Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
525 results about "Virulent characteristics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In an ecological context, virulence can be defined as the host's parasite-induced loss of fitness. Virulence can be understood in terms of proximate causes—those specific traits of the pathogen that help make the host ill—and ultimate causes—the evolutionary pressures that lead to virulent traits occurring in a pathogen strain.
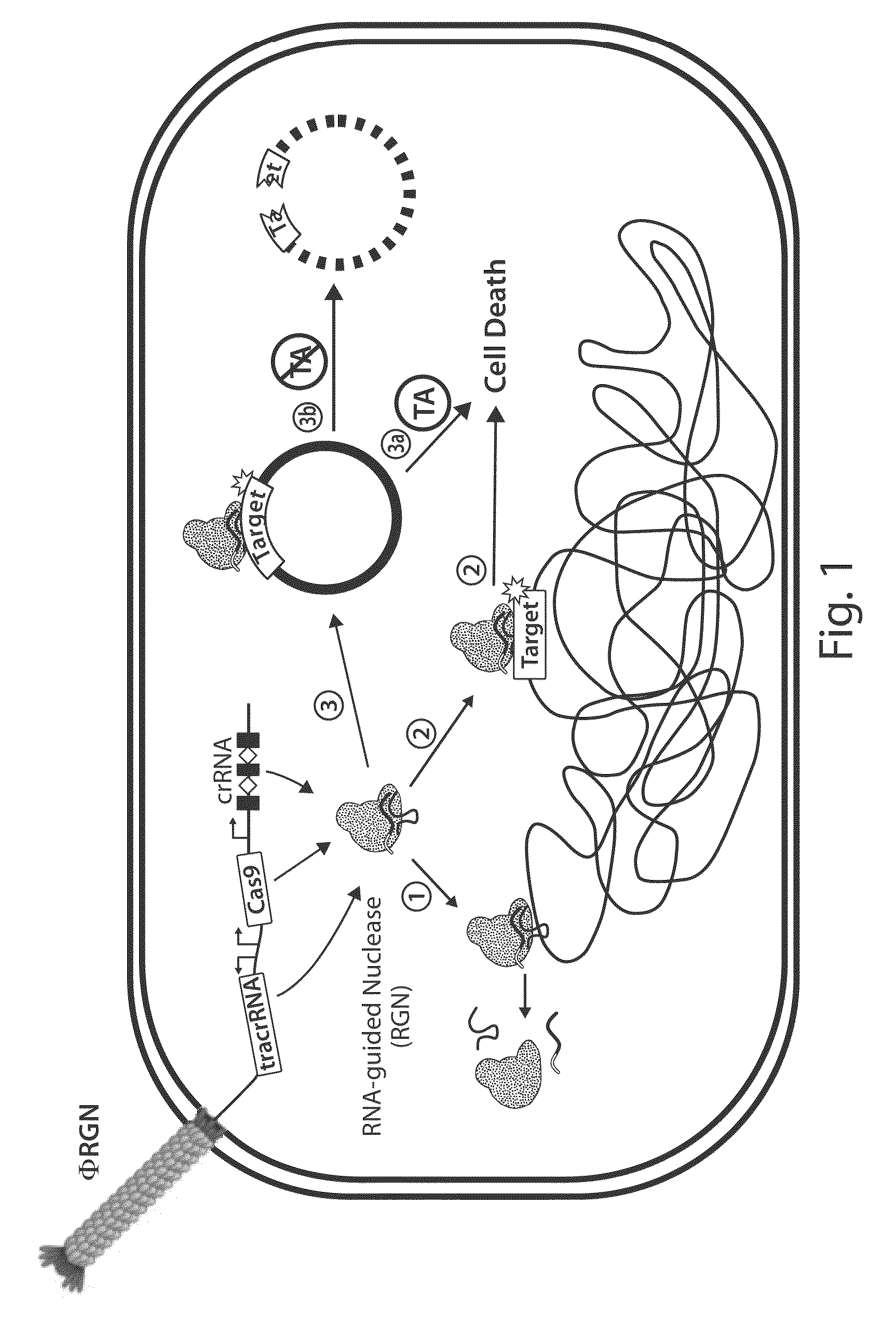
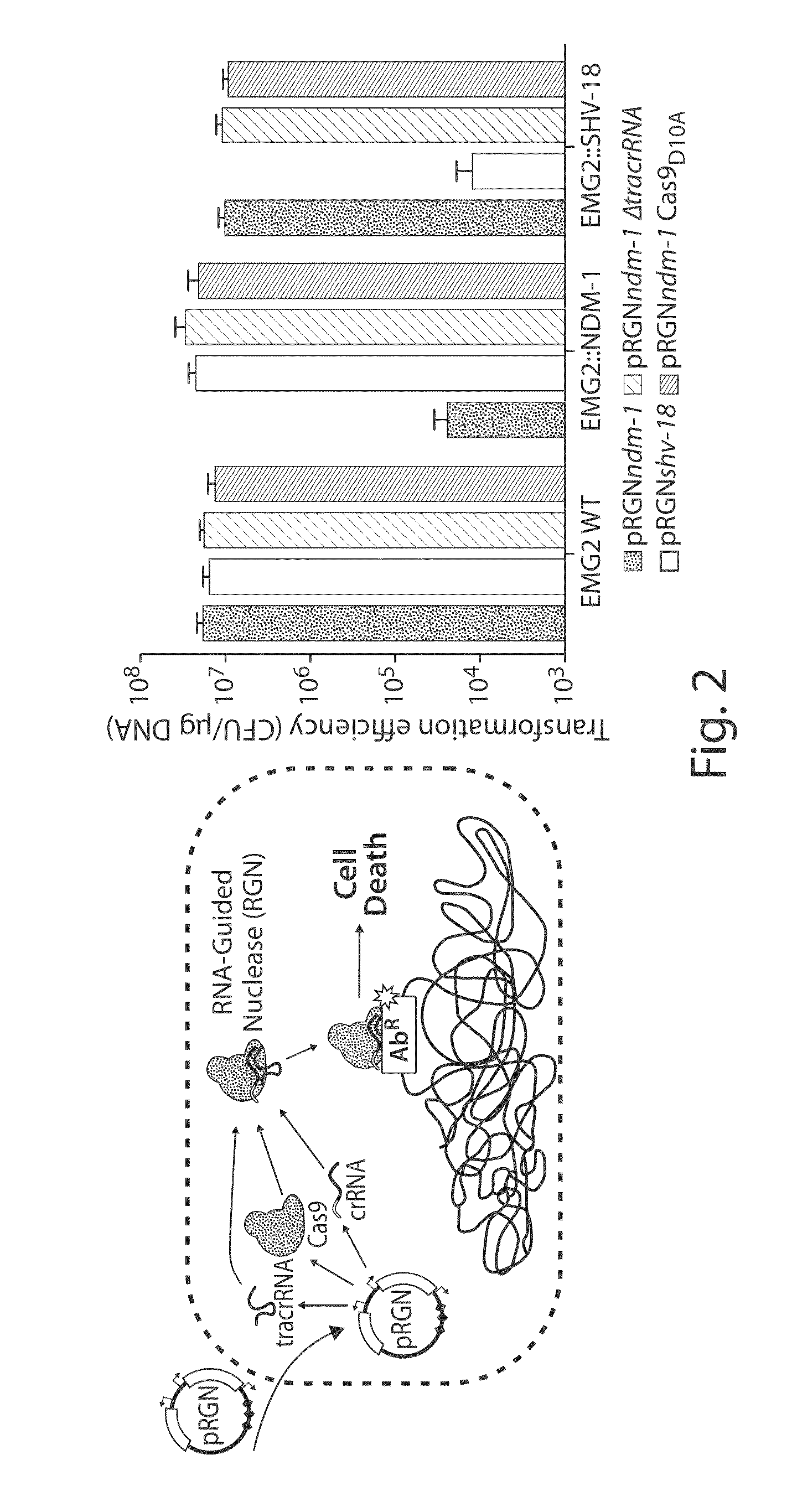
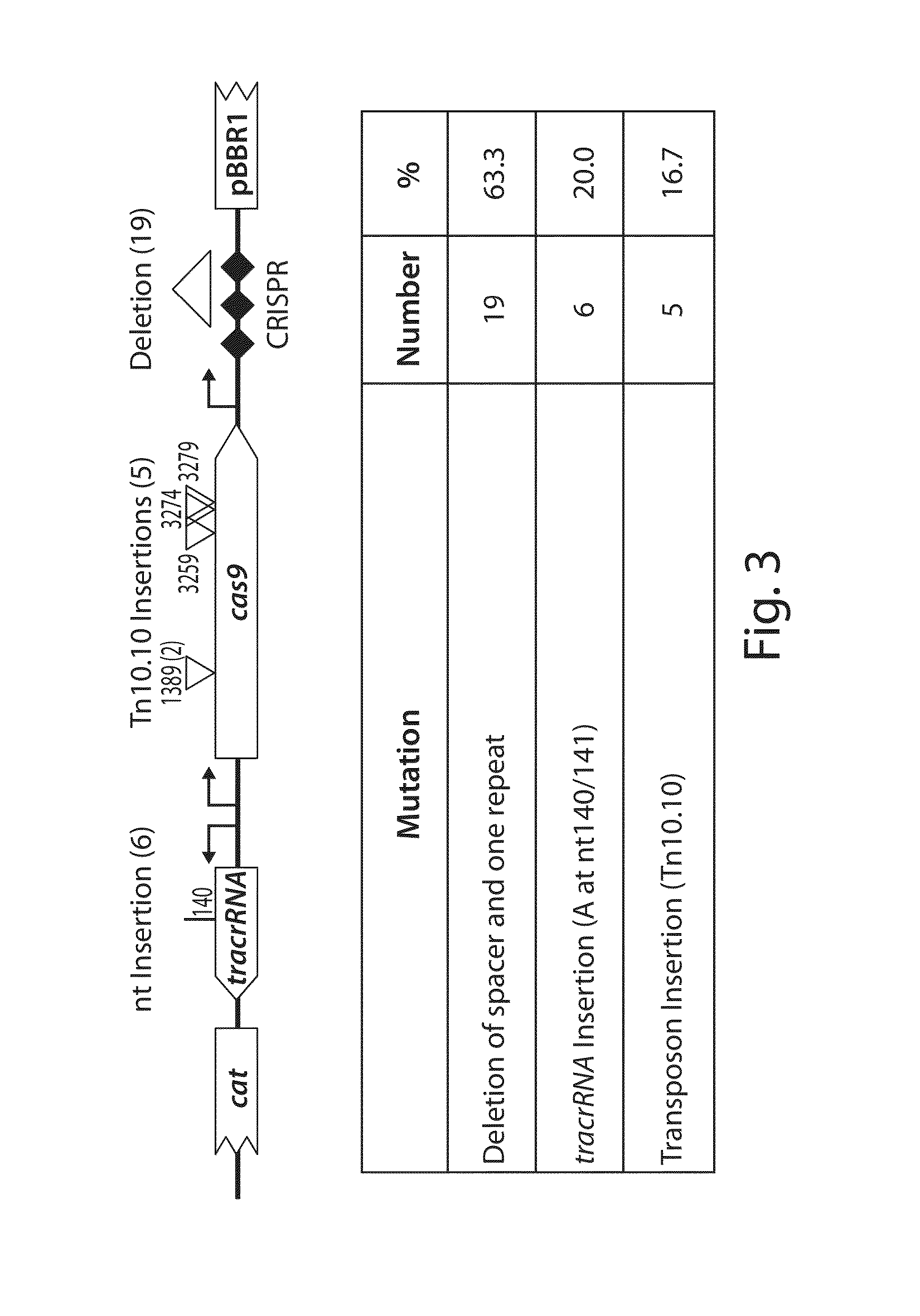
Tuning microbial populations with programmable nucleases
ActiveUS20150064138A1Efficiently and rapidly encoded into synthetic constructBroad deliveryBiocideSugar derivativesMicroorganismVirulent characteristics
Various aspects and embodiments of the invention are directed to methods and compositions for reversing antibiotic resistance or virulence in and / or destroying pathogenic microbial cells such as, for example, pathogenic bacterial cells. The methods include exposing microbial cells to a delivery vehicle with at least one nucleic acid encoding an engineered autonomously distributed circuit that contains a programmable nuclease targeted to one or multiple genes of interest.
Owner:MASSACHUSETTS INST OF TECH
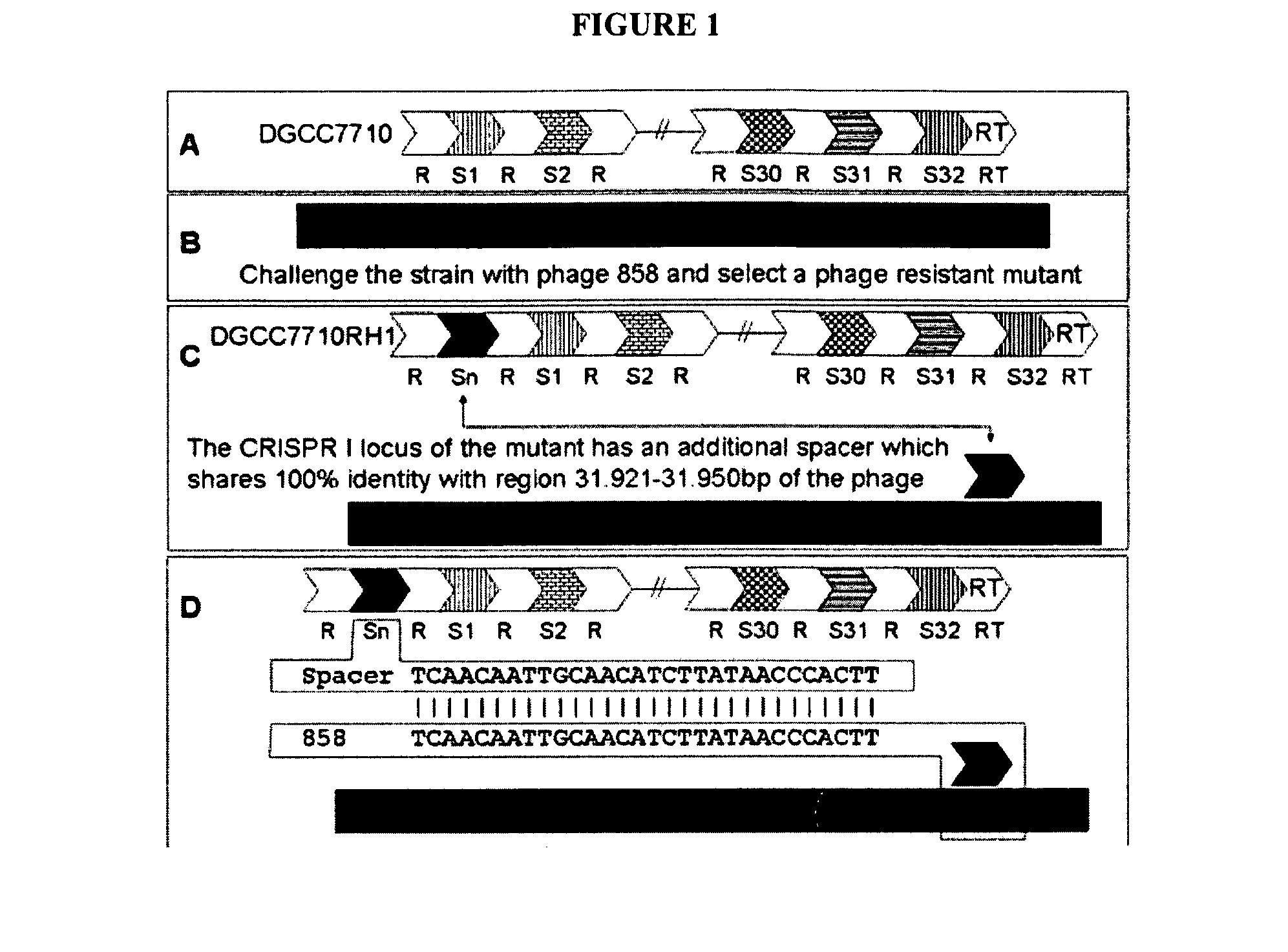
Cultures with Improved Phage Resistance
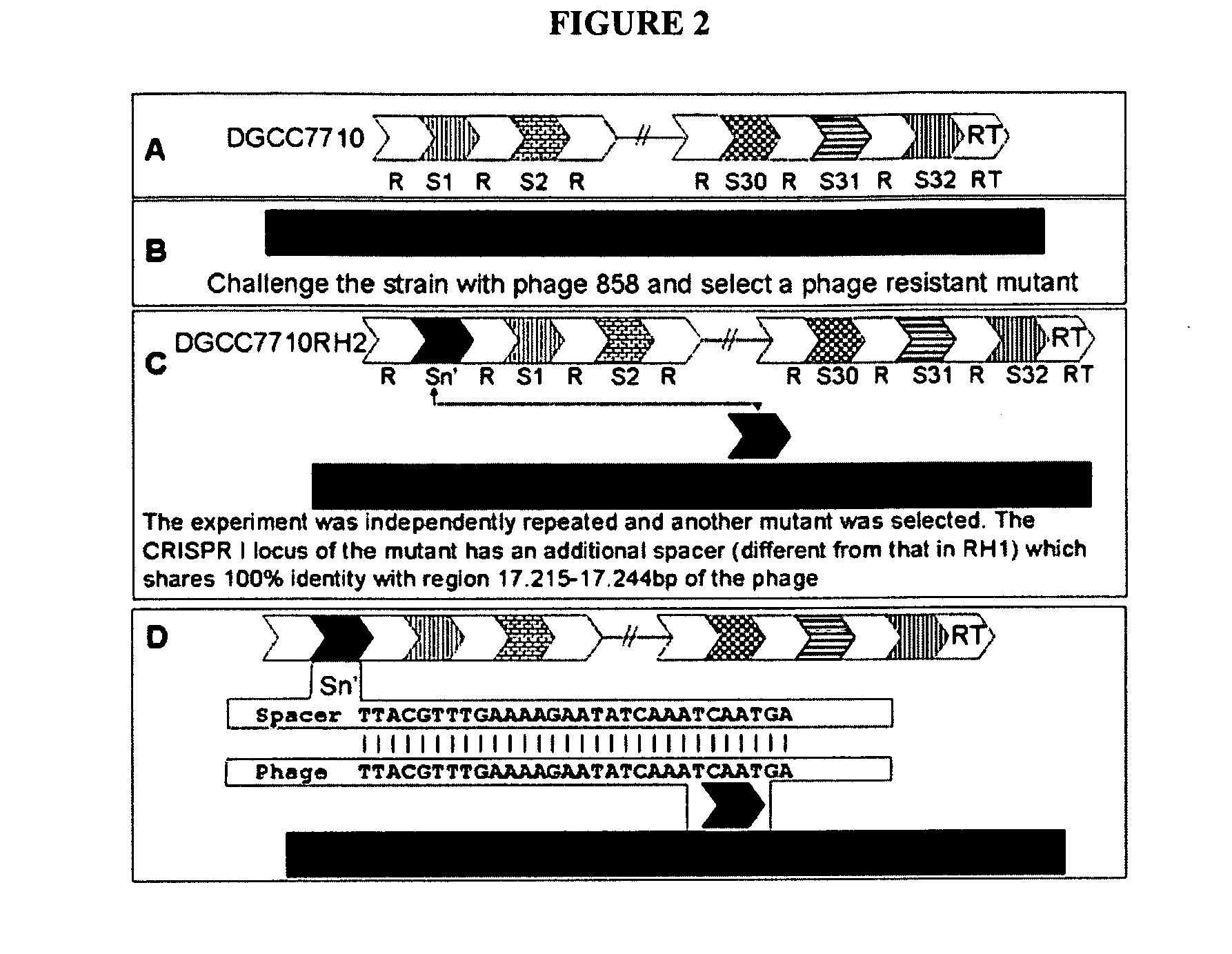
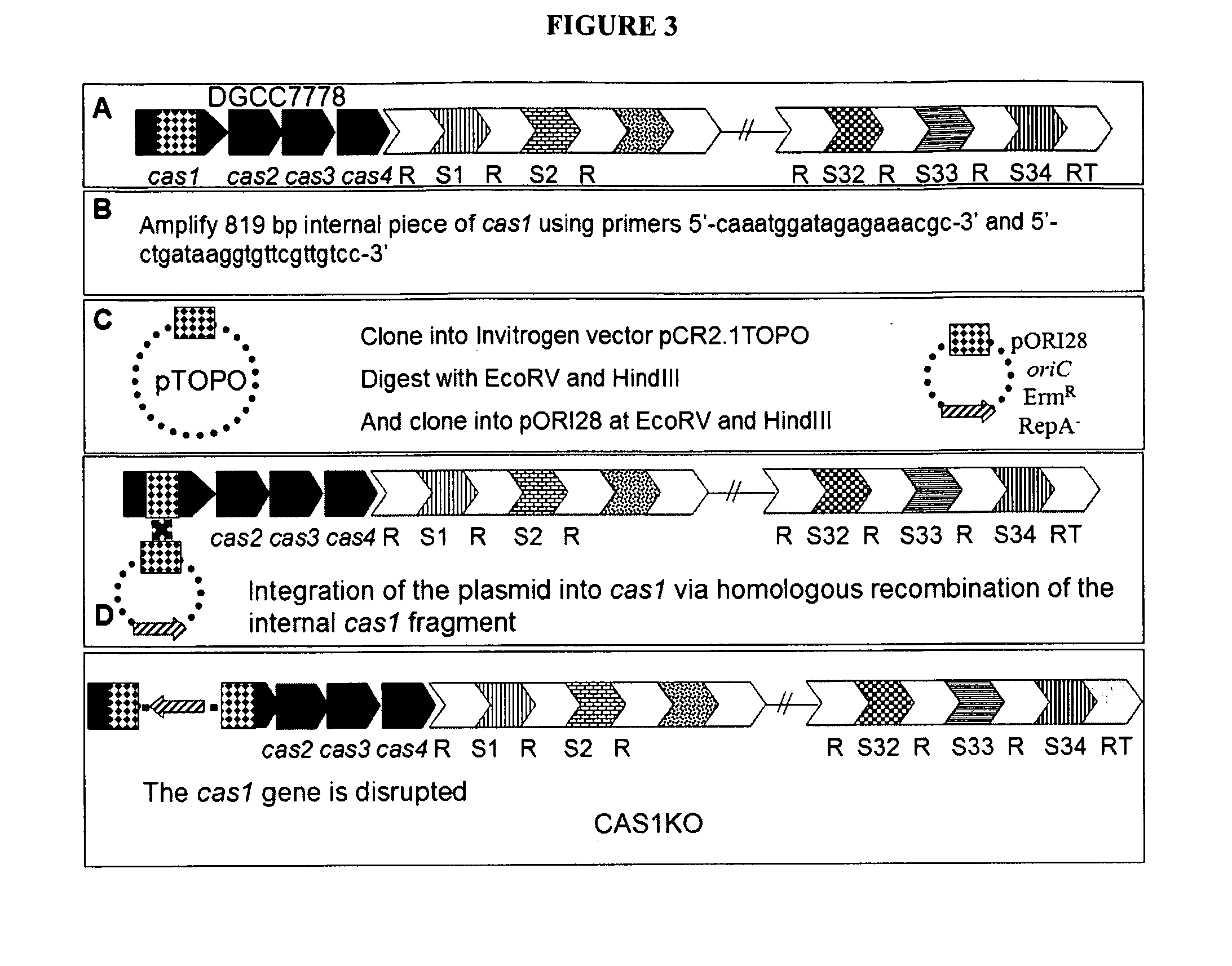
InactiveUS20110002889A1Reduced degree of homologyReduce decreaseBiocideBacteriaVirulent characteristicsBacteriophage
The present invention provides methods and compositions related to modulating the resistance of a cell against a target nucleic acid or a transcription product thereof. In some preferred embodiments, the present invention provides compositions and methods for the use of one or more cas genes or proteins for modulating the resistance of a cell against a target nucleic acid or a transcription product thereof. In some embodiments, the present invention provides methods and compositions that find use in the development and use of strain combinations and starter culture rotations. In additional embodiments, the present invention provides methods for labelling and / or identifying bacteria. In some preferred embodiments, the present invention provides methods for the use of CRISPR loci to determine the potential virulence of a phage against a cell and the use of CRISPR-cas to modulate the genetic sequence of a phage for increased virulence level. In still further embodiments, the present invention provides means and compositions for the development and use of phages as biocontrol agents.
