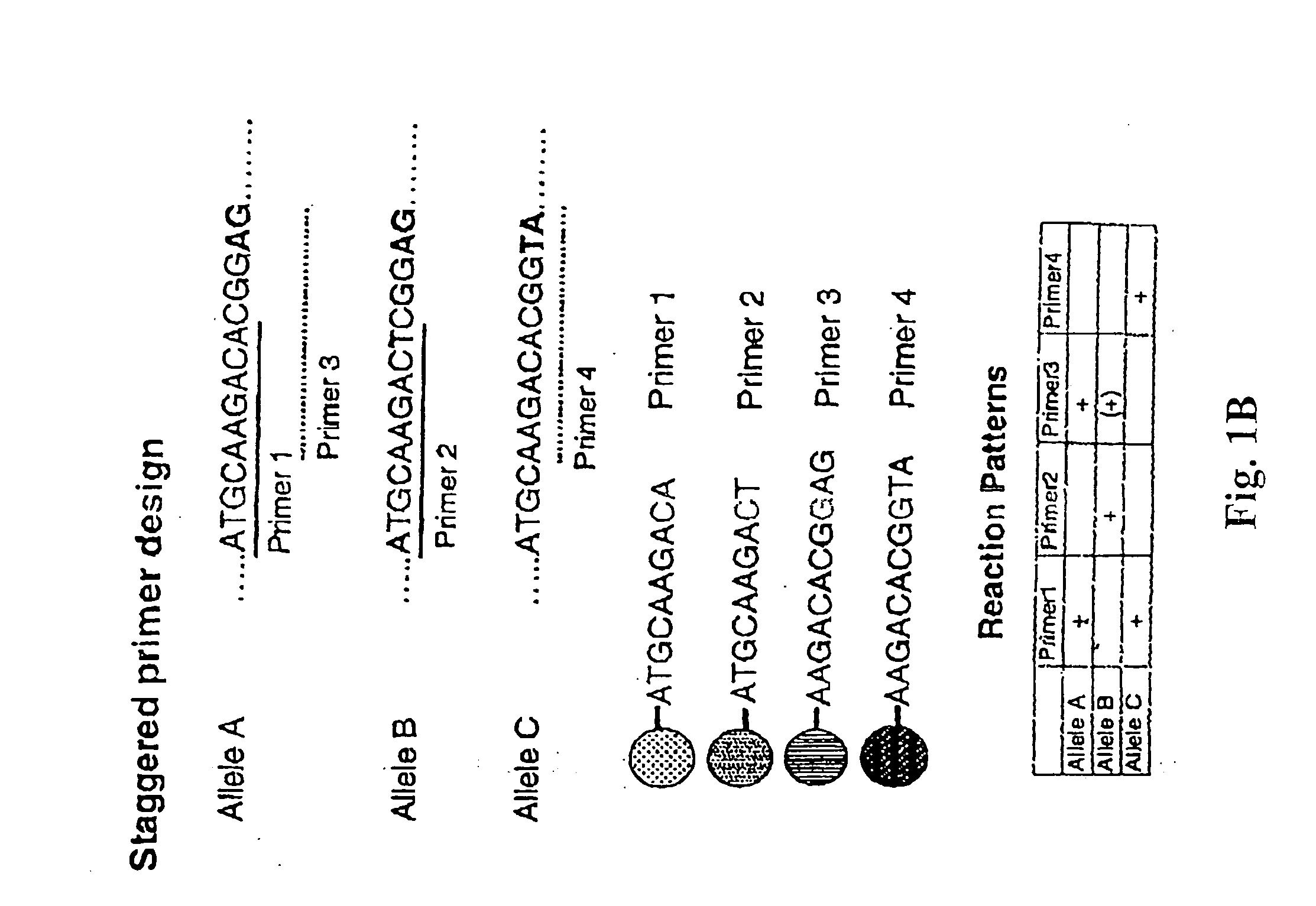
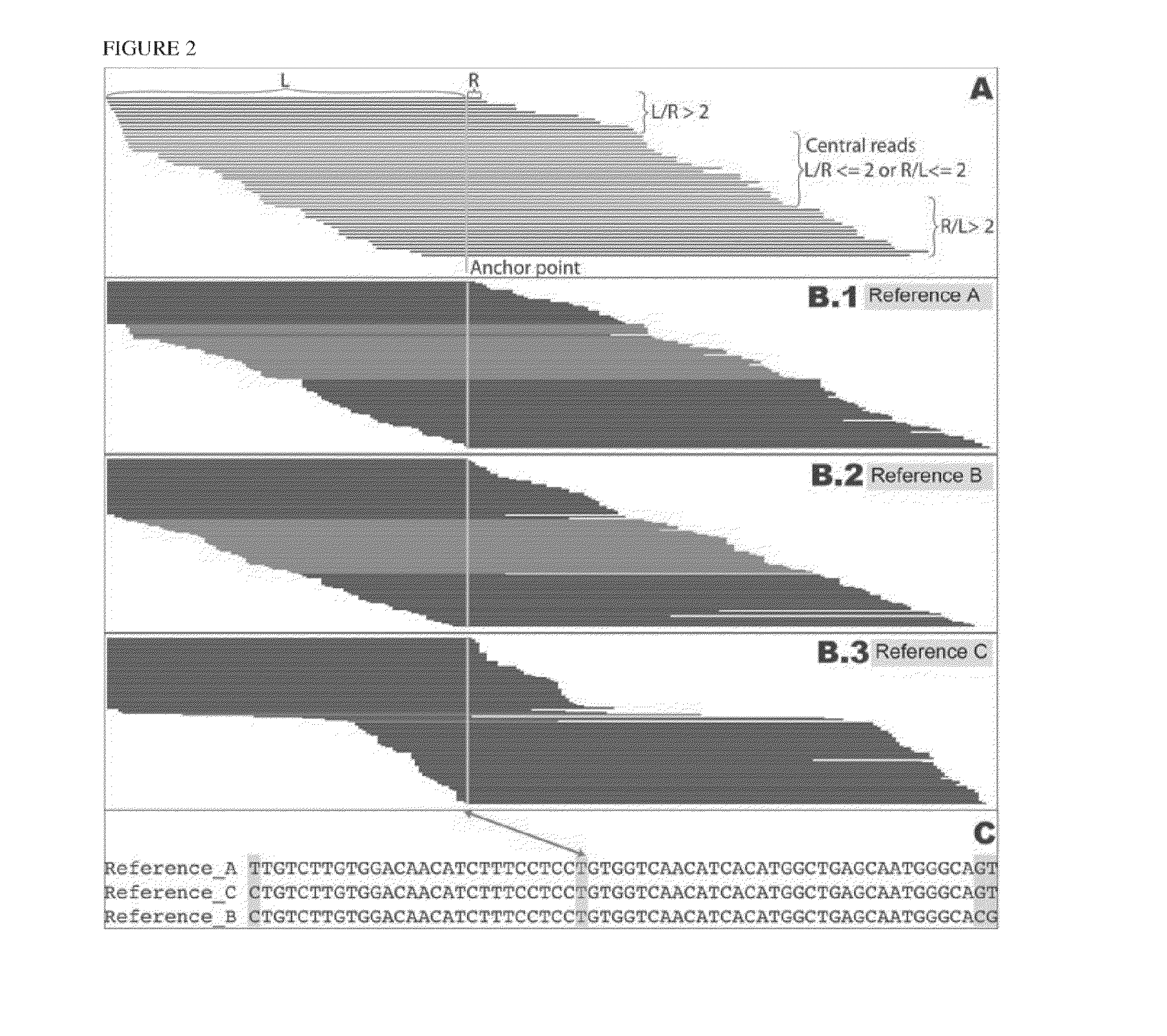
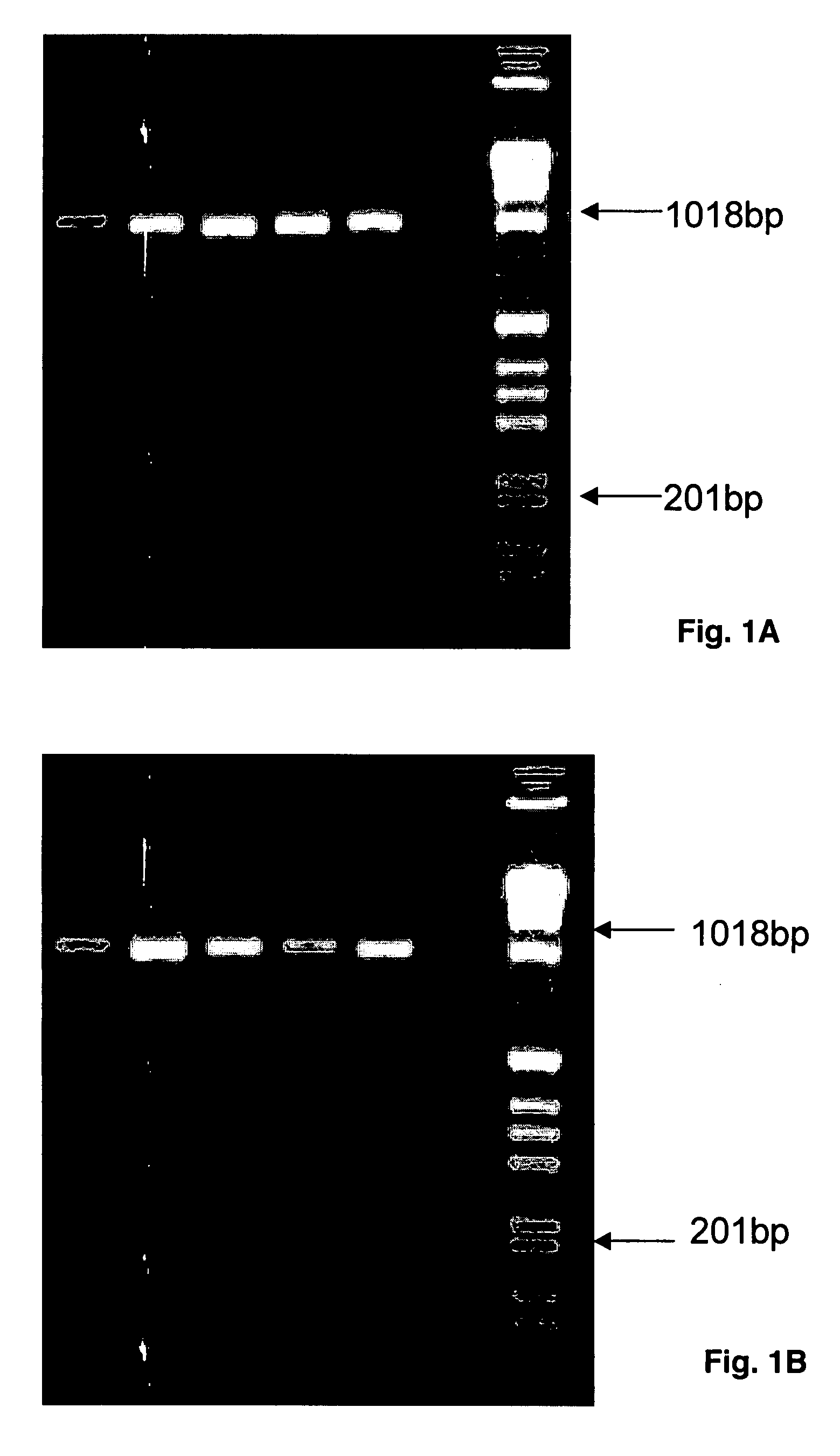
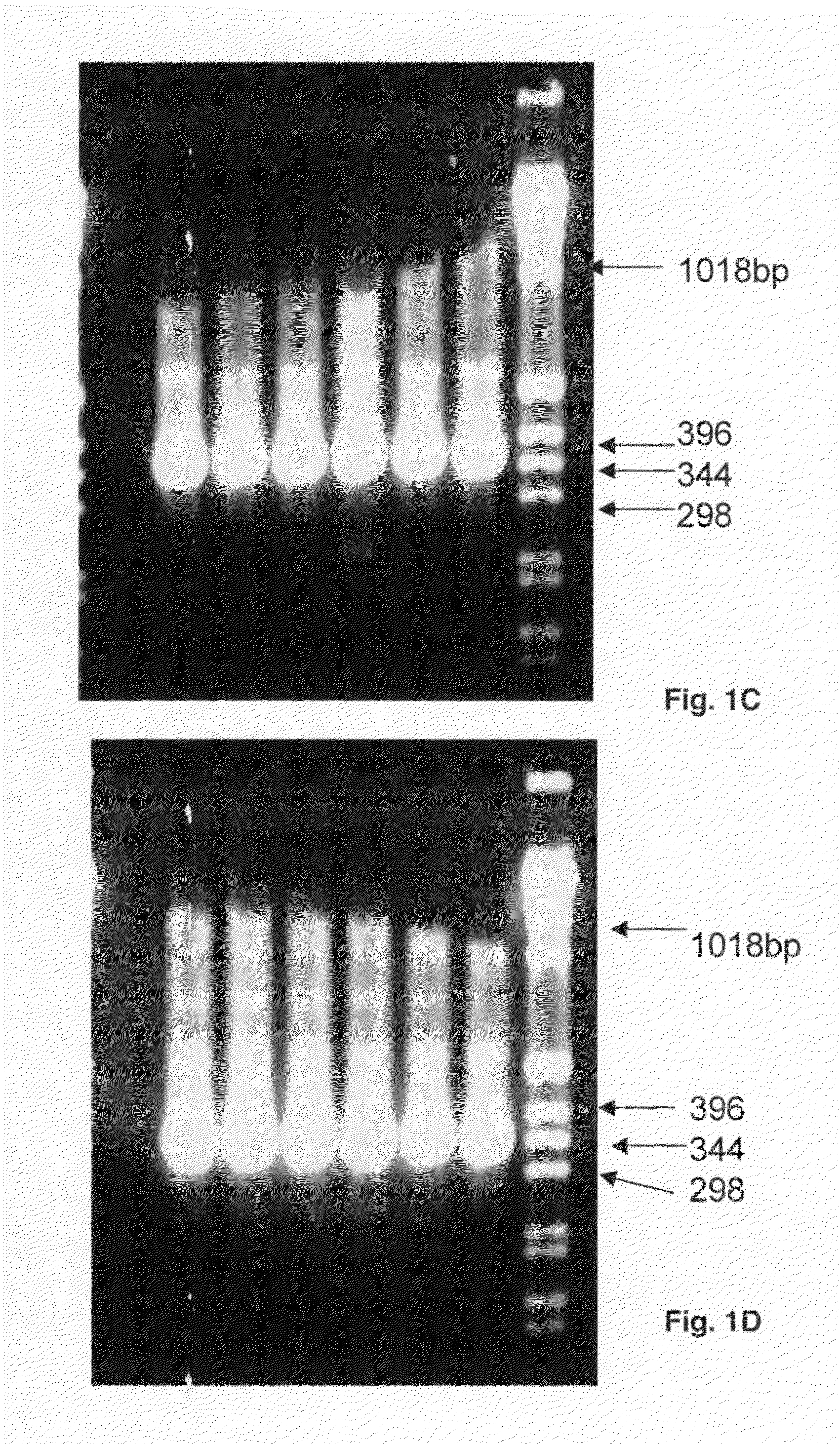
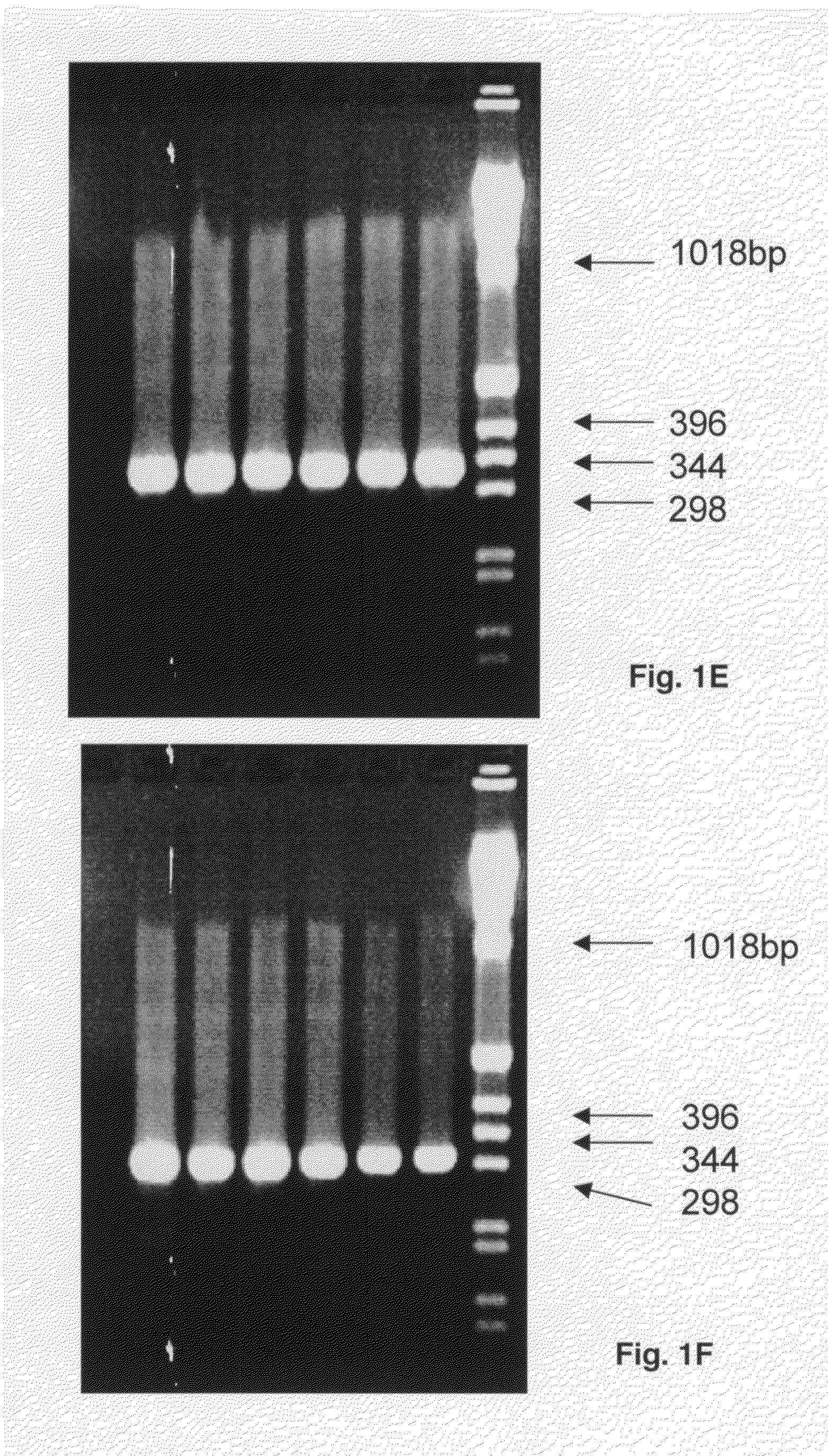
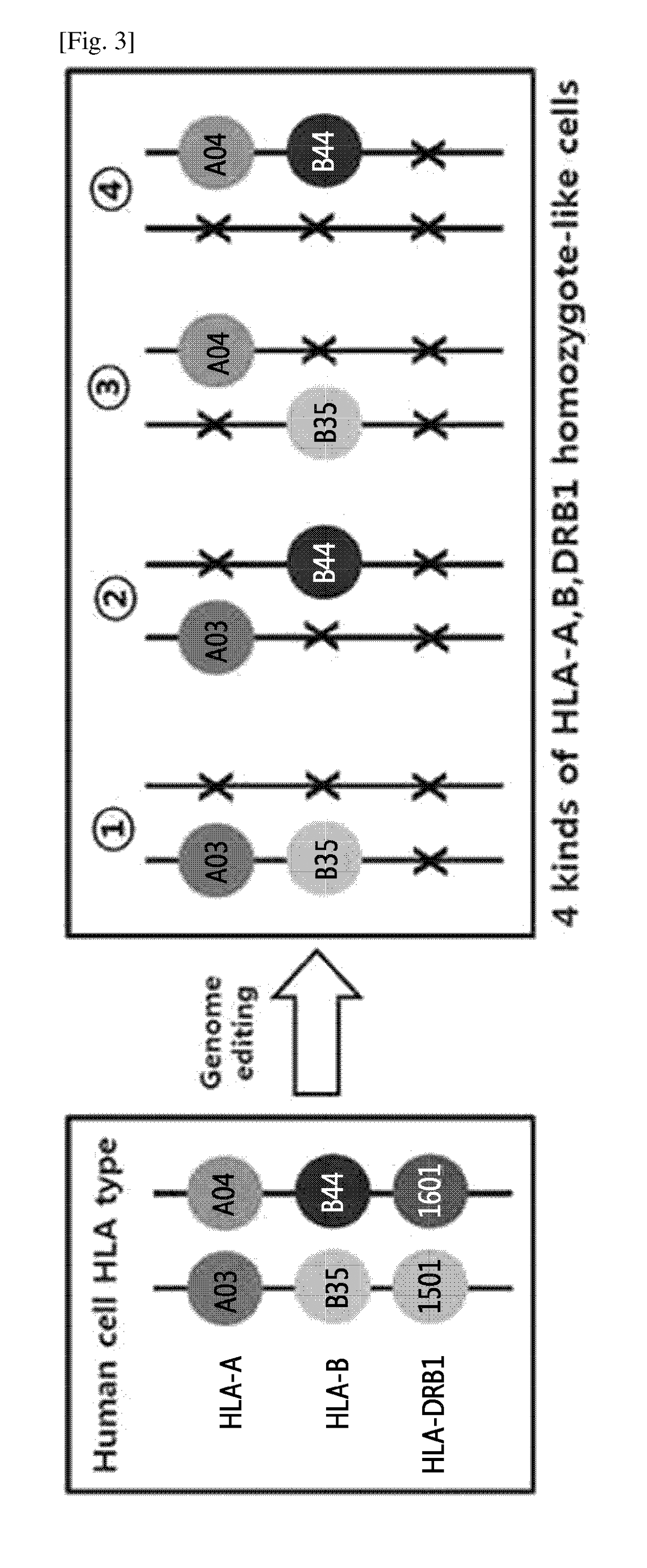
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Hla genes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
