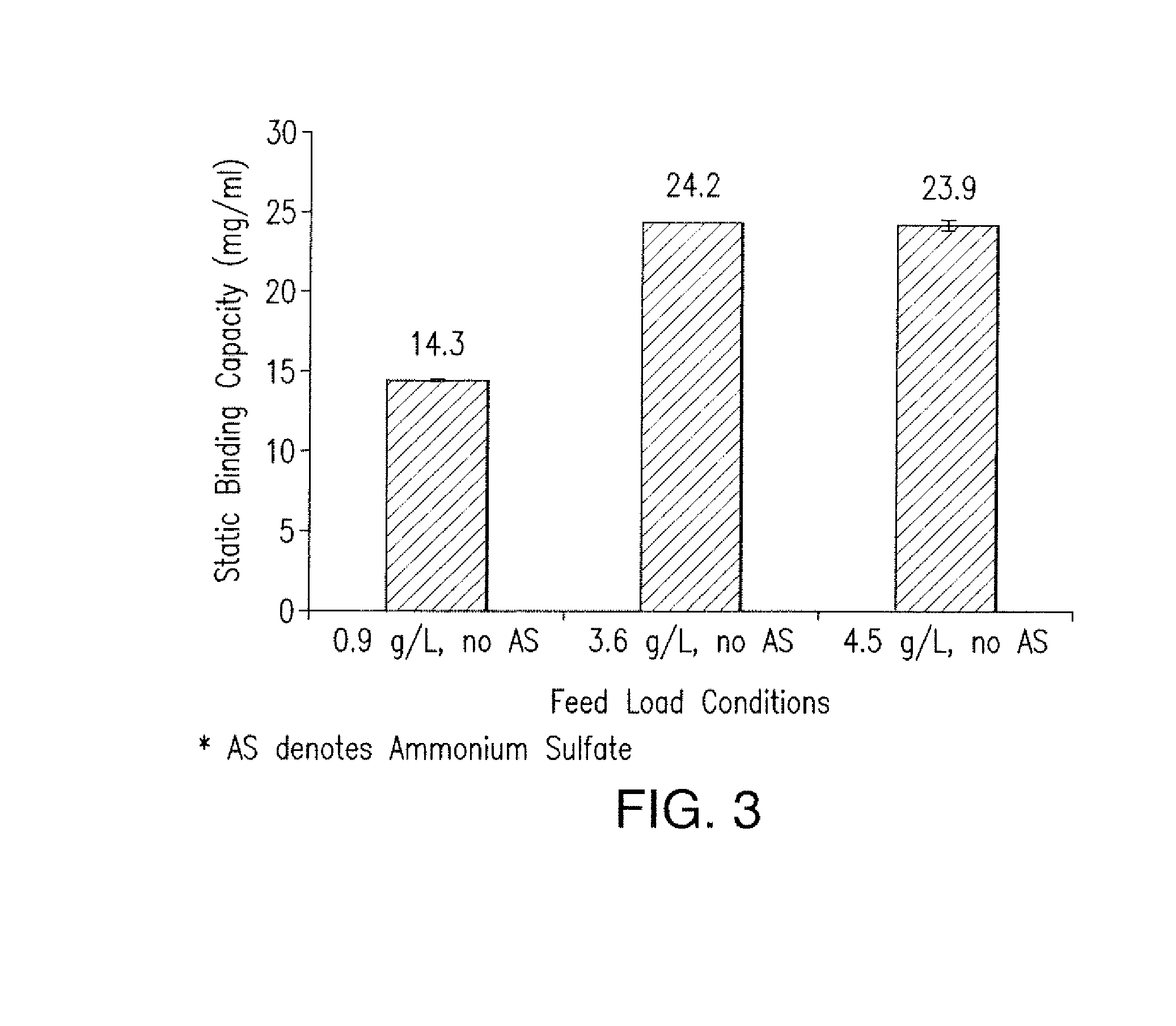
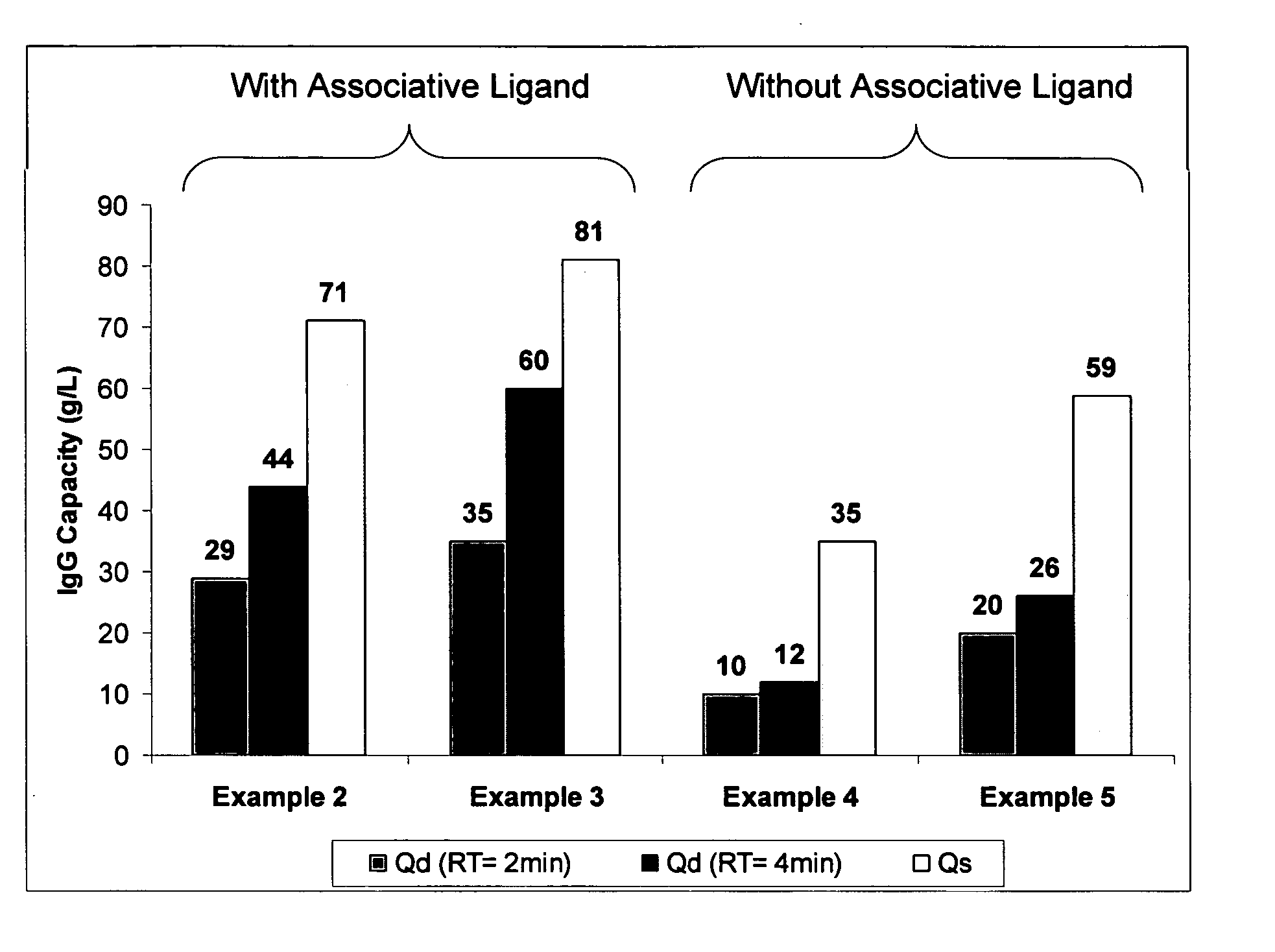
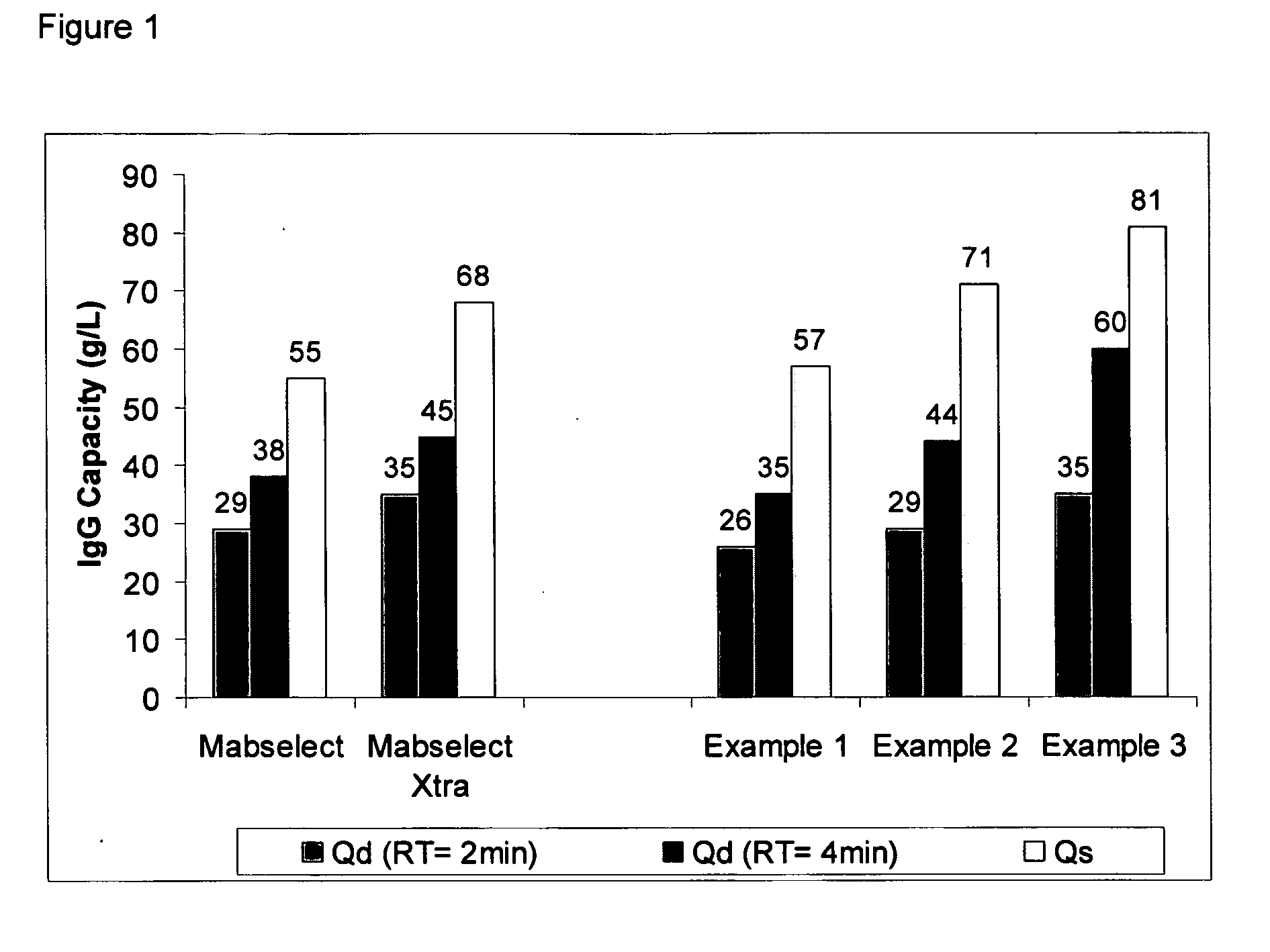
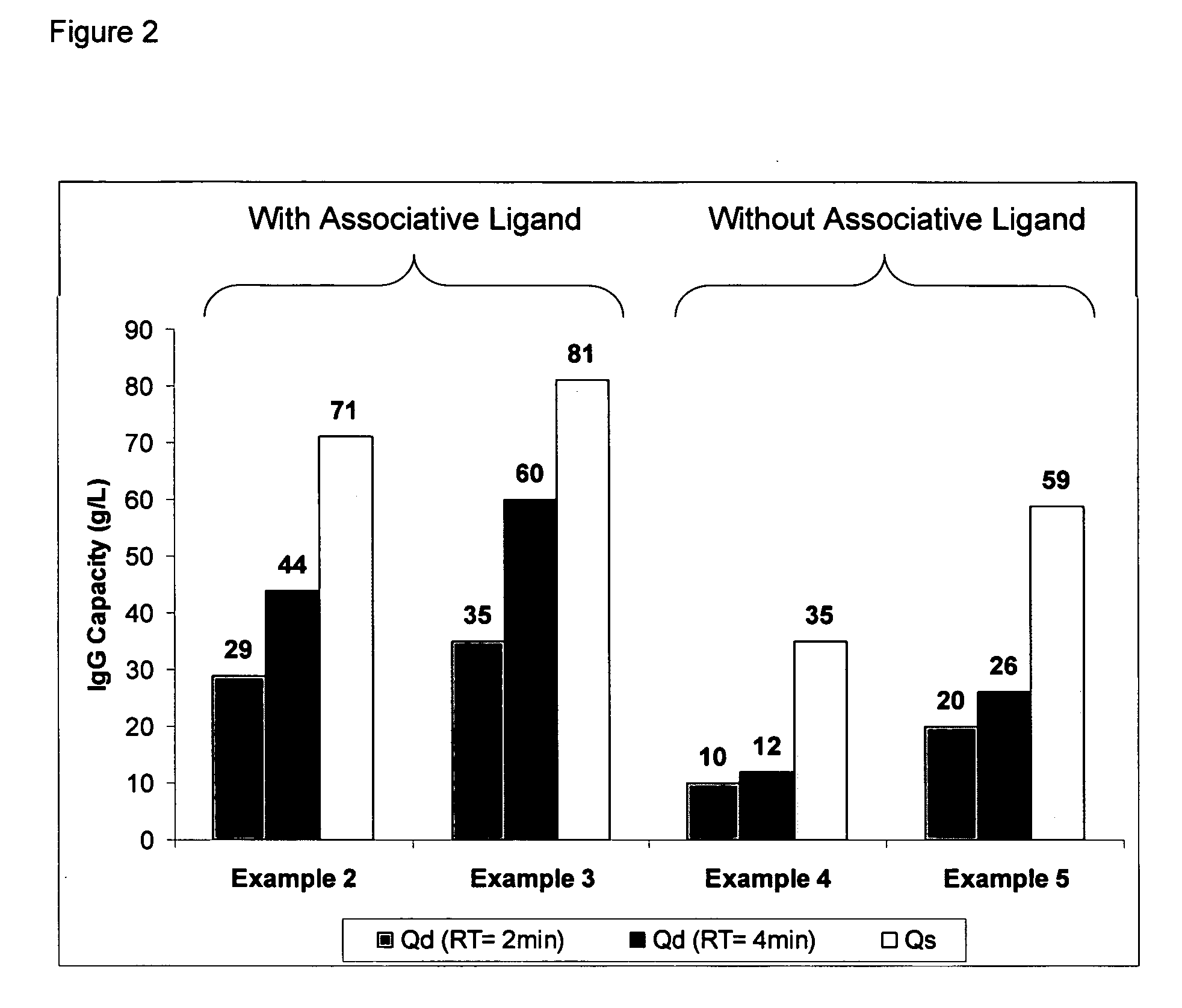
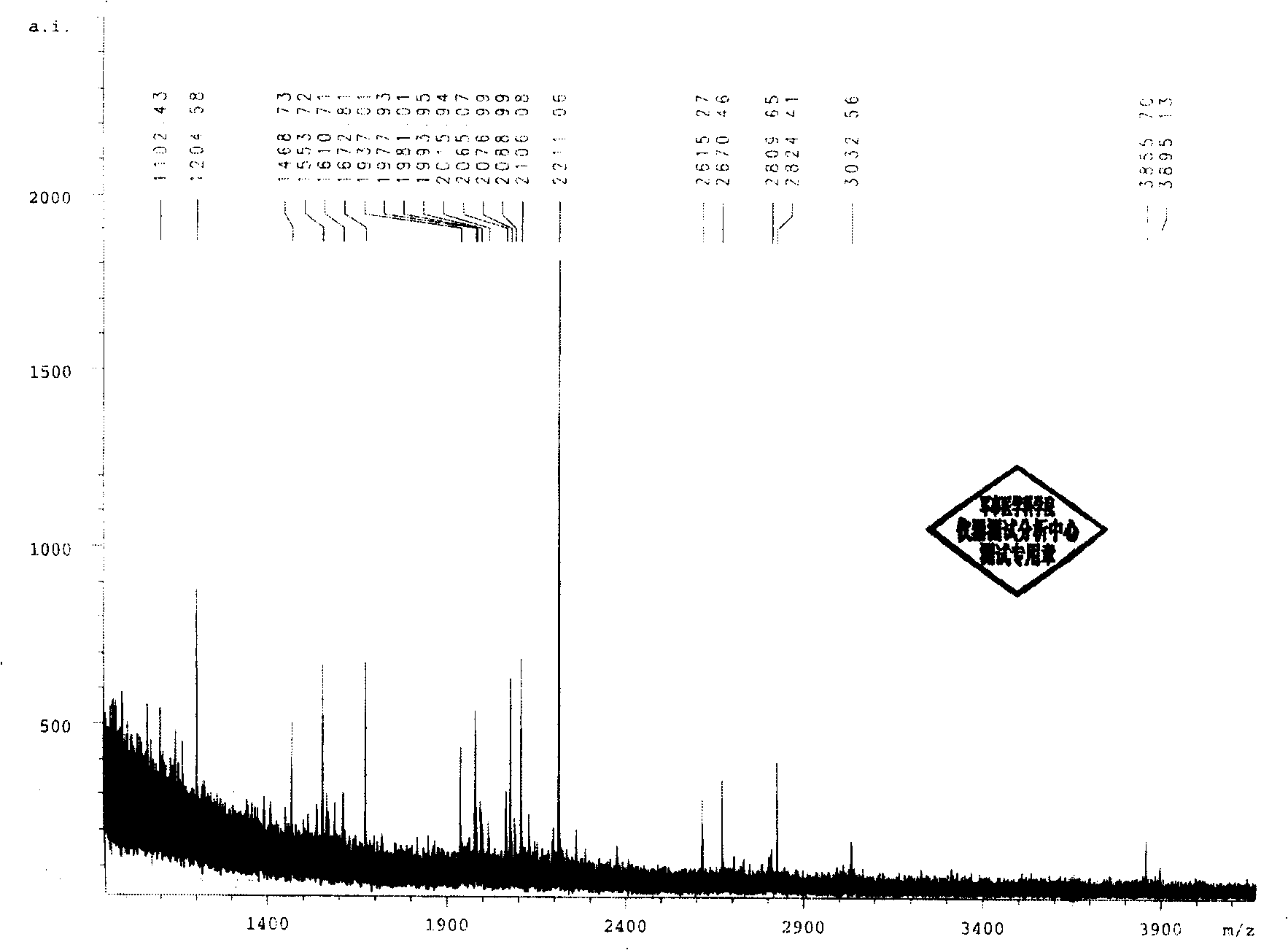
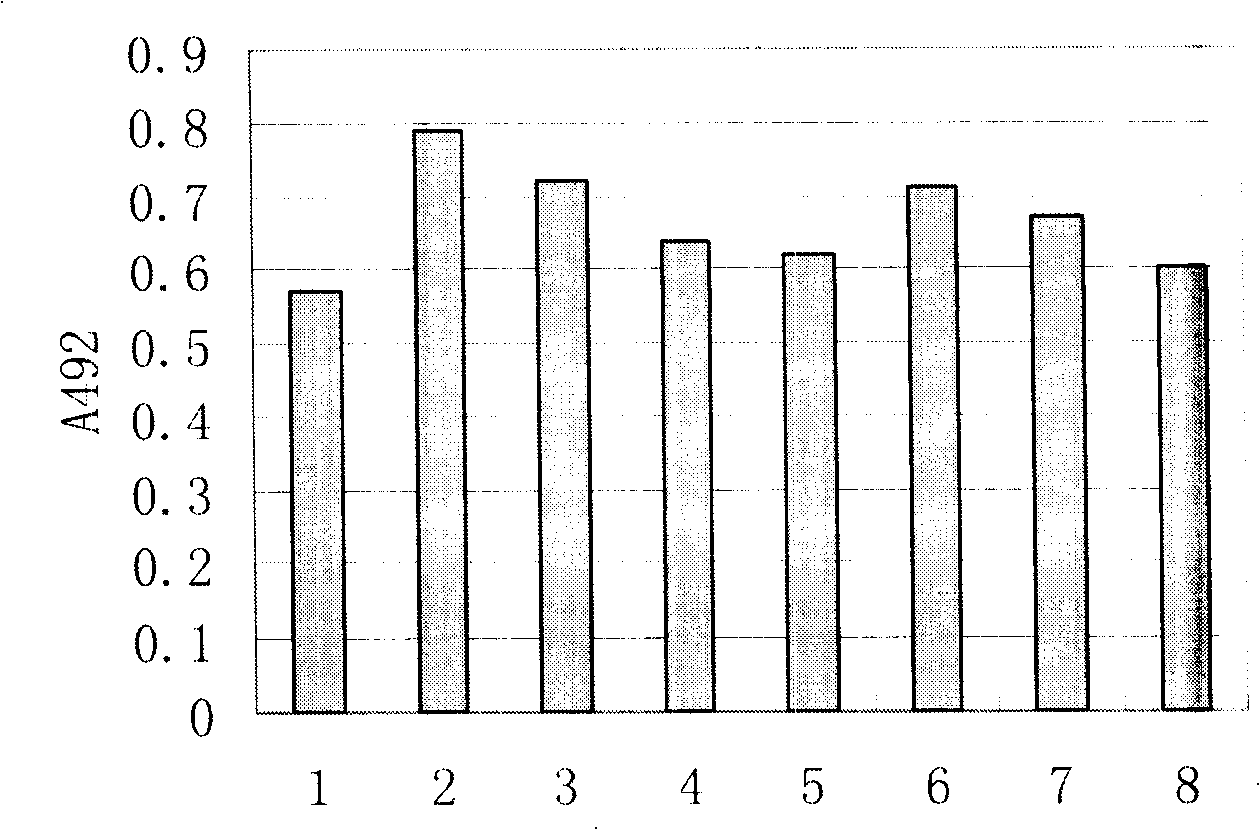
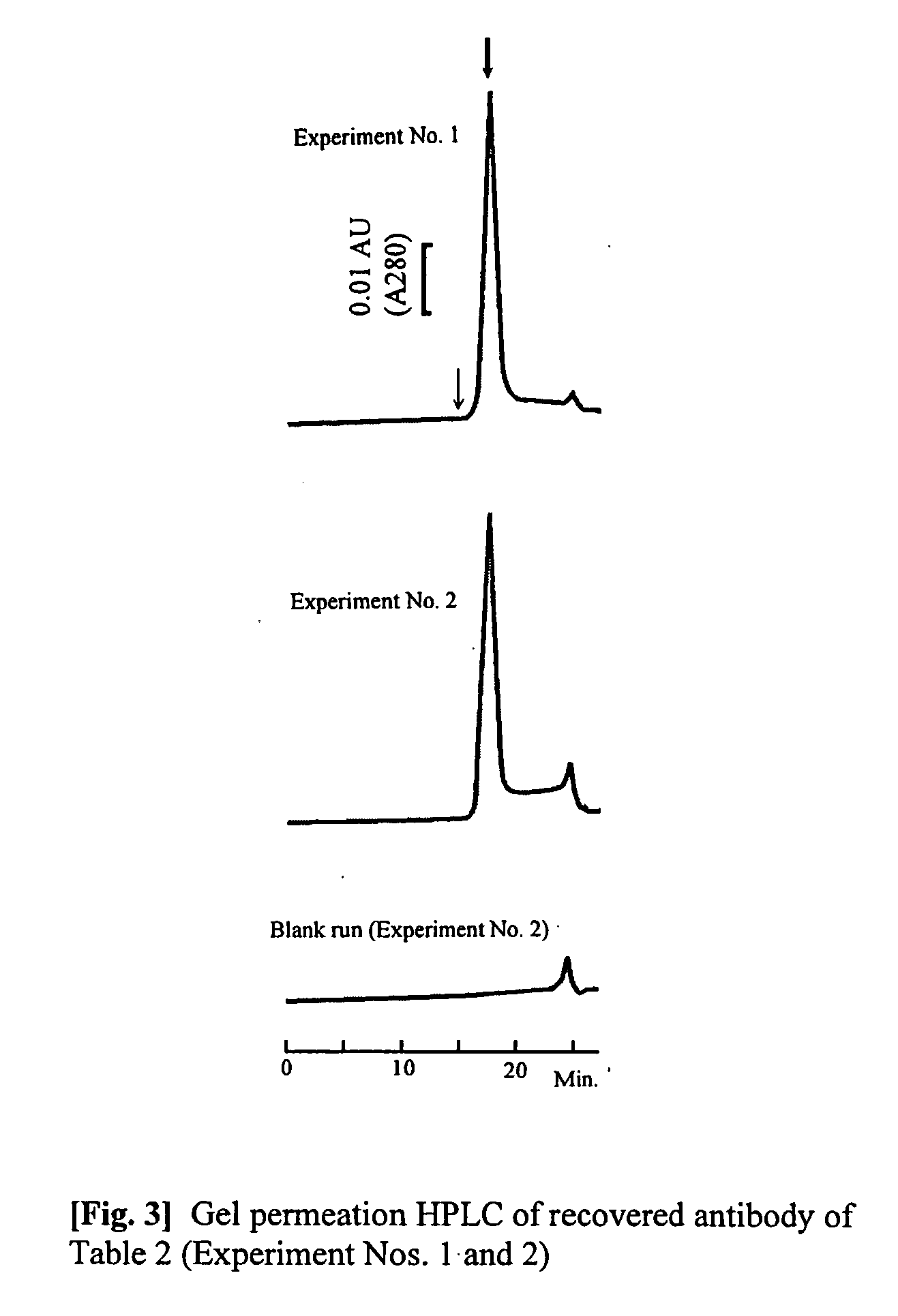
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
1839 results about "Antibody Affinity Chromatography" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Most monoclonal antibodies have been purified using affinity chromatography based on immunoglobulin-specific Protein A or Protein G, derived from bacteria. Immobilized metal ion affinity chromatography (IMAC) is based on the specific coordinate covalent bond of amino acids, particularly histidine, to metals.
Support for high performance affinity chromatography and other uses
Multilayered particulate materials are formed by coating a particulate substrate with a metal and adsorbing an organic layer comprising a recognition moiety onto the metal film. The recognition moiety interacts with an analyte of interest allowing for its detection, purification, etc. Suitable recognition moieties can be selected from a range of species including, small molecules, polymers and biomolecules and the like. The novel particulate materials of the invention can be utilized in an array of methods including, ion-exchange, ion-selective ion-exchange, assays, affinity dialysis, size exclusion dialysis, as supports in solid phase synthesis, combinatorial synthesis and screening of compound libraries and the like.
