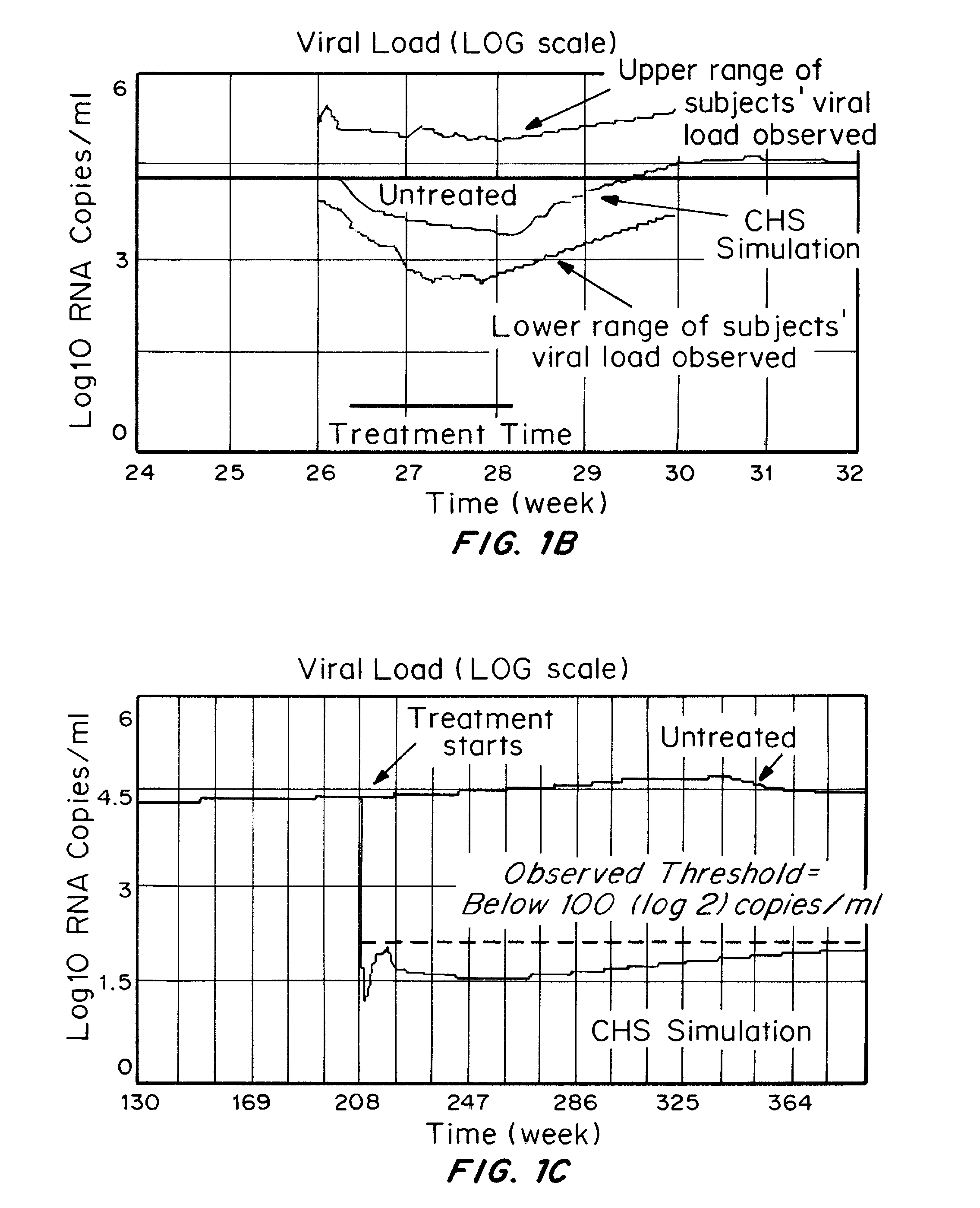
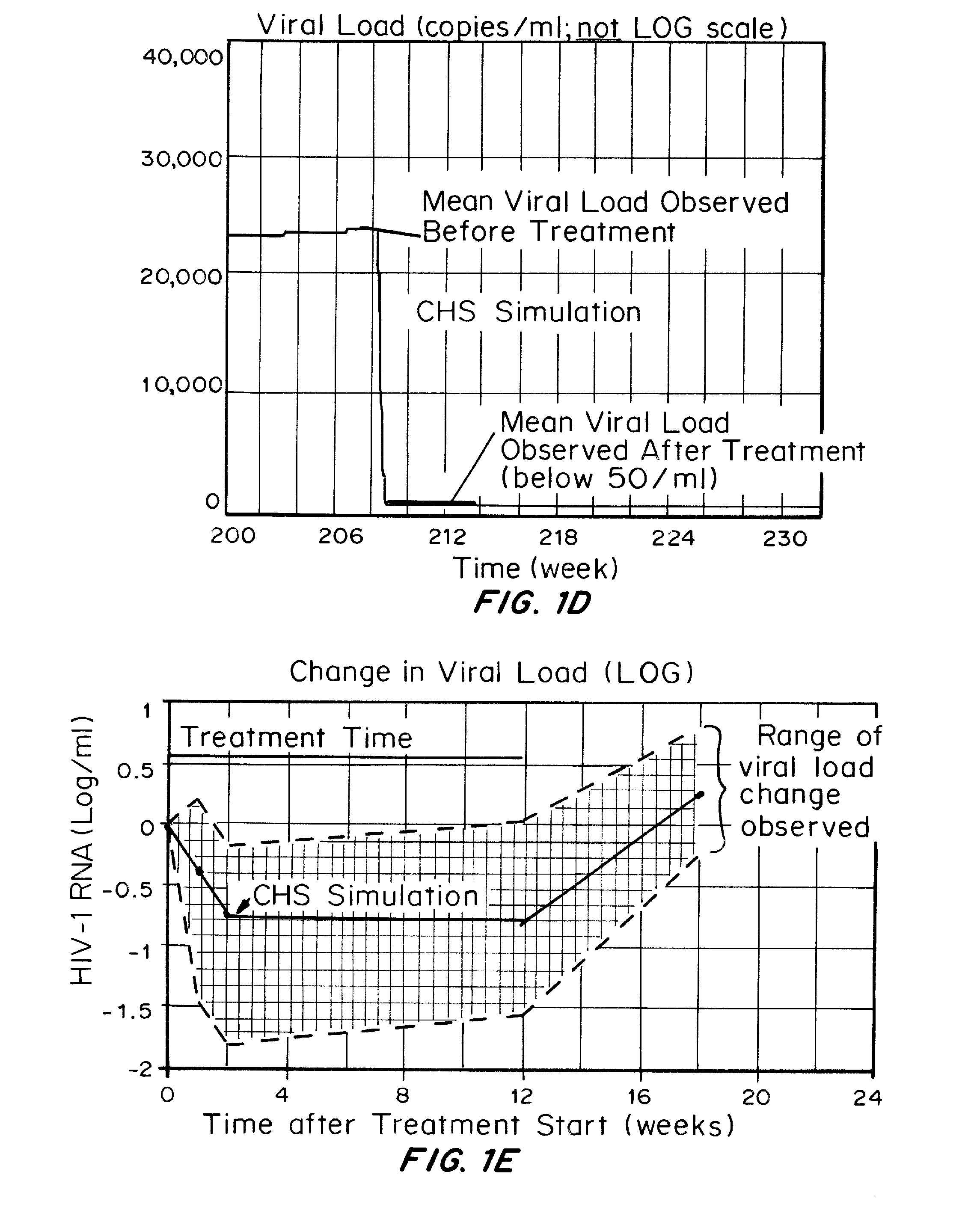
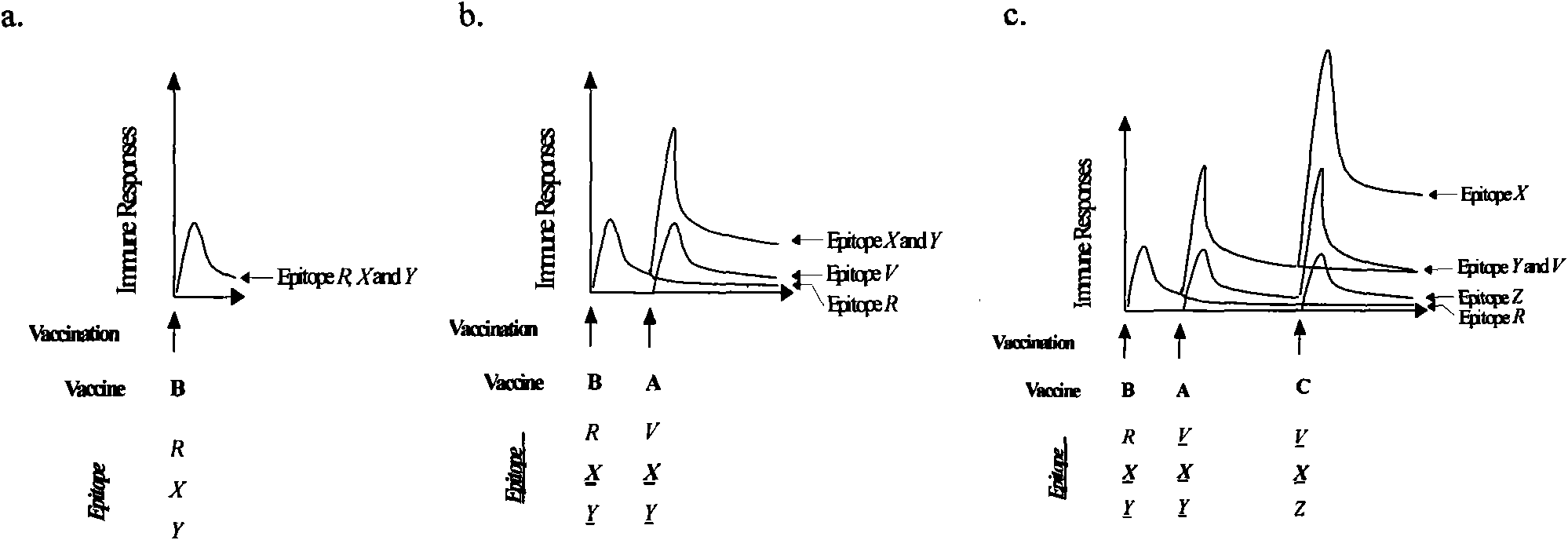
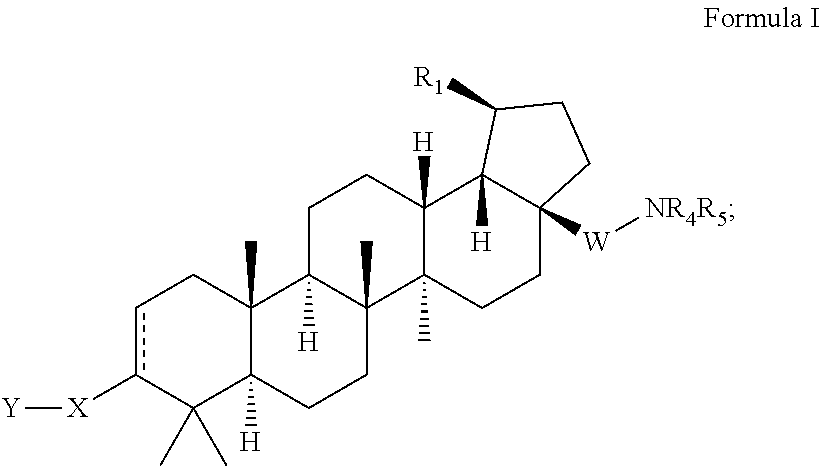
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
146 results about "HIV Coinfection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Coinfection means infection with more than one disease at the same time. Some coinfections commonly seen in people infected with HIV include: HIV/hepatitis B virus (HBV) coinfection HIV/hepatitis C virus (HCV) coinfection HIV/tuberculosis (TB) coinfection
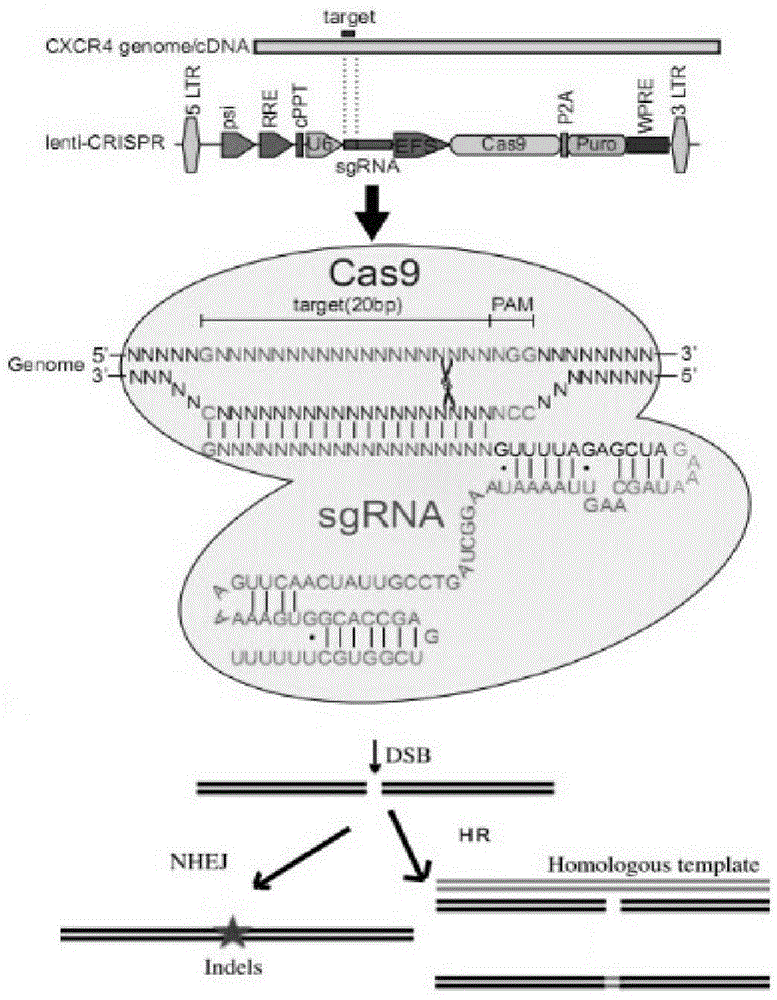
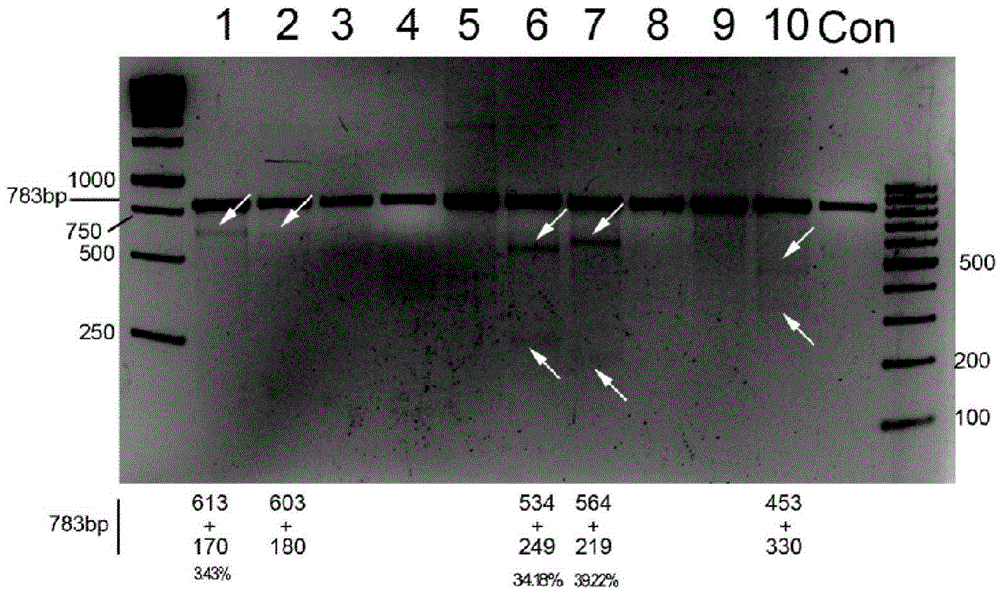
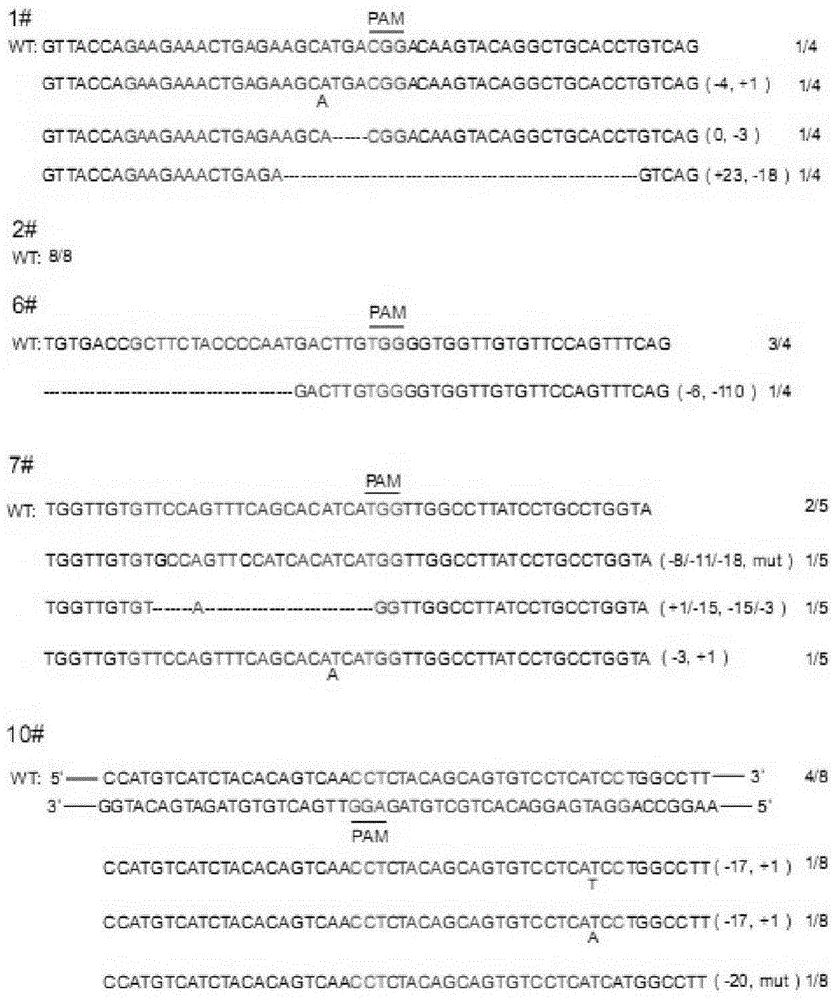
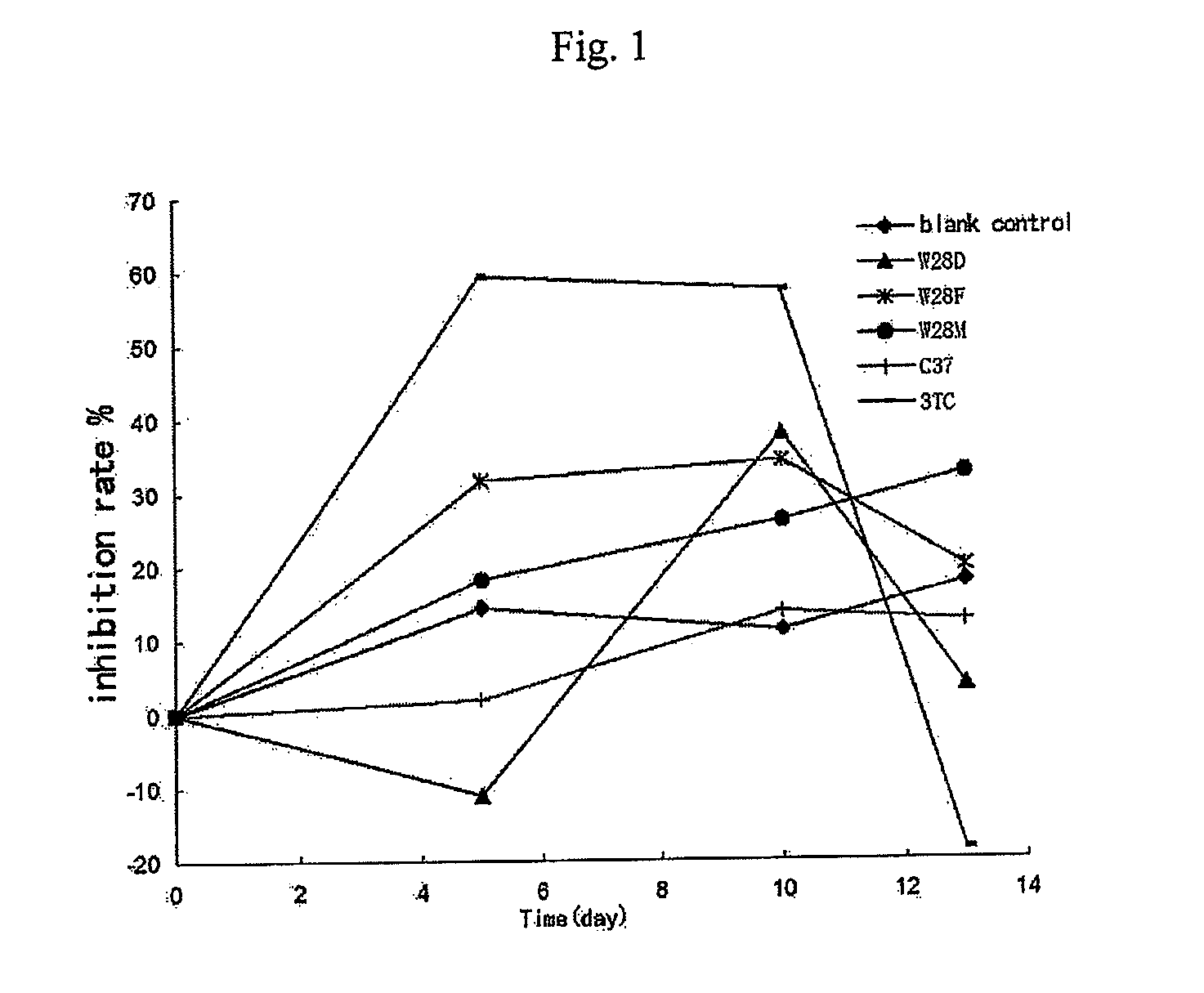
CRISPR/Cas9 recombinant lentiviral vector for human immunodeficiency virus gene therapy and lentivirus of CRISPR/Cas9 recombinant lentiviral vector
ActiveCN104480144AAvoid or delay intrusionInhibit the spread of infectionGenetic material ingredientsAntiviralsEnzyme digestionCXCR4
The invention belongs to the field of pharmaceutical and biological engineering, and relates to a CRISPR / Cas9 recombinant lentiviral vector for human immunodeficiency virus gene therapy and a lentivirus of the CRISPR / Cas9 recombinant lentiviral vector. The recombinant lentiviral vector is prepared by carrying out enzyme digestion on a lentiviral vectorlentiCRISPR by BsmBI and connecting into a BsmBI cohesive end-containing CXCR4 specific target sequence to recombine; the obtained CRISPR / Cas9 recombinant lentiviral vector is capable of mutating gene sequences at four different loci of ahuman immunodeficiency virusco-receptor CXCR4 and themutatuin rate is high and up to 25-75%. The cells transformed by the recombinant lentiviral vector cannot be infected by the human immunodeficiency virus. Compared with theRNAi-Knockdown, ZFN and TALEN technologies, the method has higher efficiency of suppressing the human immunodeficiency virus replication; the system is rapid to construct, simple and low in cost, is capable of preventing the invasion of the human immunodeficiency virus and is suitable for human immunodeficiency virus gene therapy.
