Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
101 results about "Immunologic Diagnosis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
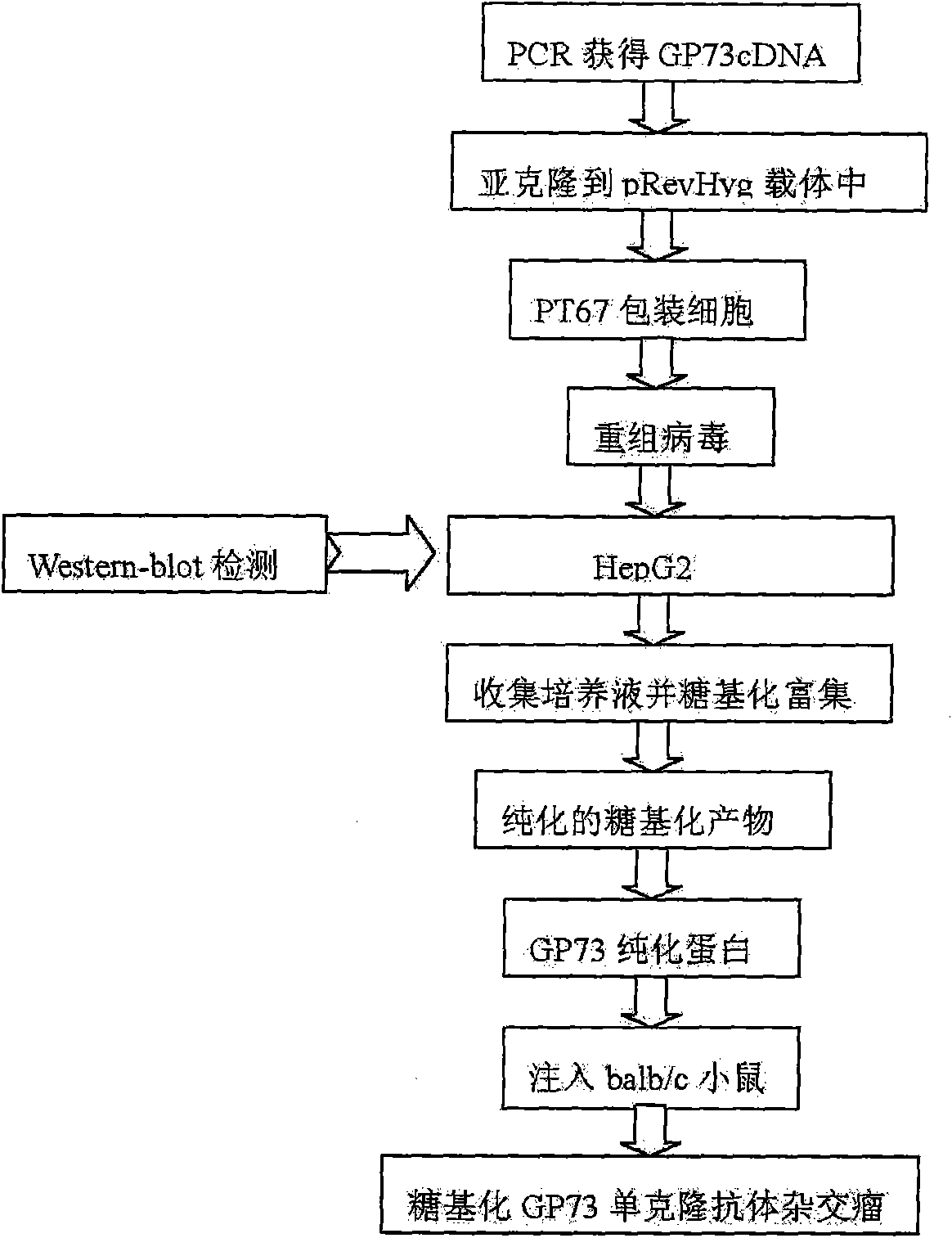
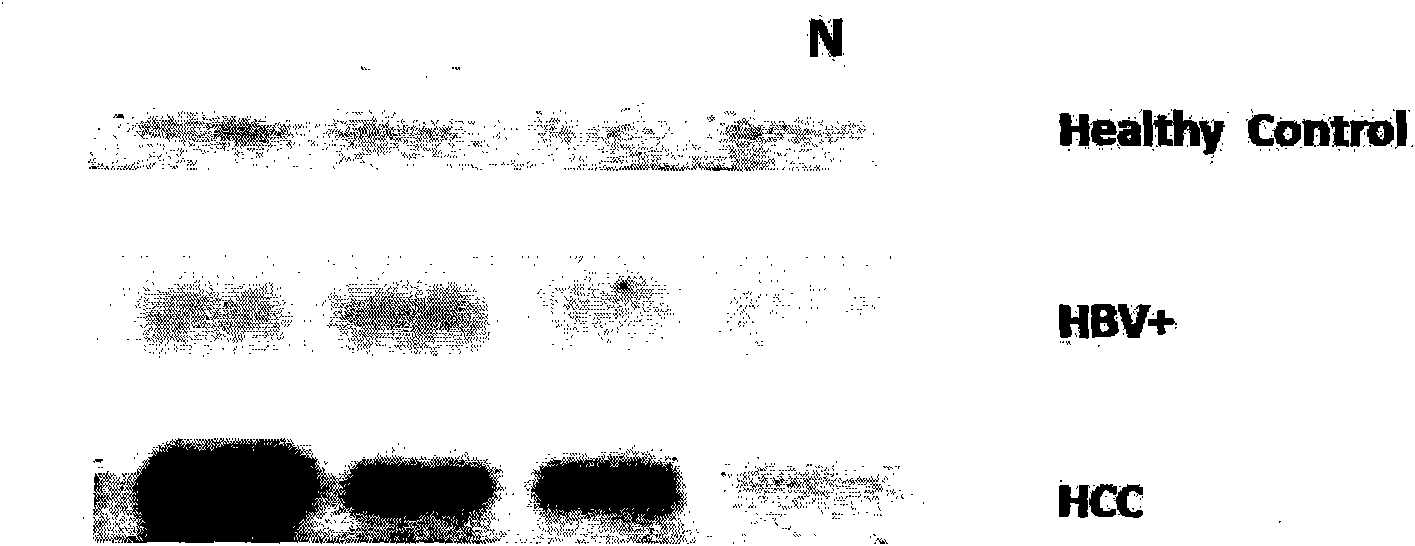
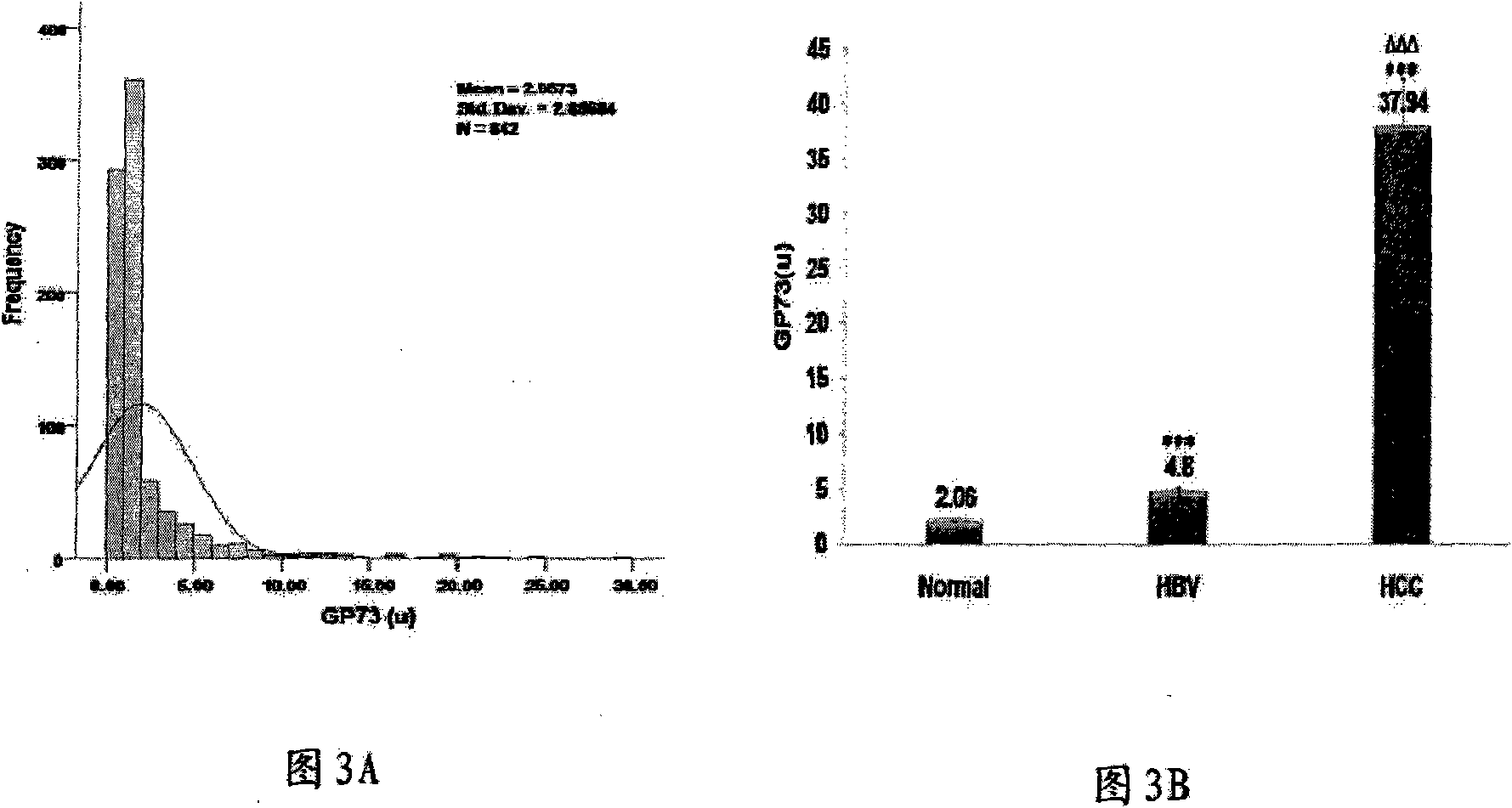
Antibody of fucosylated Golgi protein GP73 and use thereof
InactiveCN101555282AFast detection methodReduce testing costsTissue cultureImmunoglobulins against fungi/algae/lichensCDNA libraryDisease
The invention relates to an antibody against fucosylated protein GP73, a method for preparing the antibody against the fucosylated protein GP73, a reagent for diagnosing liver cancers, a kit for diagnosing the liver cancers, application of the reagent and / or the kit to preparing products for diagnosing liver diseases and a method for preparing purified fucosylated protein GP73. Occurrence, development and metastasis of liver cancers are diagnosed by detecting the fucosylated protein GP73 or the antibody. The antibody against the fucosylated protein GP73 can be more widely used for affinity chromatography, cDNA library screening, immunologic diagnosis or pharmaceutical preparation.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Nanometer carboxylated polystyrene microsphere with spacer arm and preparation method thereof
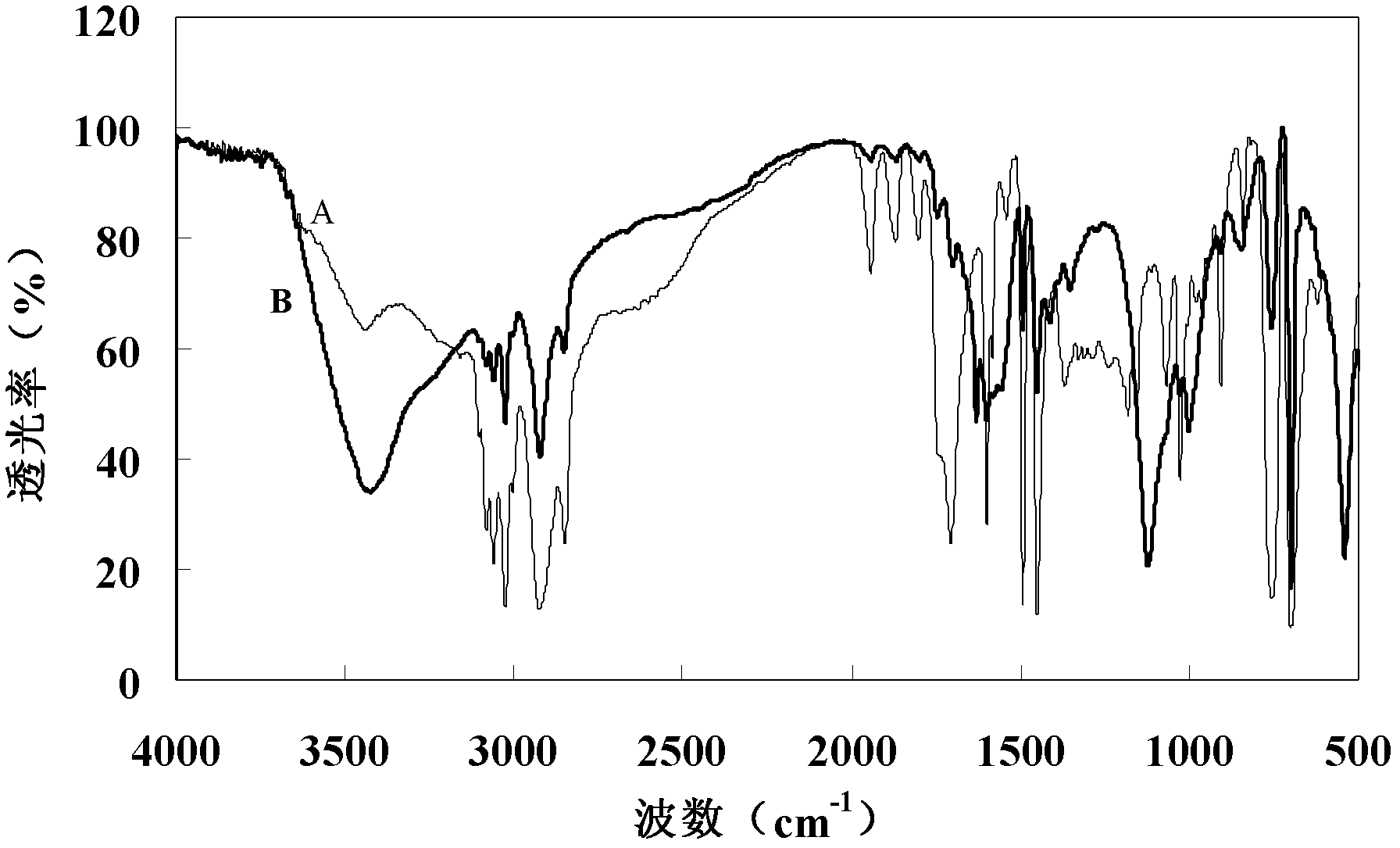
The invention relates to a nanometer carboxylated polystyrene microsphere with a spacer arm and a preparation method thereof. A nanometer carboxylated polystyrene microsphere A is prepared by taking styrene and acrylic acid as a mixed monomer, taking water as a reaction medium, taking potassium persulfate as an initiator and taking sodium dodecyl sulfate as an emulsifier with an emulsion polymerization method. In the presence of EDC (Ethylidene Dichloride) serving as a coupling agent and Sulfo NHS, 4-aminobutyric acid, 6-amidocaproic acid or 8-aminocaprylic acid is connected to the surface of the microsphere, so that the nanometer carboxylated polystyrene microsphere with the spacer arm is prepared. The spacer arm is introduced into the surface of the nanometer microsphere, so that the steric hindrance of a coupling macromolecule is lowered, the activity and utilization ratio of the macromolecule are increased, and a nonspecific interaction between the macromolecule and the polystyrene microsphere is reduced. Meanwhile, the grain diameter of the microsphere can be adjusted by adjusting the using amount of the emulsifier, the length of the space arm and surface density, so that the nanometer microsphere has a wide application prospect in the fields of immune diagnosis, solid phase extraction and the like when serving as a carrier as well as a template.
