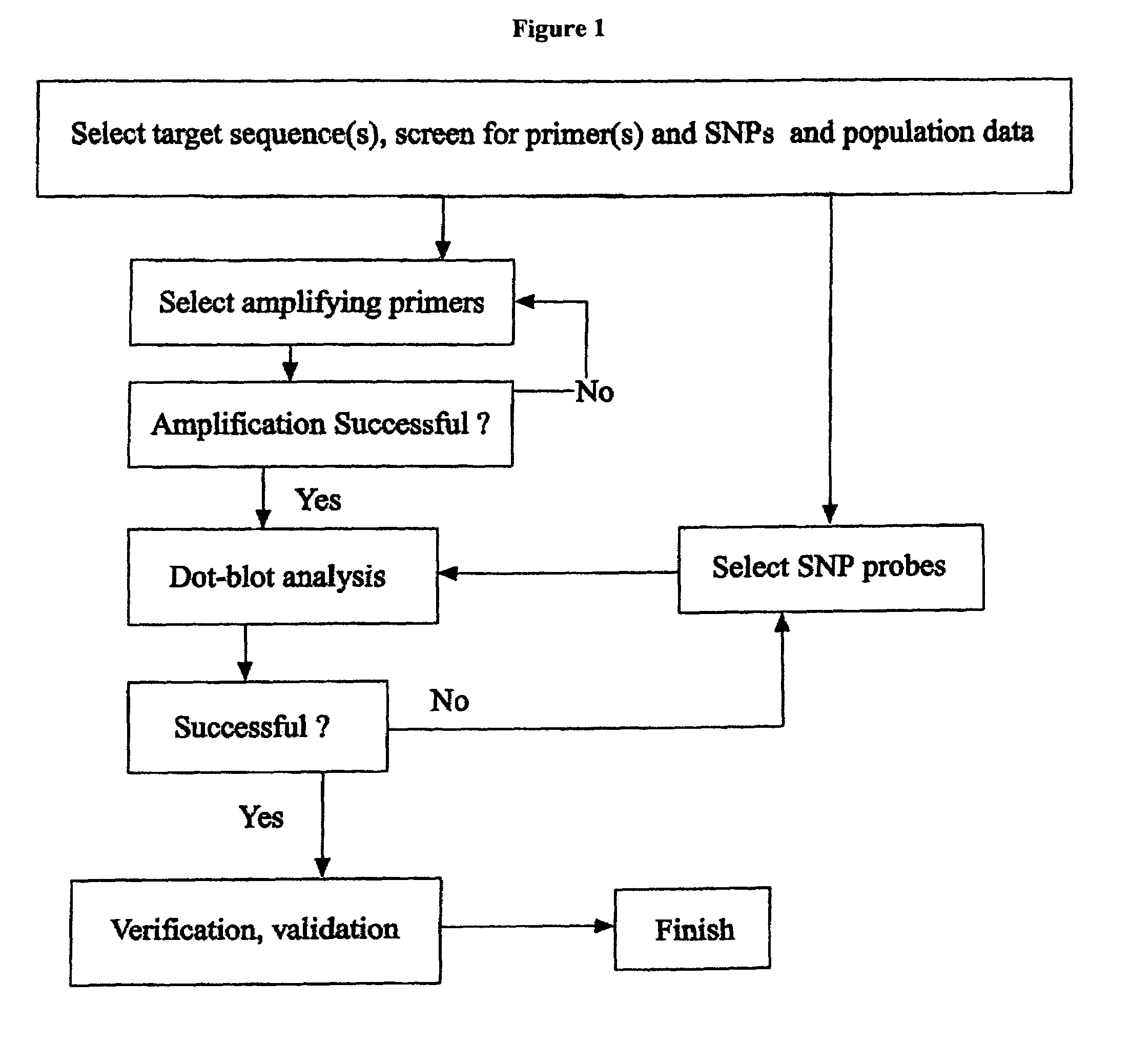
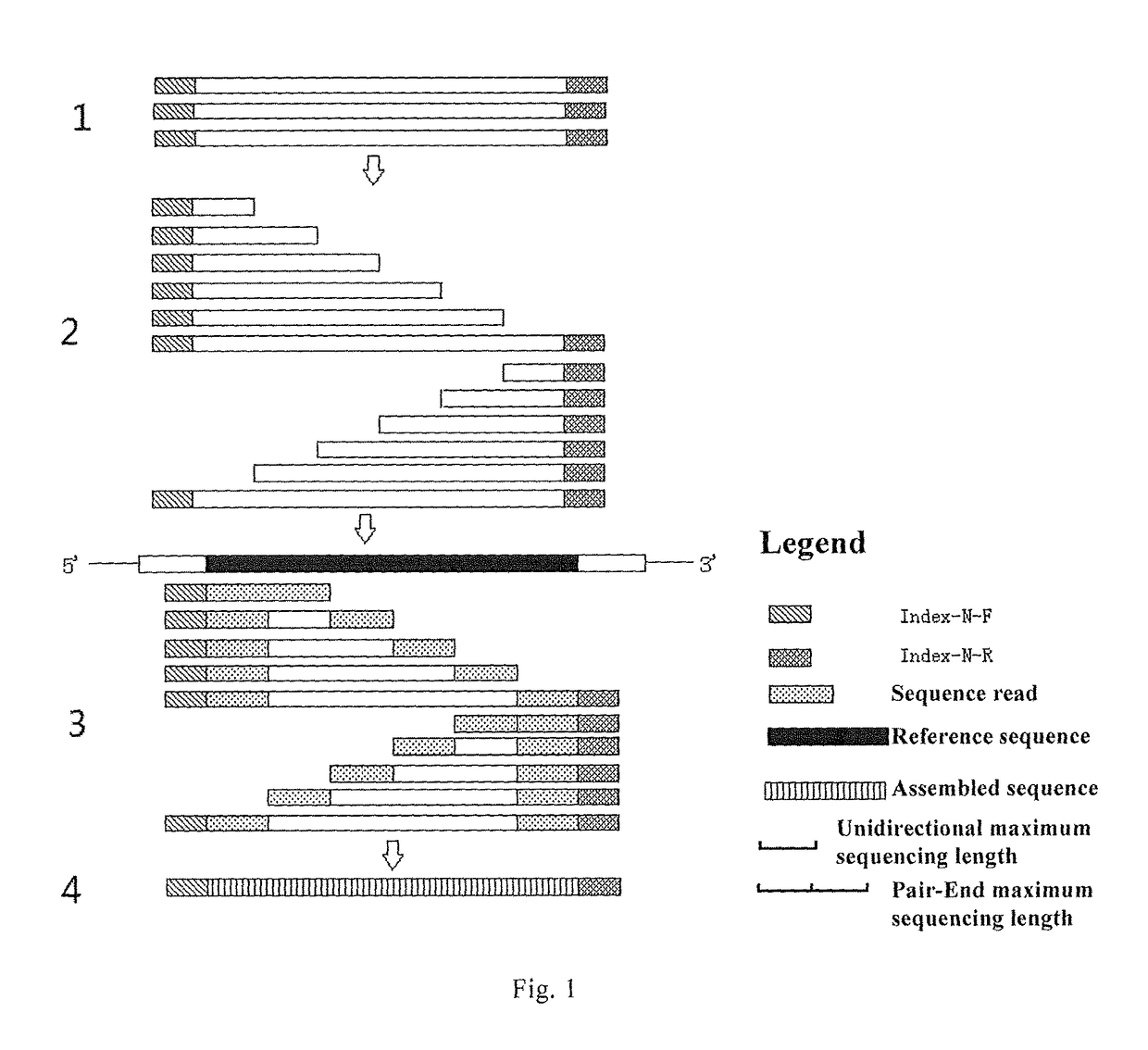
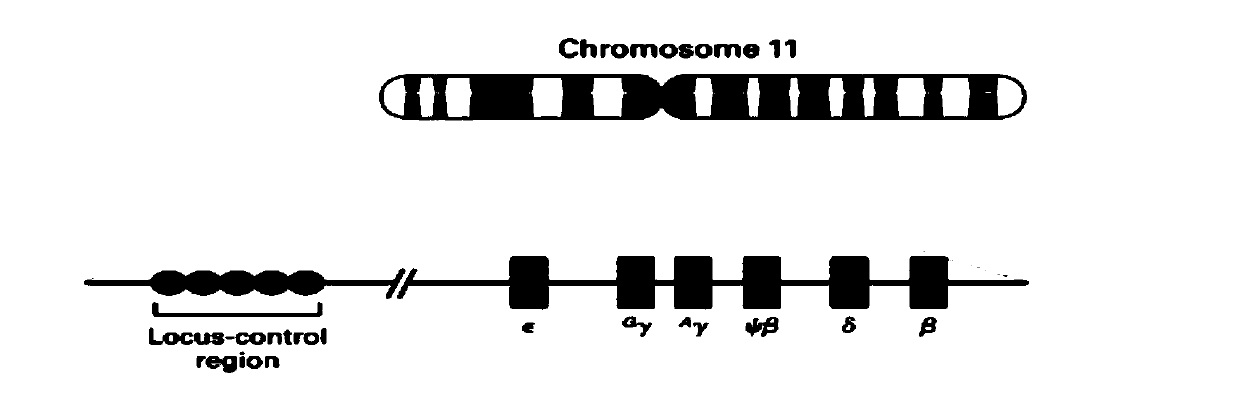
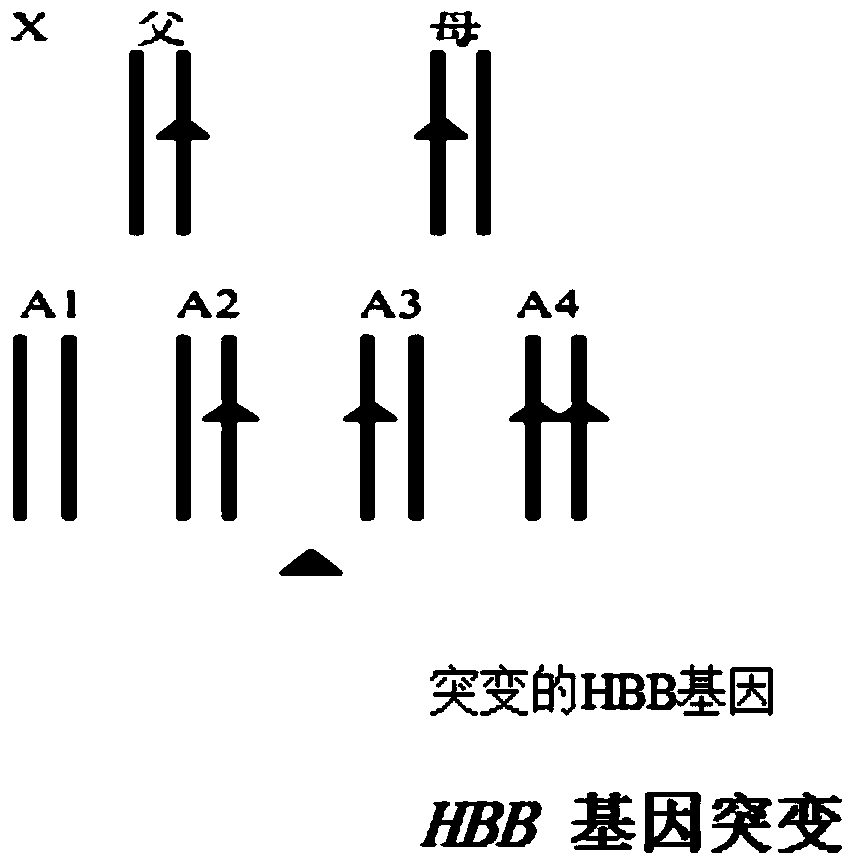
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Hla genotyping" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
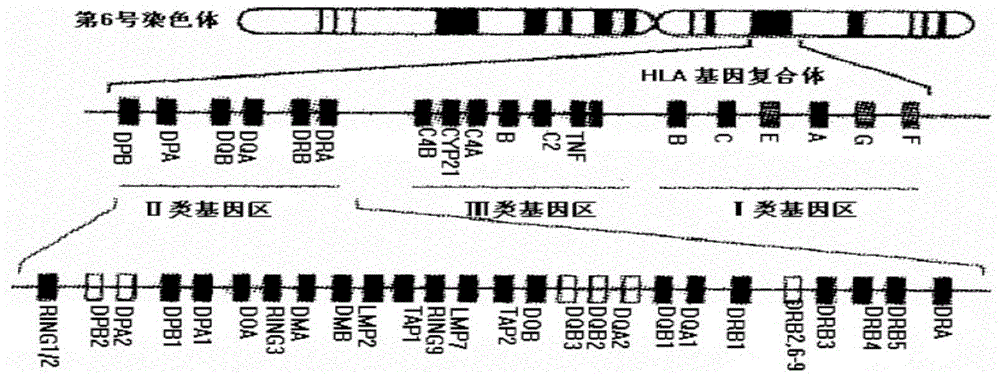
HLA genotyping is the identification of the HLA class I and class II gene polymorphisms for individuals, which is indispensable for transplant matching and disease association studies. Unambiguous HLA genotyping is technically challenging owing to high polymorphism in various genomic regions.
Method for amplifying and typing HLA gene and relevant primer thereof
ActiveCN101654691AImprove accuracyHigh resolutionMicrobiological testing/measurementFermentationTypingHla genes
Owner:BGI GENOMICS CO LTD
HLA gene specific PCR amplification primer, HLA typing method and kit
InactiveCN103045591ANo StrapReasonable and accurateMicrobiological testing/measurementDNA/RNA fragmentationNucleotideTyping methods
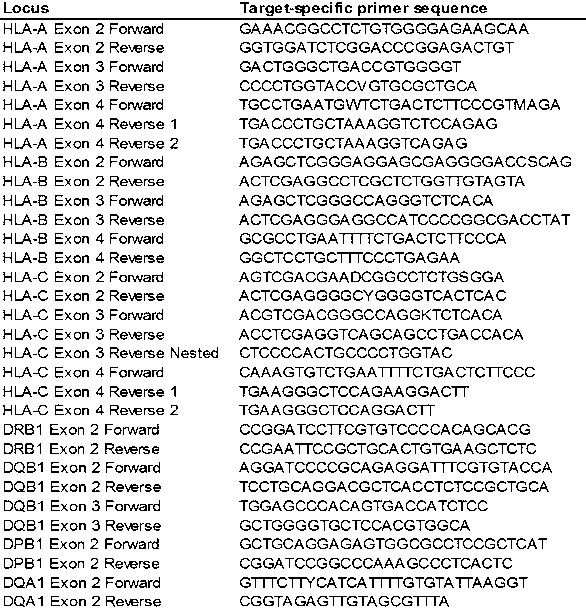
The invention discloses an HLA (human leukocyte antigen) gene specific PCR (polymerase chain reaction) amplification primer, an HLA typing method and an HLA typing kit. A sequence of the PCR amplification primer comprises a primer sequences shown as SEQ ID No. (sequence identification number) 1-2, 3-4, 5-6, 7-8 and 9-10. The HLA gene typing method comprises the steps that the HLA gene specific PCR amplification primer is used for conducting PCR amplification and purification on sample DNA (deoxyribonucleic acid); a gene exon sequencing primer is used for conducting sequencing PCR amplification, purification and sequencing on an HLA gene amplification product, the sequence of the HLA gene amplification product is compared with a standard sequence; and the type of the HLA gene is determined. The HLA gene typing kit comprises the PCR amplification primer and the sequencing primer. A qualitative leap in aspects of detection flux, data quality, cost control and the like is achieved; data is more reliable and realer; and the problem that typing cannot be conducted when nucleotide of certain alleles is positioned outside an amplification area is solved.