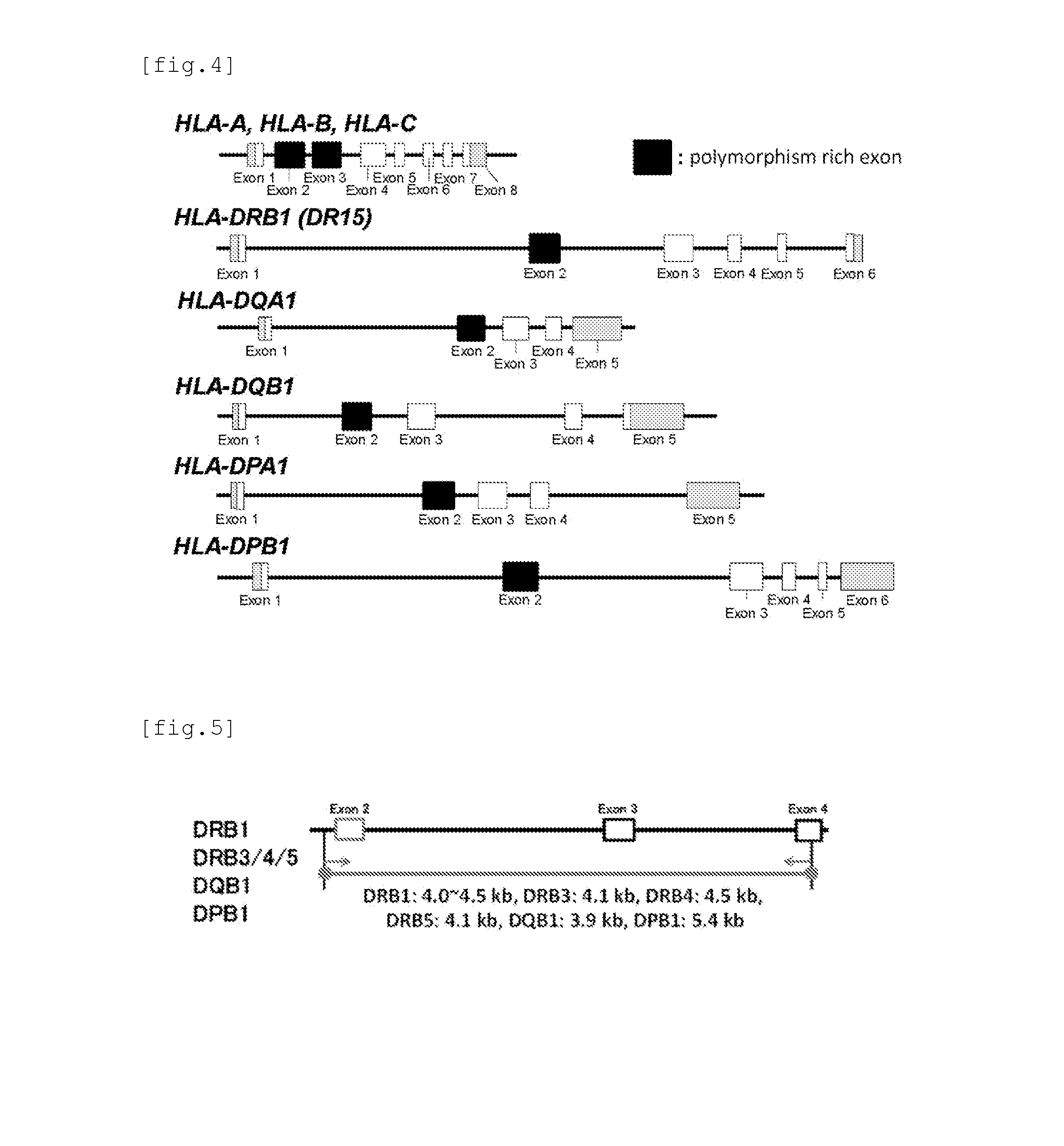
HLA is the human version of the major histocompatibility complex (MHC), a gene group that occurs in many species. In humans, the MHC complex consists of more than 200 genes located close together on chromosome 6. Genes in this complex are categorized into three basic groups: class I, class II, and class III.