Owner:TIANJIN MEDICAL UNIV
Hollow microsphere containing silicon magnetism and preparation method and use thereof
InactiveCN101337171ALow densityLarge specific surface areaMagnetic materialsMicroballoon preparationTumor targetingMicrosphere
The invention provides a hollow silicon magnetic microsphere, as well as the preparation method and the application thereof. The hollow silicon magnetic microspheres have the preparation method that a polystyrene / silicon dioxide composite microsphere is used as a core; Fe3O4 coats the periphery of the core through the layer-by-layer self-assembly method; then, a PS / SiO2 / Fe3o4 polymeric magnetic composite nanosphere is obtained, and polystyrene is removed through the high temperature sintering method, thereby forming a magnetic material with hollow structure; the inner layer of the hollow magnetic material is SiO2, and the stability is good, thereby the material is not easy to collapse at high temperature; furthermore, the specific surface area of the material can be improved, and the material is a monodisperse hollow magnetic microsphere material. The method has the advantages of low cost and good repetitiveness. The obtained product not only can be used as a magnetic targeting drug carrier material, but also can be used in the biological and the medical fields such as biosensors, immunologic diagnosis, drug delivery and tumor targeting therapy as well as DNA isolation, etc.
Owner:SHANGHAI HUAMING HI TECH GRP +1
Method for producing nano-stephanoporate intelligent photochemistry sensitization functional material
InactiveCN101220167AIncreased sensitivityImprove anti-interference abilityPhase-affecting property measurementsUltraviolet lightsPolymer thin films
The invention discloses a method for preparing nanoporous intelligent photochemical sensitive functional material, pertaining to the functional high molecular material field. A colloidal crystal template is formed by automatic assembly of colloidal particles and is wetted with solution of polymer monomer, corsslinking agent and initiator that contain imprinting molecular, and blotting polymer is prepared with ultraviolet light or through heat curing, then the template and the blotting molecular in the blotting polymer are removed and polymer membrane that has three-dimensional ordered porous structure and blotting molecular cavities is obtained. The intelligent photochemical sensitive functional material prepared by the method not only has high selectivity, sensitivity and interference rejection property but also can directly change chemical response into optical signals without any complicated precision instrument or making special treatment to analytes. The method is characterized by simple preparation technique and convenient and rapid inspection, etc., thereby being widely applicable to fields of medicine separation and identification, immunologic diagnosis and analysis and drug and exhilarant inspection, etc.
Owner:TSINGHUA UNIV
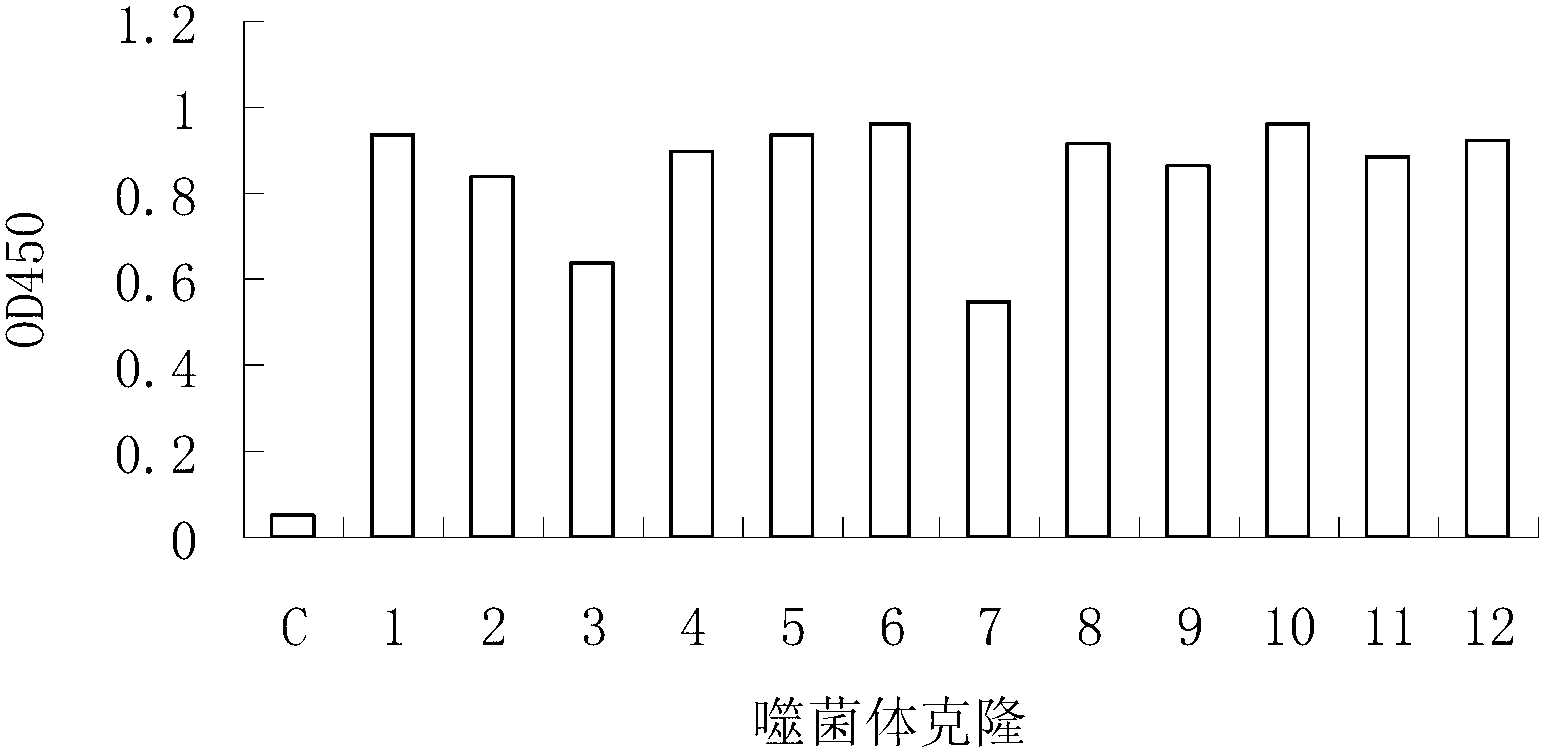
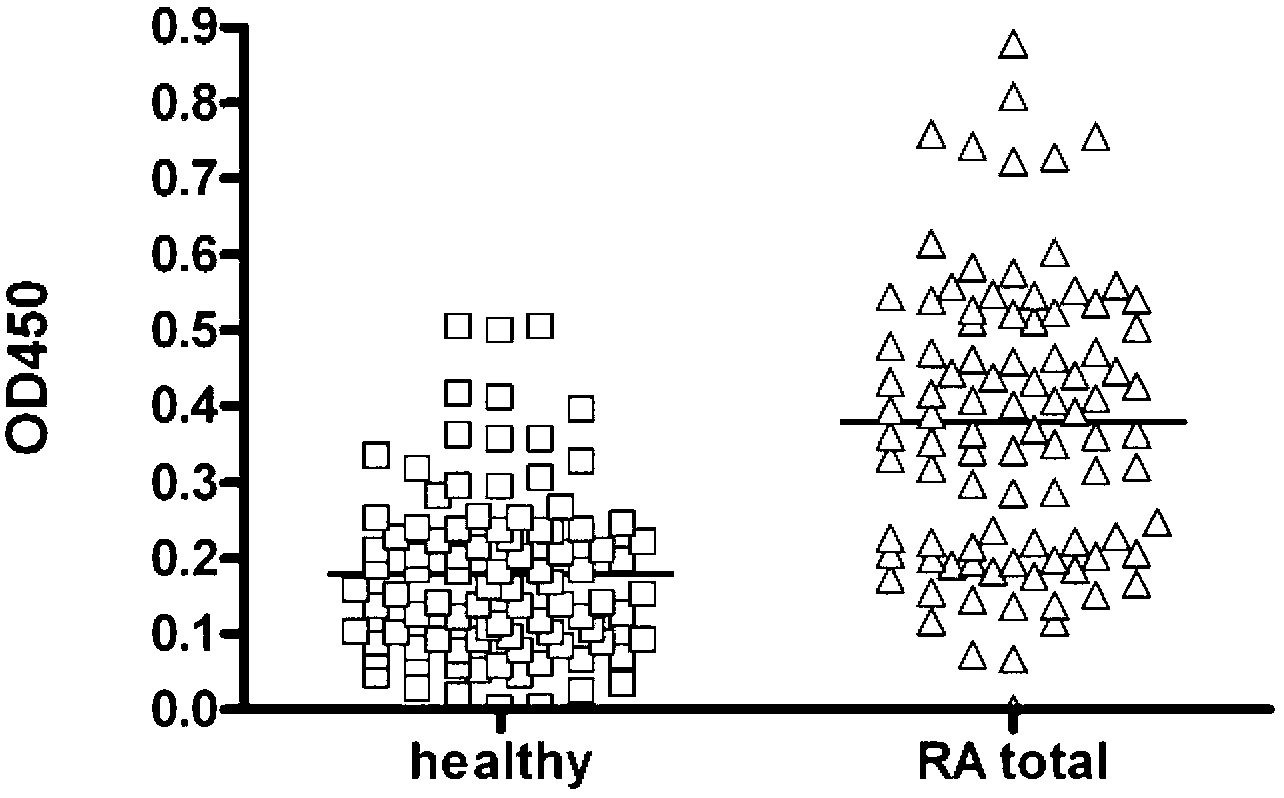
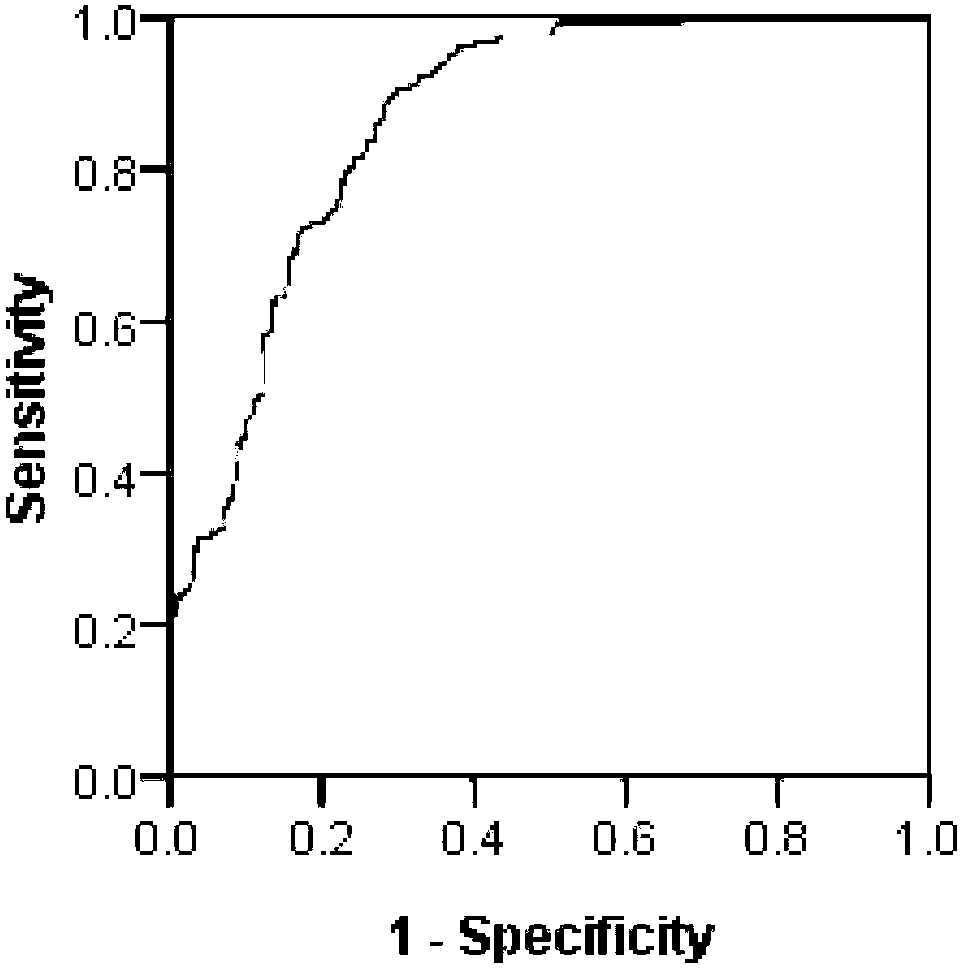
Epitope of rheumatoid arthritis and application thereof
The invention discloses an epitope of rheumatoid arthritis and application thereof, belonging to the technical field of immunologic diagnosis. The epitope is a polypeptide, and the amino acid sequence is disclosed as SEQ ID NO:1 in the sequence table. ELISA detection and patient serum reaction condition indicate that the epitope polypeptide can be specifically combined with IgG in the patient serum, and does not react with the serum of a health person. The epitope polypeptide can be used for preparing medicines for diagnosing rheumatoid arthritis.
Owner:INST OF BASIC MEDICAL SCI ACAD OF MILITARY MEDICAL SCI OF PLA +1
Treponema pallidum antibody diagnostic kit and preparation method thereof
The invention belongs to the technical field of immunologic diagnosis, in particular to a treponema pallidum antibody diagnostic kit by a chemiluminescence method and a preparation method thereof. The kit comprises an anti-TP test reaction plate, an anti-TP test enzyme complex, chemiluminescence substrate liquid, concentrated washing liquor, a negative contrast and a positive contrast. The invention also discloses a preparation method of the diagnostic kit, which adopts a chemiluminescence immunoassay technology; compared with ELISA (enzyme-linked immuno sorbent assay), the method has higher sensitivity and specificity, is suitable for the auxiliary diagnosis of clinical syphilis and screening of blood donors and fills a blank of the production of a treponema pallidum antibody diagnostic reagent detected by the domestic chemiluminescence method.
