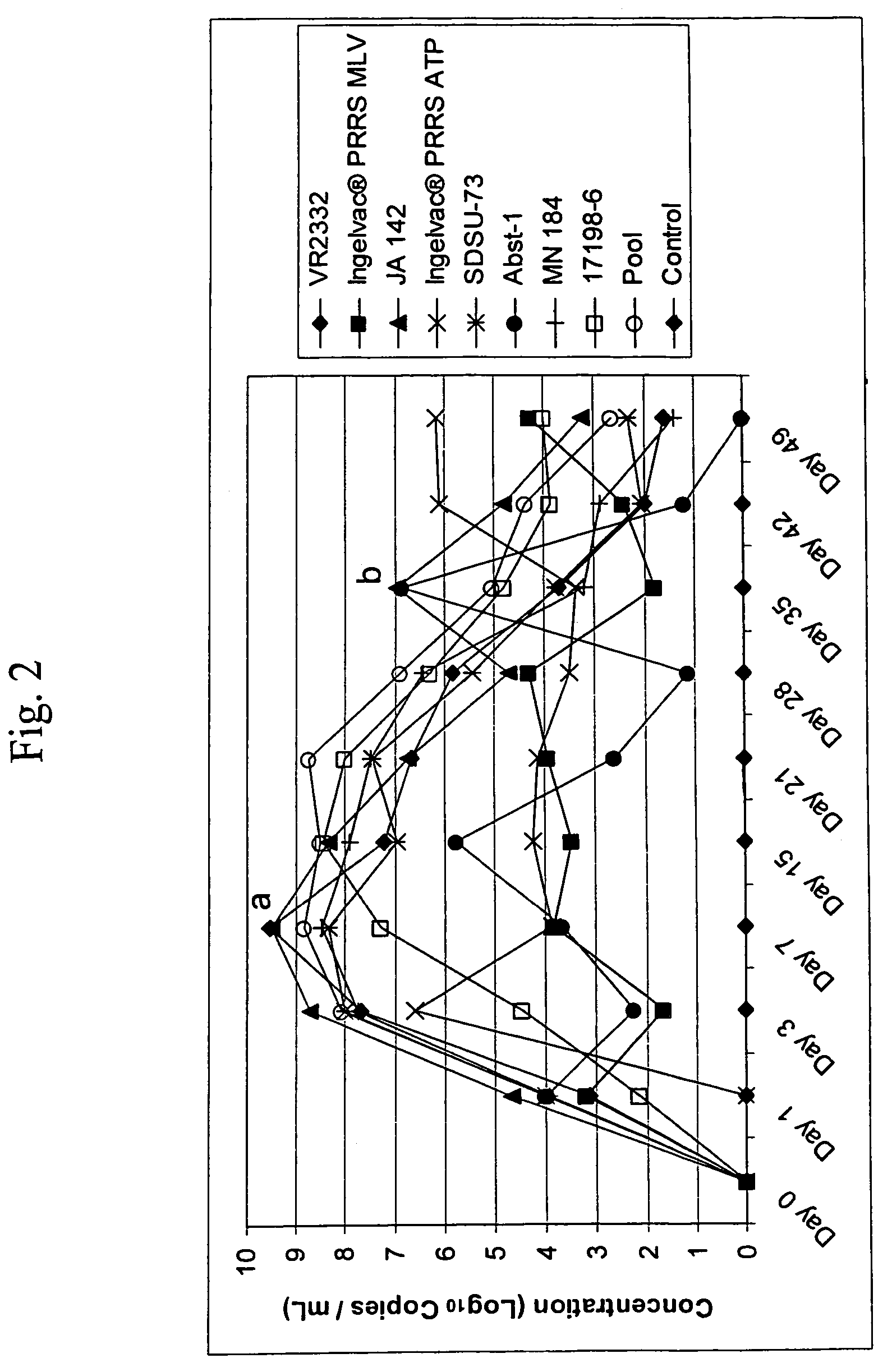
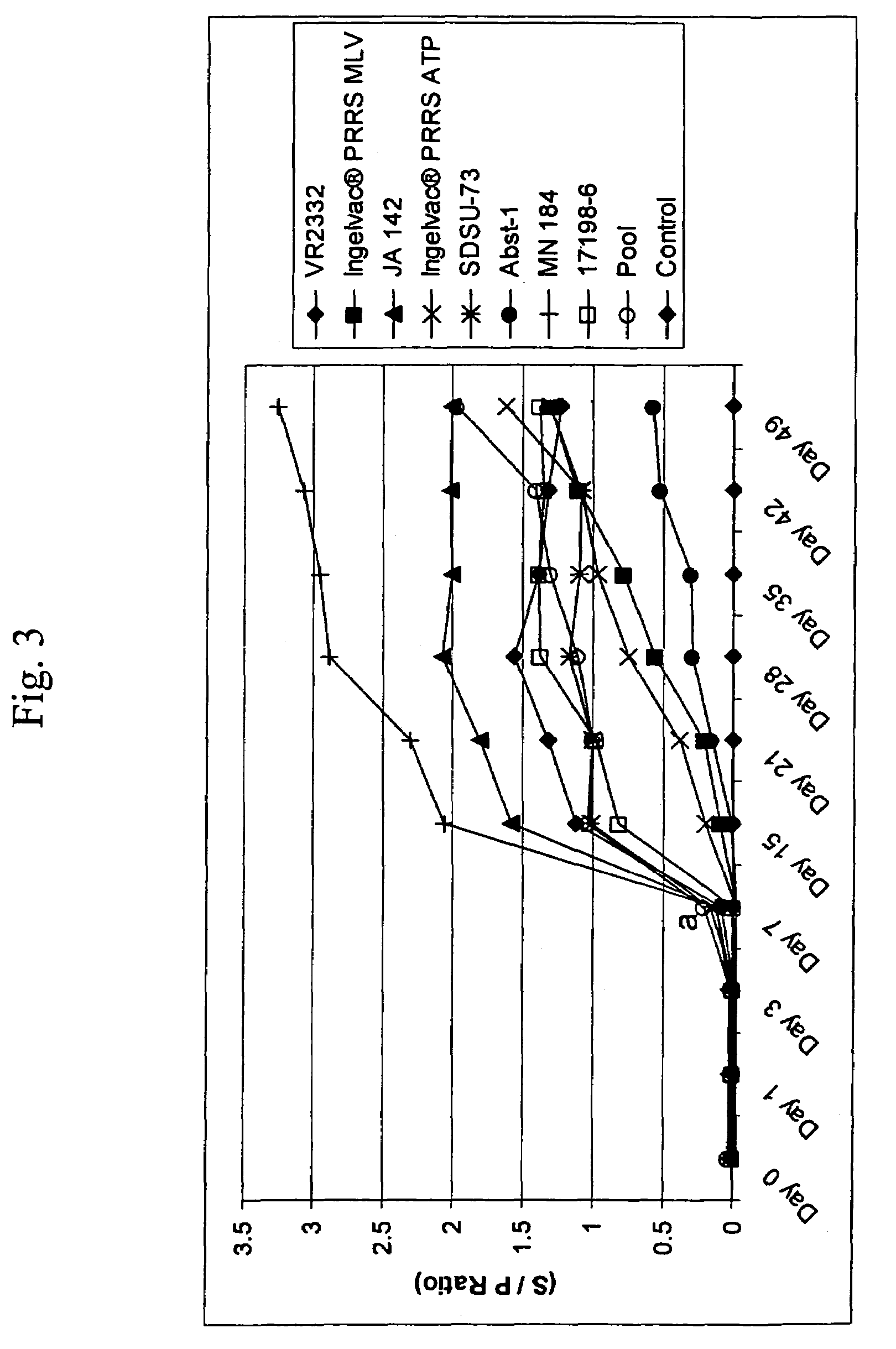
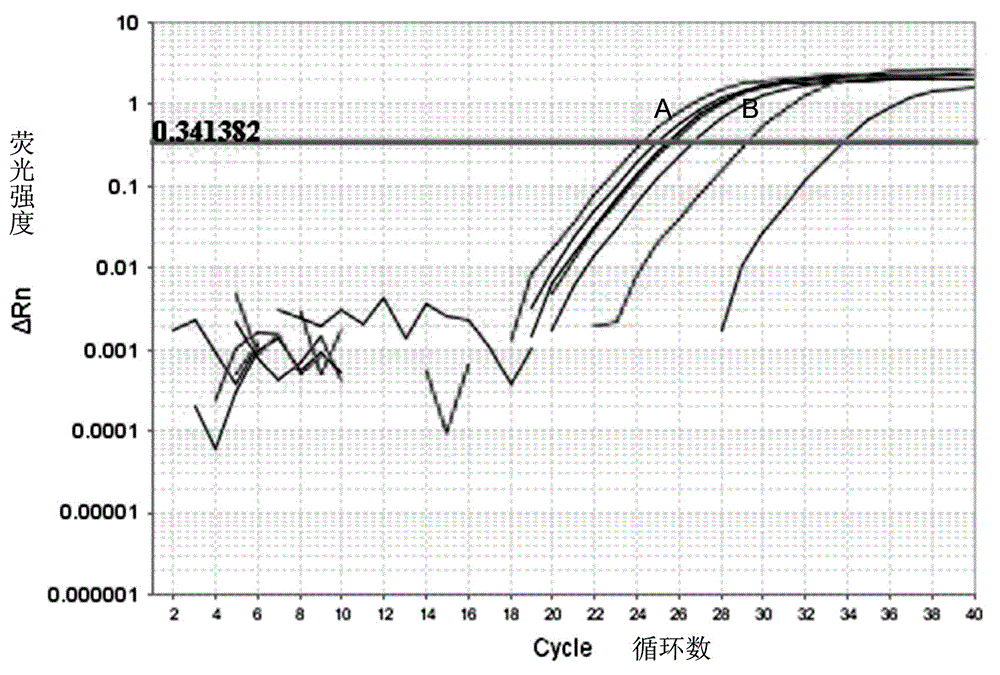
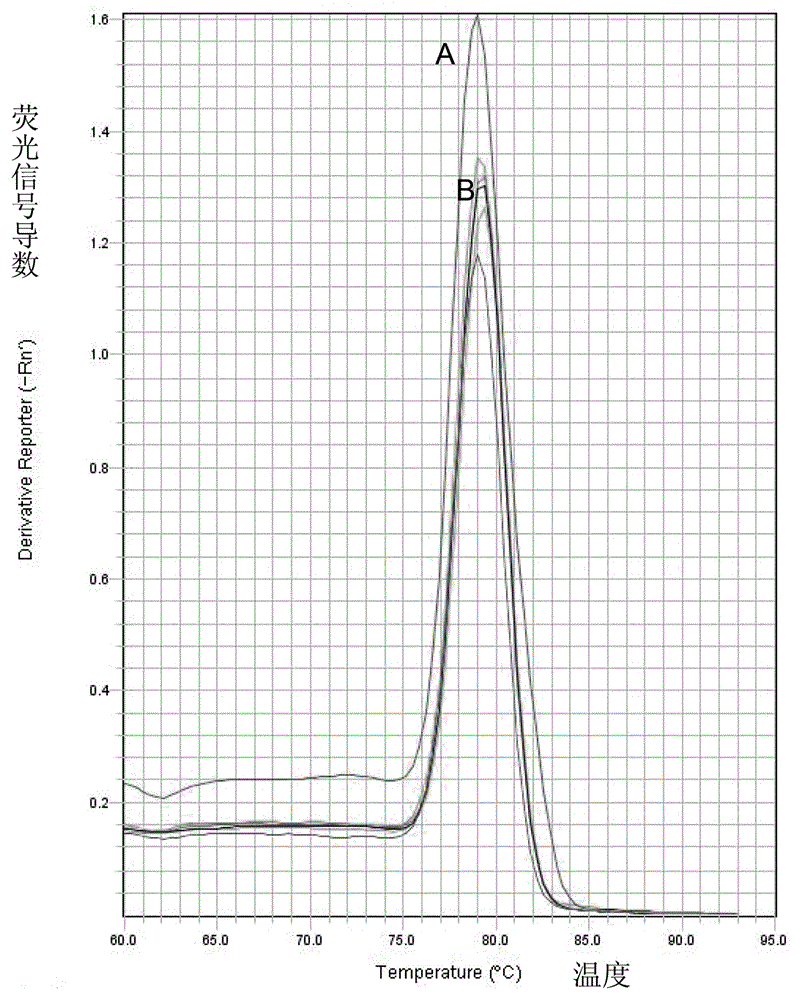
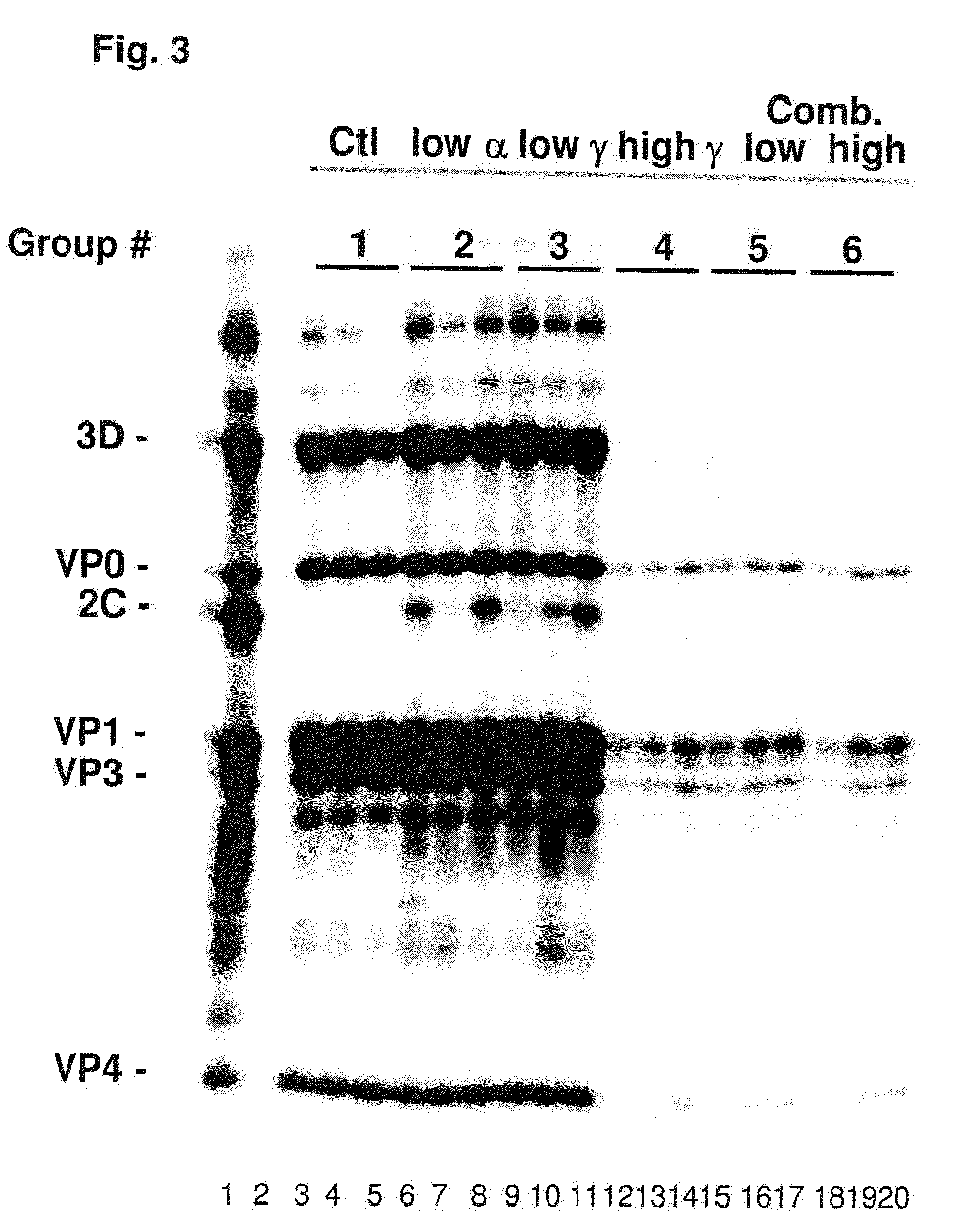
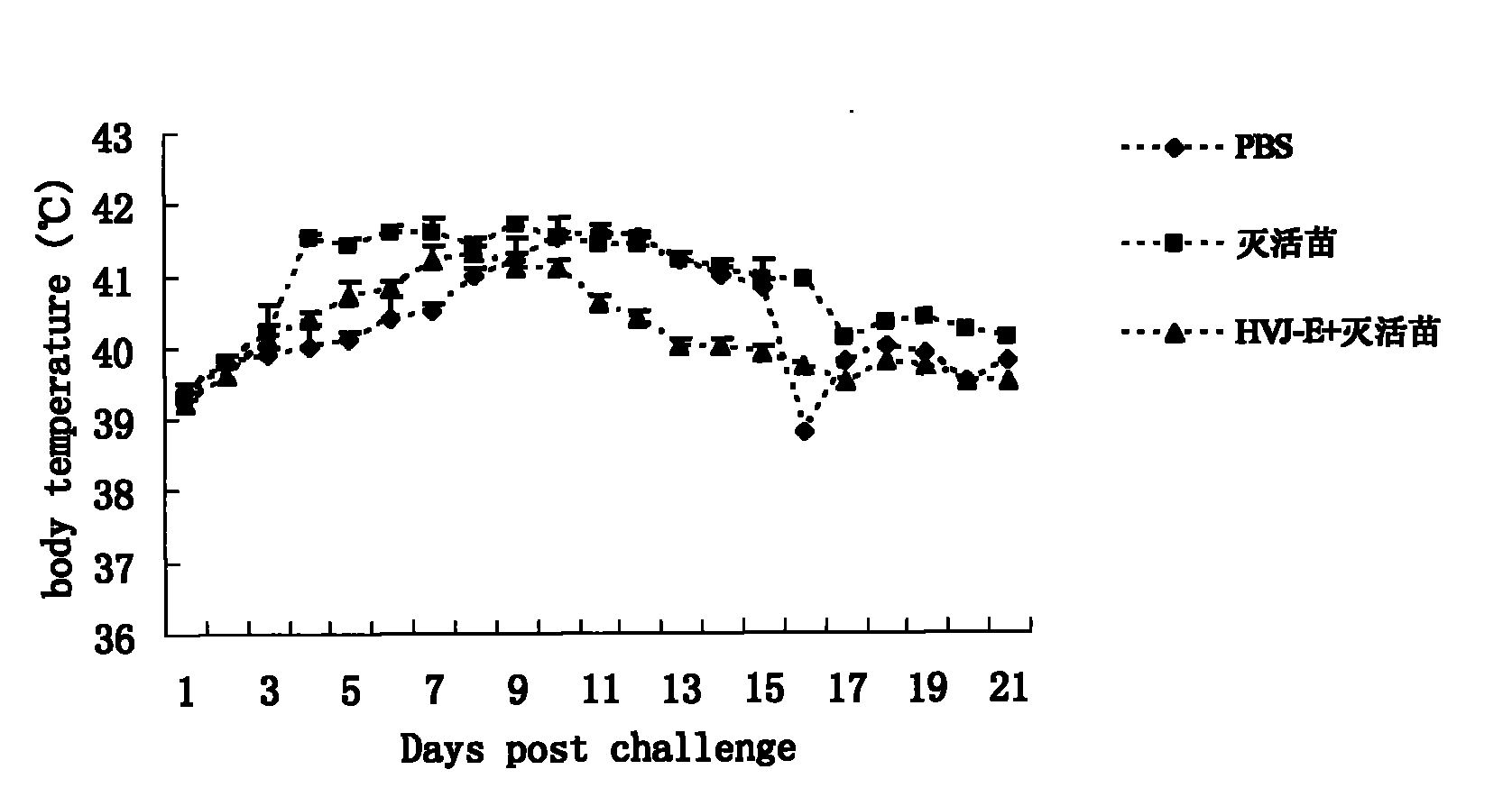Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
106 results about "Viremia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Viremia is a medical condition where viruses enter the bloodstream and hence have access to the rest of the body. It is similar to bacteremia, a condition where bacteria enter the bloodstream. The name comes from combining the word "virus" with the Greek word for "blood" (haima). It usually lasts for 4 to 5 days in the primary condition.
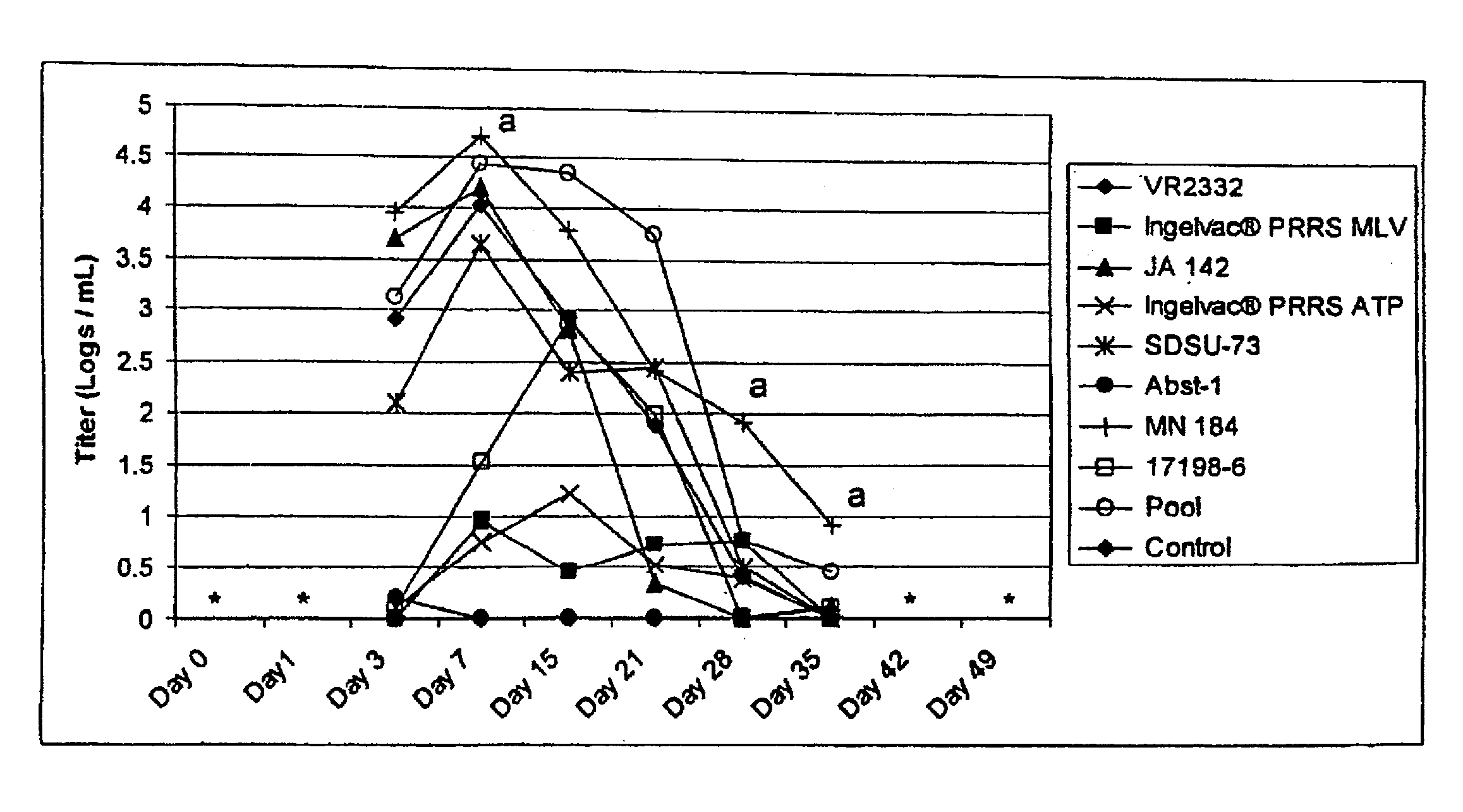
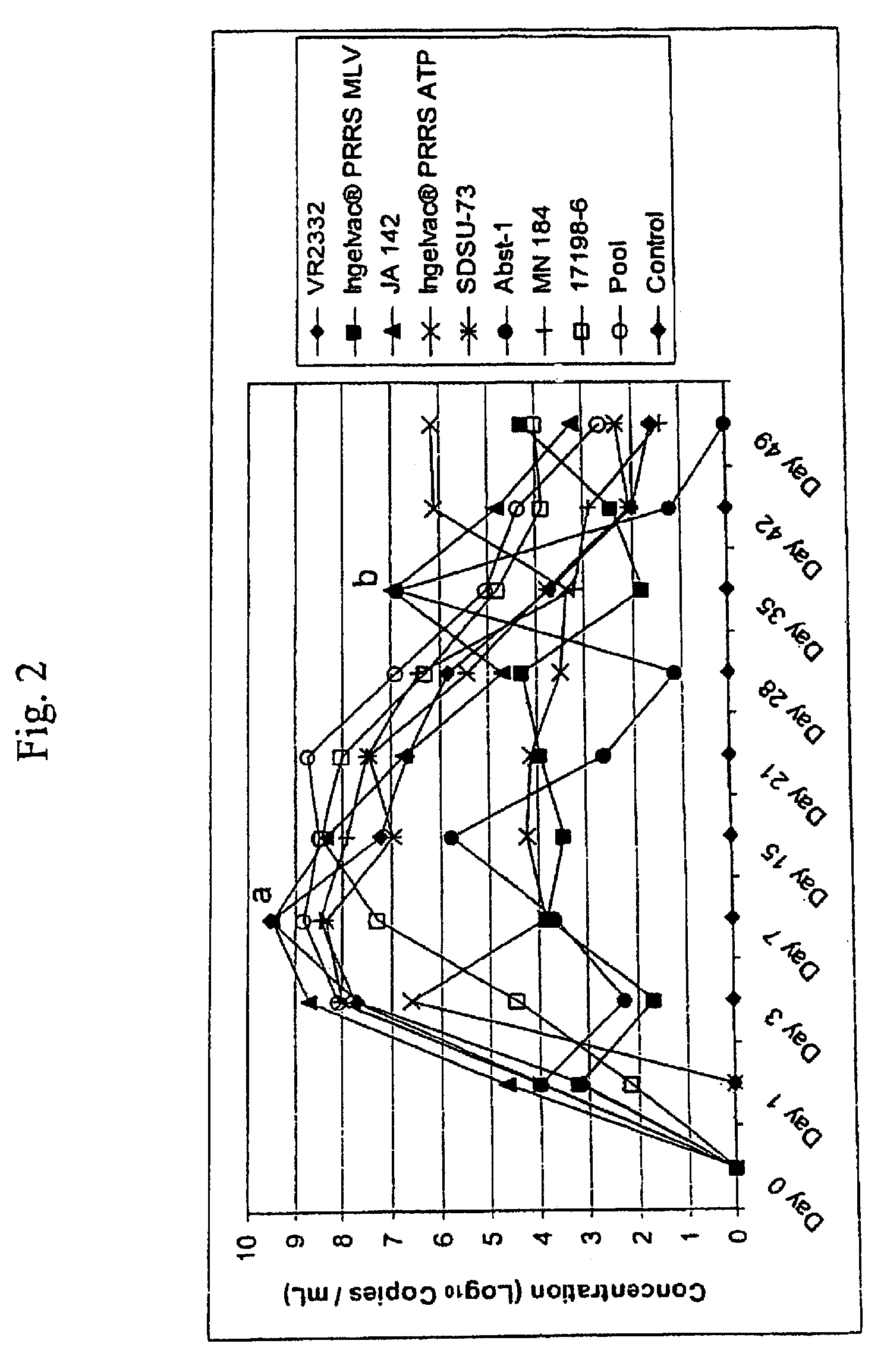
Porcine reproductive and respiratory syndrome isolates and methods of use
InactiveUS20060063151A1Improve immunityIncrease virulenceSsRNA viruses positive-senseMicrobiological testing/measurementVirulent characteristicsImmunogenicity
A method of predicting the virulence of a new or uncharacterized PRRS virus isolate is provided wherein the isolate is injected into swine and allowed to replicate for a period of from about 3-15 days. During this period, the rate of virus growth and / or the magnitude of viremia is determined, and this data is compared with a corresponding growth rate and / or viremia magnitude of a PRRS virus isolate of known virulence, as a measure of the virulence of the new or uncharacterized isolate. Additionally, a method of selecting an isolate for inclusion in an immunogenic composition based on the predicted virulence is also provided, together with compositions incorporating attenuated forms of viruses predicted to be virulent.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Porcine reproductive and respiratory syndrome isolates and methods of use
InactiveUS20090148474A1Improve immunityIncrease virulenceSsRNA viruses positive-senseViral antigen ingredientsVirulent characteristicsImmunogenicity
A method of predicting the virulence of a new or uncharacterized PRRS virus isolate is provided wherein the isolate is injected into swine and allowed to replicate for a period of from about 3-15 days. During this period, the rate of virus growth and / or the magnitude of viremia is determined, and this data is compared with a corresponding growth rate and / or viremia magnitude of a PRRS virus isolate of known virulence, as a measure of the virulence of the new or uncharacterized isolate. Additionally, a method of selecting an isolate for inclusion in an immunogenic composition based on the predicted virulence is also provided, together with compositions incorporating attenuated forms of viruses predicted to be virulent.