Owner:SHANGHAI TISSUEBANK BIOTECH +3
Rapid genotyping analysis and the device thereof
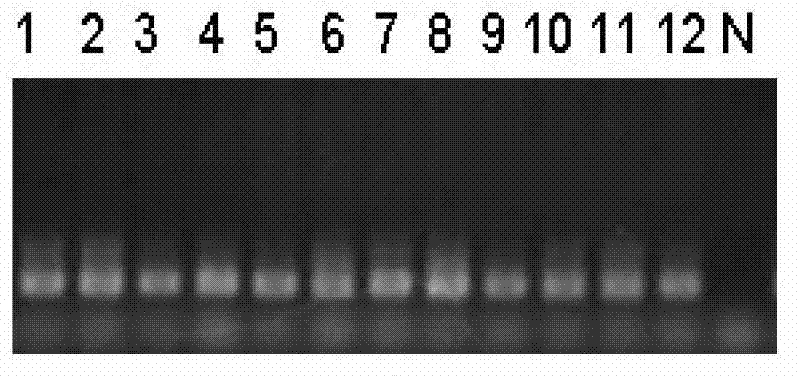
InactiveUS20060292601A1Accuracy is compromisedFacilitate SNP genotypingBioreactor/fermenter combinationsBiological substance pretreatmentsSensitive analysisDot blotting
The present invention discloses the use of Allele-Specific-oligonucleotide (ASO) as a detection assay for human HLA classification. Using Reversed-Dot-Blotting format and flow through hybridization process, more efficient, faster and less expensive HLA classification can be achieved. A simplified procedure for HLA genotyping is also described. This invention further provides a Single Nucleotide Polymorphism (SNP)-based DNA fingerprining method for rapid and accurate genotyping, identification as well as DNA analyses of genetic data from human beings and different organisms. In addition this invention also discloses a new device for rapid and sensitive analyses of nucleic acids, proteins and other analysts for diagnosis.
Owner:GUANGZHOU BAIGAO MEDICAL LAB CO LTD
HLA high-resolution gene sequencing kit
InactiveCN101892317AAvoid problems that cannot be effectively typedHigh resolutionMicrobiological testing/measurementDNA/RNA fragmentationHLA-BExon
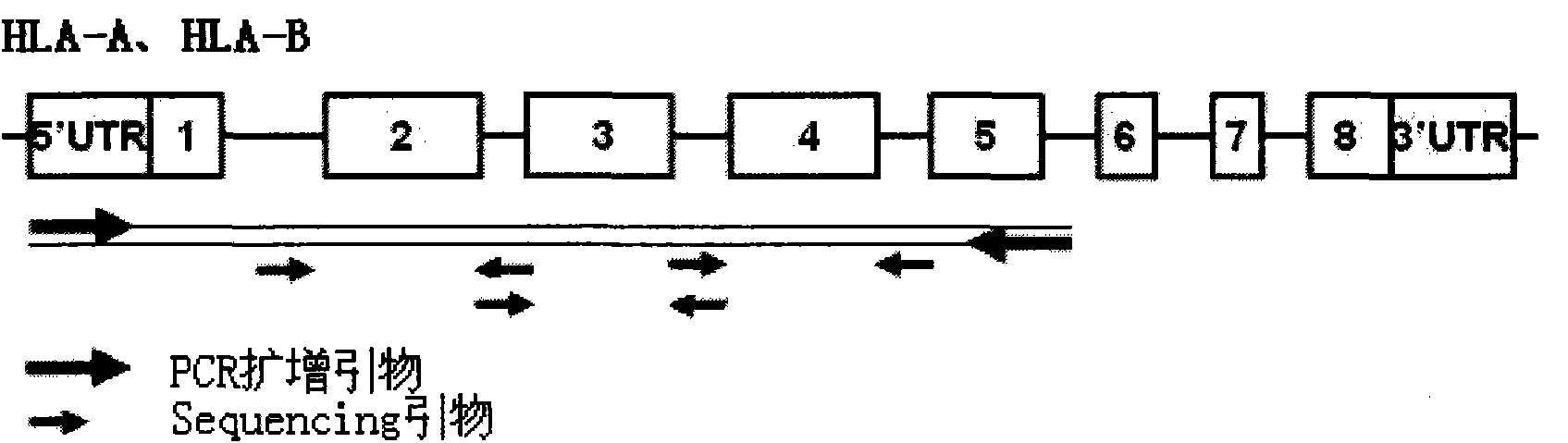
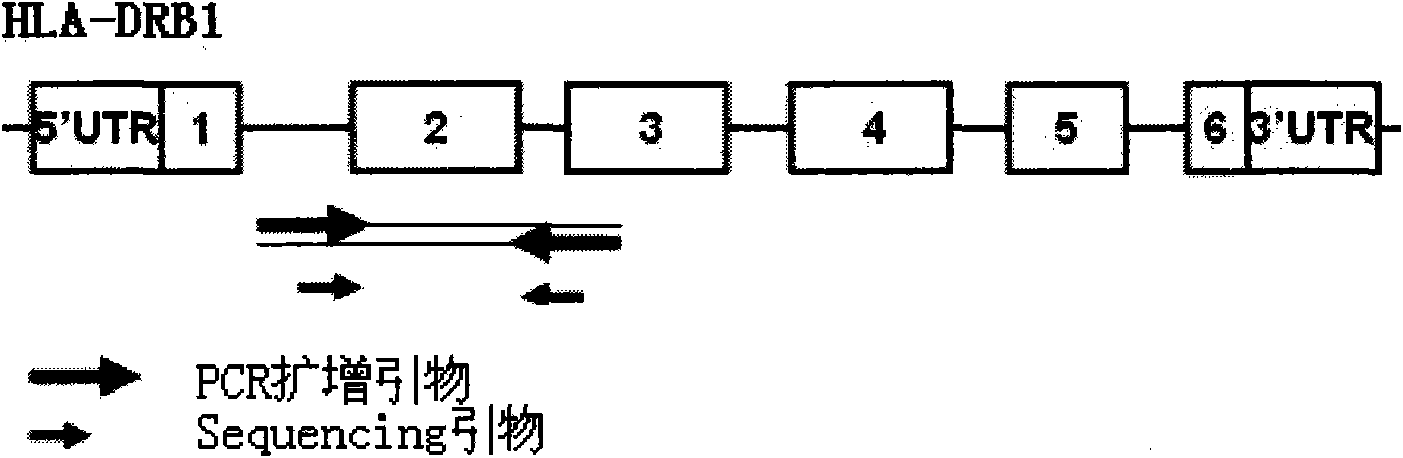
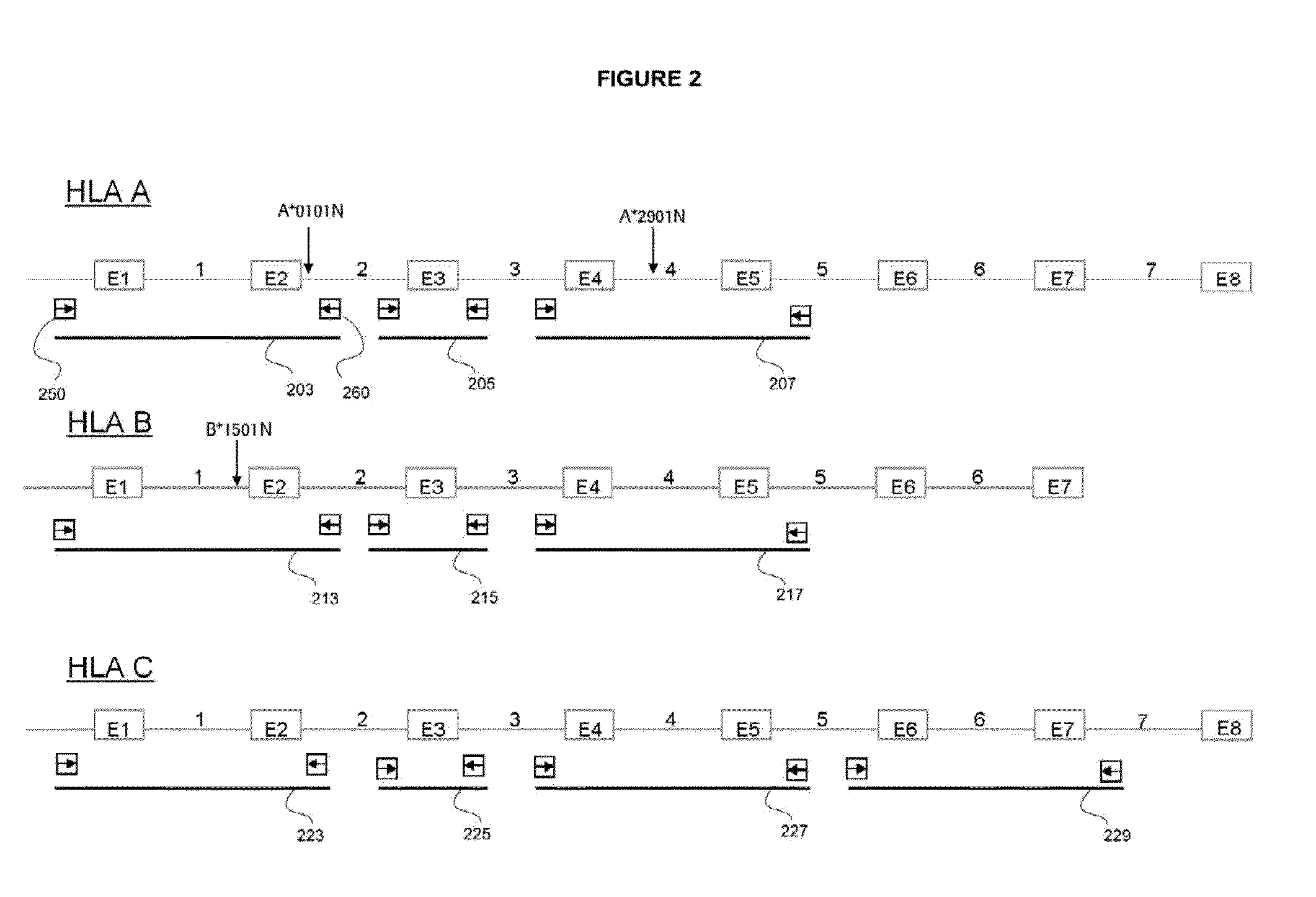
The invention discloses a parting method of leucocyte antigen gene of human being, comprising the following steps of: (1) extracting genome DNA to be tested by a regular technology, and amplifying a destination gene fragment to be analyzed by using PCR amplification primer: 2,3,4 exon of HLA-A, 2,3,4 exon of HLA-B and exon on the locus 2 of HLA-DRB; and (2) amplifying the PCR output obtained in the step (1) by using sequencing primer, amplifying the exon, sequencing the amplified exon and comparing the sequencing result with the standard sequence in a database to determine the gene parting result. As the 2,3,4 exon of HLA-A, 2,3,4 exon of HLA-B and exon on the locus 2 of HLA-DRB are effectively amplified as a result of optimized combination of the HLA gene sequencing kit and the test condition, and the corresponding exon is sequenced, the invention solves the problem that effective parting can not be performed when certain allelic gene nucleotide is located outside an amplification area during further parting, thereby improving the parting resolution and accuracy of the HLA gene.