Owner:威海威高生物科技有限公司
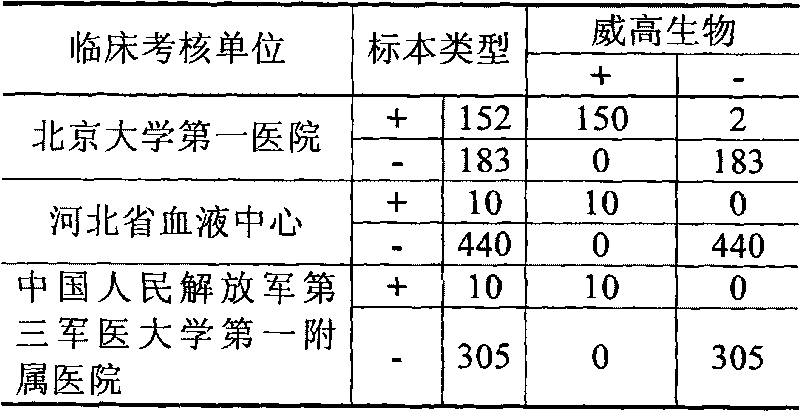
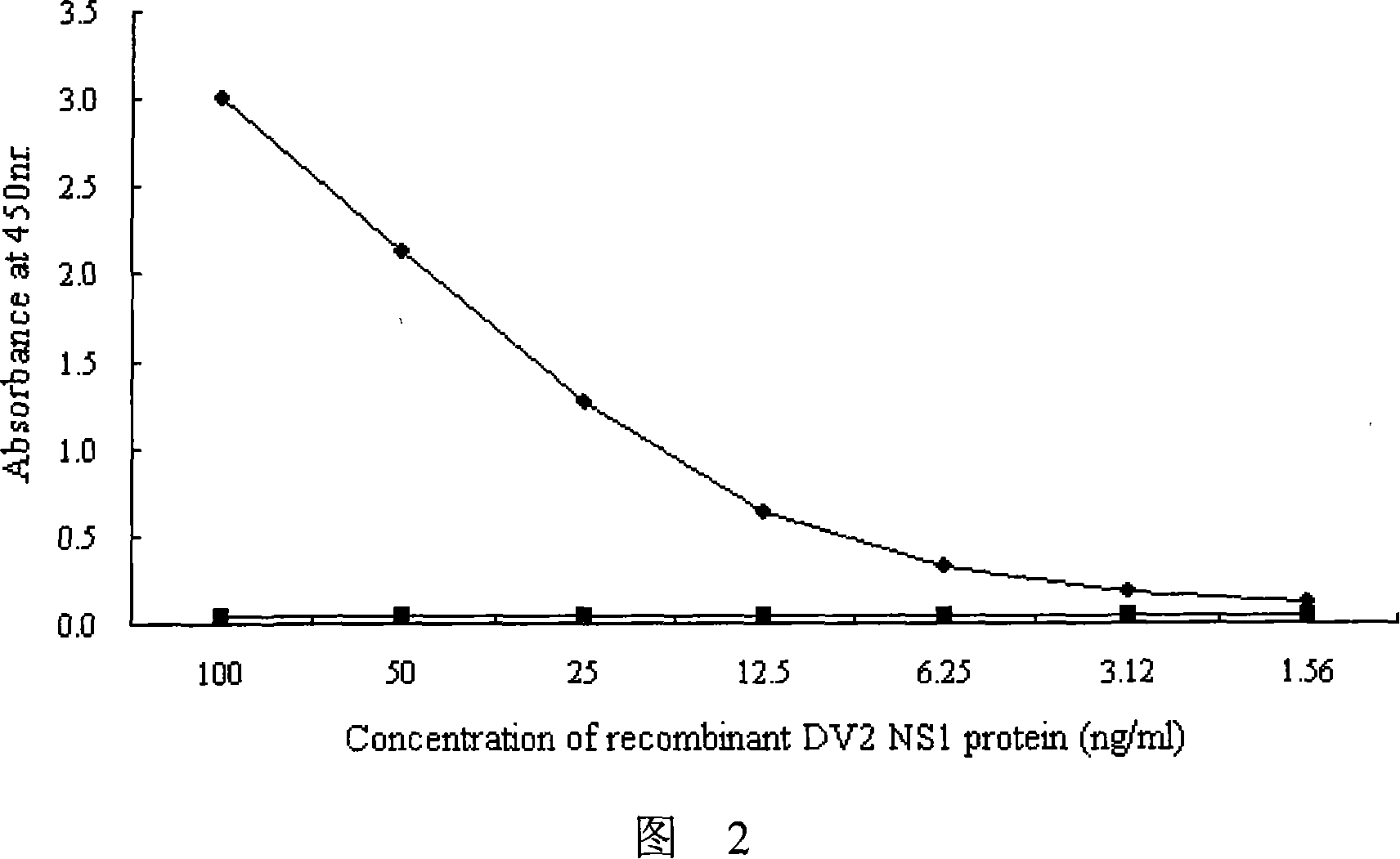
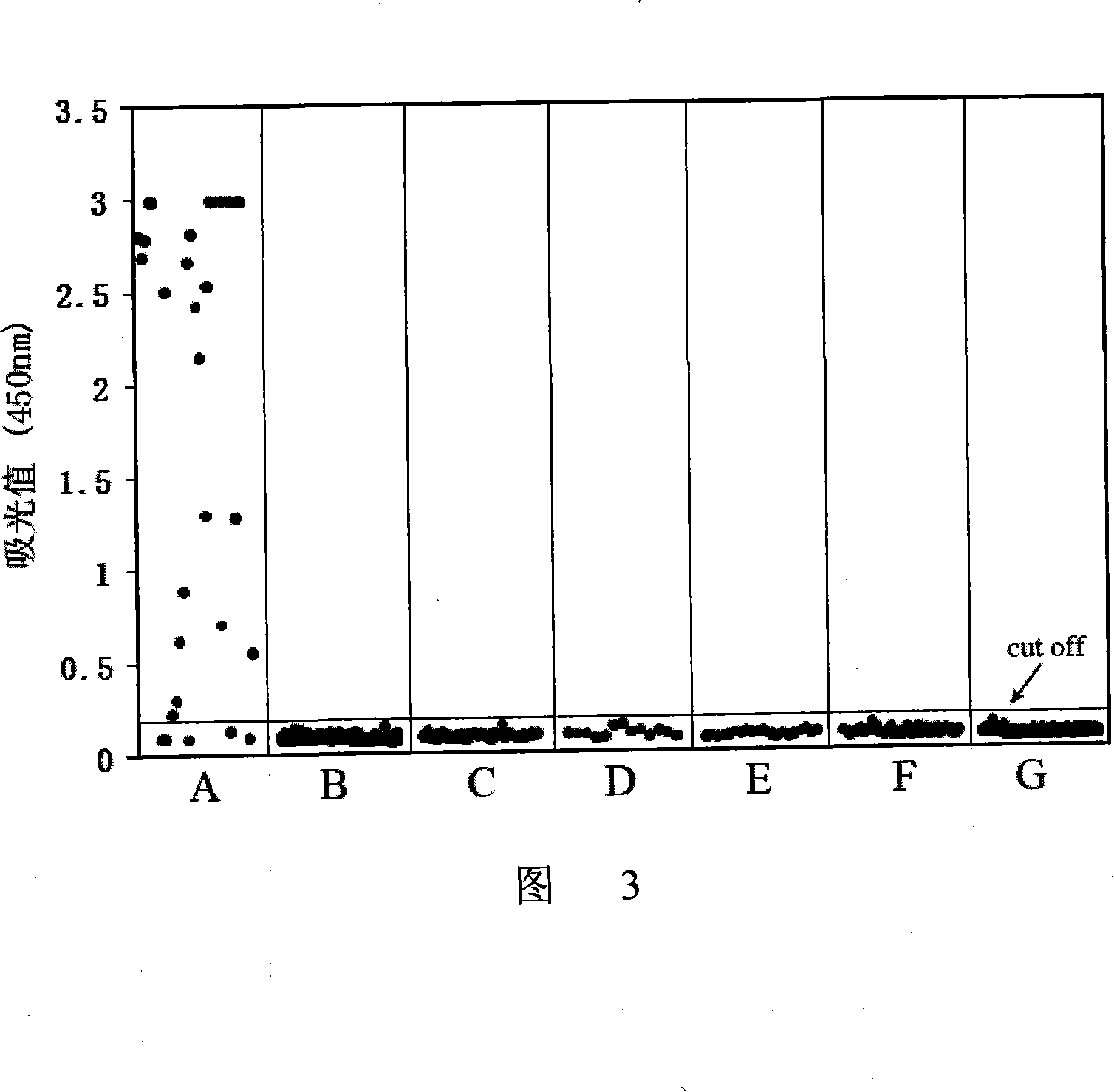
Immunologic diagnosis kit for detecting type II dengue virus NS1 antigen
ActiveCN101226196AAccurate detectionQuick checkMaterial analysisAgainst vector-borne diseasesSerotypeElisa test
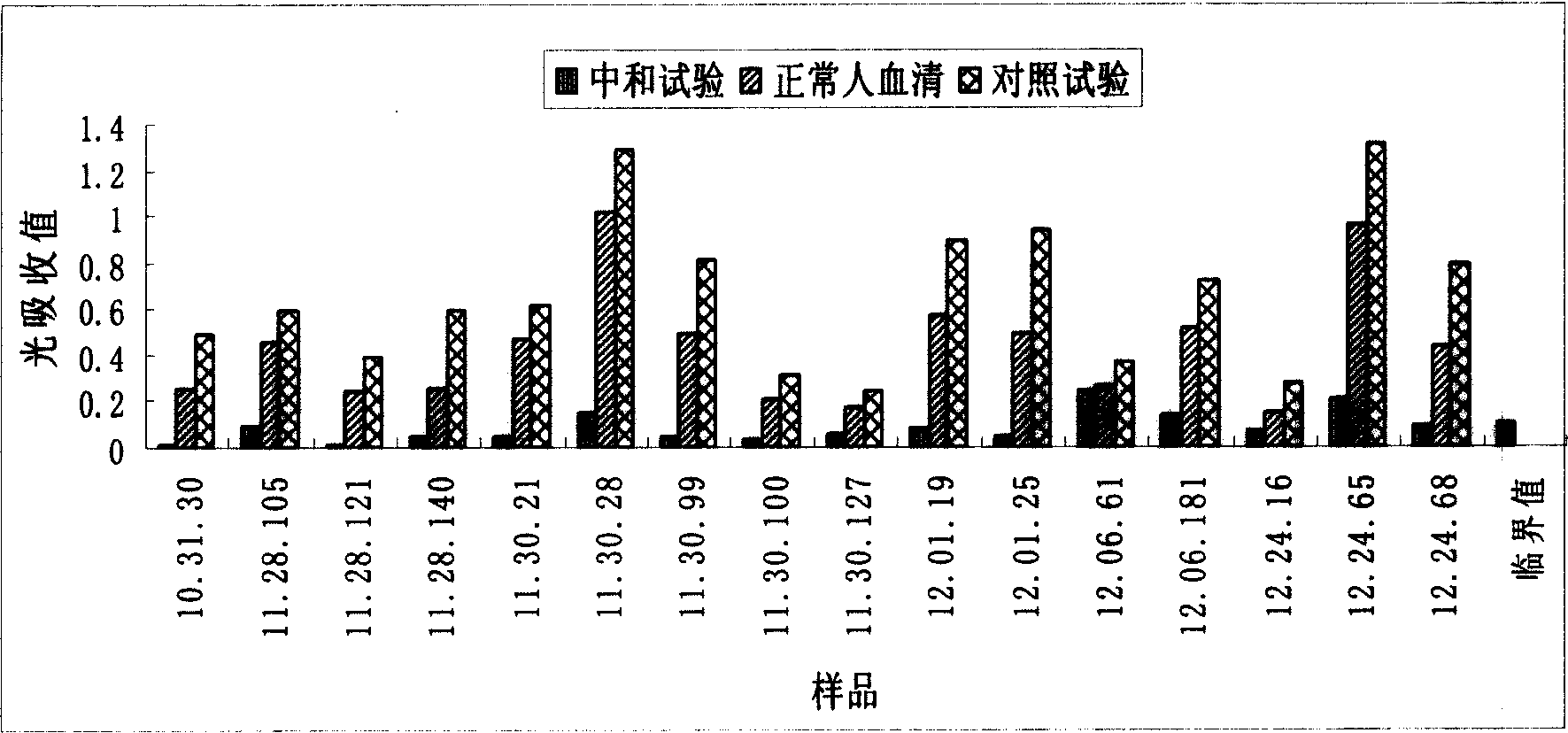
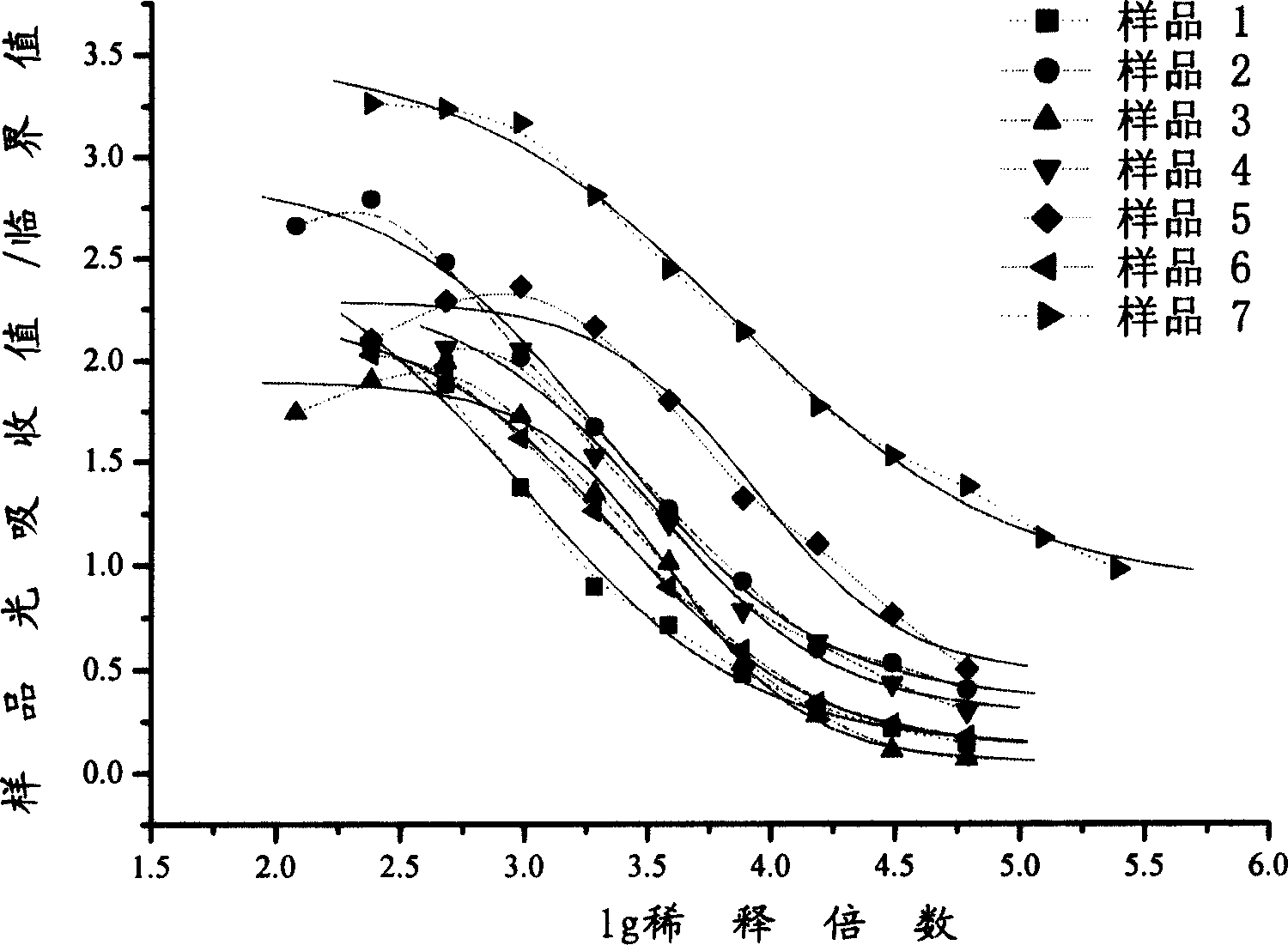
The invention provides an immunity diagnosis test kit for detecting II-type dengue virus antigen, which comprises a porous reaction plate covering monoclonal antibody DV2-M6, a sample treatment liquid, a monoclonal antibody DV2-M15 marked with a label, a positive contrast, a negative contrast, a concentration washing liquid, a develop liquid and a termination liquid, wherein the monoclonal antibodies DV2-M6 and DV2-M14 of the test kit can be specifically combined with NS1 protein of II-type dengue virus, without cross reaction with other three kinds of serotype dengue viruses NS1 and respectively combined with different antigen points of NS1, while the check sensitivity of NS1 protein of II-type dengue virus can reach 3ng / ml and the check sensitivity of culture supernatant of II-type dengue virus infection cell is 8 power of Pan-E dengue early elisa test kit, thereby improving the sensitivity of clinical serum sample check.