Owner:DUPONT NUTRITION BIOSCIENCES APS
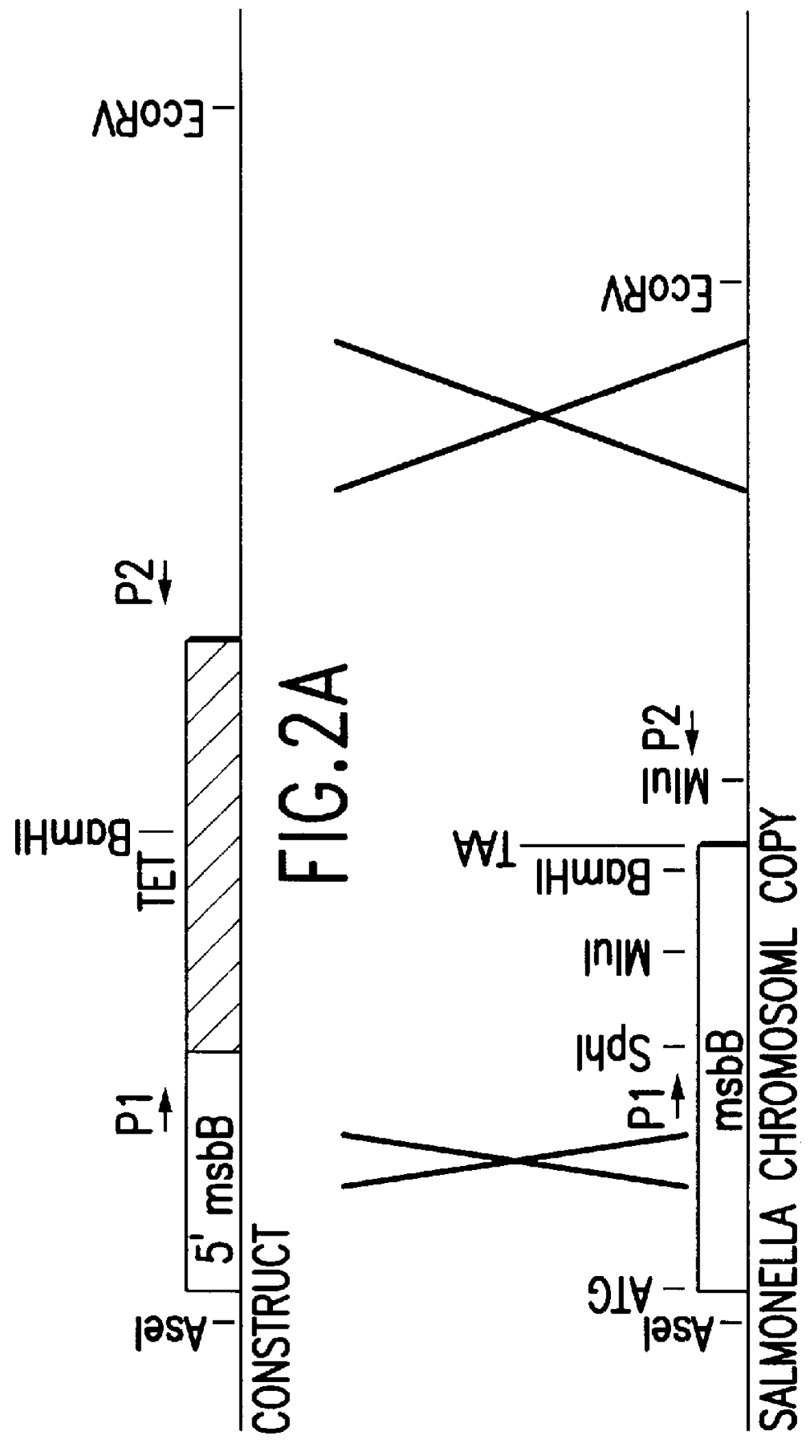
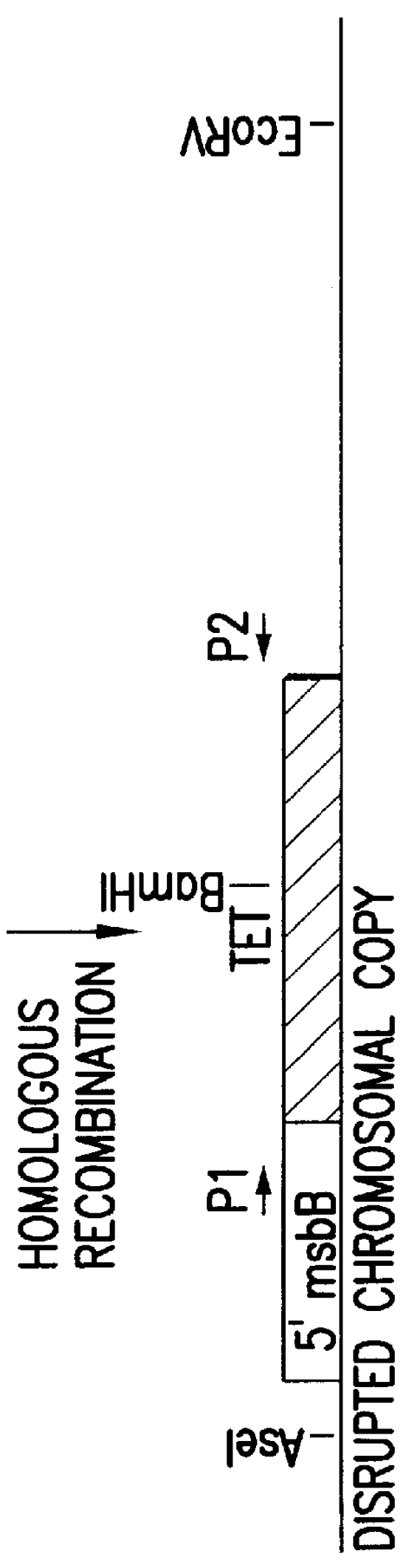
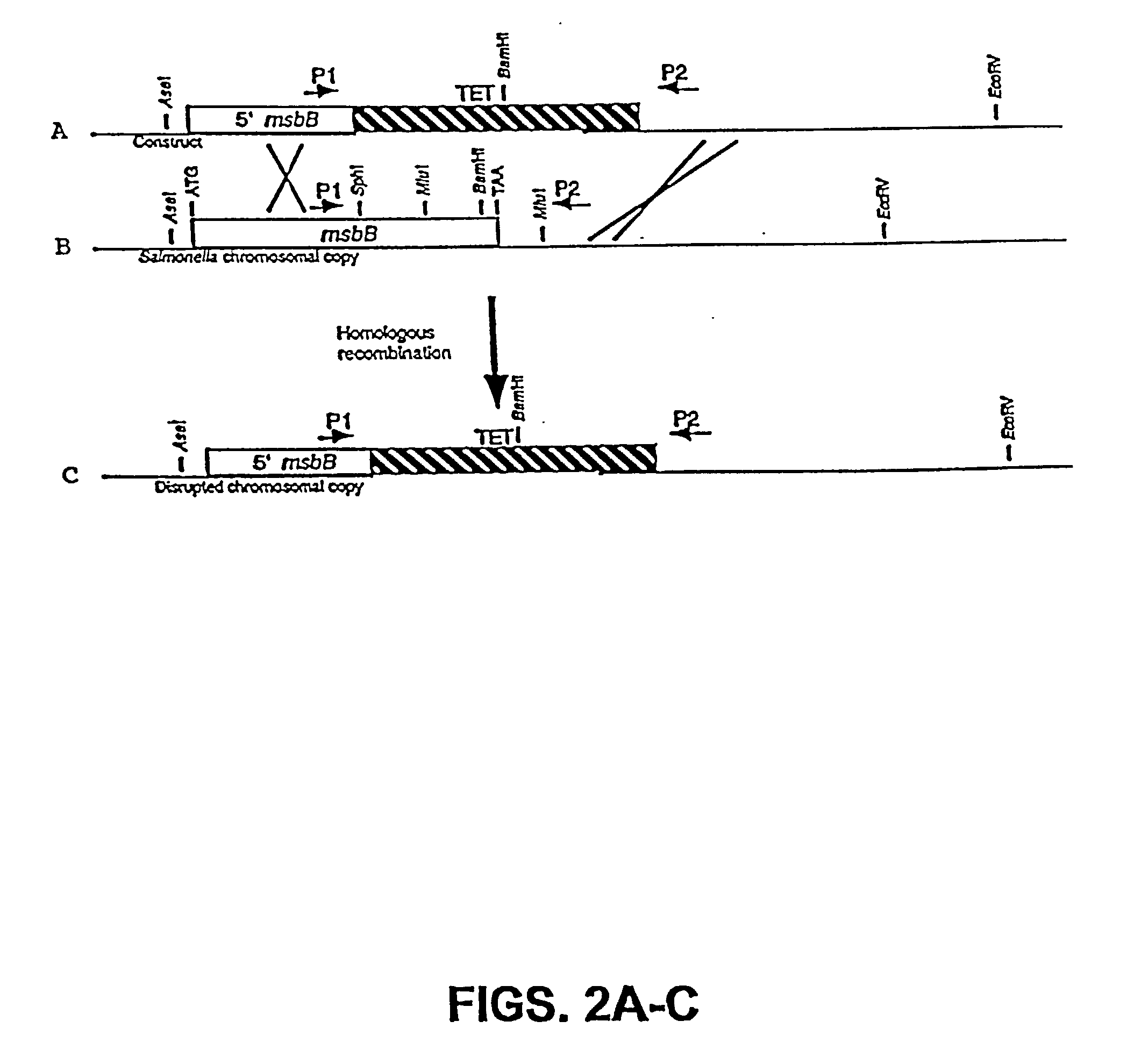
Genetically modified tumor-targeted bacteria with reduced virulence
InactiveUS6080849AImprove securityReduce capacityBiocideBacteriaTumor targetVirulent characteristics
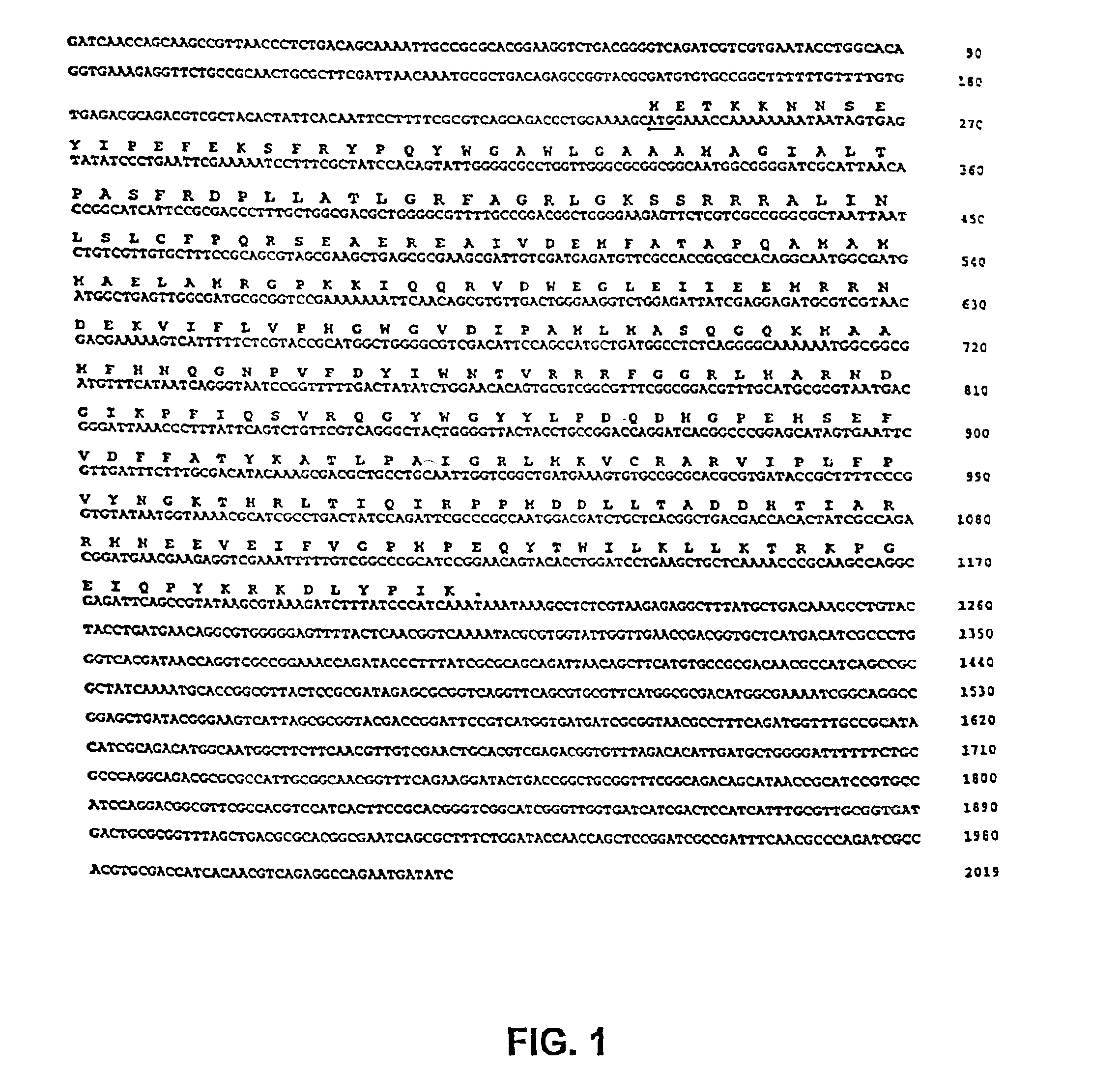
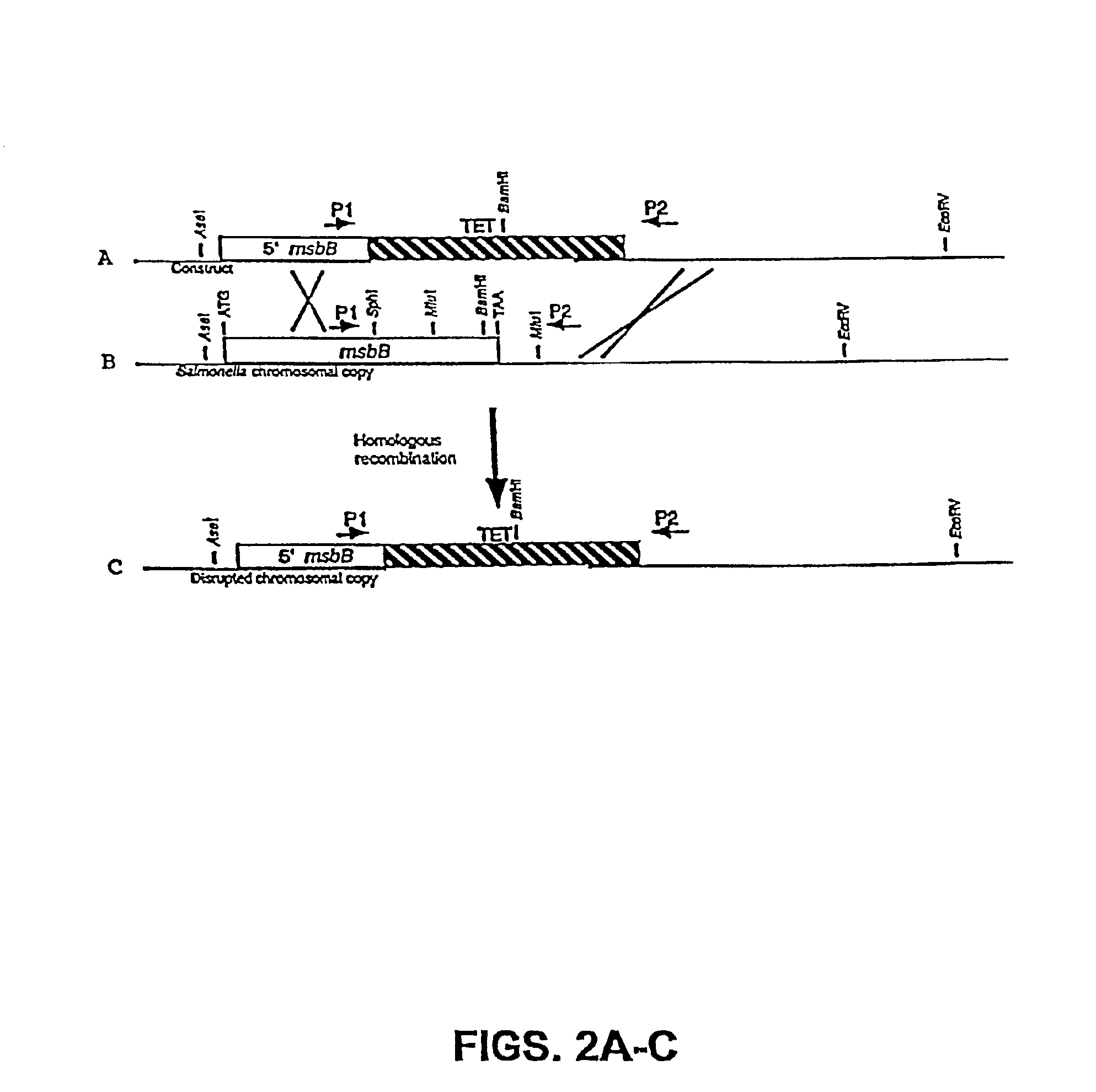
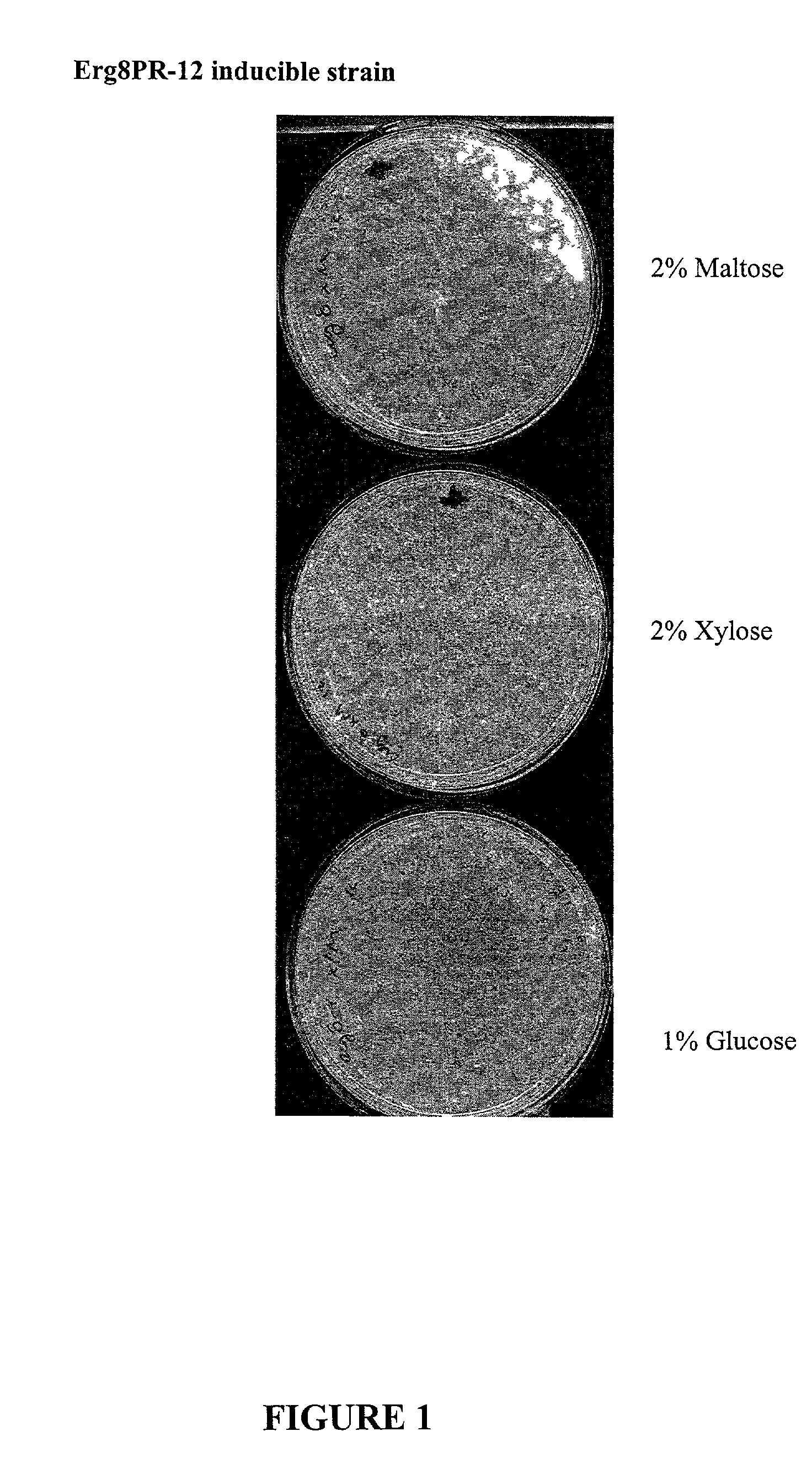
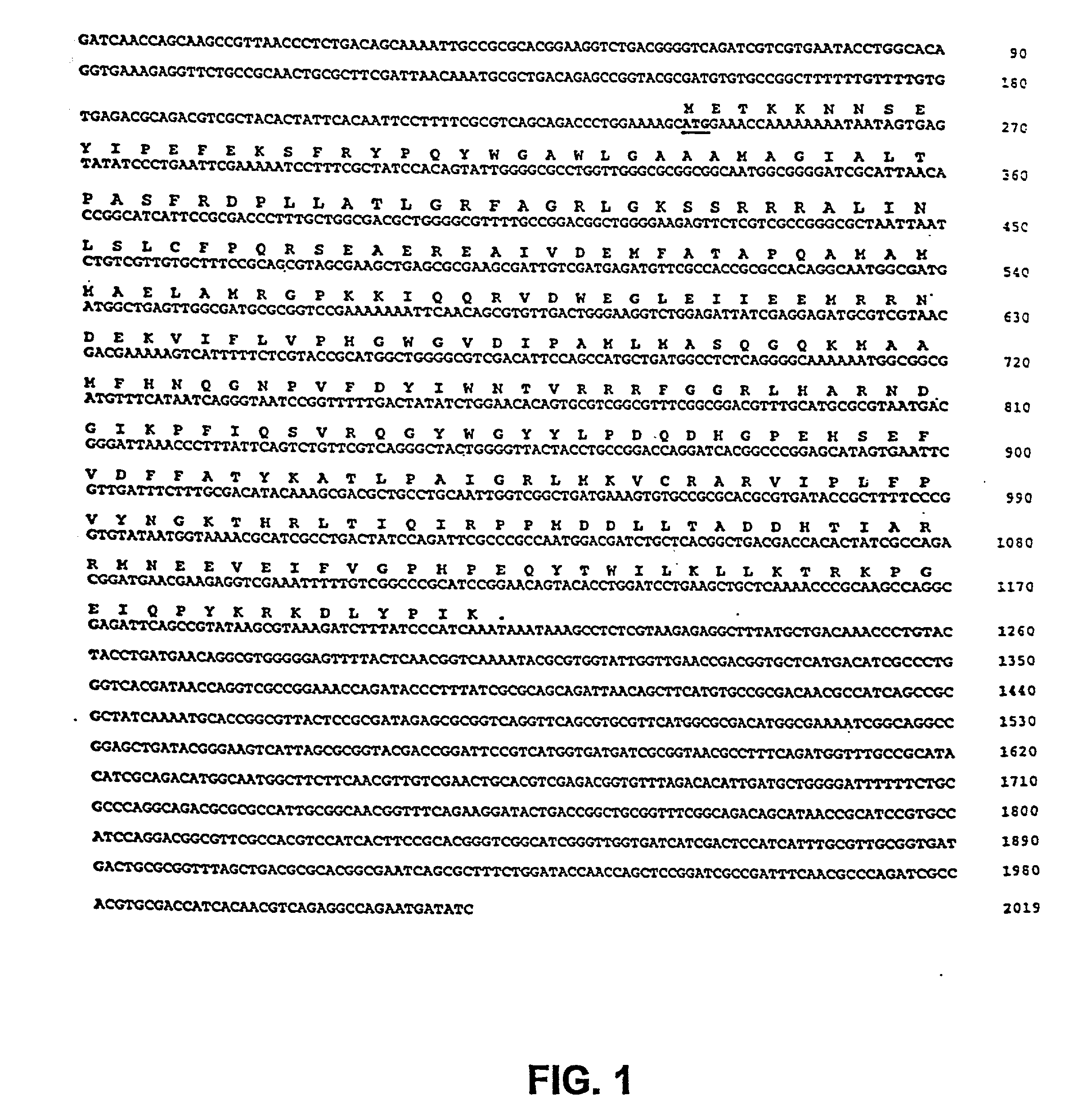
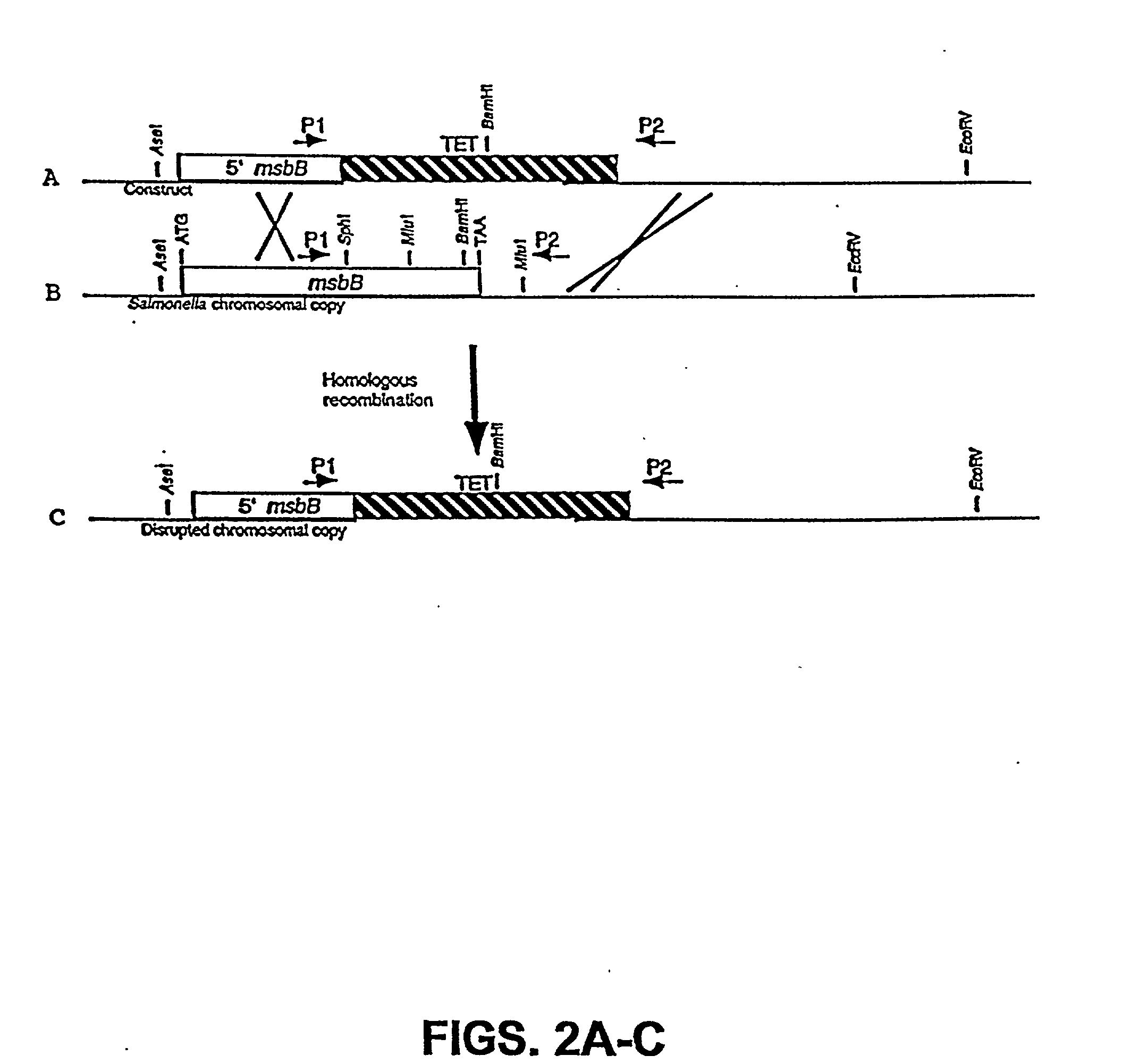
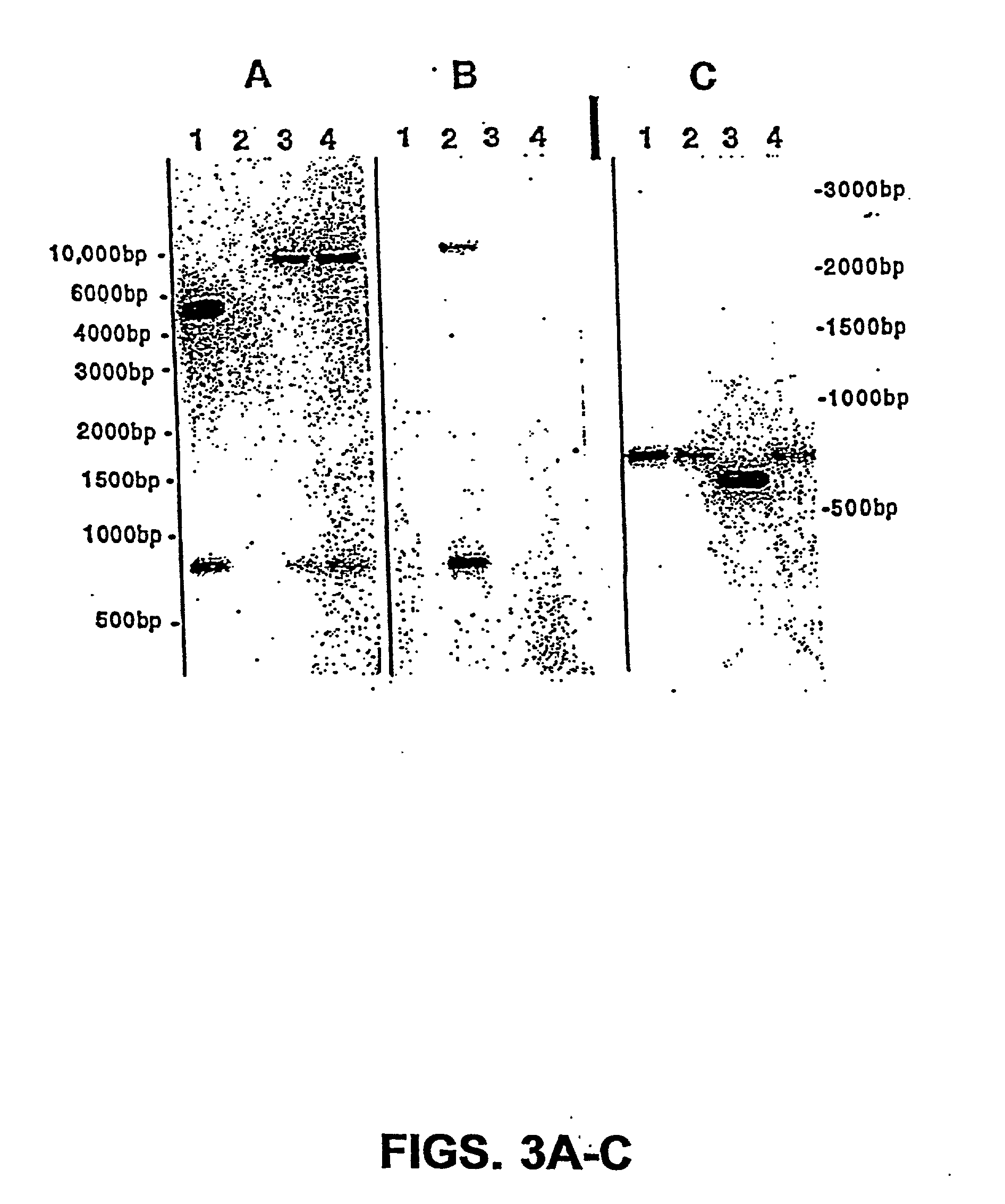
The present invention is directed to mutant Salmonella sp. having a genetically modified msbB gene in which the mutant Salmonella is capable of targeting solid tumors. The present invention further relates to the therapeutic use of the mutant Salmonella for growth inhibition and / or reduction in volume of solid tumors.
Owner:YALE UNIV +1
Immunogenic compositions for gram positive bacteria such as streptococcus agalactiae
InactiveUS20060165716A1InhibitionEasy to eliminateAntibacterial agentsBacterial antigen ingredientsBacteroidesVirulent characteristics
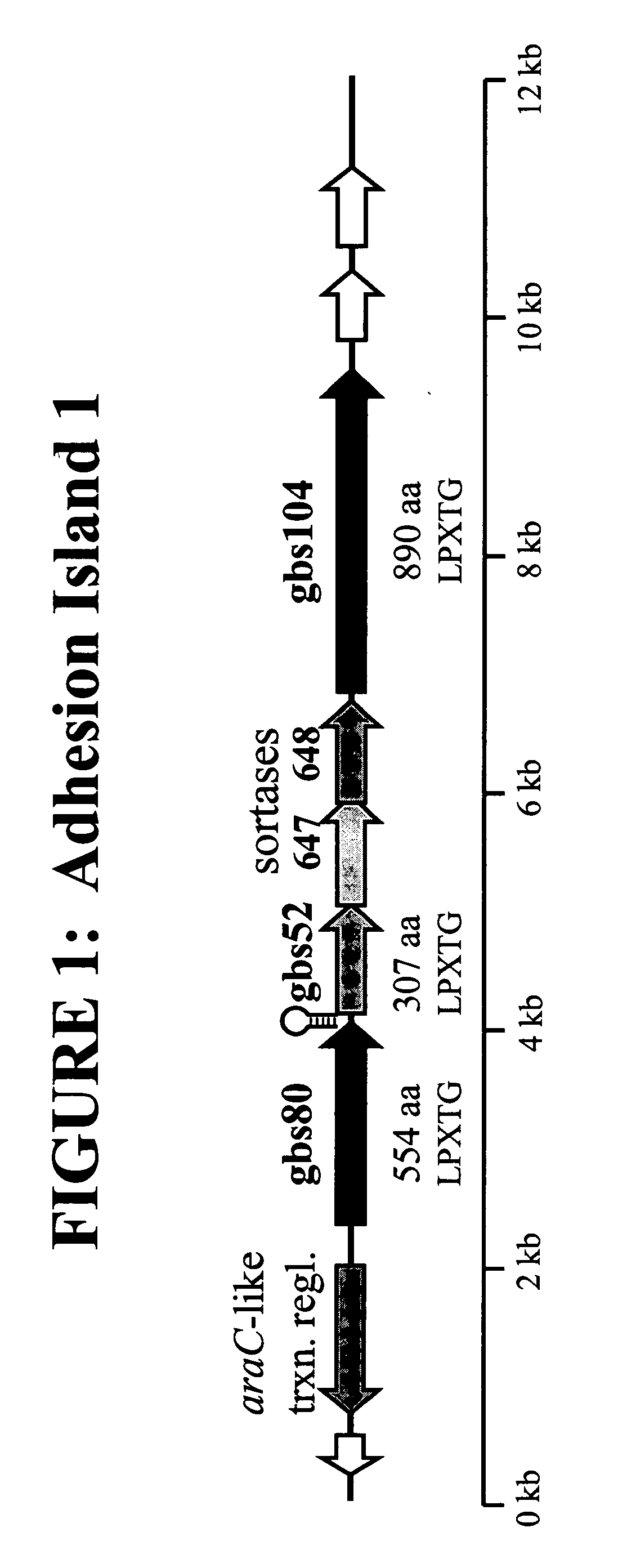
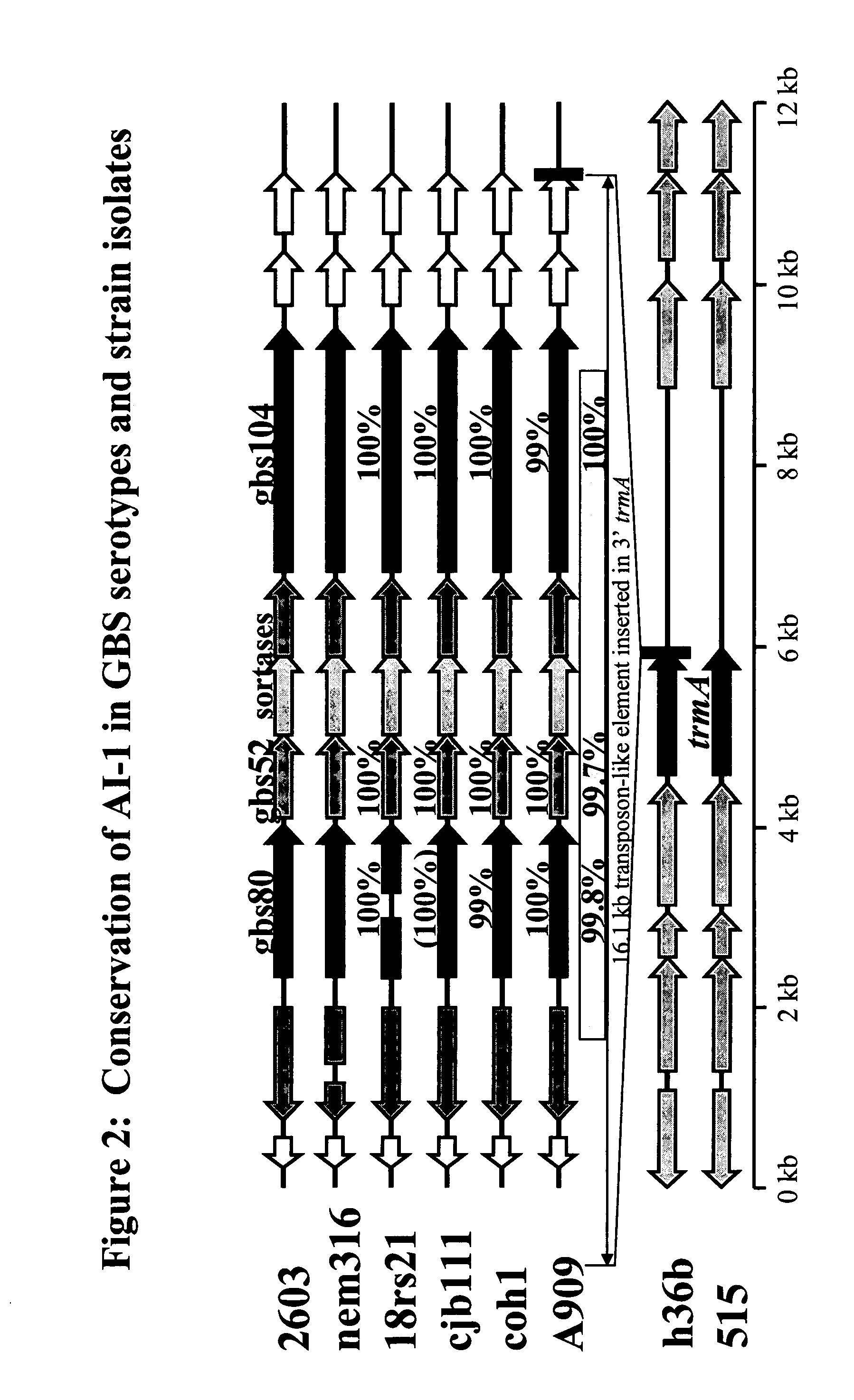
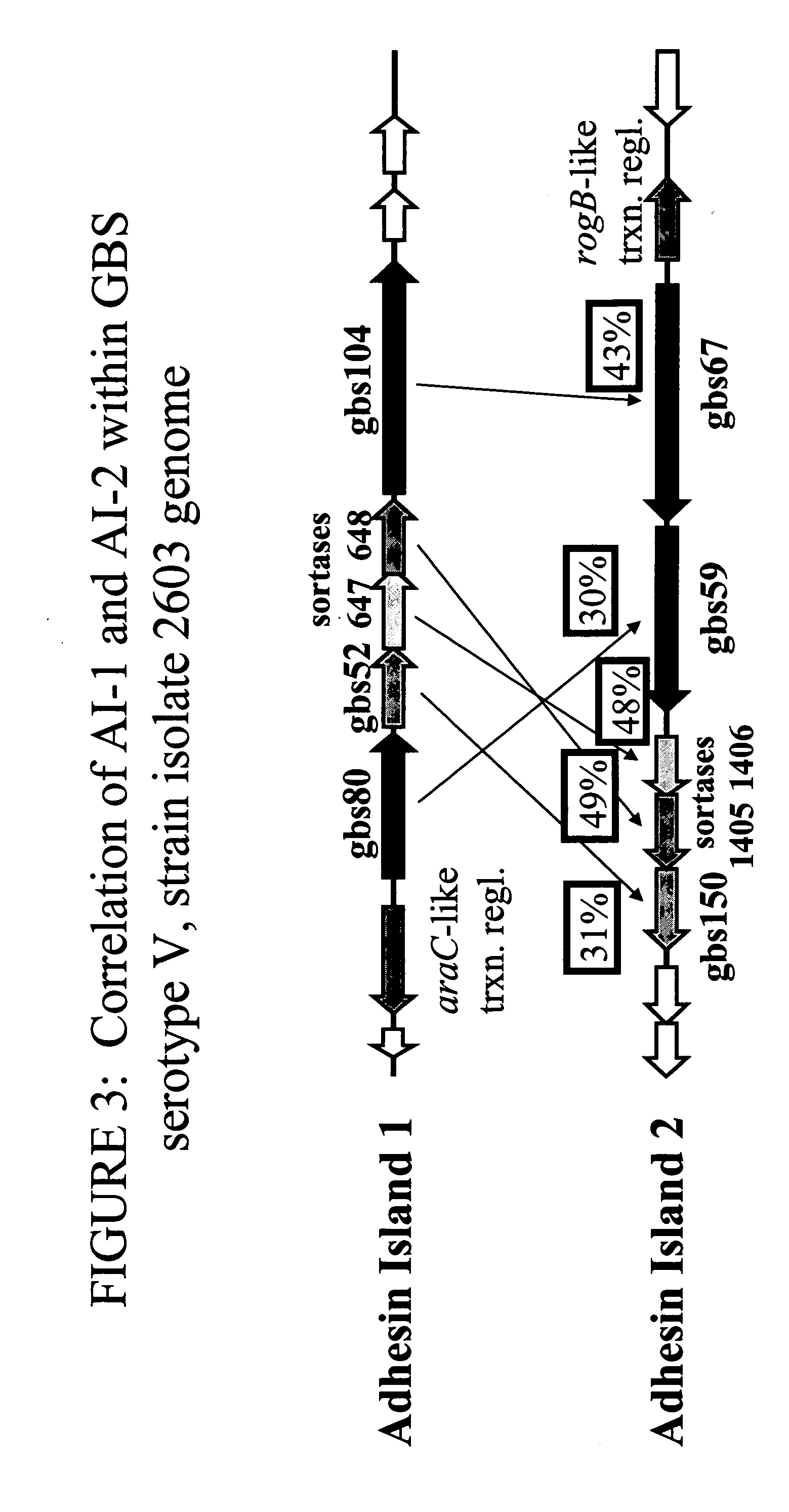
The invention relates to the identification of a new adhesin islands within the genomes of several Group A and Group B Streptococcus serotypes and isolates. The adhesin islands are thought to encode surface proteins which are important in the bacteria's virulence. Thus, the adhesin island proteins of the invention may be used in immunogenic compositions for prophylactic or therapeutic immunization against GAS or GBS infection. For example, the invention may include an immunogenic composition comprising one or more of the discovered adhesin island proteins.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Genetically modified tumor-targeted bacteria with reduced virulence
InactiveUS6863894B2Effective concentration and/or duration of the therapeutic vectorBiocideBacteriaTumor targetVirulent characteristics
The present invention is directed to mutant Salmonella sp. having a genetically modified msbB gene in which the mutant Salmonella is capable of targeting solid tumors. The invention is also directed to Salmonella sp. containing a genetically modified msbB gene as well as an genetic modification in a biosynthetic pathway gene such as the purI gene. The present invention further relates to the therapeutic use of the mutant Salmonella for growth inhibition and / or reduction in volume of solid tumors.
