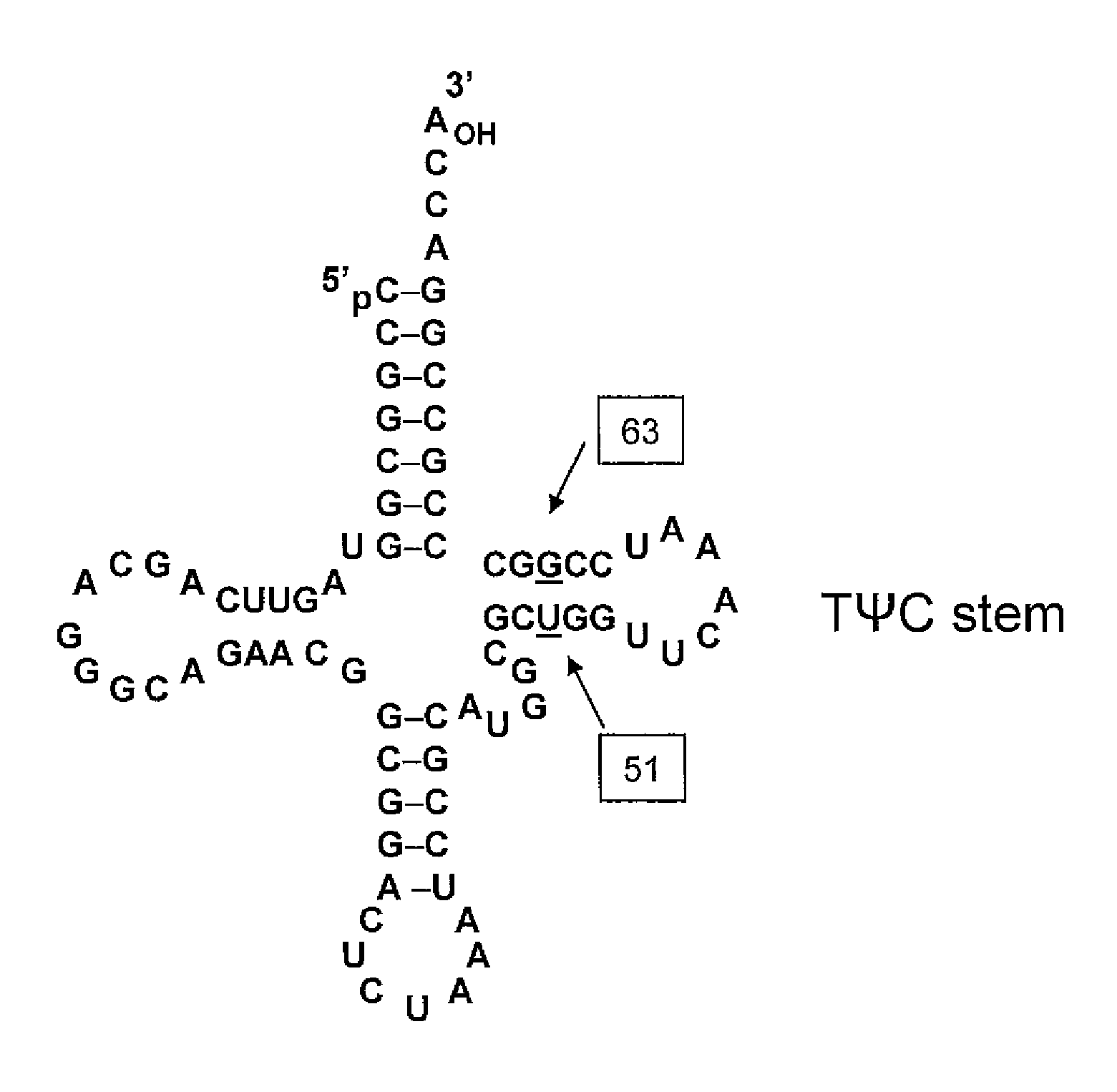Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
282results about How to "Large dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
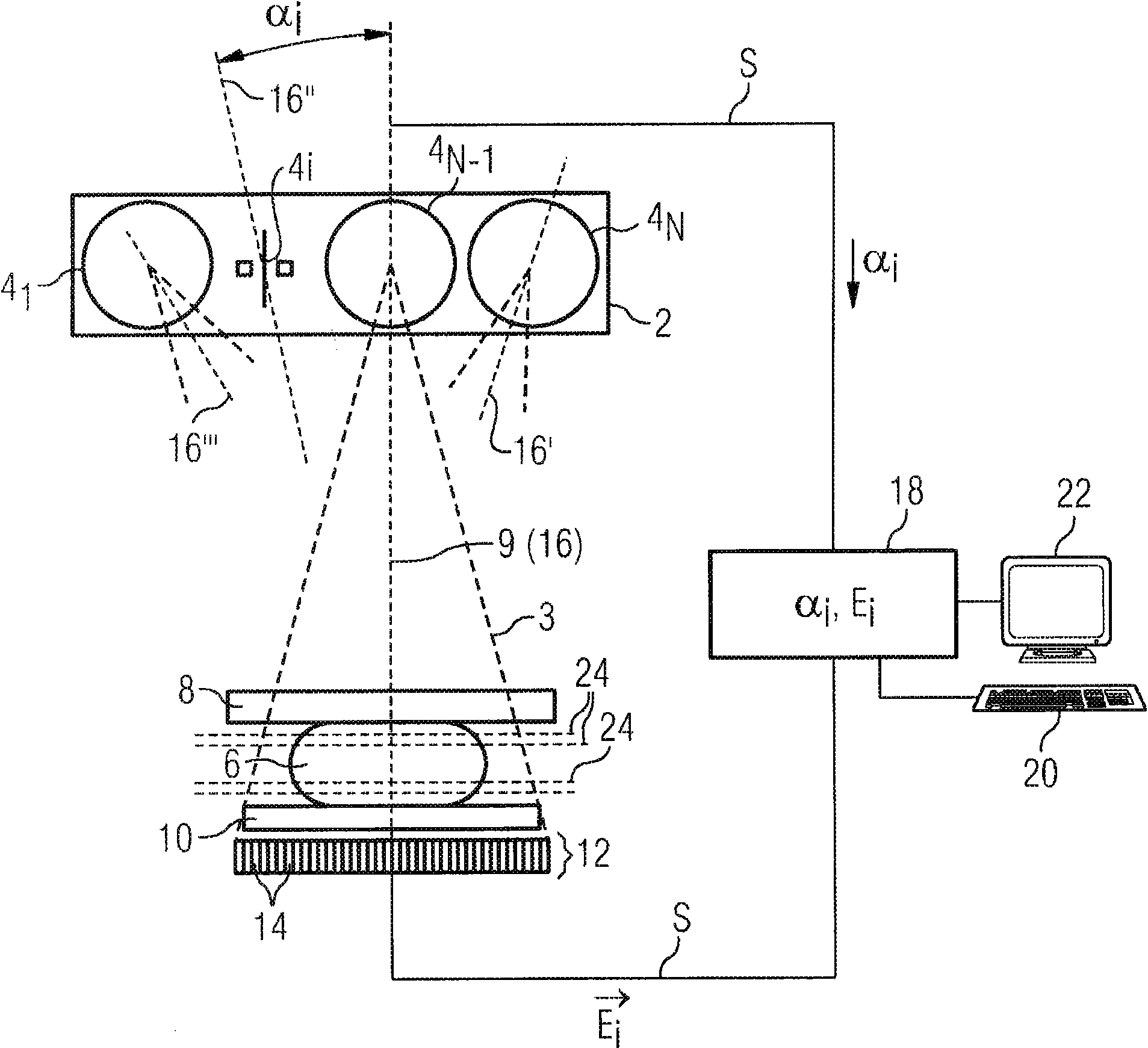
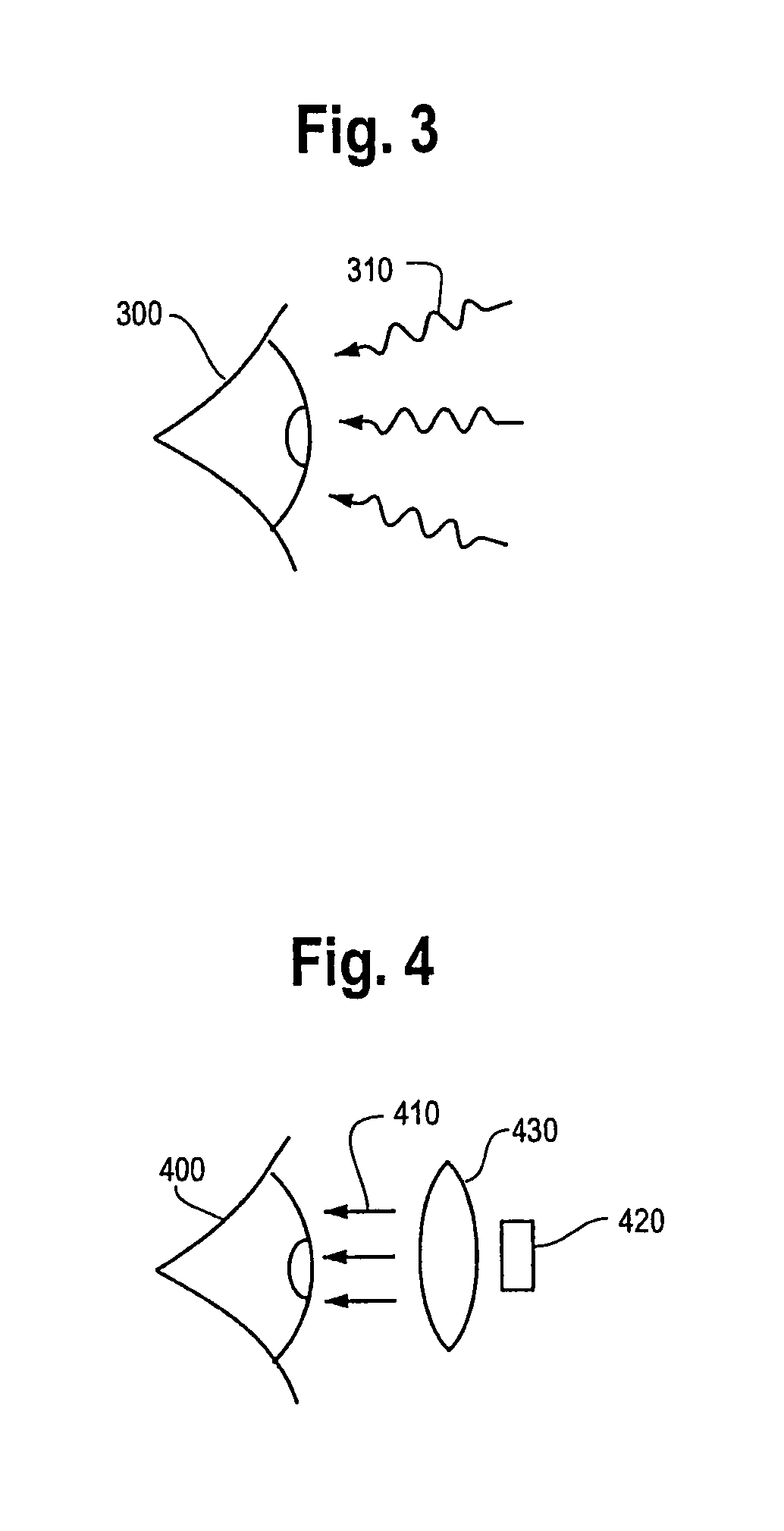
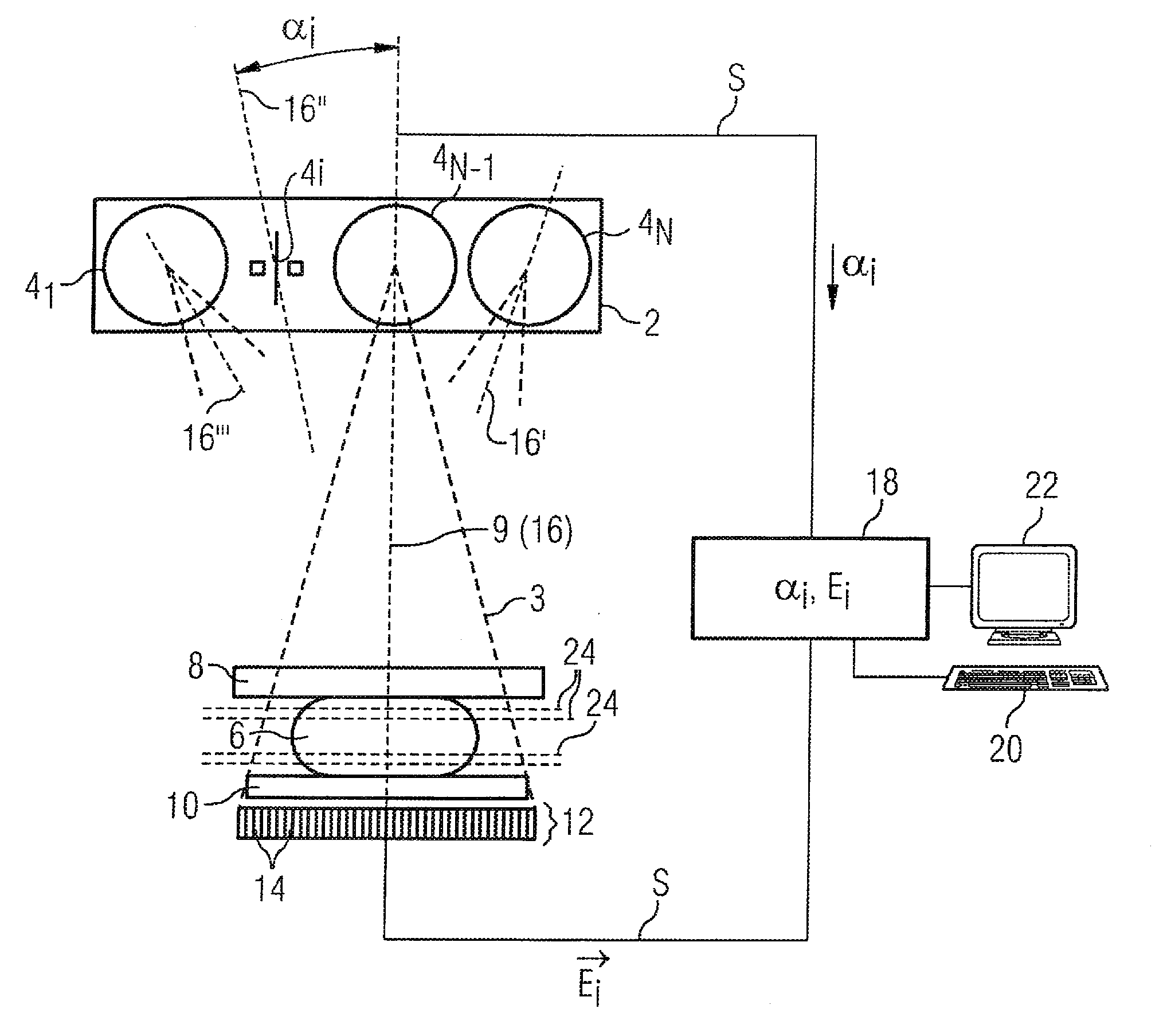
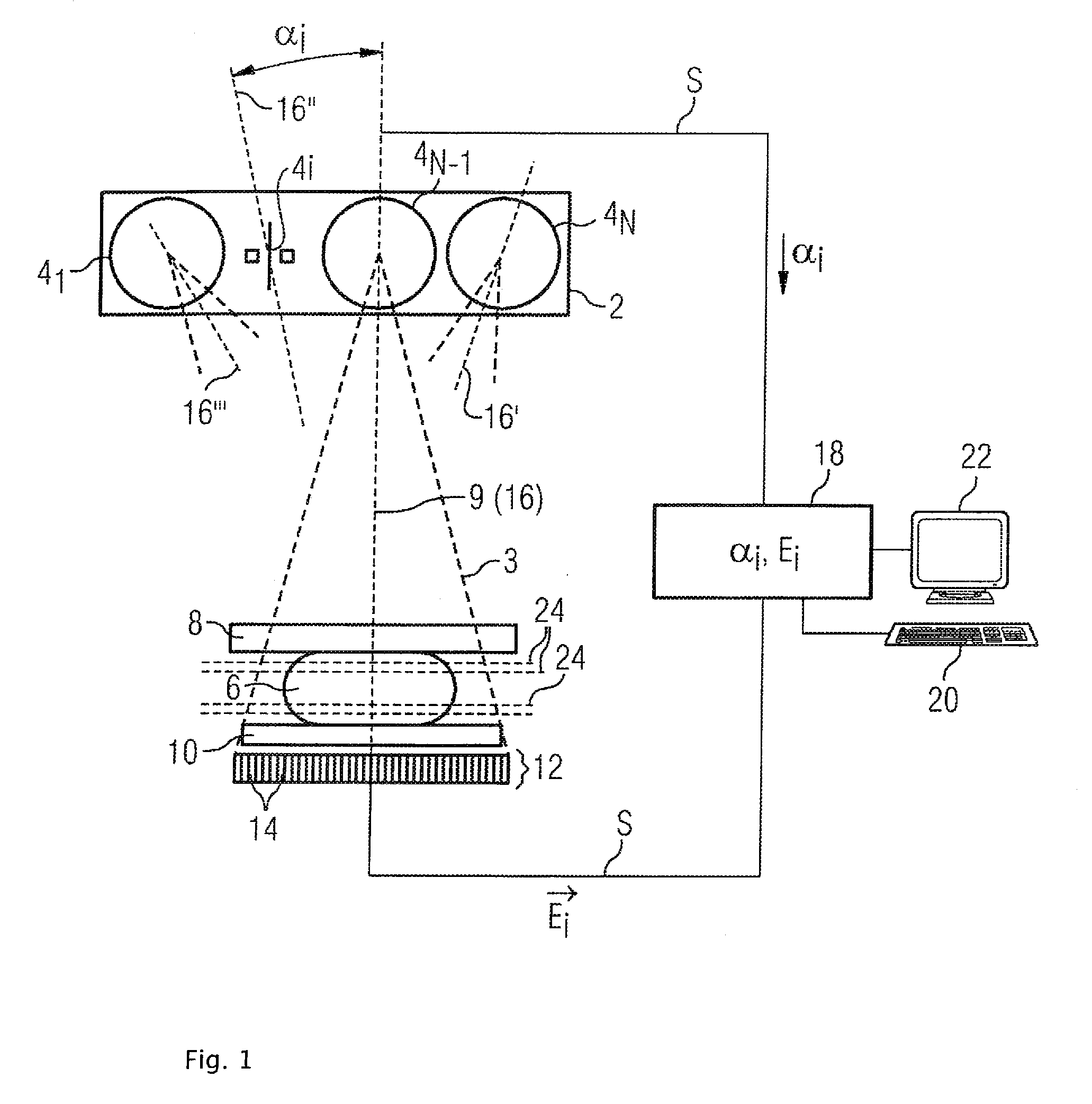
Method and device for producing a tomosynthetic 3D x-ray image
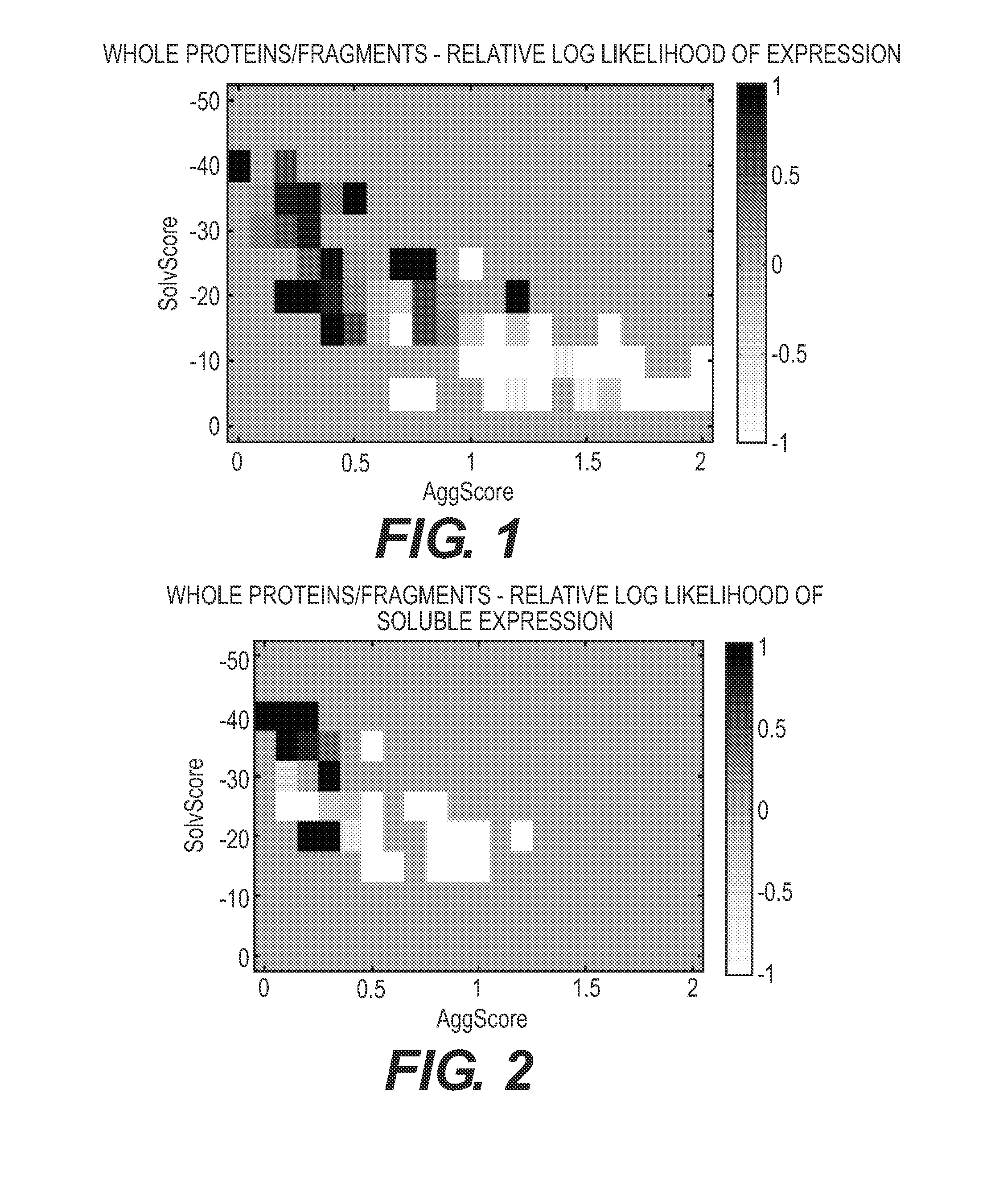
InactiveUS20100034450A1Increase doseLarge doseMaterial analysis using wave/particle radiationRadiation/particle handlingProjection image3d image
In a method and device for producing a tomosynthetic 3D x-ray image, a number of 2D projection images of an examination subject are acquired using a fixed x-ray source. The x-ray source has multiple, individually controllable emitters that respectively emit a single x-ray dose from various different directions. The tomosynthetic 3D image is reconstructed from the individual 2D projection images, and at least one 2D projection image is composed of multiple individual images.
Owner:SIEMENS AG
Non-gelatin soft capsule system
ActiveUS20060099246A1Improve stabilityImprove solubilityBiocidePharmaceutical non-active ingredientsHigh resistanceCarrageenan
A non-gelatin encapsulation system for liquid filled soft capsules, by nature of the carrier, the cationic-ionic balance of the carrier and the active ingredients, or the concentration of the active ingredients and excipients, are difficult or impossible to commercially encapsulate in gelatin capsules. In particular, the system is adapted for the encapsulation of highly basic, or alkaline, fills. The system provides for a predominantly starch and gelling carrageenan based shell, which displays high resistance to both concentrated fills and to alkaline fills, in particular, to those fills which contain the salt or salts of weak acids and strong bases.
Owner:R P SCHERER TECH INC
Modified Release Formulations of (6R)-4,5,6,7-tetrahydro-N6-propyl-2,6-benzothiazole-diamine and Methods of Using the Same
Modified release pharmaceutical compositions (controlled release, sustained release, and / or extended release) of the R-(+) enantiomer of pramipexole (RPPX) and methods of using such compositions for the treatment of neurodegenerative diseases, or those related to mitochondrial dysfunction or increased oxidative stress are disclosed.
Owner:KNOPP BIOSCIENCES LLC
Compositions and methods of using (r)-pramipexole
Pharmaceutical compositions of (R)-pramipexole and methods and kits of using such compositions for the treatment of neurodegenerative diseases, or those related to mitochondrial dysfunction or increased oxidative stress are disclosed.
Owner:KNOPP BIOSCIENCES LLC
Personal noise monitoring apparatus and method
InactiveUS7151835B2Large doseVibration measurement in solidsMaterial analysis using sonic/ultrasonic/infrasonic wavesNoise monitoringCredit card
