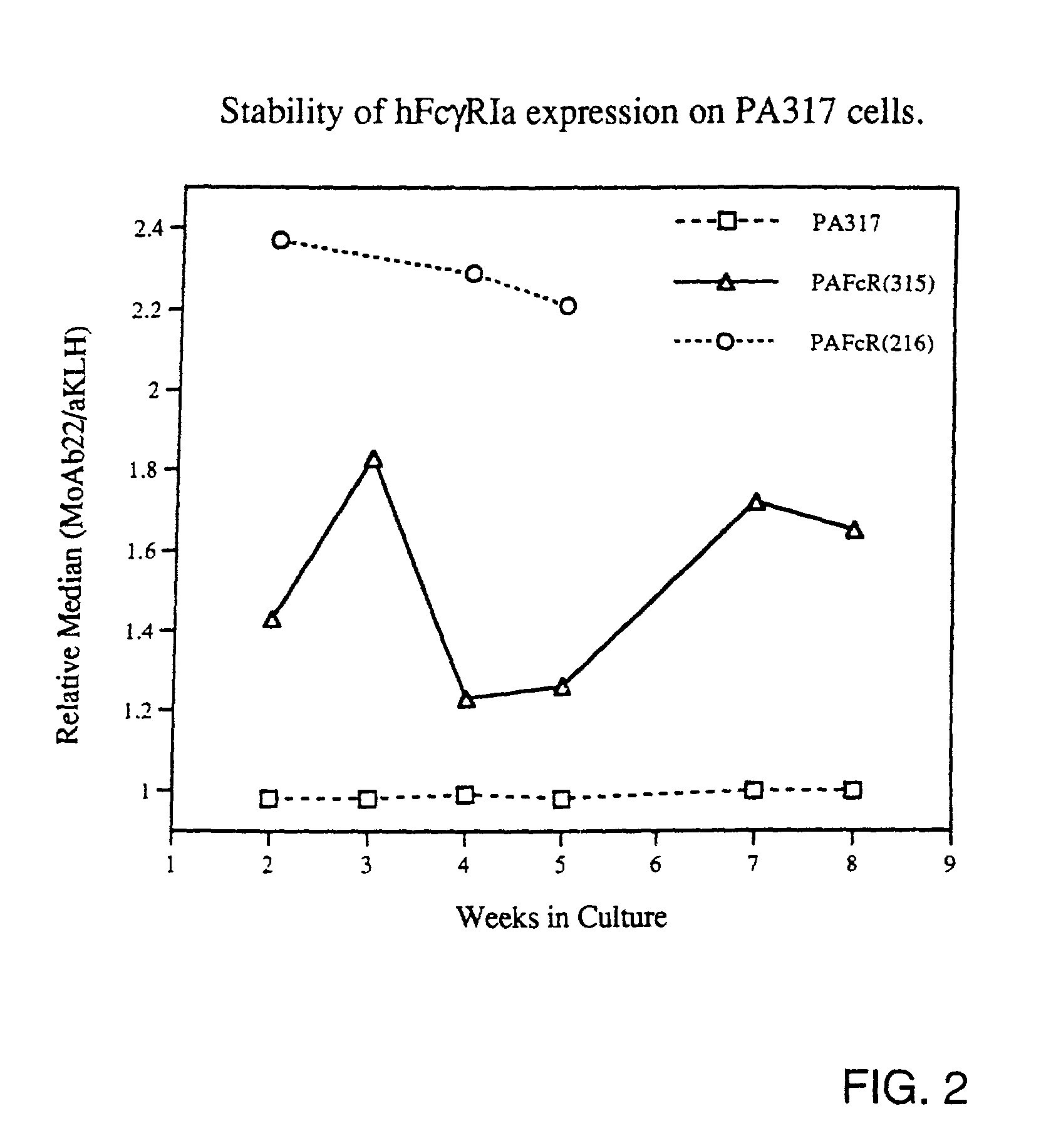
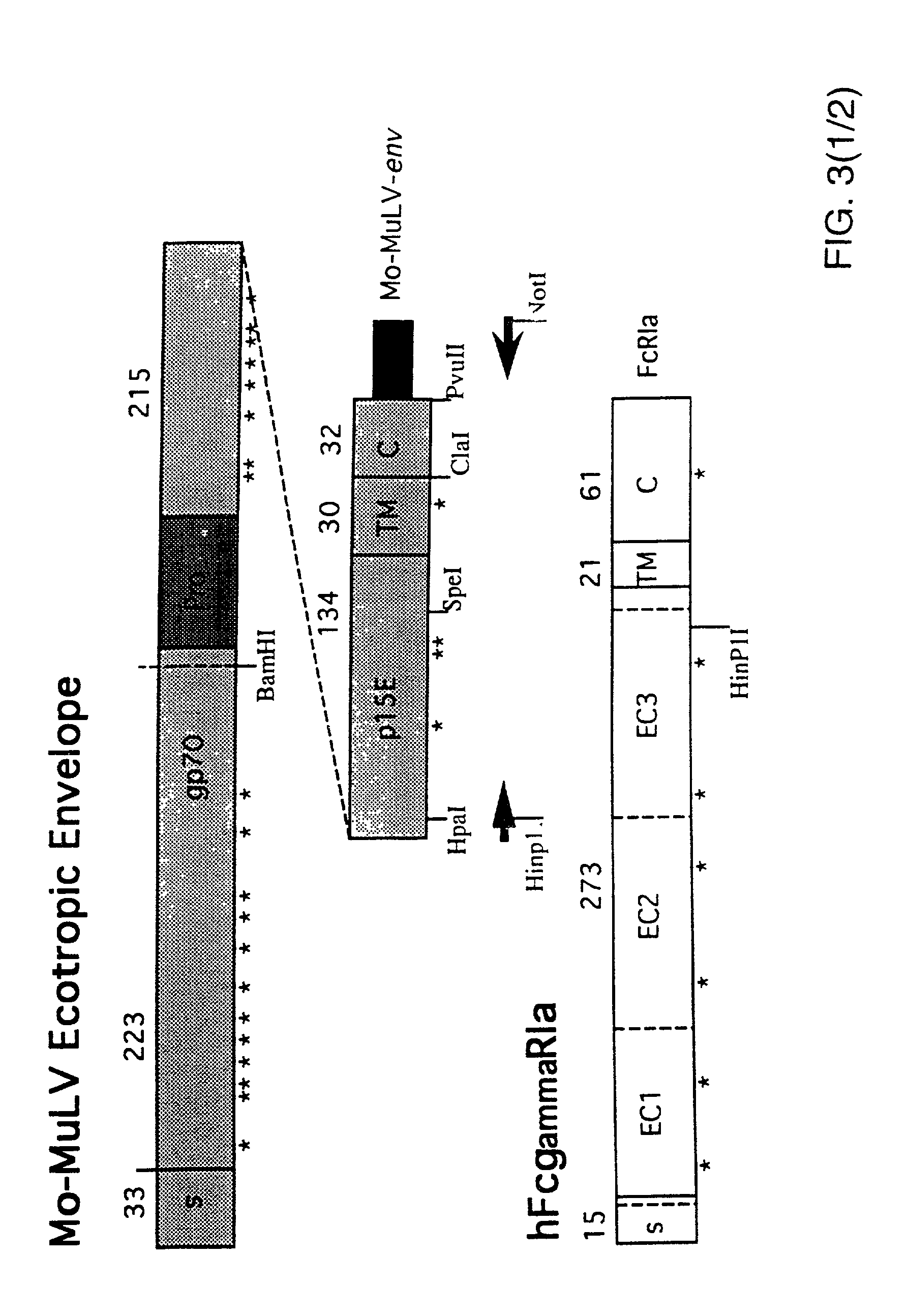
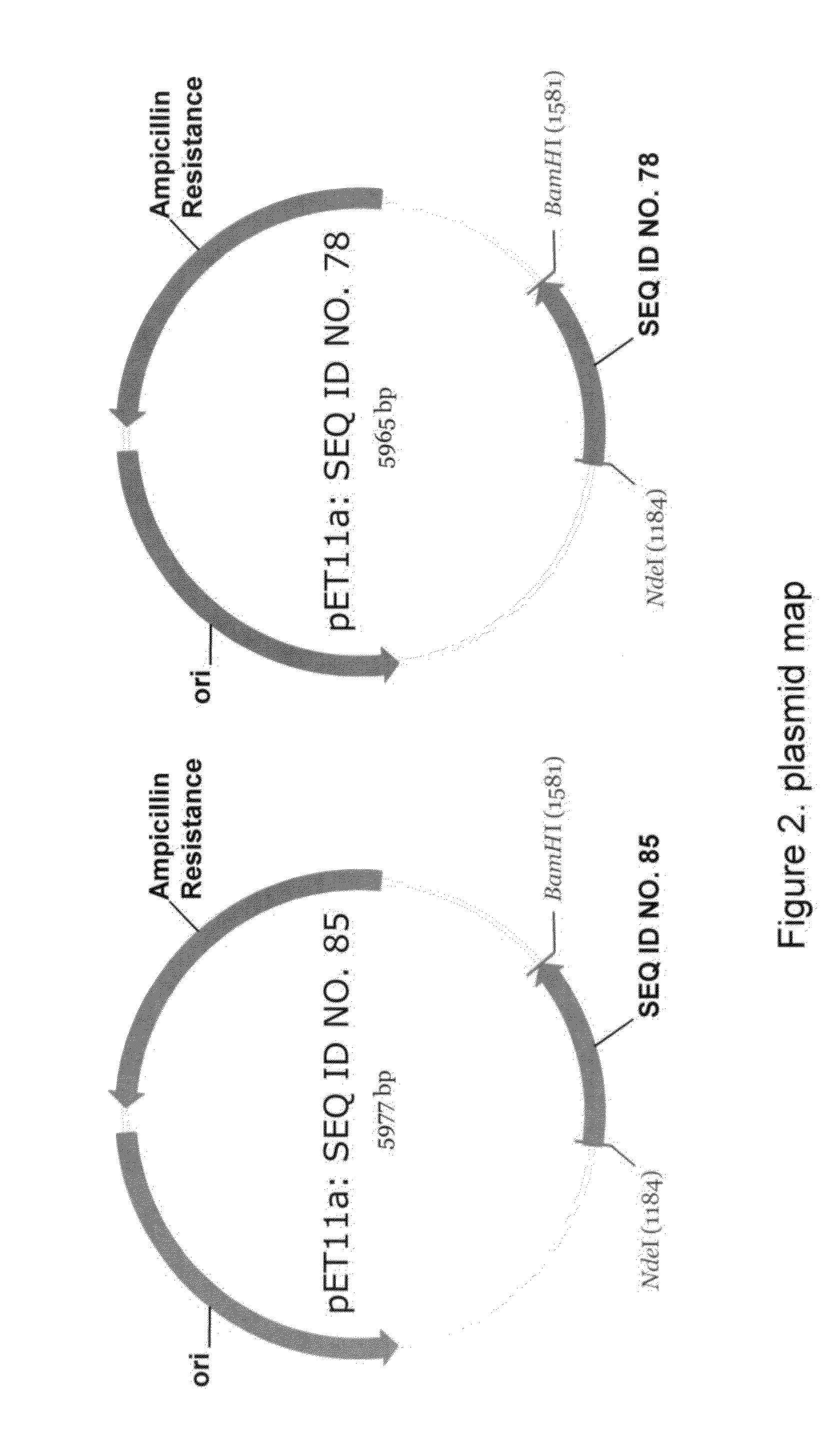
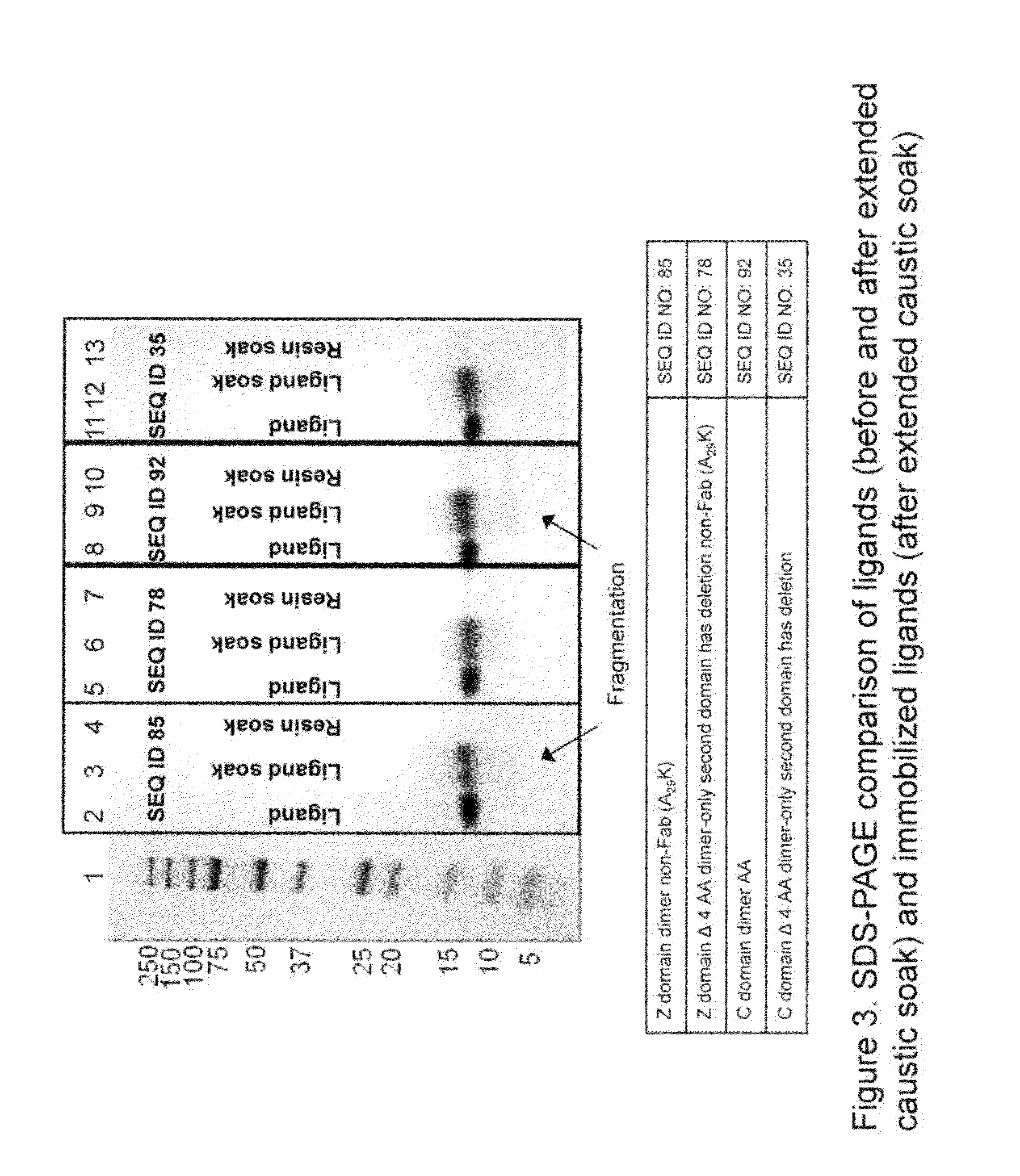
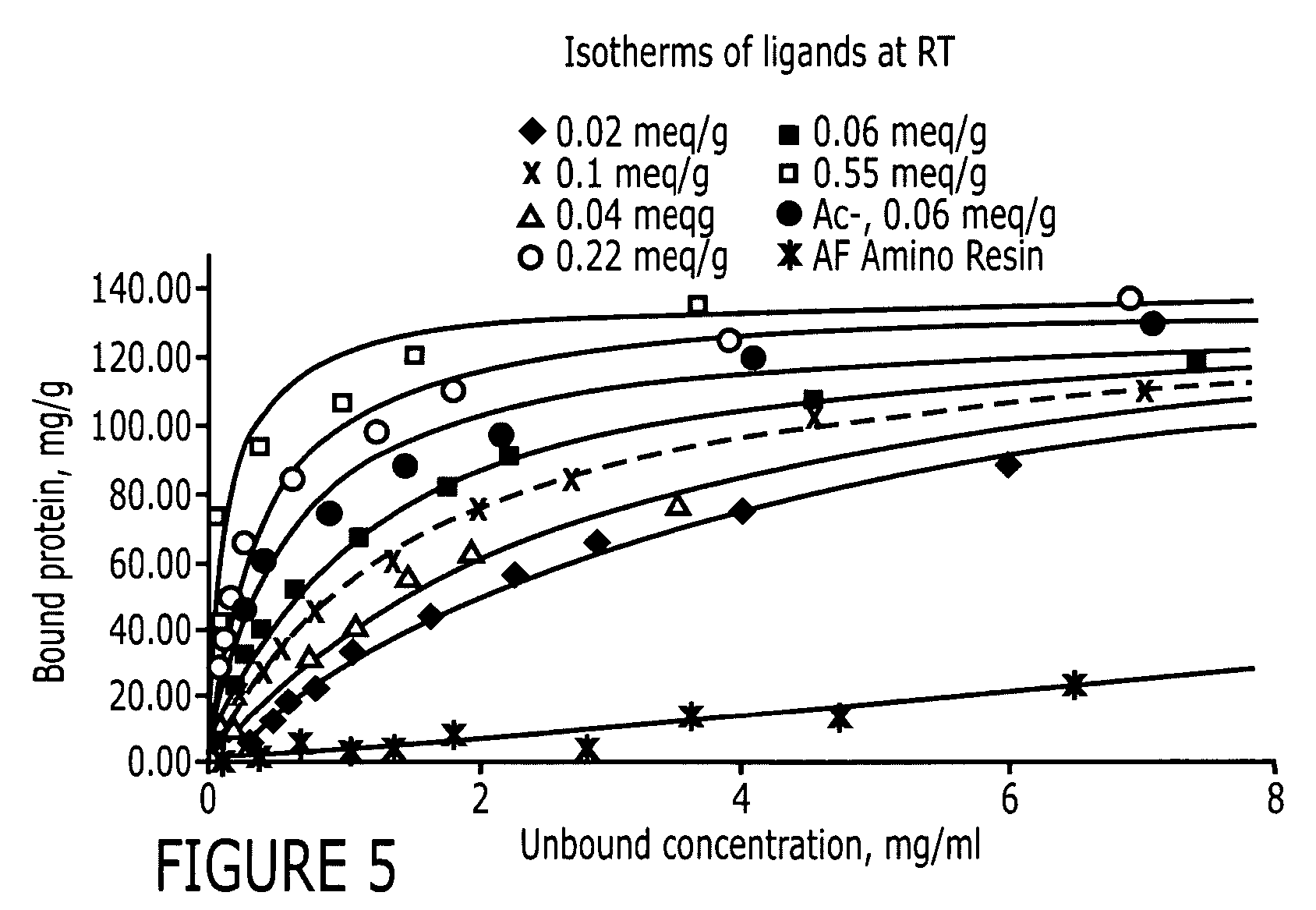
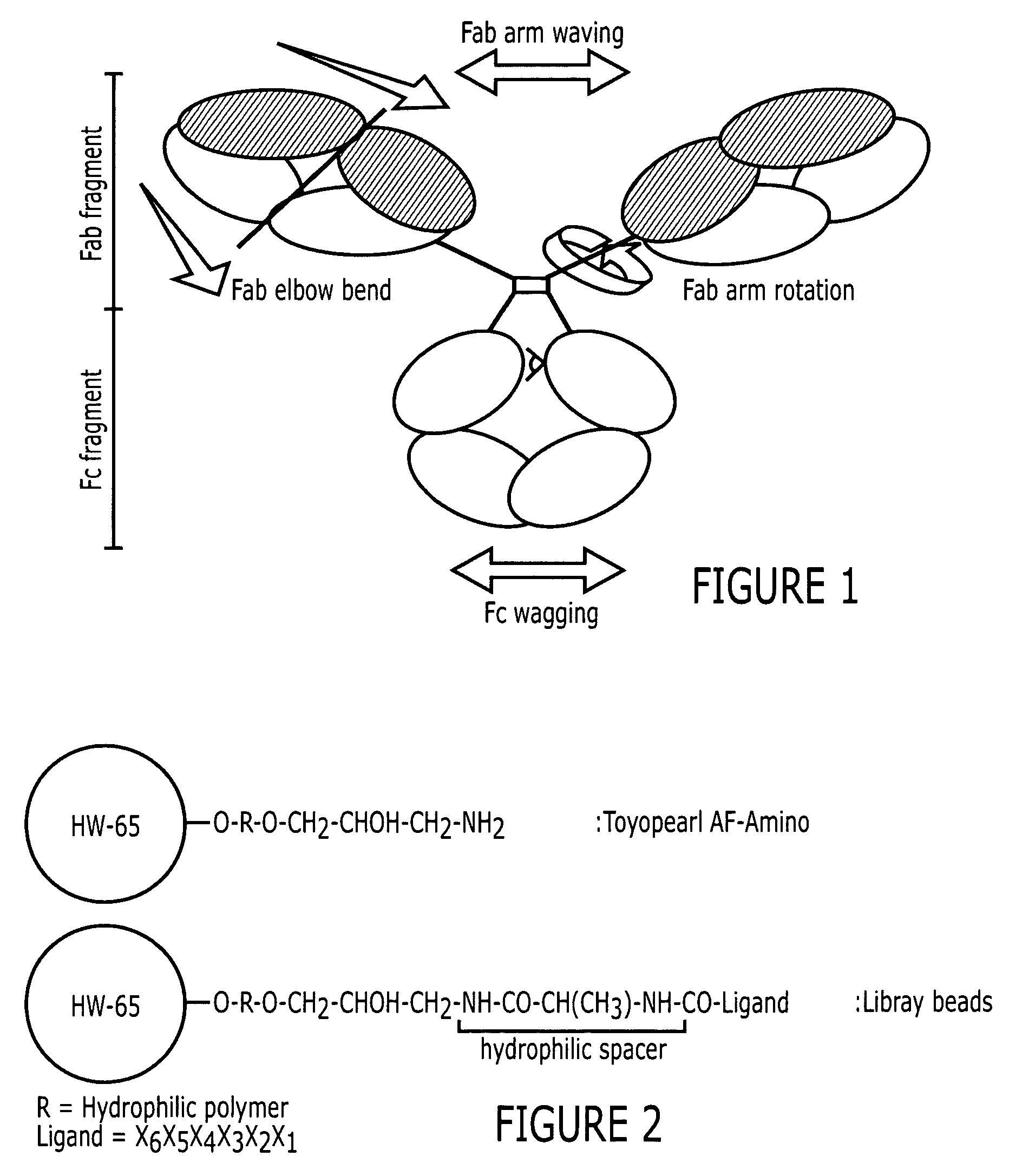
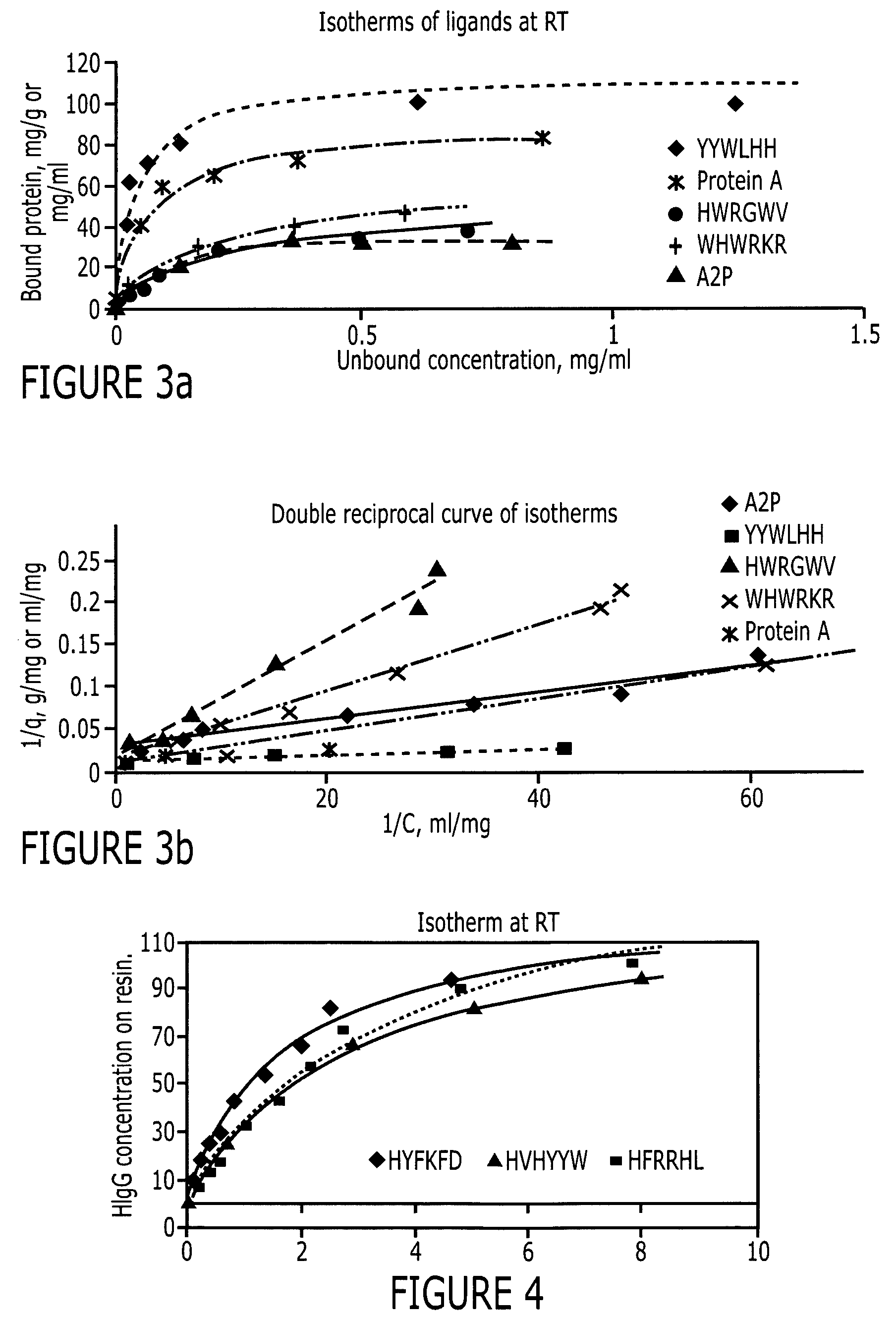
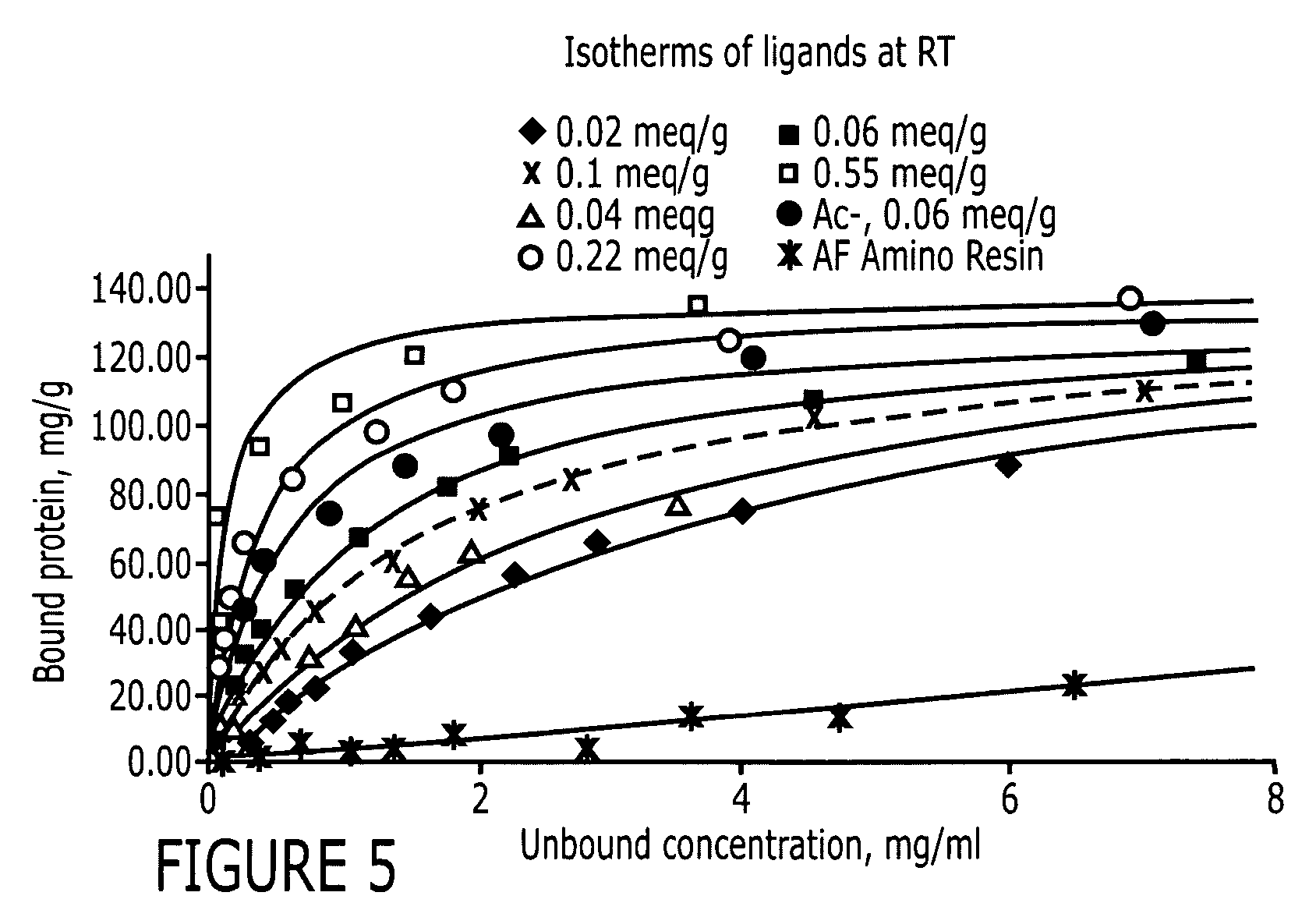
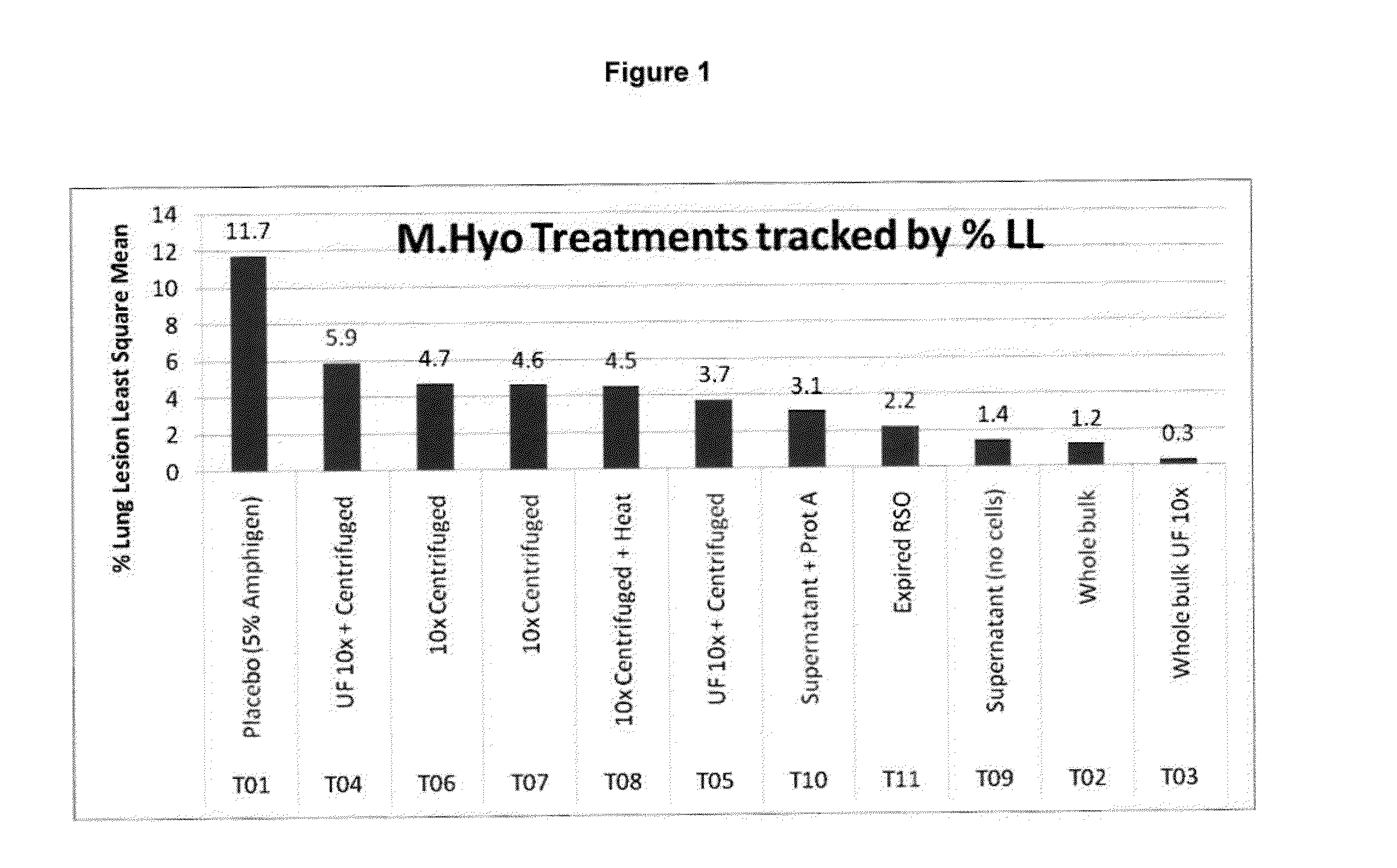
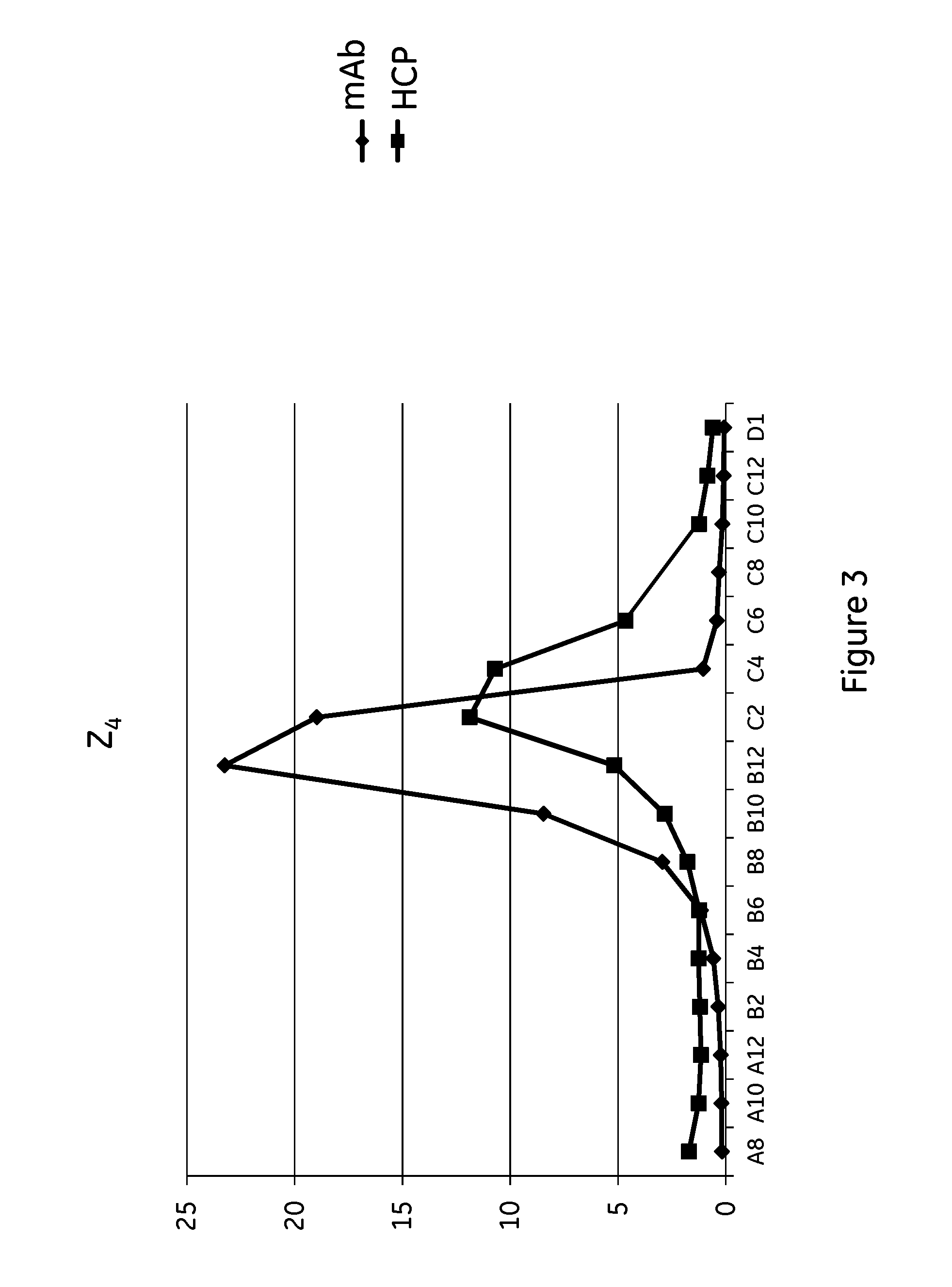
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
138 results about "Immunoglobulin binding" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Interacting selectively and non-covalently with an immunoglobulin. [GOC:ma]
Isolation of proteins
InactiveUS20050176122A1Other chemical processesSolid sorbent liquid separationSpecial classCarboxylic acid
Owner:UPFRONT CHROMATOGRAPHY
Isolation of proteins
InactiveUS6919436B2Other chemical processesSolid sorbent liquid separationSpecial classCarboxylic acid
The present invention relates to a novel method for the isolation or purification of immunoglobulins (a special class of proteins) from a solution containing immunoglobulins, e.g. hybridoma cell culture supernatants, animal plasma or sera, or colostrum. The method includes the use of a minimum of salts, such as lyotropic