Histamine-releasing factor (HRF), hrf-receptor and methods of modulating inflammation
a technology of hrf and receptor, which is applied in the field of hrf, hrf receptor and methods of modulating inflammation, can solve the problems of hrf, if any, being elusive for decades in the role of hrf in allergic and other immune diseases, and low levels of phosphorylation, so as to reduce the binding of hrf/tctp, reduce the probability, severity, frequency, duration or preventing a subject, and reduce the probability of hr
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0226]This example includes a description of various materials and methods.
Mice
[0227]C57BL / 6 and Balb / c mice were purchased from the Jackson Laboratory (Maine). FcεRIα− / − mice (Dombrowicz et al., Cell 75:969 (1993)) on a Balb / c background, FcεRIγ− / − mice (Takai et al., Cell 76:519 (1994)) on a C57BL / 6 background, μMT mice (Kitamura et al., Nature 350:6317 (1991)) on a C57BL / 6 background, and KitW-sh / W-sh (Grimbaldeston et al., Am. J. Pathol. 167:835 (2005)) on a C57BL / 6 background. Such knockout mice are usually backcrossed to Balb / c or C57BL / 6 mice for >10 generations.
Purification and Preparation of IgE and IgG
[0228]For purification of IgE, Ishizaka's method (Ishizaka, Methods Enzymol. 116:76 (1985)) was utilized such that IgE was enriched from ascites or culture supernatants by ammonium sulfate precipitation (40-55%). IgE-containing fractions were then purified by DEAE-agarose (GE Healthcare Bio-Sciences AB) column chromatography, followed by gel filtration using Sephacryl S-300 (...
example 2
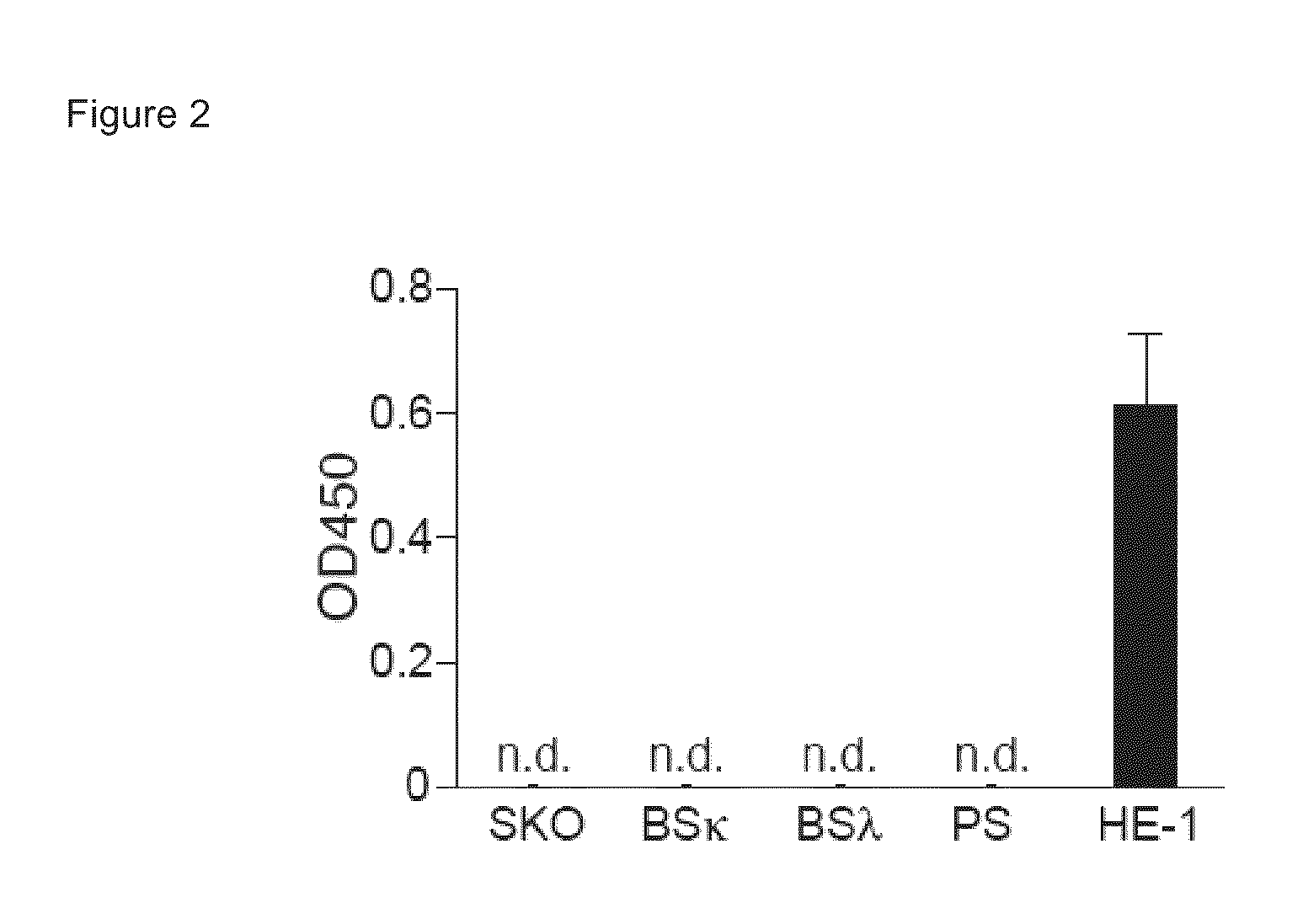
[0248]This example includes a description of a subset of mouse IgE and IgG molecules, and that human IgE binds to HRF.