The present invention relates to a method, system and device for monitoring noise received by individuals operating in noisy environments.The device comprises a substantially credit card size housing and can be worn as a “badge”. The device includes a monitoring means for monitoring sound, and a display for displaying various noise parameter values, including a percentage value of cumulative noise dose received by the device. The device includes an input key which enables a user to select one of a plurality types of hearing protection equipment, so the user can indicate to the device the hearing protection equipment they are wearing. This enables the device to monitor compliance. The device also includes an input key enabling the user to acknowledge when an unallowable noise dose level has been reached.
Owner:SOUNDSAFETY
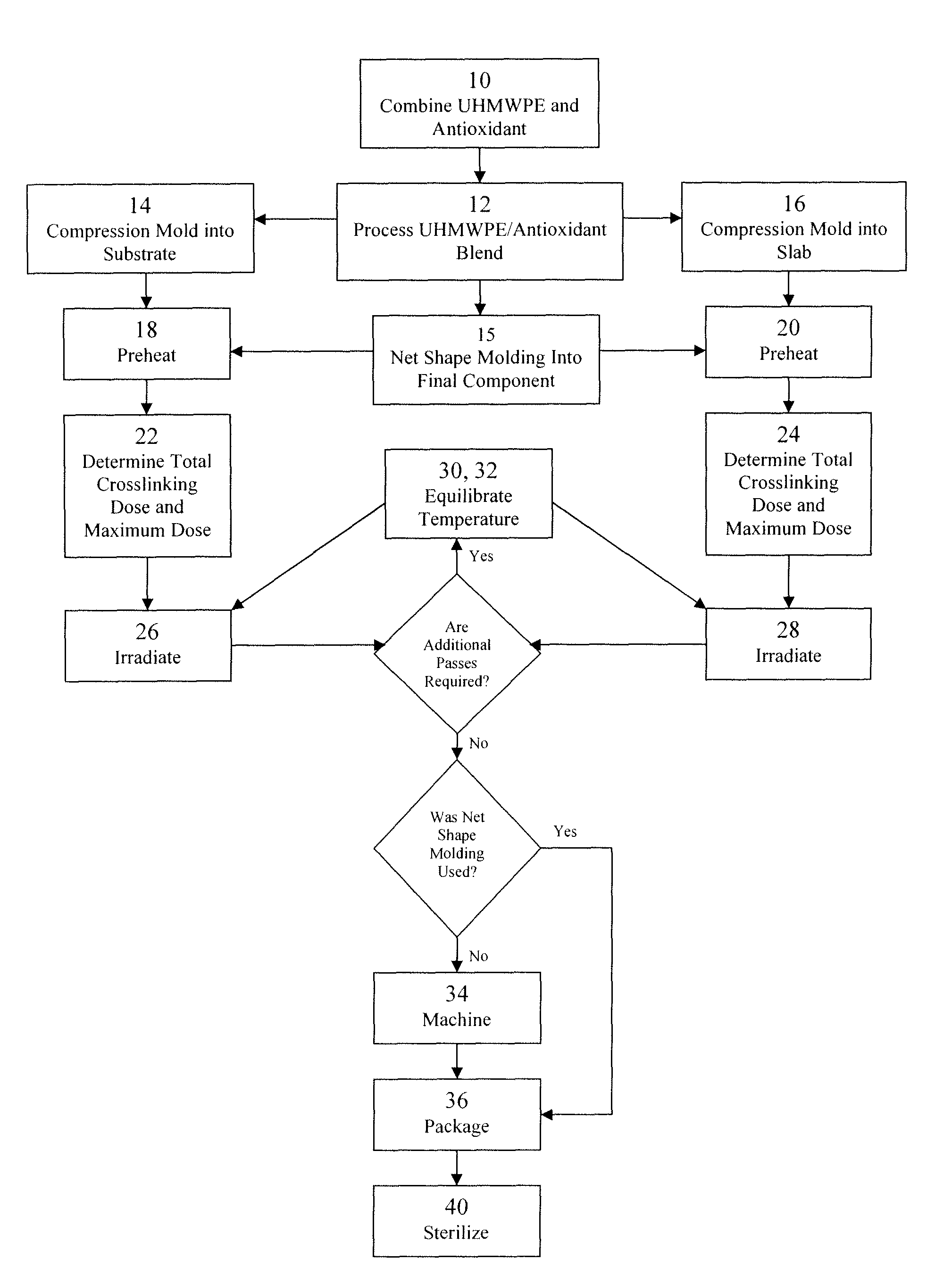
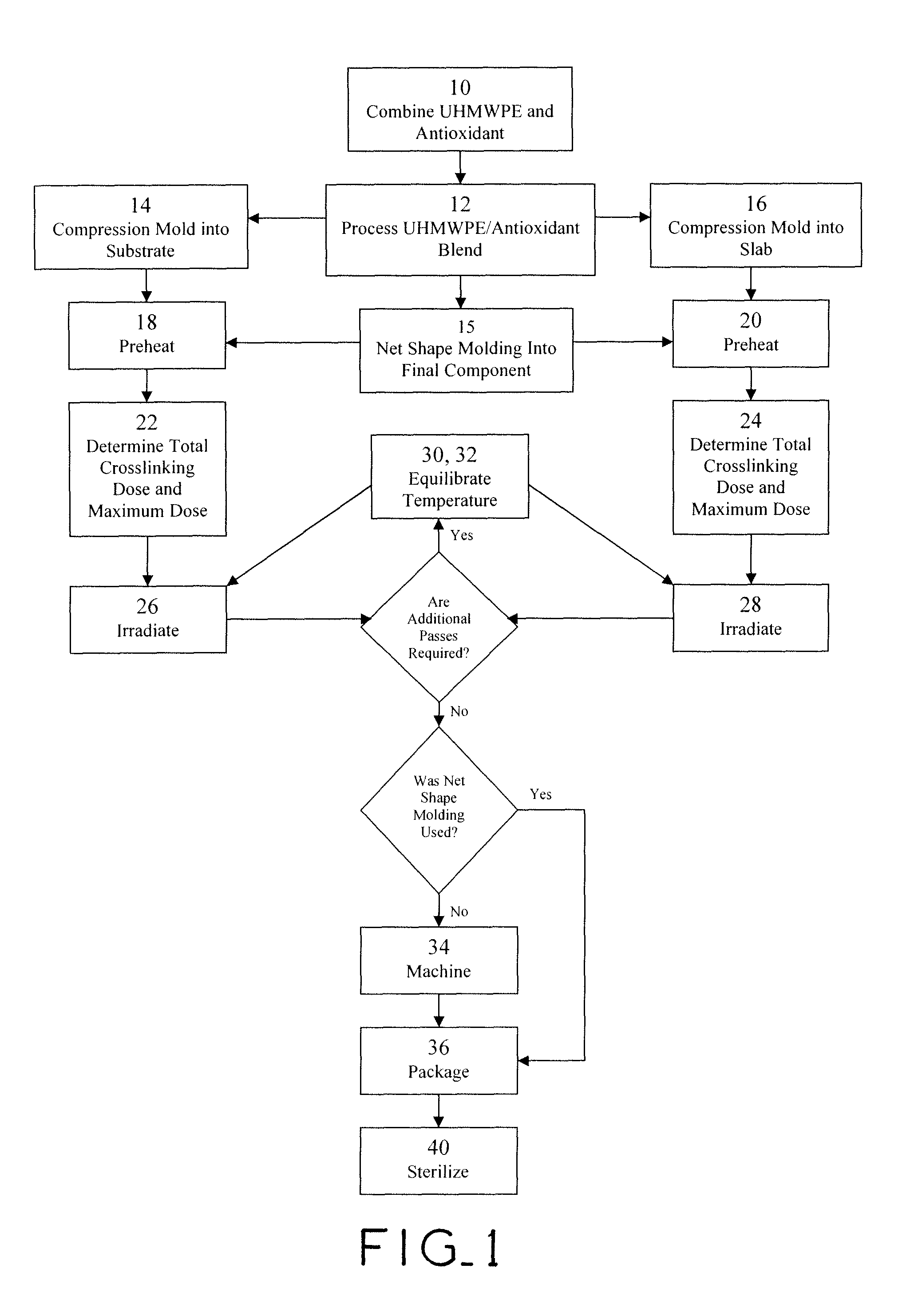
Antioxidant stabilized crosslinked ultra-high molecular weight polyethylene for medical device applications
ActiveUS20100029858A1High levelAvoid mass meltingThin material handlingProsthesisAntioxidantIrradiation
An antioxidant combined with UHMWPE prior to subjecting the UHMWPE to crosslinking irradiation. In one exemplary embodiment, the antioxidant is tocopherol. After the antioxidant is combined with the UHMWPE, the resulting blend may be formed into slabs, bar stock, and / or incorporated into a substrate, such as a metal, for example. The resulting product may then be subjected to crosslinking irradiation. In one exemplary embodiment, the UHMWPE blend is preheated prior to subjecting the same to crosslinking irradiation. Once irradiated, the UHMWPE blended product may be machined, packaged, and sterilized in accordance with conventional techniques.
Owner:ZIMMER INC
Medical paste for rheumatism and bone ache
InactiveCN1626187ALong lastingImprove efficacyAmphibian material medical ingredientsAnthropod material medical ingredientsToad VenomPoultice
A Chinese medicine in the form of plaster for treating rheumatic arthritis, hyperosteogeny, cervical spondylopathy, lumbar intervertebral disk protrusion, and muscle and bone injury is prepared from 56 Chinese-medicinal materials including camphor, pinellia tuber, hot pepper, toad venom, etc. Its advantage is high curative effect.
Owner:刘勇成
Non-gelatin soft capsule system
ActiveUS8231896B2Increase resistanceLarge doseBiocidePharmaceutical non-active ingredientsCarrageenanSoftgel
A non-gelatin encapsulation system for liquid filled soft capsules, by nature of the carrier, the cationic-ionic balance of the carrier and the active ingredients, or the concentration of the active ingredients and excipients, are difficult or impossible to commercially encapsulate in gelatin capsules. In particular, the system is adapted for the encapsulation of highly basic, or alkaline, fills. The system provides for a predominantly starch and gelling carrageenan based shell, which displays high resistance to both concentrated fills and to alkaline fills, in particular, to those fills which contain the salt or salts of weak acids and strong bases.