Owner:RGT UNIV OF CALIFORNIA
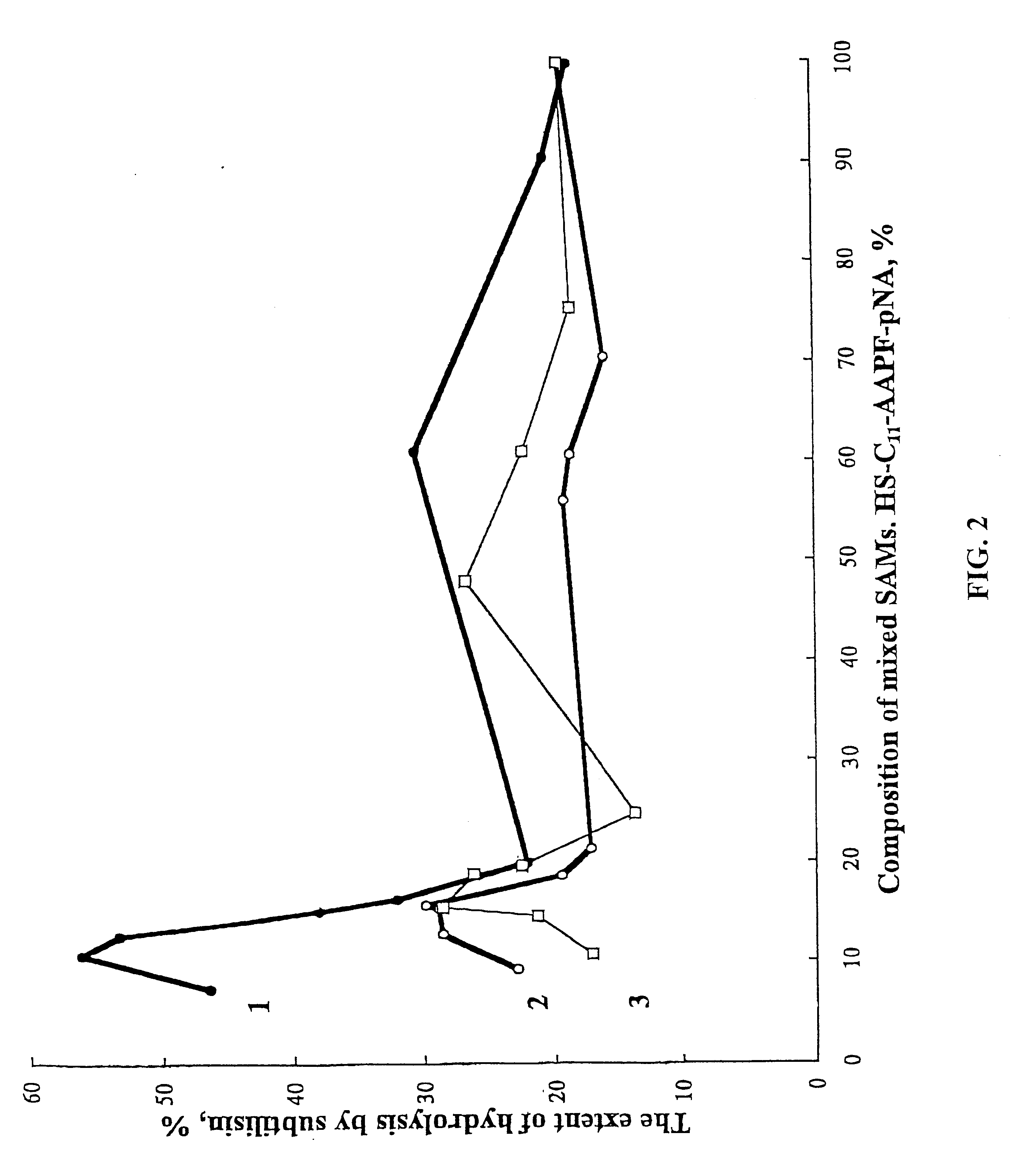
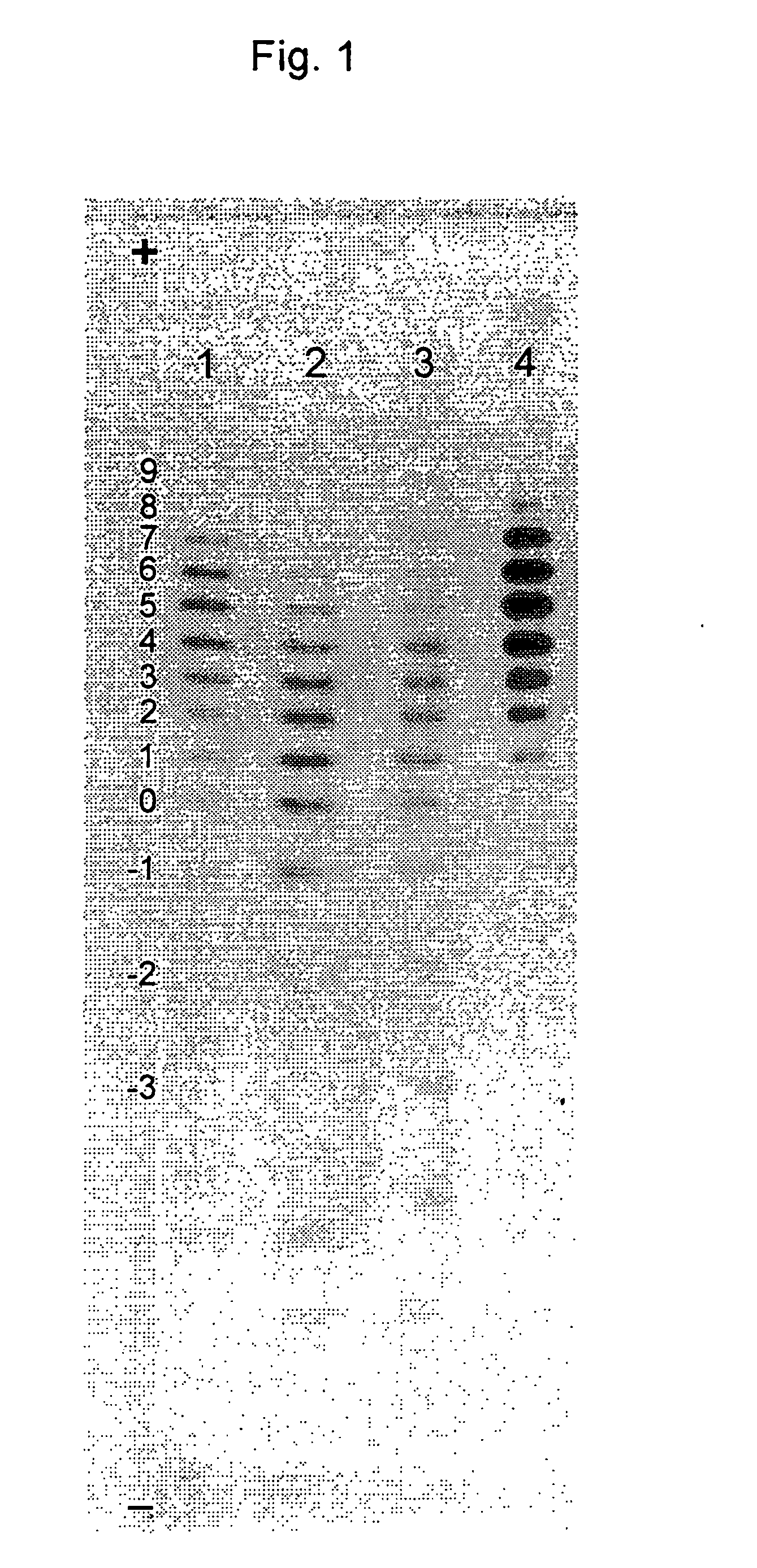
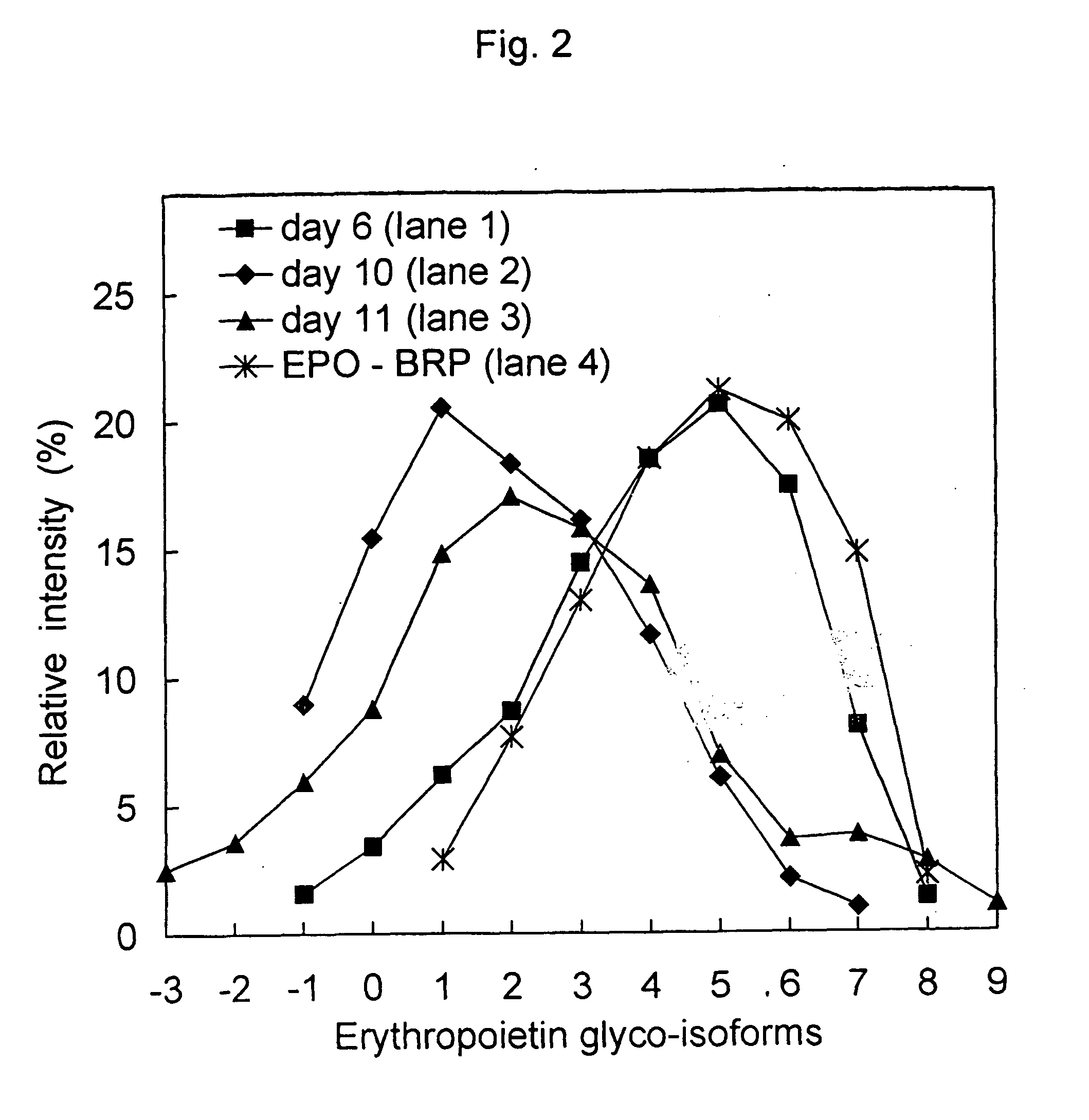
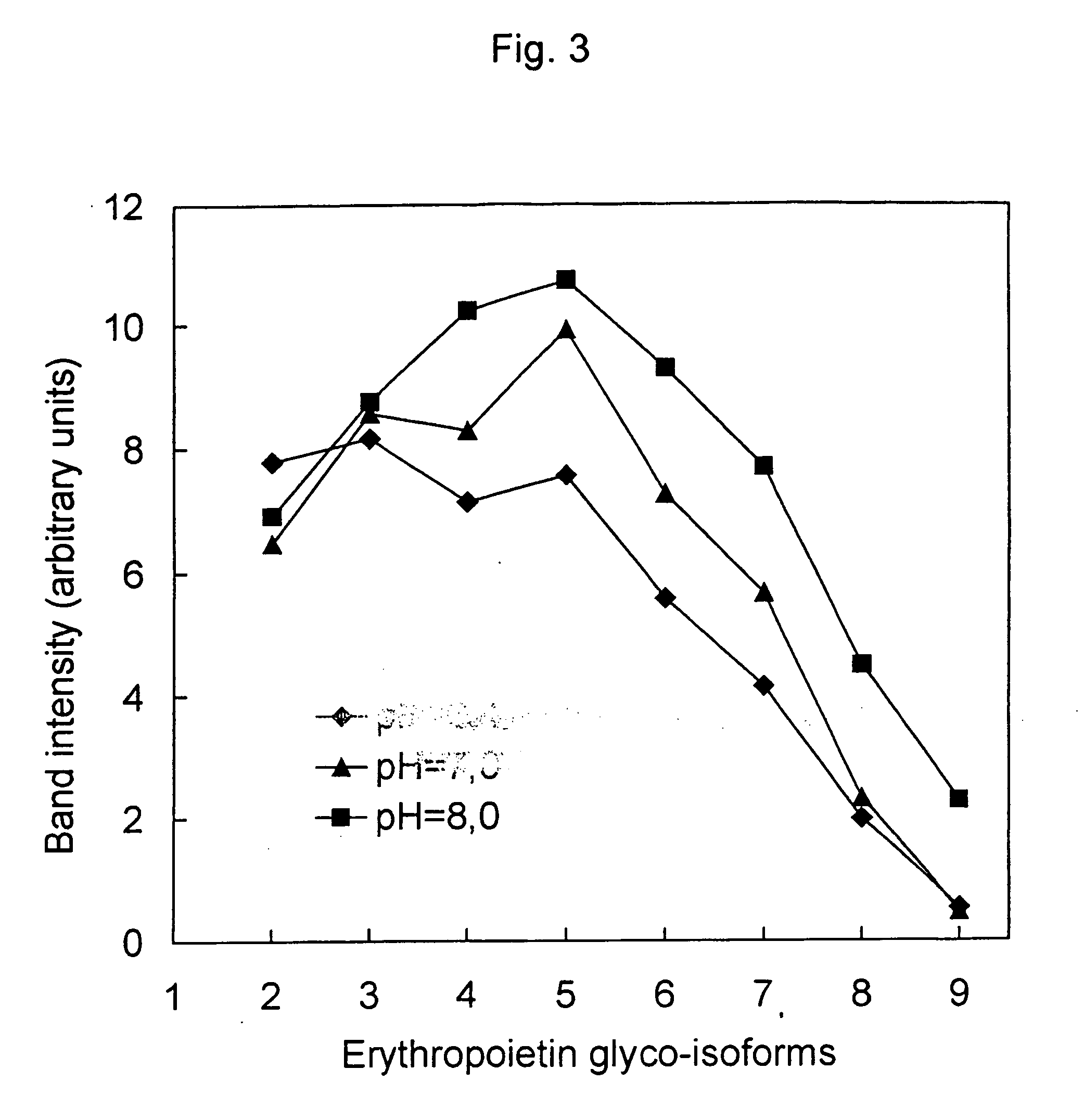
Process for the preparation of a desired erythropoietin glyco-isoform profile
InactiveUS20050153879A1High and uniform product specificityImprove product qualityPeptide/protein ingredientsComponent separationFiltrationRed blood cell
The present invention provides a process for the production of erythropoietin (EPO) with high purity and with a desired profile of EPO glycol-isoforms by using a combination of specific chromatographic steps in such a manner that the starting EPO glycol-isoform profile is changed or modified. The applied chromatographic steps includes at least (a) dye affinity chromatography, and (b) hydrophobic chromatography and / or (c) anion-exchange chromatography. In a preferred embodiment, the process further includes (d) gel filtration chromatography. The present invention also provides a process for the determination of erythropoietin (EPO) glycol-isoform profile in an EPO containing composition.
Owner:SVETINA MONICA +4
DNA amplification and subtraction techniques
InactiveUS6107023AImprove concentrationSugar derivativesMicrobiological testing/measurementHybrid speciesDna amplification
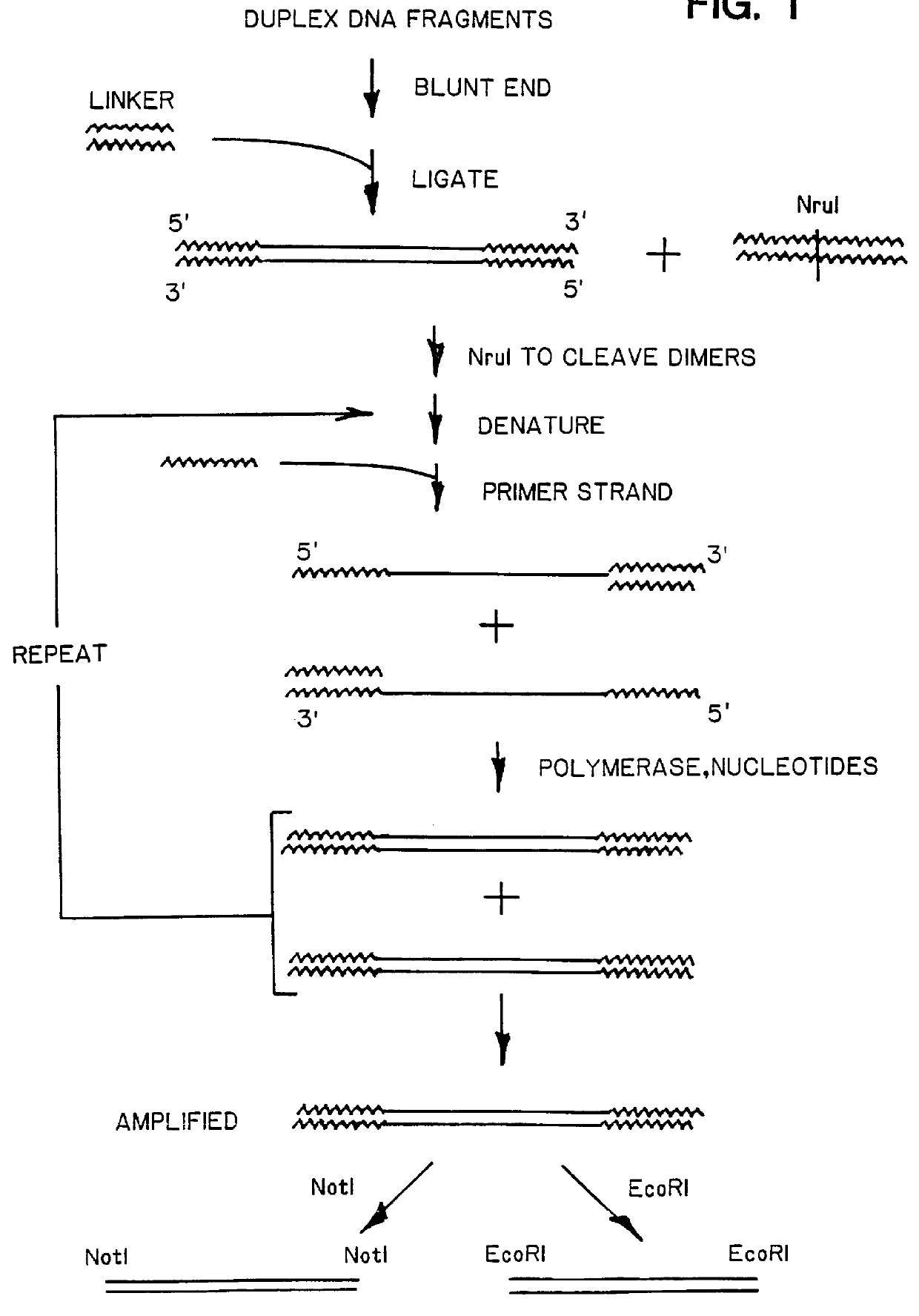
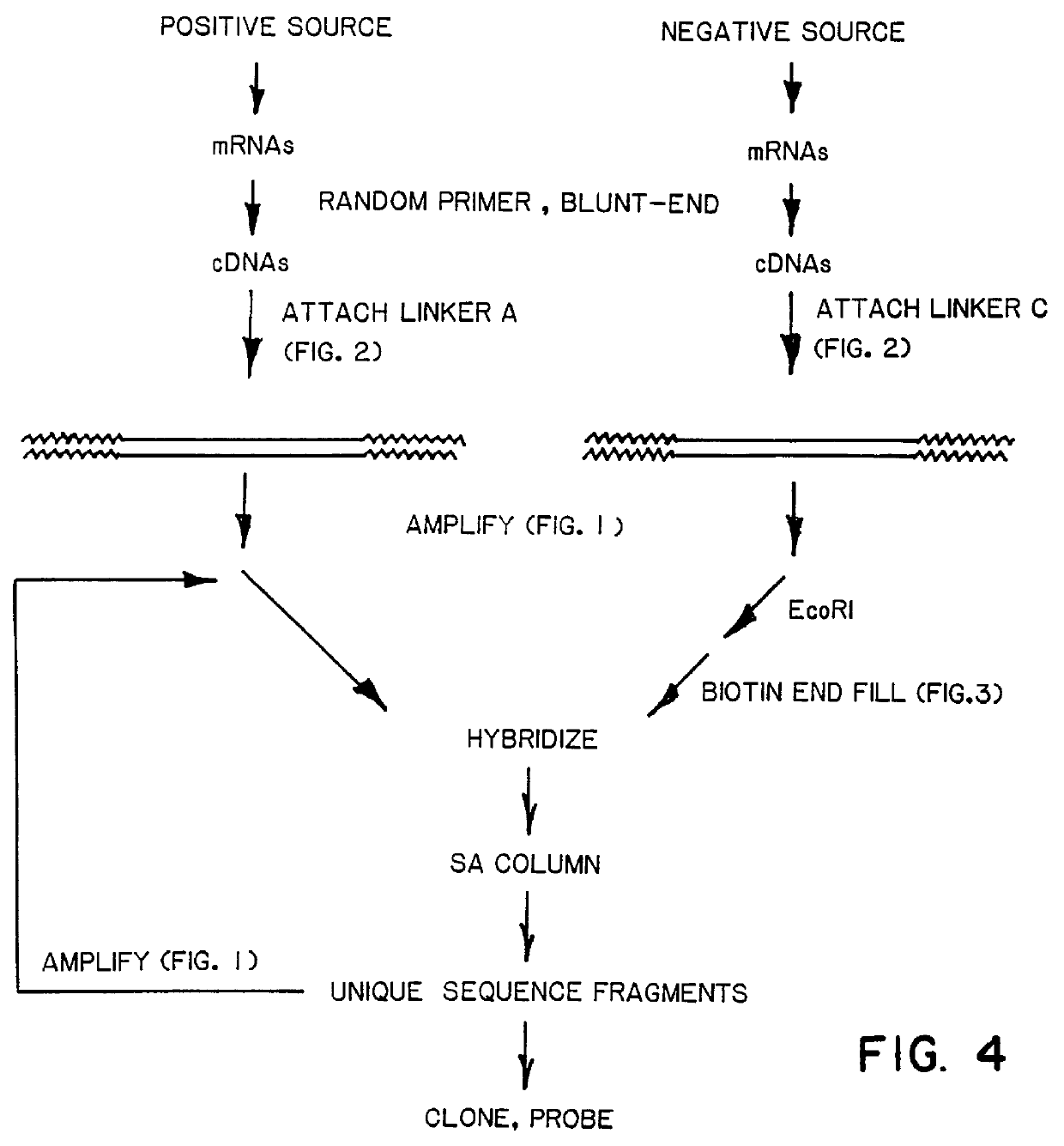
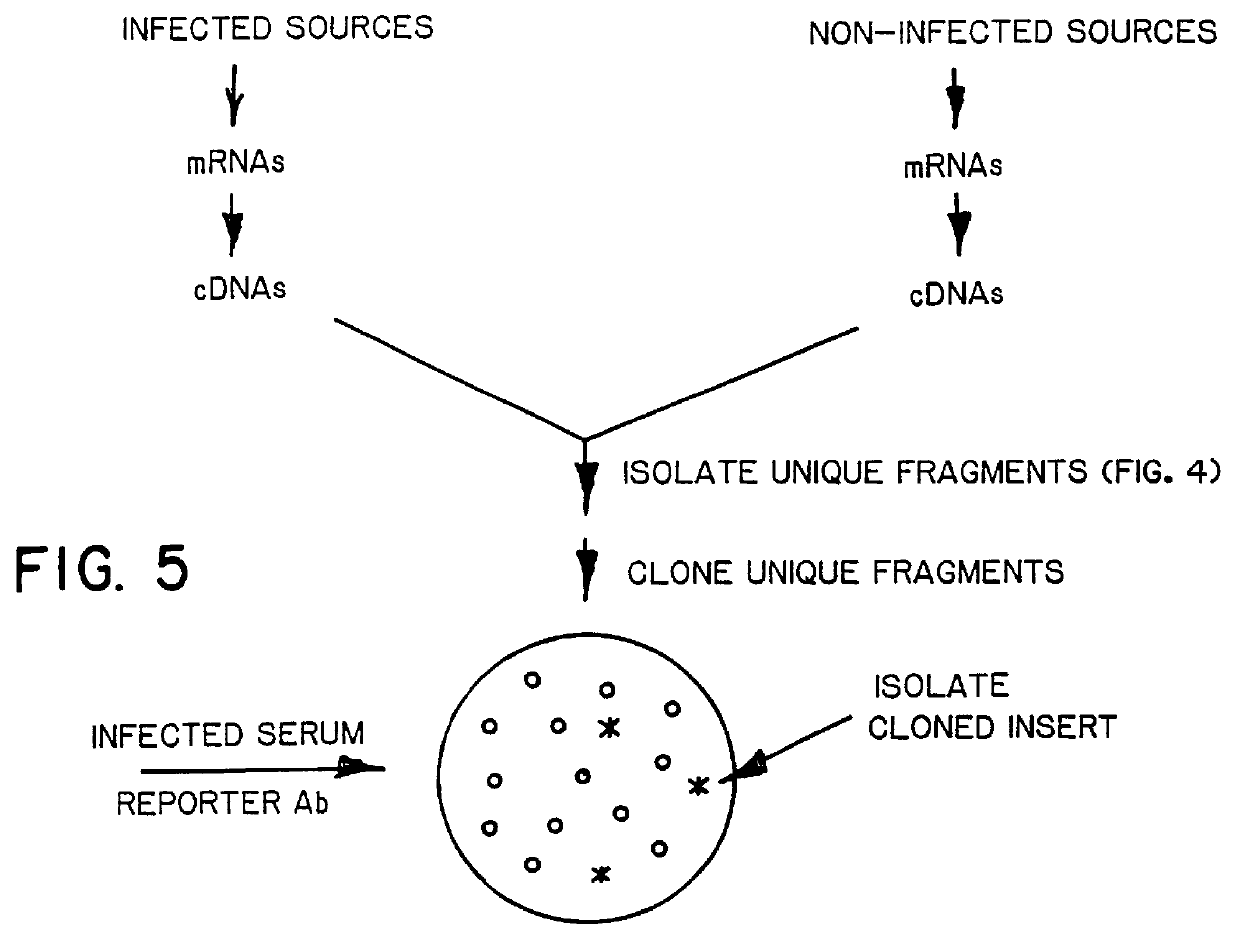
A method of isolating genomic or RNA-derived duplex fragments which are unique to one of two fragment mixtures. The fragments in positive-source and negative-source mixtures are separately equipped with end linkers, and each mixture is amplified by successive primed-strand replications, using a single primer which is homologous to the associated linker. The second-source linker is biotinylated, and the fragments in this mixture are hybridized in molar excess with the fragments in the positive-source mixture. DNA species which are not hybridized with the biotinylated species, i.e., species that are unique to the positive-source mixture, are isolated after removal of hybridized species by affinity chromatography. Also disclosed is a method of amplifying a mixture of DNA fragments by repeated linker / primer replication.
Owner:ILLUMINA INC +1
Non-affinity purification of proteins
InactiveUS7323553B2Chromatographic cation exchangersOther chemical processesProtein purificationAntibody Affinity Chromatography
The present invention relates to a method for protein purification that involves the combination of non-affinity chromatography with HPTFF.
Owner:GENENTECH INC
Methods for purifying protein
ActiveUS7122641B2Antibody mimetics/scaffoldsNGF/TNF-superfamilyAntibody Affinity ChromatographyImmunoglobulin domain
A method is provided for separating a protein from one or more other proteins using hydroxyapatite chromatography in which the protein does not bind to hydroxyapatite but the other protein(s) does. In some embodiments, a second protein affixed to a solid support has been used previously to purify the protein by affinity chromatography, and small amounts of the second protein are introduced in the sample during this process. The protein being purified can comprise at least one constant antibody immunoglobulin domain. The second protein can bind to proteins comprising such a domain.