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Porcine reproductive and respiratory syndrome isolates and methods of use
ActiveUS7632636B2SsRNA viruses positive-senseMicrobiological testing/measurementVirulent characteristicsVirus strain
A method of predicting the virulence of a new or uncharacterized PRRS virus strain is provided wherein the strain is injected into swine and allowed to replicate for a period of from about 3-15 days. During this period, the rate of virus growth and / or the magnitude of viremia is determined, and this data is compared with a corresponding growth rate and / or viremia magnitude of a PRRS virus strain of known virulence, as a measure of the virulence of the new or uncharacterized strain.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Fluorescent quantitative PCR detection method of spring viraemia of carp virus
ActiveCN105002298AStrong specificityEfficient detectionMicrobiological testing/measurementMicroorganism based processesAquatic animalFluorescence
The invention discloses a fluorescent quantitative PCR detection method of spring viraemia of carp virus, and belongs to the technical field of aquatic virus detection. According to the fluorescent quantitative PCR detection method of the spring viraemia of carp virus, a specific primer probe set used for detecting the spring viraemia of carp virus is arranged and composed of specific primer pairs aiming at the spring viraemia of carp virus and fluorescent probes. By means of the provided primers, good sensitivity and specificity for identifying the spring viraemia of carp virus are achieved. By means of the fluorescent quantitative PCR detection method of the spring viraemia of carp virus, hemorrhagic tissues infected by the spring viraemia of carp virus can be detected, asymptomatic fish carrying the spring viraemia of carp virus and cells infected by the spring viraemia of carp virus can be detected, and great application prospects for rapid detection and supervision of the epidemic disease on entry-exit aquatic animals are achieved.