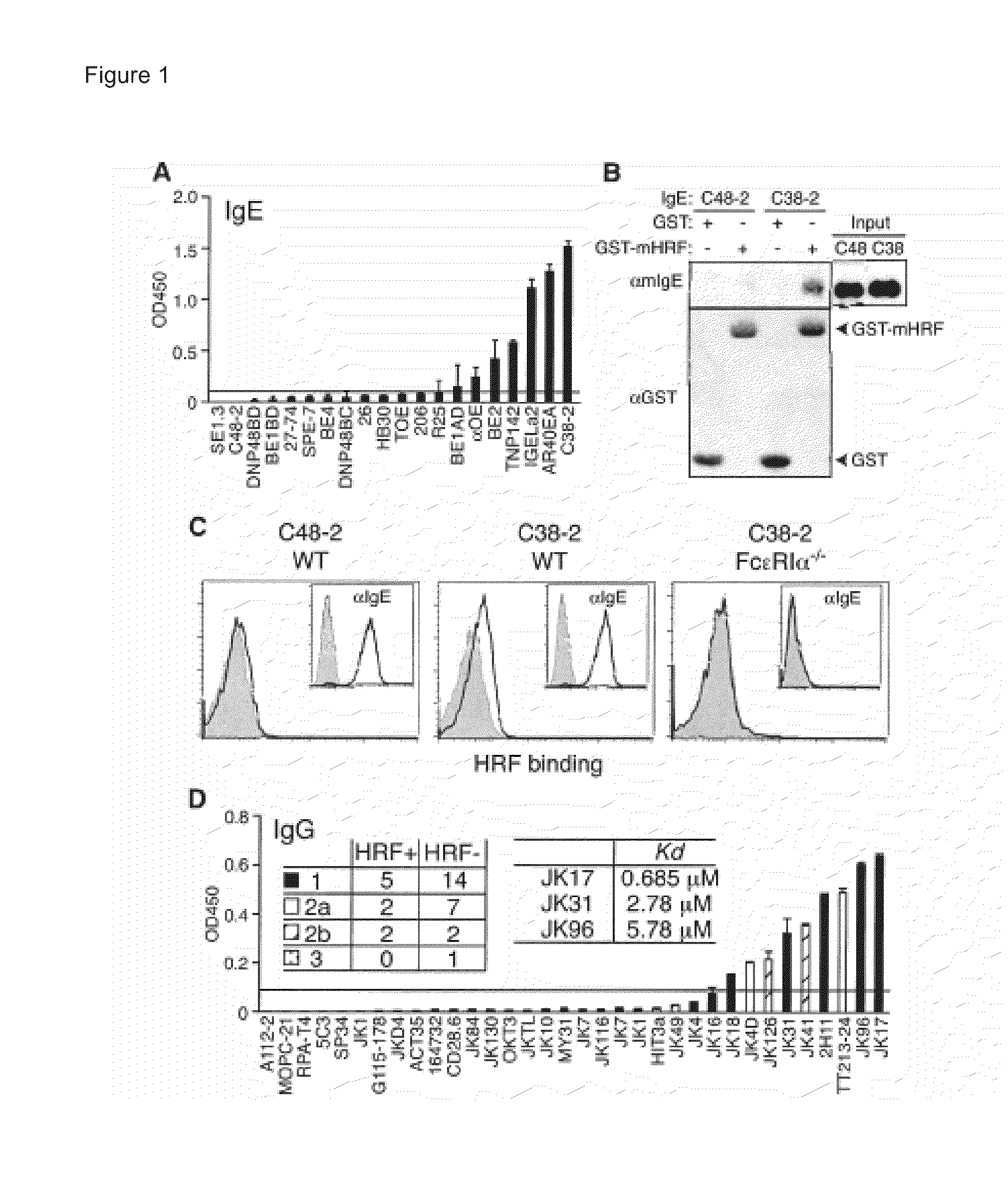
[0249]A previous report indicated that IgE polyclonal antibody does not interact with HRF (Wantke, et al., J Allergy Clin Immunol 103, 642 (April 1999)). To evaluate the possibility of IgE / HRF interactions, a panel of IgE mAbs was examined. N-terminally glutathione S-transferase (GST)-tagged mouse HRF protein (GST-mHRF) or GST were immobilized onto enzyme-linked immunosorbent assay (ELISA) plates and incubated with IgE mAbs. Bound IgE was detected with anti-IgE-biotin and streptavidin-horseradish peroxidase conjugates. GST-mHRF, but not GST, bound C38-2, IGELa2, and 5 other IgE mAbs (FIG. 1A). By contrast, C48-2 and 12 other IgE mAbs failed to bind GST-mHRF using OD4506 (C-terminally hexahistidine-tagged mHRF) was used as a capturing agent.
