNAOligonucleotide
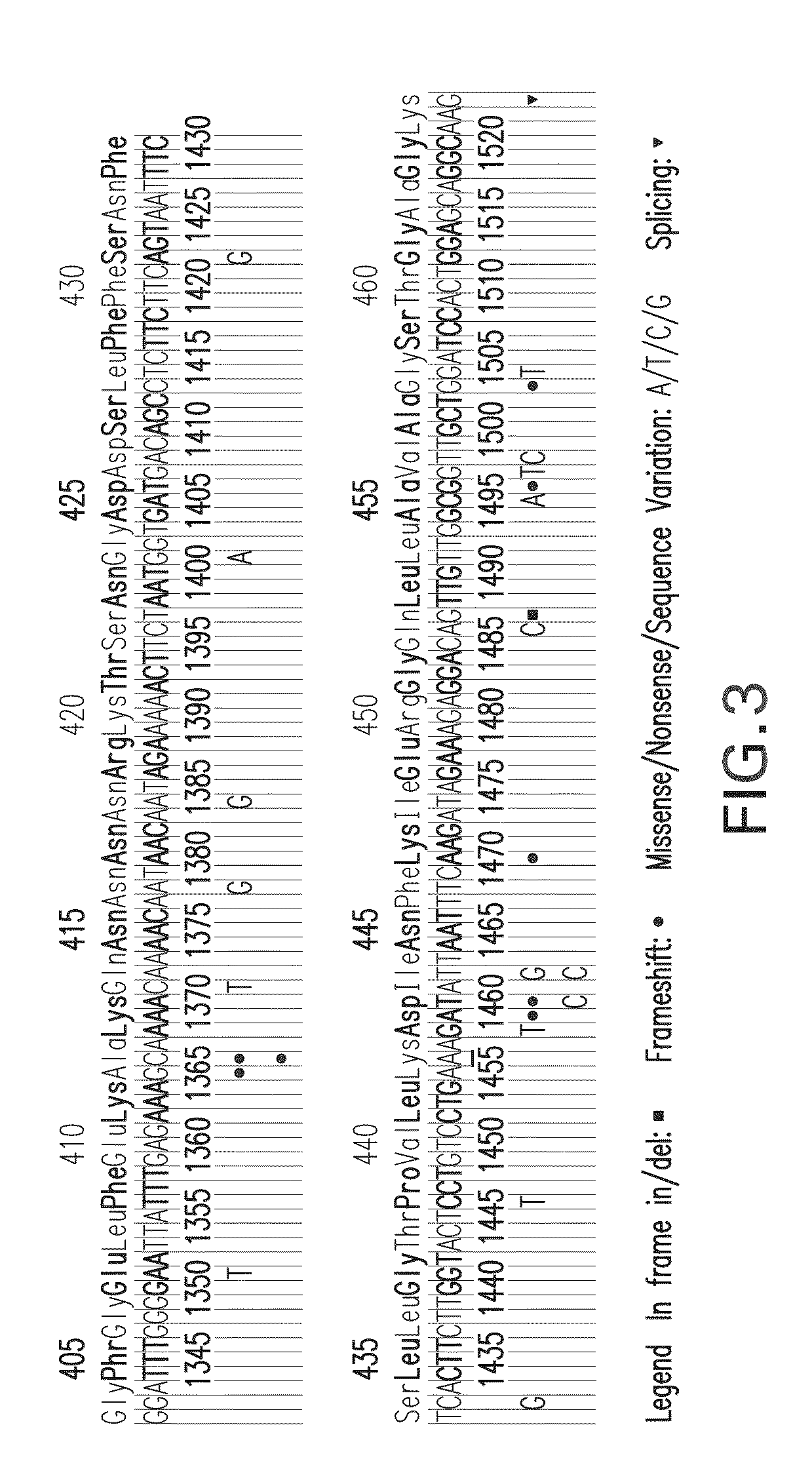
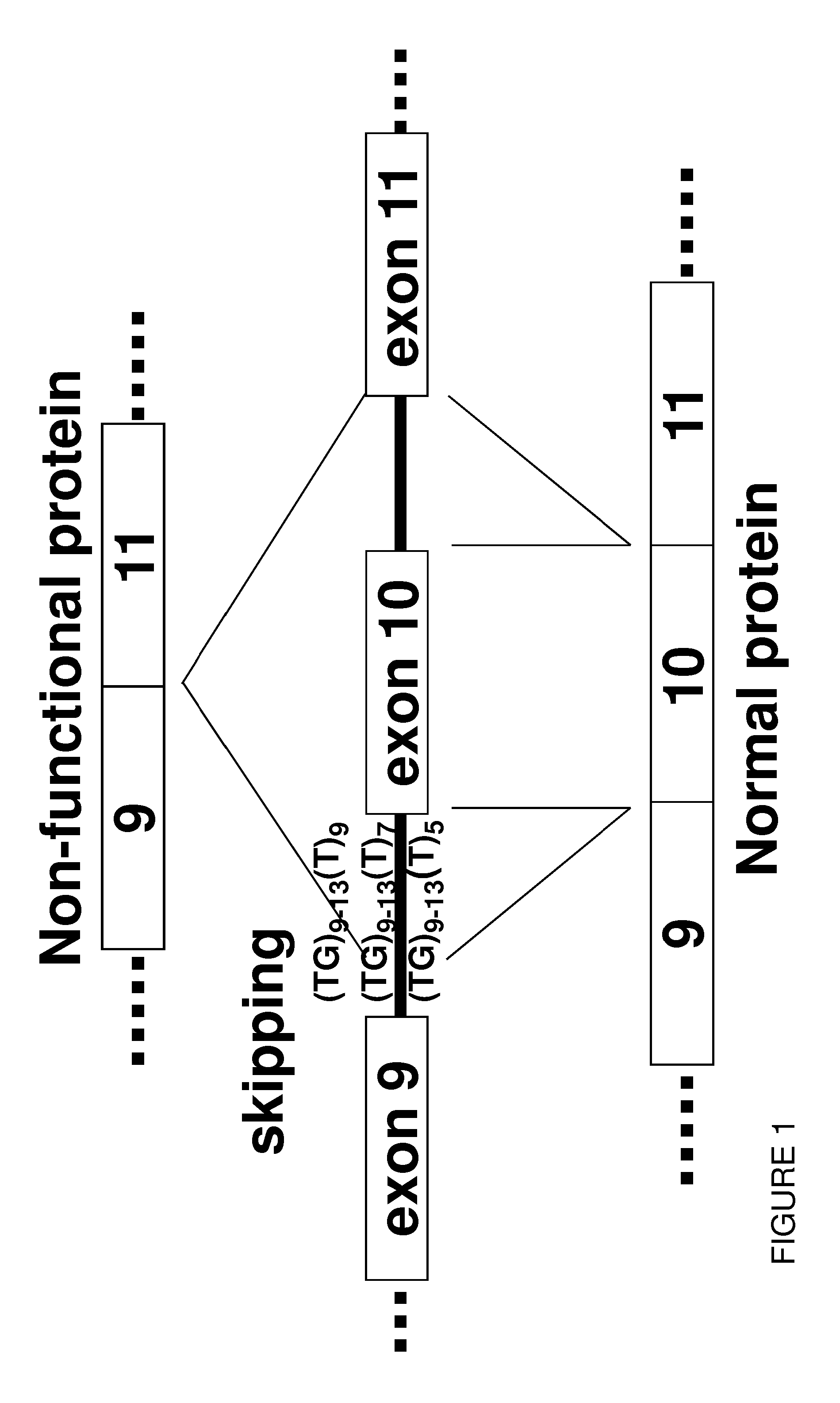
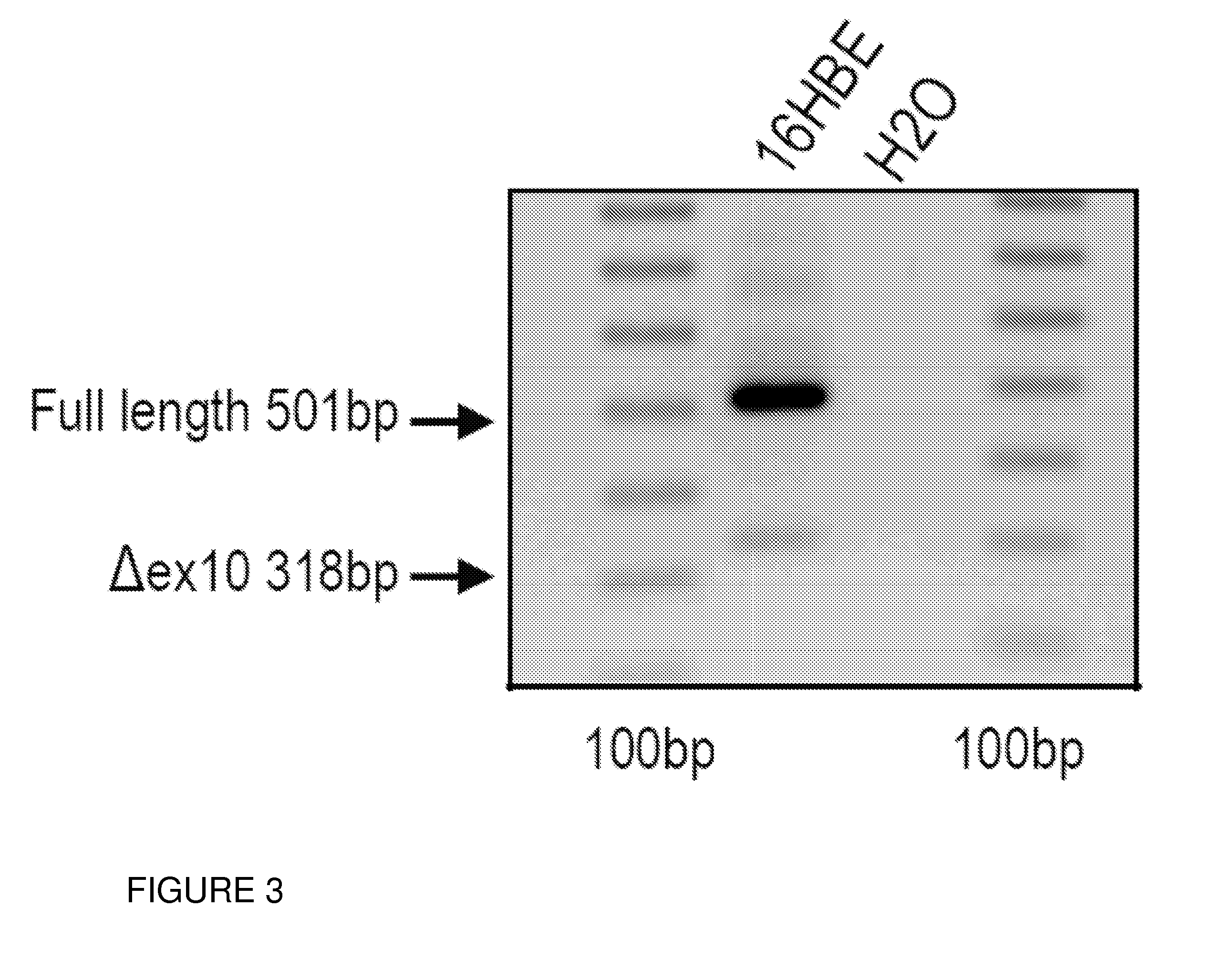
Oligonucleotides capable of binding to and modulating the splicing of the pre-mRNA of the CFTR gene, compositions including the oligonucleotides, kits including the compositions, and uses thereof. Compositions of oligonucleotides useful in methods for suppressing exon 10 skipping optionally in combination with additional CFTR therapeutics.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
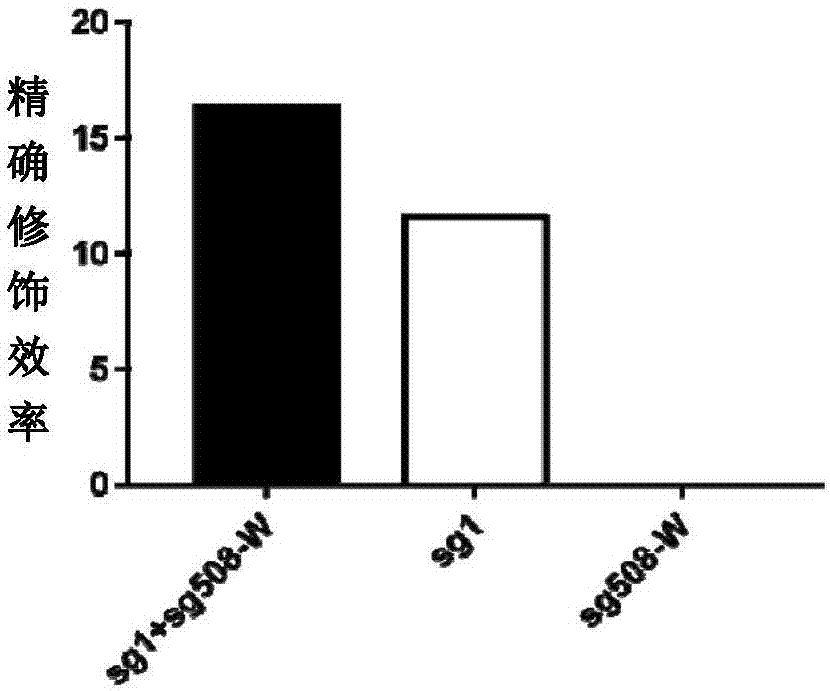
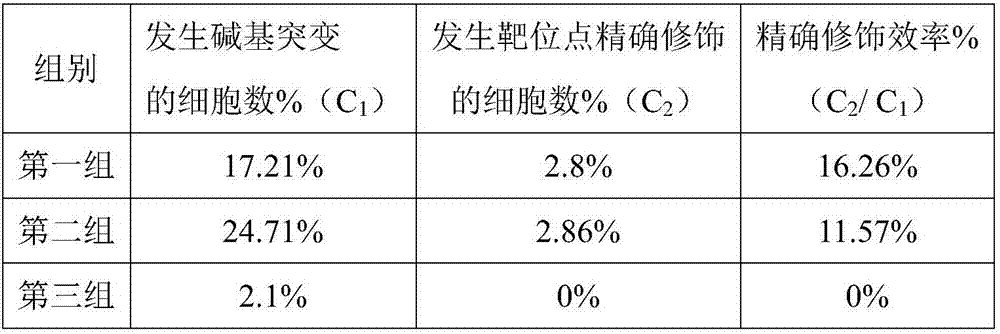
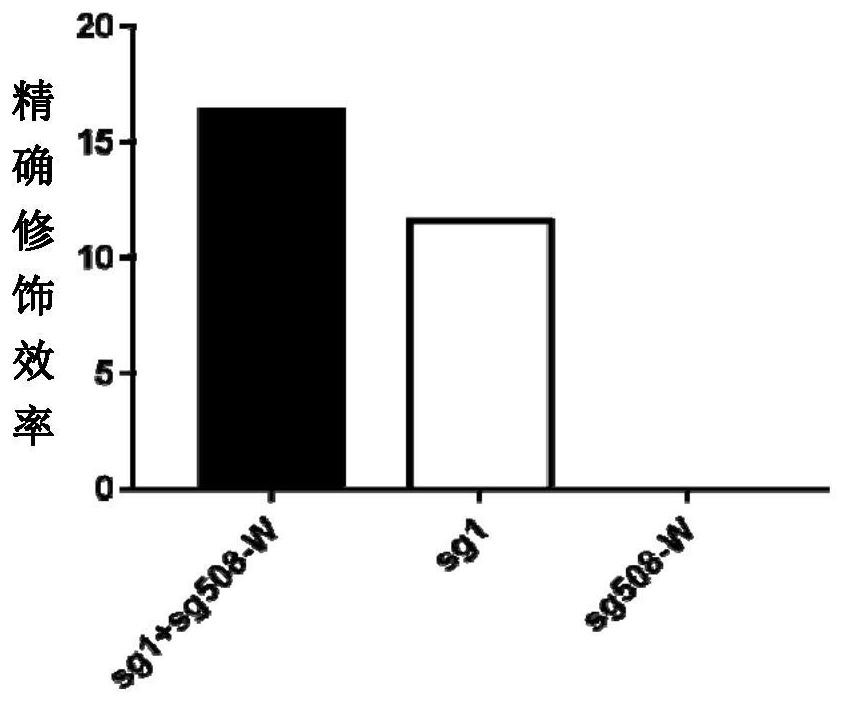
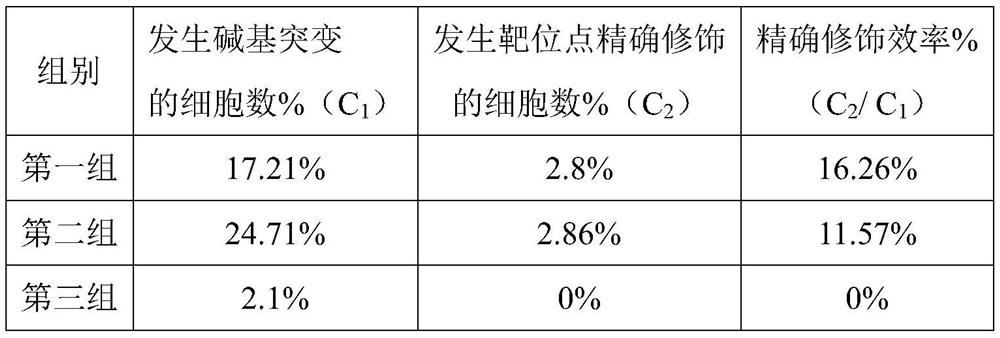
Double sgRNA-mediated gene accurate modification method and application thereof
ActiveCN107267516AImprove gene editing efficiencyStable introduction of DNAAnimals/human peptidesDiseaseNucleotide
The invention discloses a double sgRNA-mediated gene accurate modification method and an application thereof. According to the invention, two strips of sgRNA are simultaneously used, a CF cell is taken as a model, directional deletion is carried out on nucleotide of the 508th sites amino acid for coding CFTR gene, the pathogenic genotype which is same with the deletion patients of 508th sites amino acid of the CFTR can be obtained, and the method found that compared with single sgRNA, the double sgRNA has higher efficiency for editing. The invention provides the more effective method for cell model construction and gene editing for the gene disease.