Owner:上海市水产研究所(上海市水产技术推广站)
Therapeutic immunization of hiv-infected individuals
InactiveUS20060216272A1Effectively maintaining low titerReceive treatment wellBiocideGenetic material ingredientsImmunodeficiency virusMedicine
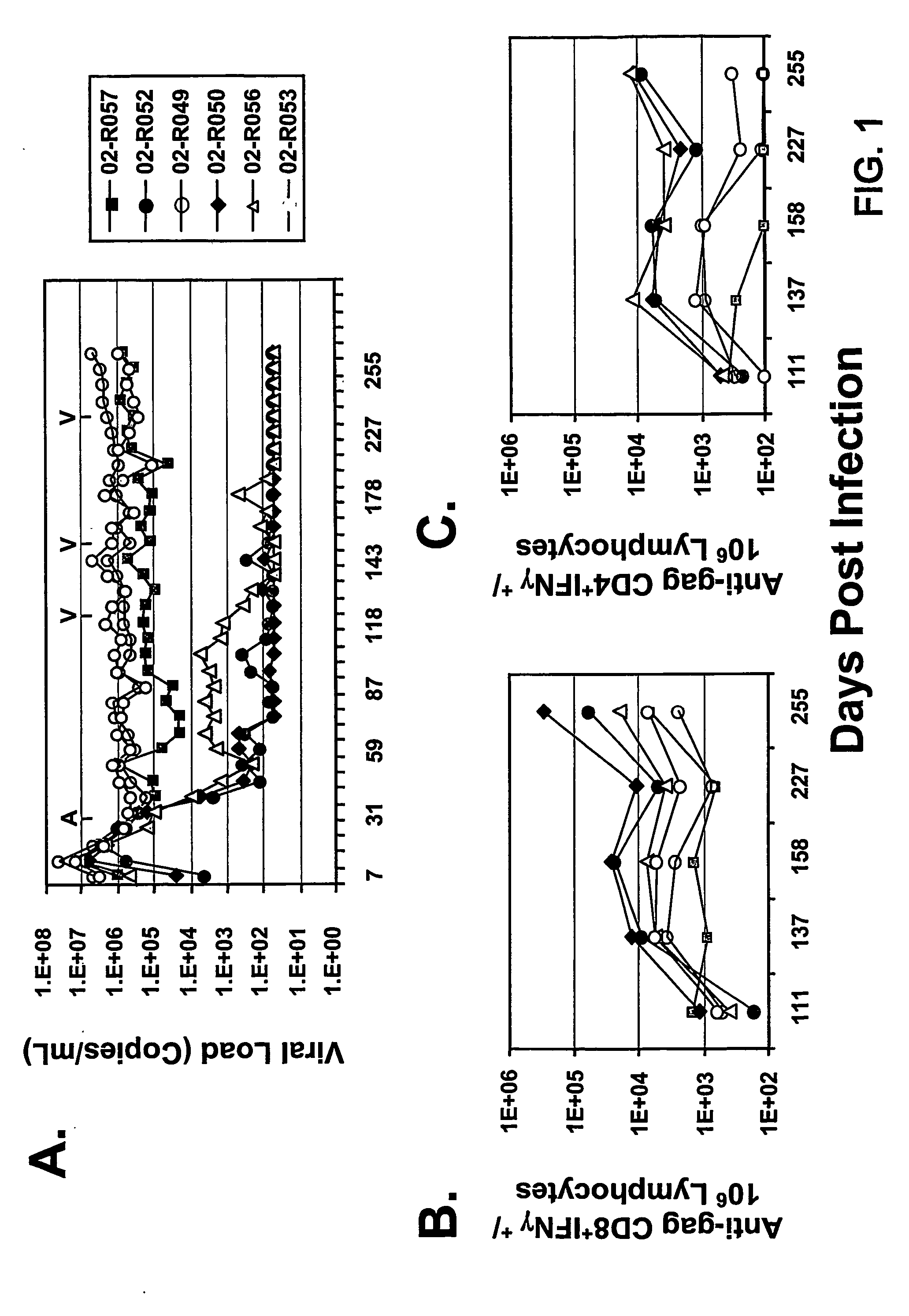
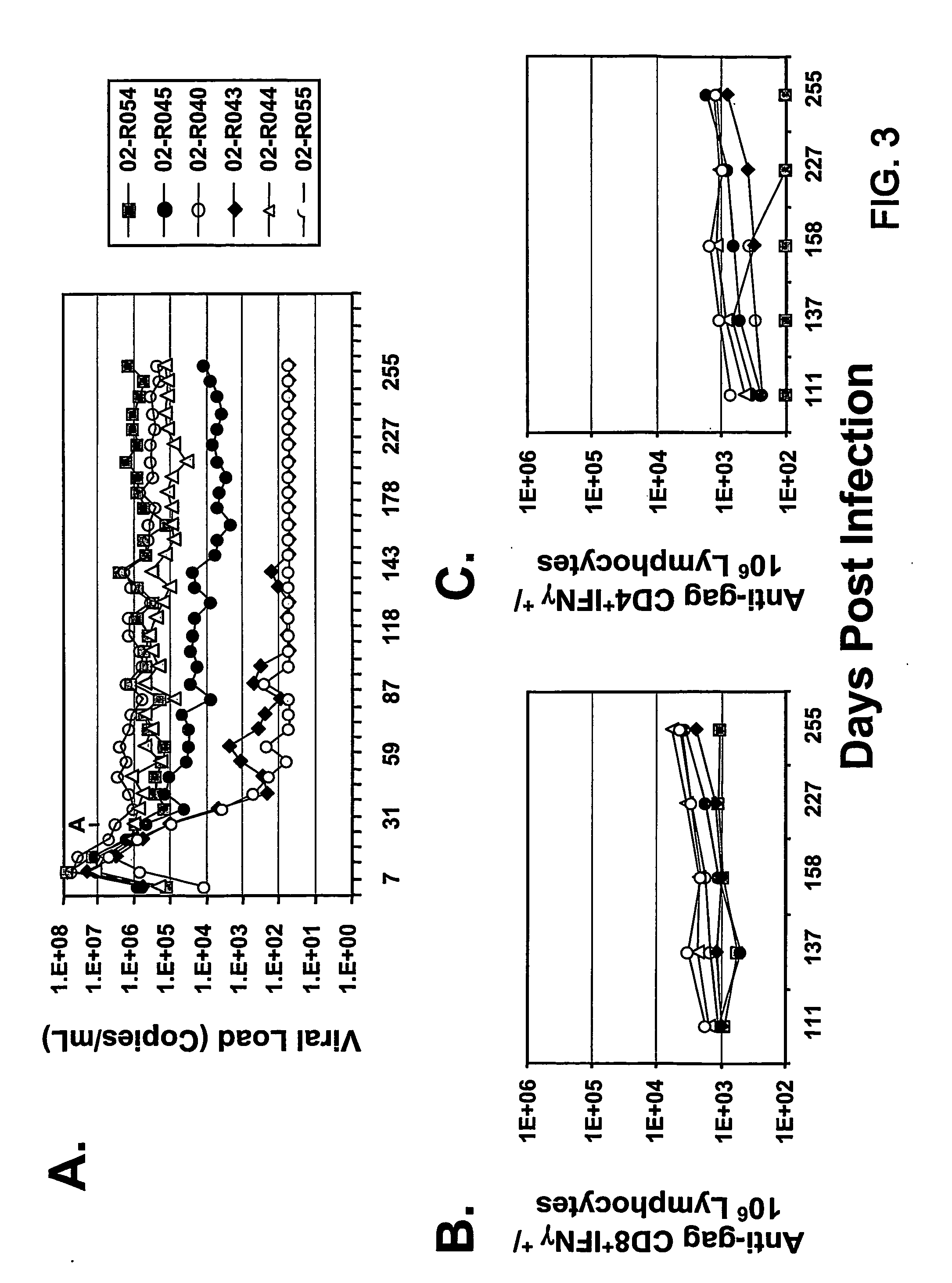
The present invention provides an improved method for eliciting a therapeutic immune response in an individual infected with human immunodeficiency virus (“HIV”). The method comprises administering an adenoviral vaccine composition expressing an HIV antigen to an individual with controlled viremia. Immunization of infected individuals in this manner elicits a cellular-mediated immune response against the virus that is significant both in the level of the response and the breadth of the response. The therapeutic immune response that ensues is capable of effectively maintaining low titers of virus and, thus, offers the prospect of reducing individual dependency on antiviral therapy.
Owner:MERCK & CO INC
Adjuvant for improving effect of porcine reproductive and respiratory syndrome inactivated vaccine, preparation method thereof and application thereof
InactiveCN101940787AImprove the immunityImprove immunityViral antigen ingredientsAntiviralsAdjuvantClinical manifestation
The invention discloses an adjuvant for improving the effect of a porcine reproductive and respiratory syndrome (PRRSV) inactivated vaccine, a preparation method thereof and application thereof and relates to the preparation method and steps of Hemagglutinating virus of Japan envelope (HVJ-E) and application of a medicament for improving the effect of the PRRSV inactivated vaccine. The preparation method of adjuvant comprises the following steps of: breeding Hemagglutinating virus of Japan (HVJ) by using an SPF chick embryo, and then concentrating, purifying, counting and inactivating the HVJ to prepare the Hemagglutinating virus of Japan envelope (HVJ-E); immunizing PRRSV negative pigs with the HVJ-E and the PRRSV inactivated vaccine together, and setting a PBS contrast group and a PRRSV inactivated vaccine group at the same time, wherein compared with the contrast group, the HVJ-E and PRRSV inactivated vaccine group can obviously improve the response level of hunoral immunity and cellular immunity of the pigs; and after immunizing, expelling toxin by using a PRRSV virulent strain (JXA1), wherein the result shows that the clinical manifestations and the weight increasing of the HVJ-E and PRRSV inactivated vaccine group are better than those of the contrast group, and the generated viremiavirusemia rate, toxin expelling rate and virus distribution rate are obviously reduced when compared with the contrast group. The adjuvant of the invention has the advantages of convenient production and low cost and is suitable for large-scale production.
Owner:朱善元 +2
Enhanced Antiviral Activity Against Foot and Mouth Disease
InactiveUS20090269372A1Effectively ensureEarly protection against FMDVSsRNA viruses positive-sensePeptide/protein ingredientsHigh dosesIn vivo
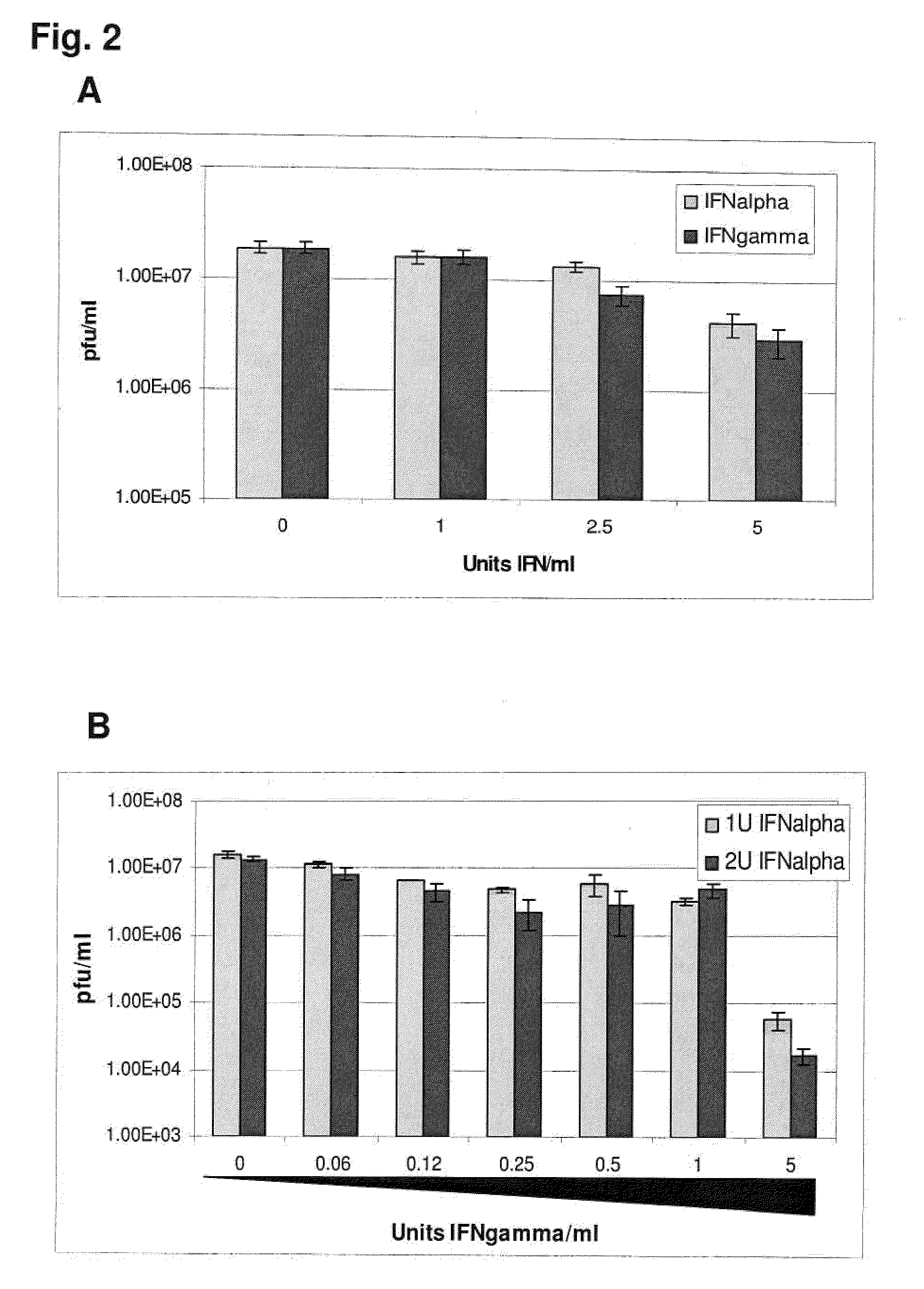
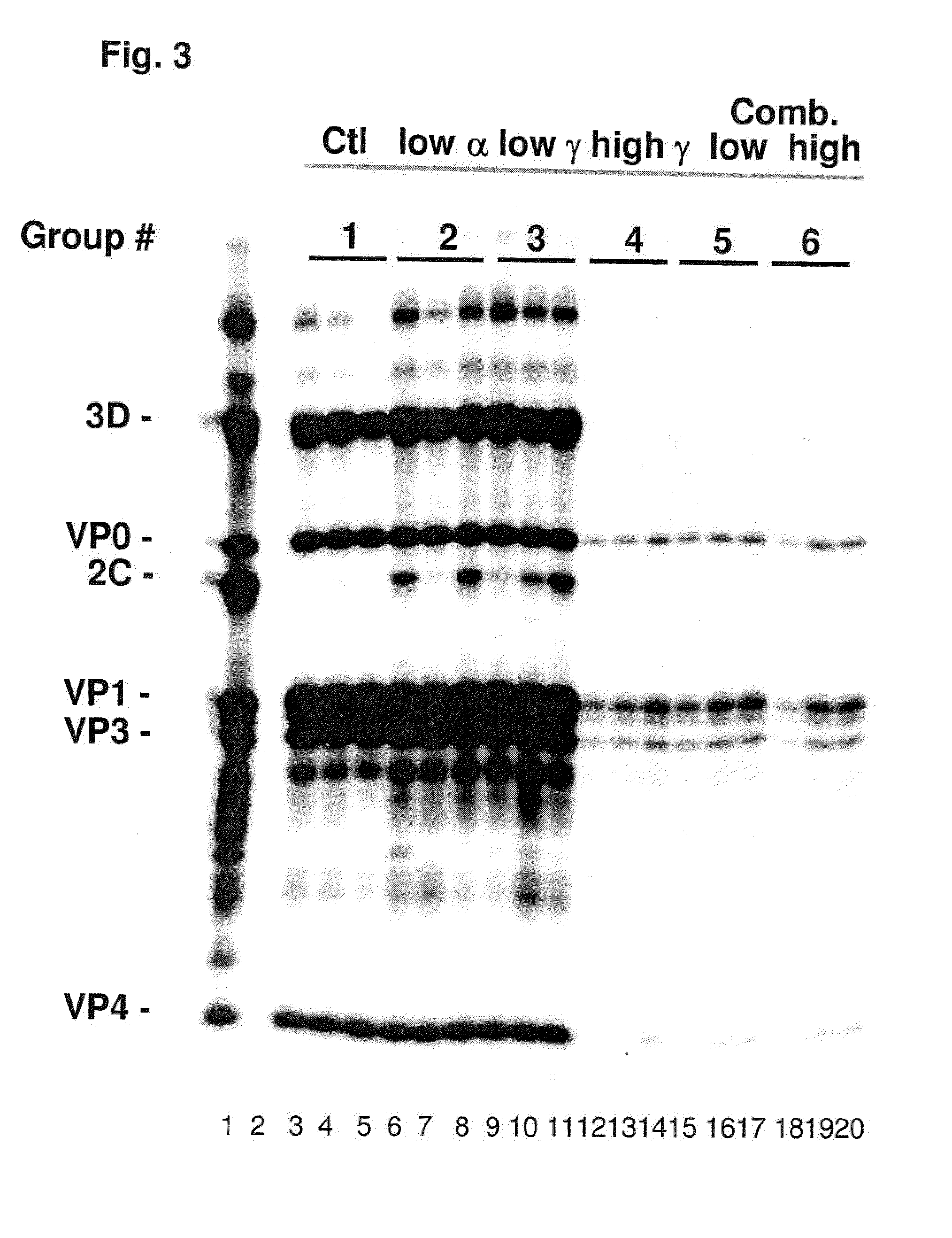
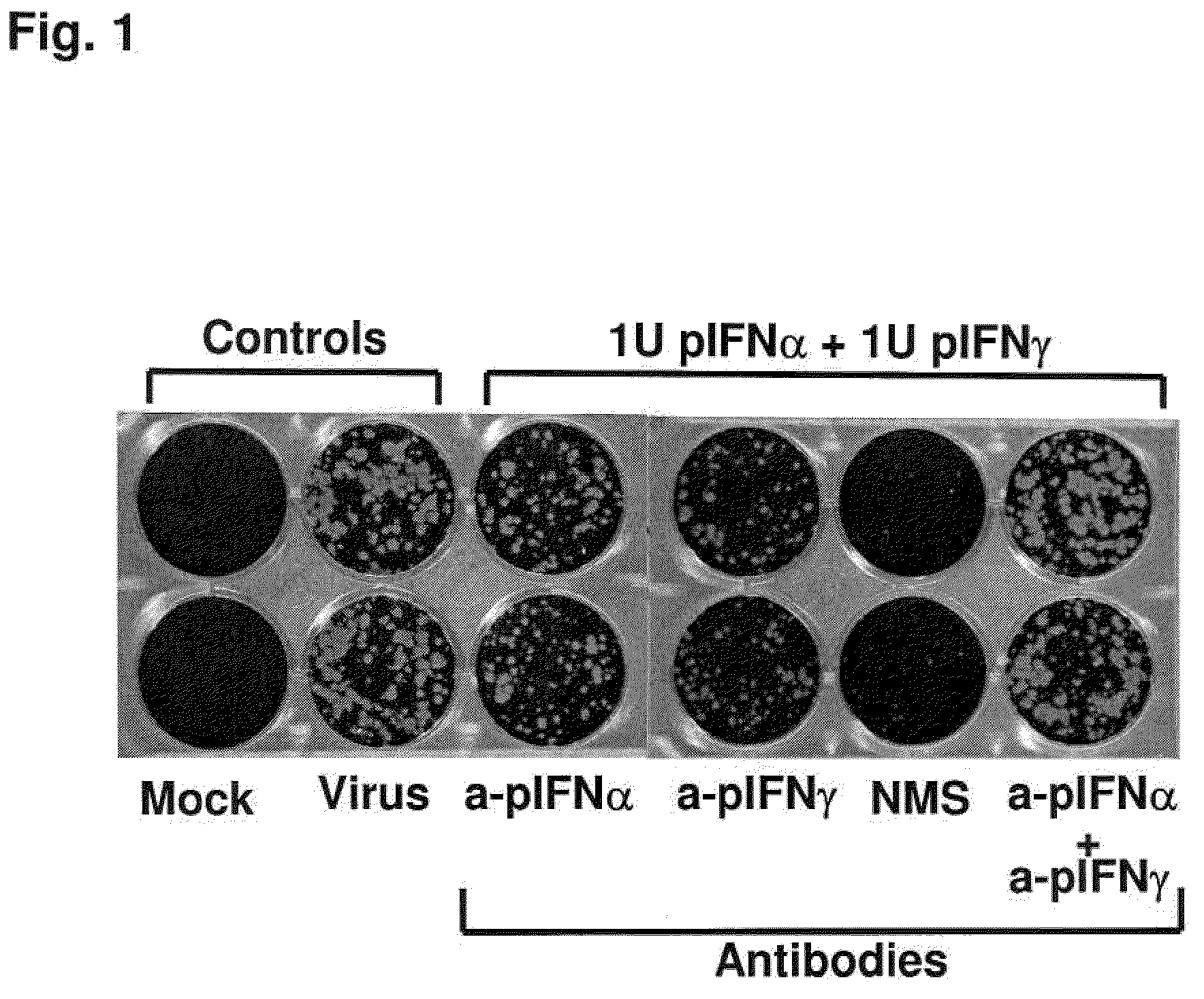
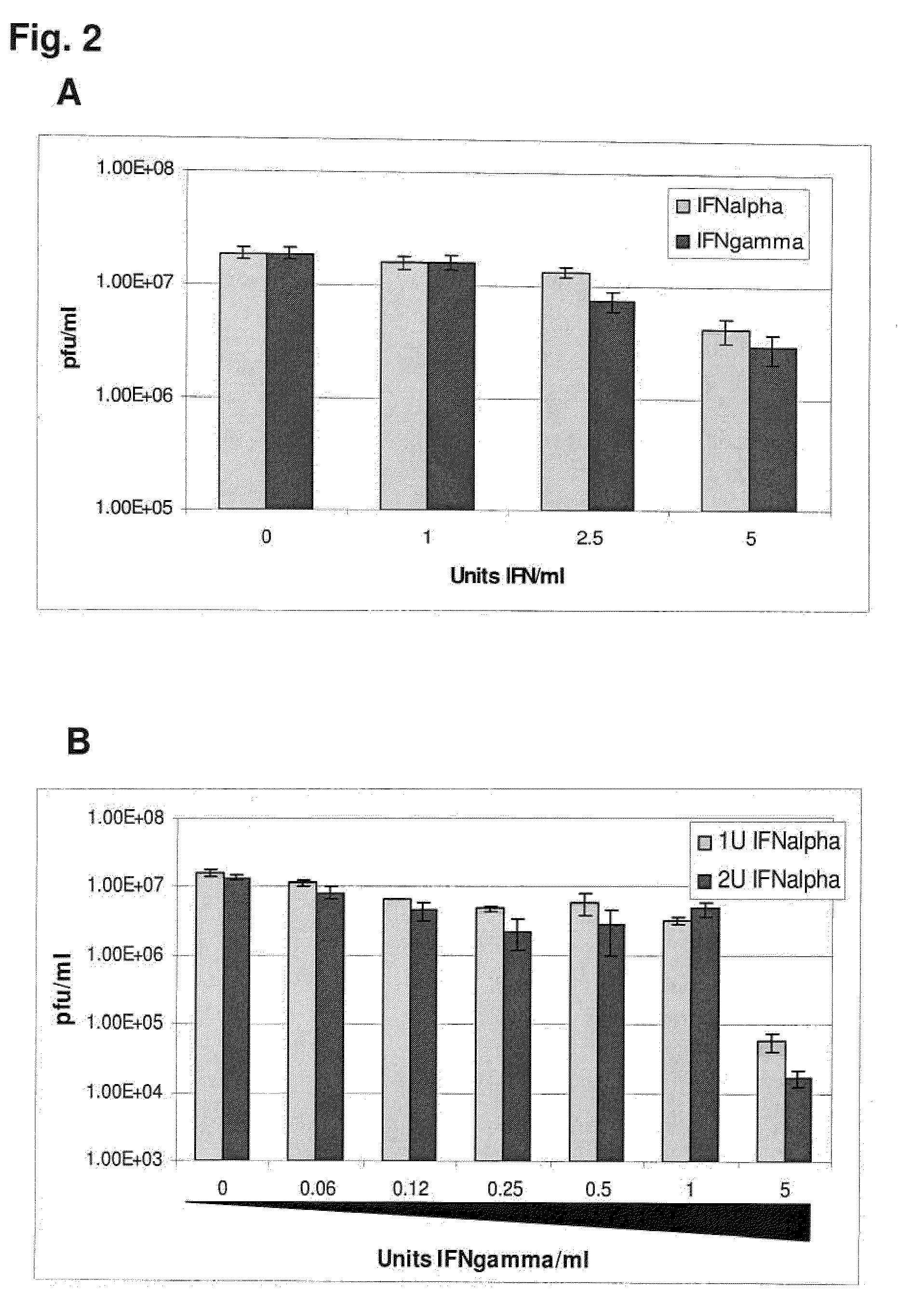