Owner:IMMUNEX CORP
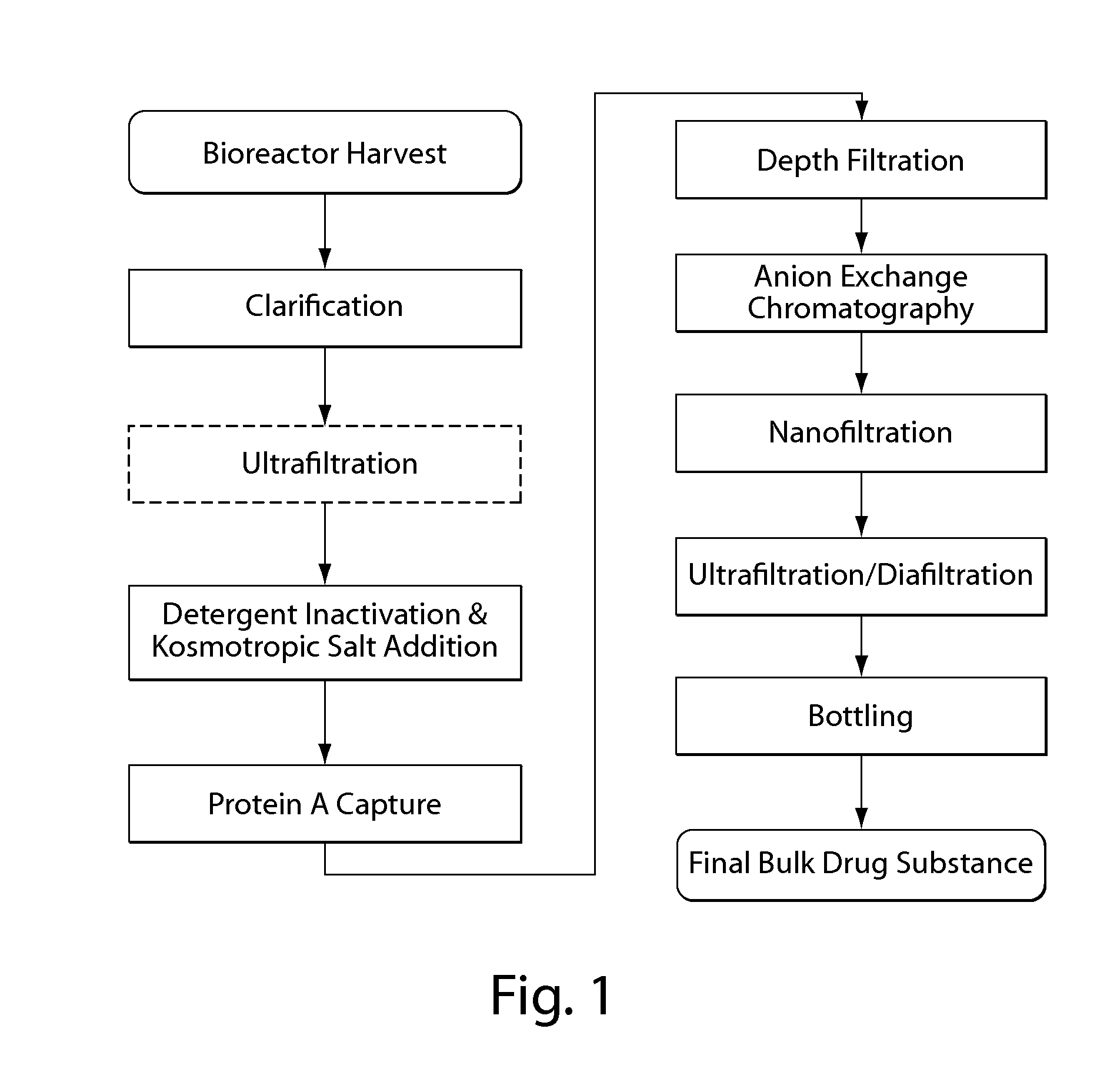
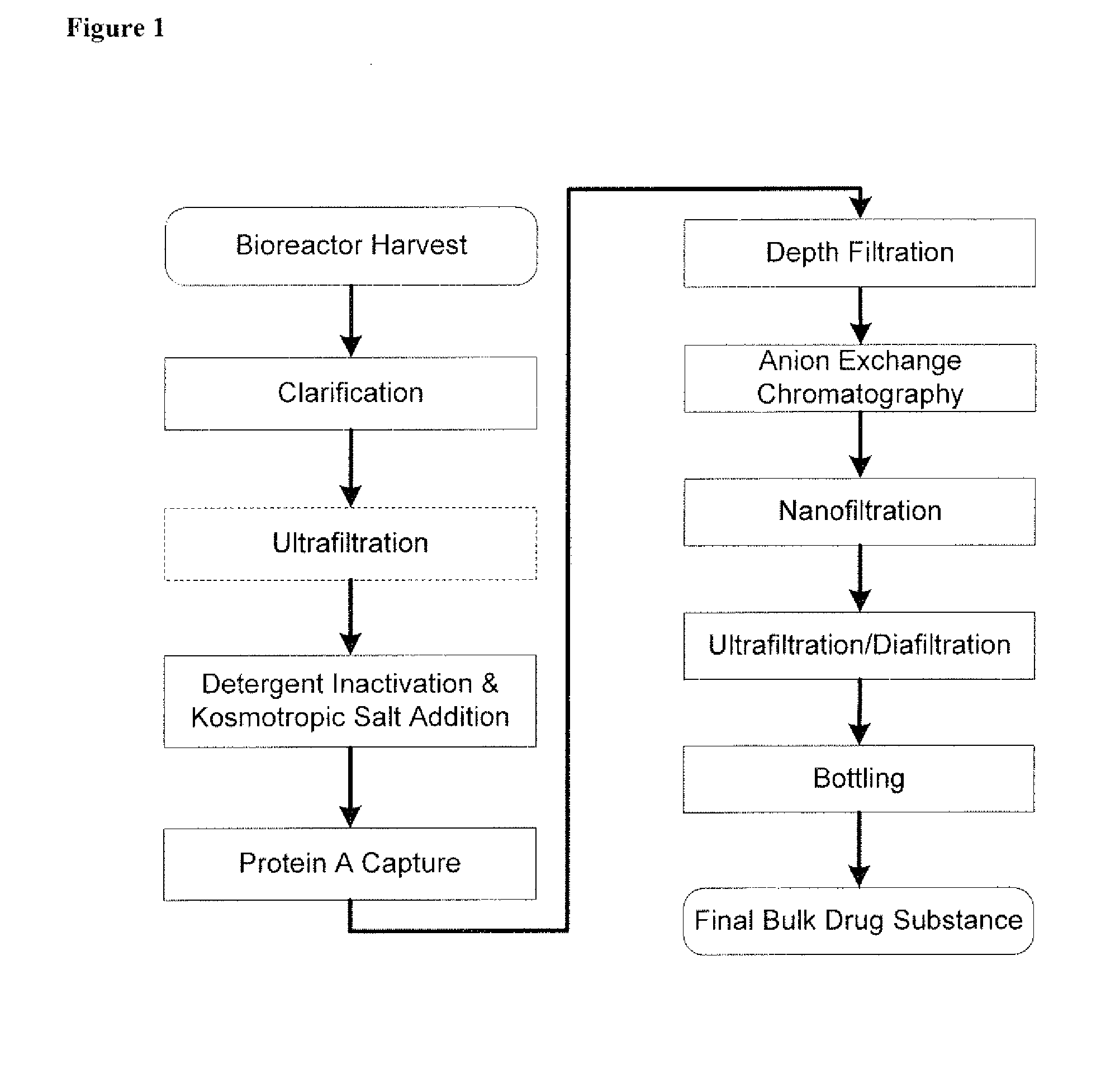
Isolation and purification of antibodies using protein a affinity chromatography
ActiveUS20100135987A1Easy to disassembleReduce or inactivate pH-sensitive virusesAntipyreticAnalgesicsIon exchangeAntibody Affinity Chromatography
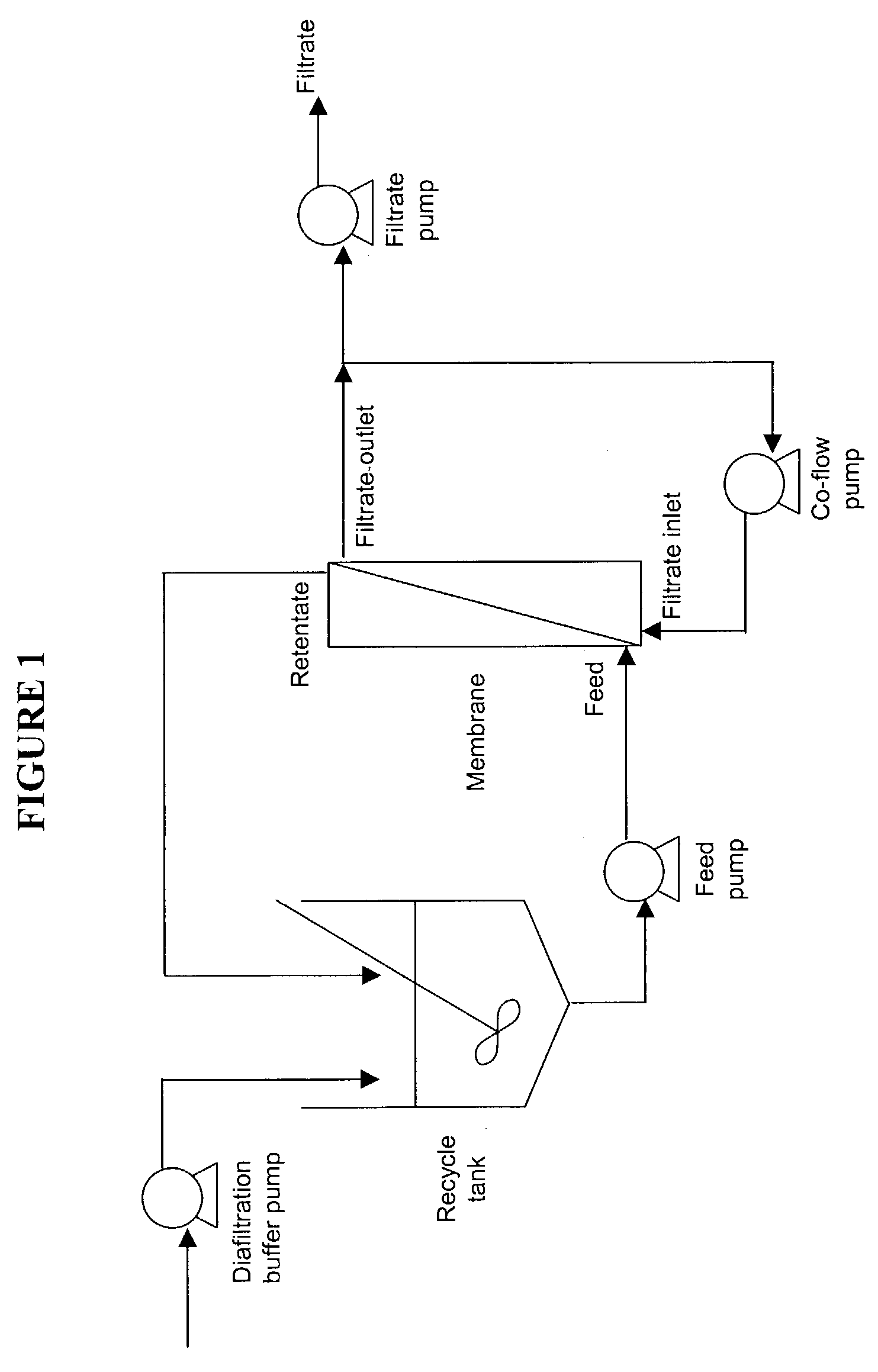
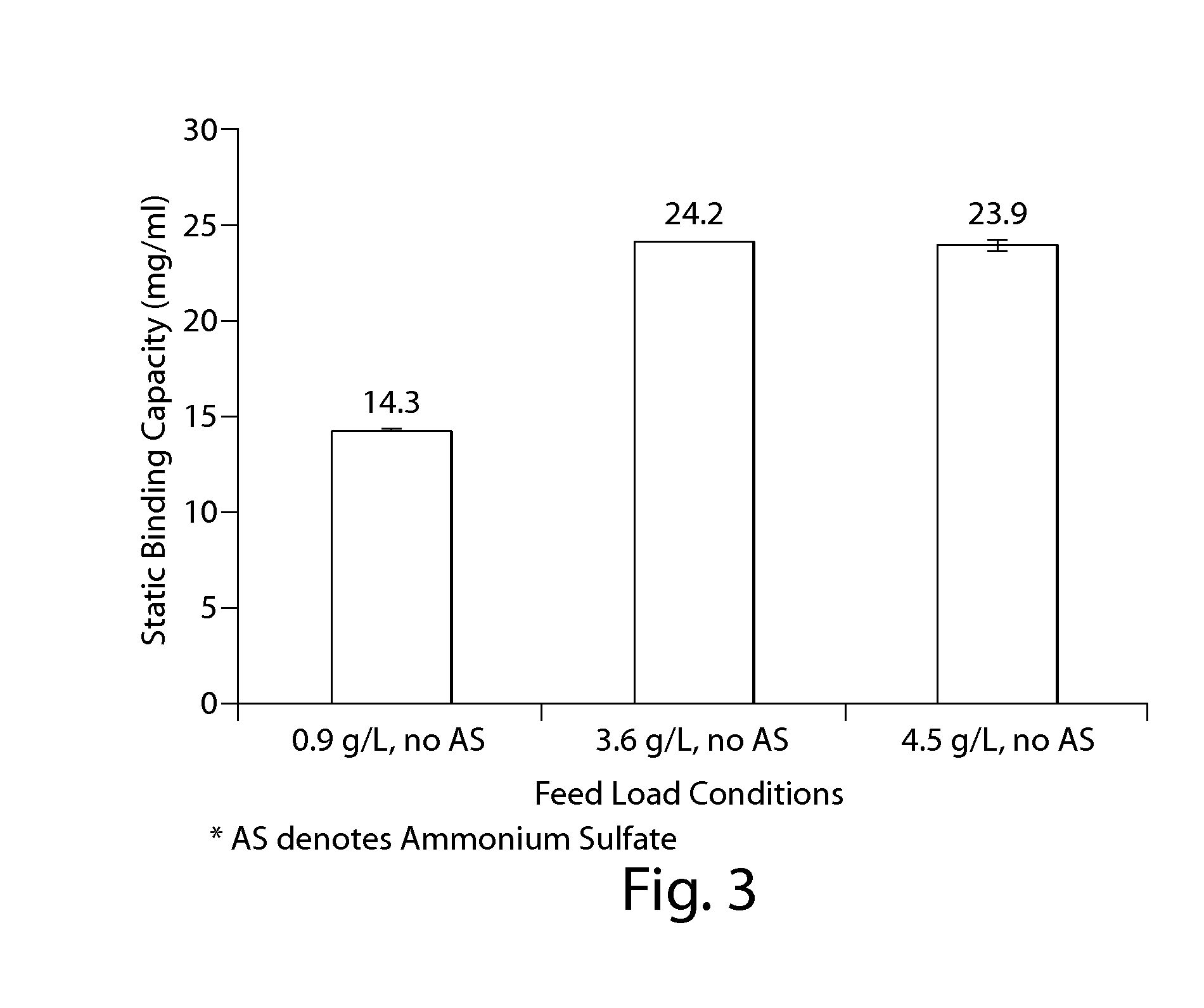
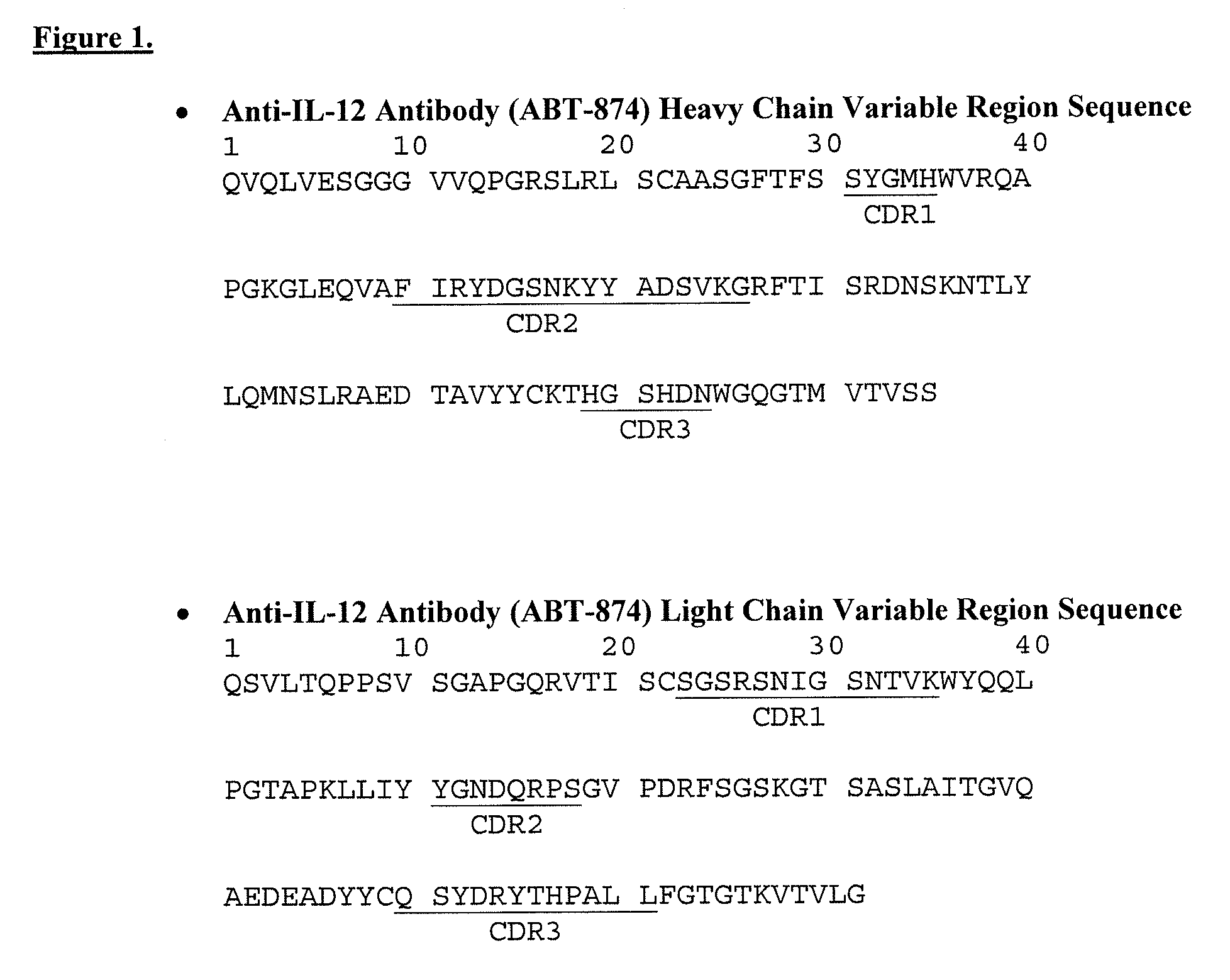
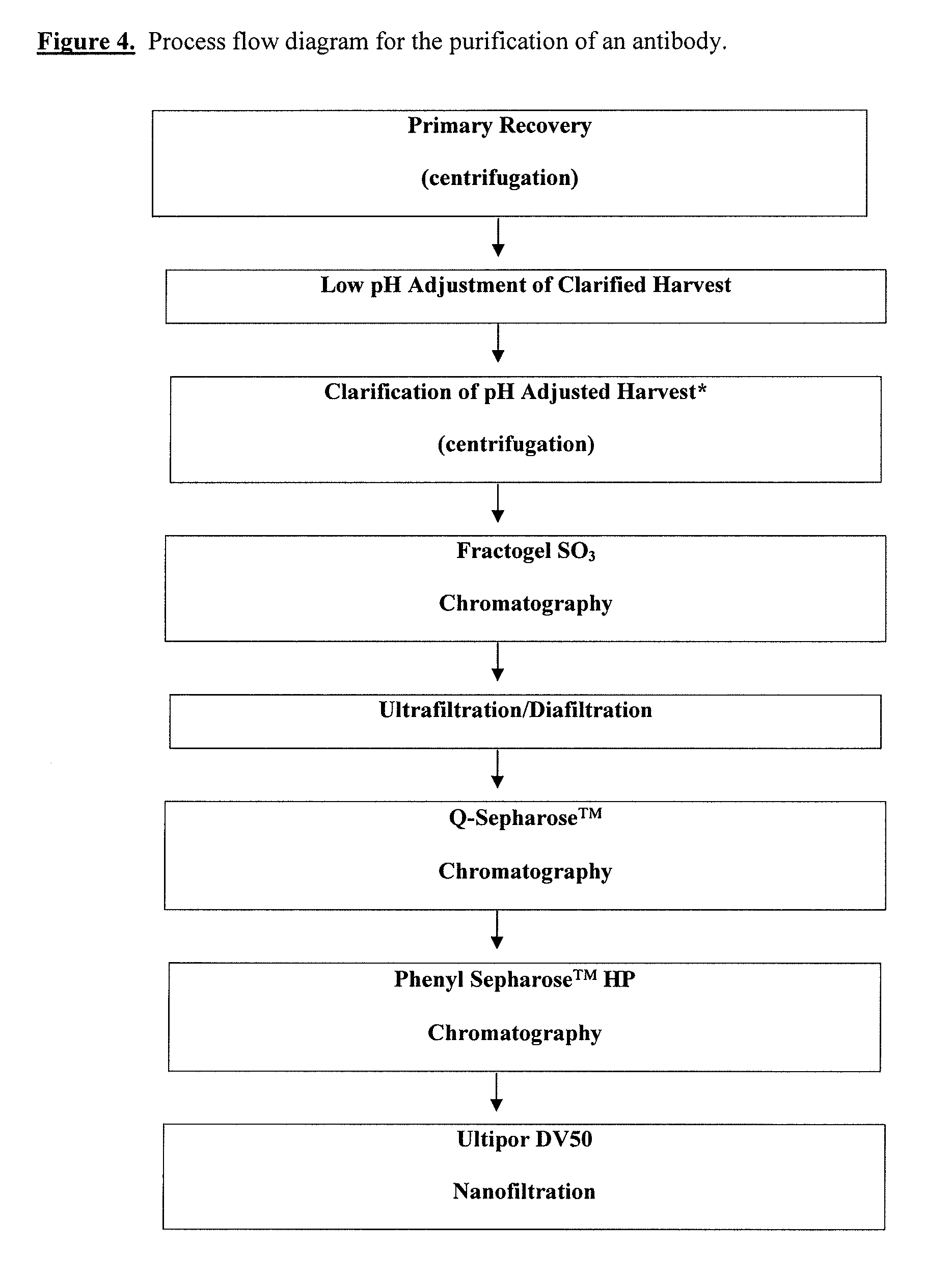
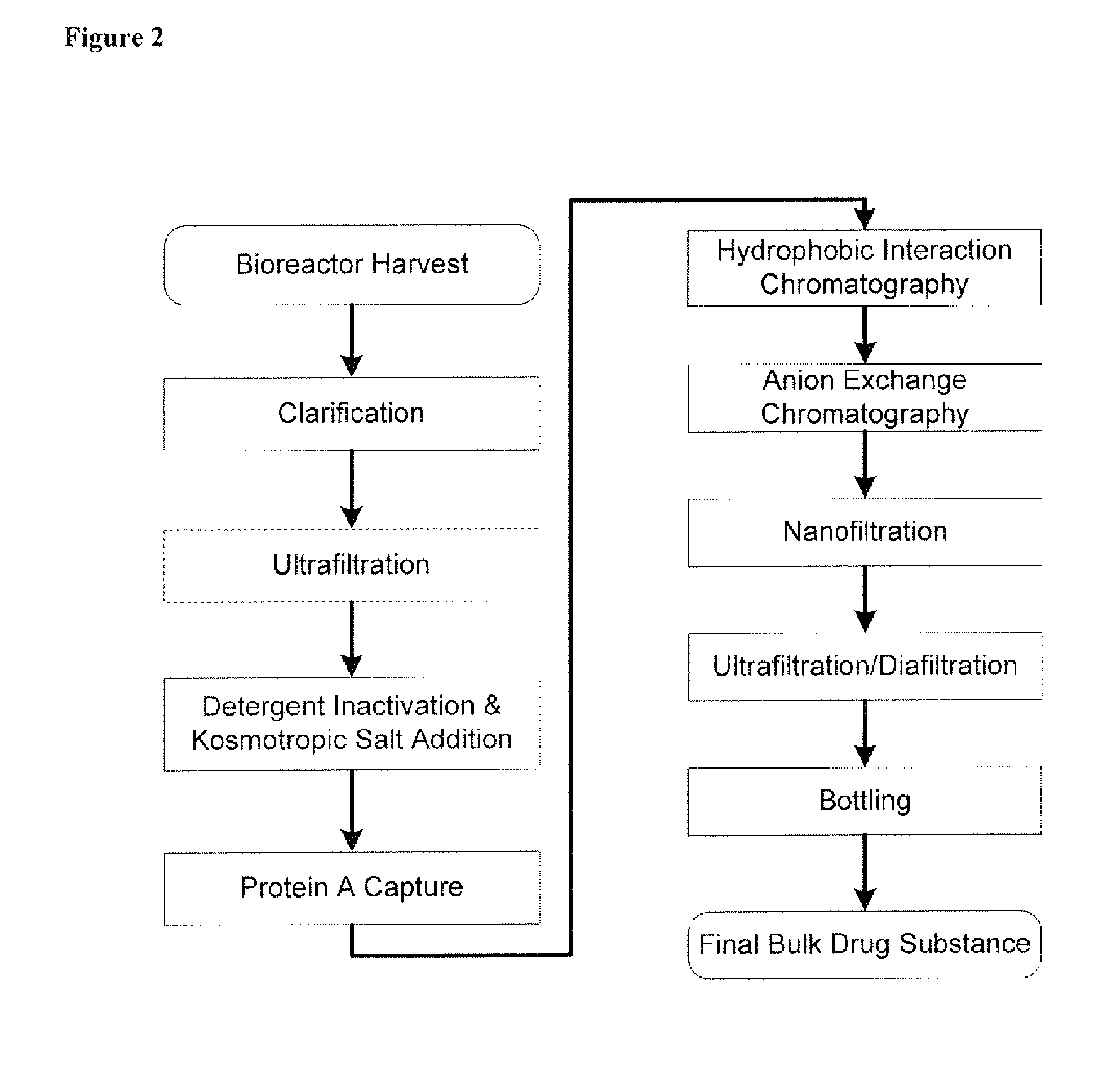
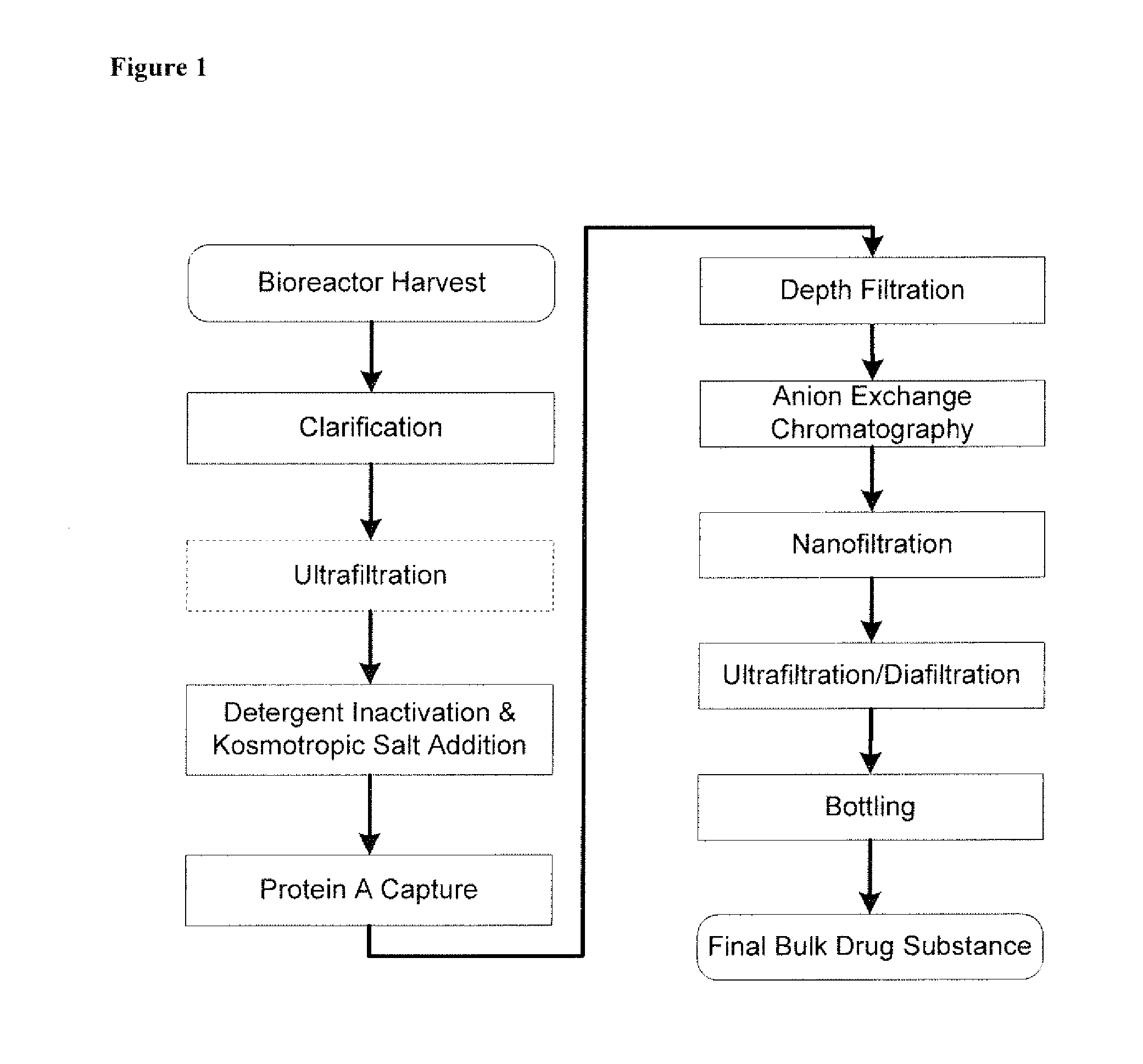
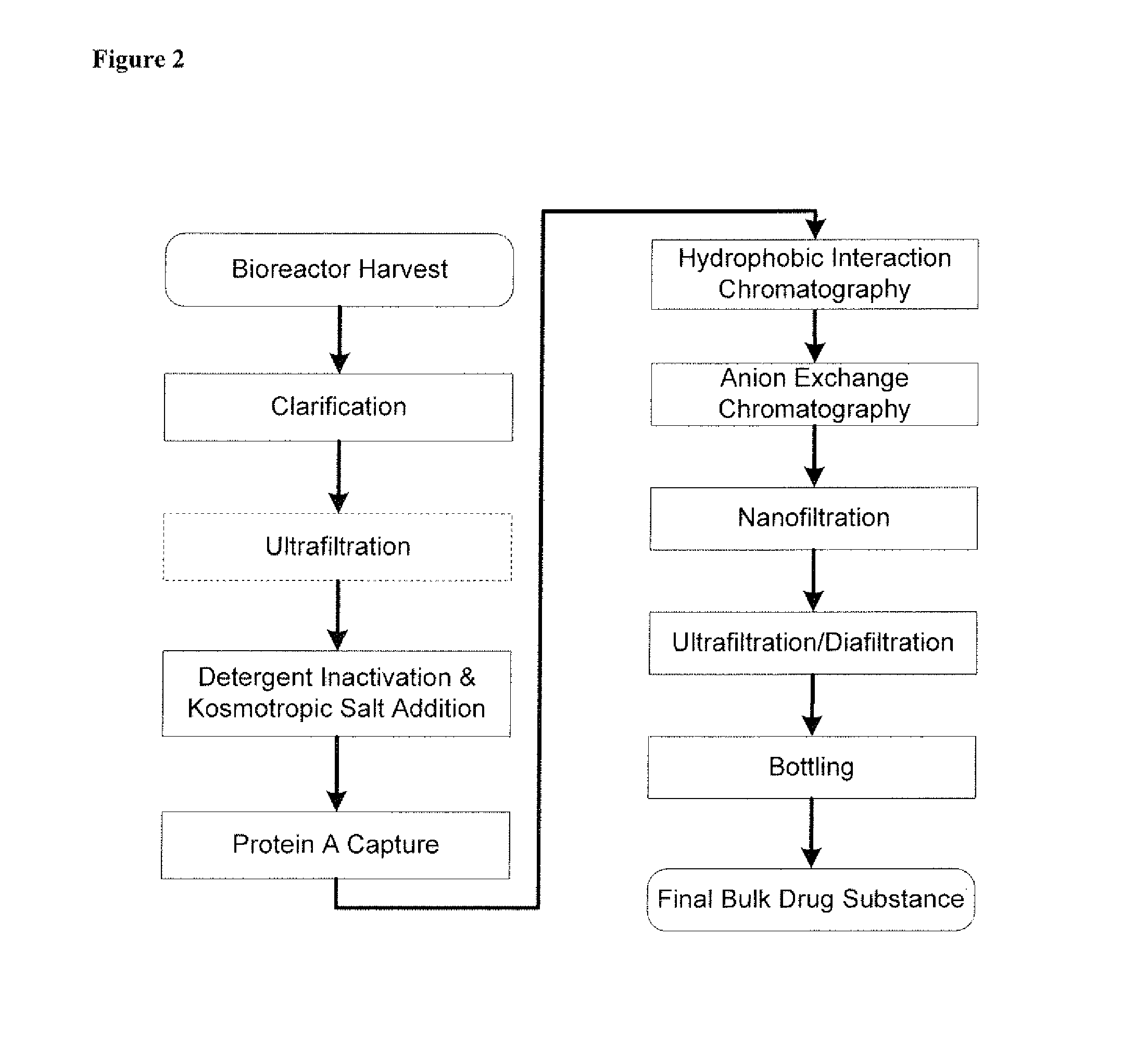
Disclosed herein are methods for the isolation and purification of antibodies wherein the use of an affinity chromatographic step results in an antibody composition sufficiently pure for pharmaceutical uses. The methods described herein comprise pH viral reduction / inactivation, ultrafiltration / diafiltration, affinity chromatography, preferably Protein A affinity, ion exchange chromatography, and hydrophobic chromatography. Further, the present invention is directed toward pharmaceutical compositions comprising one or more antibodies of the present invention.