Owner:FOSHAN UNIVERSITY
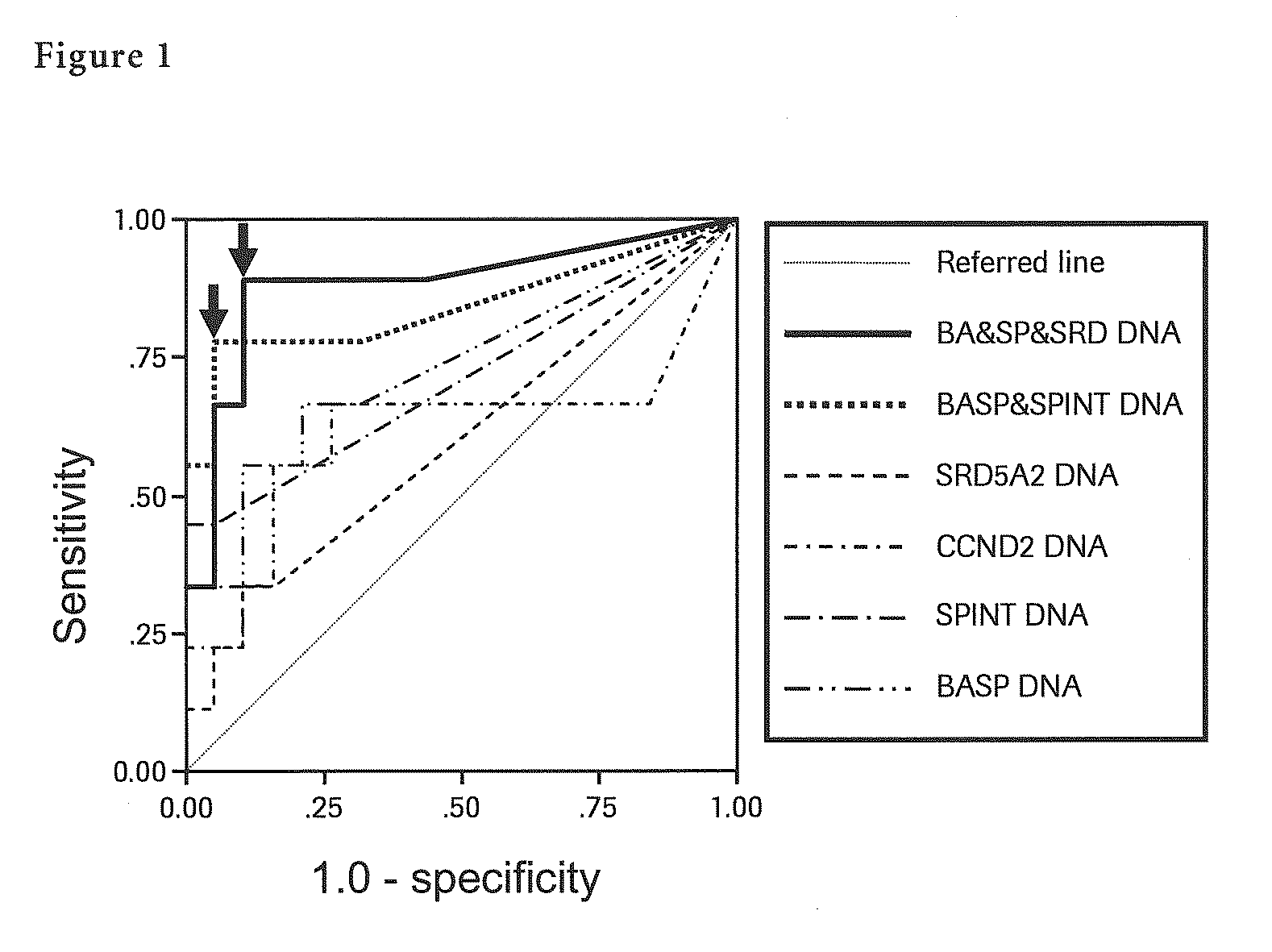
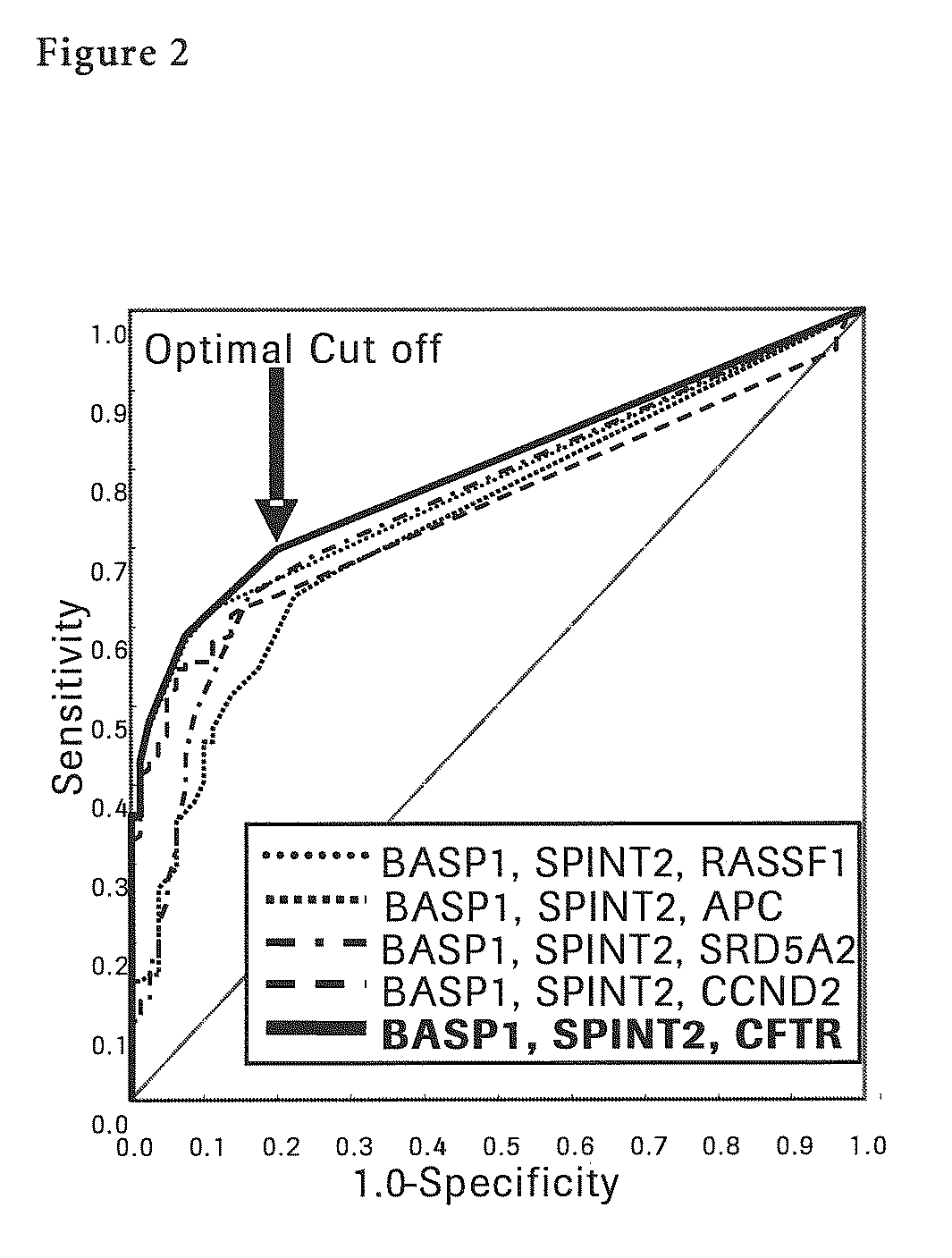
Liver Cancer Methods and Compositions
InactiveUS20100304372A1Improve automationThe process is simple and accurateMicrobiological testing/measurementMalignancyWilms' tumor
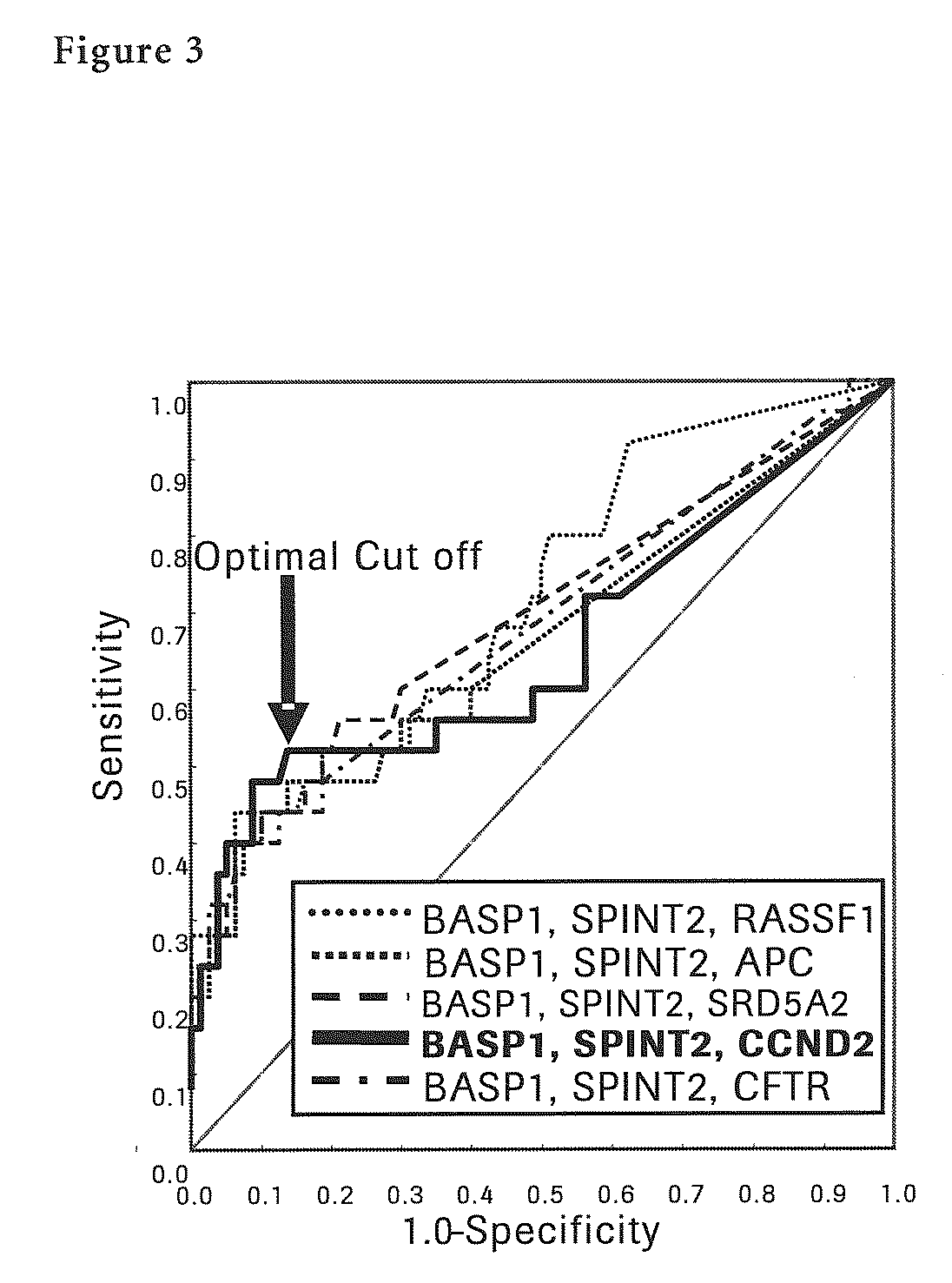
The present invention provides a novel method for detection of liver cancer. This method detects high-sensitively, high-specifically, simply and accurately liver cancer, especially that in early stage by identifying and / or quantifying methylation on particular genes and / or their DNA fragments in clinical specimens, and by combining said methylated DNA values with existing tumor marker values and / or DNA amounts in blood. This invention also detects a precancerous lesion, detects a risk of recurrence after treatment of liver cancer, detects malignancy of liver cancer and monitors progression of liver cancer with time by the same method. As for particular genes, BASP1 gene, SPINT2 gene, APC gene, CCND2 gene, CFTR gene, RASSF1 gene and SRD5A2 gene are mentioned.
Owner:ROCHE MOLECULAR SYST INC
Spinal muscular atrophy pathogenic gene detection kit based on melting curve analysis
The invention relates to a spinal muscular atrophy pathogenic gene detection kit based ona melting curve analysis, and relates to a pathogenic gene detection kit, which comprises an upstream primer F1 and a downstream primer R1 for amplifyingexon7 of SMN1 and SMN2 genes; an upstream primer F2 and a downstream primer R2 for amplifying exon4 of an internal reference CFTR gene; and a fluorescent probe for detection. In a single-tube PCR system, the copy number of exon7 of the SMN1 gene as a main pathogenic gene of SMA can be quantitatively detected, the sample genotype can be known by a fluorescence PCR melting curve analysis after PCR amplification is finished, the whole operation is completed in 2 to 3h, is simple and rapid, and is short in time-consuming; the homogeneous detection and closed tube operation are carried out; the detection flux is high; the detection specificity is high, and the results are easy to interpret. The detection kit can be rapidly and convenientlyapplied to large-scale population screening of the SMA pathogenicgene, and is especially suitable for prenatal, premaritaland pre-pregnancy screening and genetic diagnosis of patients.
Owner:夏众敏 +2
Polypeptides for increasing mutant CFTR channel activity
InactiveUS20040115770A1Reduce deliveryFacilitates uptakeBacteriaPeptide/protein ingredientsCompetitive bindingHsp70