Owner:R P SCHERER TECH INC
Green tea formulations and methods of preparation
ActiveUS7232585B2Well received naturalLarge doseTea extractionTea substituesFlavorIntracellular substance
Green tea formulations and methods for the preparation thereof are shown and described. Generally speaking, the method of preparation includes the mixing of fresh tea leaves in an amount of cold water, followed by pulverization of the leaves to release their intracellular material from the cells of the green tea leaves into the water and form an aqueous extract component. The remaining cellular material forms a leaf residue component which is removed from the mixture. Once the leaf residue is removed, the aqueous extract component is collected and may be dried or further processed to produce a final tea extract that has good natural color, robust natural flavor, and pleasant organoleptic properties, which also is high in polyphenol content, and may be used for various purposes such as the creation of a green tea beverage.
Owner:XEL HERBACEUTICALS INC
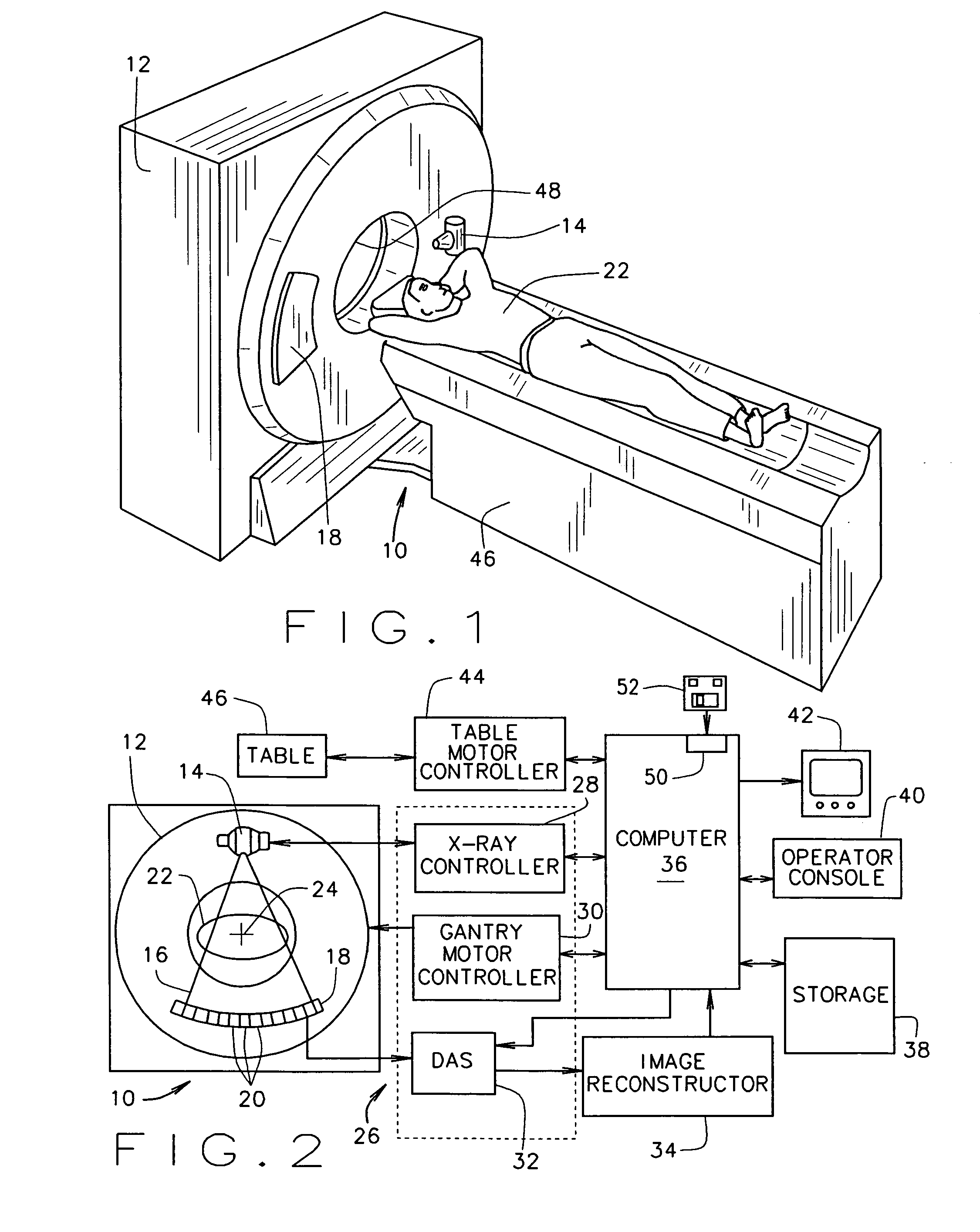
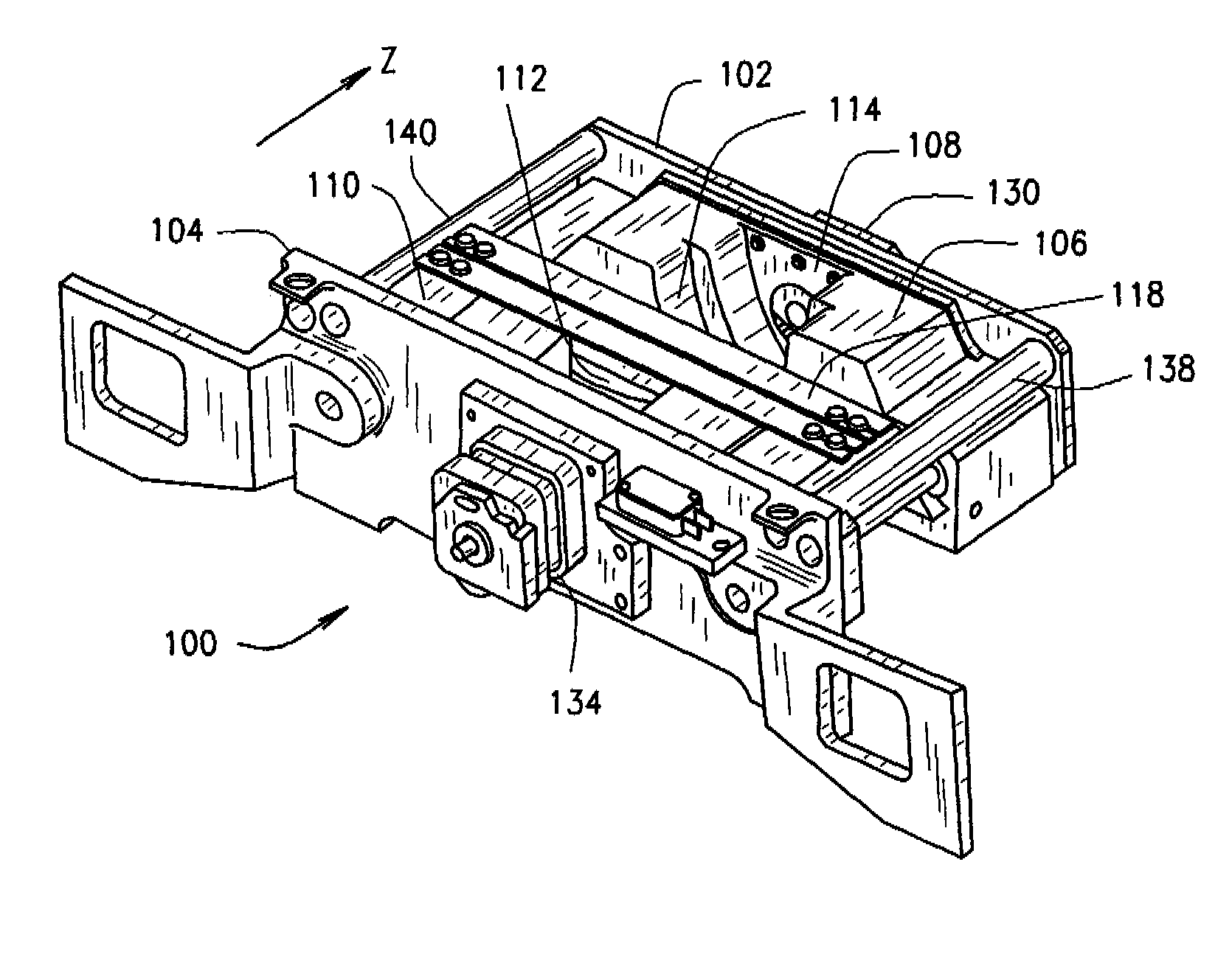
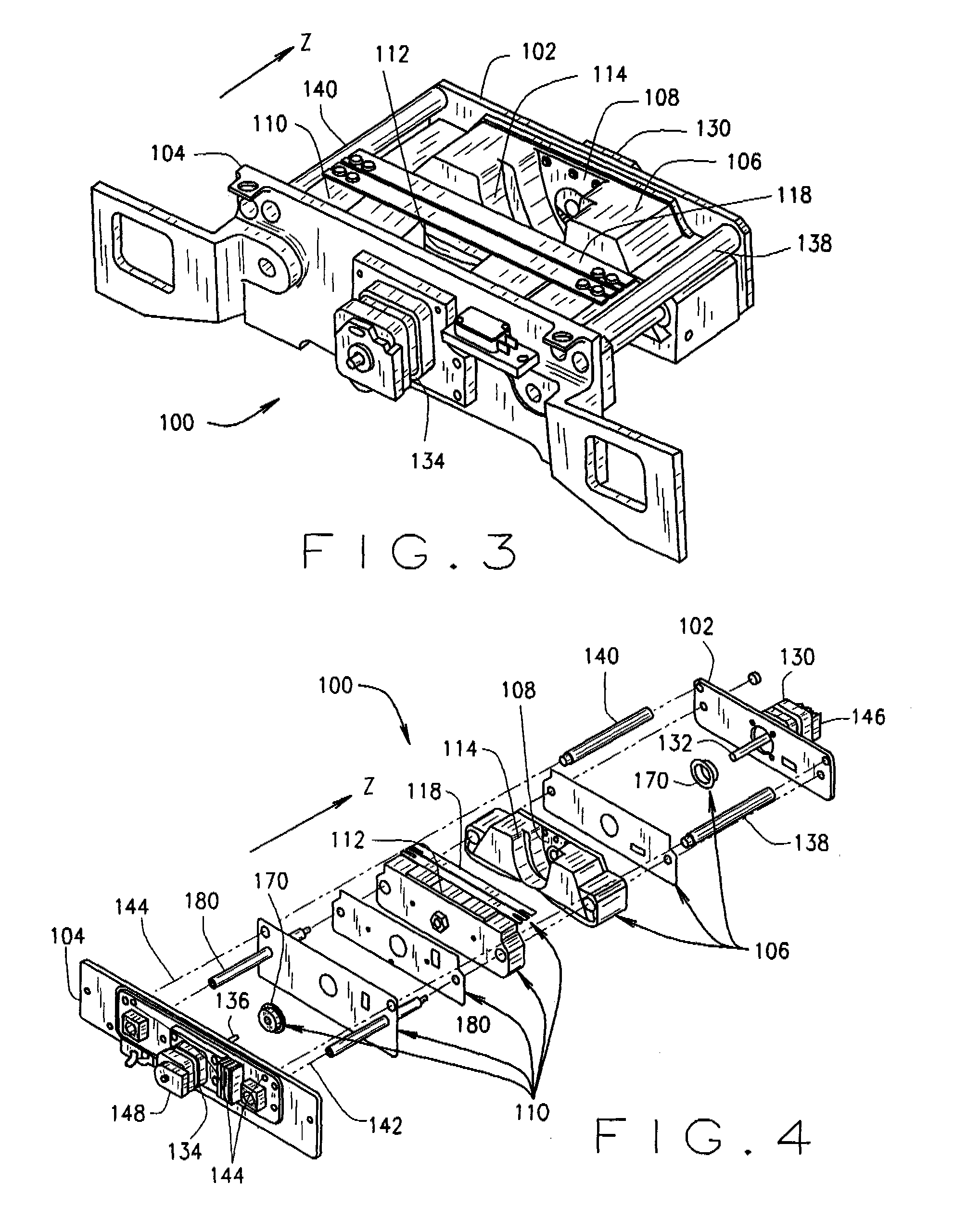
Methods and apparatus for filtering a radiation beam and CT imaging systems using same
InactiveUS7254216B2Conveniently changedLarge doseRadiation/particle handlingTomographyX-ray filterEngineering
Owner:GENERAL ELECTRIC CO
Preparation method of traditional Chinese medicine ultrafine formula particles
InactiveCN101822697AReduce dosageKeep it authenticPteridophyta/filicophyta medical ingredientsGranular deliveryDiseaseAdditive ingredient
The invention discloses a preparation method of traditional Chinese medicine ultrafine formula particles. The preparation method comprises the following steps that: single Chinese medicinal material or traditional Chinese medicine decoction pieces are partially ultrafine-crushed into ultrafine powder, the other parts are coarse-crushed, extracted, concentrated, dried and crushed to obtain dry extract powder, the ultrafine powder and auxiliary materials are added to obtain mixture, and the obtained mixture is mixed uniformly, palletized, granulated and subpackaged. The traditional Chinese medicine ultrafine formula particles is characterized in that the characteristics of the traditional Chinese medicine ultrafine decoction pieces of saving medicine materials and being controllable in quality and convenient in administration. Compared with the traditional Chinese medicine formula particles, the preparation method better preserves the medicinal properties of the traditional Chinese medicines, has the advantages of little used amount of auxiliary materials, convenient identification, benefit to measuring the content of index ingredients, preserves the advantages that the traditional Chinese medicine decoction piece can be added and reduced according to the disease and the traditional decoction has unique curative effect, also overcomes the defects of troublesome decoction and inconvenient administration, and has advanced technique and controllable quality. The invention also discloses a processing method of the Chinese medicinal material, which extracts the raw medicinal materials partially, carries out ultrafining treatment partially and then carries out mixing. The method can obvious improve the utilization ratio and leachability of the Chinese medicinal materials.
Owner:湖南省中医药研究院
Methods and apparatus for filtering a radiation beam and CT imaging systems using same
InactiveUS20070025520A1Conveniently changedLarge doseRadiation/particle handlingTomographyX-ray filterEngineering
A filter assembly for a computed tomographic imaging system includes first and second endplates at opposite ends of the filter assembly. Also provided is a first moveable subassembly that includes at least a first x-ray filter and which is configured to move along an axis perpendicular to the first endplate between the first the second endplates. A second moveable subassembly is also provided that includes at least a second x-ray filter. The second moveable subassembly is configured to move along an axis perpendicular to the second endplate between the first and second endplates. The first moveable subassembly and the second moveable subassembly are independently movable to provide at least a small bowtie x-ray filter, a large bowtie x-ray filter, a medium bowtie x-ray filter, a flat filter, and a closed position for a radiation source positioned in a fixed position relative to the filter assembly.