Owner:SUZHOU UNIV +1
PCR-SBT method for HLA genotyping and reagent thereof
ActiveCN101928776AImprove accuracyIncrease success rateMicrobiological testing/measurementSodium acetateGenomic DNA
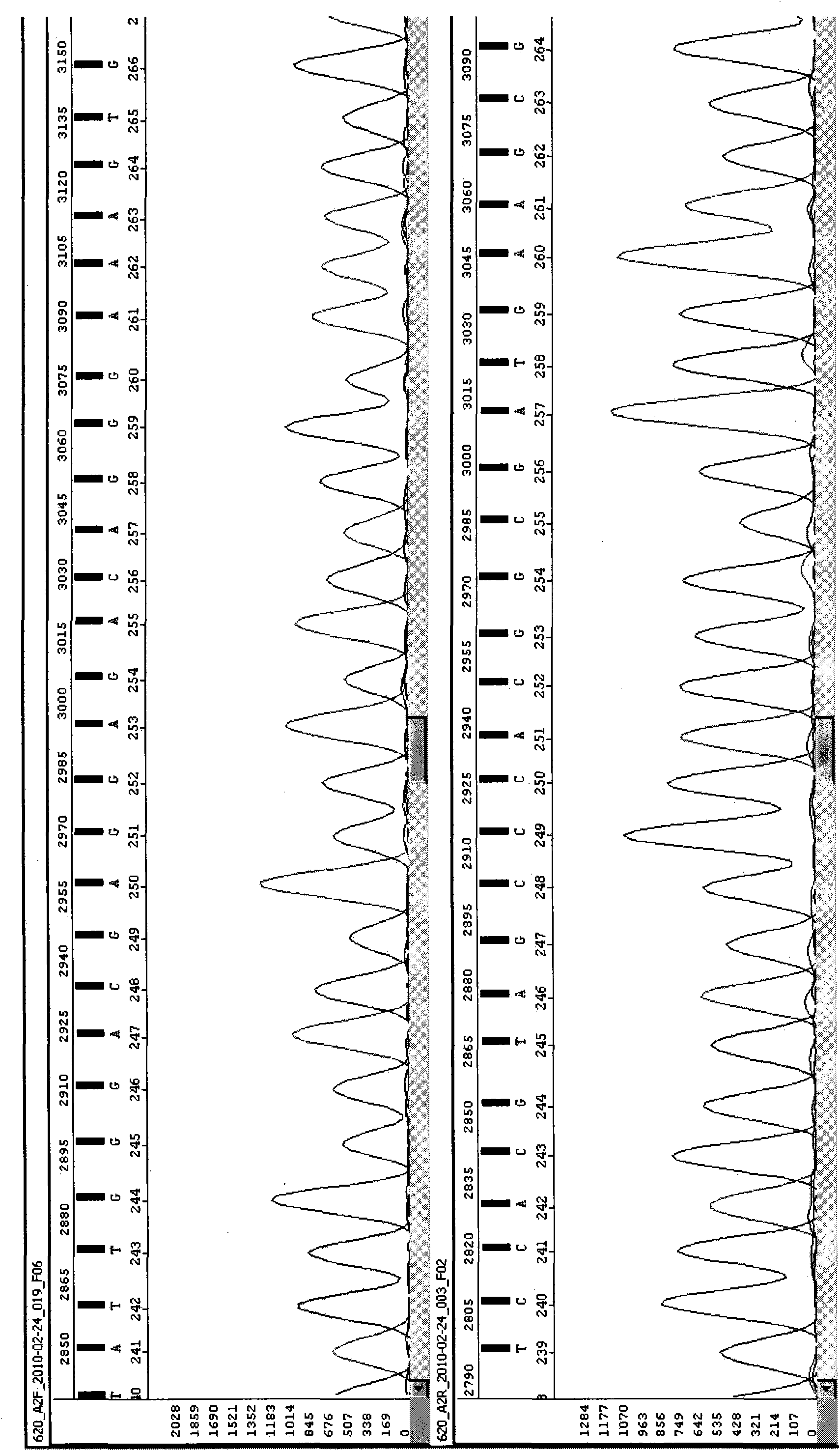
The invention provides a PCR-SBT method for HLA genotyping. Exons 1, 2, 3, 4, 5, 6, 7 of an HLA-A locus, an HLA-B locus and an HLA-C locus are subjected to genotyping. The method comprises the following steps of: (1) preparing human genomic DNA; (2) designing an amplification primer; (3) performing double digestion purification on an amplification product; (4) designing a sequencing primer and performing sequencing PCR on a purification product; (5) purifying a sequencing product by a sodium acetate-ethanol precipitation method and performing capillary electrophoresis sequencing; and (6) performing software analysis on the acquired sequence to determine genotypes thereof. The exons 1, 2, 3, 4, 5, 6, 7 of the HLA-A, B, C loci are subjected to full-length sequence sequencing, so that the full length of the related exons of the HLA-A, B, C loci and part of intron oligonucleotide sequences are obtained and the HLA genotyping is accurately performed.
Owner:浙江省血液中心
HLA (histocompatibility locus antigen) genetic typing method of HLA determinant gene through high-throughput sequencing
InactiveCN103074444AAvoid financial burdenShortcut typing methodMicrobiological testing/measurementCost ControlsRead through
The invention discloses an HLA (histocompatibility locus antigen) genetic typing method of an HLA determinant gene through high-throughput sequencing. Patterning HLA typing software based on various high-throughput sequencing platform data has important significance in clinic or biomedicine. Compared with the traditional sequencing method through a PCR-SBT (polymerase chain reaction-sequence based typing) method, the high-throughput sequencing technology has the obvious advantages in economic cost and time cost. HLA sequence data of thousands of samples can be read through an experiment and high resolution of HLA typing is achieved at one time through the high-throughput sequencing technology, and meanwhile, a new allele can be found. Qualitative leap in the aspects of flux detection, data quality, cost control and the like are achieved, 'low cost and high data' are achieved, additional economic burden of a patient caused by typing for multiple times can be avoided, the time for searching for a provider whose HLA is matched with that of the patient can also be reduced, and the precious time is saved for treating the patient.