The present invention provides methods and compositions for enhancing channel activity to the mutant cystic fibrosis trans-membrane conductance regulator protein (CFTR). The compositions of the invention comprise polypeptides containing CFTR sub-domains that are designed to mimic the folding defect of the full length mutant CFTR proteins, resulting in competitive binding to cytoplasmic chaperones such as Hsc / Hsp7O and Hdj2. The methods of the invention comprise transduction, or recombinant expression, of CFTR polypeptides in a cell expressing mutant CFTR. The presence of the CFTR polypeptide results in a dominant effect whereby the CFTR polypeptide competes with the endogenously expressed mutant CFTR for binding to cytoplasmic chaperones such as Hsc / Hsp70 and Hdj2. Mutant CFTR proteins include, but are not limited to, DeltaF508 CFTR. The present invention is based on the discovery that reduced binding of cytoplasmic chaperones to the endogenous DeltaF508 CFTR, mediated by the presence of CFTR polypeptides, results in restoration of plasma membrane localization and channel activity. The methods and compositions of the invention can be used to restore channel activity in cystic fibrosis subjects carrying genetic defects in the CFTR gene, such as for example, DeltaF508 CFTR.
Owner:PITTSBURGH UNIV OF
Long-term in vivo transgene expression
Efficient and prolonged hCFTR expression is one of the major obstacles for cystic fibrosis lung therapy. hCFTR mRNA expression levels depend on eukaryotic expression cassette components, prokaryotic backbone elements, and the gene transfer method may also influence transcriptional silencing mechanisms. A codon-optimized and CpG-reduced human CFTR gene (CO-CFTR) was made. Various vector modifications were tested to facilitate extended duration of CO-CFTR expression. Insertion of an extended 3′BGH transcribed sequence (712 bp) in an inverted orientation produced prolonged expression of CO-CFTR expression at biologically relevant levels. Further studies revealed that prolonged CO-CFTR expression is dependant on the orientation of the extended BGH 3′ BGH transcribed sequence and its transcription, is not specific to the UbC promoter, and is less dependent on other vector backbone elements.
Owner:COPERNICUS THERAPEUTICS
Compositions and methods for treatment of cystic fibrosis
InactiveUS20200308590A1Symptoms improvedEasy to correctCell receptors/surface-antigens/surface-determinantsDNA/RNA fragmentationBase JBinding peptide
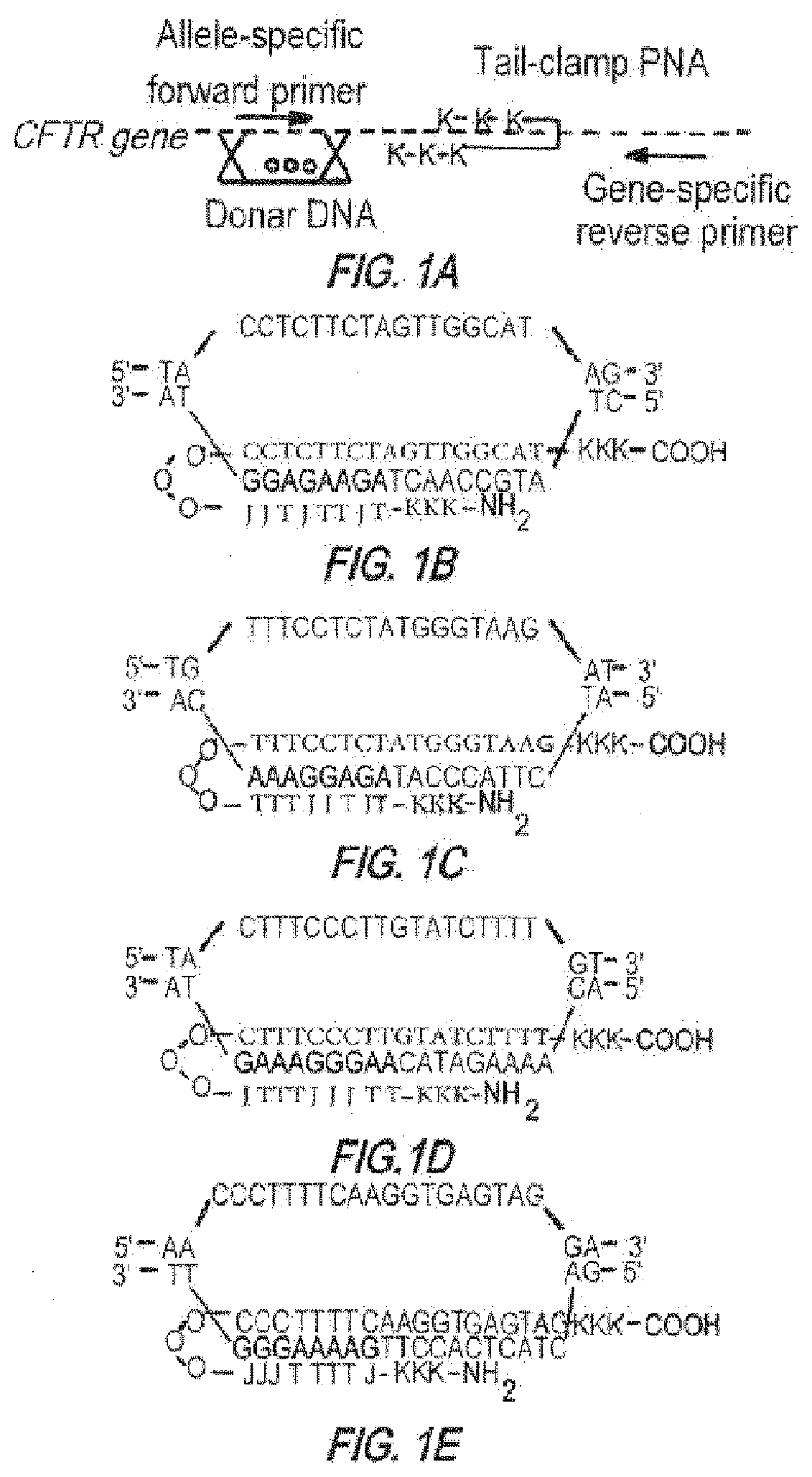
Compositions and methods of genome engineering in vitro and in vivo are provided. In some embodiments, the compositions are triplex forming molecules that bind or hybridize to a target region sequence in the human cystic fibrosis transmembrane conductance regulator (CFTR) gene. Preferably the triplex forming molecules are peptide nucleic acids that include a Hoogsteen binding peptide nucleic acid (PNA) segment and a Watson-Crick binding PNA segment collectively totaling no more than 50 nucleobases in length, wherein the two segments can binid or hybridize to a target region in the CFTR gene having a polypurine sequences and induce strand invasion, displacement, and formation of a triple-stranded molecule among the two PNA segments and the target region's sequence. Methods of using the triplex forming molecules to treat cystic fibrosis are also provided.