Human leukocyte antigen HLA-A and HLA-B gene full-length sequencing method and HLA gene sequencing and typing method
InactiveCN101962676AWide detection coverageResolving Ambiguous Results IssuesMicrobiological testing/measurementDNA/RNA fragmentationTyping methodsHLA-B gene
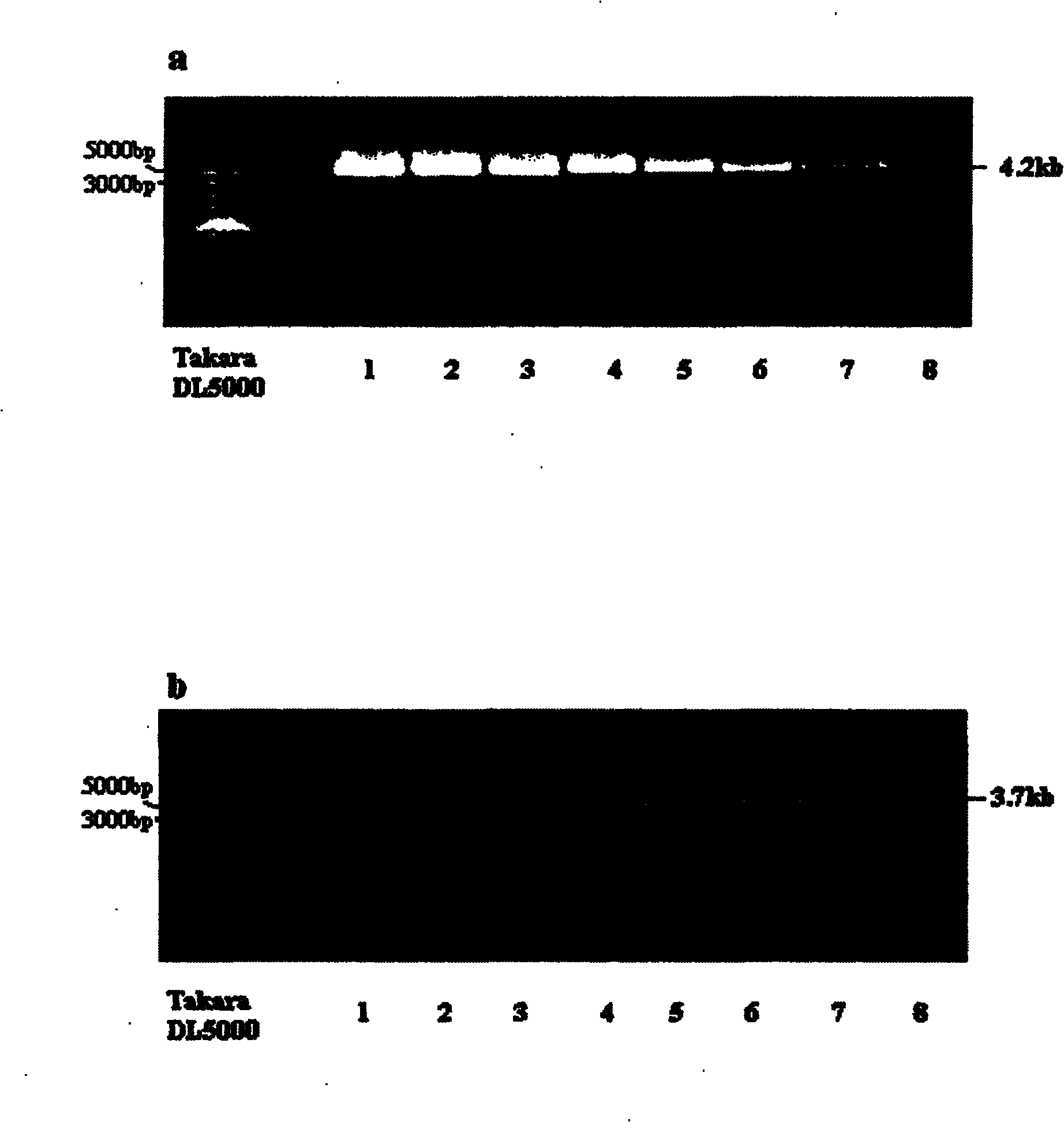
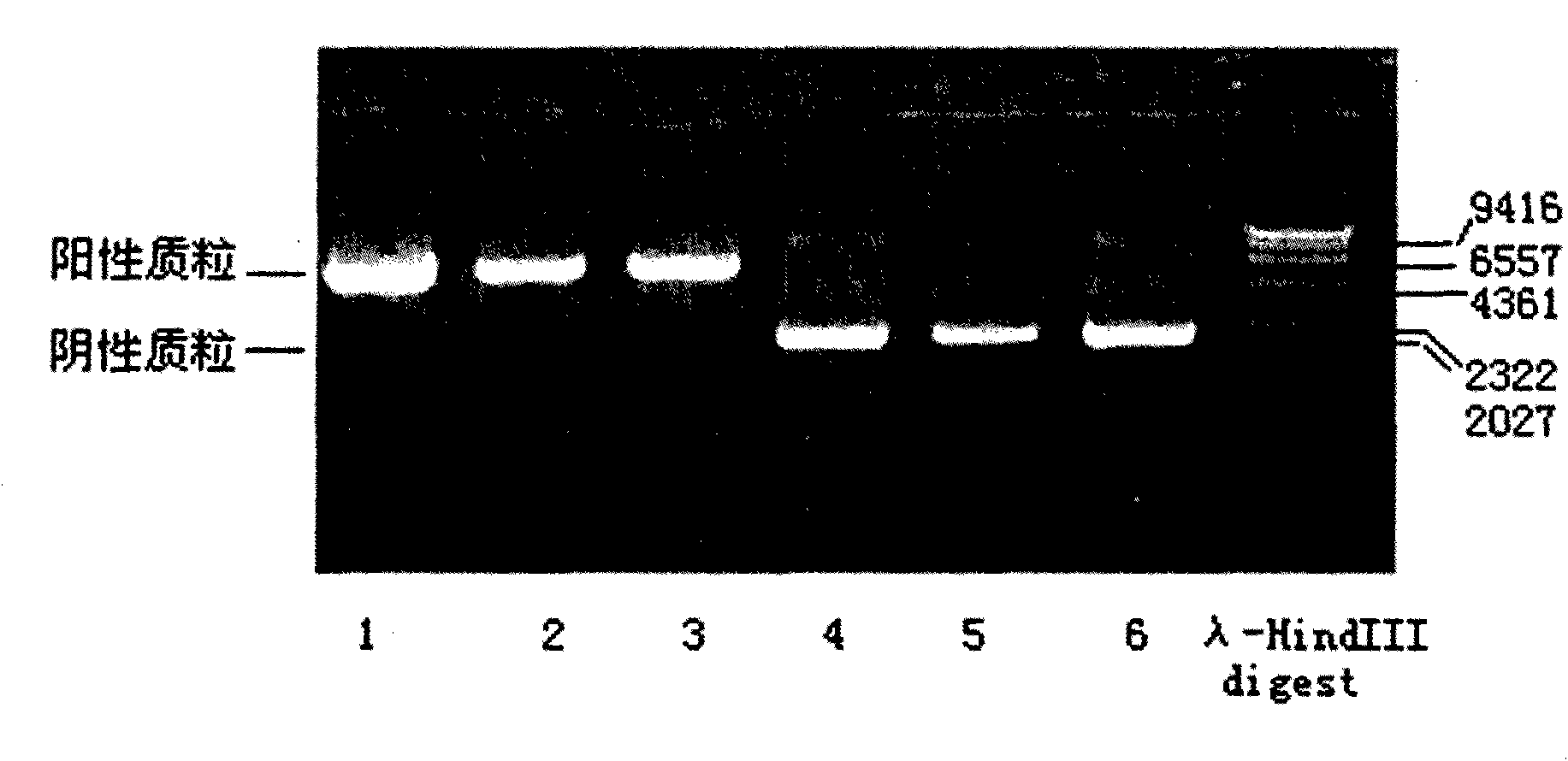
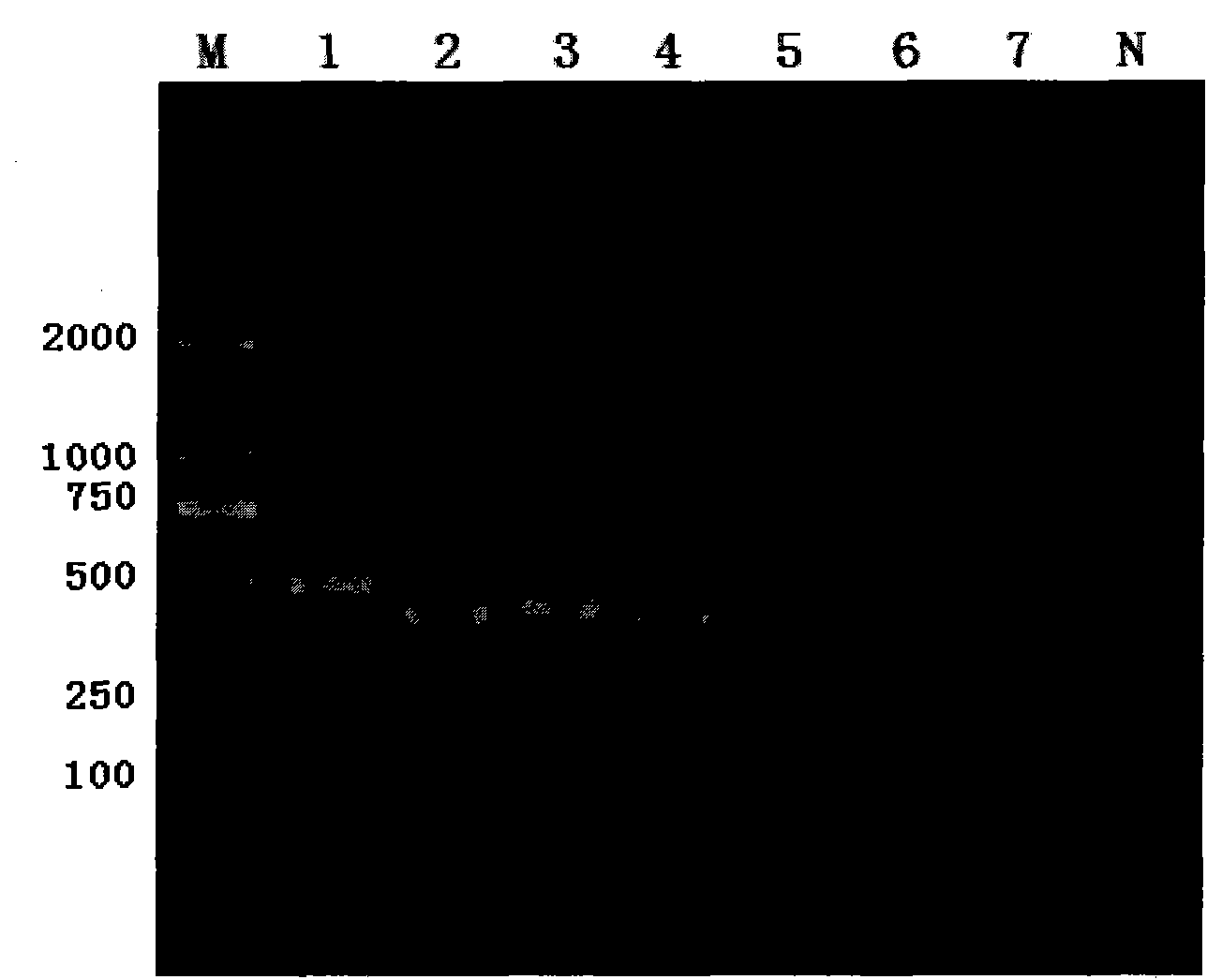
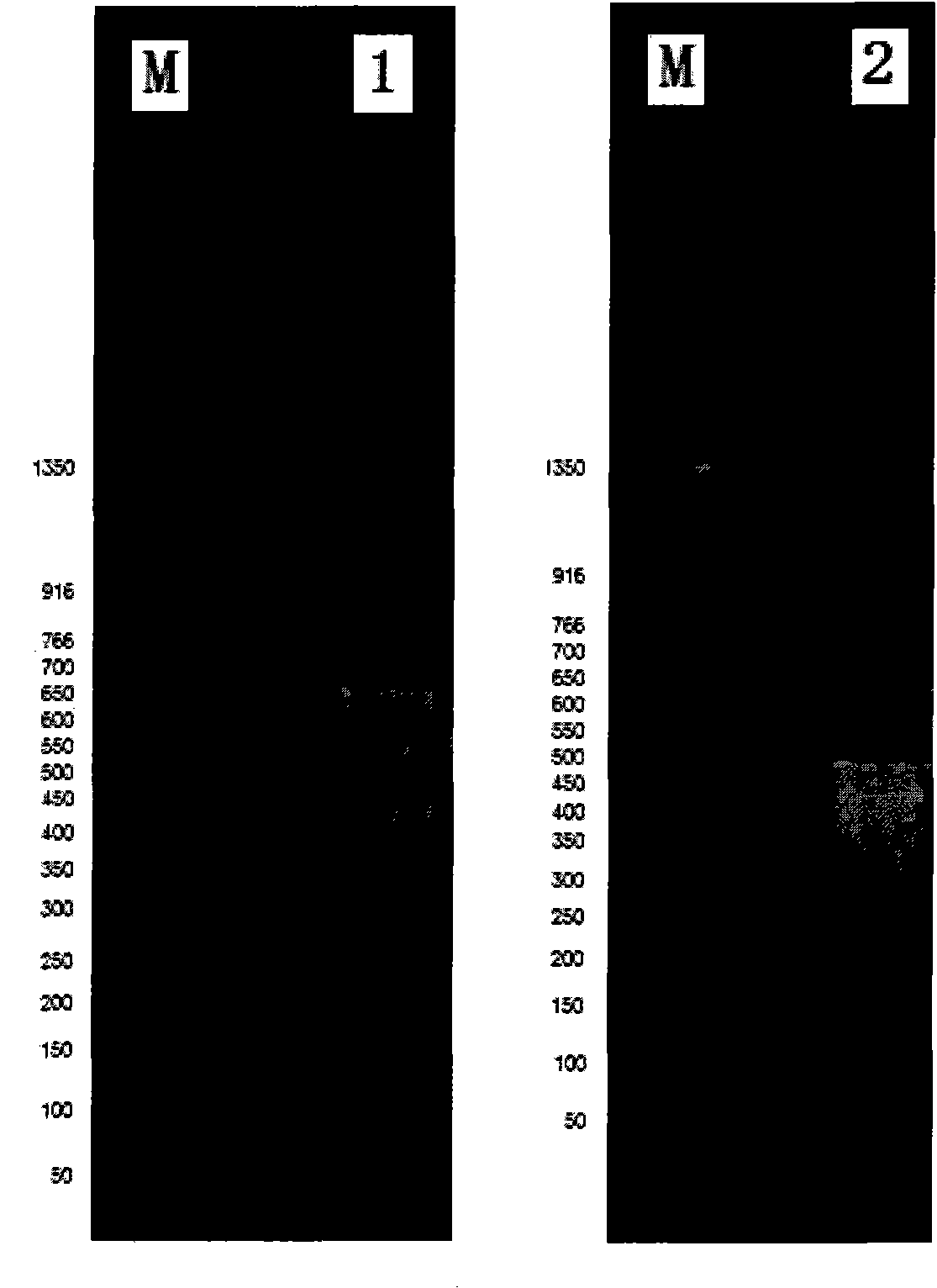
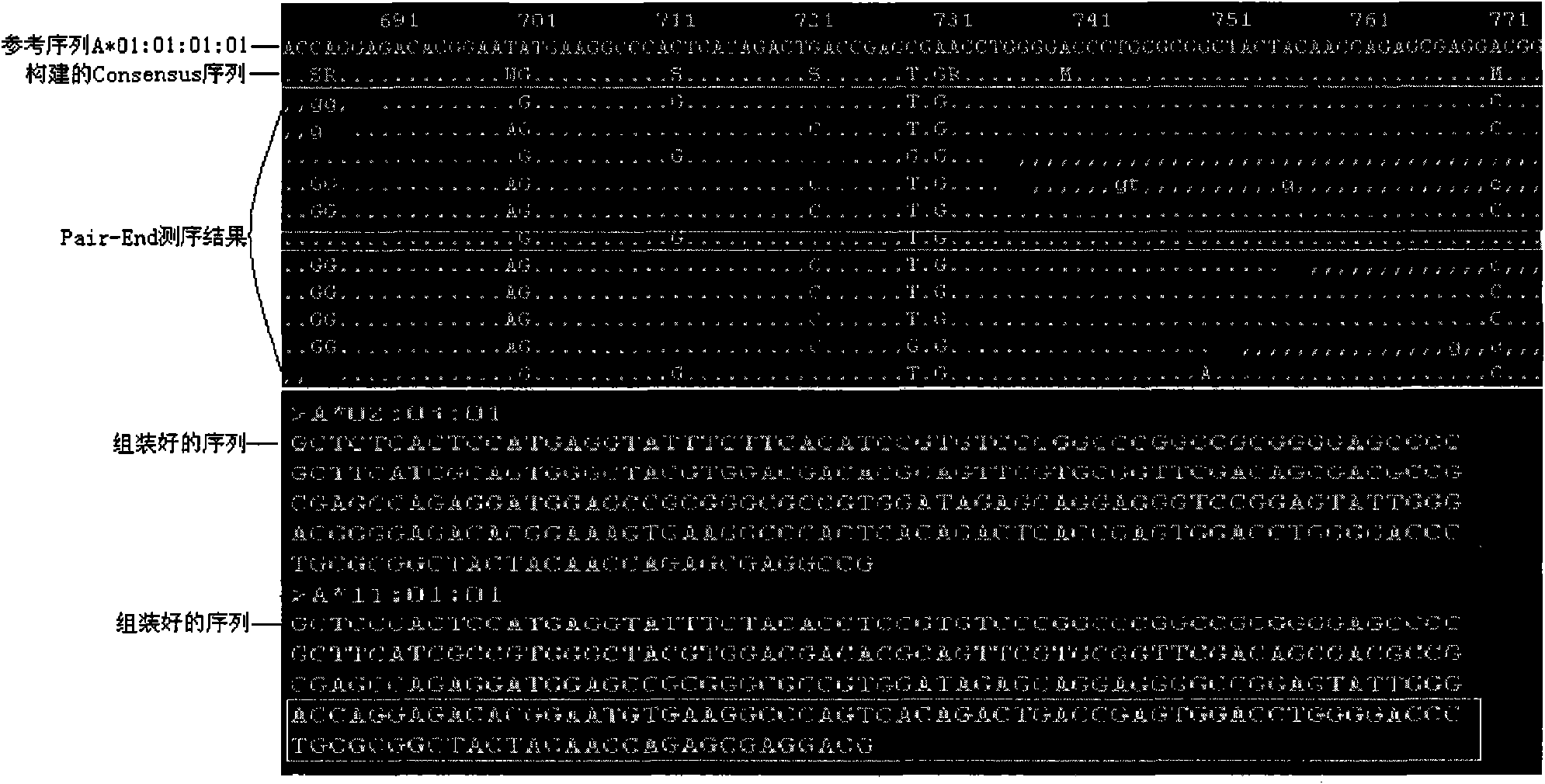
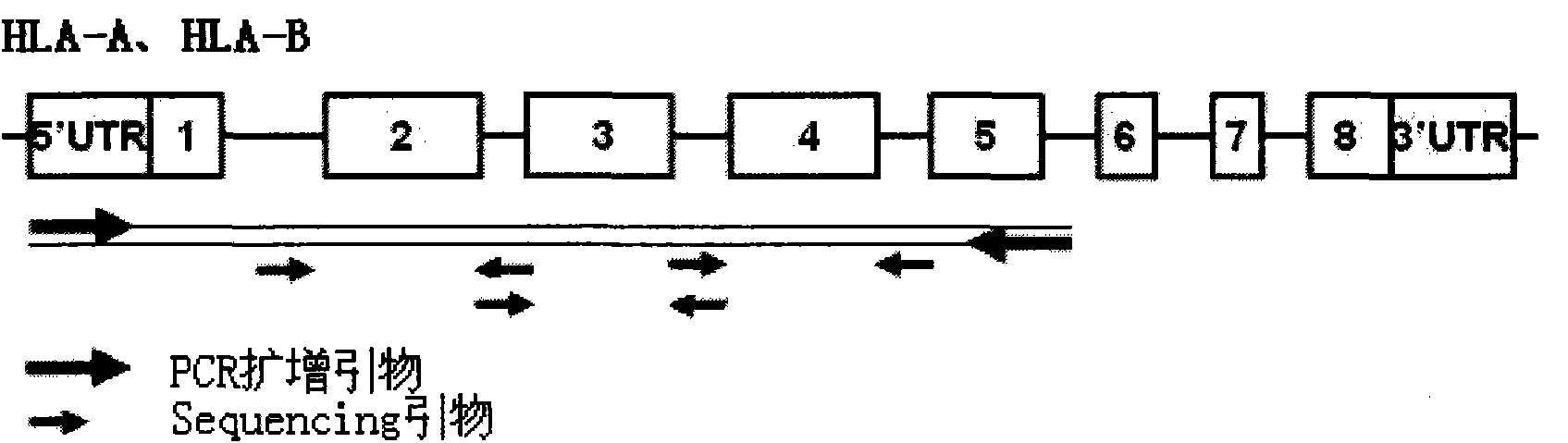
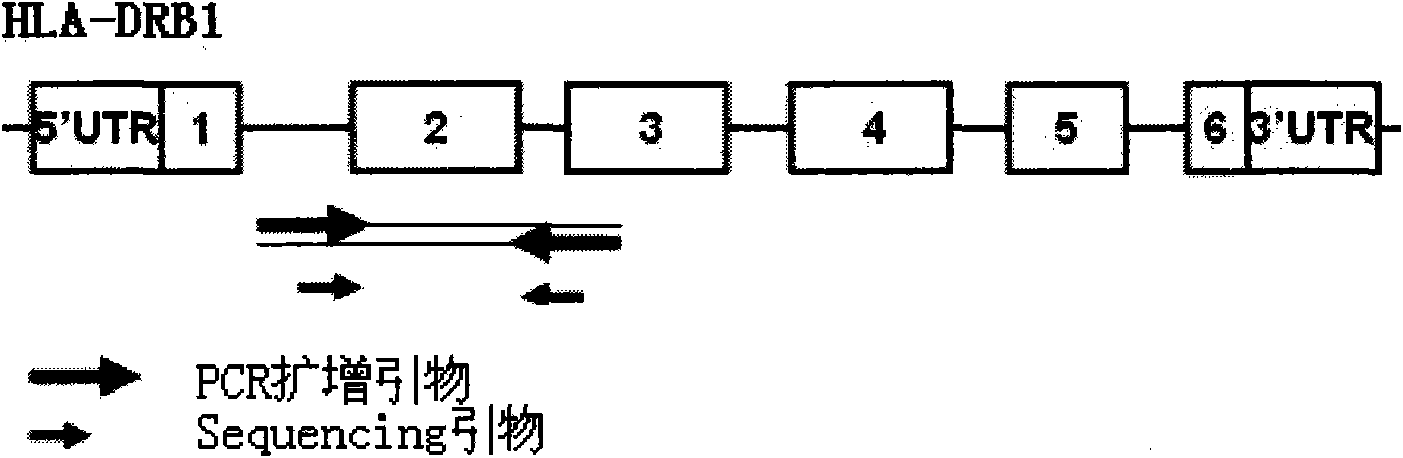
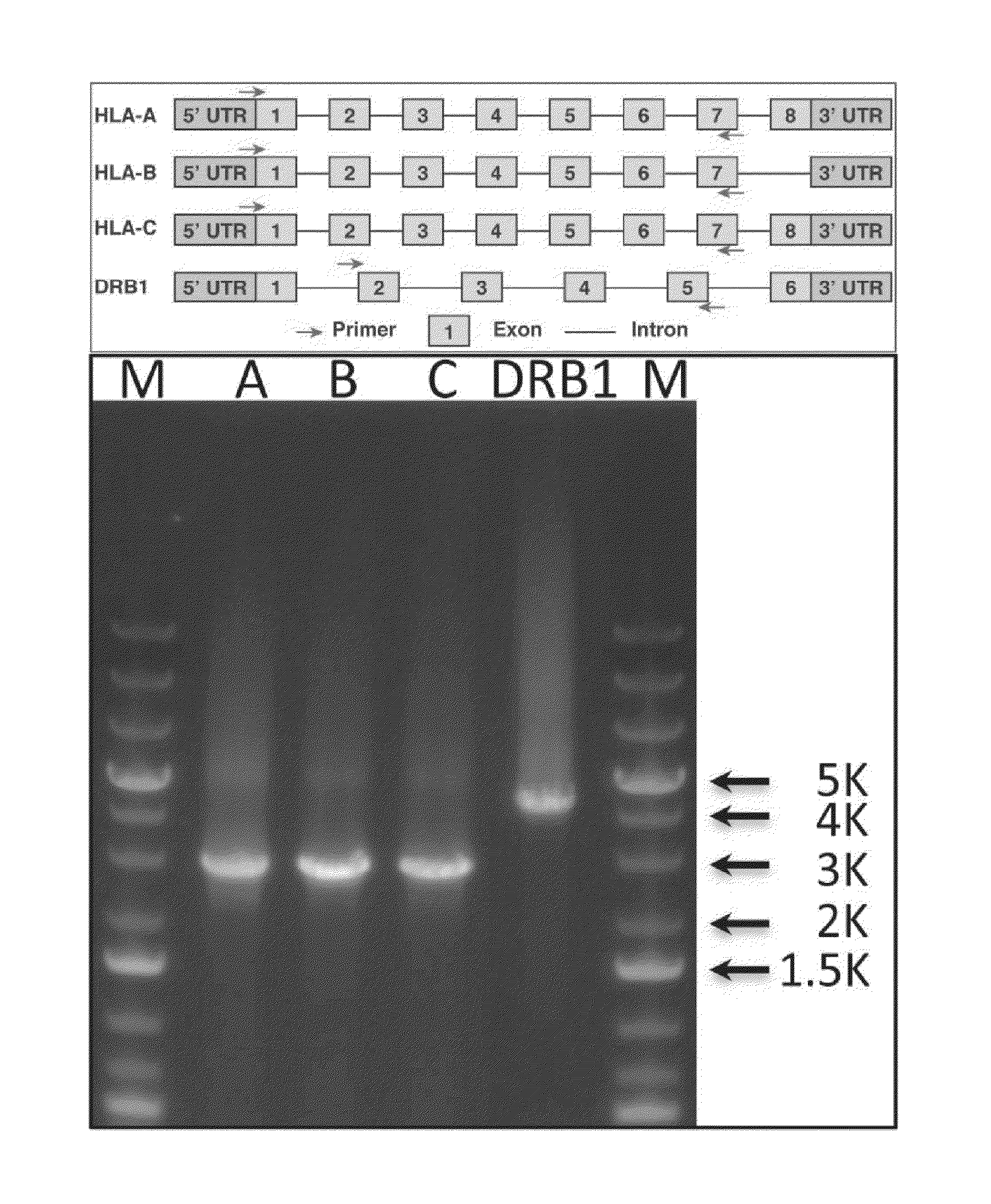
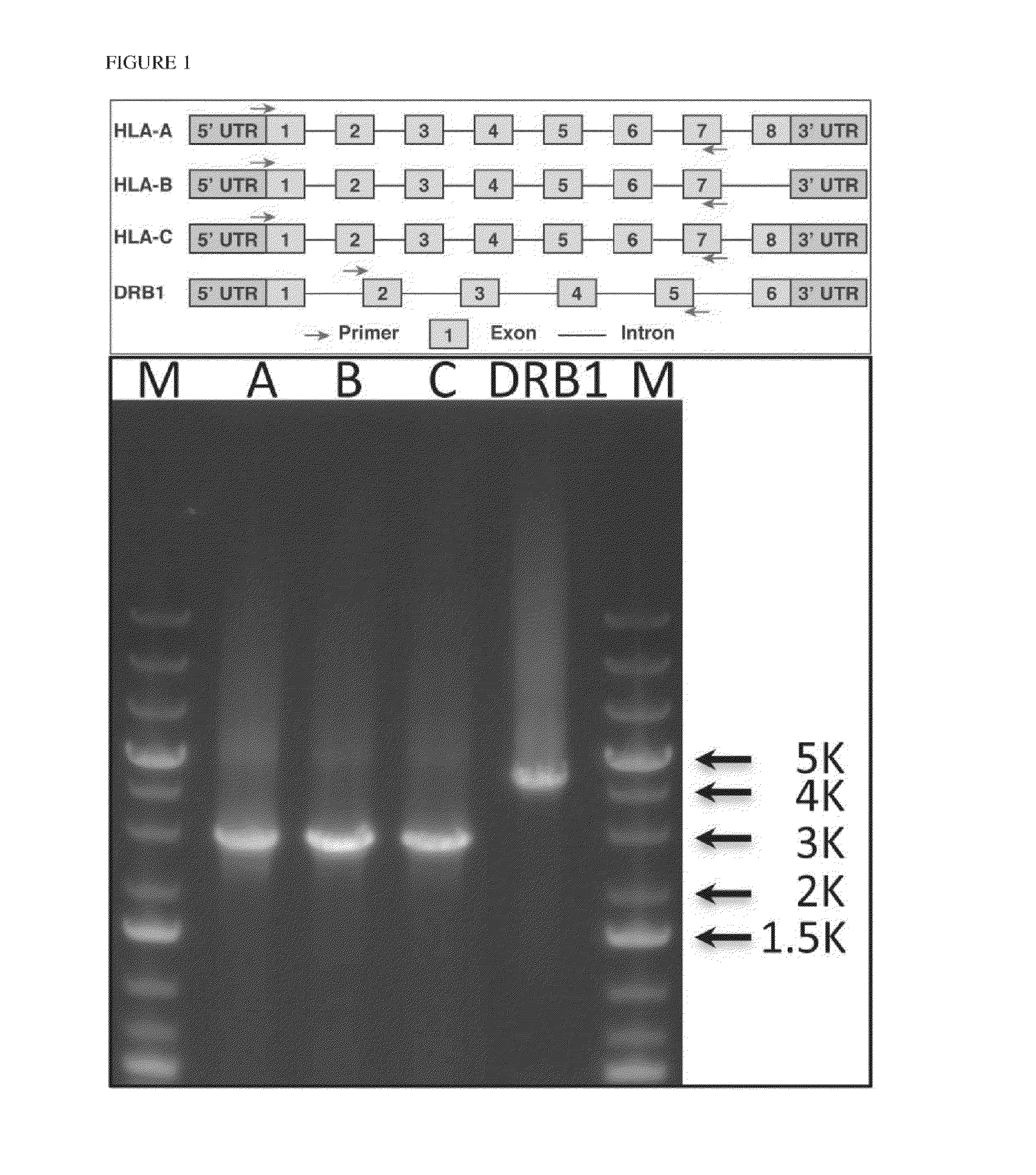
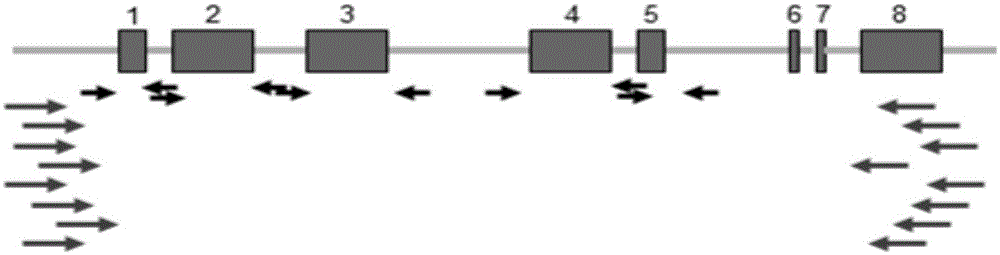
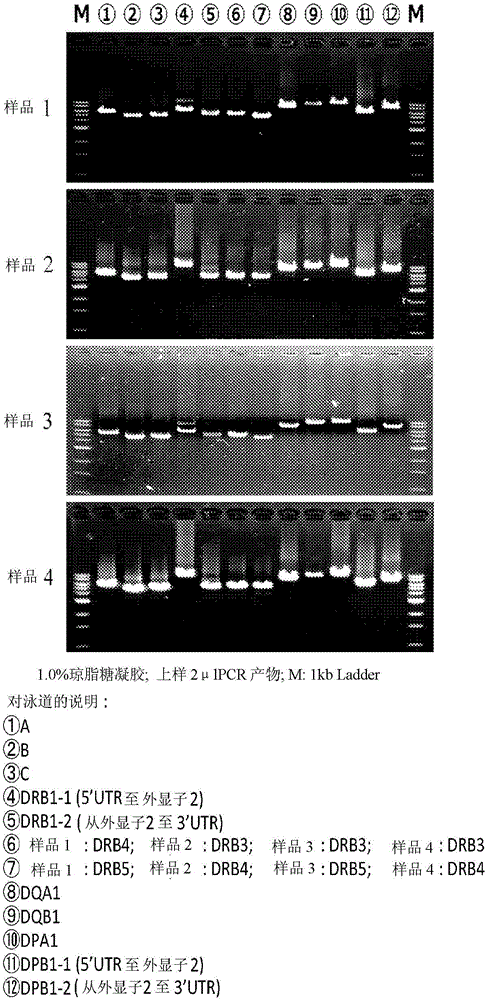
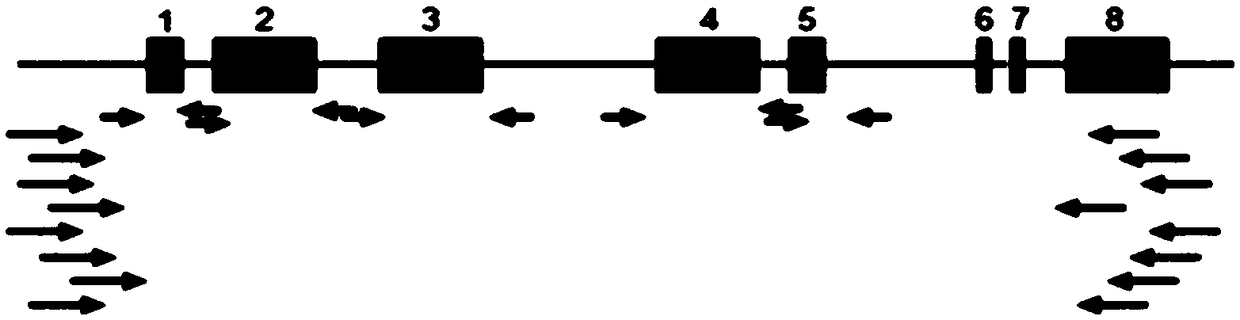
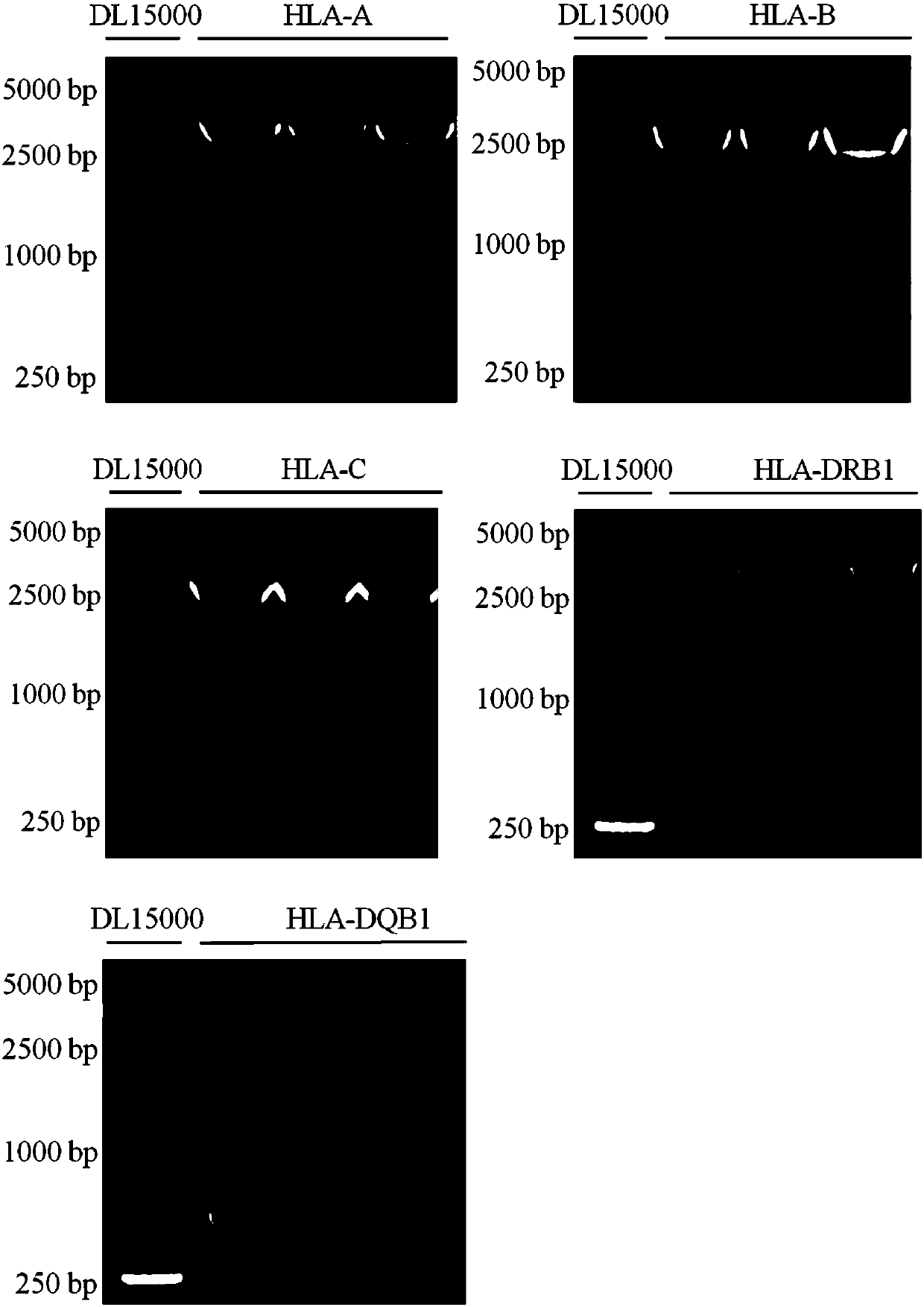
The invention discloses a human leukocyte antigen HLA-A and HLA-B gene full-length sequencing method and an HLA gene sequencing and typing method. The HLA-A and HLA-B gene full-length sequencing method comprises the following steps of: a, performing PCR amplification on about 4kb full-length sequences of HLA-A and HLA-B genes by using a pair of primers respectively; and b, cloning the amplification products to a pGEM-Tea sy vector, sequencing the full-length sequences by using ten walking sequencing primers in positive and negative directions respectively, and totally obtaining 38 allele 4.3kb full-length sequences of the HLA-A and 30 allele 3.7kb full-length sequences of the HLA-B. The HLA-A and HLA-B sequencing and typing method comprises the following steps of: performing PCR amplification on typing target areas of the HLA-A and HLA-B by using two pairs of primers respectively; and performing two directional sequencing on products by using fourteen sequencing primers respectively, wherein the HLA-DRB1 and HLA-DQB1 sequencing and typing method comprises the following steps of: amplifying sequences of second and third exons of DRB1 and DQB1 by adopting group specificity primers respectively; performing two directional sequencing on the second and third exons of the DRB1 by adopting eight group specificity primers and three sequencing primers; and performing two directional sequencing on the second and third exons of the DQB1 by adopting four sequencing primers respectively.