Owner:WUHAN UNIV
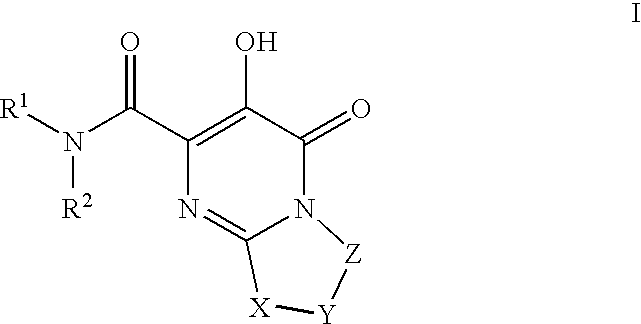
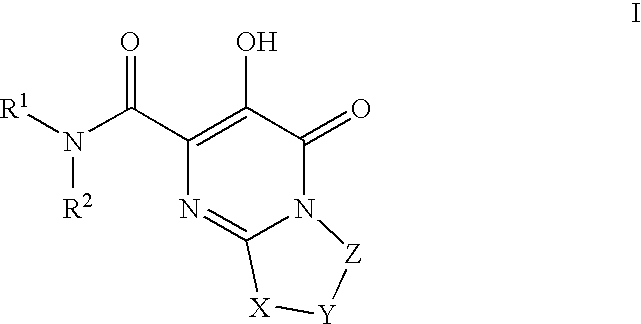
HIV Integrase Inhibitors
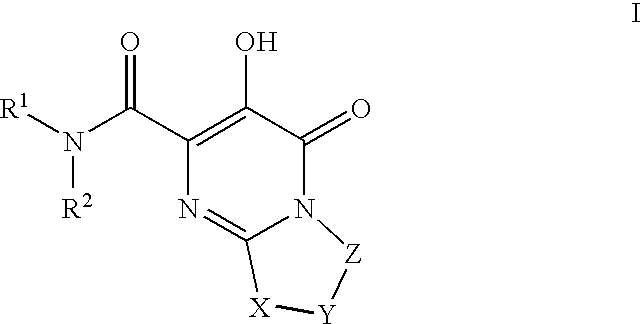
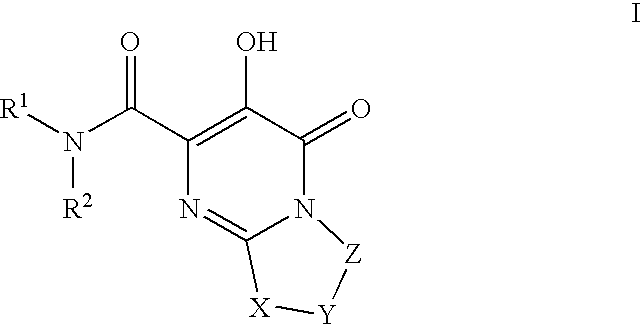
The invention encompasses series bicyclic pyrimidinone compounds of Formula I which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
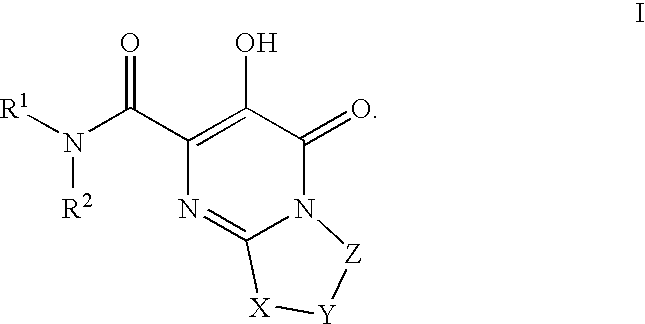
HIV integrase inhibitors: cyclic pyrimidinone compounds
The invention encompasses a series of pyrimidinone compounds which inhibit HIV integrase and thereby prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses intermediates useful for making the pyrimidone compounds. Additionally, pharmaceutical compositions and methods for treating those infected with HIV are encompassed
Owner:BRISTOL MYERS SQUIBB CO
Methods of treating HIV infection
Owner:VIIV HEALTHCARE UK (NO 5) LTD
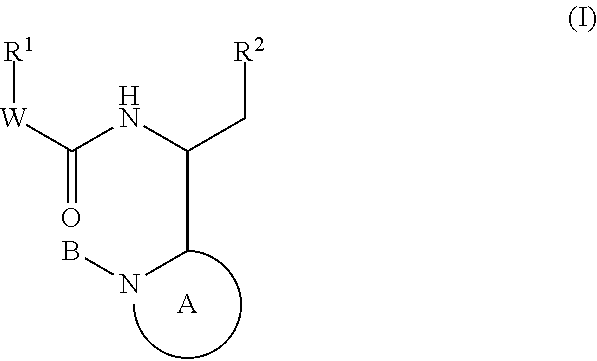
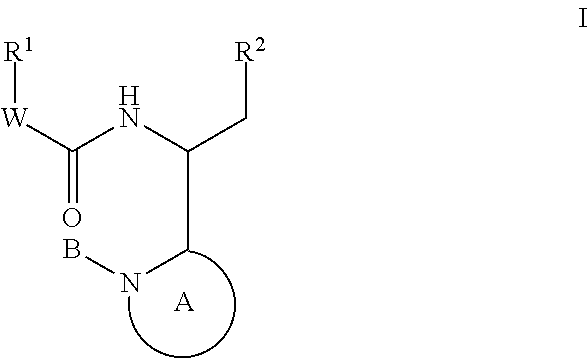
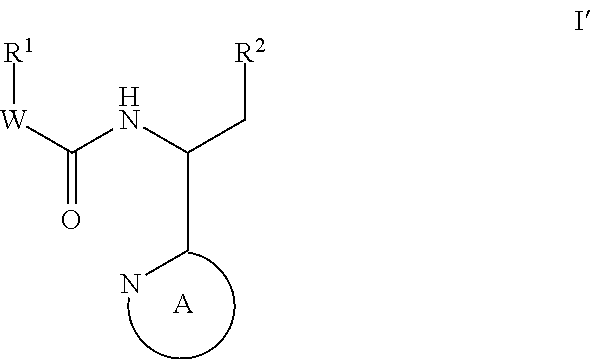
Indole, azaindole and related heterocyclic 4-alkenyl piperidine amides
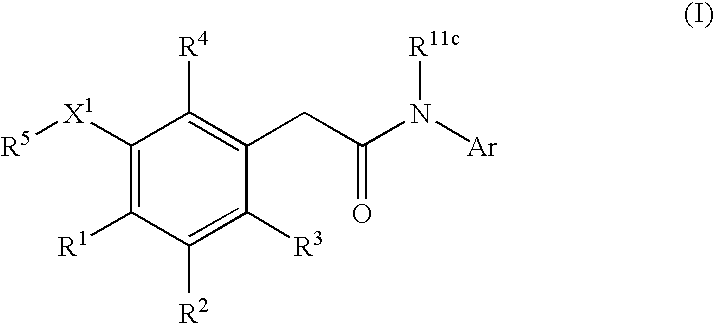
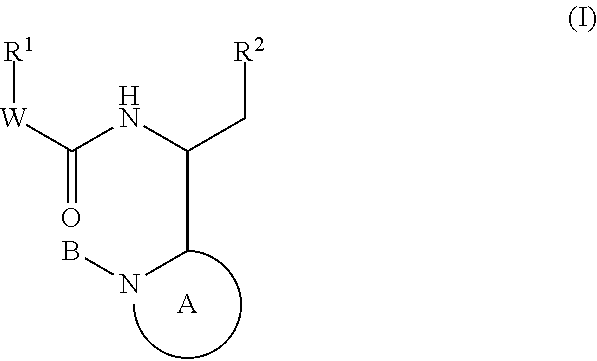
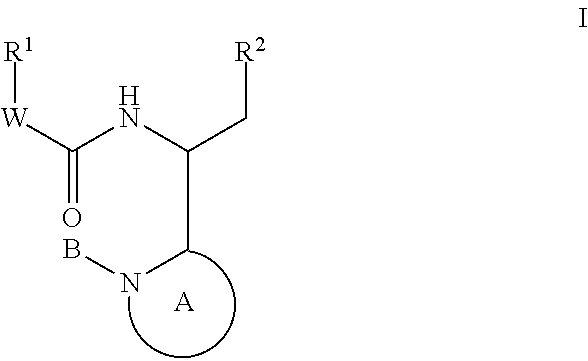
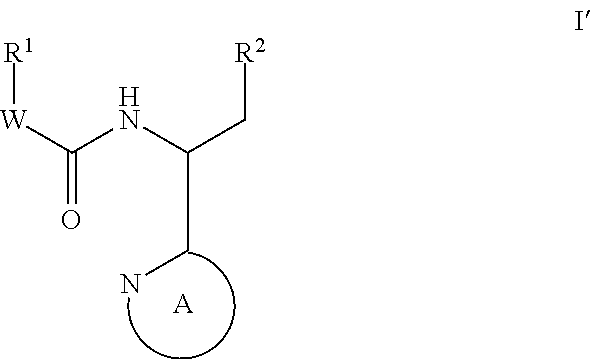
This invention provides compounds having drug and bio-affecting properties, their pharmaceutical compositions and method of use. In particular, the invention is concerned with new piperidine 4-alkenyl derivatives that possess unique antiviral activity. More particularly, the present invention relates to compounds useful for the treatment of HIV and AIDS. The compounds of the invention for the general Formula I: wherein: Z is Q is selected from the group consisting of: -W- is
Owner:VIIV HEALTHCARE UK (NO 5) LTD
Heterocyclic Non-Nucleoside Compounds, Their Preparation, Pharmaceutical Composition And Their Use As Antiviral Agents
InactiveUS20100056569A1Inhibition of replicationEasy to prepareBiocideOrganic chemistryDiseaseMedicine