salts, in the binding process and preferably also the use of small amounts of organic solvents in the elution process. The solid phase matrices, preferably epichlorohydrin activiated agarose matricees, are functionalised with mono- or bicyclic aromatic or heteroaromatic ligands (molecular weight: at the most 500 Dalton) which, preferably, comprises an acidic substituent, e.g. a carboxylic acid. The matrices utilised show excellent properties in a “Standard Immunoglobulin Binding Test” and in a “Monoclonal Antibody Array Binding Test” with respect to binding efficiency and purity, and are stable in 1M NaOH.
Owner:UPFRONT CHROMATOGRAPHY
Half immunoglobulin binding proteins and uses thereof
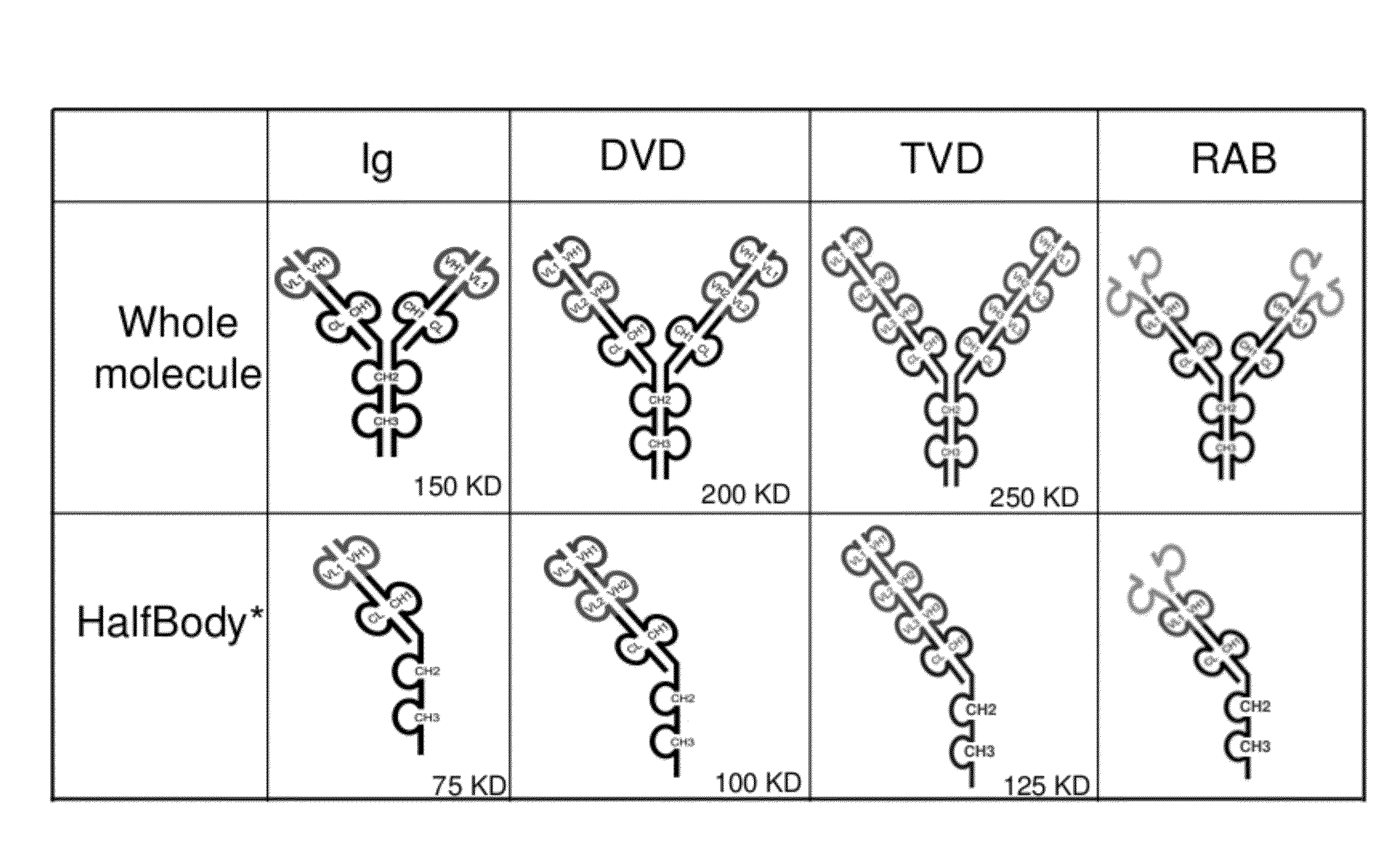
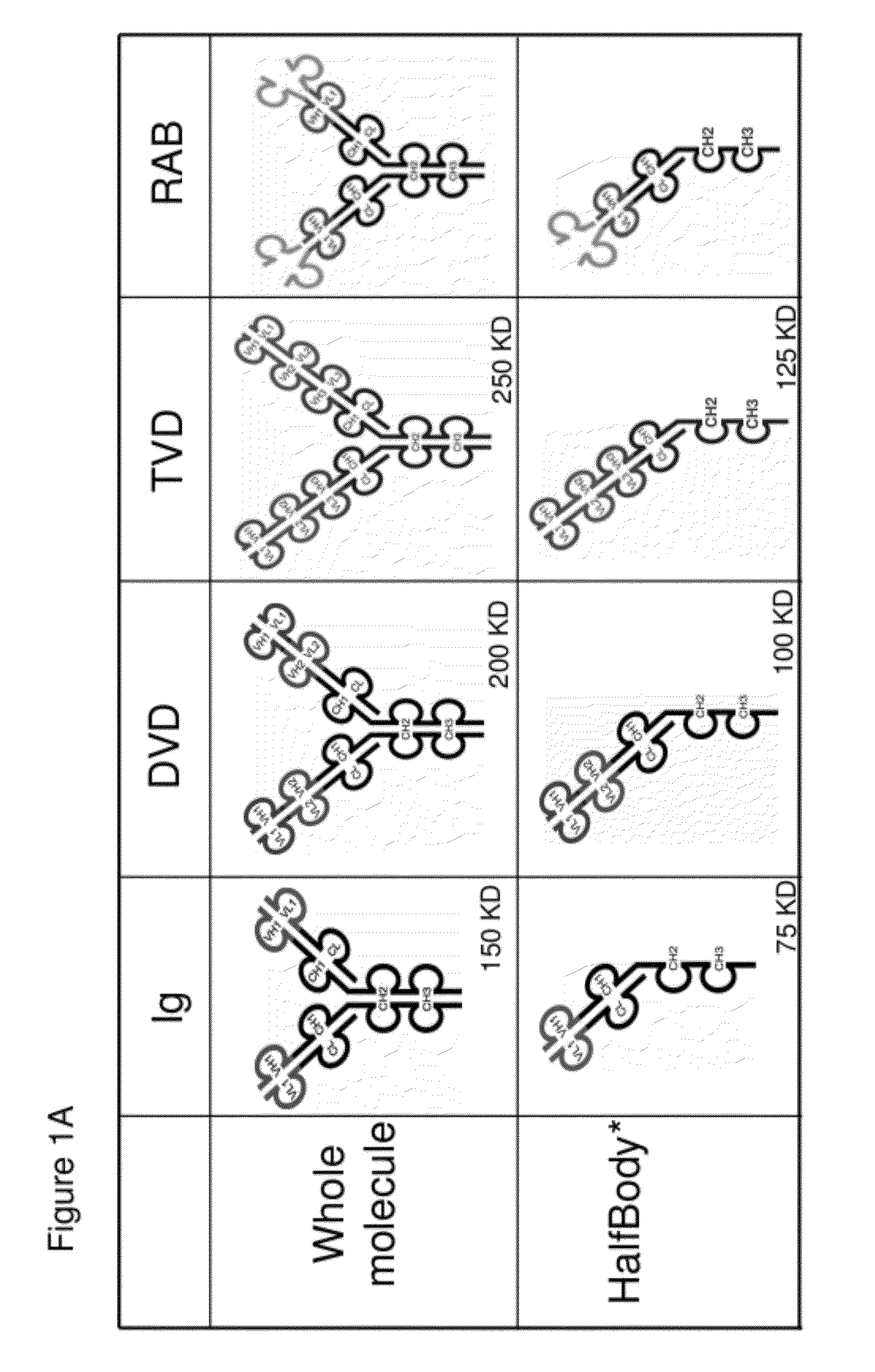
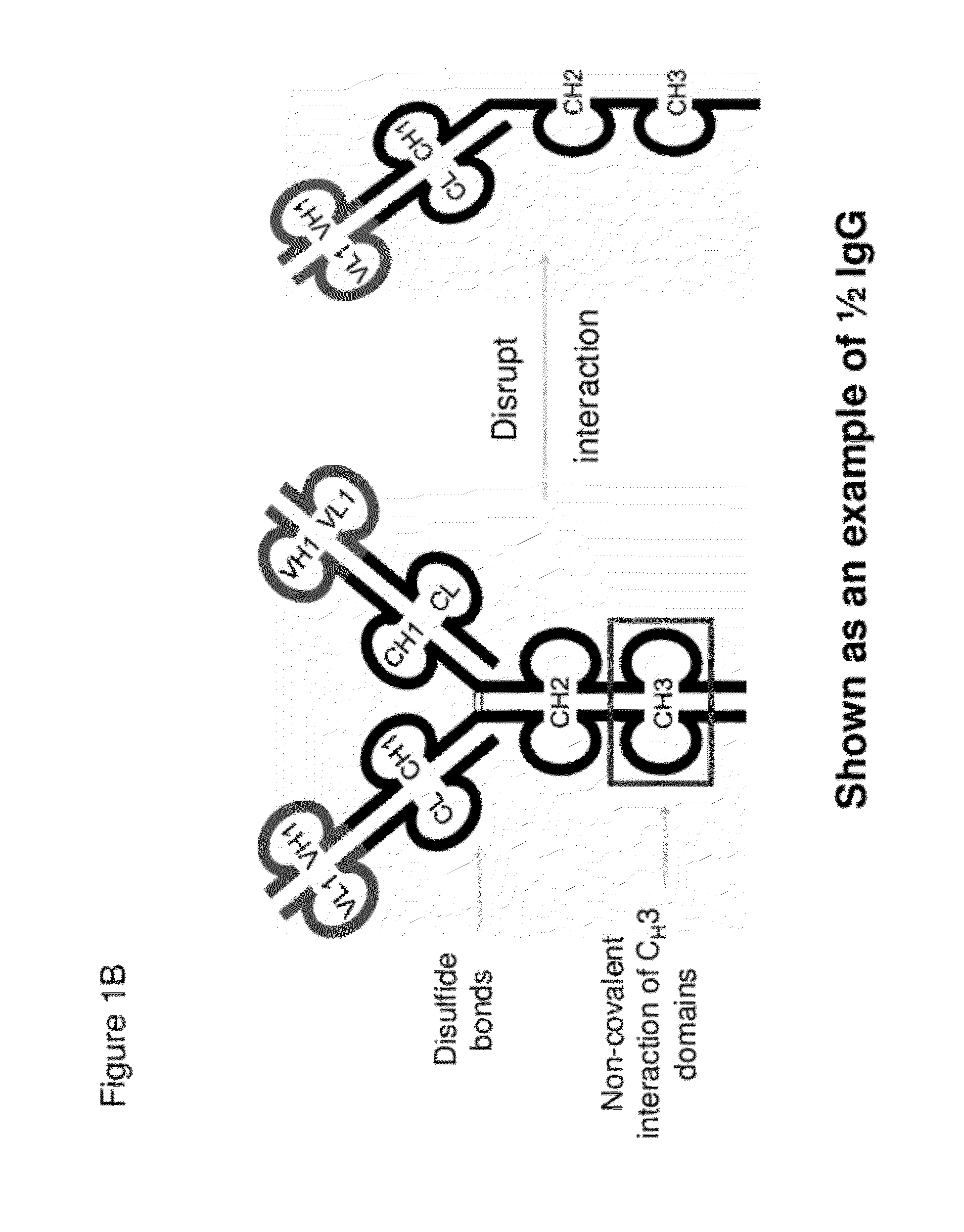
InactiveUS20120201746A1Altered pharmacokineticAltered pharmacodynamic propertyIn-vivo radioactive preparationsAntibody mimetics/scaffoldsAntibody Binding SiteBinding site
The invention provides compositions, methods, and kits related to half-Ig binding proteins that include a functional antibody binding site and a CH3 domain wherein the CH3 domain includes at least one mutation to inhibit CH3-CH3 dimerization.