Owner:SHENZHEN BLOOD CENT
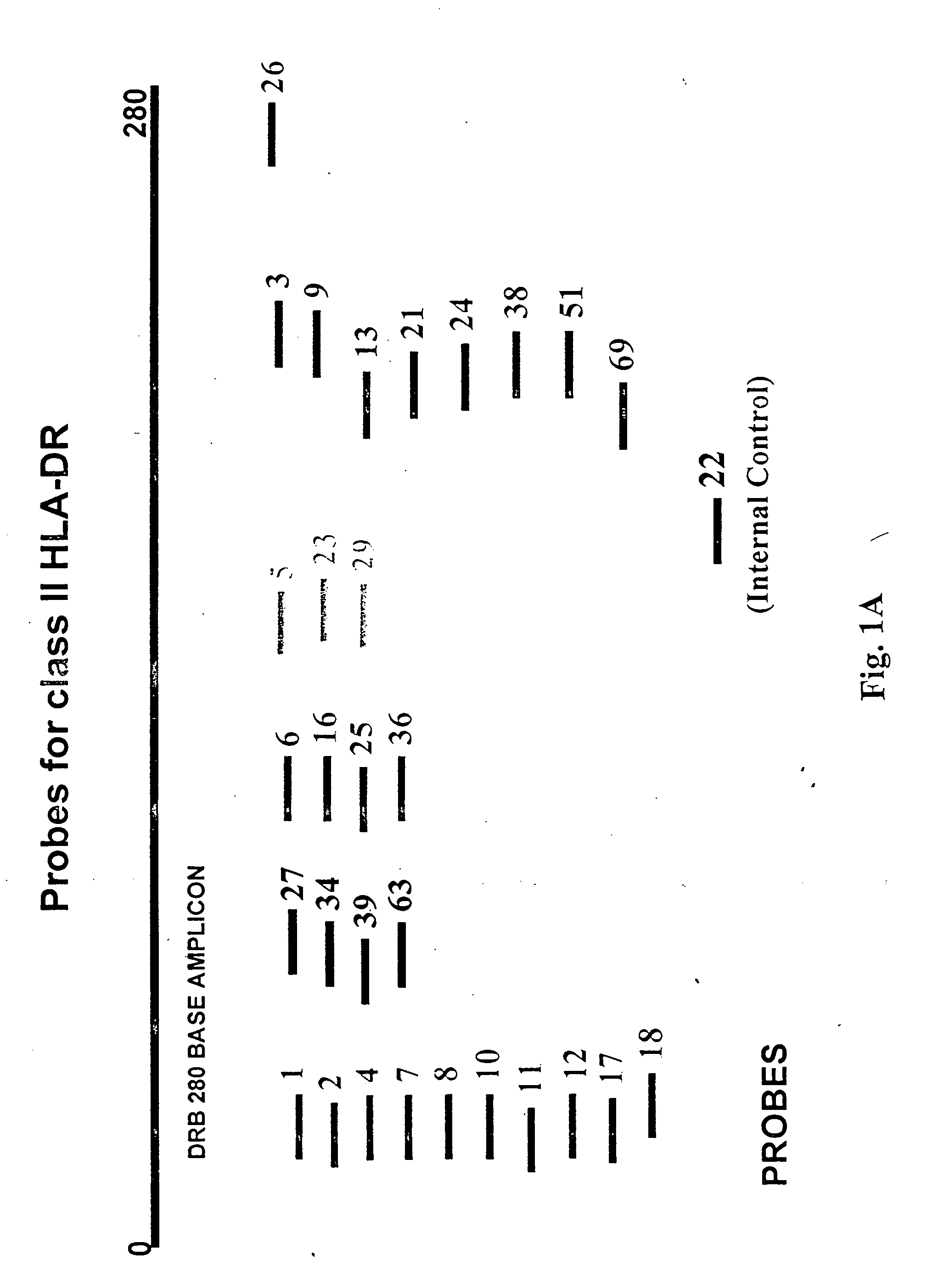
Multiplexed analysis of polymorphic loci by concurrent interrogation and enzyme-mediated detection
InactiveUS20080167195A1Strong specificityDegree of reductionMicrobiological testing/measurementLibrary member identificationHla genesEnzyme
The invention provides methods and processes for the identification of polymorphisms at one or more designated sites, without interference from non-designated sites located within proximity of such designated sites. Probes are provided capable of interrogation of such designated sites in order to determine the composition of each such designated site. By the methods of this invention, one or more mutations within the CFTR gene and the HLA gene complex can be can be identified.
Owner:BIOARRAY SOLUTIONS
System and method for detection of HLA Variants
A method for detecting one or more HLA sequence types is described that comprises the steps of: amplifying a plurality of first amplicons from a double stranded nucleic acid sample, wherein the first amplicons are amplified with a plurality of pairs of nucleic acid primers that define exons 2 and 3 of both strands of HLA loci from the group consisting of HLA-A, HLA-B, and HLA-C; amplifying the first amplicons to produce a plurality of populations of second amplicons, wherein each population of second amplicons is clonally amplified from one of the first amplicons; sequencing the plurality of populations of second amplicons to generate a nucleic acid sequence composition for each of the plurality of second amplicons; and detecting variation in the sequence composition from one or more of the second amplicons for one or more of the HLA loci.
Owner:454 LIFE SCIENCES CORP
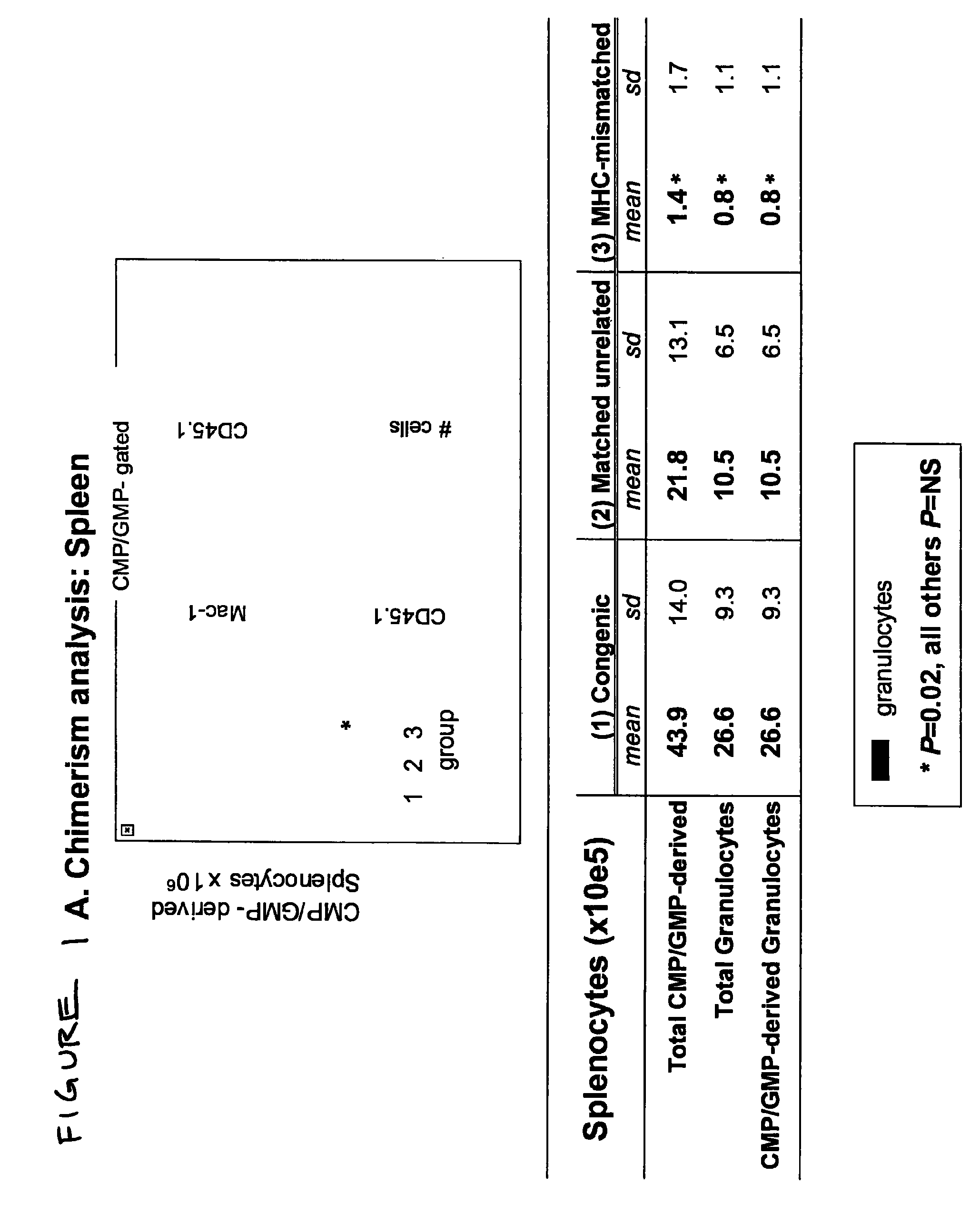
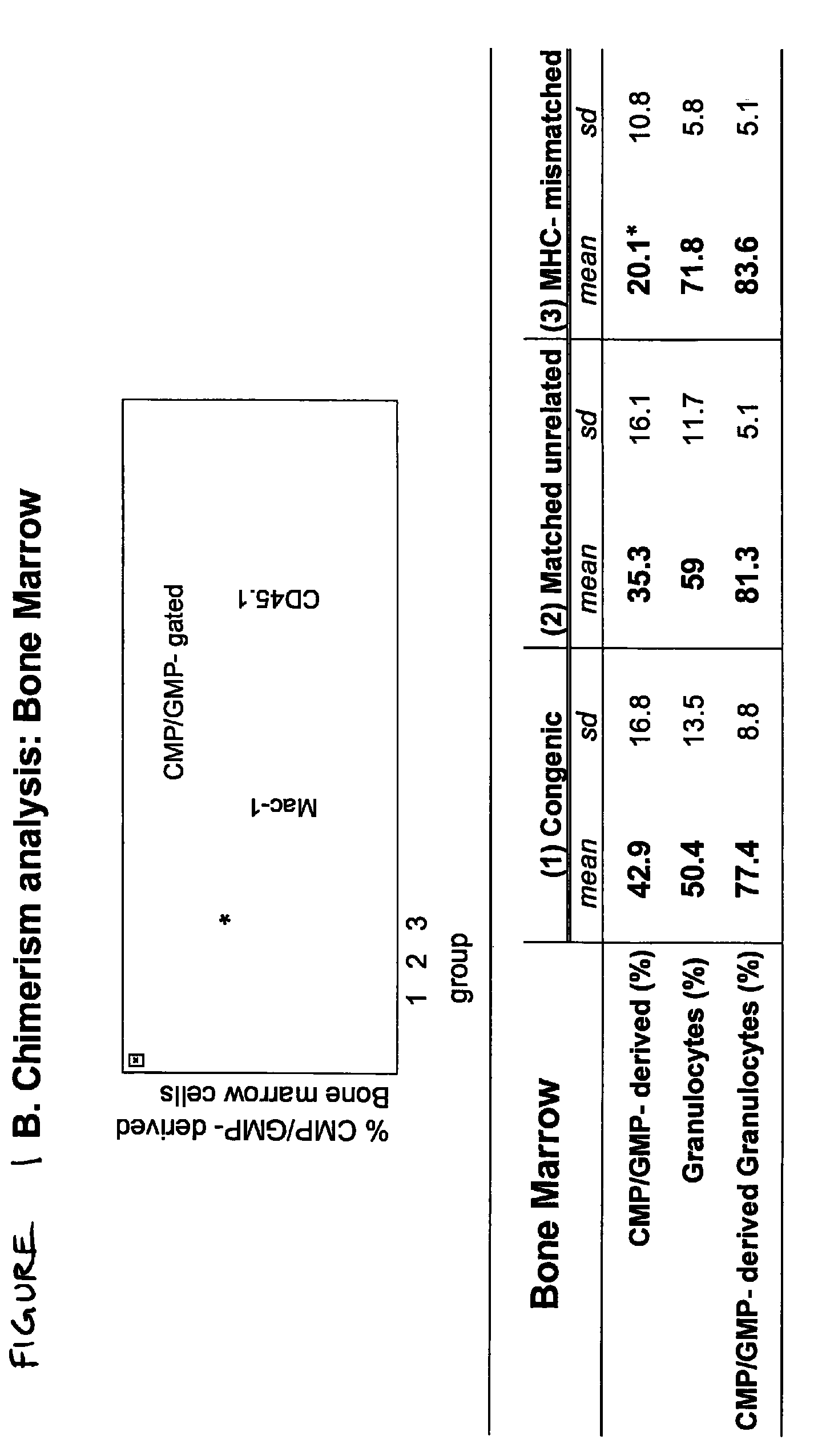
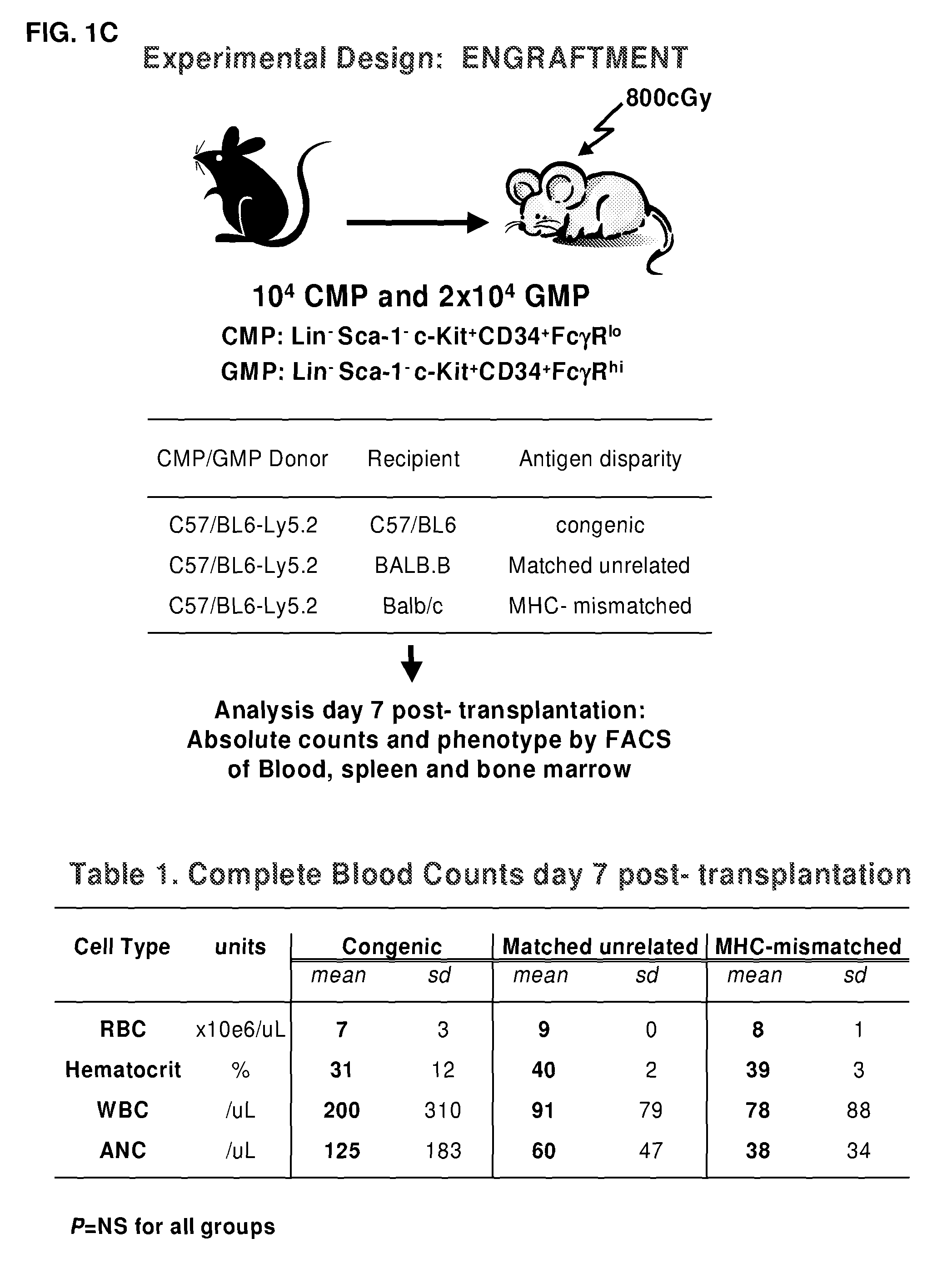
Therapeutic uses of allogeneic myeloid progenitor cells
Myeloid function is enhanced by transplantation or infusion of allogeneic myeloid progenitor cells, including CMP, GMP, MEP and MKP cell subsets. Myeloid progenitors ameliorate sequelae of anemia and thrombocytopenia, and can prevent or treat gastrointestinal mucositis associated with chemotherapy, radiotherapy, and the like. The transplantation or infusion may be performed in the absence of HLA typing, and the cells may be mismatched at one or more Class I HLA loci. The transplantation may provide for treatment of ongoing disease, or prevention of disease in high risk patients.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Method for amplifying and typing HLA gene and relevant primer thereof