[0250]Interaction of the C38-2 and IGELa2 IgE mAbs with mHRF was also demonstrated by co-immunoprecipitation from a mixture of Ig...
example 3
[0255]This example includes a description of studies indicating that the N-terminal 19 residue peptide of HRF inhibits the HRF / IgE interaction.
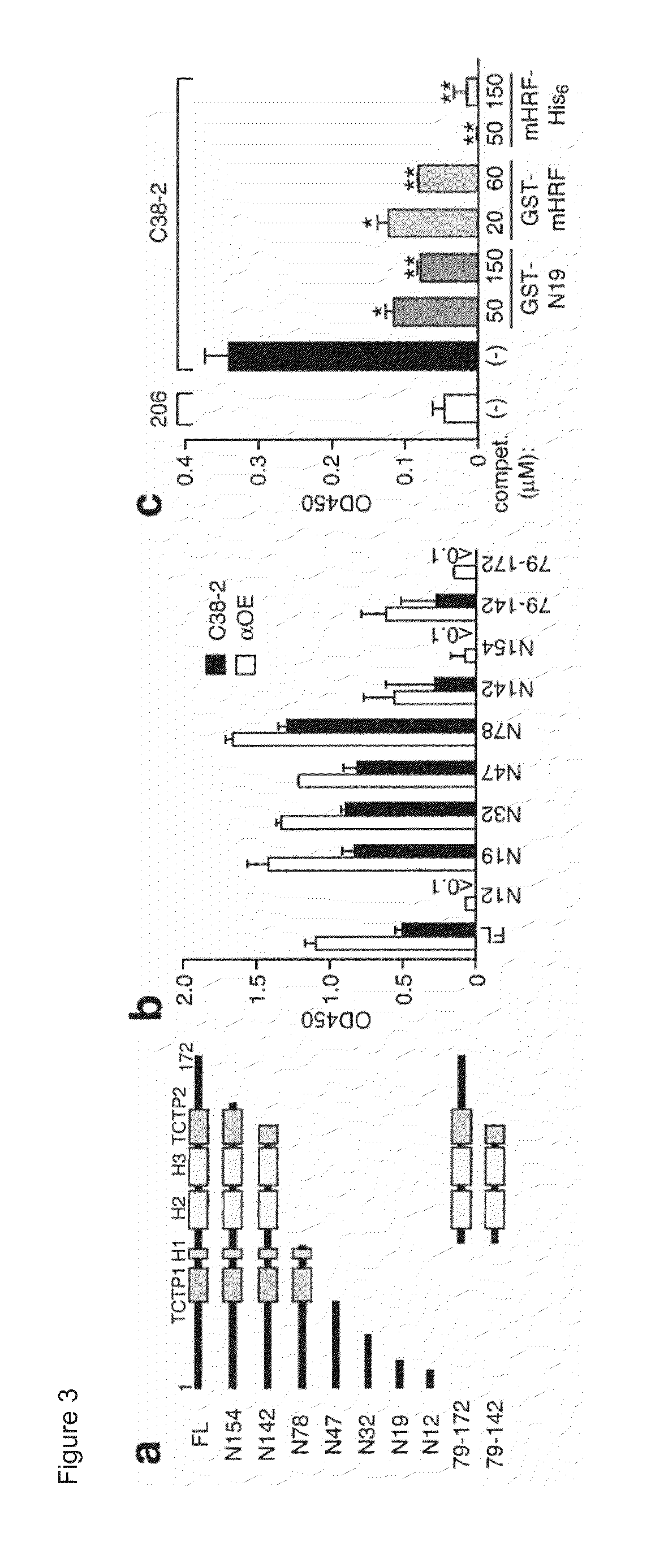
[0256]To map the Ig-interaction site within the mHRF molecule, an ELISA panel of N- or C-terminally truncated GST-mHRF proteins (FIG. 3A) was used as capturing agents and two HRF-reactive IgE mAbs (C38-2 and aOE) and two HRF-reactive IgG mAbs (JK18 and JK31) as binding probes. C38-2 and aOE IgE molecules were incubated in wells coated with full-length or truncated forms of GST-mHRF. After blocking, JK18 and JK31 IgGs were incubated. The bound IgGs were detected by incubation with horseradish peroxidase-conjugated anti-mouse IgG antibody. The absorbance at 450 nm was measured after development of the color.
[0257]HRF-reactive IgE and IgG mAbs gave similar binding patterns (FIGS. 3B and 4). C-terminal truncations of mHRF up to residue 19 did not affect Ig binding, but a further truncation abrogated Ig binding. Thus, a major Ig-binding site was m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| binding affinity | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com