Owner:ABBVIE INC
Methods and means for targeted gene delivery
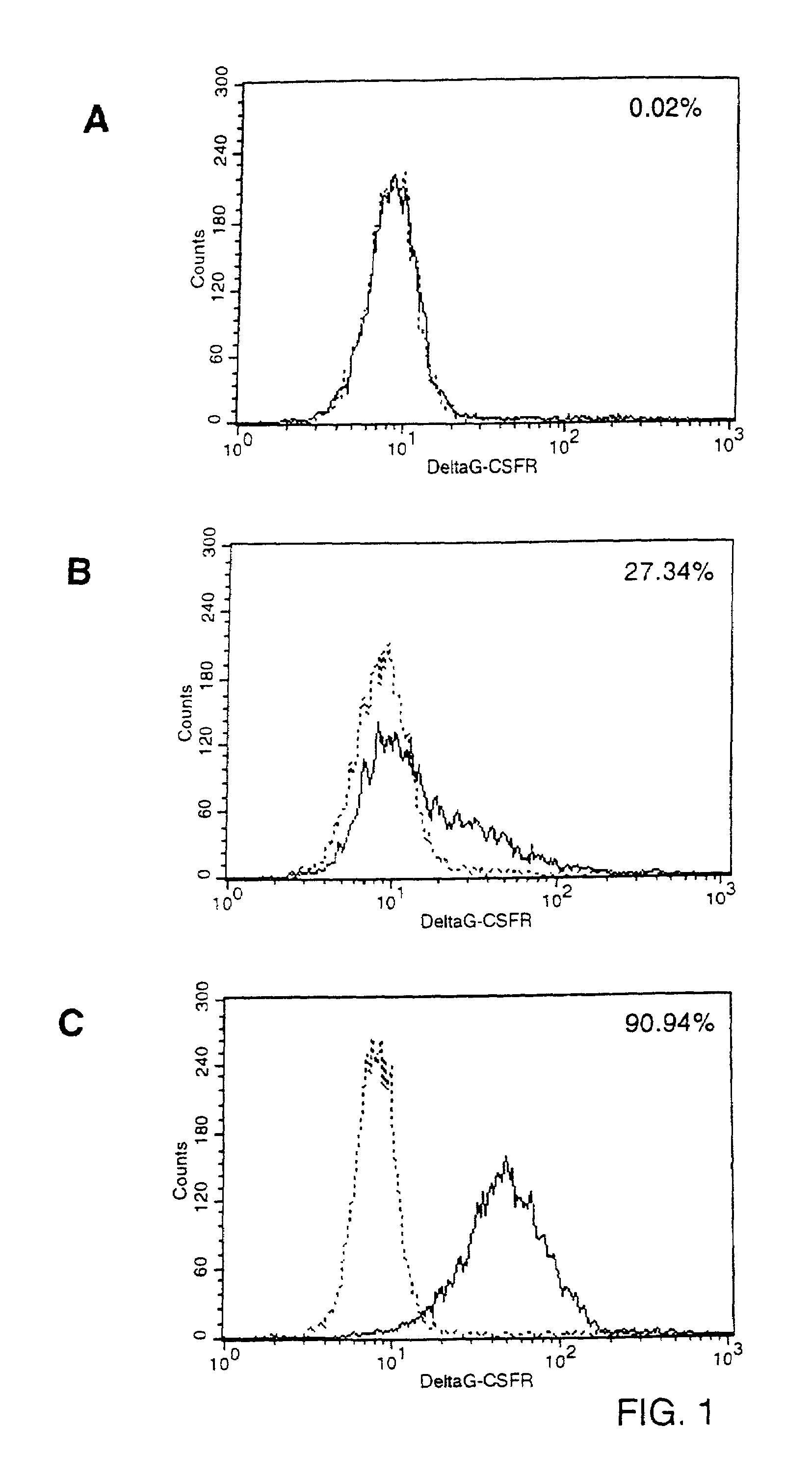
InactiveUS7033834B2Efficient introductionEfficiently introducedBiocideGenetic material ingredientsGene deliveryDelivery vehicle
A method for producing viral gene delivery vehicles which can be transferred to pre-selected cell types by using targeting conjugates. The gene delivery vehicles comprise: 1) the gene of interest; and 2) a viral capsid or envelope carrying a member of a specific binding pair, the counterpart of which is not directly associated with the surface of the target cell. These vehicles can be rendered unable to bind to their natural cell receptor. The targeting conjugates include the counterpart member of the specific binding pair, linked to a targeting moiety which is a cell-type specific ligand (or fragments thereof). The number of the specific binding pair present on the viral vehicles can be, for example, an immunoglobulin binding moiety (e.g., capable of binding to a Fc fragment, protein A, protein G, FcR or an anti-Ig antibody), or biotin, avidin or streptavidin. The virus' outer membrane or capsid may contain a substance which mediates entrance of the gene delivery vehicle into the target cell. Due to the specificity of the ligand, the binding pair's high affinity, and the gene delivery vehicle's inability to be targeted when used alone, the universality of the method for gene delivery, together with its high cell type selectively can be achieved by using various targeting conjugates.
Owner:JANSSEN VACCINES & PREVENTION BV
Chromatography matrices including novel staphylococcus aureus protein a based ligands
ActiveUS20130046056A1Reduce lossesLarge degree of fragmentationSolid sorbent liquid separationPeptide preparation methodsStaphylococcus aureusStaphylococcus aureus protein A
The present invention relates to chromatography matrices including ligands based on one or more domains of immunoglobulin-binding proteins such as, Staphylococcus aureus Protein A (SpA), as well as methods of using the same.
Owner:MILLIPORE CORP
Serum half-life extension using igbd
InactiveUS20170145062A1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsSerum igeHeavy chain
Owner:UNIV STUTTGART
Protein having affinity for immunoglobulin, and immunoglobulin-binding affinity ligand
ActiveUS20120208234A1Good chemical stabilityImprove identityBacteriaPeptide/protein ingredientsLow affinityADAMTS Proteins
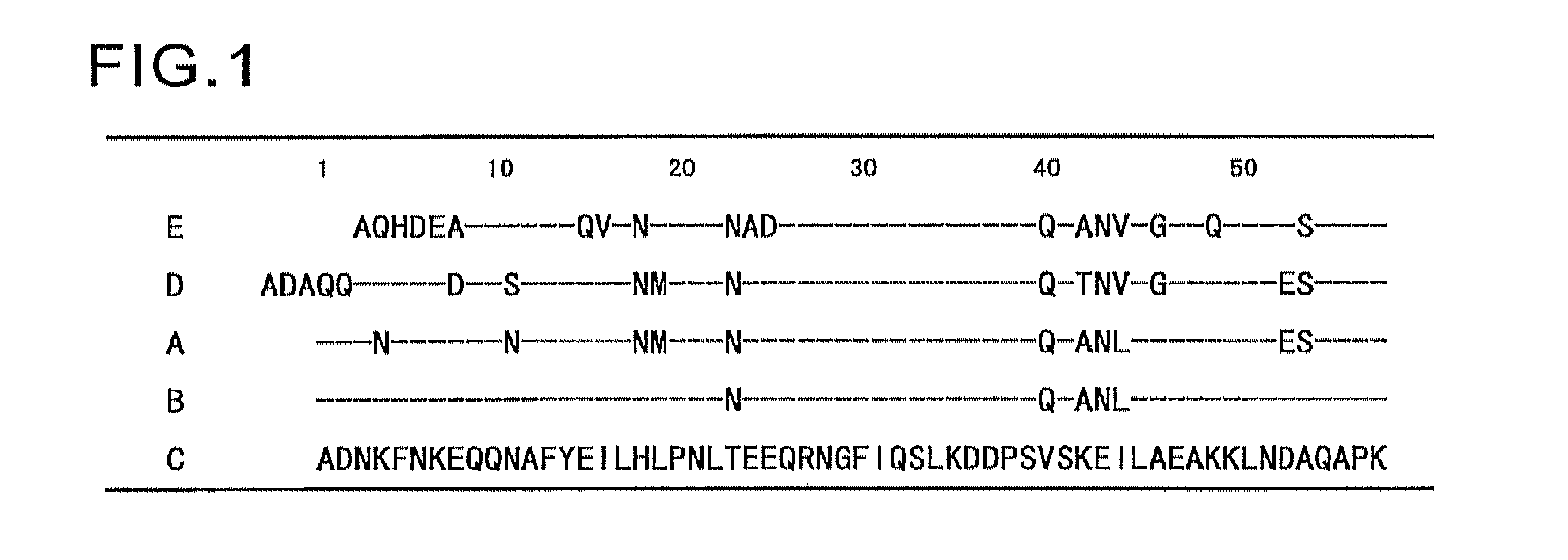
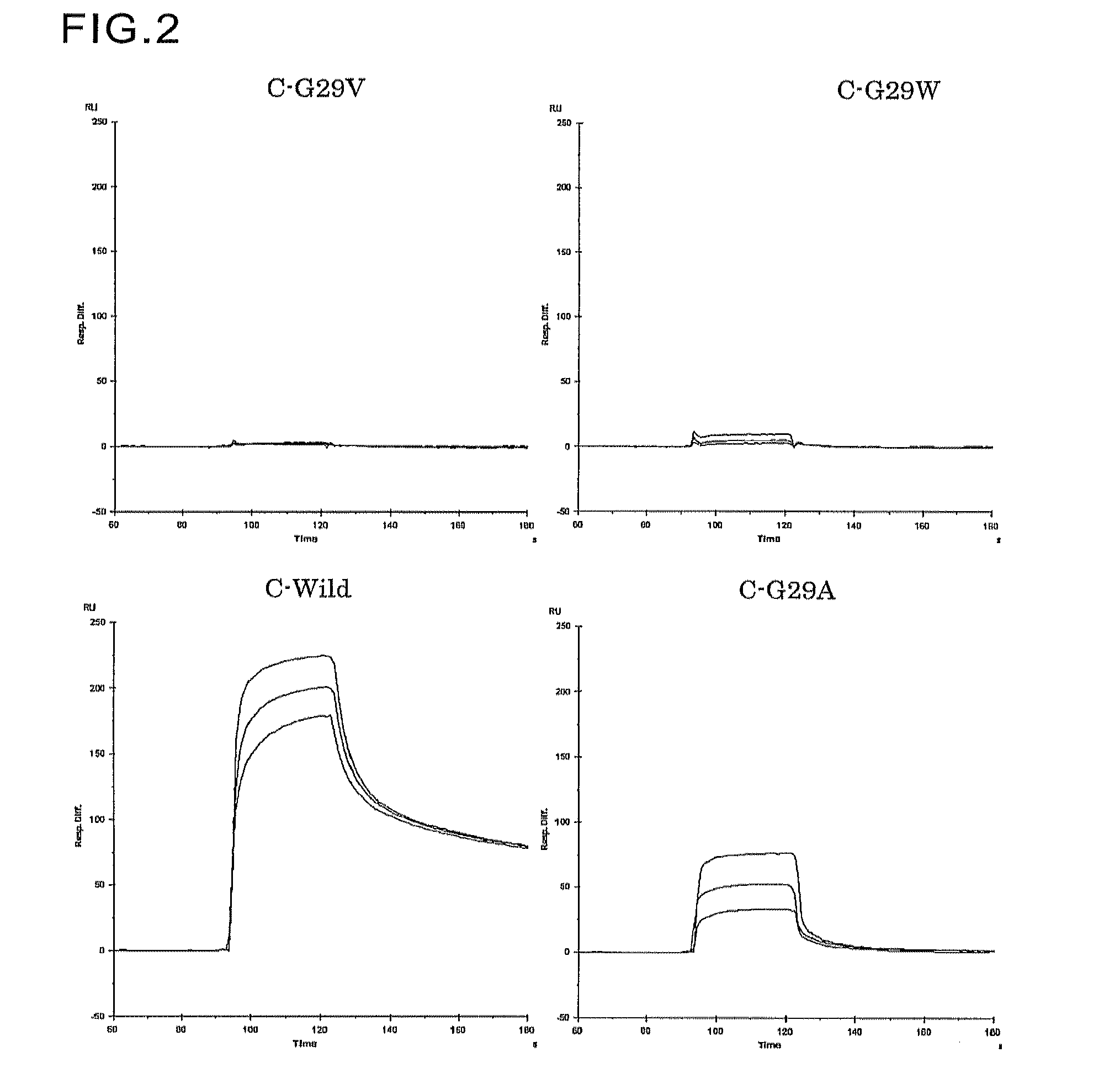
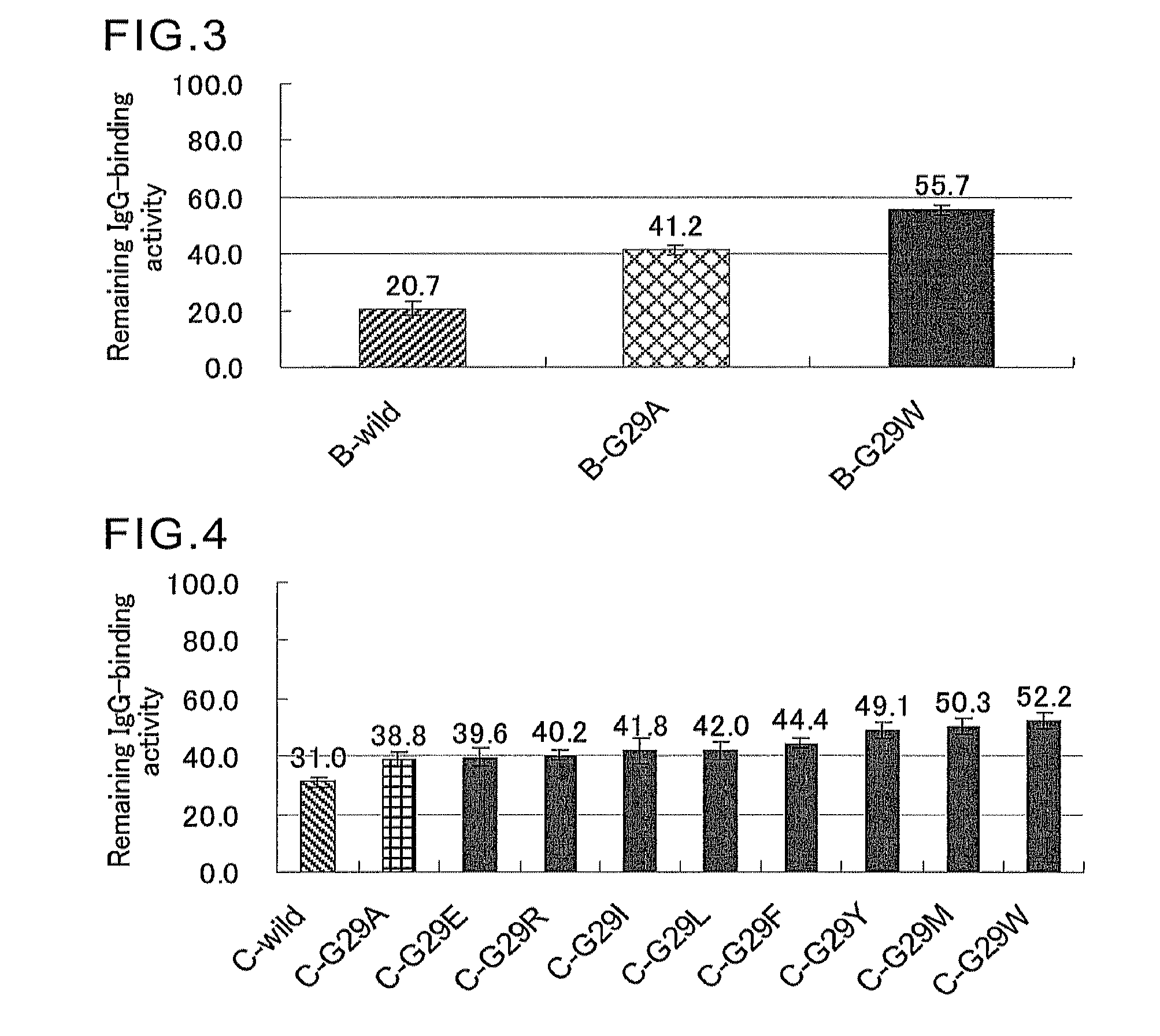
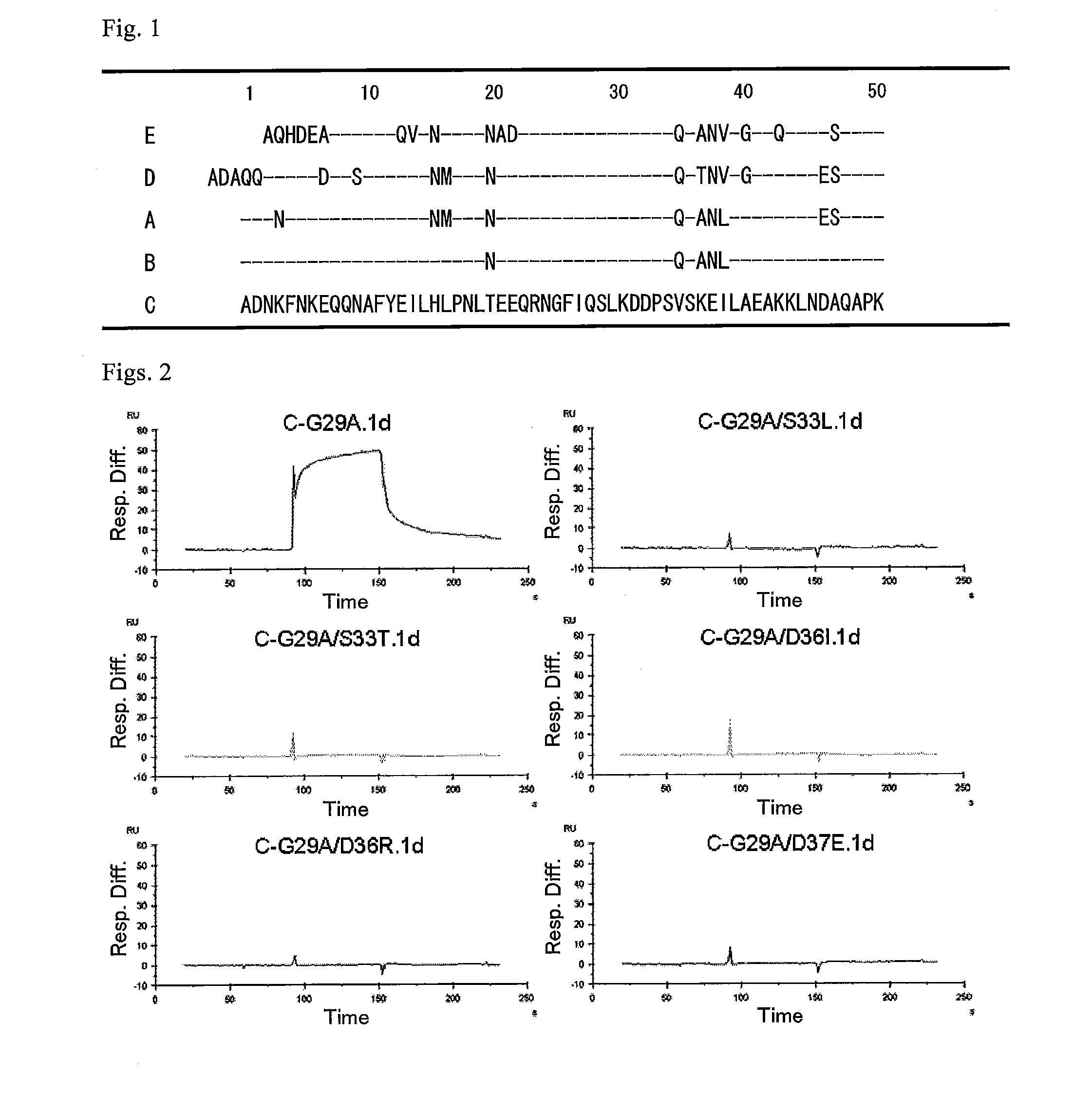
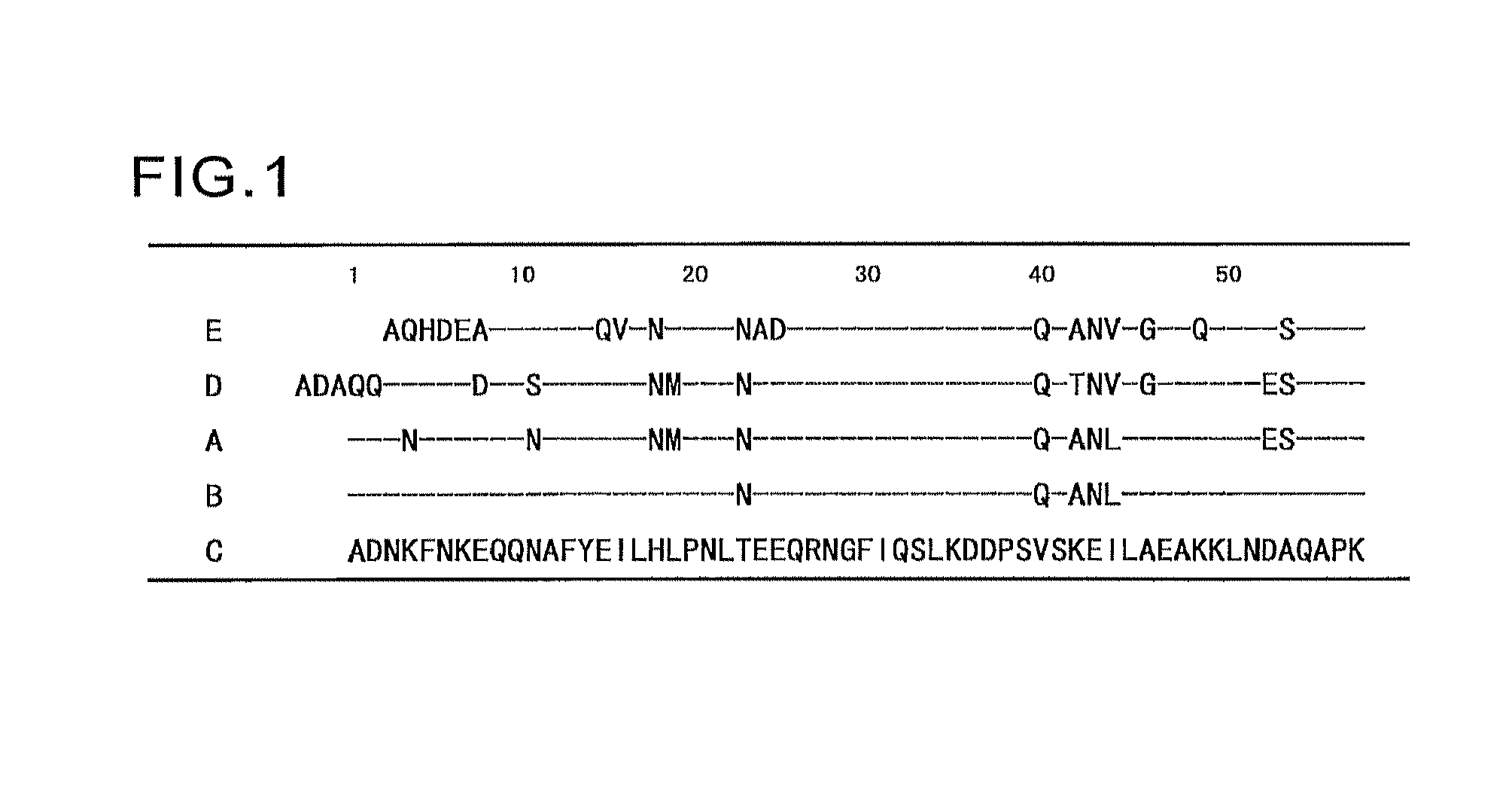
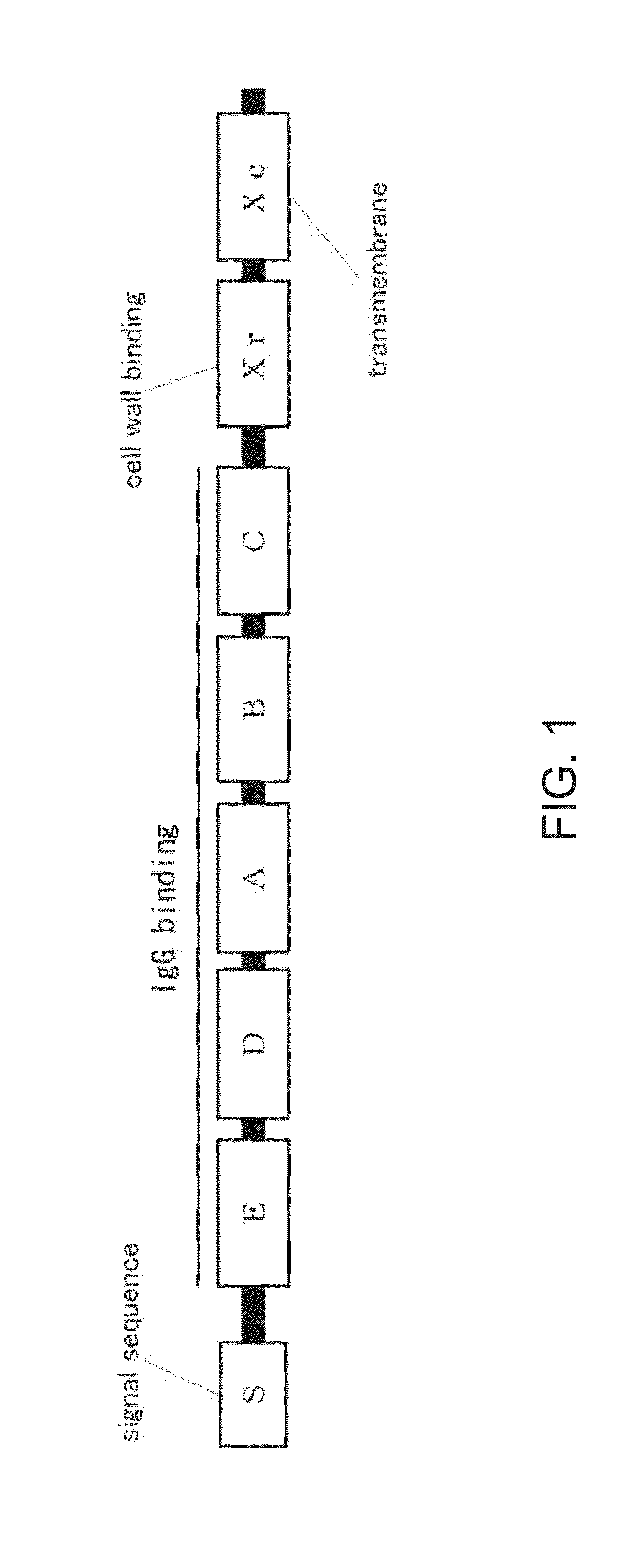
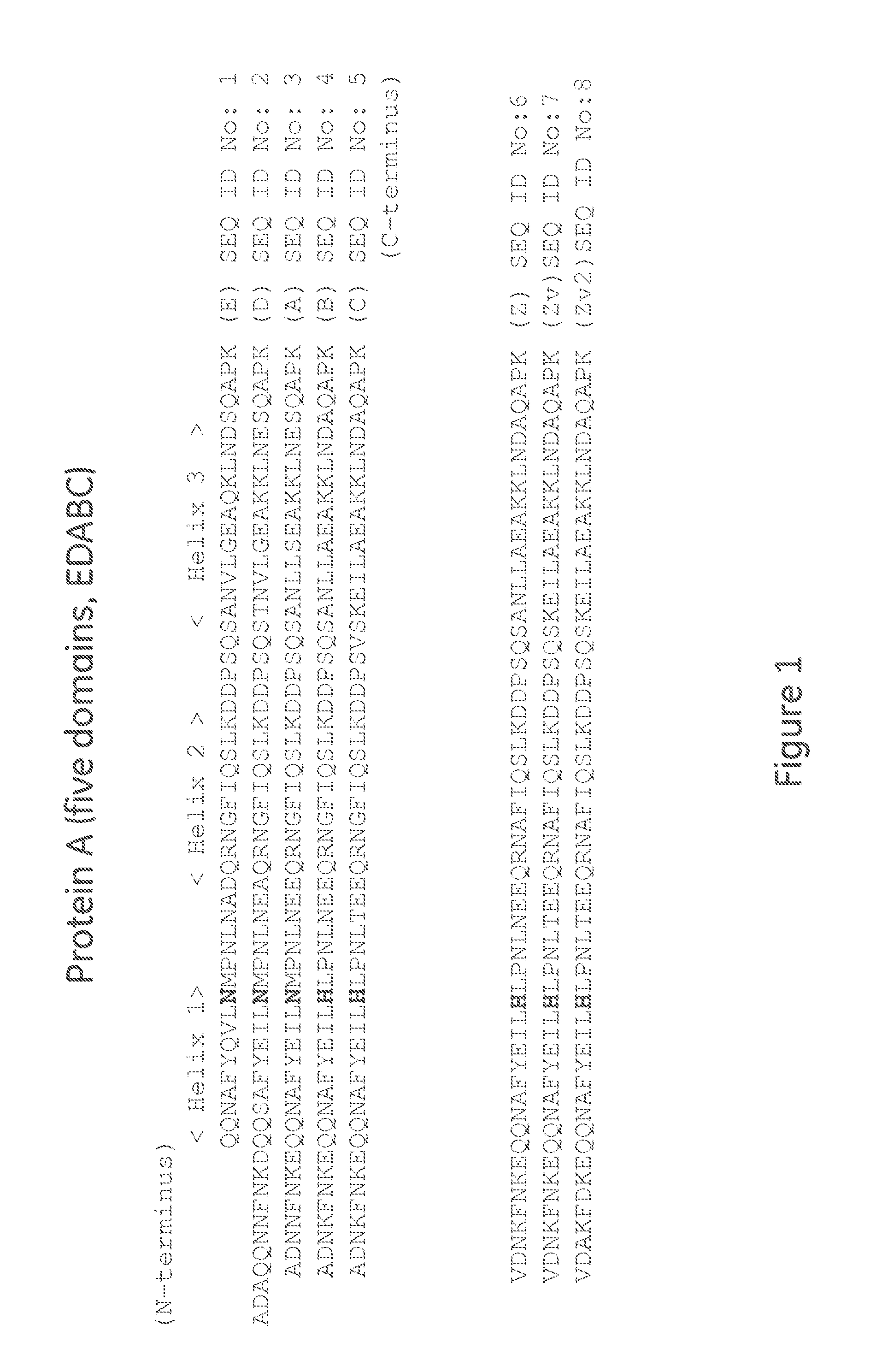