Owner:GENERAL ELECTRIC CO
Charged nutritive proteins and methods
ActiveUS8809259B2High in proteinInduce postprandial satietyOrganic active ingredientsPeptide/protein ingredientsNutrients proteinMicroorganism
Owner:AXCELLA (ASSIGNMENT FOR THE BENEFIT OF CREDITORS) LLC
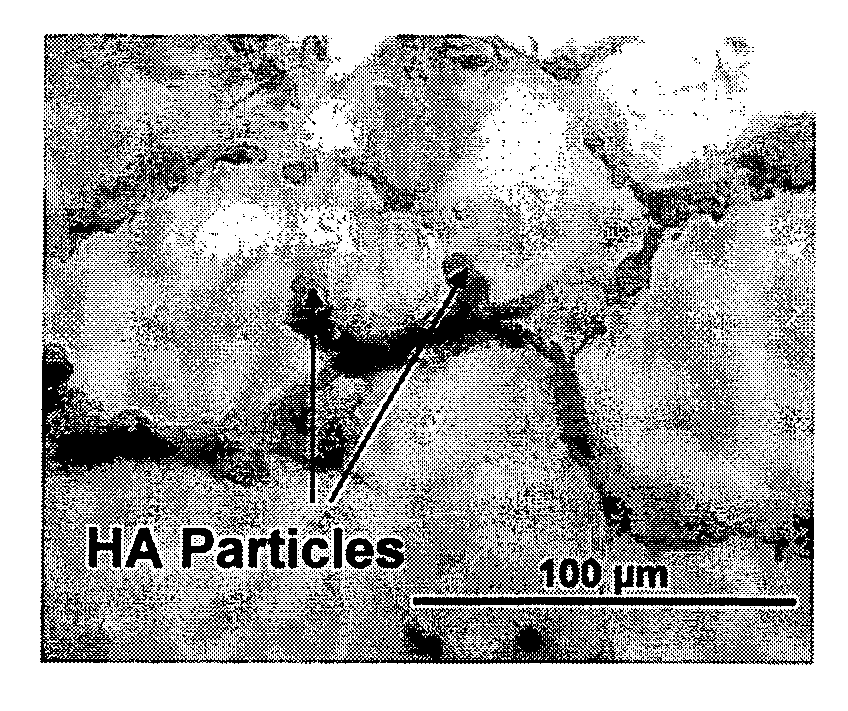
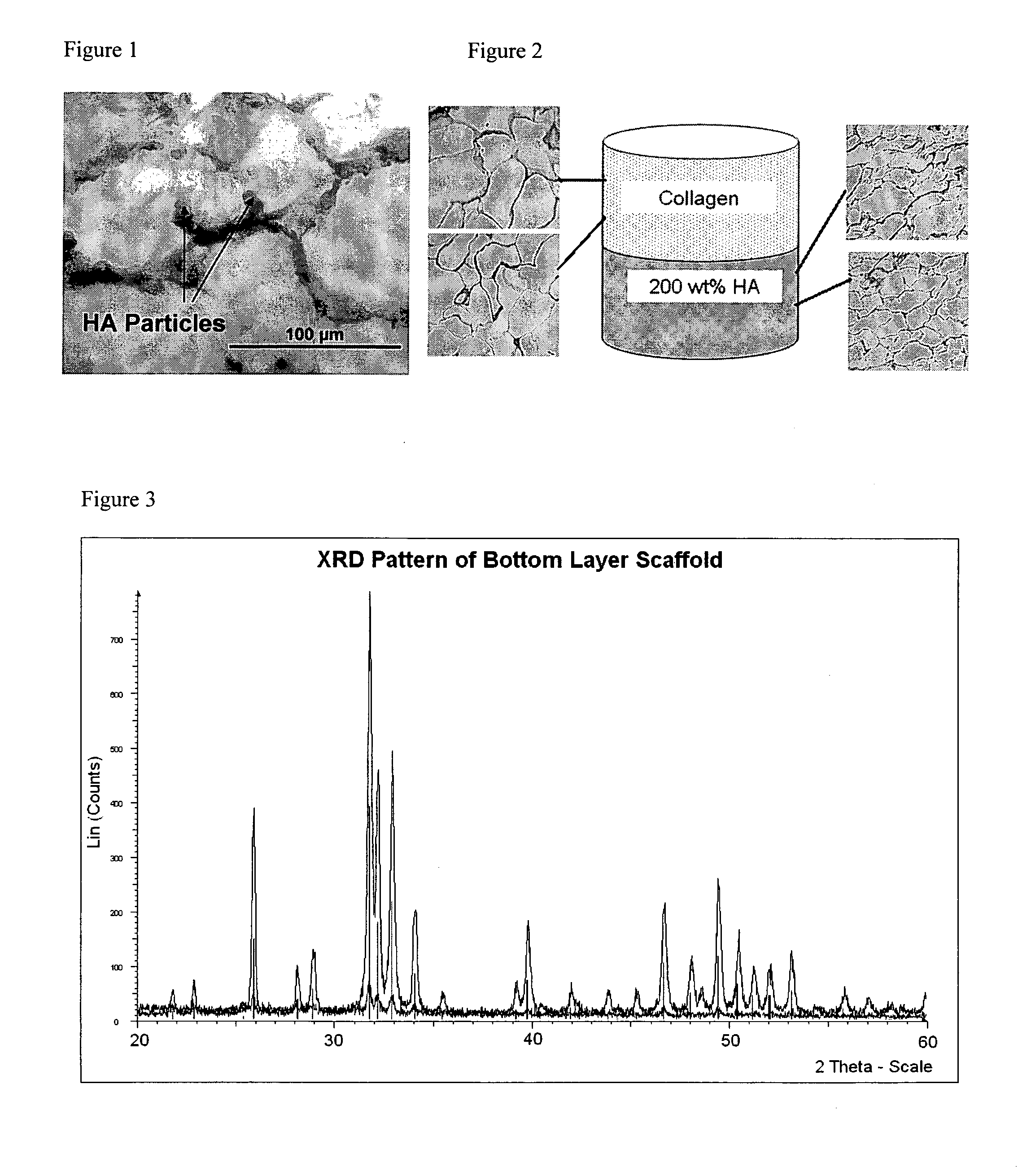
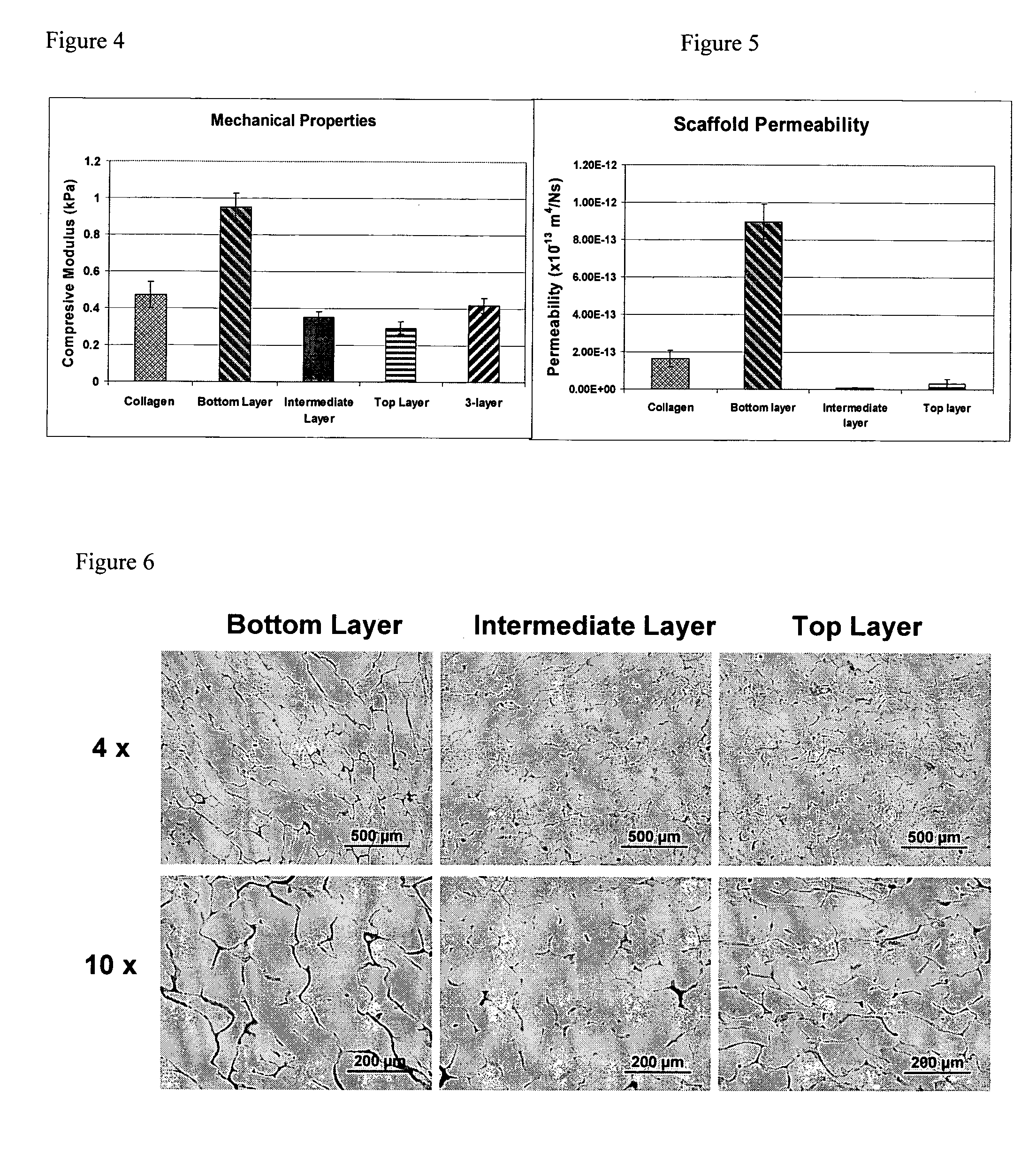
Layered Scaffold Suitable for Osteochondral Repair
ActiveUS20120015003A1High degreeReduced strengthBiocideSkeletal disorderFreeze-dryingCollagen scaffold
The invention relates to a method for producing a multi-layer collagen scaffold. The method generally comprises the steps of: preparing a first suspension of collagen and freezing or lyophilising the suspension to provide a first layer; optionally preparing a further suspension of collagen and adding the further suspension onto the layer formed in the previous step to form a further layer, and freezing or lyophilising the layers, wherein when the layer formed in the previous step is formed by lyophilisation the lyophilised layer is re-hydrated prior to addition of the next layer; optionally, repeating the aforementioned step to form one or more further layers; and preparing a final suspension of collagen and pouring the final suspension onto the uppermost layer to form a final layer, and freeze-drying the layers to form the multilayer collagen composite scaffold.
Owner:ROYAL COLLEGE OF SURGEONS & IRELAND
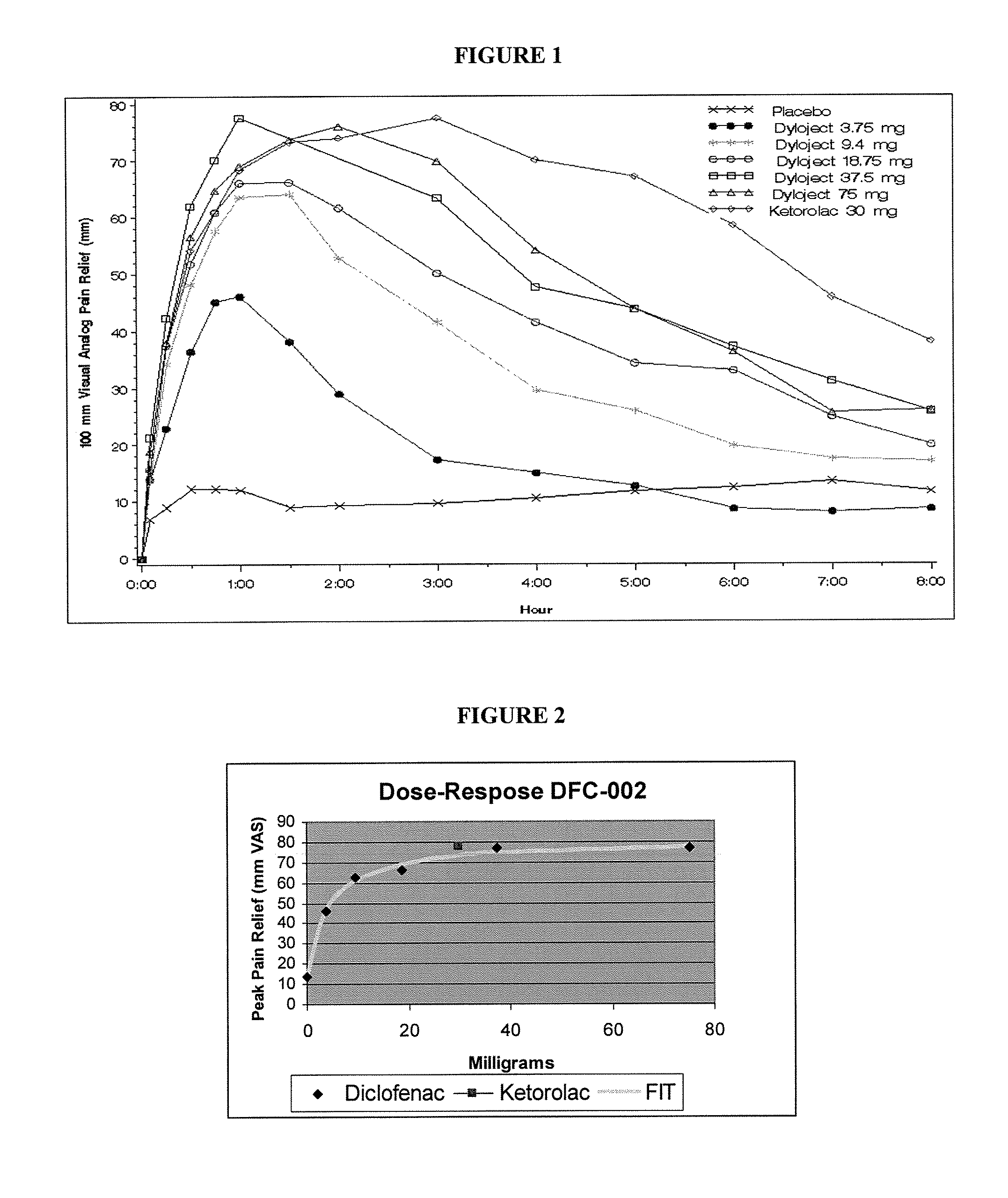
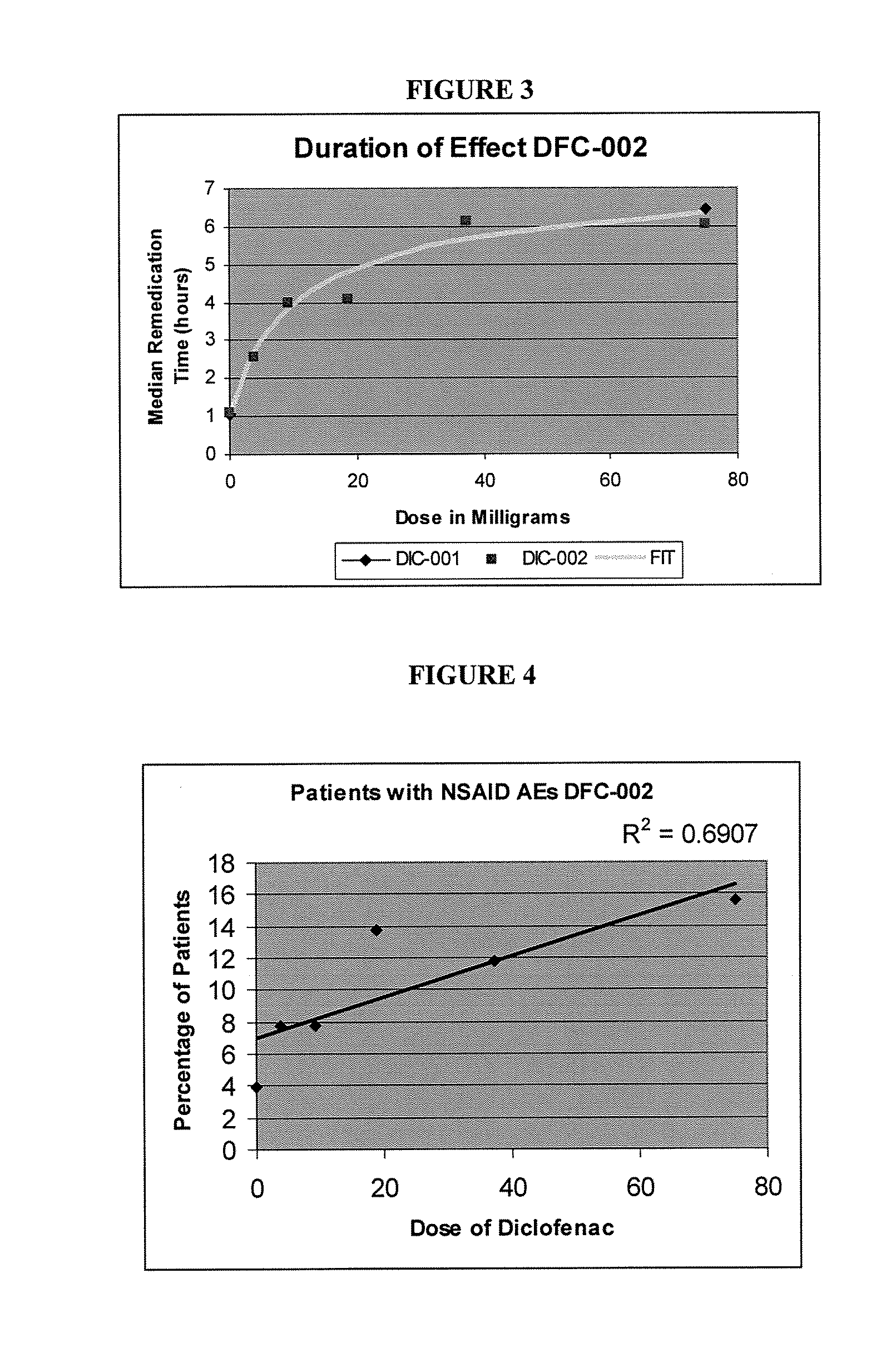
Formulations Of Low Dose Diclofenac And Beta-Cyclodextrin
InactiveUS20070232566A1Good curative effectParticular value in treating pain in a horsePowder deliveryBiocideBeta-CyclodextrinsLow dose
The present invention is directed to a pharmaceutical composition containing a unit dose of a diclofenac compound effective to induce analgesia; and a beta-cyclodextrin compound; wherein the dose of the diclofenac compound is less than 10 mg. The present invention is also directed to methods of treating a subject in need of analgesia with the pharmaceutical compositions of the invention.