Owner:VION PHARMA INC +1
Pharmaceutical compositions and methods to vaccinate against candidiasis
InactiveUS20030124134A1Treat prevent alleviateToxic reductionPeptide/protein ingredientsSnake antigen ingredientsHospitalized patientsVirulent characteristics
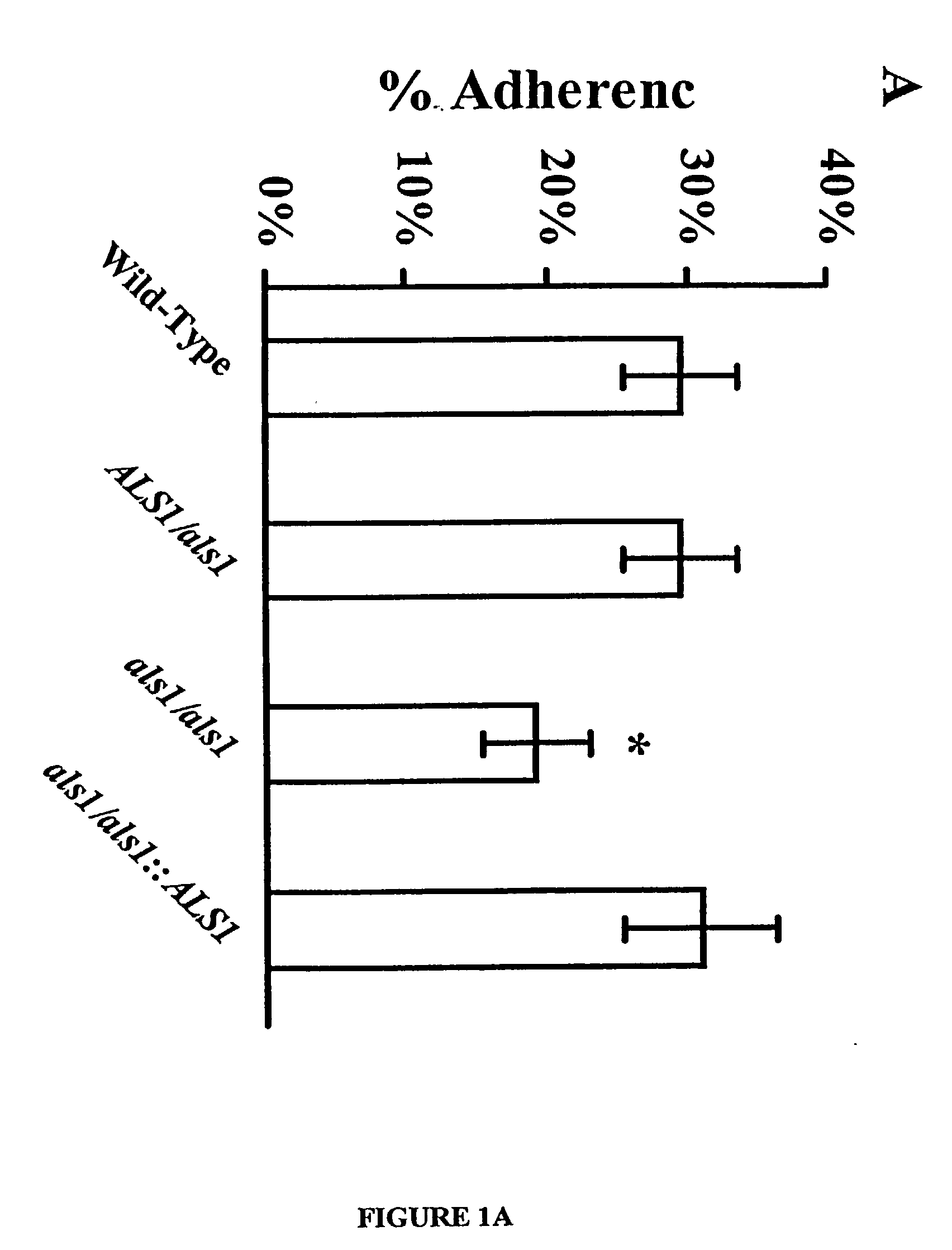
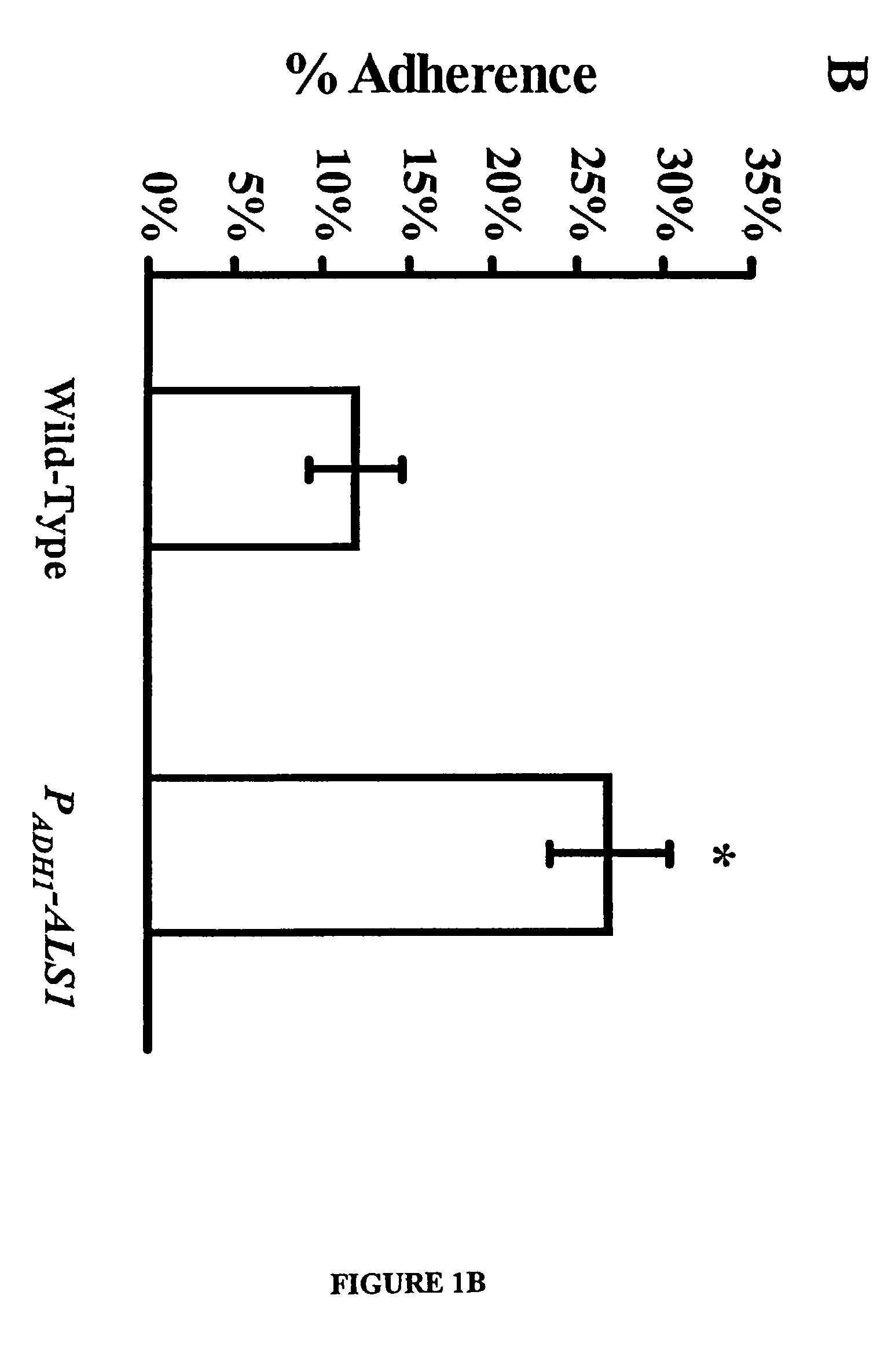
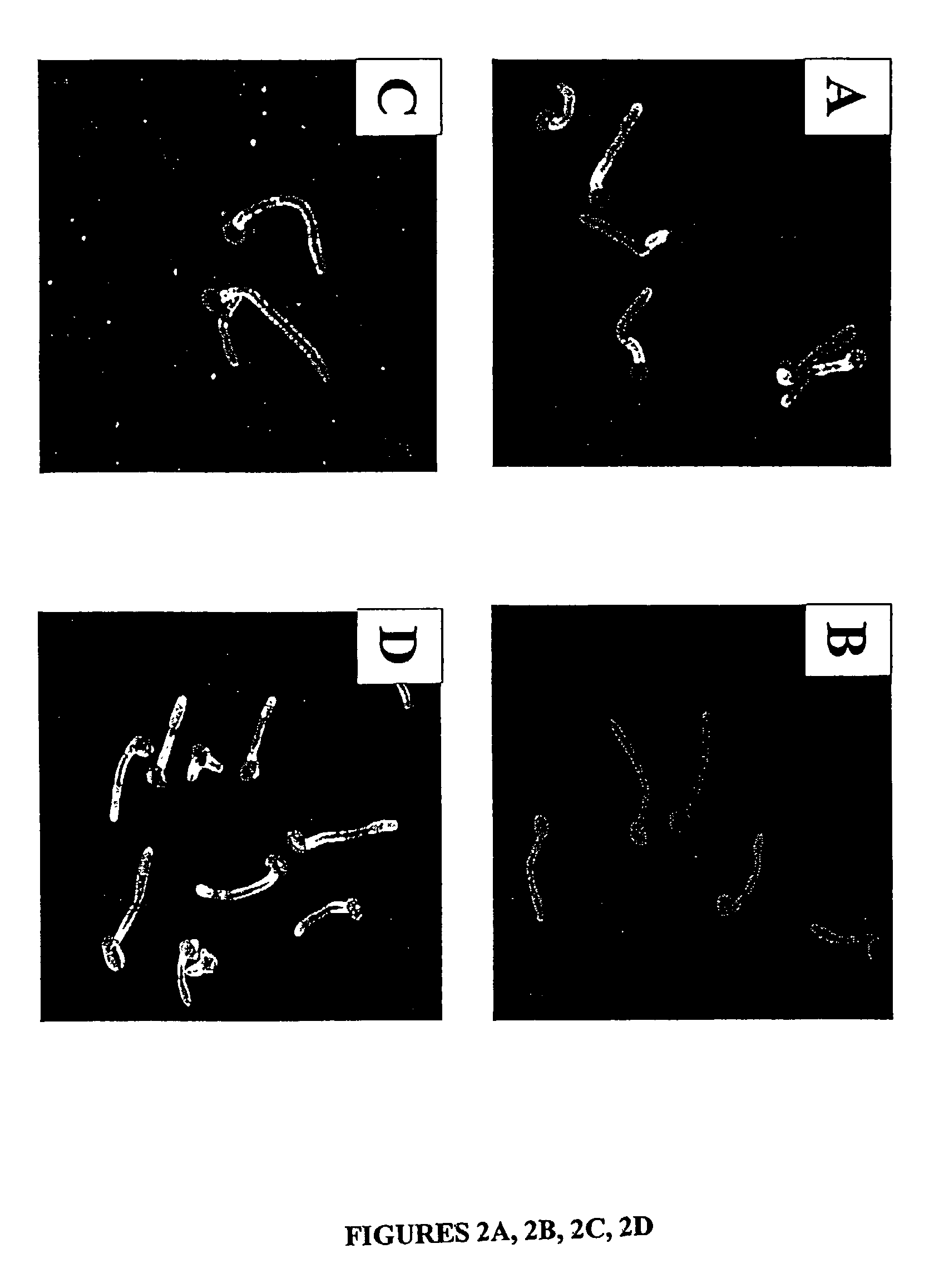
A Candida albicans bloodstream infections cause significant morbidity and mortality in hospitalized patients. Filament formation and adherence to host cells are critical virulence factors of C. albicans. Multiple filamentation regulatory pathways have been discovered, however the downstream effectors of these regulatory pathways remain unknown. The cell surface proteins in the ALS group are downstream effectors of the filamentation regulatory pathway. Particularly, Als1p mediates adherence to endothelial cells in vitro and is required for virulence. The blocking of adherence by the organism is described resulting from the use of a composition and method disclosed herein. Specifically, a pharmaceutical composition comprised of a gene, gene product, or specific antibody to the ALS gene family is administered as a vaccine to generate an immune response capable of blocking adherence of the organism.
Owner:LOS ANGELES BIOMEDICAL RES INST AT UCLA HARBOR MEDICAL CENT +1
Recombinant bacteriophage and methods for their use
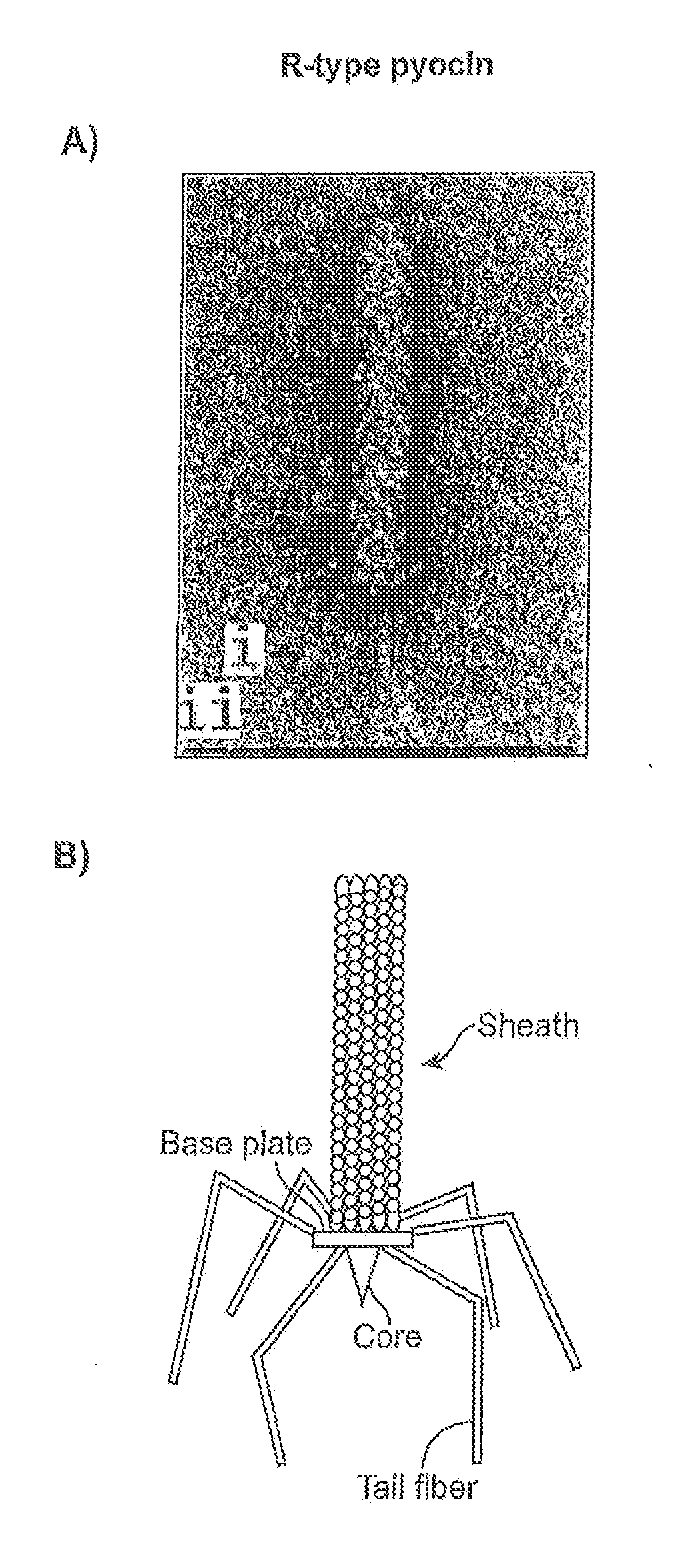
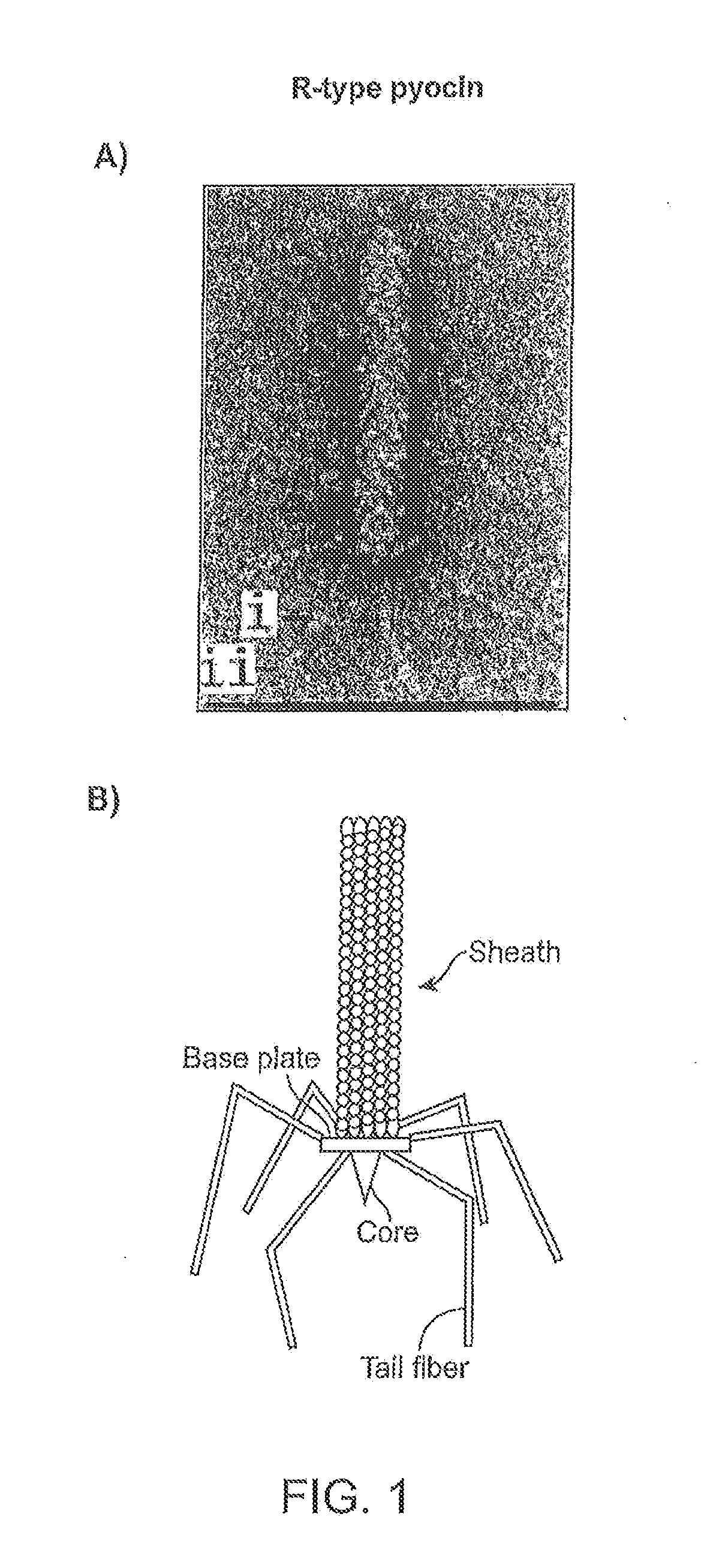
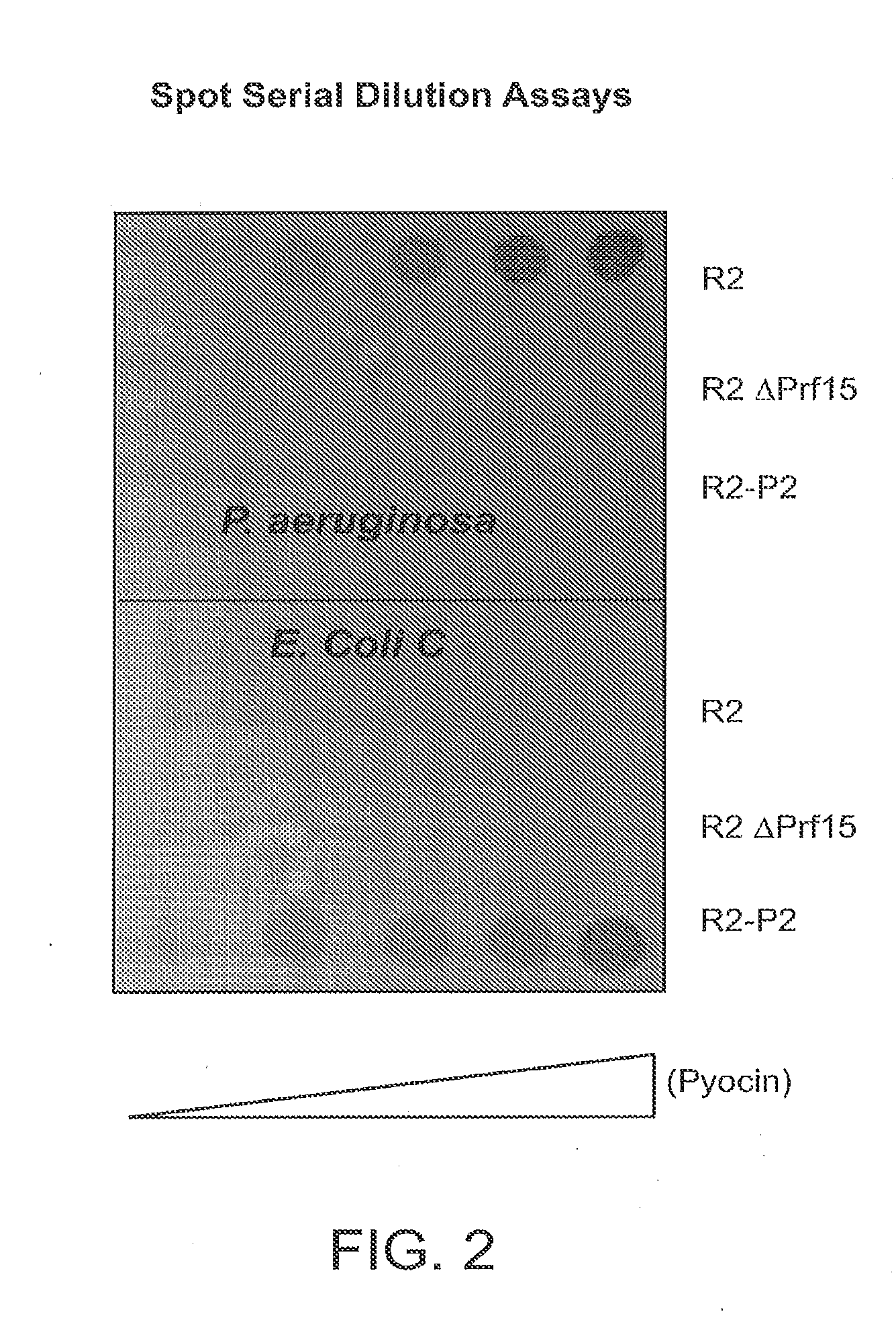
Modified forms of naturally occurring bacteriocins, such as the R-type pyocins of Pseudomonas aeruginosa, are disclosed as are methods for producing them in GRAS organisms. The bacteriocins are modified at the ends of their tail fibers in a region responsible for binding specificity and affinity to their cognate binding partners, or receptors, such as those on the surface of bacteria. Methods for the use of the modified bacteriocins, such as to bind receptors, including virulence or fitness factors, on the surfaces of bacteria, are also described.