Owner:SUZHOU JINGYIN BIOLOGICAL TECH
Methods and Compositions for Very High Resolution Genotyping of HLA
The invention is a method of determining HLA genotype for HLA-A, HLA-B, HLA-C, DQB1, DRB1, DRB3, DRB4, DRB5, DPA1 and DPB1. Reagents and kits are also disclosed.
Owner:ROCHE MOLECULAR SYST INC
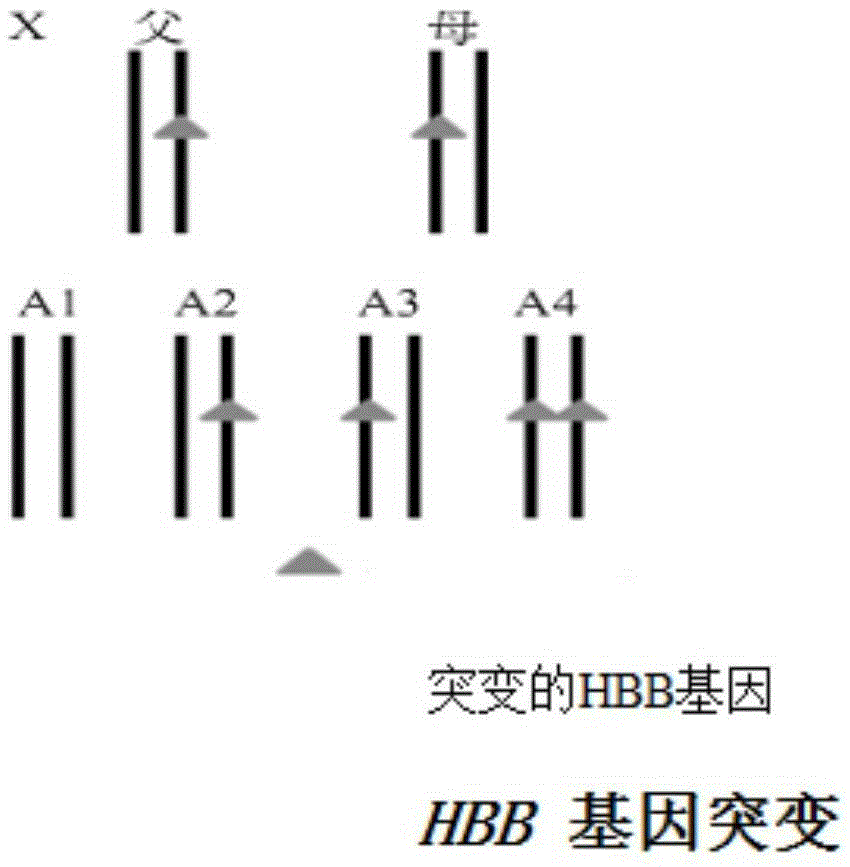
Reagent kit for detecting HBB gene mutation and HLA genotyping
ActiveCN105420233AImprove throughputLow costMicrobiological testing/measurementDNA/RNA fragmentationBeta globinBeta thalassemia
The invention provides a method for detecting HBB gene mutation and HLA genotyping based on the high throughput sequencing technology and a corresponding reagent kit. An adopted primer composition comprises a primer of closely-linked single nucleotide polymorphisms (SNP) within the 1 Mb range of the up stream and the down stream of the specific amplification human embryo beta-thalassemia HBB gene and primers of the closely-linked single nucleotide polymorphisms (SNP) within the ranges at the up stream of the LHA-A gene, between the HLA-A gene and the HLA-B gene, between the HLA-B gene and the HLA-DRA gene, between the HLA-DRA gene and the HLA-DQB1 gene and at the downstream of the HLA-DQB1 gene of the specific amplification human leucocyte antigen system. The method has the advantages of university, single nucleotide polymorphisms (SNP) sequencing, high throughput, low cost, high flexibility and strong specificity.
Owner:海南医学院附属医院 +1
High resolution, high throughput hla genotyping by clonal sequencing
The invention provides methods and reagent for performing full, multi-locus HLA genotyping for multiple individuals in a single sequencing run using clonal sequencing.
Owner:F HOFFMANN LA ROCHE & CO AG
Method for targeted capture and sequencing of HLA gene sequences
InactiveCN109913539AEasy to control the lengthIncrease abundanceMicrobiological testing/measurementTarget captureNucleic Acid Probes
The invention relates to the field of biotechnology, and particularly provides a method for targeted capture and sequencing of HLA gene sequences. The method comprises the following steps: 1) hybridizing a denatured nucleic acid sample with a nucleic acid probe library fixed on a solid-phase carrier under a hybridization condition to form a target nucleic acid molecule-probe-solid-phase carrier membrane complex; 2) cleaning the 'probe-solid-phase carrier membrane complex' bound with target nucleic acid in step 1) by using a cleaning solution, eluting the probe-solid-phase carrier membrane complex bound with the target nucleic acid from a solid-phase carrier membrane by using an eluting solution, purifying, enriching or constructing to obtain a nucleic acid library; and 3) taking the nucleic acid library obtained by enrichment or constructed after enrichment in step 2) for high-throughput sequencing. A nucleic acid probe which can rapidly generate high-fold coverage and single-base displacement and is used for enriching HLA genes can be immovably fixed on the solid-phase carrier membrane. The method has characteristics of simplicity and convenience in operation, flexible probe acquisition and low cost, is favorable for improving the HLA genotype identification accuracy, and is rapid and convenient.
Owner:ZHEJIANG UNIV
Rapid genotyping analysis and the device thereof
InactiveUS7732138B2Accurate measurementNot require expensiveBioreactor/fermenter combinationsBiological substance pretreatmentsSensitive analysisAllele-specific oligonucleotide
The present invention discloses the use of Allele-Specific-Oligonucleotide (ASO) as a detection assay for human HLA classification. Using Reversed-Dot-Blotting format and flow through hybridization process, more efficient, faster and less expensive HLA classification can be achieved. A simplified procedure for HLA genotyping is also described. This invention further provides a Single Nucleotide Polymorphism (SNP)-based DNA fingerprining method for rapid and accurate genotyping, identification as well as DNA analyses of genetic data from human beings and different organisms. In addition this invention also discloses a new device for rapid and sensitive analyses of nucleic acids, proteins and other analysts for diagnosis.
Owner:GUANGZHOU BAIGAO MEDICAL LAB CO LTD
Kit for detecting HLA genotypes through fluorescent PCR melting curve assay
PendingCN107190088ANo follow-up experiments requiredQuick Results ReferenceMicrobiological testing/measurementOrgan transplantationHLA-B
The invention provides a kit for detecting HLA genotypes through fluorescent PCR melting curve assay. The kit includes HLA-A, HLA-B, HLA-DRB1 and HLA-DQB1 genotypes, amplification is performed by using a 96-pore optical reaction plate containing primers, an amplified dual-strand DNA product is actively bonded through SYBR Green I, the low-resolution genotypes of HLA-A, HLA-B, HLA-DRB1 and HLA-DQB1 are comprehensively judged according to the result of a 96-pore fusion curve, and accordingly diagnosis of the matched types for organ transplantation is assisted.
Owner:DEBIQI BIOTECH XIAMEN
Primers, kit and method for HLA (human leukocyte antigen) genotyping
InactiveCN105861673AEfficient specificityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationTypingGene engineering
The invention belongs to the technical field of gene engineering, and discloses a primer combination for HLA (human leukocyte antigen) genotyping, a kit containing the primer combination and a method for HLA genotyping. Thus, the sequence-specific HLA genotyping (GSA-SBT) technique is quick, simple, accurate and visual. The primer combination can be synchronously used for amplification and sequencing with the commercialized kit, thereby lowering the experimentation cost. Besides, the specific design of mismatched bases are introduced to the 3' terminal of the primer, thereby enhancing the specificity of the primer amplification and ensuring the accuracy of the typing result.