Owner:JAVELIN PHARMA INC +1
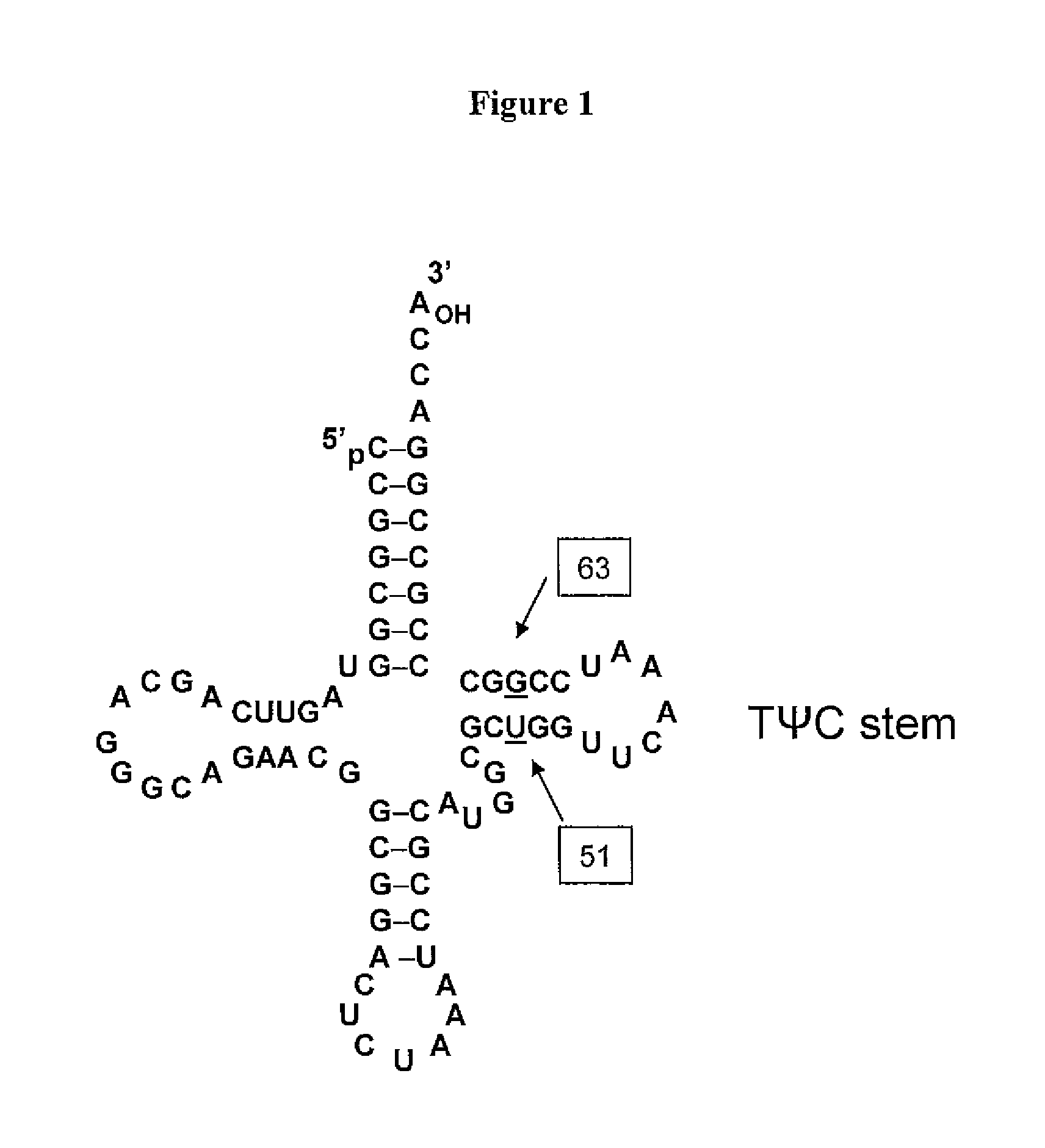
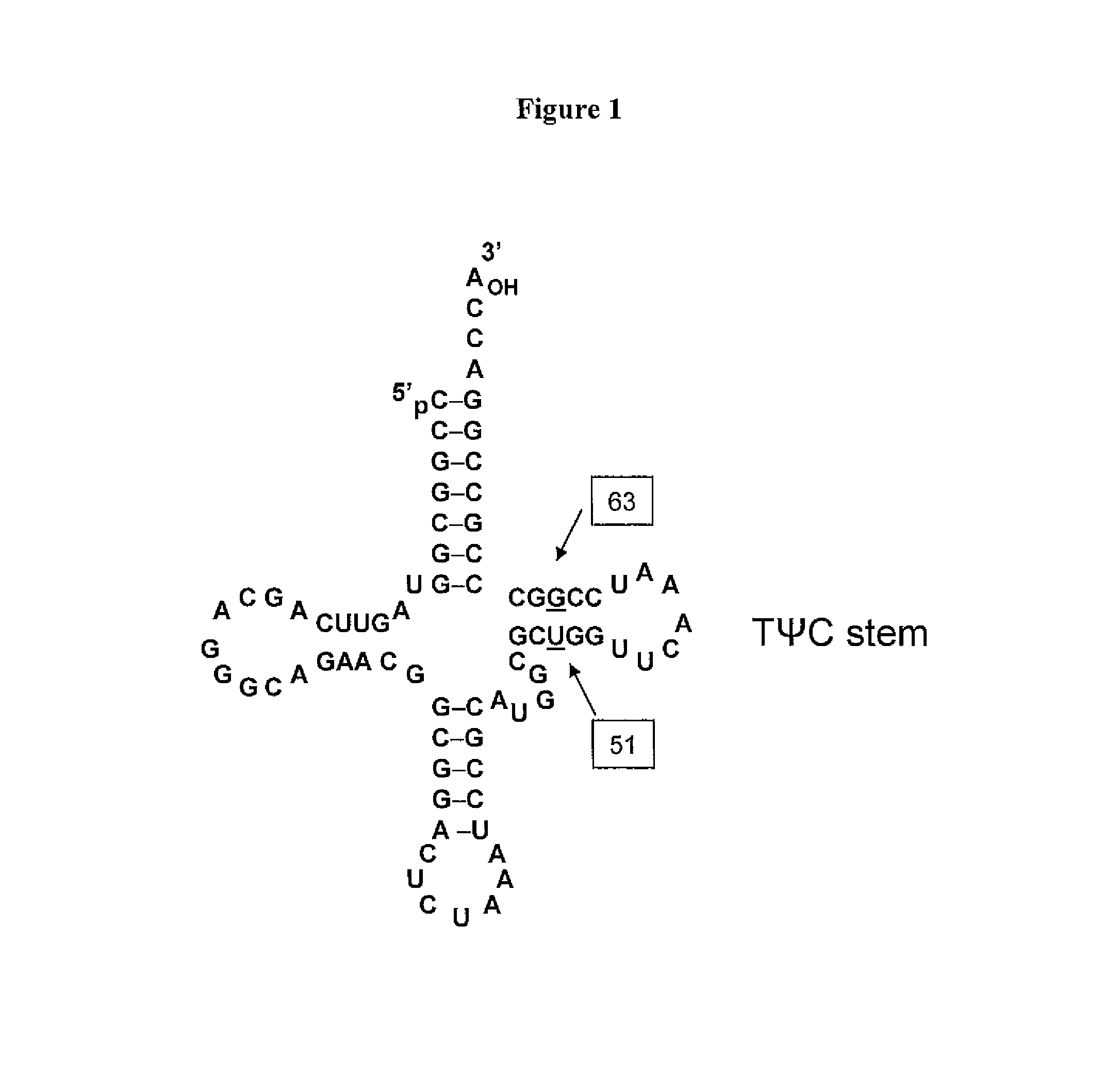
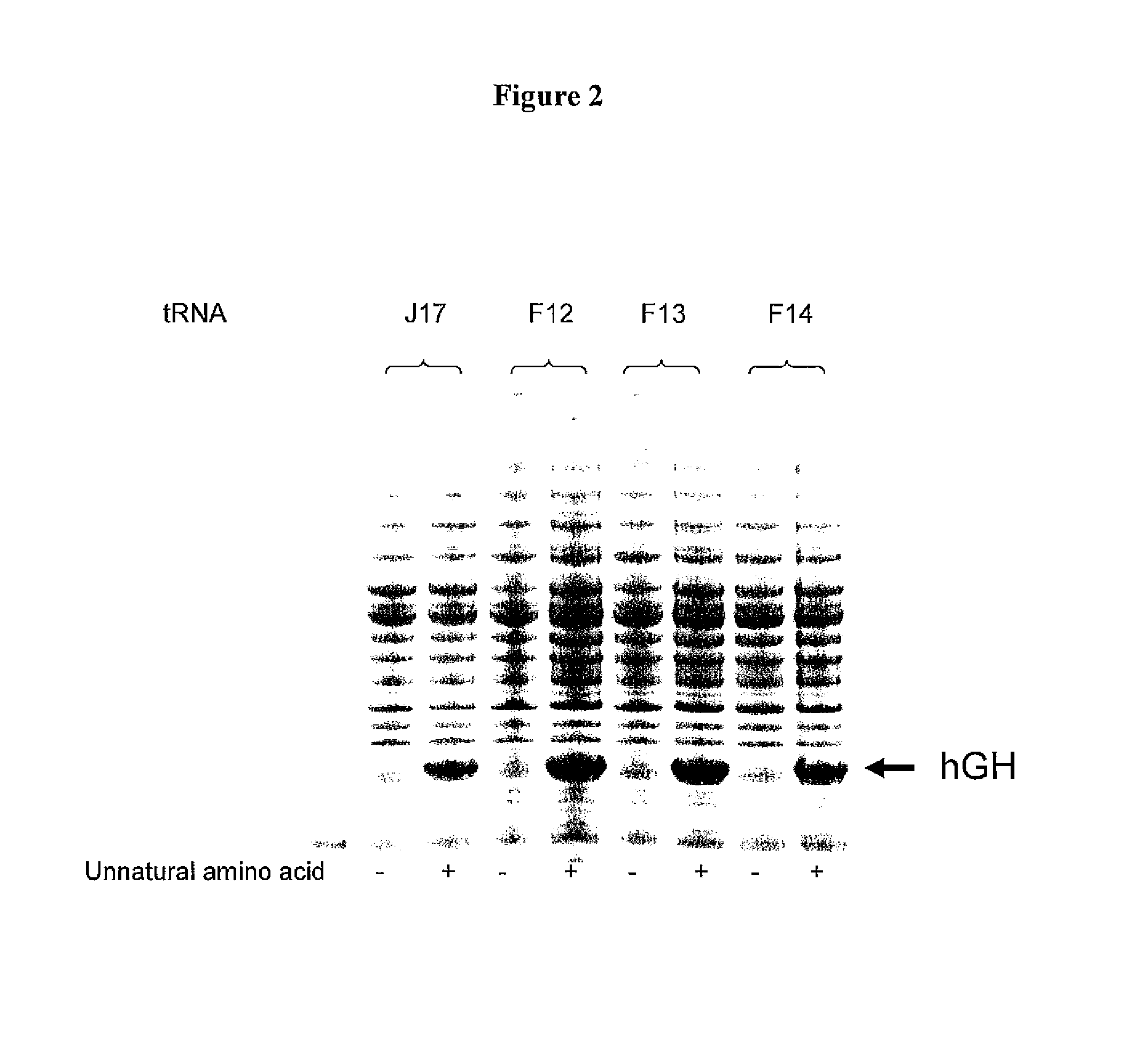
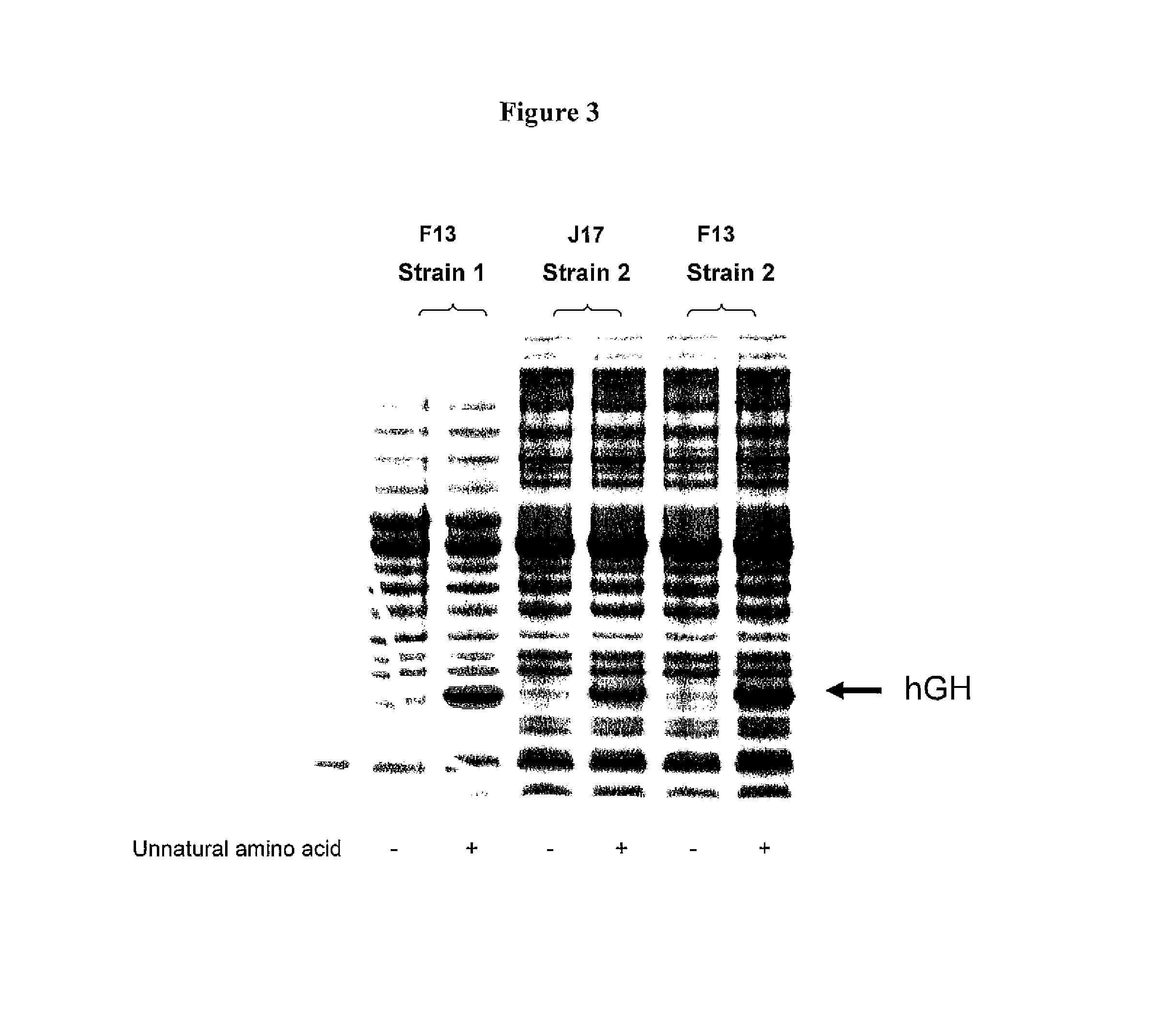
Non-Natural Amino Acid Replication-Dependent Microorganisms and Vaccines
ActiveUS20130323821A1Low efficacyEnhance antigen presentationAntibacterial agentsBacterial antigen ingredientsMicroorganismBiological body
Compositions and methods of producing vaccines, including methods wherein whole organism vaccines are provided with limited replication abilities, thereby increasing vaccine safety and efficacy, through the use of non-natural, unnatural, or non-naturally encoded amino acids.