Owner:YALE UNIV
Use of polymorphisms for identifying individuals at risk of developing autism
InactiveUS20130317006A1Increased and decreased riskBiocideOrganic active ingredientsDevelopmental disorderCvd risk
The present invention relates to nucleic-acid based diagnostics and the use of such diagnostics for the diagnosis of developmental disorders. Novel methods of assessing individuals for the risk of developing autism through the identification of mutations of the CFTR gene alone or in combination with other genes associated with the methylation pathways are identified. Methods of identifying those individuals that are at increased and / or decreased risk for developing autism are provided.
Owner:YASKO AMY
Multiplexed analysis of polymorphic loci by concurrent interrogation and enzyme-mediated detection
InactiveUS20070264641A1Degree of reductionStrong specificityMicrobiological testing/measurementEnzymeBioinformatics
The invention provides methods and processes for the identification of polymorphisms at one or more designated sites, without interference from non-designated sites located within proximity of such designated sites. Probes are provided capable of interrogation of such designated sites in order to determine the composition of each such designated site. By the methods of this invention, one or more mutations within the CFTR gene and the HLA gene complex can be can be identified.
Owner:LI ALICE XIANG +2
Diagnostic and treatment methods involving the cystic fibrosis transmembrane regulator
InactiveUS20070053879A1Smooth transmissionReduce Potential ToxicityBiocideSugar derivativesIntracellularMutant
Disclosed are full length isolated DNAs encoding cystic fibrosis transmembrane conductance regulator (CFTR) protein and a variety of mutants thereof. Also disclosed are antibodies specific for various CFTR domains and methods for their production. Expression of CFTR from cells transformed with these CFTR genes or cDNAs demonstrate surprising CFTR intracellular distributions and results thereby providing for new diagnostic and therapeutic procedures.
Owner:GENZYME CORP
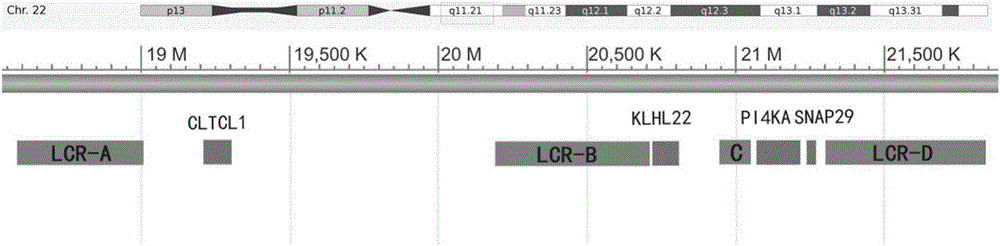
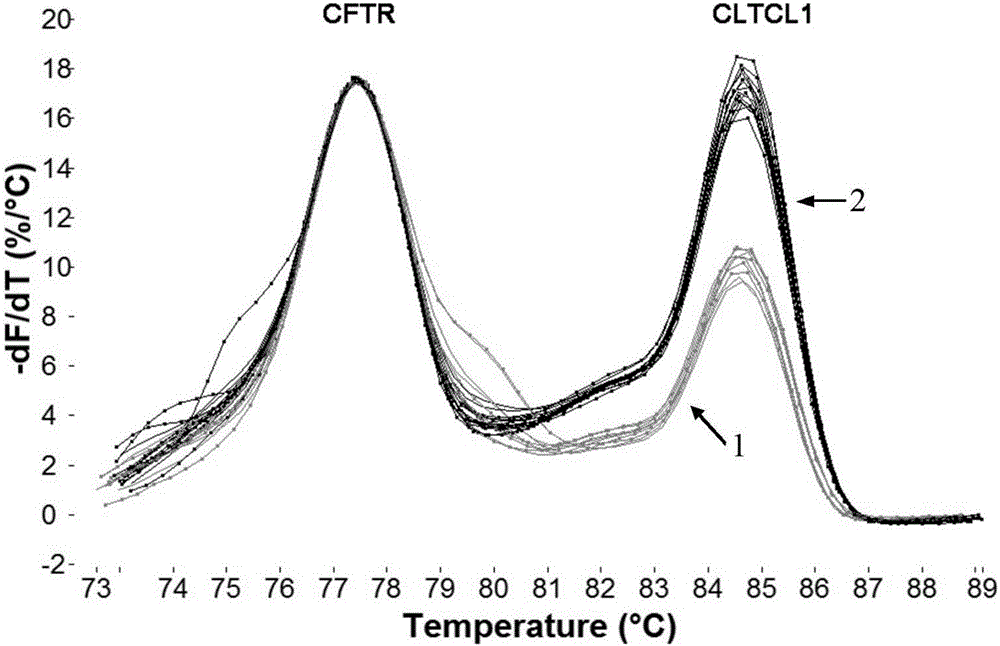
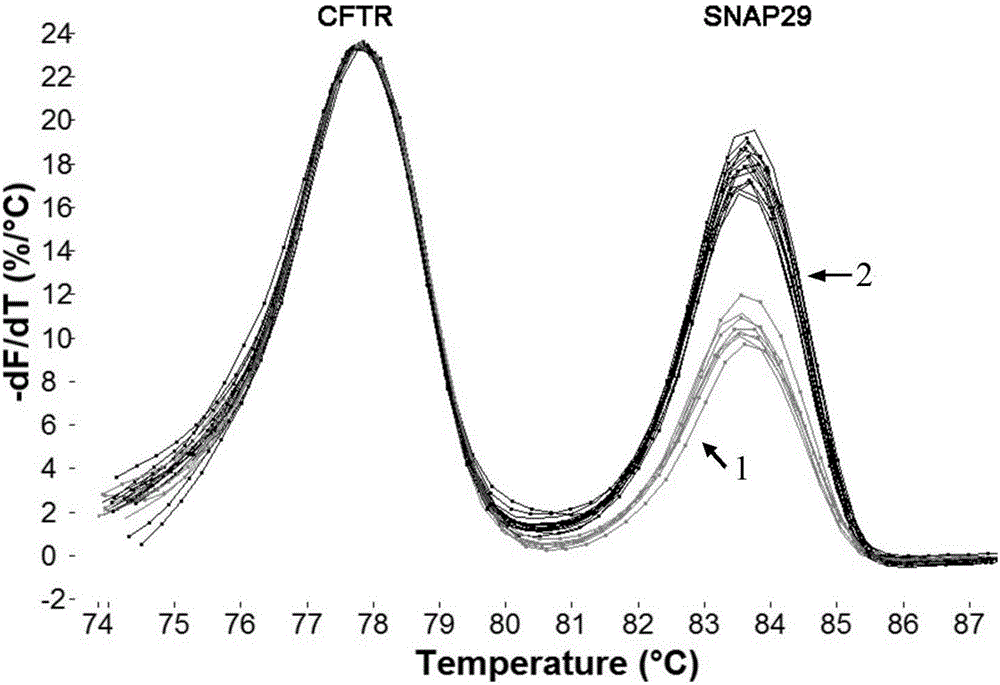
Method for detecting 22q11.2 copy number deletion
ActiveCN106319079AEasy to operateShort detection cycleMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceTrue positive rate
The invention provides a method for detecting 22q11.2 copy number deletion by limited dNTP (deoxyribonucleotide triphosphate) competitive (polymerase chain reaction) in combination with an HRM (high resolution melt) technique. The method comprises the step of detecting CLTCL1, SNAP29, KLHL22, PI4KA and CFTR genes. The invention also provides a primer combination and kit of the method. The method is simple and quick to operate, and only comprises the processes of PCR reaction and subsequent HRM analysis, thereby shortening the detection period. The method implements closed pipe operation; and since fluorescent dyes are added in the PCR, HRM curve analysis can be performed without other treatments after the detection segment amplification is completed, thereby effectively avoiding the pollution. In the reaction, only conventional PCR reagents and small amounts of fluorescent dyes are needed, and special detection and analysis apparatuses are not needed. The detection on 99 patients and normal person control samples indicates that the sensitivity and specificity respectively reach 100%, and the stability and accuracy of the system are confirmed.