ActiveCN101654691AImprove accuracyHigh resolutionMicrobiological testing/measurementFermentationTypingHla genes
Owner:BGI GENOMICS CO LTD
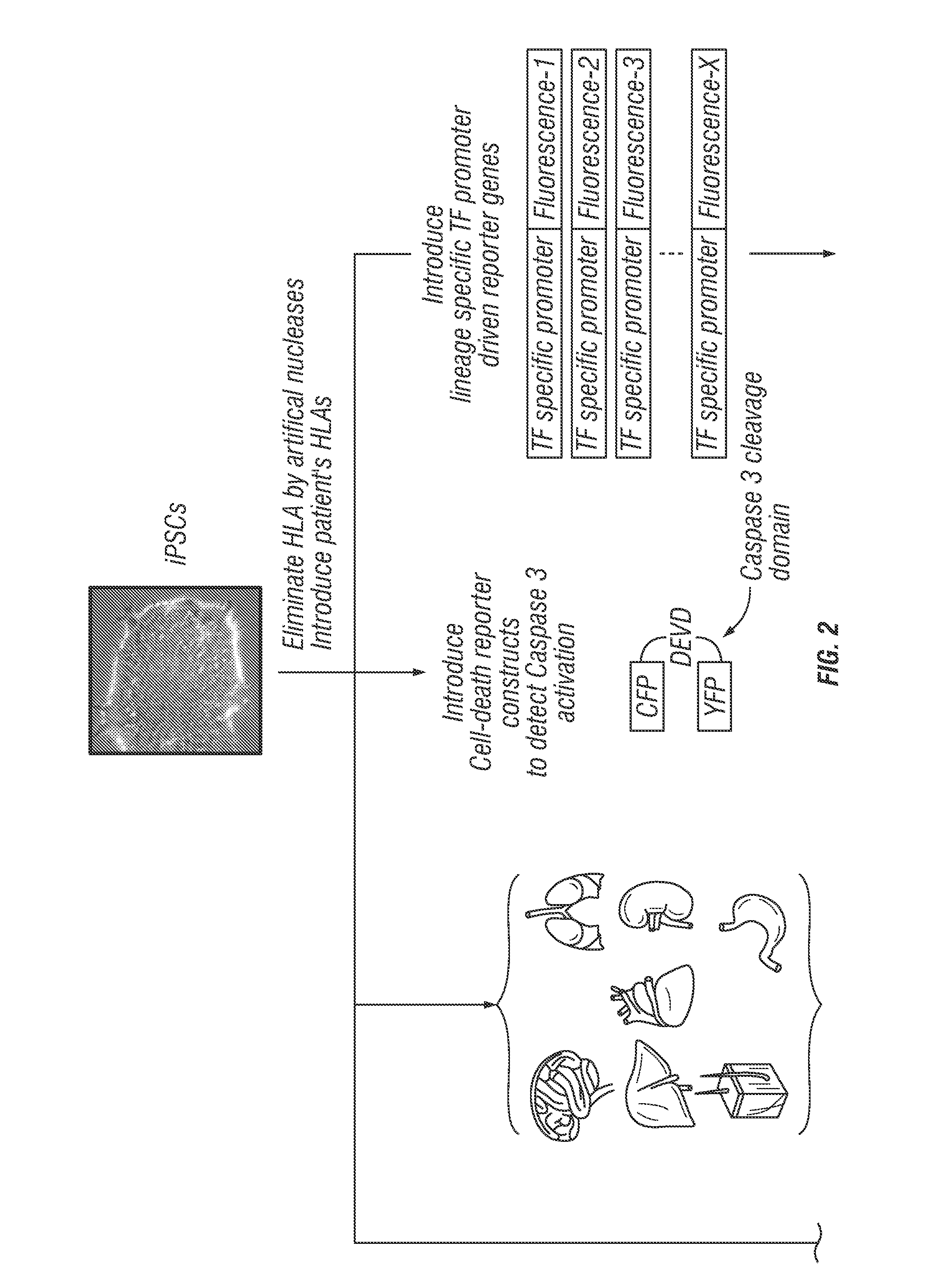
Application of induced pluripotent stem cells to generate adoptive cell therapy products
The application of induced pluripotent stem cells (iPSCs) to generate adoptive cell therapy products and usage of iPSCs for screening potential toxicity of immune receptors are described herein. In some aspects, iPSCs and cells differentiated therefrom are provide which do not express a HLA gene (e.g., HLA-A).
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
HLA gene specific PCR amplification primer, HLA typing method and kit
InactiveCN103045591ANo StrapReasonable and accurateMicrobiological testing/measurementDNA/RNA fragmentationNucleotideTyping methods
The invention discloses an HLA (human leukocyte antigen) gene specific PCR (polymerase chain reaction) amplification primer, an HLA typing method and an HLA typing kit. A sequence of the PCR amplification primer comprises a primer sequences shown as SEQ ID No. (sequence identification number) 1-2, 3-4, 5-6, 7-8 and 9-10. The HLA gene typing method comprises the steps that the HLA gene specific PCR amplification primer is used for conducting PCR amplification and purification on sample DNA (deoxyribonucleic acid); a gene exon sequencing primer is used for conducting sequencing PCR amplification, purification and sequencing on an HLA gene amplification product, the sequence of the HLA gene amplification product is compared with a standard sequence; and the type of the HLA gene is determined. The HLA gene typing kit comprises the PCR amplification primer and the sequencing primer. A qualitative leap in aspects of detection flux, data quality, cost control and the like is achieved; data is more reliable and realer; and the problem that typing cannot be conducted when nucleotide of certain alleles is positioned outside an amplification area is solved.