Owner:AMBRX
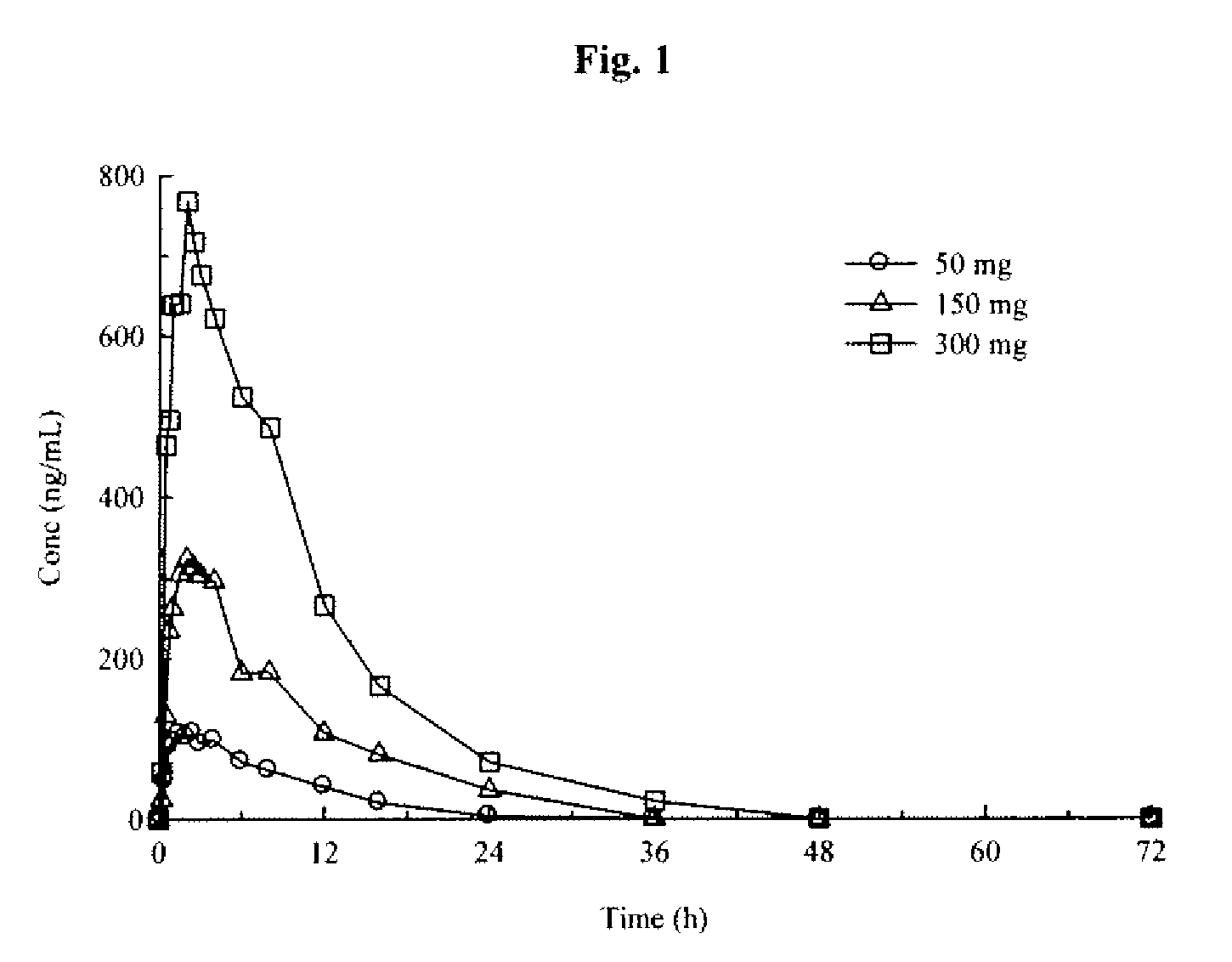
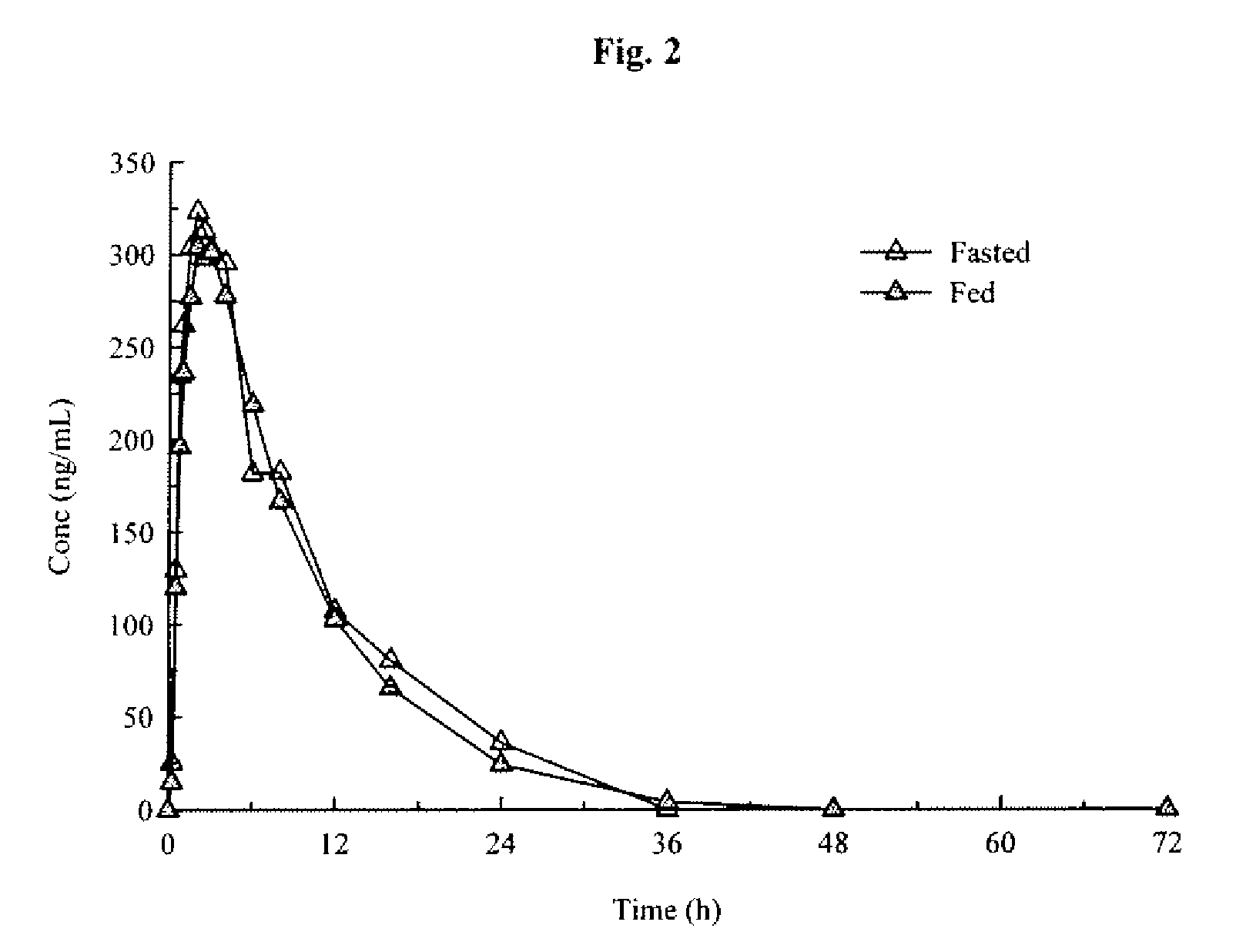
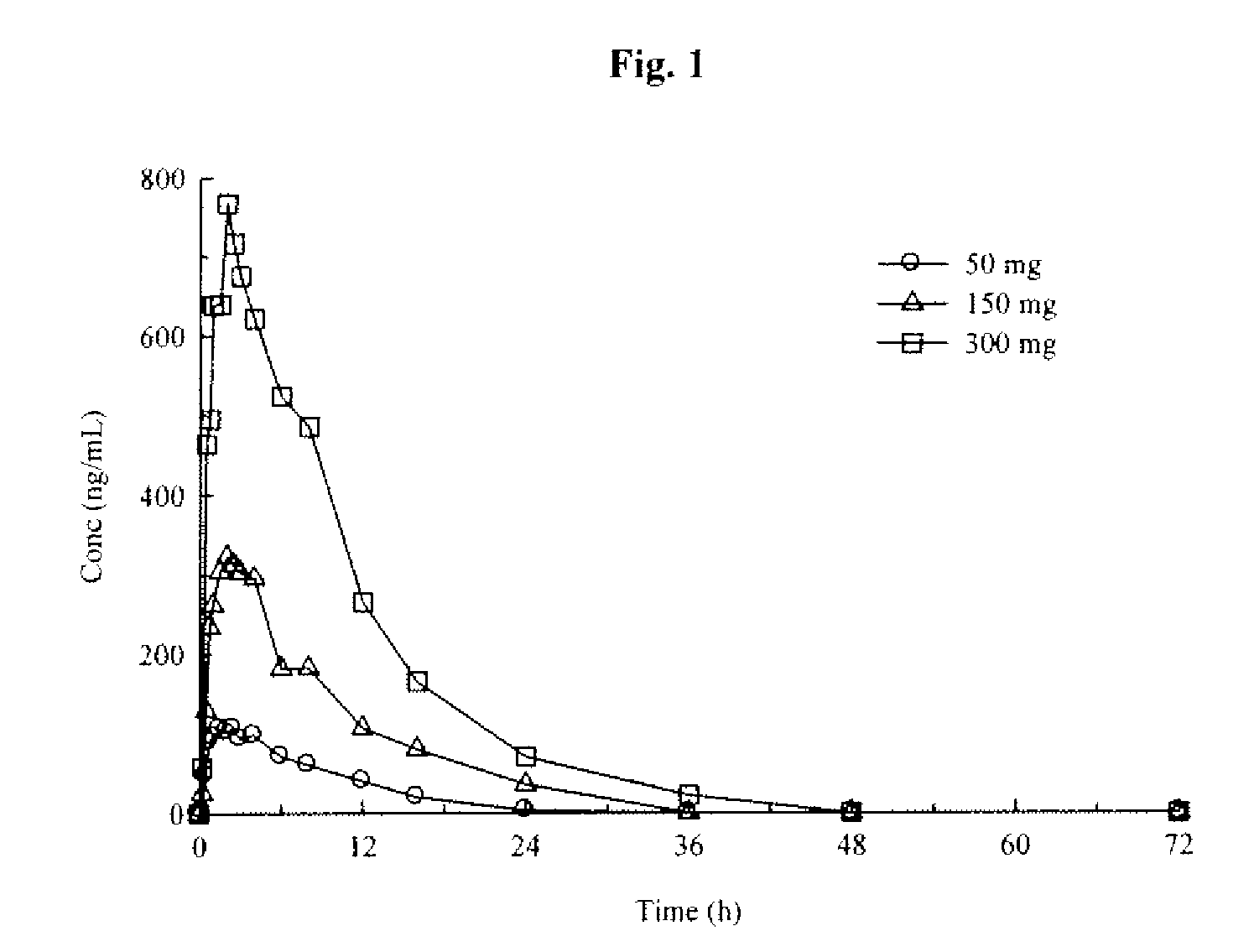
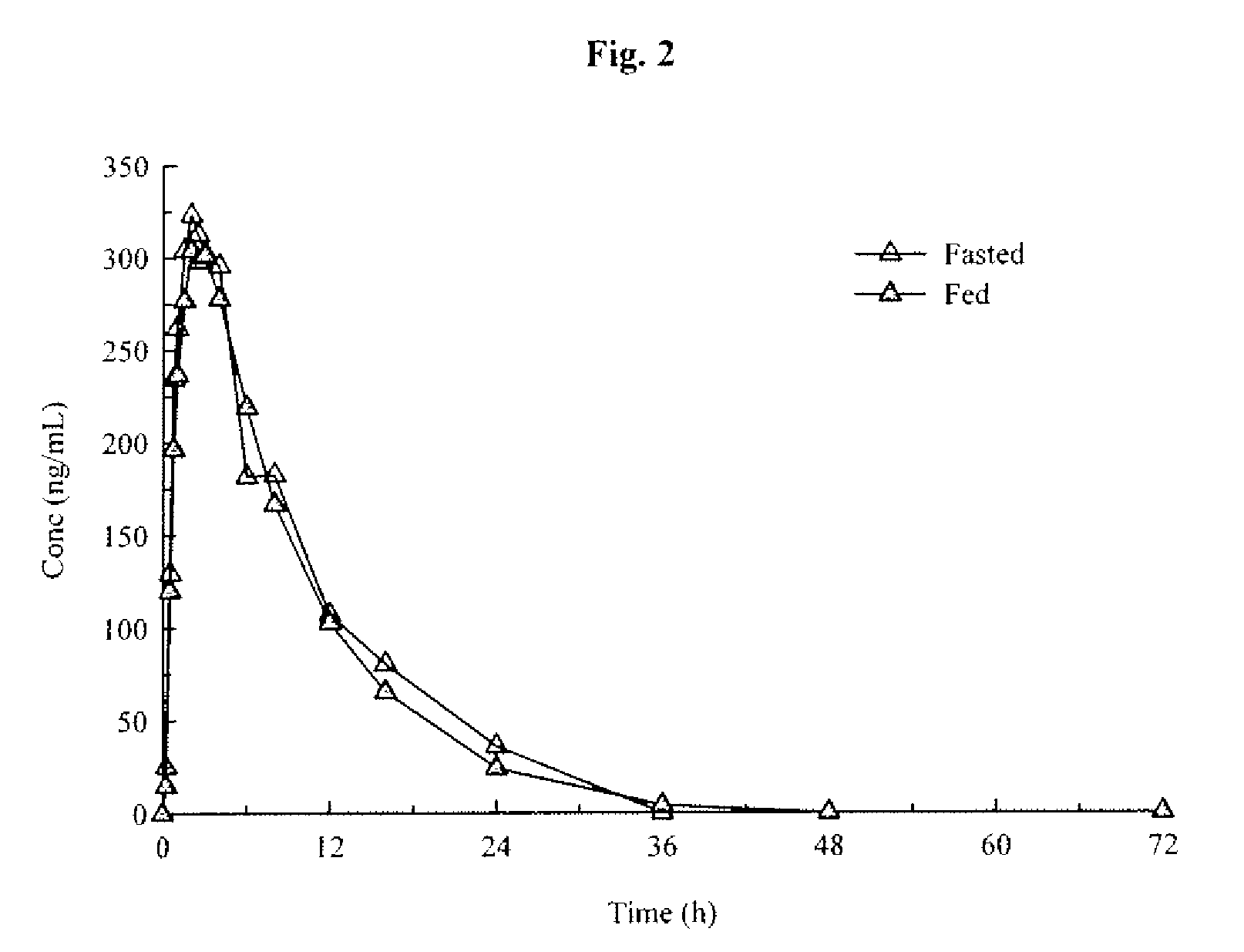
Aryl [a] indole [2,3-g] quinolizine compound as well as preparation method, pharmaceutical composition and application thereof
The invention relates to an aryl [a] indole [2,3-g] quinolizine compound as well as a preparation method, a pharmaceutical composition and application thereof, and particularly relates to a aryl [a] indole [2,3-g] quinolizine compound with a novel structure as shown in a general formula (I) and a derivative, a preparation method and a pharmaceutical composition thereof and application of the aryl [a] indole [2,3-g] quinolizine compound in preparation of a drug for treating diseases related to alpha 1-adrenoreceptor and urinary system diseases, such as benign prostatic hyperplasia, uroschesis and bladder outlet obstruction, especially.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Yinhuang powder injection for intravenous injection and its prepn process
The present invention discloses a Yinhuang powder injection for intravenous injection and its preparation process. The formula includes honeysuckle extractive in 0.1-0.3 weight portions and skullcap root extractive in 0.1-0.6 weight portions. The preparation process includes the steps of dissolution of honeysuckle extractive and skullcap root extractive in water solution of sodium hydroxide, heating to slightly boiling for 15-20 min, filtering, disinfection, refrigerating for one week, pressurized filtering, freeze drying or spray drying for the filtrate liquid to obtain the said powder.
Owner:李大鹏
Compositions and methods of using (r)-pramipexole
Pharmaceutical compositions of (R)-pramipexole and methods and kits of using such compositions for the treatment of neurodegenerative diseases, or those related to mitochondrial dysfunction or increased oxidative stress are disclosed.