Owner:ABBVIE INC
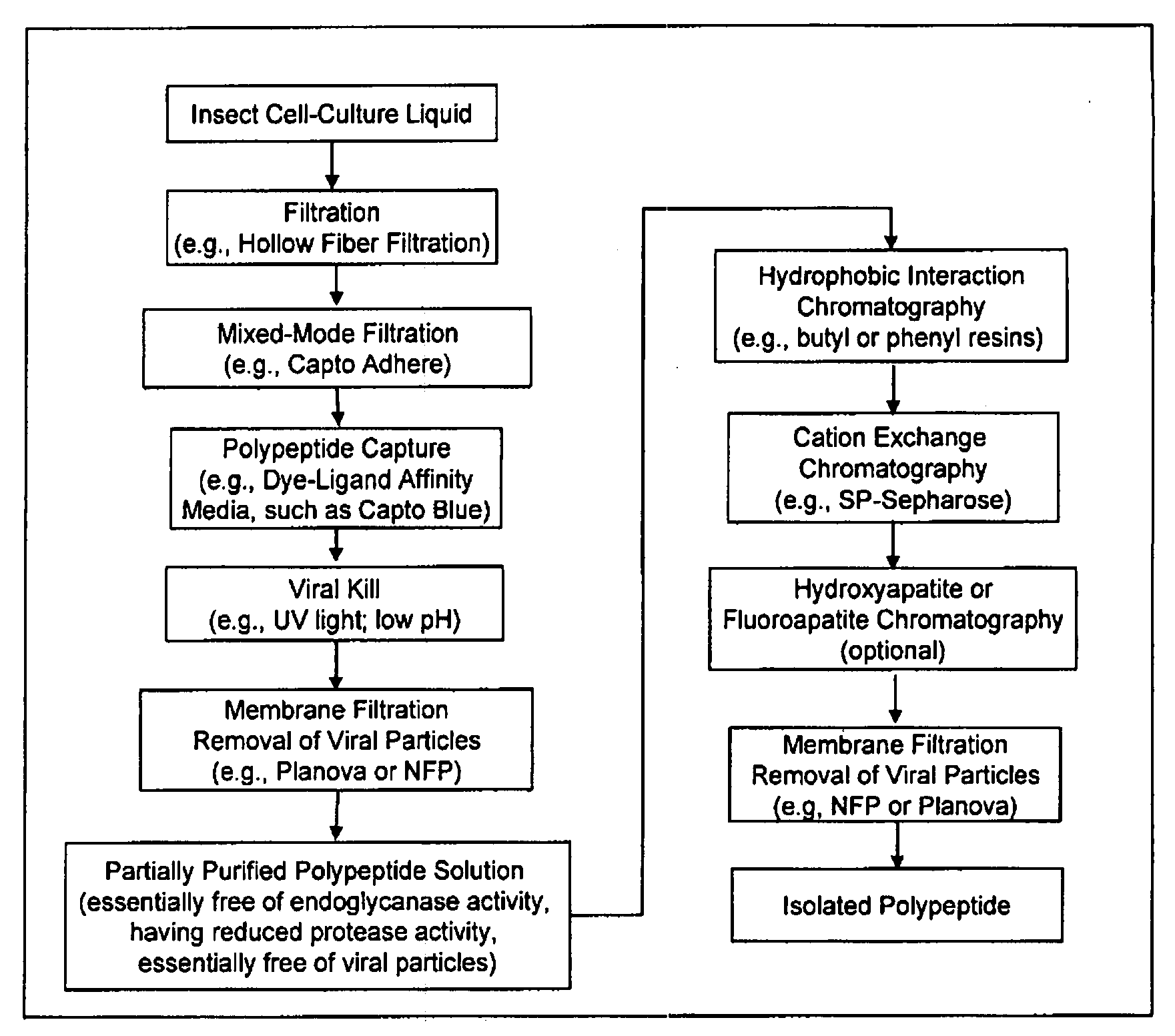
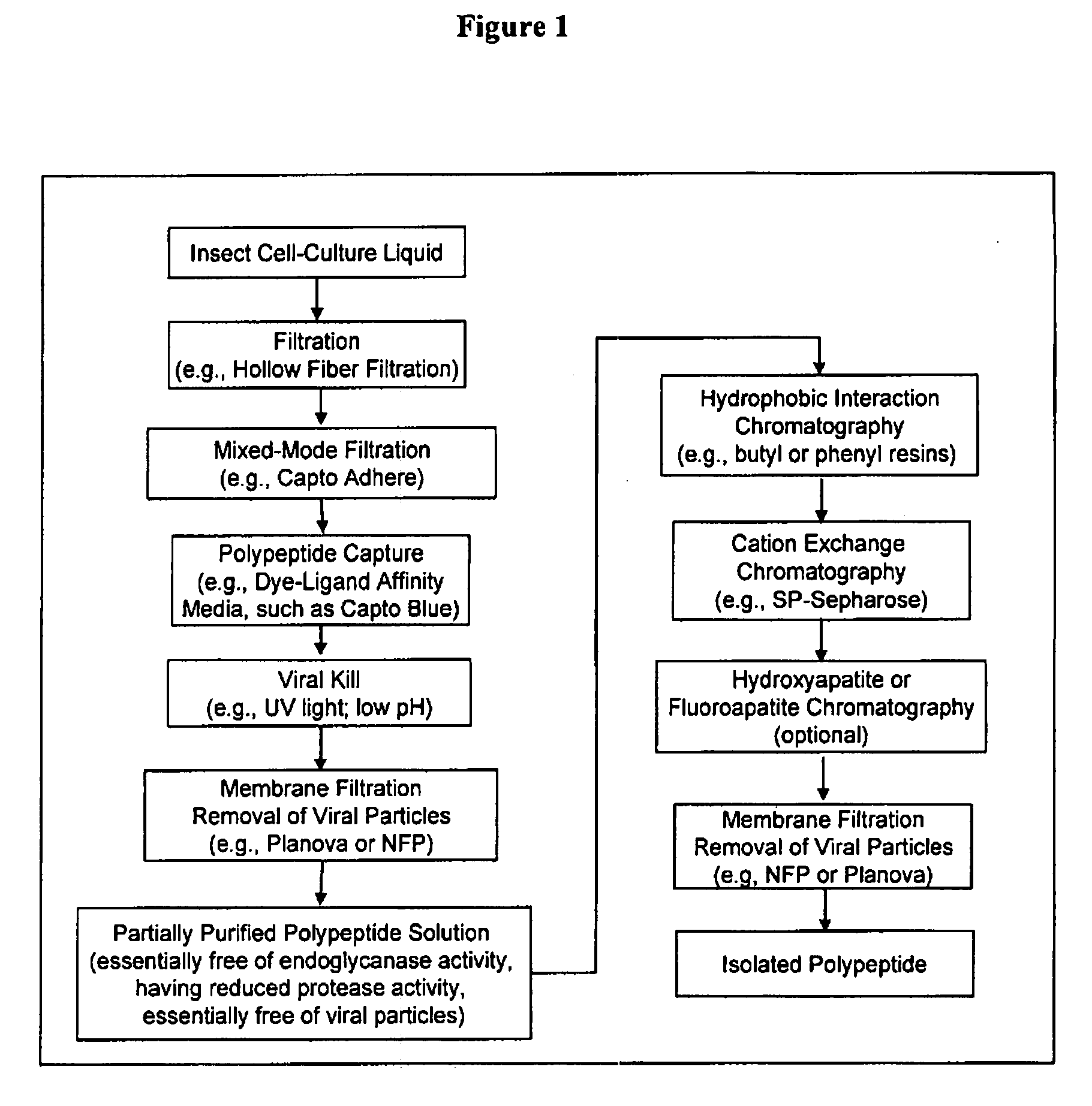
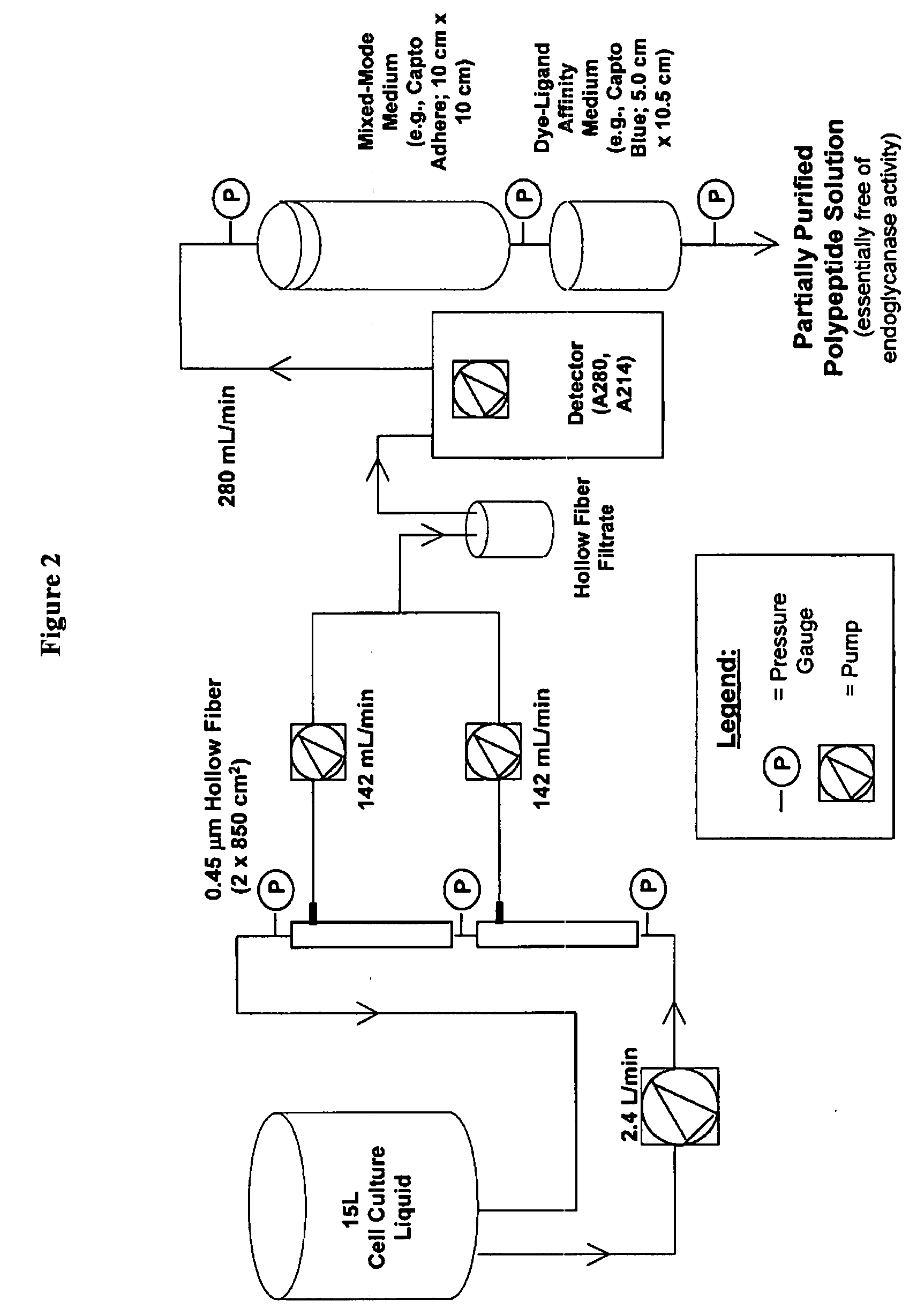
Manufacturing process for the production of polypeptides expressed in insect cell-lines
InactiveUS20080207487A1Promote recoveryReduce manufacturing costPeptide/protein ingredientsDepsipeptidesFiberCulture fluid
The present invention provides a manufacturing method for polypeptides that are produced in insect cells using a baculoviral expression system. In one example, the insect cell culture is supplemented with a lipid mixture immediately prior to infection (e.g., one hour prior to infection). The polypeptides are isolated from the insect cell culture using a method that employs anion exchange or mixed-mode chromatography early in the purification process. This process step is useful to remove insect-cell derived endoglycanases and proteases and thus reduces the loss of desired polypeptide due to enzymatic degradation. In another example, mixed-mode chromatography is combined with dye-ligand affinity chromatography in a continuous-flow manner to allow for rapid processing of the insect-cell culture liquid and capture of the polypeptide. In yet another example, a polypeptide is isolated from an insect cell culture liquid using a process that combines hollow fiber filtration, mixed-mode chromatography and dye-ligand affinity in a single unit operation producing a polypeptide solution that is essentially free of endoglycanase and proteolytic activities. In a further example, the isolated polypeptides are glycopeptides having an insect specific glycosylation pattern, which are optionally conjugated to a modifying group, such as a polymer (e.g., PEG) using a glycosyltransferase and a modified nucleotide sugar.
Owner:NOVO NORDISK AS
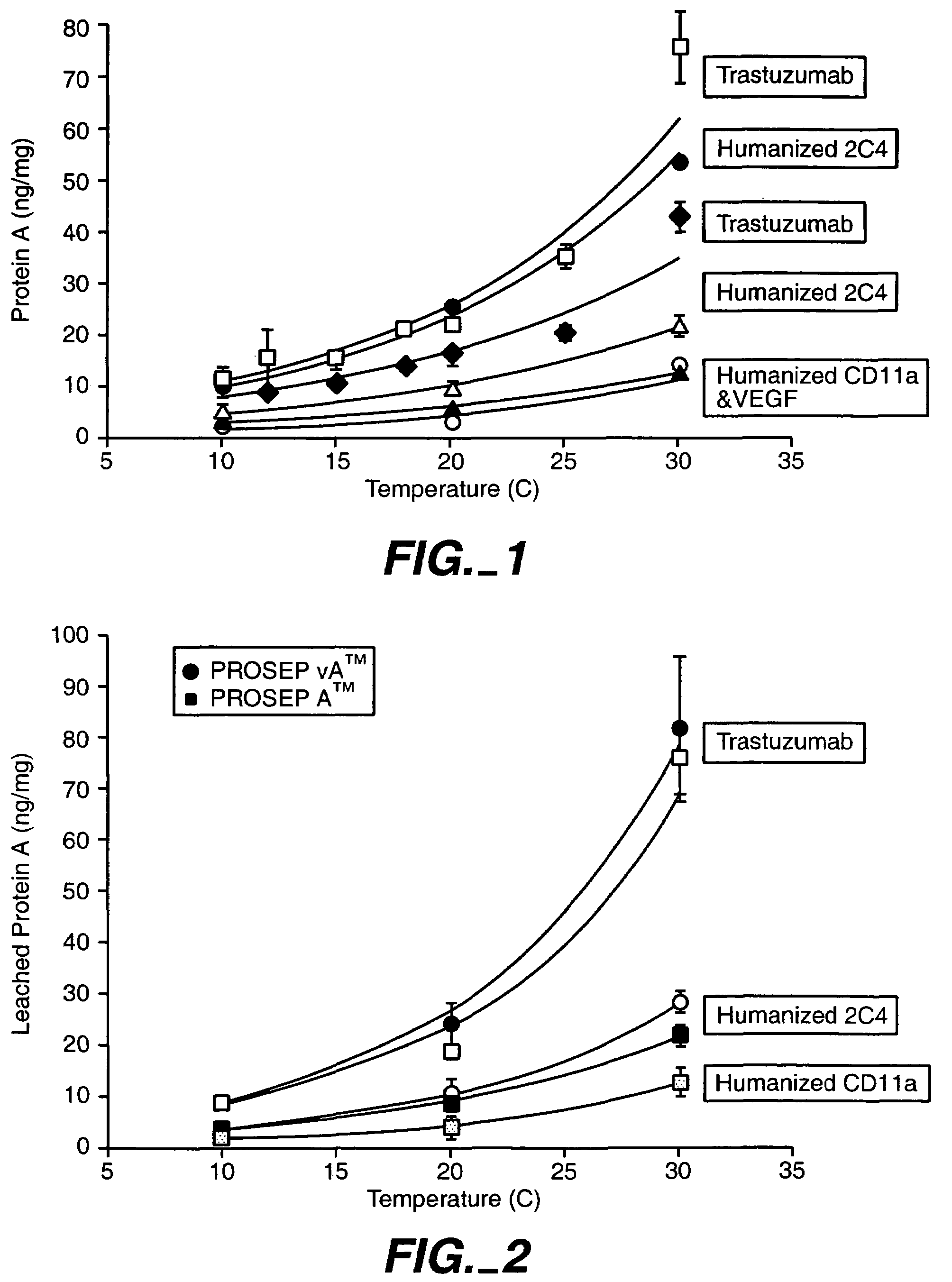
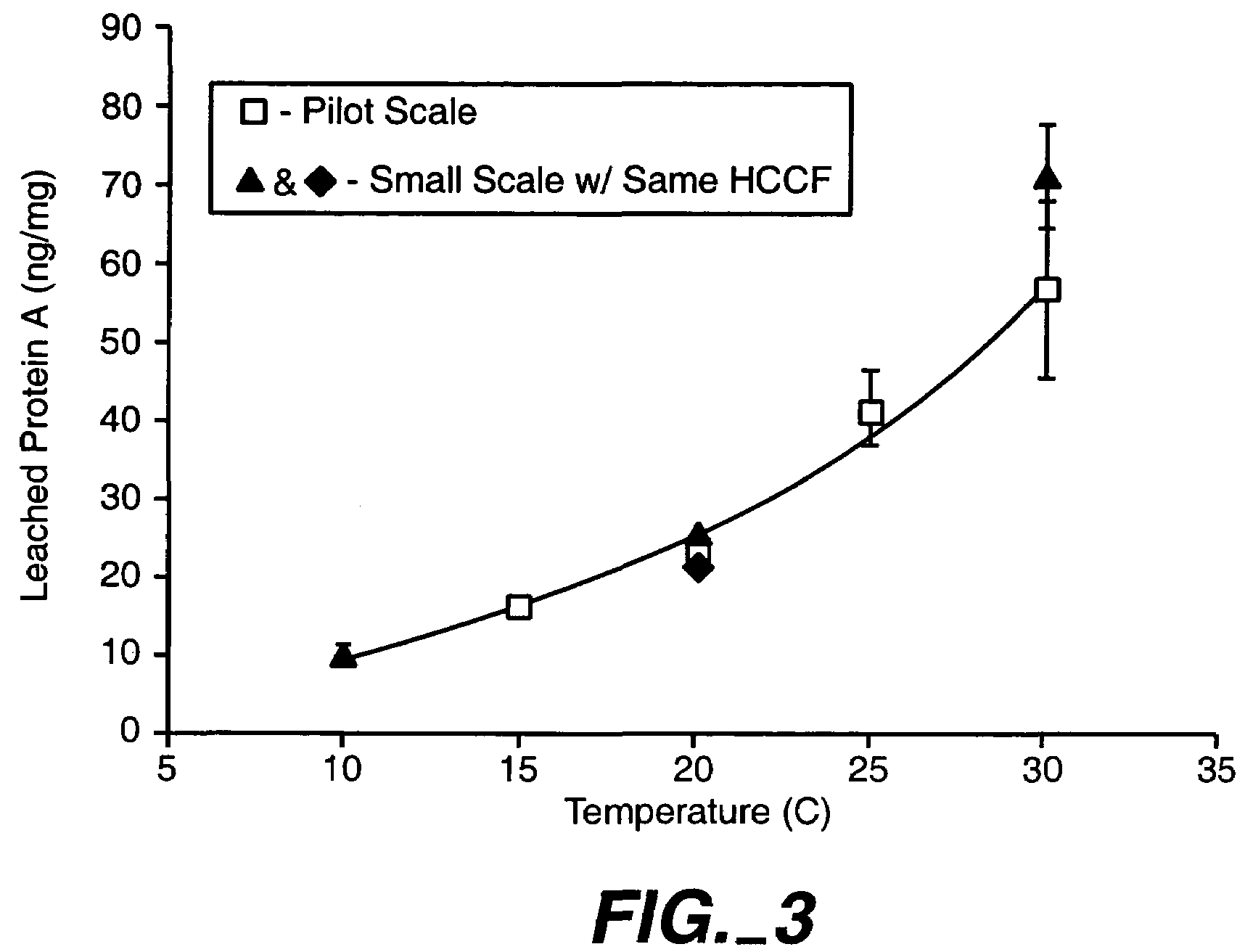
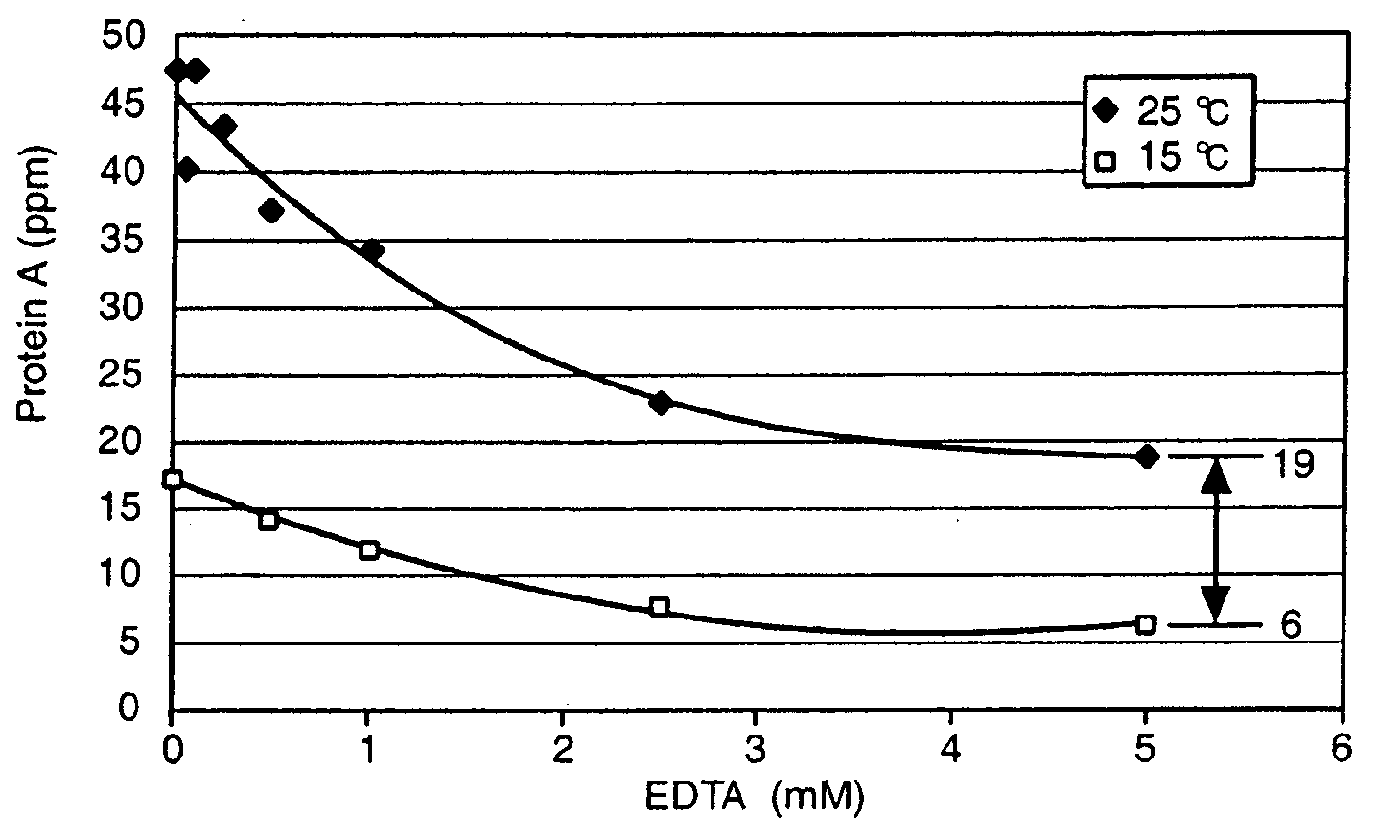
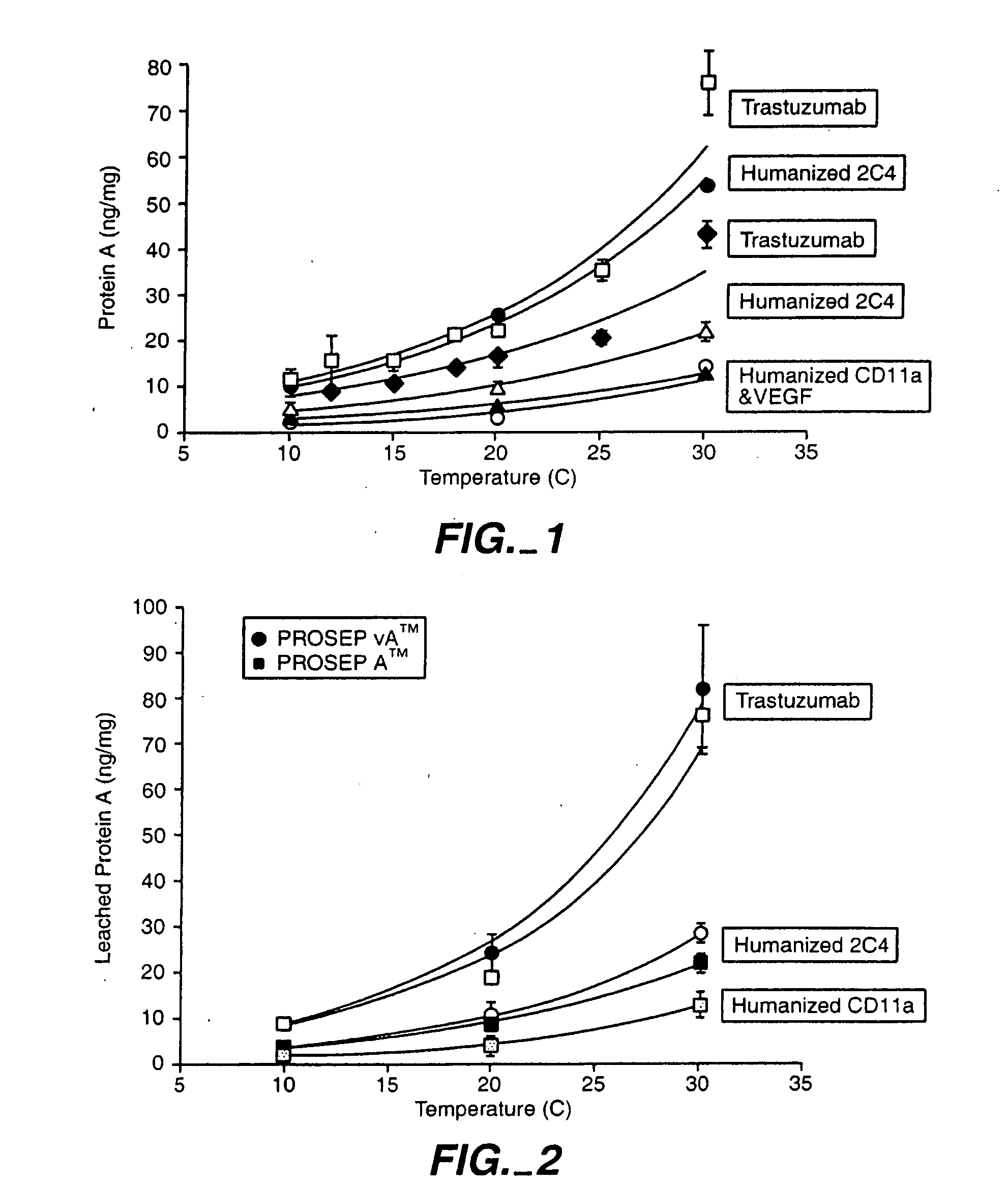
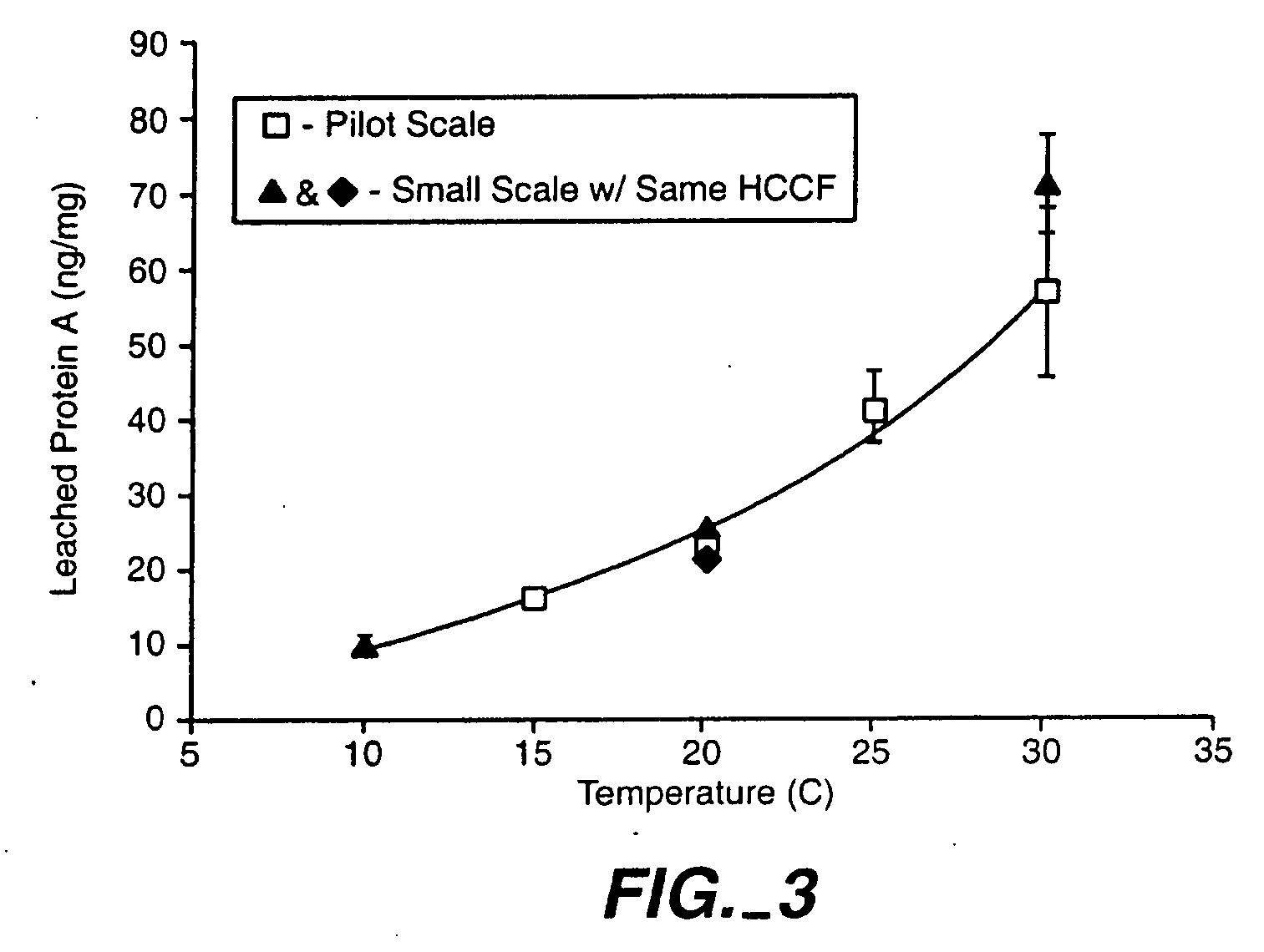
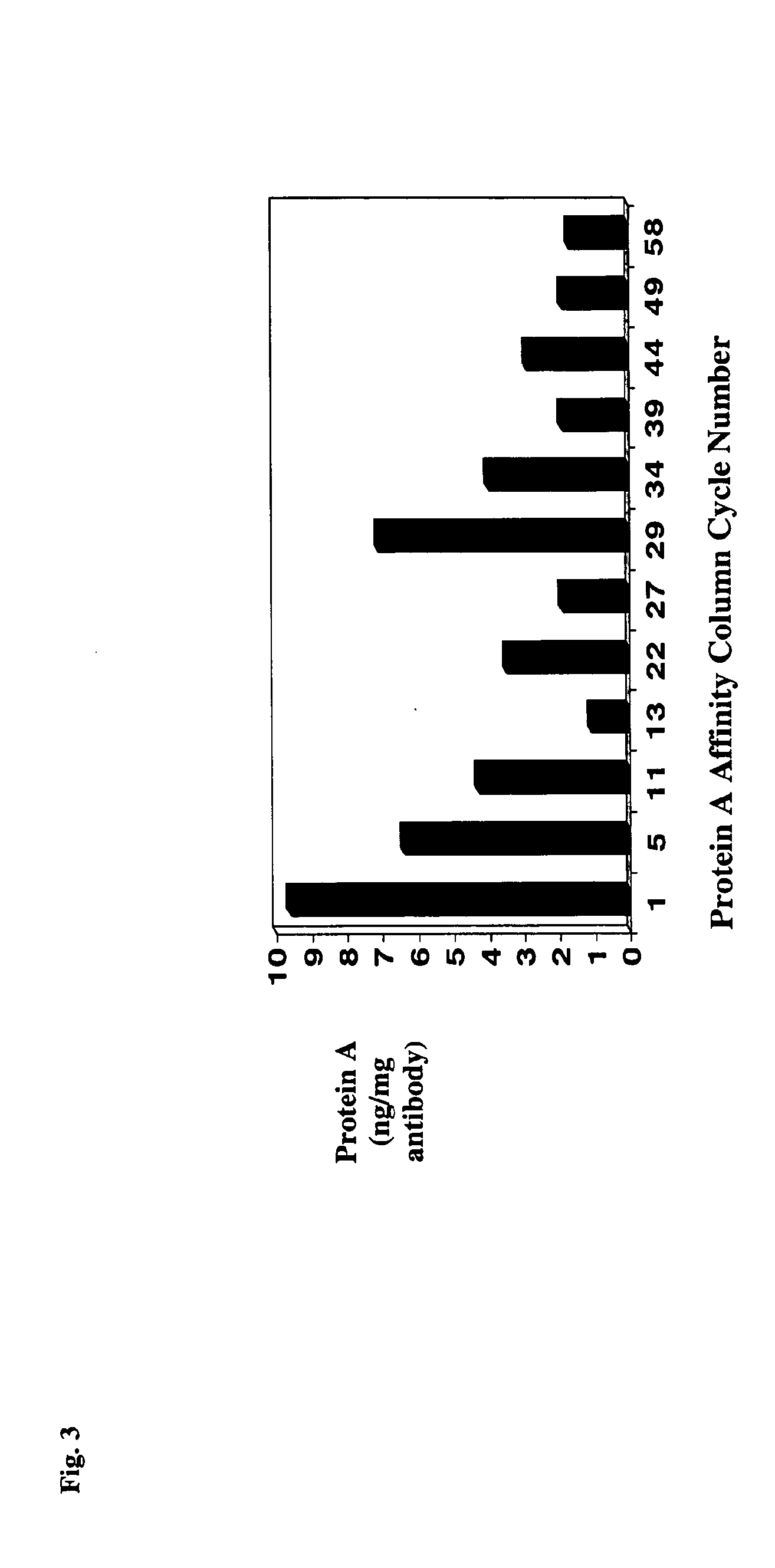
Reducing protein A leaching during protein A affinity chromatography
ActiveUS7485704B2Reduce the temperatureProtein A leaching is reduced.Serum immunoglobulinsSolid sorbent liquid separationProteinase activityAntibody Affinity Chromatography
A method for reducing leaching of protein A during protein A affinity chromatography is described which involves reducing temperature or pH of, or by adding one or more protease inhibitors to, a composition that is subjected to protein A affinity chromatography.