An object of the present invention is to create a novel engineered Protein A ligand having better antibody dissociation properties in the presence of an acid than conventional engineered Protein A ligands and a further object of the present invention is to create a novel engineered Protein A ligand having higher alkali resistance. The present invention is to provide a protein having an affinity for an immunoglobulin, including an amino acid sequence derived from any of E, D, A, B and C domains of Protein A, wherein at least one Gly residue in the amino acid sequence is replaced with an amino acid other than Ala, and the protein has a lower affinity for an Fab region of an immunoglobulin than a protein including an amino acid sequence in which the Gly residue is replaced with Ala. Also, the present invention is to provide the protein having an affinity for an immunoglobulin, which has improved chemical stability in an alkaline condition compared to the corresponding domain.
Owner:KANEKA CORP
Purification of immunoglobulins using affinity chromatography and peptide ligands
ActiveUS20060153834A1High affinityPeptide librariesPeptide/protein ingredientsCarboxyl radicalPeptide ligand
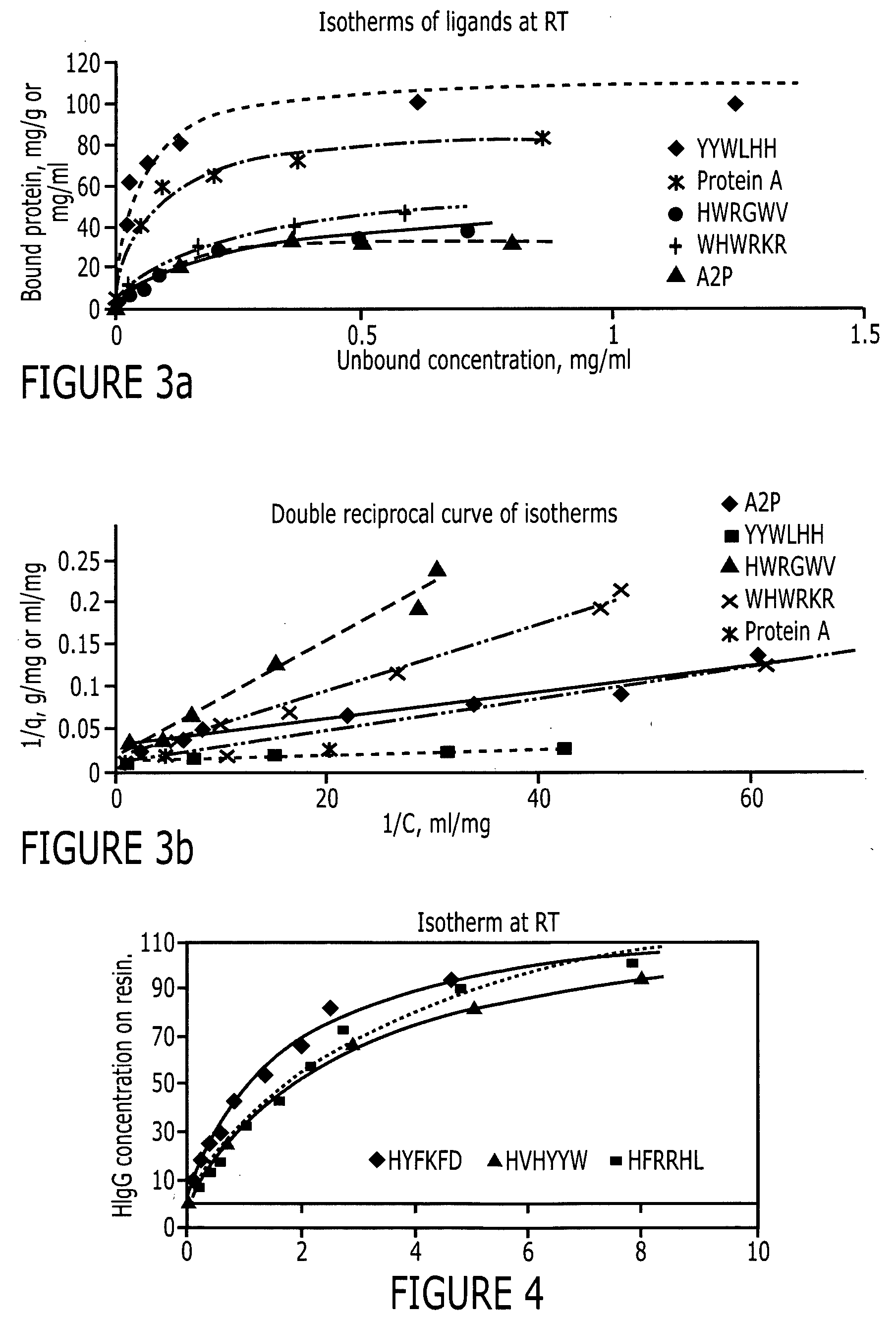
An immunoglobulin binding peptide having the general formula, from amino terminus to carboxy terminus, of Z-R1—R2—R3—R4—R5—R6—X, is described, wherein: R1 is H or Y; R2 is a hydrophobic, preferentially aromatic, amino acid (for example W, F, Y, V); R3 is a positively charged or aromatic amino acid (for example R, H, F, W); R4 is a hydrophobic or positively charged amino acid (for example G, Y, R, K, L); R5 is a positively charged or aromatic amino acid (for example W, F, R, H, Y); R6 a random amino acid but preferably hydrophobic or negatively charged (for example V, W, L, D, H); X is present or absent and when present is a linking group; and Z is present or absent and when present is a capping group bonded to the N terminus of R1; and wherein the amino acids of said peptide are in D form, L form, or a combination thereof. Methods of using such peptides for the purification of Immunoglobulins are also described
Owner:NORTH CAROLINA STATE UNIV
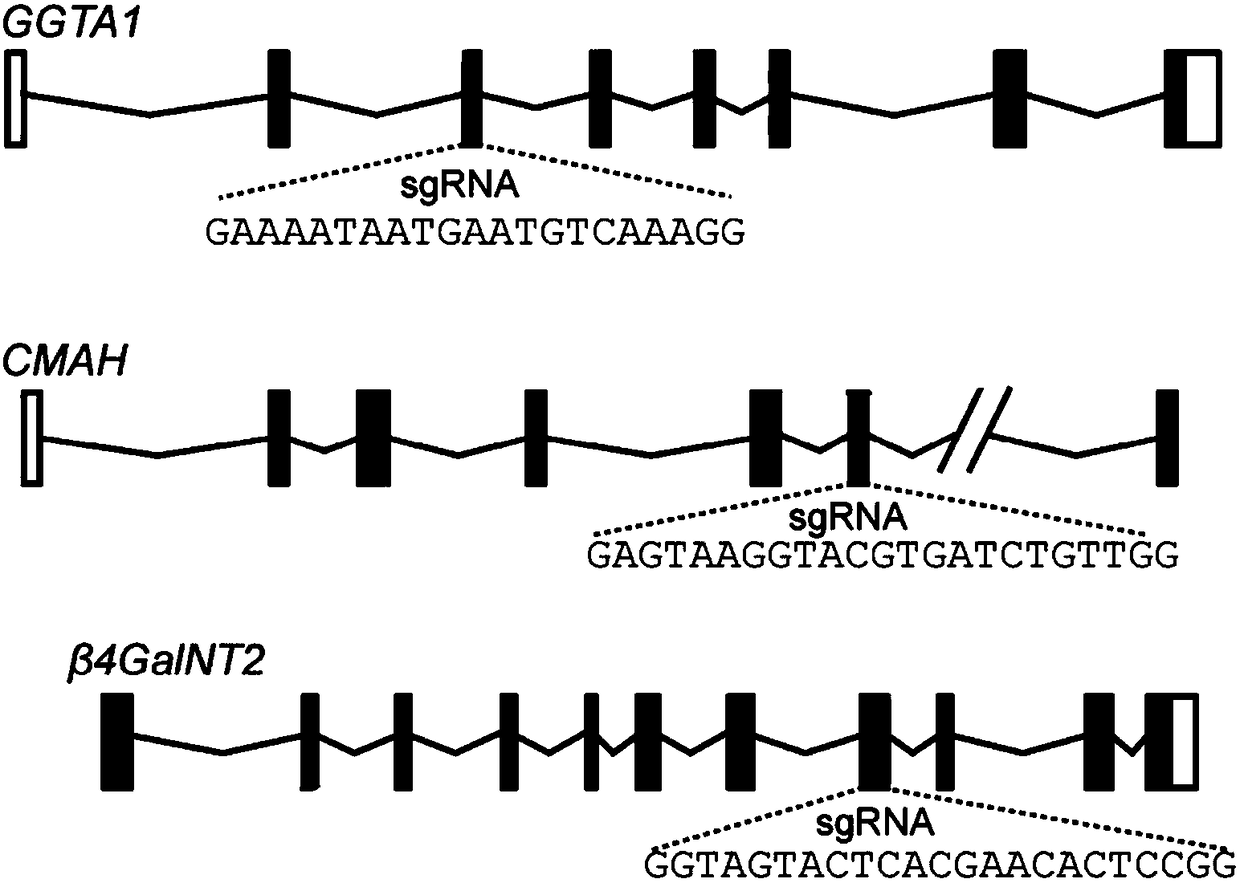
Application of CRISPR/Cas9 carrier combination in preparation of blood product of gene knockout pig
PendingCN108588123AReduce the binding forceSolve the problem of ischemiaHydrolasesStable introduction of DNAWhole blood productRed Cell
The invention discloses the application of a CRISPR / Cas9 carrier combination in preparation of a blood product of a gene knockout pig. The gene knockout pig is a pig without GGTA1 genes, CMAH genes and beta4Ga1NT2 genes. The CRISPR / Cas9 carrier combination comprises a GGTA1-CRISPR / Cas9 carrier, a CMAH-CRISPR / Cas9 carrier and a beta4Ga1NT2-CRISPR / Cas9. By designing a specifically targeted SgRNA sequence, the knockout efficiency of the three genes is respectively 56 percent, 63 percent and 41 percent; after the three genes relating to immunological rejection are knocked out, the gene knockout pig is obtained; the combination between erythrocytes and immune globulin in human serum is obviously reduced; an outstanding effect is achieved to overcome hyperacute immunological rejection; the problem of clinical ischemia is effectively solved; a precious material source is provided for clinical blood transfusion.