The invention relates a kind of antiviral agents, more concretely, relates to a kind of heterocyclic non-nucleoside compounds with the following structures, their preparation and pharmaceutical compositions including the compounds. The said compounds can be used as antiviral agents and as medicaments for treating diseases such as hepatitis B, influenza, herpes, HIV and so on.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Vaccine for prevention and treatment of HIV-infection
ActiveUS7612173B2Promote humoral and cellular responseAntibody mimetics/scaffoldsVirus peptidesImmunogenicityPolynucleotide
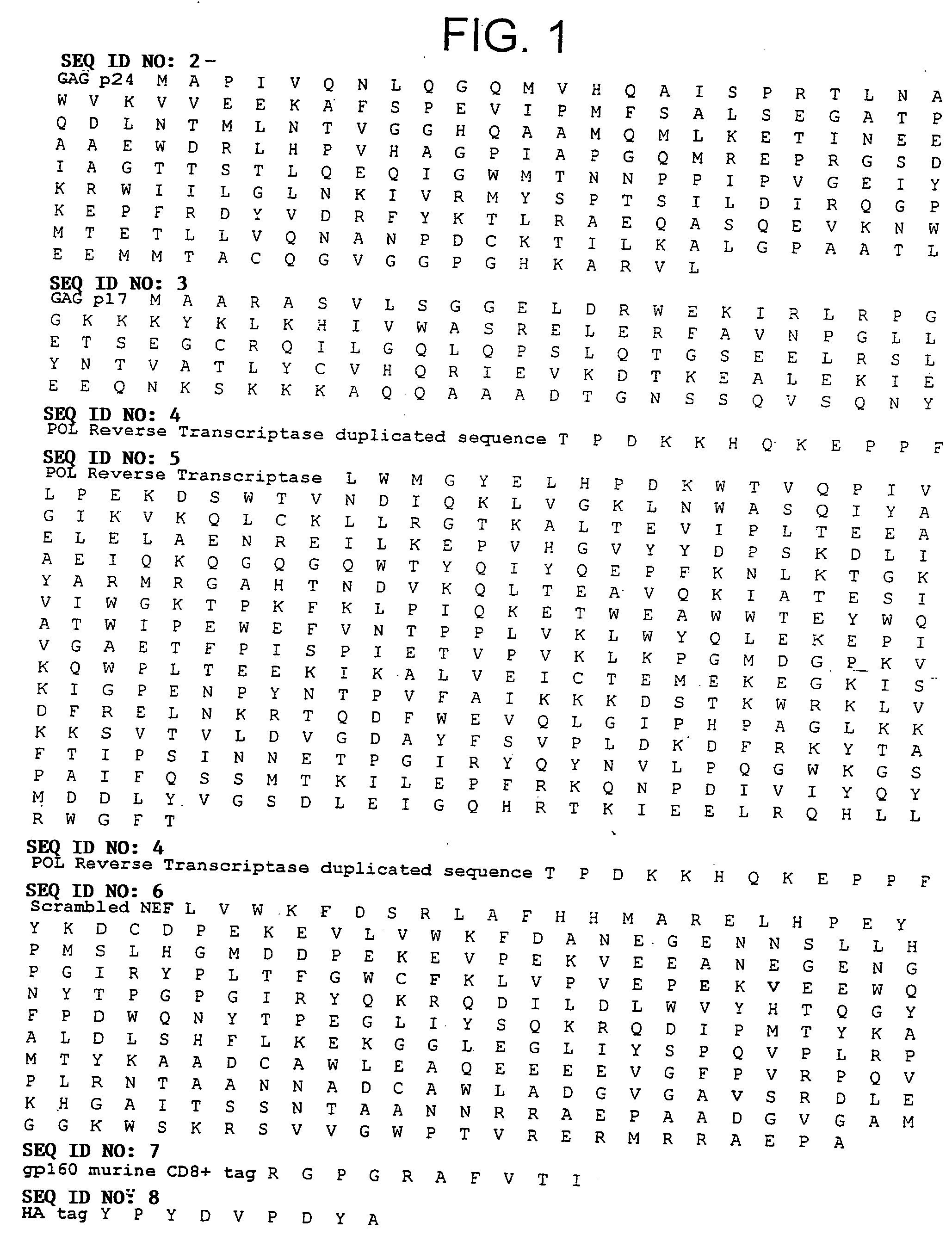
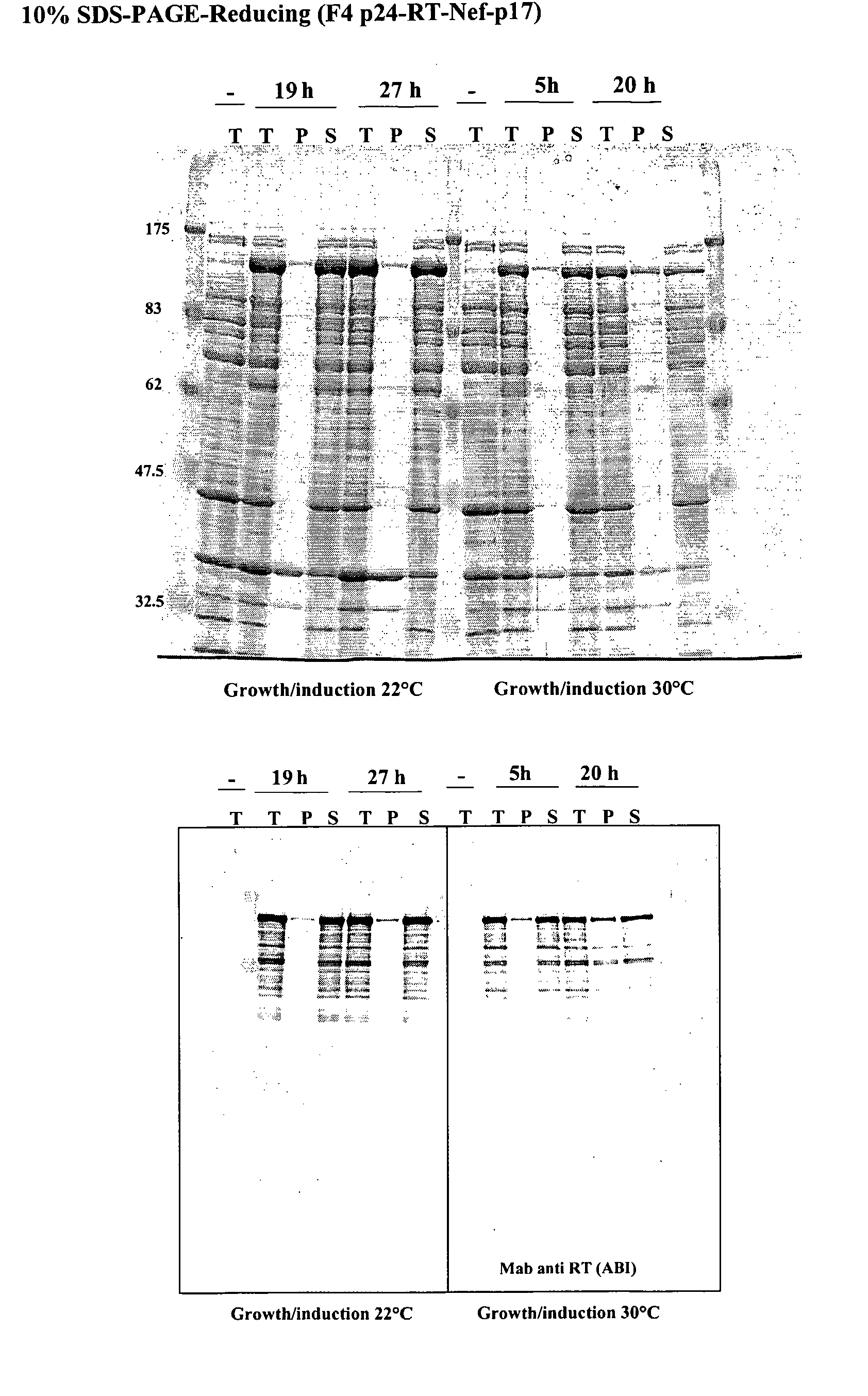
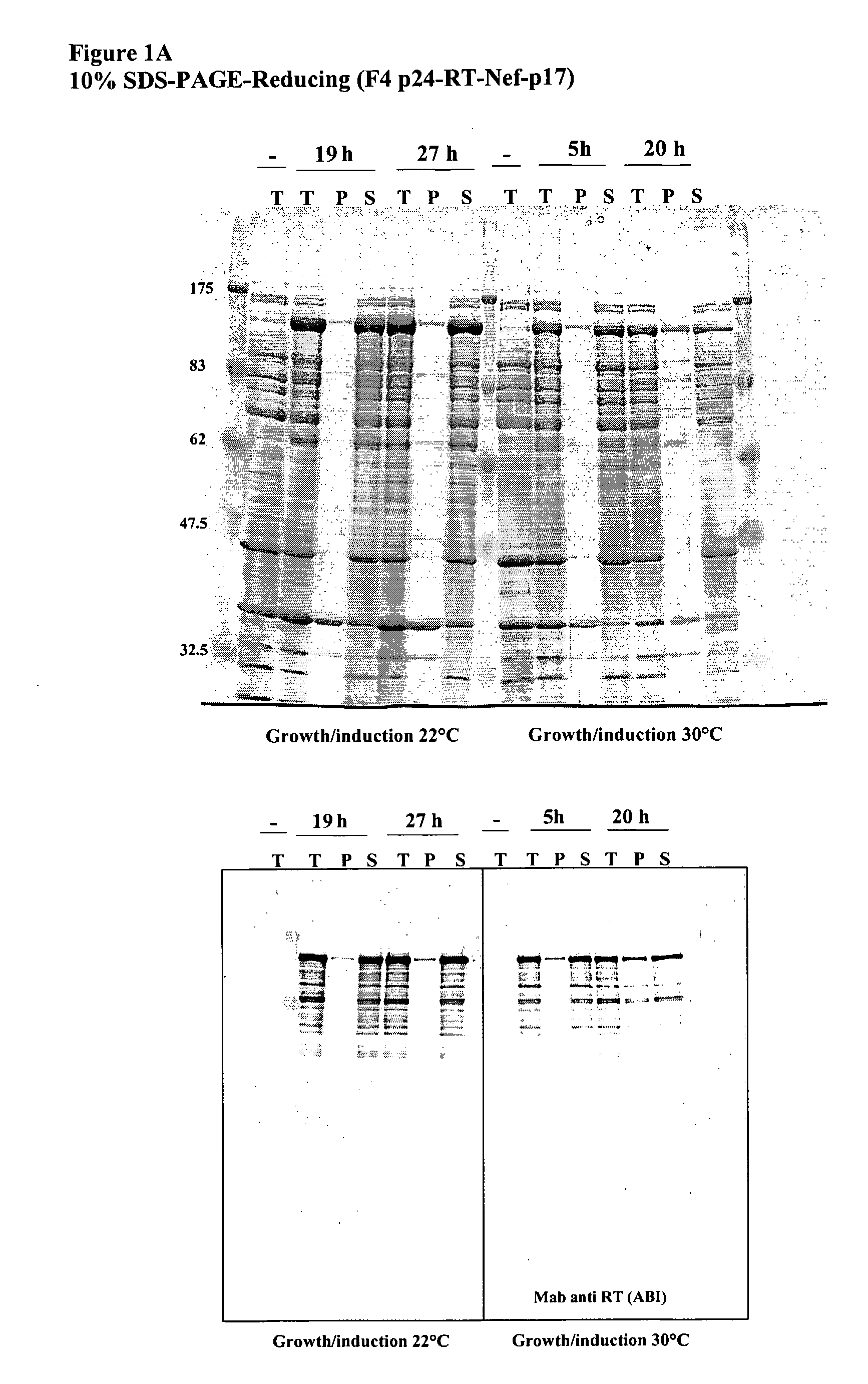
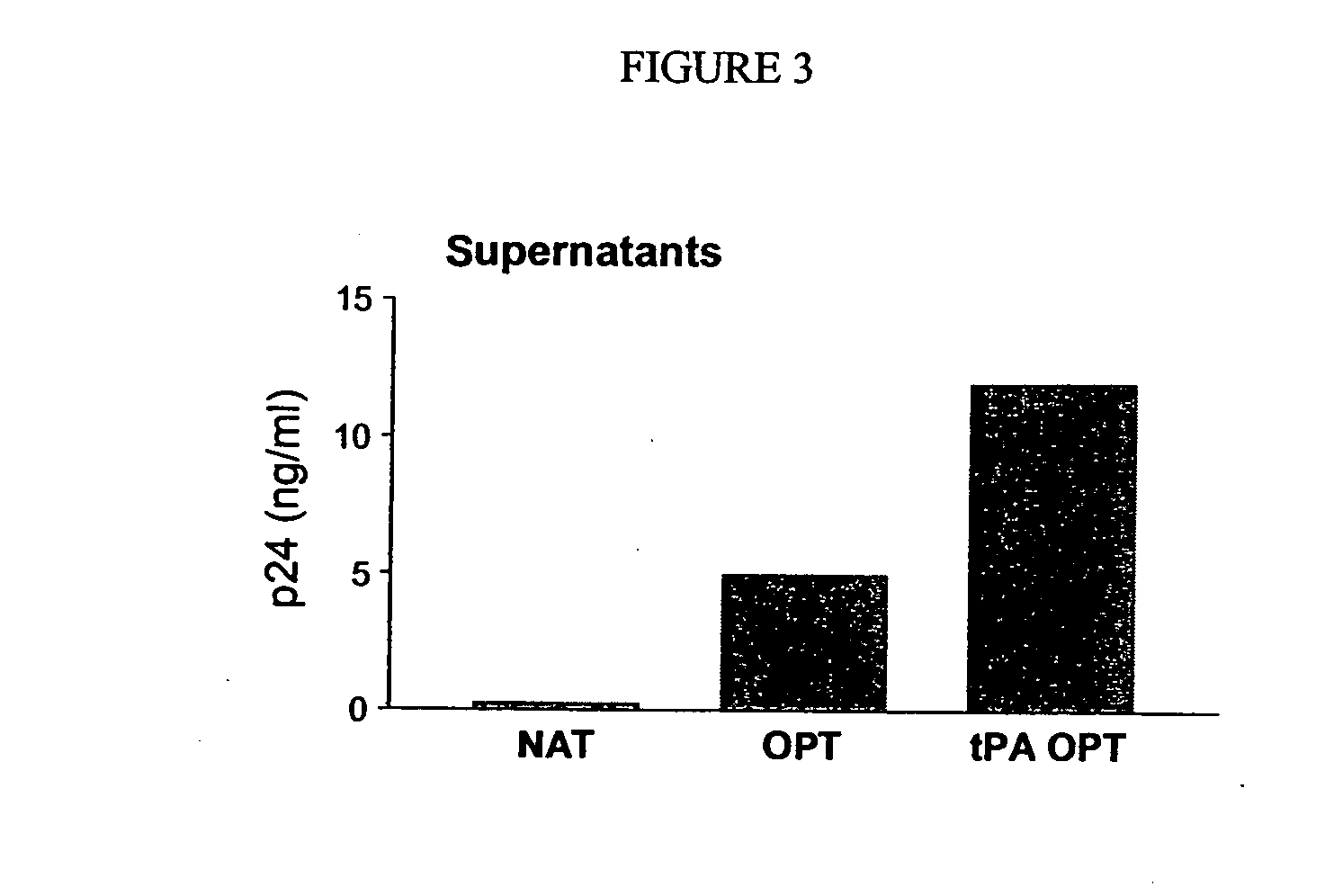
This invention relates to novel HIV polypeptide and polynucleotide fusions of Gag, Pol and Nef which are useful in immunogenic compositions and vaccines. The invention relates in particular to a polypeptide which comprises Nef or an immunogenic fragment thereof, and p17 Gag and / or p24 Gag or immunogenic fragments thereof, wherein when both p17 and p24 Gag are present there is at least one HIV antigen or immunogenic fragment between them. The polypeptide may also comprise Pol or RT or an immunogenic fragment thereof.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Disinfectants to eradicate viral diseases such as HIV and Hepatitis
InactiveUS6387384B1Efficient killingEffective distributionBiocideDead animal preservationViral diseaseDisinfectant
The present invention is directed to sodium hypochlorite solutions, solids, sprays, gels, powders and bandages to sanitize or disinfect against the harmful effects of diseases such as HIV or hepatitis.
Owner:PROBERT DAVID D +1
Methods for treating retroviral infections
Owner:ROCHE PALO ALTO LLC
Compounds for the treatment of HIV
The invention provides compounds of formula (I): or a salt thereof as described herein. The invention also provides pharmaceutical compositions comprising a compound of formula (I), processes for preparing compounds of formula (I), intermediates useful for preparing compounds of formula I and therapeutic methods for treating a Retroviridae viral infection including an infection caused by the HIV virus.