Owner:GENENTECH INC
Purification of non-human antibodies using kosmotropic salt enhanced protein a affinity chromatography
InactiveUS20140154270A1Solid sorbent liquid separationPeptide preparation methodsKosmotropicWeak binding
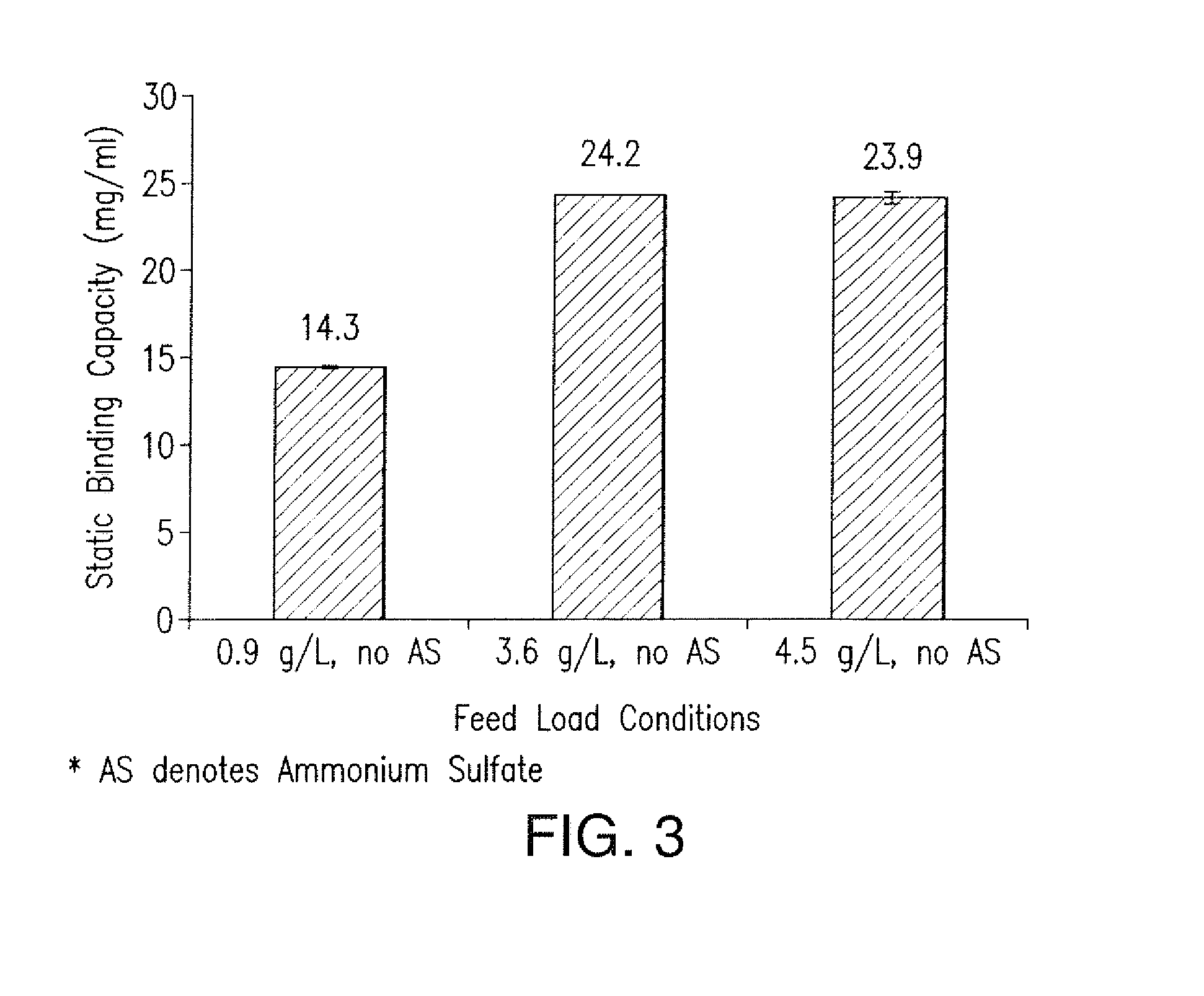
The present invention is directed to methods for purifying a non-human antibody, or antigen binding portion thereof, exhibiting weak binding strength and low binding capacity for Protein A chromatography media. In one aspect, a kosmotropic salt solution is employed to promote the hydrophobic interaction between the non-human antibody, or antigen binding portion thereof, and the Protein A ligand, thereby enhancing the binding of the non-human antibody, or antigen binding portion thereof, to the Protein A chromatography media. In another aspect, the concentration of the non-human antibody, or antigen binding portion thereof, in a sample comprising the antibody, or antigen binding portion thereof, exposed to a Protein A chromatography media is increased to enhance the binding of the non-human antibody, or antigen binding portion thereof, on the Protein A chromatography media.
Owner:ABBVIE INC
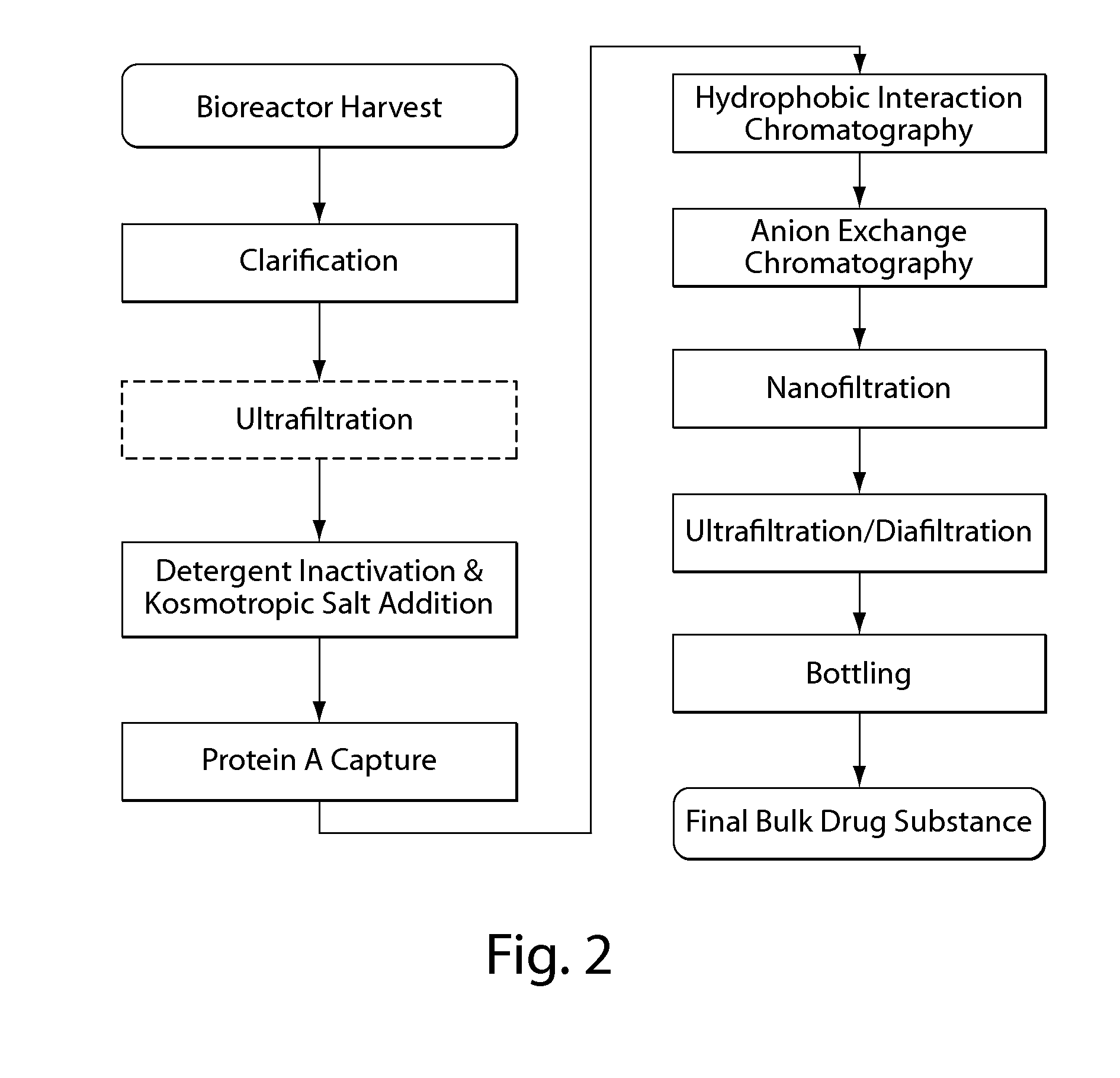
Viral inactivation during purification of antibodies cross reference to related applications
ActiveUS20100136025A1Easy to eliminateEnhanced interactionNervous disorderAntipyreticIon exchangeViral Inactivation
Described herein are methods for isolating and purifying antibodies from a sample matrix. One aspect of the present disclosure is directed to viral reduction / inactivation of samples generated in the various steps of antibody purification. In a particular aspect, methods herein employ an acidification step followed by one or more chromatography steps. The chromatography steps can include one or more of the following chromatographic procedures: ion exchange chromatography, affinity chromatography, and hydrophobic interaction chromatography.
Owner:ABBVIE INC
Reducing protein a leaching during protein a affinity chromatography
ActiveUS20090099344A1Reduce the temperatureProtein A leaching is reducedSerum immunoglobulinsSolid sorbent liquid separationProteinase activityEnzyme inhibitor
A method for reducing leaching of protein A during protein A affinity chromatography is described which involves reducing temperature or pH of, or by adding one or more protease inhibitors to, a composition that is subjected to protein A affinity chromatography.
Owner:GENENTECH INC
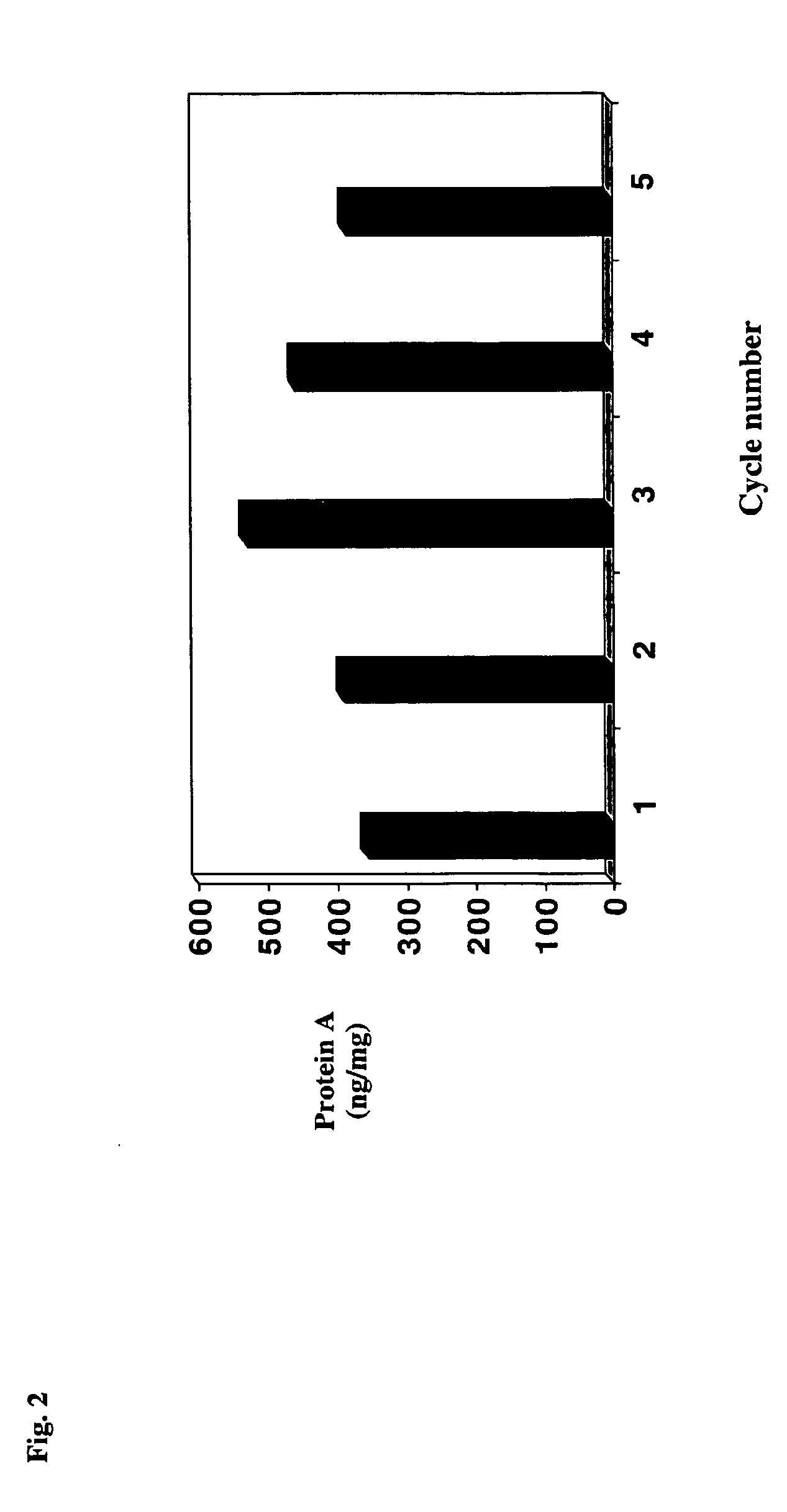
Antibody purification by protein a and ion exchange chromatography
ActiveUS20060194953A1Serum immunoglobulinsImmunoglobulins against animals/humansIon exchangeAntibody Affinity Chromatography
A novel method for selectively removing leaked protein A from antibody purified by means of protein A affinity chromatography is disclosed.
Owner:LONZA BIOLOGICS PLC
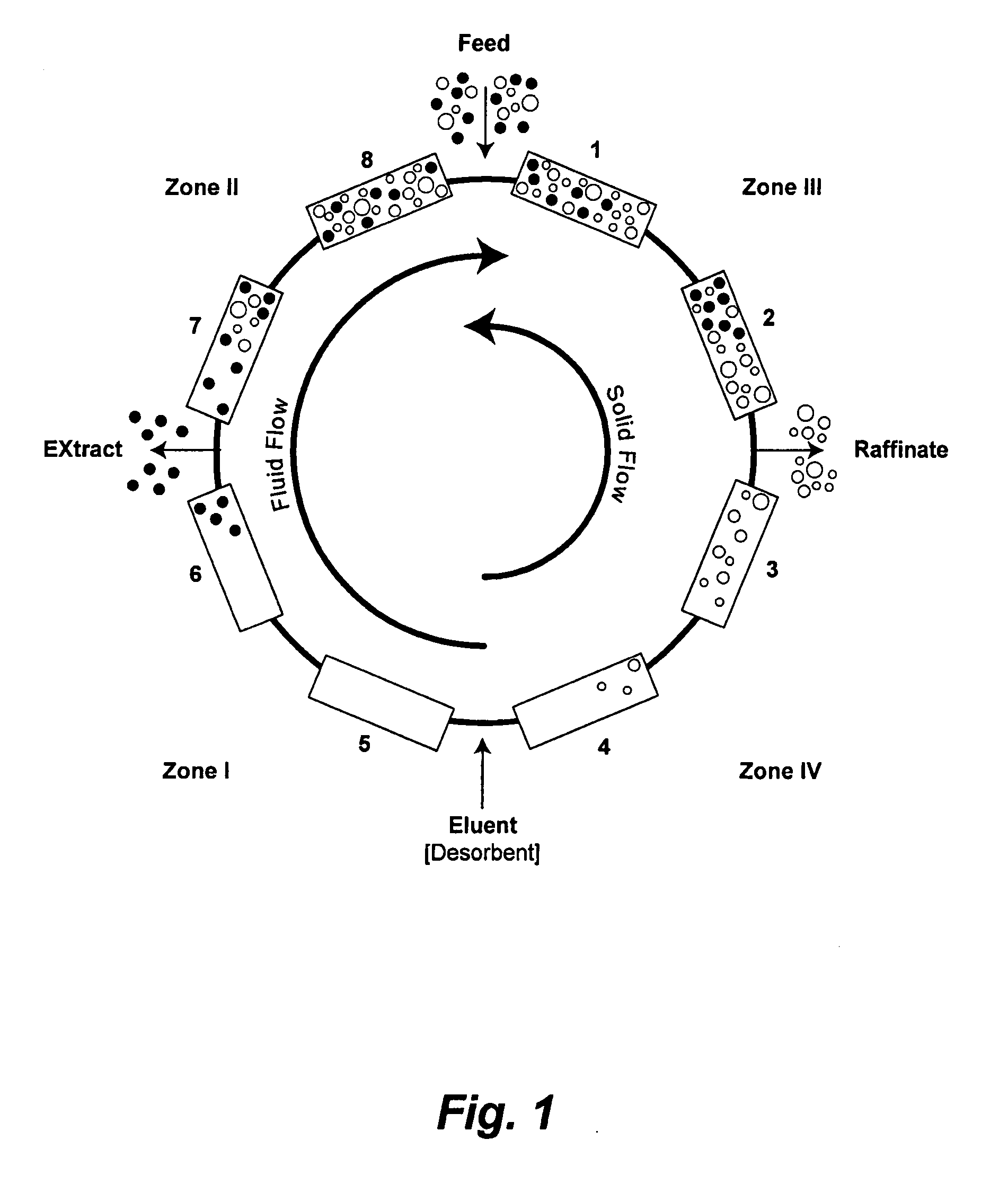
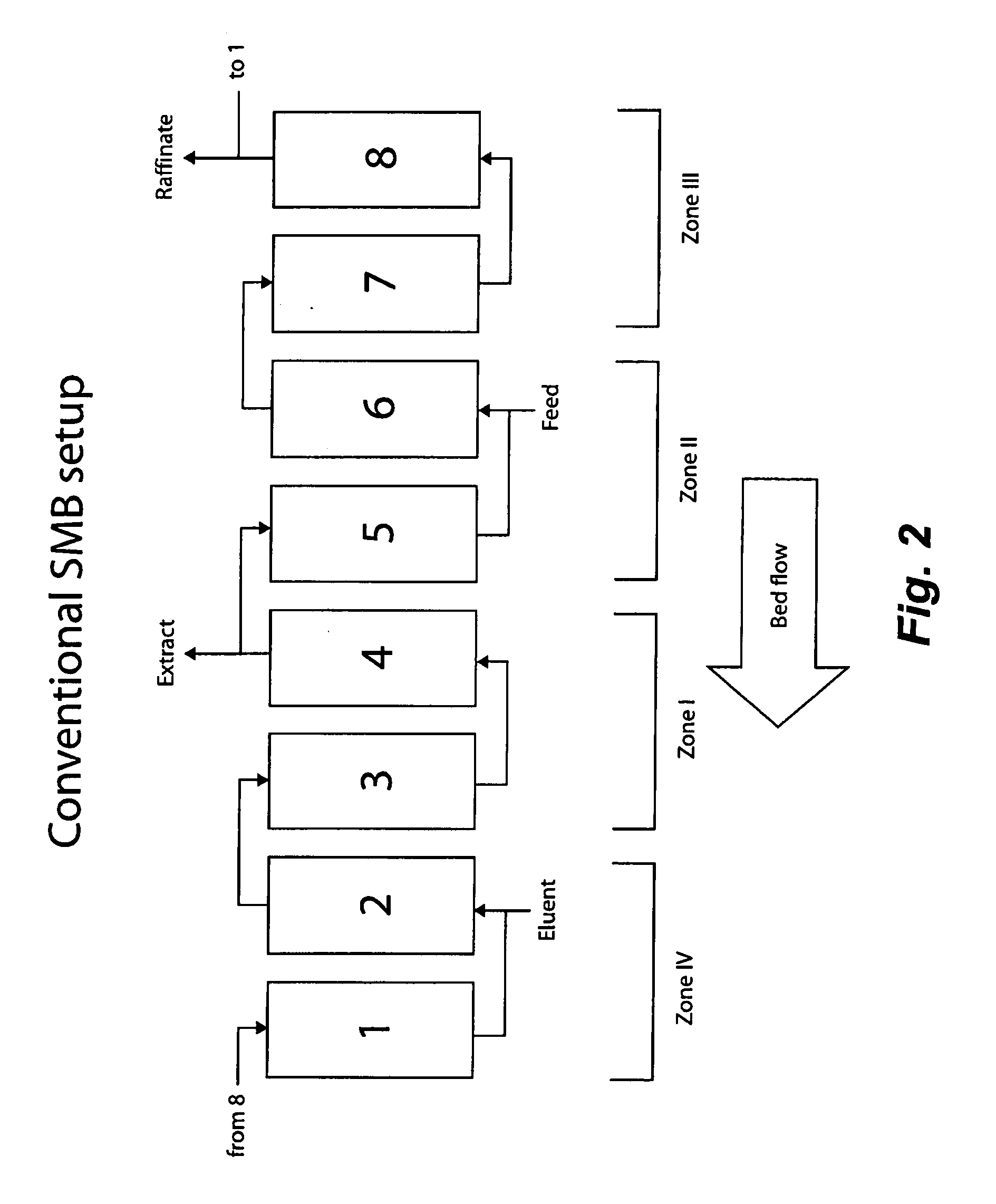
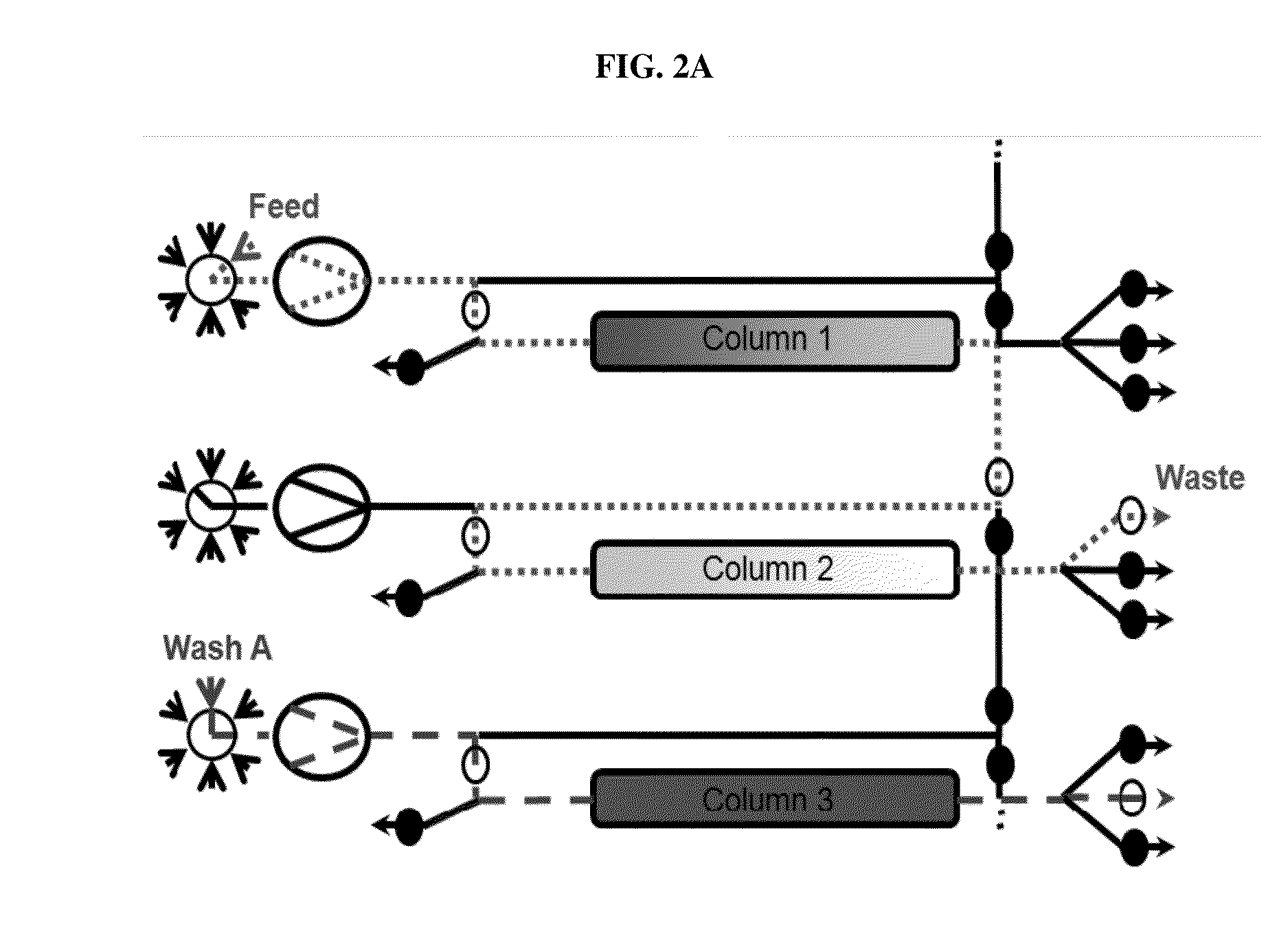
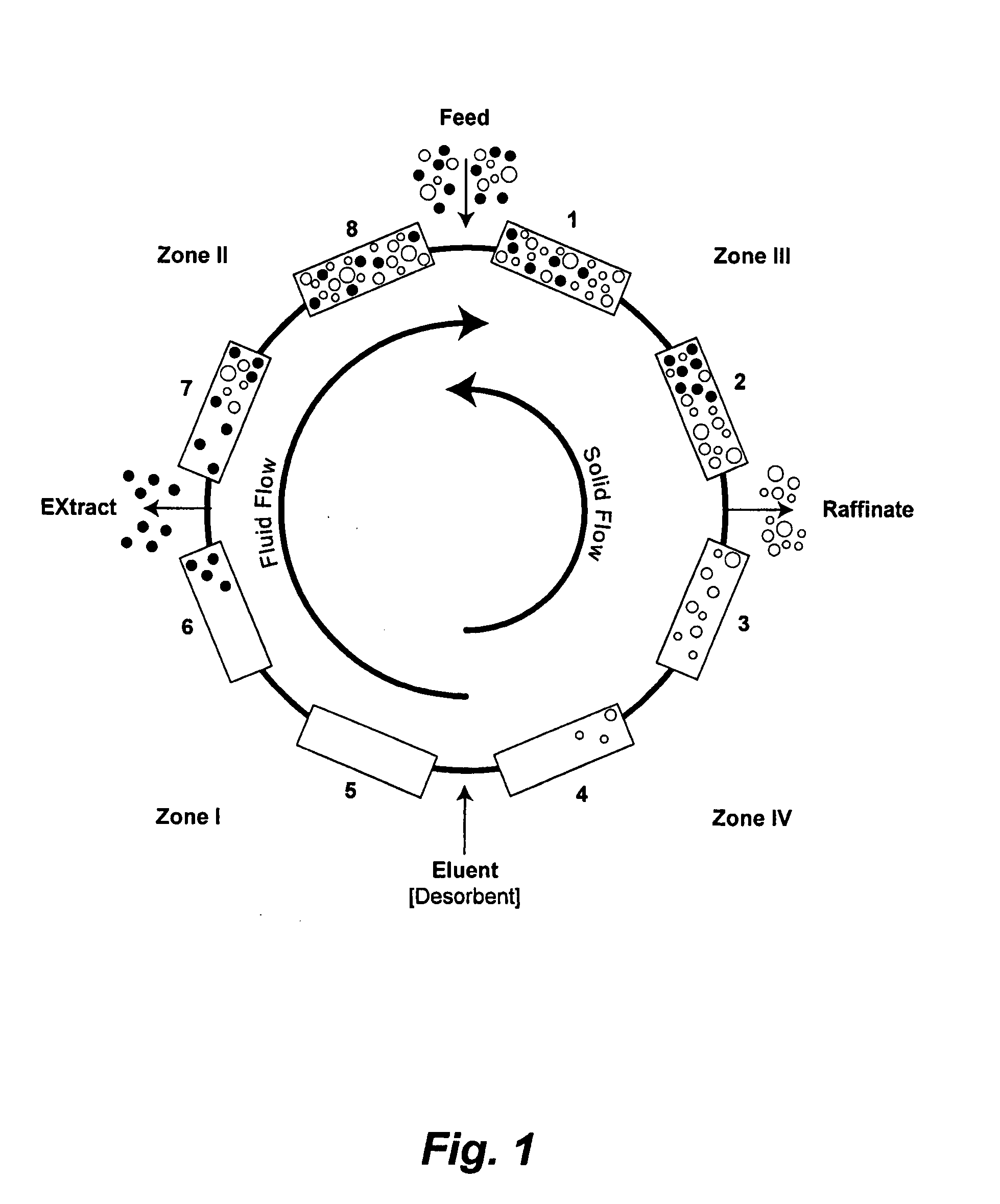
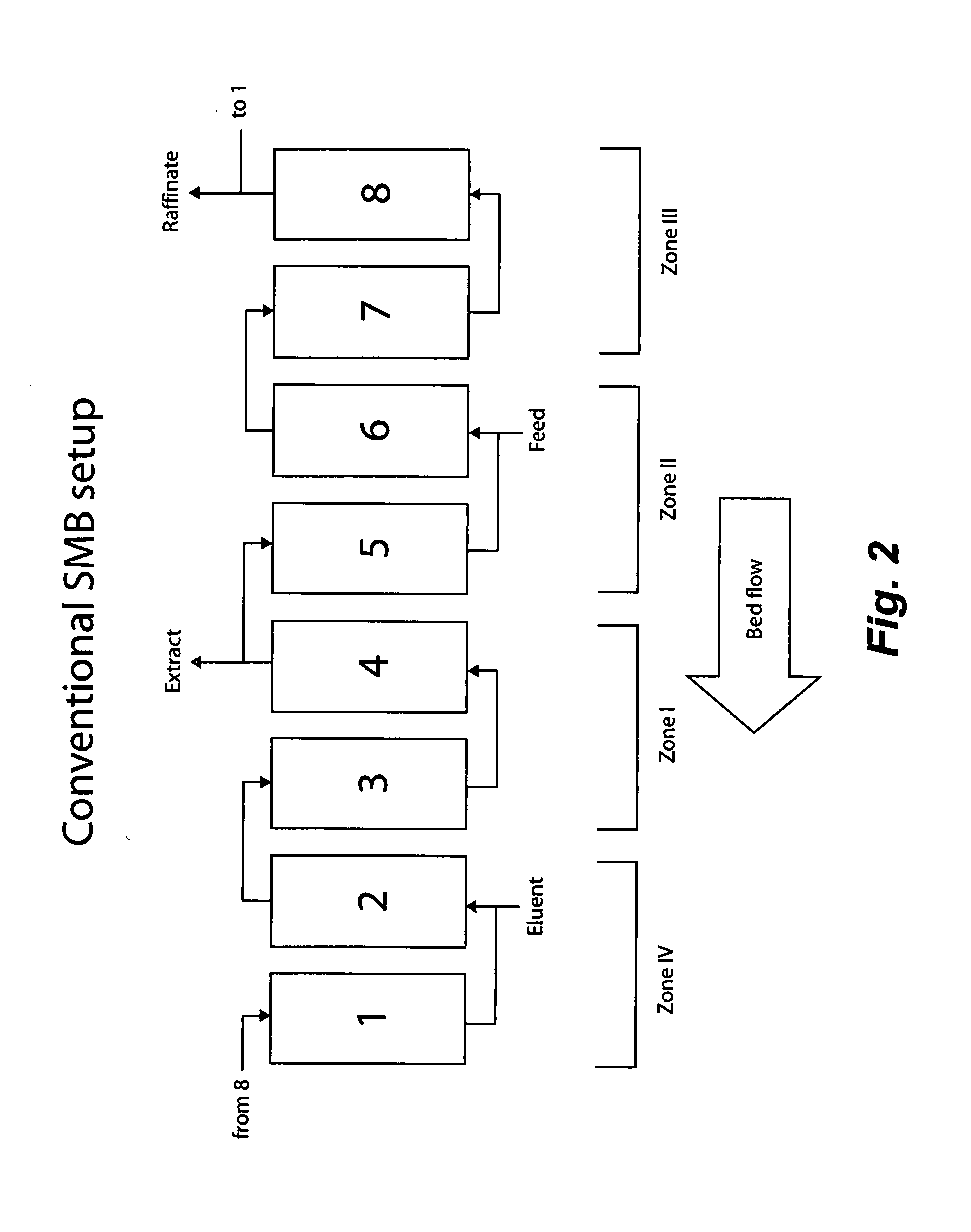
Valve Module And Methods For Simulated Moving Bed Chromatography
ActiveUS20080053543A1Simple and easily programmable controlPromote repairComponent separationSolid sorbent liquid separationSimulated moving bedMoving bed
The present invention provides devices and methods for micro-scale simulated moving bed chromatography (SMB) for continuous preparation of analytic quantities of highly pure fractions of target molecules. The present apparatus and method of the invention is adapted in a preferred embodiment to separations by affinity chromatography involving three discontinuous liquid flow loops. An alternative embodiment of affinity chromatography utilizes standard SMB operating under isocratic conditions.
Owner:TOSOH BIOSCIENCE LLC
Chromatography method
ActiveUS20130280788A1Ion-exchange process apparatusComponent separationAntibody Affinity ChromatographyChromatography detector
The present invention is directed to a continuous affinity chromatography method and to an apparatus to be used in such method. The method allows the use of high operational velocity while maintaining high binding capacities.