Previously, we showed that type I interferon (alpha / beta interferon [IFN-α / β]) can inhibit foot-and-mouth disease virus (FMDV) replication in cell culture, and swine inoculated with 109 PFU of human adenovirus type 5 expressing porcine IFN-α (Ad5-pIFN-α) were protected when challenged 1 day later. In this study, we found that type II pIFN (pIFN-γ) also has antiviral activity against FMDV in cell culture and that, in combination with pIFN-α, it has a synergistic antiviral effect. We also observed that while each IFN alone induced a number of IFN-stimulated genes (ISGs), the combination resulted in a synergistic induction of some ISGs. To extend these studies to susceptible animals, we inoculated groups of swine with a control Ad5, 108 PFU of Ad5-pIFN-α, low- or high-dose Ad5-pIFN-γ, or a combination of Ad5-pIFN-α and low- or high-dose Ad5-pIFN-γ and challenged all groups with FMDV 1 day later. The control group and the groups inoculated with either Ad5-pIFN-α or a low dose of Ad5-pIFN-γ developed clinical disease and viremia. However, the group that received the combination of both Ad5-IFNs with the low dose of Ad5-pIFN-γ was completely protected from challenge and had no viremia. Similarly the groups inoculated with the combination of Ad5s with the higher dose of Ad5-pIFN-γ or with only high-dose Ad5-pIFN-γ were protected. The protected animals did not develop antibodies against viral nonstructural (NS) proteins, while all infected animals were NS protein seropositive. No antiviral activity or significant levels of IFNs were detected in the protected groups, but there was an induction of some ISGs. The results indicate that the combination of type I and II IFNs act synergistically to inhibit FMDV replication in vitro and in vivo.
Owner:UNITED STATES OF AMERICA
Antagonistic drug against replication of porcine reproductive and respiratory syndrome virus (PRRSV) and application of antagonistic drug
ActiveCN110613706AInhibition of replicationStrong replicabilityOrganic compound preparationKetone active ingredientsPlant SourcesTherapeutic effect
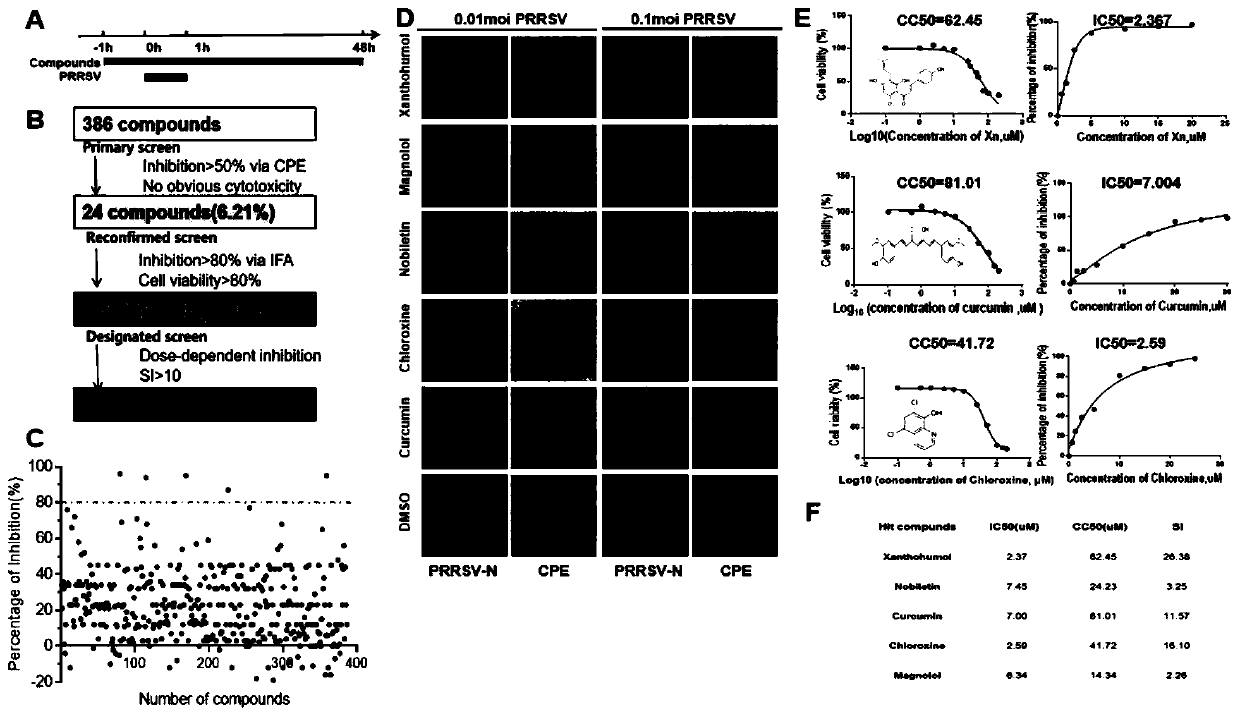
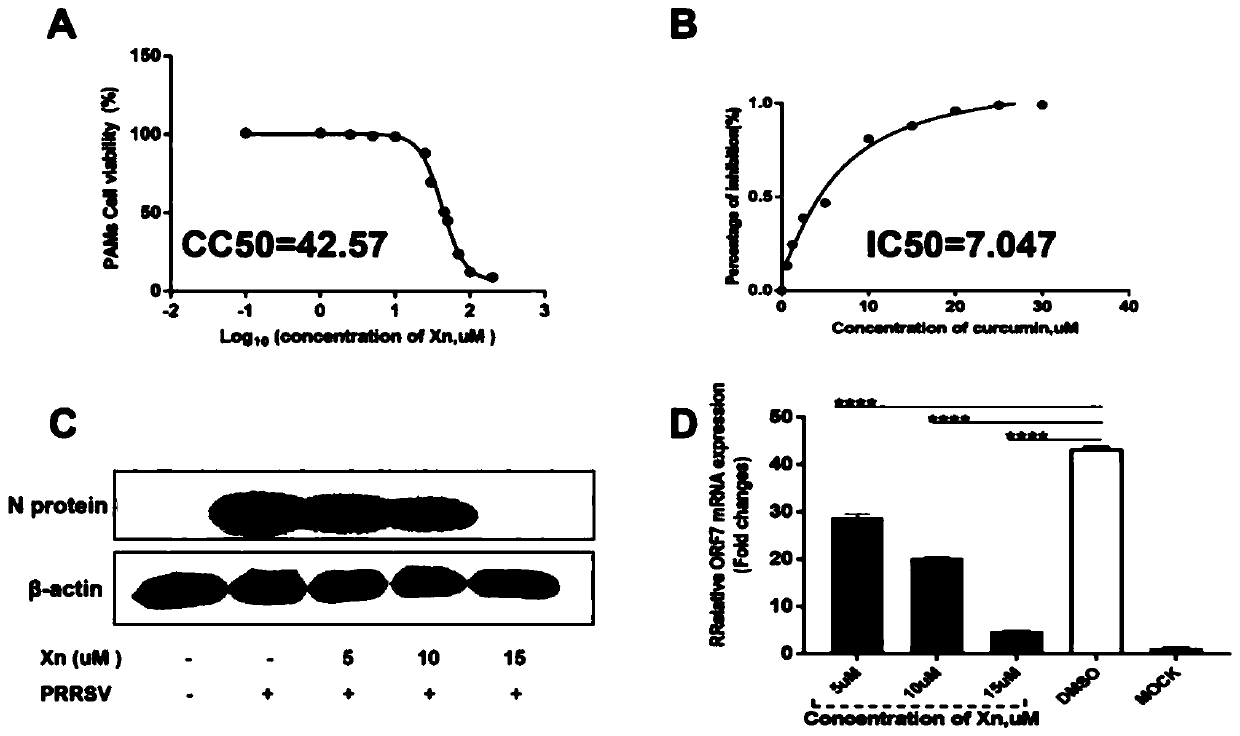
The invention discloses an antagonistic drug against replication of a porcine reproductive and respiratory syndrome virus (PRRSV) and application of the antagonistic drug. A virus infection test is adopted, and Xanthohumol (Xn), the antagonistic drug of the PRRSV, is discovered for the first time from a natural medicine library with 386 plant sources. The drug can effectively inhibit PRRSV replication in both Marc-145 and PAM cells, five xanthohumol derivatives with different molecular structures are artificially synthesized, and the viral infection test further finds that the inhibition effect to virus replication by drug derivative Xn-4 is highest in vitro; and test results of artificial infection and drug therapy in piglets show that the Xn-4 can effectively inhibit PRRSV viremia, relieves clinical symptoms of infected pigs, and significantly reduces pulmonary inflammation and pathological damage. Combined with the results, it is proved that the antagonistic drug has important preventive and therapeutic effects to a porcine reproductive and respiratory syndrome, and has important application prospects.