Owner:GILEAD SCI INC
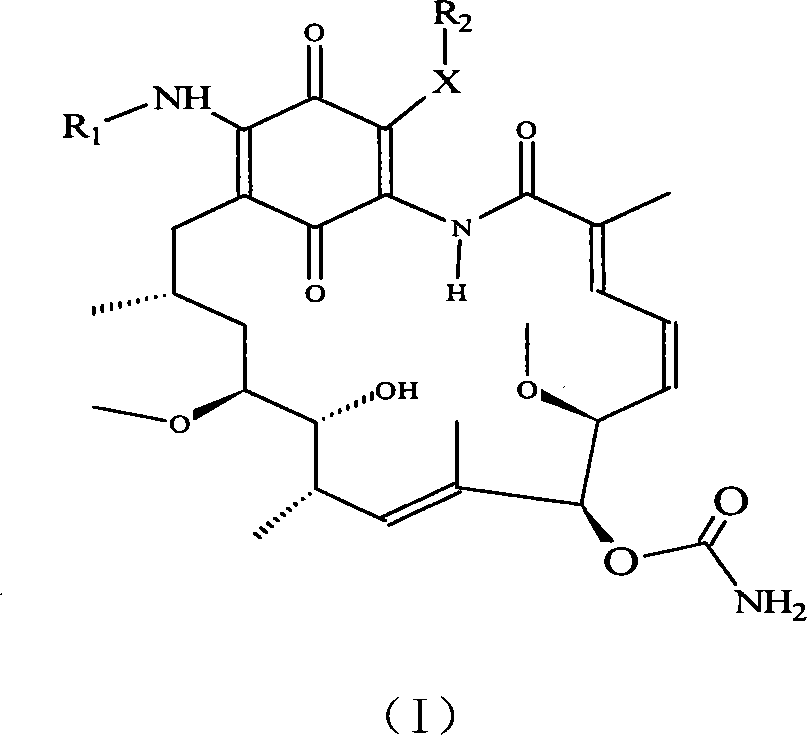
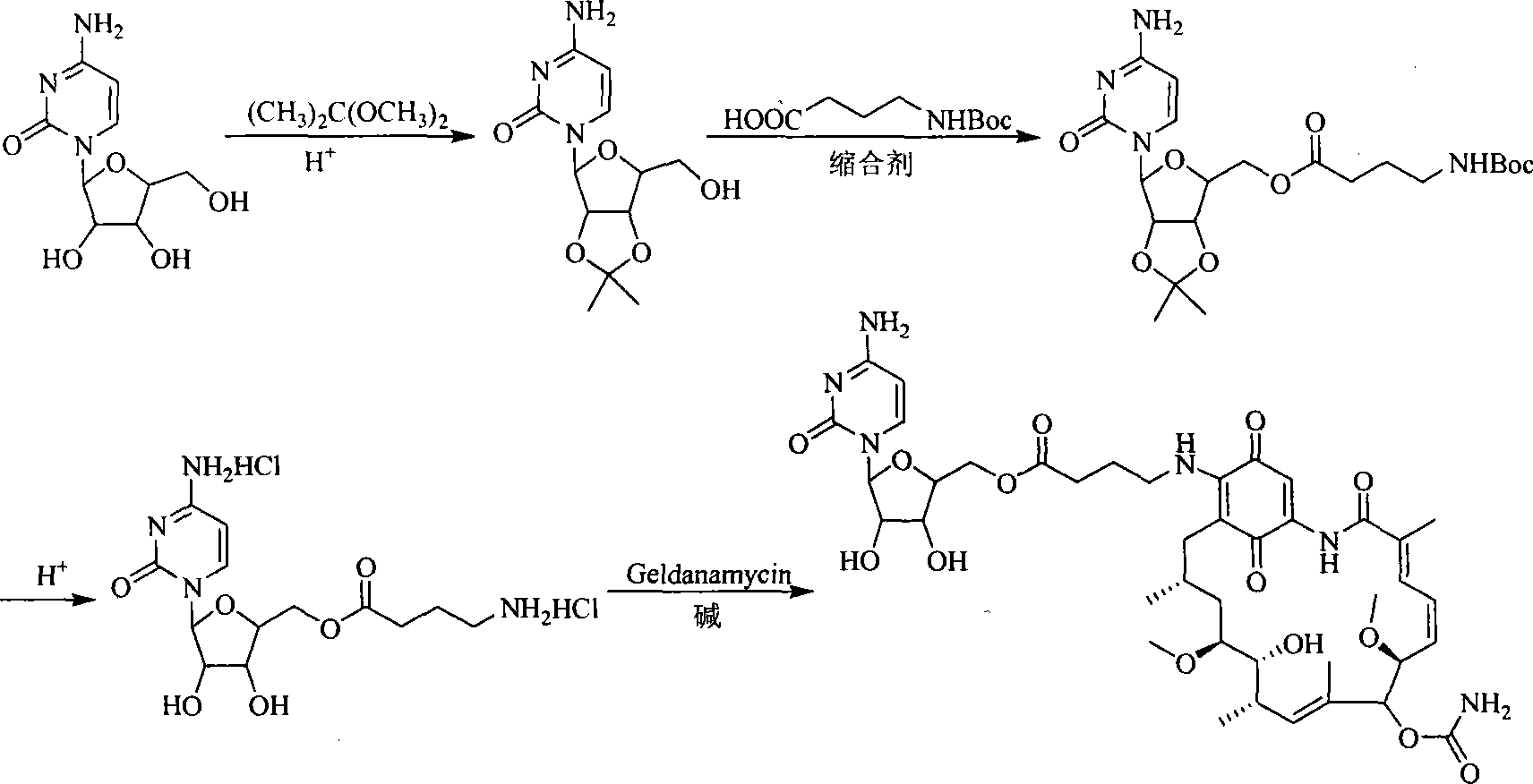
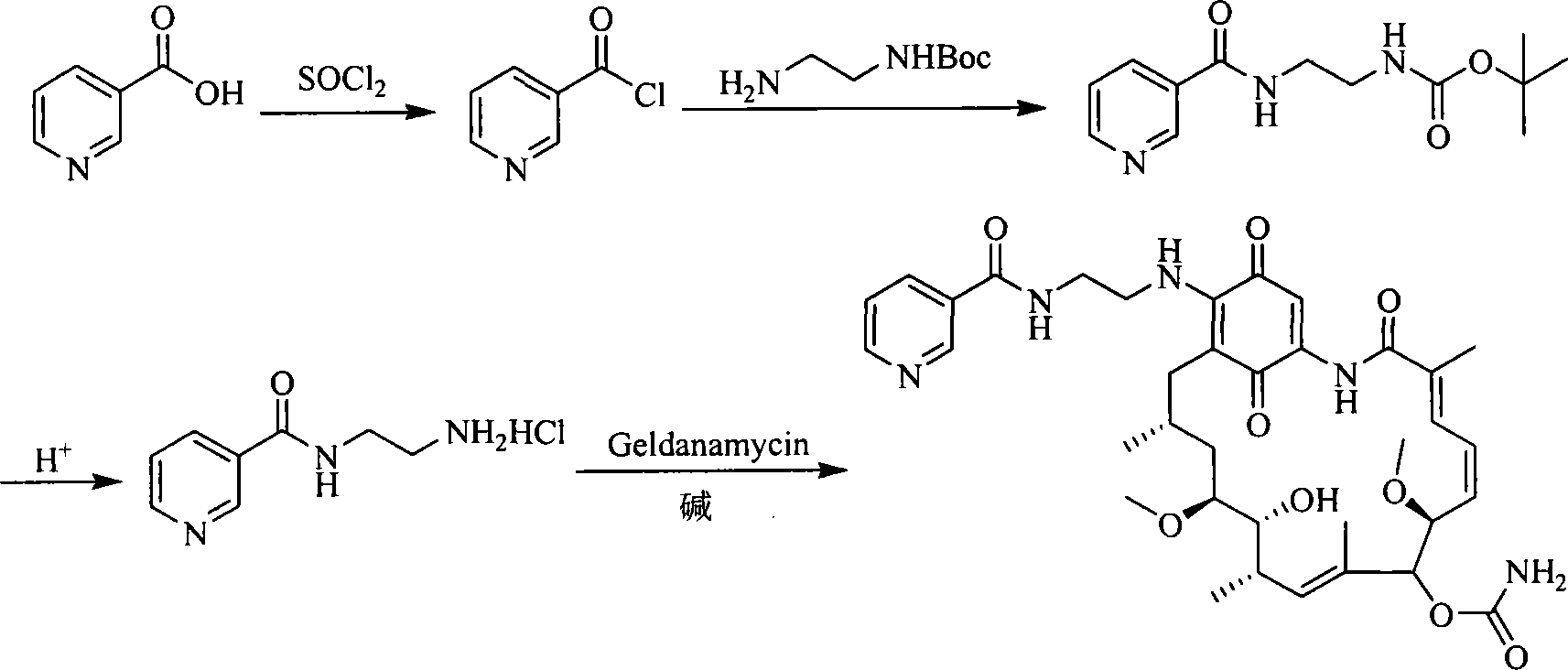
A set of geldanamycin derivant and method for preparing the same
The invention provides a set of new geldanamycin derivatives and the preparation method thereof, which also provides a drug combination using the compound as an active component. The experimental results prove that the derivatives have broad-spectrum antiviral activity, which have stronger inhibition on HIV-1 and HBV and have better inhibitory activity on herpesvirus. Owing to the inhibition on Hsp90, the compound is effective in antivirus and antitumor at the same time.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Compounds for the treatment of HIV
The invention provides compounds of formula (I): or a salt thereof as described herein. The invention also provides pharmaceutical compositions comprising a compound of formula (I), processes for preparing compounds of formula (I), intermediates useful for preparing compounds of formula I and therapeutic methods for treating a Retroviridae viral infection including an infection caused by the HIV virus.
Owner:GILEAD SCI INC
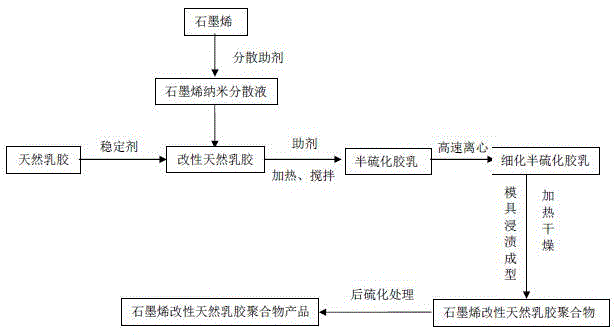
Graphene modified natural emulsion preparation method and high barrier condom
ActiveCN105542248AMeet the use requirementsHigh strengthMale contraceptivesComposite materialHuman papilloma virus
The invention discloses a graphene modified natural emulsion preparation method and a high barrier condom. The graphene modified natural emulsion preparation method and the high barrier condom have the beneficial effects that firstly graphene and a dispersing agent mixed liquor are mixed, ground and stirred and then the product is mixed in a natural emulsion aqueous solution, so that graphene can be uniformly dispersed in emulsion and the strength of a natural emulsion composite material is improved; meanwhile, the number of micropores is smaller due to the graphene / emulsion composite action, thus greatly reducing the virus penetration probability; the thickness of the graphene condom can be 5-80mu m, so that the use requirements of the condom can be met; dense high barrier packing layers are formed by the composite crosslinked nano-packing technology, so that the natural gaps among molecules are reduced; therefore not only can HIVs be blocked but also hepatitis B viruses, hepatitis C viruses, human papilloma viruses, and the like, and most harmful bacteria, which are smaller than the HIVs, can not pass through the micropores.
Owner:沈阳天地乳胶有限公司
HIV pharmaccines
InactiveUS20050175627A1Increase the number ofEnhance immune responsePeptide/protein ingredientsGenetic material ingredientsPol genesVaccination
The invention relates to a recombinant polypeptide comprising amino acid sequence derived from at least one of an HIV gag gene product; an HIV pol gene product; or an HIV nef gene product, said sequence being mutated with respect to the natural sequence of said gene product, and said sequence maintaining each of the naturally occurring CD8+ T cell epitopes of said gene product as defined in p17 and p24 (gag), amino acids 1-440 of RT (pol) and nef shown in Example 8. Furthermore the invention relates to nucleic acids encoding same, and viral vectors encoding same, and to their use in medicine and in immunisation and vaccination.
Owner:OXXON THERAPEUTICS LTD
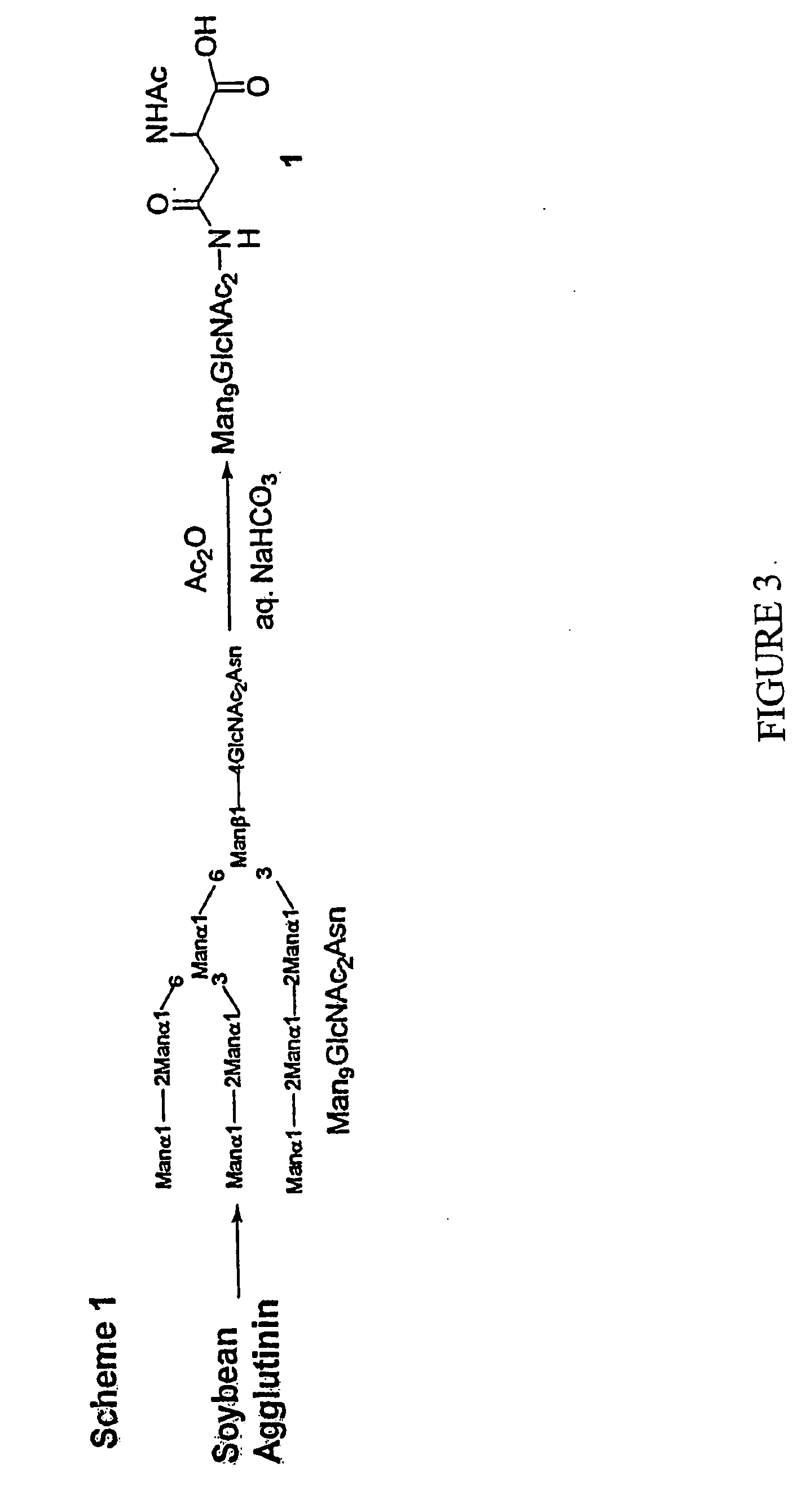
Carbohydrate-based synthetic vaccines for hiv
InactiveUS20050244424A1Increase productionBiocideOrganic active ingredientsHigh mannoseReactive site
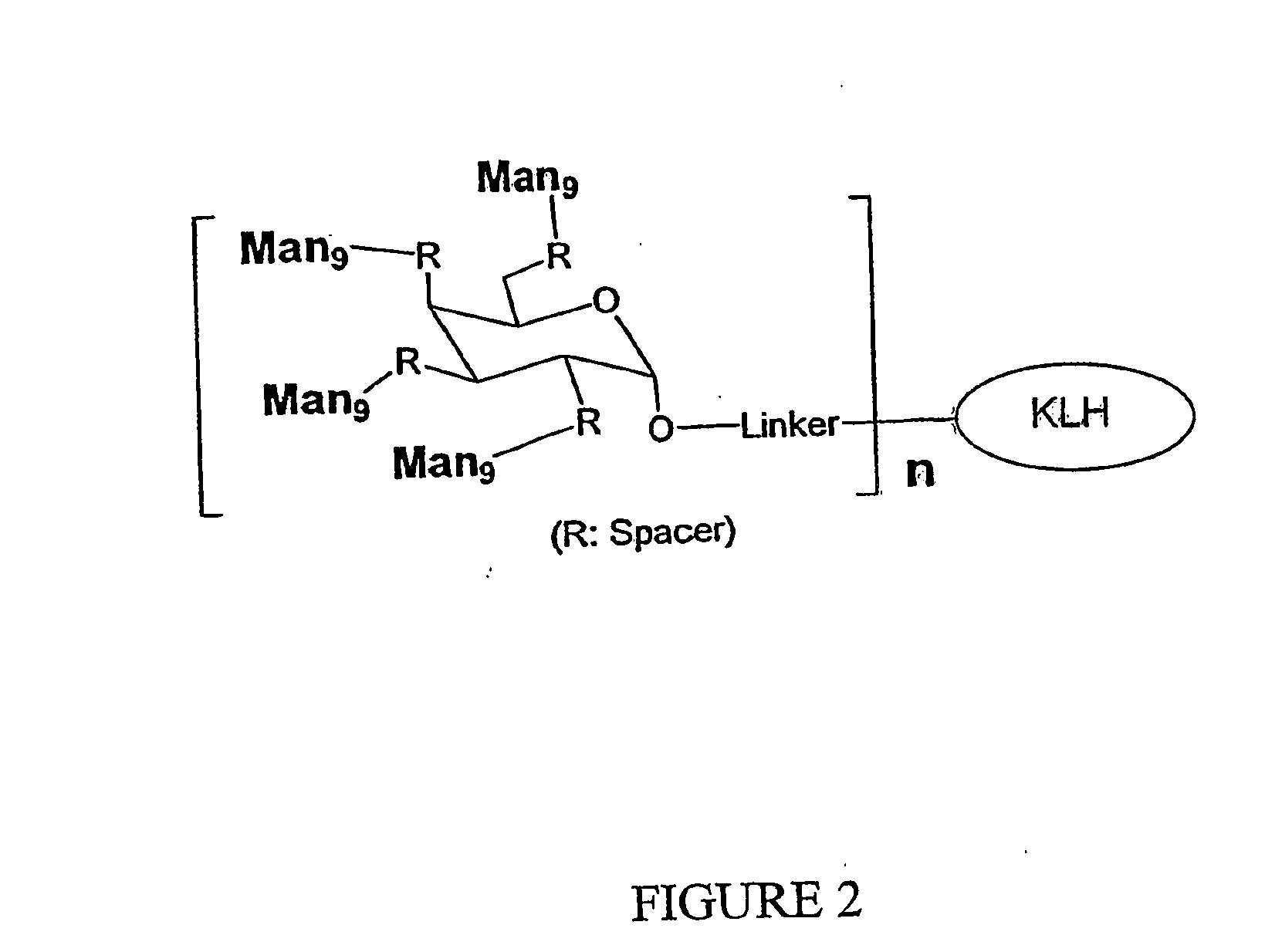
The present invention relates to a constructed to oligosaccharide cluster, optionally bonded to an immunogenic protein, that can be administered to a subject to induce an immune response for increasing production of 2G12 and / or used in assays as reactive sites for determining compounds that inactivate and / or bind the high-mannose oligosaccharide cluster. Compositions comprising these clusters, methods of using these clusters and compositions are disclosed.