Owner:PYLUM BIOSCI INC
Salmonella vaccine materials and methods
Attenuated mutant Salmonella bacteria containing inactivated virulence genes are provided for use in safe, efficacious vaccines.
Owner:PHARMACIA & UPJOHN CO
Altered strain of the modified vaccinia virus ankara (mva)
InactiveUS20030013190A1Ease of mass productionEfficient and large-scale productionBiocideGenetic material ingredientsAdjuvantVirulent characteristics
The invention provides new strains of the Modified Vaccinia Virus Ankara (MVA) that have a strongly reduced virulence for most mammals, especially humans, but nevertheless grows in cells of a continuous cell line approved for the production of a therapeutic agent such as a vaccine. The invention also provides a method for producing said adapted MVA strains. The adapted MVA can be used e.g. for parenteral immunization, as a vector system, or in the active or inactivated from as an adjuvant or as a regulator of the unspecific components of the immune system.
Owner:BAVARIAN NORDIC AS
Chimeric and/or growth-restricted flaviviruses
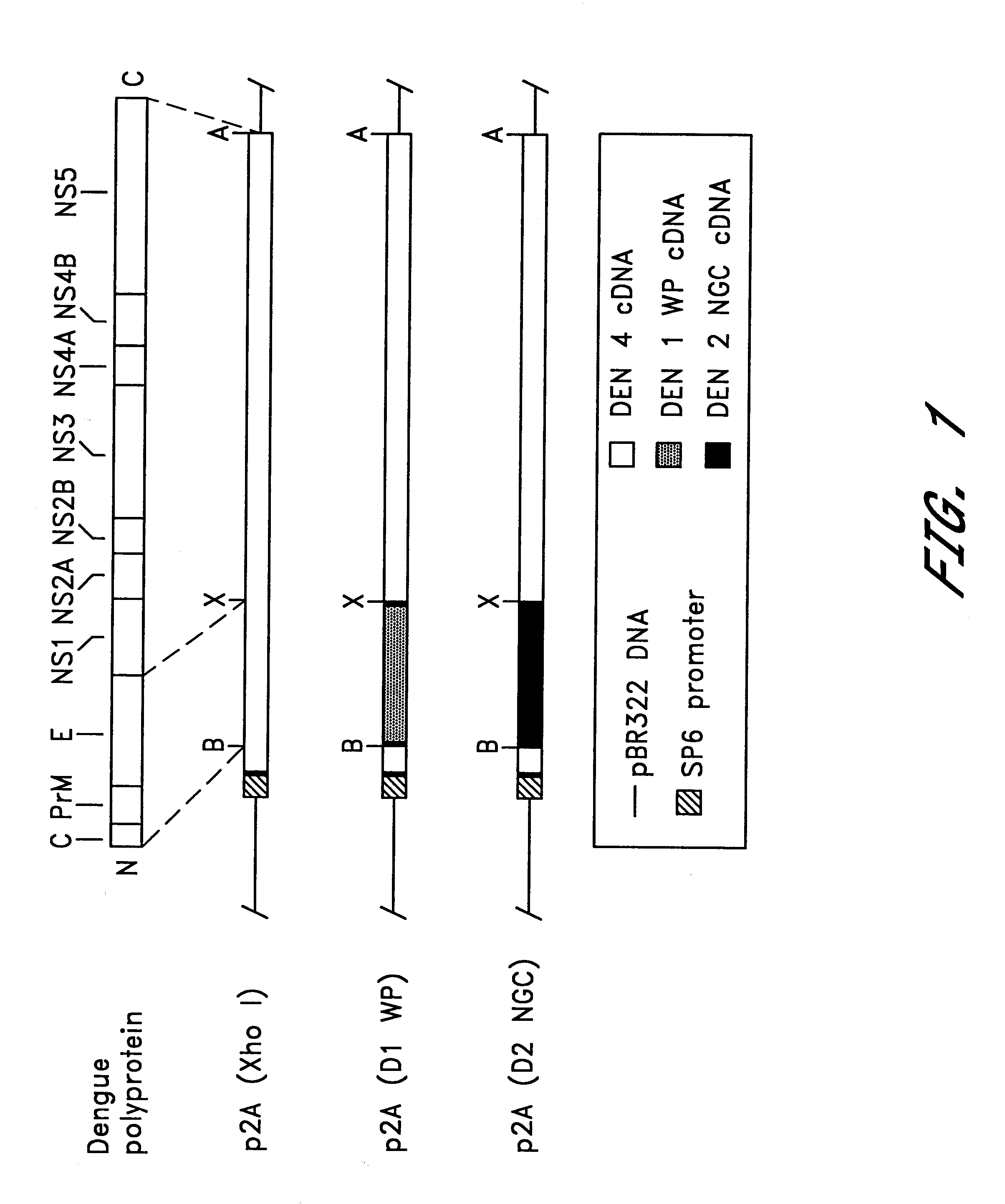
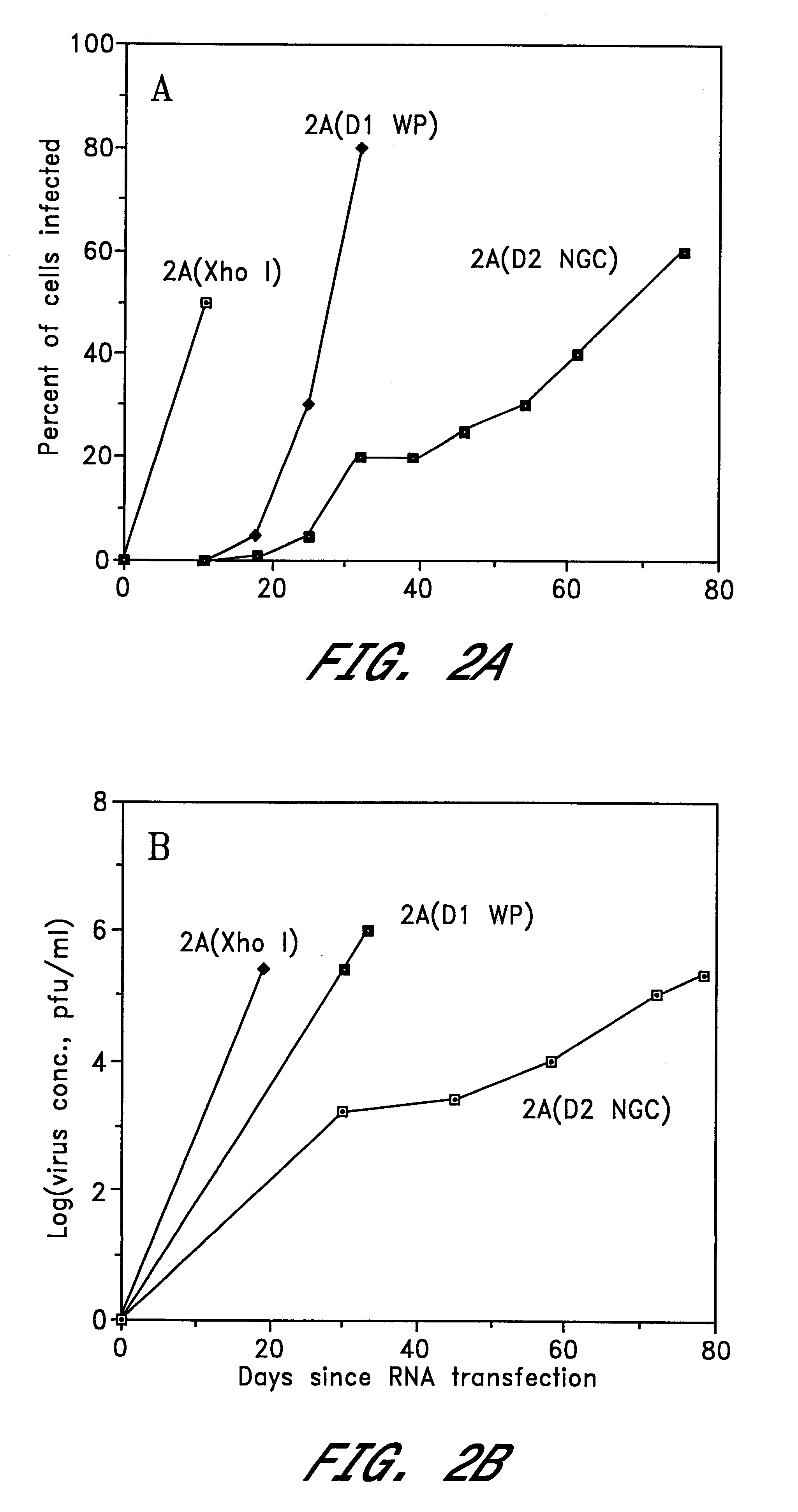
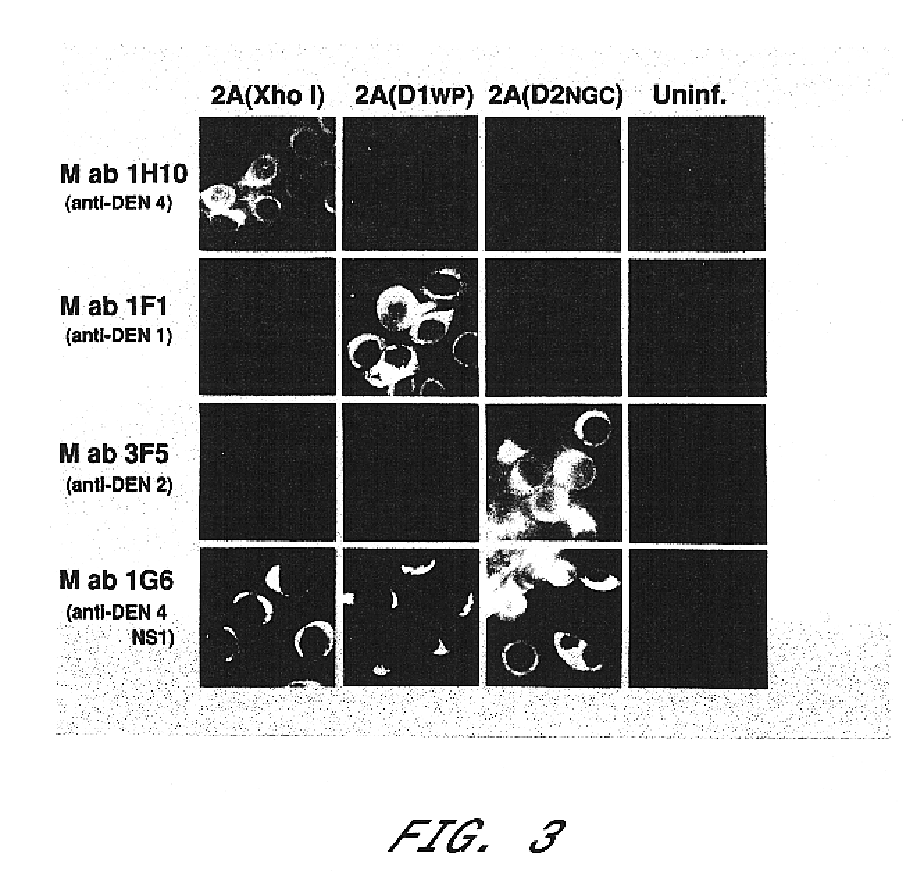
InactiveUS6676936B1BiocideSsRNA viruses positive-senseVirulent characteristicsJapanese B Encephalitis Virus
The invention includes a chimeric virus for use in a vaccine preparation having a genome comprising nucleic acid sequences encoding at least one structural protein from one flavivirus and nucleic acid sequences encoding nonstructural protein from another flavivirus. The genome preferably includes mutations within the viral genome that reduce virus virulence and in a particularly preferred embodiment these vaccines are directed to flaviviruses such as dengue virus, tick-borne encephalitis virus and Japanese encephalitis virus. The invention also includes a baculovirus having a recombinant dengue cDNA sequence which encodes: (1) dengue virus capsid protein, pre-matrix protein, envelope glycoprotein and NS1 and NS2a nonstructural proteins or (2) dengue envelope glycoprotein or (3) dengue non-structural proteins NS1 and NS2a. The invention further includes a baculovirus having a recombinant Japanese B encephalitis virus cDNA sequence which encodes the Japanese B encephalitis virus capsid protein, pre-matrix protein, envelope glycoprotein and non-structural proteins NS1 and NS2a. The invention further includes a vaccine and a method to produce that vaccine.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
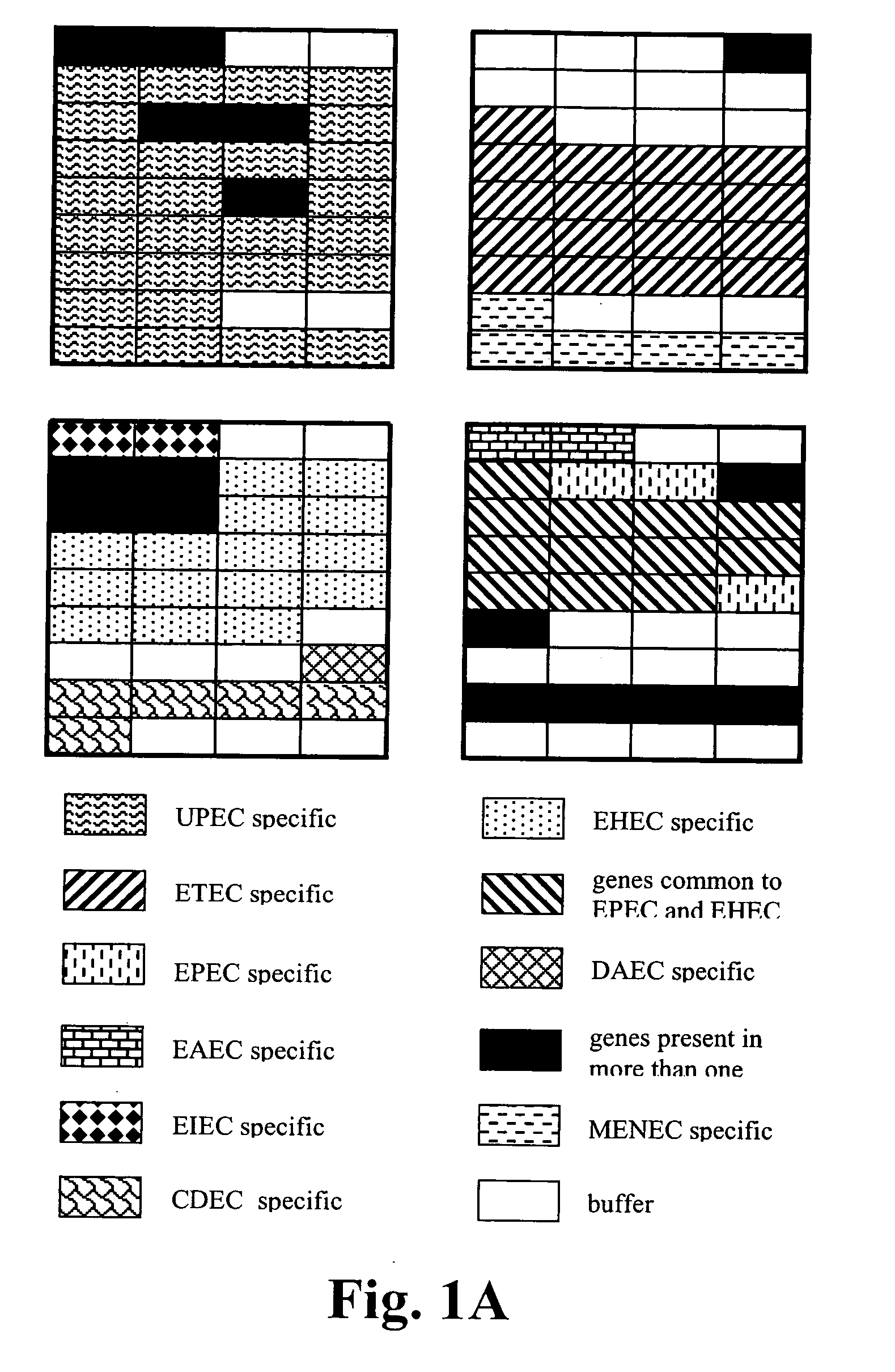
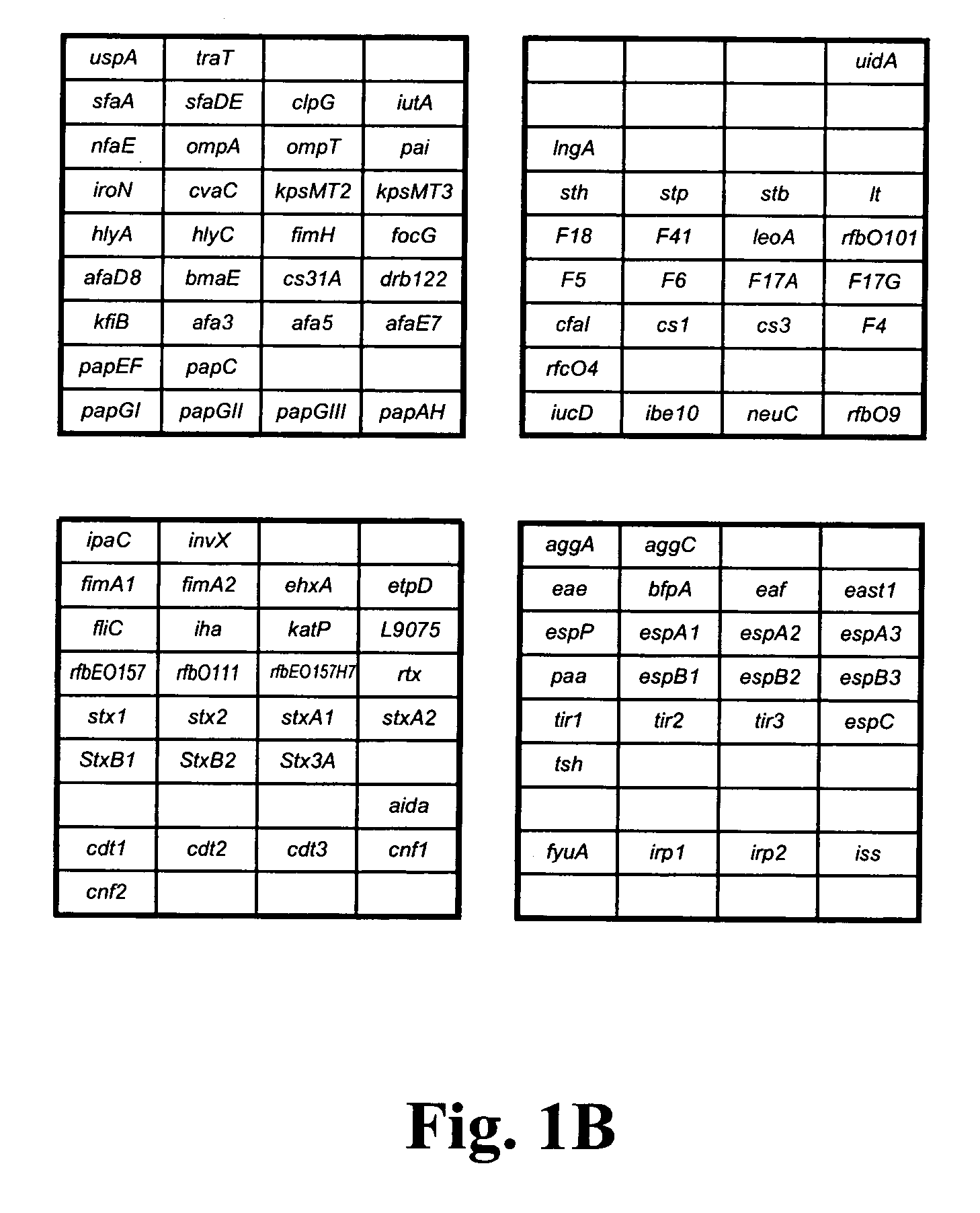
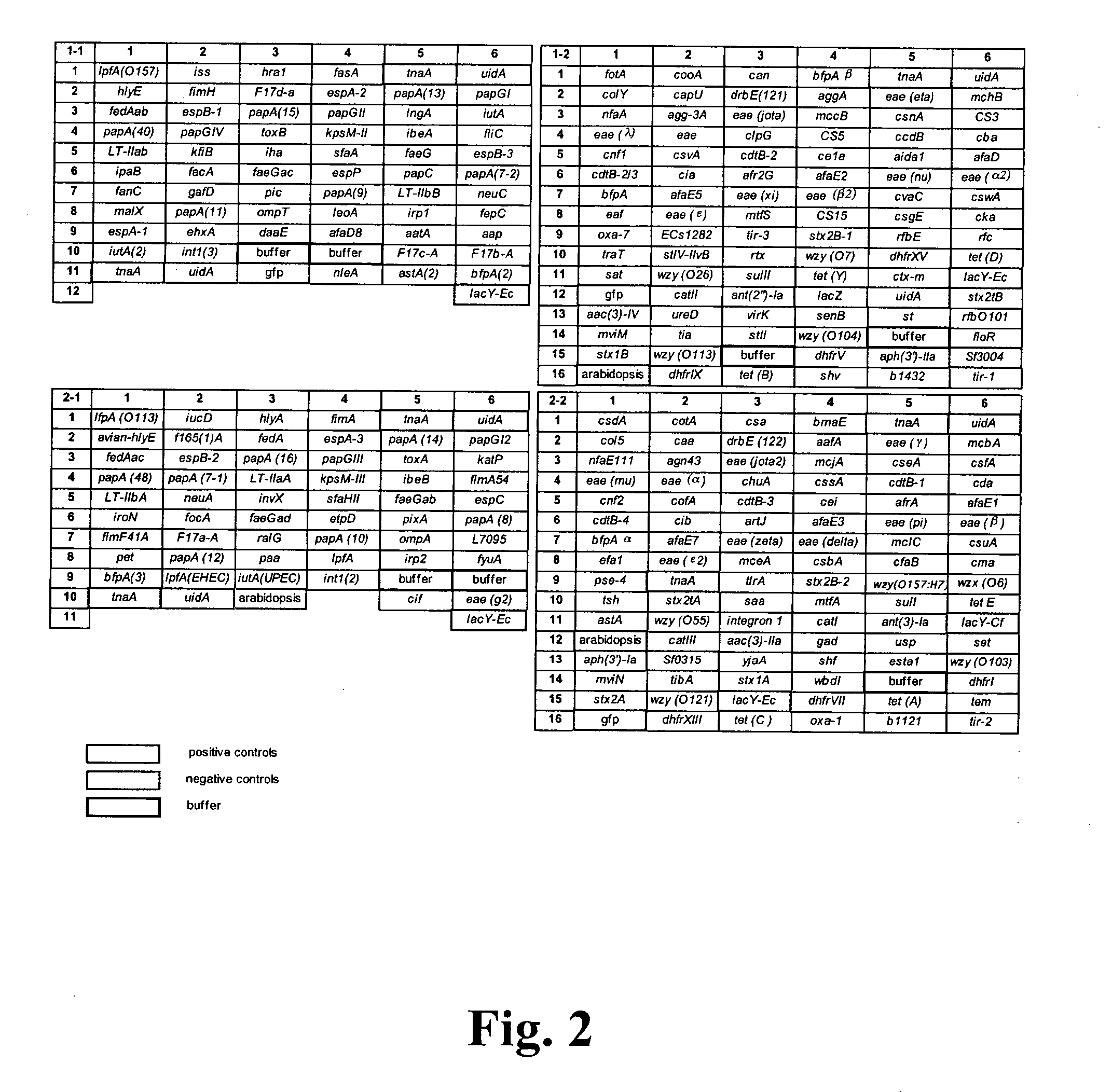
Virulence and antibiotic resistance array and uses thereof
InactiveUS20060094034A1Low costImprove reliabilityBioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismVirulent characteristics
An array of nucleic acid probes is described for simultaneously identifying or characterizing a pathotype of a microorganism and detecting antibiotic resistance of said microorganism. Methods are also described for detecting the presence of a microorganism in a sample, as well as determining its pathotype and its antibiotic resistance, using the array.