Owner:NANJING AGRICULTURAL UNIVERSITY
RT-LAMP detection primer group of spring viremia of carp virus (SVCV), kit and detection method
ActiveCN103045759ASimple and fast operationMild conditionsMicrobiological testing/measurementMicroorganism based processesReverse transcriptaseBioinformatics
The invention discloses an RT-LAMP detection primer group of spring viremia of carp virus (SVCV), a detection kit and a detection method. The detection primer group comprises a pair of outer primers, a pair of inner primers and a pair of cyclic primers; the detection kit comprises primer liquid, reaction liquid, DNA (deoxyribonucleic acid) polymerase, reverse transcriptase and a contrast; and the kit also can be provided with a color developing agent. The detection method comprises the following steps of: extracting the RNA (ribonucleic acid) of the to-be-detected virus; and performing amplification of a sample RNA template at 63-65 DEG C by adopting six specific primers and DNA polymerase with strand displacement activity according to the reverse transcription activity of reverse transcriptase, wherein the pg level of pure virus RNA can be detected, and the identification adds the detection of SYBRGreen IESE-Quant-tubeScanner instrument or the turbidity change of precipitate in a reaction tube is observed by a turbidity meter to judge amplification to determine whether a to-be-detected sample contains the RNA of SVCV. The method disclosed by the invention has the advantages of high speed and high efficiency, convenience in operation, high specificity, high sensitivity, easiness in identification, suitability for field detection and the like, and is suitable for popularization and application.
Owner:河北三狮生物科技有限公司
Enhanced antiviral activity against foot and mouth disease
InactiveUS7833533B2Early protection against FMDVEffectively ensureSsRNA viruses positive-sensePeptide/protein ingredientsHigh dosesIn vivo
Previously, we showed that type I interferon (alpha / beta interferon [IFN-α / β]) can inhibit foot-and-mouth disease virus (FMDV) replication in cell culture, and swine inoculated with 109 PFU of human adenovirus type 5 expressing porcine IFN-α (Ad5-pIFN-α) were protected when challenged 1 day later. In this study, we found that type II pIFN (pIFN-γ) also has antiviral activity against FMDV in cell culture and that, in combination with pIFN-α, it has a synergistic antiviral effect. We also observed that while each IFN alone induced a number of IFN-stimulated genes (ISGs), the combination resulted in a synergistic induction of some ISGs. To extend these studies to susceptible animals, we inoculated groups of swine with a control Ad5, 108 PFU of Ad5-pIFN-α, low- or high-dose Ad5-pIFN-γ, or a combination of Ad5-pIFN-α and low- or high-dose Ad5-pIFN-γ and challenged all groups with FMDV 1 day later. The control group and the groups inoculated with either Ad5-pIFN-α or a low dose of Ad5-pIFN-γ developed clinical disease and viremia. However, the group that received the combination of both Ad5-IFNs with the low dose of Ad5-pIFN-γ was completely protected from challenge and had no viremia. Similarly the groups inoculated with the combination of Ad5s with the higher dose of Ad5-pIFN-γ or with only high-dose Ad5-pIFN-γ were protected. The protected animals did not develop antibodies against viral nonstructural (NS) proteins, while all infected animals were NS protein seropositive. No antiviral activity or significant levels of IFNs were detected in the protected groups, but there was an induction of some ISGs. The results indicate that the combination of type I and II IFNs act synergistically to inhibit FMDV replication in vitro and in vivo.
Owner:UNITED STATES OF AMERICA
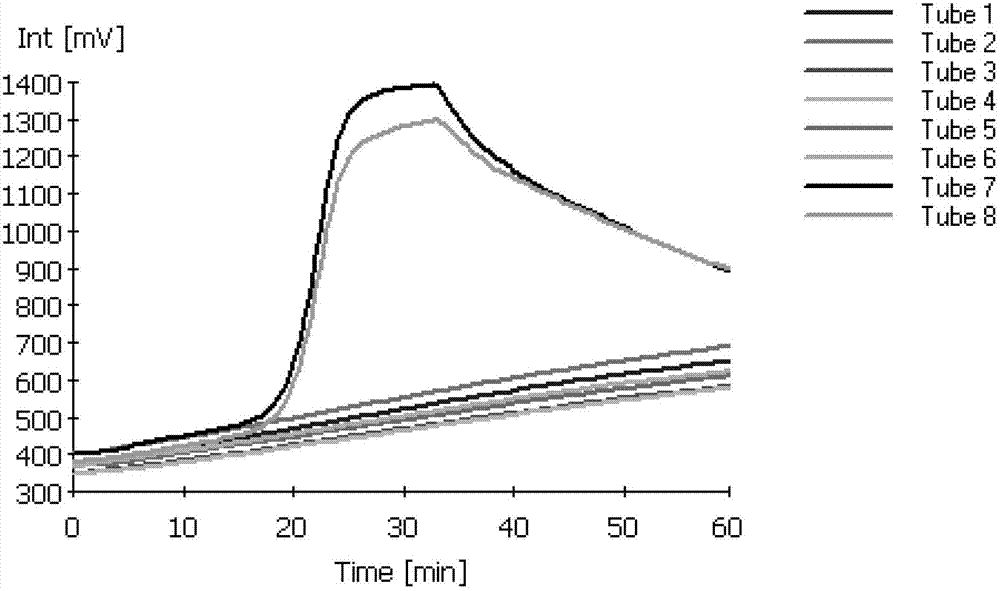
RT-LAMP (reverse transcription loop-mediated isothermal amplification) detection kit and RT-LAMP detection method for SVCV (spring viremia of carp virus)
InactiveCN102876811AEfficient detectionStrong specificityMicrobiological testing/measurementWater bathsBetaine
The invention discloses a RT-LAMP (reverse transcription loop-mediated isothermal amplification) detection kit and a RT-LAMP detection method for SVCV (spring viremia of carp virus). The RT-LAMP detection kit of SVCV comprises 10*ThermoPol Reaction buffer, Bst DNA (deoxyribonucleic acid) polymerase, dNTPs (deoxynucleoside triphosphates), AMV reverse transcriptase, outer primer F3 and B3, inner primer FIP, BIP and Betaine, MgC 12, DTT, and 1000*SYBR Green I. The RT-LAMP detection kit is simple, fast, and high in specificity and sensitivity, only needs a water bath kettle or a metal bath to accurately detect SVCV in samples in 2 hours, can detect SVCV of infected tissues of sick fish, can detect cells infected by SVCV, and is suitable for fast field detection of SVCV.
Owner:YANGTZE RIVER FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Infectious schmallenberg virus from cloned cdnas and uses thereof
The present invention belongs to the field of animal health and relates to a nucleic acid sequence which comprises the complete genome of an infectious Schmallenberg virus (SBV) useful for studying viremia and diseases caused by SBV in ruminants, and in the development of vaccines, therapeutics and diagnostics for the prophylaxis, treatment and diagnosis of viremia and diseases caused by SBV.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Porcine circovirus 2 immune protection polypeptide and vaccine comprising same
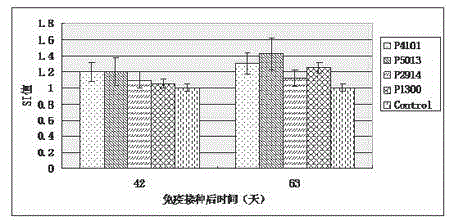
ActiveCN104098657AHigh titerRaise antibody levelsViral antigen ingredientsVirus peptidesPeptide vaccineCytokine
The invention relates to the technical field of porcine circovirus, in particular to a porcine circovirus 2 (PCV2) immune protection polypeptide, and a vaccine containing the PCV2 immune protection polypeptide. The amino acid sequence of the PCV2 immune protection polypeptide is shown in the sequence table 1-4. According to the vaccine, tests show that after 42-63 days of first immune of a vaccine of P5013 and a vaccine of P1300, the antibody titer and the neutralizing antibody titer of an ELISA antibody are remarkably increased, the lymphocyte proliferative response and IL-4 and IFN-Gamma cytokines are significantly higher than those of a control group, and the antibody level and the cellular immunity level of the vaccine group of P5013 are the highest; mice tests show that PCV2 immune response induced by the synthetic peptide vaccine of P5013 is the highest and the strongest; pig tests find that the synthetic polypeptide vaccine of P5013 can induce pigs to generate a PCV2-specific immune response, can reduce viremia caused by PCV2 virulent virus attack, alleviate the clinical symptoms and pathological lesions of viremia, and has immune protection effect on PCV2.
Owner:NANJING AGRICULTURAL UNIVERSITY
Epitope of spring viraemia of carp
The invention relates to an antigenic epitope of spring viraemia of carp and belongs to the technical field of biology. According to the invention, by virtue of phage display technique, a positive clone is screened out by the use of spring viraemia of carp virus (SVCV) monoclonal antibody, a conserved sequence binding to the SVCV monoclonal antibody is found out, a clinical diagnosis reagent is prepared by the screened antigenic epitope, and spring viraemia of carp is prevented and treated by a small molecular antigen peptide as a vaccine. The gene sequence of the invention is SEQ ID NO:1. The phage display technique is utilized to screen the positive clone by SVCV monoclonal antibody and find out the conserved sequence which can bind to the monoclonal antibody with the ability of SVCV activity neutralization in a random peptide library. The site of the antigenic determinant is determined, and the relationship of its sequence, structure and function is analyzed. The study of inhibition effect to SVCV generated from the competitive binding of small peptide to SVCV receptor will have a huge application value for taking small molecular antigen peptide as vaccine in the future.