Owner:KNOPP BIOSCIENCES LLC
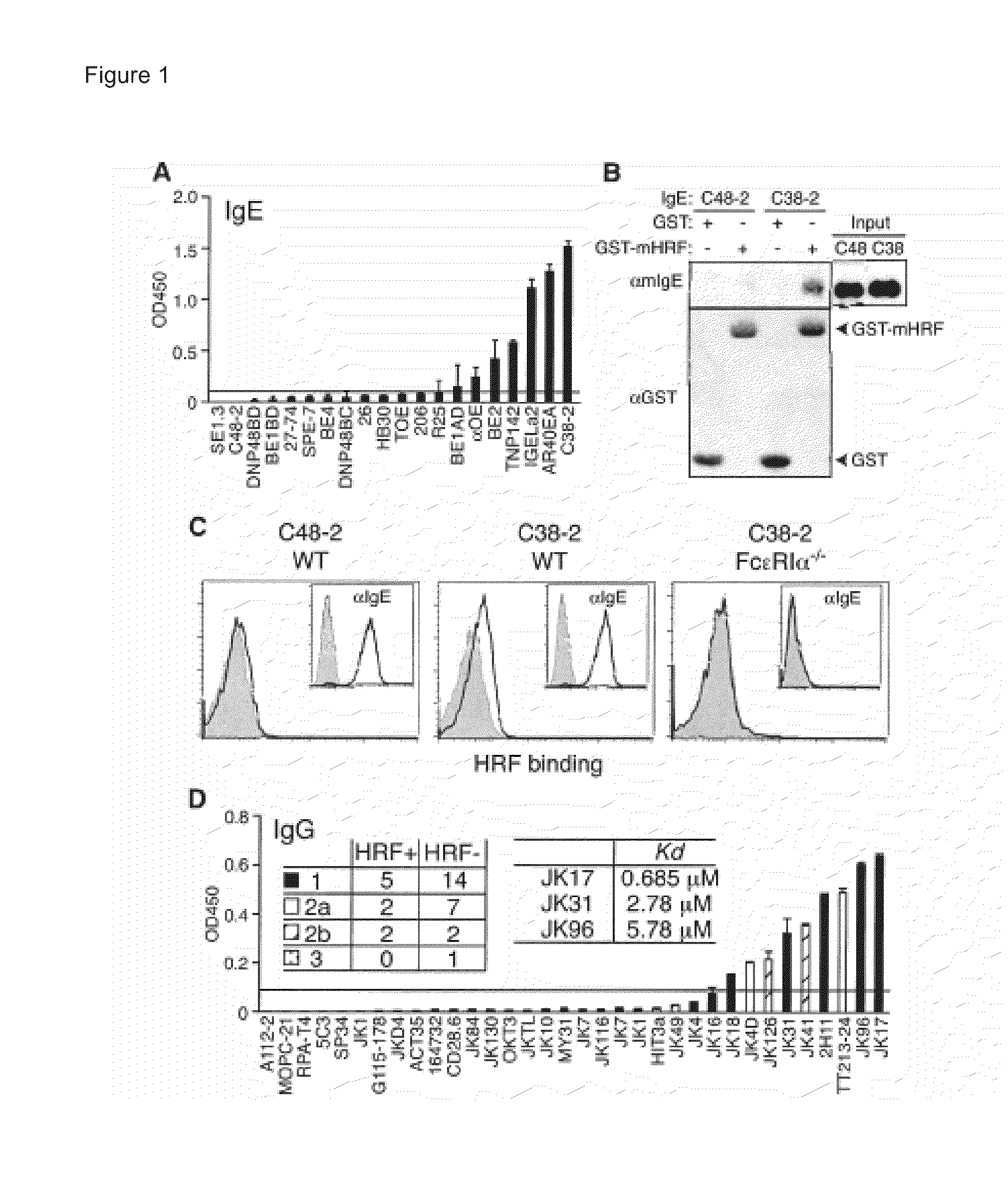
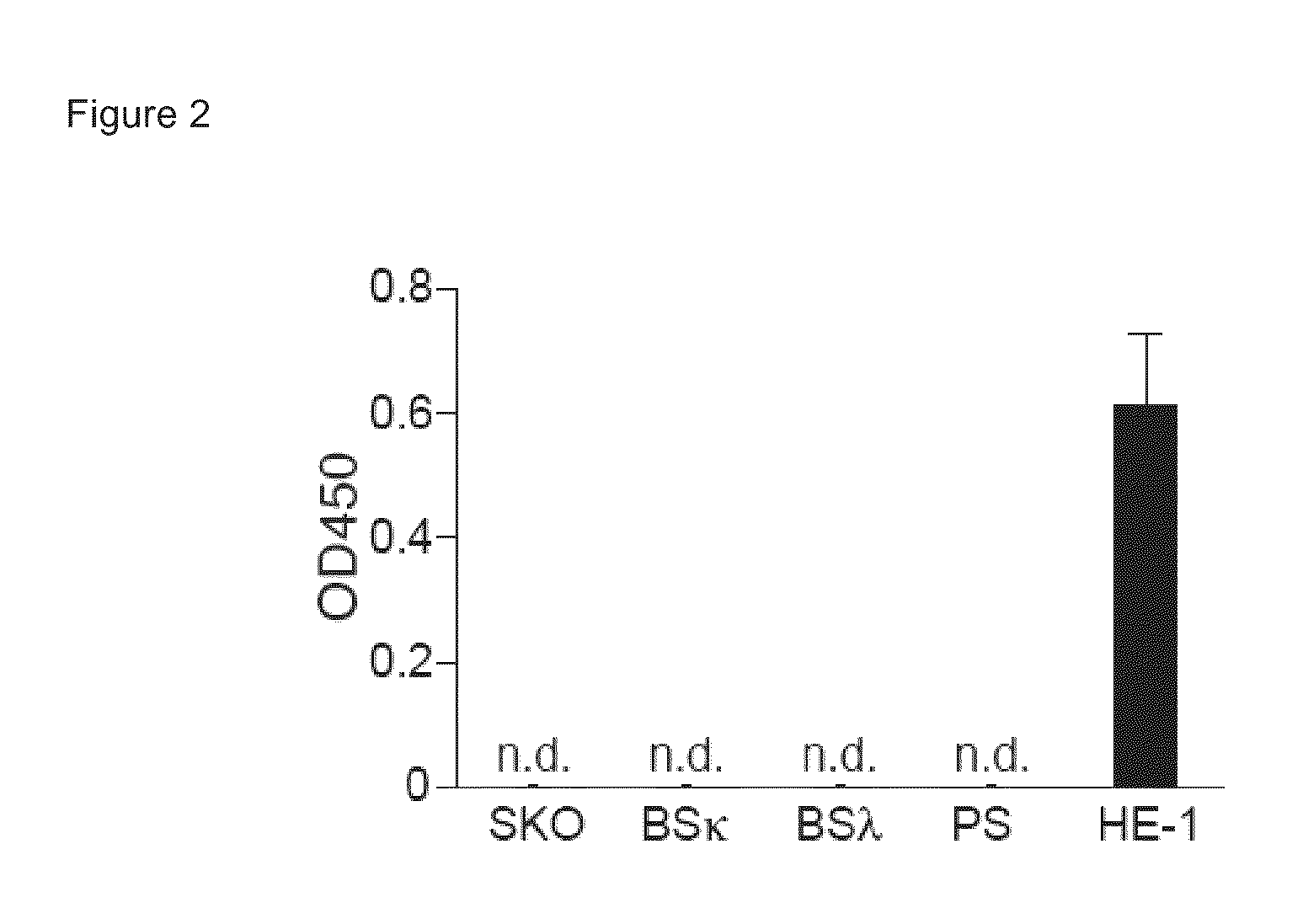
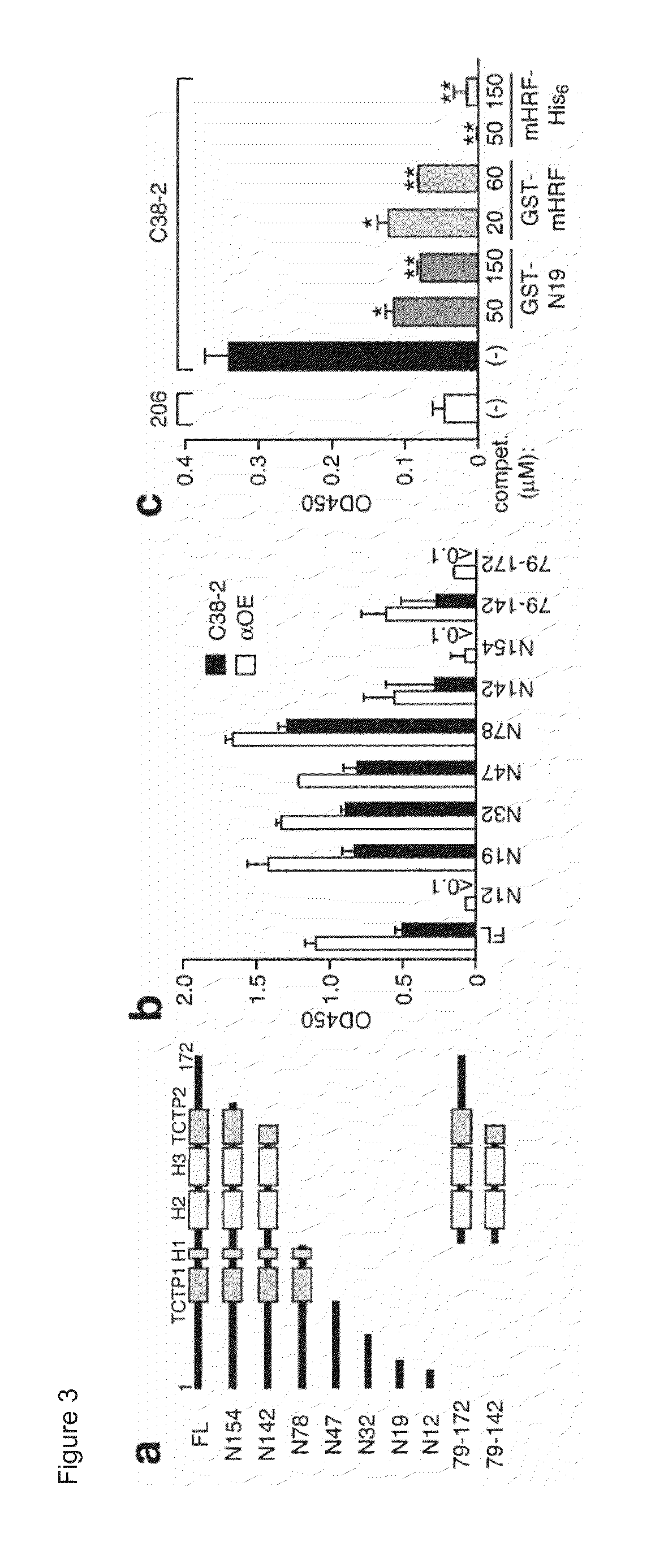
Histamine-releasing factor (HRF), hrf-receptor and methods of modulating inflammation
InactiveUS20130084293A1Shorten the durationReduce frequencyCompound screeningApoptosis detectionHistamine releasing factorHypersensitive response
Methods of treating a food allergy, allergic reactions, hypersensitivity, inflammatory responses, inflammation are provided. In one method, histamine releasing factor (HRF) / translationally controlled tumor protein (TCTP) is contacted with a compound that inhibits or reduces binding of HRF / TCTP to an immunoglobulin in order to treat the food allergy, allergic reaction, hypersensitivity, inflammatory response, or inflammation. Methods of reducing or decreasing the probability, severity, frequency, duration or preventing a subject from having an acute or chronic food allergy, allergic reaction, hypersensitivity, an inflammatory response or inflammation, are also provided. In one method, histamine releasing factor (HRF) / translationally controlled tumor protein (TCTP) is contacted with a compound that inhibits or reduces binding of HRF / TCTP to an immunoglobulin in order to reduce or decrease the probability, severity, frequency, duration or prevent a subject from having an acute or chronic food allergy, allergic reaction, hypersensitivity, an inflammatory response or inflammation.
Owner:LA JOLLA INST FOR ALLERGY & IMMUNOLOGY
Method and device for producing a tomosynthetic 3d x-ray image
InactiveCN101641589AQuality improvementLarge doseTomographyX-ray tube cold cathodesProjection image3d image
In a method and device for producing a tomosynthetic 3D x-ray image, a number of 2D projection images of an examination subject are acquired using a fixed x-ray source. The x-ray source has multiple,individually controllable emitters that respectively emit a single x-ray dose from various different directions. The tomosynthetic 3D image is reconstructed from the individual 2D projection images, and at least one 2D projection image is composed of multiple individual images.
Owner:SIEMENS AG
Submicroemulsion injection containing polyene paclitaxel and its preparing method
InactiveCN100998559AImprove solubilityImprove playbackOrganic active ingredientsEmulsion deliverySolubilityMedicine
A sub-microemulsion injection of polyene taxol with a certain target performance and low poison is prepared from polyene taxol, the oil for injection, emulsifier, stabilizer, isotonic regulator, pH regulator, and emulsifying aid. Its preparing process is also disclosed.
Owner:SHENYANG PHARMA UNIVERSITY
Anti-mosquito tensile silk and producing method thereof
InactiveCN1772978ALarge doseEasy storage and long-term releaseFilament/thread formingArtifical filament manufacturePermethrinYarn
The present invention relates to a mosquito-repelling drawn yarn and its production method. Said drawn yarn has sheath-core structure, the volume ratio of sheath-core is 2:8-7:3; its fibre formula is as follows: sheath material is linear polymer, including PTT, PBT, PA-6, PET or one kind of PP whose melting index is 25-50; the core material formula composition includes (by wt%) 93-98% of core polymer and 2-7% of mosquito-repellent; the core polymer is PE. PP or one kind of PET whose melting point is 150-200 deg.C, and the mosquito-repellent is at least one kind of Metadelphene, permethrin, allethrin, valerathrin, methothrin, allylguaiacol or plifenate. Said invention also provides the concrete steps of its production method.
Owner:HAIAN COUNTY BROS SYNTHETIC FIBER
Formulations Of Low Dose Non-Steroidal Anti-Inflammatory Drugs And Beta-Cyclodextrin
InactiveUS20070232567A1Sufficient quantitySufficient amountBiocideAntipyreticNon steroid anti inflammatory drugBeta-Cyclodextrins
The present invention is directed to pharmaceutical compositions containing (a) a dosage of a non-steroidal anti-inflammatory drug (NSAID) effective to induce analgesia an anti-inflammatory effect, or an anti-pyretic effect and (b) a beta-cyclodextrin compound; wherein the dosage of the NSAID compound is less than the minimum approved dose for the route of administration. Additionally, the present invention is directed to methods for treating a mammal in need of an analgesic, an anti-inflammatory, or an anti-pyretic agent comprising administering the pharmaceutical composition of the present invention.
Owner:MYRIAD GENETICS INC (US)
Non-Natural Amino Acid Replication-Dependent Microorganisms and Vaccines
ActiveUS20110195483A1Enhance vaccine efficiencyPromote cross presentationAntibacterial agentsBacterial antigen ingredientsWhole OrganismBiological body
Compositions and methods of producing vaccines, including methods wherein whole organism vaccines are provided with limited replication abilities, thereby increasing vaccine safety and efficacy, through the use of non-natural, unnatural, or non-naturally encoded amino acids.
Owner:AMBRX
Photodynamic therapy treatment for eye disease
InactiveUS7320786B2Reduce amountReduce lightUltrasonic/sonic/infrasonic diagnosticsBiocideDiabetic retinopathyDisease
Owner:LIGHT SCI ONCOLOGY
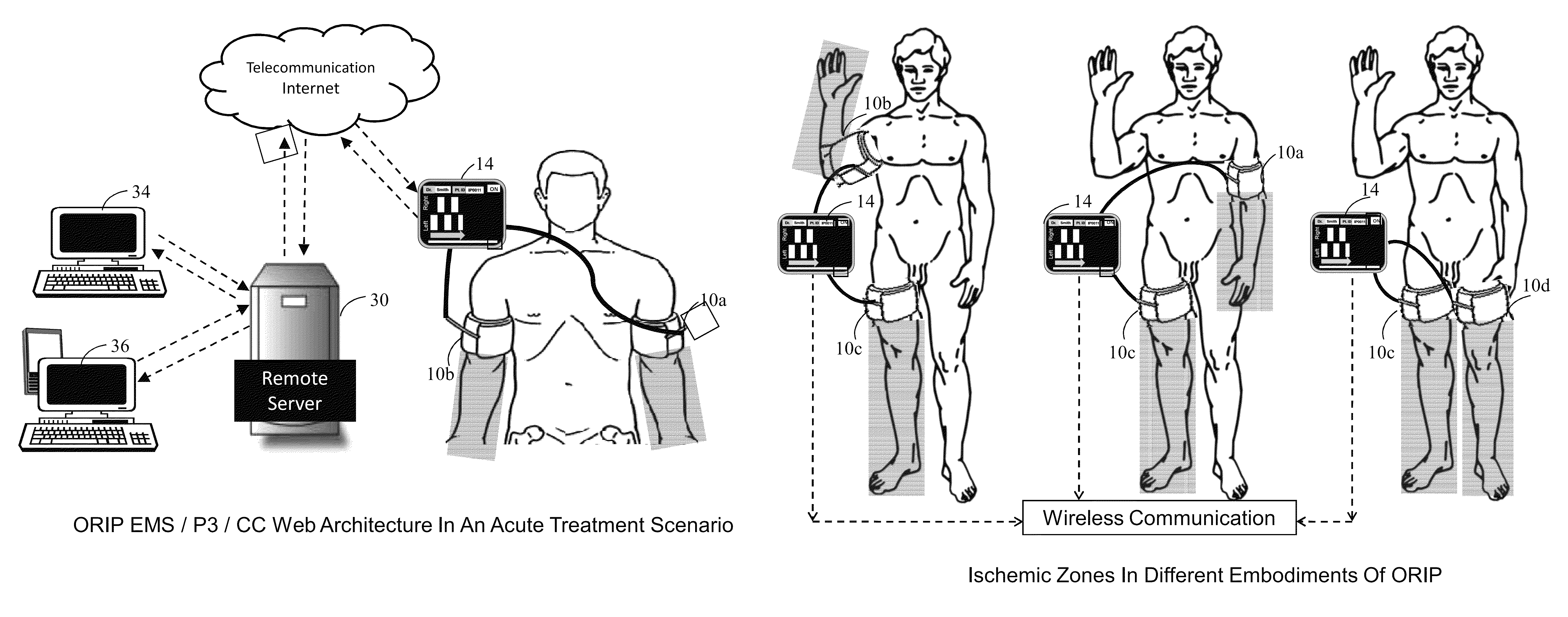
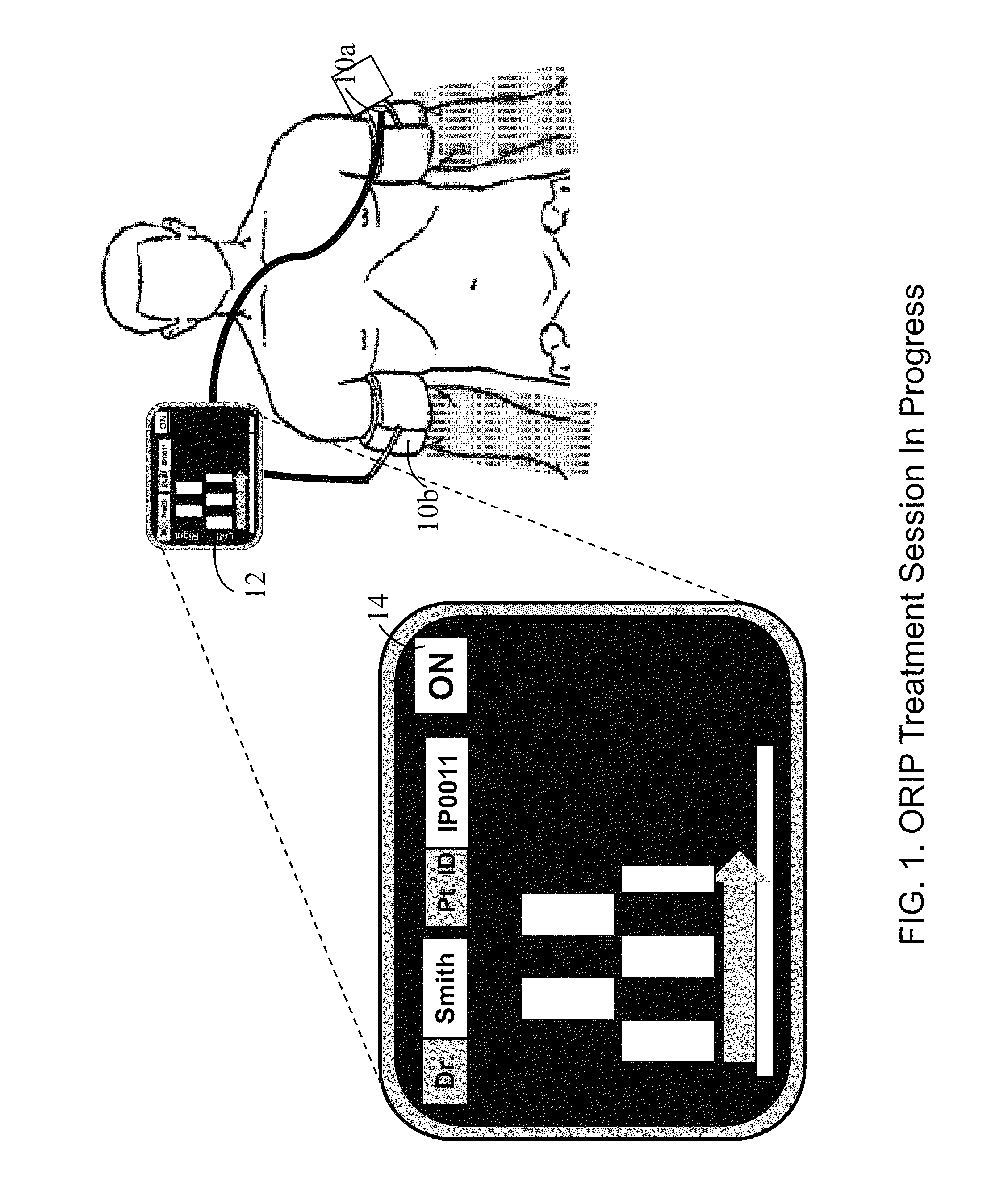
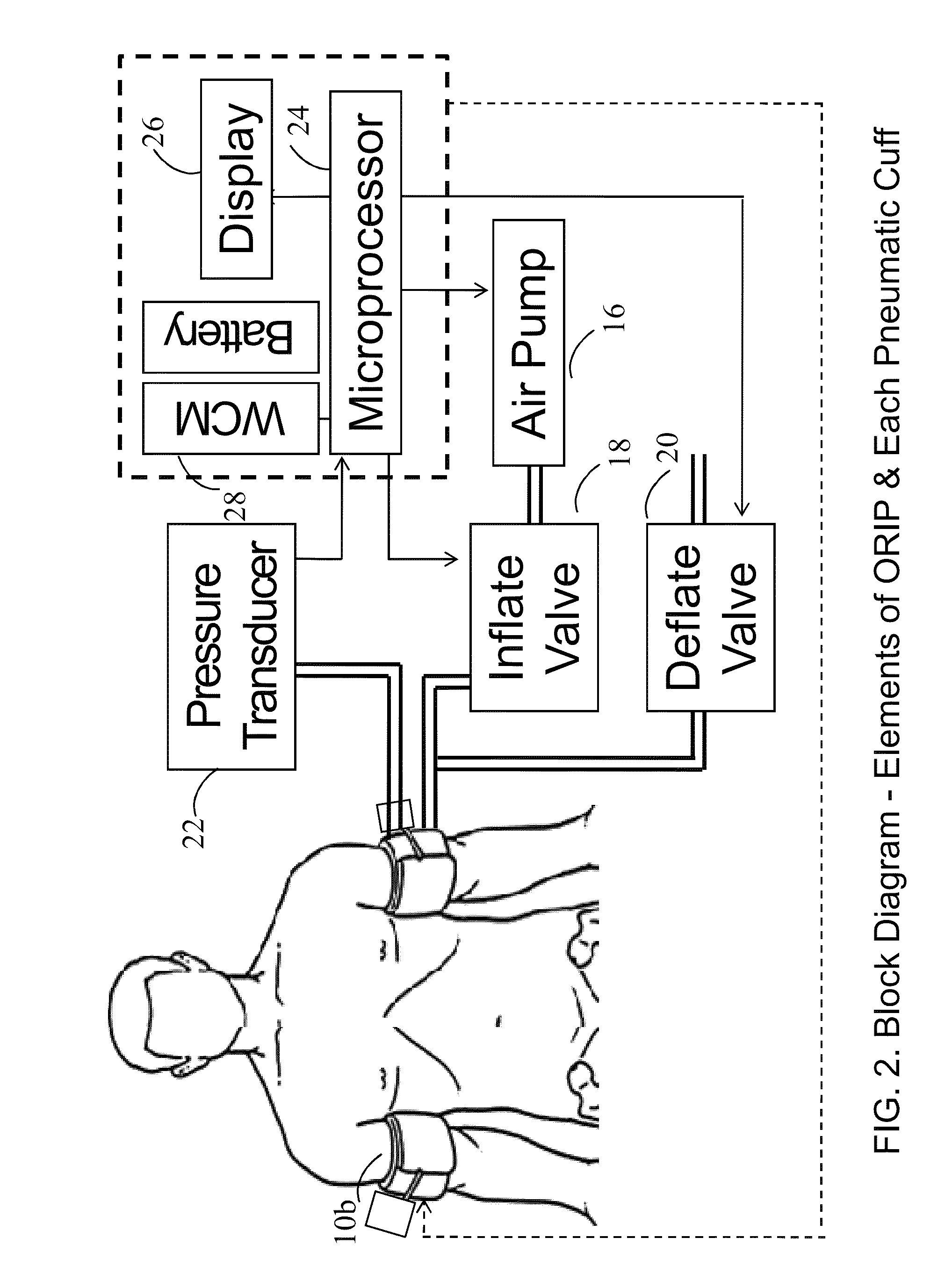
