Genetically modified tumor-targeted bacteria with reduced virulence
a tumor-targeted, genetically modified technology, applied in the field of salmonella isolation, can solve the problems of toxic consequences for the host, wide-spread infection in the cancer patient, and the inability to directly kill bacteria, so as to improve the safety of tumor-targeted bacteria, reduce the ability to kill, and improve the effect of therapeutic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
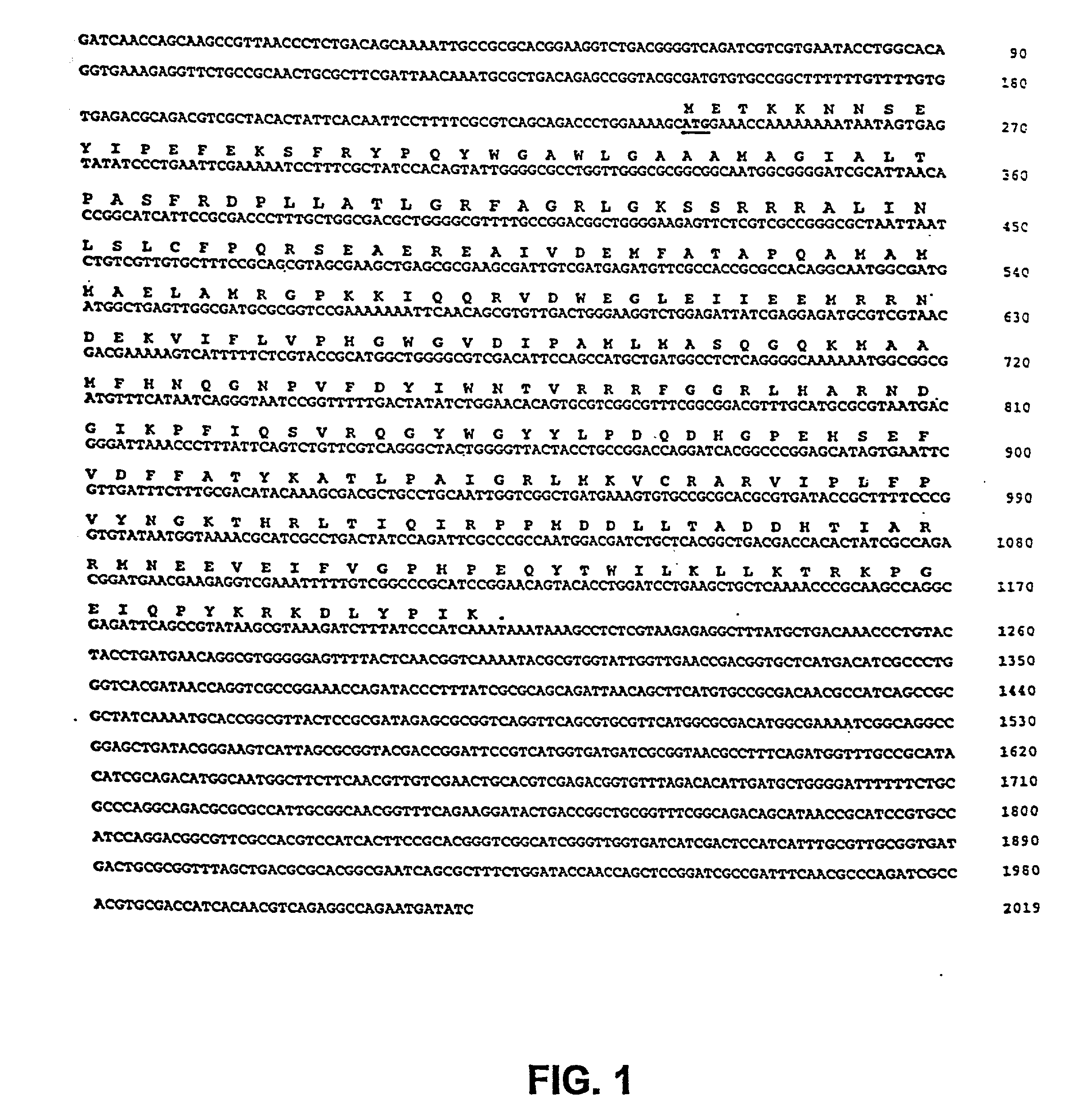
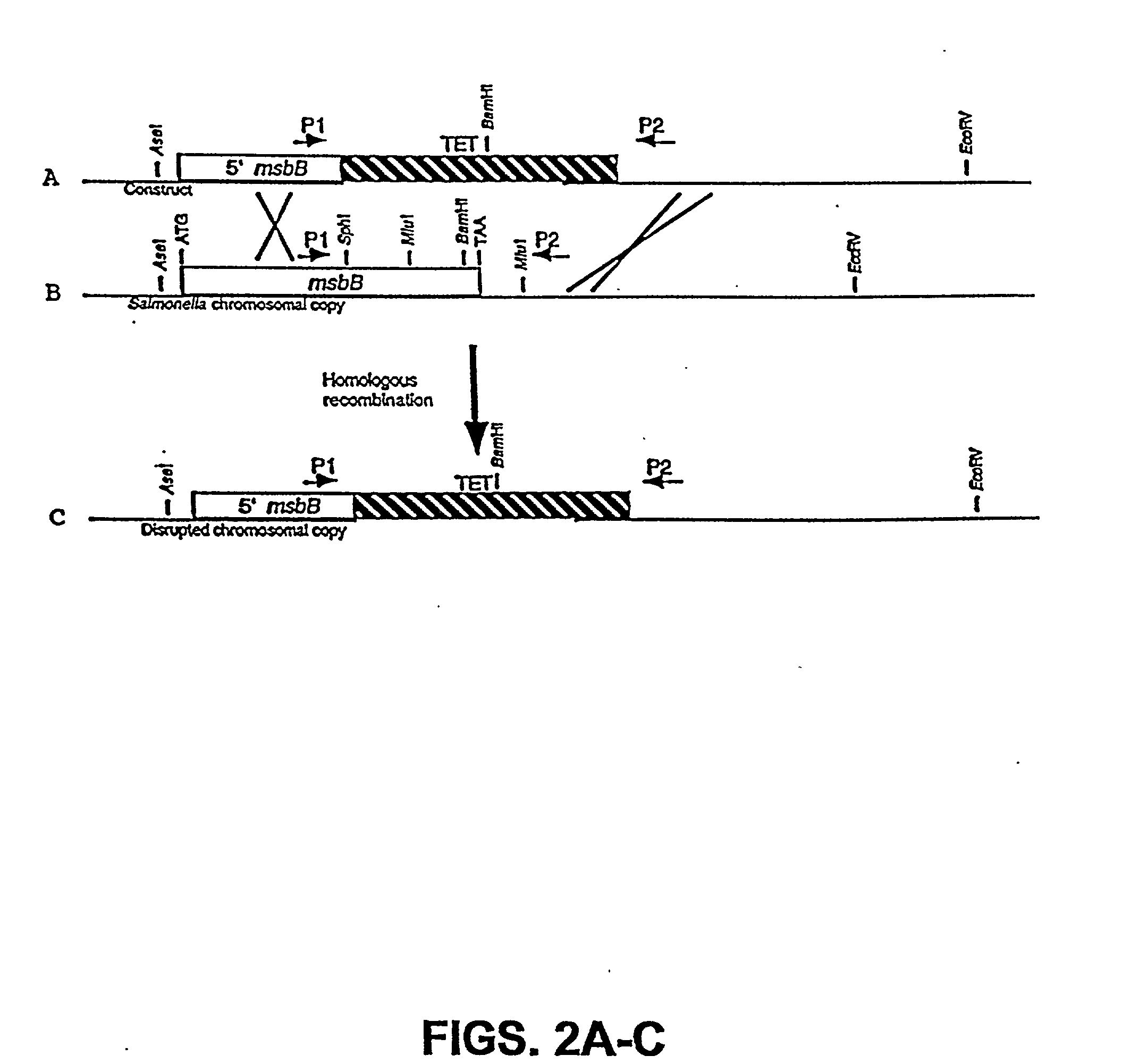