Owner:SOUTHERN MEDICAL UNIVERSITY
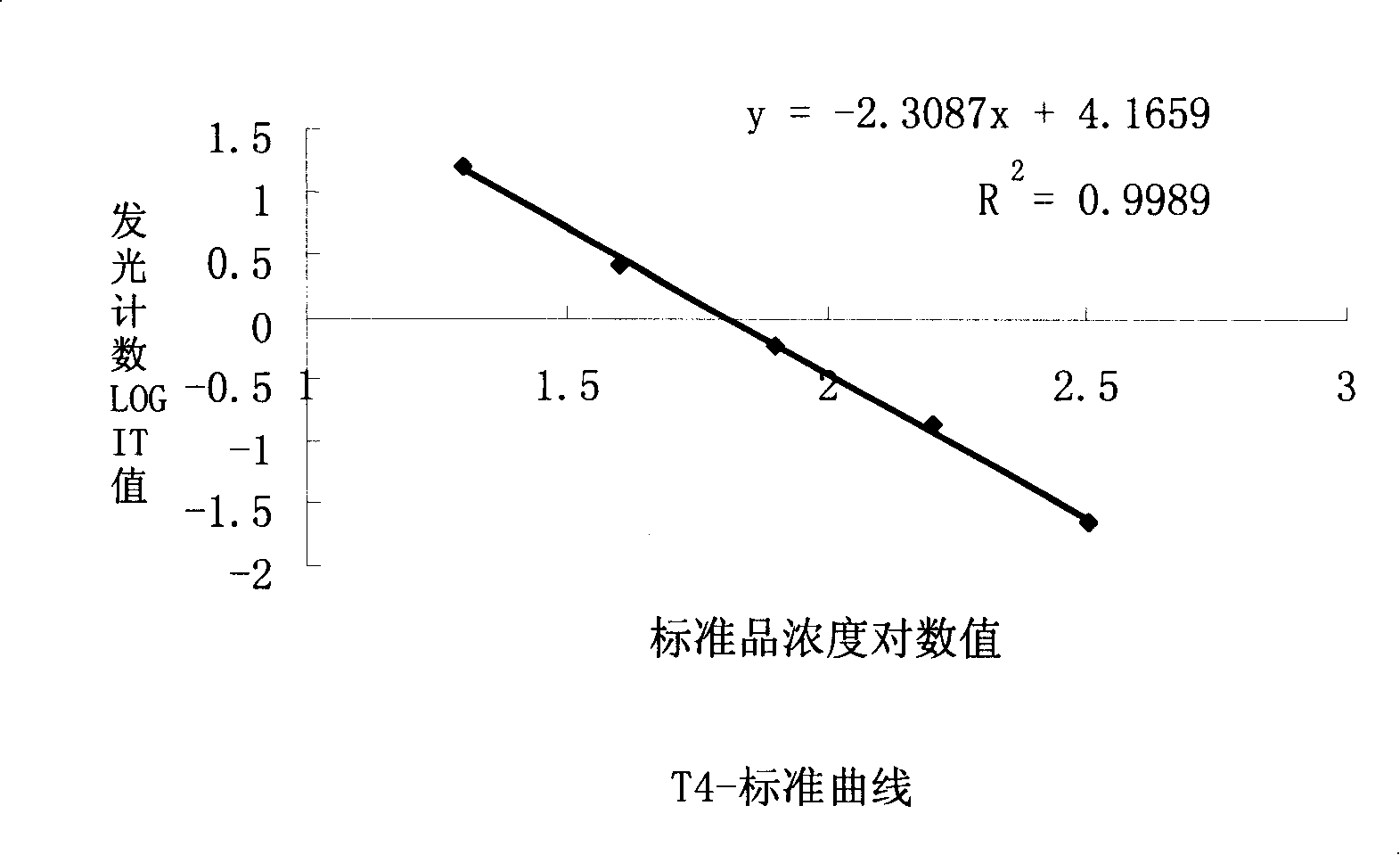
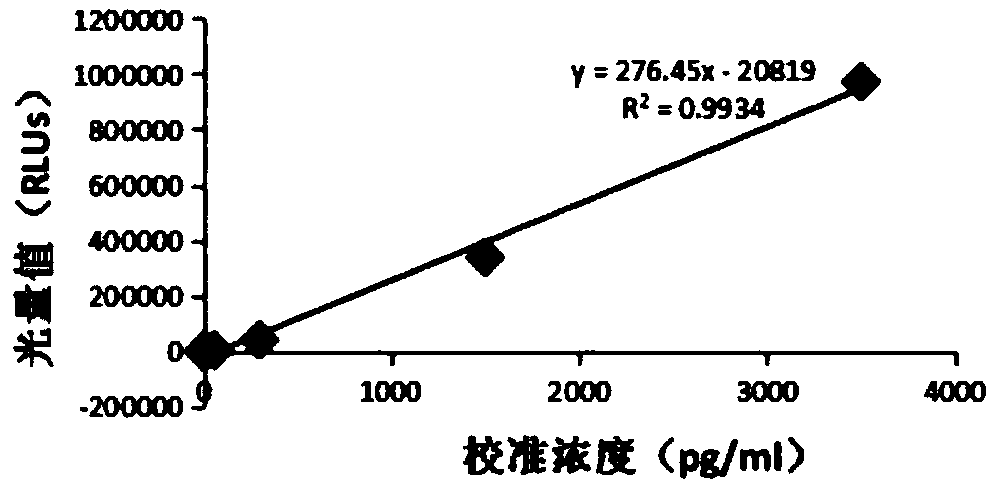
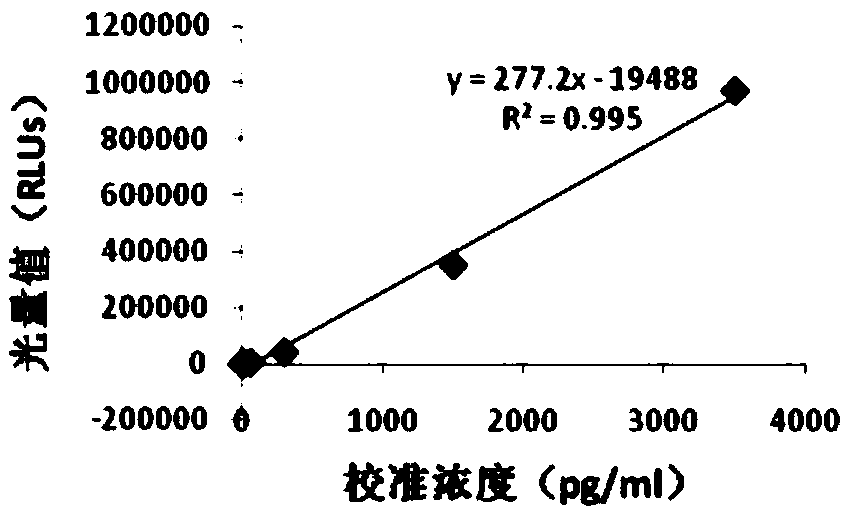
Thyroxine chemiluminescence immune analysis quantitative measuring reagent kit and method for preparing the same
ActiveCN101201354ALow feesEasy to operateChemiluminescene/bioluminescenceSevere hypothyroidismChemiluminescence
The invention belongs to the technical field of immunodiagnosis, and discloses a thyroxine (T4) enzymatic chemiluminescence quantitative determination kit. The kit disclosed by the invention consists of a T4 standard product, an antibody pre-coated reaction plate, a horseradish peroxidase marker, diluent, cleaning mixture, and chemiluminescence substrate liquid. The kit disclosed by the invention has the advantages of quickness, handiness, sensitiveness, cheapness and high repeatability, and is an important index for diagnosis and therapeutic evaluation of hyperthyroidism and hypothyroidism. The invention has high clinic use value. The invention also provides a method to prepare the kit.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Colloidal gold semi-quantitative quick immunity diagnosis test-paper stripe
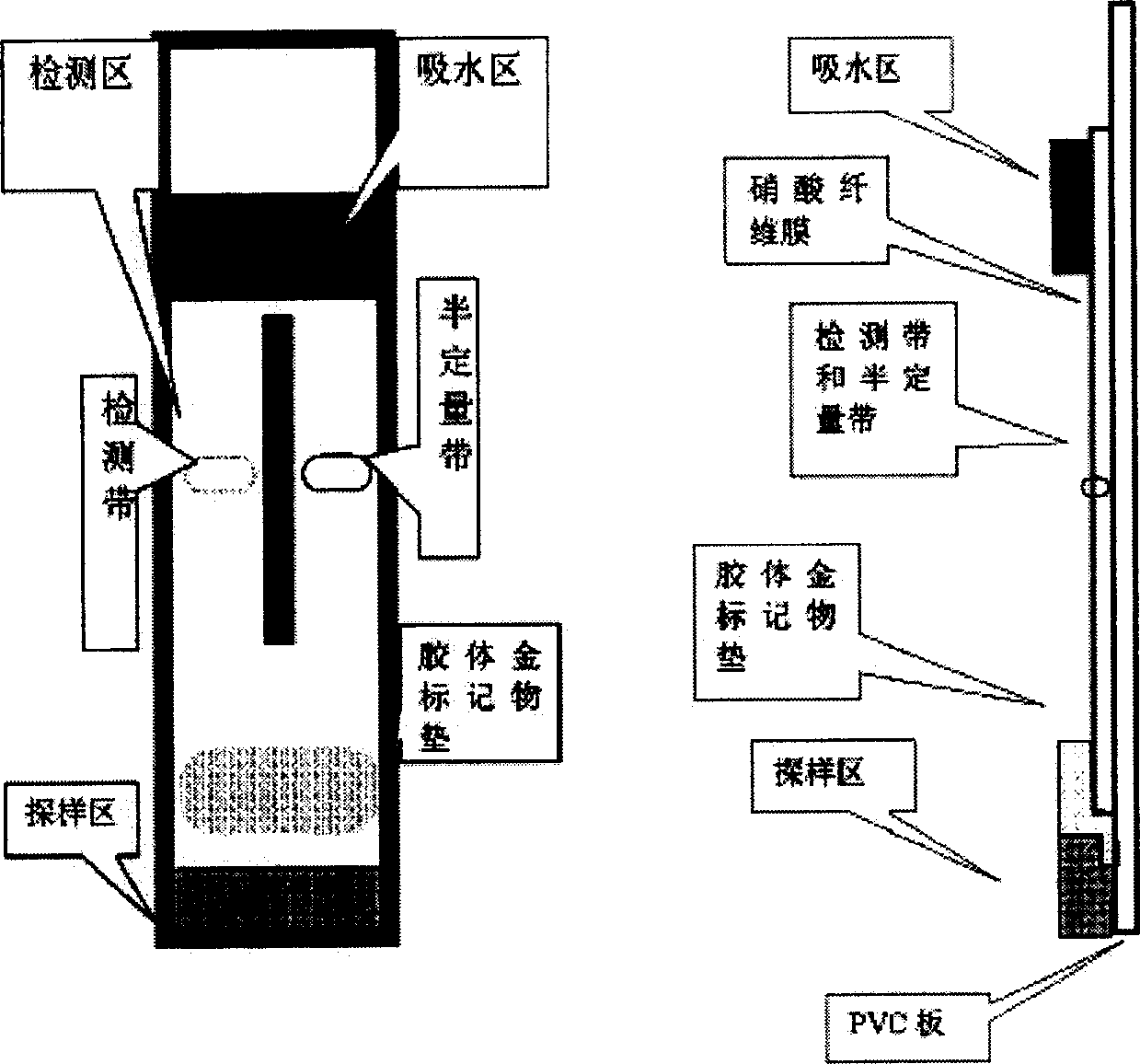
InactiveCN1892223AEasy to produceSimplify testing proceduresBiological testingVisual inspectionColloid
The present invention relates to a colloidal gold semiquantitative immunologic diagnosis test paper preparing and assembly method. Said test paper utilizes immunity chromatograph principle capable of rapid visual inspection nano colloidal gold marker and sample combined colour, judging detective semiquantitative result through test zone with parallel reference zone colour contrast.
Owner:LANZHOU UNIVERSITY
Monitoring device
Provided is a small sized, portable monitoring device capable of determining an analyte under investigation and having a system and method for providing compliance information to a user of his management of a disease, and for easily navigating a menu structure by means of manual control (s). Further provided is the provision of feedback to the user in form of a disease management information to be easily understood by a user such as the further described COMPLIANCE WINDOW (50) or the INDICATOR CATEGORIES (60,62,64,66,68). The user interface can be used in connection with a glucose diagnostic device, a coagulation diagnostic device, immunoassay diagnostic device, and other monitoring devices such as an blood pressure monitor or a pedometer.
Owner:EGOMEDICAL SWISS
Method for marking protein by carboxyl microsphere
InactiveCN104897904AEfficient couplingSimple and fast operationBiological testingMicrosphereBiological activation
The invention provides a method for marking protein by a carboxyl microsphere, belonging to the field of preparation of immunologic diagnosis kits. According to invention, in a system for marking the protein by the carboxyl microsphere, according to the content of carboxyls on the surface of the microsphere, the molar ratio of cross-linking agents EDC to -COOH is set to be (0.8-2) to 1, and the molar ratio of EDC to NHS or Sulfo to NHS is (1-3) to 1, so that the optimal ratio of the number of the carboxyls on the surface of the microsphere to the number of activated carboxyls is achieved; meanwhile, the pH is regulated, so that the reaction system is rapidly converted from condition beneficial for activation into state beneficial for antibody connection, the step of centrifugalization or tangential flow filtering is saved, and finally, the purposes of simplifying marking steps, saving cost, and marking protein by the carboxyl microsphere efficiently are achieved. The method provided by the invention can be applied to preparation of a detection kit taking the latex microsphere or the magnetic microsphere as a carrier, and has good market application prospect and economic value.
Owner:BIOSINO BIO TECH & SCI
Human immunodeficiency virus (HIV) antibody detection kit and preparation method thereof
InactiveCN104090101AHigh sensitivityStrong specificityChemiluminescene/bioluminescencePositive controlTrue positive rate
The invention belongs to the technical field of immunologic diagnosis and particularly relates to a human immunodeficiency virus (HIV) antibody detection kit using a microparticle chemiluminiscence method and a preparation method of the kit. The kit is composed of magnetic microparticles for detecting an HIV antibody, a tracing conjugate for detecting the HIV antibody, a negative control, a I type positive control, a II type positive control and an analyzing buffering solution. The invention further discloses the preparation method of the detection kit, which adopts a particle chemiluminiscence immunoassay technology; compared with ELISA (Enzyme-Linked Immunosorbent Assay), the technology has higher sensitivity and specificity and is suitable for the clinical auxiliary diagnosis of HIV.