Owner:JILIN AGRICULTURAL UNIV
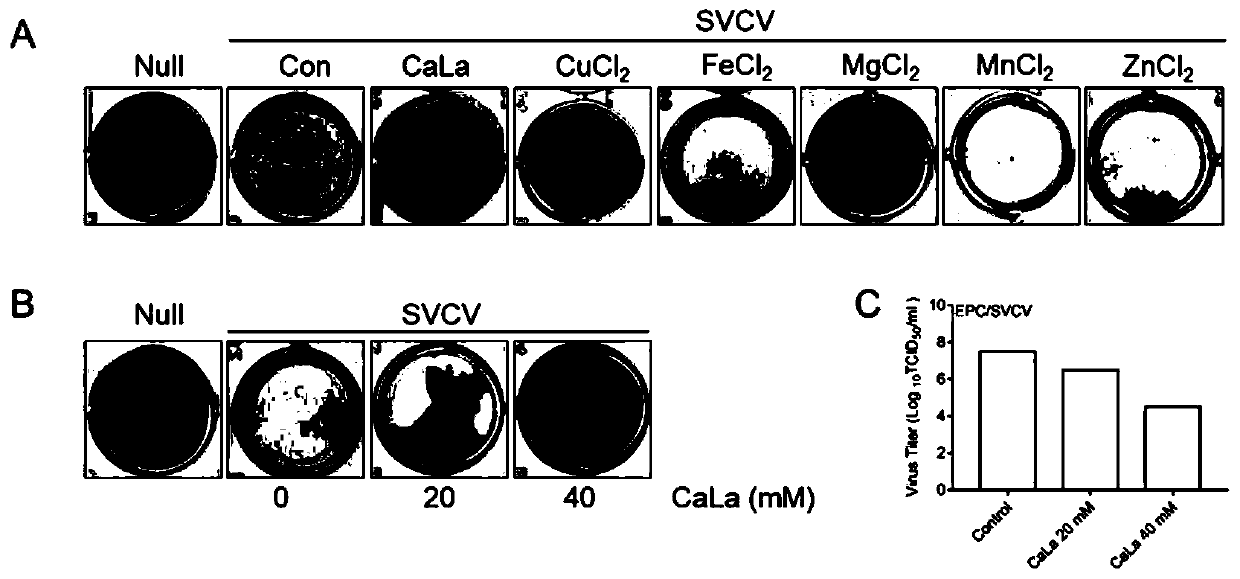
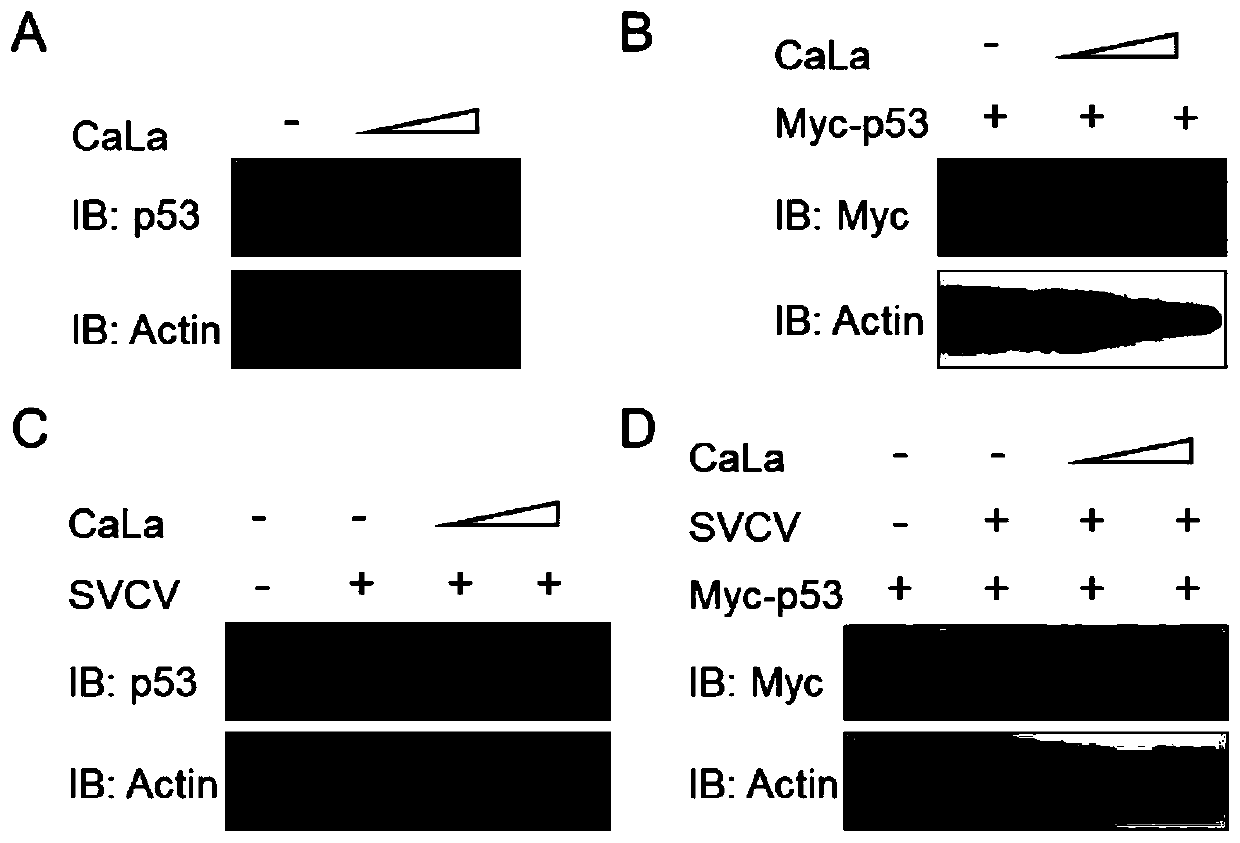
Application of calcium lactate in preparation of medicine for preventing and treating infection caused by spring viremia of carp virus (SVCV)
ActiveCN111035631AAntiviralsAluminium/calcium/magnesium active ingredientsSide effectCALCIUM LACTOBIONATE
The invention discloses an application of calcium lactate in preparation of a medicine for preventing and treating infection caused by spring viremia of carp virus (SVCV). An experimental animal infection model proves that calcium lactate can play a role in treating SVCV-caused infection of zebra fish, virus proliferation and qPCR experiments are combined to find that the expression quantity of virus-related genes after calcium lactate treatment is obviously reduced, so that calcium lactate can be used as a medicine for preventing and treating SVCV and has the advantages of low dosage, safetyand no toxic or side effect.
Owner:INST OF AQUATIC LIFE ACAD SINICA
Isolated antiviral natural immune protein TRIM32 (tripartite motif 32) for carps and antiviral activity
InactiveCN104611343AAffect proliferationImmunoglobulins against animals/humansAntiviralsNucleotideAquatic animal
Owner:HUAZHONG AGRI UNIV
Carp spring viremia virus detection kit based on pyrosequencing
InactiveCN105112568AHigh sensitivitySequencing is slowMicrobiological testing/measurementAgricultural scienceCarp
The invention discloses a carp spring viremia virus detection kit based on pyrosequencing. The carp spring viremia virus detection kit based on the pyrosequencing depends on a carp spring viremia virus fingerprint sequence, and the carp spring viremia virus fingerprint sequence is nucleotides at the 511th-526th positions of SEQ ID No.1 shown in a sequence table. The kit comprises a sequencing primer of the carp spring viremia virus fingerprint sequence and a primer pair for amplifying the carp spring viremia virus fingerprint sequence. The sequencing primer is single-stranded DNA represented by SEQ ID No.4 shown in the sequence table. The primer pair for amplifying the carp spring viremia virus fingerprint sequence consists of single-stranded DNA represented by SEQ ID No.2 shown in the sequence table and single-stranded DNA represented by SEQ ID No.3 shown in the sequence table.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
New porcine reproductive and respiratory syndrome virus ORF5 modified gene and application thereof
ActiveCN102321638AImprove immunityLow blood levelsBacteriaViral antigen ingredientsLymphocyte proliferationPorcine reproductive and respiratory syndrome virus
The invention belongs to the field of molecular biology, and discloses a new porcine reproductive and respiratory syndrome virus ORF5 modified gene and an application thereof. The porcine reproductive and respiratory syndrome virus ORF5 modified gene has a sequence as shown in SEQ IDNO. 2, SEQ IDNO. 3, SEQ IDNO. 4, or SEQ IDNO. 5. A recombinant plasmid containing the porcine reproductive and respiratory syndrome virus ORF5 modified gene is provided. Attenuated salmonella SL-pCI-SynGP5 / GP4-5 containing the recombinant plasmid is provided. Results show that the constructed recombinant plasmid can induce high-level humoral immune response, lymphocyte proliferation response, and cytokine response. the recombinant salmonella can induce high-level antibody response, and induce high-level interferon; the viremia level is low; the damage of tissues and organs after toxin attack is relatively slight, and the biosafety level is high.
Owner:NANJING AGRICULTURAL UNIVERSITY
Method for detecting spring viremia of carp virus based on liquid chip
InactiveCN103173574ANo pollutionNo health threatMicrobiological testing/measurementMicroorganism based processesAgricultural scienceAquatic animal
The invention discloses a mehtod for detecting spring viremia of carp virus based on a liquid chip. The invention provides a specific primer pair for assisting in identification of the spring viremia of the carp virus, which comprises single-stranded DNA (deoxyribonucleic acid) molecules as shown in a sequence 1 in a sequence table and the single-stranded DNA molecules as shown in a sequence 2 in the sequence table. The invention further protects a primer probe composition for assisting in identification of the spring viremia of the carp virus, which comprises the specific primer pair and a probe T1, wherein the nucleotide sequence of the probe T1 is as shown in a sequence 3 in the sequence table. The specific primer pair provided by the invention has good specificity when being used for identifying the spring viremia of the carp virus. The primer probe composition combined liquid chip provided by the invention has the advantages of good specificity, high sensitivity, simplicity and convenience in operation, short time, no environmental pollution, no threat to human health and high-throughput detection when being used for identifying the spring viremia of the carp virus. The method disclosed by the invention is very suitable for detecting import and export aquatic animals.
Owner:INSPECTION & QUARANTINE TECH CENT SHANDONG ENTRY EXIT INSPECTION & QUARANTINE BUREAU +1
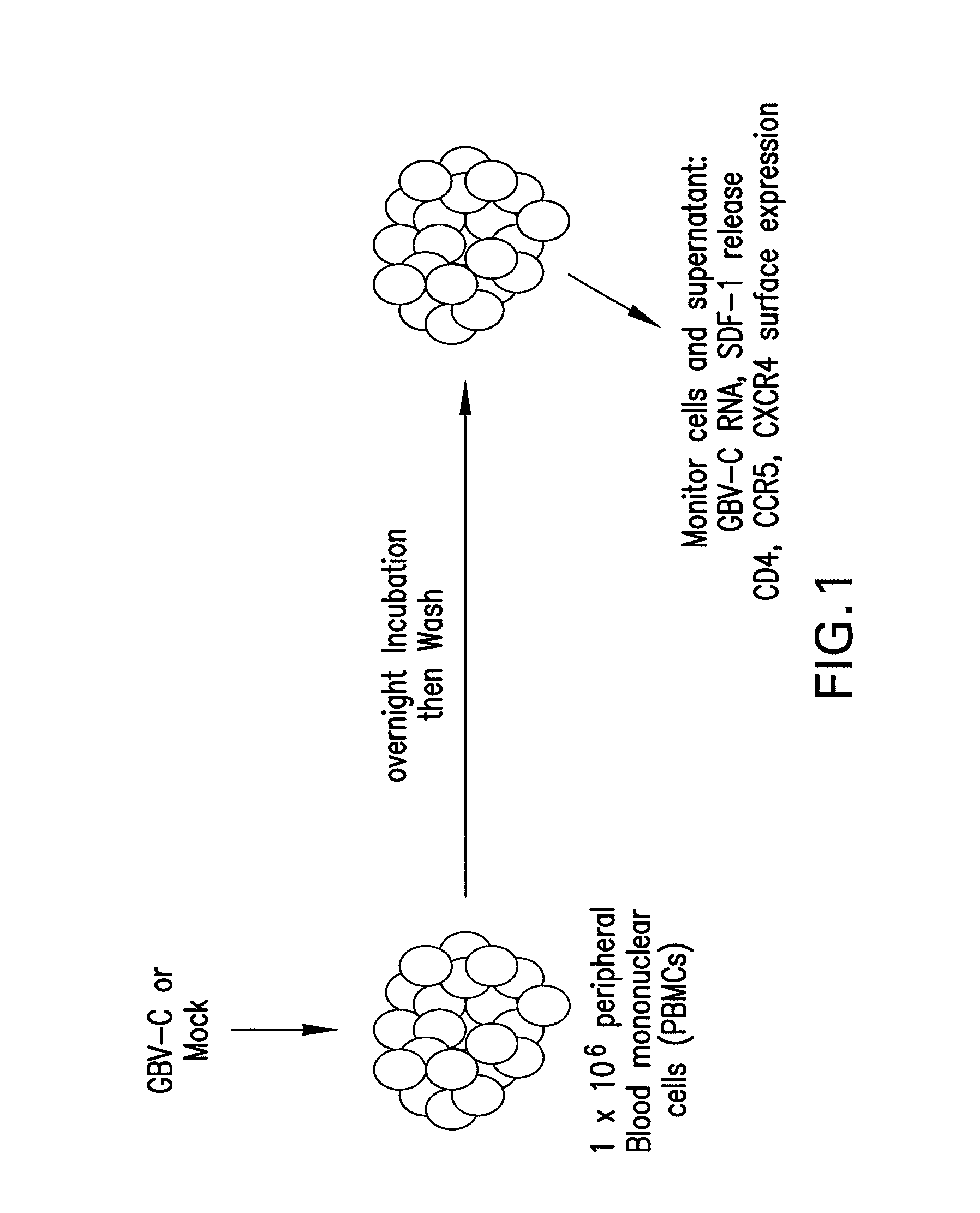
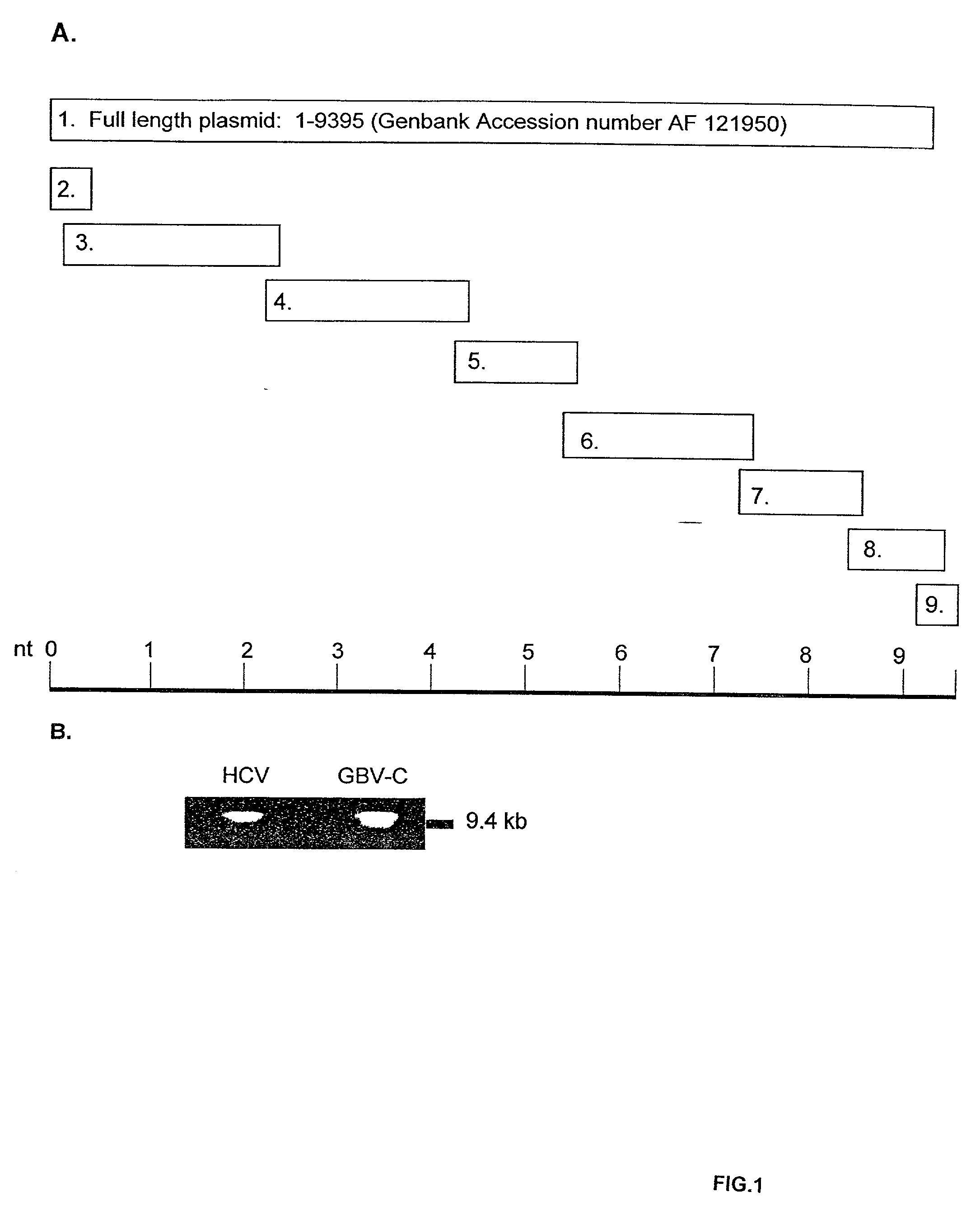
Full-length GB virus C (hepatitis G virus) RNA transcripts are infectious in primary CD4 positive T cells and methods of treating HIV
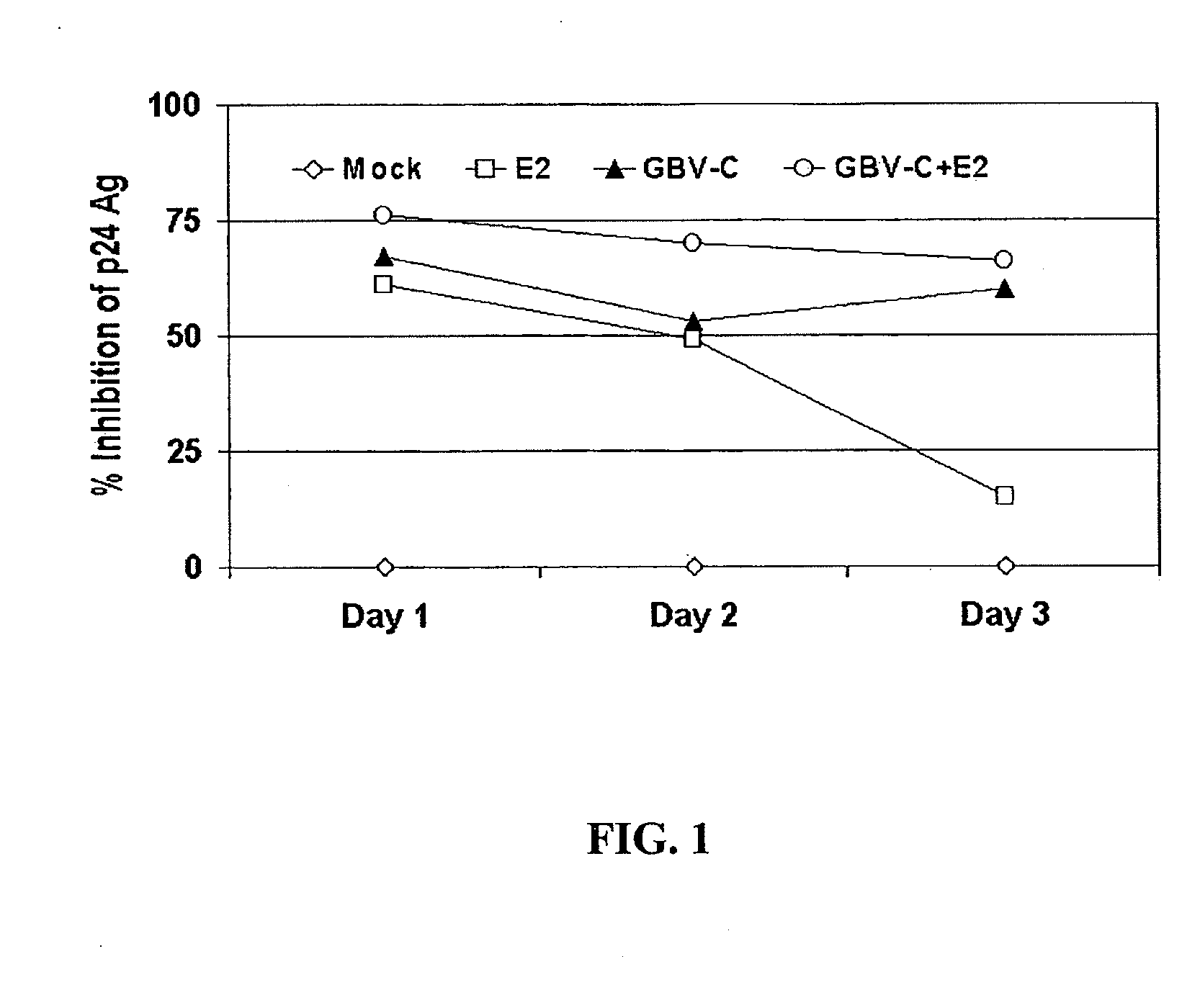
GB virus C (GBV-C or hepatitis G virus) is a recently described flavivirus that frequently leads to chronic viremia in humans. Although associated with acute post-transfusion hepatitis, it is not clear if GBV-C is pathogenic for humans. A full-length cDNA was constructed from the plasma of a person with chronic GBV-C viremia. Peripheral blood mononuclear cells (PBMCs) transfected with full-length RNA transcripts from this GBV-C clone resulted in viral replication, demonstrating an isolated infectious GBV-C nucleic acid molecule. In addition to composition involving an isolated infectious GBV-C nucleic acid molecule, the present invention concerns methods of inhibiting and treating HIV infections.