Owner:SHANGHAI TISSUEBANK BIOTECH +3
Population scale HLA-typing and uses thereof
InactiveUS7667026B2Bioreactor/fermenter combinationsBiological substance pretreatmentsHybridization probeTyping
The present invention provides a portable system for real-time population-scale HLA genotyping and / or allelotyping in a field environment and methods of such population-scale HLA genotyping. The individual components of the system are portable to and operable within a field environment thereby providing high throughput with real-time geno- or allelotyping. Also provided are HLA gene-specific primers and HLA allele-specific or single nucleotide polymorphism-specific hybridization probes. In addition the present invention provides a microarray comprising the hybridization probes. Further provided is a kit comprising the HLA gene-specific primers and the microarray.
Owner:GENOMICS USA
Method and system for calculating tumor neoantigen load
PendingCN112309502AExpand the scope of the filterMicrobiological testing/measurementBiostatisticsWhite blood cellMutation frequency
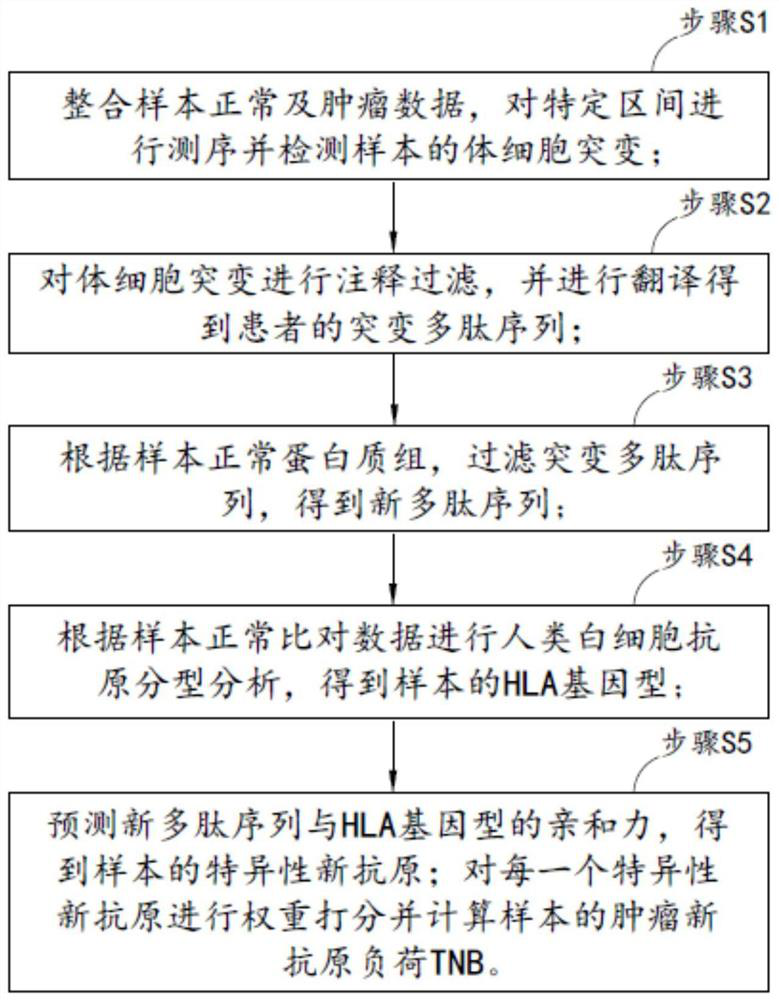
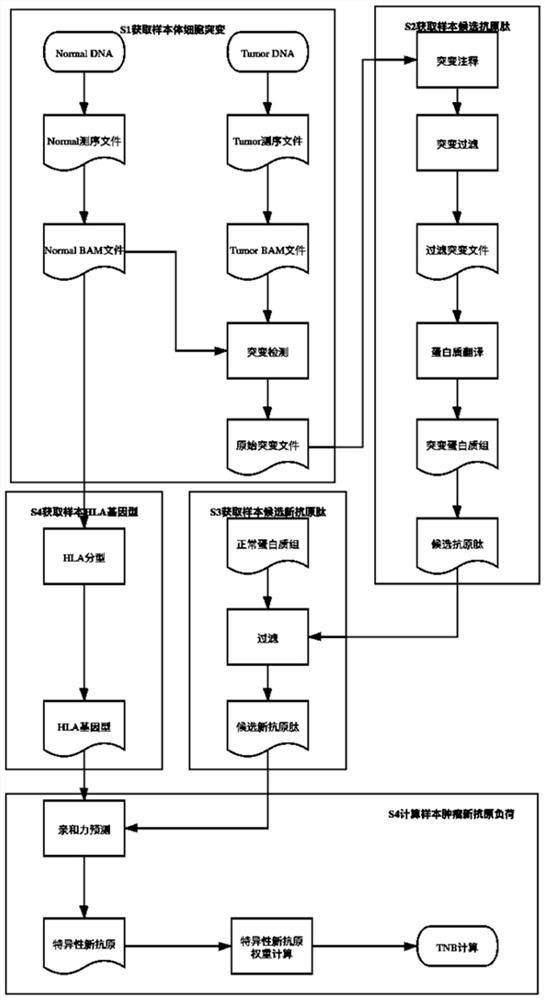
The invention provides a method and system for calculating tumor neoantigen load. The method comprises the steps: S1, integrating normal and tumor data of a sample, sequencing a specific interval, anddetecting somatic mutation of the sample; S2, annotating and filtering the somatic mutation, and performing translating to obtain a mutant polypeptide sequence of the patient; S3, according to the normal proteome of the sample, filtering the mutant polypeptide sequence to obtain a new polypeptide sequence; and S4, performing human leukocyte antigen typing analysis according to the normal comparison data of the samples to obtain HLA genotypes of the samples; According to the method for calculating the tumor neoantigen load, weight scoring is performed on each predicted neoantigen, and high-quality and low-quality neoantigens can be distinguished by considering different dimension information such as mutation quality, mutation frequency, affinity and the like, so that a numerical value capable of better reflecting the real tumor neoantigen load of the sample is obtained.
Owner:深圳市新合生物医疗科技有限公司
Kit and method for typing HLA gene
ActiveCN105255875AAccurate typingHigh resolutionMicrobiological testing/measurementDNA/RNA fragmentationTypingHLA-B gene
The invention provides a kit and method for typing an HLA gene. The kit comprises a primer for amplifying a second exon and a third exon of an HLA-B gene and a primer for amplifying a fourth exon of the HLA-B gene, wherein the primer for amplifying the second exon and the third exon of the HLA-B gene comprises an amplification primer pair composed of primer sequences as shown in SEQ ID NO.1 and SEQ ID NO.2; and the primer for amplifying the fourth exon of the HLA-B gene comprises an amplification primer pair composed of primer sequences as shown in SEQ ID NO.3 and SEQ ID NO.4. By using the kit and method provided by the invention, the typing process can be simpler, the typing resolution ratio and accuracy rate are increased while the cost is reduced, and furthermore the kit and method are further applied to scientific research and clinic work.
Owner:SUN YAT SEN UNIV CANCER CENT
hla genotype-snp linkage database, its construction method, and hla typing method
ActiveCN103221551BRapid TypingAccurate typingMicrobiological testing/measurementGenomicsTyping methods
The invention belongs to the fields of genomics and bioinformatics, relates to an HLA genotype-SNP linkage database, a construction method thereof, and a HLA typing method. Specifically, the construction method of the HLA genotype-SNP linkage database comprises the following steps: a) selecting one or more HLA loca sequences as a reference sequence; b) comparing the known type HLA genes in a conventional HLA database with the reference sequence to find out a difference site of the reference sequence i.e. a SNP site, and to obtain an SNP linkage relationship relative to the reference sequence for constructing the HLA genotype-SNP linkage database. The present invention also relates to a method for determining SNP linkage relationship of HLA gene, and an HLA typing device. The method of the present invention achieves low-cost, high-throughput, high-accuracy and high-resolution typing for HLA.