Owner:威海威高生物科技有限公司
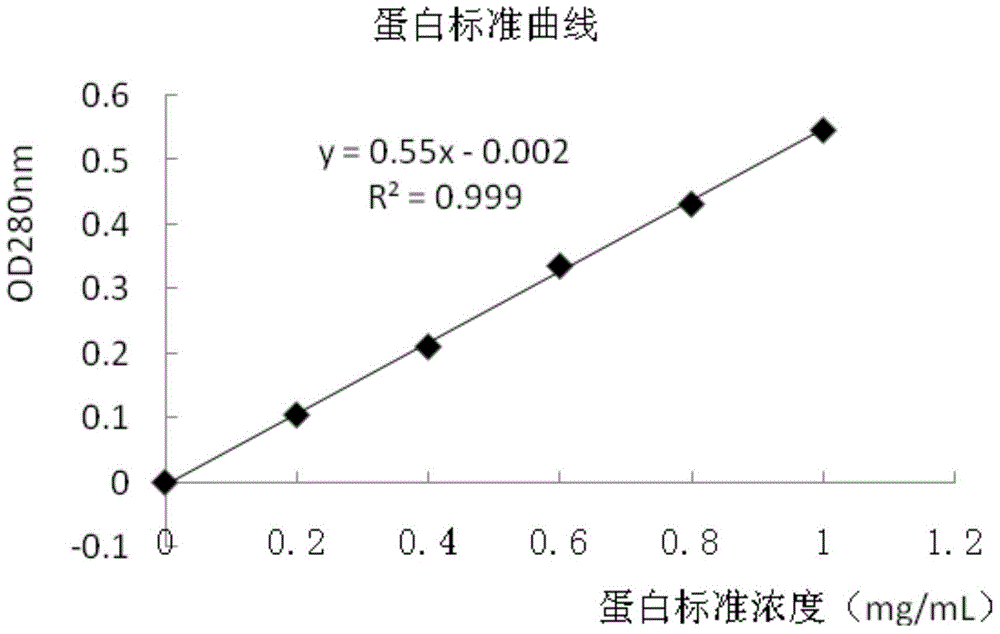
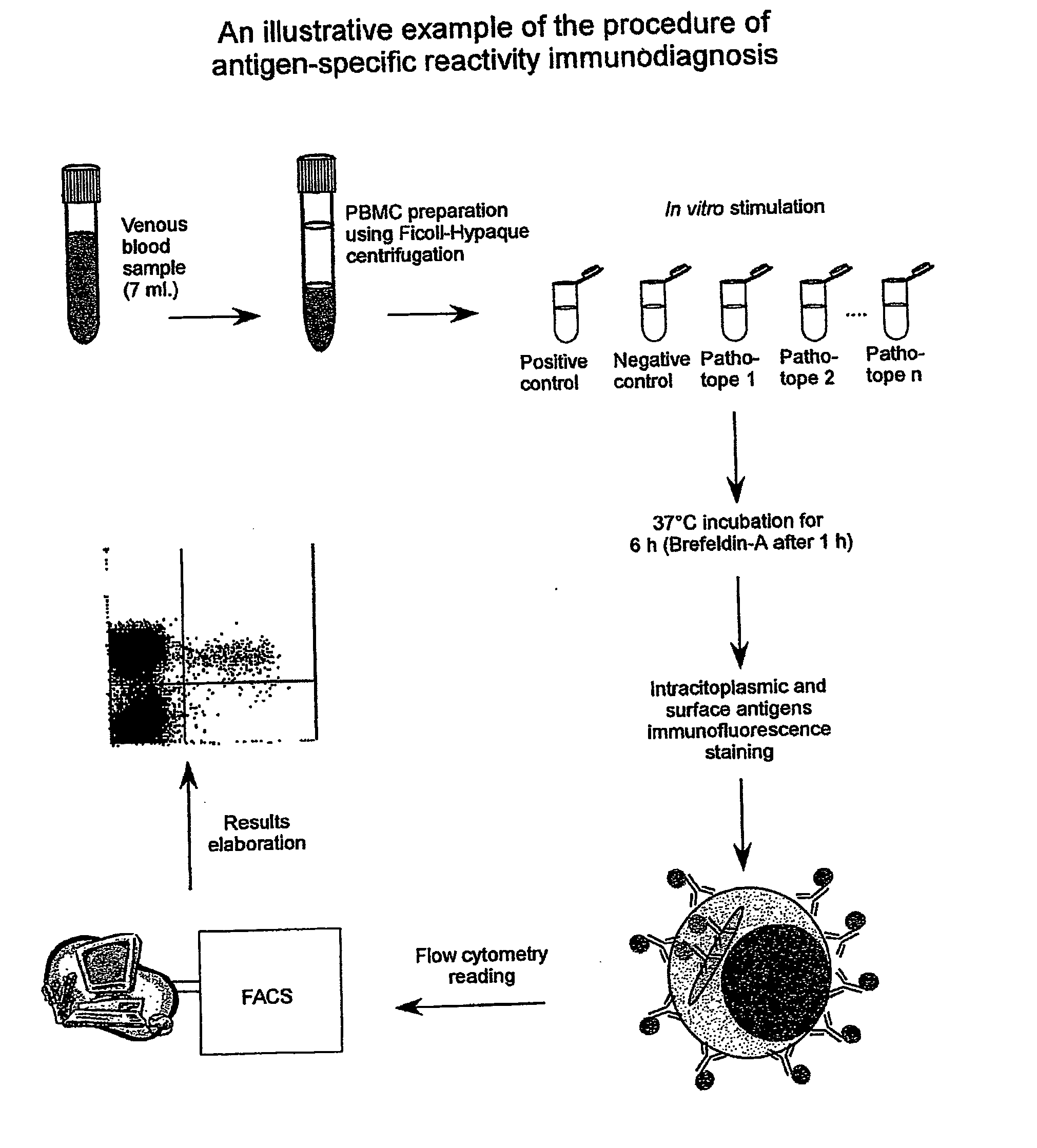
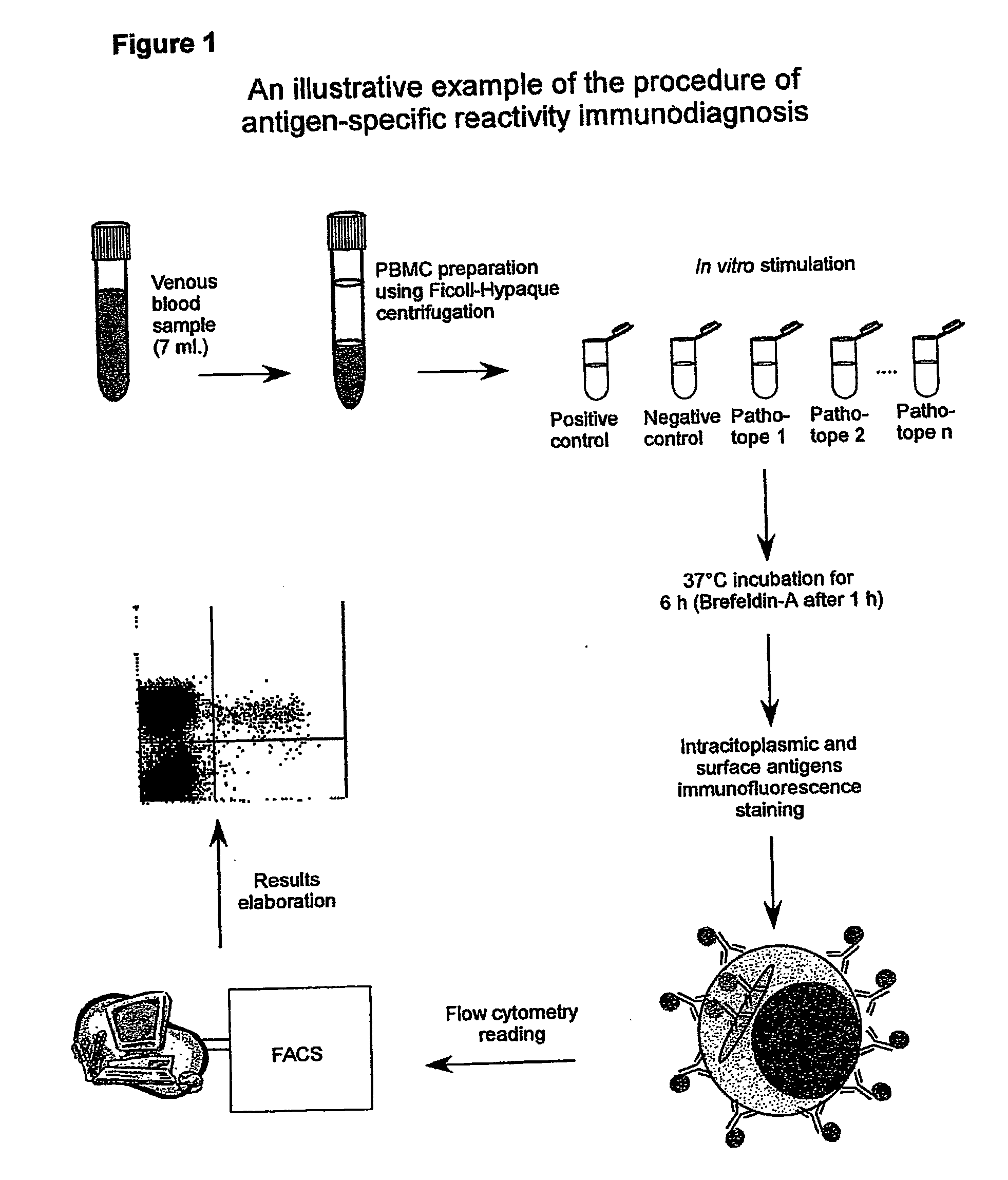
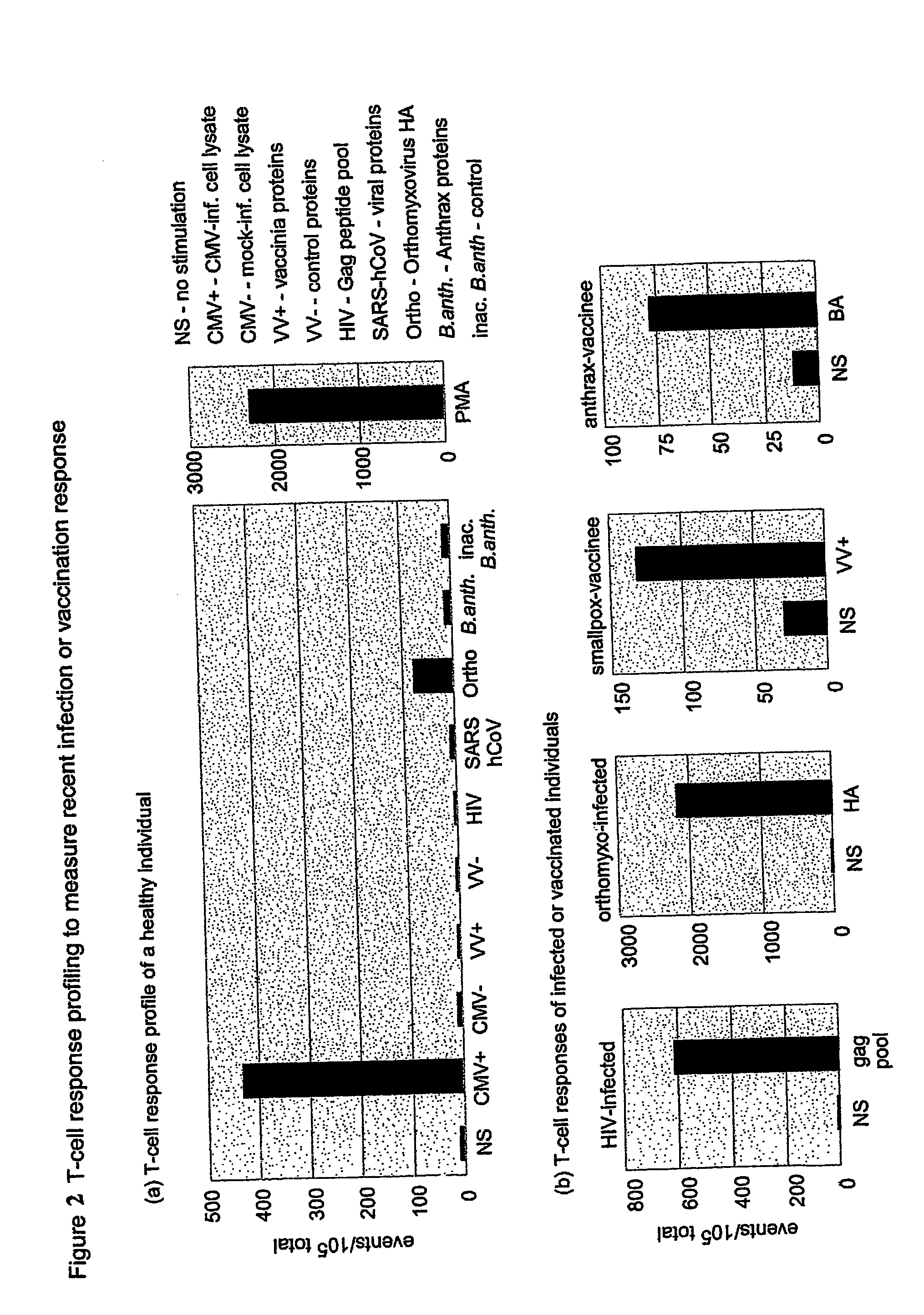
Method and diagnostic tests based on flow cytometric analysis of antigen-specific t lymphocytes
The present invention provides a method for the immuno-diagnosis of diseases with different aetiology (infectious diseases, tumors etc) by measurement of the T cell response J, B and NK lymphocytes) induced by a set of diseasespecific antigens. The method is based on the quantitative determination of antigenspecific T lymphocytes (referred as Ag-Sp), stimulated by using a newly devised pathology-specific antigen or epitope compositions which represent further embodiments of the invention. After stimulation, the selective measurement of the Ag-Sp T lymphocytes is performed by: A) monoclonal antibodies recognizing membrane structures of T lymphocytes and of their sub-populations; B) monoclonal antibodies binding to cytokines accumulating at intracellular level after the stimulation with the antigen; or C) mixtures of A) and B). The flow cytometric detection of the presence of markers of differentiation on T lymphocytes and of intracytoplasmic cytokines allows the acquisition of both qualitative and quantitative results. The invention also provides diagnostic kits for performing the method of the invention.
Owner:INST NAT PER LE MALATTIE INFETTIVE LAZZARO SPALLANZANI IRCCS
Enzyme-linked immunologic diagnosis kit for core antigen of C type hepatitis virus and method for preparing same
Disclosed are a hepatitis C virus antigen enzyme-linked immunoassay reagent box and a method for making the same. The invention obtains the cell strain of excretive anti HCV core antigen by analyzing the core antigen array of the hepatitis C virus different type and cloning the core antigen gene of HCV, purifying out the high activity monoclonal antibody with four core aa expression sites of HCV, wherein the Cab1 and Cabs are used as coating antibodies, the Cab3 and Cab4 are used as enzyme labeled antibodies; employing double antibodies sandwich technology to prepare HCV-cAg ELISA diagnosing reagent box.