Owner:MARYLAND UNIV OF
Vaccine for Prevention and Treatment of Hiv-Infection
ActiveUS20070243203A1Improve solubilityImprove expression levelPeptide/protein ingredientsAntibody mimetics/scaffoldsImmunogenicityHIV Antigens
This invention relates to novel HIV polypeptide and polynucleotide fusions of Gag; Pol and Nef which are useful in immunogenic compositions and vaccines. The invention relates in particular to a polypeptide which comprises Nef or an immunogenic fragment thereof, and p17 Gag and / or p24 Gag or immunogenic fragments thereof, wherein when both p17 and p24 Gag are present there is at least one HIV antigen or immunogenic fragment between them. The polypeptide may also comprise Pol or RT or an immunogenic fragment thereof.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Washing free gel for disinfection
A non-washing disinfecting gel for disinfecting human skin and surface of object is prepared from triclosan, alcohol, moistening agent, thickening agent, pH regulator and deionized water through dissolving triclosan in alcohol, and sequentially adding others while stirring. It can be used to prevent SARS, hepatitis A, hepatitis B, and AIDS.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Human immunodeficiency virus (HIV) antibody detection kit and preparation method thereof
InactiveCN104090101AHigh sensitivityStrong specificityChemiluminescene/bioluminescencePositive controlTrue positive rate
The invention belongs to the technical field of immunologic diagnosis and particularly relates to a human immunodeficiency virus (HIV) antibody detection kit using a microparticle chemiluminiscence method and a preparation method of the kit. The kit is composed of magnetic microparticles for detecting an HIV antibody, a tracing conjugate for detecting the HIV antibody, a negative control, a I type positive control, a II type positive control and an analyzing buffering solution. The invention further discloses the preparation method of the detection kit, which adopts a particle chemiluminiscence immunoassay technology; compared with ELISA (Enzyme-Linked Immunosorbent Assay), the technology has higher sensitivity and specificity and is suitable for the clinical auxiliary diagnosis of HIV.
Owner:威海威高生物科技有限公司
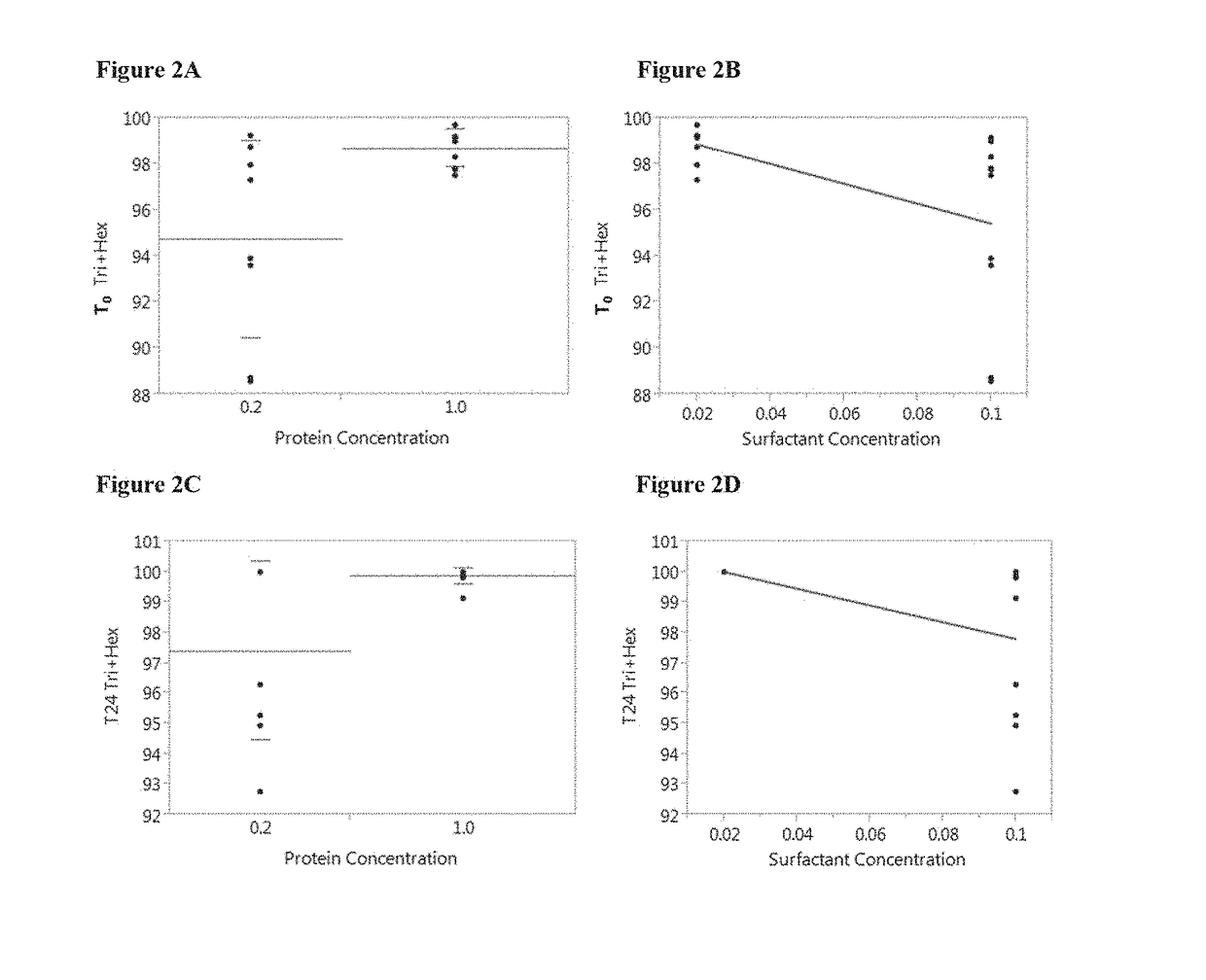
HIV vaccine formulation
ActiveUS20170362280A1Improve stabilityGood for clinical useHydroxy compound active ingredientsViral antigen ingredientsAdjuvantEngineering
Immunogenic compositions containing a human immunodeficiency virus (HIV) gp140 protein, sorbitol, polysorbate 20, and histidine buffer are described. The described immunogenic compositions are advantageous in that they are stable at refrigerated temperature for extended periods of time, and are compatible with an adjuvant. Also described are methods of using the immunogenic compositions to induce an immune response against an HIV in a subject. The immunogenic compositions can be administered alone, or in combination with one or more additional HIV antigens, or one or more adenovirus vectors encoding the one or more additional HIV antigens.
Owner:JANSSEN VACCINES & PREVENTION BV
HIV vaccine composition
An anti-HIV vaccine composition is disclosed. The vaccine comprises an combination of immunogenic peptide mixtures, which mixtures may be prepared in a single synthesis. The composition collectively represents the in vivo variability seen in immunogenic epitopes from highly variable regions of HIV. Immunization with the vaccine elicits broadly reactive immunity (CTL and T helper cell responses) against the divergent strains of HIV upon which it is based. The vaccine may be formulated to target regionally distinct variability based on an HIV clade predominant in a geographical region.
Owner:VARIATION BIOTECHNOLOGIES INC
Methods and compositions for treatment of HIV infection
InactiveUS20160095850A1Reduce viral loadBiocidePeptide/protein ingredientsImmunodeficiency virusCD4 antigen
Methods and compositions for treatment of human immunodeficiency virus (HIV) infections have been developed which dampen immune activation with a bias more on the CD4 T cells relative to the CD8 T cell response, inhibit HIV replication, reactivate latent HIV, and inhibit infection of cells by HIV. Pushing latent HIV into active infections with hindrance of cell infection by the reactivated HIV can substantially reduce the number of cells infected with HIV and the viral load of HIV, which is not achieved using just the combination of ART and compounds which activate latent HIV. The methods involve administering to an HIV-infected subject three or more compounds which collectively dampen immune activation with a bias more on the CD4 T cells relative to the CD8 T cell response, inhibit HIV replication, reactivate latent HIV, and inhibiting infection of CD4 T cells by HIV.
Owner:COOPER HUMAN SYST
Combination vaccine against various HIVs and combination method thereof
InactiveCN101569745ANew Vaccine FeaturesGenetic material ingredientsAntiviralsHIV vaccineCell Membrane Proteins
The invention discloses a combination vaccine against various HIVs and a combination method thereof. The combination vaccine consists of two or more HIV vaccines, different AIDS vaccines contain different HIV membrane proteins or membrane protein encoding genes, and each HIV vaccine is independently inoculated and inoculated at least one time, namely the total times of inoculation are at least twice. The core of the technology is that the different HIV membrane proteins are used to carry out sequential immunization, namely in sequential repeated immune processes of the vaccines, the HIV membrane proteins used in the immunization at different times are different so as to obtain a broad-spectrum neutral antibody against the HIVs with high titer. The HIV preventing vaccine developed by the method can be used for preventing various subtype HIV infections.