Owner:GCREATENE SUZHOU BIOTECH CO LTD
Mutated Immunoglobulin-Binding Polypeptides
ActiveUS20170334954A1Improved alkaline stabilityImprove stabilityHybrid immunoglobulinsSolid sorbent liquid separationArginineFc binding
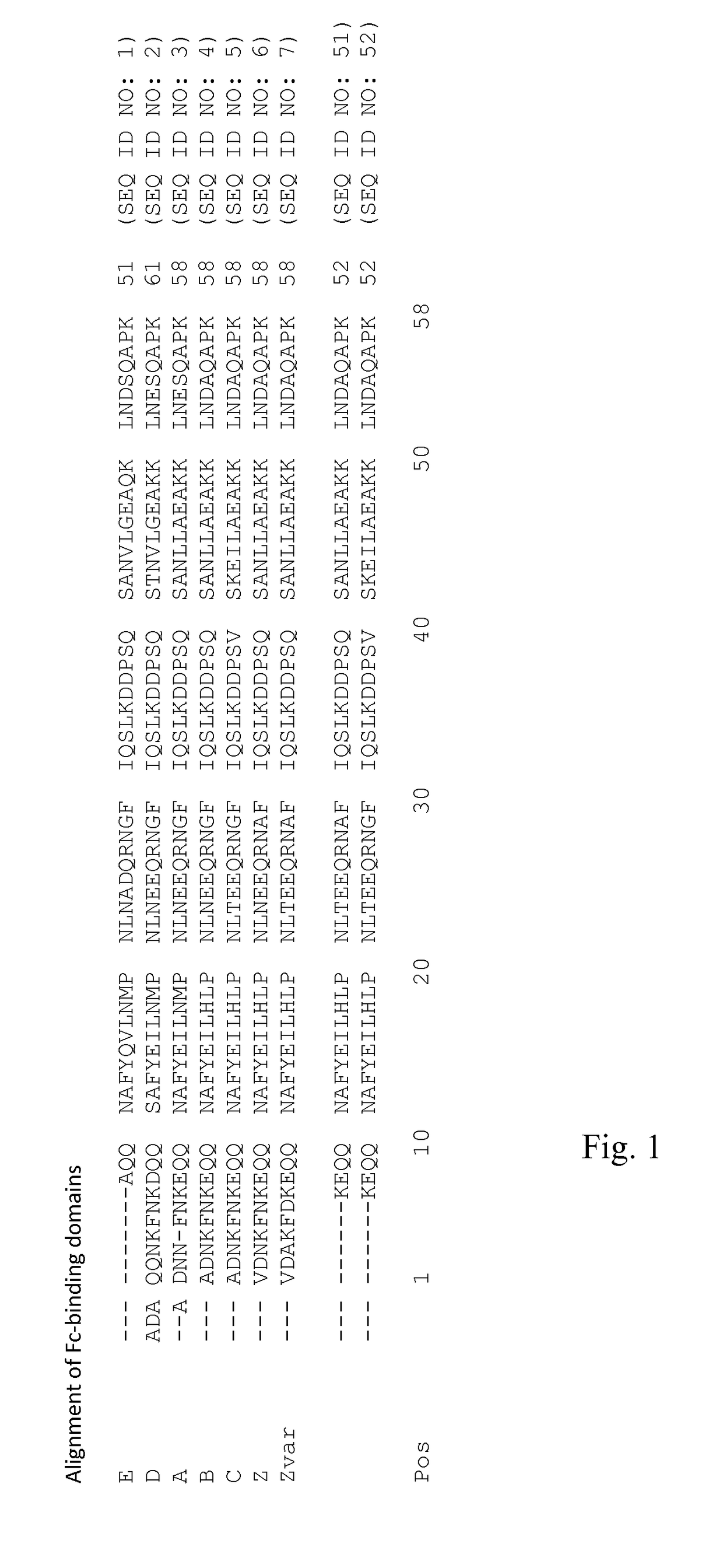
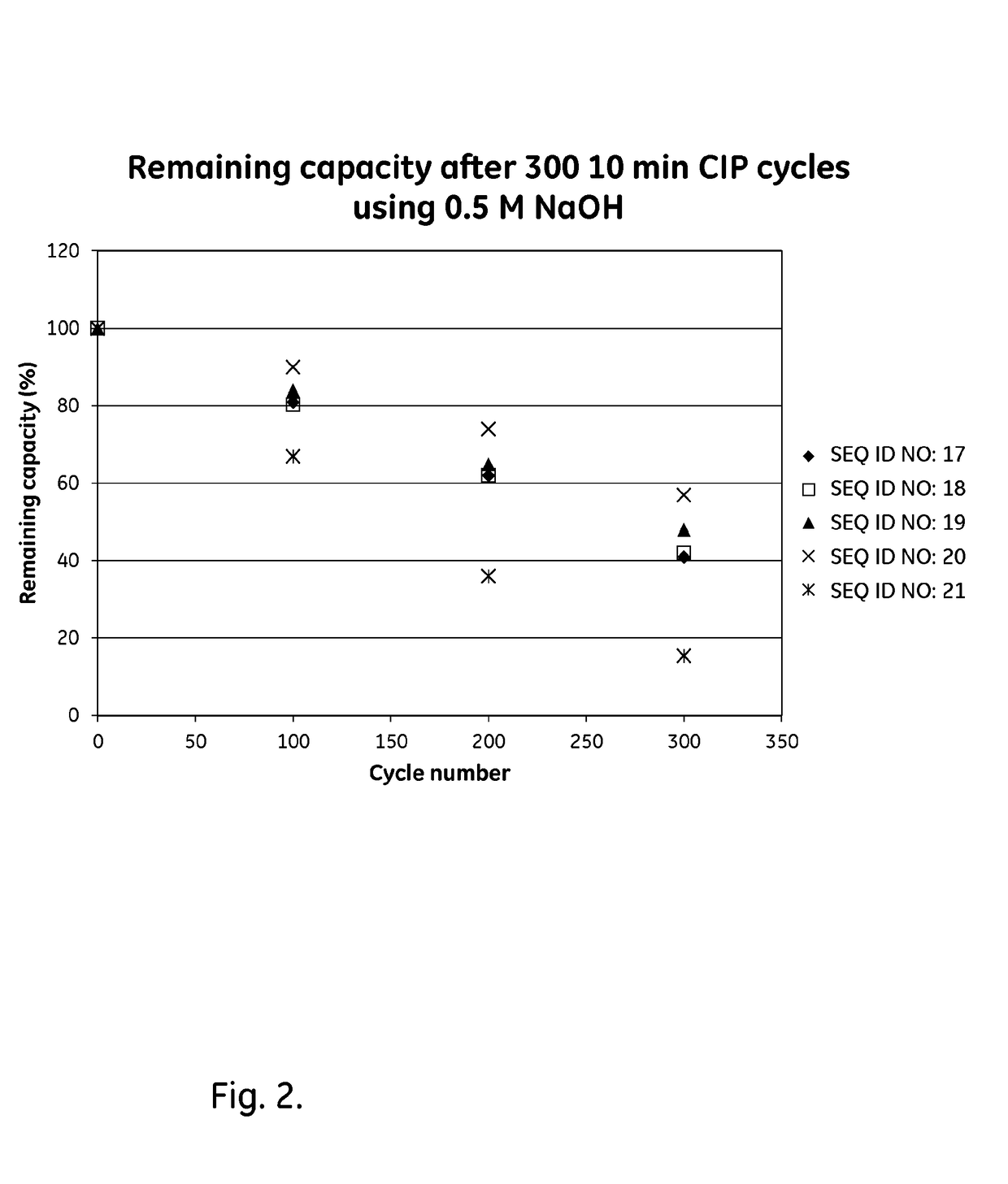
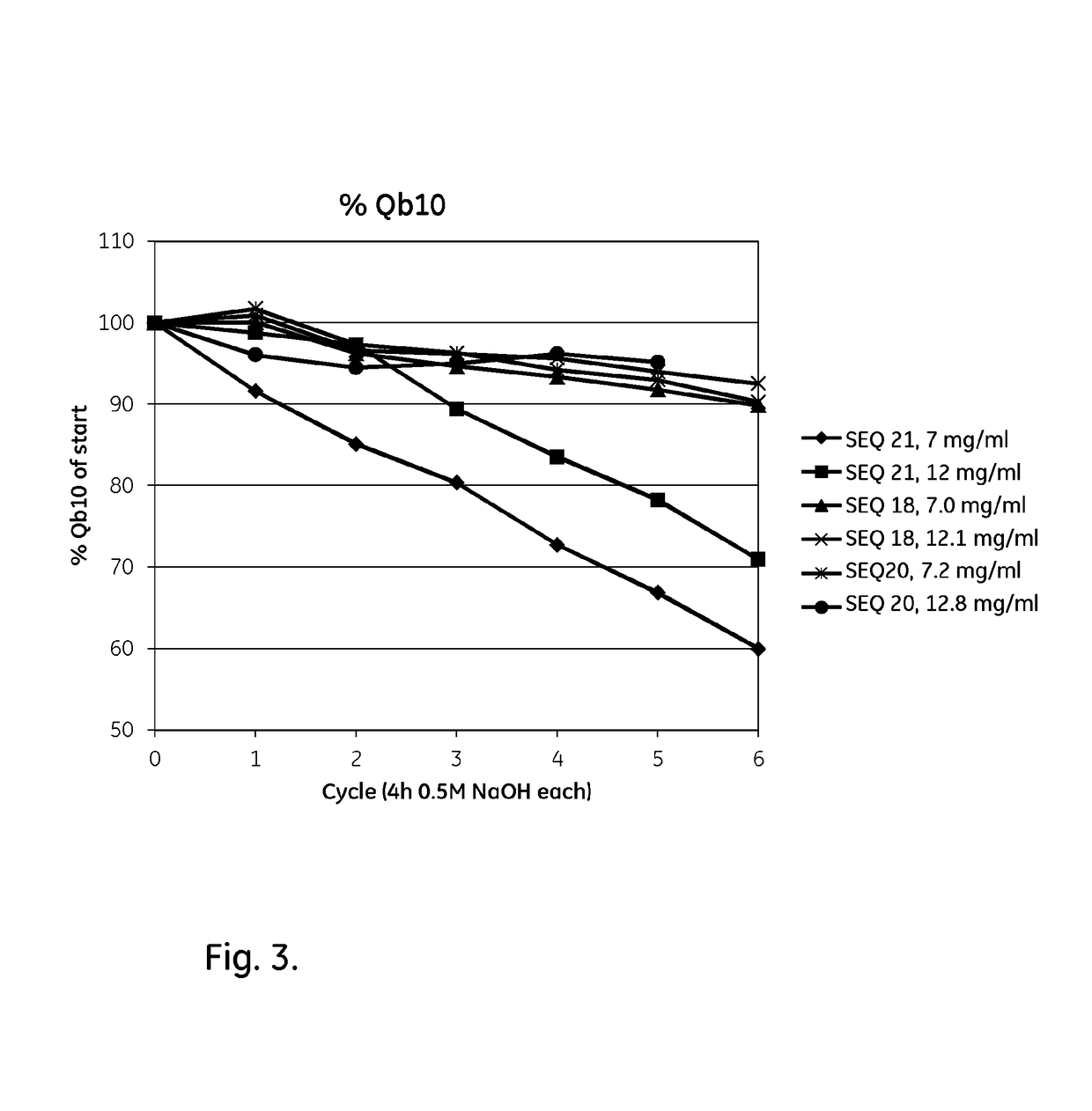
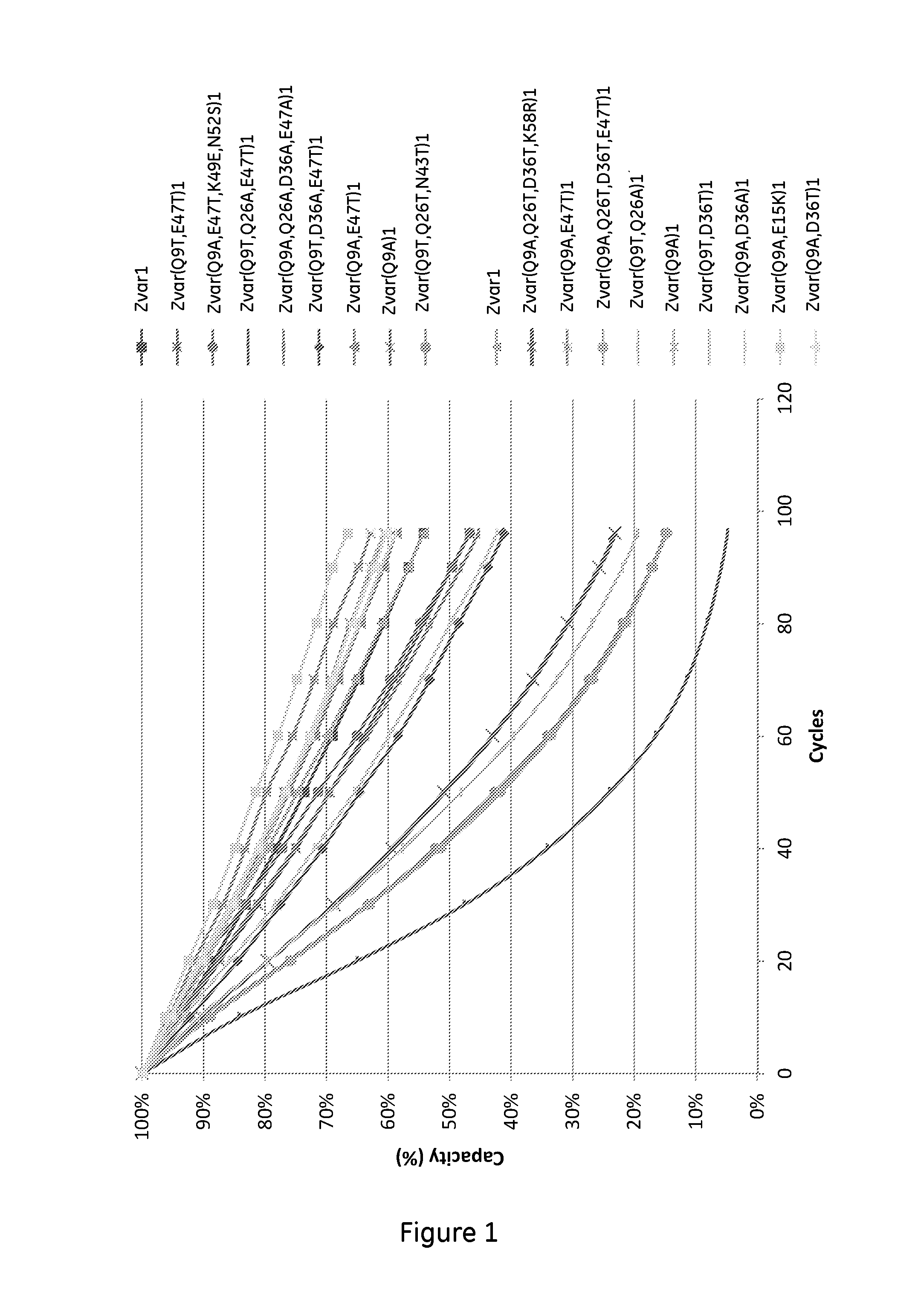
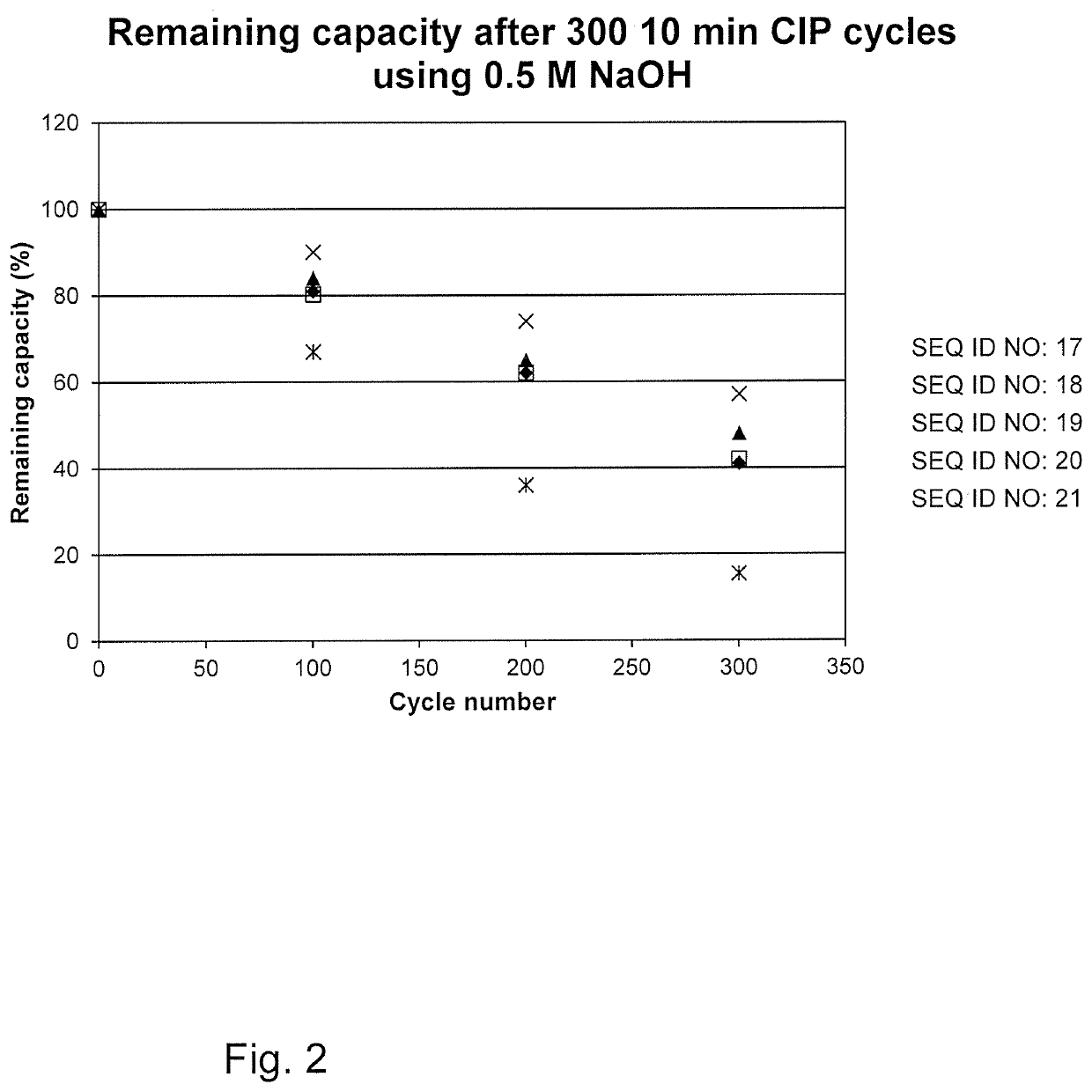
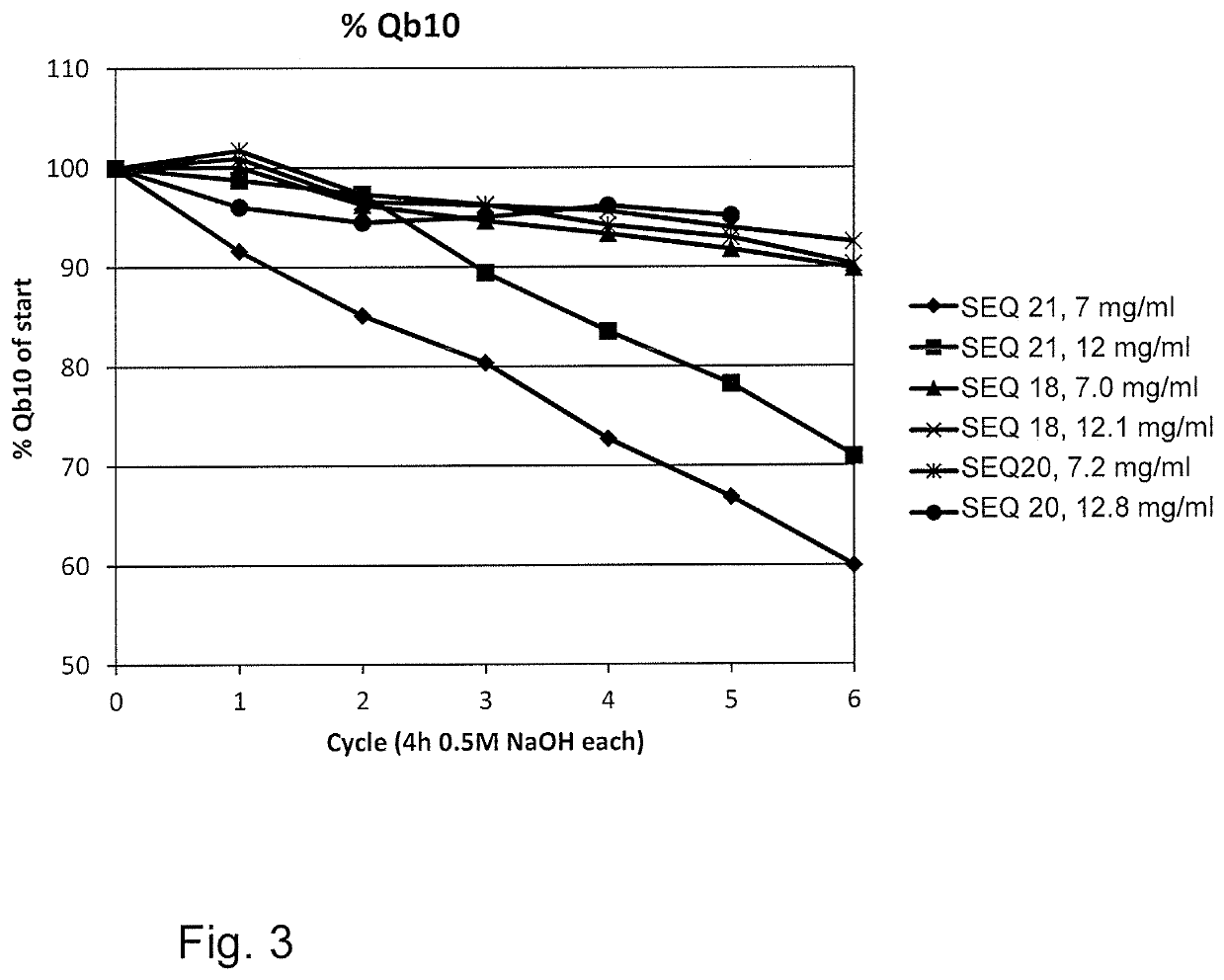
An Fc-binding polypeptide of improved alkali stability, comprising a mutant of an Fc-binding domain of Staphylococcus Protein A (SpA), as defined by SEQ ID NO: 1, SEQ ID NO: 2, SEQ ID NO:3, SEQ ID NO: 4, SEQ ID NO: 5, SEQ ID NO:6, SEQ ID NO:7, SEQ ID NO:22, SEQ ID NO 51 or SEQ ID NO 52 wherein at least the asparagine or serine residue at the position corresponding to position 11 in SEQ ID NO:4-7 has been mutated to an amino acid selected from the group consisting of glutamic acid, lysine, tyrosine, threonine, phenylalanine, leucine, isoleucine, tryptophan, methionine, valine, alanine, histidine and arginine.
Owner:CYTIVA BIOPROCESS R&D AB
Protein capable of binding specifically to immunoglobulin, and immunoglobulin-binding affinity ligand
ActiveUS20130096276A1Excellent antibody dissociation propertyEasy to separateBacteriaSugar derivativesLow affinityAmino acid substitution
An object of the present invention is to create a novel engineered Protein A ligand having better antibody dissociation properties in the acidic condition compared with known engineered Protein A ligands. The present invention provides a protein having an affinity for an immunoglobulin, including an amino acid sequence obtained by introducing, into an amino acid sequence derived from any of E, D, A, B and C domains of Protein A, at least one amino acid substitution at any one or more of amino acid residues corresponding to positions 31 to 37 of the A, B and C domains (positions 29 to 35 of the E domain, positions 34 to 40 of the D domain), which are conserved in all the domains, the protein having a lower affinity for an Fab region of an immunoglobulin than a protein having the amino acid sequence before introduction of the substitution.
Owner:KANEKA CORP
Mutated Immunoglobulin-Binding Polypeptides
ActiveUS20160159857A1Highly selective bindingImproved alkaline stabilitySolid sorbent liquid separationDepsipeptidesTryptophanMutant
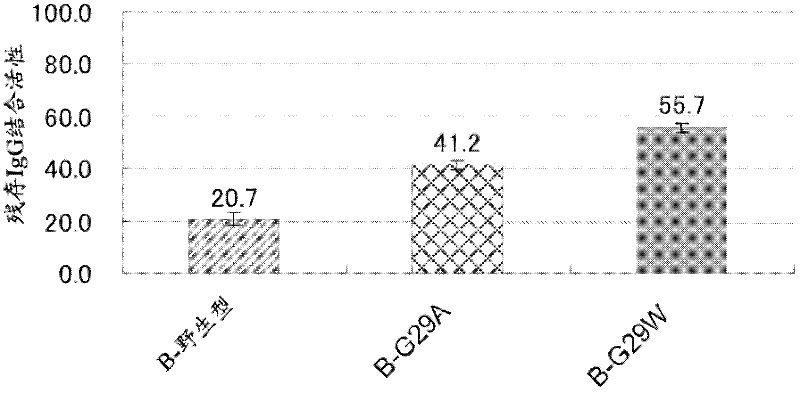
The invention discloses a polypeptide with improved alkaline stability, which polypeptide comprises a mutant of a B or C domain of Staphylococcus Protein A (SpA), as specified by SEQ ID NO 1 or SEQ ID NO 2, or of Protein Z, as specified by SEQ ID NO 3, comprising at least the mutation wherein the glutamine residue at position 9 has been mutated to a tryptophan, leucine, glutamic acid, valine or lysine. The invention also discloses multimers of the polypeptide, as well as separation matrices comprising the multimers or polypeptides.