Owner:湖南景达基因有限公司
Tuberculosis antigen specific whole blood IFN-gamma diagnosis kit, method for producing the same and method for using same
InactiveCN101493454ADiagnostic advantageShorten the timeBiological testingEscherichia coliEnzyme linked immunoassay
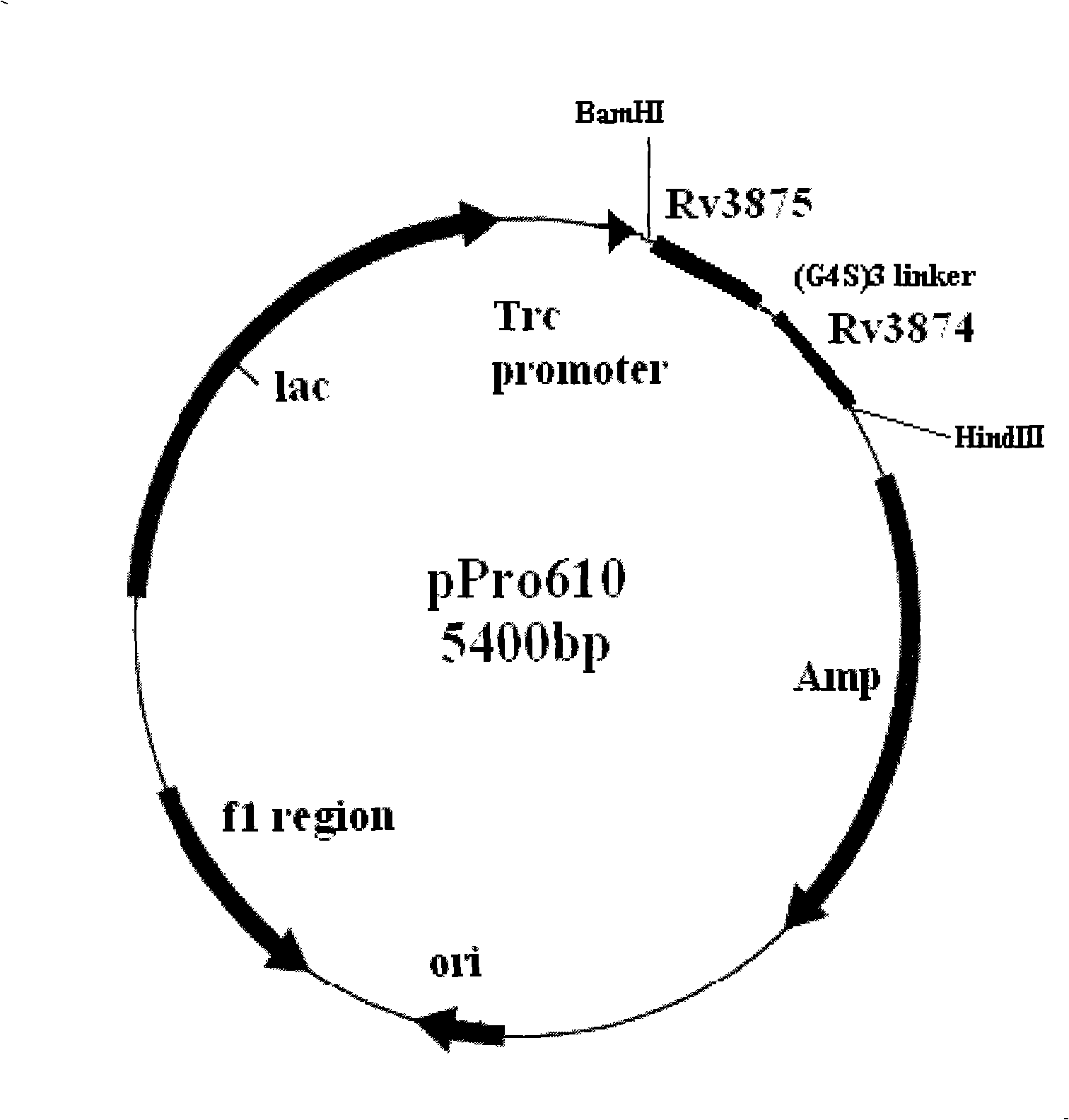
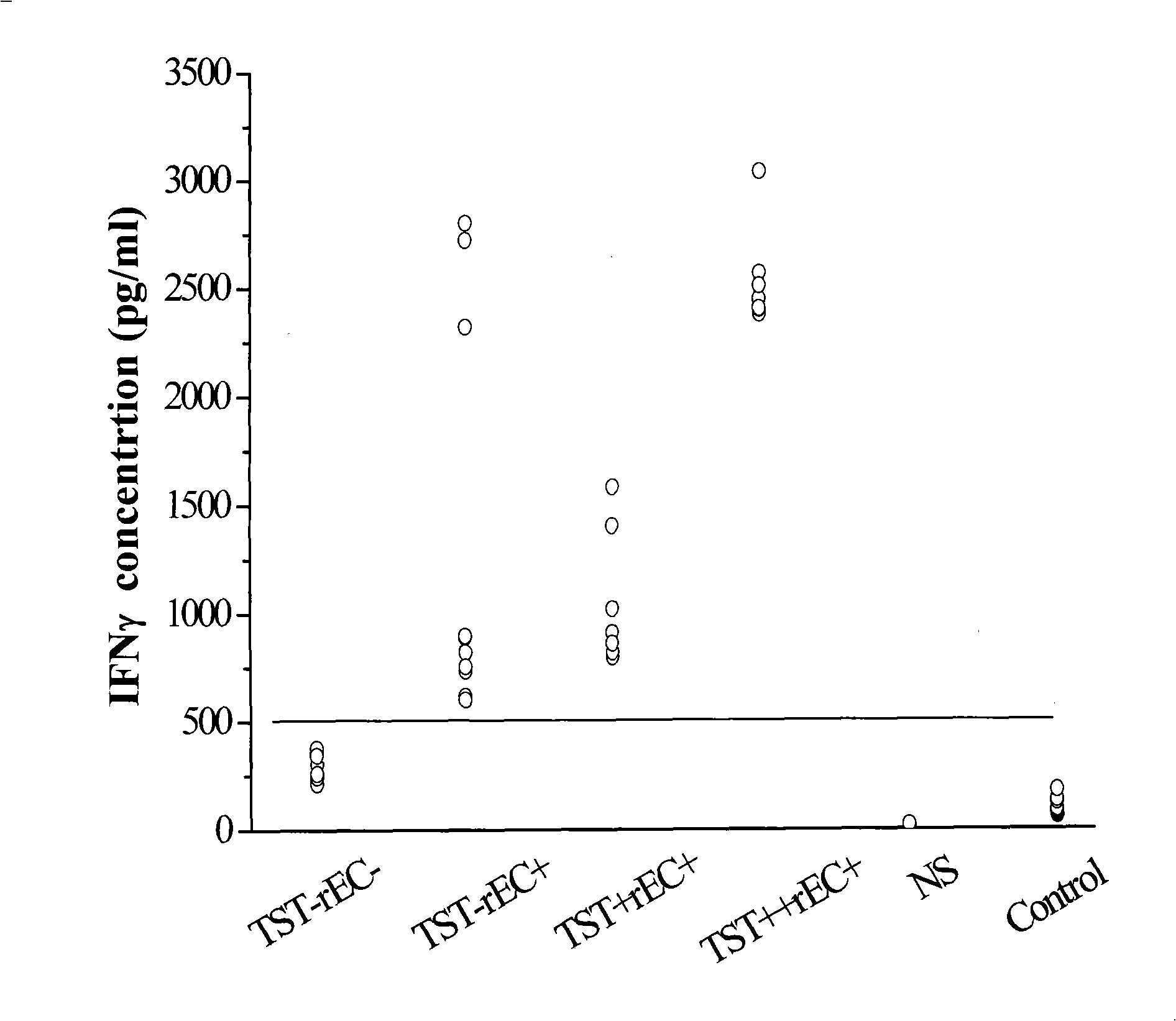
The invention relates to a diagnostic kit for tuberculosis and mycobacterium tuberculosis infectors, a preparation method and an application method thereof. By using the linker for encoding 15 amino acid (G4S1) 3, the encoding genes (SEQ.ID.NO.6) of a mycobacterium tuberculosis specific antigen Rv3875 and Rv3874 are connected in series, and then inserted into an E. coli expression vector, and the high-efficiency expression and purification for the fusion protein of Rv3875 and Rv3874 in the E. coli are achieved. The recombinant protein at least comprises 8 T cell epipositions which can be used for cell immunity diagnosis, and a diagnostic kit and diagnostic method for a whole blood IFN-Gamma release analysis method are established by taking the protein as the basis and combining the human IFN-Gamma enzyme-linked immunoassay technology, and can be used for the early, specific diagnosis and screening of tuberculosis and mycobacterium tuberculosis infectors.
Owner:范雄林
Immune-chromatographic test strip for fluorescence quantitative detection of INHB (inhibin B) and preparation method of immune-chromatographic test strip
An immunochromatographic test strip for fluorescence quantitative detection of INHB and a preparation method thereof. The invention belongs to the technical field of immunodiagnosis. The purpose of the invention is to address the deficiencies in the prior art. The invention adopts the following technical solutions: a fluorescent The immunochromatographic test strip for quantitative detection of INHB is composed of a sample pad, a marker pad, a coating film, and an absorbent paper sequentially lapped on a PVC bottom plate. The marker pad is sprayed with avidin; the coating of the quality control line has specific identification Rabbit anti-avidin antibody for avidin. The invention has the following advantages: the detection line and the quality control line adopt an independent reaction system without mutual interference and influence, and adopt the T / C value method for calibration, which ensures the accuracy of the test results. Fluorescence immunochromatography is adopted, and the detection method has high sensitivity, simple operation and low cost. It can detect inhibin B in blood samples with a concentration as low as 10 pg / mL. The detector used does not require professional operators, and the detection result can be obtained in 15 minutes.
Owner:SHENZHEN YHLO BIOTECH
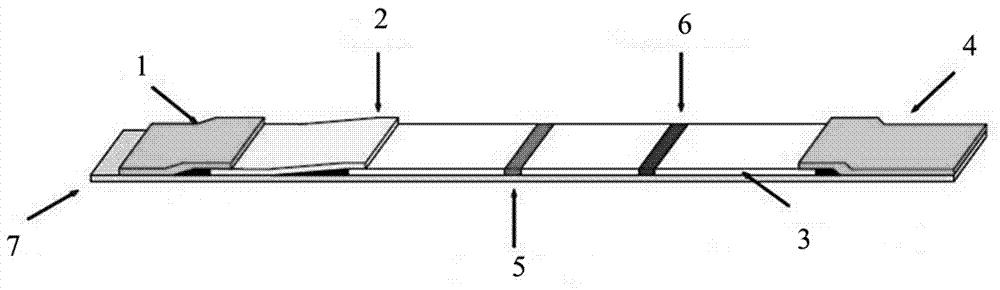
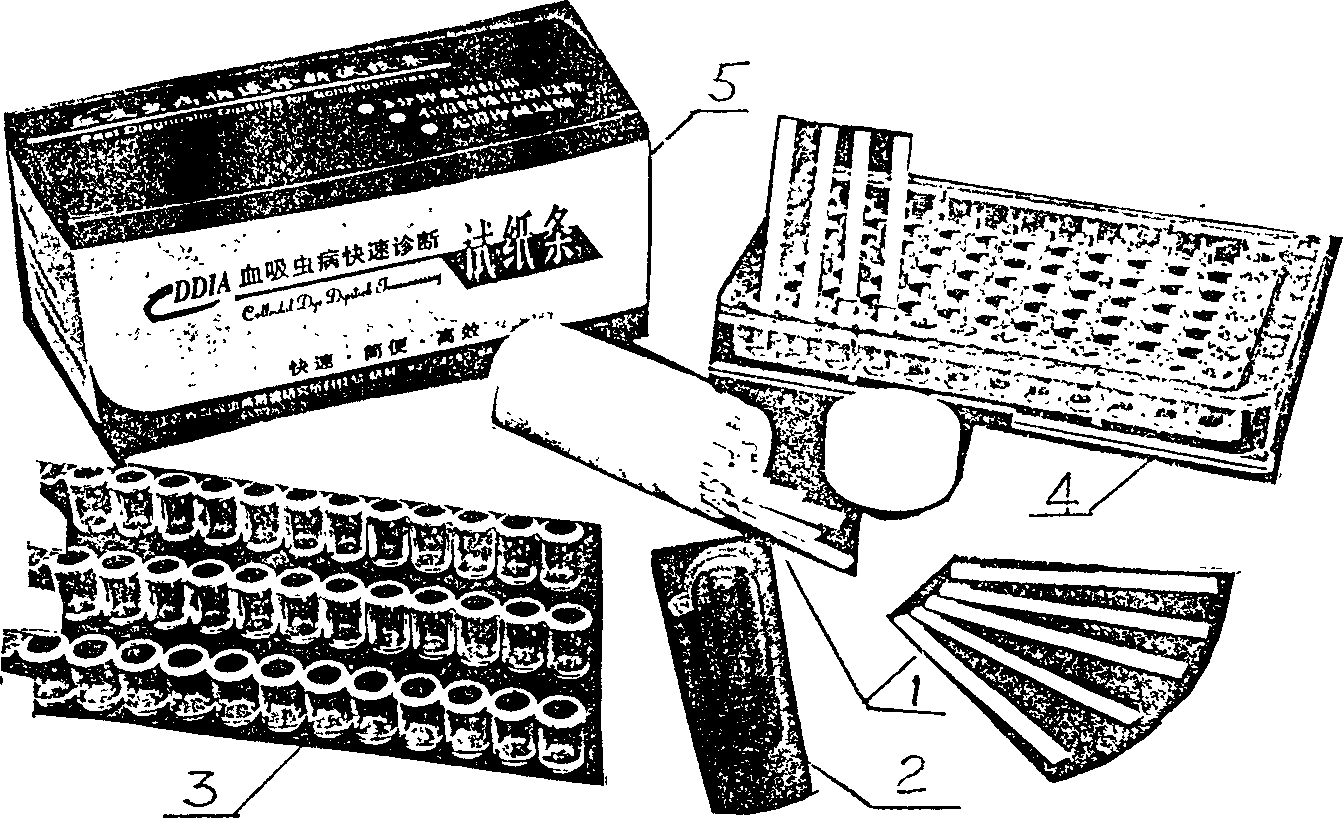
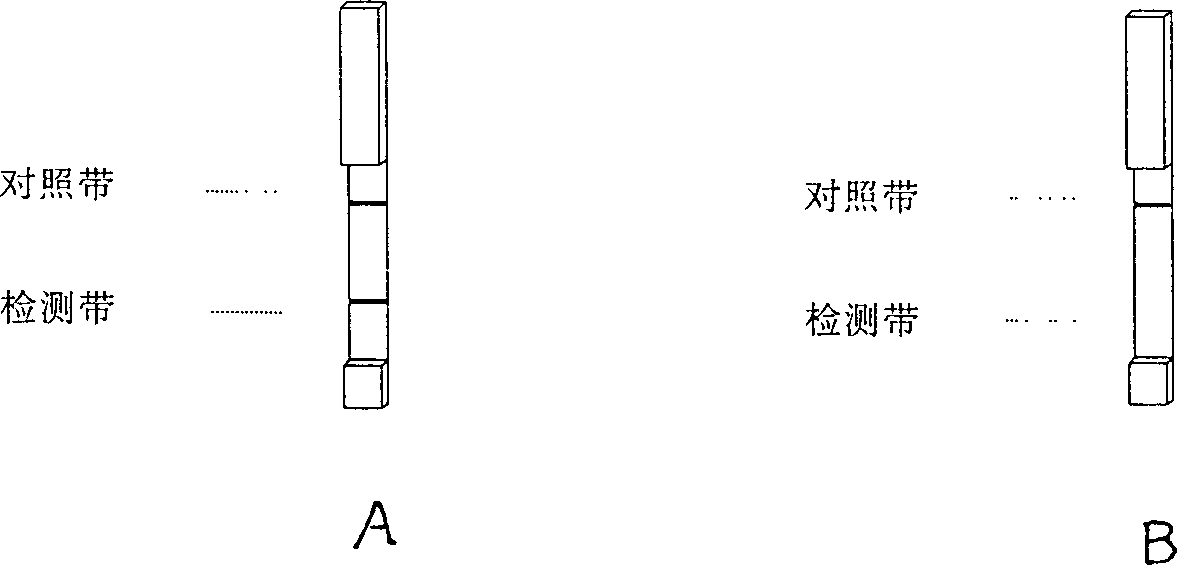
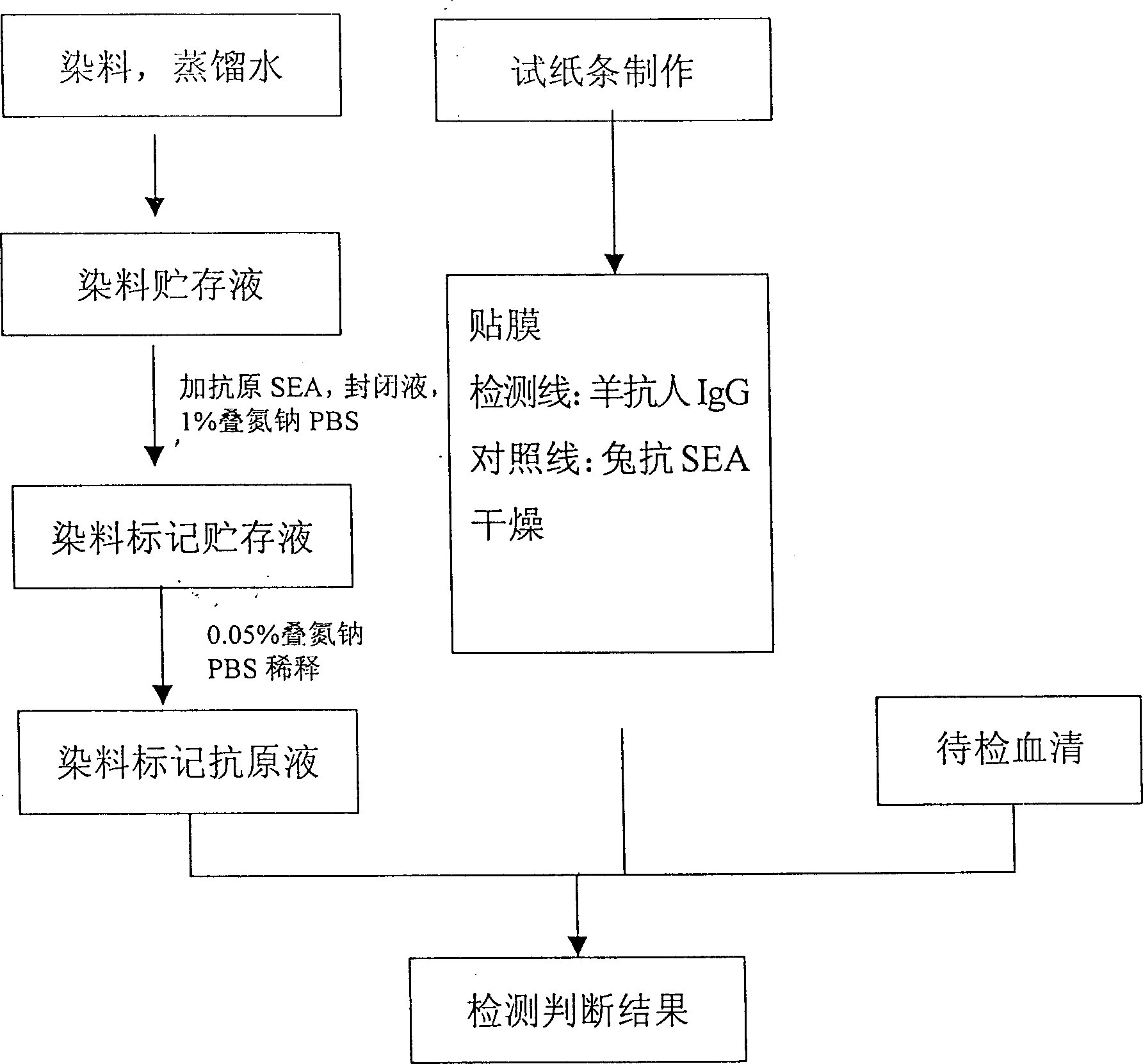
Quick test paper strip diagnostic reagent kits for bilharziasis and its preparing method and use
InactiveCN1425918AEasy to markQuality improvementBiological testingNitrocelluloseBovine serum albumin
The present invention belongs to immunological parasitic disease diagnosis technology. The reagent kit is prepared via dye labeling process and includes labeling antigen solution compounded with 2BLN disperse blue dye, soluble schistosoma japoncium egg antigen, 1% sodium azide and bow serum albumen blocking liquid; and test paper strip with nitrocellulose as chromatographic film detection lines of human sheep antigen IgG and contrast lines of soluble schistosoma japonium egg resisting rabbit antigen IgG on the chromatographic film. The serum to be tested is mixed with the labeling antigen solution and the mixture liquid is tested with the test paper strip.