Owner:BROUSU ROLAND +4
Genetically modified tumor-targeted bacteria with reduced virulence
InactiveUS20030109026A1Improve securityReduce capacityBacteriaPeptide/protein ingredientsTumor targetVirulent characteristics
The present invention is directed to mutant Salmonella sp. having a genetically modified msbB gene in which the mutant Salmonella is capable of targeting solid tumors. The invention is also directed to Salmonella sp. containing a genetically modified msbB gene as well as an genetic modification in a biosynthetic pathway gene such as the purI gene. The present invention further relates to the therapeutic use of the mutant Salmonella for growth inhibition and / or reduction in volume of solid tumors.
Owner:VION PHARMA INC +1
Attenuated Pasteurella piscicida vaccine for fish
Live-attenuated vaccines against Edwardsiella ictaluri or against Pasteurella piscicida are disclosed. Both vaccines are incapable of reversion to virulence, because both are made by deletion mutations in the aroA gene, the purA gene, or both. These vaccines may be used not only to vaccinate fish against Edwardsiella ictaluri or Pasteurela piscicida, but also to serve as vectors to present antigens from other pathogens to the fish, thereby serving as vaccines against other pathogens as well, with no risk of infection by reversion to the virulent form of the pathogen in which the antigen occurs naturally.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
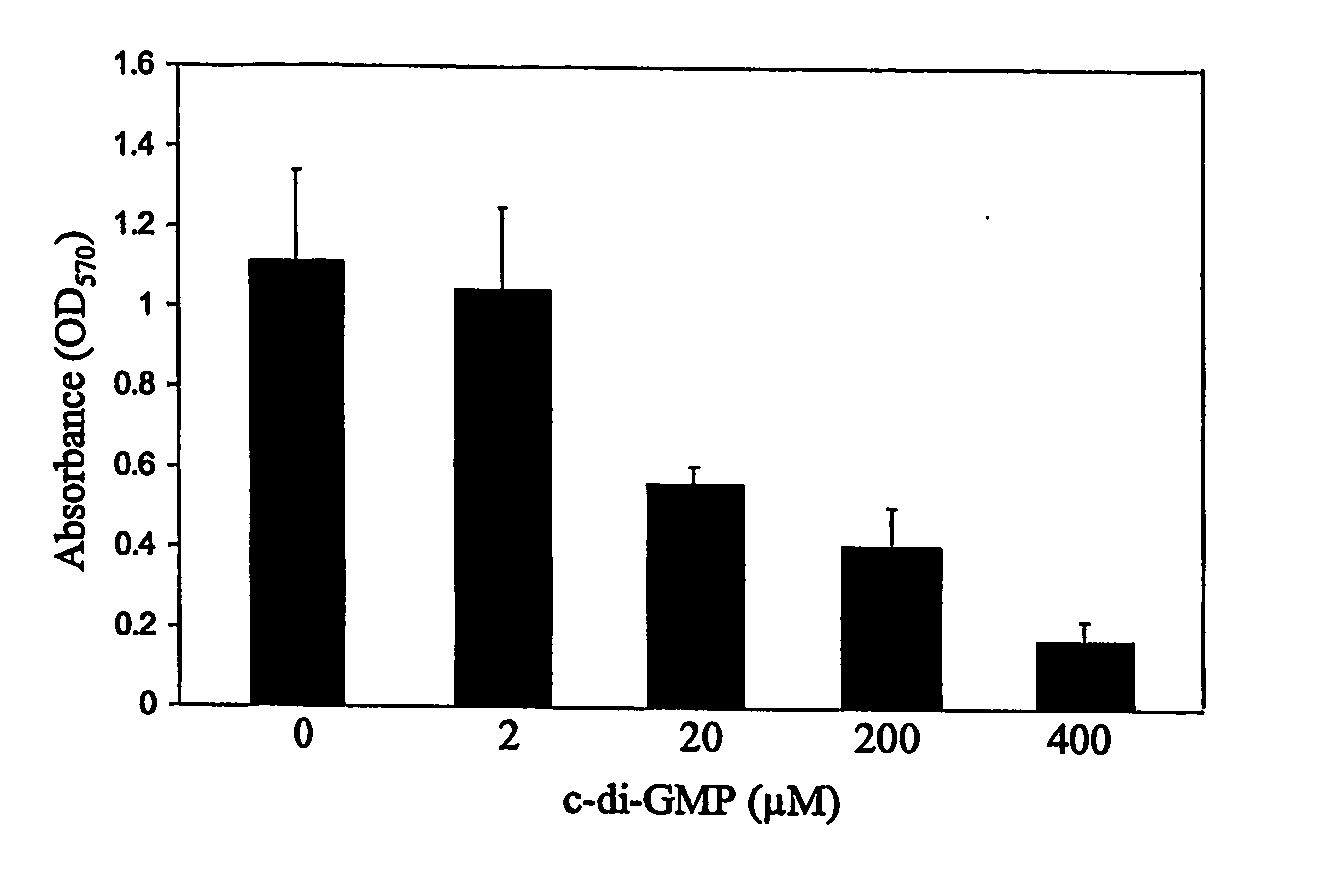
Method For Attentuating Virulence Of Microbial Pathogens And For Inhibiting Microbial Biofilm Formation
ActiveUS20070244059A1Reduced virulenceInhibiting and reducing colonizationAntibacterial agentsBiocideMicroorganismVirulent characteristics
The present invention relates to the use of the cyclic dinucleotide c-di-GMP and cyclic dinucleotide analogues thereof in a method for attenuating virulence of a microbial pathogen or for inhibiting or reducing colonization by a microbial pathogen. This method further inhibits microbial biofilm formation and is capable of treating bacterial infections. The microbial colonization or biofilm formation inhibited or reduced may be on the skin or on nasal or mucosal surface. The microbial colonization or biofilm formation inhibited can also be on the surfaces of medical devices, especially those in close contact with the patient, as well on the surfaces of industrial and construction material where microbial colonization and biofilm formation is of concern.
Owner:KARAOLIS DAVID K R
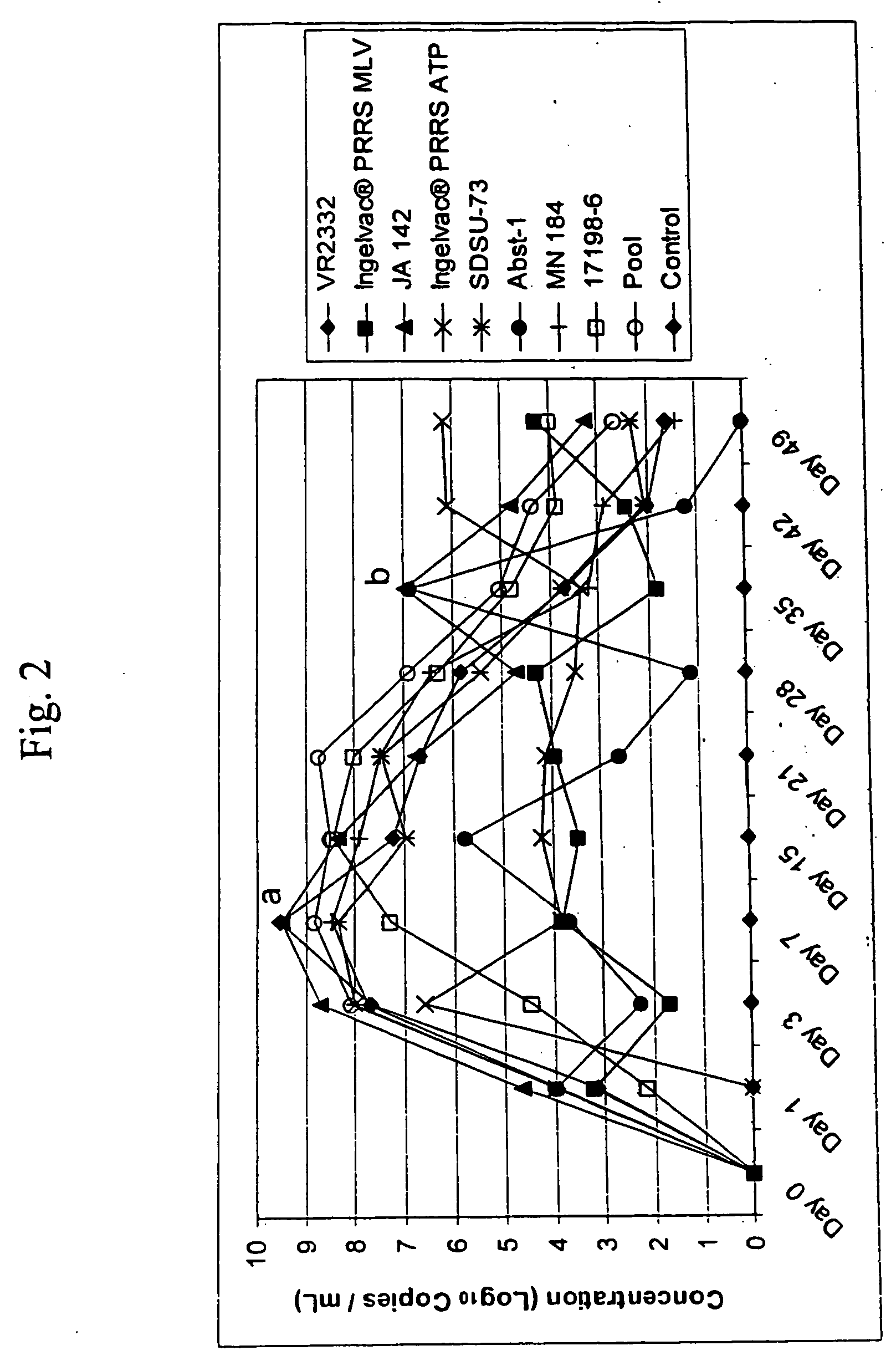
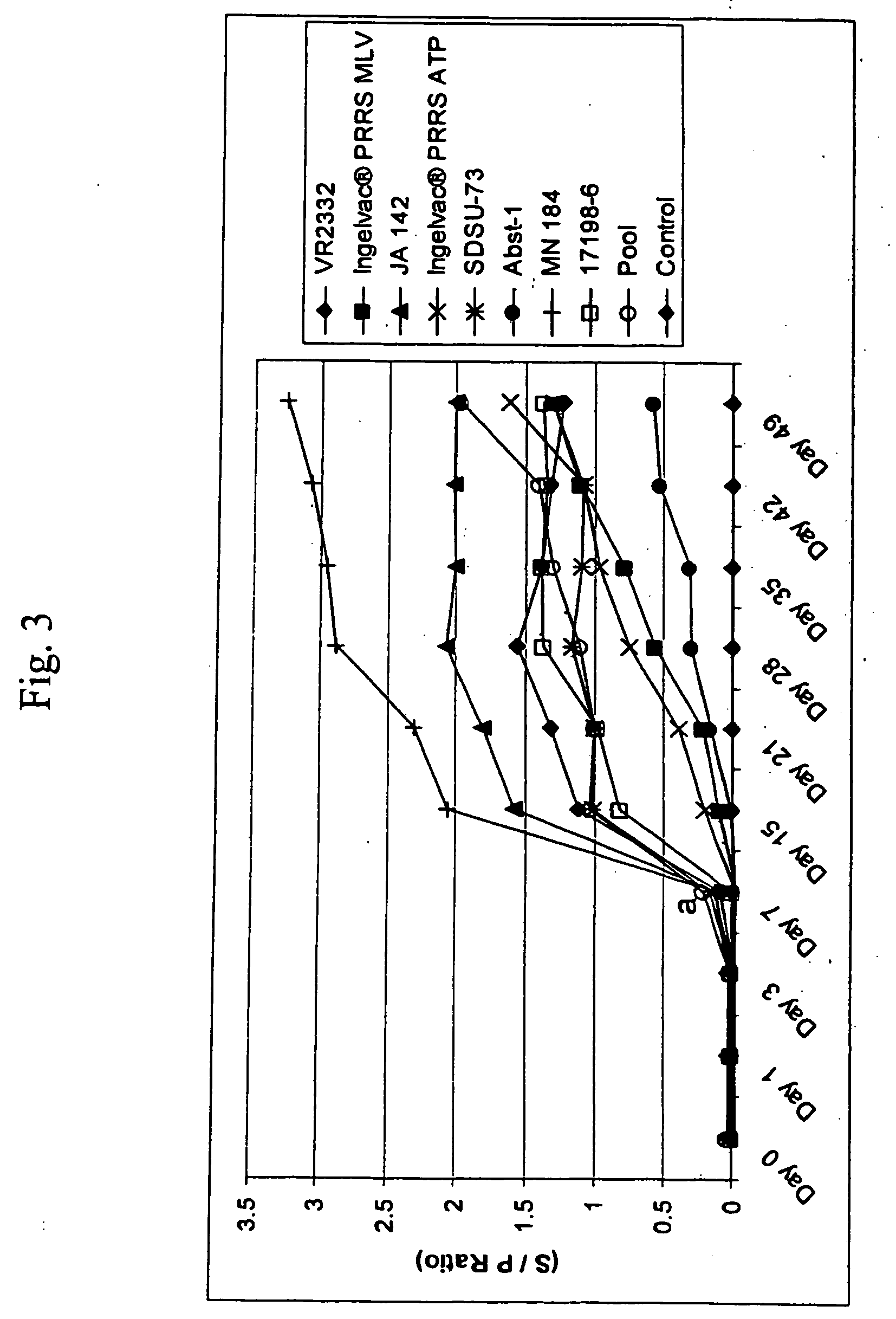
Porcine reproductive and respiratory syndrome isolates and methods of use
InactiveUS20060063151A1Improve immunityIncrease virulenceSsRNA viruses positive-senseMicrobiological testing/measurementVirulent characteristicsImmunogenicity
A method of predicting the virulence of a new or uncharacterized PRRS virus isolate is provided wherein the isolate is injected into swine and allowed to replicate for a period of from about 3-15 days. During this period, the rate of virus growth and / or the magnitude of viremia is determined, and this data is compared with a corresponding growth rate and / or viremia magnitude of a PRRS virus isolate of known virulence, as a measure of the virulence of the new or uncharacterized isolate. Additionally, a method of selecting an isolate for inclusion in an immunogenic composition based on the predicted virulence is also provided, together with compositions incorporating attenuated forms of viruses predicted to be virulent.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Gene detection kit for hereditary hearing loss
ActiveCN103352080AWide range of usesImprove detection success rateMicrobiological testing/measurementHybrid typeVirulent characteristics
The invention discloses a fluorescence detection kit capable of detecting 17 non-syndromic hereditary hearing loss susceptibility genes. The kit adopts 17 pairs of specific primers to conduct genetic typing on the hearing loss susceptibility genes, and 17 hotspot mutations in four most common Chinese hearing loss related genes can be detected at the same time in a single tube within 3 hours. The kit comprises primer combinations of 17 polymorphic sites of hereditary hearing loss on a GJB2 (CX26) gene, an SLC26A4 (PDS) gene, a GJB3 gene and a 12SrRNA (MTRNR1) gene, can be used for accurately judging the wild type, the pure mutant type or the hybrid type of the 17 sites, and achieves diagnosis and screening of the hearing loss genes. The kit provided by the invention can be applied to rapidly and efficiently detecting the hearing loss genes and is a rapid, convenient, economical and efficient screening kit for hearing loss virulence genes.
Owner:AGCU SCIENTECH +1
Identification of essential genes of Aspergillus fumigatus and methods of use
InactiveUS20030119013A1Antibacterial agentsOrganic active ingredientsVirulent characteristicsNucleotide
The present invention provides nucleotide sequences, methods and compositions that enable the experimental determination as to whether any gene in the genome of Aspegillus fumigatus is essential, and whether that gene is required for virulence or pathogenicity. The methods involve the construction of genetic mutants in which a target gene is placed under conditional expression. The identification of essential genes and those genes critical to the development of virulent infections, provides a basis for the development of screens for new drugs against Aspergillus fumigatus. The present invention further provides Aspergillum fumigatus genes that are essential and are potential targets for drug screening. The nucleotide sequence of the target genes can be used for various drug discovery purposes, such as expression of the recombinant protein, hybridization assay and construction of nucleic acid arrays. The uses of proteins encoded by the essential genes, and genetically engineered cells comprising modified alleles of essential genes in various screening methods are also encompassed by the invention.
Owner:MERCK & CO INC
Genetically modified tumor-targeted bacteria with reduced virulence
InactiveUS20020026655A1Improve securityReduce capacityBacteriaPeptide/protein ingredientsTumor targetVirulent characteristics
The present invention is directed to mutant Salmonella sp. having a genetically modified msbB gene in which the mutant Salmonella is capable of targeting solid tumors. The invention is also directed to Salmonella sp. containing a genetically modified msbB gene as well as an genetic modification in a biosynthetic pathway gene such as the purl gene. The present invention further relates to the therapeutic use of the mutant Salmonella for growth inhibition and / or reduction in volume of solid tumors.