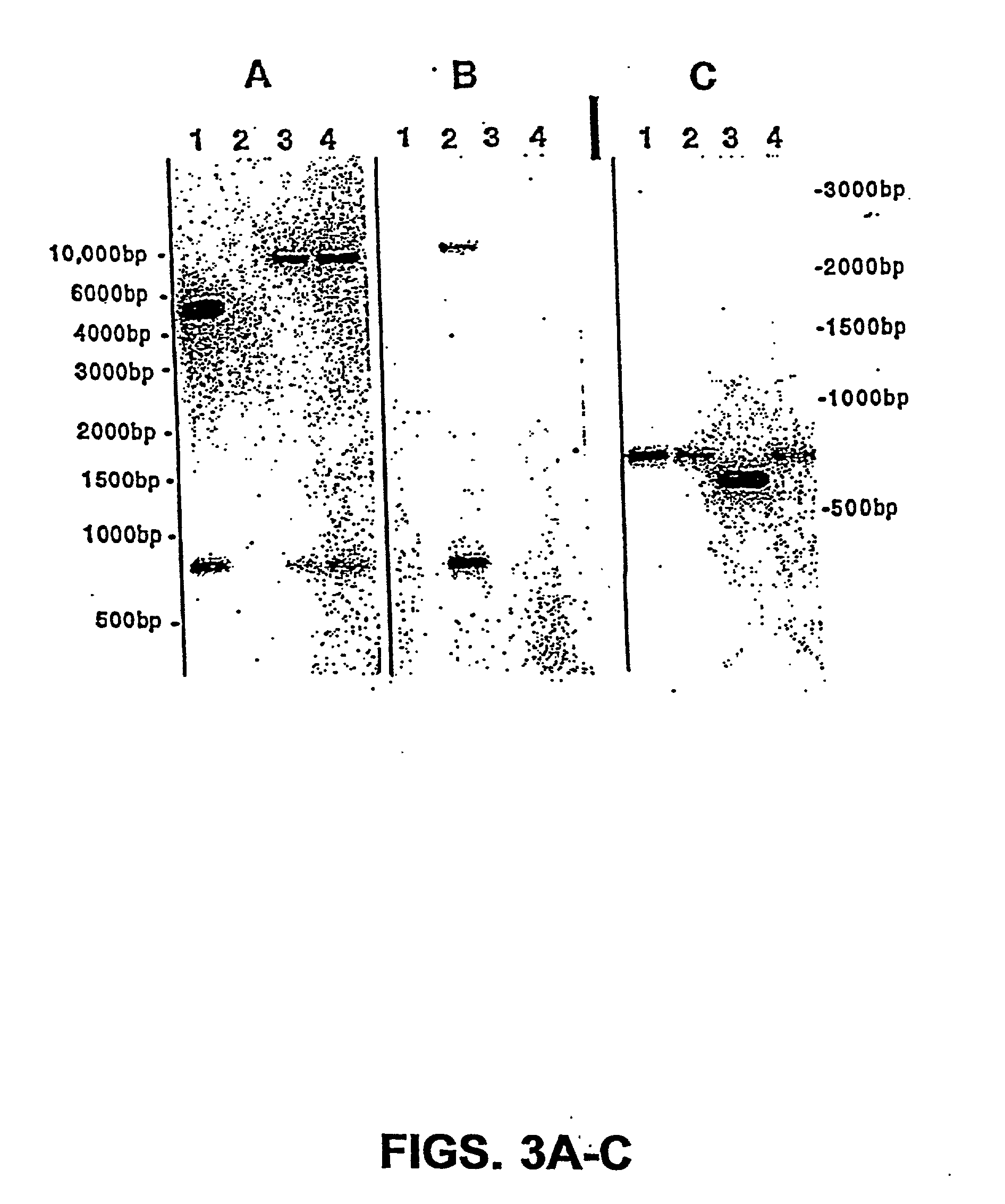
[0054] The present invention is based on the isolation of a gene of Salmonella, i.e., msbB, which, when present in its normal form, contributes to TNF.alpha. induction, general virulence, survival within macrophages, and insensitivity to certain agents which promote eradication of the bacteria. The present invention is directed to the genetic modification of the gene which results in disrupting the normal function of the product of the gene, and the incorporation of the genetic modification into tumor-targeted bacteria, including Salmonella, for therapeutic use. In a preferred embodiment, the bacteria have a genetic modification of the msbB gene as well as genetic modification of a gene in a biosynthetic pathway, such as the purI gene, resulting in an auxotrphic strain.
[0055] In a preferred embodiment, the genetically modified bacteria are used in animals, including humans, for reduction of volume and / or growth inhibition of solid tumors.
[0056] In an additional preferred embodiment,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com