Owner:北京六合华大基因科技有限公司
Management of osteoarthritis using pooled allogeneic mesenchymal stem cells
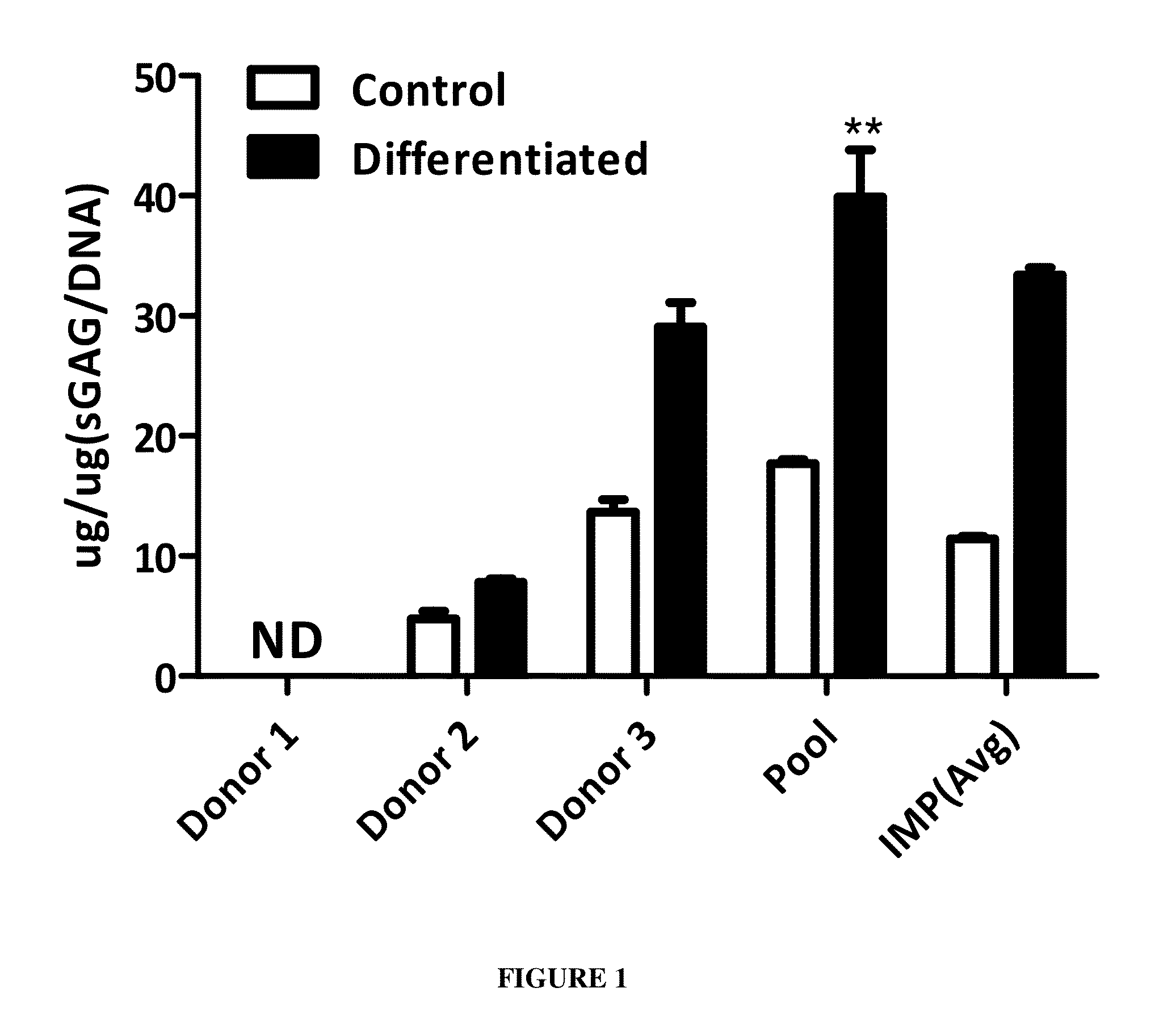
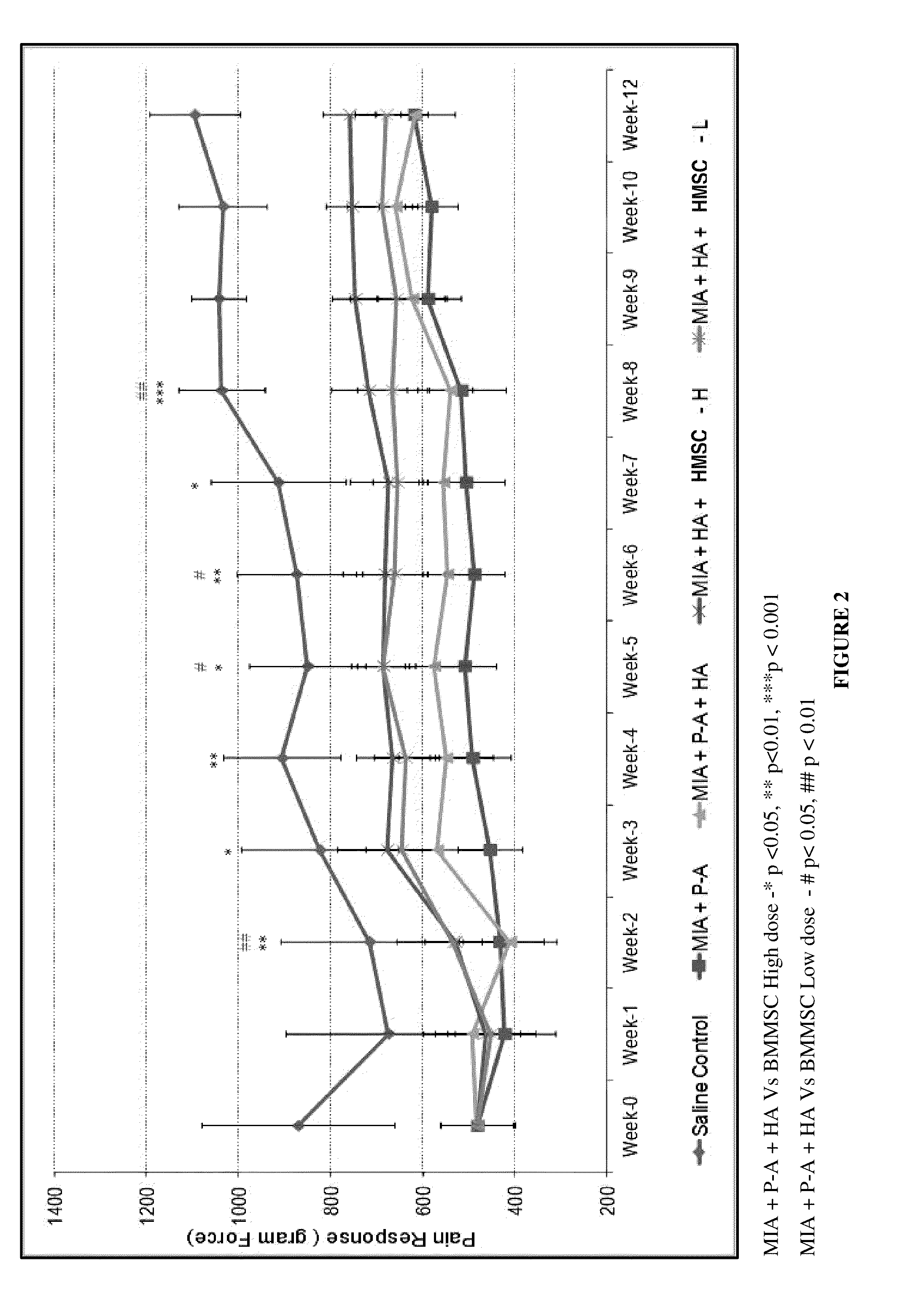
The present disclosure relates to a composition and method for management of Osteoarthritis, including improvement in pain and cartilage regeneration of knee joint affected by osteoarthritis. The present disclosure also relates to a kit for treating Osteoarthritis and the method of assembling the same. The present disclosure relates to a pooled allogeneic mesenchymal stromal cell composition from multiple donors with diverse HLA genotypes suitable for transplantation into a diverse population without the risk of rejection. The pooled cell composition of the present disclosure shows increased potency and higher chondrogenic differentiation potential.