Owner:SHANGHAI TISSUEBANK BIOTECH +3
HLA (Human Leukocyte Antigen) gene high-resolution genotyping method based on Illumina GA sequencing technology
ActiveCN101921841AExperiment operation is simpleImprove throughputSugar derivativesMicrobiological testing/measurementBioinformaticsHla genes
The invention provides an HLA (Human Leukocyte Antigen) gene high-resolution genotyping method based on an Illumina GA sequencing technology and a primer label for the method.
Owner:BGI GENOMICS CO LTD
HLA (Human Leukocyte Antigen)-A,B genotyping PCR (Polymerase Chain Reaction) primer and application method thereof
ActiveCN101921842AImprove featuresImproved conservatismMicrobiological testing/measurementDNA/RNA fragmentationHla genesBiology
The invention provides an HLA (Human Leukocyte Antigen)-A,B genotyping PCR (Polymerase Chain Reaction) primer, and a method of applying the PCR primer to HLA gene analysis based on a sequencing method.
Owner:BGI GENOMICS CO LTD
HLA high-resolution gene sequencing kit
InactiveCN101892317AAvoid problems that cannot be effectively typedHigh resolutionMicrobiological testing/measurementDNA/RNA fragmentationHLA-BExon
The invention discloses a parting method of leucocyte antigen gene of human being, comprising the following steps of: (1) extracting genome DNA to be tested by a regular technology, and amplifying a destination gene fragment to be analyzed by using PCR amplification primer: 2,3,4 exon of HLA-A, 2,3,4 exon of HLA-B and exon on the locus 2 of HLA-DRB; and (2) amplifying the PCR output obtained in the step (1) by using sequencing primer, amplifying the exon, sequencing the amplified exon and comparing the sequencing result with the standard sequence in a database to determine the gene parting result. As the 2,3,4 exon of HLA-A, 2,3,4 exon of HLA-B and exon on the locus 2 of HLA-DRB are effectively amplified as a result of optimized combination of the HLA gene sequencing kit and the test condition, and the corresponding exon is sequenced, the invention solves the problem that effective parting can not be performed when certain allelic gene nucleotide is located outside an amplification area during further parting, thereby improving the parting resolution and accuracy of the HLA gene.