Owner:CYTIVA BIOPROCESS R&D AB
Support for affinity chromatography and method for isolating immunoglobulin
InactiveUS9040661B2Peptide/protein ingredientsSerum immunoglobulinsImmunglobulin eImmobilized protein
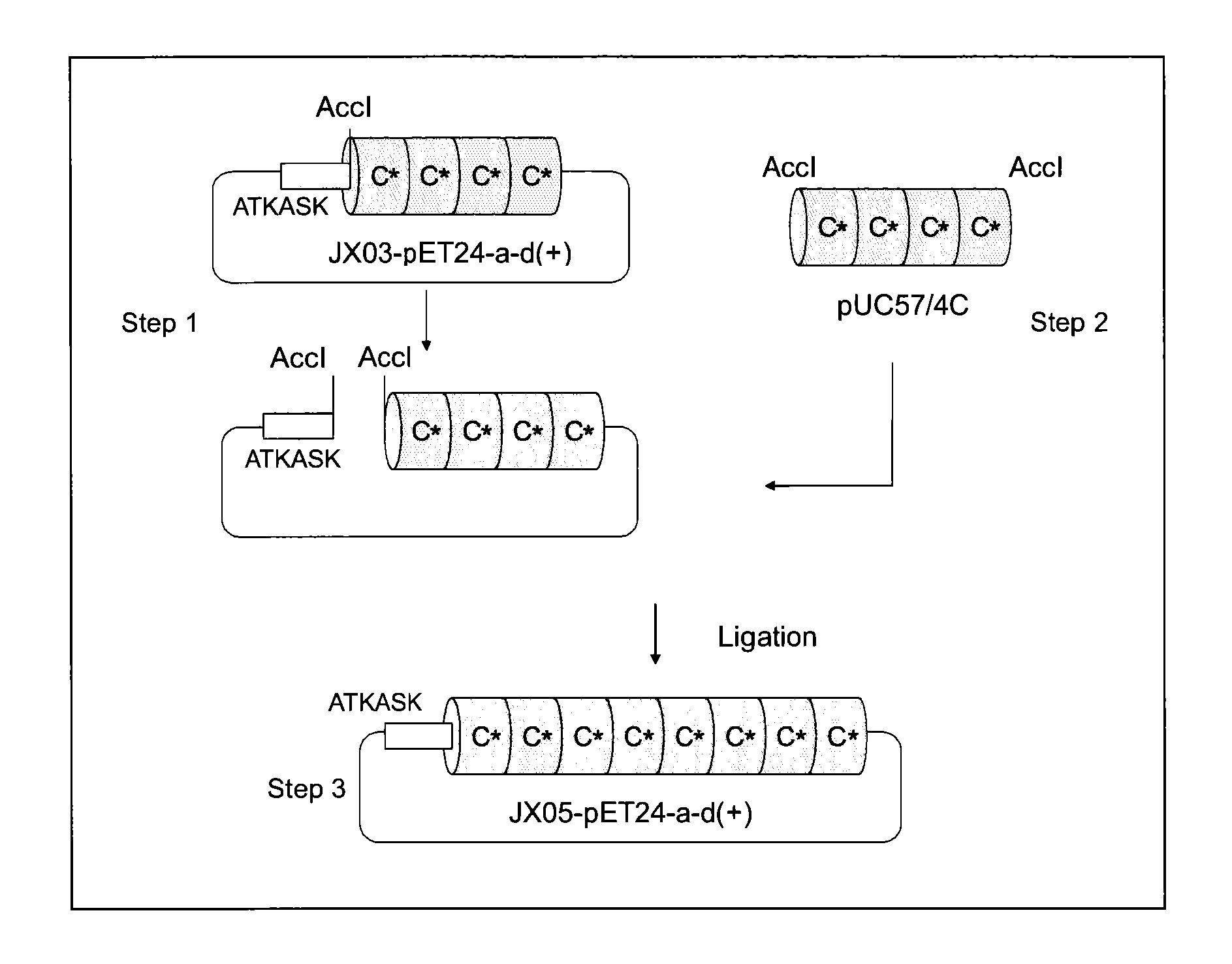
Provided are a support for affinity chromatography which has excellent alkali resistance, and a method for isolating immunoglobulin. A support for affinity chromatography, containing an immobilized protein ligand represented by the following formula (1):R—R2 (1)wherein R represents a polypeptide consisting of 4 to 30 amino acid residues that contains an amino acid sequence represented by ATK or ASK; and R2 represents a polypeptide consisting of 50 to 500 amino acid residues containing an immunoglobulin-binding domain consisting of an amino acid sequence represented by SEQ ID NO: 1 or SEQ ID NO: 2, the partial sequence thereof, or an amino acid sequence having 70% or more identity to these sequences; with the proviso that a terminus at which R2 binds to R is C-terminus or N-terminus of the immunoglobulin-binding domain.
Owner:JSR CORPORATIOON +1
Protein having affinity for immunoglobulin, and immunoglobulin-binding affinity ligand
Disclosed are: a novel modified protein A ligand having a superior property of causing the acid dissociation of antibodies compared to those of existing modified protein A ligands; and a novel modified protein A ligand having high alkali resistance. Specifically disclosed are: a protein having an affinity for an immunoglobulin, which is characterized by comprising an amino acid sequence produced by substituting at least one Gly residue by an amino acid residue other than an Ala residue in an amino acid sequence derived from any one of E-domain, D-domain, A-domain, B-domain and C-domain of protein A, and which is also characterized by having a reduced affinity for an Fab region contained in the immunoglobulin compared to a protein comprising an amino acid sequence produced by substituting the Gly residue by an Ala residue in the amino acid sequence derived from any one of E-domain, D-domain, A-domain, B-domain and C-domain of protein A; and a protein having an affinity for an immunoglobulin, which is characterized by having improved chemical stability under alkaline conditions compared to a corresponding domain.
Owner:KANEKA CORP
Mutated immunoglobulin-binding polypeptides
ActiveUS20160159855A1Improved alkaline stabilityHighly selectiveSugar derivativesPeptide/protein ingredientsImmunoglobulin IgEMutant
A polypeptide with improved alkaline stability, which polypeptide comprises a mutant of a B or C domain of Staphylococcus Protein A, as specified by SEQ ID NO 1 or SEQ ID NO 2, or of Protein Z, as specified by SEQ ID NO 3, wherein at least the glutamine residue at position 9 has been mutated to an amino acid other than asparagine. The invention also discloses multimers of said polypeptide, as well as separation matrices comprising the multimers or polypeptides.
Owner:CYTIVA BIOPROCESS R&D AB
Antibodies and uses thereof
The present invention provides antibodies or fragments thereof that bind to cancer cells and is important in physiological phenomena, such as cell rolling and metastasis. Therapeutic and diagnostic methods and compositions using such antibody fragments thereof are also provided. The methods and compositions according to the present invention can be used in targeting therapeutic agents and in diagnosis, prognosis, and staging of and therapy for such diseases as cancer, including tumor growth and metastasis, leukemia, auto-immune disease, and inflammatory disease. Also provided is a library of immunoglobulin binding domains having a diverse antigen-binding domain for complementary binding, wherein the library has diversity only in heavy chain CDR3.
Owner:BIO TECH GENERAL ISRAEL
Purification of immunoglobulins using affinity chromatography and peptide ligands
ActiveUS7408030B2Peptide/protein ingredientsSolid sorbent liquid separationImmunglobulin ePeptide ligand