Owner:STEMPEUTICS RES PRIVATE
HLA high-resolution gene sequencing kit
InactiveCN101892317BAvoid problems that cannot be effectively typedHigh resolutionMicrobiological testing/measurementDNA/RNA fragmentationHLA - Human leukocyte antigen geneHla genes
The invention discloses a parting method of leucocyte antigen gene of human being, comprising the following steps of: (1) extracting genome DNA to be tested by a regular technology, and amplifying a destination gene fragment to be analyzed by using PCR amplification primer: 2,3,4 exon of HLA-A, 2,3,4 exon of HLA-B and exon on the locus 2 of HLA-DRB; and (2) amplifying the PCR output obtained in the step (1) by using sequencing primer, amplifying the exon, sequencing the amplified exon and comparing the sequencing result with the standard sequence in a database to determine the gene parting result. As the 2,3,4 exon of HLA-A, 2,3,4 exon of HLA-B and exon on the locus 2 of HLA-DRB are effectively amplified as a result of optimized combination of the HLA gene sequencing kit and the test condition, and the corresponding exon is sequenced, the invention solves the problem that effective parting can not be performed when certain allelic gene nucleotide is located outside an amplification area during further parting, thereby improving the parting resolution and accuracy of the HLA gene.
Owner:SUZHOU UNIV +1
Method for genotyping HLA (Human Leucocyte Antigen) by using serum trace genome DNA
ActiveCN101914622AEasy and time-saving identificationGood effectMicrobiological testing/measurementGenotypeHLA antigen typing
The invention discloses a method for genotyping an HLA (Human Leucocyte Antigen) by using a serum trace genome DNA with serum as a sample, comprising the following steps of: cracking and extracting DNA from small amount of serum and designing a specific primer; subjecting to two-turn net-PCR applying a net-PCR (Polymerase Chain Reaction) technology; and identifying three genotypes of HLA-A through electrophoresis by successfully using the sizes of PCR product segments. By using the serum as the analysis sample, the invention greatly increases convenience to genotyping the HLA.
Owner:北京科卫临床诊断试剂有限公司
Application of a PCR sequencing method, based on DNA barcoding technique and DNA incomplete shearing strategy, in HLA genotyping
ActiveUS9957564B2Improve throughputSimple procedureNucleotide librariesMicrobiological testing/measurementAgricultural scienceDNA barcoding
Owner:BGI GENOMICS CO LTD
Restenosis/atherosclerosis diagnosis, prophylaxis and therapy
InactiveUS6183752B2Accelerate the accumulation processBiocideAntibody mimetics/scaffoldsSkin testPercent Diameter Stenosis
Disclosed and claimed are compositions and methods for therapy and / or prevention of restenosis and / or atherosclerosis. The compositions can include an agent for decreasing viral load of cytomegalovirus, such as an immunological composition or vaccine against cytomegalovirus (CMV) containing at least one epitope of interest of CMV and / or an expression system which expresses at least one epitope of interest of CMV. Such compositions can include at least one epitope of p53. Alternatively, the compositions can include at least one epitope of p53 and / or an expression system which expresses the epitope. The methods can include administering the compositions to a patient in need of such therapy and / or prevention. Additionally, compositions and methods for diagnosing atherosclerosis and / or restenosis, or susceptibility thereto, including screening a sample from a patient for antibodies to CMV and / or CMV proteins and / or screening a sample from a patient for specific viral proteins that predict whether the virus has been reactivated and / or antibodies thereto and / or detecting whether CMV nucleic acid, e.g., mRNA is present in peripheral blood monocytes (PBMCs) and / or detecting a cellular-mediated immune response to CMV peptides or proteins is present and / or HLA phenotyping and / or HLA genotyping. Embodiements can include a skin test.
Owner:PASTEUR MERIEUX SERUMS & VACCINS SA +1
Neoantigen treatment prioritization using multivariate analysis based on: HLA genotype, self-similarity, similarity to known antigens, antigen expression levels and mutant allele frequency
ActiveUS10563266B2Shorten the timeSave moneyMolecular entity identificationMolecular designMutant alleleCancer immunology
Owner:PERSONAL GENOME DIAGNOSTICS INC
Method for detecting tumor neoantigen load, computing device and computer storage medium
The invention relates to a method for detecting tumor neoantigen load, computing equipment and a computer storage medium. The method comprises the following steps: acquiring a transcriptome sequence related to a tumor sample, and first comparison result information and splicing site information which are compared with a reference genome; obtaining a whole exome sequence of the paired normal sample and a whole exome sequence of the paired normal sample in second comparison result information compared with the reference genome; generating somatic mutation information based on the first comparison result information and the second comparison result information; determining a specific HLA genotype; generating a first result about the specific neoantigen based on the splicing site information and the specific HLA genotype; generating a second result about the specific neoantigen based on the somatic mutation information and the specific HLA genotype; and generating the tumor neoantigen load of the detected object based on the first result and the second result. According to the invention, the comprehensiveness and reliability of tumor neoantigen load detection can be effectively improved.
Owner:CARRIER GENE TECH SUZHOU CO LTD +1
Methods and algorithm for selecting allogenic hematopoietic cell donor based on kir and HLA genotypes
ActiveUS20160034666A1Improve clinical outcomesImmunoglobulin superfamilyMedical data miningCandidate donorHematopoietic cell
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Genotype stratification in diabetes treatment and prevention
PendingUS20210077587A1Peptide/protein ingredientsMetabolism disorderAutoimmune conditionAutoimmune disease
The present invention relates to a method for treatment or prevention of an autoimmune disease in a patient, comprising: (a) Determining the HLA genotype of the patient; and (b) Subjecting the patient to a treatment regimen based on said genotype.
Owner:DIAMYD MEDICAL
Detection of HLA genotype
Disclosed is a rapid genetic method for detection of HLA genotype based on the loop-mediated isothermal amplification (LAMP) principles, and in particular, disclosed are a method and a kit for detection of HLA-B*1502 allele based on LAMP principles.
Owner:THE CHINESE UNIVERSITY OF HONG KONG +1
HLA genotyping method and device and storage medium
InactiveCN113345516AReduce the problem of low accuracyImprove accuracyProteomicsGenomicsMedicineTyping
The invention discloses an HLA genetic typing method and a device and a storage medium. The method comprises an HLA prediction typing acquisition step, an HLA typing marking step and an HLA typing integration step. According to the HLA genotyping method, the weight scores of the HLA prediction types obtained by different genotyping tools are marked, and the HLA prediction types obtained by the different genotyping tools are integrated according to the weight scores, so that the detection advantages of each genotyping tool can be fully utilized; and the problems of low accuracy of some genetic typing tools and the like caused by factors such as sequencing depth are reduced, so that the accuracy of HLA typing is improved.
Owner:深圳裕泰抗原科技有限公司
hbb gene mutation and hla typing detection kit
ActiveCN105420233BImprove throughputLow costMicrobiological testing/measurementDNA/RNA fragmentationAntigenHuman leucocyte antigen
The invention provides a method for detecting HBB gene mutation and HLA genotyping based on the high throughput sequencing technology and a corresponding reagent kit. An adopted primer composition comprises a primer of closely-linked single nucleotide polymorphisms (SNP) within the 1 Mb range of the up stream and the down stream of the specific amplification human embryo beta-thalassemia HBB gene and primers of the closely-linked single nucleotide polymorphisms (SNP) within the ranges at the up stream of the LHA-A gene, between the HLA-A gene and the HLA-B gene, between the HLA-B gene and the HLA-DRA gene, between the HLA-DRA gene and the HLA-DQB1 gene and at the downstream of the HLA-DQB1 gene of the specific amplification human leucocyte antigen system. The method has the advantages of university, single nucleotide polymorphisms (SNP) sequencing, high throughput, low cost, high flexibility and strong specificity.
Owner:海南医学院附属医院 +1
Reagent for PCR-SBT method for HLA genotyping
ActiveCN101928776BImprove accuracyIncrease success rateMicrobiological testing/measurementSodium acetateEthanol precipitation
The invention provides a PCR-SBT method for HLA genotyping. Exons 1, 2, 3, 4, 5, 6, 7 of an HLA-A locus, an HLA-B locus and an HLA-C locus are subjected to genotyping. The method comprises the following steps of: (1) preparing human genomic DNA; (2) designing an amplification primer; (3) performing double digestion purification on an amplification product; (4) designing a sequencing primer and performing sequencing PCR on a purification product; (5) purifying a sequencing product by a sodium acetate-ethanol precipitation method and performing capillary electrophoresis sequencing; and (6) performing software analysis on the acquired sequence to determine genotypes thereof. The exons 1, 2, 3, 4, 5, 6, 7 of the HLA-A, B, C loci are subjected to full-length sequence sequencing, so that the full length of the related exons of the HLA-A, B, C loci and part of intron oligonucleotide sequences are obtained and the HLA genotyping is accurately performed.
Owner:浙江省血液中心
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com