Owner:SUZHOU UNIV +1
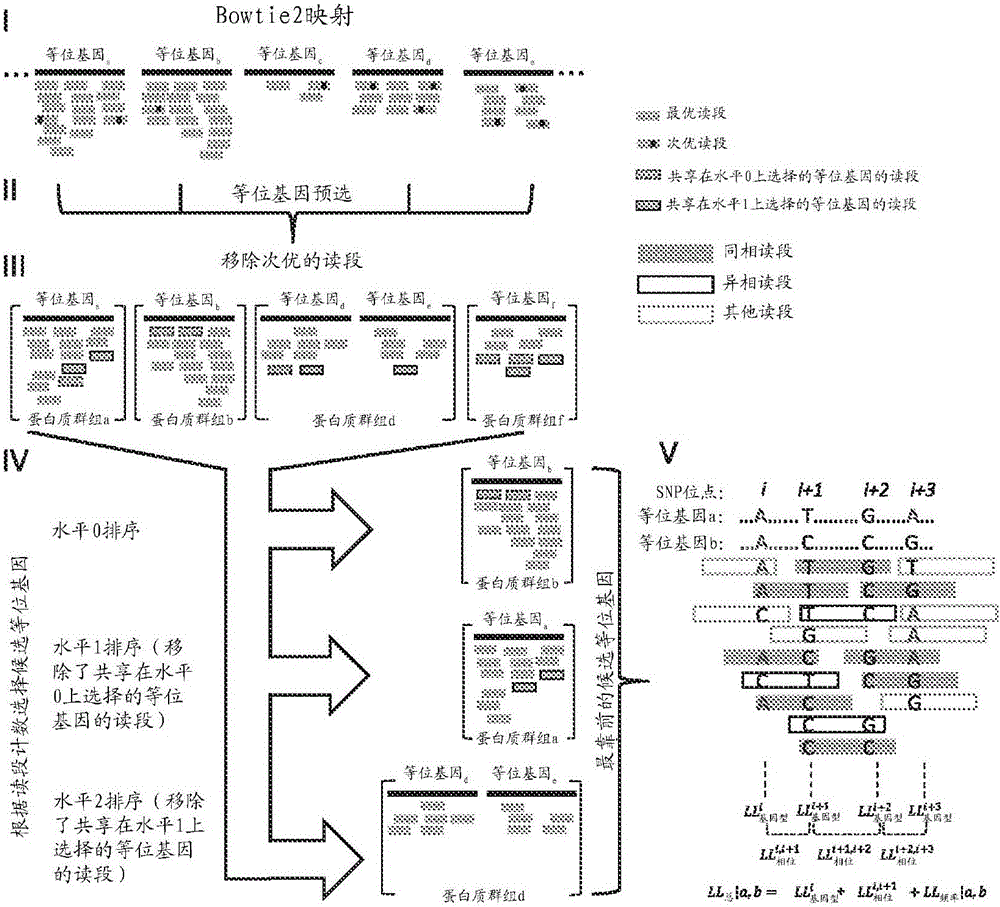
Software haplotying of HLA loci
InactiveUS20150379195A1Accurate genotype callingAccurate callMicrobiological testing/measurementLibrary member identificationHla haplotypesExon
Methods are provided to determine the genomic sequence of the alleles at the HLA gene. The resultant sequences provide linkage information between different exons, and produces the unique sequence at each gene from the two alleles of the individual sample being typed. The sequence information provides an accurate HLA haplotype. Methods to decrease allele dropout during long range PCR reactions are also disclosed.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Genotyping Hla Loci
InactiveUS20080182252A1Faster turn around timeEasy accessMicrobiological testing/measurementGeneticsGenotyping
Owner:LINKAGE BIOSCI
Group-specific amplification primer, sequence-based typing method and kit for HLA genes
InactiveCN105039332AAvoid missing detectionEasy to analyzeMicrobiological testing/measurementDNA/RNA fragmentationAmbiguityImmunogenetics
The invention relates to biomedicines and immunological genetics and particularly relates to a group-specific amplification primer, sequence-based typing method and a kit for HLA genes. Sequences of a PCR amplification primer are selected from sequences represented by SEQ ID NO.1-28, sequences represented by SEQ ID NO.29-64 and sequences represented by SEQ ID NO.65-94. By designing the group-specific amplification primer by carrying out serum classification on the HLA genes, all allelic genes including HLA-A, -B and -C can be subjected to sequence-based typing; by carrying out amplification sequence on full-length sequences of genes HLA-A, -B and -C, the ambiguity can be effectively solved, an absolute high score is achieved, and data is relatively accurate and reliable.
Owner:SHENZHEN GENEBIOHEALTH
High resolution allele identification
Provided herein are methods for accurately determining the alleles present at a locus that is broadly applicable to any locus, including highly polymorphic loci such as HLA loci, BGA loci and HV loci. Embodiments of the disclosed methods are useful in a wide range of applications, including, for example, organ transplantation, personalized medicine, diagnostics, forensics and anthropology.
Owner:REGENERON PHARM INC
Method for targeted capture and sequencing of HLA gene sequences
InactiveCN109913539AEasy to control the lengthIncrease abundanceMicrobiological testing/measurementTarget captureNucleic Acid Probes
The invention relates to the field of biotechnology, and particularly provides a method for targeted capture and sequencing of HLA gene sequences. The method comprises the following steps: 1) hybridizing a denatured nucleic acid sample with a nucleic acid probe library fixed on a solid-phase carrier under a hybridization condition to form a target nucleic acid molecule-probe-solid-phase carrier membrane complex; 2) cleaning the 'probe-solid-phase carrier membrane complex' bound with target nucleic acid in step 1) by using a cleaning solution, eluting the probe-solid-phase carrier membrane complex bound with the target nucleic acid from a solid-phase carrier membrane by using an eluting solution, purifying, enriching or constructing to obtain a nucleic acid library; and 3) taking the nucleic acid library obtained by enrichment or constructed after enrichment in step 2) for high-throughput sequencing. A nucleic acid probe which can rapidly generate high-fold coverage and single-base displacement and is used for enriching HLA genes can be immovably fixed on the solid-phase carrier membrane. The method has characteristics of simplicity and convenience in operation, flexible probe acquisition and low cost, is favorable for improving the HLA genotype identification accuracy, and is rapid and convenient.
Owner:ZHEJIANG UNIV
Non-invasive early detection of solid organ transplant rejection by quantitative analysis of mixtures by deep sequencing of HLA gene amplicons using next generation systems
ActiveUS20140336056A1Microbiological testing/measurementLibrary member identificationOrgan transplant rejectionNon invasive
The invention is a method of detecting or assessing solid organ graft (transplant) rejection by detecting donor-specific HLA alleles in a blood sample of a graft (transplant) recipient. The invention further comprises a method of detecting the presence of maternal cells in a blood sample of an offspring.
Owner:THE SCRIPPS RES INST +1
Method and device for HLA genotyping, storage medium and processor
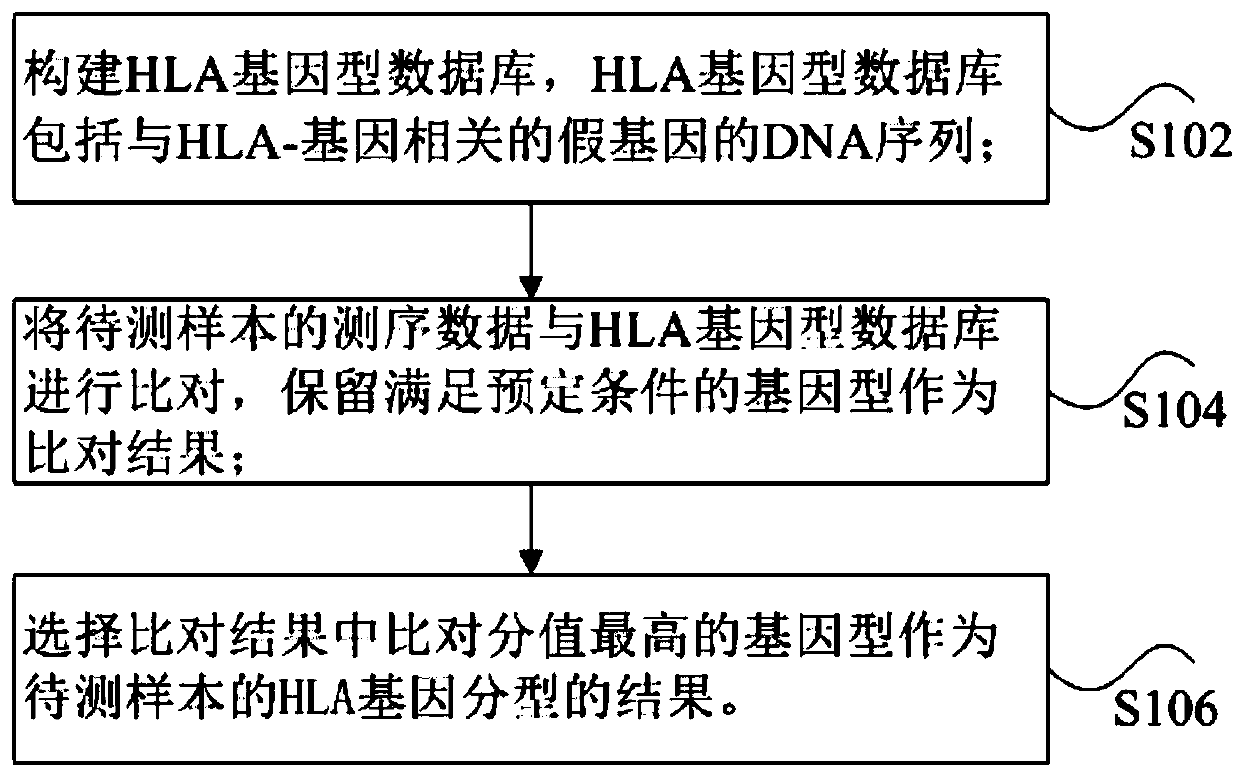
PendingCN110033827AReduce false positive resultsSolve the technical problem of high typing false positiveSequence analysisInstrumentsSequencing dataHla genes
The invention discloses a method and device for HLA genotyping, a storage medium and a processor. The method for HLA genotyping includes the steps: constructing an HLA genotype database, wherein the HLA genotype database comprises a DNA sequence of pseudogenes associated with HLA-A genes; comparing the sequencing data of the sample to be tested with the HLA genotype database, and retaining the genotype satisfying the predetermined condition as a comparison result; and selecting the genotype with the highest comparison value in the comparison result as the result of HLA genotyping of the sampleto be tested. For the method for HLA genotyping, adding an HLA pseudogene sequence very similar to the HLA gene sequence in the HLA genotyping database helps to reduce the false positive results of typing, thus solving the technical problem of high false positive HLA genotyping in the prior art.
Owner:ZHENYUE BIOTECHNOLOGY JIANGSU CO LTD
Hla gene multiplex dna typing method and kit
ActiveCN105339508AHigh precisionRapid typingMicrobiological testing/measurementRecombinant DNA-technologyHuman DNA sequencingTest sample
The purpose is to provide a high-precision DNA typing method and kit that make full use of a high-throughput sequencer and eliminate the ambiguity derived from phase ambiguity. The invention provides an HLA DNA typing method characterized by including: (1) a step for providing a set of primers that each hybridize specifically to an upstream region and a downstream region of at least two genes selected from genes belonging to HLA class I and class II in the nucleotide sequence of the human genome and amplify under identical PCR conditions; (2) a step for amplifying these at least two genes in a test sample (DNA) simultaneously in the same container under identical PCR conditions using this set of primers; (3) a step for determining the nucleotide sequence of the PCR product; and (4) an optional step for conducting a homology search of a database.
Owner:GENODIVE PHARMA
Population scale HLA-typing and uses thereof
InactiveUS7667026B2Bioreactor/fermenter combinationsBiological substance pretreatmentsHybridization probeTyping
The present invention provides a portable system for real-time population-scale HLA genotyping and / or allelotyping in a field environment and methods of such population-scale HLA genotyping. The individual components of the system are portable to and operable within a field environment thereby providing high throughput with real-time geno- or allelotyping. Also provided are HLA gene-specific primers and HLA allele-specific or single nucleotide polymorphism-specific hybridization probes. In addition the present invention provides a microarray comprising the hybridization probes. Further provided is a kit comprising the HLA gene-specific primers and the microarray.
Owner:GENOMICS USA
Immune-compatible cells created by nuclease-mediated editing of genes encoding HLA
The present invention relates to a method for producing immune-compatible cells or a cell population which comprises a step of editing one or two alleles of one or more immune-compatible antigen genes by gene deletion or modification in an isolated cell comprising at least one of the immune-compatible antigen genes selected from HLA (human leukocyte antigen)-A, HLA-B and HLA-DR, to immune-compatible cells produced by the method, and to a cell population comprising the immune-compatible cells produced by the method
Owner:COLLEGE OF MEDICINE POCHON CHA UNIV IND +1
Method for determining HLA-A*24 group
InactiveCN104024410AEasy to judgeMicrobiological testing/measurementRecombinant DNA-technologyStart codonNucleotide
Owner:TOPPAN PRINTING CO LTD
Method of screening for drug hypersensitivity reaction
ActiveUS20070148641A9High incidenceIncreased riskSugar derivativesMicrobiological testing/measurementHypersensitive responseDrug allergy
Methods of assessing the risk of clinical signs of hypersensitivity reaction to nucleoside antiviral compounds, including abacavir, are described. The methods include genotyping subjects for polymorphisms in the TNFα gene, the class 1 HLA genes, or a combination of both the TNFα and HLA genes.