Owner:SHANGHAI CHILDRENS MEDICAL CENT AFFILIATED TO SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Multiplexed Analysis Of Polymorphic Loci By Concurrent Interrogation And Enzyme-Medicated Detection
InactiveUS20120214681A1Strong specificityDegree of reductionSugar derivativesMicrobiological testing/measurementHla genesEnzyme
The invention provides methods and processes for the identification of polymorphisms at one or more designated sites, without interference from non-designated sites located within proximity of such designated sites. Probes are provided capable of interrogation of such designated sites in order to determine the composition of each such designated site. By the methods of this invention, one or more mutations within the CFTR gene and the HLA gene complex can be identified.
Owner:BIOARRAY SOLUTIONS
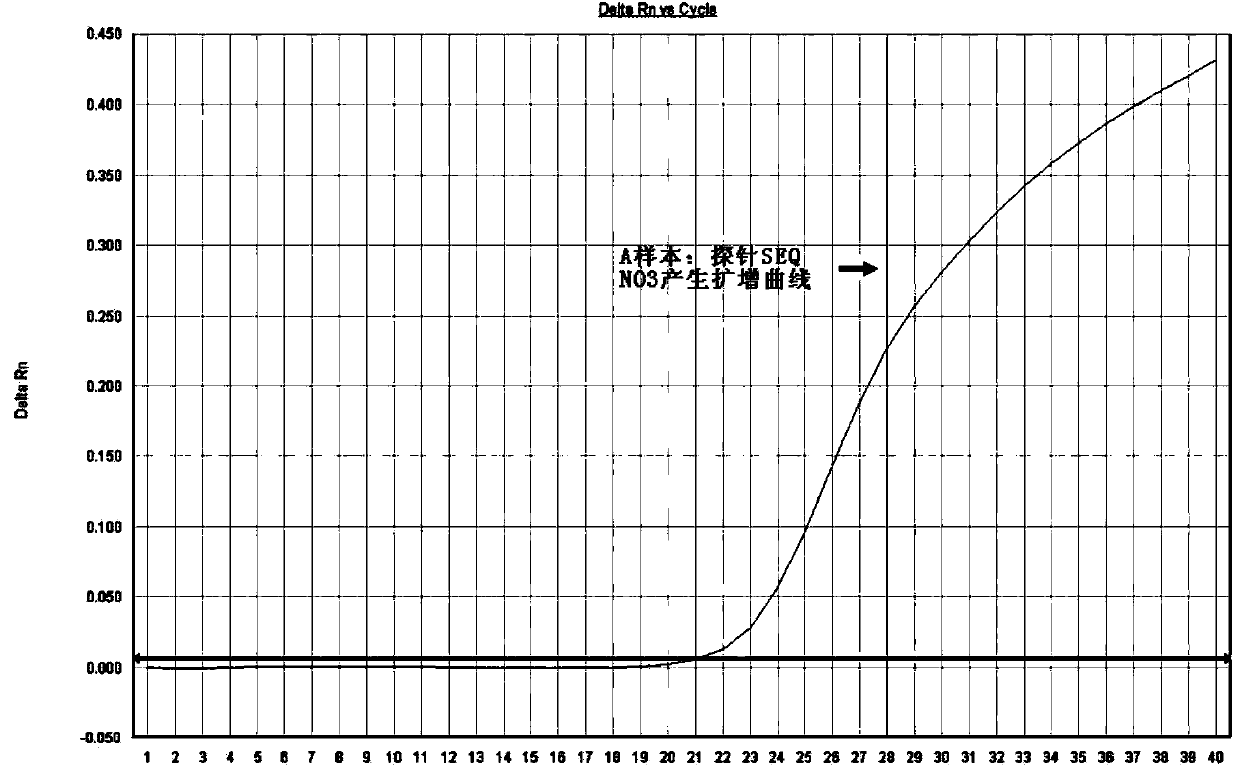
Method for detecting mutation on locus deltaF508 of CFTR (Cystic Fibrosis Transmembrane Regulator) gene and oligonucleotide
ActiveCN103789415AIncrease the Tm valueSimplify the design processMicrobiological testing/measurementDNA/RNA fragmentationMale infertilitySpecific detection
The invention discloses a method for detecting mutation on the locus deltaF508 of a CFTR (Cystic Fibrosis Transmembrane Regulator) gene and an oligonucleotide, relating to a pair of specific amplification primers SEQ NO 1 and SEQ NO 2 and a pair of specific detection probes SEQ NO 3 and SEQ NO4. The method has the advantages of short detection period, high specificity, high accuracy, high sensitivity, low conditional dependency, low pollution risk and the like, and can be used for assisting in accurately and rapidly diagnosing cystic fibrosis and male infertility.
Owner:广州艾迪康医学检验所有限公司
CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) gene deletion mutation form of cystic fibrosis patients and application of CFTR gene deletion mutation form
ActiveCN106674344AMicrobiological testing/measurementGenetic engineeringHuman DNA sequencingNucleotide
The invention discloses a CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) gene deletion mutation form of cystic fibrosis patients and application of the CFTR gene deletion mutation form. The CFTR gene deletion mutation form is used for protecting proteins including: (a1) a protein obtained by deleting a 508th amino acid residue of a CFTR; and (a2) a protein obtained by deleting 476th to 478th amino acid residues of the CFTR. The CFTR gene deletion mutation form is also used for protecting application of substances for detecting mutation A and / or B to preparation of a kit, wherein the mutation A: 128th to 130th nucleotide deletion of a sequence 3 in a human genome; and the mutation B: 34th to 42nd nucleotide deletion of the sequence 3 in the human genome. Functions of the kit comprise: (c1) evaluation of risks of cystic fibrosis of a person to be detected; (c2) evaluation of the risks of the cystic fibrosis of the person to be detected or offspring of parents to be detected; and (c3) diagnosis or auxiliary diagnosis for judging whether the person to be detected is a patient suffering from the cystic fibrosis or not. The CFTR gene deletion mutation form has an important application value on the diagnosis of the patient suffering from the cystic fibrosis.
Owner:BEIJING CHILDRENS HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
Method for detecting deficiency of 7q11.23
InactiveCN106967837AJudgment missingShort detection cycleMicrobiological testing/measurementDNA/RNA fragmentationReference genesMicrobiology
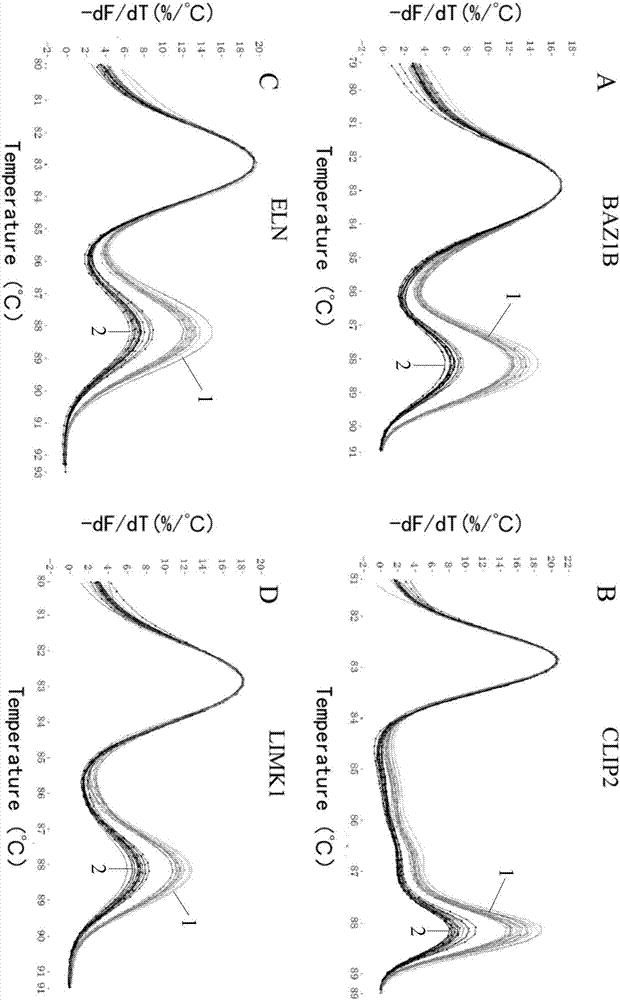
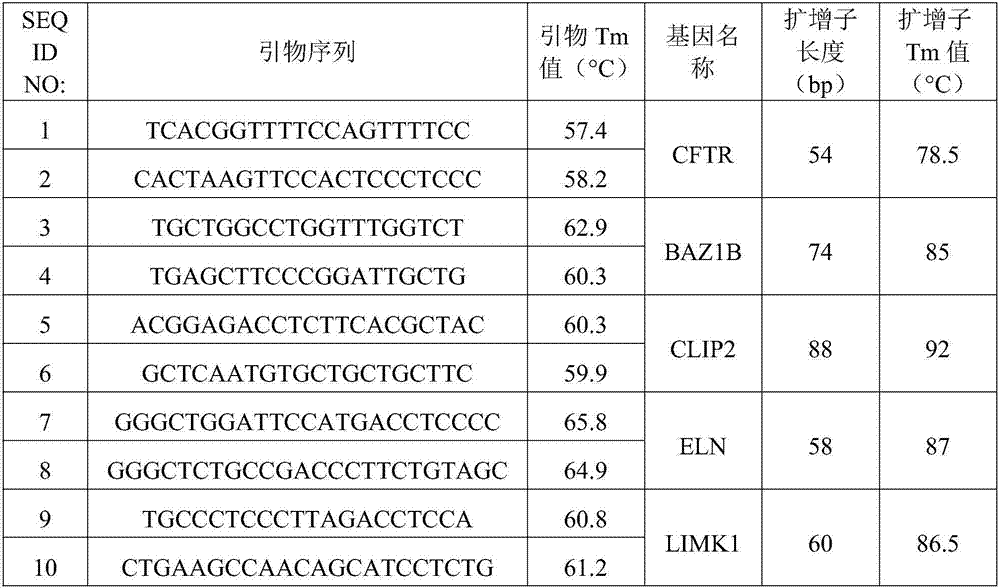
The invention relates to a method for detecting deficiency of 7q11.23 on the basis of limited dNTP (Diethyl-Nitrophenyl Thiophosphate) competitive PCR (Polymerase Chain Reaction) and HRM. According to the method, a CFTR (Cystic Fibrosis Transmembrane Conductance Regulator) gene is adopted as a reference gene, and copy numbers of genes BAZ1B, CLIP2, ELN and LIMK1 are detected. The invention further provides a primer combination, a kit and the like related to the method. The method has the advantages that the method is implemented under a same reaction condition, detection can be implemented simultaneously by using one PCR tester, only 79 minutes are taken from PCR amplification to HRM detection, and the detection period can be shortened. Pipe opening operation is not needed after PCR amplification, and operation steps and possible pollution can be reduced. Appropriate genes are selected, proper primer sequences are designed, and PCR reaction systems and procedures are set reasonably. The method provided by the invention is very high in accuracy, and has great advantages in 7q11.23 micro deficiency detection and large-scale population screening.