An immunoglobulin binding peptide having the general formula, from amino terminus to carboxy terminus, of Z-R1—R2—R3—R4—R5—R6—X, is described, wherein: R1 is H or Y; R2 is a hydrophobic, preferentially aromatic, amino acid (for example W, F, Y, V); R3 is a positively charged or aromatic amino acid (for example R, H, F, W); R4 is a hydrophobic or positively charged amino acid (for example G, Y, R, K, L); R5 is a positively charged or aromatic amino acid (for example W, F, R, H, Y); R6 a random amino acid but preferably hydrophobic or negatively charged (for example V, W, L, D, H); X is present or absent and when present is a linking group; and Z is present or absent and when present is a capping group bonded to the N terminus of R1; and wherein the amino acids of said peptide are in D form, L form, or a combination thereof. Methods of using such peptides for the purification of Immunoglobulins are also described.
Owner:NORTH CAROLINA STATE UNIV
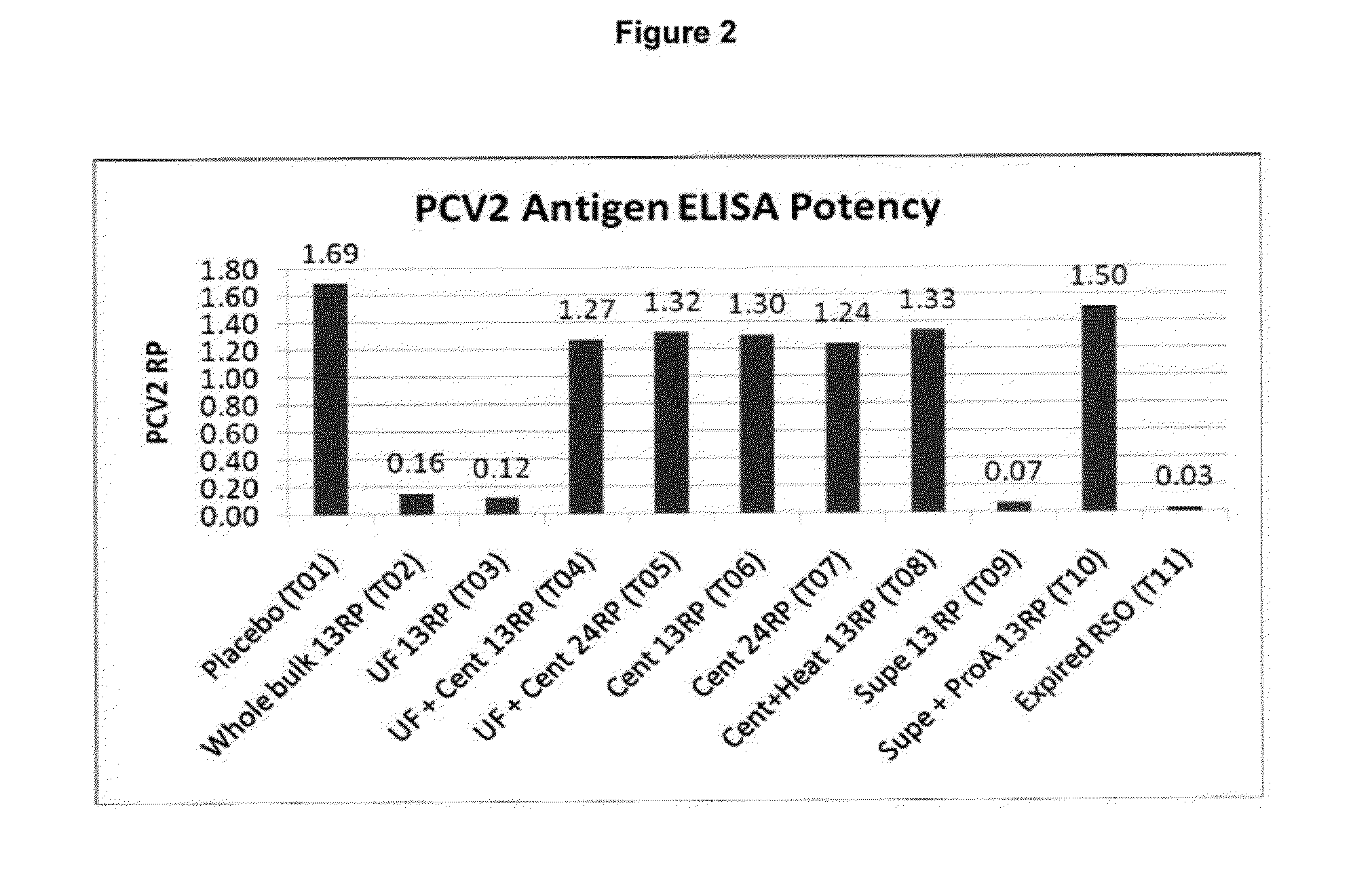
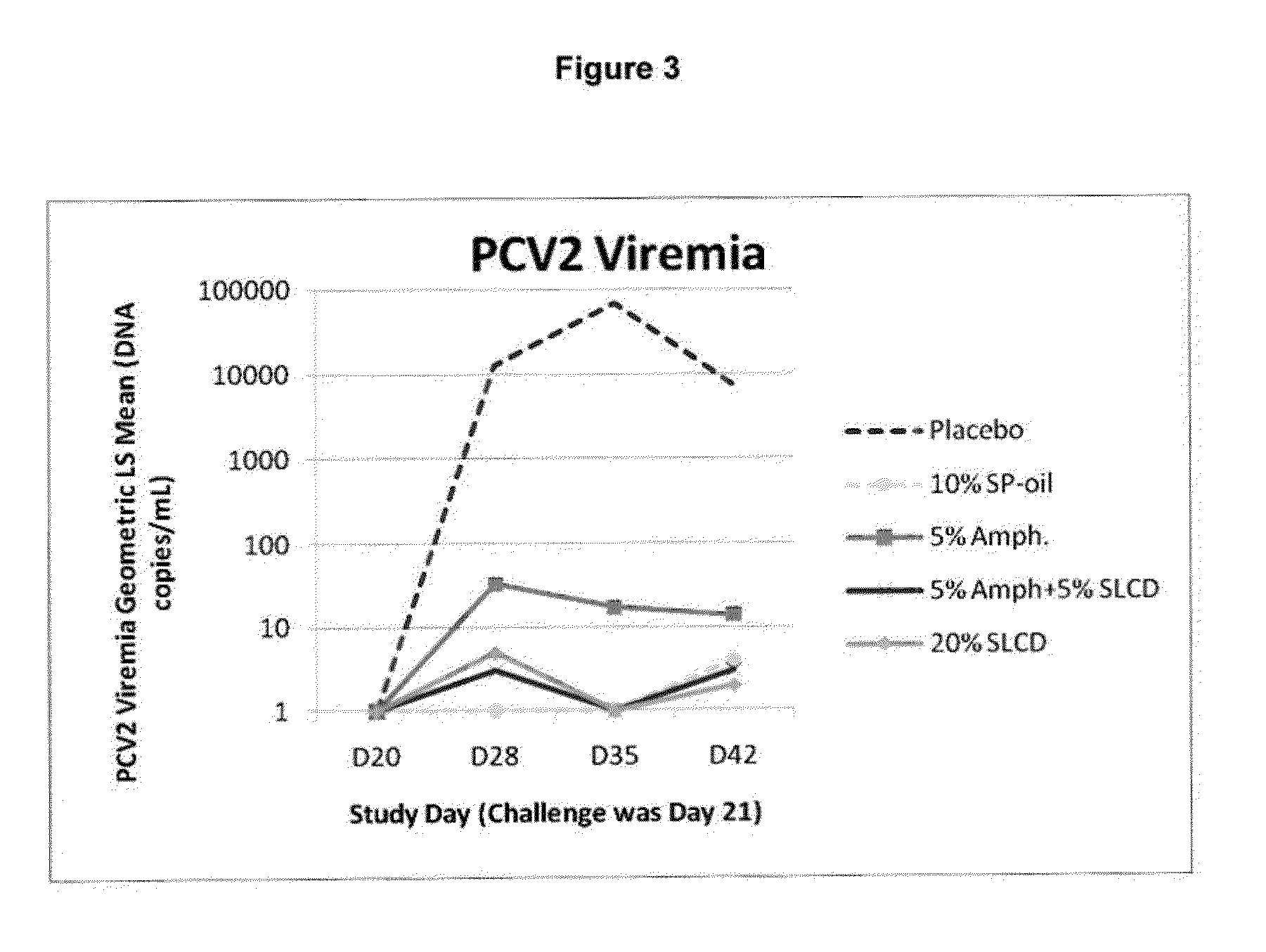
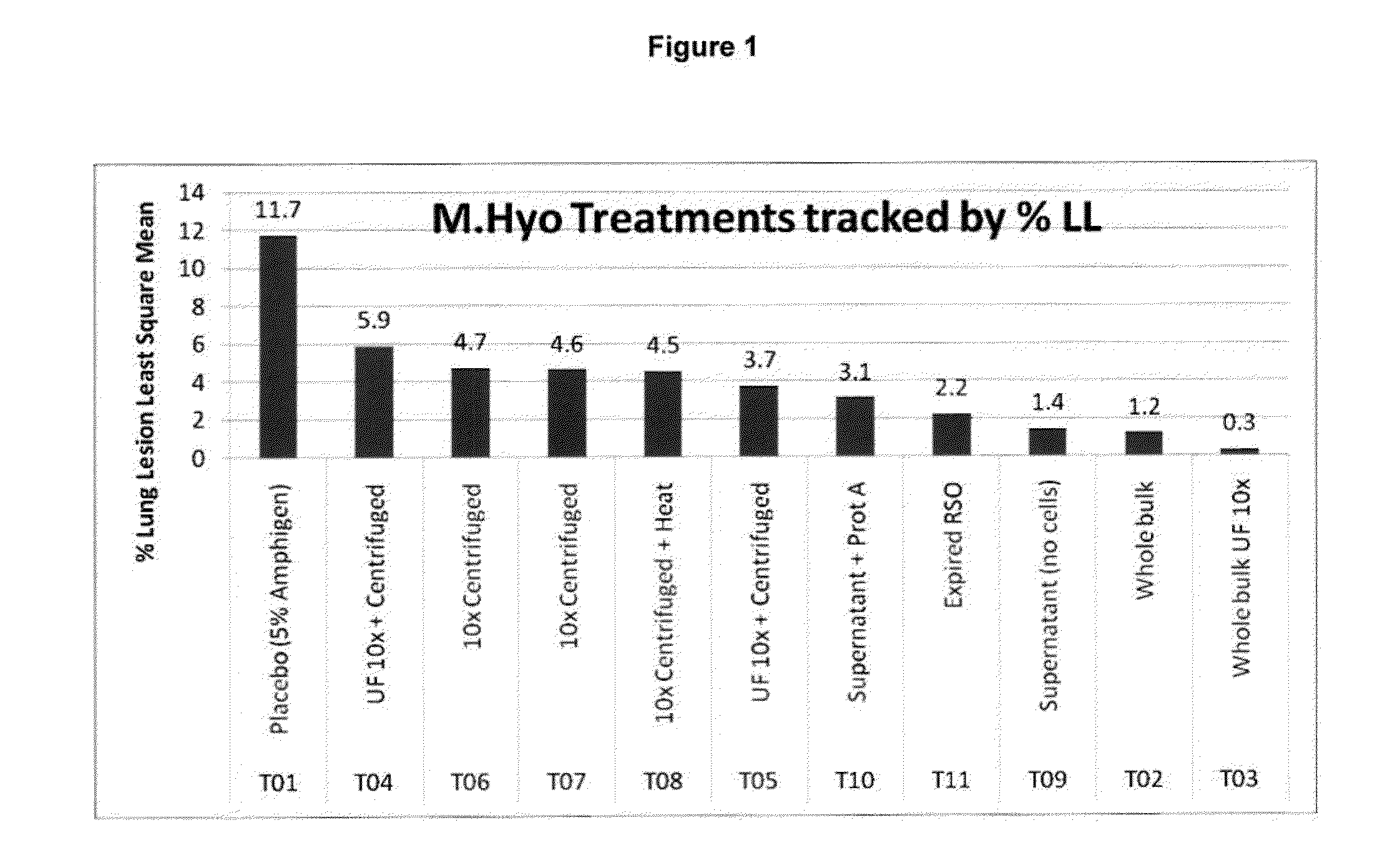
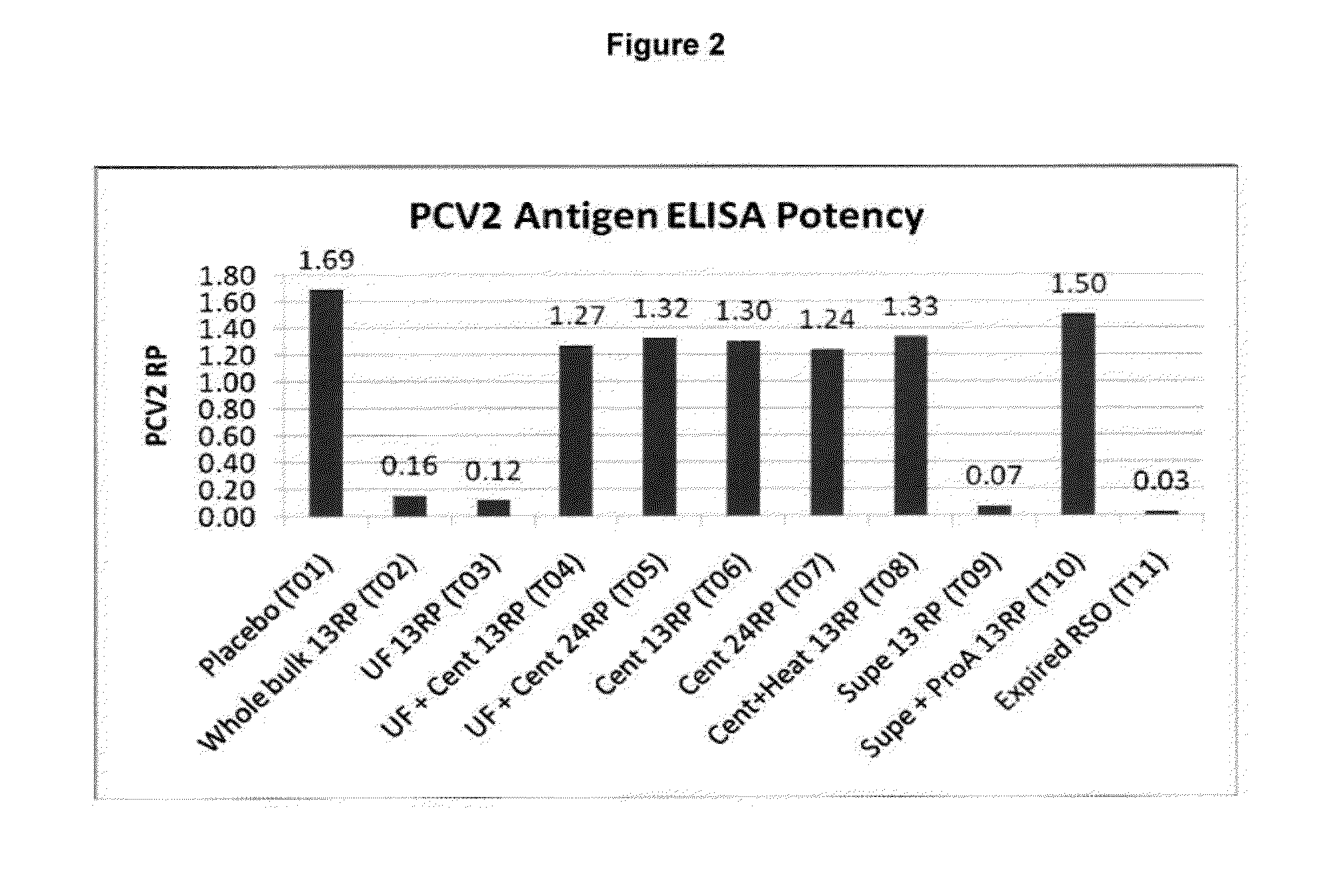
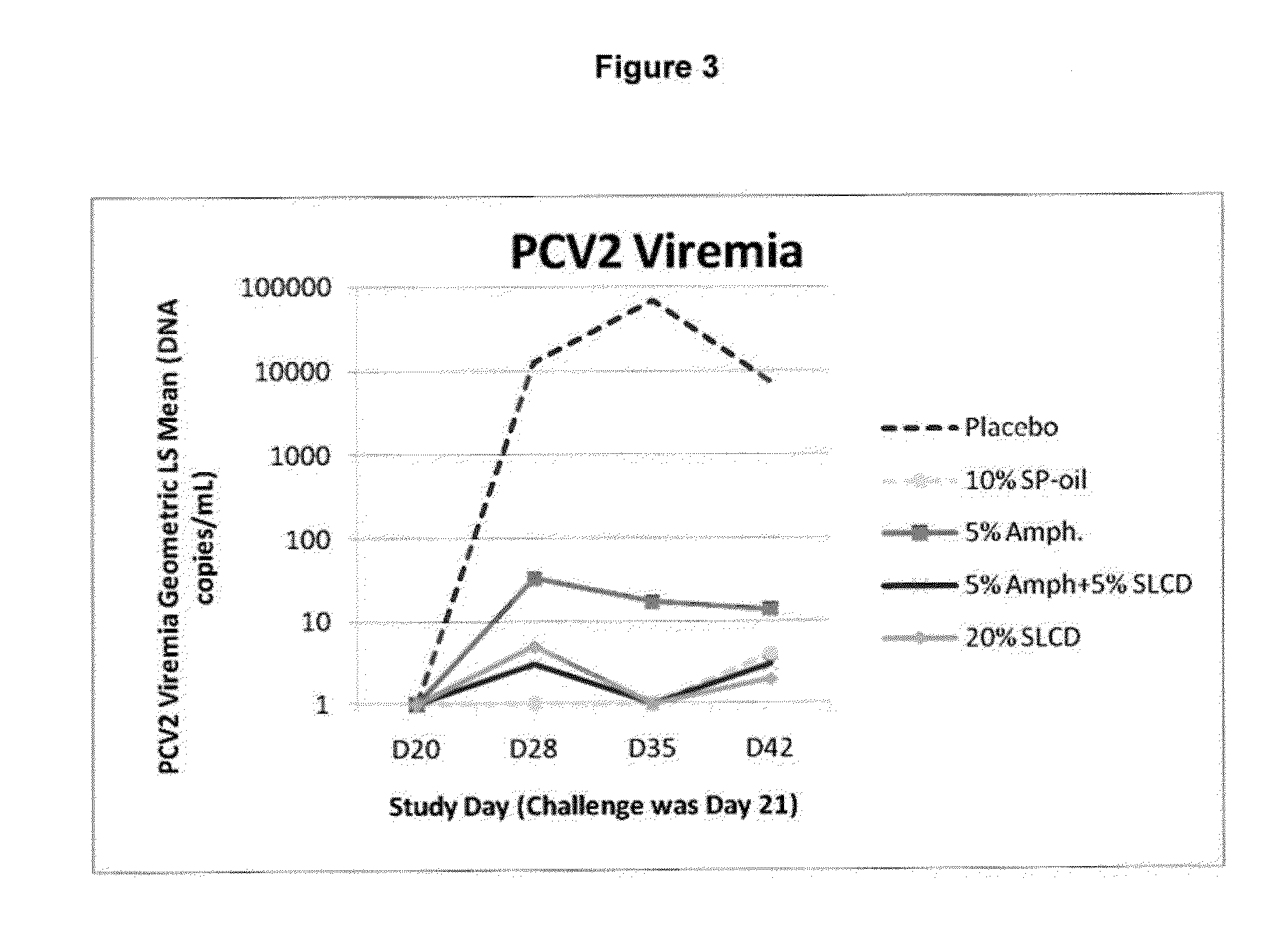
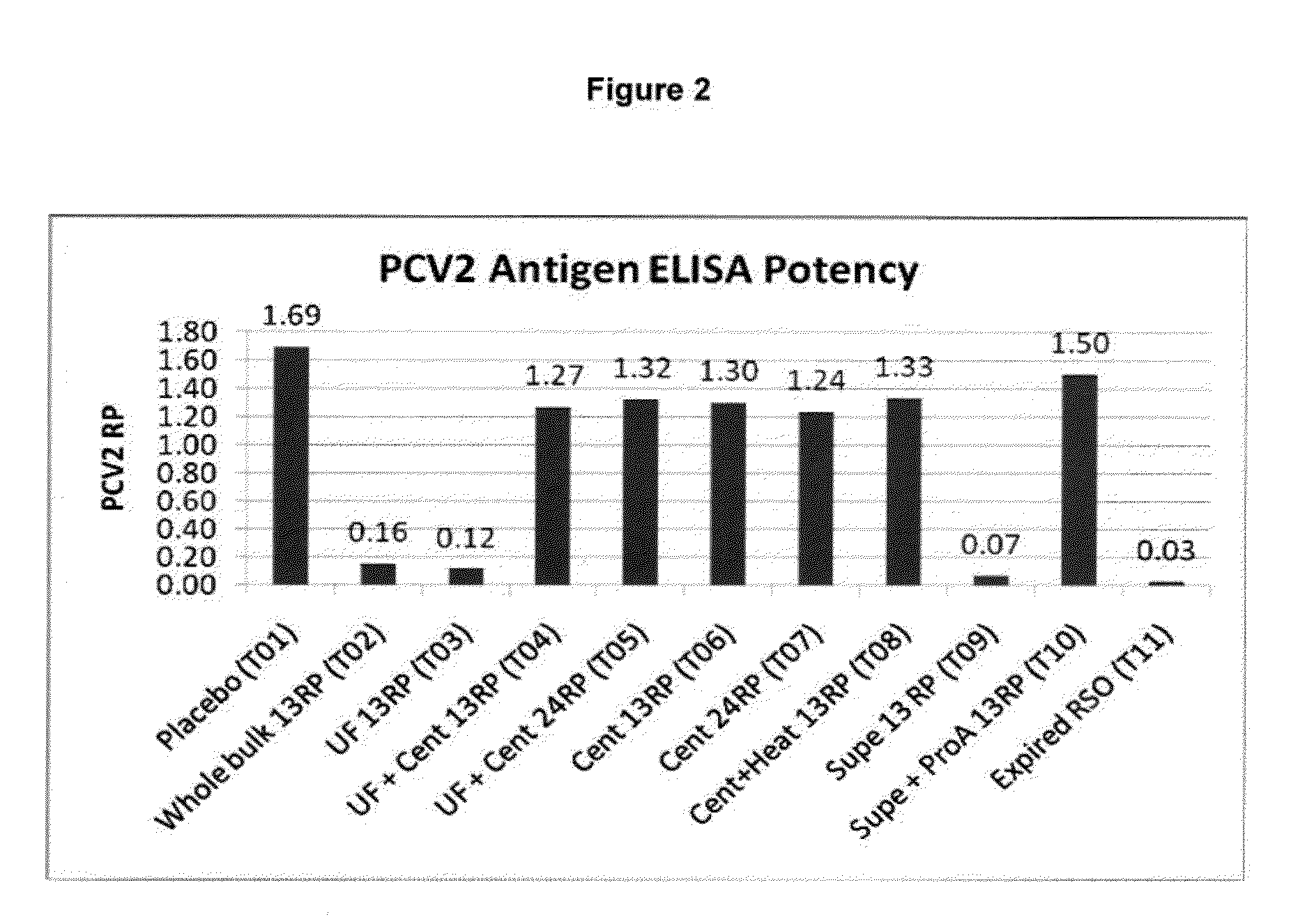
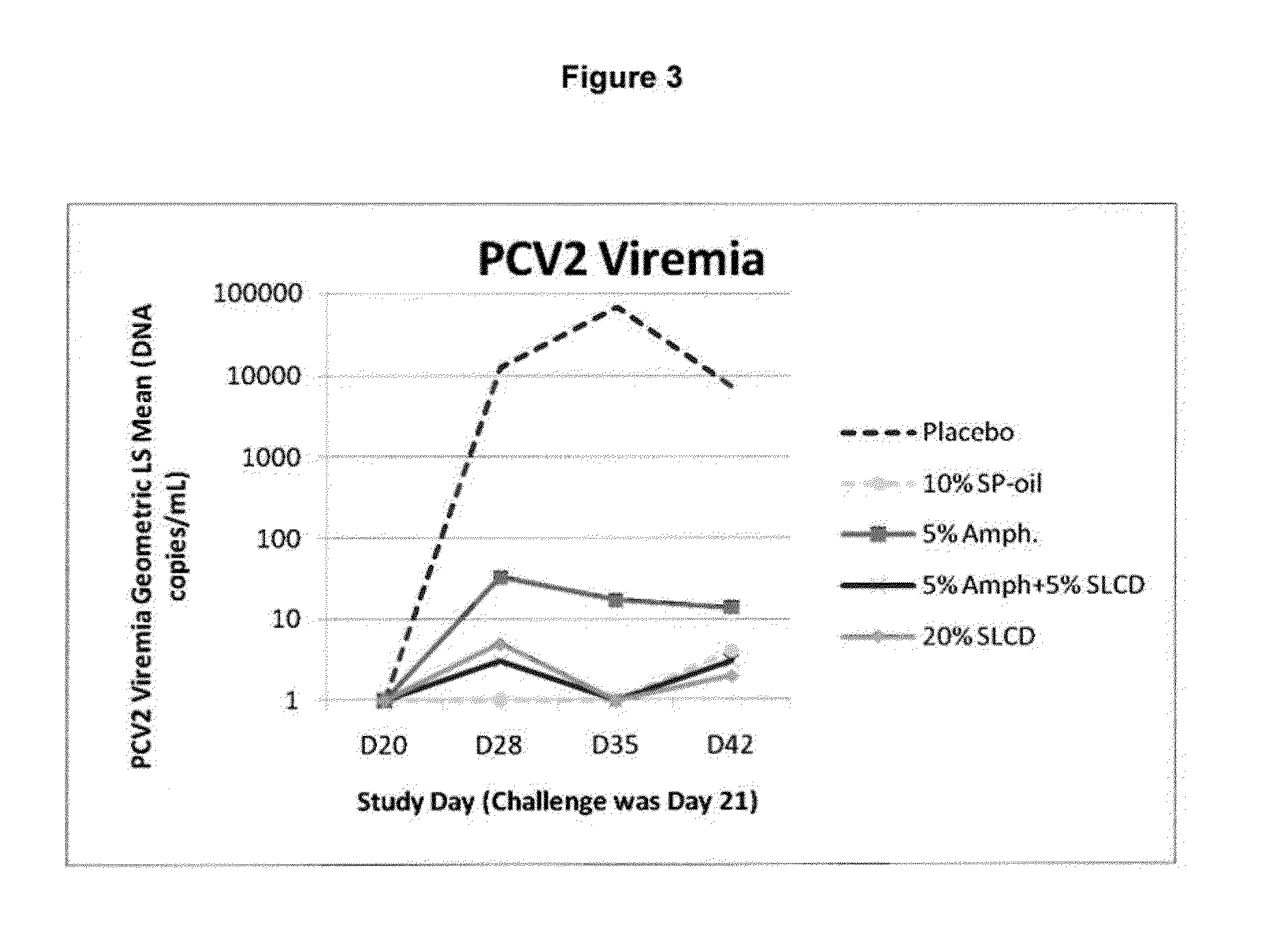
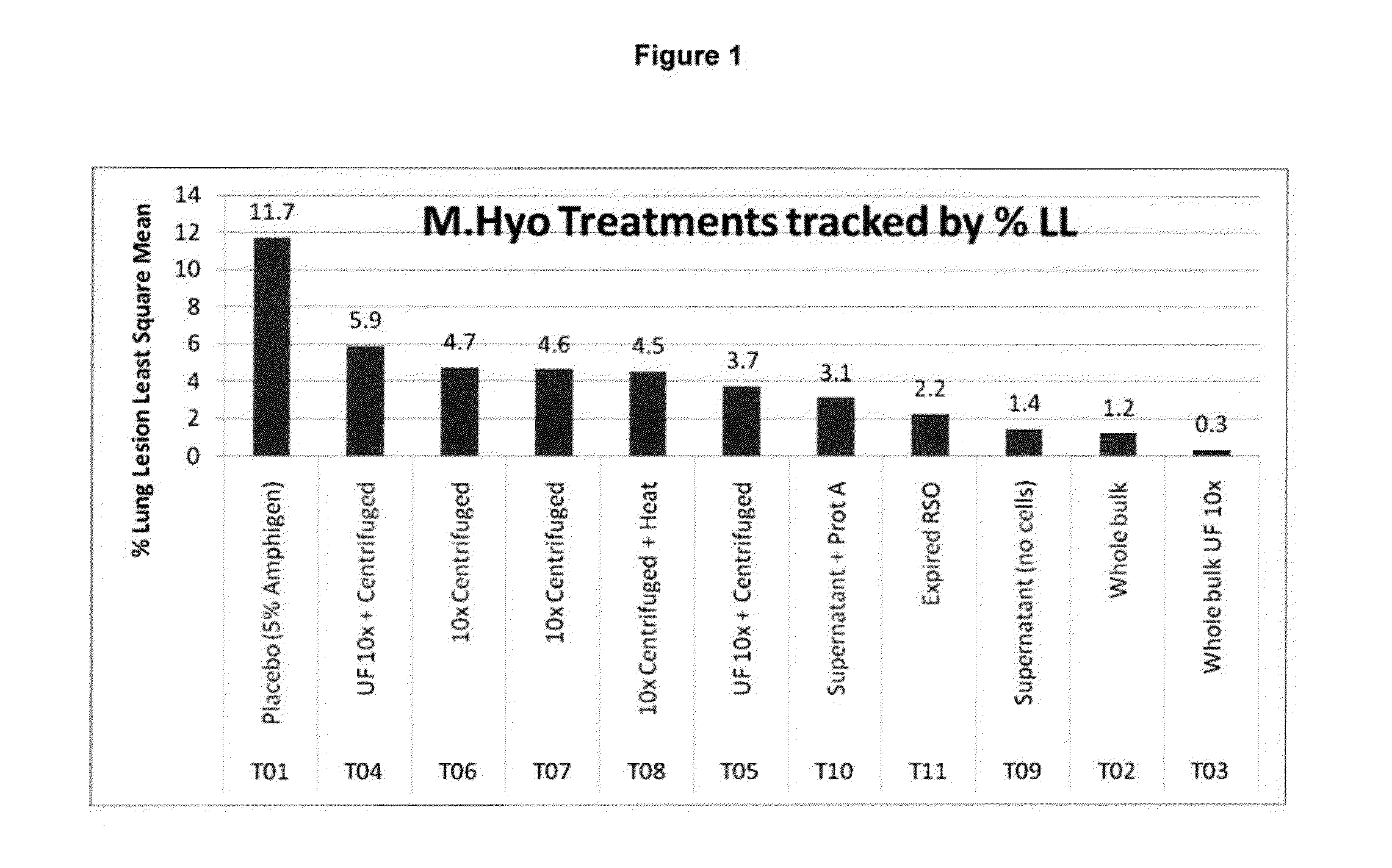
PCV/Mycoplasma Hyopneumoniae/PRRS Combination Vaccine
ActiveUS20130266603A1Protective immune responseAntibacterial agentsBacterial antigen ingredientsImmune complexVirus antigen
This invention provides a trivalent immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M. hyo) whole cell preparation; a porcine circovirus type 2 (PCV2) antigen; and a PRRS virus antigen, wherein the soluble portion of the M. hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
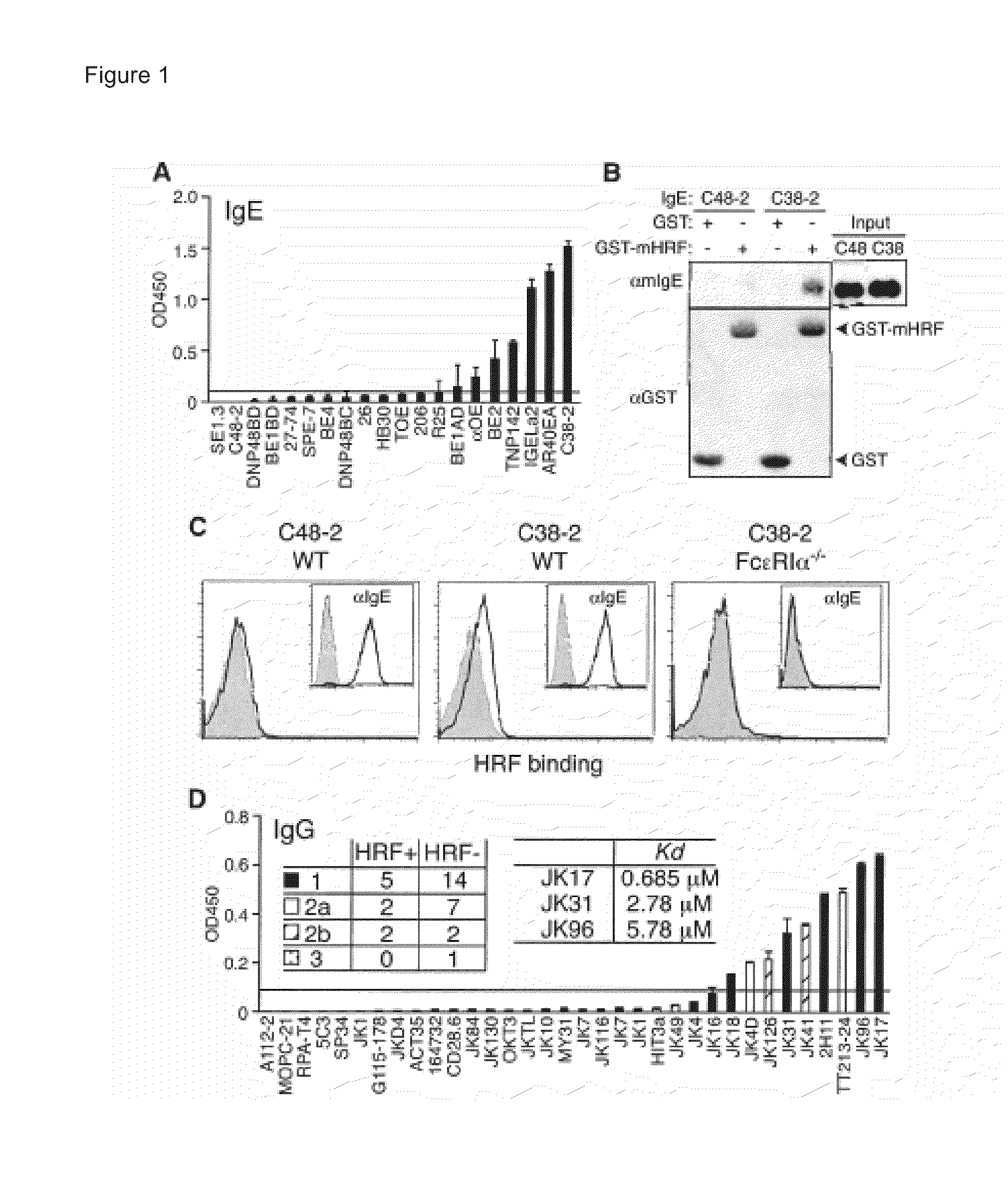
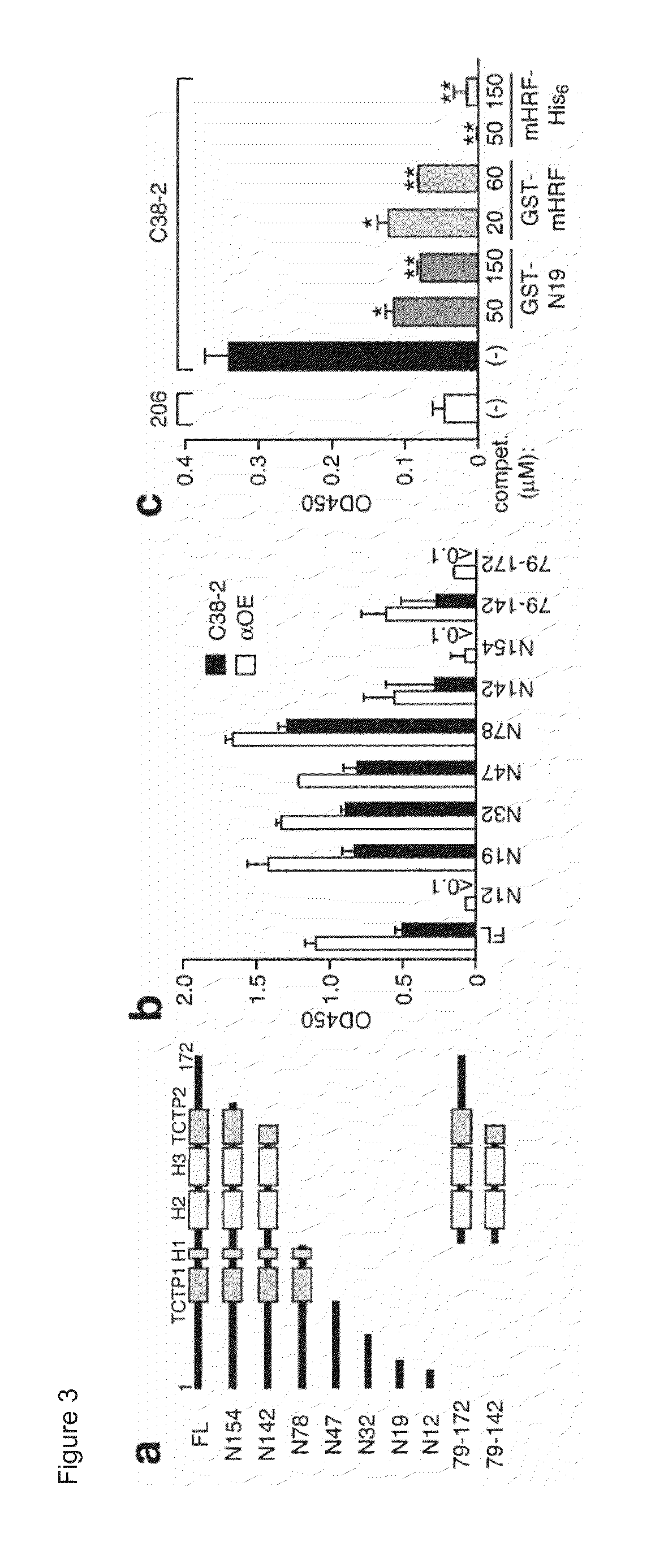
Histamine-releasing factor (HRF), hrf-receptor and methods of modulating inflammation
InactiveUS20130084293A1Shorten the durationReduce frequencyCompound screeningApoptosis detectionHistamine releasing factorHypersensitive response
Methods of treating a food allergy, allergic reactions, hypersensitivity, inflammatory responses, inflammation are provided. In one method, histamine releasing factor (HRF) / translationally controlled tumor protein (TCTP) is contacted with a compound that inhibits or reduces binding of HRF / TCTP to an immunoglobulin in order to treat the food allergy, allergic reaction, hypersensitivity, inflammatory response, or inflammation. Methods of reducing or decreasing the probability, severity, frequency, duration or preventing a subject from having an acute or chronic food allergy, allergic reaction, hypersensitivity, an inflammatory response or inflammation, are also provided. In one method, histamine releasing factor (HRF) / translationally controlled tumor protein (TCTP) is contacted with a compound that inhibits or reduces binding of HRF / TCTP to an immunoglobulin in order to reduce or decrease the probability, severity, frequency, duration or prevent a subject from having an acute or chronic food allergy, allergic reaction, hypersensitivity, an inflammatory response or inflammation.
Owner:LA JOLLA INST FOR ALLERGY & IMMUNOLOGY
PCV/Mycoplasma Hyopneumoniae Combination Vaccine
This invention provides a multivalent immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M. hyo) whole cell preparation; and a porcine circovirus type 2 (PCV2) antigen, wherein the soluble portion of the M. hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
Protein having affinity for immunoglobulin, and immunoglobulin-binding affinity ligand
ActiveUS9403883B2Good chemical stabilityImprove identityBacteriaPeptide/protein ingredientsLow affinityLower affinity
Owner:KANEKA CORP
Mycoplasma Hyopneumoniae Vaccine
ActiveUS20130266601A1Antibacterial agentsSsRNA viruses positive-senseImmunoglobulin IgEMycoplasma hyopneumoniae
This invention provides an immunogenic composition including a soluble portion of a Mycoplasma hyopneumoniae (M.hyo) whole cell preparation, wherein the soluble portion of the M.hyo preparation is substantially free of both (i) IgG and (ii) immunocomplexes comprised of antigen bound to immunoglobulin.