Methods and apparatus for optimal remote ischemic preconditioning (ORIP) for preventing ischemia-reperfusion injuries to organs
ActiveUS8911469B2Strong responsePreventing ischemia-reperfusionEvaluation of blood vesselsCatheterSurgical departmentHeart bypass
Ischemia-reperfusion injury commonly results from any surgical procedure requiring stopping of blood supply to an organ followed by reperfusion such as in heart bypass, angioplasty or organ transplant. The invention discloses a method to harness the innate power of repetitive transient ischemia in protecting organs against imminent ischemia-reperfusion, or any patho-physiological insults. This method of optimal remote ischemic preconditioning (ORIP) comprises of utilizing a pair of programmable pneumatic cuffs that inflate / deflate alternately occluding blood circulation to each of the limbs for pre-defined time intervals. The apparatus delivers maximal ORIP dose in shortest possible time either as an EMS procedure during patient transportation to hospital, as elective pre-surgery treatment, or in critical care for preventing multiple-organ-dysfunction-syndrome. ORIP can be self-administered and remotely monitored by clinician especially in chronic patients for homeostasis of malfunctioning target organs. ORIP may also be deployed as adjunct in angioplasty, gene / stem cell heart repair therapies.
Owner:NEOCARDIUM
Implants and methods for treating inflammation-mediated conditions of the eye
InactiveUS8063031B2Increase ratingsSlowing exposureOrganic active ingredientsSenses disorderDexamethasoneOphthalmology
Methods for treating inflammation-mediated conditions of the eye are described, comprising: implanting into the vitreous of the eye of an individual a bioerodible implant comprising a steroidal anti-inflammatory agent and a bioerodible polymer, wherein the implant delivers the agent to the vitreous in an amount sufficient to reach a concentration equivalent to at least about 0.05 μg / ml dexamethasone within about 48 hours and maintains a concentration equivalent to at least about 0.03 μg / ml dexamethasone for at least about three weeks.
Owner:ALLERGAN INC
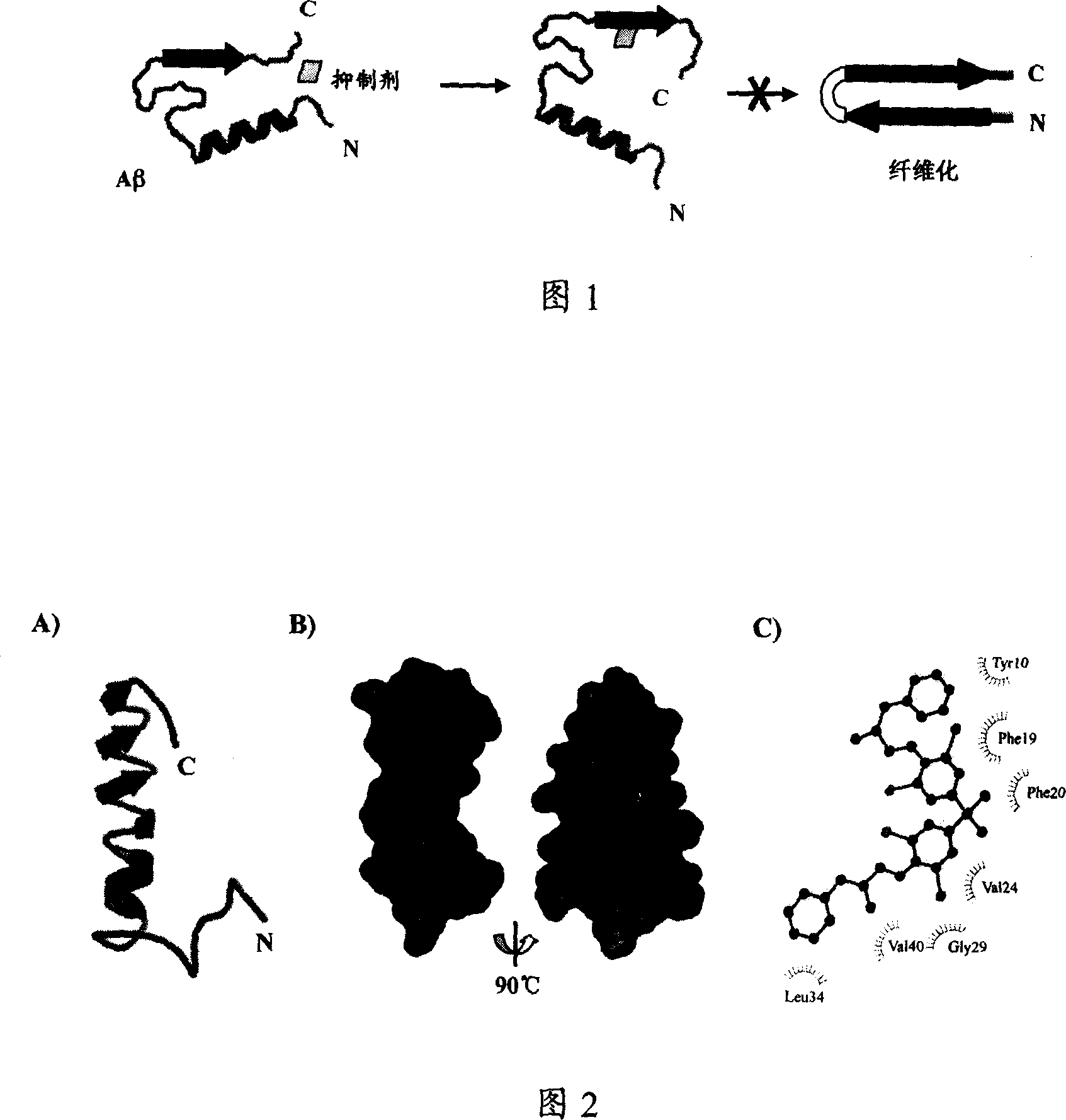
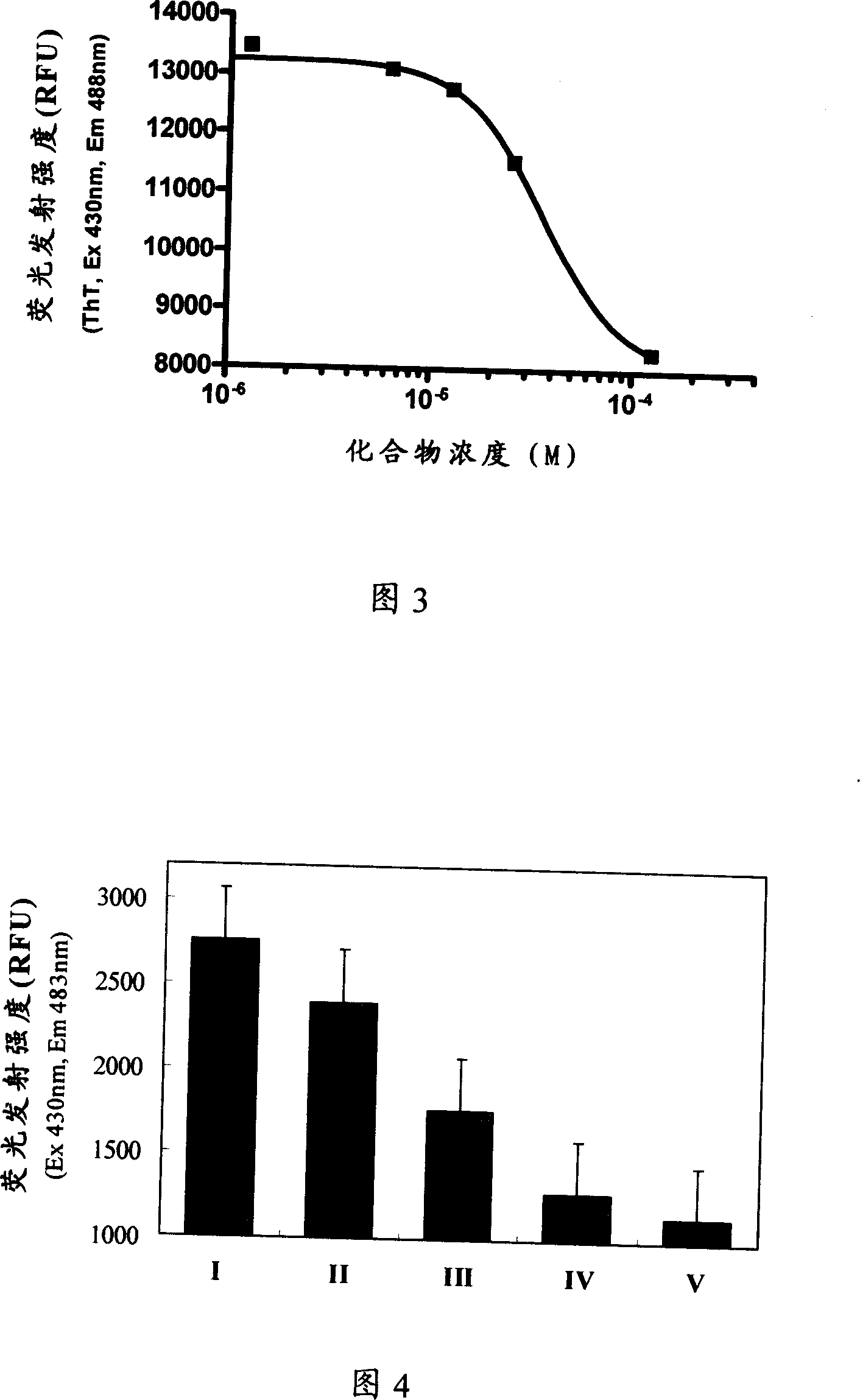
Small molecule inhibitor for preventing Alzheimer's disease Abeta polypeptide from fiberizing and its preparation method, pharmaceutical composition and application
A compound for inhibiting beta-starch protein aggregation and fibrillation or its medicinal accepted slat, composition, its production and use in prevention and treatment of Alzheimer's disease are disclosed. The compound or medicinal accepted slat combines with Alpha-beta polypeptide and inhibits Alpha-beta polypeptide aggregation and fibrillation.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Charged nutritive proteins and methods
ActiveUS20130296231A1Sufficient amountImprove heat generationOrganic active ingredientsBacteriaNutritionTotal amino acids
Charged nutritive proteins are provided. In some embodiments the nutritive proteins an aqueous solubility of at least 12.5 g / L at pH 7. In some embodiments the nutritive proteins an aqueous solubility of at least 50 g / L at pH 7. In some embodiments the nutritive proteins an aqueous solubility of at least 100 g / L at pH 7. In some embodiments the nutritive proteins comprise at least one of a level of a) a ratio of branch chain amino acid residues to total amino acid residues present in the nutritive protein equal to or greater than the ratio of branch chain amino acid residues to total amino acid residues present in a benchmark protein; b) a ratio of leucine residues to total amino acid residues present in the nutritive protein equal to or greater than the ratio of leucine residues to total amino acid residues present in a benchmark protein; and c) a ratio of essential amino acid residues to total amino acid residues present in the nutritive protein equal to or greater than the ratio of essential amino acid residues to total amino acid residues present in a benchmark protein. Also provided are nucleic acids encoding the proteins, recombinant microorganisms that make the proteins, methods of making the proteins using recombinant microorganisms, compositions that comprise the proteins, and methods of using the proteins, among other things.
Owner:AXCELLA HEALTH INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Aryl [a] indole [2,3-g] quinolizine compound as well as preparation method, pharmaceutical composition and application thereof Aryl [a] indole [2,3-g] quinolizine compound as well as preparation method, pharmaceutical composition and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4455fa22-256c-4bbc-a9a0-2e2e27785703/BDA00002149557400041.PNG)
![Aryl [a] indole [2,3-g] quinolizine compound as well as preparation method, pharmaceutical composition and application thereof Aryl [a] indole [2,3-g] quinolizine compound as well as preparation method, pharmaceutical composition and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4455fa22-256c-4bbc-a9a0-2e2e27785703/BDA00002149557400071.PNG)
![Aryl [a] indole [2,3-g] quinolizine compound as well as preparation method, pharmaceutical composition and application thereof Aryl [a] indole [2,3-g] quinolizine compound as well as preparation method, pharmaceutical composition and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4455fa22-256c-4bbc-a9a0-2e2e27785703/BDA00002149557400081.PNG)