Owner:SHANGHAI CHILDRENS MEDICAL CENT AFFILIATED TO SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
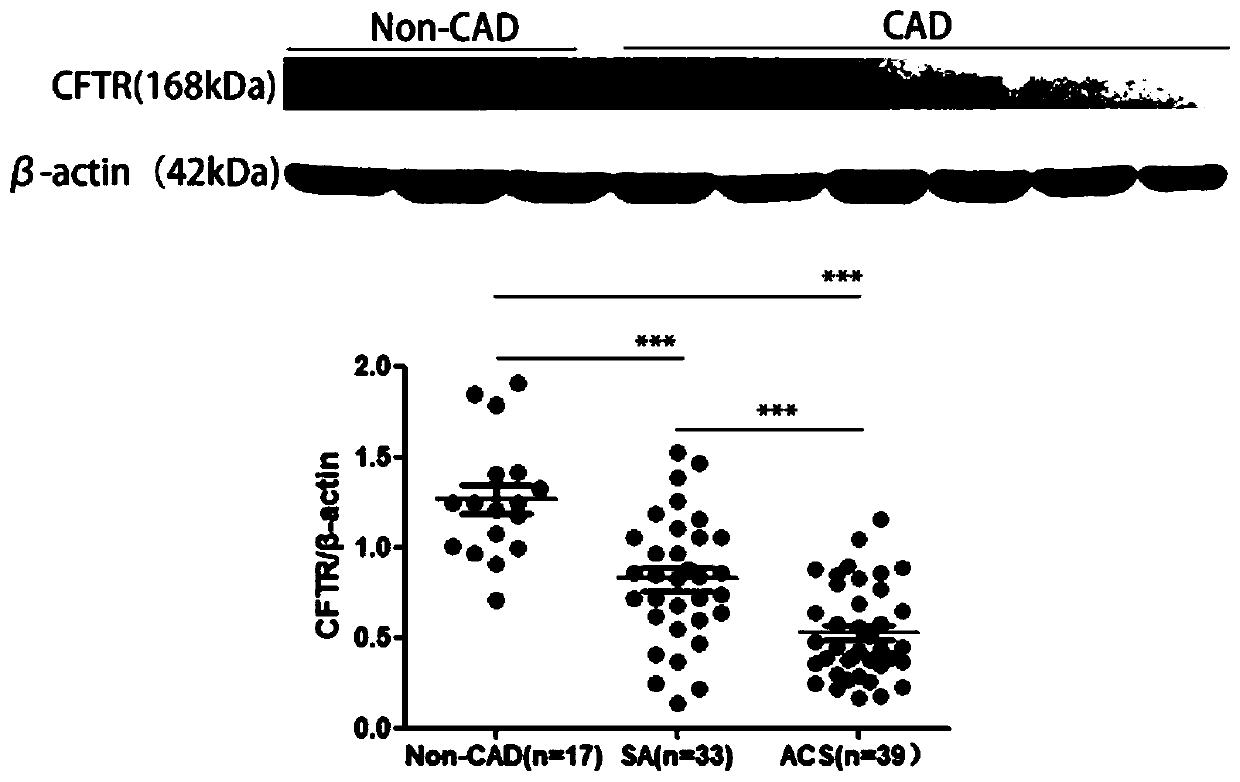
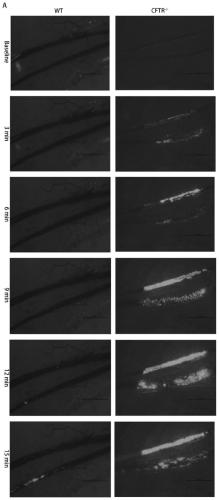
Application of CFTR gene to preparation of thrombotic disease early warning or diagnosing medicine
The invention discloses application of a CFTR gene to preparation of thrombotic disease early warning or diagnosing medicine. The CFTR gene codes a chloride ion channel with cAMP dependency, and it isfound that the expression of the CFTR gene on the peripheral blood platelets and mononuclear cells of a patient with a coronary heart disease is reduced remarkably; statistics difference is shown among patient groups with stable angina pectoris and acute coronary syndromes. On an animal cell model, the specificity restraining CFTR gene is found to remarkably promote thrombosis, and has the effects of promoting platelet aggregation and up regulating a P2Y12 receptor-mediated platelet aggregation signal channel. The new medical application of the CFTR gene to thrombotic disease early warning and diagnosing is mined, the new application field of gene treatment is expanded, and the new clinical diagnosis marker is provided for early warning and diagnosing of a critical thrombotic disease.
Owner:SUN YAT SEN UNIV
Multiplexed analysis of polymorphic loci by concurrent interrogation and enzyme-mediated detection
InactiveUS20080138800A1Strong specificityDegree of reductionMicrobiological testing/measurementHla genesEnzyme
The invention provides methods and processes for the identification of polymorphisms at one or more designated sites, without interference from non-designated sites located within proximity of such designated sites. Probes are provided capable of interrogation of such designated sites in order to determine the composition of each such designated site. By the methods of this invention, one or more mutations within the CFTR gene and the HLA gene complex can be can be identified.