Owner:ZOETIS SERVICE LLC
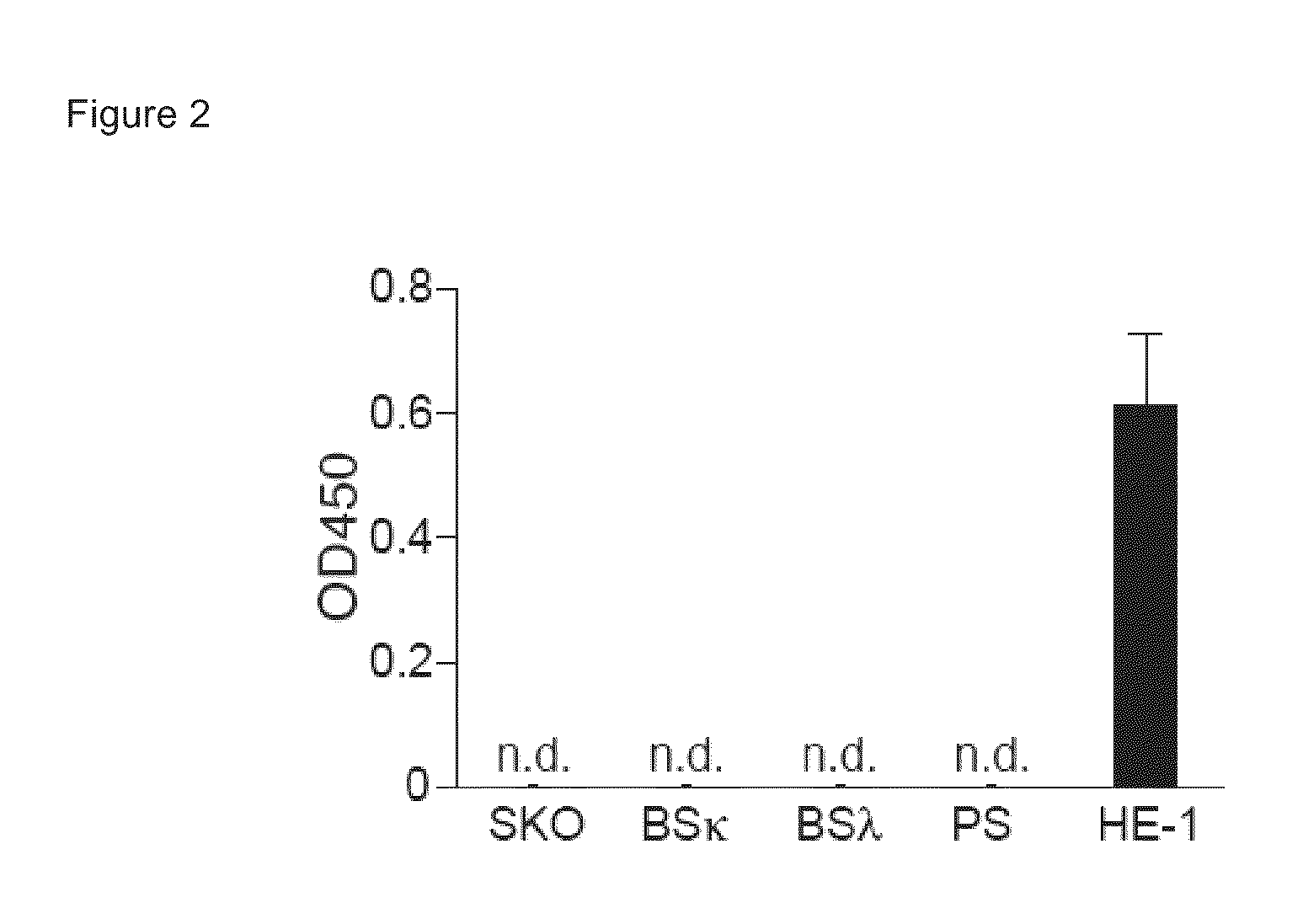
Native immunoglobulin binding reagents and methods for making and using same
InactiveUS20050142609A1Promote resultsPreferentially detect native immunoglobulinAnimal cellsPeptide librariesGlobin bindingReagent
Isolated native immunoglobulin binding reagents including antibodies are provided, along with articles of manufacture, compositions and kits that include the native immunoglobulin binding reagents. Labeled reagents and substrates that comprise samples or the reagents are provided. Methods of screening for, making and using the reagents are also provided.
Owner:EBIOSCIENCE (US)
Mutated protein of protein a having reduced affinity in acidic region and antibody-capturing agent
ActiveUS20140179898A1Reduced ability to bindEasy to eluteBacteriaPeptide/protein ingredientsMutated proteinProteome
A modified protein of an extracellular domain of protein A, which has the reduced ability to bind to immunoglobulin in an acidic region, compared with the wild-type extracellular domain of protein A, without impairing a selective antibody-binding activity in a neutral region. On the basis of three-dimensional structure coordinate data on a complex of the extracellular domain of protein A bound with the Fc region of immunoglobulin G, the modified protein is obtained by the substitution of amino acid residues that are located within the range of 10 angstroms from the Fc region and have a 20% or more ratio of exposed surface area, by histidine residues. Preferably, the modified protein is obtained by the substitution of amino acid residues at sites identified from the analysis of sequences selected from a library constituted by the protein group, by histidine residues. These substitutions may be combined.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Chromatography matrices including novel Staphylococcus aureus protein A based ligands
ActiveUS8754196B2Reduce lossesLarge degree of fragmentationPeptide/protein ingredientsSolid sorbent liquid separationStaphylococcus aureusStaphylococcus aureus bacteria
Owner:MILLIPORE CORP
Mycoplasma hyopneumoniae vaccine
Owner:ZOETIS SERVICE LLC
Separation method
ActiveUS20180094024A1Improve stabilityOther chemical processesImmunoglobulins against bacteriaArginineFc binding
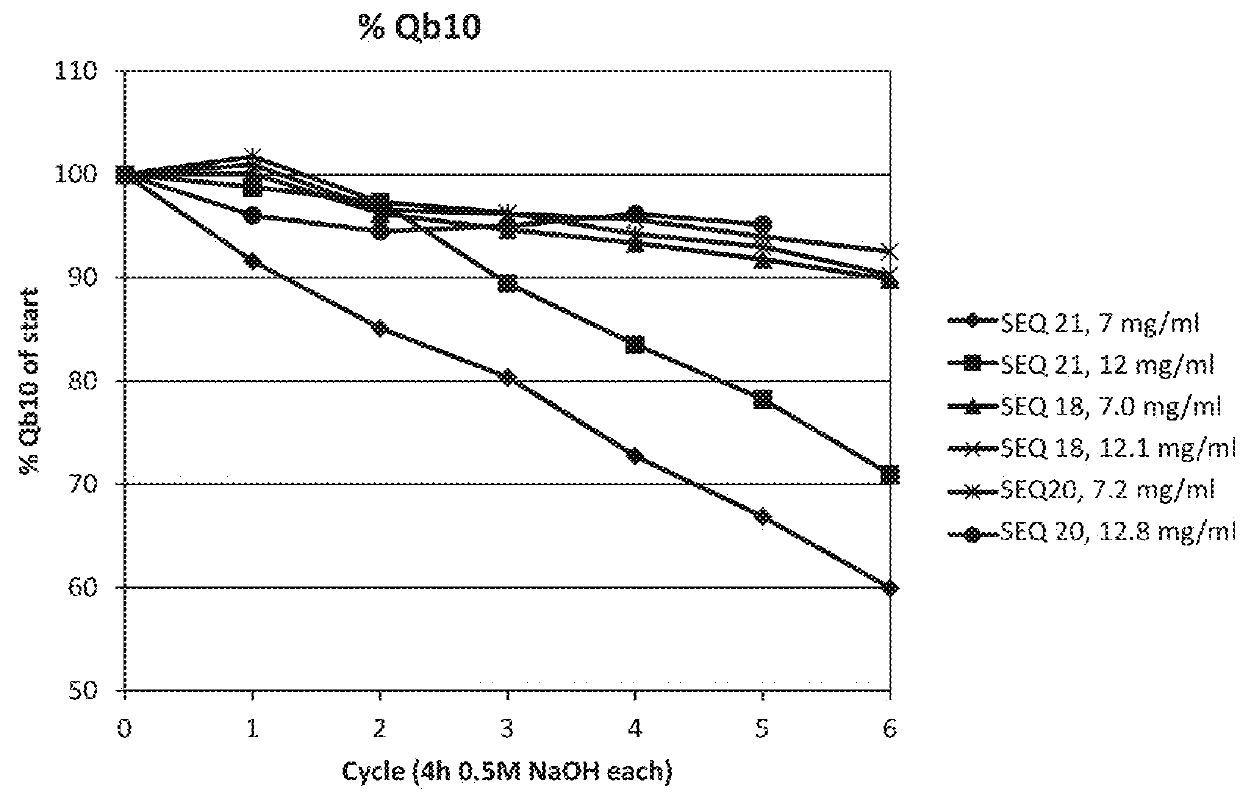
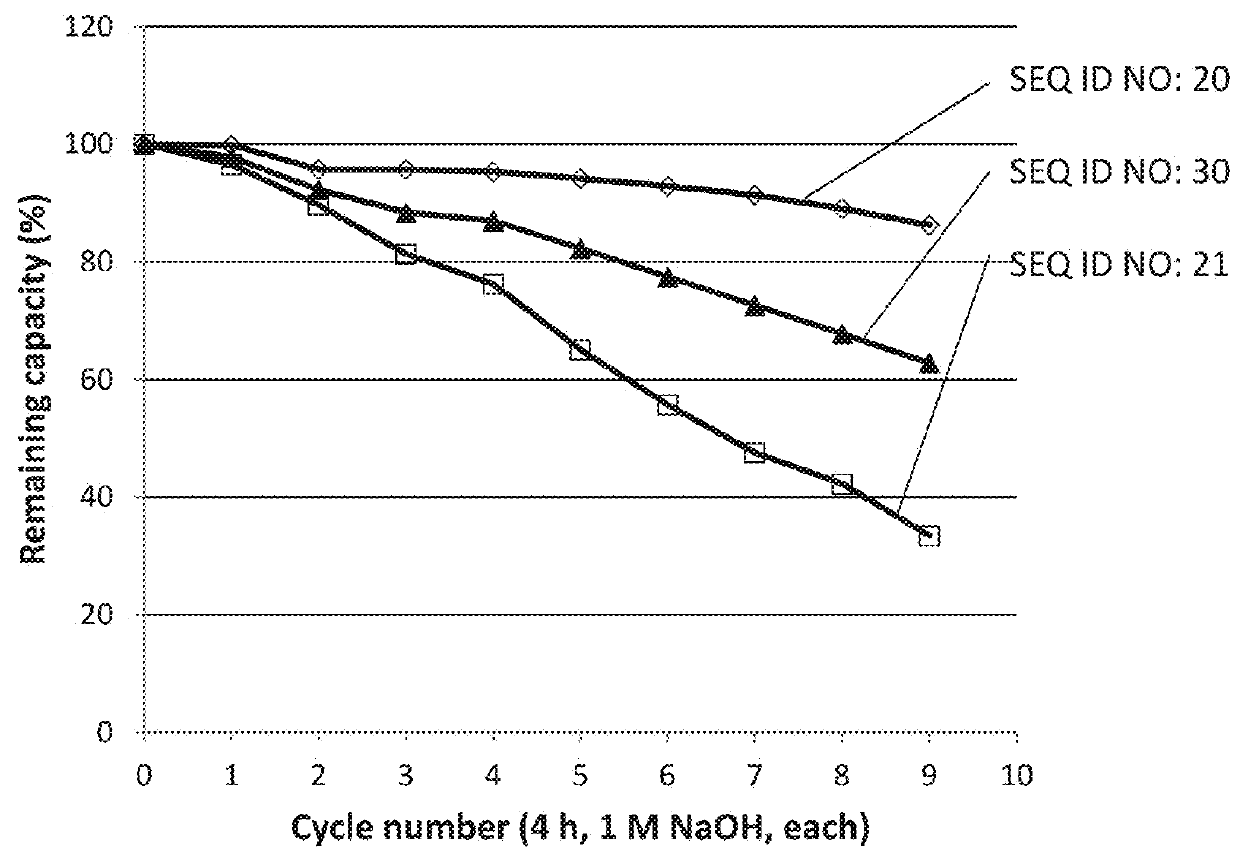
The invention relates to a method of isolating an immunoglobulin, comprising the steps of:a) providing a separation matrix comprising multimers of immunoglobulin-binding alkali-stabilized Protein A domains covalently coupled to a porous support,b) contacting a liquid sample comprising an immunoglobulin with the separation matrix,c) washing said separation matrix with a washing liquid,d) eluting the immunoglobulin from the separation matrix with an elution liquid, ande) cleaning the separation matrix with a cleaning liquid,wherein the alkali-stabilized Protein A domains comprise mutants of a parental Fc-binding domain of Staphylococcus Protein A (SpA), as defined by, or having at least 80% such as at least 90%, 95% or 98% identity to, SEQ ID NO 51 or SEQ ID NO 52, wherein the amino acid residues at positions 13 and 44 of SEQ ID NO 51 or 52 are asparagines and wherein at least the asparagine residue at position 3 of SEQ ID NO 51 or 52 has been mutated to an amino acid selected from the group consisting of glutamic acid, lysine, tyrosine, threonine, phenylalanine, leucine, isoleucine, tryptophan, methionine, valine, alanine, histidine and arginine.
Owner:CYTIVA BIOPROCESS R&D AB
Affinity chromatography matrix
ActiveUS20150080554A1Optimize purification stepsInhibition formationOther chemical processesSolid sorbent liquid separationThreonineHistidine
The invention discloses an immunoglobulin-binding protein comprising one or more mutated immunoglobulin-binding domains (monomers) of staphylococcal Protein A (E, D, A, B, C) or protein Z or a functional variant thereof, wherein in at least one of the one or more mutated monomers, the asparagine or histidine at the position corresponding to H18 of the B domain of Protein A or of Protein Z has been deleted or substituted with a first amino acid residue which is not proline or asparagine and wherein, if the amino acid residue at position 57 is proline and the amino acid residue at position 28 is asparagine, then the amino acid residue at the position corresponding to H18 of the B domain of protein A or of protein Z is not serine, threonine or lysine.
Owner:CYTIVA BIOPROCESS R&D AB
Nucleic acids encoding ara h 3 polypeptides
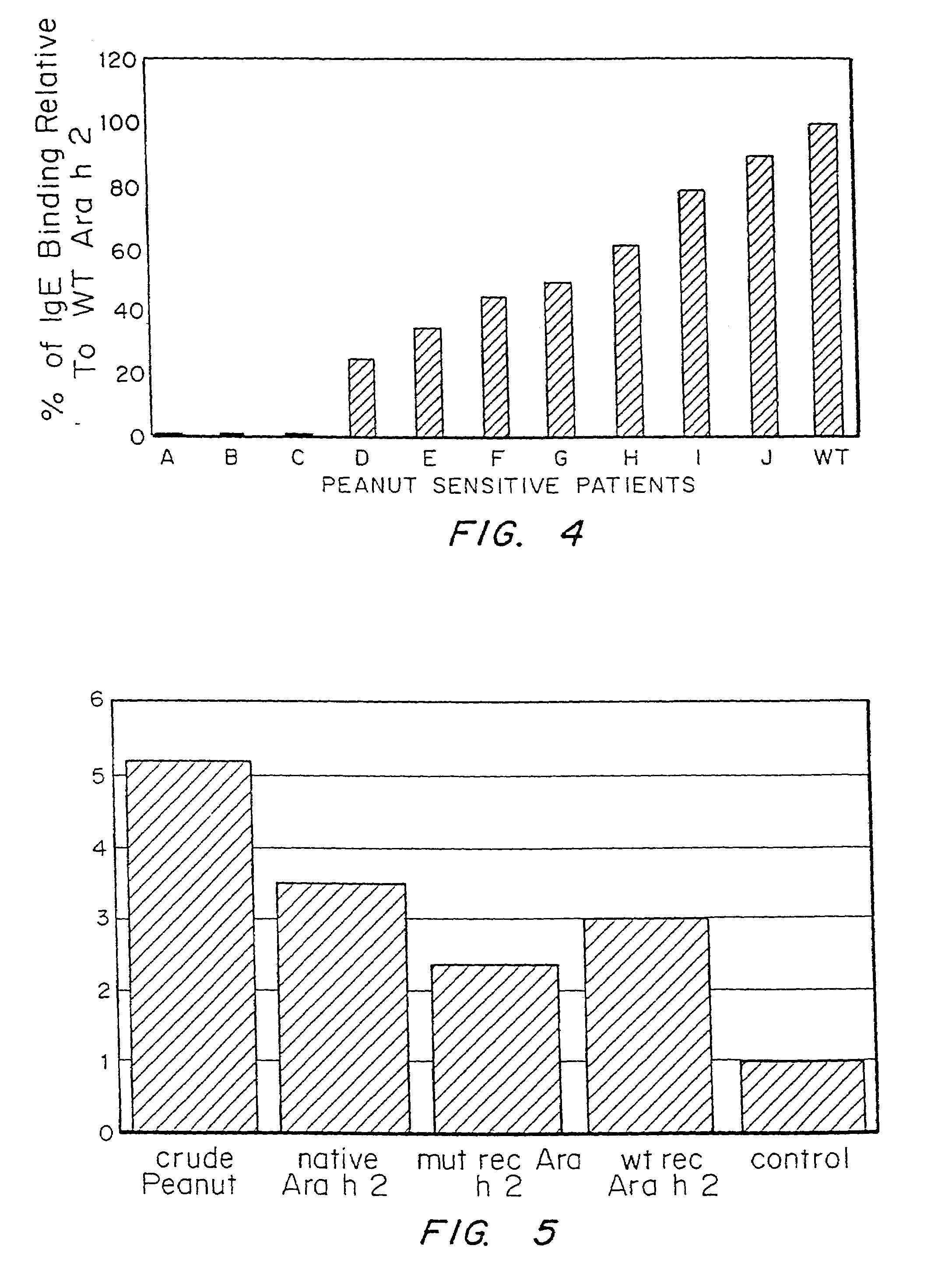
It has been determined that allergens, which are characterized by both humoral (IgE) and cellular (T cell) binding sites, can be modified to be less allergenic by modifying the IgE binding sites. The IgE binding sites can be converted to non-IgE binding sites by masking the site with a compound that prevents IgE binding or by altering as little as a single amino acid within the protein, most typically a hydrophobic residue towards the center of the IgE binding epitope, to eliminate IgE binding. The method allows the protein to be altered as minimally as possible, other than within the IgE-binding sites, while retaining the ability of the protein to activate T cells, and, in some embodiments by not significantly altering or decreasing IgG binding capacity. The examples use peanut allergens to demonstrate alteration of IgE binding sites. The critical amino acids within each of the IgE binding epitopes of the peanut protein that are important to immunoglobulin binding have been determined. Substitution of even a single amino acid within each of the epitopes led to loss of IgE binding. Although the epitopes shared no common amino acid sequence motif, the hydrophobic residues located in the center of the epitope appeared to be most critical to IgE binding.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Separation method
ActiveUS10730908B2Improve stabilityHighly selectiveIon-exchange process apparatusOther chemical processesPhysical chemistryIntravenous gammaglobulin
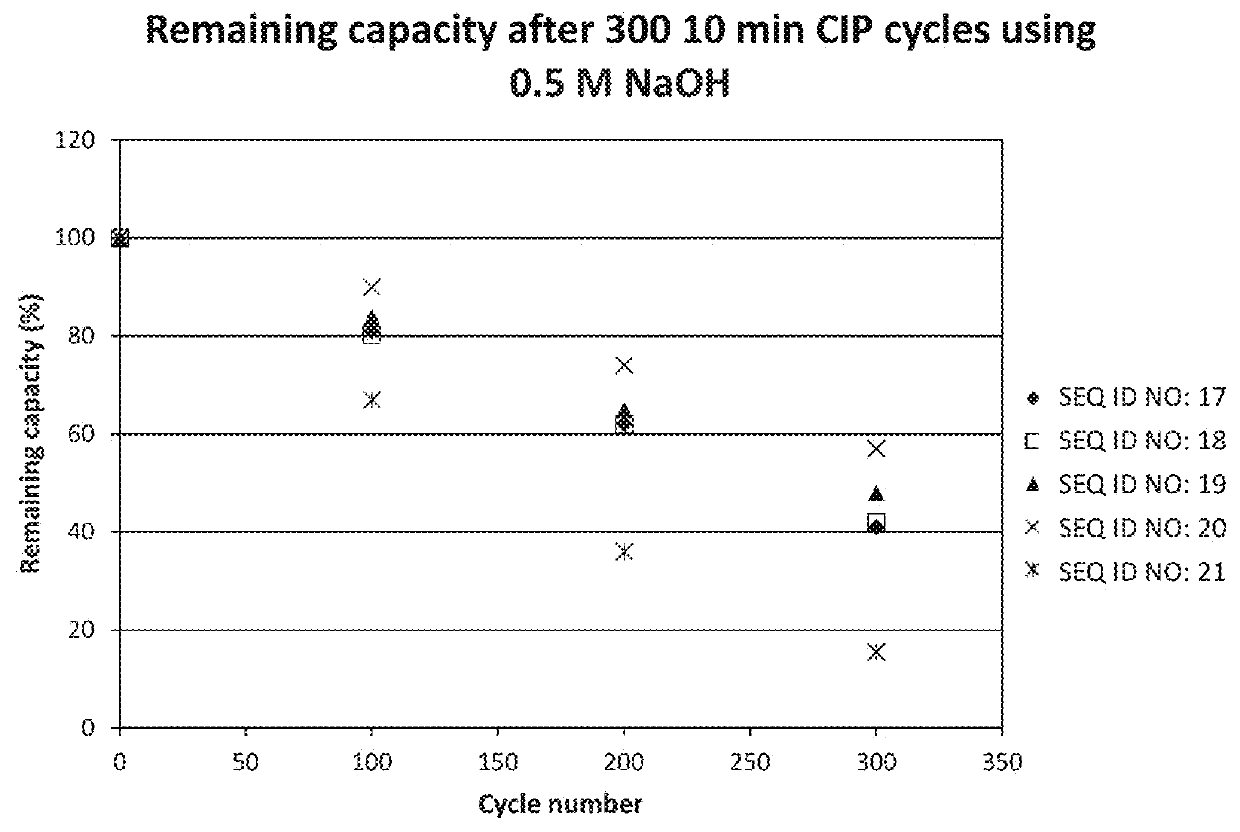
The invention relates to a method of isolating an immunoglobulin, comprising the steps of:a) providing a separation matrix comprising at least 15 mg / ml multimers of immunoglobulin-binding alkali-stabilized Protein A domains covalently coupled to a porous support, wherein the porous support comprises cross-linked polymer particles having a volume-weighted median diameter (d50,v) of 56-70 micrometers and a dry solids weight of 55-80 mg / ml;b) contacting a liquid sample comprising an immunoglobulin with the separation matrix;c) washing the separation matrix with a washing liquid;d) eluting the immunoglobulin from the separation matrix with an elution liquid; ande) cleaning the separation matrix with a cleaning liquid comprising at least 0.5 M NaOH.
Owner:CYTIVA BIOPROCESS R&D AB
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com