Owner:JIANGSU INST OF PARASITIC DISEASES
High-throughput nucleotide library sequencing
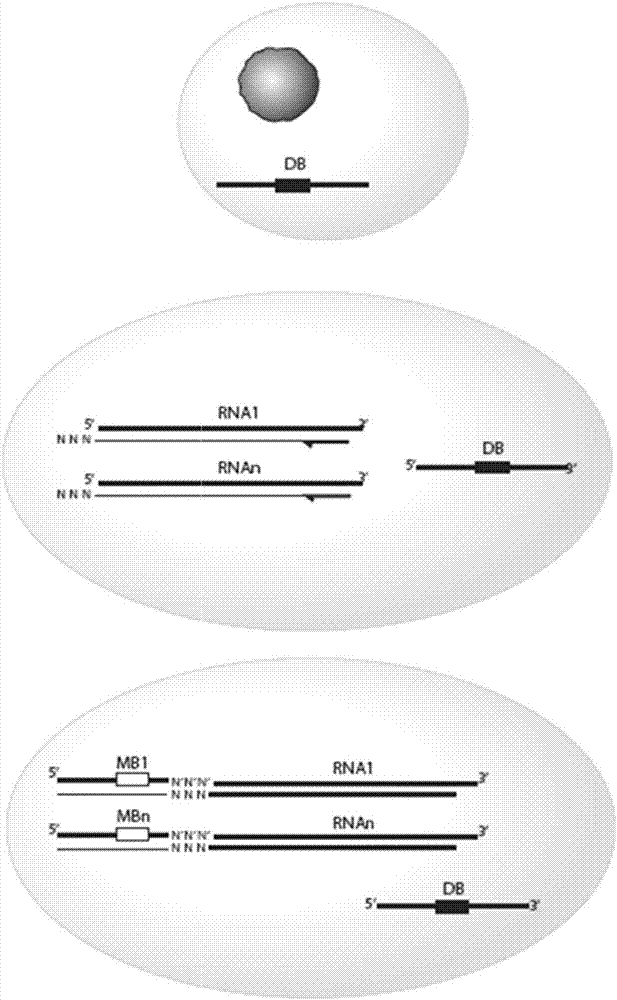
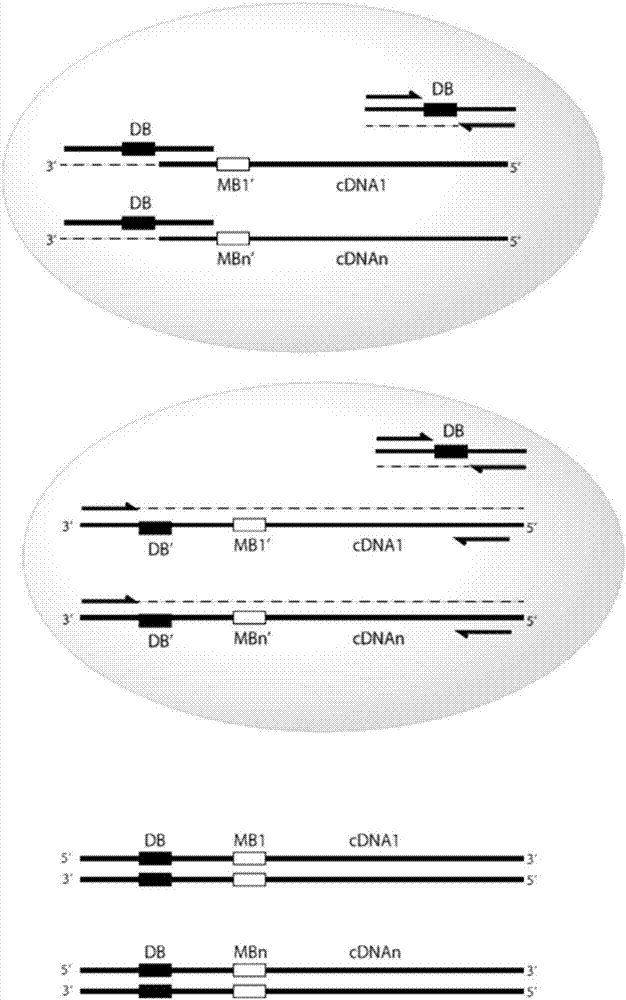
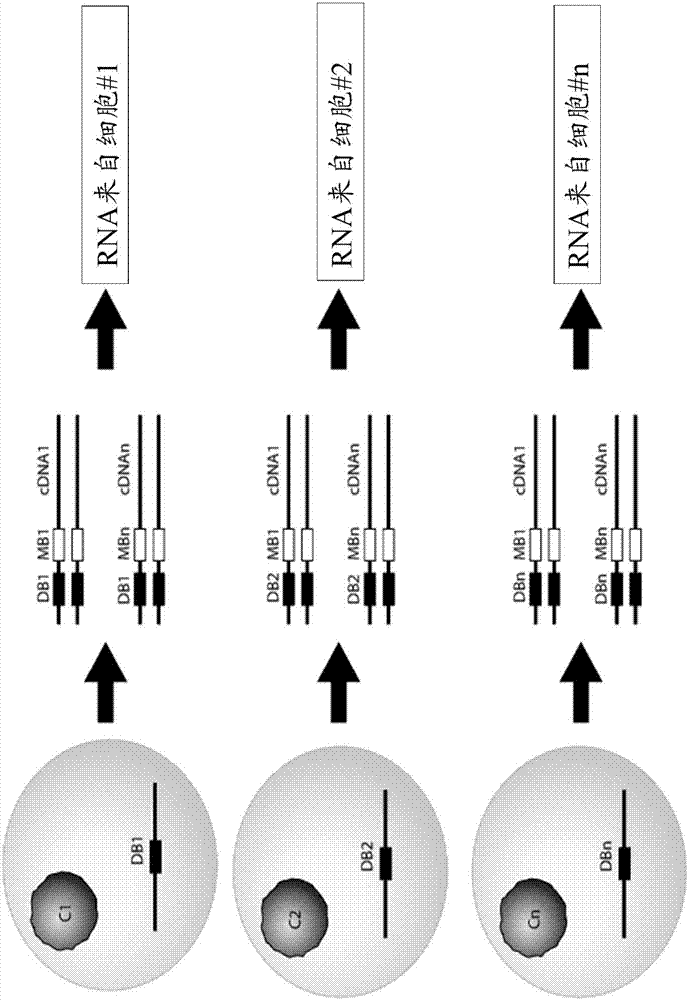
Provided herein are methods and composition for immune repertoire sequencing and single cell barcoding. The methods and compositions can be used to pair any two sequences originating from a single cell, such as heavy and light chain antibody sequences, alpha and beta chain T-cell receptor sequences, or gamma and delta chain T-cell receptor sequences, for antibody and T-cell receptor discovery, disease and immune diagnostics, and low error sequencing.
Owner:ABVITRO LLC
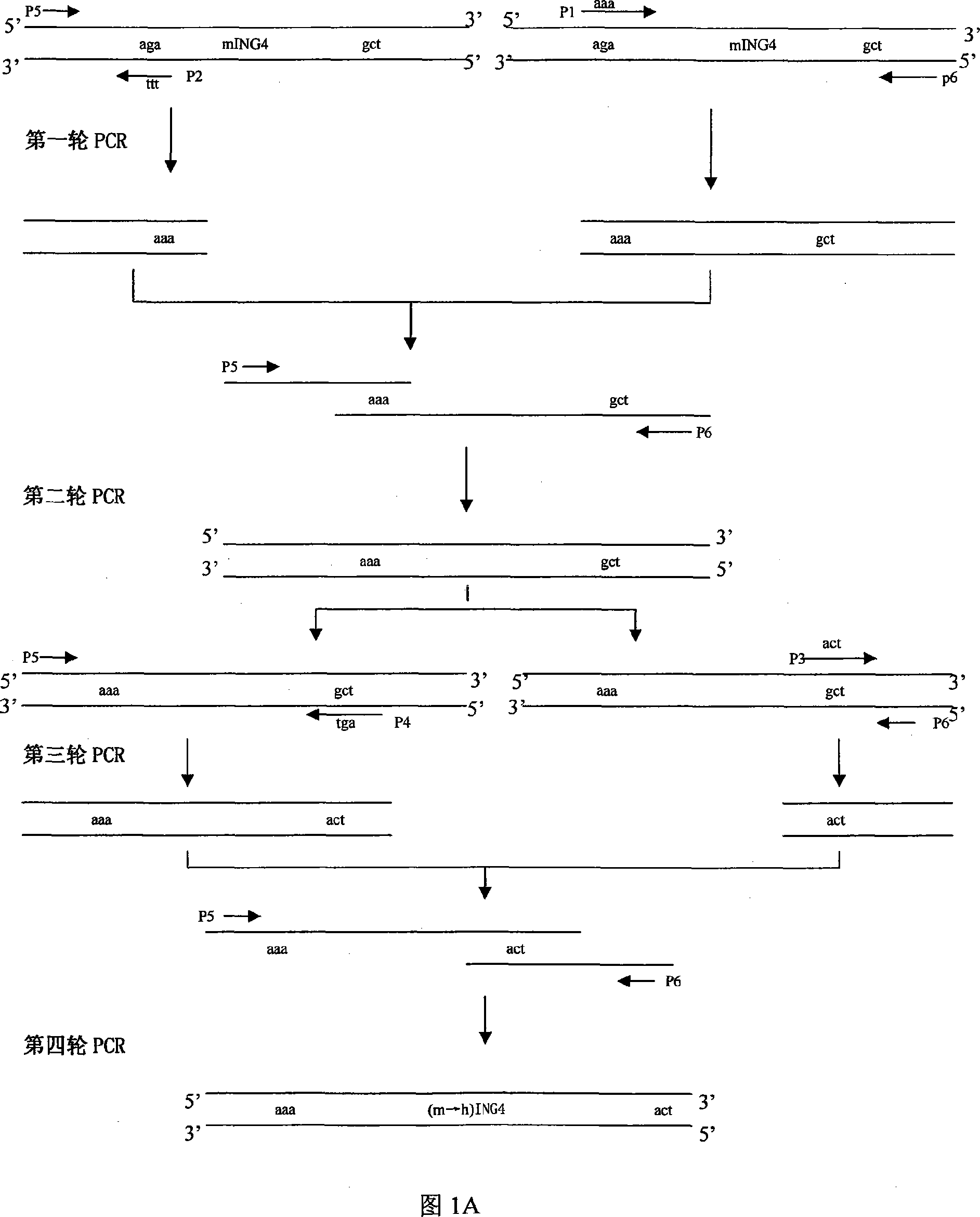
Humanization modified rat ING4 gene and adenovirus expression vectors thereof
ActiveCN101058809AGrowth inhibitionIncrease apoptosis rateBacteriaBacteria material medical ingredientsGenes mutationDisease
The invention relates to a human reforming mouse ING4 (tumor growth inhibition factor) gene and recombinant adenovirus (adenovirus hominis 5 shape Ad-ING4-DeltaGFP), wherein preservation cooperation is Chinese typical culture preservation centre, preservation number is CCTCC-V200701. The invention also relates to a human reforming gene mutation technique, adenovirus transition carrier removing GFP and homologous recombinant adenovirus carrier. The ING4 gene and recombinant adenovirus carrier provide new immunodiagnosis and target therapeutic approach for treating neoplastic disease, development disease, immune disease, the other disease owing to abnormal expression of tumour growth inhibition factor ING4, special cancer of the lungs and leukocythemia, which is provided with a giant application prospect.
Owner:SUZHOU UNIV
Enzyme linked immunity diagnose reagent kit for HB core antigen detecting in two sandwich method and application thereof
The disclosed dual-sandwich enzyme-linked immunologic diagnosis agent box for hepatitis-B core antibody comprises: a micro-porous reaction plate composed by the hepatitis-B core antigen recombinant with purity more than 90% and 5*104~5*105u / mg titer, and the enzyme compound composed by the hepatitis-B core antigen recombinant marked by HRP. This invention has high specificity and sensitivity and convenient to qualitative or semi-qualitative detection.