Owner:UNIV OF IOWA RES FOUND
African swine fever gene deletion attenuated virus and live vaccine thereof
PendingCN111925996AReduce feverReduce viremiaViral antigen ingredientsVirus peptidesClassical swine fever virus CSFVAfrican swine fever
The invention discloses an African swine fever gene deletion attenuated virus, further provides an African swine fever gene deletion live vaccine prepared by utilizing the African swine fever gene deletion attenuated virus, and provides a novel attenuated African swine fever virus with gene deletion. By deleting partial or all gene functions of L7L, L8L, L9R, L10L and L11L, compared with a parentstrain, the obtained recombinant strain has the advantages that after a pig is infected, fever and viraemia do not appear or are remarkably relieved, and the test pig is healthy and alive; vaccines prepared from the gene deletion live virus can protect susceptible pigs from ASFV virulent infection or artificial challenge, and can be used for preventing African swine fever.
Owner:ACAD OF MILITARY SCI PLA CHINA ACAD OF MILITARY MEDICAL SCI INST OF MILITARY VETERINARY MEDICINE
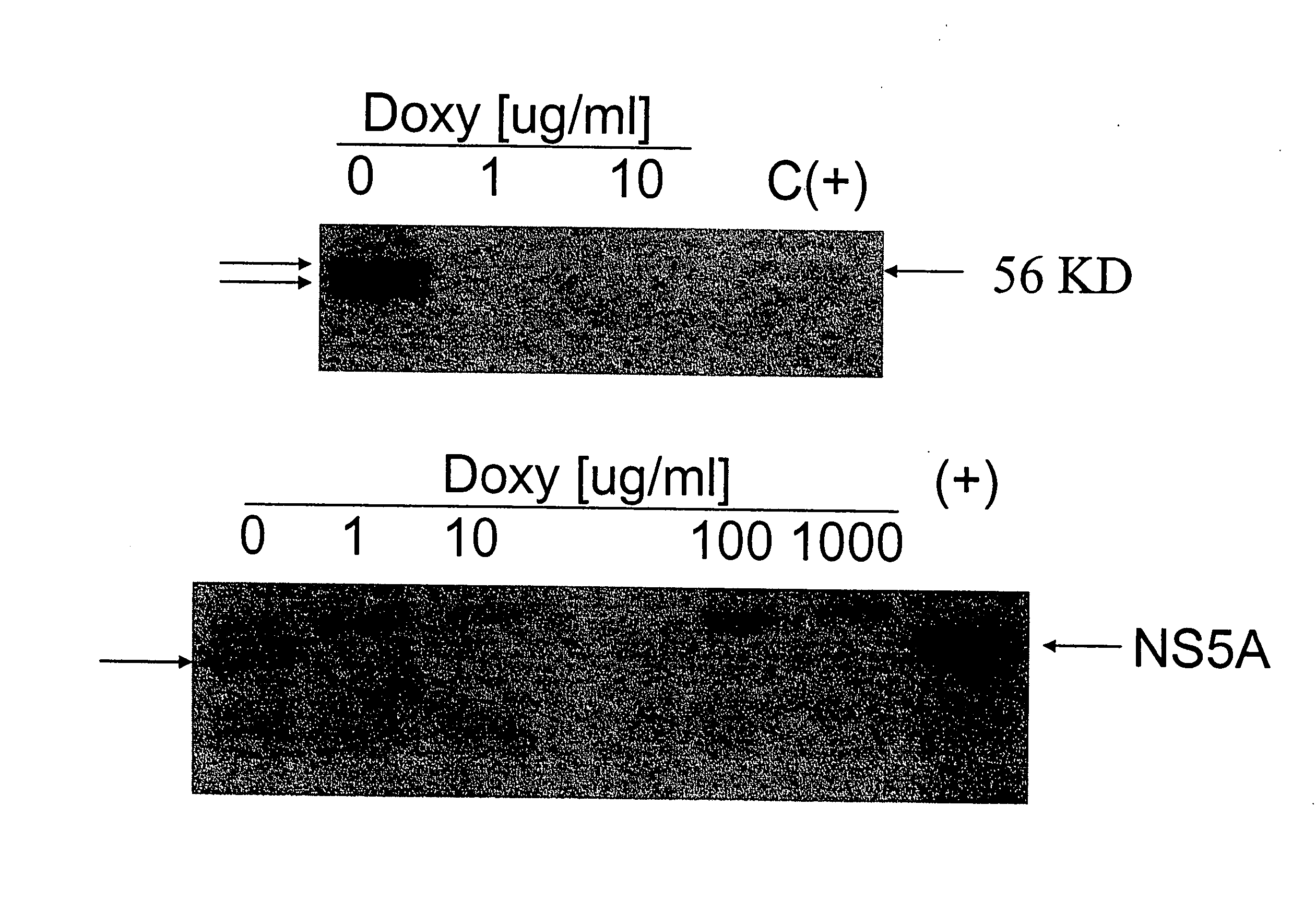
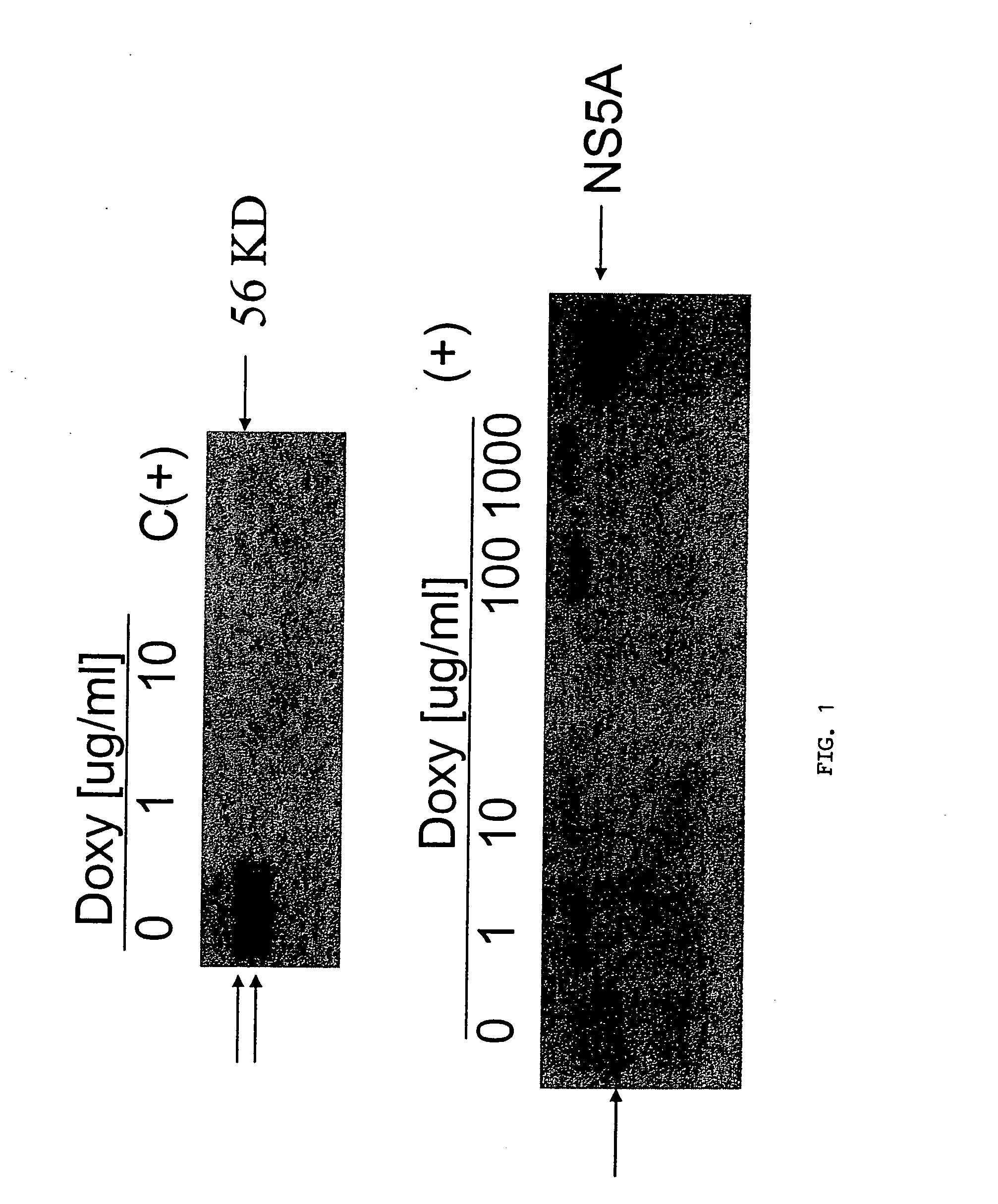
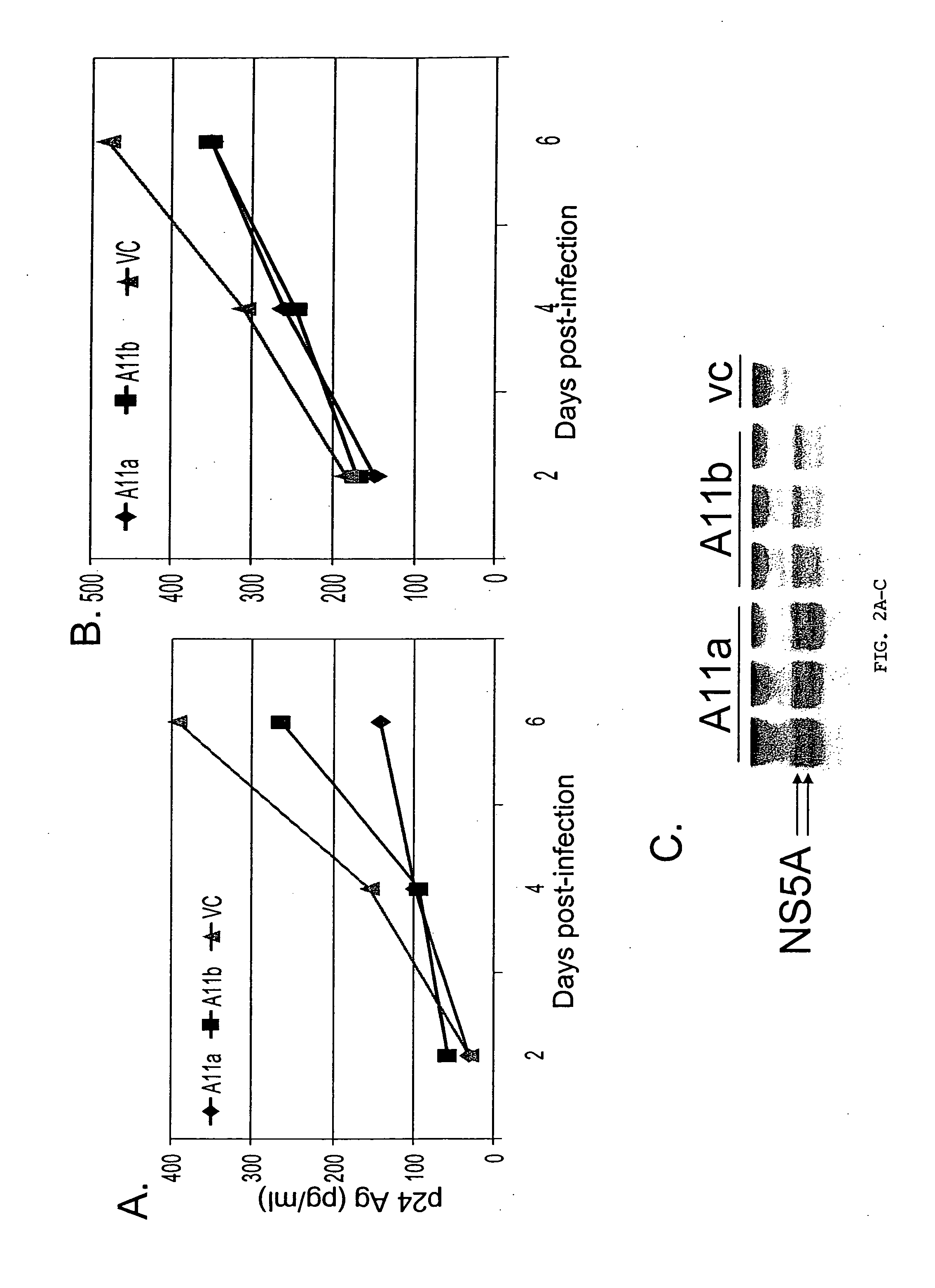
Flavivirus NS5A proteins for the treatment of HIV
InactiveUS20070036825A1Reduce HIV replicationPreventing HIV infectionSsRNA viruses positive-sensePeptide/protein ingredientsGB virus CHepatitis G virus RNA
Owner:IOWA RES FOUND UNIV OF
Anti-SVCV (Spring Viraemia of Carp Virus) monoclonal antibody 3E1 as well as preparation and application thereof
InactiveCN103509758AStrong specificityHigh potencyImmunoglobulins against virusesDepsipeptidesAntigenMicrosphere
The invention discloses an anti-SVCV (Spring Viraemia of Carp Virus) monoclonal antibody 3E1 as well as preparation and application thereof. The monoclonal antibody 3E1 is secreted by a hybridoma cell strain SVCV-3E1, and the preservation number of the cell strain is CCTCC C201366. The hybridoma cell strain SVCV-3E1 can stably generate the monoclonal antibody 3E1, and the antibody has the advantages of high antibody titer, high sensitivity, strong specificity, strong affinity with natural antigen and the like. The invention also provides a rapid detection test strip containing the monoclonal antibody and a kit thereof. The rapid detection test strip adopts a fluorescent nanometer microsphere immunochromatography assay technology and has the characteristics of good sensitivity, specificity, stability, repeatability, reproducibility and the like; meanwhile, the test strip and the kit have the advantages of simplicity and convenience in use and rapid detecting speed, can meet the detection requirements for food safety, slaughter, detection mechanisms and the like, and are suitable for field detection.