Owner:VIIV HEALTHCARE UK LTD
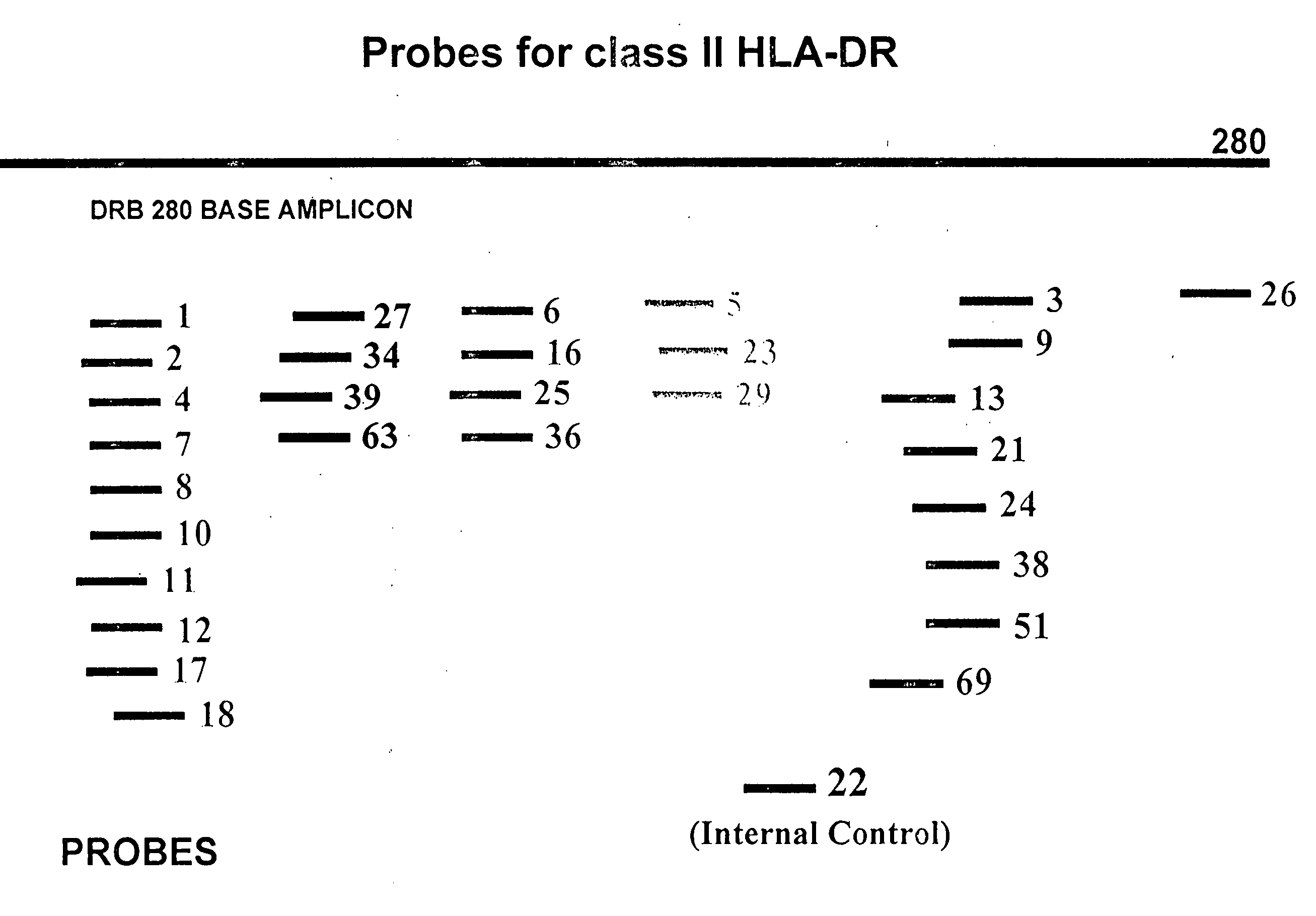
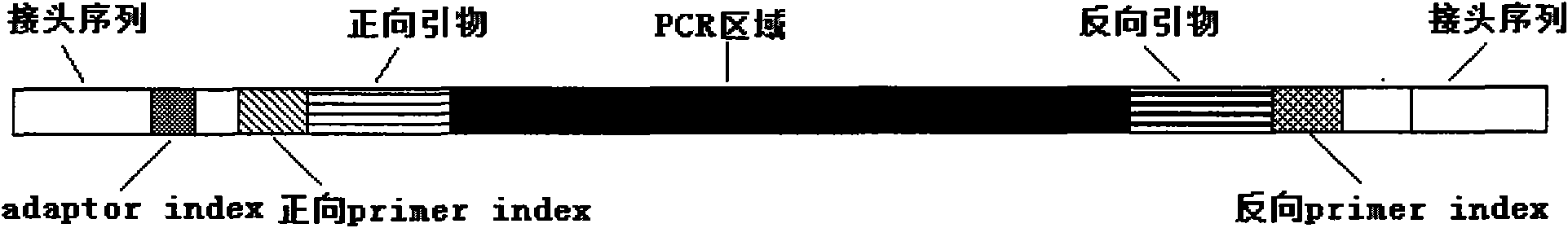
Simple Method and Kit for DNA Typing of HLA Genes by High-Throughput Massively Parallel Sequencer
ActiveUS20170029885A1Shorten the timeReduce data sizeMicrobiological testing/measurementHuman DNA sequencingIntein
The present invention addresses the problem of providing a method and kit for the DNA profiling of HLA genes using a high-throughput massively parallel sequencer. The present invention pertains to a method for the DNA profiling of HLA genes, said method being characterized by including: (1) a step for preparing a primer set that anneals specifically to exon 4 and intron 1 and includes exon 2 and exon 3 of at least one target gene selected from the group consisting of HLA-DRB1, HLA-DRB3, HLA-DRB4, HLA-DRB5, HLA-DQB1 and HLA-DPB1 in the base sequence of the human genome; (2) a step for amplifying a sample (DNA) by PCR using the primer set; (3) a step for determining the base sequence of the amplified PCR product; and (4) a step for carrying out a homology search against a database.
Owner:GENODIVE PHARMA
Non-invasive early detection of solid organ transplant rejection by quantitative analysis of mixtures by deep sequencing of HLA gene amplicons using next generation systems
The invention is a method of detecting or assessing solid organ graft (transplant) rejection by detecting donor-specific HLA alleles in a blood sample of a graft (transplant) recipient. The invention further comprises a method of detecting the presence of maternal cells in a blood sample of an offspring.
Owner:THE SCRIPPS RES INST +1
Multiplexed Analysis Of Polymorphic Loci By Concurrent Interrogation And Enzyme-Medicated Detection
InactiveUS20120214681A1Strong specificityDegree of reductionSugar derivativesMicrobiological testing/measurementHla genesEnzyme
The invention provides methods and processes for the identification of polymorphisms at one or more designated sites, without interference from non-designated sites located within proximity of such designated sites. Probes are provided capable of interrogation of such designated sites in order to determine the composition of each such designated site. By the methods of this invention, one or more mutations within the CFTR gene and the HLA gene complex can be identified.
Owner:BIOARRAY SOLUTIONS
hla genotype-snp linkage database, its construction method, and hla typing method
ActiveCN103221551BRapid TypingAccurate typingMicrobiological testing/measurementGenomicsTyping methods
The invention belongs to the fields of genomics and bioinformatics, relates to an HLA genotype-SNP linkage database, a construction method thereof, and a HLA typing method. Specifically, the construction method of the HLA genotype-SNP linkage database comprises the following steps: a) selecting one or more HLA loca sequences as a reference sequence; b) comparing the known type HLA genes in a conventional HLA database with the reference sequence to find out a difference site of the reference sequence i.e. a SNP site, and to obtain an SNP linkage relationship relative to the reference sequence for constructing the HLA genotype-SNP linkage database. The present invention also relates to a method for determining SNP linkage relationship of HLA gene, and an HLA typing device. The method of the present invention achieves low-cost, high-throughput, high-accuracy and high-resolution typing for HLA.
Owner:北京六合华大基因科技有限公司
Primer group, kit and method for HLA (human leukocyte antigen) gene amplification and gene parting
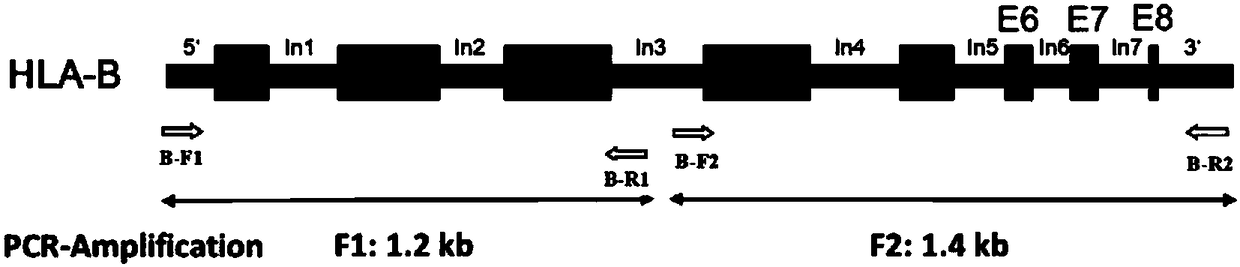
ActiveCN108441547AReduced integrity requirementsLow costMicrobiological testing/measurementDNA/RNA fragmentationHLA-B geneHla genes
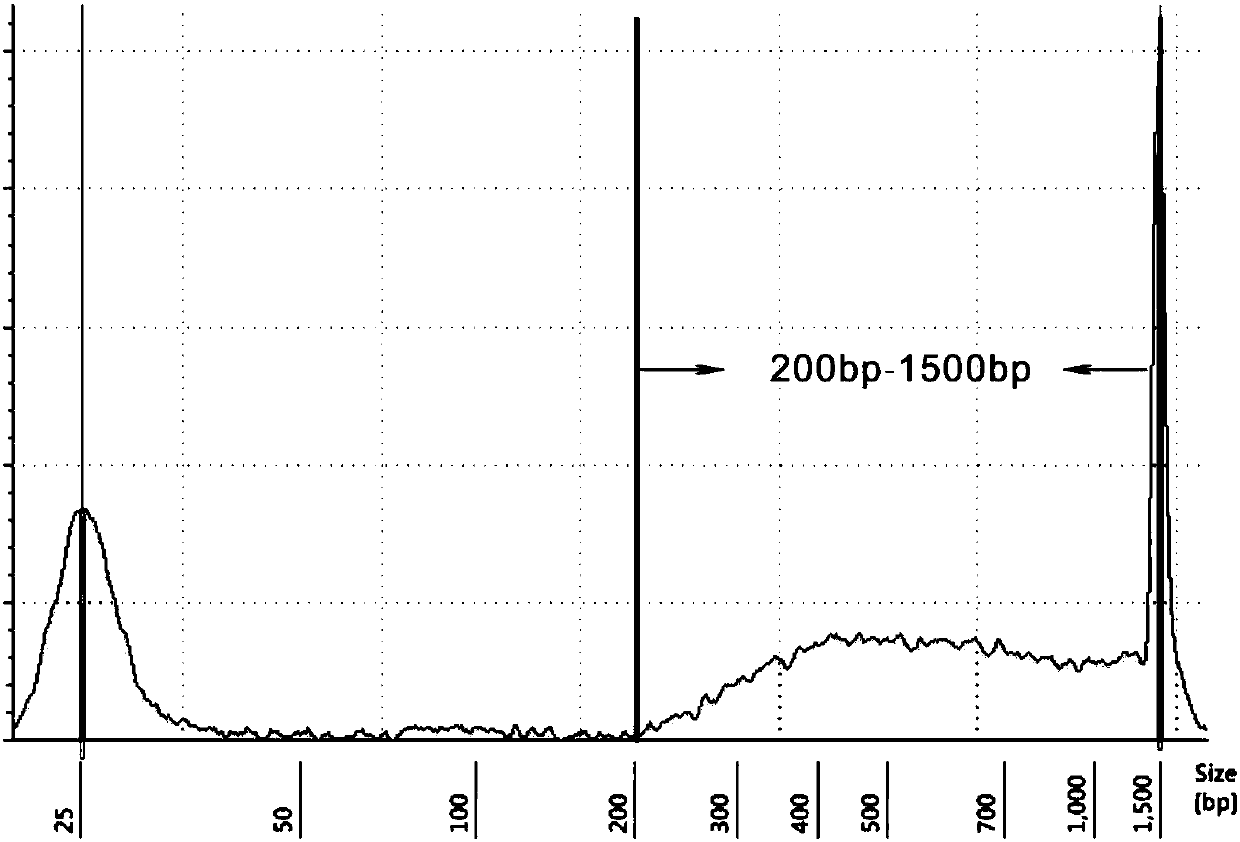
The invention provides a primer group, a kit and a method for HLA (human leukocyte antigen) gene amplification and gene parting and belongs to the field of gene detection. According to the primer group for HLA gene amplification, primers are designed according to 8 exon zones of an HLA-A gene, 8 exon zones of an HLA-B gene and an exon 2 and an exon 3 of an HLA-DRB1 gene, PCR amplification is performed on the HLA gene by use of the primers, and a product with a gene segment length larger than 400 bp and smaller than 1.5 kb is obtained. By means of the PCR amplified HLA genes, the gene segment length of the amplification product is larger than 400 bp and smaller than 1.5 kb, the requirement for completeness of a template is reduced, the amplification efficiency is high, the amplification reaction time is short, the cost is reduced, and the demands of large-scale amplification or gene parting of sample HLA genes can be met.
Owner:BEIJING NUOSHI KANGYING MEDICAL TECH
Group-specific amplification primers, sequencing typing method and kit for hla gene
InactiveCN105039332BAvoid missing detectionEasy to analyzeMicrobiological testing/measurementDNA/RNA fragmentationTyping methodsImmunogenetics
The present invention provides a group-specific amplification primer, sequence-based typing method, and kit for HLA genes. A sequence of the PCR amplification primer is selected from a sequence represented by SEQ ID NO.1-28, a sequence represented by SEQ ID NO.29-64, and a sequence represented by SEQ ID NO.65-94. By means of the present invention, a group-specific amplification primer is designed by performing serum classification on HLA genes, so as to perform sequence-based typing on all allelic genes of HLA-A, -B, and -C, and perform amplification sequencing on full-length sequences of HLA-A, -B, and -C.
Owner:SHENZHEN GENEBIOHEALTH
Kit for detecting lung cancer susceptibility genes
InactiveCN106191221AReduce morbidityImprove survival rateMicrobiological testing/measurementLung cancer susceptibilityHla genes
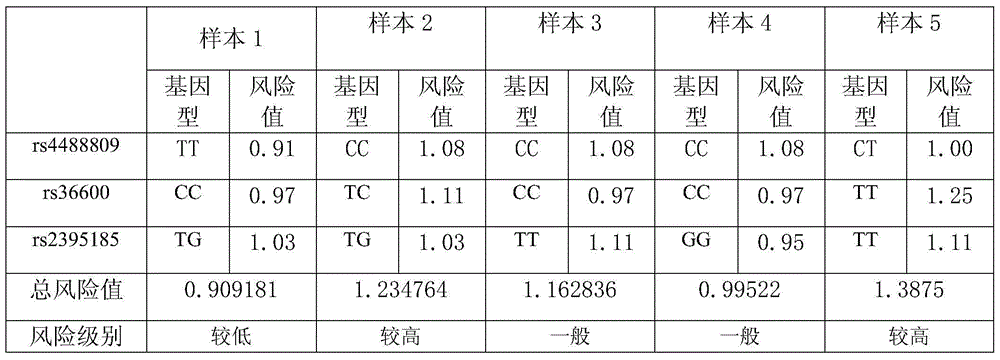
The invention provides a kit for detecting lung cancer susceptibility genes. The kit comprises a primer aiming at a single nucleotide polymorphism site rs4488809 (T-C) on a TP63 gene, a primer aiming at a single nucleotide polymorphism site rs36600 (T-C) on an MTMR3 gene, and a primer aiming at a single nucleotide polymorphism site rs2395185 (G-T) on an HLA gene.
Owner:GUANGZHOU TOPGENE TECH CO LTD
Primers, kit and method for high-resolution typing of HLA gene
InactiveCN108048546AQuality improvementImprove throughputMicrobiological testing/measurementDNA/RNA fragmentationTypingGene engineering
The invention belongs to the technical field of gene engineering, and discloses a primer combination for high-resolution typing of HLA gene, a kit containing the primer combination, and a method for high-resolution typing of HLA gene. Compared to the traditional methods such as SBT, SSO and the like, the method of the present invention has the following advantages that the sequencing reaction cansimultaneously determine 480 samples so as to provide high-throughput characteristic, the HLA typing results obtained by using the method of the present invention contain the low-resolution typing results and the high-resolution typing results so as to provide high-resolution characteristic, and the HLA typing detection results of 3000 random samples show that the typing accuracy can achieve 100%so as to provide high accuracy characteristic.
Owner:SHANGHAI TISSUEBANK MEDICAL LAB CO LTD +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com