Owner:MERCK PATENT GMBH
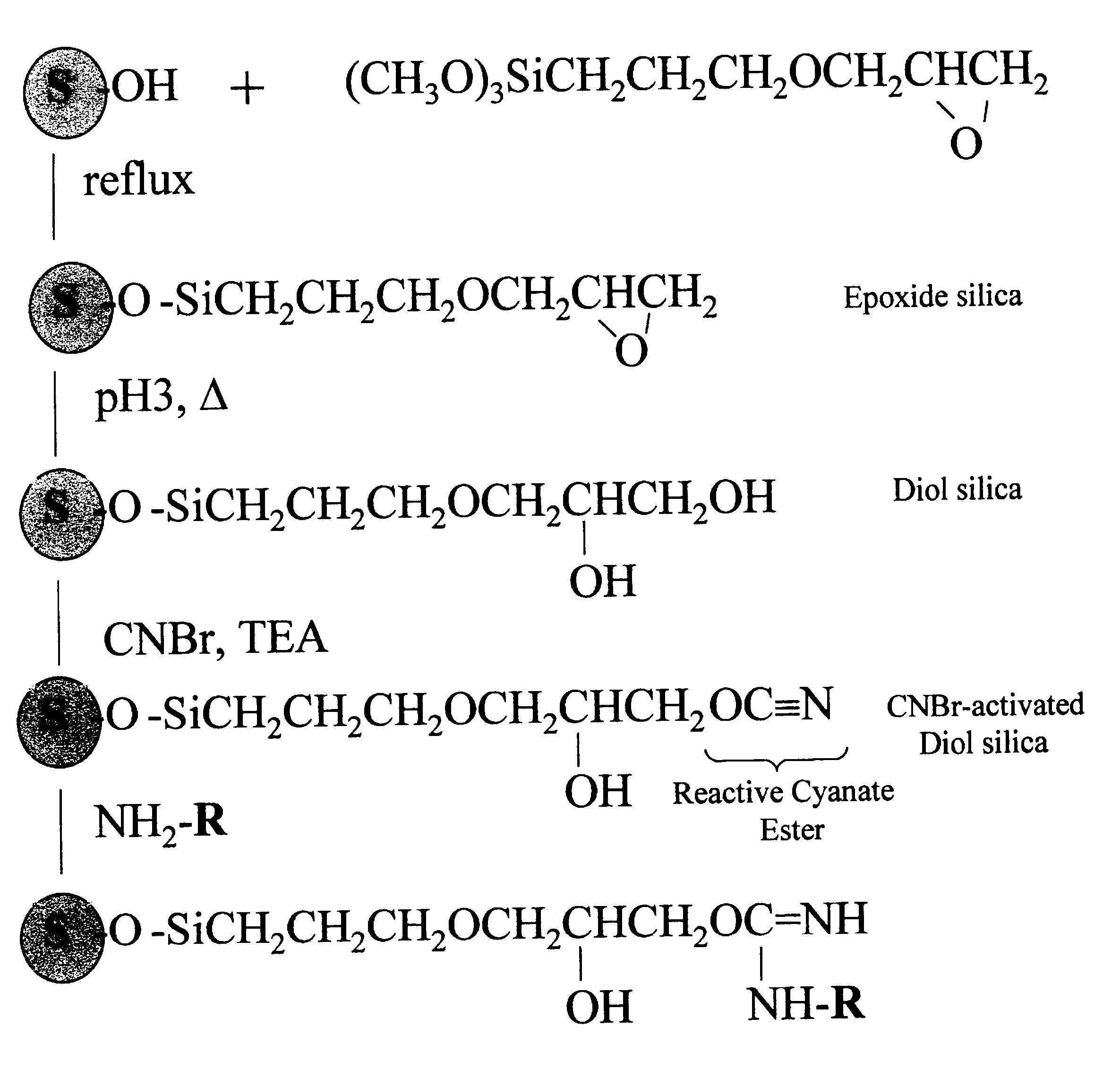
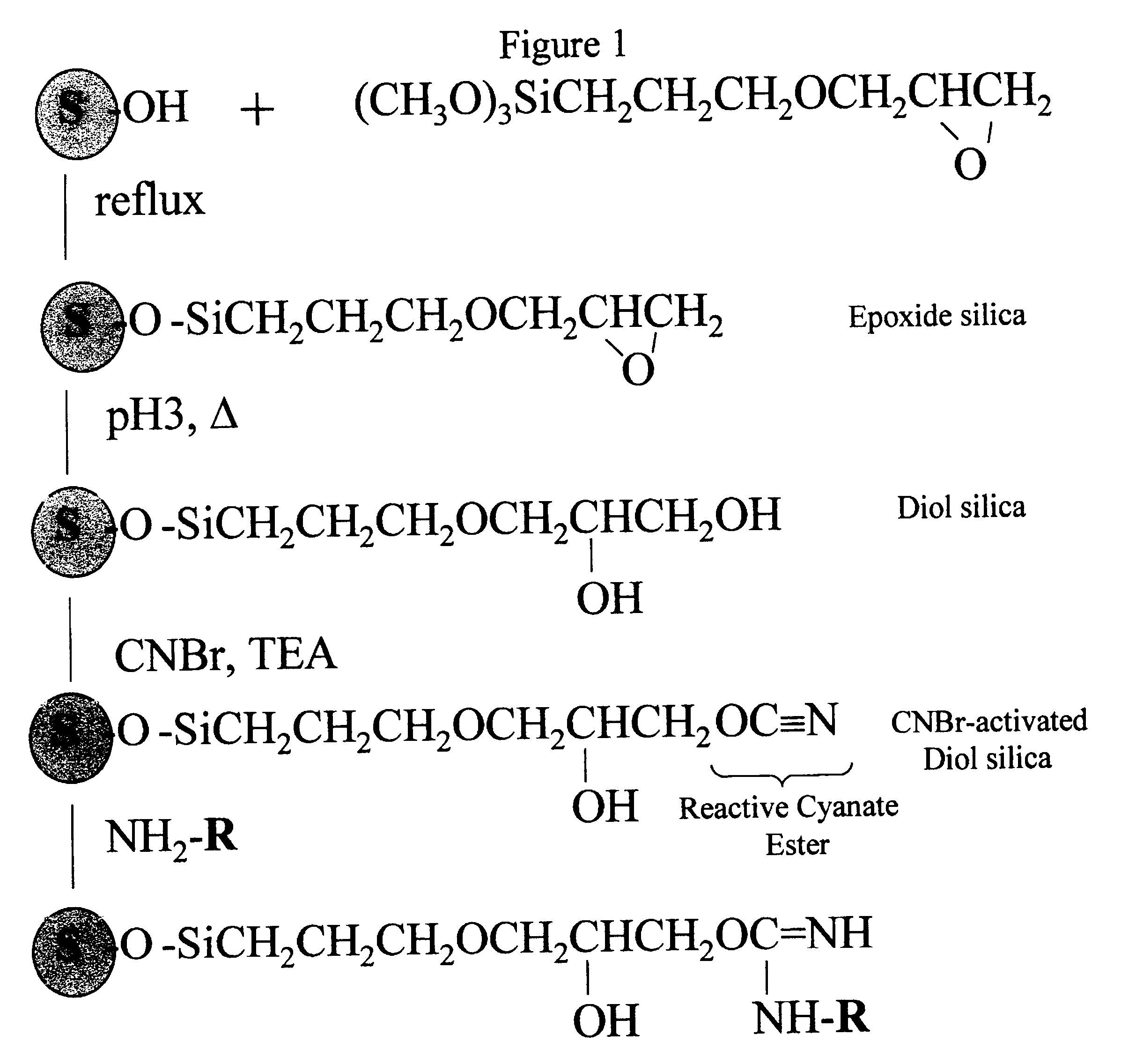
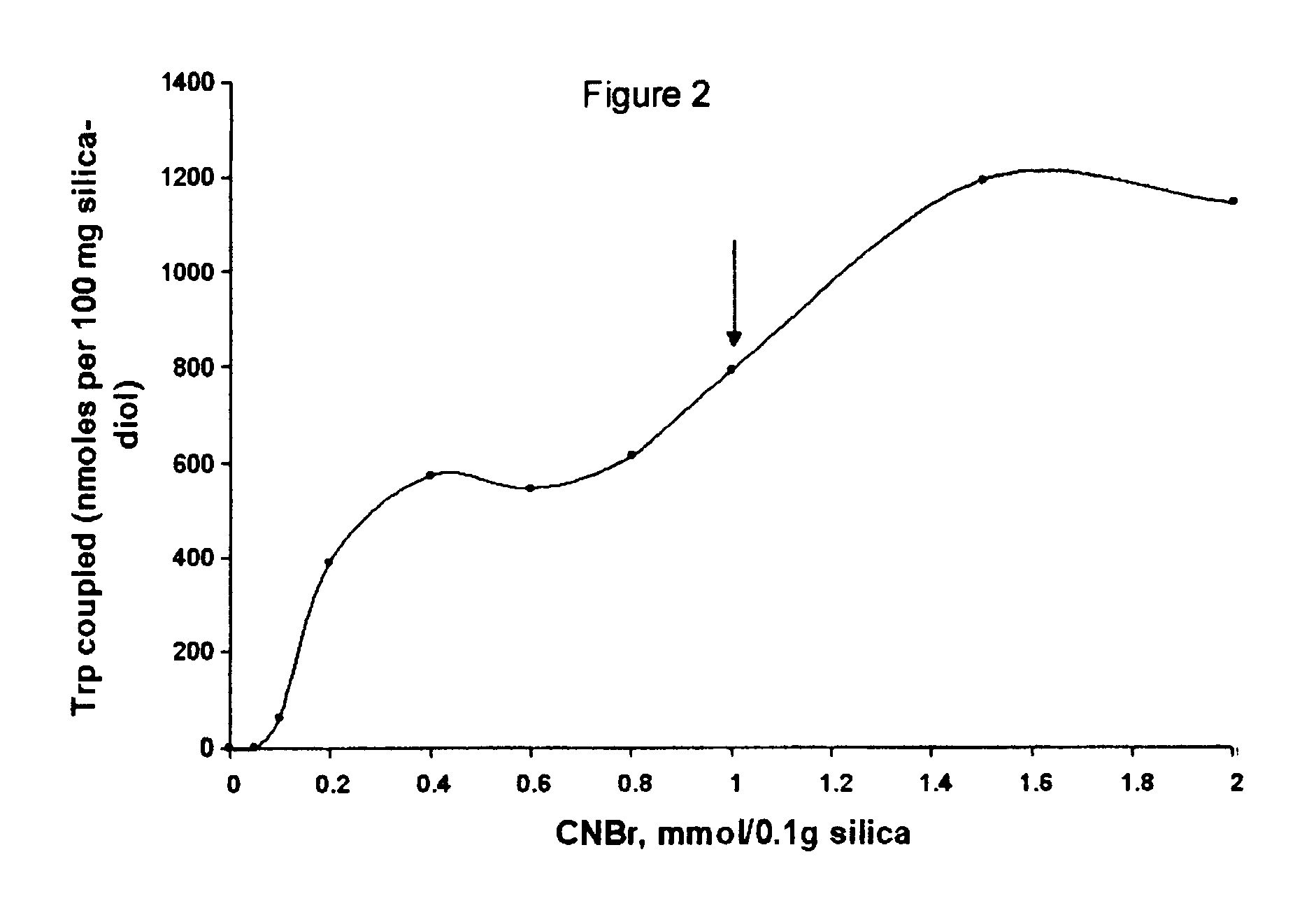
Cyanogen bromide-activation of hydroxyls on silica for high pressure affinity chromatography
InactiveUS6375846B1Excellent chromatographic performanceIon-exchange process apparatusOther chemical processesCyanogen halideCyanogen bromide
The present invention provides for a method to prepare a pressure stable and pH stable medium for use in high pressure (performance) affinity chromatography. The method includes the steps of treating hydroxyalkyl-silica with cyanogen halides or other cyanogen transfer reagents in the presence of an organic base in anhydrous solvents at temperatures in the range of from about -15° C. to about 20° C. for a period of time in the range of from about 1 minute to about 5 minutes, and washing the resulting medium in anhydrous solvent.
Owner:JARRETT HARRY WELLINGTON +1
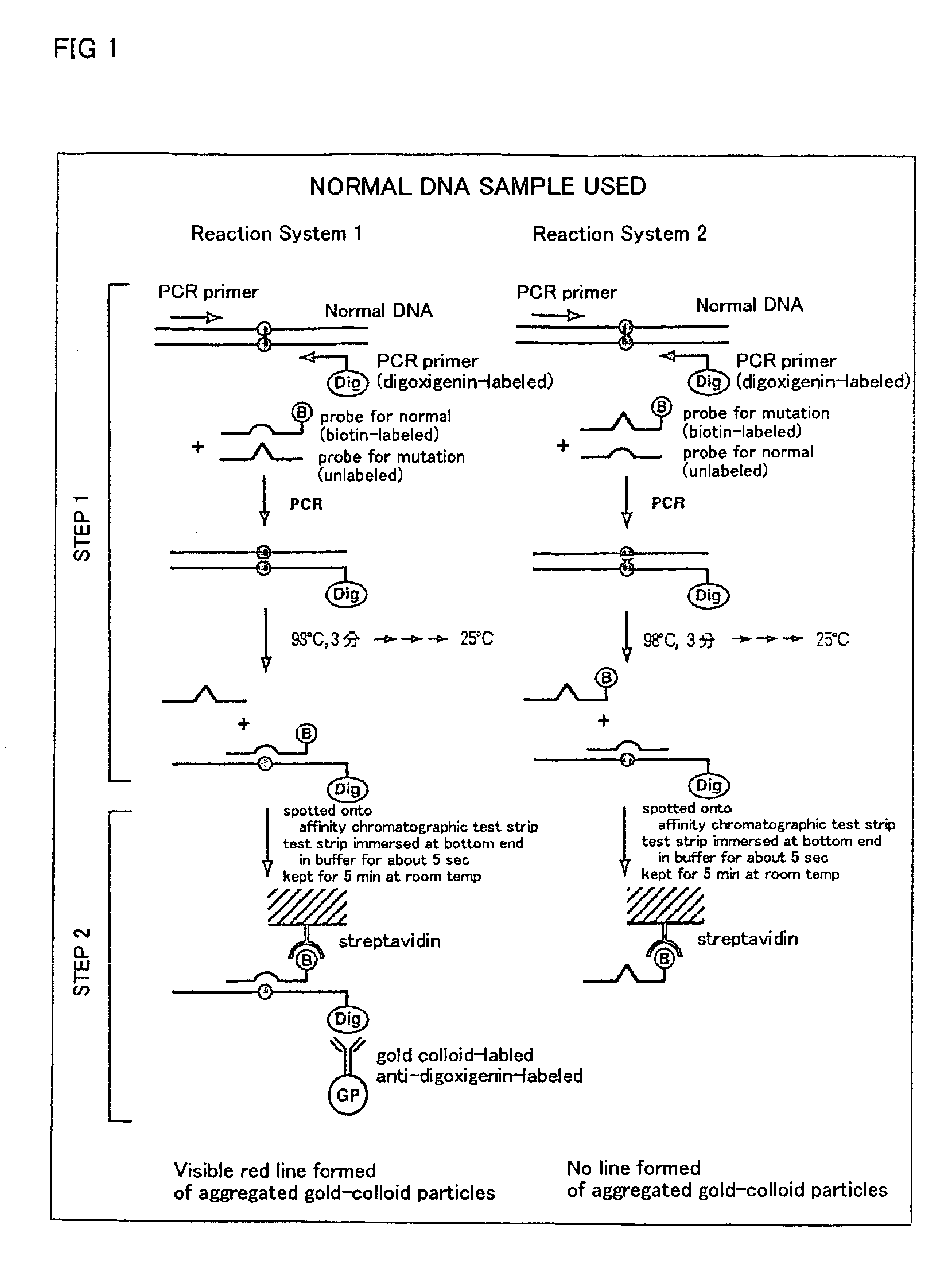
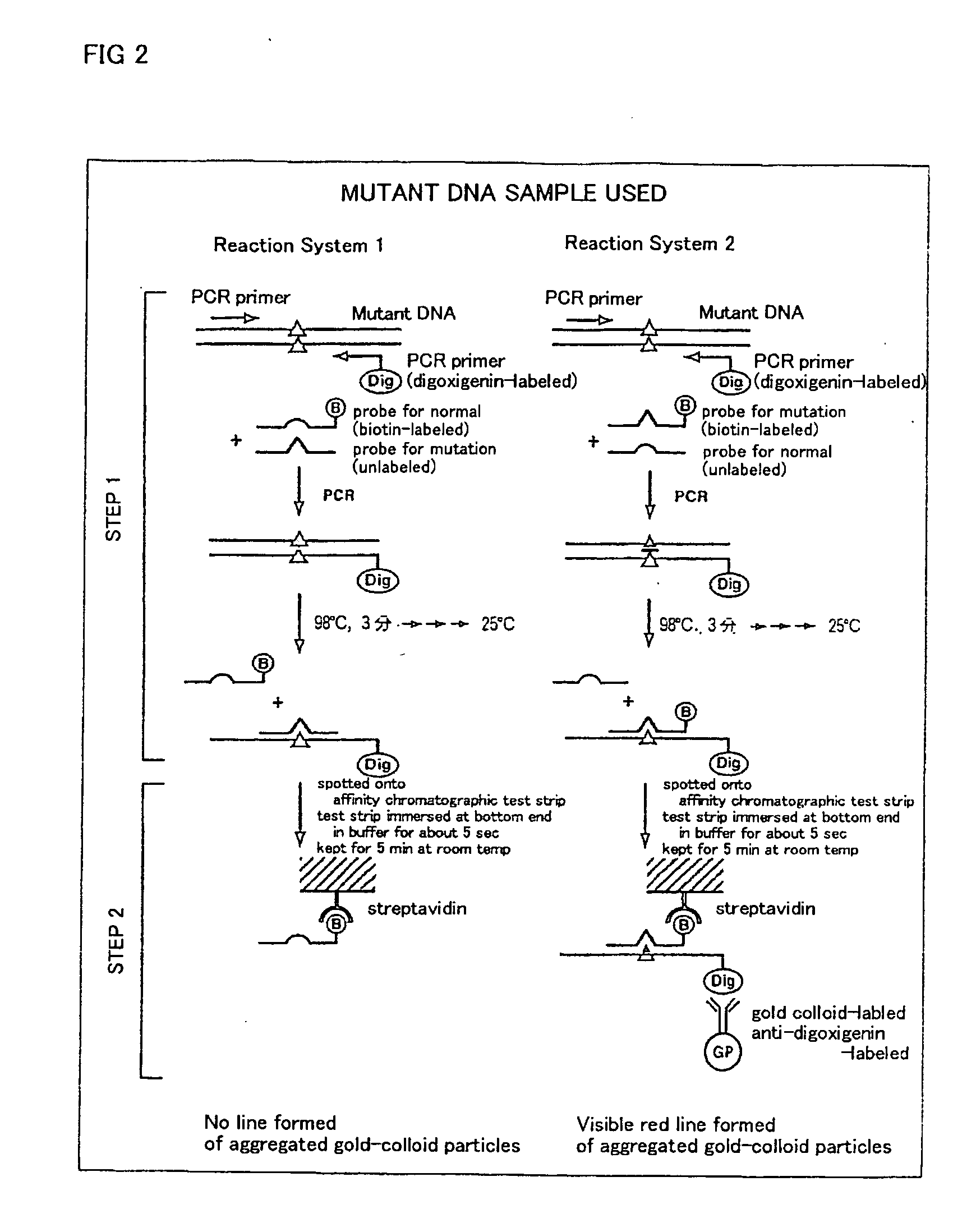
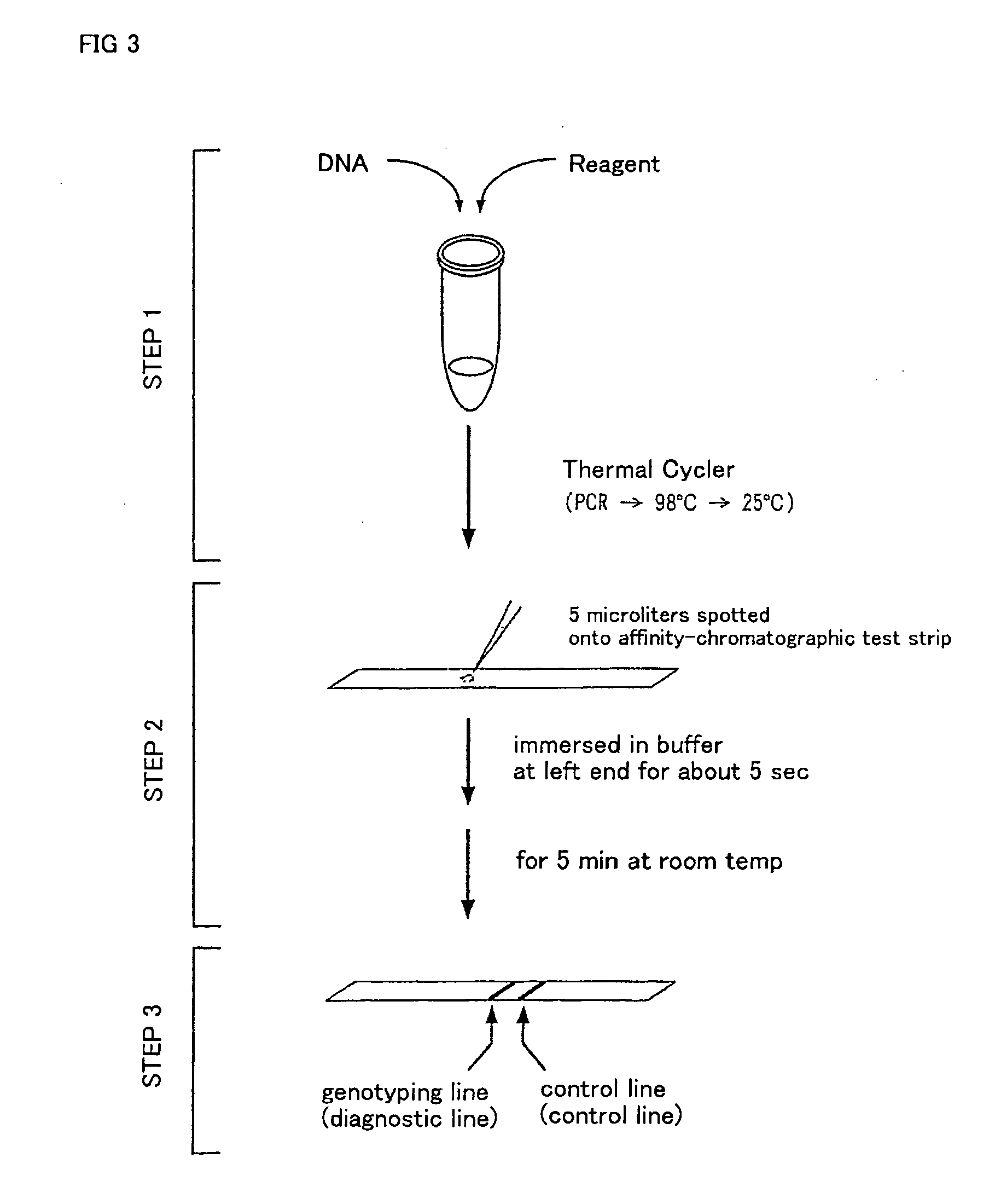
Method of detecting gene mutation
InactiveUS20060127907A1Simply and quickly detectingEasy to detectSugar derivativesMicrobiological testing/measurementHybridization probeDna amplification
DNA amplification and hybridization are successively carried out in a reaction system containing primers for the DNA amplification and hybridization probes, followed by detecting the hybrid in the reaction solution by affinity chromatography, wherein at least one of the primers to be used in the DNA amplification is labeled with a first labeling agent so that the amplified DNA will be labeled with the first labeling agent, a hybridization probe is labeled with a second labeling agent and contained in a reaction solution for effecting the DNA amplification, the base sequence of the hybridization probe is designed not to inhibit the DNA amplification, and a hybrid is detected by affinity chromatography with the use of the first and second labeling agents.
Owner:MATSUBARA YOICHI
Isolation and purification of antibodies using protein A affinity chromatography
ActiveUS8895709B2Easy to disassembleReduce or inactivate pH-sensitive virusesAntipyreticAnalgesicsIon exchangeAntibody Affinity Chromatography
Disclosed herein are methods for the isolation and purification of antibodies wherein the use of an affinity chromatographic step results in an antibody composition sufficiently pure for pharmaceutical uses. The methods described herein comprise pH viral reduction / inactivation, ultrafiltration / diafiltration, affinity chromatography, preferably Protein A affinity, ion exchange chromatography, and hydrophobic chromatography. Further, the present invention is directed toward pharmaceutical compositions comprising one or more antibodies of the present invention.
Owner:ABBVIE INC
Novel purification of human, humanized, or chimeric antibodies using protein a affinity chromatography
InactiveUS20130336957A1Improving Protein A performanceIncrease volumeSolid sorbent liquid separationAntibody ingredientsKosmotropicMedicine
Disclosed herein are compositions and methods for the isolation and purification of antibodies from a sample matrix. In particular, the present invention relates to compositions and methods for isolating and purifying antibodies exhibiting low or high binding capacity for Protein A resin. In certain embodiments, the methods herein employ a kosmotropic salt solution, an affinity chromatographic step, and may include one or more additional chromatography and / or filtration steps to achieve the desired degree of purification. The present invention is also directed toward pharmaceutical compositions comprising one or more antibodies purified by a method described herein.
Owner:ABBVIE INC
Novel purification of non-human antibodies using protein a affinity chromatography
InactiveUS20140010820A1Enhanced hydrophobic interactionsHigh retention rateSolid sorbent liquid separationPeptide preparation methodsKosmotropicPharmaceutical drug
Disclosed herein are compositions and methods for the isolation and purification of antibodies from a sample matrix. In particular, the present invention relates to compositions and methods for isolating and purifying antibodies exhibiting weak binding strength and low binding capacity for Protein A resin. In certain embodiments, the methods herein employ a kosmotropic salt solution, an affinity chromatographic step, and may include one or more additional chromatography and / or filtration steps to achieve the desired degree of purification. The present invention is also directed toward pharmaceutical compositions comprising one or more antibodies purified by a method described herein.
Owner:ABBVIE INC
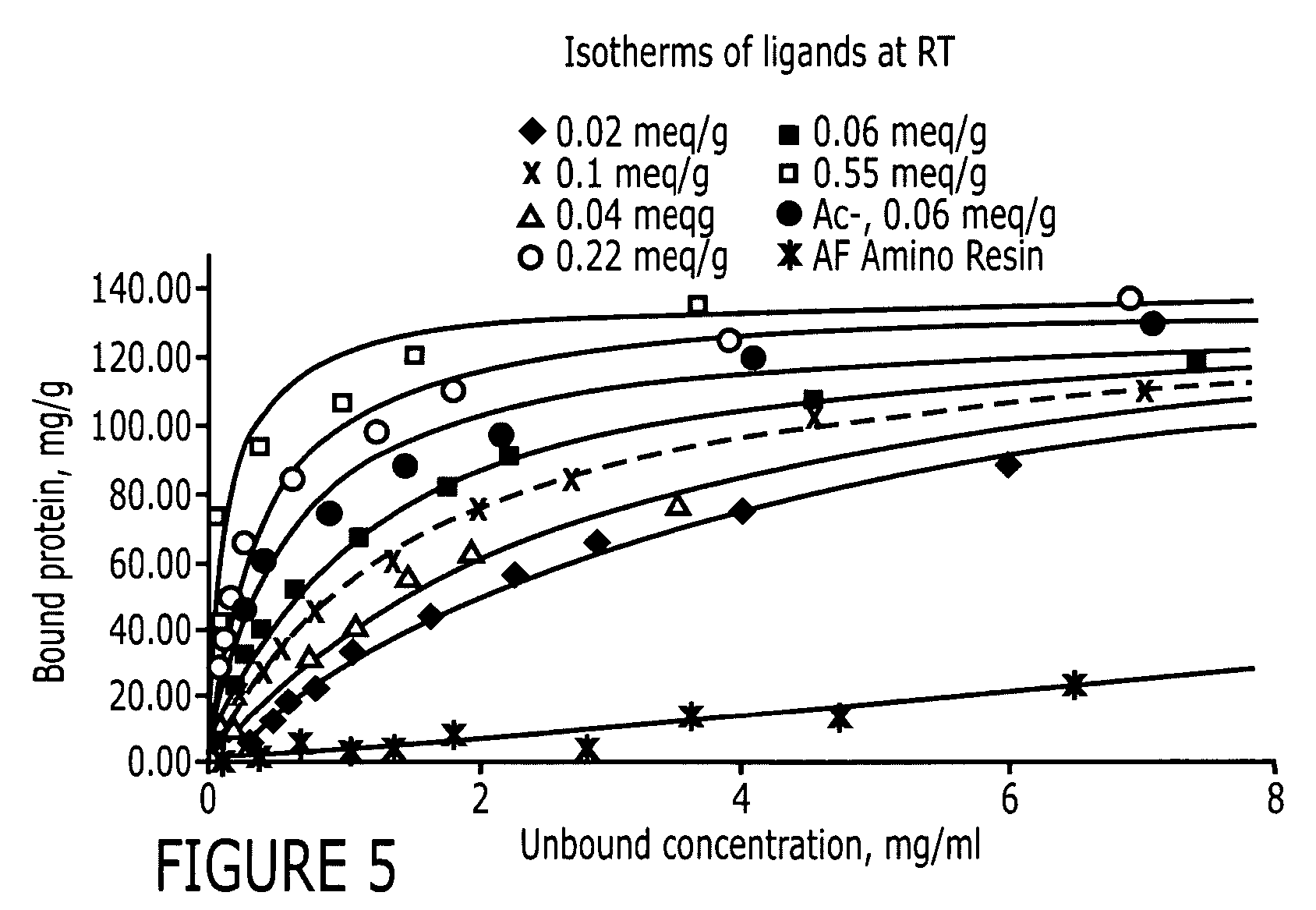
Affinity chromatography matrices and methods of making and using the same
ActiveUS20070207500A1Highly specificFast and economicalPeptide/protein ingredientsOther chemical processesAntibody Affinity ChromatographyChromatography
The invention provides methods of coupling protein ligands to a solid support. The invention also provides affinity chromatography matrices and methods of using affinity chromatography matrices to purify a target molecule.