Owner:SHENZHEN AUDAQUE DATA TECH +1
RT-PCR (reverse transcription-polymerase chain reaction) detection kit for spring viremia of carp virus as well as detection method
InactiveCN108004349AQuick checkWide Application MonitoringMicrobiological testing/measurementPositive controlReverse transcriptase
The invention relates to the technical field of aquaculture, and in particular discloses a reverse transcription-polymerase chain reaction (RT-PCR) detection kit for spring viremia of carp virus. TheRT-PCR detection kit for the spring viremia of carp virus comprises a 5*reverse transcription buffer solution, reverse transcriptase, a random primer, a 2*reaction mixed buffer solution, preferably designed upstream and downstream primers, Taq DNA polymerase, positive control liquid, negative control liquid and ddH2O. The invention provides a novel RT-PCR rapid detection kit and a detection methodso as to overcome the defects in the existing method for detecting the spring viremia of carp virus. Clinical diagnosis on the spring viremia of carp virus is practicable, the advantages of rapidness, accuracy and specificity are achieved, the requirements of clinical diagnosis are met and convenient conditions are providing for detecting the spring viremia of carp virus.
Owner:广州利洋水产科技股份有限公司
A gene-deleted attenuated African swine fever virus strain and its construction method and application
ActiveCN113122511BNo joint swellingIncrease virulenceViral antigen ingredientsVirus peptidesImmunogenicityTGE VACCINE
The invention discloses a gene-deleted attenuated African swine fever virus strain, a construction method and application thereof, and belongs to the technical field of biological vaccine products. The gene-deleted attenuated African swine fever virus strain constructed by the homologous recombination method of the present invention is based on the type II African swine fever virus genome and simultaneously deletes CD2v, MGF (12L, 13L, 14L) and I177L gene fragments The gene deletion virus strain is obviously attenuated relative to the parental strain, and will not affect the stable replication and immunogenicity of the gene deletion virus strain. After being inoculated into experimental pigs, there will be no obvious rise in body temperature, joint swelling, disease or death of experimental pigs, and no viremia, showing good safety and good immune challenge protection effect. Therefore, the gene-deleted attenuated African swine fever virus strain provided by the present invention can be used as a candidate vaccine strain with good safety and immune protection effect.
Owner:JINYUBAOLING BIO PHARMA CO LTD +2
Gb virus c (hepatitis g virus) for the treatment of HIV
InactiveUS20090010932A1Decreased and delayed mortalitySimple methodBiocideSsRNA viruses positive-senseAntigenHepacivirus
Owner:UNIV OF IOWA RES FOUND +1
Reagent for multiple detection of salmon trout virus and application thereof
ActiveCN110387440ARapid single-use assayEffective one-time analysis and detectionMicrobiological testing/measurementDNA/RNA fragmentationViral haemorrhagic septicaemia virusTrout
The invention discloses a reagent for multiple detection of salmonidae virus and an application thereof. The reagent provided by the invention comprises a specific primer group, wherein the specific primer group comprises at least one pair from a first primer pair to a sixth primer pair; the first primer pair to the sixth primer pair are sequentially primer pairs of: nucleic acid of specifically-amplified spring viraemia of carp virus, nucleic acid of infectious hematopoietic necrosis virus, nucleic acid of viral haemorrhagic septicaemia virus, nucleic acid of infectious salmon anaemia virus,nucleic acid of salmonid alphavirus, and nucleic acid of infectious pancreatic necrosis virus. The upstream and downstream primers of the first primer pair to the sixth primer pair are sequentially the sequences as shown in Seq ID No.1 to Seq ID No.12. The reagent can carry out multiple detection on six salmonidae viruses, is simple and convenient to use, shortens the detection time of the six salmonidae viruses, improves the detection efficiency and quality, and has great significance for rapid detection and prevention and control of the six salmonidae viruses.
Owner:SHENZHEN CUSTOMS ANIMAL & PLANT INSPECTION & QUARANTINE TECH CENT
Droplet digital PCR kit for detecting carp edema virus
InactiveCN110819740ANo cross reactionHigh sensitivityMicrobiological testing/measurementMicroorganism based processesCarp edema virusFluoProbes
The invention relates to a droplet digital PCR (Polymerase Chain Reaction) kit for detecting carp edema virus. The kit includes a primer group and a fluorescent probe. The primer group includes an upstream primer of the sequence shown in SEQ ID NO.8 and a downstream primer of the sequence shown in SEQ ID NO.9. The fluorescent probe includes the fluorescent probe sequence shown in SEQ ID NO.5. Whenusing the kit to perform droplet digital PCR, the lowest detection limit of CEV (carp edema virus) is 1.8 copies / muL which is better than the sensitivity (17.6 copies / muL) of the fluorescent quantitation PCR detection method of the CEV; and no cross reaction is found with the nucleic acids of important aquatic animal pathogens like andriasda vidianus ranaviurs (ADRV), bohle virus (BIV), channel catfish virus (CCV) and spring viremia of carp virus (SVCV). The droplet digital PCR kit provided by the invention is of good specificity.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Detecting and quantifying cryptic HIV replication
InactiveUS20150024381A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBlood plasmaAntiviral therapy
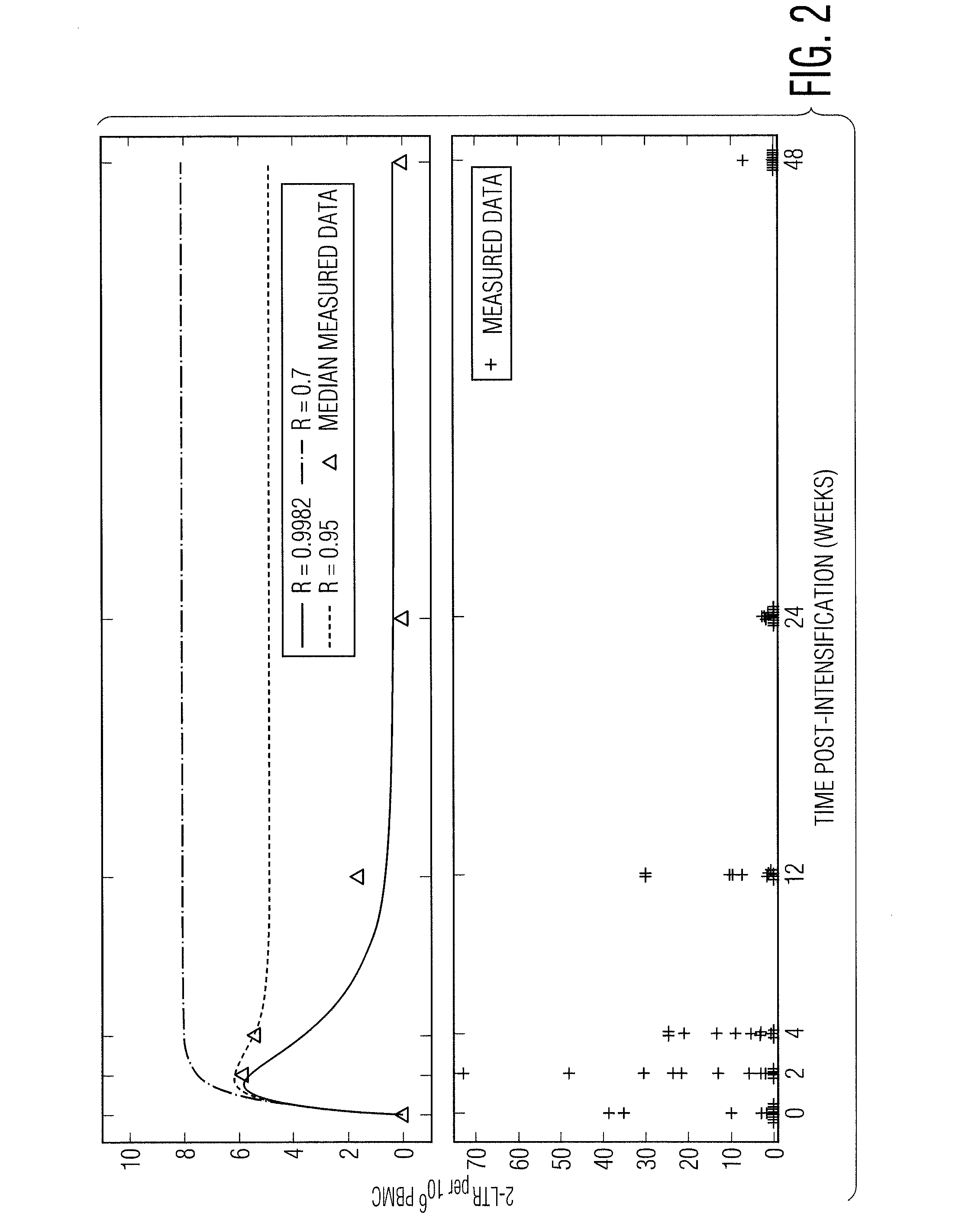
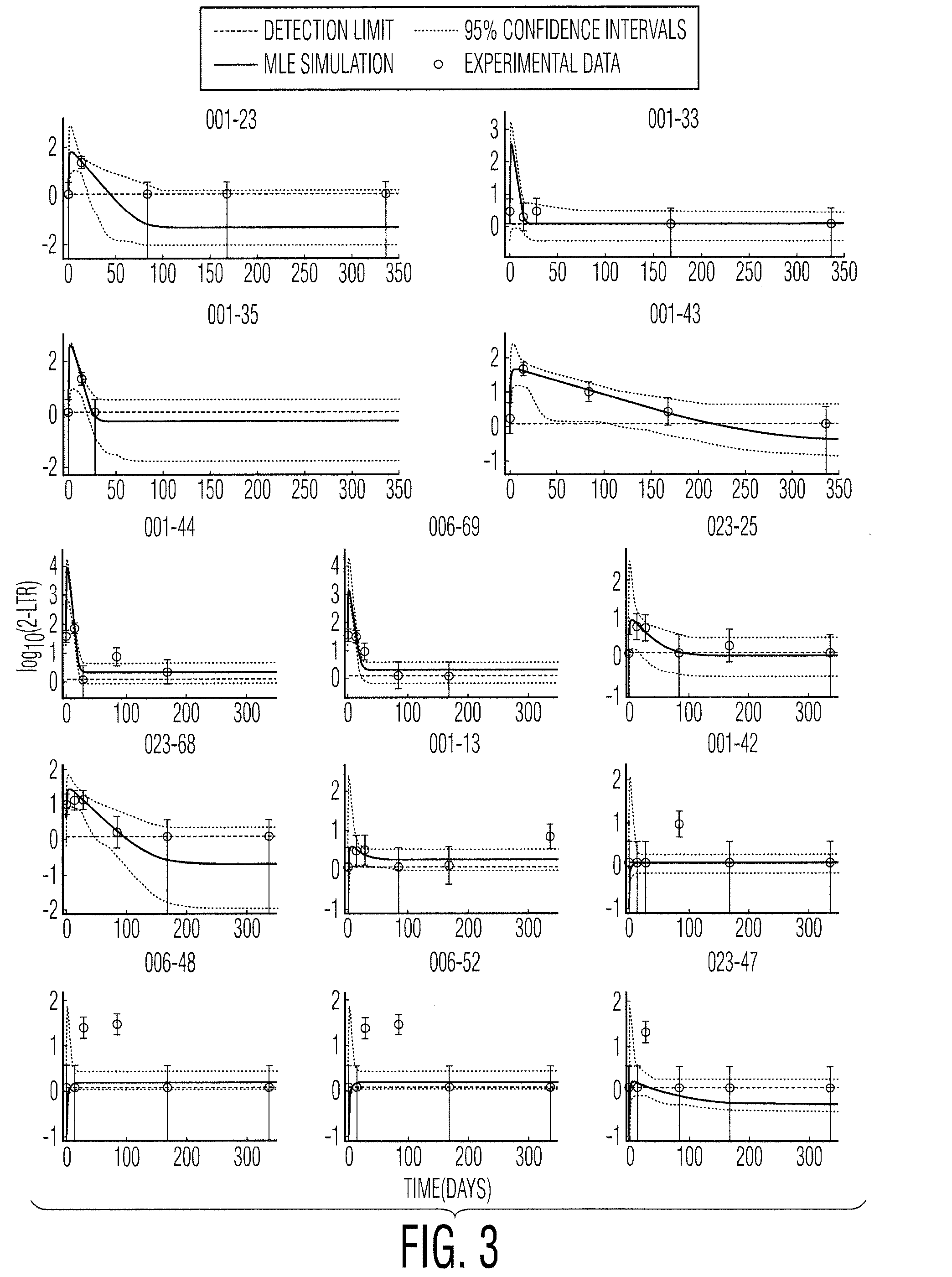
The present invention relates to a novel method for detecting efficient cryptic HIV replication in a patient who receives a suppressive antiviral therapy followed by administration of the HIV integrase inhibitor in an effective amount for intensifying the suppressive antiviral therapy, and has undetectable plasma viremia prior to the administration of the HIV integrase inhibitor. The method comprises making a pre-intensification measurement and one or more post-intensification measurements of the concentration of an episomal artifact in samples from the patient, and computing a pre-intensification HIV infection success ratio (R). A pre-intensification HIV infection success ratio (R) sufficiently close to 1 indicates that the patient has the efficient cryptic HIV replication. The method may further comprise quantifying the efficient cryptic HIV replication.
Owner:ZURAKOWSKI RYAN
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com