Owner:VACDIAGN BIOTECH
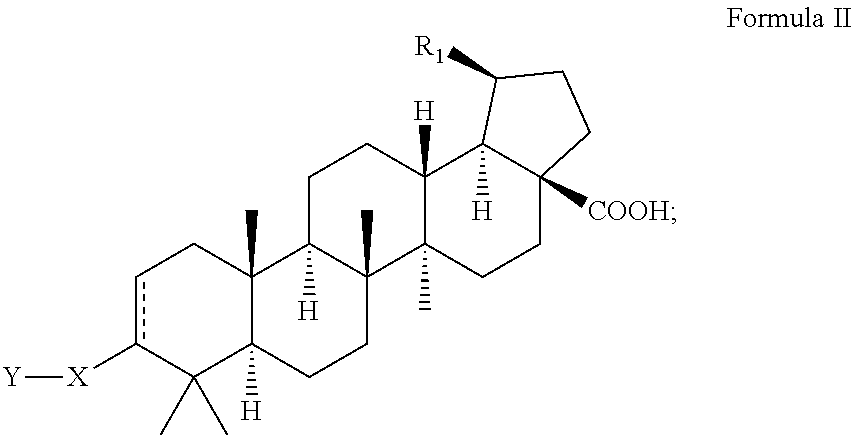
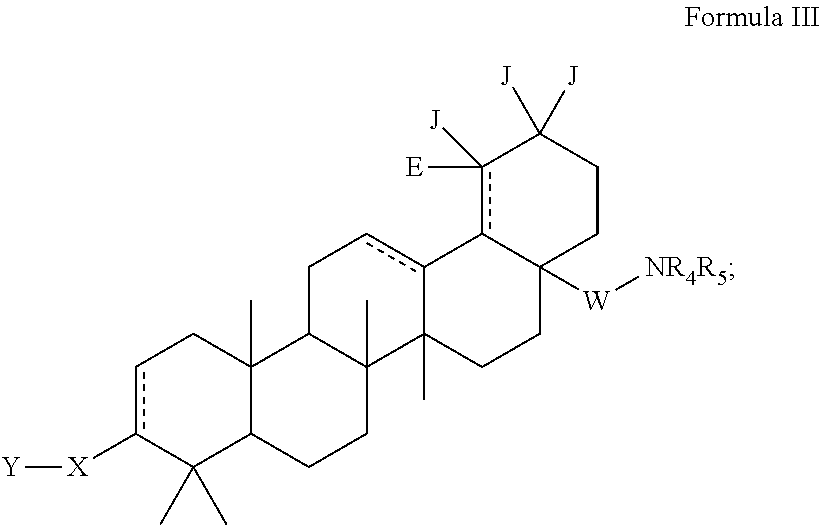
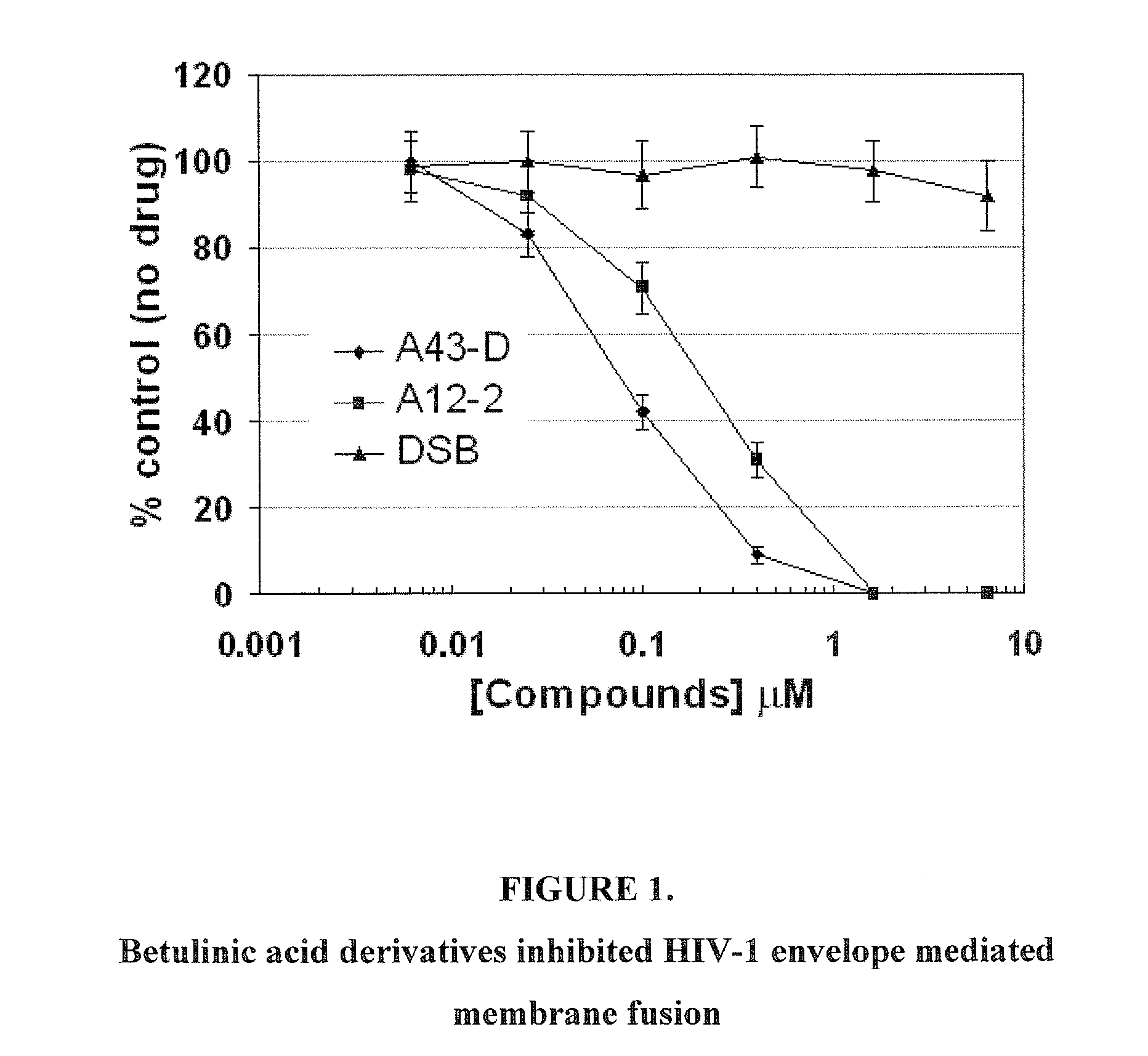
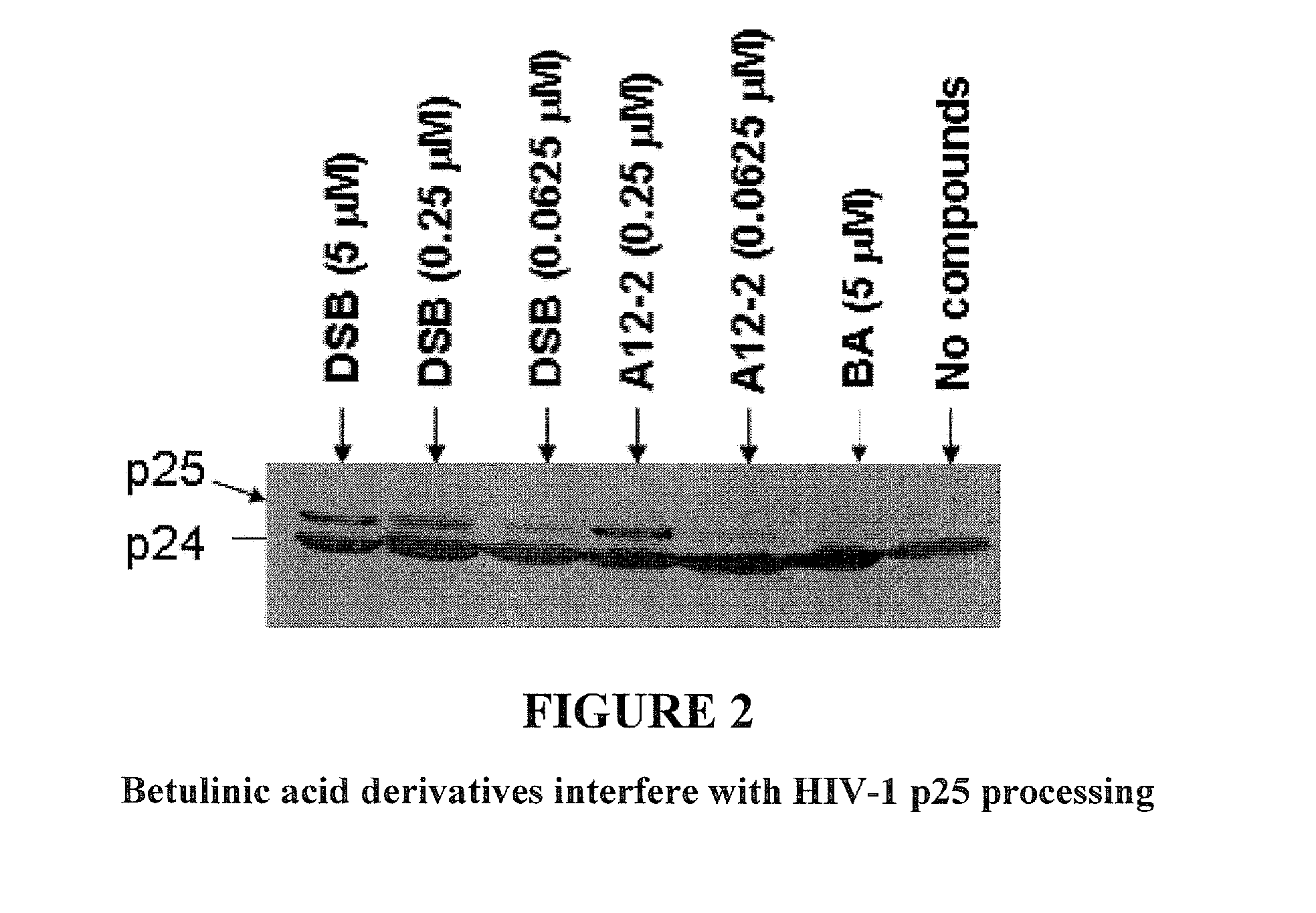
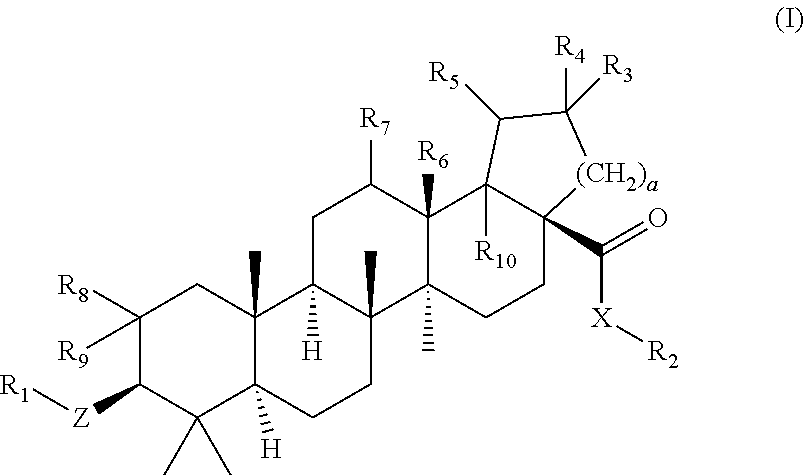
C-3 alkyl and alkenyl modified betulinic acid derivatives
Compounds having drug and bio-affecting properties, their pharmaceutical compositions and methods of use are set forth. In particular, alkyl and alkenyl C-3 modified betulinic acid derivatives that possess unique antiviral activity are provided as HIV maturation inhibitors, as represented by compounds of Formulas I, II, III and IV:a compound of Formula Ia compound of Formula IIa compound of Formula IIIand a compound of Formula IVThese compounds are useful for the treatment of HIV and AIDS.
Owner:VIIV HEALTHCARE UK (NO 5) LTD
Pharmaceutical being used for treating HIV infection, the composition and uses thereof
InactiveUS6962900B2Reduce loadMaintain antiviral activityBiocideOrganic active ingredientsChemistryPeptide sequence
A pharmaceutical being used for treating HIV infection is provided which has the following peptide sequence: X-SWETWEREIENYTKQIYKILEESQQDRN-EKDRNEKDLLE-Z, where S is Serine, W is Trytophan, E is Glutamate, T is Threonine, R is Argine, I is Isoleucine, N is Asparagine, Y is Tyrosine, K is Lysine, Q is Glutamine, L is Leucine, D is Aspartic acid, X is amino group, acetyl-hydrophobic group, or macromolecule carrier group, Z is carboxyl group, amino group, amido group, tert-nutyloxycarbonyl group, hydrophobic group, or macromolecule carrier group. The drug inhibits strongly the infection of HIV.
Owner:TIANJIN FUSOGEN BIOTECH CO LTD
Betulinic acid derivatives as Anti-hiv agents
InactiveUS20110152229A1Useful in treatmentOrganic active ingredientsAntiviralsAnti-HIV AgentMedicine
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL +1
Chitosan glycyrrhizic acid nano particle and its preparing method
InactiveCN1586488AHigh encapsulation efficiencyEasy to operatePowder deliveryOrganic active ingredientsIntestinal absorptionChemistry
The present invention relates to preparation process of nano chitosan-glycyrrhizic acid particle. Glycyrrhizic acid has the effects of resisting viral hepatitis and chronic hepatitis and destroying HIV cell in blood vessel, but available clinically applied glycyrrhizic acid preparations have the problem of hard absorption. The present invention prepares powdered nano chitosan-glycyrrhizic acid particle through mixing chitosan dissolved in acid aqua and glycyrrhizic acid dissolved in ammonia solution, synthesis under mild condition and freeze drying. The product of the present invention may be re-dispersed in water to form nano particle and has certain targeting and delayed releasing performance. Compared with available marketed oral preparation, the present invention has obviously raised intestinal absorption and bioavailability.