Owner:MILLIPORE CORP
Affinity chromatography matrix
ActiveUS20130274451A1Increased elution pHGuaranteed cost-effective operationSolid sorbent liquid separationPeptide preparation methodsElutionA domain
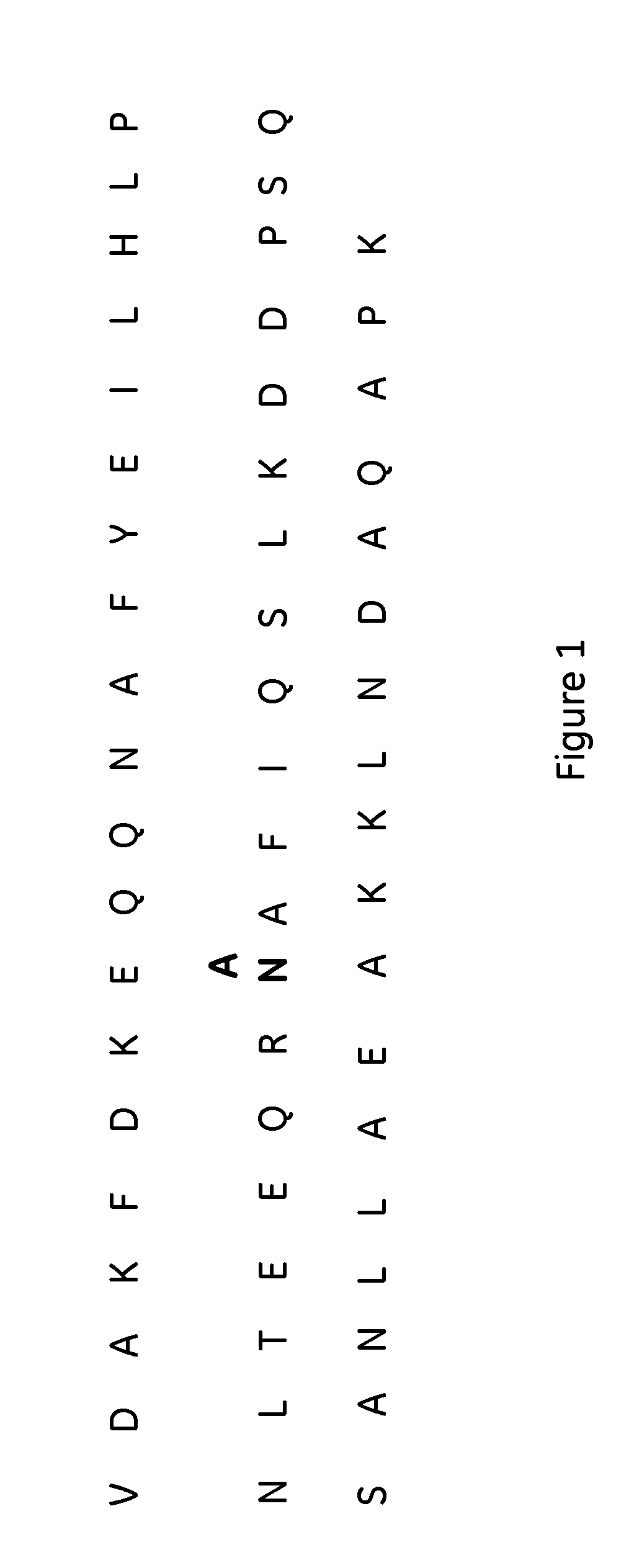
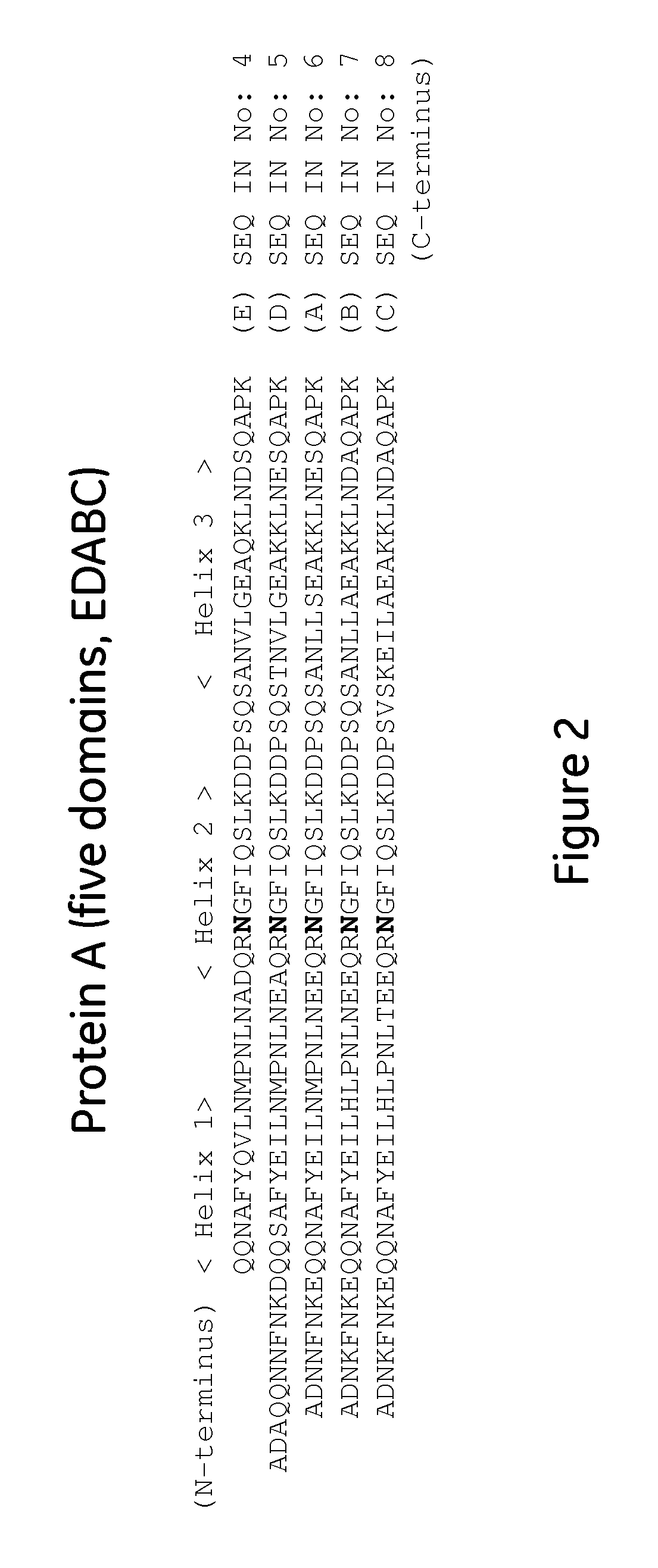
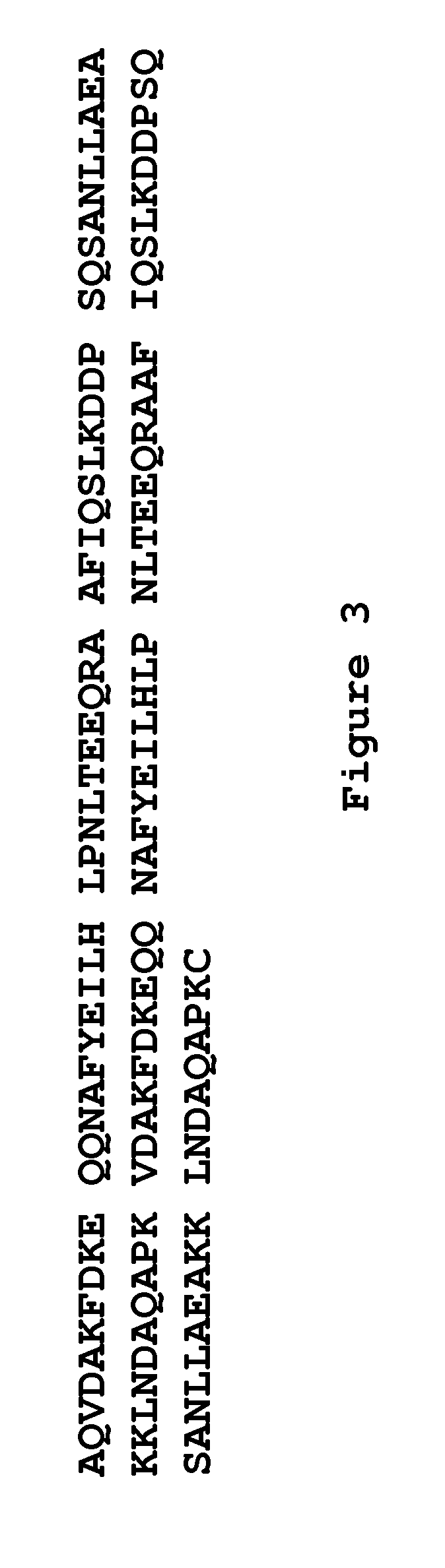
The present invention relates to a method of separating one or more immunoglobulin containing proteins from a liquid. The method includes first contacting the liquid with a separation matrix comprising ligands immobilised to a support; allowing the immunoglobulin containing proteins to adsorb to the matrix by interaction with the ligands; followed by an optional step of washing the matrix containing the immunoglobulin containing proteins adsorbed thereon; and recovering said immunoglobulin containing proteins by contacting the matrix with an eluent which releases the proteins. The method improves upon previous separation methods in that each of the ligands comprises one or more of a protein A domain (E, D, A, B, C), or protein Z, or a functional variant thereof, with at least one of the monomers having a substitution of the Asparagine at the position corresponding to N28 of B domain of Protein A or Protein Z, and wherein the ligand provides an increase in elution pH compared to non-substituted ligand.
Owner:CYTIVA BIOPROCESS R&D AB
Wash solution and method for affinity chromatography
ActiveUS20120283416A1Efficient and robust wash solutionHigh yieldSolid sorbent liquid separationImmunoglobulins against virusesArginineImpurity
The invention provides a washing method for affinity chromatography in which a wash solution comprising arginine, or an arginine derivative, and a nonbuffering salt, preferably at high pH, greater than 8.0, is effective in removing impurities, such as high molecular weight species and host cell proteins, while also increasing product concentration in the eluate and maintaining a high percent yield of recovered product.
Owner:NOVARTIS AG
Antihuman transferrin acceptor human source antibody and uses thereof
InactiveCN101245107AAvoiding Immunogenicity IssuesImprove expression levelNervous disorderBacteriaSingle-Chain AntibodiesDrug administration
The invention relates to an anti-human transferrin receptor human antibody and the application, which pertains to the field of molecular biology. The anti-human transferrin receptor human antibody has a single-chain antibody (scFv) amino acid sequence which is shown in a sequence table SEQ ID No.1. The anti-human transferrin receptor human antibody and the application have the advantages that: the human antibody solves the problem of immunogenicity of the target antibody, thus allowing the human antibody to carry out the long-term repeated drug administrations in the human body; the method for screening a genetic engineering antibody in a large-capacity antibody library which is adopted by the invention is easier than the monoclonal antibody preparation, the large-scale production is easy, the molecule of the antibody is small, and the antibody can enter tissues deeply, thus having obvious advantages. A pET-22b (plus) vector which is adopted by the invention contains a stronger T7 promoter, which can ensure the higher expression level of the target antibody. The induced expression conditions after the optimization can ensure that the antibody is expressed in a soluble form and the activity of protein is higher. The 3' end of the pET-22b (plus) vector is provided with six His(histidine) labels, which can easily use the Ni-NTA agarose to carry out affinity chromatography and purification.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
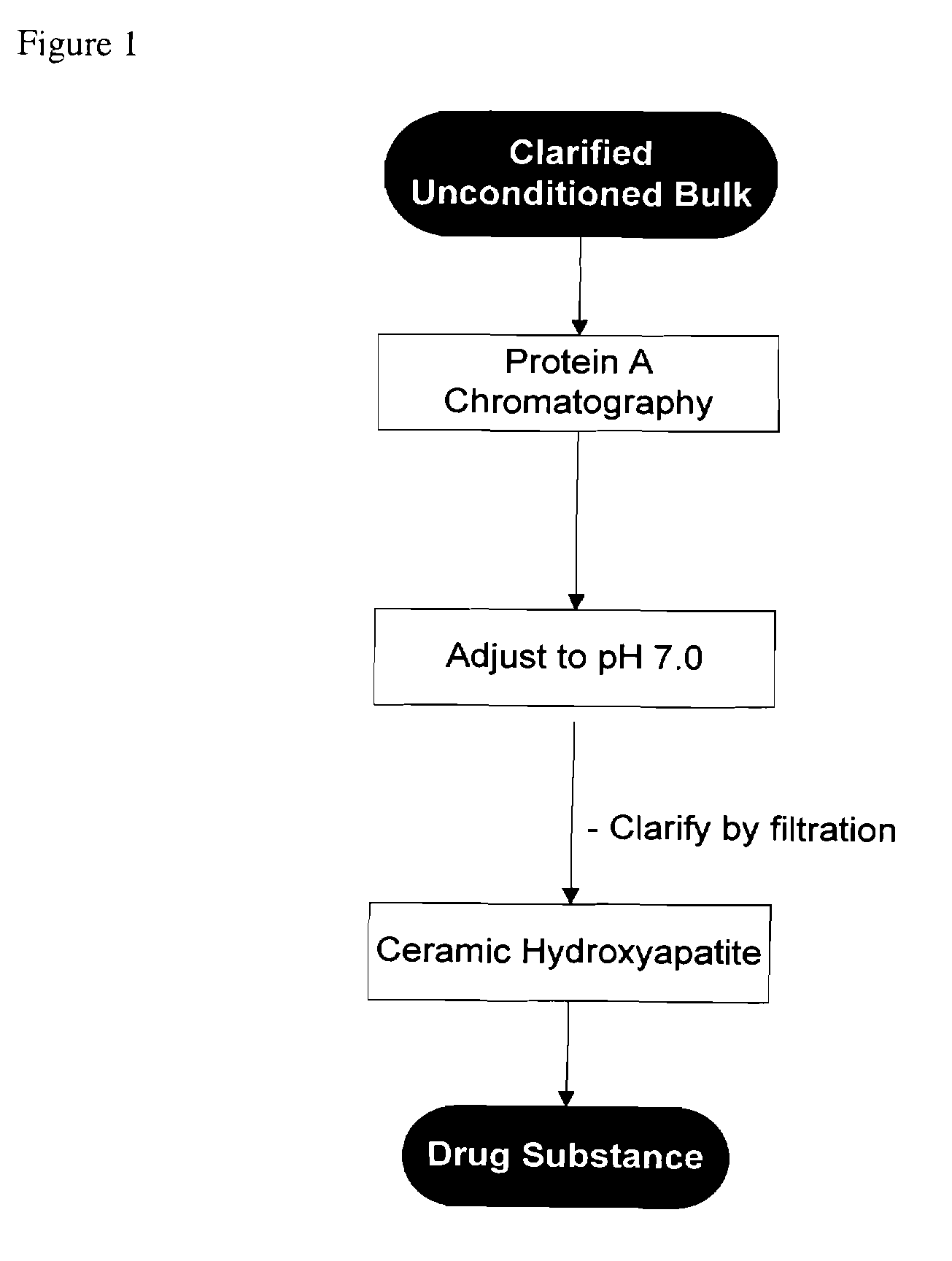
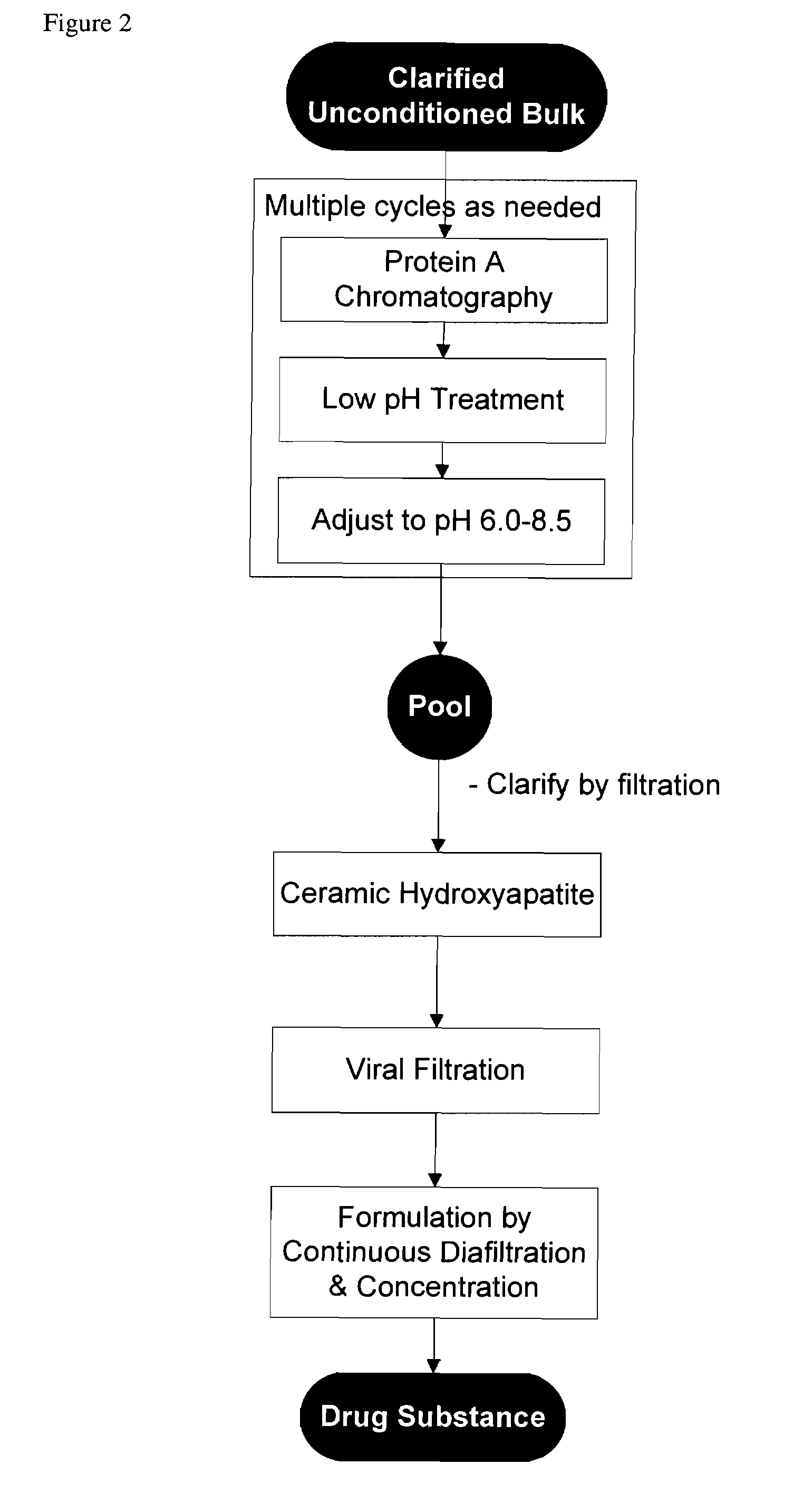
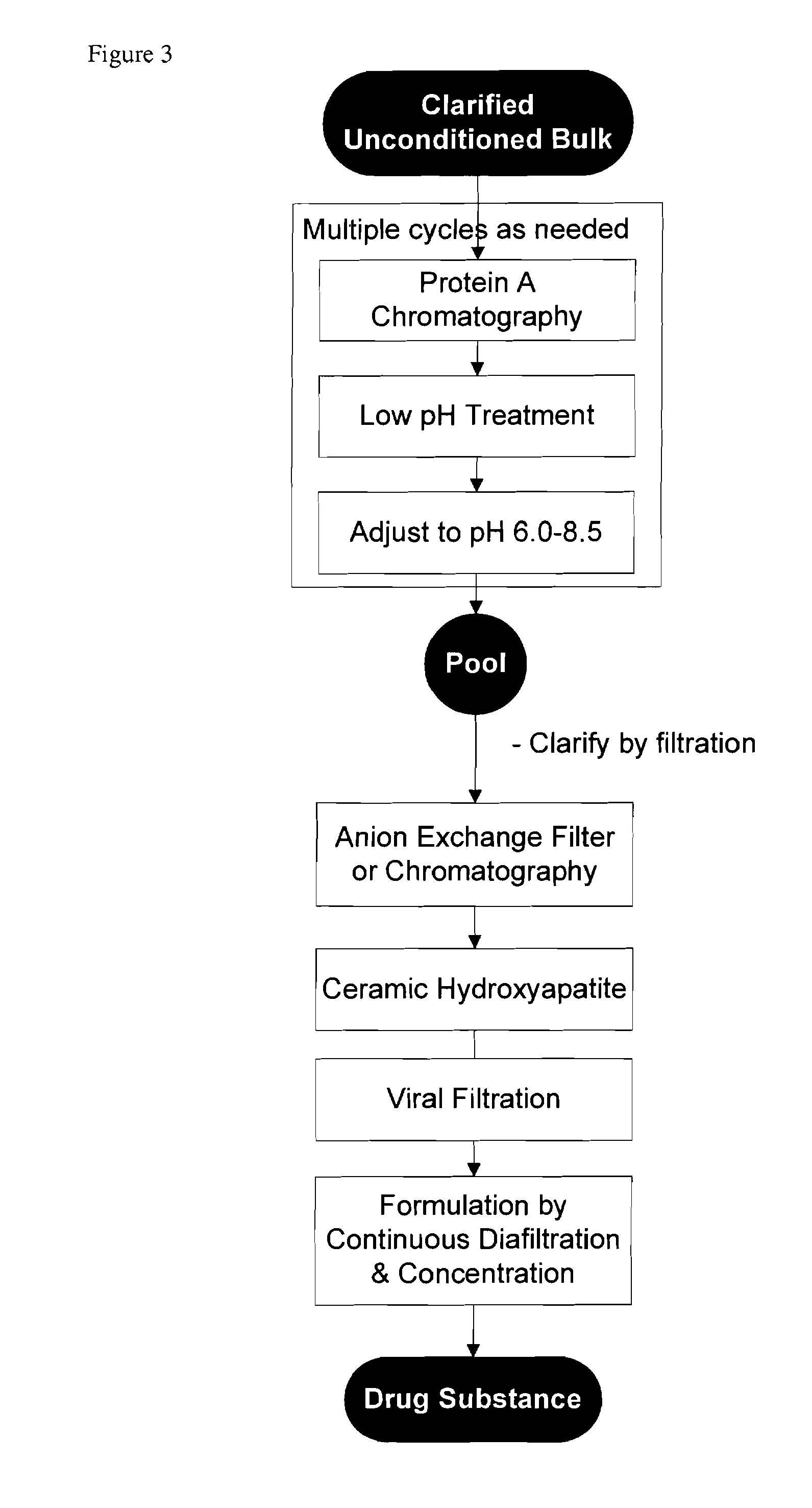
Methods for purifying antibodies using ceramic hydroxyapatite
InactiveUS20100234577A1Increase productionPeptide preparation methodsImmunoglobulinsApatiteCulture fluid
This invention relates to the purification of monoclonal antibodies from mammalian cell culture fluid utilizing sequential, orthogonal chromatography and filtration techniques resulting in material of high purity and quality that is suitable for human administration. The method involves capturing an IgG product using immobilized protein A affinity chromatography, followed by at least one ion exchange technique prior to adsorbing the IgG to hydroxyapatite and selectively eluting the product in a single isocratic step to achieve purification from impurities and simultaneously reducing multiple types of impurities including but not limited to IgG aggregates, residual protein A, non-IgG proteins, host cell proteins, viral particles, and DNA
Owner:SMITHKLINE BECKMAN CORP
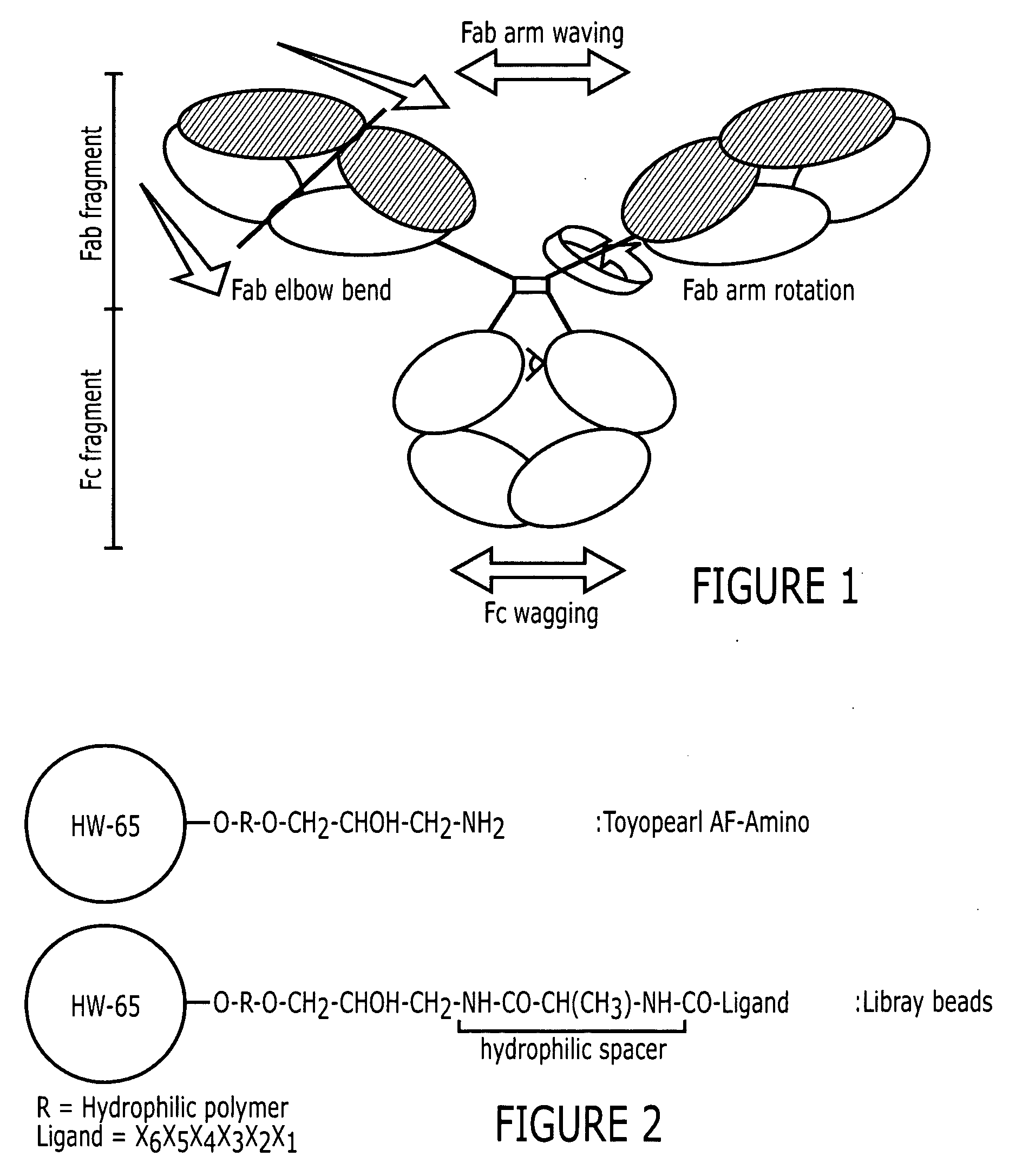
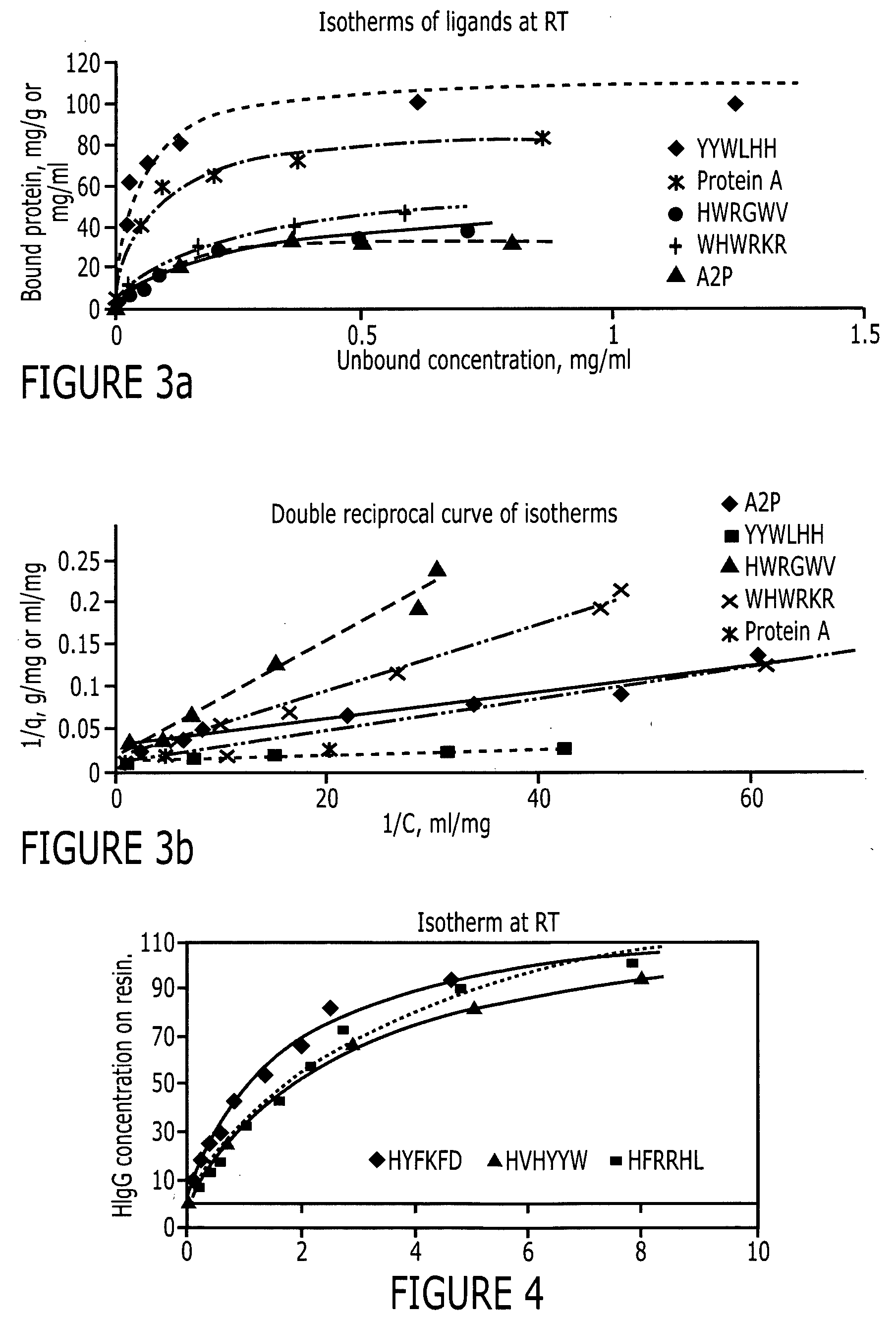
Purification of immunoglobulins using affinity chromatography and peptide ligands
ActiveUS20060153834A1High affinityPeptide librariesPeptide/protein ingredientsCarboxyl radicalPeptide ligand
An immunoglobulin binding peptide having the general formula, from amino terminus to carboxy terminus, of Z-R1—R2—R3—R4—R5—R6—X, is described, wherein: R1 is H or Y; R2 is a hydrophobic, preferentially aromatic, amino acid (for example W, F, Y, V); R3 is a positively charged or aromatic amino acid (for example R, H, F, W); R4 is a hydrophobic or positively charged amino acid (for example G, Y, R, K, L); R5 is a positively charged or aromatic amino acid (for example W, F, R, H, Y); R6 a random amino acid but preferably hydrophobic or negatively charged (for example V, W, L, D, H); X is present or absent and when present is a linking group; and Z is present or absent and when present is a capping group bonded to the N terminus of R1; and wherein the amino acids of said peptide are in D form, L form, or a combination thereof. Methods of using such peptides for the purification of Immunoglobulins are also described
Owner:NORTH CAROLINA STATE UNIV
Antibody purification by protein a and ion exchange chromatography
InactiveUS7847071B2Serum immunoglobulinsSolid sorbent liquid separationIon chromatographyProtein insertion
A novel method for selectively removing leaked protein A from antibody purified by means of protein A affinity chromatography is disclosed.