Owner:YALE UNIV +1
HEK (human embryonic kidney) 293 cell line applicable to serum-free culture and application thereof
InactiveCN102604889AMicroorganism based processesViruses/bacteriophagesHEK 293 cellsVirulent characteristics
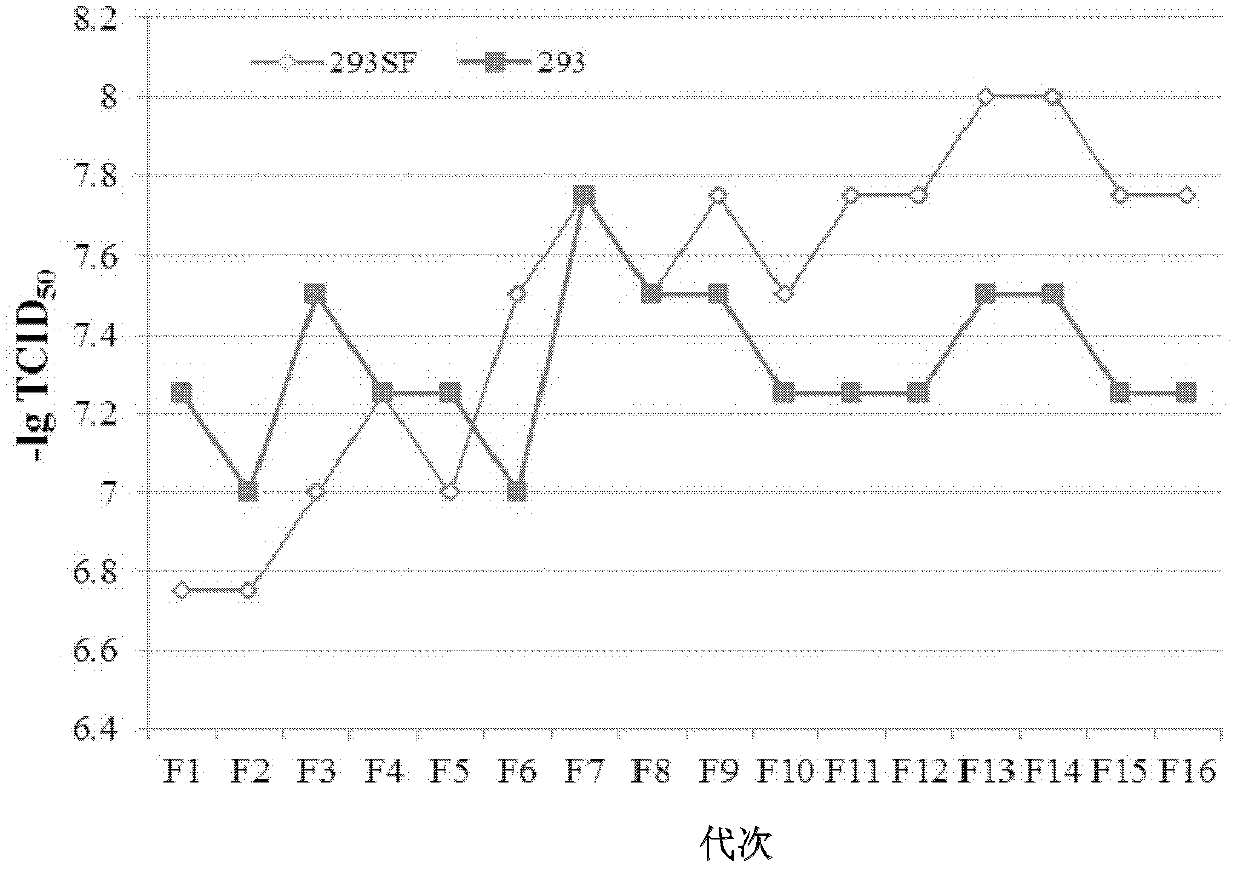
The invention discloses an HEK (human embryonic kidney) 293 cell line applicable to serum-free culture and an application thereof. According to the invention, the HEK 293 cell (293SF) applicable to serum-free culture is obtained by adopting a culture solution progressive substitution method, wherein the preservation number of the HEK 293 cell is CGMCC (China General Microbiological Culture Collection Center) No.5824. Detection shows that the 293SF cell has stable adenovirus proliferation capacity and foreign protein expressing ability; average lesion time is 97 hours and is reduced by 12.9% compared with the HEK 293 cell; virus virulence reaches up to 107.48TCID50 / mL and is improved by 43.3% compared with the HEK293 cell; and stability is good while generation number is improved, and the state and susceptibility of the 293SF cell are not changed after the 293SF cell is continuous passage is carried out for sixteen times. The cell line disclosed by the invention can be used for serum-free production of an adenovirus vector vaccine.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Altered strain of the modified vaccinia virus ankara (MVA)
InactiveUS6682743B2Ease of mass productionEfficient and large-scale productionBiocideGenetic material ingredientsVector systemVirulent characteristics
The invention provides new strains of the Modified Vaccinia Virus Ankara (MVA) that have a strongly reduced virulence for most mammals, especially humans, but nevertheless grows in cells of a continuous cell line approved for the production of a therapeutic agent such as a vaccine. The invention also provides a method for producing said adapted MVA strains. The adapted MVA can be used e.g. for parenteral immunization, as a vector system, or in the active or inactivated from as an adjuvant or as a regulator of the unspecific components of the immune system.
Owner:BAVARIAN NORDIC AS
Gene chip for screening various ophthalmological hereditary diseases as well as preparation and usage method of gene chip
InactiveCN102978284AImprove efficiencyAccurate diagnosisNucleotide librariesMicrobiological testing/measurementVirulent characteristicsIntein
The invention relates to the technical field of biological gene chips, and particularly relates to a gene chip for screening various ophthalmological hereditary diseases as well as preparation and a usage method of the gene chip. Specific oligonucleotide probes of gene sequences which are related to 261 ophthalmological hereditary diseases are fixed on the surface of a carrier of the gene chip for screening various ophthalmological hereditary diseases; and the gene chip which is related to the 261 ophthalmological hereditary diseases comprises the sequences of all coding region sequences of 954 related virulence genes or disease predisposing genes and introne sequences adjacent to the coding regions. As the specific oligonucleotide probes of the gene sequences which are related to 261 ophthalmological hereditary diseases and the sequences of all the coding region sequences of the related virulence genes or disease predisposing genes and the introne sequences adjacent to the coding regions are fixed on the surface of the carrier of the gene chip, the gene chip provided by the invention is capable of screening a plurality of ophthalmological hereditary diseases at the same time and then the efficiency is greatly improved.
Owner:金子兵
Identification of essential genes of cryptococcus neoformans and methods of use
InactiveUS20040014955A1Simple and reasonable designSugar derivativesMicrobiological testing/measurementBiotechnologyVirulent characteristics
The present invention provides C. neoformans genes that are essential and are potential targets for drug screening. The nucleotide sequence of the target genes can be used for various drug discovery purposes, such as expression of the recombinant protein, hybridization assay and construction of nucleic acid arrays. The uses of proteins encoded by the essential genes, and genetically engineered cells comprising modified alleles of essential genes in various screening methods are also encompassed by the invention. The present invention also provides methods and compositions that enable the experimental determination as to whether any gene in the genome of Cryptococcus neoformans is essential, and whether that gene is required for virulence or pathogenicity. The identification of essential genes and those genes critical to the development of virulent infections, provides a basis for the development of screens for new drugs against C. neoformans.
Owner:MERCK & CO INC
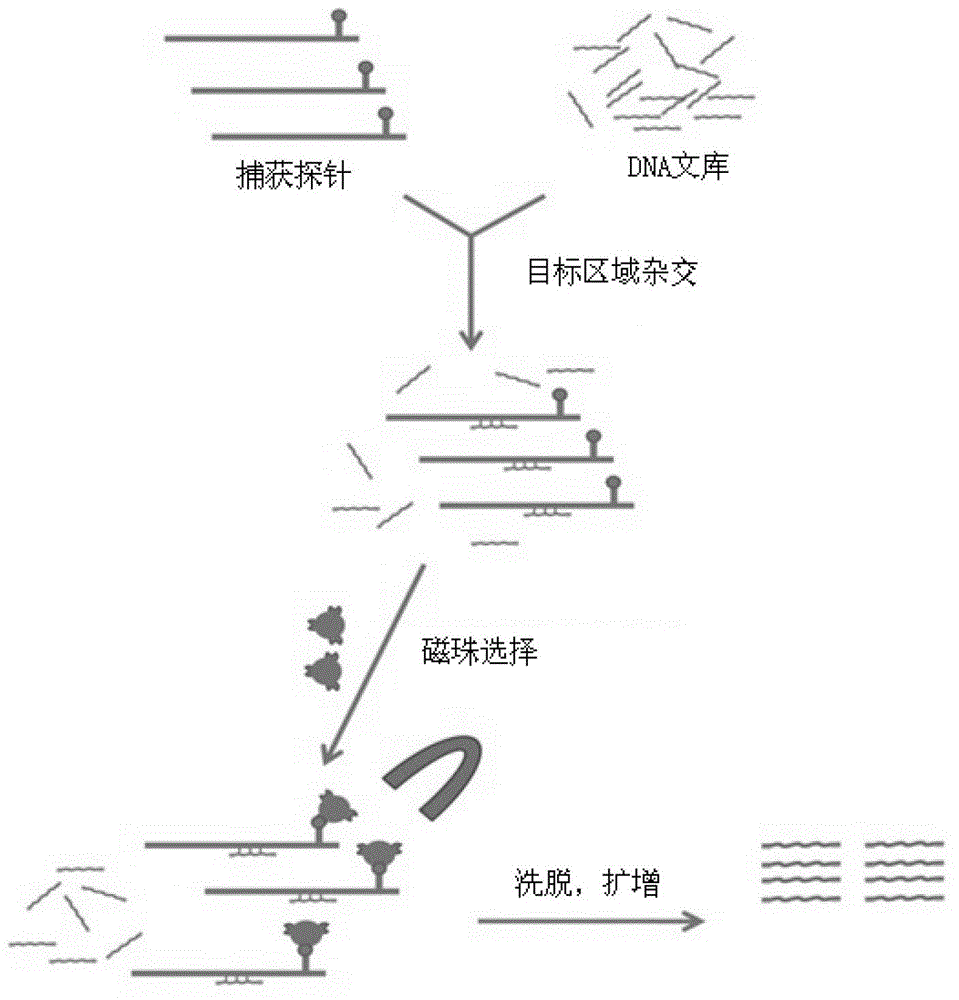
Rapid analysis method and system for genomes of pathogenic microorganisms
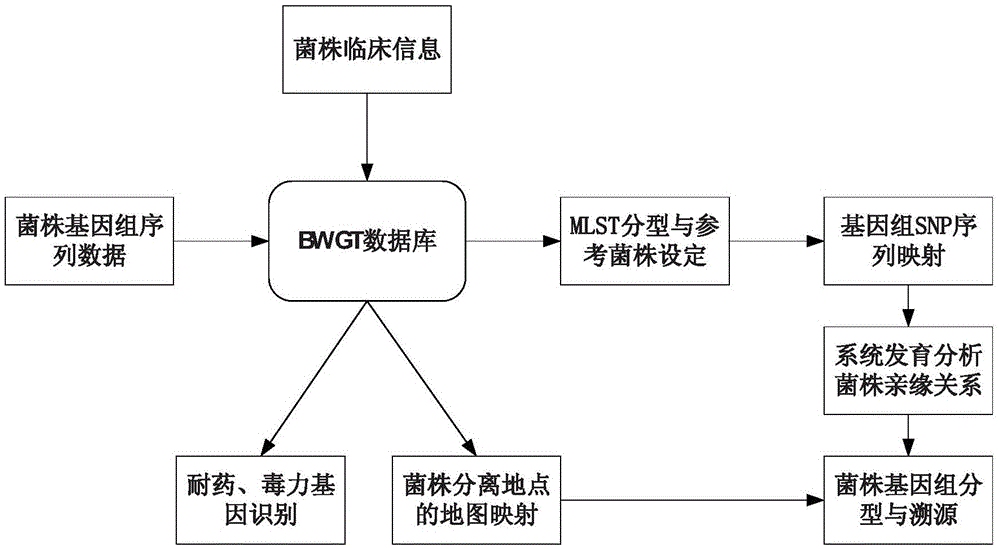
ActiveCN106886689AHigh resolutionAccurate distinctionSequence analysisSpecial data processing applicationsGenomicsVirulent characteristics
The invention discloses a rapid analysis method for genomes of pathogenic microorganisms and a data analysis platform. The method provides typing and source-tracing functions of bacterial genome sequences based on a bacterial strain database system. A user can only set simple parameters and upload bacterial strain genome sequences. The system can feed back the MLST parting result of bacterial strains, medicine resistance and distribution of virulence genes within the shortest time and make reference to names of bacterial strains and provide phylogenetic trees of all bacterial strains which are uploaded by a user and arranged in a database. The method is suitable for the establishment of a data analysis platform for pathogeny microbiology which integrates study of genetics, genomics and phylogenetics. Compared with the conventional typing technology, the rapid analysis method and system for genomes of pathogenic microorganisms have the following beneficial effects: higher resolution is obtained; bacterial strains of the same clone can be accurately detected; the feedback speed of results is high; and the technology of efficient reference strain setting and genome SNP sequence mapping, data analysis results can be quickly obtained by a user, thereby facilitating real-time tracking and fast source-tracing of clonal spreads in pathogenic microorganisms.
Owner:ZHEJIANG UNIV
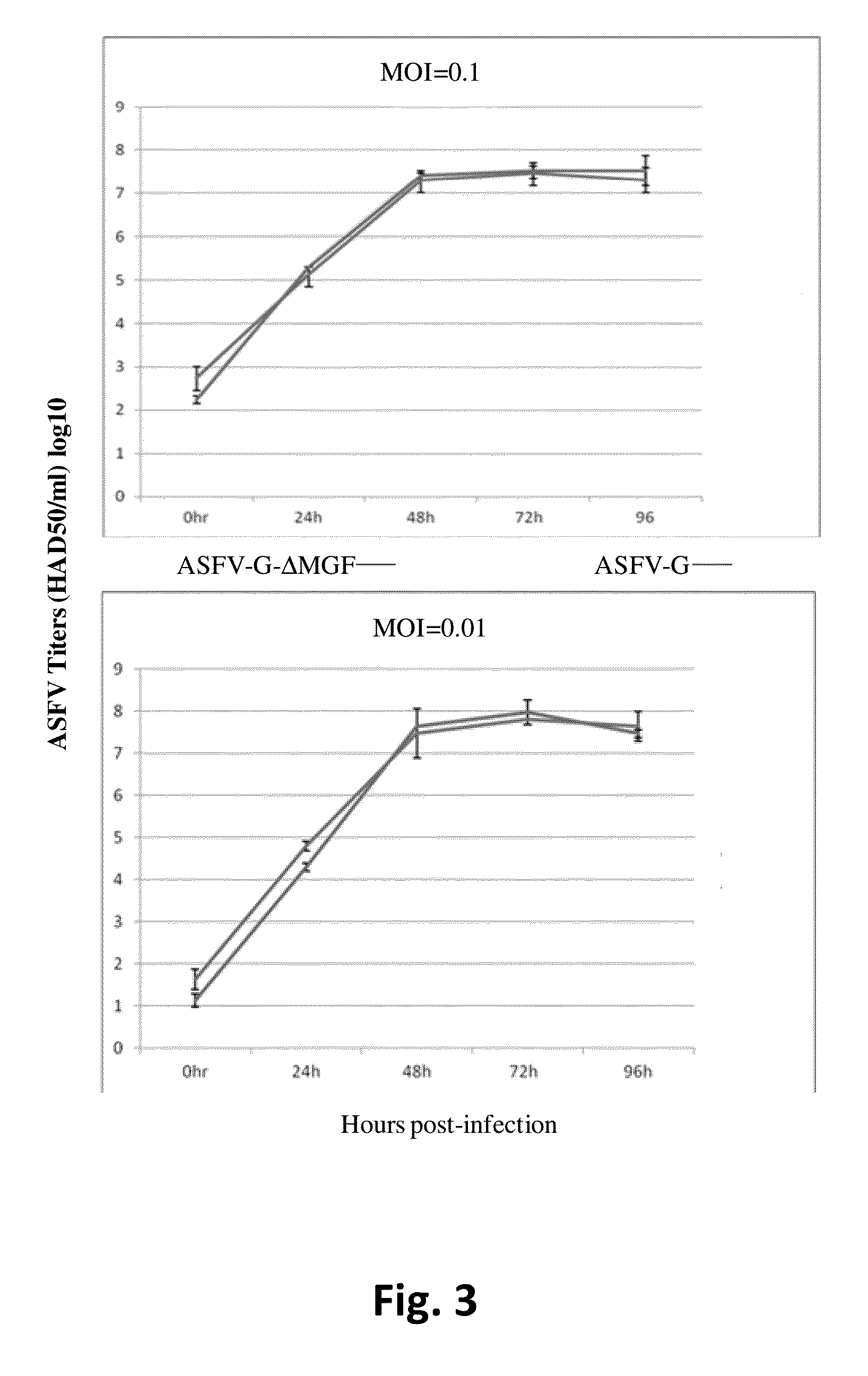
Attenuated African Swine Fever Virus Vaccine Based in the Deletion of MGF Genes
ActiveUS20160130562A1Viral antigen ingredientsMicrobiological testing/measurementVirulent characteristicsDomestic pig
African swine fever virus (ASFV) is the etiological agent of a contagious and often lethal viral disease of domestic pigs. Control of ASF has been hampered by the unavailability of vaccines. Experimental vaccines have been derived from naturally occurring, cell culture-adapted, or genetically modified live attenuated ASFVs; however, these vaccines are only successful when protecting against homologous viruses. Among viral genes reported to be involved in virulence are components of the multi gene family (MGF). Here we report the construction of a recombinant ΔMGF virus derived from the highly virulent ASFV Georgia 2007 (ASFV-G) isolate. In vivo, ASFV-G ΔMGF administered intramuscularly (IM) to swine at either 102 or 104 HAD50 are completely attenuated; the inoculated animals are completely asymptomatic. Animals infected with 102 or 104 HAD50 of ASFV-G ΔMGF are protected against the presentation of clinical disease when challenged at 28 days post infection with the virulent parental strain Georgia 2007.
Owner:US SEC AGRI +1
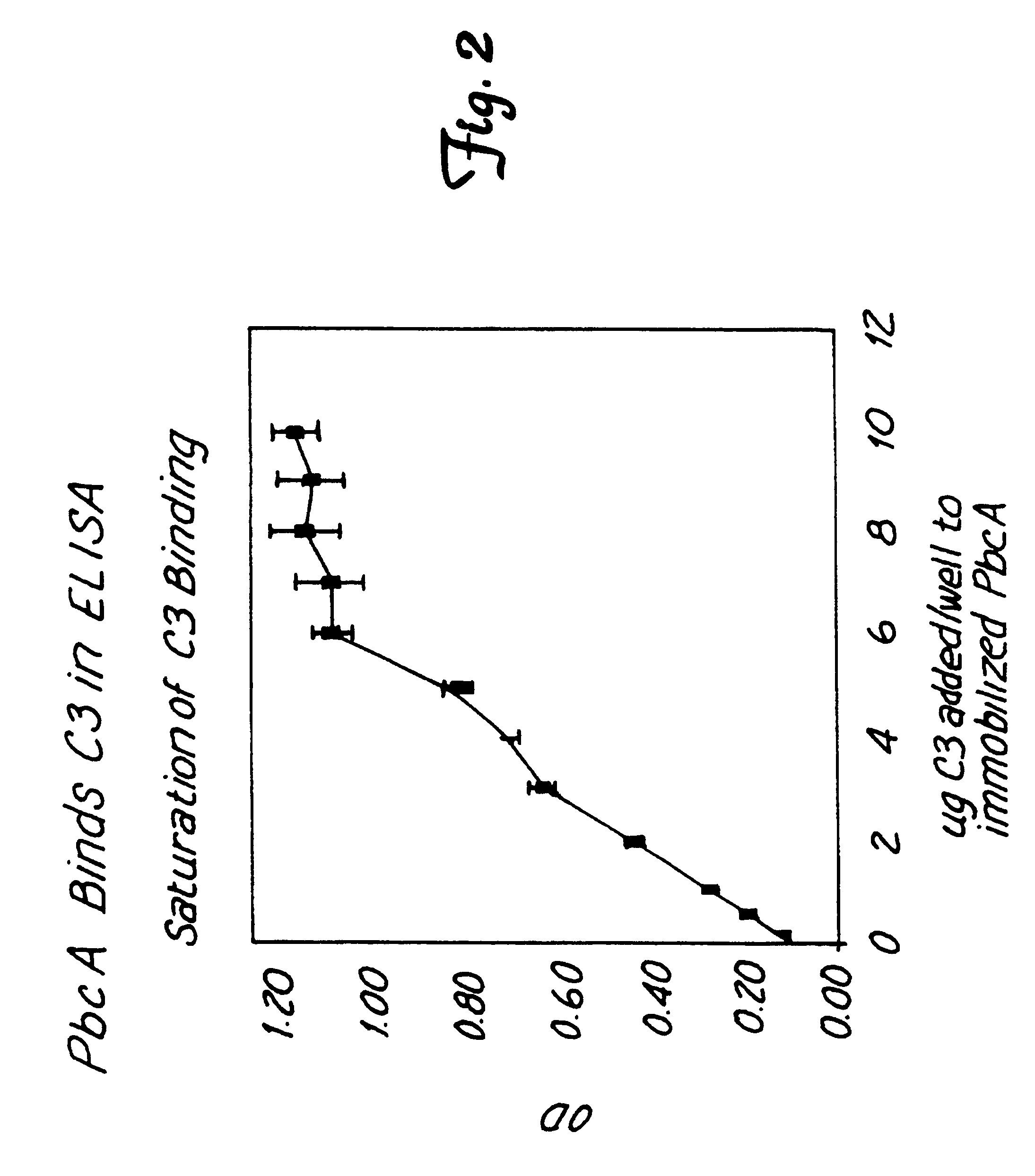
Method for isolating a C3 binding protein of streptococcus pneumoniae
This invention relates to the identification of a human complement C3 binding protein from Streptococcus pneumoniae and to its sequence and to methods for its purification and use. The protein binds but does not degrade or cleave C3 and is implicated in S. pneumoniae virulence. The protein is recognized by antibodies produced by humans recovering from pneumococcal infection.