Owner:SHENYANG HUIMIN BIOTECH CO LTD
Immunologic diagnosis kit for detecting dengue virus NS1 antigen and application thereof
ActiveCN101726593AImprove featuresIncreased sensitivityMaterial analysisAgainst vector-borne diseasesBiotinSerotype
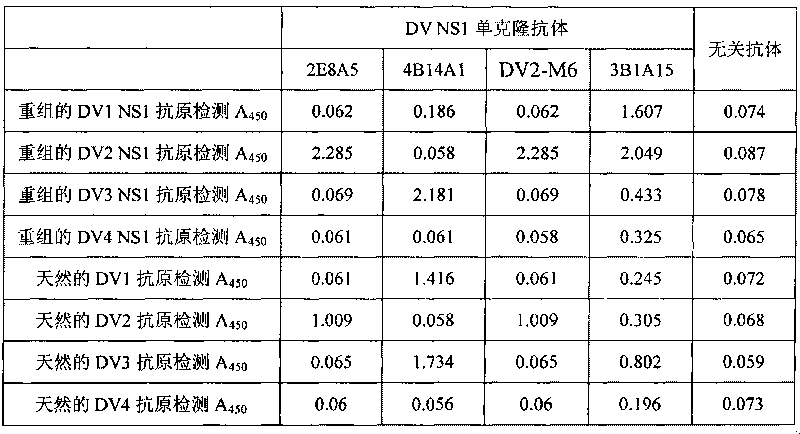
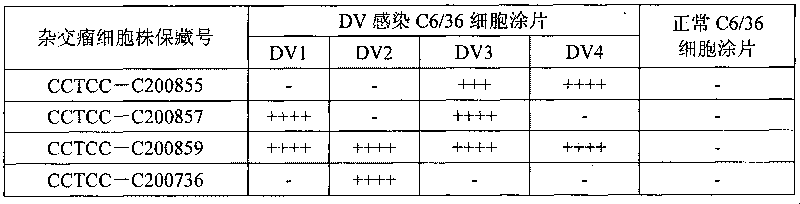
The invention discloses an immunologic diagnosis kit for detecting dengue virus NS1 antigen and application thereof. The kit comprises a capturing antibody and a detecting body combined with a marker, and the kit is characterized in that the capturing antibody consists of a monoclonal antibody 2E8A5, a monoclonal antibody DV2M6 and a monoclonal antibody 4B14A1, the detecting antibody is a monoclonal antibody 3B1A15, and the marker can be biotin, horseradish peroxidase, alkaline phosphatase, colloidal gold or fluorescein. The kit can specifically detect NS1 proteins of four serotype dengue viruses and does not have cross reaction with other viruses of flavivirus system, such as flavivirus and encephalitis B virus, the sensitivity of the kit is at least four times higher than that of detection of foreign commodity kits, and the kit can efficiently reduce the omission factor of the clinical application.
Owner:SOUTHERN MEDICAL UNIVERSITY
Preparation method for gold labeled immucochromatographic test strip jointly marked through colloidal gold and latex microsphere
InactiveCN104991058AHigh detection sensitivityExpand the scope of detectionBiological material analysisBiological testingMicrosphereProtein molecules
The invention discloses a preparation method for a gold labeled immucochromatographic test strip jointly marked through colloidal gold and latex microsphere. The preparation method comprises the following steps that 1 chloroauric acid is reduced through trisodium citrate to prepare colloidal gold solution; 2 the colloidal gold solution is used for marking protein molecules; 3 50 nm red latex microsphere is prepared or bought directly; 4 the protein molecule is marked by the latex microsphere; 5 quality testing is conducted on the marking colloidal gold solution and latex marking solution which are obtained through the above steps; 6 a nitrocellulose membrane is pasted on a PVC plastic bottom plate; 7 a sample pad, a combining pad, a water-absorbing pad, handle paper and Max glue are assembled on the PVC plastic bottom plate; 8 quality testing is conducted on the prepared gold labeled test strip according to the quality standard of the product. The preparation method for the gold labeled immucochromatographic test strip jointly marked through the colloidal gold and the latex microsphere has the advantages that the detecting sensitivity and detecting range of the gold labeled immucochromatographic test strip are improved, and the application area and quantitative application of colloidal gold immunologic diagnosis are enlarged in this way.
Owner:宋晓峰 +1
B-cell antigenic multi-epitope peptide linked in tandem in OmpU of vibrio mimicus, making method and application thereof
InactiveCN101747416AEasy accessAccurately obtainedAntibacterial agentsMicroorganism based processesDiseaseMolecular Immunology
The present invention relates to an antigenic B-cell multi-epitope peptide linked in tandem in the outer membrane protein(Omp) U gene of vibrio mimicus, a making method and an application thereof, which belong to the field of molecular immunology. The B-cell multi-epitope peptide linked in tandem can induce fish to make protective immunity response to vibrio mimicus infection. In the present invention, the amino acid sequence of the antigenic B-cell multi-epitope peptide linked in tandem in the OmpU is shown in SEQIDNO:10. The B-cell multi-epitope peptide linked in tandem in the OmpU of vibrio mimicus is made by a genetic engineering technique. The verification of immune blotting analysis, specific antibody detection and immune animal protective experiments shows that the peptide can elicit efficient and specific protective humoral immunity response to vibrio mimicus infection. The B-cell multi-epitope peptide linked in tandem can be used for the immune diagnosis and the immune prevention and treatment of aquatic animal ascitic diseases. The present invention has high social benefit and economic benefit.
Owner:ANHUI AGRICULTURAL UNIVERSITY
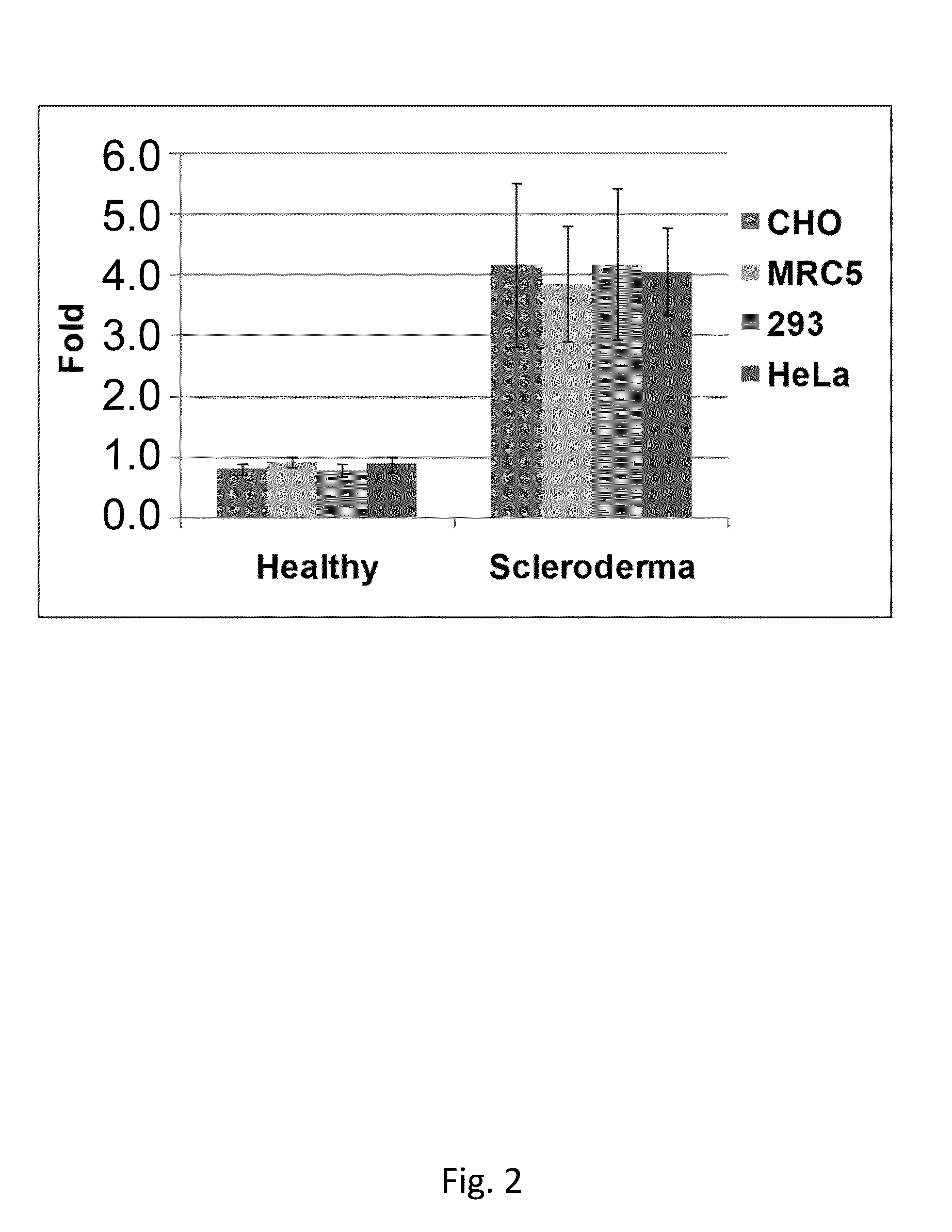
Immunodiagnostic method for diagnosing auto-immune systemic sclerosis (SSC) and systemic lupus erythematosus (SLE)
The invention relates to a method for the identification of patients affected by Systemic Sclerosis (SSc) (early, limited cutaneous, diffuse forms) or Systemic Lupus Erythematosus (SLE). Among SSc and SLE patients, this method allows identification of presence of vascular ulcerations in SSc, and Raynaud's phenomenon in SLE, respectively.
Owner:VIVABIOCELL
Recombinant protein used as NT-proBNP immunodiagnosis reagent standard as well as preparation method and use thereof
InactiveCN101230101AHigh puritySimple preparation processHormone peptidesBiological testingNucleotideNucleotide sequencing
The invention relates to NT-proBNP recombinant albumin. The NT-proBNP recombinant albumin is provided with the amino acid sequence chosen from a) or b): a). the amino acid sequence showed in SEQ ID NO: 1; b). the amino acid sequence with NT-proBNP recombinant albumin reactivity after one or more amino acids are deleted, replaced or inserted. The invention also relates to a nucleotide sequence for coding the NT-proBNP recombinant albumin and a preparation method of the NT-proBNP recombinant albumin. The NT-proBNP recombinant albumin is provided with the immunogenicity the same as the NT-proBNP albumin, and high purity and better stability, which can replace the e natural NT-proBNP polypeptide, is used as a NT-proBNP immunologic diagnosis reagent standard, and establishes basis for further researching and developing the NT-proBNP detection kit.
Owner:ARMY MEDICAL UNIV
Preparation method of carbon quantum dot test paper strip for detecting P24 antigen
InactiveCN103344756AEnabling immunodiagnosticsHigh sensitivityMaterial analysisCelluloseNitrocellulose
The invention relates to a test paper strip for detecting a P24 antigen and particularly relates to a preparation method of a carbon quantum dot test paper strip for detecting a P24 antigen. The preparation method comprises the following steps of: bonding a sample pad and a water absorption pad respectively at two ends of a bottom plate, preparing a P24 carbon quantum dot pad and a P24 nitrocellulose membrane, bonding the sample pad, the P24 carbon quantum dot pad, the P24 nitrocellulose membrane and the water absorption pad in a lapping way in sequence on the bottom plate to obtain a test paper plate, cutting the test paper plate into a plurality of test paper strips conforming to defined widths, and hermetically storing the test paper strips. According to the invention, a known specific antibody or antigen is fixed in a zone of the cellulose membrane at first, a sample to be detected is added on the sample pad, a carbon quantum dot marking reagent on the carbon quantum dot pad is dissolved, and a large amount of carbon quantum dots are gathered on a detection band, so that bright light is emitted and specific immunologic diagnosis is achieved. The method is high in sensitivity and low in toxicity or free from toxicity in a detection process.
Owner:湖南美生医疗健康产业股份有限公司
Mouse anti-MCR-1 protein hybridoma cell strain, monoclonal antibody and application
ActiveCN113416710AMicroorganism based processesImmunoglobulins against cell receptors/antigens/surface-determinantsMonoclonalPcr method
The invention provides a mouse anti-MCR-1 protein hybridoma cell strain, a monoclonal antibody and application. Ig variable region genes are cloned through mouse hybridoma monoclonal antibody screening and an RT-PCR method, and a hybridoma cell strain capable of stably secreting a mouse anti-MCR-1 protein antibody and a variable region sequence of the hybridoma cell strain are obtained. Systematic evaluation shows that the mouse anti-MCR-1 protein antibody has better performance in all aspects, so that the mouse anti-MCR-1 protein antibody is suitable for being used as an immunodiagnostic reagent for in-vitro diagnosis of MCR-1 protein antigen, the titer reaches 1: 1280000 or above, and the mouse anti-MCR-1 protein antibody is used for developing related in-vitro diagnostic reagents.
Owner:TIANJIN ERA BIOLOGY TECH CO LTD +1
Parathyroid hormone kit and preparation method thereof
InactiveCN109212197AHigh detection sensitivityAccurate quantitative detectionChemiluminescene/bioluminescenceBiological testingAntibody typesMonoclonal antibody
The invention relates to a parathyroid hormone kit and a preparation method thereof, belongs to the technical field of immunologic diagnosis, and solves the technical problems that a parathyroid hormone method in the prior art is low in accuracy, short in shelf life, complicated in operation, unstable in test result and high in cost. The kit disclosed by the invention comprises a reagent R1, a reagent R2 and a reagent R3; the reagent R1 is a buffer solution I containing streptavidin magnetic particles; the reagent R2 is a parathyroid hormone monoclonal antibody solution labeled with a chemiluminescent marker diluted by the buffer solution I; and the reagent R3 is a coupled labeled parathyroid hormone monoclonal antibody solution diluted by the buffer solution I. The kit detects parathyroidhormone by adopting a chemiluminescence immunoassay, and has the advantages of high detection sensitivity, accurate quantitative detection, simple operation, no radioactive risk, short detection timeand capability of detecting antigen and antibody type target substances.
Owner:DIRUI MEDICAL TECH CO LTD
Novel mycobacterium tuberculosis specific fusion protein as well as preparation and application thereof
InactiveCN102702360AStrong specificityIncreased sensitivityMicroorganism based processesBiological testingNucleotideMycobacterium
The invention relates to a novel mycobacterium tuberculosis specific fusion protein as well as preparation and application thereof and belongs to the technical field of tuberculosis medical immunologic diagnosis. The fusion protein is formed by sequentially connecting antigenic epitopes of two proteins Rv0057 and Rv1352, wherein the nucleotide sequence of the antigenic epitope of the protein Rv0057 is shown in a sequence 1 in a sequence table; and the nucleotide sequence of the antigenic epitope of the protein Rv1352 is shown in a sequence 2 in a sequence table. Compared with the current commercial antibody detection kit, the mycobacterium tuberculosis specific fusion protein disclosed by the invention has the advantages of high sensitivity, strong specificity and complementarity with other antigens in the aspect of tuberculosis serodiagnosis, and can be used for detecting specific anti-tuberculosis antibodies in body fluid samples such as blood serum and pleural effusion.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY
Portable immune-chromatography analysis meter
ActiveCN103776998AImproved Handheld PortabilityReduce thicknessMaterial analysis by optical meansBiological testingMirror reflectionPoint-of-care testing
The invention relates to the technical field of immunologic diagnosis instruments, and particularly relates to a portable immune-chromatography analysis meter. The portable immune-chromatography analysis meter comprises a detection card fixing device, a large reflective mirror used for reflecting a detection area, a small reflective mirror used for reflecting a detection area image formed in the large reflective mirror, and an imaging sensor used for capturing the detection area image reflected by the small reflective mirror. According to the portable immune-chromatography analysis meter disclosed by the invention, effective image information of the overall detection area can be finally captured by the imaging sensor through two times of mirror reflection under a condition that the direct distance between the imaging sensor and the detection card is 2-6 cm, so that the thickness of the overall instrument is reduced, and the characteristic that POCT (Point Of Care Testing) detection is handheld and portable is reflected; an LED (Light-Emitting Diode) light source unit is arranged inside a shell of the diagnosis instrument, and the light emitted from the LED light source unit can be uniformly distributed on a detection card arranged in the fixing device, thus an effective image of the detection area can be rapidly captured by the imaging sensor.
Owner:常州思康立生物科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com