Owner:LONZA BIOLOGICS PLC
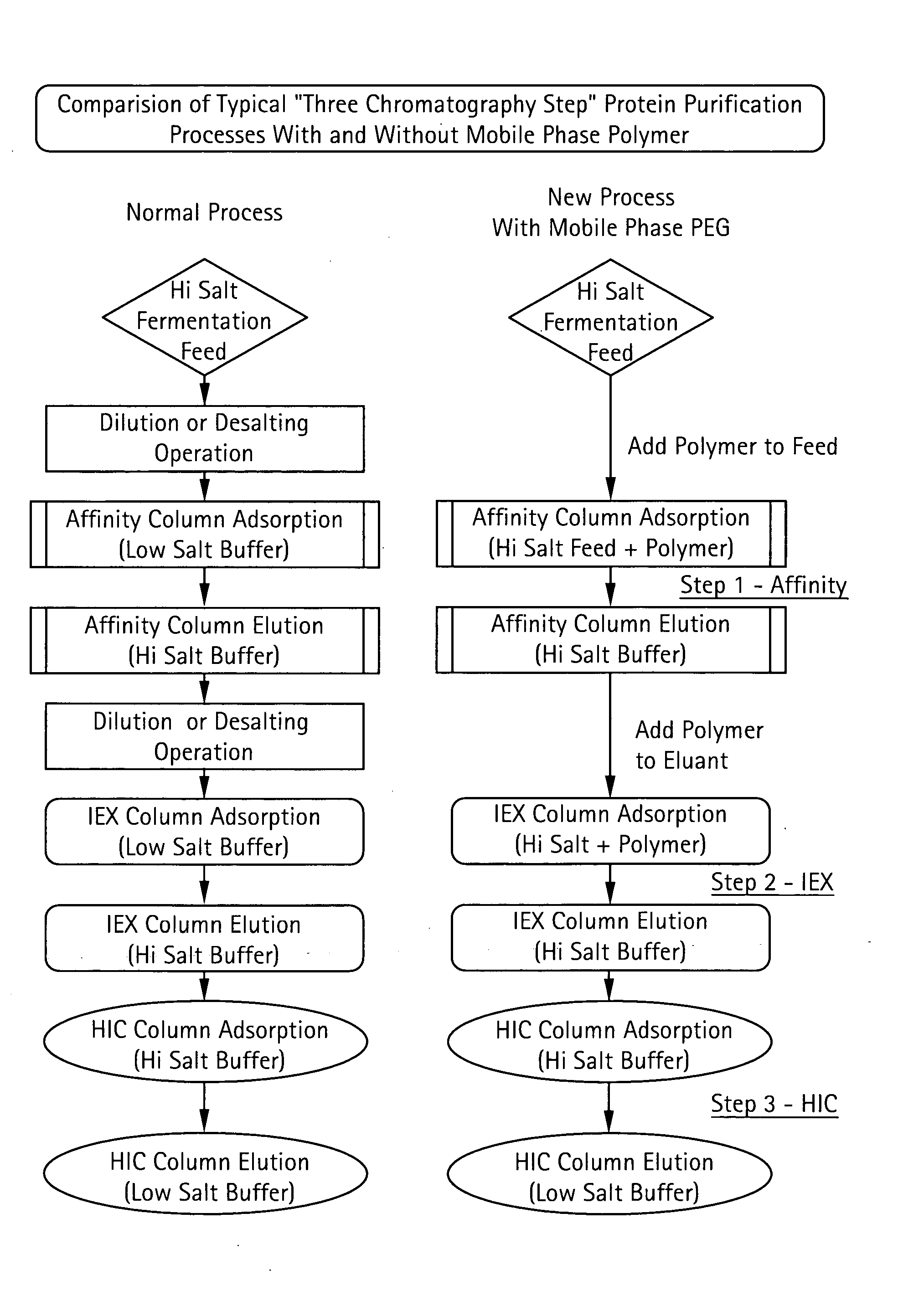
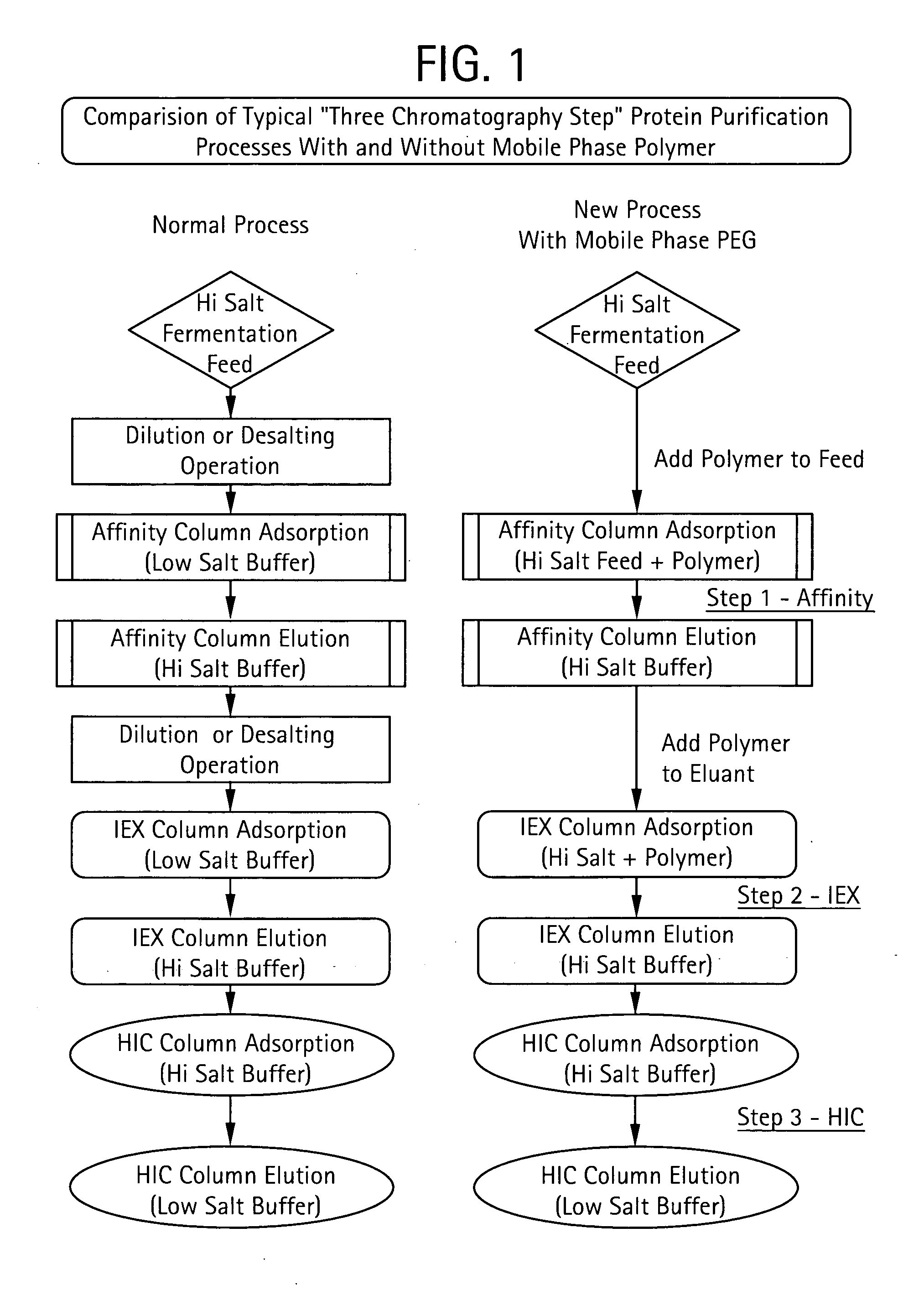
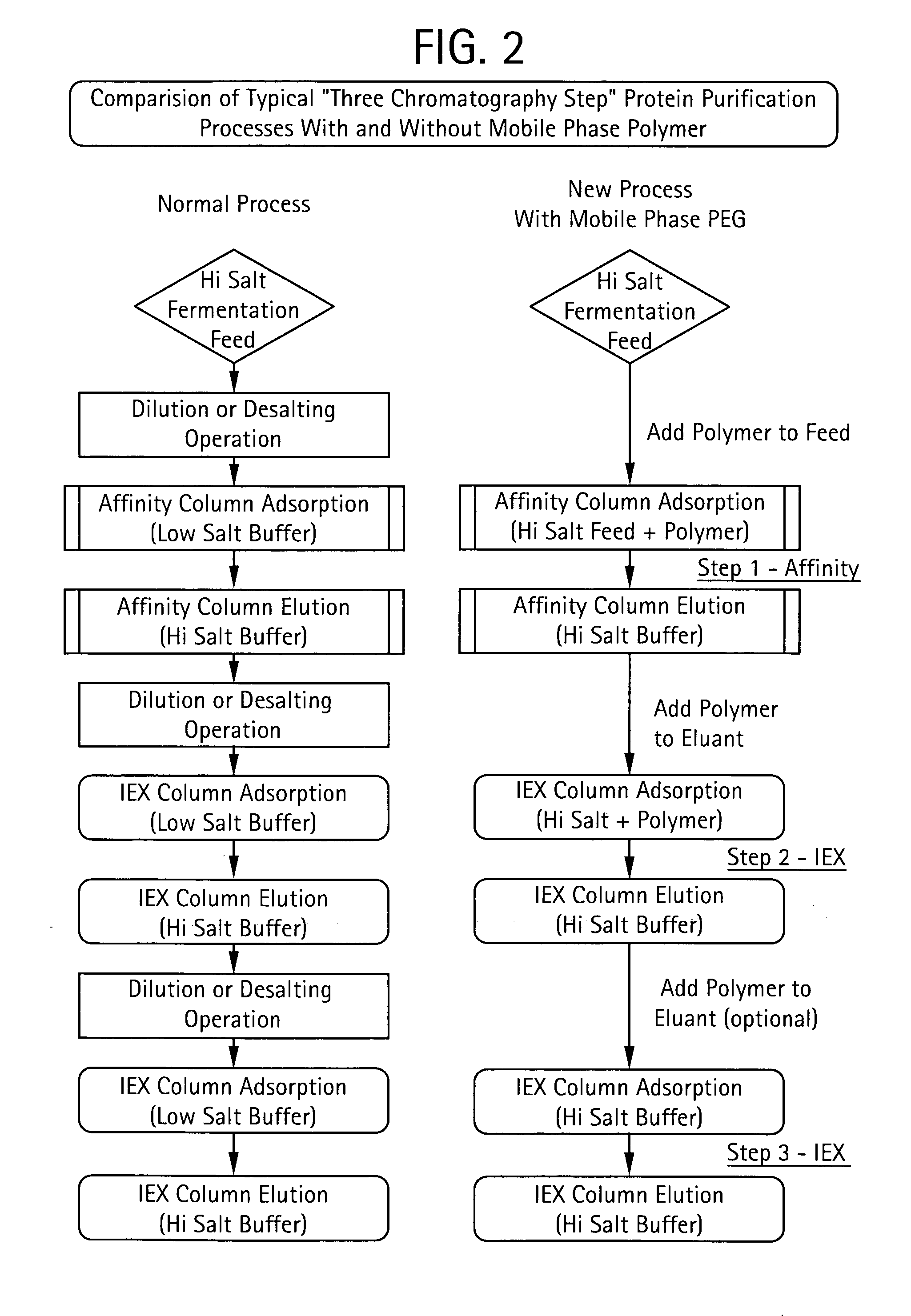
Method for Chromatographic Purification
InactiveUS20070213513A1Improve efficiencyReduce stepsCation exchanger materialsComponent separationIon exchangePolyethylene glycol
The present invention relates to a method of isolating a target compound from other components of a liquid, which method comprises at least two chromatographic steps, in any sequence of order, wherein the mobile phase is contacted with an affinity chromatography matrix and / or an ion-exchange chromatography matrix and / or a hydrophobic interaction chromatography matrix, wherein the contacting with at least one of the matrices takes place in the presence of at least one non-ionic polyether; and obtaining the target compound(s) in a separate fraction from the last chromatographic step. In the most preferred embodiment, the non-ionic polyether is poly(ethylene glycol) (PEG).
Owner:GE HEALTHCARE BIO SCI CORP
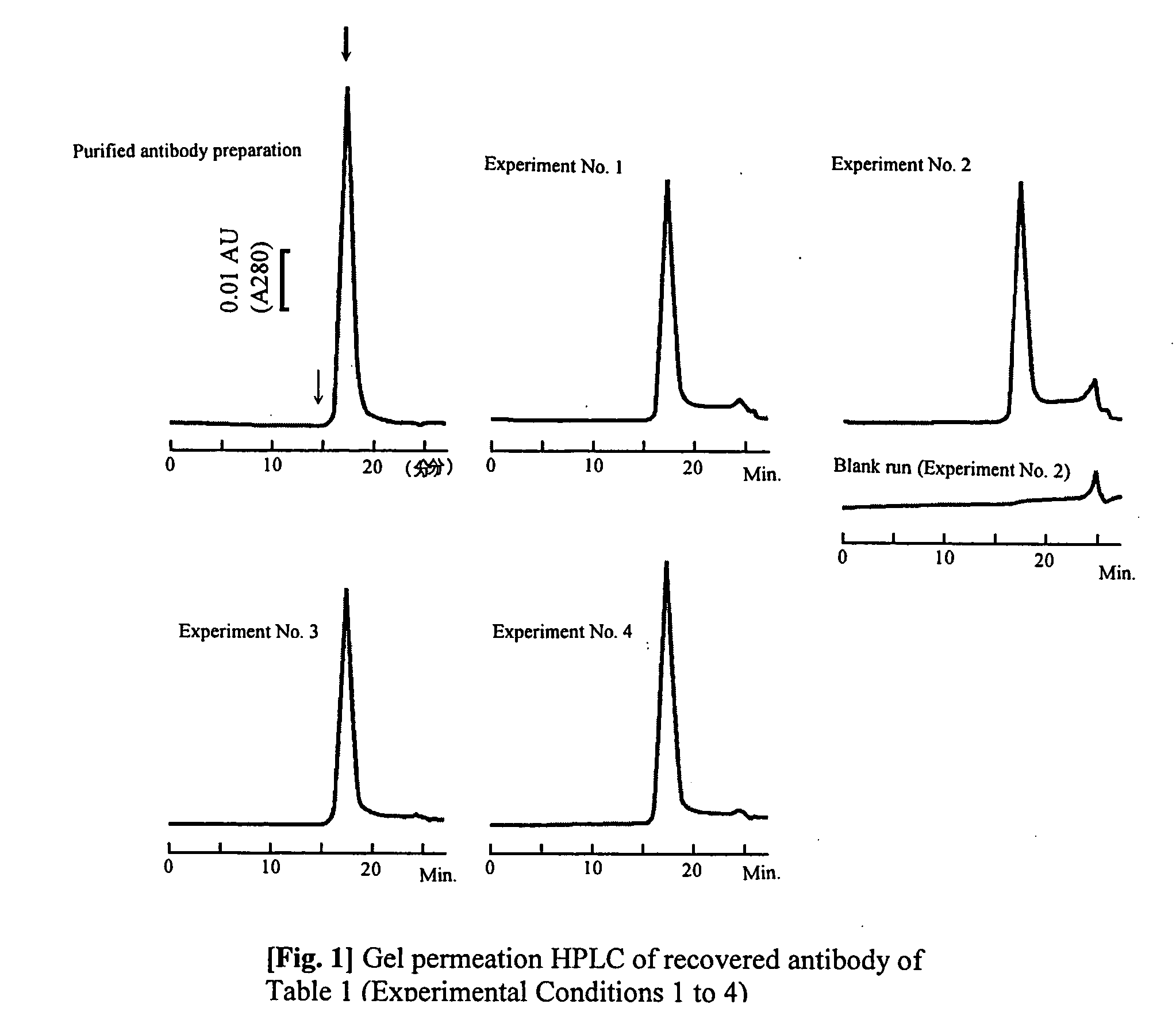
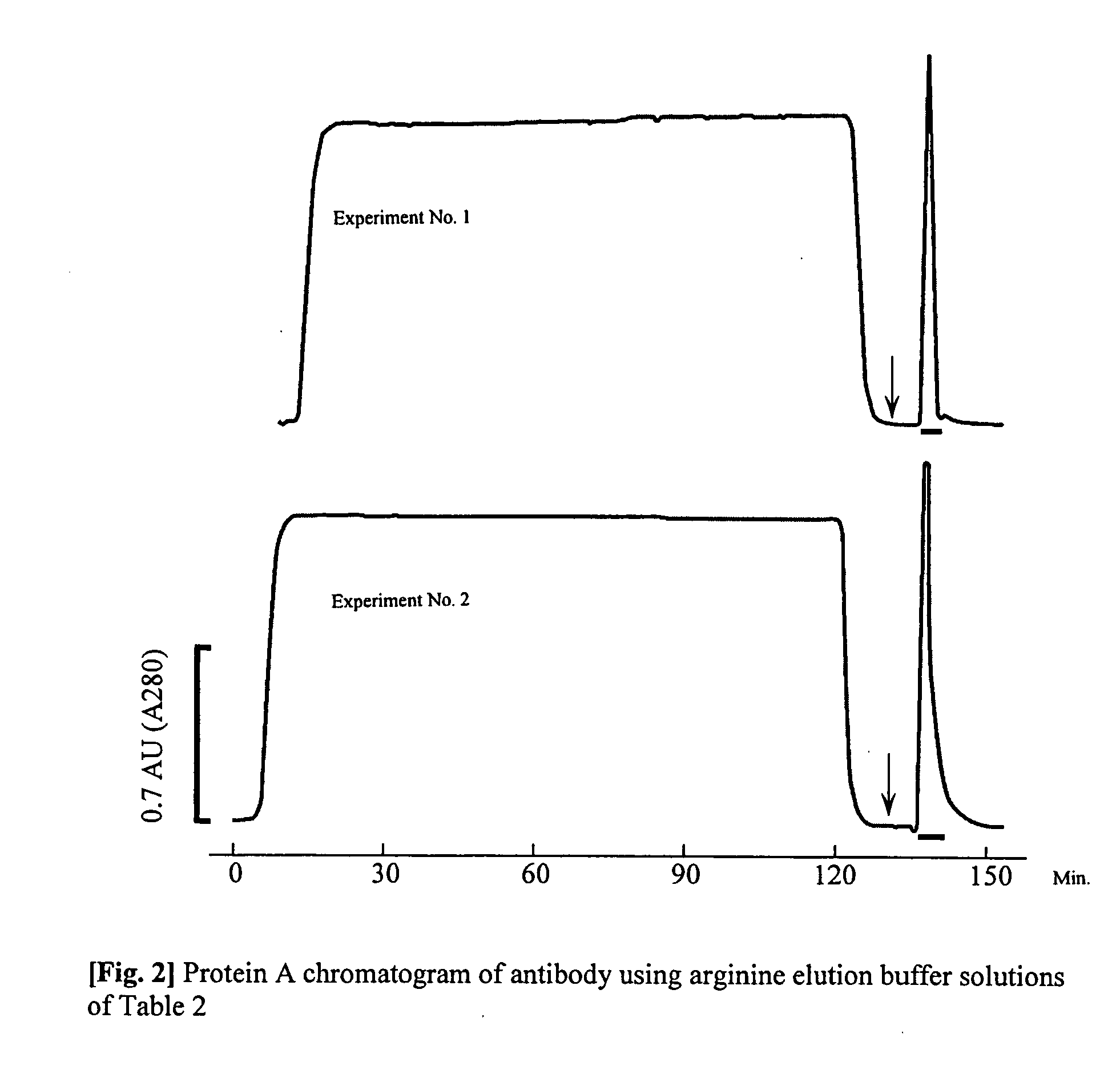
Purification method which prevents denaturation of an antibody
ActiveUS20050176109A1Improve efficiencyInhibit purificationImmunoglobulins against blood coagulation factorsSerum immunoglobulinsPurification methodsElution
The present invention provides a method of purifying an antibody by protein A affinity chromatography. More specifically, the present invention provides a technique relating to an elution buffer solution which provides a good antibody recovery rate without denaturation.
Owner:AJINOMOTO CO INC
Control System For Simulated Moving Bed Chromatography
ActiveUS20080053917A1Simple and easily programmable controlPromote repairSolid sorbent liquid separationWater/sewage treatmentControl systemSimulated moving bed
The present invention provides devices and methods for micro-scale simulated moving bed chromatography (SMB) for continuous preparation of analytic quantities of highly pure fractions of target molecules. The present apparatus and method of the invention is adapted in a preferred embodiment to separations by affinity chromatography involving three discontinuous liquid flow loops. An alternative embodiment of affinity chromatography utilizes standard SMB operating under isocratic conditions.
Owner:TOSOH BIOSCIENCE LLC
Nucleic acid separation using immobilized metal affinity chromatography
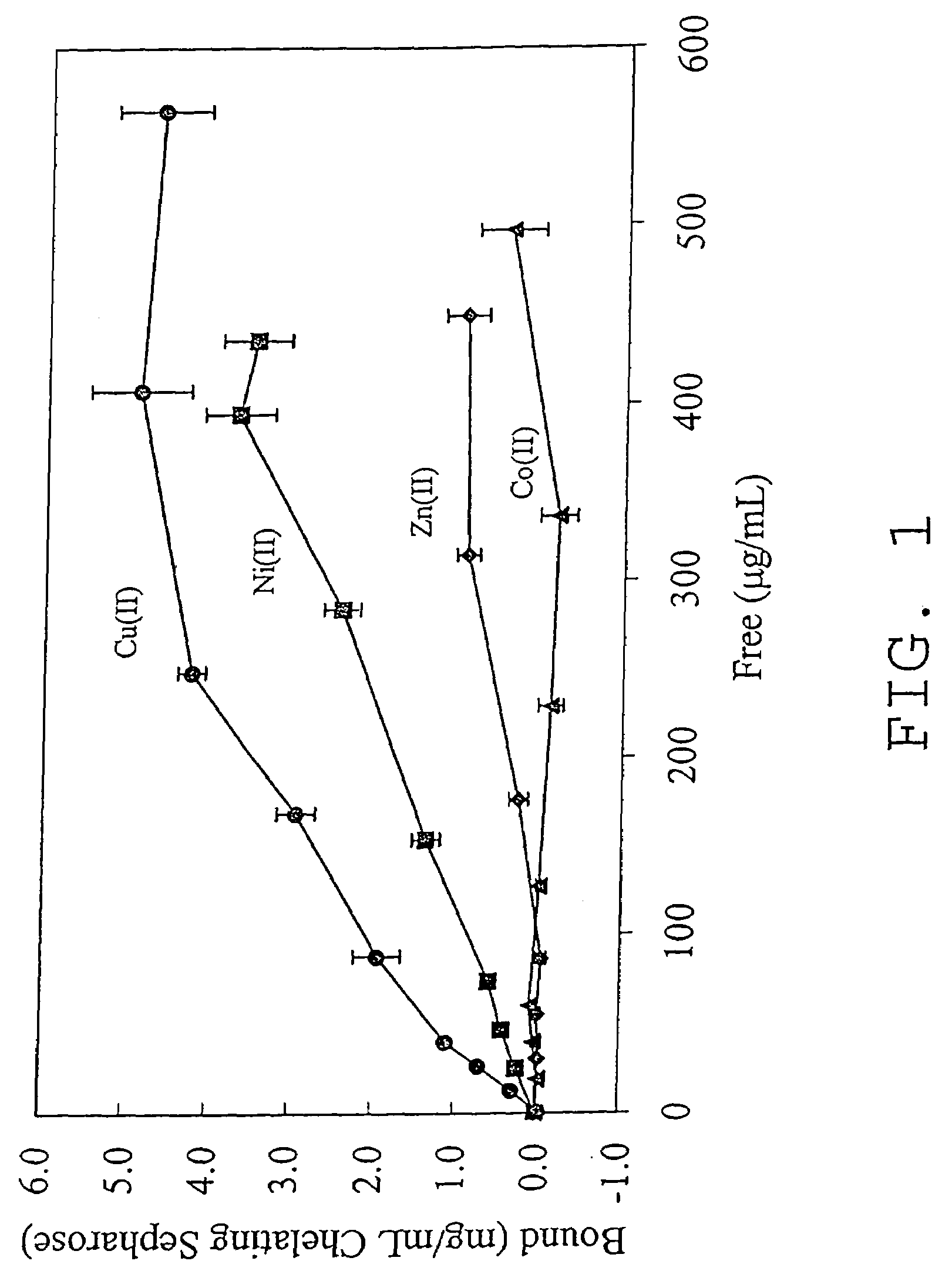
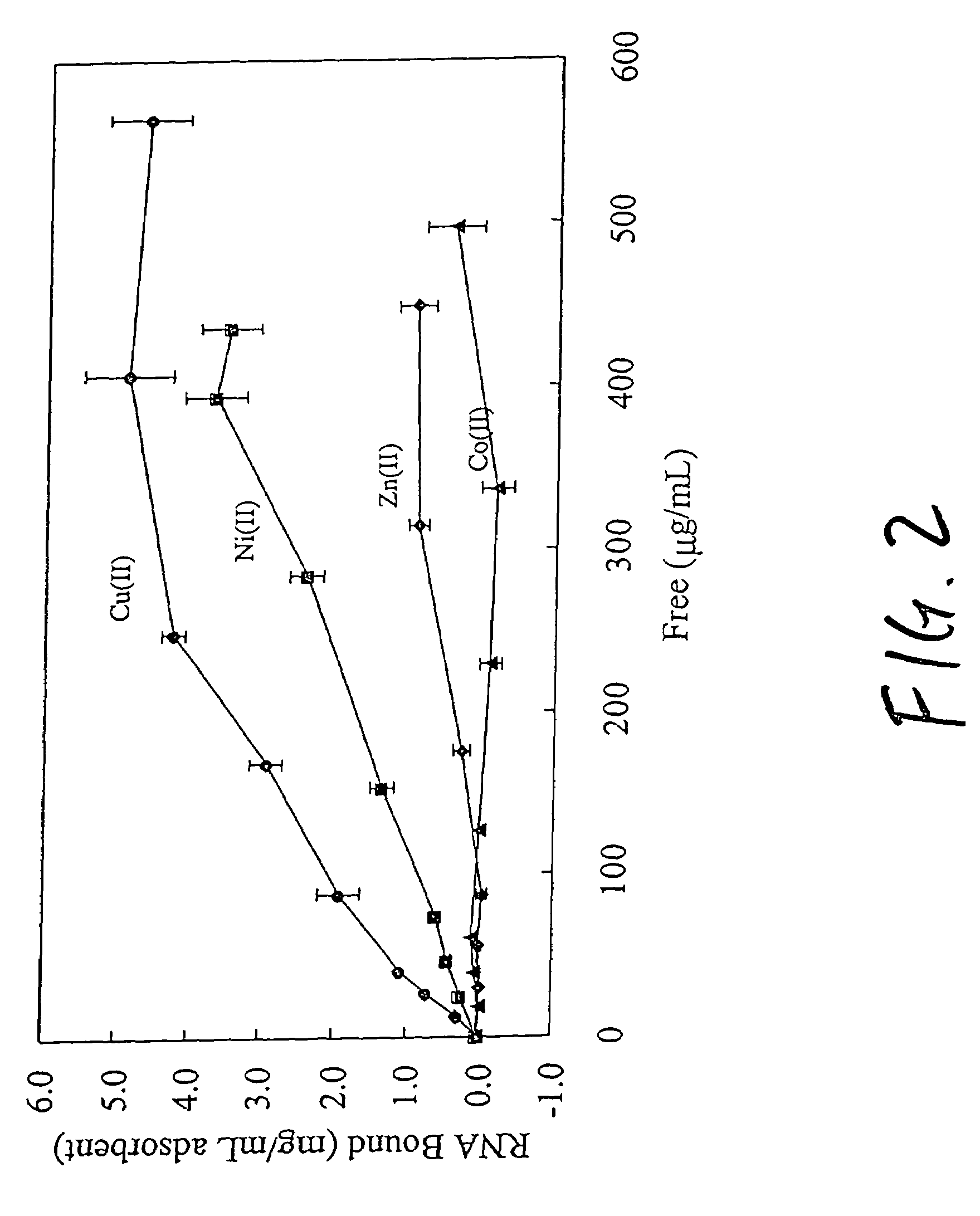
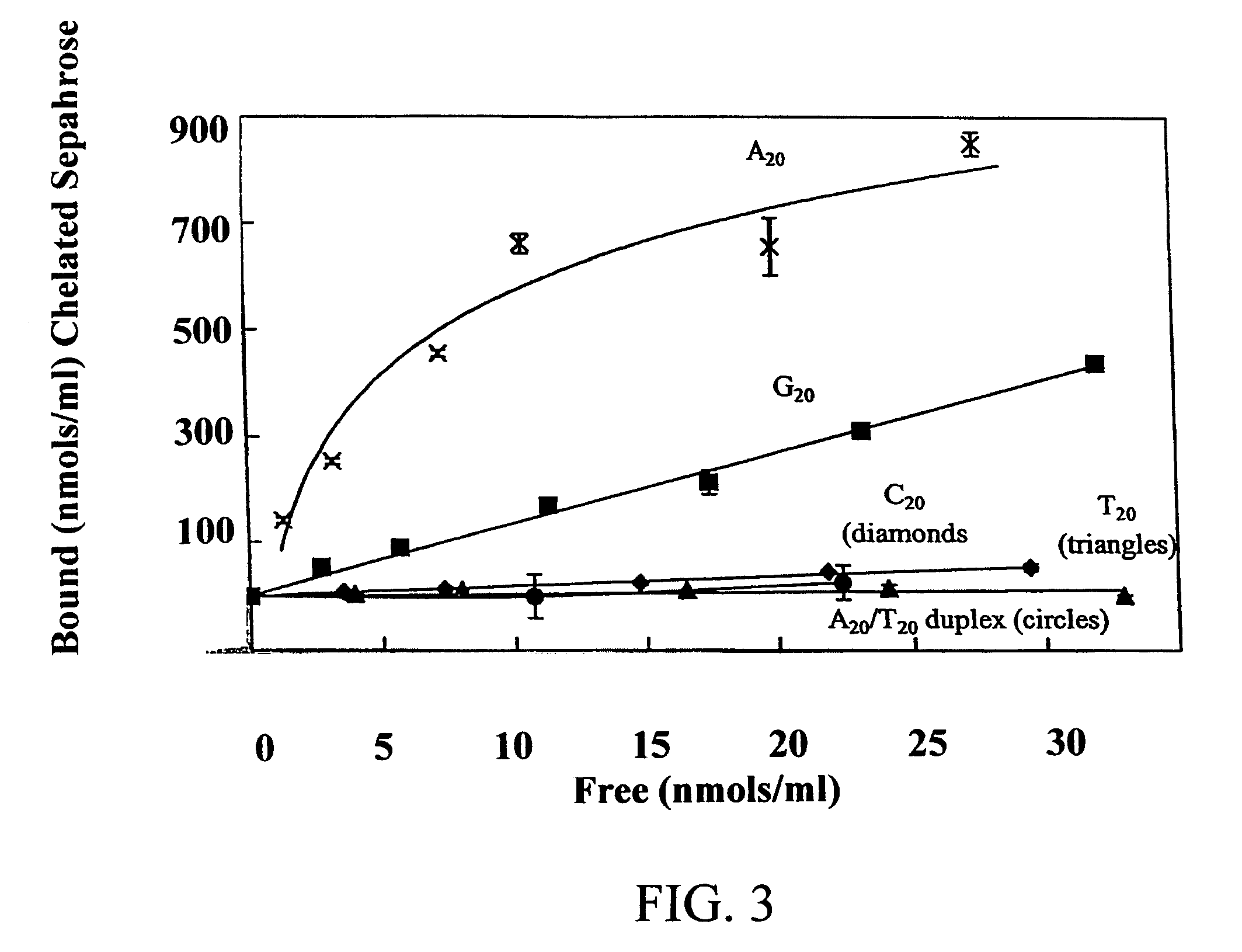
An immobilized metal affinity chromatography (IMAC) method for separating and / or purifying compounds containing a non-shielded purine or pyrimidine moiety or group such as nucleic acid, presumably through interaction with the abundant aromatic nitrogen atoms in the purine or pyrimidine moiety. The method can also be used to purify compounds containing purine or pyrimidine moieties where the purine and pyrimidine moieties are shielded from interaction with the column matrix from compounds containing a non-shielded purine or pyrimidine moiety or group. Thus, double-stranded plasmid and genomic DNA, which has no low binding affinity can be easily separated from RNA and / or oligonucleotides which bind strongly to metal-charged chelating matrices. IMAC columns clarify plasmid DNA from bacterial alkaline lysates, purify a ribozyme, and remove primers and other contaminants from PCR reactions. The metal ion affinity of yeast RNA decreases in the order: copper (II), nickel (II), zinc (II), and cobalt (II).
Owner:UNIV HOUSTON SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com