Owner:BIOARRAY SOLUTIONS
Double sgRNA-mediated precise genetic modification method and application
ActiveCN107267516BImprove gene editing efficiencyStable introduction of DNAAnimals/human peptidesDiseaseNucleotide
Owner:FOSHAN UNIVERSITY
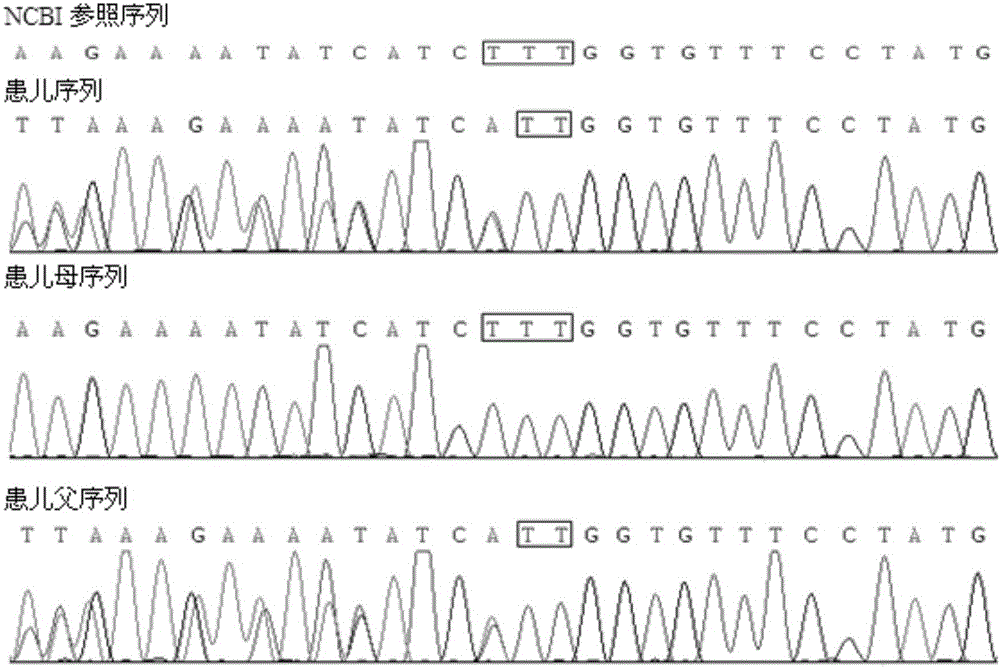
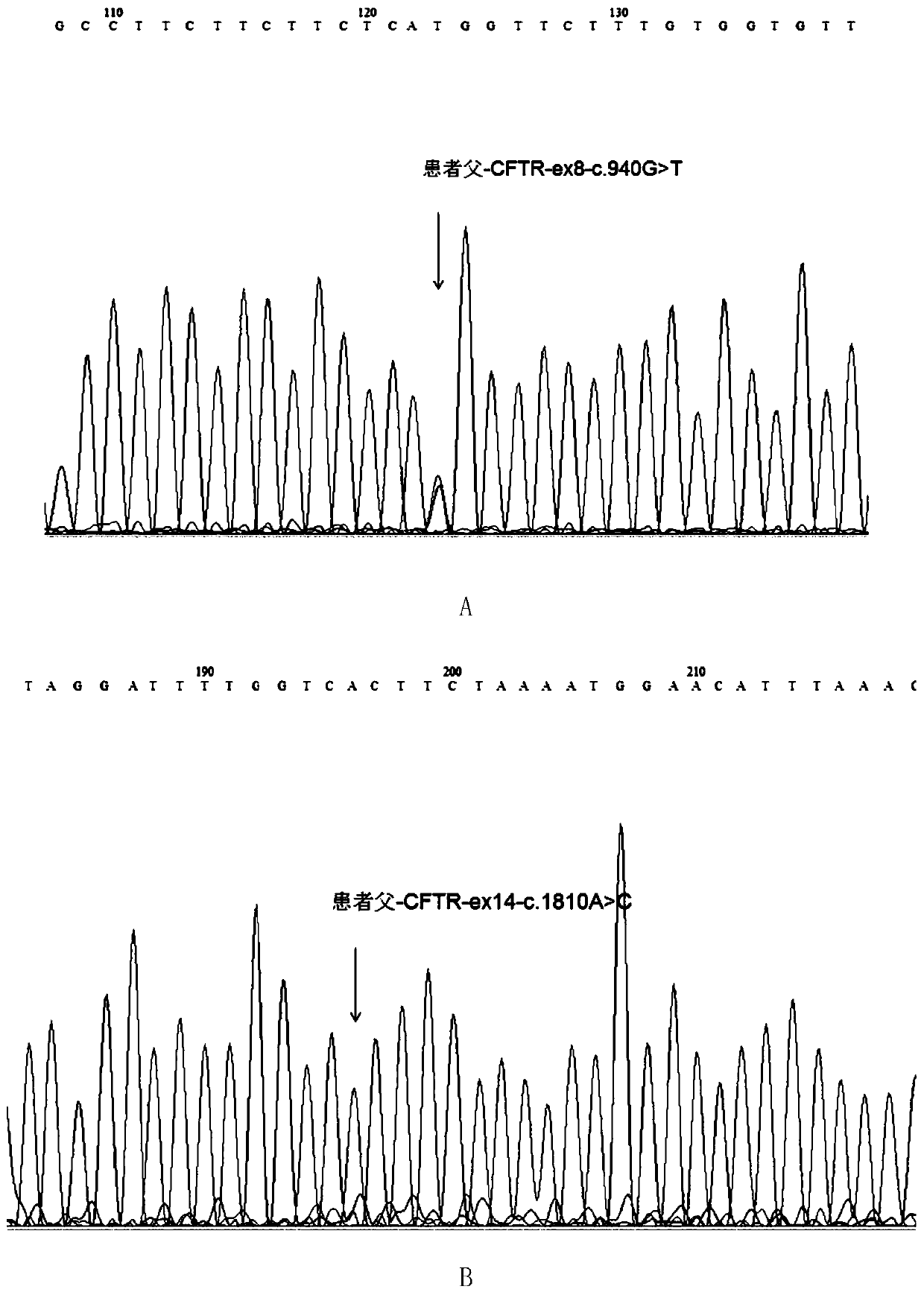
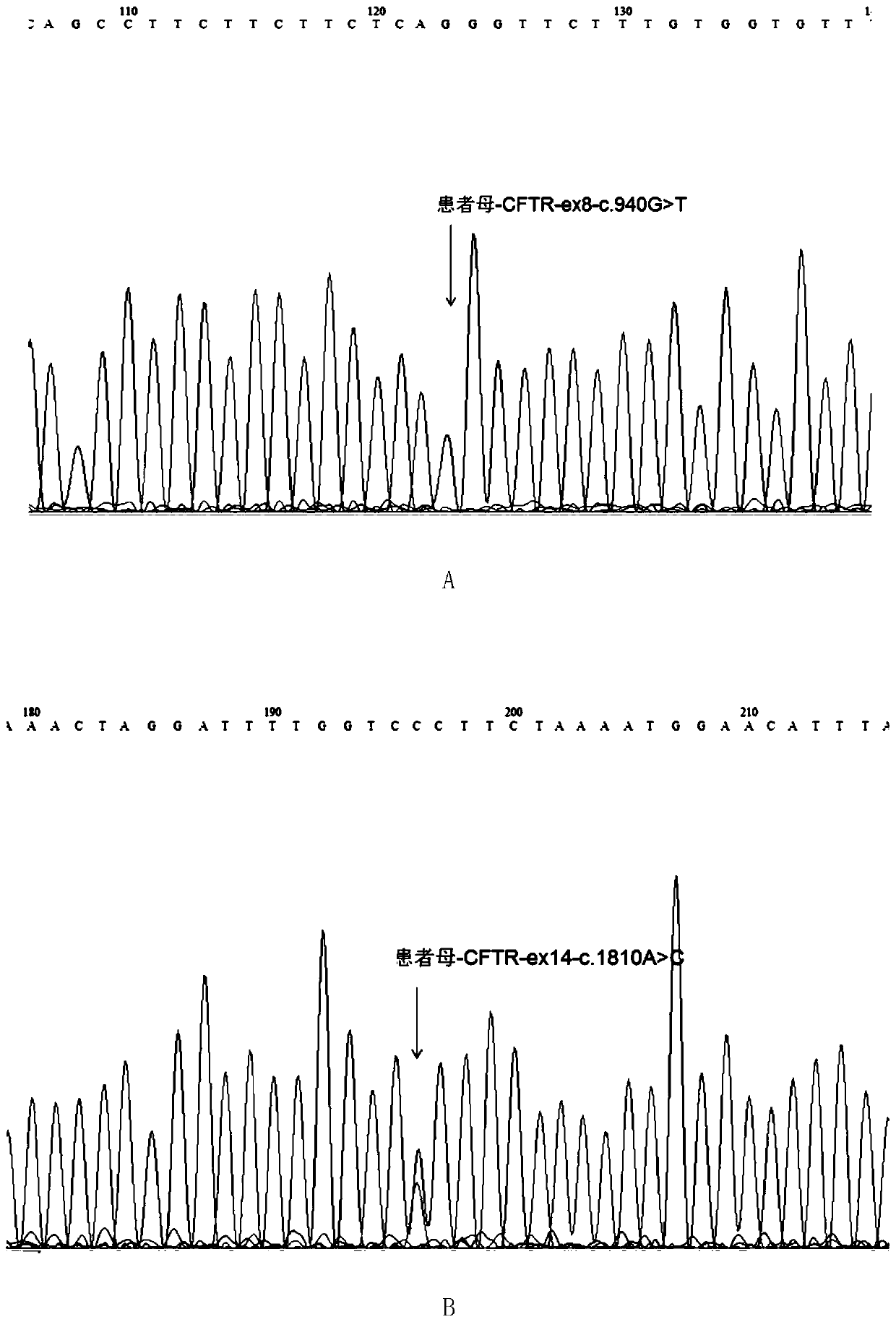
Mutated form of cftr gene in cystic fibrosis patients and its application
The invention discloses a CFTR (cystic fibrosis transmembrane regulator) gene mutation form of patients suffering from cystic fibrosis, and application thereof. Protected protein provided by the invention is as follows: (a1) protein obtained by mutating the 314th amino acid residue of the CFTR from glycine to tryptophane; and (a2) protein obtained by mutating the 604th amino acid residue of the CFTR from threonine to proline. The invention also protects application of a substance for detecting mutant A and / or mutant B to preparation of a kit; in the mutant A, the 71st position of the sequence 3 in a human genome is mutated from G to T; in the mutant B, the 44th position of the sequence 4 in the human genome is mutated from A to C; and the kit has the following functions: (c1) evaluating the cystic fibrosisrisk of a to-be-tested person; (c2) evaluating the cystic fibrosis risk of the offspring of the to-be-tested person or to-be-tested couple; and (c3) diagnosing or assisting in diagnosing whether the to-be-tested person is the patient suffering from the cystic fibrosis or not. An important application value on diagnosis of the patient suffering from the cystic fibrosis is achieved.
Owner:BEIJING CHILDRENS HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com