Owner:RGT UNIV OF MINNESOTA
Nontypeable Haemophilus influenzae virulence factors
InactiveUS7306805B2Translation is prevented and reducedAvoid stickingAntibacterial agentsSenses disorderVirulent characteristicsOperon
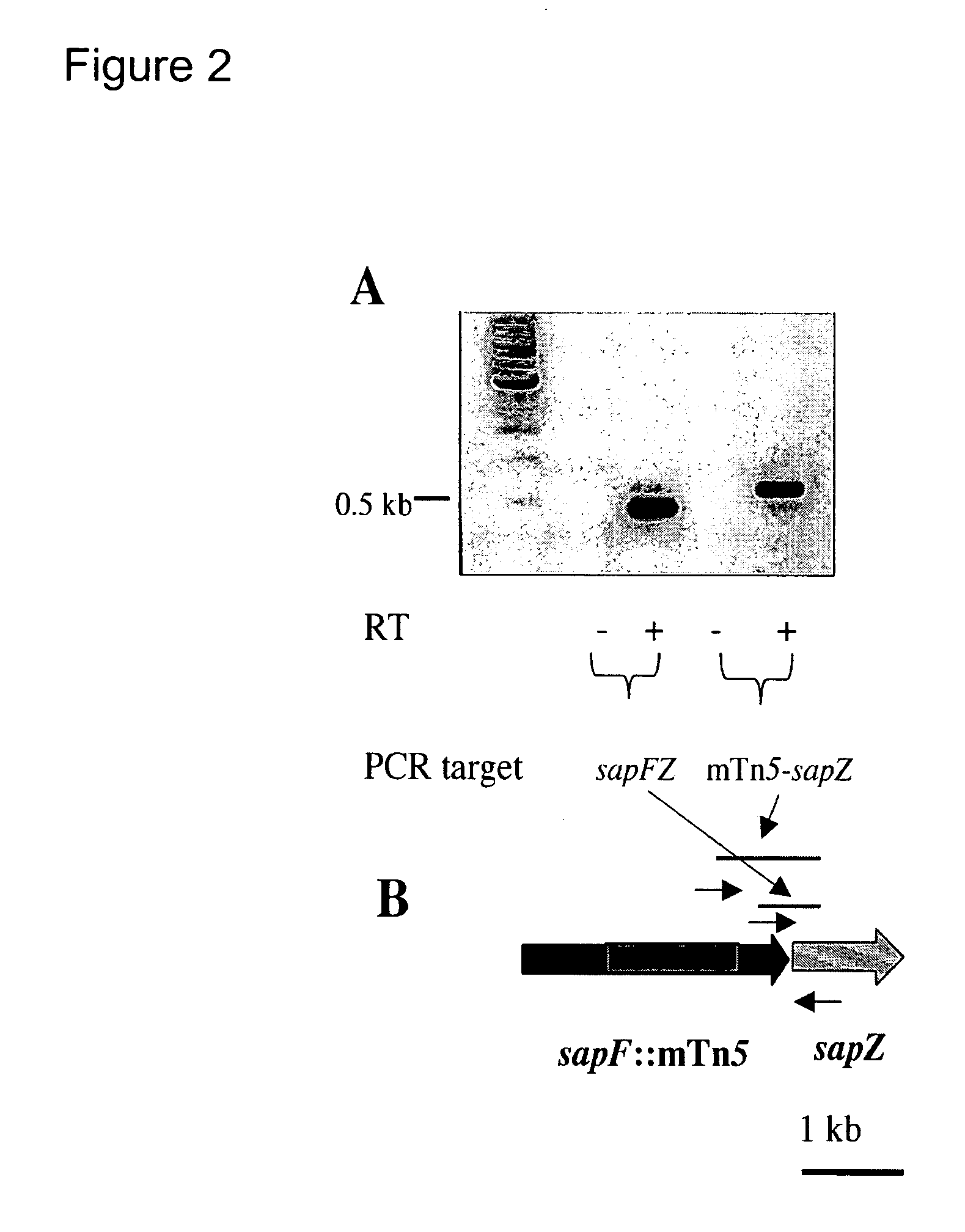
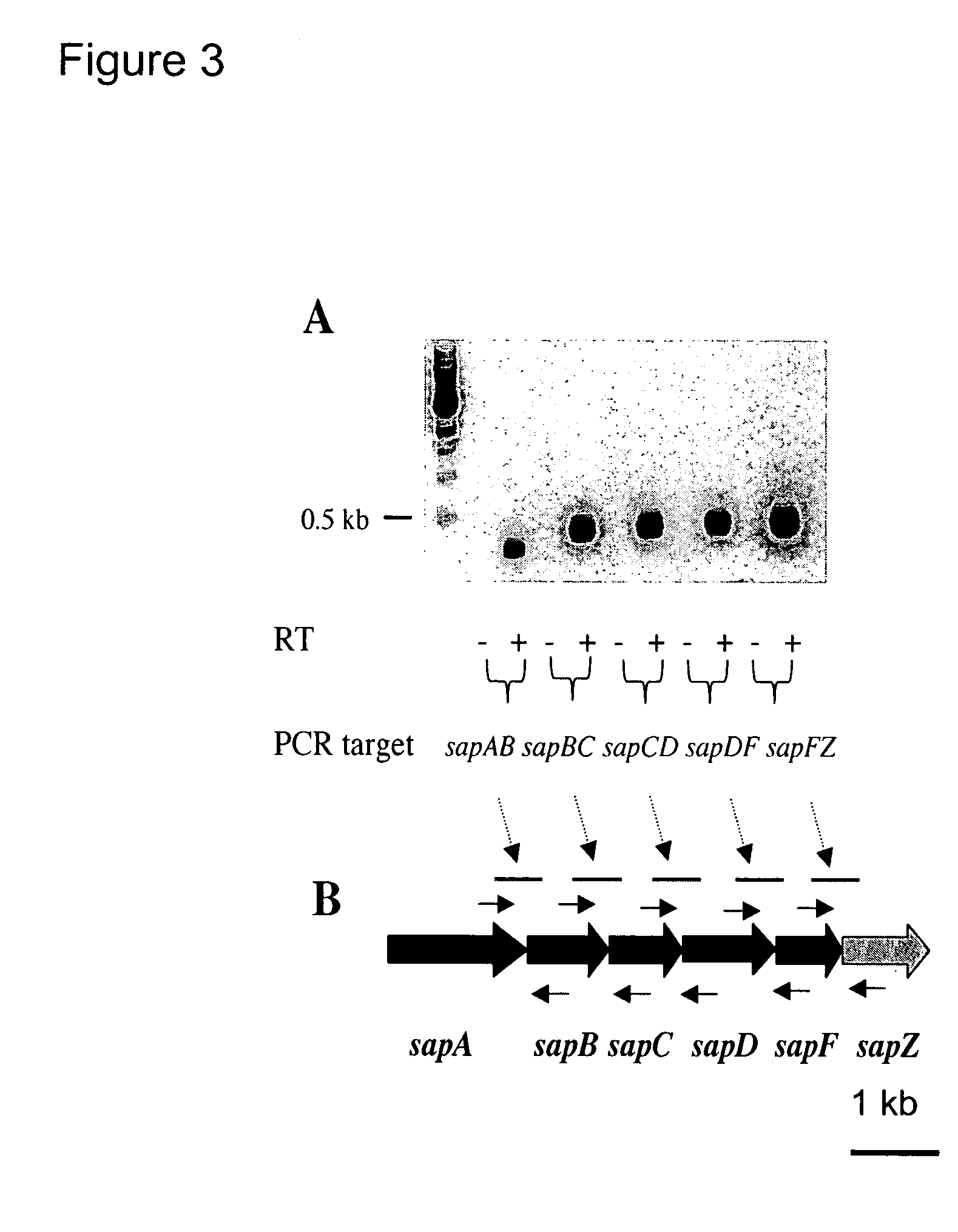
The invention relates to a mutation within the sap operon of an avirulent clone of a nontypeable strain of Haemophilus influenzae (NTHi). The invention also relates to the NTHi sap operon genes and the polypeptides encoded by these polynucleotide sequences. The invention also relates to a novel 110 kDa NTHi outer membrane protein and the polynucleotide that encodes this outer membrane protein. Methods of screening for NTHi infection, and treating and preventing NTHi related disorders are also contemplated.
Owner:NATIONWIDE CHILDRENS HOSPITAL
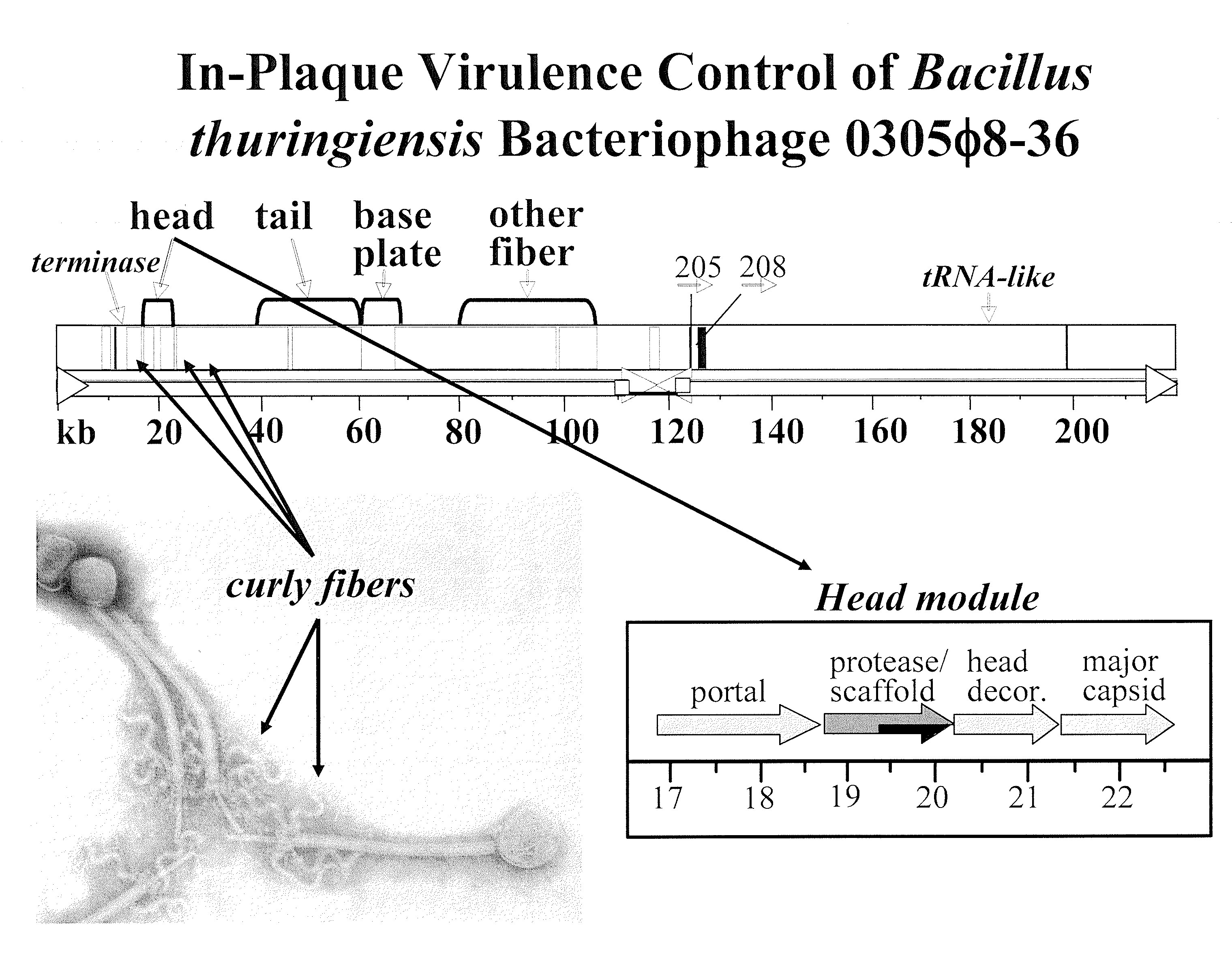
Methods and compositions involving bacteriophage isolates
InactiveUS20090081173A1Easy to detectIncreasing phage lytic potentialBiocideBacteria material medical ingredientsBiotechnologyVirulent characteristics
Disclosed are methods of increasing the virulence of a bacteriophage, comprising contacting a bacteriophage with a composition comprising a bacterium or bacterial extract; and a polymer. Also disclosed are methods of propagating and isolating therapeutic bacteriophages that involve use of dilute polymer compositions. Also disclosed are pharmaceutical compositions the bacteriophage for which virulence has been increased by the methods set forth herein or which have been isolated by the methods set forth herein.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Compounds and methods for modulating communication and virulence in quorum sensing bacteria
The present invention provides compositions and methods for modulating the communication and virulence of quorum sensing bacteria. In various exemplary embodiments, the invention provides a combinatorial library of quorum sensing compounds including synthetic analogs of naturally occurring and non-naturally occurring acyl-homoserine lactone (AHL) analogs, and methods of synthesizing and using these compounds.
Owner:WISCONSIN ALUMNI RES FOUND
Chip for gene detection of multiple vibrios at the same time, and detection and use thereof
ActiveCN101475986AEffective guidanceEffectively guide productionMicrobiological testing/measurementMicroorganism based processesForward primerVirulent characteristics
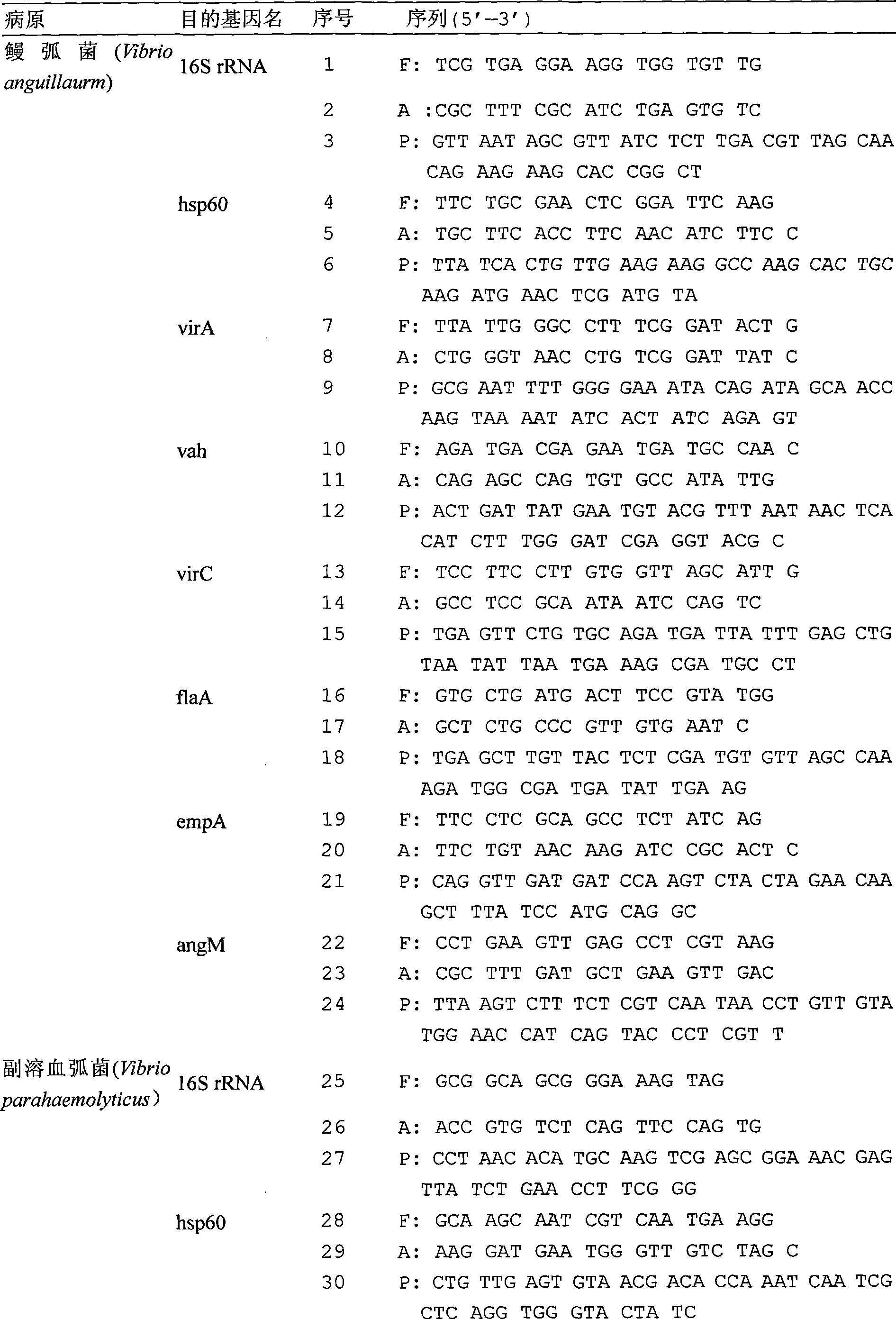
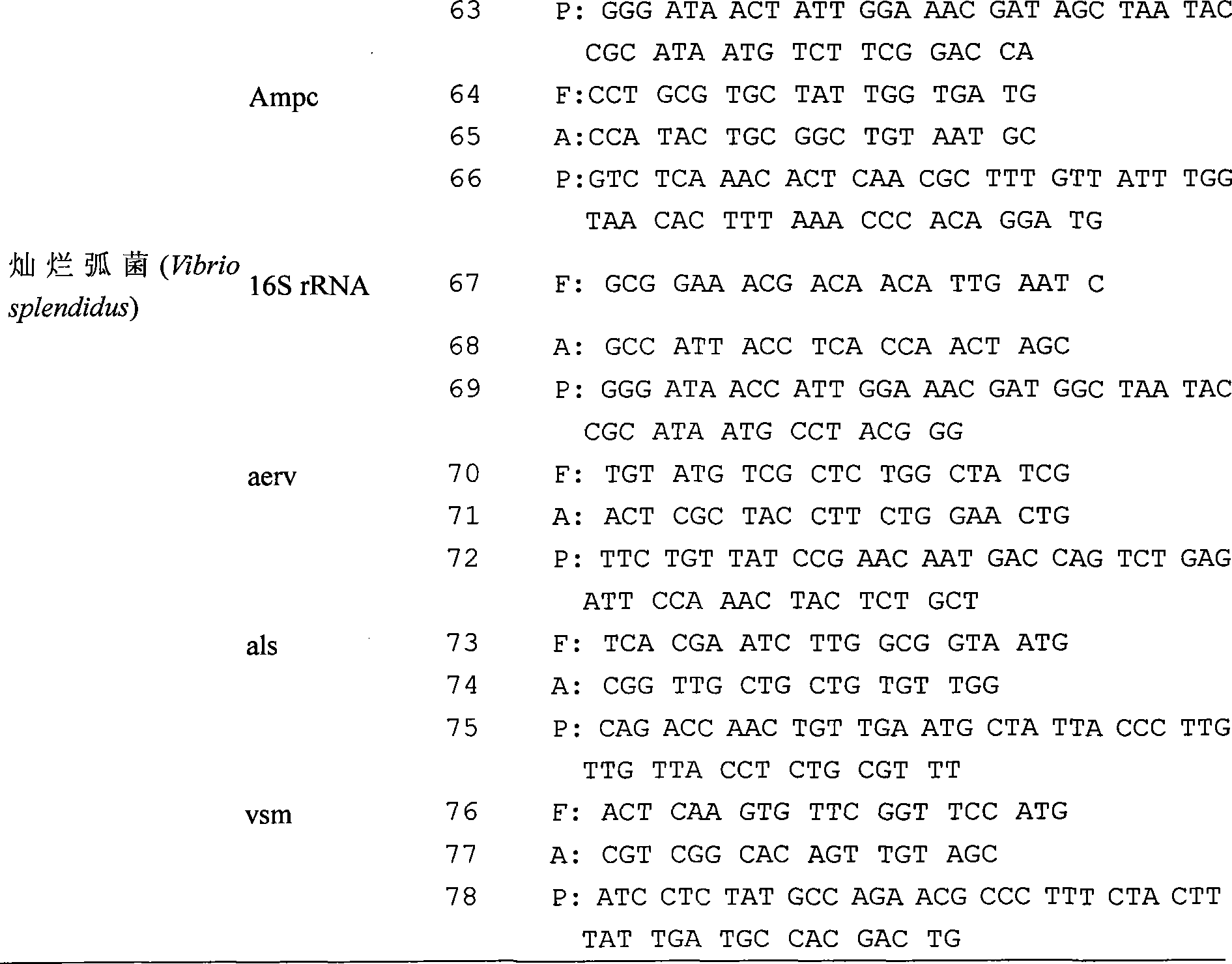
The present invention relates to a detection chip for performing gene detection to various vibrio and its detection and applications. The invention provides 16S rRNA sequences corresponding to each vibrio of vibrio anguillarum, vibrio harveyi, vibrio alginolyticus, vibrio parahaemolyticus, brilliant vibrio and Fisher vibrio; heat shock protein hsp60 probe sequence; virulence gene probe sequence; 16S rRNA forward primer sequence; 16S rRNA reverse primer sequence; heat shock protein hsp60 forward primer sequence; heat shock protein hsp60 reverse primer sequence; virulence gene forward primer sequence and virulence gene reverse primer sequence. The present invention has specific, sensitive and high-throughput features, can simultaneously detect six kinds of bacteria virulence genes, and the invention will effectively guide the production as an important disease early-warning detection method used in clinical diagnosis of aquatic animals.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Fluorescent quantitative PCR kit for detecting copy number of virulence gene of human spinal muscular atrophy
InactiveCN108048548AAvoid interferenceEliminate interfering fluorescent signalsMicrobiological testing/measurementDNA/RNA fragmentationReference genesVirulent characteristics
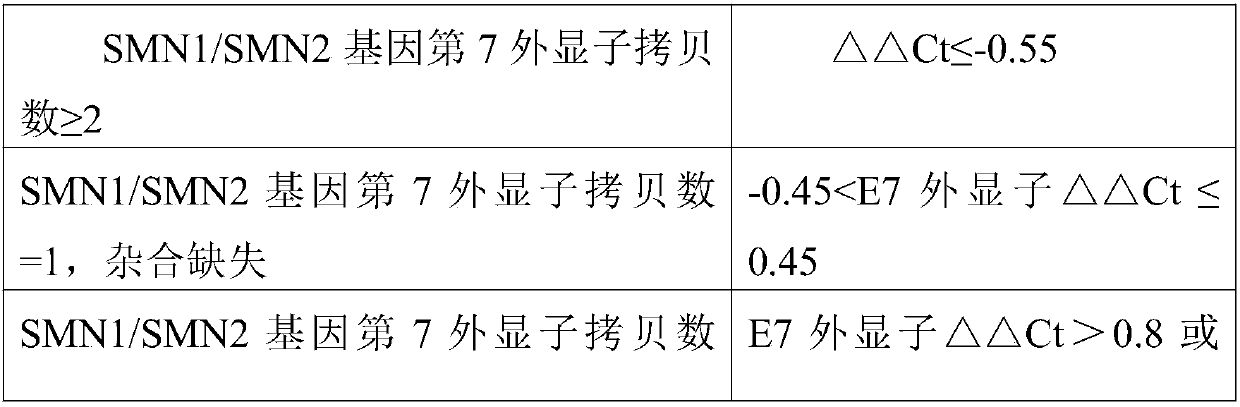
The invention discloses a fluorescent quantitative PCR kit for detecting the copy number of a virulence gene of human spinal muscular atrophy. The fluorescent quantitative PCR kit comprises amplimersand fluorescent probes, wherein the amplimers consist of a pair of shared primers for specific amplification of the seventh exon of SMN1 and SMN2 genes, a pair of shared primers for specific amplification of the eighth exon of the SMN1 and SMN2 genes, and a pair of specific primers for specific amplification of a reference gene, i.e., a CFTR gene; and the fluorescent probes consist of a fluorescent probe for specific detection of the seventh exon of the SMN1 gene, a fluorescent probe for specific detection of the eighth exon of the SMN1 gene, a fluorescent probe for specific detection of the seventh exon of the SMN2 gene, a fluorescent probe for specific detection of the eighth exon of the SMN2 gene and a fluorescent probe for specific detection of the reference gene CFTR. The fluorescentquantitative PCR kit for detecting the copy number of the virulence gene of human spinal muscular atrophy is directed at problems and insufficiencies in quantitative detection of the copy numbers of human motoneuron genes and is rapid and simple in detection and reliable in detection results.
Owner:北京华瑞康源生物科技发展有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com