Owner:FUDAN UNIV
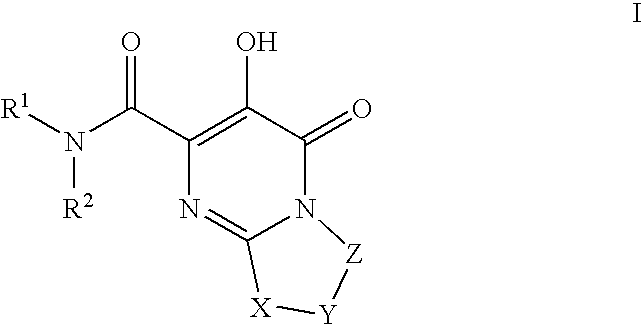
HIV integrase inhibitors
The disclosure generally relates to the novel compounds of formula I, including their salts, which inhibit HIV integrase and prevent viral integration into human DNA. This action makes the compounds useful for treating HIV infection and AIDS. The invention also encompasses pharmaceutical compositions and methods for treating those infected with HIV.
Owner:BRISTOL MYERS SQUIBB CO
Methods and compositions for immunization against hiv
The present invention relates to nucleic acid and attenuated vaccinia vectors for prophylactic use against HIV infection, as well as methods of eliciting immune responses in subjects susceptible to HIV infection. The prophylactic vaccine regimen of the invention involves immunological priming with an inoculum comprising two novel DNA vectors, followed by boosting with a Modified Vaccinia Ankara (MVA) recombinant viral vector expressing the corresponding HIV proteins.
Owner:HUANG YAOXING +2
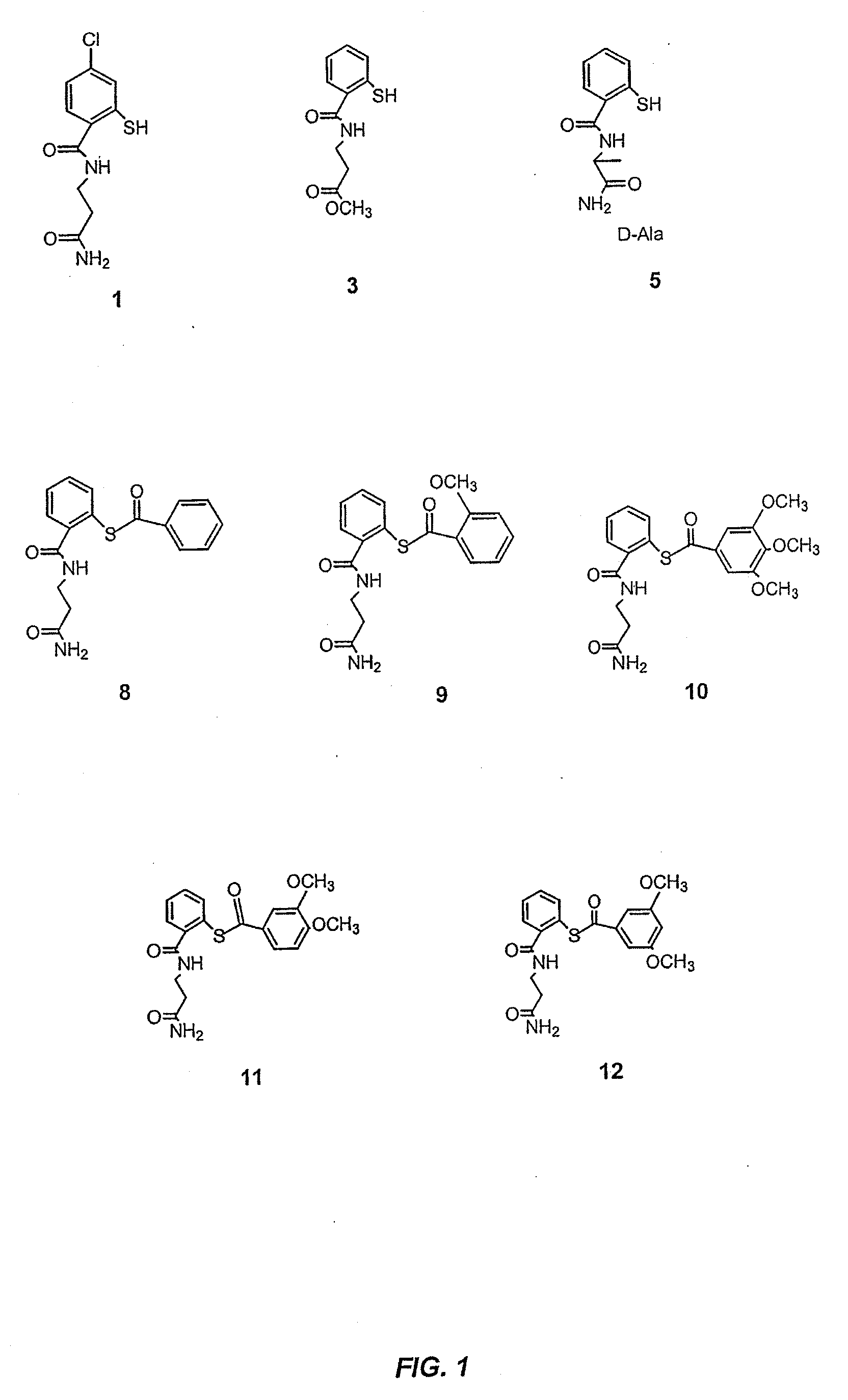
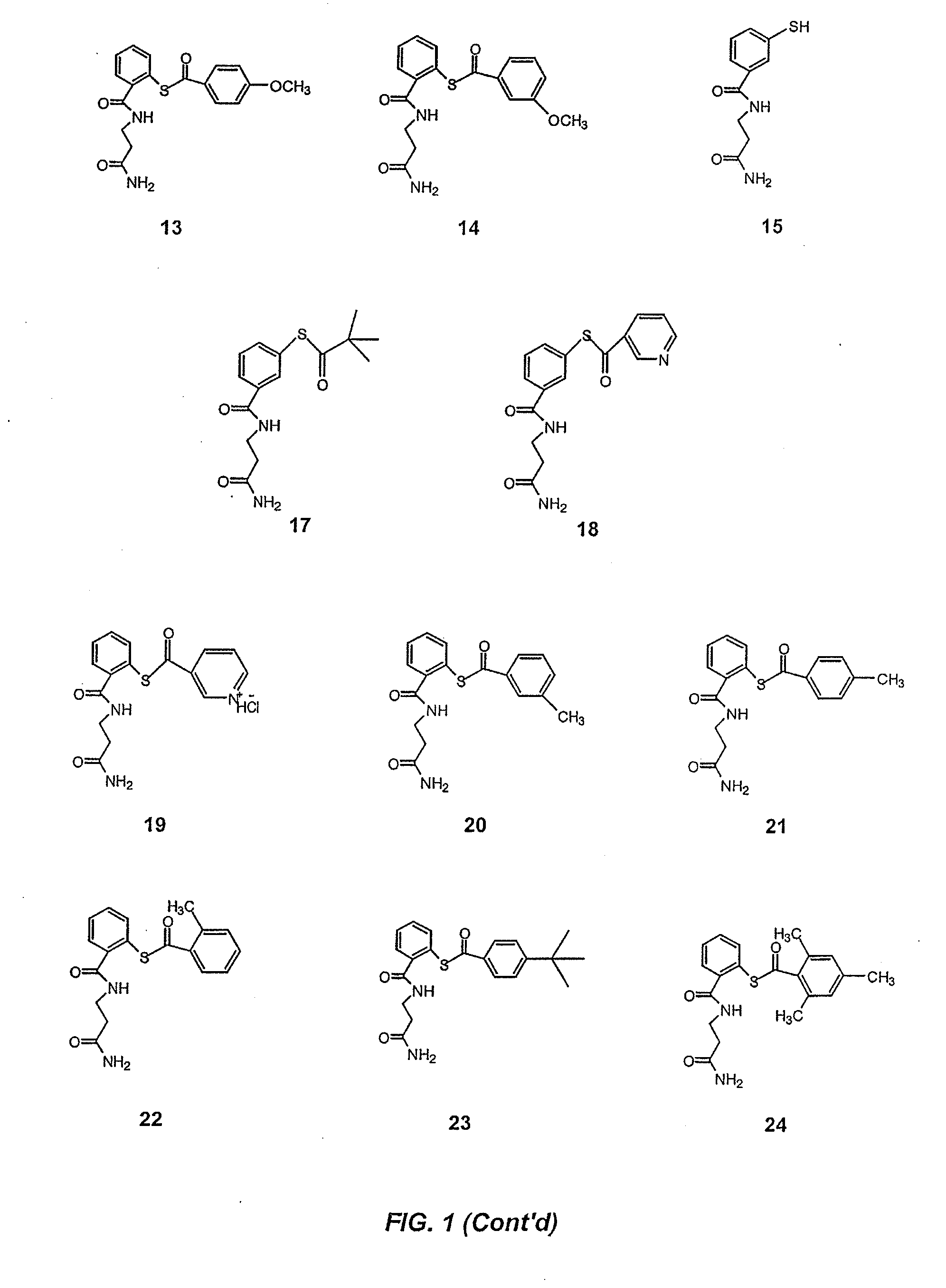
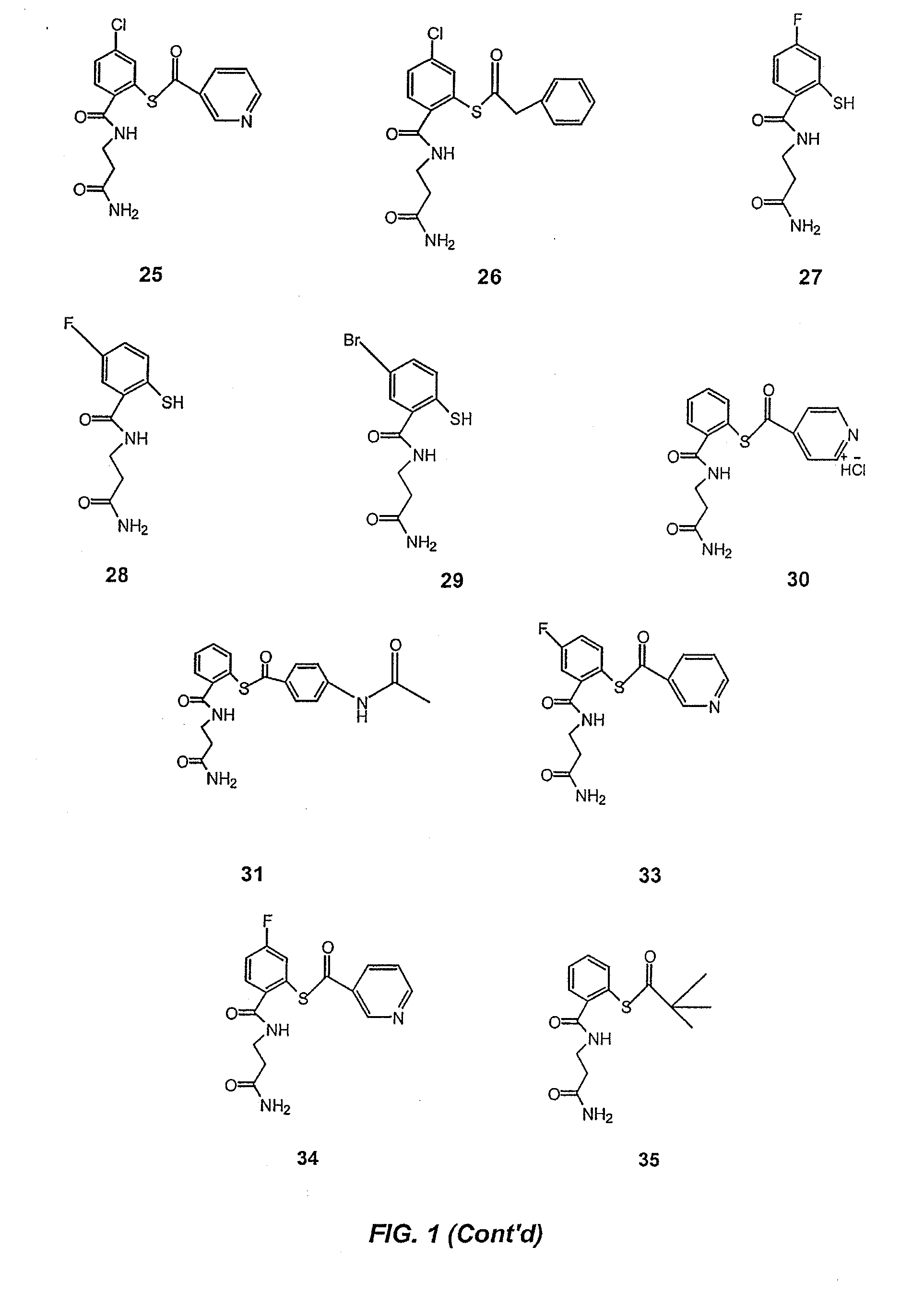
Acythiols and component thiol compositions as Anti-hiv and Anti-retroviral agents
Certain thiol and acylthiol compounds inhibit retrovirus growth by attacking the highly conserved zinc finger regions of essential viral proteins. These compounds, compositions containing them, and methods of using them to treat retroviral infections such as HIV are described. These compounds are also useful for preparation of vaccines comprised of inactivated retroviruses such as HIV, prevention of the transmission of such retroviruses, and detection of retroviral proteins.
Owner:UNITED STATES OF AMERICA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com